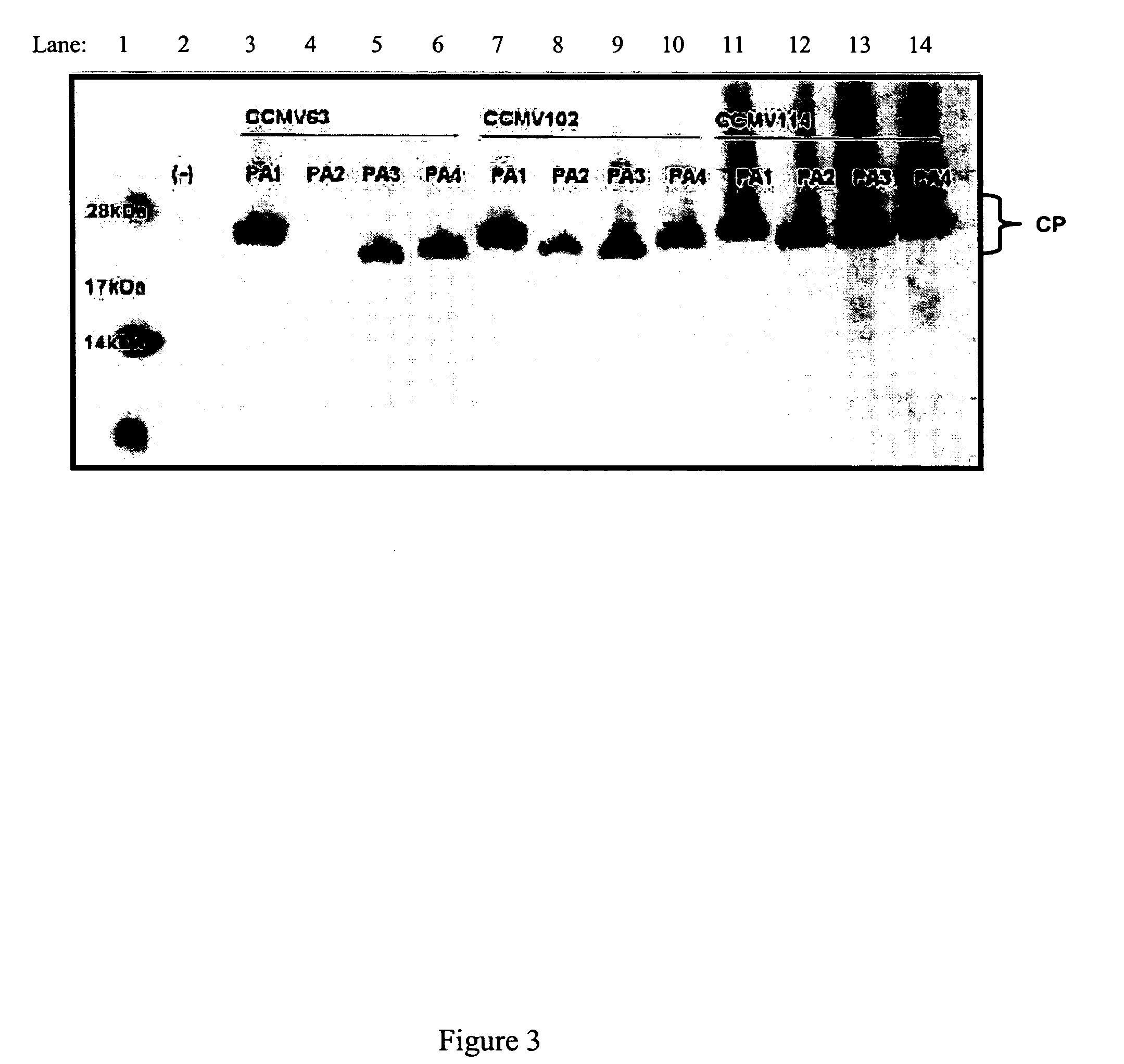
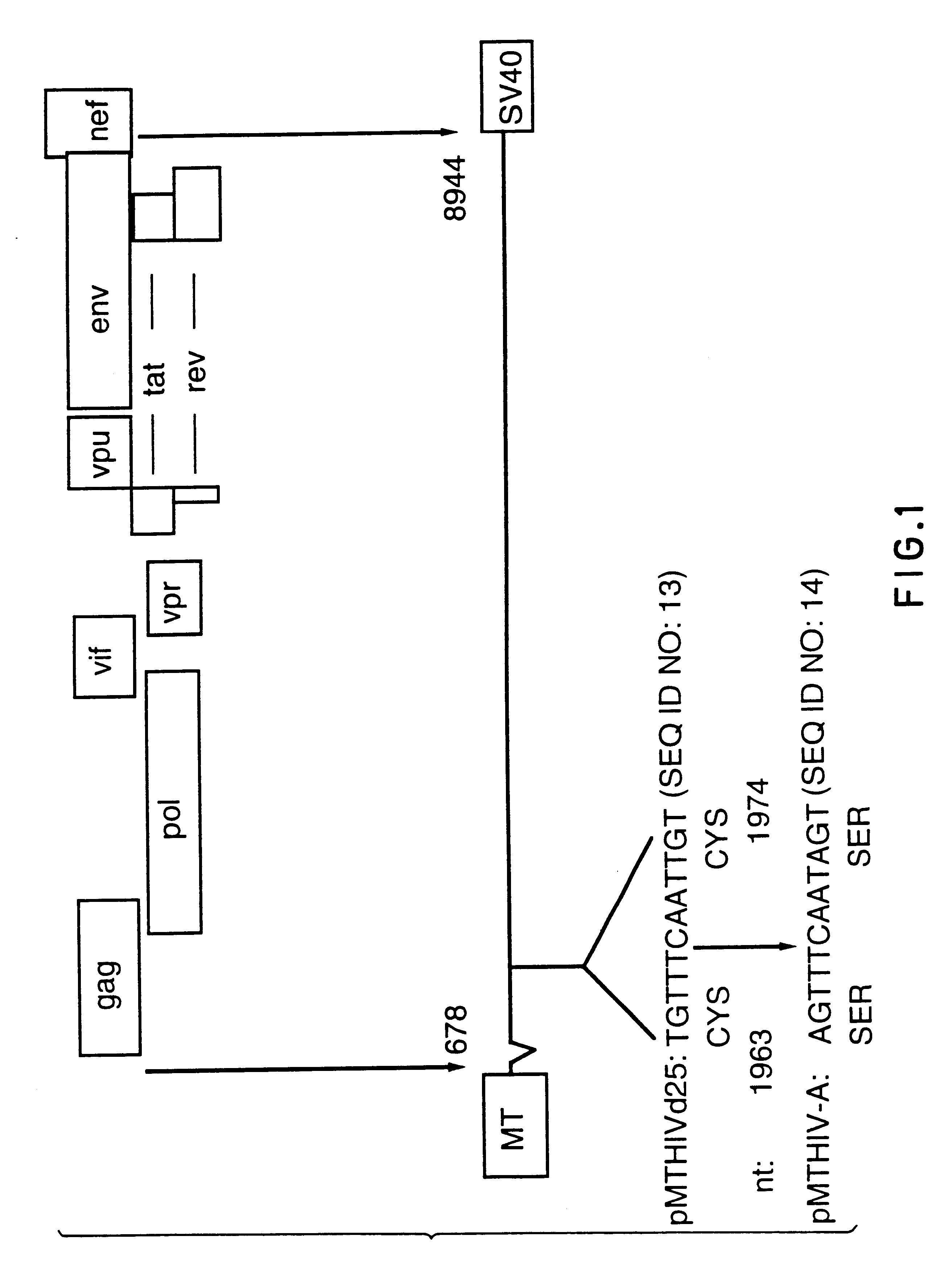
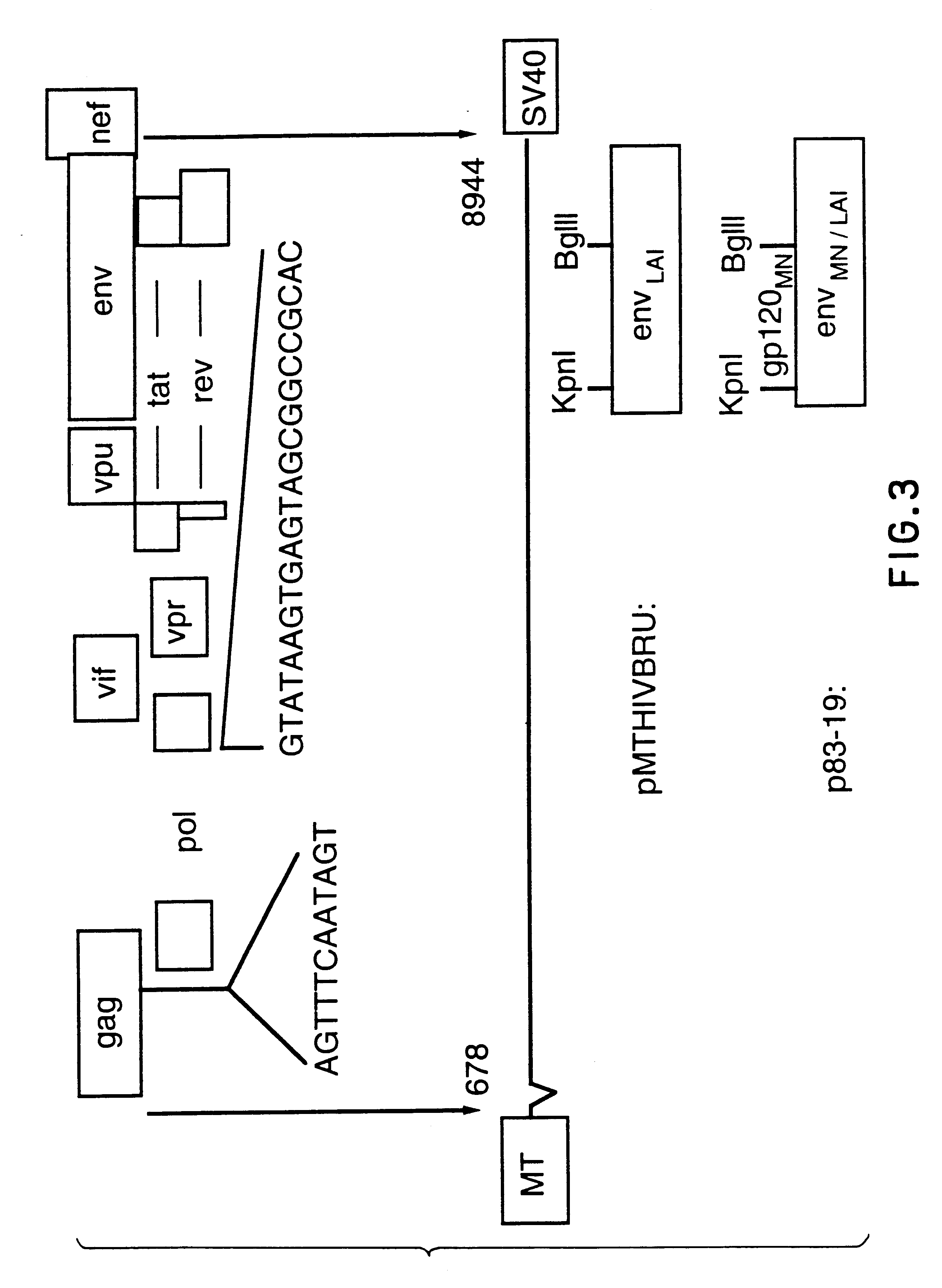
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
176 results about "Non infectious" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of non-infectious in English: non-infectious. adjective. 1(of a disease or disease-causing organism) not liable to be transmitted through the environment.
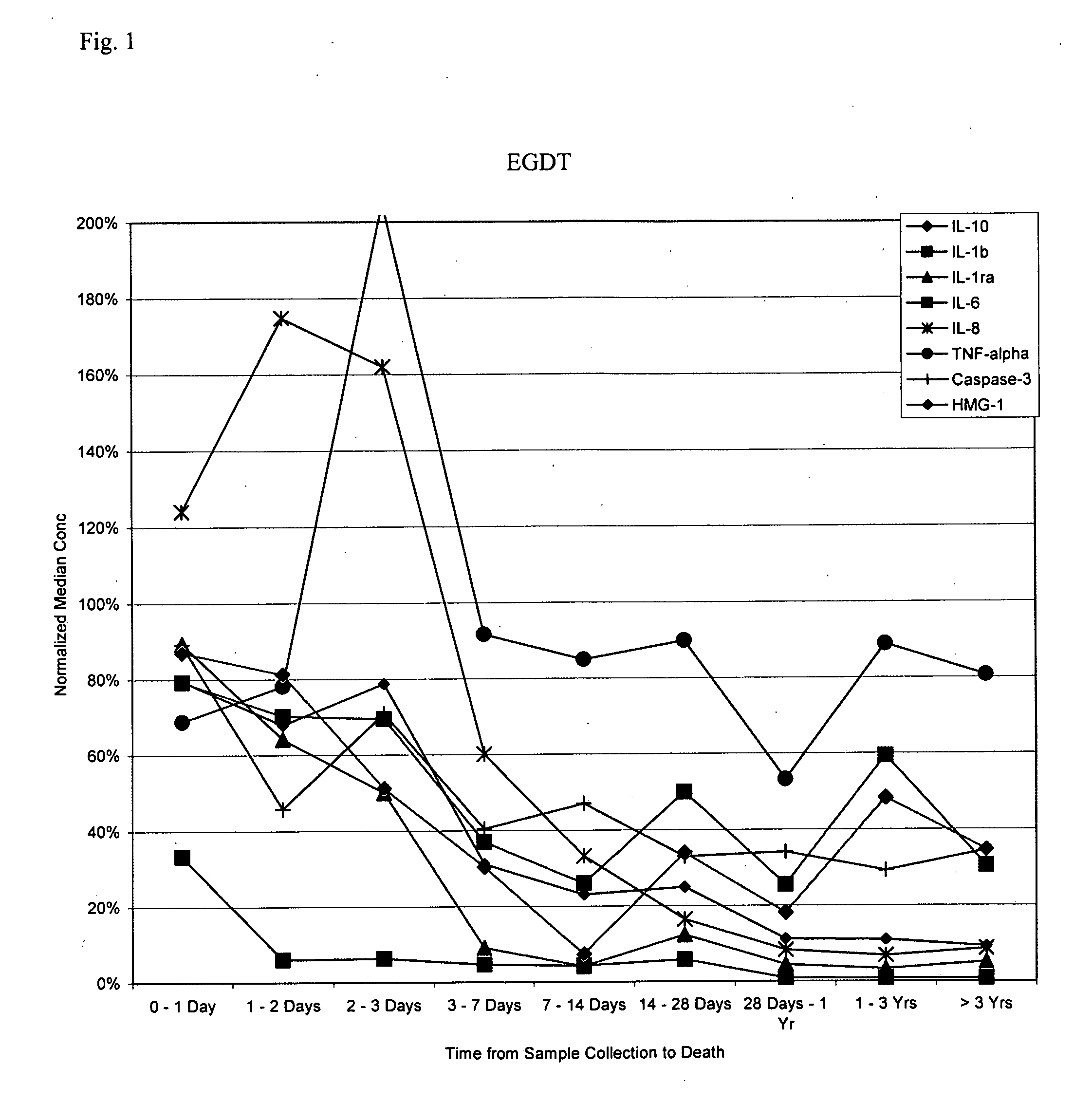
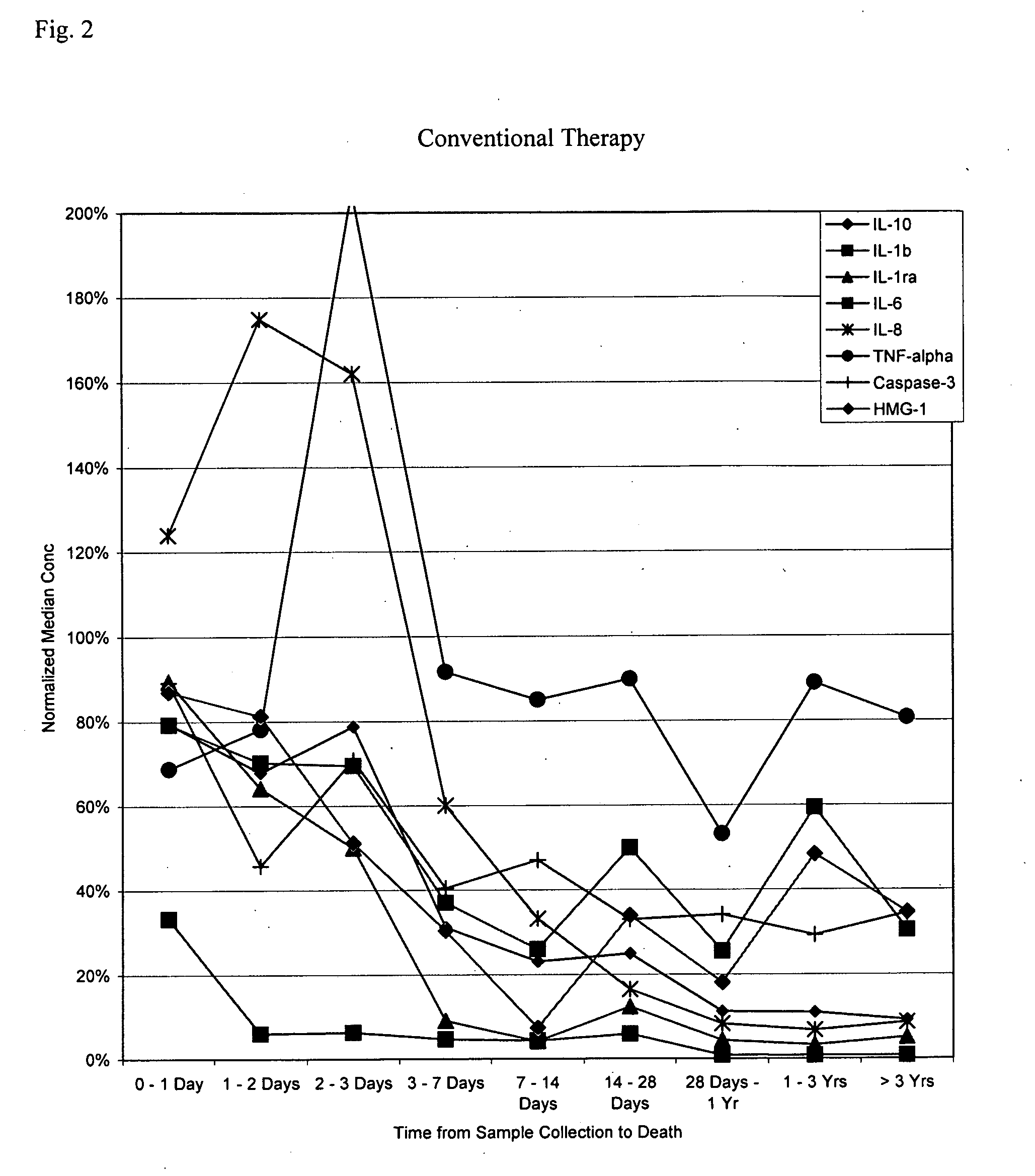
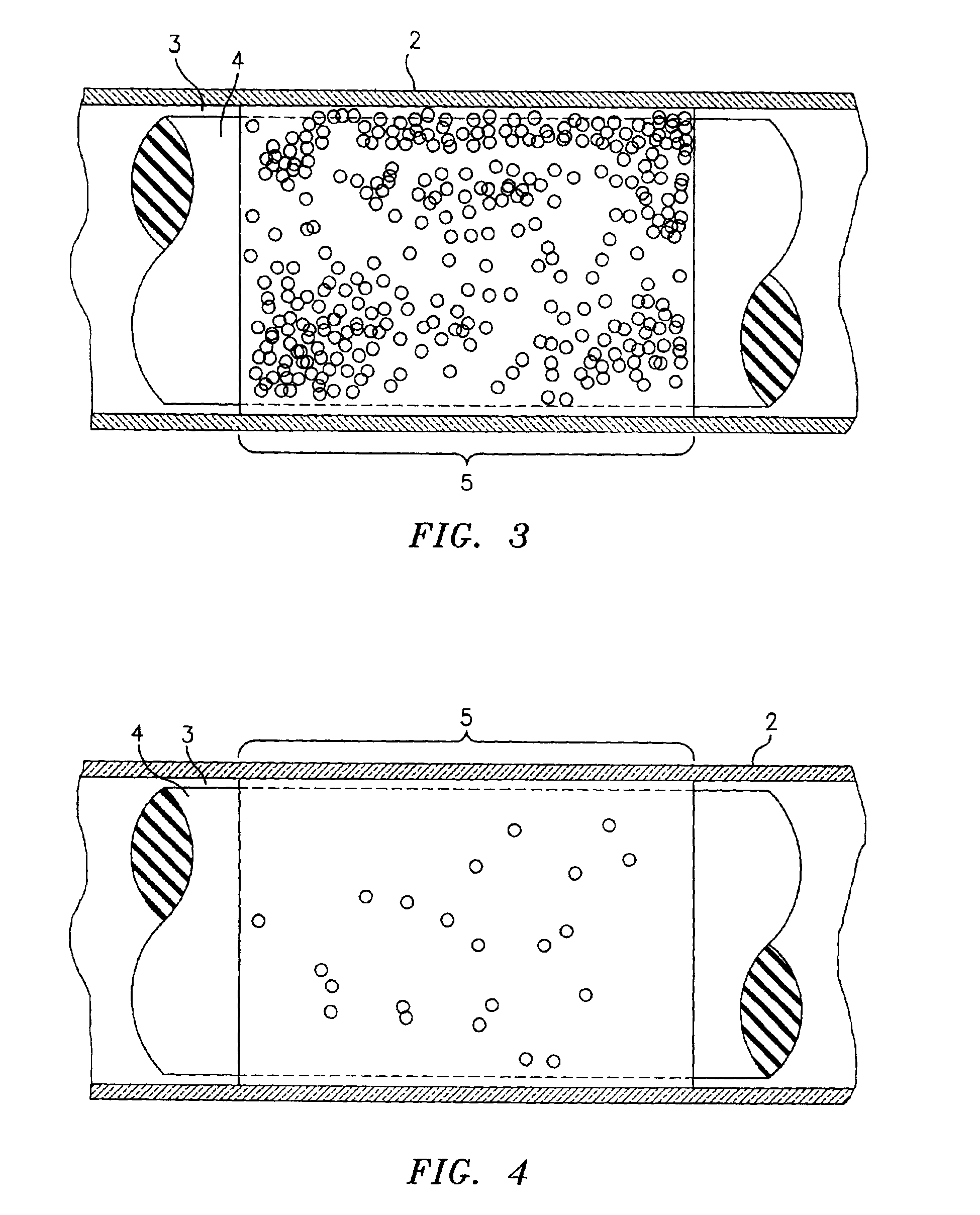
Methods and compositions for determining treatment regimens in systemic inflammatory response syndromes
InactiveUS20050148029A1Improve discriminationMicrobiological testing/measurementDisease diagnosisMedicineWhole body
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to methods and compositions selected to rule in or out SIRS, or for differentiating sepsis, severe sepsis, septic shock and / or MODS from each other and / or from non-infectious SIRS.
