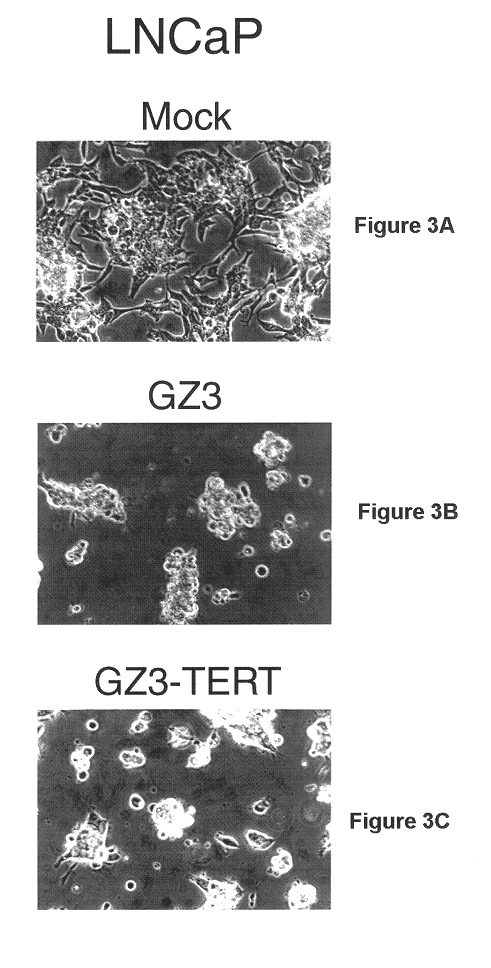
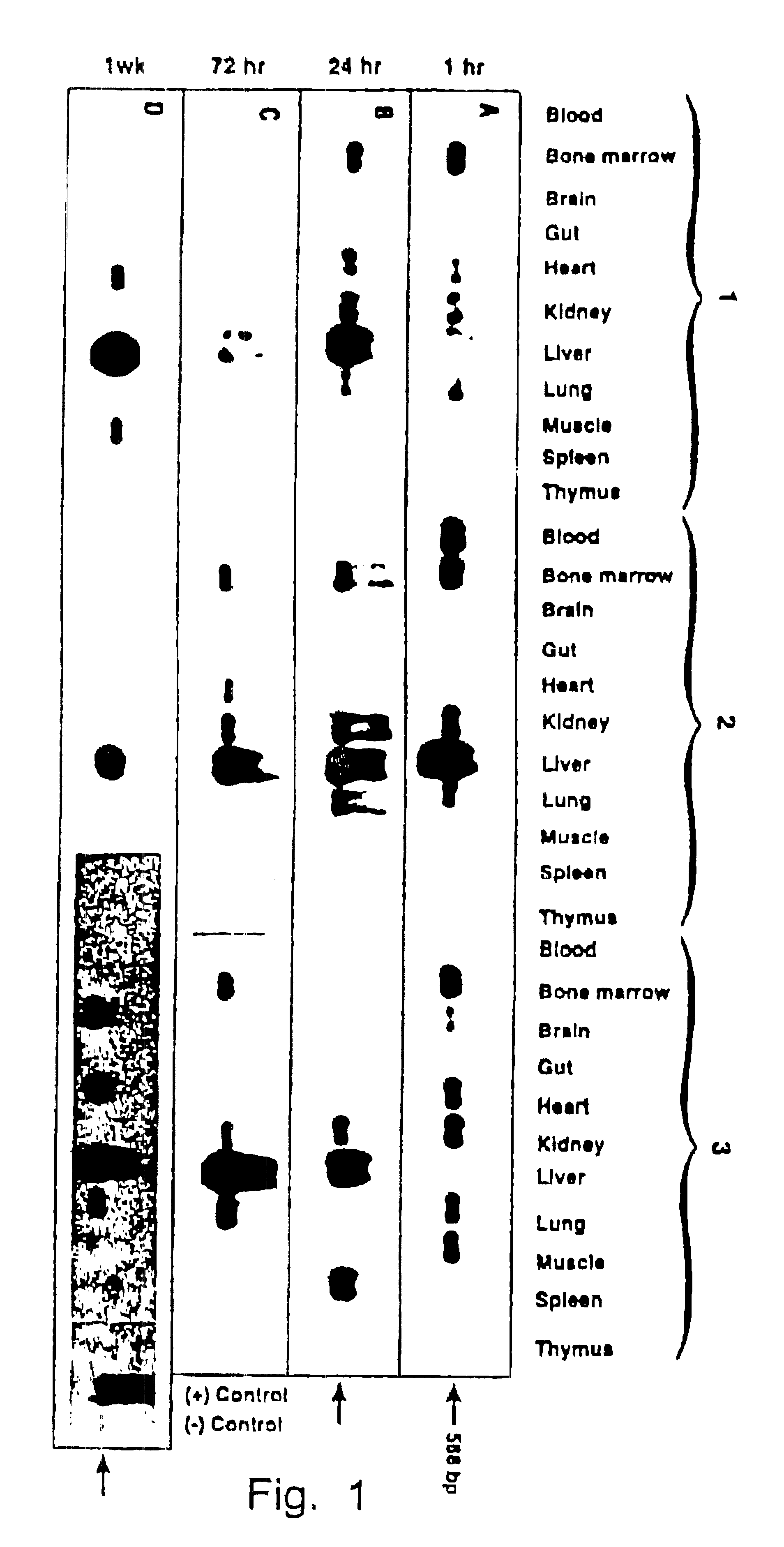
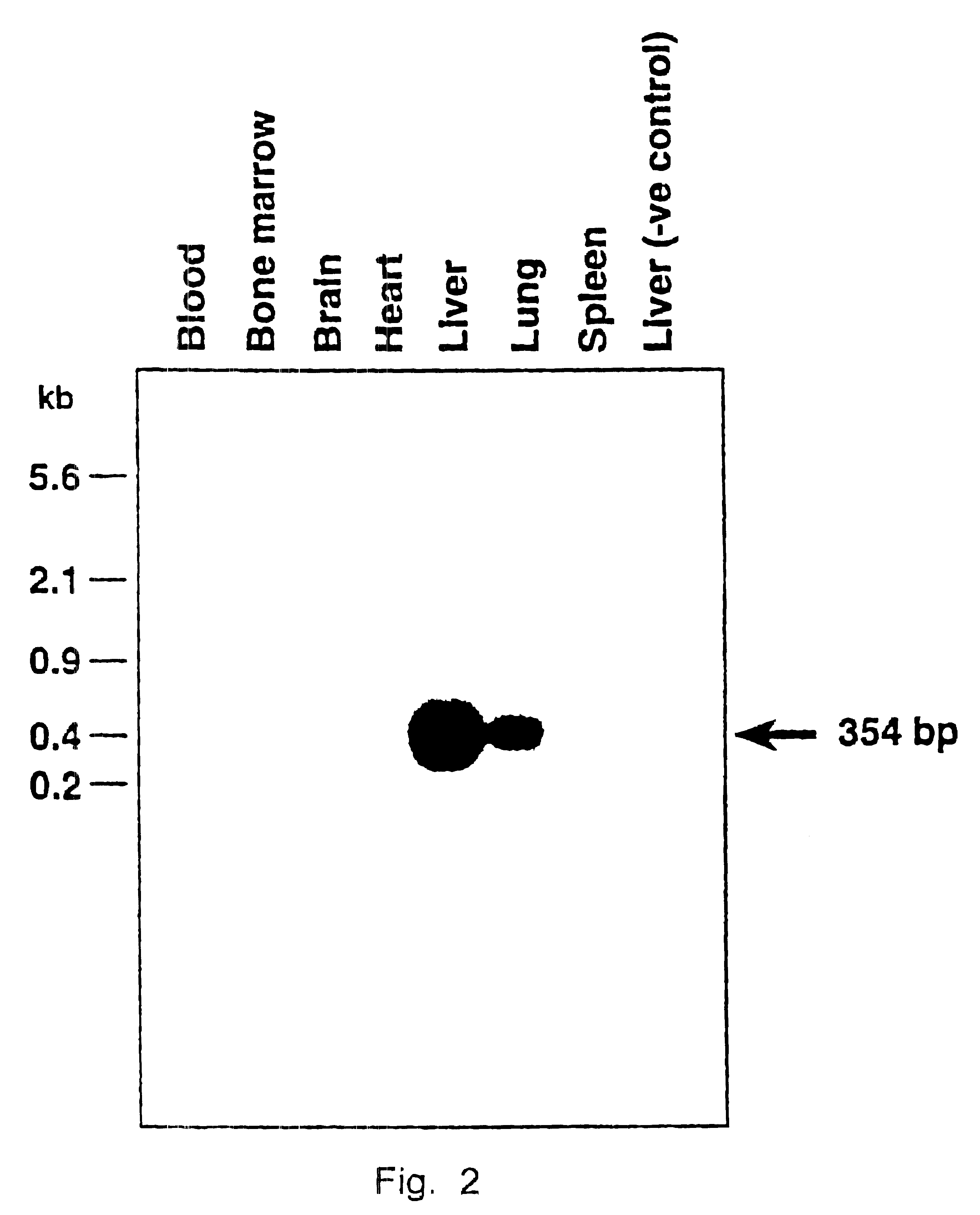
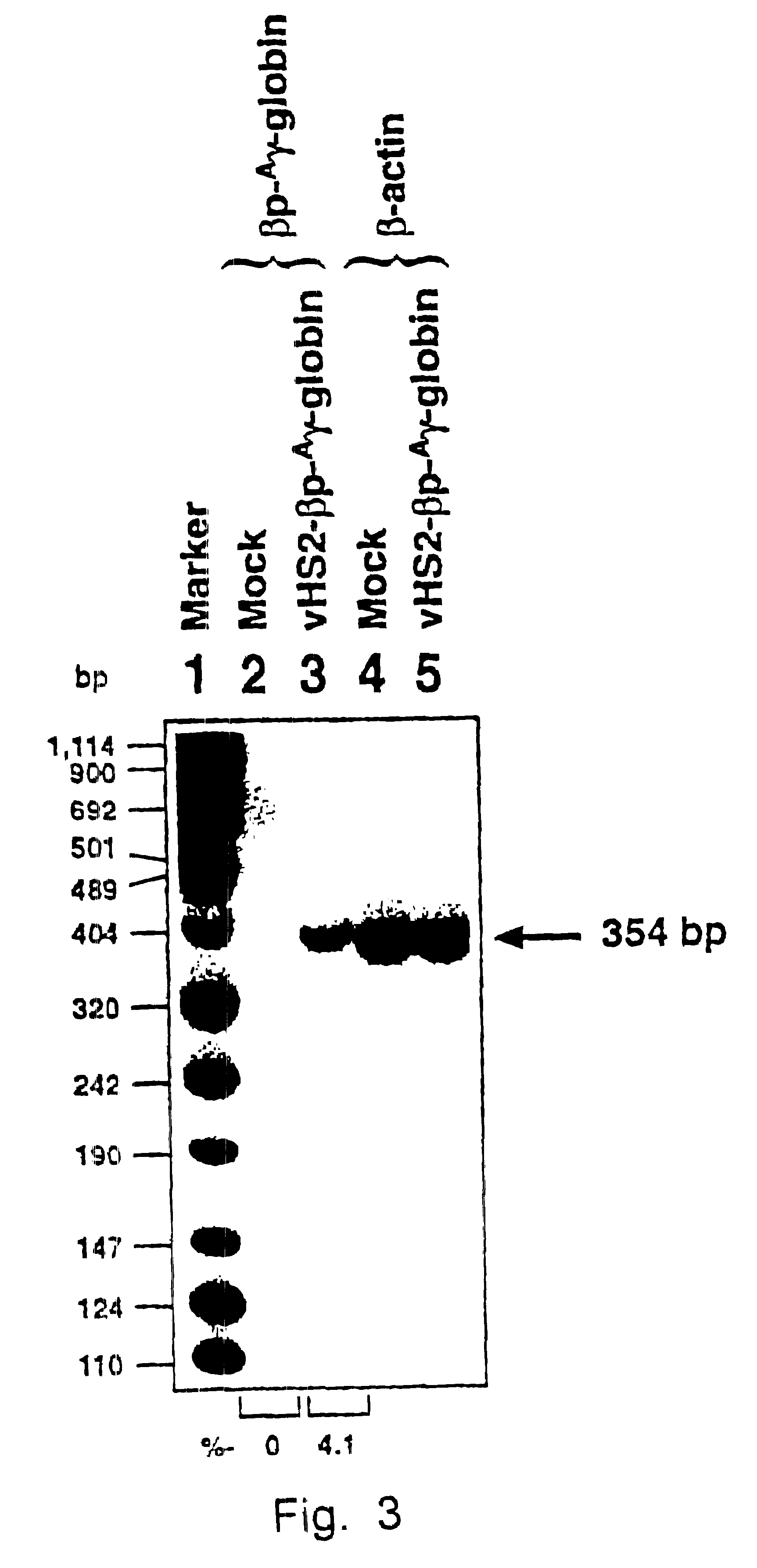
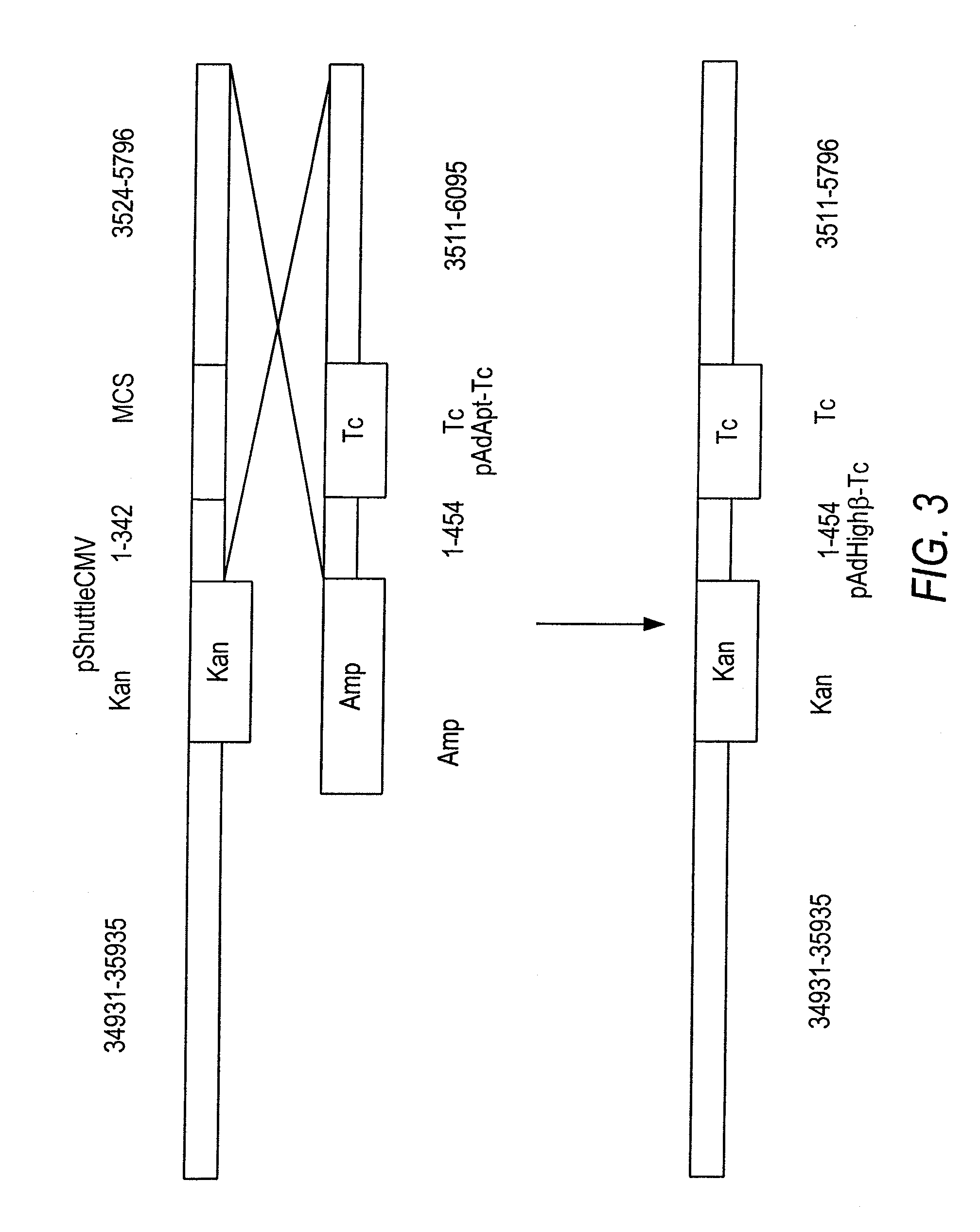
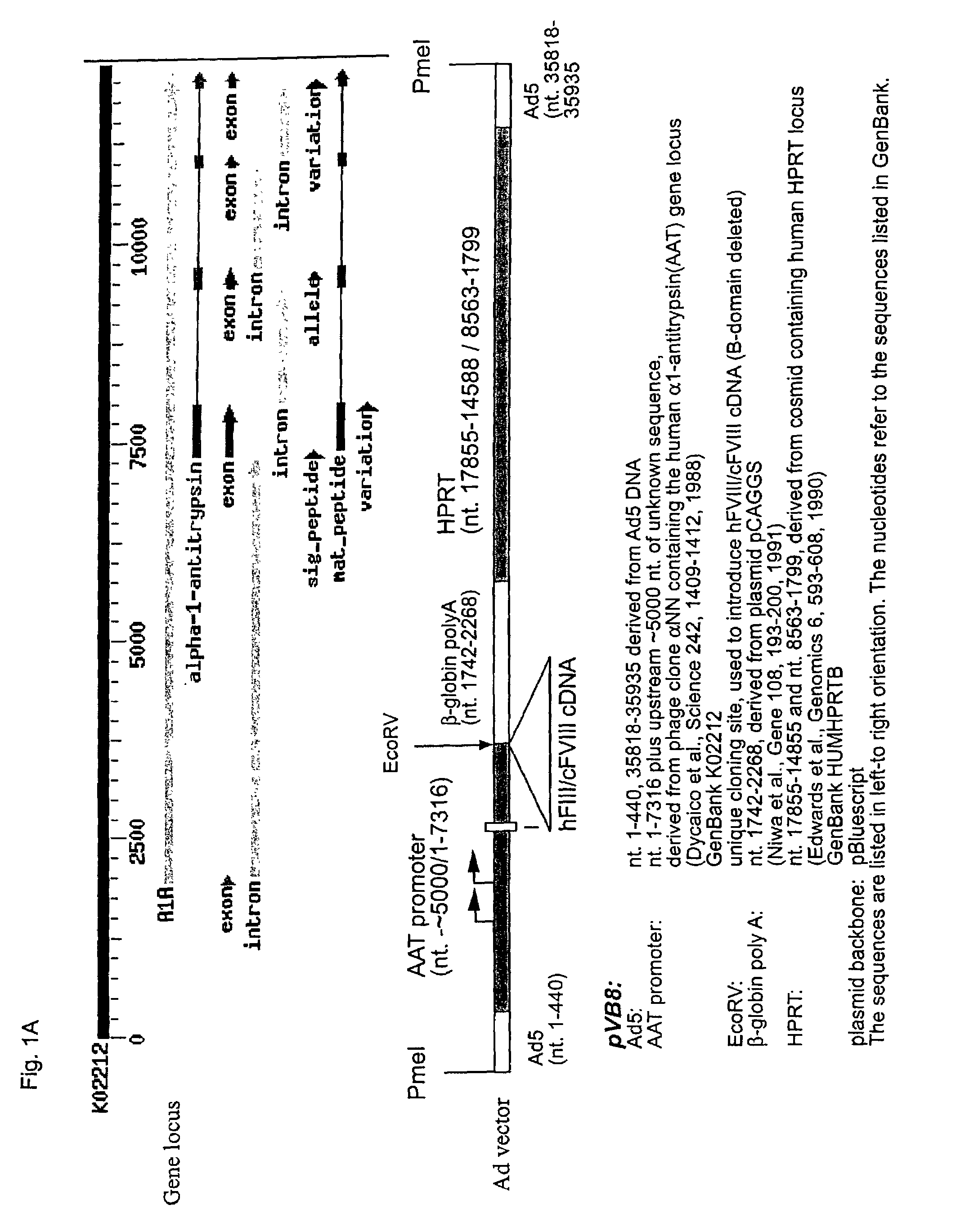
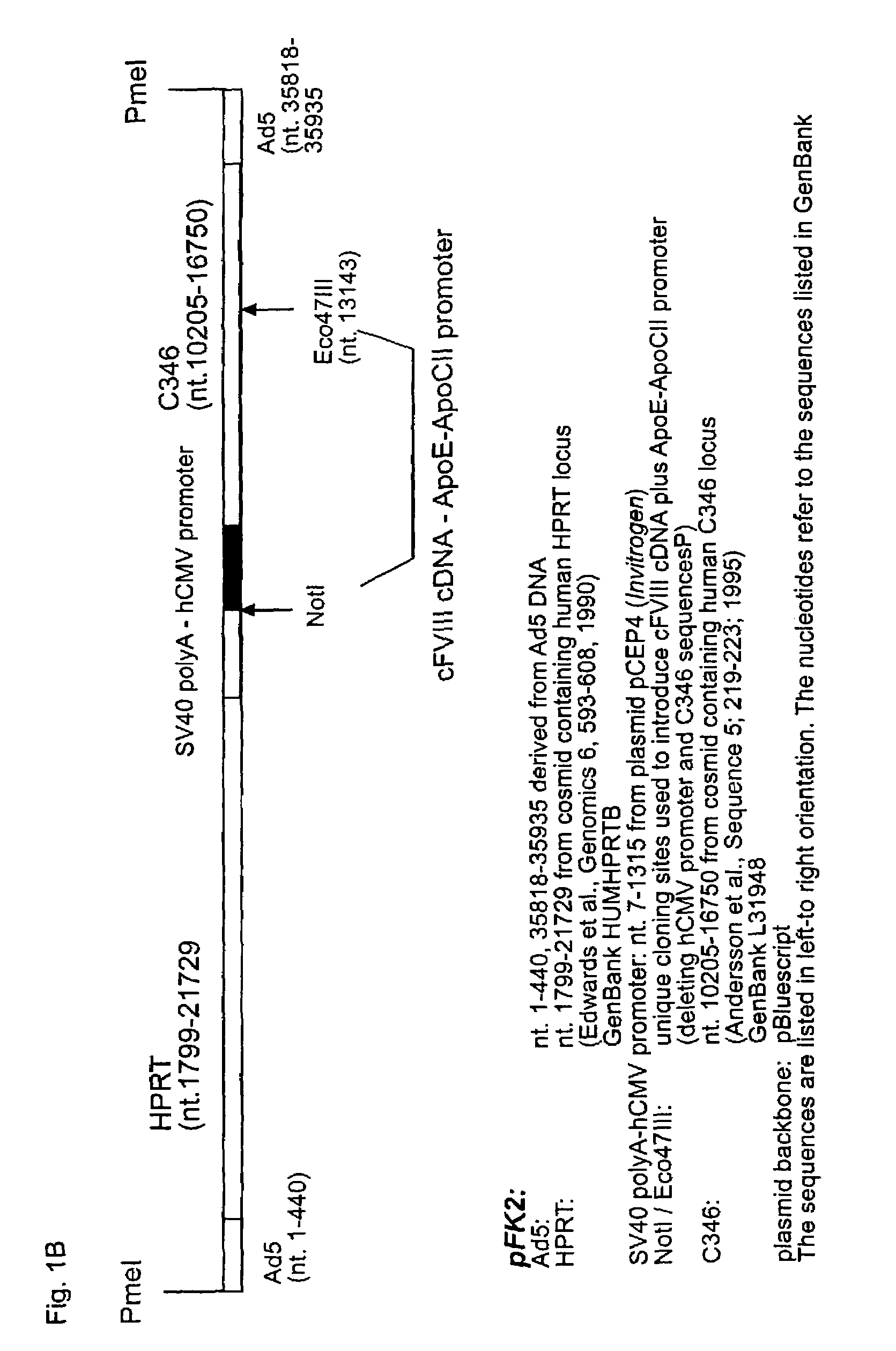
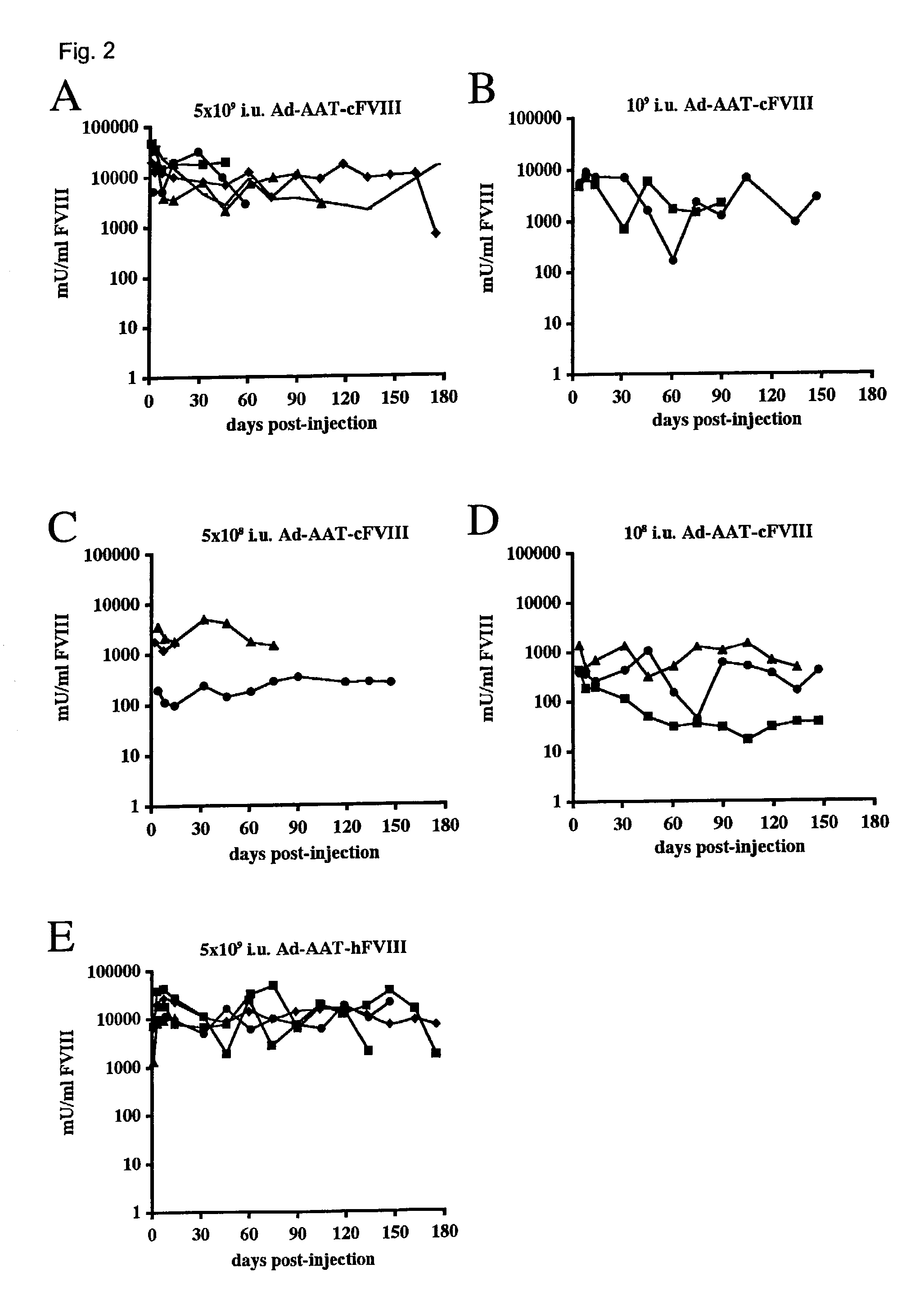
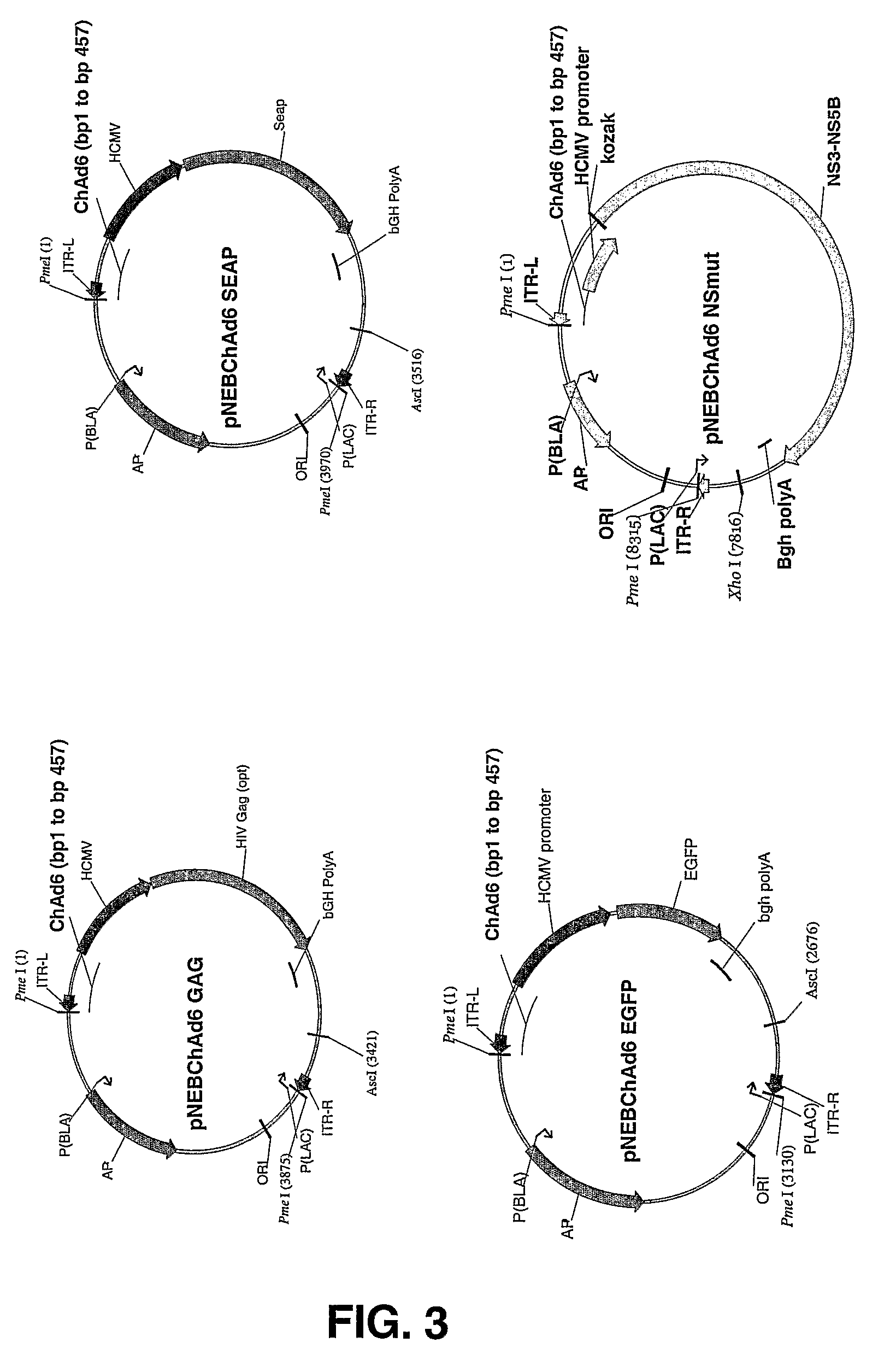
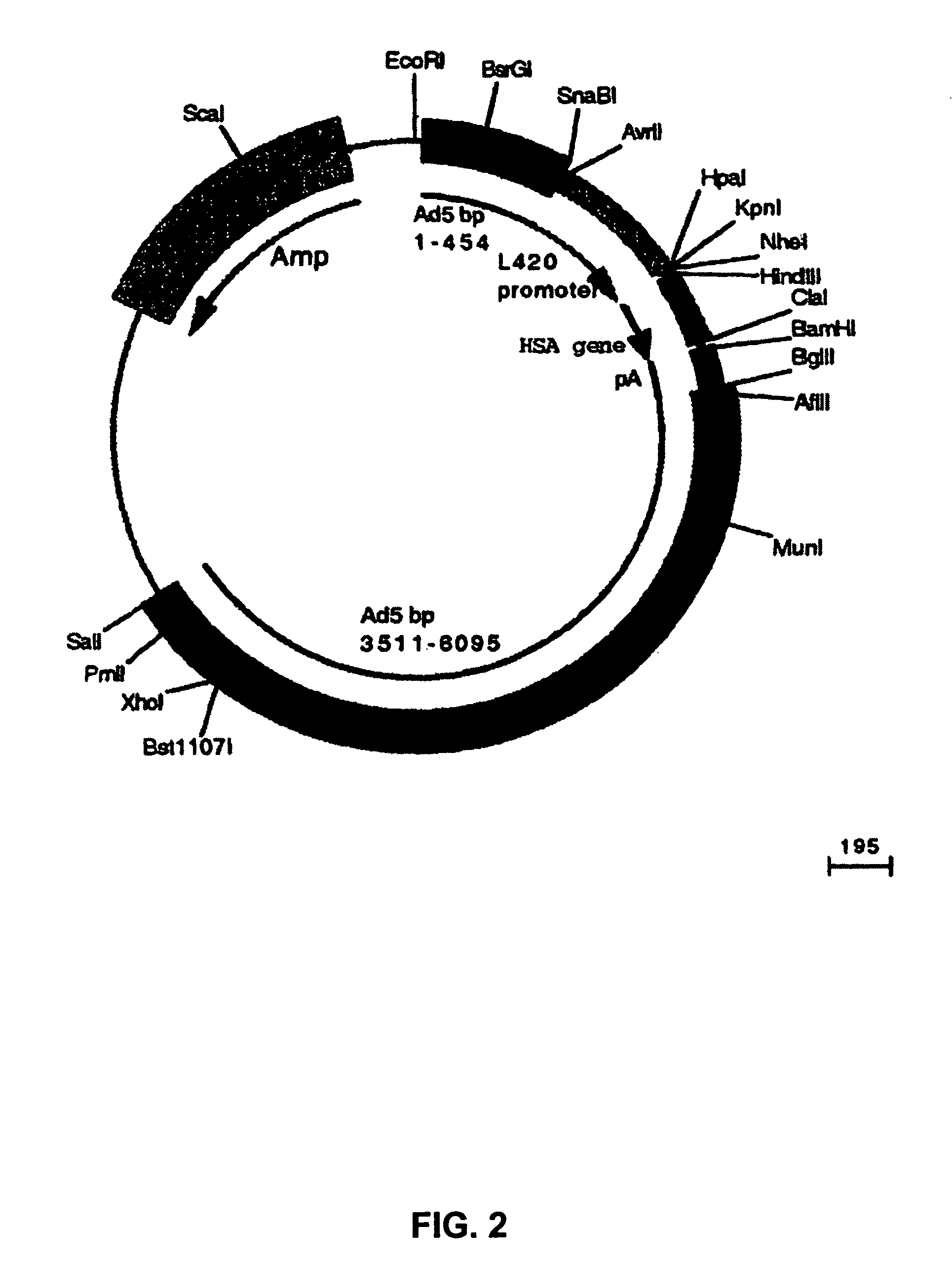
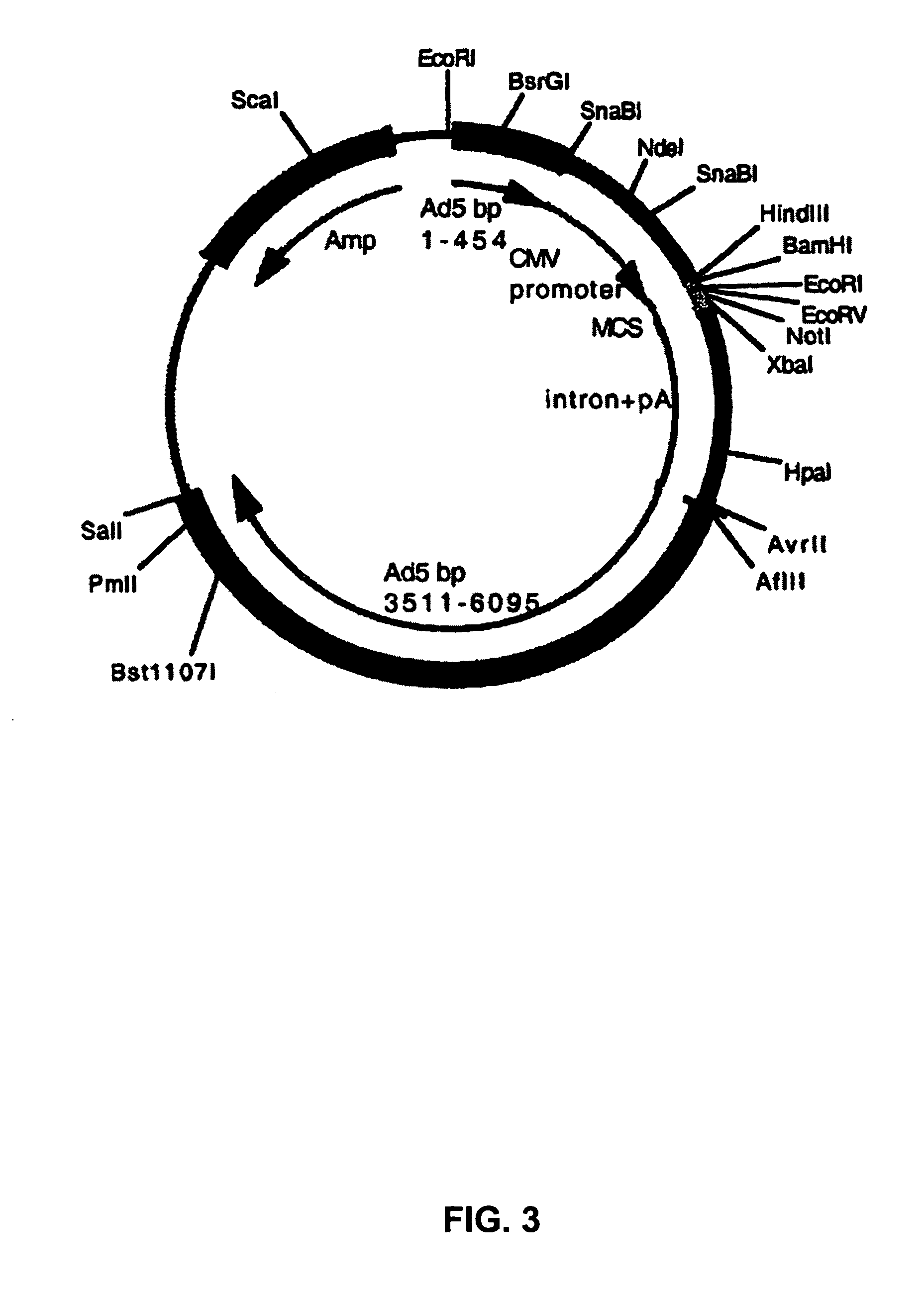
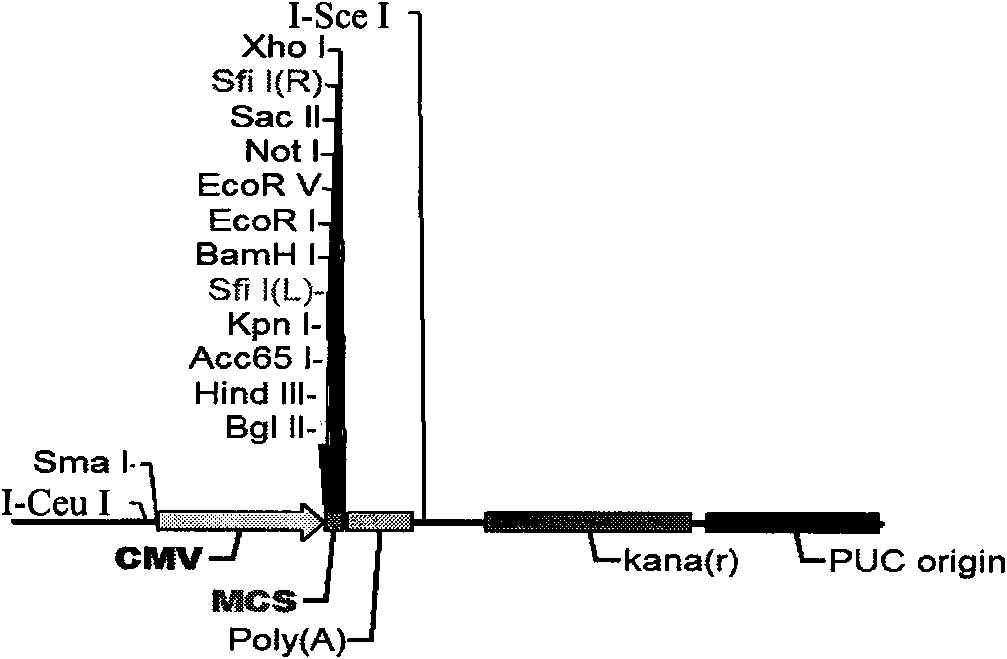
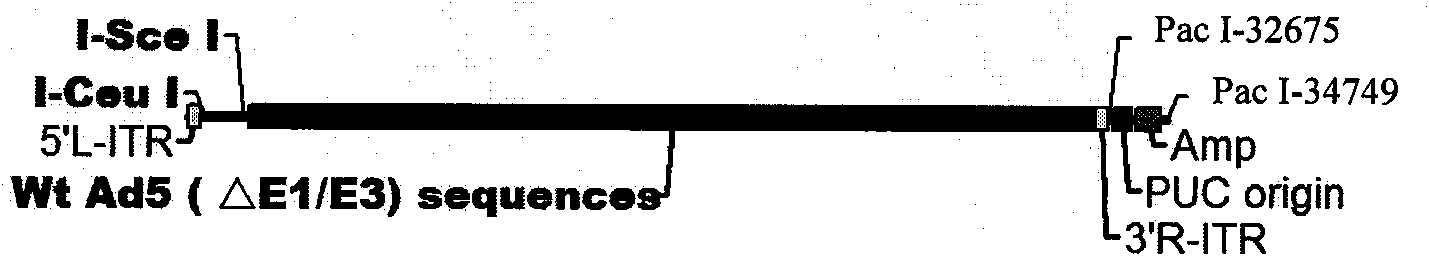Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
782 results about "Recombinant adenoviruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
Adenoviral vector and methods for making and using the same
In vitro methods for making a recombinant adenoviral genome, as well as kits for practicing the same and the recombinant adenovirus vectors produced thereby, are provided. In the subject methods, the subject genomes are prepared from first and second vectors. The first vector includes an adenoviral genome having an E region deletion and three different, non-adenoviral restriction endonuclease sites located in the E region. The second vector is a shuttle vector and includes an insertion nucleic acid flanked by two of the three different non-adenoviral restriction endonucleases sites present in the first vector. Cleavage products are prepared from the first and second vectors using the appropriate restriction endonucleases. The resultant cleavage products are then ligated to produce the subject recombinant adenovirus genome. The subject adenoviral genomes find use in a variety of application, including as vectors for use in a variety of applications, including gene therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
