Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Clinical grade" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
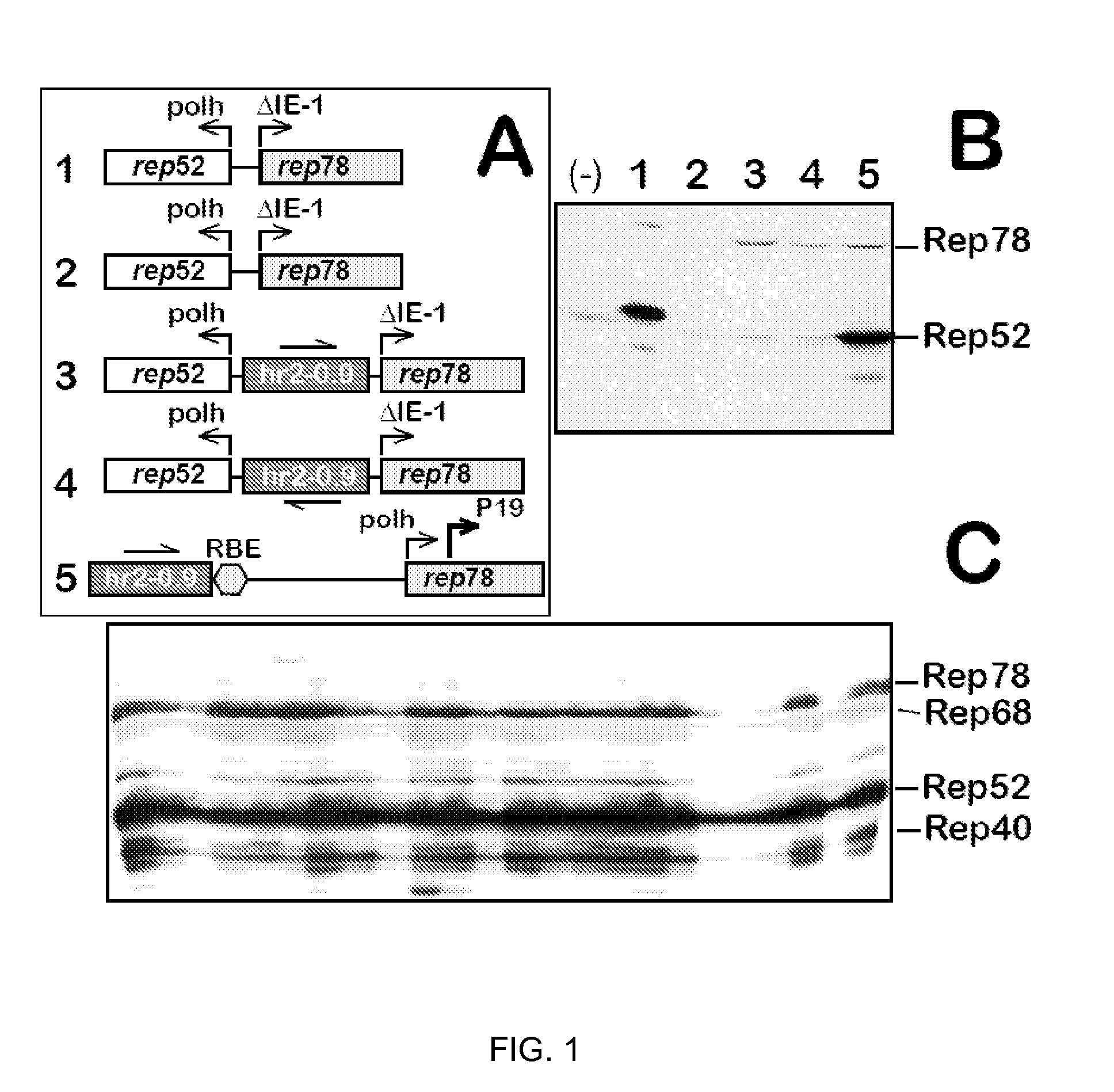
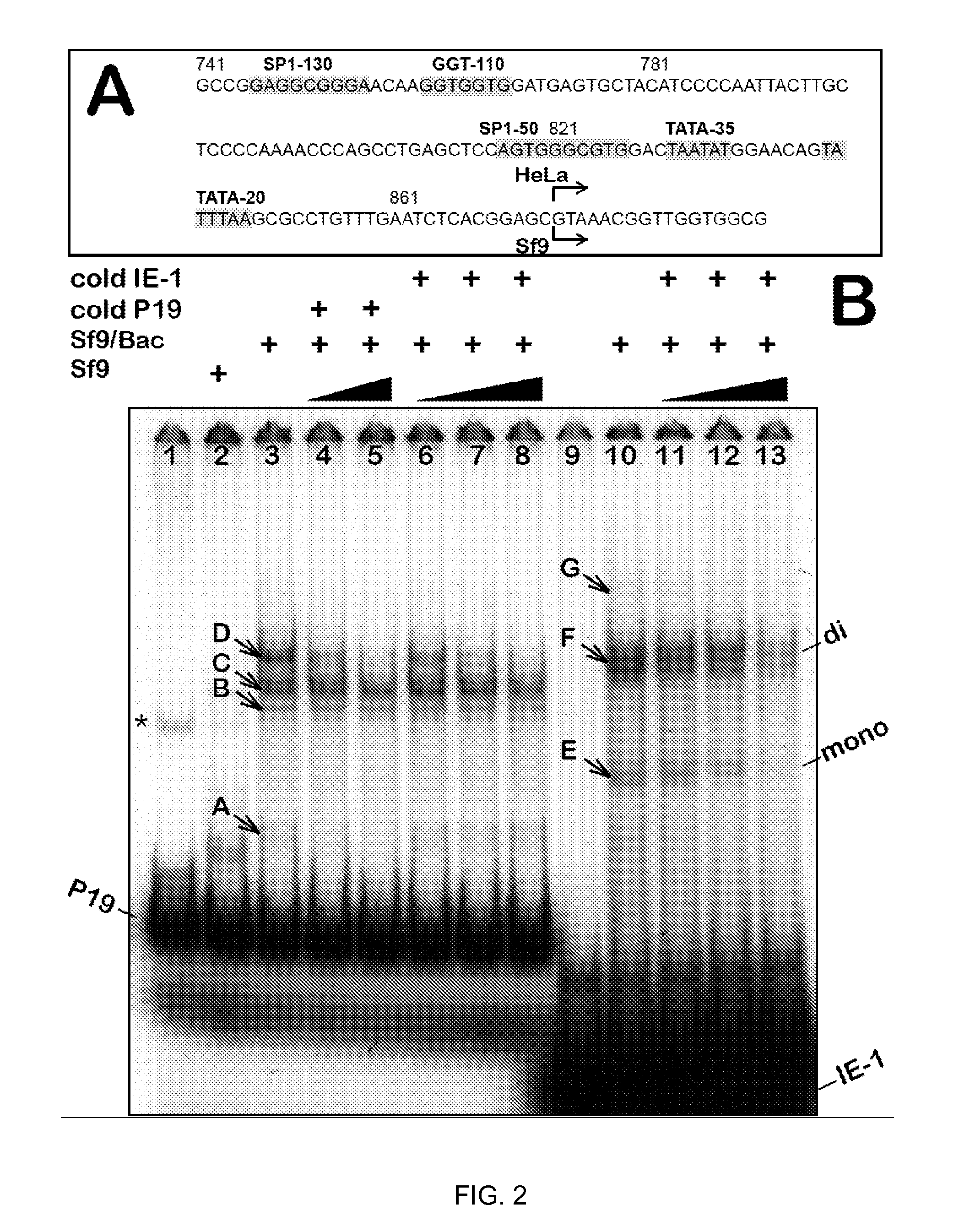
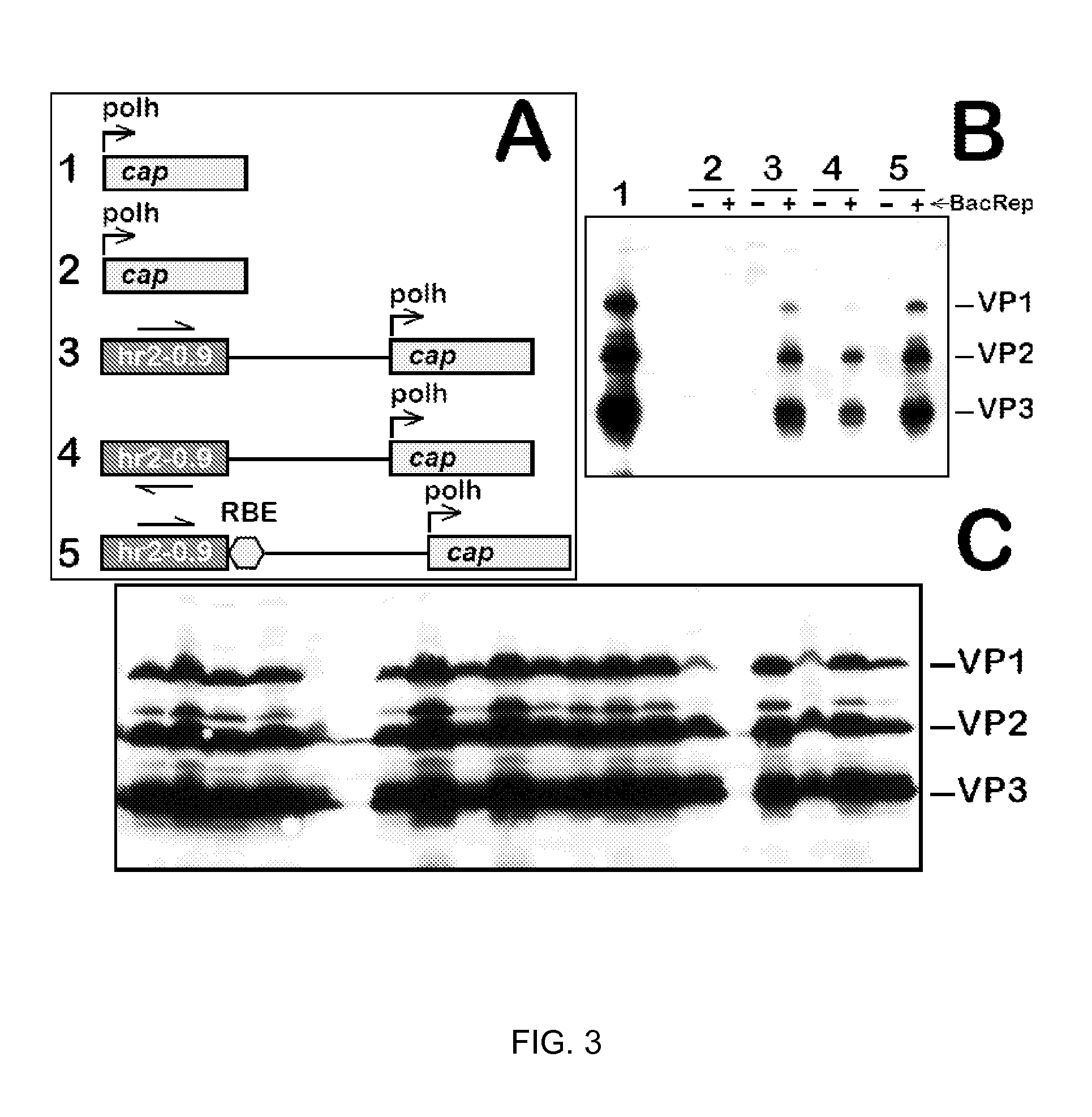
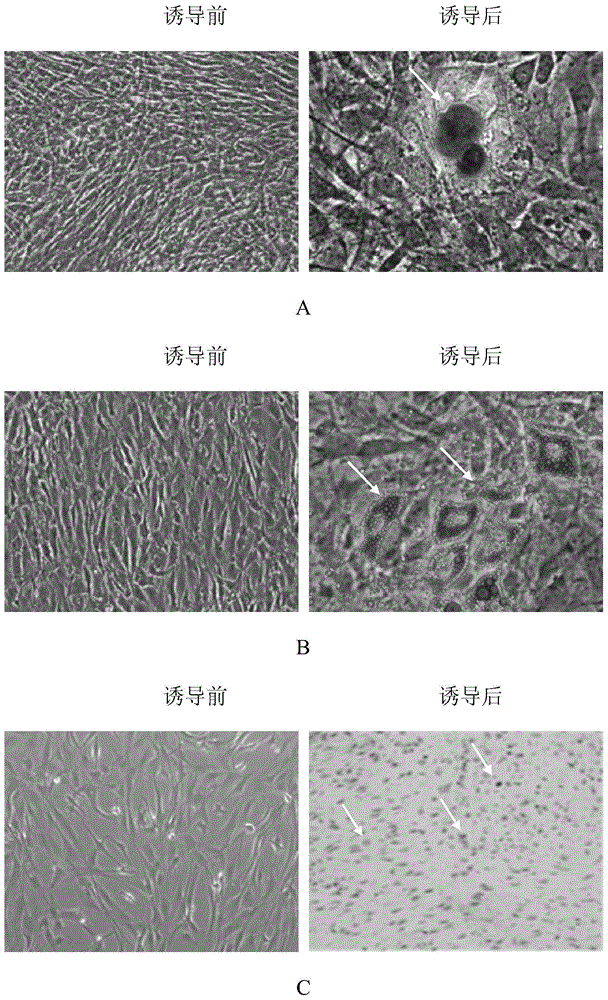
Inducible System for Highly Efficient Production of Recombinant Adeno-Associated Virus (rAAV) Vectors
ActiveUS20120100606A1High production costHigh yieldAnimal cellsNucleic acid vectorDiseaseClinical grade
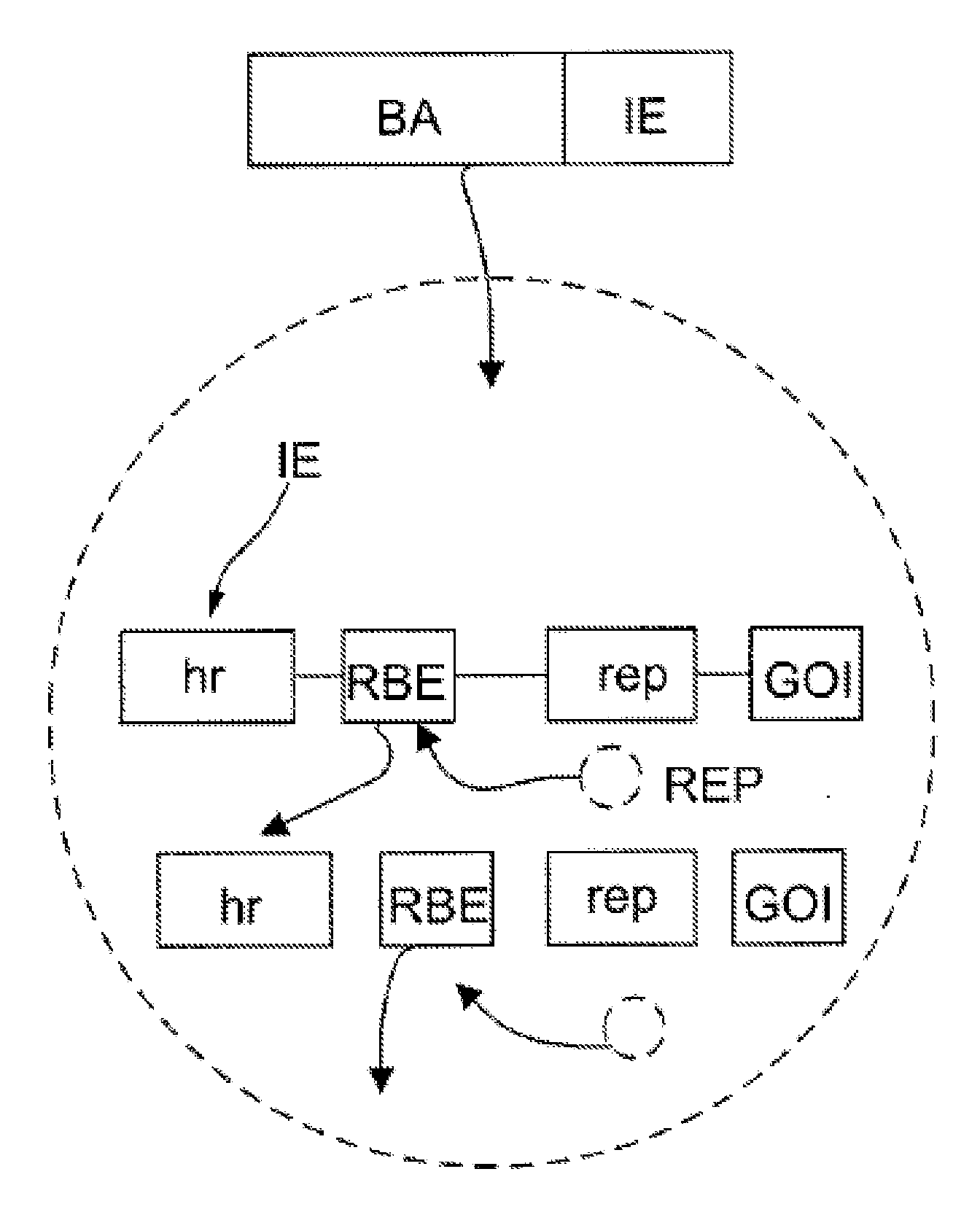
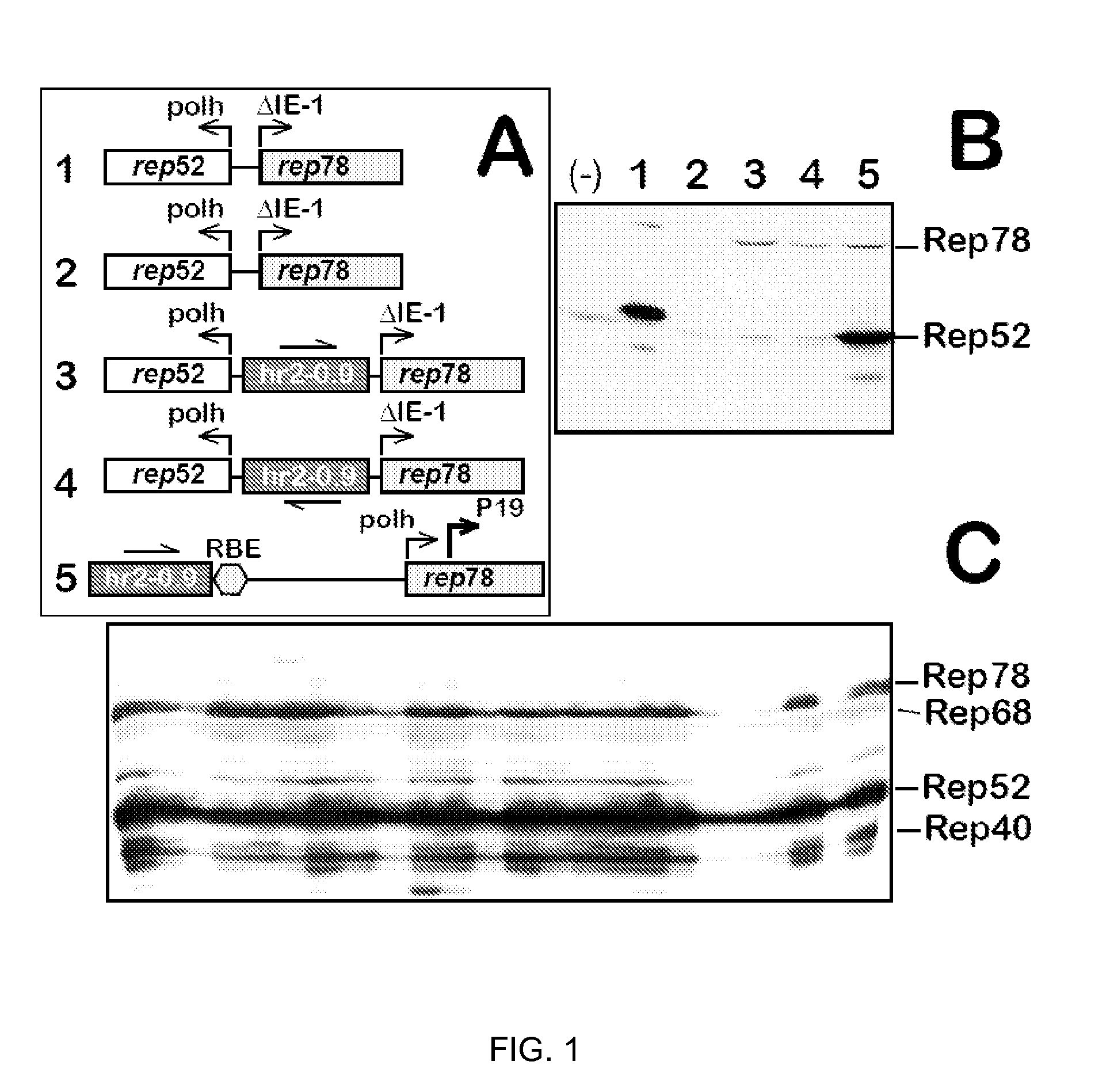
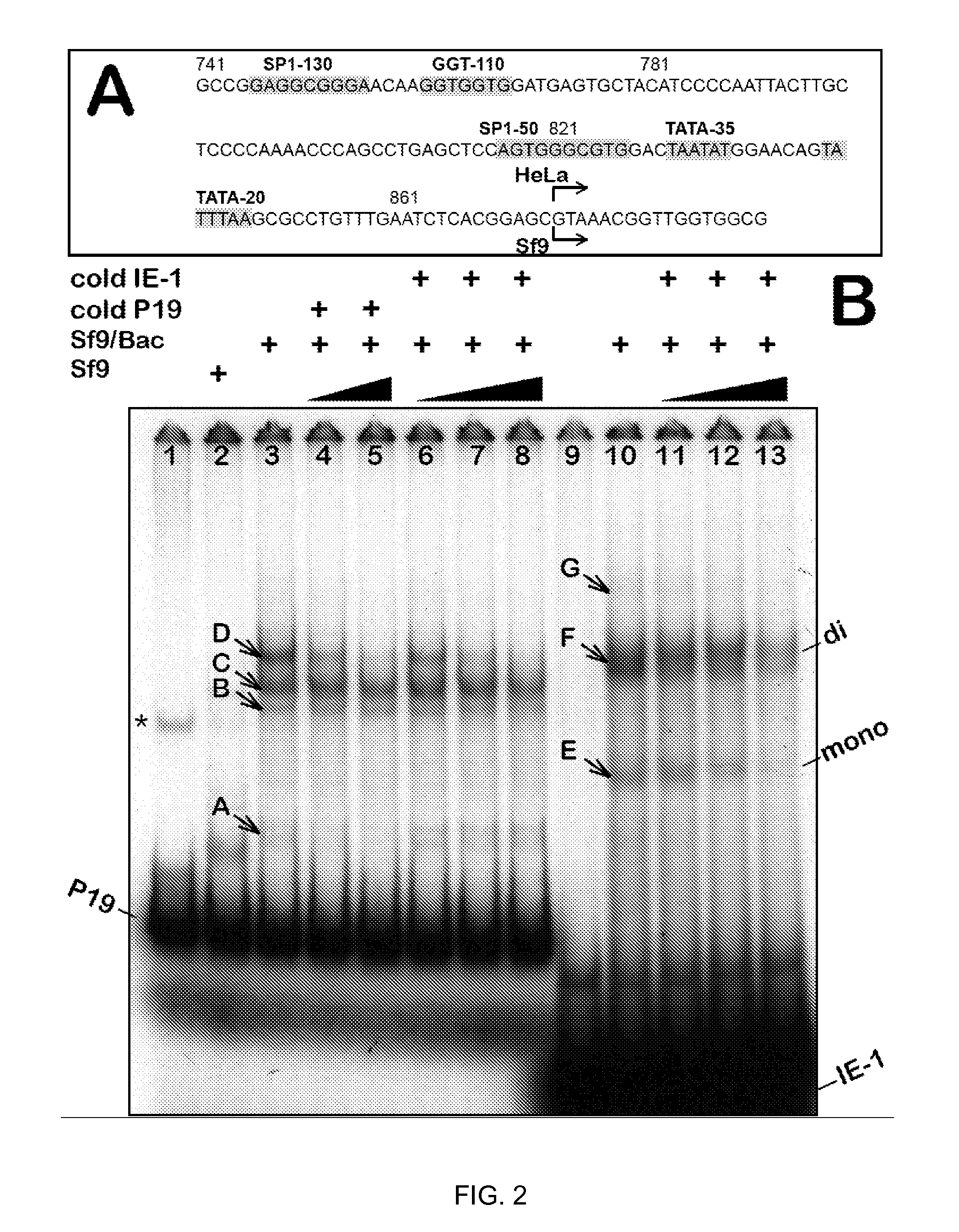
Production of clinical grade gene therapy vectors for human trials remains a major hurdle in advancing cures for a number of otherwise incurable diseases. Disclosed herein are systems based on a stably trans formed insect cell lines harboring helper genes required for vector production. Specifically exemplified are system embodiments that take advantage of DNA regulatory elements from two unrelated viruses—AcMNPV and AA V2. System embodiments utilize rep and / or cap genes either stably transfected in cell lines or which are introduced into cells as an expression cassette in a vector. Rep and cap genes that are designed to remain silent until the cell is infected with a viral vector. Infection with viral initiates rescue / amplification of integrated AAV helper genes resulting in dramatic induction of the expression and assembly of rAAV. The arrangement of this specific embodiment provides high levels of Rep and Cap proteins in every cell thus improving rAAV yields by 10-fold. The described vectors are modular in design and may be utilized for the production of other multiprotein complexes.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
