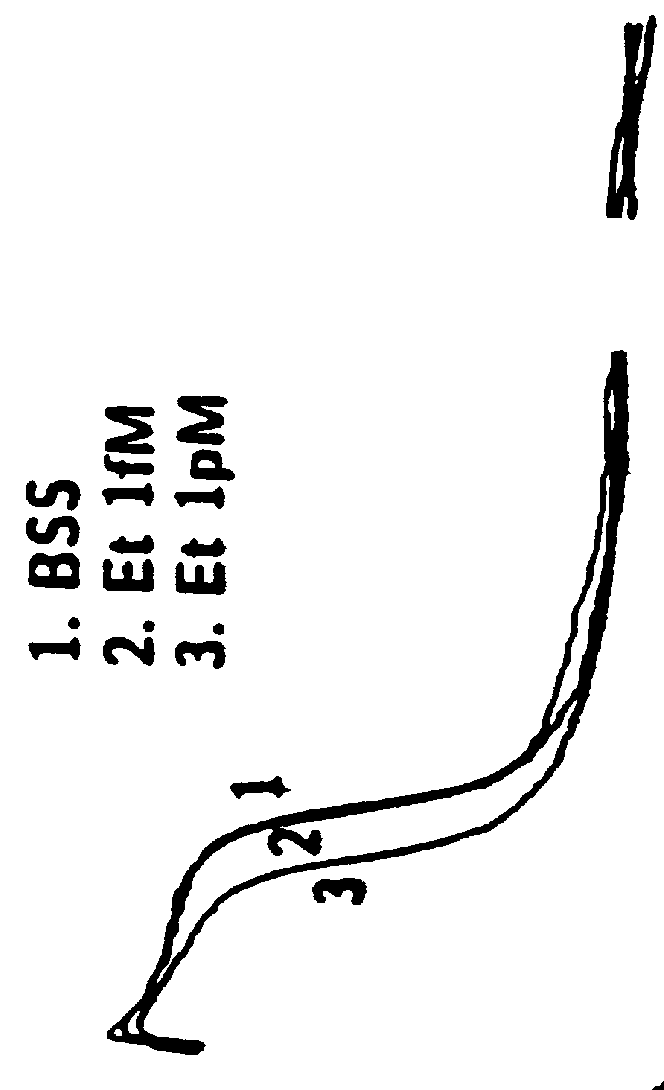
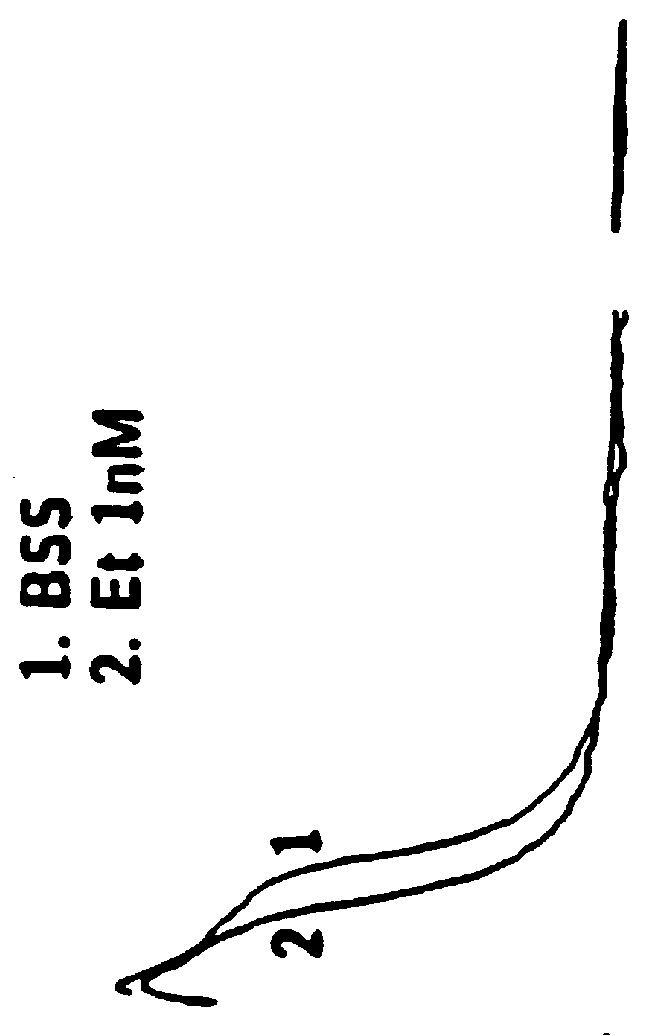
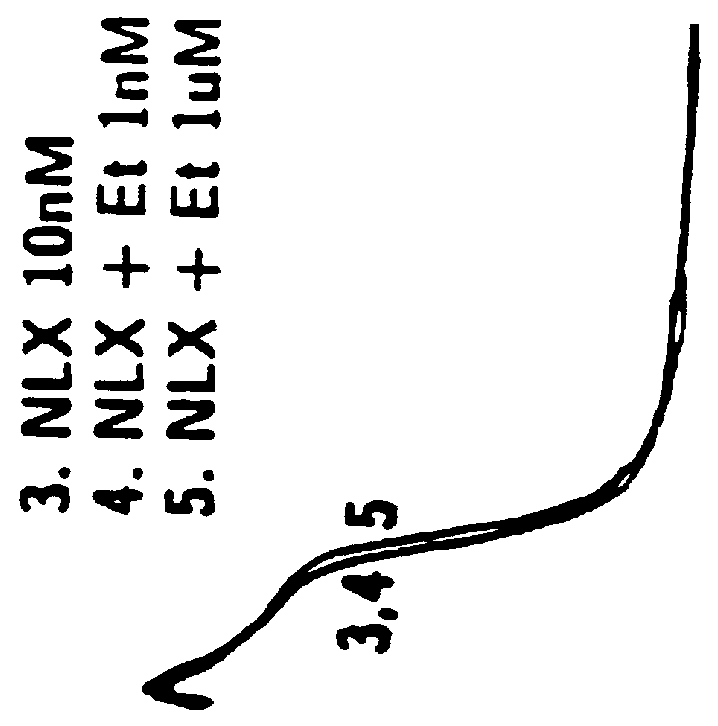
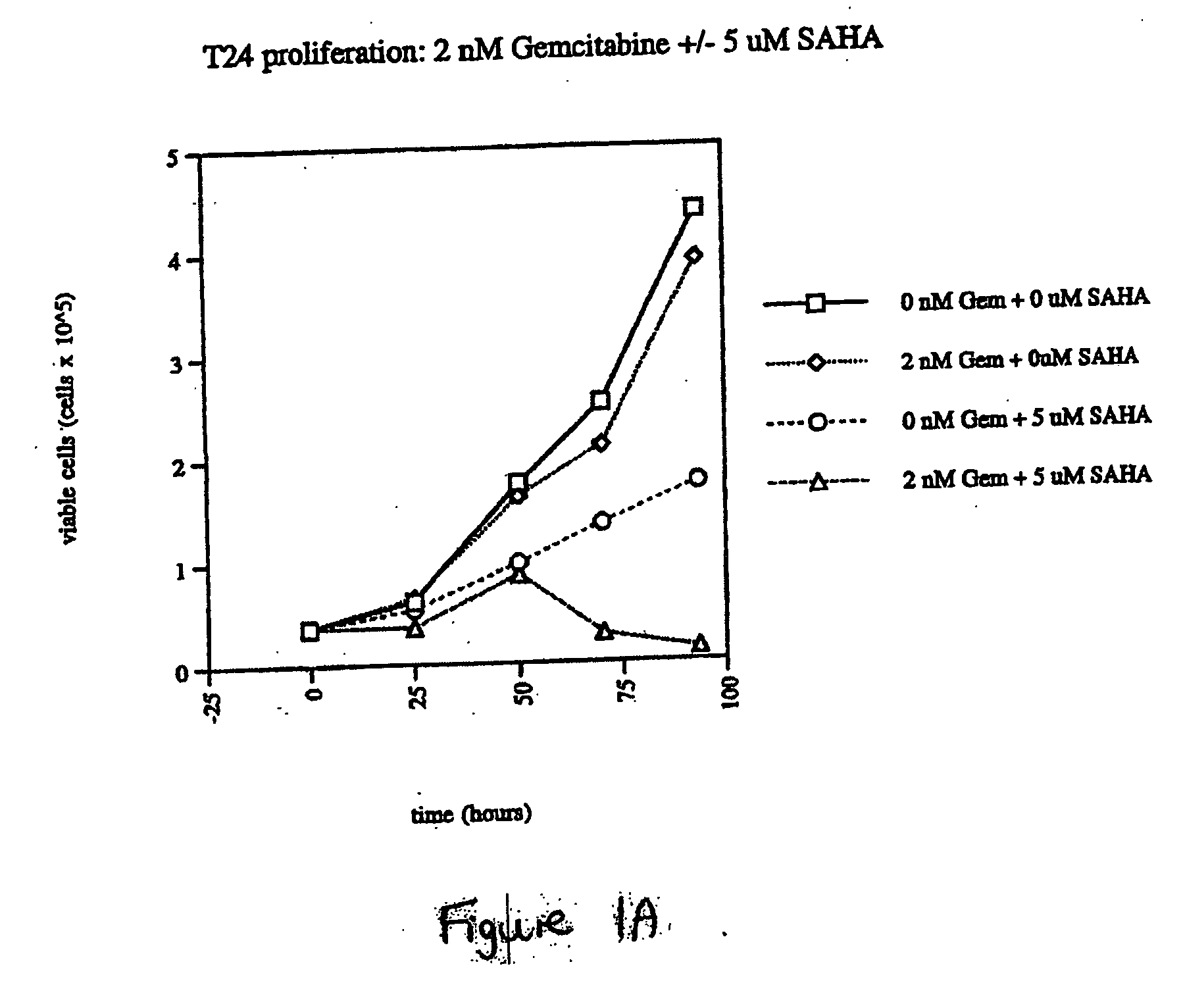
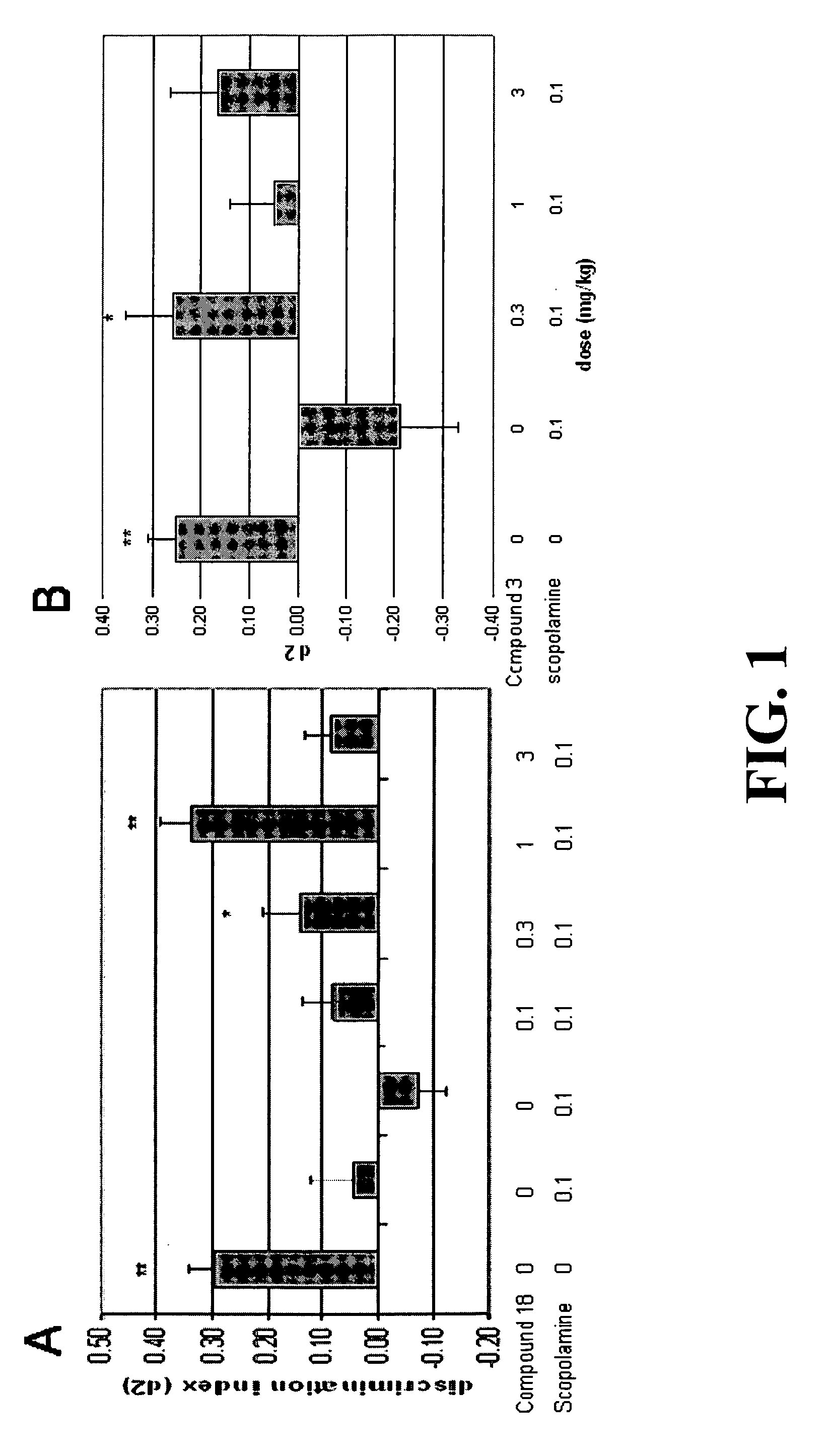
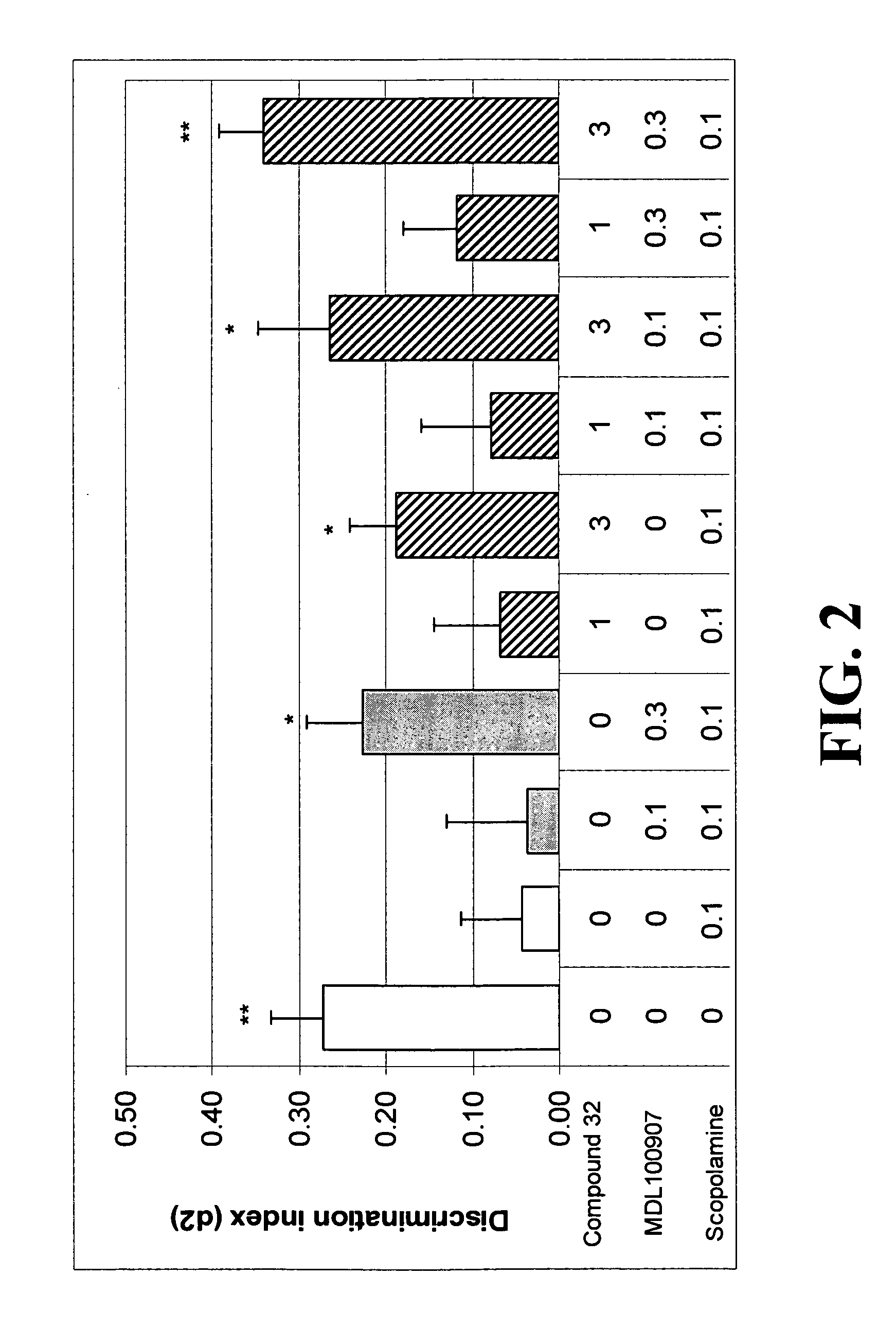
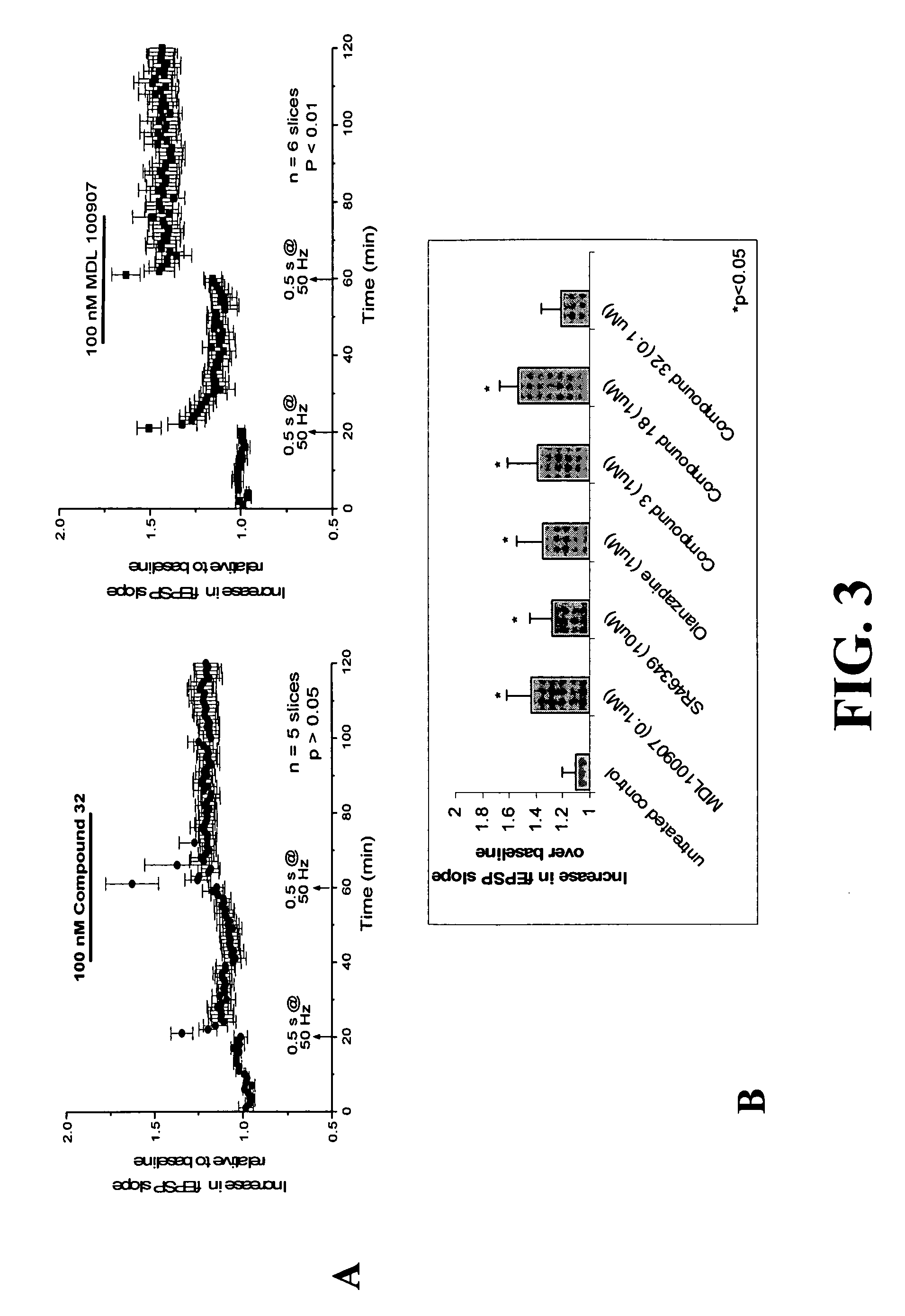
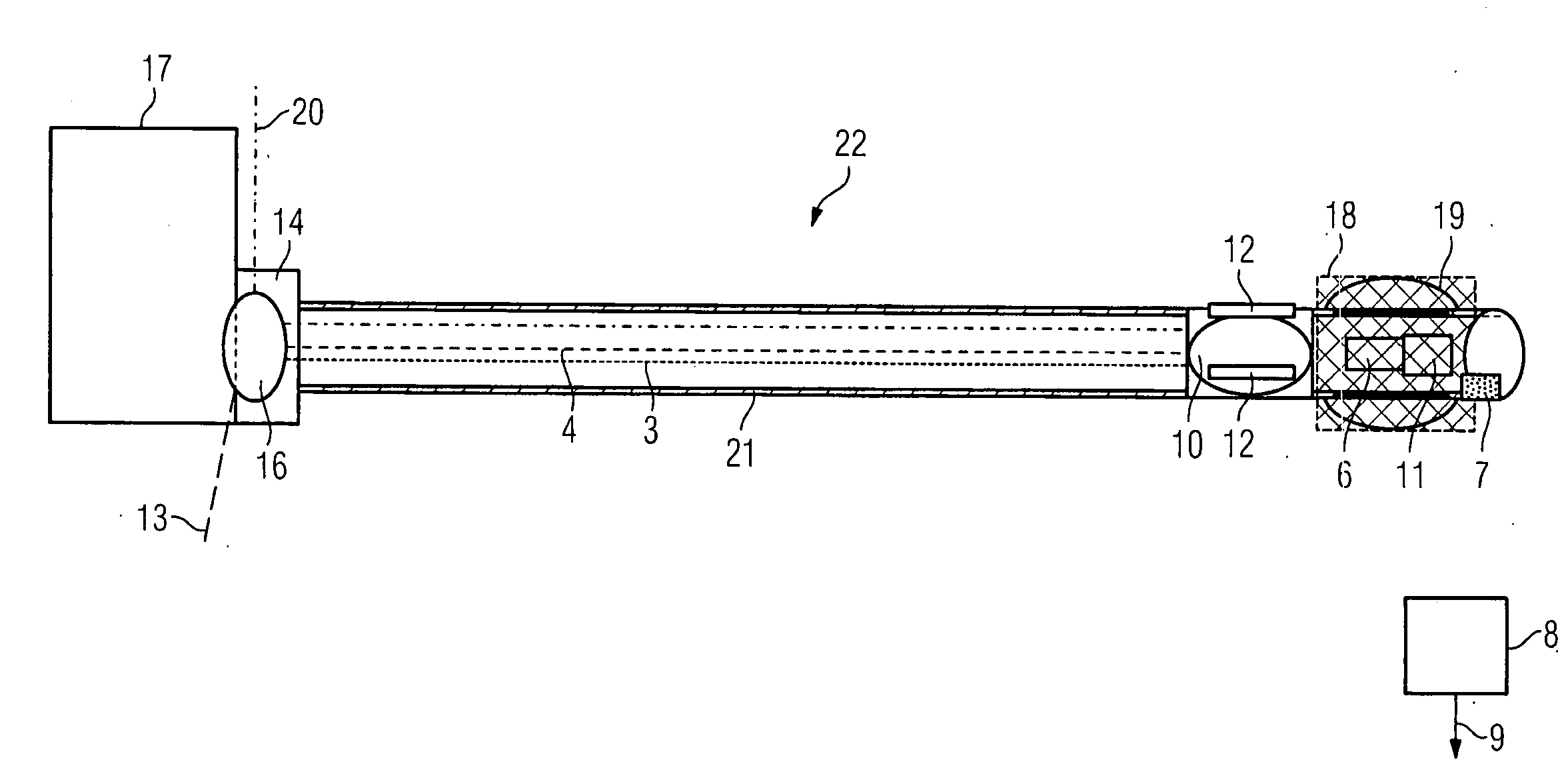
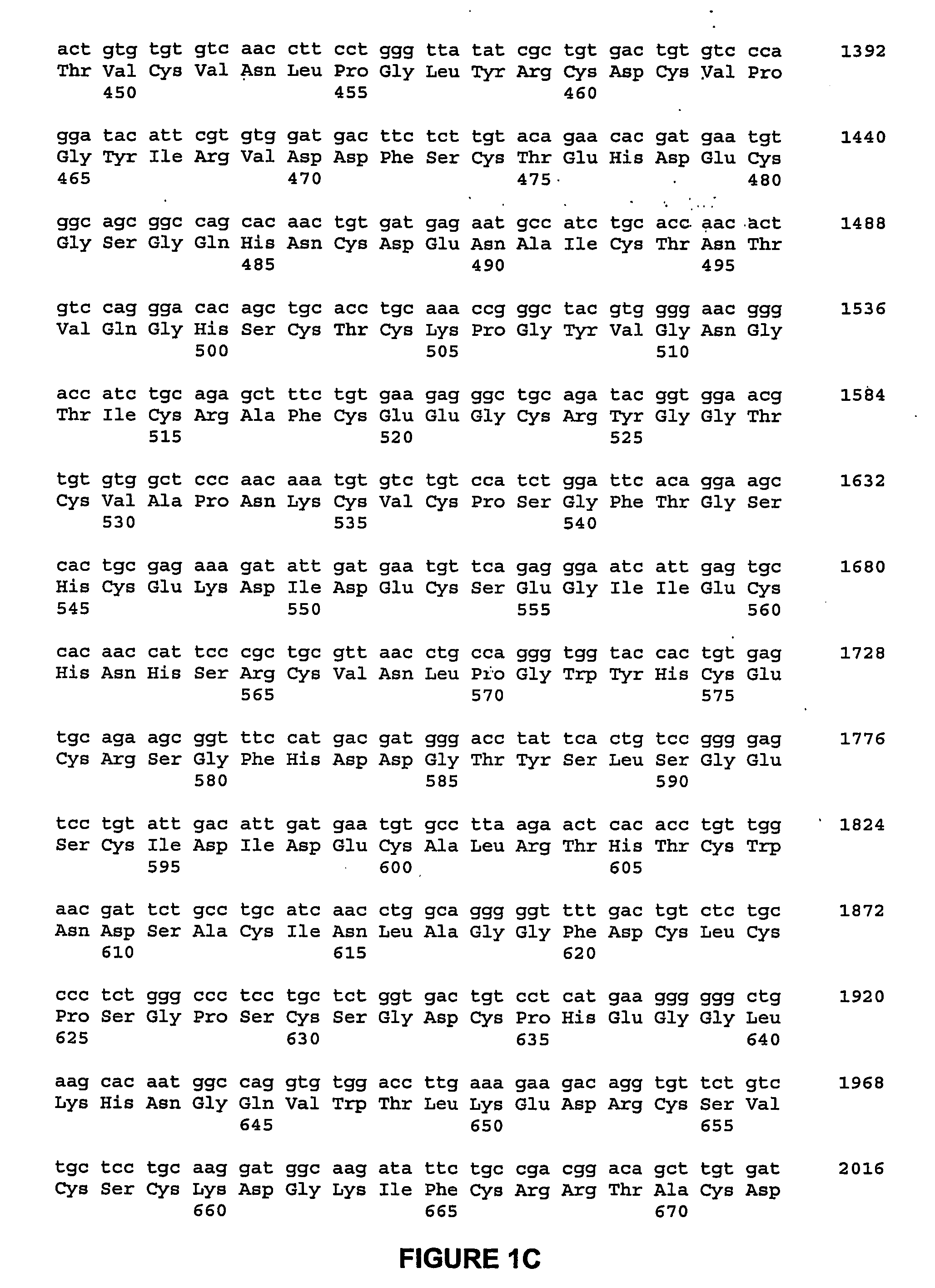
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
226results about How to "Reduced dos" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS20030124185A1Analgesic and euphoric effect be reduce and eliminateCompromise integrityPowder deliveryPill deliveryOpioid antagonistDrug
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
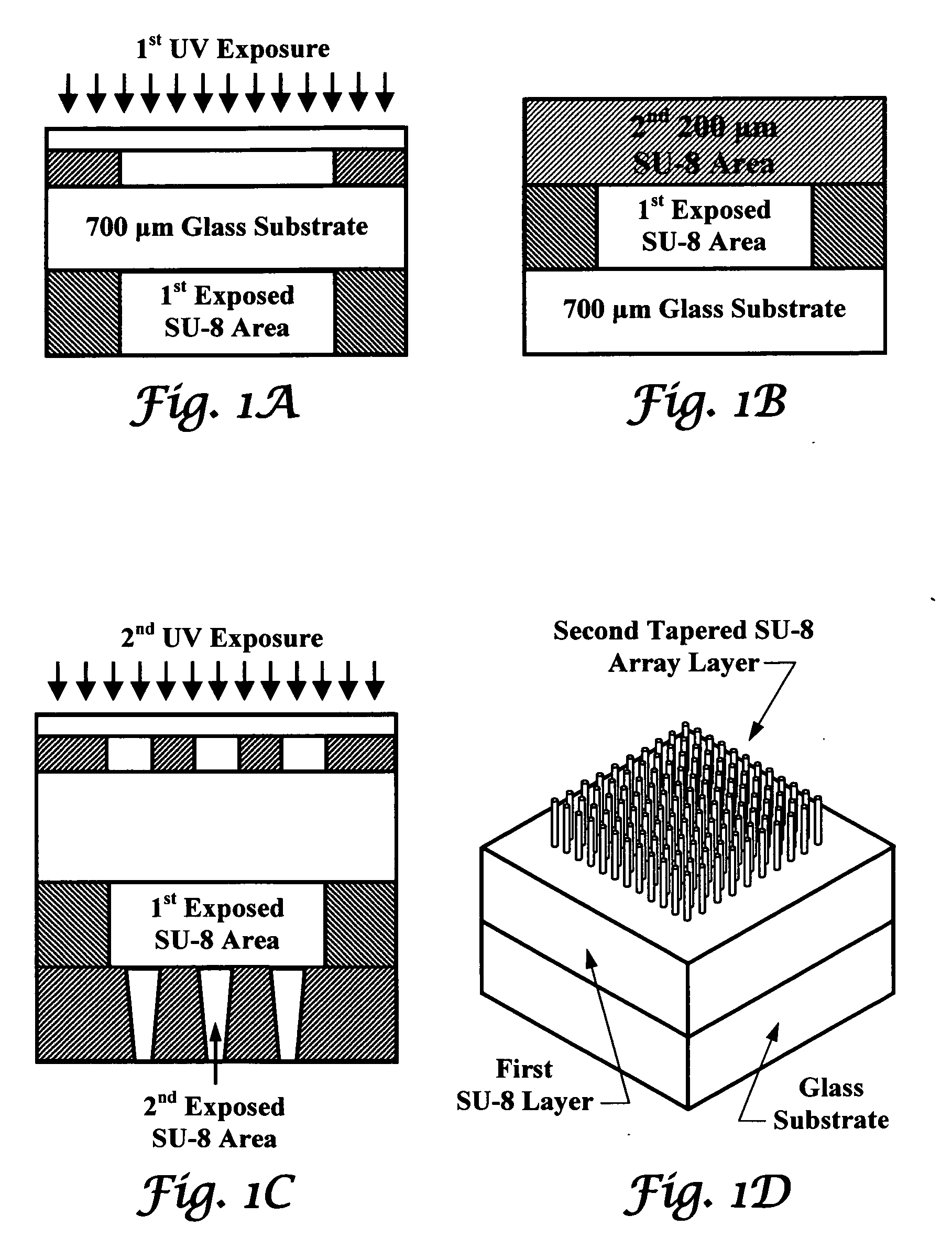
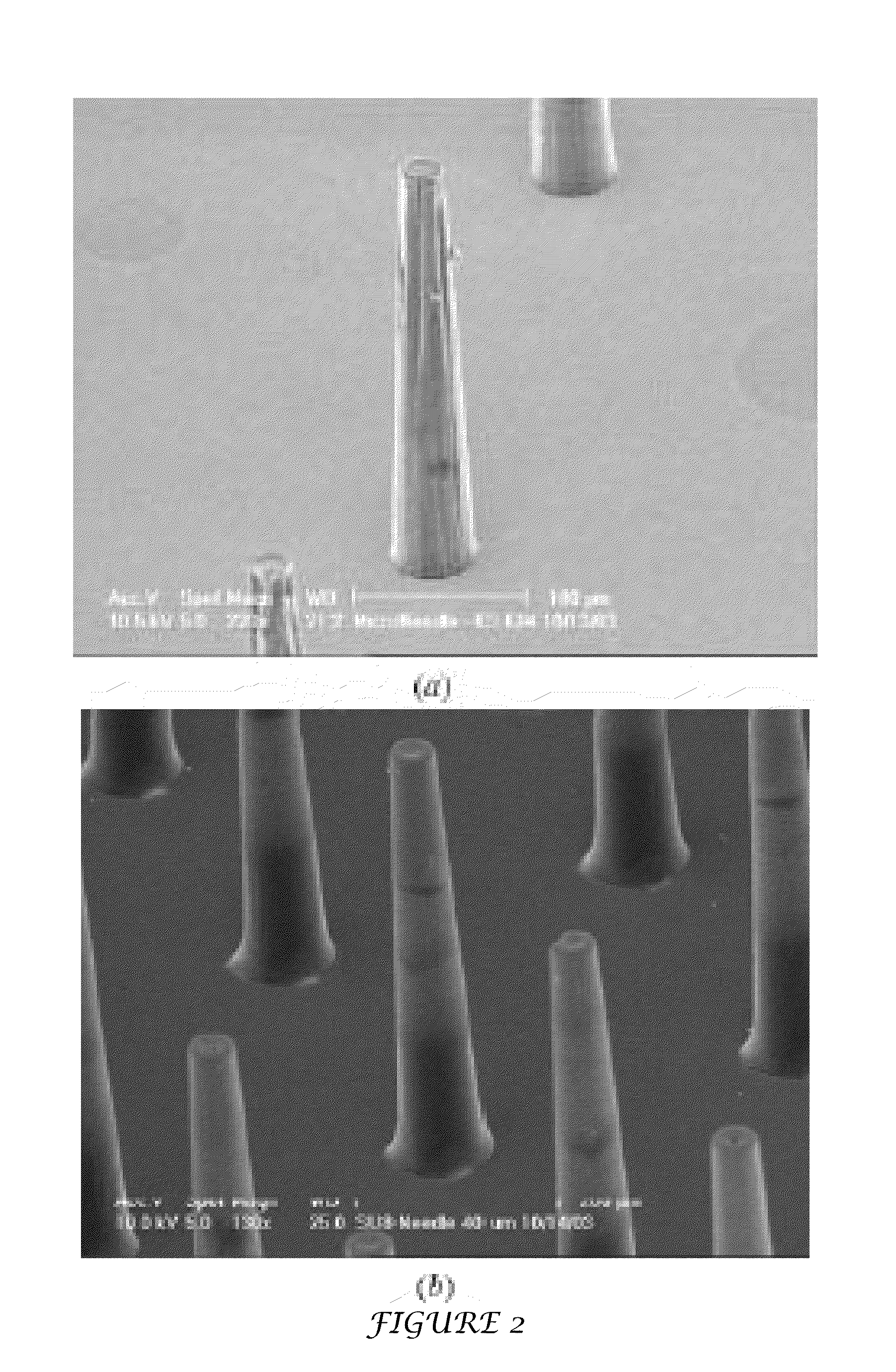
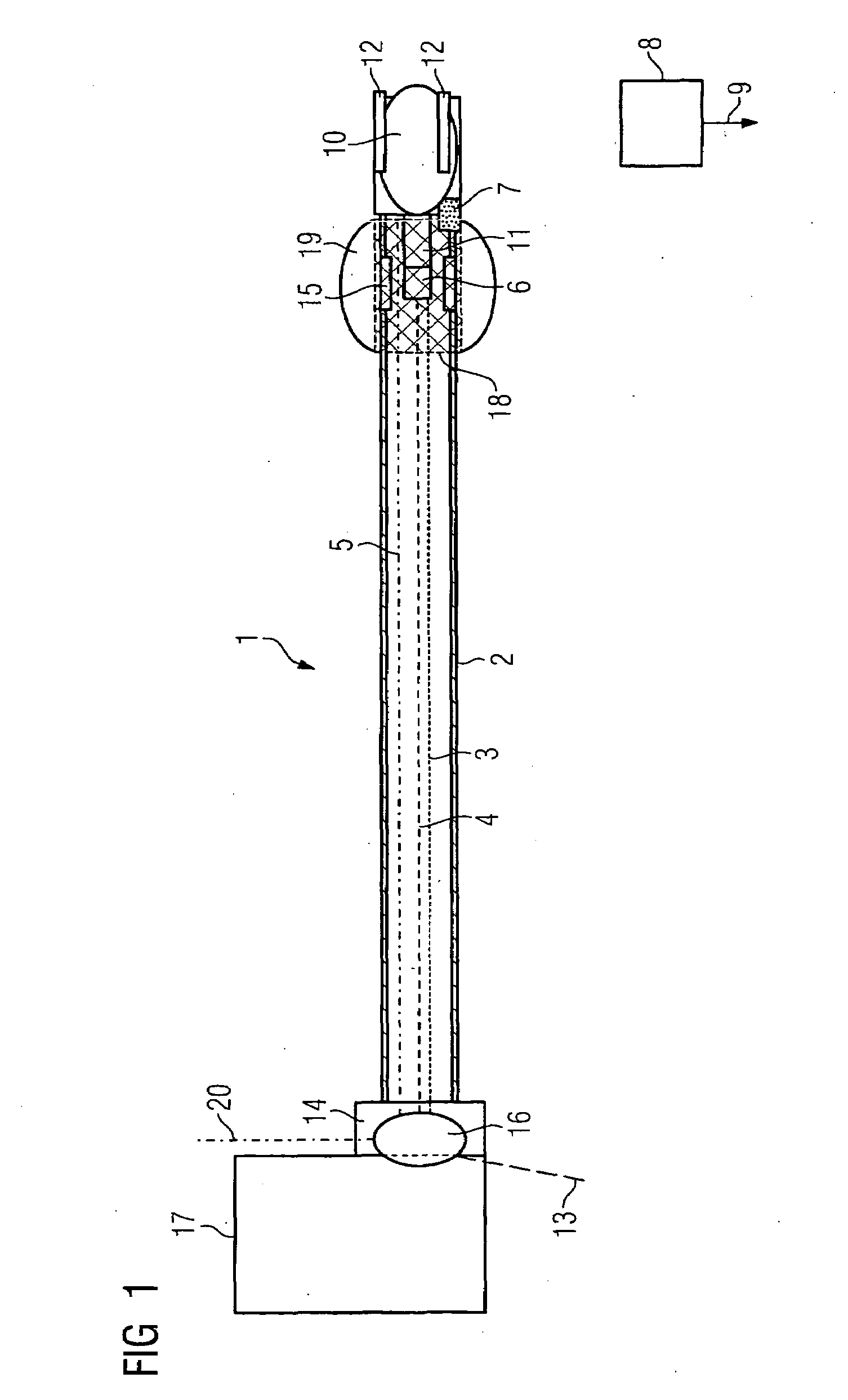
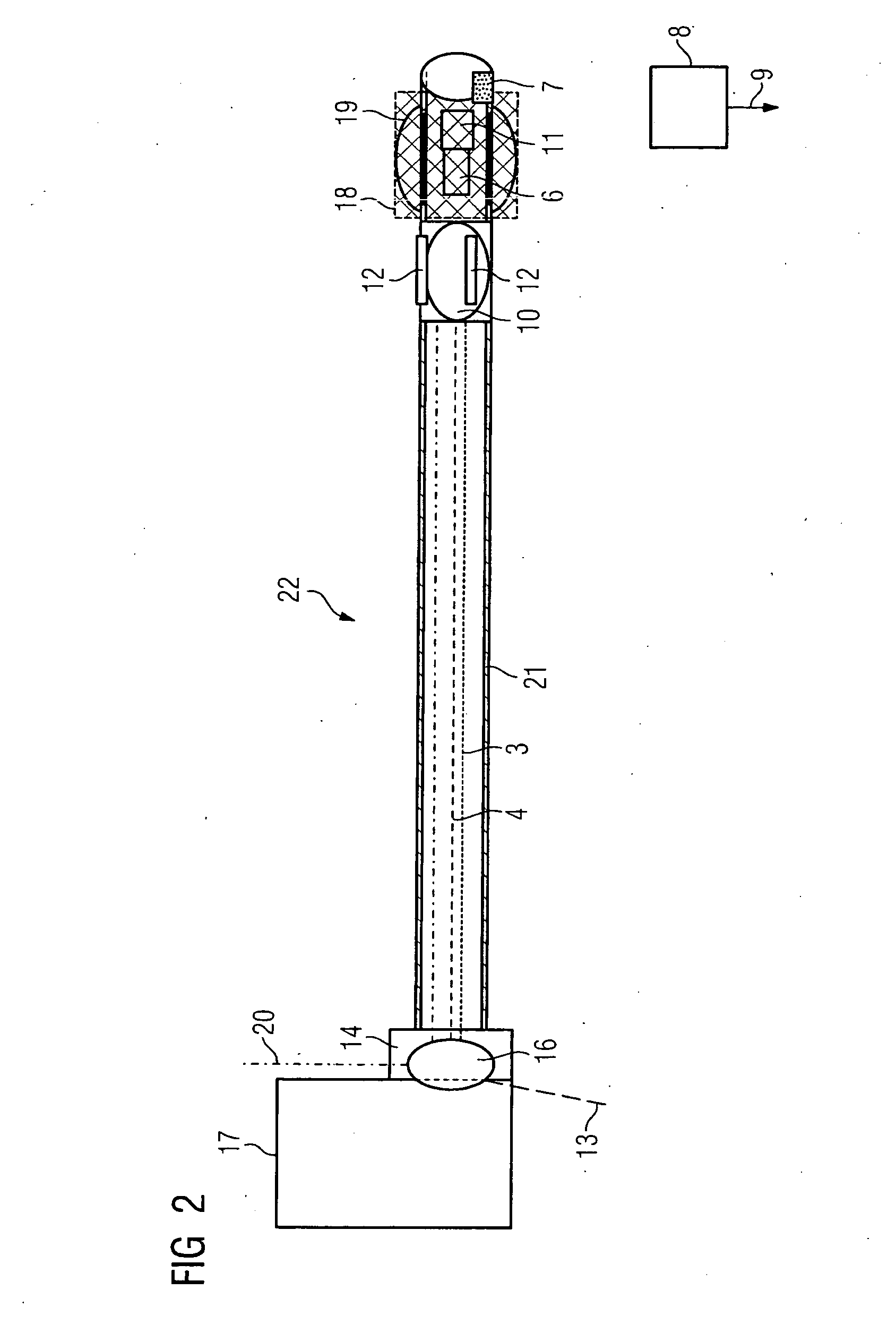
Tapered hollow metallic microneedle array assembly and method of making and using the same
InactiveUS20060084942A1Minimal and damageReduce riskLaminationPharmaceutical delivery mechanismBiomedical engineeringMultiple layer
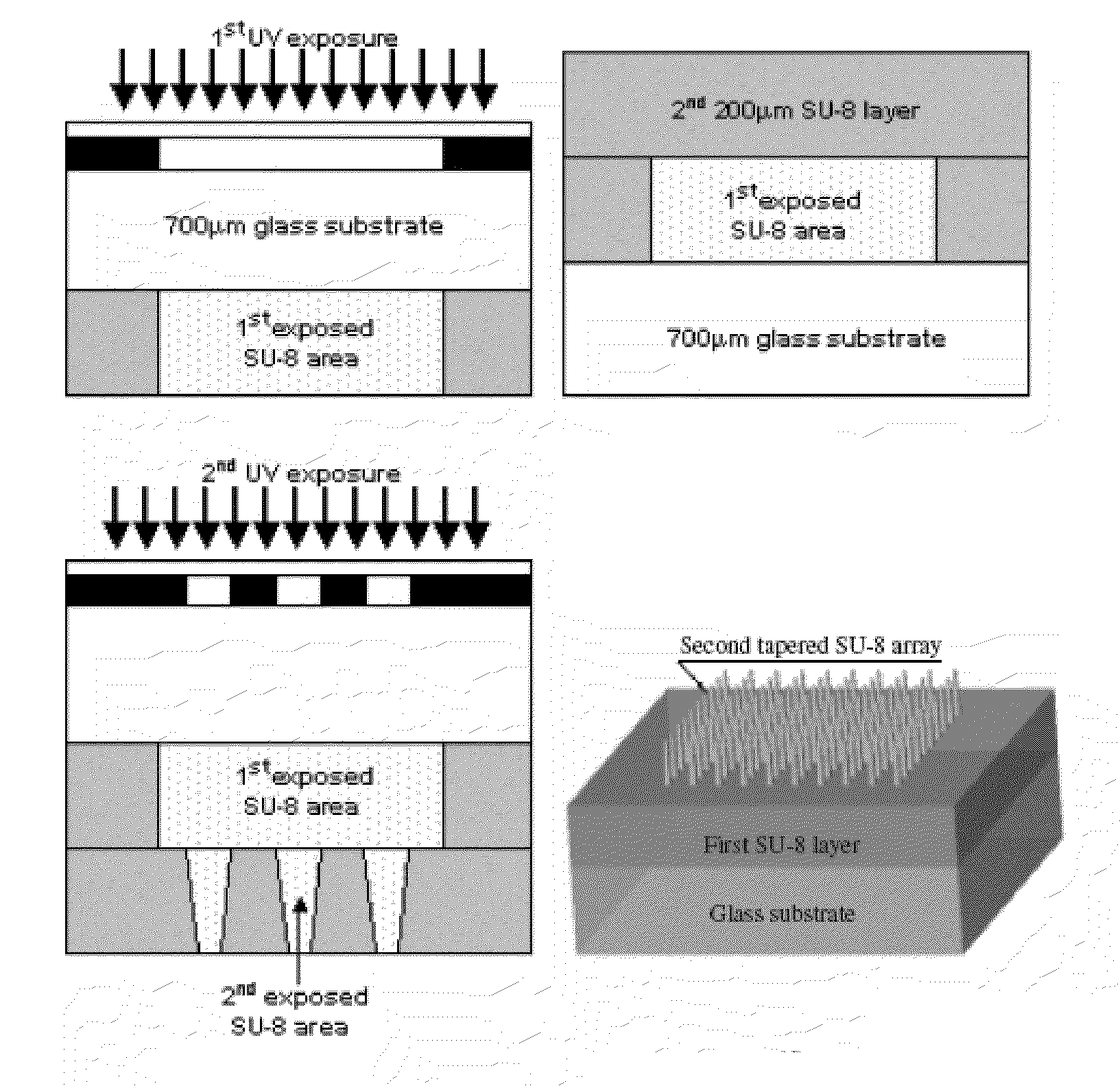
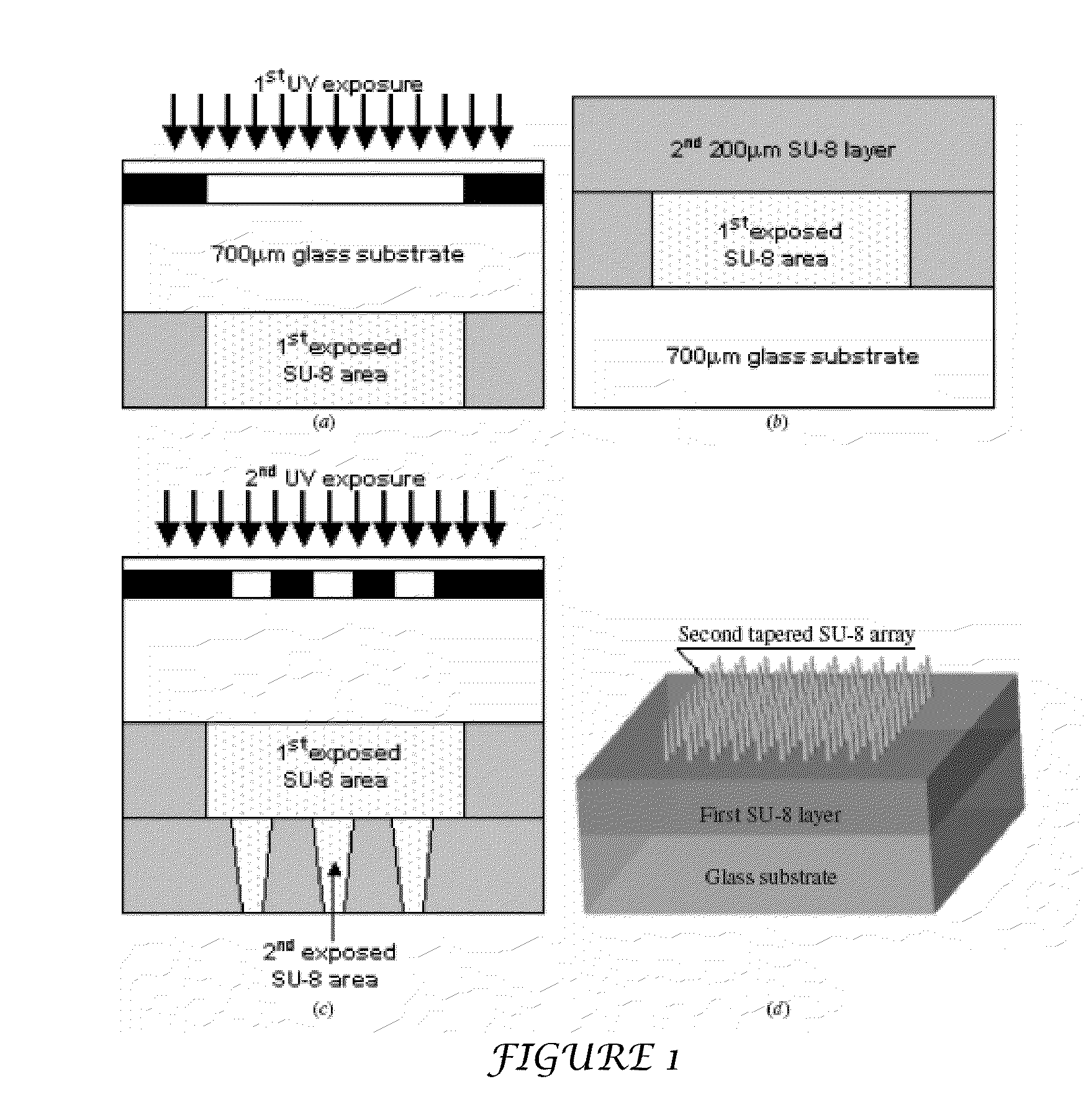