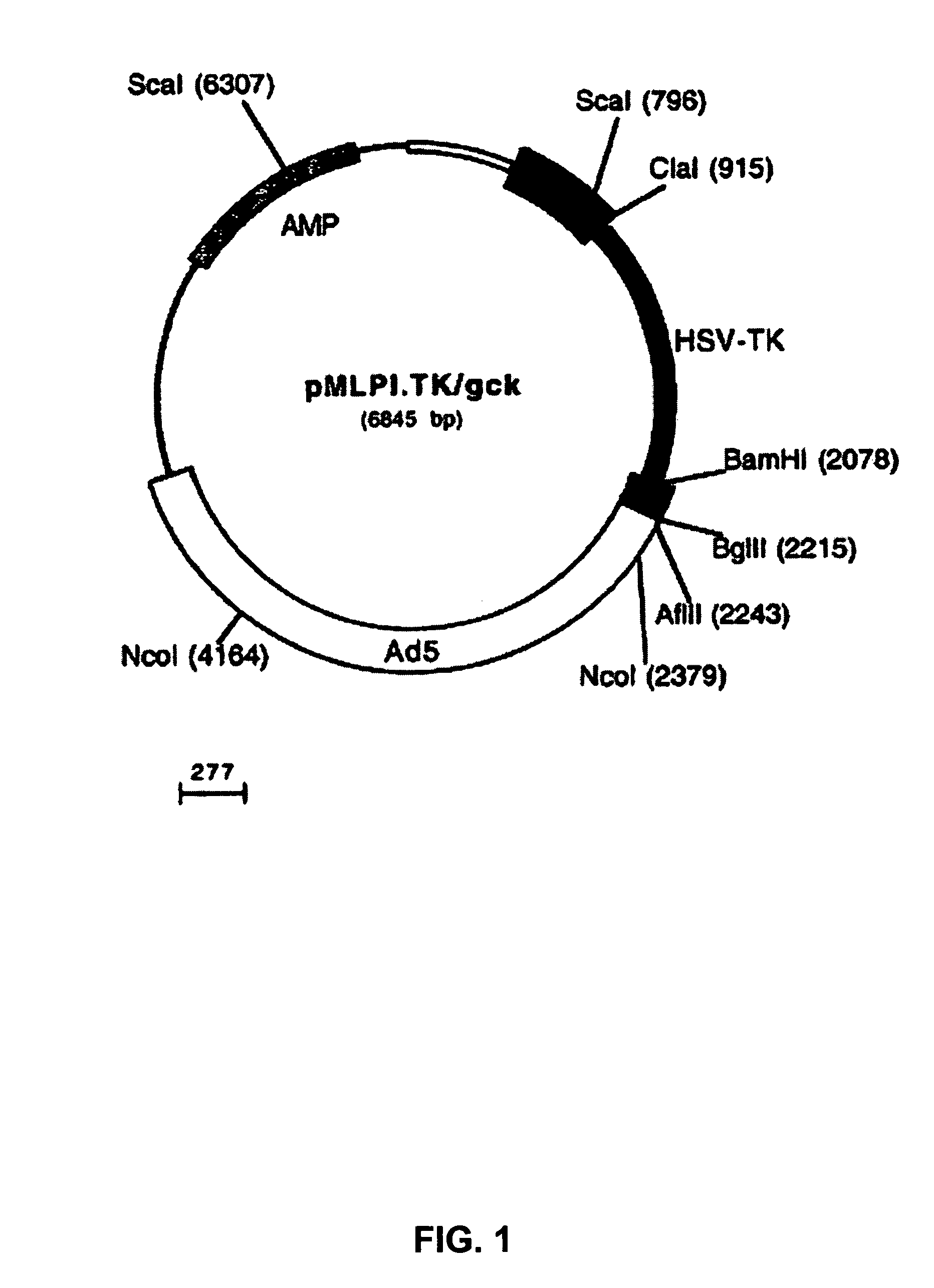
Helper-free, totally defective adenovirus for gene therapy
InactiveUS6228646B1Encapsidation efficiencyMaximal recombinationInactivation/attenuationNucleic acid vectorIn vivoHelper virus
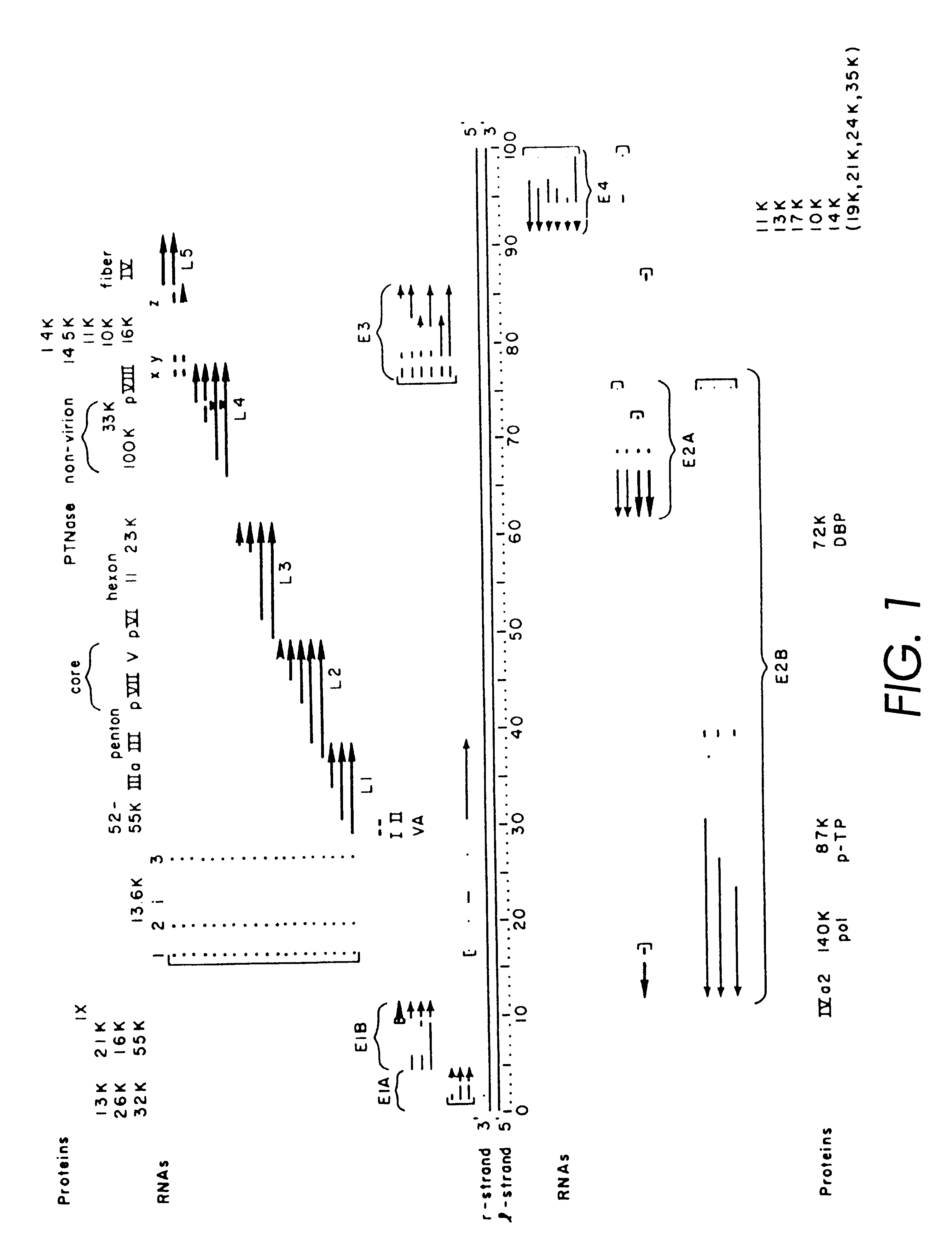
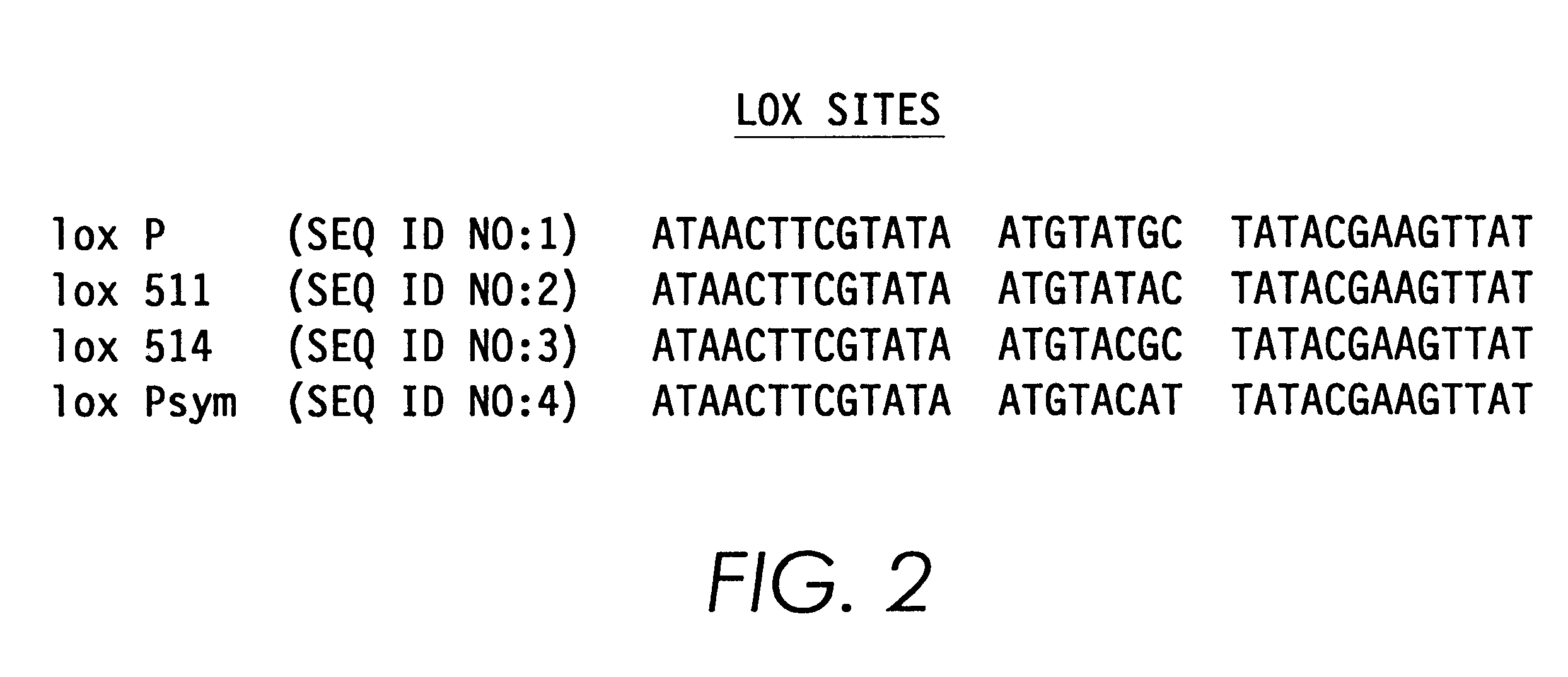
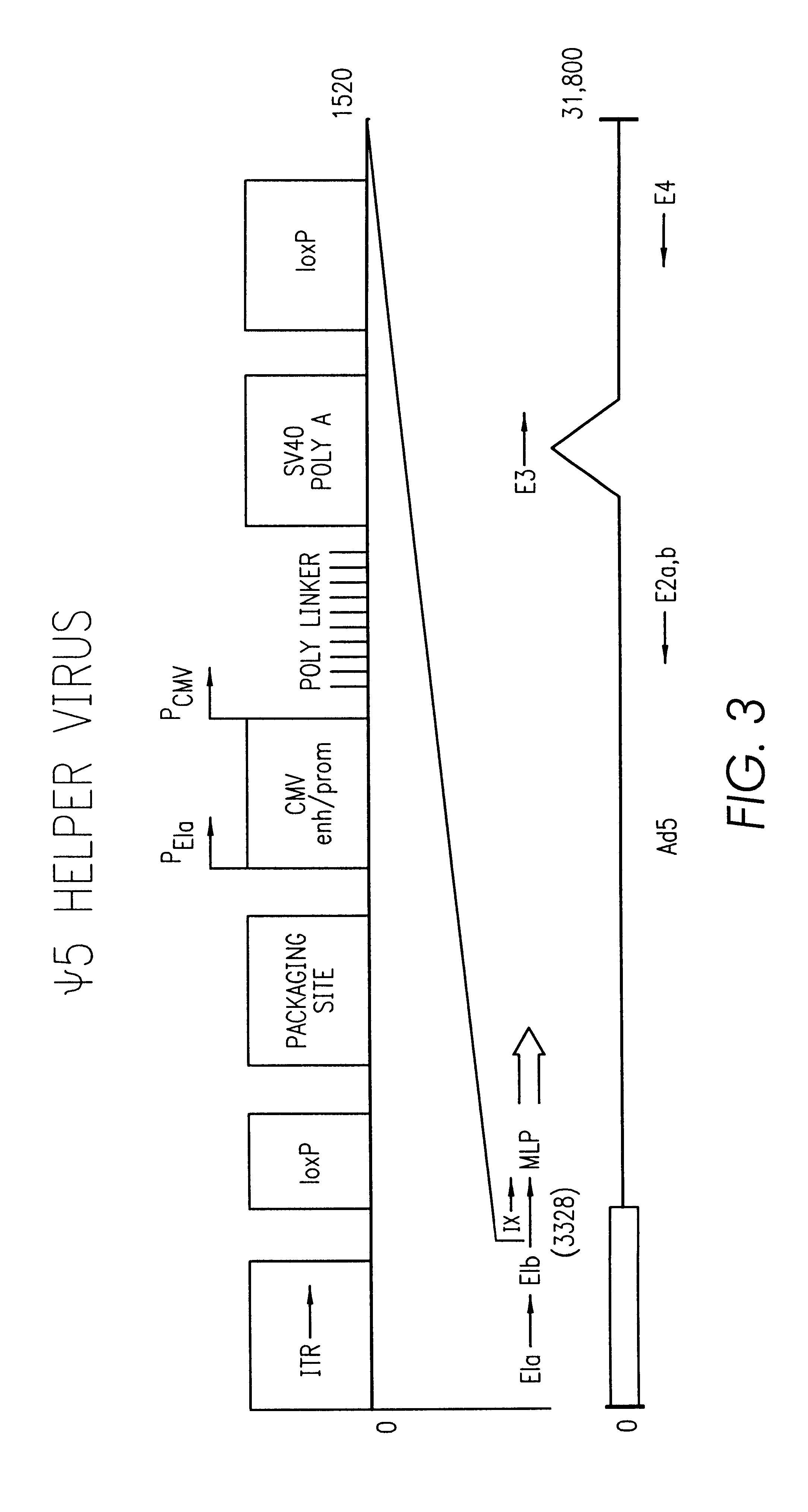
A method for producing in vivo packaged recombinant adenovirus vectors is provided. The recombinant Ad vectors do not contain any Adenovirus genes and are therefore useful for gene therapy. The recombinant Adenovirus vectors are packaged in vivo using a helper virus which is itself very inefficiently packaged, providing a recombinant viral preparation with very little or no contamination with helper virus. In particular, the method makes use of a helper virus in which the packaging site can be easily excised in vivo by recombination mediated by a recombinase. The helper virus is also useful for the in vivo construction of new recombinant adenovirus vectors containing substitutions in the E1 or other adenoviral region.
Owner:RGT UNIV OF CALIFORNIA
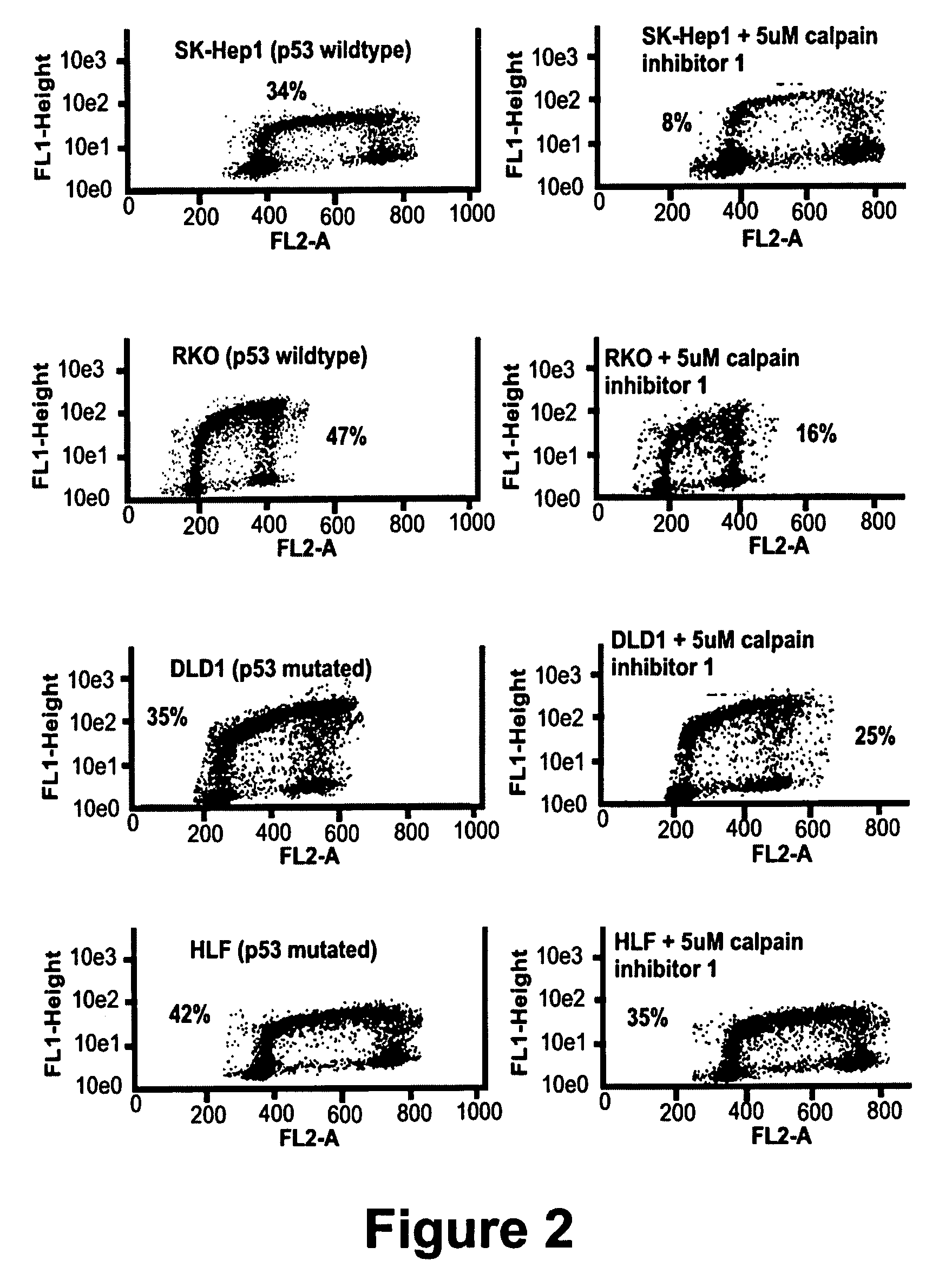
Calpain inhibitors and their applications
InactiveUS7001770B1Enhance p53-mediated apoptosisIncrease infectivityPeptide/protein ingredientsFermentationCo administrationApoptosis
The present invention provides a method to enhance apoptosis in a cell by the administration of p53 in combination with a calpain inhibitor. The present invention provides a method of increasing the infectivity of a cell to a viral vector by treatment of the cell with a calpain inhibitor. the present invention further