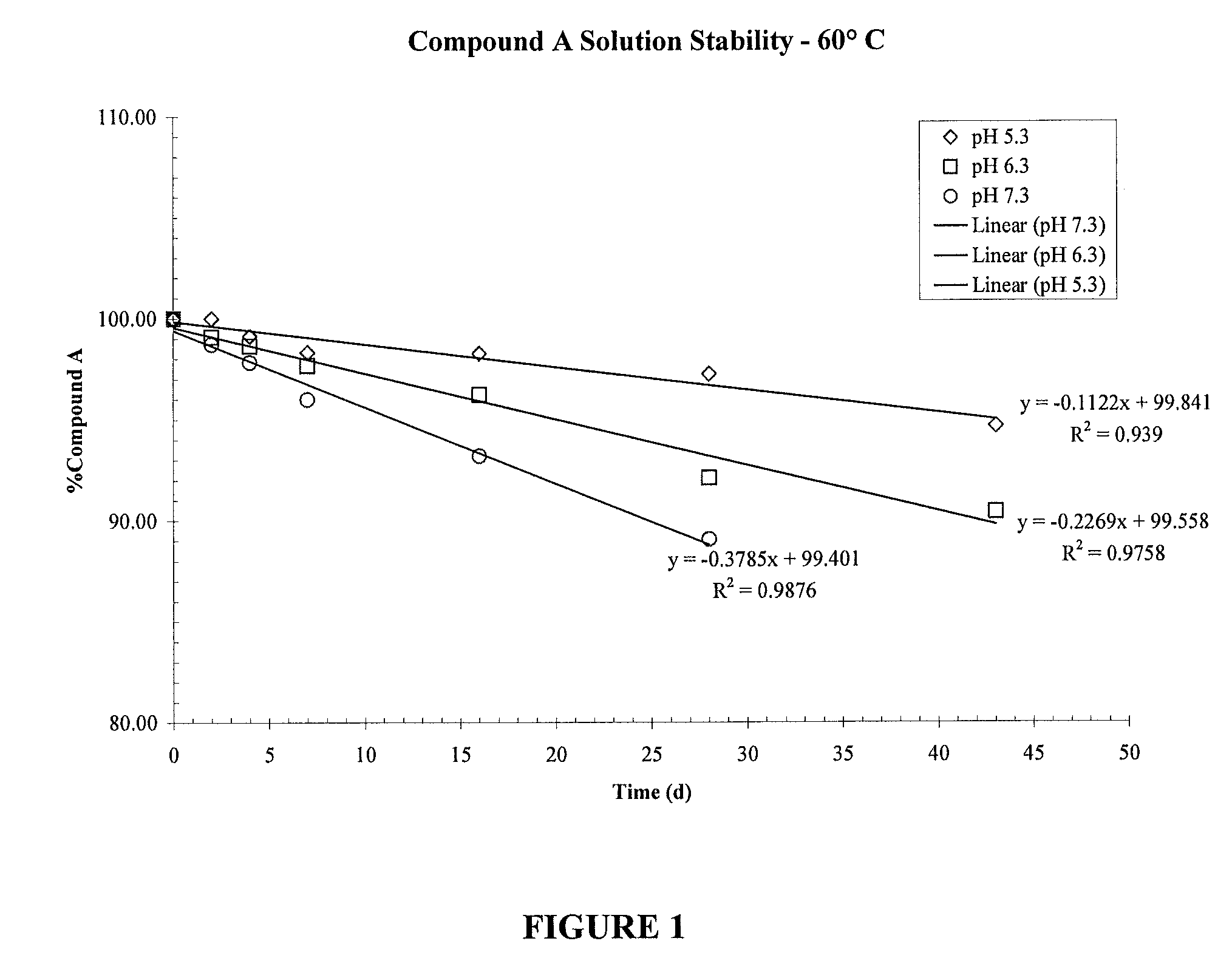
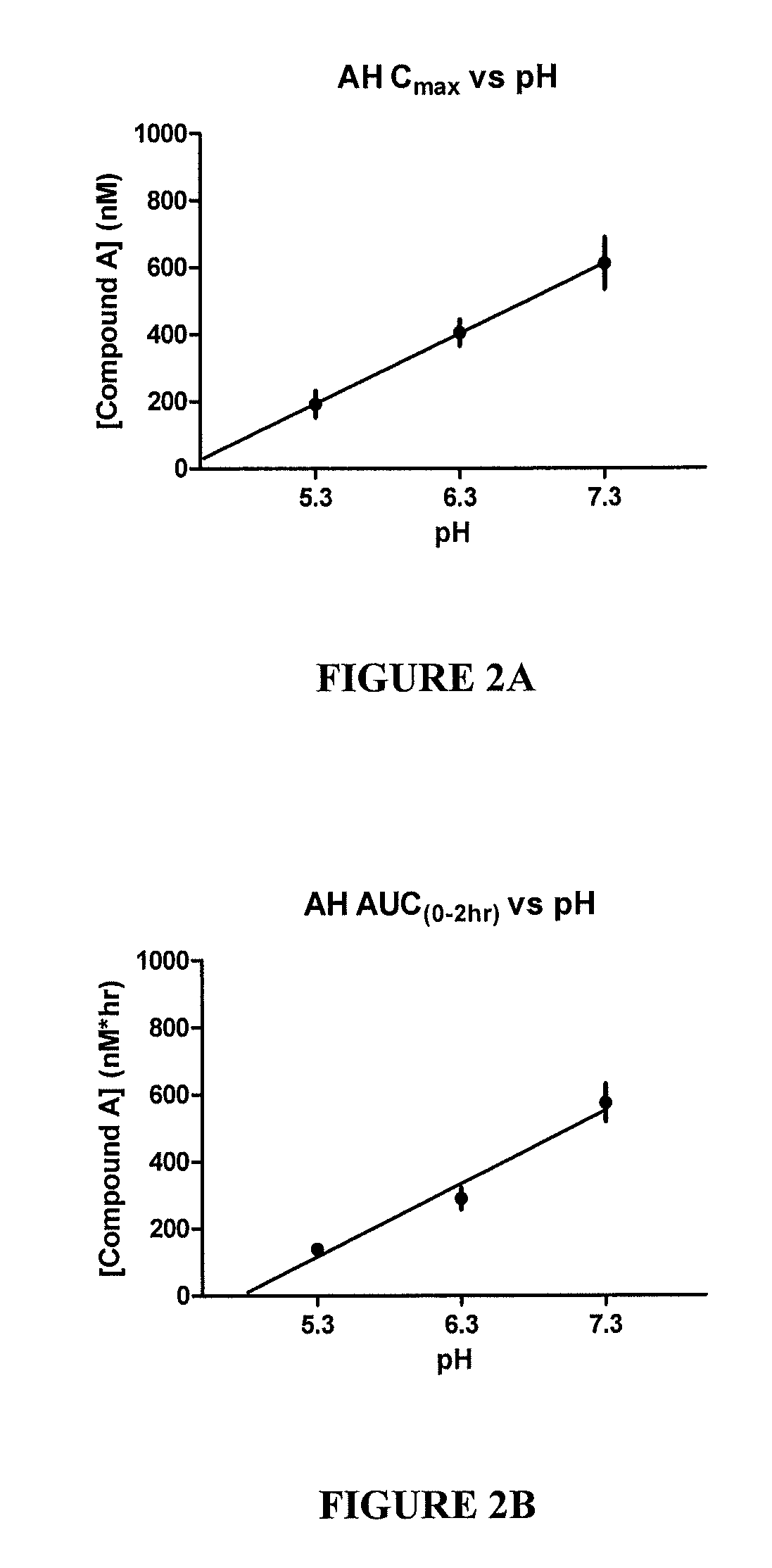
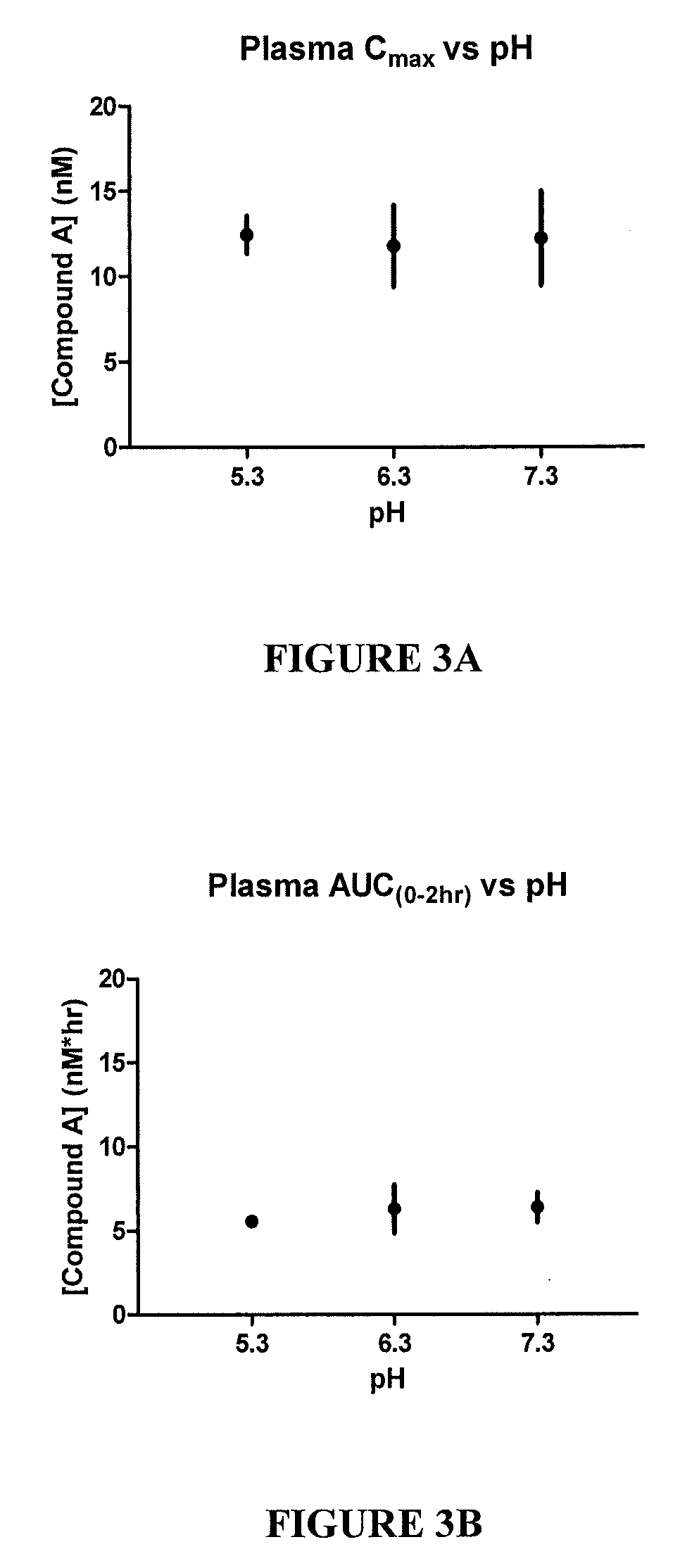
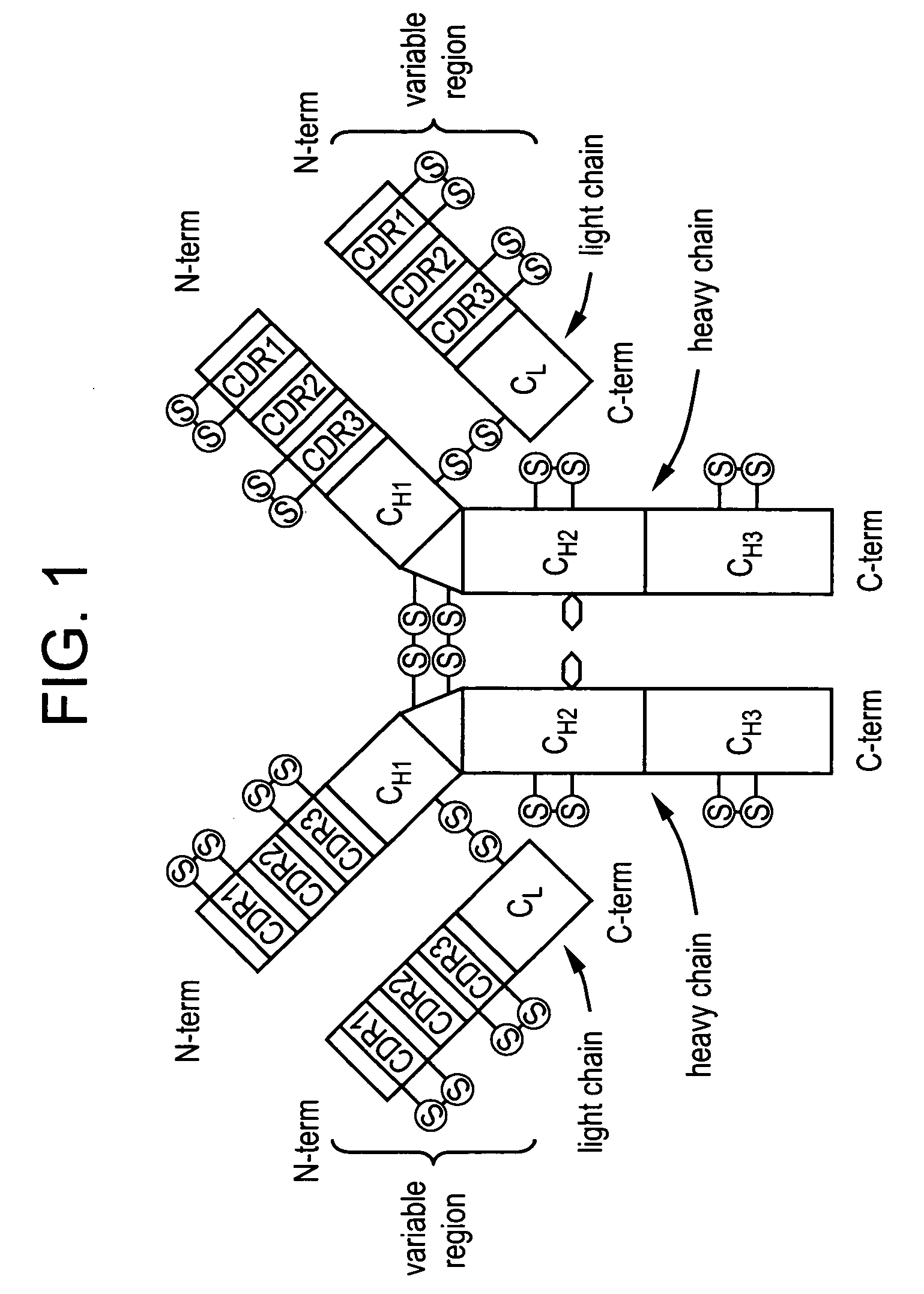
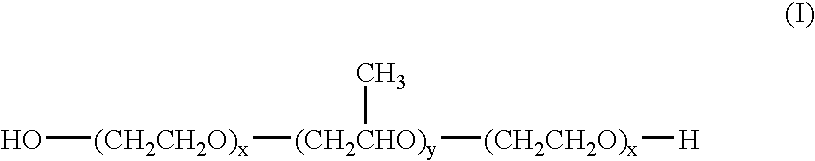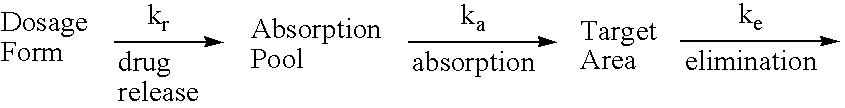Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
208 results about "Tonicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a semipermeable membrane cell membrane. In other words, tonicity is the relative concentration of solutes dissolved in solution which determine the direction and extent of diffusion. It is commonly used when describing the response of cells immersed in an external solution.
