Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
147 results about "Pharmaceutical Solutions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
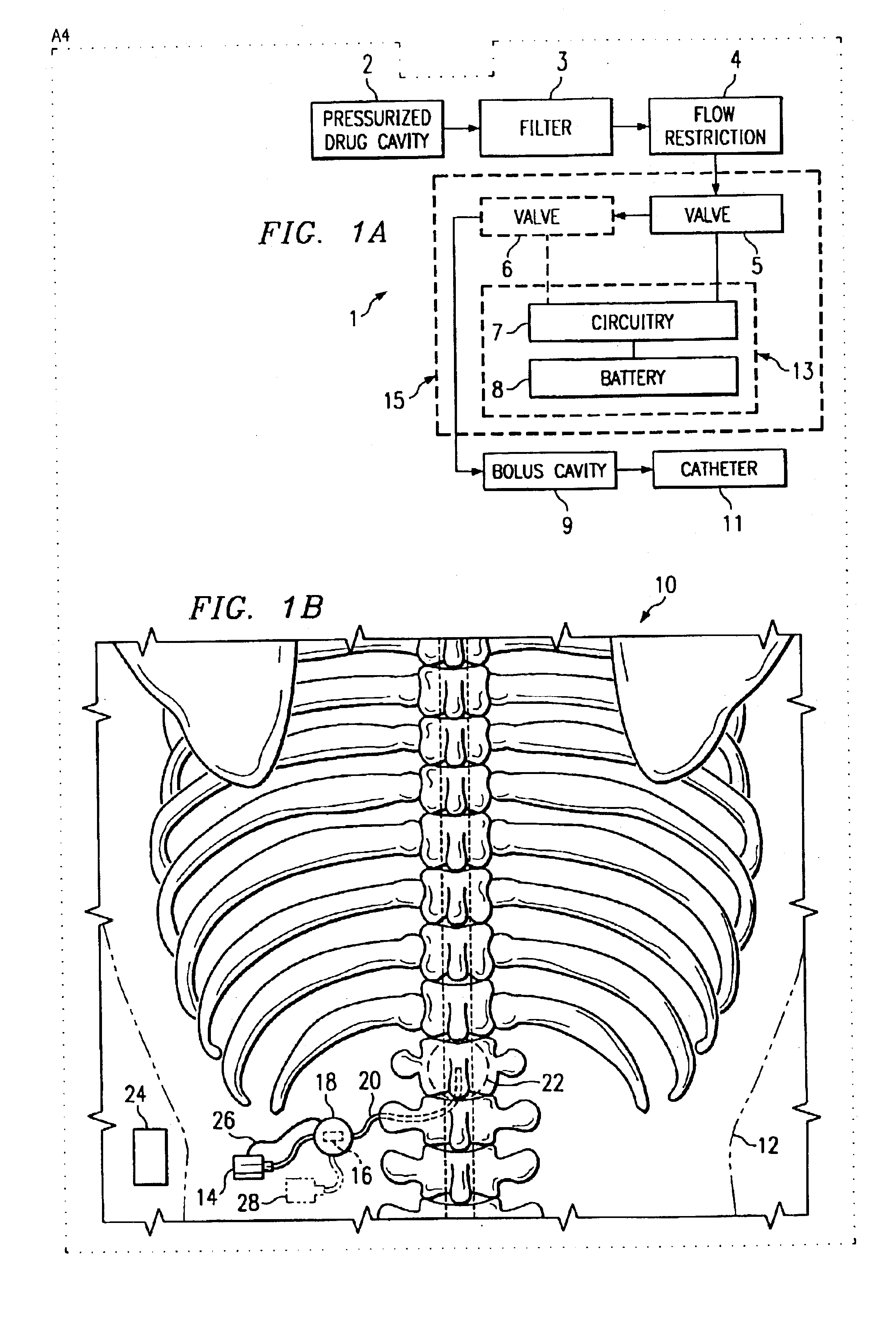
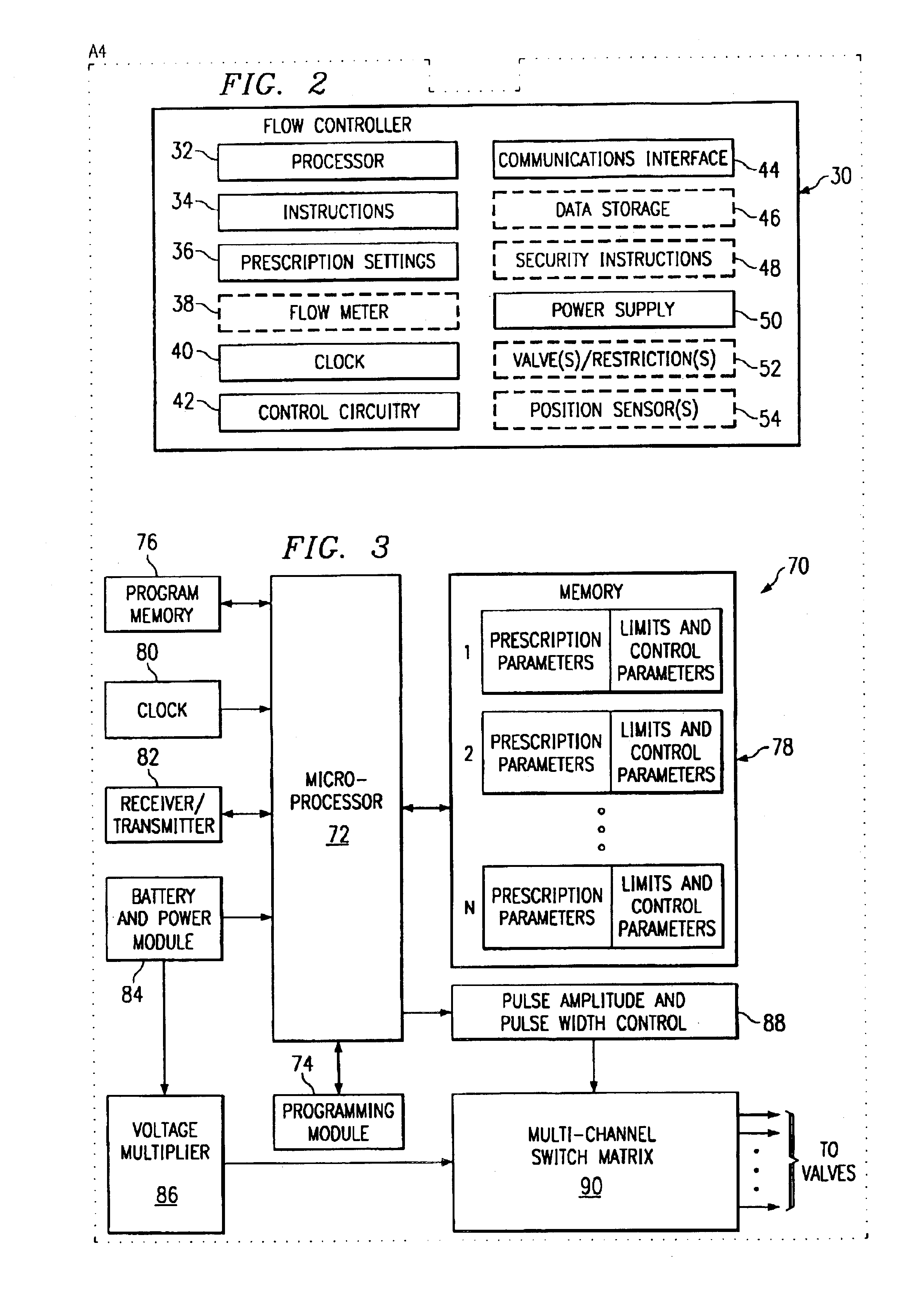
Programmable dose control module
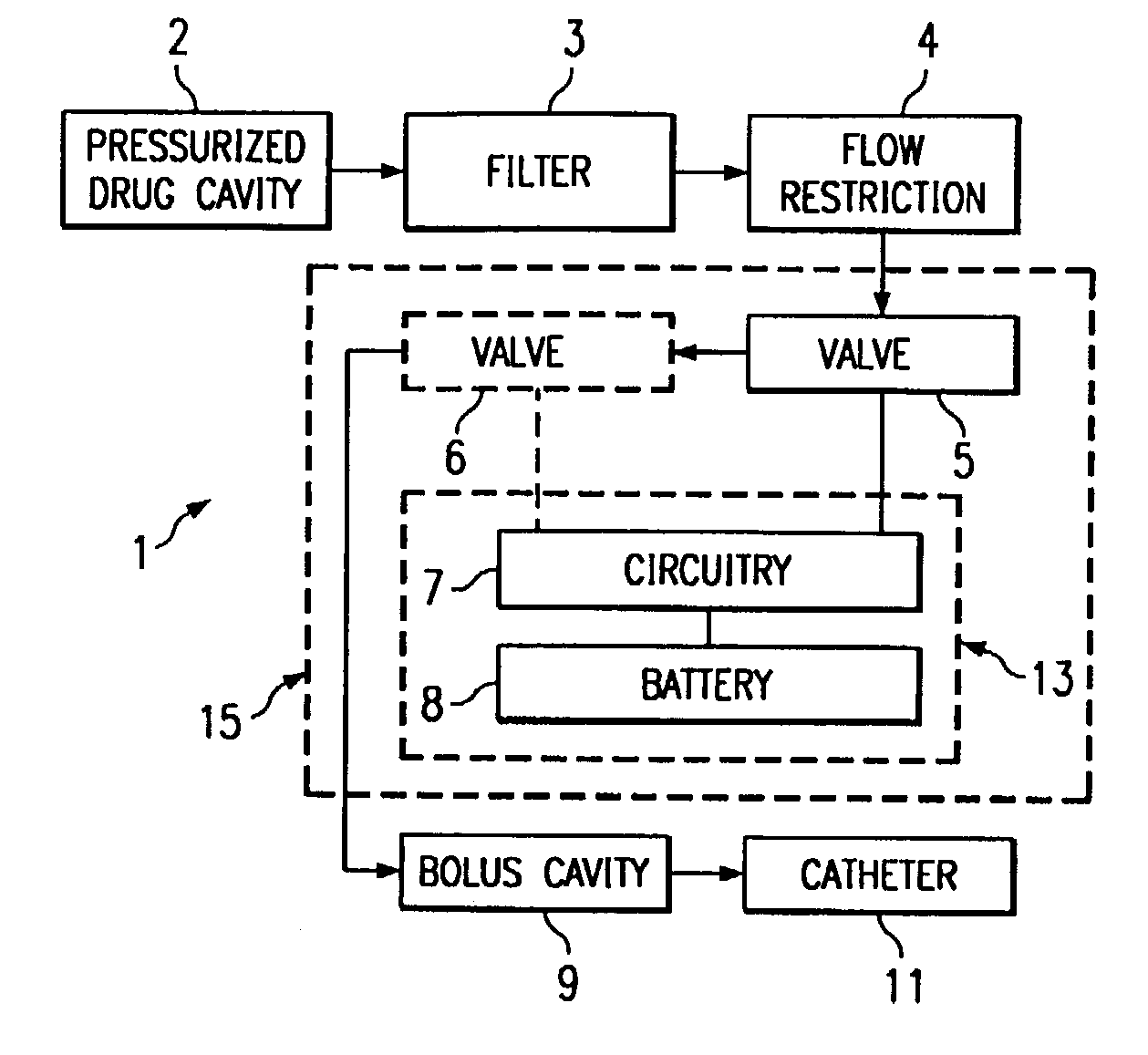
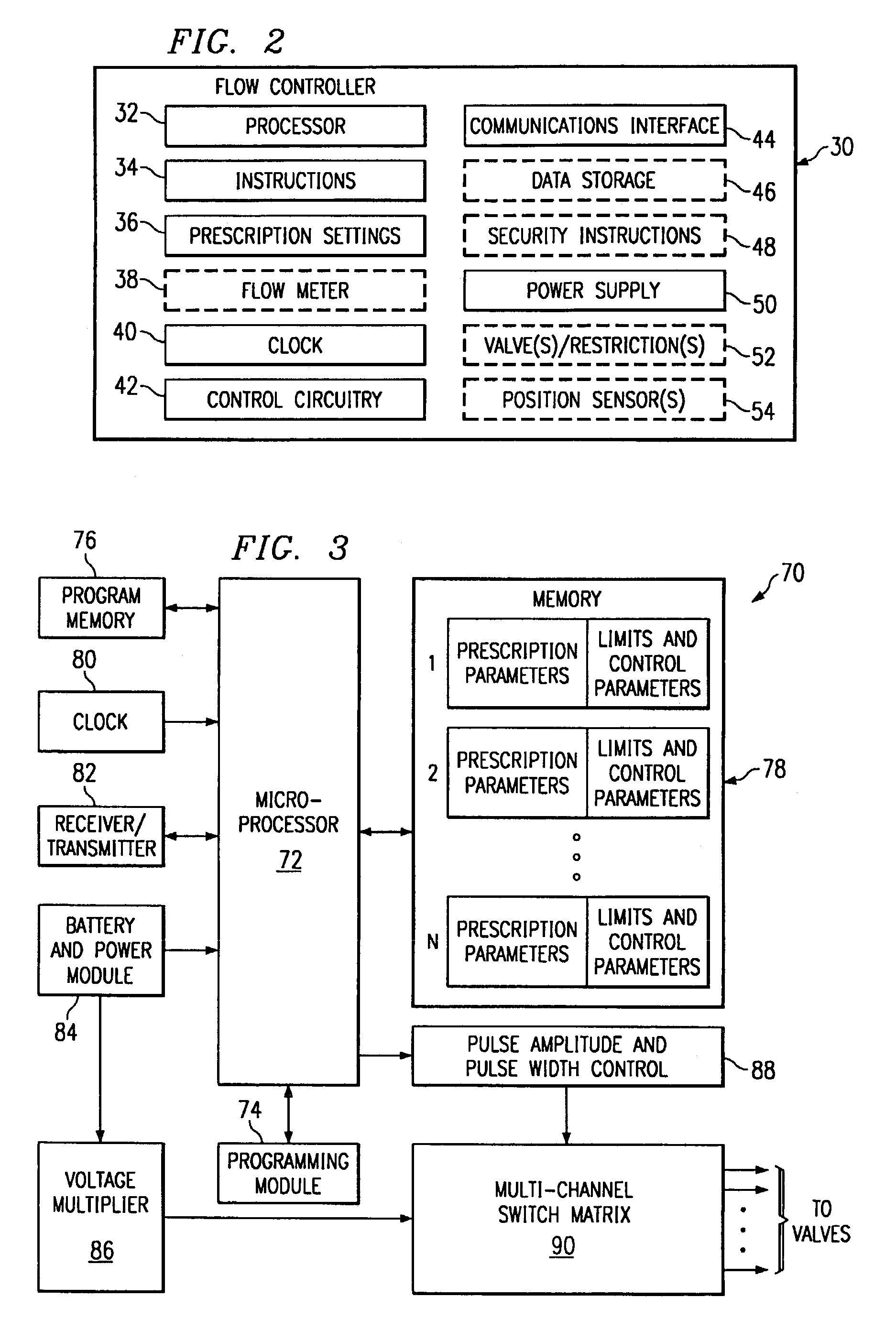
The invention is directed to a dose control module for manipulating the dosage of one or more pharmaceutical solutions emanating from an implantable drug infusion pump. The dose control module has a processor and other circuitry for manipulating flow valves and other dose manipulators. Effluent catheters from one or more implantable drug infusion pumps may be connected to the dose control module. The dose control module may direct the effluent pharmaceutical solution to one or more catheters. These catheters direct the pharmaceutical solutions to treatment locations. The invention is also directed to induction coil valves for use in or with the dose control module. The use of induction coils permits the dose control module to determine valve position and counteract large directional magnetic fields produced by MRIs.