provides a method of enhancing transciption of a therapeutic transgene from the CMV promoter. The present invention also provides a method of suppress the in vivo CTL response to viral vectors by the use of calpain inhibitors. The present invention further provides a pharmaceutical formulations of p53 and a calpain inhibitor in a pharmaceutically acceptable carrier. The present invention provides a method of ablating neoplastic cells in a mammalian organism in vivo by the co-administration of a calpain inhibitor and p53. The present invention also provides a method of ablating neoplastic cells in a population of normal cells contaminated by said neoplastic cells ex vivo by the administration of a recombinant adenovirus in combination with a calpain inhibitor to said population.
Owner:CANJI
Packaging systems for human recombinant adenovirus to be used in gene therapy
The problem of replication competent adenovirus in virus production is solved in that we have developed packaging cells that have no overlapping sequences with a new basic vector and, thus, are suited for safe large scale production of recombinant adenoviruses. One of the additional problems associated with the use of recombinant adenovirus vectors is the host-defense reaction against treatment with adenovirus. Another aspect of the invention involves screening recombinant adenovirus vector lots, especially those intended for clinical use, for the presence of adenovirus E1 sequences, as this will reveal replication competent adenovirus, as well as revertant E1 adenoviruses. It is also an aspect of the present invention to molecularly characterize the revertants that are generated in the newer helper / vector combinations.
