Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Cannabidivarin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
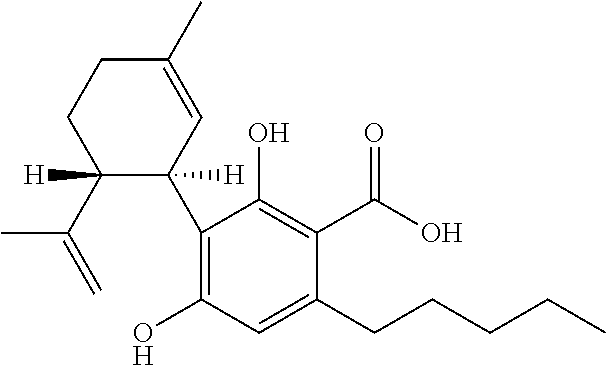
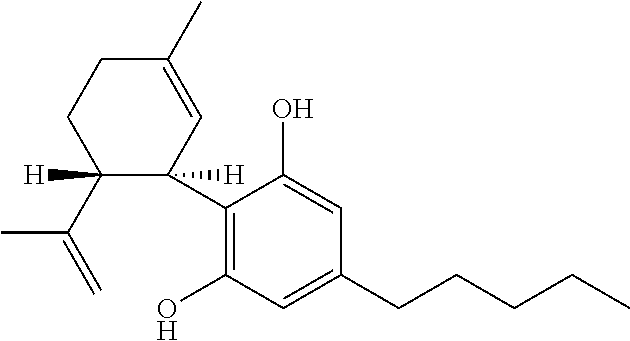
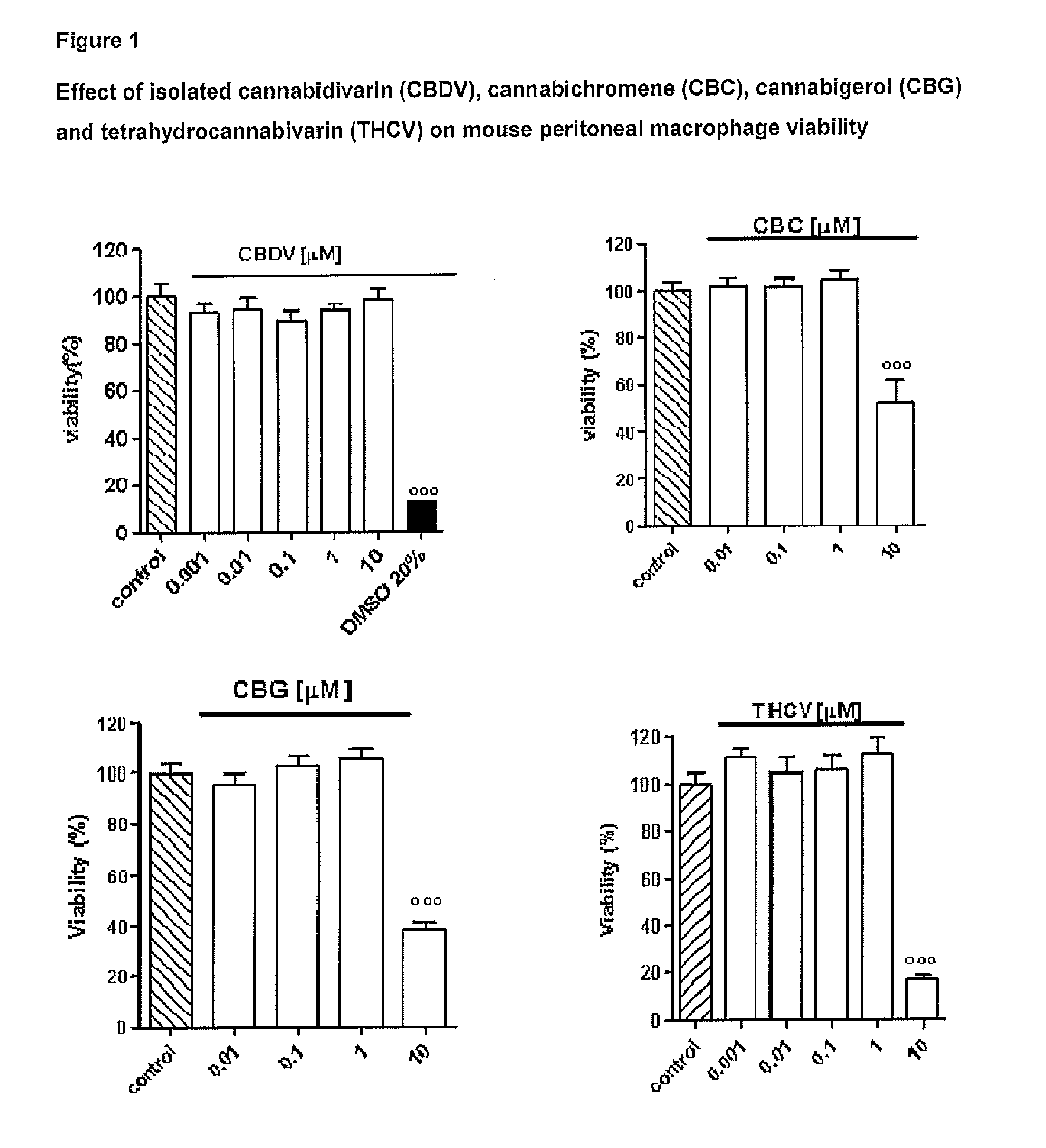
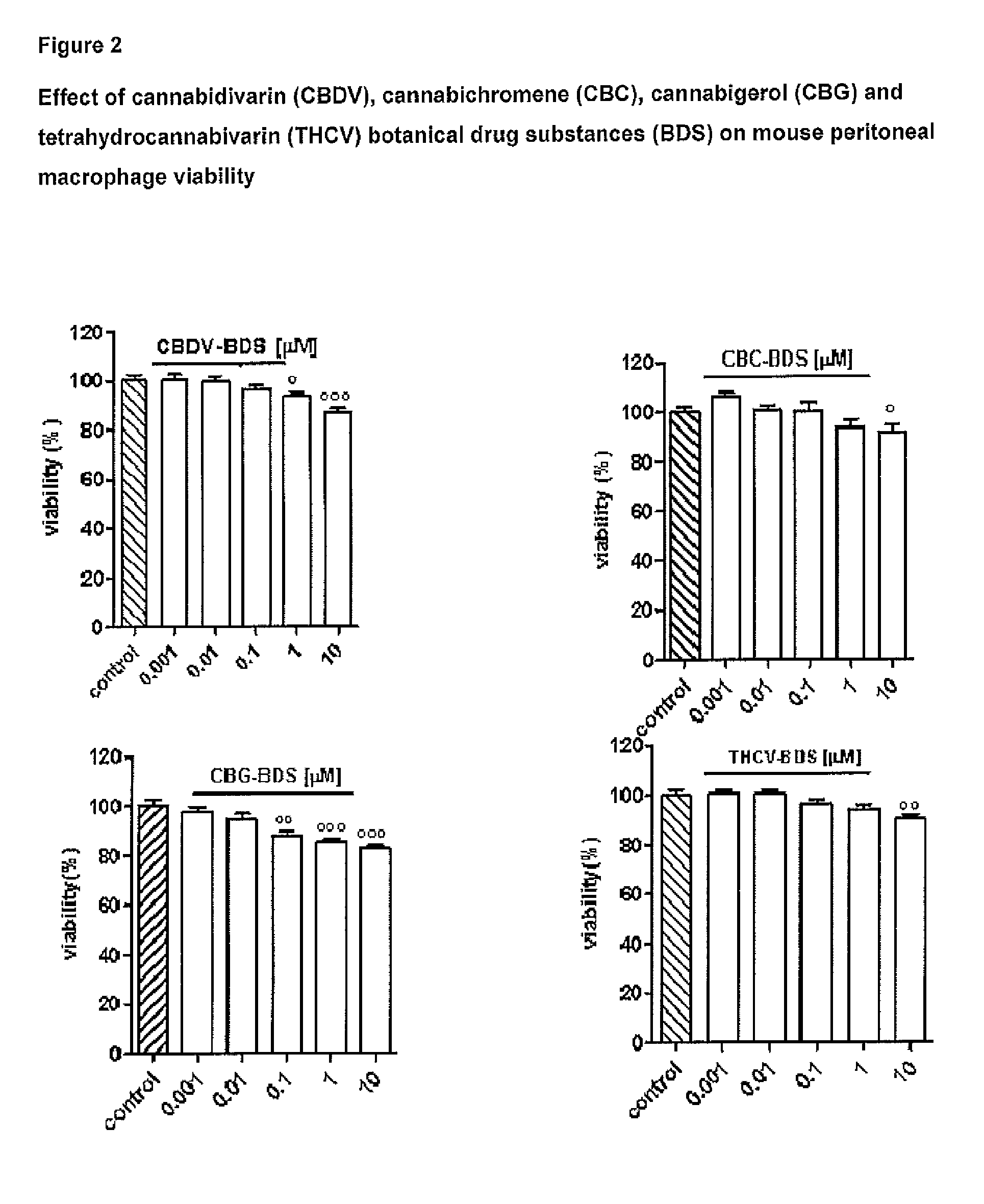
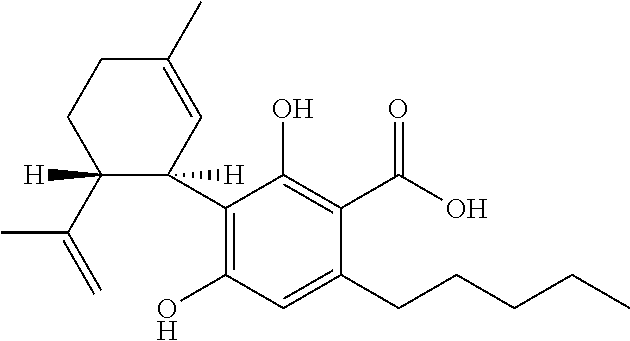
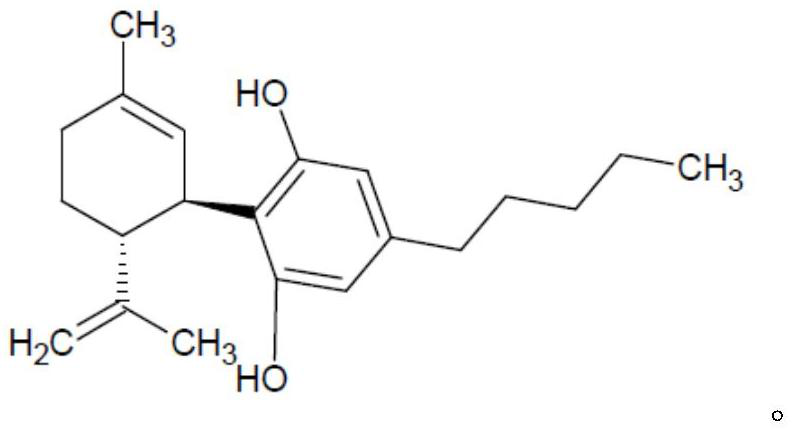
Cannabidivarin (CBDV) is a non-psychoactive cannabinoid found in Cannabis. It is a homolog of cannabidiol (CBD), with the side-chain shortened by two methylene bridges (CH₂ units). Plants with relatively high levels of CBDV have been reported in feral populations of C. indica ( = C. sativa ssp. indica var. kafiristanica) from northwest India, and in hashish from Nepal. CBDV has anticonvulsant effects. It was identified for the first time in 1969 by Vollner et al.
