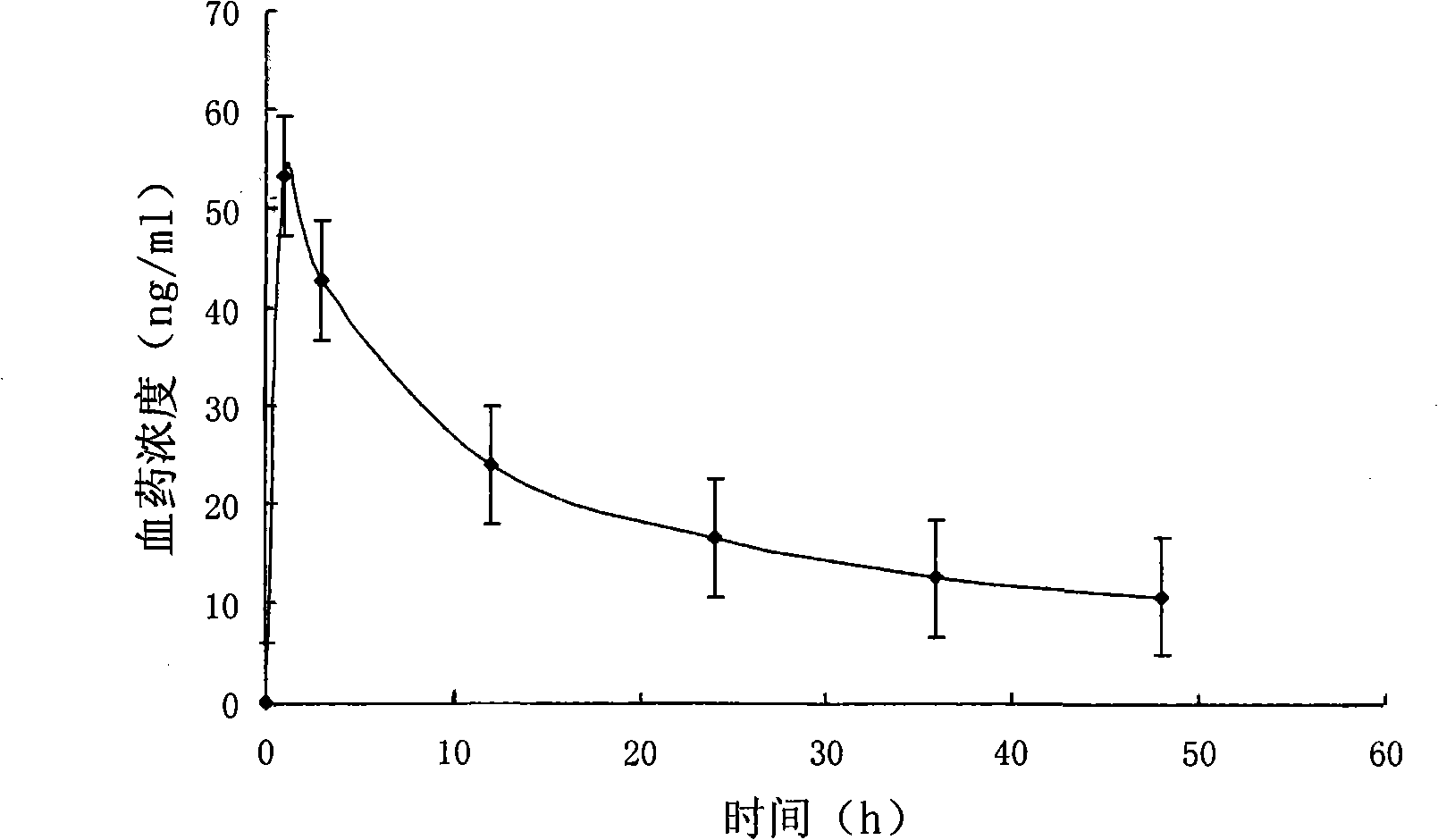
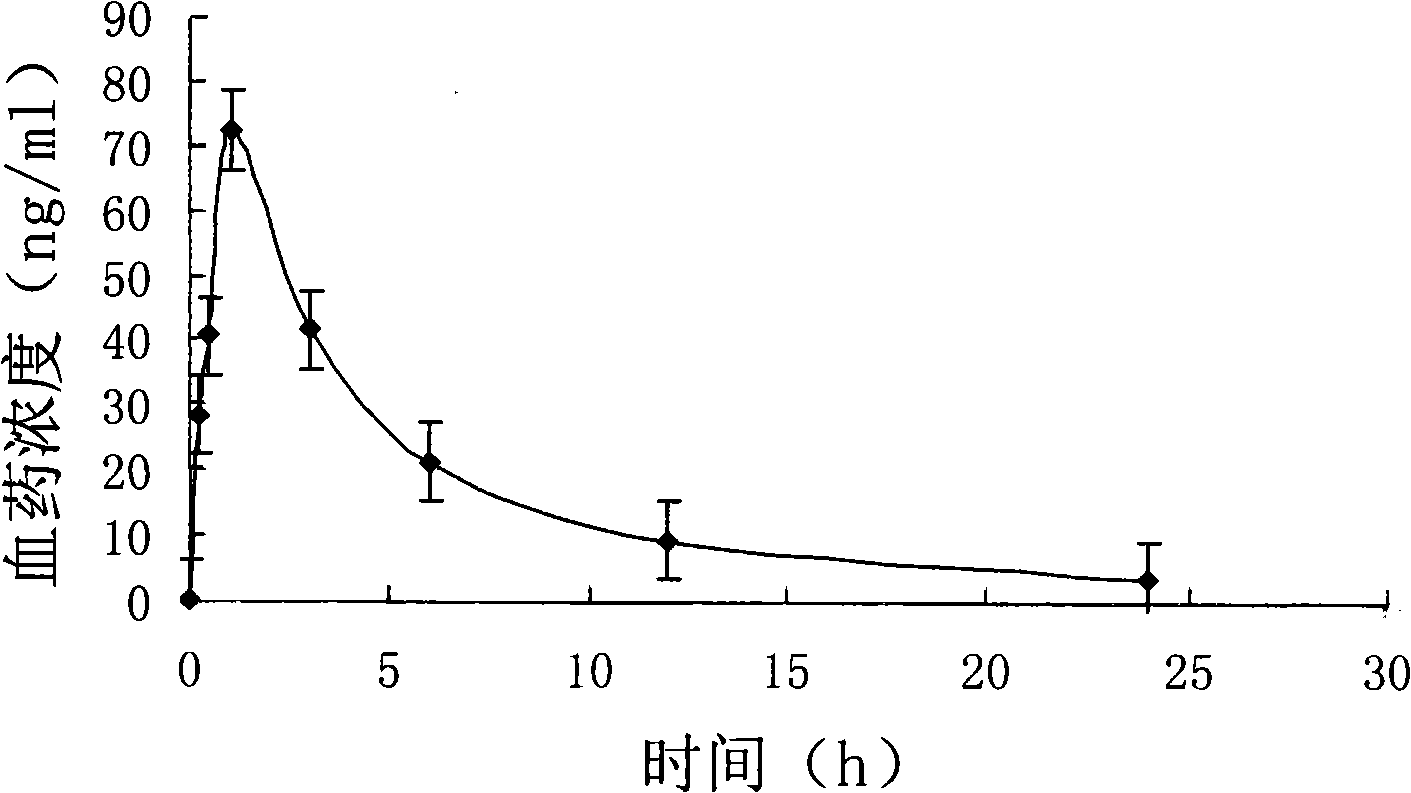
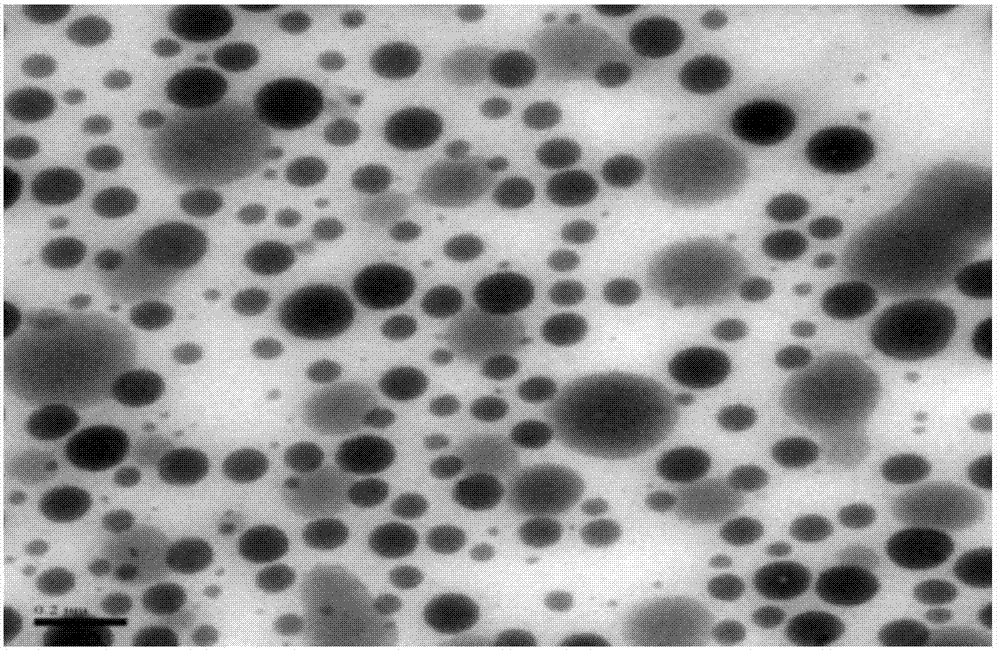
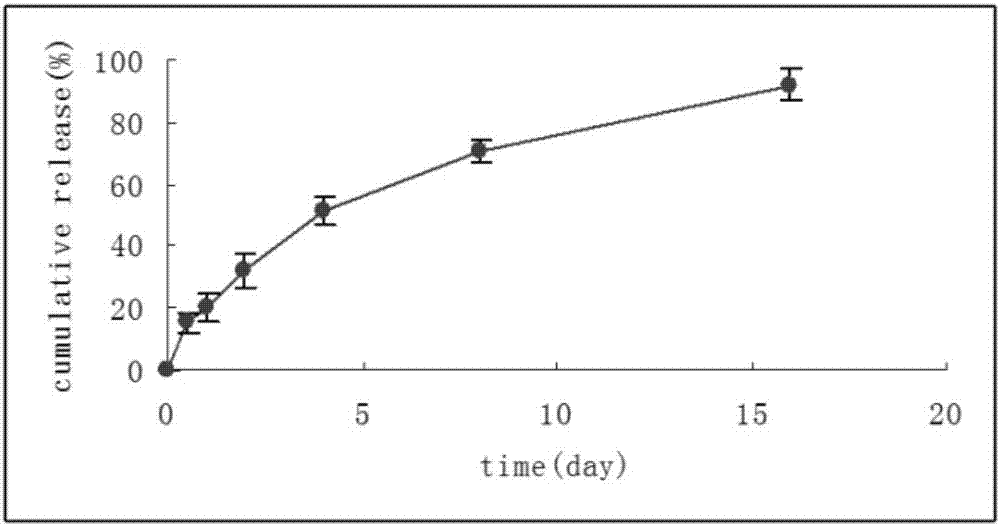
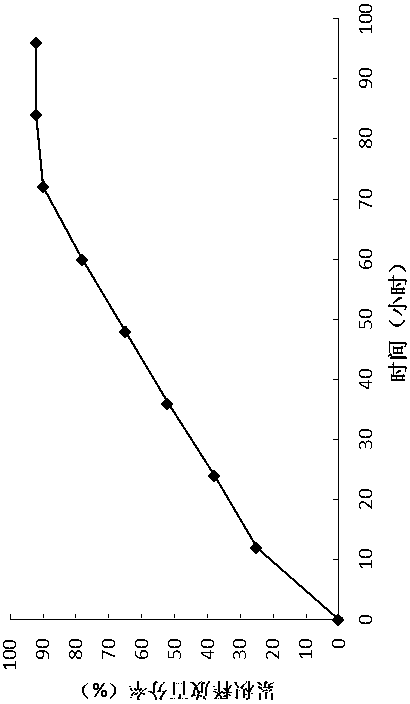
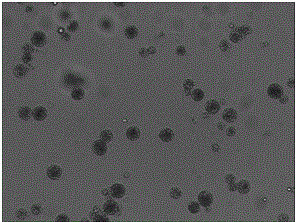
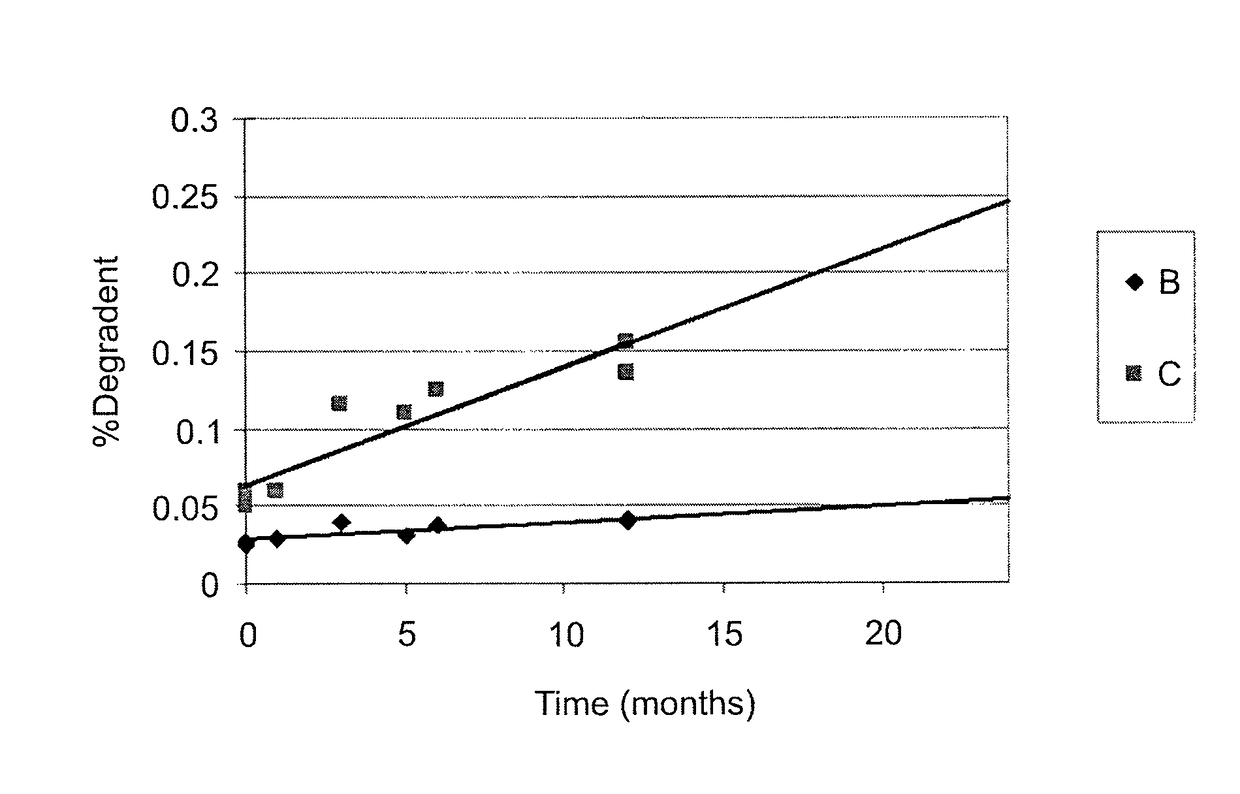
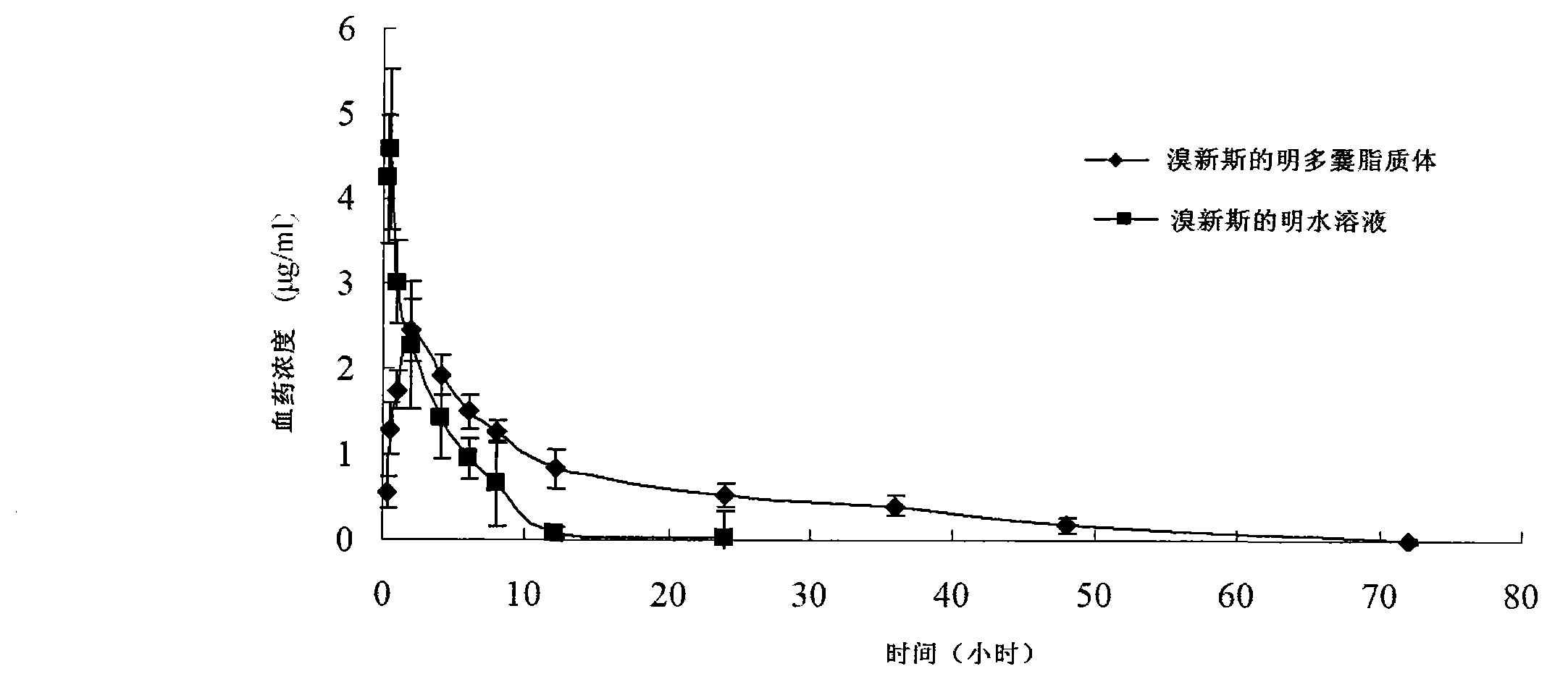
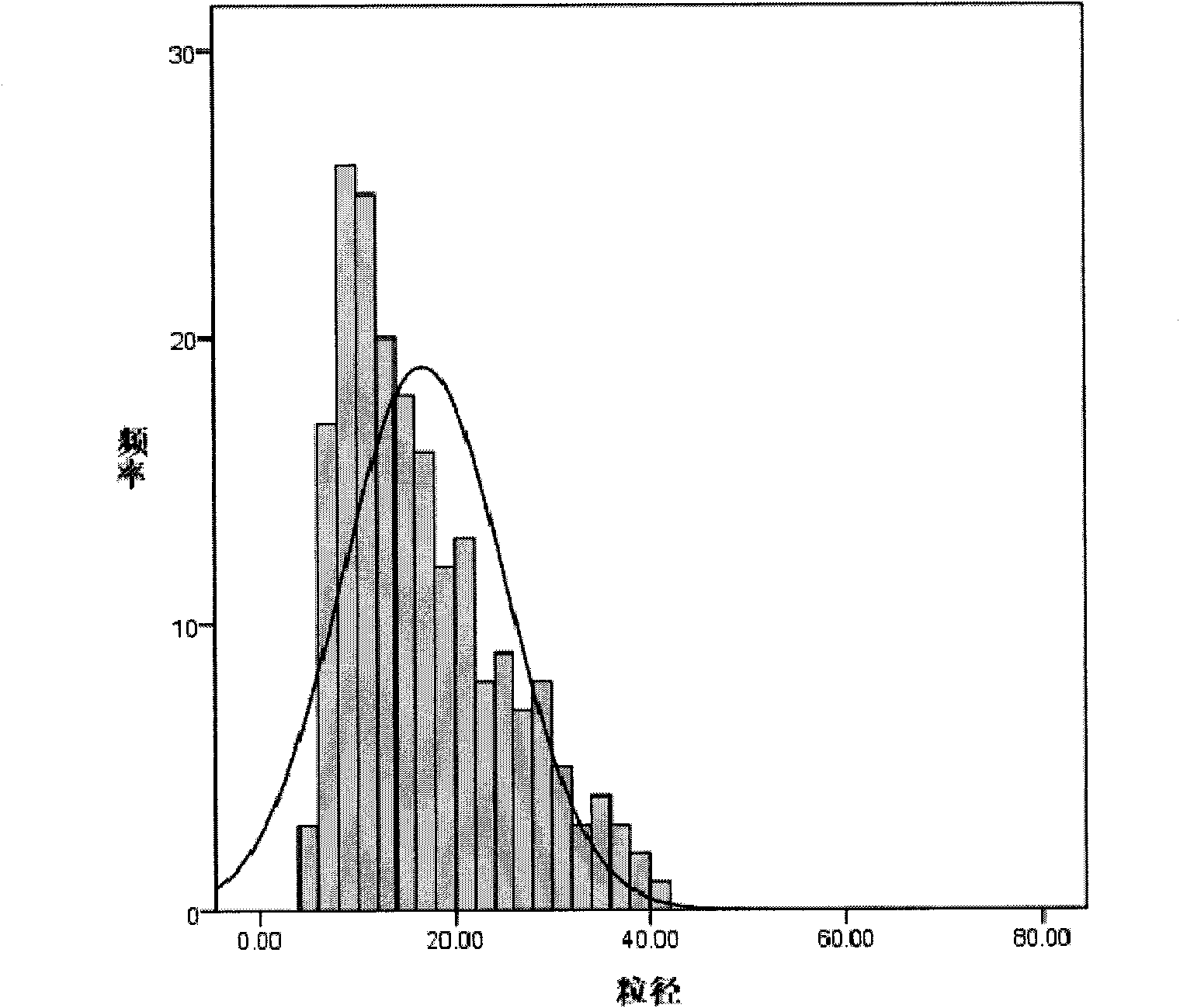
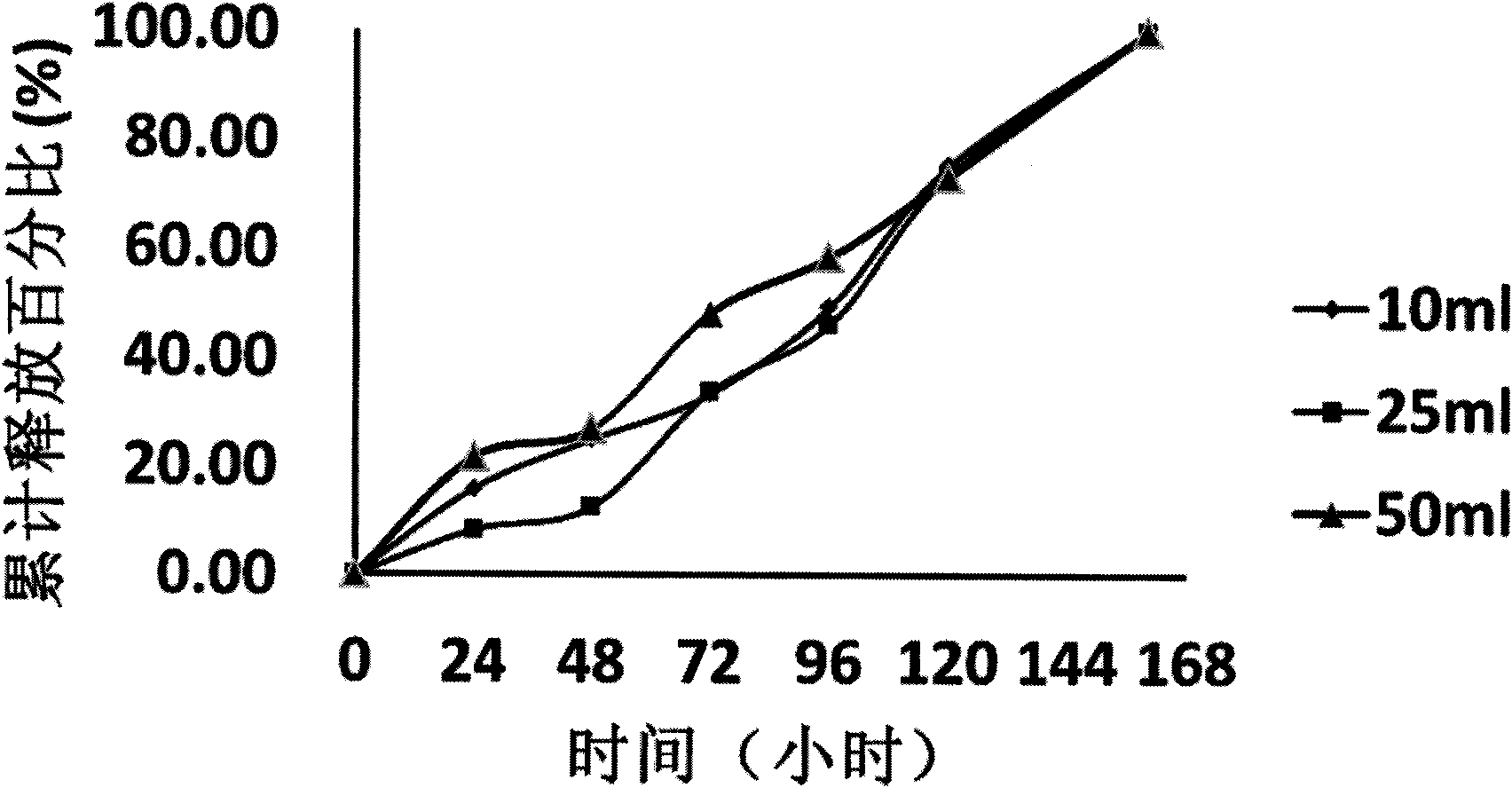
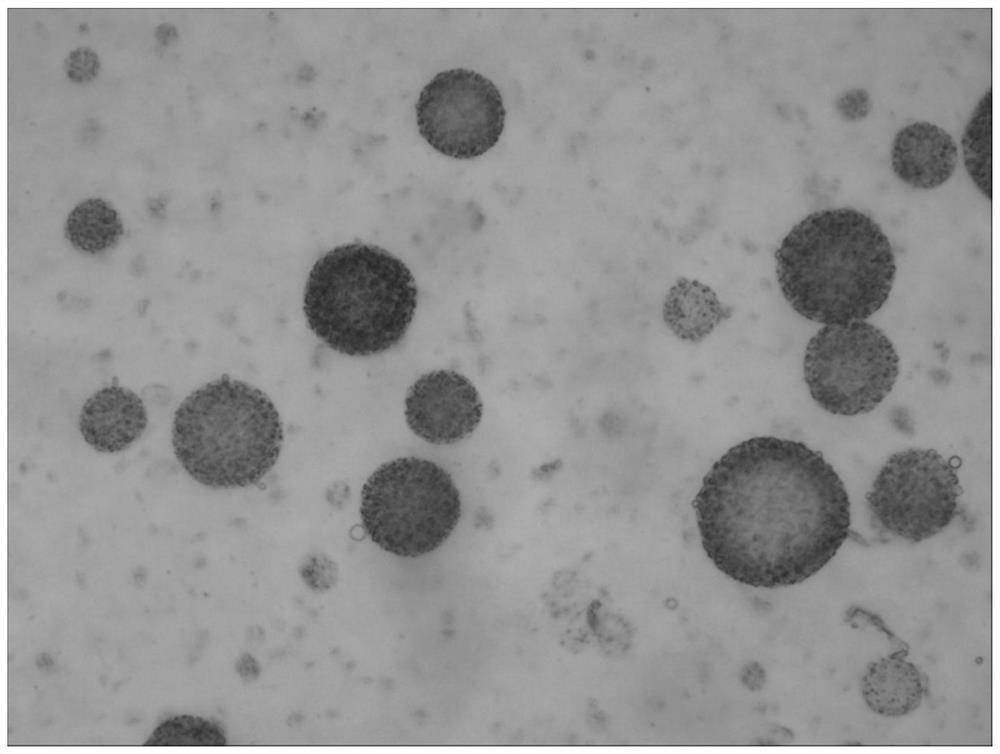
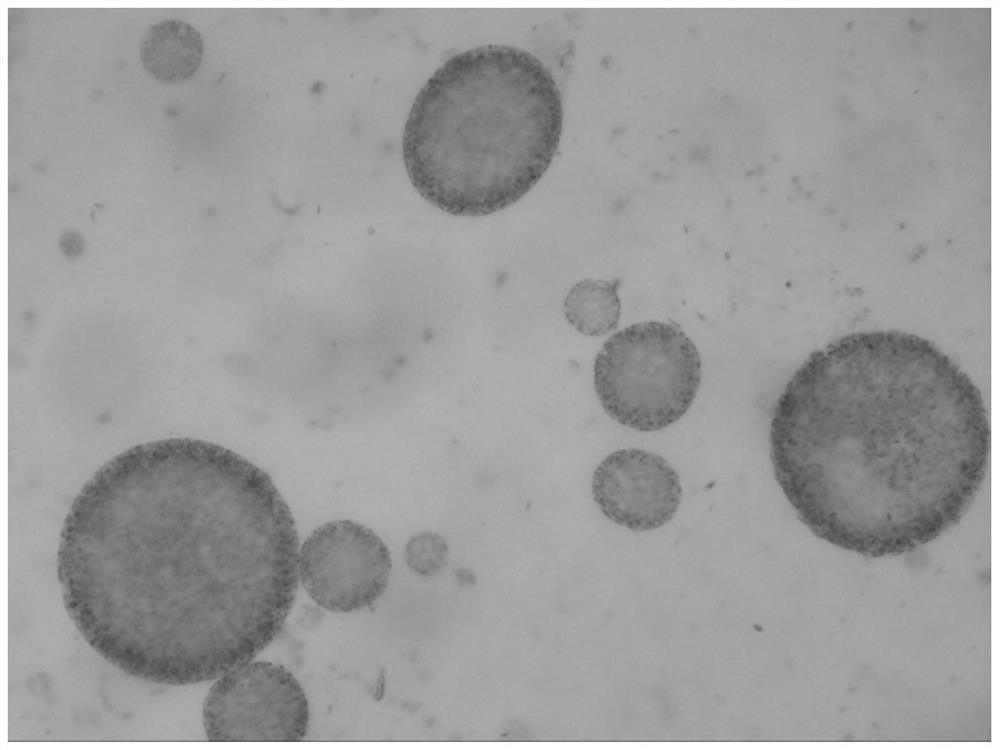
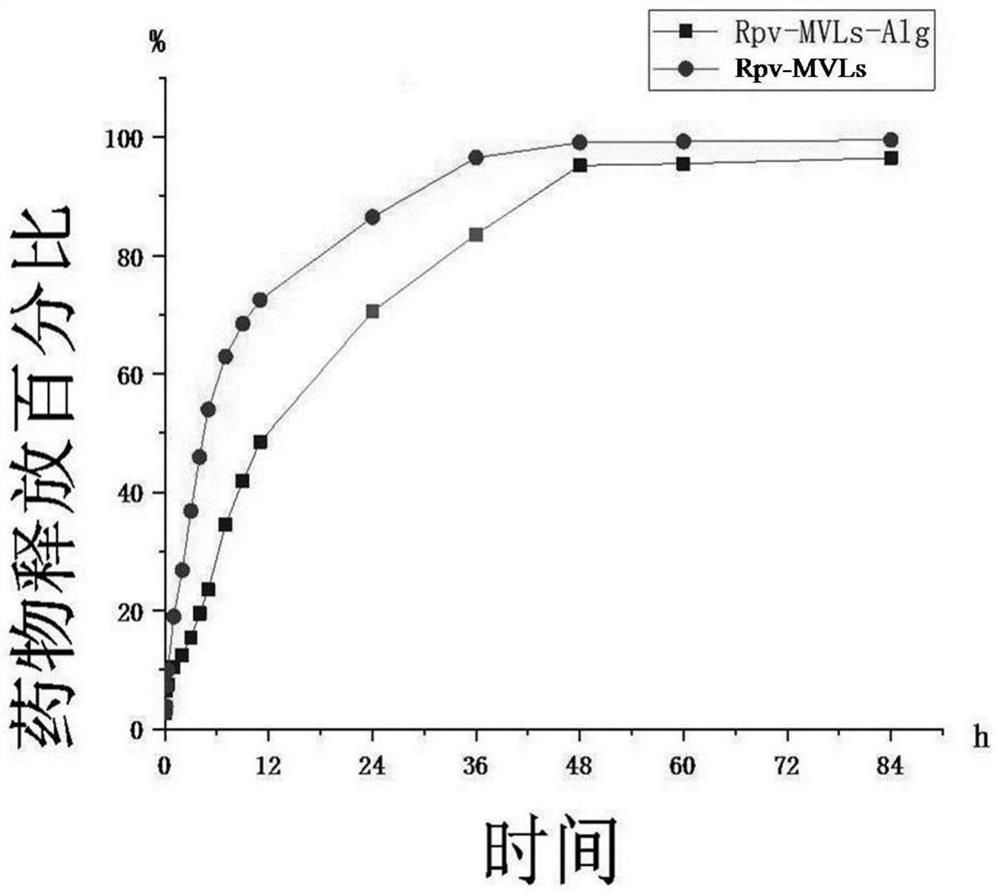
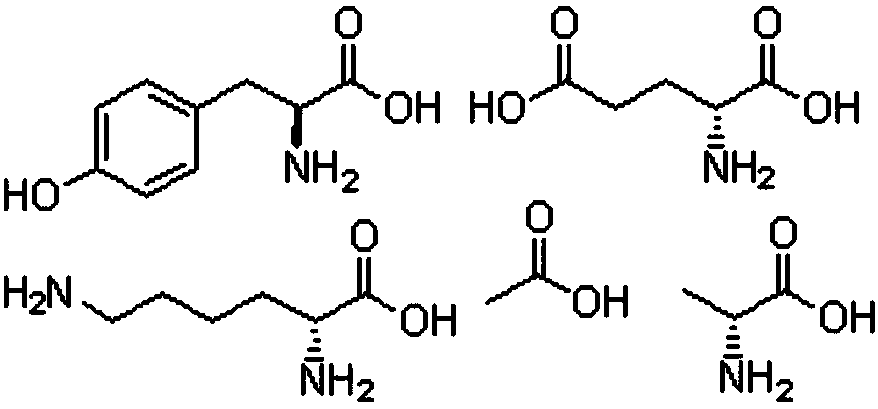
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Multivesicular liposomes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
