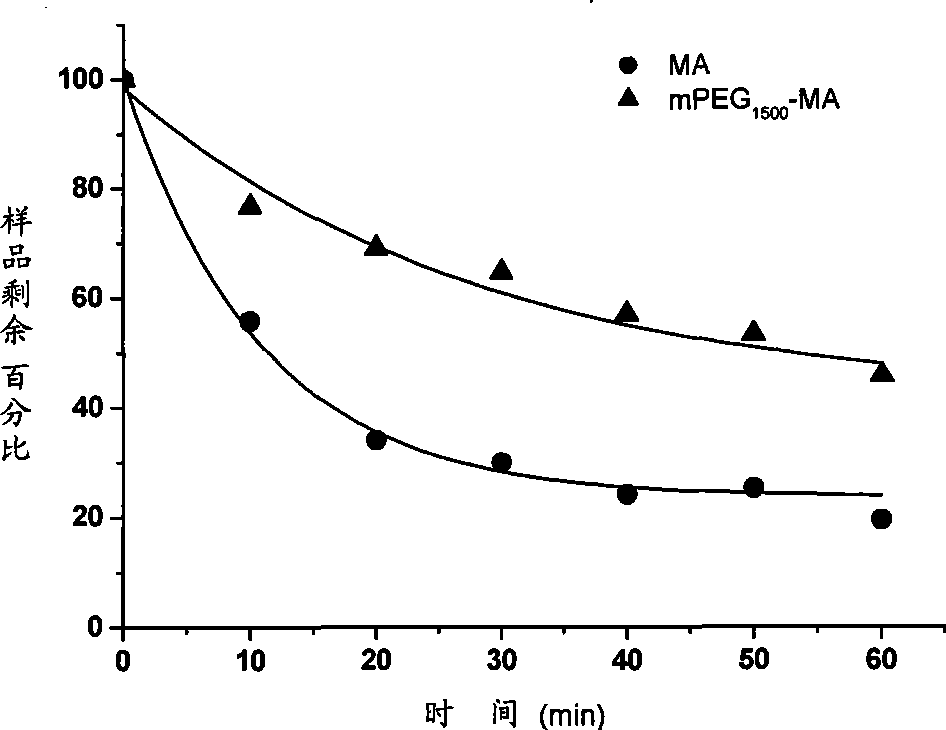
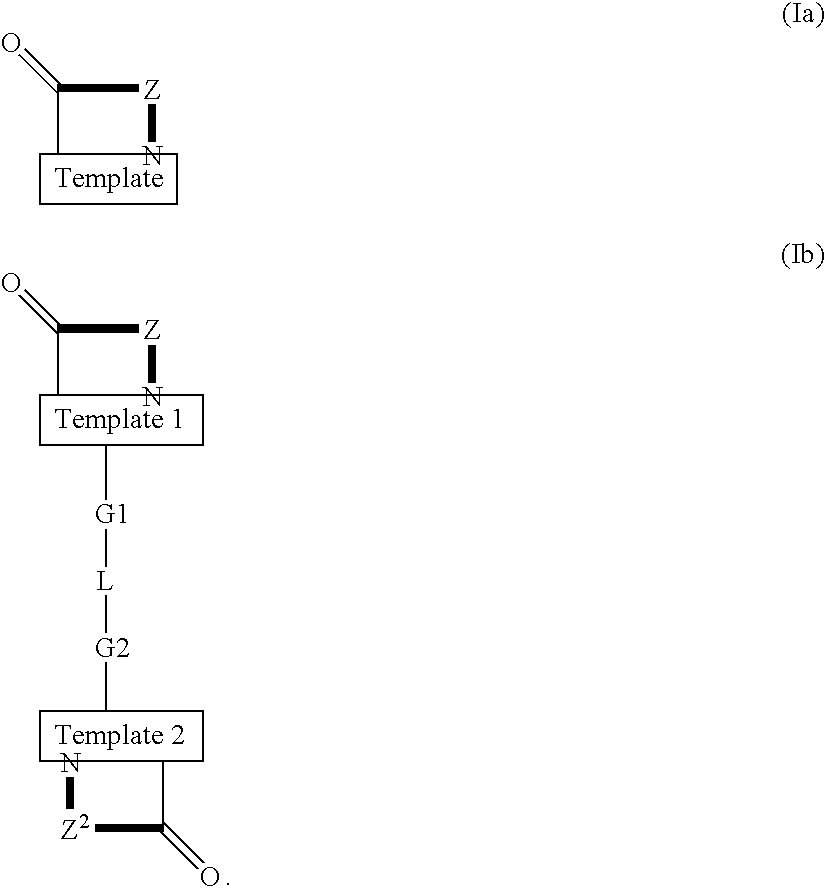
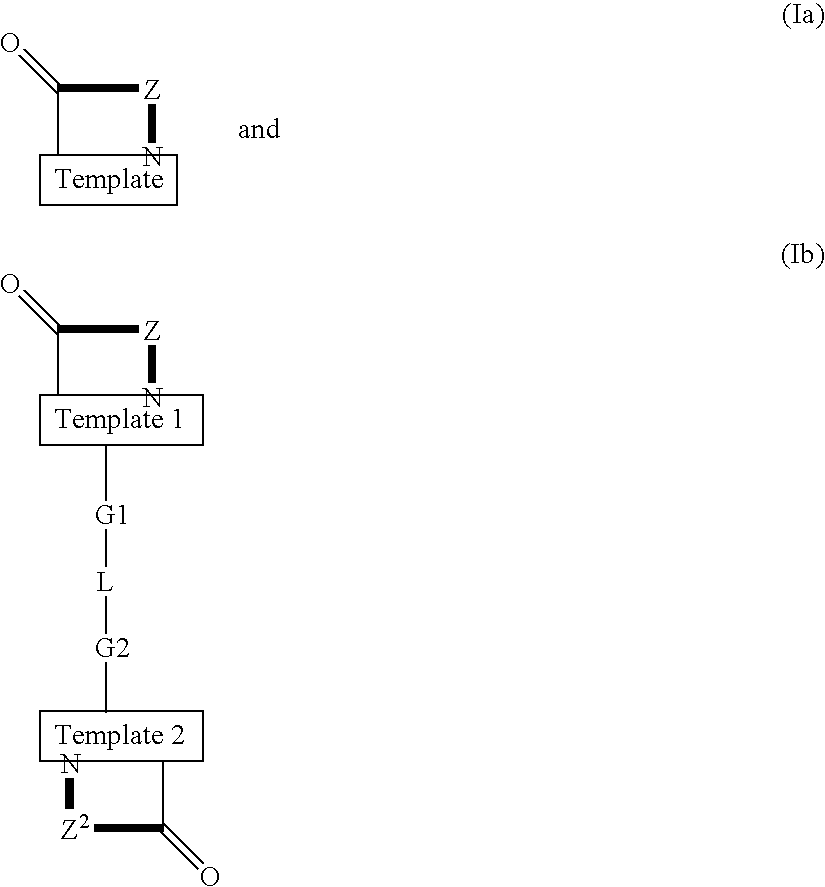
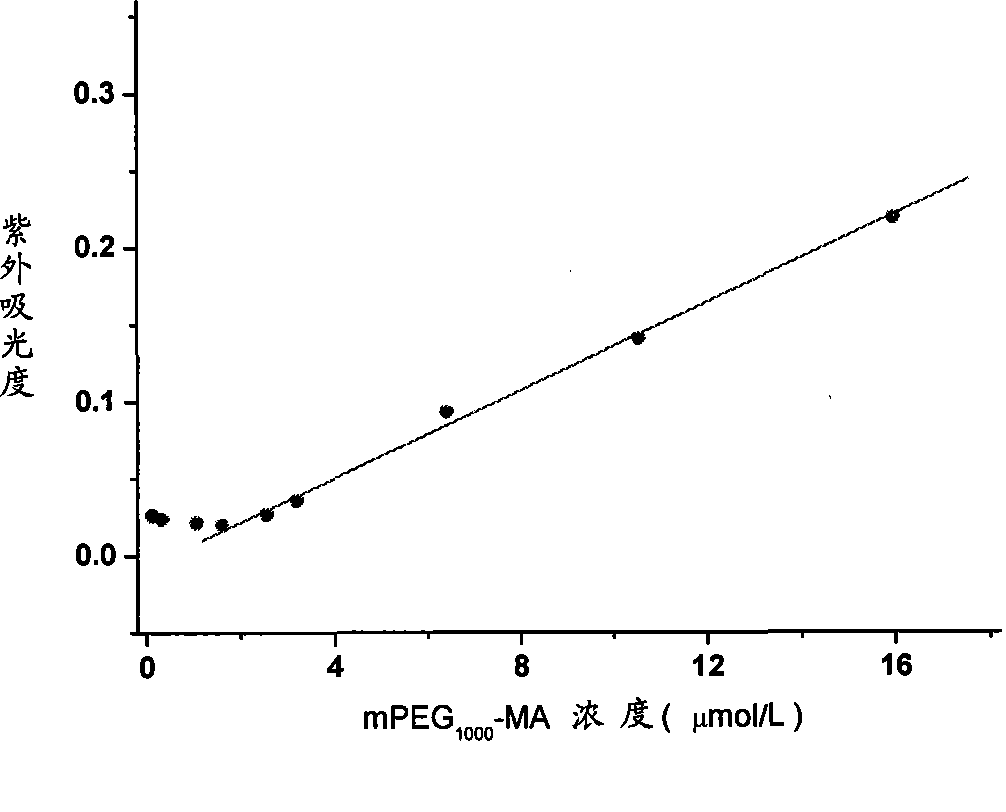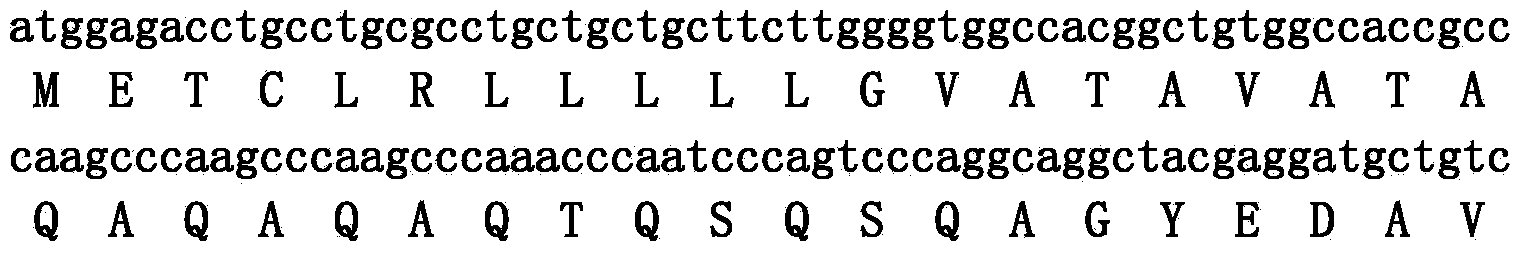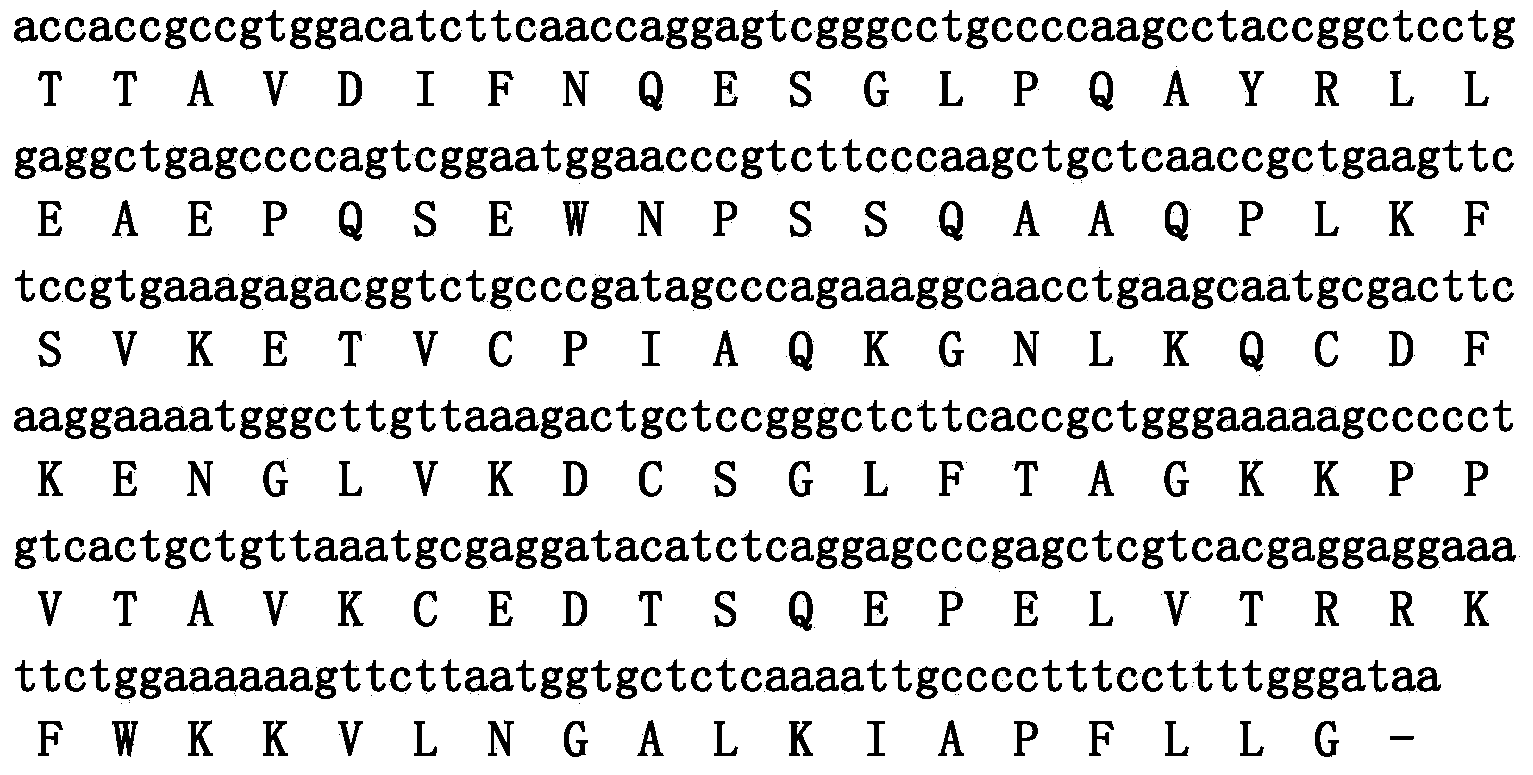Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
149results about How to "Low hemolytic activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
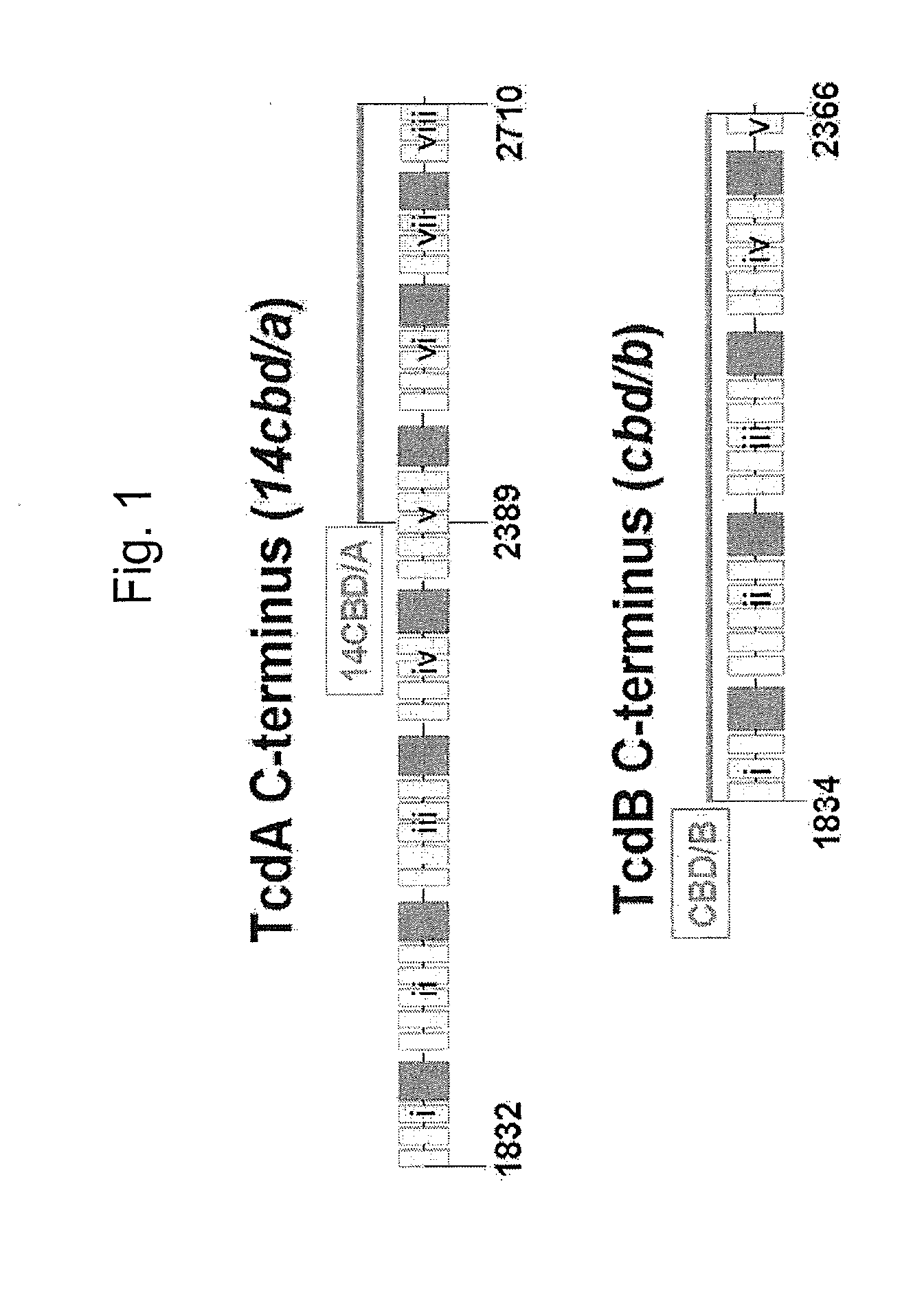
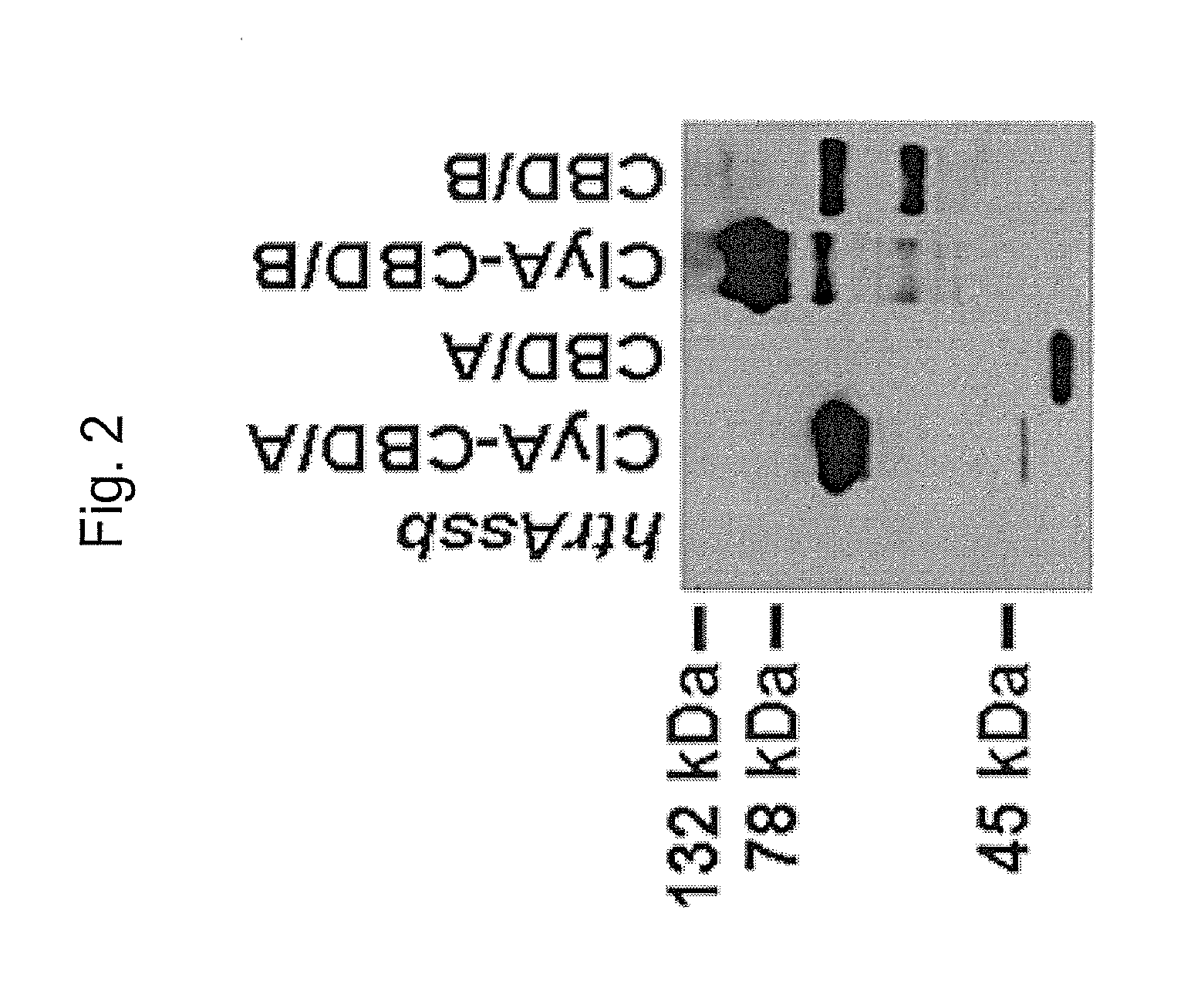
Multivalent Live Vector Vaccine against Clostridium difficile-Associated Disease
ActiveUS20120282293A1Easy to exportLow hemolytic activityAntibacterial agentsBacterial antigen ingredientsClostridium difficile (bacteria)Live vector vaccine
The invention relates to a multivalent Clostridium difficile vaccine comprising a Salmonella Typhi live vector comprising the cell binding domain of TcdA toxin (CBD / A) of Clostridium difficile or an antigenic fragment thereof and the cell binding domain of TcdB toxin (CBD / B) of Clostridium difficile or an antigenic fragment thereof and optionally the cell-binding subunit component (CdtB) of binary toxin of Clostridium difficile or an antigenic fragment thereof. The invention further provides methods of inducing an immune response and methods of preventing recurrence of C. difficile infections in subjects.
Owner:UNIV OF MARYLAND BALTIMORE
Polyglycol modified antimicrobial peptide and uses thereof
InactiveCN101429233AReduce hemolysisSmall toxicityPeptide preparation methodsBulk chemical productionAntimicrobial peptidesAntibacterial activity
The invention relates to a series of polyethylene glycol modified cationic antibacterial peptide with different molecular weight. The modified antibacterial peptide is self-assembled into a nanometer micelle in an aqueous medium. The formation of the micelle can play a role in protecting the antibacterial peptide and weakening the degradation effect of protease on the antibacterial peptide, and simultaneously improves the stability and antibacterial activity of polypeptide in serum. In addition, the modification of polyethylene glycol obviously reduces the hemolytic toxic side effects of the antibacterial peptide.