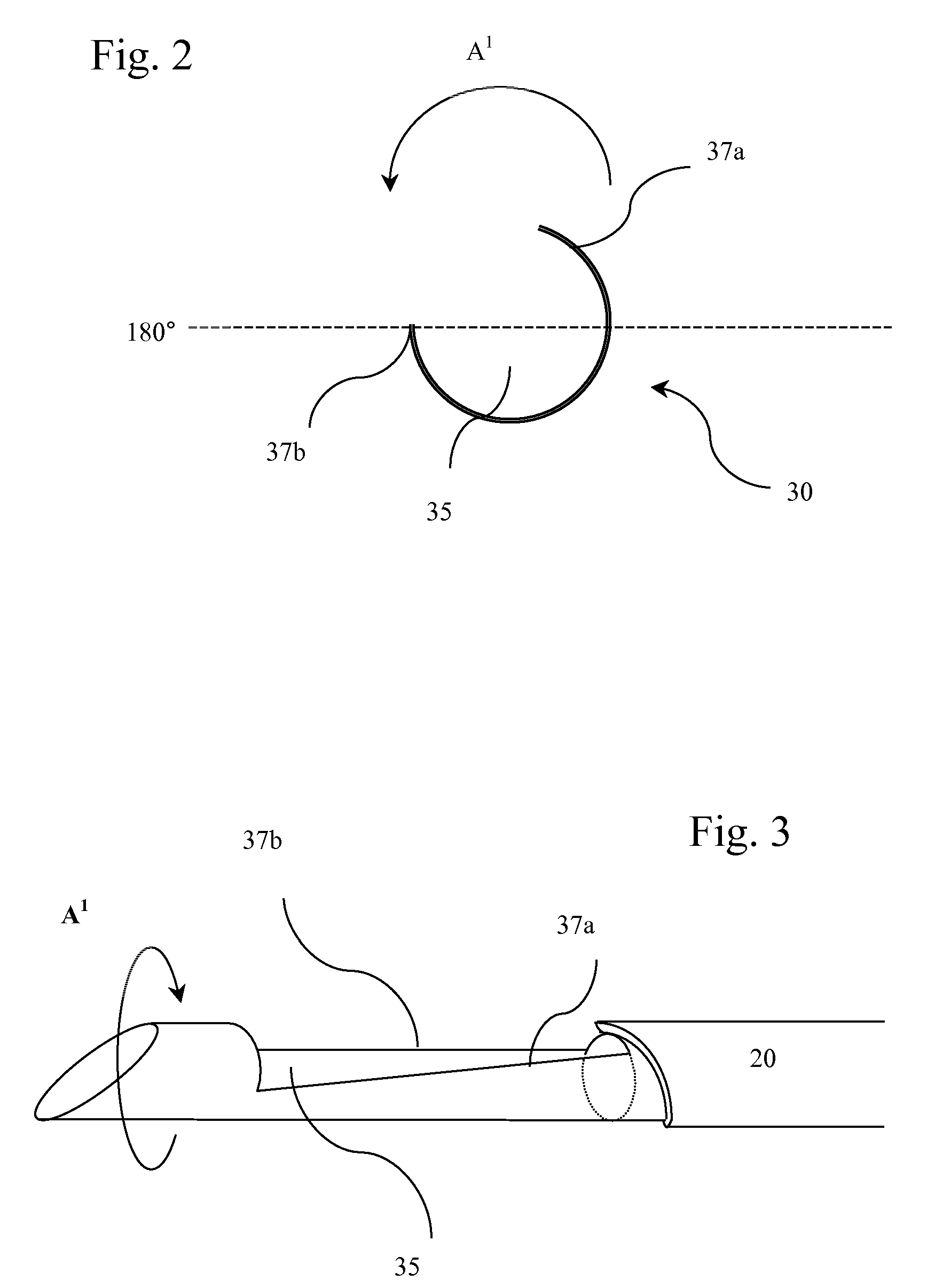
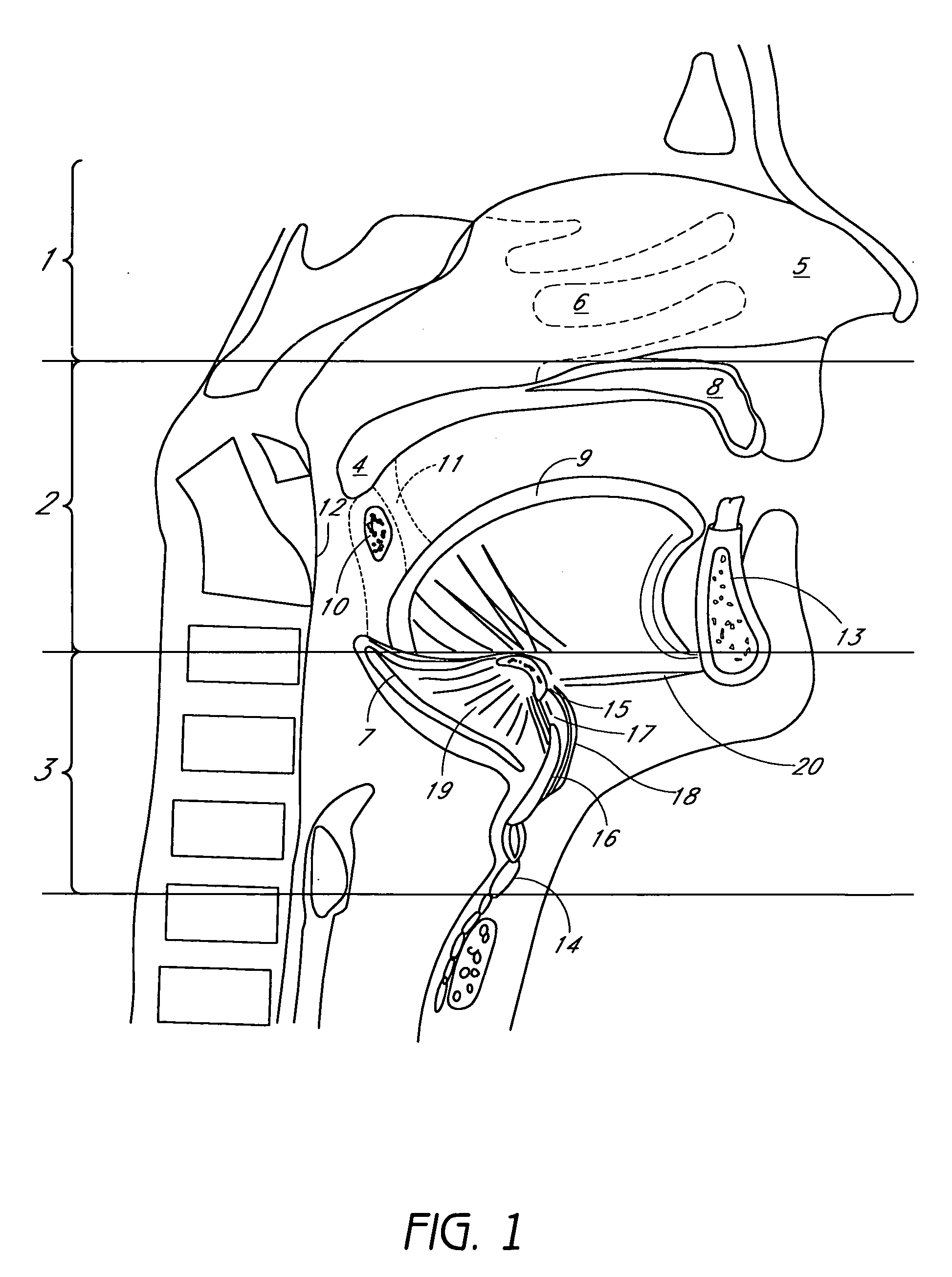
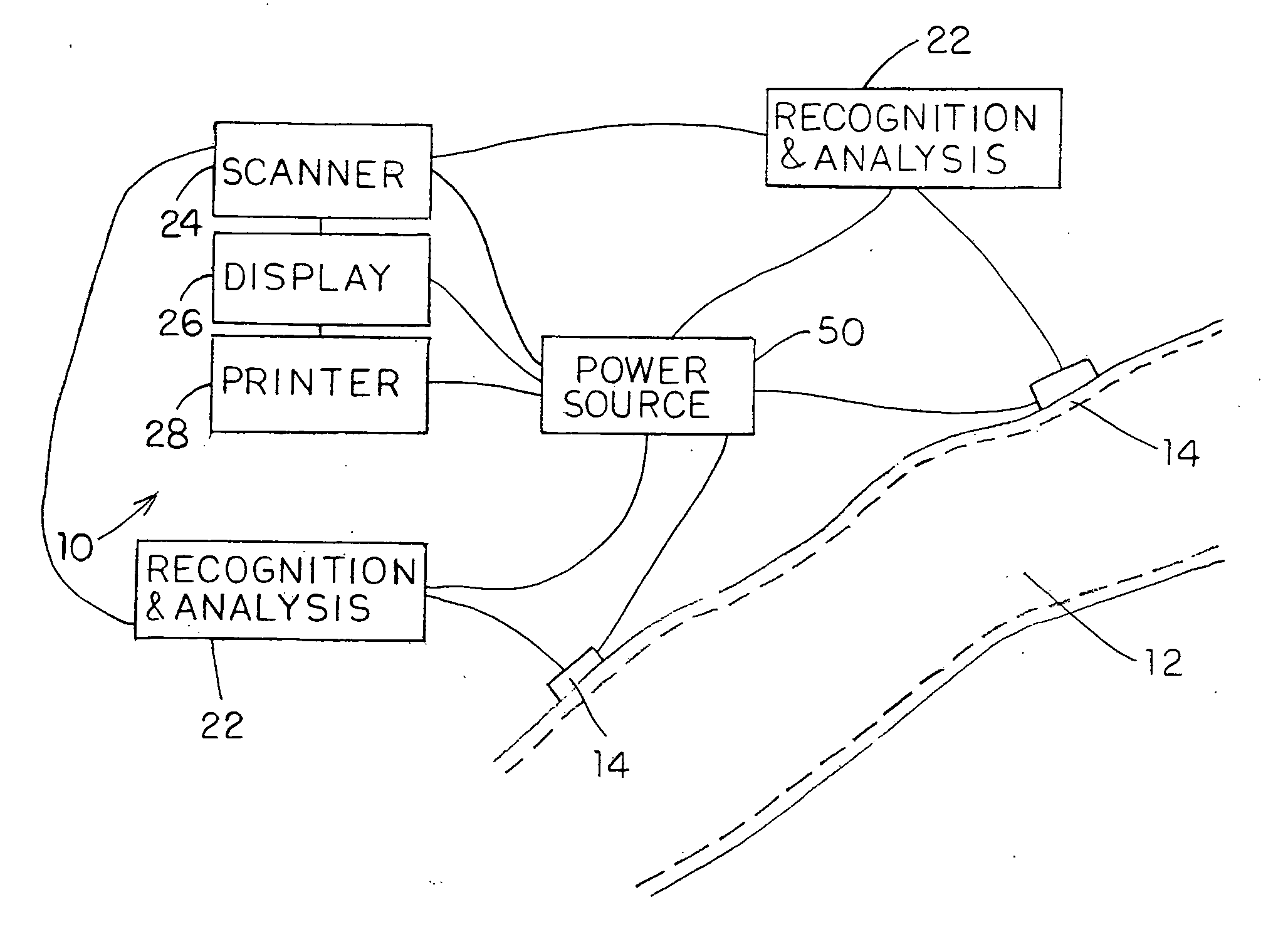
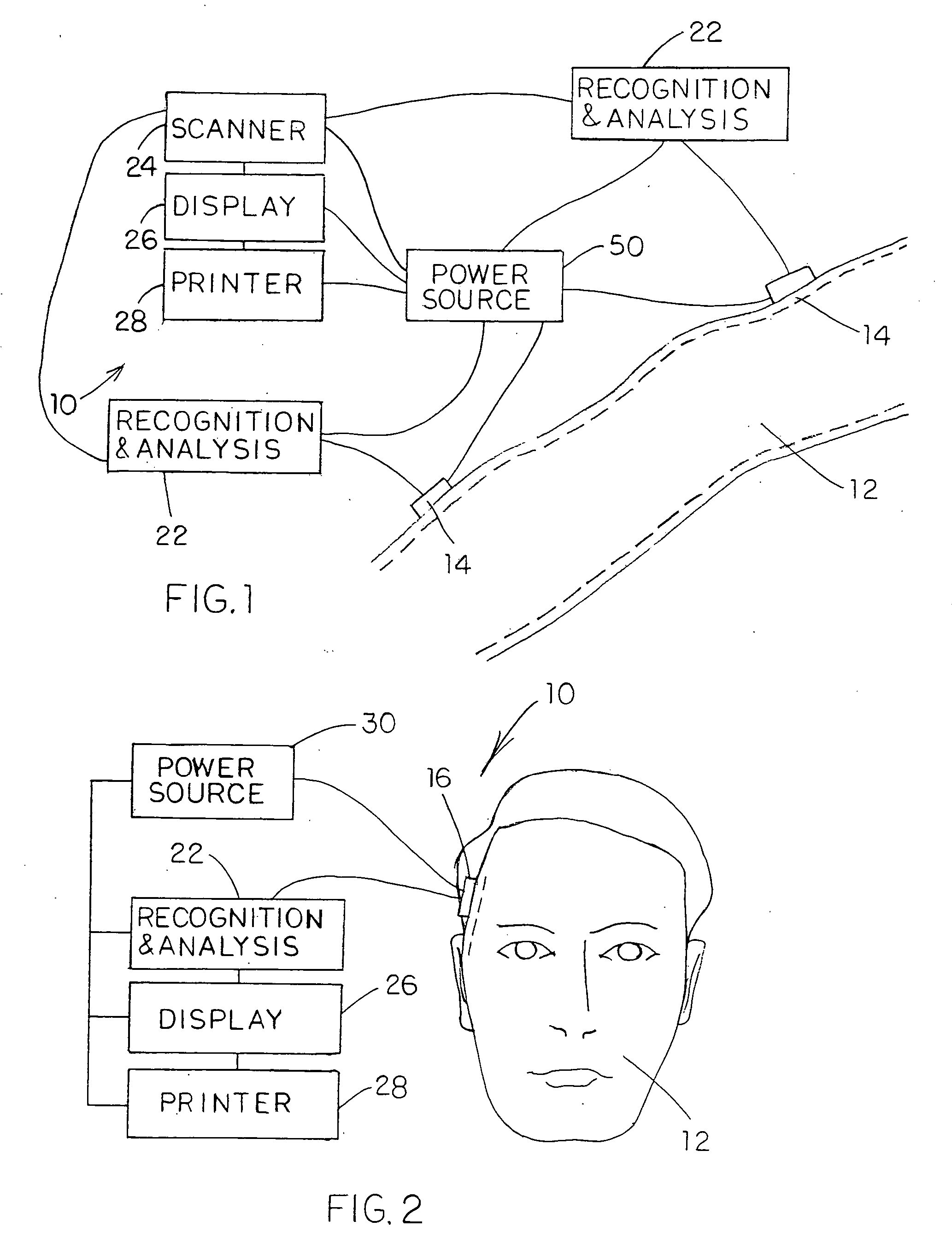
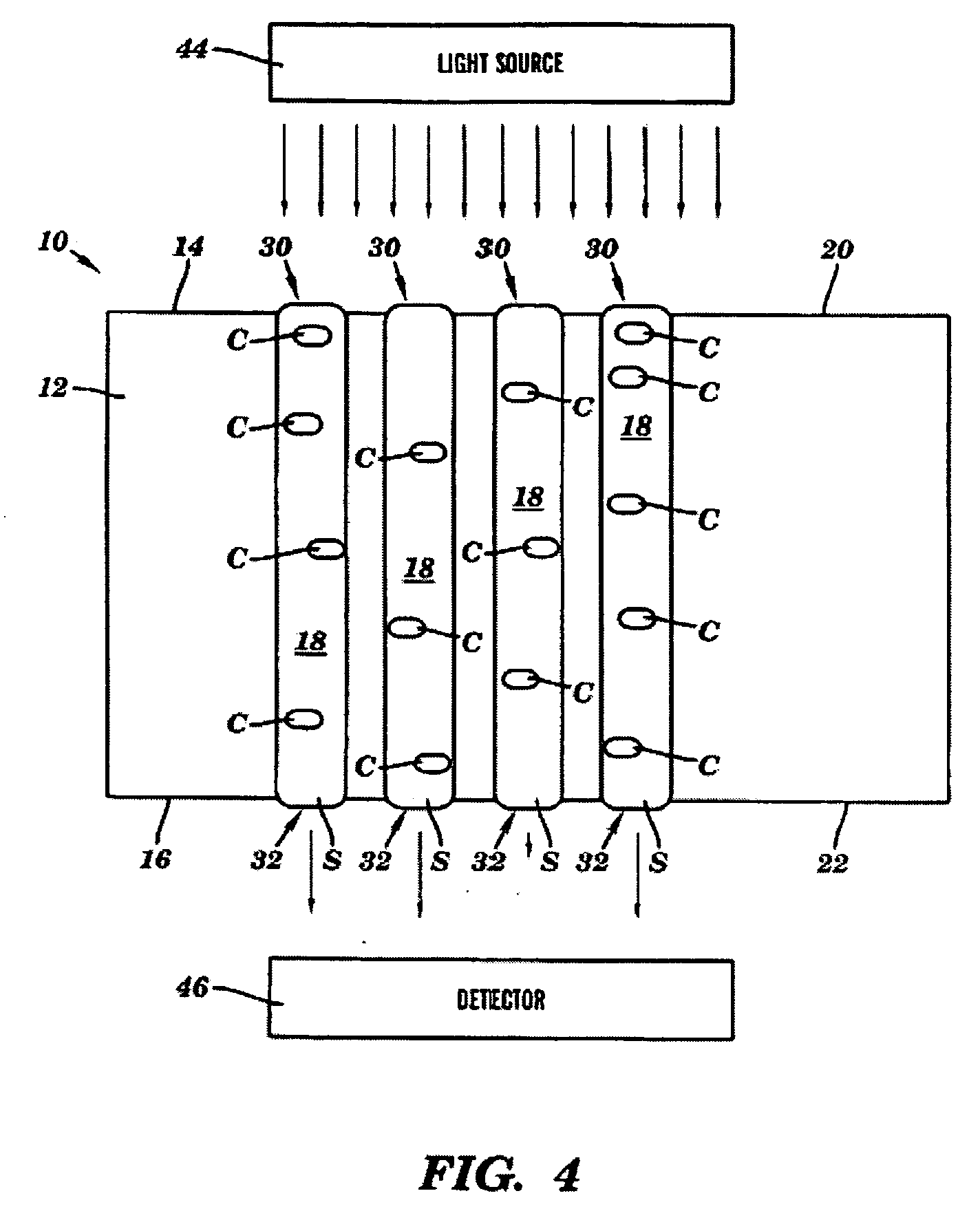
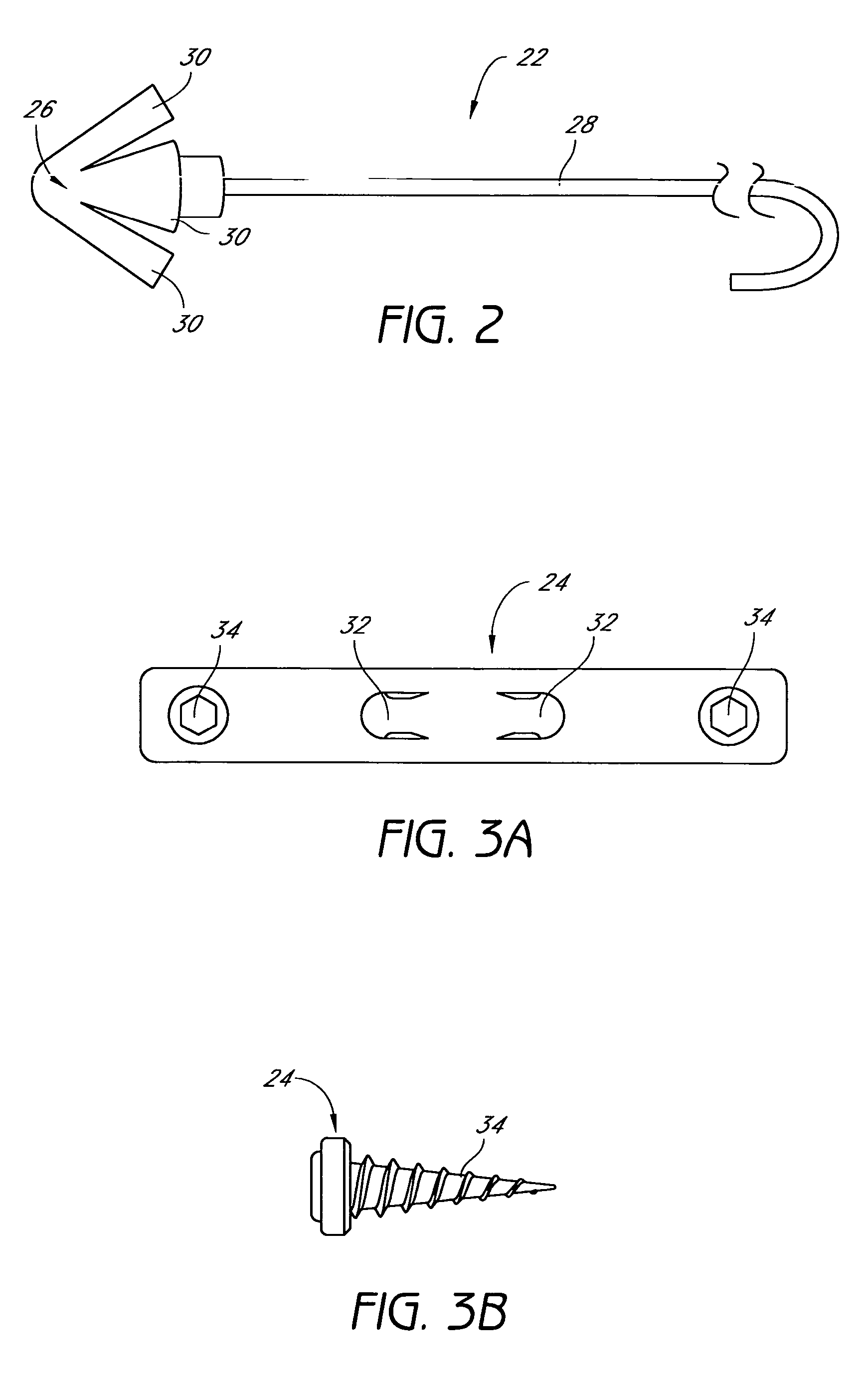
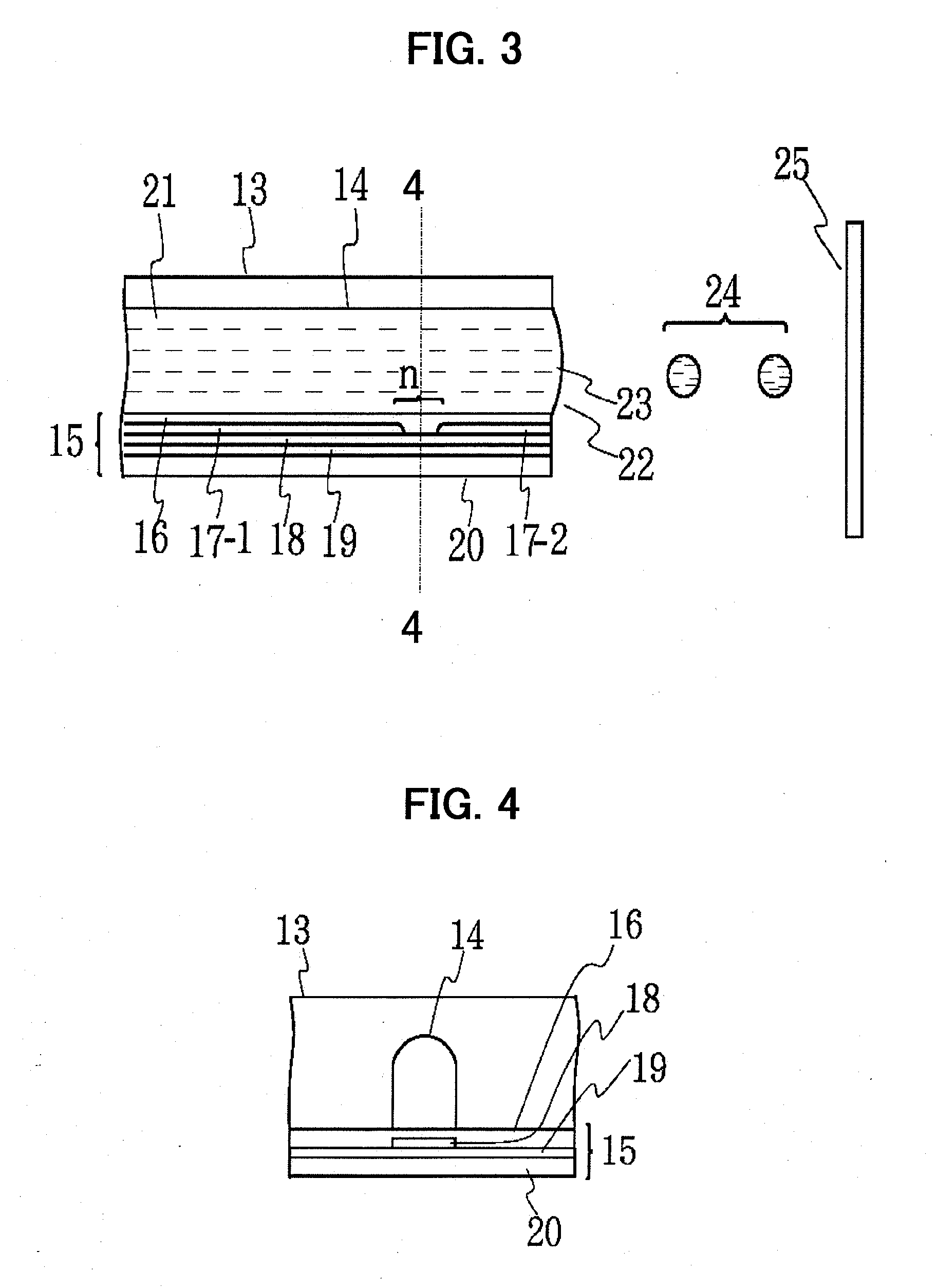
Device for treating mitral valve regurgitation
InactiveUS20070066863A1Reduce lateral distanceReduce fatigueSuture equipmentsHeart valvesHeart chamberTonicity
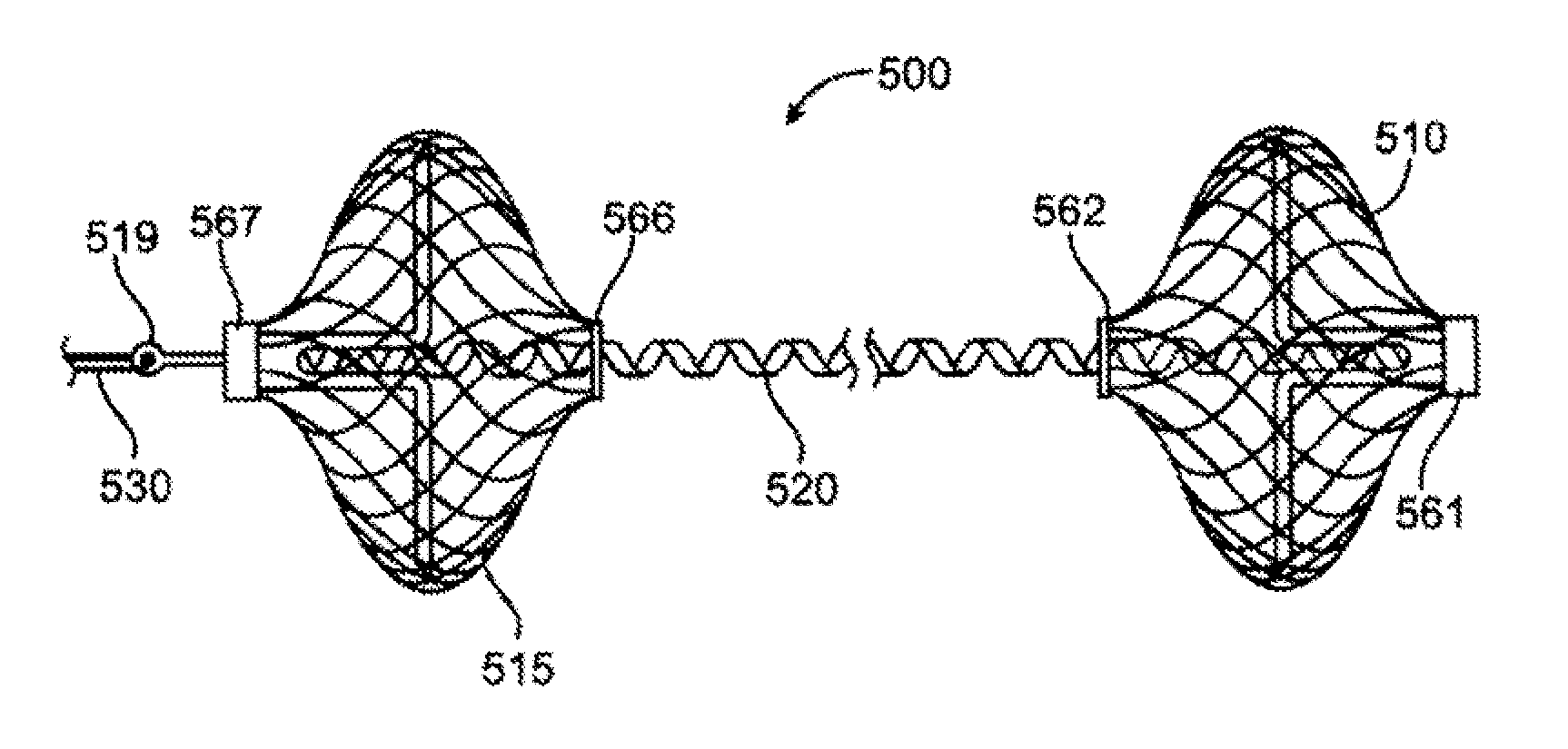
A system for treating mitral valve regurgitation comprises tensioning device that can be deployed using a delivery catheter. The device includes tension member linking a proximal anchor and distal anchor. The device is constructed from a material having suitable elastic properties such that the device applies a constant tension force between the anchors, while stretching or flexing in response to a heartbeat when positioned across a chamber of a heart. The anchors may include a plurality of arms. In some embodiments, the arms may also flex in response to a heart beat. When positioned across the left ventricle of a heart, the device can reduce the lateral distance between the walls of the ventricle and thus allow better coaption of the mitral valve leaflets thereby reducing mitral regurgitation.
Owner:MEDTRONIC VASCULAR INC
Apparatus and method for skin treatment with compression and decompression
InactiveUS20070255355A1Reduce tensionCavity massageSurgical instrument detailsOptical radiationSkin treatments