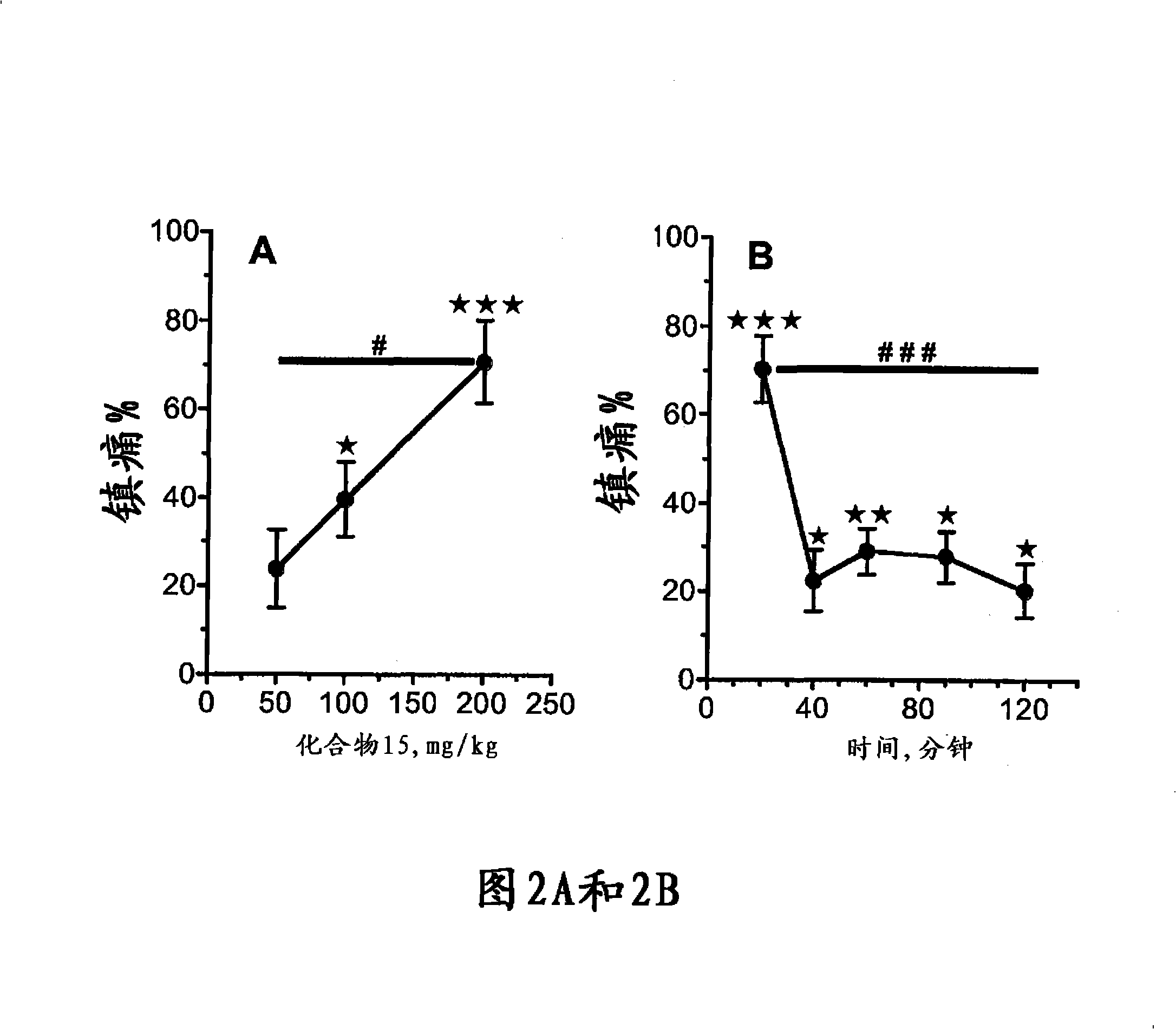
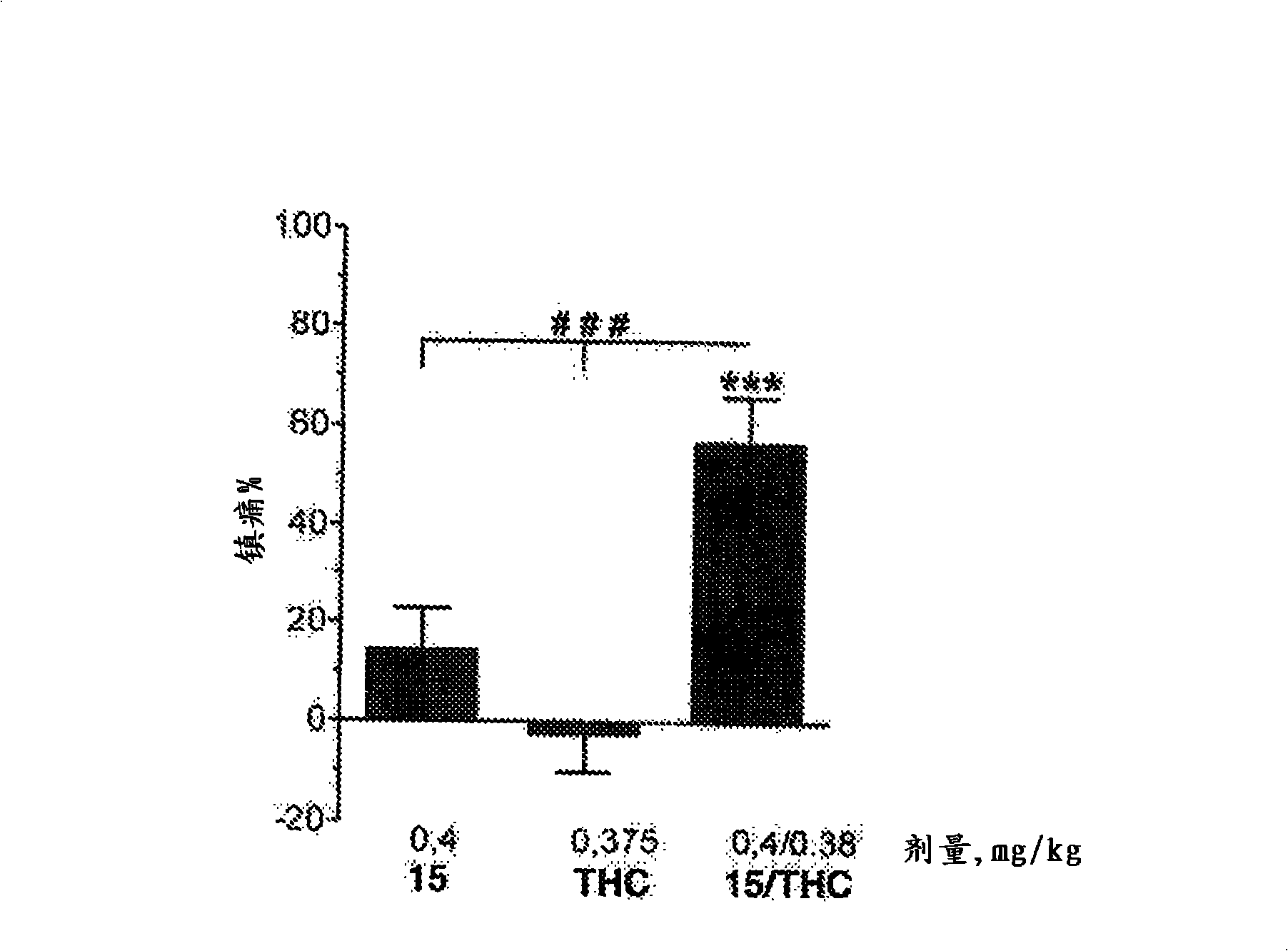
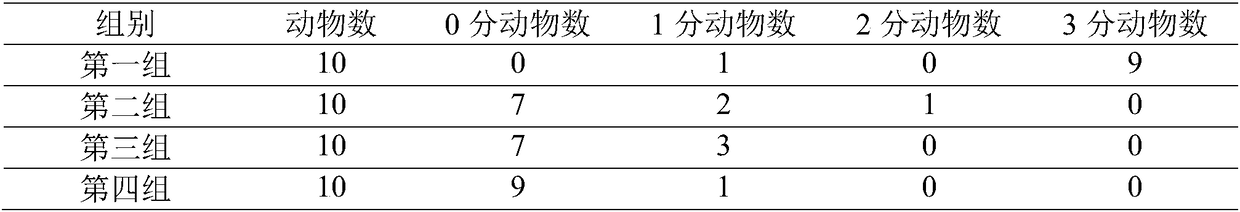
Use of the phytocannabinoid cannabidivarin (CBDV) in the treatment of epilepsy
ActiveUS9125859B2Growth inhibitionReduces high-frequency neuronal firingBiocideNervous disorderValproic AcidPhenobarbital
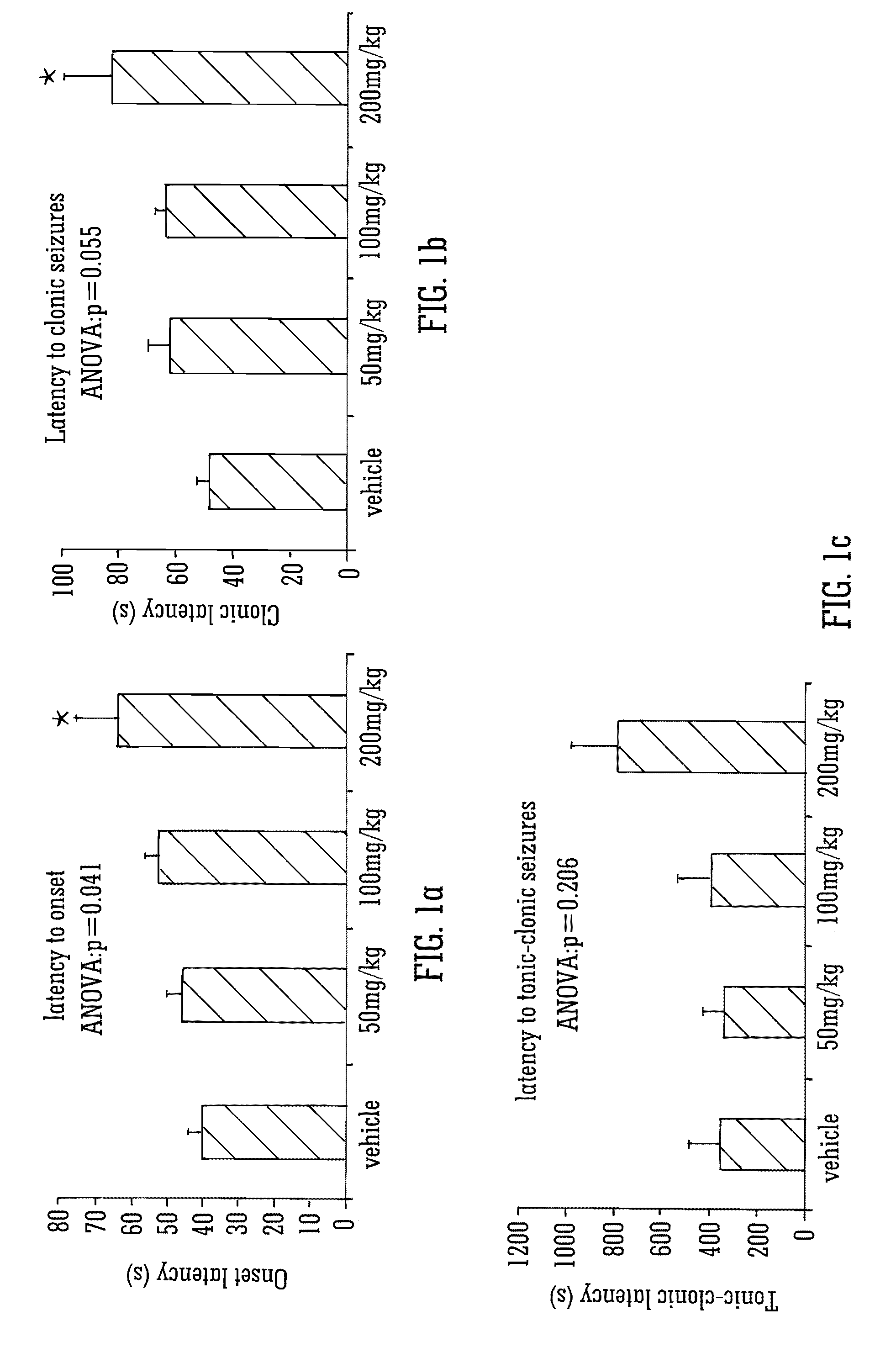
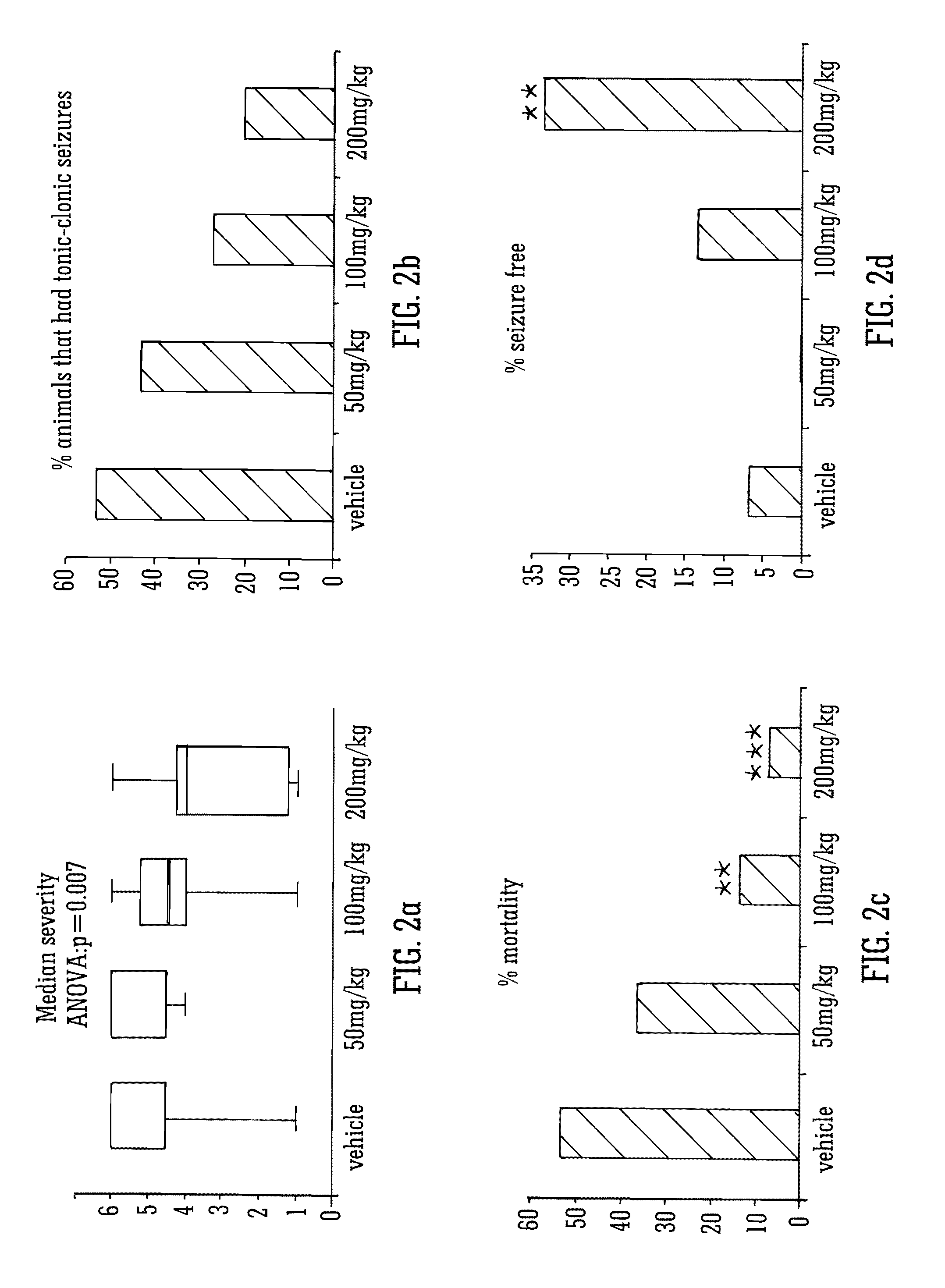
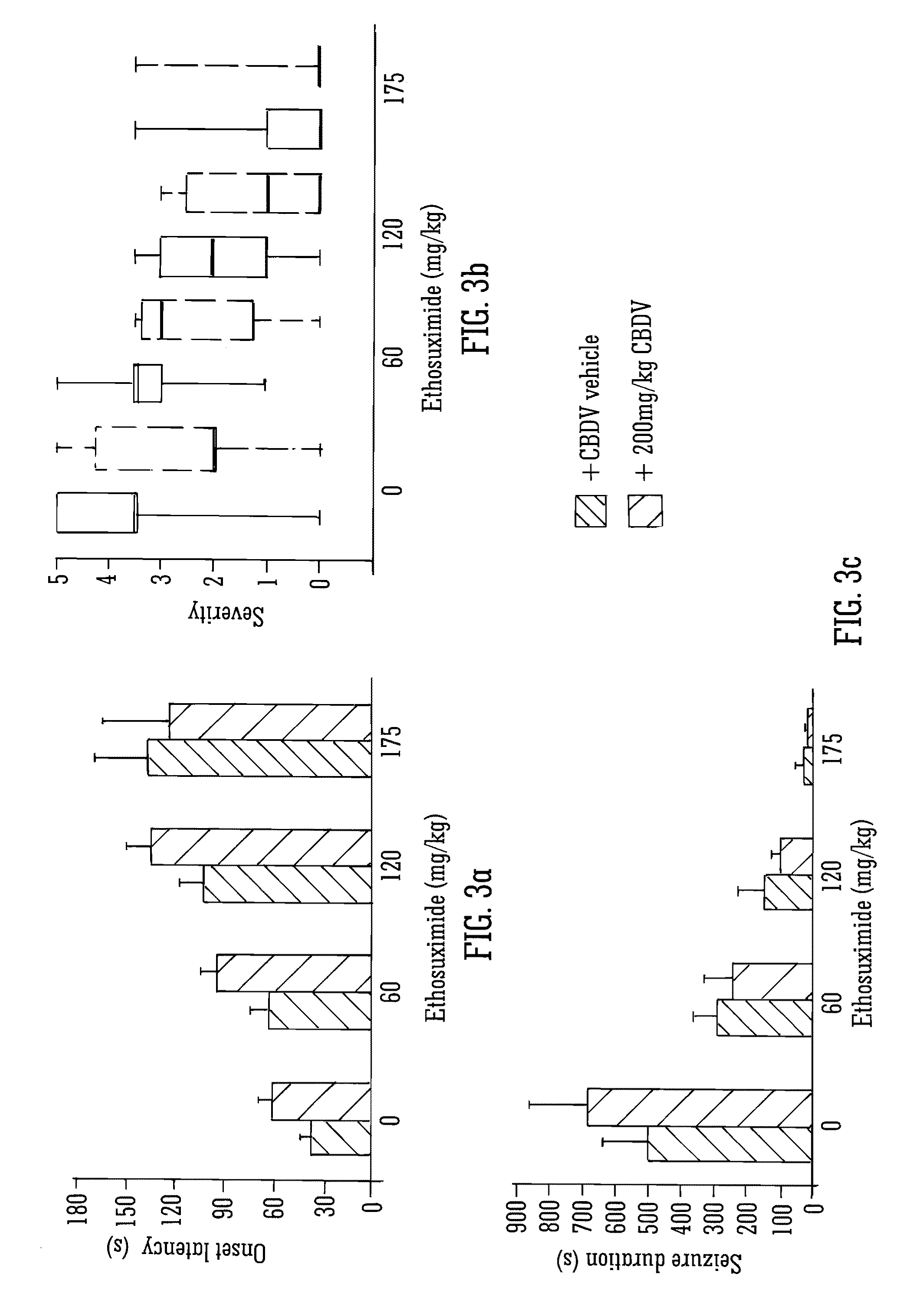
This invention relates to the use of the phytocannabinoid cannabidivarin (CBDV) and combinations of the phytocannabinoid CBDV with tetrahydrocannabivarin (THCV) and cannabidiol (CBD) in the treatment of epilepsy. The invention further relates to the use of the phytocannabinoid CBDV in combination with standard anti-epileptic drugs (SAEDs). Preferably the SAED is one of ethosuximide, valproate or phenobarbital.
Owner:GW PHARMA LTD
Use of cannabinoids in the treatment of epilepsy
ActiveUS20190083418A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsSturge–Weber syndromeMedicine
The present invention relates to the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Sturge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Parenteral formulations
ActiveUS20190314296A1Improve solubilityLow toxicityHydroxy compound active ingredientsPharmaceutical delivery mechanismSURFACTANT BLENDPharmacology
The present invention relates to parenteral cannabinoid formulations, and more particularly to cannabinoid containing intravenous (IV) formulations. Preferably the parenteral containing formulation comprises a cannabinoid; an isotonic agent; a surfactant; and one or more stability enhancers. Furthermore the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerolpropyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
Oral cannabinoid formulations