Owner:ADVANCED NEUROMODULATION SYST INC
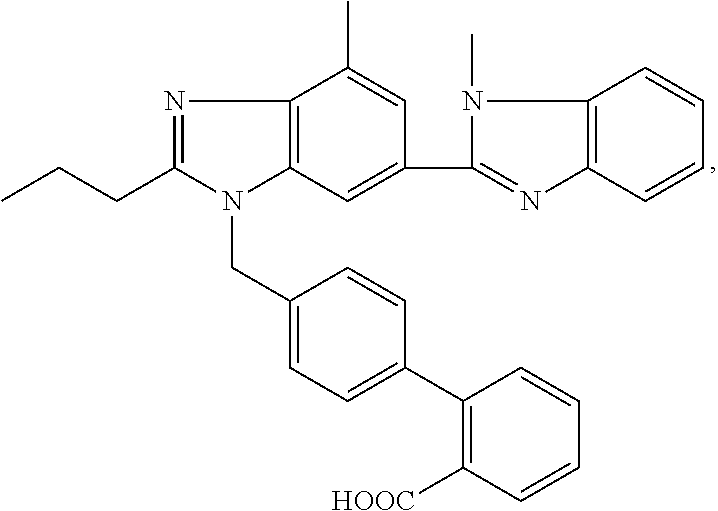
Aripiprazole oral solution
The present invention provides for a pharmaceutical solution suitable for oral administration comprising aripiprazole, a pharmaceutically suitable solvent system, one or more taste-enhancing / masking agents and one or more agents selected from the group consisting of lactic acid, acetic acid, tartaric acid and citric acid, wherein said solution has a pH from 2.5 to 4.5.
Owner:OTSUKA PHARM CO LTD
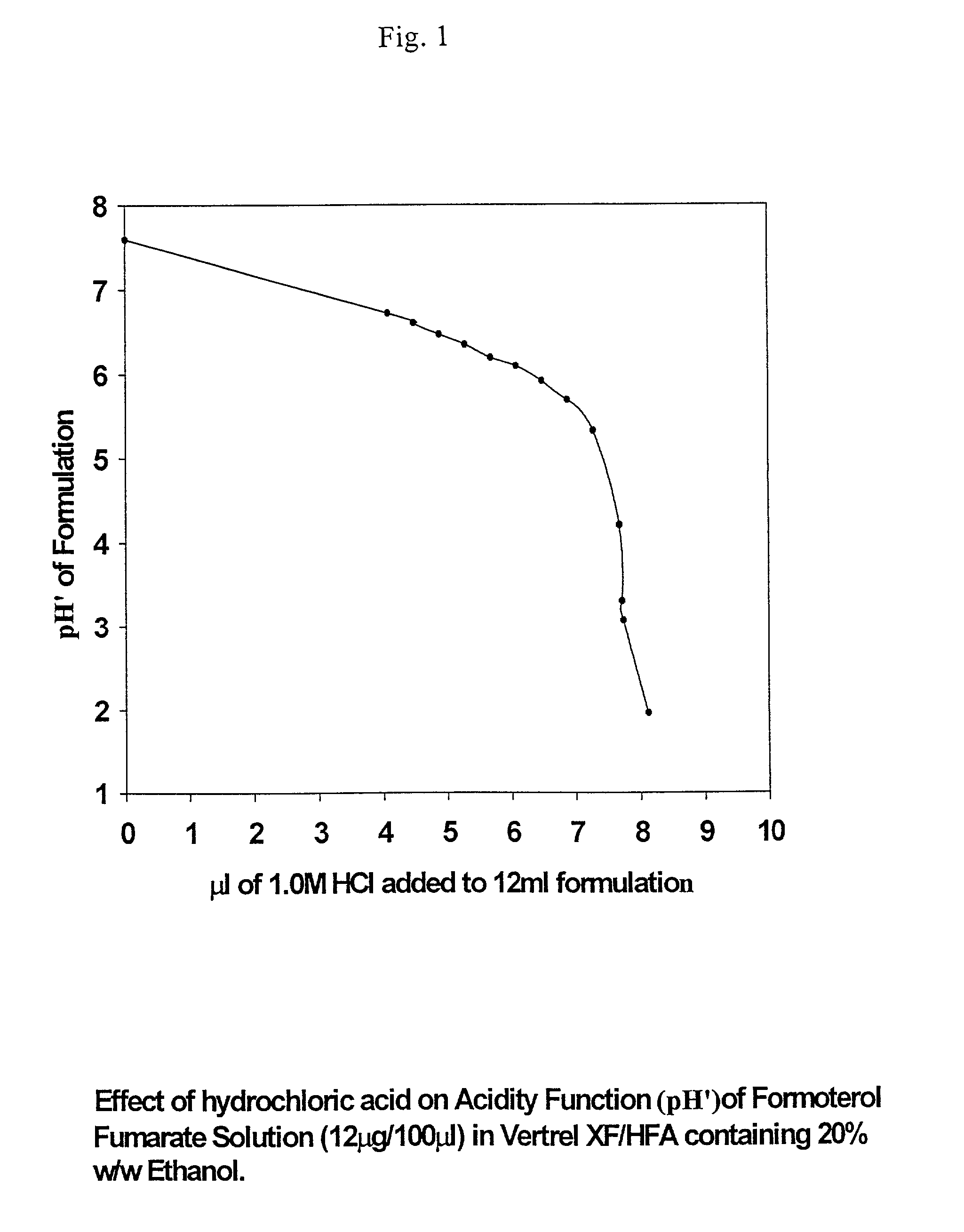
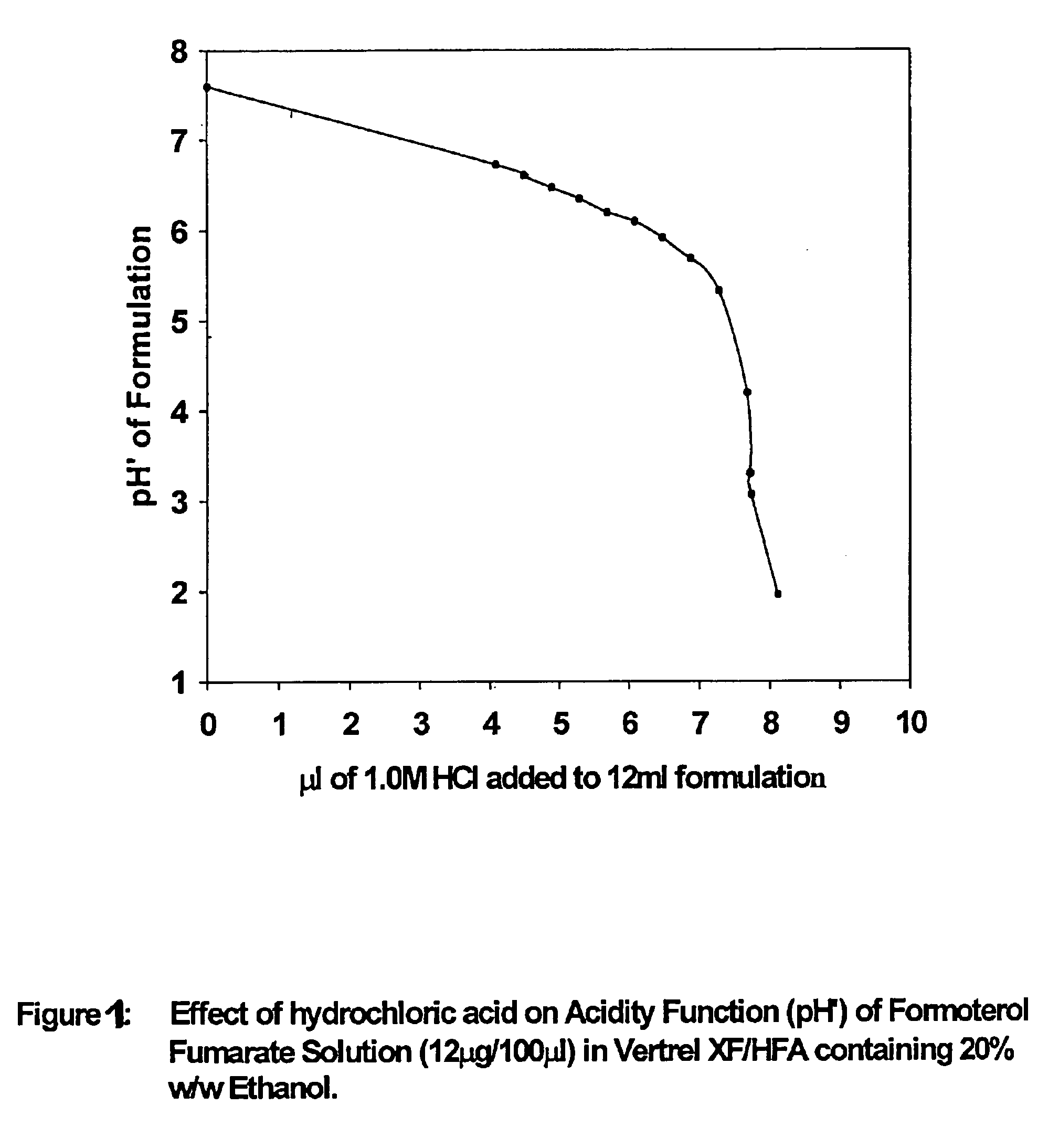
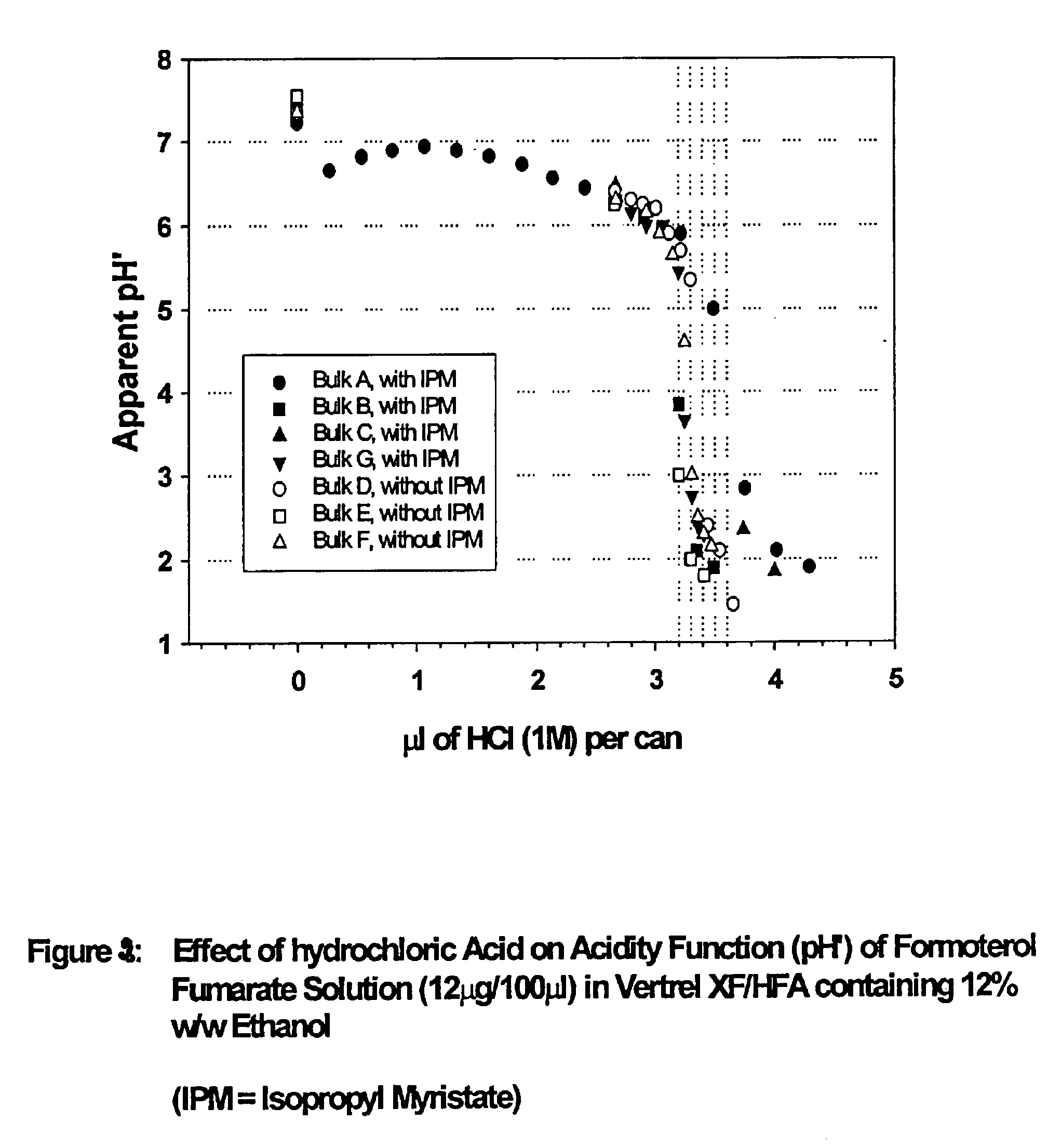
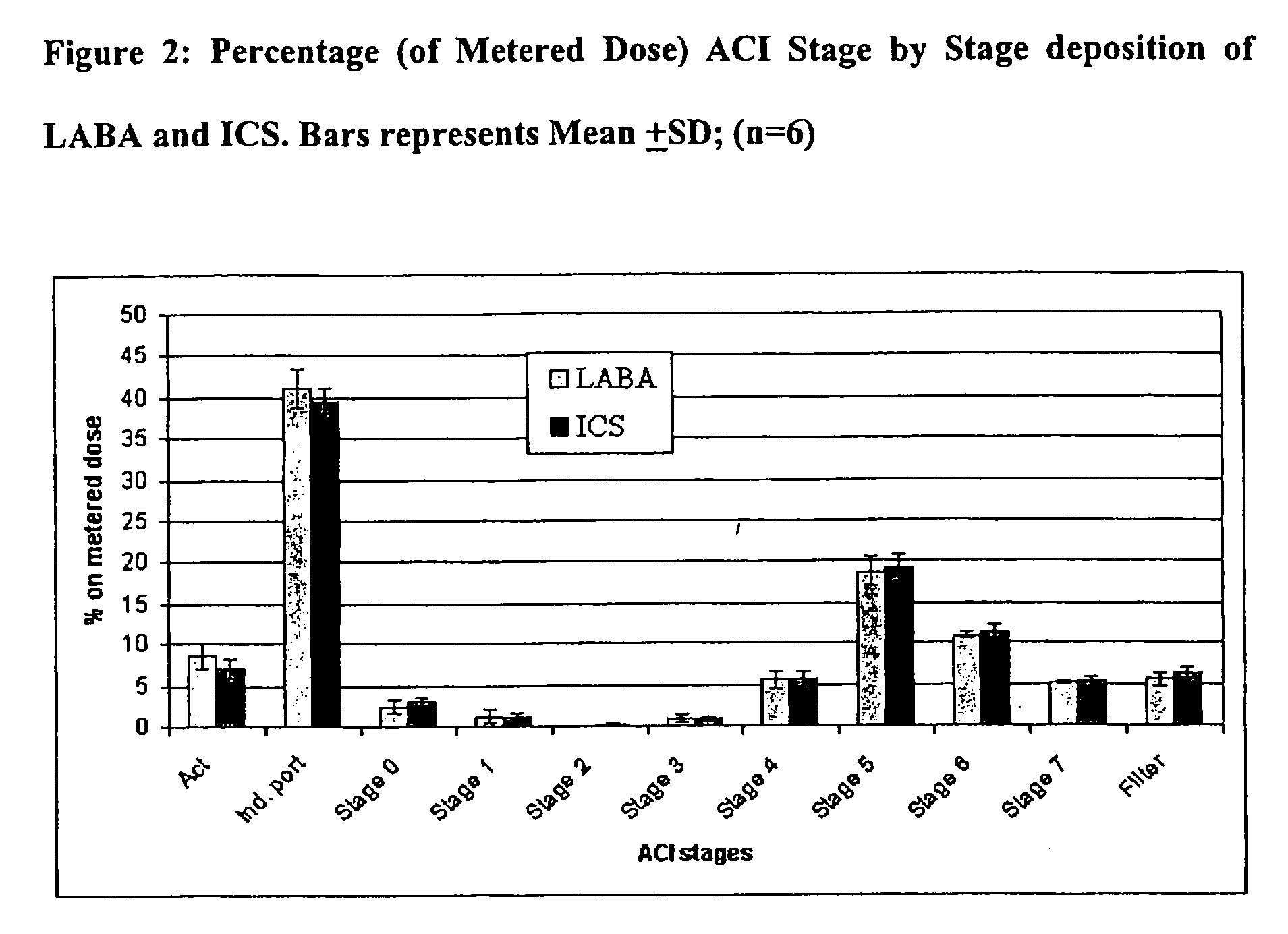
Stable pharmaceutical solution formulations for pressurised metered dose inhalers
An aerosol solution composition for use in an aerosol inhaler comprises an active material, a propellant containing a hydrofluoroalkane, a cosolvent and optionally a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler. The composition is stabilized by using a small amount of mineral acid and a suitable can having part or all of its internal metallic surfaces made of stainless steel, anodized aluminium or lined with an inert organic coating.