The present invention generally provides methods and devices that allow more efficient delivery of a stimulus, such as optical radiation, to the skin. In many embodiments, negative and / or positive pressure is applied to one or more skin regions in order to maintain a skin target under tension so as to redistribute blood volume between the skin target and other skin segments. In many cases, such tension can cause a depletion of the volumetric blood content in the skin target (that is, in the blood vessels beneath a surface of the skin target), thereby facilitating delivery of radiation to the skin target.
Owner:PALOMAR MEDICAL TECH
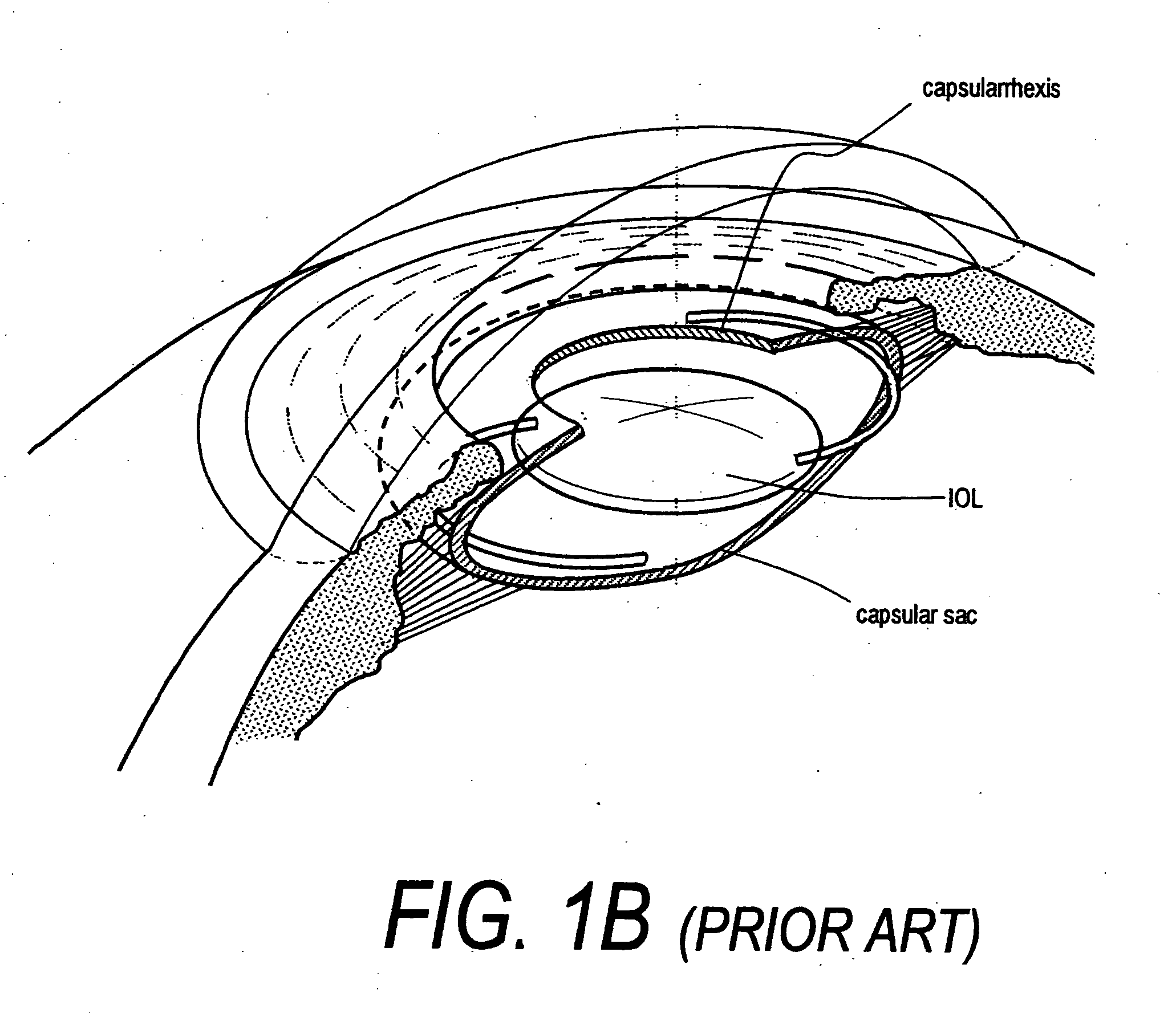
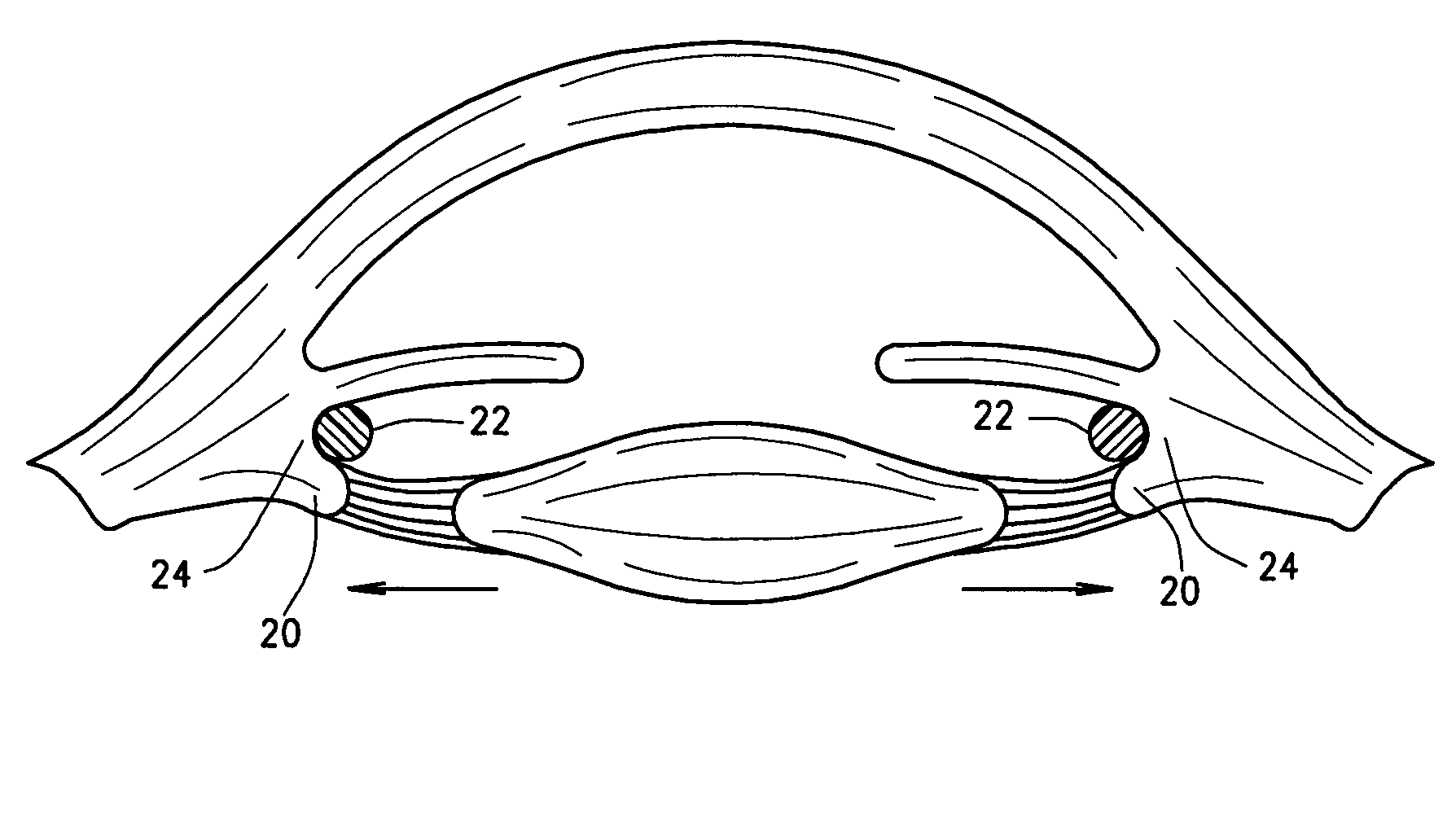
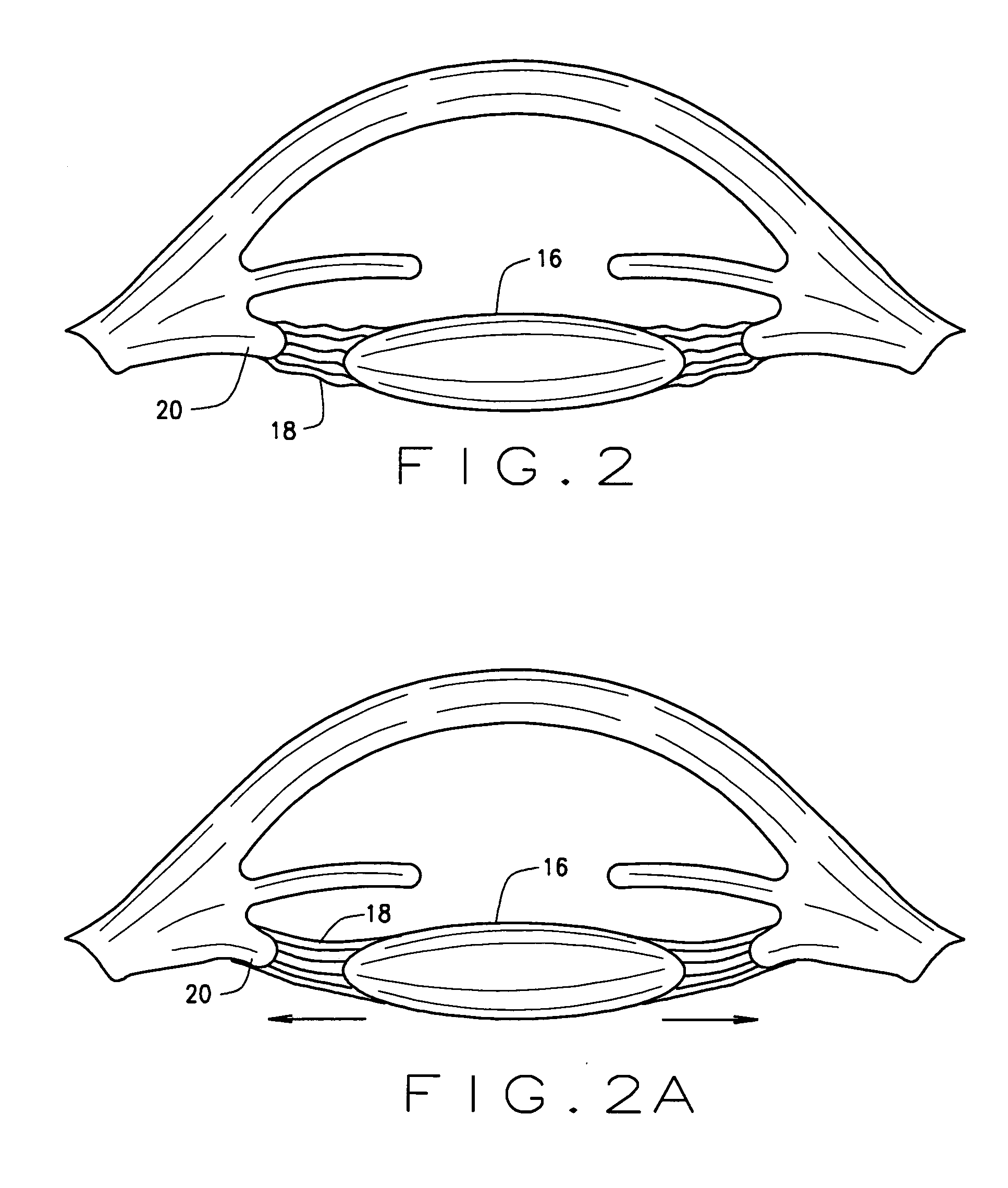
Intraocular lenses and business methods
An intraocular accommodating lens comprising a resilient polymer monolith with a peripheral component that has a Young's modulus and an equilibrium memory shape that imparts to the capsular sac's periphery the natural shape of the capsule in an accommodated state. The intraocular lens includes a central deformable optic that provides accommodated and disaccommodated shapes. The peripheral component is deformable to a disequilibrium, stressed shape in responsive to equatorial tensioning-and is capable of applying restorative forces to move the lens toward the accommodated shape from a disequilibrium disaccommodated shape. In one embodiment, the central optic portion includes a displaceable media than can be displaced by very low forces of zonular excursion, wherein the displaceable media can comprise a very low modulus polymer or an index-matched fluid.
