Reduced Volume Formulation of Glatiramer Acetate and Methods of Administration
a technology of glatiramer acetate and glatiramer acetate, which is applied in the direction of drug compositions, peptide/protein ingredients, immunological disorders, etc., can solve the problems of reducing the frequency of relapse, affecting the effect of relapse, and unable to predict the effect of any modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
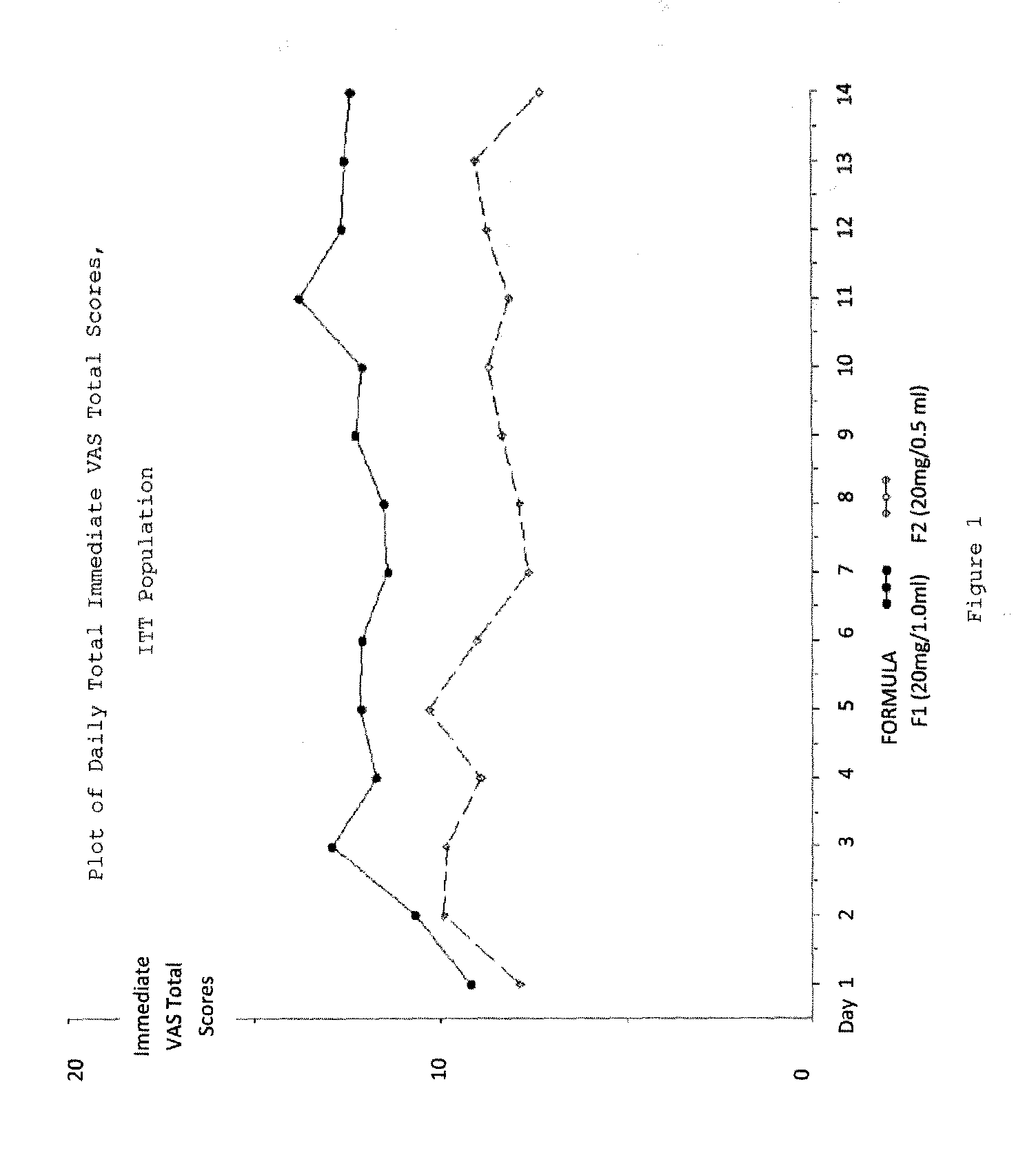
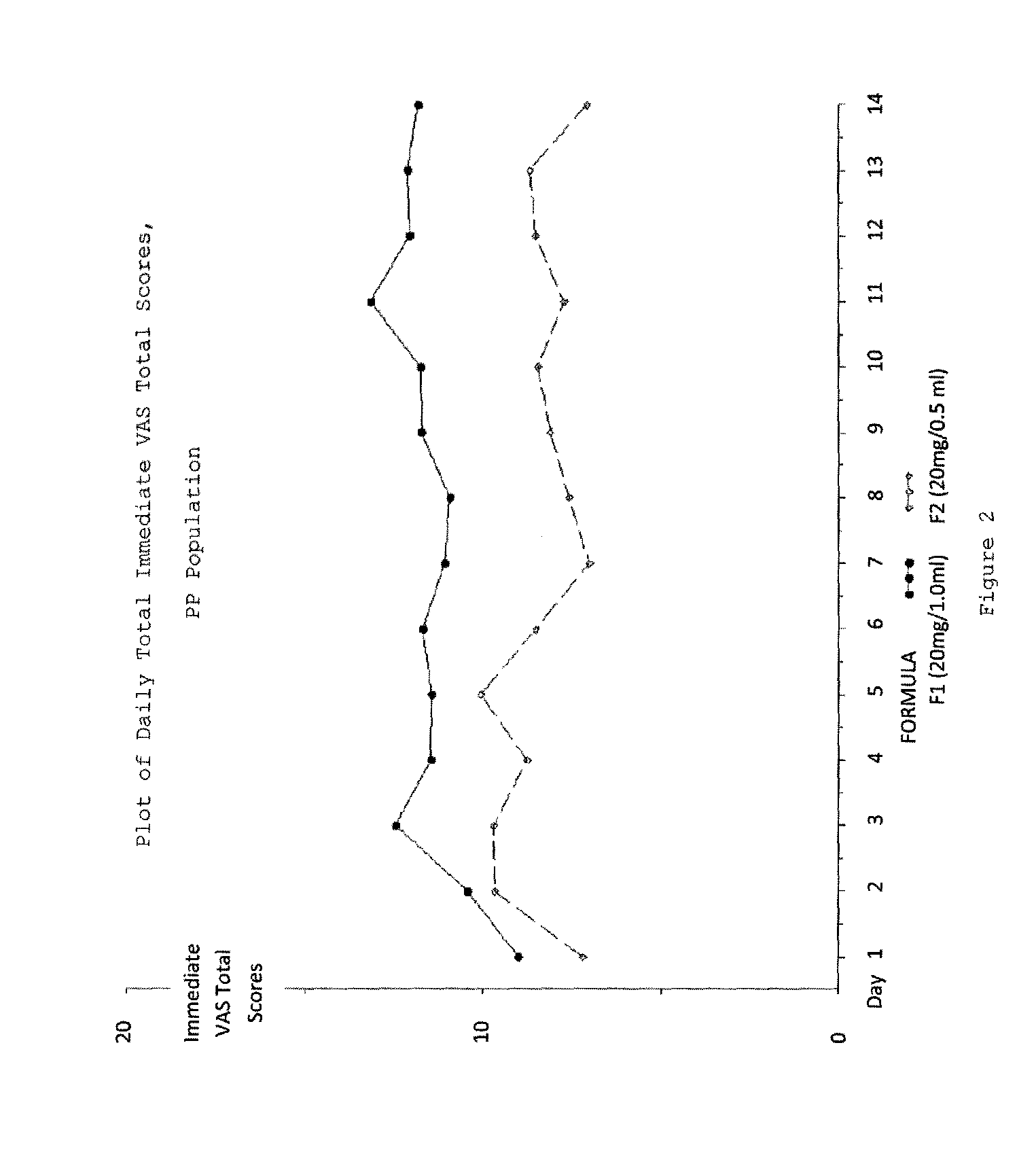
Evaluating Subject-Reported Injection Pain Associated with Injections of a 20 mg / 0.5 ml Formulation of GA
[0196]A multicenter, randomized, two arm, single crossover study was undertaken to compare the subject-reported pain of GA 20 mg / 1.0 ml (F1) versus GA 20 mg / 0.5 ml (F2) administered subcutaneously in subjects with RRMS. Safety and tolerability of the F2 formulation were also assessed. Subjects received both doses once daily in a cross over fashion, for a total treatment duration of five (5) weeks. Subject-reported injection pain was recorded in a daily diary. The primary endpoint was the difference in daily subject-reported injection pain occurring immediately after the injection, for the two GA formulations (F1 versus F2), as recorded on a 100 mm VAS. Secondary objectives included:[0197]To compare subject-reported injection pain associated with injections of F1 versus F2 5 minutes following injection.[0198]To compare the subject-reported presence or absence of Local Injection Si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com