Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
163 results about "Local injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
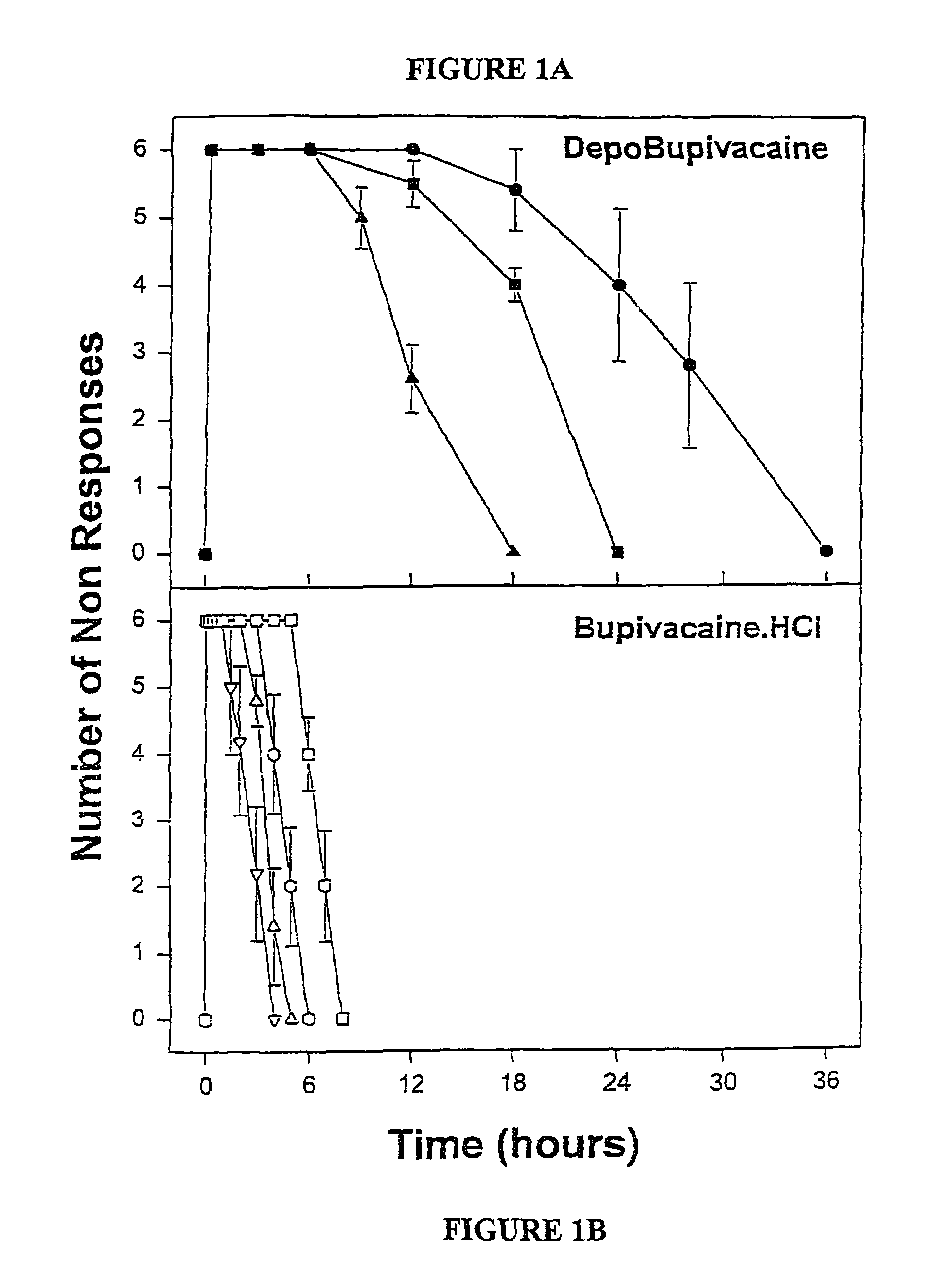
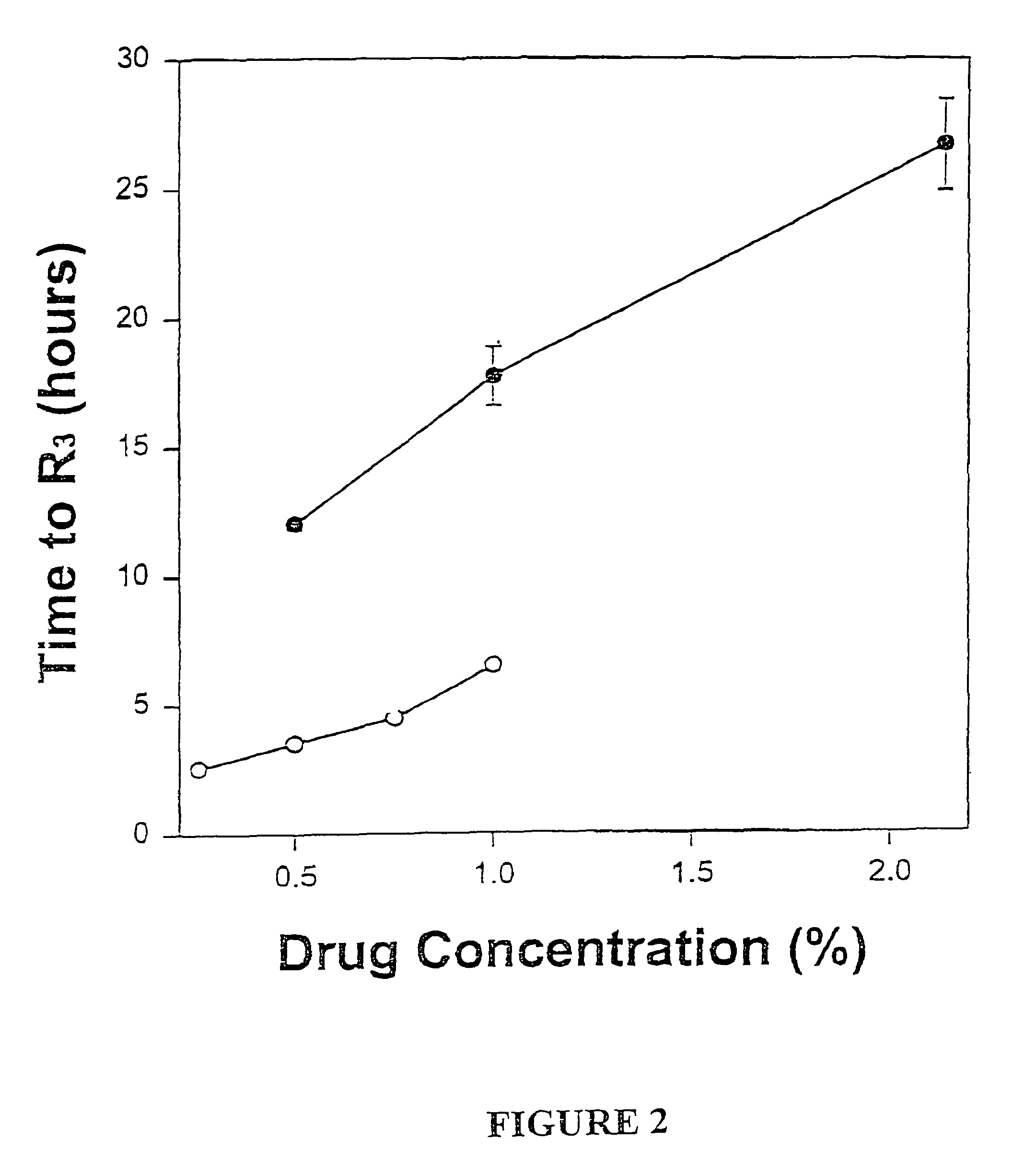
Local or regional anesthesia involves the injection or application of an anesthetic drug to a specific area of the body, as opposed to the entire body and brain as occurs during general anesthesia. Purpose. Local anesthetics are used to prevent patients from feeling pain during medical, surgical, or dental procedures.
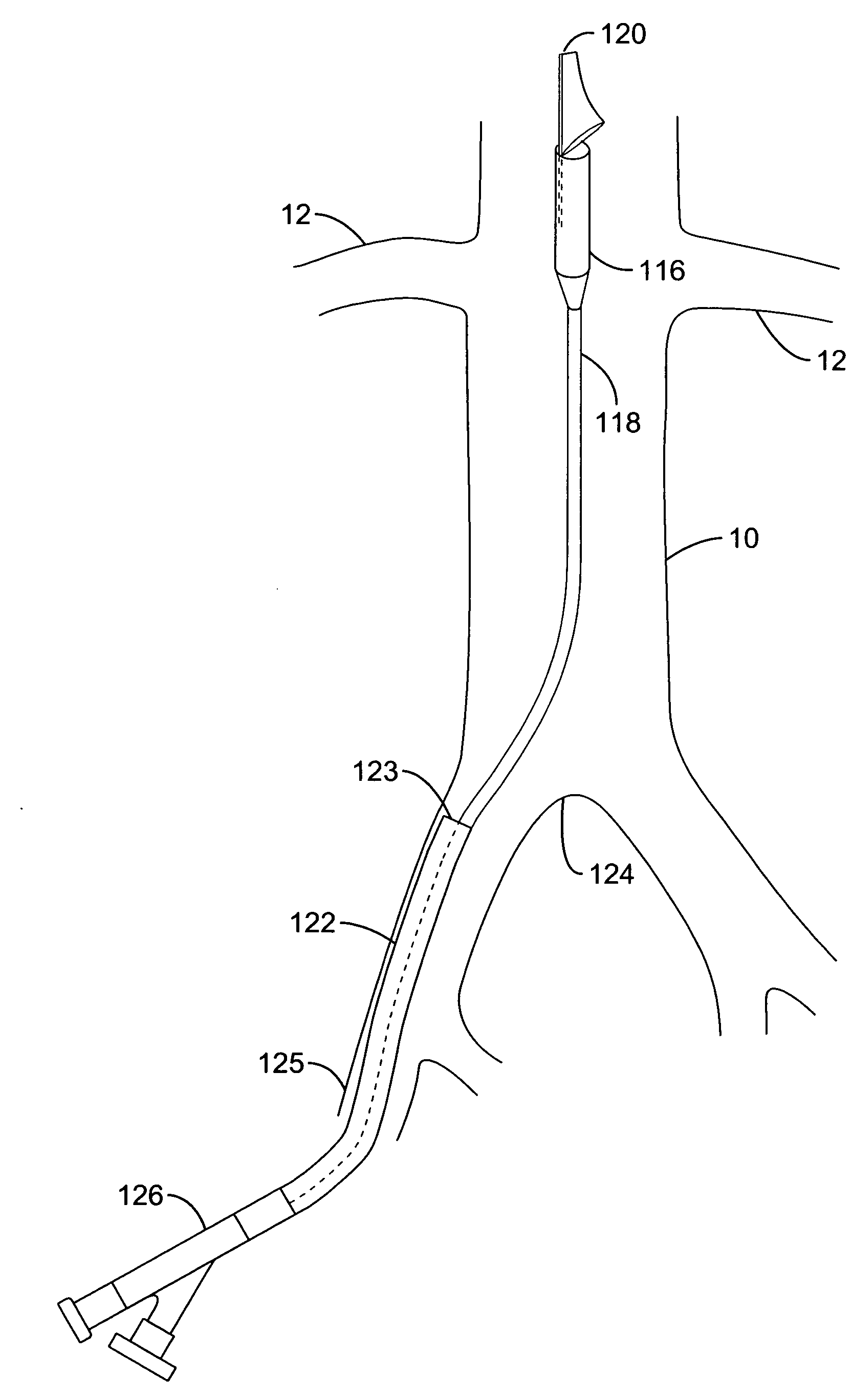
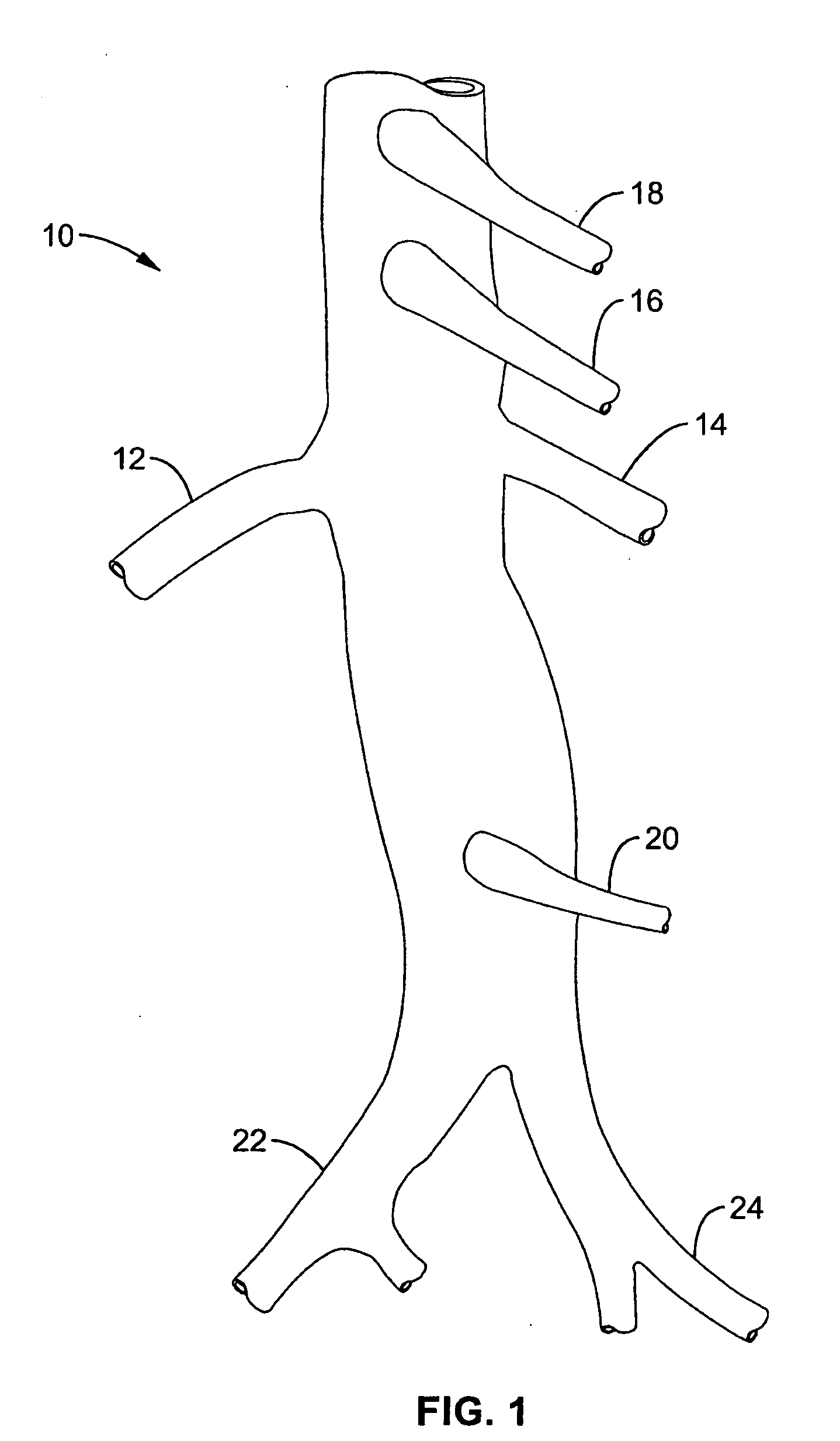
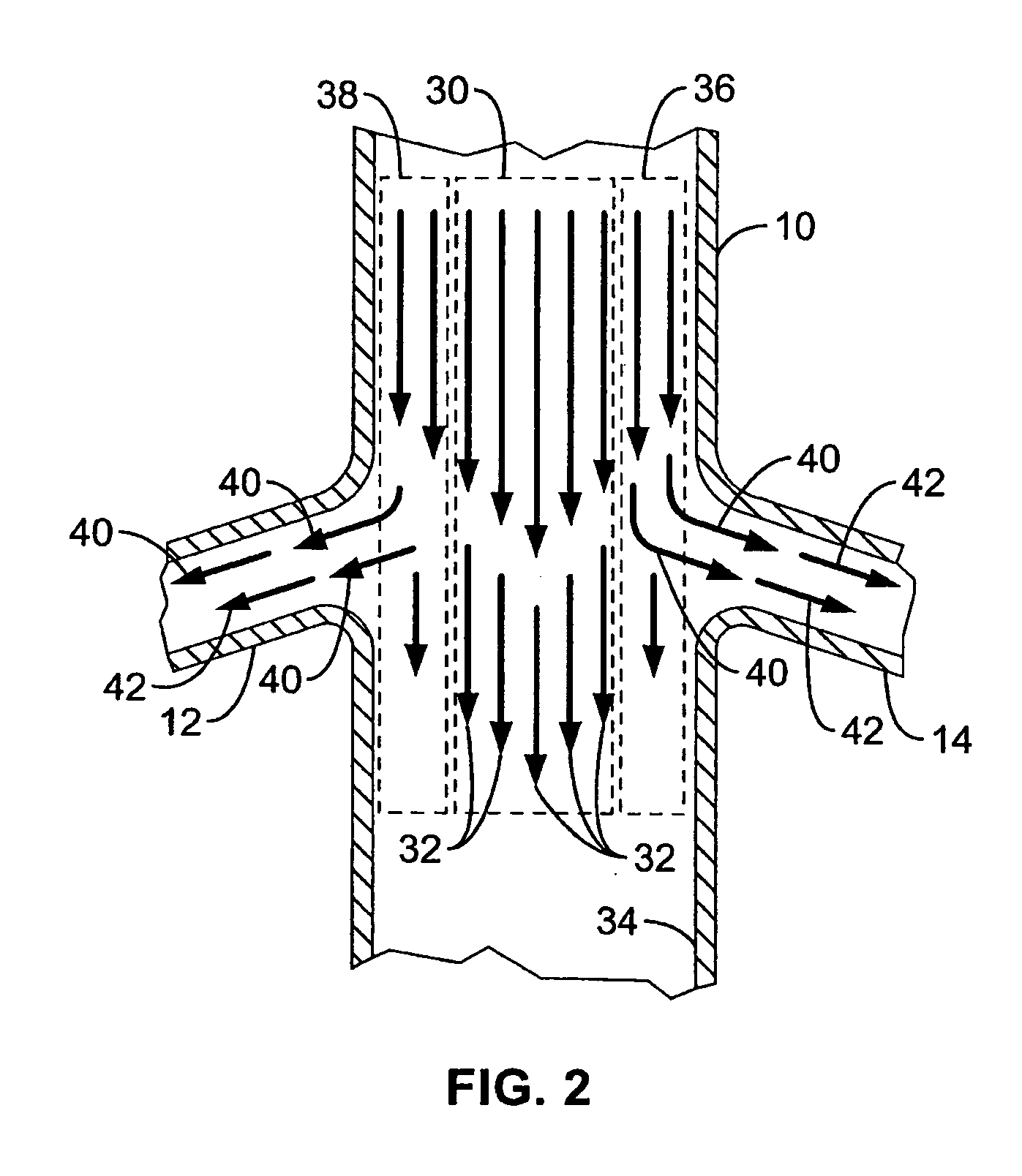
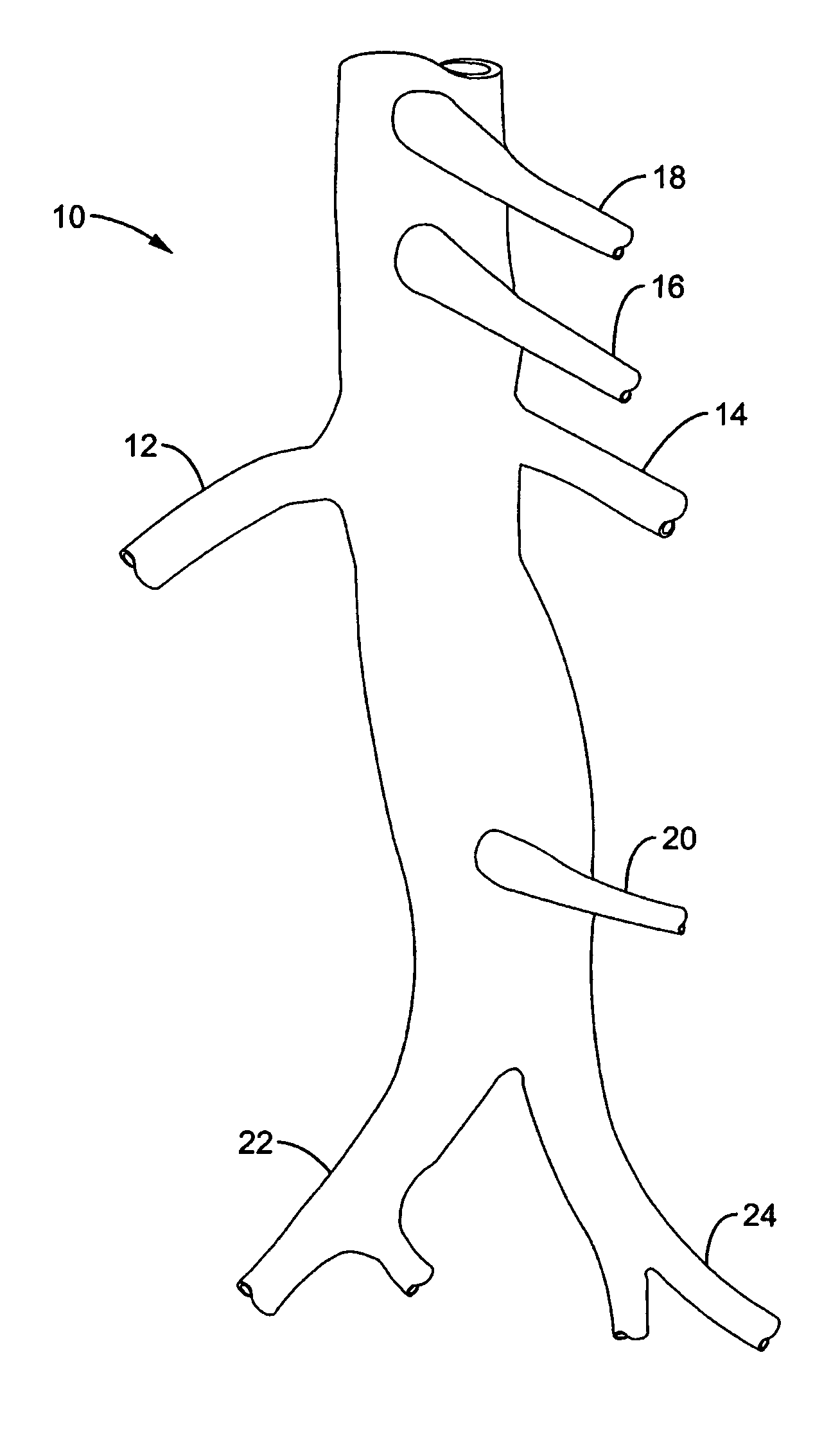