Owner:BIOSITE INC
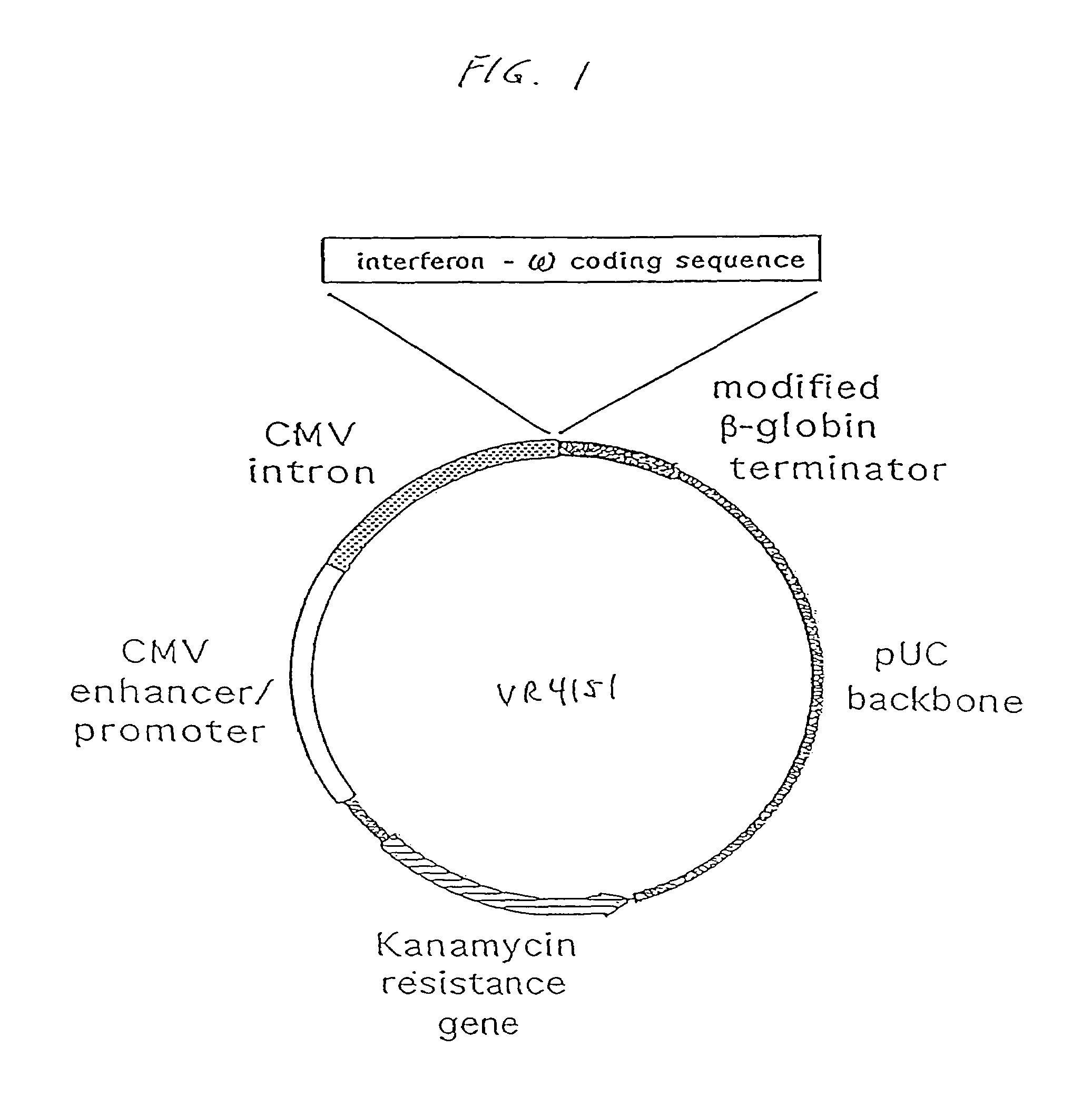
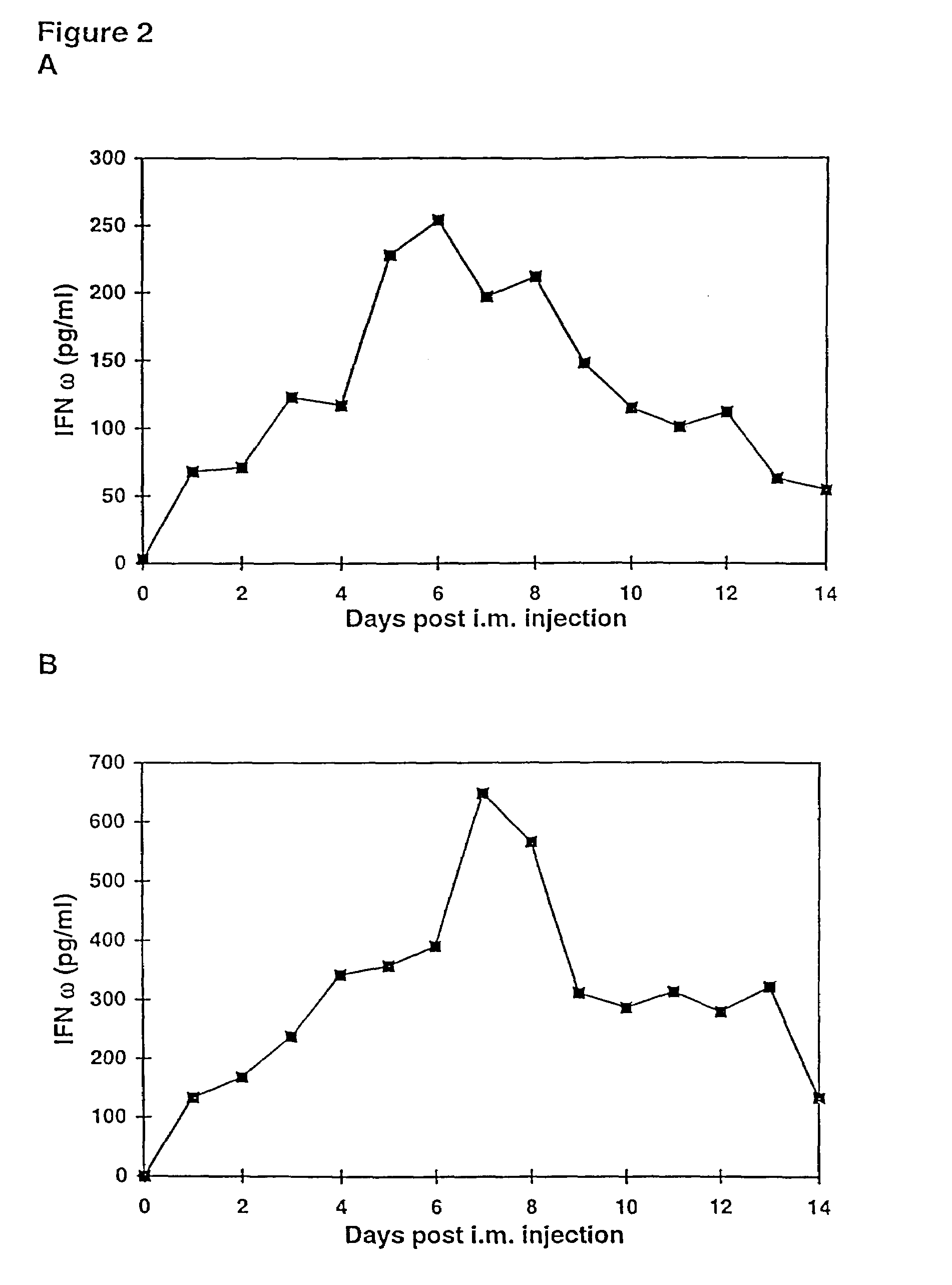
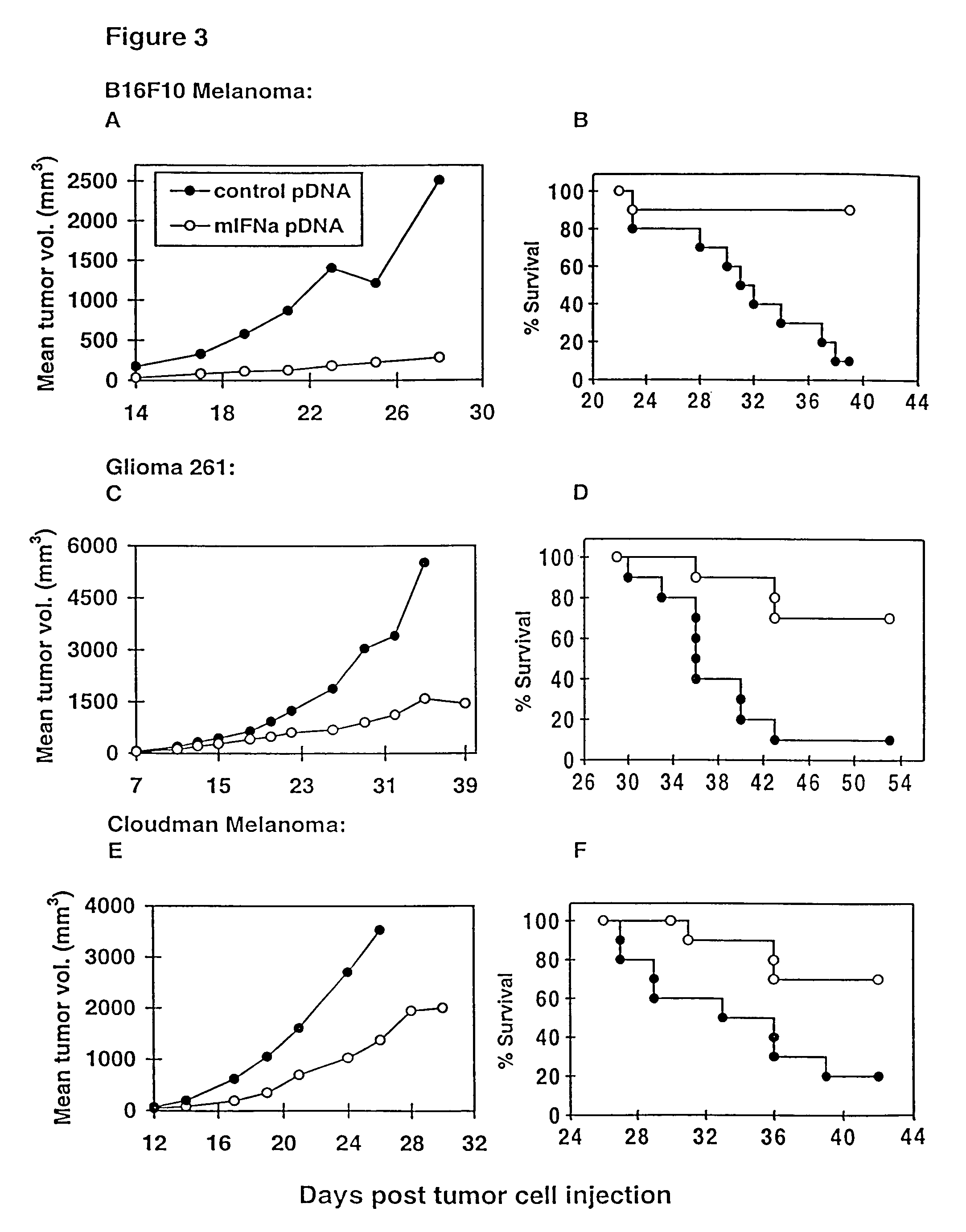
Methods for treating cancer using cytokine-expressing polynucleotides
InactiveUS7268120B1Improved in vivo polypeptide expressionMinimizing adverse side effectBiocideOrganic active ingredientsMammalSodium phosphates
The present invention provides a pharmaceutical composition, comprising a non-infectious, non-integrating polynucleotide construct comprising a polynucleotide encoding an interferon ω and one or more cationic compounds. The present invention also provides methods of treating cancer in a mammal, comprising administering into a muscle of the mammal a non-infectious, non-integrating DNA polynucleotide construct comprising a polynucleotide encoding a cytokine. In addition, the present invention also relates to the methodology for selective transfection of malignant cells with polynucleotides expressing therapeutic or prophylactic molecules in intra-cavity tumor bearing mammals. More specifically, the present invention provides a methodology for the suppression of an intra-cavity dissemination of malignant cells, such as intraperitoneal dissemination. Furthermore, the invention relates to compositions and methods to deliver polynucleotides encoding polypeptides to vertebrate cells in vivo, where the composition comprises an aqueous solution of sodium phosphate.
Owner:VICAL INC
Methods and compositions for diagnosis and /or prognosis in systemic inflammatory response syndromes
InactiveUS20070092911A1Improve discriminationDisease diagnosisBiological testingWhole bodyDifferential diagnosis
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to methods and compositions selected to rule in or out SIRS, or for differentiating sepsis, severe sepsis, septic shock and / or MODS from each other and / or from non-infectious SIRS.
