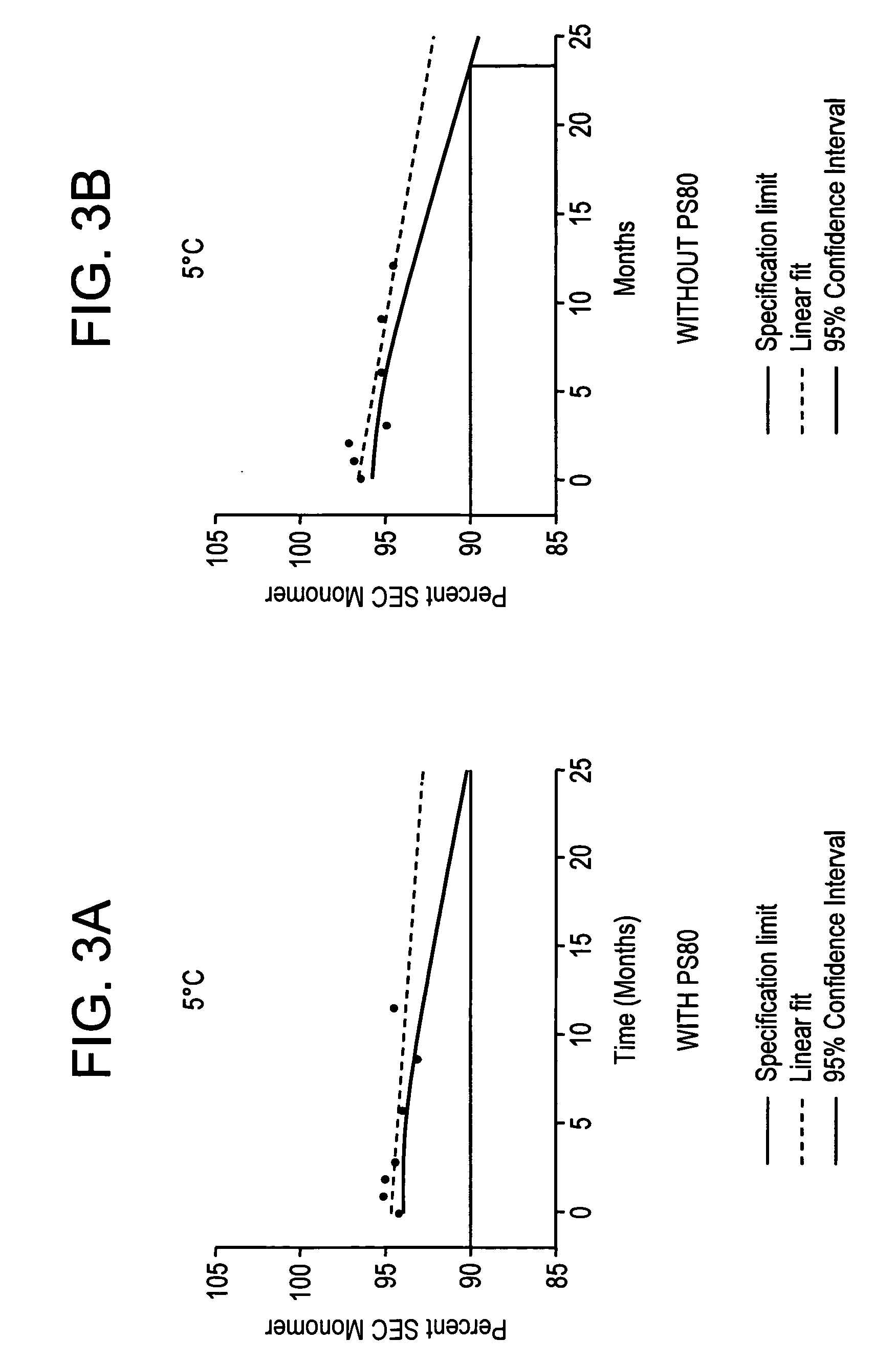
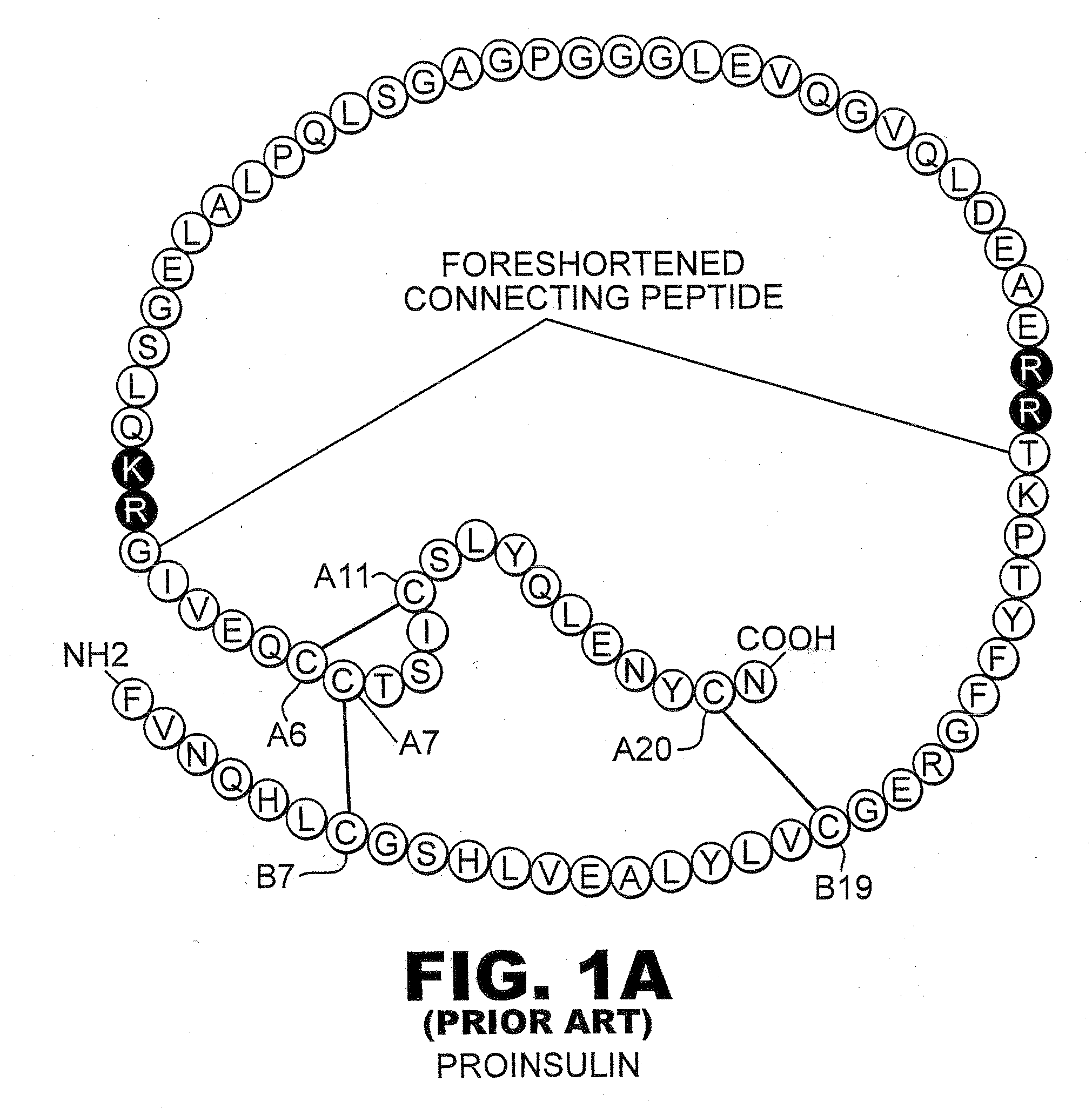
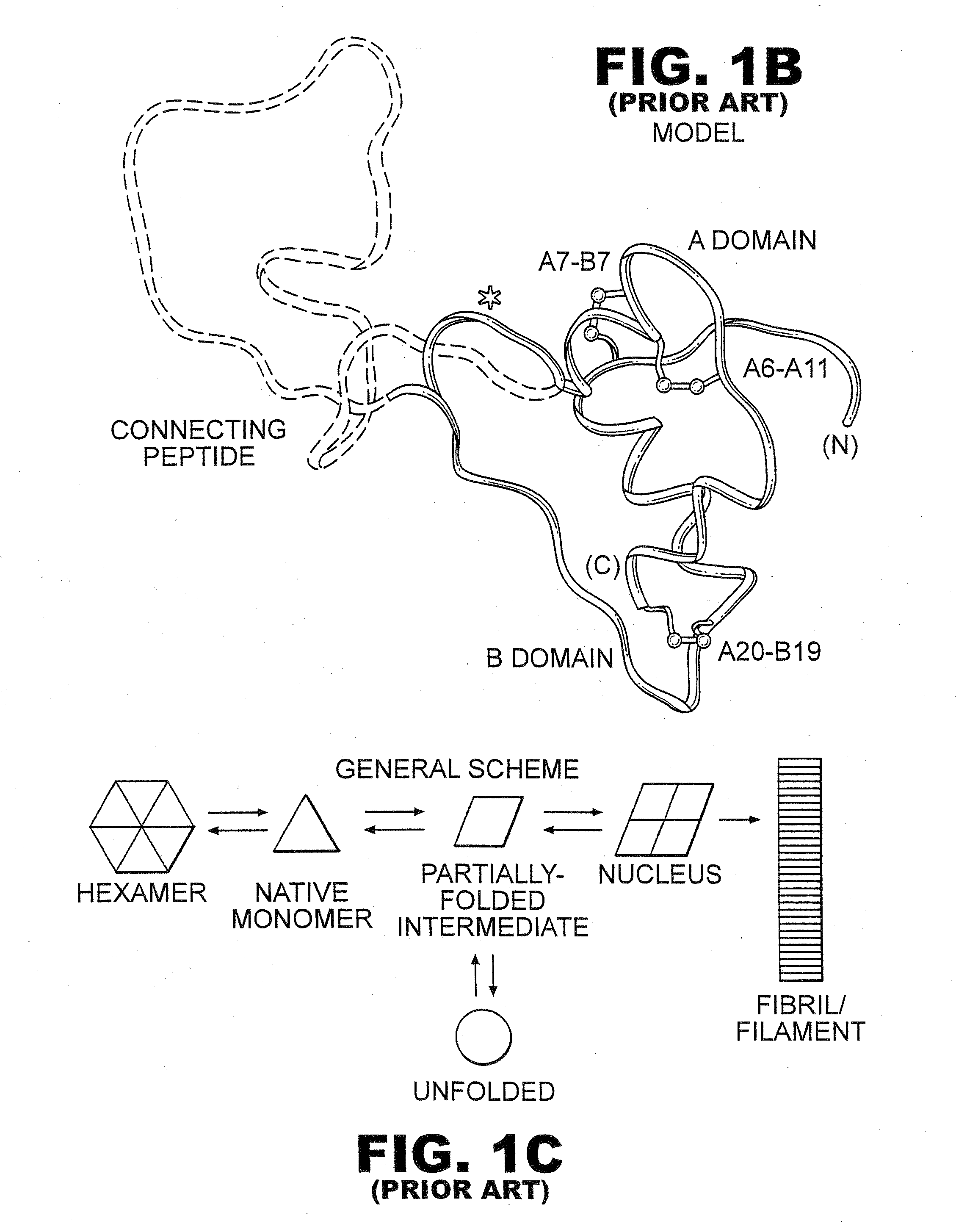
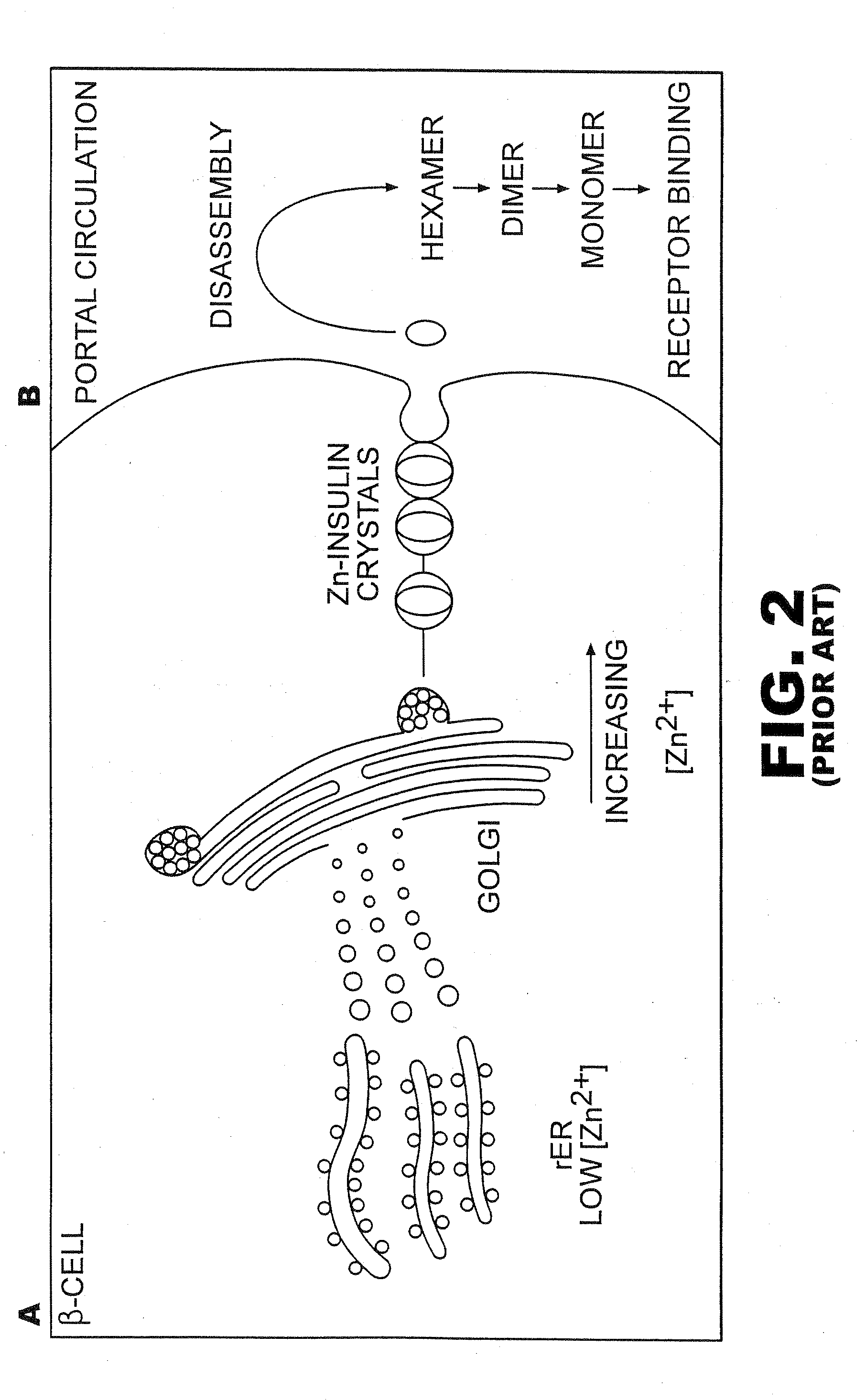
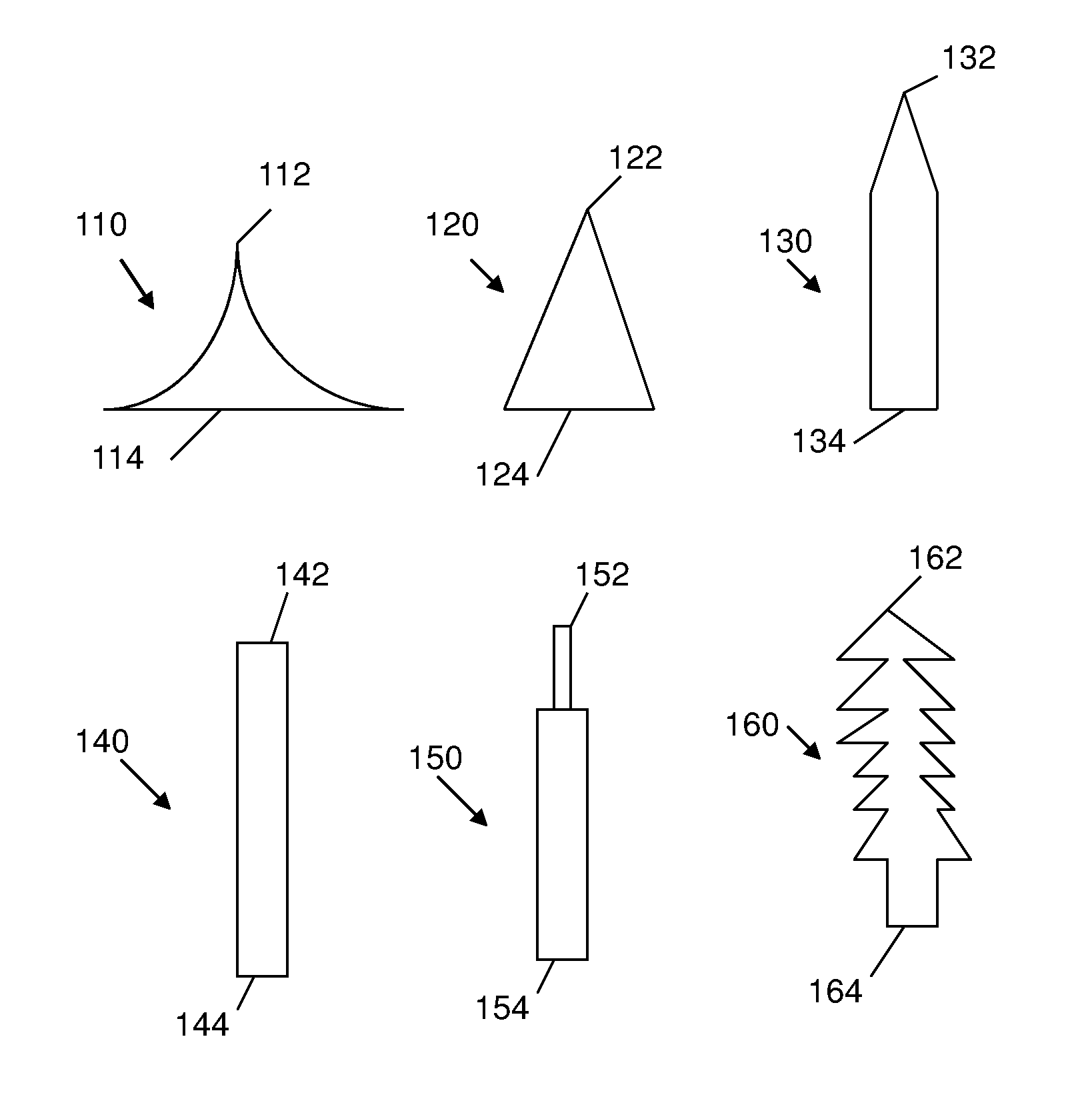
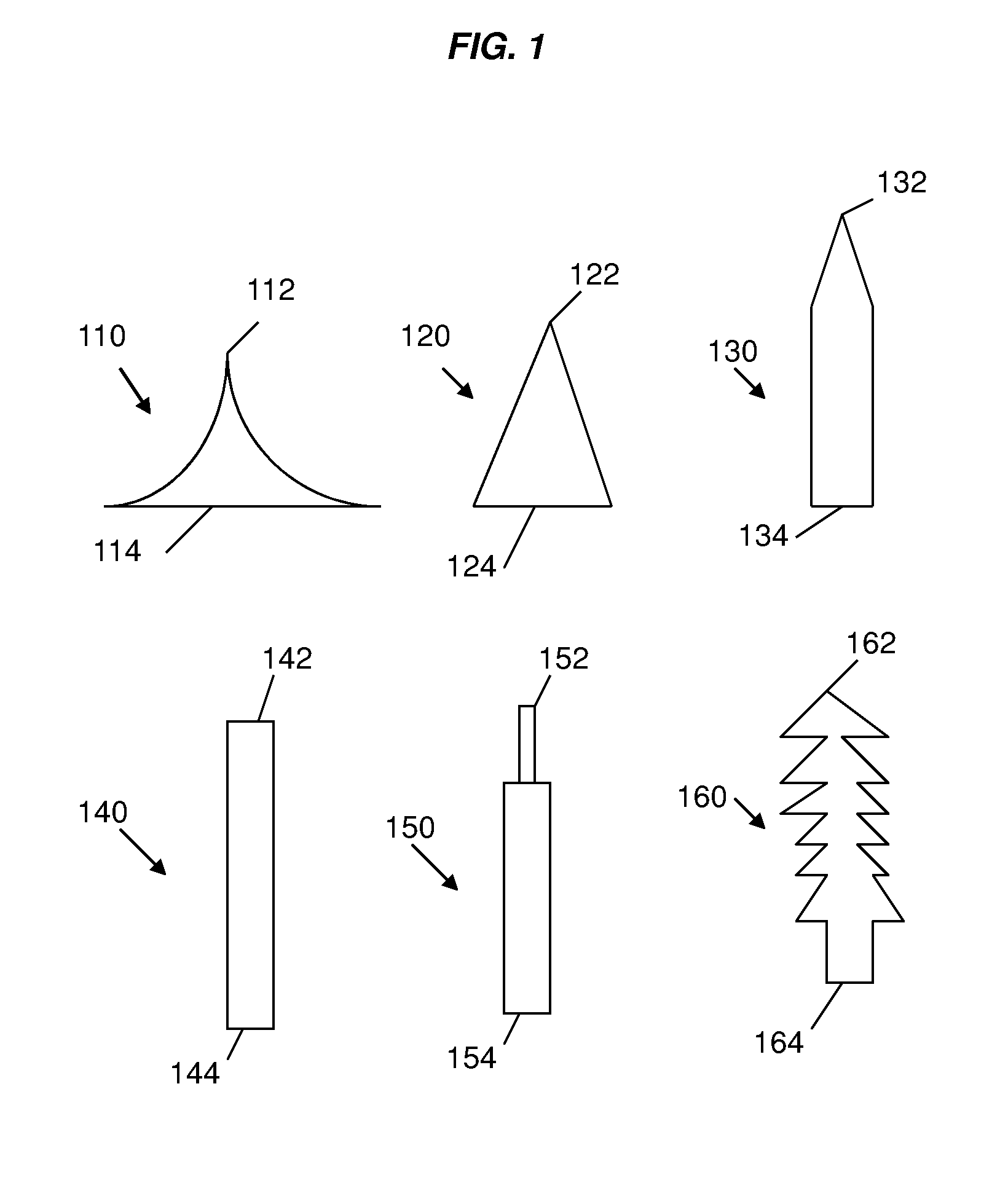
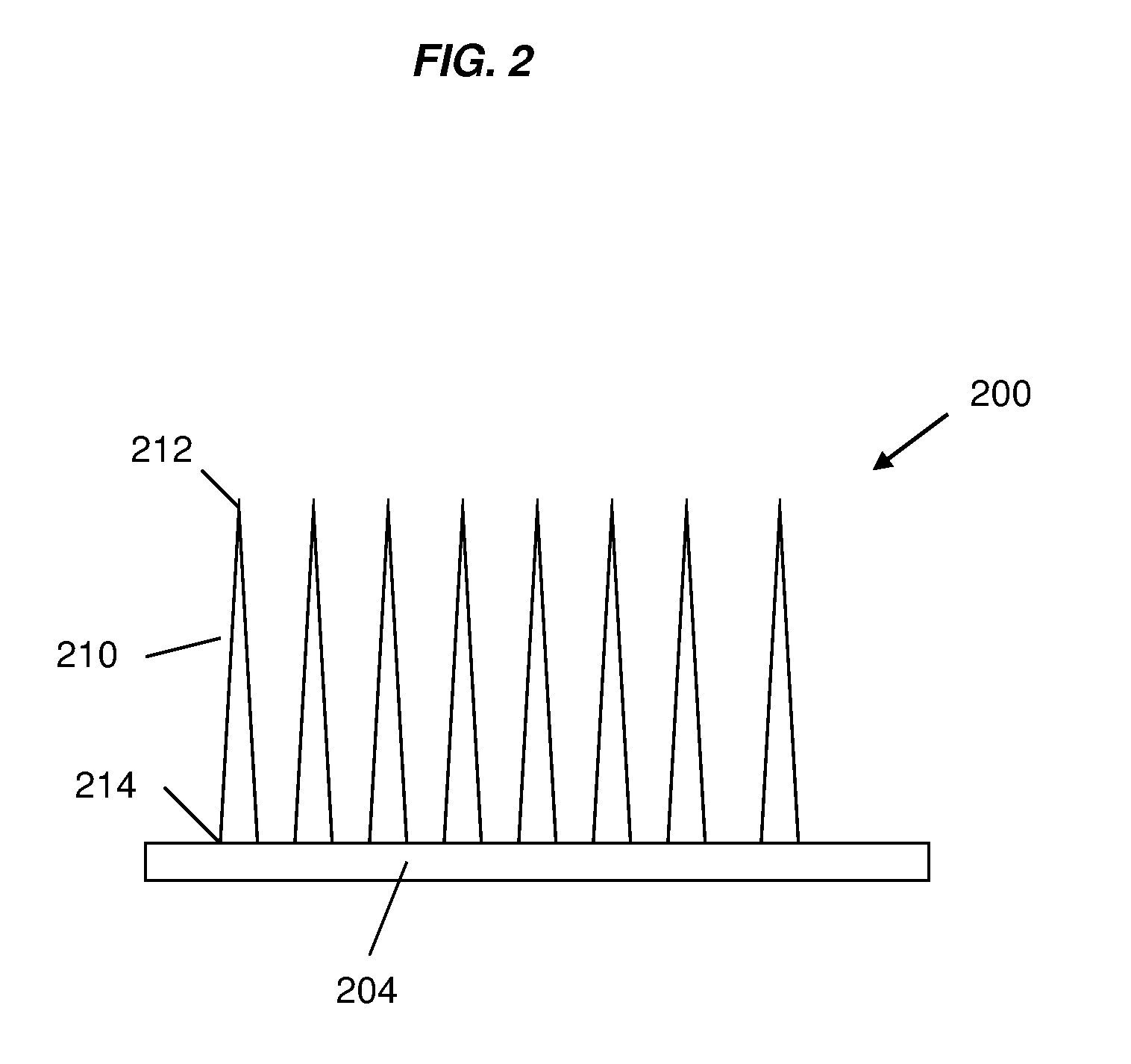
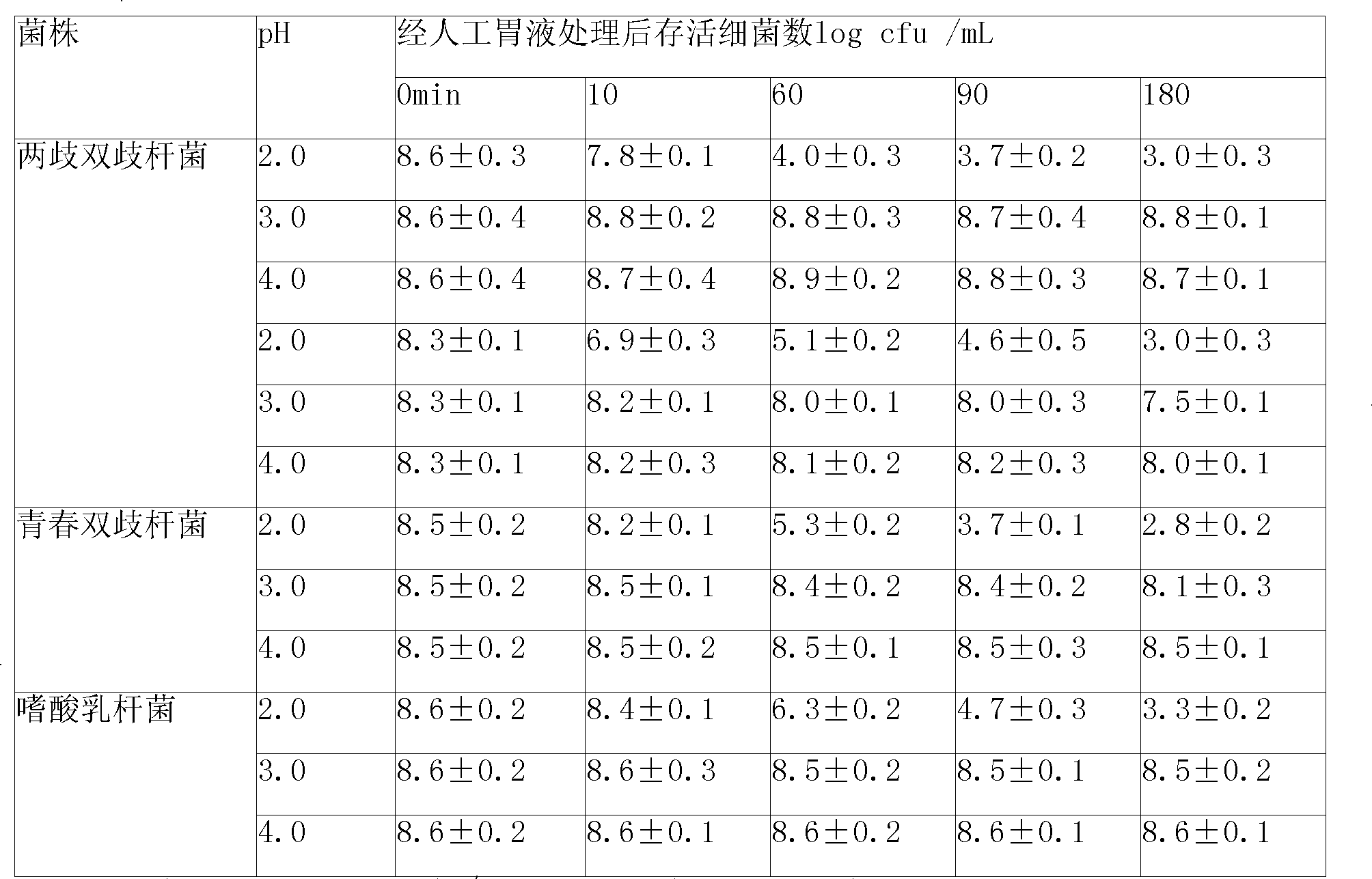Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1342results about How to "Maintain biological activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
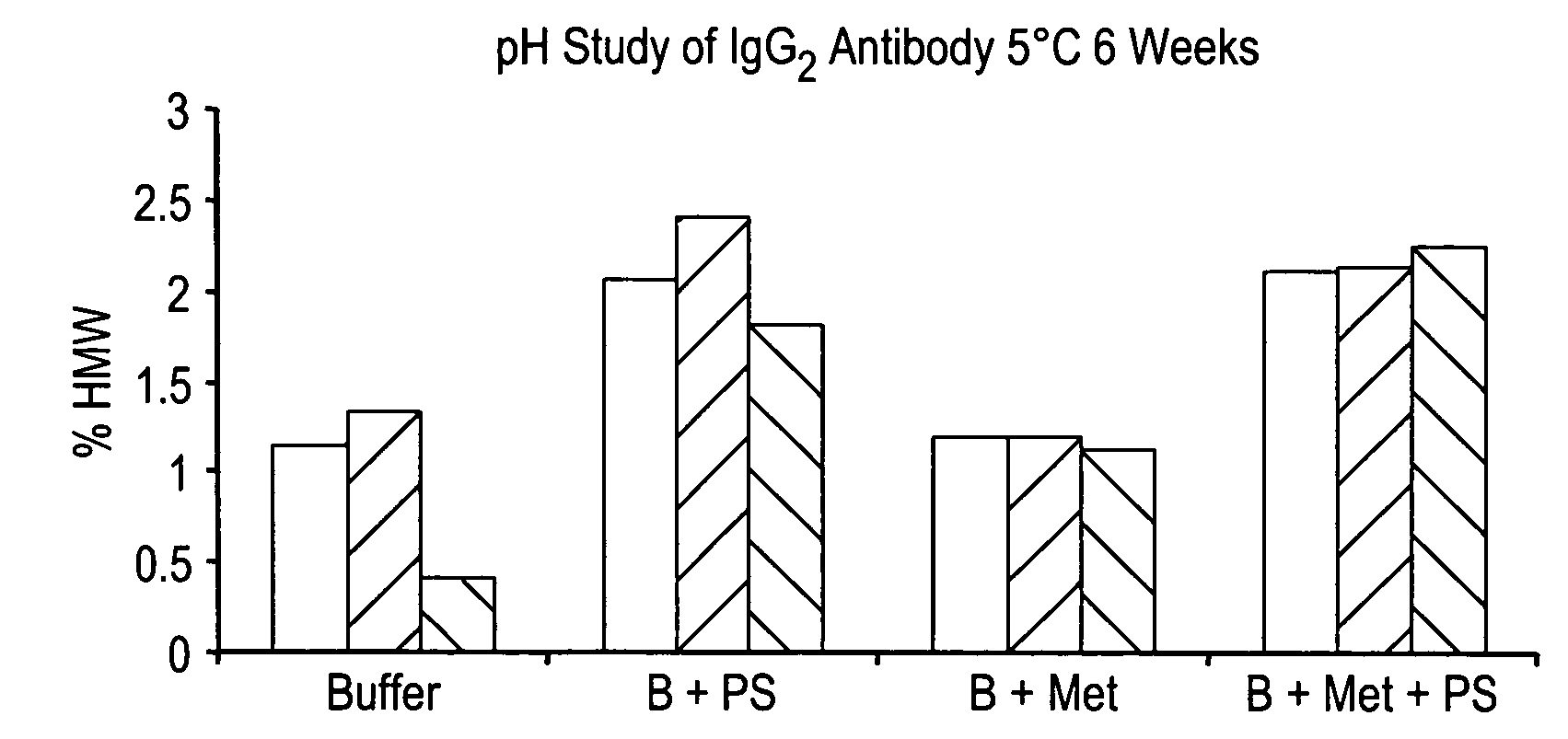
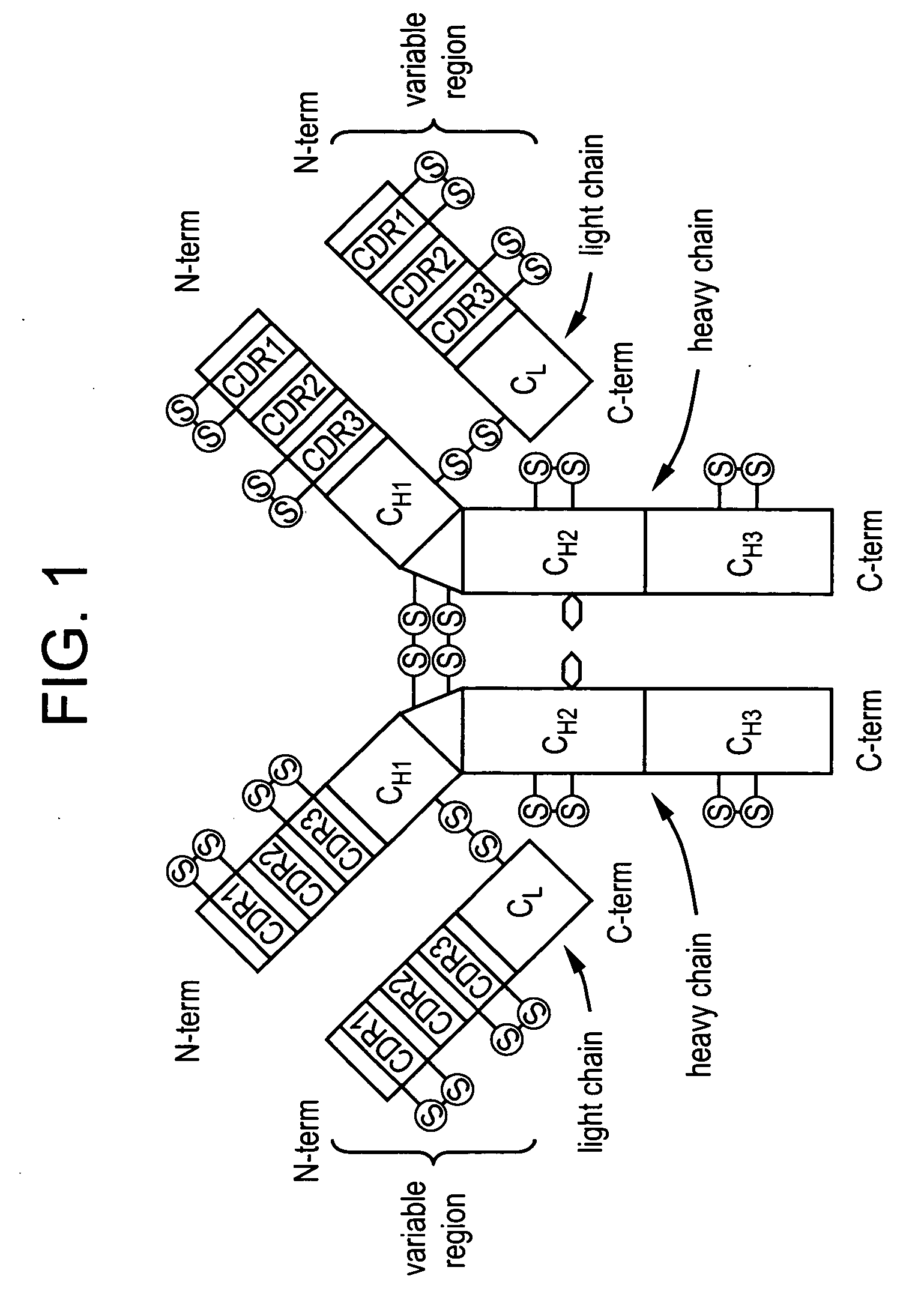
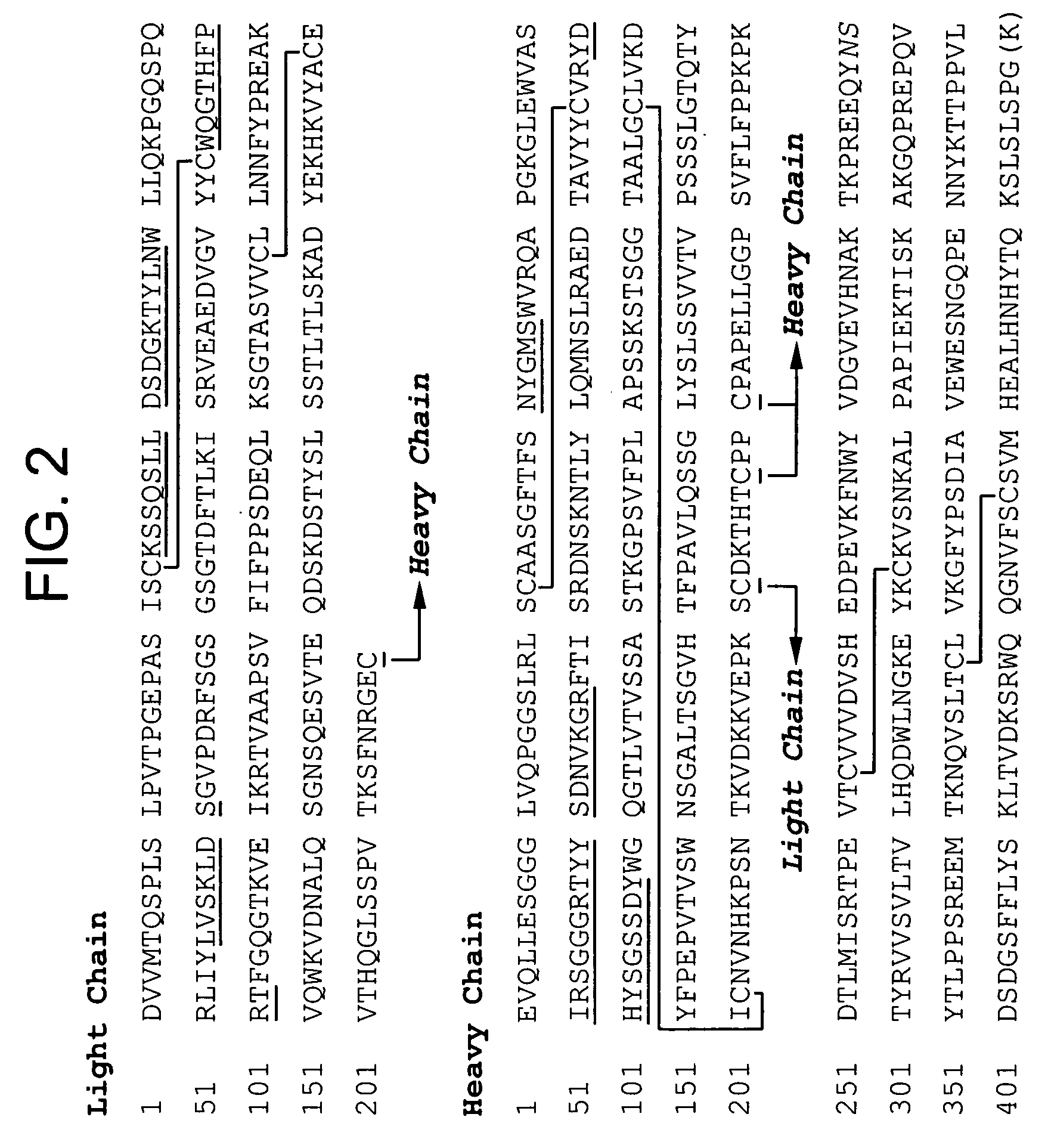