Owner:POWERVISION
Method of using skin compositions including tensioning polymers
InactiveUS20060210513A1Improving skin functionReduce appearance problemsCosmetic preparationsToilet preparationsWrinkle skinThird generation
The invention features a method of treating at least one sign of aging on the skin selected from the group consisting of (i) thickening the skin, (ii) enhancing the barrier function of skin, and / or (iii) treating at least one sign of aging on the skin selected from the group consisting of enhancing the elasticity of said skin, enhancing the firmness of said skin, smoothing the surface of the skin, and reducing the appearance of wrinkles on the skin, wherein the method includes applying to skin in need of such treatment a skin care composition including a tensioning polymer, wherein the tensioning polymer has a contractile force greater than about 3 g / mg and a water resistance index from about 0.9 to about 1.9.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Hair treatment composition and hair cosmetic for damaged hair
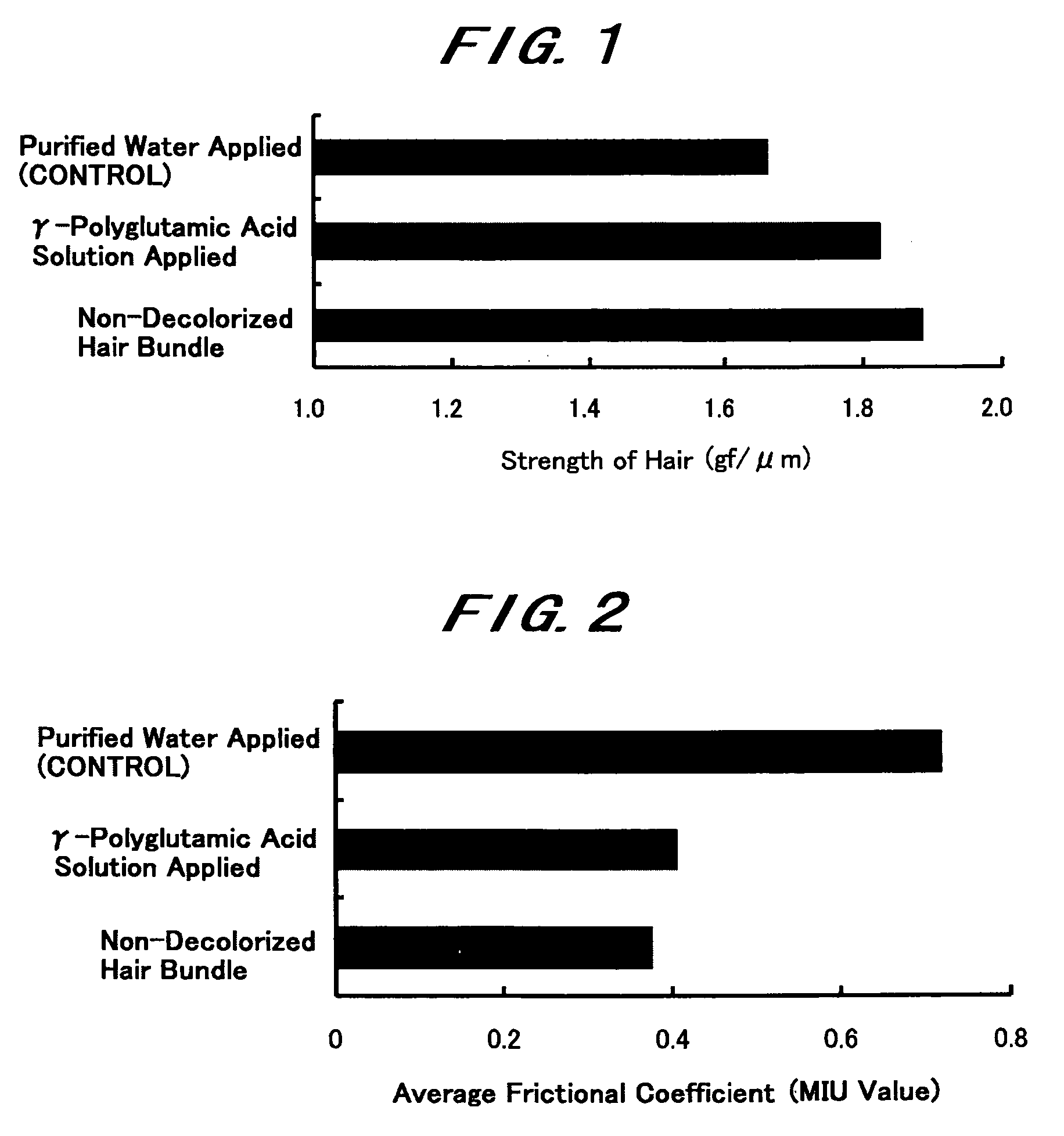
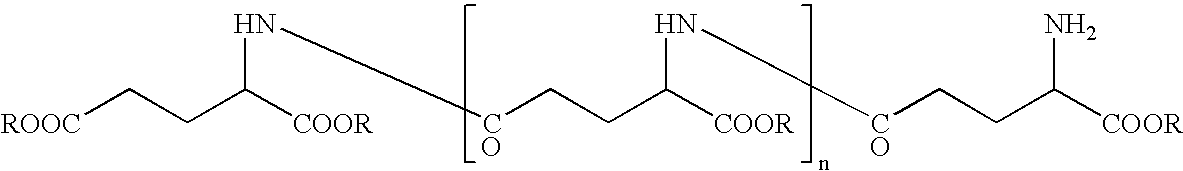
InactiveUS20060165636A1Impaired strengthReduce frictionCosmetic preparationsHair cosmeticsTonicitySplit hair
The present invention intends to provide a composition for hair treatment containing γ-polyglutamic acid or a salt thereof, a hair cosmetic for damaged hair containing such a composition, and their uses. The composition for hair treatment containing γ-polyglutamic acid or a salt thereof and the hair cosmetic for damaged hair of the present invention have excellent improvement effects on the strength and frictional force of hair, so that they can provide tension, elasticity, or the like to damage hair to prevent or alleviate split hair and broken hair as well as improvements in combing and touch. Furthermore, they also exert effects of moisture retention inherent to γ-polyglutamic acid or a salt thereof, preventing or improving effects on the generation of dandruff on the basis of such effects, preventing effects on the feeling of stickiness or creak, and various effectiveness including appropriate residual tendency to hair in a simultaneous manner, respectively.
Owner:MEIJI SEIKA KAISHA LTD
Core Biopsy Device
InactiveUS20070016101A1Quantity maximizationSurgeryVaccination/ovulation diagnosticsRadiologyTissue sample
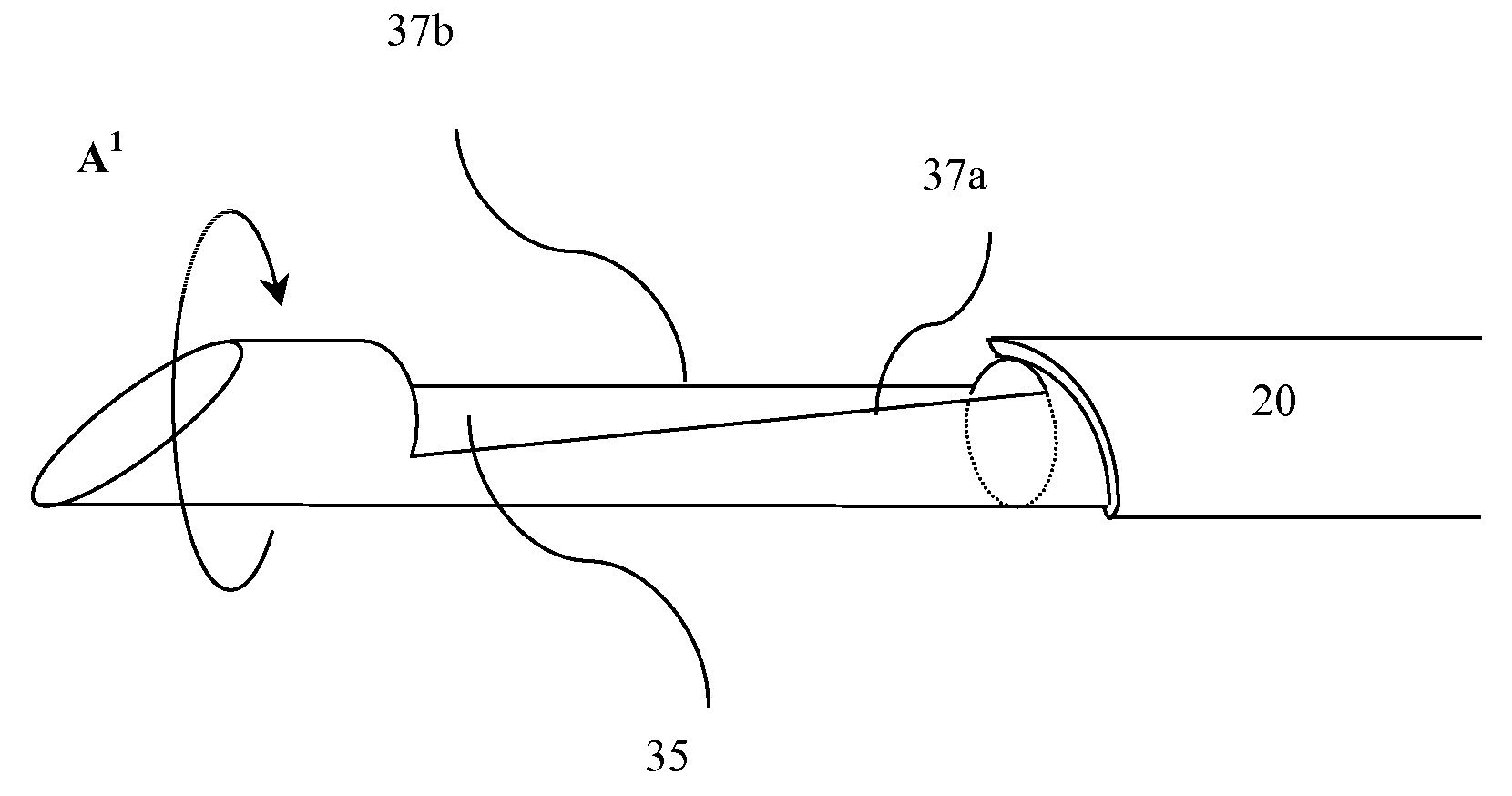
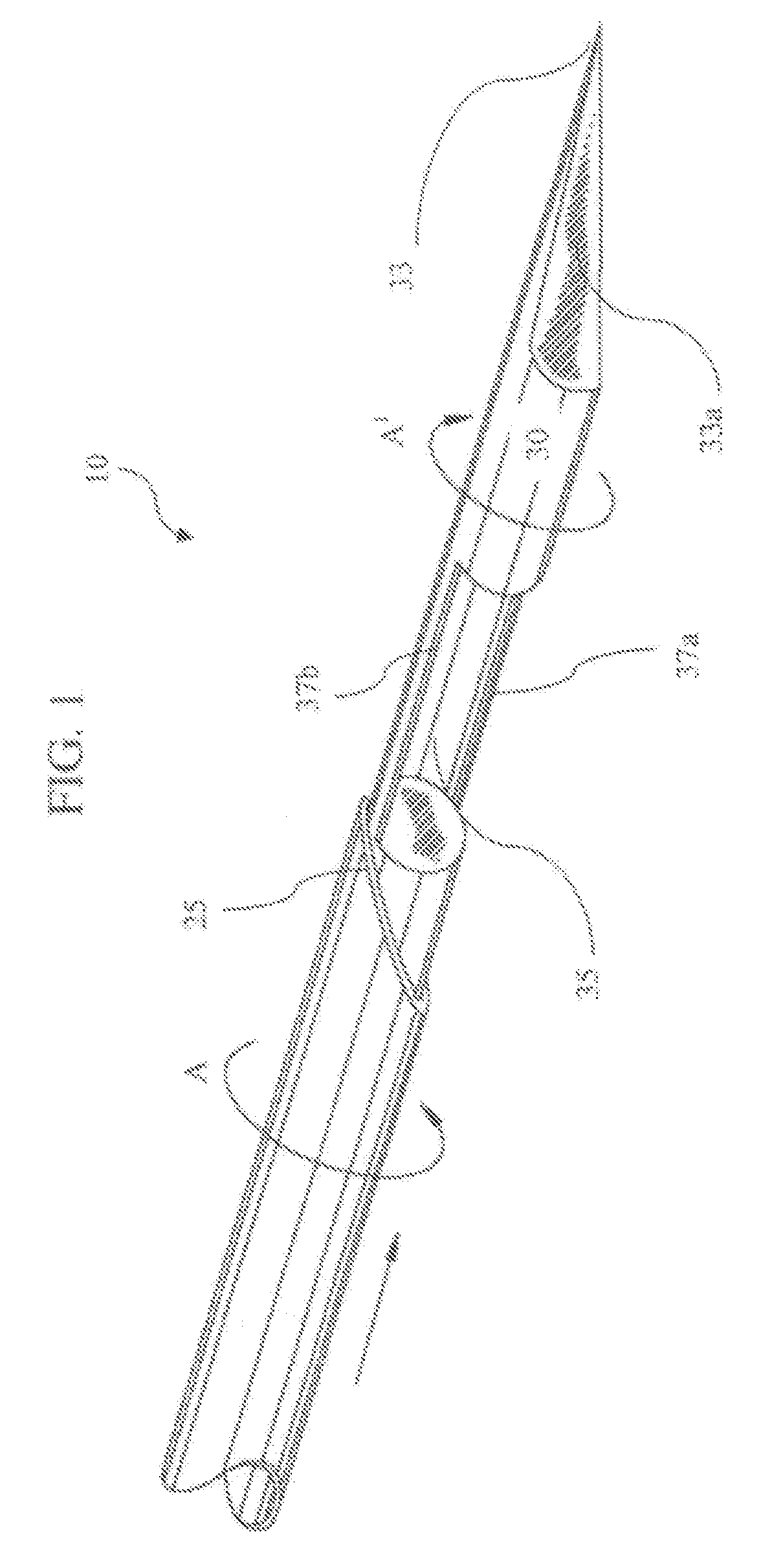
A biopsy device used to capture a tissue sample by placing the tissue under tension, thus allowing a greater sized sample with increased quality. The device uses a rotating cannula and stylet with a concave “sample notch.” The rotation of the stylet places the tissue under tension while the rotation of the cannula as it is fired severs the sample.