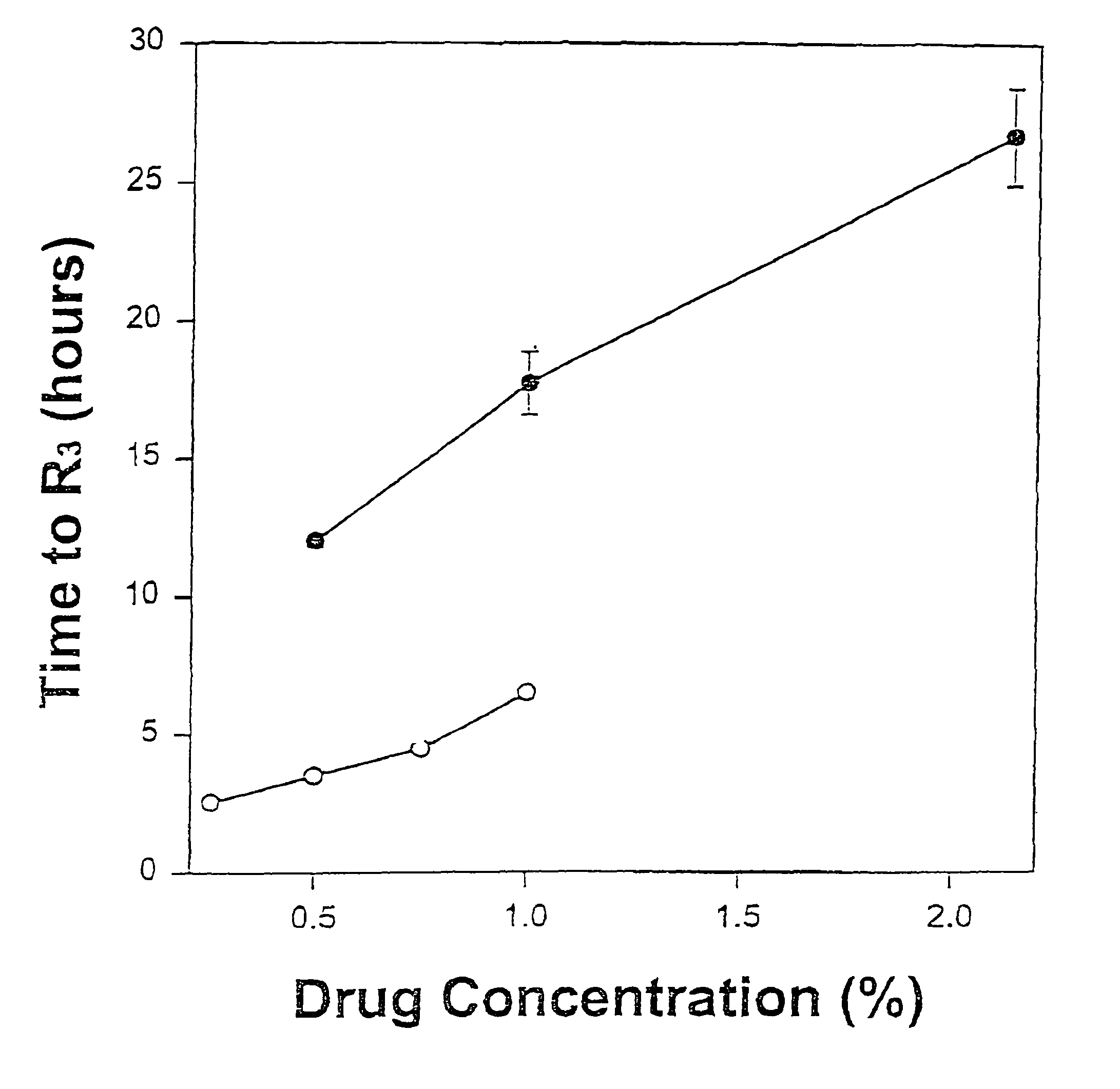
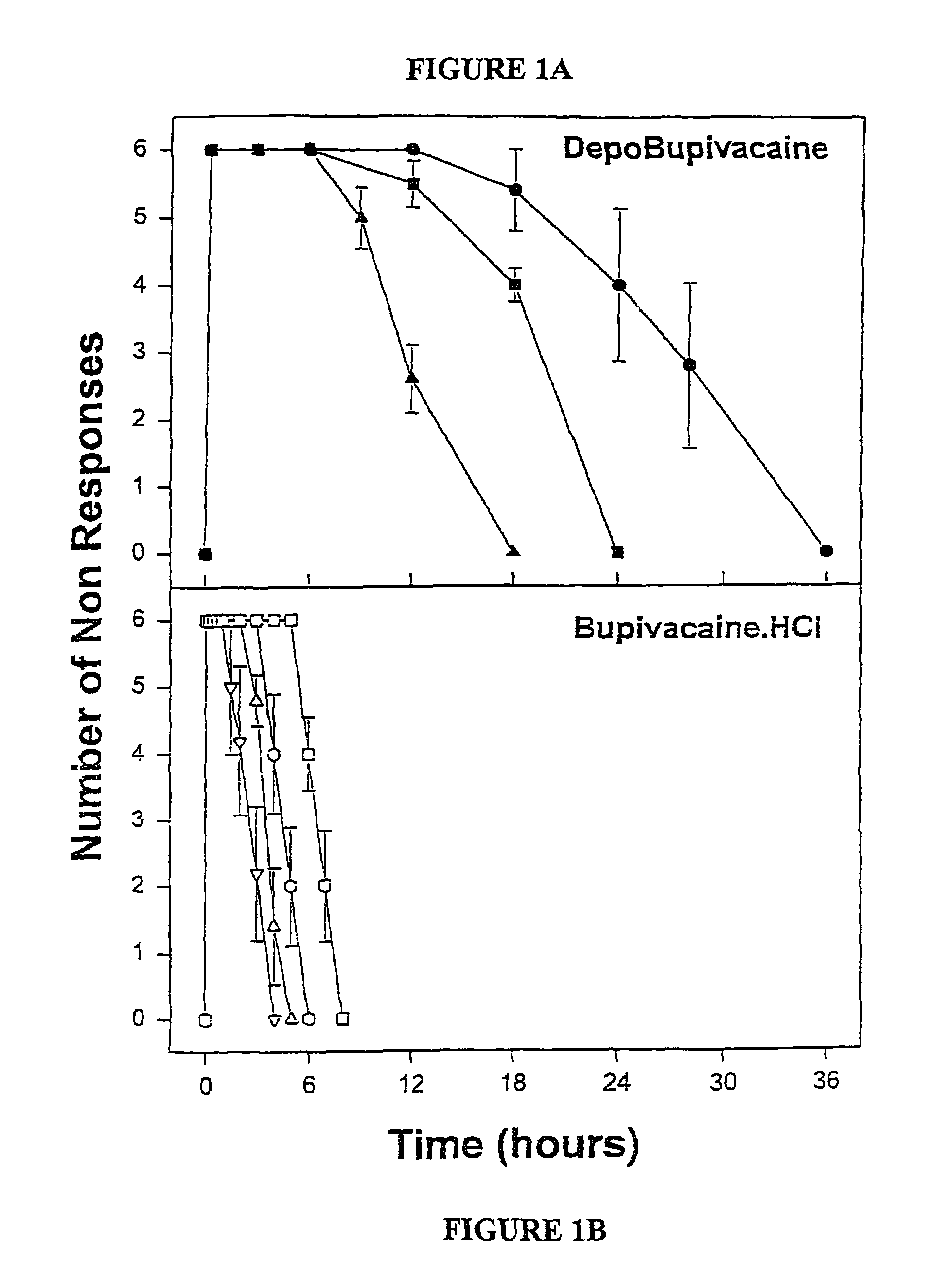
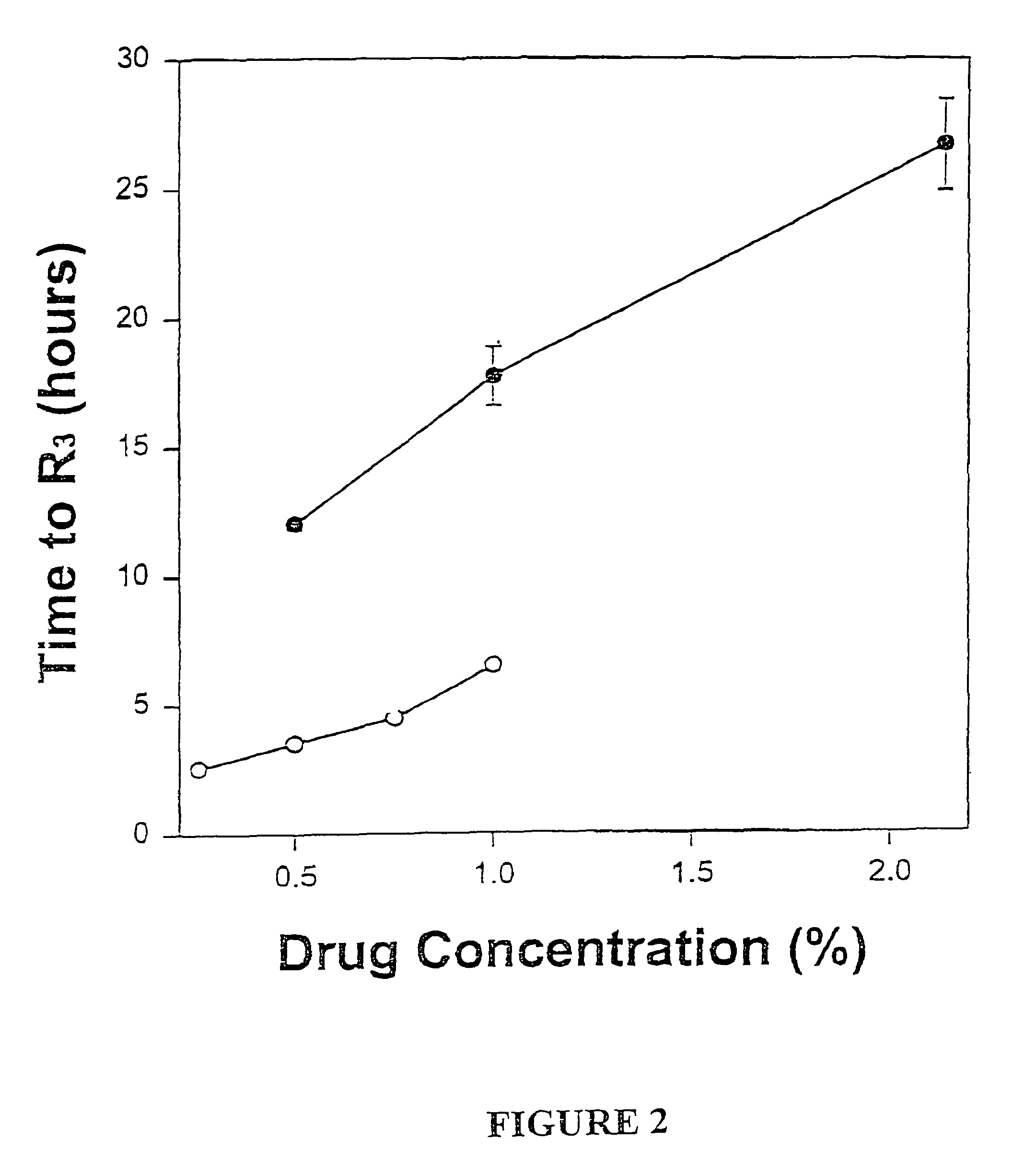
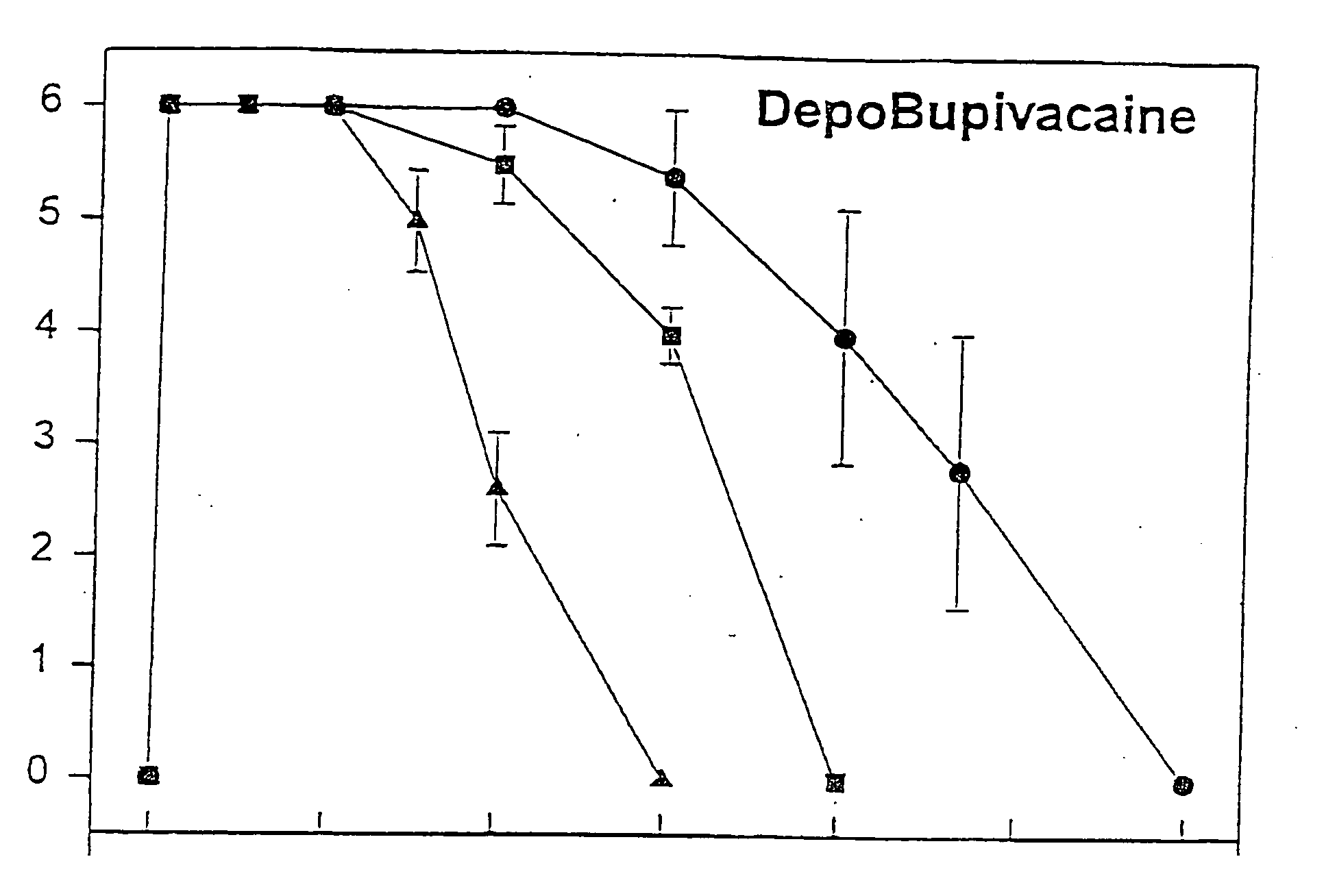
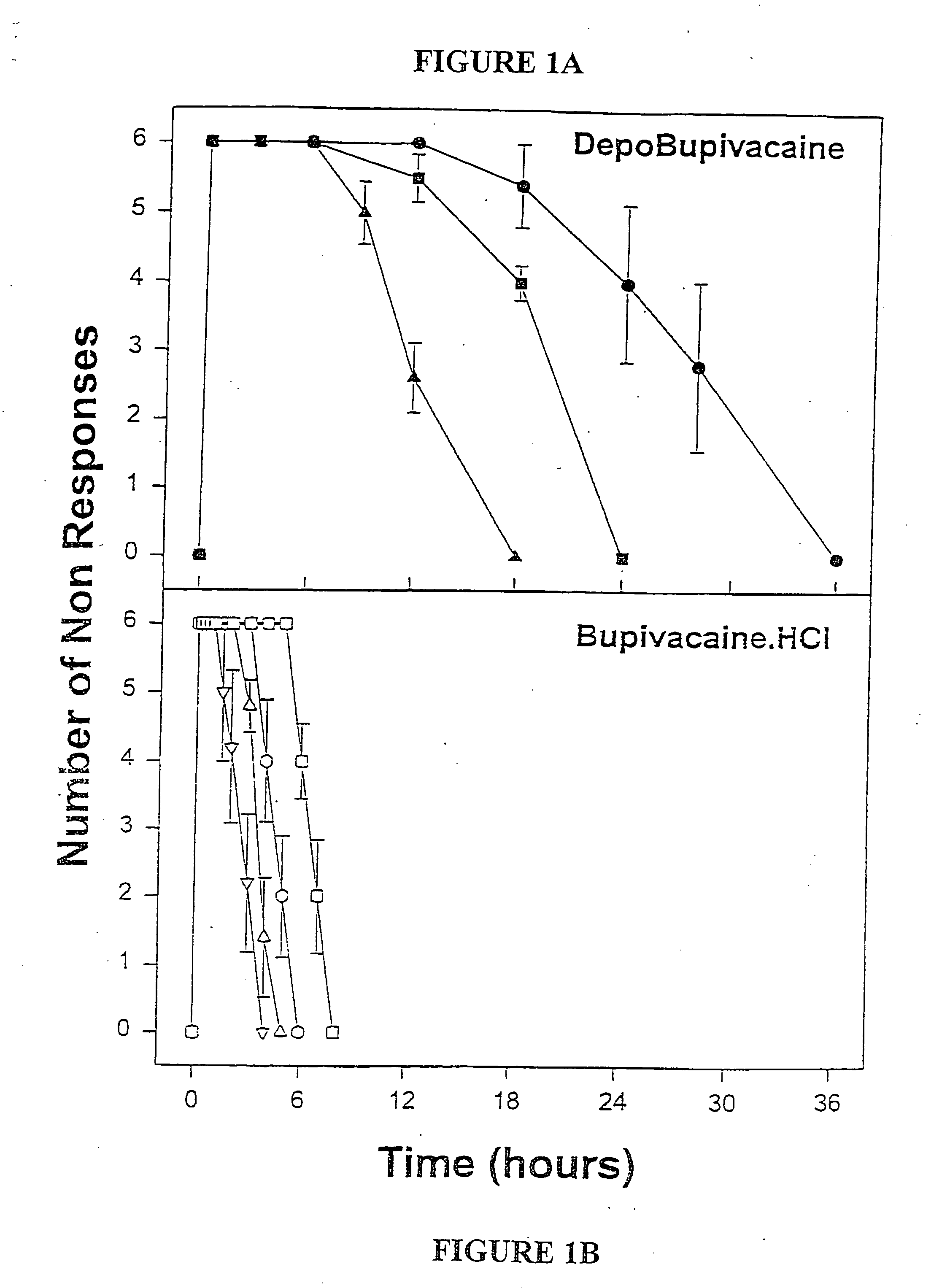
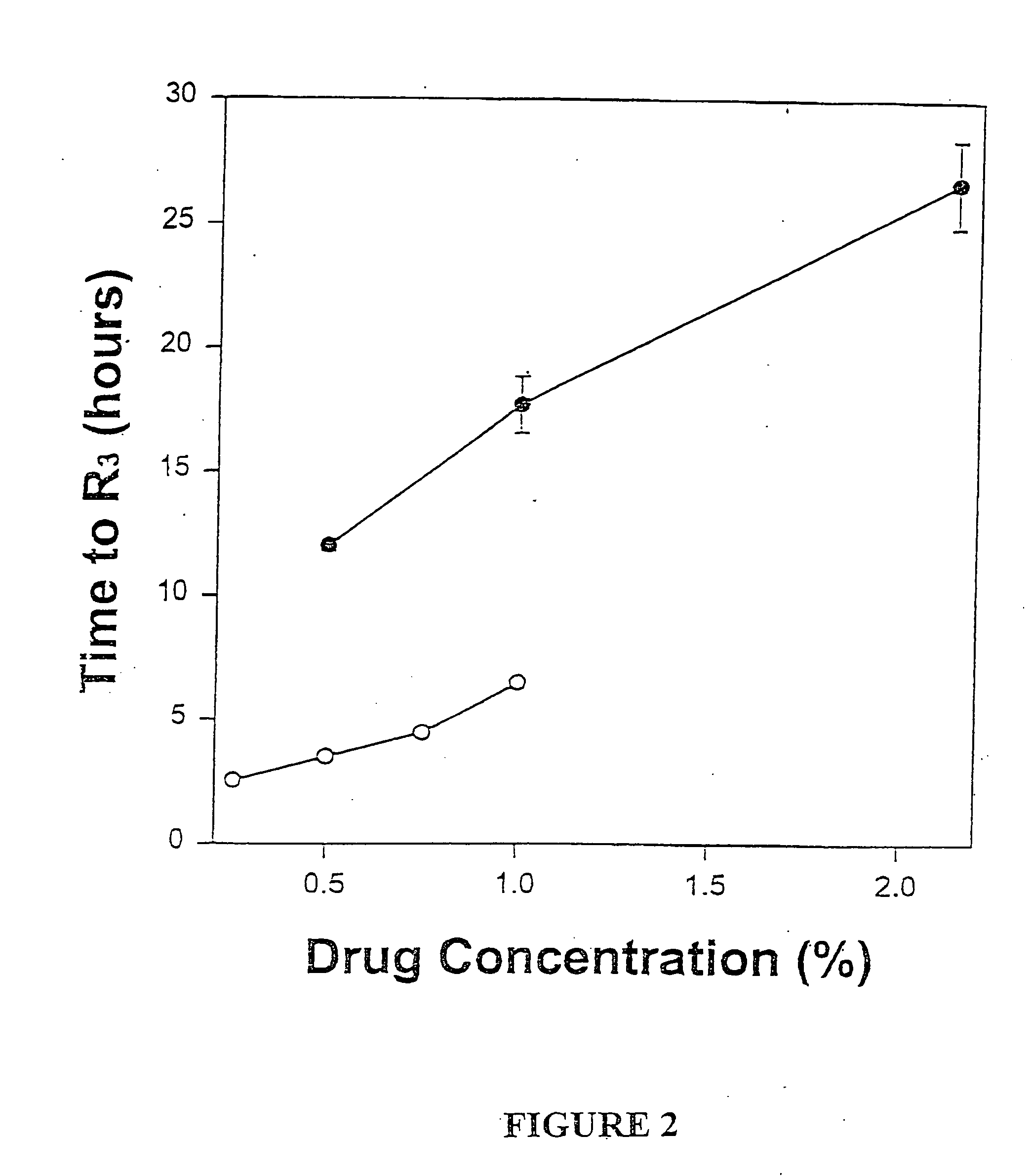
Sustained-release liposomal anesthetic compositions
InactiveUS8182835B2High acceptabilityImprove encapsulationInorganic non-active ingredientsAnaestheticsHalf-lifeMaximum tolerated dose
Owner:PACIRA PHARMA INC
Production of multivesicular liposomes
InactiveUS20070235889A1Accurately and quickly scale up reactionAccurately and quickly up reactionOrganic active ingredientsPeptide/protein ingredientsEmulsionCross-flow filtration