Owner:NANKAI UNIV
Natural antimicrobial peptide Alligatorin4 and application thereof
ActiveCN104151415ABroad-spectrum high-efficiency antibacterial effectSmall molecular weightAntibacterial agentsCosmetic preparationsPreservativeHeat stability
The invention discloses a natural antimicrobial peptide Alligatorin4 and an application thereof in the antimicrobial aspect. The antimicrobial peptide Alligatorin4 disclosed by the invention is derived from Alligator sinensis. The mature peptide sequence of the antimicrobial peptide is obtained by retrieving a gene database of the Alligator sinensis, screening and analyzing, and the amino acid sequence is as shown in SEQ ID NO. 1 in a sequence table. By comparative analysis, the natural antimicrobial peptide Alligatorin4 is obviously different from the amino acid sequences of all the existing antimicrobial peptides, and belongs to a novel antimicrobial peptide. Antimicrobial experimental results show that the antimicrobial peptide Alligatorin4 disclosed by the invention has very strong antimicrobial activity against Gram-positive bacteria, Gram-negative bacteria and fungi, has broad-spectrum and efficient antimicrobial effect, has the characteristics of low hemolytic activity, good salt tolerance, good heat tolerance, good heat stability and the like, and can be applied in preparation of medicines for resisting bacteria and inhibiting the growth of bacteria, preservatives, veterinary medicines, animal feeds and cosmetics.
Owner:SUZHOU UNIV
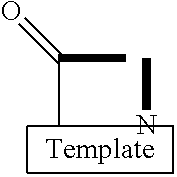
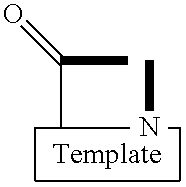
Template-fixed peptidomimetics with antimicrobial activity
InactiveUS7253146B2Facilitates structure-activity studiesPotent anticancer activityBiocideImmunoglobulinsDiseaseCancer cell
Template-fixed β-hairpin peptidomimetics of the general formulae (I) and (II) wherein Z, Z1 and Z2 are template-fixed chains of 8 to 16 α-amino acid residues which, depending on their positions in the chain (counted starting from the N-terminal amino acid) are Gly, or Pro, or of certain types which, as the remaining symbols in the above formulae, are defined in the description and the claims, and salts thereof, have the property to inhibit the growth of or to kill microorganisms and cancer cells. They can be used as disinfectants for foodstuffs, cosmetics, medicaments or other nutrient-containing materials or as medicaments to treat or prevent infections or diseases related to such infections and / or cancer. These β-hairpin peptidomimetics can be manufactured by a process which is based on a mixed solid- and solution phase synthetic strategy
Owner:POLYPHOR AG
Novel synthesis antibacterial peptides and application thereof
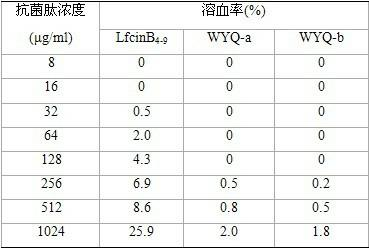
InactiveCN102432672ABroad-spectrum killing activityEnhance killing activityAntibacterial agentsPeptide/protein ingredientsHemolysisCombinatorial chemistry
The invention provides novel synthesis antibacterial peptides and application thereof. The novel synthesis antibacterial peptides are designed and synthesized on the basis of analyzing sequences and structures of natural antibacterial peptides, and the sequences of the novel synthesis antibacterial peptides comprise WYQ-a: ArgArgTrpTrpArg and WYQ-b: PheArgTrpTrpArg. The synthesis antibacterial peptides have the broad spectrum killing activity on gram-positive bacteria and gram-negative bacteria, have higher bactericidal activity than that of the natural antibacterial peptides, and do not have a toxic effect on animal and plant cells. The synthesis antibacterial peptides have broad antibacterial spectra and simple structures, are not needed to be modified and connected and are convenient to synthesize artificially. Experiments prove that: for G+bacteria (the gram-positive bacteria) and G-bacteria (the gram-negative bacteria), the novel synthesis antibacterial peptides have the obvious bacteriostatic and bactericidal activity and do not have hemolysis activity, so the synthesis antibacterial peptides have significant value in the development and application of antibacterial medicines.
Owner:CHONGQING UNIV OF TECH
Transformed body HC-15 of antimicrobial peptide Hc-CATH (cathelicidins) of hydrophis cyanocinctus and preparation method and application of transformed body
ActiveCN103665111ABroad-spectrum high-efficiency antimicrobial activitySmall molecular weightPeptide/protein ingredientsPeptidesTailAntimicrobial peptides
The invention belongs to the technical field of biomedicine, and particularly relates to a transformed body antimicrobial peptide HC-15 of antimicrobial peptide Hc-CATH (cathelicidins) of hydrophis cyanocinctus and a preparation method and application of the transformed body. The transformed body antimicrobial peptide is the transformed body HC-15 of antimicrobial peptide Hc-CATH (cathelicidins) of hydrophis cyanocinctus, is linear chain polypeptide and comprises 15 amino acid residues, the tail end of C- is amidated, the molecular weight is 1974.46Da, and the isoelectric point is 12.61. The transformed body antimicrobial peptide HC-15 has high-potency antimicrobial activity; furthermore, the transformed body antimicrobial peptide HC-15 has beneficial characteristics of small molecular weight, simple structure, low hemolytic activity and simple preparation method.
Owner:SHANDONG INT BIOTECH PARK DEV +2
Antibacterial peptide LZ1 and application of antibacterial peptide in preparation of antibacterial medicament
InactiveCN102924574AEasy to synthesizeSmall molecular weightAntibacterial agentsPeptide/protein ingredientsArginineLysine arginine
An antibacterial peptide LZ1 is an artificially designed and synthesized active polypeptide and contains 15 amino acid residues, the molecular weight is 2,228.77Da, and the isoelectric point is 12.05; and the full sequence of the antibacterial peptide is valine-lysine-arginine-tryptophan-lysine-lysine-tryptophan-tryptophan-arginine-lysine-tryptophan-lysine-lysine-tryptophan-valine-NH2. The antibacterial peptide LZ1 is small in molecular weight, strong in bactericidal effect and wide in antibacterial spectrum, almost does not have hemolytic activity or eukaryocyte toxicity, is a small molecular antibacterial peptide with application value, is convenient to artificially synthesize, and can be used for preparing an antibacterial medicament.
Owner:SUZHOU KANGER BIOLOGICAL MEDICAL +1
Derived peptide IR2 of pig-derived antibacterial peptide as well as preparation method and application thereof
ActiveCN103923189ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsArginineAntibacterial activity
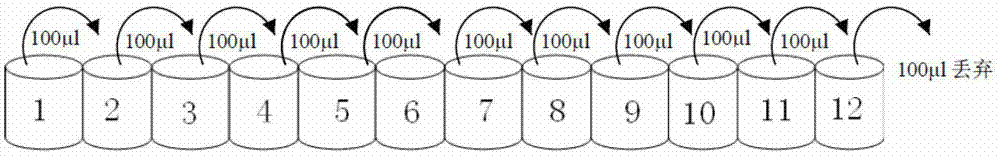
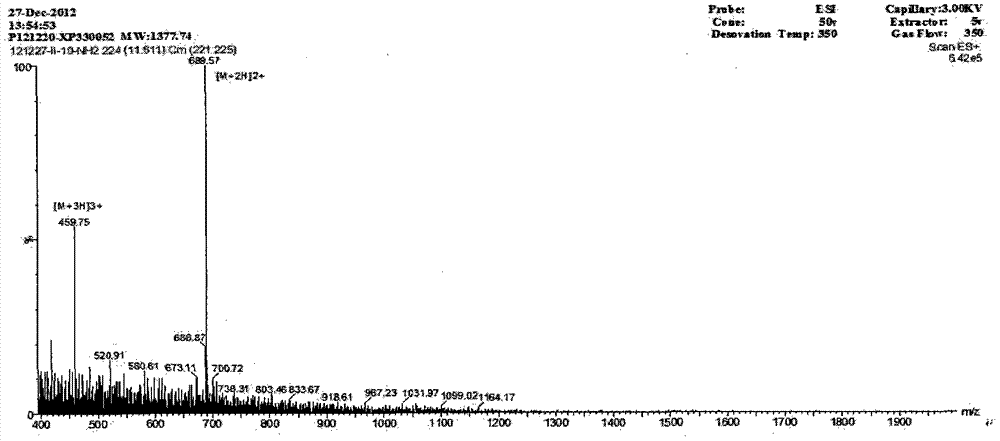
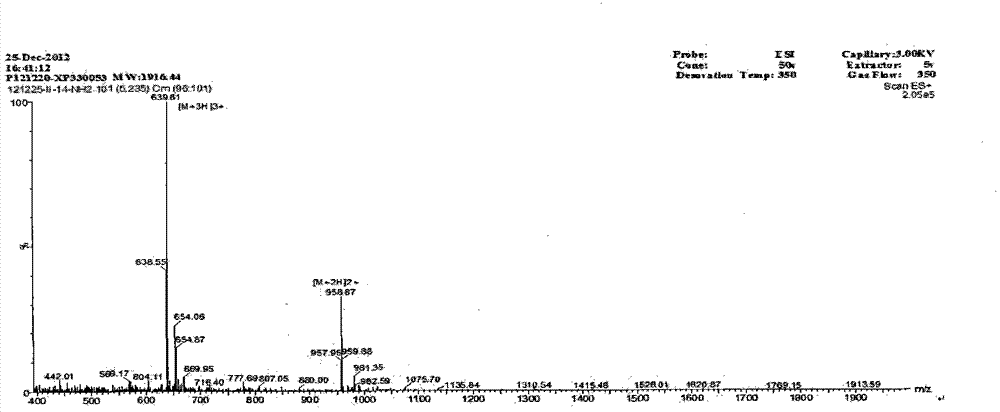
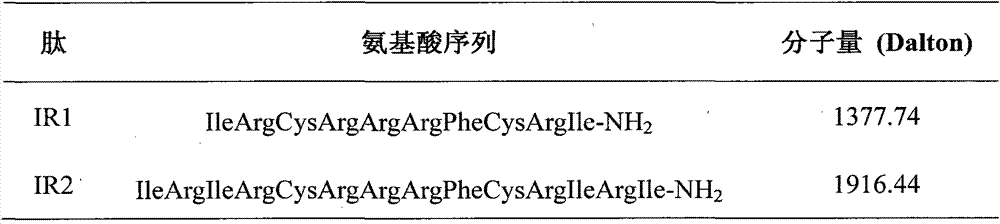
The invention provides a derived peptide IR2 of a pig-derived antibacterial peptide as well as a preparation method and application thereof. The sequence of the derived peptide IR2 of the pig-derived antibacterial peptide is as shown in SEQ ID No.1. Antibacterial peptides IR1 and IR2 are obtained by using a fixed point amino acid fragment interception and binary amino acid sequence superimposing method for intercepting six amino acid fragments of a pig-derived PG-1 corner part and symmetrically and circularly arranging charged amino acid arginine and hydrophobic amino acid isoleucine serving as repeated binary sequence units at the two sides of the corner. The antibacterial activity of the antibacterial peptide IR2 is higher than that of the antibacterial peptide IR1. The therapeutic index of the antibacterial peptide IR2 reaches up to 43.2 and is 216 times of that of the pig-derived antibacterial peptide RG-1. By virtue of the method, the hemolytic activity of the antibacterial peptide is greatly reduced under the condition of increasing the antibacterial activity of the antibacterial peptide, the selectivity of the antibacterial peptide between bacterial cells and mammalian cells is increased, and the development potential of the antibacterial peptide serving as antibiotic substitutes is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Structure and application of three-dimensional fluorescence standard spectrum library used for recognizing toxic-to-fish algae
ActiveCN104316505AUnderstand the characteristics of toxic productionDiscrimination results are stableFluorescence/phosphorescenceSpecial data processing applicationsRed tideBiology
The invention discloses a structure and an application of a three-dimensional fluorescence standard spectrum library used for recognizing toxic-to-fish algae. The hemolytic activity and the change of the toxic-to-fish algae in different growing periods and under the control of environmental factors, and a chlorophyll three-dimensional fluorescence spectrum of the toxic-to-fish algae are researched and contrasted with a chlorophyll three-dimensional fluorescence spectrum of large sample nontoxic-to-fish algae, three-dimensional fluorescence spectrum analysis and recognition methods are screened, a fluorescence characteristic spectrum closely related to the toxic-to-fish algae and the hemolytic activity of the toxic-to-fish algae is extracted, and three-dimensional fluorescence standard spectrum libraries of the toxic-to-fish algae and the nontoxic-to-fish algae are screened by a clustering method; based on the three-dimensional fluorescence standard spectrum libraries, a Fisher discrimination function for recognizing the toxic-to-fish algae and a function for discriminating the degree of the hemolytic activity of the toxic-to-fish algae are established respectively. Discrimination results obtained by utilizing the discrimination functions are more stable, accuracy and reliable. The method provided by the invention realizes highly correct diagnosis and recognition functions for the toxic-to-fish algae in an in-place red tide water body and the hemolytic activity of the toxic-to-fish algae.