ActiveUS20190365667A1Ensure palatabilityEnsure stabilityNervous disorderDispersion deliveryNon ionicSURFACTANT BLEND
The present invention relates to an oral formulation containing one or more cannabinoids. Preferably one or more cannabinoids dissolved in a solvent system consisting essentially of: a non-ionic surfactant and water together with other components which ensure the cannabinoids stability and the formulations palatability. Furthermore, the cannabinoid may be selected from one or more of cannabichromene (CBC), cannabichromenic acid (CBCV), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabigerol (CBG), cannabigerol propyl variant (CBGV), cannabicyclol (CBL), cannabinol (CBN), cannabinol propyl variant (CBNV), cannabitriol (CBO), tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV) and tetrahydrocannabivarinic acid (THCVA).
Owner:GW RES LTD
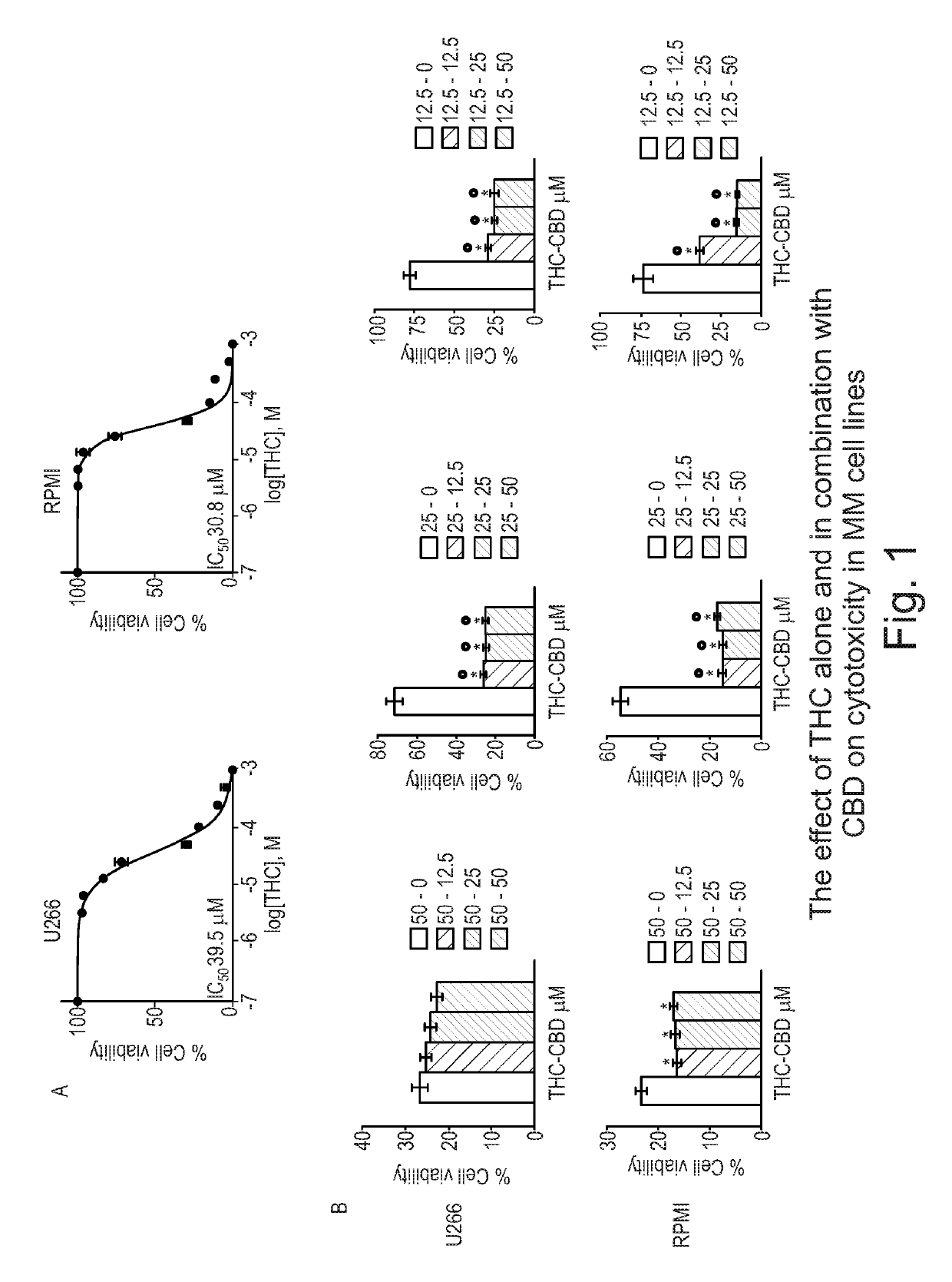
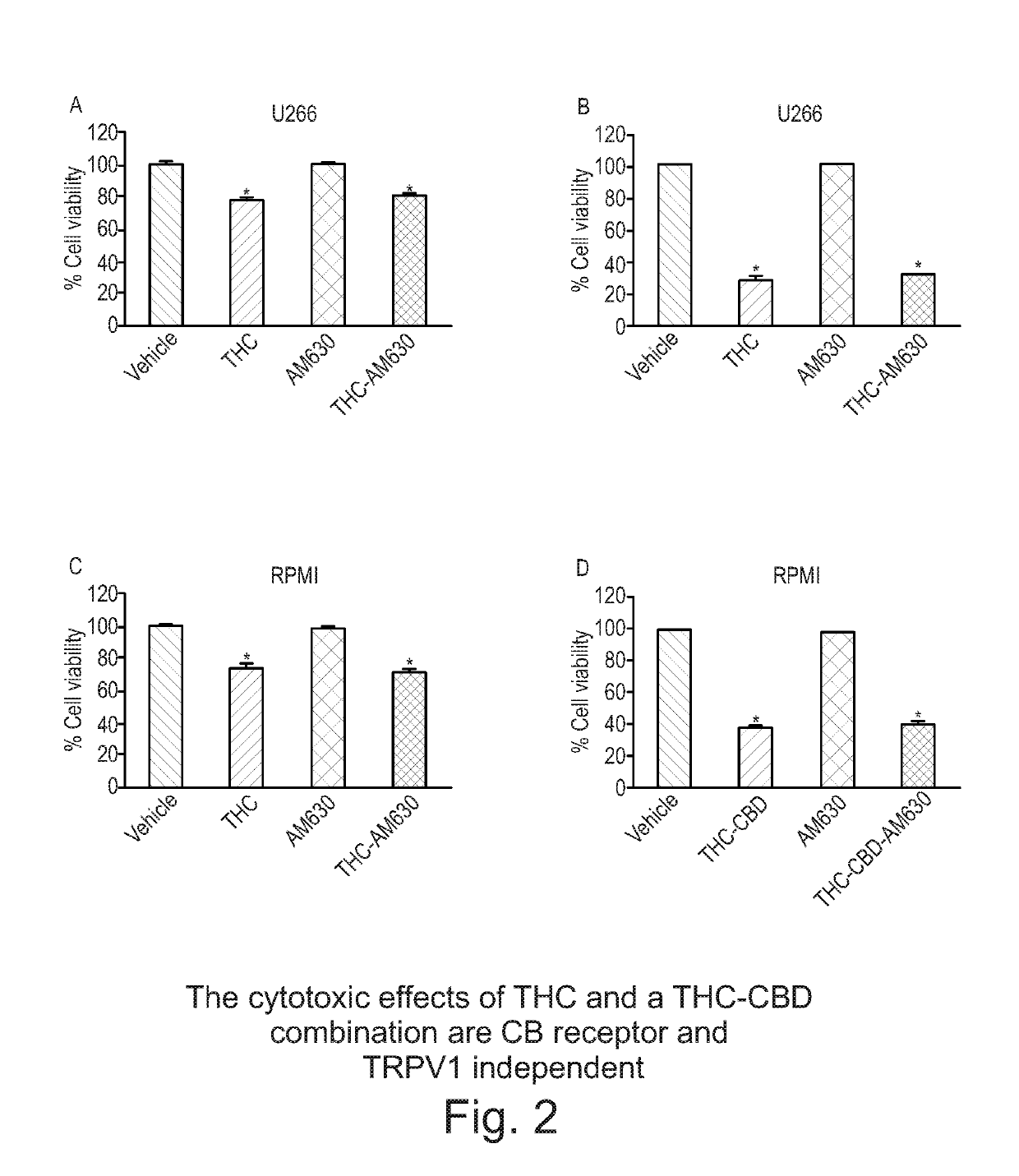
Use of cannabinoids in the treatment of multiple myeloma
ActiveUS20190175547A1Low effective doseHydroxy compound active ingredientsBoron compound active ingredientsDiseaseCell migration
The present invention relates to the use of a combination of tetrahydrocannabinol (THC) and cannabidiol (CBD) in the treatment of multiple myeloma. The combination of THC and CBD appears to be particularly effective in reducing cell viability and cell migration in this disease. Preferably the THC and CBD are used is in the form of a highly purified extract of cannabis such that the cannabinoids are present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the THC and CBD may be a synthetically produced cannabinoid.
Owner:GW RES LTD
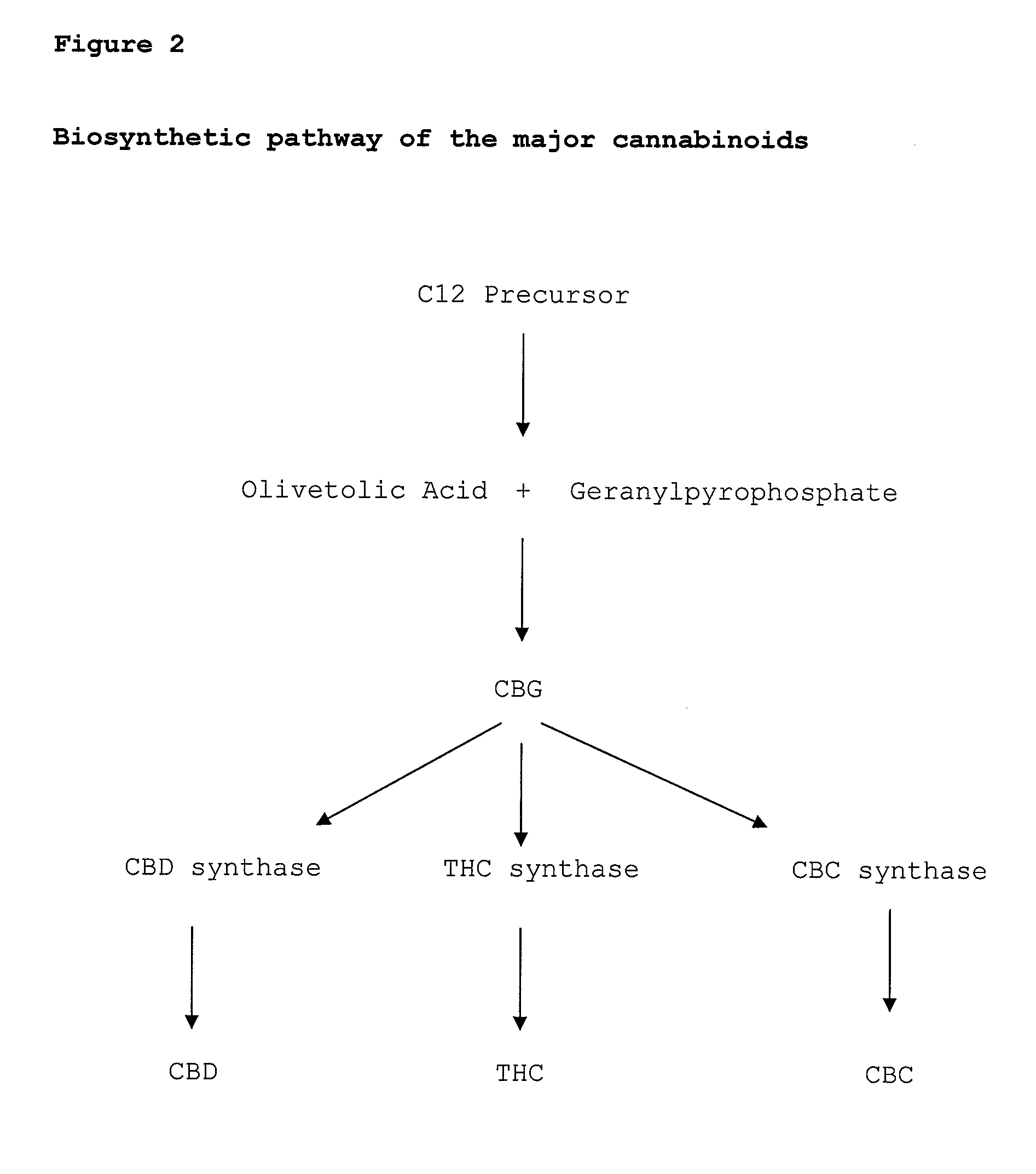
Pharmaceutical compositions comprising cannabigerol
ActiveUS8481085B2High activityLess degree of activityBiocideNervous disorderCannabigerolCannabidivarin