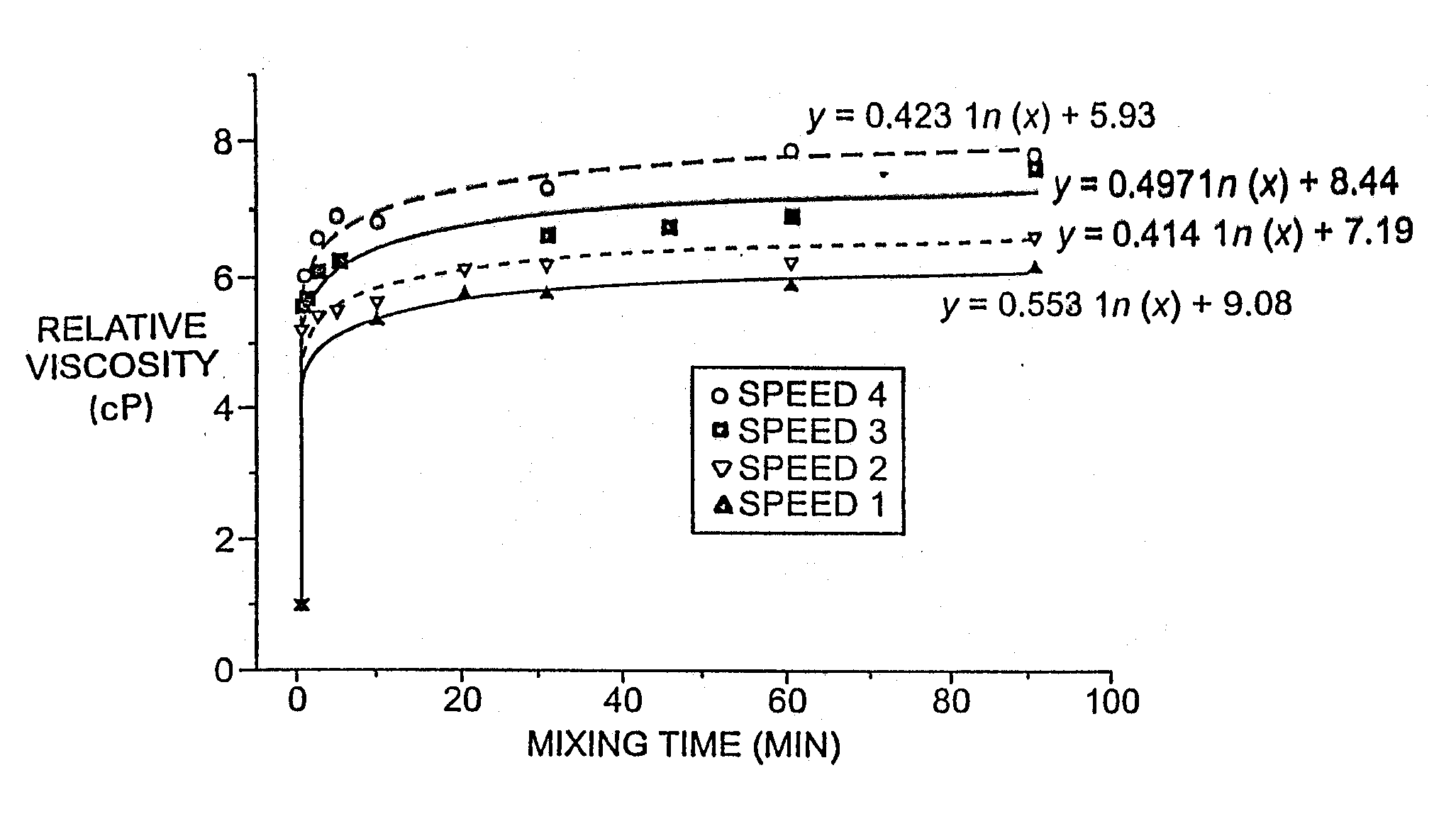
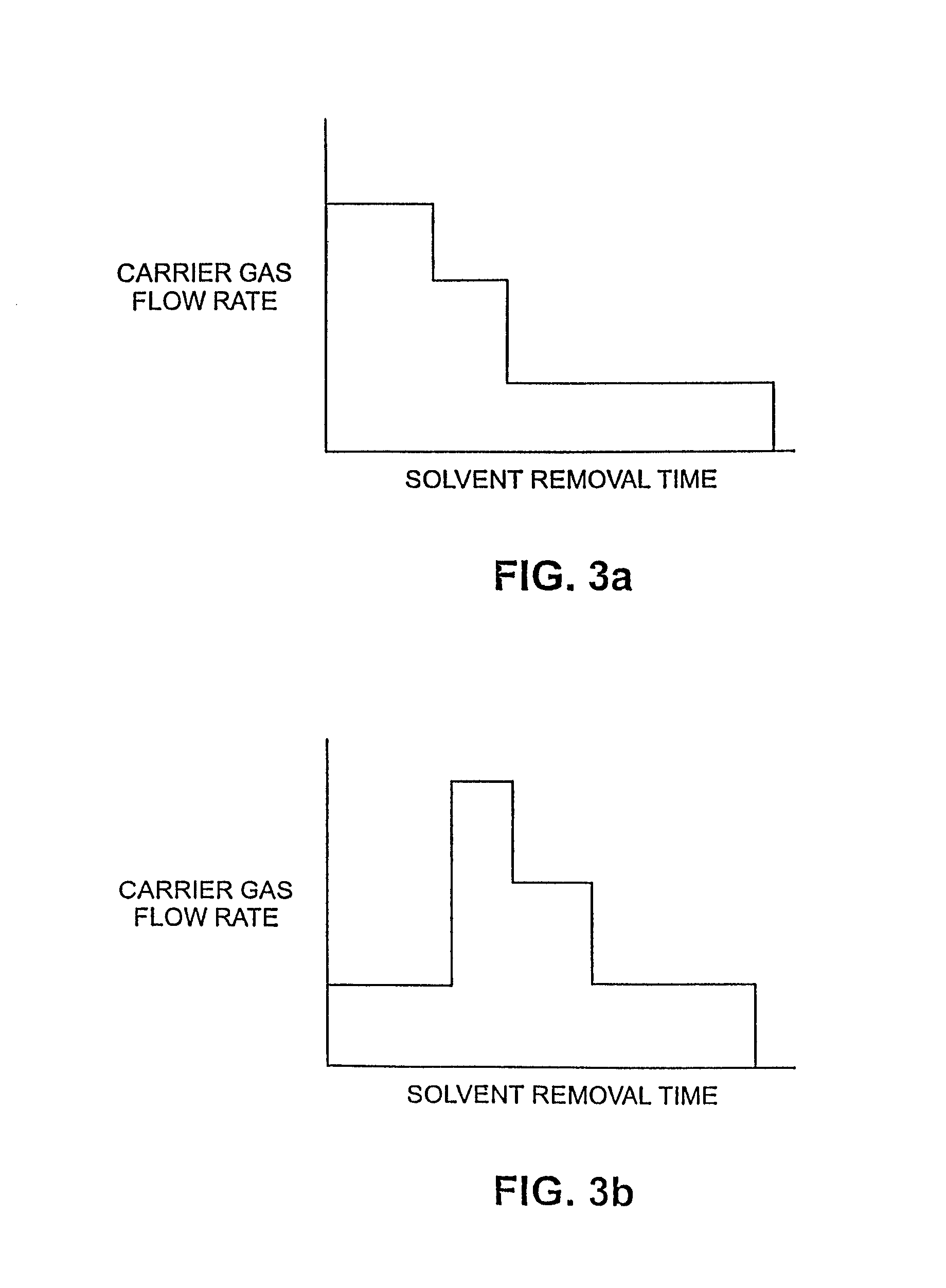
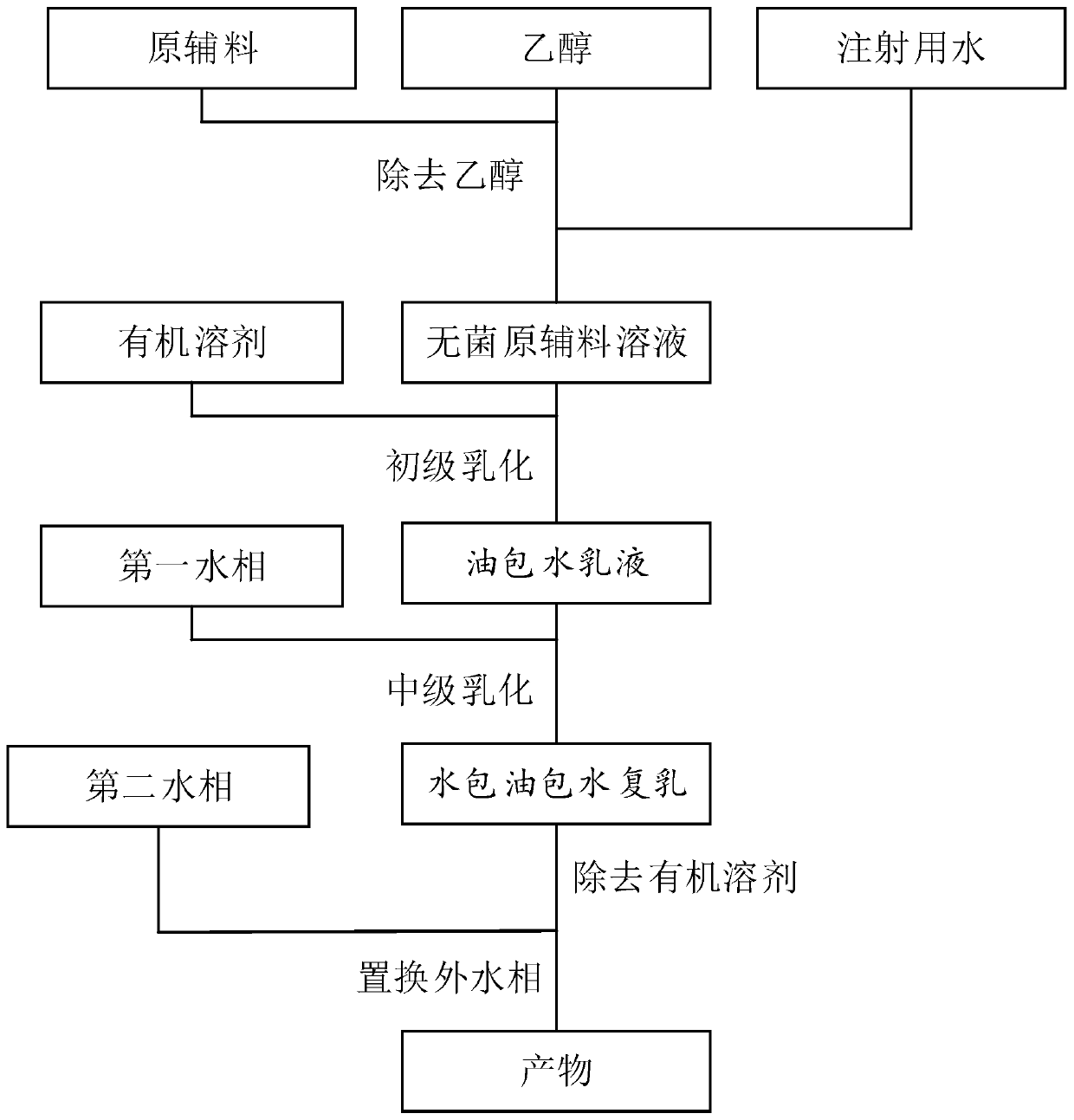
Multivesicular liposomes are prepared at commercial scales by combining a first w / o emulsion with a second aqueous solution to form a w / o / w emulsion using a static mixer. Solvent is removed from the resulting emulsion to form multivesicular liposome-containing compositions. Further optional process steps include primary filtration and secondary cross-flow filtration. The products produced according to the processes of the invention can be produced through a series of aseptic steps.
Owner:PACIRA PHARMA INC
Production of multivesicular liposomes
InactiveUS9585838B2Accurately and quickly up reactionOrganic active ingredientsPeptide/protein ingredientsEmulsionCross-flow filtration
Multivesicular liposomes are prepared at commercial scales by combining a first w / o emulsion with a second aqueous solution to form a w / o / w emulsion using a static mixer. Solvent is removed from the resulting emulsion to form multivesicular liposome-containing compositions. Further optional process steps include primary filtration and secondary cross-flow filtration. The products produced according to the processes of the invention can be produced through a series of aseptic steps.