Owner:SHENZHEN LIGHTSUN TECH CO LTD +1
Ultra-short peptide Purin-WH for boosting skin repairing as well as preparation method and application thereof
ActiveCN107344959APromote repairSmall molecular weightCosmetic preparationsPeptide/protein ingredientsHemolysisCuticle
The invention discloses an ultra-short peptide Purin-WH for boosting skin repairing as well as a preparation method and application thereof and belongs to the field of biological medicine. The ultra-short peptide Purin-WH is a straight-chain peptide; the amino acid structure of the ultra-short peptide Purin-WH is Val-Val-Pro-Thr-Val-Val-Cys-Pro-Lys; the ultra-short peptide Purin-WH can be synthesized by an automatic peptide synthesizer and is desalted and purified through HPLC anti-phase column chromatography. The ultra-short peptide Purin-WH disclosed by the invention is low in molecular weight, has a simple structure, is ultralow in compounding cost, is capable of obviously quickening the skin wound healing, effectively removing active oxygen and boosting the generation of skin collagen and is beneficial to skin repairing; the ultra-short peptide Purin-WH is free from cytotoxicity and hemolysis; the ultra-short peptide can be used for preparing the drug for repairing and regenerating injured epidermis, such as, the drugs for treating burn, scald and skin ulcer; the ultra-short peptide can be used for replacing the EGF used in the fields of anti-ageing, antioxidant skincare products for repairing skin and stimulating skin collagen hyperplasia; the ultra-short peptide Purin-WH is not limited to such a function.
Owner:于海宁
Multi-stranded beta-hairpin short-peptide with tolerant protease and preparation method and application
InactiveCN106749532ASimple experimental techniqueStable structureAntibacterial agentsPeptide/protein ingredientsBeta hairpinCrystallography
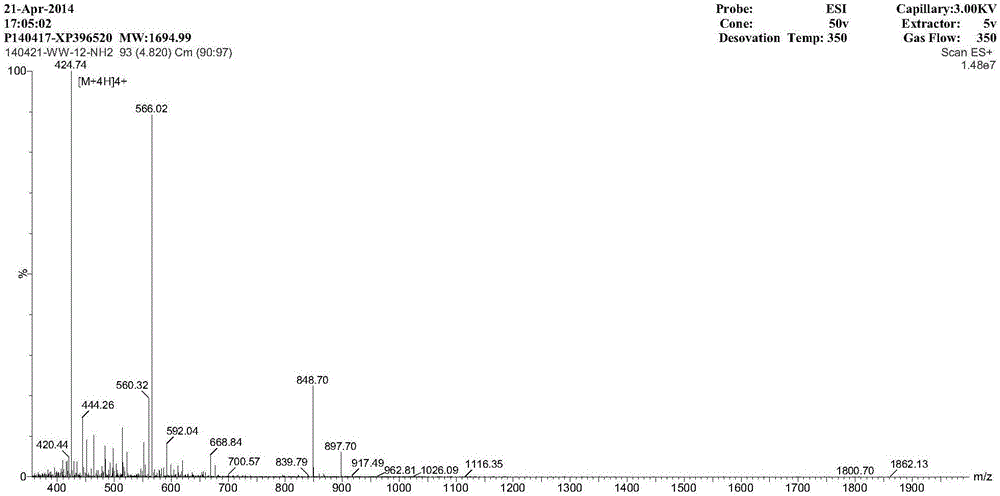
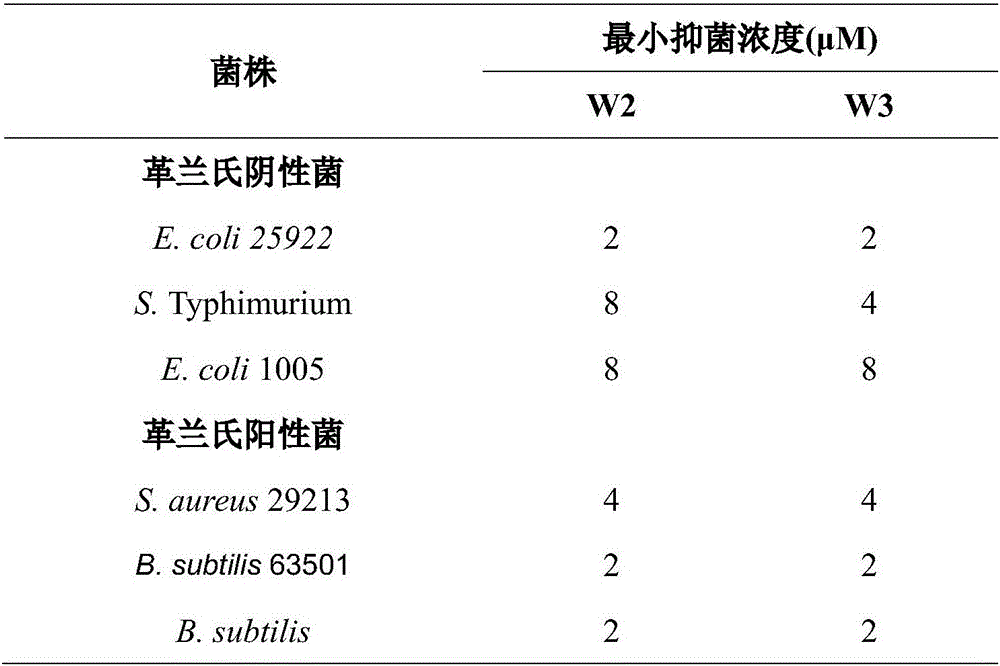
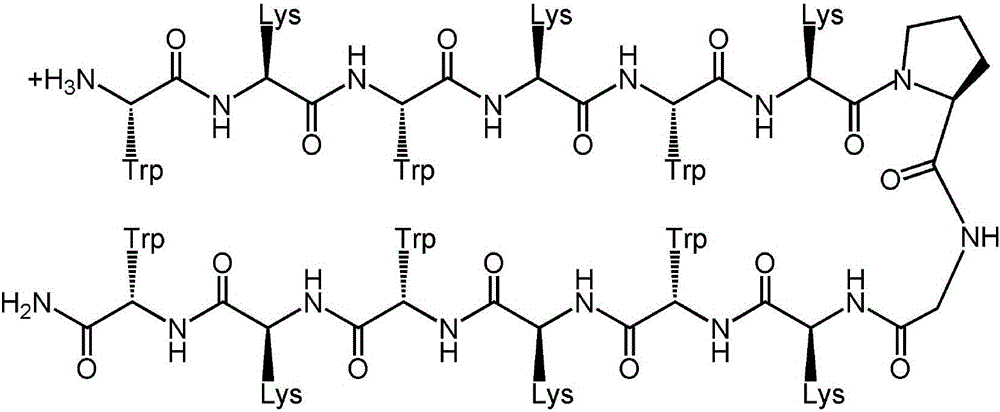
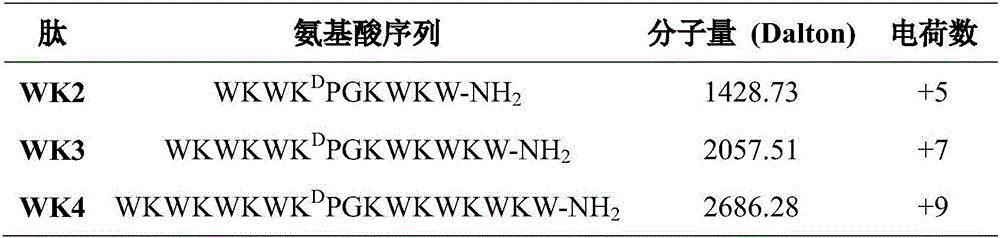
The invention provides a multi-stranded beta-hairpin short-peptide with tolerant protease and a preparation method and an application. A sequence is as shown in a SEQ ID No.1. The short-peptide provided by the invention fully exerts the structural advantages of a beta-hairpin structure to obtain a multi-stranded beta-hairpin structural antibacterial peptide. The stability is further improved while the activity is ensured by using a common amino, and composition of a corner amino acid is changed, so that a series of multi-stranded beta-hairpin antibacterial peptides of brand new structures: (WRXxRW)n-NH2, wherein n is 1, 2, 3 and 4; PG is selected as a corner to describe; when n is equal to 2, the antibacterial peptide is named W2; and when n is equal to 3, the antibacterial peptide is named W3. The peptide verifies relatively high cell selectivity and salt ion tolerance, and moreover, based on the activity of the antibacterial peptide with structural stability, the obtained antibacterial peptide is also relatively short, and relatively strong tolerance of in vivo protease is also represented.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Tryptophan tension chain-beta-hairpin antibacterial peptide and preparation method and application thereof
InactiveCN106749531ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsCell selectivityCytotoxicity
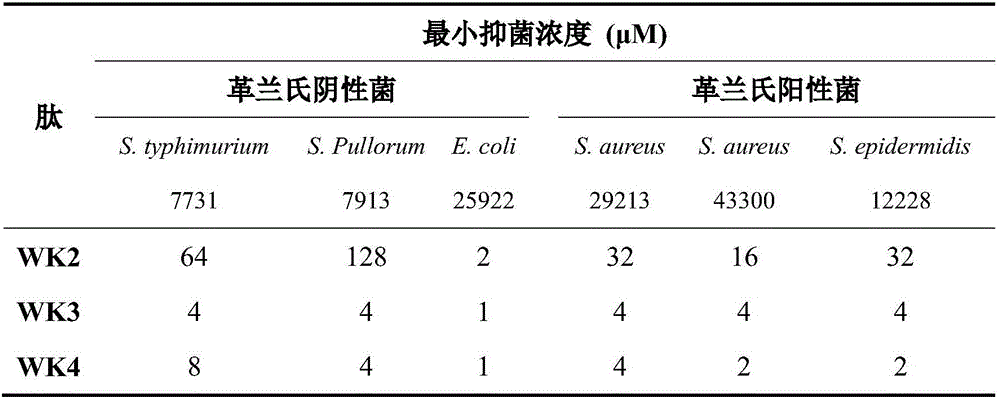
The invention provides a tryptophan tension chain-beta-hairpin antibacterial peptide and a preparation method and application thereof. A sequence of the antibacterial peptide is as shown in a sequence table SEQ ID No.1. The preparation method comprises the following steps: by taking a tryptophan tension chain structure as a template, combining amino acid composition and structural characteristics of the antibacterial peptide; and by using interaction of cross-chain W-W as a structural stable factor, symmetrically and circularly arranging an amino acid lysine with charges and a hydrophobic amino acid tryptophan as repeated binary sequence units on two arms of the beta-hairpin to obtain the antibacterial peptide WK3. The antibacterial peptide has relatively high bacteriostatic activity and cell selectivity, and the treatment index reaches up to 161.27. The antibacterial peptide designed by the method needs not to bind a disulfide bond but has extremely high structural stability, so that the selectivity of the antibacterial peptide between bacterial cells and mammalian cells is improved, and the antibacterial peptide has an application potential as an antibiotic substituent. The antibacterial peptide is relatively high in activity and relatively low in cytotoxicity.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
High-efficiency and low-toxicity antibacterial peptide derivative and application thereof in preparation of antibacterial infection drug
PendingCN111892646ALow cytotoxicityLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsBactericidal effectBroad spectrum
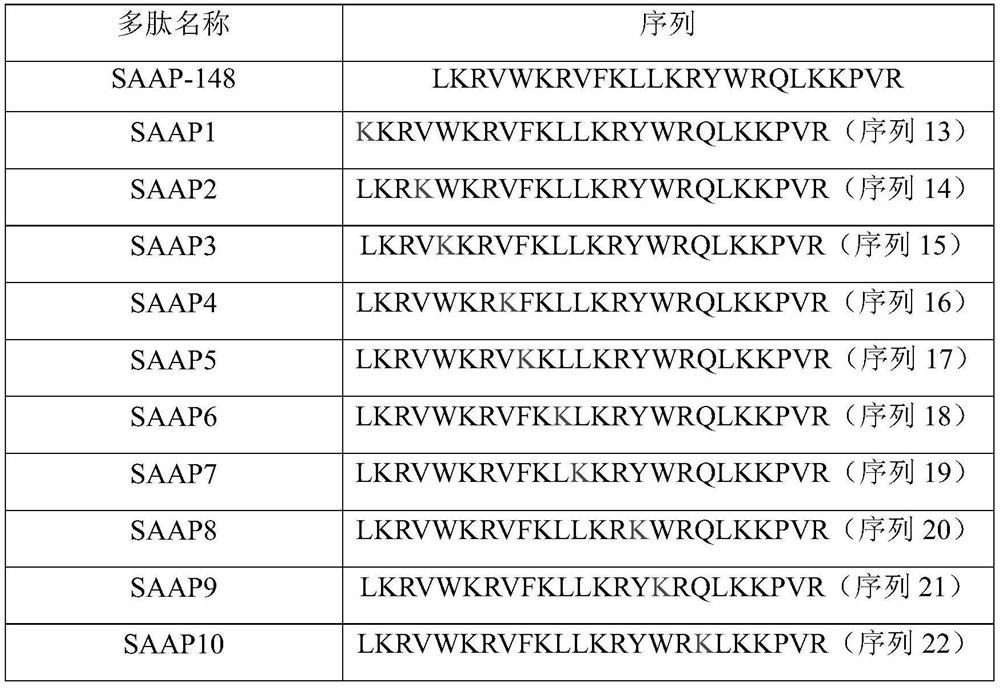
The invention discloses a high-efficiency and low-toxicity antibacterial peptide derivative and an application thereof in preparation of an antibacterial infection drugs. Any amino acid residue, except lysine Lys and arginine Arg, in the GF-17 antibacterial peptide and the SAAP-148 antibacterial peptide is replaced with lysine Lys, and two groups of novel antibacterial peptide derivatives which are low in cytotoxicity, high in treatment index and good in biocompatibility are obtained. Experiments prove that compared with natural antibacterial peptide, the novel antibacterial peptide derivativedisclosed by the invention has higher selective toxicity to bacteria, that is, the novel antibacterial peptide derivative has the same or stronger bactericidal effect as the natural antibacterial peptide, the cytotoxicity to human is obviously reduced, and the influence on red blood cells is extremely low. The novel antibacterial peptide derivative disclosed by the invention has a broad-spectrumkilling effect on gram-positive bacteria or gram-negative bacteria, can be used for treatment of diseases caused by infection of antibiotic-resistant gram-positive bacteria or gram-negative bacteria,and has a bright application prospect.