The present invention includes device, system, method of using and making a microneedle array including the steps of forming one or more pins on a substrate, depositing one or more layers on the one or more pins and the substrate, exposing a portion of the one or more pins, and separating the one or more pins from the one or more layers to form the hollow microneedle array.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
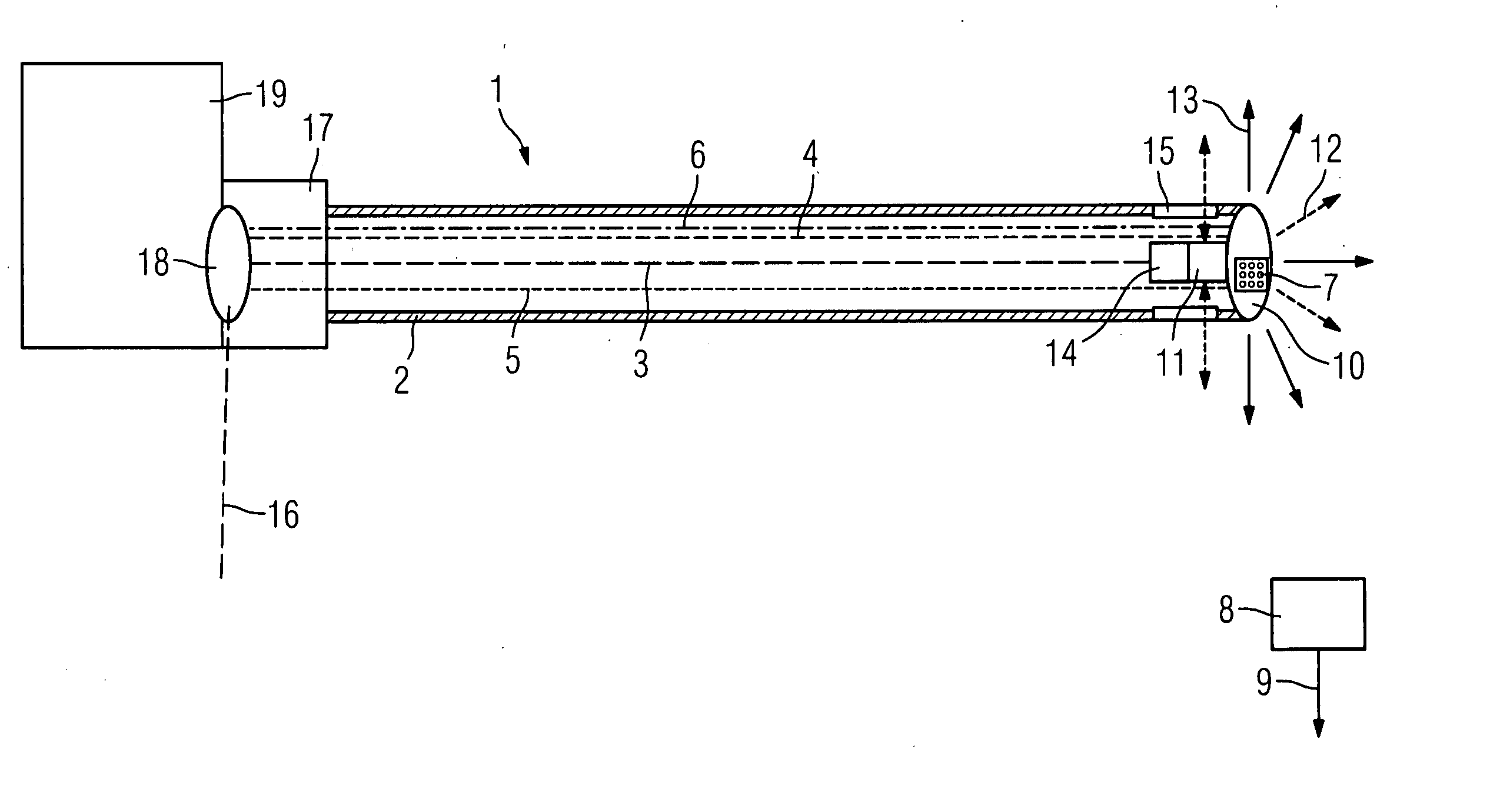
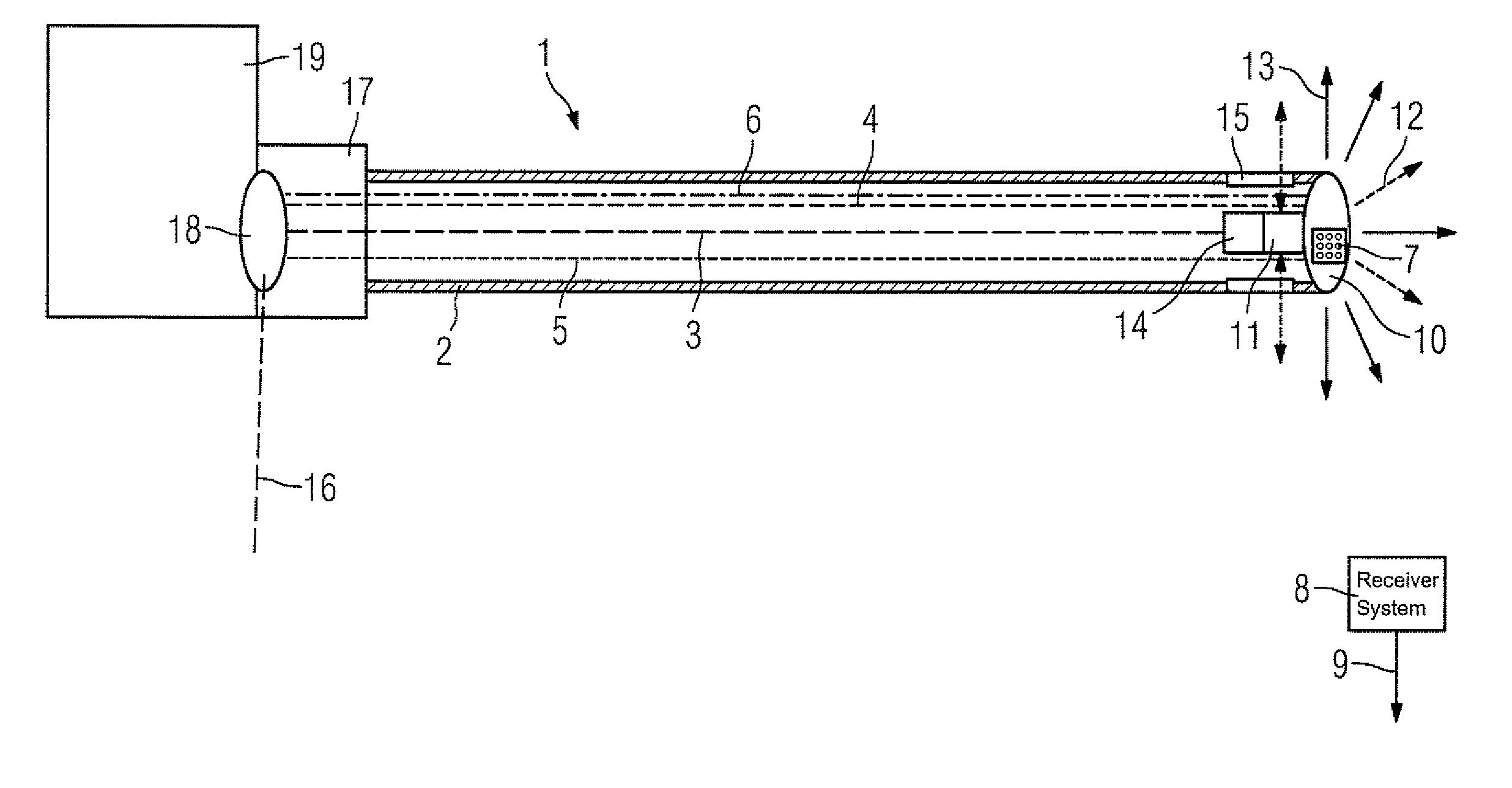
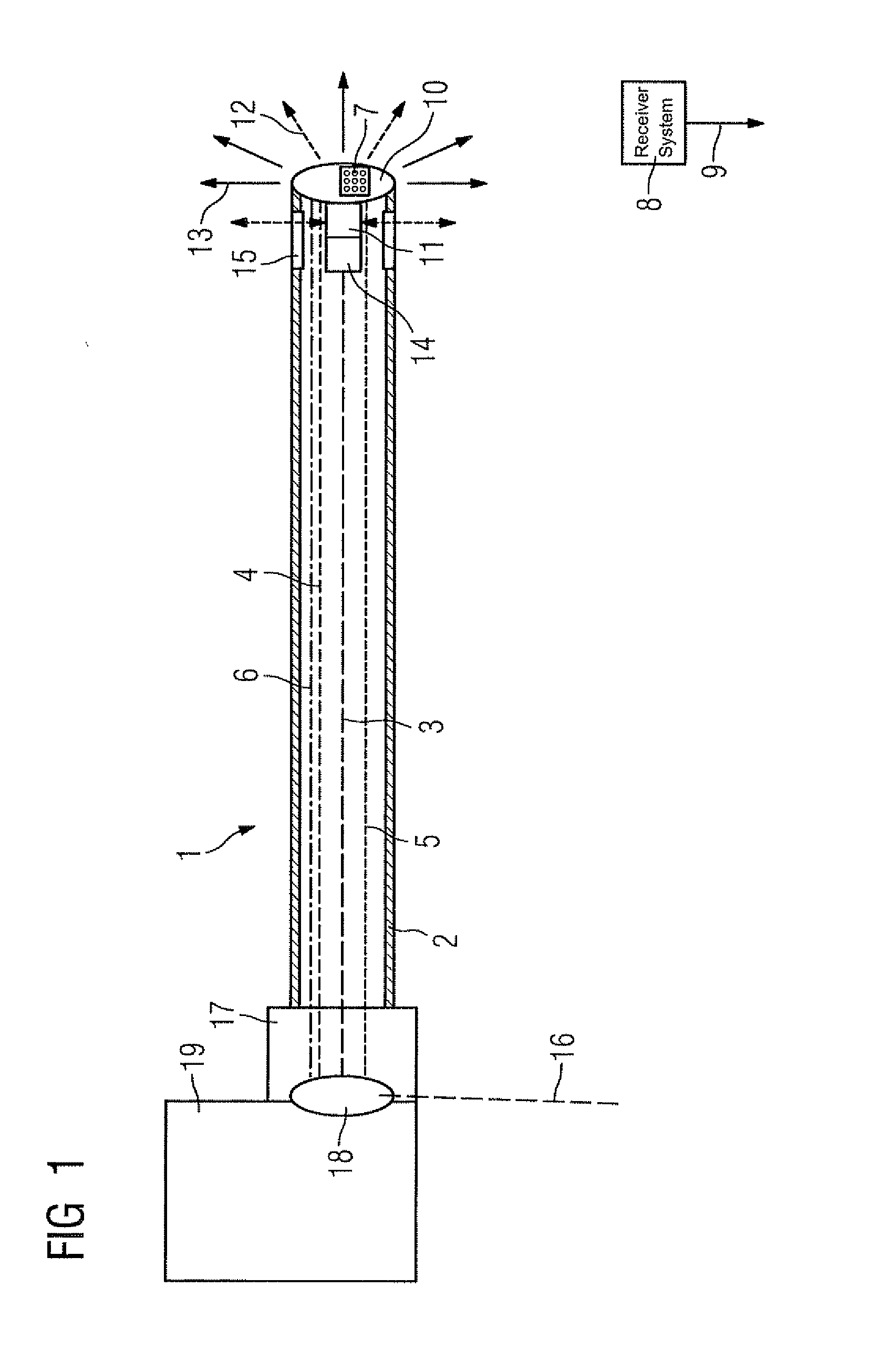
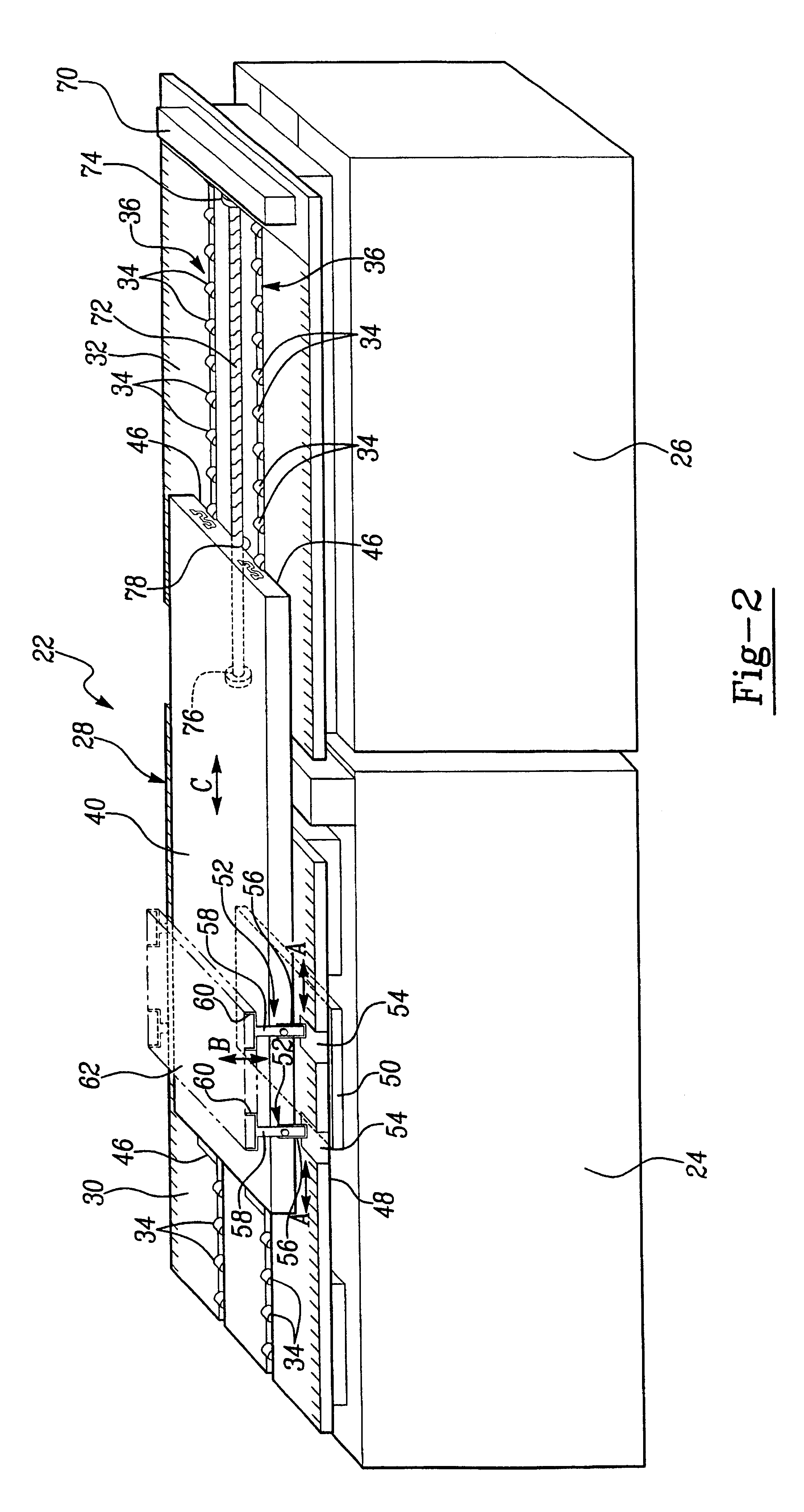
Catheter device with a position sensor system for treating a vessel blockage using image monitoring
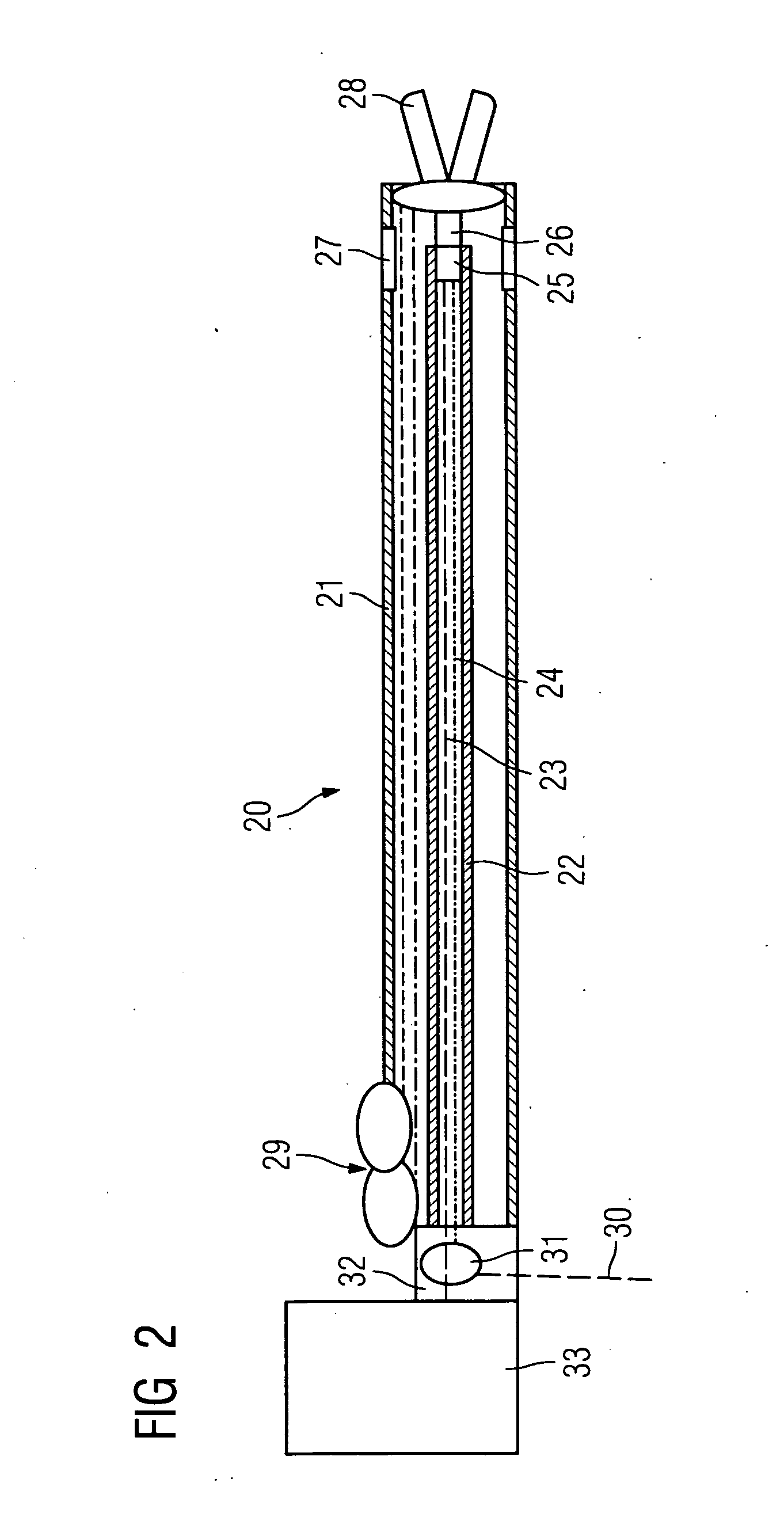
InactiveUS20070066888A1Reduce x-ray radiationGood imageUltrasonic/sonic/infrasonic diagnosticsSurgical navigation systemsSensor systemPosition sensor
The invention relates to a catheter device, with a position sensor system, for treatment of a partial or complete vessel blockage under image monitoring, with the catheter device featuring a treatment catheter of a vessel blockage, especially by removal or destruction of plaque and / or expansion of the vessel,, which is embodied as an integrated unit, especially as a combination catheter, with an OCT catheter and an IVUS catheter for image monitoring and with the position sensor system.
Owner:SIEMENS HEALTHCARE GMBH
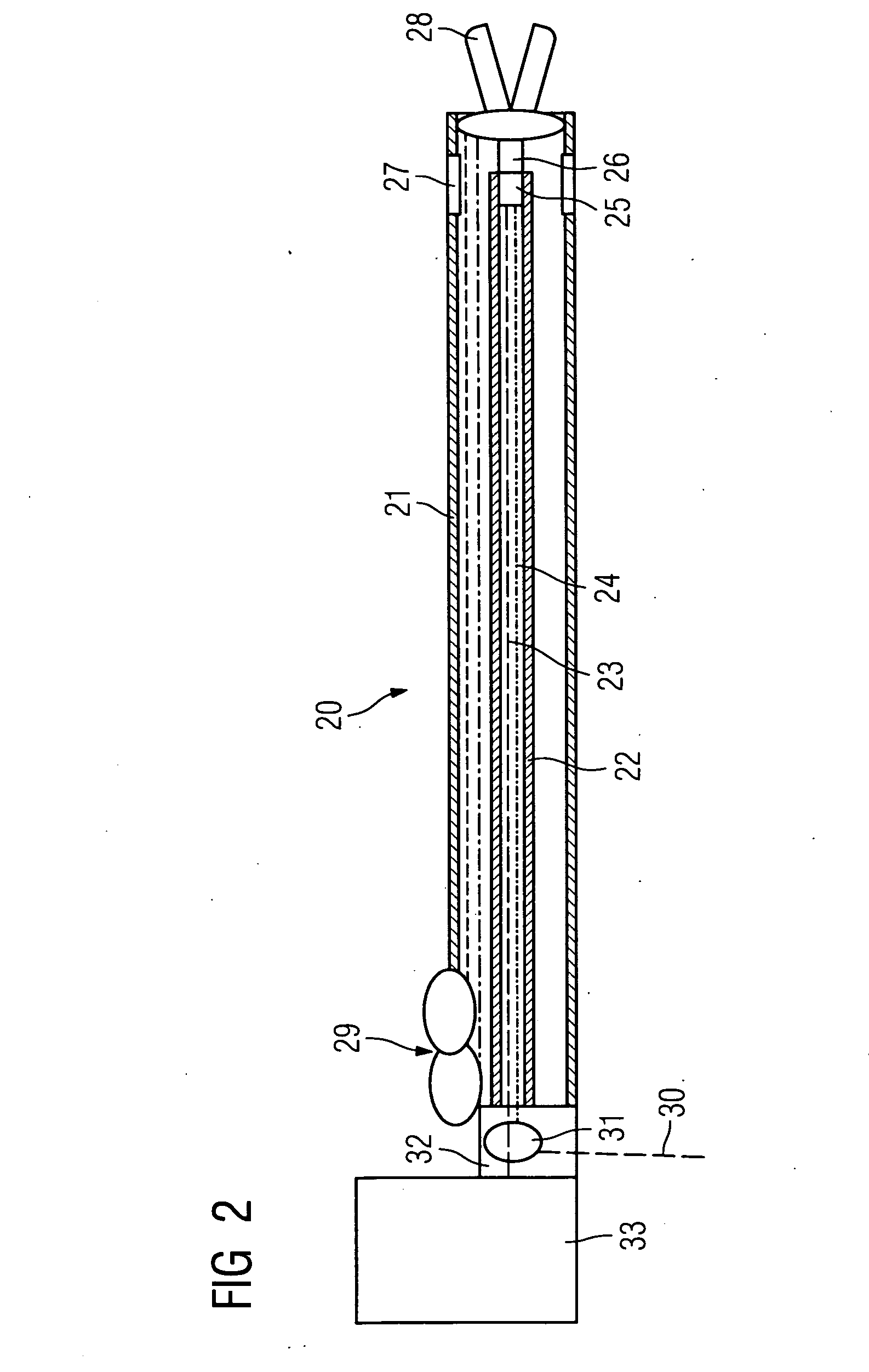
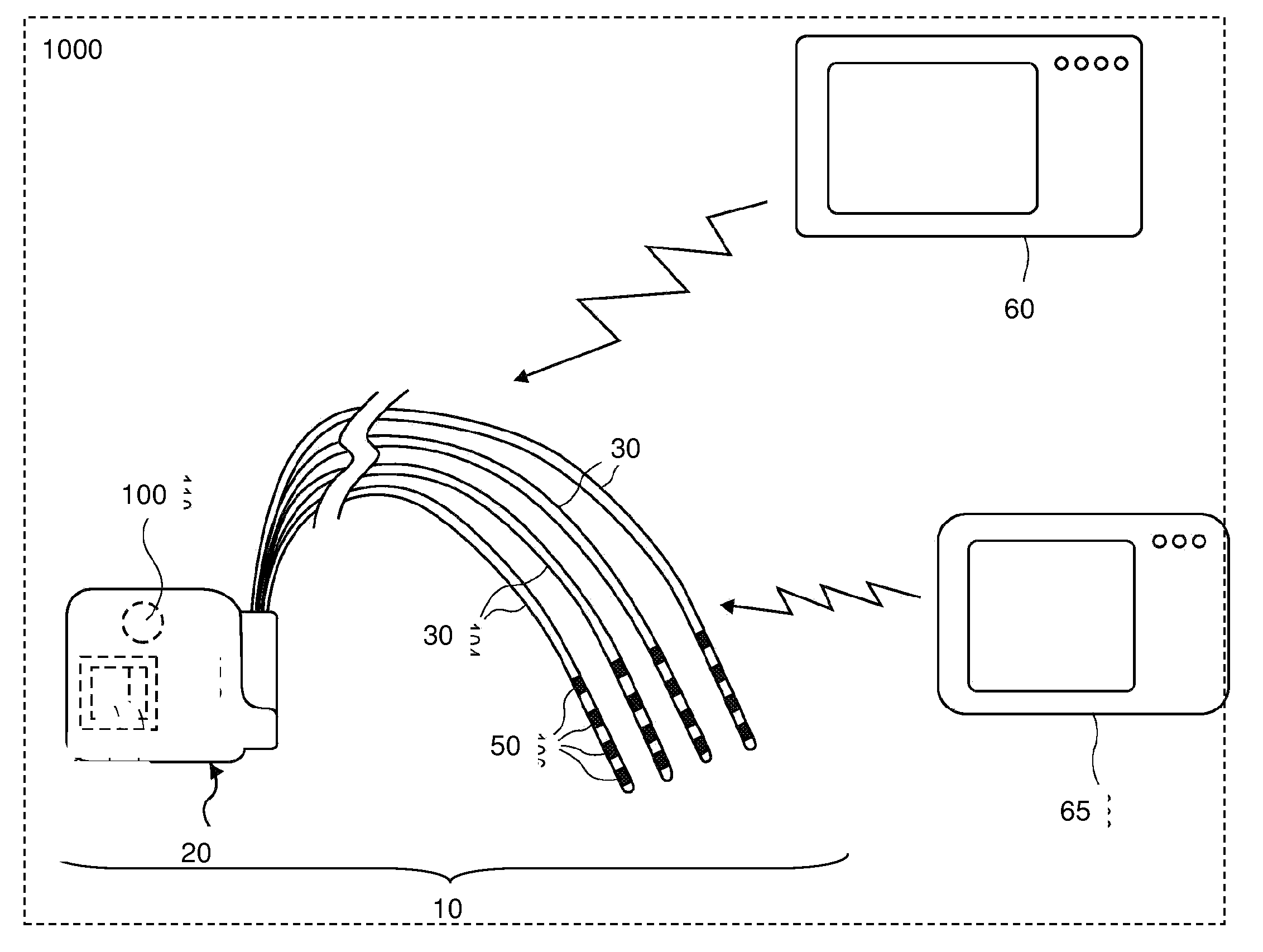
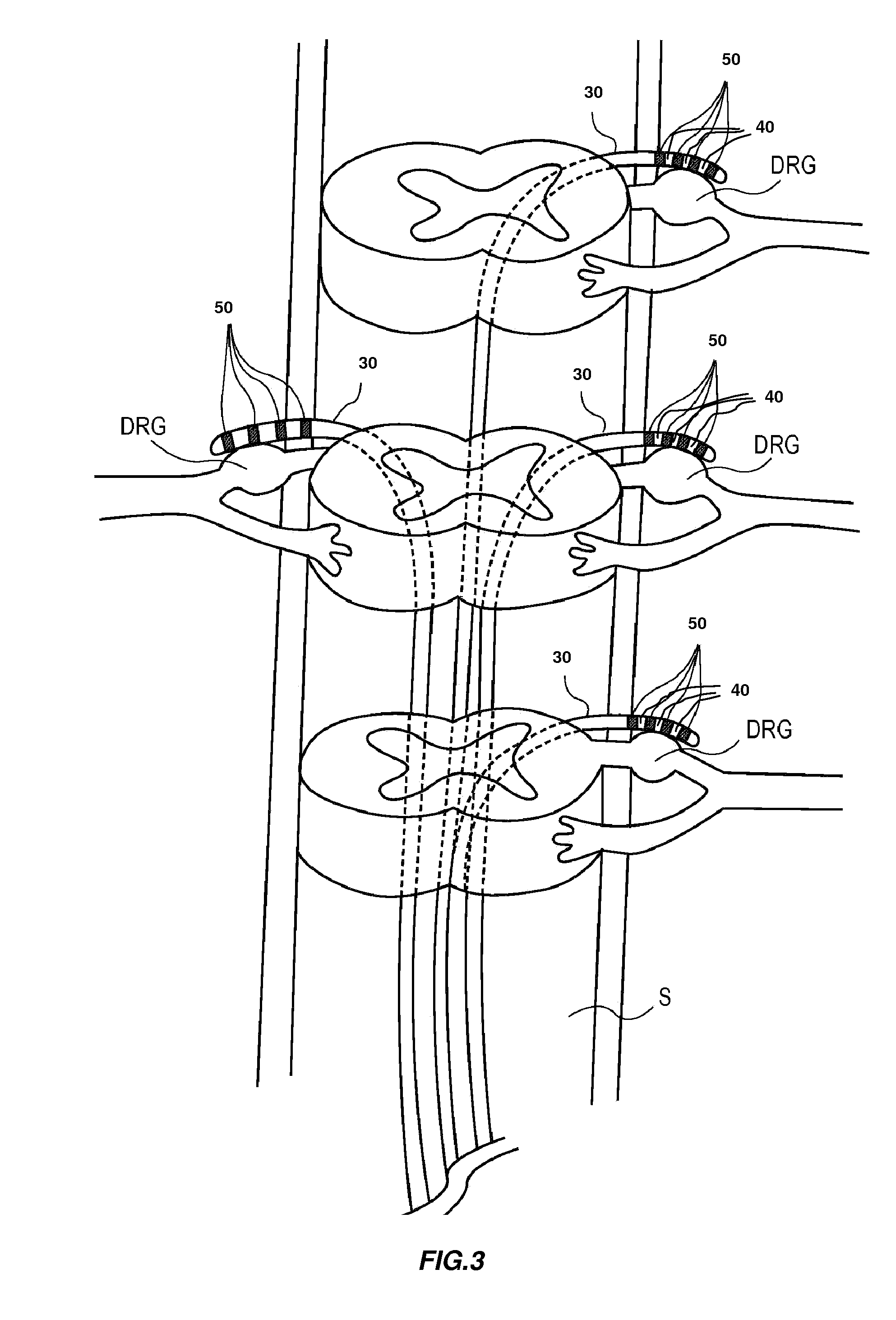
Directed delivery of agents to neural anatomy
InactiveUS20120310140A1Effective pain managementAdequate pain reliefSpinal electrodesPharmaceutical delivery mechanismDiseaseAutomatic control
The present invention is directed generally to systems, devices and methods for direct delivery of agents, e.g., pharmaceutical agents, to target spinal and neuronal anatomies, e.g., the dorsal root ganglia (DRG), for the treatment of various disorders, particularly pain and pain related disorders, such as chronic itch, sensory disorders, multiple sclerosis, post-herpetic neuralgia and the like. The system, devices and methods of the invention encompass the agents to be delivered to the target anatomy alone or in combination with electrical stimulation. The delivery device and systems and methods as disclosed herein place the distal end of the delivery element, which comprises at least one agent delivery structure, and optionally at least one electrode, in close proximity, or in contact with or next to the target spinal anatomy, e.g., DRG. A variety of agents can be delivered using the device, including sodium channel blockers, biologics, neuroinflammatory modulators, toxins etc., to selectively neuromodulate the neurons. Agent delivery and / or electrical stimulation can be automated and / or can be controlled automatically or by a pre-determined program, or by a patient control pump (PCA).