Owner:PACIRA PHARMA INC
A kind of preparation method and application of multivesicular liposome
ActiveCN102274183AHigh encapsulation efficiencyGood sustained release effectOrganic active ingredientsSaccharide peptide ingredientsHydroxyethyl starchArginine
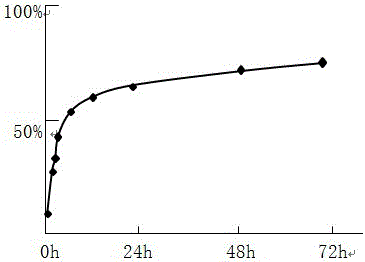
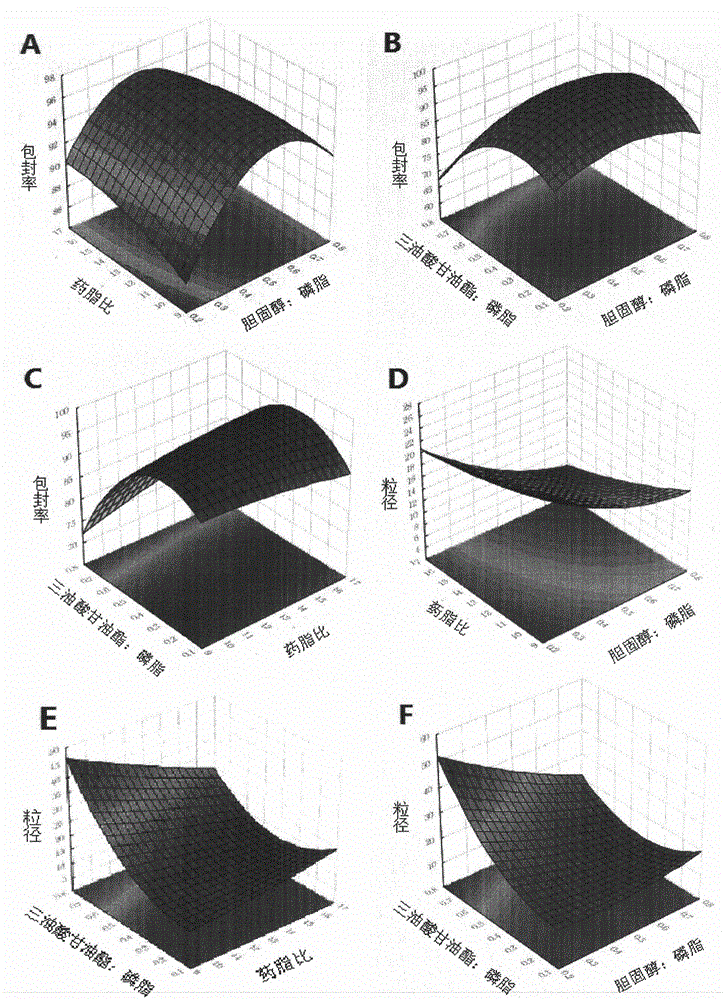
The invention relates to a multi-vesicular liposome, a blank multi-vesicular liposome and a preparation method and application thereof. The multi-vesicular liposome contains the following components in part by weight: 1 part of liposome, 0.01 to 20 parts of auxiliary emulsifier, 1 to 50 parts of osmotic pressure regulator and medicinal active ingredients; the medicament-to-lipid ratio of the multi-vesicular liposome is 1:(0.1-1):200; the lipid contains of a specific amount of neutral phospholipid, cholesterol and triglyceride; and the auxiliary emulsifier is selected from dextran, polyvinyl pyrrolidone, hydroxyethyl starch, gelatin, albumin, arginine and hydroxymethyl starch. The blank multi-vesicular liposome contains the following components in part by weight: 1 part of lipid, 0.01 to 20 parts of auxiliary emulsifier, 1 to 50 parts of osmotic pressure regulator and 0.1 to 50 parts of ion gradient regulator; the osmotic pressure of the in vivo water phase of the multi-vesicular liposome is equal to the osmotic pressure of human plasma; and the auxiliary emulsifier is as previously mentioned. The multi-vesicular liposome has high entrapment rate, and can achieve good sustained-release effect on in vivo and in vitro experiments.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Sustained-release liposomal anesthetic compositions
InactiveUS20060078606A1High acceptabilityImprove encapsulationInorganic non-active ingredientsRotary piston pumpsHalf-lifeMaximum tolerated dose
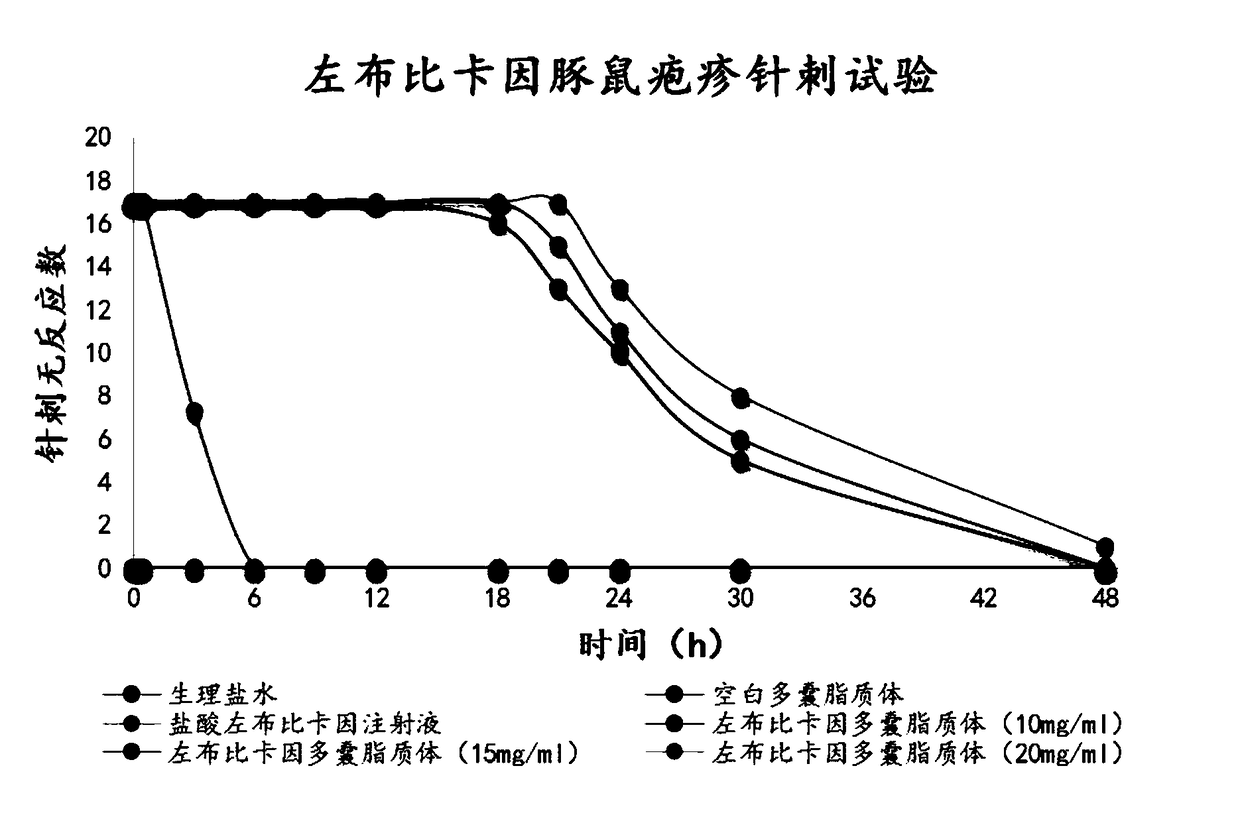
The invention provides a method for obtaining local anesthetics encapsulated in liposomes, such as multivesicular liposomes, with high encapsulation efficiency and slow release in vivo. When the encapsulated anesthetic is administered as a single intracutaneous dose, the duration of anesthesia and half-life of the drug at the local injection site is increased as compared to injection of unencapsulated anesthetic. The maximum tolerated dose of the encapsulated anesthetic is also markedly increased in the liposomal formulation over injection of unencapsulated anesthetic. These results show that the liposomal formulation of local anesthetic is useful for sustained local infiltration and nerve block anesthesia.
Owner:PACIRA PHARMA INC
Clonidine hydrochloride multivesicular liposome and preparation method thereof
InactiveCN101536981AHigh encapsulation efficiencyGood sustained release effectOrganic active ingredientsAntipyreticLipid formationCholesterol
The invention discloses clonidine hydrochloride multivesicular liposome and a preparation method thereof. The clonidine hydrochloride multivesicular liposome comprises the following components by weight portion: 1 portion of active ingredient (clonidine hydrochloride), 1 to 10 portions of lipid component, 0.1 to 3.5 portions of acid regulator, 10 to 50 portions of osmotic pressure regulator, and 1.0 to 6.0 portions of lysine, wherein the lipid component comprises neutral phospholipid and cholesterol in a mol ratio of 1: 0.5-1: 2, and neutral lipid which accounts for 1 to 2 mol percent of the weight of the lipid component. The clonidine hydrochloride multivesicular liposome has high encapsulation rate and stability, and is shown in vivo and in vitro experiments to have a good slow release effect.
Owner:SHANGHAI INST OF PHARMA IND
Sustained release formulation of a non-steroidal Anti-inflammatory drug
Disclosed are formulations comprising multivesicular liposomes and one or more non-steroidal anti-inflammatory drugs which minimize the side effects of unencapsulated non-steroidal anti-inflammatory drugs while maintaining or improving efficacy. Methods of making and administering the formulations comprising multivesicular liposomes and one or more non-steroidal anti-inflammatory drugs and their use as medicaments are also provided.
Owner:PACIRA PHARMA INC
Local anesthesia pain relieving and slow-release medicine delivery system as well as preparation method and application thereof
InactiveCN108354903AHigh encapsulation efficiencyHigh drug loadingAnaestheticsPharmaceutical non-active ingredientsMultivesicular liposomesOil phase
The invention discloses a novel local anesthesia pain relieving and slow-release medicine delivery system, which comprises an inner water phase, an outer water phase, an oil phase, an organic solvent,an isotonic regulator and a pH regulator, wherein the inner phase comprises a pain relieving agent, a medicine solvent and a medicine solubilizing agent; the pain relieving agent is selected from onekind of materials from bupivacaine, levobupivacaine, ropivacaine, lidocaine, bupivacaine or mepivacaine; the pain relieving agent is in a free alkali form or acid salt form; the medicine solvent is selected from N or P containing inorganic acid; the medicine solubilizing agent is selected from one or several kinds of materials from sugar or annular organic acid. The prepared muhivescular liposomes have high encapsulation rate, high medicine carrying capacity, uniform particle diameter and good slow release effect.
Owner:XIAN LIBANG BIOMEDICAL TECH CO LTD
Method for formulating large diameter synthetic membrane vesicles
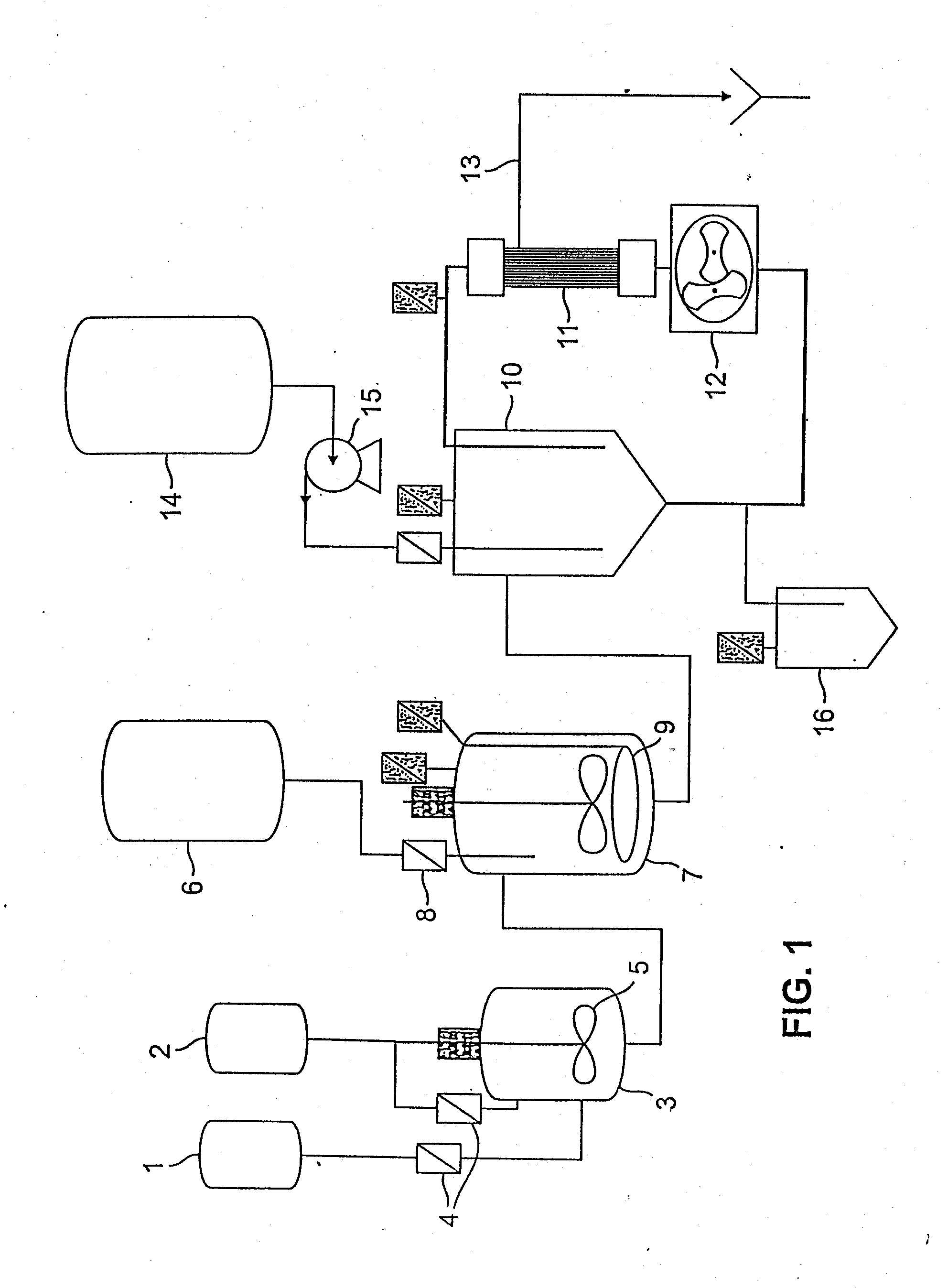
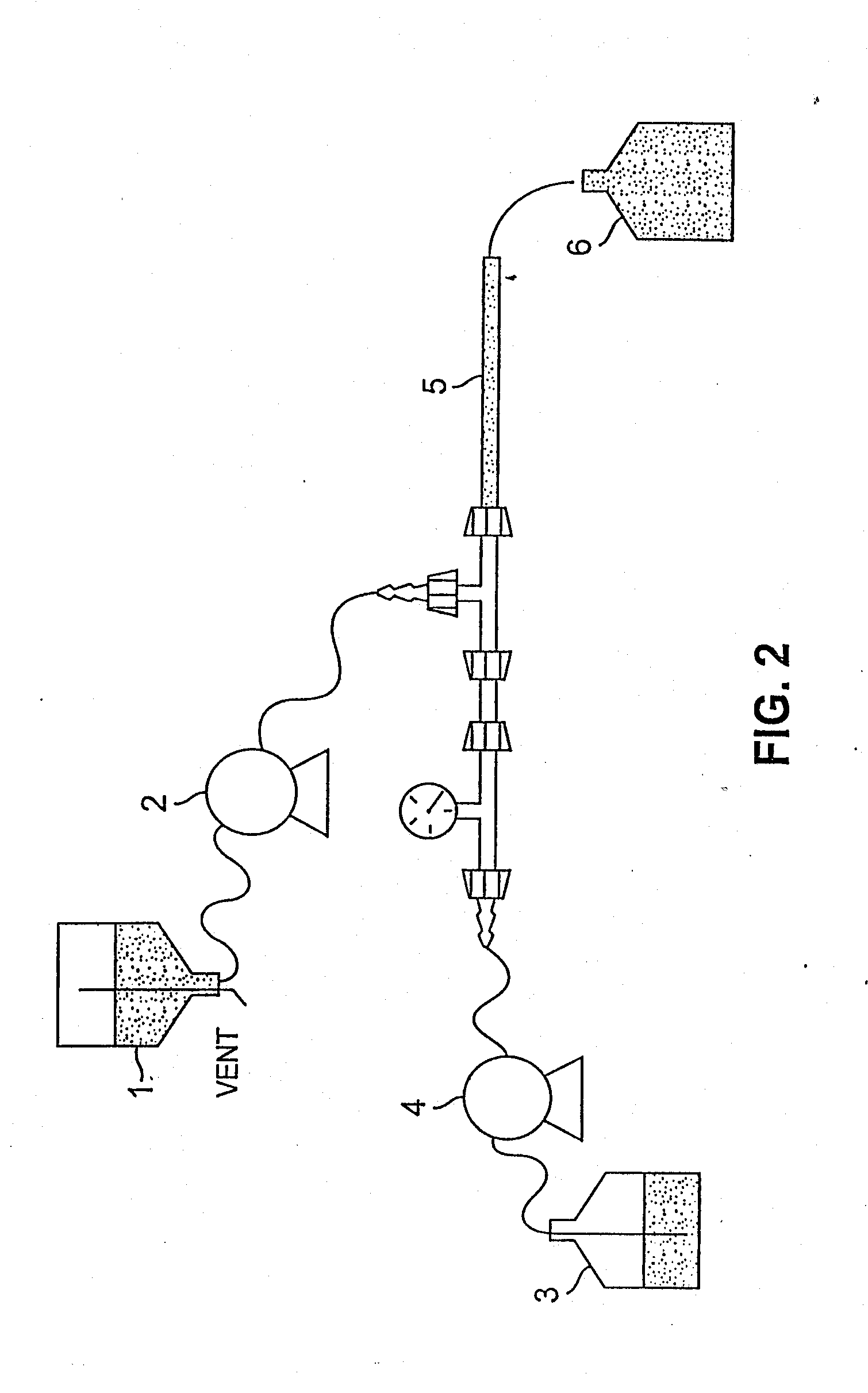
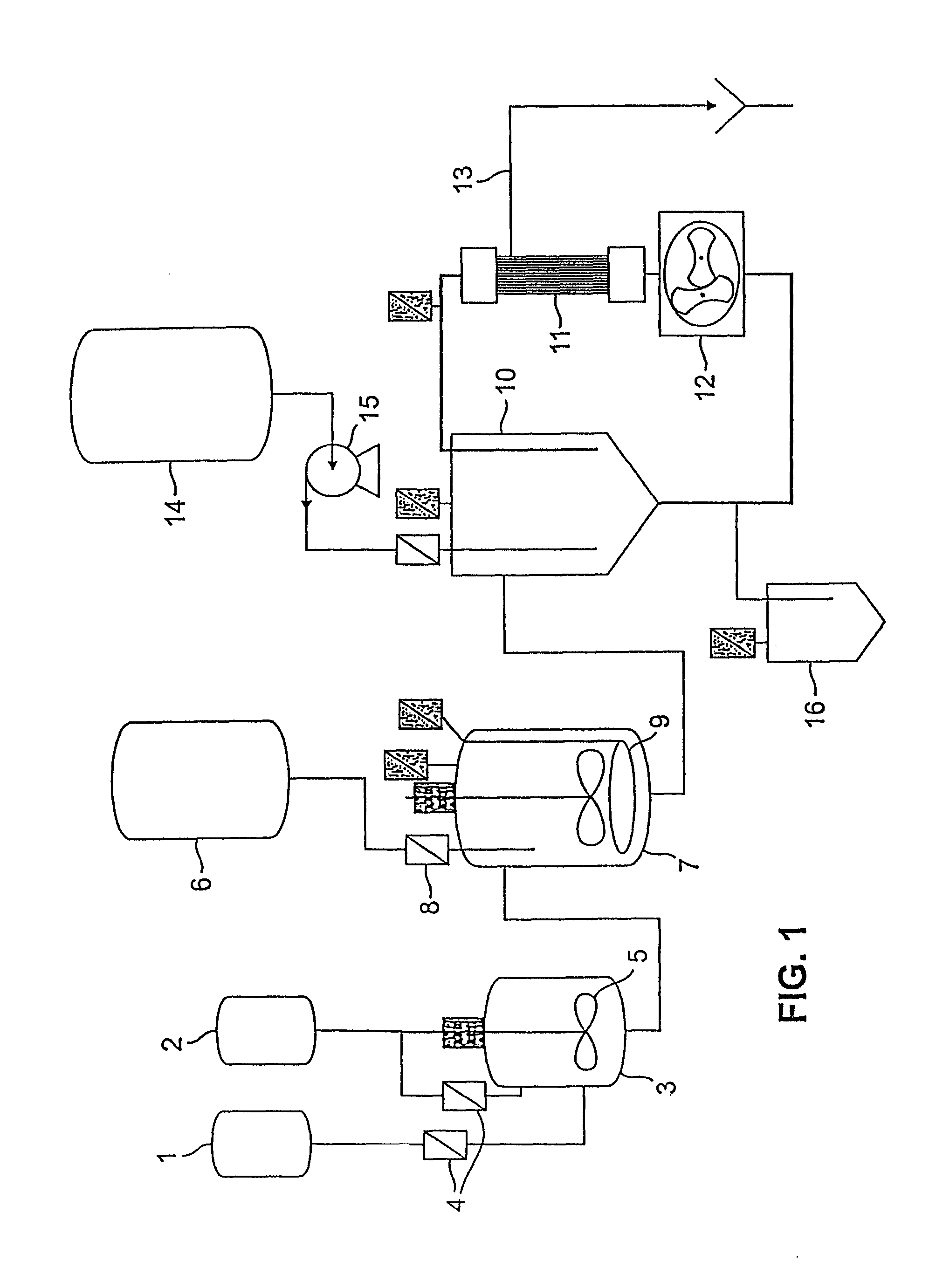
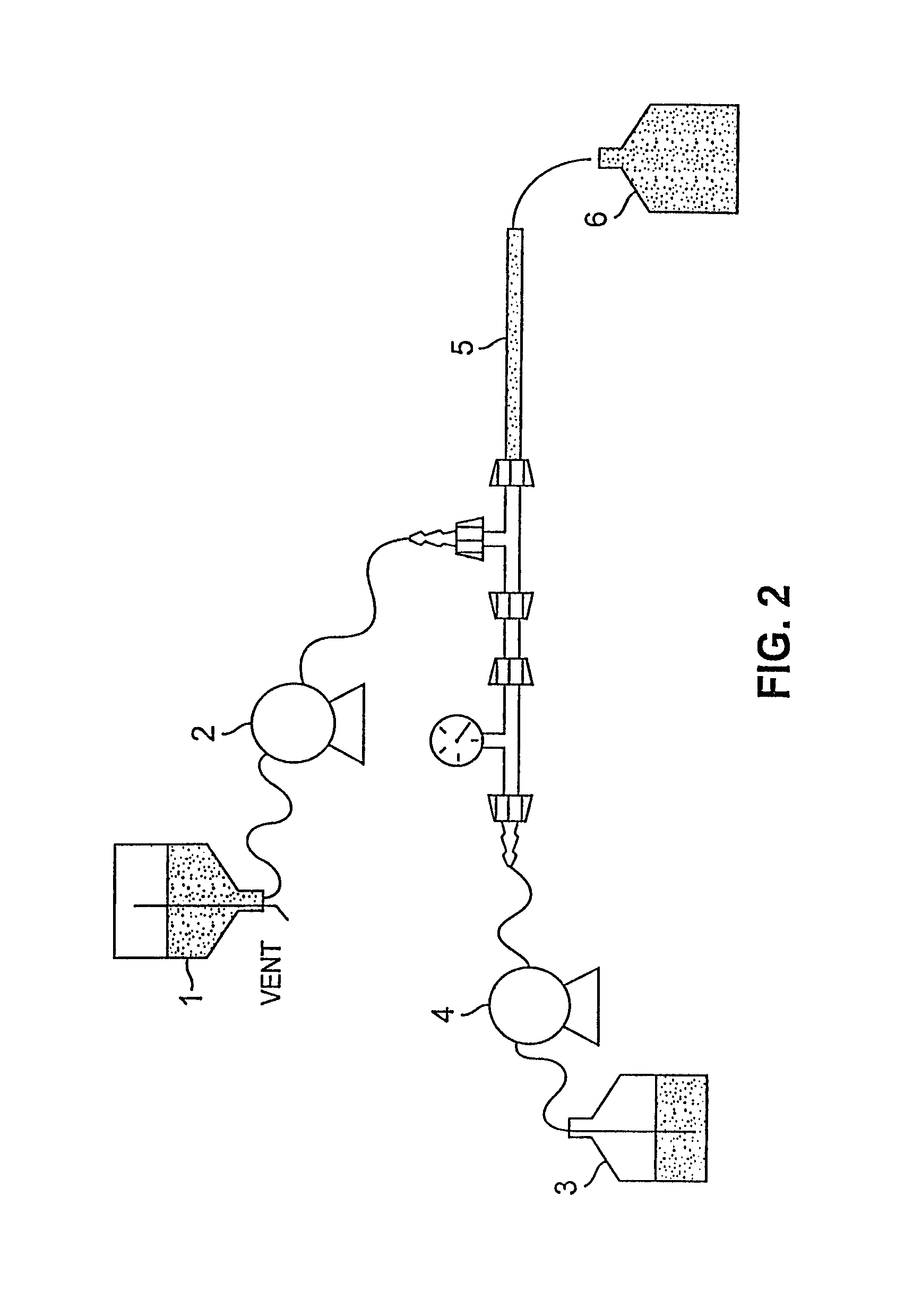
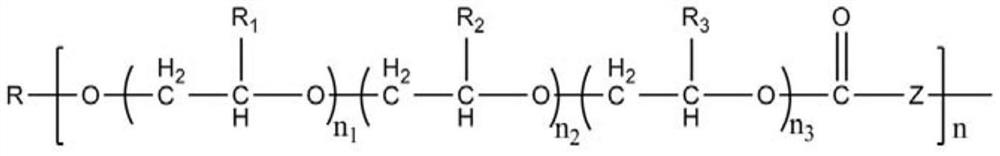
The present invention generally relates to the field of pharmaceutical sciences. More specifically, the present invention includes apparatus and devices for the preparation of pharmaceutical formulations containing large diameter synthetic membrane vesicles, such as multivesicular liposomes, methods for preparing such formulations, and the use of specific formulations for therapeutic treatment of subjects in need thereof. Formation and use of the pharmaceutical formulations containing large diameter synthetic membrane vesicles produced by using the apparatus and devices for therapeutic treatment of subjects in need thereof is also contemplated.
Owner:PACIRA PHARMA INC
Breviscapine internal phase gelating polycystic lipidosome freeze-dried powder and preparation method thereof
InactiveCN104800171AReduce releaseImprove stabilityPowder deliveryOrganic active ingredientsSucroseFreeze-drying
The invention aims to provide breviscapine internal phase gelating polycystic lipidosome freeze-dried powder which is stable in internal and external properties, high in packaging rate and low in medicine release, and a preparation method thereof. The breviscapine internal phase gelating polycystic lipidosome disclosed by the invention comprises the following raw and auxiliary materials in parts by weight: 1 part of breviscapine, 0.95-1.18 parts of neutral phospholipid, 0.025-0.25 part of negative-charge phospholipid, 0.25-1 part of cholesterol, 0.15-0.25 part of triglyceride, 0.125-1 part of cane sugar, 0.163-0.263 part of an internal water phase assisting emulsifying agent, 9.5-10 parts of poloxamer 407, 0.25-1.75 parts of poloxamer 188, 0.5-1.25 parts of glucose, 0.1-0.15 part of an external water phase assisting emulsifying agent and 6-14 parts of a supporting agent.
Owner:LINYI UNIVERSITY
Method for preparing nicotinamide-coated multivesicular liposomes
InactiveCN106619149AImprove stabilityLess irritatingCosmetic preparationsToilet preparationsOrganic solventEmulsion
