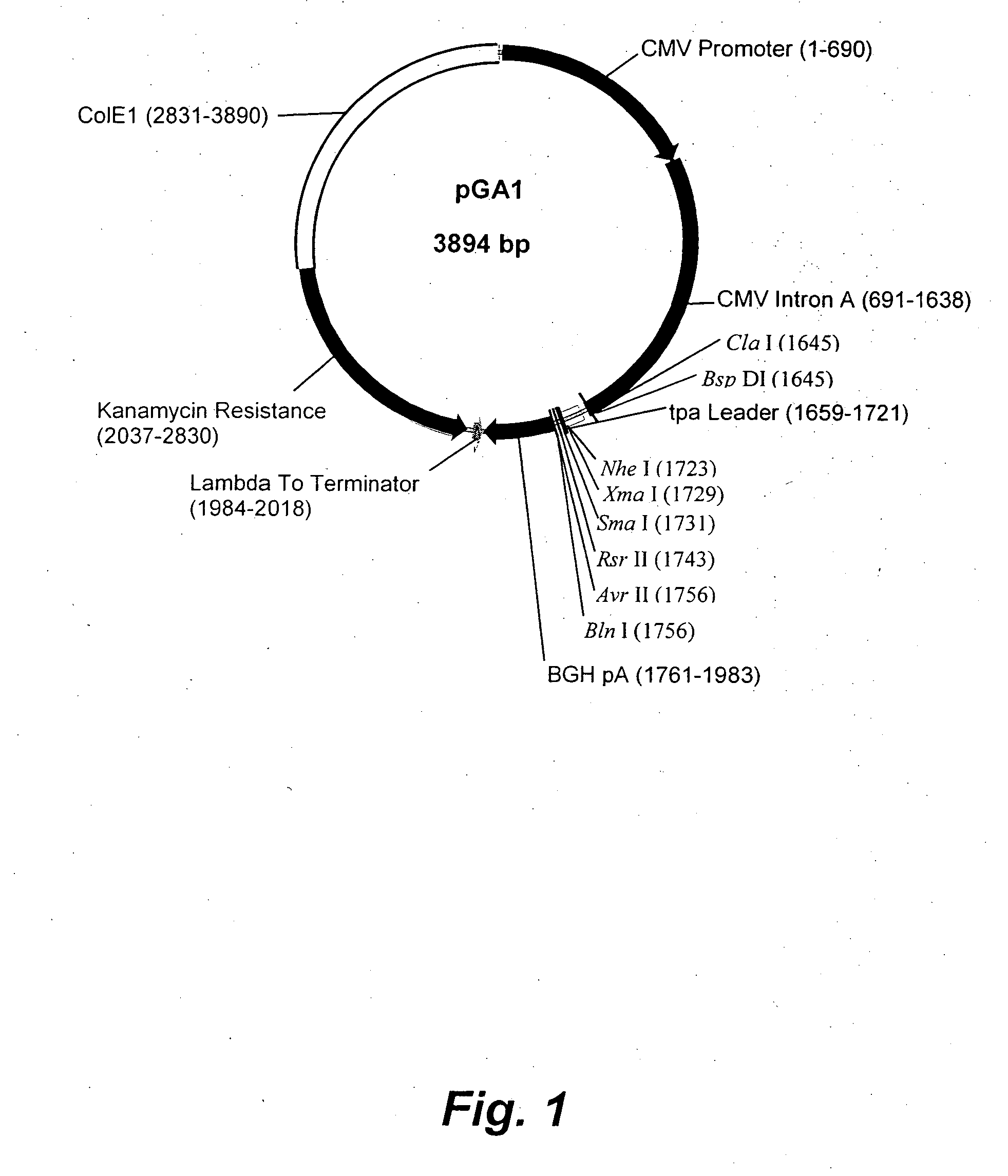
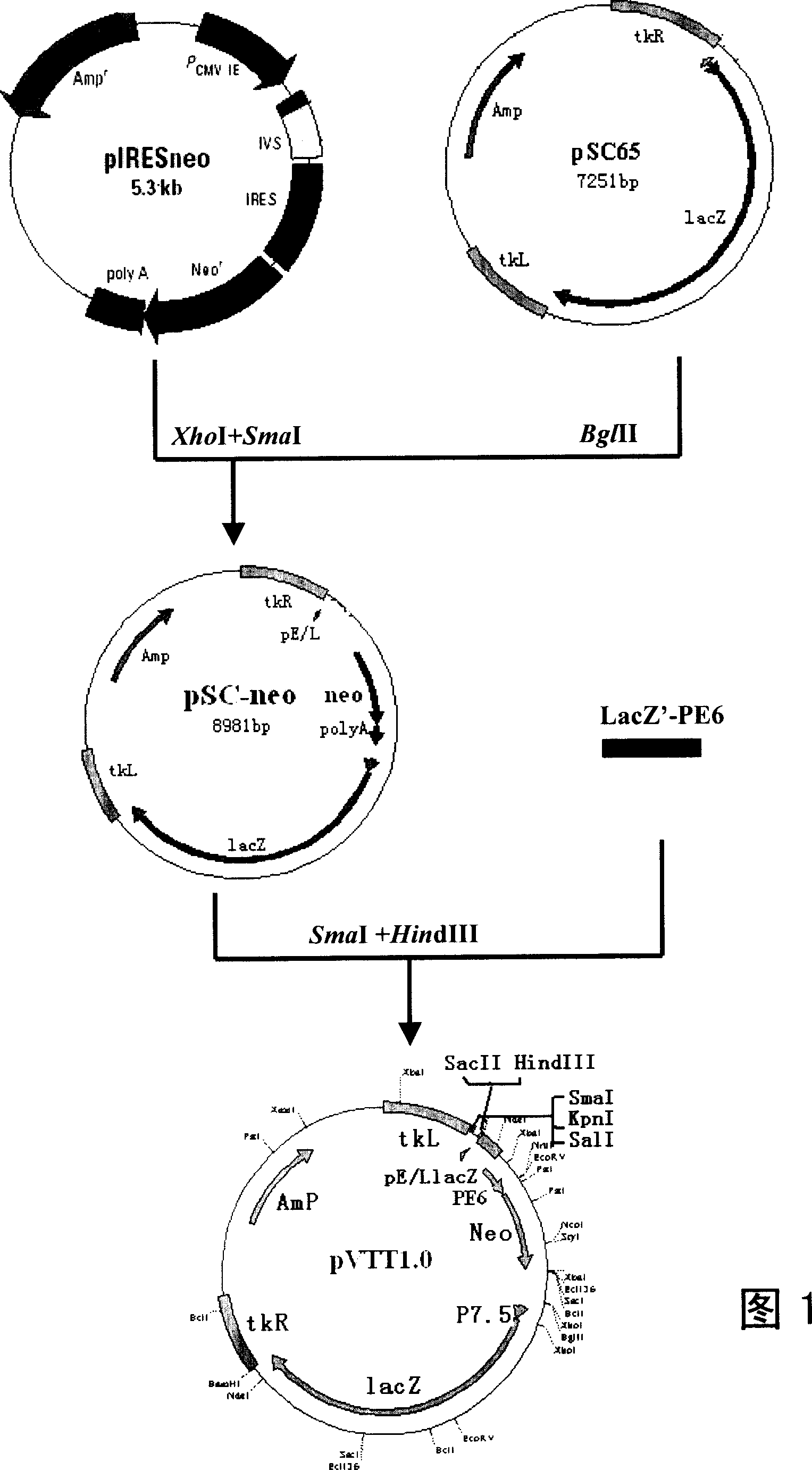
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Live vector vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A live vector vaccine is a vaccine that uses a chemically weakened virus to transport pieces of the pathogen in order to stimulate an immune response. The genes used in this vaccine are usually antigen coding surface proteins from the pathogenic organism. They are then inserted into the genome of a non-pathogenic organism such as adenovirus where they are expressed on the cell's surface and can elicit an immune response.