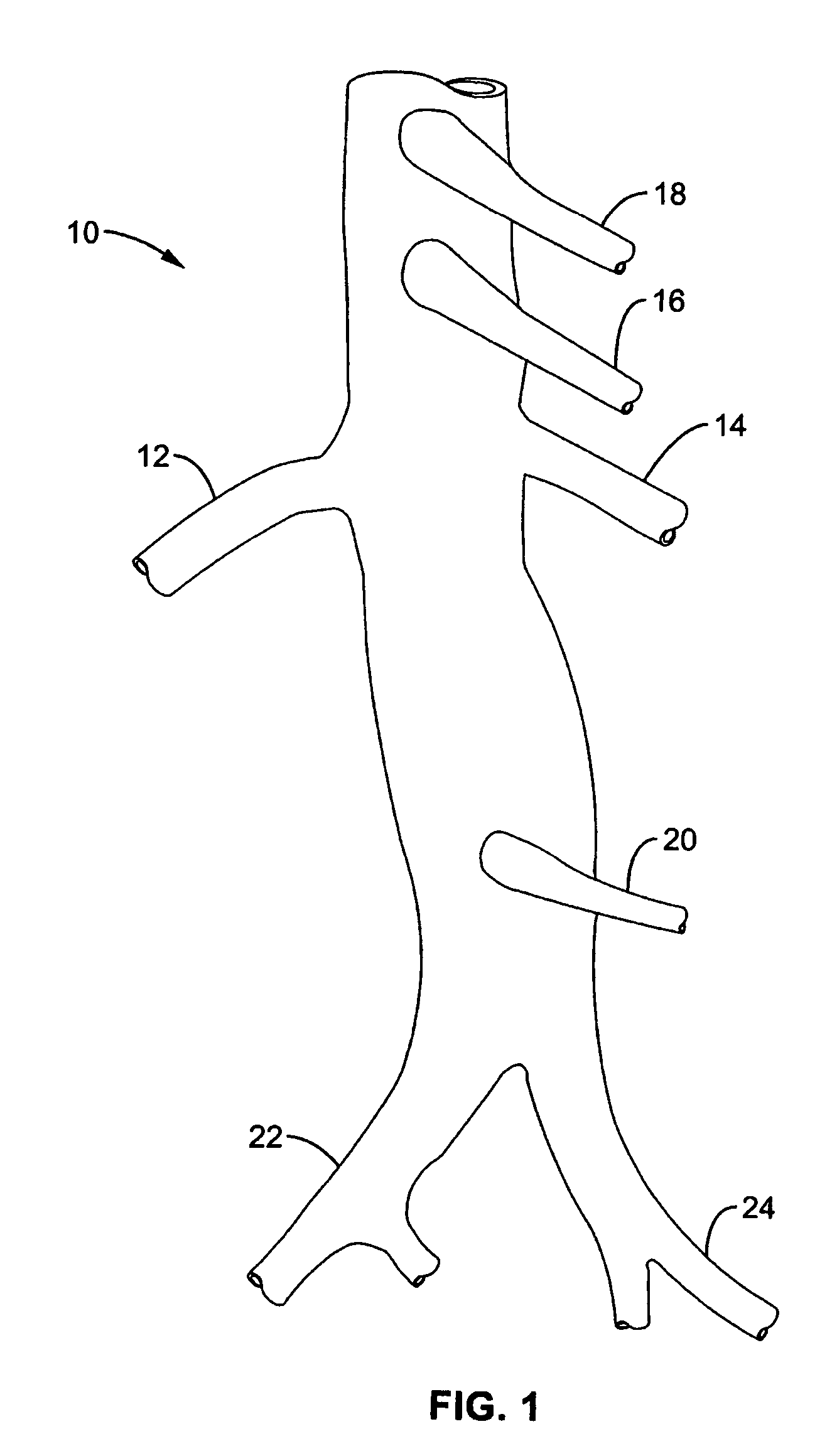
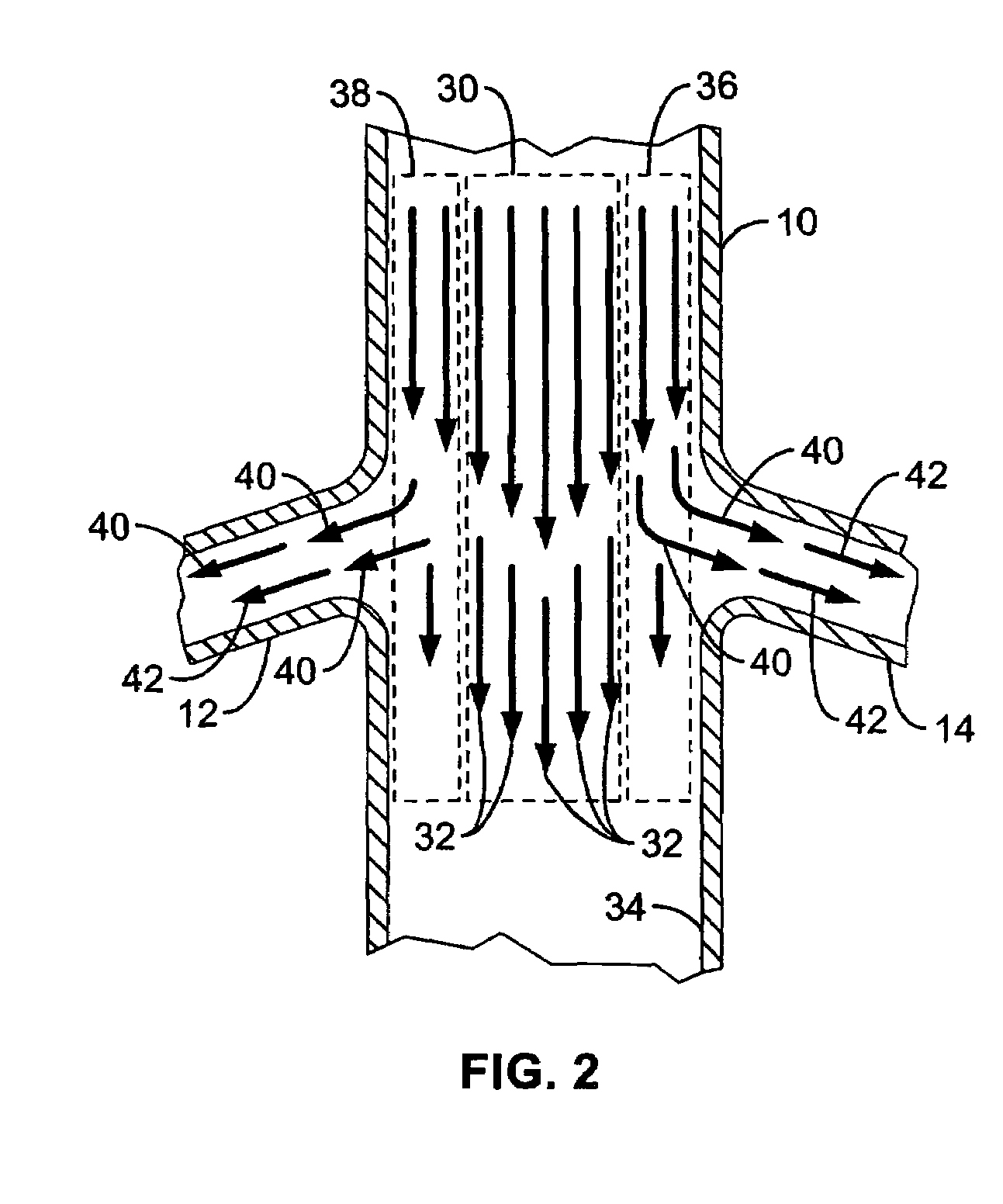
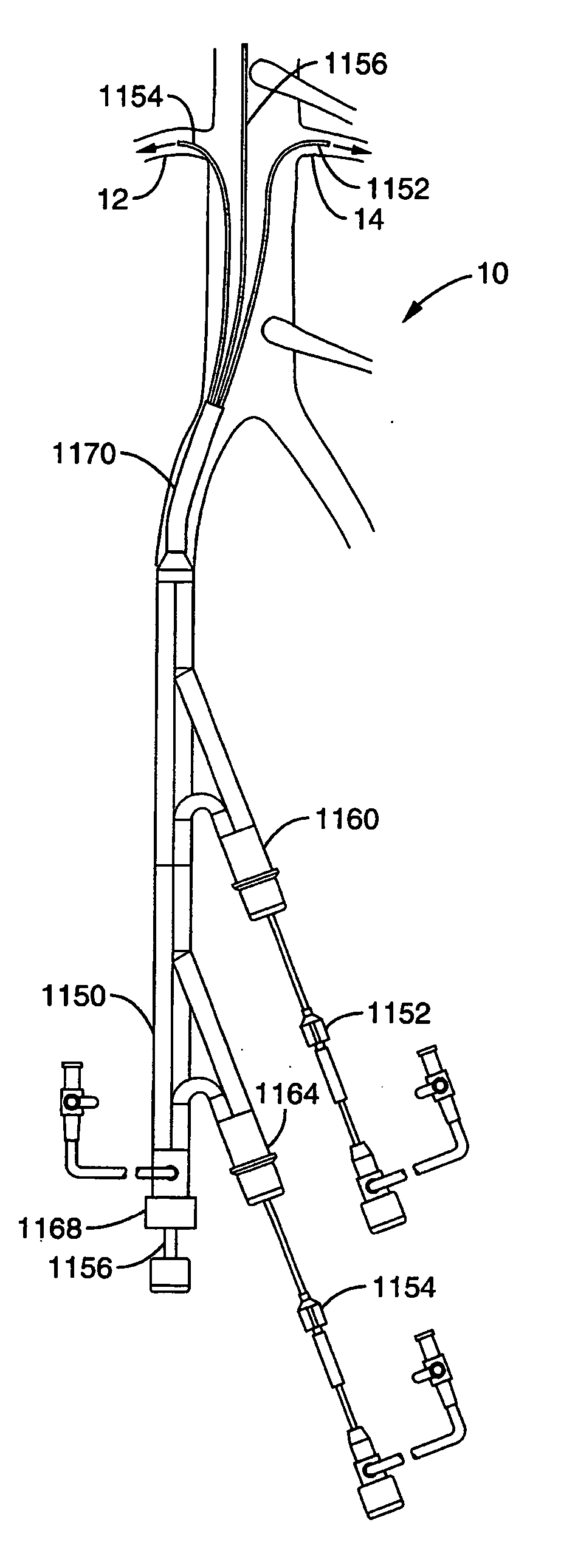
Method and apparatus for selective drug infusion via an intra-aortic flow diverter delivery catheter
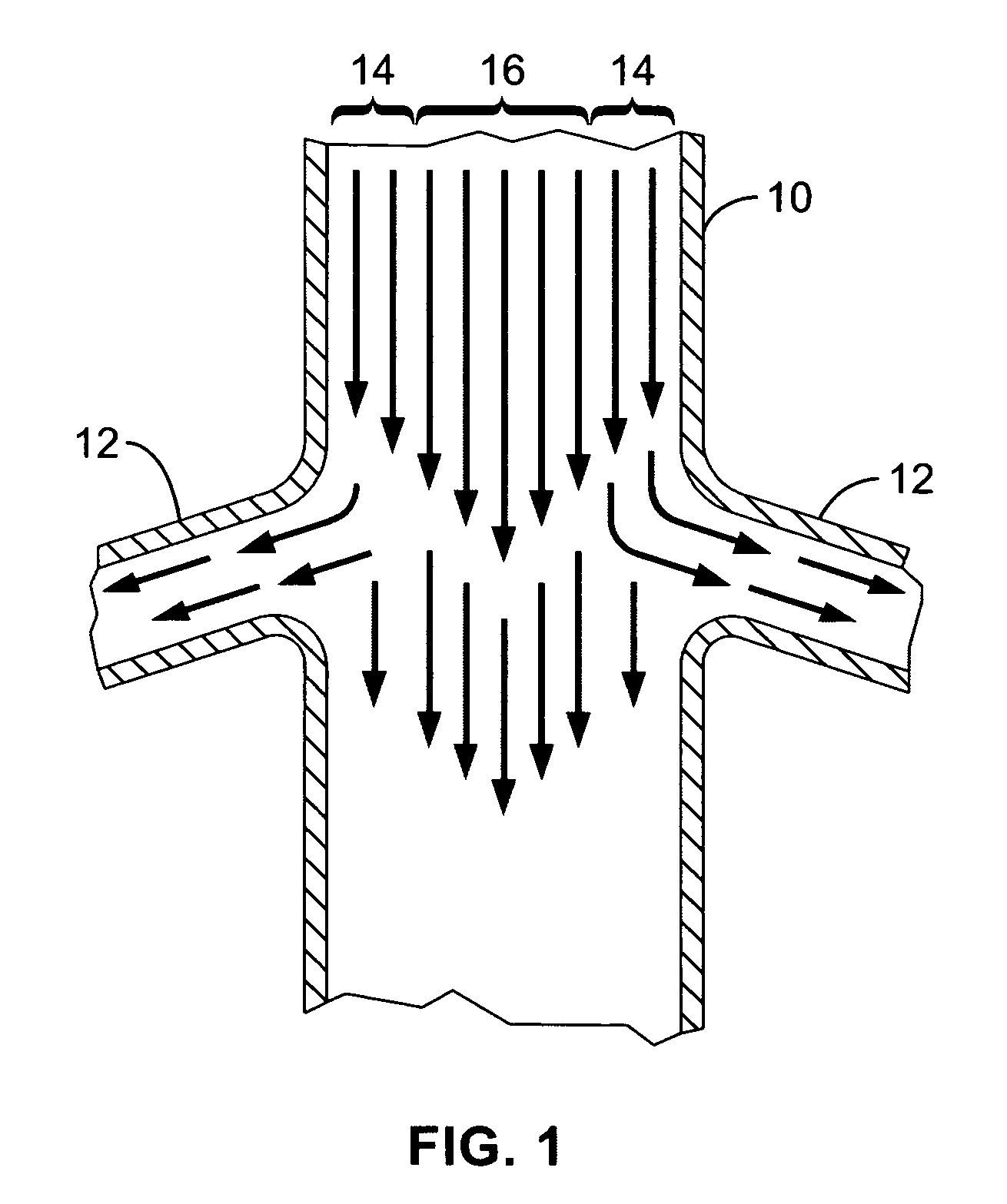
A local renal delivery system includes a flow isolation assembly and a local injection assembly. The flow isolation assembly in one mode is adapted to isolate only a partial flow region along the outer circumference along the aorta wall such that fluids inject there are maintained to flow substantially into the renal arteries. Various types of flow isolation assemblies and local injection assemblies are described.