Owner:BIOSITE INC
Methods and compositions for the diagnosis of sepsis
InactiveUS20050164238A1Improve discriminationMicrobiological testing/measurementDisease diagnosisDifferential diagnosisNon infectious
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to methods and compositions selected to rule in or out SIRS, or for differentiating sepsis, severe sepsis, and / or septic shock from each other and / or from non-infectious SIRS.
Owner:BIOSITE INC
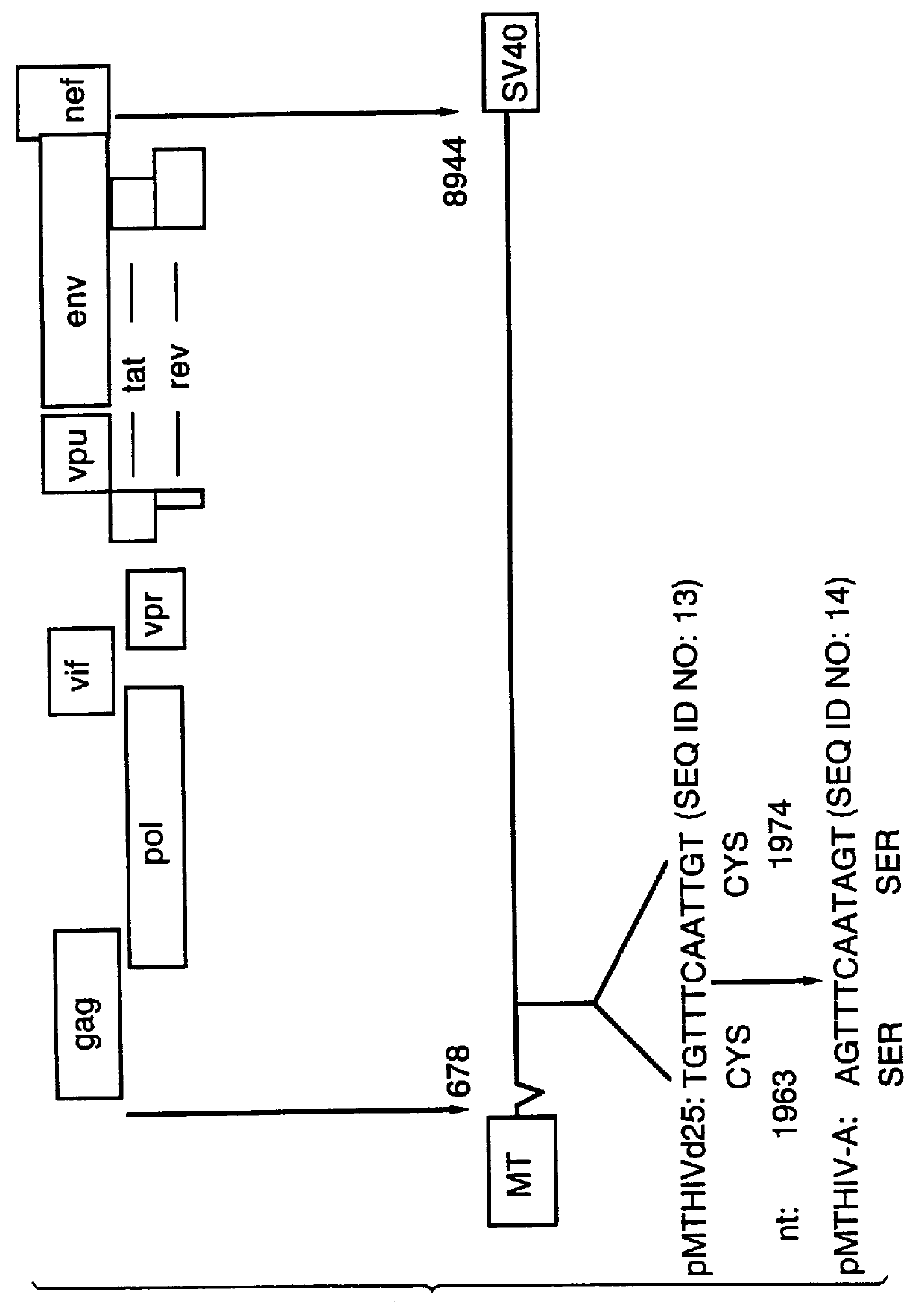
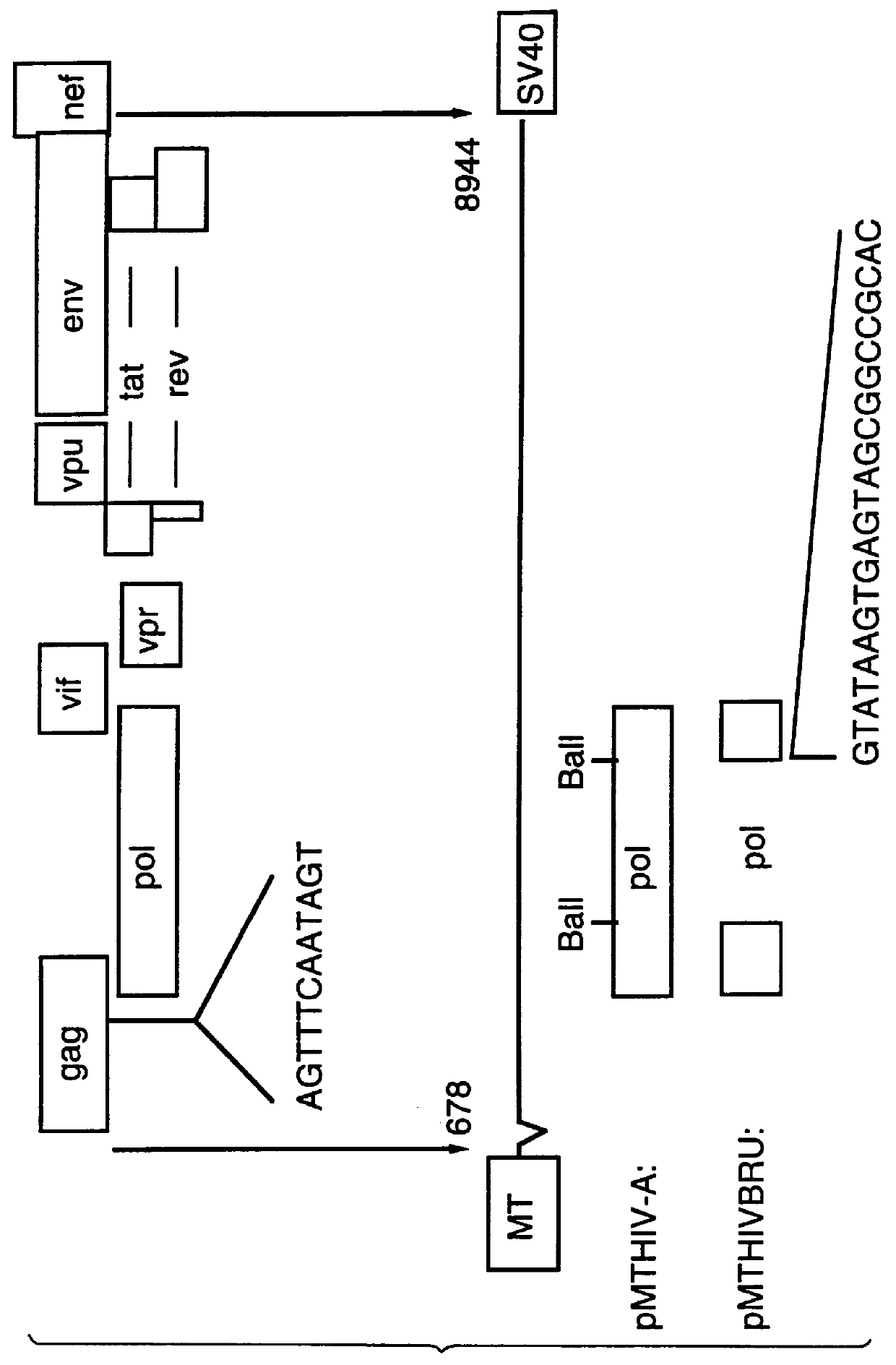
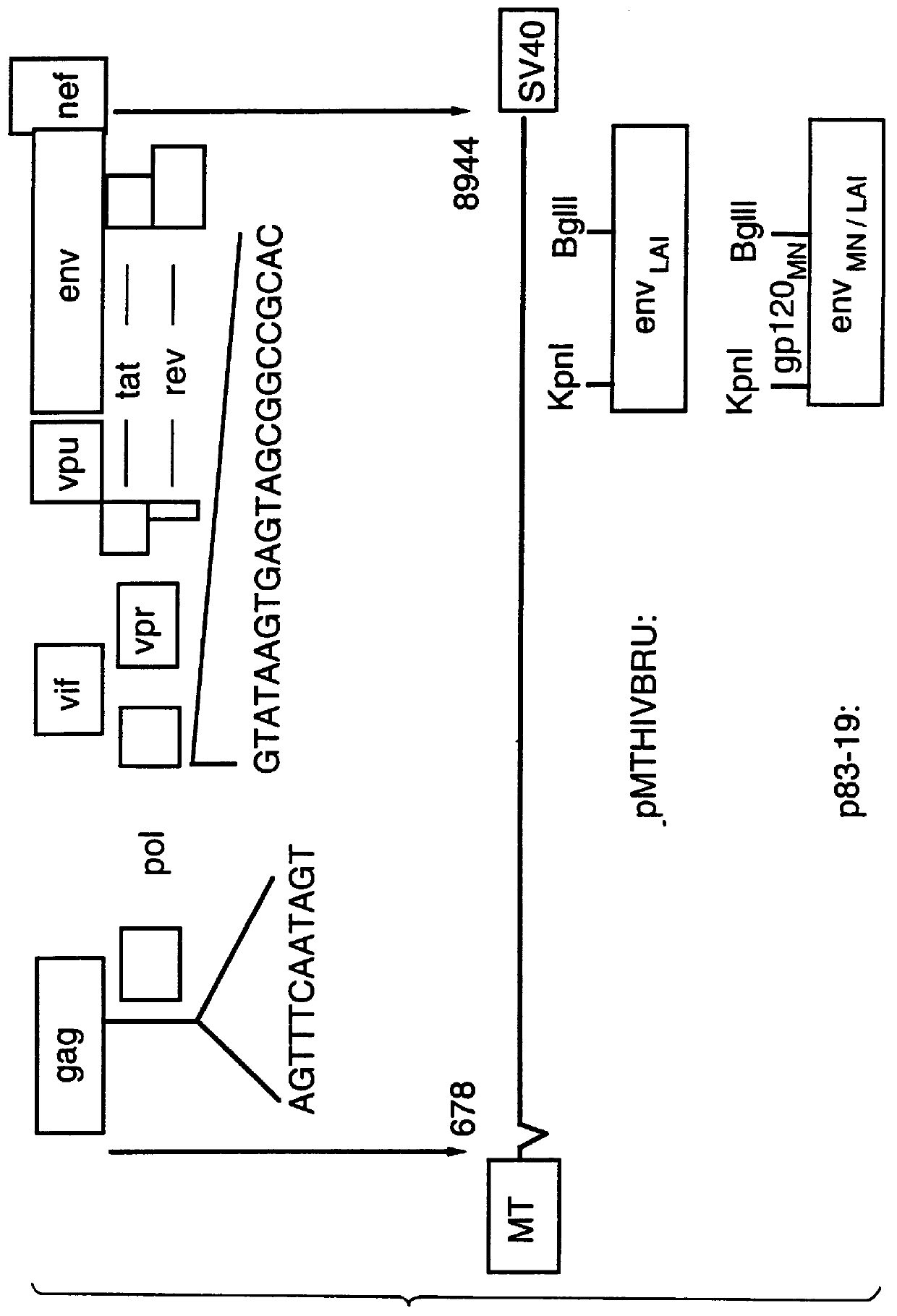
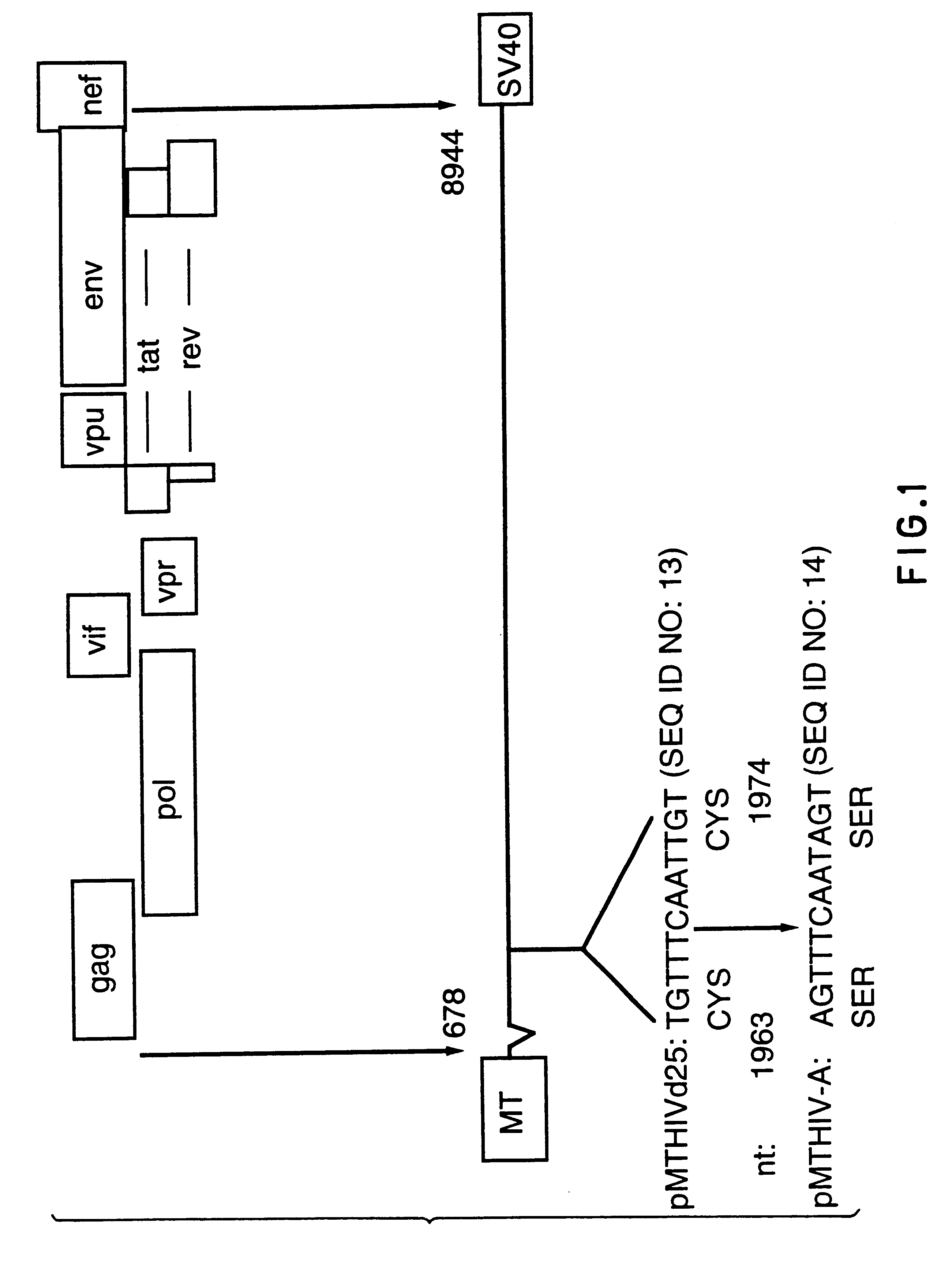
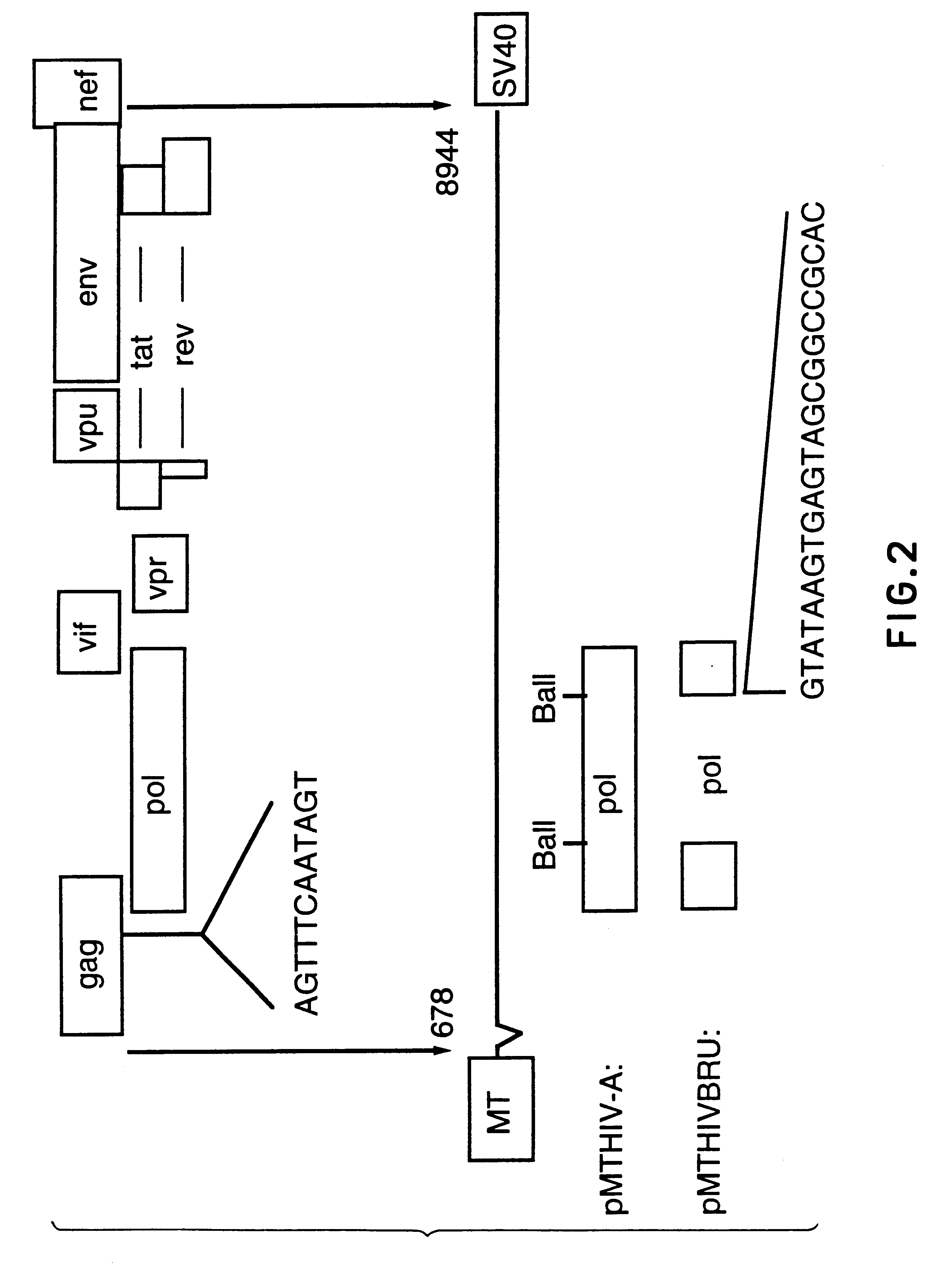
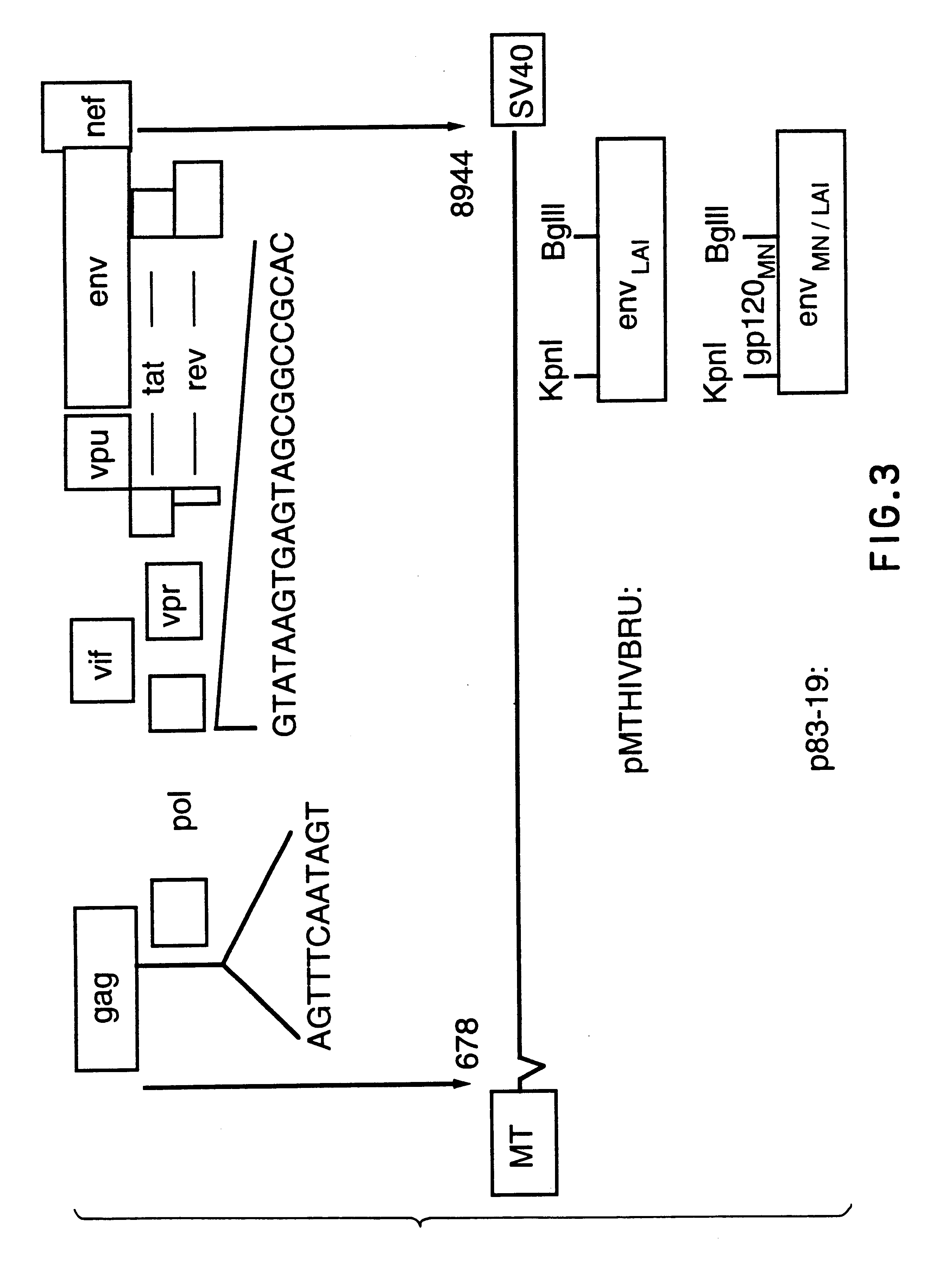
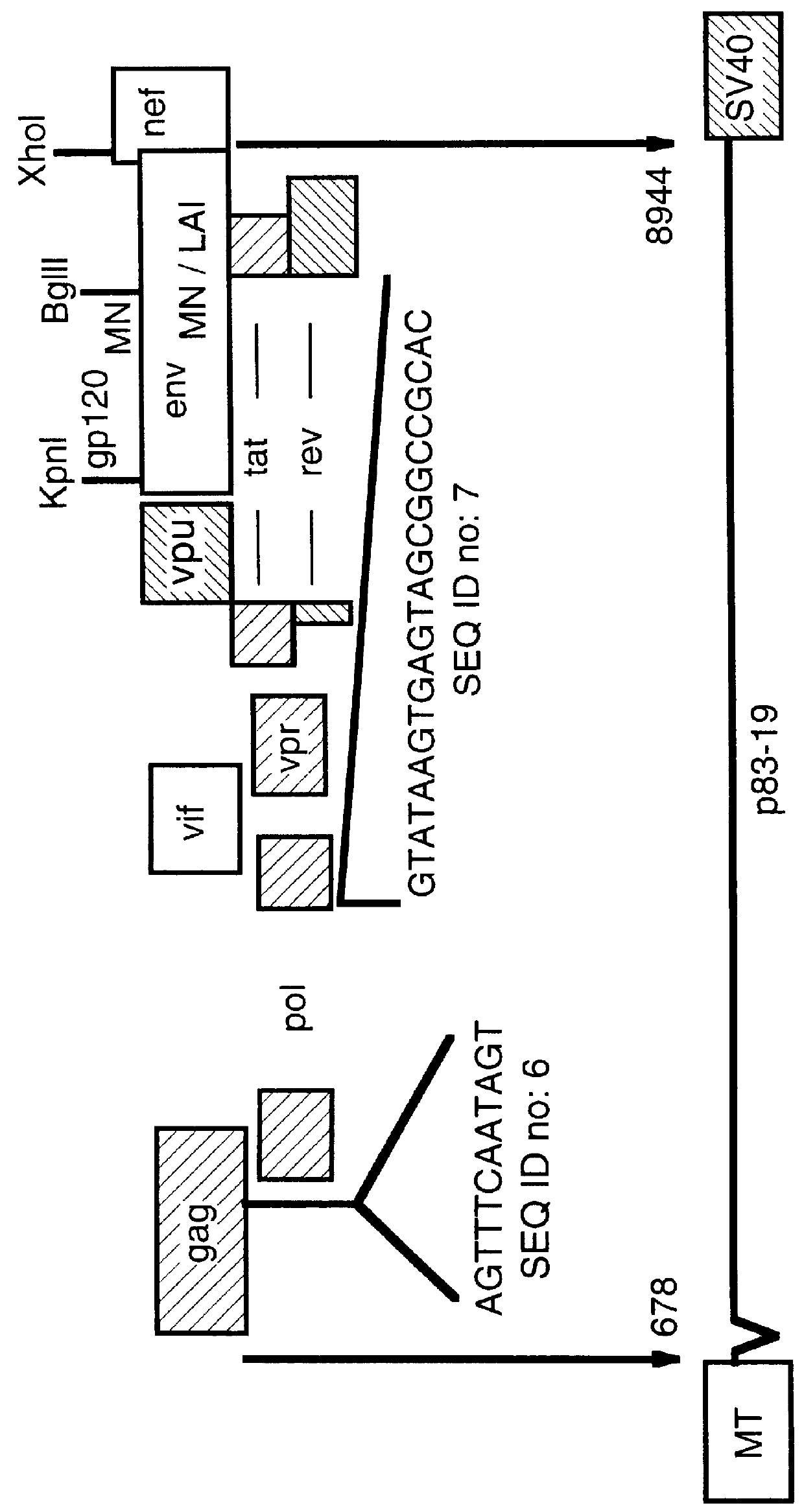
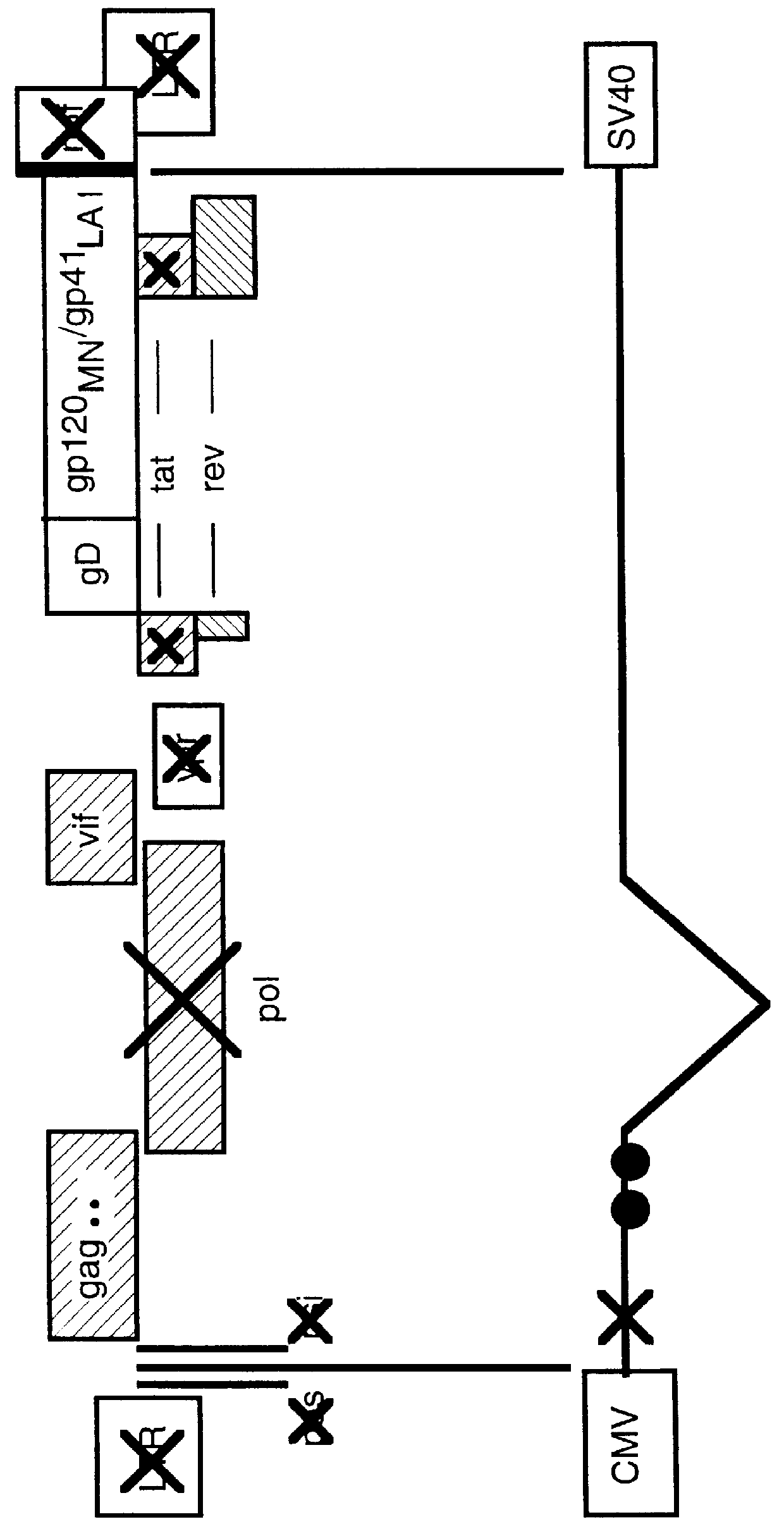
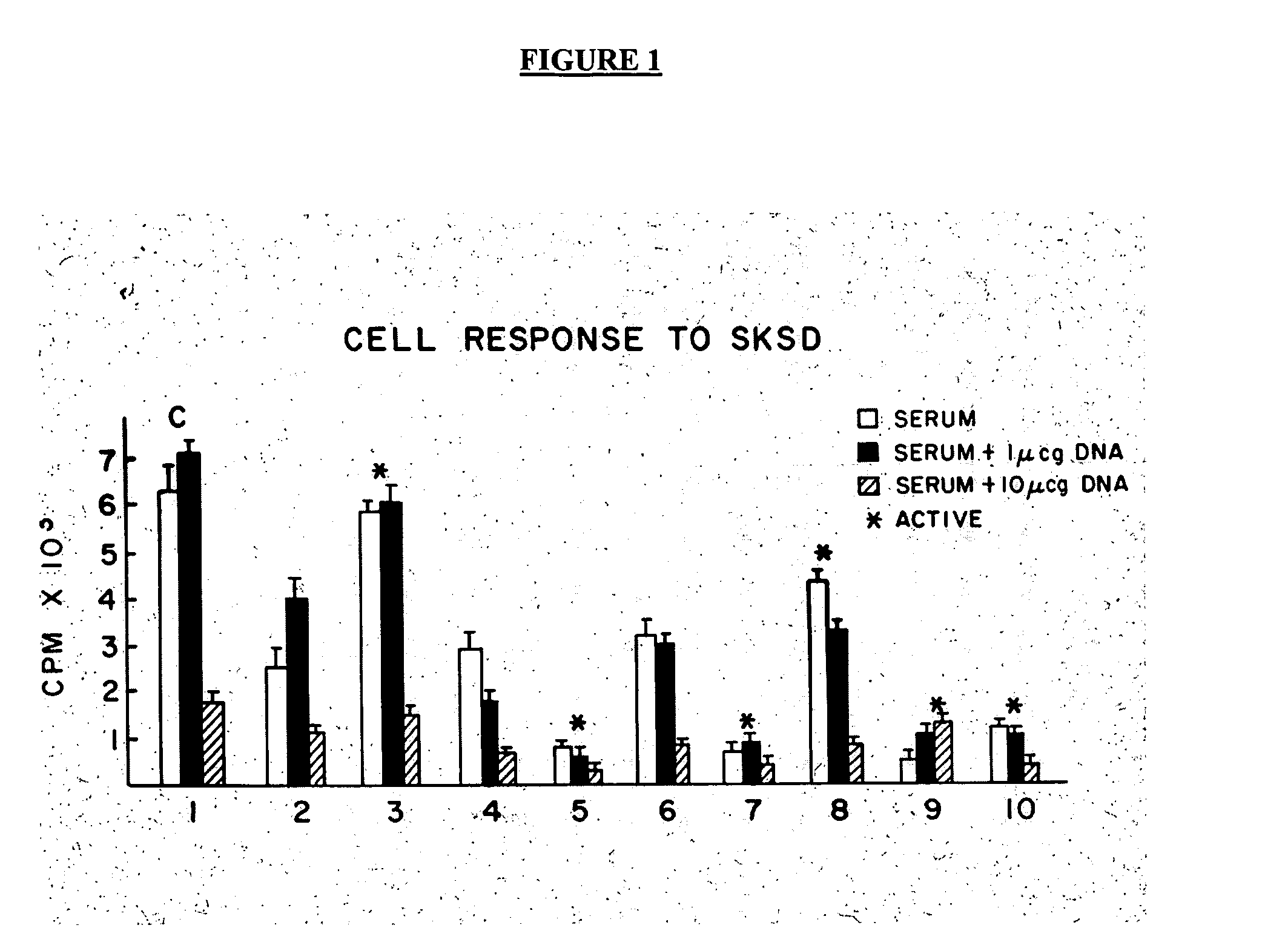
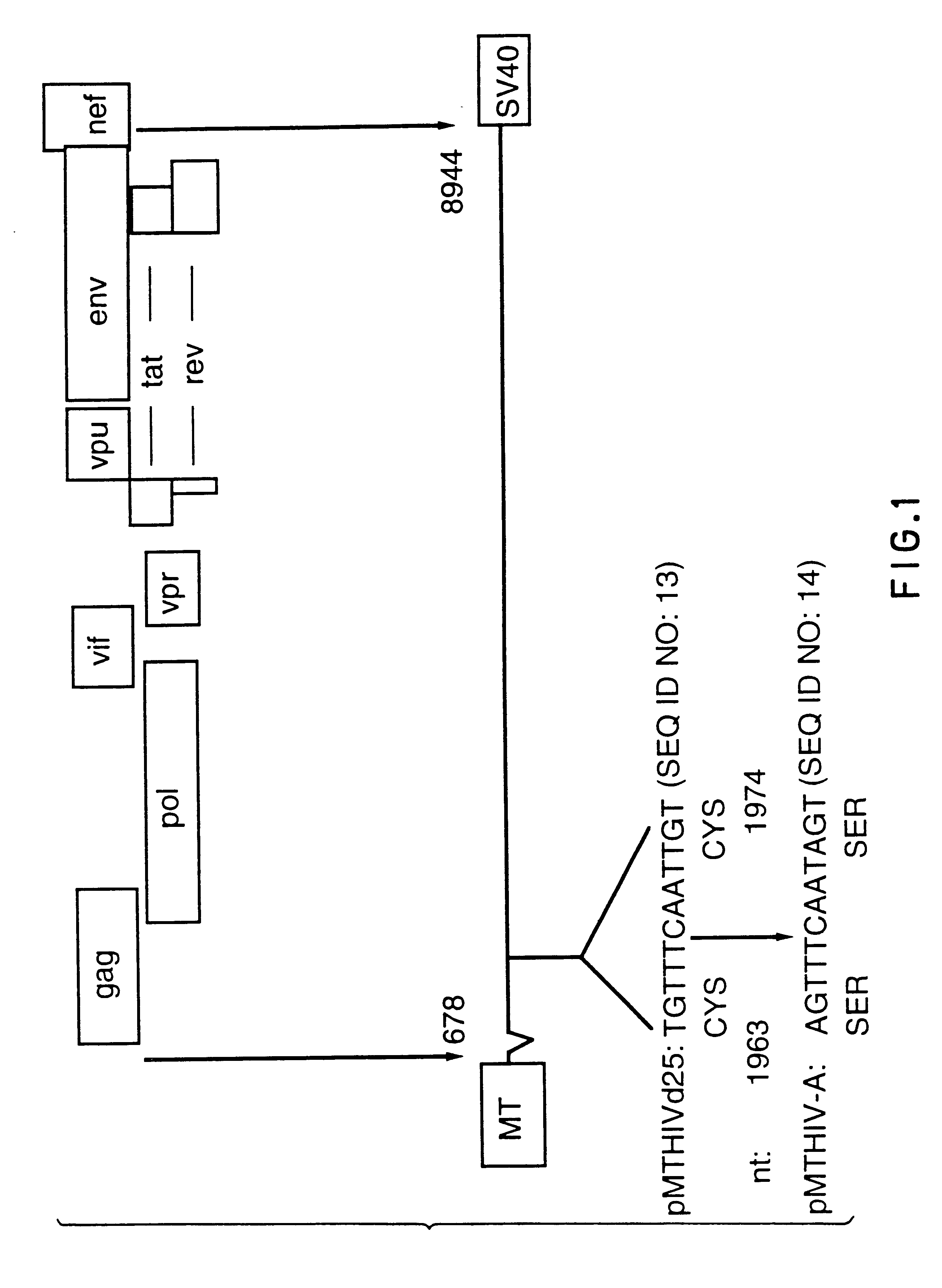
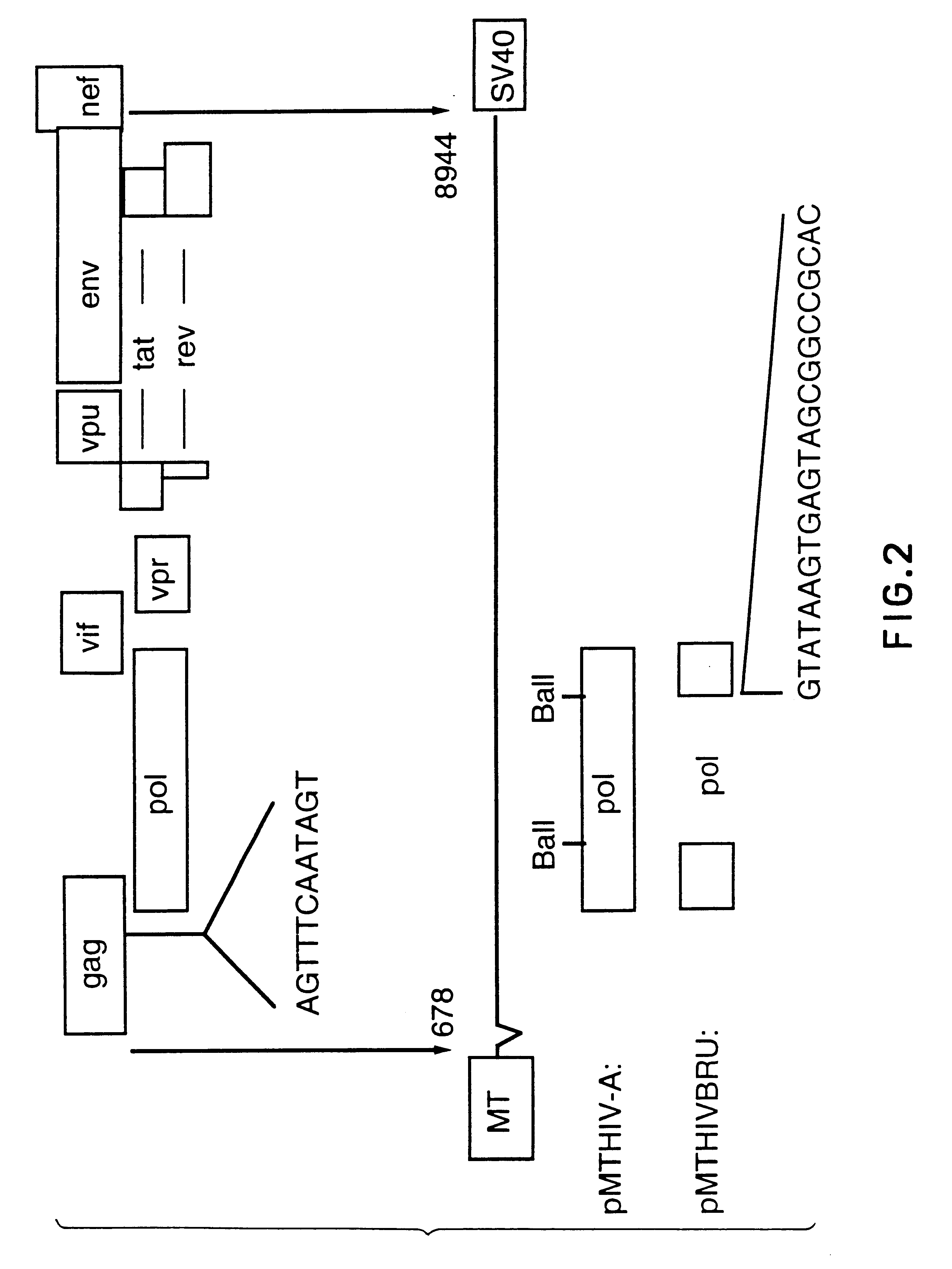
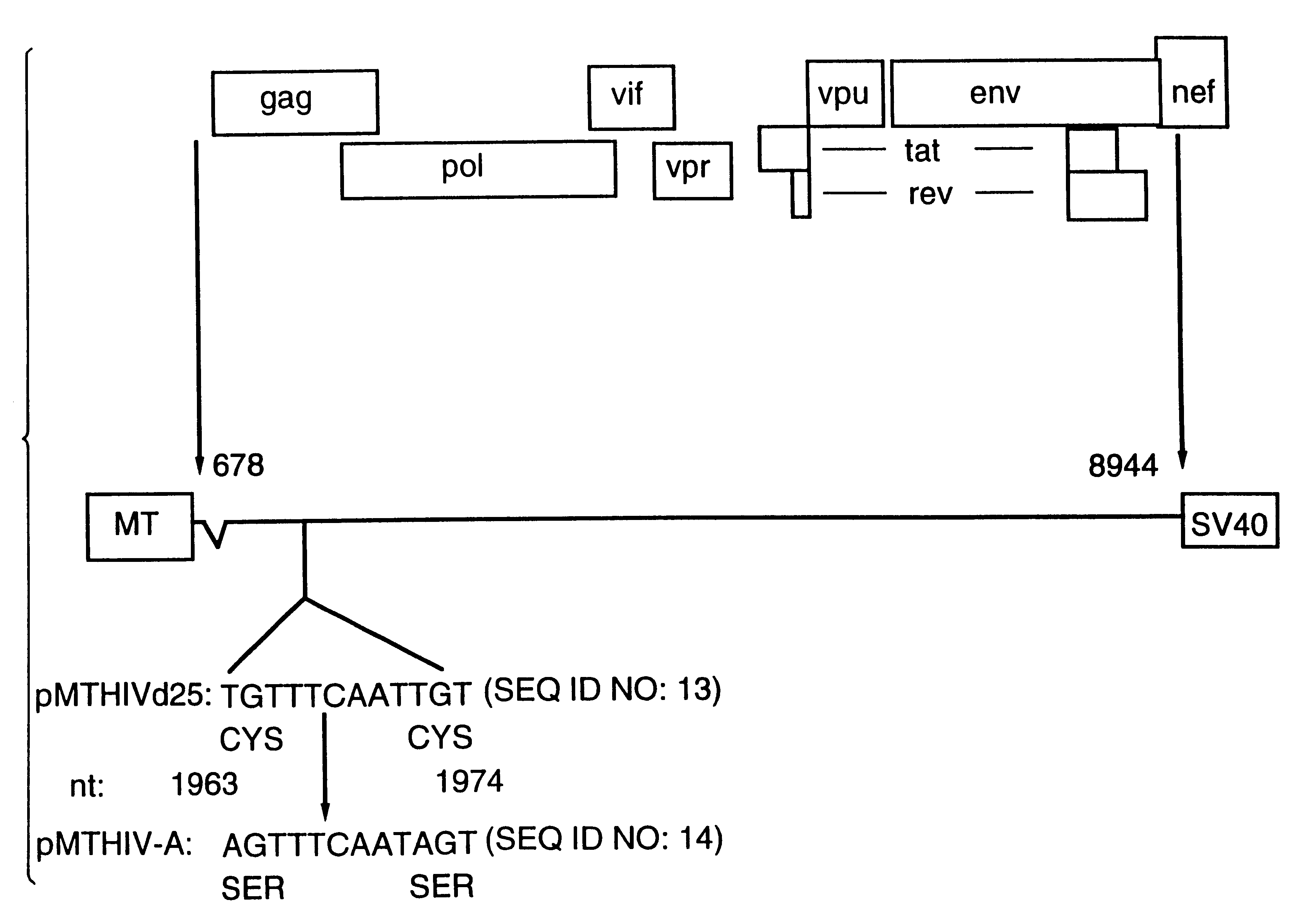
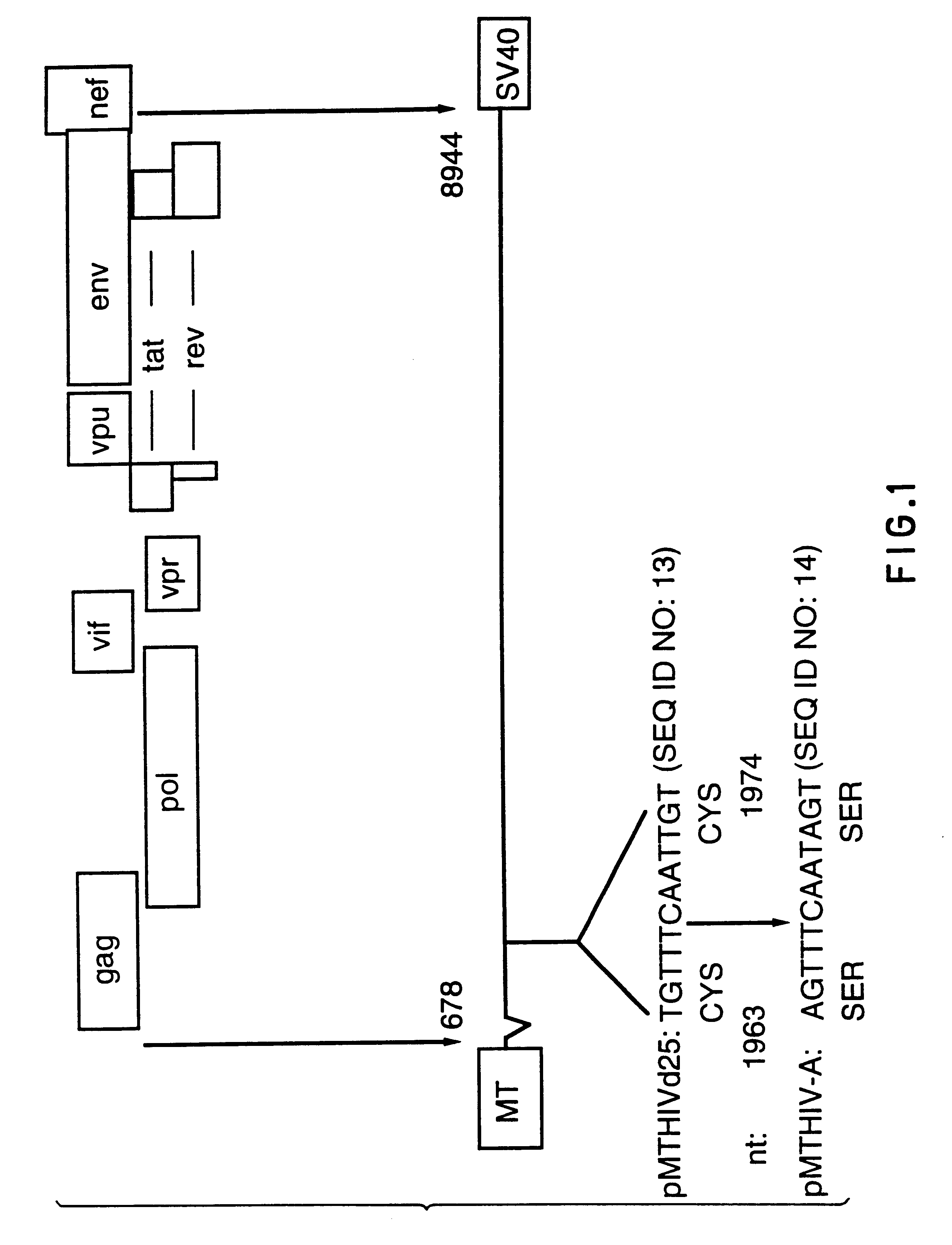
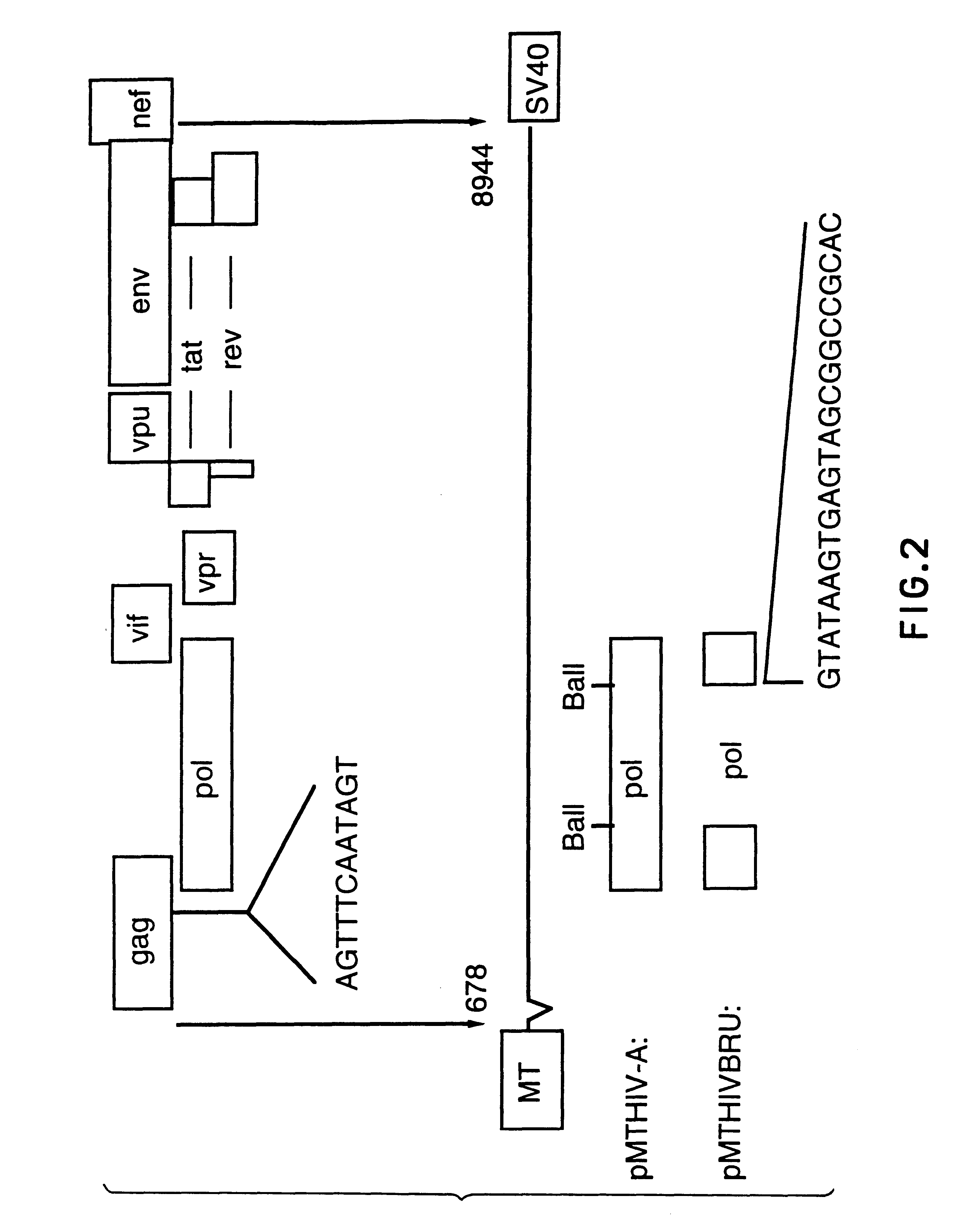
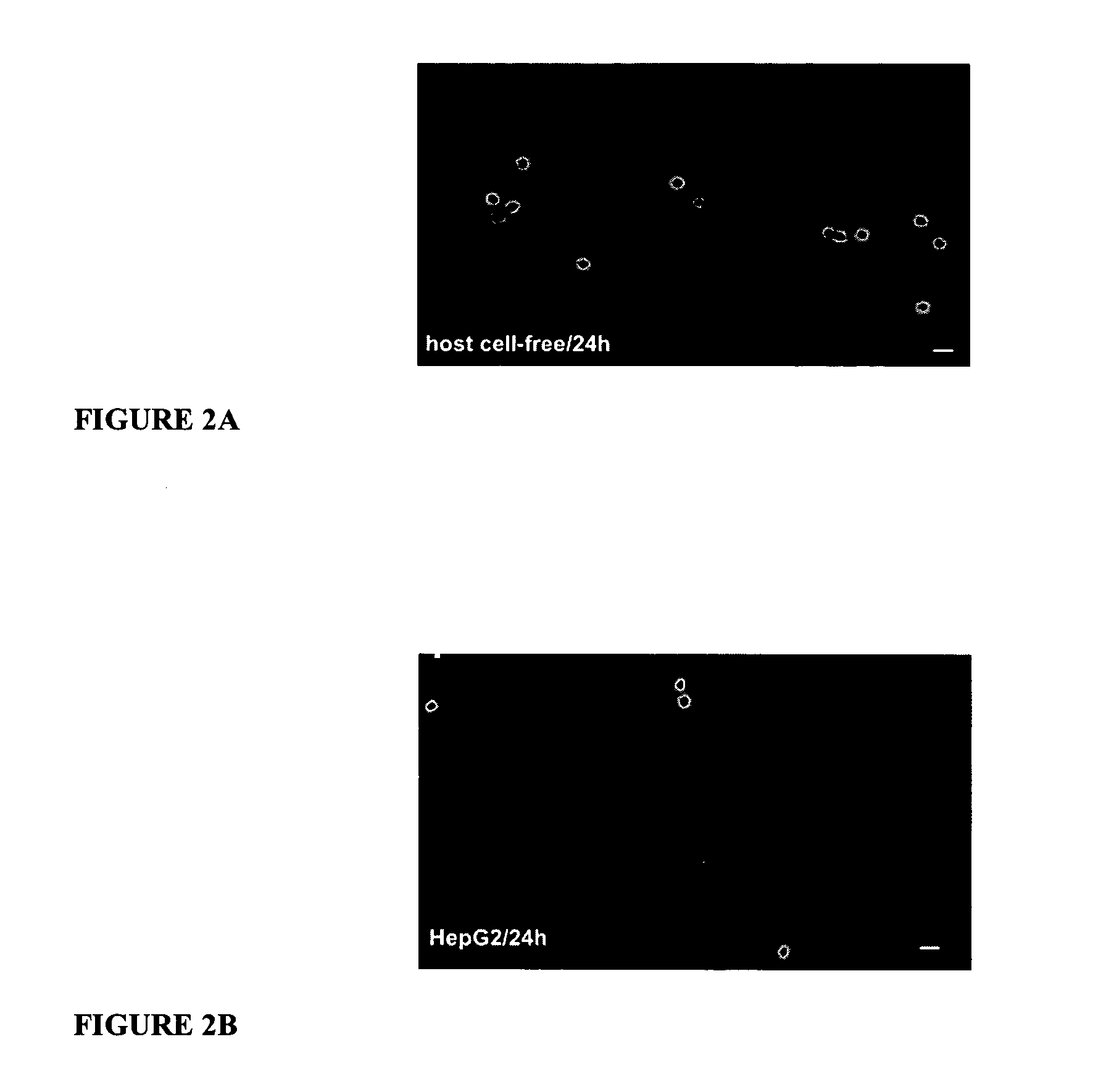
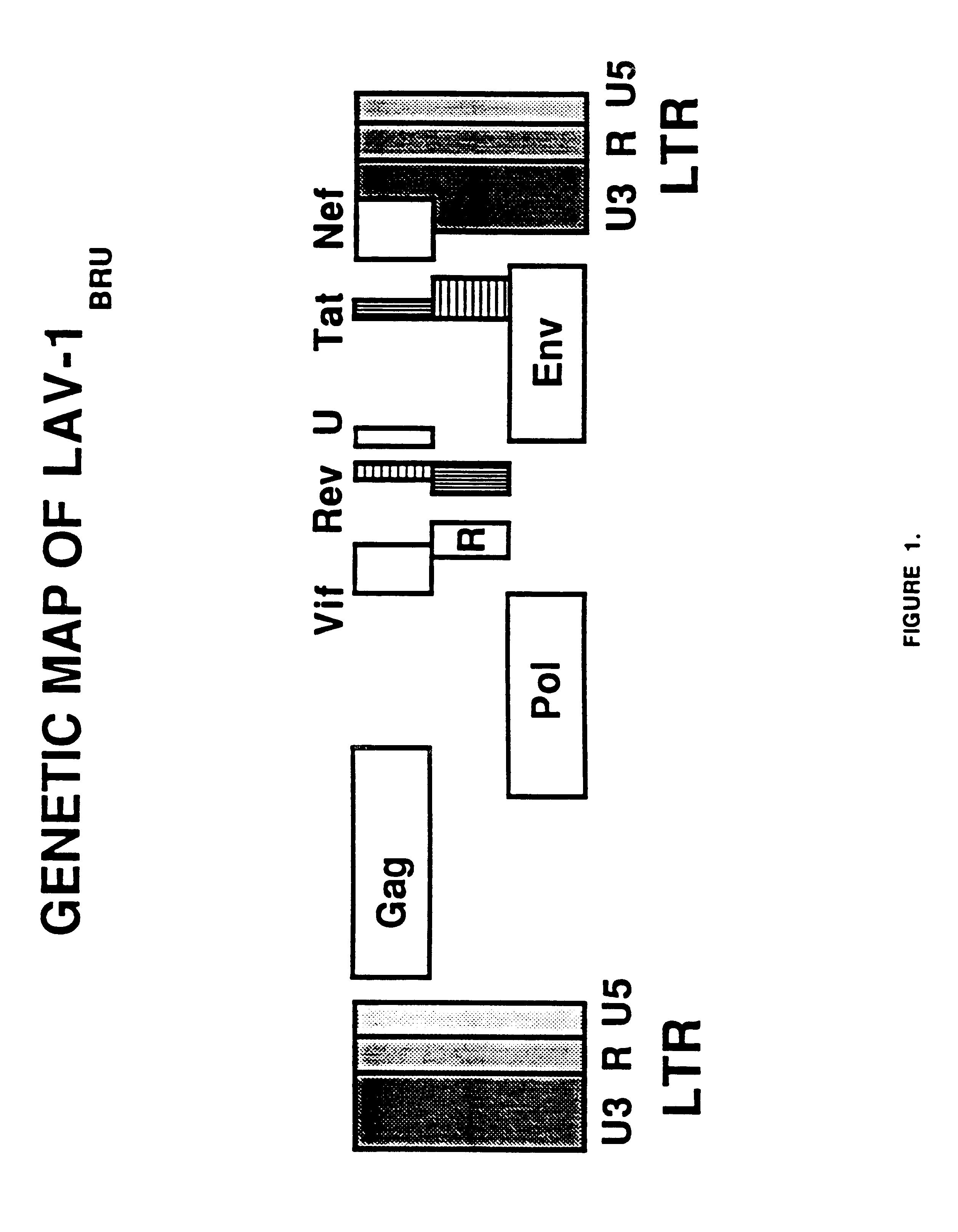
Human immunodeficiency virus type 1 nucleic acids devoid of long terminal repeats capable of encoding for non-infectious, immunogenic, retrovirus-like particles
InactiveUS6080408AImprove efficiencyLow backgroundSugar derivativesViral antigen ingredientsPol genesReverse transcriptase activity
Non-infectious, retrovirus-like particles contain mutations to reduce gag-dependent RNA-packaging of the gag gene product, eliminate reverse transcriptase activity of the pol gene product, eliminate integrase activity of the pol gene product and eliminate RNase H activity of the pol gene product through genetic manipulation of the gag and pol genes. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in diagnosis.
Owner:CONNAUGHT LAB
Methods to treat undesirable immune responses
