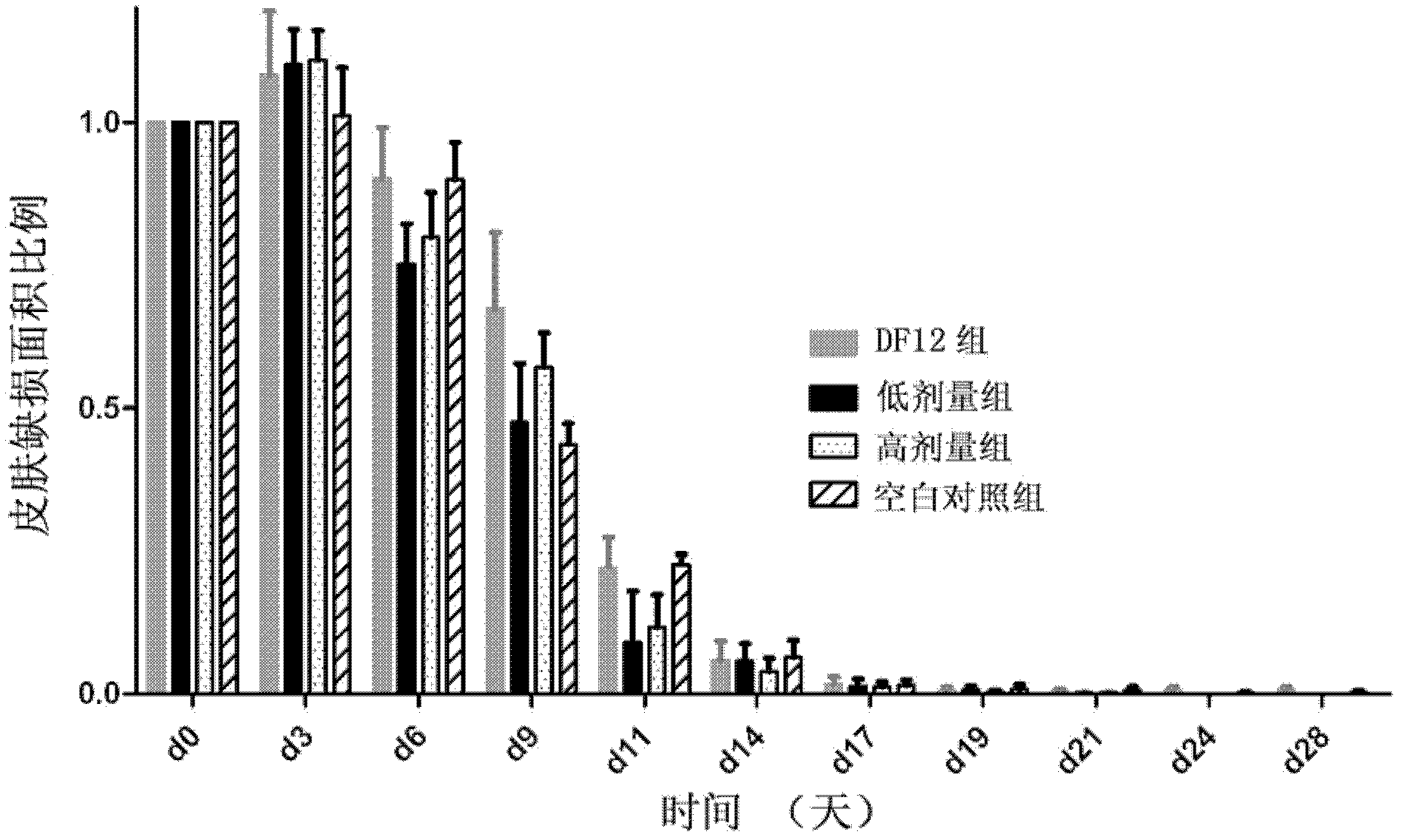
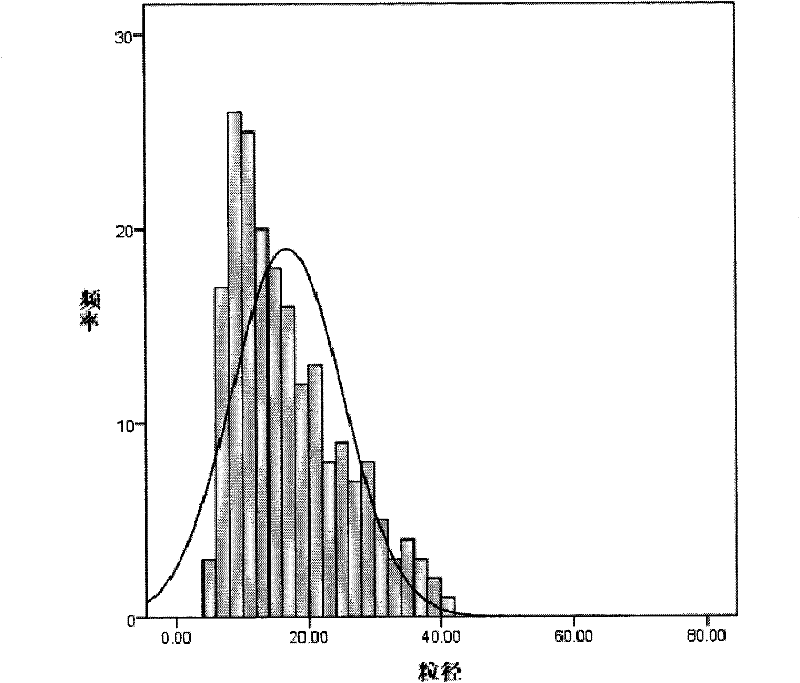
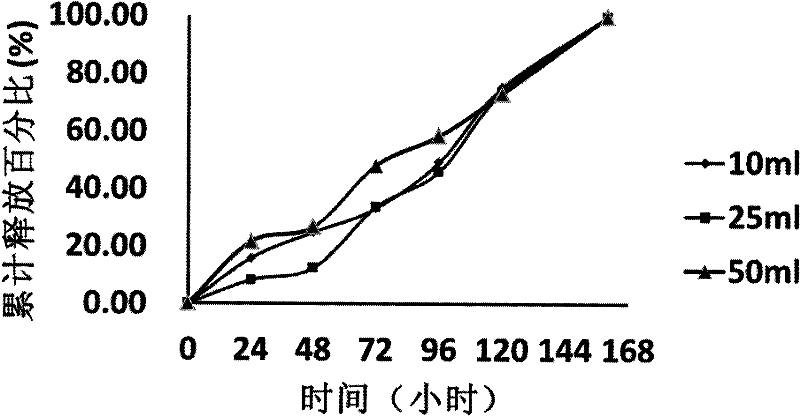
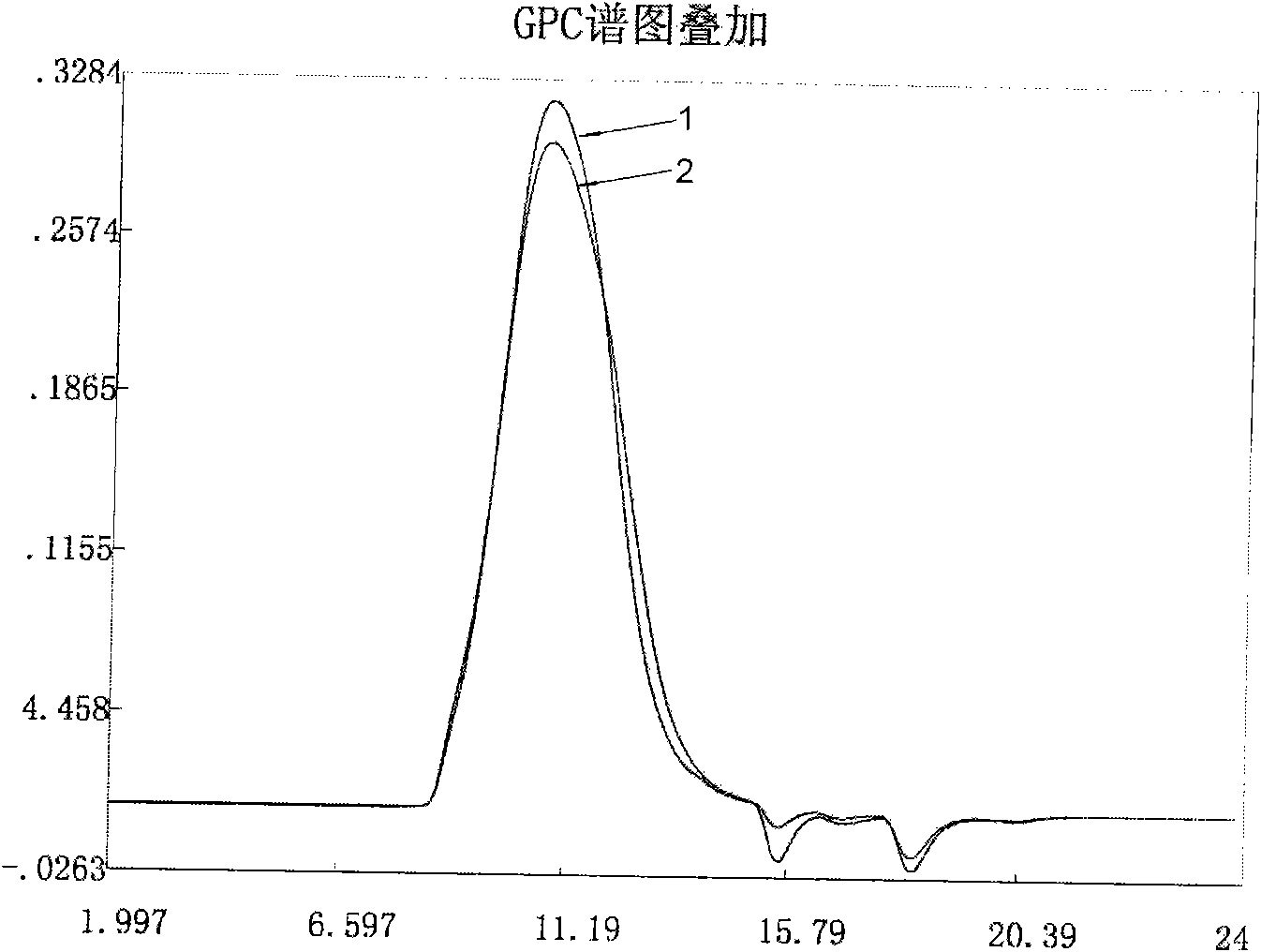
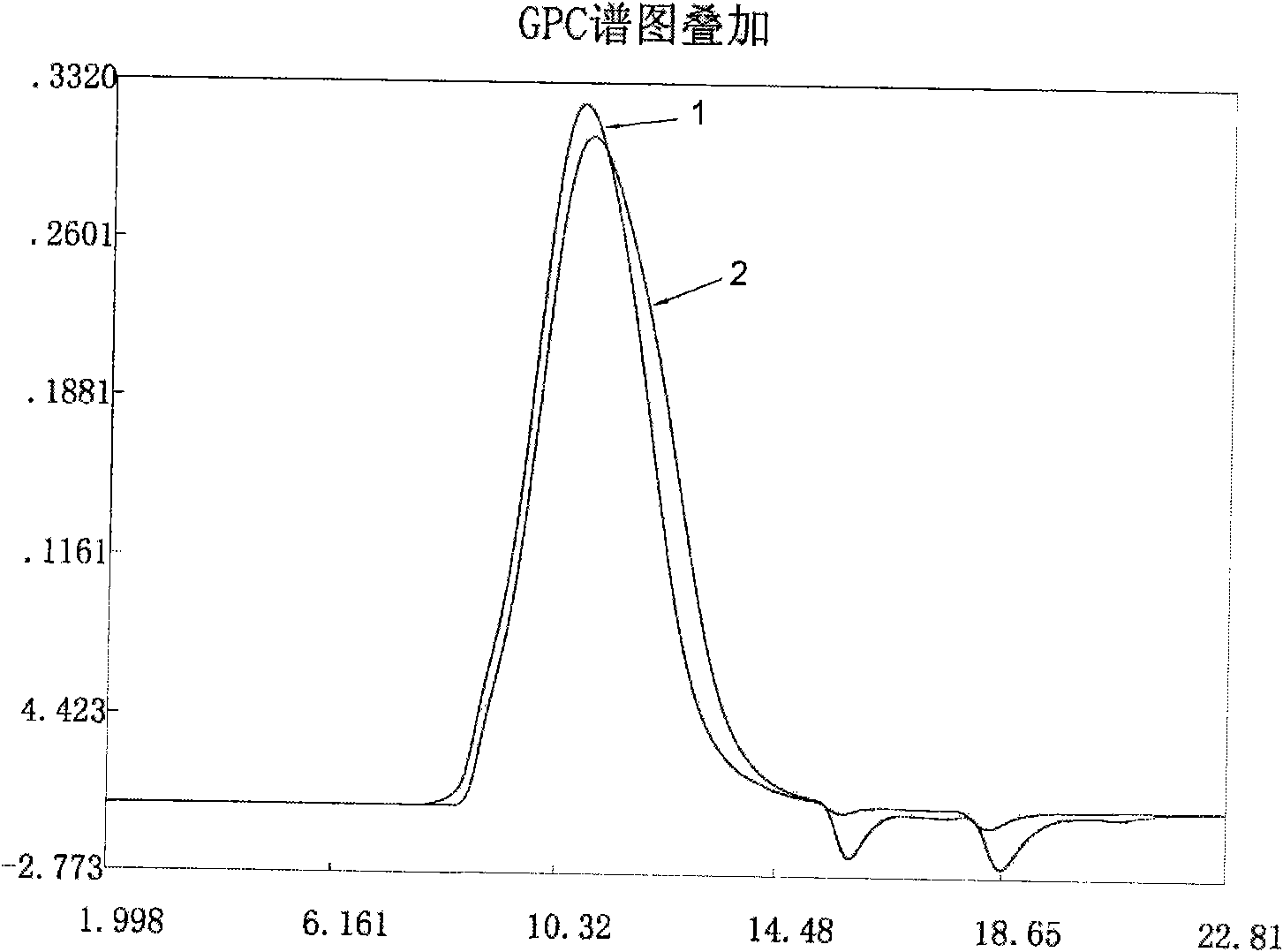
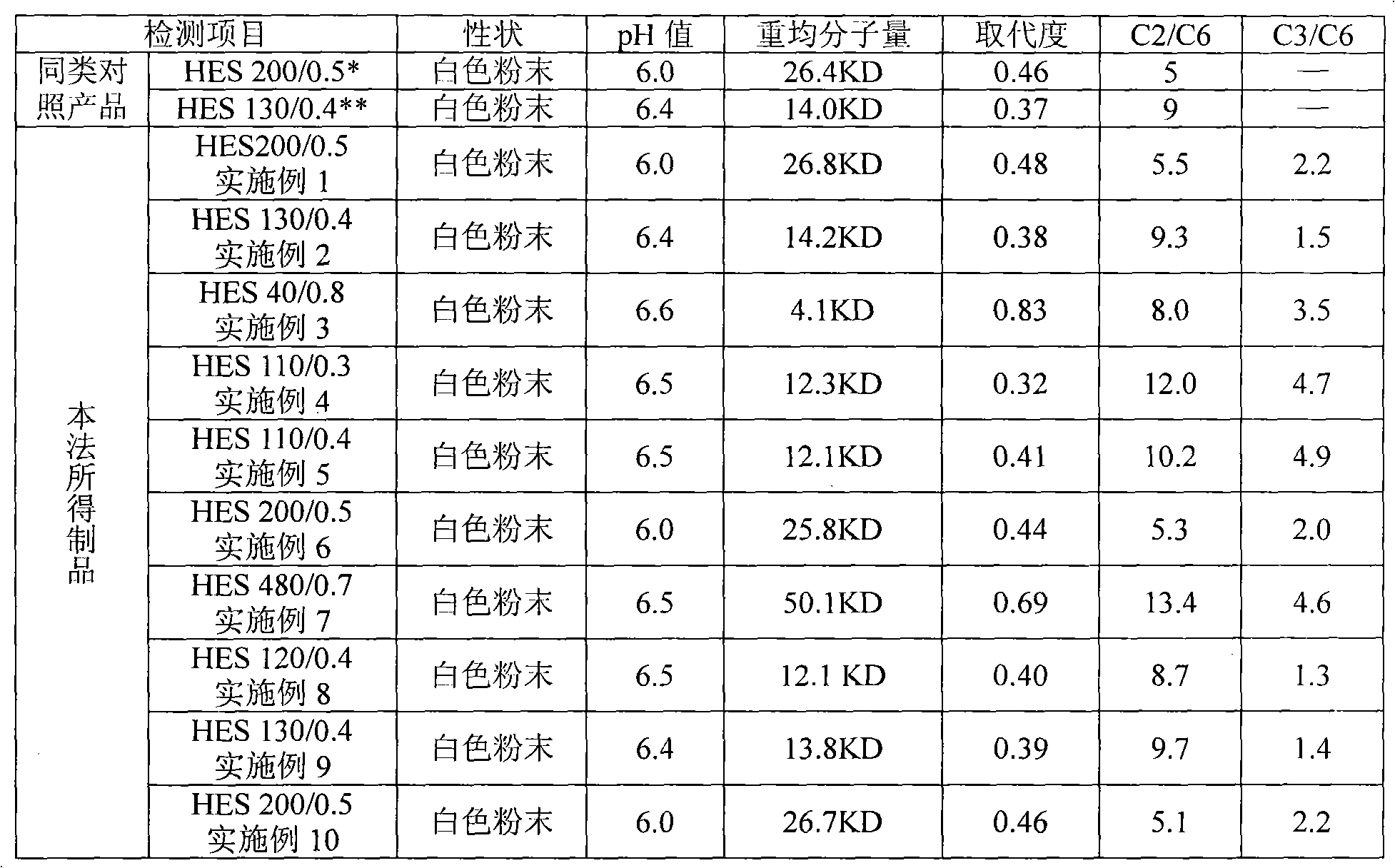
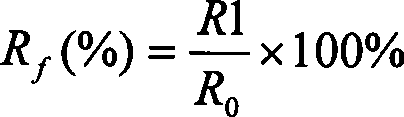Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
502 results about "Hydroxyethyl starch" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydroxyethyl starch (HES/HAES), sold under the brand name Voluven among others, is a nonionic starch derivative, used as a volume expander in intravenous therapy. The use of HES on critically ill patients is associated with an increased risk of death and kidney problems.
Dialysis solution for peritoneal dialysis
InactiveUS6284140B1Easy to degradeDwell timeBiocideSolvent extractionHydroxyethyl starchUltrafiltration
The present invention relates to dialysis solutions for peritoneal dialysis, containing hydroxyethyl starch as the osmotically-active substance, electrolytes and, optionally, conventional additives, where the hydroxyethyl starch has a molecular weight Mw in the range from 10,000 to 150,000, a substitution MS in the range from 0.10 to 0.40, a substitution DS in the range from 0.09 to 0.35 and a substitution ratio C2 / C6>=8. With this peritoneal dialysis solution it is possible, with an outstanding ultrafiltration, to maintain a longer dwell time, for example the dialysis solution can be utilized for a period of 12 hours in the CAPD without replacement. In addition, the inventive dialysis solution is also particularly advantageous for patients with residual kidney function. The resorption of the osmotically active substance is clearly diminished and even after a dwell time of 12 hours it amounts to a maximum of 60-70%.
Owner:FRESENIUS AG
Plasma-like substance
InactiveUS6110504AEliminate circulationDelay EliminationBiocideSulfur/selenium/tellurium active ingredientsHydroxyethyl starchWater soluble polysaccharides
An artificial plasma-like substance having at least one water soluble polysaccharide oncotic agent selected from the group consisting of high molecular weight hydroxyethyl starch, low molecular weight hydroxyethyl starch, dextran 40 and dextran 70, and albumin which is buffered by lactate and has a pre-administration pH of between 5 and 6.5 is disclosed. Also disclosed is an artificial plasma-like solution having at least two water soluble polysaccharide oncotic agents one of which is eliminated from the circulation slowly and the other of which is eliminated from the