The invention relates to a method for preparing nicotinamide-coated multivesicular liposomes. The nicotinamide-coated multivesicular liposomes are prepared from phospholipid, neutral lipid, cholesterol, an organic solvent, a hydrophilic emulsifier, nicotinamide, amino acid, an osmotic pressure regulator and deionized water. The method comprises the steps: dissolving the phospholipid, the cholesterol and the neutral lipid in the organic solvent, so as to obtain an organic phase; uniformly mixing the nicotinamide, the amino acid, the osmotic pressure regulator and the deionized water, so as to obtain an internal water phase; homogenizing the organic phase component, and adding the internal water phase component into the organic phase component, so as to prepare a W / O primary emulsion; mixing the hydrophilic emulsifier, the amino acid, the osmotic pressure regulator and the deionized water, and carrying out dispersing, so as to obtain an external water phase; homogenizing the external water phase component, adding the W / O primary emulsion component into the external water phase component, carrying out homogenizing, then, carrying out ultrasonic treatment, and then, cooling the mixture to room temperature with stirring, so as to form a W / O / W multiple emulsion; dispersing the multiple emulsion into an amino acid solution, introducing nitrogen gas into the solution to remove the organic solvent, thereby obtaining a nicotinamide-coated multivesicular liposome suspension. According to the method, the stability of the nicotinamide is improved.
Owner:SHANGHAI INST OF TECH
Preparation method of squalene and astaxanthin composite multivesicular liposome and radical lowering and harm reducing method thereof
ActiveCN103859588AEasy to manufactureHigh encapsulation efficiencyTobacco smoke filtersFiberCellulose acetate
The invention discloses a preparation method of a squalene and astaxanthin composite multivesicular liposome and a radical lowering and harm reducing method thereof. The multivesicular liposome is prepared from 12-18 parts of hydrogenated phospholipid, 10-20 parts of stabilizer, 10-15 parts of surfactant and the balance of squalene and astaxanthin composite. Through microencapsulating the squalene and astaxanthin composite, the defect that the squalene and astaxanthin composite is easily oxidized in the air and loses effectiveness is overcome, and the effect of increasing the stability of a squalene and astaxanthin blending synergy composite is achieved. The multivesicular liposome is built in a cigarette filter tip made of cellulose acetate fiber in the forming process of a cigarette filter tip bar, and 0.5-1.5g of multivesicular liposomes is arranged in each cigarette filter tip, so that a cigarette filter tip capable of lowering the harm of cigarettes is obtained; the surface area of the multivesicular liposome is large, so that anti-oxidant makes full contact with main stream smoke, gas-phase free radical in the main stream smoke can be effectively removed, and powerful technical support is provided for solving the smoking and health problem.
Owner:CHINA TOBACCO GUANGXI IND +2
Preparation method of vesicle-loaded multivescular liposome
ActiveCN106924185AAddress common cases of sudden releaseMaintain biological activityOrganic active ingredientsSenses disorderLipid formationExocytic vesicle
The invention relates to a preparation method of a vesicle-loaded multivescular liposome. The preparation method comprises the steps of adopting nanoscale vesica and an osmotic pressure regulator as an inner water phase and an organic solvent of a lipid with good biocompatibility as an oil phase, dispersing the inner water phase into the oil phase to form a W / O primary emulsion; dispersing the primary emulsion into an outer water phase containing the osmotic pressure regulator and an auxiliary emulsifier to form a W / O / W compound emulsion; and transferring the compound emulsion to the same outer water phase and removing an organic solvent through rotary evaporation or nitrogen introduction to obtain a multivescular liposome suspension. According to a multivescular liposome preparation, the encapsulation efficiency of a loaded drug can be ensured, the biological activity of biomacromolecules can be improved, the immunogenicity is reduced, the burst effect is reduced and sustained slow release of the drug is ensured.
Owner:YANTAI UNIV
Selexipag polycystic lipidosome and preparation method thereof
ActiveCN107811970AReduce leakageExtension of timeOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolIn vitro test
The invention belongs to the field of pharmaceutic preparations and particularly relates to a selexipag polycystic lipidosome and a preparation method thereof. The selexipag polycystic lipidosome comprises selexipag, lipids, a surfactant, an osmotic pressure regulator and an auxiliary emulsifier, wherein the lipids comprise amphiphilic lipids, neutral lipids and cholesterol. The selexipag polycystic lipidosome has relatively high encapsulation efficiency and stability, and shows a good slow release action in an in vitro test.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Clonidine multivesicular liposome and preparation method thereof
InactiveCN101756902AReduce releaseReduce wasteOrganic active ingredientsNervous disorderLipid formationMultivesicular liposomes
The invention discloses a clonidine multivesicular liposome and a preparation method thereof. The clonidine multivesicular liposome comprises clonidine serving as a medicinal active ingredient and an empty multivesicular liposome serving as a carrier. The empty multivesicular liposome comprises the following components in part by mass: 1 part of lipid component and 0.1 to 50 parts of ion gradient regulator, wherein the lipid component comprises amphiphilic lipids and neutral lipids in a molar ratio of 5-36:1. The clonidine multivesicular liposome is obtained by adopting the empty multivesicular liposome enveloped with the gradient regulator and using a transmembrane gradient active loading method to enable the clonidine to enter the aqueous phase of the empty multivesicular liposome through a phospholipid membrane. The clonidine multivesicular liposome has high utilization rate of raw materials and high loading concentation, shows good slow release effect in in vivo and in vitro experiments, and has better analgesic effect.
Owner:SHANGHAI INST OF PHARMA IND
Berberine sulfate or hydrochloride multi-vesicular liposome and preparation method thereof
ActiveCN106361702AEfficient storageEffective encapsulationOrganic active ingredientsSenses disorderSucroseCholesterol
The invention discloses a berberine sulfate or hydrochloride multi-vesicular liposome and a preparation method thereof. The preparation method comprises the following steps: dissolving lecithin, cholesterol, oleic acid and triolein into a mixed solvent to form an oil phase; dissolving berberine sulfate or hydrochloride into a cane sugar solution to form an inner water phase; preparing primary emulsion through the inner water phase and the oil phase; adding the primary emulsion into a specific mixed solution so as to obtain compounded emulsion; and finally performing preparation, thereby obtaining the berberine sulfate or hydrochloride multi-vesicular liposome which is high in berberine sulfate or hydrochloride encapsulation efficiency, large in drug loading capacity, remarkable in slow-release effect, stable in drug release concentration, high in vesicle content and uniform in appearance.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Oleanolic acid polycystic lipidosome and preparation method and application thereof
InactiveCN104983684AImprove complianceReduce releaseOrganic active ingredientsAntineoplastic agentsOrganic solventCholesterol
The invention provides oleanolic acid polycystic lipidosome and a preparation method and application thereof. The method comprises the following steps: 1 weighing granulesten, cholesterol, olein, oleanolic acid and stearic acid by mass, and dissolving the ingredients into organic solvent, wherein oil phase is formed through the dissolution; 2 taking and adding a specified amount of oil phase into internal water phase, and using an emulsifying machine for conducting shearing and dispersing for forming primary emulsion; 3 taking and adding a specified amount of primary emulsion into external water phase, and conducting vortex oscillation for forming compound emulsion; 4 transferring the compound emulsion rapidly into a round-bottom flask, conducting rotary evaporation for removing the organic solvent, and obtaining the oleanolic acid polycystic lipidosome. According to the oleanolic acid polycystic lipidosome and the preparation method and application thereof, the oleanolic acid is loaded into a lipoid layer of polycystic lipidosome for preparing the oleanolic acid polycystic lipidosome (OA-MVLs), and it is expected to improve the bioavailability and achieve the purposes of releasing medicine slowly, reducing administration times and improving the adaptability of a patient.