Owner:GW PHARMA LTD
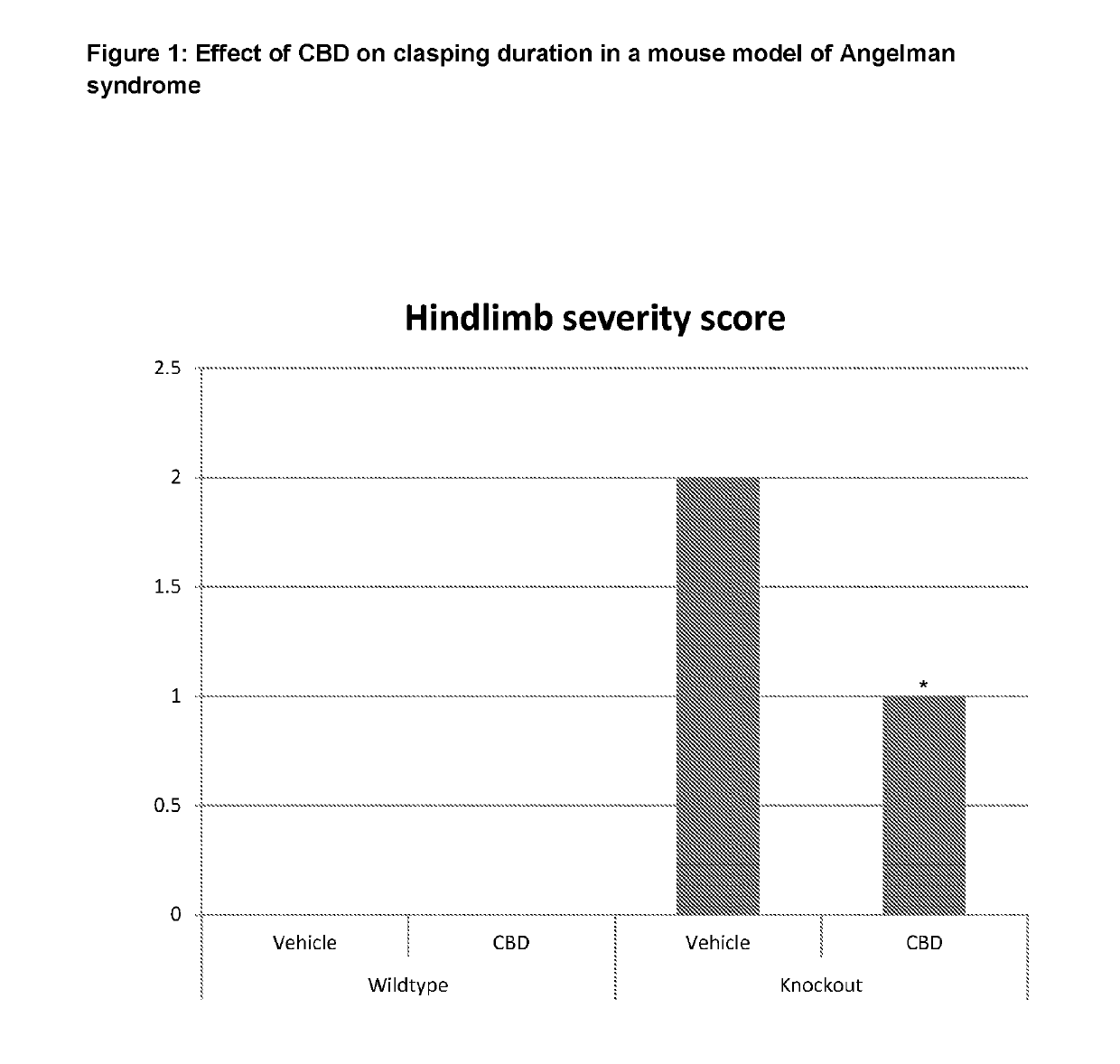
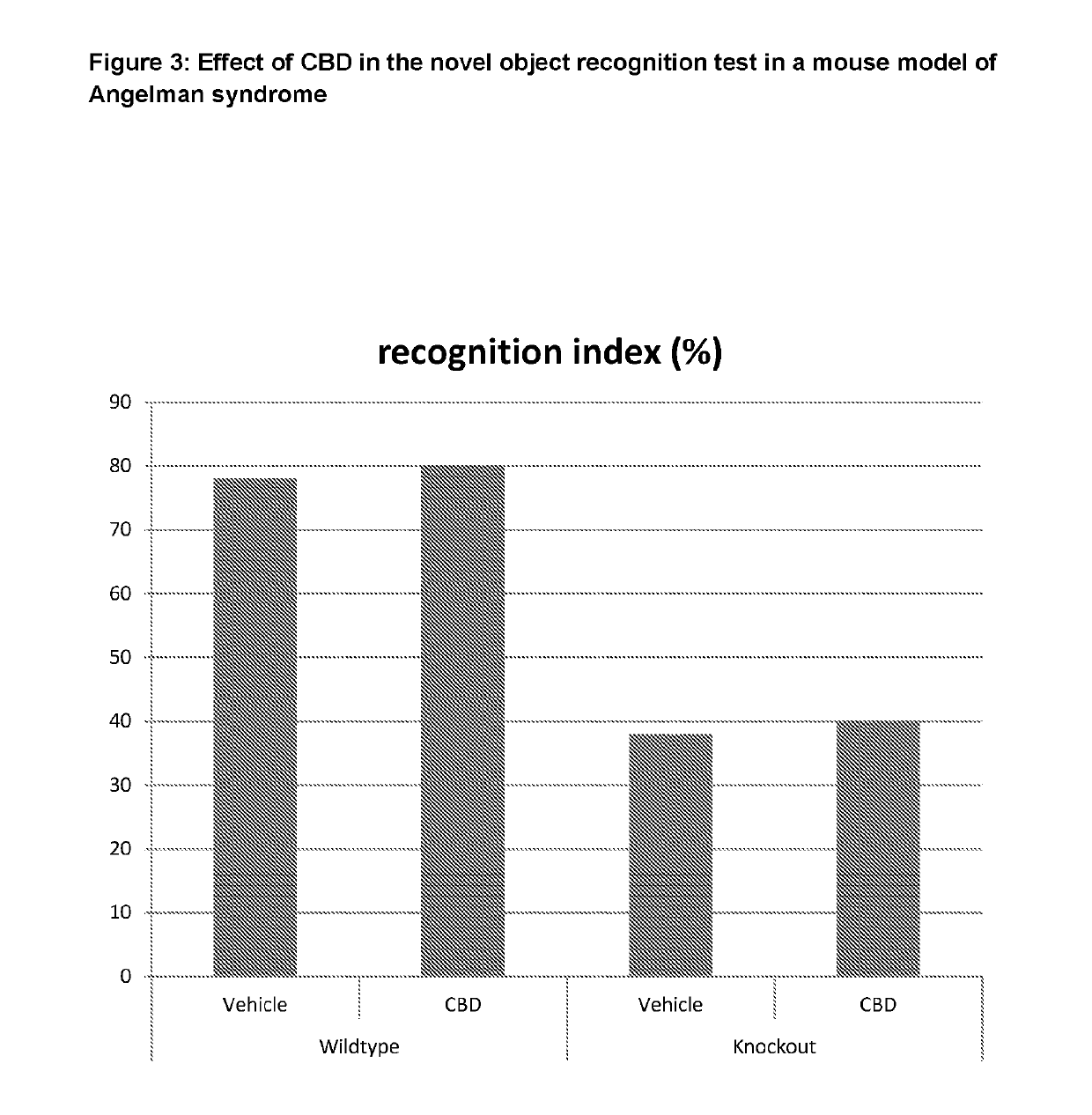
Use of cannabinoids in the treatment of angelman syndrome
ActiveUS20190321307A1Symptoms improvedNervous disorderHydroxy compound active ingredientsRodent modelAnxiety
The present invention relates to the use of cannabidiol (CBD) in the treatment of Angelman syndrome (AS). CBD has It been shown to be particularly effective in improving anxiety in rodent models of AS. The CBD is preferably substantially pure. It may take the form of a highly purified extract of Cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBD is synthetically produced. Alternatively, the CBD may be used as a botanical drug substance (BDS) from a Cannabis plant in which CBD is the predominant cannabinoid. The CBD may also be present in combination with other cannabinoids and non-cannabinoid components such as terpenes. In use the CBD may be used concomitantly with one or more other medicaments. Alternatively, the CBD may be formulated for administration separately, sequentially or simultaneously with one or more medicaments or the combination may be provided in a single dosage form. Where the CBD is formulated for administration separately, sequentially or simultaneously it may be provided as a kit or together with instructions to administer the one or more components in the manner indicated. It may also be used as the sole medication, i.e. as a monotherapy.
Owner:GW RES LTD
Composition containing cannabidiol and/or cannabidivarin and application of composition in treatment of dysmenorrhea
InactiveCN109498606ANervous disorderHydroxy compound active ingredientsMedicineFeminine Hygiene Products