circulation quickly. Supplimentation of the plasma-like solution with certain ions is described. A system for administration of the plasma-like solution to a subject wherein the system comprises a first and second solution each having particular buffers is described. The plasma-like solution including cryoprotective adducts is also disclosed. The use of the plasma-like solution in organ transplant, novel chemotherapy procedures, and tissue, organ and organism cryopreservation are also disclosed.
Owner:LINEAGE CELL THERAPEUTICS INC
Solutions for use as plasma expanders and substitutes
InactiveUS20030022147A1Eliminate circulationDelay EliminationPharmaceutical delivery mechanismDead animal preservationHydroxyethyl starchPlasma expander
An artificial plasma-like substance having at least one water soluble polysaccharide oncotic agent selected from the group consisting of high molecular weight hydroxyethyl starch, low molecular weight hydroxyethyl starch, dextran 40 and dextran 70, which is buffered by lactate and has a pre-administration pH of between 4 and 6.5 is disclosed. In one embodiment, the artificial plasma-like solution may have at least two water soluble polysaccharide oncotic agents one of which is eliminated from the circulation slowly and the other of which is eliminated from the circulation quickly. Supplementation of the plasma-like solution with certain ions is described. A system for administration of the plasma-like solution to a subject wherein the system comprises a first and second solution each having particular buffers is described. Methods for the administration of the plasma-like solution are also disclosed.
Owner:LINEAGE CELL THERAPEUTICS INC
Stem cells preparation for repairing wound surface and preparation method thereof
InactiveCN102670654AImprove survival rateProlong survival timePharmaceutical delivery mechanismMammal material medical ingredientsHigh cellHydroxyethyl starch
The invention discloses a stem cells preparation for repairing a wound surface and preparation method thereof, wherein the stem cells preparation uses umbilical cords, placentae or mesenchymal stem cells between amniotic membranes as living cells, and is added with high polymer material sodium alginate with excellent biocompatibility, sodium hyaluronate, chitosan and hydroxyethyl starch as cell stabilizer. The stem cells preparation for repairing the wound surface has higher cell viability and is durable in action, and breaks through technical limitations of effectiveness of the mesenchymal stem cells for treating.