Owner:ACADEMY OF MILITARY MEDICAL SCI
A kind of antibacterial peptide and preparation method thereof
InactiveCN102276691ASimple experimental techniqueImprove therapeutic indexAntibacterial agentsPeptide preparation methodsCell selectivityArginine
The invention relates to antibacterial peptides and a preparation method thereof. An amino acid sequence of the antibacterial peptide is IleTrpArgIlePheArgArgIlePhe. The method comprises the following steps of: 1) designing a group of antibacterial peptides consisting of isoleucine, arginine and phenylalanine which serve as basic units by utilizing the bacteriostatic mechanism of the antibacterial peptides and the characteristic of amino acid composition; 2) synthesizing a polypeptide crude product by using a polypeptide synthesizer in a solid-phase synthetic process; 3) purifying the synthesized polypeptide by reversed phase high performance liquid chromatography, and identifying by electrospray mass spectrometry; and 4) performing an in-vitro activity test, wherein a result of the activity test indicates that antibacterial peptides B containing 3 basic units have high bacteriostatic activity and weak hemolytic activity, a treatment index is the highest, and cell selectivity is optimum, so the antibacterial peptides have high application value.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
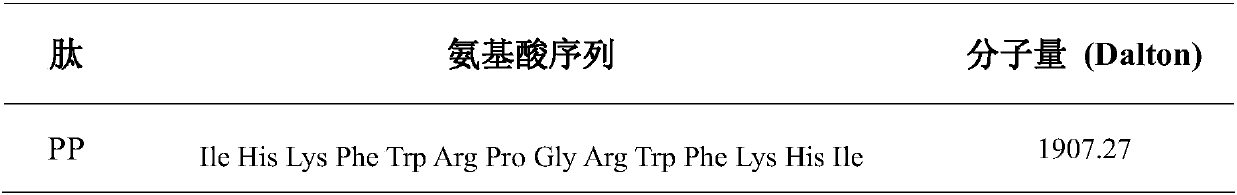
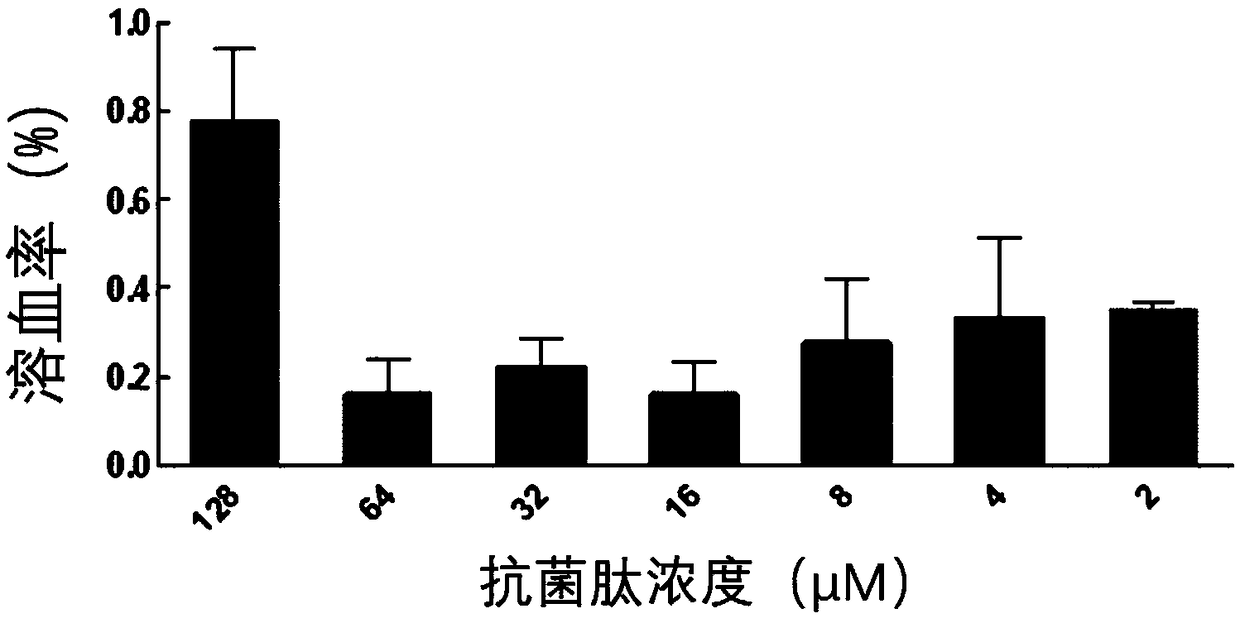
Symmetrical-terminals antibacterial peptide PP and preparation method and application thereof
InactiveCN107746429AEnhanced inhibitory effectLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsChemistryPathogen
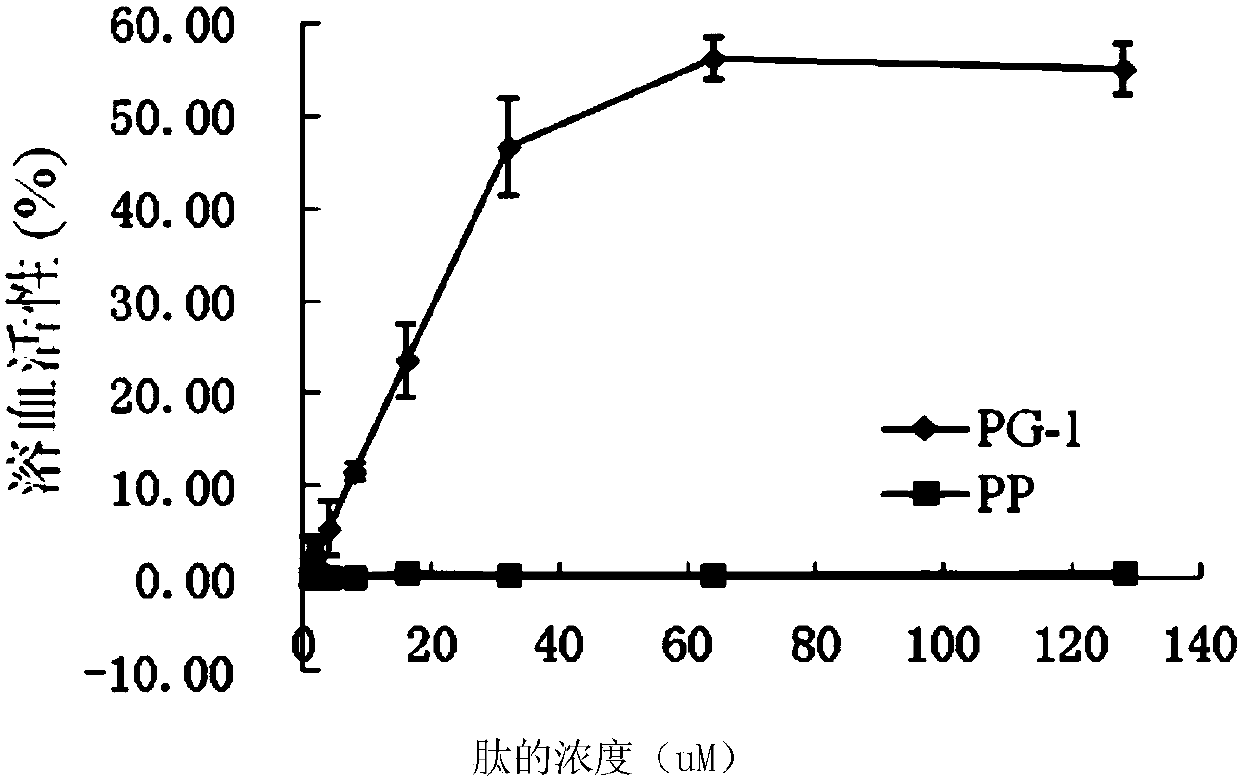
The invention provides symmetrical-terminals antibacterial peptide PP and a preparation method and an application thereof. A sequence of antibacterial peptide PP is shown as a sequence table SEQ ID No.1. The preparation method comprises the following steps: employing two symmetrical alpha spiral units, a beta-corner part amino acid sequence CRRRFC of PG-1 is embedded in the symmetrical alpha spiral units to form the symmetrical-terminals antibacterial peptide, and the symmetrical-terminals antibacterial peptide PP is designed. The invention also provides an application of the symmetrical-terminals antibacterial peptide PP in preparation of a medicine for treating gram-positive bacteria or gram-negative bacteria infectious diseases, the antibacterial peptide PP has high bacteriostatic activity and low toxicity, a therapeutic index is 80 times of PG-1, and the antibacterial peptide PP has high cell selectivity. The antibacterial peptide PP can purposely kill pathogen in organism, and increases the development potential and biological value of the antibacterial peptide.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Targeted antibacterial peptide against Gram-negative bacteria and preparation method and application
ActiveCN109232717ASimple experimental techniqueHigh application potentialAntibacterial agentsPeptide/protein ingredientsStatistical analysisCytotoxicity
The invention provides a targeted antibacterial peptide against Gram-negative bacteria and a preparation method and an application. A sequence of the targeted antibacterial peptide is as shown in a sequence table SEQ ID No. 1, and in the preparation method, an R language is utilized for statistical analysis of amino acid sequence characteristics of a natural antimicrobial peptide library, variouspeptide chain parameters affecting antibacterial activity of a peptide chain having the antibacterial activity only against Gram-negative bacteria are statistically analyzed, and the optimal amino acid composition is obtained by screening, namely K, G, L, with the number of positive charges being 4, and the hydrophobicity being 30%-50%. Tryptophan is inserted into the center of a screening sequence to obtain a centrally symmetric short peptide sequence through design, so as to obtain a short-chain narrow-spectrum antibacterial peptide sequence which is low in toxicity and highly effective, andnot easy to cause disorder of immune regulation in a body. The targeted antibacterial peptide is beneficial to maintaining micro-ecological balance while killing harmful bacteria. The antibacterial peptide has relatively high activity, high stability, relatively low cytotoxicity, and substitute antibiotics, thereby having an application potential for becoming a safer and environmentally-friendlynovel antibacterial product.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
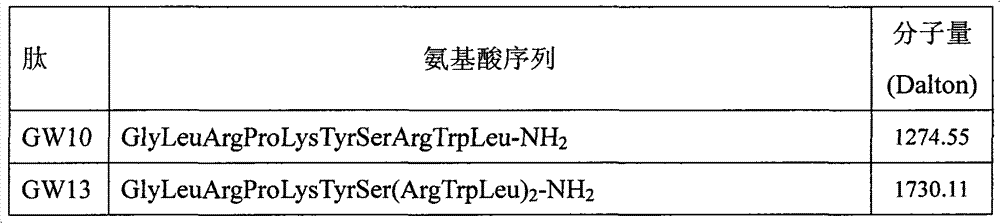
Antibacterial peptide GW13 and its preparation method and use
ActiveCN102827255ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsBeta defensinCombinatorial chemistry
The invention relates to an antibacterial peptide GW13 and its preparation method and use. The antibacterial peptide GW13 has an amino acid sequence of GlyLeuArgProLysTyrSer(ArgTrpLeu)2-NH2. The preparation method comprises the following steps of 1, cutting out 13 amino acid fragments from a linear chicken beta-defensin 4 carboxyl end by a fixed-point amino acid fragment cutting method, removing disulfide bonds to obtain a GW10 (GlyLeuArgProLysTyrSerArgTrpLeu-NH2) fragment, and repeating connecting characteristic three-residue complexes ArgTrpLeu to obtain the antibacterial peptide GW13, and 2, synthesizing a peptide resin in a polypeptide synthesizer by a solid-phase synthesis method, carrying out TFA cutting to obtain a polypeptide, and carrying out reversed-phase high-performance liquid chromatography purification. The antibacterial peptide GW13 obtained by the preparation method has strong bacteriostatic activity, low hemolytic activity, the highest therapeutic index and a large development potential.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Yangtze alligator antimicrobial peptide Alligatorin 6 and application thereof