InactiveUS6929796B1Reduced activityReduce the amount requiredBacterial antigen ingredientsPeptide/protein ingredientsViral vectorGene replacement therapy
Owner:MINNESOTA RGT UNIV OF A CORP OF MN
Antigenically-marked non-infectious retrovirus-like particles
InactiveUS6291157B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:CONNAUGHT LAB
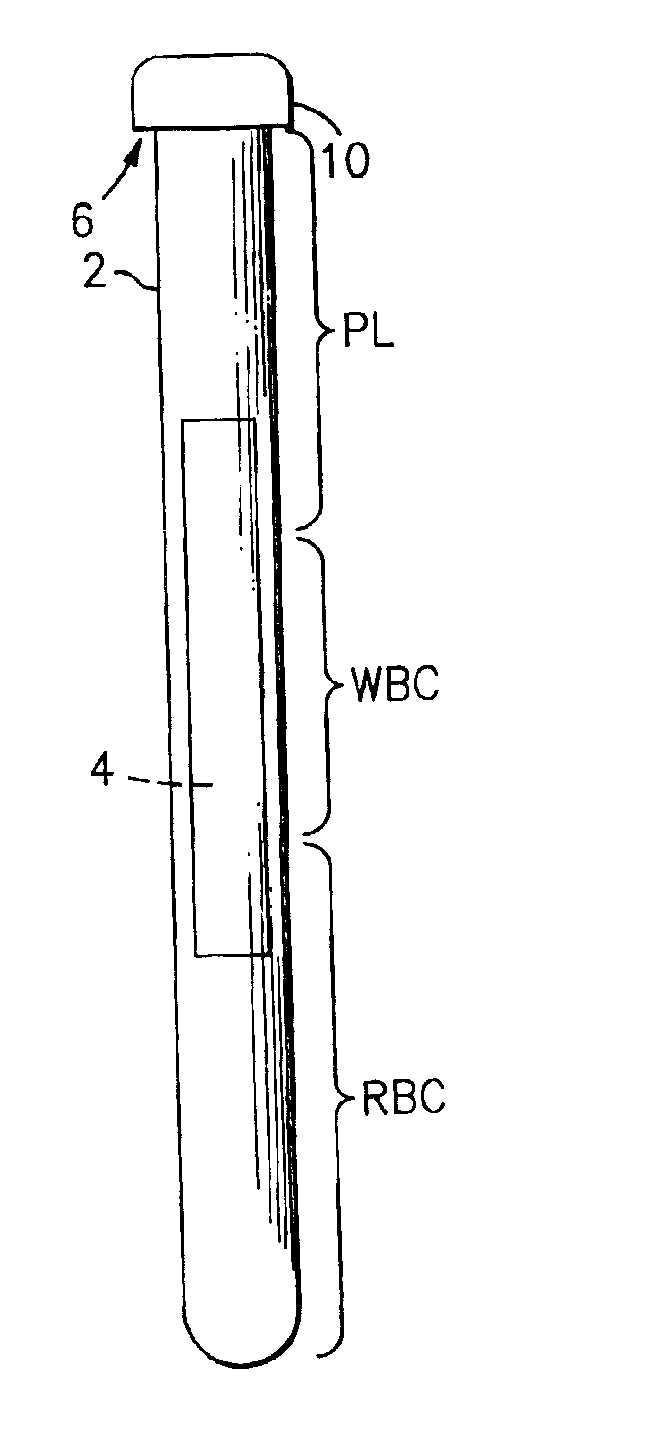
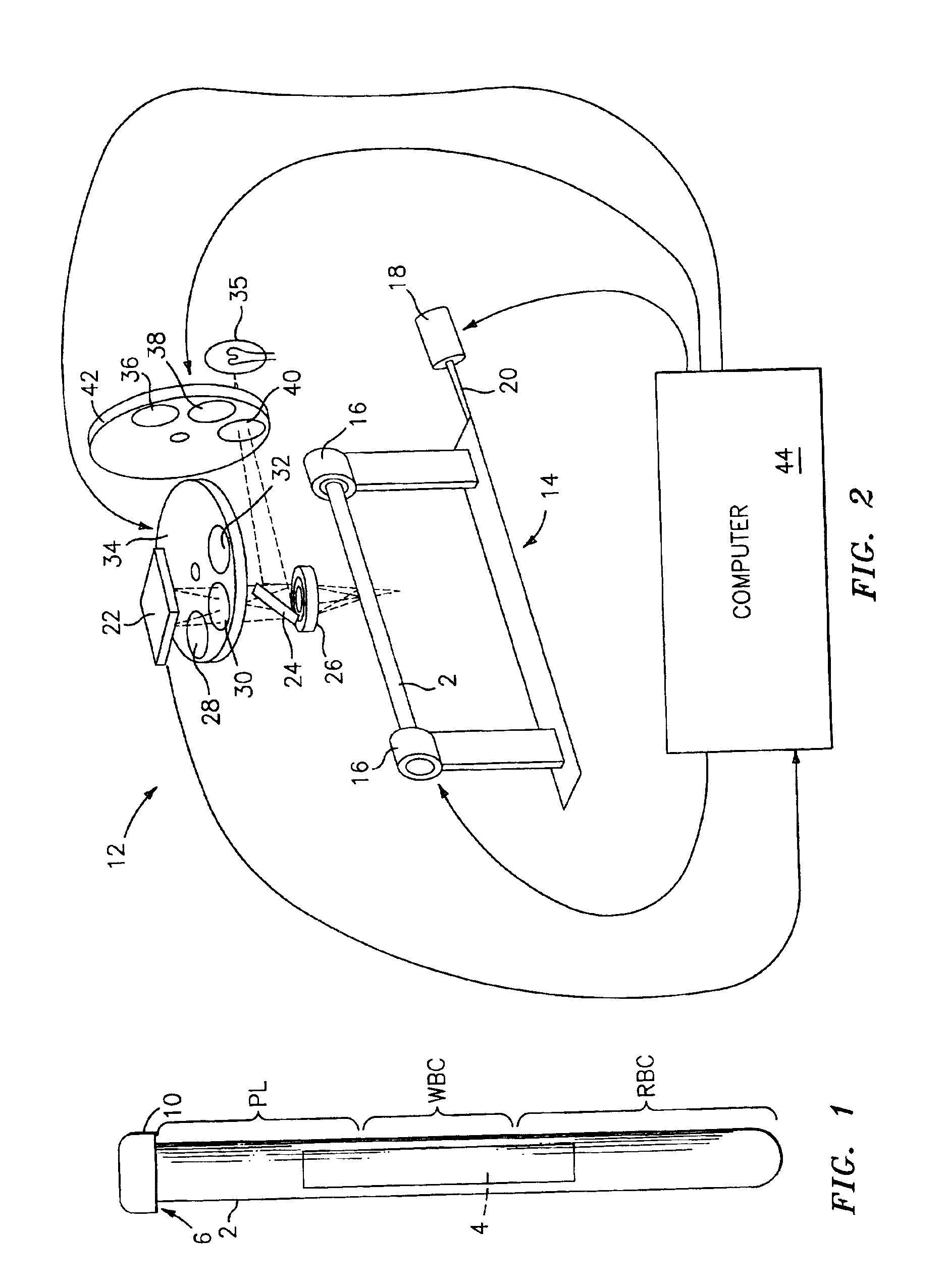
Method for the detection, identification, enumeration and confirmation of virally infected cells and other epitopically defined cells in whole blood
InactiveUS6911315B2Reduce the possibilityAvoid pollutionMicrobiological testing/measurementImmunoglobulins against virusesInfected cellFluorophore
A method for analyzing blood enables one to isolate, detect, enumerate and confirm under magnification the presence of target cells which have expressed surface epitopes that indicate intracellular infection by various viruses or other infectious agents, and also cells which have expressed surface epitopes that indicate the presence of non-infectious medical conditions. The analysis involves the examination of cells in the blood sample for the presence or absence of particular surface epitopes while the blood sample is disposed in a centrifuged blood sampling container. The epitopic analysis for the presence or absence of infected cells, or cells which indicate the presence of non-infectious medical conditions relies on the detection of known target expressed epitopes. The target epitopes on the target cell types are epitopes which are also known to be absent on normal circulating cells in the blood. Fluorophores or other labels with distinct wavelength emissions are coupled with specific binding agents such as lectins, antibodies, aptamers, or the like, which are directed against the target expressed epitopes. The epitopic analyses may be performed