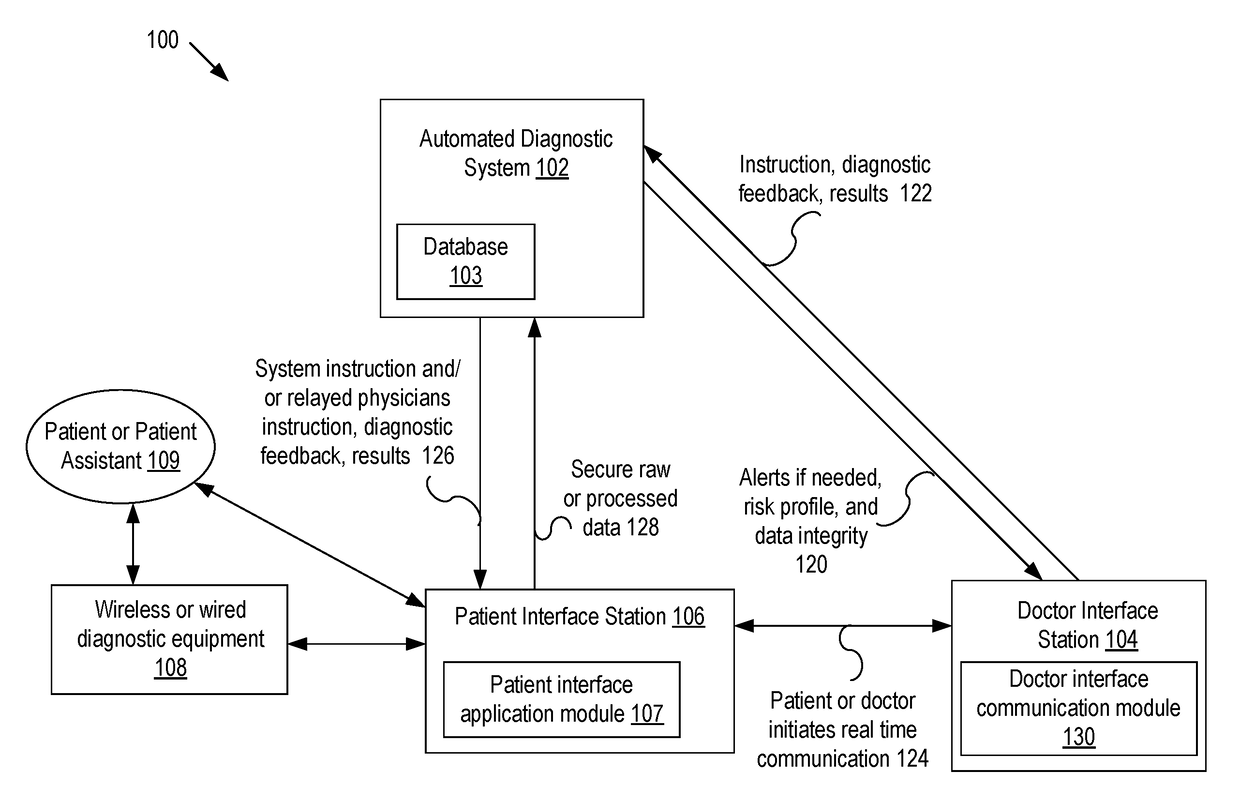
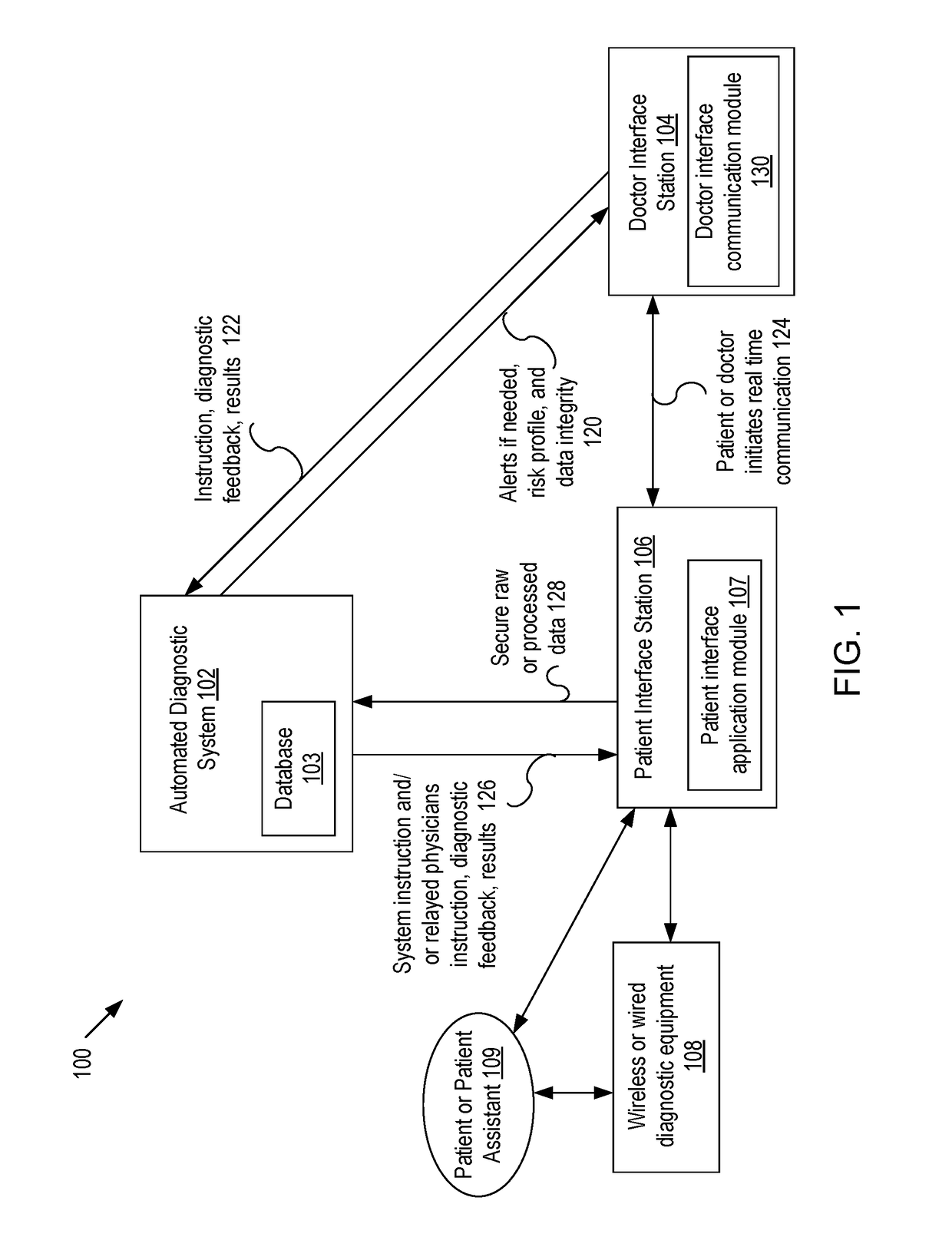
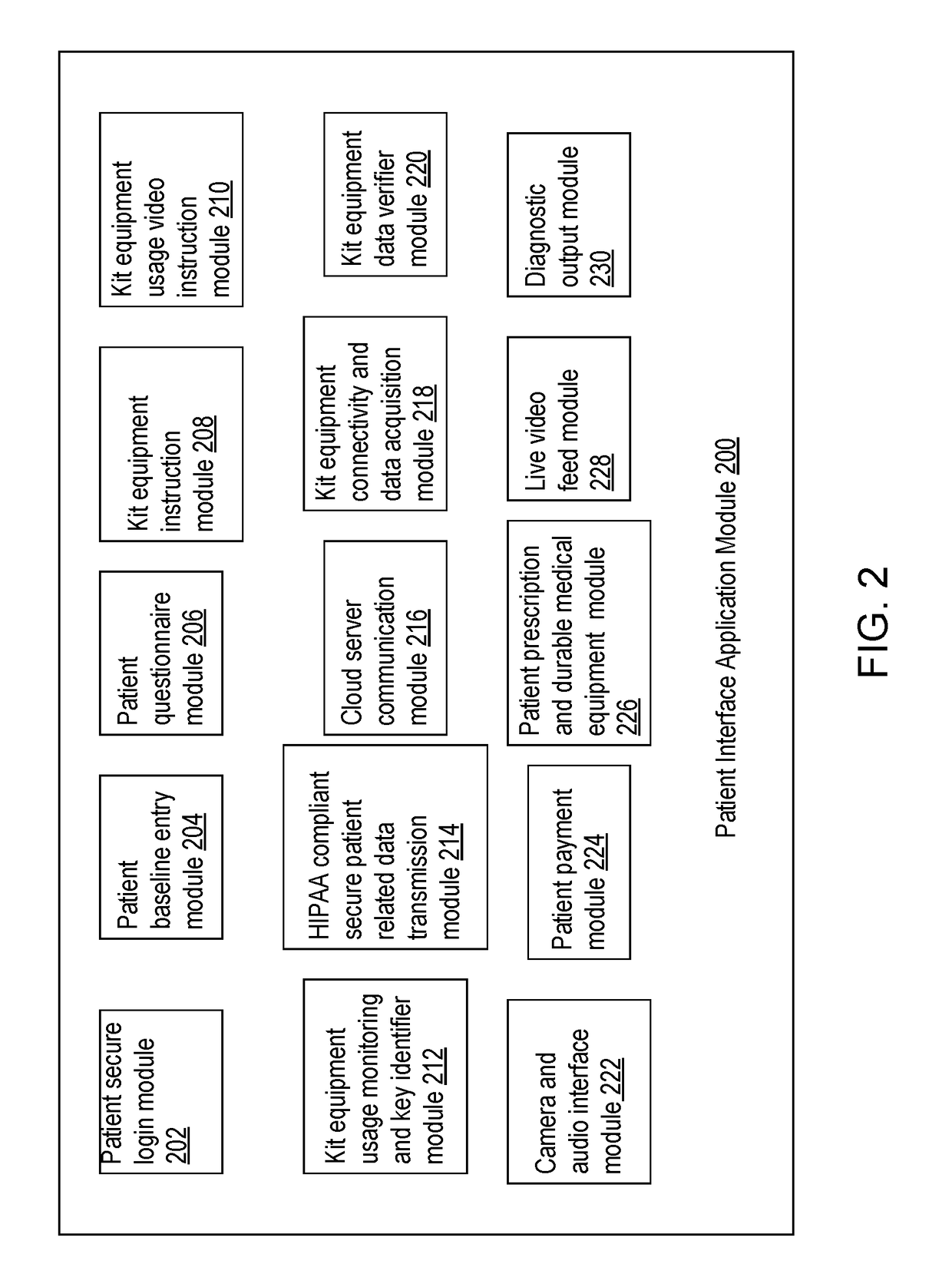
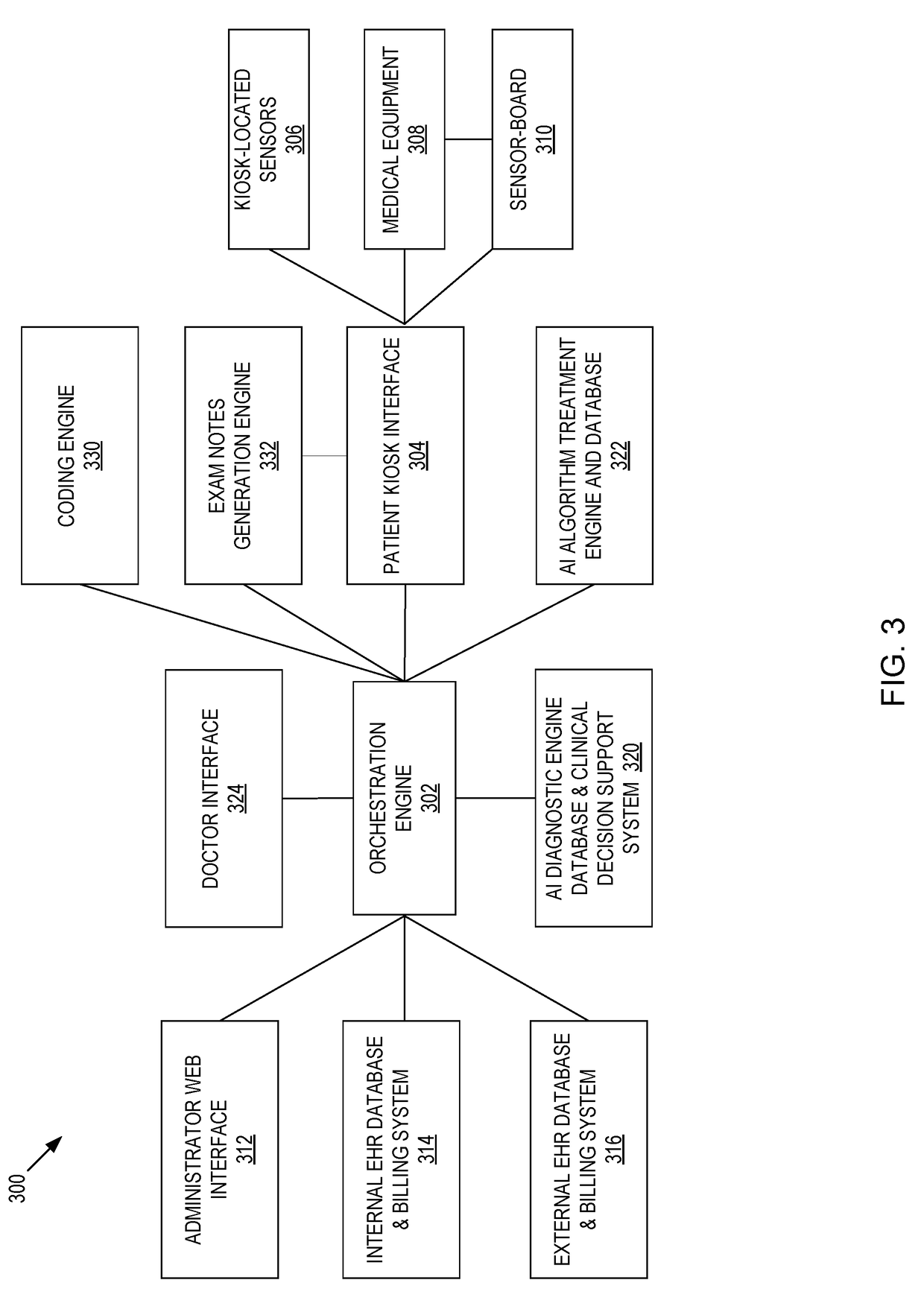
Systems and methods for automated medical diagnostics
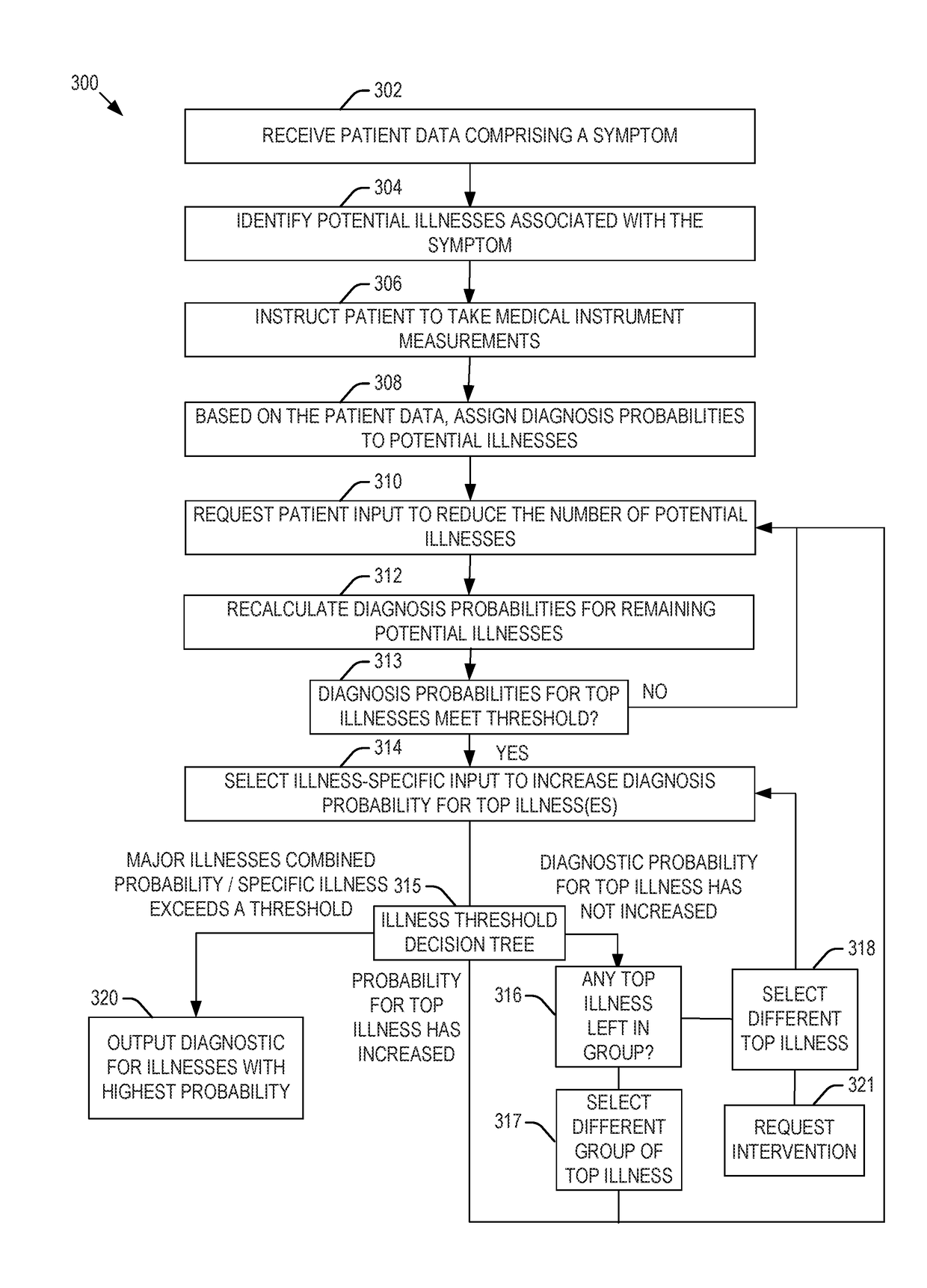
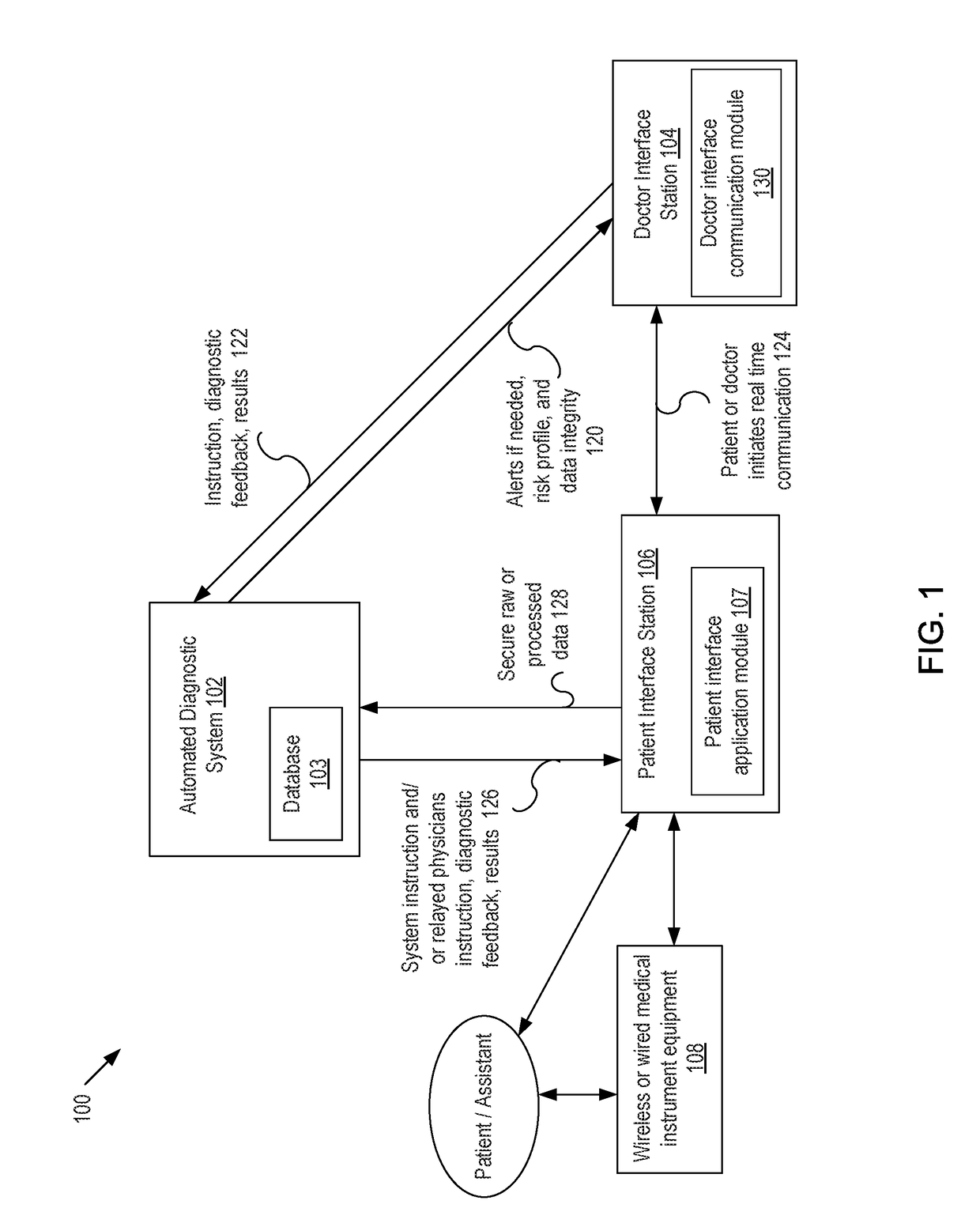
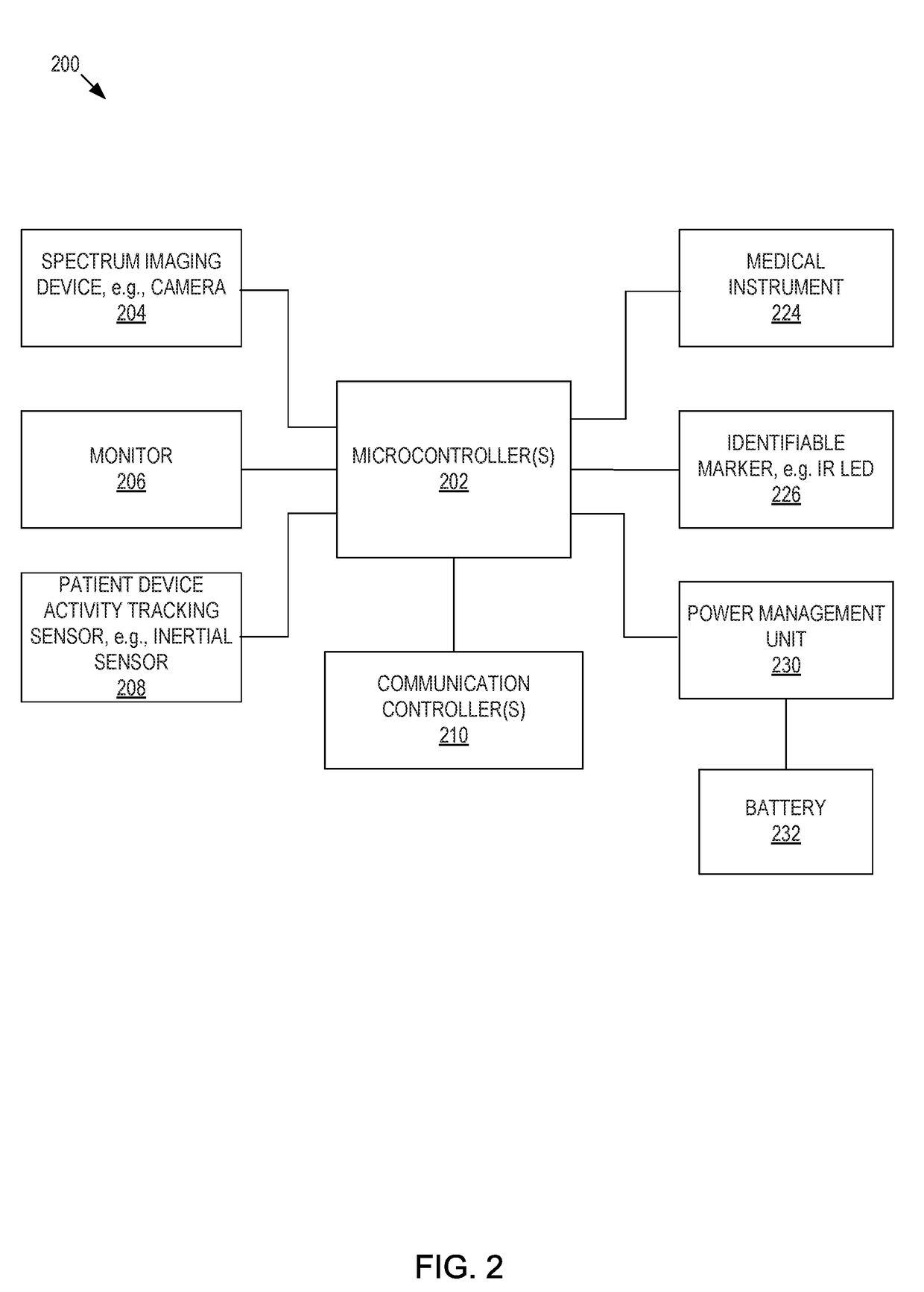
Presented are systems and methods that provide patients with diagnostic measurement tools and clear and concise audio / video guidance to reliably and accurately perform clinical grade diagnostic measurements of key vital signs. In various embodiments, this is accomplished by using an automated remote (or local, e.g., in the form of a kiosk) end-to-end medical diagnostic system that monitors equipment usage for accuracy. The diagnostic system analyzes patient responses, measurement data, and patient-related information to generate diagnostic and / or treatment information that may be shared with healthcare professionals and specialists, as needed. Automation provides for timely, hassle-free, and cost-effective health care management that takes the stress out of doctor visits, while delivering personalized health care. The high accuracy of generated diagnostic data improves health care to patients and reduces the risk of medical malpractice for treating physicians.
