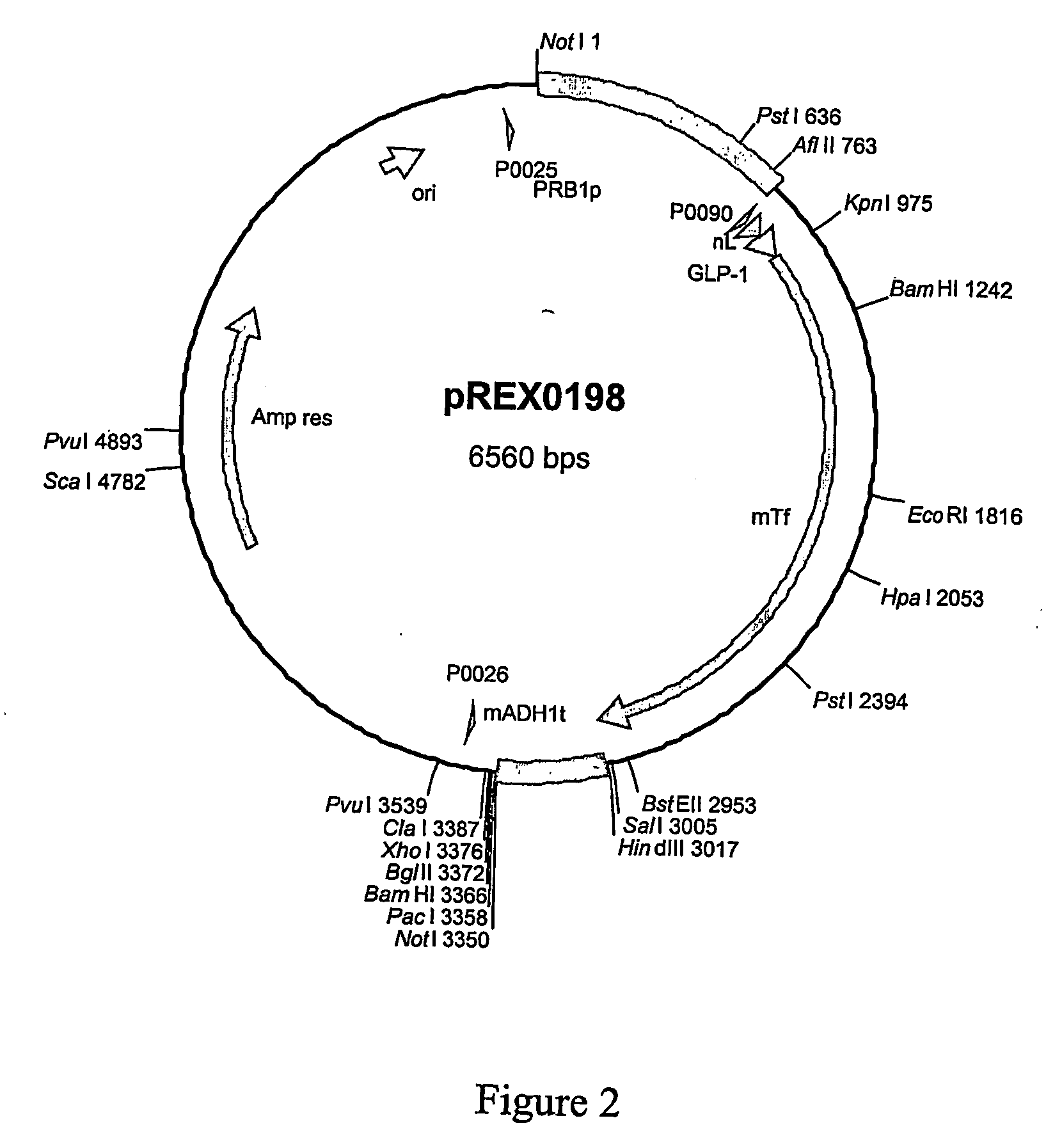
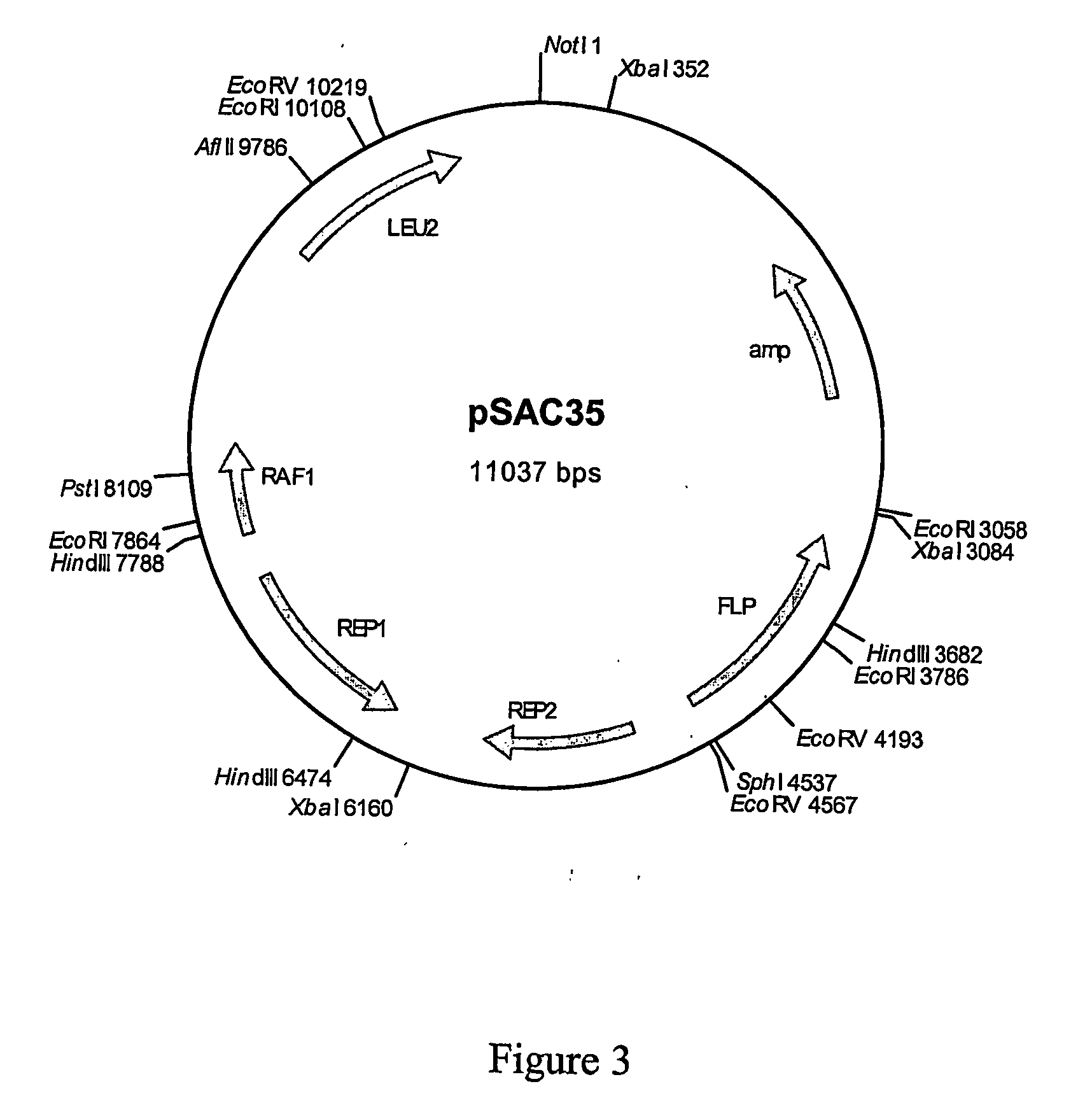
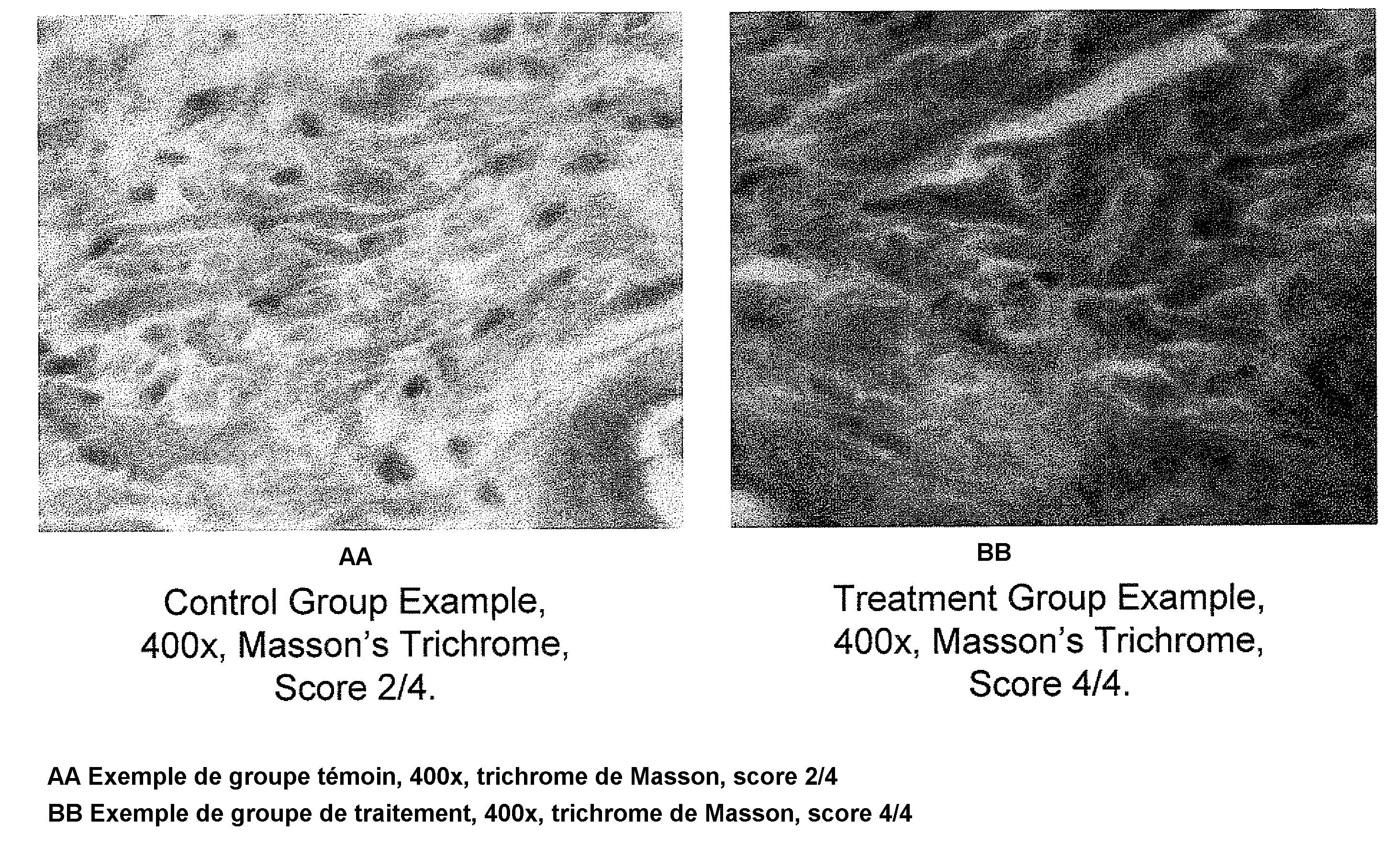
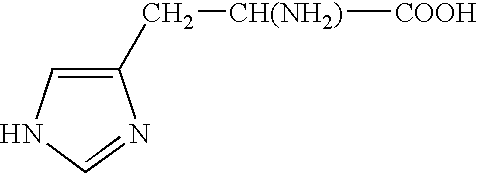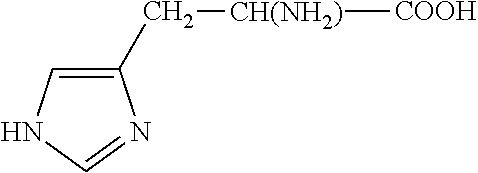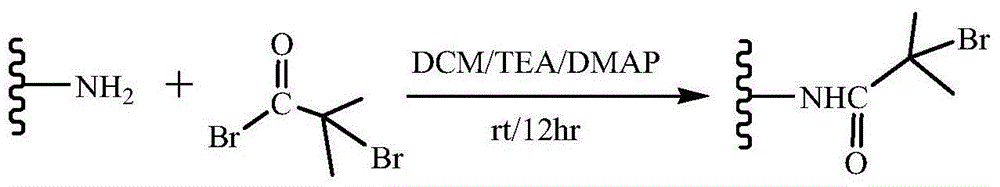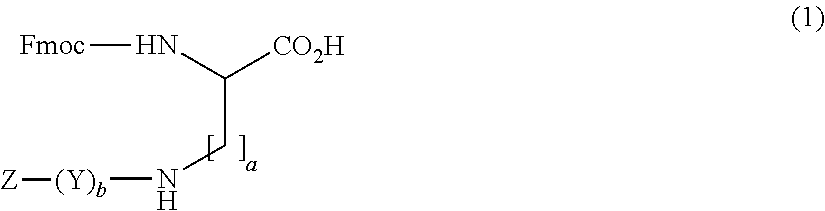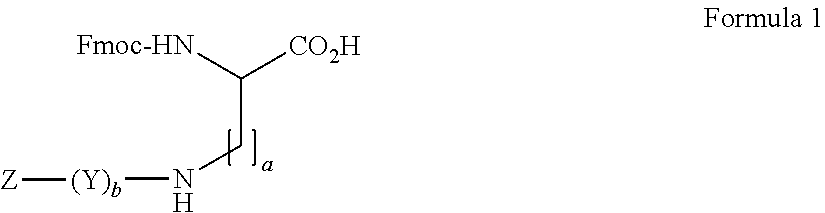Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
136 results about "Peptide modification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The covalent alteration of one or more amino acid residues within a peptide, resulting in a change in the properties of that peptide. [GOC:mah]
Modifications of peptide compositions to increase stability and delivery efficiency
ActiveUS8067532B2Increased stability and potencyReduce probabilityPeptide/protein ingredientsTransferasesPhysical stabilityDisulfide bond
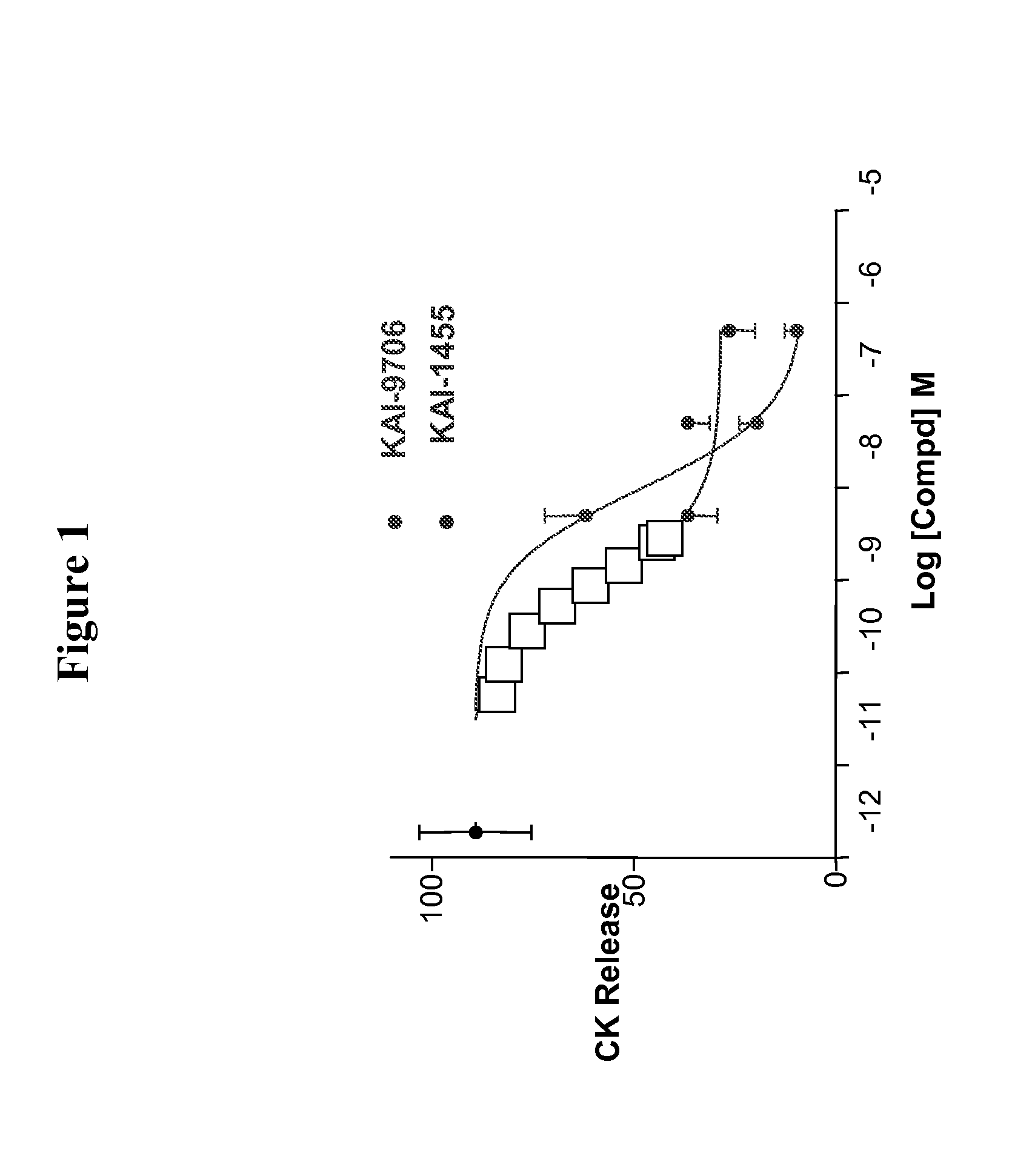
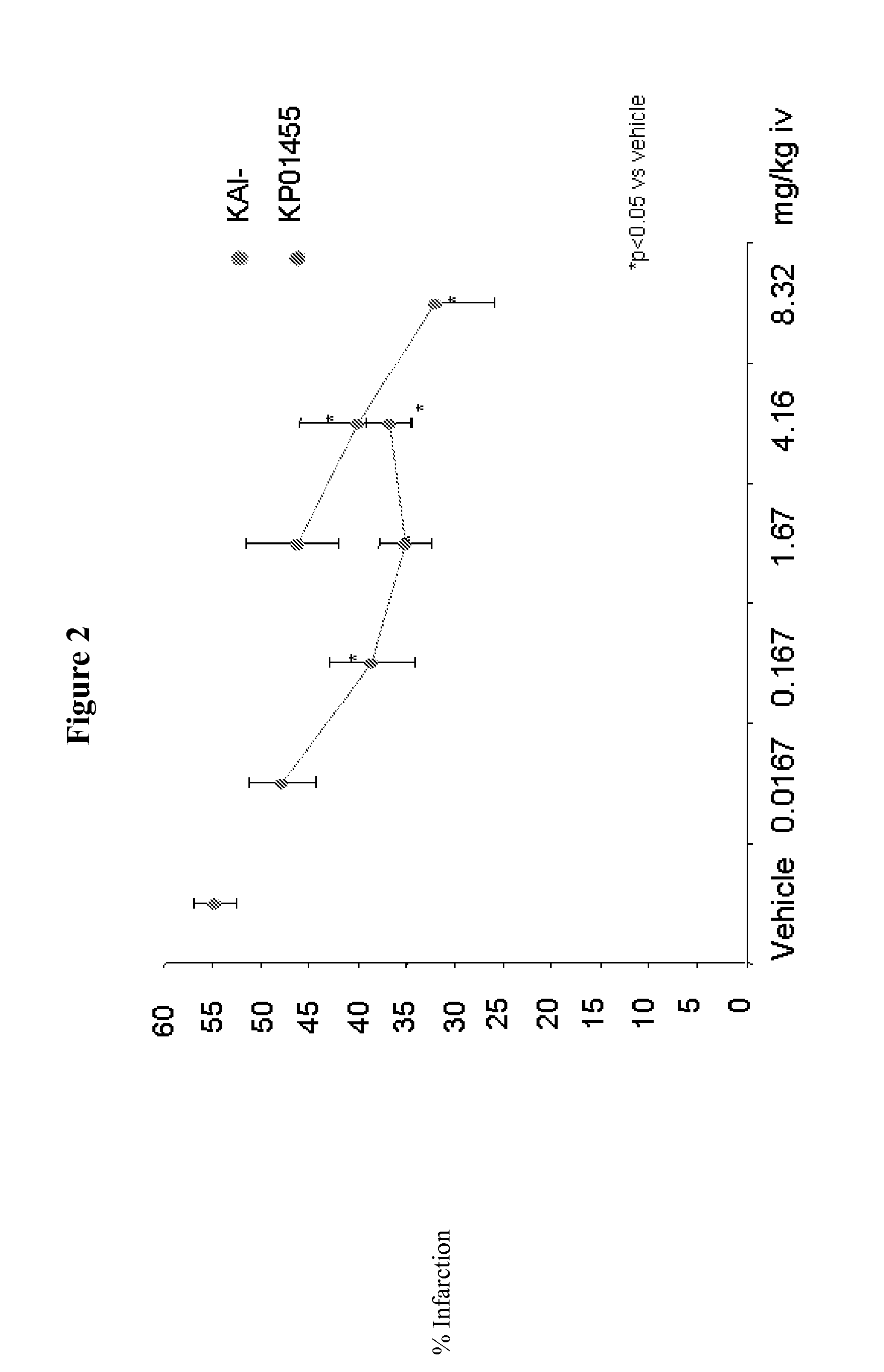
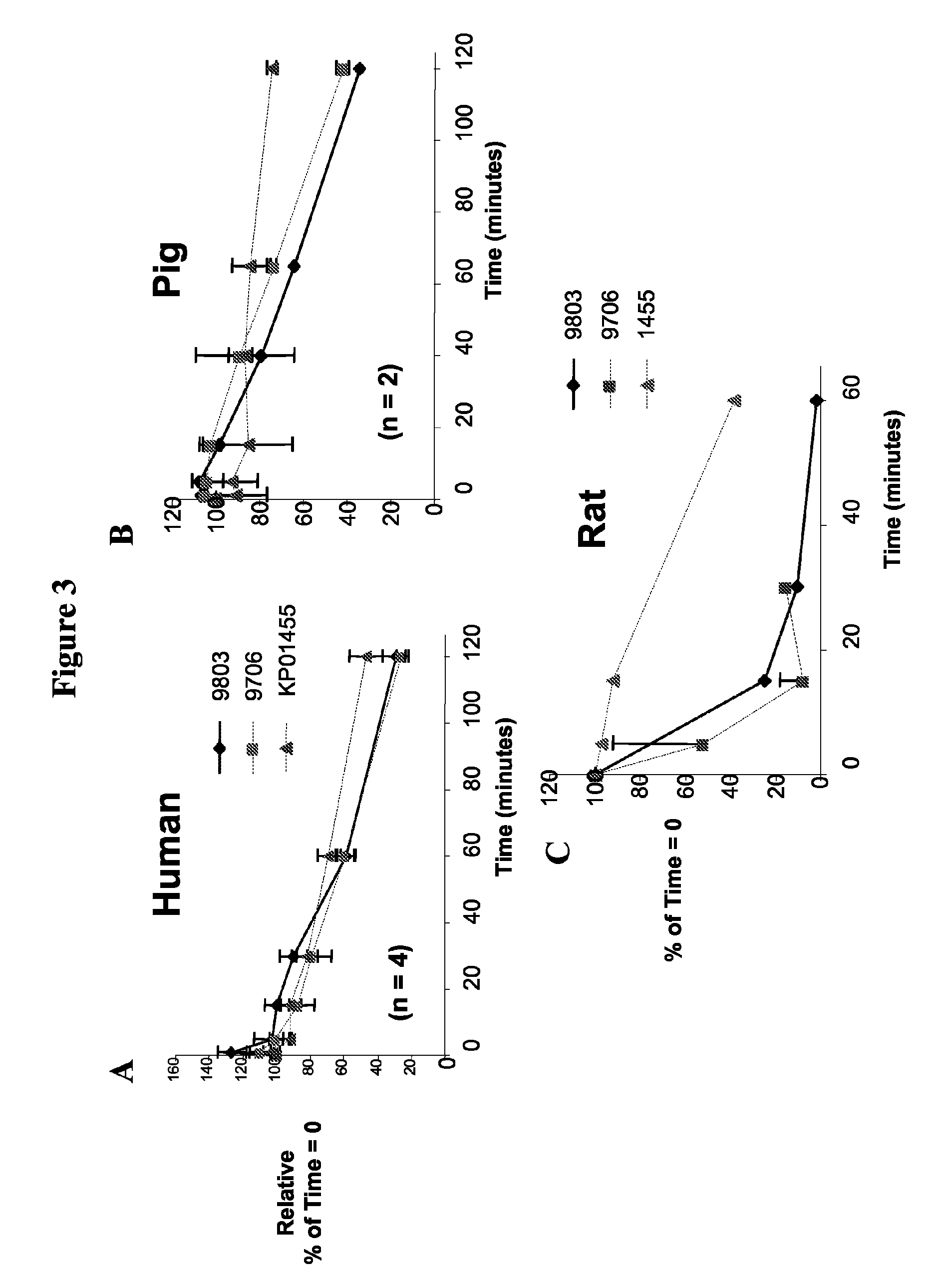
The disclosed invention relates to methods of modifying peptide compositions to increase stability and delivery efficiency. Specifically, the disclosed invention relates to methods to increase the stability and delivery efficiency of protein kinase C (PKC) modulatory peptide compositions. A “therapeutic peptide composition” comprises a “carrier peptide” and a “cargo peptide.” A “carrier peptide” is a peptide or amino acid sequence within a peptide that facilitates the cellular uptake of the therapeutic peptide composition. The “cargo peptide” is a PKC modulatory peptide. Peptide modifications to either the carrier peptide, the cargo peptide, or both, which are described herein increase the stability and delivery efficiency of therapeutic peptide compositions by reducing disulfide bond exchange, physical stability, reducing proteolytic degradation, and increasing efficiency of cellular uptake.
Owner:KAI PHARMA
Dipeptidyl-peptidase protected protein