Owner:钟志容
High-concentration bupivacaine multivesicular liposome, and preparation method and liquid distribution system thereof
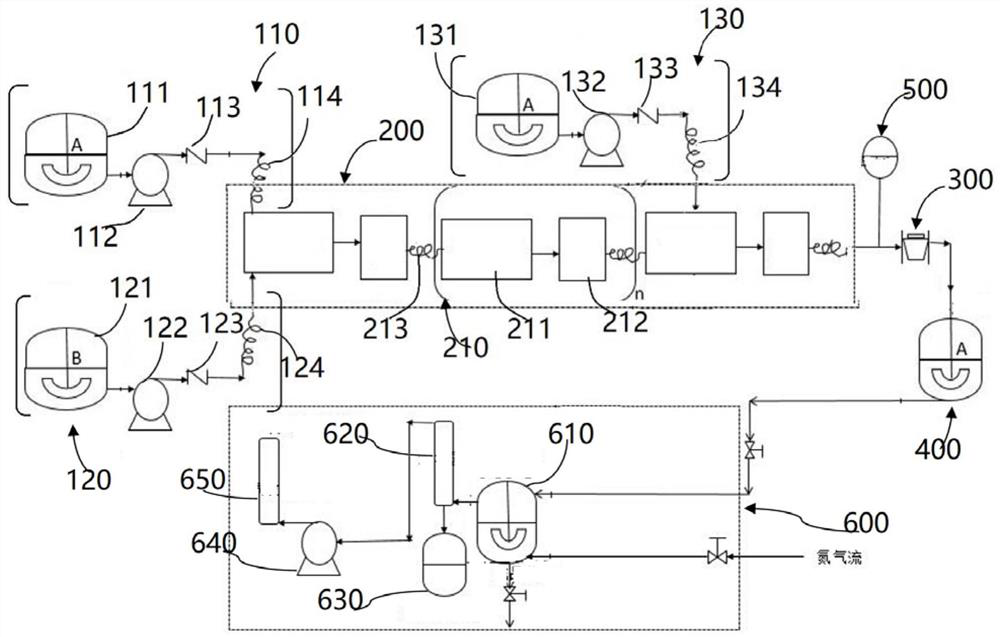
PendingCN110179752AReduce in quantityReduce control difficultyTransportation and packagingMixer accessoriesHigh concentrationNitrogen generator
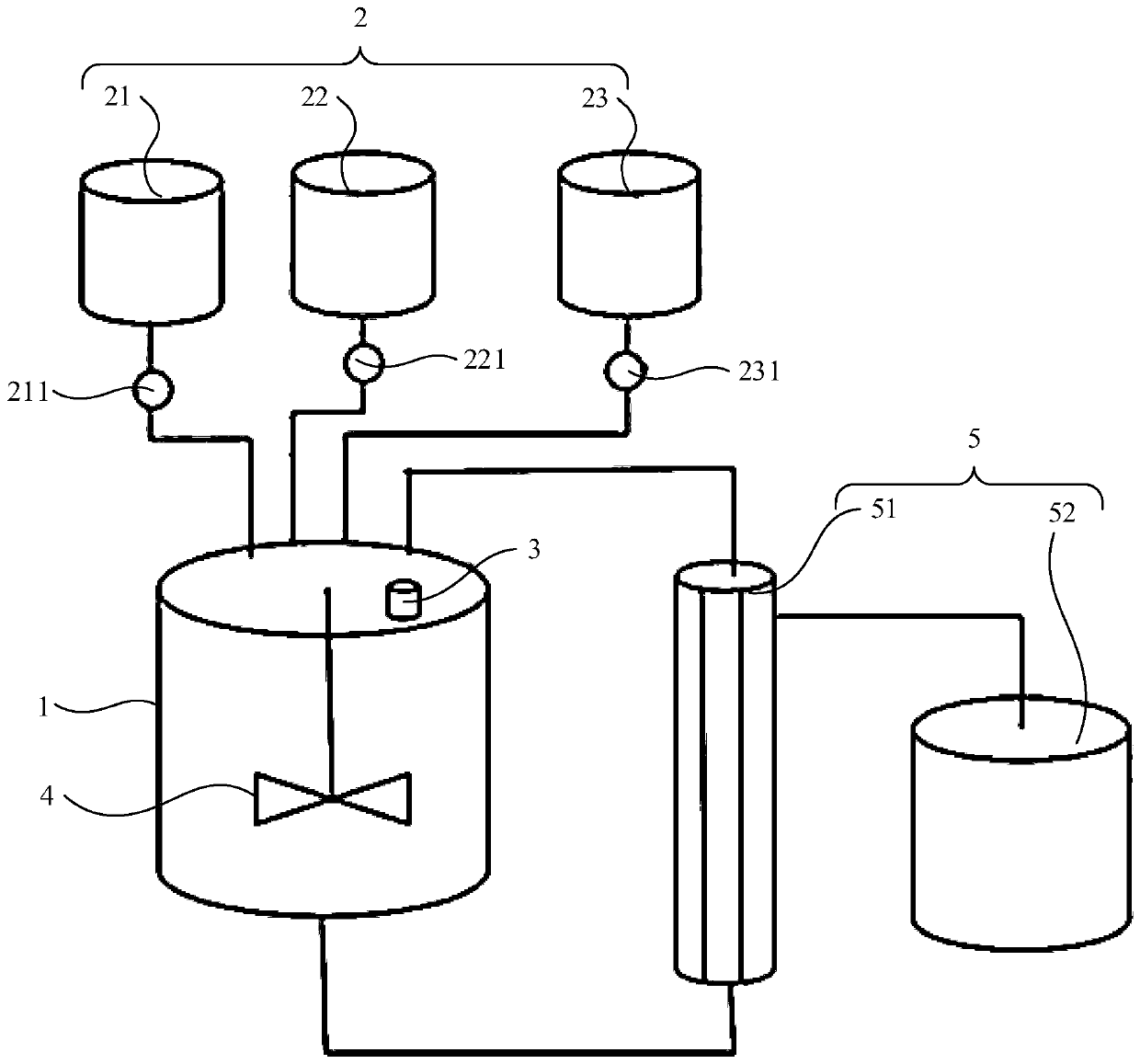
The invention belongs to the technical field of preparations, and specifically relates to a high-concentration bupivacaine multivesicular liposome as well as a preparation method and a liquid distribution system thereof. The liquid distribution system comprises a main liquid distribution tank, a plurality of auxiliary liquid distribution tanks which are arranged in parallel on the main liquid distribution tank, and a heating apparatus and an air exhaust apparatus which are respectively located on the main liquid distribution tank, wherein the heating apparatus is suitable for heating test liquid in the main liquid distribution tank and the air exhaust apparatus is suitable for maintaining vacuum degree in the main liquid distribution tank, thereby allowing extraction of volatile organic solvents from the test liquid; and thus, nitrogen generator and gas sterile filtration are not required while sterility control of gas is not needed, so that, difficulty of control of aseptic process isreduced with aseptic environment in liquid distribution process improved. Therefore, the liquid distribution system is suitable for industrial production.
Owner:常州吾合生物医药有限责任公司
Microencapsulated multivesicular liposome drug carrier and preparation method thereof
ActiveCN104622847AReduced release rateAchieving co-releasePharmaceutical non-active ingredientsMicrocapsulesSide effectCationic polymerization
The invention provides a microencapsulated multivesicular liposome drug carrier and a preparation method thereof. The method comprises the following steps: with a sucrose solution as an inner water phase, glucose and an L-lysine solution as outer water phases, and a dichloromethane solution dissolved with lipid as an oil phase, carrying out high-speed homogenate emulsification on the inner water phase and the oil phase to form colostrums; adding the outer water phase, carrying out vortex emulsification to form a compound emulsion, carrying out rotary evaporation to remove dichloromethane, so as to obtain a multivesicular liposome suspension; dispersing the multivesicular liposome into a sodium alginate solution, dispersing uniformly, and then dropping into a calcium chloride solution through a high-pressure microcapsule molding device and an injection pump, so as to prepare calcium alginate gel beads embedded with the multivesicular liposome; and finally forming a film from the gel beads and a cationic polymer solution, so as to form a sample, wherein the drug is carried on at least one part in the multivesicular liposome or out of the multivesicular liposome in the microcapsule. According to the microencapsulated multivesicular liposome drug carrier, space allocation of different drugs is ensured; the long-acting release and absorption of the drug are improved; and toxic and side effects caused by simple use of the drug are reduced.
Owner:HUAQIAO UNIVERSITY
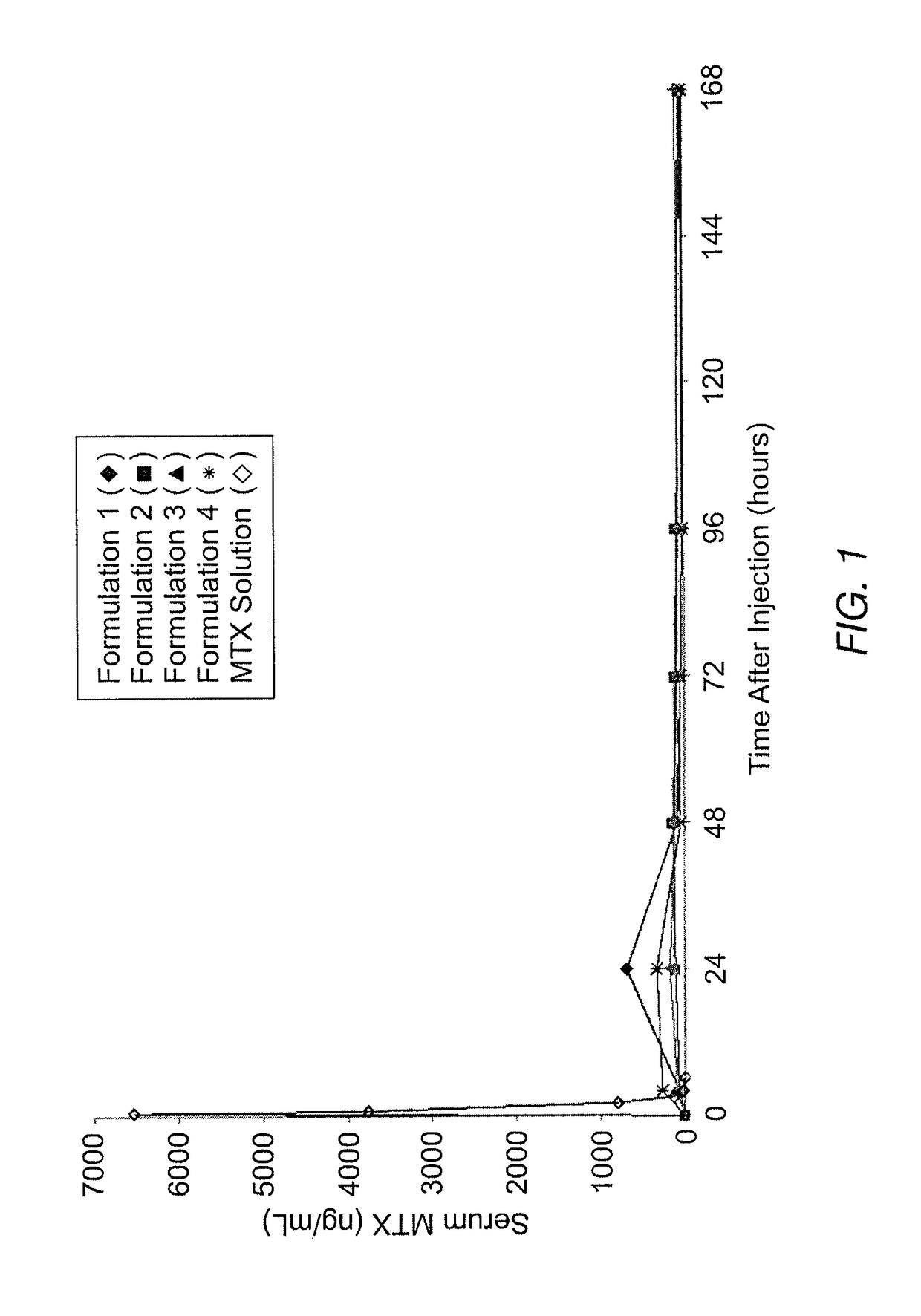
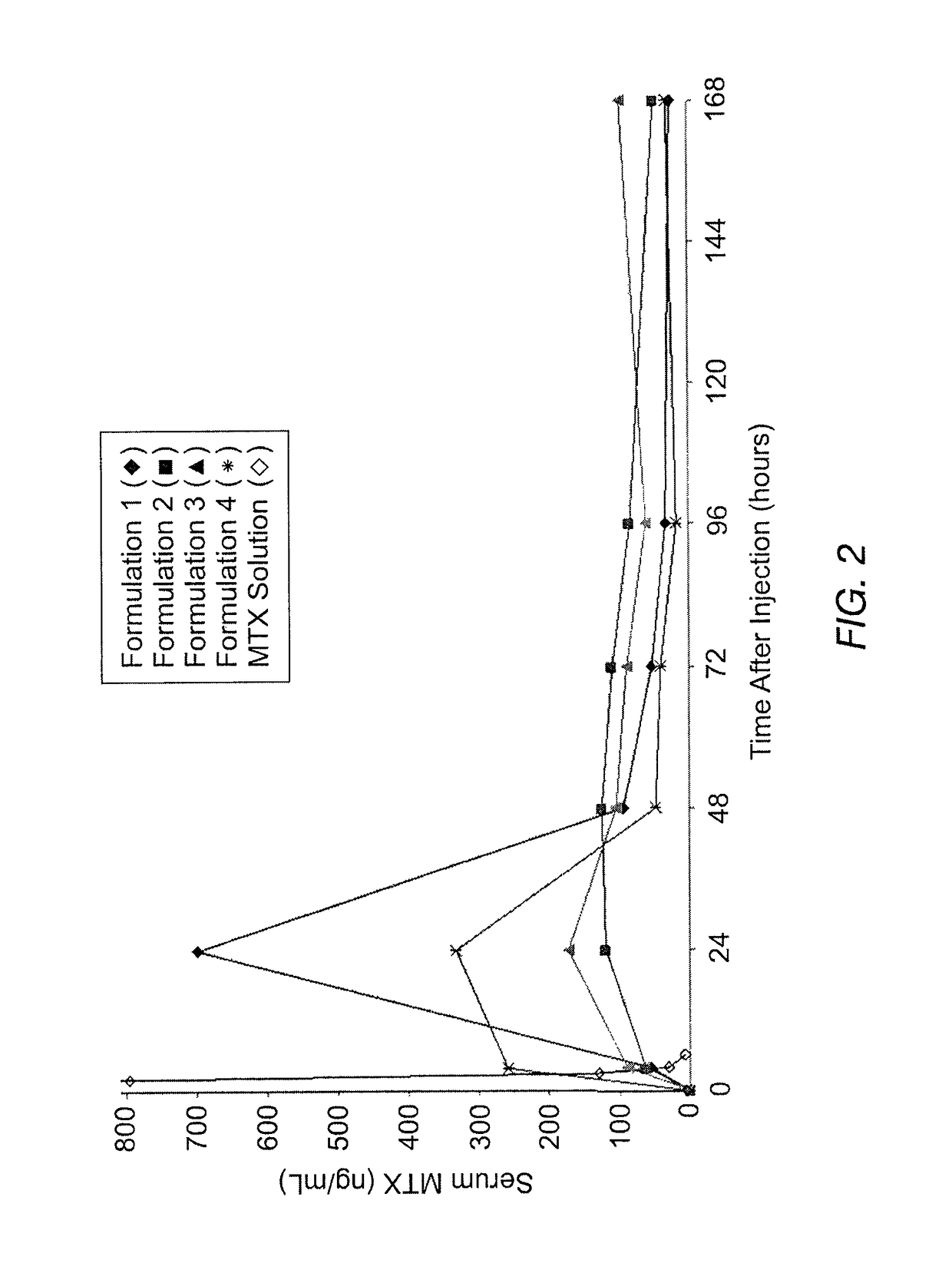
Sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an anti-cancer agent
Disclosed is are formulations comprising a multivesicular liposome and MTX, the administration of which results in a Cmax of MTX between 5% and 50% of the Cmax of an immediate release dosage form of MTX, the duration of which lasts from about 1 to about 30 days. Also disclosed are methods of treating autoimmune diseases and cancer by administering these formulations of MTX.
Owner:PACIRA PHARMA INC
Neostigmine bromide muhivescular liposome and preparation method thereof
ActiveCN103191059AThe sustained release effect in the body is obviousTo achieve the purpose of sustained releaseDigestive systemMuscular disorderMedicineMultivesicular liposomes
The invention belongs to the field of a pharmaceutic preparation, and relates to a neostigmine bromide muhivescular liposome formula and a preparation method thereof. The neostigmine bromide muhivescular liposome prepared by the method can achieve slow release of a medicine, and prolongs the medicine effect time and the effective medicine duration.
Owner:CHONGQING MEDICAL UNIVERSITY
Sustained release formulation of a non-steroidal Anti-inflammatory drug
Disclosed are formulations comprising multivesicular liposomes and one or more non-steroidal anti-inflammatory drugs which minimize the side effects of unencapsulated non-steroidal anti-inflammatory drugs while maintaining or improving efficacy. Methods of making and administering the formulations comprising multivesicular liposomes and one or more non-steroidal anti-inflammatory drugs and their use as medicaments are also provided.
Owner:PACIRA PHARMA INC
A kind of bupivacaine multivesicular liposome preparation device
ActiveCN108158998BAvoid breakingRound shapePharmaceutical product form changePharmaceutical non-active ingredientsMedicineOrganosolv
Owner:GUANGZHOU BOSITAO CONTROLLED RELEASE PHARMA CO LTD
Blank multivesicular liposome as well as preparation method and device thereof
InactiveCN112120022ACompact structureSmall particle sizeBiocideChemical/physical/physico-chemical microreactorsPESTICIDE ADJUVANTSMultivesicular liposomes
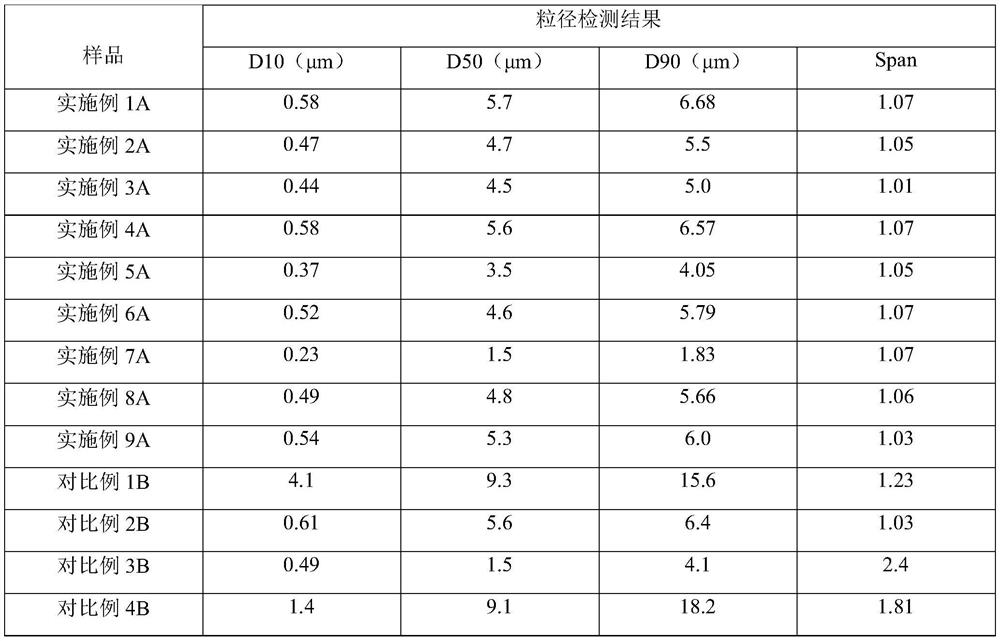
The invention discloses a blank multivesicular liposome and a preparation method and device thereof, and belongs to the field of pesticide adjuvants. The preparation raw materials of the polycystic lipidosome provided by the invention comprise phospholipid substances, sterol substances, neutral lipidosome, fatty acid ester substances and vitamins; the polycystic lipidosome containing vesicles withhigh electronegativity and steric hindrance can be obtained; coagulation of the multivesicular liposome can be effectively inhibited, the drug release time is prolonged, and the drug effect is improved. The invention provides a blank multivesicular liposome preparation method and device. According to the blank multivesicular liposome preparation method and device, a continuous flow micro-channelreactor is used for replacing traditional high-speed shearing equipment; by means of the device, the vesicle stability and particle size uniformity of the multivesicular liposome can be obviously improved, the vesicle size is reduced, the environment-friendly multivesicular liposome can wrap pesticide active matters and is applied to the field of pesticides.