The invention discloses a composition for preventing and / or treating female dysmenorrhea. The composition is prepared from cannabidiol and / or cannabidivarin, a penetration enhancer and a carrier, wherein the mass ratio of the cannabidiol and / or cannabidivarin to the penetration enhancer is 1:(0.1-0.8). The composition has the advantages that the female dysmenorrheal can be effectively relieved, and the problem that in the prior art, a cannabidiol and / or cannabidivarin composition capable of effectively preventing and / or treating the female dysmenorrhea is in shortage can be solved; and meanwhile, the composition can also be used for preparing female hygienic aids for preventing and / or treating the female dysmenorrhea.
Owner:HANYI BIO TECH CO LTD
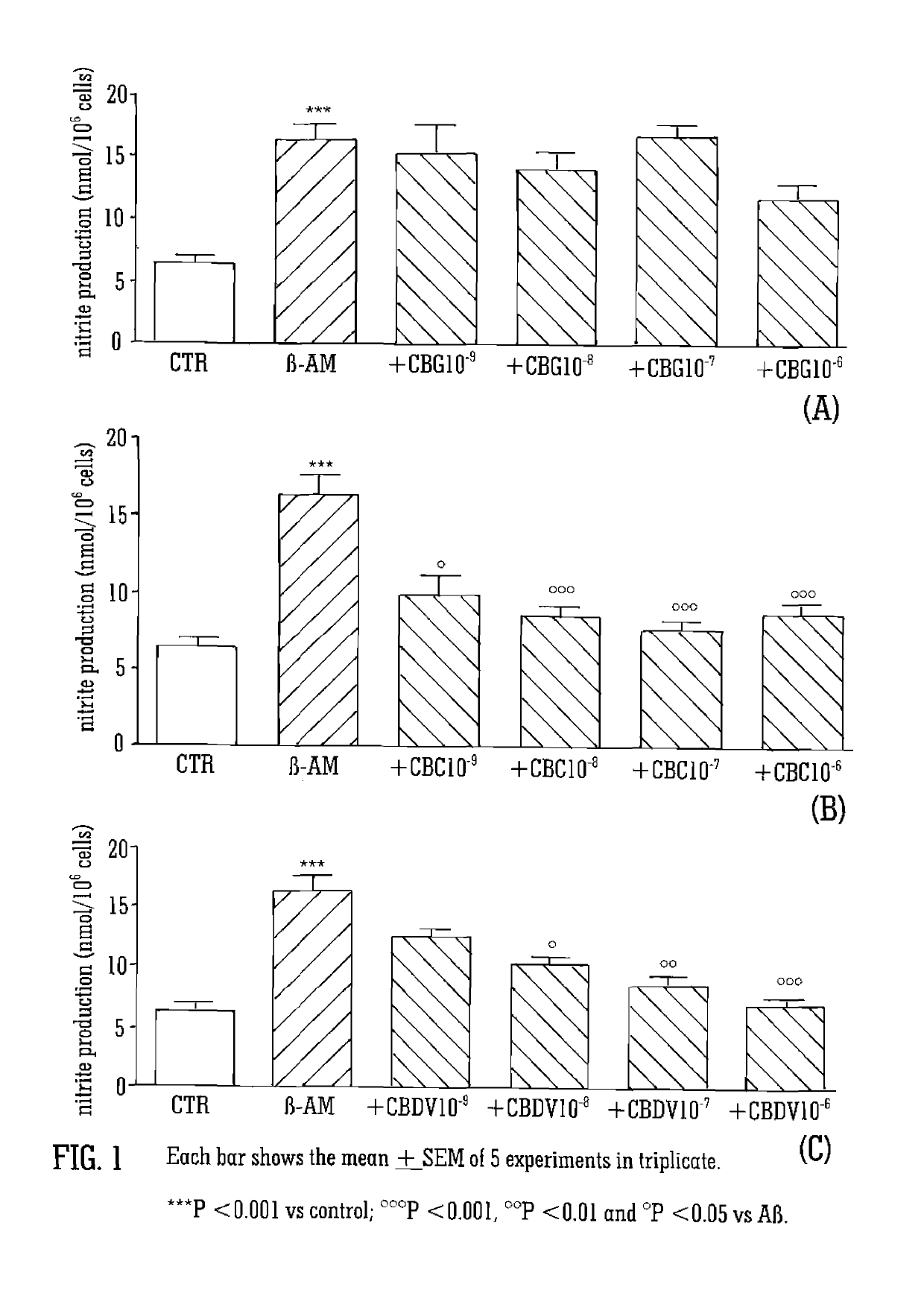
Cannabinoids for use in the treatment of neurodegenerative diseases or disorders
ActiveUS20140228438A1Reduce usageUseful in treatmentBiocideNervous disorderNeuro-degenerative diseasePsychiatry
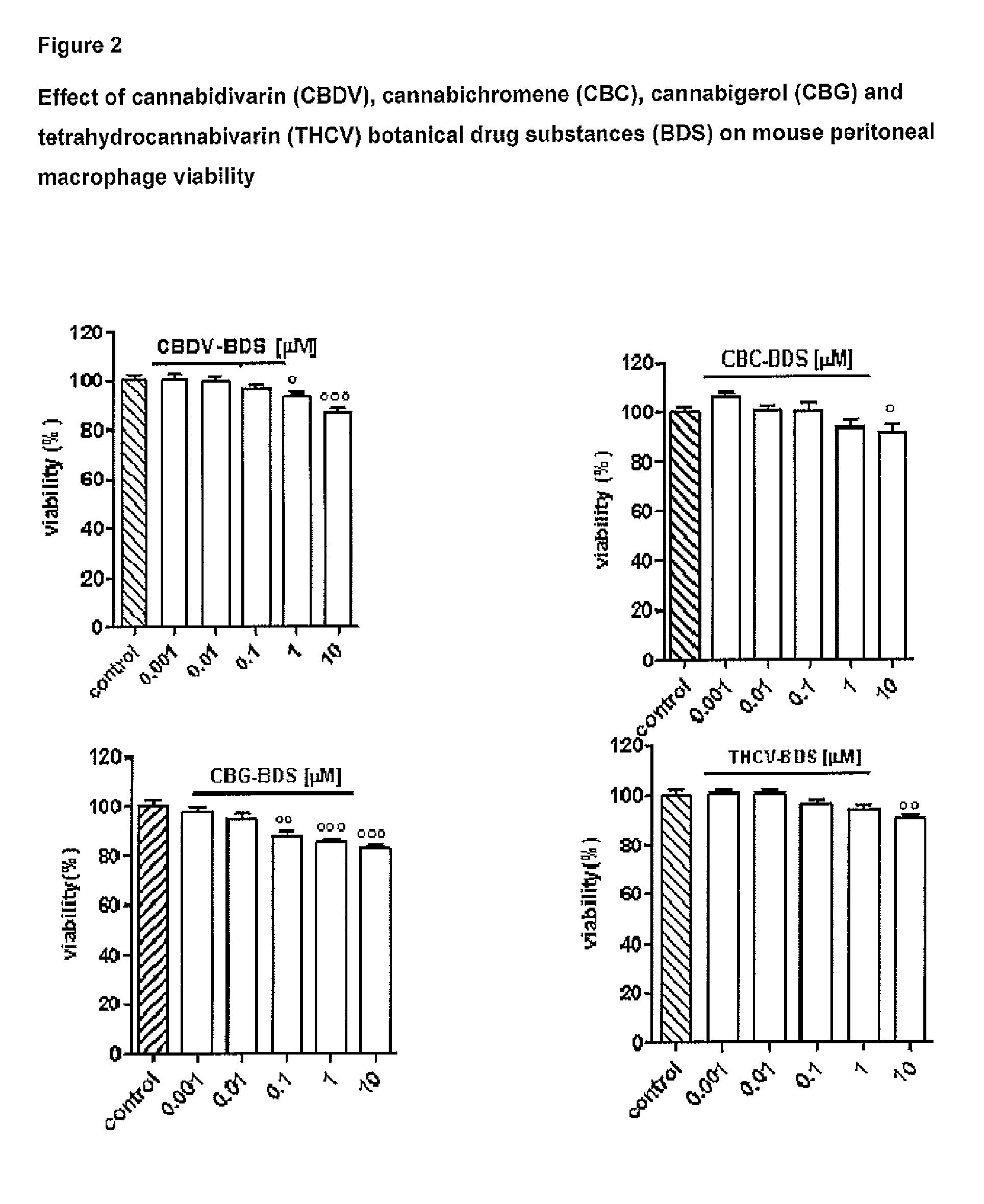
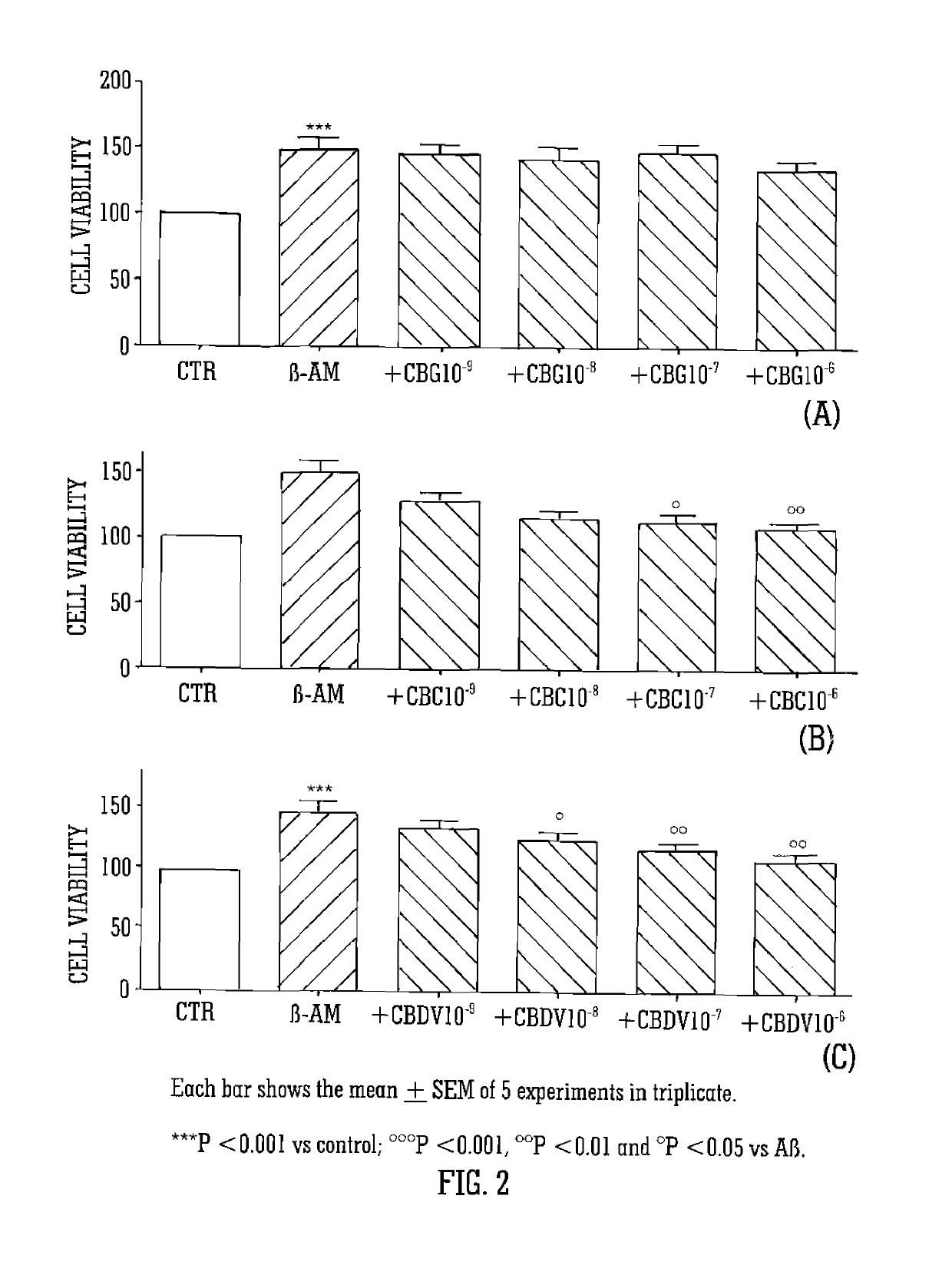
The present invention relates to cannabinoids for use in the prevention or treatment of neurodegenerative diseases or disorders. Preferably the cannabinoids are cannabichromene (CBC) cannabidivarin (CBDV) and / or cannabidivarin acid (CBDVA). More preferably the neurodegenerative disease or disorder to be prevented or treated is Alzheimer's disease.
Owner:GW PHARMA LTD
Phytocannabinoids for use in the treatment of intestinal inflammatory diseases
The present invention relates to one or more of the phytocannabinoids tetrahydrocannabivarin (THCV); cannabigerol (CBG); cannabichromene (CBC); and cannabidivarin (CBDV) for use in the treatment of intestinal inflammatory diseases. Preferably the intestinal inflammatory disease is either ulcerative colitis or Crohn's disease.
Owner:GW PHARMA LTD
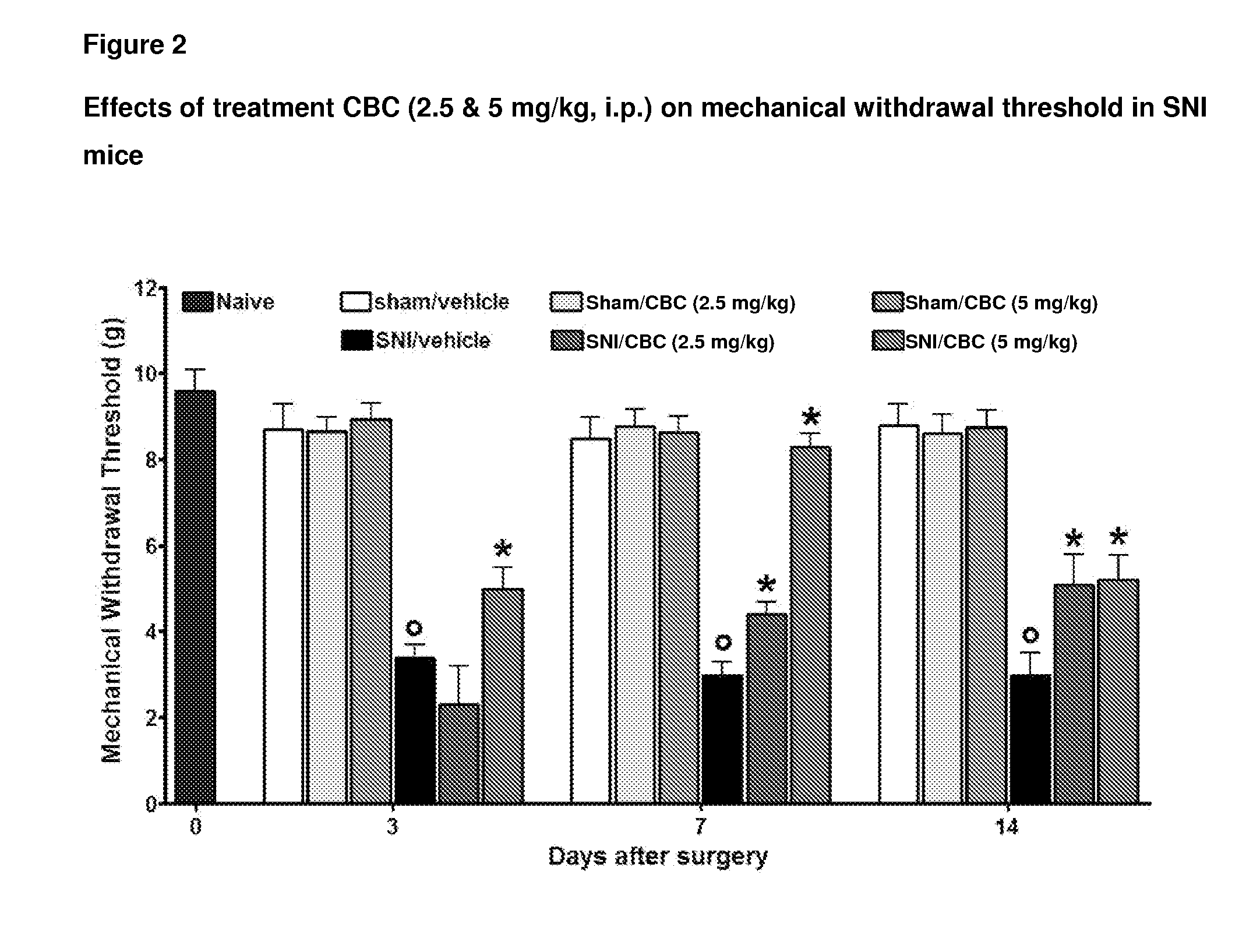