Multivalent Live Vector Vaccine against Clostridium difficile-Associated Disease
ActiveUS20120282293A1Easy to exportLow hemolytic activityAntibacterial agentsBacterial antigen ingredientsClostridium difficile (bacteria)Live vector vaccine
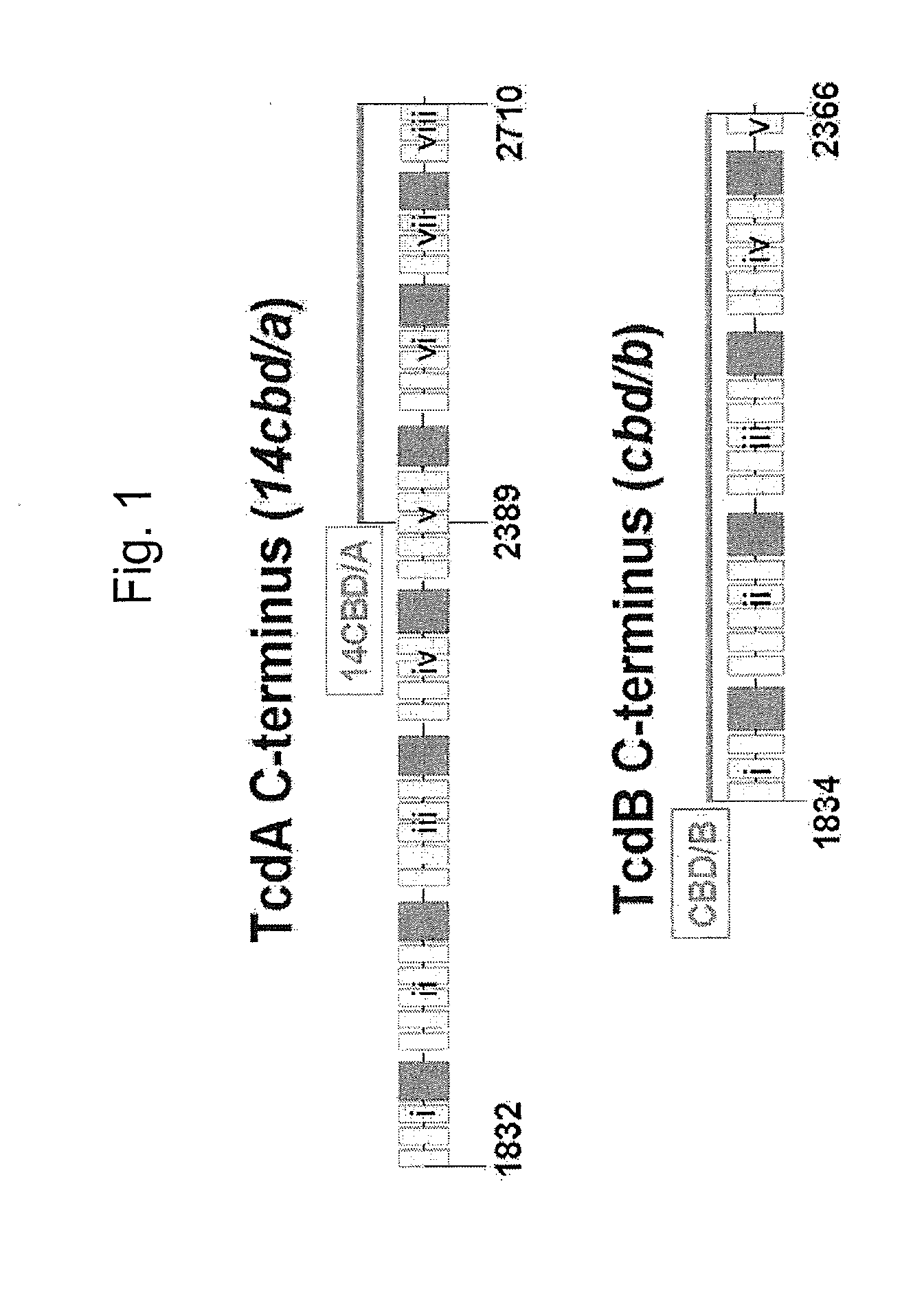
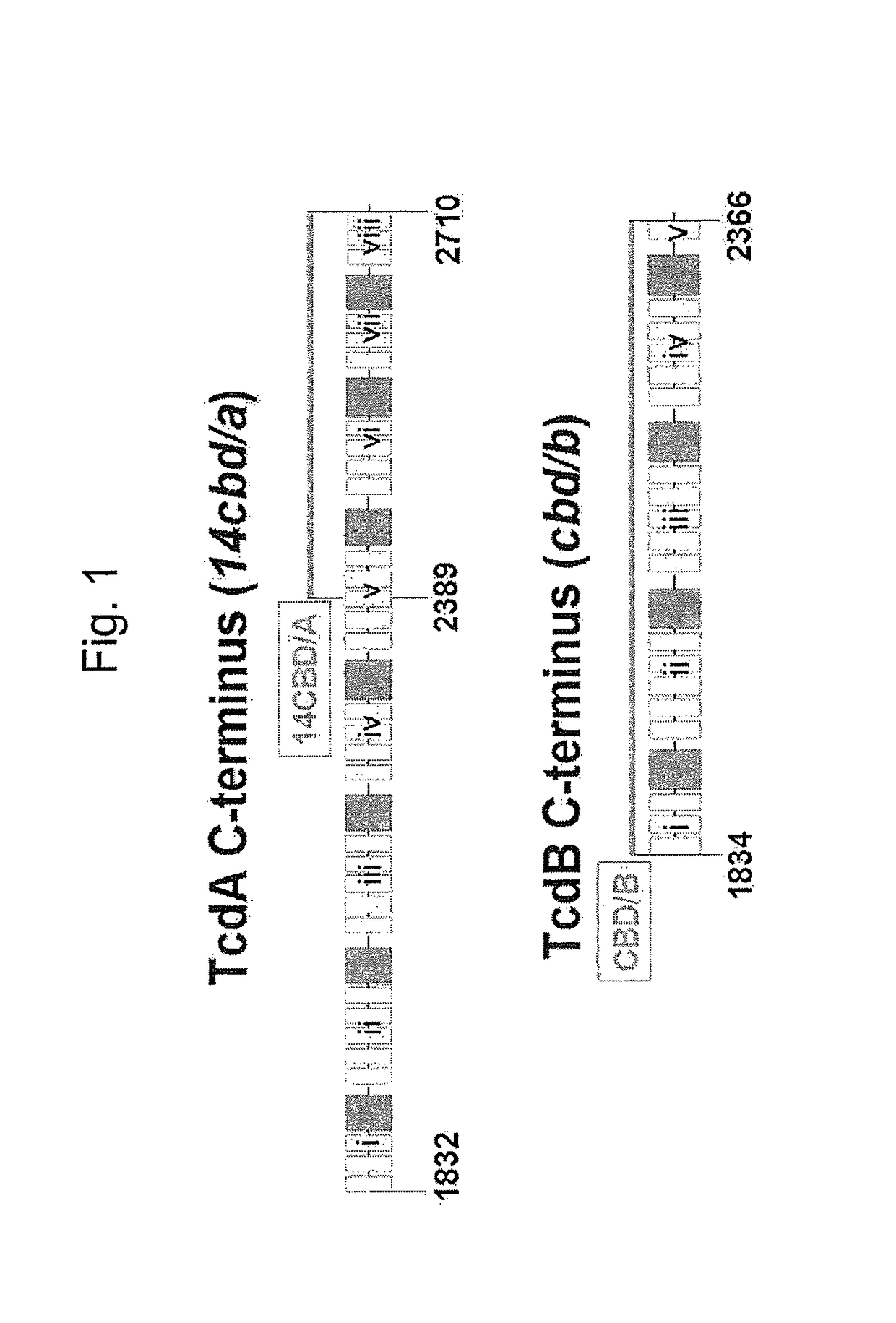
The invention relates to a multivalent Clostridium difficile vaccine comprising a Salmonella Typhi live vector comprising the cell binding domain of TcdA toxin (CBD / A) of Clostridium difficile or an antigenic fragment thereof and the cell binding domain of TcdB toxin (CBD / B) of Clostridium difficile or an antigenic fragment thereof and optionally the cell-binding subunit component (CdtB) of binary toxin of Clostridium difficile or an antigenic fragment thereof. The invention further provides methods of inducing an immune response and methods of preventing recurrence of C. difficile infections in subjects.