Owner:ANGIODYNAMICS INC
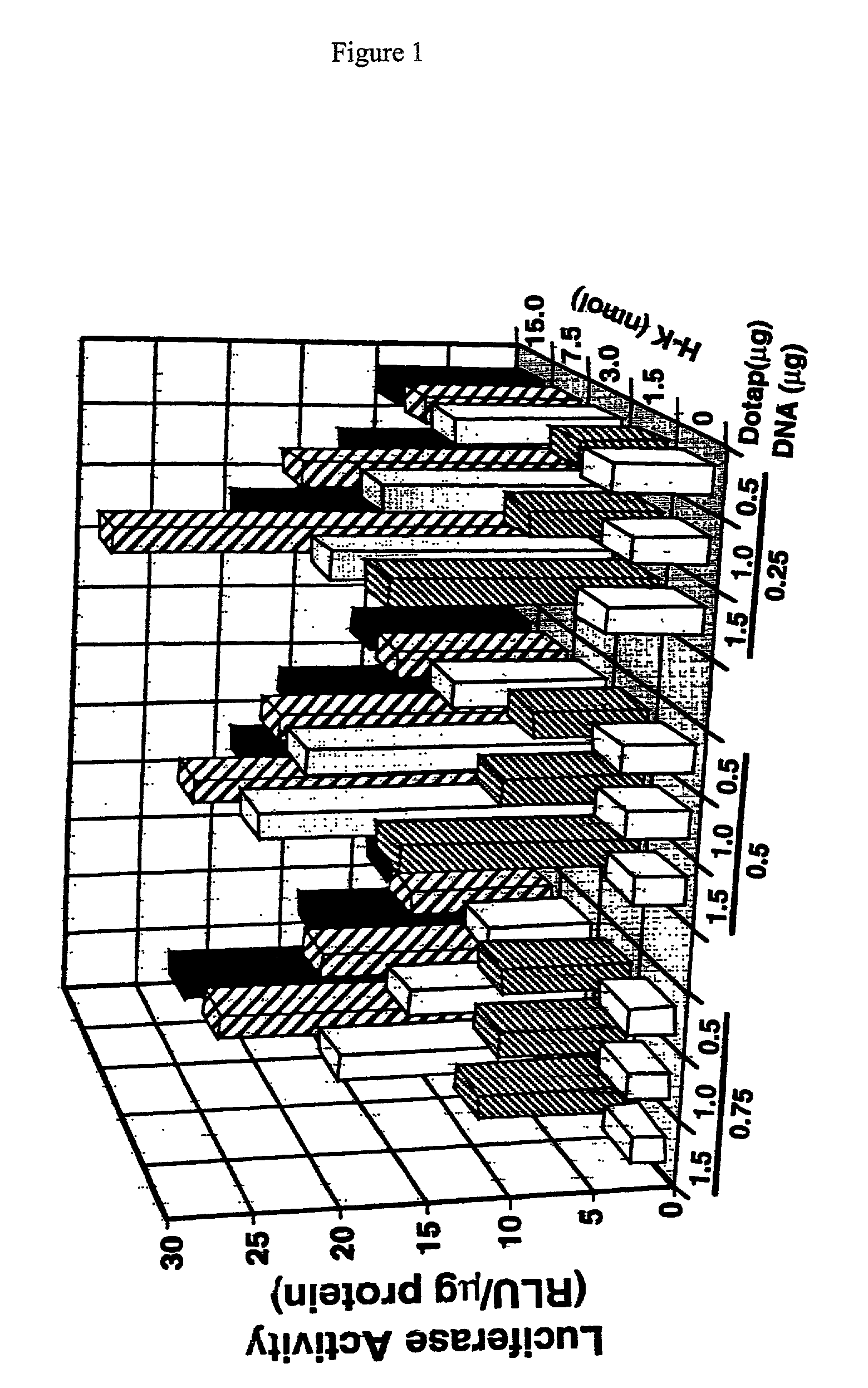
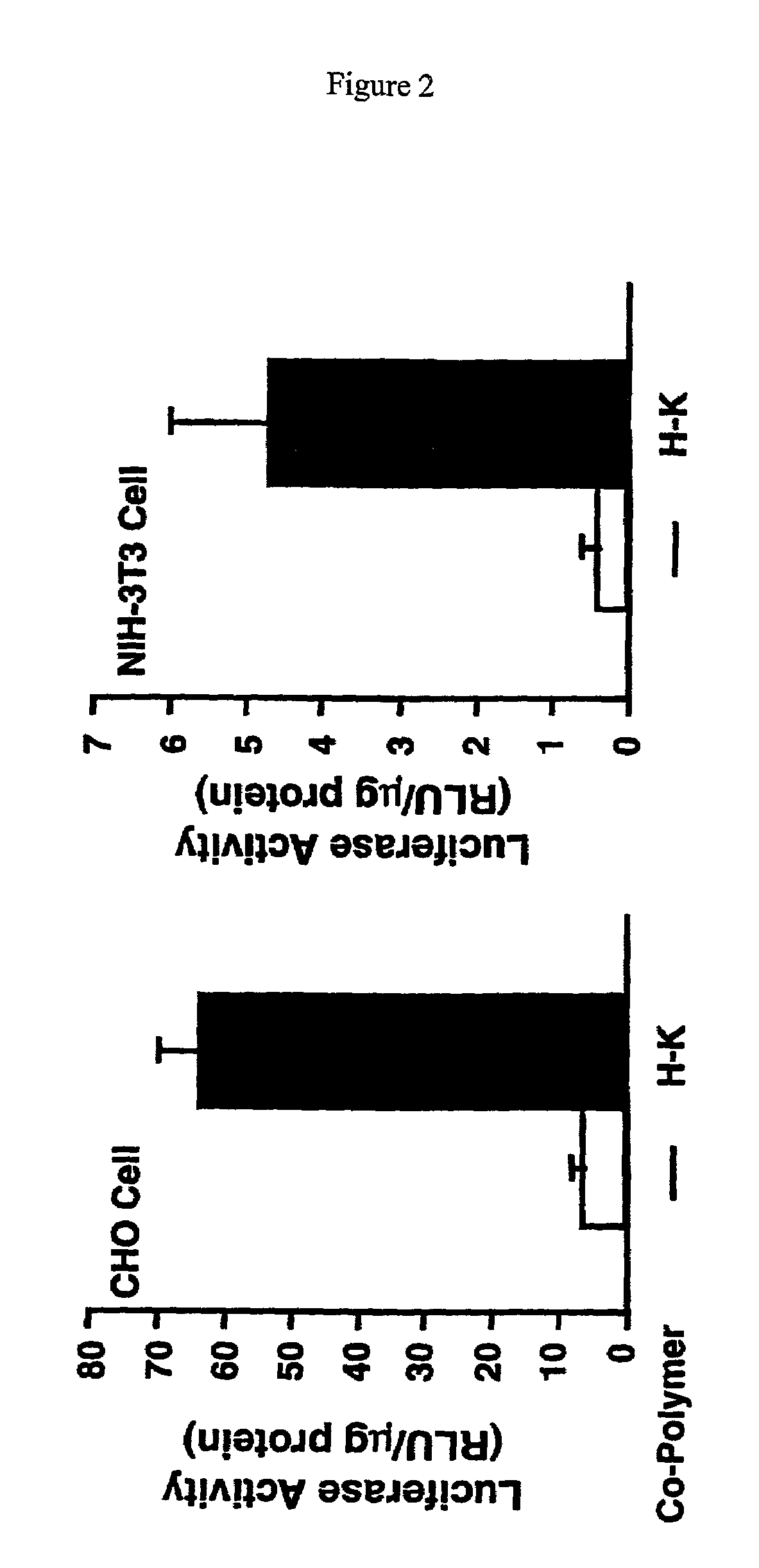
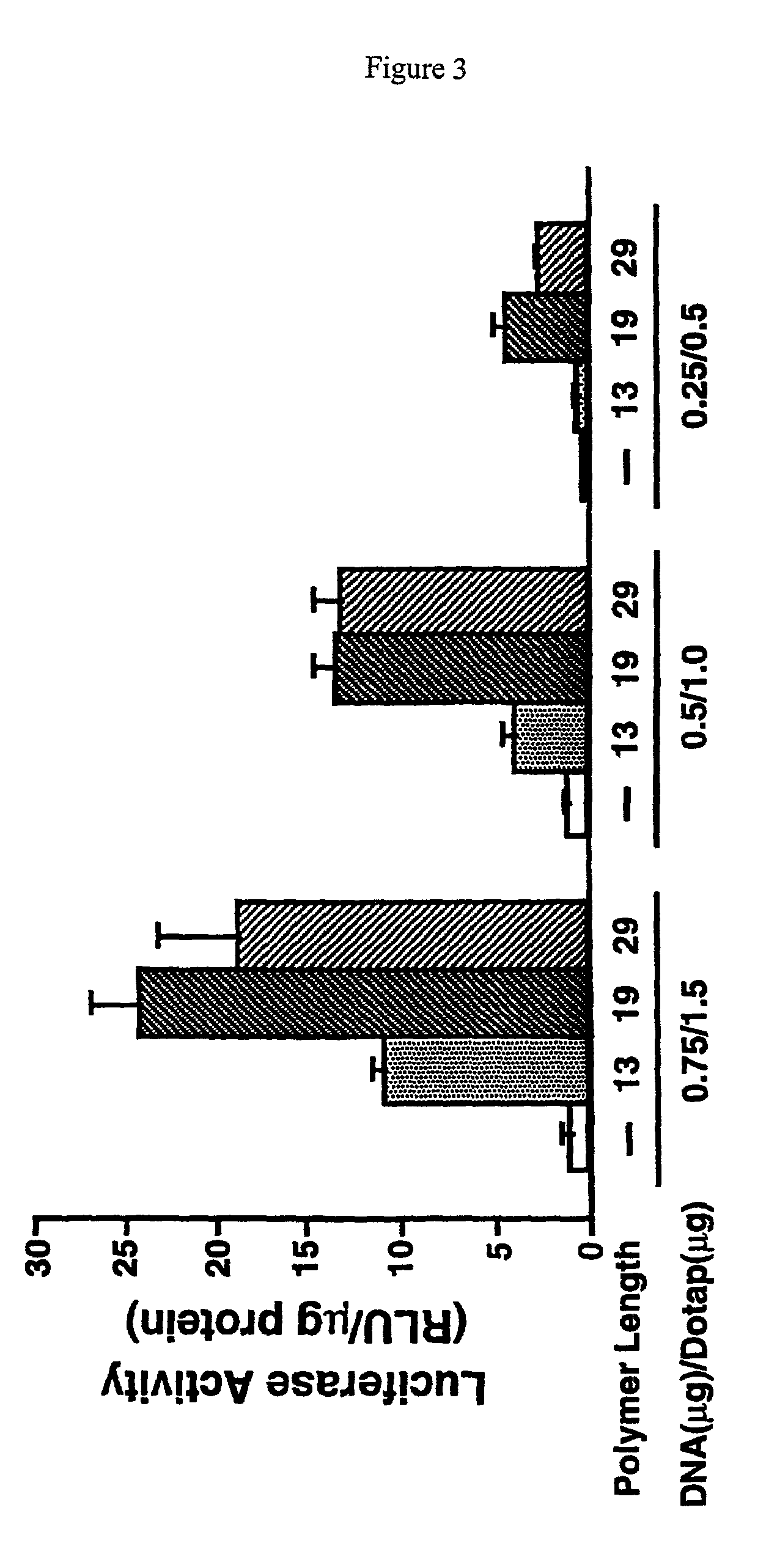
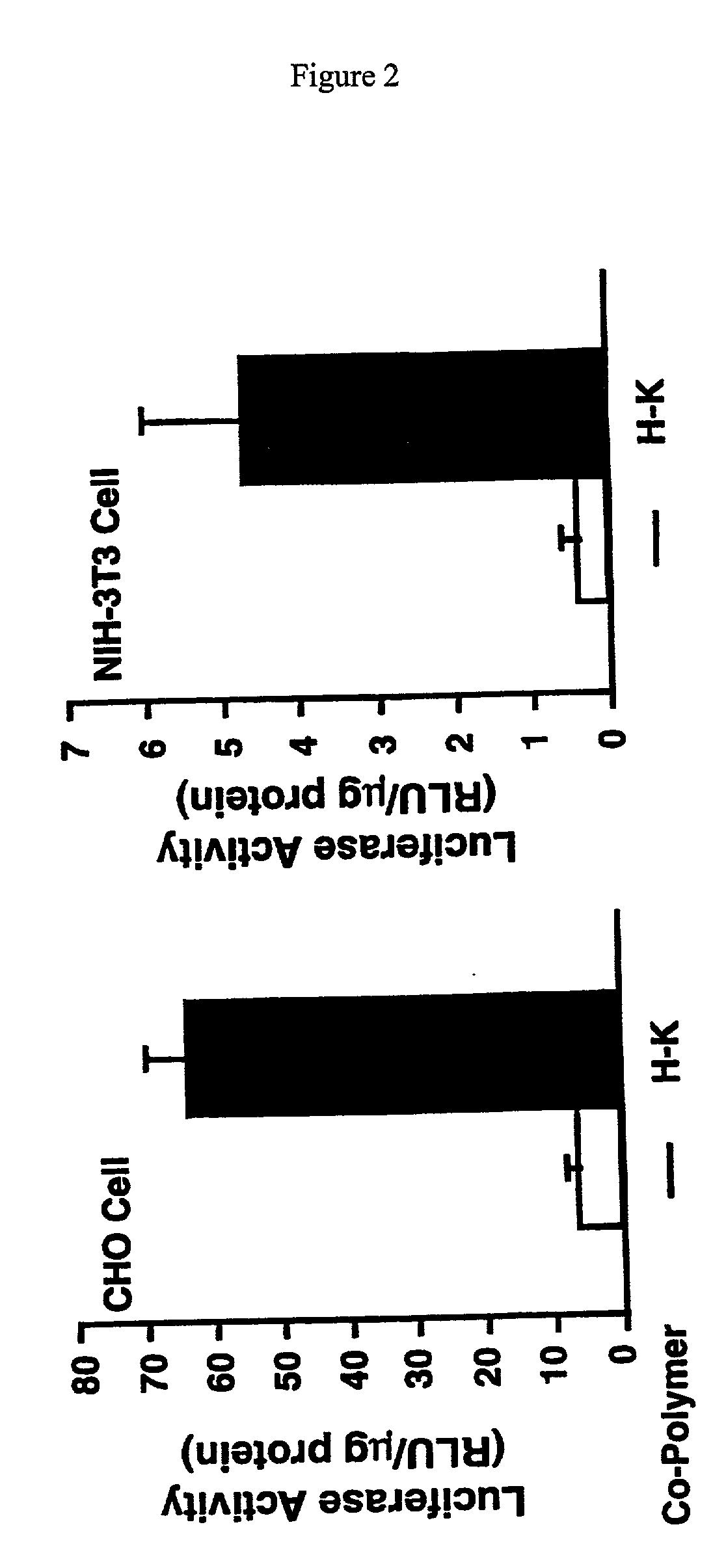
Branched histidine copolymers and methods for using same
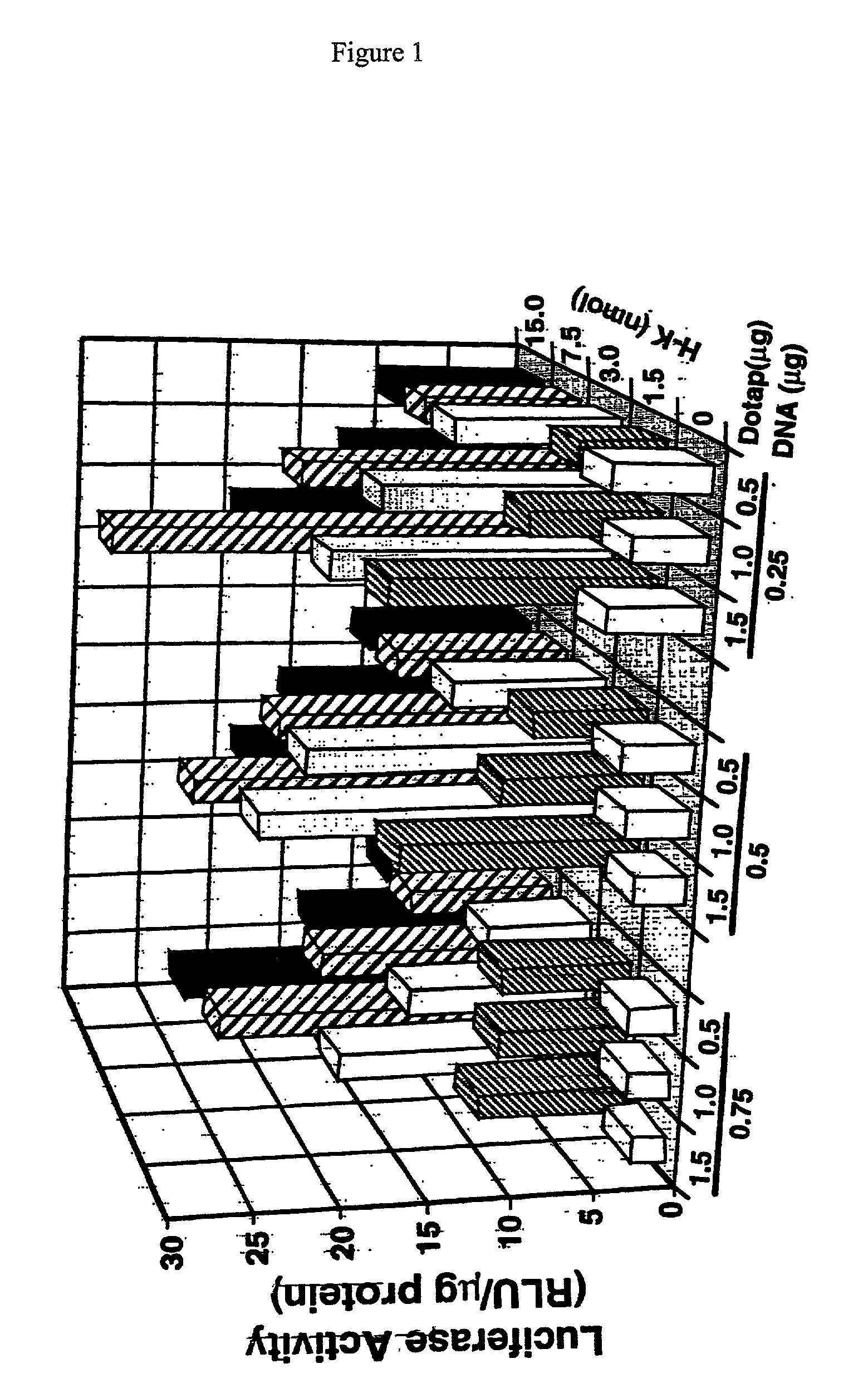
InactiveUS7070807B2Prolong half-life in vivoEnhanced transfectionPowder deliveryPeptide/protein ingredientsIn vivoLocal injection
The invention provides a branched transport polymer characterized as having at least 10 amino acids and a ratio of histidine to non-histidine amino acids greater than 1.5, said branched transport polymer comprising one or more backbones, one or more terminal branches, and optionally, one or more non-terminal branches. The branched transport polymer may be associated with a pharmaceutical agent to form a pharmaceutical agent delivery composition useful for in vivo therapies based on local injection.