Cannabinoids for use in the treatment of neuropathic pain
ActiveUS20140107192A1Relieve neuropathic painExtended maintenance periodBiocideNervous disorderNeuropathic painCannabichromene
The present invention relates to cannabinoids for use in the treatment of neuropathic pain. Preferably the cannabinoids are one or more phytocannabinoids of: cannabigerol (CBG), cannabichromene (CBC), cannabidivarin (CBDV) or tetrahydrocannabivarin (THCV). More preferably the phytocannabinoids are isolated and / or purified from cannabis plant extracts.
Owner:GW PHARMA LTD
Aminoacid derivatives containing a disulfanyl group in the form of mixed disulfanyl and aminopeptidase n inhibitors
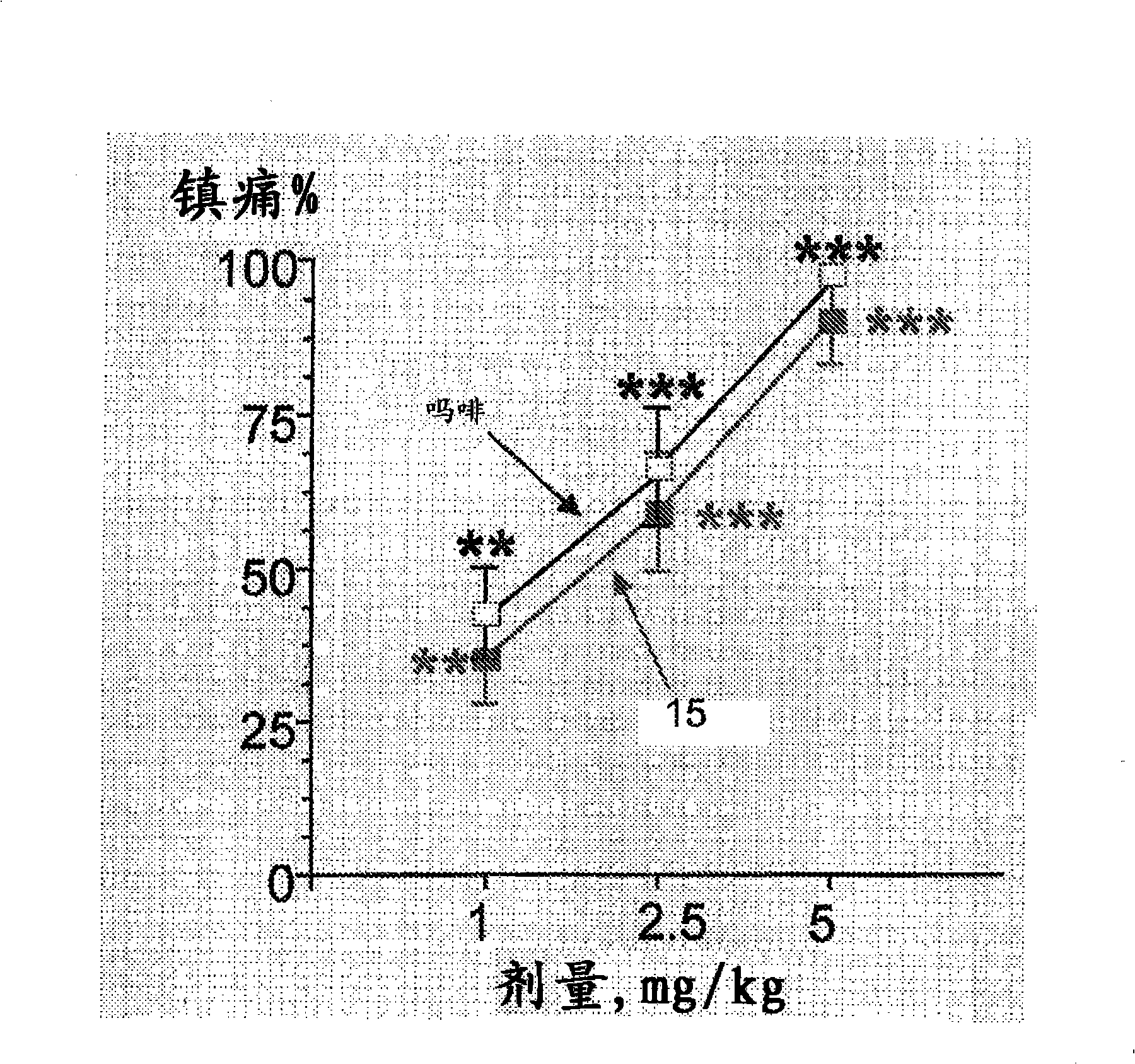
The invention relates to novel compounds of formula (I): H2N-CH(R1)-CH2-S-S- CH2-CH(R2)-CONH-R5, wherein R1 is a hydrocarbon chain, phenyl or benzyl radical, methylene radical substituted by a 5 or 6 atom heterocycle; R2 is a phenyl or benzyl radical, a 5 or 6 atom aromatic heterocycle, methylene group substituted by a 5 or 6 atom heterocycle; R5 is a CH(R3)-COOR4 radical, wherein R3 is hydrogen, an OH or OR group, a saturated hydrocarbon group, a phenyl or benzyl radical and OR4 is hydrophile ester, or 5 or 6 membered heterocycle comprising several heteroatoms selected from a group consisting of nitrogen, sulphur and oxygen, with at least two nitrogene atoms, wherein said heterocycle is substitutable by an alkyl C1-C6, phenyl or benzyl radical.; The use of the inventive compounds in the form of drugs, a pharmaceutical composition comprising said compounds, a pharmaceutically acceptable excipient, the use in conjunction of at least one type of cannabinoid derivative for potentiating the analgesic and antidepressant effect of the novel compounds of formula (I) and / or morphine or the derivatives thereof are also disclosed.