Owner:CHIESI FARM SPA
Pharmaceutical solution formulations for pressurised metered dose inhalers
A method for delivering two or more active drug substances to the lungs by inhalation from a single pressurized metered dose inhaler product, said inhaler containing a HFA / cosolvent based solution formulation wherein all the active drug substances are fully dissolved in the formulation.
Owner:CHIESI FARM SPA
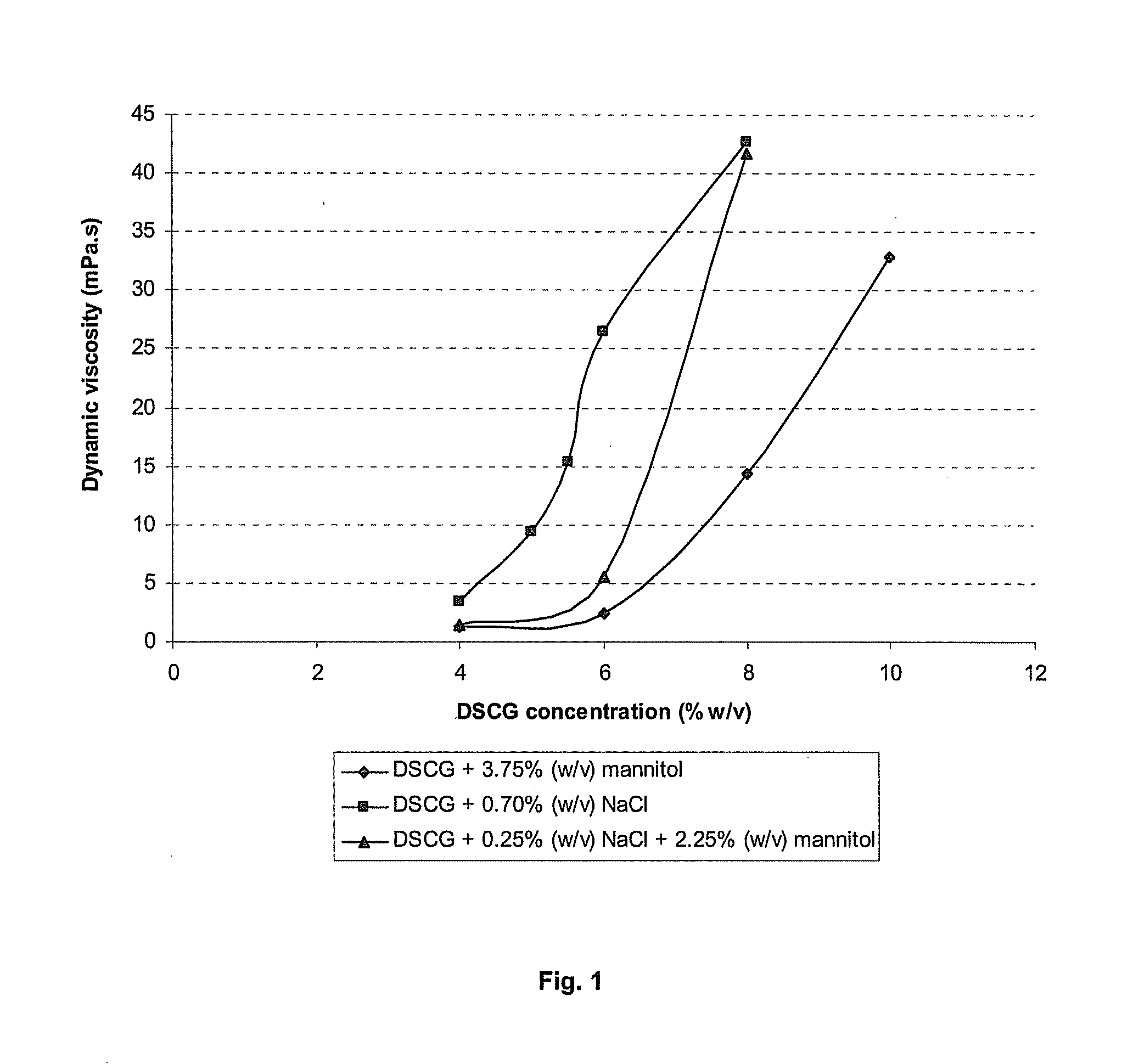
Concentrated mast cell stabilizing pharmaceutical formulations
ActiveUS20120118991A1Favourable aerosolisation propertyEasy to solveBiocideDispersion deliveryMast cellActive agent
Liquid aqueous pharmaceutical solutions, containing a mast cell stabilizing active agent for application to the upper and lower respiratory tract or in the eye are provided. The solutions comprise both a non-ionic and an ionic tonicity-adjusting excipient. They are particularly useful for the aerosol treatment of respiratory diseases such as asthma. Furthermore, methods for nebulization of these solutions and methods of packaging the solutions are provided.
Owner:PARI PHARMA GMBH
Pharmaceutical solution formulations containing 17-AAG
A pharmaceutical solution formulation containing 17-AAG in an amount of up to 15 mg / mL dissolved in a vehicle comprising (i) a first component that is ethanol, in an amount of between about 40 and about 60 volume %; (ii) a second component that is a polyethoxylated castor oil, in an amount of between about 15 to about 50 volume %; and (iii) a third component that is selected from the group consisting of propylene glycol, PEG 300, PEG 400, glycerol, and combinations thereof, in an amount of between about 0 and about 35 volume %.
Owner:KOSAN BIOSCI
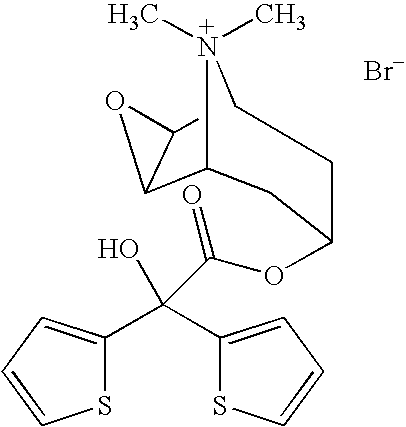
Tiotropium containing HFC solution formulations
This invention relates to tiotropium containing stable pharmaceutical solution formulations suitable for aerosol administration. More particularly, this invention relates to tiotropium containing stable pharmaceutical solution formulations suitable for aerosol administration wherein either an inorganic acid or an organic acid is added to the aerosol solution formulation which contains a tiotropium salt, preferably tiotropium bromide in solution with an environmentally safe hydrofluorocarbon (HFC) as a propellant, together with an organic compound as a cosolvent. The acid provides stability against degradation or decomposition of the medicament resulting largely from interaction of the medicament with the cosolvent and / or water present in the solution formulation.
Owner:BOEHRINGER INGELHEIM PHARM KG
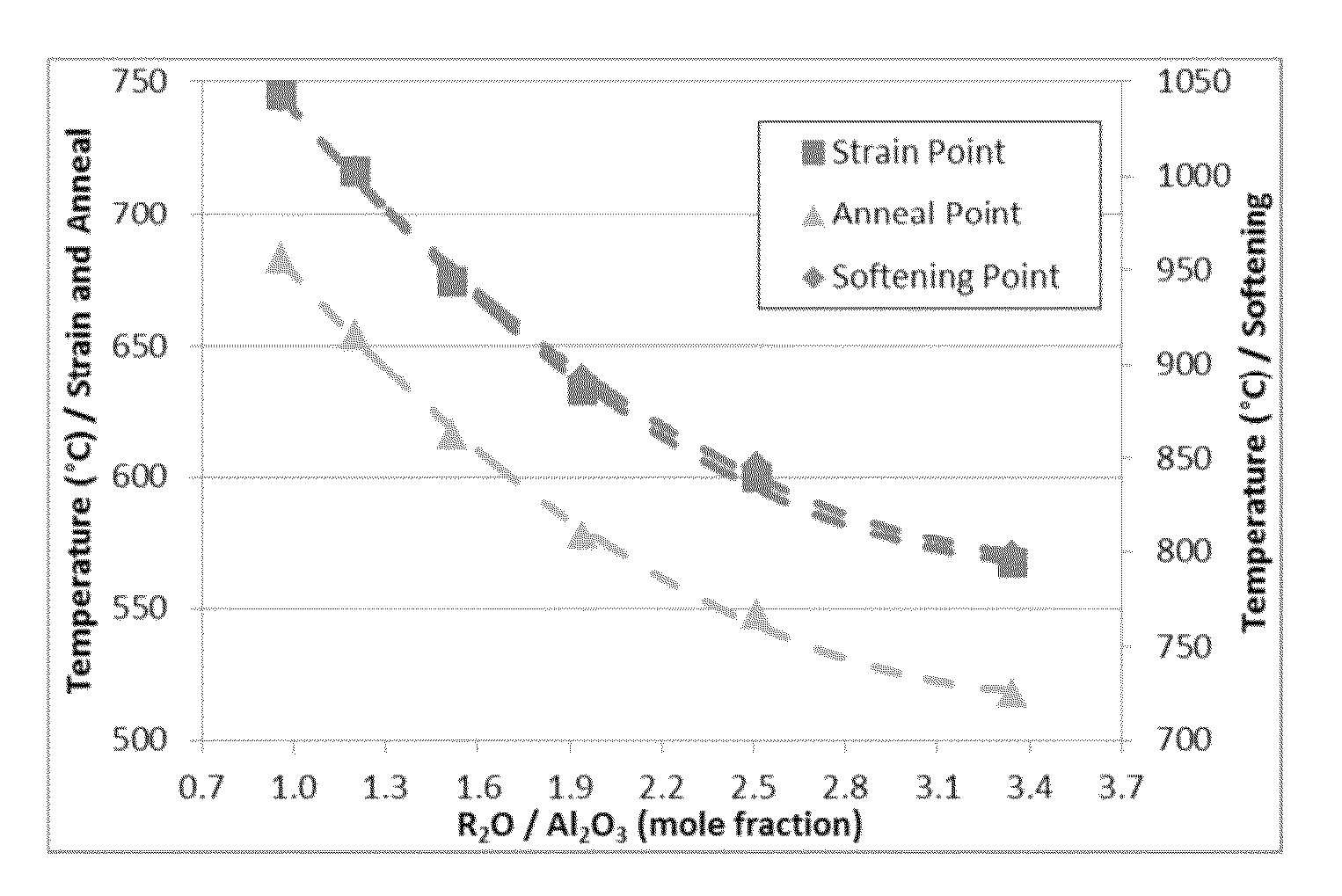
Delamination resistant pharmaceutical glass containers containing active pharmaceutical ingredients
ActiveUS20130196094A1Organic active ingredientsPeptide/protein ingredientsParticulatesBULK ACTIVE INGREDIENT
The present invention is based, at least in part, on the identification of a pharmaceutical container formed, at least in part, of a glass composition which exhibits a reduced propensity to delaminate, i.e., a reduced propensity to shed glass particulates. As a result, the presently claimed containers are particularly suited for storage of pharmaceutical compositions and, specifically, a pharmaceutical solution comprising a pharmaceutically active ingredient, for example, NEUPOGEN (filgrastim), NEULASTA (pegfilgrastim), EPOGEN (epoetin alfa) or ENBREL (etanercept).