in or near the expanded buffy coat layer in the centrifuged blood sample. The epitopic analysis may be performed under magnification either visually and / or photometrically. The blood sampling container is sized to hold between about 1 and about 20 ml, preferably about 10 ml of blood, and contains an insert that occupies about 90-98% of the volume of the container bore in the area of the container where the target cells will, if present, be detected. The insert forces the target cells in question to reside in an annular space in the container which is adjacent to the circumference of the container bore. The entire analysis can be performed in a relatively short period of time which is typically a matter of minutes to single digit hours.
Owner:LEVINE ROBERT A +1
Method for Treating Oncological, Virulent and Somatic Diseases, Method for Controlling Treatment Efficiency, Pharmaceutical Agents and Composition for Carrying Out Said Treatment
The invention relates to medicine and veterinary science and discloses a novel method for treating oncological, virulent and somatic diseases whose main target for therapeutic action is embodied in the form of DNA which freely circulates in blood plasma (and other liquid media) and originates from tumoral and mutant cells or cells infected by bacteria, fungi or protozoan and from different microorganisms which reside in the organism thereof. Said invention also relates to novel pharmaceutical compositions and to the use thereof for treating oncological diseases and infectious states provoked by bacteria, fungi and protozoa and non-infectious somatic diseases and states produced by accumulation of somatic mutations in cells of organism. Medicinal and immunological compositions, sorption and physico-chemical engineering and the method for using them in order to treat malignant tumors and other diseases are also disclosed.