ActiveCN104177485ABroad-spectrum high-efficiency antibacterial effectSmall molecular weightAntibacterial agentsCosmetic preparationsAntimicrobial actionHeat stability
The invention discloses a Yangtze alligator antimicrobial peptide Alligatorin 6 as well as an amino acid sequence thereof and an application of the Yangtze alligator antimicrobial peptide Alligatorin 6 in antibiosis. The antimicrobial peptide Alligatorin 6 is a mature peptide sequence of the antimicrobial peptide which is obtained by searching a Yangtze alligator gene database by virtue of screening and analyzing, wherein an amino acid sequence of the mature peptide sequence is as shown in a sequence table SEQ ID NO.1. By virtue of analysis and comparison, the Yangtze alligator antimicrobial peptide Alligatorin 6 has obvious difference from the amino acid sequences of all of the existing known antimicrobial peptides, and belongs to a novel antimicrobial peptide. According to an antimicrobial test, the Yangtze alligator antimicrobial peptide Alligatorin 6 has very strong antimicrobial activity on gram positive bacteria and gram negative bacteria, is broad-spectrum and efficient in antimicrobial action. Besides, the Yangtze alligator antimicrobial peptide Alligatorin 6 has the characteristics of low hemolytic activity, good salt tolerance, good heat tolerance and good heat stability, and can be applied to the fields of medicines, cosmetics, food fresh keeping and breeding industry.
Owner:宜肌坊(厦门)生物科技有限公司
Antibacterial peptide VK-21 and application
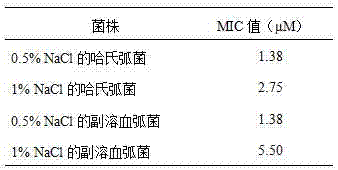
ActiveCN107383175AGood antibacterial effectGood inhibitory effectAntibacterial agentsPeptide-nucleic acidsEscherichia coliVibrio parahaemolyticus
The invention discloses an antibacterial peptide VK-21 in the technical field of biology. The amino acid sequence of the antibacterial peptide is Val-Lys-Arg-Lys-Lys-Lys-Pro-Gln-Ser-Trp-Lys-Thr-Trp-Trp-Thr-Lys-Trp-Trp-Thr-Lys-Lys. The antibacterial peptide has an obvious inhibiting effect on Escherichia coli, pseudomonas aeruginosa, staphylococcus aureus and four methicillin-resistant staphylococcus aureus, has an excellent antibacterial effect on vibrio parahaemolyticus and vibrio harveyi frequently existing in aquatic products, has low hemolytic activity, good stability, strong antibacterial activity and efficient broad-spectrum bacteriostasis functions, and can be used as an antibiotic substitute.
Owner:ZUNYI MEDICAL UNIVERSITY
Reptile antibacterial peptide Alligatorin5 and applications thereof
ActiveCN104163861ABroad-spectrum high-efficiency antibacterial effectSmall molecular weightAntibacterial agentsCosmetic preparationsChinese alligatorAntibacterial activity
The invention discloses a reptile-derived antibacterial peptide Alligatorin5, an amino acid sequence thereof and applications of the antibacterial peptide in the antibacterial field. The antibacterial peptide Alligatorin5 is derived from Chinese alligator. By searching a Chinese alligator gene database, screening and analyzing, a mature peptide sequence of the antibacterial peptide is obtained. The amino acid sequence of the antibacterial peptide Alligatorin5 is shown as SEQ ID NO.1 in a sequence table. By comparative analysis, the antibacterial peptide Alligatorin5 is obviously different from amino acid sequences of antibacterial peptides known at present, and belongs to a novel antibacterial pepetide. Antibacterial experiments show that: the antibacterial peptide Alligatorin5 has high antibacterial activity for gram positive bacteria, gram negative bacteria and funguses, and is broad in antibacterial spectrum and efficient; the antibacterial peptide Alligatorin5 has characteristics of low hemolytic activity, salt tolerance, heat tolerance, good thermal stability, and the like, and can be used for the fields of medicines, cosmetics, food fresh-keeping and the breeding industry.
Owner:SUZHOU UNIV
Template-fixed beta-hairpin peptidomimetics with CXCR4 antagonizing activity
ActiveUS8895695B2High antagonistic activityEfficient collectionBiocideAntipyreticCXCR4Organic chemistry
Template-fixed β-hairpin peptidomimetics of the general formula (I)wherein Z is a template-fixed chain of 12, 14 or 18 α-amino acid residues which, depending on their positions in the chain (counted starting from the N-terminal amino acid), are Gly, NMeGly, Pro or Pip, or of certain types which, as the remaining symbols in the above formula, are defined in the description and the claims, and salts thereof, have CXCR4 antagonizing properties. These β-hairpin peptidomimetics can be manufactured by a process which is based on a mixed solid- and solution phase synthetic strategy.
Owner:POLYPHOR AG +1
Antibacterial peptide and application thereof
ActiveCN112940082ABroad-spectrum antimicrobial activityHigh antibacterial activityAntibacterial agentsCosmetic preparationsBiotechnologyEscherichia coli
The invention discloses an antibacterial peptide and application thereof. The antibacterial peptide is an antibacterial peptide YHX-1 with an amino acid sequence as shown in SEQ ID NO. 1. The antibacterial peptide has broad-spectrum antibacterial activity, can inhibit gram-positive bacteria such as listeria monocytogenes, staphylococcus aureus and streptococcus mutans and gram-negative bacteria such as escherichia coli and salmonella, and has high antibacterial activity. In addition, the antibacterial peptide is relatively low in hemolytic activity, relatively low in toxicity, short in synthetic sequence, small in molecular weight and low in chemical synthesis difficulty, the safety is ensured while pathogenic bacteria in a living body can be well and specifically killed, and in addition, the large-scale production cost can also be saved.