Owner:智腾大中华区有限公司
Use of cannabis to treat fibromyalgia, methods and compositions thereof
InactiveUS20180116998A1Reduce riskAvoid seizuresNervous disorderHydroxy compound active ingredientsCannabisSynthetic cannabinoids
The present invention discloses a composition comprising cannabis natural extract, or synthetic cannabinoids, for use in treating a subject suffering from fibromyalgia. The present invention further discloses methods and uses of the aforementioned composition.
Owner:ONE WORLD CANNABIS LTD
Use of phytocannabinoids in the treatment of ovarian carcinoma
ActiveUS10098867B2Hydroxy compound active ingredientsAntineoplastic agentsOvarian cancerCannabigerolic acid
The present invention relates to the use of phytocannabinoids in the treatment of ovarian cancer. Preferably the phytocannabinoid is selected from the group consisting of: cannabidiol (CBD); cannabidiol acid (CBDA); cannabigerol (CBG); cannabigerolic acid (CBGA); cannabigerol propyl variant (CBGV); and tetrahydrocannabivarin (THCV). In a further embodiment the one or more phytocannabinoids are used in combination with each other. Preferably the combination of cannabinoids consists of CBD and CBG.
Owner:GW PHARMA LTD +1
Composition for treating gynecologic inflammation and application thereof to external use composition
The invention discloses a composition for preventing and / or treating gynecologic inflammation. The composition comprises cannabidiol, cannabidivarin and a carrier, wherein the mass ratio of the cannabidiol to the cannabidivarin is (0.2 to 10):1. The defect of shortage of a composition containing cannabidiol substances for effectively preventing and / or treating gynecologic inflammation in the priorart is overcome. Meanwhile, the composition provided by the invention can also be used for preparing a female sanitation article for preventing and / or treating gynecologic inflammation.
Owner:HANYI BIO TECH CO LTD
Cannabinoid-Containing Fatty Acid Formulations for Treating Disorders of the Nervous System
Pharmaceutical compositions comprising cannabidiol (CBD), tetrahydrocannabinol (THC), and select omega-3 fatty acids are provided. The CBD and THC are present in a weight ratio of CBD:THC of 10:1 to 20:1. The omega-3 fatty acids comprise eicosapentaenoic acid and / or docosahexaenoic acid. Such compositions are useful in the treatment and prevention of traumatic brain injuries, concussions, post-concussive brain injury (PCS), and complications arising from such injuries.
Owner:BODHI RES & DEV INC
Pharmaceutical compositions for the treatment of disease and/or symptoms in arthritis
InactiveUS20160361290A1Powder deliveryNervous disorderDelta-9-tetrahydrocannabinolPharmaceutical drug
The invention relates to the use of a combination of cannabinoids for the treatment of pain, inflammation and / or disease modification in arthritis. Preferably the cannabinoids are selected from cannabidiol (CBD) or cannabidivarin (CBDV) and delta-9-tetrahydrocannabinol (THC) or tetrahydrocannabinovarin (THCV). More preferably the cannabinoids are in a predefined ratio by weight of less than or equal to 19:1 of CBD or CBDV to THC or THCV.
Owner:GW PHARMA LTD
Cannabinoids for use in the treatment of neuropathic pain
Owner:GW PHARMA LTD
Use of cannabidivarin in the treatment of autism spectrum disorder, associated disorders and schizophrenia
InactiveUS20190070128A1Symptoms improvedNervous disorderHydroxy compound active ingredientsCannabisDisease
The present invention relates to the use of cannabidivarin (CBDV) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). In a further embodiment the invention relates to the use of CBDV in the treatment of schizophrenia. CBDV has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS, AS and schizophrenia. The CBDV is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDV is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDV is synthetically produced.
Owner:GW RES LTD
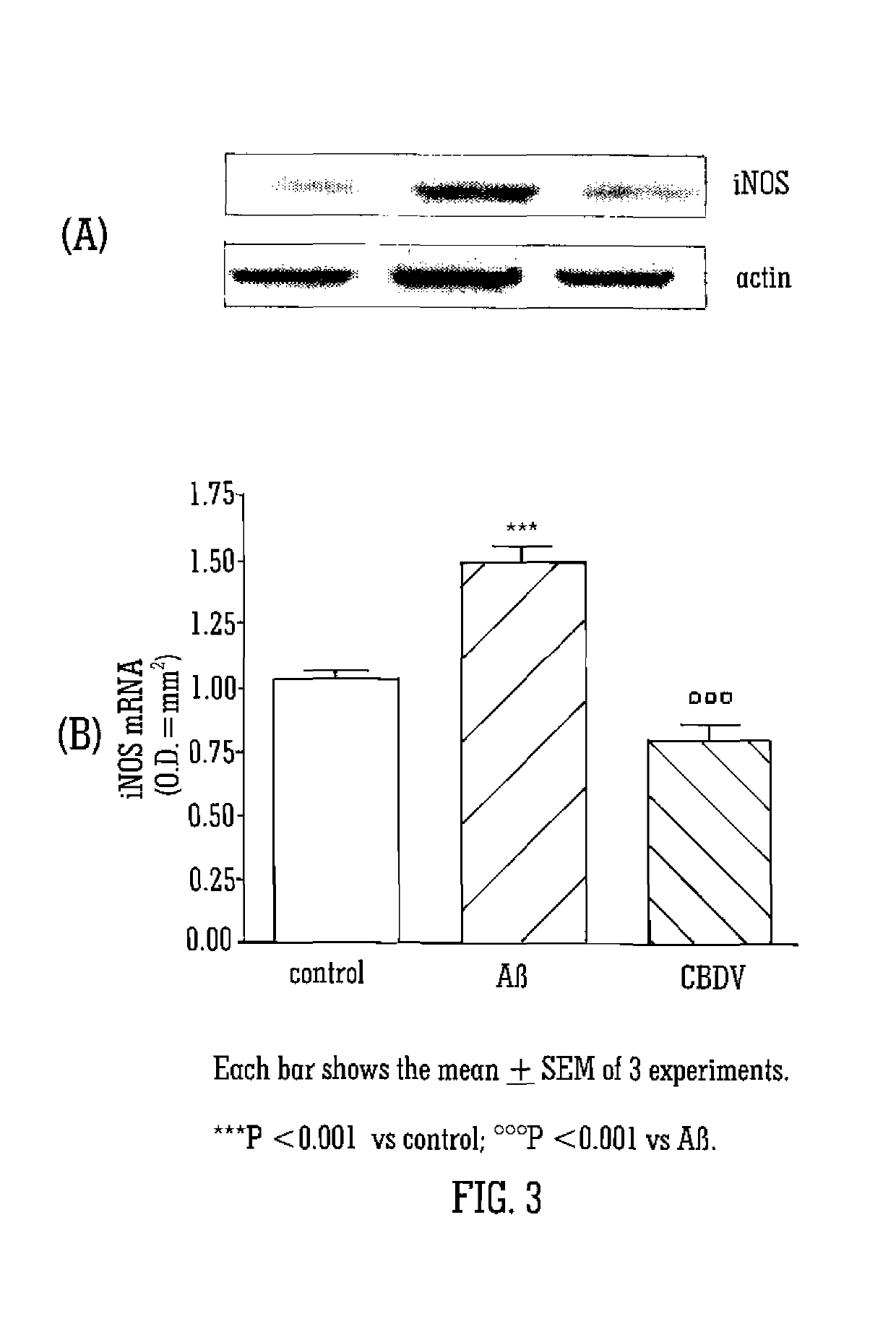
7-oh-cannabidiol (7-oh-cbd) and/or 7-oh-cannabidivarin (7-oh-cbdv) for use in the treatment of epilepsy
The present invention relates to the use of 7-hydroxy-cannabidol (7-OH-CBD) and / or 7-hydroxy-cannabidivarin (7-OH-CBDV) in the treatment of epilepsy. Preferably the cannabinoid metabolites are isolated from plants to produce a highly purified extract or can be reproduced synthetically.
Owner:GW RES LTD
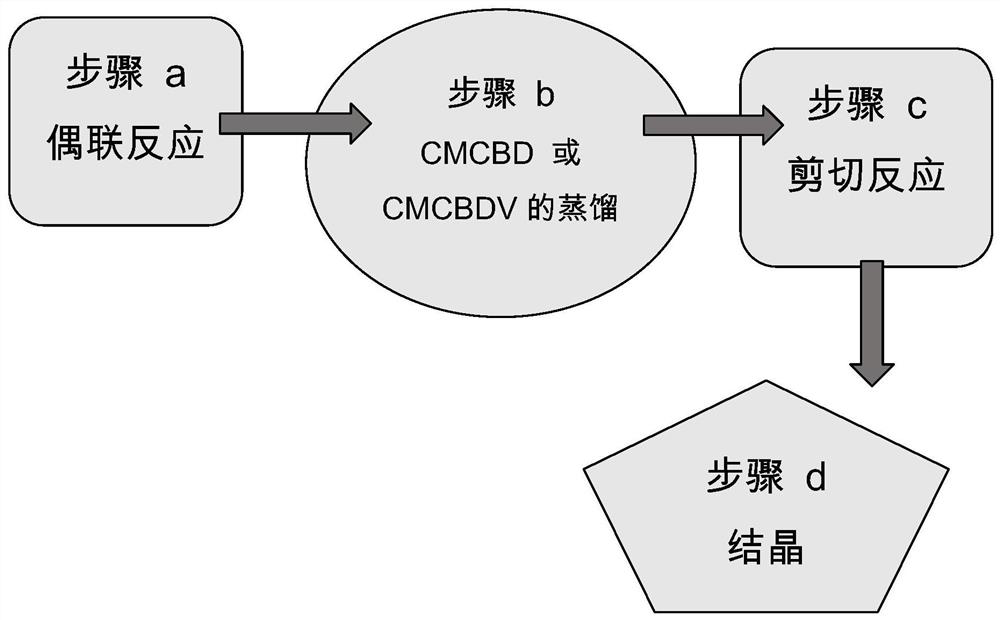
Preparation method of cannabidivarin crystal
ActiveCN113683490AHigh extraction rateImprove recycling ratesOrganic compound preparationOrganic chemistry methodsAlkaline waterOrganic solvent
The invention relates to a preparation method of a cannabidivarin crystal. The method comprises the steps of: (1) extracting industrial cannabis sativa powder with a first solvent, and concentrating a leaching solution containing cannabidivarin obtained after extraction to obtain a concentrated extract; (2) dissolving the concentrated extract in an alkaline aqueous solution, and carrying out saponifying; (3) adding a second solvent into the mixture in the step (2) for first extraction, adding a third solvent into the lower-layer water phase for second extraction, and concentrating an upper-layer extract obtained from the second extraction to obtain a refined substance; (4) dissolving the refined substance in a fourth solvent, performing chromatographic purification, and concentrating the eluent to obtain a cannabidivarin oily substance; and (5) mixing the oily substance with a crystallization solvent, and crystallizing. According to the method, a high-purity cannabidivarin crystal can be obtained at a high total extraction rate, the energy consumption is low, the consumption of the organic solvent is low, the method is more energy-saving and environment-friendly, the method is particularly suitable for industrial application, and the crystal product does not contain addictive components.