Owner:GENKIN DMITRY DMITRIEVICH +1
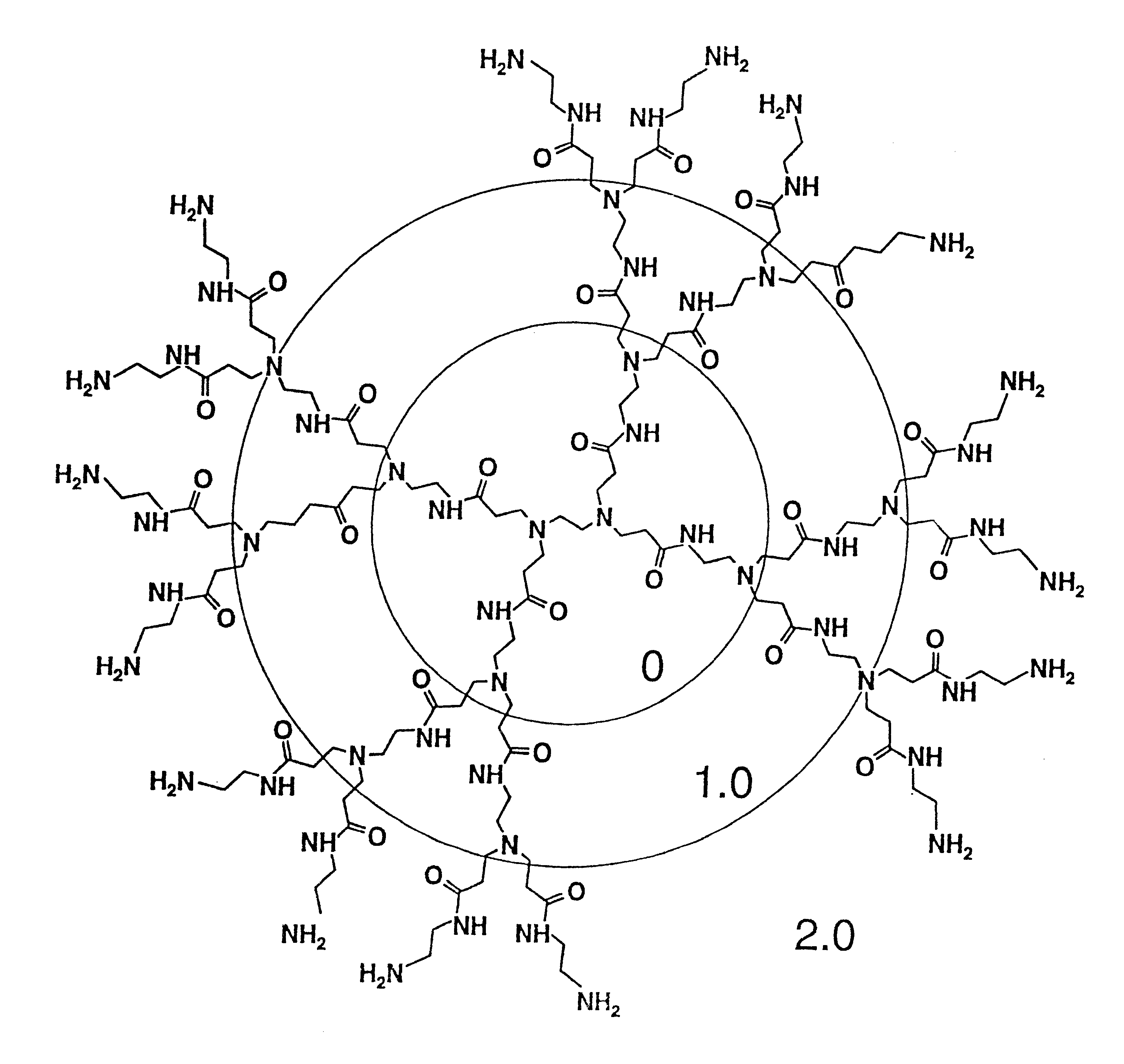
Method of sterilizing
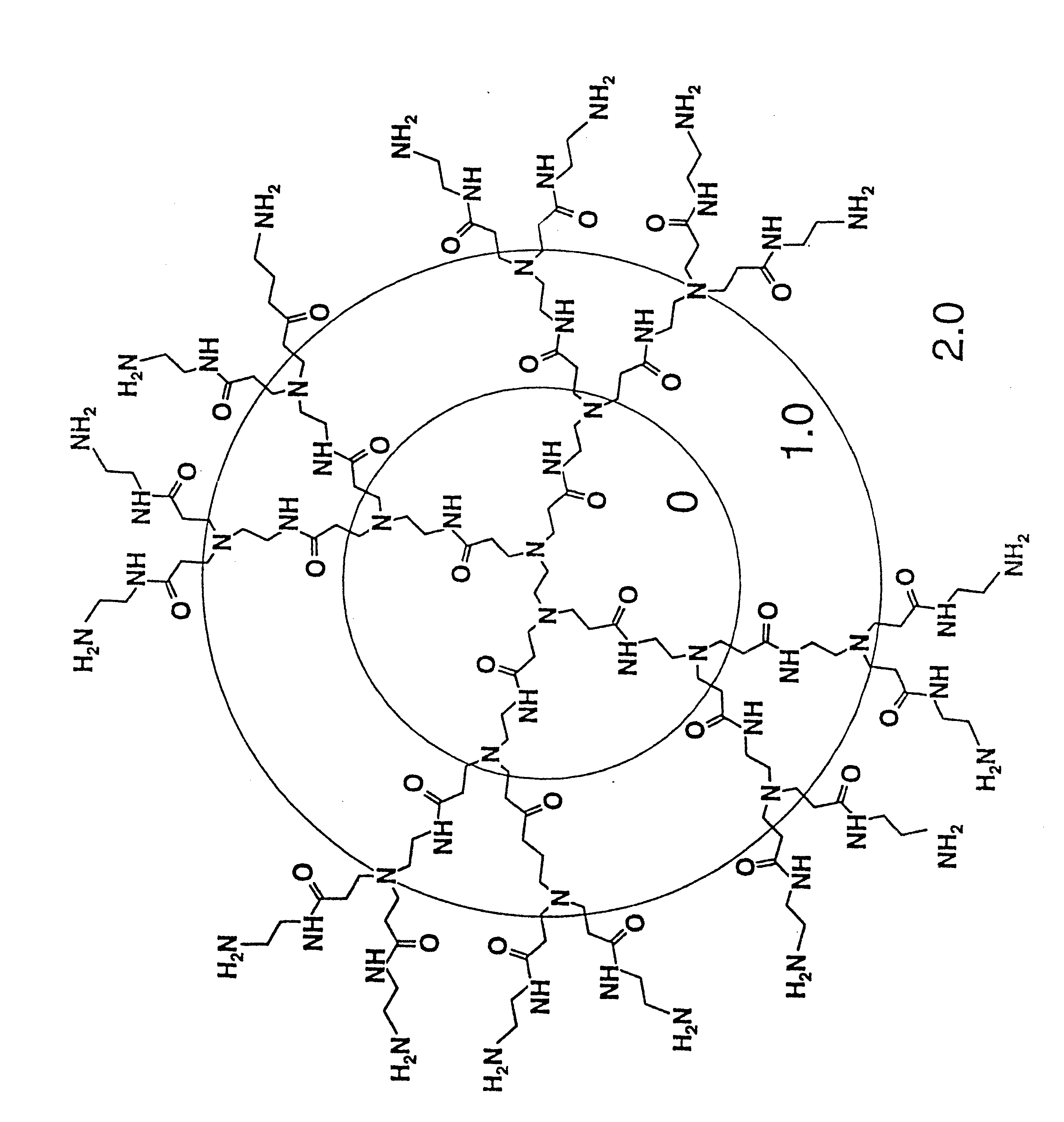
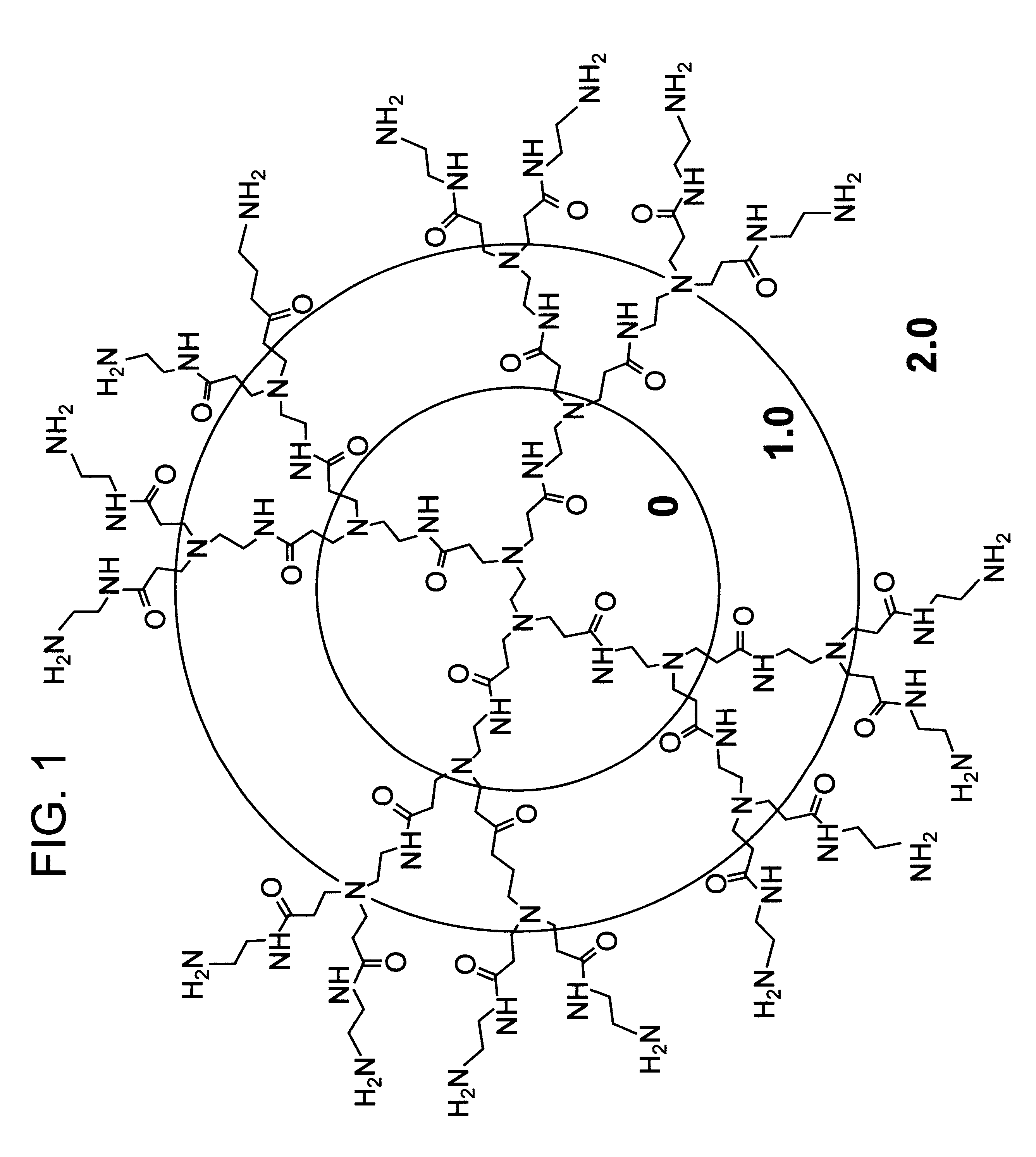
A method of sterilizing objects as well as the sterilized objects obtained from the method are disclosed. The method involves contacting an object such as a medical device to be reused with polycationic dendrimer under conditions which result in rendering a conformationally altered protein (e.g. a prion) non-infectious. A disinfecting agent or surgical scrub composition which comprises the dendrimers is also disclosed as are gelatin capsules treated with polycationic dendrimers.
Owner:RGT UNIV OF CALIFORNIA
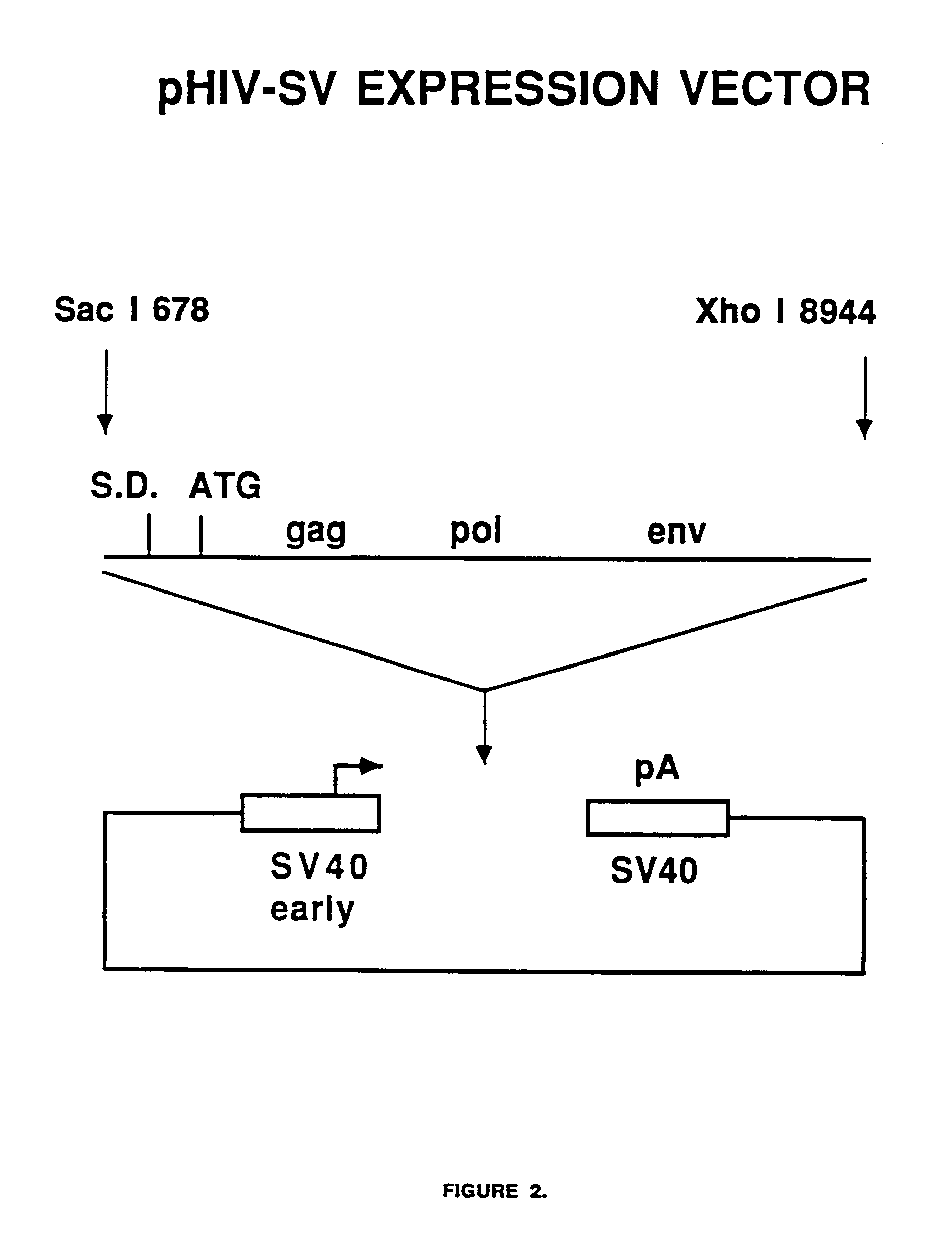
Constitutive expression of non-infectious HIV-like particles
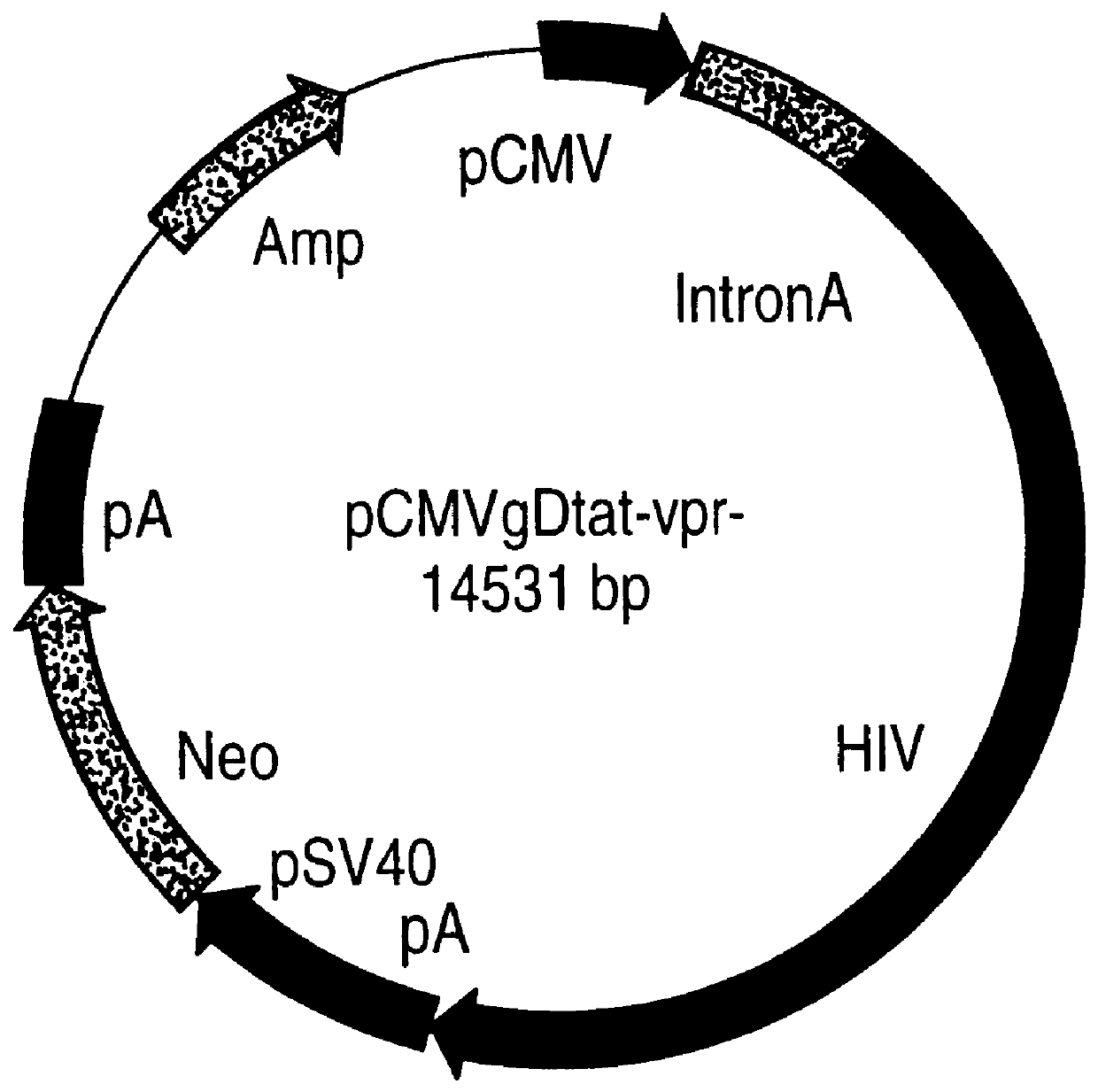
Non-infectious, non-replicating immunogenic HIV-like particles are produced by stable longn-term constitutive expression in mammalian cells by eliminating elements toxic to the mammalian cells. An expression vector contains a nucleic acid molecule comprising a modified HIV genome devoid of long terminal repeats and wherein Tat and vpr sequences are functionally disabled and a constitutive promoter operatively connected to the modified HIV genome for constitutive expression of the modified genome to produce the HIV-like particles.
Owner:CONNAUGHT LAB
Treatment of inflammatory, non-infectious, autoimmune, vasculitic, degenerative vascular, host-v-graft diseases, Alzheimers disease, and amyloidosis using mammalian, dsDNA vaccination
The present invention relates generally to compositions and methods using mammalian, dsDNA (Double Stranded Deoxyribonucleic Acid) vaccination for the induction and maintenance of regulator suppressor T cells resulting in suppression of non infectious, and post infectious, inflammatory, allergic, auto-immune, vasculitic, certain degenerative vascular, and graft versus host diseases, with or without the use of IL-10, and with or without the use or TGFβ, with or without the use of anti-IL 6 receptor antibody, anti TNF antibody and or Plasmapheresis, IVIG, Corticosteroids, Methotrexate, Bromocriptine, and or vitamin D analogues.
Owner:LAWLESS OLIVER J
Diagnostic kits comprising genetically engineered human immunodeficiency virus-like particles containing heterologous antigenic markers
InactiveUS6342228B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesHeterologous
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
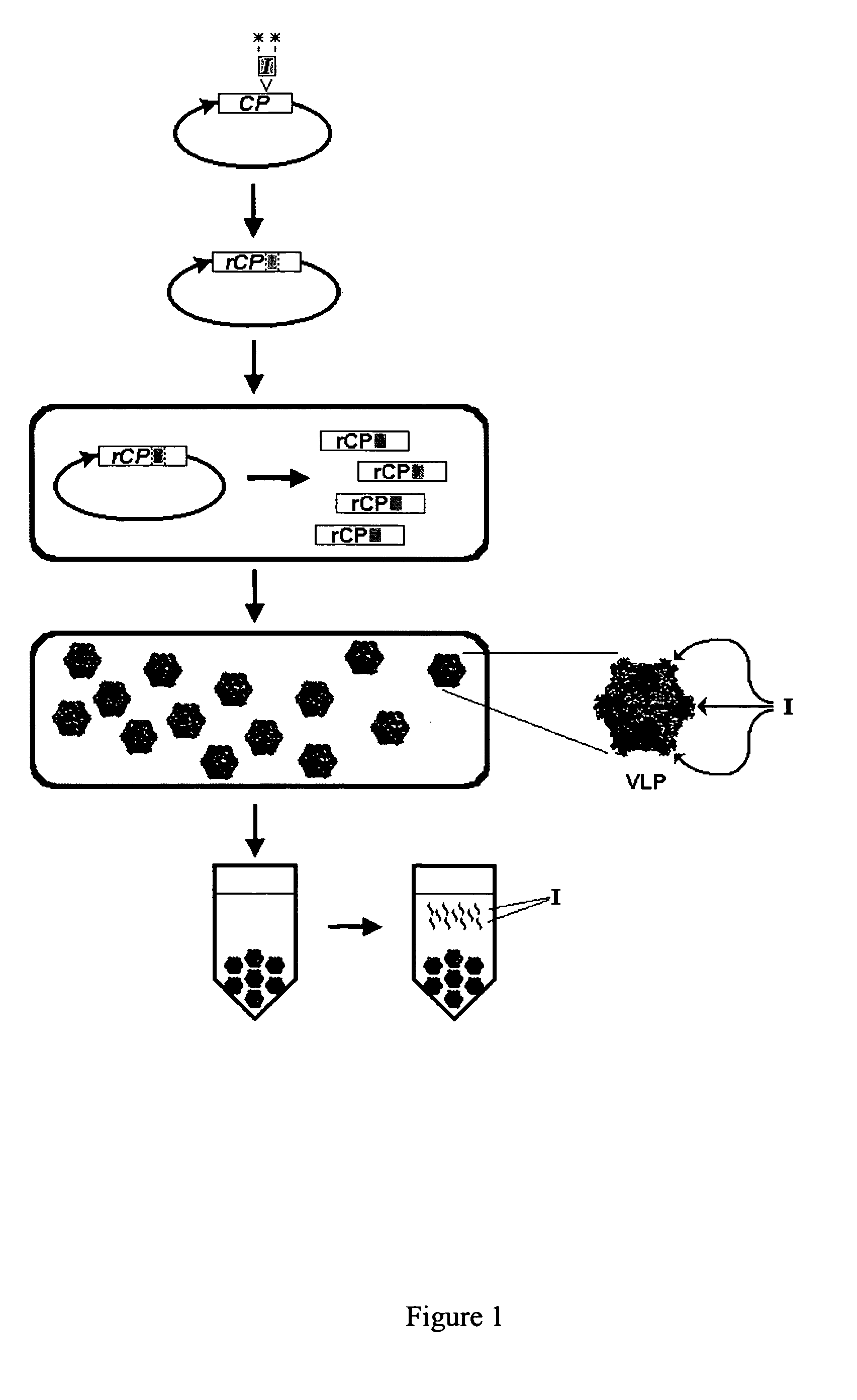
High efficiency peptide production in plant cells
ActiveUS20050260758A1Less variableMore stablyAntibacterial agentsSsRNA viruses positive-sensePlant cellEukaryotic plasmids
The present invention provides an improved process for the production of recombinant peptides. In particular, the present invention provides an improved process for the production of recombinant peptides in the form of viral capsid fusion proteins which can be assembled in vivo in plant cell suspension cultures. The invention also includes plasmids, sequences, and plant cells which allow for non-infectious viral capsid fusion peptide production.
Owner:DOW AGROSCIENCES LLC
Antigentically-marked non-infectious retrovirus-like particles
InactiveUS6518030B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:AVENTIS PASTEUR LTD
Schistosome infectious oncomelania detection kit and detection method thereof
InactiveCN101457258ASuitable for useSimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceOncomelaniaBiology
A blood fluke infectious oncomelania detection kit and a detection method thereof belongs to the verminosis transmission medium detecting field. The invention provides a kit and a detection method for detecting blood fluke infectious oncomelania based on a loop-mediated isothermal DNA amplification technology (LAMP). According to the LAMP technology principle, six pairs of specific primers for amplifying a DNA fragment between the 20bp and 231bp of the schistosoma japonicum non-long terminal repeated inverse transcription transposon gene (AF412214) are designed. The LAMP method is constructed for detecting blood fluke gene in the body of the infectious oncomelania and the objective for distinguishing the blood fluke infectious oncomelania from the non-infectious oncomelania is reached. Comparing to the conventional blood fluke infectious oncomelania detection method the method of the invention has advantages of easy and fast operation, sensitive and specific without especial equipment and is suitable for bilharziasis controlling personnel use on site.
Owner:JIANGSU INST OF PARASITIC DISEASES
Retrovirus like particles made non infectious by a plurality of mutations
InactiveUS6451322B1Improve efficiencyLow backgroundSugar derivativesViral antigen ingredientsPol genesReverse transcriptase activity
Non-infectious, retrovirus-like particles contain mutations to reduce gag-dependent RNA-packaging of the gag gene product, eliminate reverse transcriptase activity of the pol gene product, eliminate integrase activity of the pol gene, product and eliminate RNase H activity of the pol gene product through genetic manipulation of the gag and pol genes. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis.