InactiveUS20070060512A1Improve biostabilityLower blood sugar levelsPeptide/protein ingredientsMetabolism disorderDiabetes mellitusDipeptidyl peptidase
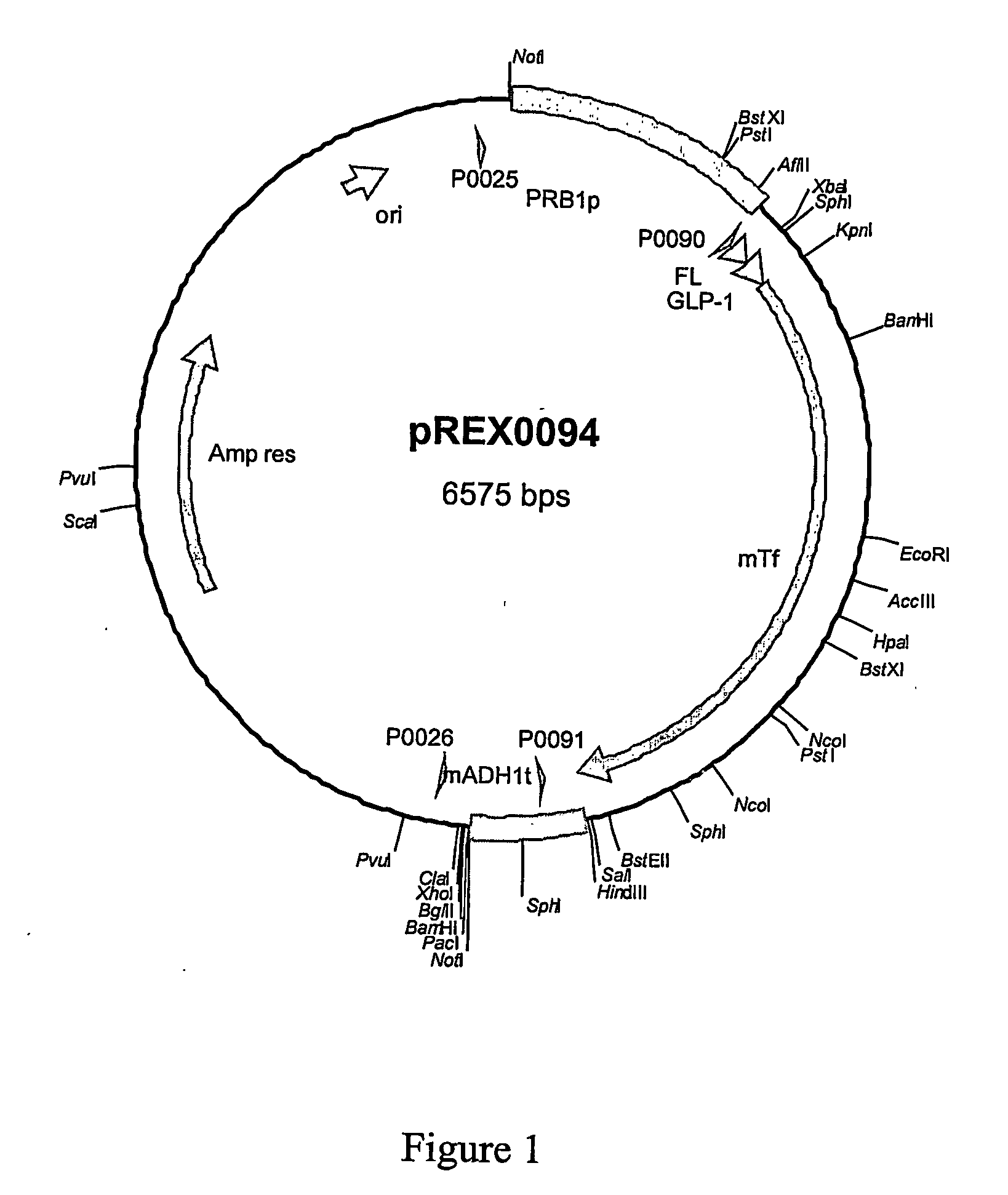
The present invention provides modified therapeutic polypeptides or peptides partially or completely protected from DPP activity. The modified polypeptides or peptides comprise at least one additional amino acid at the amino terminus. The modified therapeutic polypeptides or peptides are useful in the treatment of diseases such as diabetes.
Owner:BIOREXIS PHARMA CORP
Peptide nanoparticles and uses therefor
InactiveUS20100172943A1Low production costMaintain biological activityCosmetic preparationsPowder deliveryMedicineNanoparticle
The present invention provides nanoparticle compositions including one or more peptides. The present invention achieves transdermal delivery of such peptides without the need for peptide modification, or for use of chemical or mechanical abrasion or disruption of skin.
Owner:ANTERIOS INC
Polyglycol modified antimicrobial peptide and uses thereof
InactiveCN101429233AReduce hemolysisSmall toxicityPeptide preparation methodsBulk chemical productionAntimicrobial peptidesAntibacterial activity
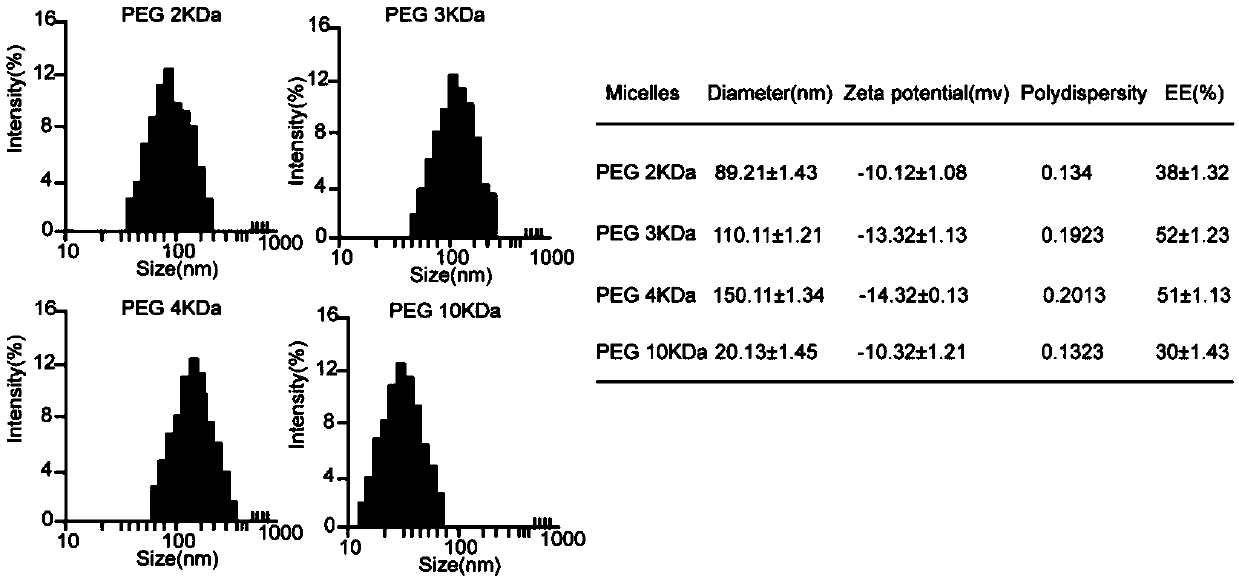
The invention relates to a series of polyethylene glycol modified cationic antibacterial peptide with different molecular weight. The modified antibacterial peptide is self-assembled into a nanometer micelle in an aqueous medium. The formation of the micelle can play a role in protecting the antibacterial peptide and weakening the degradation effect of protease on the antibacterial peptide, and simultaneously improves the stability and antibacterial activity of polypeptide in serum. In addition, the modification of polyethylene glycol obviously reduces the hemolytic toxic side effects of the antibacterial peptide.
