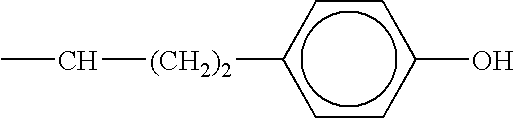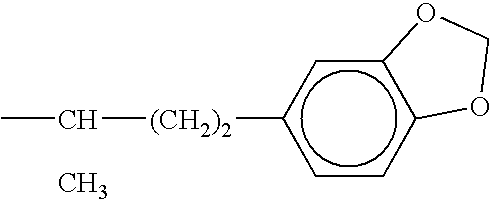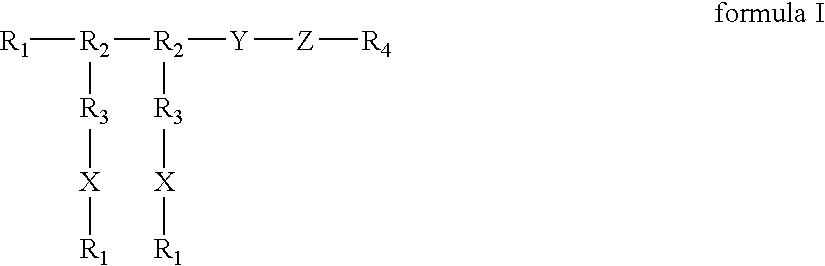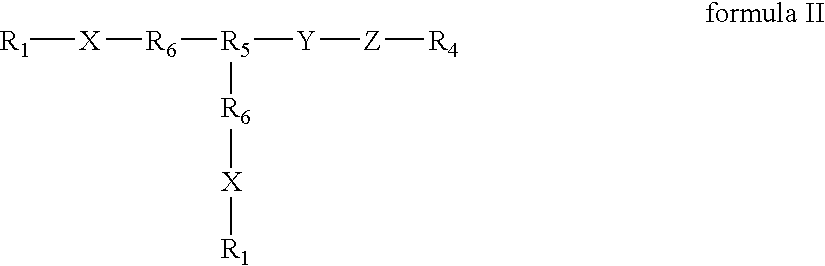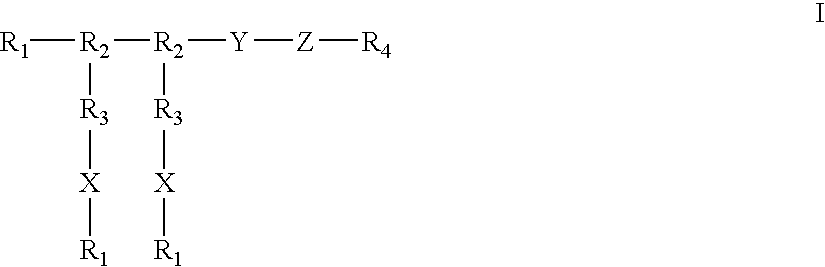Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
210results about How to "Low effective dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
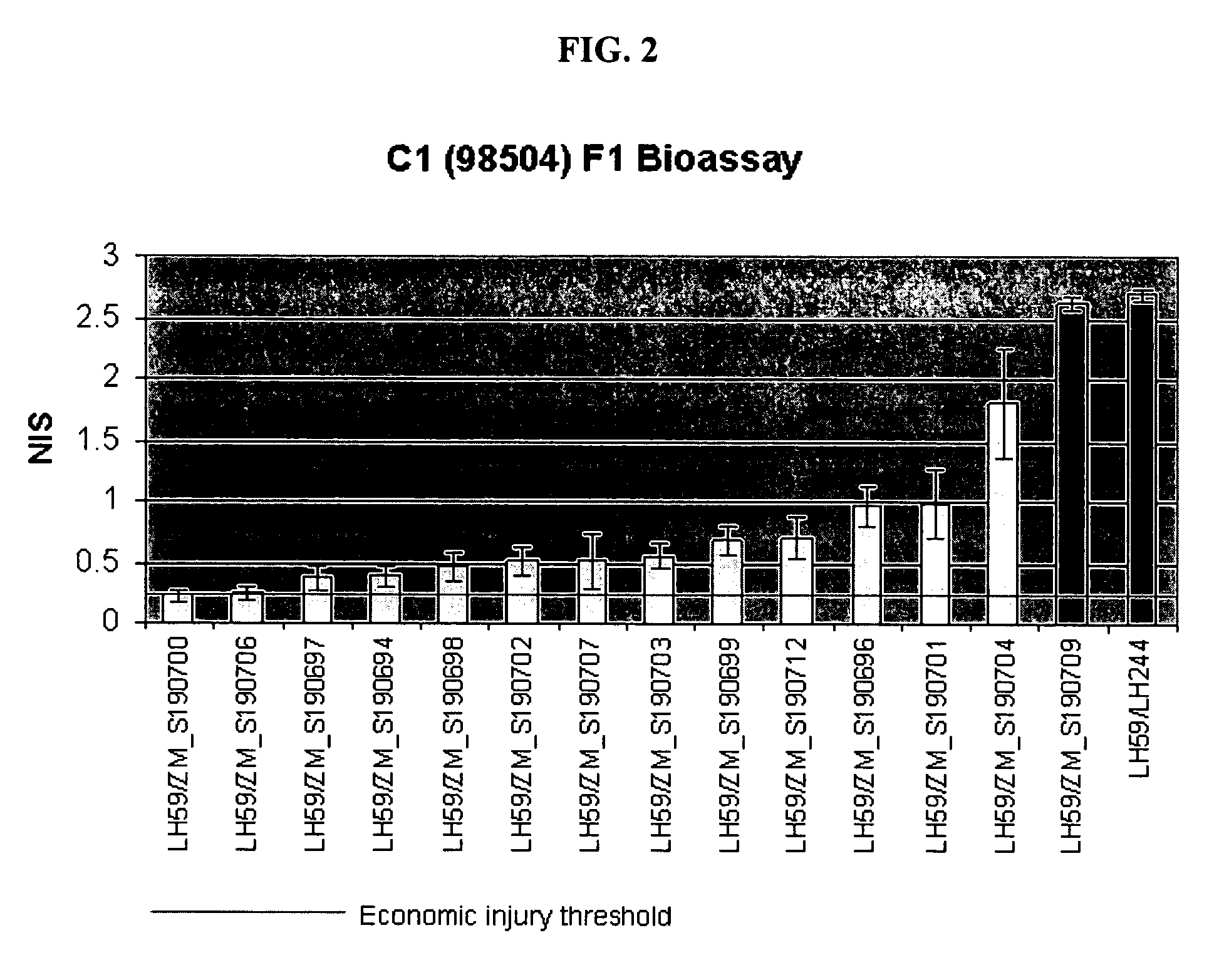
Compositions and methods for control of insect infestations in plants