Owner:UNIV OF MARYLAND BALTIMORE
Compositions and methods for generating an immune response
InactiveUS20070048861A1Increase typeSsRNA viruses negative-senseAntibody mimetics/scaffoldsImmunodeficiency virusEukaryotic plasmids
The present invention relates to novel plasmid constructs useful for the delivery of DNA vaccines. The present invention provides novel plasmids having a transcription cassette capable of directing the expression of a vaccine nucleic acid insert encoding immunogens derived from any pathogen, including fungi, bacteria and viruses. The present invention, however, is particularly useful for inducing in a patient an immune response against pathogenic viruses such as HIV, measles or influenza. Immunodeficiency virus vaccine inserts of the present invention express non-infectious HIV virus-like particles (VLP) bearing multiple viral epitopes. VLPs allow presentation of the epitopes to multiple histocompatability types, thereby reducing the possibility of the targeted virus escaping the immune response. Also described are methods for immunizing a patient by delivery of a novel plasmid of the present invention to the patient for expression of the vaccine insert therein. Optionally, the immunization protocol may include a booster vaccination that may be a live vector vaccine such as a recombinant pox virus or modified vaccinia Arbora vector. The booster live vaccine vector includes a transcription cassette expressing the same vaccine insert as the primary immunizing vector.
Owner:ROBINSON HARRIET L +11
Recombinant fusion protein vaccine and attenuated live vector vaccine for treating and preventing helicobacter pylori (Hp) infection
The invention relates to a recombinant fusion protein vaccine and an attenuated live vector vaccine for treating and preventing helicobacter pylori (Hp) infection, and belongs to the field of biopharmaceutics. The recombinant fusion protein vaccine and the attenuated live vector vaccine for expressing the recombinant fusion protein are characterized in that: the recombinant fusion protein is formed by connecting immune protective function fragments of helicobacter pylori cytotoxin relevant gene protein CagA from helicobacter pylori, vacuolization cytotoxin VacA and urease subunit UreB linearly, and the immunogenicity and immune protection of the recombinant fusion protein are verified through animal experiments. The live vaccine has the advantages that: 1, an Hp fusion protein gene can beexpressed stably; 2, mucosa and systemic immune response can be induced after immunity; and 3, the method is convenient, the cost is low, and the economic benefit is obvious. Therefore, the attenuated live vector vaccine can be used as candidate vaccines for treating and preventing the Hp infection.
Owner:ARMY MEDICAL UNIV
Peste des petits ruminants virus (PPRV) reverse genetic operating system and application thereof
ActiveCN102071218ALow costReduce labor costsInactivation/attenuationVector-based foreign material introductionComplementary deoxyribonucleic acidFull length cdna
The invention relates to a peste des petits ruminants virus (PPRV) reverse genetic operating system and an application thereof. The PPRV reverse genetic operating system comprises a transcription plasmid and one or more helper plasmids, wherein the transcription plasmid can express the genome full-length cDNA (complementary deoxyribonucleic acid) sequence of the PPRV; and the helper plasmid(s) can express nucleoprotein (N), phosphoprotein (P) and polymerase large protein (L) of the PPRV, and virus replication-permitting host cells of the PPRV. By using the PPRV reverse genetic operating system, the recombined PPRV is successfully saved. The establishment of the PPRV reverse genetic operating technical platform provides an excellent technical platform for the development of PPRV live vector vaccines and the fundamental research related to PPRV.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
DNA expression vectors and methods of use
Owner:EMORY UNIVERSITY
DNA expression vectors and methods of use
The present invention relates to novel plasmid constructs useful for the delivery of DNA vaccines. The present invention provides novel plasmids having a transcription cassette capable of directing the expression of a vaccine nucleic acid insert encoding immunogens derived from any pathogen, including fungi, bacteria and viruses. The present invention, however, is particularly useful for inducing in a patient an immune response against pathogenic viruses such as HIV, measles or influenza. Immunodeficiency virus vaccine inserts of the present invention express non-infectious HIV virus-like particles (VLP) bearing multiple viral epitopes. VLPs allow presentation of the epitopes to multiple histocompatability types, thereby reducing the possibility of the targeted virus escaping the immune response. Also described are methods for immunizing a patient by delivery of a novel plasmid of the present invention to the patient for expression of the vaccine insert therein. Optionally, the immunization protocol may include a booster vaccination that may be a live vector vaccine such as a recombinant pox virus or modified vaccinia Arbora vector. The booster live vaccine vector includes a transcription cassette expressing the same vaccine insert as the primary immunizing vector.
Owner:EMORY UNIVERSITY
Salmonella enteritidis double knockout attenuated mutant and preparation as well as application thereof
The invention discloses a salmonella enteritidis double knockout attenuated mutant and preparation as well as application thereof. The salmonella enteritidis double knockout attenuated mutant is obtained by knocking out crp gene and spiC gene of salmonella enteritidis C50041. The invention further discloses a preparation method as well as application of the salmonella enteritidis double knockout attenuated mutant. Further attenuation of the salmonella enteritidis attenuated mutant is realized. A foundation is laid for researching salmonella enteritidis attenuated live vaccines and live vector vaccines.
Owner:YANGZHOU UNIV
Pig viral infectious disease gene recombined live vaccine using canine II type adenovirus as carrier and preparation process thereof