Owner:YANGZHOU SPED CHEM
Preparation method and application of multi-vesicular liposome
ActiveCN102274183BHigh encapsulation efficiencyGood sustained release effectLiposomal deliveryHydroxyethyl starchArginine
The invention relates to a multi-vesicular liposome, a blank multi-vesicular liposome and a preparation method and application thereof. The multi-vesicular liposome contains the following components in part by weight: 1 part of liposome, 0.01 to 20 parts of auxiliary emulsifier, 1 to 50 parts of osmotic pressure regulator and medicinal active ingredients; the medicament-to-lipid ratio of the multi-vesicular liposome is 1:(0.1-1):200; the lipid contains of a specific amount of neutral phospholipid, cholesterol and triglyceride; and the auxiliary emulsifier is selected from dextran, polyvinyl pyrrolidone, hydroxyethyl starch, gelatin, albumin, arginine and hydroxymethyl starch. The blank multi-vesicular liposome contains the following components in part by weight: 1 part of lipid, 0.01 to 20 parts of auxiliary emulsifier, 1 to 50 parts of osmotic pressure regulator and 0.1 to 50 parts of ion gradient regulator; the osmotic pressure of the in vivo water phase of the multi-vesicular liposome is equal to the osmotic pressure of human plasma; and the auxiliary emulsifier is as previously mentioned. The multi-vesicular liposome has high entrapment rate, and can achieve good sustained-release effect on in vivo and in vitro experiments.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
A kind of preparation method of bupivacaine multivesicular liposome and bupivacaine multivesicular liposome preparation
ActiveCN108078929BAvoid breakingRound shapePharmaceutical non-active ingredientsLiposomal deliveryOrganosolvDrugs preparations
The invention discloses a preparation method of bupivacaine multivesicular liposome and a bupivacaine multivesicular liposome preparation, and relates to the field of bupivacaine medicinal preparations. The preparation method of the bupivacaine multivesicular liposome comprises the following steps: adding a neutral fat into a preemulsion formed from an organic solvent-containing oil phase and a bupivacaine-containing first water phase to obtain a first solution for forming a compound emulsion. Compared with the conventional preparation method of the bupivacaine multivesicular liposome, the preparation method provided by the invention has the advantages as follows: through the step of adding the neutral fat into the preemulsion formed from the organic solvent-containing oil phase and the bupivacaine-containing first water phase, the problem of breakage of the multivesicular liposome in the subsequent organic solvent removing step can be overcome, so that the prepared multivesicular liposome is round in shape and basically free of phospholipid fragments, and thus the long-acting slow-release effect of a bupivacaine medicine is improved.
Owner:GUANGZHOU BOSITAO CONTROLLED RELEASE PHARMA CO LTD
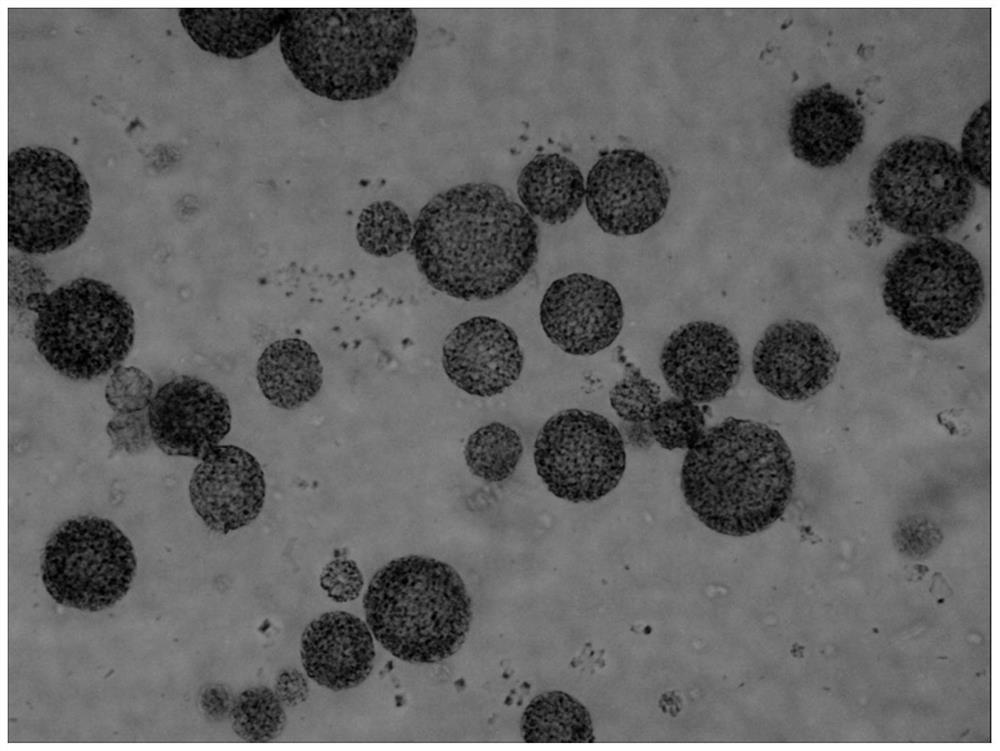
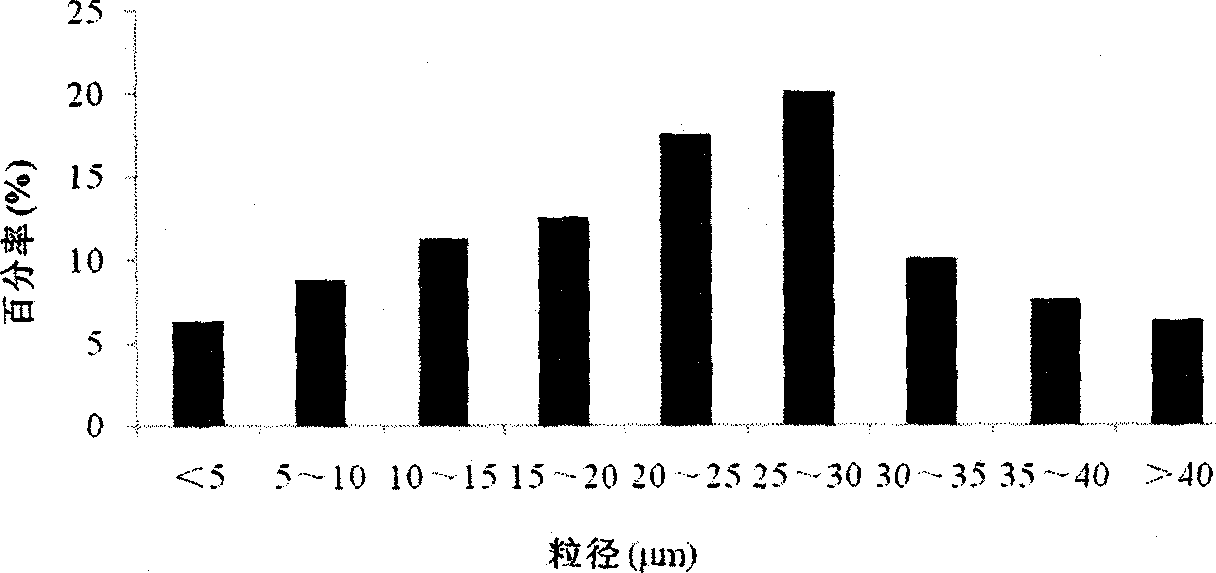
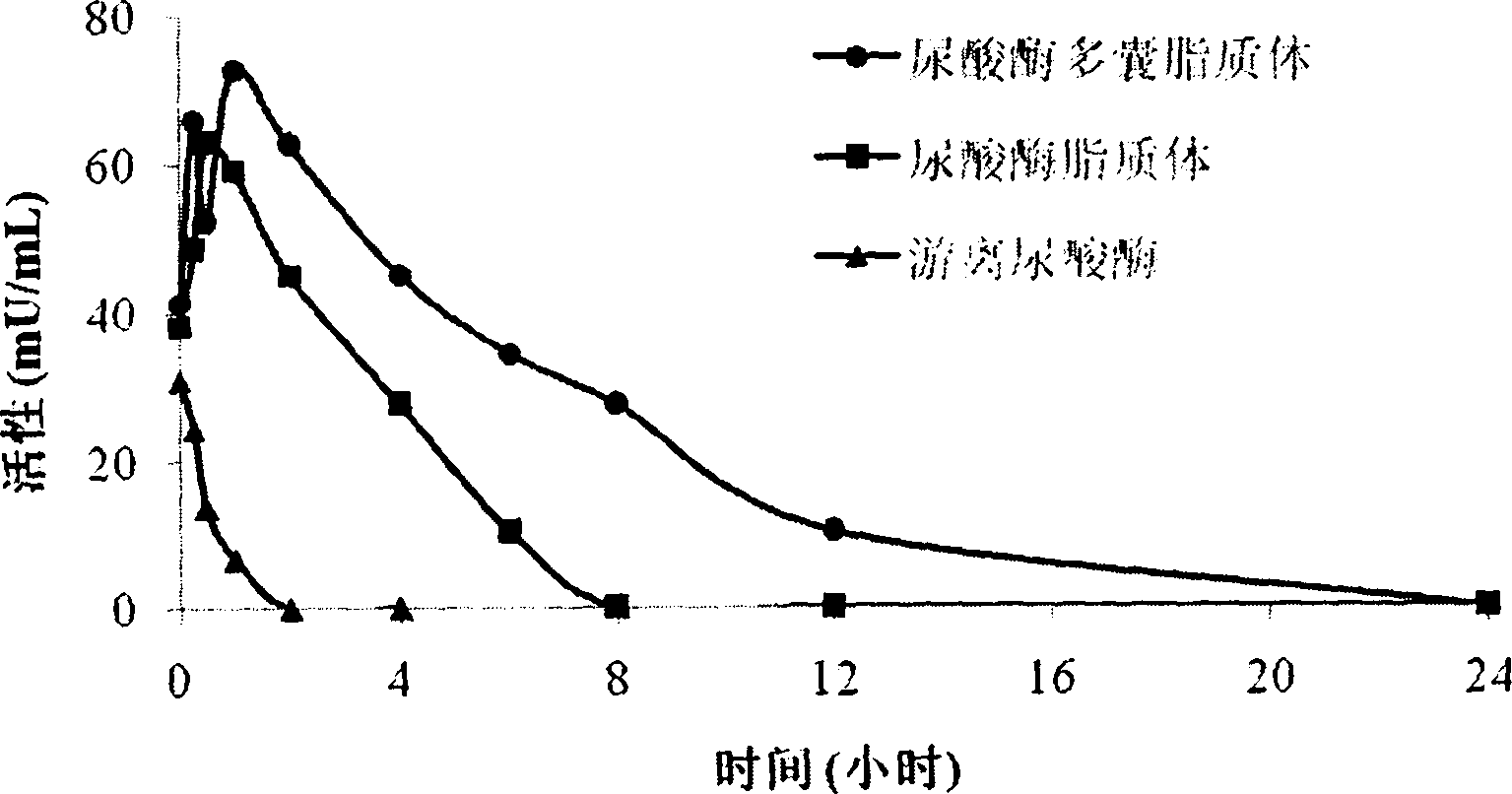
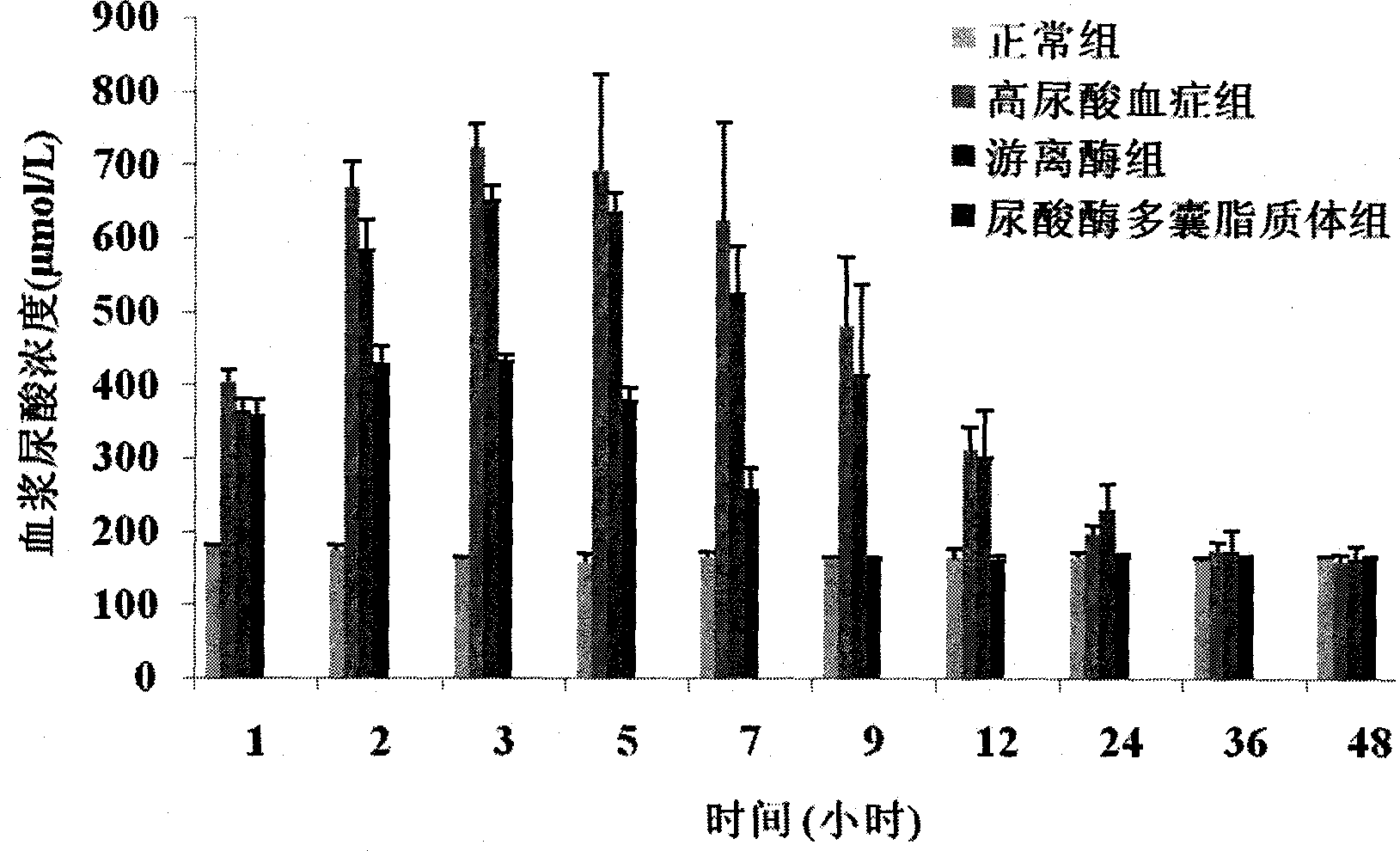
Uricase multivesicular liposome and preparation method thereof
ActiveCN104013576AGood slow releaseReduced activityPeptide/protein ingredientsSkeletal disorderMedicineMultivesicular liposomes
The invention belongs to the field of pharmaceutical preparations, and relates to a formula and a preparation method of uricase multivesicular liposome. The prepared uricase multivesicular liposome can improve the activity of uricase and reduce the immunogenicity thereof; moreover, the uricase can be slowly released to prolong the action time and efficacy time of a drug.
Owner:CHONGQING MEDICAL UNIVERSITY
Sodium alginate modified ropivacaine hydrochloride multivesicular liposome microsphere as well as preparation method and application thereof
ActiveCN113171354AGuarantee structureEvenly dispersedAnaestheticsPharmaceutical non-active ingredientsMicrosphereOrganosolv
The invention discloses a sodium alginate modified ropivacaine hydrochloride multivesicular liposome microsphere as well as a preparation method and an application thereof. The preparation method comprises the following steps: firstly, uniformly mixing and dispersing a nano calcium carbonate gel initiator into liquid paraffin, adding multivesicular liposome under the condition of uniform stirring, then adding a sodium alginate solution, and uniformly mixing; and then adjusting the pH value to 3.0-5.8, and rapidly initiating gelation reaction by free calcium ions to form the uniform gel-coated multi-vesicular liposome microspheres with a net-shaped cross-linked structure. The multi-vesicular liposome microsphere has the characteristics of clear structure, uniform particle size, stable state, long slow release time and the like. Compared with an existing preparation method, the preparation method has the advantages that the encapsulation effect of the final multi-vesicular liposome is guaranteed, the surface of the multi-vesicular liposome is uniformly covered with the sodium alginate, the problem that the sodium alginate is connected into a sheet is solved, the stability of the multi-vesicular liposome is enhanced, and therefore the slow release effect is improved. Meanwhile, the use amount of chloroform in the organic solvent can be reduced.
Owner:SOUTH CHINA UNIV OF TECH
Ophthalmic injectable formulation preparing and oculopathy treating and preventing
Disclosed are an ophthalmic injectable sustained-release formulation, a process for preparing the same and a method for treating and preventing oculopathy with the same. The ophthalmic injectable sustained-release formulation comprises a delivery system and a pharmaceutically acceptable excipient, wherein the delivery system is selected from the group consisting of microspheres, microcapsules, microparticles, liposomes, multivesicular liposomes, nanocrystals and nanoparticles.
Owner:LIU YUNXIANG
Granatilide acetate multivescular liposome and preparation method thereof
The invention relates to a granatilide acetate multivescular liposome. The granatilide acetate multivescular liposome is a liposome membrane material composed of common phospholipid, triglyceride andcharged phospholipid, and active component granatilide acetate is encapsulated in the multivescular liposome. The liposome membrane material also comprises cholesterol. The mass ratio of the common phospholipid to the charged phospholipid to the triglyceride is (3-10):1:(0.8-1.2). The granatilide acetate liposome provided by the invention has a simple preparation method and a high drug loading rate, and a slow release effect can be achieved.
Owner:HYBIO PHARMA WUHAN CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com