Owner:BEIJING HEALTH & BIOTECH STEM CELL INST CO LTD
A kind of preparation method and application of multivesicular liposome
ActiveCN102274183AHigh encapsulation efficiencyGood sustained release effectOrganic active ingredientsSaccharide peptide ingredientsHydroxyethyl starchArginine
The invention relates to a multi-vesicular liposome, a blank multi-vesicular liposome and a preparation method and application thereof. The multi-vesicular liposome contains the following components in part by weight: 1 part of liposome, 0.01 to 20 parts of auxiliary emulsifier, 1 to 50 parts of osmotic pressure regulator and medicinal active ingredients; the medicament-to-lipid ratio of the multi-vesicular liposome is 1:(0.1-1):200; the lipid contains of a specific amount of neutral phospholipid, cholesterol and triglyceride; and the auxiliary emulsifier is selected from dextran, polyvinyl pyrrolidone, hydroxyethyl starch, gelatin, albumin, arginine and hydroxymethyl starch. The blank multi-vesicular liposome contains the following components in part by weight: 1 part of lipid, 0.01 to 20 parts of auxiliary emulsifier, 1 to 50 parts of osmotic pressure regulator and 0.1 to 50 parts of ion gradient regulator; the osmotic pressure of the in vivo water phase of the multi-vesicular liposome is equal to the osmotic pressure of human plasma; and the auxiliary emulsifier is as previously mentioned. The multi-vesicular liposome has high entrapment rate, and can achieve good sustained-release effect on in vivo and in vitro experiments.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Preparation method and preservation method of clinical application-level placental hematopoietic stem cells
InactiveCN103789262AIncrease contentEnhance cell viabilityDead animal preservationBlood/immune system cellsHydroxyethyl starchEnzyme digestion
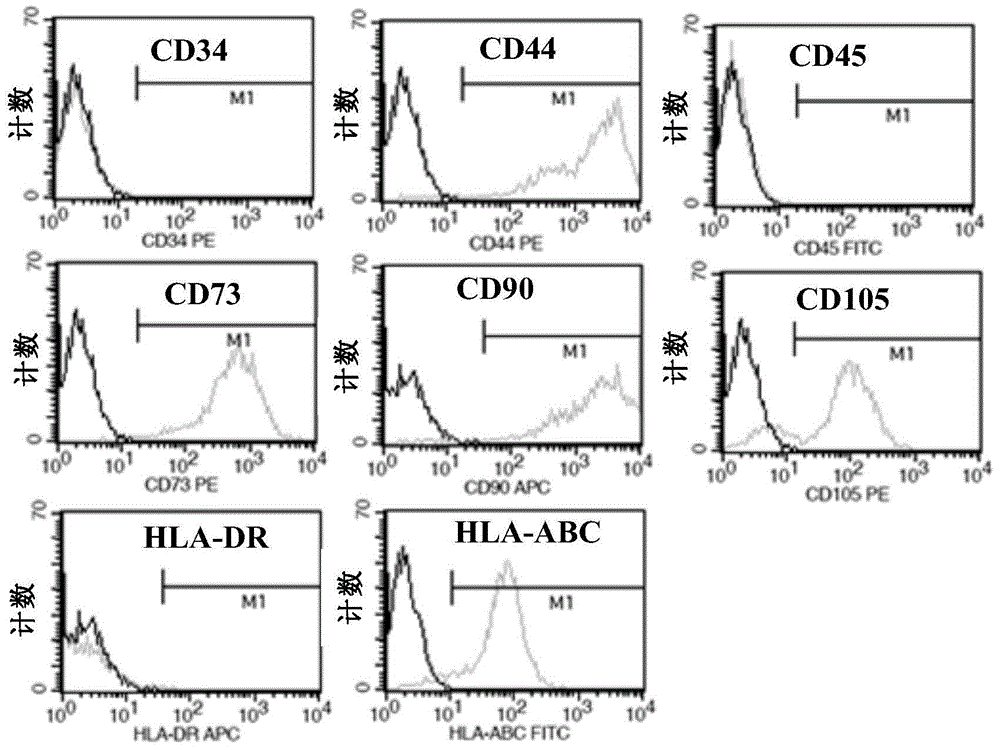
The invention provides a preparation method of clinical application-level placental hematopoietic stem cells. The preparation method comprises the steps of pretreating a fresh delivered placenta immediately and then separating out umbilical arteries and umbilical veins, pouring a cleaning fluid into the chorionic vessels of the placenta and colleting the cleaning fluid after pouring, pouring an enzyme digestion fluid into the chorionic vessels of the placenta and digesting at 37 DEG C for 5-20min, pouring the collected fluid into the placenta and collecting the liquid after pouring, centrifuging the obtained fluid and re-suspending cells after removing the supernatant, and separating the resuspended cell suspension through a two-step process with hydroxyethyl starch and a lymphocyte separation medium to obtain the clinical application-level placental hematopoietic stem cells. The method is capable of increasing the number and the activity of the prepared placental hematopoietic stem cells and the content of CD34 positive cells, and the prepared placental hematopoietic stem cells are at the clinical application level and free of potential pathogenic contamination, and moreover, the method is low in preparation cost.
Owner:台州恩源生物科技有限公司
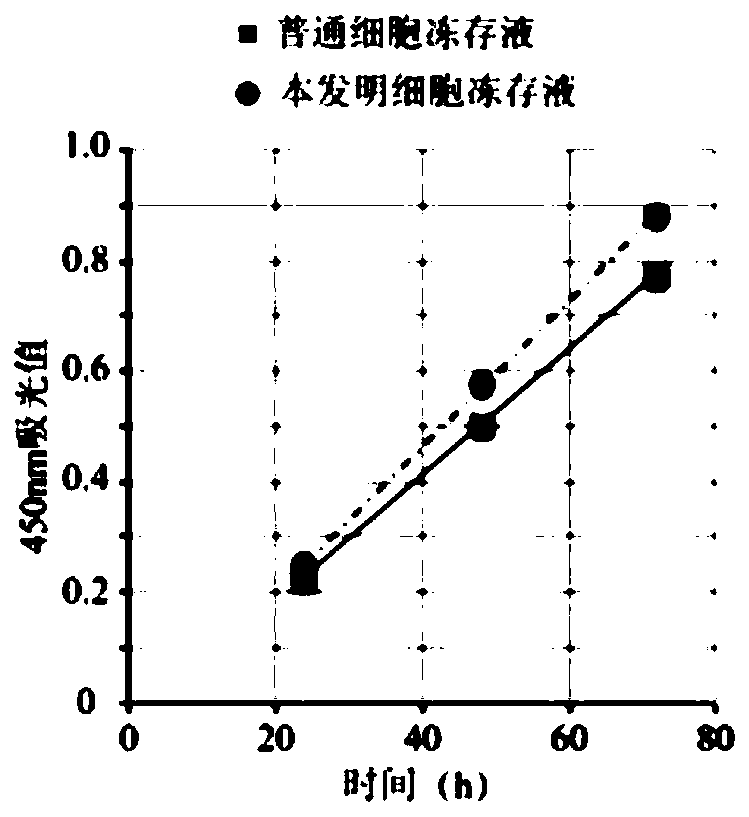
Mesenchymalstem cellfrozen stock solution as well as preparation method and using method thereof
InactiveCN109090100AKeep aliveGood freezing effectDead animal preservationHydroxyethyl starchMesenchymal stem cell
The invention relates to a mesenchymalstem cellfrozen stock solution as well as a preparation method and a using method thereof. The mesenchymalstem cellfrozen stock solution comprises a Rock inhibitor Y27632, dimethyl sulfoxide, human serum albumin, hydroxyethyl starch and the like. The mesenchymalstem cellfrozen stock solution disclosed by the invention is applicable to a slow cryopreservation method; not only is the mesenchymalstem cellfrozen stock solution simple to operate, but also the formula has the advantages of low toxicity and low economic cost; the activity of mesenchymalstem cellscan also be kept for long time; and the mesenchymalstem cellfrozen stock solution has an excellent cryopreservation effect and is safer and more reliable.
Owner:深圳市浊安认证生物技术有限公司
MSC (mesenchymal stem cell) injection as well as preparation and application thereof
InactiveCN104857022AImprove survival rateAvoid gatheringPharmaceutical delivery mechanismUnknown materialsClinical gradeHydroxyethyl starch
The invention relates to the field of biology, in particular to MSC (mesenchymal stem cell) injection as well as a preparation and an application thereof. The injection comprises MSCs and a cell cryopreservation solution, wherein the cryopreservation solution comprises components in percentage by volume as follows: 25%-70% of an electrolyte balance solution, 5%-20% of clinical-grade DMSO (dimethyl sulfoxide), 1%-50% of 20% human serum albumin, 1%-10% of hydroxyethyl starch 130 / 0.4 and 5%-20% of triphosadenine-disodium magnesium chloride freeze-drying powder. The injection is free of animal serum, has clear ingredients and good cell cryopreservation effect and is safe and controllable, long-term storage and long-distance transport are facilitated, the survival rate of cells after recovery is higher than 95%, the vitality is high, and the injection can effectively relieve injury and inflammation symptoms of lesion tissue of lungs and promote tissue regeneration of the lungs, so that acute lung injury can be fundamentally and comprehensively treated.
Owner:北京青藤谷禧干细胞科技研究院有限公司
A hemopoietic stem cell cryopreserving solution, and a hemopoietic stem cell cryopreserving method
InactiveCN107094753AImprove protectionDrop in recovery rateDead animal preservationHydroxyethyl starchCell culture media
The invention relates to the technical field of cytobiology, and particularly relates to a hemopoietic stem cell cryopreserving solution, and a hemopoietic stem cell cryopreserving method. The cryopreserving solution includes following raw materials by weight: 10-20 mL of dimethyl sulfoxide, 6-10 g of hydroxyethyl starch, 4-8 mL of a cell culture medium, 6-10 mL of human serum albumin, and 60-100 mL of human autologous plasma. The formula of the cryopreserving solution is simple, and the raw materials are cheap. The cryopreserving solution can effectively protect hemopoietic stem cells from freezing injuries, is high in safety and low in cell injuries, can increase the resuscitation survival rate of hemopoietic stem cells. The survival rate after resuscitation can be 96% or above, and after resuscitation, the cell viability is high, the quantity of cell proliferation is high, the cells are not liable to age, physiological functions and biological characteristics of the hemopoietic stem cells after resuscitation can be ensured, and microbe detection indexes meet requirements. The survival time of the hemopoietic stem cells is prolonged.
Owner:DONGGUAN BOALAI BIOLOGICAL TECH CO LTD
Method for preparing hydroxyethyl starch
InactiveCN101775076AAvoid destructionSolve problems such as removal difficultiesBlood disorderExtracellular fluid disorderHydroxyethyl starchStarch gelatinization
The invention discloses a method for preparing hydroxyethyl starch. The starch raw materials are subjected to hydroxyethylation and acid hydrolysis treatment in a solvent medium respectively. The method for preparing the hydroxyethyl starch comprises the following steps: firstly, under the alkaline condition that the pH is 13-14, carrying out hydroxyethylation reaction by using ethylene oxide or chlorethanol in an amount which is 0.2 to 1.8 times mol of the starch as a hydroxyethylation reagent; secondly, hydrolyzing the starch raw materials which are subjected to the hydroxyethylation under the acidic condition that the pH is 1-3 to obtain the hydroxyethylation starch, wherein the weight-average molecular weight is 20 to 500kD; the molar degree of substitution is 0.1 to 0.9, and the substituted ratio at the C2 / C6 position is 3 to 20 and the substituted ratio at the C3 / C6 position is 0.1 to 8. The method for preparing the hydroxyethyl starch can prevent the starch structure from being damaged without pasting the starch, solve the problems that the control of the molecular weight of the products is inaccurate during acid hydrolysis, an organic residual solvent which is subjected to the hydroxyethylation is hardly removed and the like, and is favorable for industrialized production. Therefore, hydroxyethylation starch products with different molecular weights and / or degrees of substitution can be obtained.
Owner:CHENGDU QINGSHAN LIKANG PHARMA CO LTD
Culturing method of mesenchymal stem cells of menstrual blood
ActiveCN104560871APollution prevention methodsReduced Chances of ContaminationSkeletal/connective tissue cellsHydroxyethyl starchSurface marker
The invention provides a method for preparing mesenchymal stem cells of menstrual blood. The method comprises steps as follows: female menstrual blood is collected and taken as a raw material and is subjected to sterile processing; stem cells in the menstrual blood are separated; the mesenchymal stem cells of the menstrual blood are cultured; the mesenchymal stem cells of the menstrual blood are cryopreserved; and the mesenchymal stem cells of the menstrual blood are recovered. The method has the technical characteristics as follows: the female menstrual blood is collected and stored in a preservative fluid; by means of a bacterial pollution prevention method, the pollution probability is reduced from a collection source, a collecting cup is repeatedly washed by sterile water, and the pollution probability is effectively reduced; with the adoption of a differential centrifugation method, bacteria in the menstrual blood are removed as far as possible; a sample is repeatedly separated by HES (hydroxyethyl starch), and the maximum quantity of mesenchymal stem cells of the menstrual blood can be obtained; a serum-free medium is utilized for culturing, components of animal origin are reduced, the cell performance is stable, the in-vitro long-term culturing process of the mesenchymal stem cells of the menstrual blood can be kept, and cellular morphology, multiplication capacity, MSC (mesenchymal stem cell) surface marker expression, differentiation capacity and the like are maintained. The method is simple, practical and convenient to operate, the maximum quantity of required stem cells can be obtained, and the stem cells are successfully cultured.