Owner:NANKAI UNIV
Modifications of peptide compositions to increase stability and delivery efficiency
ActiveUS20090042769A1Extended half-lifeGreat propensityPeptide/protein ingredientsPeptide sourcesPhysical stabilityDisulfide bond
The disclosed invention relates to methods of modifying peptide compositions to increase stability and delivery efficiency. Specifically, the disclosed invention relates to methods to increase the stability and delivery efficiency of protein kinase C (PKC) modulatory peptide compositions. A “therapeutic peptide composition” comprises a “carrier peptide” and a “cargo peptide.” A “carrier peptide” is a peptide or amino acid sequence within a peptide that facilitates the cellular uptake of the therapeutic peptide composition. The “cargo peptide” is a PKC modulatory peptide. Peptide modifications to either the carrier peptide, the cargo peptide, or both, which are described herein increase the stability and delivery efficiency of therapeutic peptide compositions by reducing disulfide bond exchange, physical stability, reducing proteolytic degradation, and increasing efficiency of cellular uptake.
Owner:KAI PHARMA
Peptide nanoparticles and uses therefor
ActiveUS9486409B2Low production costMaintain biological activityPowder deliveryCosmetic preparationsNanoparticleMedicine
The present invention provides nanoparticle compositions including one or more peptides. The present invention achieves transdermal delivery of such peptides without the need for peptide modification, or for use of chemical or mechanical abrasion or disruption of skin.
Owner:ANTERIOS INC
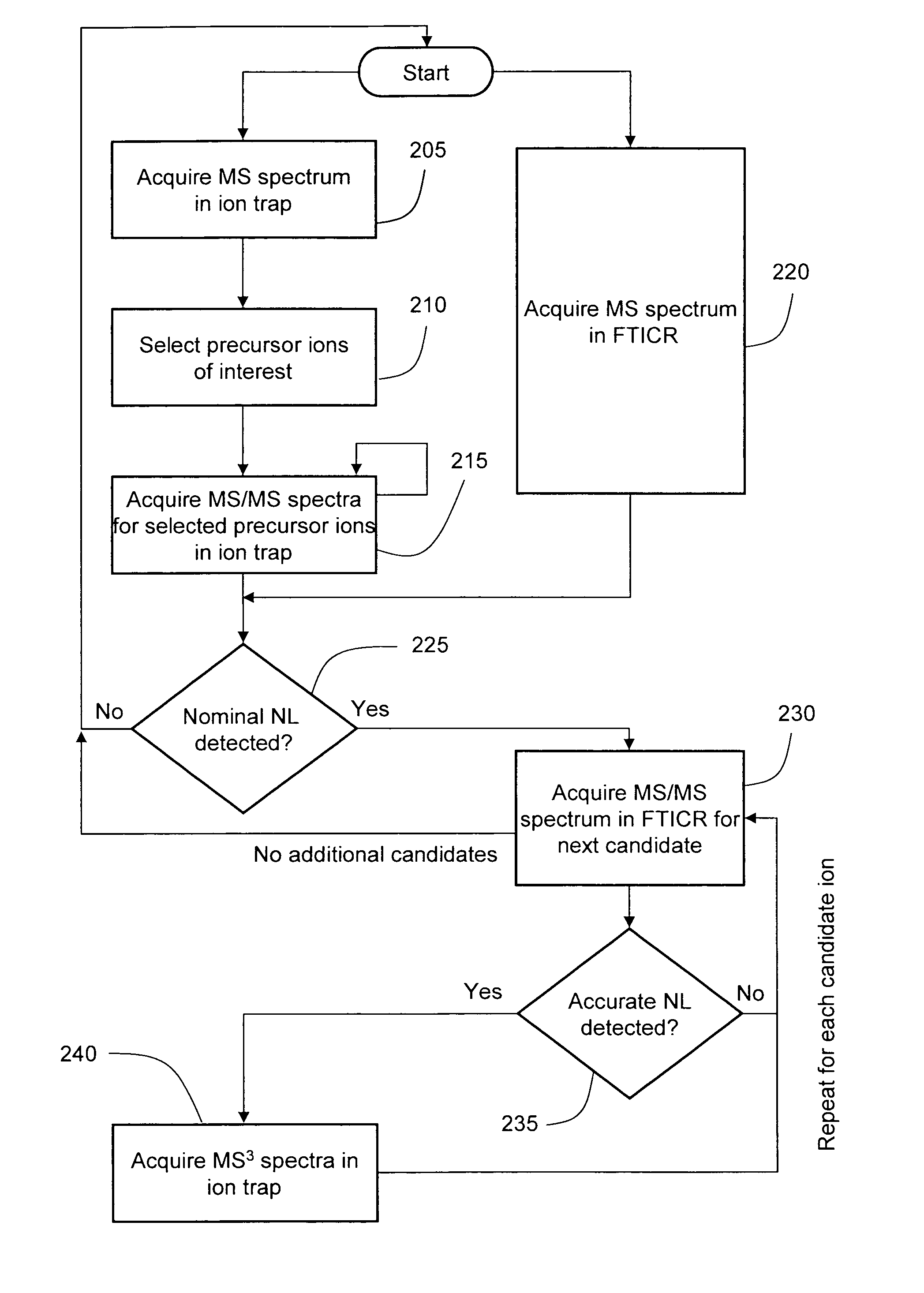
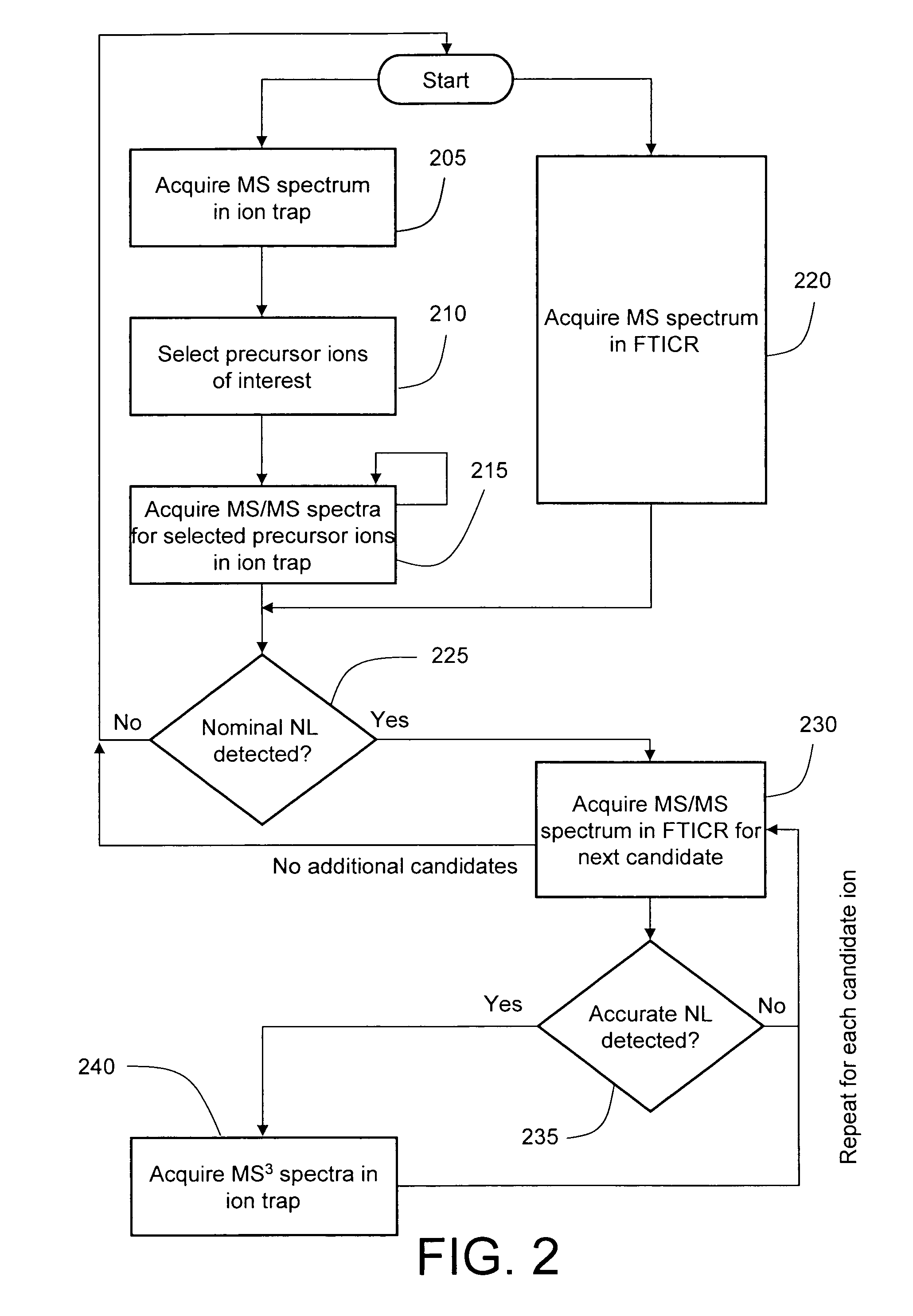
Data-dependent accurate mass neutral loss analysis
InactiveUS20080111068A1High selectivityReduce spurious MS scanIsotope separationMass spectrometersPhosphateMass analyzer
A method is disclosed for data-dependent neutral loss analysis of biomolecules and other materials in a hybrid mass spectrometer. Candidate product ions are selected by identifying peaks in a MS / MS spectrum acquired in a first mass analyzer that exhibit a specified nominal neutral loss value, which may be representative of the mass of a peptide modification, such as phosphate. High mass accuracy MS and MS / MS spectra acquired at a second mass analyzer, such as an FTICR or Orbitrap, are processed to determine if the mass difference between a candidate product ion and its corresponding precursor matches an accurate neutral loss value. In one embodiment, MS3 analysis is performed only on the candidate product ions that satisfy the accurate mass neutral loss value test.
Owner:THERMO FINNIGAN
Data-dependent accurate mass neutral loss analysis
InactiveUS7511267B2High selectivityImprove accuracyIon sources/gunsIsotope separationPhosphateMass analyzer
Owner:THERMO FINNIGAN
Peptide nanoparticles and uses therefor
ActiveUS20140234382A1Low production costMaintain biological activityPowder deliveryCosmetic preparationsMedicineNanoparticle
The present invention provides nanoparticle compositions including one or more peptides. The present invention achieves transdermal delivery of such peptides without the need for peptide modification, or for use of chemical or mechanical abrasion or disruption of skin.
Owner:ANTERIOS INC
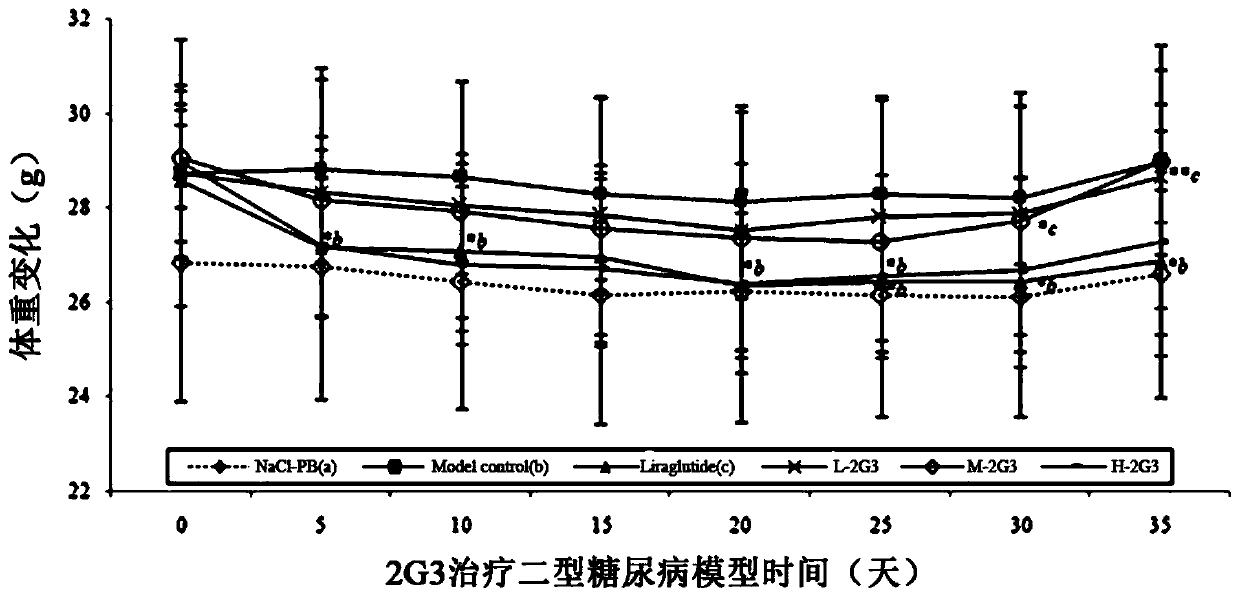
GLP-1 similar peptide modified dimer different in configuration and application of preparation method thereof in treating II-type diabetes
ActiveCN110845601AProlonged hypoglycemic effectHigh specific activityPeptide/protein ingredientsMetabolism disorderDisulfide bondingDimer
The invention provides application of novel glucagon peptide 1 fatty acid modified or unmodified dimer different in configuration in pancreas protection or hypoglycemic effect during treatment of II-type diabetes. The dimer is formed by connecting two identical GLP-1 monomers containing cysteine through disulfide bond formed by cysteine oxidation. Hypoglycemic duration of GLP-1 dimer is remarkablyprolonged without lowering activity of H-shaped GLP-1 homodimer (the disulfide bond is formed inside peptide chain), in-vivo continuous activity of GLP-1 analogue dimer can last for 19d while in-vivoactivity of liraglutide which is positive control drug is 3d, or in-vivo activity duration is remarkably prolonged when compared with long-acting GLP1 similar peptide which has already been reportedat present, so that technical progress of long-acting GLP1 drug is greatly promoted while convenience is brought to clinical application and popularization of the same. U-shaped homodimer (the disulfide bond is formed at the terminal C of the peptide chain) does not have impact on blood glucose but can obviously protect exocrine portion cells like pancreatic acini and catheters, thereby having a pancreas protecting function.