Owner:CORNING INC
Apparatus for dosage control
Owner:ADVANCED NEUROMODULATION SYST INC
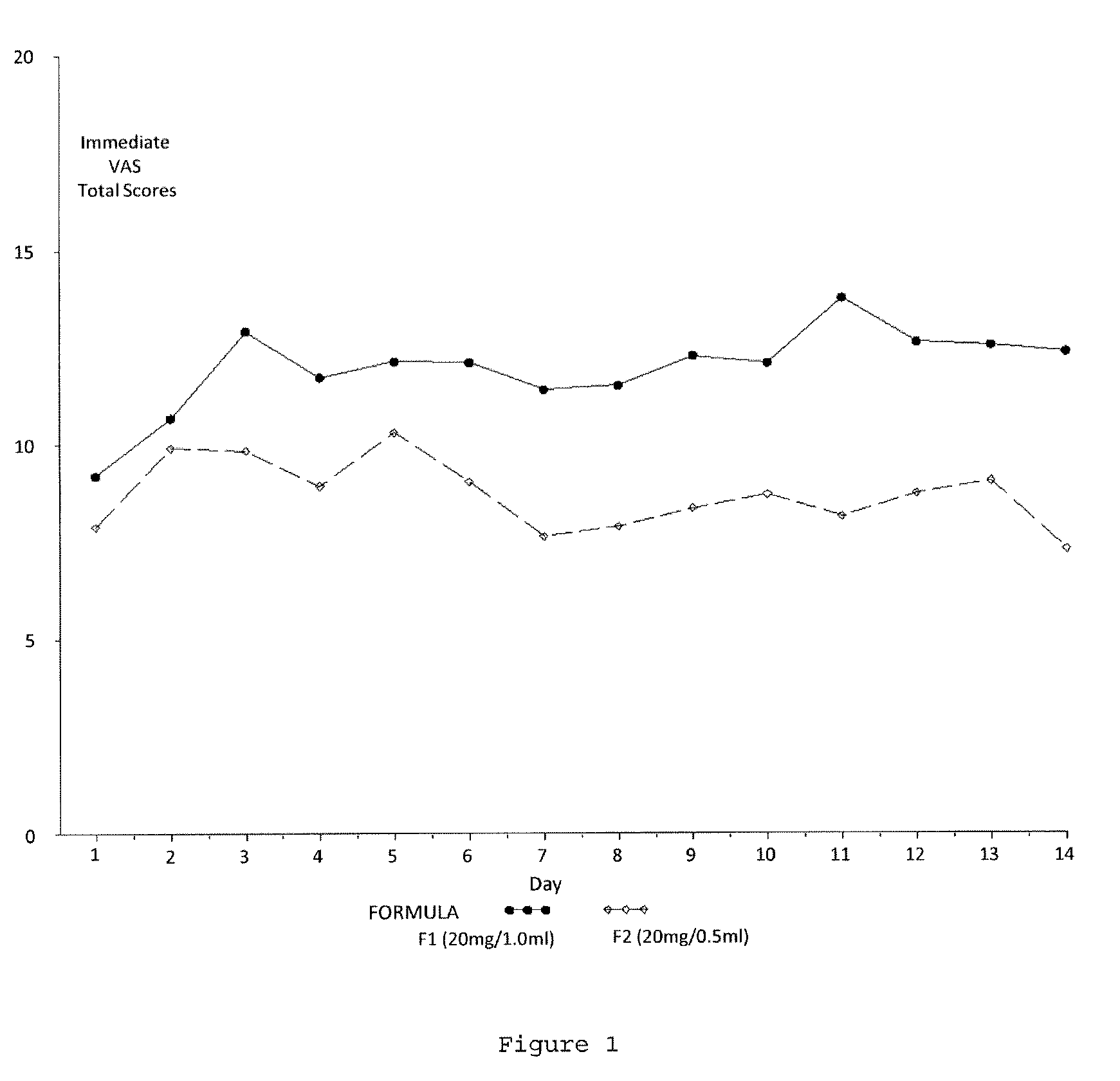
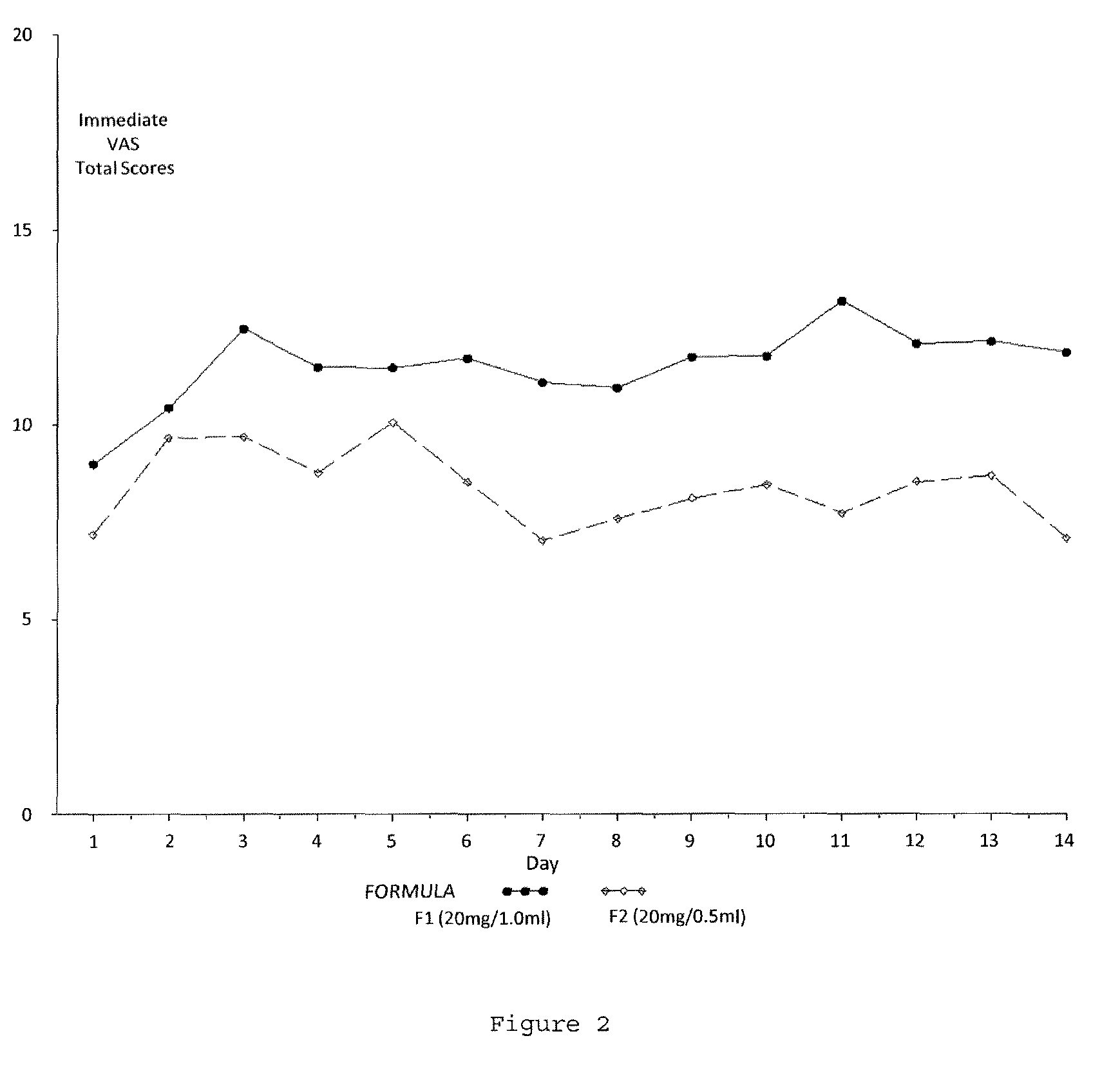
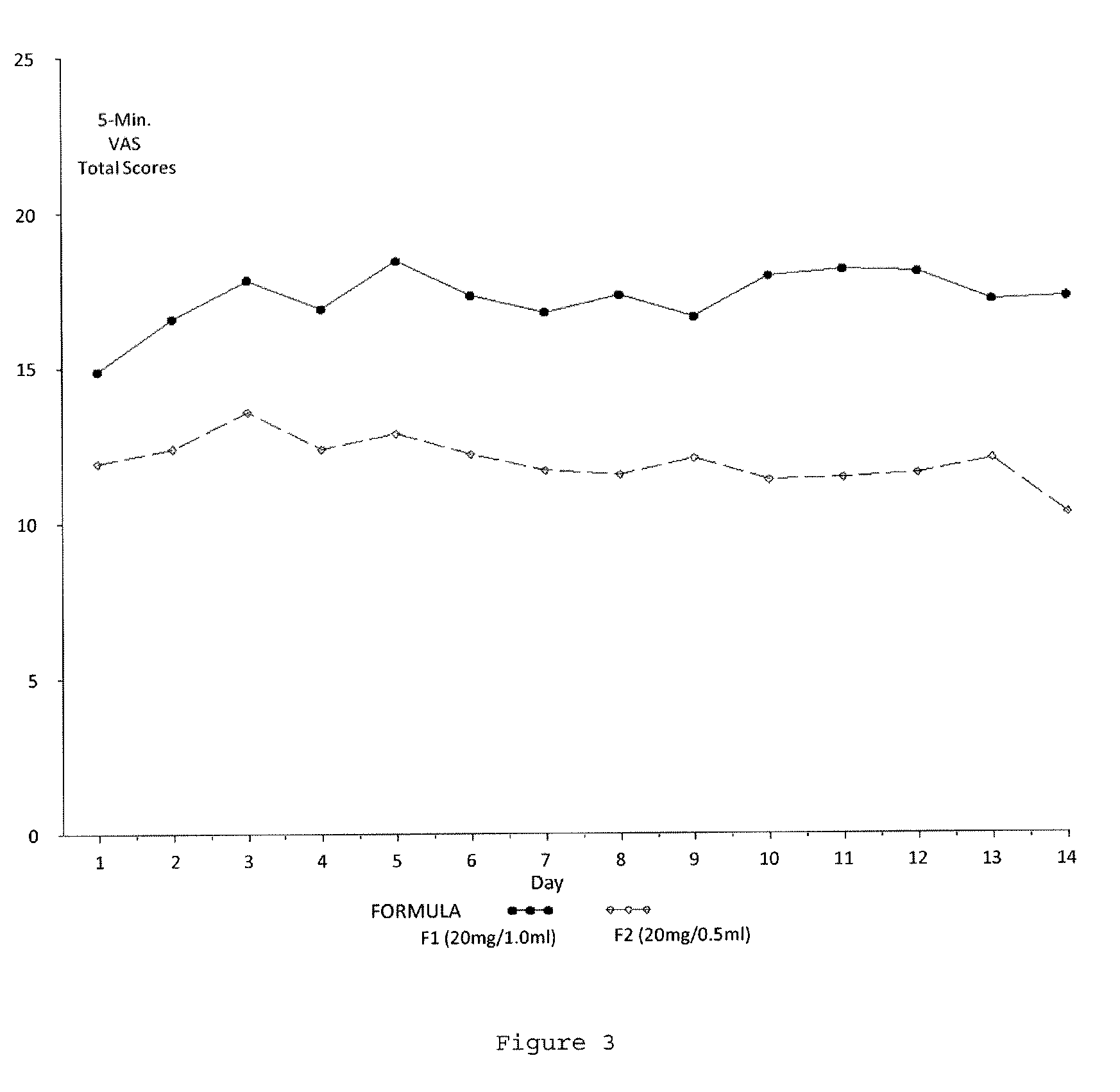
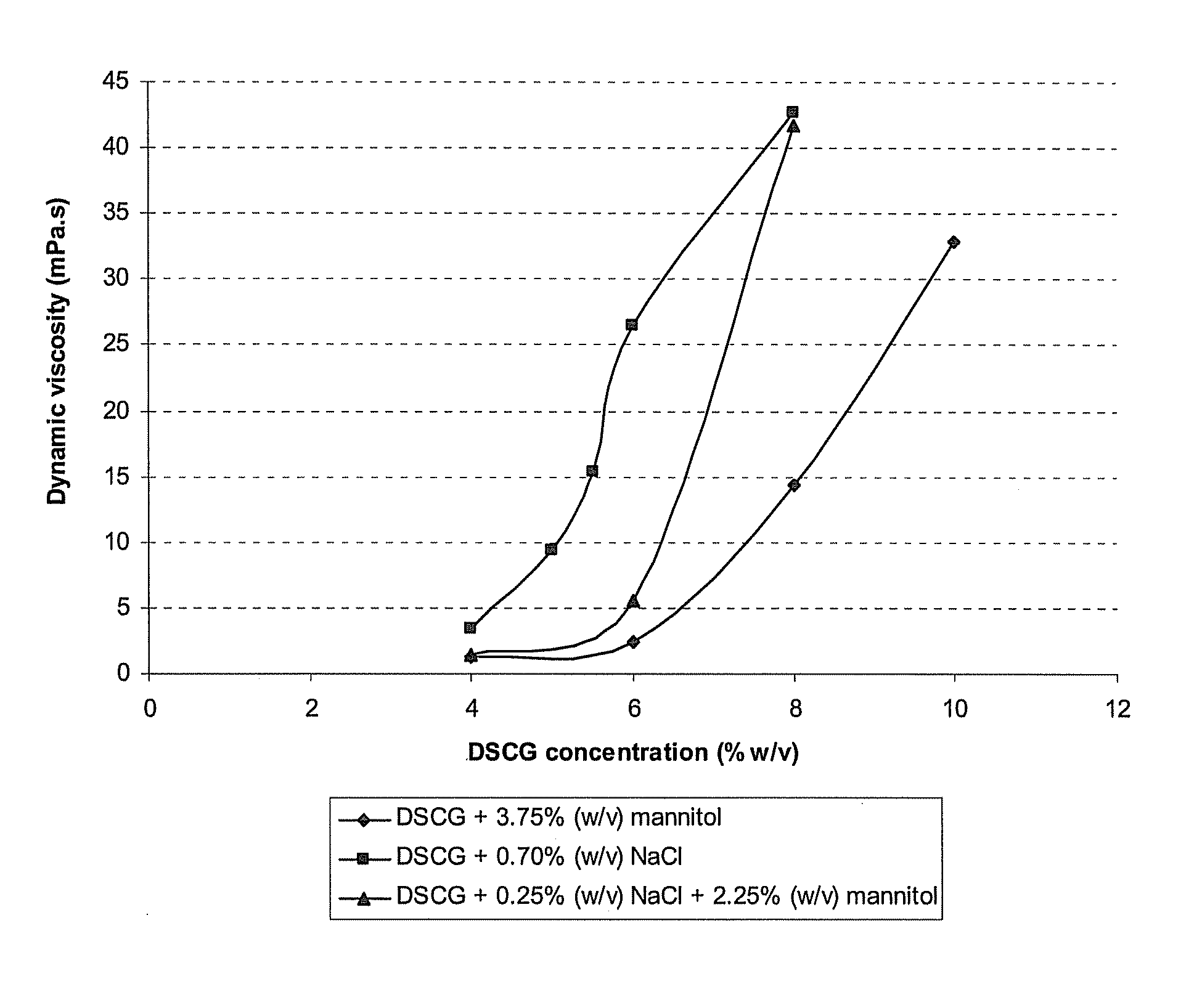
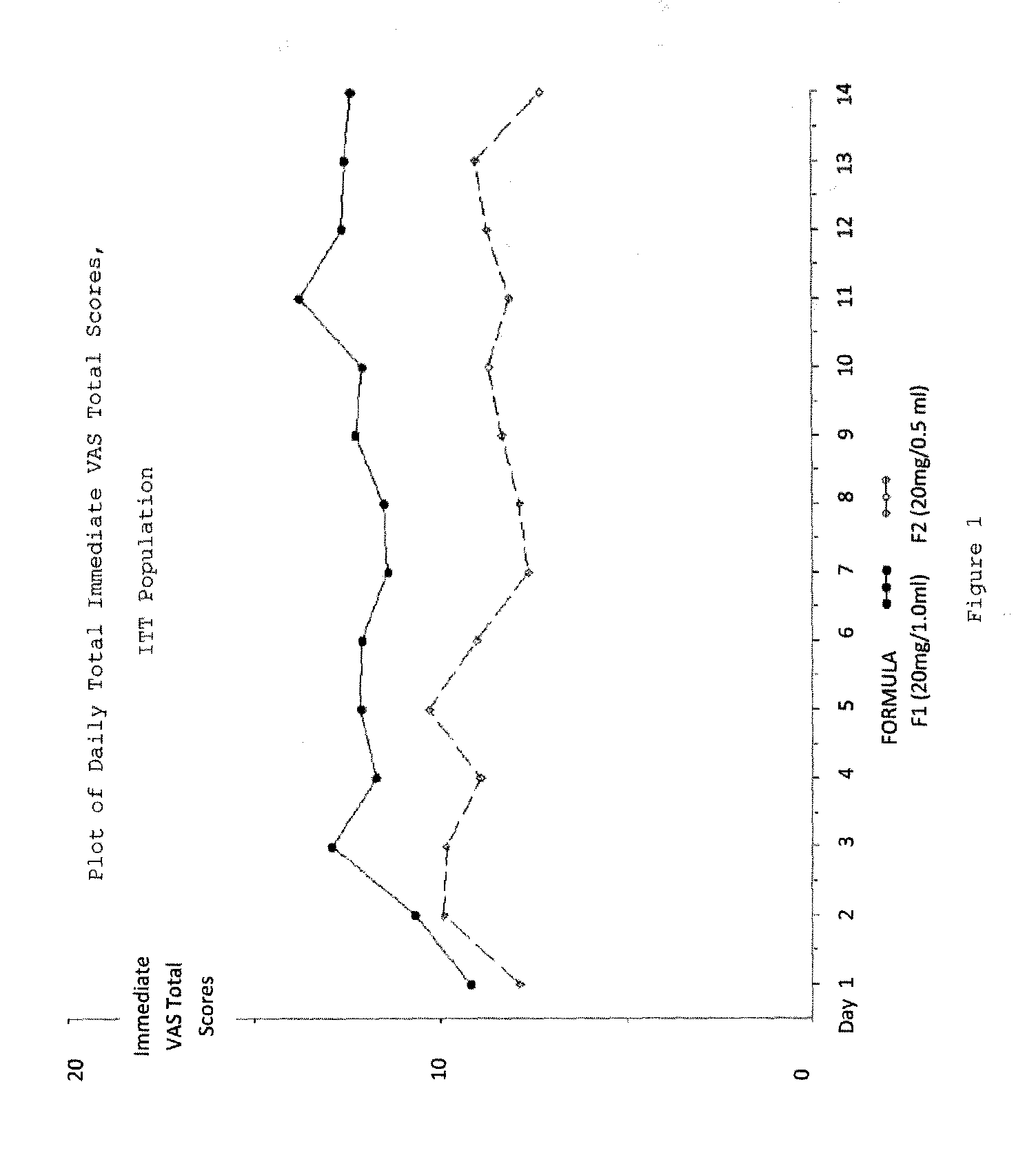
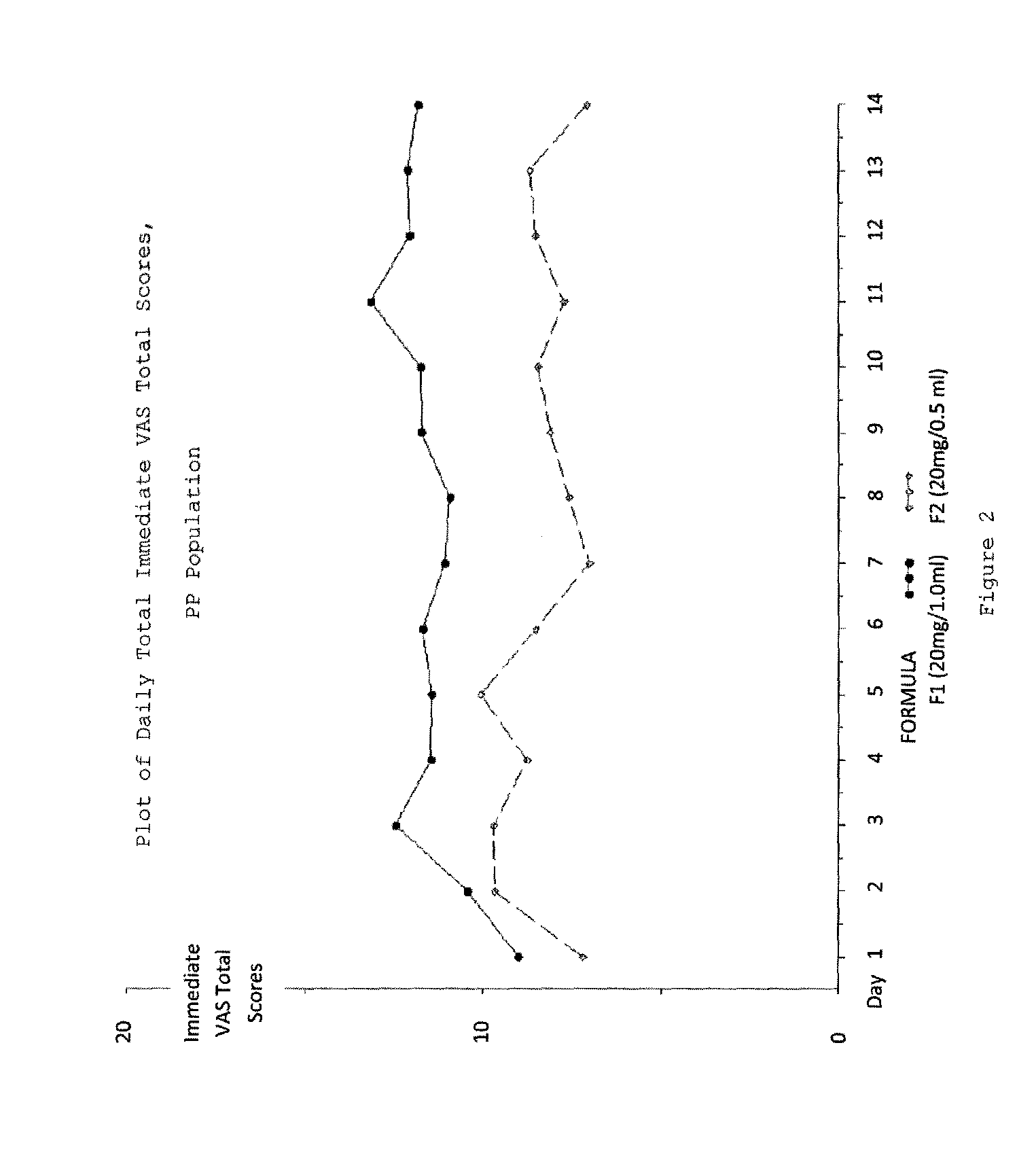
Reduced volume formulation of glatiramer acetate and methods of administration
A method for reducing frequency of relapses in a human patient afflicted with relapsing-remitting multiple sclerosis (RRMS) comprising administering to the patient 0.5 ml of an aqueous pharmaceutical solution of 20 mg glatiramer acetate and 20 mg mannitol.
Owner:TEVA PHARMA IND LTD
Concentrated mast cell stabilizing pharmaceutical formulations
ActiveUS9198859B2Improve securityIncrease concentrationBiocideDispersion deliveryMast cellActive agent
Liquid aqueous pharmaceutical solutions, containing a mast cell stabilizing active agent for application to the upper and lower respiratory tract or in the eye are provided. The solutions comprise both a non-ionic and an ionic tonicity-adjusting excipient. They are particularly useful for the aerosol treatment of respiratory diseases such as asthma. Furthermore, methods for nebulization of these solutions and methods of packaging the solutions are provided.
Owner:PARI PHARMA GMBH
Stable pharmaceutical solution formulations for pressurised metered dose inhalers
InactiveUS20040047809A1Avoid leachingGood formulation stabilityBiocideDispersion deliveryDrugs solutionAlkane
An aerosol solution composition for use in an aerosol inhaler comprises an active material, a propellant containing a hydrofluoroalkane, a cosolvent and optionally a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler. The composition is stabilized by using a small amount of mineral acid and a suitable can having part or all of its internal metallic surfaces made of stainless steel, anodized aluminium or lined with an inert organic coating.
Owner:CHIESI FARM SPA