Owner:JANSSEN VACCINES & PREVENTION BV
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
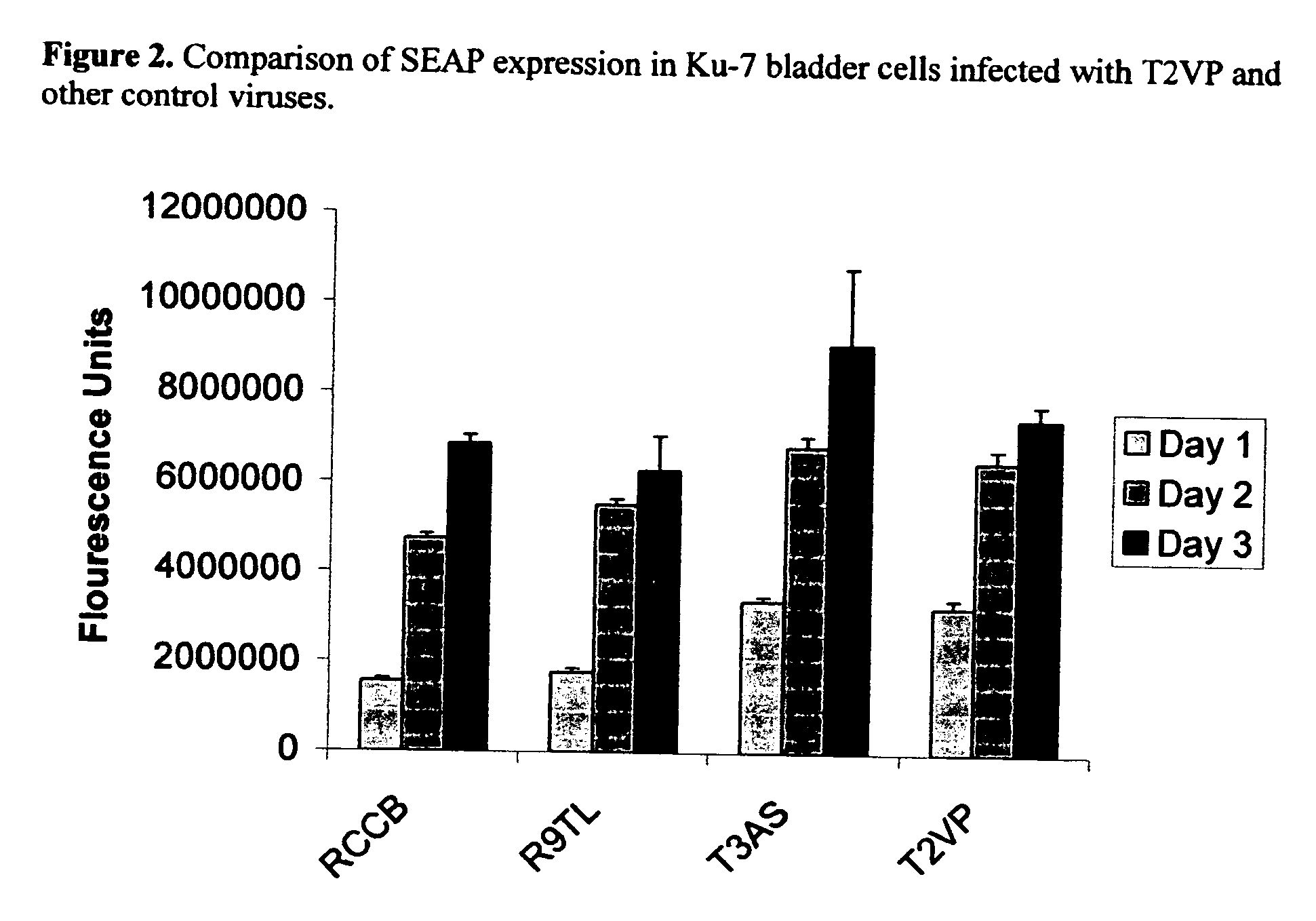
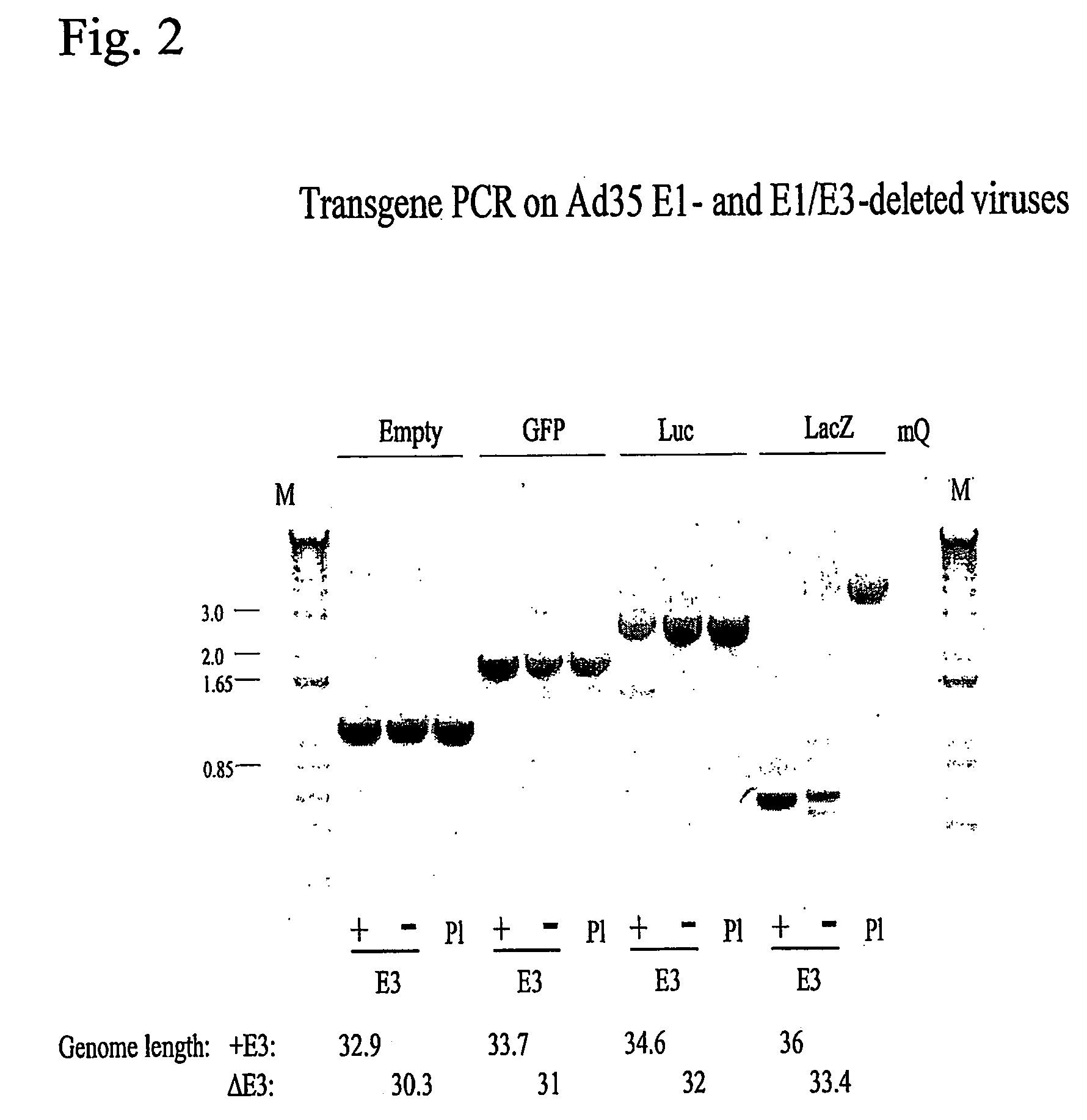
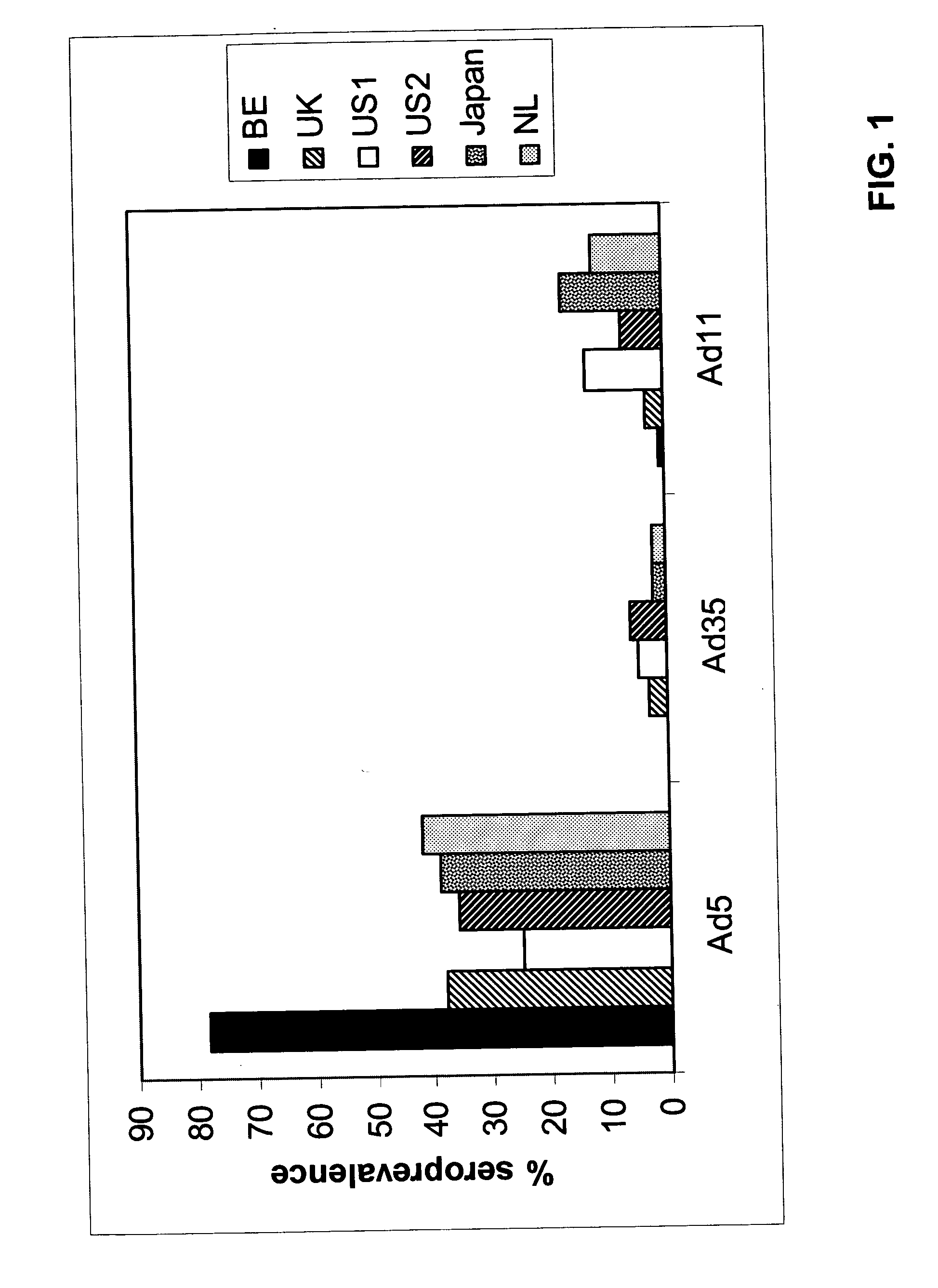
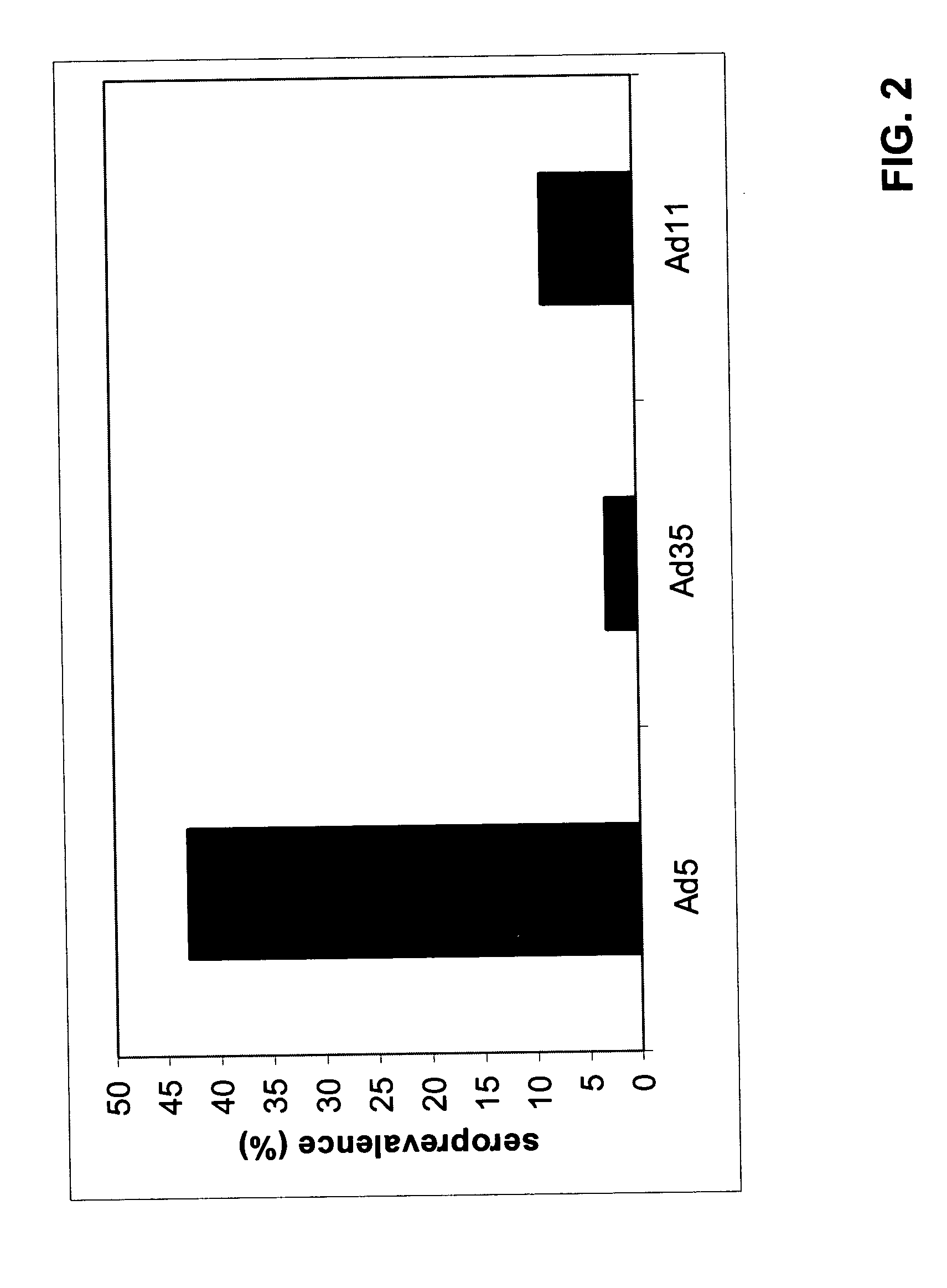
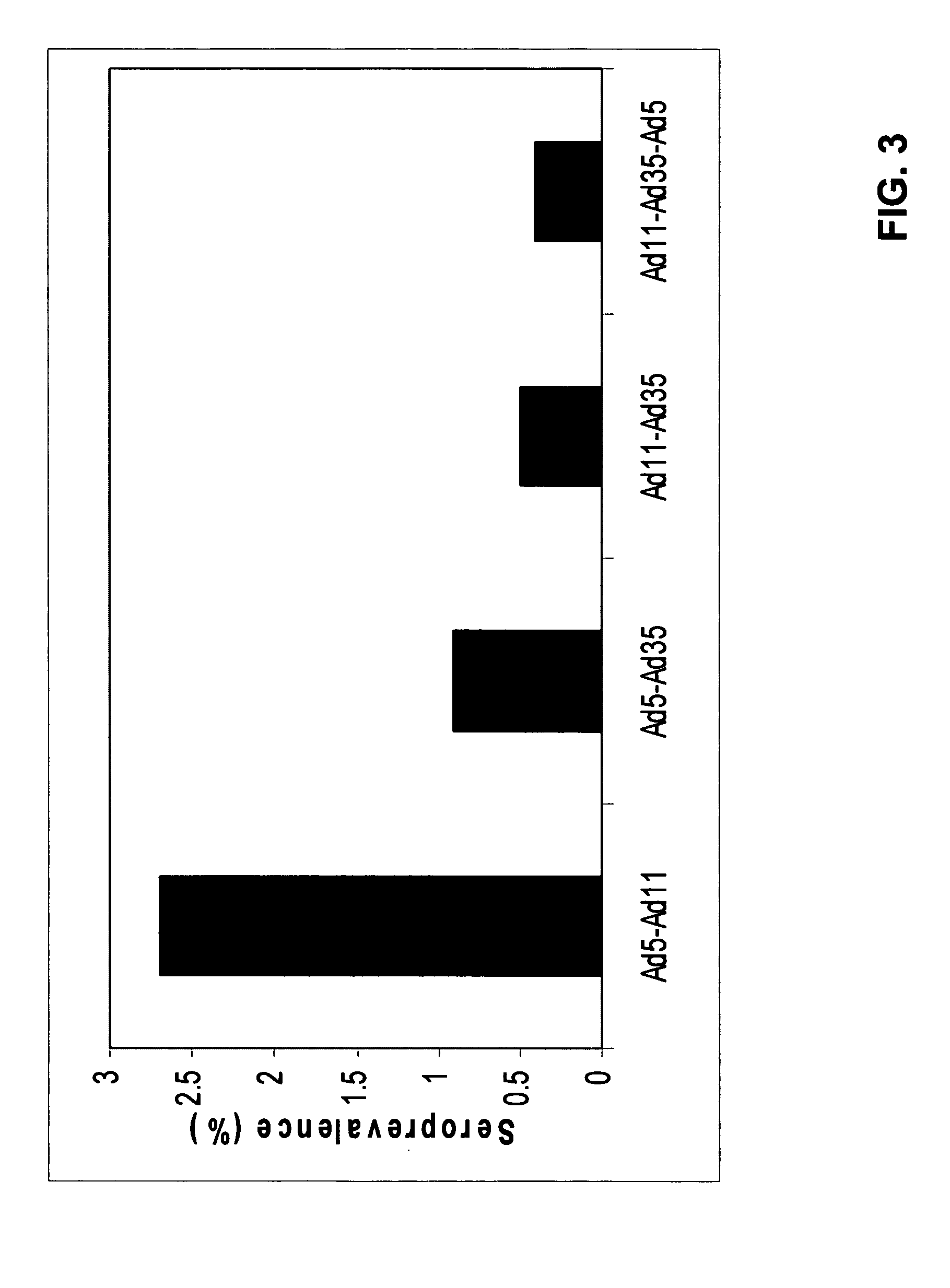
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
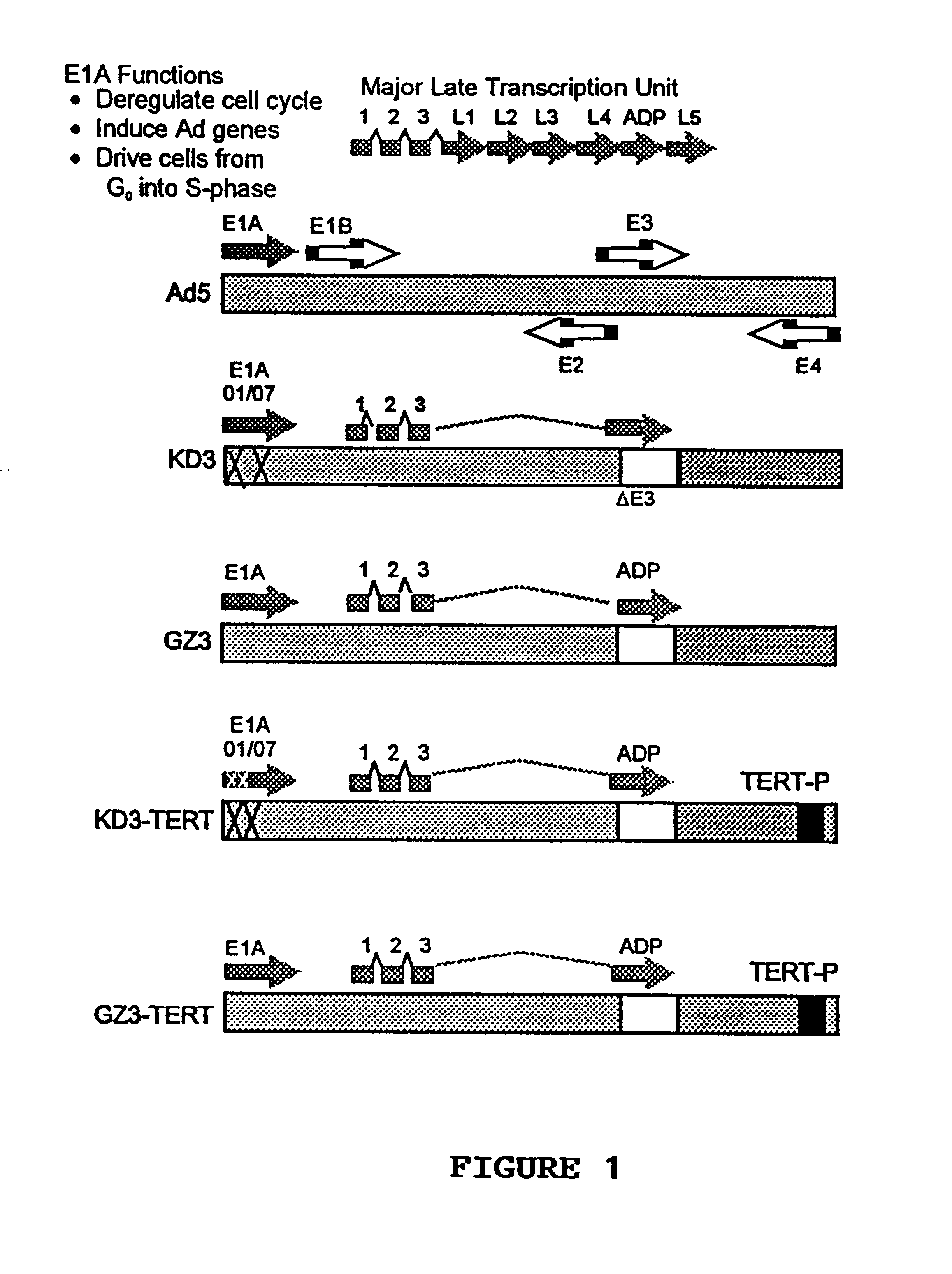
Recombinant adenovirus vectors that are replication-competent in tert-expressing cells
Novel adenovirus vectors which overexpress an adenovirus death protein and which are replication-competent in and, preferably, replication-restricted to cells expressing telomerase. One embodiment provides for efficient destruction and removal of viral-infected host cells expressing telomerase. Still further, another embodiment provides for additional restriction and safety by disrupting E1A's ability to bind p300 and / or members of the Rb family members. Compositions of the novel vectors and methods for promoting death of cells expressing telomerase with these vectors are also disclosed.
Owner:SAINT LOUIS UNIVERSITY
AAV vectors
InactiveUS6521225B1Prevent and limit expressionPrevents terminal glycosylation and translationBiocideOrganic active ingredientsLong terminal repeatNucleotide
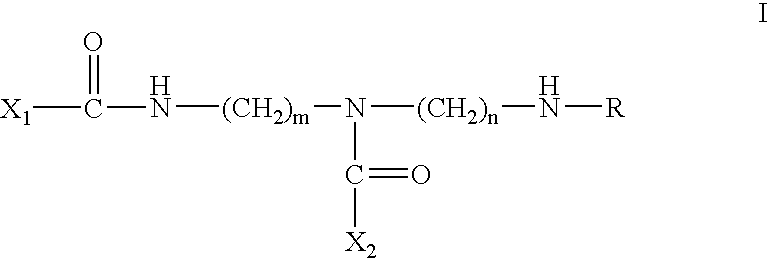
The present invention is directed to a recombinant adenovirus vector comprising two inverted terminal repeats (ITRs) each of which comprises a D-sequence having (i) from 5 to 15 native nucleotides and (ii) one or more deletions or substitutions therein.
Owner:ADVANCED RES TECH INST +1
CAS9-carrying recombinant adenovirus and application thereof
ActiveCN104178461ARapid knockoutImprove efficiencyViruses/bacteriophagesFermentationNucleotideGene Modification
The invention discloses a CAS9-carrying recombinant adenovirus and application thereof. The invention protects a recombinant adenovirus carrying coding gene of CAS9 protein; and the CAS9 protein is disclosed as Sequence 7 in the sequence table. The coding gene of the CAS9 protein is disclosed as 789th-5045th nucleotides from the 5' terminal of Sequence 6 in the sequence table. The invention also protects application of the recombinant adenovirus in preparing a kit for knocking out target gene. The CRISPR (clustered regularly interspaced short palindromic repeat) method has the characteristics of high efficiency, high speed and low cost. The CAS9 recombinant adenovirus disclosed by the invention can be used in a protein function cell platform (1) for quick gene modification and high flux screening, a liver protein function platform (2) for implementing quick and specific gene knock-out at the liver, and a mouse gene modification platform (3) for preparing a gene knock-out / knock-in mouse.