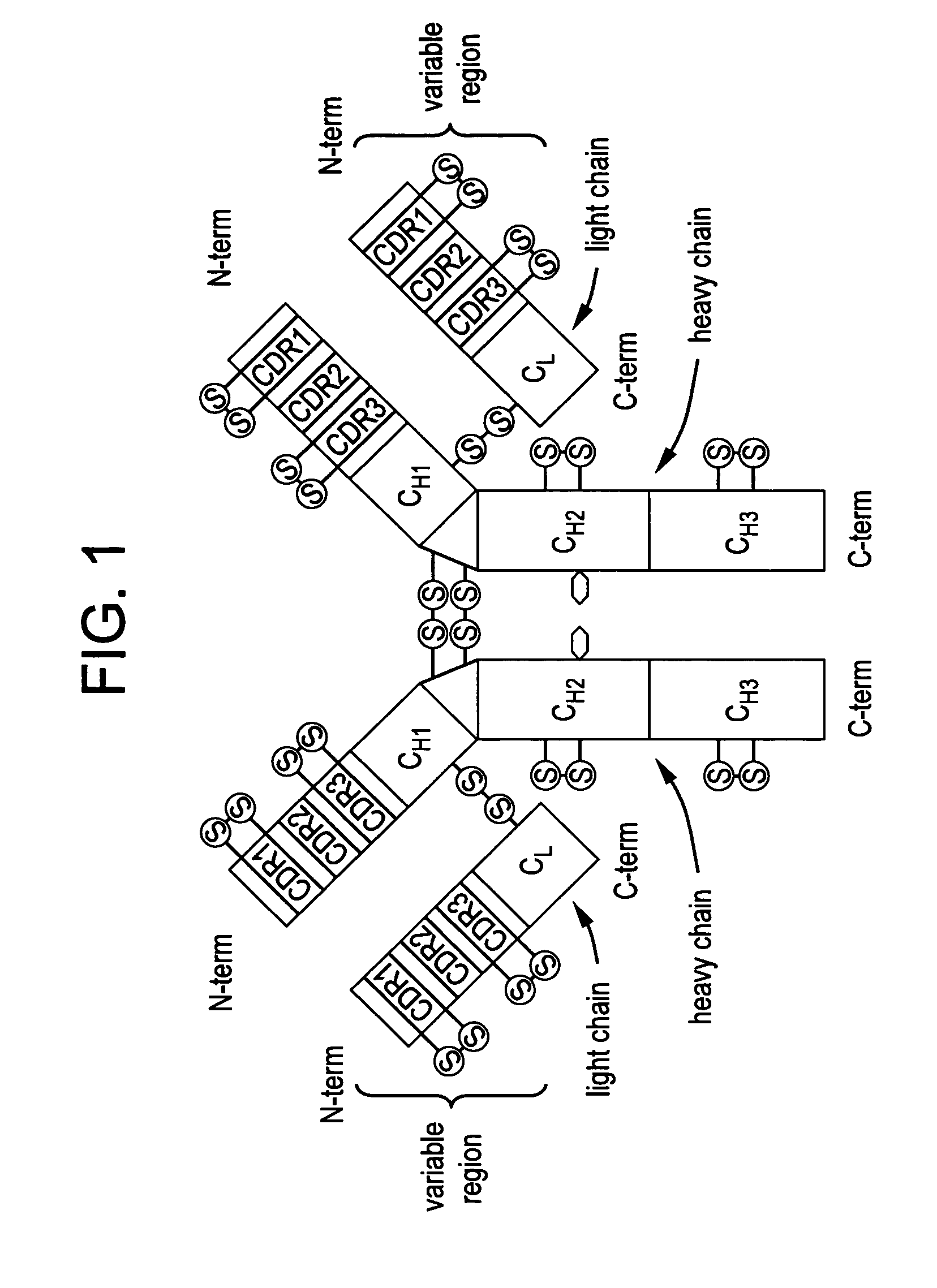
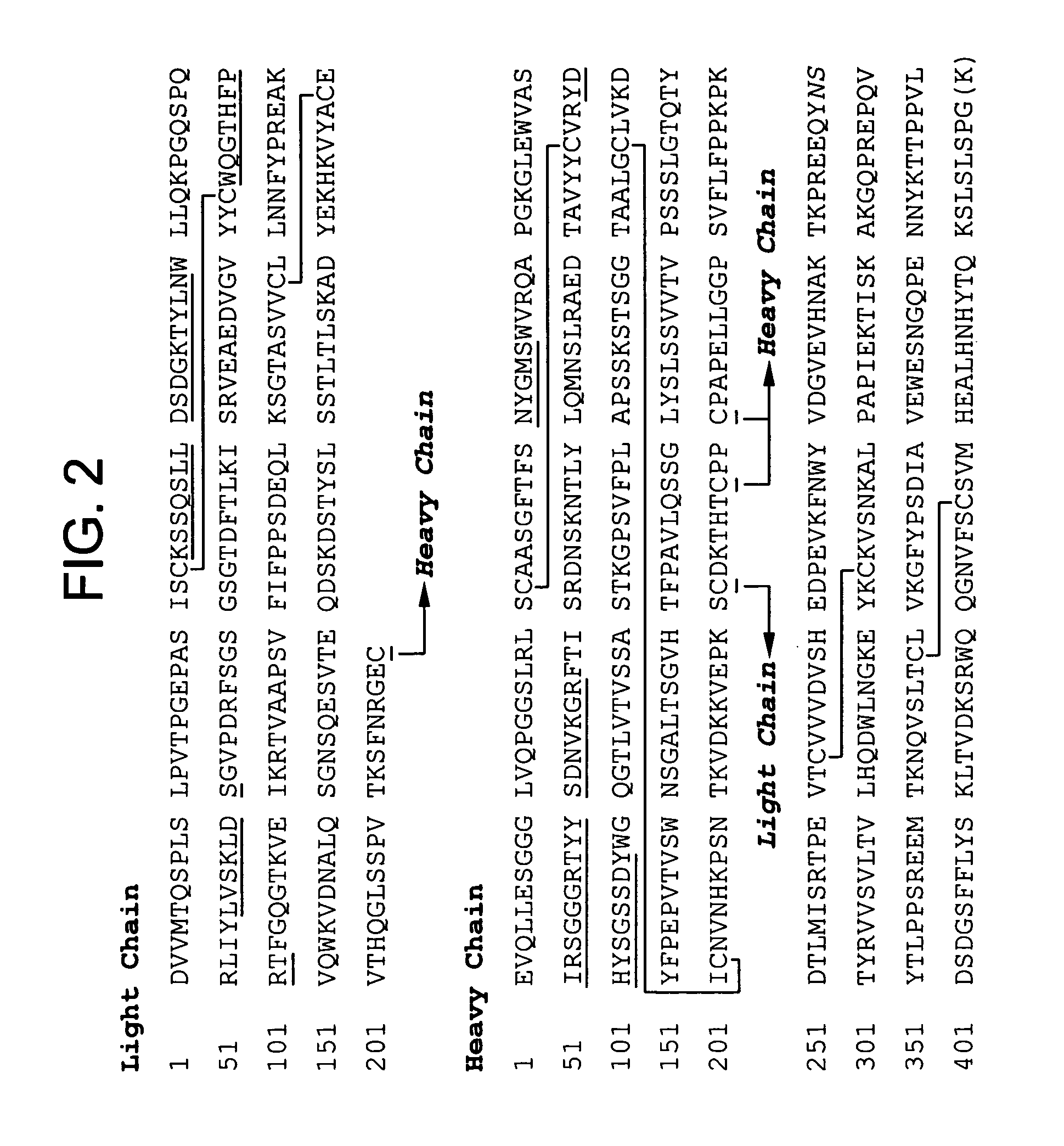
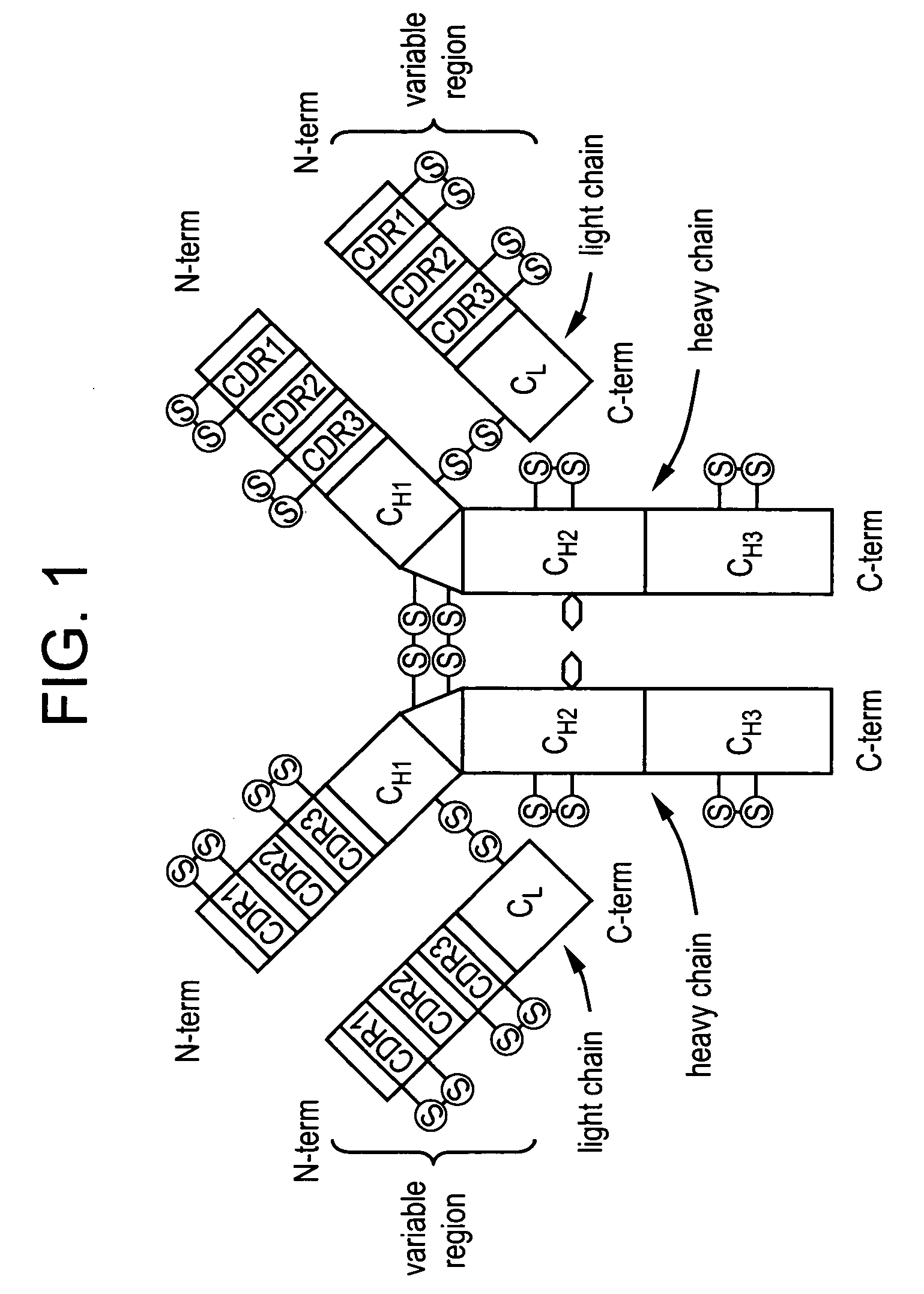
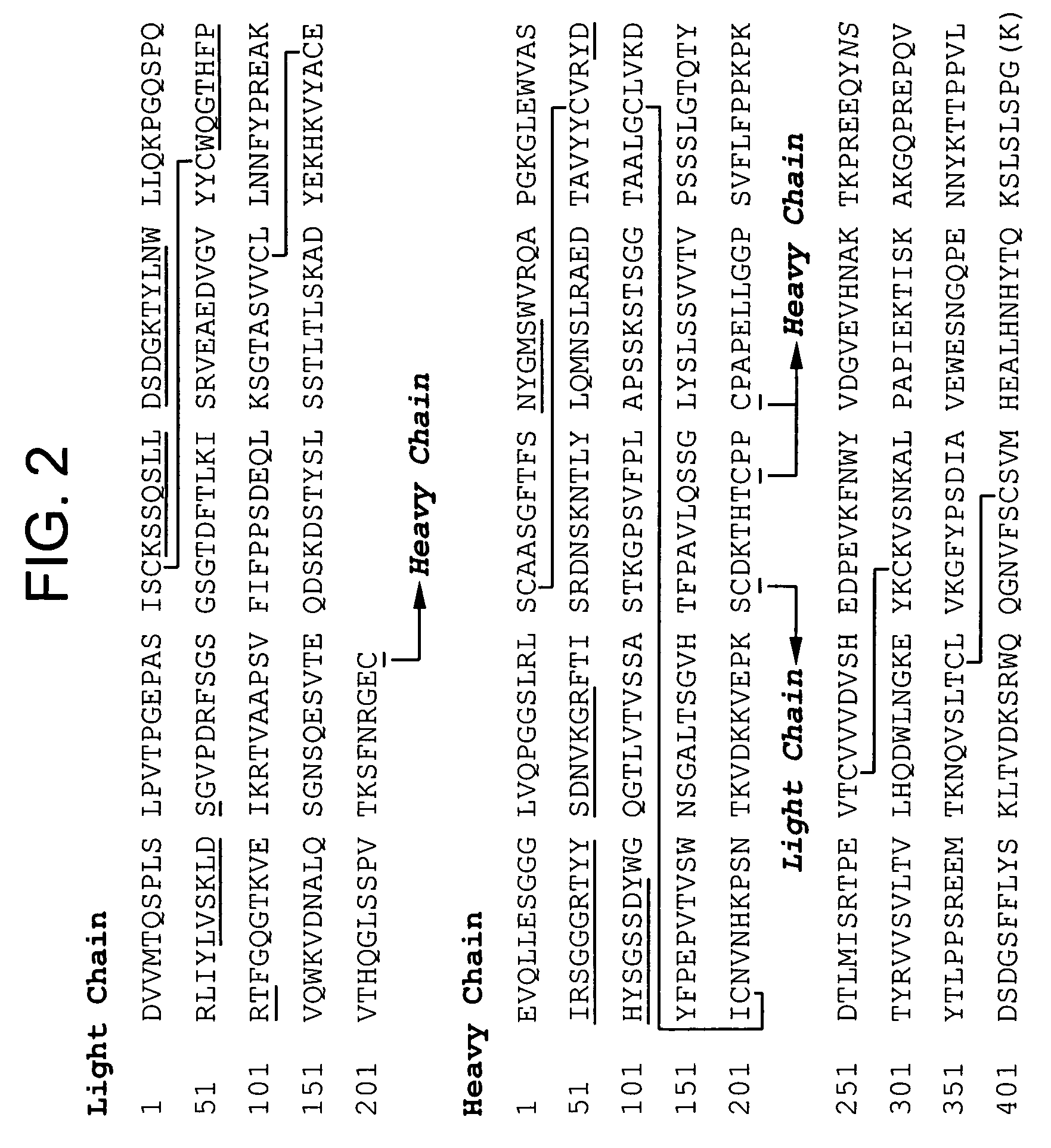
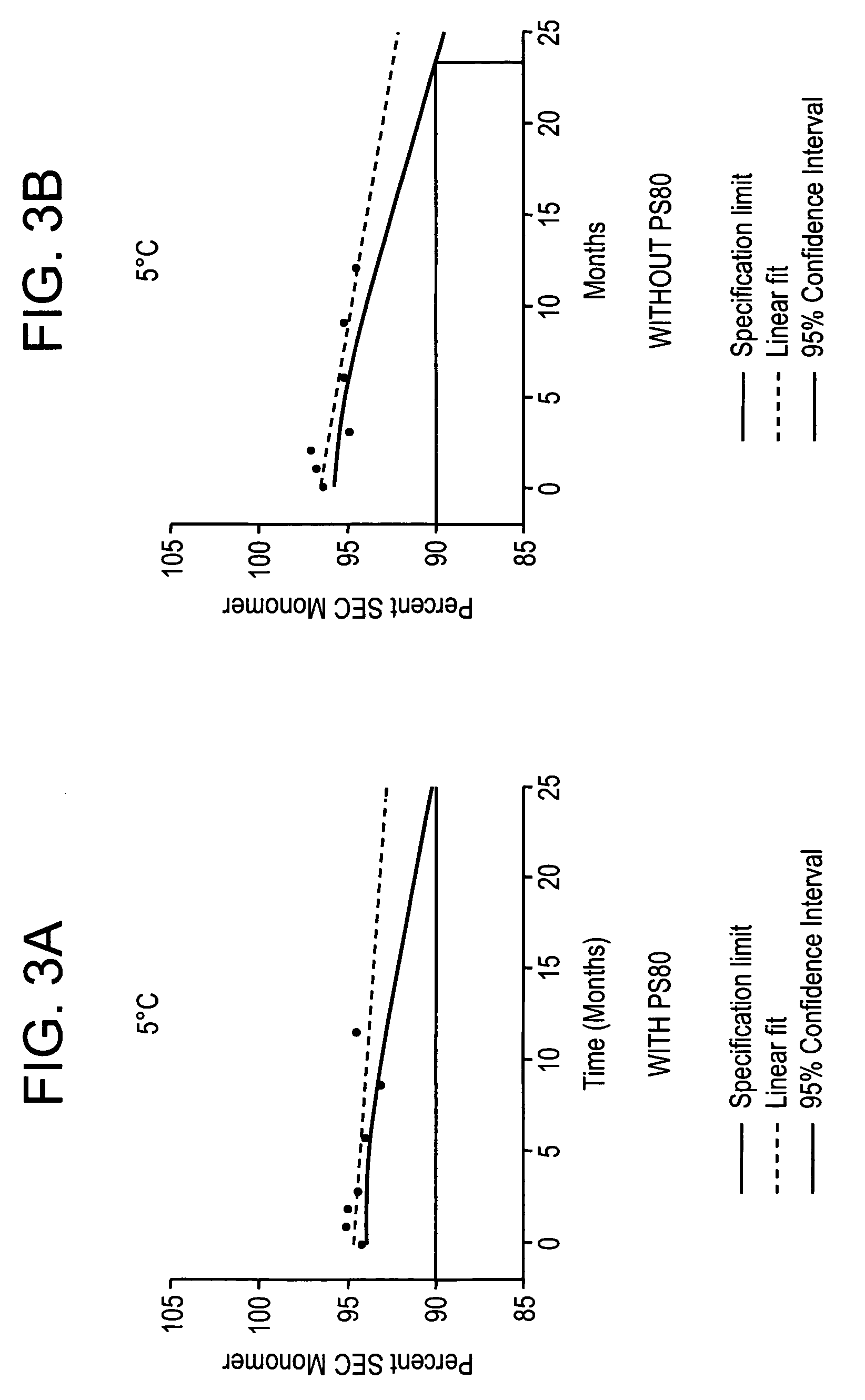
Anti a beta antibody formulation
ActiveUS20060193850A1Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
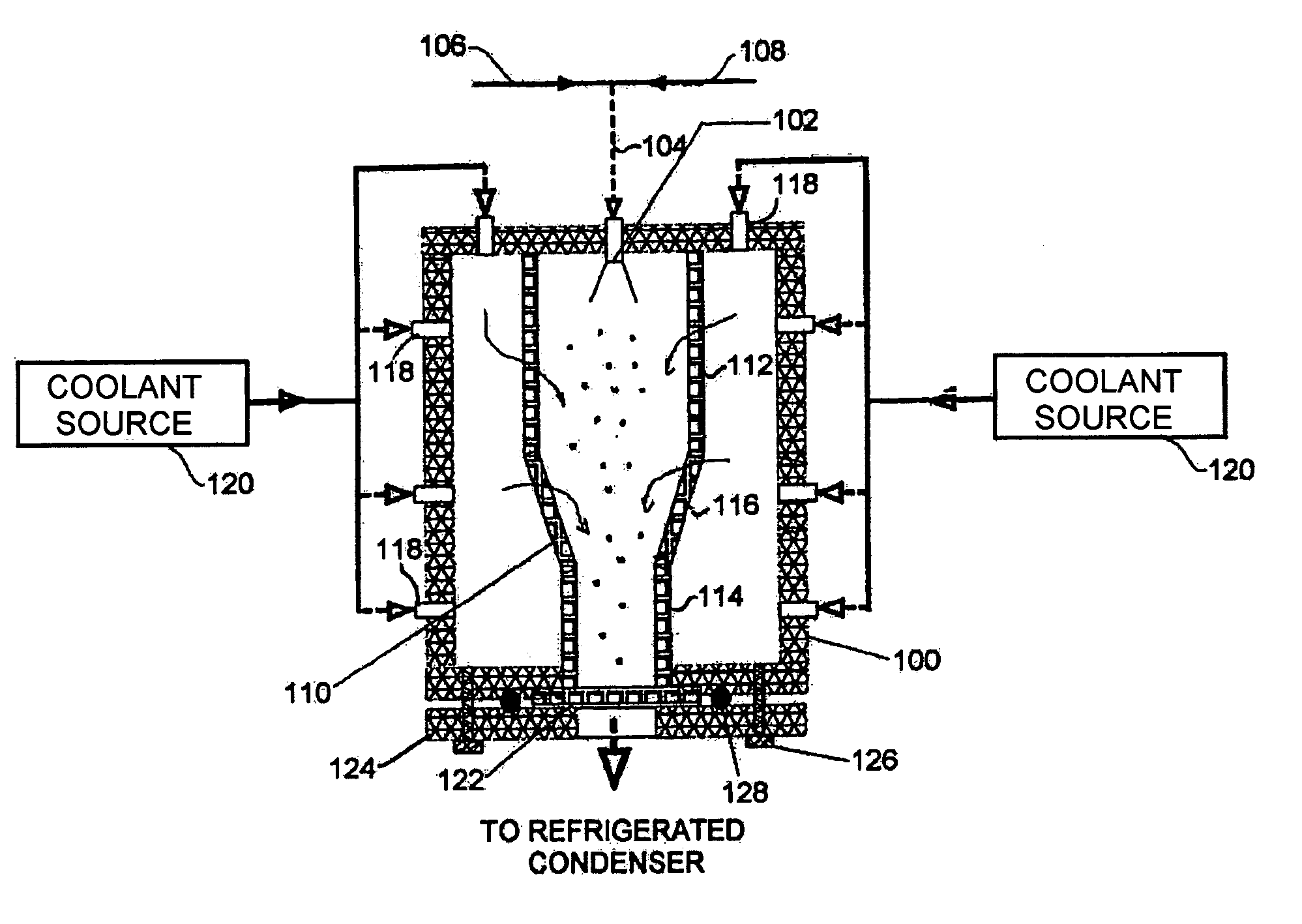
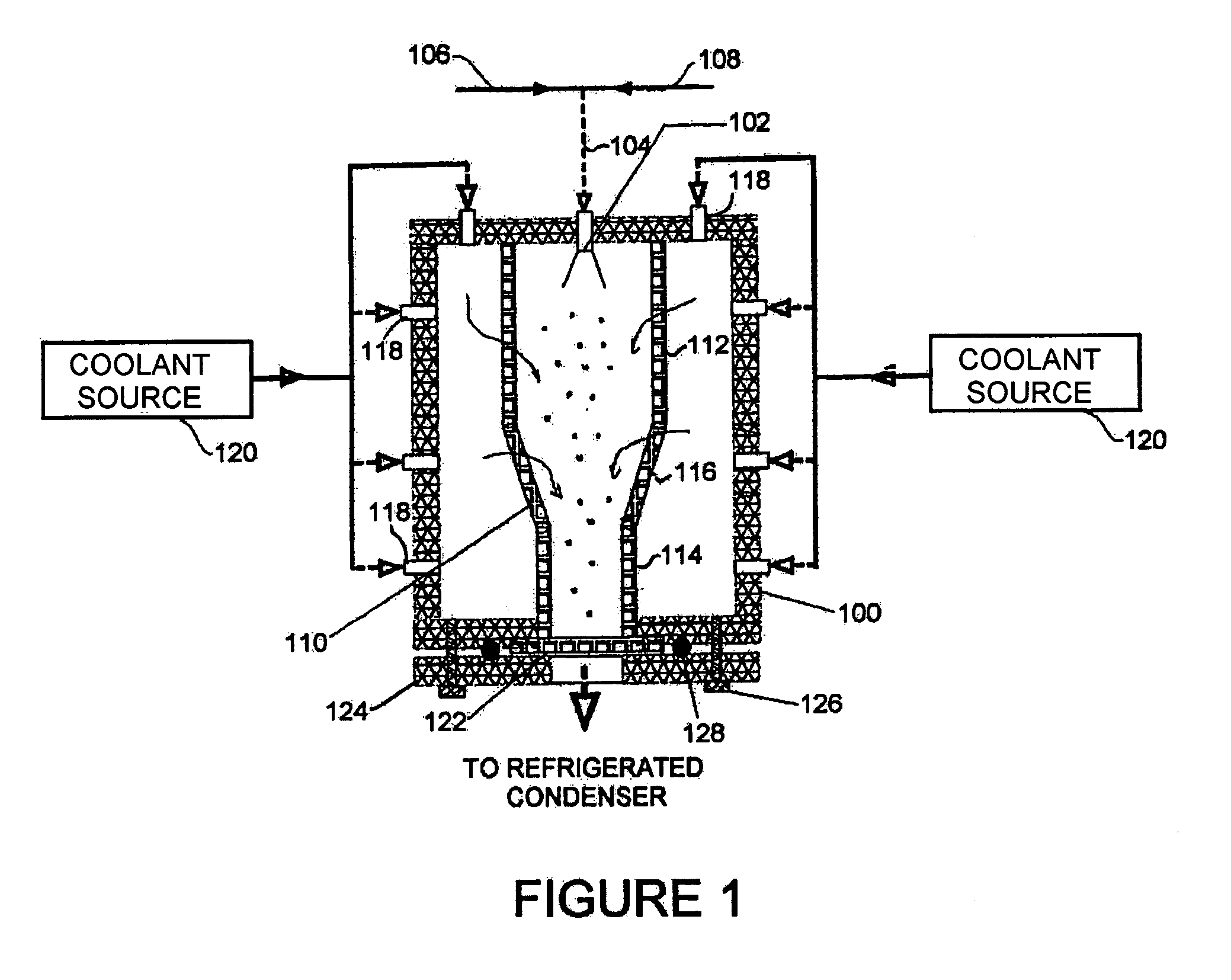
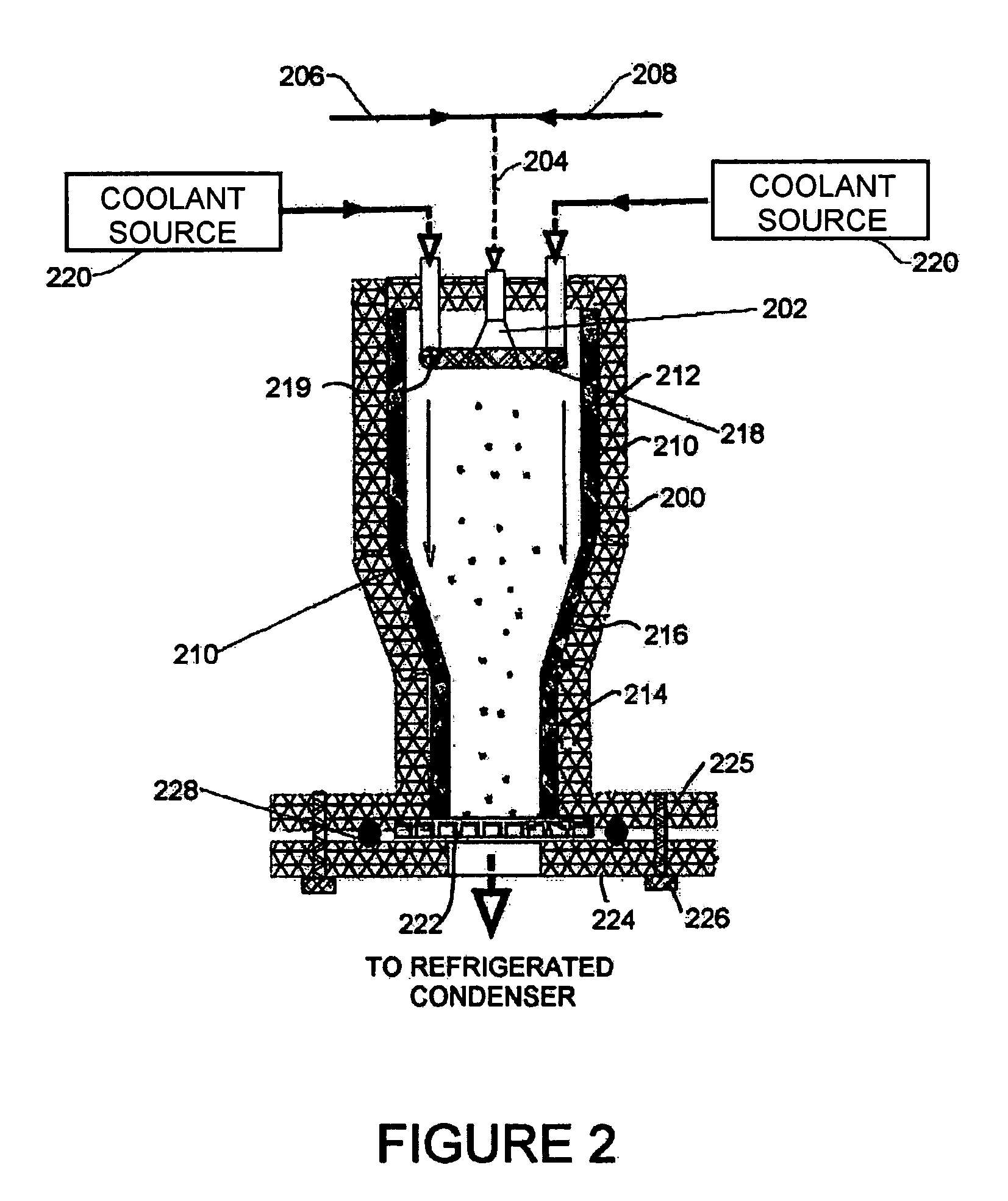
Powder formation by atmospheric spray-freeze drying
ActiveUS7007406B2High emitted doseEasy to storeDrying solid materials without heatDrying machines with progressive movementsFreeze-dryingAtomizer nozzle
A method of manufacturing heat-sensitive pharmaceutical powder is disclosed. The original pharmaceutical substances are dissolved in a solution or suspended in a suspension, which is sprayed through an atomizing nozzle and frozen in a cold gas phase or liquid nitrogen atomized directly in the spray-freeze chamber or gas jacket at the same time (for cooling purposes). The particles are freeze-dried at roughly atmospheric pressure in a down-stream fluid flow with exit filter thereby to remove moisture entrapped on or inside the frozen particles. The system has applicability for forming other powders.