Owner:MIXSON A JAMES
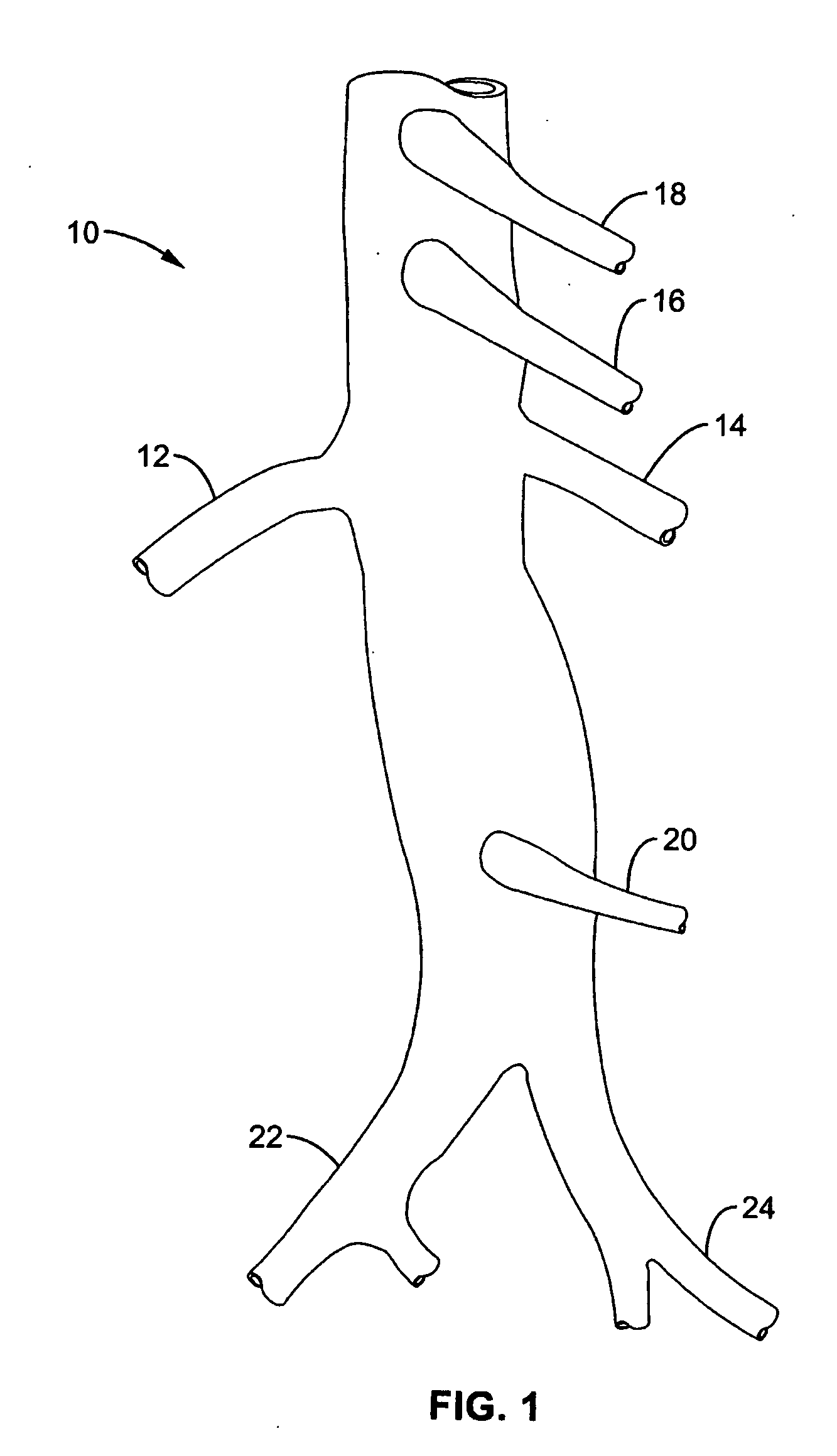
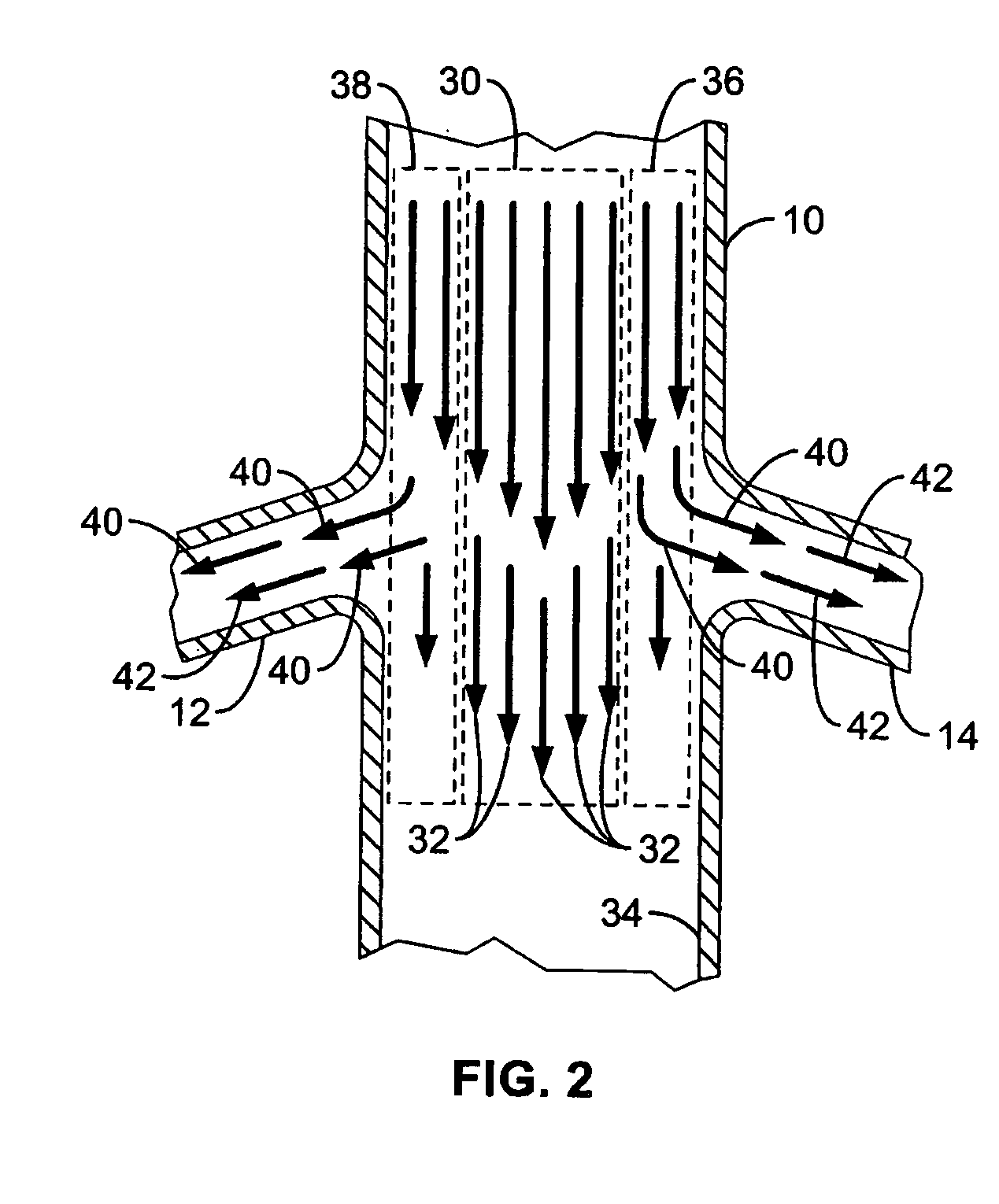
Method and apparatus for intra aortic substance delivery to a branch vessel
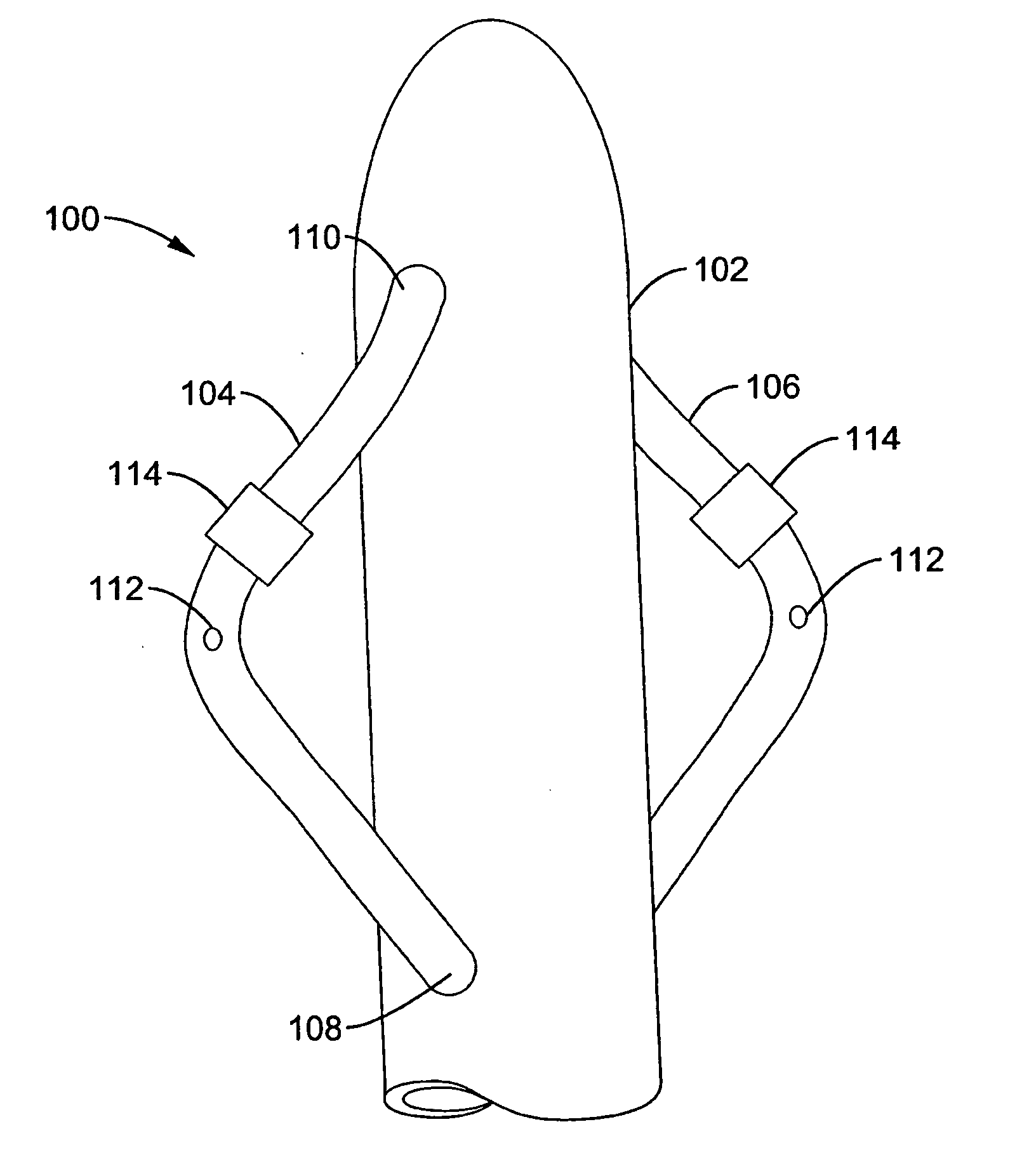
A renal flow system injects a volume of fluid agent into a location within an abdominal aorta in a manner that flows bilaterally into each of two renal arteries via their respectively spaced ostia along the abdominal aorta wall. A local injection assembly (100) includes two injection members (104, 106), each having an injection port (112) that couples to a source of fluid agent externally of the patient. The injection ports may be positioned within an outer region of blood flow along the abdominal aorta wall perfusing the two renal arteries.
Owner:ANGIODYNAMICS INC
Method and apparatus for intra-aortic substance delivery to a branch vessel
A renal flow system injects a volume of fluid agent into a location within an abdominal aorta in a manner that flows bi-laterally into each of two renal arteries via their respectively spaced ostia along the abdominal aorta wall. A local injection assembly includes two injection members, each having an injection port that couples to a source of fluid agent externally of the patient. The injection ports may be positioned with an outer region of blood flow along the abdominal aorta wall perfusing the two renal arteries. A flow isolation assembly may isolate flow of the injected agent within the outer region and into the renals. The injection members are delivered to the location in a first radially collapsed condition, and bifurcate across the aorta to inject into the spaced renal ostia. A delivery catheter for upstream interventions is used as a chassis to deliver a bilateral local renal injection assembly to the location within the abdominal aorta.
Owner:ANGIODYNAMICS INC
Method and apparatus for intra-aortic substance delivery to a branch vessel
A renal flow system injects a volume of fluid agent into a location within an abdominal aorta in a manner that flows bi-laterally into each of two renal arteries via their respectively spaced ostia along the abdominal aorta wall. A local injection assembly includes two injection members, each having an injection port that couples to a source of fluid agent externally of the patient. The injection ports may be positioned with an outer region of blood flow along the abdominal aorta wall perfusing the two renal arteries. A flow isolation assembly may isolate flow of the injected agent within the outer region and into the renals. The injection members are delivered to the location in a first radially collapsed condition, and bifurcate across the aorta to inject into the spaced renal ostia. A delivery catheter for upstream interventions is used as a chassis to deliver a bilateral local renal injection assembly to the location within the abdominal aorta.
Owner:ANGIODYNAMICS INC
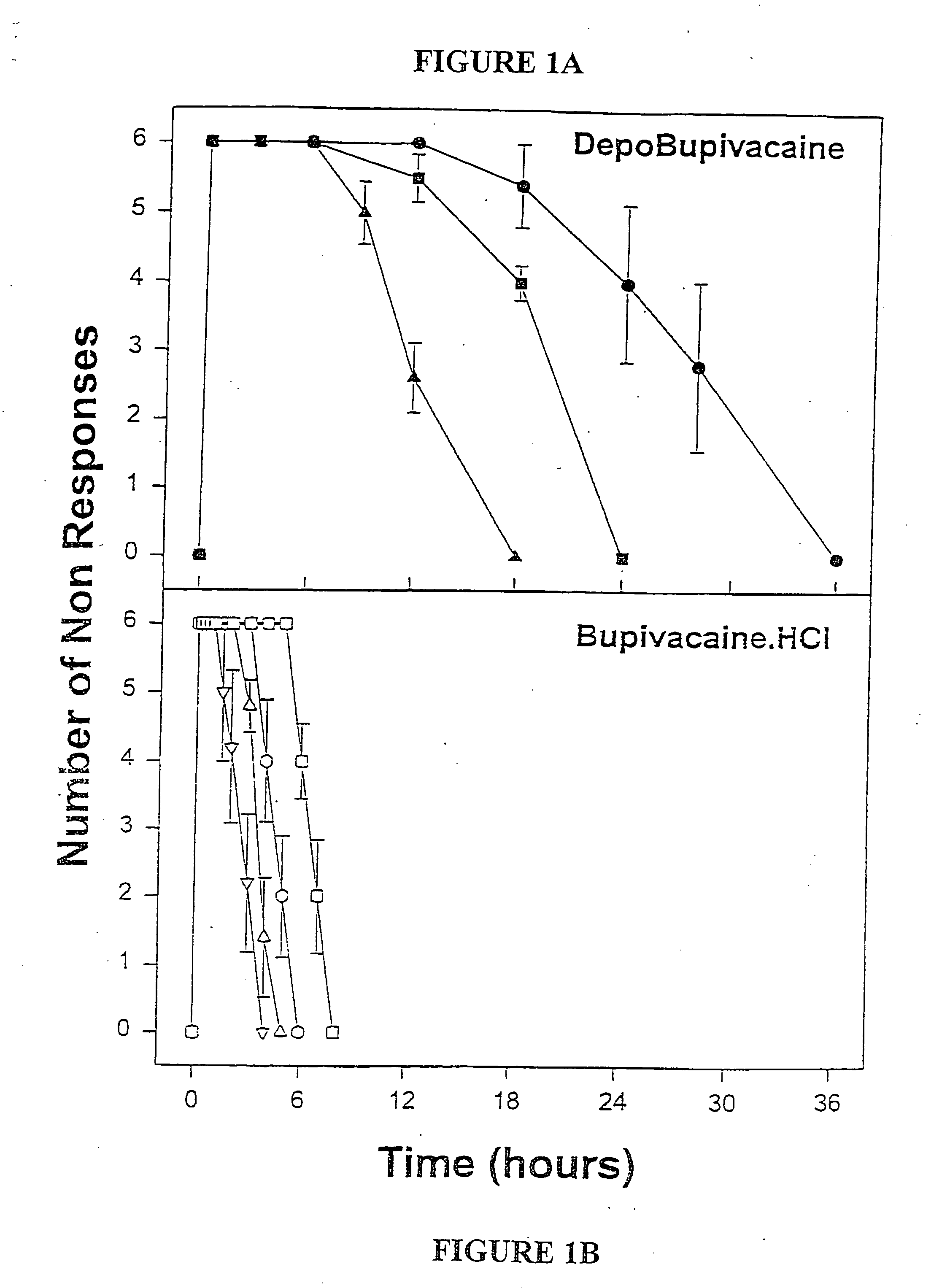
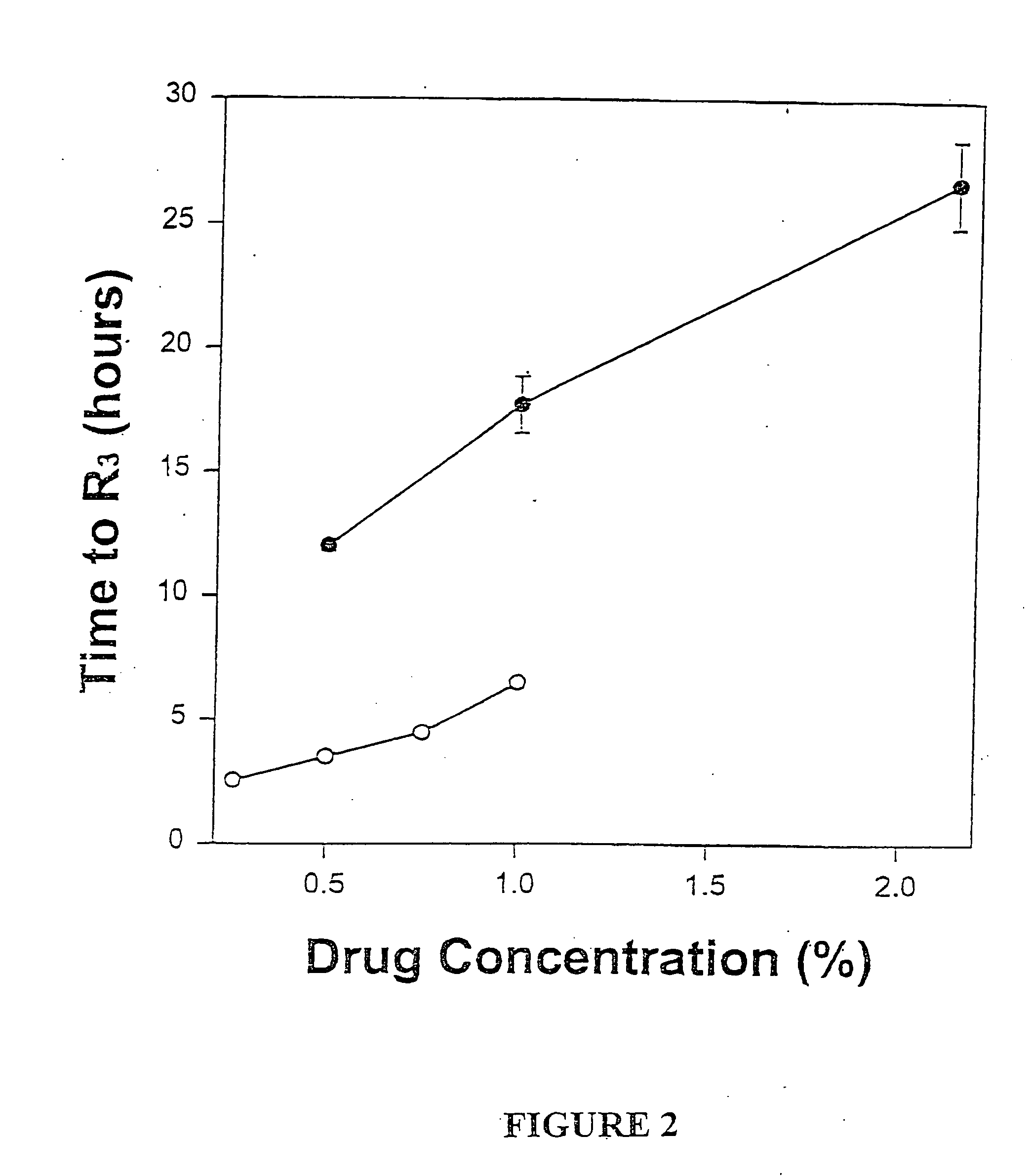
Sustained-release liposomal anesthetic compositions