Owner:ADVINOW INC
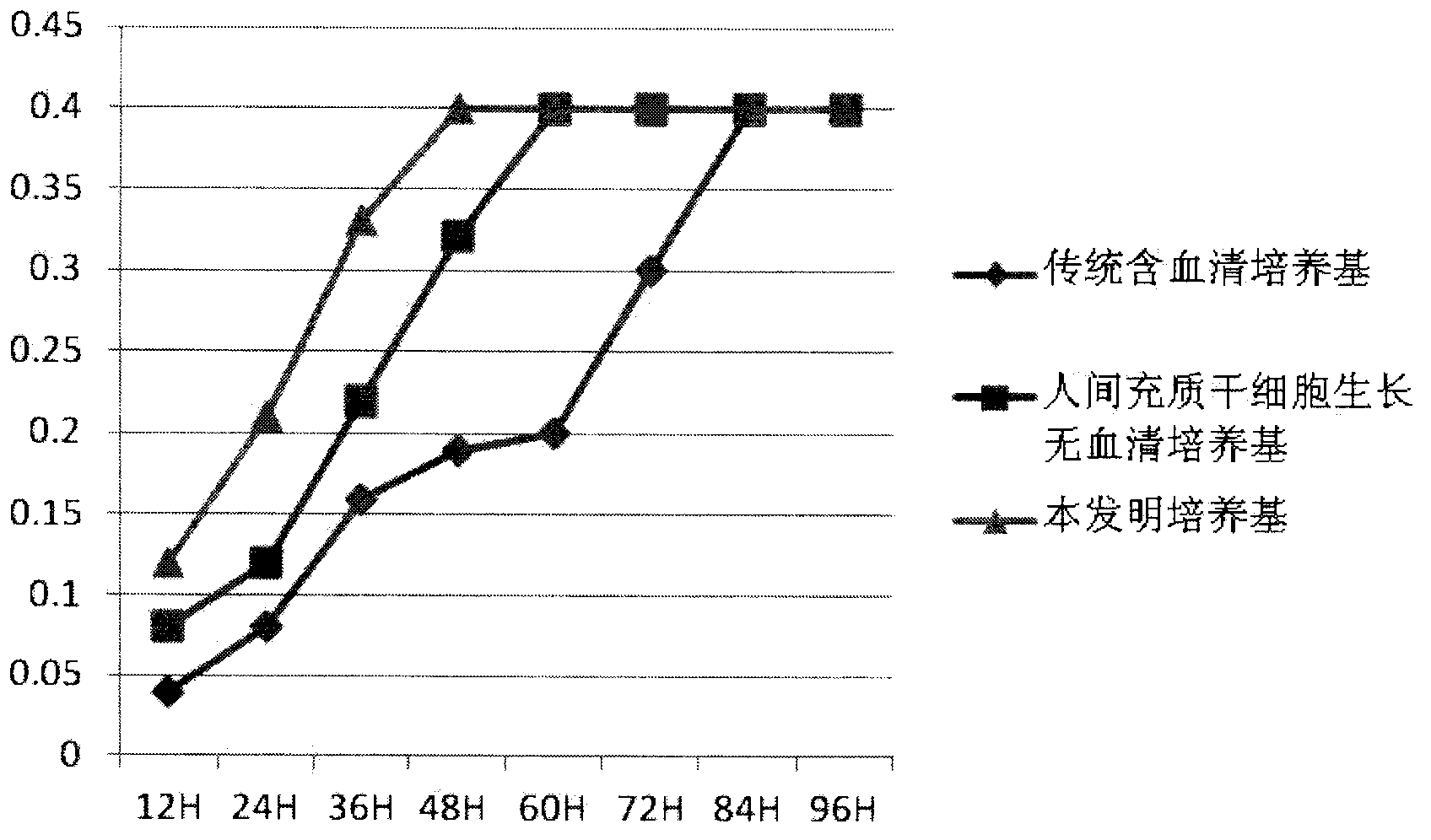
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Inducible system for highly efficient production of recombinant Adeno-associated virus (rAAV) vectors
ActiveUS8679837B2High production costHigh yieldBiocideGenetic material ingredientsClinical gradeDisease
Production of clinical grade gene therapy vectors for human trials remains a major hurdle in advancing cures for a number of otherwise incurable diseases. Disclosed herein are systems based on a stably trans formed insect cell lines harboring helper genes required for vector production. Specifically exemplified are system embodiments that take advantage of DNA regulatory elements from two unrelated viruses—AcMNPV and AA V2. System embodiments utilize rep and / or cap genes either stably transfected in cell lines or which are introduced into cells as an expression cassette in a vector. Rep and cap genes that are designed to remain silent until the cell is infected with a viral vector. Infection with viral initiates rescue / amplification of integrated AAV helper genes resulting in dramatic induction of the expression and assembly of rAAV. The arrangement of this specific embodiment provides high levels of Rep and Cap proteins in every cell thus improving rAAV yields by 10-fold. The described vectors are modular in design and may be utilized for the production of other multiprotein complexes.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
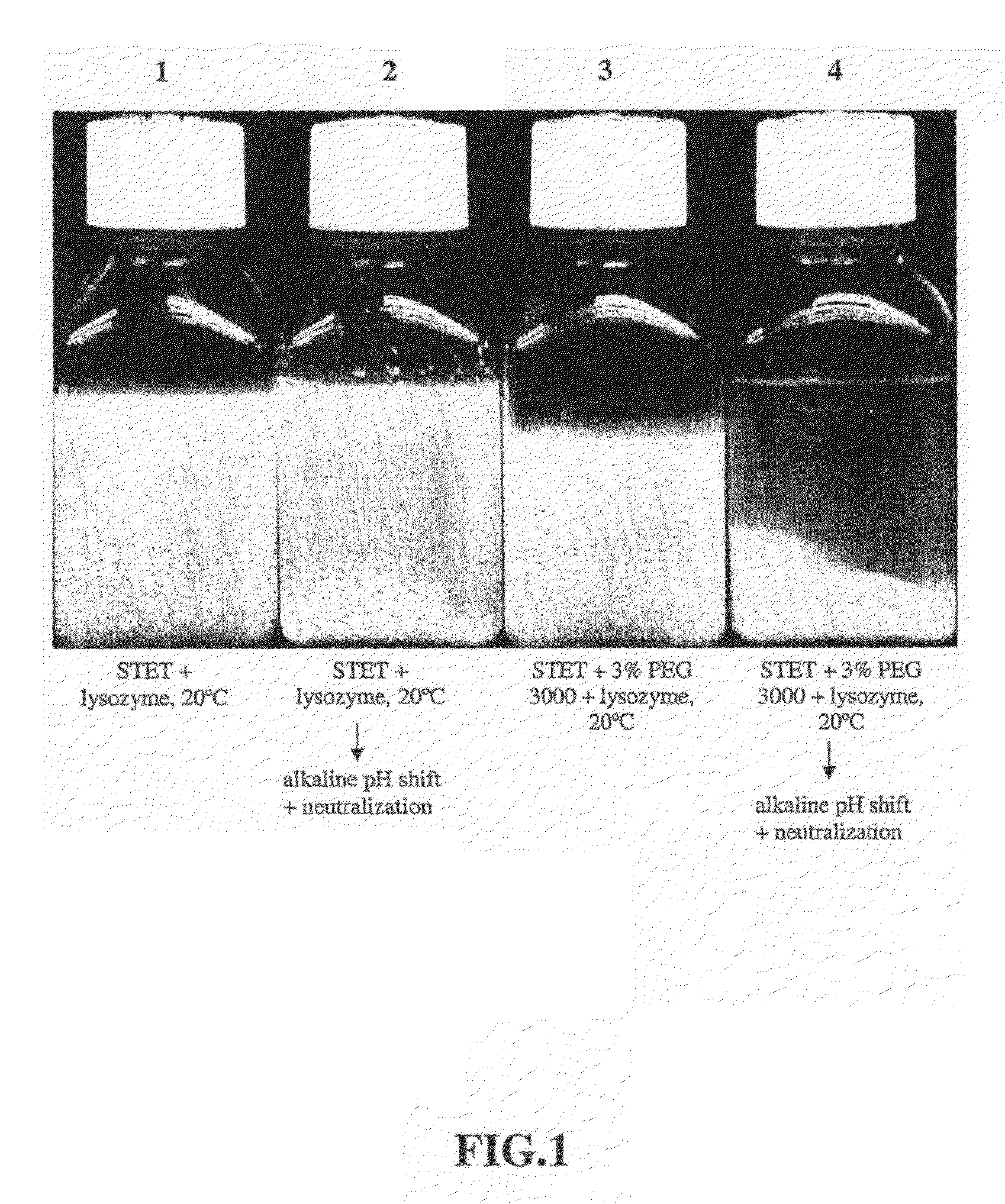
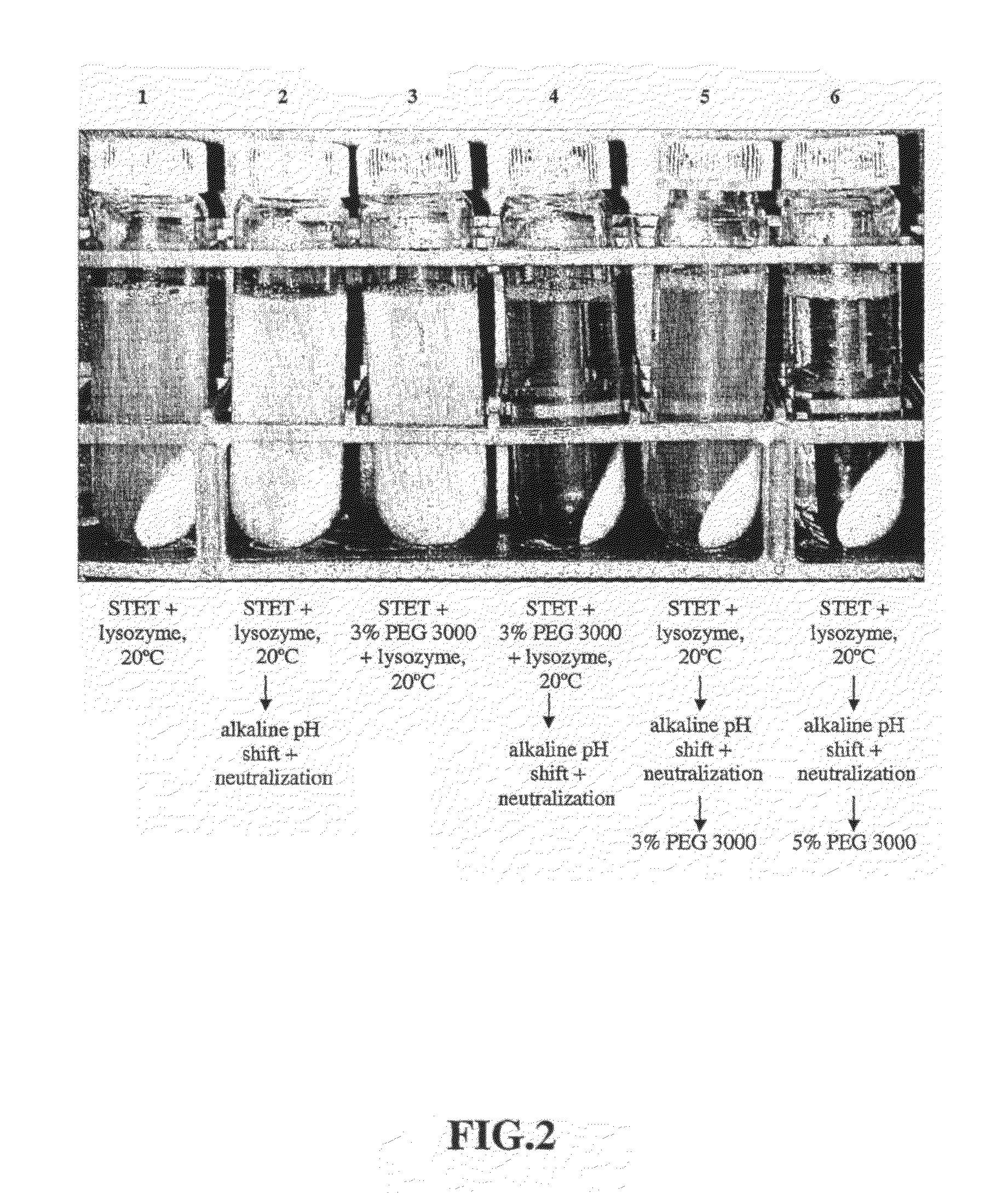
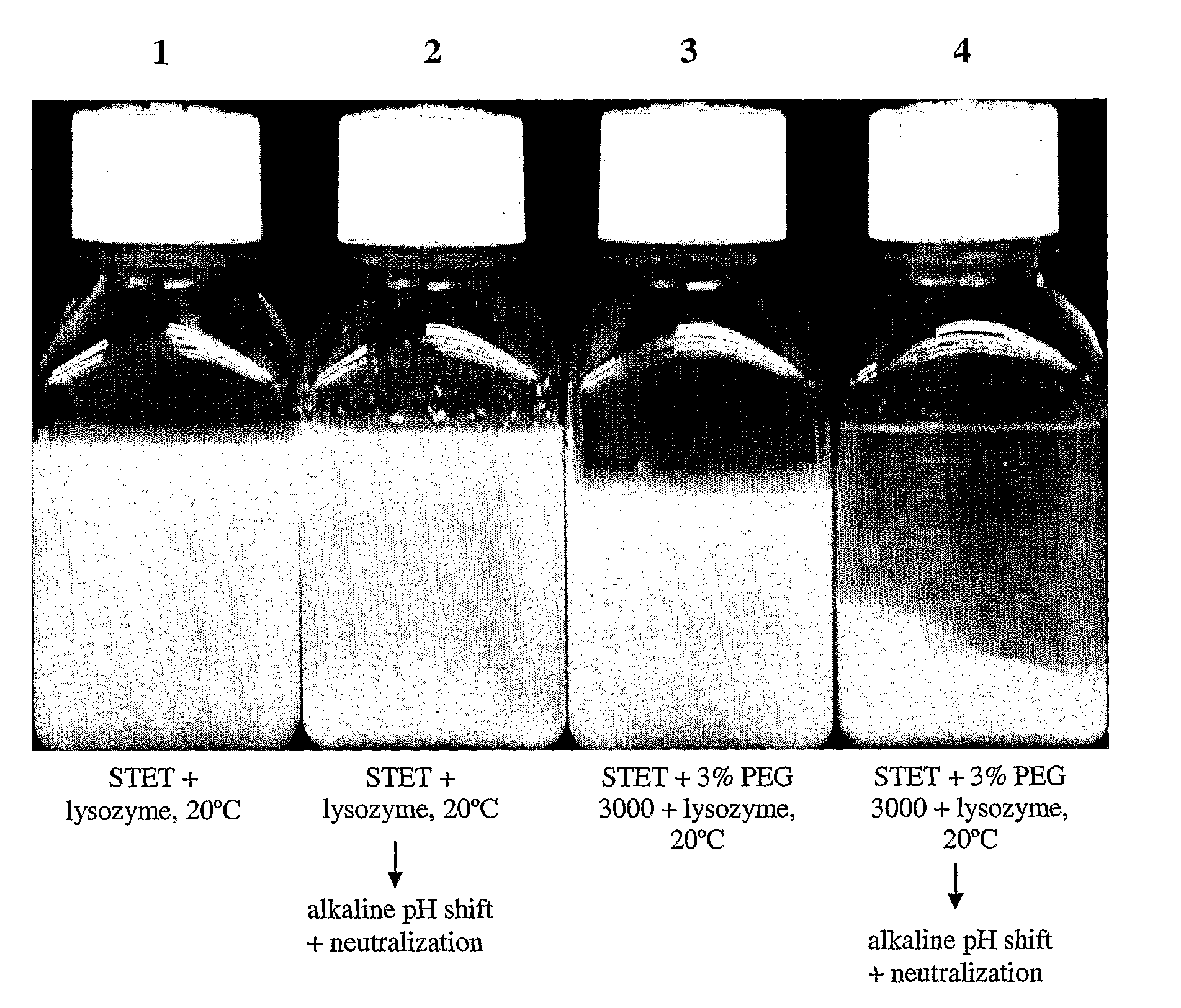
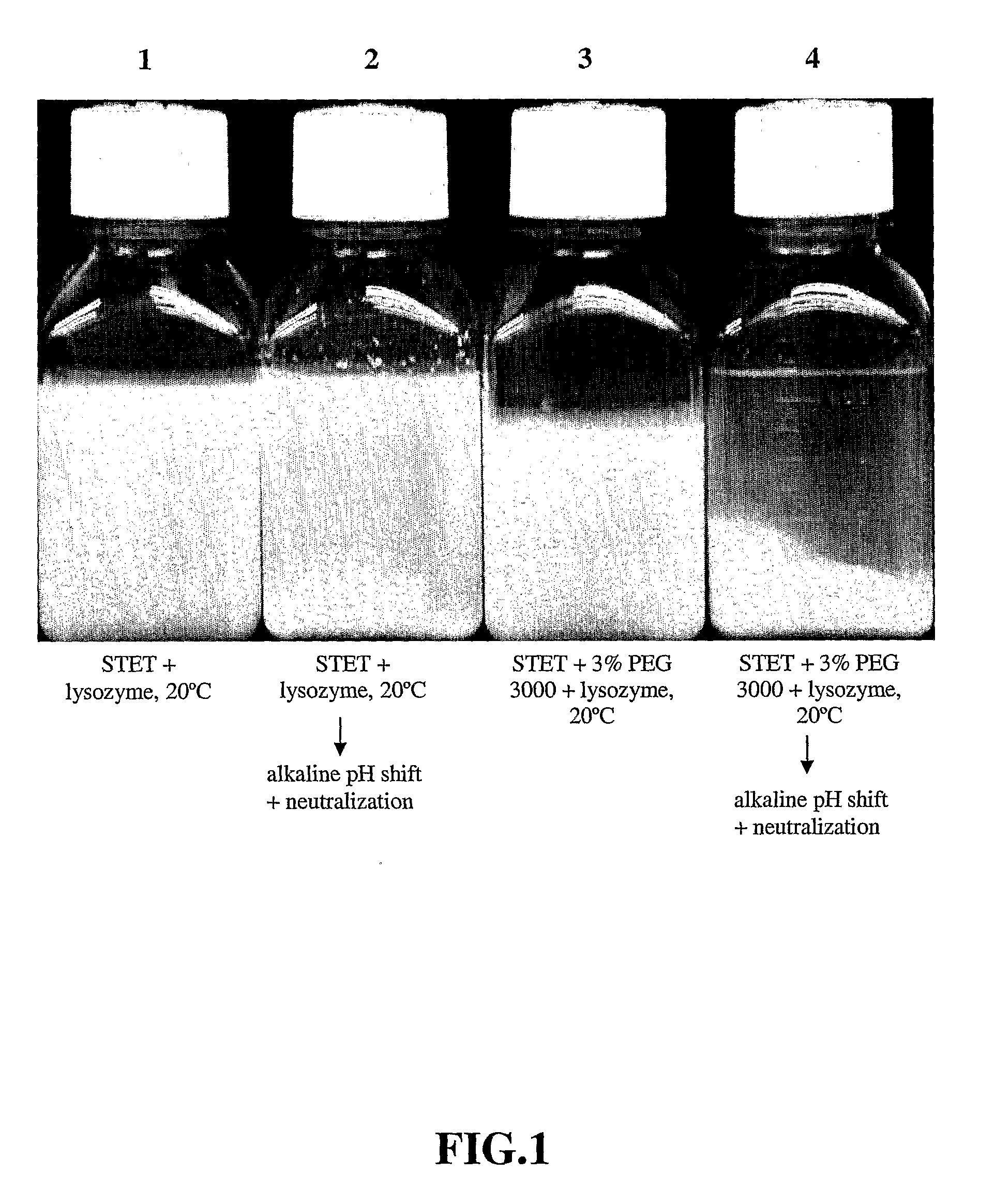
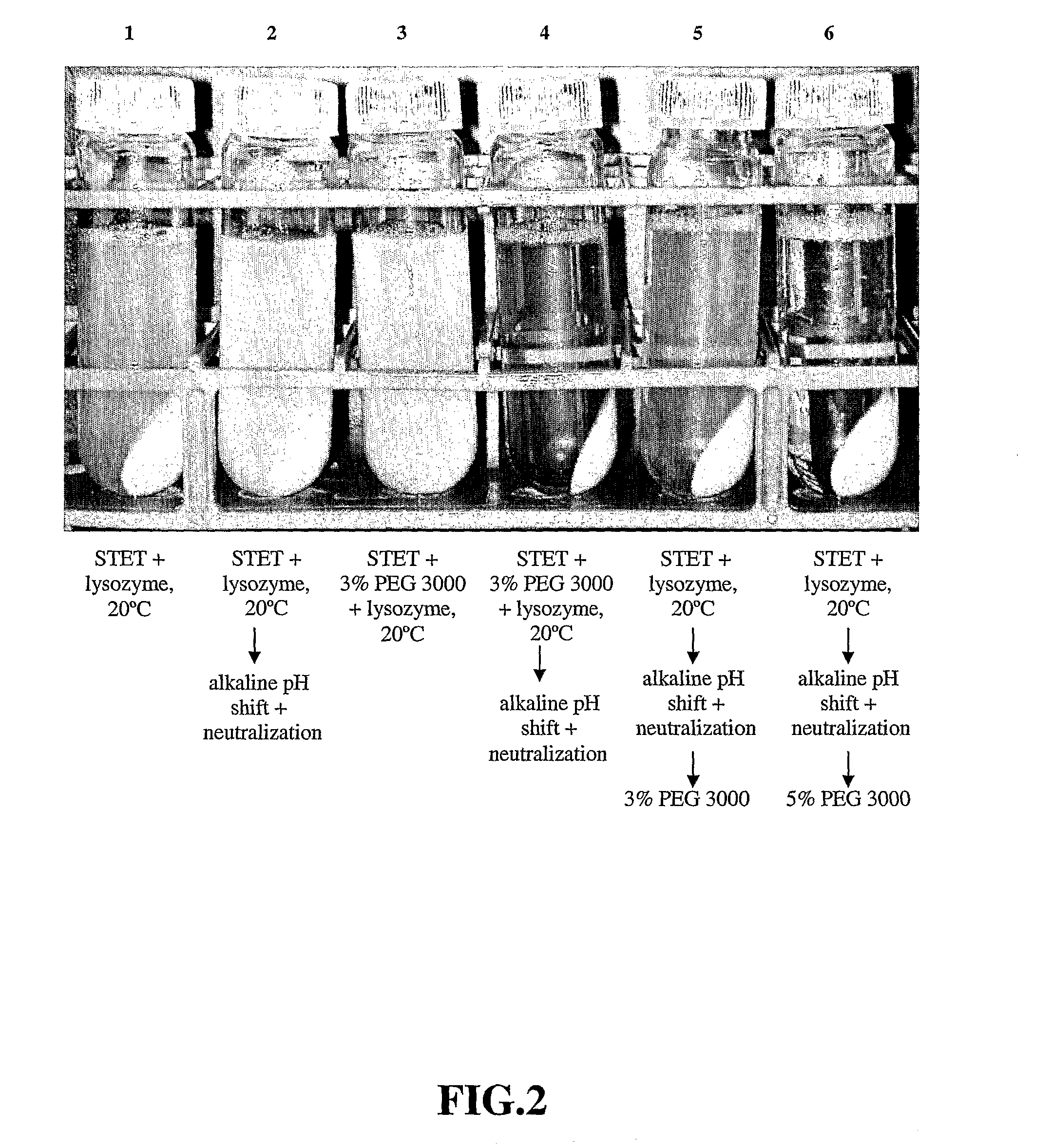
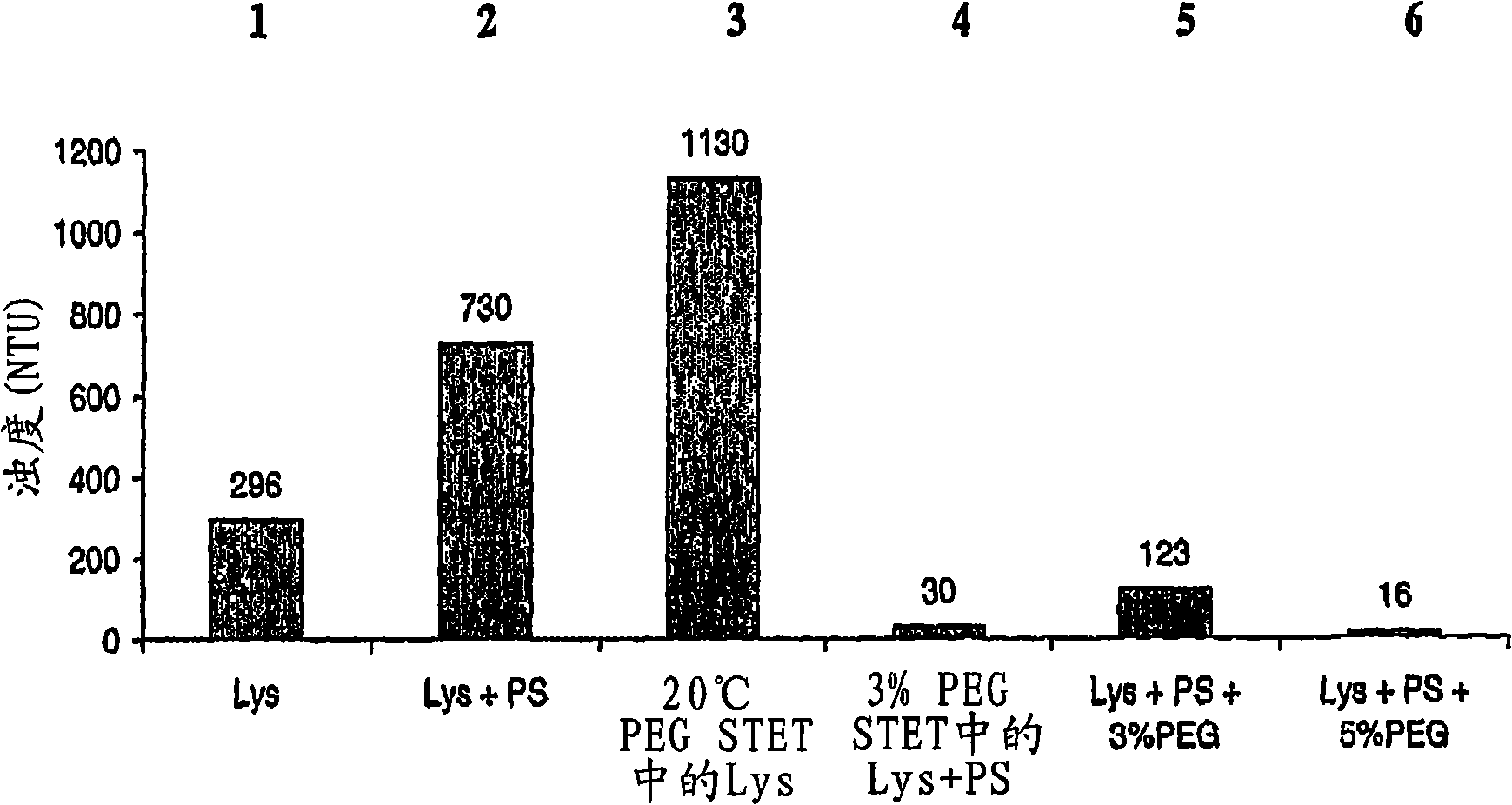