Owner:深圳纳福生物医药有限公司
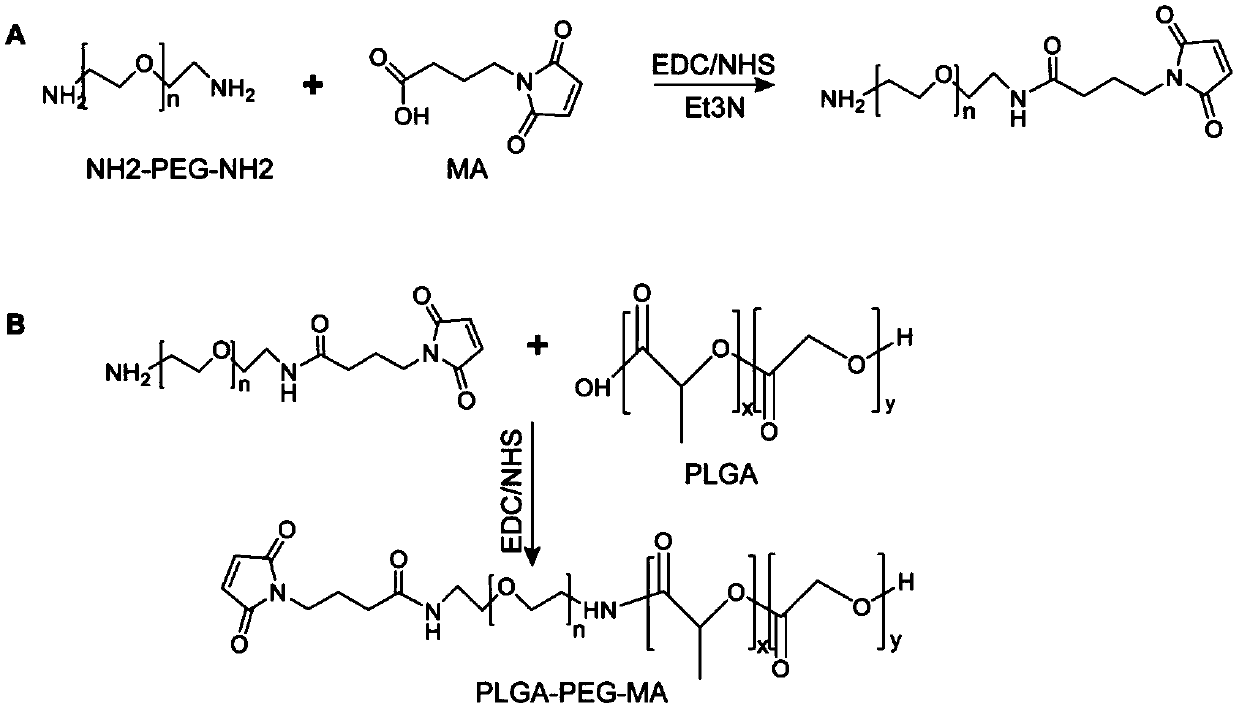
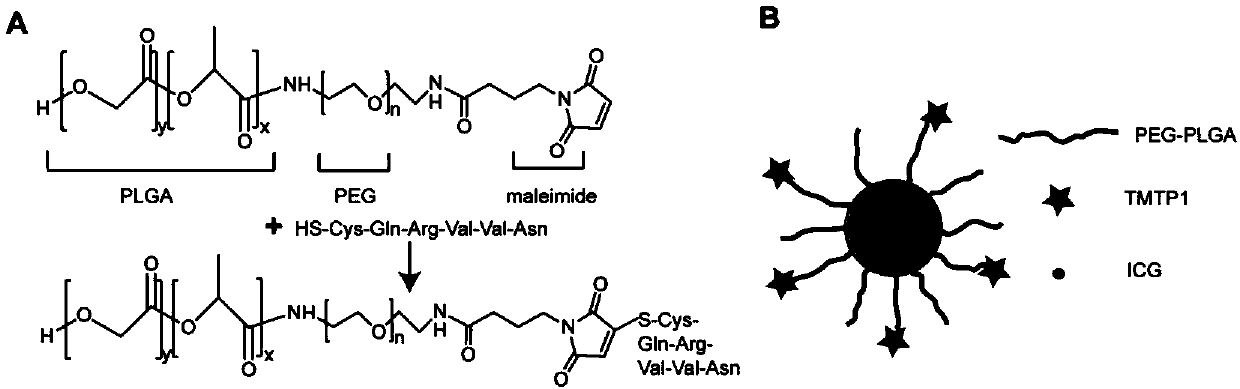
Preparation and application of nano-micelle imaging agent for cervical cancer sentinel node
ActiveCN110787306AReduce quenchingImprove stabilityEmulsion deliveryIn-vivo testing preparationsIndividualized treatmentImaging agent
The invention relates to preparation and application of a nano-micelle imaging agent for a cervical cancer sentinel node. A nanometer imaging agent high polymer PEG-PLGA is used as a carrier, micelleparticles are formed through polymerization, indocyanine green is loaded inside, TMTP1 targeting peptide is modified on the surface of a micelle, and maleic amide connected with a surface hydrophilicgroup PEG end of the micelle is connected with a sulfhydryl group of TMTP1 with ring formation by amido bonds at the head and the tail. The near infrared fluorescence nano-micelle diagnosis reagent provided by the invention can image the cervical cancer sentinel node in real time, can distinguish whether tumor cell metastasis occurs or not, and offers the assistance for the imaging diagnosis of intraoperative lymph nodes of cervical cancer in clinic in the future and the guidance of individualized treatment of tumor patients.
Owner:WUHAN KDWS BIOLOGICAL TECH CO LTD
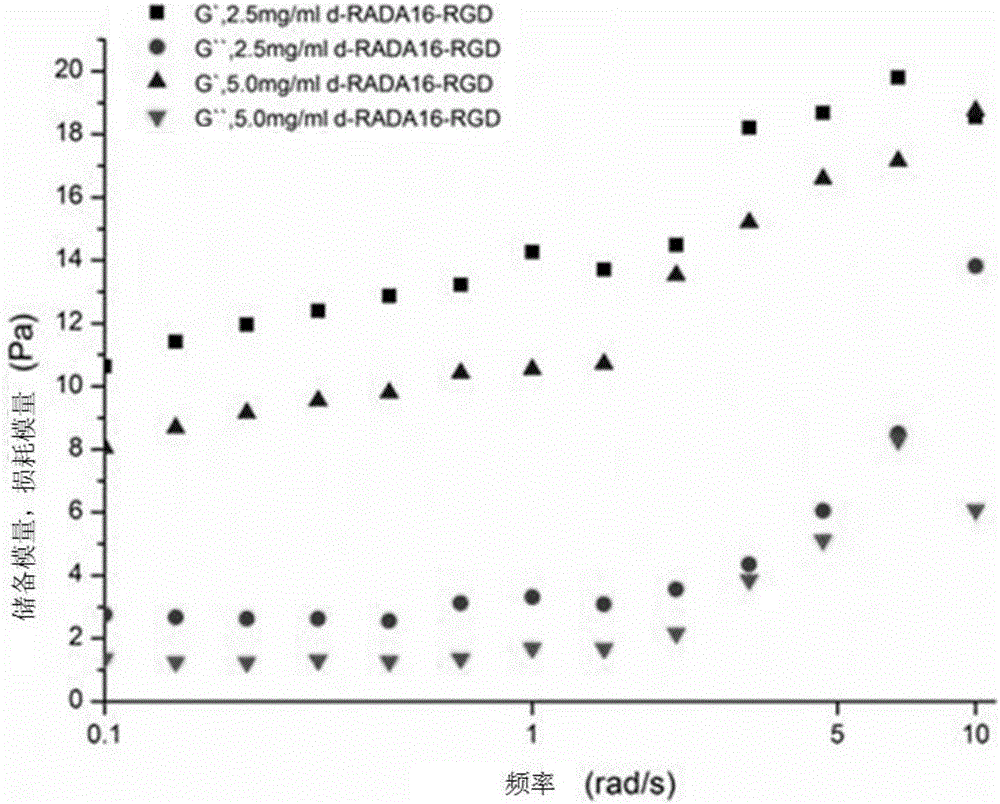
Self-assembly polypeptide d-RADA16-RGD and preparation method and application thereof
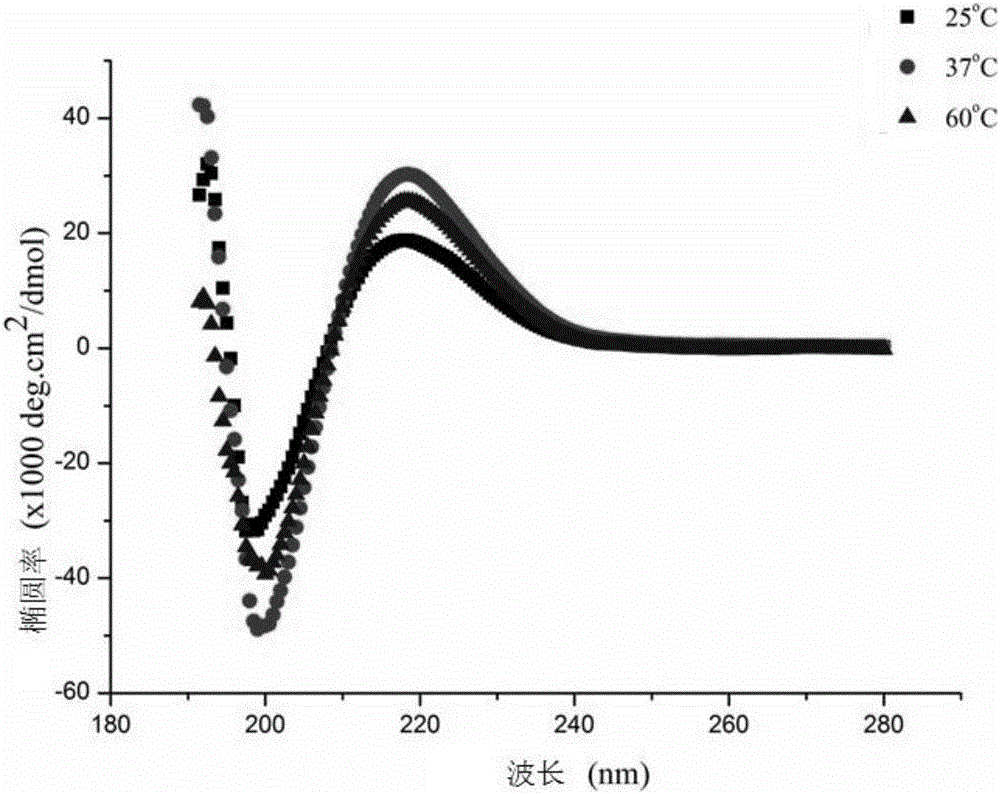
InactiveCN105669870AGood tissue compatibilityImprove biological activityAntibody mimetics/scaffoldsPeptide preparation methodsDiseaseGly-Gly-Arg
The invention discloses a self-assembly polypeptide d-RADA16-RGD and a preparation method and application thereof. A polypeptide sequence is Ac-(Arg-Ala-Asp-Ala-Arg-Ala-Asp-Ala)2-Gly-Gly-Arg-Gly-Asp-Ser-CONH2. The self-assembly polypeptide d-RADA16-RGD is modified by a functional motif (FM) RGD and is obtained through the synthesis of amino acids, and a formed three dimensional stent has good histocompatibility and biological activity. The self-assembly polypeptide can serve as a carrier for slowly releasing growth factors and medicines, and the functional motif, such as the RGD, is also applied to short peptide modification, and the bone inductivity and osteogenic capability can be significantly improved. The preparation method is simple, convenient to operate and good in bone repair effect, has an important promoting role on bone repair and can be widely applied to clinical bone defect diseases.
Owner:THE FIRST AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIVERSITY
A Peptide Modified Segment and Its Application in the Modification of Peptide Molecular Imaging Probes
InactiveCN102276688AGood pharmacokinetic propertiesIncrease contrastRadioactive preparation carriersPeptidesMolecular imagingImaging quality
The invention discloses a peptide modified segment, the structural formula is as follows: (L)m-(A)n-(S)o, wherein L is a connecting segment, m is 0-10; A is a PN or NP amino acid pair , n is 1-10; S is a spacer segment, and o is 0-10. The beneficial effects of the present invention are: the peptide modified segment provided by the present invention and its application in the modification of peptide molecular imaging probes can significantly improve the pharmacokinetic properties of targeting peptides, increase the tumor uptake value and tumor The contrast with the background optimizes the image quality. The target peptide product can be conveniently prepared by using an automatic peptide synthesizer through Fmoc protection chemistry, which is suitable for large-scale production.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Application of transdermal peptide modified pueraria thomsonii exosome nano preparation in preparation of anti-skin aging products
ActiveCN112656836APrevent agingEasy to produceCosmetic preparationsToilet preparationsMedical treatmentCutis
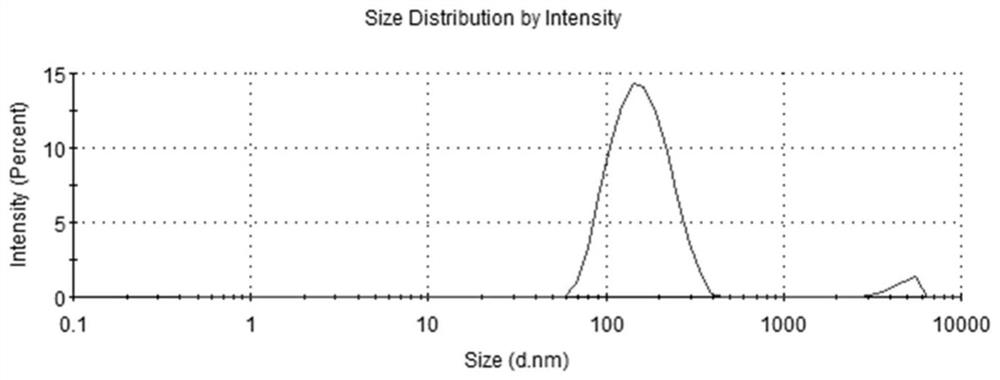
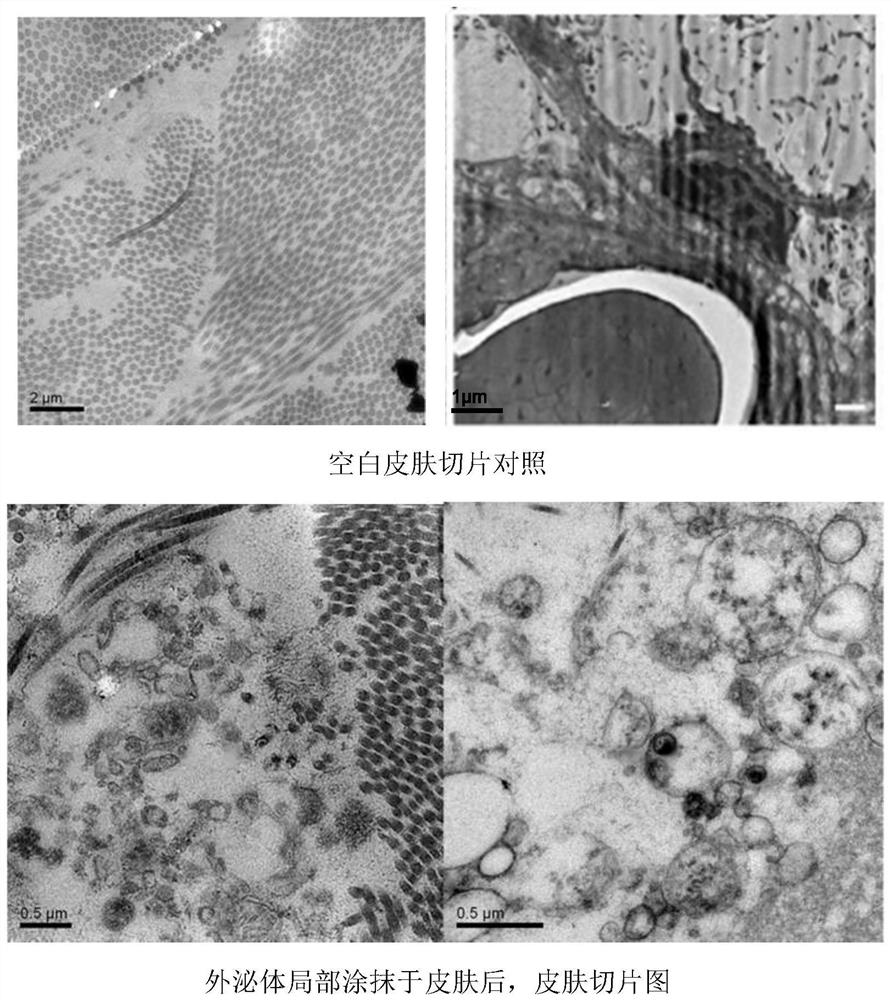
The invention discloses an application of a transdermal peptide modified pueraria thomsonii exosome nano preparation in preparation of an anti-skin aging product. The pueraria thomsonii exosome nano preparation modified by the transdermal peptide can effectively penetrate through the cuticle of the skin and is efficiently taken by skin cells such as skin fibroblasts, so that the obvious function of resisting skin (cell) aging is exerted, and a new strategy is provided for skin aging resistance; The transdermal peptide modified pueraria thomsonii exosome can be used as a novel skin anti-aging beauty product which is efficient, safe and convenient to prepare, can also be applied to aging resistance of other cells and tissues, and is used for delaying aging and function decline of body tissues and organs; therefore, great market development prospects are realized in the fields of medical products and beauty cosmetics.