Owner:BEIJING PROTEOME RES CENT
Capsid-modified recombinant adenovirus and methods of use
InactiveUS6955808B2Low yieldEfficiencyBiocideAntibody mimetics/scaffoldsSingle-Chain AntibodiesNoninvasive imaging
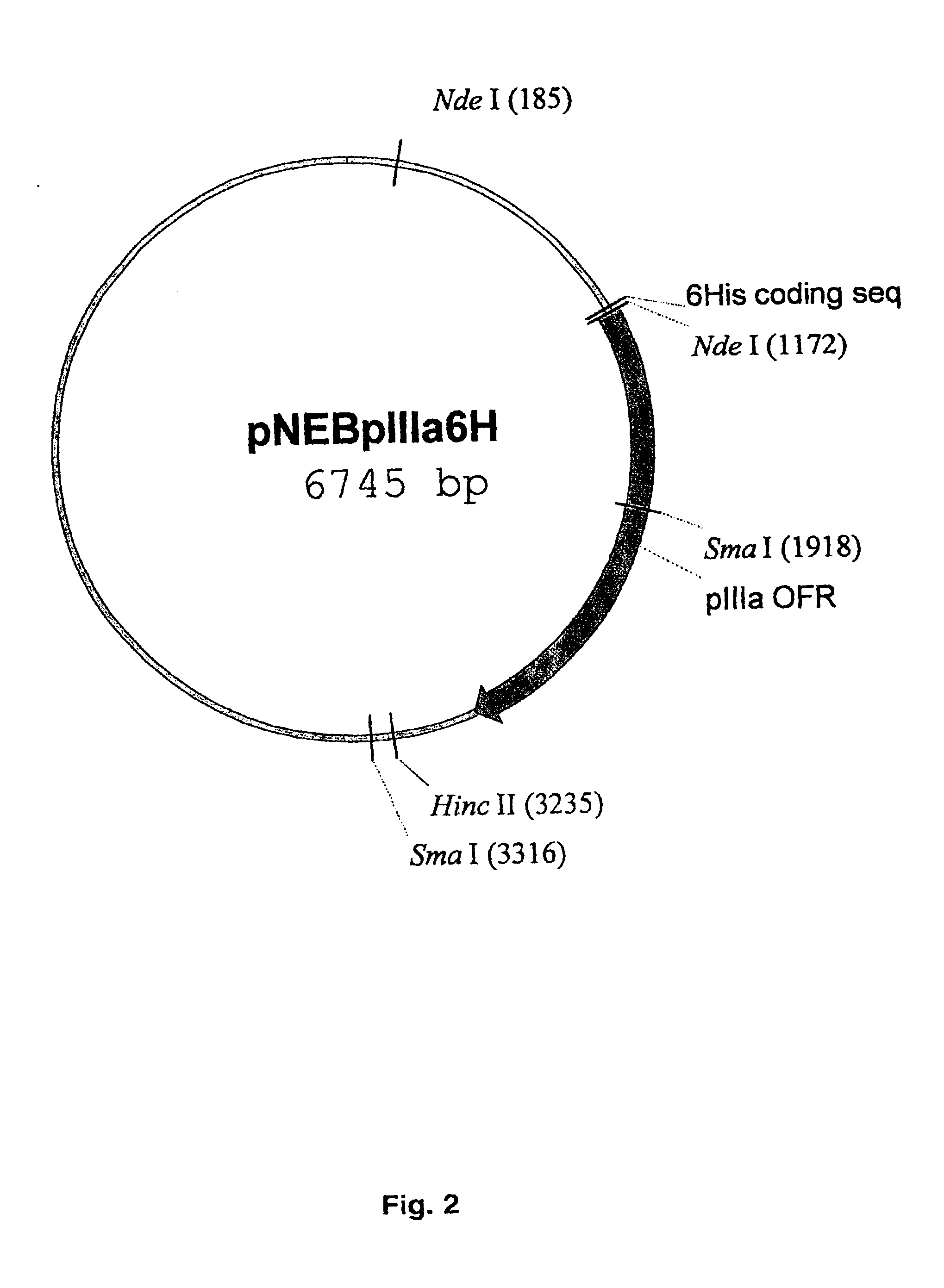
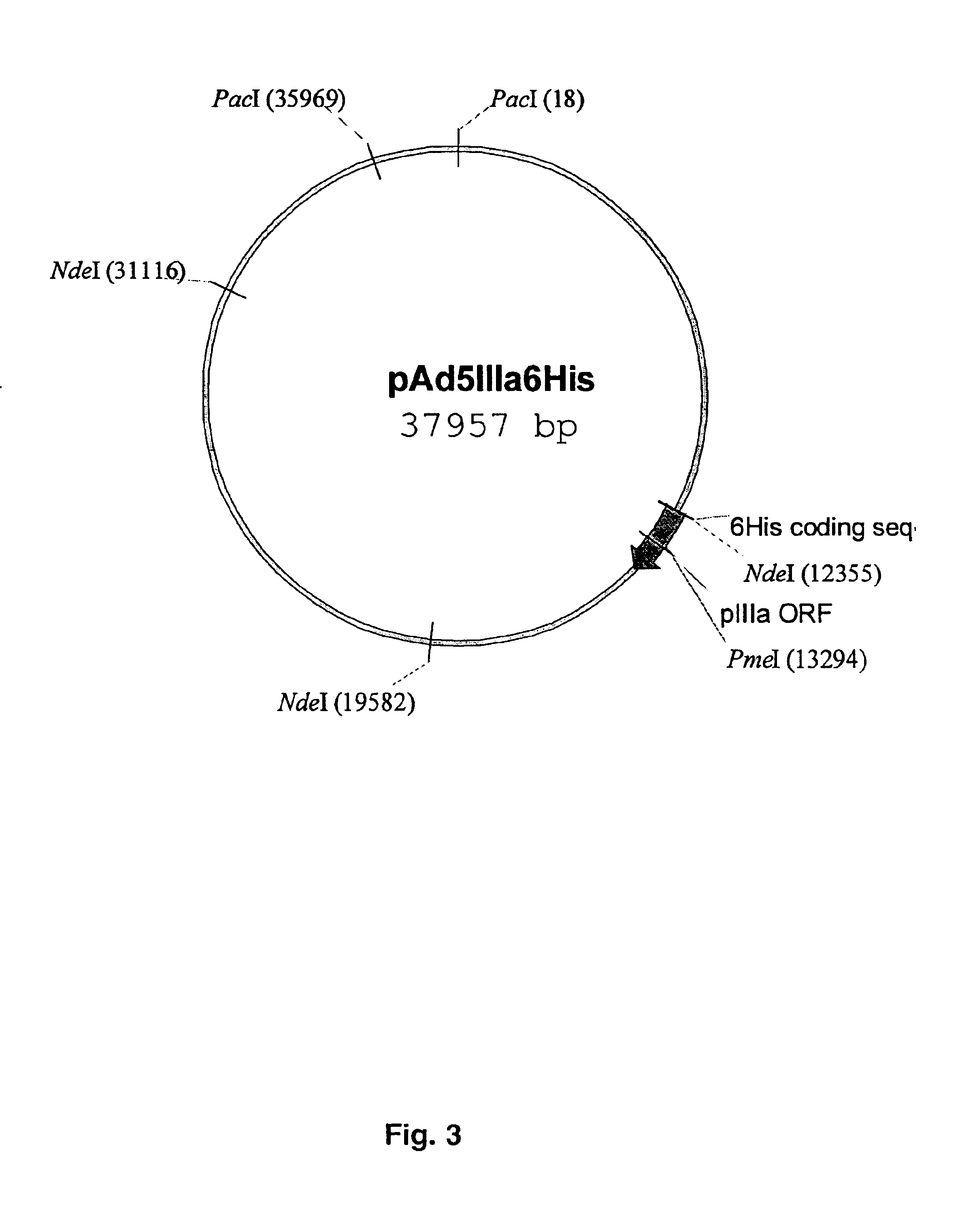
The present invention describes recombinant adenoviral vectors modified by incorporating targeting ligands or label into viral capsid or structural proteins. In one embodiment, single-chain antibody was introduced into the minor capsid proteins pIIIa or pIX so that the adenoviral vector can be targeted to a particular cell type. In another embodiment, there is provided a noninvasive imaging strategy useful for monitoring the replication and spread of conditionally replicative adenoviral vectors. Viral structural proteins such as pIX capsid protein, core proteins mu, V and VII were expressed as fusion protein with a fluorescent label. Once incorporated into the virions, detection of the structural fusion protein label would indicate the localization of the disseminated viral progeny. The detected fluorescent signals also closely correlate with the level of viral replication and progeny production.
Owner:UAB RES FOUND
Recombinant adenoviruses and use thereof
ActiveUS20150291935A1Diminishment of extentAvoid spreadingAntibacterial agentsAntimycoticsSimian AdenovirusesDisease
The present invention relates to recombinant adenoviruses and vectors thereof. In particular, the adenoviruses are novel simian adenoviruses having a low seroprevalence and high immunogenicity relative to other adenoviruses and vectors thereof. The invention also provides methods for production of the adenoviruses and for the treatment of diseases by administering the adenoviral vector(s) to a subject (e.g., a human).
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Site specific recombinase based method for producing adenoviral vectors
Site-specific recombinase based methods for making a recombinant adenoviral genome, as well as kits for practicing the same and the recombinant adenovirus vectors produced thereby, are provided. In the subject methods, the subject genomes are prepared from donor and acceptor vectors that each include at least one site recombinase recognition site, where in certain preferred embodiments, one of the donor and acceptor vectors includes a single recombinase recognition site while the other includes two recombinase recognition sites. The acceptor vector includes an adenoviral genome having an E region deletion. The donor vector includes an insertion nucleic acid. In the subject methods, the donor and acceptor vectors are combined in the presence of a recombinase to produce an adenoviral genome that includes the insertion nucleic acid. The subject adenoviral genomes find use in a variety of applications, including as vectors for use in a variety of applications.
Owner:LIFE TECH CORP
CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats-Cas9) homing sequences and primers thereof, and transgenic expression vector and establishment method thereof
ActiveCN105907758AEasy and fast to buildHigh efficiency of editing functionNucleic acid vectorFermentationEnzyme digestionNucleotide
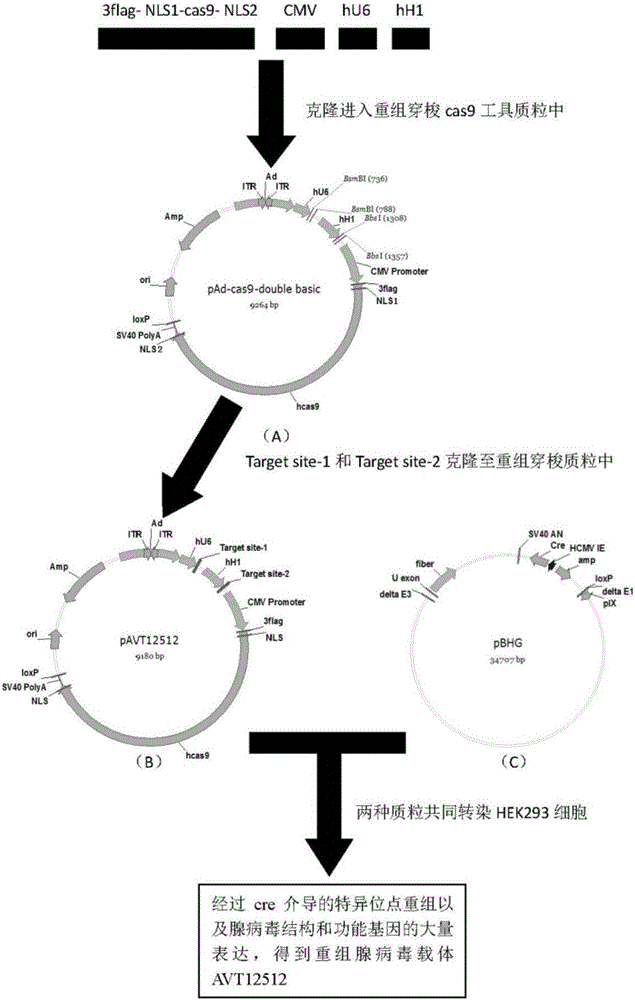
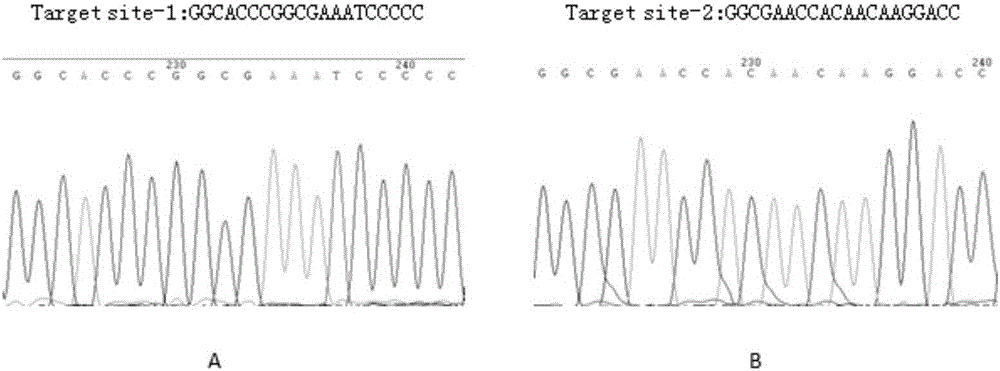
The invention belongs to the technical field of biomedicine, and particularly relates to CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats-Cas9) homing sequences and primers thereof, and a transgenic expression vector and an establishment method thereof. The CRISPR-Cas9 homing sequence sgRNA comprises SIDT1-gene-specific Target site-1 of which the target is positioned on E1 exon, and SIDT1-gene-specific Target site-2 of which the target is positioned on E2 exon, wherein the nucleotide sequence of the Target site-1 is disclosed as SEQ ID NO.8, and the nucleotide sequence of the Target site-2 is disclosed as SEQ ID NO.9. The CRISPR-Cas9 transgenic expression vector is formed by connecting the Cas9 homing sequence Target site-1 and Target site-2 to the BsmB I and Bbs I enzyme digestion sites of a recombinant shuttle Cas9 tool plasmid. The recombinant shuttle Cas9 tool plasmid can be quickly assembled with homing sequences of the two target sites of the genome, thereby packaging the recombinant adenovirus vector specifically for the two target sites; and the recombinant shuttle Cas9 tool plasmid can independently complete the editing of the large-segment genome without dependence on the synergistic actions. The establishment process is simple and quick, and has high efficiency for performing the genome large-segment editing function.
Owner:世翱(上海)生物医药科技有限公司
Modified adenoviral vectors and methods of treatment using same
InactiveUS20140348791A1Improve abilitiesLow immunogenicityBiocideGenetic material ingredientsDiseaseFiber
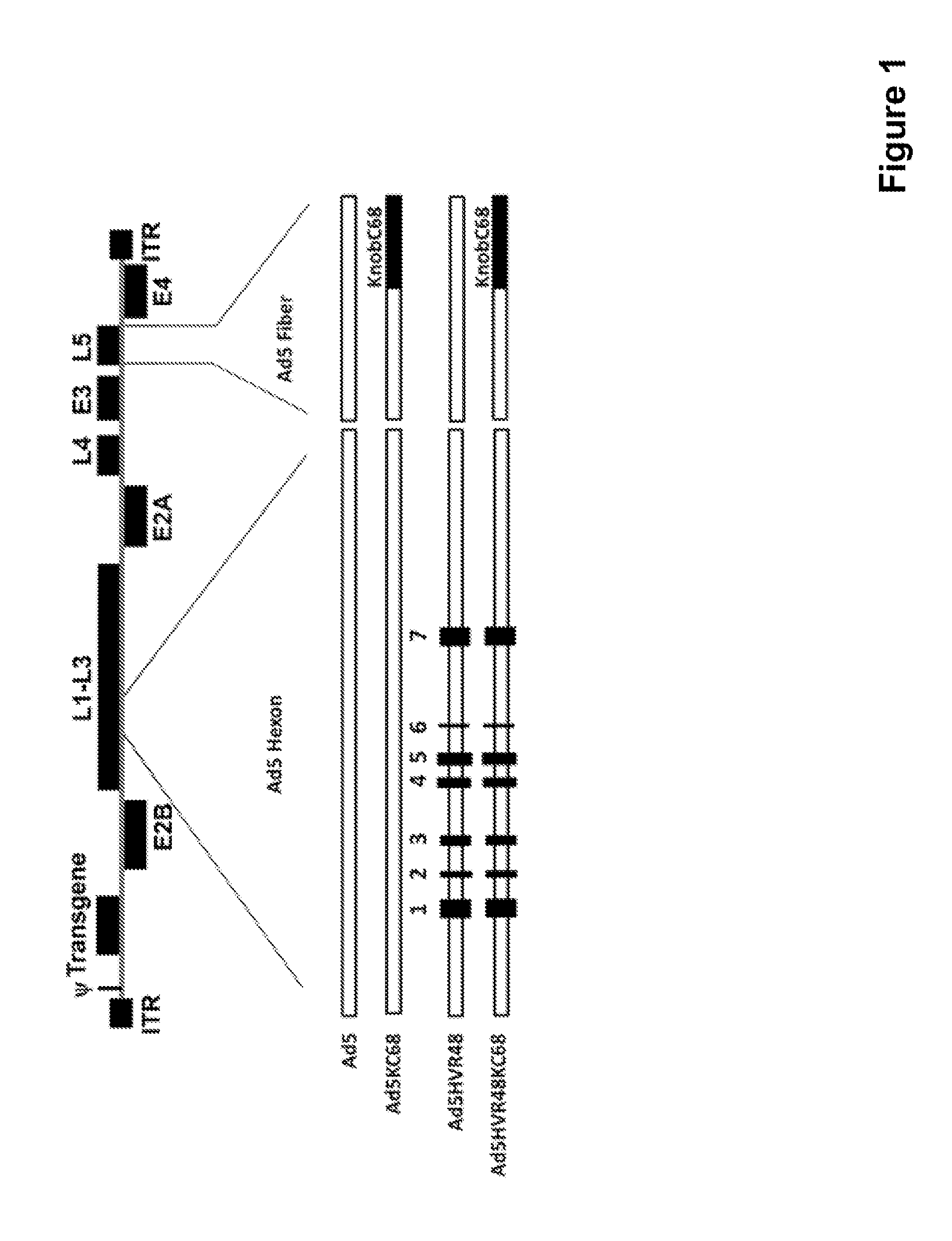
The present invention relates to recombinant adenovirus serotype 5 (Ad5) vectors which harbor chimeric capsid proteins including substitutions of the corresponding regions from adenovirus serotypes having a lower seroprevalence relative to Ad5. In particular, the chimeric capsid includes modifications of both the adenoviral hexon and fiber proteins. The invention also provides methods for the treatment of diseases or disorders caused by infective agent(s) by administering the adenoviral vector(s) to a subject (e.g., a mammal, such as a human).
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
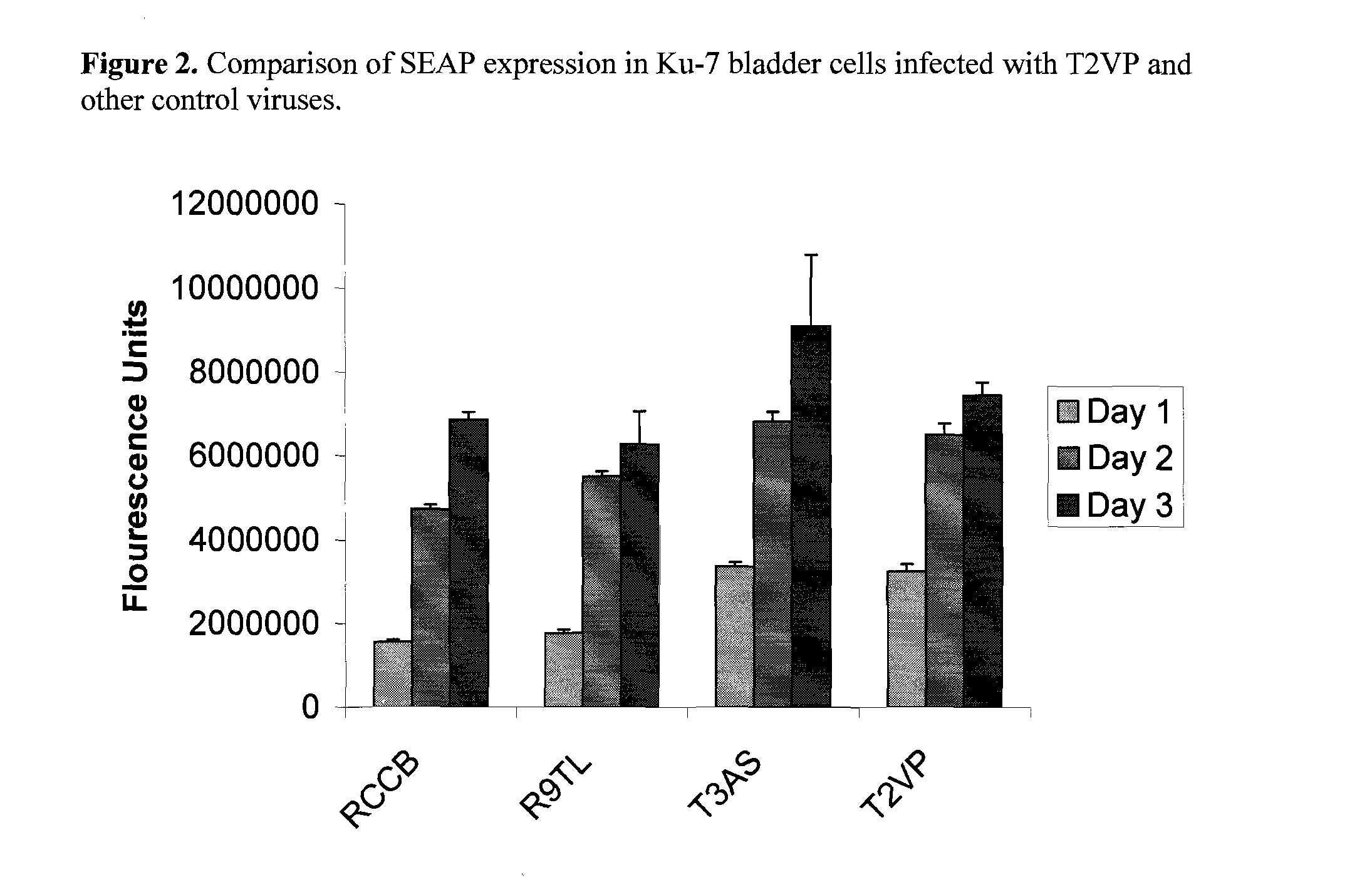
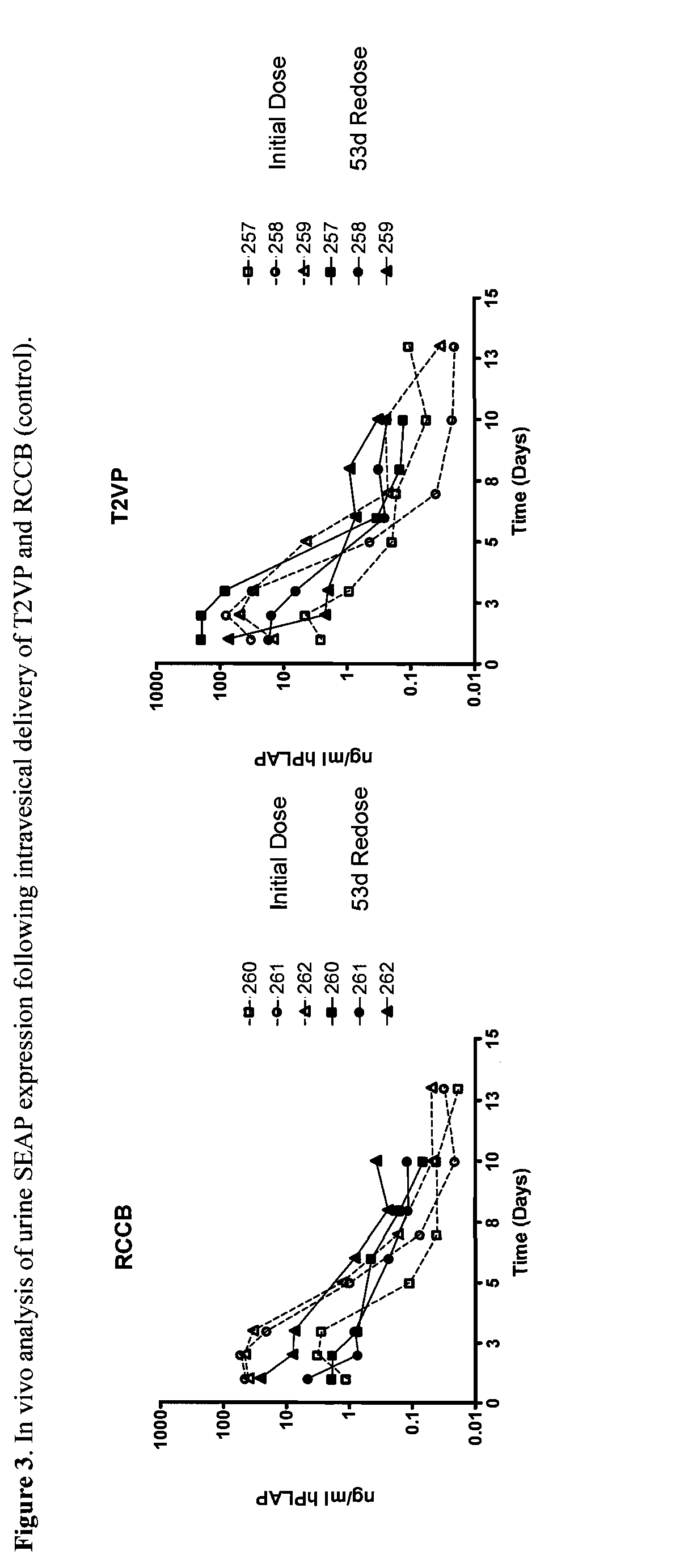
System for rapid production of high-titer and replication-competent adenovirus-free recombinant adenovirus vectors
InactiveUS20090175897A1Shorten production timeLow costSsRNA viruses negative-senseBiocideHeterologousImmunologic function
The present invention relates generally to the fields of gene therapy, immunology, and vaccine technology. More specifically, the invention relates to a novel system that can rapidly generate high titers of adenovirus vectors that are free of replication-competent adenovirus (RCA). Also provided are methods of generating these RCA-free adenoviral vectors, immunogenic or vaccine compositions comprising these RCA-free adenovirus vectors, methods of expressing a heterologous nucleic acid of interest in these adenovirus vectors and methods of eliciting immunogenic responses using these adenovirus vectors.