Delamination resistant pharmaceutical glass containers containing active pharmaceutical ingredients
The present invention is based, at least in part, on the identification of a pharmaceutical container formed, at least in part, of a glass composition which exhibits a reduced propensity to delaminate, i.e., a reduced propensity to shed glass particulates. As a result, the presently claimed containers are particularly suited for storage of pharmaceutical compositions and, specifically, a pharmaceutical solution comprising a pharmaceutically active ingredient, for example, NEUPOGEN® (filgrastim), NEULASTA® (pegfilgrastim), (epoetin alfa) or ENBREL® (etanercept).
Owner:CORNING INC
Reduced Volume Formulation of Glatiramer Acetate and Methods of Administration
InactiveUS20110060279A1Reduce frequencyNervous disorderPeptide/protein ingredientsMANNITOL/SORBITOLHuman patient
A method for reducing frequency of relapses in a human patient afflicted with relapsing-remitting multiple sclerosis (RRMS) comprising administering to the patient 0.5 ml of an aqueous pharmaceutical solution of 20 mg glatiramer acetate and 20 mg mannitol.
Owner:TEVA PHARMA IND LTD
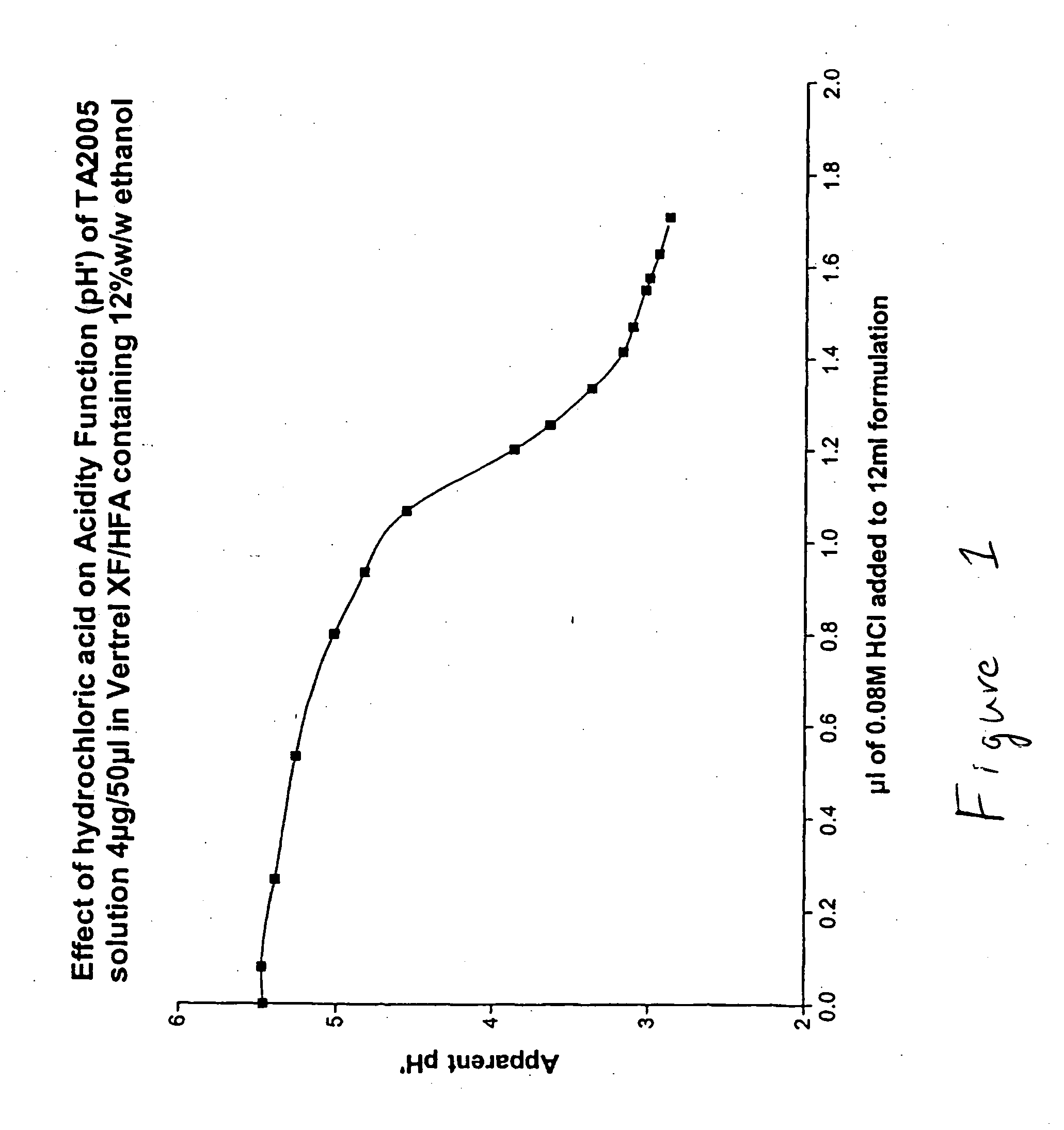
Stable pharmaceutical solution formulations for pressurized metered dose inhalers
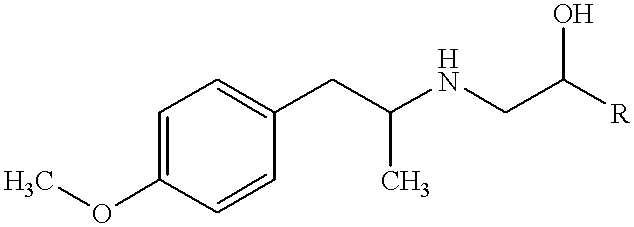
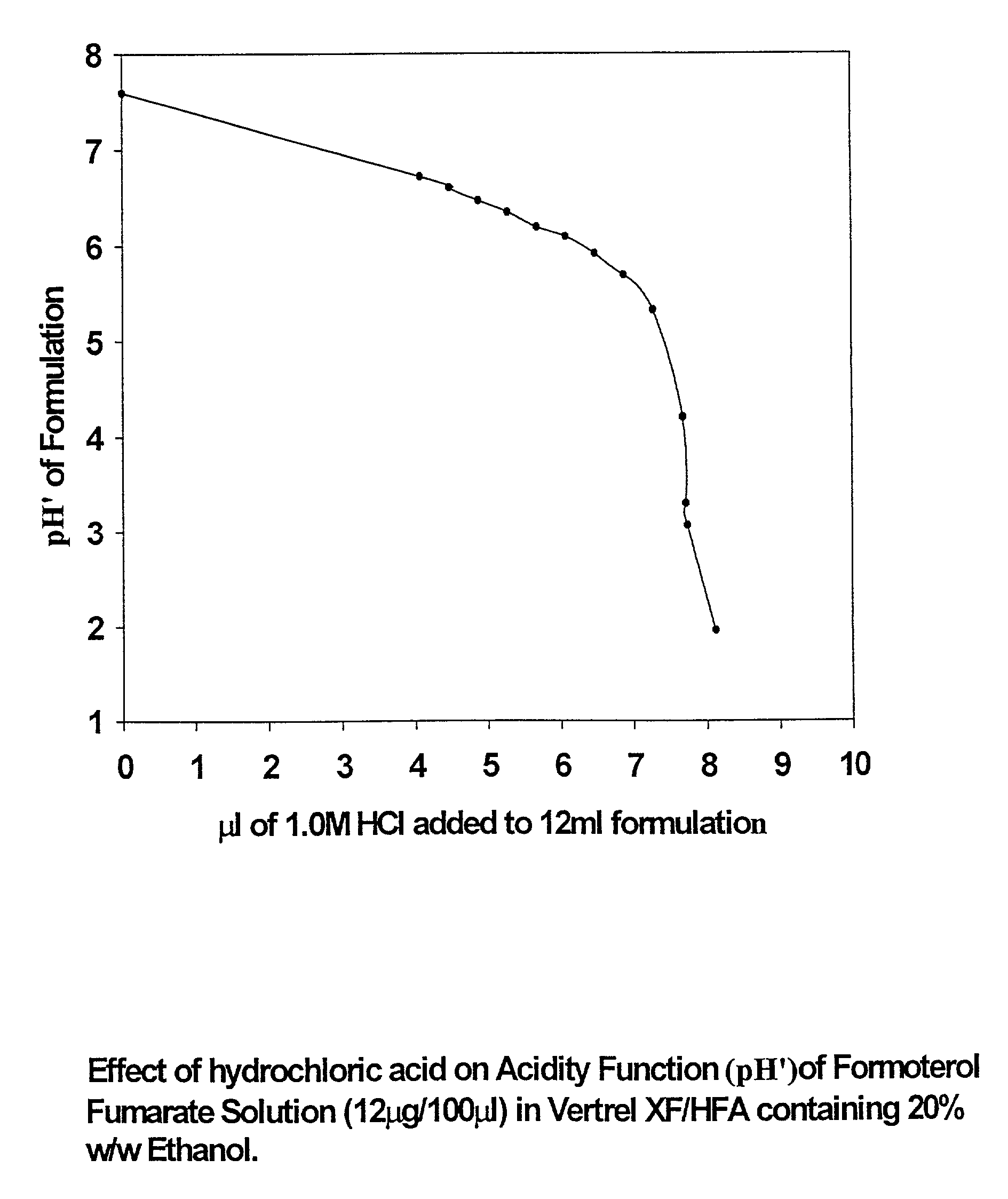
InactiveUS20050220718A1Extended shelf lifeAdequate shelf lifeBiocideDispersion deliveryO-Phosphoric AcidBULK ACTIVE INGREDIENT
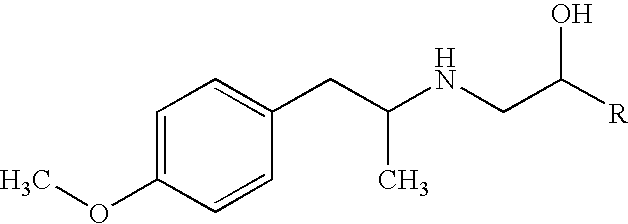
Aerosol solution formulations for use in an aerosol inhaler which comprise 8-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]-ethyl]-2(1H)-quinolinone or a salt thereof, in particular the hydrochloride salt (TA 2005), as an active ingredient, a propellant containing a hydrofluoroalkane, and a cosolvent, stabilized by addition of a specific small amount of a high concentrated phosphoric acid exhibit improved shelf life. The formulation may be optionally contained in a can having part or all of its internal metallic surfaces lined with an inert organic coating.