Purification process for plasmid DNA
ActiveUS7767399B2Low production costImprove robustnessMicrobiological testing/measurementMicroorganism lysisClinical gradePurification methods
Methods of isolating clinical-grade plasmid DNA from manufacturing processes, including large-scale fermentation regimes, are disclosed which encompass alternatives to two core unit operations common to plasmid DNA purification processes. The novel upstream and downstream purification processes disclosed herein provide for reduced production costs and increase process robustness. Either or both of the purification processes disclosed herein may be used in combination with additional purification steps known in the art that are associated with DNA plasmid purification technology.
Owner:MERCK SHARP & DOHME LLC
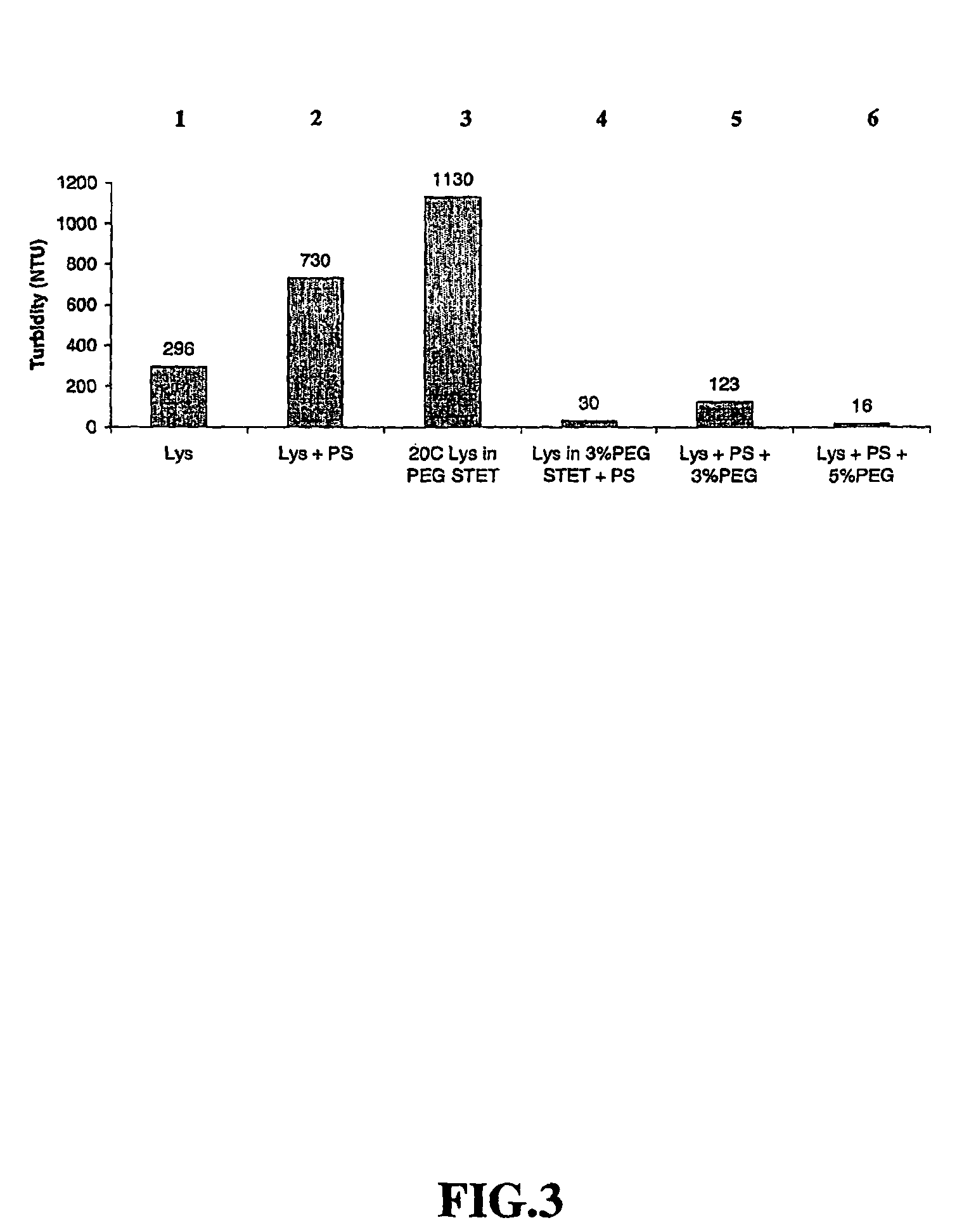
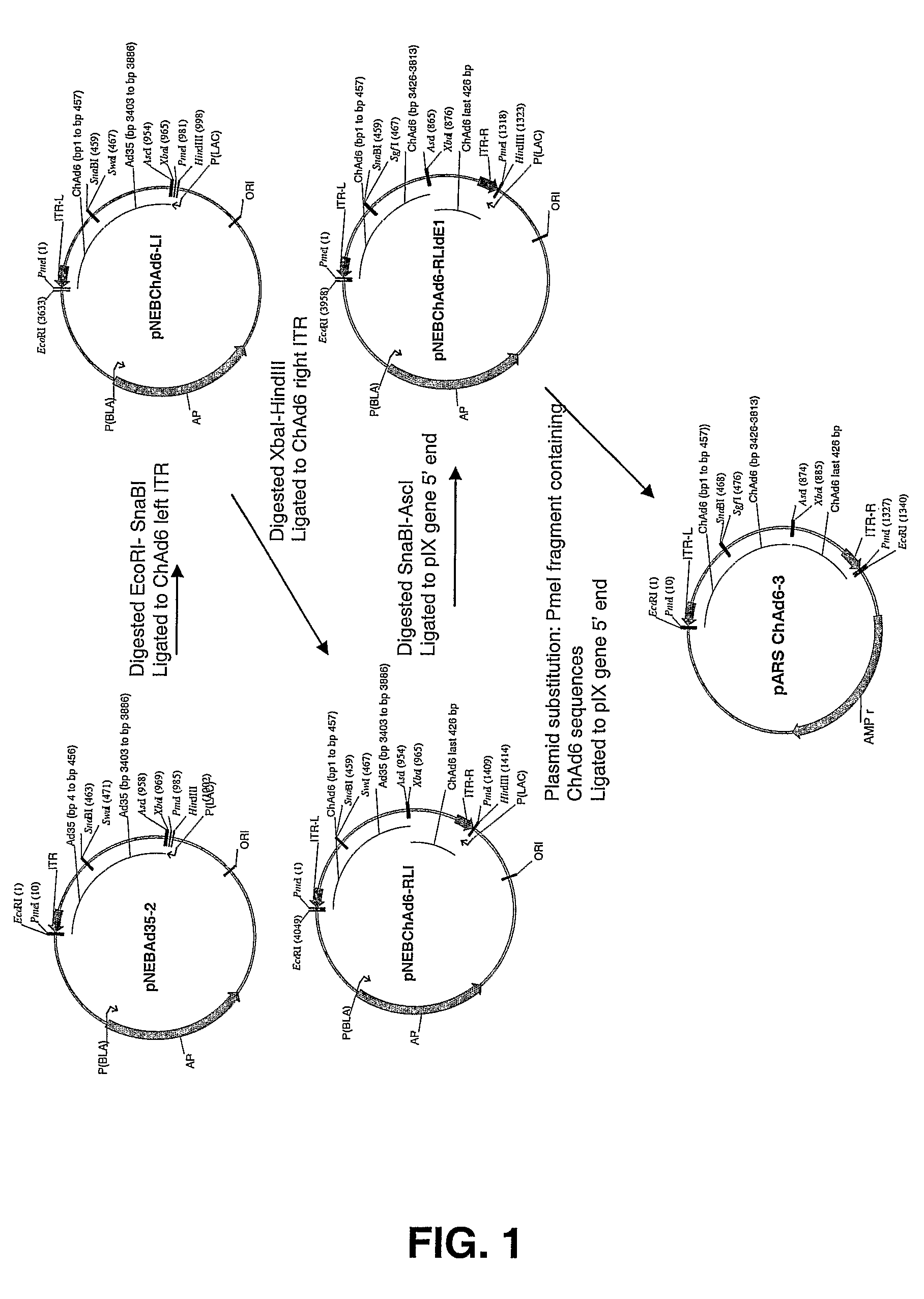
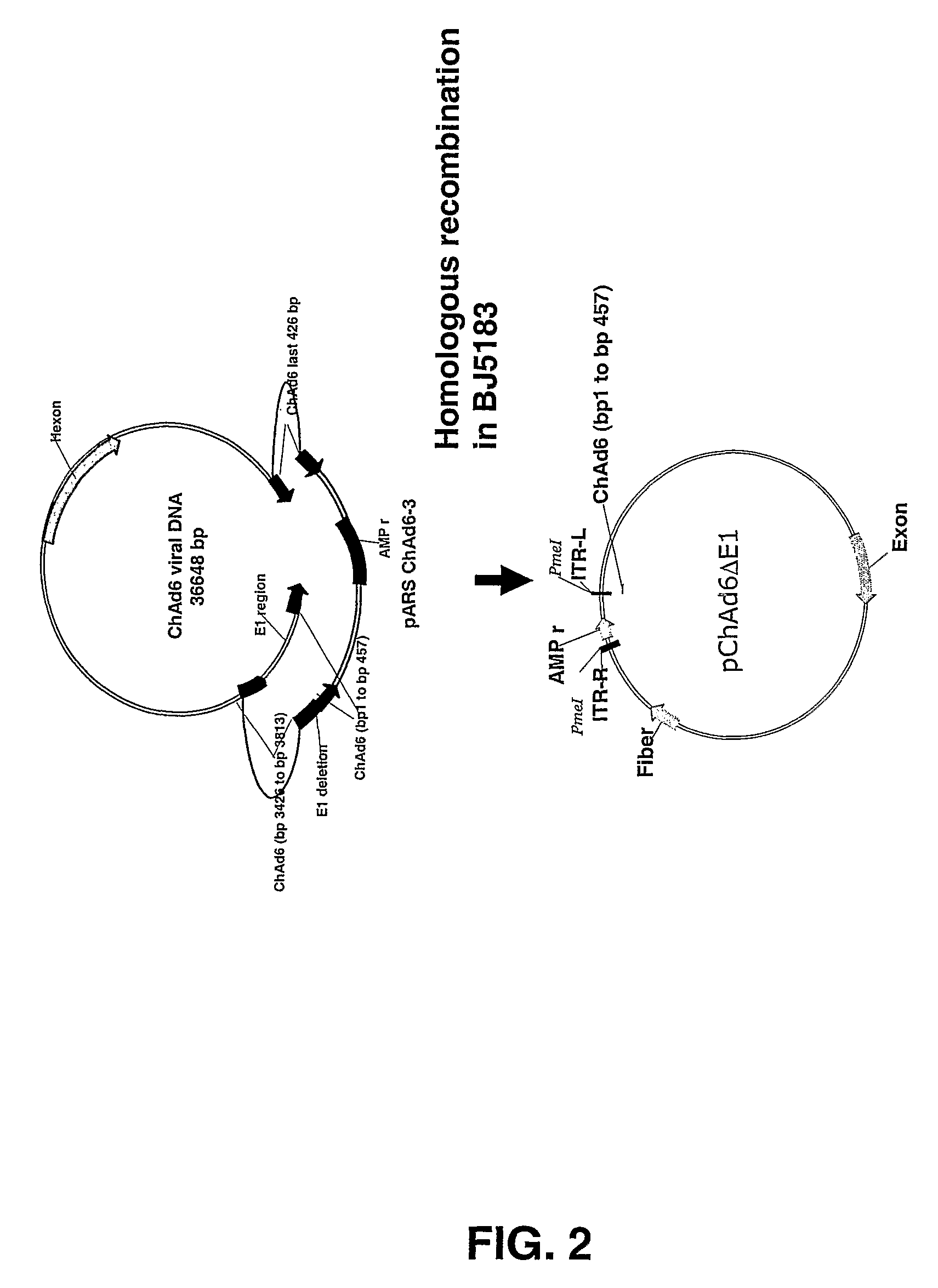
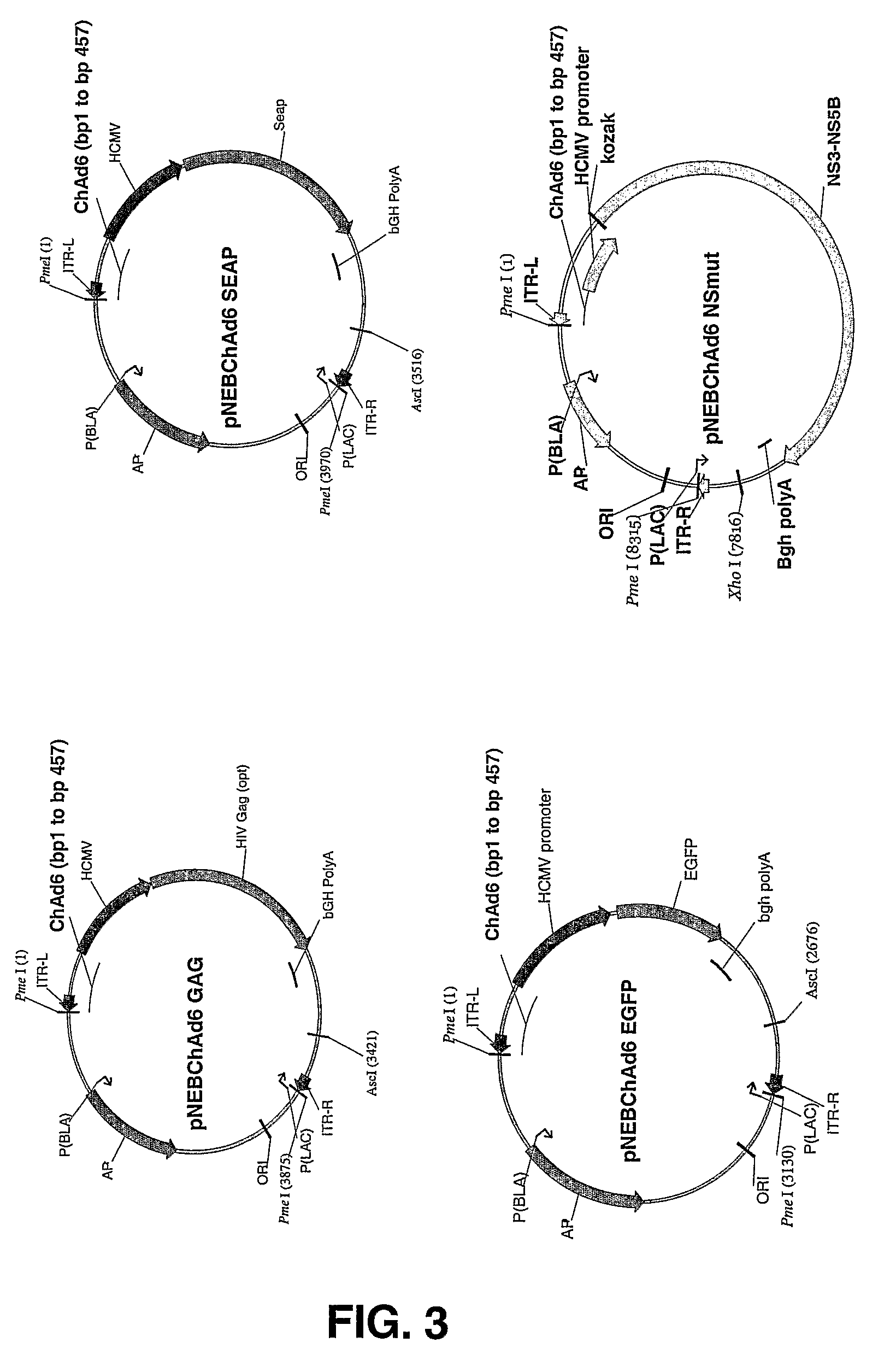
Chimpanzee adenovirus vaccine carriers
The present invention provides recombinant replication-defective adenoviral vectors derived from chimpanzee adenoviruses and methods for generating recombinant adenoviruses in human E1-expressing cell lines. The invention also provides compositions and methods suitable for use for the delivery and expression of transgenes encoding immunogens against which a boosted immune response is desired. The invention further provides methods of generating clinical grade vector stocks suitable for use in humans. In a particular embodiment the invention contemplates the use of vectors comprising transgenes which encode tumor associated antigens in vaccines and pharmaceutical compositions for the prevention and treatment of cancer.
