Umbilical cord mesenchymal stem cell injection as well as preparation method and application thereof
A stem cell and injection technology, applied in the field of biomedicine, can solve the problems of unclear pathogenesis of inflammatory bowel disease, reduced or ineffective drug response, serious drug toxicity and side effects, etc. The effect of increasing the rate and reducing the toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
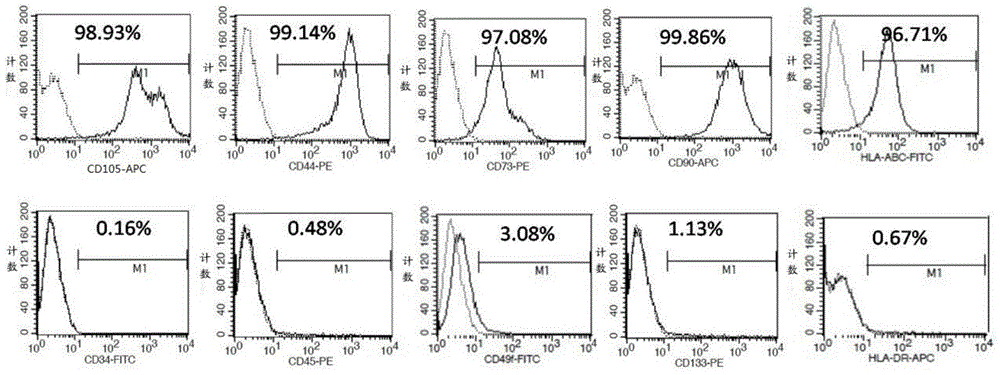
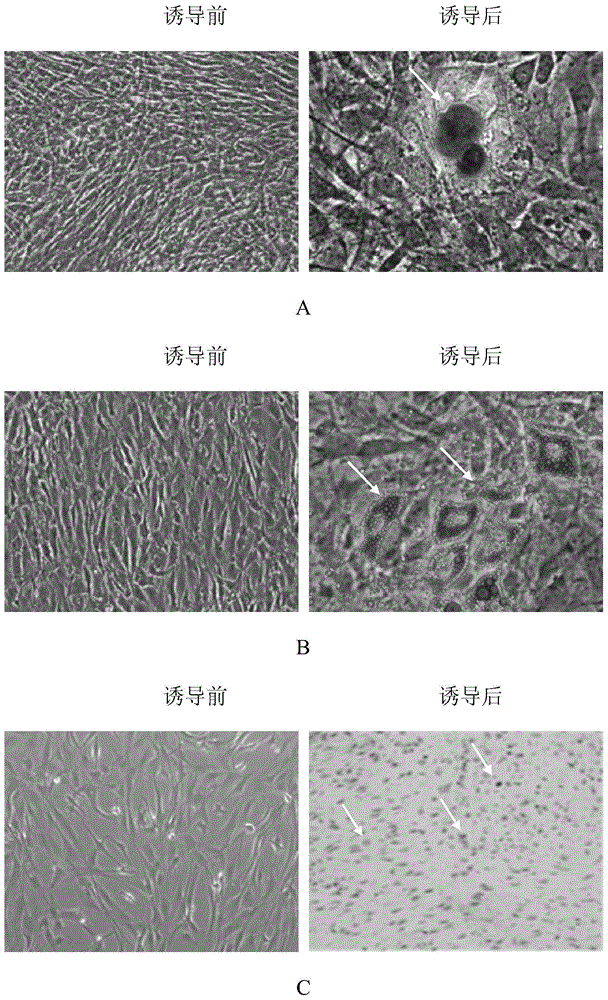
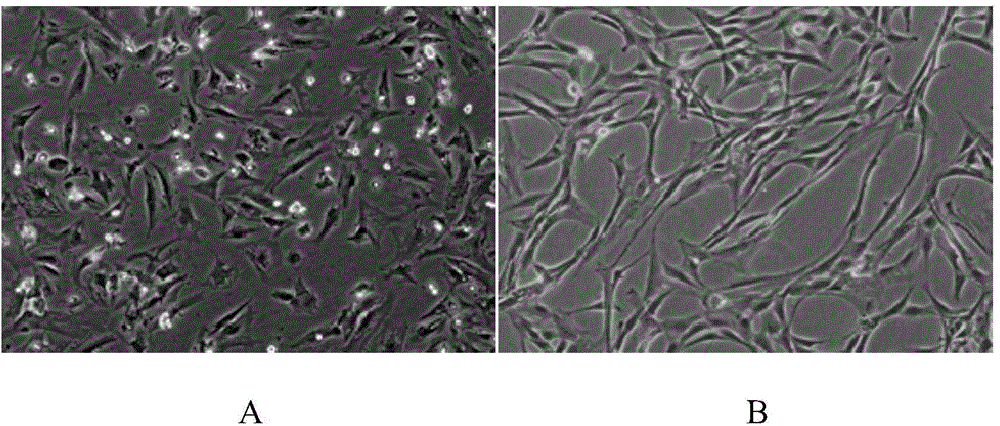
[0076] In this example, the umbilical cord mesenchymal stem cells were isolated and cultured by the following method, and the specific steps were as follows:
[0077] Take fresh umbilical cords that have left the mother’s body for no more than 2 hours after cesarean section, and check whether the mother is carrying infectious disease pathogens (hepatitis B surface antigen, hepatitis C antibody, syphilis antibody, AIDS antibody, negative), and place the fresh umbilical cord containing D-Hanks balance Stored in a special storage bottle for the liquid, transported in a low-temperature ice box;
[0078] The biosafety cabinet was irradiated with ultraviolet rays for 30 minutes, and the water bath shaker was preheated at 37°C in advance, and the umbilical cord sample bottle was placed in the biosafety cabinet for operation. Take out the sample with long hemostatic forceps and place it in a 150mm sterile glass dish, and wash away the blood on the surface of the umbilical cord with D-...
Embodiment 2
[0089] In this example, the umbilical cord mesenchymal stem cell injection is prepared by the following method, the injection contains mesenchymal stem cells and the cryopreservation solution, wherein the cryopreservation solution contains the following components:
[0090] Bomaili A injection with a volume percentage of 44%;
[0091] 6% hydroxyethyl starch 130 / 0.4 with a mass volume percentage in g / mL;
[0092] Adenosine triphosphate disodium magnesium chloride with a mass volume percentage of 10% in g / mL;
[0093] 10% clinical grade DMSO by volume;
[0094] 20% human albumin at 30% by volume.
[0095] Prepared by the following method, specifically comprising the following steps:
[0096] (1) Bomaili A injection (Shanghai Baite Medical Supplies Co., Ltd.), hydroxyethyl starch 130 / 0.4 (Qidu Pharmaceutical), adenosine triphosphate disodium magnesium chloride (Yabao Pharmaceutical Group Co., Ltd.) and 20 % Human serum albumin (Shanghai Raas Blood Products Co., Ltd.) was mixe...
Embodiment 3
[0114] In this example, the umbilical cord mesenchymal stem cell injection is prepared by the following method, the injection contains mesenchymal stem cells and the cryopreservation solution, wherein the cryopreservation solution contains the following components:
[0115] Volume percentage content is 40% Naringer acetate injection;
[0116] 6% hydroxyethyl starch 130 / 0.4 with a mass volume percentage in g / mL;
[0117] Adenosine triphosphate disodium magnesium chloride with a mass volume percentage of 10% in g / mL;
[0118] 10% clinical grade DMSO by volume;
[0119] 20% human albumin at 34% by volume.
[0120] Prepared by the following method, specifically comprising the following steps:
[0121] (1) Mix Nalinger Acetate Injection (Hunan Kangyuan Pharmaceutical Co., Ltd.), hydroxyethyl starch 130 / 0.4, adenosine triphosphate disodium magnesium chloride and 20% human serum albumin according to the above volume percentage, and use a 0.22 μm bacterial filter filter, reserve; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com