Owner:AEROSOL THERAPEUTICS
Stabilized liquid polypeptide formulations
InactiveUS20060210557A1Provide stabilityMaintain biological activityBiocideOrganic active ingredientsMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of polypeptides, in particular, therapeutic antigen-binding polypeptides such as antibodies and the like, for example, anti-Aβ antibodies. The formulations generally include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine, and thus, the formulations are suitable for several different routes of administration.
Owner:WYETH LLC
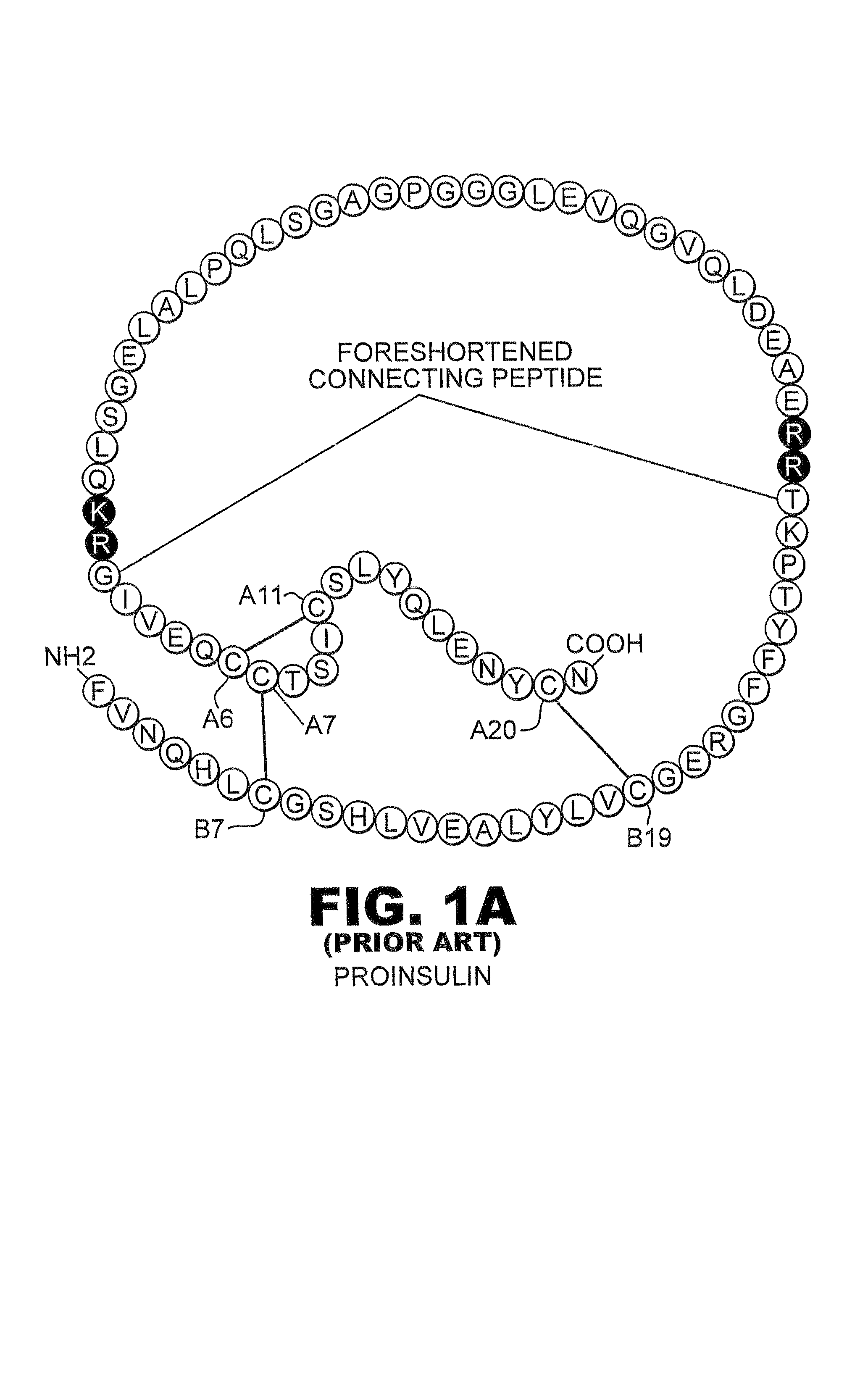
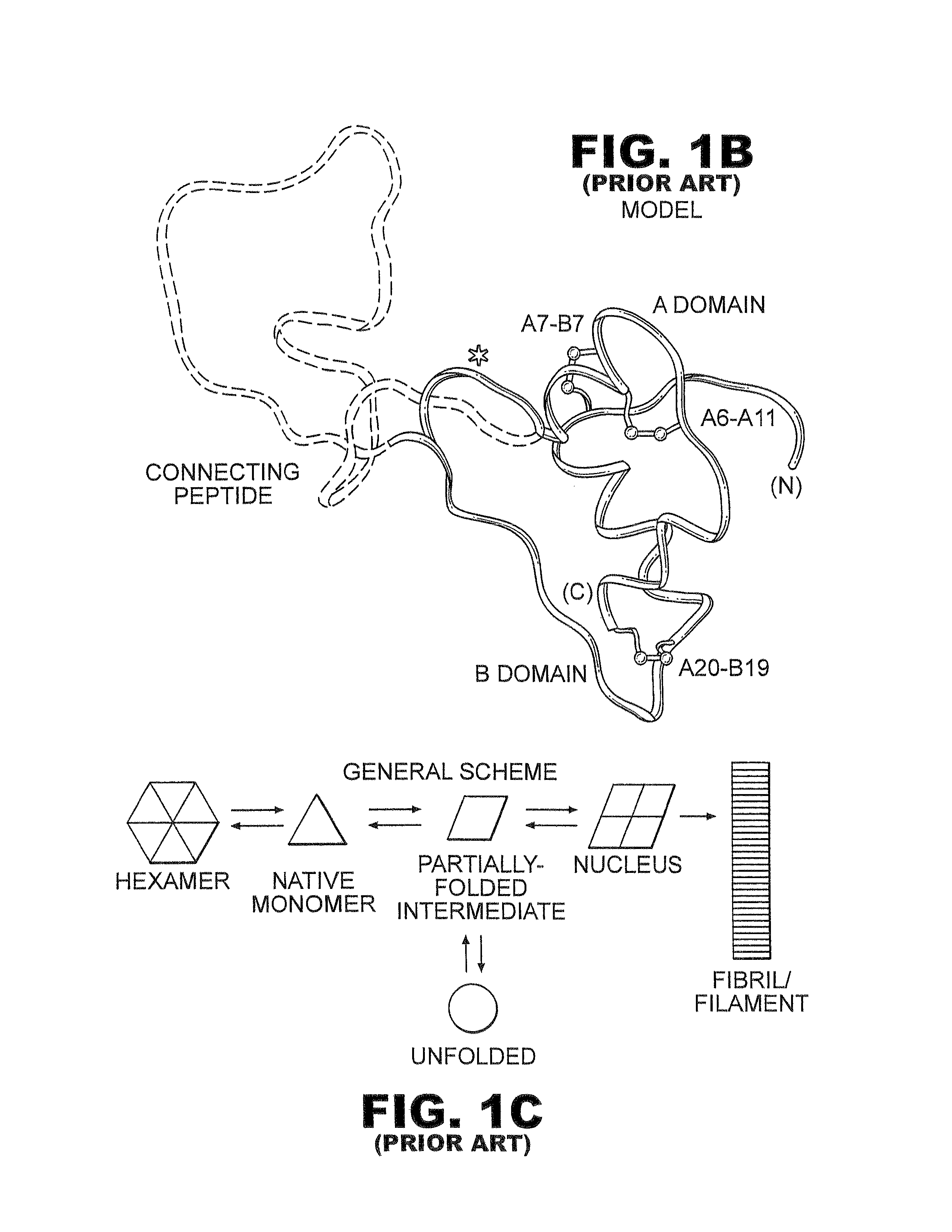
Fibrillation-resistant insulin and insulin analogues
InactiveUS20100099601A1Promote absorptionRapidity of absorptionFungiSugar derivativesInsulin pumpInsulin receptor binding
A fibrillation-resistant insulin analogue may be a single-chain insulin analogue or a physiologically acceptable salt thereof, containing an insulin A chain sequence or an analogue thereof and an insulin B chain sequence or an analogue thereof connected by a polypeptide of 4-10 amino acids. The fibrillation-resistant insulin analogue preferably displays less than 1 percent fibrillation with incubation at 37° C. for at least 21 days. A single-chain insulin analogue displays greater in vitro insulin receptor binding than normal insulin while displaying less than or equal binding to IGFR than normal insulin. The fibrillation-resistant insulin may be used to treat a patient using an implantable or external insulin pump, due to its greater fibrillation resistance.
Owner:CASE WESTERN RESERVE UNIV
Rapid acting and long acting insulin combination formulations
ActiveUS20080039368A1Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
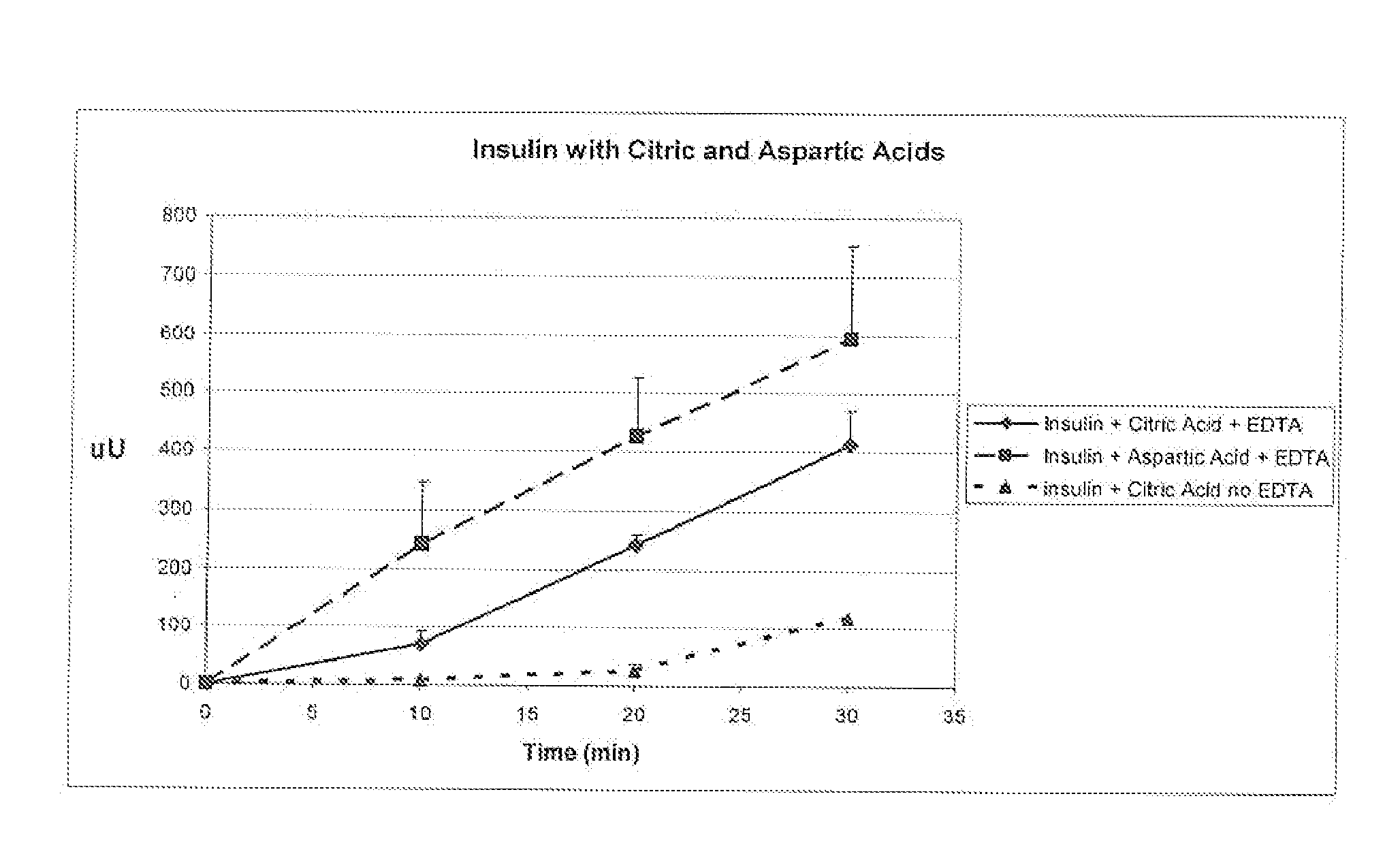
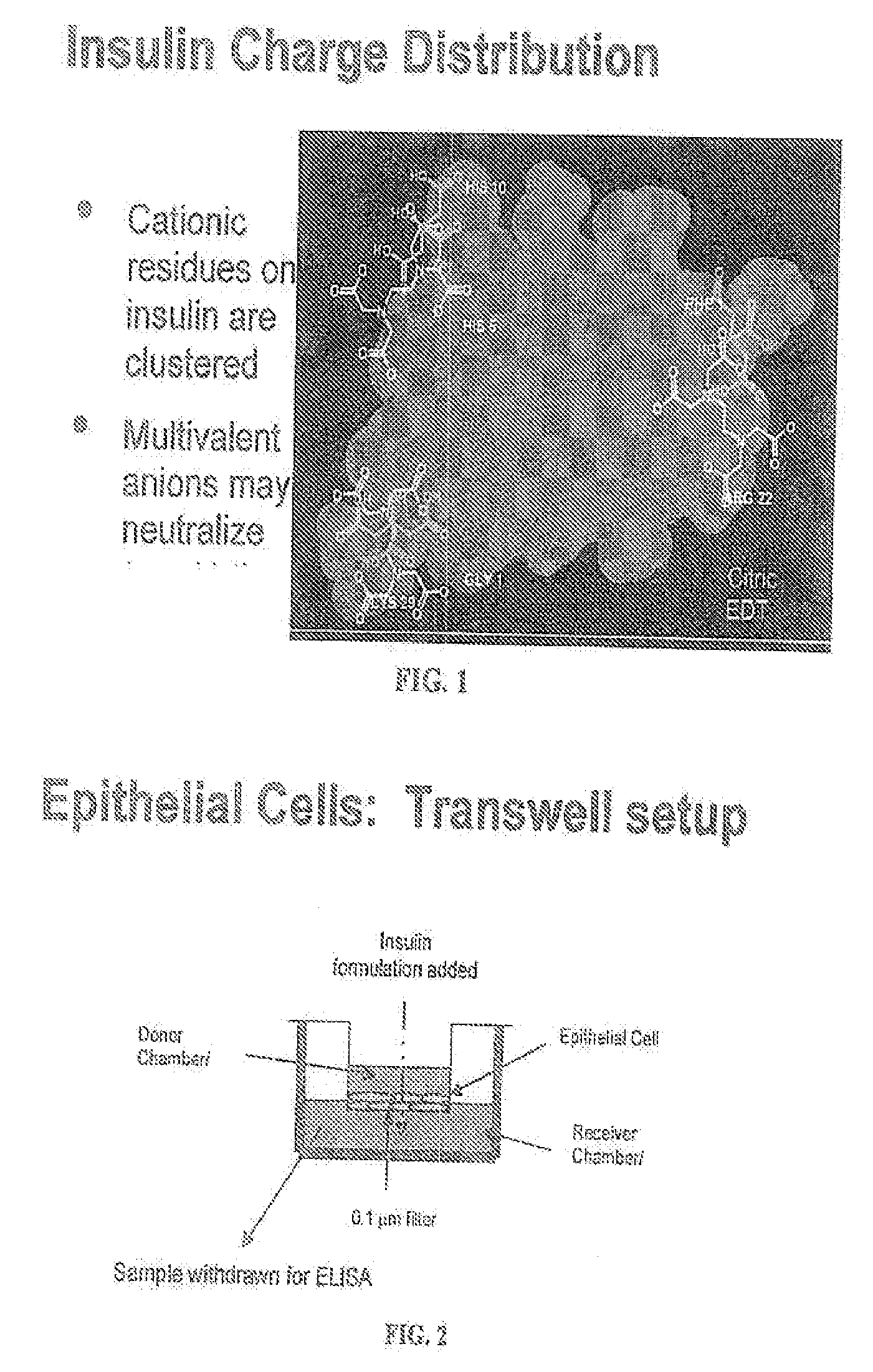
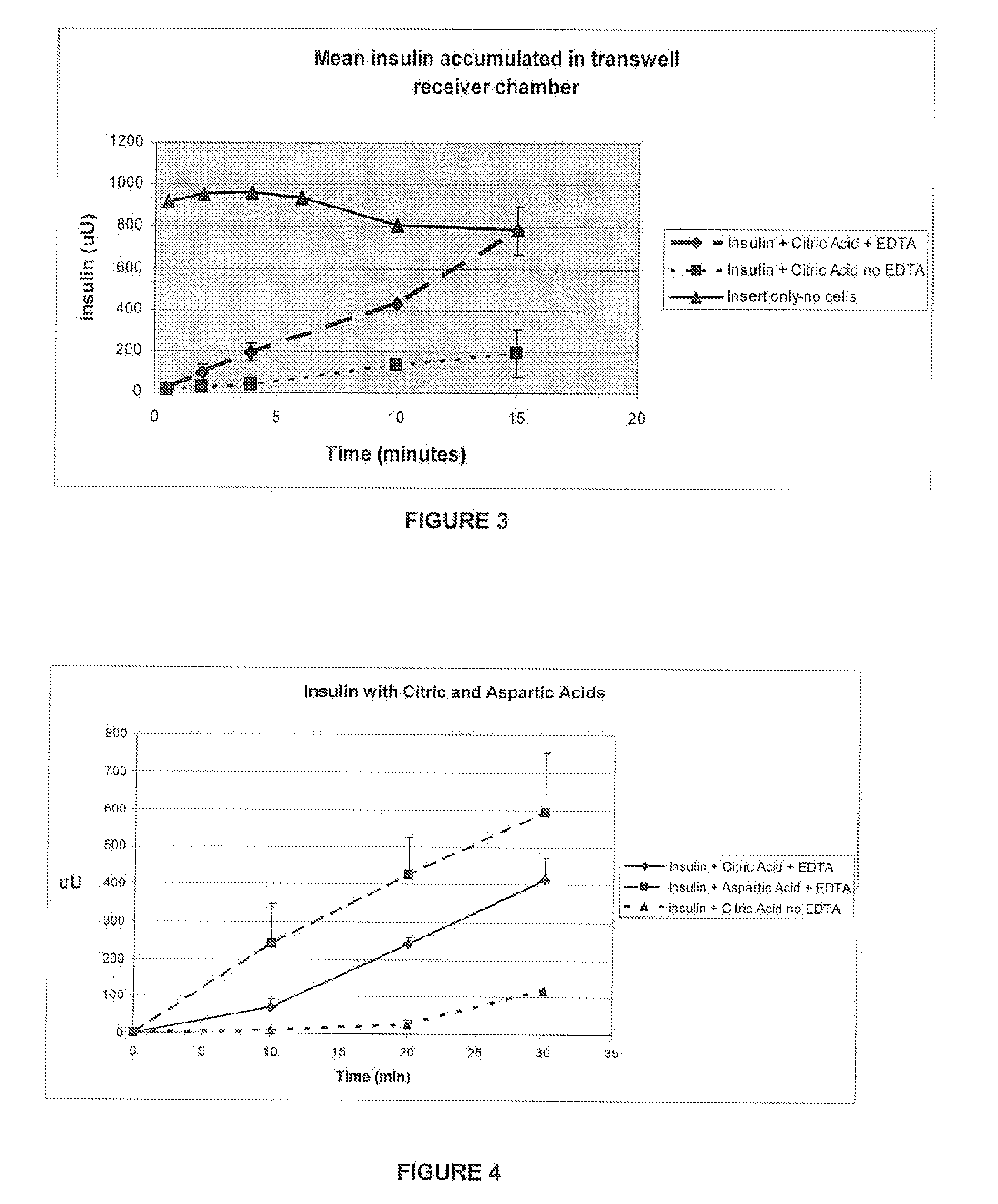
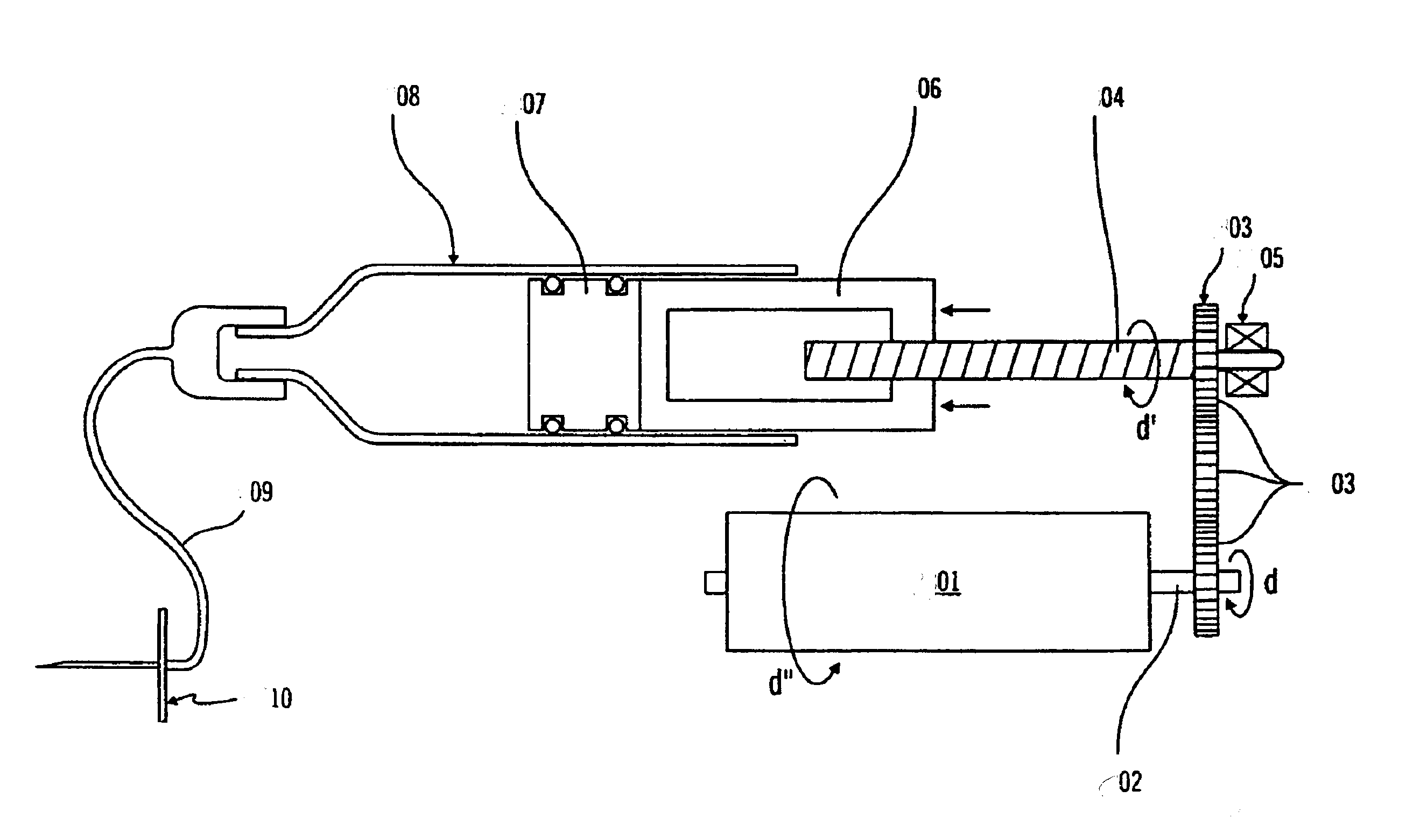
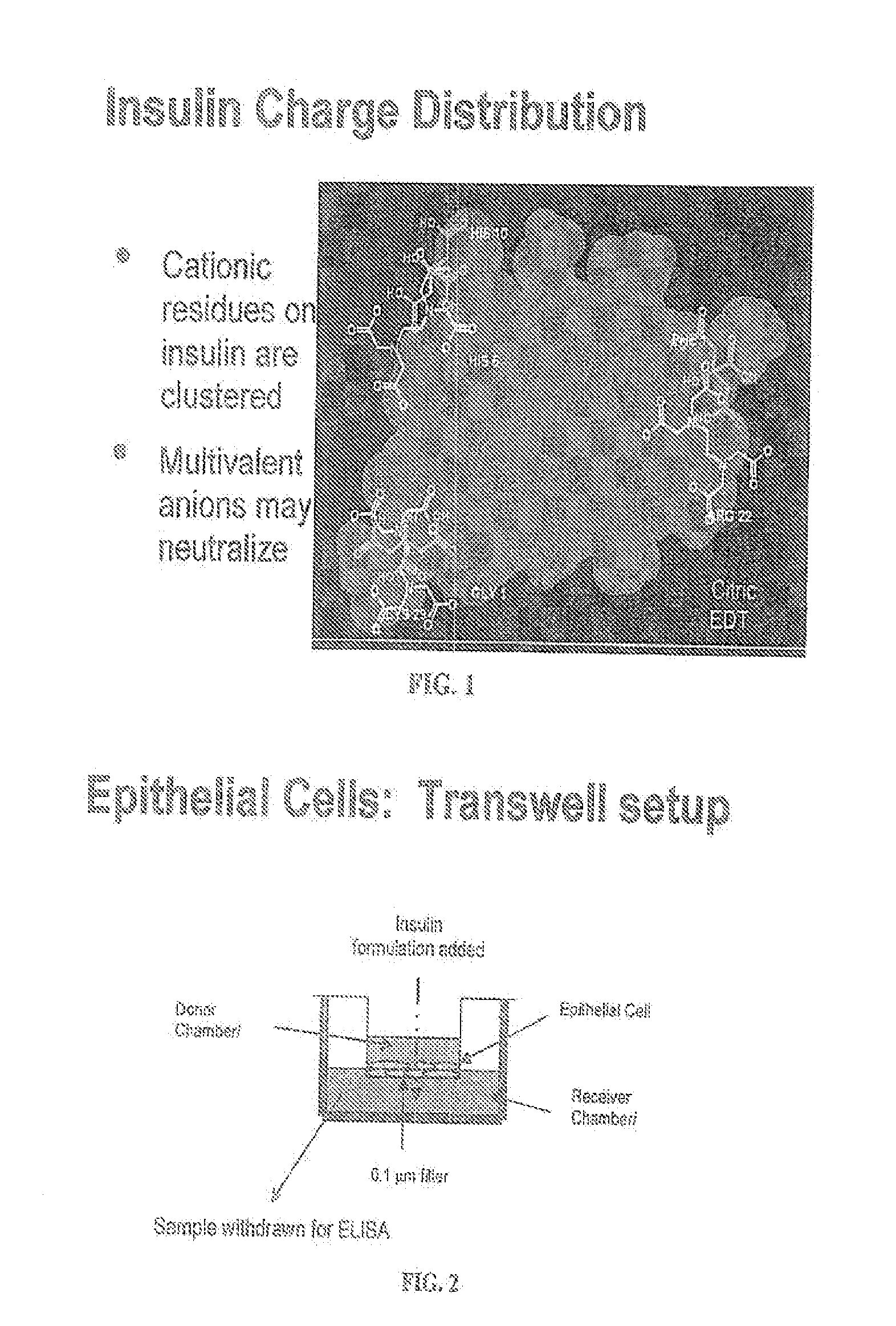
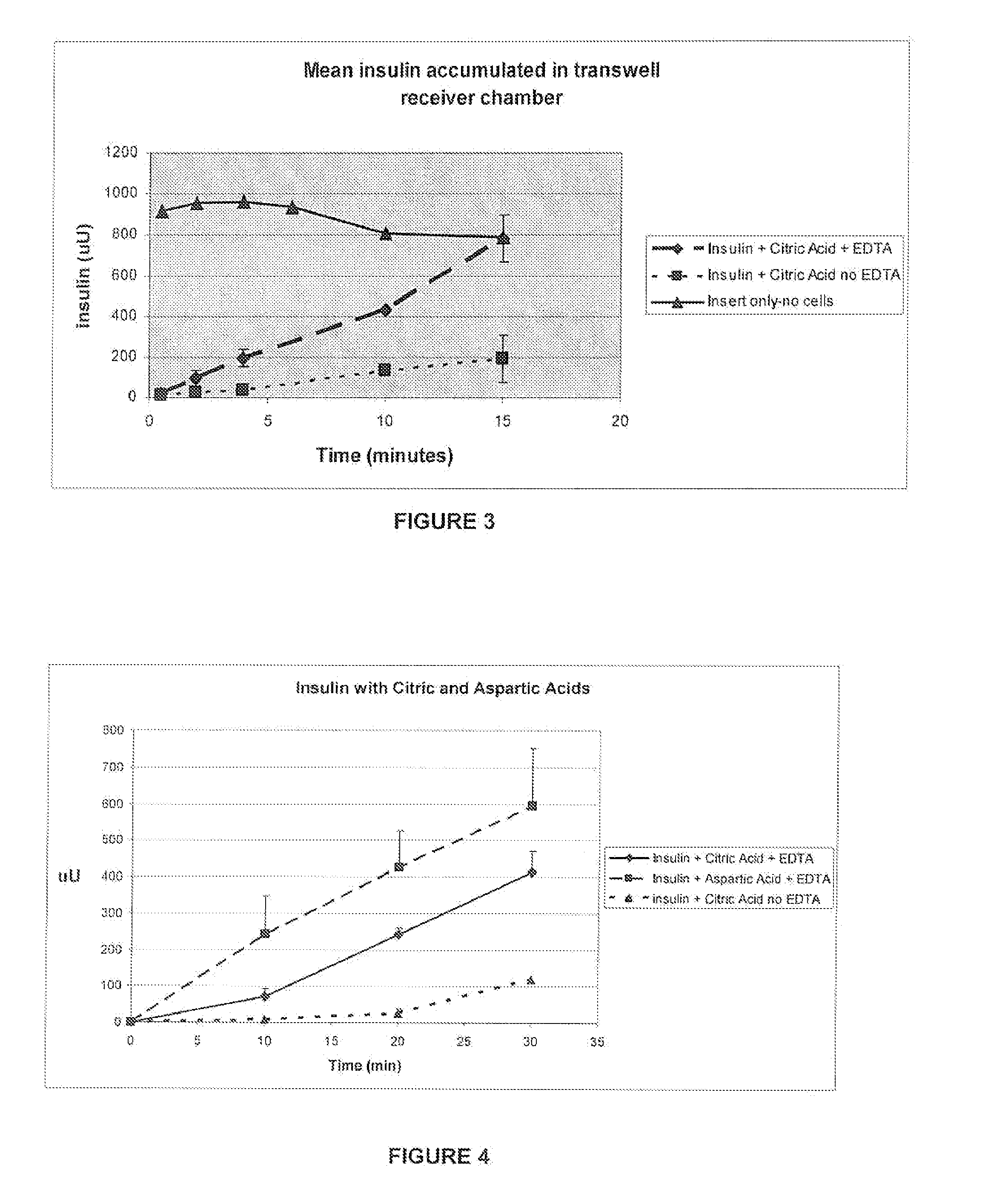
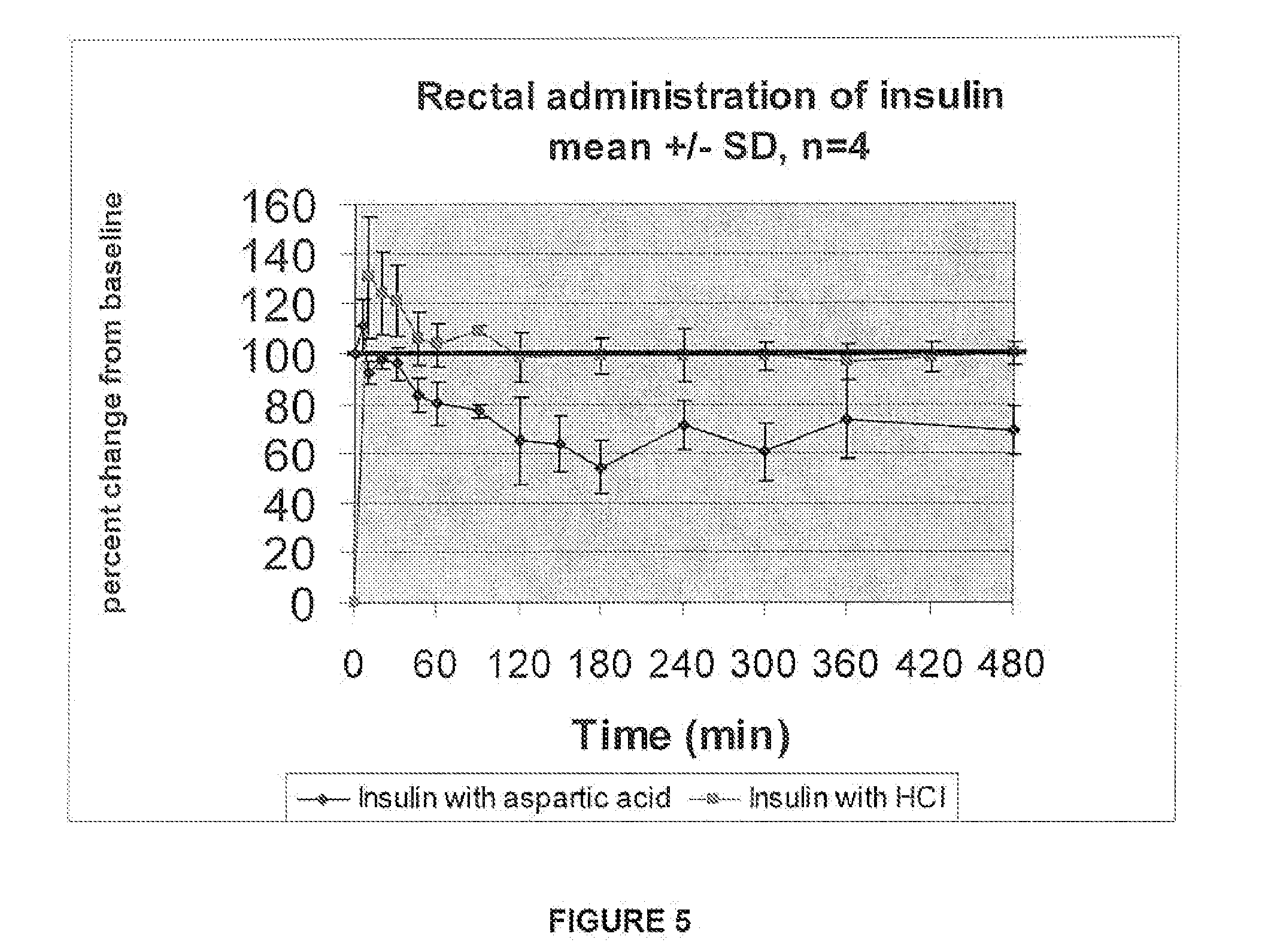
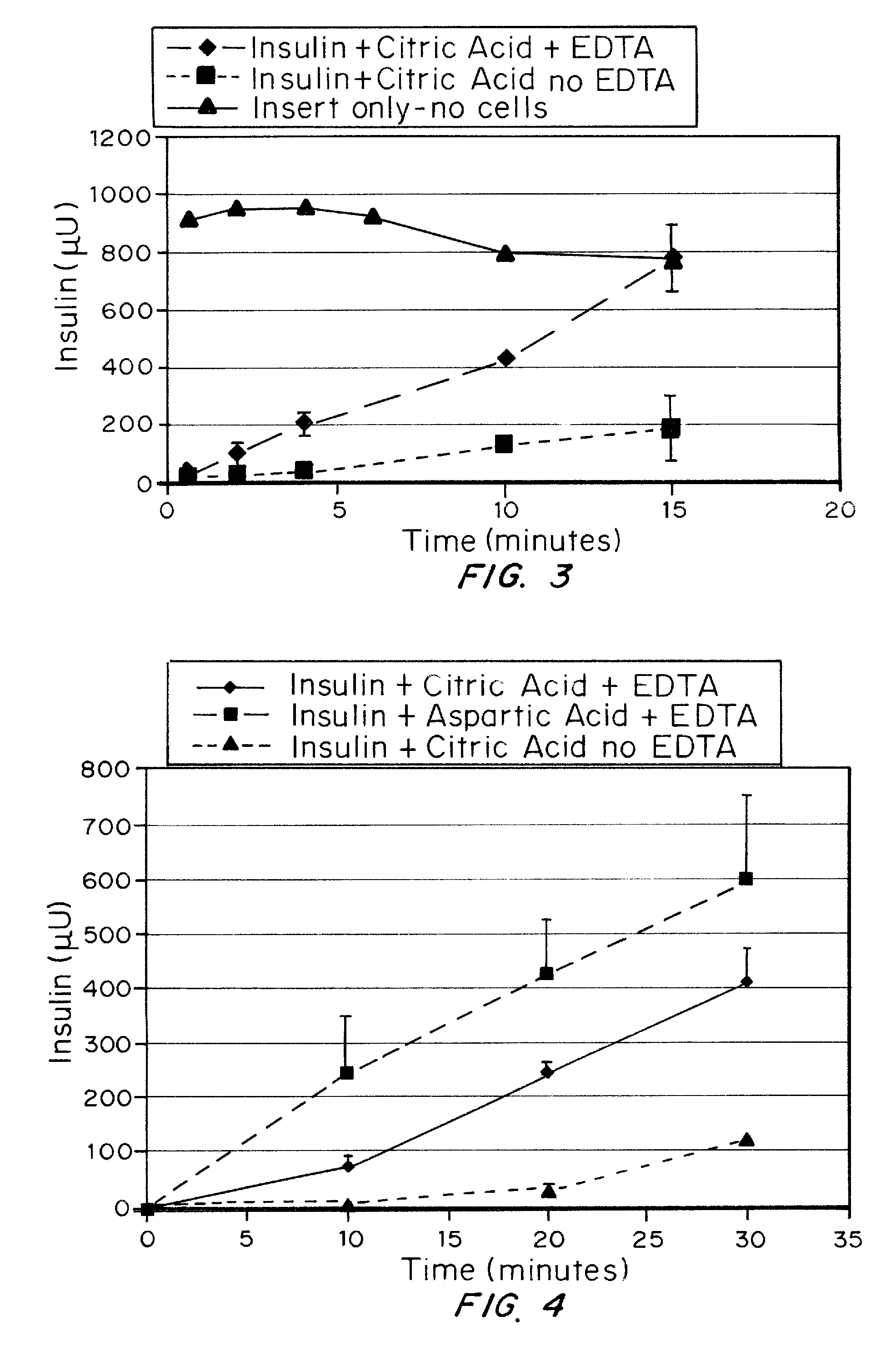
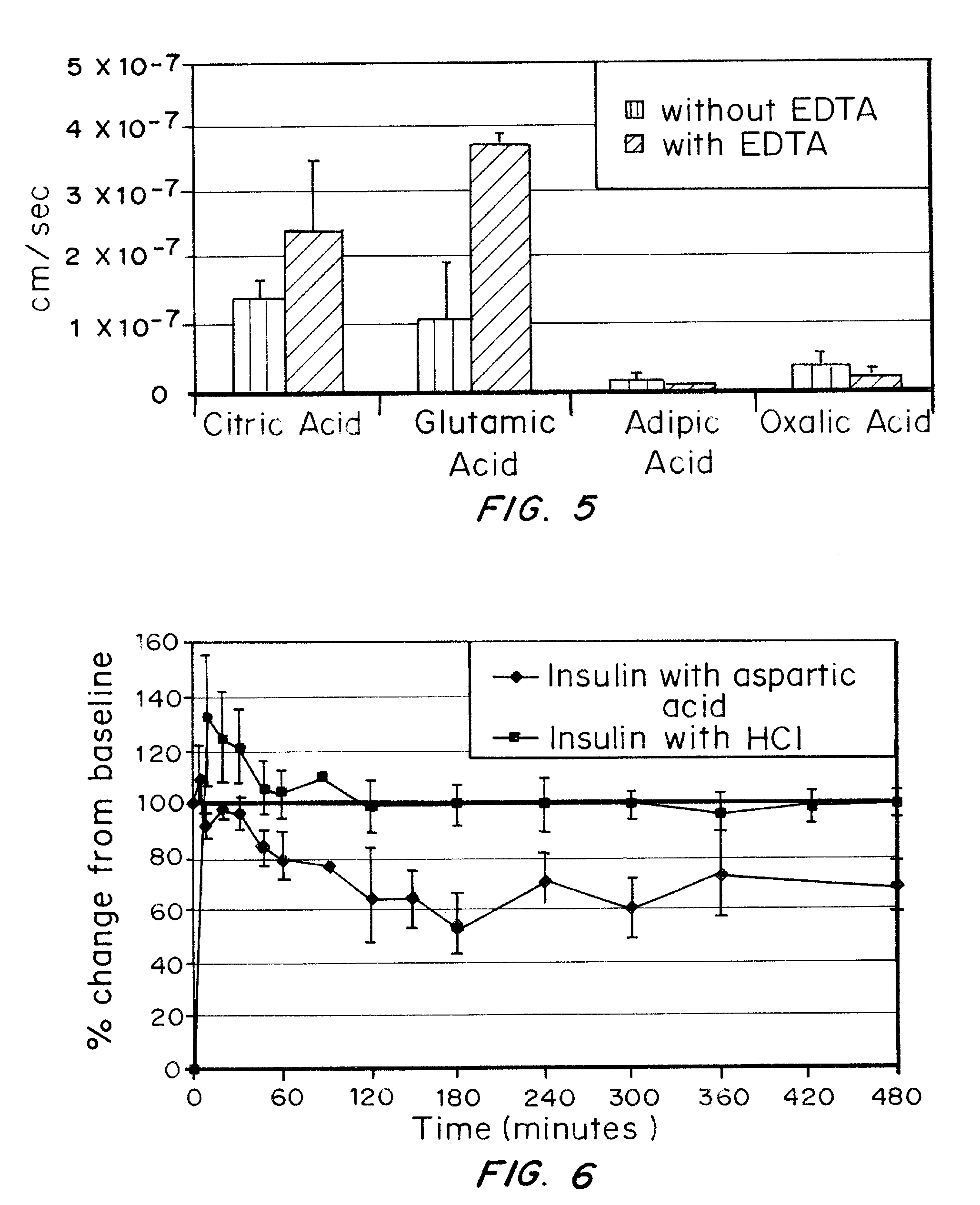
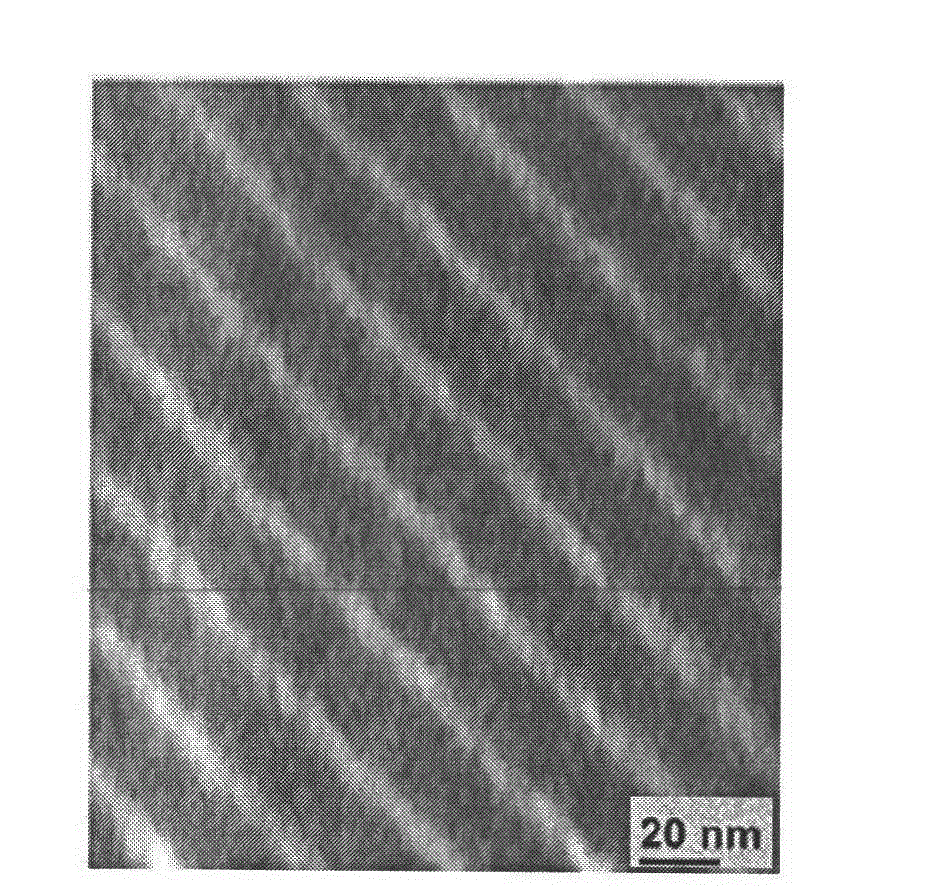
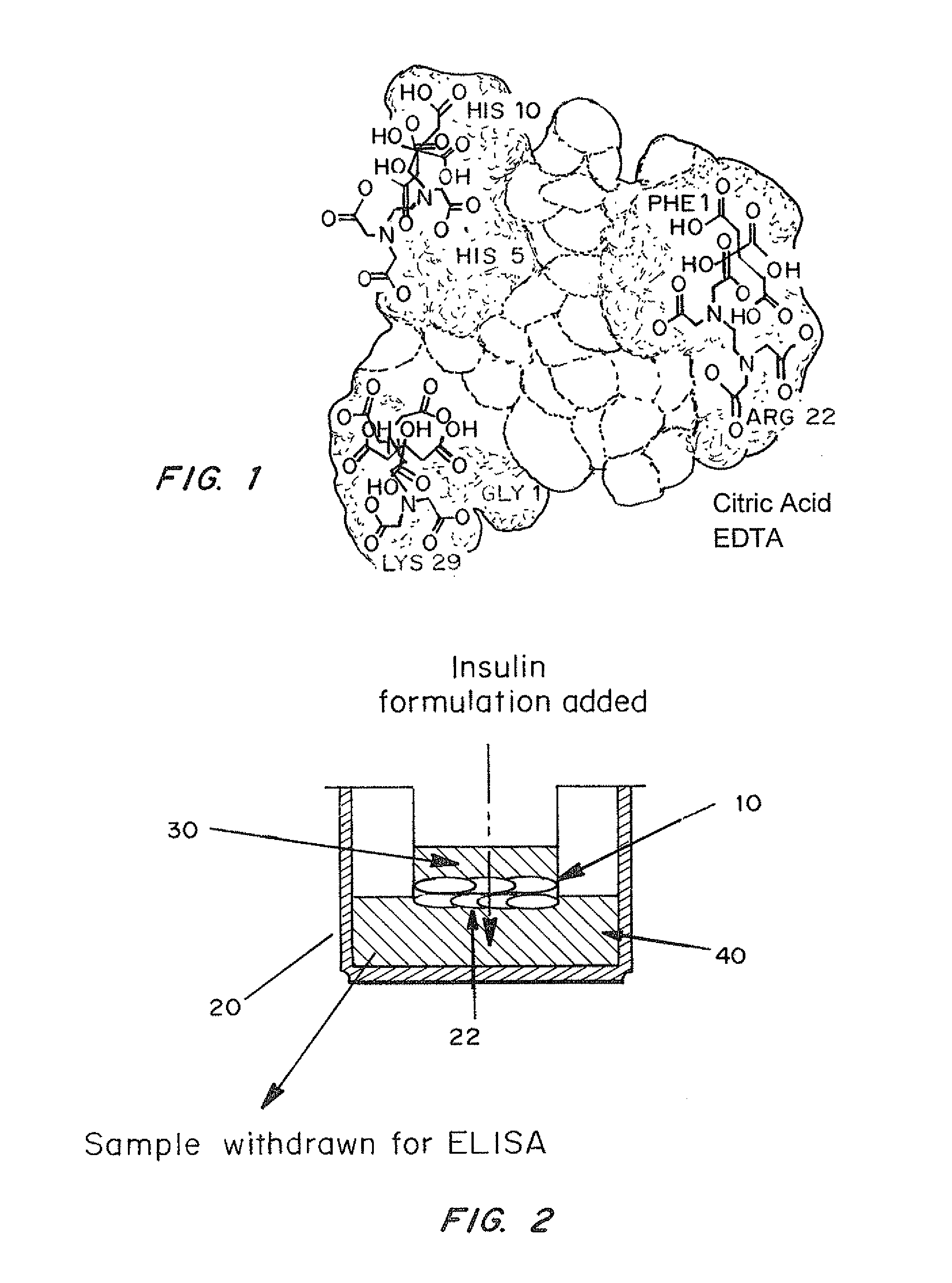
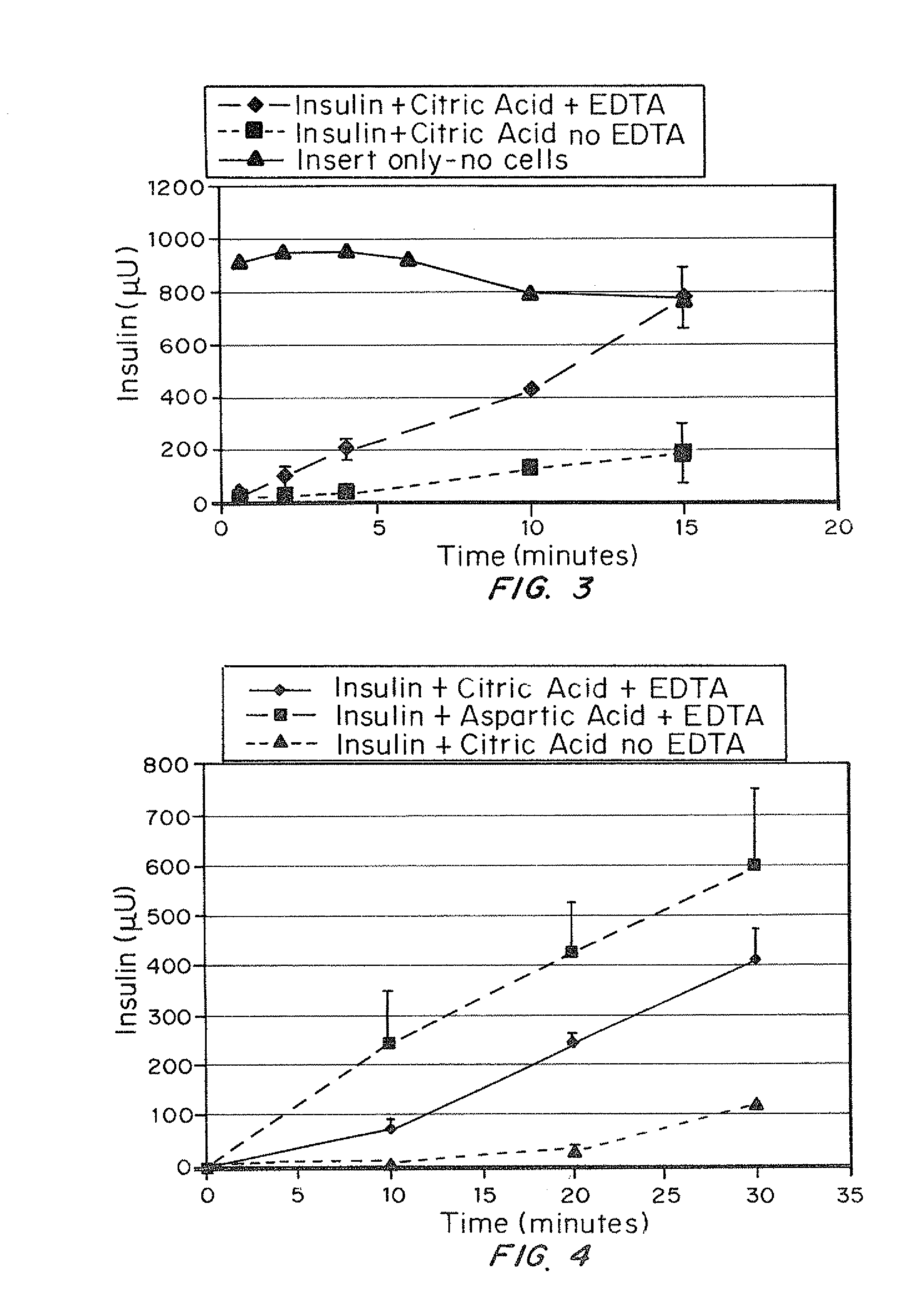
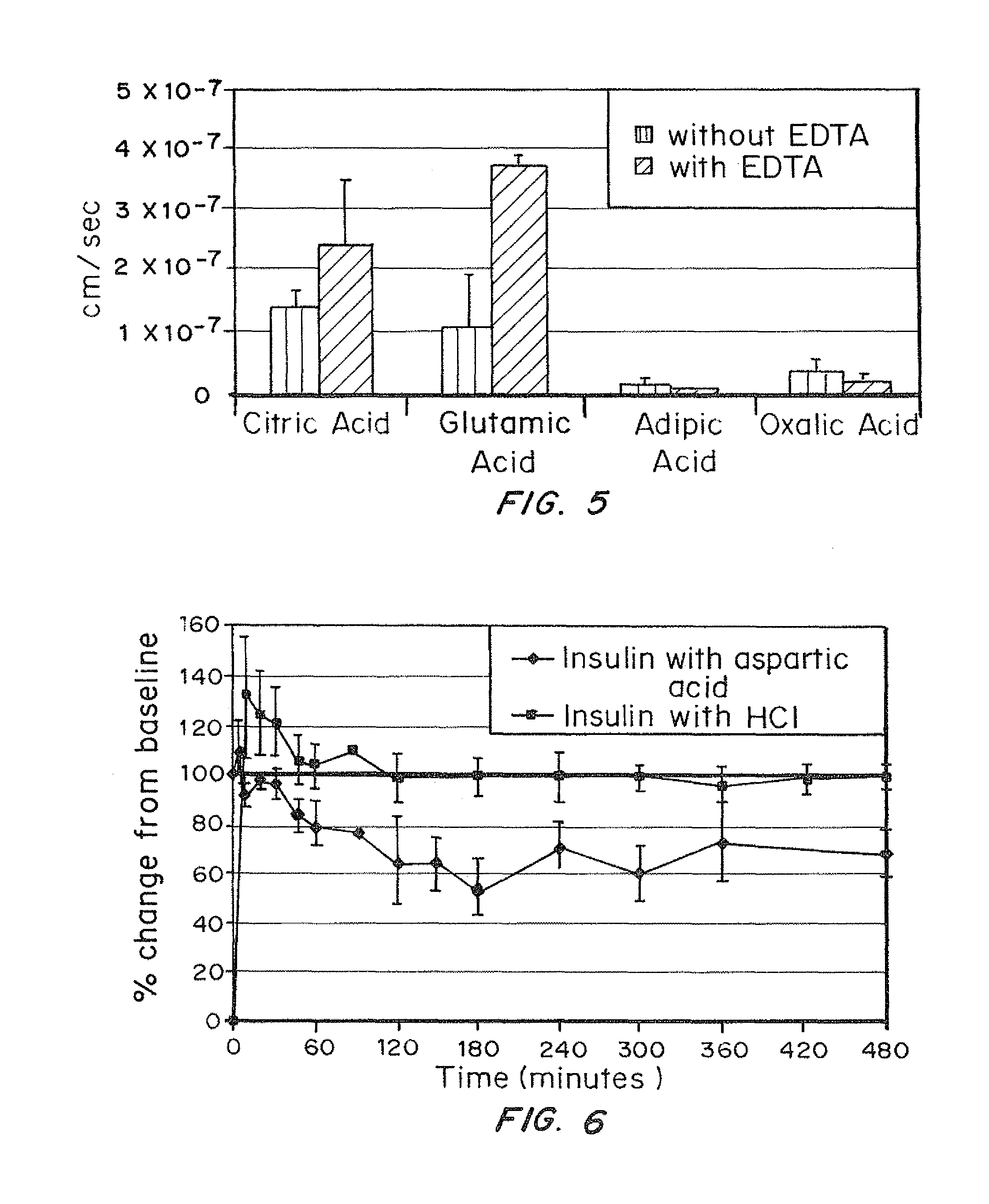
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate, the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, maleic, succinic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
Barrier coatings for fluids contacting medical devices
The invention relates to methods and materials that, for example, function to increase the barrier properties of containers including polymeric drug medication reservoirs and related containers such as infusion set tubing. Embodiments of the invention include aqueous container systems having containers coated with a composition selected to have one or more material properties including an ability to reduce the diffusion or permeation of compounds such as oxygen, carbon dioxide, and preservatives (e.g. phenol, benzyl alcohol and m-cresol) into or through a wall of the container.