Owner:ST JUDE MEDICAL LUXEMBOURG HLDG SMI S A R L SJM LUX SMI
Method of simultaneously enhancing analgesic potency and attenuating dependence liability caused by exogenous and endogenous opioid agonists
InactiveUSRE36547E1Enhance analgesic potencyDecrease dependence liabilityCompound screeningBiocideEndogenous OpiatesNervous system
This invention relates to a method of selectively enhancing the analgesic potency of morphine and other clinically used bimodally-acting opioid agonists and simultaneously attenuating development of physical dependence, tolerance and other undesirable side effects caused by the chronic administration of said bimodally-acting opioid agonists comprising the co-administration of a bimodally-acting opioid agonist which activates both inhibitory and excitatory opioid receptor-mediated functions of neurons in the nociceptive (pain) pathways of the nervous system and an opioid receptor antagonist which selectively inactivates excitatory opioid receptor-mediated side effects. This invention also relates to a method of using excitatory opioid receptor antagonists alone to block the undesirable excitatory side effects of endogenous bimodally-acting opioid agonists which may be markedly elevated during chronic pain. This invention further relates to a method of long-term treatment of previously detoxified opiate, cocaine and alcohol addicts utilizing said excitatory opioid receptor antagonists, either alone or in combination with low-dose methadone, to prevent protracted physical dependence, and to compositions comprising an excitatory opioid receptor antagonist of the invention and a bimodally-acting opioid agonist.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Combination methods of treating cancer
InactiveUS20070190022A1Dosage of each agent in a combination therapy can be reducedAntitumor effectBiocidePeptide/protein ingredientsAnticarcinogenOncology
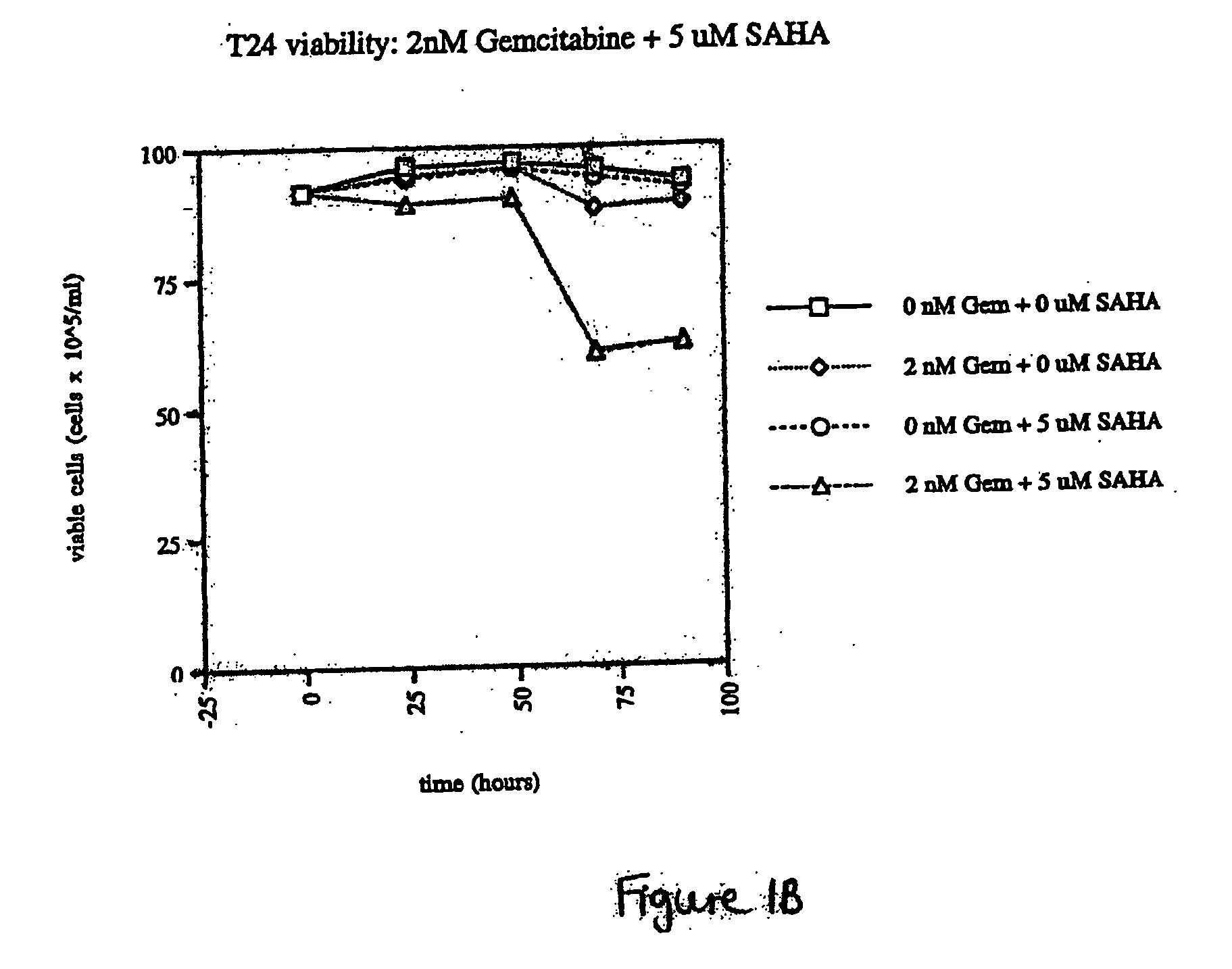
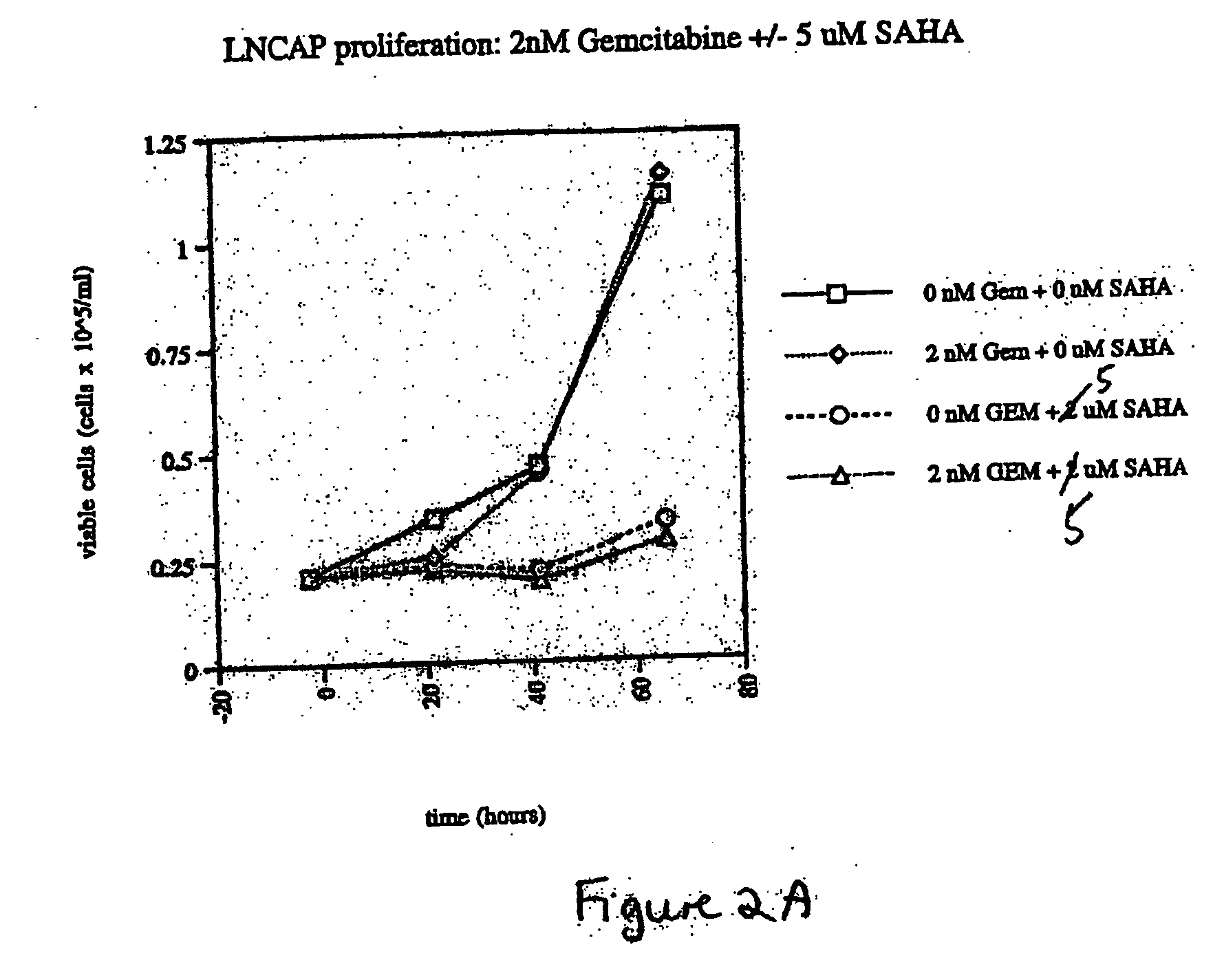
The present invention relates to a method of treating cancer in a subject in need thereof, by administering to a subject in need thereof a first amount of a histone deacetylase (HDAC) inhibitor or a pharmaceutically acceptable salt or hydrate thereof, in a first treatment procedure, and a second amount of an anti-cancer agent in a second treatment procedure. The first and second amounts together comprise a therapeutically effective amount. The effect of the HDAC inhibitor and the anti-cancer agent may be additive or synergistic.
Owner:SLOAN KETTERING INST FOR CANCER RES +1
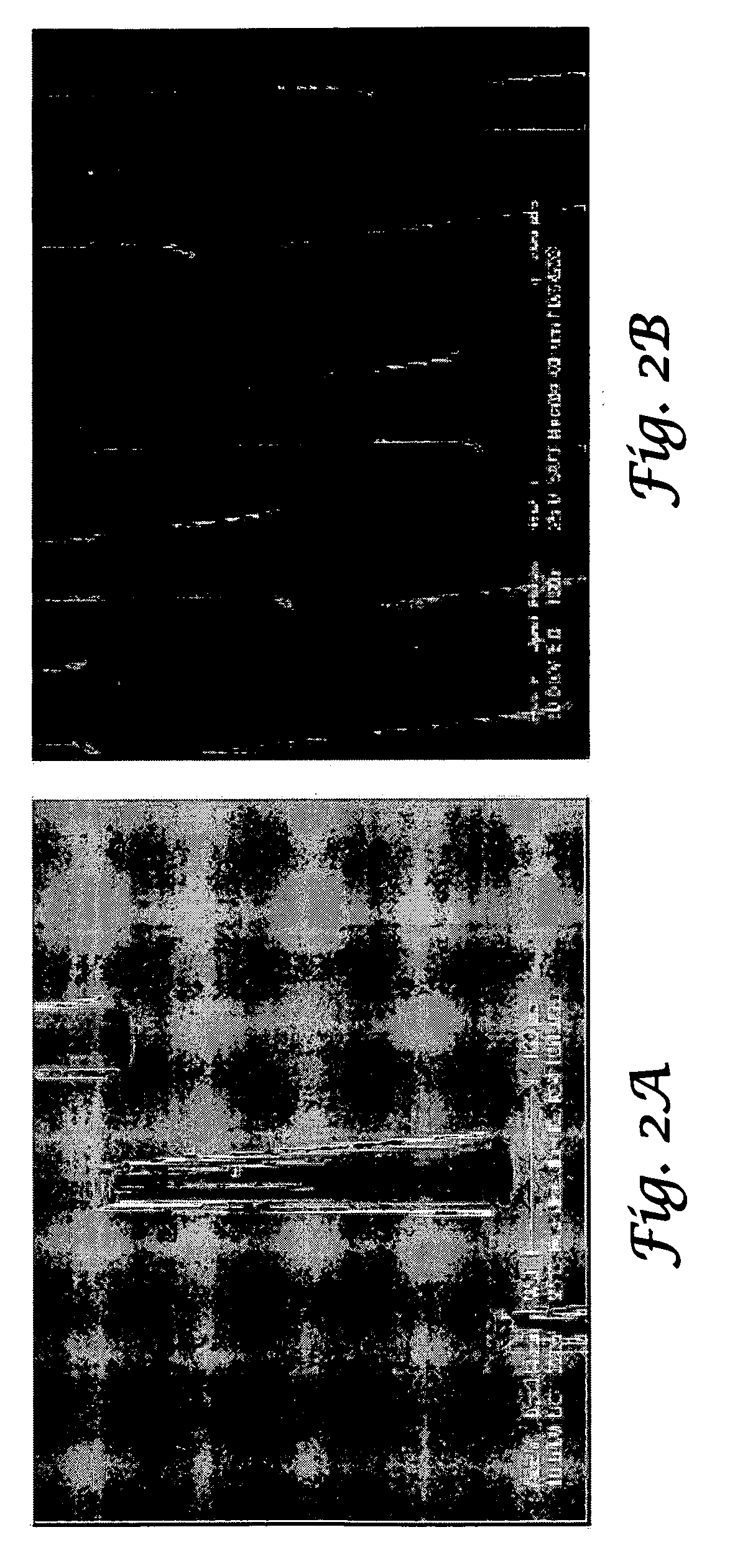
Tapered hollow metallic microneedle array assembly and method of making and using the same
InactiveUS7627938B2Minimal and no damage and painReduce riskLaminationPharmaceutical delivery mechanismBiomedical engineering