Owner:ZHEJIANG UNIV
Targeting peptide modified traditional Chinese medicine multi-component exosome-like fusion nanoparticle as well as preparation method and application thereof
ActiveCN113908293ATake full advantage of hydrophilicityMake full use of spaceMaterial nanotechnologyNervous disorderPolyethylene glycolPhospholipid
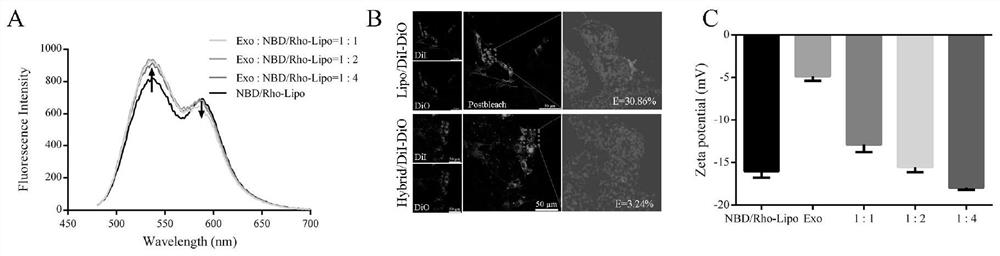
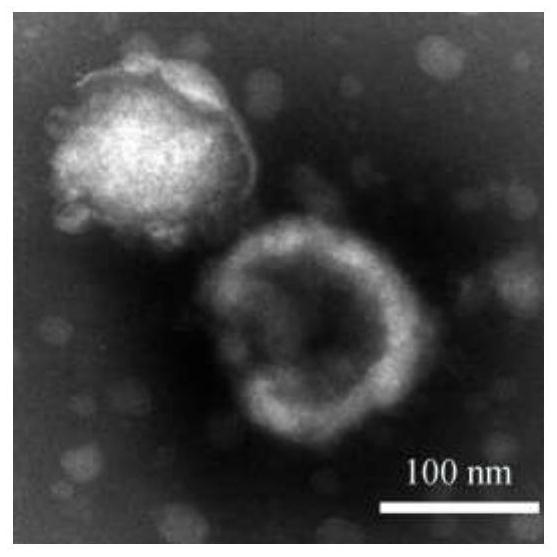
The invention discloses a preparation method and application of a targeting peptide modified traditional Chinese medicine multi-component exosome-like fusion nanoparticle, the nanoparticle is mainly formed by fusing a functionalized liposome and an exosome, the functionalized liposome is mainly composed of a targeted peptide, phospholipid and a traditional Chinese medicine fat-soluble active drug, the exosome is loaded with a traditional Chinese medicine water-soluble active medicine. The preparation method of the fusion nanoparticles comprises a polyethylene glycol induction method, a repeated freezing and thawing method, an extrusion method, an ultrasonication method and an incubation method. The preparation process is simple, the condition is mild, the cost is low, and the prepared nanoparticles have the advantages of various drug loading types, high penetrability, biological targeting regulation, therapeutic mechanism complementation, biological safety and the like. According to the nano-drug delivery platform, co-delivery, combined administration and collaborative precise treatment of multiple components of traditional Chinese medicine can be achieved, collaborative targeted treatment of complex progressive diseases such as tumors and neurodegenerative diseases can be achieved, and the nano-drug delivery platform has good application prospects.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Trp-Trp-Trp pentapeptide modified beta-carboline, preparation therefor, nanostructure, activity and application thereof
InactiveCN105218635AAvoid stickingAbility to inhibit migrationMaterial nanotechnologyPeptide/protein ingredientsInvasion and migrationBeta-Carboline
The invention discloses 1-(4-isopropyl propyl)-beta-carboline-3-formyl-Trp-Trp-Trp-Lys-Glu structured as follows, a preparation method therefor, a nanostructure thereof, antitumor action thereof, action thereof in resisting adhesion and invasion and migration of tumor cells, and illustrates application thereof in medical science. The formula is shown in the description.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Intein-modified proteases, their production and industrial applications
ActiveCN104822830AFusion with post-translational modification motifAccessory food factorsProteinase activityIntein
Methods for expressing multiple proteins by constructing transformation vectors that include multiprotein expression cassettes and transforming hosts with vectors and by engineering hosts expressing multiprotein units are provided. Multiprotein units that include multiple proteins fused to modified inteins capable of effecting splicing of the multiprotein units are described. Expression cassettes that include nucleic acids encoding multiprotein units and hosts including the expression cassettes are also provided.
Owner:AGRIVIDA
Preparation method and application of high-density RGD peptide modified material
ActiveCN104789547APreserve integrityPreserve activityOther chemical processesOn/in organic carrierHigh concentrationComputational chemistry
The invention discloses a preparation method and application of a high-density RGD peptide modified material. The preparation method comprises the following steps: dispersing a material, modified in the way that the surface is provided with an active group, into methylbenzene, adding a catalytic amount of 4-dimethylamino-pyridine, and slowly dropwise adding triethylamine and 2-bromoisobutyryl bromide under ice-bath, so as to prepare an initiator; adding cuprous bromide, 2,2'-dipyridyl and poly(ethylene glycol) methyl ether methacrylate into the initiator, so as to obtain a macromolecular polymer; adding the macromolecular polymer into a dimethylformamide solution containing an activating agent and 4-dimethylamino-pyridine to obtain an activated polymer brush, and adding RGD peptide for performing reaction in the dark at the room temperature for 18 hours; transferring a cell into the RGD peptide modified polymer brush, and performing reaction on a shaking table at the temperature of 4 DEG C for 20 minutes by adopting a phosphate buffer solution as a suspension liquid, so as to obtain the high-density RGD peptide modified material. The method is moderate in reaction conditions, simple, convenient and feasible in procedures, and achieves the purpose of fixing a cell on the surface of a solid material with high concentration and high density.
Owner:NORTHWEST UNIV(CN)
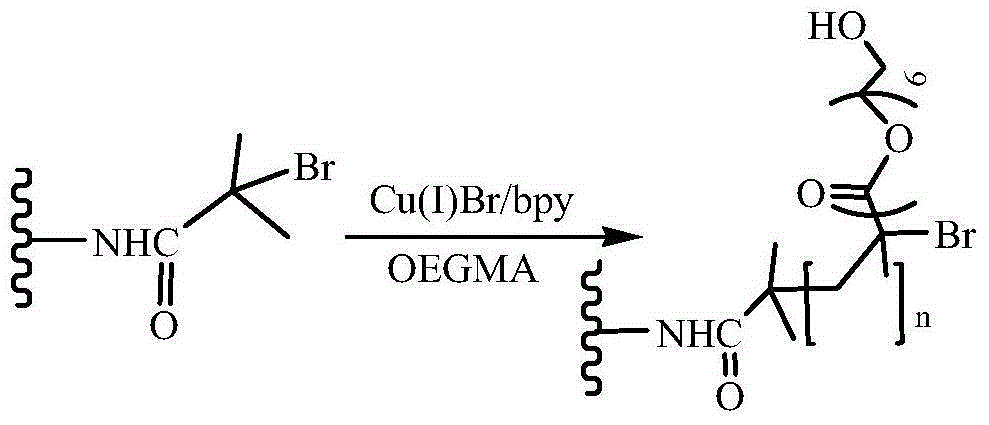
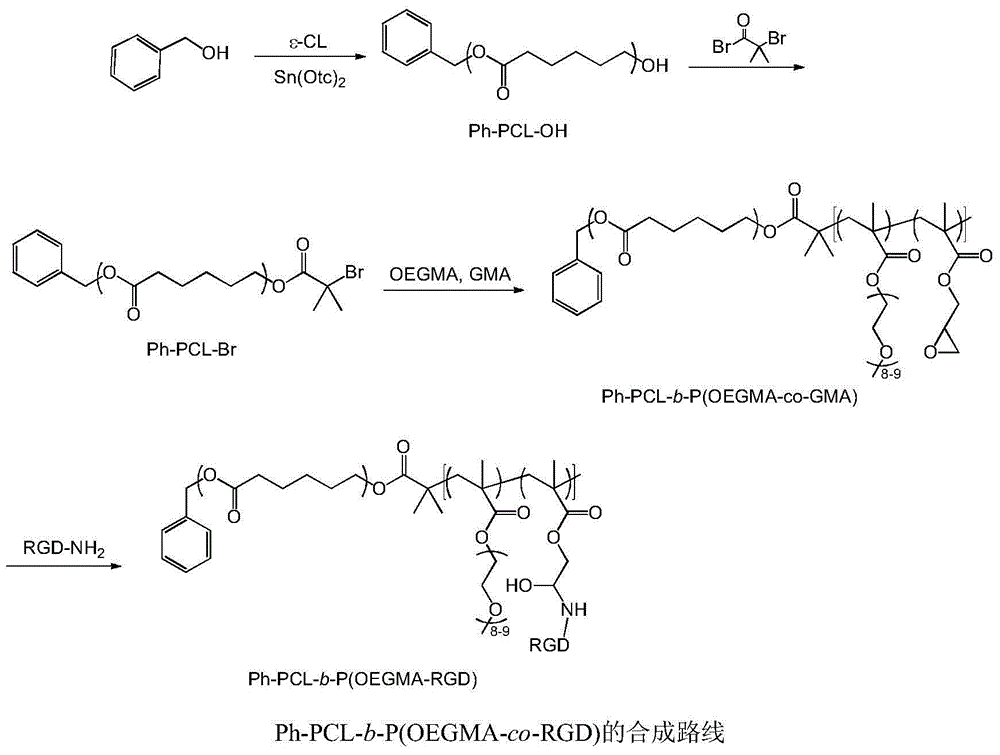
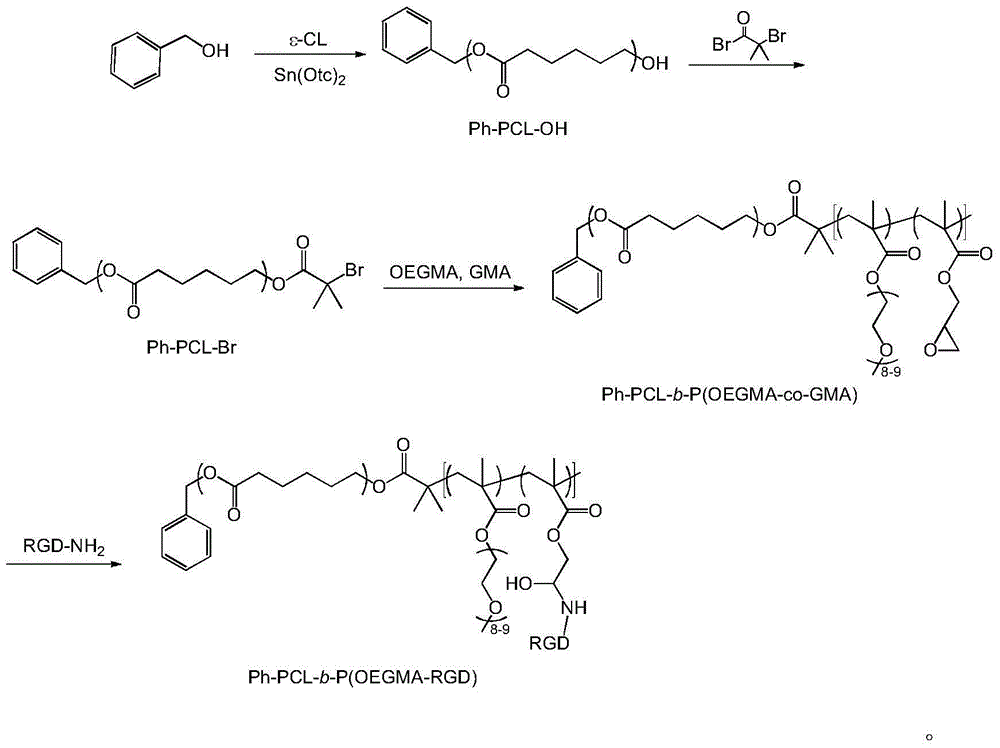
Bufalin-loaded polypeptide-modified poly(oligo(ethylene glycol)methacrylate)-polycaprolactone (Ph PCL b P(OEGMA co RGD) bufalin) nanometer preparation
InactiveCN105709231AEasy to prepareSuitable for mass productionPharmaceutical non-active ingredientsEmulsion deliveryMethacrylateCyclic peptide
The invention belongs to the technical field of a tumor targeting deliverying and slow release administration system. The invention relates to a bufalin-loaded valine-arginine-glycine-aspartic acid-glutamic acid cyclopeptide (cRGD)-modified poly(oligo(ethylene glycol)methacrylate)-polycaprolactone (Ph PCL b P(OEGMA co RGD) bufalin) nanometer particle and a preparation method thereof. Ph-PCL-b-P(OEGMA-co-GMA) as a base material is modified through cRGD to form Ph-PCL-b-P(OEGMA-co-RGD) and bufalin is wrapped by micelle of the Ph-PCL-b-P(OEGMA-co-RGD) so that (Ph PCL b P(OEGMA co RGD) bufalin) nanometer particles are prepared. The nanometer preparation can effectively reduce toxic or side effect of bufalin and improve water solubility and tumor targeting ability. The bufalin is slowly released along with material degradation so that long-term treatment effects are obtained.
Owner:SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE PUTUO DISTRICT CENT HOSPITAL
Method for preparing adriamycin-dipeptide complexes and applications
InactiveCN101274100ALow toxicityHigh selectivityOrganic active ingredientsMacromolecular non-active ingredientsDipeptideReaction rate
The invention discloses a preparation method of an adriamycin-dipeptide compound. Dipeptide or polypeptide containing proline residue is connected to amino of the adriamycin by the acylation through the technique of peptide modification; the toxicity of the adriamycin can be reduced and at the same time the pro-drug can release the adriamycin under the action of tumor interstitial cells so as to ensure that the adriamycin can enrich around tumor tissue to improve the selectivity of the adriamycin to the tumor tissue. The preparation method of the invention has the advantages of fast reaction rate, short term, relatively high productive rate and purity.