Owner:MEDTRONIC MIMIMED INC
Synbiotics medicament composition
InactiveCN101366734AAdd flavorHigh nutritional valueBacteriaBacteria material medical ingredientsIntestinal structureMicroecosystem
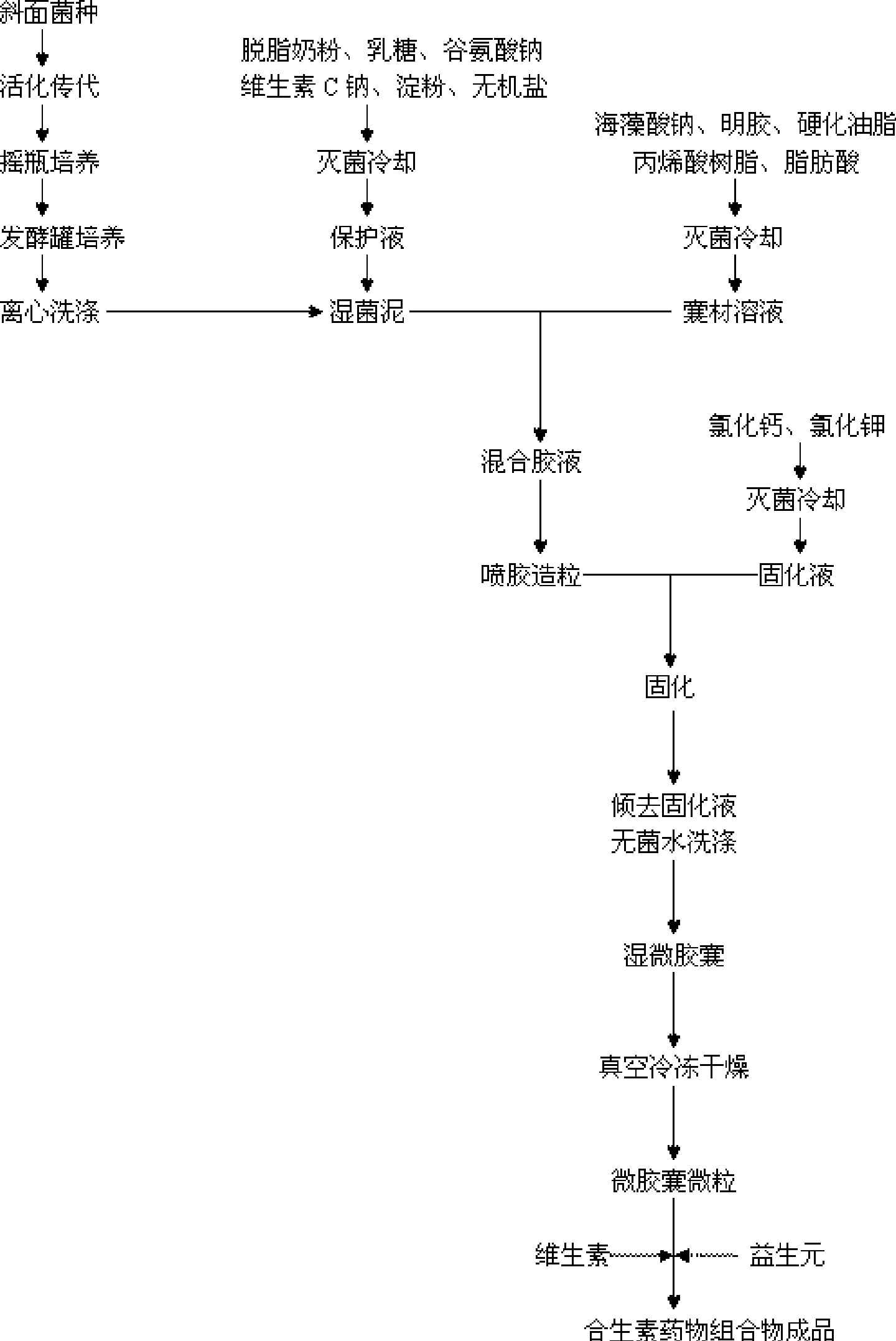
The invention relates to a micro-ecological regulator, which belongs to the category of micro-ecological preparations. The product contains Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium adolesentis, lactobacillus acidophilus, fructo-oligosaccharides, galacto-oligosaccharides, vitamins and the like. A concrete production process comprises the following steps: 1. bacterial strains are respectively or jointly subjected to liquid deep high-density cultu, so as to obtain fermentation liquid; 2. after the fermentation liquid is centrifuged, bacterial sludge is collected, added to protective agents and made into freeze-dried powder; 3. the freeze-dried powder is added to microcapsule materials and made into microcapsules through air suspension treatment; and 4. the microcapsules are mixed with oligosaccharides, vitamins and the like and then are made into a synbiotics pharmaceutical composition. By directly replenishing human intestines with Bifidobacterium and lactobacillus acidophilus, and by simultaneously providing prebiotics for ensuring that beneficial bacteria after entering intestines can be activated rapidly and proliferate, the product adjusts and improves intestinal micro-ecosystem, so as to achieve the aims of regulating organism immunity, delaying senility, inhibiting tumors, regulating blood lipid and improving intestines and stomach. A preparation method of the product can solve the technical problem that the prior probiotics medicine is difficult to keep the stability of probiotics at normal temperature and unstable under acidic conditions, and provides a pharmaceutical composition which can be stably stored at normal temperature.
Owner:辽宁大生商贸有限公司
Rapid Acting and Long Acting Insulin Combination Formulations
InactiveUS20080039365A1Promote absorptionAct quicklyBiocidePeptide/protein ingredientsBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is decreased so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
Method for extracting refined cordycepin and cordycepin polysaccharide from cordyceps mititaris
ActiveCN101124988ALow costReduce pollutionSugar derivativesSugar derivatives preparationAmylaseAdditive ingredient
The present invention relates to a method for extracting the purificatory cordycepin and the cordyceps amylase from the cordyceps militaris, belonging to the fields of medicines and health protection and chemical engineering. The cordyceps militaris powder is extracted and filtrated by a cooling seep method, the filter residue is mixed with ethanol, the supersonic wave assists to extract and offcenter, the supernatant has the processes of concentration and alcohol-precipitating, then the alcohol-precipitated supernatant has the processes of