The present invention includes device, system, method of using and making a microneedle array including the steps of forming one or more pins on a substrate, depositing one or more layers on the one or more pins and the substrate, exposing a portion of the one or more pins, and separating the one or more pins from the one or more layers to form the hollow microneedle array.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
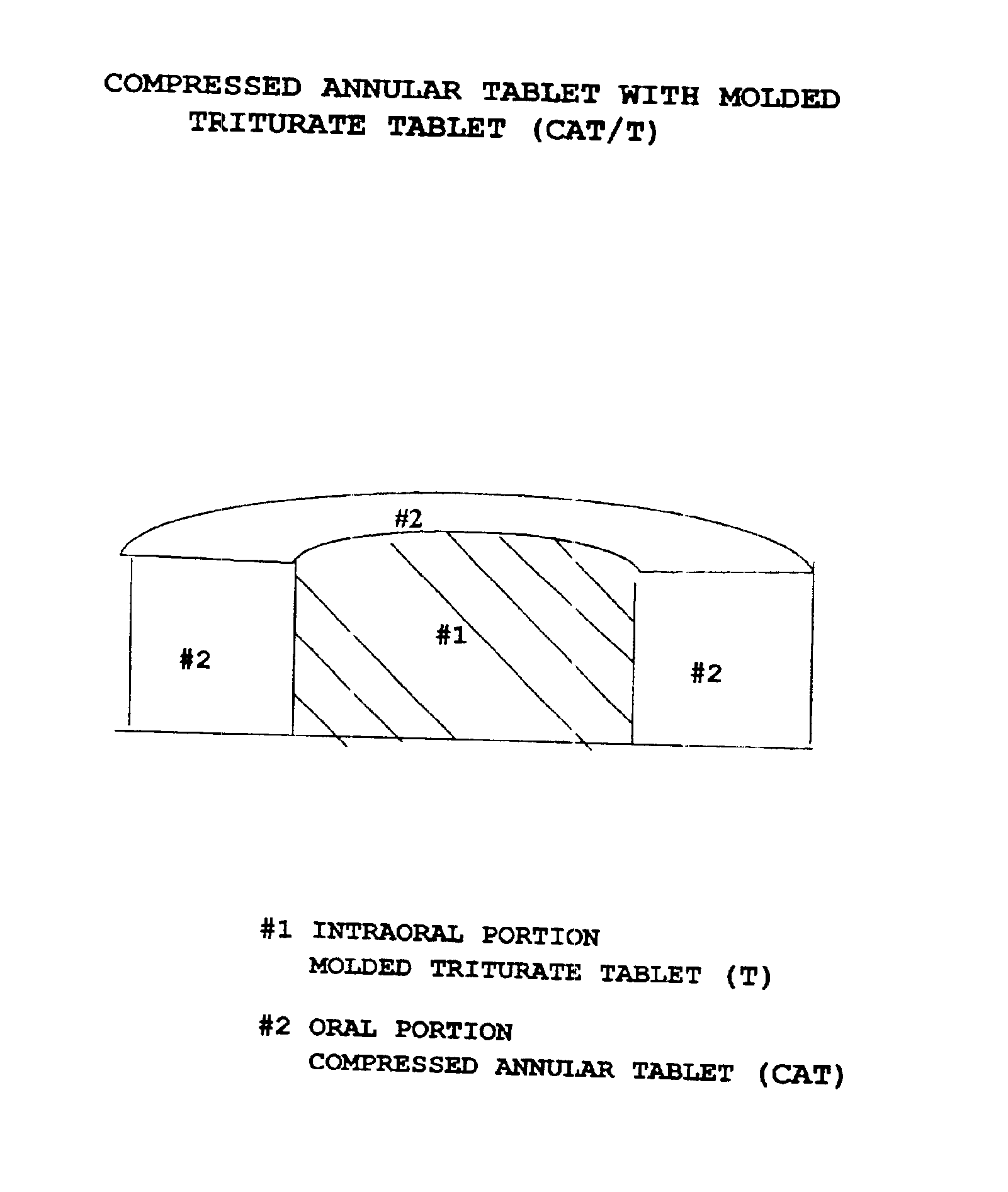
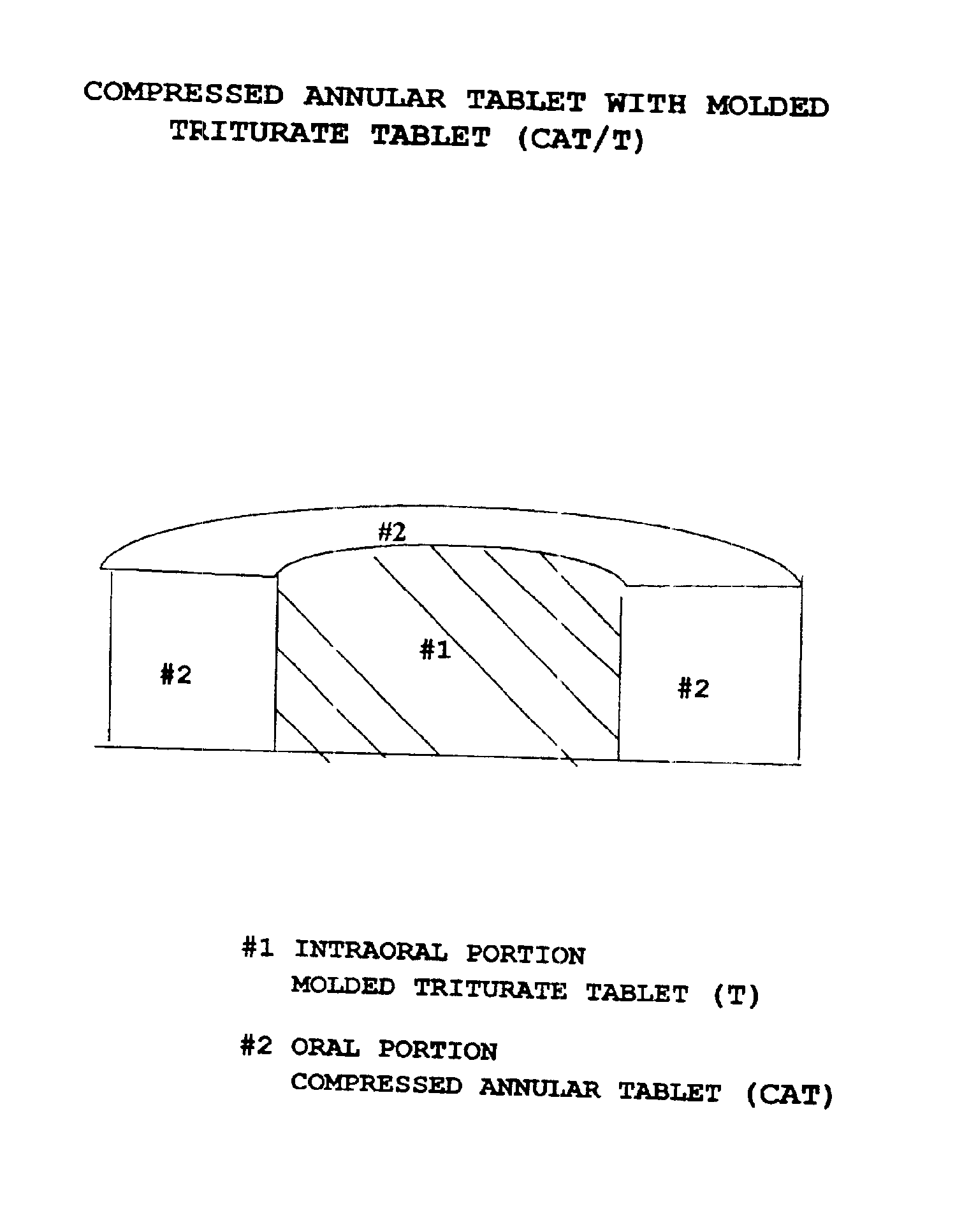
Pharmaceutical composition for compressed annular tablet with molded triturate tablet for both intraoral and oral administration
InactiveUS6863901B2Easily administrable to a patientMaximize the effect of treatmentNervous disorderSkeletal disorderOral medicationPharmaceutical drug
New pharmaceutical compositions in unit dosage form are disclosed for both intraoral and oral administration to a patient, said unit dosage form configured to be placed intraorally of said patient, which comprises:(a) as a first portion, at least one discrete molded triturate tablet comprising a therapeutically effective amount of at least one pharmaceutically active ingredient capable of intraoral administration; and(b) as a second portion located around the said first portion, a therapeutically effective amount of at least one pharmaceutically active ingredient capable of oral administration and which is releasable and orally ingestible by the patient after the molded triturate tablet has disintegrated or has dissolved intraorally.
Owner:HIRSH JANE +1
Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators
ActiveUS8216997B2Increased formationImprove the level ofOrganic active ingredientsPeptide/protein ingredientsPrimatePhysiology
Owner:ACCELERON PHARMA INC
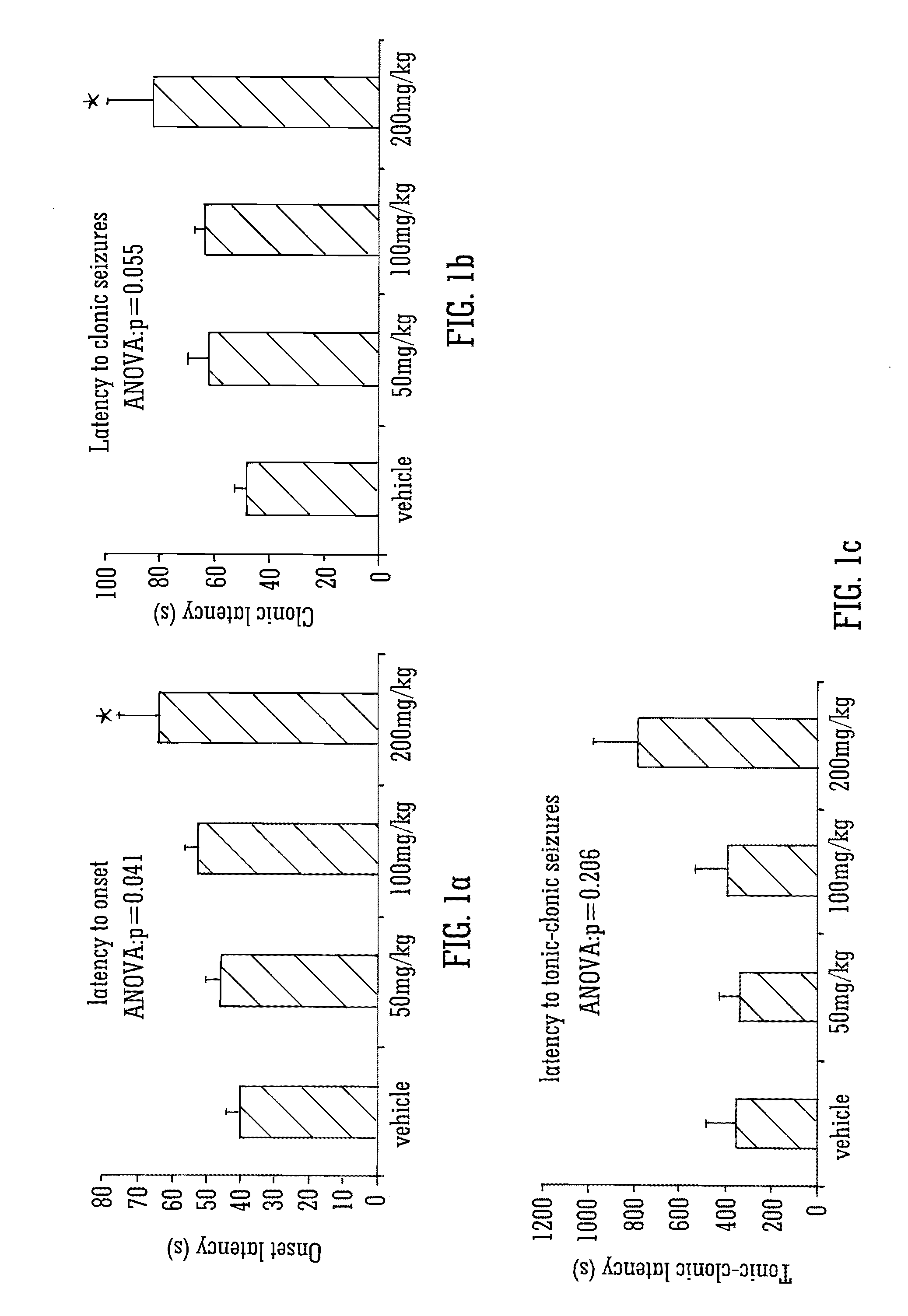
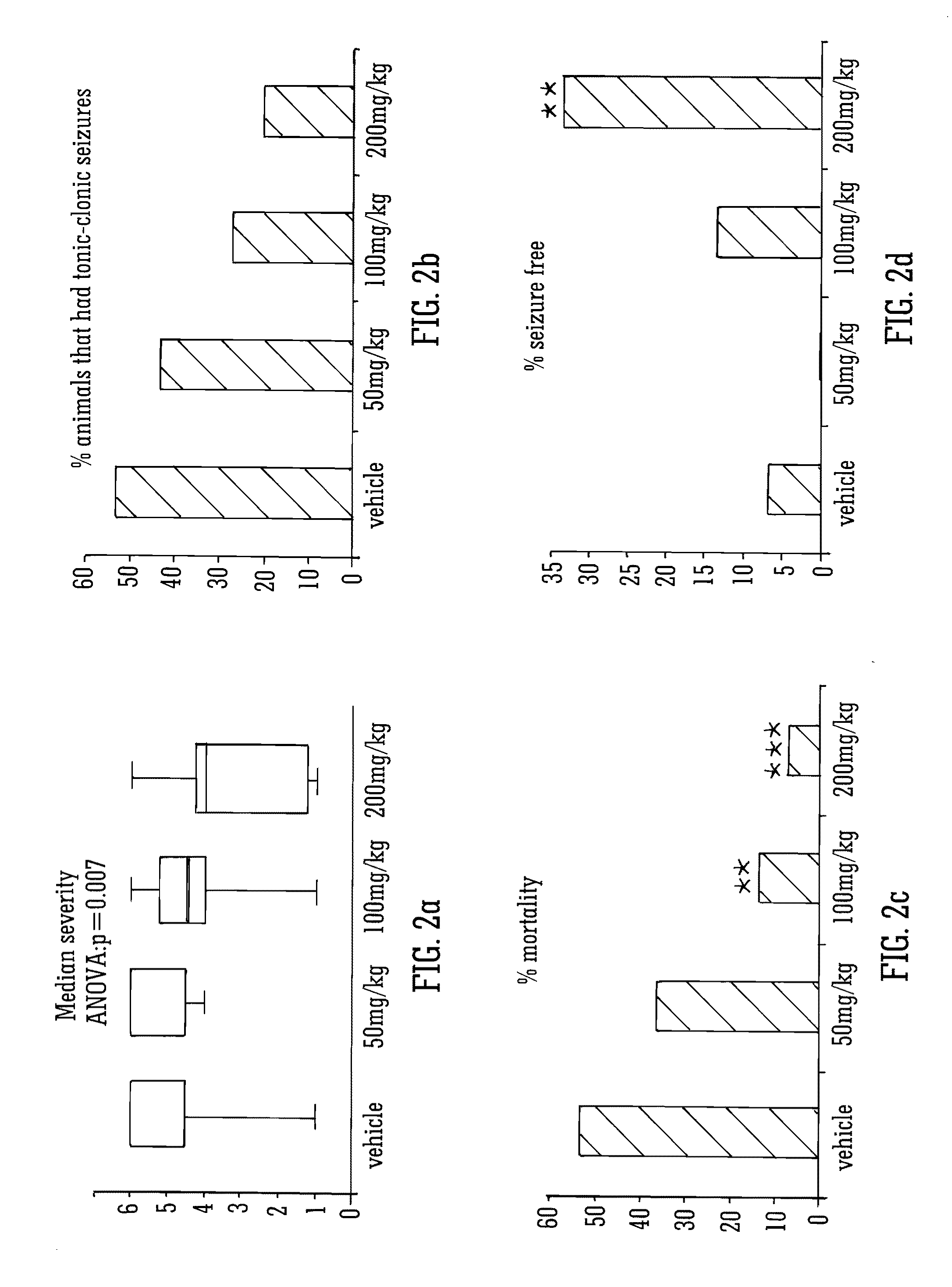
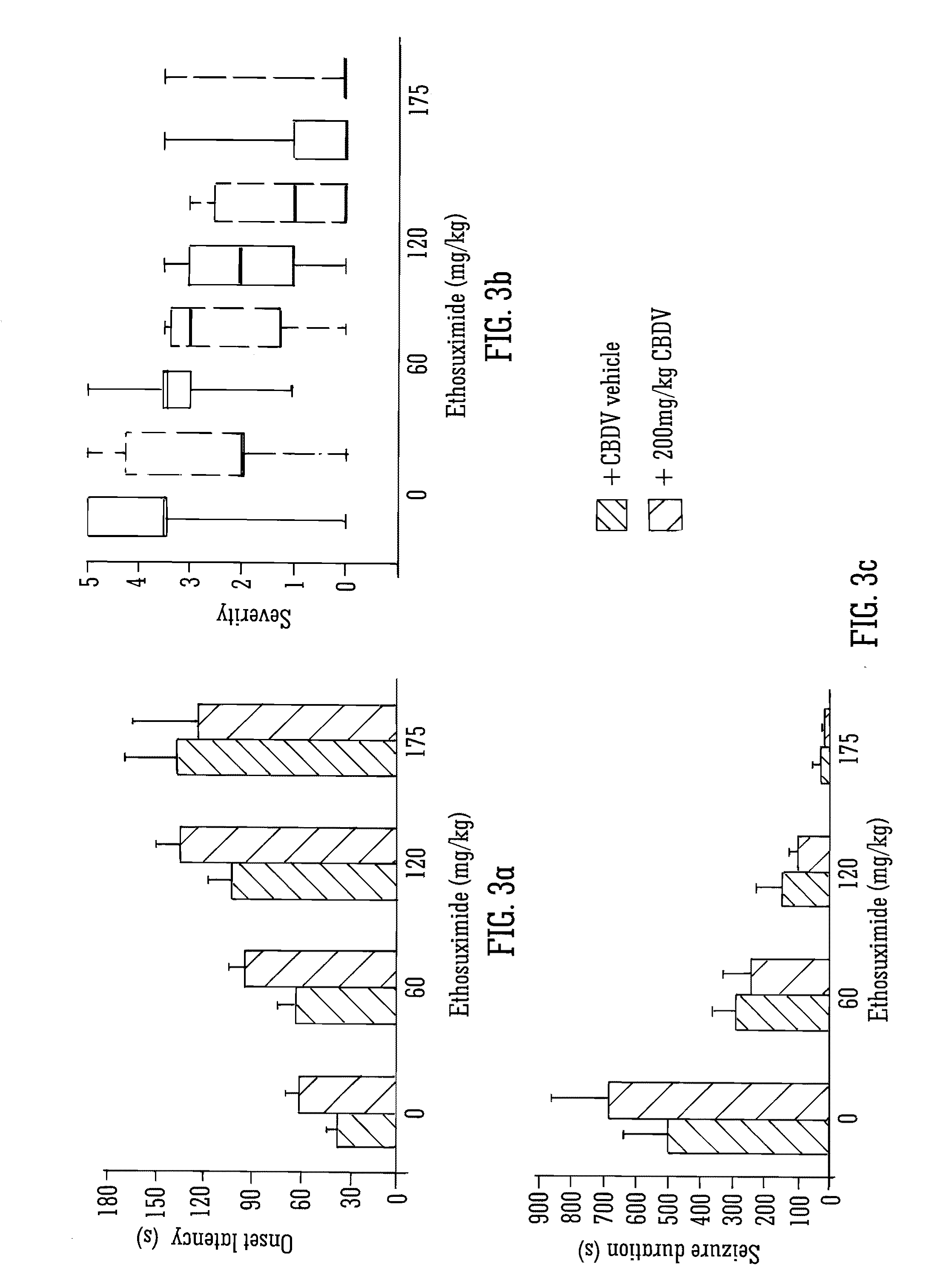
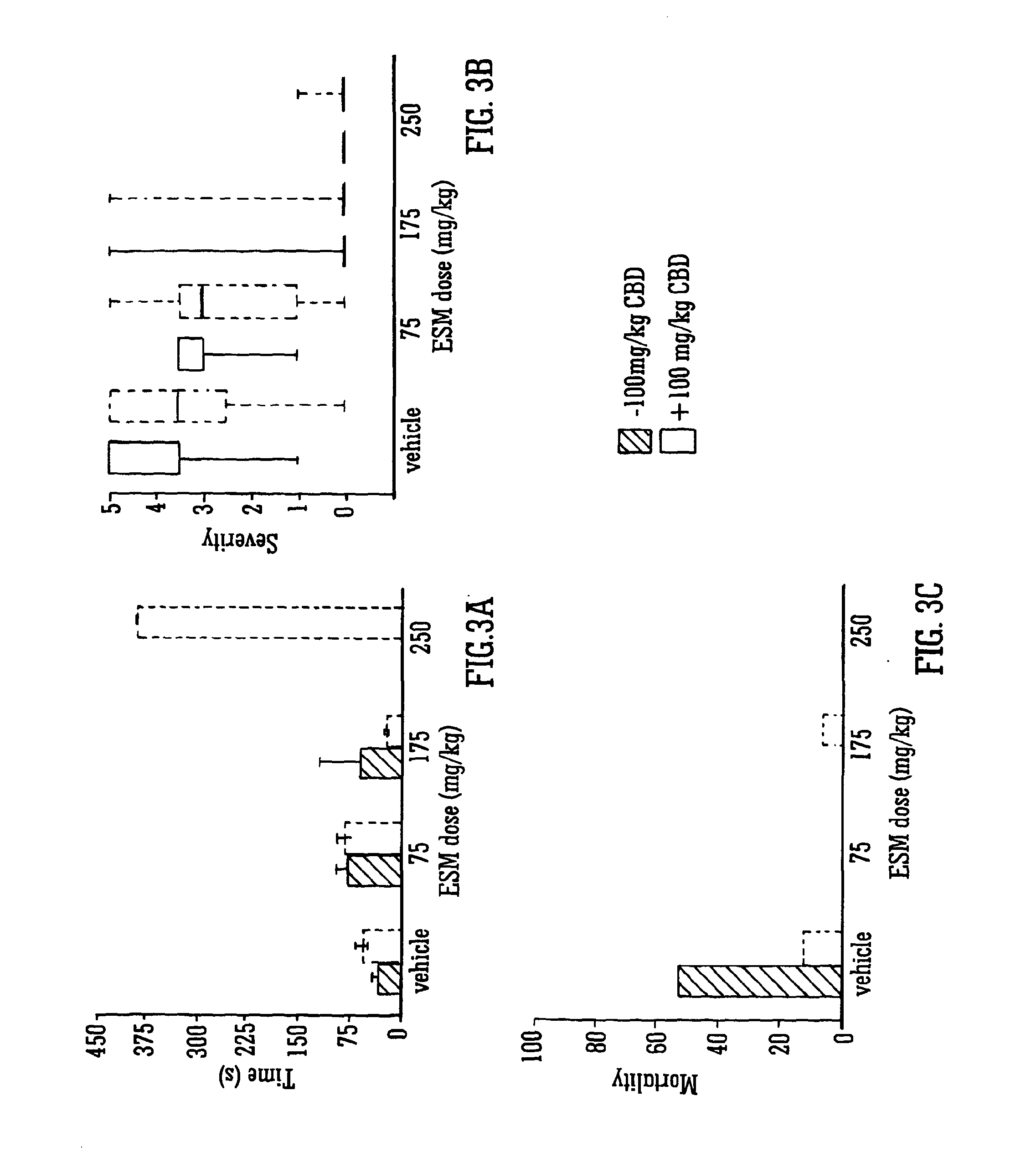
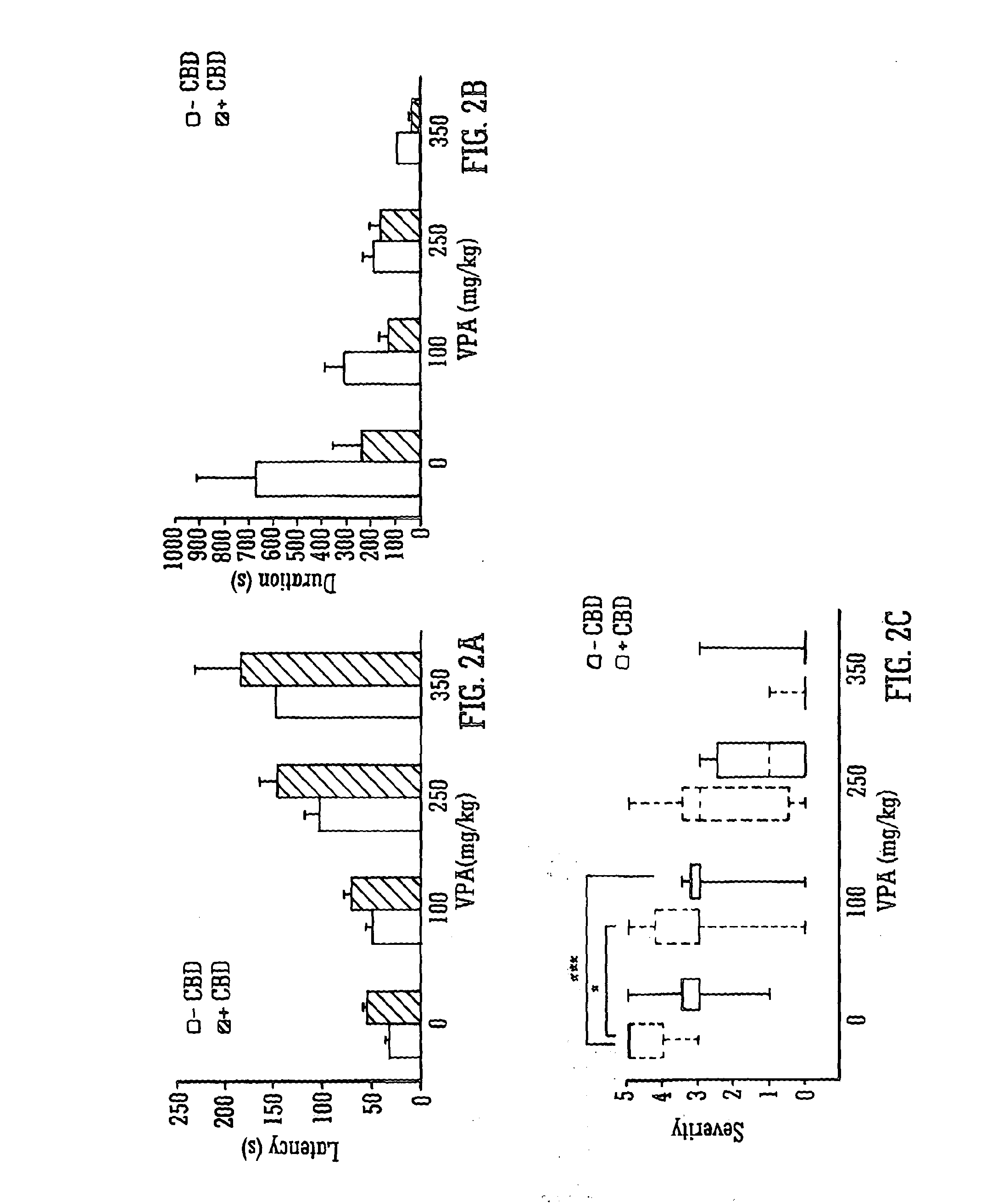
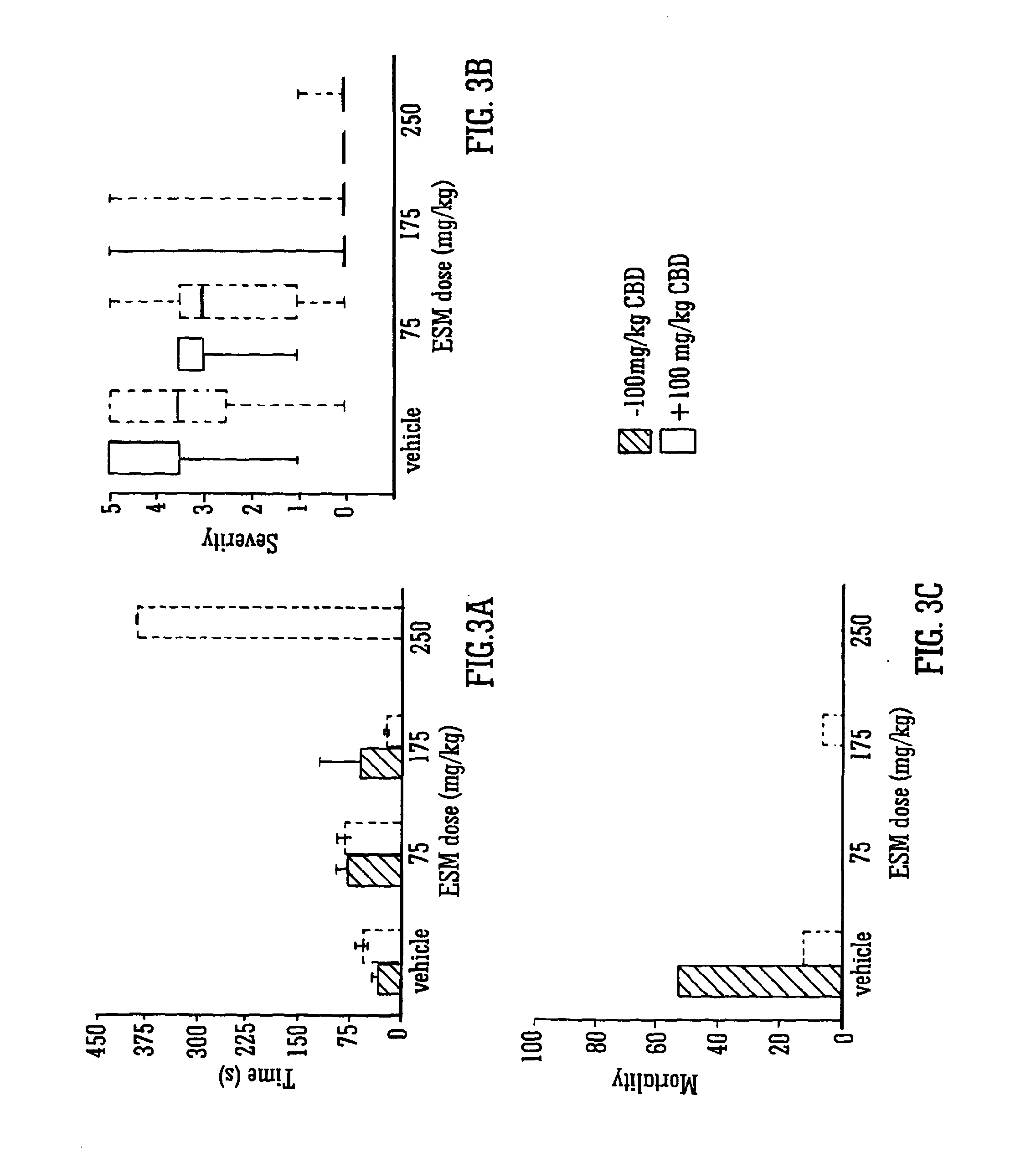
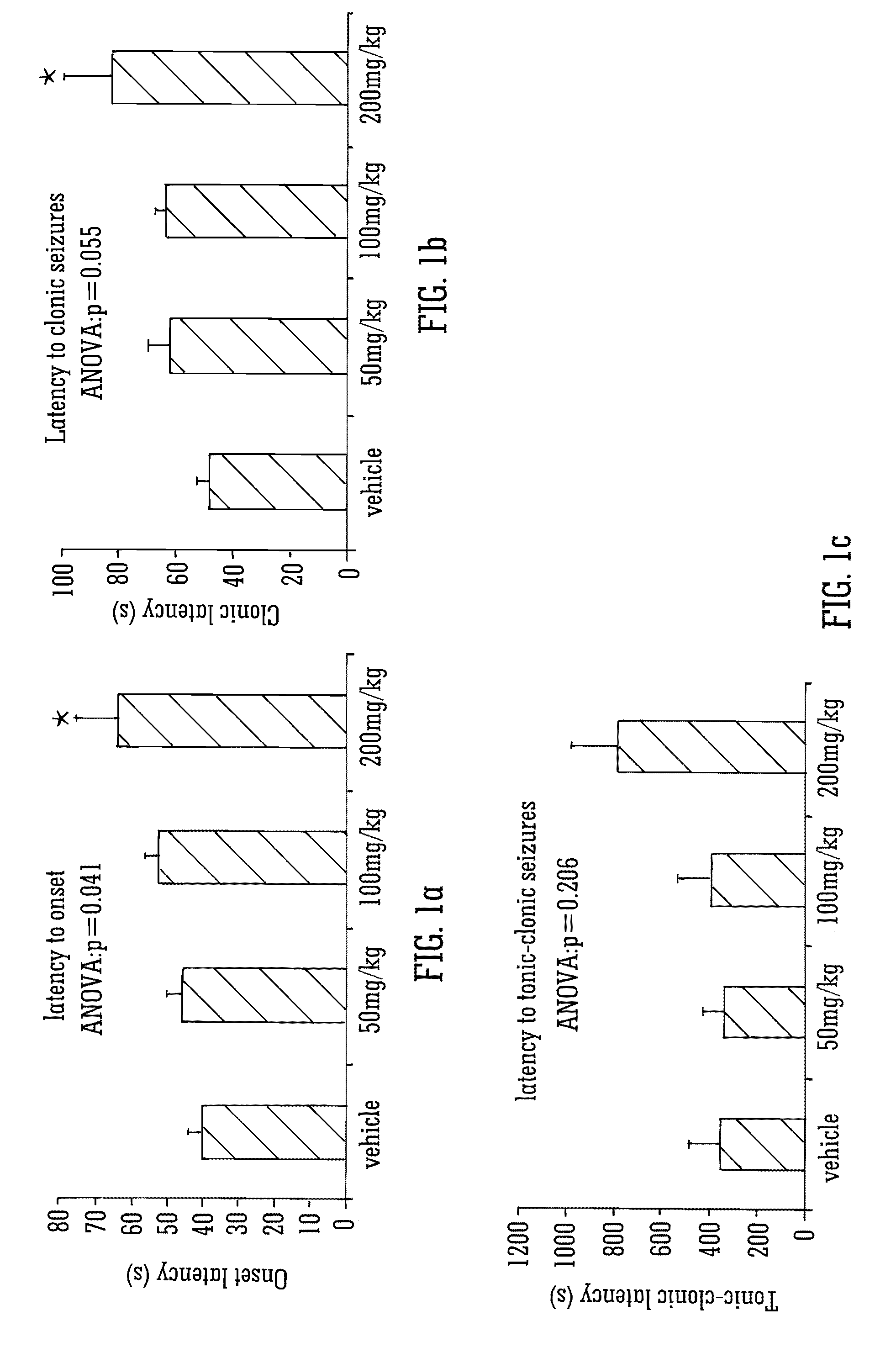
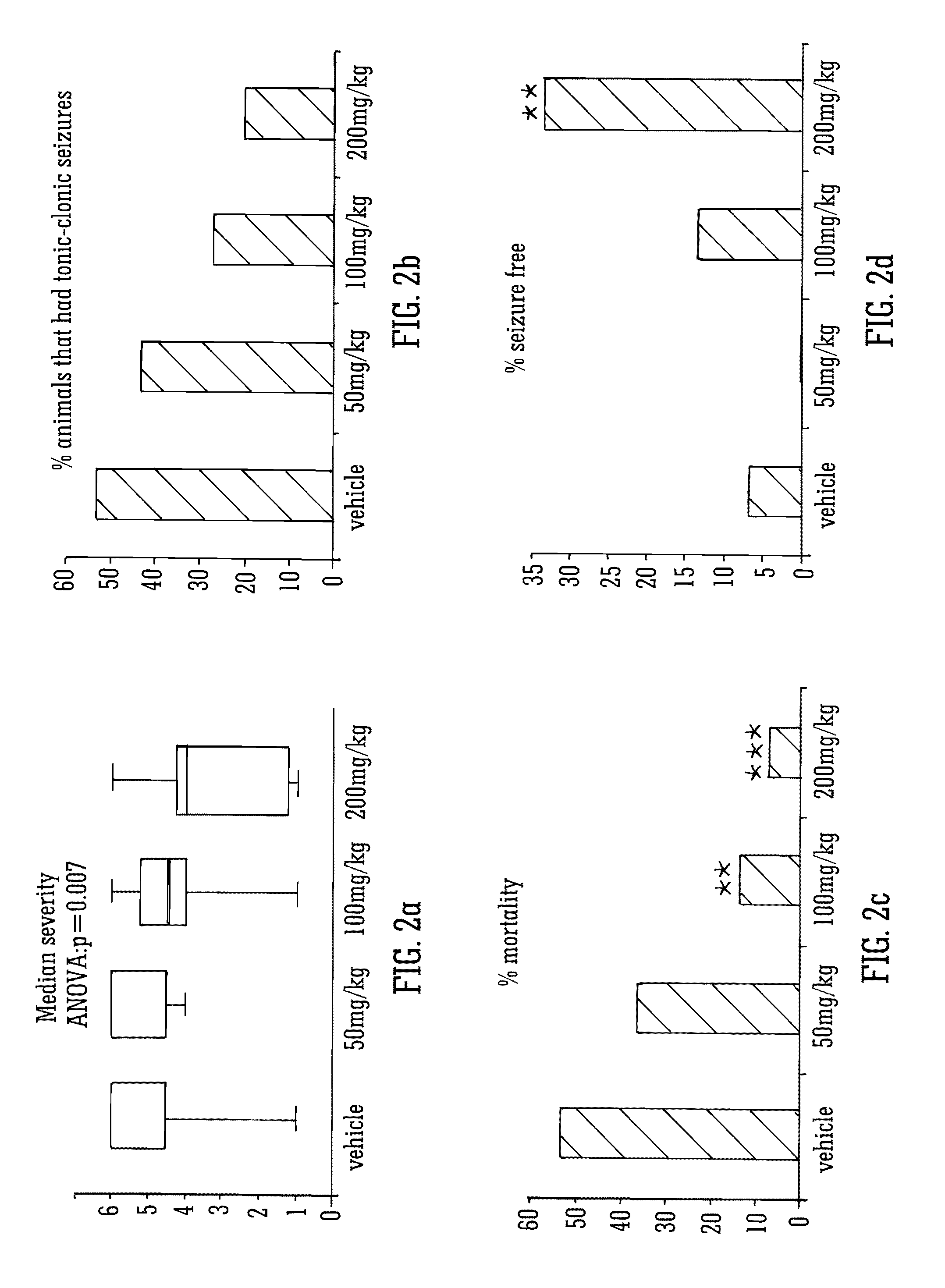
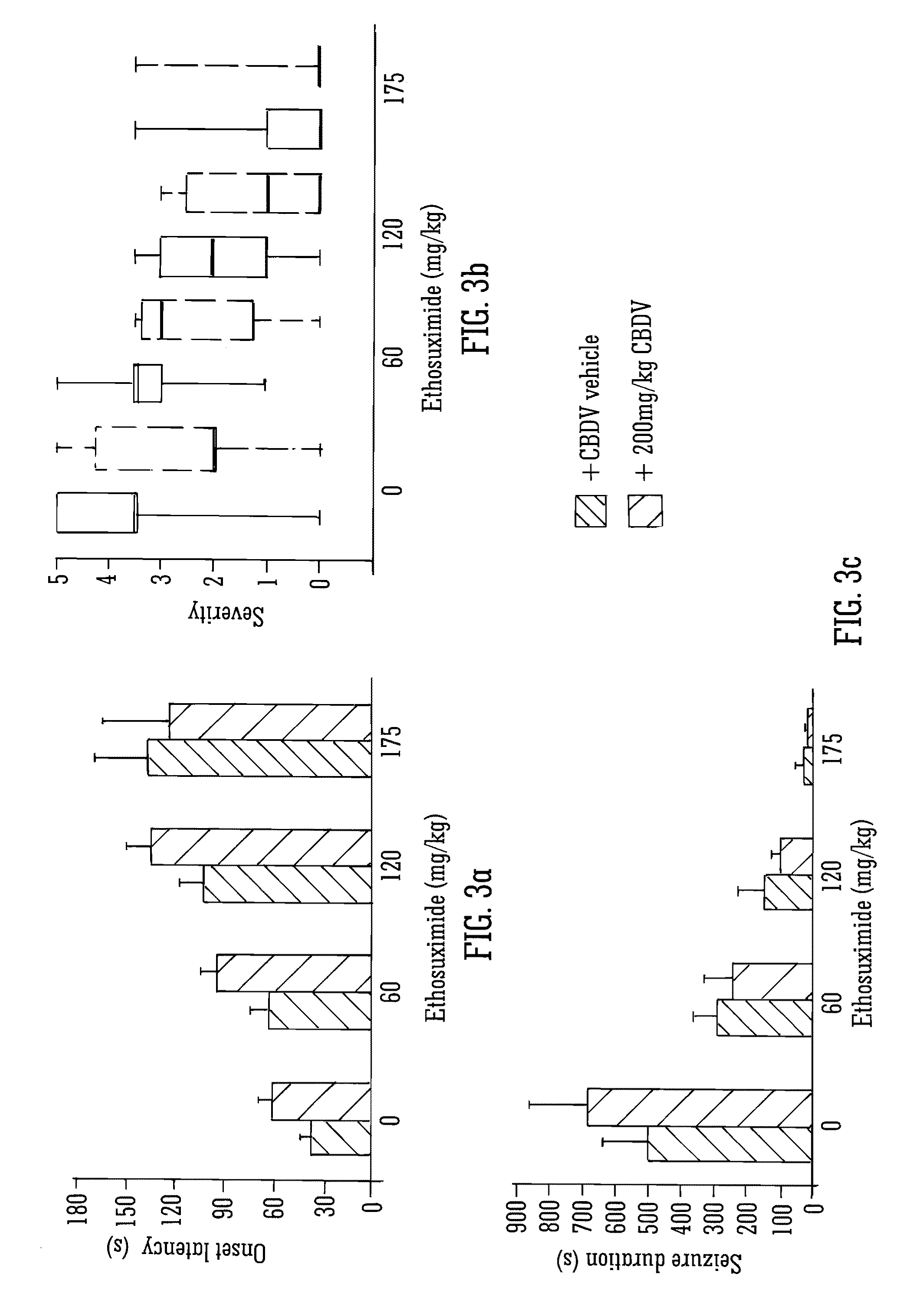
Use of the phytocannabinoid cannabidivarin (CBDV) in the treatment of epilepsy
ActiveUS20120004251A1Well side effect profileGrowth inhibitionBiocideNervous disorderPhenobarbitalDrug
This invention relates to the use of the phytocannabinoid cannabidivarin (CBDV) and combinations of the phytocannabinoid CBDV with tetrahydrocannabivarin (THCV) and cannabidiol (CBD) in the treatment of epilepsy. The invention further relates to the use of the phytocannabinoid CBDV in combination with standard anti-epileptic drugs (SAEDs). Preferably the SAED is one of ethosuximide, valproate or phenobarbital.