Owner:MSD ITAL
MSC (mesenchymal stem cell) injection as well as preparation and application thereof
InactiveCN104857022AImprove survival rateAvoid gatheringPharmaceutical delivery mechanismUnknown materialsClinical gradeHydroxyethyl starch
The invention relates to the field of biology, in particular to MSC (mesenchymal stem cell) injection as well as a preparation and an application thereof. The injection comprises MSCs and a cell cryopreservation solution, wherein the cryopreservation solution comprises components in percentage by volume as follows: 25%-70% of an electrolyte balance solution, 5%-20% of clinical-grade DMSO (dimethyl sulfoxide), 1%-50% of 20% human serum albumin, 1%-10% of hydroxyethyl starch 130 / 0.4 and 5%-20% of triphosadenine-disodium magnesium chloride freeze-drying powder. The injection is free of animal serum, has clear ingredients and good cell cryopreservation effect and is safe and controllable, long-term storage and long-distance transport are facilitated, the survival rate of cells after recovery is higher than 95%, the vitality is high, and the injection can effectively relieve injury and inflammation symptoms of lesion tissue of lungs and promote tissue regeneration of the lungs, so that acute lung injury can be fundamentally and comprehensively treated.
Owner:北京青藤谷禧干细胞科技研究院有限公司
Umbilical cord mesenchymal stem cell injection as well as preparation method and application thereof
InactiveCN104873542AImprove the quality of lifeReduce releaseDigestive systemPharmaceutical delivery mechanismHydroxyethyl starchClinical grade
The invention provides umbilical cord mesenchymal stem cell injection as well as a preparation method and application thereof. The umbilical cord mesenchymal stem cell injection comprises mesenchymal stem cells and a frozen stock solution, wherein the frozen stock solution comprises the following components: a balanced electrolyte solution with the volume percentage of 25-70 percent, hydroxyethyl starch 130 / 0.4 with the mass / volume percentage (g / ml) of 1-10 percent, triphosadenine disodium magnesium chloride with the volume percentage of 5-20 percent, clinical-grade DMSO with the volume percentage of 5-20% and 20% human albumin with the volume percentage of 1-50 percent. The prepared umbilical cord mesenchymal stem cell injection is free of animal serum, safe, high in cell viability after cryopreservation resuscitation, can be used for treating inflammatory bowel disease, has a good effect and has no toxic or side effects.
Owner:北京青藤谷禧干细胞科技研究院有限公司
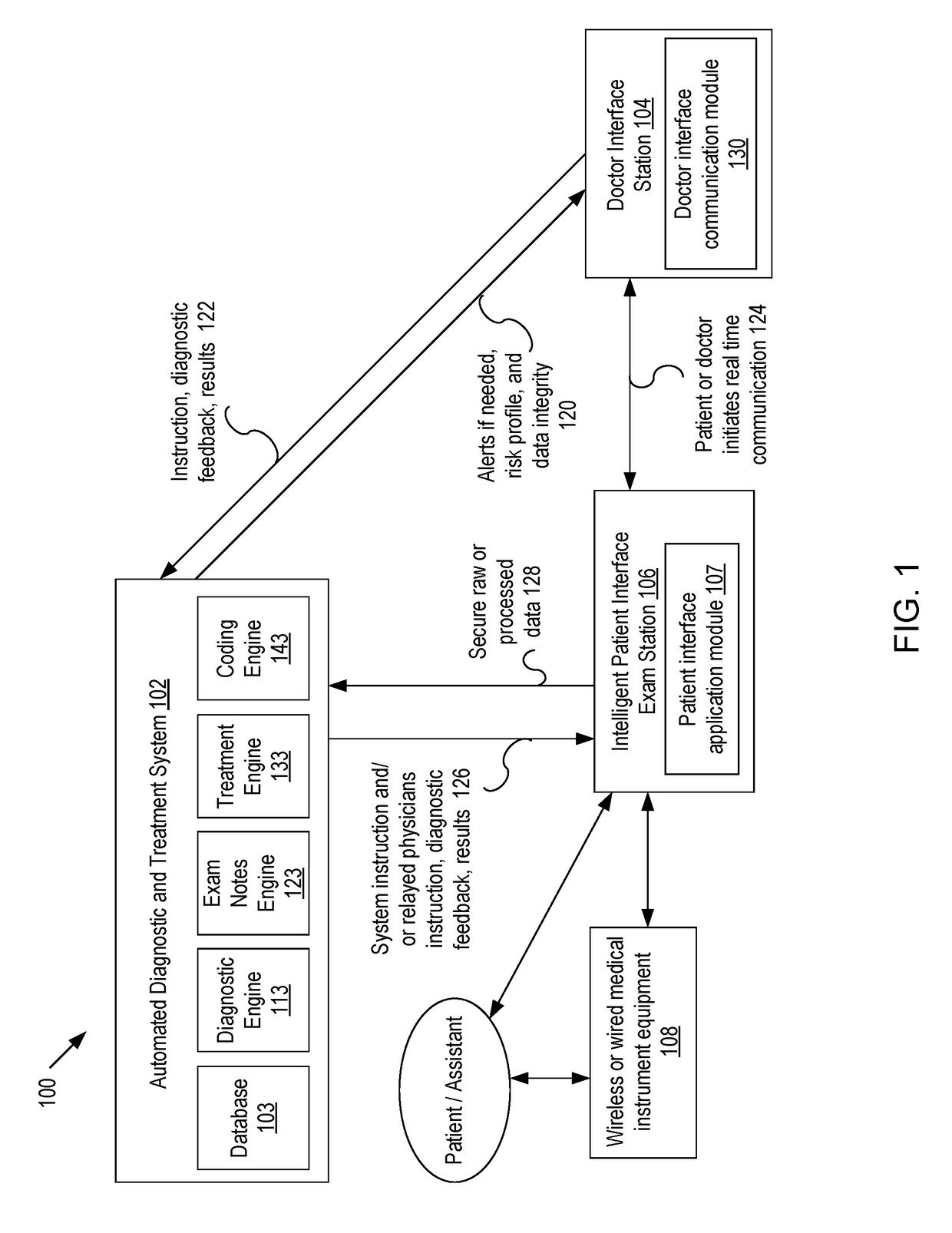
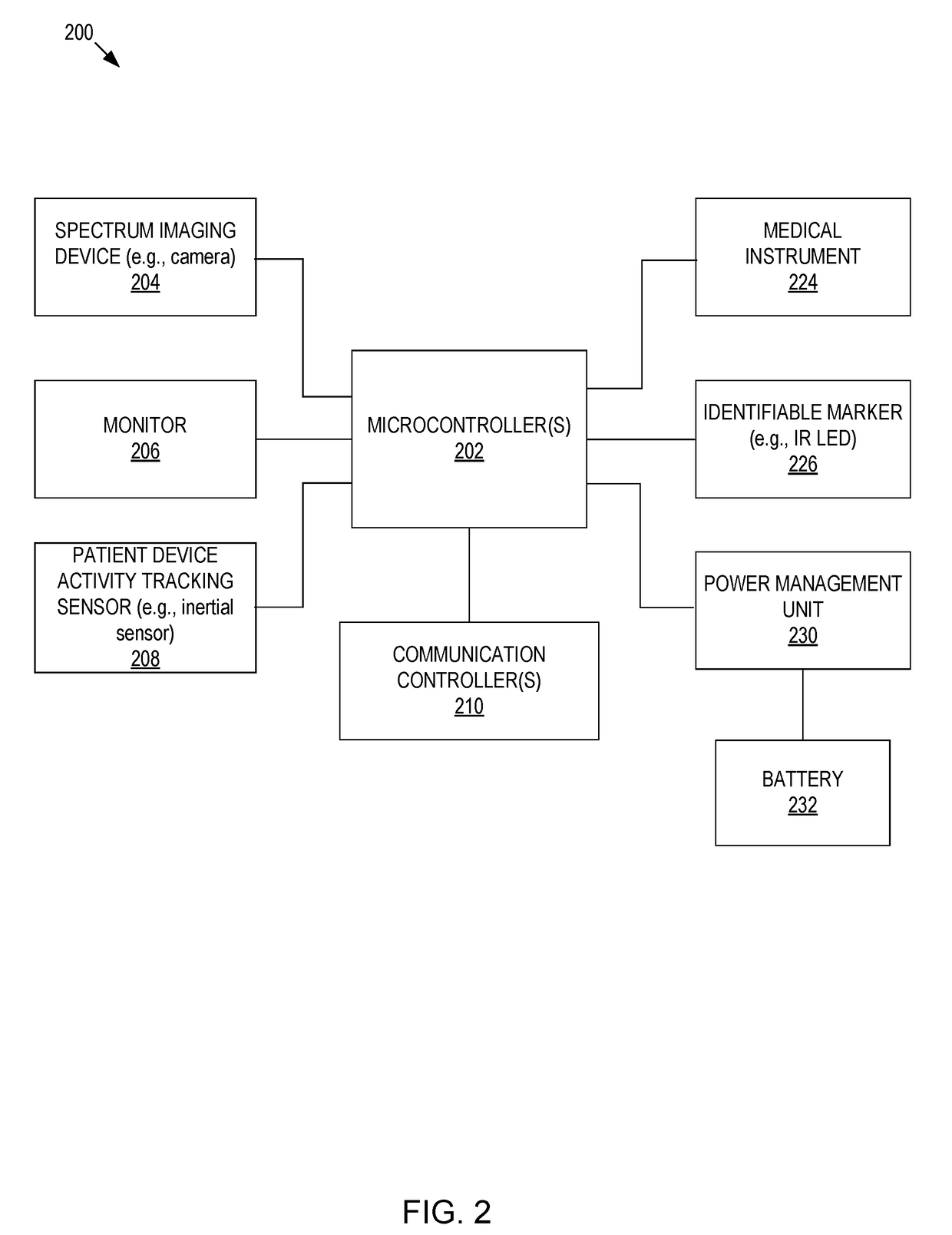
Systems and methods for intelligent patient interface exam station
Presented are systems and methods for examining patients and generating diagnostic medical data to make automated diagnoses and treatment recommendations to patients and doctors. Various embodiments of the present invention provide patients with diagnostic tools and audio / video guidance to reliably and accurately perform clinical grade diagnostic measurements of key vital signs. In embodiments, this is accomplished by using an automated remote (or local, e.g., in the form of a kiosk) end-to-end medical diagnostic system that monitors equipment usage for accuracy. The diagnostic system analyzes patient responses, measurement data, and patient-related information to generate diagnostic and / or treatment information that may be shared with healthcare professionals and specialists, as needed. Automation provides for timely, hassle-free, and cost-effective health care management that takes the stress out of doctor visits, while delivering personalized health care. The high accuracy of generated diagnostic data improves health care to patients and reduces the risk of medical malpractice for treating physicians.
Owner:ADVINOW INC
Mesenchymal stem cell injection, as well as preparation method and application in preparing medicament for treating children dilated cardiomyopathy
InactiveCN102908364AIncrease productionEasy quality controlPeptide/protein ingredientsMammal material medical ingredientsClinical gradeLife quality
The invention provides mesenchymal stem cell injection, as well as a preparation method and application in preparing a medicament for treating children dilated cardiomyopathy. The mesenchymal stem cell injection comprises 2*10<5>-1*10<7> mesenchymal stem cells in every milliliter, clinical grade DMSO (dimethylsulfoxide) with volume ratio of 5 to 8 percent, human albumin with mass volume ratio of 1 to 6 percent, and multi-electrolyte liquid. The mesenchymal stem cell injection is prepared from placenta and umbilical cord, has the advantages of being high in yield, and being easy to realize industrialization, the preparation system quality is easy to control; the injection can be directly frozen through program-controlled freezing procedure and directly used in clinical injection after being recovered, and is safe and reliable. According to the injection, the dilated cardial structure can be changed, the function of heart can be recovered, the water supply state of each tissue in the whole body can be improved, the living quality of a patient can be improved, and the dilated cardiomyopathy can be treated fundamentally, so that the patient can get rid of serious complications caused by massive medicine administration, living action limitation and poor disease control.
Owner:青岛奥克生物开发有限公司
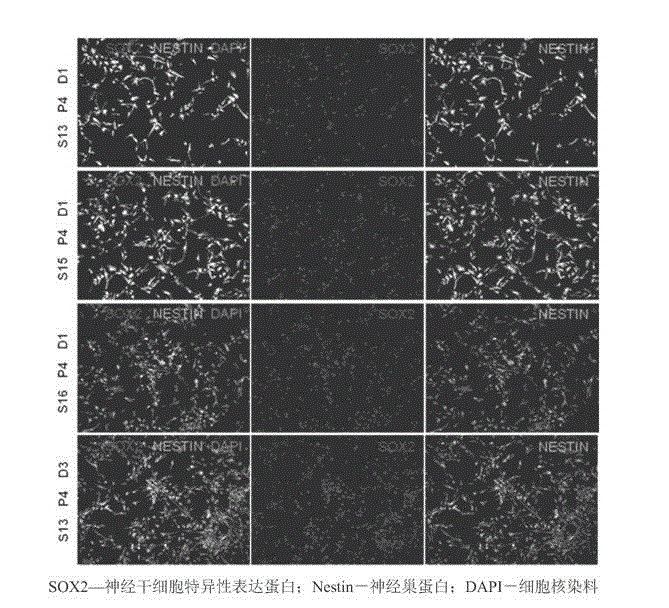
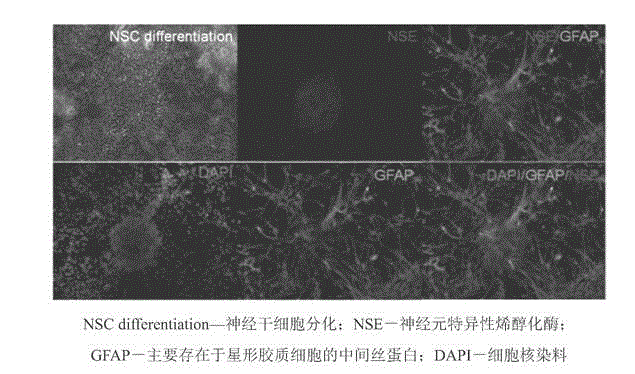
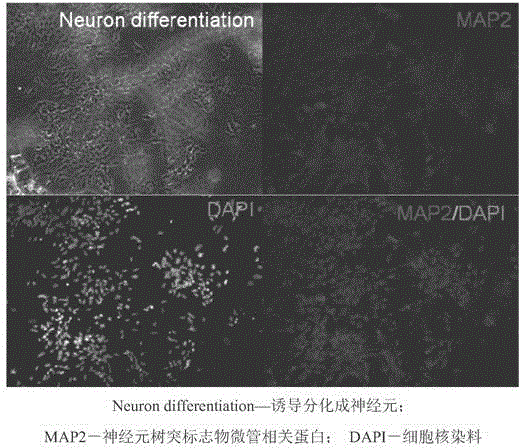
Establishment method and application of clinical-grade neural stem cell line
InactiveCN103451153ALow tumorigenicityAvoid Tumor RiskNervous disorderMicroorganism based processesClinical gradeNeurulation
The invention belongs to the field of cytobiology and neurobiology, and provides an establishment method and application of a clinical-grade neural stem cell line. The method under GMP (Good Manufacturing Practice) control comprises two steps, namely, primary culturing of neural stem cells, and subculturing of neural stem cells. The identification result shows that the neural stem cell line established by the method can express a plurality of stem cell markers, can be differentiated into nerve cells, astrocyte and oligodendrocyte, has high stability, and is suitable for long-term culturing; a large amount of sufficient clinical-grade neural stem cells can be obtained by the method. The method can be used for establishing the neural stem cell line based on a plurality of neural stem cells and even single neural stem cell, and thus the error in study can be reduced as far as possible. By adopting the neural stem cell line established by the method and the cells obtained by differentiating the neural stem cell line, the problems of the safety and effectiveness risk in cell therapy of nervous system disease can be solved effectively, and safe and effective treatment on such diseases becomes possible.