Owner:AVENTIS PASTEUR LTD
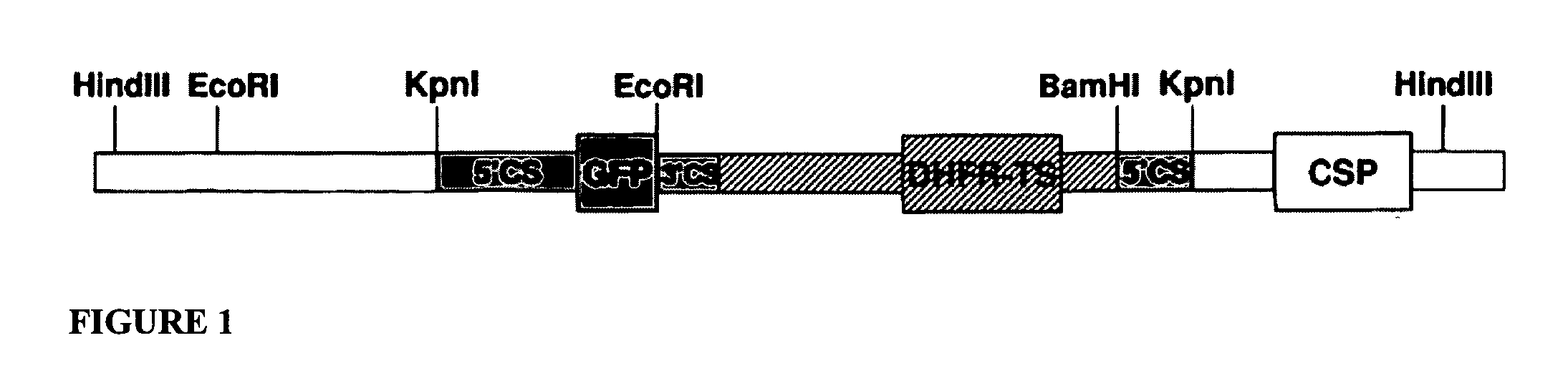
Plasmodium axenic liver stages as a noninfectious whole organism malaria vaccine
The present invention relates to the treatment and prevention of malaria infection. In particular, the present invention provides novel noninfectious, whole organism vaccines for malaria, which vaccines comprise a Plasmodium axenic liver stage. The invention also provides methods to treat and prevent malaria by administering such Plasmodium axenic liver stage vaccines, as well as methods to generate Plasmodium axenic liver stages.
Owner:NYU MEDICAL CENT
Compositions and methods for generating an immune response
InactiveUS20070048861A1Increase typeSsRNA viruses negative-senseAntibody mimetics/scaffoldsImmunodeficiency virusEukaryotic plasmids
The present invention relates to novel plasmid constructs useful for the delivery of DNA vaccines. The present invention provides novel plasmids having a transcription cassette capable of directing the expression of a vaccine nucleic acid insert encoding immunogens derived from any pathogen, including fungi, bacteria and viruses. The present invention, however, is particularly useful for inducing in a patient an immune response against pathogenic viruses such as HIV, measles or influenza. Immunodeficiency virus vaccine inserts of the present invention express non-infectious HIV virus-like particles (VLP) bearing multiple viral epitopes. VLPs allow presentation of the epitopes to multiple histocompatability types, thereby reducing the possibility of the targeted virus escaping the immune response. Also described are methods for immunizing a patient by delivery of a novel plasmid of the present invention to the patient for expression of the vaccine insert therein. Optionally, the immunization protocol may include a booster vaccination that may be a live vector vaccine such as a recombinant pox virus or modified vaccinia Arbora vector. The booster live vaccine vector includes a transcription cassette expressing the same vaccine insert as the primary immunizing vector.
Owner:ROBINSON HARRIET L +11
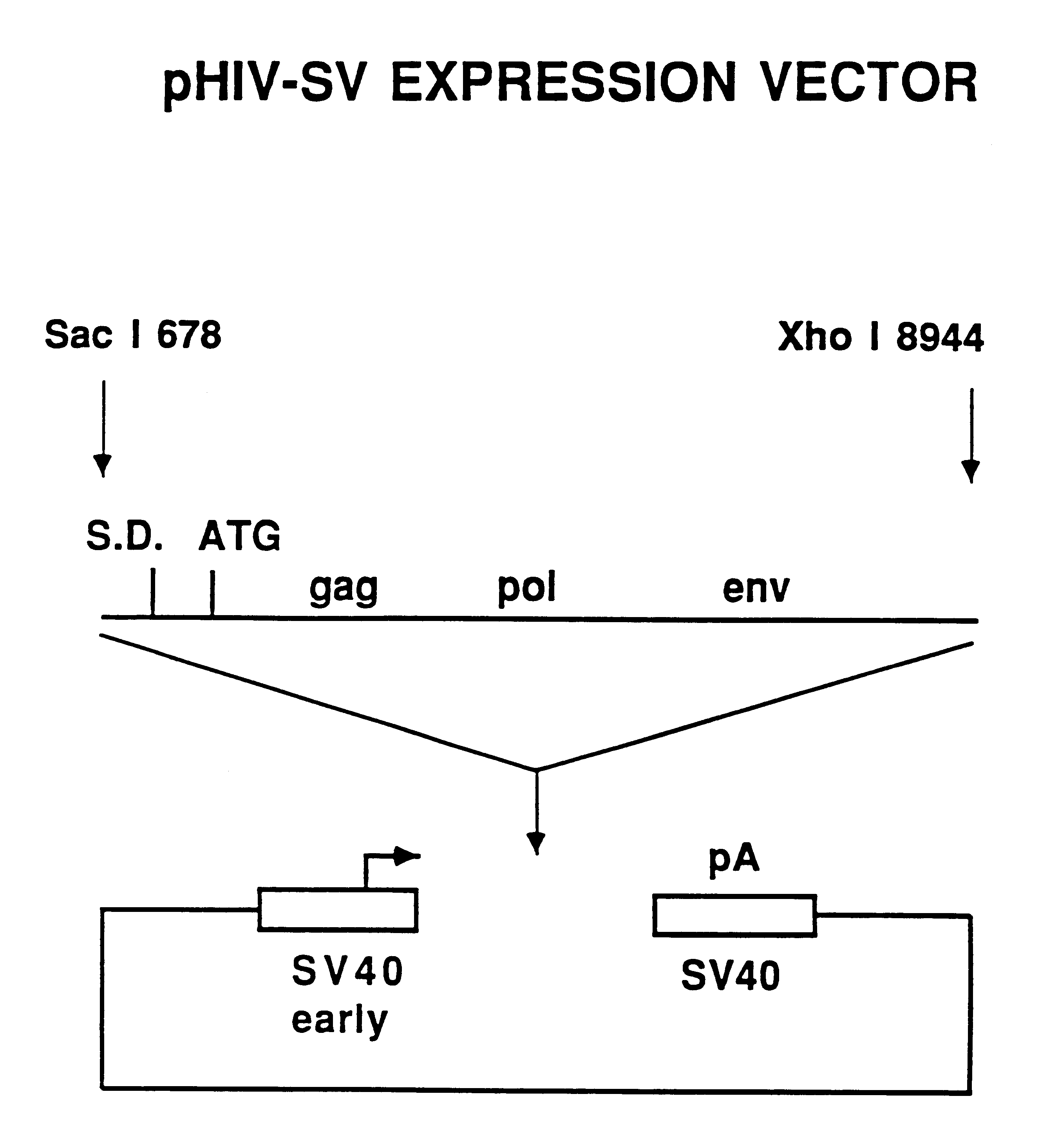
Non-infectious, non-replicating, immunogenic human immunodeficiency virus-like particles
InactiveUS7008784B1Improving immunogenicitySafe preparationNanotechVirus peptidesHeterologousPol genes
The present invention is directed toward methods for the production of non-infectious, replication-deficient, immunogenic human immunodeficiency virus (HIV)-like particles. These particles are prepared from a recombinant expression vector comprising a heterologous promoter operatively connected to a DNA molecule comprising a modified HIV genome devoid of the long terminal repeat (LTR) regulatory regions but containing at least the gag and pol genes in their natural genomic arrangement. This vector is introduced into mammalian cells to produce the particles of interest. These particles should prove useful in a number of diagnostic, virologic, and immunologic applications.
Owner:AVENTIS PASTEUR LTD
Interleukin 37 containing drug, preparation method and application thereof
InactiveCN104083755AGood treatment effectPeptide/protein ingredientsAntipyreticAutoimmune responsesWhite blood cell
The invention discloses application of an interleukin in pharmacy, and in particular discloses interleukin 37 (IL-37) as an active ingredient of a drug and preparation thereof. At the same time, the invention discloses IL-37, its derivative, variant and / or truncated polypeptide as the active ingredient, or coding nucleotide sequences of the substances as the active ingredients, discloses a method for expression and purification of IL-37 and its active ingredients by prokaryotic, eukaryotic and mammalian cells, and discloses cloning of the coding nucleotide sequences of the IL-37 and its active ingredients to various expression vectors to conduct gene therapy. The invention also discloses application of the active ingredients in treatment of autoimmunity or chronic non-infectious inflammatory diseases. The drug provided by the invention can be used for treatment of rheumatoid arthritis, systemic lupus erythematosus, atopic dermatitis, psoriasis, Crohn's disease, multiple sclerosis, asthma, diabetes, transplant rejection, atherosclerosis or inflammatory bowel disease, etc.
Owner:SHENZHEN UNIV
Vaccine for chikungunya virus infection
ActiveUS20130022631A1Easy to be validatedSsRNA viruses positive-sensePeptide/protein ingredientsProtection sexIn vivo
The present invention relates to vaccine formulation capable of eliciting protective immune response against Chikun-gunya virus infection in humans and other mammalian hosts. The immunogenic formulation comprises purified inactivated Chikun-gunya virus in a stable formulation. Methods of propagation and purification of the virus are discussed. The inactivated virus formulation is non-infectious, immunogenic and elicits protective immune response in mammalian host. The immunogenic composition is formulated for in vivo administration to humans. The invention also discusses the strategy of developing a subunit vaccine using the recombinant viral proteins as antigens for immunization. The recombinant virus antigens that are potentially immunogenic can be used in diagnosing for the presence of the virus.
Owner:BHARAT BIOTECH INTERNATIONAL
Method of sterilizing
InactiveUS20020041862A1Reduce concentrationBiocideCosmetic preparationsDendrimerDisinfection methods
A method of sterilizing objects as well as the sterilized objects obtained from the method are disclosed. The method involves contacting an object such as a medical device to be reused with polycationic dendrimer under conditions which result in rendering a conformationally altered protein (e.g. a prion) non-infectious. A disinfecting agent or surgical scrub composition which comprises the dendrimers is also disclosed as are gelatin capsules treated with polycationic dendrimers.