Owner:GW PHARMA LTD +1
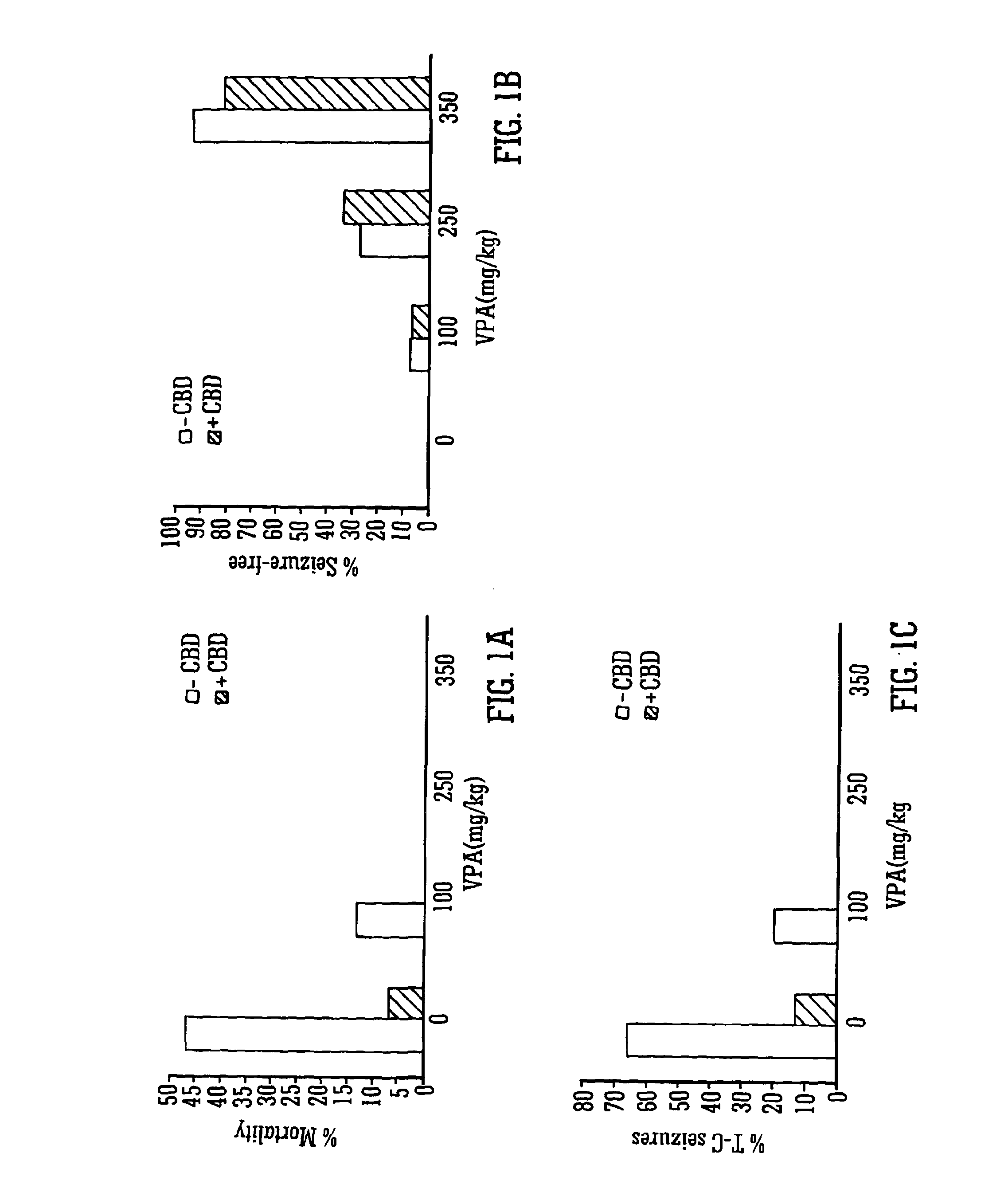
Use of the phytocannabinoid cannabidiol (CBD) in combination with a standard Anti-epileptic drug (SAED) in the treatment of epilepsy
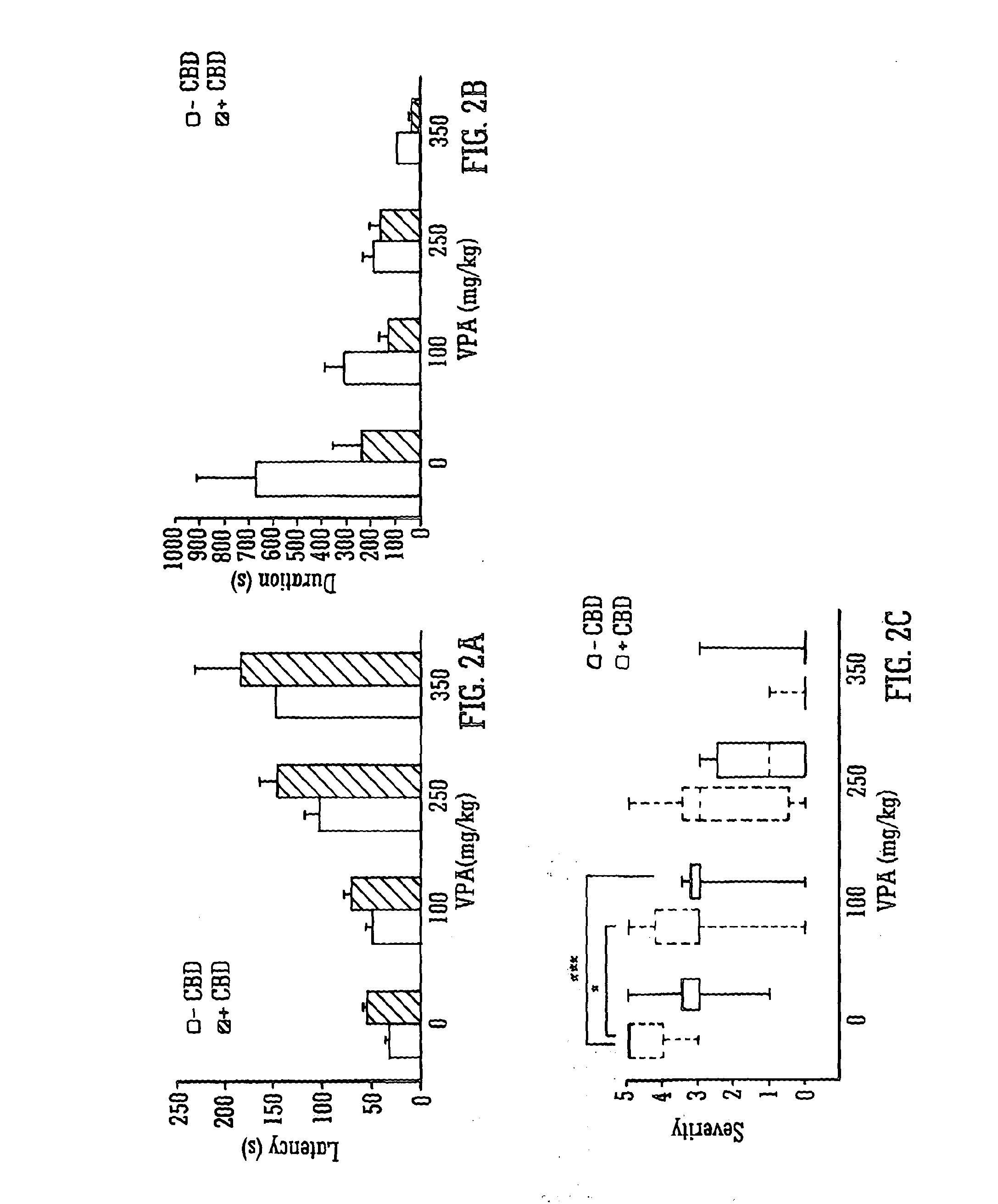
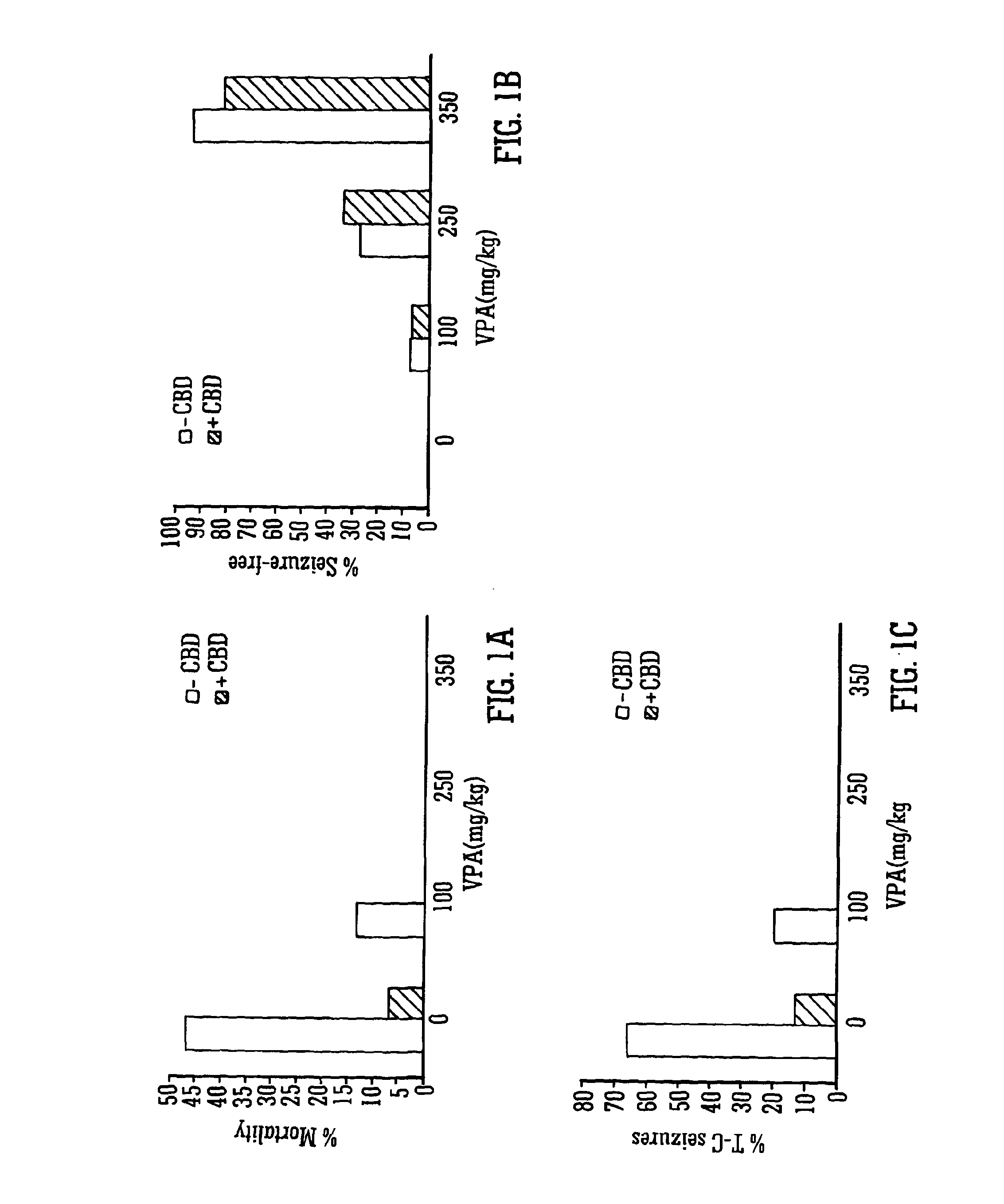
InactiveUS20130296398A1Reduce severityReduce mortalityBiocideNervous disorderValproic AcidCannabidiol
The invention relates to the use of cannabidiol (CBD), at a dose of greater than 300 mg / day, in combination with a standard anti-epileptic drug (SAED) which acts via sodium or calcium channels, for use in the treatment of epilepsy. The SAED is preferably one which•modifies low-threshold or transient neuronal calcium currents,or•reduces high-frequency neuronal firing and sodium-dependent action potentials and enhances GABA effects. Preferred SAEDs are ethosuximide and valproate.
Owner:GW PHARMA LTD +1
Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels
ActiveUS20110038831A1Increase formationHigh levelOrganic active ingredientsPeptide/protein ingredientsCellular levelRodent
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Use of the phytocannabinoid cannabidiol (CBD) in combination with a standard Anti-epileptic drug (SAED) in the treatment of epilepsy
InactiveUS20140155456A9Reduces high-frequency neuronal firingGood effectBiocideNervous disorderValproic AcidCannabidiol
The invention relates to the use of cannabidiol (CBD), at a dose of greater than 300 mg / day, in combination with a standard anti-epileptic drug (SAED) which acts via sodium or calcium channels, for use in the treatment of epilepsy. The SAED is preferably one which•modifies low-threshold or transient neuronal calcium currents,or•reduces high-frequency neuronal firing and sodium-dependent action potentials and enhances GABA effects. Preferred SAEDs are ethosuximide and valproate.
Owner:GW PHARMA LTD +1
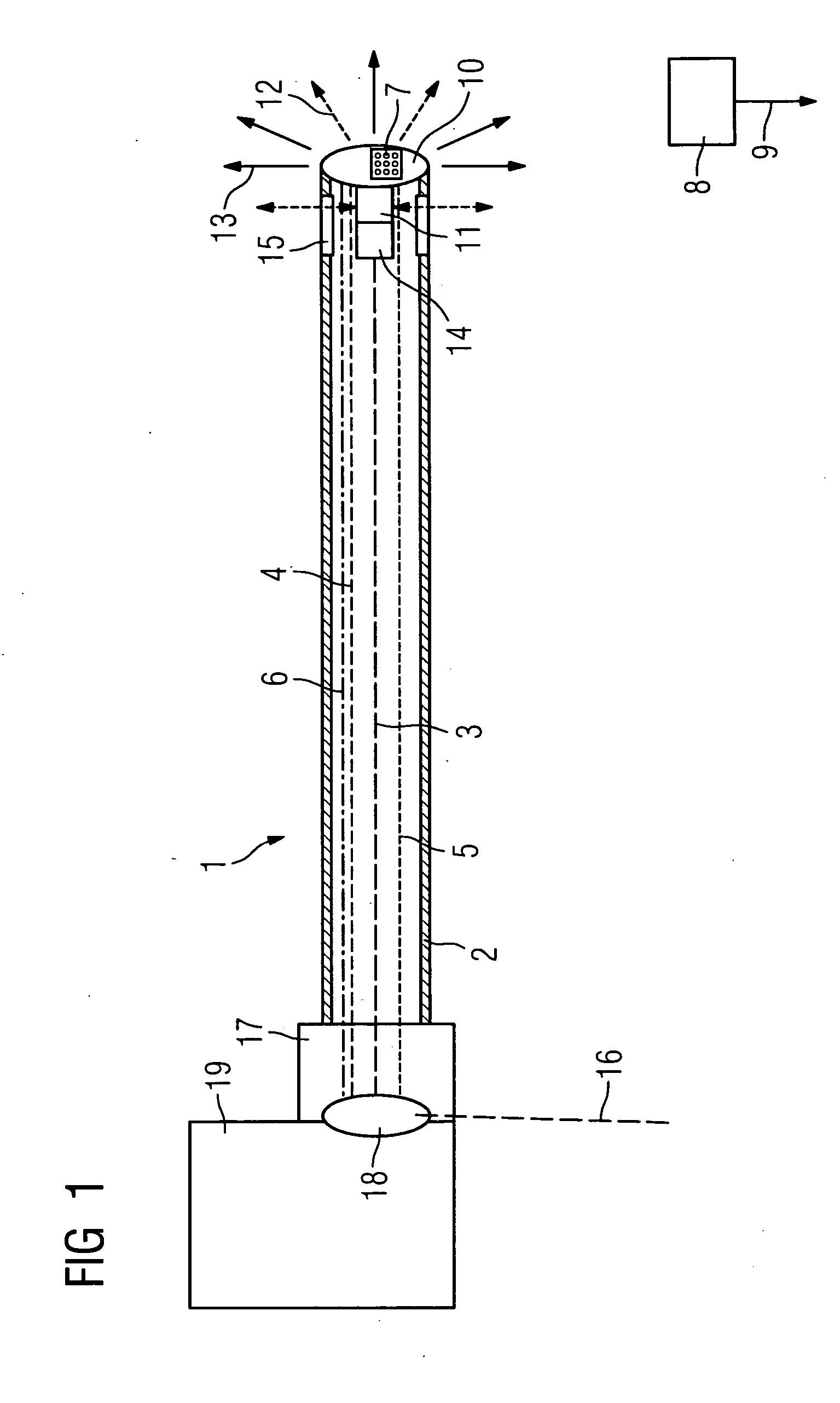
Catheter device with a position sensor system for treating a vessel blockage using image monitoring
InactiveUS20090149739A9High resolutionEnhance the imageUltrasonic/sonic/infrasonic diagnosticsSurgical navigation systemsPosition sensorSensor system
The invention relates to a catheter device, with a position sensor system, for treatment of a partial or complete vessel blockage under image monitoring, with the catheter device featuring a treatment catheter of a vessel blockage, especially by removal or destruction of plaque and / or expansion of the vessel,, which is embodied as an integrated unit, especially as a combination catheter, with an OCT catheter and an IVUS catheter for image monitoring and with the position sensor system.
Owner:SIEMENS HEALTHCARE GMBH
Use of the phytocannabinoid cannabidivarin (CBDV) in the treatment of epilepsy
ActiveUS9125859B2Growth inhibitionReduces high-frequency neuronal firingBiocideNervous disorderValproic AcidPhenobarbital
This invention relates to the use of the phytocannabinoid cannabidivarin (CBDV) and combinations of the phytocannabinoid CBDV with tetrahydrocannabivarin (THCV) and cannabidiol (CBD) in the treatment of epilepsy. The invention further relates to the use of the phytocannabinoid CBDV in combination with standard anti-epileptic drugs (SAEDs). Preferably the SAED is one of ethosuximide, valproate or phenobarbital.
Owner:GW PHARMA LTD
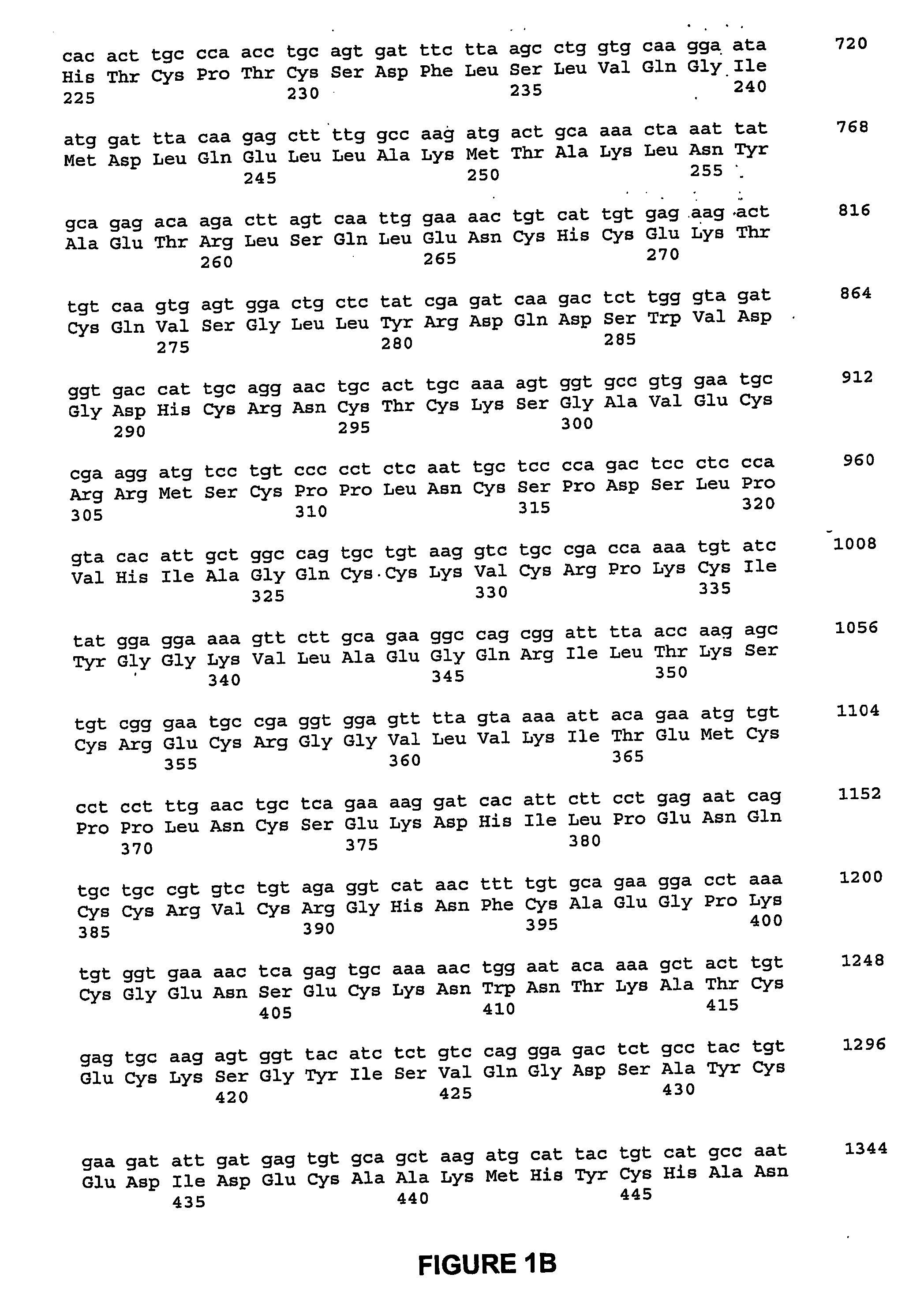
Albumin binding molecules and uses thereof
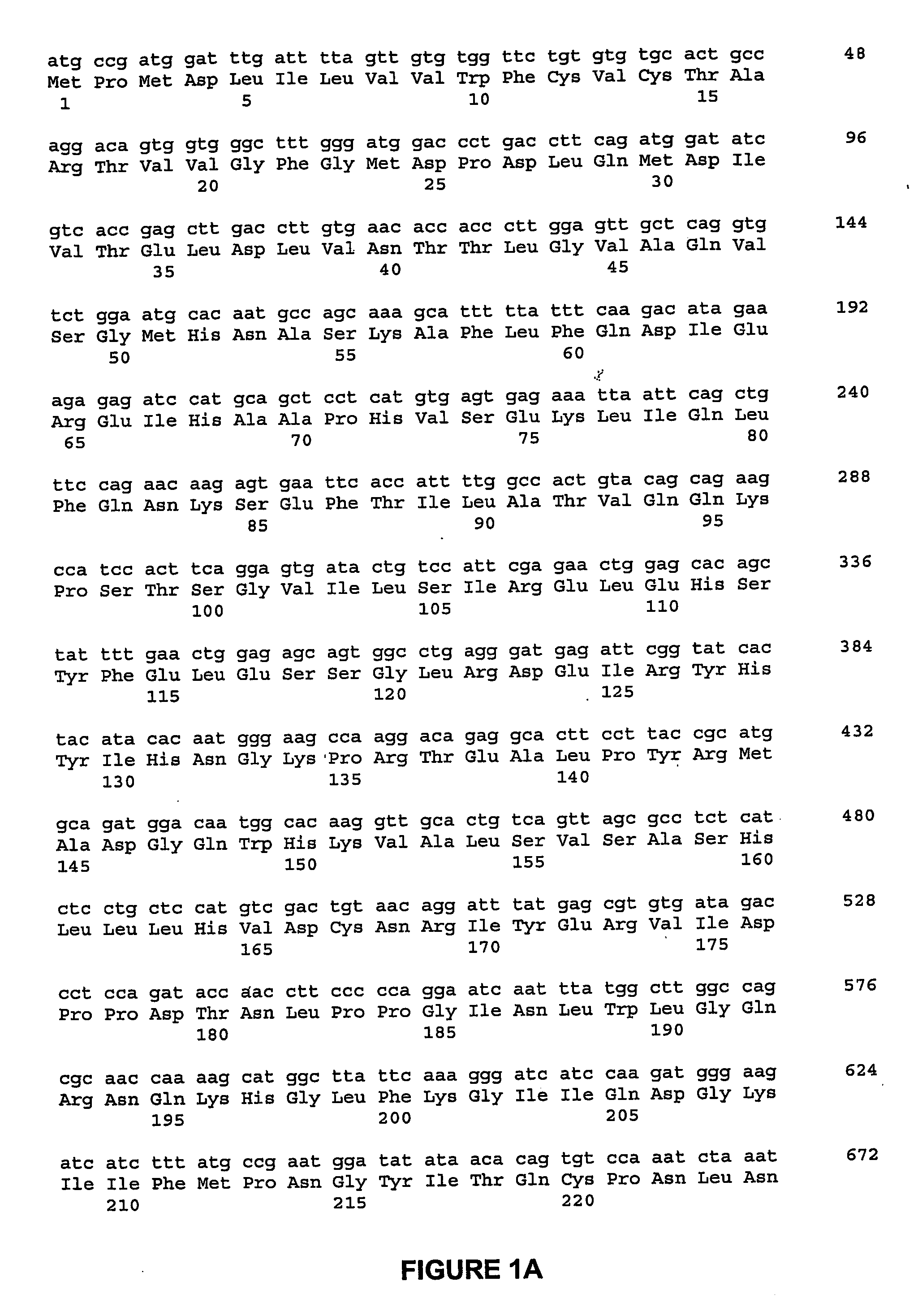
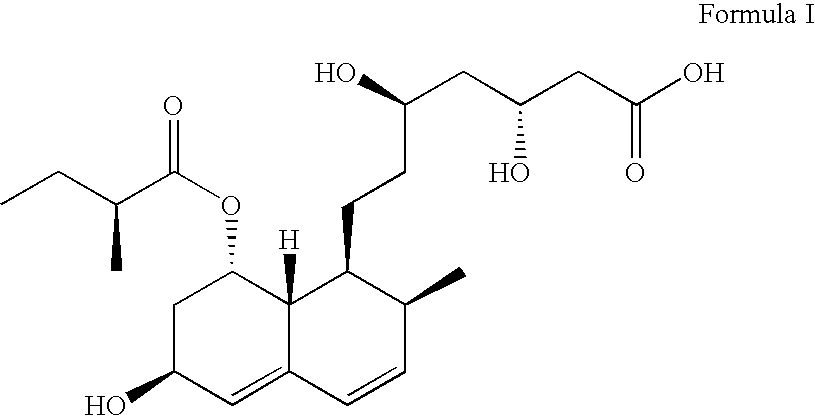
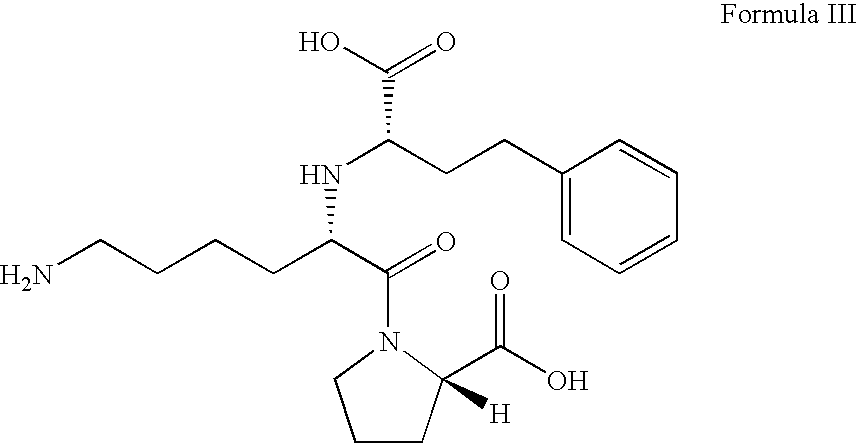
ActiveUS8344177B2Lessen risk of and severityReduced dosBiocidePeptide/protein ingredientsMedicineBlood Circulation Time
The invention relates to portable albumin binders, which are useful for improving the pharmacokinetic properties of diagnostic or therapeutic agents, in particular increasing the blood circulations time and / or the tissue penetration capacity of such agents.