Owner:FELDMAN DENNIS D +3
Suspension formulations of nepafenac and other ophthalmic drugs for topical treatment of ophthalmic disorders
Topical aqueous suspension compositions of sparingly soluble ophthalmic drugs are disclosed. The compositions comprise a combination of a poloxamer or meroxapol surfactant and a glycol tonicity-adjusting agent such as propylene glycol.
Owner:NOVARTIS AG
Topically applied Glucosamine Sulfate and all its related, precursor, and derivative compounds significantly increases the skin's natural produciton of hyaluronic acid for the rejuvenation of healthier younger-looking skin; while PhosphatidylCholine is required to replace its deficiency caused by topical Dimethylaminoethanol (DMAE)
InactiveUS20070092469A1Reducing eczemaReducing psoriasisBiocideCosmetic preparationsWrinkle skinPhysiology
A topical skin rejuvenation preparation to relieve wrinkles, increase the skin's natural production of hyaluronic acid, reverse the lack of suppleness, hydrate from within, erase spider veins, reduce varicose veins, lighten aging dark blotches (“liver spots” / Lentigos, Senile Lentigines), decrease acne, and reduce under eye puffiness includes Glucosamine (2-amino-2-deoxy-alpha-D-glucose), a hexosamine (6 carbon amino sugar), including its derivative and precursor compounds: Glucosamine Sulfate, Glucosamine Hydrochloride, Glucose-6-Phosphate, Acetyl Glucosamine, Fructose-6-phosphate, Glucosamine-6-Phosphate, to increase production of Hyaluronic acid and collagen from Glucosamine Sulfate, its precursors and derivatives and to increase skin muscle tone by Dimethylaminoethanol (DMAE) while over coming deficiency it creates in each cell's production of PhosphatidylCholine, whose deficiency damages cell membranes, as well as mitochondrial and lysosome membranes.
Owner:JACOBS ERIC
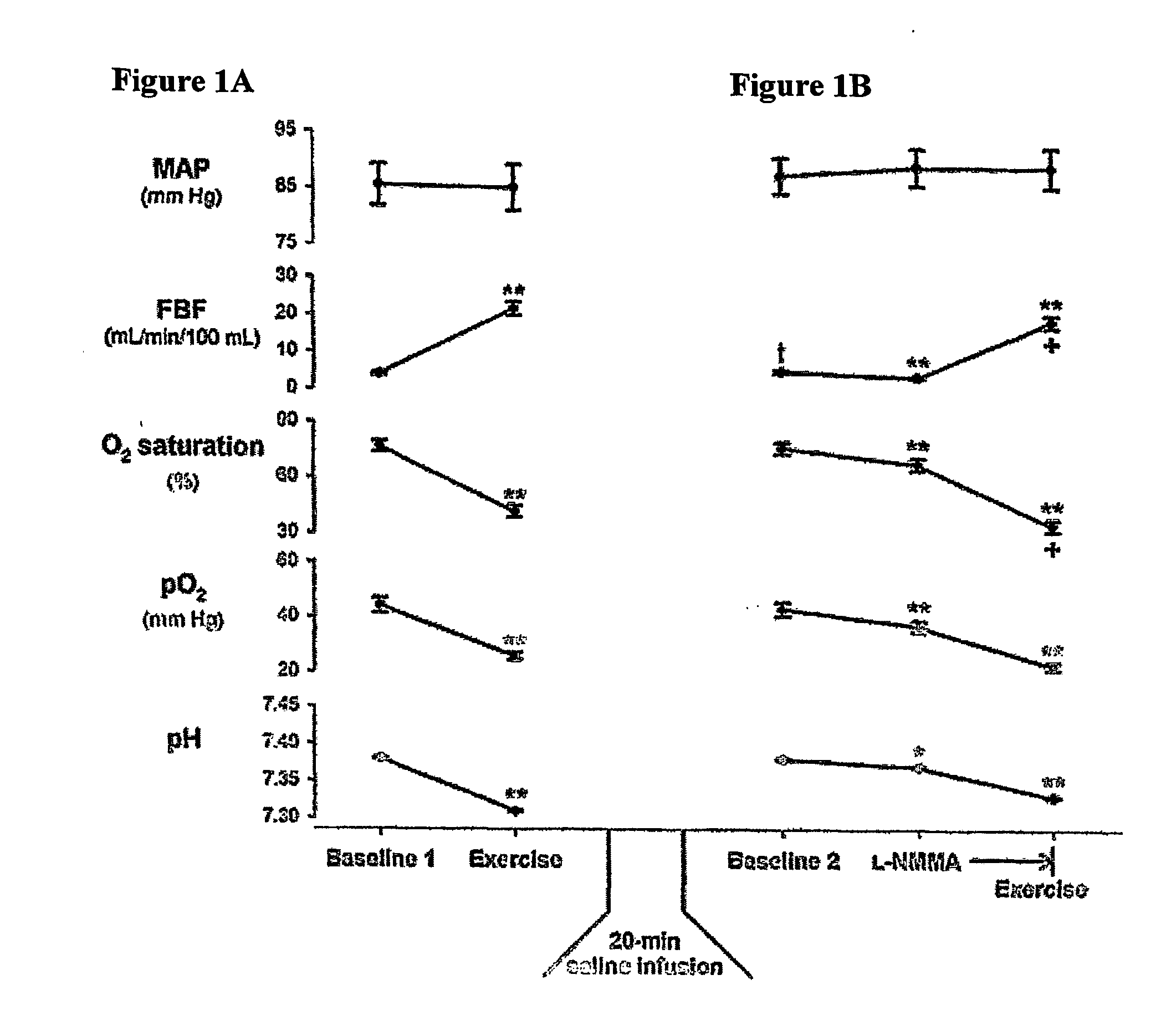
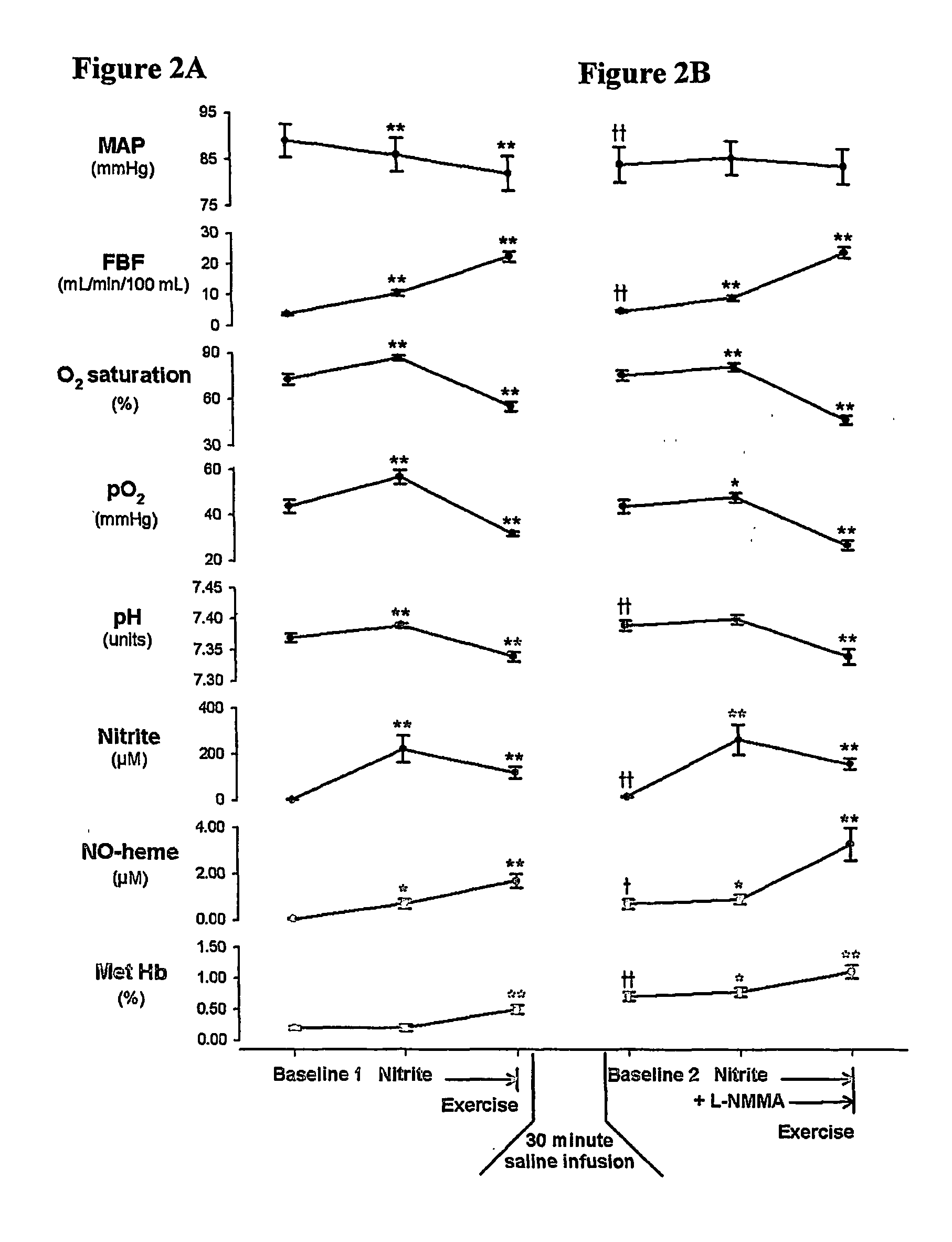
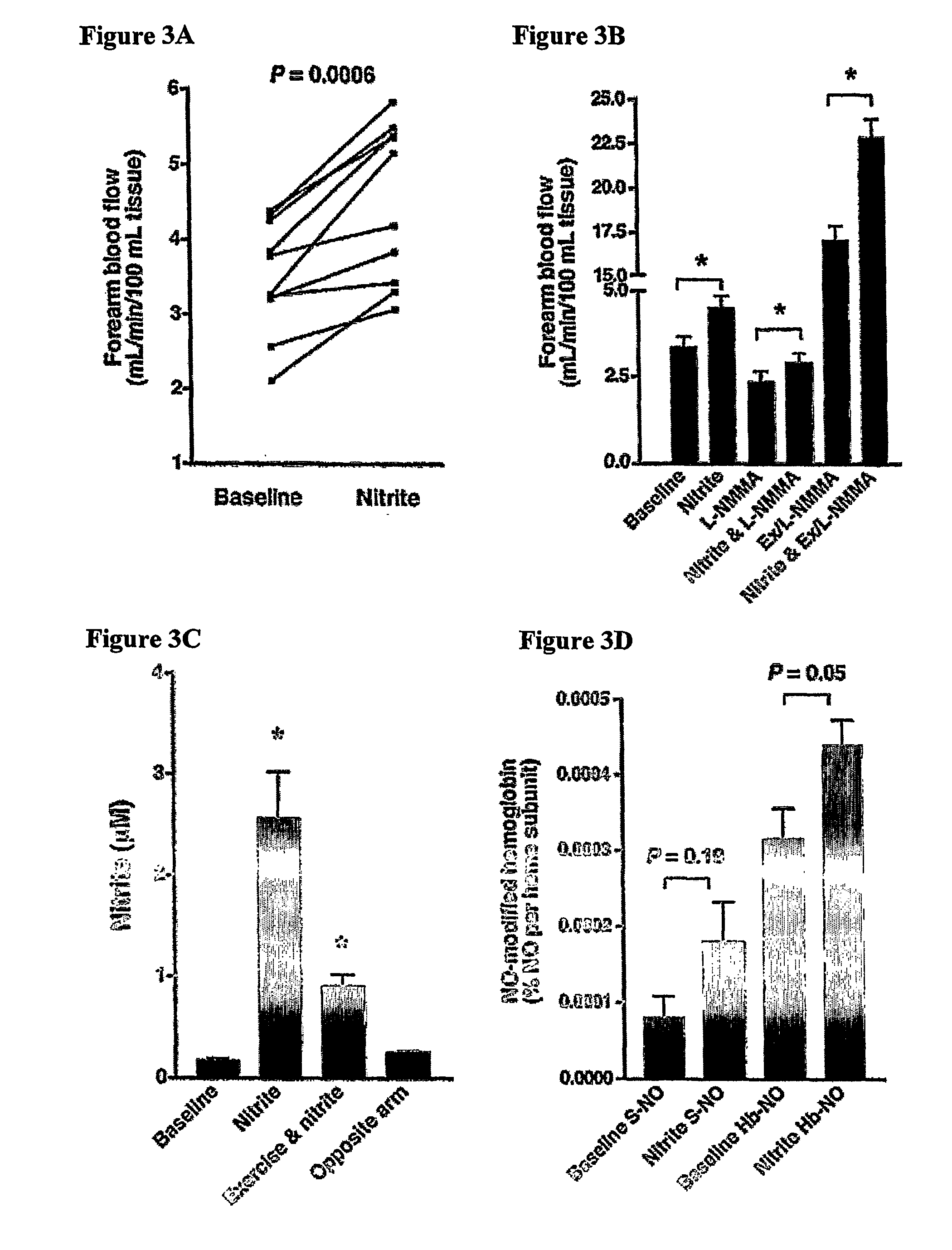
Use of nitrite salts for the treatment of cardiovascular conditions
ActiveUS20070154569A1Increase blood flowEffective vasodilatorBiocideNervous disorderReperfusion injuryBlood flow
It has been surprisingly discovered that administration of nitrite to subjects causes a reduction in blood pressure and an increase in blood flow to tissues. The effect is particularly beneficial, for example, to tissues in regions of low oxygen tension. This discovery provides useful treatments to regulate a subject's blood pressure and blood flow, for example, by the administration of nitrite salts. Provided herein are methods of administering a pharmaceutically-acceptable nitrite salt to a subject, for treating, preventing or ameliorating a condition selected from: (a) ischemia-reperfusion injury (e.g., hepatic or cardiac or brain ischemia-reperfusion injury); (b) pulmonary hypertension (e.g., neonatal pulmonary hypertension); or (c) cerebral artery vasospasm.