Owner:SHENZHEN BEIKE BIOTECH
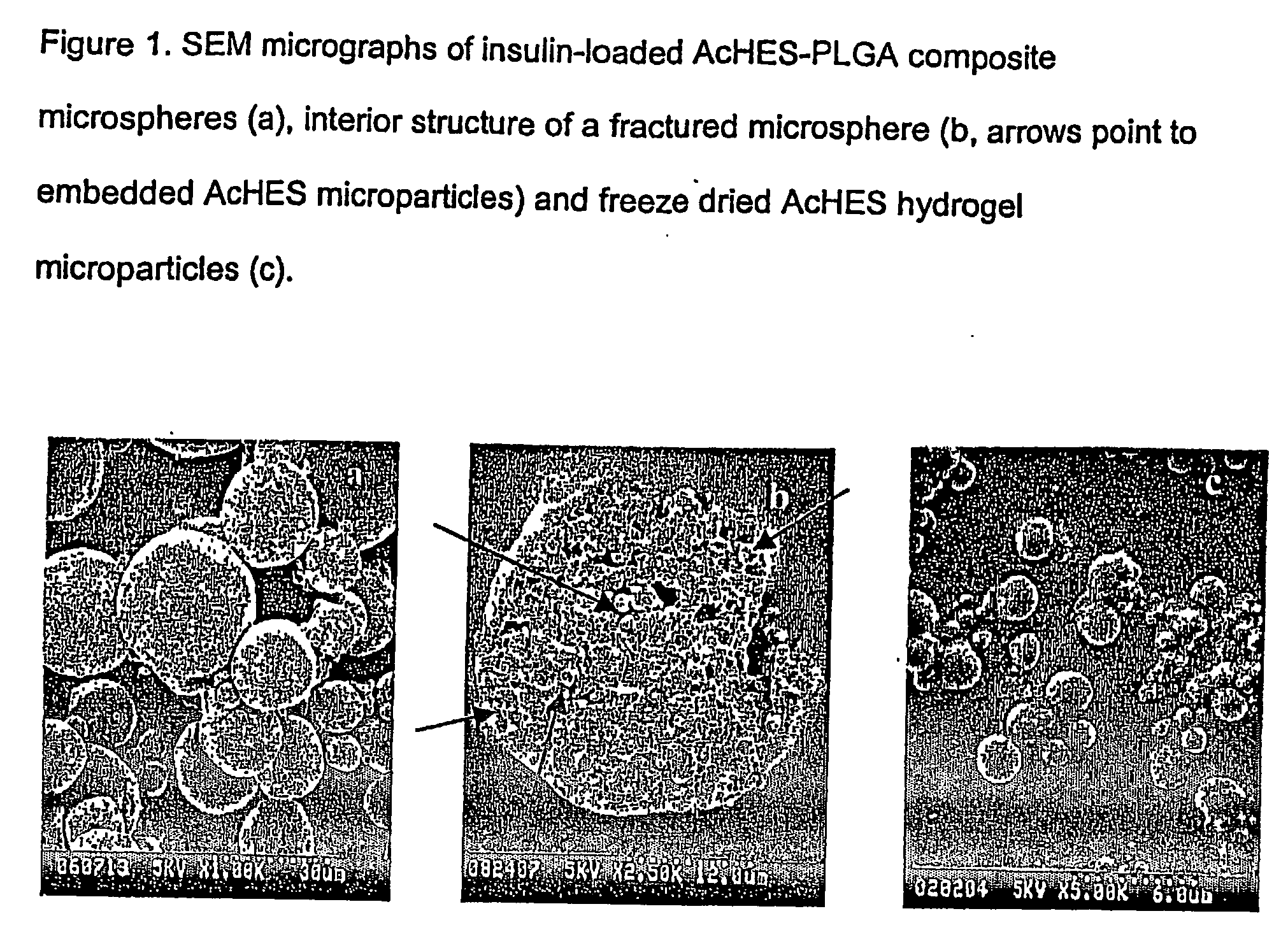
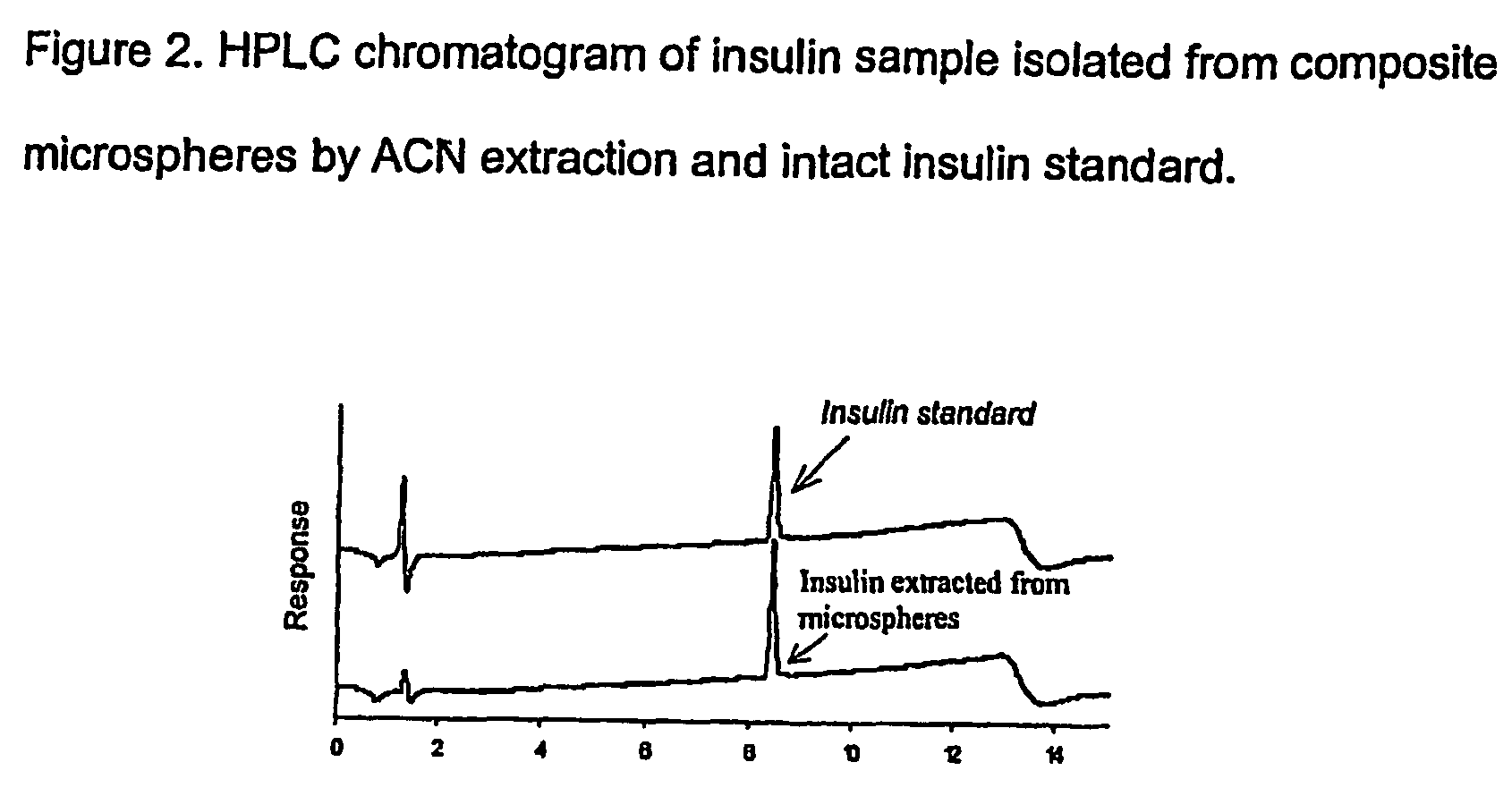
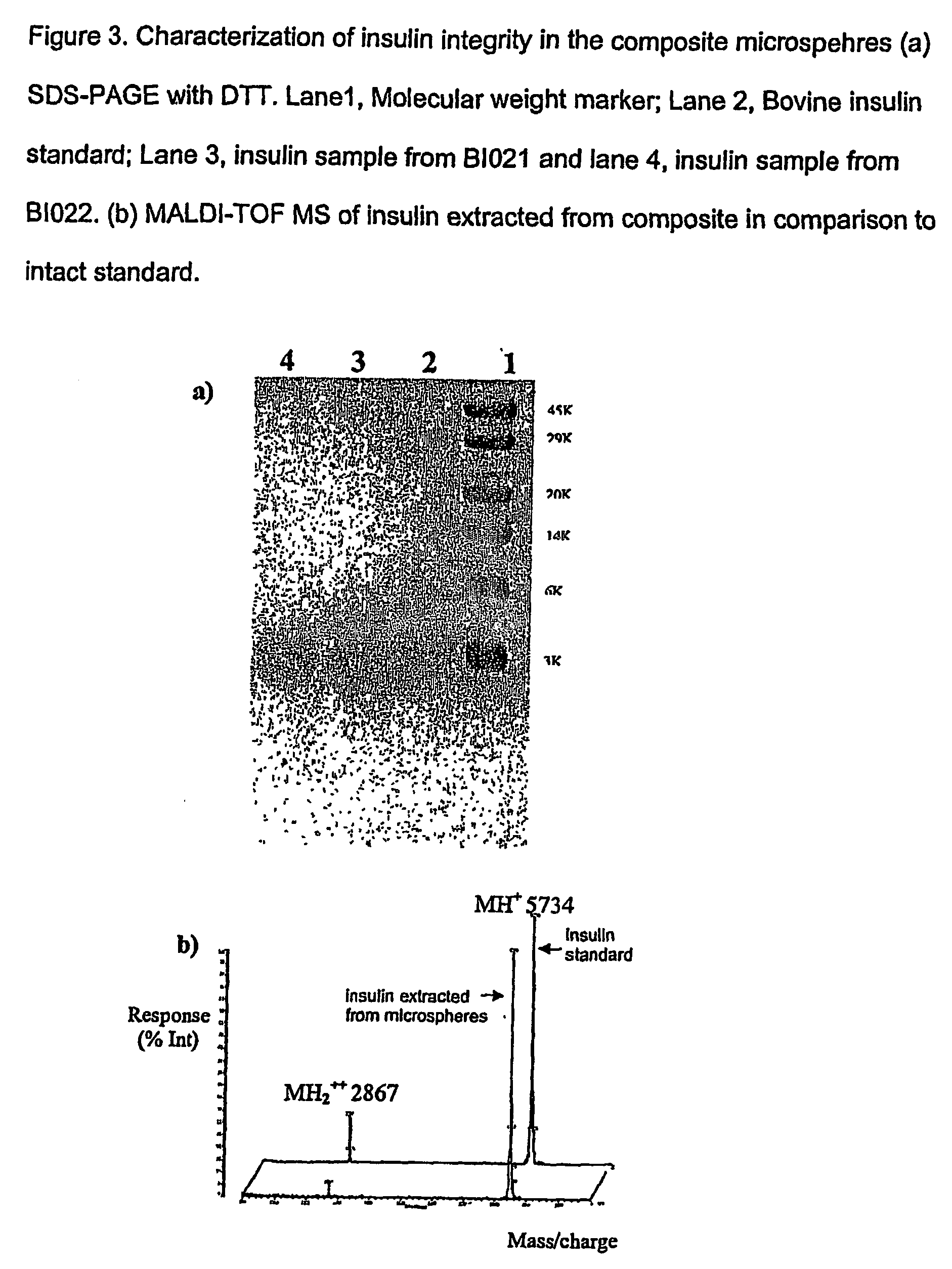
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
Umbilical cord mesenchymal stem cell injection as well as preparation method and application thereof
InactiveCN104873542AImprove the quality of lifeReduce releaseDigestive systemPharmaceutical delivery mechanismHydroxyethyl starchClinical grade
The invention provides umbilical cord mesenchymal stem cell injection as well as a preparation method and application thereof. The umbilical cord mesenchymal stem cell injection comprises mesenchymal stem cells and a frozen stock solution, wherein the frozen stock solution comprises the following components: a balanced electrolyte solution with the volume percentage of 25-70 percent, hydroxyethyl starch 130 / 0.4 with the mass / volume percentage (g / ml) of 1-10 percent, triphosadenine disodium magnesium chloride with the volume percentage of 5-20 percent, clinical-grade DMSO with the volume percentage of 5-20% and 20% human albumin with the volume percentage of 1-50 percent. The prepared umbilical cord mesenchymal stem cell injection is free of animal serum, safe, high in cell viability after cryopreservation resuscitation, can be used for treating inflammatory bowel disease, has a good effect and has no toxic or side effects.
Owner:北京青藤谷禧干细胞科技研究院有限公司
Peripheral blood stem cell preserving fluid and preparation method thereof
InactiveCN107156108ALife sustaining metabolismEquilibrium osmotic pressureDead animal preservationHydroxyethyl starchDextran
The invention relates to the technical field of stem cells and in particular relates to a peripheral blood stem cell preserving fluid. The peripheral blood stem cell preserving fluid comprises the following components in parts by weight: 3-8 parts of human serum albumin, 2-4 parts of trehalose, 1-3 parts of hydroxyethyl starch, 5-15 parts of nutrients, 1-3 parts of dextran, 1-3 parts of dimethyl sulfoxide and a proper amount of water. The preserving liquid is used to preserve the peripheral blood stem cells, so that the stem cells keep relatively good stability and the survival rates of the stem cells are greatly increased (over 98%).