Owner:栾佐
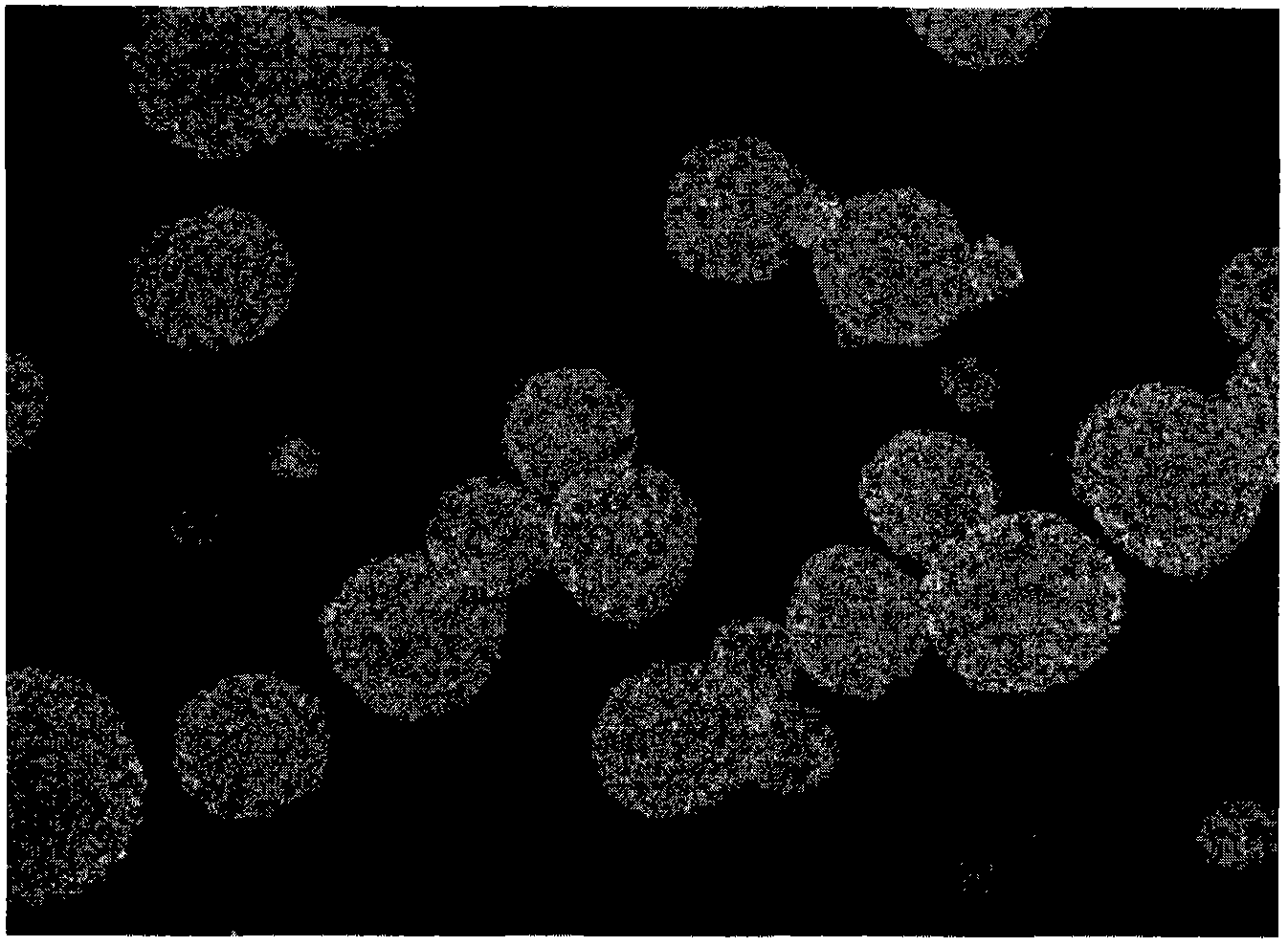
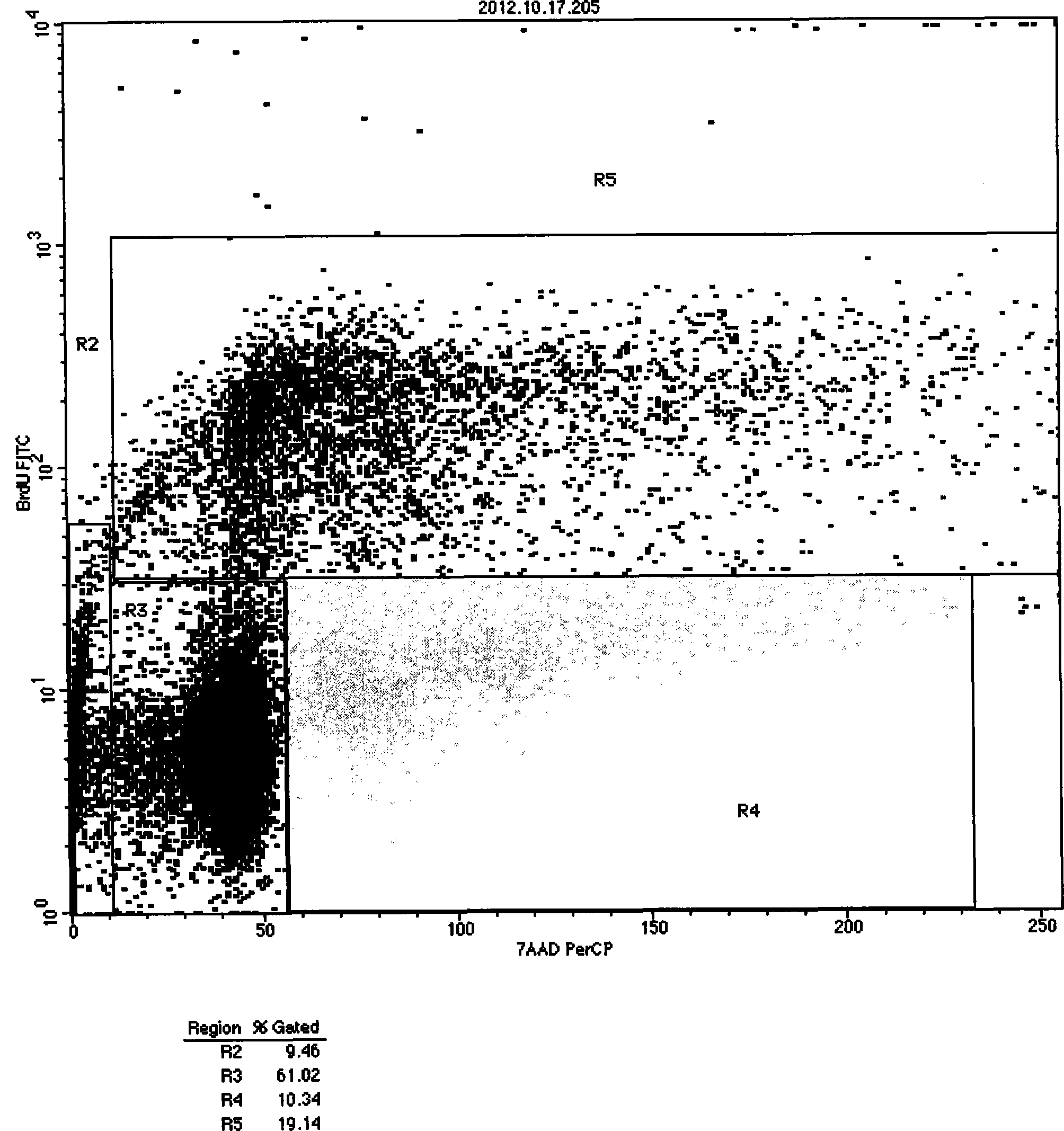
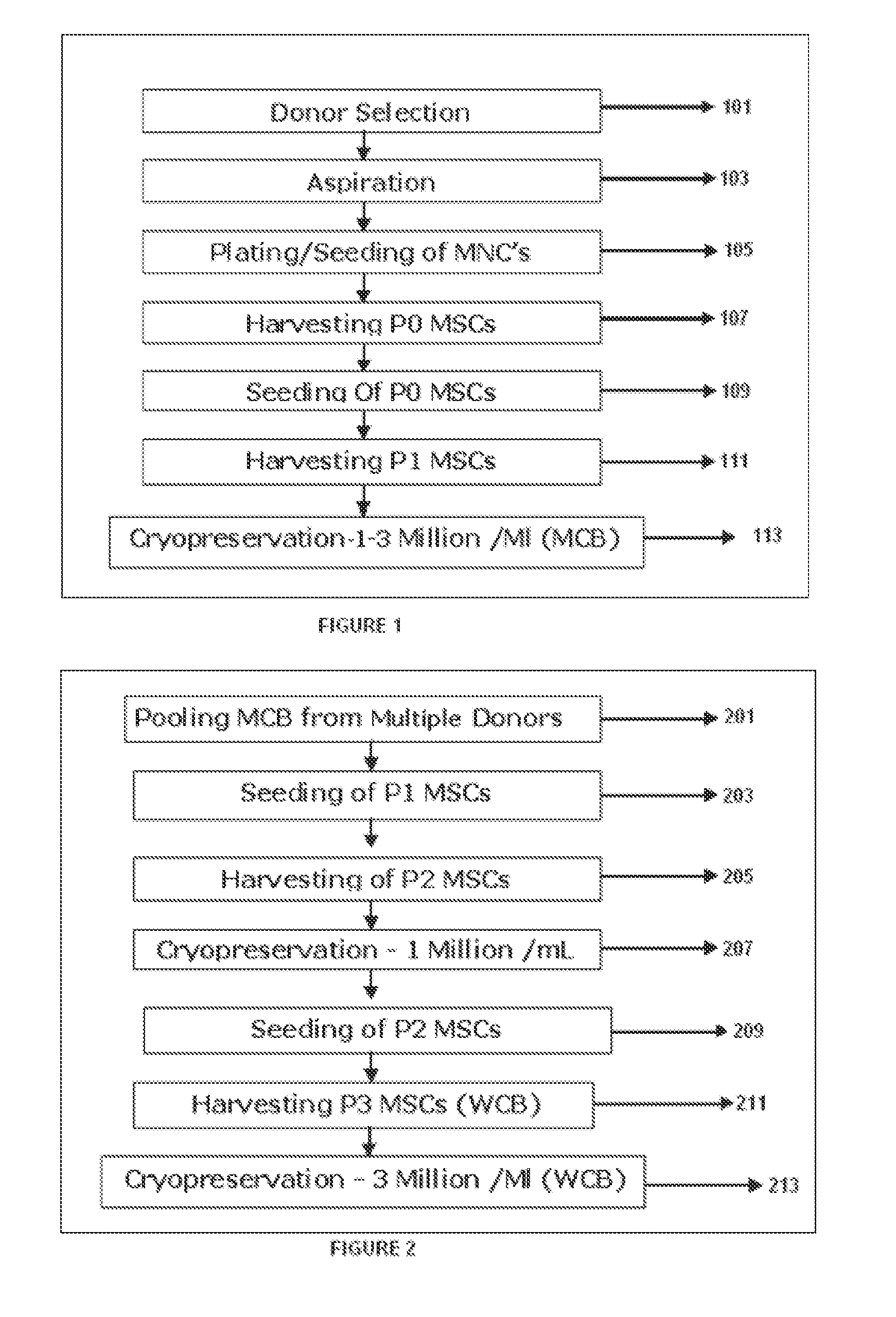
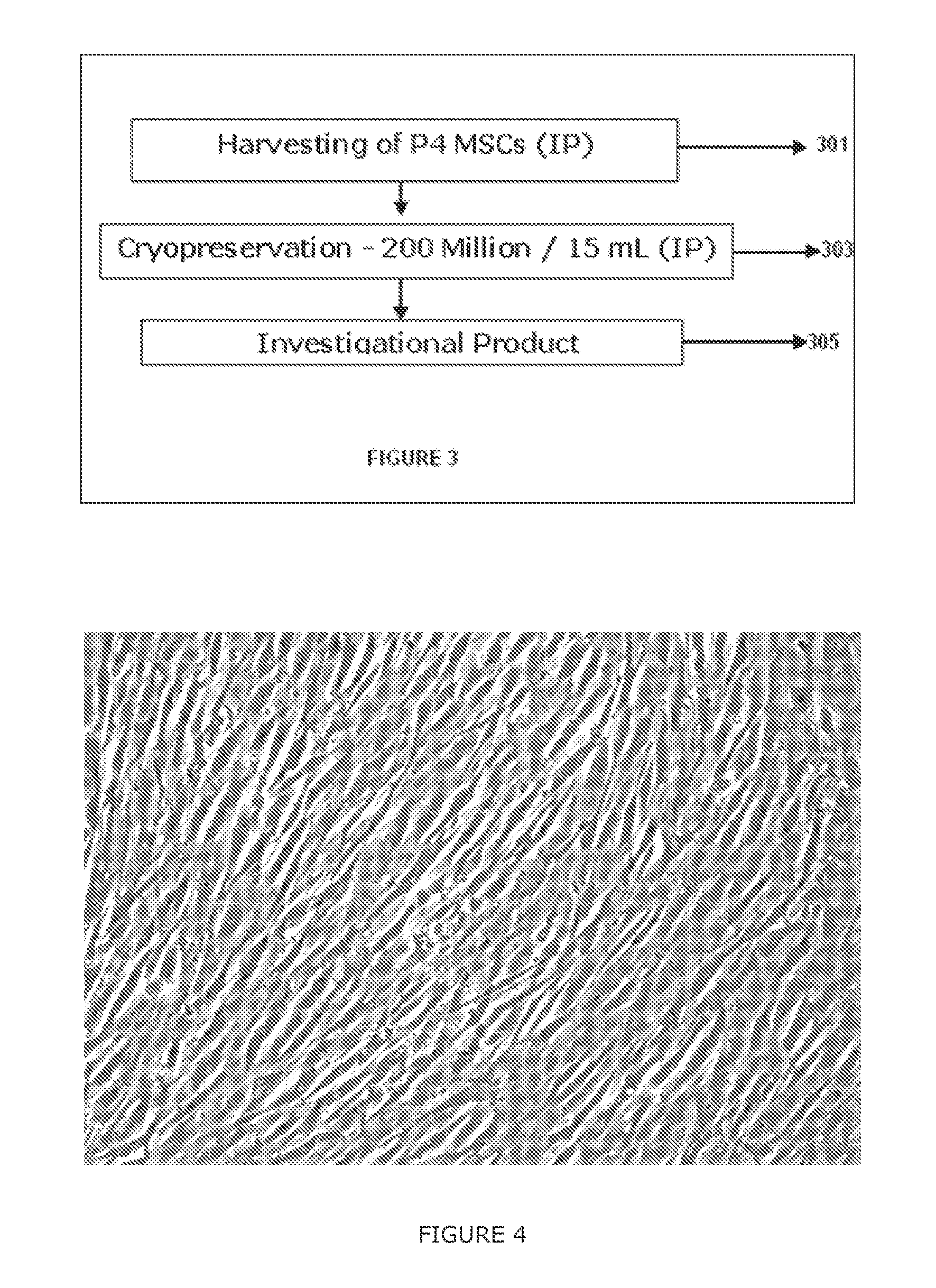
Methods of upscaling mesenchymal stromal cell production, compositions and kit thereof
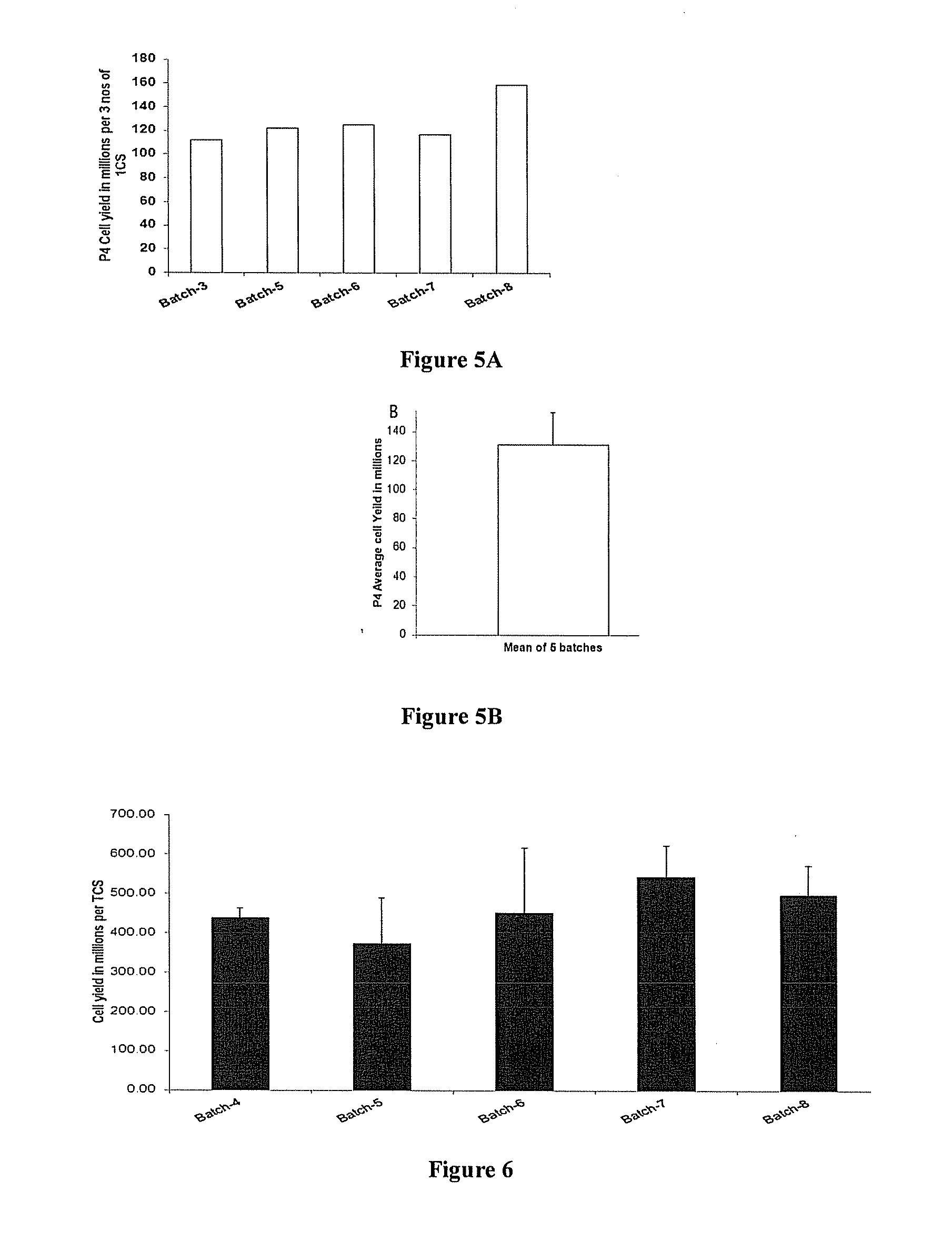
InactiveUS20160002601A1Dead animal preservationSkeletal/connective tissue cellsHigh cellClinical grade
The present invention discloses a method of isolation, pooling and further culturing of Mesenchymal Stem cells (MSC) for clinical application. Present invention also discloses the method of establishing Master Cell bank, followed by Working Cell Bank from which the final therapeutic composition referred to as Investigational Product / Investigational Medicinal Product comprising of allogenic bone marrow-derived MSC is formulated for clinical applications. Present disclosure also discloses a robust manufacturing process for consistent production of clinical grade Mesenchymal Stromal cells (MSCs). The process enables production of highly viable potent cells. The process steps relating to preparation of media, cell seeding, harvesting are fine tuned to achieve consistency in cell yield, superior cell viability, purity, improved cell proliferation, high cell recovery, low HLA-DR expression, reduction in culture duration. The viability and purity of cells are further improved by optimized wash process without cell loss / cell stress. The disclosure further provides a method of cyrostoring MSCs at high cell density without affecting the viability of cells. It further provides economical means to store and transport at −80° C.
Owner:STEMPEUTICS RES PRIVATE
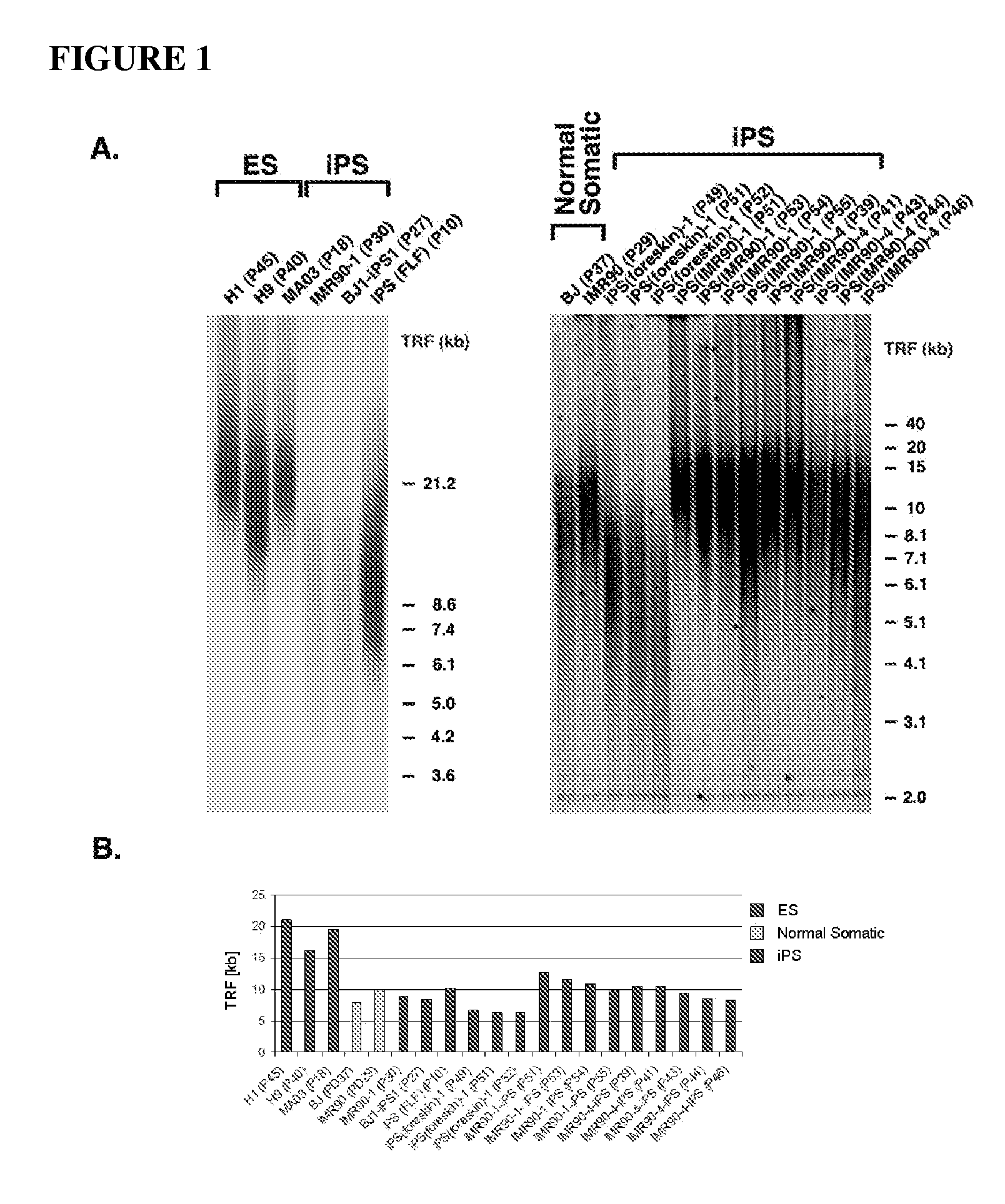
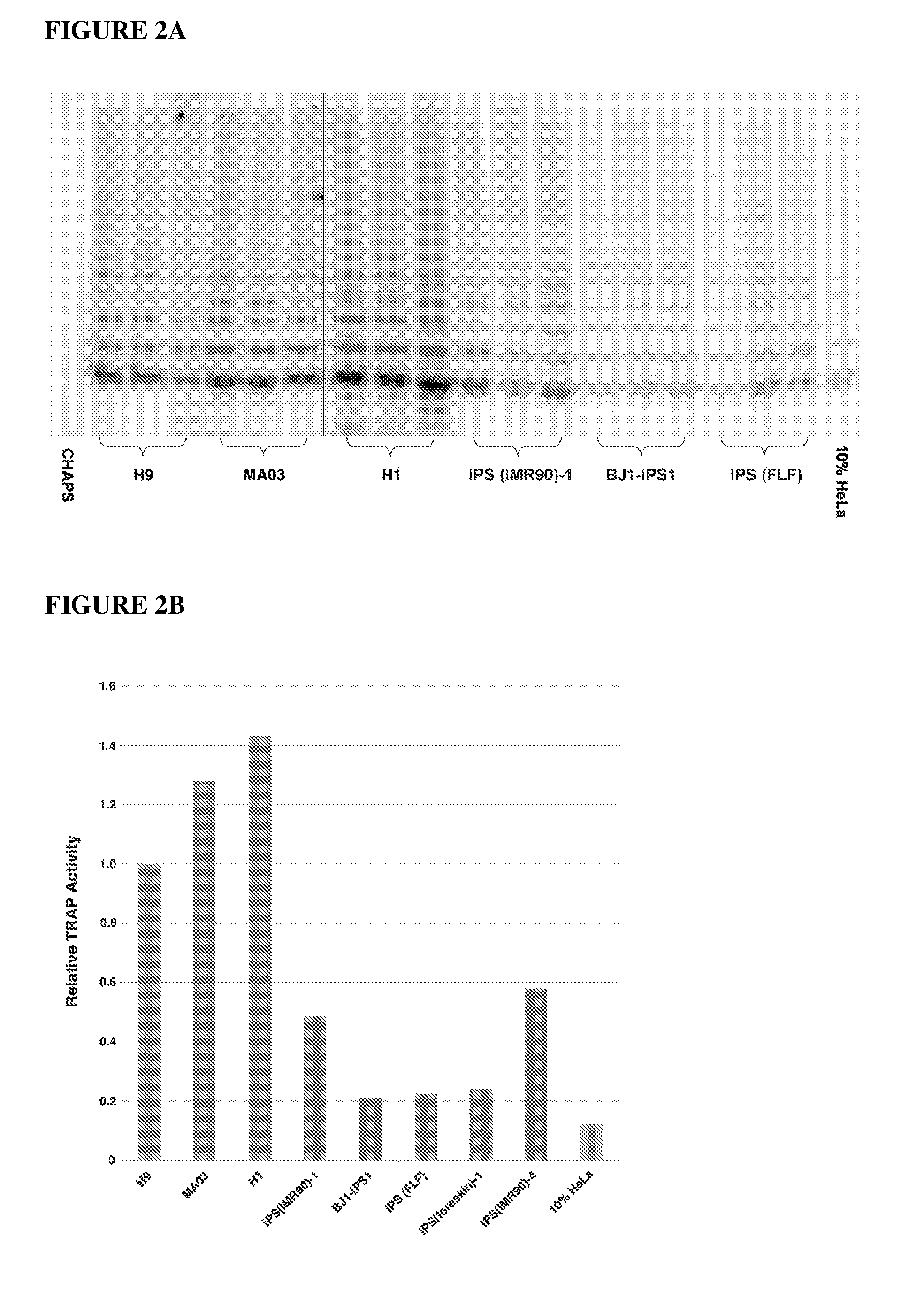
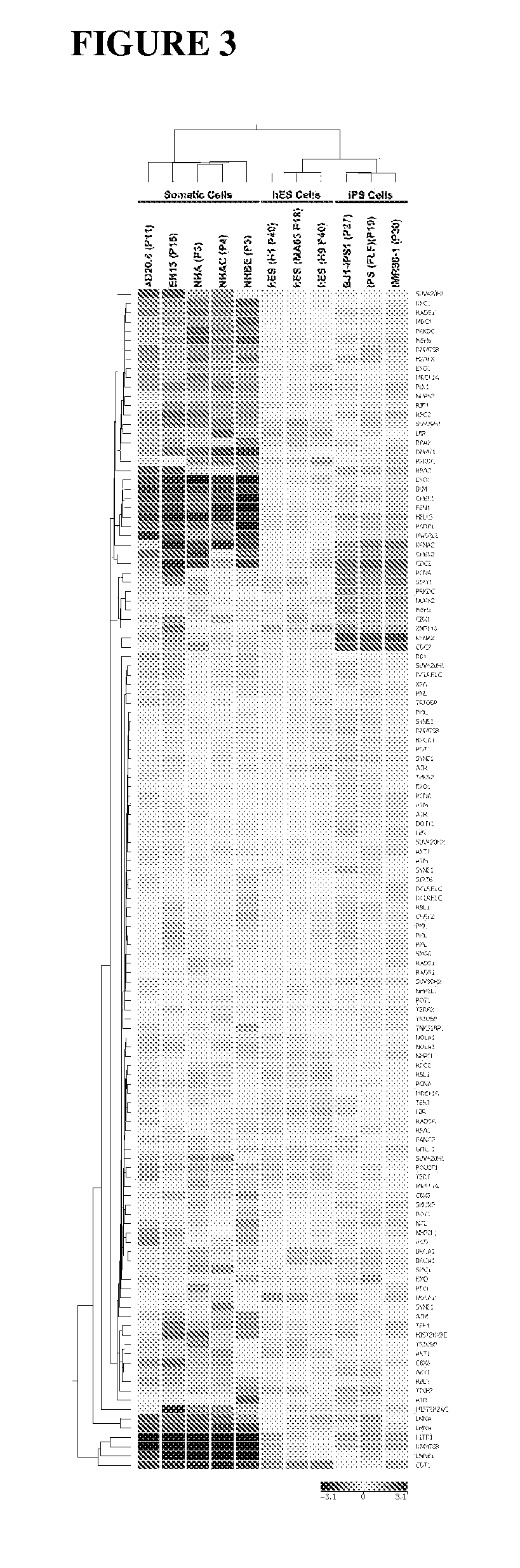
Methods for telomere length and genomic DNA quality control and analysis in pluripotent stem cells
InactiveUS20130011918A1Reduce riskDesired phenotypeMicrobiological testing/measurementLibrary screeningPluripotential stem cellClinical grade
The generation of clinical-grade cell-based therapies from human embryonic stem cells or cells reprogrammed to pluripotency from somatic cells, requires stringent quality controls to insure that the cells have long enough telomeres and resulting cellular lifespan to be clinically useful, and normal gene expression and genomic integrity so as to insure cells with a desired and reproducible phenotype and to reduce the risk of the malignant transformation of cells. Assays useful in identifying human embryonic stem cell lines and pluripotent cells resulting from the transcriptional reprogramming of somatic cells that have embryonic telomere length are described as well as quality control assays for screening genomic integrity in cells expanded and banked for therapeutic use, as well as assays to identify cells capable of abnormal immortalization,
Owner:LINEAGE CELL THERAPEUTICS INC
Upstream and a Downstream Purification Process for Large Scale Production of Plasmid Dna
ActiveUS20080138886A1Low production costImprove robustnessMicrobiological testing/measurementMicroorganism lysisClinical gradePurification methods
Methods of isolating clinical-grade plasmid DNA from manufacturing processes, including large-scale fermentation regimes, are disclosed which encompass alternatives to two core unit operations common to plasmid DNA purification processes. The novel upstream and downstream purification processes disclosed herein provide for reduced production costs and increase process robustness. Either or both of the purification processes disclosed herein may be used in combination with additional purification steps known in the art that are associated with DNA plasmid purification technology.