Owner:WAKE FOREST UNIV +4
Treatment of female sexual dysfunction with vasoactive intestinal polypeptide agonists
InactiveUS7226910B2Good for healthImprove the lubrication effectBiocidePeptide/protein ingredientsObstetricsActive agent
Owner:VIVUS
Anti a beta antibody formulation
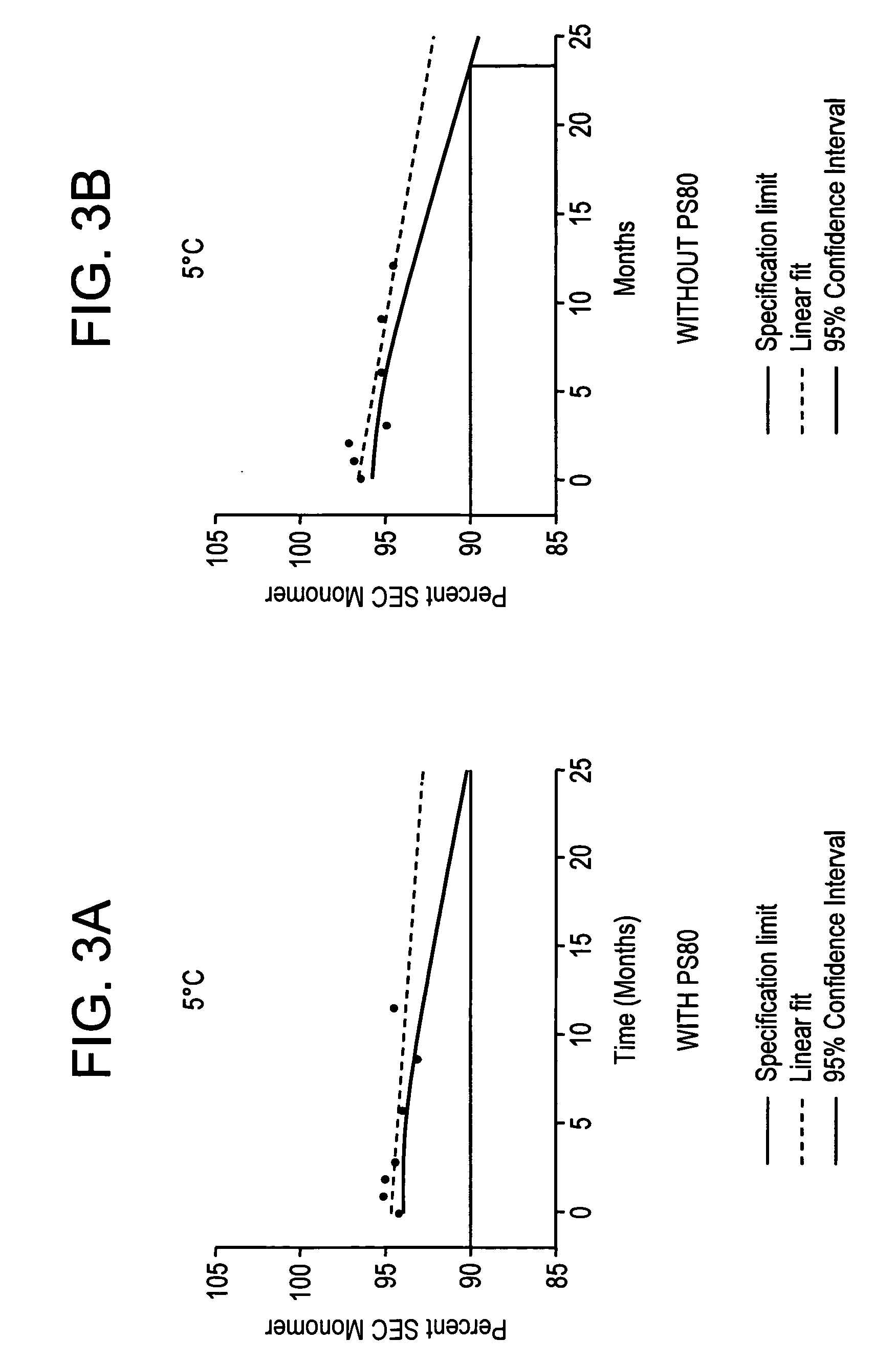
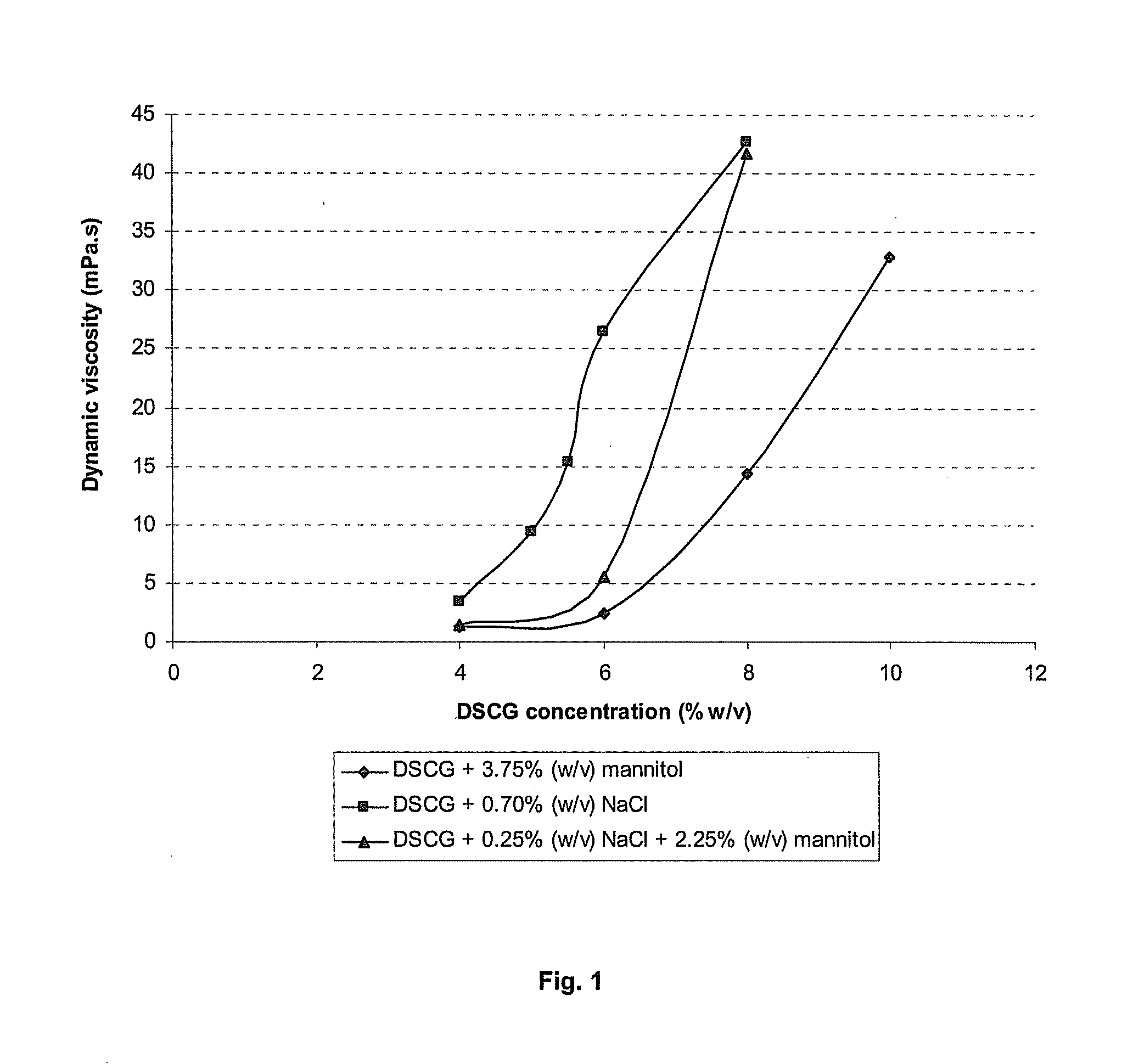
ActiveUS20060193850A1Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
Particulate water absorbent agent and production method thereof, and water absorbent article
ActiveUS20050209352A1Improve liquidityImprove water absorptionOther chemical processesAbsorbent padsParticulatesSaline water
A particulate water absorbing agent of the present invention includes a water absorbent resin, having a cross-linking structure, whose surface has been cross-linked by adding a surface treatment agent, wherein: (i) a mass average particle diameter (D50) ranges from 200 to 600 μm and 95 to 100 wt % of a particulate water absorbing agent whose particle diameter ranges from less than 850 μm to not less than 150 μm is contained with respect to 100 wt % of whole the particulate water absorbing agent, and (ii) a logarithmic standard deviation (σζ) of particle size distribution ranges from 0.25 to 0.45, and (iii) a compressibility rate defined by a following equation ranges from 0 to 18%, and (iv) a surface tension of a supernatant liquid obtained in 4 minutes after dispersing 0.5 g of the particulate water absorbing agent in 50 ml of physiological saline whose temperature is 20° C. is 55 mN / m or more, the compressibility rate (%)=(P−A) / P×100 where P represents a tapped bulk density of the particulate water absorbing agent and A represents a loose bulk density of the particulate water absorbing agent.
Owner:NIPPON SHOKUBAI CO LTD
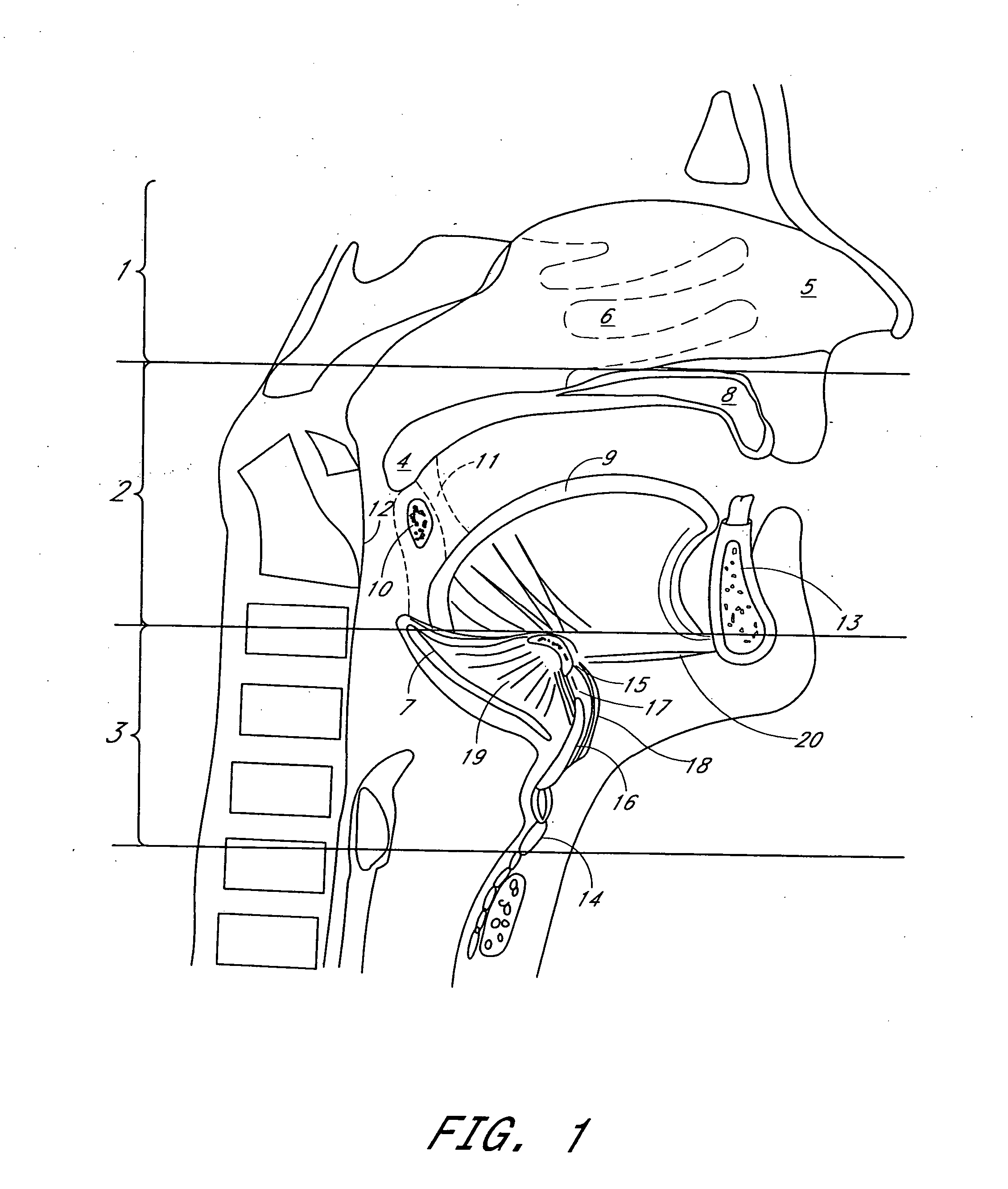
Method for adjusting tissue anchors
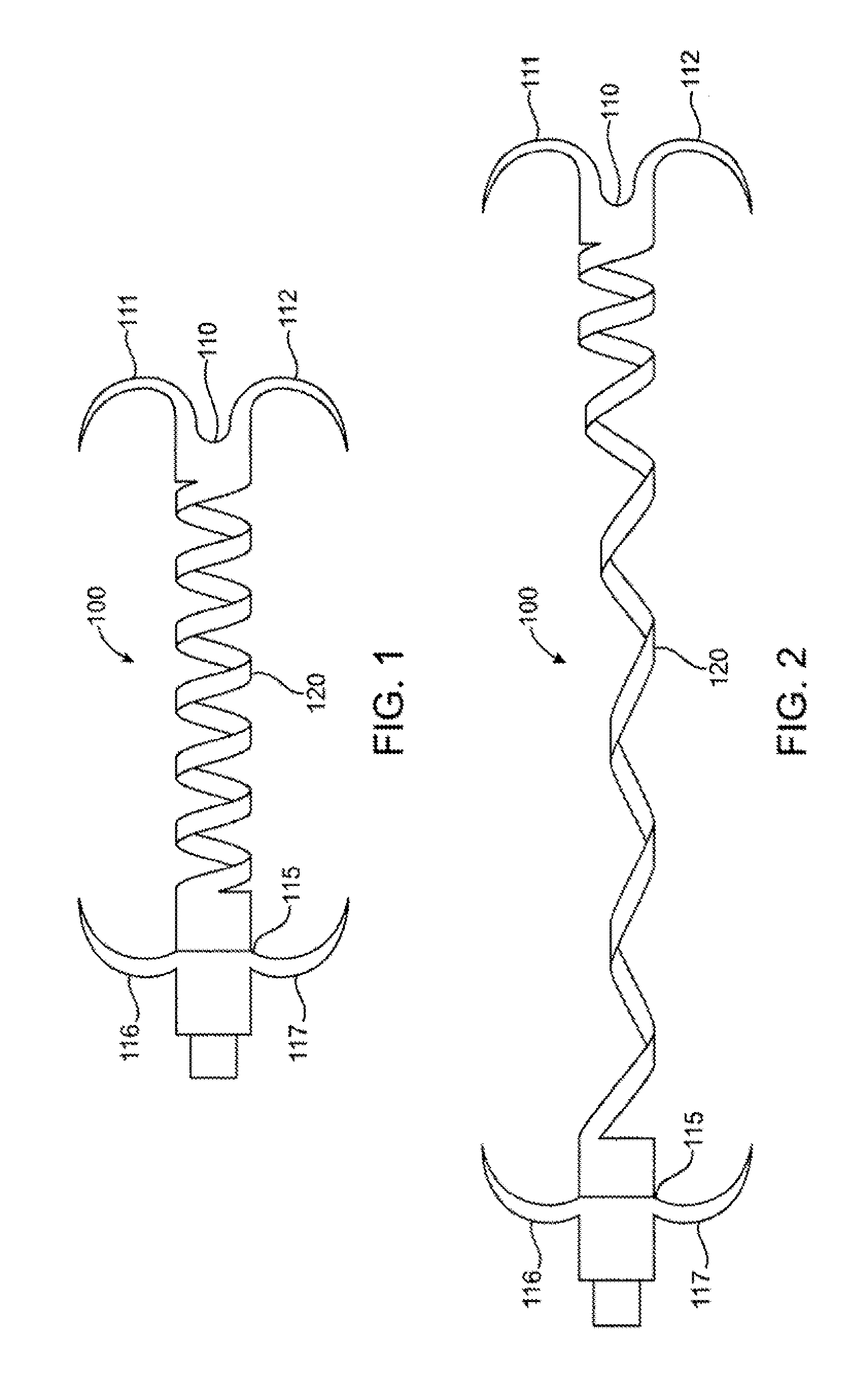
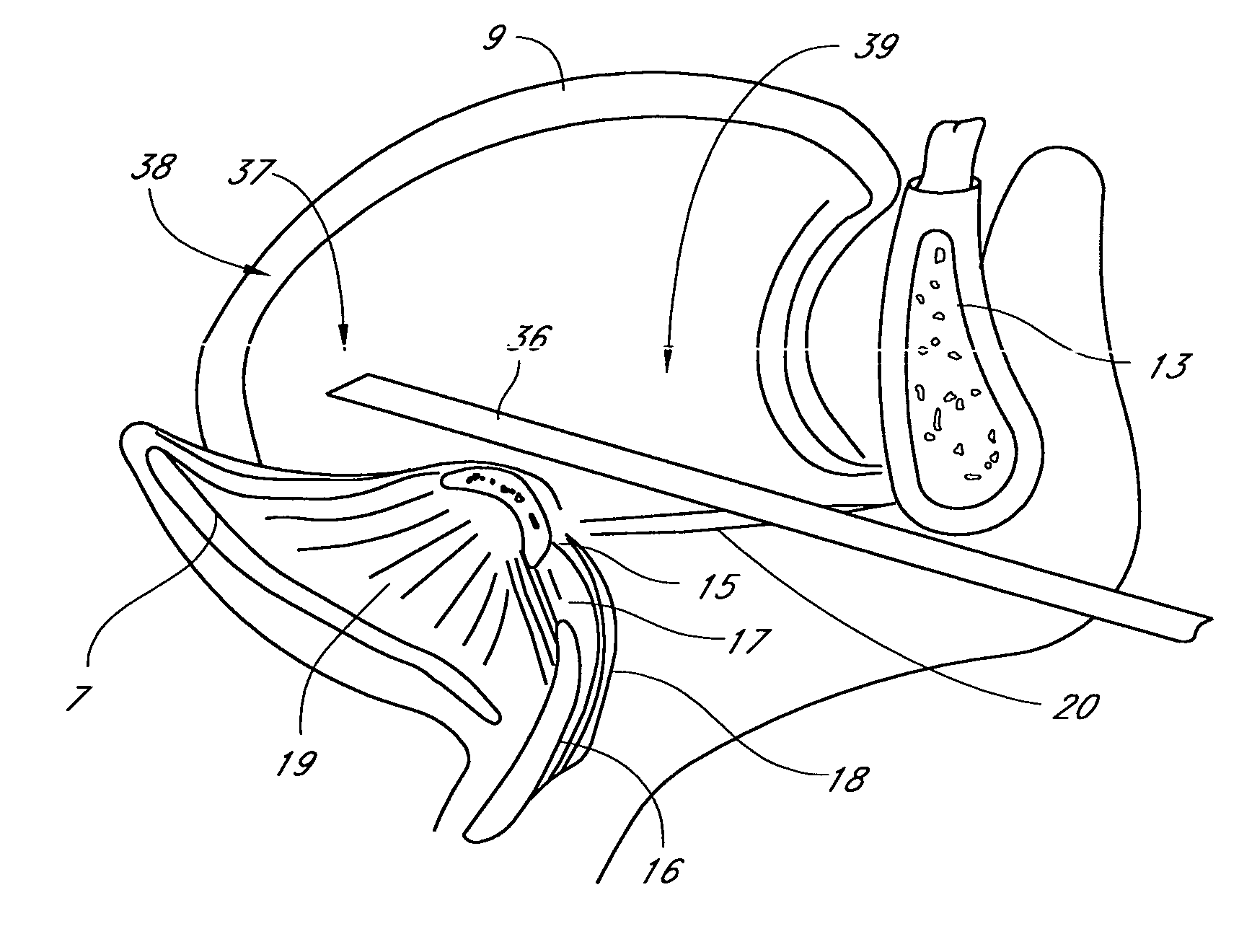
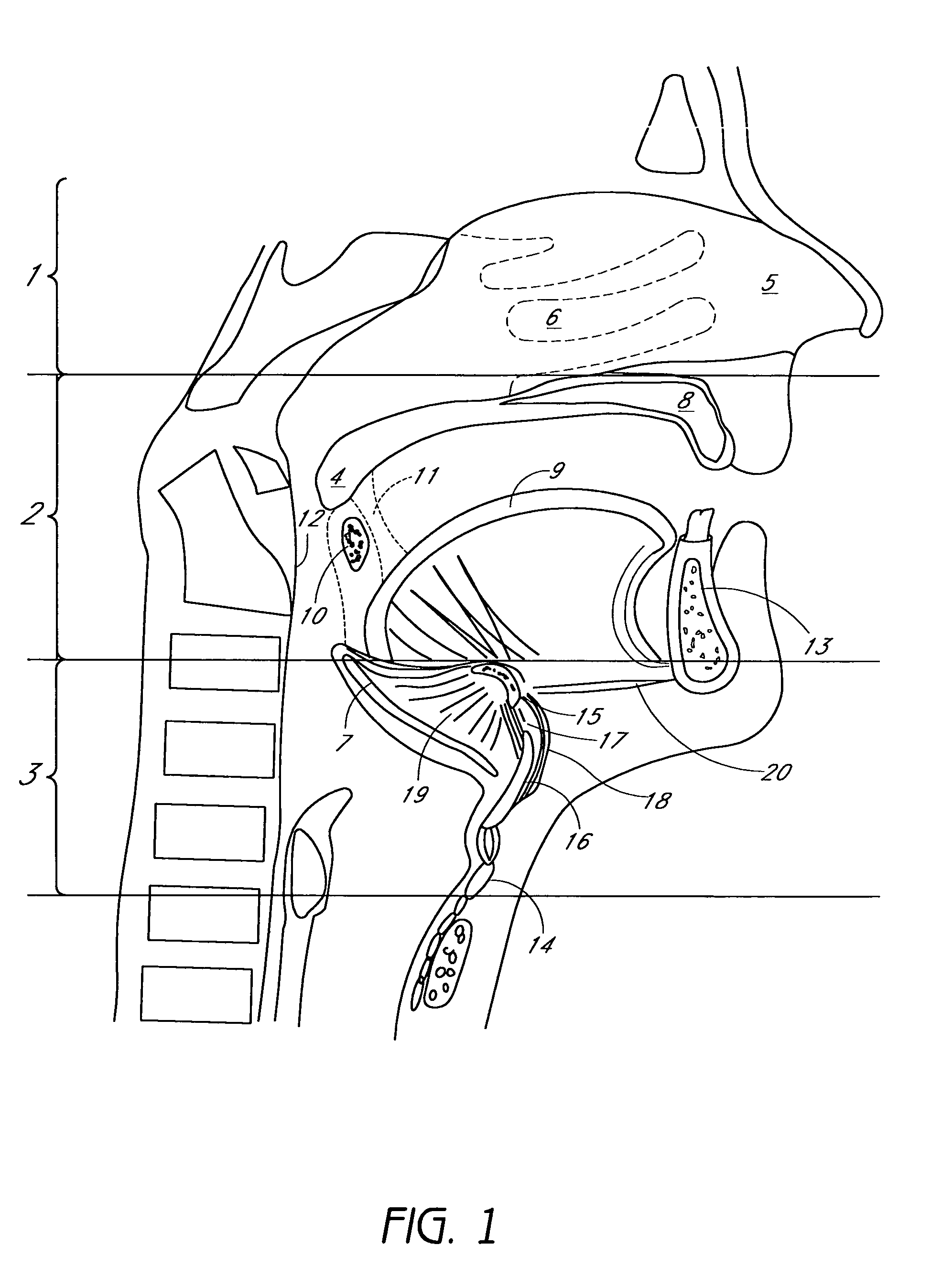
Methods and devices are disclosed for manipulating the tongue. An implant is positioned within at least a portion of the tongue and may be secured to other surrounding structures such as the mandible and / or hyoid bone. In general, the implant is manipulated to displace at least a portion of the posterior tongue in an anterior or lateral direction, or to alter the tissue tension or compliance of the tongue. Methods and devices are also disclosed for manipulating other soft tissue structures, including the soft palate and pharyngeal airway.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Stabilized liquid polypeptide formulations
InactiveUS20060210557A1Provide stabilityMaintain biological activityBiocideOrganic active ingredientsMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of polypeptides, in particular, therapeutic antigen-binding polypeptides such as antibodies and the like, for example, anti-Aβ antibodies. The formulations generally include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine, and thus, the formulations are suitable for several different routes of administration.
