High-efficiency and low-toxicity antibacterial peptide derivative and application thereof in preparation of antibacterial infection drug
An antibacterial peptide, the technology in the sequence table, applied in the application field of high-efficiency and low-toxicity antibacterial peptide derivatives, the preparation of anti-bacterial infection drugs, can solve the problems of cytotoxicity, hindering clinical application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1, the modification method of antimicrobial peptide and the preparation of antimicrobial peptide derivative
[0115] 1. Modification method of antimicrobial peptides
[0116] The antimicrobial peptide modification method of the present invention is to replace any amino acid residue in the antimicrobial peptide except lysine Lys and arginine Arg with lysine Lys. Antimicrobial peptides with single amino acid residues substituted are referred to as antimicrobial peptide derivatives.
[0117] 2. Preparation of Antimicrobial Peptide Derivatives
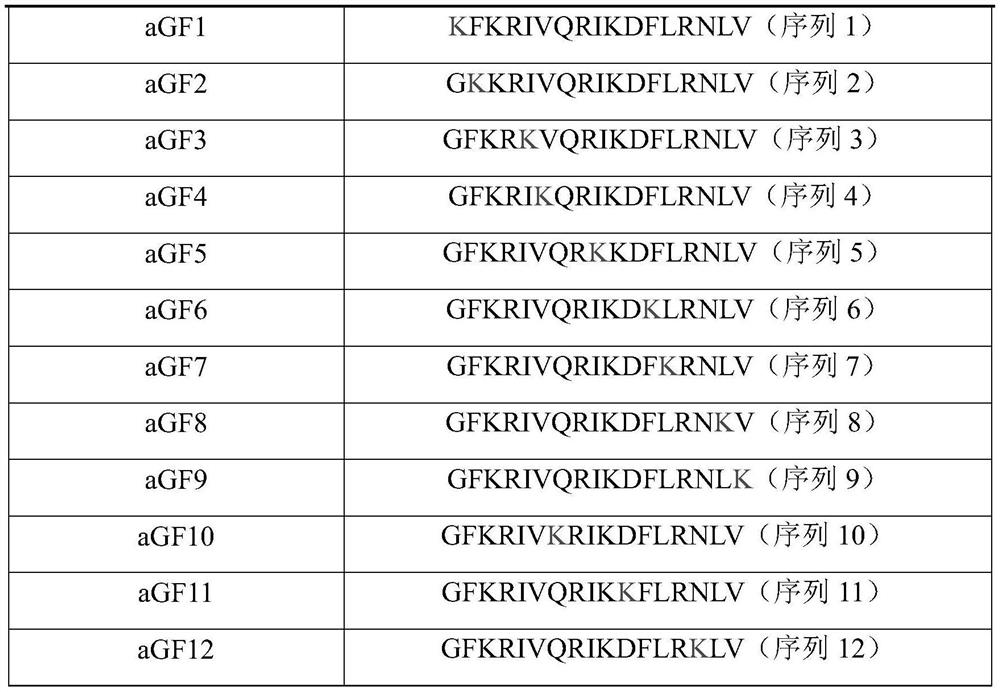
[0118] 1. Preparation of GF-17 antimicrobial peptide derivatives
[0119] Taking GF-17 antimicrobial peptide as an example, the GF-17 antimicrobial peptide was modified according to the modification method in step 1 to obtain a group of GF-17 antimicrobial peptide derivatives, named aGF1-aGF12. The amino acid sequences of GF-17 antimicrobial peptide and GF-17 antimicrobial peptide derivatives aGF1-aGF12 are shown in Ta...
Embodiment 2
[0128] Embodiment 2, the application of antimicrobial peptide derivative
[0129] 1. Application of GF-17 Antibacterial Peptide Derivatives
[0130] 1. Determination of hemolytic ability of GF-17 antimicrobial peptide derivatives
[0131] Antimicrobial peptide derivatives to be tested: GF-17 antimicrobial peptide derivatives aGF1-aGF12 prepared in Example 1, and GF-17 antimicrobial peptide as a control.
[0132] The hemolytic ability of the GF-17 antimicrobial peptide and the GF-17 antimicrobial peptide derivative (the ability to lyse erythrocytes) was determined according to the following method. Specific steps are as follows:
[0133] 1) Human fresh whole blood was washed with PBS buffer (135mM NaCl, 2.7mM KCl, 1.5mM KH 2 PO 4 , and 8mMK 2 HPO 4 , pH 7.2) and washed three times, and centrifuged at 450×g for 5 min each time, then the compacted erythrocytes were made into a 1.25% packed erythrocyte suspension with PBS buffer, and 160 μl of the erythrocyte suspension was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com