Owner:YANGZHOU YANGDA KANGYUAN DAIRY
Swine derived hybrid antimicrobial peptide MDP-2 and preparation method and application thereof
ActiveCN110283253APerfect biological activityHigh antibacterial activityAntibacterial agentsPeptide/protein ingredientsHemolysisMinimum inhibitory concentration
The invention provides a swine derived hybrid antimicrobial peptide MDP-2 and a preparation method and application thereof. The sequence of the peptide is as shown in a sequence table SEQ ID No.2. The preparation method comprises the following steps: carrying out truncation and residue replacement on a natural antimicrobial peptide according to the composition characteristic and distribution of amino acids of the antimicrobial peptide PMAP-23 or PMAP-36 to obtain a swine derived peptide; connecting the derived peptide to a bounded sequence of lipopolysaccharide of myeloid differentiation protein-2 to further enhance the bacteria targeting ability of the derived peptide and finally obtaining four derived hybrid peptides, the lengths of amino acids of which are 20-24; then synthesizing a polypeptide; carrying out purification and identification; and finally, testing the bacteriostatic activity and the cytotoxicity of the derived antimicrobial peptide through the minimal inhibitory concentration and a hemolysis test to screen the polypeptide with relatively ideal activity. The swine derived hybrid antimicrobial peptide has the beneficial effects that the polypeptide is a natural derivative which is relatively good in safety, has broad-spectrum antibacterial activity and low toxicity, and is sophisticated in preparation method and preparation technology and low in synthetic cost.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Efficient alpha-helix antibacterial peptide GV and preparation method and application thereof
InactiveCN106366162ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsChemical synthesisAlpha helix
The invention relates to an efficient alpha-helix antibacterial peptide GV and a preparation method and application thereof. The antibacterial peptide GV is an antibacterial peptide with high cell selectivity and achieves an optimal point of junction of the antibacterial activity and cell toxicity of the antibacterial peptide. A sequence of the antibacterial peptide GV is represented by a sequence table SEQ ID No. 1. The preparation method comprises the following steps: (1) carrying out designing in accordance with alpha-helix peptide GRX2RX3RX2RG serves as a template, so as to obtain a brand-new antibacterial peptide GV, wherein X= V; (2) synthesizing a polypeptide crude product through a polypeptide synthesizer by a solid-phase chemical synthesis method; purifying the synthesized polypeptide by using out-phase high-performance liquid chromatography, and carrying out identification on the synthesized polypeptide by using electrospray mass spectrography, thereby preparing the polypeptide. The antibacterial peptide GV provided by the invention has relatively high bacteriostatic activity and relatively low hemolytic activity and is the highest in therapeutic index, thereby having great development potential.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Alpha helical antibacterial peptide RL as well as preparation method thereof and application thereof
ActiveCN107266533ALow hemolytic activityHigh inhibitory effectAntibacterial agentsPeptide/protein ingredientsEscherichia coliSalmonella Gallinarum
The invention provides alpha helical antibacterial peptide RL as well as a preparation method thereof and application thereof. The sequence of antibacterial peptide RL is as shown in SEQ ID No.1. The preparation method comprises the following step: designing an imperfect amphipathic alpha helical peptide template WXKYWXZZYKXWYK-NH2 containing a turn unit on the basis of imperfect amphipathic alpha helical multi-peptide folding principle, wherein X is positive charge amino acid, Y is hydrophobic amino acid, Z is the turn unit, and the antibacterial peptide is named as RL when X is equal to R, Y is equal to L and ZZ is equal to <D>PG. The preparation method is simple in technology, and antibacterial and hemolytic activity detection is performed on the obtained antibacterial peptide and proves that RL has efficient inhibiting effect on seven bacteria of escherichia coli, pseudomonas aeruginosa, staphylococcus aureus, staphylococcus epidermidis, salmonella typhimurium, salmonella gallinarum and bacillus subtilis, and has very low hemolytic activity.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
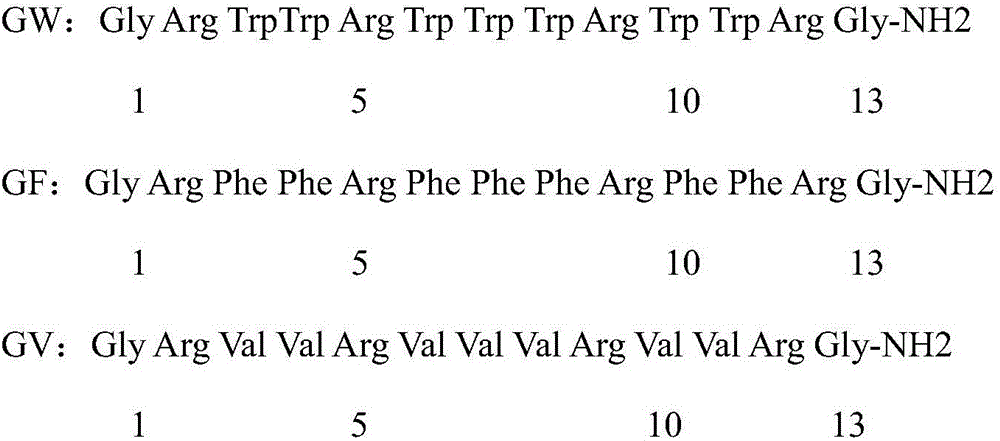
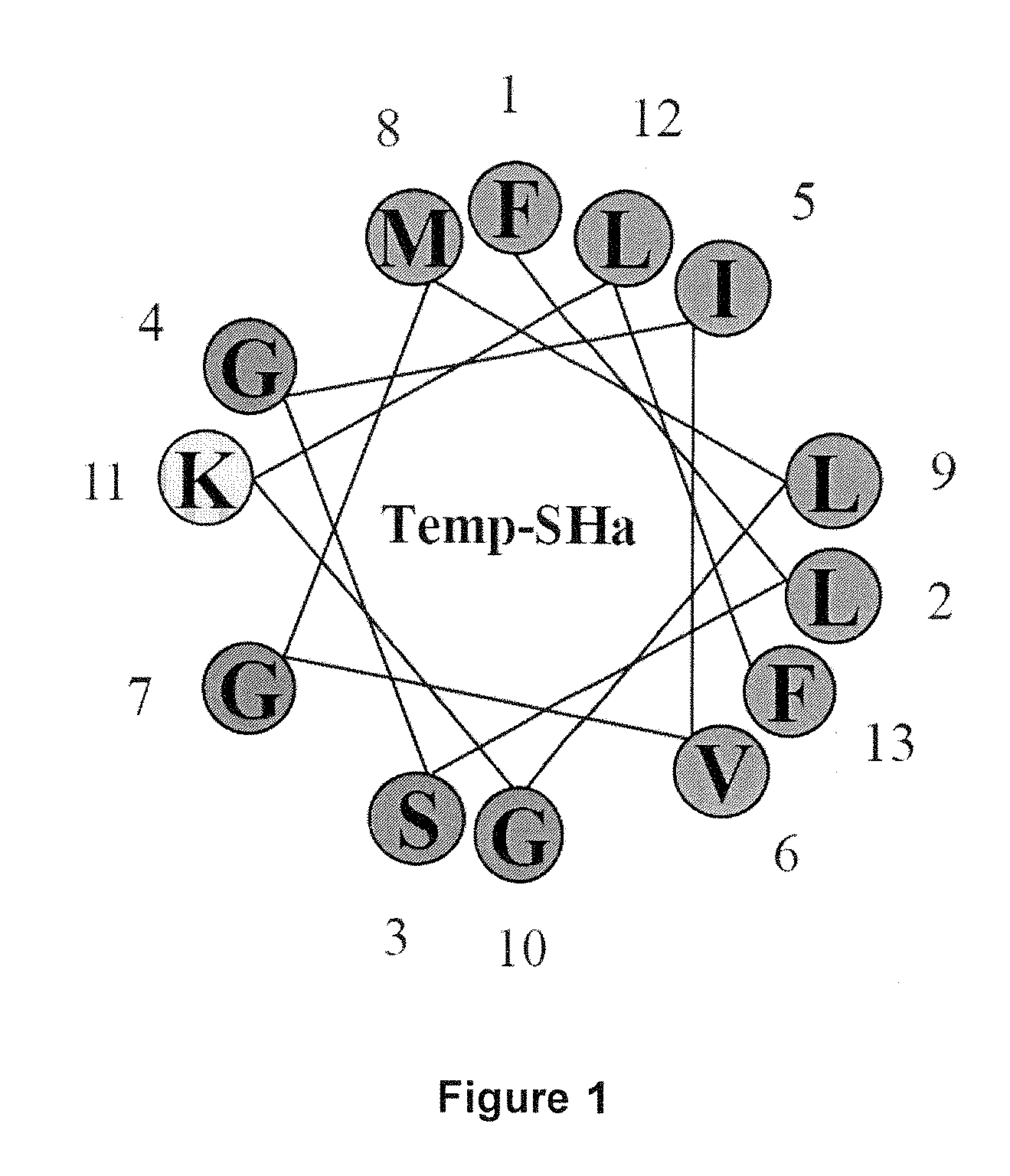
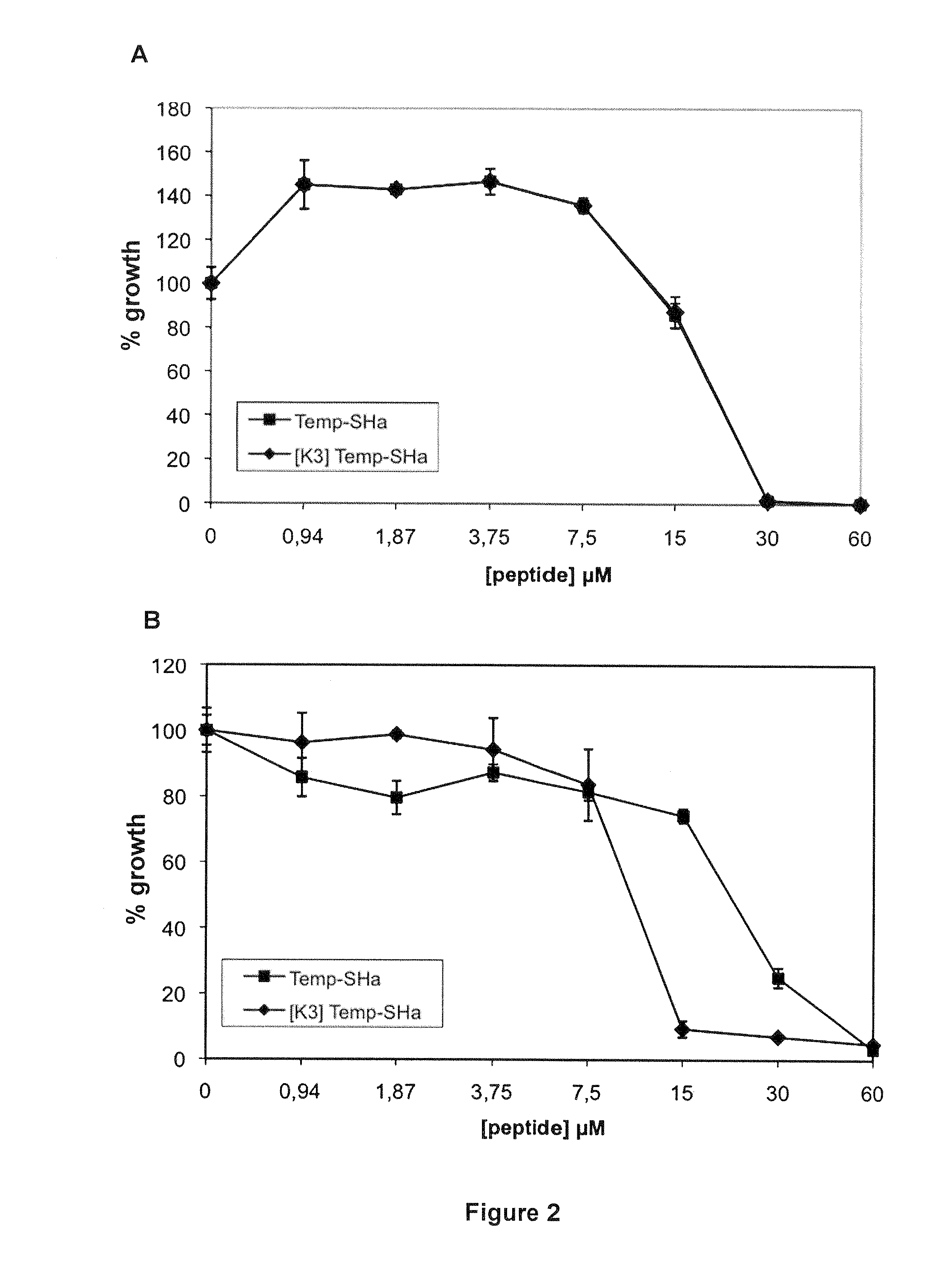
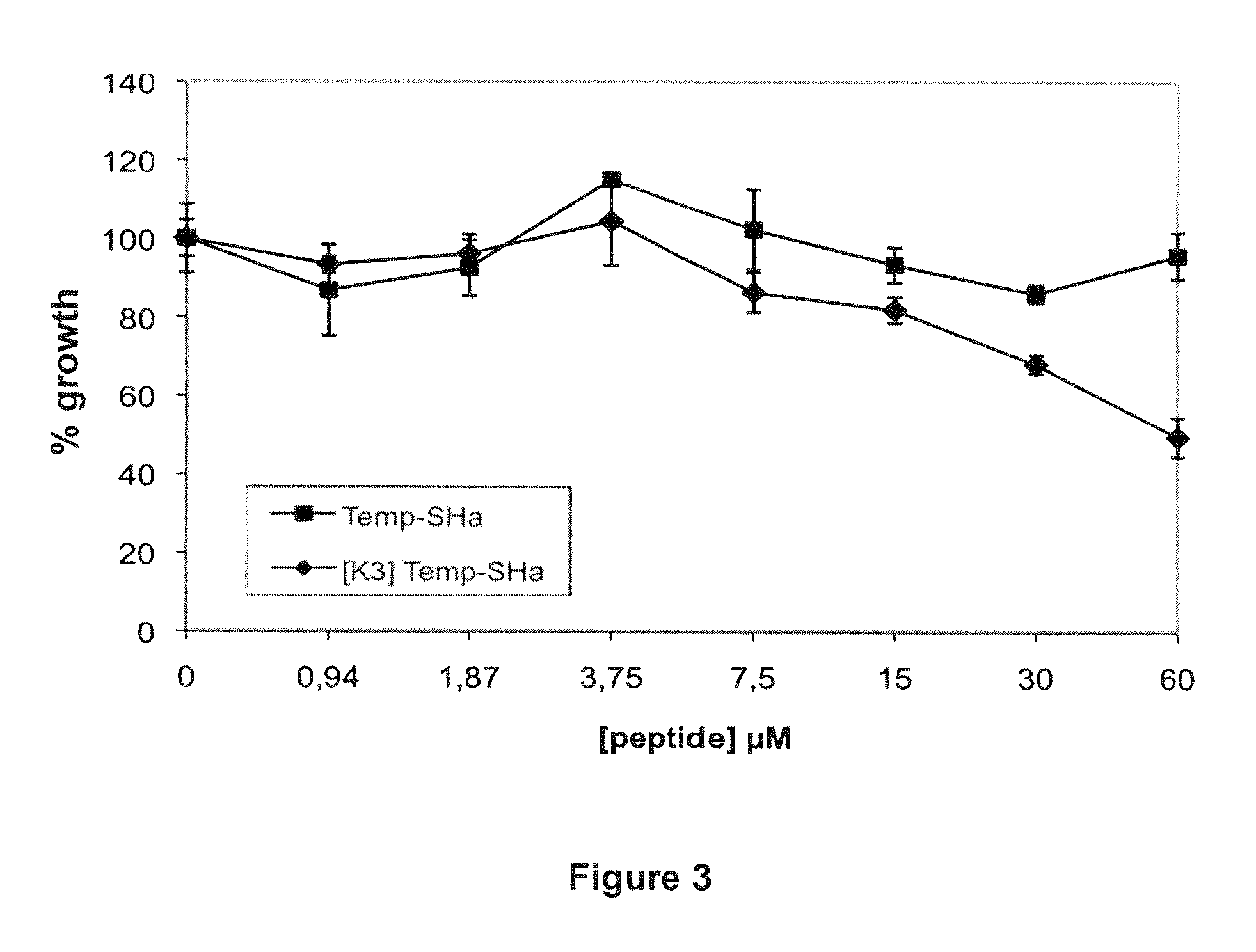
Analogs of temporin-sha and uses thereof
InactiveUS20120005790A1Enhanced anti-microbial activityLow hemolytic activityAntibacterial agentsFungiGMO PlantsDrug
The present invention relates to novel antimicrobial peptides, to pharmaceutical compositions comprising said peptides, and to the uses thereof, in particular as antimicrobial drugs, disinfectants, pesticides or preservatives. The present invention also relates to a transgenic plant expressing said novel peptides.