Owner:WYETH LLC
Multiple-layered liposome and preparation method thereof
InactiveUS20070082042A1Good skin permeabilityImprove stabilityDermatological disorderLiposomal deliverySterolIntercellular space
Disclosed are multilayered liposomes for transdermal absorption and a method of preparing the liposomes. The multilayered liposomes are prepared using a mixture of oil-phase components comprising squalane, sterols, ceramides, neutral lipids or oils, fatty acids and lecithins, is 200 to 5000 nm in particle size, and is capable of entrapping a physiologically active substance. The multilayered liposomes entrap a larger amount of a physiologically active substance and are structurally stable when encapsulating the physiologically active substance, compared to unilamellar liposomes. Also, they are prepared by a simple and cost-effective process not using a high-pressure homogenizer but using a general homo mixer. Further, since the multilayered liposomes are prepared in a larger size than the intercellular spaces in the stratum corneum, they overcome the tension of surrounding cells when passing through the intercellular spaces and are thus able to penetrate into the dermal layer, compared to nano-sized unilamellar liposomes. Thus, the multilayered liposomes are useful for enhancing the transdermal absorption of physiologically active substances.
Owner:BIOSPECTRUM
Multiparameter whole blood monitor and method
ActiveUS20060287600A1Continuous and precise measurementBlood flow measurement devicesEvaluation of blood vesselsPulse pressureIntravascular catheter
The present invention provides an apparatus and methods for continuous intravascular measurement of whole blood concentration, blood pressure, and pulse pressure. The intravascular catheter incorporates a sensor to measure whole blood sound velocity, attenuation, backscatter amplitude, and blood flow velocity and also incorporates existing technologies for multiple physiologic measurements of whole blood. Pulse wave velocity and wave intensity are derived mathematically for purposes of estimating degree of local vascular tone.
Owner:NEW PARADIGM CONCEPTS
Concentrated mast cell stabilizing pharmaceutical formulations
ActiveUS20120118991A1Favourable aerosolisation propertyEasy to solveBiocideDispersion deliveryMast cellActive agent
Liquid aqueous pharmaceutical solutions, containing a mast cell stabilizing active agent for application to the upper and lower respiratory tract or in the eye are provided. The solutions comprise both a non-ionic and an ionic tonicity-adjusting excipient. They are particularly useful for the aerosol treatment of respiratory diseases such as asthma. Furthermore, methods for nebulization of these solutions and methods of packaging the solutions are provided.
Owner:PARI PHARMA GMBH
Non-invasive modulation of the autonomic nervous system
InactiveUS20060293719A1Easy accessReduction of urinary hesitancyUltrasound therapyElectrotherapyLaryngospasmDisease
The present invention is directed to methods and apparatus for modulation of the sympathetic-parasympathetic balance by application of heat, carotid and / or ocular message to reduce sympathetic tone or increase parasympathetic tone in a target muscle system to relieve a symptom of urinary hesitancy, shy bladder syndrome, DESD, urinary retention, or laryngeal spasm, as well as to monitor the efficacy of treatments for bladder conditions and to assist in the passage of medical devices through bodily sphincters as well as to treat congestive heart failure.
Owner:THERMARX
System and method for airway manipulation
ActiveUS20060070626A1DistanceReduce distanceSuture equipmentsRespiratorsSpatial OrientationsTracheal wall
Methods and devices are disclosed for manipulating the airway, such as to treat obstructive sleep apnea. An implant is positioned within the body with respect to the airway. The spatial orientation of the airway is manipulated, directly or indirectly, to affect the configuration of the airway. In general, the implant is manipulated to displace the trachea in an inferior direction, resist superior displacement of the trachea and / or to alter the tracheal wall tension. The implant restrains the trachea in the manipulated configuration.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Foamable alcohol
InactiveUS20060104919A1Reduce surface tensionReduced effectivenessCosmetic preparationsToilet preparationsAdditive ingredientDecreased surfactant
The present invention is a foamable alcohol health, beauty, skin care, nail care and / or haircare product. Alcohol, such as ethyl alcohol is combined with a fluoro-surfactant, at least one active ingredient and water. Other ingredients can be added. The fluoro-surfactant reduces the surface tension of the alcohol allowing it to be foamed without reducing the effectiveness of the alcohol as a carrier for the active ingredient or the efficacy of the active ingredient(s). The inventive mixture foams when dispensed as an aerosol or pumped through a foam pump. The pump or other dispenser doses the alcohol. The foam is easy to apply, and liquefies as a user smears it on a surface.
Owner:NOVAK JOHN T
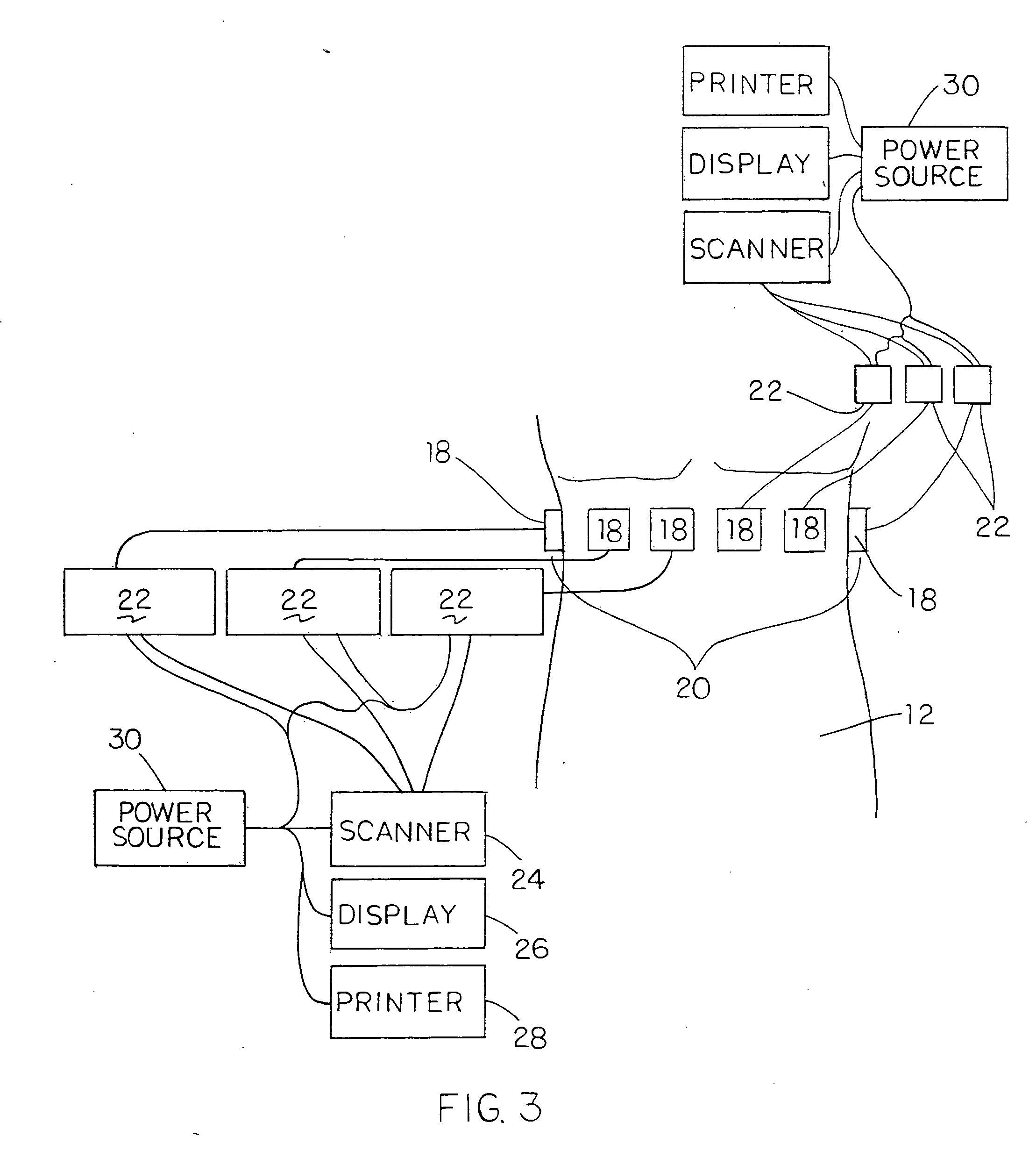
Cell analysis in multi-through-hole testing plate
InactiveUS20050059074A1Simplify the test procedureEasy constructionSequential/parallel process reactionsLibrary tagsGenetic diversityTonicity
A spatially fixed library of mutated cells and a method for creating such a library. The library is created by creating a set of cells having a genetic diversity, causing the cells to multiply and express phenotypes, preparing a solution containing the cells diluted in a medium, populating a plurality of through-holes of a testing plate with the solution such that surface tension holds the solution in respective through-holes, and analyzing the phenotypes of the cells in the solution on a hole-by-hole basis. Cells expressing specified phenotypes may then be retrieved.
Owner:LIFE TECH CORP
System and method for percutaneous palate remodeling
Methods and devices are disclosed for manipulating the tongue. An implant is positioned within at least a portion of the tongue and may be secured to other surrounding structures such as the mandible and / or hyoid bone. In general, the implant is manipulated to displace at least a portion of the posterior tongue in an anterior or lateral direction, or to alter the tissue tension or compliance of the tongue. Methods and devices are also disclosed for manipulating other soft tissue structures, including the soft palate and pharyngeal airway.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Ophthalmic formulation of rho kinase inhibitor compound
InactiveUS20100022517A1Improve bioavailabilityIncrease concentrationBiocideSenses disorderOcular bioavailabilityAqueous humor
The present invention relates to an aqueous pharmaceutical formulation comprising at least one inhibitor of Rho-associated protein kinase (ROCK). The aqueous pharmaceutical formulation comprises 0.01-0.4% w / v of ROCK inhibitor(s), a non-ionic surfactant in an amount of 0.01-2% w / v, and a tonicity agent to maintain a tonicity between 220-360 mOsm / kG, at a pH between 6.3 to 7.8, wherein the ROCK inhibitor, the surfactant, and the tonicity agent are compatible in the formulation. The aqueous ophthalmic formulations of this invention have an increased ocular bioavailability and / or aqueous humor concentrations without a concomitant increase in systemic concentrations. The present invention further provides a method of reducing intraocular pressure, particularly a method of treating glaucoma, by administering the aqueous pharmaceutical formulation to a subject.
Owner:INSPIRE PHARMA
Methods of treating anxiety disorders
Disclosed are methods of treating an anxiety disorder, e.g., obsessive compulsive disorder, in an individual, comprising identifying an individual in need thereof and treating that individual to antagonize opioid receptor activity and to restore normal monoaminergic tone within the synapse.
Owner:OREXIGEN THERAPEUTICS INC
Anti Abeta antibody formulation
ActiveUS7635473B2Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
Aqueous ink, ink jet recording method, ink cartridge, recording unit and ink jet recording apparatus
ActiveUS20080018722A1Efficient aggregationEffective diffusionMeasurement apparatus componentsDuplicating/marking methodsOrganic solventWater insoluble
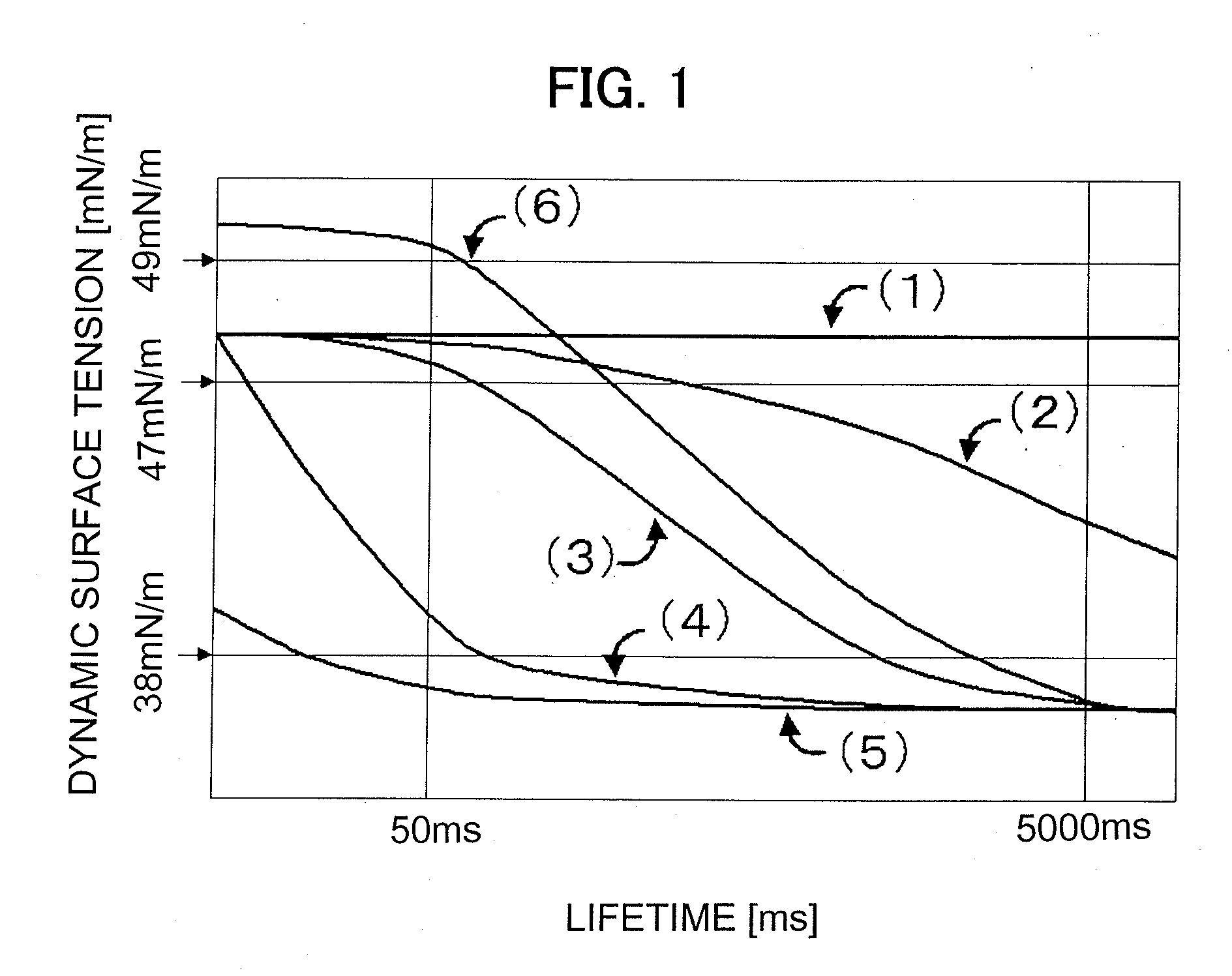
Provided herein is an aqueous ink, which is excellent in both image density and fixing ability irrespective of the kind of a recording medium even when the volume of an ink droplet is small and has such excellent properties that white stripes are not caused even when high-speed recording is conducted. The aqueous ink comprises at least water, a water-soluble organic solvent, a water-insoluble coloring material, a surfactant and a poor medium for the water-insoluble coloring material and / or a salt. The dynamic surface tension of the aqueous ink at a lifetime of 50 milliseconds determined by a maximum bubble pressure method is higher than 47 mN / m, and the dynamic surface tension at a lifetime of 5,000 milliseconds determined by the maximum bubble pressure method is 38 mN / m or lower.