InactiveCN1827172AGood genetic stabilityEasy to storePowder deliveryGenetic material ingredientsAntigenVp4 gene
This invention supplies a series of production techniques of gene recombination live vaccine of swine virus contagion with canine ó� adenovirus as carrier and finished goods. The viral live vectors vaccine takes swine important virus zymad protective antigens gene as object gene, which are chosen from HCV-E1, E2 gene, FMDV-VP1íóVP2íóVP3íóVP4 gene, TGEV-SíóNíóM gene, PEDV-SíóNíóM geneú¼SIV-HAíóNA geneú¼RV-GíóN gene, etc. The produced vaccines contain recombined swine influenza virus HA gene adenovirus carrier live vaccine, swine plague virus E2 gene adenovirus carrier live vaccine and recombination swine AsiaI foot-and-mouth disease virus VPI gene adenovirus carrier live vaccine. Recombination virus has good inheritance stability, and vaccine immunization can induct pig develop differential antiviral neutralization antibody. It has good immune protection effect and has no toxic side effect. The goods are facilitating for preserve and transportú”it has long storage life and simple technics, and it fits for commercial manufacture.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
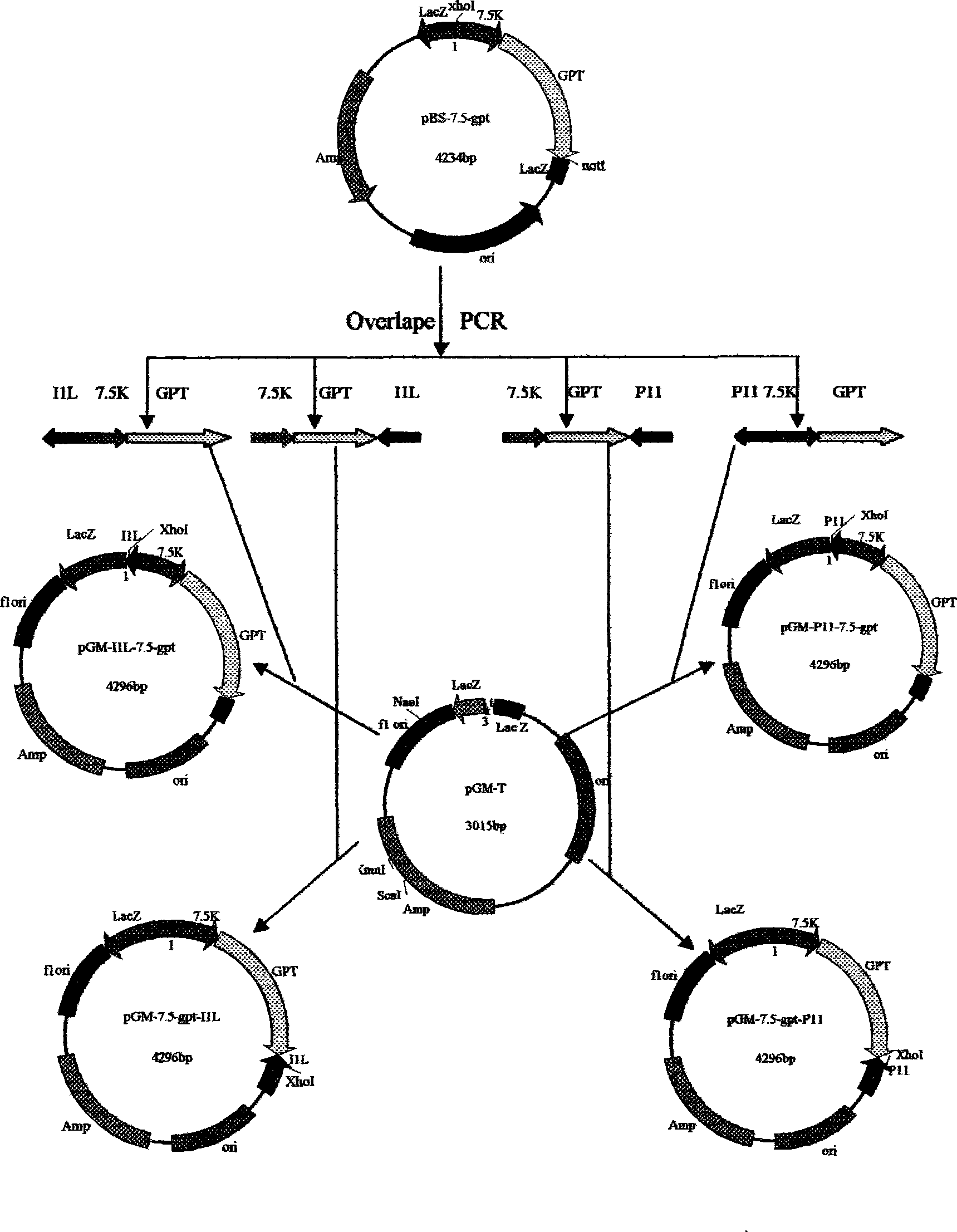
Goat pox vaccine strain expression vector
The invention provides an expression vector used for expressing foreign protein in the recombination of goat pox virus. A foreign gene is inserted in the down stream of a VVI1L promoter or a VVP11 promoter in the expression vector, by the homologous recombination with the parent plant of the goat pox virus, 12 recombined sheep pox viruses for expressing the foreign protein are obtained, a flanking sequence in the vector, which can make the homologous recombination with the parent plant of the goat pox virus, is a sheep pox virus G14-STV-44-55 vaccine strain which contains TK gene, IFNR-gamma gene or RR small subunit gene, by utilizing the expression vectors, the recombined goat pox viruses expressing different foreign proteins can be constructed by different methods, and the recombined goat pox viruses can be used as recombined live vector vaccines to prevent and treat diseases and study relations of expression, processing, structure and function of different biological active proteins.
Owner:SHIHEZI UNIVERSITY
Preparation method of avian influenza virus HA gene recombinant adenovirus
InactiveCN104404005ASolve technical problems with low expression efficiencyViruses/bacteriophagesGenetic engineeringHemagglutininAvian influenza virus
The invention provides a preparation method of avian influenza virus HA gene recombinant adenovirus, which creatively comprises the following steps: carrying out a series of intermediate processes on plasmid pCAGGS, adenovirus shuttle plasmid pShuttle, adenovirus framework plasmid pAdEasy-1 and the like to obtain a gene expression plasmid and other intermediate products, and transfecting the obtained recombinant adenovirus plasmid with 293 cell; and carrying out immunohistochemical screening on the recombinant virus according to the adenovirus-infected cytopathy and specific cells. By using the CAG as the promoter to express the target gene, the method obviously enhances the expression level of the target gene. The hemagglutinin recombinant adenovirus for respectively expressing H5N1 and H9N2 subtype avian influenza viruses provides a virus model for development of the H5 / H9 subtype avian influenza virus bivalent nucleic acid vaccine, and also lays the foundation for development of the AIV (avian influenza virus) adenovirus live vector vaccine.
Owner:TIANJIN RINGPU BIO TECH
Multivalent live vector vaccine against Clostridium difficile-associated disease
ActiveUS10046040B2Antibacterial agentsBacterial antigen ingredientsDiseaseClostridium difficile toxin A
Owner:UNIV OF MARYLAND BALTIMORE
Marek's disease virus infectivity recombinant cloning system, and construction method and application thereof
ActiveCN104946678AImprove securityEasy to insertMicroorganism based processesAntiviralsMarek's disease virus MDVGenome
The invention discloses a Marek's disease virus (MDV) infectivity recombinant cloning system, and a construction method and application thereof and belongs to the field of biotechnologies. The MDV infectivity recombinant cloning system comprises a plurality of cosmids, wherein an MDV vaccine strain gene segment is cloned in each cosmid; the MDV vaccine strain gene segment contains mutually superposed regions and can be spliced to cover an integral MDV vaccine strain genome; an exogenous gene is inserted into a non-essential region for replication of at least one of the cosmids. The MDV infectivity recombinant cloning system can quickly and efficiently clone an exogenous gene expression cassette into the MDV genome to further realize rescue of the recombinant MDV. The recombinant cloning system can be used for very conveniently inserting different foreign genes into the corresponding regions so as to construct an MDV living-vector multi-vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes and construction method thereof
ActiveCN104195116AViral antigen ingredientsMicroorganism based processesF proteinNewcastle disease virus NDV
The invention discloses a recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes and a construction method thereof, belonging to the field of recombinant virus vaccines. The recombinant Newcastle disease virus is a goose isolate, the cleavage site amino acid sequence of F protein of the recombinant Newcastle disease virus is GRQGRL, and the P3 gene is positioned in a noncoding region between the P gene and M gene of Newcastle disease virus. The transcription plasmid pCI-NA-VP3(SEQ ID NO.1) and the transcription helper plasmid pcDNA-N, pcDNA-P and pcDNA-L(SEQ ID NO. 2-4) cotransfect a host cell licensed by application of the Newcastle disease virus to culture a transfected host cell, and the recombinant Newcastle disease virus can be saved from a cell suspension of the transfected host cell. The recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes can be used as a bivalent living-vector vaccine for preventing Newcastle disease virus and goose parvovirus.