decompression, concentration and elution, the eluted liquid has the processes of concentration and crystallization and recrystallization using the n-butyl alcohol, at last the purificatory amylase is obtained after freezing and drying. The preparation progress of the present invention only uses water and ethanol as the solvent, thus reducing the pollution, the resin is capable of being reused a plurality of times with low cost. The present invention fully exploits the biological activity ingredients of the cordyceps militaris, realizes the coextraction of the cordycepin and the cordyceps amylase, guarantees the biological activities of the cordycepin and the cordyceps amylase, farthest increases the utilization rate of the raw material to the largest extent, reduces the manufacturing cost. The present invention is suitable for the large-scale industrialized manufacture.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Stable storage of proteins
InactiveUS20070117173A1Effect digestionRisk minimizationBioreactor/fermenter combinationsBiological substance pretreatmentsStable storageStorage protein
The present invention provides a method of stably storing a protein, the method comprising applying a protein to be stored to a substrate which has been treated with a polyhydric compound and dried, wherein the amount of the polyhydric compound present in the substrate is sufficient to stabilise the protein, and wherein the substrate does not consist of glass. In one embodiment the protein to be stored is trypsin.
Owner:WHATMAN PLC
Bionic three-dimensional tissue engineering scaffold and preparation method thereof
InactiveCN103006359APromote proliferationImprove regenerative functionStentsProsthesisDirected differentiationComposite scaffold
The invention discloses a bionic three-dimensional tissue engineering scaffold, which is formed by high molecular fibrous membrane-loaded active growth factors, or active growth factors loaded on a composite scaffold formed by a high molecular fibrous membrane and a macropore spongy layer. With the adoption of the bionic three-dimensional tissue engineering scaffold, the problem that the concentration of active molecules loaded with an emulsion electricity texture fibrous membrane is low; the emulsion electricity texture fibrous membrane is combined with a macropore spongy or a mixed electricity texture process, so that the load rate of the active factors can be greatly improved; parts of factors are retained in the fiber through an emulsion electricity texture core-shell structure, so that the effective control of releasing time is realized, and a repairing process is monitored for a long time; and the introduction of active molecules in the scaffold plays guiding and promoting functions for proliferating regenerative cells, directionally differentiating, migrating and adhering cells, and capturing stem cells to introduce regenerative functions of newly born tissues, so that a new path is provided for development of regenerative medicine industries.