Owner:魏方萌
Umbilical cord mesenchymal stem cell injection and preparation method and application thereof
InactiveCN104922059ANo immune rejectionNon-tumorigenicPharmaceutical delivery mechanismUnknown materialsHydroxyethyl starchFiltration
The invention relates to umbilical cord mesenchymal stem cell injection and a preparation method and application thereof. The injection comprises umbilical cord mesenchymal stem cells and mixed solution; the mixed solution comprises balanced electrolyte solution, hydroxyethyl starch, adenosine disodium triphosphate-magnesium chloride, dimethyl sulfoxide and human serum albumin. The preparation method comprises the following steps: (1) fully and evenly mixing the balanced electrolyte solution, the hydroxyethyl starch and the human serum albumin, and performing filtration sterilization; (2) adding the umbilical cord mesenchymal stem cells; (3) adding the dimethyl sulfoxide; (4) performing split charging and frozen preservation to finish preparation; the invention also provides the application of the injection in the aspect of drugs for treating chronic ischemic heart diseases. The injection disclosed by the invention has a very good resuscitation effect after the frozen preservation, can be directly injected after being unfrozen and resuscitated, and has a remarkable effect on treatment on the chronic ischemic heart diseases.
Owner:北京青藤谷禧干细胞科技研究院有限公司
Method for inducing megakaryoblast and megakaryocyte in vitro
ActiveCN101864396AHigh yieldImprove settlement efficiencyCulture processBlood/immune system cellsFicollSerum free media
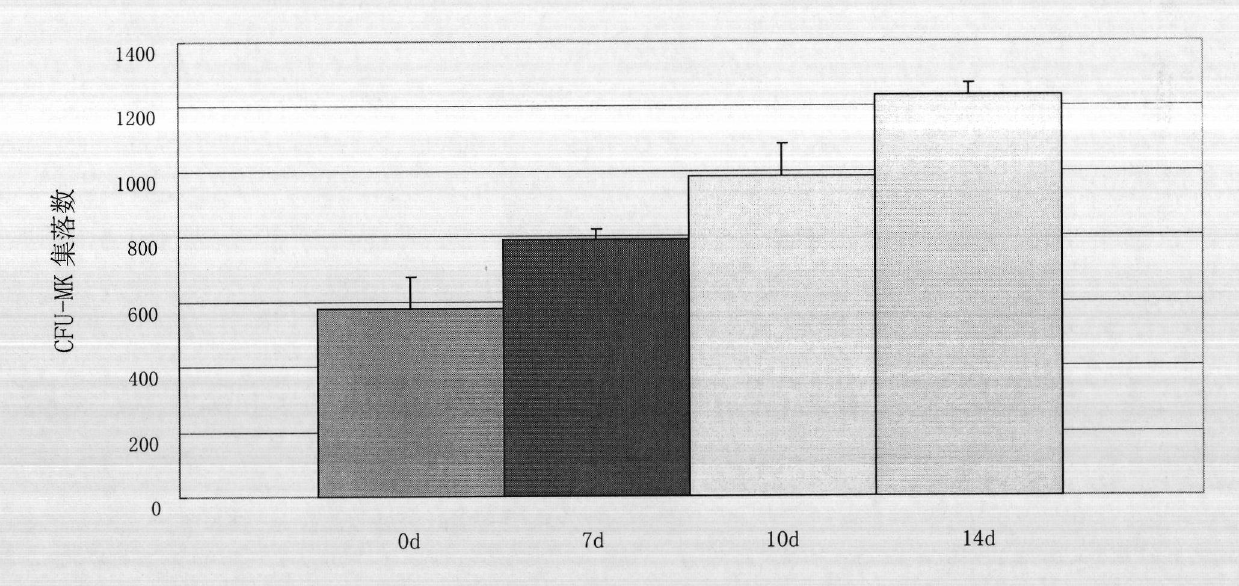
The invention discloses a method for inducing megakaryoblast and megakaryocyte in vitro and a special culture medium thereof. The special culture medium is a serum free medium containing 50ng / mL of SCF, 50ng / mL of TPO, 20ng / mL of IL-3 and 50ng / mL of IL-6. The method comprises the following steps of: 1) separating single karyocyte from umbilical cord blood by using the conventional Ficoll density gradient centrifugation method, wherein red blood cells in the umbilical cord blood are settled by 6 percent hydroxyethyl starch; and 2) culturing the single karyocyte in vitro for 4 to 14 days by using the special culture medium at 37 DEG C in the presence of 5 percent CO2 to obtain a mixture of the megakaryoblast and the megakaryocyte. The invention provides a source for supplementing the megakaryoblast and the megakaryocyte, and has the advantages of simple operation, low cost, high differentiation efficiency and the like. The method is about to play an important role in the field of medicaments, and has wide application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Kit for processing human marrow, cord blood and peripheral blood cells and cell processing method
The invention relates to a kit for processing human marrow, cord blood and peripheral blood stem cells and a stem cell processing method. The kit radically solves the problems of high manufacture cost, low cell activity, indefinite reaction of markers in a human body, mobilizing agent injection causing patient pain, long obtainment time, complication, clinical unsuitability, and the like in the prior cell separation technology. The invention has the technical scheme that the kit comprises the following three reagents: (1) a diluent comprising 0.9 percent of sodium chloride injection or PBS solution, (2) a precipitator comprising 6 percent of hydroxyethyl starch or 0.2-1 percent of methyl cellulose and (3) a separating solution which has the density of 1.074-1.076 and is prepared by saccharosan and diatrizoate. The kit is easy to store and transport and convenient and rapid to use and can be produced in industrialization.
Owner:BEI ZHENG STEM CELLS BIOLOGICAL TECH CO LTD BEIJING
General serum-free crypreservation solution for MSCs (mesenchymal stem cells) and preparation method of crypreservation solution
InactiveCN109090102AResuscitative activity is not lowAvoid reactionDead animal preservationSerum free mediaHydroxyethyl starch
The invention discloses a general serum-free crypreservation solution for MSCs (mesenchymal stem cells) and a preparation method of the crypreservation solution. The crypreservation solution is prepared from components in percentage by volume as follows: 0.5%-10% of a compound dextran 40 injection , 1%-10% of hydroxyethyl starch (130 / 0.4), 5%-30% of glycerin, 25%-70% of a serum-free medium, 0.5%-1% of a serum substitute, 20%-40% of human albumin, 0.05%-1% of vitamin C, 0.2%-1% of vitamin E and 0.5%-3% of nonessential amino acid. By the aid of the crypreservation solution, on the premise that the cell activity of MSCs under the deep hypothermia cryopreservation condition is not lower than that in existing methods, potential risks caused by low toxicity and complement reactions of certain components in existing general methods are avoided.
Owner:成都汇欣生命科技有限公司
Preserving fluid of hepatic cells for biological artificial liver and preparation method thereof
ActiveCN101919381APrevent acidificationImprove buffering effectDead animal preservationArtificial liverHydroxyethyl starch
The invention provides preserving fluid of hepatic cells for a biological artificial liver and a preparation method thereof. The preserving fluid is a solution compounded by ultrapure water. The solution contains the following components within the concentration range: 15-25mmol / L of disodium hydrogen phosphate, 1-10mmol / L of sodium hydrogen phosphate dehydrate, 4-6mmol / L of potassium citrate monohydrate, 10-30mmol / L of sodium chloride, 5-10mmol / L of magnesium chloride hexahydrate, 3-10mmol / L of disodium adenosine triphosphate, 1-5mmol / L of reducing glutathione, 0.1-0.5mmol / L of alpha-lipoic acid, 100-150mmol / L of trehalose (C6H12O5), 200 / 0.510-50g / L of hydroxyethyl starch and 2-10mg / L of matrine. The preparation method of the preserving fluid comprises the following steps of: accurately weighing all components according to the concentration requirements of the components, wherein the alpha-lipoic acid is weighed in a dark place; completely dissolving the other components except the alpha-lipoic acid by using the right amount of ultrapure water; sufficiently dissolving the alpha-lipoic acid in the dark place; and adding the ultrapure water to full dose. The preserving fluid can well protect the cell activity of the hepatic cells for the biological artificial liver and the special functions of the hepatic cells at low temperature so as to satisfy the short-term low temperature preservation of a large-scale hepatic cell bank for the biological artificial liver and / or the hepatic cell protection in the long-distance transportation process.
Owner:ZHUJIANG HOSPITAL SOUTHERN MEDICAL UNIV
Placenta-derived mesenchymal stem cell freezing medium and freezing method thereof
InactiveCN107183008AAvoid damageImprove survival rateDead animal preservationHydroxyethyl starchPolyvinyl alcohol
The invention relates to the technical field of stem cells, in particular to a placenta-derived mesenchymal stem cell freezing medium. The placenta-derived mesenchymal stem cell freezing medium comprises the following components in parts by weight: 40-70 parts of DEME culture medium, 2-6 parts of sodium glycerophosphate solution, 1-5 parts of hydroxyethyl starch, 1-4 parts of mannitol solution, 0.3-0.8 part of ethanediol, 0.5-2 parts of polyvinyl alcohol, 0.5-1.8 parts of trehalose, and 0.5-1.5 parts of polyvinylpyrrolidone. With the adoption of the freezing medium for storing placenta-derived mesenchymal stem cells, the damage on the stem cells in the freezing process can be reduced, and the survival rate of the stem cells after thawing is increased.