Owner:JILIN UNIV
AIDS vaccine based on replicative vaccinia virus vector
The present invention relates to replicative live AIDS carrier vaccine expressing HIV antigen and its use. The vaccine is constructed based on replicative vaccinia virus, such as vaccinia virus Tiantan strain. The replicative live AIDS carrier vaccine can induce high level HIV resisting body fluid and cellular immune response. The present invention provides the carrier for constructing the AIDS vaccine, and also relates to immunizing process with the AIDS vaccine.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
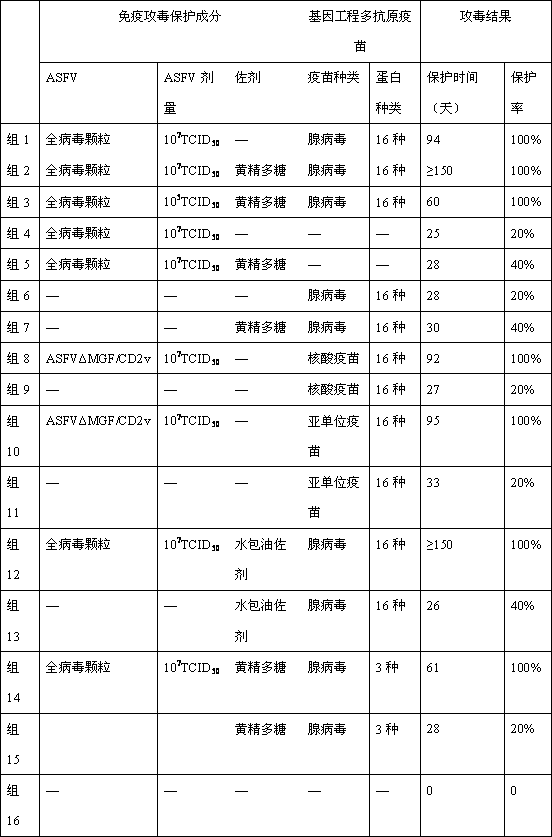
Application of immune attacking protection component for inactivated ASFV (African swine fever virus) serving as complex vaccine
ActiveCN110302371AEnhance the effect of immune attack protectionSolve protection problemsViral antigen ingredientsAntiviralsAdjuvantAfrican swine fever
The invention discloses an application of an immune attacking protection component for an inactivated ASFV (African swine fever virus) serving as a complex vaccine, and relates to an African swine fever gene engineering recombinant living vectored vaccine, subunit vaccine or nucleic acid vaccine immune attacking protection component, namely, an inactivated African swine fever virus or mixture of the inactivated African swine fever virus and adjuvants such as polysaccharides. The inactivated African swine fever virus or the mixture of the inactivated African swine fever virus and the adjuvantssuch as the polysaccharides can improve the immune attacking protection effect of an African swine fever gene engineering recombinant living vectored vaccine, a subunit vaccine or a nucleic acid vaccine, and can realize 100% protection. Besides, the invention further provides the complex vaccine jointly prepared from the immune attacking protection component and the gene engineering recombinant living vectored vaccine, the subunit vaccine or the nucleic acid vaccine.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Live vector vaccine for expressing peste des petits ruminants virus (PPRV) H gene and preparation method thereof
InactiveCN102178947AGood genetic stabilityEasy to storeGenetic material ingredientsMicroorganism based processesSide effectNeutralizing antibody
The invention provides a live vector vaccine for expressing a peste des petits ruminants virus (PPRV) H gene and a preparation method thereof. In the invention, the prepared recombinant virus has good genetic stability so that a goat can be induced to generate a specific antiviral neutralizing antibody after being immunized by the vaccine prepared from the virus. The live vector vaccine has the advantages of good immune protective effect, no toxic side effect, long shelf life and simple production process, and is easy for preservation and convenient for transportation, thus being applicable to industrialized production.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
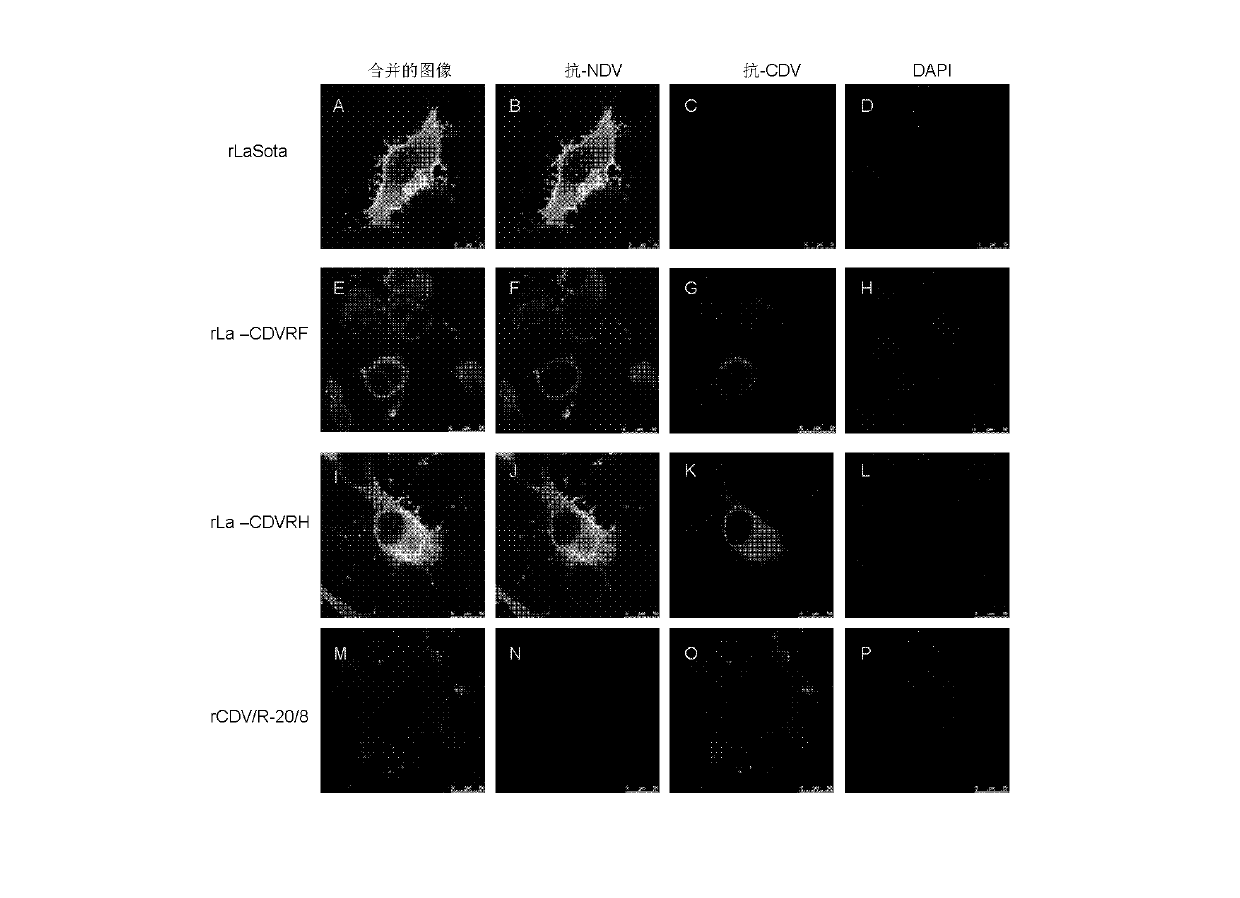
Preparation method and application of newcastle disease virus living-vector vaccine through gene recombination of canine distemper attenuated vaccine strains F and H
InactiveCN102816741AHigh growth titerReduce pathogenicityViral antigen ingredientsMicroorganism based processesCanine distemper virus CDVVector vaccine
The invention relates to a recombination newcastle disease LaSota attenuated vaccine for expressing canine distemper virus fusion protein (F) or canine distemper virus hemagglutinin protein (H). Particularly, the recombination newcastle disease LaSota attenuated vaccine is rLa-CDVR-F or rLa-CDVR-H. The invention further discloses a method for preparing the recombination newcastle disease LaSota attenuated vaccine and application of the recombination newcastle disease LaSota attenuated vaccine in preparation of vaccines / kits.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +2
Preparation method of live vector vaccine for controlling chicken coccidiosis and application thereof
The invention discloses a preparation method of a live vector vaccine for controlling chicken coccidiosis, comprising the following steps of obtaining a recombinant expression vector by connecting a mature peptide of an eimeria tenella sporozoite antigen gene 3-1E gene and an expression vector of bacillus subtilis, obtaining a recombinant strain by transferring the recombinant expression vector into a bacillus subtilis host, and culturing the recombinant strain to finally obtain the live vector vaccine for controlling the chicken coccidiosis by. The live vector vaccine can be used for controlling and / or treating the chicken coccidiosis. The invention also discloses an oral biological preparation for controlling the chicken coccidiosis, prepared by utilizing the live vector vaccine. The oral biological preparation comprises the live vector vaccine and a vector. The oral biological preparation per gram contains the live vector vaccine with a living bacteria number of 108-109cfu.
Owner:HANGZHOU BAODELI BIOTECH
Living-vector vaccine of H5N1 subtype of avian influenza virus and duck enteritis virus
ActiveCN103881981AShort cycleThere is no cross-species transferBacteriaMicroorganism based processesDiseaseAnimal Genetics
The invention belongs to the technical field of animal genetic engineering, and particularly relates to a living-vector vaccine of an H5N1 subtype of avian influenza virus and a duck enteritis virus. A vaccine strain rDEV-HA5 is preserved at the typical culture preservation centre in China, wherein the preservation number is CCTCC NO:V201404. The building method disclosed by the invention comprises the following steps: inserting a segment of an H5N1 subtype of avian influenza virus ha gene into an artificial chromosome plasmid pBAC-C-KCE of the duck enteritis virus, so as to obtain recombinant plasmids pBAC-C-KCE-HA5, wherein the gene structure composition of the plasmid pBAC-C-KCE is shown in a figure 7; the gene structure composition of the plasmids pBAC-C-KCE-HA5 is shown in a figure 14. Biological function verification proves that the vaccine disclosed by the invention has the effect of simultaneously preventing duck enteritis and the H5N1 subtype of avian influenza, and can achieve the target of simultaneously preventing two diseases by a needle.