Owner:河南省健康伟业生物医药研究股份有限公司
Adriamycin-carrying lipid nanoscale ultrasound contrast agent targeting tumor-related fibroblasts and preparation method of adriamycin-carrying lipid nanoscale ultrasound contrast agent
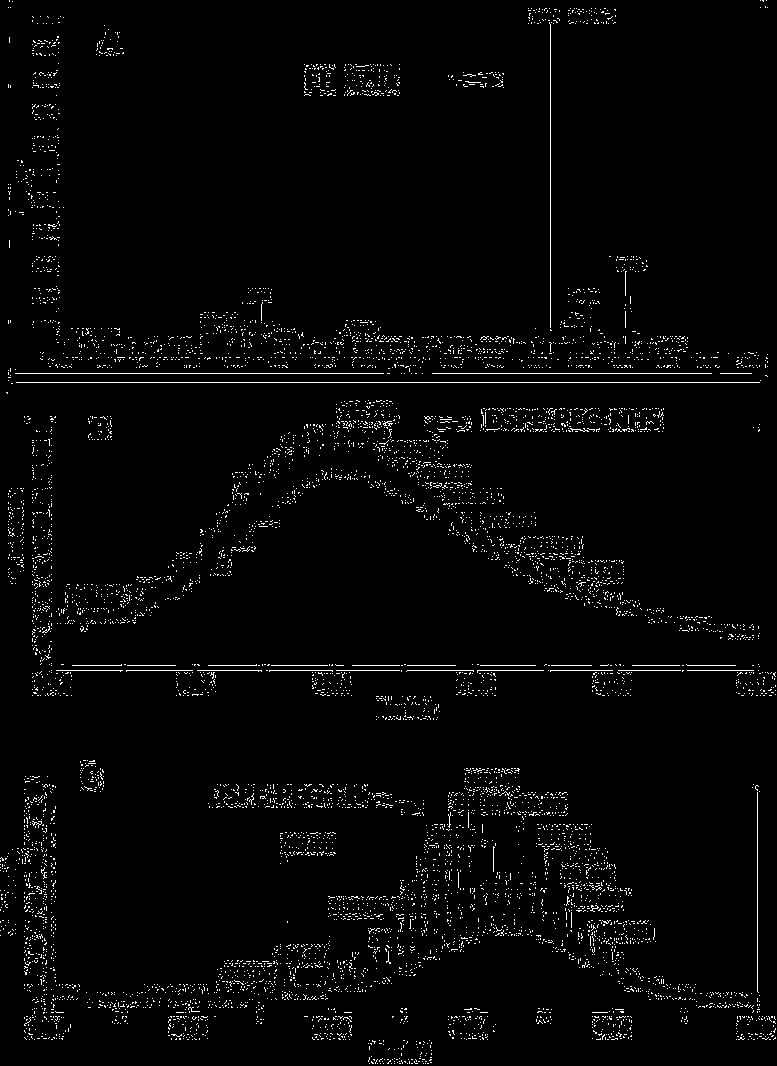
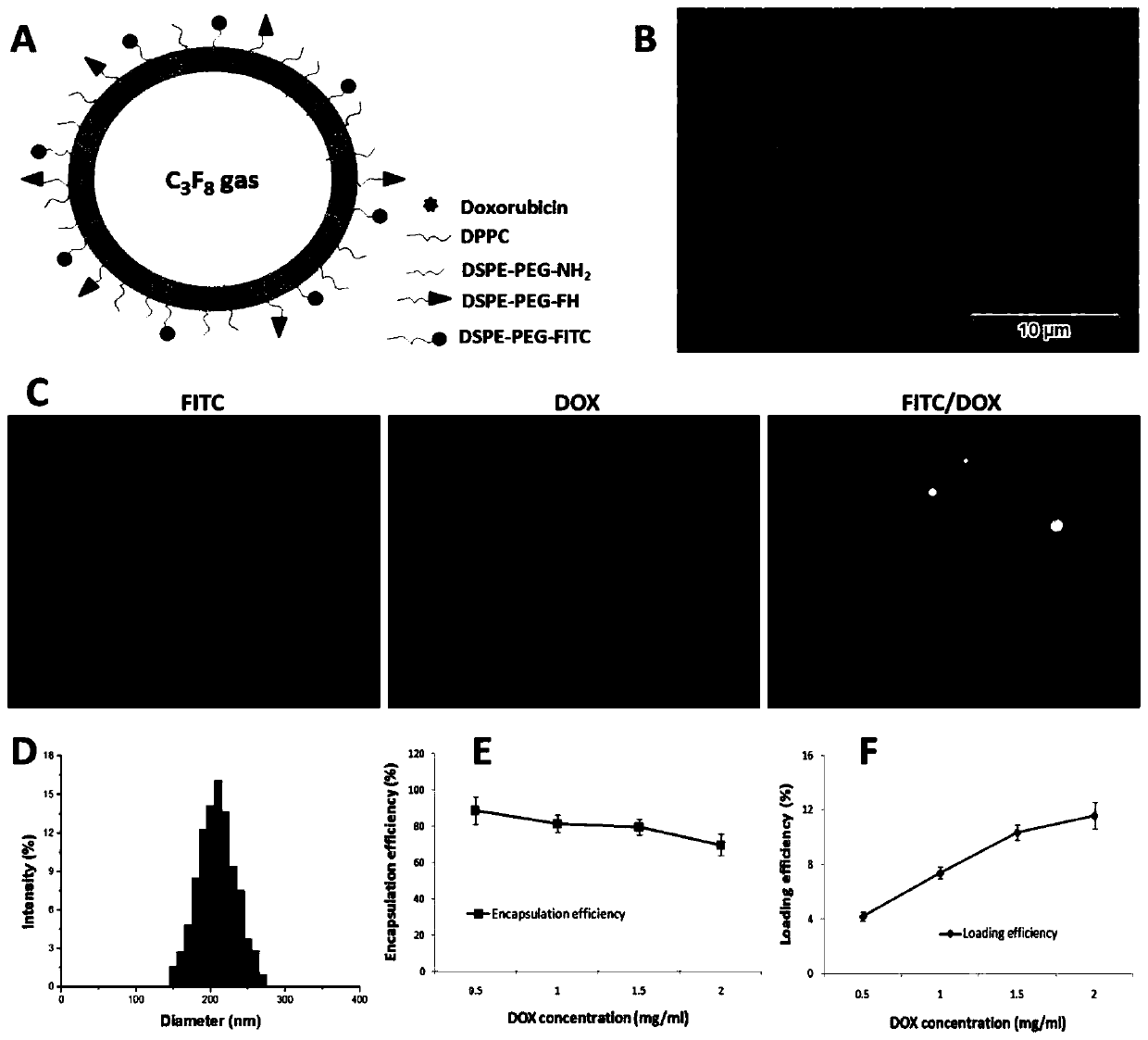
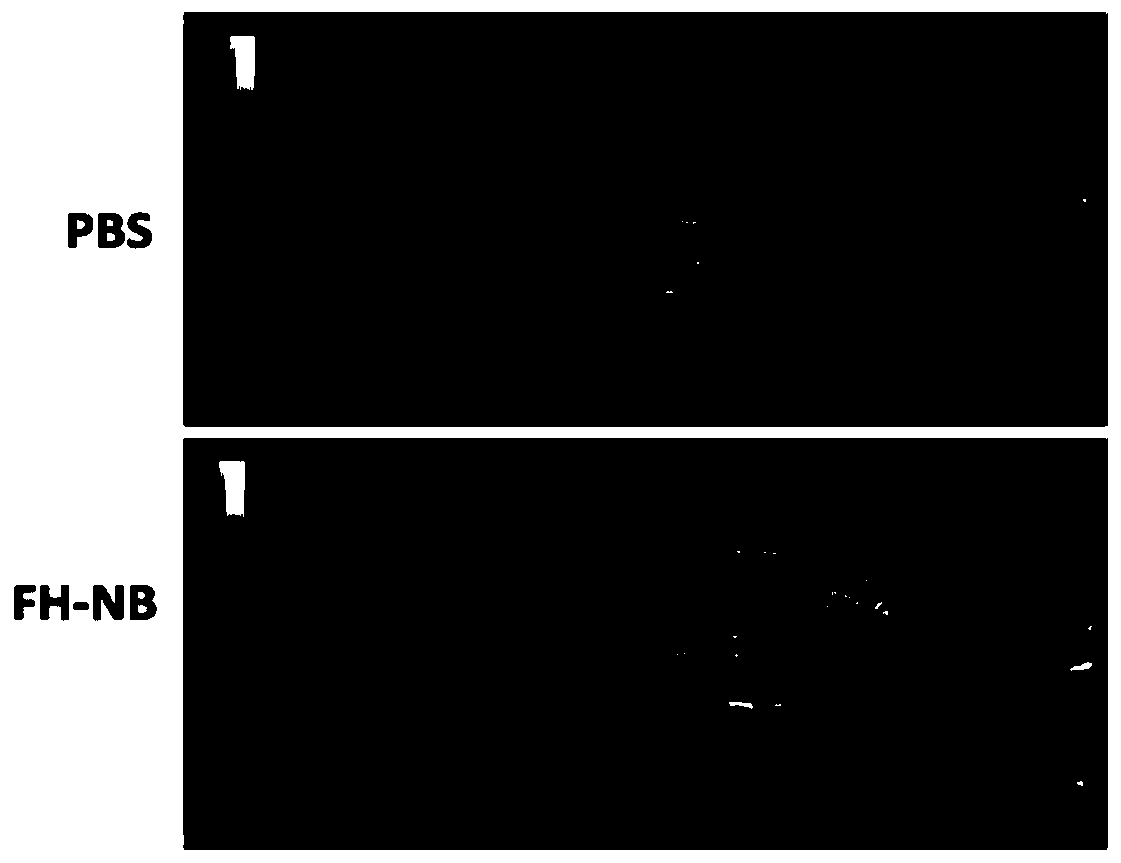
ActiveCN109908370ATargetedEfficientOrganic active ingredientsEchographic/ultrasound-imaging preparationsUltrasound imagingUltrasound contrast media
The invention provides an adriamycin-carrying lipid nanoscale ultrasound contrast agent targeting tumor-related fibroblasts and a preparation method of the adriamycin-carrying lipid nanoscale ultrasound contrast agent. The surface of the contrast agent is modified by a high affinity ligand (FH short peptide) for specifically expressing a TNC protein by the tumor-related fibroblasts, the FH short peptide is used as a targeting ligand, lipid is used as a shell membrane material, and adriamycin and gaseous fluorocarbon are wrapped inside the shell membrane. The targeted ultrasound contrast agentis in nanoscale, has ultrasound imaging capability, can be used for targeted delivery of drugs into the tumor-related fibroblasts, and specifically kills the tumor-related fibroblasts, and a tumor treatment effect is achieved through intervention on a tumor microenvironment.
Owner:青岛孚嘉康达智能科技有限公司
Amino diacids containing peptide modifiers
InactiveUS20160207957A1Carbamic acid derivatives preparationPeptide/protein ingredientsCombinatorial chemistryPerylene derivatives
The present invention relates to peptide modifier compounds of Formula (1), or a salt thereof, wherein: a is an integer from 1 to 10, more preferably from 1 to 3; b is an integer from 0 to 7; Z is a terminal group and Y is a bivalent group. Further aspects of the invention relate to intermediates in the preparation of compounds of Formula (1), and the use of compounds of Formula 1 in the synthesis of peptide derivatives.
Owner:CHEM & BIOPHARML LAB OF PATRAS
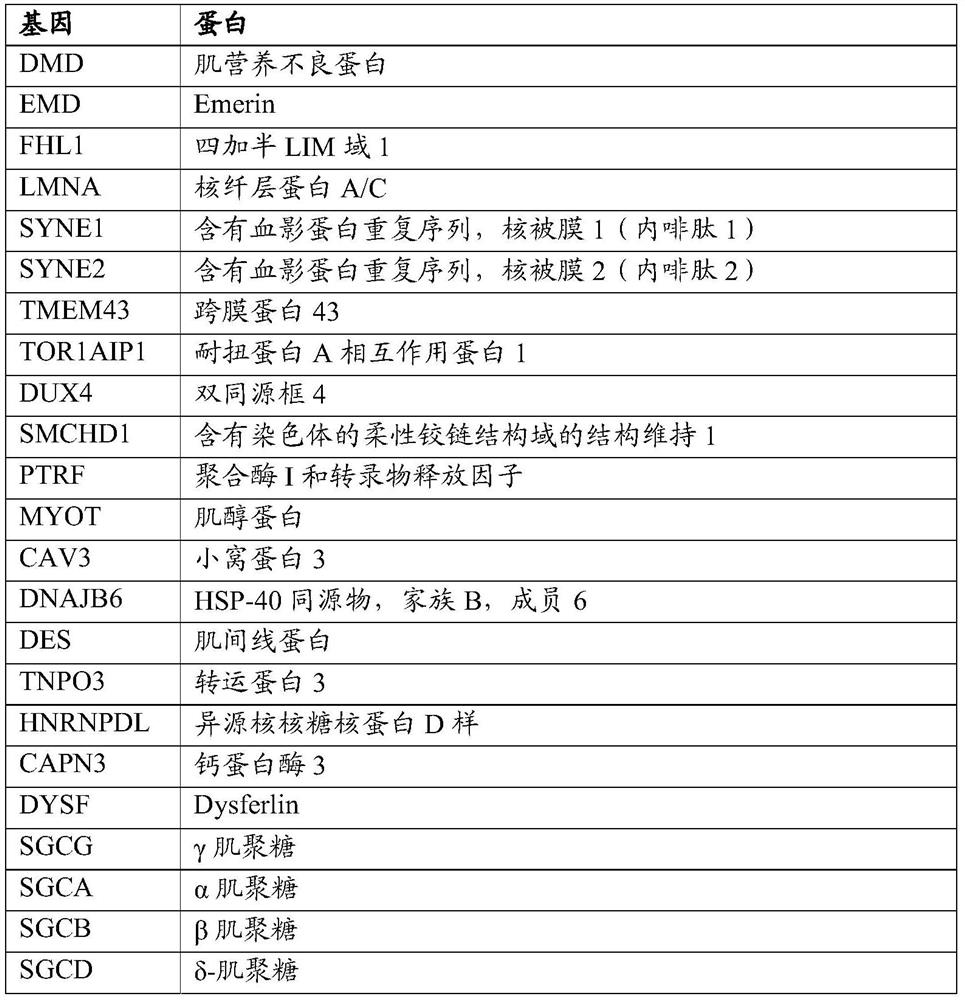
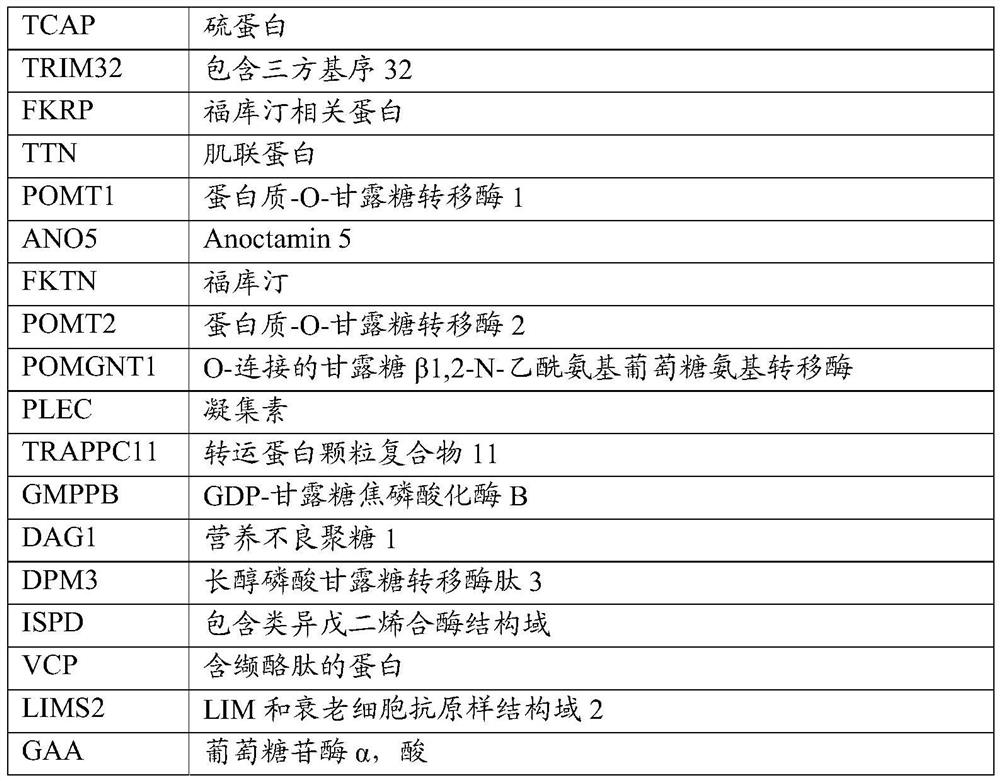
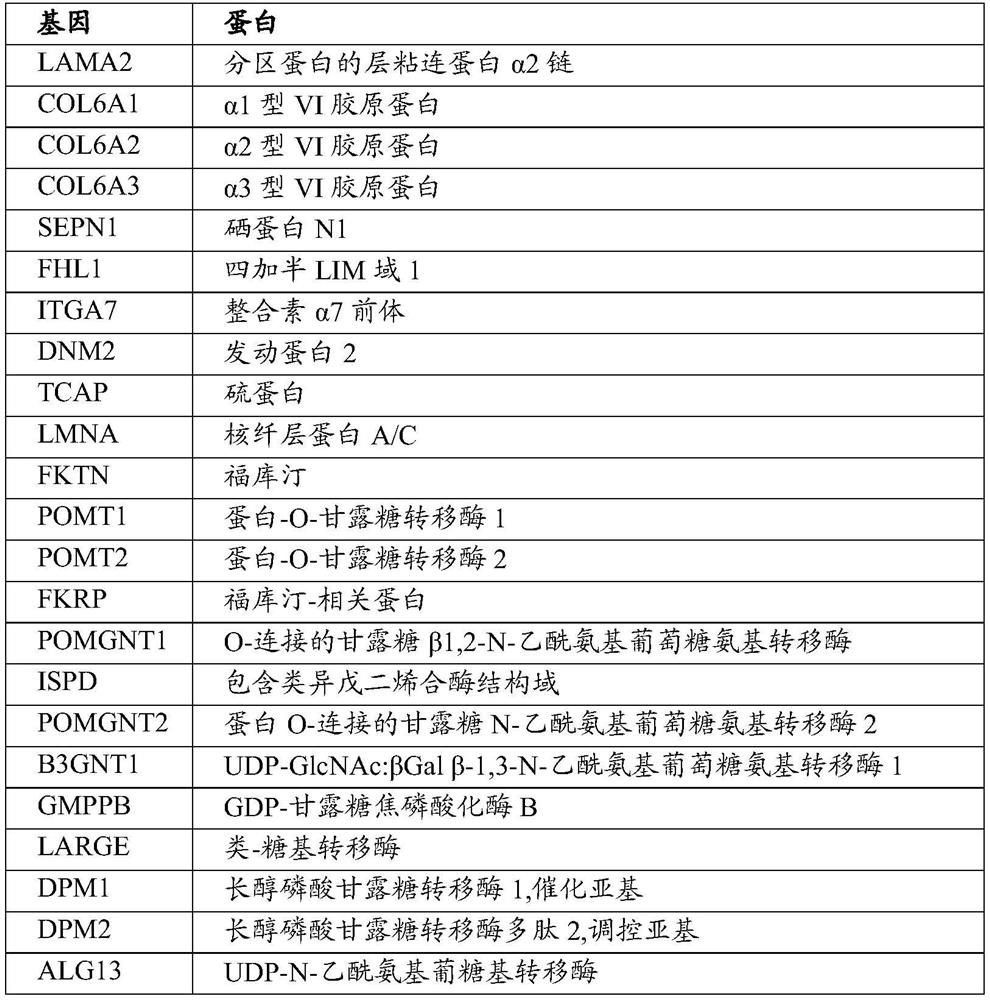
PEPTIDE-MODIFIED HYBRID RECOMBINANT ADENO-ASSOCIATED VIRUS SEROTYPE BETWEEN AAV9 AND AAVrh74 WITH REDUCED LIVER TROPISM AND INCREASED MUSCLE TRANSDUCTION
The invention relates to a recombinant adeno-associated virus (AAV) capsid protein, which is a peptide-modified hybrid between AAV serotype 9 (AAV9) and AAV serotype 74 (AAVrh74) capsid proteins comprising at least one copy of a peptide comprising the RGD motif, wherein said recombinant peptide-modified hybrid AAV capsid protein has a further reduced liver tropism and an increased muscle transduction compared to the recombinant hybrid AAV capsid protein not having said peptide. The invention relates also to the derived peptide-modified hybrid AAV serotype vector particles packaging a gene of interest and their use in gene therapy, in particular for treating neuromuscular genetic diseases, in particular muscular genetic diseases.