InactiveUS8182835B2High acceptabilityImprove encapsulationInorganic non-active ingredientsAnaestheticsHalf-lifeMaximum tolerated dose
Owner:PACIRA PHARMA INC
Agents and methods for enhancing bone formation
ActiveUS20060270645A1Improve impactPromotes bone formationBiocidePeptide/protein ingredientsMedicineOxysterol
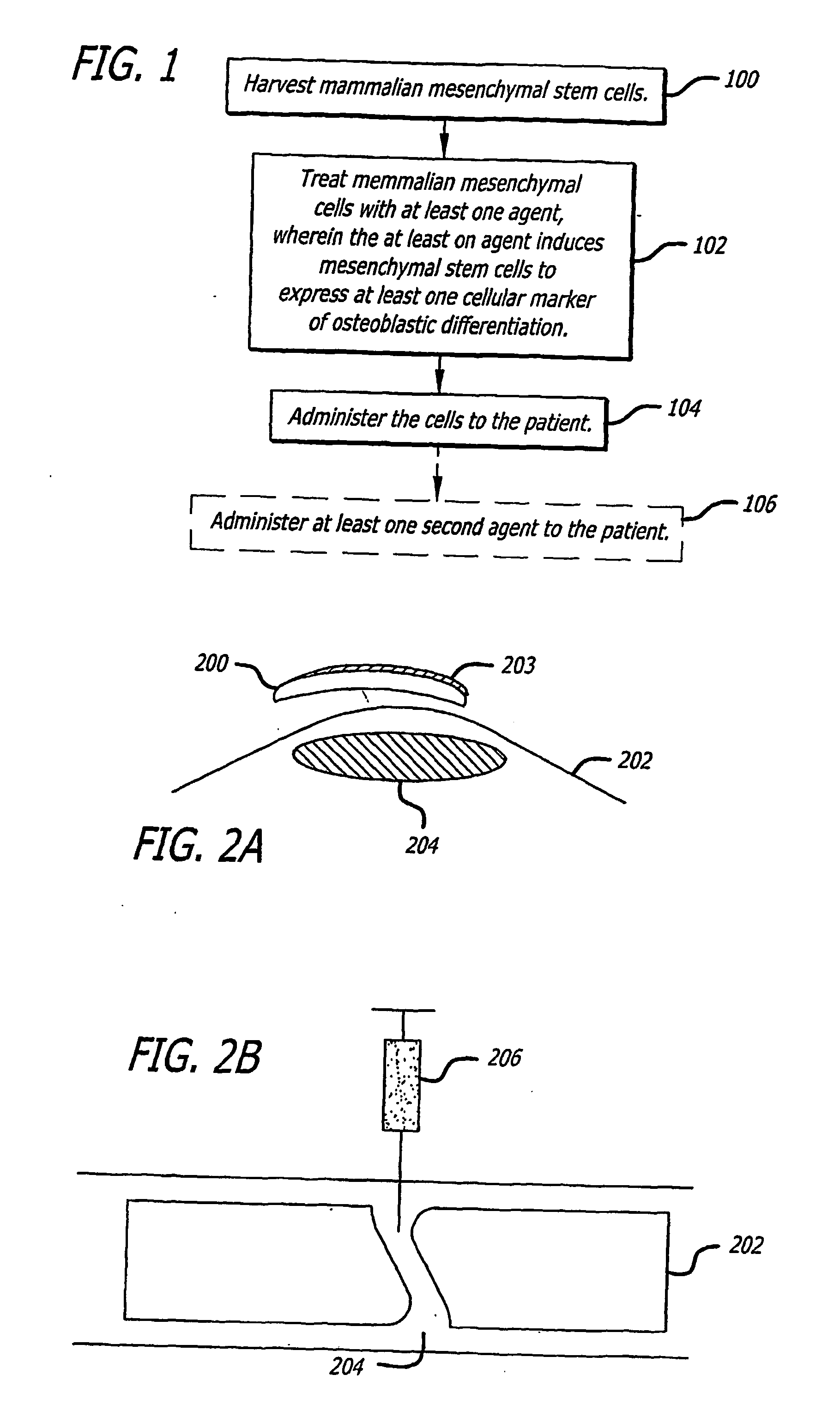
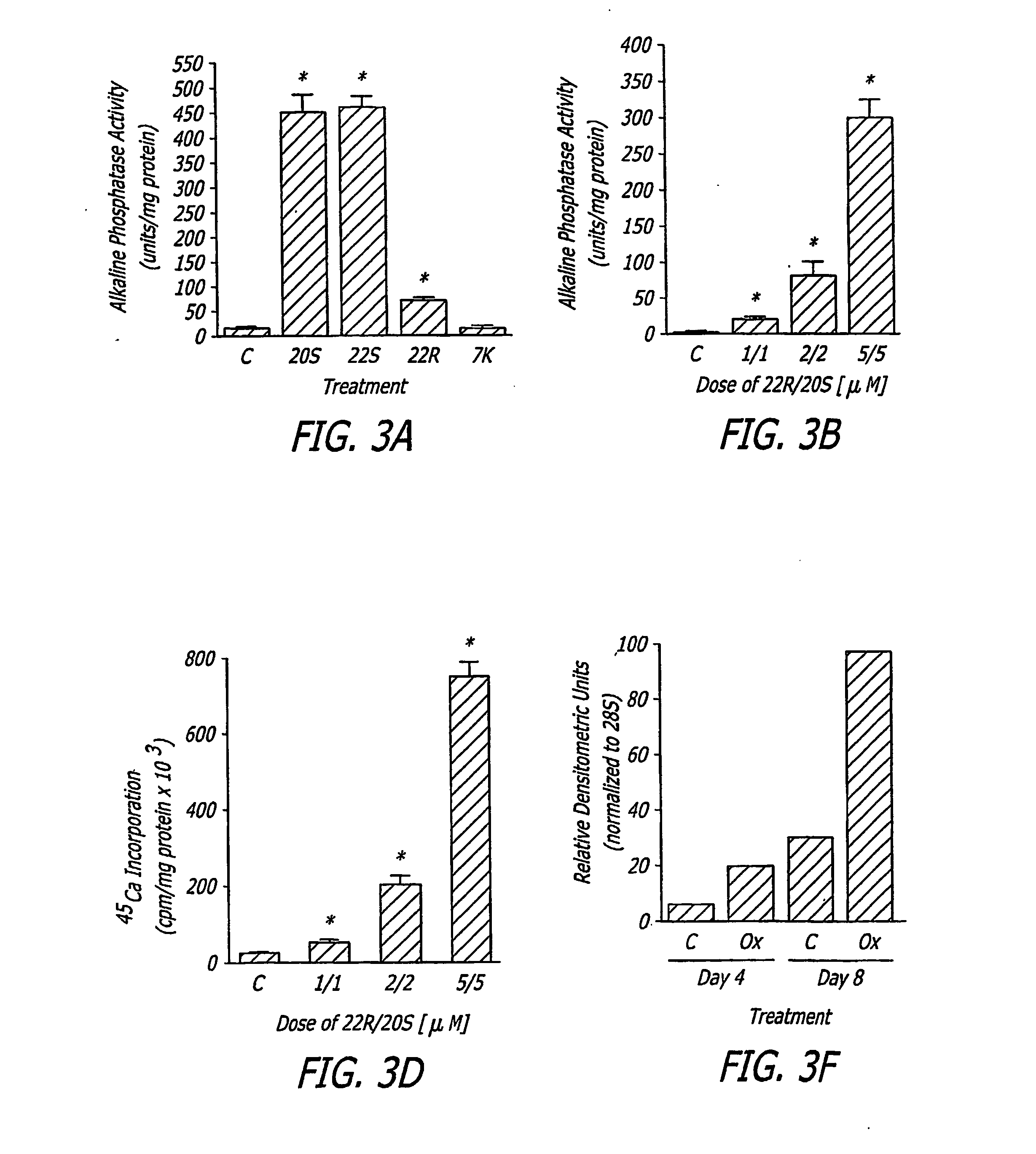
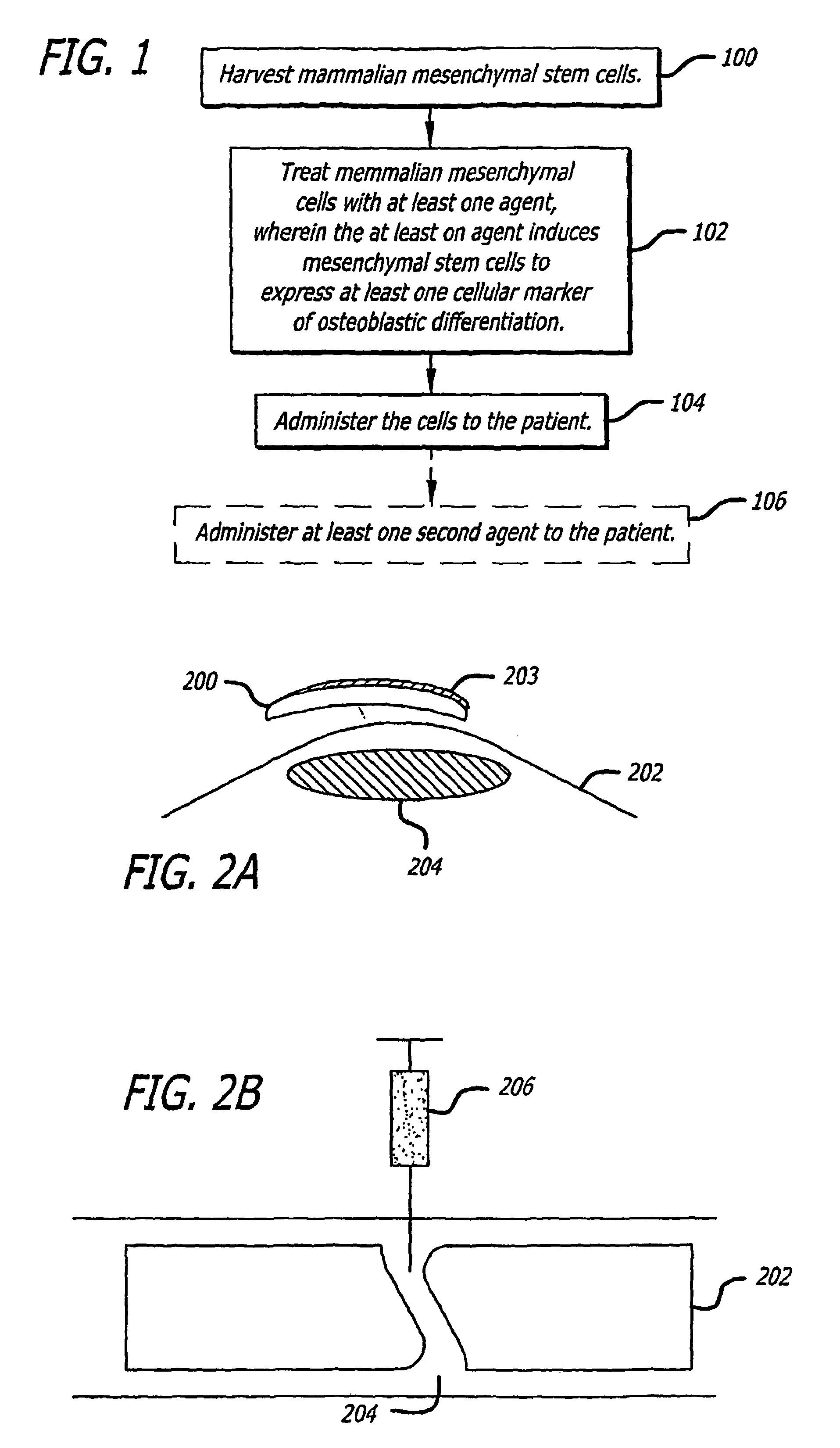
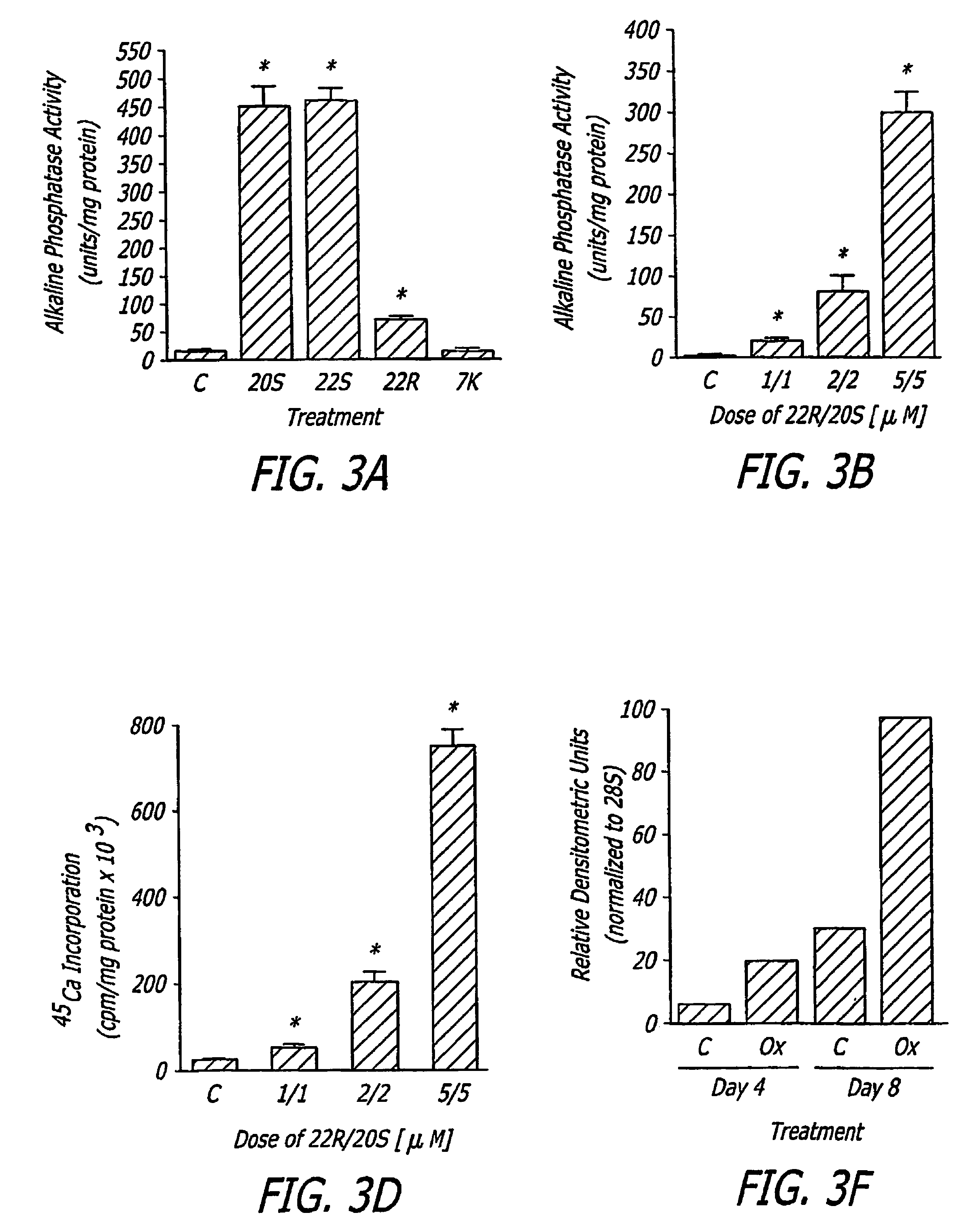
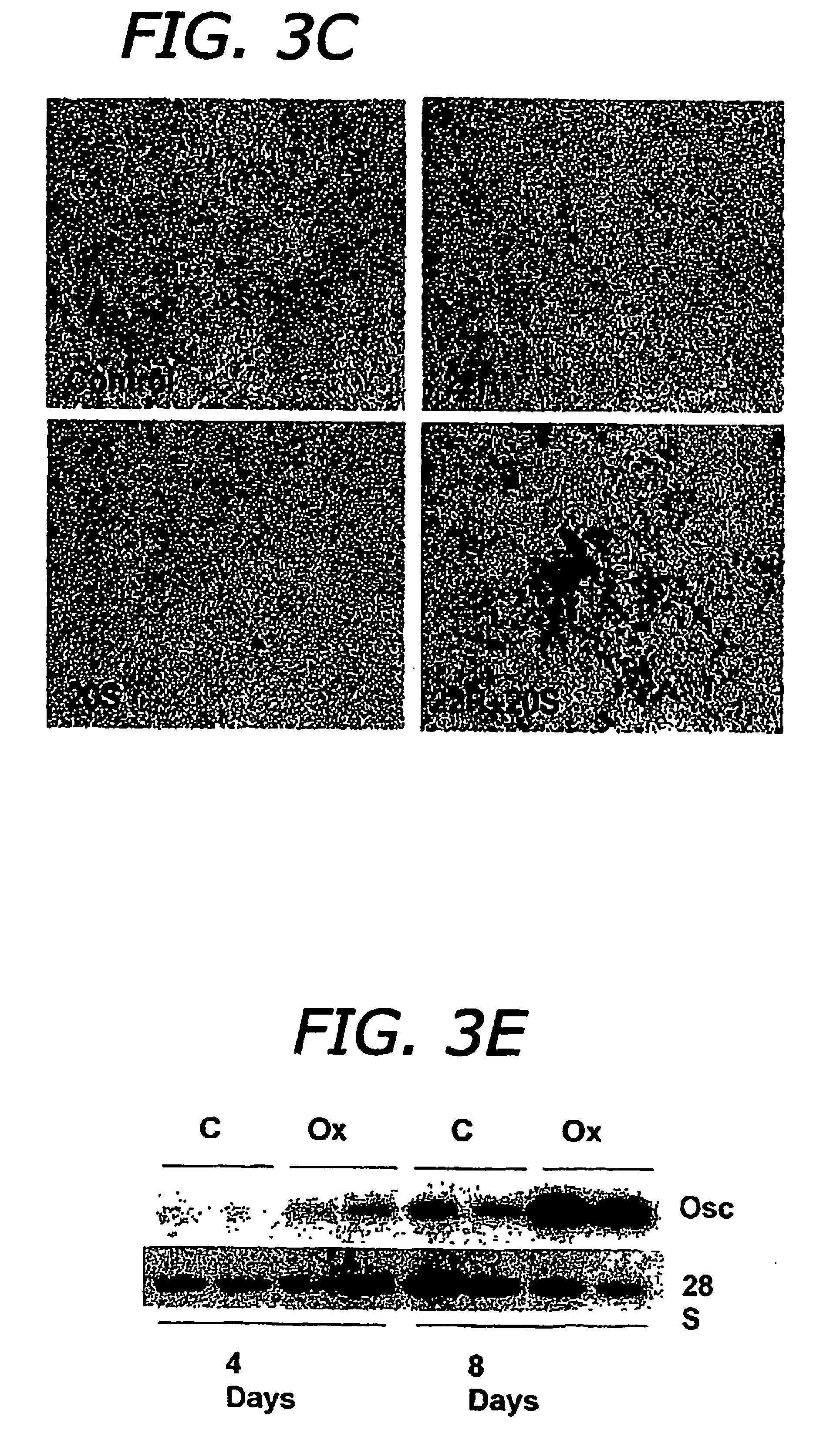
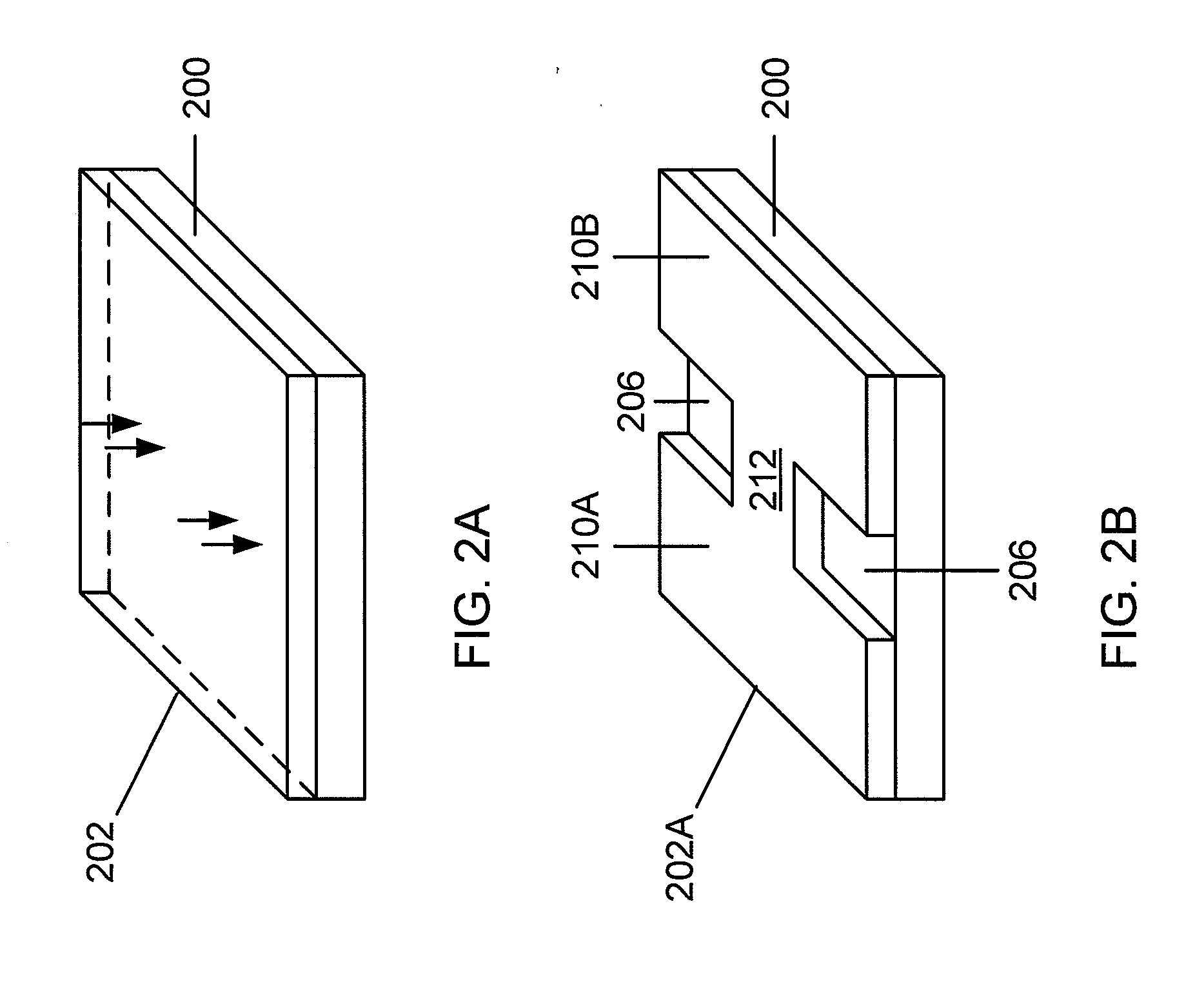
The present invention discloses agents and methods for inducing osteoblastic cellular differentiation, as well as the use of such agents and method to treat patients to maintain bone mass, enhance bone formation and / or bone repair. Exemplary agents include oxysterols, alone or in combination with particular oxysterols, or other agents known to assist in bone formation. The invention further includes medicaments including oxysterols for the treatment of bone disorders, local injections of oxysterols or cells (206) and implants (202) having agents or cells (203) to facilitate bone repair.
Owner:RGT UNIV OF CALIFORNIA
Formation testing and evaluation using localized injection
ActiveUS20100264915A1Electric/magnetic detection for well-loggingFluid removalProximateLocal injection
Evaluating a formation by lowering a downhole tool in a wellbore penetrating the formation, injecting a fluid into the formation at an injection zone via the downhole tool, and using a formation evaluation sensor to perform a measurement at each of a plurality of locations in the wellbore each proximate the injection zone. At least two of the plurality of measurements are compared, and a formation property is determined based on the comparison.
Owner:SCHLUMBERGER TECH CORP
Applications of HIFU and chemotherapy
InactiveUS20070088345A1Promote absorptionUltrasonic/sonic/infrasonic diagnosticsSonopheresisAbnormal tissue growthMilia
A method, using high intensity ultrasound, which may also be combined with a chemotherapy agent, that can result the direct destruction of tumor cells and in the reduction or elimination of local reoccurrence of cancer after removal of cancerous tissue, such as a surgical breast lumpectomy or surgical excision of a brain tumor. The method comprises either (1) the treatment of the tumor directly with High Intensity Focused Ultrasound, or (2) the treatment of the margins of the tissue surrounding the surgical cavity or void with ablative continuous wave high intensity ultrasound and a combination of a locally delivered chemotherapy agent and high intensity ultrasound, termed sonoporation. The present invention permits both the direct destruction (ablation) of tumor tissue as well as the destruction of tissue around the surgical margin and may include the enhanced local cellular uptake of locally injected chemotherapeutic drugs, all of which can be accomplished by a therapeutic ultrasound device used during surgery.