Owner:CHIESI FARM SPA
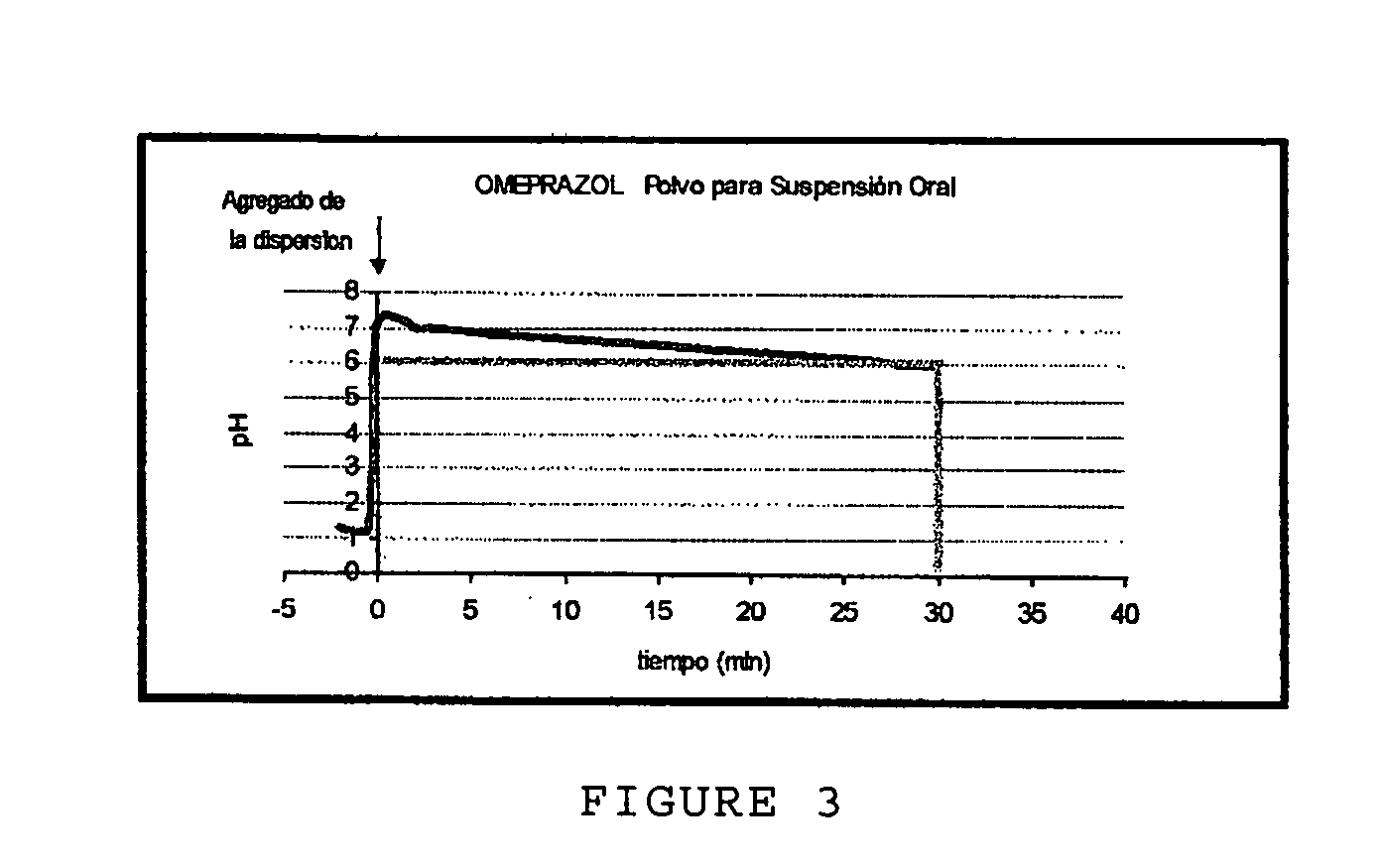
Anti-acid pharmaceutical composition in powder form and process for making it
ActiveUS20070281015A1Easy to controlImprove stabilityBiocideDispersion deliveryDrugs solutionDiluent
An anti-acid pharmaceutical composition for the rapid and prolonged neutralization of gastric acidity with mucosa-protecting activity in powder form to prepare, by dispersion in water, a pharmaceutical solution or suspension for oral use characterized in that the composition includes sodium alginate; an anti-acid soluble agent or a combination of anti-acids; an inhibitor of proton pump; diluent and sweetening agents, wherein a) at least 30% of sodium alginate present in the formulation along with the total of the inhibitor of proton pump are homogeneously distributed over the surface of the total soluble anti-acid agent or of the combination of anti-acids of the composition; and b) the rest, about 70%, of sodium alginate present in the formulation contains a percentage of humidity of less than 2%.
Owner:LAB BAGO
Antibiotic compound recipe comprising piperacillin
The invention involves antibiotic compound recipe containing piperacillin, sulbactam or clavulanic acid, ion chelator which can inhibit the formation of particles chelator, the recipe can be further added in buffering ingredient as stability system, the characteristics of the recipe is that it can be prepared to stable pharmaceutical solutions, and with aminoglycoside antibiotics in the same container are re-prepared to drugs used for complex anti-microbial infection.
Owner:ガンゾウ ヘメイ ファーマスーティカル カンパニー リミテッド
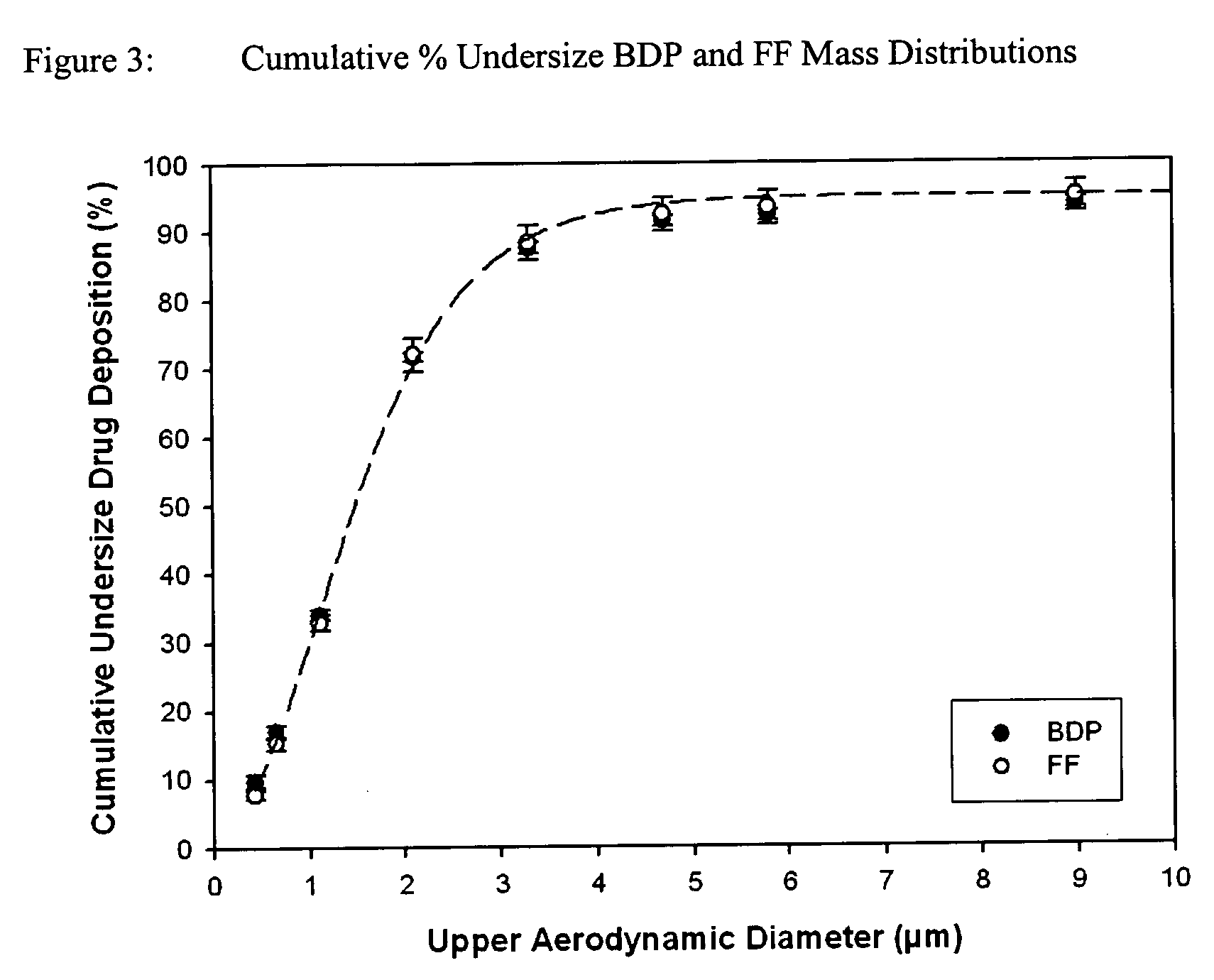
Pharmaceutical solution formulations for pressurised metered dose inhalers
The invention relates to the prevention and / or treatment of a severe broncho-pulmonary disease by administering a solution formulation from a pressurized metered dose inhaler capable of providing therapeutical doses of two or more active drug substances to the lung, wherein all the active drug substances are fully dissolved in the formulation as well as the two or more active drug substances are delivered with substantially the same particle size distribution.
Owner:CHIESI FARM SPA
Liquid dosage forms of isotretinoin
Owner:SUN PHARMA INDS
Glycopeptide compositions
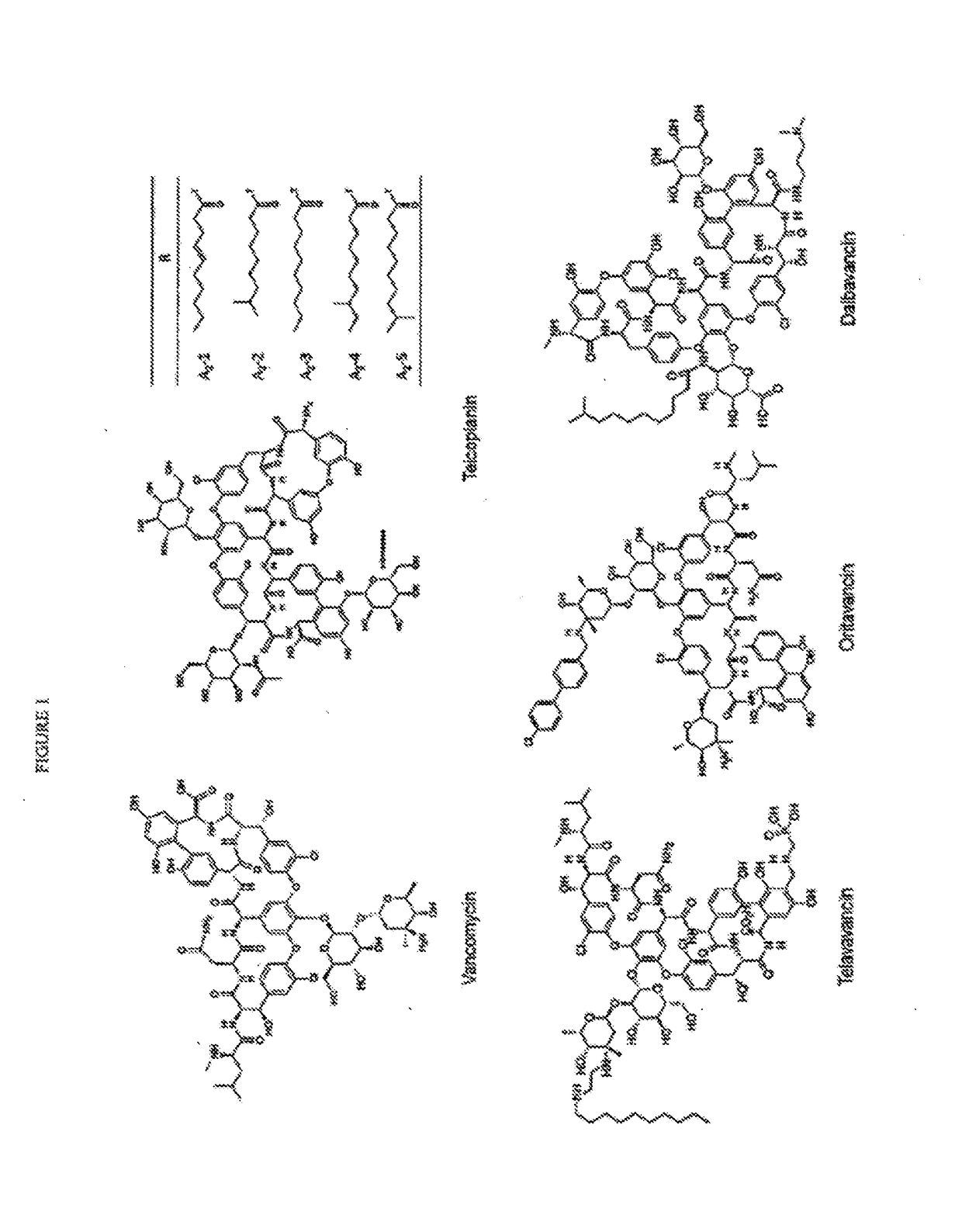
Solutions comprising a glycopeptide antibiotic, for example Vancomycin, and an amino acid or amino acid derivative such as N-acetyl-Glycine or N-acetyl-D-Alanine are provided. These solutions are stable or stabilized for long-term periods at conditions of normal use and storage, and can be formulated as pharmaceutical solutions for use in subjects. Methods of manufacturing and using these solutions are also provided, as are methods of stabilizing a glycopeptide antibiotic, for example Vancomycin, using amino acids or amino acid derivatives such as N-acetyl-Glycine or N-acetyl-D-Alanine.
Owner:AXELLIA PHARMA APS
Penicillin bottle dispensing method and penicillin bottle dispensing robot
InactiveCN107822877ASave human effortSave manpower, work efficiencyPharmaceutical containersMedical packagingDrugs solutionPenicillin
The invention discloses a vial dispensing robot, which comprises a conveying device for transporting the vials, a cap-lifting device for lifting the caps of the vials, and a cap-lifting device for sterilizing the mouth of the vials are sequentially arranged along the transporting device. Disinfecting device, air blowing device and dissolving and dispensing device; the air blowing device includes an air pipe connected with the air source, and the mouth of the air pipe is aligned with the vial on the conveying device to blow off the residual disinfectant at the mouth of the vial; dissolving and dispensing The device is used for injecting the solvent in the liquid bag into the vial and injecting the liquid medicine in the vial after dissolving the drug into the liquid bag. Application of the vial dispensing robot provided by the present invention can realize fully automatic and semi-automatic operation of dispensing vials, save manpower, and have high work efficiency. Simple structure, convenient operation, small footprint. It is suitable for hospitals and other institutions with a large amount of medicines to dissolve and dispense large quantities of medicines. The invention also discloses a medicine dispensing method for vials, which also has the above-mentioned technical effect.