Owner:云南翰谷生物科技有限公司
Phytocannabinoids for use in the treatment of intestinal inflammatory diseases
The present invention relates to one or more of the phytocannabinoids tetrahydrocannabivarin (THCV); cannabigerol (CBG); cannabichromene (CBC); and cannabidivarin (CBDV) for use in the treatment of intestinal inflammatory diseases. Preferably the intestinal inflammatory disease is either ulcerative colitis or Crohn's disease.
Owner:GW PHARMA LTD
Method for preparing cannabidiol
PendingCN114057551AHigh extraction rateLess impuritiesOrganic chemistryOrganic compound preparationAlcoholSorbent
The invention discloses a method for preparing secondary cannabidiol, which at least comprises the following steps: a) pretreating a raw material containing industrial hemp to obtain a pretreated material; b) performing alcohol extraction on the pretreated material, concentrating an extracting solution I to obtain a concentrated solution, adding water for decarboxylation, and concentrating II to obtain a dry extract; c) mixing the dry extract and an adsorbent I to form a solid dispersion system, and performing solid-phase extraction to obtain a secondary cannabidiol primary product solution; d) subjecting the crude cannabidiol solution to concentration III, decoloration and crystallization so as to obtain the secondary cannabidiol. The preparation method is simple in process, high in product transfer rate, time-saving, labor-saving, large in treatment capacity, high in product purity and suitable for industrial large-scale production.
Owner:YUNNAN HEMPMON PHARMA CO LTD
The use of phytocannabinoids in the treatment of ovarian carcinoma
ActiveUS20160136128A1Reduce ovarian cancer cell viabilityBiocideHydroxy compound active ingredientsOvarian cancerCannabigerolic acid
The present invention relates to the use of phytocannabinoids in the treatment of ovarian cancer. Preferably the phytocannabinoid is selected from the group consisting of: cannabidiol (CBD); cannabidiol acid (CBDA); cannabigerol (CBG); cannabigerolic acid (CBGA); cannabigerol propyl variant (CBGV); and tetrahydrocannabivarin (THCV). In a further embodiment the one or more phytocannabinoids are used in combination with each other. Preferably the combination of cannabinoids consists of CBD and CBG.
Owner:GW PHARMA LTD +1
Methods of manufacturing cannabidiol or cannabidivarin and intermediates of manufacturing cannabidiol or cannabidivarin
PendingCN113950467AOrganic compound preparationOrganic chemistry methodsOrganic chemistryMedicinal chemistry
Methods of manufacturing cannabidiol (CBD) and cannabidivarin (CBDV); intermediates of the methods of manufacturing CBD and CBDV; and crystallized CBD and CBDV obtained via described methods.
Owner:베누비아 매뉴팩처링 엘엘씨
Use of cannabidivarin in the treatment of autism spectrum disorder, associated disorders and schizophrenia
The present invention relates to the use of cannabidivarin (CBDV) in the treatment of autism spectrum disorder (ASD) and ASD-associated disorders such as Fragile X syndrome (FXS); Rett syndrome (RS); or Angelman syndrome (AS). In a further embodiment the invention relates to the use of CBDV in the treatment of schizophrenia. CBDV has been shown to be particularly effective in improving cognitive dysfunction in rodent models of ASD, FXS, RS, AS and schizophrenia. The CBDV is preferably substantially pure. It may take the form of a highly purified extract of cannabis such that the CBDV is present at greater than 95% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBDV is synthetically produced.
Owner:GW RES LTD
Cannabinoids for use in the treatment of neurodegenerative diseases or disorders
ActiveUS10258580B2Nervous disorderHydroxy compound active ingredientsNeuro-degenerative diseasePsychiatry
The present invention relates to cannabinoids for use in the prevention or treatment of neurodegenerative diseases or disorders. Preferably the cannabinoids are cannabichromene (CBC) cannabidivarin (CBDV) and / or cannabidivarin acid (CBDVA). More preferably the neurodegenerative disease or disorder to be prevented or treated is Alzheimer's disease.
Owner:GW PHARMA LTD
Preparation method of special chromatographic medium for separating cannabidiol
PendingCN114602438AHigh puritySimple processOrganic chemistryOrganic compound preparationChromatographic separationPtru catalyst
The invention relates to a preparation method of a special chromatographic medium for separation of sec-cannabidiol, which comprises the following steps: (1) reacting elemental bromine, sec-cannabidiol and a catalyst to obtain a brominated sec-cannabidiol intermediate; (2) adding a certain amount of porous amino silicon balls into an organic solvent, and fully stirring to completely wet the porous amino silicon balls; and (3) adding the brominated cannabidiol intermediate and a catalyst into the solution, and heating to react. And after the reaction is finished, filtering and drying to obtain the special chromatographic separation medium. According to the invention, secondary cannabidiol is bonded to a silicon ball to form the surface modified silica gel chromatographic filler; the medium has high-selectivity adsorption retention on the cannabidiol, has the advantages of good selectivity and low cost, and is suitable for industrial purification of the cannabidiol.
Owner:SOOCHOW HIGH TECH CHROMATOGRAPHY
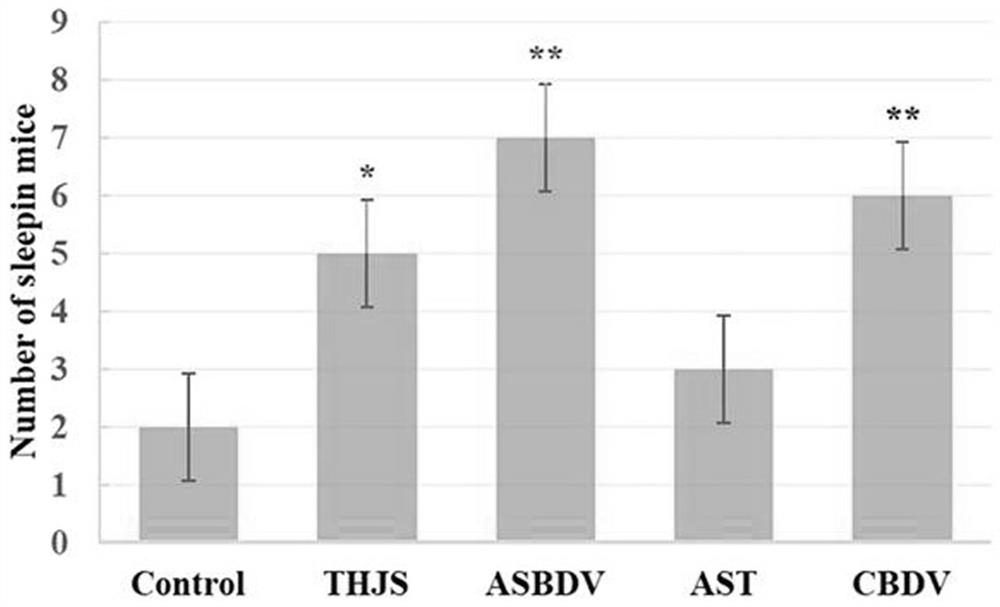
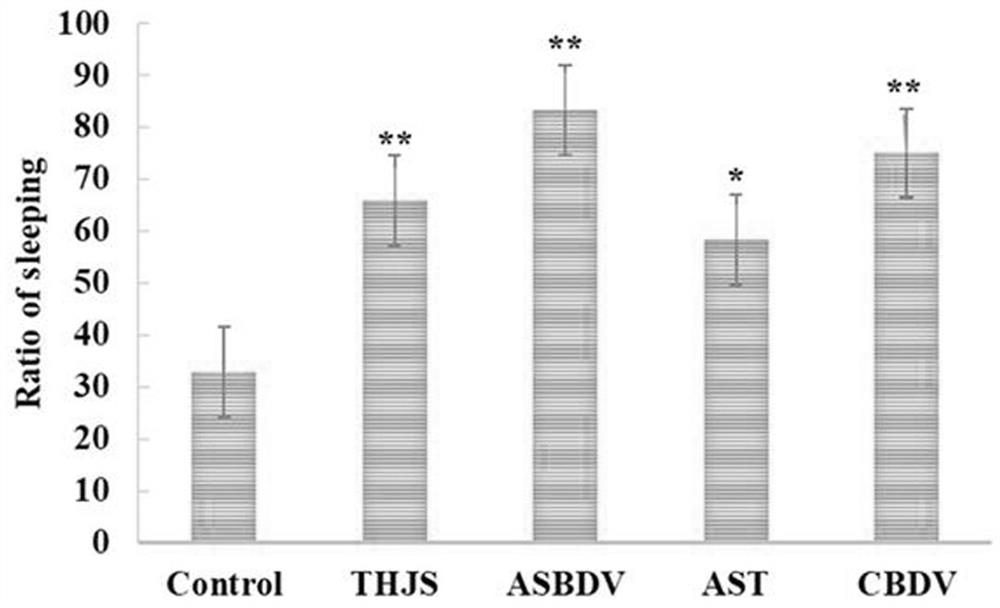
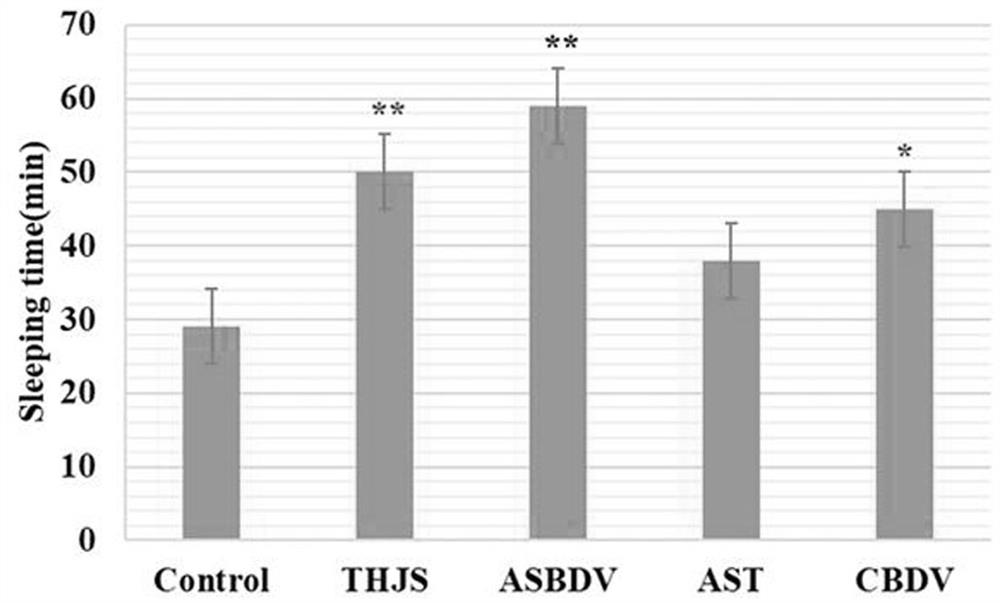
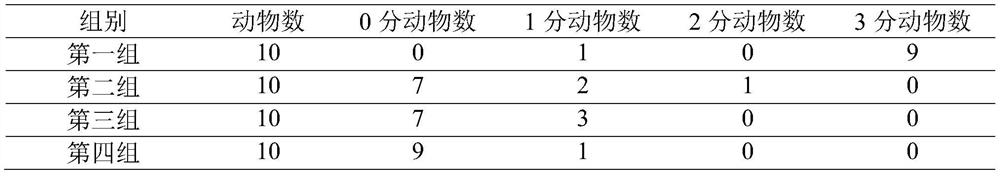
ASBDV composition for improving sleep disorders, and preparation and application thereof
The invention discloses an ASBDV composition for improving sleep disorders, and a preparation and an application thereof. The ASBDV composition for improving sleep disorders is composed of astaxanthin and cannabidiol. The preparation of the ASBDV composition for improving sleep disorders is a tablet, an oral liquid, a capsule, a granule, a powder, a pill, an injection, a film or a patch prepared by adding auxiliary materials to the ASBDV composition. The application is an application of the ASBDV composition for improving sleep disorders in the preparation of products for preventing / improving sleep disorders. The astaxanthin and cannabidivarol composition (ASBDV) has an obvious improvement effect on sleep disorders.
Owner:云南维他源生物科技有限公司
A composition for treating gynecological inflammation and its application in external composition
Owner:HANYI BIO TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com