Owner:PHILOCHEM AG
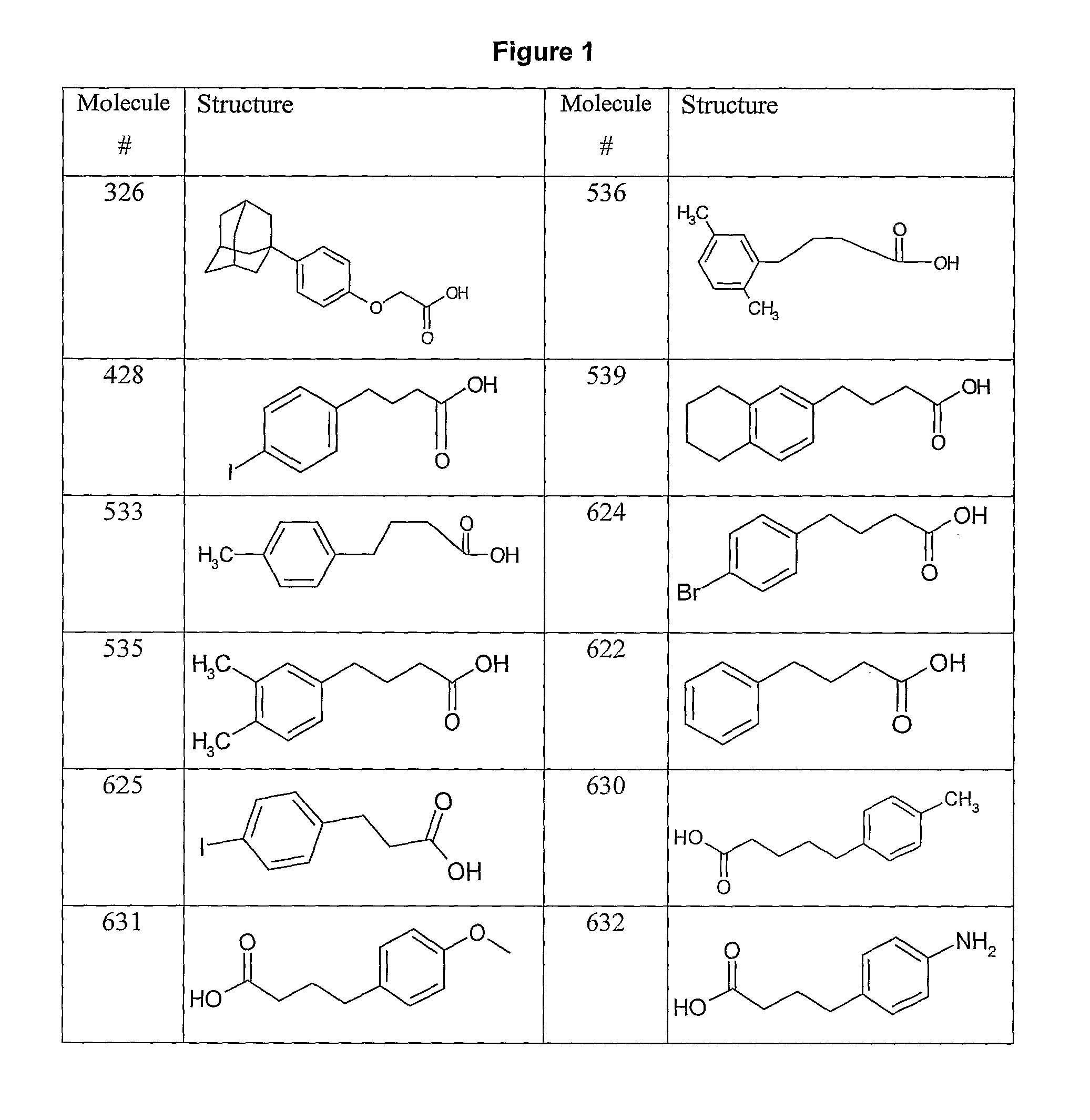
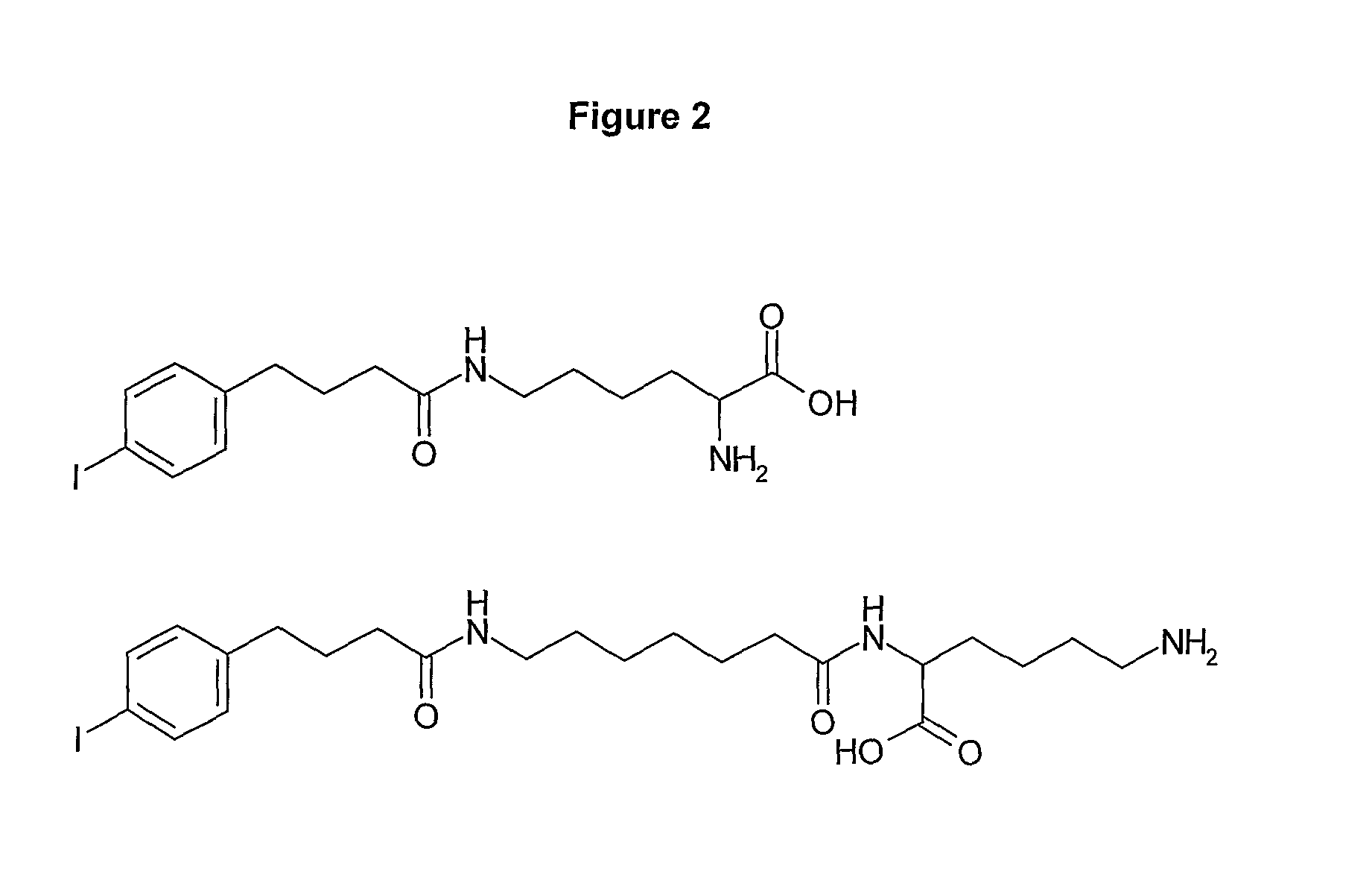
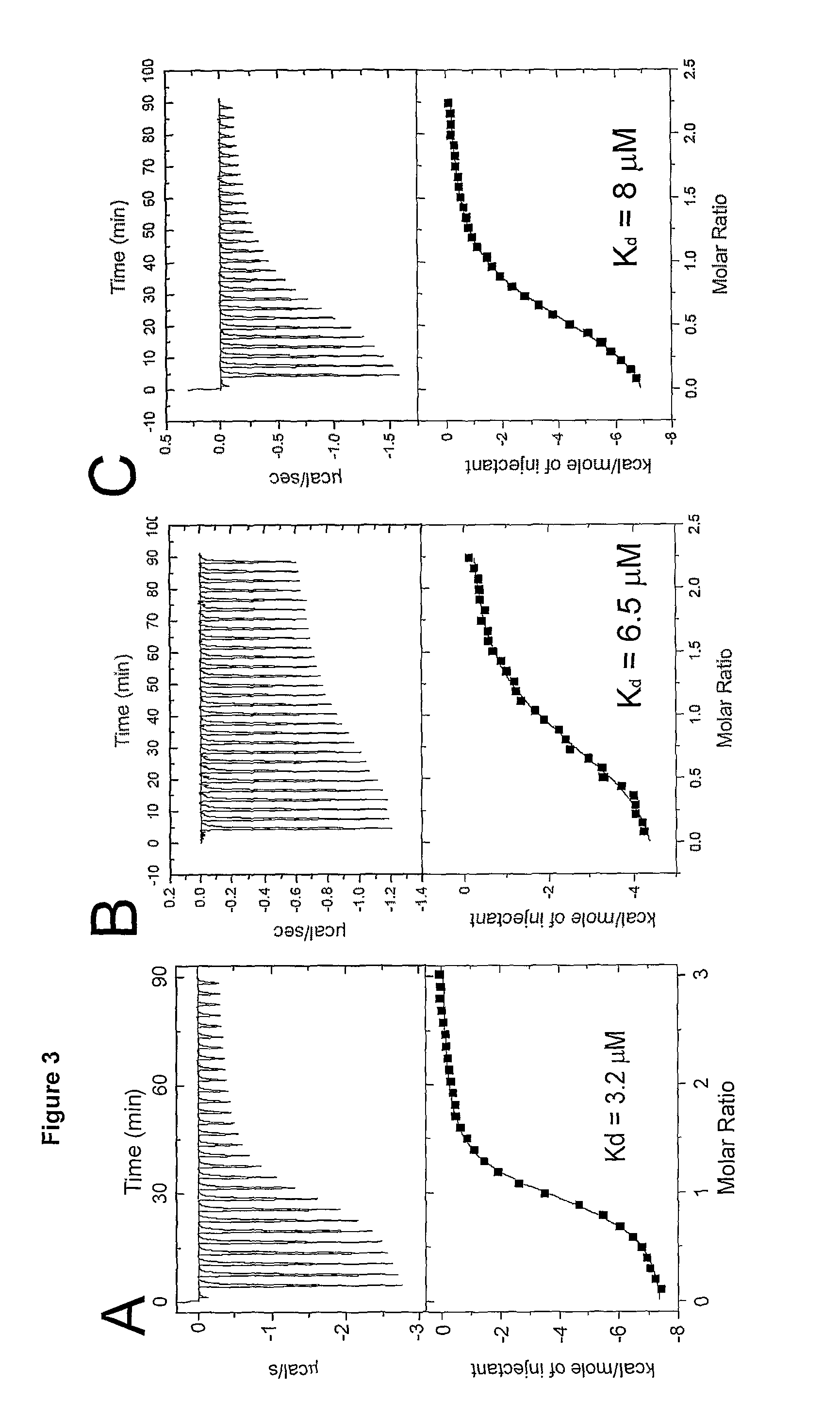
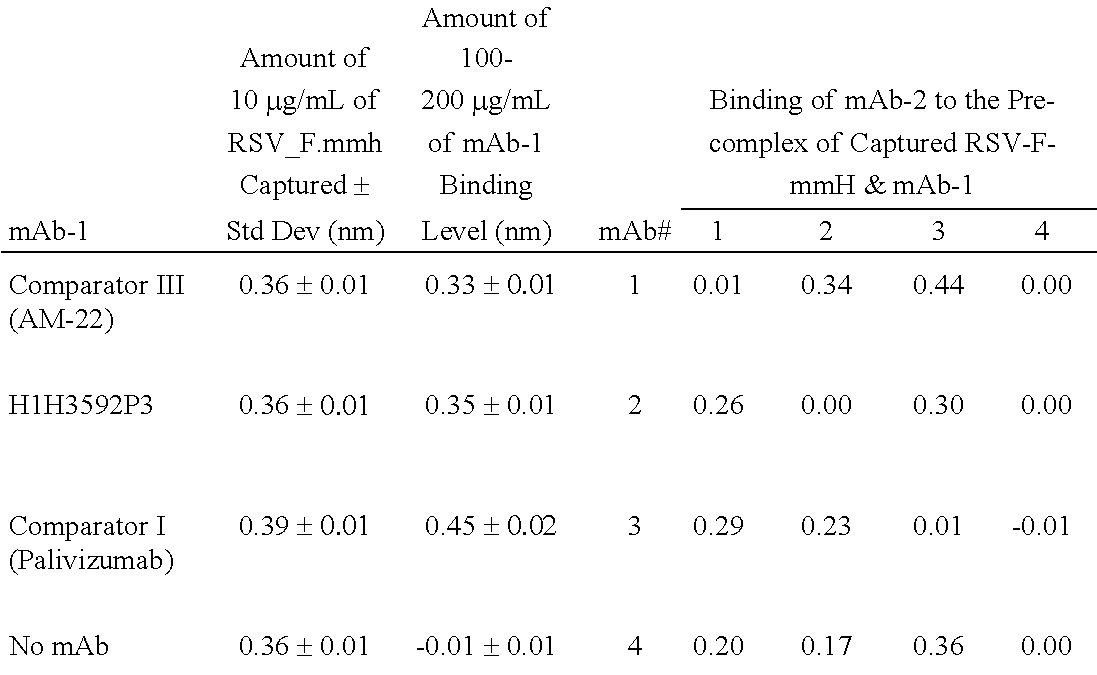
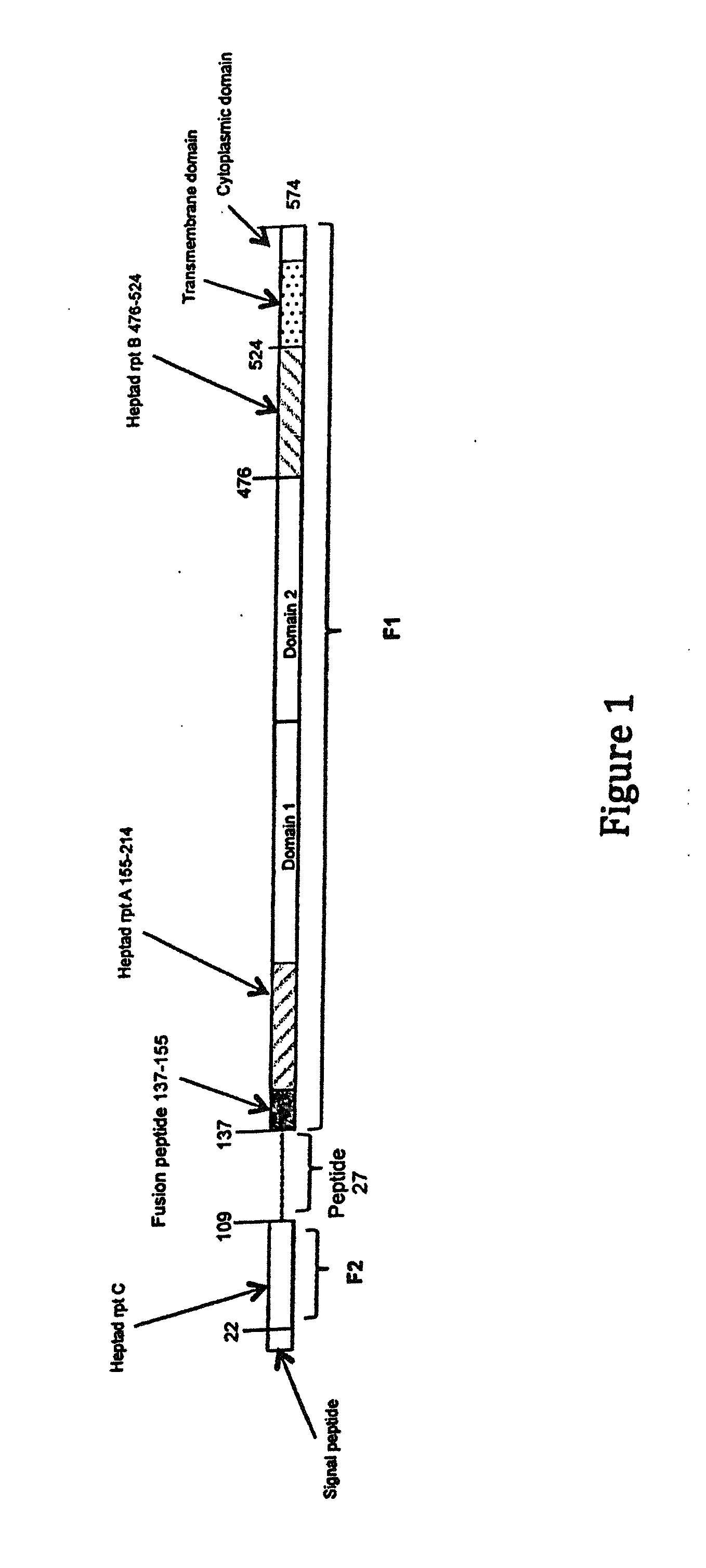
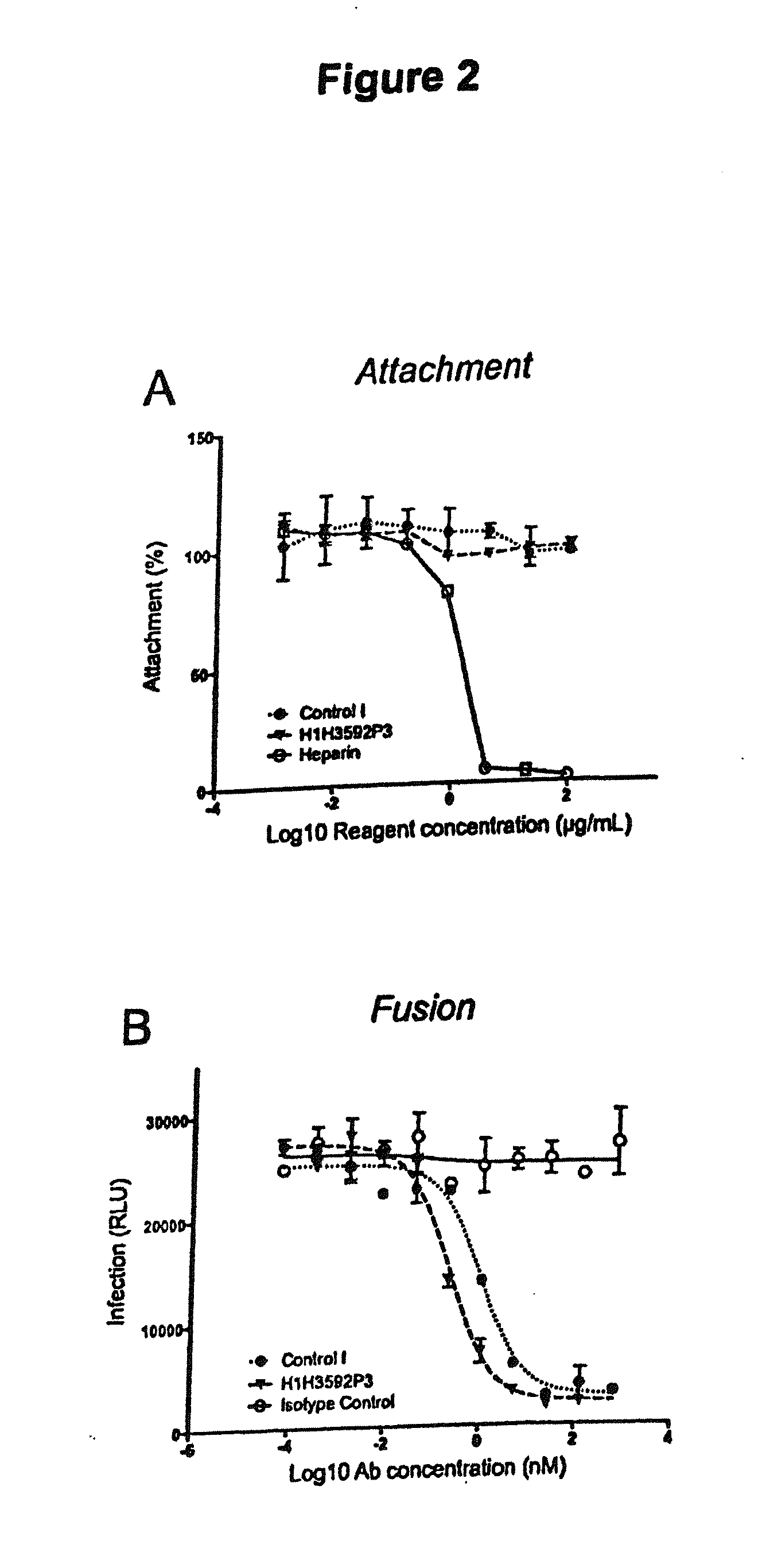
Human antibodies to respiratory syncytial virus f protein and methods of use thereof
ActiveUS20140271653A1Efficiently neutralizedGood protection levelVirusesAntipyreticF proteinCell membrane
The present invention provides fully human antibodies that bind to respiratory syncytial virus F protein, compositions comprising the antibodies and methods of use. The antibodies of the invention are useful for preventing fusion of the virus with the cell membrane and preventing cell to cell spread of the virus, thereby providing a means of preventing the infection, or treating a patient suffering from the infection and ameliorating one or more symptoms or complications associated with the viral infection. The antibodies may also be useful for diagnosis of an infection by RSV.
Owner:REGENERON PHARM INC
Rapid magnetic resonance imaging and magnetic resonance angiography of multiple anatomical territories
InactiveUS6493571B1Reduce osmotic pressureHigh resolutionBedsDiagnostic recording/measuringIodinated Contrast AgentSingle injection
A procedure and apparatus are provided which allow rapid positional change in the patient centering in order to facilitate the imaging of blood vessels in a series of different views. This procedure and apparatus can also facilitate the imaging of other tissues of the body at different spatial locations as well. The procedure and apparatus reduce the time required for obtaining the necessary images for a medical imaging examination using a single injection of an MRI or iodinated contrast agent.
Owner:WILLIAM BEAUMONT HOSPITAL
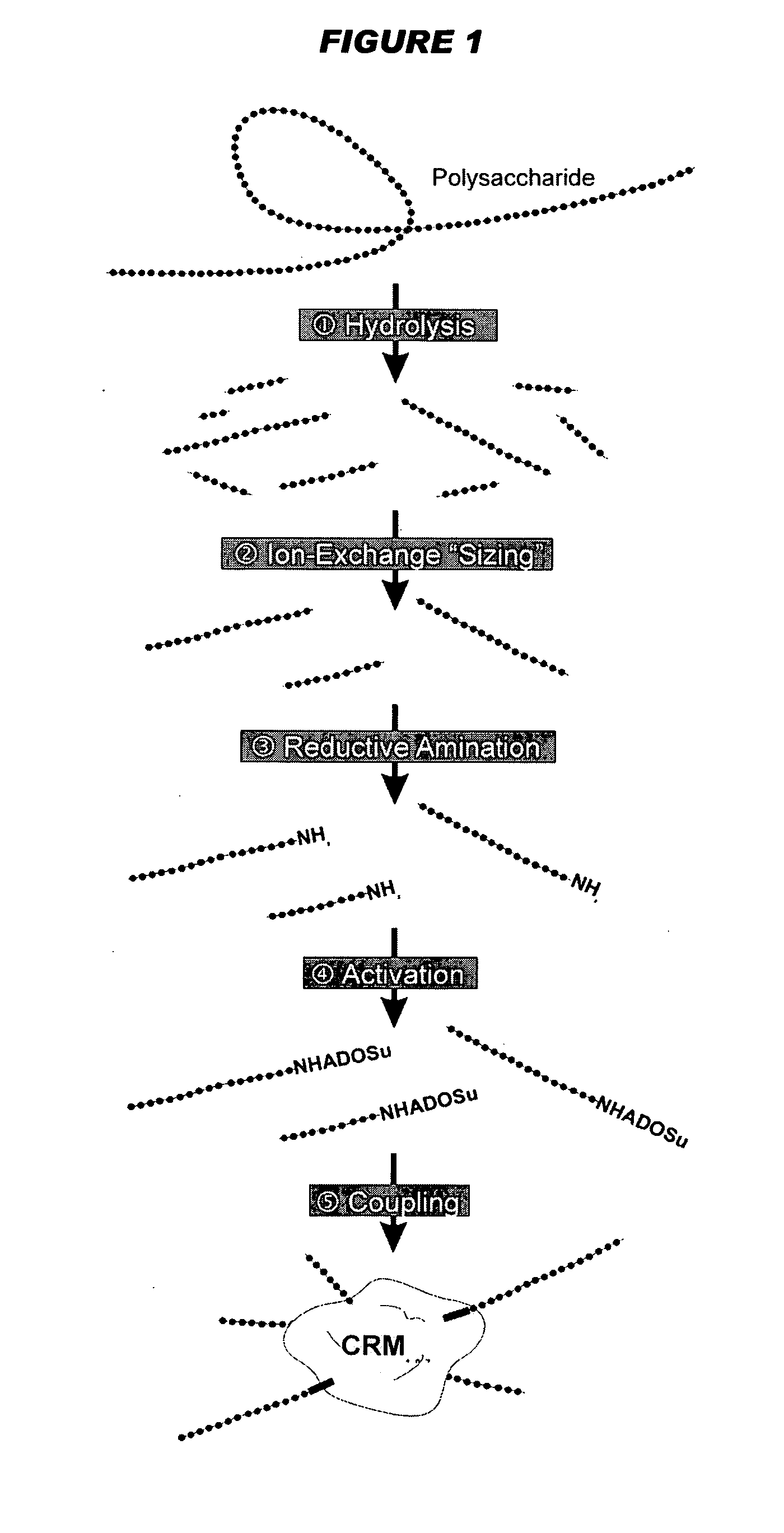
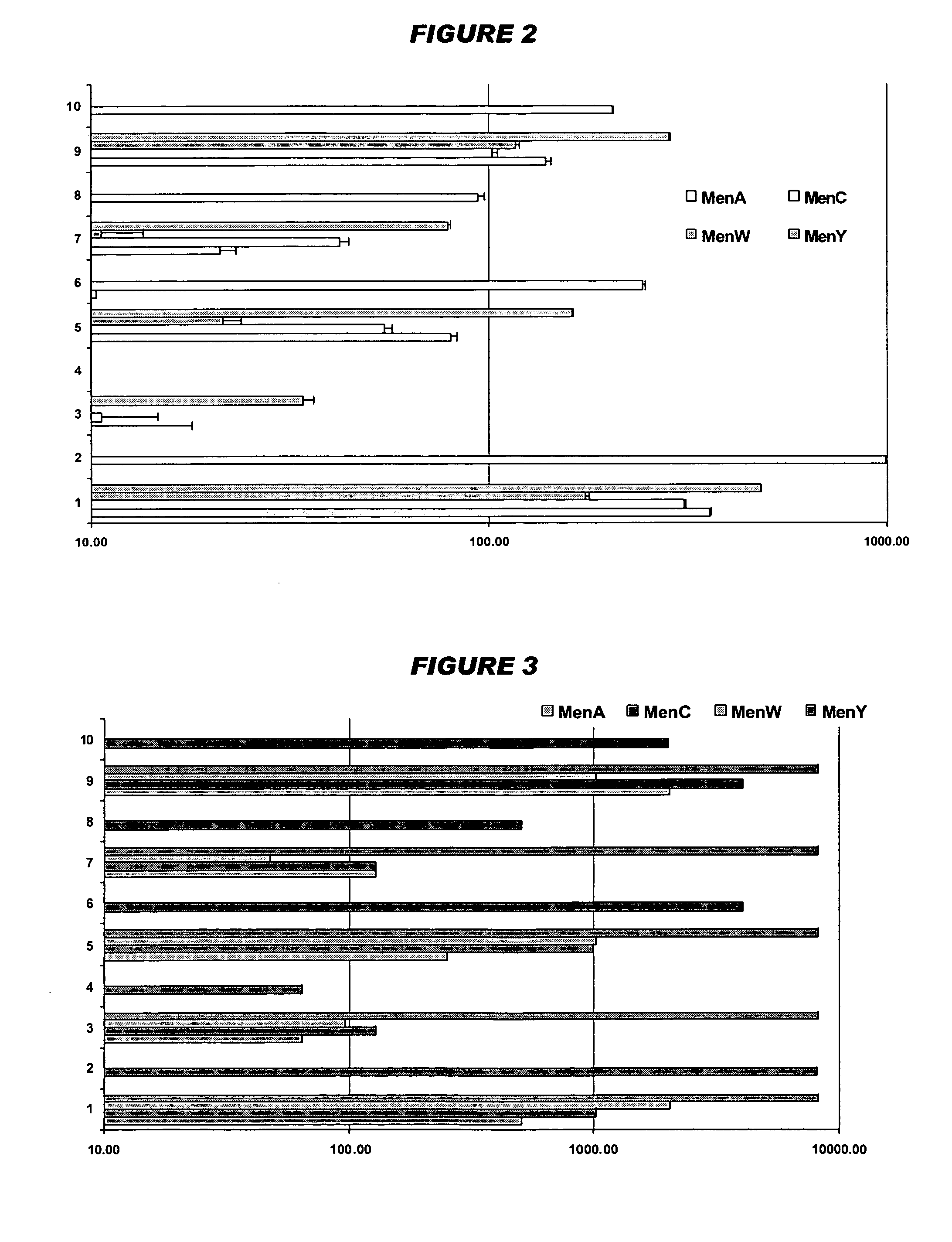
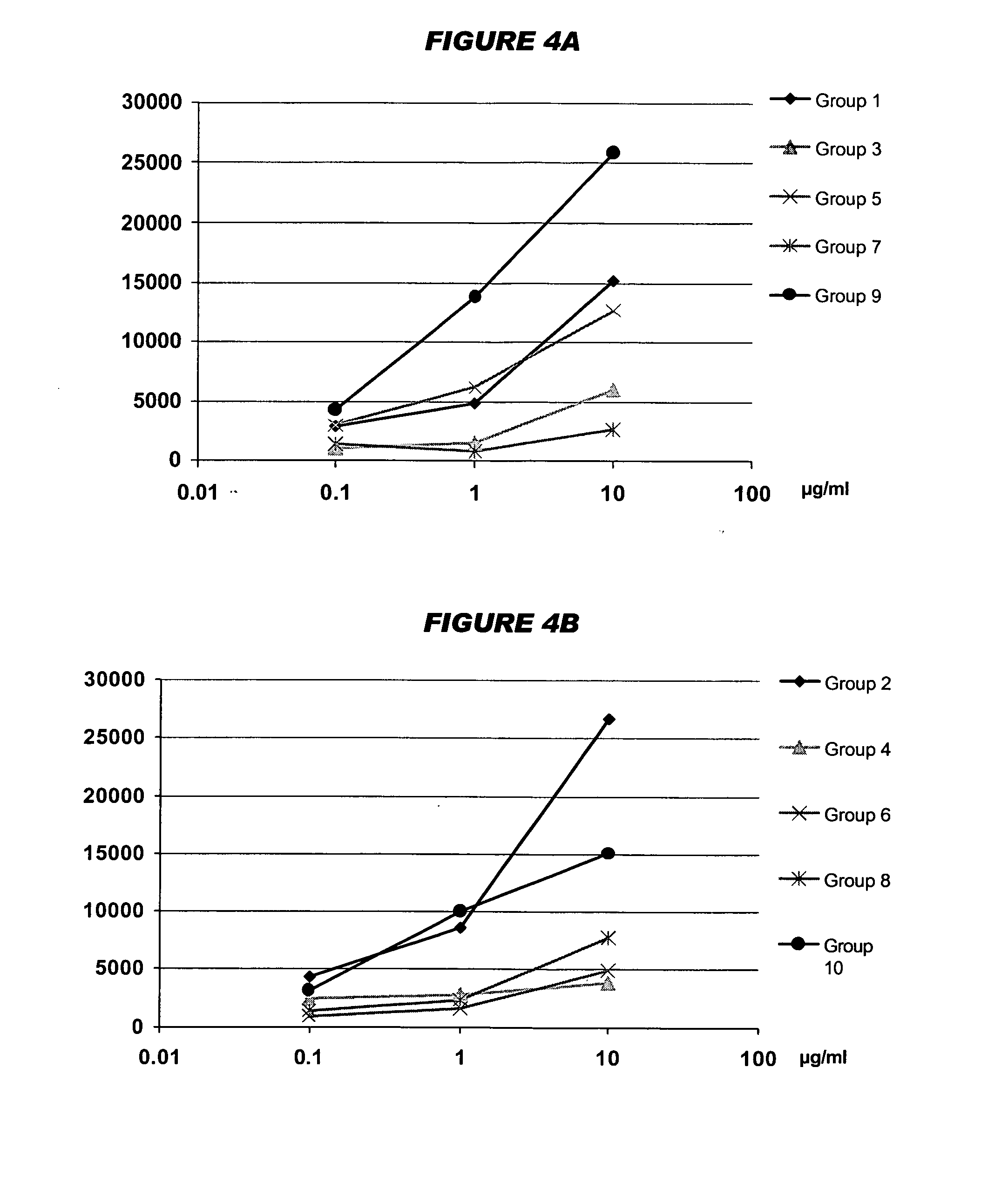
Mucosal meningococcal vaccines
InactiveUS20070207090A1Improve securityReduced dosAntibacterial agentsBacterial antigen ingredientsMucosal Immune ResponsesAdjuvant
The invention provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred. The invention also provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The use of chitosan and / or detoxified ADP-ribosylating toxin adjuvants enhances anti-meningococcal mucosal immune responses and can shift the Th1 / Th2 bias of the responses.
Owner:NOVARTIS AG
Compositions and methods for treating cognitive disorders
ActiveUS20060069094A1Reduce riskLack of efficacyBiocideOrganic chemistry5-HT6 receptorClinical psychology
Owner:ROCHE PALO ALTO LLC
Device for performing a cutting-balloon intervention
InactiveUS20070173919A1Reduce riskConvenient recordingComputerised tomographsRespiratory organ evaluationBalloon catheterCatheter device
Owner:SIEMENS HEALTHCARE GMBH
Nell peptide expression systems and bone formation activity of nell peptide
ActiveUS20060292670A1Facilitate protein traffickingFacilitate post production modificationOrganic active ingredientsBacteriaBone formationBone growth factor
The invention generally relates to a bone growth factor, and more particularly to compositions including NELL1, articles of manufacture including NELL1 and methods of using NELL1 to induce bone formation. This invention also provides methods for the expression and purification of NELL1 and NELL2 peptides.