Owner:TANG DE CHU C +2
High capacity recombinant adenoviral vector for treatment of hemophilia A
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
Dendrite cells transduced with recombinant adenovirus AdVCEA which generate CEA-specific cytotoxic T lymphocytes, vaccine and pharmaceutical composition comprising the same
The present invention relates to an antitumor immune response, and in more detail, to a method for inducing cytotoxic T lymphocytes specific to a tumor-associated antigen that acts specifically on tumor cells.Immunotherapy using the present invention may be most effective among immune therapies that use immunity of our body, because in the present invention, CEA-specific cytotoxic T lymphocytes can be induced in vitro by using a dendritic cell transduced with a recombinant adenovirus.Further, immunotherapy using the present invention can function as a powerful tool for tumor prevention or treatment, if being used in combination with antitumor vaccines or other treatments.
Owner:OXELLBIOMEDICAL
Methods and compositions comprising DNA damaging agents and p53
InactiveUS20050089511A1Improve the level ofLow cellular uptakeOrganic active ingredientsElectrotherapyRegimenCancer cell
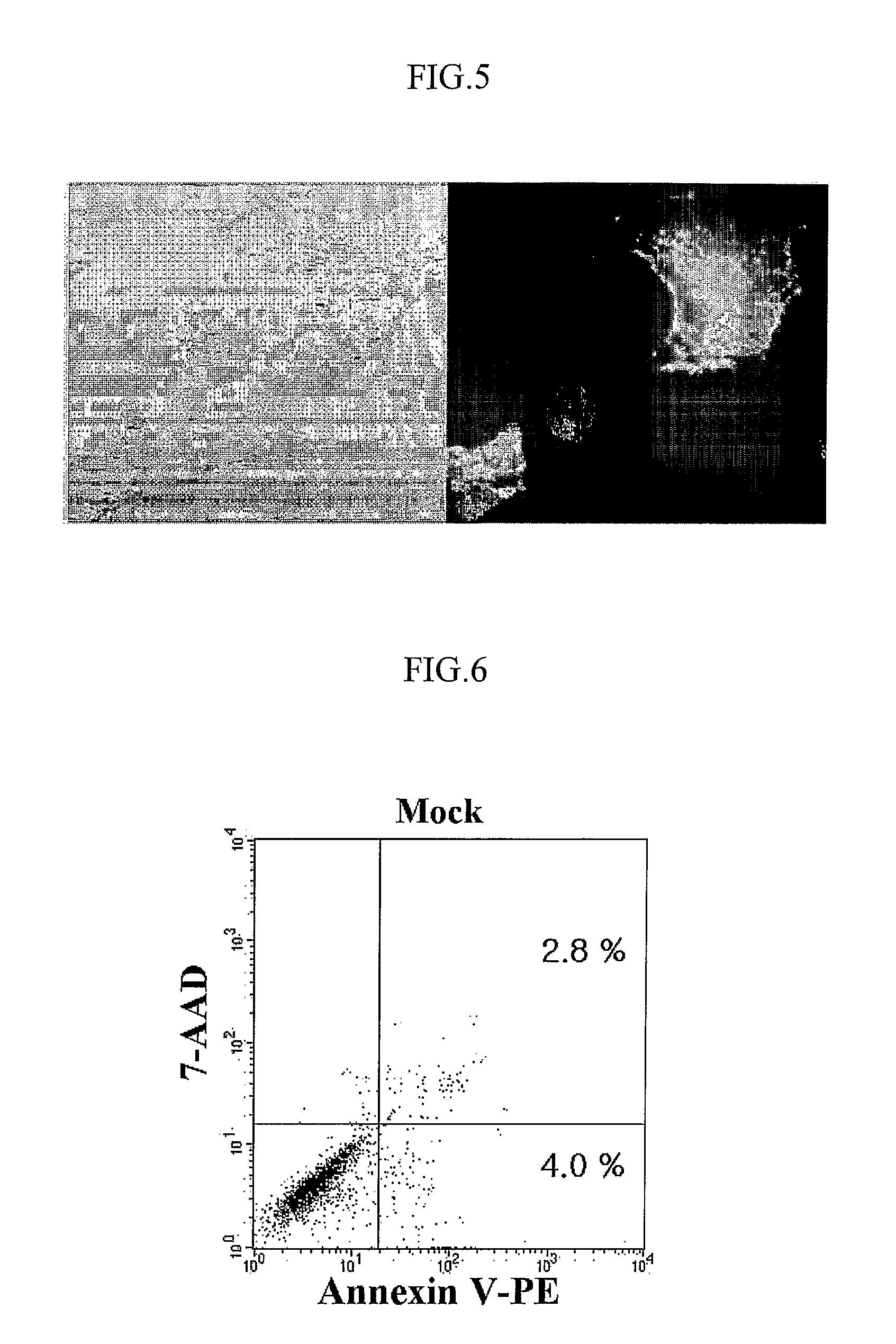
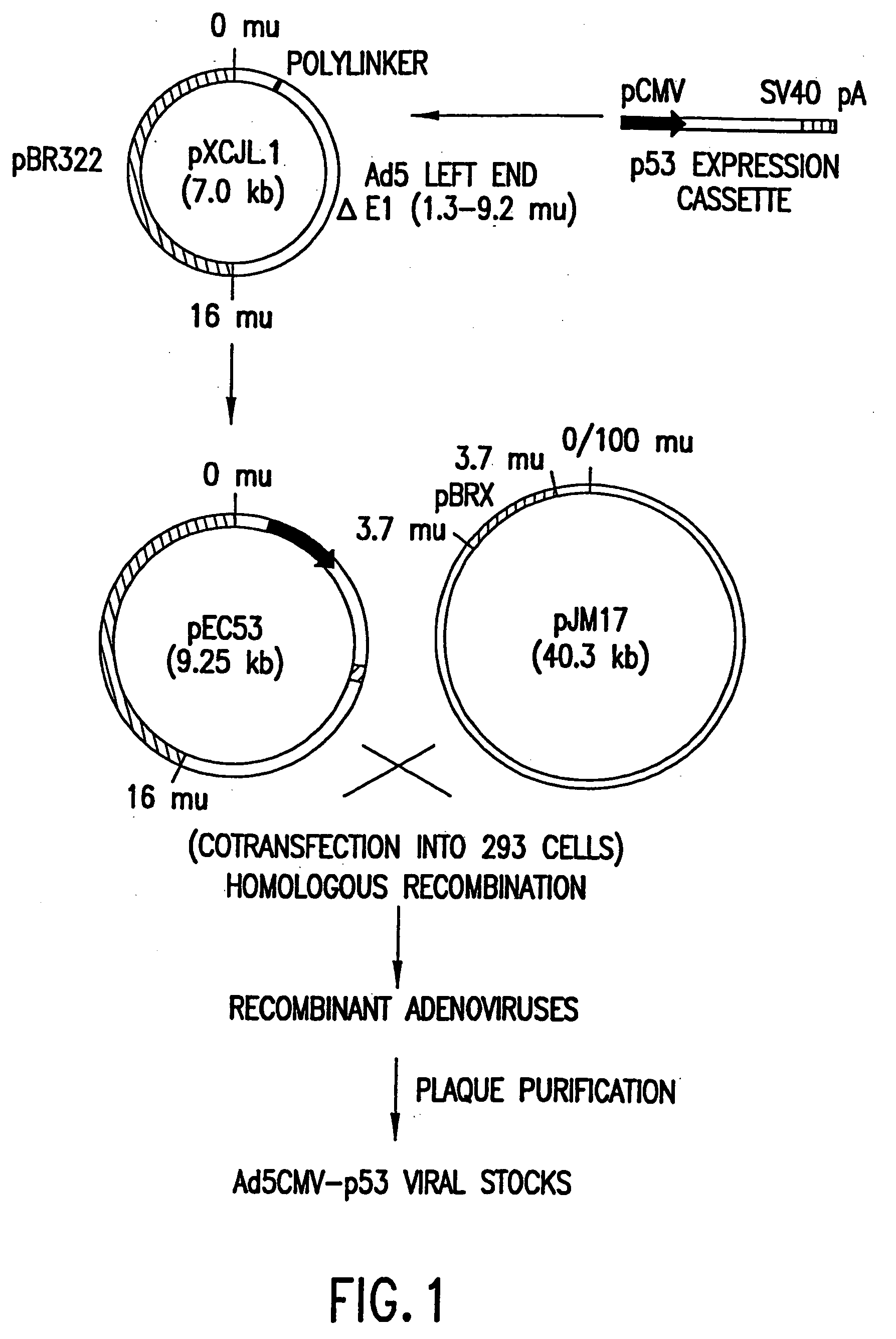
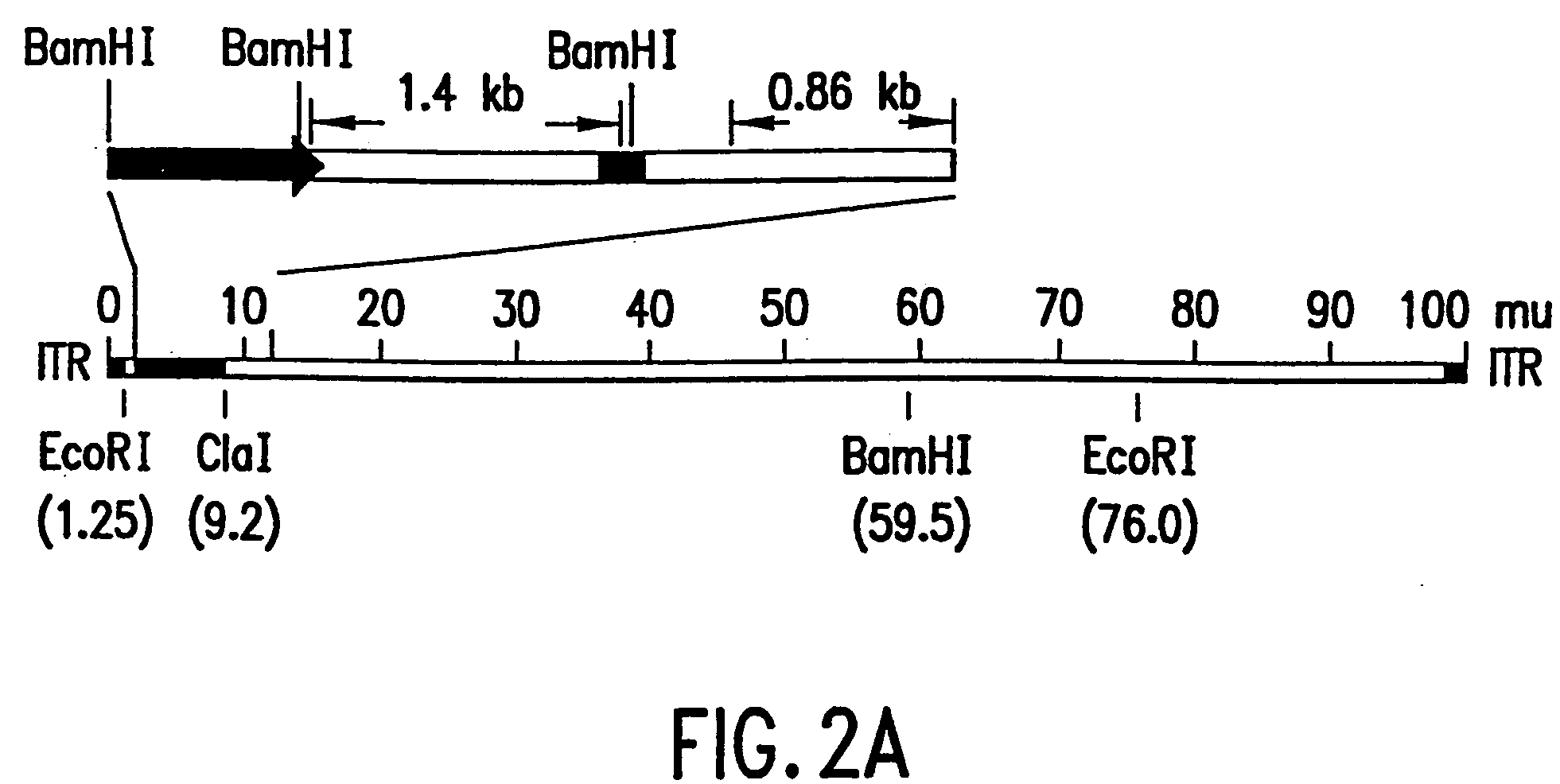
The present invention relates to the use of tumor suppressor genes in combination with a DNA damaging agent or factor for use in killing cells, and in particular cancerous cells. A tumor suppressor gene, p53, was delivered via a recombinant adenovirus-mediated gene transfer both in vitro and in vivo, in combination with a chemotherapeutic agent. Treated cells underwent apoptosis with specific DNA fragmentation. Direct injection of the p53-adenovirus construct into tumors subcutaneously, followed by intraperitoneal administration of a DNA damaging agent, cisplatin, induced massive apoptotic destruction of the tumors. The invention also provides for the clinical application of a regimen combining gene replacement using replication-deficient wild-type p53 adenovirus and DNA-damaging drugs for treatment of human cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Composition and method for stimulating immune response to pathogen using complex adenoviral vector
InactiveUS6964762B2Improving immunogenicityStrong immune responseSsRNA viruses negative-senseAntibacterial agentsHeterologousProgenitor
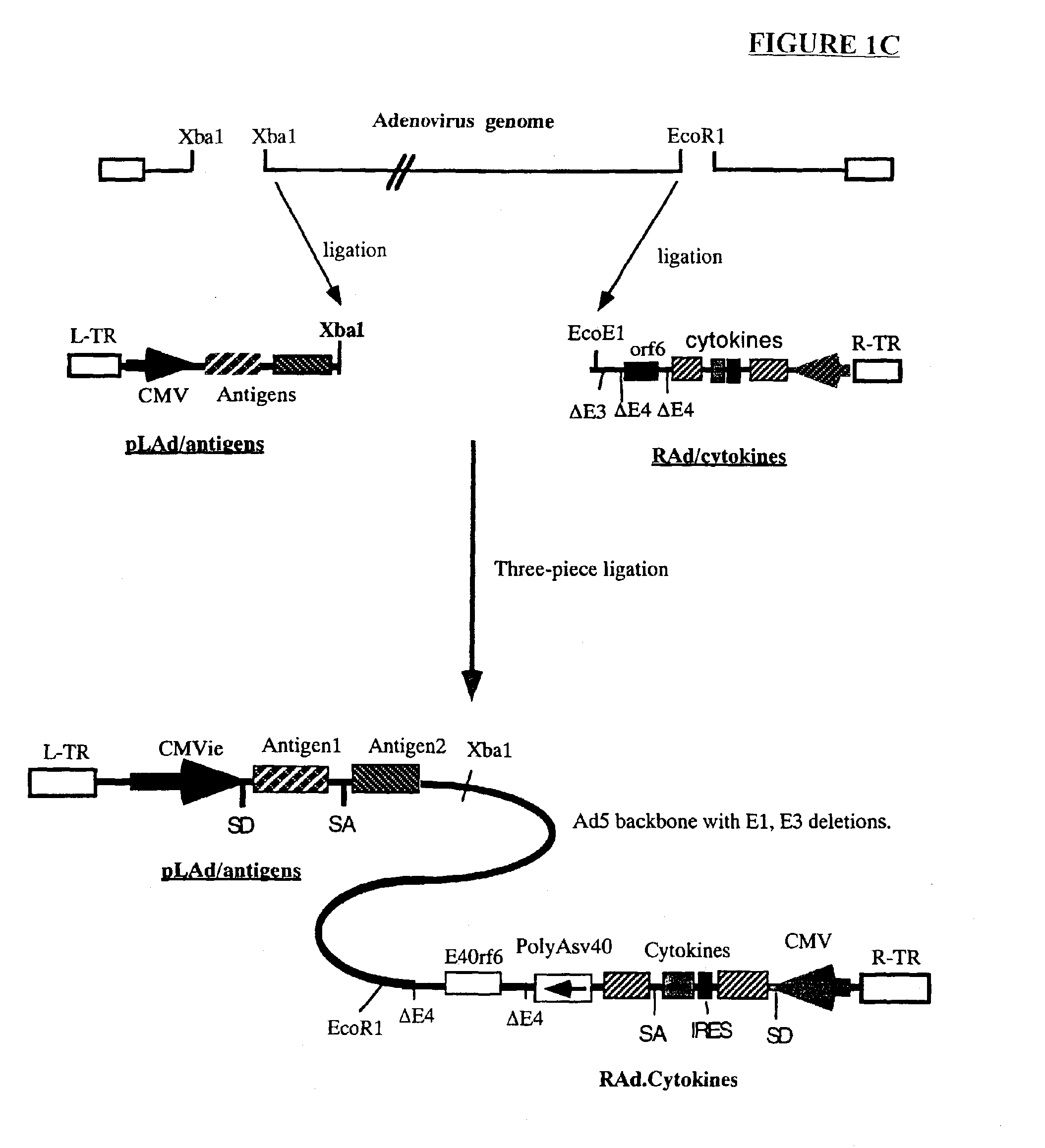
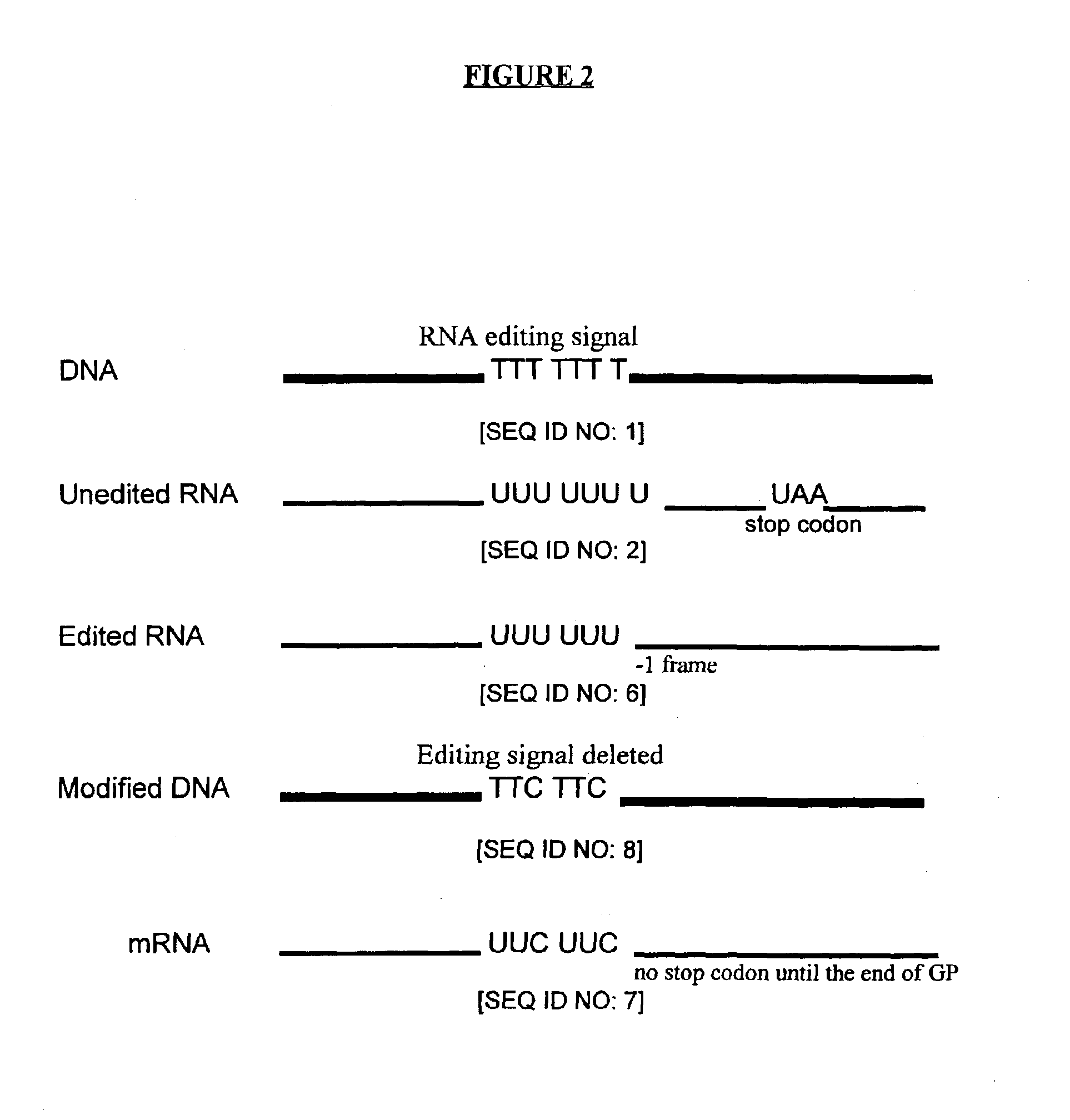
Genetic vaccines and methods are provided for enhancing the immunity of a host such as a human to one or more pathogens. In one aspect, a method of enhancing the immunity of a host to a pathogen is provided. The method comprises administering to the host a recombinant virus comprising an antigen sequence that is heterologous to a native progenitor of the recombinant adenovirus and encodes a viral antigen from a pathogenic virus, expression of which is under the transcriptional control of a first promoter; and a cytokine sequence that is heterologous to the native progenitor of the recombinant adenovirus and encodes a cytokine, expression of which is under the transcriptional control of a second promoter. Expression of the antigen and cytokine sequences elicits an immune response directed against the viral antigen upon infection of the host by the recombinant virus. The method can be used for immunizing a host against a wide variety of pathogen viruses, such as HIV, Ebola virus, Marburg virus, hepatitis B virus, hepatitis C virus, influenza virus, human simplex virus, human papilloma virus and respiratory syncytial virus.