Owner:RGT UNIV OF CALIFORNIA
Method of sterilizing
A method of sterilizing objects as well as the sterilized objects obtained from the method are disclosed. The method involves contacting an object such as a medical device to be reused with polycationic dendrimer under conditions which result in rendering a conformationally altered protein (e.g. a prion) non-infectious. A disinfecting agent or surgical scrub composition which comprises the dendrimers is also disclosed as are gelatin capsules treated with polycationic dendrimers.
Owner:RGT UNIV OF CALIFORNIA
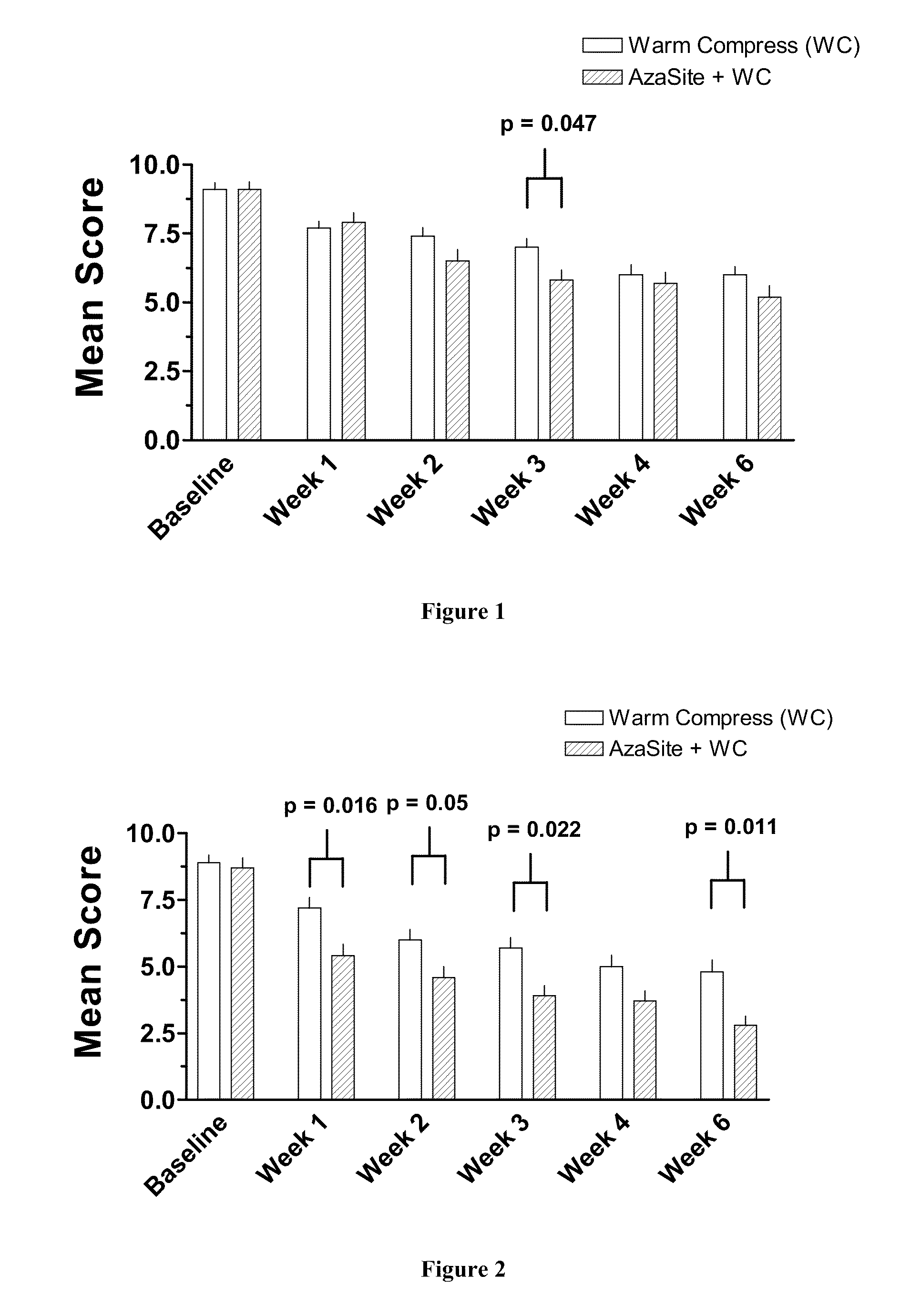
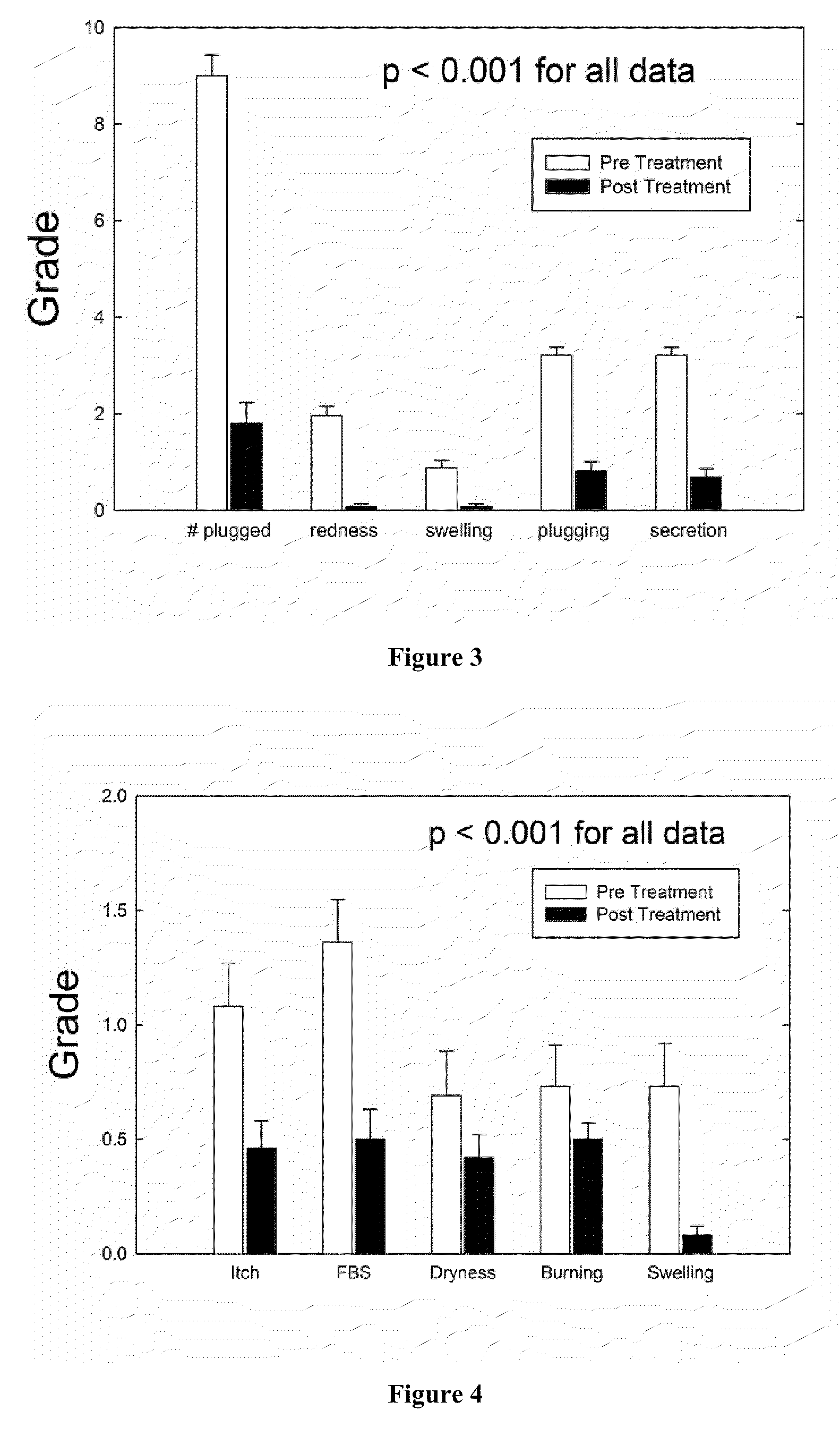
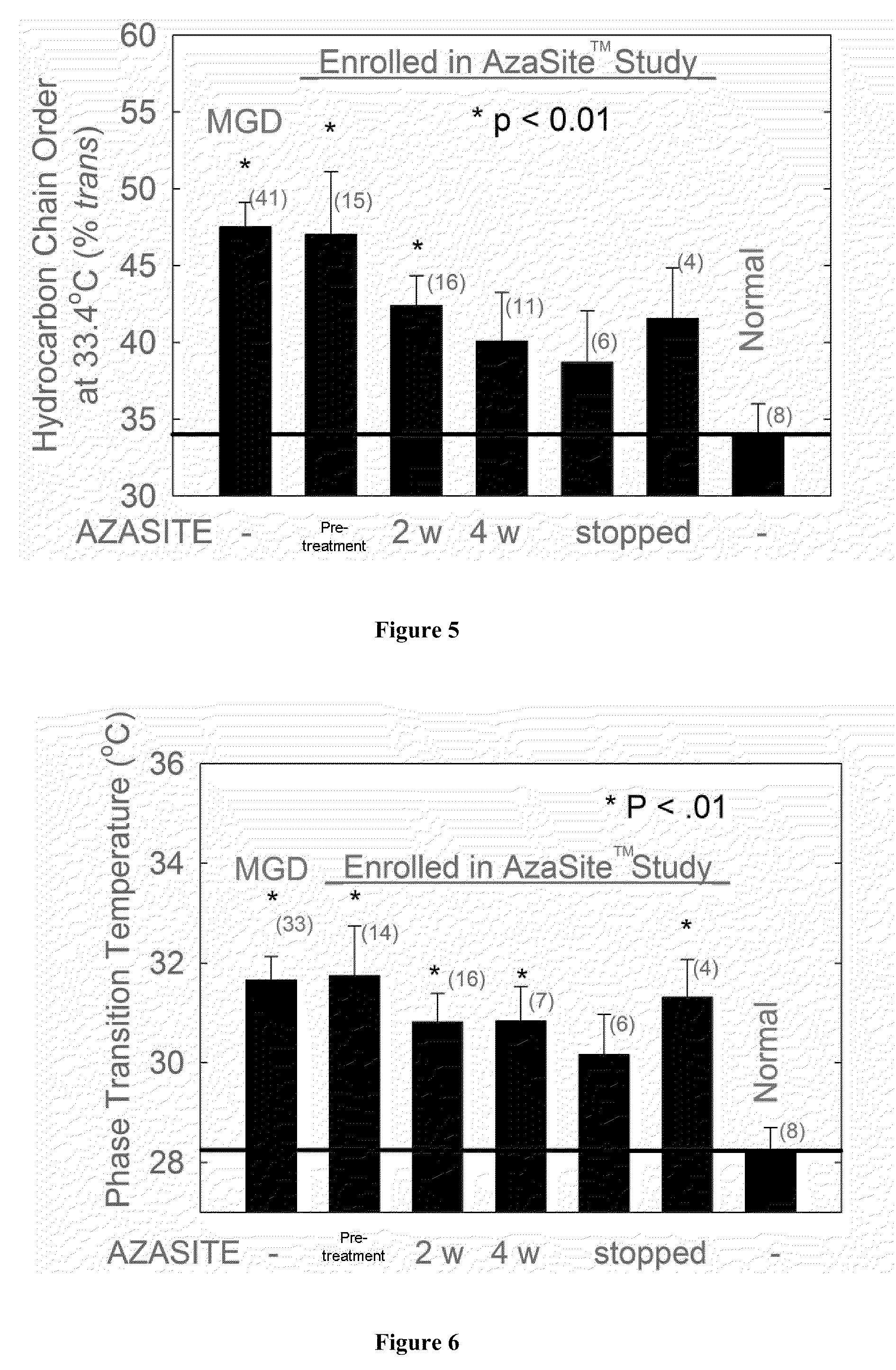
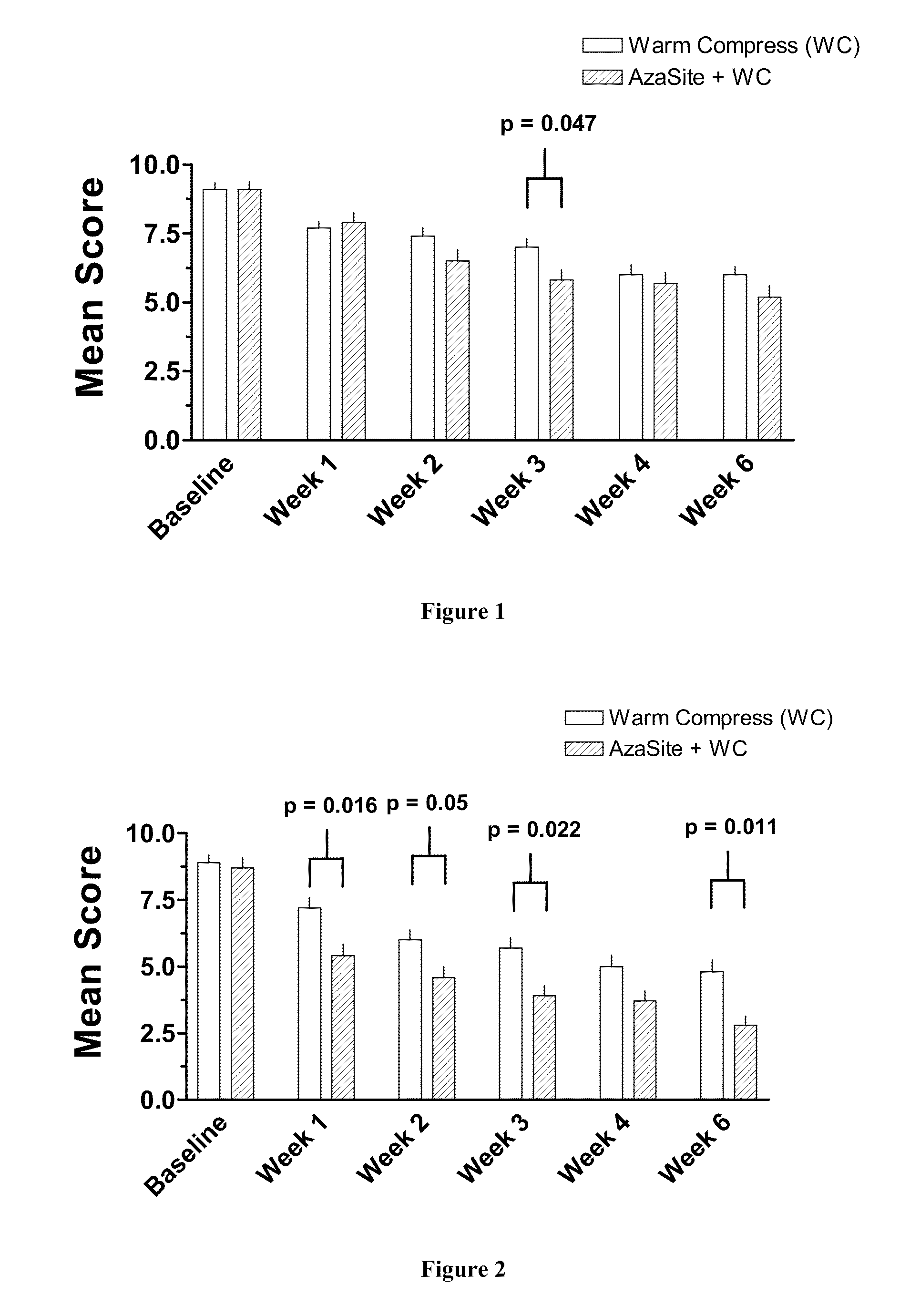
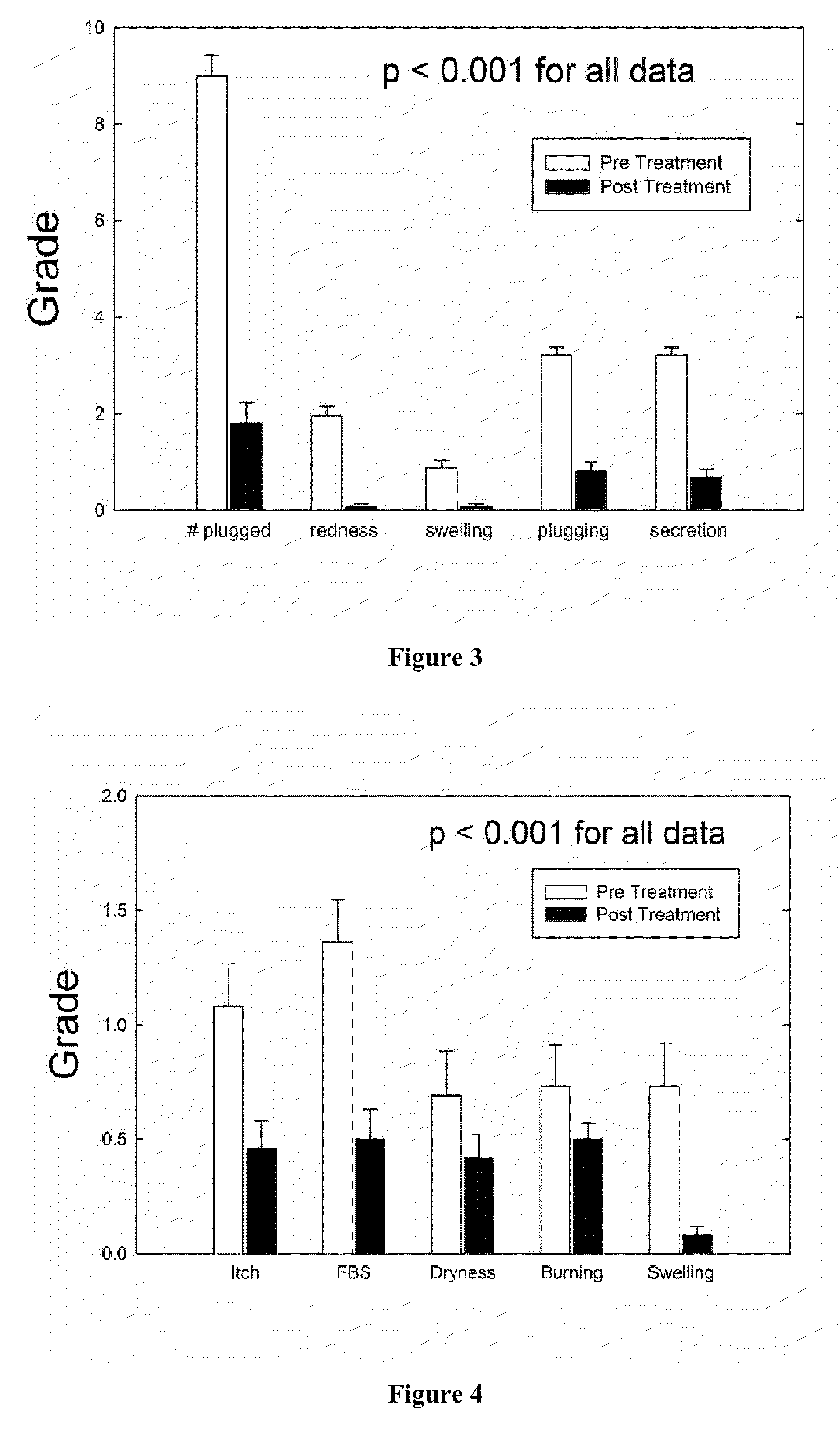
Method of treating blepharitis
ActiveUS20100022465A1Relieve symptomsReducing clinical signBiocideSenses disorderContact lens intoleranceAzithromycin
The present invention relates to a method for treating non-infectious, inflammatory chronic posterior blepharitis in a subject. The present invention also relates to a method for treating chronic posterior blepharitis in a subject for over two weeks. The method comprises identifying a subject in need thereof, and topically administering to the eye of the subject a pharmaceutical formulation consisting essentially of an effective amount azithromycin. The present invention further relates to a method for treating dry eye secondary to blepharitis in a subject. The method comprises the steps of: identifying a subject suffering from dry eye secondary to posterior blepharitis, and topically administering to the eye of the subject a pharmaceutical formulation comprising an effective amount of azithromycin. The present invention further relates to method for reducing contact lens intolerance of a subject due to blepharitis or dry eye secondary to blepharitis.
Owner:INSPIRE PHARMA
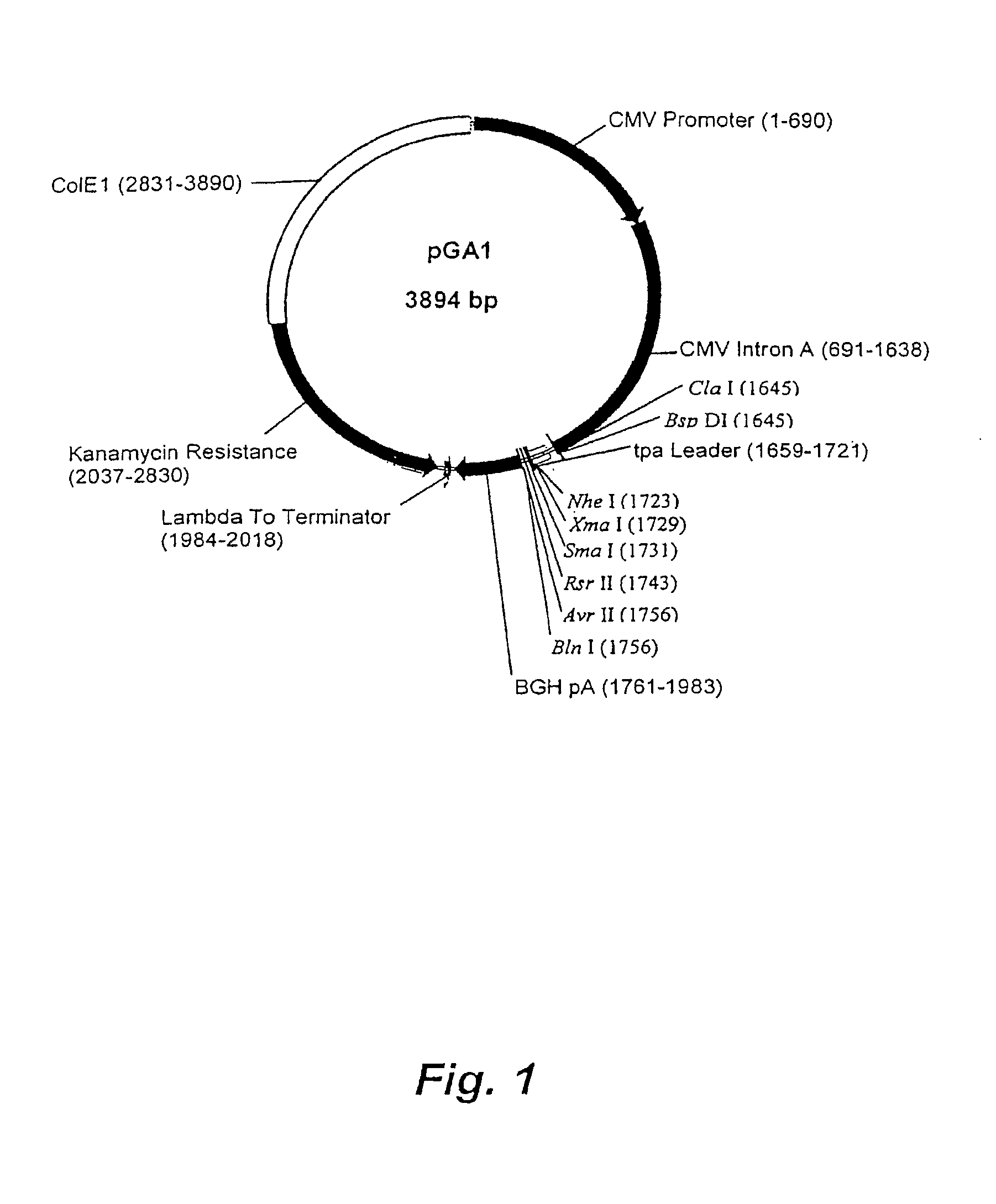
DNA expression vectors and methods of use
Owner:EMORY UNIVERSITY
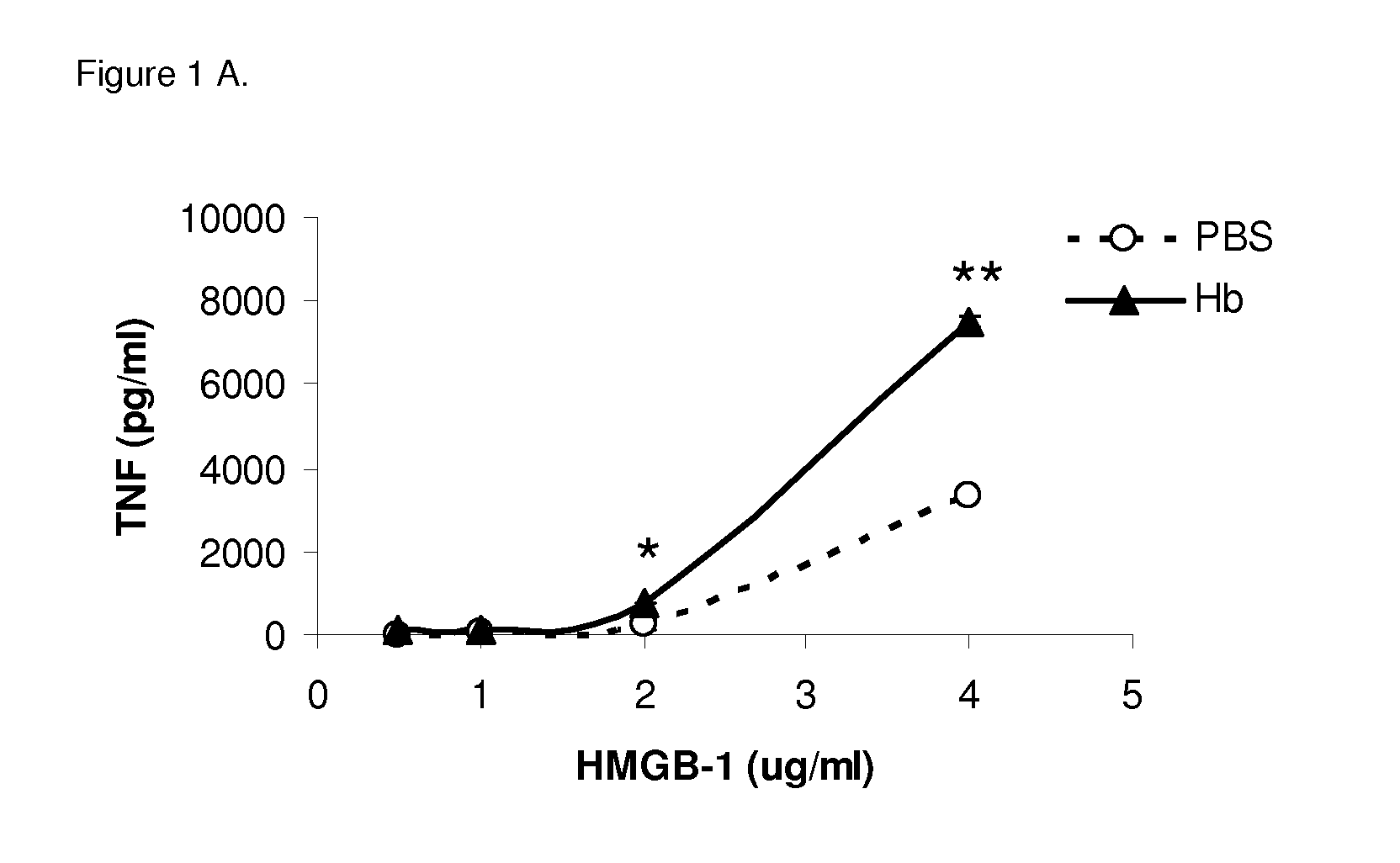
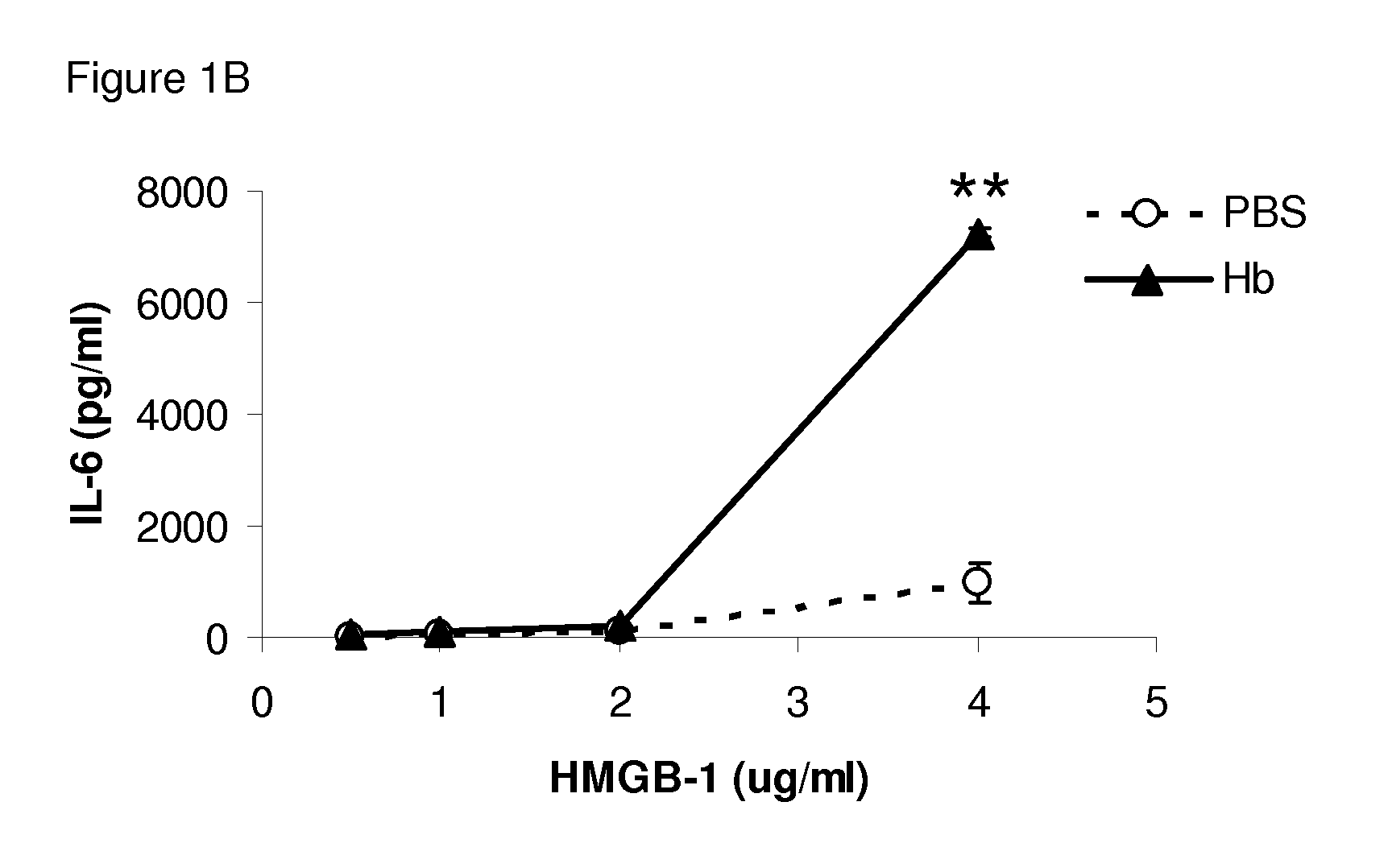
Use of hemopexin to sequester hemoglobin
The invention relates to use of hemopexin (Hx) to sequester extravascular hemoglobin and thereby reduce or prevent inflammation of non-infectious etiology in a subject (e.g., a human).
Owner:THE GENERAL HOSPITAL CORP
DNA expression vectors and methods of use
The present invention relates to novel plasmid constructs useful for the delivery of DNA vaccines. The present invention provides novel plasmids having a transcription cassette capable of directing the expression of a vaccine nucleic acid insert encoding immunogens derived from any pathogen, including fungi, bacteria and viruses. The present invention, however, is particularly useful for inducing in a patient an immune response against pathogenic viruses such as HIV, measles or influenza. Immunodeficiency virus vaccine inserts of the present invention express non-infectious HIV virus-like particles (VLP) bearing multiple viral epitopes. VLPs allow presentation of the epitopes to multiple histocompatability types, thereby reducing the possibility of the targeted virus escaping the immune response. Also described are methods for immunizing a patient by delivery of a novel plasmid of the present invention to the patient for expression of the vaccine insert therein. Optionally, the immunization protocol may include a booster vaccination that may be a live vector vaccine such as a recombinant pox virus or modified vaccinia Arbora vector. The booster live vaccine vector includes a transcription cassette expressing the same vaccine insert as the primary immunizing vector.
Owner:EMORY UNIVERSITY
Method of treating blepharitis
ActiveUS8349806B2Reducing symptom and clinical signReduce exposureBiocideSenses disorderPosterior blepharitisAzithromycin
The present invention relates to a method for treating non-infectious, inflammatory chronic posterior blepharitis in a subject. The present invention also relates to a method for treating chronic posterior blepharitis in a subject for over two weeks. The method comprises identifying a subject in need thereof, and topically administering to the eye of the subject a pharmaceutical formulation consisting essentially of an effective amount azithromycin. The present invention further relates to a method for treating dry eye secondary to blepharitis in a subject. The method comprises the steps of: identifying a subject suffering from dry eye secondary to posterior blepharitis, and topically administering to the eye of the subject a pharmaceutical formulation comprising an effective amount of azithromycin. The present invention further relates to method for reducing contact lens intolerance of a subject due to blepharitis or dry eye secondary to blepharitis.
Owner:INSPIRE PHARMA
Use of regularly scheduled high dose intravenous methotrexate therapy, with interim administration of immunomodulatory agents, to treat multiple sclerosis and other diseases of the central nervous system
InactiveUS6903100B2Halted deteriorationImprove scoreBiocidePeptide/protein ingredientsCytotoxicityHigh doses
The present invention is directed to the treatment of multiple sclerosis by periodically administering a high dose of methotrexate at a level sufficiently high to cross the blood brain barrier. The methotrexate administration is accompanied by leucovorin rescue of the periphery. The high dose methotrexate is preferably administered at 1 to 4 month intervals. The periodic high dose methotrexate treatment may be used in conjunction with interim treatments using a therapeutic agent that is effective in treating MS, but does not cross the BBB in cytotoxic amounts. It is contemplated that the method of the present invention may be employed to treat other non-infectious, non-neoplastic inflammatory conditions of the CNS.
Owner:MIDAMERICA NEUROSCI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com