Owner:HUAZHONG AGRI UNIV
Salmonella enteritidis strain with ssrAB gene deletion and construction method thereof
InactiveCN105018400ADecreased colonization rateReduce the chance of infection with the pathogenBacteriaMicroorganism based processesBacteroidesIn vivo
The invention discloses a salmonella enteritidis strain with ssrAB gene deletion. The strain is obtained after knockout of altogether 915 base pairs, from amino acid located at site 689 of ssrA gene to amino acid located at site 63 of ssrB gene. The invention further discloses a construction method for the salmonella enteritidis strain with ssrAB gene deletion. The construction method comprises the following steps: (1) acquiring SN and SC genes of ssrAB and connecting upstream and downstream homologous arms through overlap PCR; (2) constructing recombinant suicide plasmid pWM91-ssrAB; (3) carrying out solid phase joint of bacteria; and (4) screening and acquiring G9-2012 (delta ssrAB). The invention has the following beneficial effects: the G9-2012 (delta ssrAB) strain has a decreased colonization rate and obviously weakened virulence in vivo, can be used for development of attenuated live vaccine or live vector vaccine against salmonella enteritidis, and thus reduces the rate of infection of human beings or animals by salmonella enteritidis and guarantees health of the mankind.
Owner:史记生物技术有限公司
Recombinant canine adenovirus type 2 transfer vector, construction method and application thereof
InactiveCN101358202AReduce workloadStable biological propertiesViruses/bacteriophagesFermentationPurification methodsFluorescence
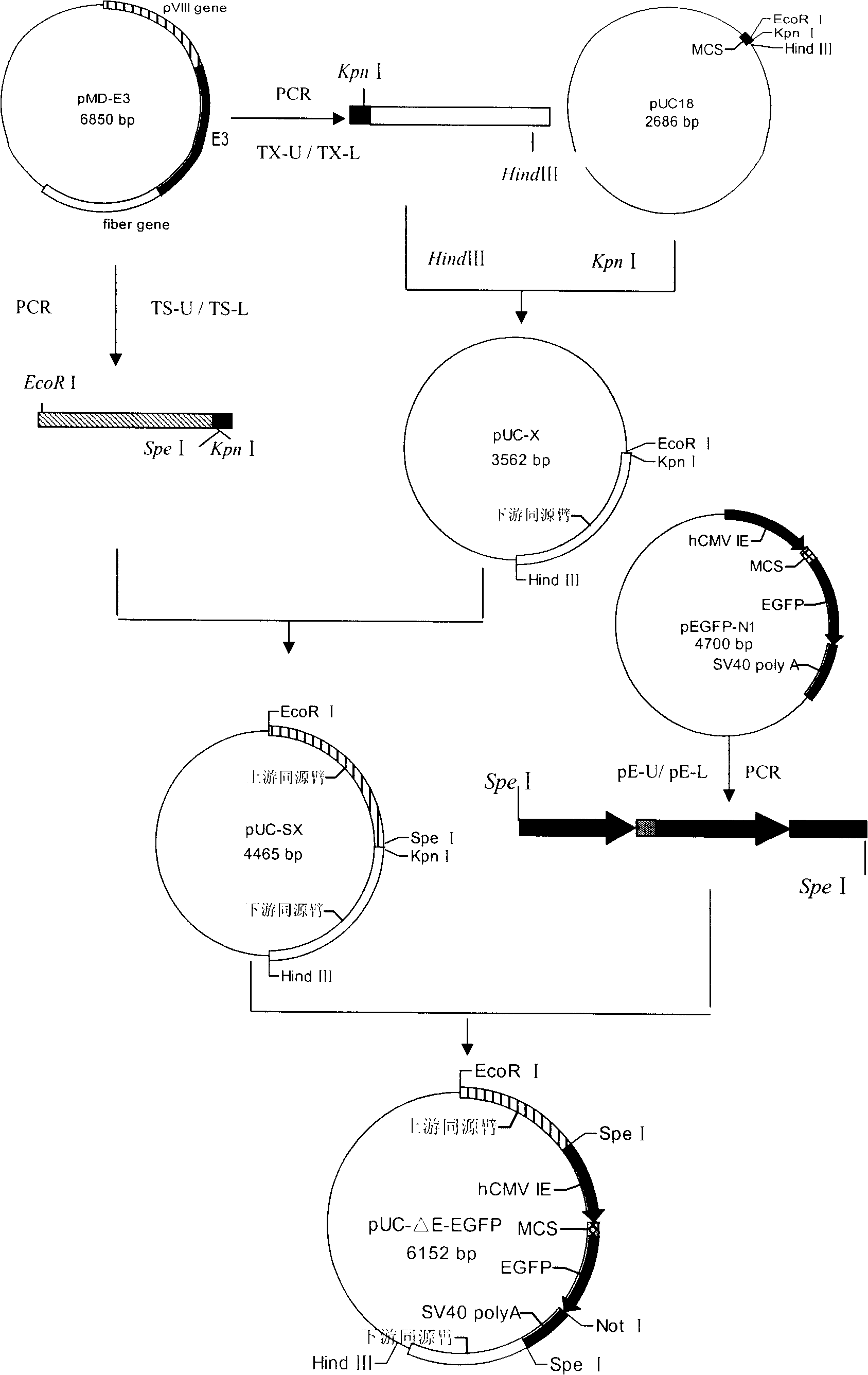
The present invention discloses a recombinant canine adenovirus-2 transfer vector, a method for constructing the recombinant canine adenovirus-2 transfer vector and an applications thereof. The recombinant canine adenovirus-2 transfer vector loses the 1412bp fragment of the E3 region, a large fragment of exogenous gene can be inserted, and moreover, a hCMV IE promoter, a multiple cloning site, an enhanced green fluorescent protein gene and a SV40 early transcribed Poly A signal sequence can be inserted in the position of the lost fragment of the E3 region. The method successfully constructs the lost recombinant CAV-2 transfer vector of the E3 region, obtains a purified canine adenovirus-2 strain containing EGFP reporter gene and optimizes the cloning and purification method thereof, an evaluation indicates that the biological property of the recombinant virus is stable, and therefore the present invention provides a technical platform for the further development of CAV-2 live vector vaccine and related fundamental researches.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Construction of recombinant pseudorabies virus vector expressing foreign protein and preparation method of recombinant pseudorabies virus
The invention provides a method for constructing pseudorabies virus replication non-essential gene insertion mutation by utilizing a CRISPR / Cas9 gene editing system and obtaining a recombinant pseudorabies virus expressing a foreign gene. After the CRISPR / Cas9 gene editing system is introduced into a cell, a viral genome is identified through a pre-screened target sequence, and a pseudorabies virus gene infecting the cell is edited and recombined. The recombinant pseudorabies virus constructed by the method is inserted into a target foreign gene at a specific gene part without affecting replication of the virus, and the recombinant virus is screened forwards or backwards by utilizing a marker in the construction process, so that the obtaining efficiency of the recombinant virus is remarkably improved, and a foundation is laid for constructing a recombinant pseudorabies virus vaccine expressing the foreign gene. The method for constructing the recombinant pseudorabies virus based on theCRISPR / Cas9 gene editing system can be used for quickly constructing a recombinant virus live vector vaccine and has important application value.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Recombination attenuated salmonella typhimurium carrier vaccine of expression IBDV (Infectious Bursal Disease Virus) immunogenic gene and preparation method thereof
InactiveCN101920011AImproving immunogenicityImprove protectionBacteriaViral antigen ingredientsVector vaccineInfectious bursitis
The invention discloses a recombination attenuated salmonella typhimurium carrier vaccine of expression IBDV (Infectious Bursal Disease Virus) immunogenic gene, a preparation method and application thereof, in particular relates to oral attenuated salmonella typhimurium. The recombination attenuated salmonella typhimurium comprises a sequence disclosed by SEQ ID NO.1, and the recombination virus can express IBDV capsid protein VP2. The preparation method comprises the following steps of: inoculating the attenuated salmonella typhimurium in an LB substrate for culturing 18 hours; regulating bacterial concentration as 1010CFU / ml; and preparing the safe and effective oral attenuated salmonella typhimurium carrier vaccine for preventing IBD (Infectious Bursal Diseases).