Owner:魏方萌
Freeze drying protective agent for rubricyte and use method in freeze drying process
InactiveCN101368172AImprove recovery rateDead animal preservationTissue cultureHydroxyethyl starchFreeze-drying
The invention relates to a rythrocyte lyophilized protective agent and a using method thereof during lyophilizing. The prescription of the protective agent before being lyophilized is as follows: glucose solution, adenine, NaCl, mannitol, KCl, polymer hydroxyethyl starch, bovine serum albumin and sodium citrate. The prescription of the lyophilized rehydration protective agents is as follows: trehalose, ascorbic acid, NaCl, KH2PO4, Na2 HPO4, dextran, bovine serum albumin, KCl, inosine and adenine. The protective liquid before being lyophilized and rythrocyte suspension liquid are mixed according to the volume ratio of 4:1, are frozen and dried to obtain lyophilized rythrocyte, then the lyophilized rythrocyte is rehydrated by the rehydration protective agent. The recovery rate of the rythrocyte lyophilized protective agent and the using method thereof during lyophilizing of the invention can reach as high as 71.1 percent through the measurement of a cell counter, and is improved by 12.3 percent by comparing with the lyophilized protective agent and the lyophilized method thereof in the prior art, the highest recovery rate of which is 58.5 percent.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Novel albumin-free factor VIII formulations
Owner:UNIV OF CONNECTICUT +1
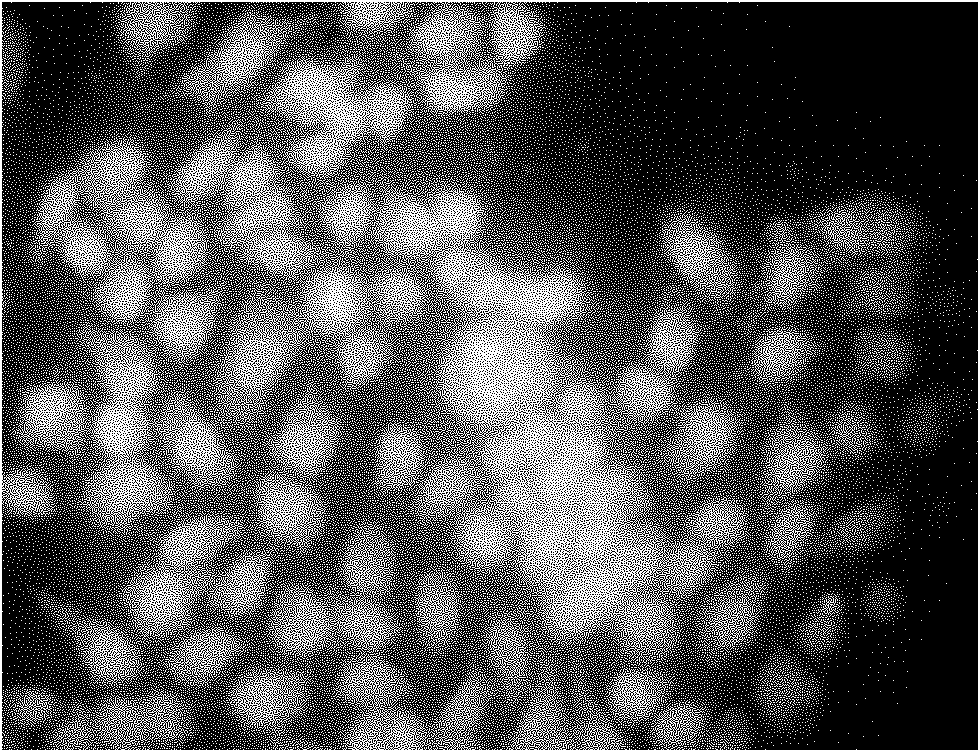
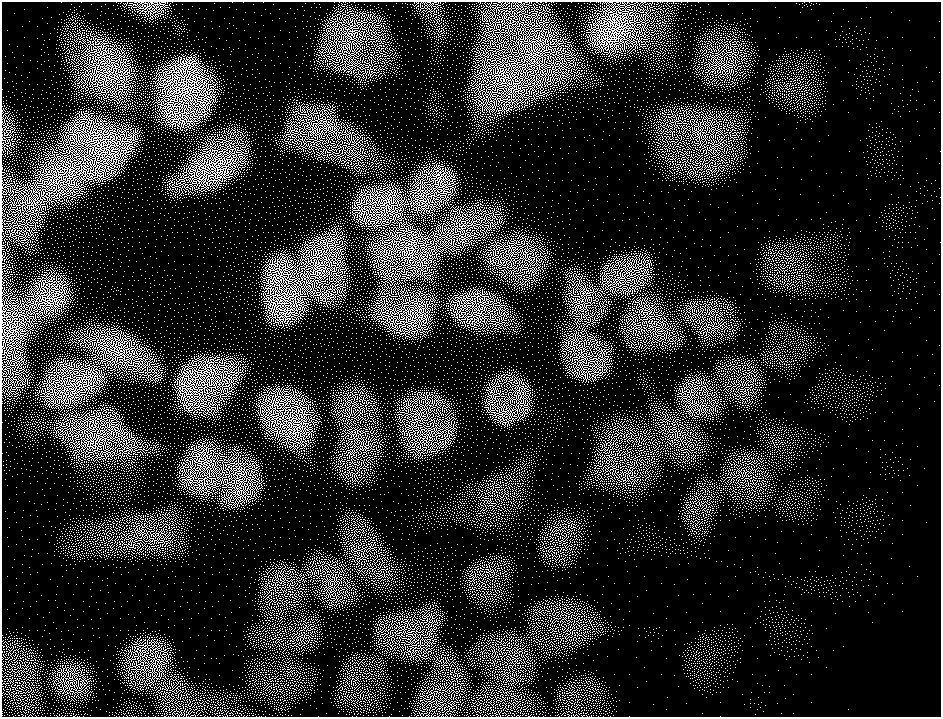
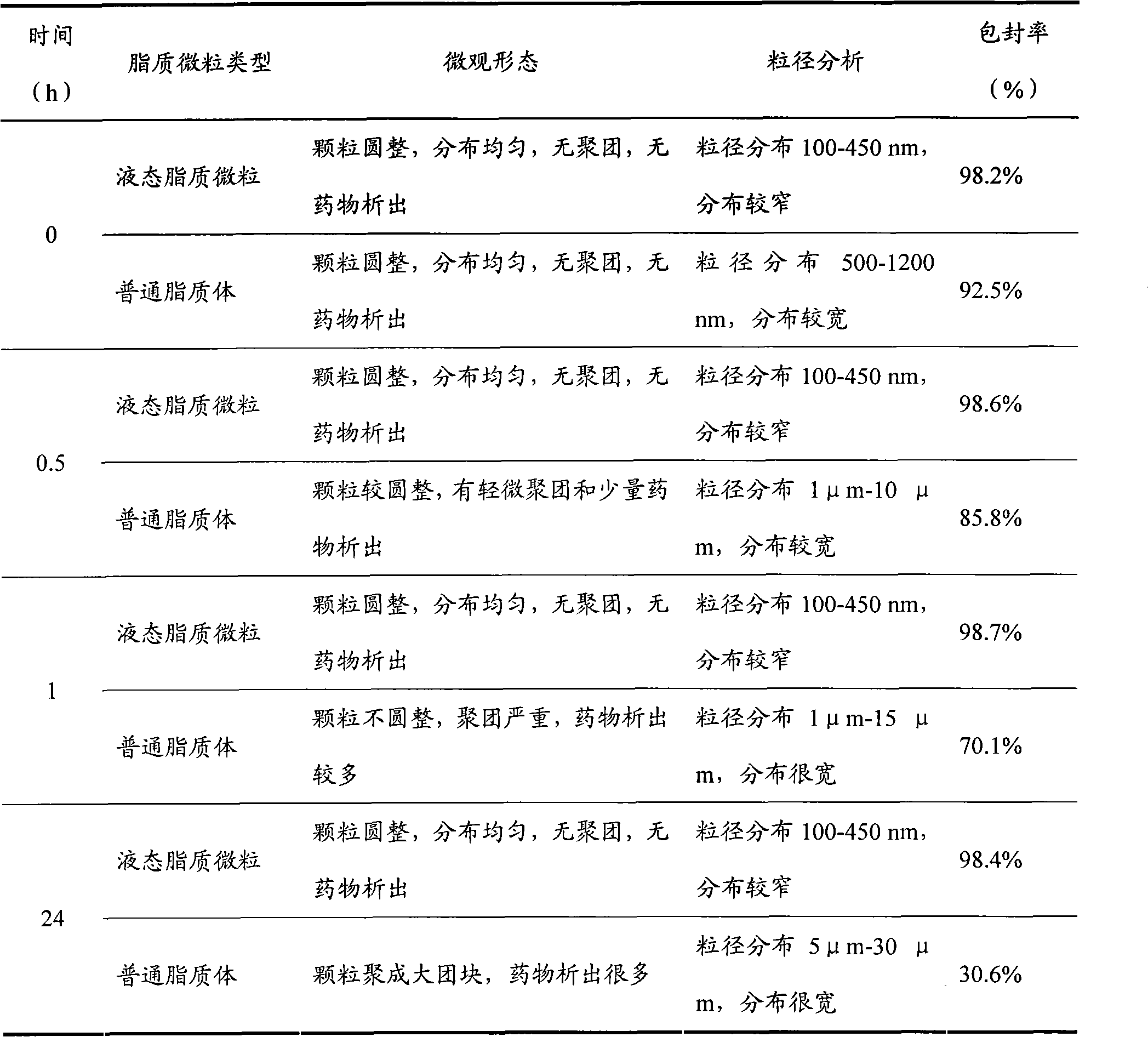
Liquid-state lipid micro-particles used for delivering cerebric medicine through olfactory pathway, preparation method thereof, and preparation thereof
InactiveCN102370623AStable structureImprove stabilityNervous disorderAerosol deliveryHydroxyethyl starchDisease
The invention relates to liquid-state lipid micro-particles used for delivering a cerebric medicine through a nose olfactory pathway, a preparation method thereof, and a preparation thereof. The liquid-state lipid micro-particles at least comprise a medicine, a lipid material, a penetrating agent, hydroxyethyl starch, propylene glycol and water. The mass ratio of the medicine to the lipid material is 1:1 to 1:10. The volume ratio of propylene glycol to water is 2:1 to 1:10. The liquid-state lipid micro-particles are advantaged in large drug load, high deformation capacity, simple preparation method, and high entrapment rate. The micro-particles are beneficial for the medicine to be delivered into the brain through the nose olfactory pathway and to provide curative effects. The range of appropriate medicines of the liquid-state lipid micro-particles is large, and the liquid-state lipid micro-particles are especially suitable for hormone, polypeptide, gene or vaccine medicines. With the liquid-state lipid micro-particles, preparations such as aerosols, nasal drops, and gels can be produced, such that requirements of cerebric disease medication can be satisfied.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Stem cell freezing medium and freezing method of stem cells