Owner:GENPHAR INC
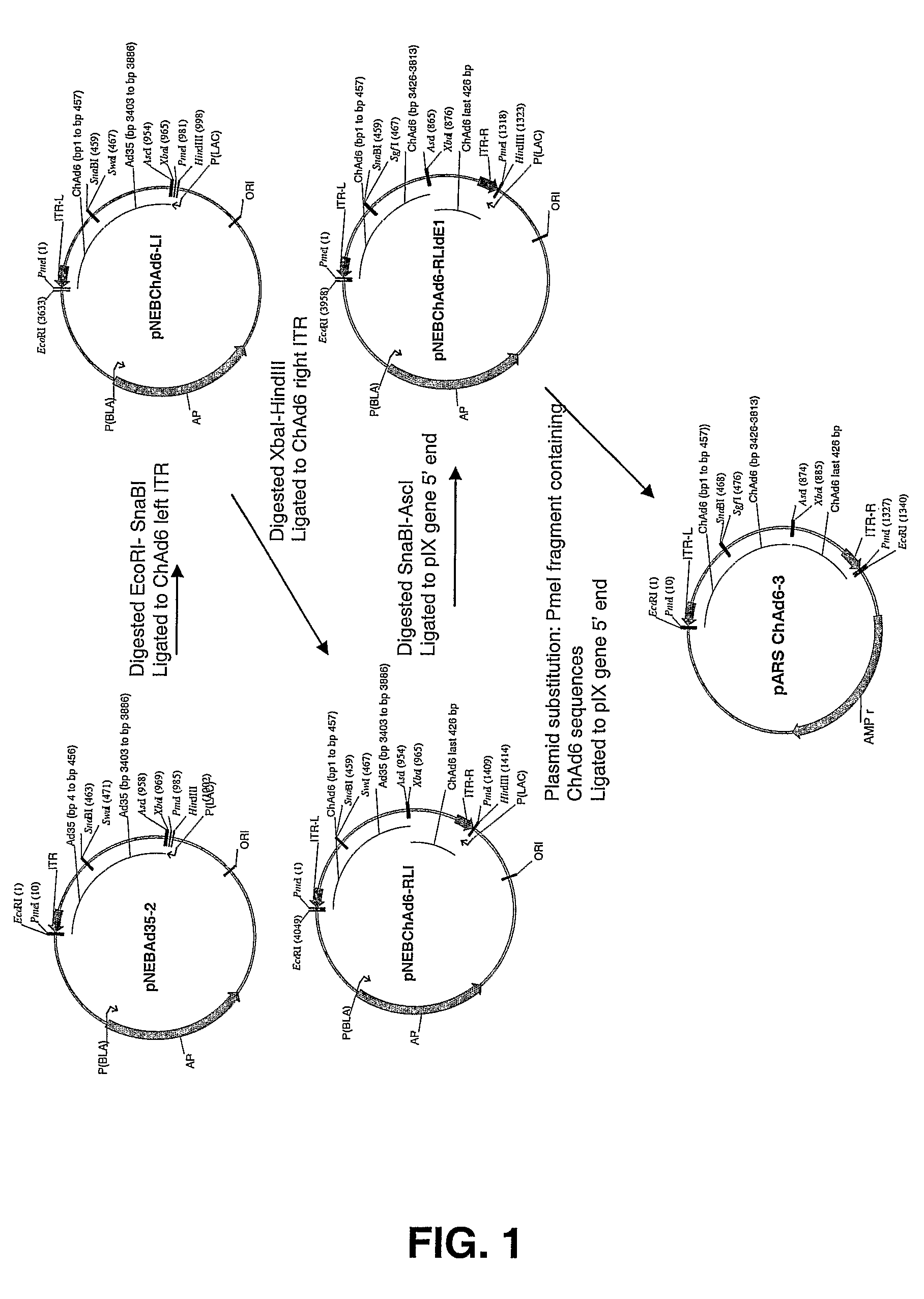
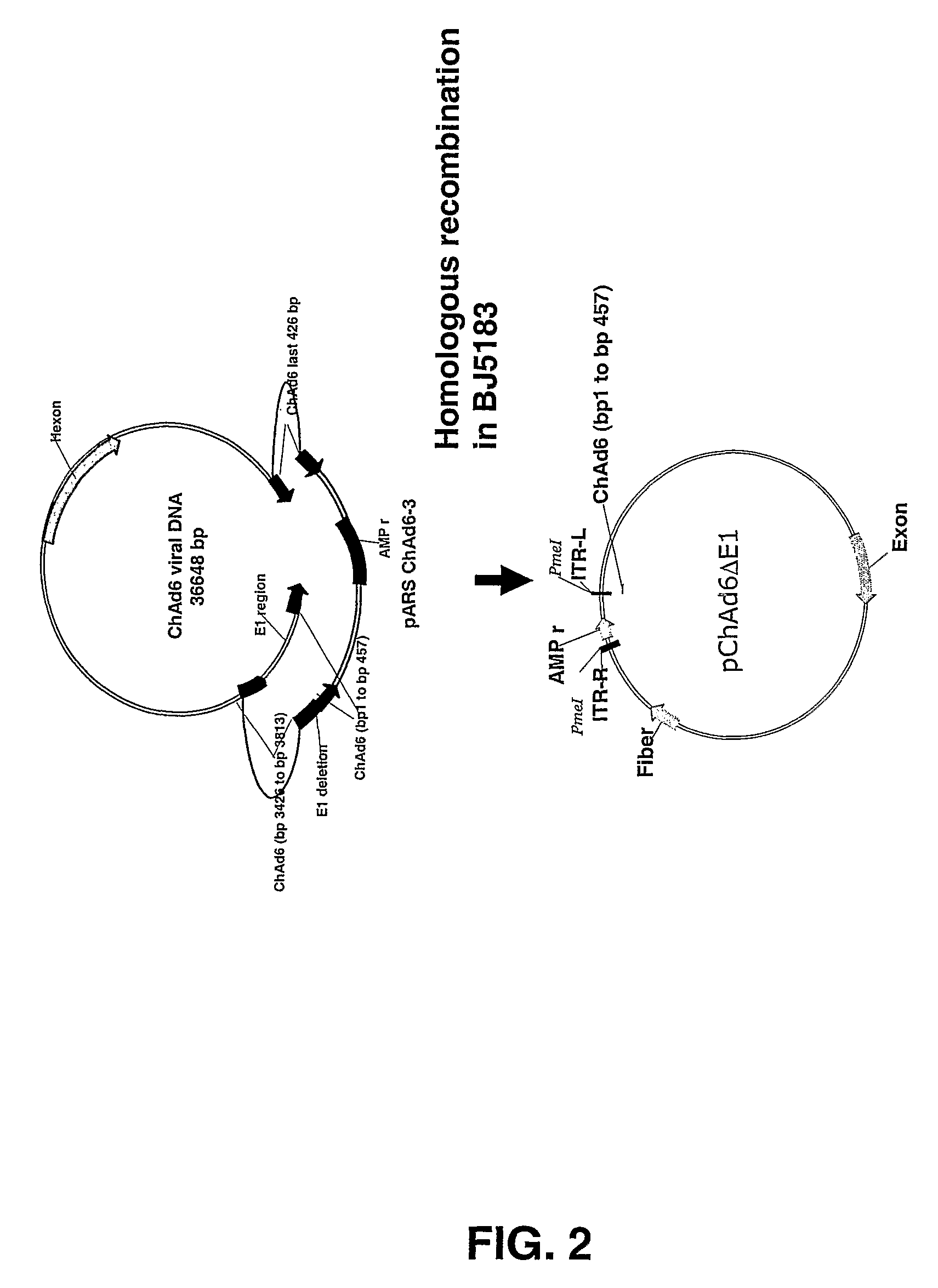
Chimpanzee adenovirus vaccine carriers
The present invention provides recombinant replication-defective adenoviral vectors derived from chimpanzee adenoviruses and methods for generating recombinant adenoviruses in human E1-expressing cell lines. The invention also provides compositions and methods suitable for use for the delivery and expression of transgenes encoding immunogens against which a boosted immune response is desired. The invention further provides methods of generating clinical grade vector stocks suitable for use in humans. In a particular embodiment the invention contemplates the use of vectors comprising transgenes which encode tumor associated antigens in vaccines and pharmaceutical compositions for the prevention and treatment of cancer.
Owner:MSD ITAL
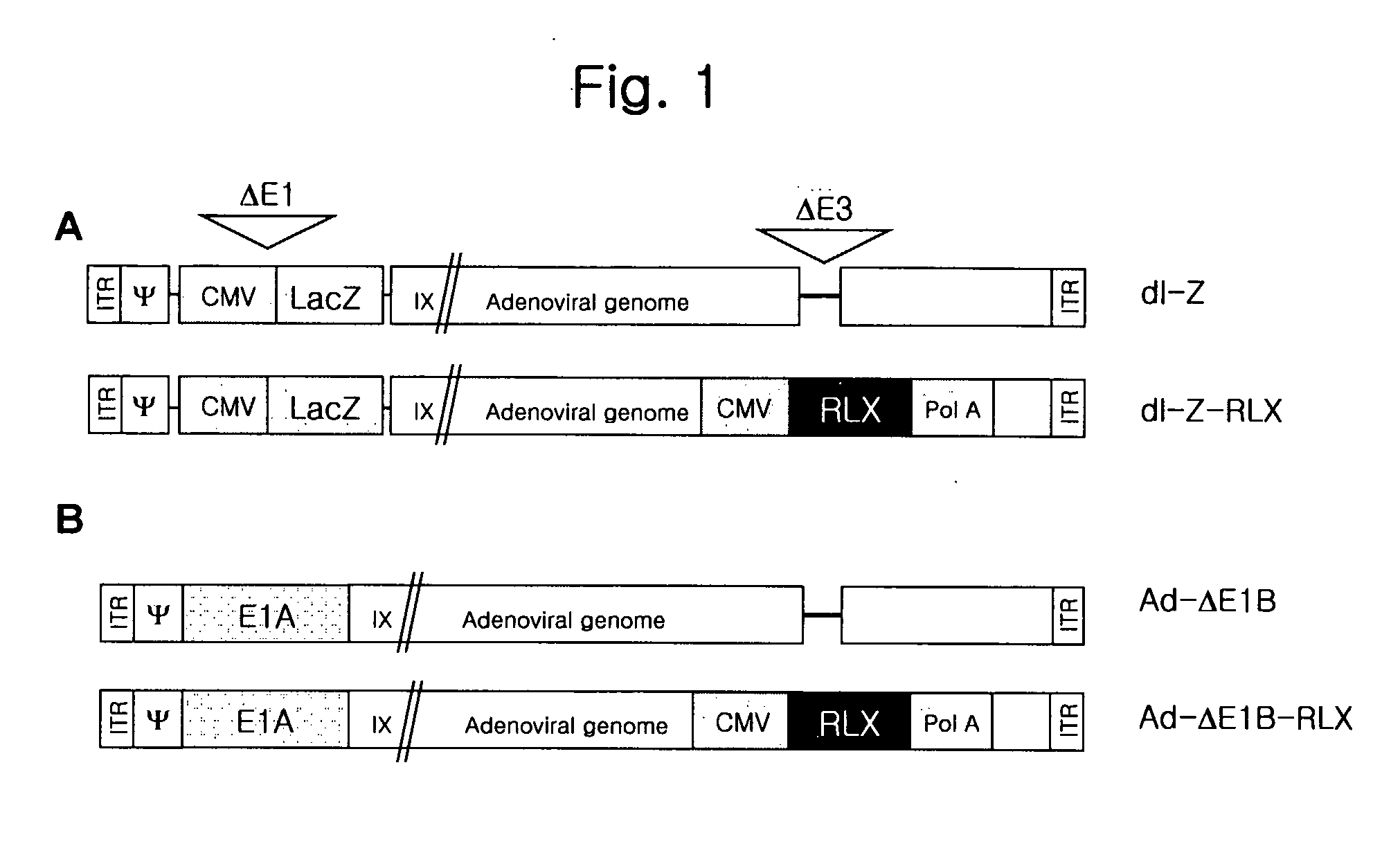
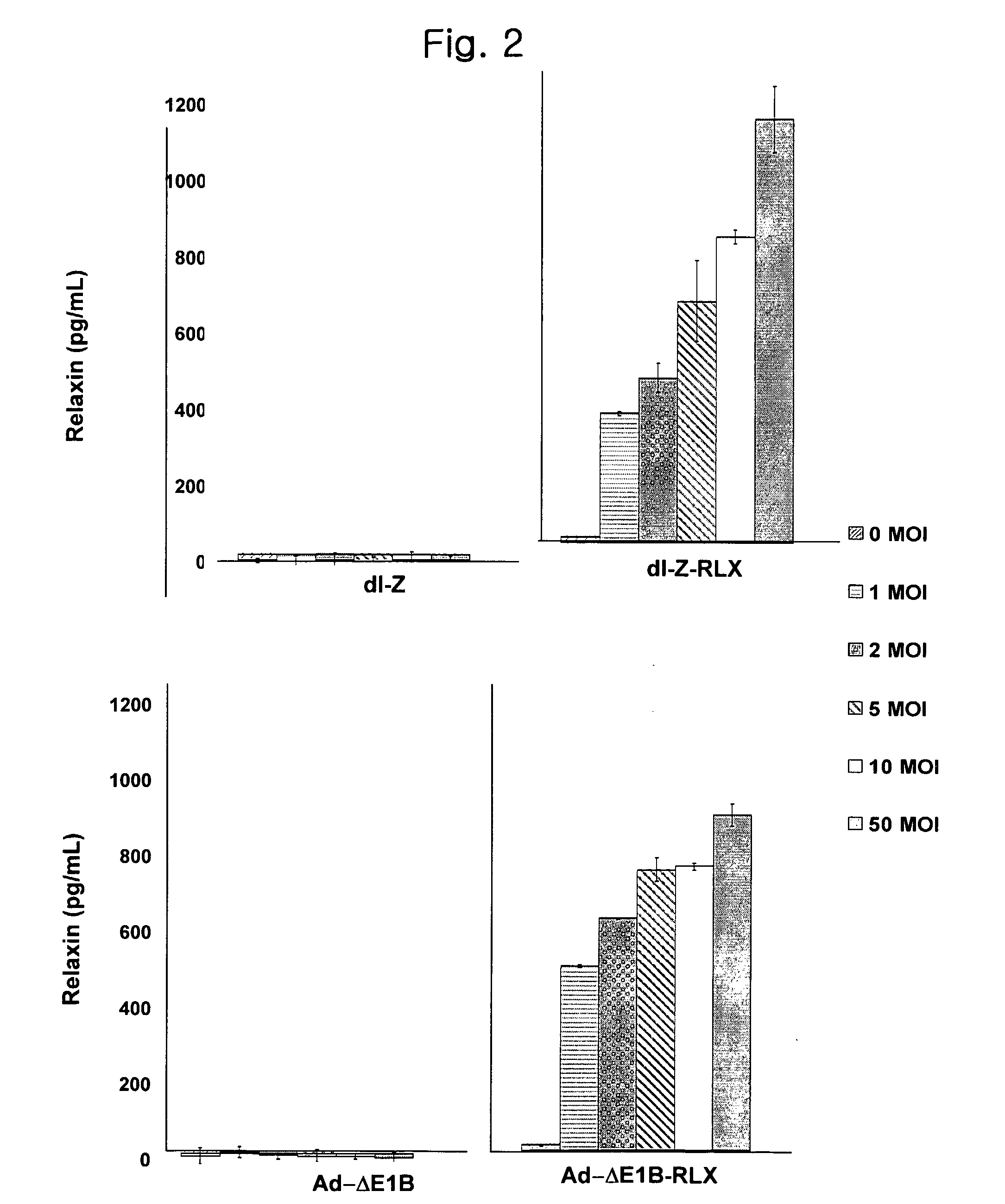
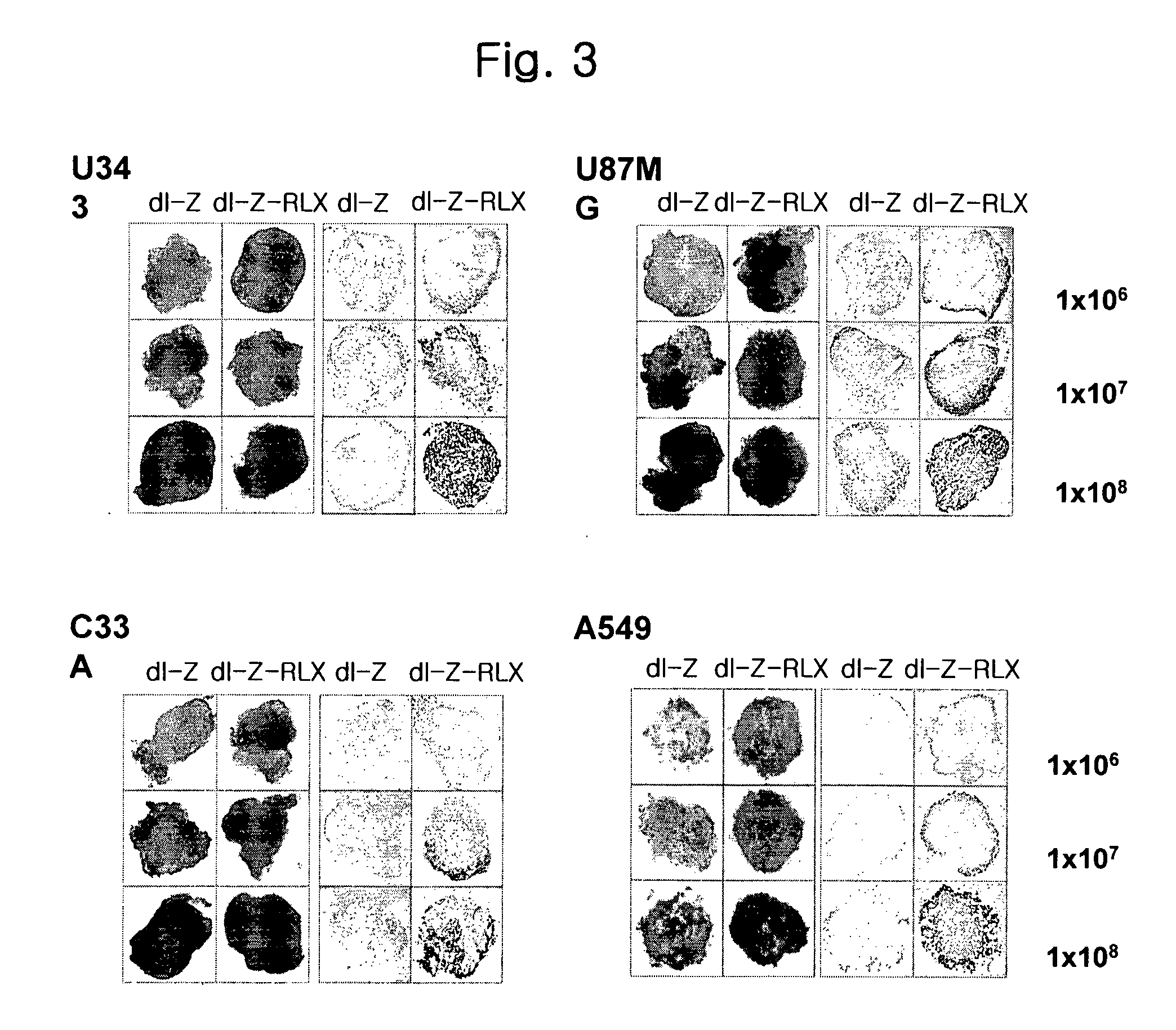
Gene Delivery System Containing Relaxin Gene And Pharmaceutical Composition Using Relaxin
InactiveUS20070202080A1High transduction efficiencyEnhance transduction (or spreading) efficiencyBiocidePeptide/protein ingredientsBiotechnologyGene delivery
The present invention relates to a novel gene delivery system and recombinant adenovirus comprising the relaxin-encoding sequence to enhance transduction efficiency of transgenes, a pharmaceutical anti-tumor composition comprising the recombinant adenovirus, a pharmaceutical composition having improved tissue penetration potency and a pharmaceutical composition for treating a disease or disorder associated with accumulation of excess extracellular matrix.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Adenoviral expression vectors
InactiveUS20070249043A1Reduce generationHigh expressionVectorsGenetic material ingredientsHeterologousCytotoxicity
The present invention provides a recombinant adenovirus vector characterized by the partial or total deletion of adenoviral E2B function and having an expression cassette containing a heterologous sequence encoding a protein of interest inserted into the E1 region. Such vectors are designed to reduce or eliminate the occurrence of replication competent adenovirus contamination. Additionally, the expression cassette of the vector may contain one or more regulatory elements capable of increasing the expression of the heterologous sequence and / or reducing the expression of viral proteins. Such a reduction in expression of viral proteins reduces the cytotoxicty and immunogenicity of the adenovirus vectors when administered in vivo. Transformed production host cells and a method of producing recombinant proteins and gene therapy also are included within the scope of this invention.