Owner:MERCK SHARP & DOHME LLC
Clinical-grade serum-free medium for adherent culture of human neural stem cells
InactiveCN104560876AImprove antioxidant capacityIncrease profitNervous system cellsLipid formationNutrition
The invention discloses a clinical-grade serum-free medium for adherent culture of human neural stem cells. The medium disclosed by the invention comprises a basic medium, basic nutrition additives, plant-based human serum albumins, saccharides, lipid, hormones, antioxidants and related substances for promoting metabolism; the basic medium is prepared from commercial DMEM / F12 and a commercial neurobasal medium according to a ratio of 1:1; the basic nutrition additives comprise insulin, holo-transferrin, apo-transferrin, putrescine, progesterone and sodium selenite; the hormones comprise biotin, corticosterone, lipoic acid, Ve and Ve acetic ester; the antioxidants comprise human-derived catalase, human-derived superoxide dismutase, glutathione and Vc; the related substances for promoting metabolism comprise carnitine, T3 and ethanol amine. The medium can improve a cell expansion speed by two to three times, well keeps stem properties of the neural stem cells, keeps the cell differentiation potential of the neural stem cells and eliminates potential animal-origin endotoxin and viral pollution.
Owner:广州吉帝生物科技有限公司
Preparation method of clinical-grade human umbilical cord mesenchymal stem cell complex factor and lyophilized powder for repairing
PendingCN108823156ACan maintain normal stateGuarantee statusPowder deliveryCulture processClinical gradeSerum ige
The invention provides a preparation method of a clinical-grade human umbilical cord mesenchymal stem cell complex factor and lyophilized powder for repairing. The preparation method comprises preparation of a clinical-grade stem cell complete culture medium, extracting and culturing human umbilical cord mesenchymal stem cells, preparing the human umbilical cord mesenchymal stem cell complex factor, lyophilizing the human umbilical cord mesenchymal stem cell complex factor and the like. The prepared human umbilical cord mesenchymal stem cell complex factor and lyophilized powder do not containallogeneic serum components; the components of the culture medium are determined and the human umbilical cord mesenchymal stem cells have a rapid cell multiplication speed when being cultured by utilizing a culture medium system and have a strong bioactive substance secretion capability; the production cost is extremely reduced, so that the human umbilical cord mesenchymal stem cell complex factor and the lyophilized powder are suitable for industrialized large-scale production and can be applied to clinical wound healing and repairing type biomedicine products.
Owner:陕西神州生物技术有限公司
Methods of producing rpe cells
ActiveUS20150299653A1Fast and economically efficient scale-upFacilitate scientificBiocideCulture processClinical gradeLaminin
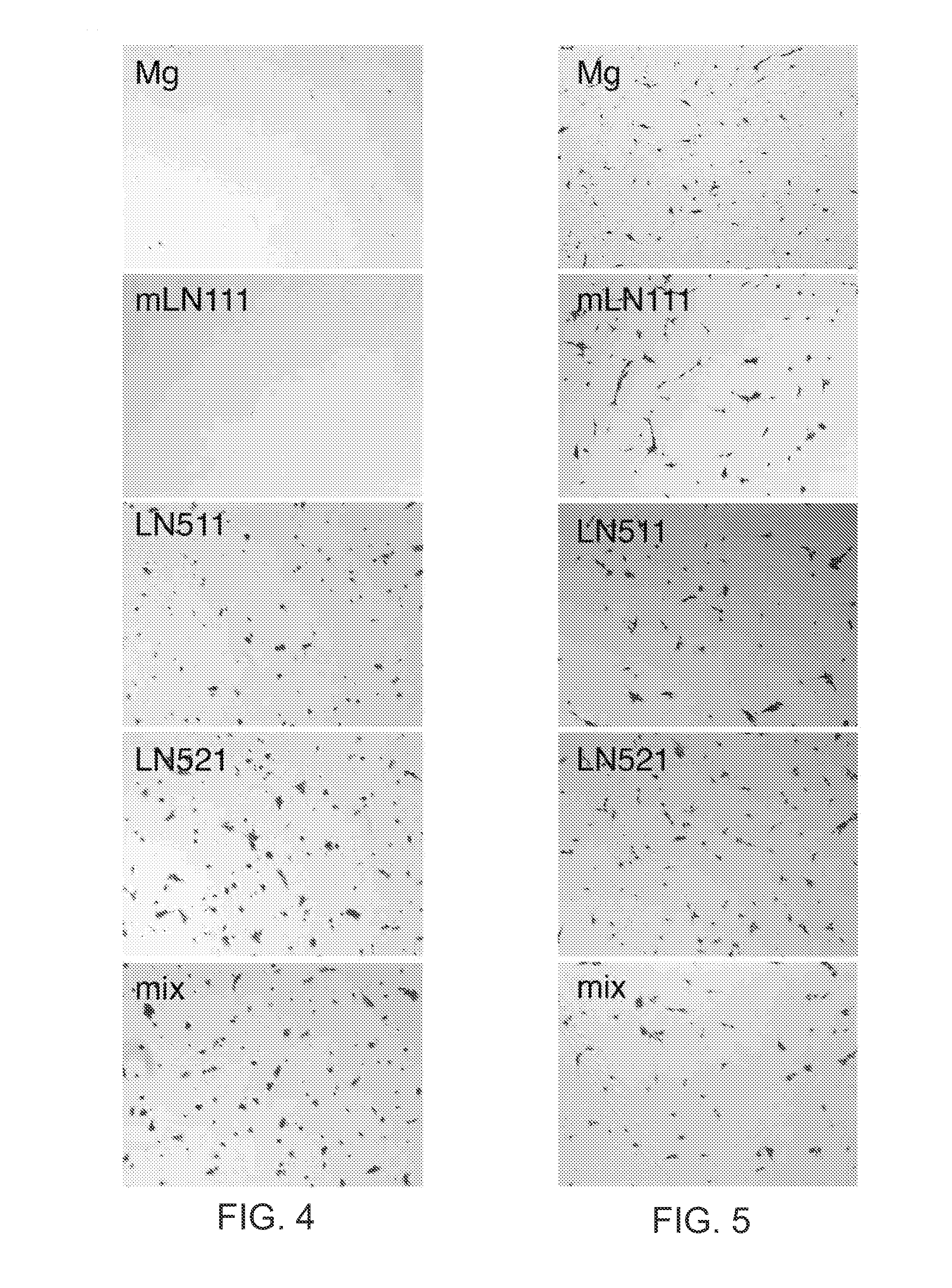
The present disclosure relates to the use of laminin-521 in obtaining retinal pigment epithelium (RPE) cells. Pluripotent human embryonic stem cells are cultured on plates coated with recombinant laminin-521 (laminin-11), in totally defined and xeno-free conditions. A first cell culture medium contains a growth factor, and a second cell culture medium does not contain growth factor. The stem cells are first exposed to the first cell culture medium, then exposed to the second cell culture medium for a longer time period. After a number of weeks, clinical grade RPE cells are obtained from the stem cells.
Owner:BIOLAMINA
Cryopreserving solution for clinical-grade CAR-T cryopreservation and capable of being directly reinfused through intravenous infusion
InactiveCN108552159AAvoid lostReduce workloadInorganic non-active ingredientsPharmaceutical delivery mechanismClinical gradeHydroxyethyl starch
The invention discloses a cryopreserving solution for clinical-grade CAR-T cryopreservation and capable of being directly reinfused through intravenous infusion. The cryopreserving solution comprisesthe following raw materials: dimethyl sulfoxide, a glycerol fructose sodium chloride injection, an invert sugar electrolyte injection, a dextran 40 glucose injection, a hydroxyethyl starch 130 / 0.4 electrolyte injection, a vitamin C injection, a human serum albumin injection and a 0.9 % sodium chloride injection; the cryopreserving solution has the pH value of 6.8-7.0. When a formula for the clinical-grade CAR-T cryopreservation and capable of being directly reinfused through the intravenous infusion is used for cryopreserving CAR-T cells, the thawed cells and the thawed cryopreserving solutiondo not need centrifugation, resuspension, fluid exchange and other processes and can be directly reinfused through the intravenous infusion, so that the loss of cell varieties due to pollution in-vitro repeated proliferation of the CAR-T cells, or changes of cell morphologies and cell functions are effectively avoided, medical equipment can be also significantly simplified and the workload of medical workers can be reduced.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Mesenchymal-like stem cells derived from human embryonic stem cells, methods and uses thereof
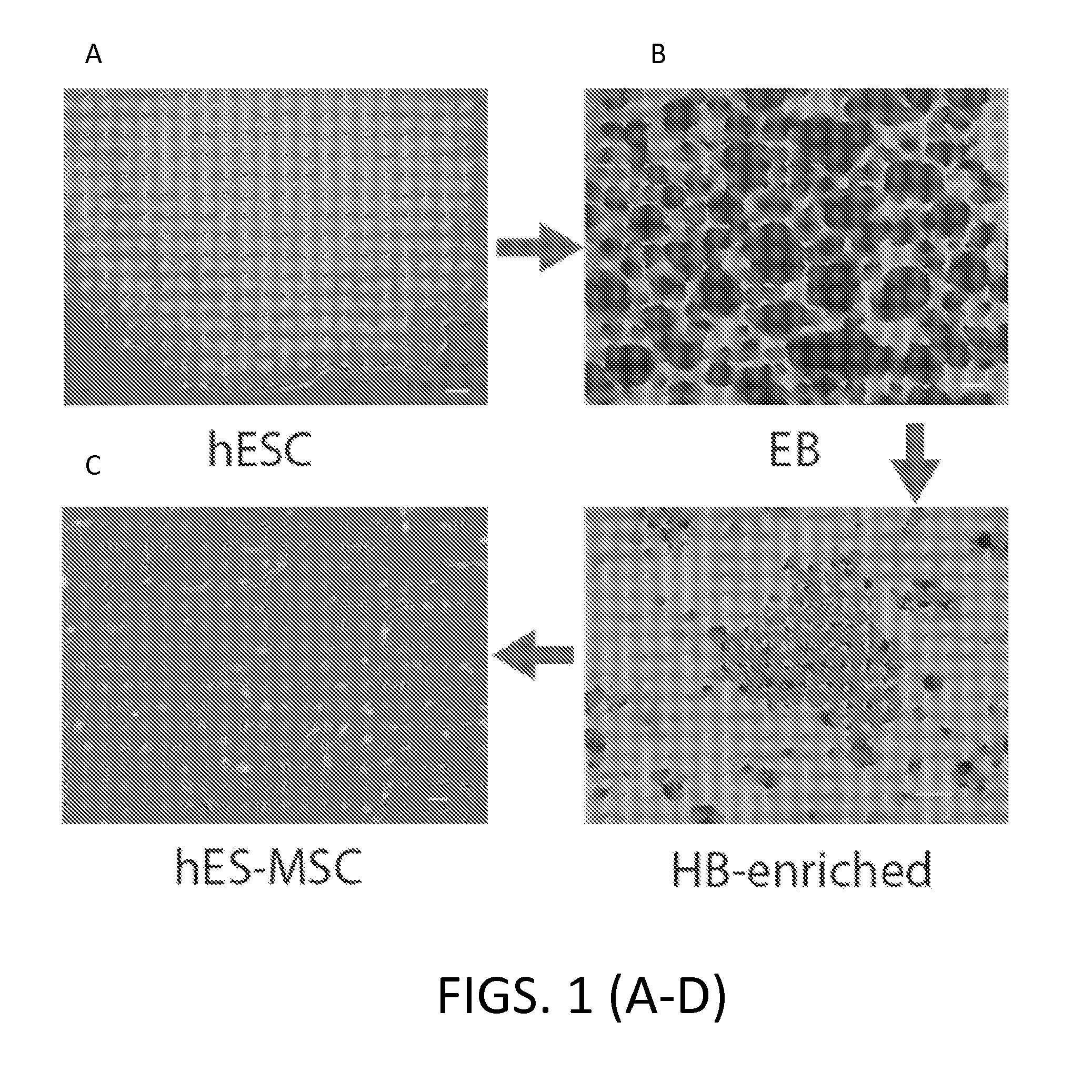
ActiveUS20150203820A1Improve efficiencyImprove consistencyBiocideMutant preparationClinical gradeAutoimmune condition
The present invention relates to methods of generating and expanding hitman embryonic stem eel! derived mesenchymal-like stem / siromal cells. These hES-MSCs are characterized at least in part by the low level of expression of IL-6. These cells are useful for the prevention and treatment of T cell related autoimmune disease, especially multiple sclerosis, as well as for delivering agents across the blood-brain barrier and the blood-spinal cord barrier. Also provided is a method of selecting clinical grade hES-MSC and a method of modifying MSC to produced a MSC with specific biomarker profile. The modified MSC are useful for treatment of various diseases.
Owner:ADVANCED CELL TECH INC
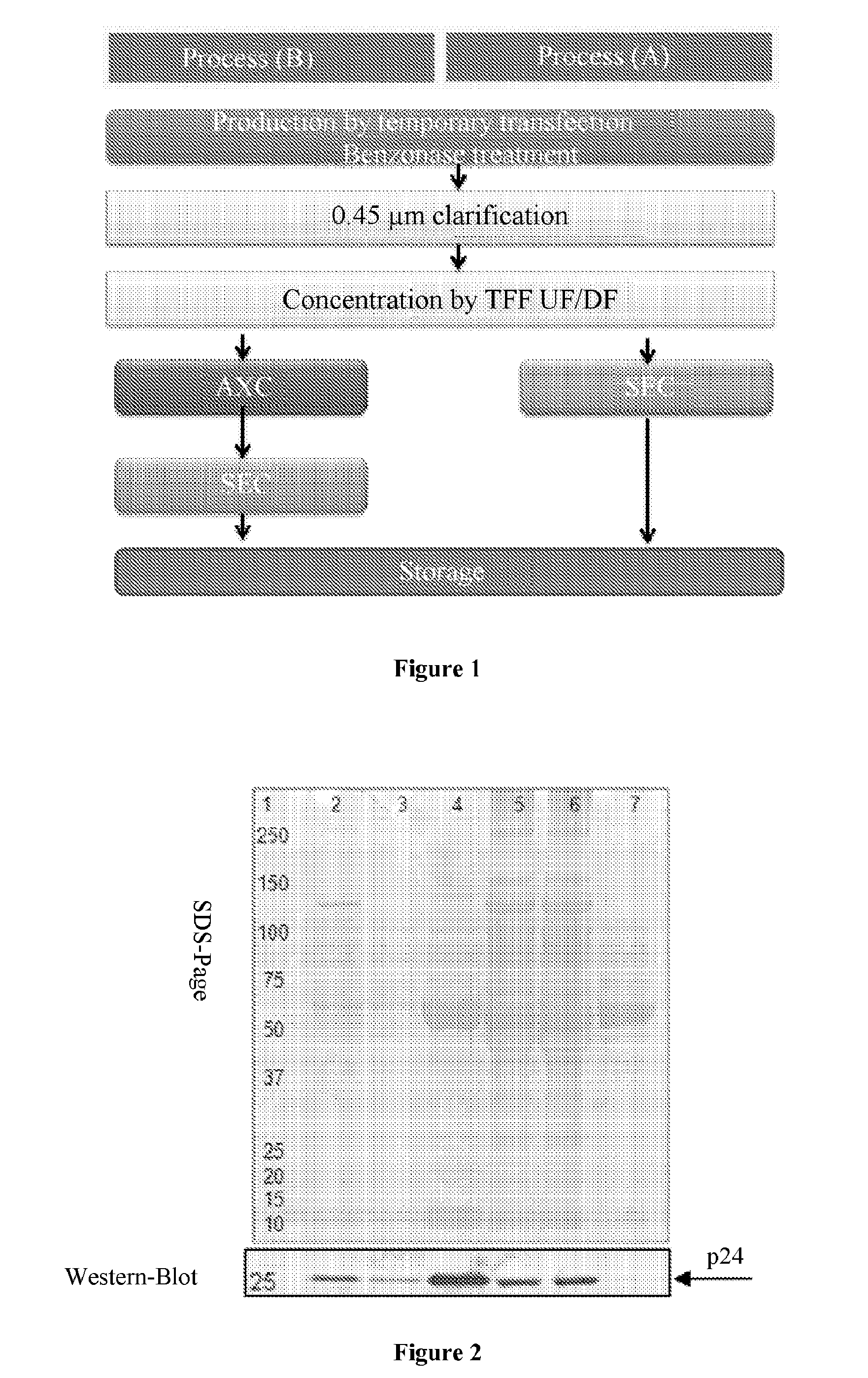
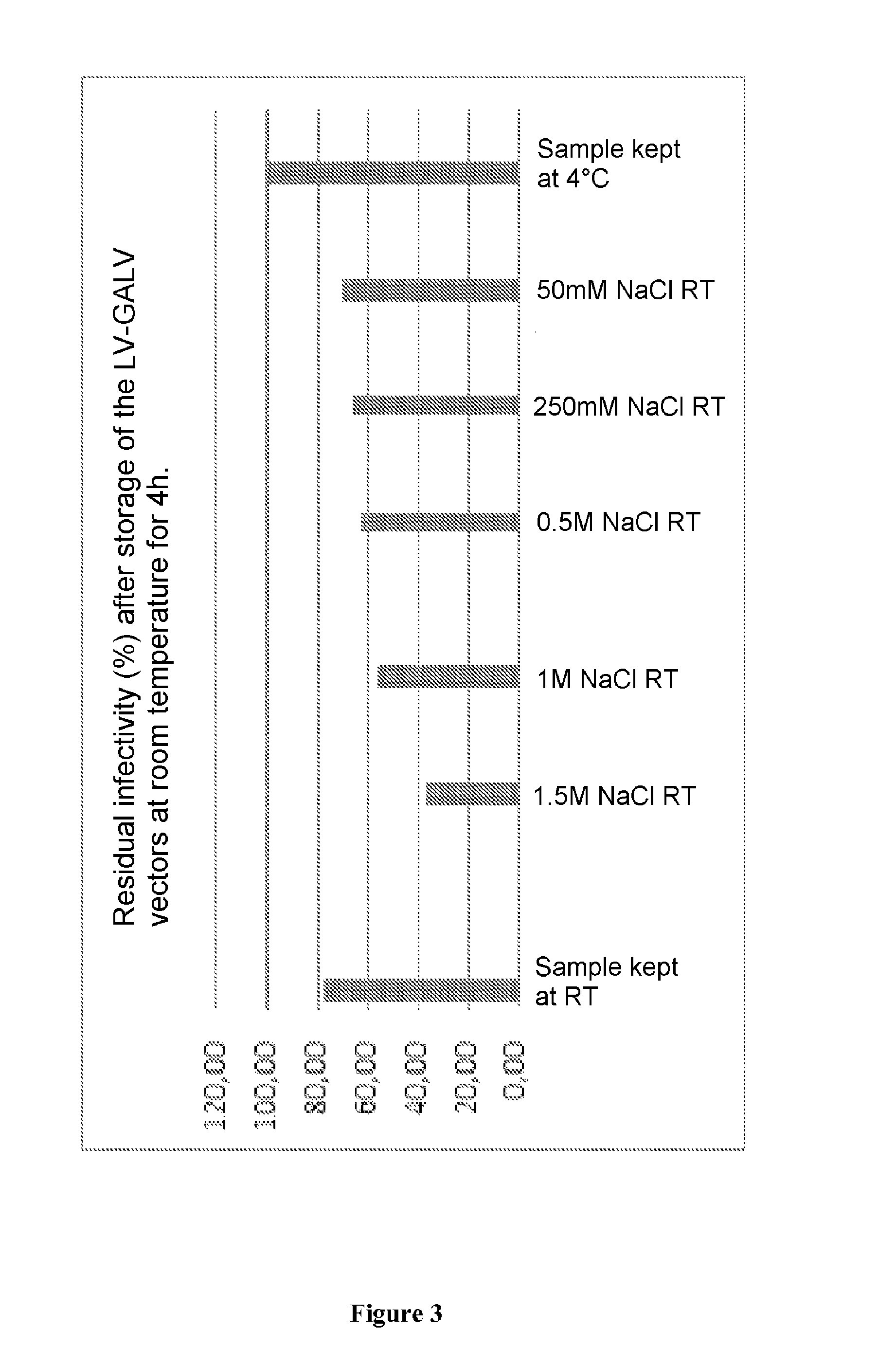
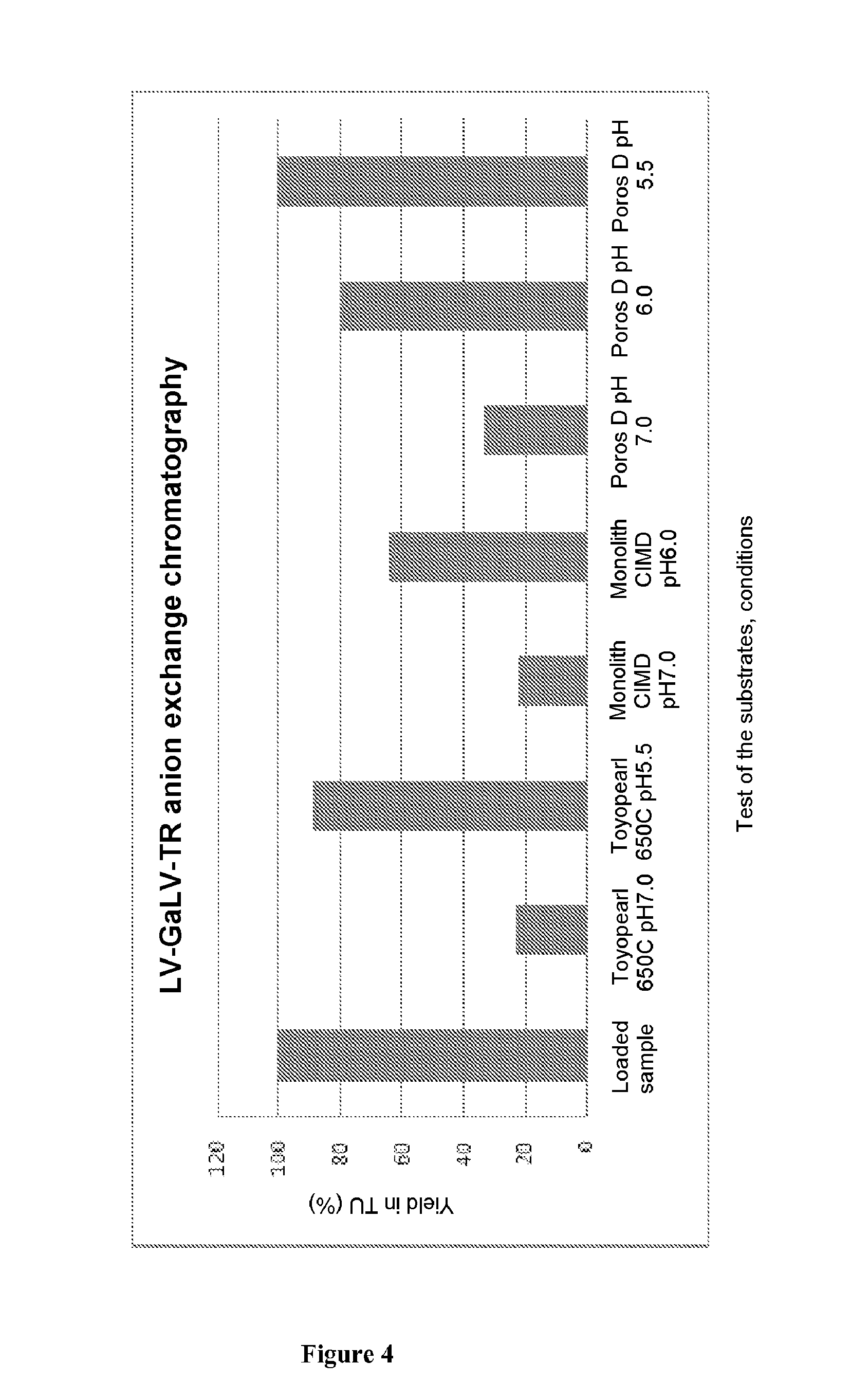
Method for purifying enveloped viruses or viral vectors
ActiveUS20170002332A1High purification yieldIncrease productionVector-based foreign material introductionReverse transcribing RNA virusesClinical gradeViral vector
The invention relates to a process for purifying enveloped viruses. The process of the invention is useful for recovering at a large scale enveloped viruses under conditions complying with good manufacturing practices and allowing viruses of a clinical grade to be obtained.