Owner:韩秋霞
Stable pharmaceutical solution formulations for pressurized metered dose inhalers
InactiveUS7381402B2Adequate shelf lifeBiocideDispersion deliveryPhosphoric acidBULK ACTIVE INGREDIENT
Owner:CHIESI FARM SPA
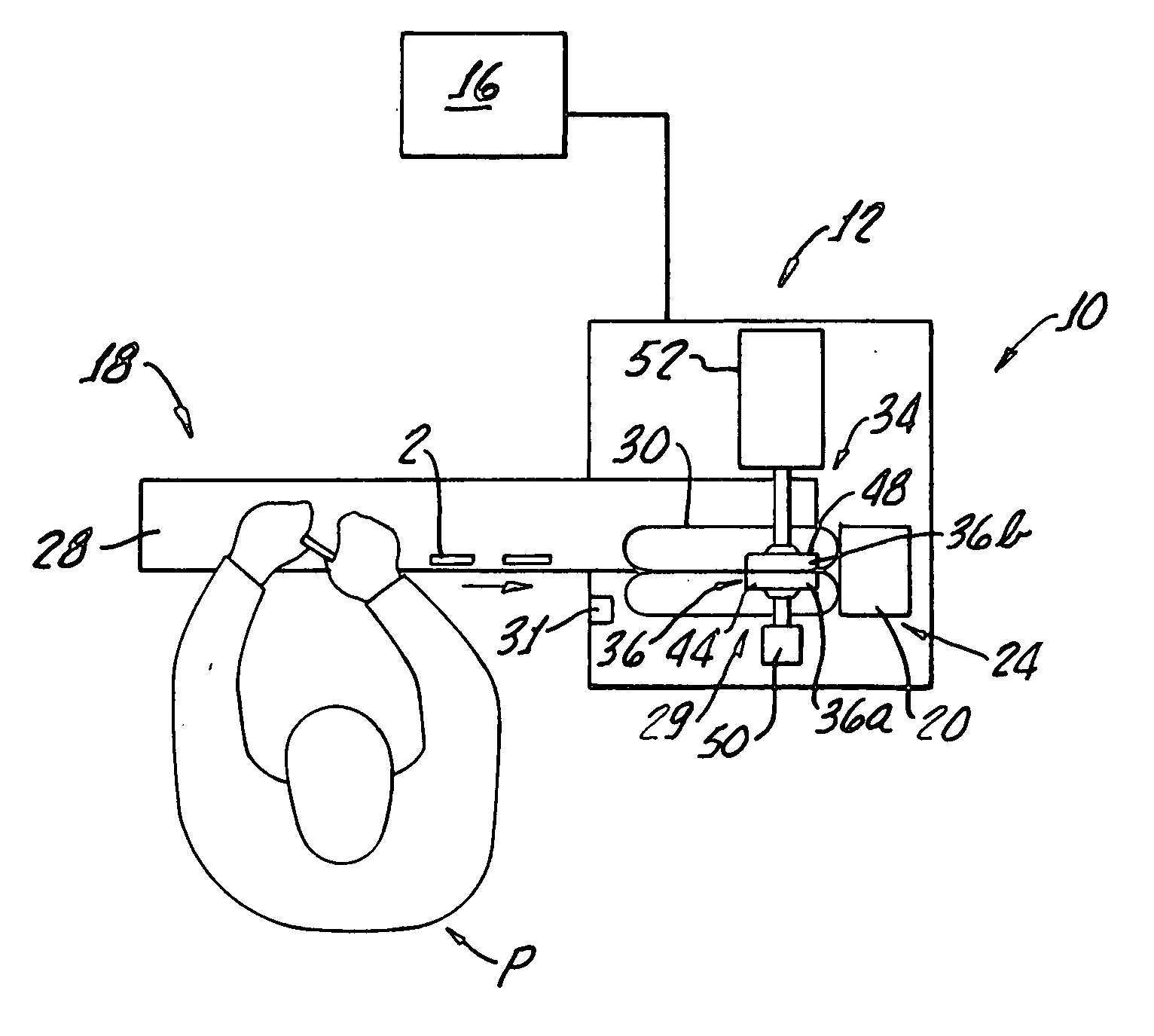
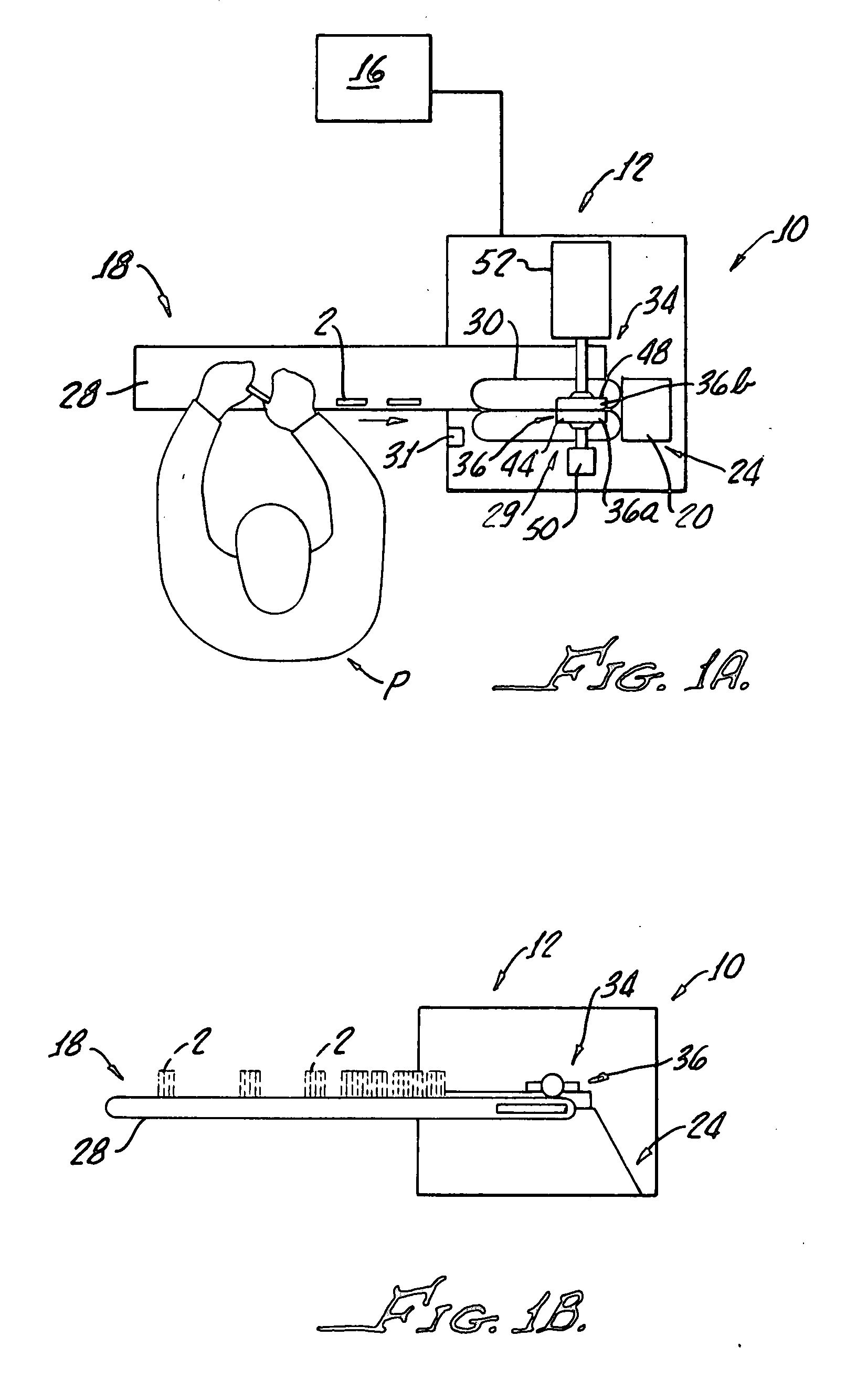
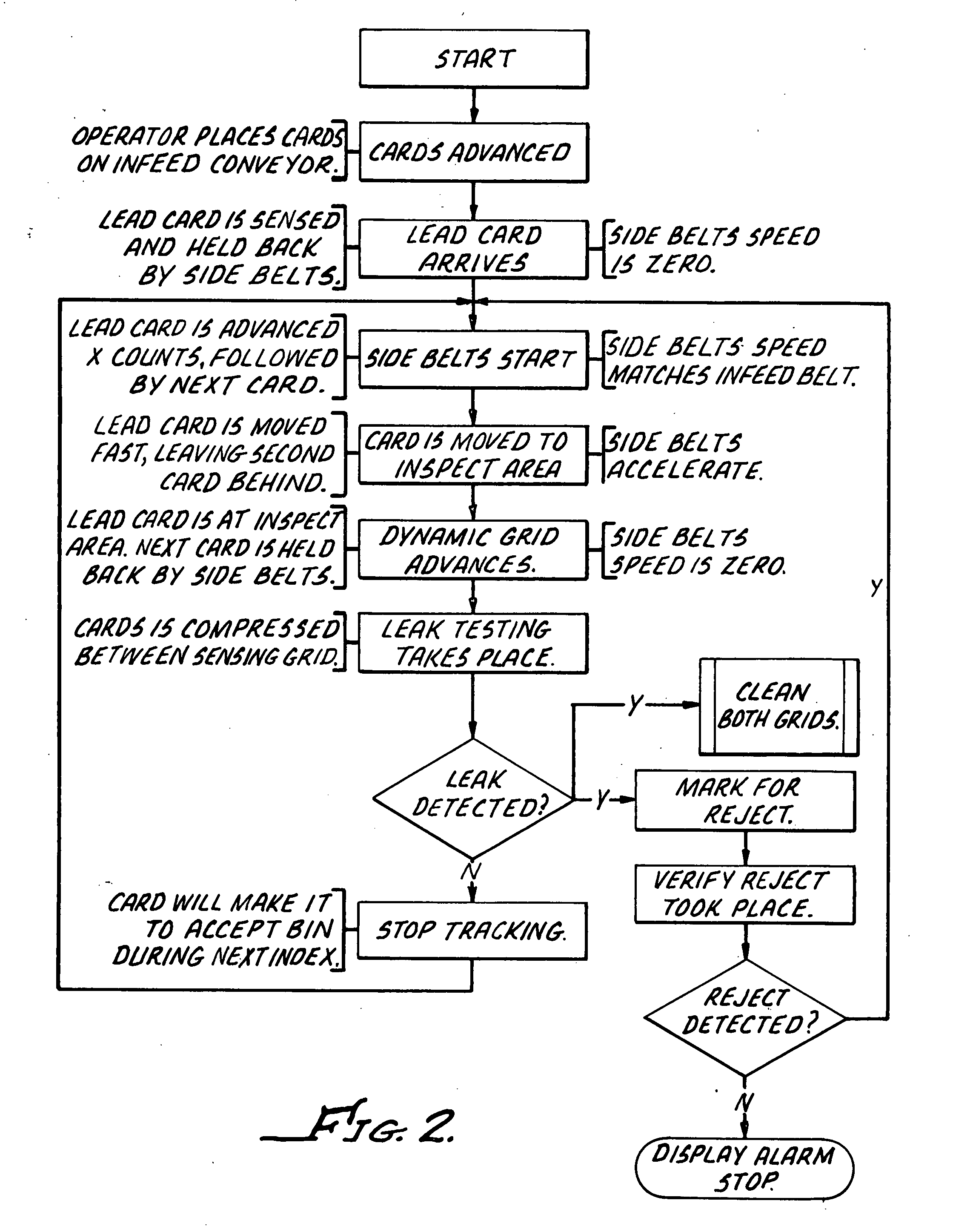
Ampoule card leak detector assembly
InactiveUS20070295060A1Soft and flexibleConvenience to workMeasurement of fluid loss/gain rateEngineeringMaterial Perforation
Systems and methods are provided for inspecting ampoule packages for the presence of leaks. The systems and methods may be part of a manufacturing line for ampoule packages, particularly flexible ampoule cards that contain sterile pharmaceutical solutions. The solution-containing packages are compressed in an amount sufficient to cause leaking of solution from a pre-existing tear or perforation in an ampoule. The amount of compression applied is less than that required to cause significant package damage such as bursting. The packages are compressed using surfaces of sensing grids which are effective in detecting presence of a liquid outside the package during the compression. By using the conductive properties of the solution, sensing grids and appropriate circuitry can be used to signal or cause an alarm in the event a package has been determined to contain a leak.
Owner:ALLERGAN INC
Drug administration preparations of ligustrazine for nasal mucosa and method for preparing the same
InactiveCN101152182AImprove distributionImprove treatment efficiencyOrganic active ingredientsAerosol deliveryNasal cavitySide effect
The invention belongs to medical technical field and discloses nasal mucosa medication agent of tetramethylpyrazine and the preparation method. The agent contains tetramethylpyrazine, pharmaceutical excipient and absorption enhancer. The weight percentage is 1:0.1 to 100:0.1 to 10. The tetramethylpyrazine includes tetramethylpyrazine prepared in various methods and salts of tetramethylpyrazine. Formula dosage of tetramethylpyrazine is put into absorption enhancer and additional pharmaceutical solutions of suitable concentration prepared in advance to produce nasal mucosa medication agent, such as nasal drops, spray, aerosol, microsphere agent, liposomes, etc. Via the special physiological structure of nasal cavity, the agent medicates via nasal mucosa and allows tetramethylpyrazine bypasses the blood-brain barrier to enhance the distribution of medicine in brain tissue. The drug can be used to treat migraine, cerebral ischemia, cerebral embolism and emergencies such as angina pectoris, coronary heart disease, myocardial infarction, etc, and the drug has the advantages of complete and rapid absorption, high treating effects, high stability, and few side effects.