Owner:江西欧芮槿生物科技有限公司 +1
Anti Abeta antibody formulation
ActiveUS7635473B2Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
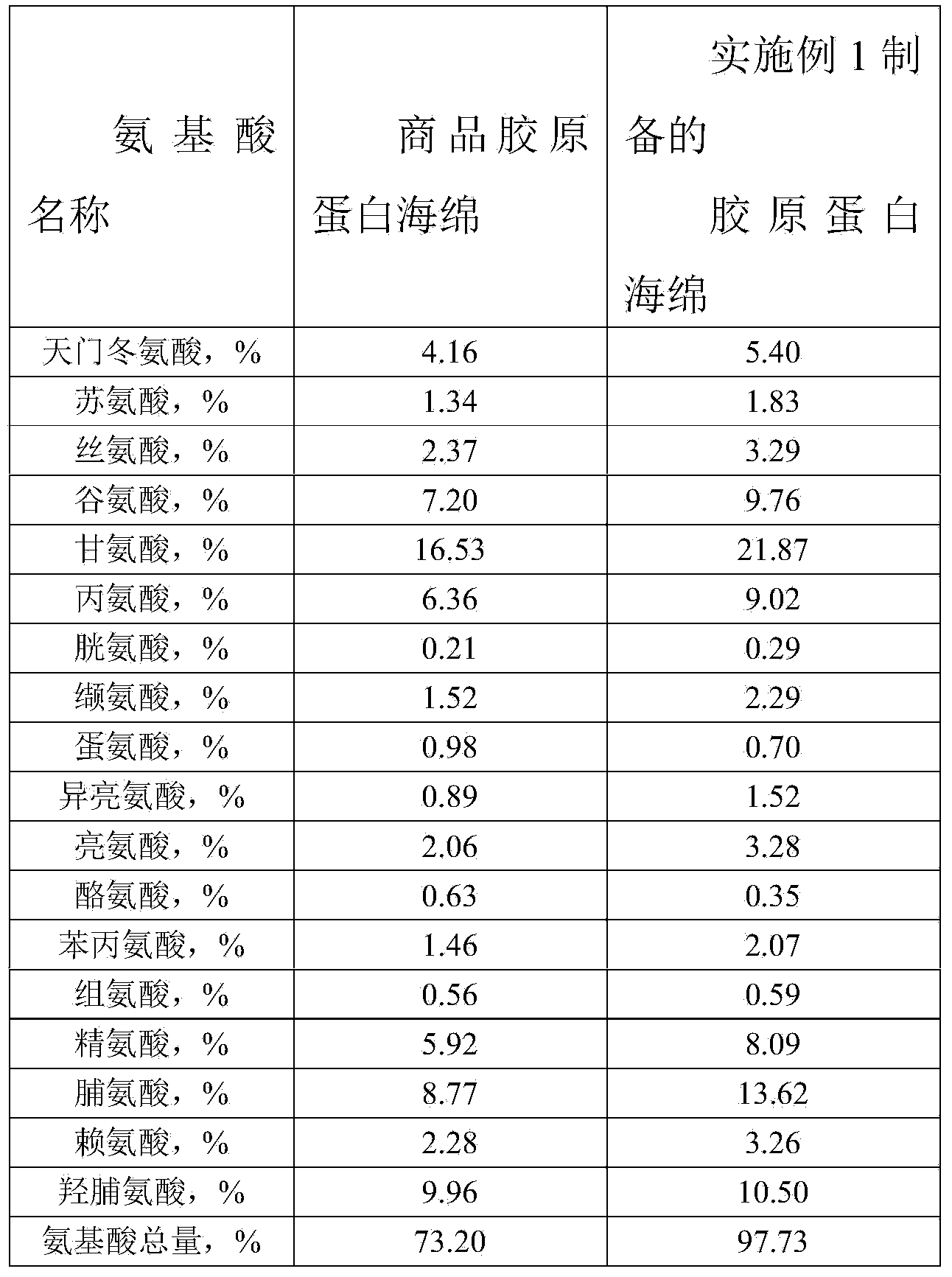
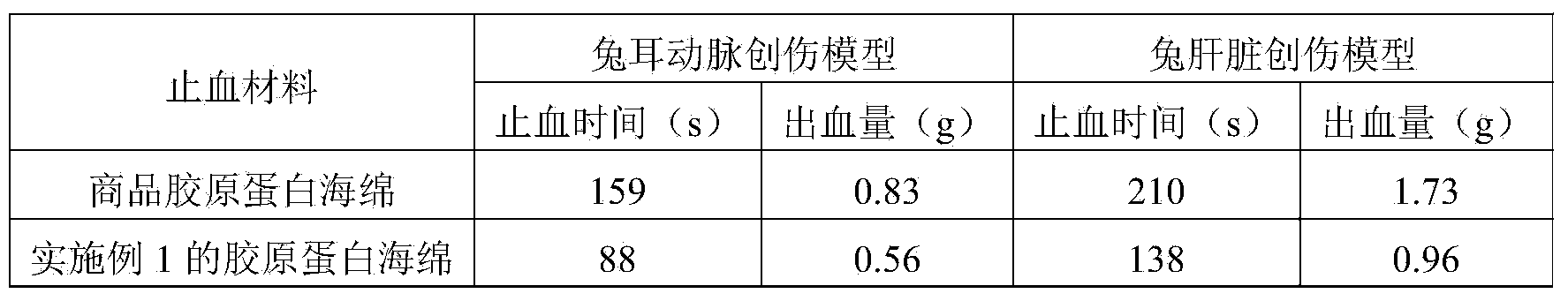
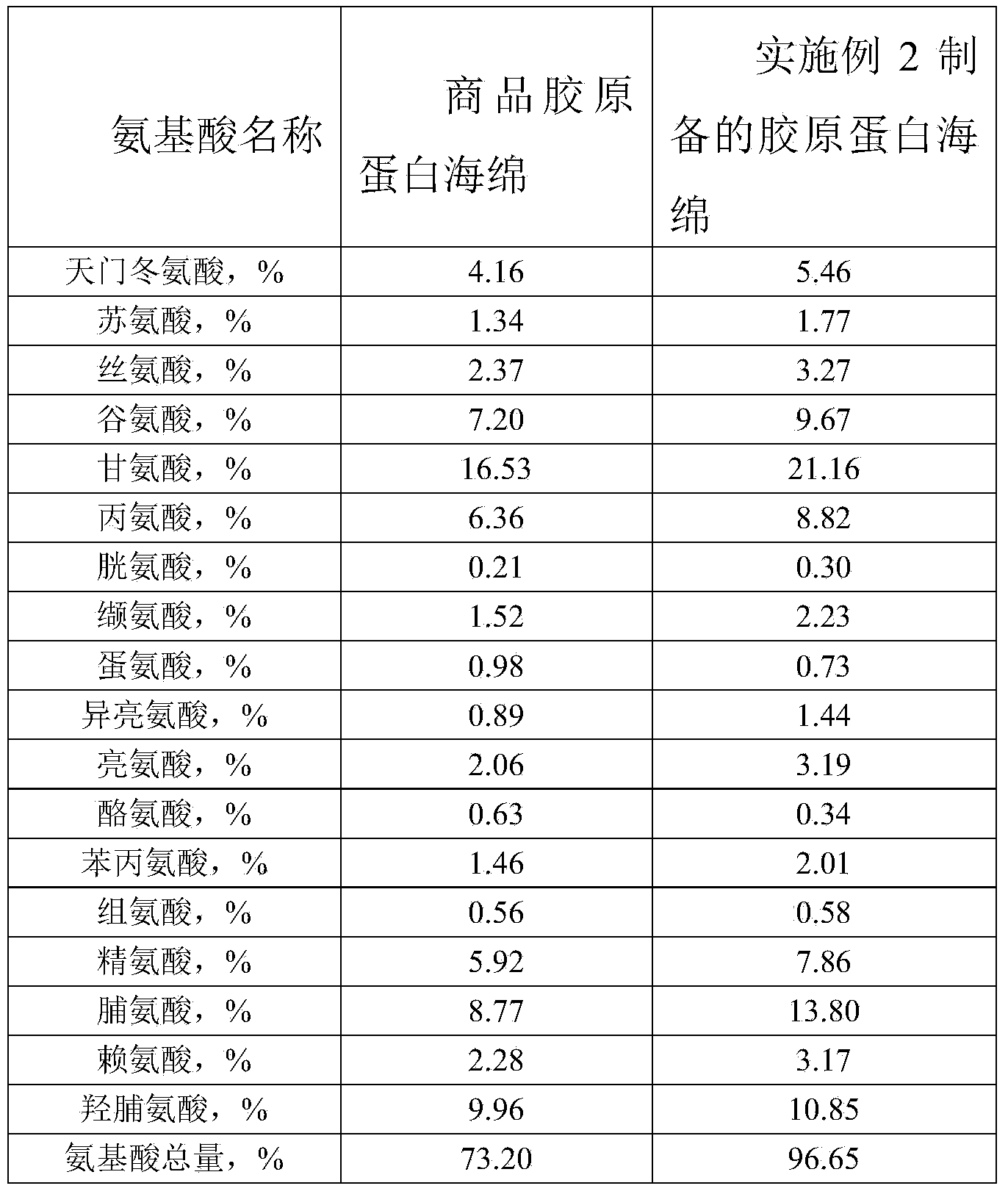
Preparation method of high-purity collagen protein sponge
InactiveCN103772734AGood removal effectHigh purityConnective tissue peptidesPeptide preparation methodsFreeze-dryingCollagen sponge
The invention provides a preparation method of high-purity collagen protein sponge, and relates to a preparation method of collagen protein sponge. The preparation method of the high-purity collagen protein sponge is used for solving the problems that the collagen protein sponge prepared by using a conventional method is long in production cycle and low in yield and purity and has poor hemostasis performance. The preparation method of the high-purity collagen protein sponge comprises the following steps: step one. pretreating fresh bovine heel tendons; step two. extracting collagen protein; step three. centrifuging; step four. salting out; step five. dissolving; step six. carrying out gradient dialysis; step seven. pre-freezing; step eight. carrying out freeze drying; and step nine. sterilizing. The final product prepared by using the method has a smooth and flat surface and relatively good hemostatic performance and is uniform in pore size distribution. The product has relatively high purity (the total amount of amino acids reaches 97.73%), an obvious hemostatic effect and no abnormal taste, is safe, non-toxic, high in yield and short in time; liquid is clear without impurities; the production cycle is shortened; the whole preparation process is carried out at a room temperature; the biological activity of the collagen protein is maintained; and the application of the high-purity collagen protein sponge in clinical is improved.
Owner:HARBIN INST OF TECH
Peptide nanoparticles and uses therefor
InactiveUS20100172943A1Low production costMaintain biological activityCosmetic preparationsPowder deliveryMedicineNanoparticle
The present invention provides nanoparticle compositions including one or more peptides. The present invention achieves transdermal delivery of such peptides without the need for peptide modification, or for use of chemical or mechanical abrasion or disruption of skin.
Owner:ANTERIOS INC
Veterinarian virus kind biological product heat resisting freeze drying protective agent and its preparation technique
A freeze-dried high-temp. resistant protecting agent for virus-type biologic products for veterinary medicine is prepared from several components through proportionally mixing. It features that each of its components is sterilized separately. For the components which can be sterilized under high temp., they are dissolved in distilled water according to the proportion of formulation and are sterilized at 116 deg.C for 30-40 min; and for those which do not resist the high temp., they are also dissolved in distilled water according the proportion of formulation and are filtered with 0.22 micron pore diameter filter membrane to remove bacteria; and then the two parts are mixed to obtain the freeze-dried high-temp. resistant, protecting agent. The mentioned two parts are added to the virus antigen liquid according to a proportion of 1:1 and after packaging, they are freeze-dried. The product can be stored at 2-8 deg.C for 24 months.
Owner:卫广森
Silk fibroin-based microneedles and methods of making the same
ActiveUS20130338632A1Limited drug load capacityDecrease in drug activityMicroneedlesSurgeryActive agentRoom temperature
A microneedle or microneedle device includes a microneedle body extending from a base to a penetrating tip formed from a silk fibroin based material, which is easy to fabricate and highly biocompatible. The microneedle device can include one or more microneedles mounted to a substrate. The silk fibroin can include active agents to be transported into or across biological barriers such as skin, tissue and cell membranes. The silk fibroin microneedles can be fully or partially biodegradable and / or bioerodible. The silk fibroin is highly stable, affords room temperature storage and is implantable. The silk fibroin structure can be modulated to control the rate of active agent delivery.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
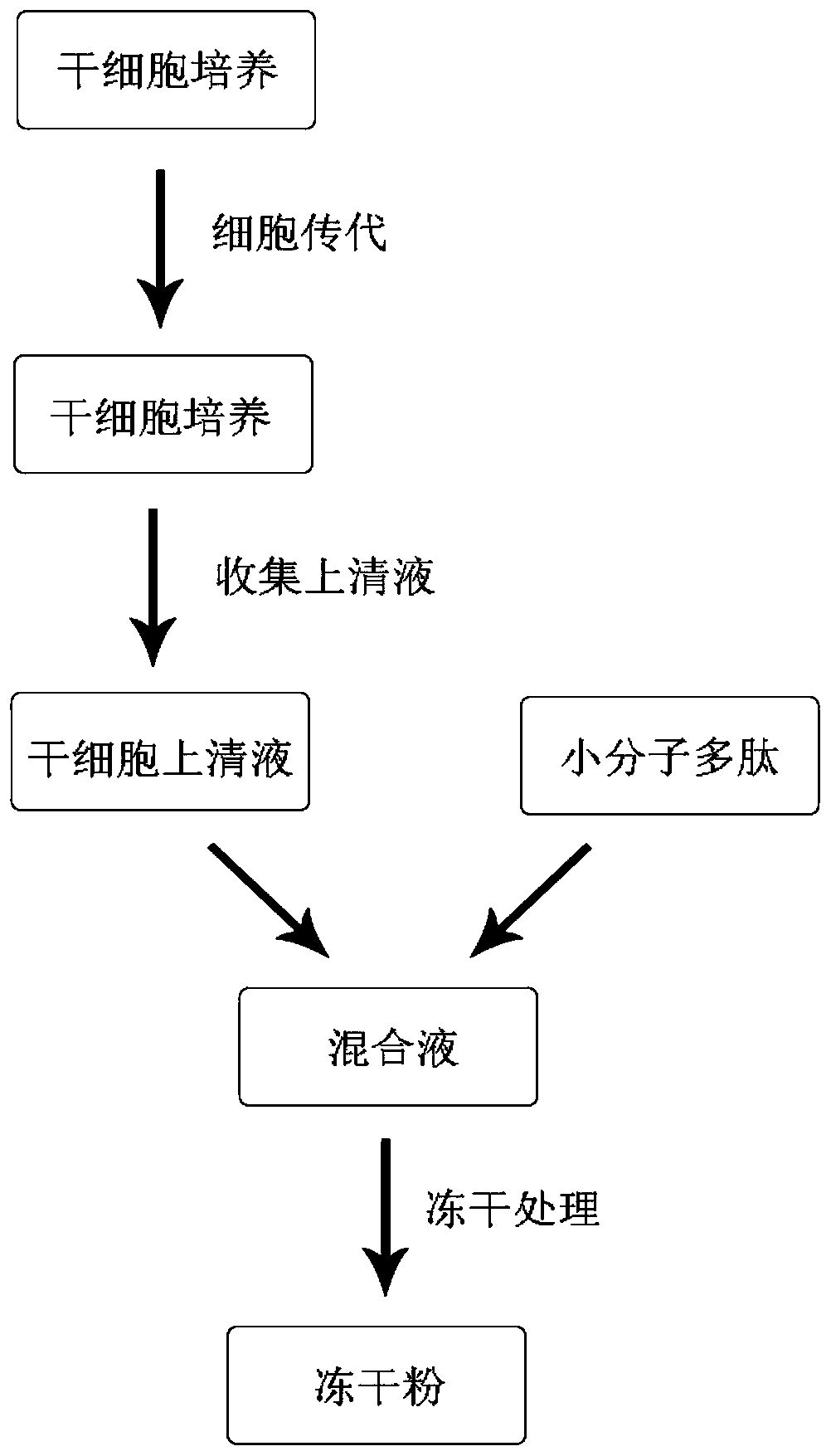
Freeze-dried powder, solvent and application of freeze-dried powder and solvent
PendingCN110237022AHigh activityGood effectCosmetic preparationsToilet preparationsWrinkle skinFreeze-drying
The invention belongs to the technical field of cosmetics, and particularly discloses a freeze-dried powder containing a stem cell supernate and micro-molecule polypeptide, a solvent containing a transdermal penetration enhancer and a cosmetic set containing the freeze-dried powder and the solvent. According to the prepared freeze-dried powder, the stem cell supernate and the micro-molecule polypeptide are compounded, so that the stem cell supernate and the micro-molecule polypeptide exert a synergistic effect, and the prepared freeze-dried powder has the functions of significantly delaying aging, reducing fine wrinkles, moisturizing and tendering the skin and the like; the solvent used for dissolving the freeze-dried powder specifically contains the transdermal penetration enhancer, the situation is promoted that active components of cell factors, micro-molecule polypeptide and the like in the freeze-dried powder permeate into the dermis through the transdermal penetration enhancer, and therefore the skin protection effect of the solvent is further improved. According to the freeze-dried powder, the solvent and the cosmetic set, the freeze-dried powder and the solvent are individually packaged, the quality guaranteeing period of the cosmetic set is prolonged, no preservative is added into the freeze-dried powder, and it is effectively ensured that the active components in the freeze-dried powder are not influenced.
Owner:STEMIRNA THERAPEUTICS CO LTD
Preparation method for high-activity lepidium meyenii extract
ActiveCN102526161AMaintain biological activityFully extractedAntinoxious agentsUrinary disorderSexual functionAlcohol
The invention provides a preparation method for high-activity lepidium meyenii extract. The preparation method is characterized by comprising the following steps of: crushing fresh or dry lepidium meyenii roots, and performing primary ultrasonic reinforced extraction, primary solid-liquid separation and primary low-temperature supernatant concentration under the conditions of certain liquid / solid ratio and 20 to 50 DEG C by taking water as a solvent; performing secondary ultrasonic reinforced extraction, secondary solid-liquid separation and secondary low-temperature supernatant concentration on filter residues under the conditions of certain liquid / solid ratio and 20 to 50 DEG C by using an ethanol-water solution; and mixing the two concentrated solutions, and drying the mixture at low temperature or quickly to obtain powdery lepidium meyenii extract. The preparation method has the characteristics of high speed, high efficiency, completeness of functional component extraction, capability of preventing the damage of heat-sensitive functional components of lepidium meyenii, high bioactivity of the extract, low energy consumption, low cost and the like. The lepidium meyenii extract prepared by the preparation method provided by the invention has the effects of eliminating fatigue, resisting pressure, improving immunity, alleviating climacteric syndromes, resisting prostatic hyperplasia, enhancing sexual function, and the like.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Rapid acting and long acting insulin combination formulations
ActiveUS7718609B2Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed in which the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
Protease resistant mutants of stromal cell derived factor-1 in the repair of tissue damage
ActiveUS20080095758A1Increase concentrationObviates abilityNervous disorderPeptide/protein ingredientsDipeptidyl peptidaseMutant
The present invention is directed stromal cell derived factor-1 peptides that have been mutated to make them resistant to digestion by the proteases dipeptidyl peptidase IV (DPPIV) and matrix metalloproteinase-2 (MMP-2) but which maintain the ability of native SDF-1 to attract T cells. The mutants may be attached to membranes formed by self-assembling peptides and then implanted at sites of tissue damage to help promote repair.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Preparation method and application of environmental estrogen electrochemical immunosensor
ActiveCN102749373AHigh sensitivityGood biocompatibilityMaterial electrochemical variablesAntigenBovine serum albumin
The invention relates to a preparation method and application of an environmental estrogen electrochemical immunosensor, belonging to the technical field of electrochemical detection. The sensitivity of the sensor is obviously improved by adopting the characteristics that the conductivity of chitosan dispersed graphene is high, the stability is high, the specific surface area of a gold hybrid mesoporous silicon dioxide nano composite material is large, the biocompatibility is good, the catalytic efficiency is high and the like. The immunosensor is constructed by adopting the method of layer-by-layer self-assembly of the chitosan dispersed graphene, cross-linking agent, composite solution of nano materials and antibodies, and bovine serum albumin. In combination with specificities of antigens and antibodies, by virtue of an electrochemical workstation instrument, by recording current change before and after corresponding antigens modify the sensor, the environmental estrogens in water and food can be directly detected. The environmental estrogen electrochemical immunosensor prepared by adopting the preparation method has the advantages that the sensitivity is high, the specificity is good, the operation is easy to conduct and the detection limit is low, and the sensitive, rapid and accurate detection of various environmental estrogens in actual samples can be realized.
Owner:UNIV OF JINAN
Fibrillation-resistant insulin and insulin analogues
InactiveUS8192957B2High thermodynamic stabilityMaintain biological activityBacteriaSugar derivativesInsulin A ChainNormal insulin
A fibrillation-resistant insulin analogue may be a single-chain insulin analogue or a physiologically acceptable salt thereof, containing an insulin A chain sequence or an analogue thereof and an insulin B chain sequence or an analogue thereof connected by a polypeptide of 4-10 amino acids. The fibrillation-resistant insulin analogue preferably displays less than 1 percent fibrillation with incubation at 37° C. for at least 21 days. A single-chain insulin analogue displays greater in vitro insulin receptor binding than normal insulin while displaying less than or equal binding to IGFR than normal insulin. The fibrillation-resistant insulin may be used to treat a patient using an implantable or external insulin pump, due to its greater fibrillation resistance.
Owner:CASE WESTERN RESERVE UNIV
Separating, purifying and inspecting method of anthocyanin in blueberry wine dregs
InactiveCN102321062AMaintain biological activityImprove antioxidant capacityOrganic chemistryColor/spectral properties measurementsFreeze-dryingSlurry
The invention relates to a separating, purifying and inspecting method of anthocyanin in blueberry wine dregs, which belongs to the technical field of functional active constituent extraction and purification. In the separating method, blueberry wine dregs or frozen blueberry wine dregs are ground into slurry or are freeze-dried and crushed into powder; and the slurry or the powder is separated in a warm extraction method in an acidified ethanol solution, and blueberry anthocyanin coarse extracting liquid is obtained through reduced pressure concentration. The blueberry anthocyanin coarse extracting liquid is purified and concentrated to prepare a blueberry anthocyanin concentrated solution. The concentrated solution is freeze-dried to obtain blueberry wine dreg anthocyanin freeze-dried powder. In the invention, the anthocyanin is separated out from waste produced by blueberry wine, which shows the research and development of simple, efficient, environment-friendly and low-cost production technology; and the content of the anthocyanin in the blueberry anthocyanin freeze-dried powder is 10-40%. The anthocyanin concentrated solution or coarse extracting liquid can be directly manufactured into oral liquid, tablets and other functional foods. The anthocyanin freeze-dried powder can be manufactured into food stain with an obvious antioxidation to be widely used in the fields of beverages, wines and the like.
Owner:吉林云尚保健食品有限公司
Method for producing fish scale collagen protein
InactiveCN101418328AReduce churnHigh yieldConnective tissue peptidesPeptide preparation methodsProtein solutionFreeze-drying
The invention relates to a method for preparing fish scale collagen. The method comprises the following steps: fresh fish scales are taken as a material, undergo cleaning, drying and crushing, are soaked in NaOH solution for degreasing, and soaked in hydrochloric acid solution for deashing; the fresh fish scale is added with water of which the weight is 5 to 10 times of that of the fish scales, the pH value is adjusted to between 2 and 4 by acid solution, pepsin of which the weight accounting for the weight of the mixing solution is between 1 and 5 percent is added, the mixing solution undergoes enzymolysis at a low temperature for 4 to 10 hours, and enzyme is killed; enzymatic liquid is filtered twice to obtain extracting solutions, the extracting solutions are mixed, the mixing solution is decolored, sodium chloride of which the weight accounting for the weight of the mixing solution is between 5 and 15 percent is added into the mixing solution for overnight salting out, deposit is separated and collected, and acetic acid of which the weight accounting for the weight of the mixing solution is between 5 and 20 percent is added into the deposit for dissolution to obtain protein solution; the protein solution is dialyzed and desalted to obtain purified collagen liquid; and the collagen liquid is subjected to freeze-drying or spray-drying and micro-crushing to obtain solid particle. The method has the advantages of reasonable process, simple operation, safety, low cost, little loss of nutrient matters of the product, low ash content and high yield and purity of the collagen.
Owner:SHANDONG HOMEY AQUATIC DEV +1
Method for producing soybean polypeptide powder
InactiveCN102511648AAdvanced technologyIncrease productivityProtein foodstuffs working-upSolubilityNeutral protease
The invention relates to a method for processing soybean isolated protein by utilizing a biological enzyme technology. The method comprises the following steps of: during preparation, selecting high-quality soy protein isolate, and dissolving; performing thermal denaturation; adjusting the pH value to 8.0 to 9.0; adding composite hydrolase of alkali protease, neutral protease, papain and trypsin, and reacting for 2 to 3 hours; adjusting the pH value to 4.0 to 6.0; inactivating enzyme; when cooling to a temperature below 50 DEG C, adding filter aid enzyme; collecting filtrate to dissolve filter residues; adding composite hydrolase of acidic protease, neutral protease, papain and trypsin, and reacting for 1 to 2 hours; inactivating the enzyme; when cooling to temperature of below 50 DEG C, adding the filter aid enzyme; filtering by a paperboard; performing ultrafiltration; drying by microwaves under vacuum; and crushing and sieving. The soybean polypeptide powder prepared by the method can improve the solubility of albumen powder to a maximum limit and keep the color and luster of appearance, mouthfeel, flavors and the bioactivity of active ingredients, offers a good mouthfeel, is easy to absorb and can be widely applied to industry of dairy products, drinks and health-care products.