The invention provides a stem cell freezing medium and a freezing method of stem cells. The freezing medium contains dimethyl sulfoxide and hydroxyethyl starch and has great clinical application value. The invention also provides the freezing method of stem cells.The stem cell freezing medium in the invention is adopted by the method.In the invention,the cells frozen with the stem cell freezing medium has the advantage of high recovery / survival rate.
Owner:SHENZHEN BEIKE BIOTECH
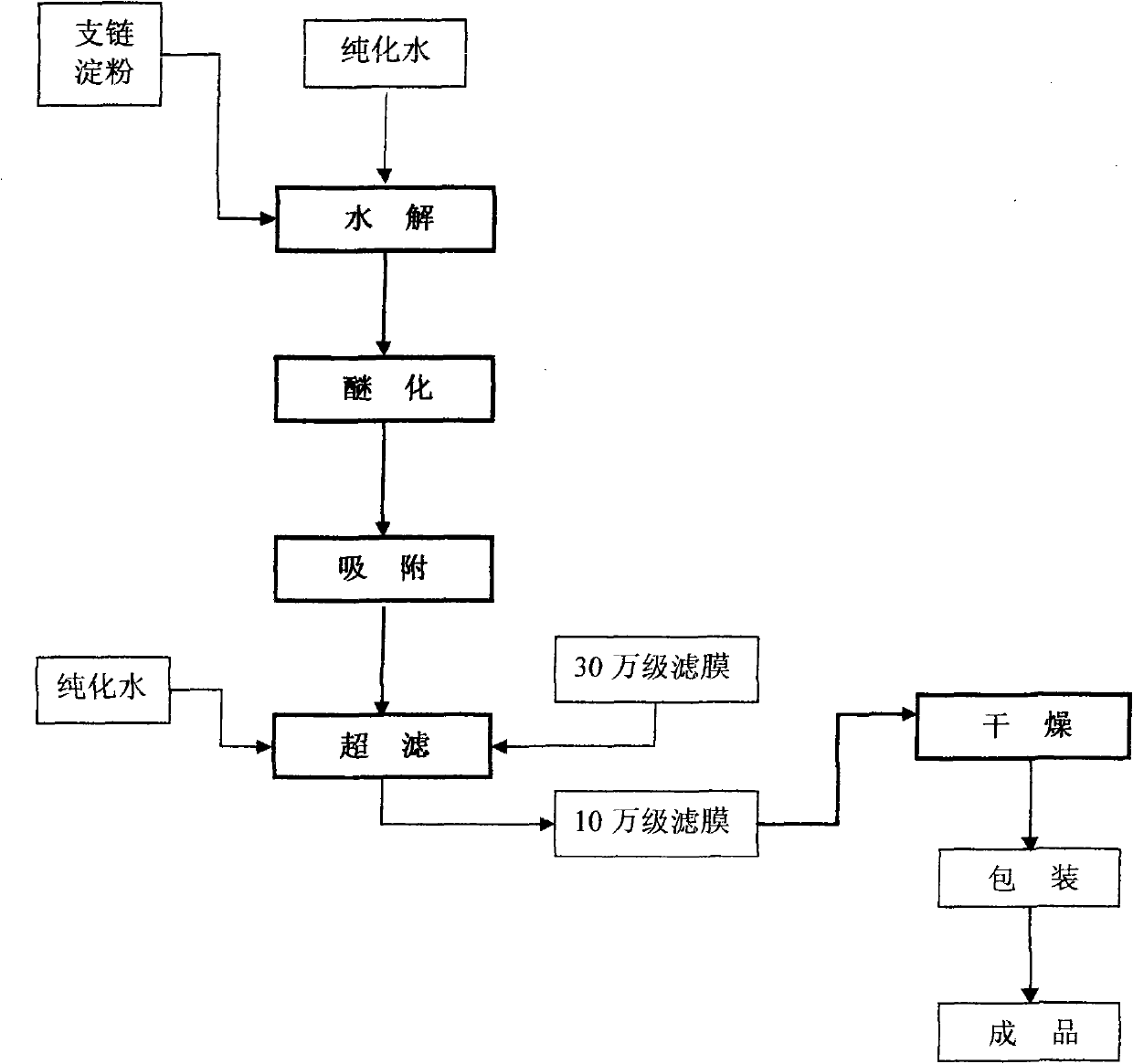
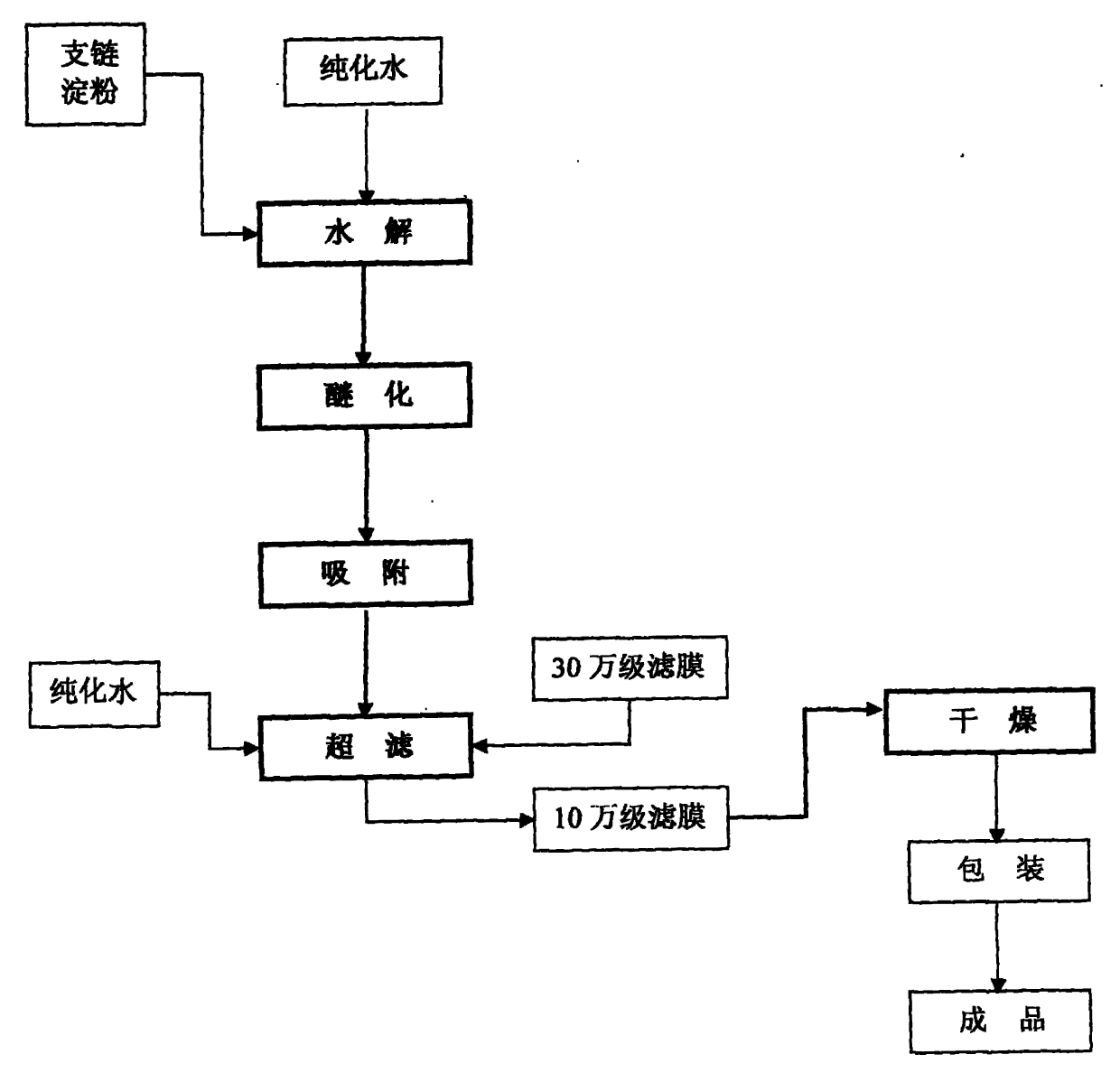
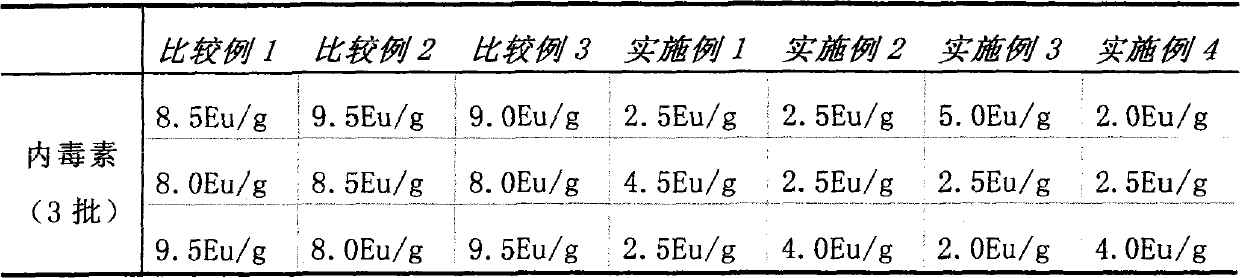
Preparation method for improving quality of medium molecular weight hydroxyethyl starch
The invention provides a new process for preparing medium molecular weight hydroxyethyl starch materials. In the ultrafiltration step of the production process flow, a filter membrane with the molecular mass cut-off of 300,000 and a filter membrane with the molecular mass cut-off of 100,000 are used in turn for ultrafiltration, and an ultrafiltration process of only using a filter membrane with the molecular mass cut-off of 100,000 is replaced, so that the content of endotoxin in the hydroxyethyl starch is effectively reduced, the molecular weight of the hydroxyethyl starch is more concentrated in a medium molecular weight range, the safety and quality of the product are improved, and the produced medium molecular weight hydroxyethyl starch (including hydroxyethyl starch 130 / 0.4 and hydroxyethyl starch 200 / 0.5) materials and preparations meet the standard of state pharmacopoeia.
Owner:CHONGQING DAXIN PHARMA +1
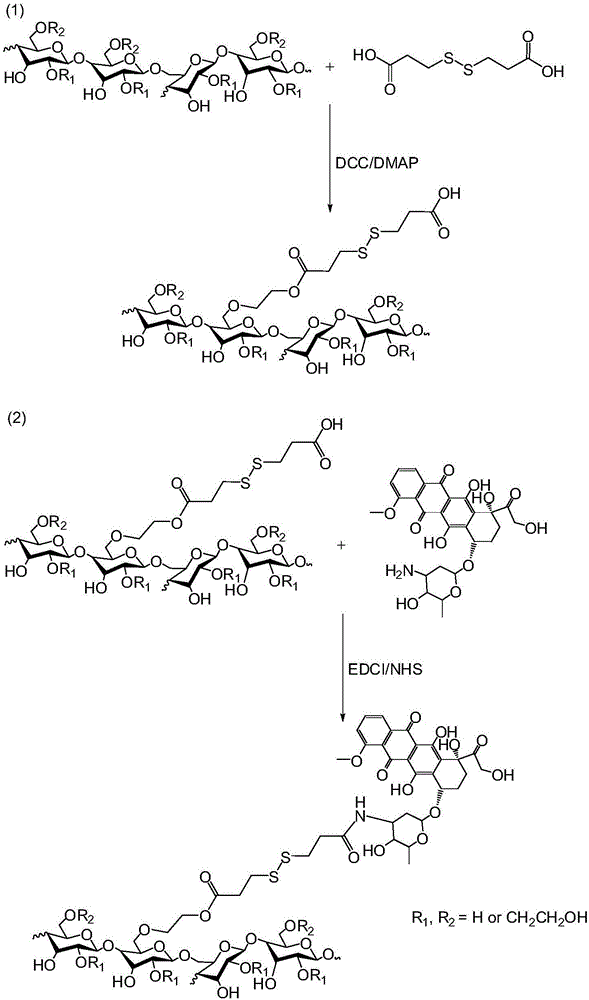
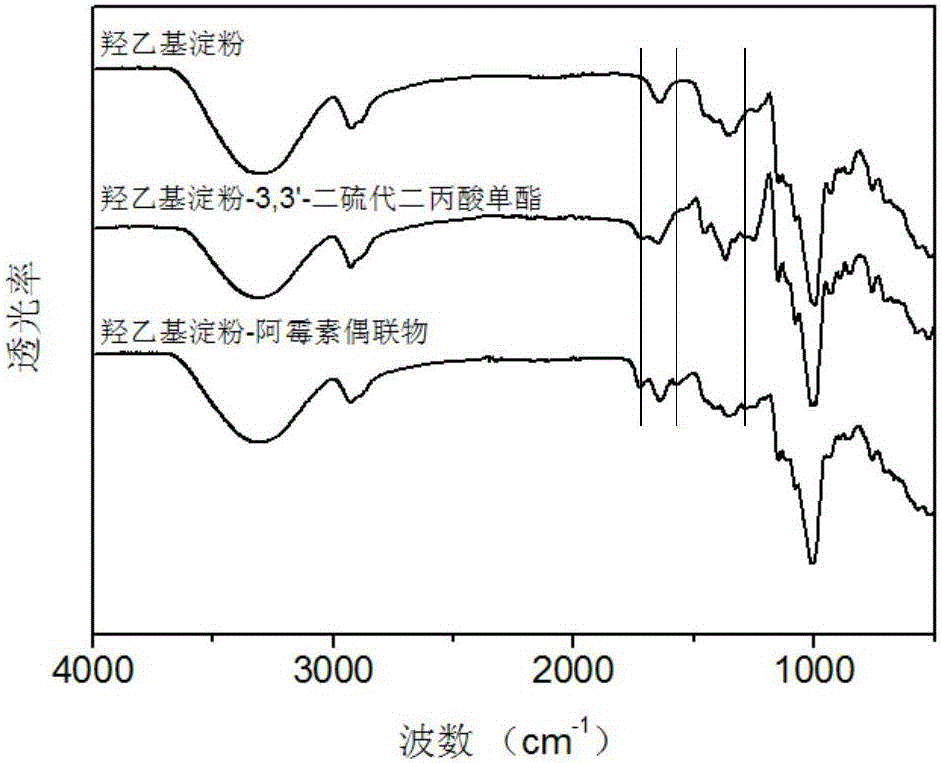
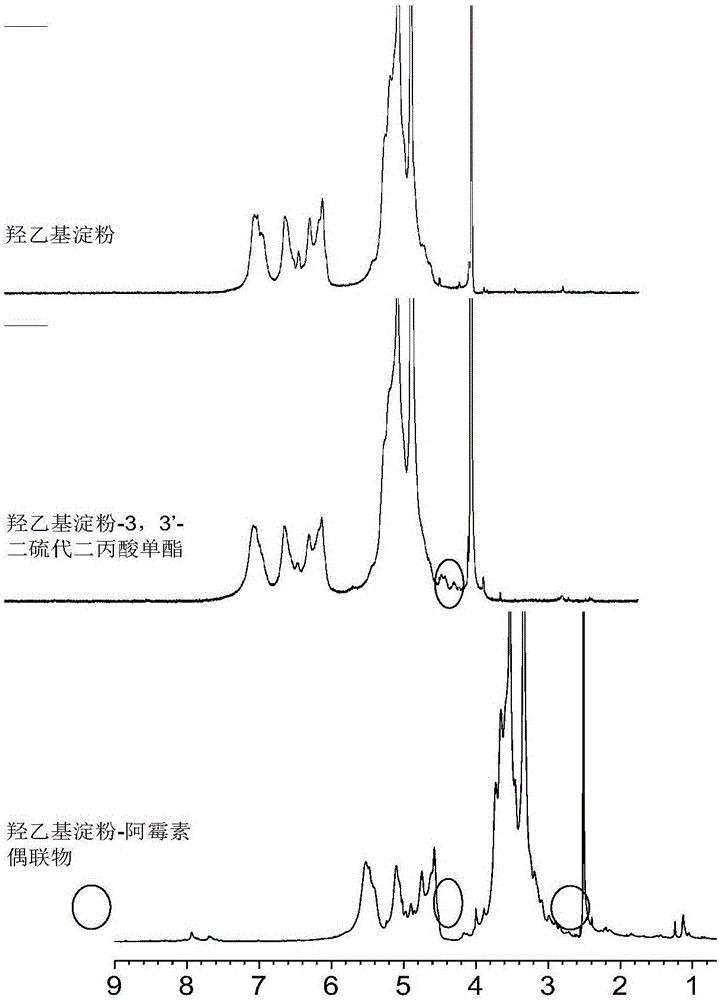
Anticancer hydroxyethyl starch-doxorubicin conjugate and preparation method thereof
InactiveCN106334195AExtend cycle timePromote enrichmentOrganic active ingredientsPharmaceutical non-active ingredientsHydroxyethyl starchTreatment effect
The invention discloses a hydroxyethyl starch-doxorubicin conjugate and preparation method and application thereof. The preparation method comprises the following steps: with hydroxyethyl starch as a medicine carrier, coupling 3,3'-dithiobispropionic acid onto hydroxyethyl of the hydroxyethyl starch through monoester, and coupling doxorubicin onto carboxyl of hydroxyethyl starch-3,3'-dithiobispropionic acid monoester through an amido bond in a dark condition to obtain the hydroxyethyl starch-doxorubicin conjugate with reducing responsiveness. The hydroxyethyl starch-doxorubicin conjugate prepared by the preparation method provided by the invention can prolong the cycle time of the doxorubicin in blood, enhance enrichment of the doxorubicin on a tumor site and improve the therapeutic effect of doxorubicin on a tumor; meanwhile, the hydroxyethyl starch-doxorubicin conjugate can be reduced by glutathione to release the doxorubicin in a tumor cell, and after release of the doxorubicin, the medicine carrier does not contain potentially toxic groups and can be metabolized, so that the hydroxyethyl starch-doxorubicin conjugate is high in safety; the preparation method provided by the invention is short in process, simple in operation and mild in condition.