Owner:SHENYANG PHARMA UNIVERSITY
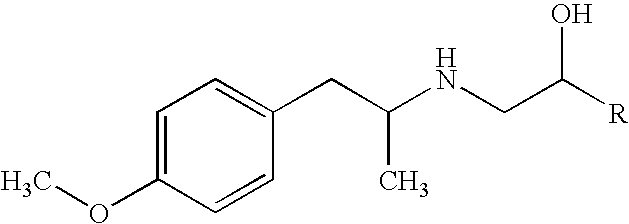
Aerosol pharmaceutical solution formulation containing glucocorticoids stable to the storage; method for stabilizing formulations and use of a stabilizer
Aerosol pharmaceutical solution formulations containing glucocorticosteroids stabilized by adding water or a mixture of water and citric acid, avoiding corrosion of the elements of container under standard storage conditions are described. The formulations comprise: between 0.05 and 1.0% by weight of a glucocorticoid having a C-20 ketone and OH group in carbons 17 and / or 21 as active substance; between 0.10 and 3% by weight of a selected stabilizer selected between water, or a mixture of water and organic acid selected between citric acid and tartaric acid; a cosolvent in amount sufficient to solubilize the active substance; optionally a surfactant; and propellant in sufficient amount to achieve100% by weight of the finished solution. Glucocorticosteroids having a C-20 ketone and an OH group at the C-17 and / or 21 position with varying substituents, have many well-known therapeutic uses, especially based upon their anti-inflammatory activity. This types of steroids, glucocorticosteroids, and their pharmaceutical formulations are useful in the treatment of several diseases including bronchial disorders and inflammatory conditions. Preferably, the glucocorticoid is selected between Triamcinolone Acetonide, budesonide, Dexamethasone and betamethasone 17-valerate. A method for stabilizing aerosol pharmaceutical solution formulations containing glucocorticoids susceptible to oxidative degradation and use of a stabilizer selected between water and a mixture of water and organic acid selected between citric acid and tartaric acid are also described
Owner:LAB PABLO CASSARA
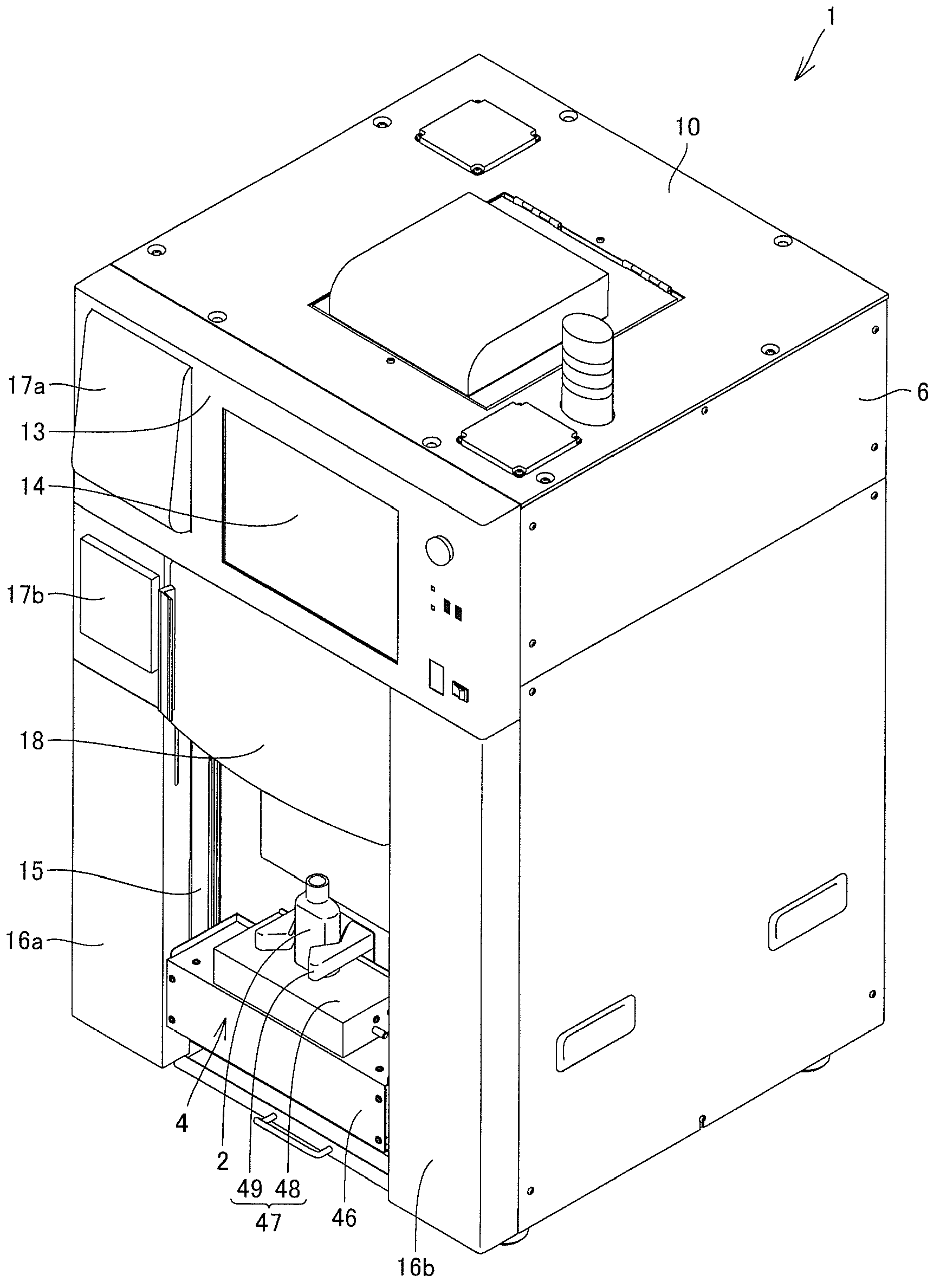
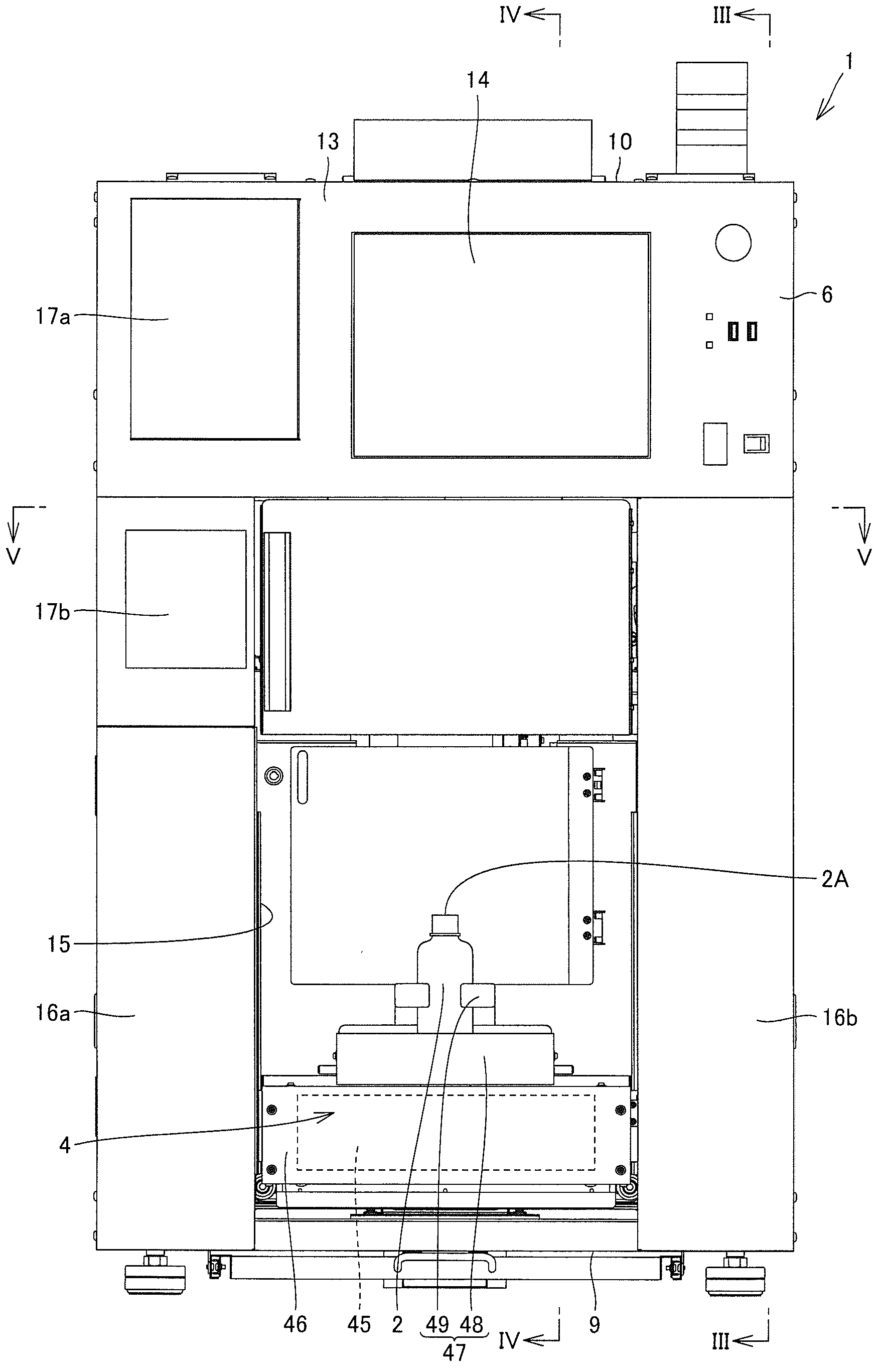
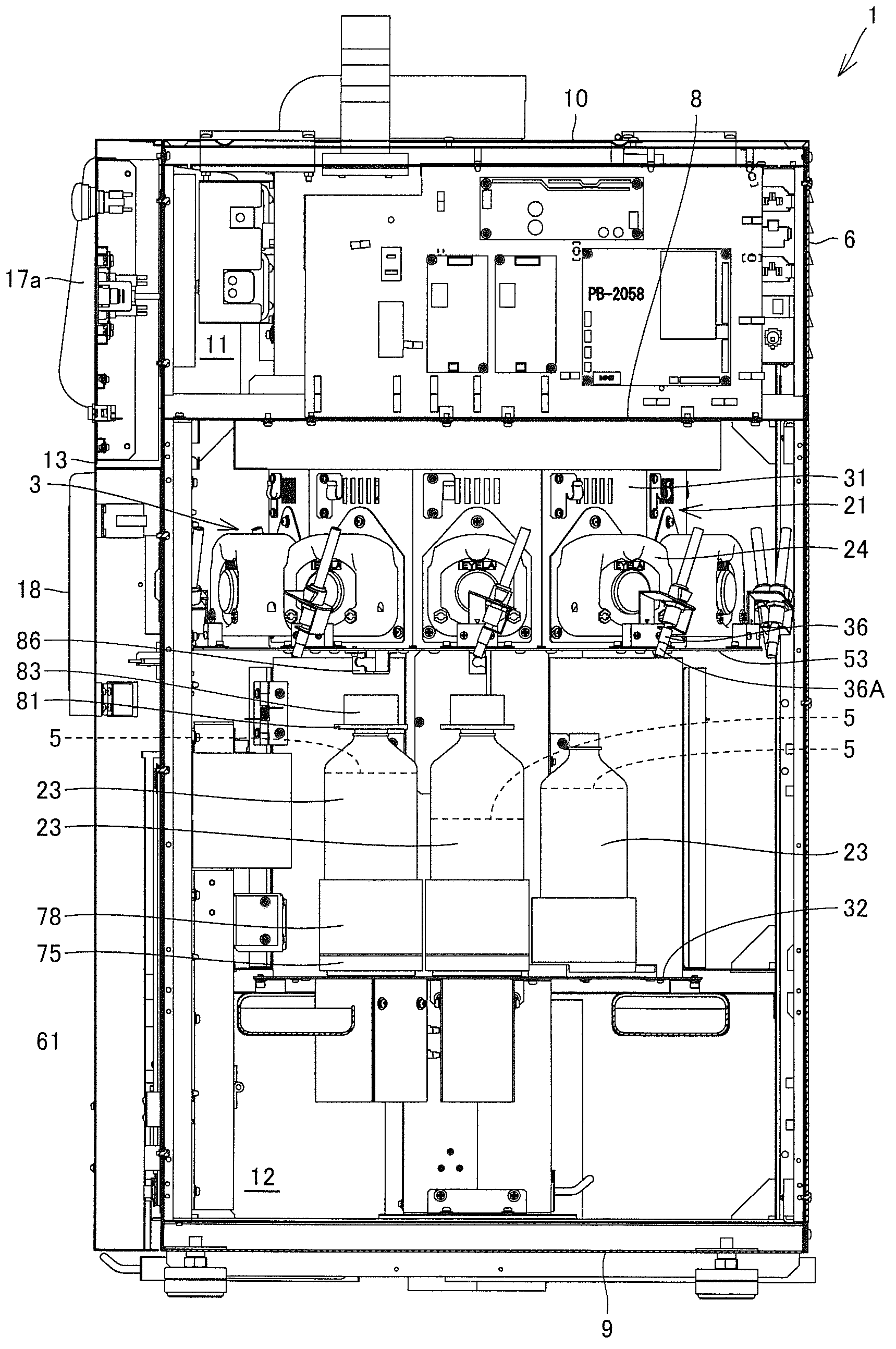
Pharmaceutical solution-dispensing device
Provided is a pharmaceutical solution-dispensing device capable of shortening the time for dispensing pharmaceutical solutions into a dosing bottle. The pharmaceutical solution-dispensing device, which dispenses pharmaceutical solutions accommodated in pharmaceutical solution bottles into a dosing bottle, is provided with: a pharmaceutical solution stirrer that stirs a pharmaceutical solution inside a pharmaceutical solution bottle; a bottle holder that holds a plurality of pharmaceutical solution bottles including a first bottle accommodating a pharmaceutical solution (G) and a second bottle accommodating a pharmaceutical solution (B); and a control unit that controls the actions of the pharmaceutical solution-dispensing device. The control unit operates the pharmaceutical solution stirrer to stir the pharmaceutical solution (B) while the pharmaceutical solution (G) is being dispensed from the first bottle into the dosing bottle.
Owner:TAKAZONO TECH
Oral suspension comprising telmisartan
ActiveUS20140364473A1Improve subjective overall impressionIncrease contact timeOrganic active ingredientsBiocideOral suspensionsAlcohol sugars
A pharmaceutical solution with a pH value of 10 or higher contains an angiotensin II receptor antagonist, where one or more sugar alcohols are present up to a total concentration of 40 wt. % to 70 wt. %.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Method of preparing multiple doses of a pharmaceutical solution from a single-dose
ActiveUS20100288393A1Dead space is minimizedLow cost of treatmentIntravenous devicesEye treatmentMedicineSingle use
A method of preparing multiple doses of a pharmaceutical solution, such as ranibizumab, from a single-dose container, includes providing a sterile enclosed area with a plurality of unused sterile syringes, a decapper, and a plurality of sterile bags, opening a single-use container of a pharmaceutical solution in the enclosed area, withdrawing a first portion of the pharmaceutical solution using one of the sterile syringes, withdrawing a second portion of the pharmaceutical solution using a second of the sterile syringes, repeating the previous step for the remaining pharmaceutical solution using the remaining sterile syringes, and placing the sterile syringes containing portions of the pharmaceutical solution individually in the sterile bags.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Drug dissolving and dispensing integrated machine of hard drug bottle
InactiveCN107737015ADissolve fastFast dissolutionPharmaceutical containersMedical packagingPhysical well beingSolvent
The invention discloses an all-in-one machine for dissolving and dispensing hard medicine bottles, which comprises a driving part, a moving assembly, a liquid bag clamping device for fixing a liquid bag, a dissolving and dispensing needle clamping device for fixing a dissolving and dispensing needle; The dispensing needle includes a long needle and a short needle. The needle tube of the long needle communicates with the liquid medicine buffer device through the connecting tube, and the needle tube of the short needle communicates with the liquid bag through the dispensing tube; the driving part is used to send the solvent of the liquid bag through the needle of the short needle. into the hard medicine bottle and the needle of the short needle draws the medicine in the hard medicine bottle back into the liquid bag; the moving component is used to drive one of the dispensing needle clamping device and the hard medicine bottle to move to the other, In order to insert or pull out the hard medicine bottle with the medicine dissolving needle. The all-in-one machine for dissolving and dispensing hard medicine bottles provided by the present invention is suitable for dissolving and dispensing large quantities of medicines in hospitals and other institutions with a large amount of medicine, which can greatly reduce labor intensity, significantly improve work efficiency, and can protect the health of dispensing personnel to the greatest possible extent. .
Owner:韩秋霞
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com