Owner:GENETHON
Purification process for piasmid DNA
ActiveCN101310022AMicrobiological testing/measurementMicroorganism lysisClinical gradePurification methods
Methods of isolating clinical-grade plasmid DNA from manufacturing processes, including large-scale fermentation regimes, are disclosed which encompass alternatives to two core unit operations common to plasmid DNA purification processes. The novel upstream and downstream purification processes disclosed herein provide for reduced production costs and increase process robustness. Either or both of the purification processes disclosed herein may be used in combination with additional purification steps known in the art that are associated with DNA plasmid purification technology.
Owner:MERCK SHARP & DOHME BV
Systems and methods for generating medical diagnosis
Presented are systems and methods that provide diagnostic measurement tools that enable even laymen to reliably and accurately perform clinical-grade diagnostic measurements of their key medical instrument measured data with little or no intervention by a health care professional and to engage in some level of self-diagnosis to detect acute conditions, previously the exclusive domain of health care professionals. In various embodiments, this is accomplished by using an automated remote (or local, e.g., in the form of a kiosk) medical diagnostic system that provides clear and concise audio / video guidance to the patient and monitors the patient's equipment usage to generate high-accuracy measurement data that utilizes a diagnostic engine to provide an output of potential diagnosis that may be analyzed locally and shared with health care professionals and specialists, as needed.
Owner:ADVINOW INC
Clinical-grade serum-free cell cryopreservation solution
InactiveCN109122670AImprove recovery rateFree from damageDead animal preservationClinical gradeMammal
The invention discloses a serum-free cell cryopreservation solution which comprises an amino acid component, an inorganic salt component, an extracellular cryoprotectant, an intracellular cryoprotectant and the like. The cell cryopreservation solution disclosed by the invention has the advantages that double protective components are contained and a cell is simultaneously protected from ice crystal damage during cryopreservation intracellularly and extracellularly, so that the recovery rate of the cell is greatly increased; the cell cryopreservation solution is suitable for extensive cell linepreservation, can be used for long-term preservation of cells and can be applied to establishment of cell banks, scientific cell line preservation and long-term preservation of clinical mesenchymal stem cells, monocytes, immune cells and all mammalian cells involved; and meanwhile, the cell cryopreservation solution is a completely serum-free, protein-free and dimethylsulfoxide (DMSO)-free cryopreservation solution, so that heterologous exogenous components introduced in clinical applications are avoided and the solution is safer.
Owner:YOCON BIOLOGY TECH CO
Clinical grading diagnosis and treatment data management system for diabetic retinopathy patients
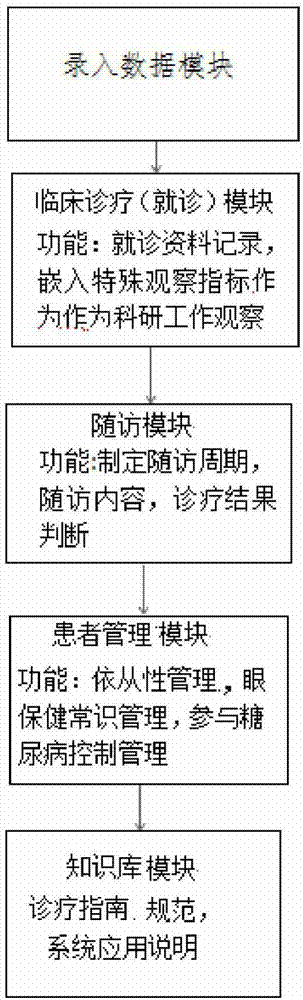
InactiveCN107330260ARealize informatizationImprove the effect of disease preventionSpecial data processing applicationsClinical gradePatient questionnaire
The invention discloses a clinical grading diagnosis and treatment data management system for diabetic retinopathy patients, and belongs to the technical field of computer database software application. A database module is used for storing all information data and classifying and managing information, and is used for compressing, transmitting and reading the information data by means of information security confidentiality technology. A clinical diagnosis and treatment module is used for providing a complete clinical diagnosis and treatment process and conducting follow-up records according to a follow-up period. A clinical follow-up module is used for providing clinical specialized medical service and accumulating an original scientific research data platform. A patient management module is used for guiding the patients to participate in the clinical diagnosis and treatment process, evaluating the patient eye care present situation, including eye care present situation questionnaires and life quality records, and conducting supervision on self-management in the patient diagnosis process. A knowledge base module is used for storing a guide of current diabetic retinopathy treatment and relevant knowledge of the patient questionnaires, so that medical workers standardize their diagnosis and treatment activities according to the guide and provide the specialized medical service for the patients.
Owner:THE 306TH HOSPITAL OF PLA
Midbrain dopaminergic nerve precursor cells and preparation method and application thereof
InactiveCN109880800AFunctionalClinical majorNervous disorderNervous system cellsClinical gradeMidbrain
The invention relates to the field of stem cell biology, in particular to midbrain dopaminergic neural precursor cells and a preparation method and application thereof. The midbrain dopaminergic neural precursor cell expresses Lmx1a+, Foxa2+, En1+ and Otx2+, wherein the midbrain dopaminergic neural precursor cell does not express one or more of Nkx2.1, DBX1 and GBX2. The method for preparing the mesencephalic dopaminergic neural precursor cell comprises the following steps: embryoid bodies are formed, midbrain floor cells are obtained, and midbrain dopaminergic neural precursor cells are obtained. By adopting the preparation method, the mesencephalic dopaminergic nerve cells can be rapidly and efficiently differentiated from the human pluripotent stem cells, and the problems are solved that an existing differentiation method is long in time, unstable in effect and low in efficiency, the culture system containing serum or the trophoblast cells are not conducive to the production of subsequent clinical-grade cell preparations and the like.
Owner:安徽中盛溯源生物科技有限公司
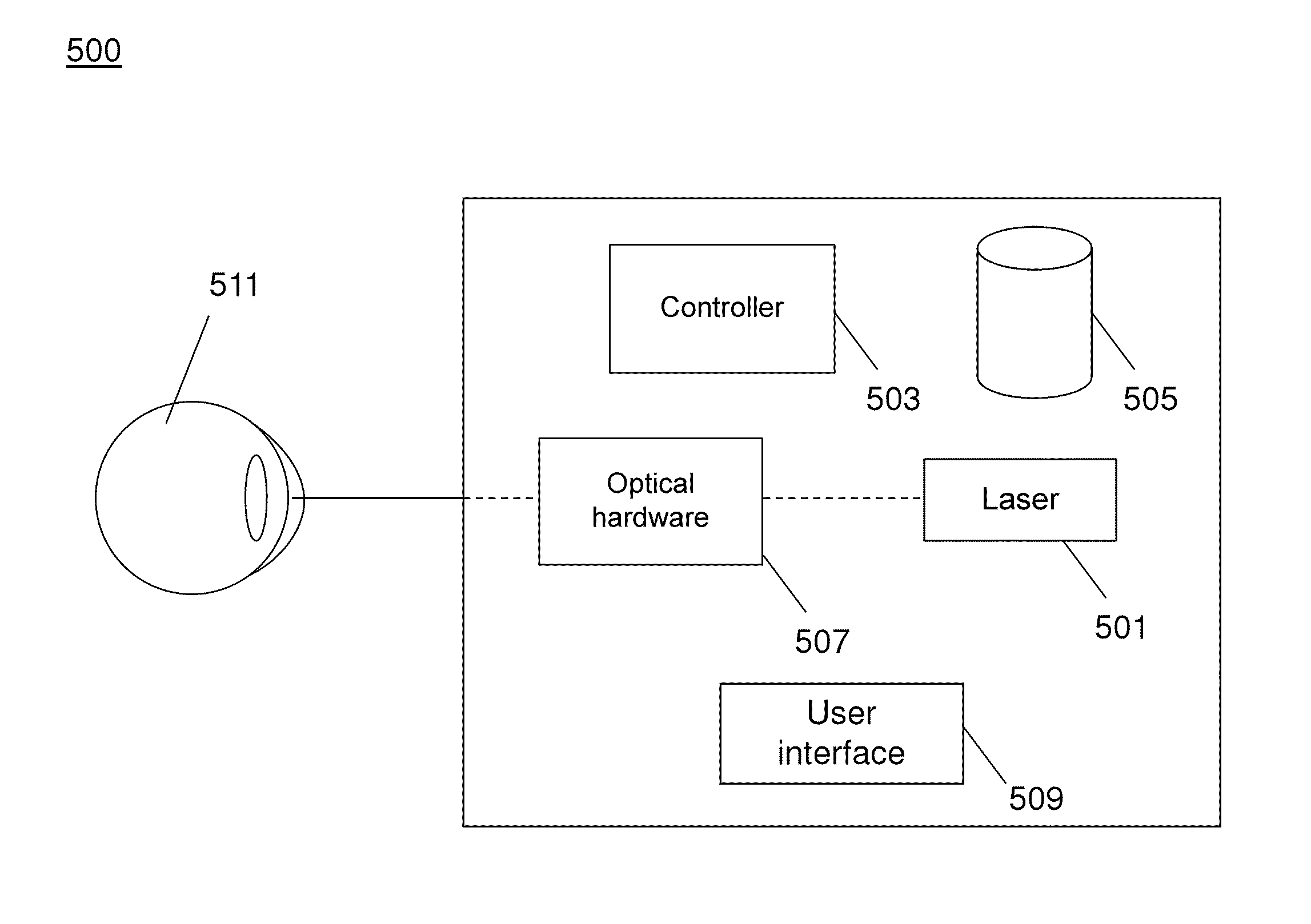
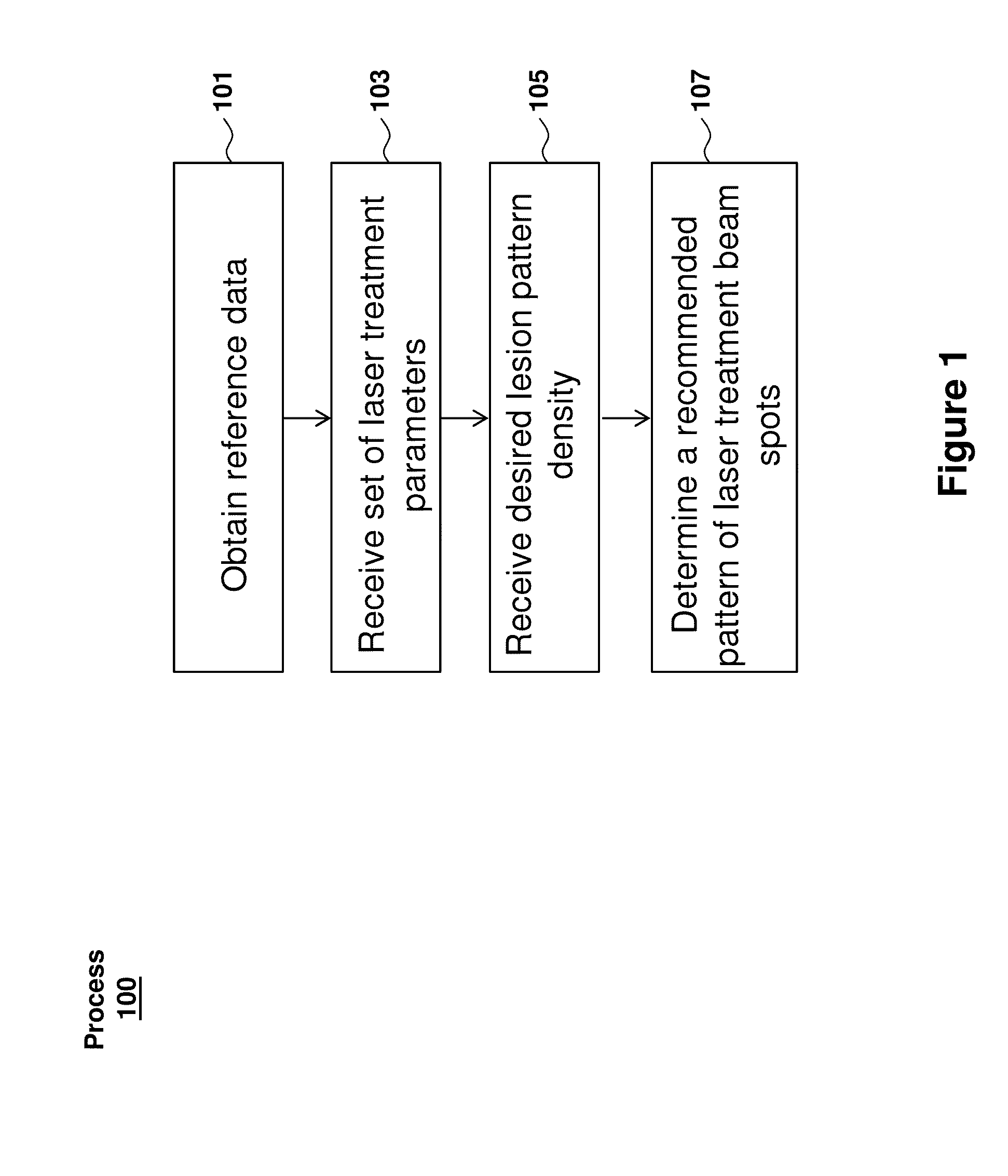
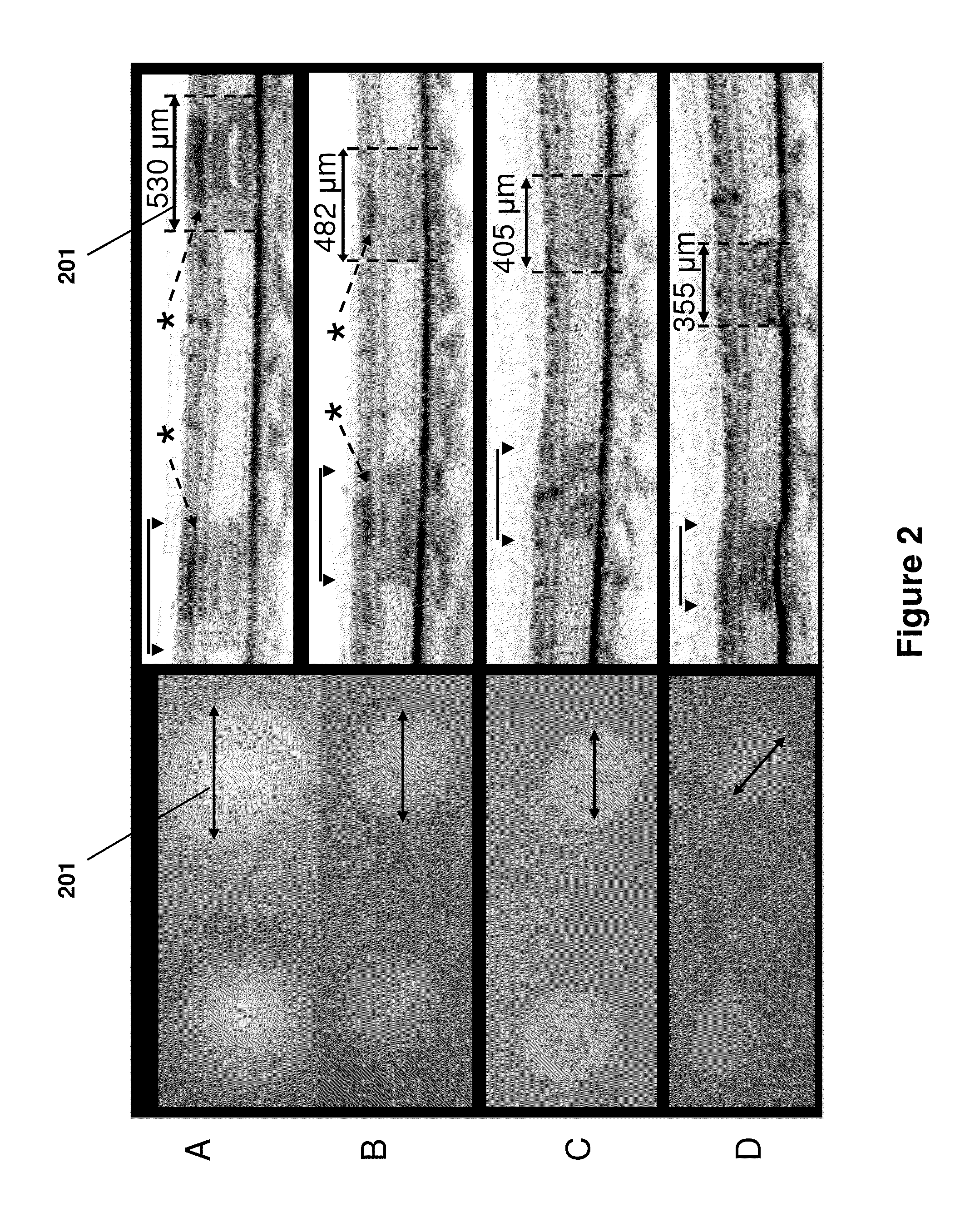
Optimization of laser therapy
Systems and processes for the optimization of laser treatment of an eye are disclosed. The process can include receiving a set of parameters of a laser treatment (e.g., an aerial beam size, contact lens, pulse duration, and the desired clinical grade), determining an estimated size of a lesion to be generated by the laser treatment beam, receiving a lesion pattern density (e.g., full grid, mild grid, or other), and determining a recommended pattern of laser treatment beam spots. The recommended pattern of laser treatment beam spots may include a recommended number of laser treatment spots and a spacing between the spots.
Owner:IRIDEX CORP
Culture method of DC cell for enhancing CTL immune response
ActiveCN105018427AGood effectGood curative effectBlood/immune system cellsClinical gradeSerum free media
The invention discloses a culture method of DC cell for enhancing CTL immune response. According to the method, expression of Ube1L in the protein ubiquitination pathway is knocked out by sh-RNA, and tumor antigen-presenting ability of DC cell is effectively enhanced. More effective in-vivo tumor-killing capacity is exerted. A clinical-grade serum-free medium is used for culture of DC cell. By adding 1% human albumin and changing dose proportion of cytokines, culture time is shortened to 4 days, and time, space and personnel occupancy are minimized. By the method, types and dosage of a maturity-accelerating cytokine combination are adjusted, and the mature DC cell obtained can more effectively promote CD8+CTL cell proliferation. Therefore, antineoplastic curative effect is enhanced.
Owner:丛秀丽
Method for reproducible differentiation of clinical-grade retinal pigment epithelium cells
InactiveUS20190169569A1Efficiency sometimes variesNot scalableCulture processNervous system cellsClinical gradeSingle cell suspension
Provided herein are methods of producing an RPE cell population from a starting cell suspension, such as a single cell suspension, of pluripotent stem cells (PSCs). Such a method may comprise culturing the starting single cell suspension of PSCs in differentiation media to produce human RPE cells.
Owner:FUJIFILM CELLULAR DYNAMICS INC +1
Preparation method of clinical-grade neural stem cell
ActiveCN108070558ANervous system cellsCell culture active agentsClinical gradeTumor necrosis factor alpha
The disclosure provides a preparation method of a clinical-grade neural stem cell. The preparation method comprises the following steps of a), providing a single neural stem cell; b), inoculating theneural stem cell into a conventional culture flask, and subsequently replacing with a low-adsorption culture flask; c), adding a tumor necrosis factor-alpha, albumin, B27 and an animal-free protogonocyte factor into a culture medium to stimulate the multiplication of the neutral stem cell; d), carrying out half-amount medium change according to a growth status; e), carrying out digestion and passage according to the growth status; f), harvesting to obtain a high-purity, high-activity and further high-differentiation-potential clinical-grade neural stem cell. The method provided by the disclosure is simple to operate; the obtained cell is high in purity and further high in multiplication capacity, and the neutral stem cell prepared through the method provided by the disclosure meets a clinical-grade requirement.
Owner:JILIN TUO HUA BIOTECH
Genetically engineered natural killer cell product for treating tumor
InactiveCN108531458AFunction increaseImprove securityGenetically modified cellsMammal material medical ingredientsClinical gradeAllogeneic cell
Provided are genetically engineered natural killer cell products for treating tumors. The cell products are genetically engineered to stably transduce human IL-2 (Interleukin-2) and HSV-tk (herpes simlex virus-thymidine kinase) genes into natural killer cells (NK). The cell products are allogeneic cell products with good stability, anti-tumor effect and safety and can be produced on a large scalewithout the adding of the IL-2. The genetically engineered natural killer cell products (NK) can be prepared into clinical grade gene cell therapeutic products for the treatment and prevention of various malignant tumors in humans.
Owner:SYNO SHENZHEN BIOMEDICAL RES CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com