Owner:RGT UNIV OF CALIFORNIA
Stable pharmaceutical compositions
InactiveUS20070116756A1Stable compositionReduce chanceSalicyclic acid active ingredientsBiocideDrugDosage form
A pharmaceutical dosage form, comprising an outer capsule containing at least one capsule, tablet, and / or particles comprising different drug substances.
Owner:DR REDDYS LAB LTD
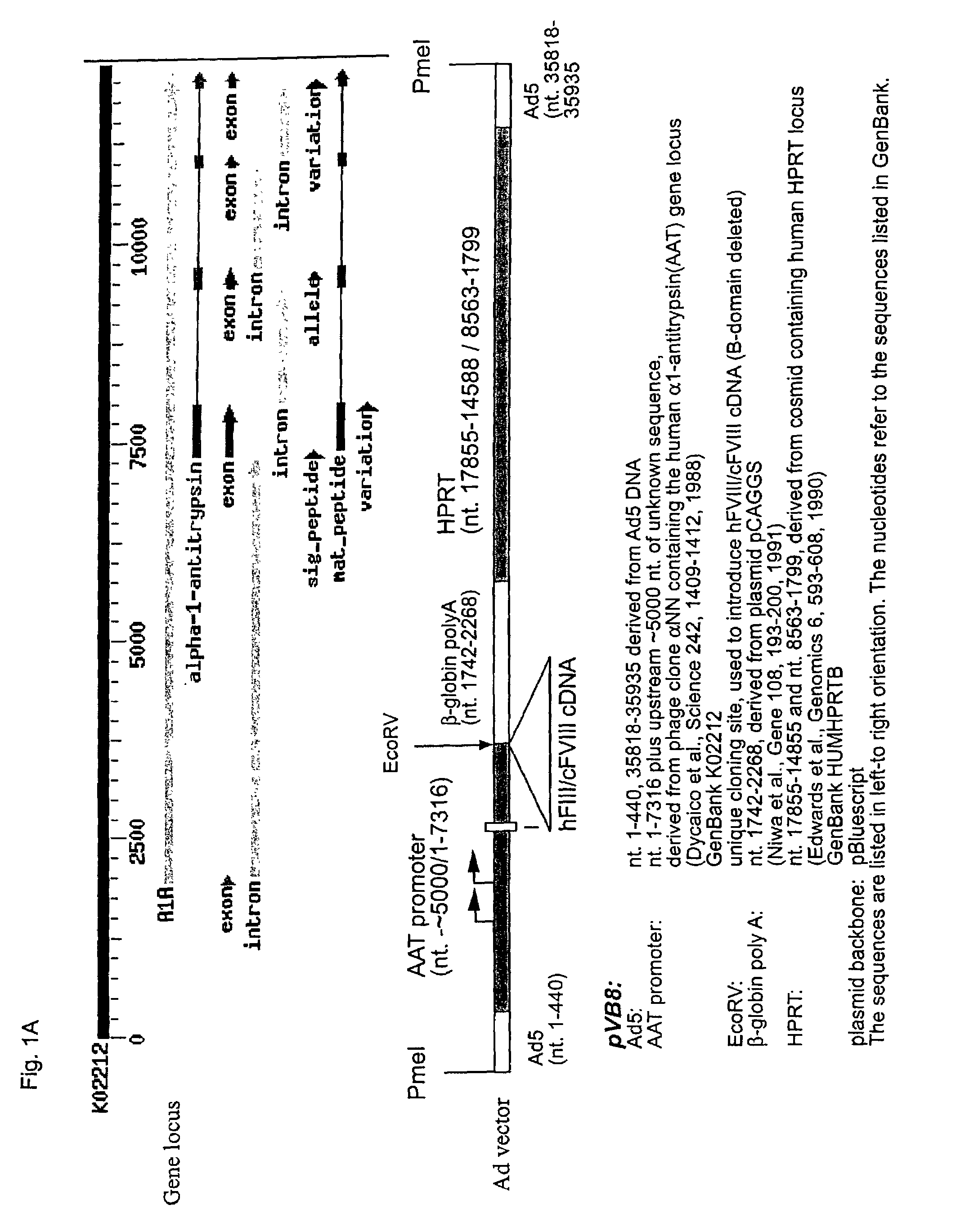
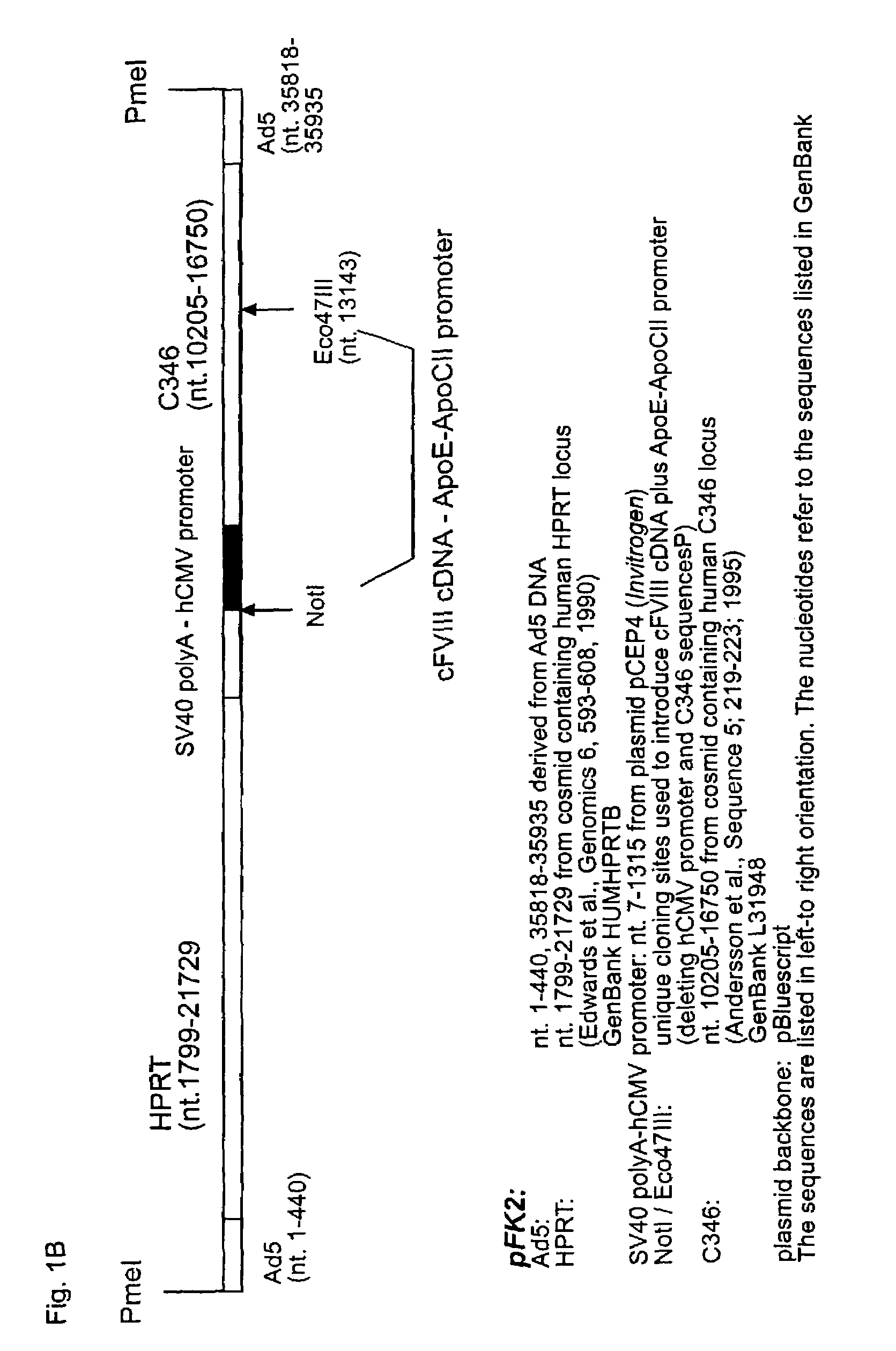
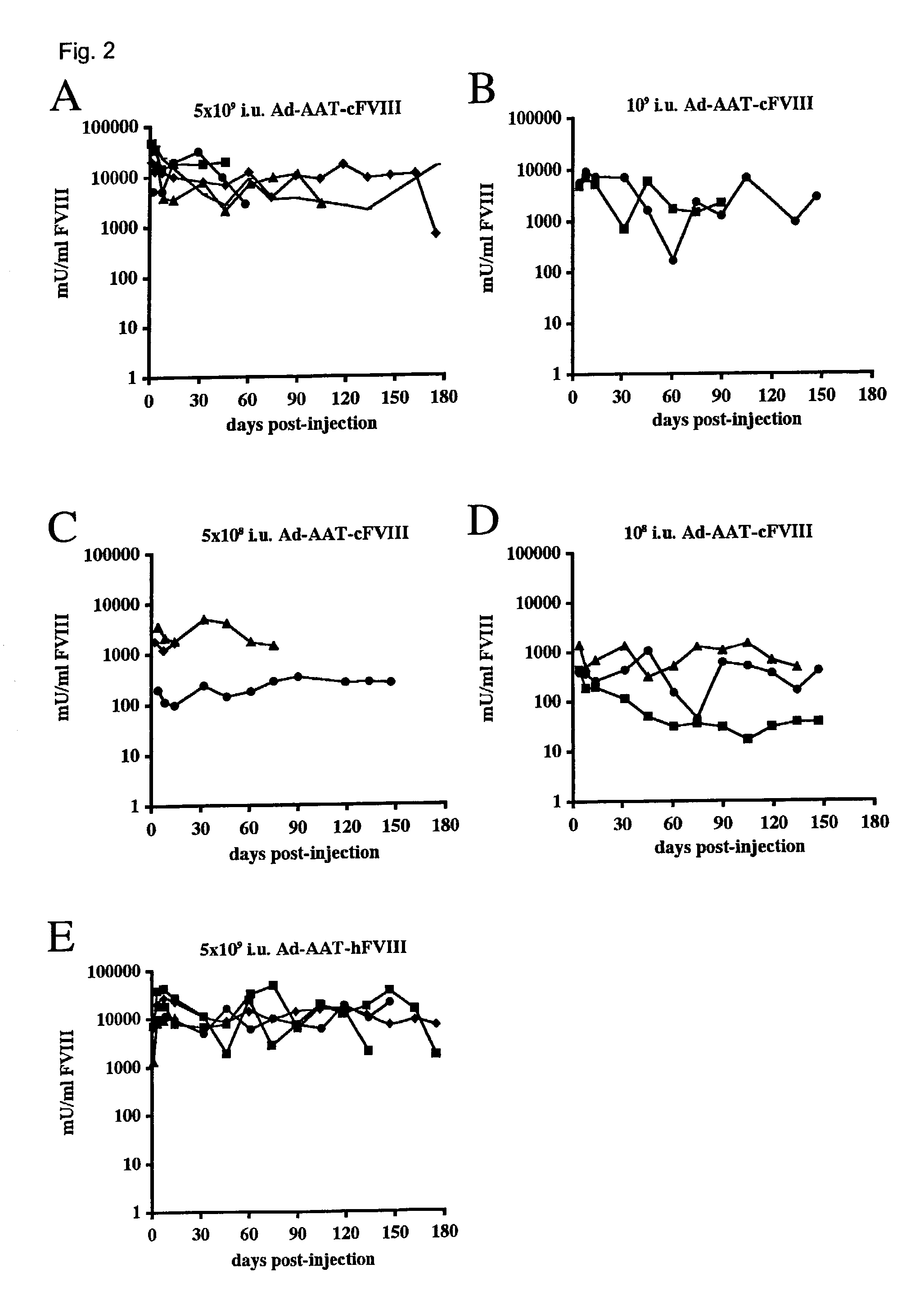
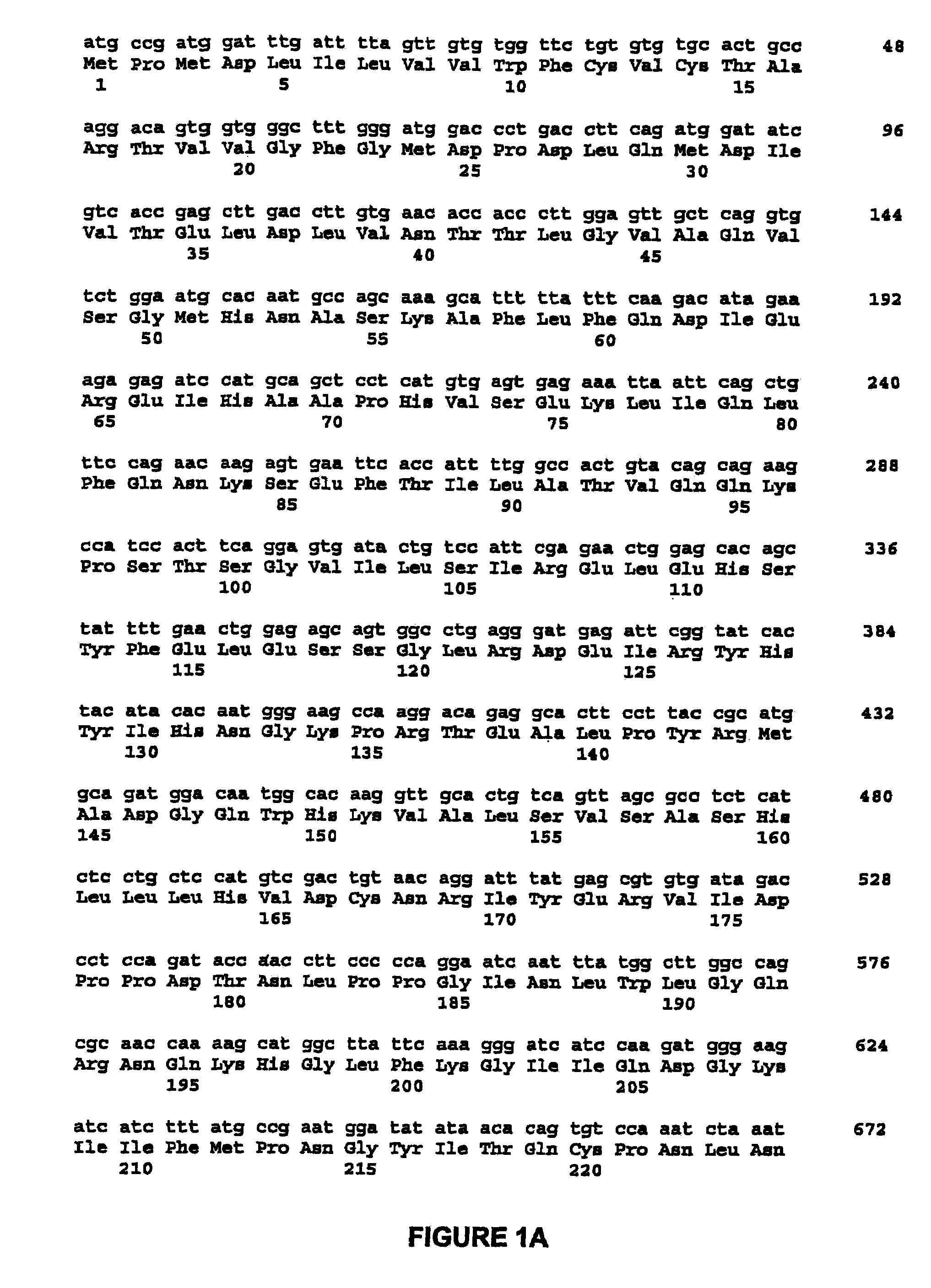
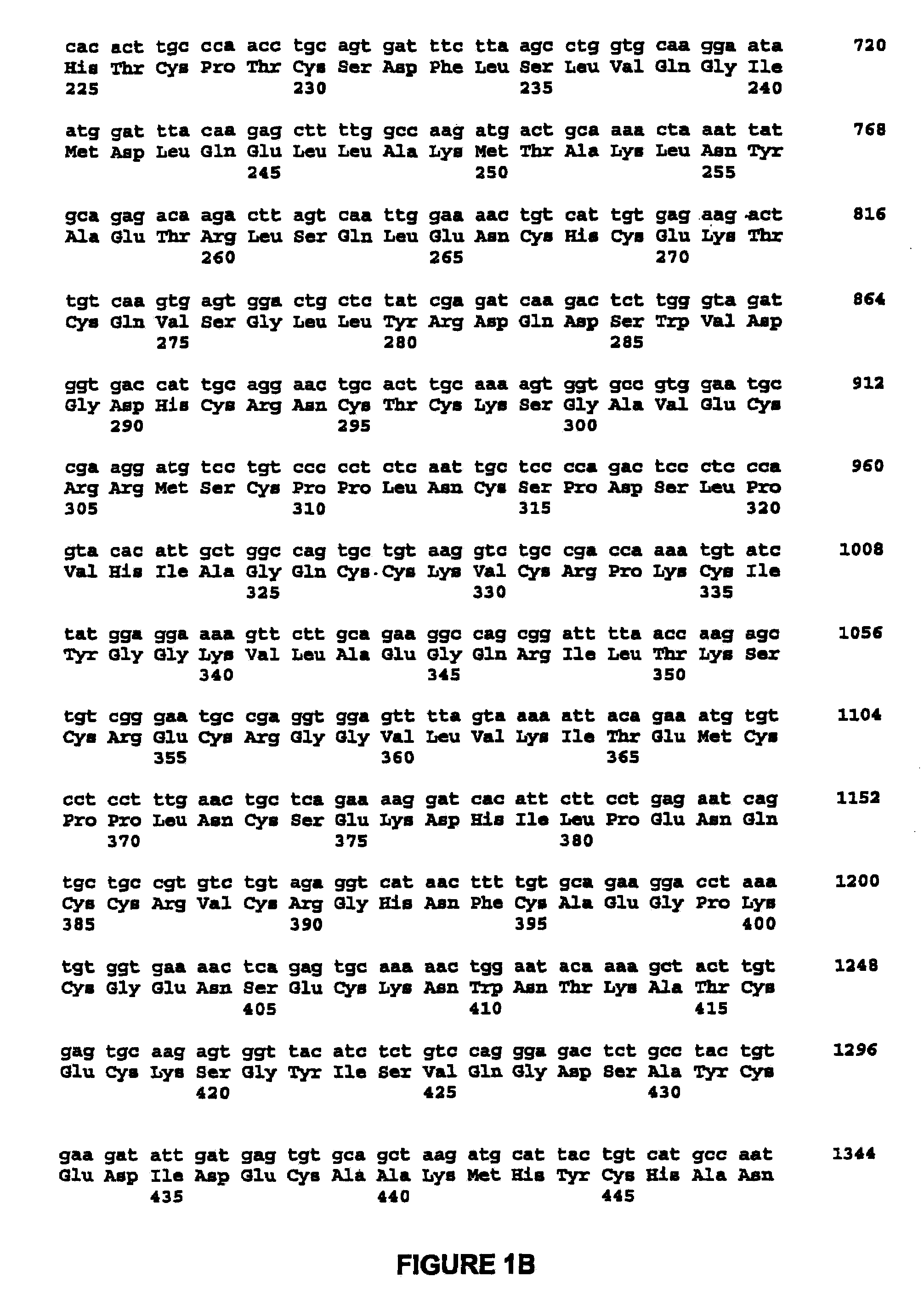
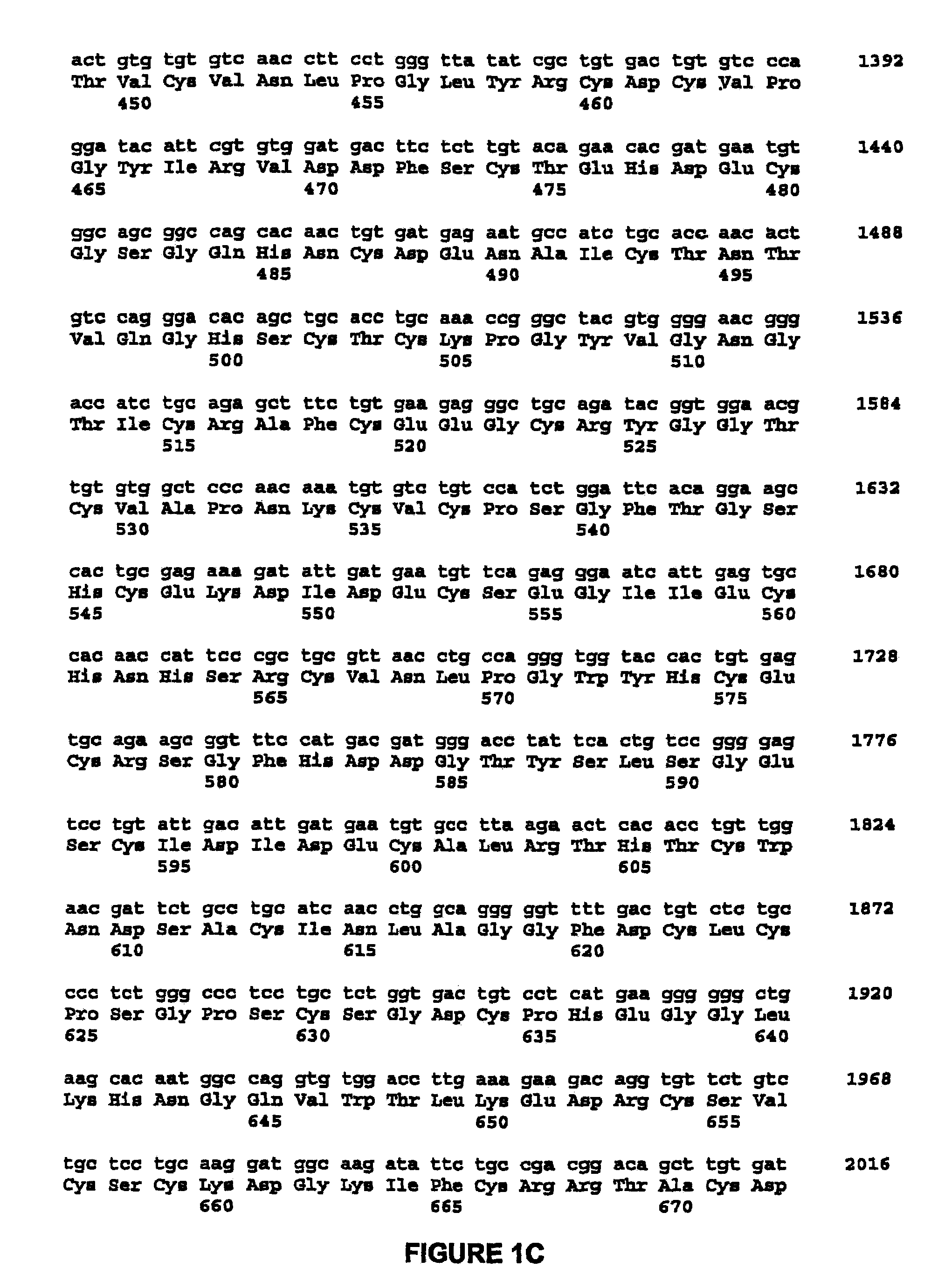
High capacity recombinant adenoviral vector for treatment of hemophilia A
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
Treatment of cancer with active vitamin D compounds in combination with radiotherapeutic agents and treatments
InactiveUS20060177374A1Treating and ameliorating cancerReduced hypercalcemicBiocideOrganic active ingredientsCompound (substance)Treated animal
The present invention relates to a method for treating cancer in an animal by administering to the animal an active vitamin D compound or a mimic thereof in combination with a radiotherapeutic agent or treatment.
Owner:NOVACEA INC
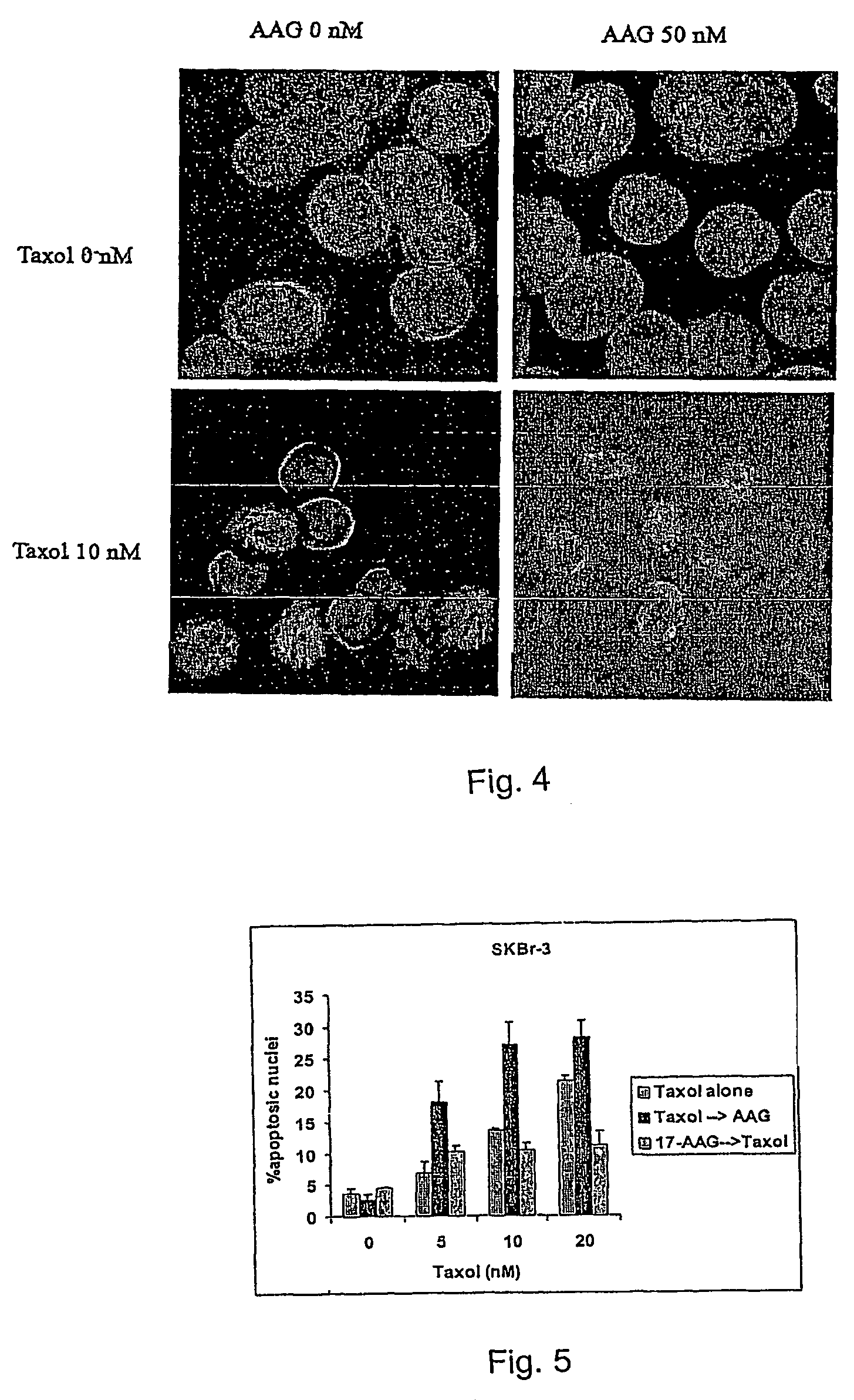
Methods for enhancing the efficacy of cytotoxic agents through the use of HSP90 inhibitors
InactiveUS7211562B2Avoid or reduce their respective toxicity to patientsGrowth inhibitionHeavy metal active ingredientsPeptide/protein ingredientsBinding siteRadicicol
The administration of cytotoxic agents followed by the administration of heat shock protein 90 inhibitors, such as ansamycins, has a synergistic effect on the growth inhibition of cells. This synergy occurs at doses of each cytotoxic agent that normally only causes minimal growth inhibition of cells. Such combination therapy thus allows one to use lower doses of cytotoxic agents to avoid or reduce their respective toxicity to patients without compromising their growth inhibitory effects. Thus, these combinations can be used for the treatment of an animal, preferably a mammal, that has a cell proliferative disorder, whether the cells have wild-type Rb or are Rb deficient or Rb negative. One such method, directed to treating cell proliferative disorders includes the step of administering a therapeutic effective amount of a cytotoxic agent followed by administering a therapeutic effective amount of a heat shock protein 90 inhibitor. The cytotoxic agent may be a microtubule-affecting agent, topoisomerase II inhibitor, a platinum complex, paclitaxel, or a paclitaxel derivative. The HSP90 inhibitor may be an ansamycin, radicicol or a synthetic compound that binds to the ATP-binding site of HSP90.
Owner:SLOAN KETTERING INST FOR CANCER RES
Nell peptide expression systems and bone formation activity of nell peptide
ActiveUS7544486B2Facilitate protein trafficking and post production modificationPromote secretionOrganic active ingredientsBacteriaBone formationBone growth factor
The invention generally relates to a bone growth factor, and more particularly to compositions including NELL1, articles of manufacture including NELL1 and methods of using NELL1 to induce bone formation. This invention also provides methods for the expression and purification of NELL1 and NELL2 peptides.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com