Owner:NORTHWEST A & F UNIV
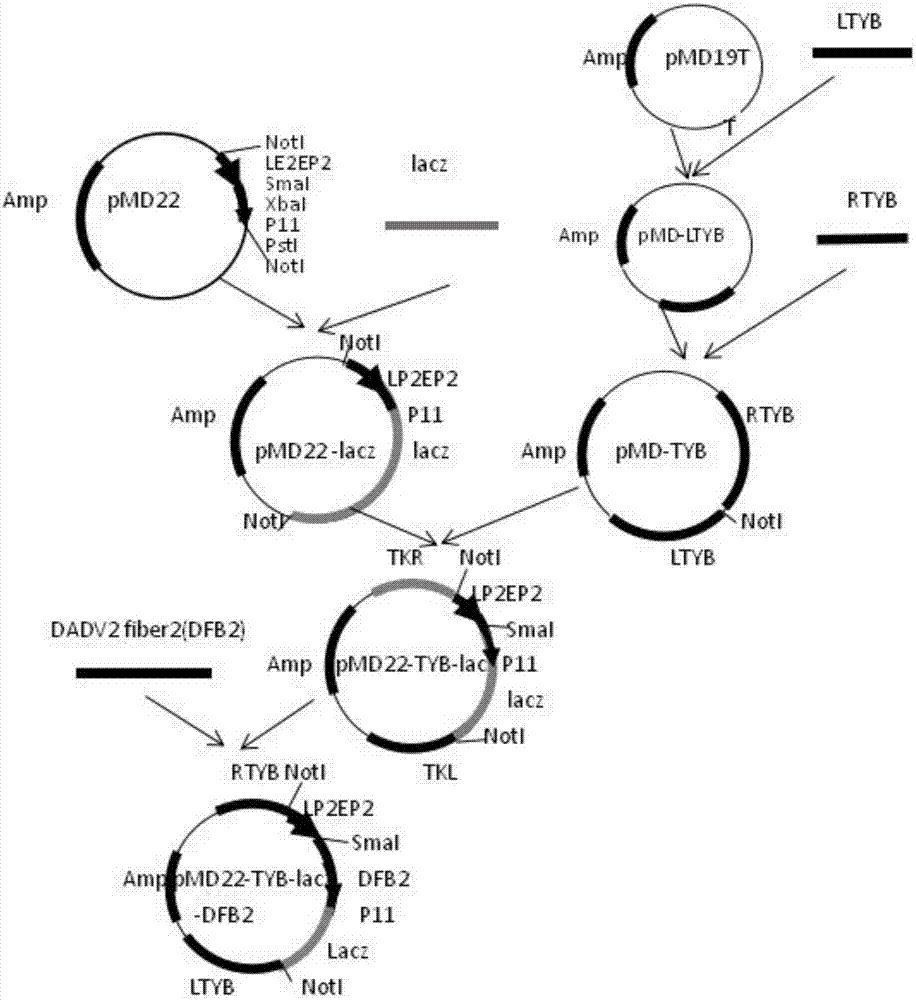
A recombinant fowlpox virus transfer vector expressing a duck adenovirus serotype-2 fiber2 gene, a constructing method thereof and applications of the transfer vector
ActiveCN107475297AAvoid conflict situationsImprove build efficiencyViral antigen ingredientsVirus peptidesGenetic engineeringLive vector vaccine
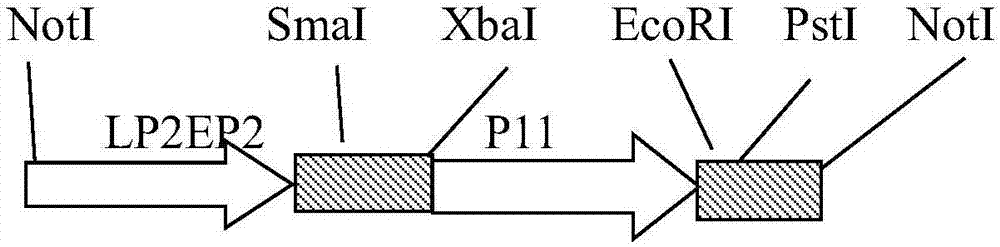
The invention belongs to the technical field of biology, and mainly relates to a recombinant fowlpox virus transfer vector expressing a duck adenovirus serotype-2 (DADV2) fiber2 gene, a constructing method thereof and applications of the transfer vector. According to the transfer vector, a DADV2 fiber2 gene promoted by a fowlpox-virus-containing early-late promoter LP2EP2, a lacz gene promoted by a P11 promoter, and fowlpox virus genome replicated non-essential fragments which are LTYB and RTYB used for homologous recombination are inserted in a TA cloning site of a pMD19T-Simple vector. The method includes constructing a plasmid pMD-TYB; constructing a plasmid pMD22; constructing a plasmid pMD22-lacz; constructing an intermediate vector pMD22-TYB-lacz; constructing the transfer vector pMD22-TYB-lacz-DFB2; and subjecting the transfer vector pMD22-TYB-lacz-DFB2 to effect verification. The recombinant fowlpox virus transfer vector constructed by the method lays a foundation for development of an efficient recombinant fowlpox virus genetic engineering living-vector vaccine expressing the duck adenovirus serotype-2.
Owner:WENS FOOD GRP CO LTD
AIDS vaccine of N1L and B8R gene deletion-based vaccinia virus vector
The invention relates to a replicating type AIDS live vector vaccine expressing a human immunodeficiency virus (HIV) antigen and application thereof. The vaccine is constructed based on a replicatingtype vaccinia virus such as a vaccinia virus Tiantan strain. The replicating type AIDS live vector vaccine can induce high-level anti-HIV humor immune response and cellular immune response, and provides a vector for constructing the AIDS vaccine. The invention also relates to an immunization scheme using the AIDS vaccine.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Genetically engineered bacteria strain expressing porcine transmissible gastroenteritis virus
InactiveCN103060250AAvoid stressAvoid absorptionBacteriaVirus peptidesLive vector vaccineBiomedical engineering
The invention discloses a genetically engineered bacteria strain expressing a porcine transmissible gastroenteritis virus. The strain is Lactococcus lactis MG1363 / pMG36e-S preserved in China center for type culture collection, with a preservation number of CCTCC M 2012355. Lactococcus lactis is a common bacteria in intestinal tracts of human and majority of animals, has characteristics of bacterial resistance and diarrhoea resistance, and can be widely used for researches of viable cell oral vaccines. The recombinant Lactococcus lactis MG1363 / pMG36e-S constructed in the invention can stably and reliably express S protein of active TGEV, can be used for producing genetically engineering subunit vaccines and genetically engineered live vector vaccines, provides a new method for preventing TGE, and is relatively low in cost.