Owner:GENETHON +4
KRGD peptide-modified carbolino-hexahydropyrazine-1,4-diketones and their preparation method, antithrombotic effect and use
InactiveCN103450336AOrganic active ingredientsPeptide preparation methodsAntithrombotic AgentDiketone
The invention discloses KRGD peptide-modified carbolino-hexahydropyrazine-1,4-diketones, which are three novel conjugates of carbolino-hexahydropyrazine-1,4-diketone and RGD peptide and are shown in the general formula I. In the general formula I, R represents Lys-Arg-Gly-Asp-Val, Lys-Arg-Gly-Asp-Phe or Lys-Arg-Gly-Asp-Ser. The invention also discloses heterocyclic nucleuses of the novel conjugates, wherein R represents OH. The invention also discloses a preparation method and in-vitro anti-platelet aggregation effects of the novel conjugates, and also discloses an antithrombotic use of the novel conjugates in a rat thrombus formation model. A result shows that the three novel conjugates of carbolino-hexahydropyrazine-1,4-diketone and RGD peptide (wherein R represents Lys-Arg-Gly-Asp-Val, Lys-Arg-Gly-Asp-Phe or Lys-Arg-Gly-Asp-Ser) and their heterocyclic nucleuses (wherein R represents OH) have good antithrombotic activity and clear application prospects in antithrombotic agent preparation.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Specific polypeptide modified colorimetric sensor and making method thereof
InactiveCN104165855AEfficient detectionHigh selectivityColor/spectral properties measurementsChemical physicsCu2 ions
The invention relates to a specific peptide modified colorimetric sensor and a making method thereof. The colorimetric sensor is used for detecting copper ions. The sensor uses a gold-mercapto key to make the surface of gold nanoparticles to be modified by a specific peptide, the addition of copper ions to be measured changes the conformation of the specific polypeptide from alpha-helix to beta-folding, and the gold nanoparticles mutually aggregate by using beta-folding fragments formed by the surface modification specific polypeptide in order to change the absorbance of a solution and generate color difference. The copper ions are detected by the gold nanoparticles modified by the specific peptide for the first time, and the colorimetric sensor of the gold nanoparticles modified by the specific polypeptide has very high sensitivity, simplicity and specificity on the copper ions, has a wide detection range, has potential application values in the detection of the content of the copper ions in drinking water, serum, rivers, sewage and other solutions, and also has the advantages of convenient operation, high sensitivity and wide application prospect.
Owner:SHANGHAI UNIV
Extended recombinant polypeptide-modified c-peptide
InactiveUS20130316946A1Reduce riskReduce incidenceBacteriaPeptide/protein ingredientsC-peptideIn vivo
The present invention relates to modified forms of C-peptide, and methods for their use. In one aspect, the modified forms of C-peptide comprise modified C-peptide derivatives which exhibit superior pharmacokinetic and biological activity in vivo.
Owner:CEBIX
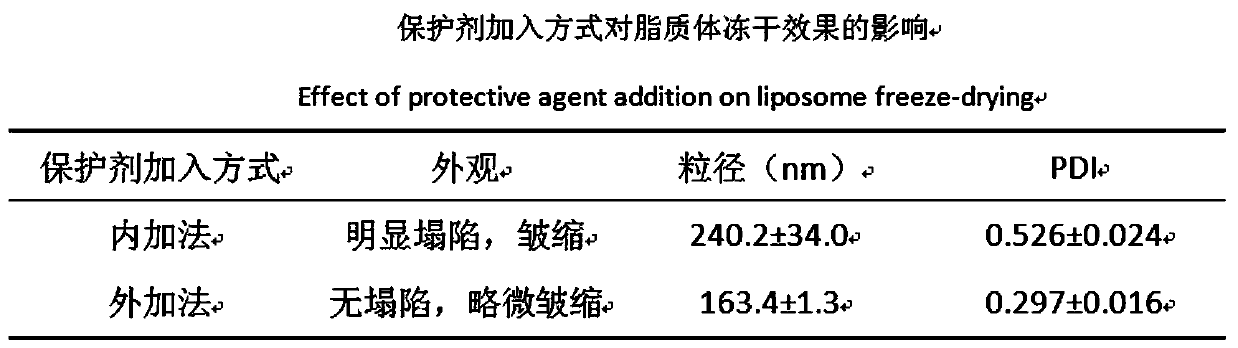
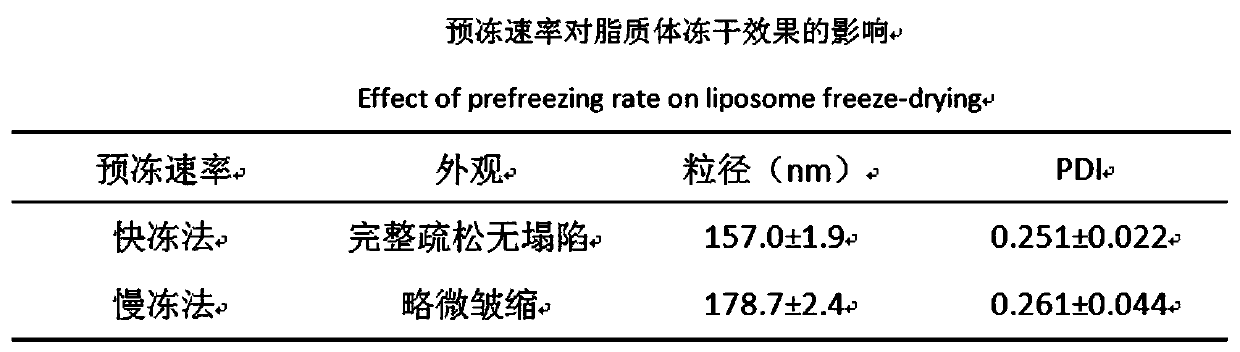
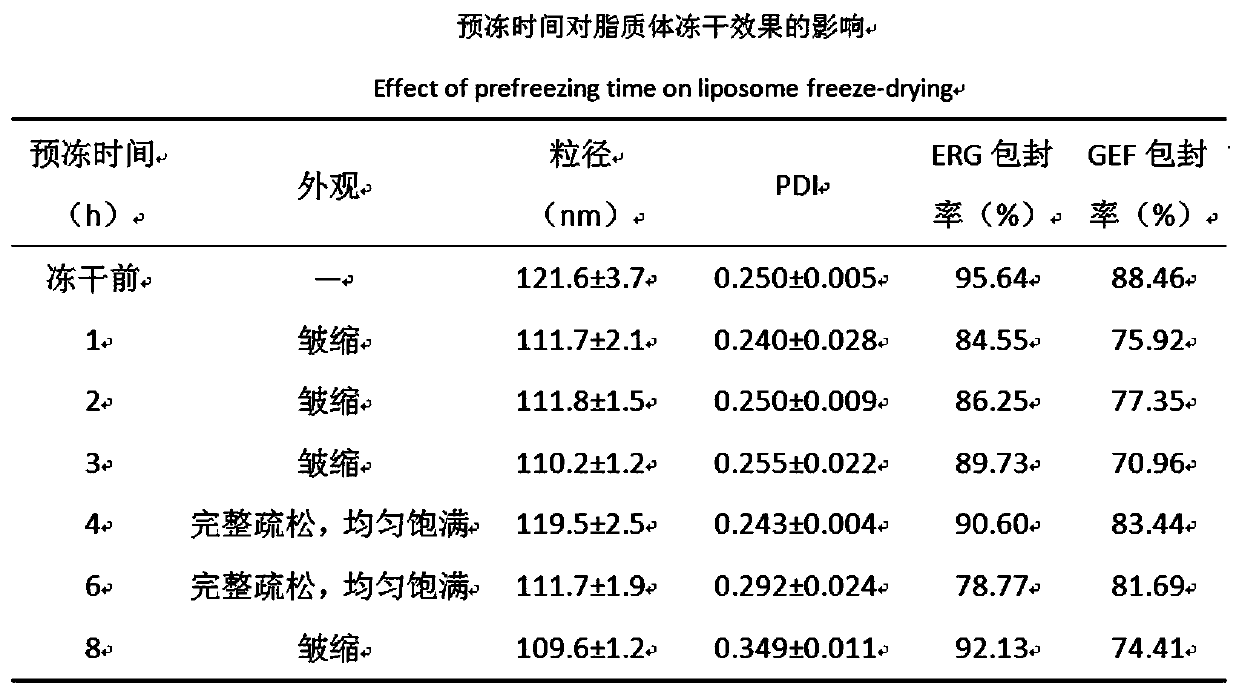
Preparation method of ergosterol and gefitinib combined compound liposome freeze-dried powder, liposome and application thereof
ActiveCN110623964AStrong proliferation inhibitory effectGood apoptosis rateOrganic active ingredientsPowder deliveryCyclic peptideFluorescence
The invention relates to a preparation method of RGD cyclic peptide R8 peptide modified ergosterol and gefitinib combined compound liposome freeze-dried powder. The preparation method comprises the following steps: adding a freeze-drying protective agent into a pre-prepared RGD / R8-ERG / GEF-LIP liposome suspension in an external addition manner; and finally, preparing the freeze-dried powder of thecompound liposome by adopting a freeze-drying method. The RGD / R8-ERG / GEF-LIP lipidosome suspension is prepared by adopting the following method: firstly, preparing ERG / GEF-LIP, and then, preparing theRGD / R8-ERG / GEF-LIP lipidosome suspension by adopting a post-insertion method. According to the invention, an RGD / R8-ERG / GEF-LIP active drug-loading liposome drug delivery system is successfully constructed; ERG / GEF-LIP is used for investigating a freeze-drying process and a prescription, and after an optimal prescription process is screened out, the optimal prescription process is applied to RGD / R8-ERG / GEF-LIP for verification. An in-vitro test result of RGD / R8-ERG / GEF-LIP freeze-dried powder proves that the RGD / R8-ERG / GEF-LIP freeze-dried powder has a relatively strong tumor cell proliferation inhibition effect, the fluorescence uptake intensity and the good cell apoptosis rate.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