Owner:UST INC
Method for treating primary and secondary forms of glaucoma
InactiveUS20070197491A1Organic active ingredientsSenses disorderOpen angle glaucomaAngiostatic Agents
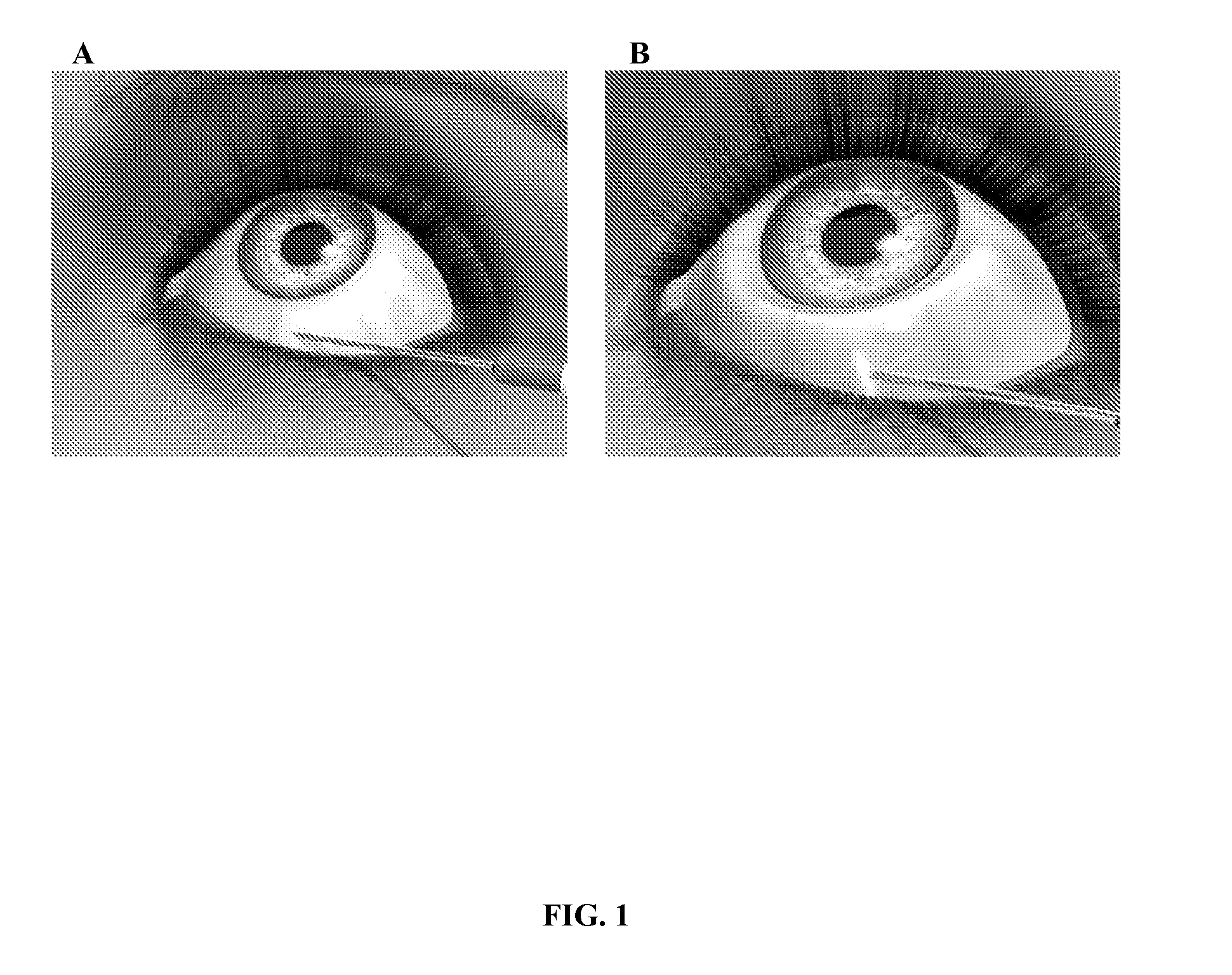
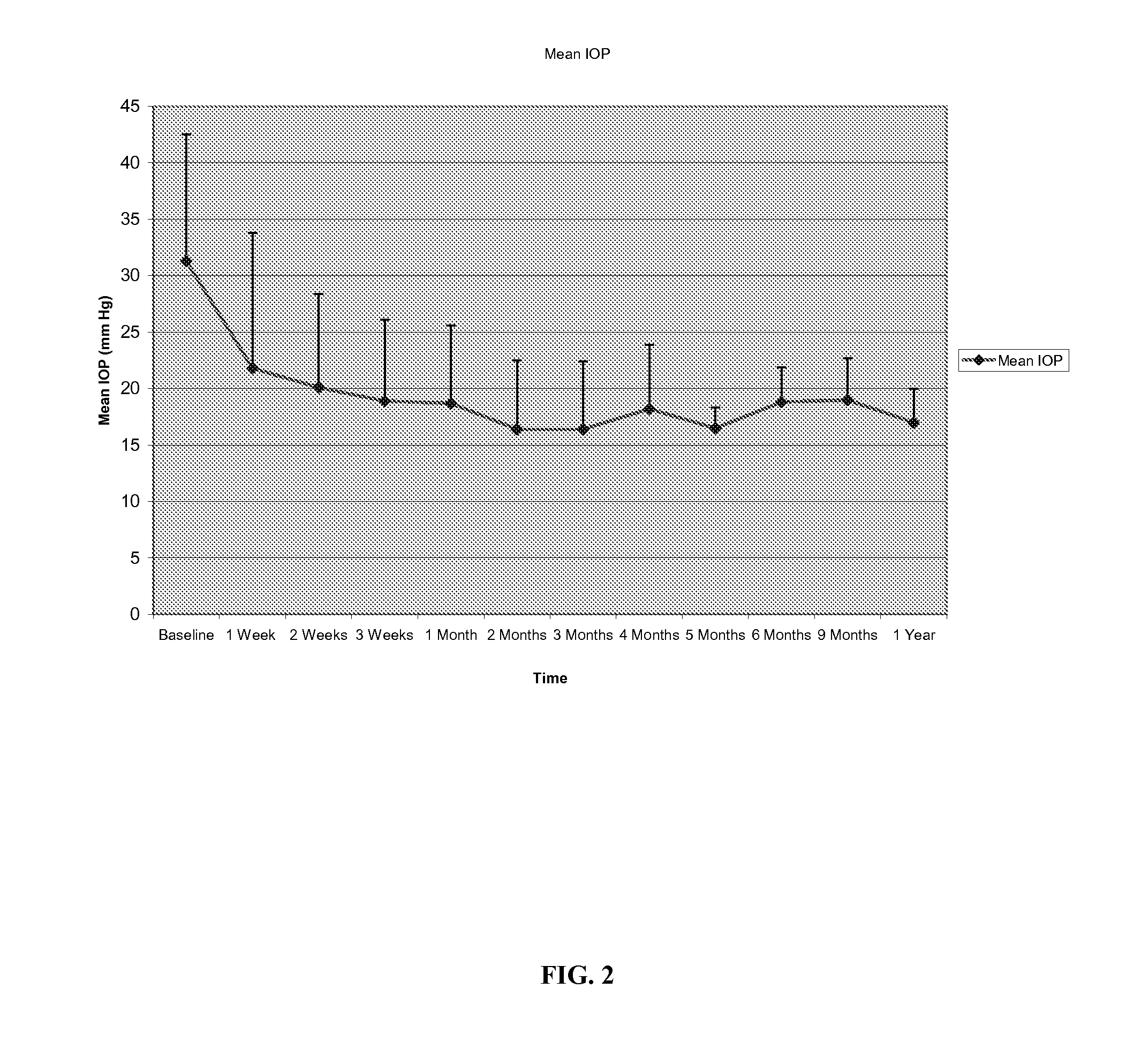
Methods and compositions for controlling ocular hypertension associated with (i) primary open angle glaucoma (POAG), (ii) other forms of glaucoma, or (iii) glucocorticoid therapy are disclosed. The methods involve administration of angiostatic agents and other IOP-lowering agents via local injections in the anterior segment of the eye. The most preferred IOP-lowering agents are angiostatic steroids, particularly anecortave acetate, and the most preferred route of administration is an anterior juxtascleral injection or implant. The invention is based in part on the discovery that anterior juxtascleral injections of anecortave acetate are capable of controlling intraocular pressure for sustained periods of from one to several months or more. This result is believed to be attributable to facilitation of access of the anecortave acetate to the trabecular meshwork via the anterior juxtascleral route of administration. This route of administration is also believed to be advantageous for other types of IOP-lowering agents, particularly molecules that cannot readily penetrate the cornea due to size or other physical properties.
Owner:ALCON INC
Suspension of hyaluronic acid or salt thereof containing macromolecule hydrogel for injection and preparation method thereof
The invention relates to hyaluronic acid which contains giant molecule hydrogel and is applied to injection or turbid liquor of salt thereof and a preparation method thereof; the turbid liquor of the invention is characterized in that hyaluronic acid or isosmotic solution of salt thereof serves as a carrier; water-insoluble macromolecular compound aqueous gel particle fully swelling in isosmotic solution is added. In the invention, the preparation of the turbid liquor has the steps as follows: preparing cross linking macromolecule compound or polymer particle so as to facilitate the compound or polymer particle to fully swelling in the isosmotic solution to form gel particles, then being mixed with hyaluronic acid or salting liquid dissolved in the isosmotic solution. The hyaluronic acid containing giant molecule hydrogel or the turbid liquor of salt thereof are applied to preparing injection for beauty care or medical treatment, which has the characteristics of easy injection, long-term local effect, good plasticity and fine biocompatibility; in local injection part, the invention is used as an isolating and lubricating pad which enables surrounding tissues to be fully repaired; the characteristic is even more pronounced in treating arthritis by bone joint cavity injection.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Histidine copolymer and methods for using same
InactiveUS20030165567A1Enhanced transfectionProlong half-life in vivoPeptide-nucleic acidsPowder deliveryIn vivoLocal injection
The invention provides a branched transport polymer characterized as having at least 10 amino acids and a ratio of histidine to non-histidine amino acids greater than 1.5, said branched transport polymer comprising one or more backbones, one or more terminal branches, and optionally, one or more non-terminal branches. The branched transport polymer may be associated with a pharmaceutical agent to form a pharmaceutical agent delivery composition useful for in vivo therapies based on local injection.
Owner:MIXSON A JAMES
Octreotide acetate freeze-dried combination for injection and preparation method thereof
ActiveCN102526700AImprove stabilityConvenient transportation and distributionPeptide/protein ingredientsDigestive systemSodium bicarbonateSolubility
The invention provides an octreotide acetate freeze-dried combination for injection, which comprises octreotide acetate, mannitol and a proper amount of buffer substances, wherein the mass ratio of the octreotide acetate to the mannitol is 1:(450-500); and the buffer substances are lactic acid and sodium bicarbonate and can be tartaric acid and sodium tartrate. The invention also provides a preparation method of the composition. The composition provided by the invention is produced by adopting an aseptic technique; by striving to make the technological breakthrough in optimal pH range and optimal preparation temperature in a liquid medicine preparation process, the product stability is improved; and finally, a freeze-dried product is prepared. By adopting a quick-freezing mode for pre-freezing, a finished product has low related substance content and good re-solubility, can be preserved at room temperature for two months and can be refrigerated for two years, thereby facilitating the product transportation and delivery. By clinically matching with a solution, the combination has good stability and low local injection irritation. The invention provides a safe and effective octreotide acetate freeze-dried combination for injection, which has good and controllable quality, for clinics.
Owner:西藏嘉信景天药业有限公司 +1
Agents and methods for enhancing bone formation
The present invention discloses agents and methods for inducing osteoblastic cellular differentiation, as well as the use of such agents and method to treat patients to maintain bone mass, enhance bone formation and / or bone repair. Exemplary agents include oxysterols, alone or in combination with particular oxysterols, or other agents known to assist in bone formation. The invention further includes medicaments including oxysterols for the treatment of bone disorders, local injections of oxysterols or cells (206) and implants (202) having agents or cells (203) to facilitate bone repair.
Owner:RGT UNIV OF CALIFORNIA
Temperature-sensitive amphiphilic cyclodextrin polymer as well as preparation method and application
ActiveCN102321250AThe synthesis steps are simpleThe synthesis steps are accurate and controllablePharmaceutical non-active ingredientsChemical structureControl release
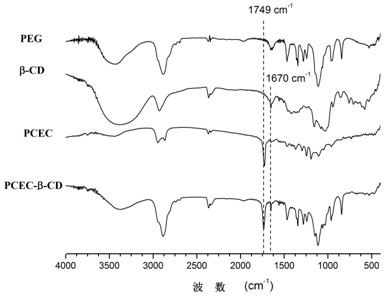
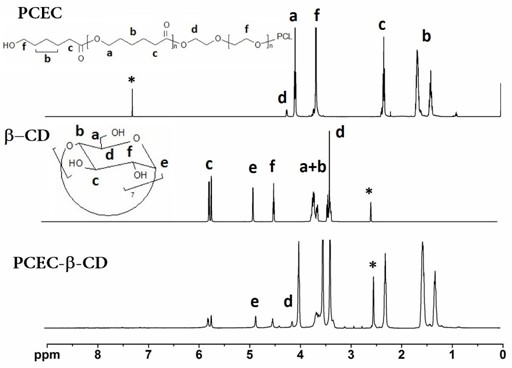
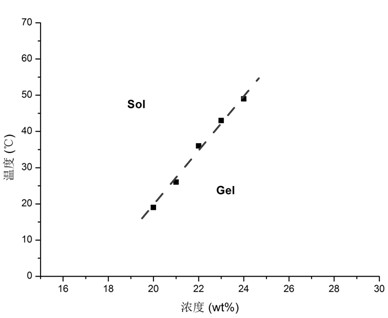
The invention provides a temperature-sensitive amphiphilic cyclodextrin polymer PCEC (polycaprolactone-polyethylene glycol-polycaprolactone, PCL-PEG-PCL). The polymer provided by the invention is the amphiphilic cyclodextrin polymer which is obtained by linking a PCL-PEG-PCL molecule with beta-CD (cyclodextrin) through utilizing the temperature-sensitive characteristic of a PCL-PEG-PCL triblock copolymer and the inclusion effect of beta-CD on a hydrophobic medicament, can effectively load and slowly release a hydrophobic or water-soluble medicament on the basis of the traditional hydrogel encapsulated water-soluble medicament, has proper critical gel temperature and can form in situ temperature-sensitive hydrogel after injection. According to the invention, the hydrophobic zone of the polymer is obviously enlarged, thereby effectively improving the loading capacity of the hydrogel on the hydrophobic medicament and obtaining high medicament encapsulation rate and medicament loading amount; and simultaneously, the controlled-release effect of the medicament after local injection delivery is realized by utilizing the temperature-sensitive gel performance of the polymer. The polymer provided by the invention has the following chemical structure shown in the specification.
Owner:ZHEJIANG UNIV
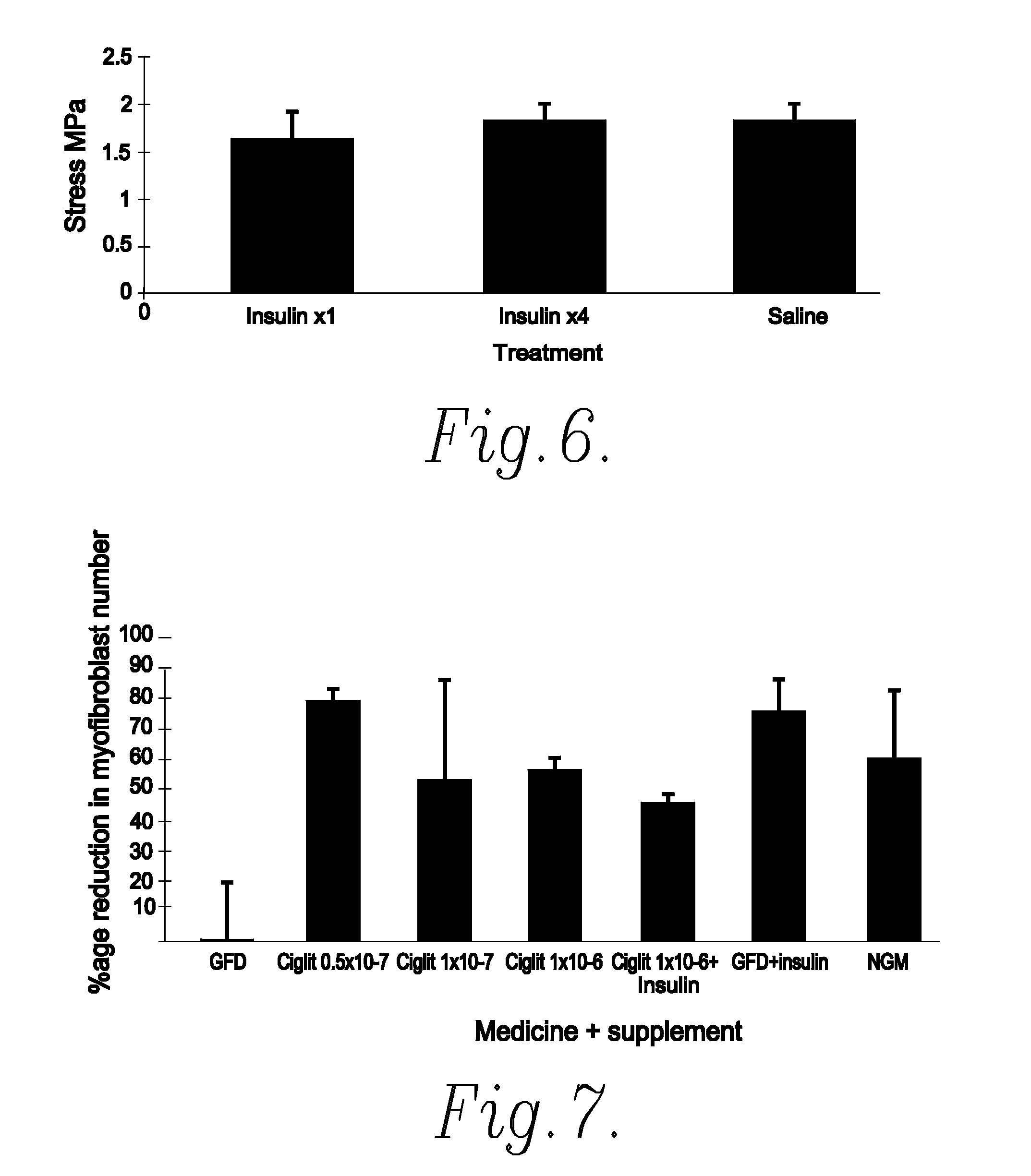
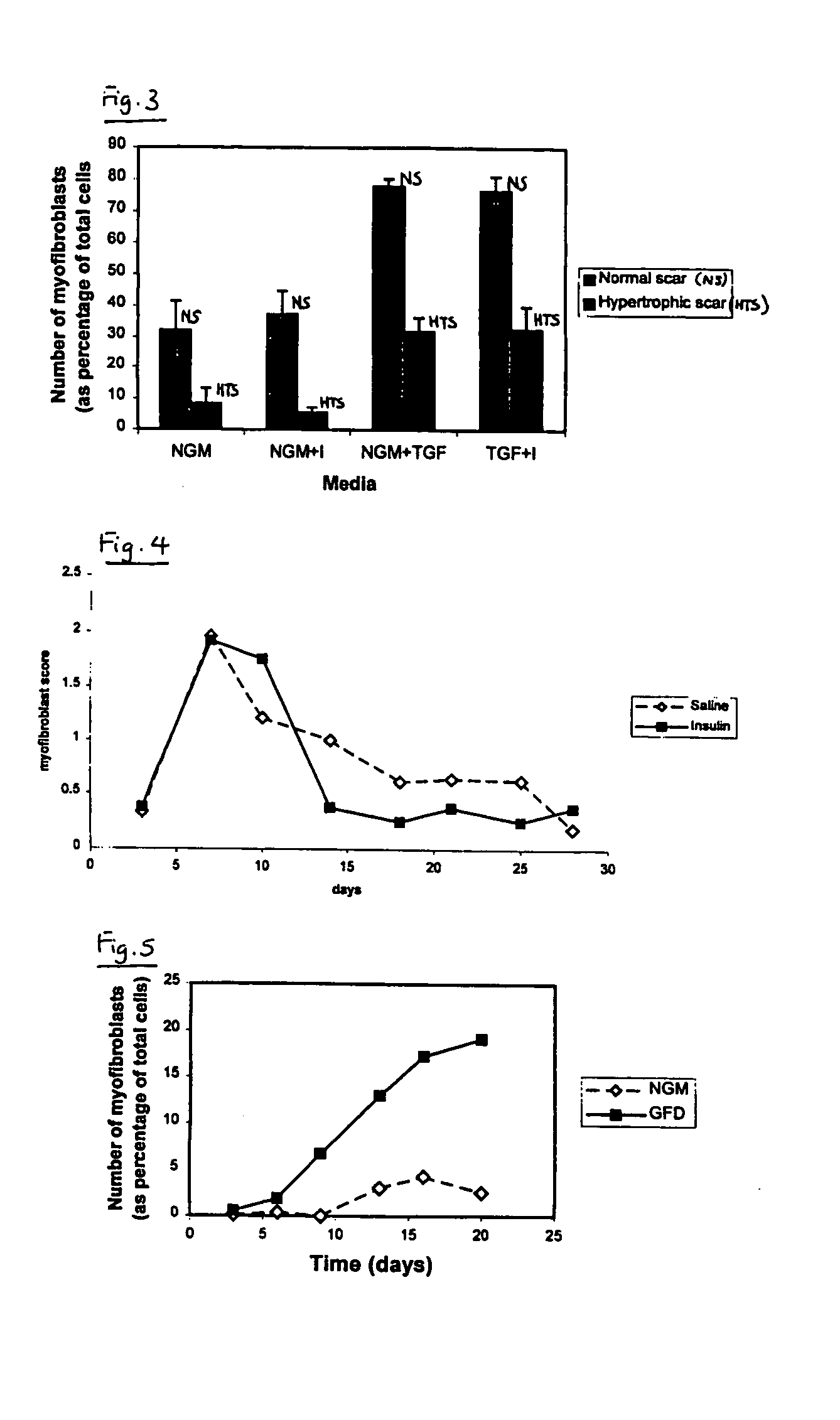
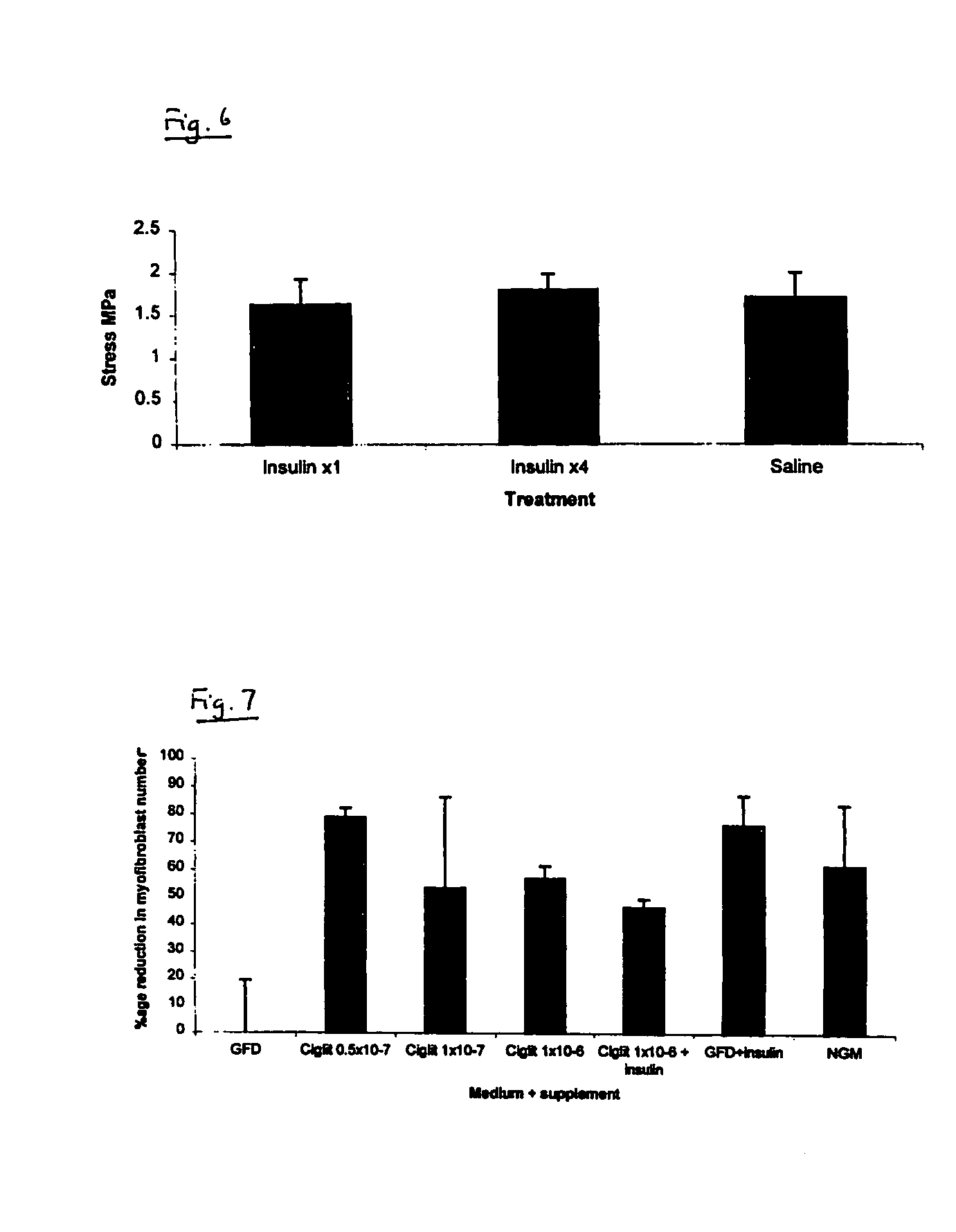
Method of Preventing or Reducing Scarring of Human Skin
InactiveUS20080182780A1Prevents and reduces formation of scar tissueIncrease speedBiocidePeptide/protein ingredientsHuman skinRe-epithelialisation
Insulin or a peroxisome proliferator-activated receptor (PPAR) agonist provides reliable and effective prevention of scarring in human skin, or at least a reduction in the severity of scarring. The application of insulin or the PPAR agonist to wounds topically or by local injection is particularly advantageous since it simultaneously reduces / prevents scarring whilst enhancing re-epithelialisation of the wound and thus provides a dual action wound healing treatment. The present invention accordingly provides a highly effective prophylactic treatment for any individual suffering tissue trauma to reduce and / or prevent normal and / or pathological scarring.