Owner:SHANDONG SINDER TECH
Preparation method and application of live vector vaccine for expressing duck Tembusu virus (DTMUV) prm and E protein recombinant Newcastle disease virus (NDV)
ActiveCN106520710AReduce detoxificationSsRNA viruses negative-senseSsRNA viruses positive-senseTembusu virusNewcastle disease virus NDV
The invention discloses a preparation method for expressing the duck Tembusu virus (DTMUV) prm and the E protein recombinant Newcastle disease virus (NDV). The preparation method comprises the following steps: 1) constructing full-length plasmids of an attenuated ND GM strain; 2) constructing plasmids for expressing the DTMUV prm and the E protein recombinant NDV; 3) rescuing and identifying the recombinant virus aGM-prm / E. The invention also discloses the virus aGM-prm / E prepared by the method. The virus aGM-prm / E is collected at the China Center for Type Culture Collection (CCTCC), with collection number of CCTCC V201644. A vaccine prepared by utilizing the virus can prevent the ND and the DTMUV disease and conduce to reducing virus removal after virulent NDV infection.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI +1
PRRSV and PCV-2bivalent recombinant fowl pox virus disease live vector vaccines
InactiveCN101112621AEffective immunityNo pathologyGenetic material ingredientsRespiratory disorderDiseaseFowl
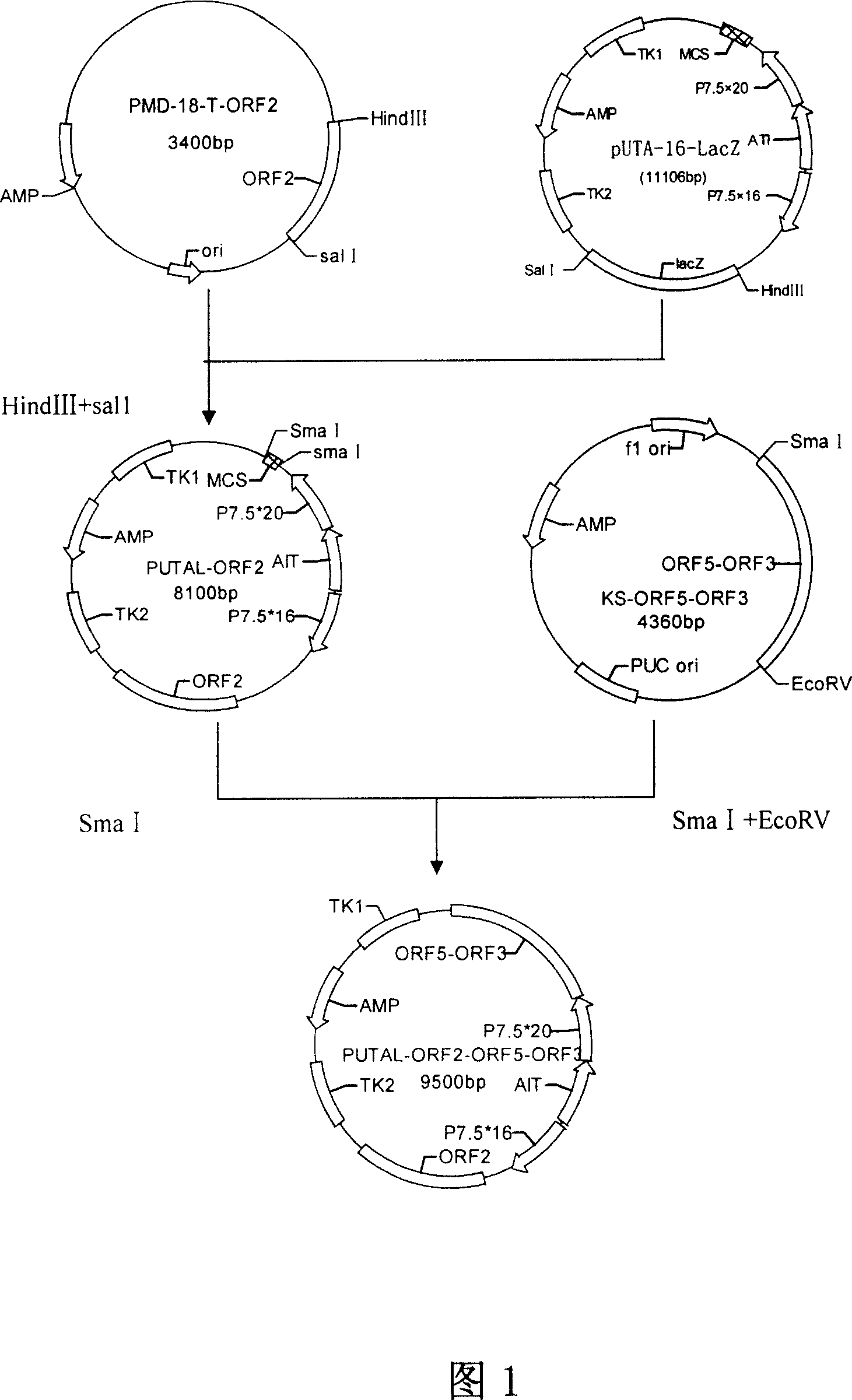
The present invention relates to a PRRSV and PCV-2 bivalent recombinant fowlpox live vector vaccine, which pertains to the field of biotechnology. The present invention aims at providing the PRRSV and PCV-2 bivalent recombinant fowlpox live vector vaccine which has ORF5, ORF3 genes to express the structural glycoprotein of the PRRSV and ORF2 gene to express PCV-2 nucleocapsid protein and can be used as the live vector vaccine for the prevention of PRRS and PCV-2 infection in our country. The present invention constructs the pMD18T-ORF2, pMD18T-ORF5 and pMD18T-ORF3 plasmids, the plasmids are inserted to the downstream of the compound promoter ATI-P7.5 multiplied by 20 of the fowlpox virus expression vector pUTAL, at the same time, the ORF2 gene of the PCV2 are inserted to the downstream of the linking promoter P7.5 multiplied by 16, and the present invention further constructs the recombinant fowlpox virus gene transfer plasmid pUTAL-ORF2-ORF5-ORF3 which contains the ORF3-ORF3 gene of the PRRSV and the ORF2 gene of the PCV2. The present invention can be used as the live vector vaccine for the prevention of PRRS-PCV2 in our country.
Owner:金宁一
Newcastle disease virus rNDV-H52AH9 and construction method and application thereof
InactiveCN103074306AImprove reproductive performanceMicroorganism based processesViruses/bacteriophagesNewcastle disease virus NDVAvian influenza virus
The invention relates to a Newcastle disease virus rNDV-H52AH9, accession number CGMCC (China General Microbiological Culture Collection Center) No: 6654. An attenuated strain AI4 of the Newcastle disease virus is used as a carrier, HA genes of H5 and H9 subtype avian influenza virus are connected in series through FMDV (Foot and mouth disease virus) 2A and are inserted into the genome AI4 containing full-length transcript carrier pNDV / AI4 to obtain recombinant NDV genome full-length clone pNDV / AI4-H52AH9. The recombinant virus rNDV-H52AH9 is obtained by reverse genetic operation, has higher reproductive titer on embryonated eggs, and simultaneously expresses the HA protein of H5 and H9 subtype avian influenza virus stably, and lays a foundation for the development and application of multi-joint recombinant live vector vaccine of AI and ND.
Owner:YANGZHOU UNIV
A recombinant fowlpox virus transfer vector expressing a fowl adenovirus serotype-4 fiber2 gene, a constructing method thereof and applications of the transfer vector
ActiveCN107475296AEliminate conflictImprove efficiencyViral antigen ingredientsVirus peptidesVector vaccineEmbryo
The invention belongs to the technical field of biology, and mainly relates to a recombinant fowlpox virus transfer vector expressing a fowl adenovirus serotype-4 (FADV4) fiber2 gene, a constructing method thereof and applications of the transfer vector. A pMD19T-Simple vector is adopted as a base of the transfer vector. The FADV4 fiber2 gene, a lacz gene, and fowlpox virus genome replicated non-essential fragments which are LTYB and RTYB used for homologous recombination are inserted in a TA cloning site. The method includes constructing a plasmid pMD-TYB; constructing a plasmid pMD22; constructing a plasmid pMD22-lacz; constructing an intermediate vector pMD22-TYB-lacz; amplifying the FADV4 fiber2 gene; constructing a recombinant fowlpox virus transfer vector pMD22-TYB-lacz-F4; subjecting a chicken embryo Fibroblast to cotransfection with the transfer vector pMD22-TYB-lacz-F4 and a fowlpox virus; performing identification to select a positive product; performing subculture continuously; and identifying expression effects of the recombinant fowlpox virus. The recombinant fowlpox virus transfer vector constructed by the method lays a foundation for development of an efficient recombinant fowlpox virus genetic engineering living-vector vaccine expressing the fowl adenovirus serotype-4.
Owner:WENS FOODSTUFF GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com