Owner:UNIV PIERRE & MARIE CURIE +2
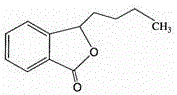
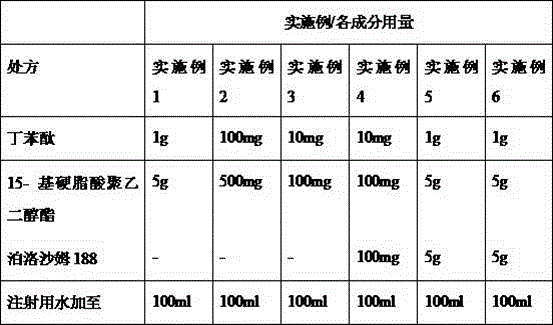
Pharmaceutical composition containing butylphthalide and novel solubilizer
InactiveCN105688220AImprove securityLow hemolytic activityOrganic active ingredientsPowder deliverySolubilityButylphthalide
The invention relates to the field of medicine, in particular to a pharmaceutical composition containing butylphthalide and a novel solubilizer to improve the water solubility of butylphthalide by means of the novel solubilizer, clinically required solid form, or semisolid form or liquid form of butylphthalide is developed, so that the treatment effect of butylphthalide can be better realized. The composition can be used for preparing various drug forms such as tablets, capsules, particles, powder, ointment, cream, gel, infusion, squirt cut, powder filling and oral liquid. Compared with the prior art, the pharmaceutical composition is better in safety performance and water solubility of butylphthalide.
Owner:SICHUAN MANSAISI MEDICINE TECH CO LTD
Template-fixed beta-hairpin peptidomimetics with protease inhibitory activitiy
InactiveUS20140213531A1Facilitates structure-activity studiesPotent activityNervous disorderPeptide/protein ingredientsCathepsin GCathepsin
Template-fixed β-hairpin peptidomimetics of the general formulaewherein Z is a chain of 11 α-amino acid residues which, depending on their positions in the chain (counted starting from the N-terminal amino acid) are Gly, or Pro, or Pro(4NHCOPhe), or of certain types which, as the remaining symbols in the above formula, are defined in the description and the claims, and salts thereof, have the property to inhibit proteases, in particular serine proteases, especially Cathepsin G or Elastase or Tryptase. These β-hairpin peptidomimetics can be manufactured by processes which are based on a mixed solid- and solution phase synthetic strategy.
Owner:UNIV ZURICH +1
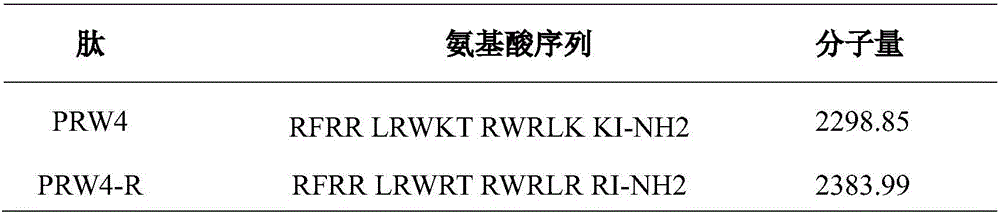
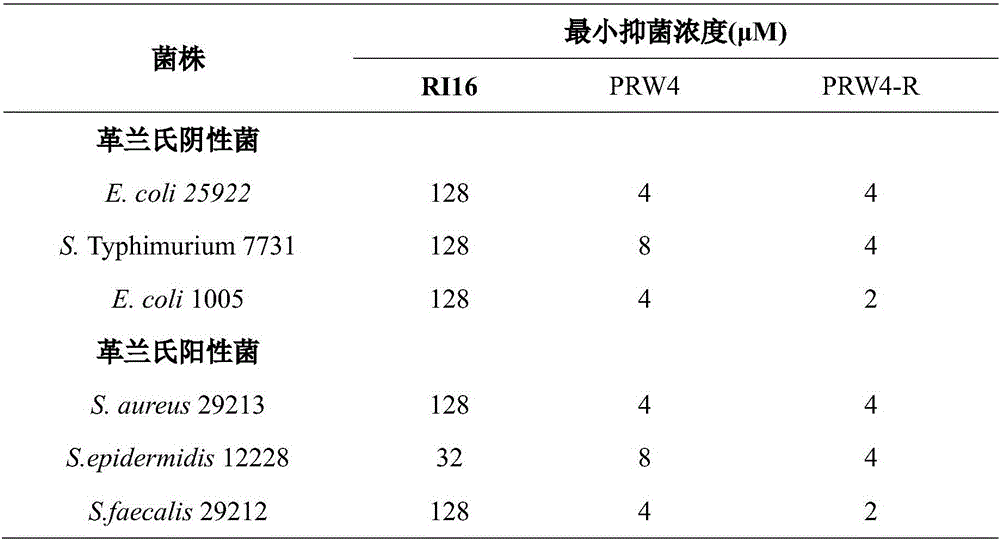
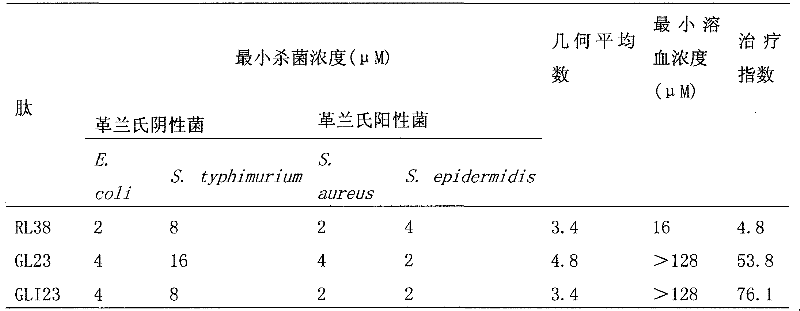
Imperfect amphiphilic antimicrobial peptide PRW4-R and preparation method and application thereof
InactiveCN106554400ASimple experimental techniqueImprove cell selectivityAntibacterial agentsPeptide/protein ingredientsCrystallographyArginine
The invention provides an imperfect amphiphilic antimicrobial peptide PRW4-R and a preparation method and application thereof. The sequence of the imperfect amphiphilic antimicrobial peptide PRW4-R is shown as SEQ ID No. 1. The preparation method comprises the following steps: cutting out 16 amino acid sequences at a PMAP-36N end to obtain RI16 with a perfect amphiphilic structure; based on an Alpha-helix protein folding principle, substituting Trp for paired Lys in the polar face of the amphiphilic structure to obtain imperfect amphiphilic antimicrobial peptide PRW4; and substituting Arg for all lysine in the sequence of the PRW4 to obtain the PRW4-R. The therapeutic index of the PRW4-R is up to 161.5; and the peptide PRW4-R shows high cell selectivity and has low hemolytic activity, so that the development potential of the peptide PRW4-R in becoming a substituent of antibiotics is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
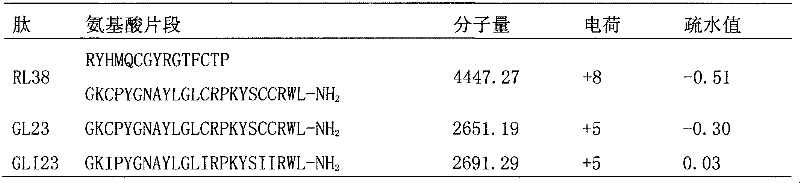
Antibacterial peptide GLI23 derived from linear chicken beta-phylaxin4 (RL38) and preparation method thereof
InactiveCN102382186ALow hemolytic activityHigh selectivityDepsipeptidesAmino acid replacementMammalian cell
The invention provides an antibacterial peptide GLI23 derived from linear chicken beta-phylaxin4 (RL38) and a preparation method thereof. According to the invention, methods of fixed-point amino acid segment interception and amino acid replacement are adopted so as to simplify the linear chicken beta-phylaxin4 and obtain two polypeptides; the antibacterial and the hemolytic activities the polypeptides are measured; and the therapeutic index of the polypeptides are calculated so as to evaluate the selectivity of the polypeptides to cells. Found by researches, the intercepted peptide GLI23 and the peptide GLI23 after being subject to amino acid replacement have obvious bactericidal activity and obviously-decreased hemolytic activity compared with the chicken beta-phylaxin4, especially the GLI23 of which the therapeutic index is as high as 76.1. The method provided by the invention has the advantages that: under the circumstance that the bactericidal activity of the antibacterial peptideis not decreased, the hemolytic activity of the antibacterial peptide is decreased; the selectivity of the antibacterial peptide between bacterial cells and mammalian cells is improved; and the development potential of substituting for antibiotic is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com