Owner:RAFT TRUSTEES
Temp-sensitive, slow-releasing gel used for local injection, and its prepn. method
InactiveCN1861041AImprove stabilityEasy to administerAntipyreticAnalgesicsInjection sitePEG-PLGA-PEG
A temp-sensitive slow-release gel for local injection features that when its temp is lower than that of human body, it exists in liquid state and after it is injected into human body, it becomes gel for slowly releasing its medicine component. Its preparing process includes such steps preparing the temp-sensitive polymer as carrier from PLGA-PEG-PLGA block copolymer, PEG-PLGA-PEG block copolymer and poloxamer, dissolving it in water, and dissolving or dispersing the medicine in said aqueous solution.
Owner:SHENYANG PHARMA UNIVERSITY
Method of preventing or reducing scarring of human skin
InactiveUS20050054608A1Prevents and reduces formation of scar tissueIncrease speedBiocideOrganic active ingredientsHuman skinDual action
Insulin or a peroxisome proliferator-activated receptor (PPAR) agonist provides reliable and effective prevention of scarring in human skin, or at least a reduction in the severity of scarring. The application of insulin or the PPAR agonist to wounds topically or by local injection is particularly advantageous since it simultaneously reduces / prevents scarring whilst enhancing re-epithelialisation of the wound and thus provides a dual action wound healing treatment. The present invention accordingly provides a highly effective prophylactic treatment for any individual suffering tissue trauma to reduce and / or prevent normal and / or pathological scarring.
Owner:RAFT TRUSTEES
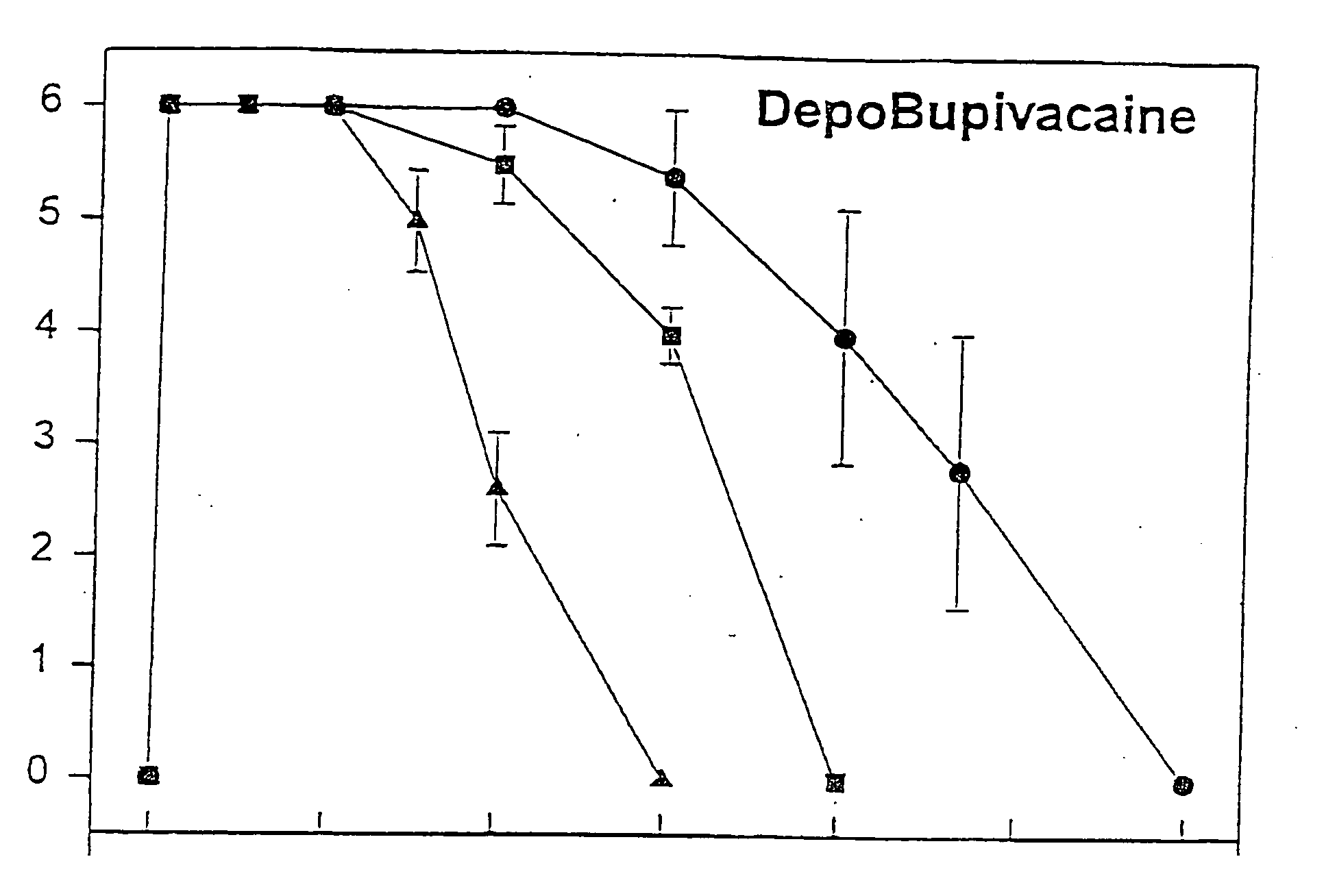
Sustained-release liposomal anesthetic compositions
InactiveUS20060078606A1High acceptabilityImprove encapsulationInorganic non-active ingredientsRotary piston pumpsHalf-lifeMaximum tolerated dose
The invention provides a method for obtaining local anesthetics encapsulated in liposomes, such as multivesicular liposomes, with high encapsulation efficiency and slow release in vivo. When the encapsulated anesthetic is administered as a single intracutaneous dose, the duration of anesthesia and half-life of the drug at the local injection site is increased as compared to injection of unencapsulated anesthetic. The maximum tolerated dose of the encapsulated anesthetic is also markedly increased in the liposomal formulation over injection of unencapsulated anesthetic. These results show that the liposomal formulation of local anesthetic is useful for sustained local infiltration and nerve block anesthesia.
Owner:PACIRA PHARMA INC
Medicinal composition containing insoluble medicament
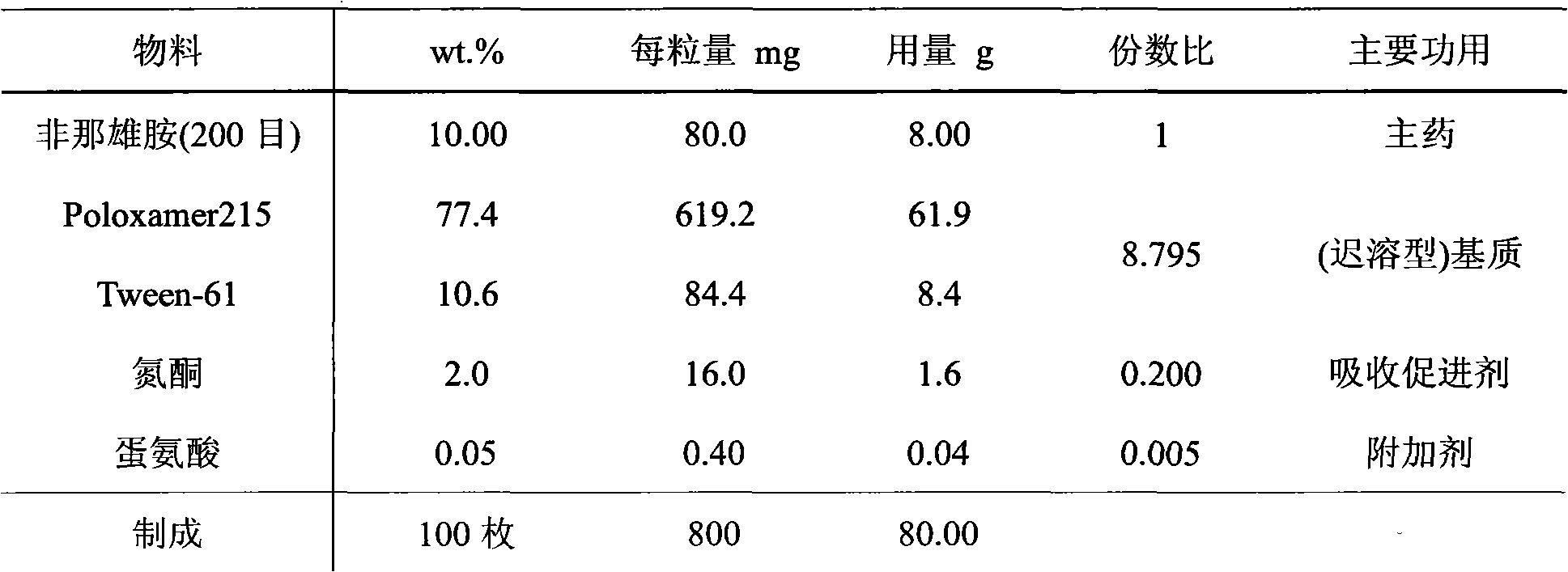
InactiveCN102145003AAvoid degradationAvoid Absorption IrregularitiesUrinary disorderAmide active ingredientsPoor complianceSide effect
The invention discloses a medicinal composition containing an insoluble alpha-receptor retardant and / or 5alpha-reductase inhibitor with an effective dose, which comprises the following components of: principal ingredients, a substrate, a solubilizer, a sorbefacient and an additive in a weight ratio of 1:(8-7,000):(0-460):(0-150):(0-150), wherein the medicinal composition at least contains one of the solubilizer and the sorbefacient. The effective dose of the insoluble alpha-receptor retardant and / or the 5alpha-reductase inhibitor is in 0.05 to 80 milligrams of parent compounds of the insoluble alpha-receptor retardant and / or the 5alpha-reductase inhibitor, the weight of a preparation per unit is between 0.8 and 4.2 grams. The medicinal composition can be subjected to oral administration or rectum administration, so the defects of poor curative effect and large toxic and side effect in the conventional oral administration and systemic administration of injection and large side effect and poor compliance of patients in local injection administration can be overcome, the lasting time of the medicinal effect can be increased, and better treatment means can be provided for medical care personnel and patients; and a product process is simple and is suitable for industrial batch production.
Owner:张立英
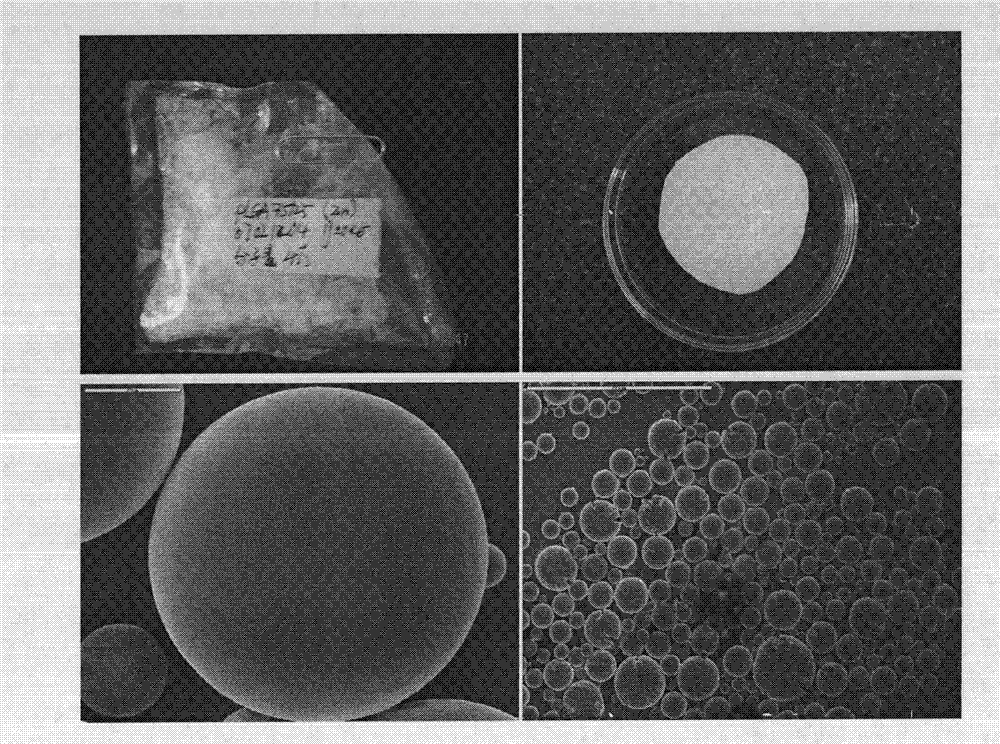
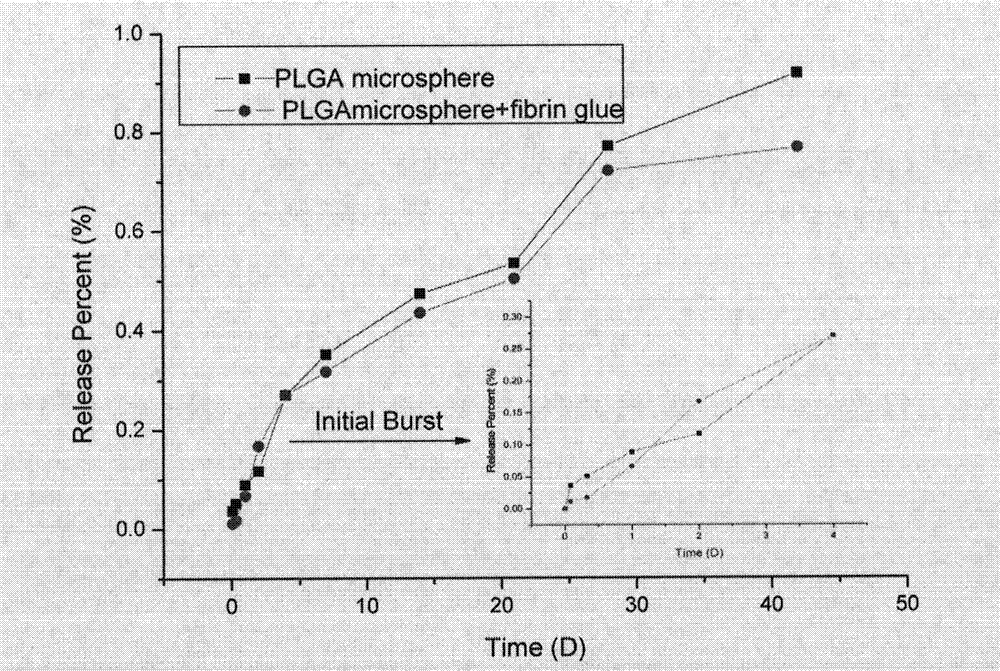
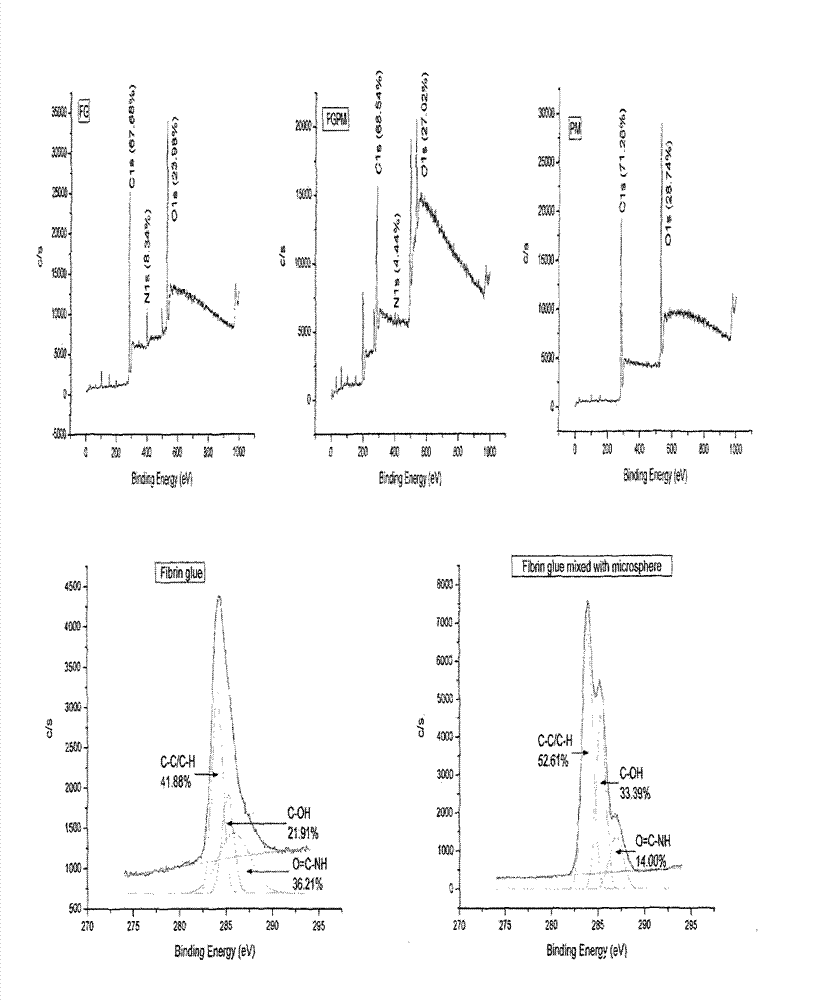
Preparation method and application of fibrin glue composite recombinant human bone morphogenetic protein-2 (rhBMP-2) microsphere
The invention relates to a preparation method and application of a fibrin glue composite recombinant human bone morphogenetic protein-2 (rhBMP-2) microsphere. The preparation method comprises the steps of: preparing a slow release microsphere with a proper particle diameter; and then constructing an rhBMP-2 / PLGA microsphere / fibrin glue composite material. The rhBMP-2 / PLGA microsphere fibrin glue composite material can be used through local injection, surgical trauma can be reduced, a healing process of bone fracture and nonunion is accelerated by continuously supplementing the local bone morphogenetic protein, thus the fibrin glue composite recombinant human bone morphogenetic protein-2 is a bone repair material with excellent degradability and osteogenic activity.