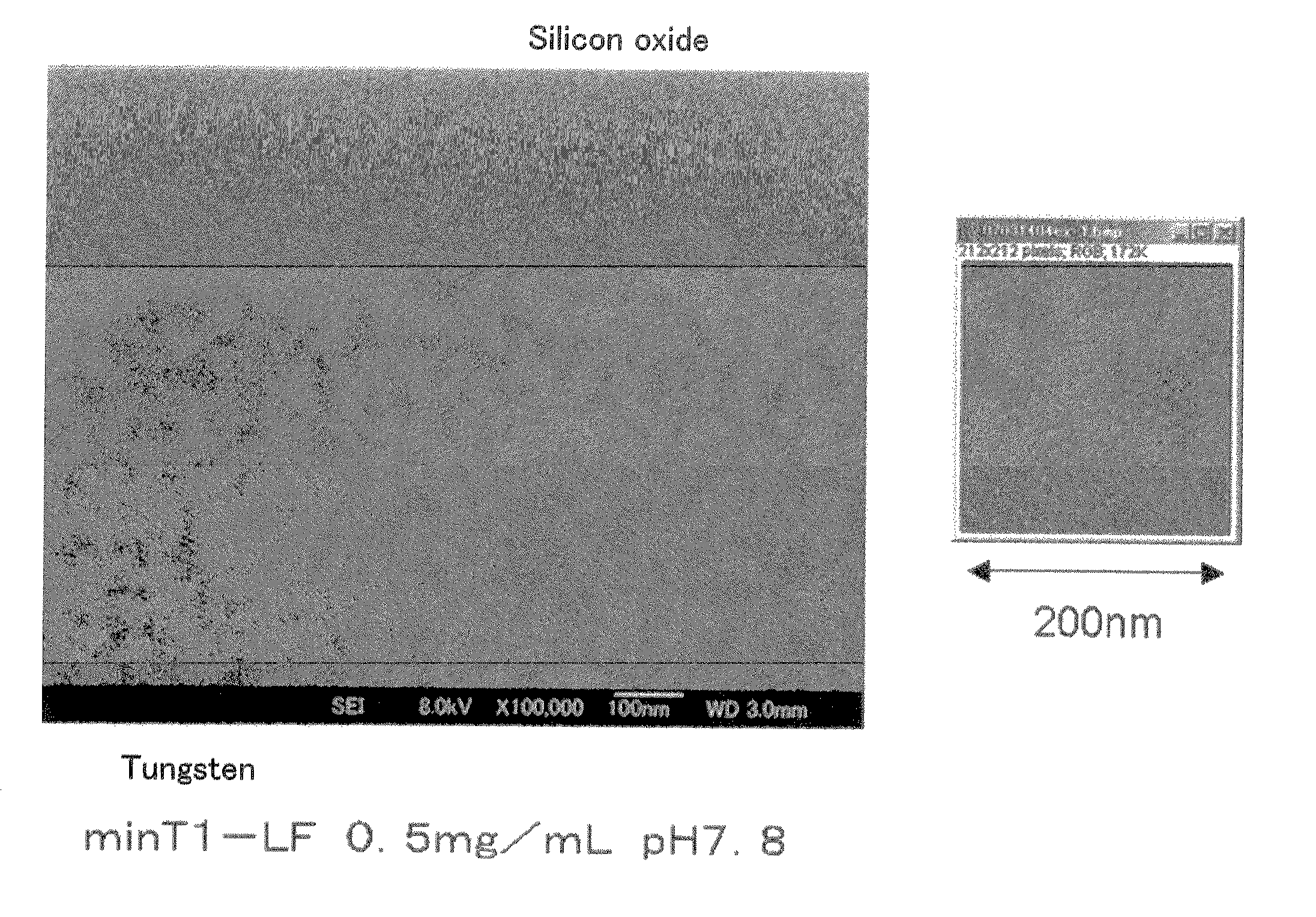
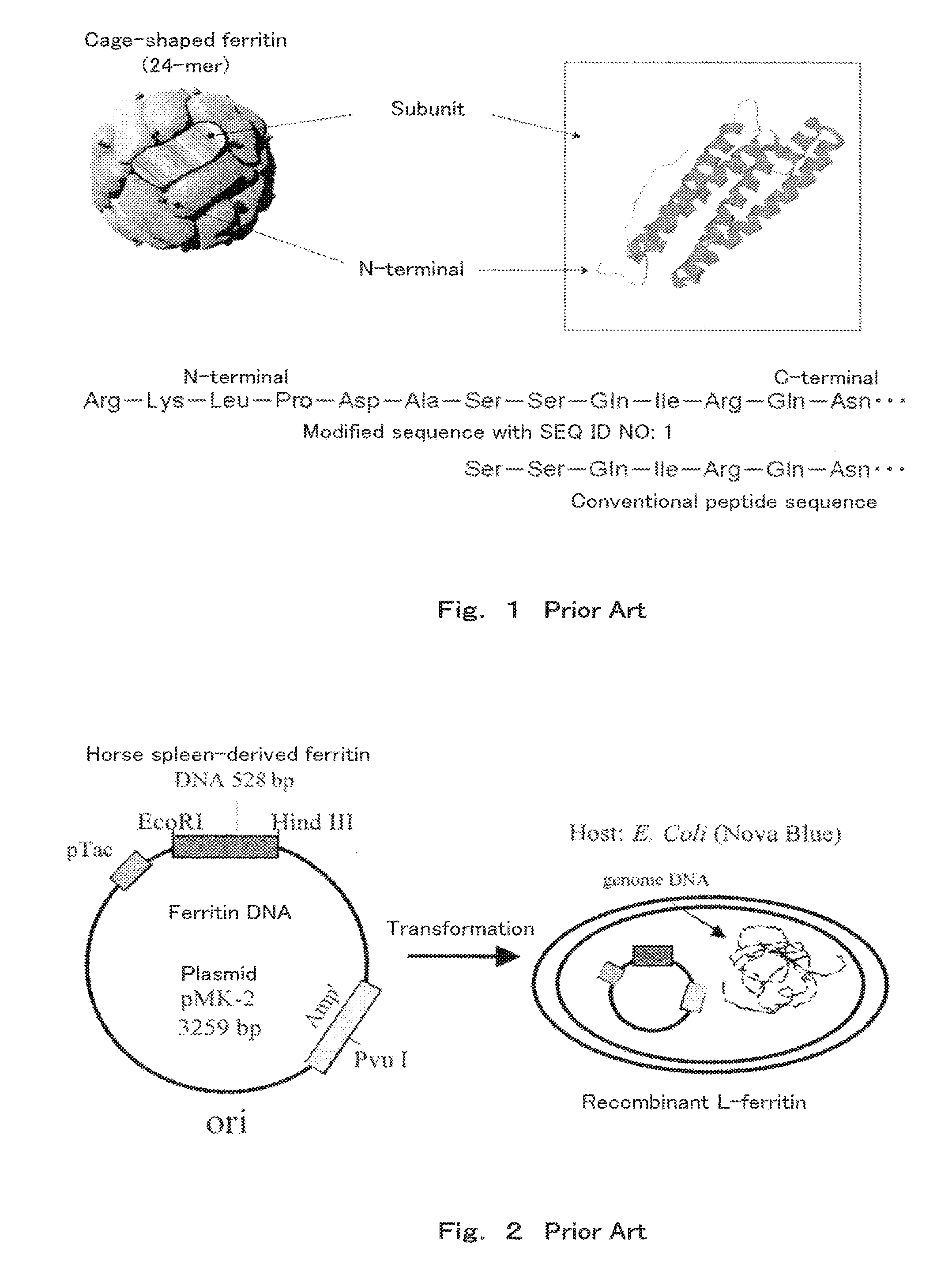
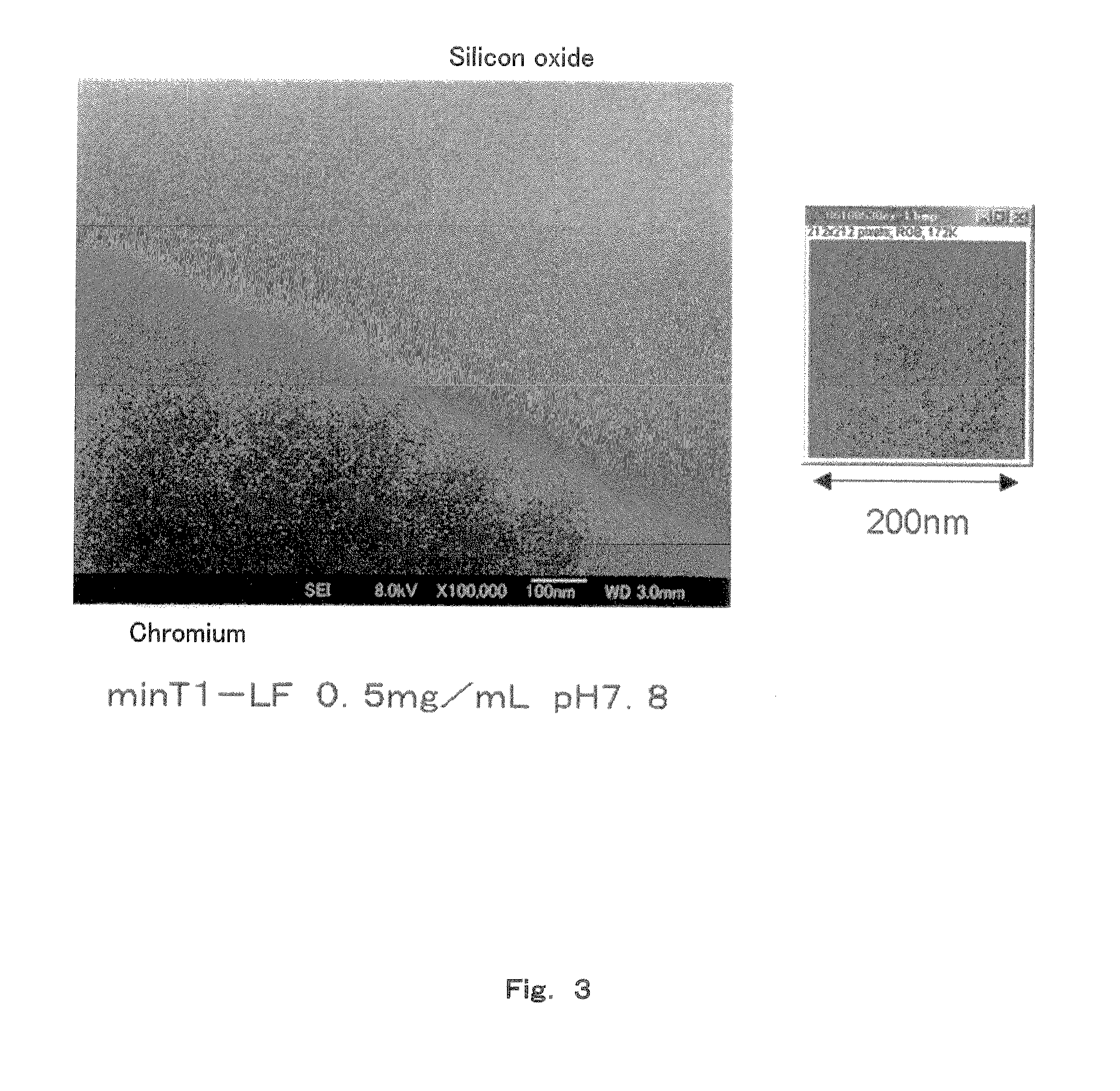
Method of arraying ferritin
A method of selectively arraying ferritin and inorganic particles on a silicon oxide substrate having a chromium, niobium or tungsten portion. An aspect of the method includes steps of: preparing a solution which contains ferritin modified at an N-terminal part of a subunit with a peptide set out in SEQ ID NO: 1, and a nonionic surfactant; and a binding step of bringing the solution in contact with the silicon oxide substrate to selectively array peptide-modified ferritin to the chromium, niobium or, tungsten portion. Another aspect of the method includes selectively arraying ferritin modified with the peptide set out in SEQ ID NO: 1, and the inorganic particles contained in ferritin at the chromium, niobium, or tungsten portion by removing the solution.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
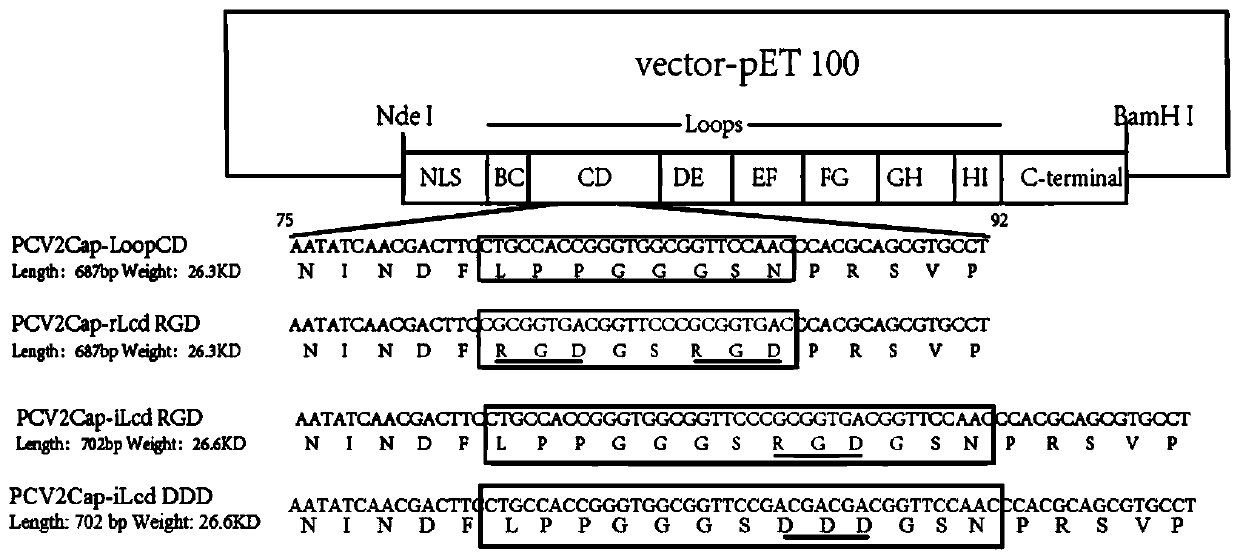
Arg-gly-asp (RGD)-fused porcine circovirus 2 (PCV2) virus-like particles (VLPs), mutant infectious clone and preparation method of RGD-fused PCV2 VLPs, and application of RGD-fused PCV2 VLPs and infectious clone
ActiveCN110669142AImprove the level ofEnhance immune responseAntibody mimetics/scaffoldsViral antigen ingredientsVirus-like particleSpecific igm
The invention discloses arg-gly-asp (RGD)-fused porcine circovirus 2 (PCV2) virus-like particles (VLPs), an infectious clone and a preparation method of the RGD-fused PCV2 VLPs, and application of theRGD-fused PCV2 VLPs and the infectious clone. According to the RGD-fused PCV2 VLPs, RGD oligopeptides are fused on PCV2 Cap proteins. According to the RGD-fused PCV2 VLPs, the PCV2 Cap proteins are subjected to RGD polypeptide sequence oligopeptide modification, the RGD oligopeptides are fused on the PCV2 Cap proteins, and the PCV2 VLPs with RGD-fused on the surfaces are assembled in vitro. Compared with wild PCV2 VLPs, the levels of PCV2 specific IgM and IgG antibodies generated by RGD-fused PCV2 VLP immune mice are significantly increased, high-titer PCV2 antibodies can be generated in an induced mode, and the RGD-fused PCV2 VLPs enhance humoral immunity response. The RGD-fused PCV2 VLPs can be applied to development of enhanced VLPs and differential diagnosis molecular label VLPs vaccines, and a new idea is provided for research and development of PCV2 vaccines.
Owner:湖南派智生物科技有限公司
Mesenchymal stem cell exosome agent based on DNA tetrahedron, preparation method and application of mesenchymal stem cell exosome agent
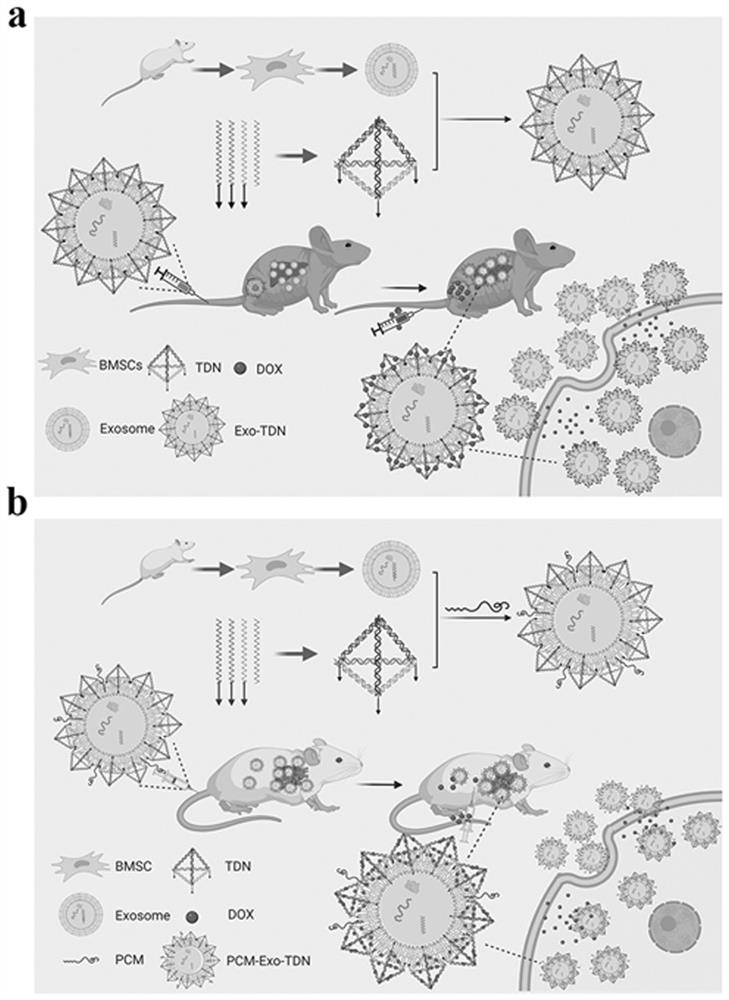
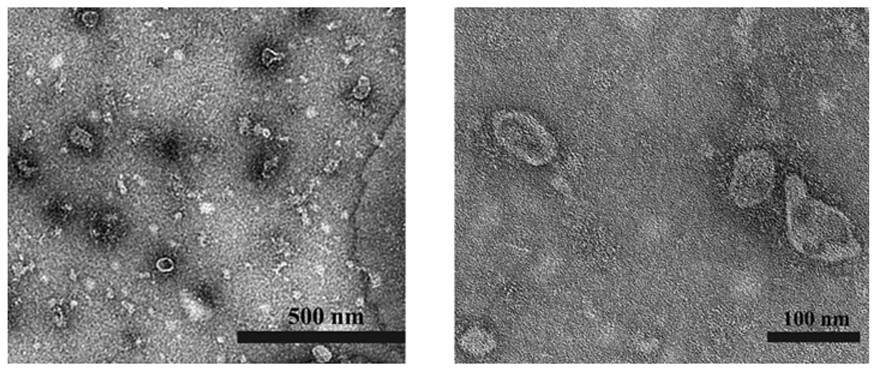
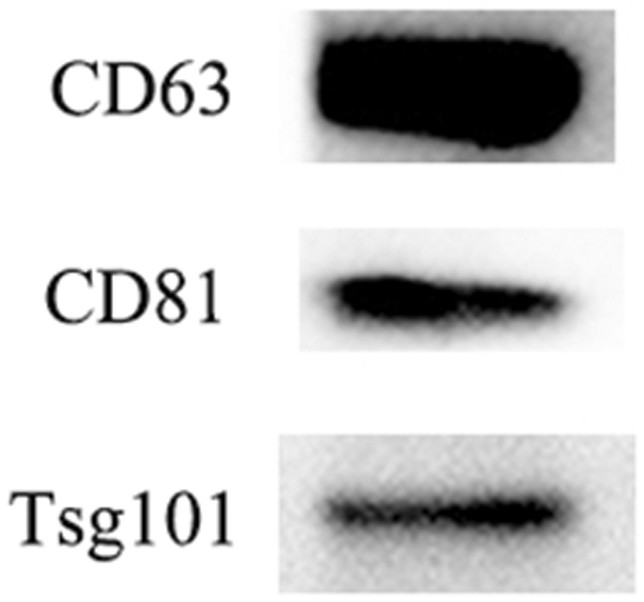
The invention provides a mesenchymal stem cell exosome agent based on a DNA tetrahedron, a preparation method and application of the mesenchymal stem cell exosome agent. The exosome preparation is an exosome agent Exo-TDN which is obtained by loading a DNA tetrahedral nano material on the surface of a mesenchymal stem cell exosome; or the exosome agent is an exosome preparation PCM-Exo-TDN which is obtained by loading a DNA tetrahedral nano material on a mesenchymal stem cell exosome and modifying a myocardial specific targeting peptide PCM on the surface of the exosome through a cross-linking agent phospholipid polyethylene glycol maleimide. Exo-TDN is mainly enriched in a liver area, the chemotherapeutic drug DOX can be captured by the Exo-TDN when reaching the liver, the DOX is inhibited from entering a cell nucleus, and the function of protecting cells is achieved; a myocardial specific targeting peptide PCM is further modified, and PCM-Exo-TDN is synthesized and used for relieving myocardial damage caused by DOX.
Owner:HEBEI UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com