Owner:CANJI
Stable adenoviral vectors and methods for propagation thereof
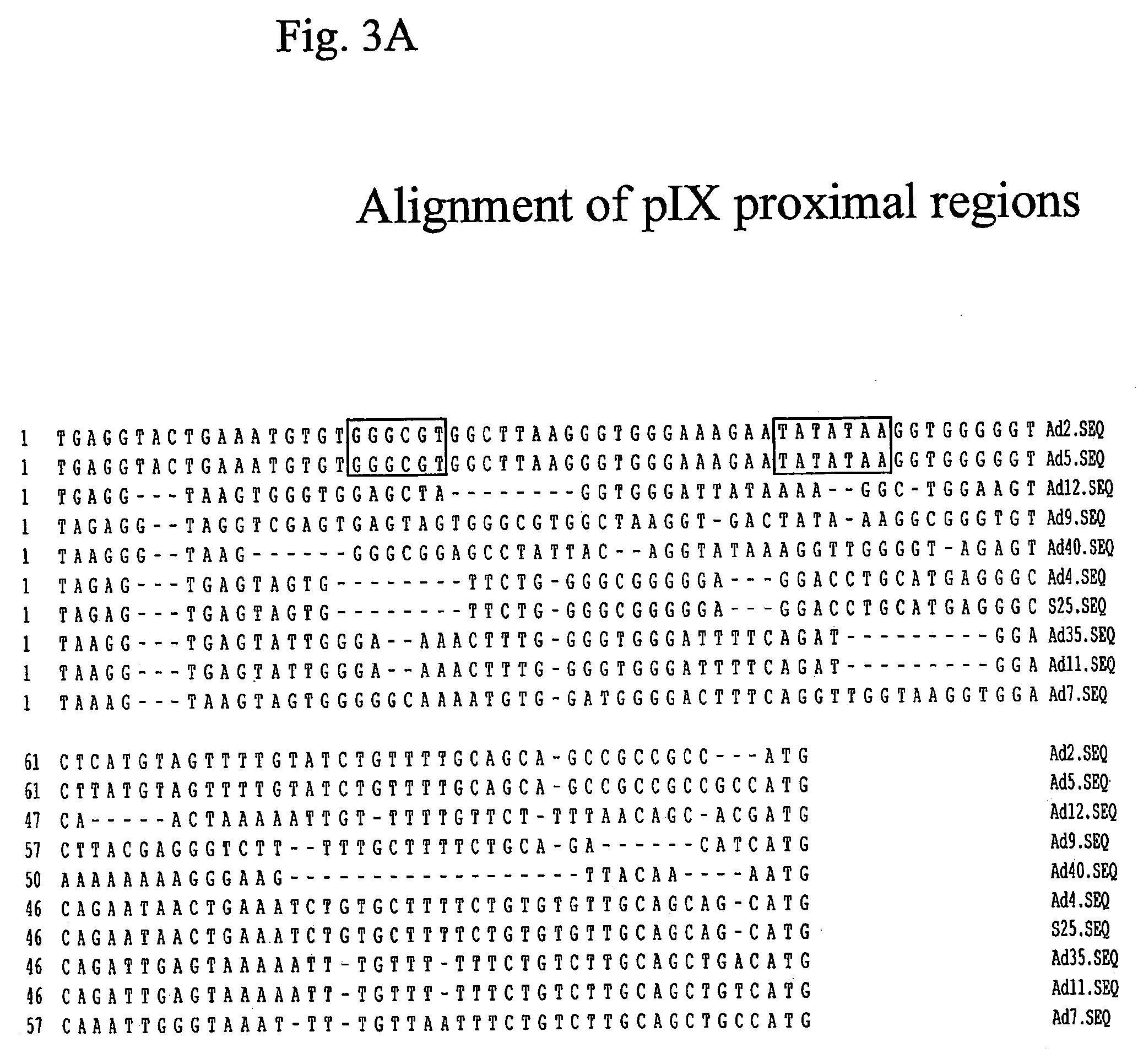
ActiveUS7285265B2Increase capacityImprove stabilityBiocideGenetic material ingredientsHeterologousOpen reading frame
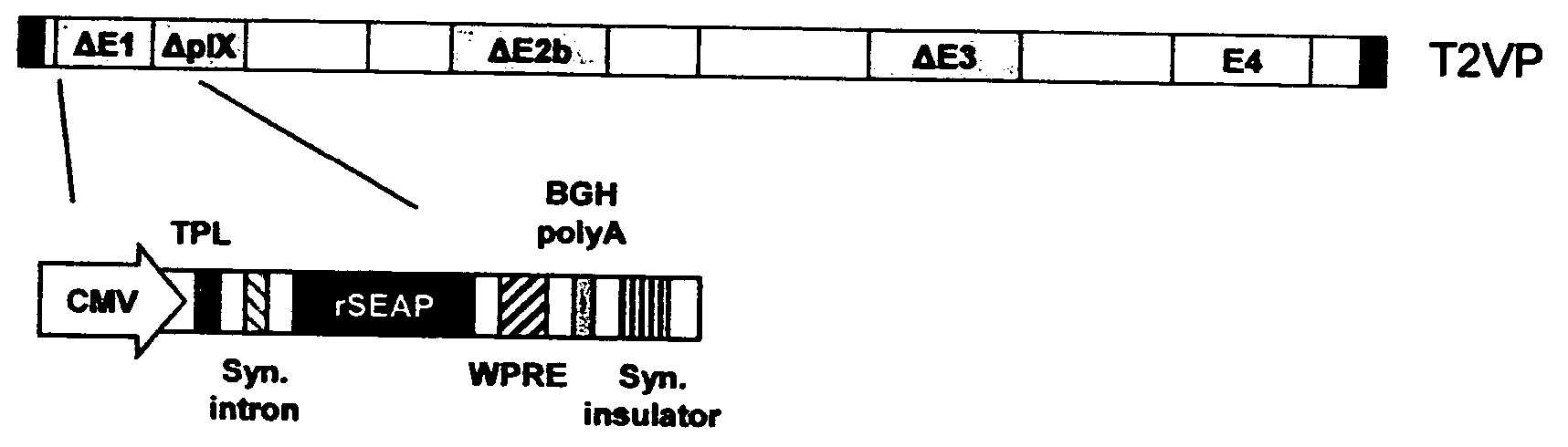
The present invention provides methods and means to increase the stability and / or the packaging capacity of recombinant adenoviruses, by overexpression of pIX in an adenoviral packaging cell, by retaining at least a part of the E1B 55K region in the recombinant adenoviral vector or by regulating pIX with a heterologous promoter. The invention further relates to methods and means for the production of such adenoviruses on complementing cell lines, wherein the early region 4 open reading frame 6 (E4-orf6) encoding nucleic acid is present in the adenovirus and wherein the E4-orf6 gene product is compatible with one or more products of the E1 gene products in the complementing cell, such that the adenoviral vector can be efficiently produced by the complementing cell.
Owner:JANSSEN VACCINES & PREVENTION BV
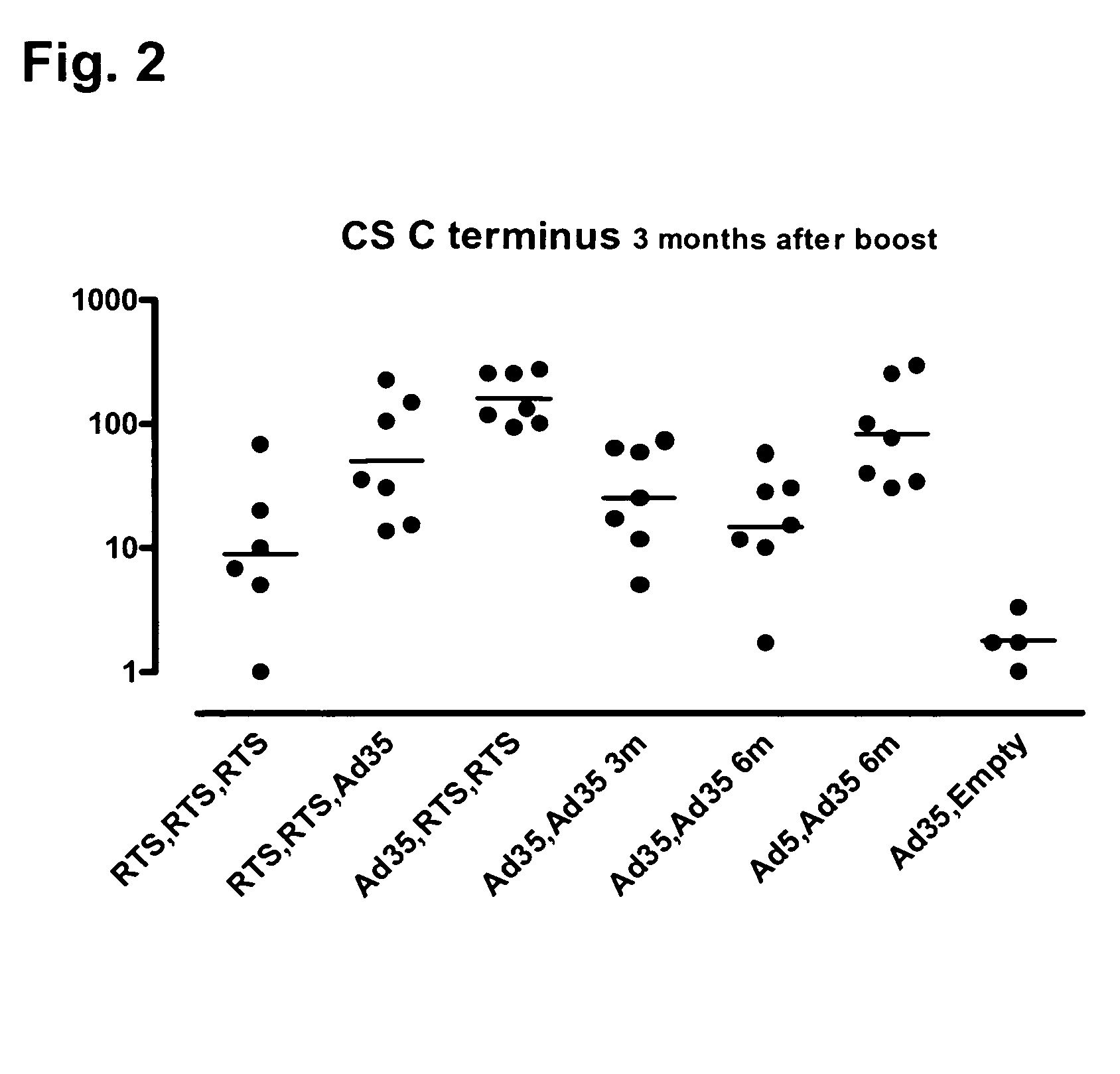
Malaria prime/boost vaccines
InactiveUS20090285879A1High potencyIncrease volumeViral antigen ingredientsAntiparasitic agentsAntigenAdjuvant
Described are vaccine regimens in which specific prime / boost regimens are applied using low-neutralized recombinant adenoviral vectors harboring nucleic acids encoding antigens from Plasmodium falciparum and purified recombinant protein vaccines such as RTS,S, in the context of appropriate adjuvants.
Owner:WALTER REED ARMY INST OF RES THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE ARMY ON BEHALF OF THE +2
Adenoviral expression vectors
The present invention provides a recombinant adenovirus vector characterized by the partial or total deletion of adenoviral E2B function and having an expression cassette containing a heterologous sequence encoding a protein of interest inserted into the E1 region. Such vectors are designed to reduce or eliminate the occurrence of replication competent adenovirus contamination. Additionally, the expression cassette of the vector may contain one or more regulatory elements capable of increasing the expression of the heterologous sequence and / or reducing the expression of viral proteins. Such a reduction in expression of viral proteins reduces the cytotoxicty and immunogenicity of the adenovirus vectors when administered in vivo. Transformed production host cells and a method of producing recombinant proteins and gene therapy also are included within the scope of this invention.
Owner:CANJI
Chimeric adenoviruses
InactiveUS7749493B2Low antigenicityExact fitSsRNA viruses negative-senseBiocideVaccinationDendritic cell
The present invention provides methods and vector systems for the generation of chimeric recombinant adenoviruses. These hybrid adenoviruses contain a genome that is derived from different adenovirus serotypes. In particular, novel hybrid adenoviruses are disclosed with improved properties for gene therapy purposes. These properties include: a decreased sensitivity towards neutralizing antibodies, a modified host range, a change in the titer to which adenovirus can be grown, the ability to escape trapping in the liver upon in vivo systemic delivery, and absence or decreased infection of antigen presenting cells (APC) of the immune system, such as macrophages or dendritic cells. These chimeric adenoviruses thus represent improved tools for gene therapy and vaccination since they overcome the limitations observed with the currently used serotype subgroup C adenoviruses.
Owner:JANSSEN VACCINES & PREVENTION BV
Recombinant adenovirus carrier and application thereof
InactiveCN101892261AHas gene therapy valueGenetic material ingredientsMicroorganism based processesDNA fragmentationEukaryotic plasmids
The invention discloses a recombinant adenovirus carrier and application thereof. A plasmid of the recombinant adenovirus carrier is a pAdxsi plasmid carrying a DNA fragment (a) or (b): (a) a DNA fragment of a coding gene which contains a CMV promoter and DENND2D protein from the upstream to the downstream, and (b) a DNA fragment of a coding gene which contains a tetracycline reactive factor, a promoter, and a DENND2D protein from the upstream to the downstream. Two recombinant adenoviruses both have inhibiting effects on various tumor cell lines in vitro and in vivo and have a certain genetic therapy value.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
Settings for recombinant adenoviral-based vaccines
The present invention provides new uses of recombinant adenoviral vectors in vaccination regimens, such as prime / boost set-ups and subsequent vaccinations and applications for gene therapy. Moreover, the invention provides new assays to determine the best regimen for applying the most suitable recombinant viral vector in a vaccination or gene therapy setting.
Owner:JANSSEN VACCINES & PREVENTION BV
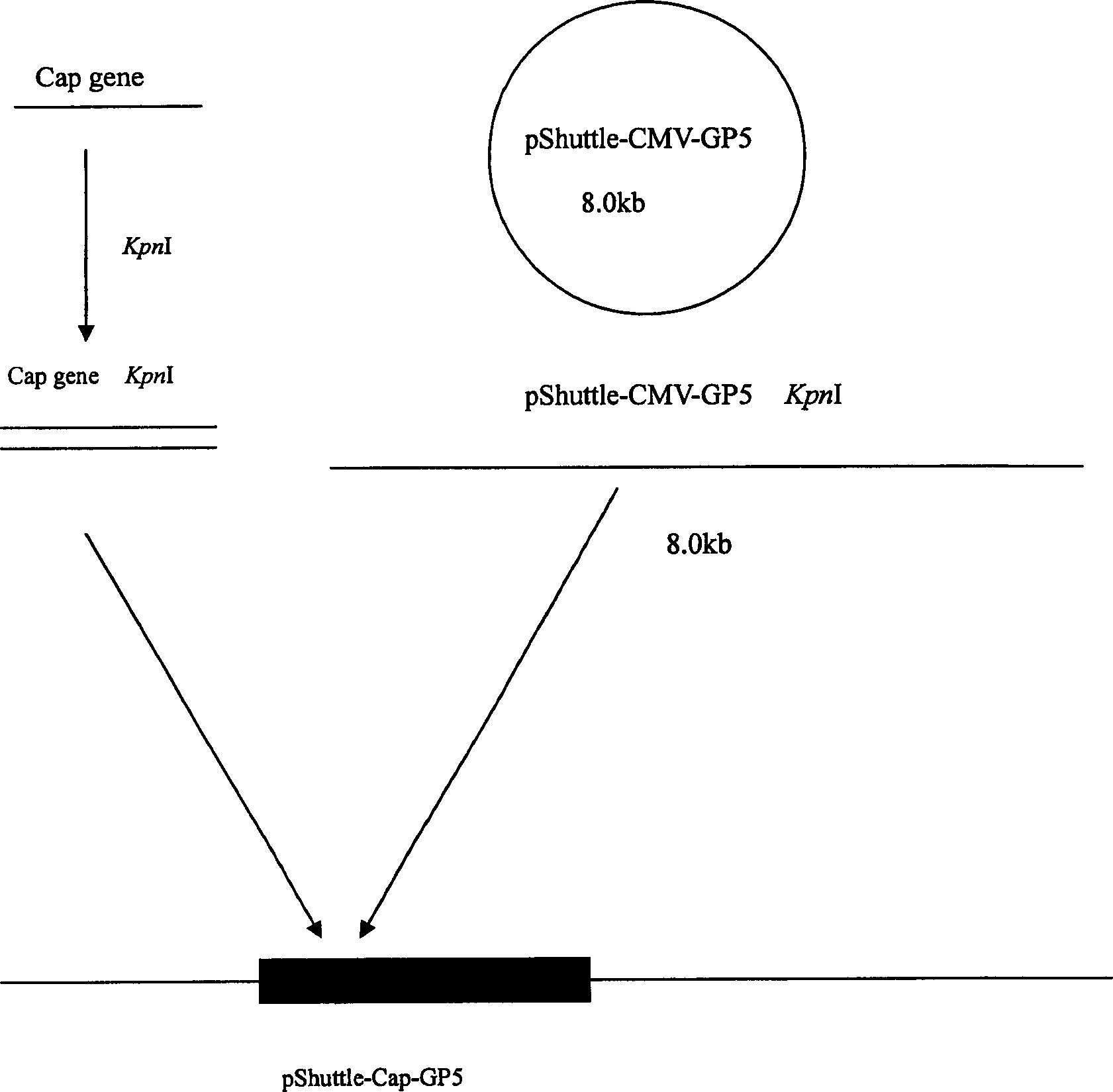
Recombinant adenovirus of porcine reproductive and respirator syndrome virus and porcine Circovirus, and vaccine
InactiveCN1800375AHas the copy featureStable potencyViruses/bacteriophagesAntibody medical ingredientsEscherichia coliCircovirus
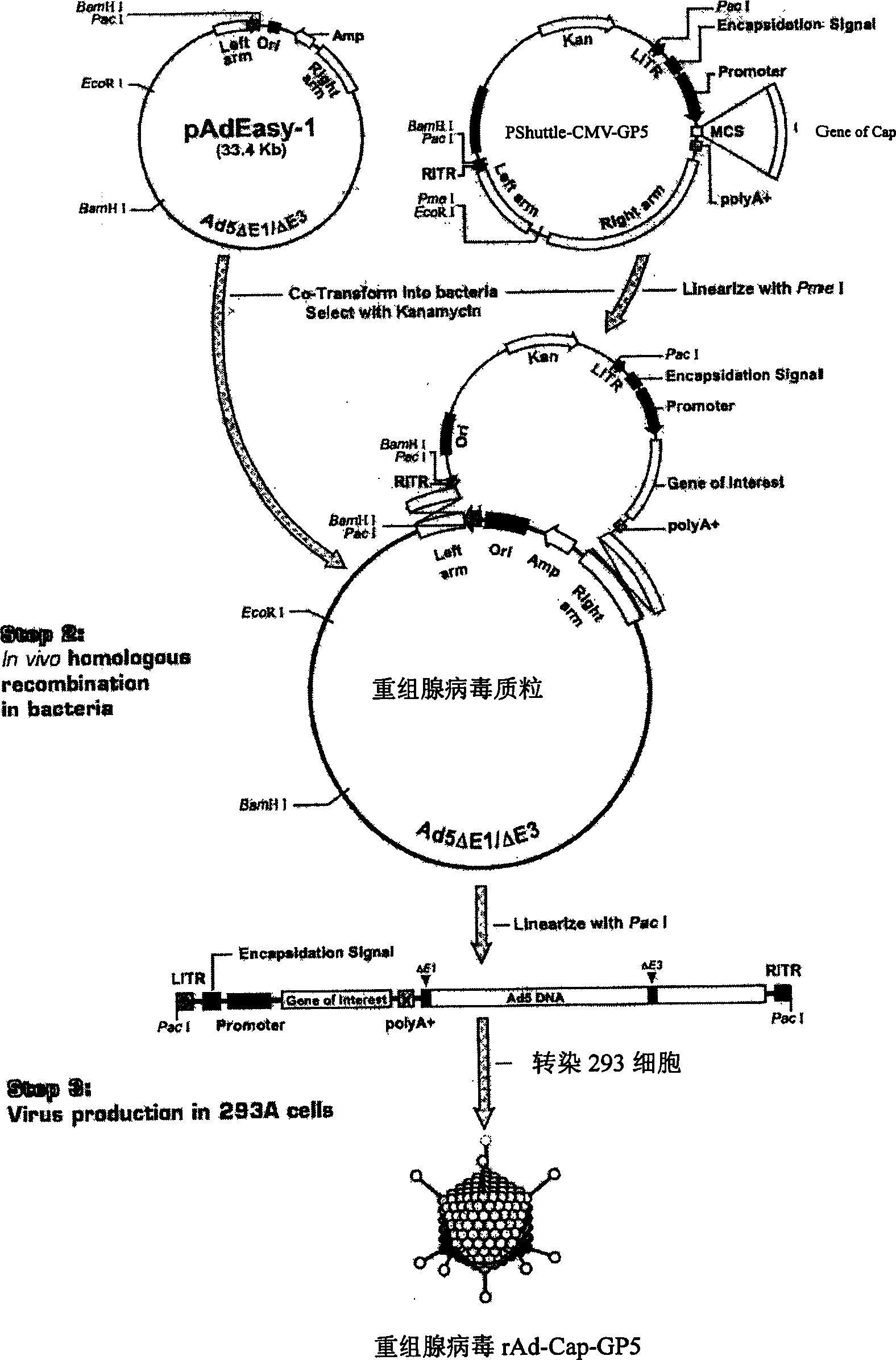
The invention relates to a pig breeding and breathing complex virus (PPRSV) and pig 2-type loop virus (PCV 2) series gene recombination adenovirus and vaccine in the field of high and new biotechnology. It uses PCR technology to clone the Cap protein gene into the plasmid carrier pShuttle-CMV-GP5 by open type reading rule and transforms tobacillus coli BJ5183strain with cage carrier pAdEasy-1 to capture the recombination plasmid; it uses recombination plasmid HEK293-A cell to capture recombination adenovirus and purifies it to express the recombination adenovirus rAd-Cap-GP5 of PRRSV GP5protein and PCV-2 Cap protein.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com