InactiveUS20060021087A1Limiting and eliminating invertebrateInhibit expressionBiocideSugar derivativesInvertebrateOrganism
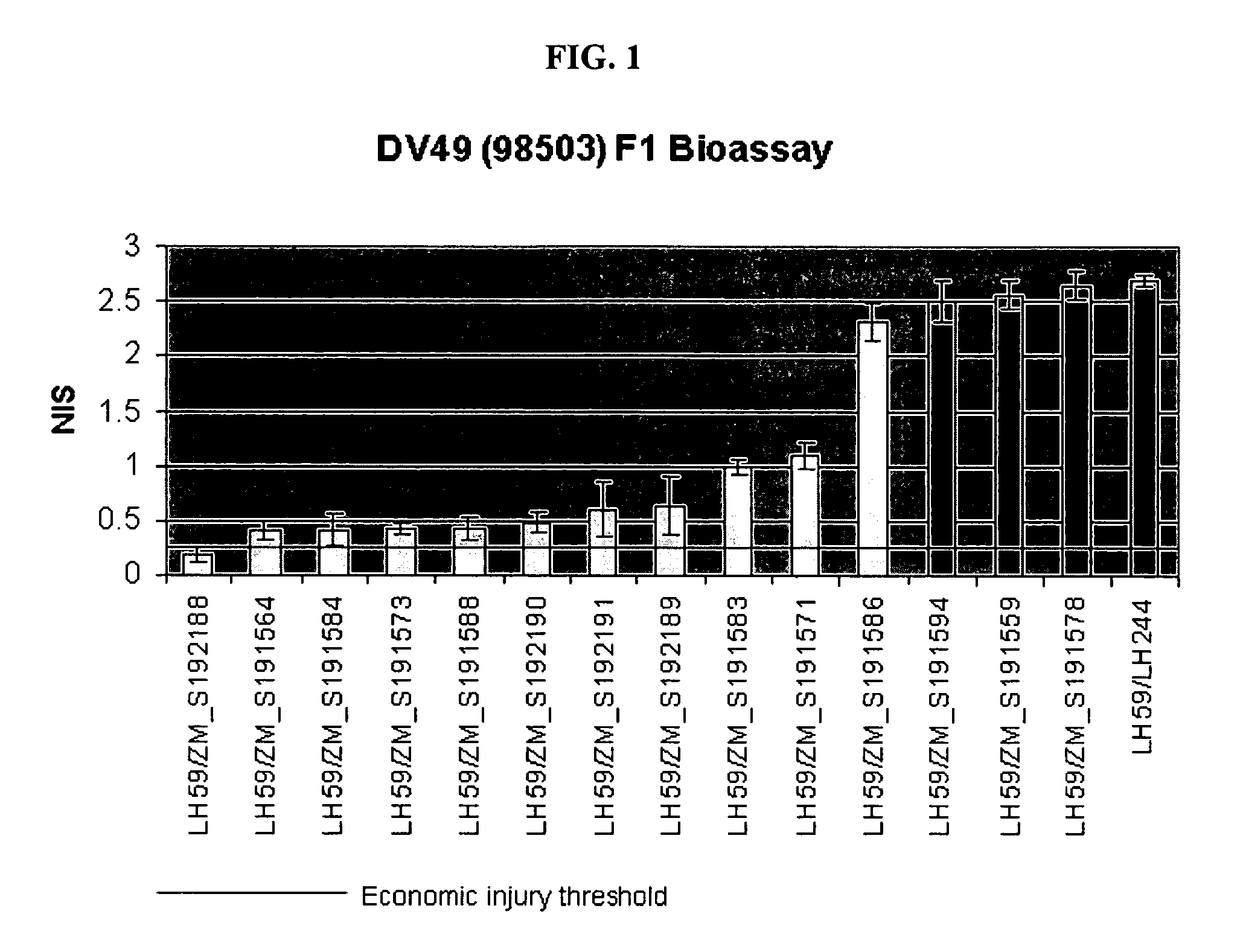
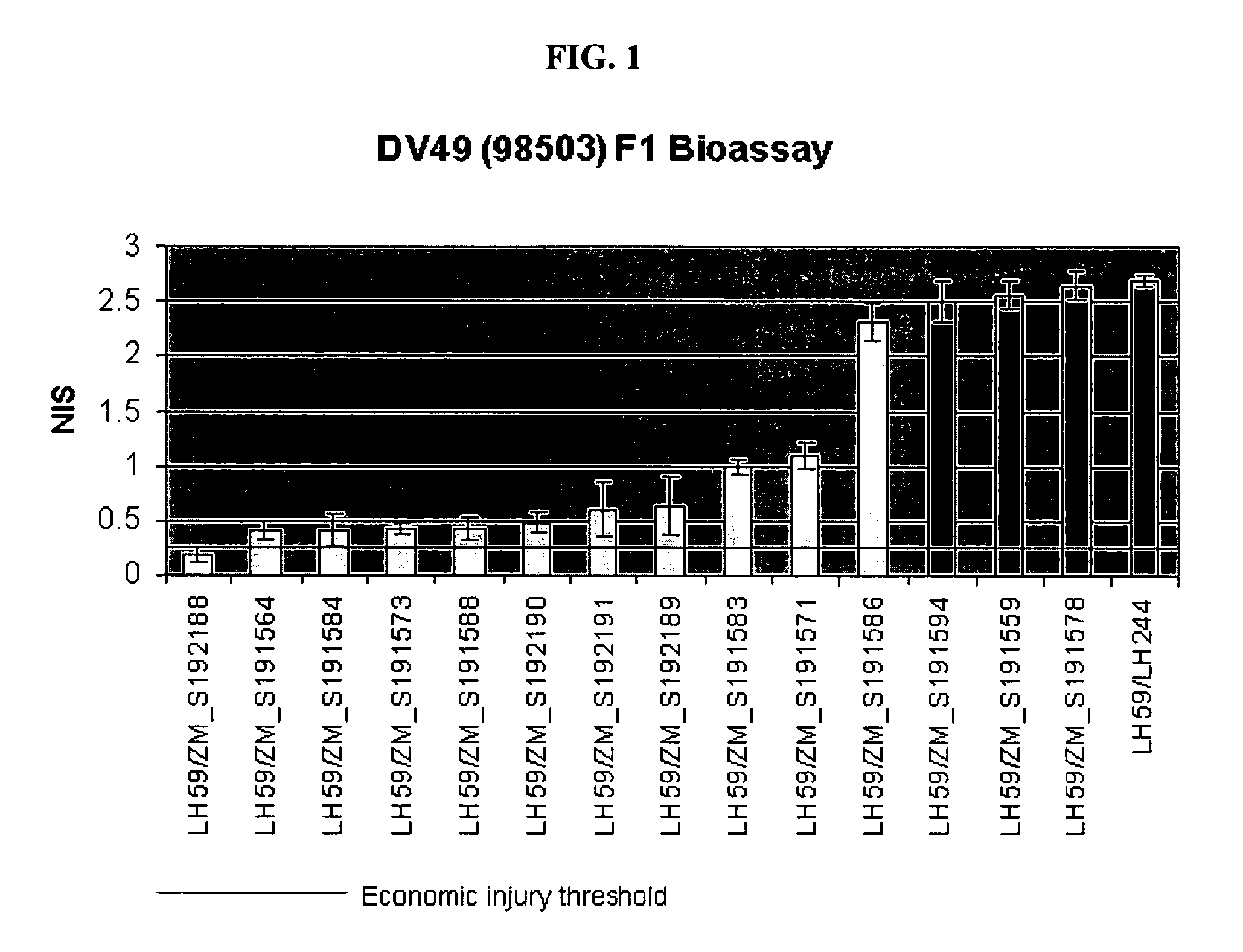
The present invention is directed to controlling pest infestation by inhibiting one or more biological functions in an invertebrate pest. The invention discloses methods and compositions for use in controlling pest infestation by feeding one or more different recombinant double stranded RNA molecules to the pest in order to achieve a reduction in pest infestation through suppression of gene expression. The invention is also directed to methods for making transgenic plants that express the double stranded RNA molecules, and to particular combinations of transgenic pesticidal agents for use in protecting plants from pest infestation.