Owner:姚琦 +2
Low profile system for joining optical fiber waveguides
InactiveUS20060266082A1Low profileAccurately inferredGlass making apparatusCoupling light guidesFiberActive optics
A compact, low profile splicing system for joining optical fibers produces durable, low transmission loss fusion splices. The system employs active optical techniques such as profile alignment or local injection and detection to achieve optimized alignment of the fibers prior to fusion. Light injected into one fiber is propagated across the interface to a second fiber. A detector senses the intensity of the injected light in the second fiber. After the relative position of the fibers is manipulated to maximize the transmitted intensity, the fibers are fusion spliced using an electric arc discharge. The accurate alignment achievable using the local injection and detection system to drive adaptive fiber positioning affords a method for reliably producing low loss splices. The present system is compact and low in profile, making it operable in cramped quarters with limited clearance to adjacent equipment and structures and with only a minimal amount of free fiber slack available. Simplicity of design and operation make the system rugged and enable accurate alignment and low loss fusion of fibers under adverse working conditions.
Owner:AURORA INSTR
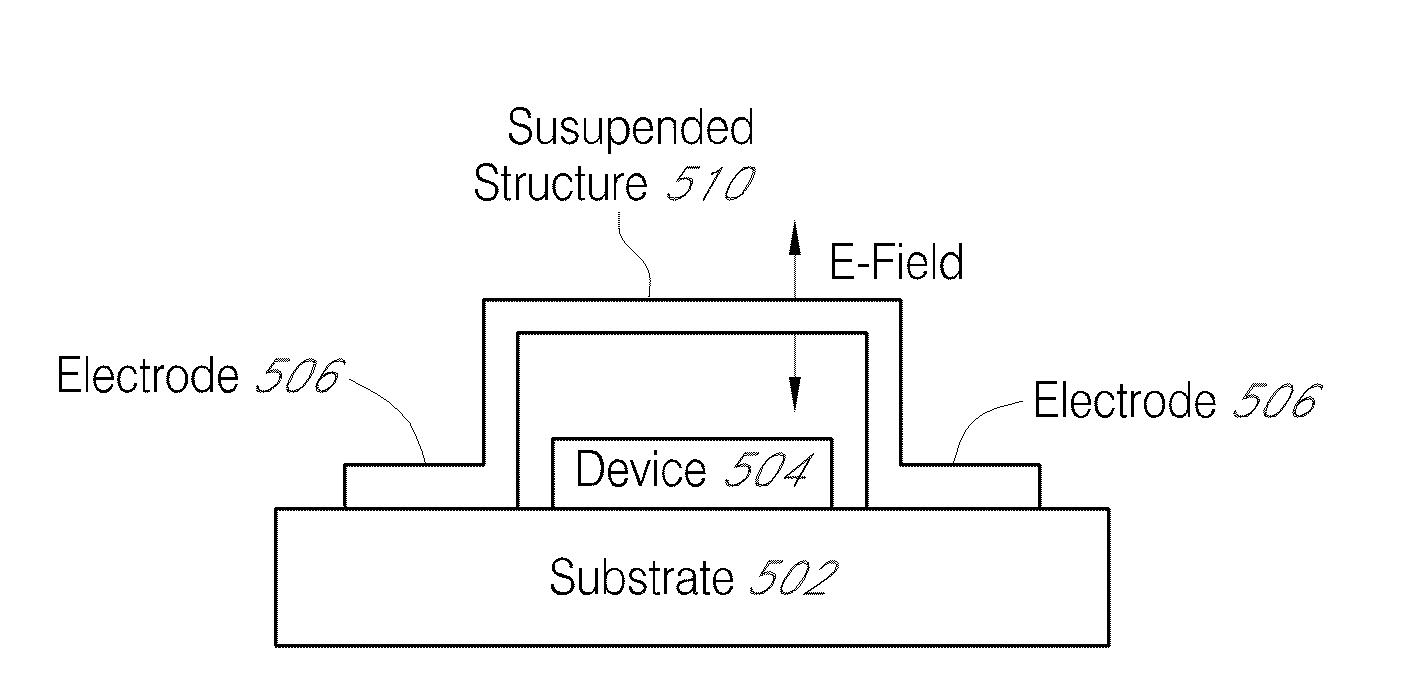
Suspended structures
InactiveUS20090225592A1Acceleration measurement using interia forcesFluid pressure measurement by electric/magnetic elementsLithography processManufacturing technology
A multi-level lithography processes for the fabrication of suspended structures are presented. The process is based on the differential exposure and developing conditions of several a plurality of resist layers, without harsher processes, such as etching of sacrificial layers or the use of hardmasks. These manufacturing processes are readily suited for use with systems that are chemically and / or mechanically sensitive, such as graphene. Graphene p-n-p junctions with suspended top gates formed through these processes exhibit high mobility and control of local doping density and type. This fabrication technique may be further extended to fabricate other types of suspended structures, such as local current carrying wires for inducing local magnetic fields, a point contact for local injection of current, and moving parts in microelectromechanical devices.
Owner:RGT UNVIVERSITY OF CALIFORNIA THE
Joint lubricating material and preparation method thereof
InactiveCN106046397AControllable gelation timeIncrease moisture contentSurgeryPharmaceutical delivery mechanismO carboxymethyl chitosanPolymer science
The invention discloses a joint lubricating material and a preparation method thereof. The material is covalent crosslinked O-carboxymethyl chitosan hydrogel, wherein the gelation time is 0.5-10min, the water content is higher than 70%, and the water retention rate is higher than 65%; and the material is prepared by carrying out gelation reaction between O-carboxymethyl chitosan of which the degree of deacetylation is greater than 25% and glutaric dialdehyde. The prepared covalent crosslinked O-carboxymethyl chitosan hydrogel has good biocompatibility, can serve as a joint lubricating agent, is suitable for local injection of joints, and can enable the joints to keep certain capacity and elastic properties within certain time so as to promote cartilage tissue repair.
Owner:SHENZHEN BRIGHT WAY NOVEL BIO MATERIALS TECH CO LTD
Disinfectant, antibiotic and anesthetic containing device for injections and incisions
InactiveUS20050085791A1Reduce the risk of infectionMinimize movementAntisepticsSurgeryDisinfectantDevice form
Devices, kits and methods for reducing the risk of infection at an injection or incision site are described herein. The device contains a bioadhesive, biocompatible and bioerodable material and one or more disinfectant agents. In the preferred embodiment, the material is formed of one ore more hydrogels Optionally, the device also contains one or more anesthetics to decrease discomfort. The device may be marked or calibrated to facilitate localized injection or incision at a pre-specified site on the skin or mucus membrane. After identifying or selecting the injection or incision site, the device is placed on the site for a time sufficient to achieve localized disinfection, and optionally localized anesthesia. Then the needle or surgical instrument is inserted through the composition into the site. Thereafter, the drug is administered, fluid is removed, in the case of an injection, or the surgical instrument is placed at the site, in the case of an incision. Then the needle or surgical instrument is removed from the site, and the device forms a continuous seal over the site. The disinfectant and / or anesthetic is delivered before, during, and / or after the treatment. Optionally, the disinfectant and / or anesthetic is released in a controlled-release manner. Optionally, the disinfectant and / or anesthetic may de delivered following a time-delay. In a second embodiment, the device may be placed at a site following the injection or incision at the site to reduce the risk of infection, provide anesthesia, and / or prevent reflux of blood or fluid following the injection or incision.
Owner:SHAW SHARON M
System for joining polarization-maintaining optical fiber waveguides
InactiveUS6984077B2Easy alignmentCoupling light guidesOptical waveguide light guidePolarization-maintaining optical fiberEngineering
A splicing system for joining polarization-maintaining, single mode optical fibers produces durable fusion splices that have low transmission loss and maintain mode integrity. The system employs active optical techniques such as profile alignment or local injection and detection to achieve optimized lateral alignment of the fibers prior to fusion. Azimuthal alignment is performed using a transverse, polarized light illumination and detection system. Each fiber is rotated azimuthally to determine a transverse intensity function. The transverse intensity functions of the respective fibers are cross-correlated to determine a relative orientation that matches the polarization axes of the fibers. After the relative position of the fibers is manipulated laterally, axially, and azimuthally, the fibers are fusion spliced using an electric arc discharge. The accurate alignment achievable using the transverse illumination mechanism to drive adaptive fiber positioning affords a method for reliably producing low loss, mode-matched splices. Simplicity of design and operation make the system rugged and enable accurate alignment and low loss fusion of fibers under adverse working conditions.
Owner:AURORA INSTR
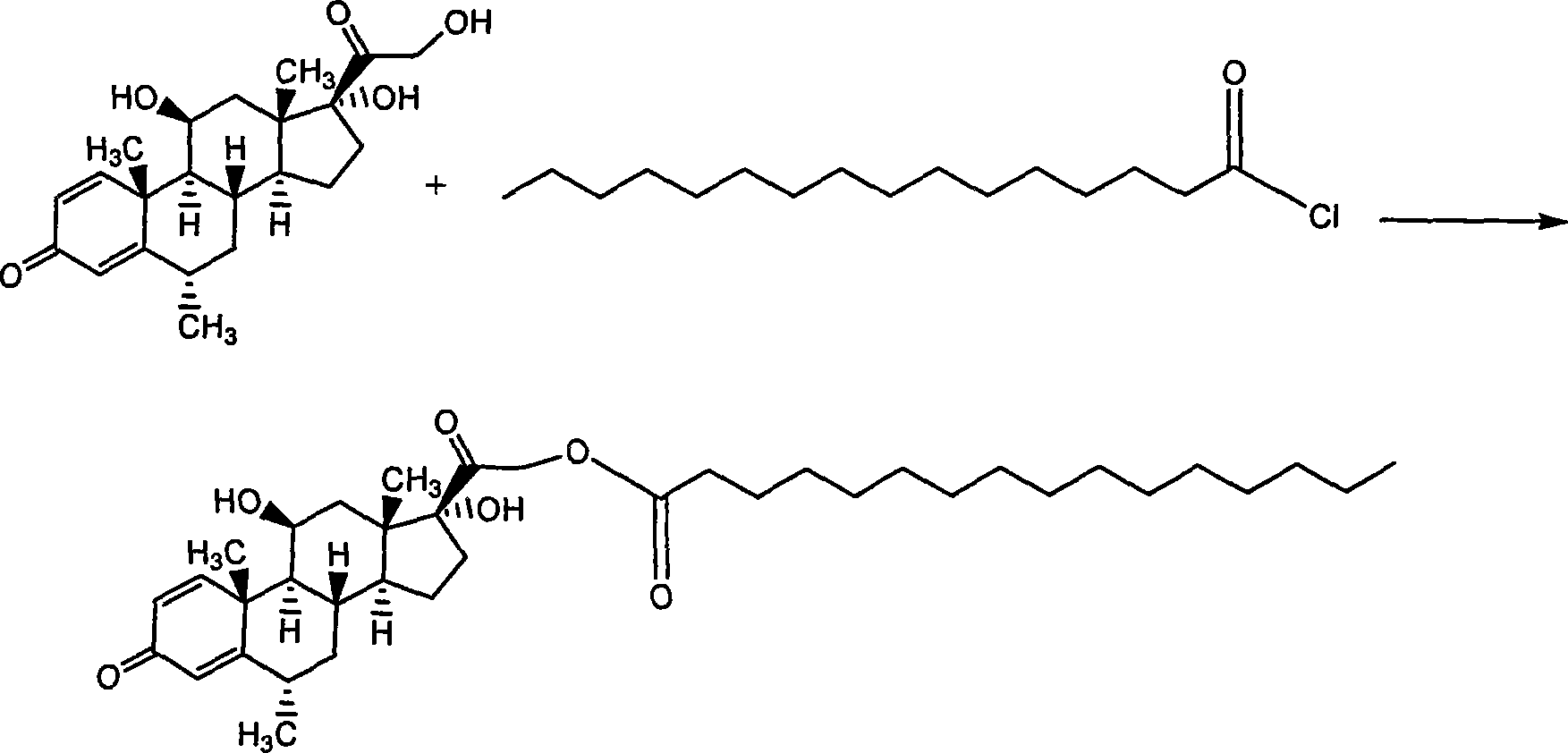
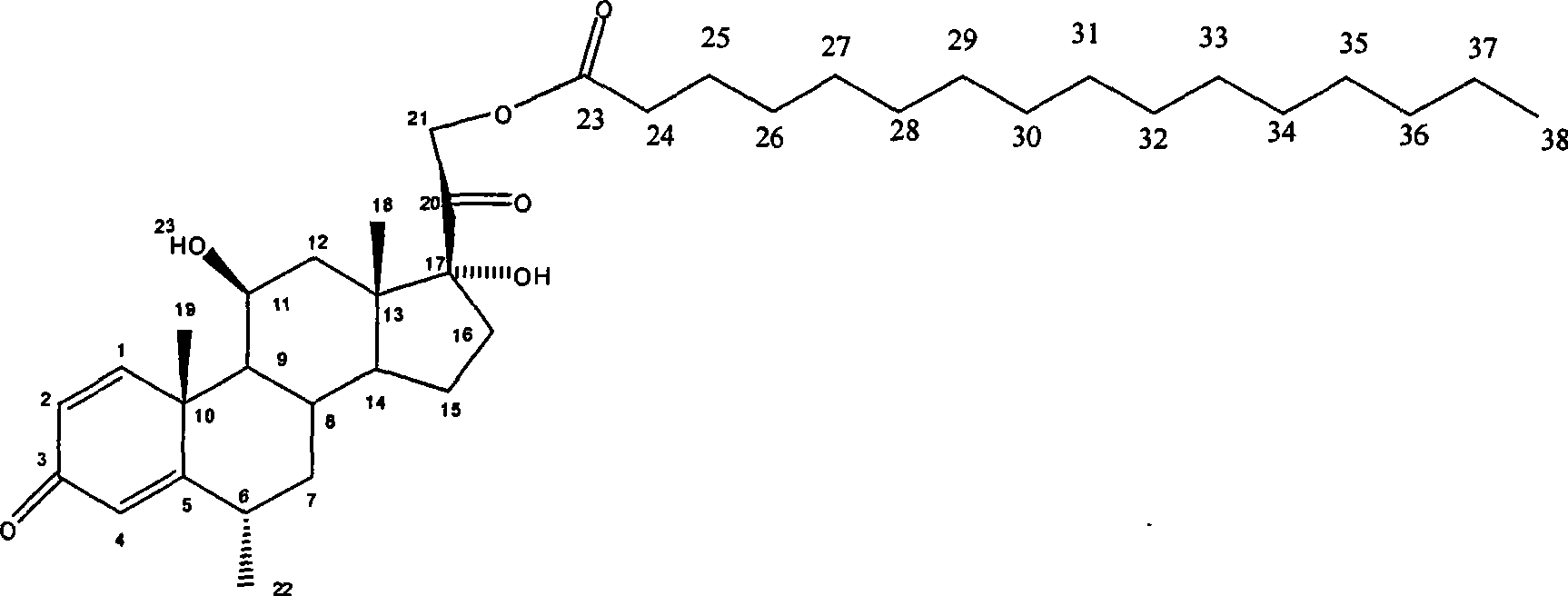
Composition with methylprednisolone palmitate as active component for treating local inflammation
InactiveCN101412741AEnhanced inhibitory effectOrganic active ingredientsAntipyreticActive componentMethylprednisolone
The invention relates to a drug composition for treating local inflammation, which uses methylprednisolone palmitate as an active composition. The drug composition consists of the methylprednisolone palmitate as the active composition and an inactive composition suitable for local injection, and is used for treating local inflammation of people or mammal through local injection.
Owner:TIANJIN JINYAO GRP
Stem cell injection for treating osteoarthritis cartilage defect and preparation and application thereof
The invention provides a stem cell injection for treating osteoarthritis cartilage defect and preparation and application thereof. The injection comprises fifth-generation umbilical cord mesenchymal stem cells and a liquid solvent, wherein the fifth generation umbilical cord mesenchymal stem cells are suspended in the liquid solvent, and the concentration is (2.0-3.0)x10<7> cells / 4.5-5.0ml of the liquid solvent. The liquid solvent is normal saline, or autologous platelet-rich plasma, or a mixed solution of normal saline and human albumin, or a mixed solution of normal saline and autologous platelet-rich plasma. The human albumin accounts for 5-8% in volume by percentage. The normal saline accounts for 10% or below in volume by percentage. The injection is administered by intravenous injection or bone joint cavity local injection or the combination of the two, the cell injection amount of the intravenous injection is (1.0-1.5)x10<8> cells / one time, and the cell injection amount of the bone joint cavity injection is (2.0-3.0)x10<7> cells / one time / one knee. The effect of the stem cell injection for treating the integrity degree of osteoarthritis cartilage is obviously superior to that of a traditional hyaluronic acid (HA) method.
Owner:WUHAN HAMILTON BIOTECH
Microsphere-containing gel for injection and preparation method thereof
InactiveCN110327497AImprove liquidityAvoid problems such as unevennessPharmaceutical delivery mechanismProsthesisPolyesterMicrosphere
The invention relates to a microsphere-containing gel for injection. The gel comprises a carrier and degradable polyester microspheres dispersed in the carrier and used for carrying out filling and supporting functions, wherein the carrier is prepared from high polymer material particles and a physiological buffer solution. The invention also relates to a preparation method of the microsphere-containing gel for injection. The method comprises the following steps: S1, preparing the carrier by using the high polymer material particles and the physiological buffer solution; and S2, uniformly dispersing the degradable polyester microspheres in the carrier to form the microsphere-containing gel for injection. The microsphere-containing gel for injection provides a short-term filling and supporting function through the high polymer material particles, and provides a long-term filling and supporting function through the degradable polyester microspheres. In addition, the degradable polyestermicrospheres are uniformly dispersed in the carrier, so that the problems of uneven filling caused by local injection can be solved.
Owner:YIPURUN SHANGHAI BIOTECH CO LTD
Sustained-release injection and preparation method thereof
InactiveCN101380291APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectSuspending Agents
A slow release injection contains bioactive components, slow release adjuvants, a suspending agent and a dissolvant, wherein, the dissolvant is a common dissolvant or a special dissolvant containing the suspending agent. The slow release adjuvants are chosen from copolymer of polylactic acid, polyglycolic acid and hydroxyacetic acid, copolymer of ethylene vinyl acetate, polifeprosan, etc. The injection can be slowly released to the local focuses of tumors, inflammation and tuberculosis during the decomposition and absorption of the slow release adjuvants, therefore, the slow release adjuvants can not only extremely reduce general toxic reactions thereof but also keep the effective drug concentration at the chronic local focuses of tumors, etc. The suspending agent is chosen from sodium carboxymethyl cellulose, mannitol, and the like, and is used for suspending the active components or suspending the slow release particles or microspheres containing anticancer active components. The slow release injection has the advantages of good injectivity, seldom blockage, strong stability, infrequent demixing, and little general toxic reaction of local injection; furthermore, the slow release injection can selectively increase the drug concentration in local focuses, and enhance the curative effects of non-operative therapies such as radiotherapy, chemotherapy, and the like.
Owner:孔庆忠
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com