Owner:CANON KK
Ophthalmological zonular stretch segment for treating presbyopia
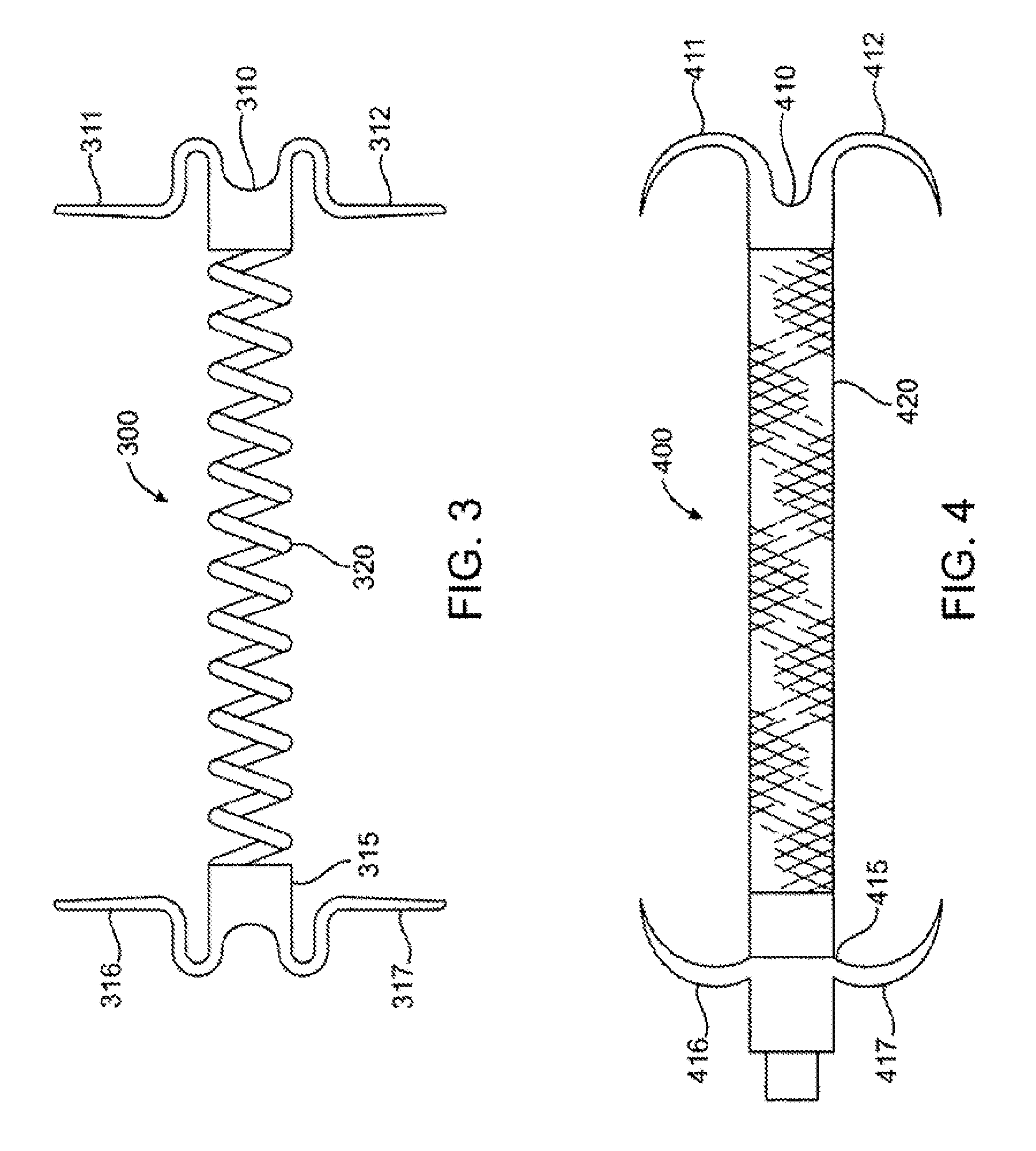
The invention comprises a device for treating presbyopia. A stretch segment is provided for intraocular implantation into the annular sulcus region defined by the intersection of the iris and ciliary body. The stretch segment engages and exerts an outward radial tension against the ciliary body. The segment is designed to take up slack in the equatorial zonules in the presbyopic eye, such that their effective working distance is enhanced. This aids in the accommodation process which affects the curvature of the lens for near viewing. The segment may a closed ring, or may be open ended.
Owner:BOXER WACHLER BRIAN S
Lens care composition and method
ActiveUS20040142829A1Improve effectivenessLens cleaning compositionsSurface-active detergent compositionsChlorideTonicity
An buffered aqueous contact lens disinfecting solution having much lower concentration of disinfecting agents, and less irritating to the eye. The solutions have a tonicity of 200 to 450 mOsm / kg, a pH of between 6 and 8, and a concentration of chloride ions below 1500 ppm. Despite having less than 1 ppm of antimicrobial, the solutions are effective against C. albicans within 15 minutes of contact.
Owner:ALCON INC
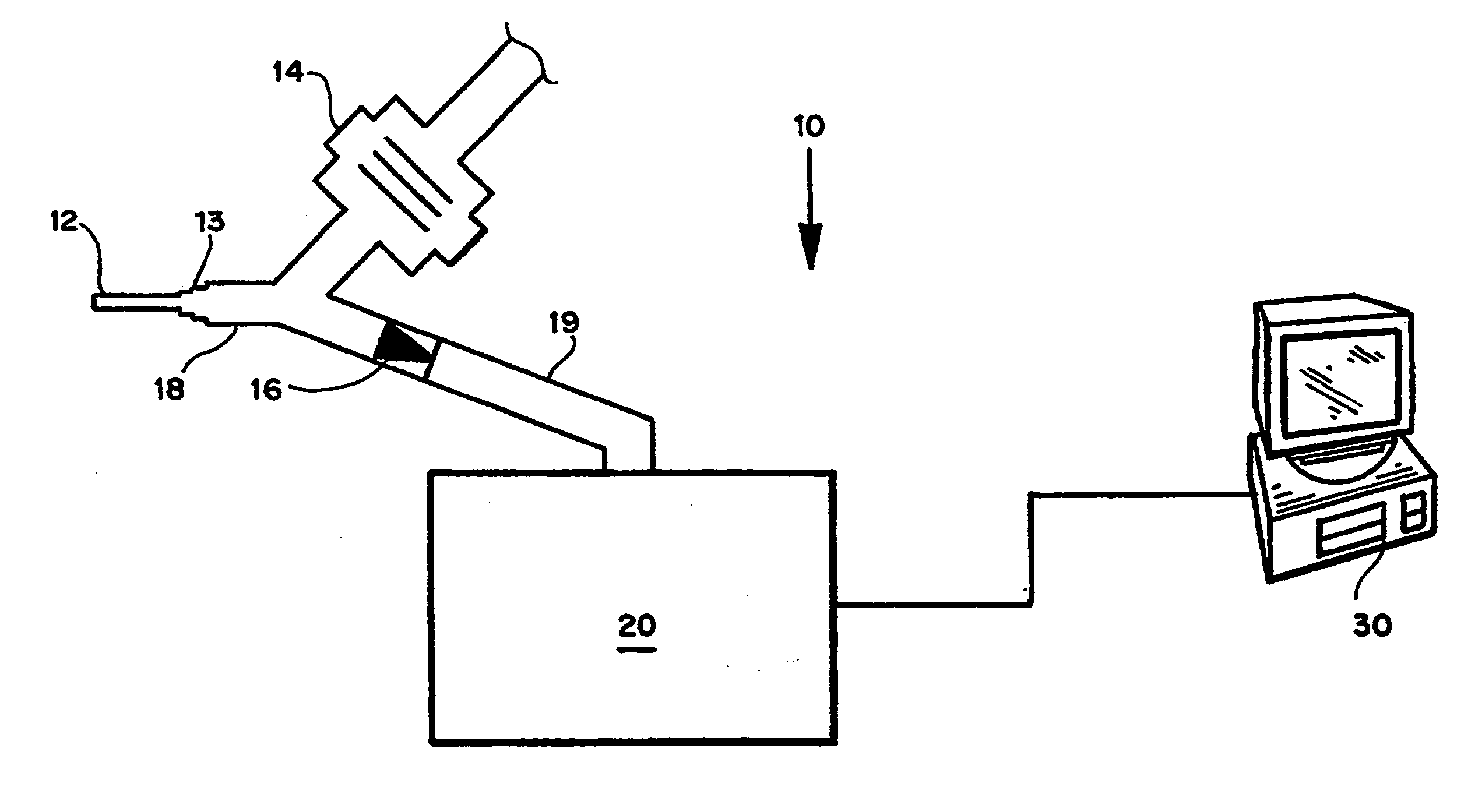
Method and device for decreasing contamination
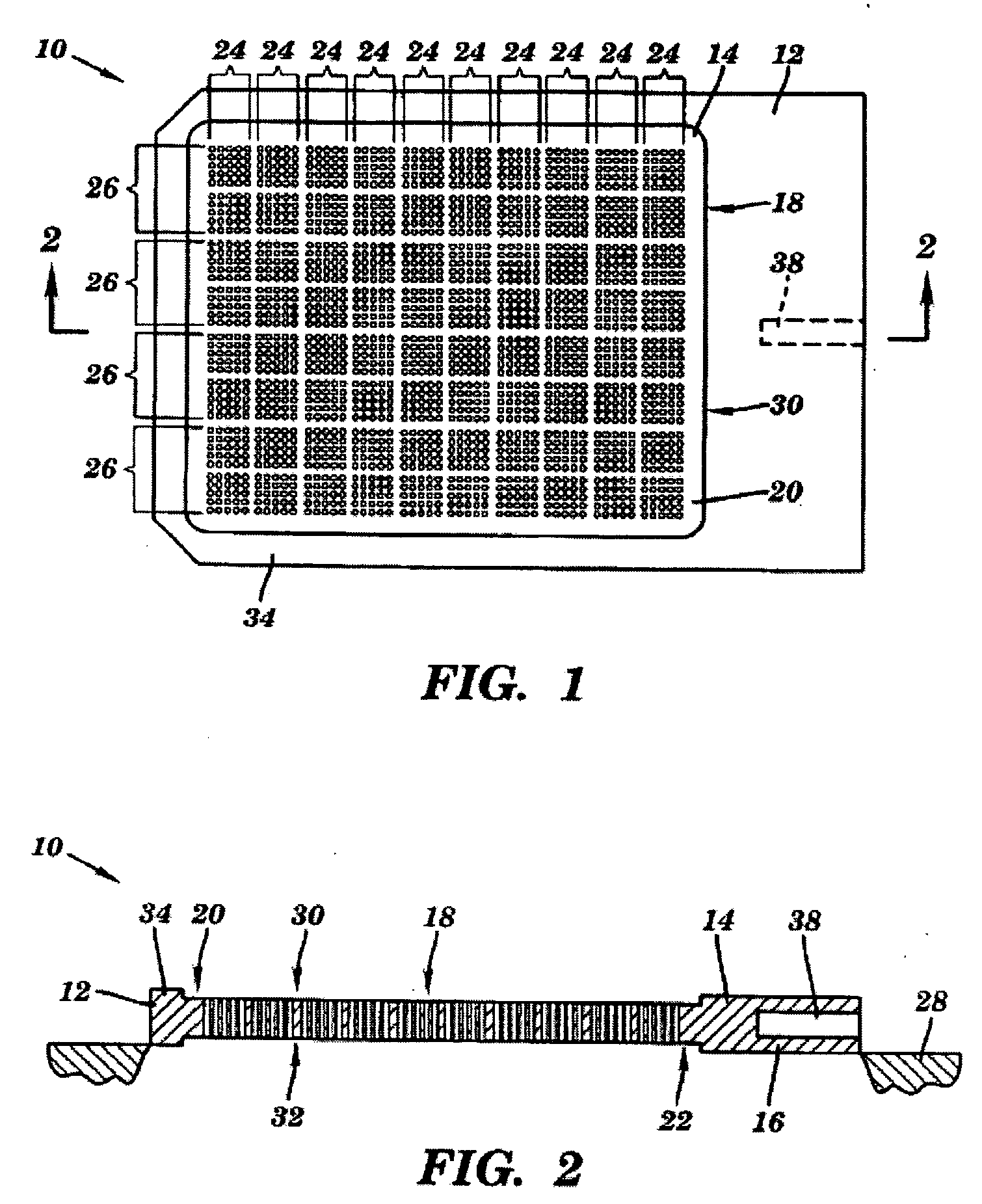
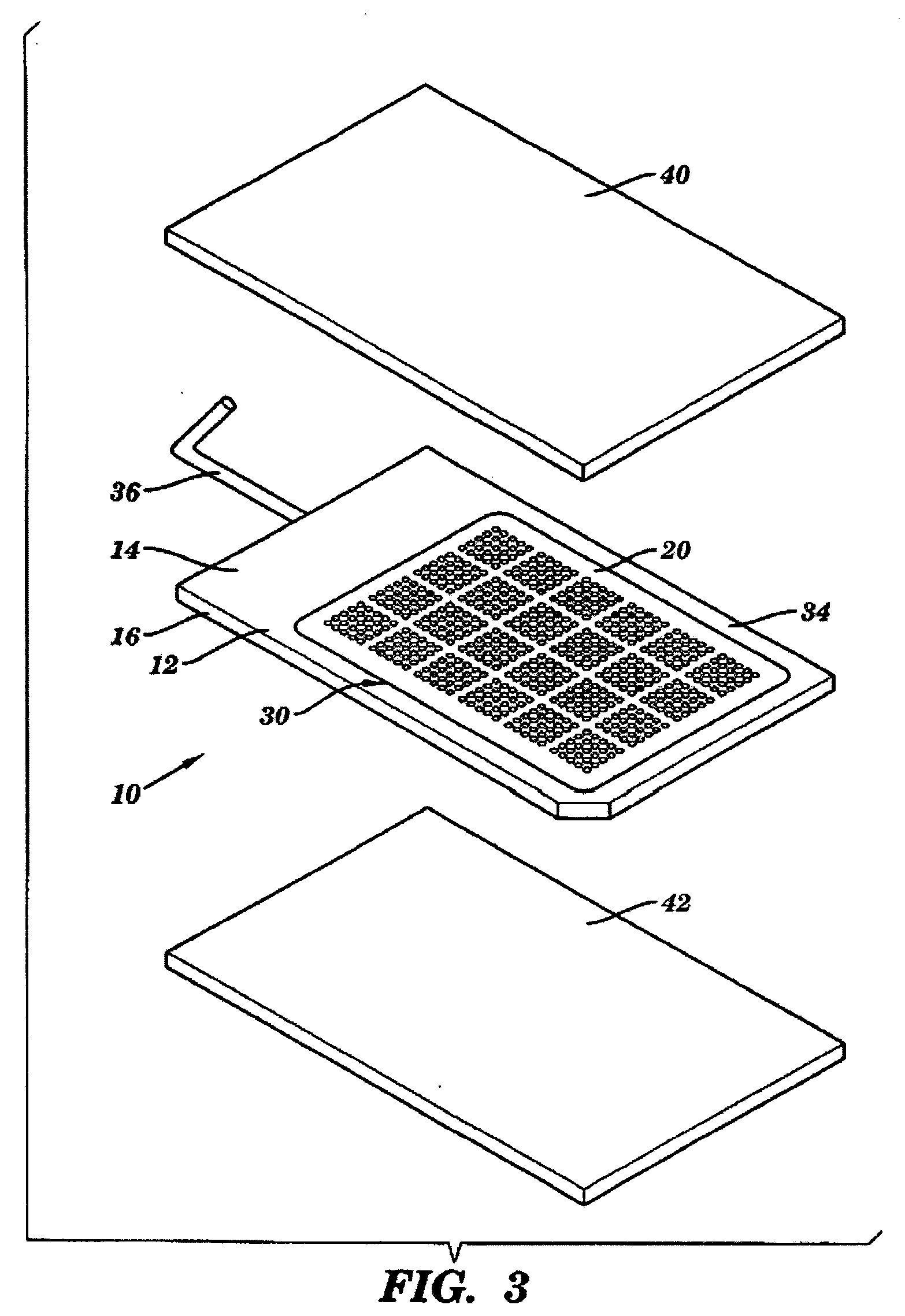
ActiveUS20080038207A1Reduce Particle GenerationAltered propertyBiocidePeptide/protein ingredientsViscoelasticityContamination
Methods and devices to determine rate of particle production and the size range for the particles produced for an individual are described herein. The device (10) contains a mouthpiece (12), a filter (14), a low resistance one-way valve (16), a particle counter (20) and a computer (30). Optionally, the device also contains a gas flow meter (22). The data obtained using the device can be used to determine if a formulation for reducing particle exhalation should be administered to an individual. This device is particularly useful prior to and / or following entry in a cleanroom to ensure that the cleanroom standards are maintained. The device can also be used to identify animals and humans who have an enhanced propensity to exhale aerosols (referred to herein as “over producers”, “super-producers”, or “superspreaders”). Formulations to reduce particle production are also described herein. The formulation is administered in an amount sufficient to alter biophysical properties in the mucosal linings of the body. When applied to mucosal lining fluids, the formulation alters the physical properties such as the gel characteristics at the air / liquid interface, surface elasticity, surface viscosity, surface tension and bulk viscoelasticity of the mucosal lining. The formulation is administered in an effective amount to minimize ambient contamination due to particle formation during breathing, coughing, sneezing, or talking, which is particularly important in cleanroom applications. In one embodiment, the formulation for administration is a non-surfactant solution. In one embodiment, the formulations are conductive formulations containing conductive agents, such as salts, ionic surfactants, or other substances that are in an ionized state or easily ionized in an aqueous or organic solvent environment. Preferably the formulation is administered in the form of an aerosol.
Owner:PULMATRIX OPERATING CO
Sodium chloride solution for drug reconstitution or dilution
InactiveUS20070135343A1Prevent agglutinationIncrease ionic strengthBiocidePeptide/protein ingredientsHemolysisPresent method
The invention provides methods for preparing pharmaceutical formulations for injection such that upon injection the formulation does not cause erythrocyte agglutination, hemolysis, and / or cell shrinkage. To prevent agglutination, a pharmaceutical formulation ready for injection needs to have a sufficient ionic strength. To prevent hemolysis or cell shrinkage, a pharmaceutical formulation ready for injection needs to be about isotonic with respect to plasma. The invention provides methods that prepare pharmaceutical formulations for injection that have both the sufficient ionic strength to prevent agglutination and the requisite tonicity to prevent significant hemolysis or cell dehydration or shrinkage. The present methods involve the use of sodium chloride solutions that are about 25 mM to about 150 mM for reconstituting lyophilized cakes (or other non-liquid pharmaceutical formulations) into solution or for diluting pharmaceutical formulation solutions.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com