Owner:天津诺奥酶生产力促进有限公司
Biomarker preserving liquid, biomarker reagent and method
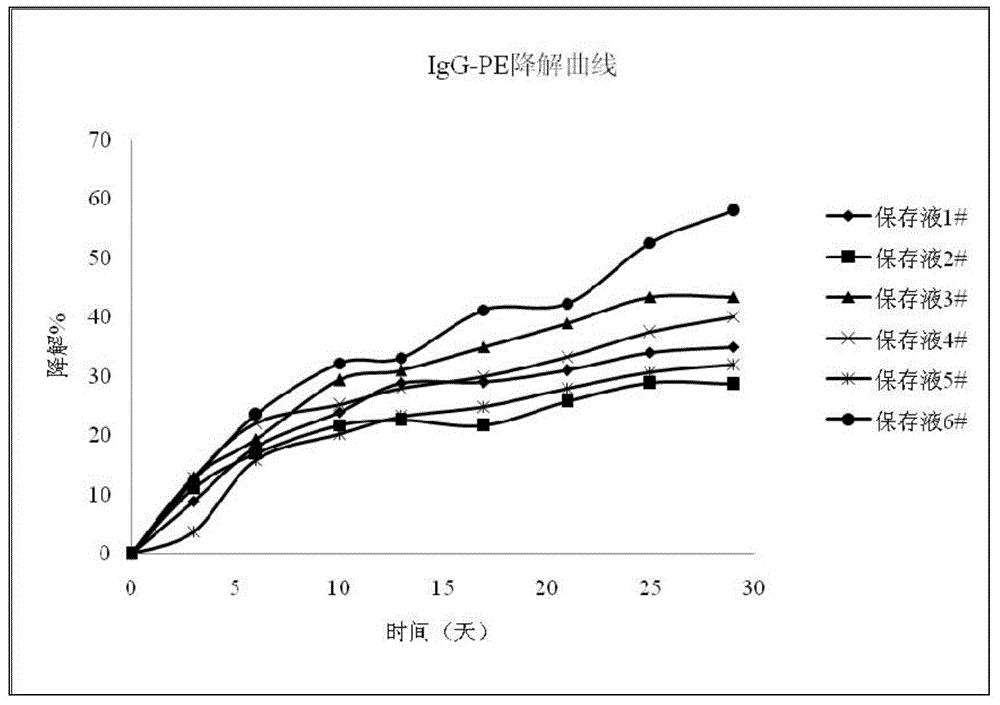
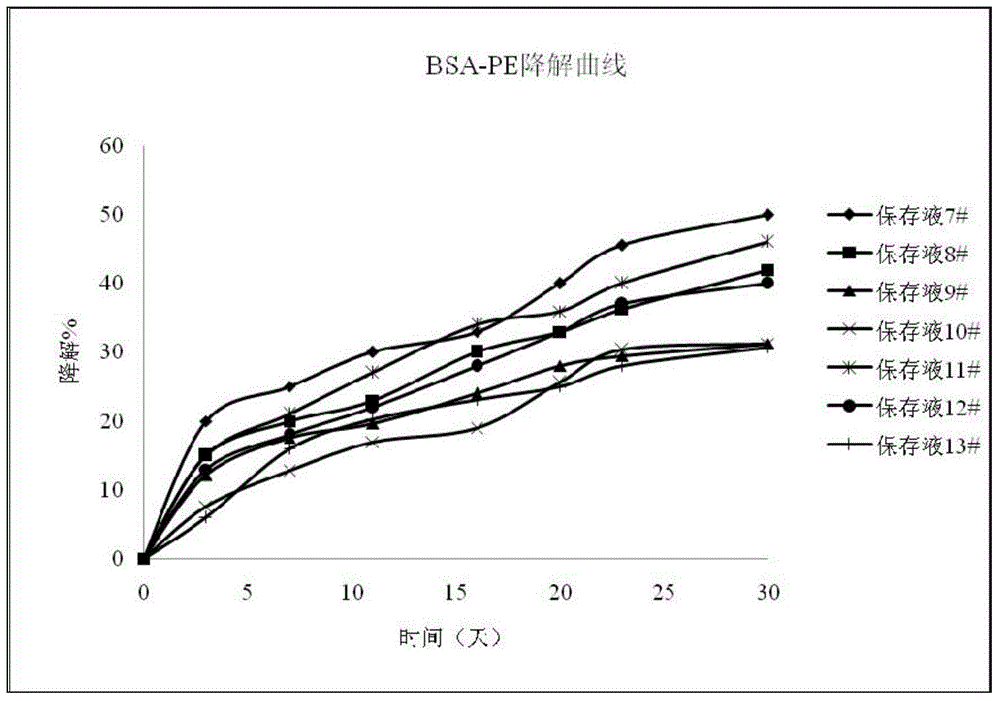
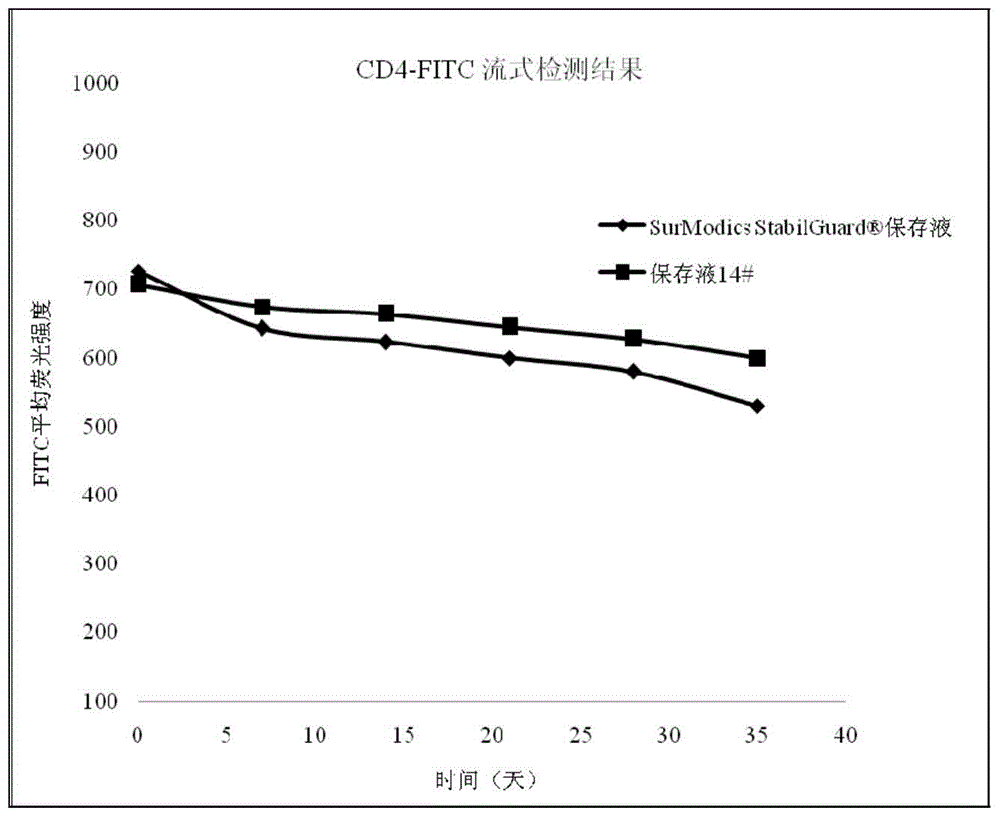
InactiveCN104459147AEasy to useLow costDead animal preservationEnzyme stabilisationCitric acidPolymer
The invention discloses a biomarker preserving liquid. The biomarker preserving liquid comprises at least one protein stabilizer, at least one alkaline or neutral amino acid, at least one polyol or polymer, and citrate. The invention also discloses a fluorescent marker reagent prepared by using the preserving liquid, a preparation method of the reagent, and a use of the preserving liquid. The preserving liquid has a very good preserving effect on a fluorescent tracer-biomarker conjugate in the fluorescent marker reagent, can keep the activity and stability of a fluorescent tracer, the biomarker and the conjugate to make the fluorescent marker reagent form a liquid commercial reagent which can be directly used, so the preserving liquid is convenient to use; and the fluorescent marker reagent prepared by using the preserving liquid can tolerate short time high-temperature transportation, so the cost reduction is facilitated.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
Preparation method of immune base and antigen or antibody immunoassay method
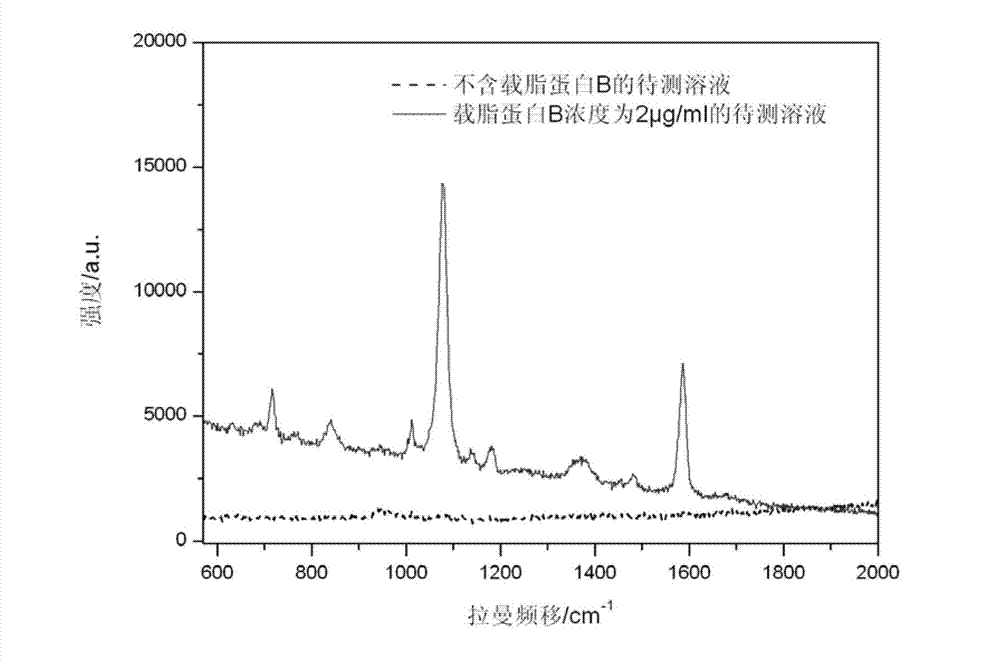
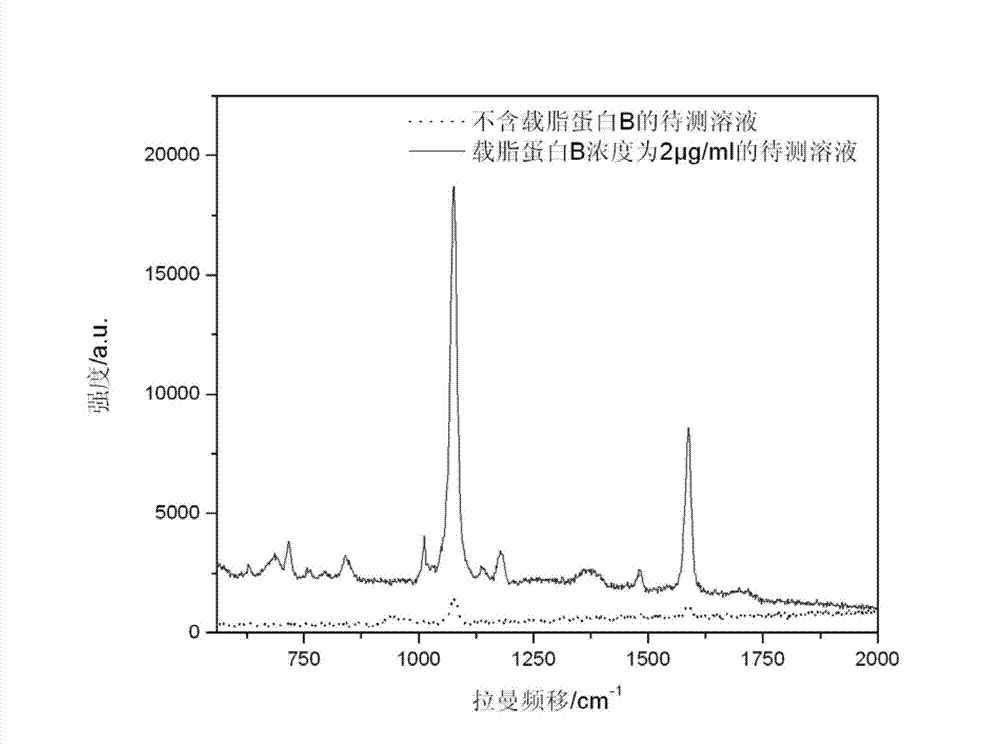
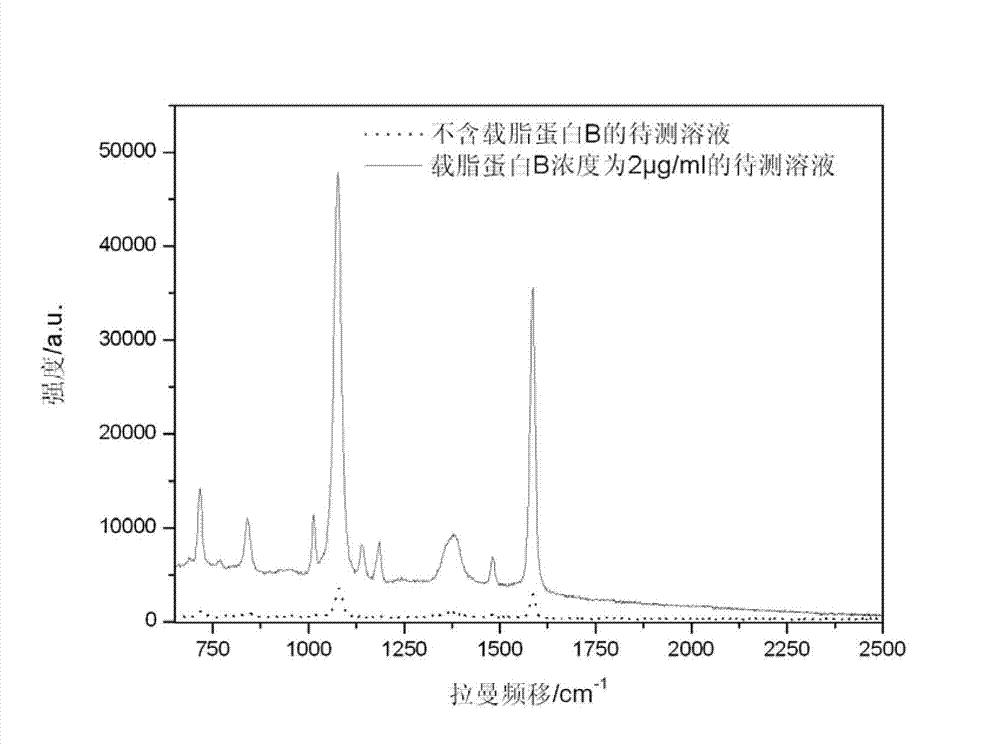
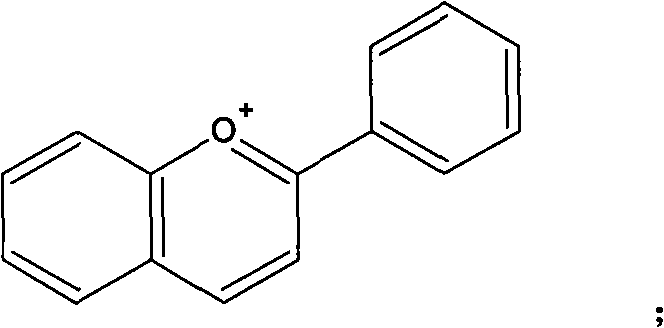
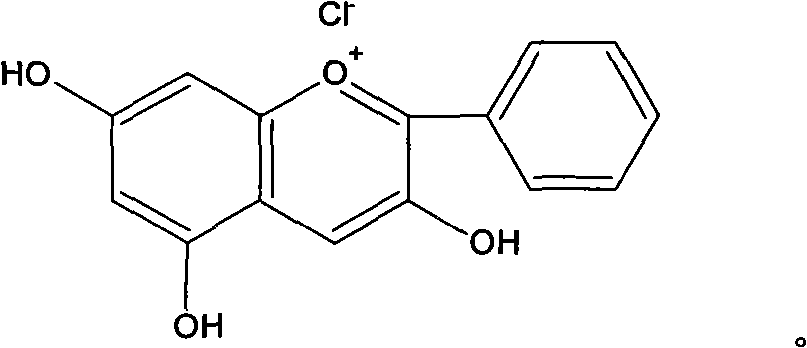
ActiveCN103116019AThe process is simpleLow costRaman scatteringImmune complex depositionRaman scattering
The invention discloses an immune base preparation method and an antigen or antibody immunoassay method. According to the immune base preparation method, a silicon slice is modified by using gold or silver nanoparticles, and antibodies / antigens are modified on the surfaces of the gold or silver nanoparticles, thereby being used for capturing antigens / antibodies to be assayed. According to the antigen or antibody immunoassay method, an immune base which is prepared by using the immune base preparation method and gold or silver nanoparticle immunoprobes is used, an immune base-antigen / antibody-immunoprobe three-layer sandwich structure is formed through an antigen-antibody immune complex reaction, and the assay on the antigens / antibodies to be assayed is realized in a manner that the characteristic dactylograms of Raman markers on the surfaces of the immunoprobes are assayed by using a surface-enhanced Raman scattering effect of the gold or silver nanoparticles. The method has the advantages that the sensitivity of the immunoassay can be greatly improved and the assay on high-flux multiple antigens / antibodies can be carried out.
Owner:NINGBO UNIV
Vaccinium uliginosum cyanidin and separation and purification method thereof
InactiveCN101265252AGuaranteed AntioxidantMaintain biological activityOrganic chemistryBiotechnologyEngineering
The invention relates to an anthocyanidin made from Vaccinium uliginosum L. and separation and purification methods thereof, which belong to the technology field of active component extraction and purification and functional food development. The content of anthocyanidin in Vaccinium uliginosum L. is 29.8-40%, wherein the content of malvidin accounts for 4.68-5.48% of the anthocyanidin; and antioxidant activity of the anthocyanidin is as 7.8-15 times as that of Vc. The preparation method includes pre-treating raw material, cold soaking and concentrating, purifying and concentrating, and freeze dying. The ultrasonic and microwave-assisted cold soaking method is employed for separation, and an AB-8 macroporous resin static-dynamic combined absorption purification method is employed. The invention provides an environmental friendly, high-efficiency and low-cost separation and purification technology of anthocyanidin made from Vaccinium uliginosum L., which maintains product bioactivity such as antioxidant property of product and ideal separation effect, establishes the basis for wide utilization of abundant resource of Vaccinium uliginosum L. and industrialized development promotion of a series of Vaccinium uliginosum L. health food, and embodies great economic benefit and social benefit in deep processing of the Vaccinium uliginosum L.
Owner:吉林云尚保健食品有限公司
Rapid acting and long acting insulin combination formulations
ActiveUS8084420B2Short durationImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderBefore BreakfastInsulin injection
An injectable formulation containing a rapid acting insulin and a long acting insulin has been developed. The pH of the rapid acting insulin is adjusted so that the long acting insulin, remains soluble when they are mixed together. Preferably, the formulation is administered before breakfast, provides adequate bolus insulin levels to cover the meal and basal insulin for up to 24 hours, and does not produce hypoglycemia after the meal. Lunch and dinner can be covered by two bolus injections of a fast, rapid, or very rapid acting insulin. Alternatively, by adjusting the ratio of rapid to long acting insulin, the long acting insulin may be shortened to a 12 hour formulation, and re-administered to the patient at dinner time, providing a safe and effective basal insulin level until morning. As a result, a patient using intensive insulin therapy should only inject three times a day.
Owner:ELI LILLY & CO
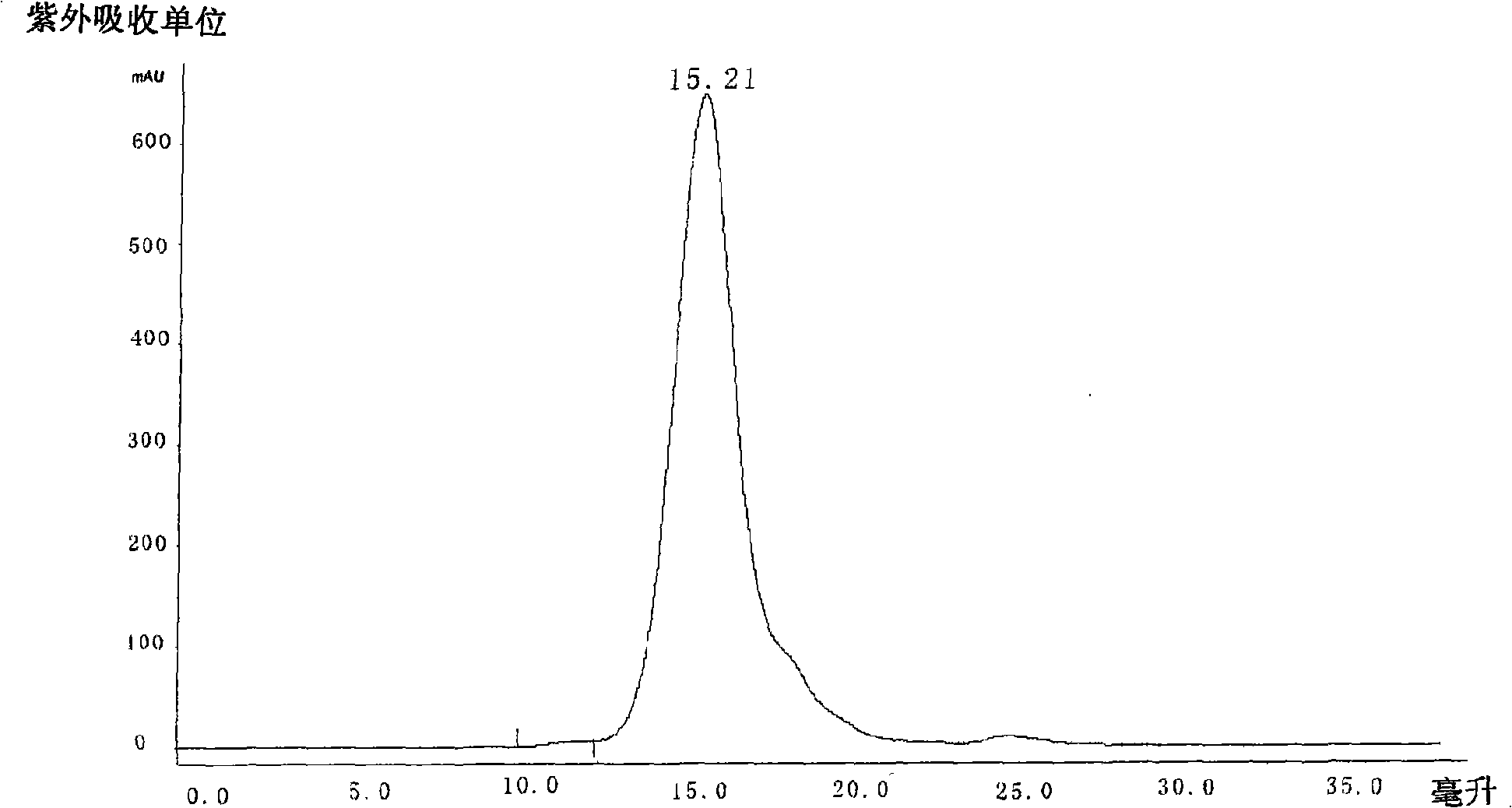
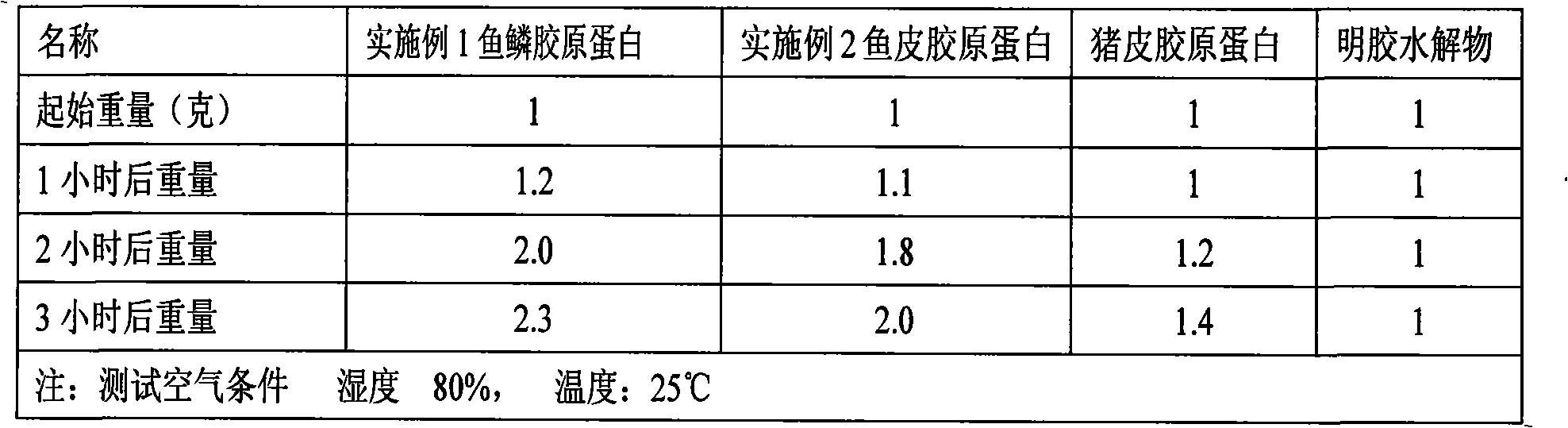
Collagen protein and collagen polypeptides, preparation thereof and applications
ActiveCN101289507AMolecular weight can be controlledControllable distributionOrganic active ingredientsCosmetic preparationsProcess equipmentArthritis
The invention provides a collagen and a collagen polypeptide, wherein, the weight percentage of hydroxylysine is more than 1.3 percent. The invention also provides the preparation technology and the application of the collagen and the collagen polypeptide. The invention can make full use of inexpensive raw materials including the fish skin and fish scale of tilapias to produce products with high added value. The collagen polypeptide is used for treating arthritis with a definite drug effect, and the clinical dose is considerably less than other collagen polypeptides. The collagen polypeptide can solve the pollution problem of fish skin wastes, and at the same time avoid the risk of infectious diseases which can be caused by the collagen of land animals. The collagen and the collagen polypeptide have the advantages of considerable economic benefits, energy conservation and environmental protection, simple process equipment, low cost and easy industrialization.
Owner:史宗洁
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com