Owner:HUAZHONG UNIV OF SCI & TECH
Amniotic membrane long-term preserving fluid and preparation method thereof
ActiveCN102132697AKeep aliveEasy batch processingDead animal preservationHydroxyethyl starchCulture fluid
The invention relates to an amniotic membrane long-term preserving fluid which is characterized in that 1000ml of preserving fluid contains 10g-200g of chondroitin sulfate and low-molecular-weight dextranum, 10g-50g of hydroxyethyl starch, 5ml-20ml of amino acid, 5ml-20ml of tobramycin, 10ml-50ml of HEPES, 5ml-30ml of ascorbic acid, 5ml-20ml of sodium pyruvate, and 860ml-970ml of culture solution. A preparation method of the amniotic membrane long-term preserving fluid comprises the following steps: adding the chondroitin sulfate, the low-molecular-weight dextranum, the amino acid, the tobramycin, the hydroxyethyl starch, the ascorbic acid and the sodium pyruvate into the culture solution and then uniformly mixing the mixture; using 1%-5% of HEPES to adjust pH value till the pH value is 7.2-7.4; using an osmotic pressure buffering agent to adjust the osmotic pressure till the osmotic pressure is 350-380mOsm / L; and performing membrane filtration and degerming, thereby acquiring the amniotic membrane long-term preserving fluid.
Owner:周海华
Serum-free and DMSO-free cell cryopreservation solution and preparation method thereof
InactiveCN110934132AHigh biosecurityHigh clinical application prospectsDead animal preservationHydroxyethyl starchCytokine
The invention discloses a serum-free and DMSO-free cell cryopreservation solution, which comprises a 16.762-16.825 g / L DMEM / F-12 basal culture medium, 32-34 mg / L cytokines, 0.2-0.8 mg / L hydroxyethyl starch, 1-3 mg / L vitamins, 0.15-0.16 g / L antibiotics, 5-6 mg / L protein polypeptide, 1-4 mg / L lipids and a 0.1-0.41 g / L intracellular and extracellular cryoprotectant. According to the technical scheme,the obtained cell cryopreservation solution does not contain serum or DMSO components, the components of the formula are determined, the cell cryopreservation solution has high biological safety andclinical application prospects, and the cell cryopreservation liquid has a cell recovery survival rate of more than 85%, can maintain good cell activity and good physiological characteristics, and issuitable for the related research fields of primary cells and stem cells.
Owner:苏州君欣生物科技有限公司
Separation method of human peripheral blood mononuclear cells
InactiveCN104480070AHigh purityOvercoming disadvantages in large volume blood samplesBlood/immune system cellsFicollHydroxyethyl starch
The invention discloses a separation method of human peripheral blood mononuclear cells. The separation method combines the advantages of a hydroxyethyl starch precipitation method and a Ficoll density gradient centrifugation method. The PBMC with a purity of greater than 95% and a vitality of greater than 85% is obtained by removing most of red blood cells by the hydroxyethyl starch precipitation method, then naturally settling in air, and then further purifying the mononuclear cells by the Ficoll density gradient centrifugation method, aiming at the separation characteristic of a high-capacity blood sample. Compared with a method without natural setting in air, the vitality and the purity of the cells obtained by the separation method are higher.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
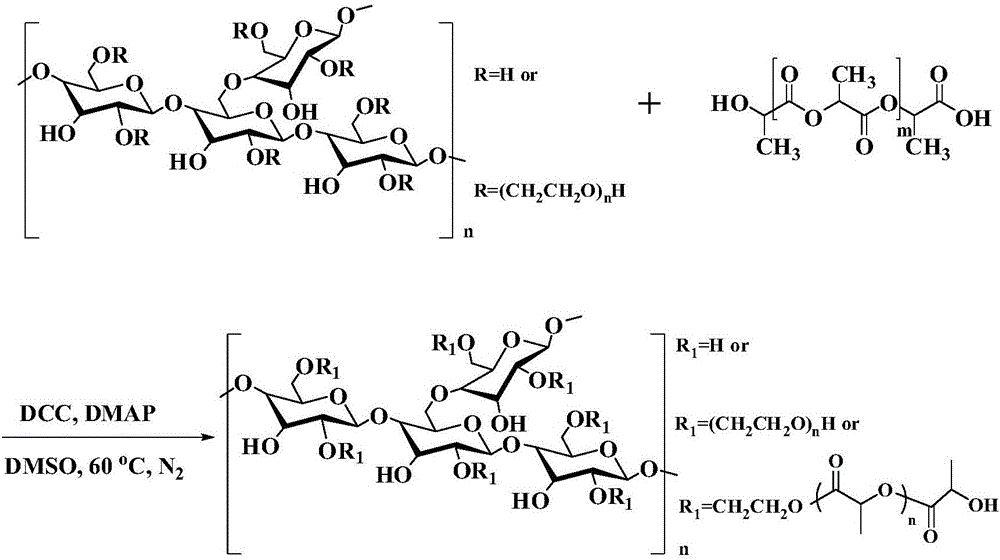
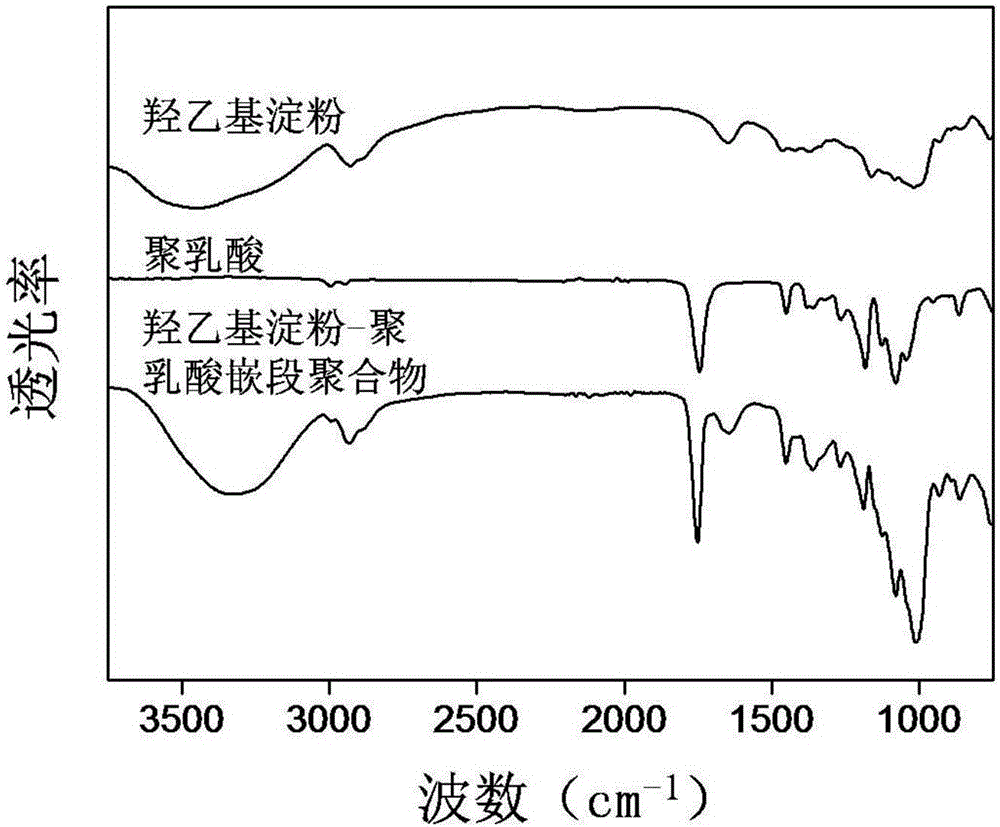
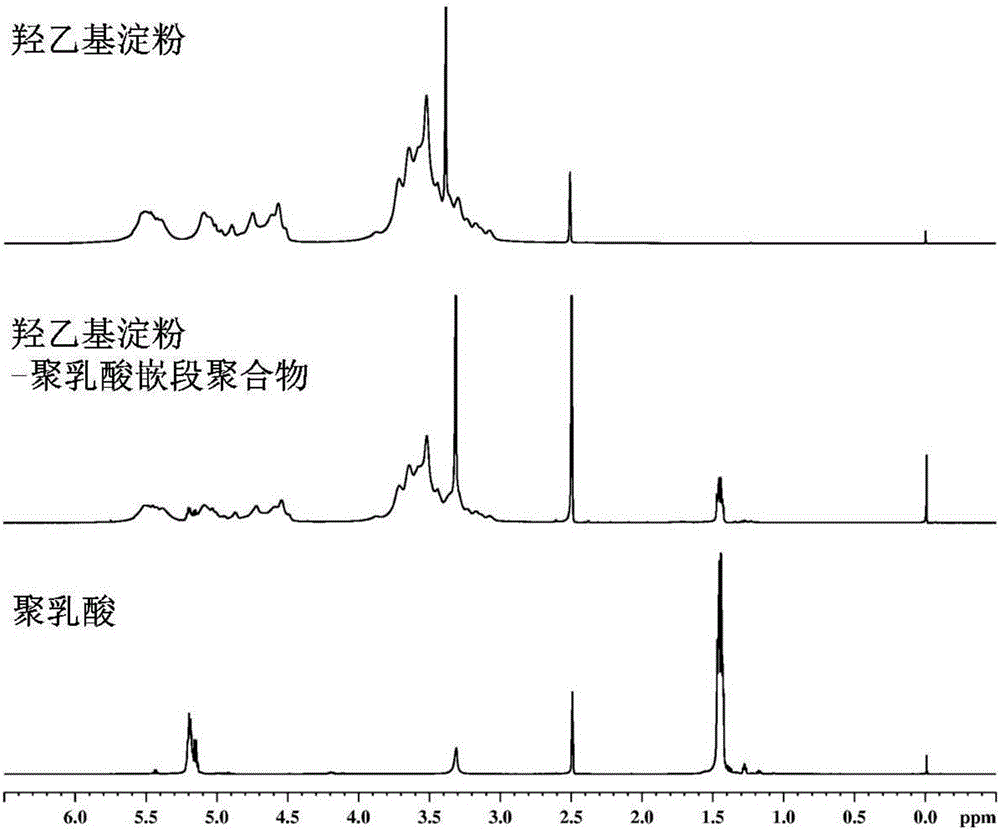
Amphipathy hydroxyethyl-starch-coupled-polylactic-acid copolymer and preparing method and application thereof
ActiveCN106432746AUniform chain lengthHigh degree of polymerizationOrganic active ingredientsPharmaceutical non-active ingredientsHydroxyethyl starchSide effect
The invention discloses an amphipathy block-polymer hydroxyethyl-starch-olylactic-acid copolymer and a preparing method and nanometer medicine loading system thereof. The method includes the steps that 4-dimethylaminopyridine serves as a catalyst, N-N'-dicyclohexylcarbodiimide serves as a dehydrating agent, carboxyl at the tail end of polylactic acid and hydroxyl on hydroxyethyl-starch sugar ring are subjected o an esterification reaction, hydrophobic polylactic acid is coupled on hydrophilic hydroxyethyl starch accordingly, and the amphipathy hydroxyethyl-starch-polylactic-acid block polymer is synthesized. Debydrochlorination adriamycin amycin is loaded to a hydrophobic core with the emulsified solvent evaporation method and the high pressure homogenization technology, evenly-distributed medicine loading polymer nanometer particles with the particle-size of about 140 nm are formed, and are applied in preparing antitumor medicine, the medicine in-vivo cycling time is prolonged, the toxic and side effects of the medicine are reduced, and the good antitumor effect is achieved.
Owner:HUAZHONG UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com