Owner:MONSANTO TECH LLC
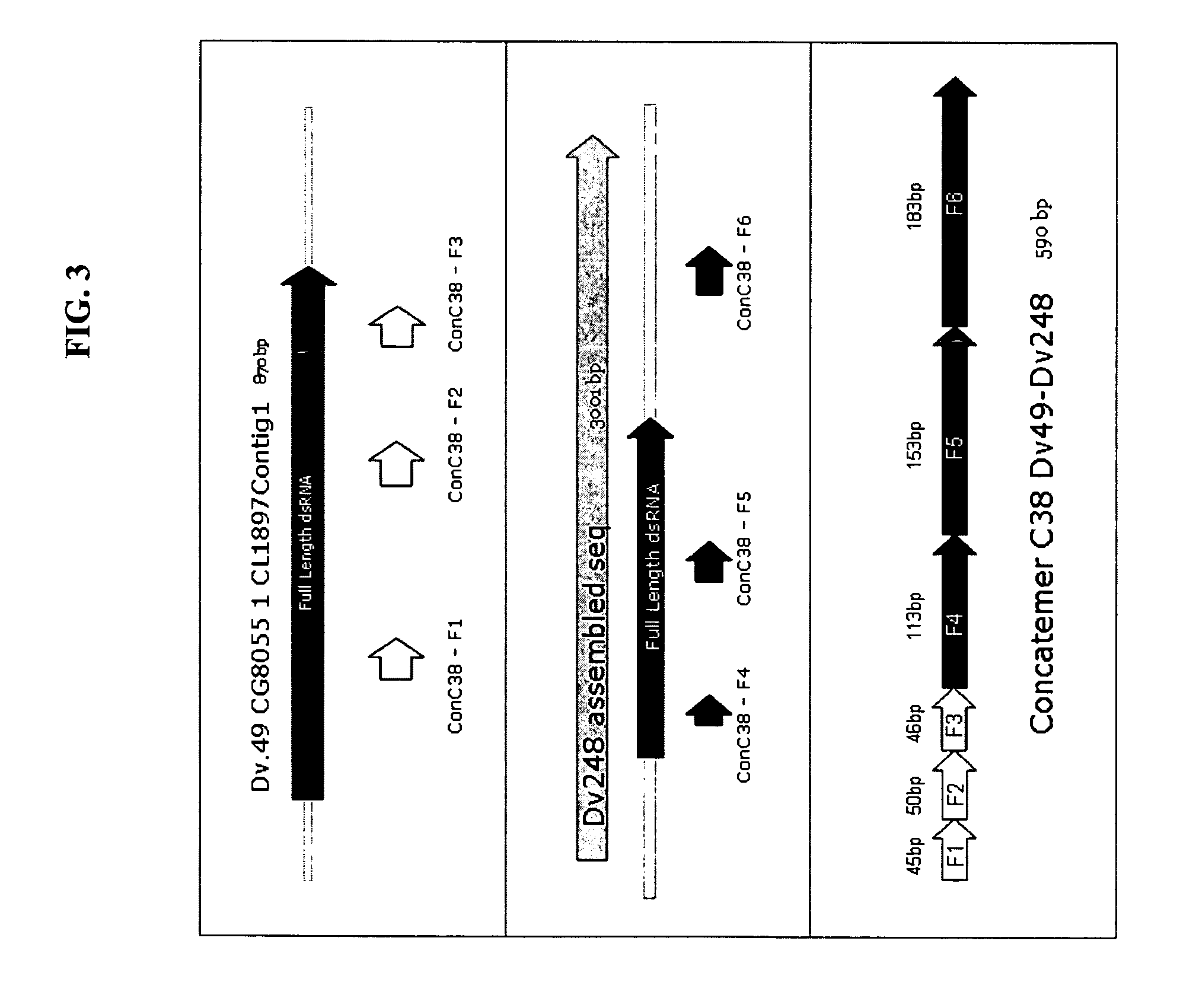
Methods for genetic control of insect infestations in plants and compositions thereof
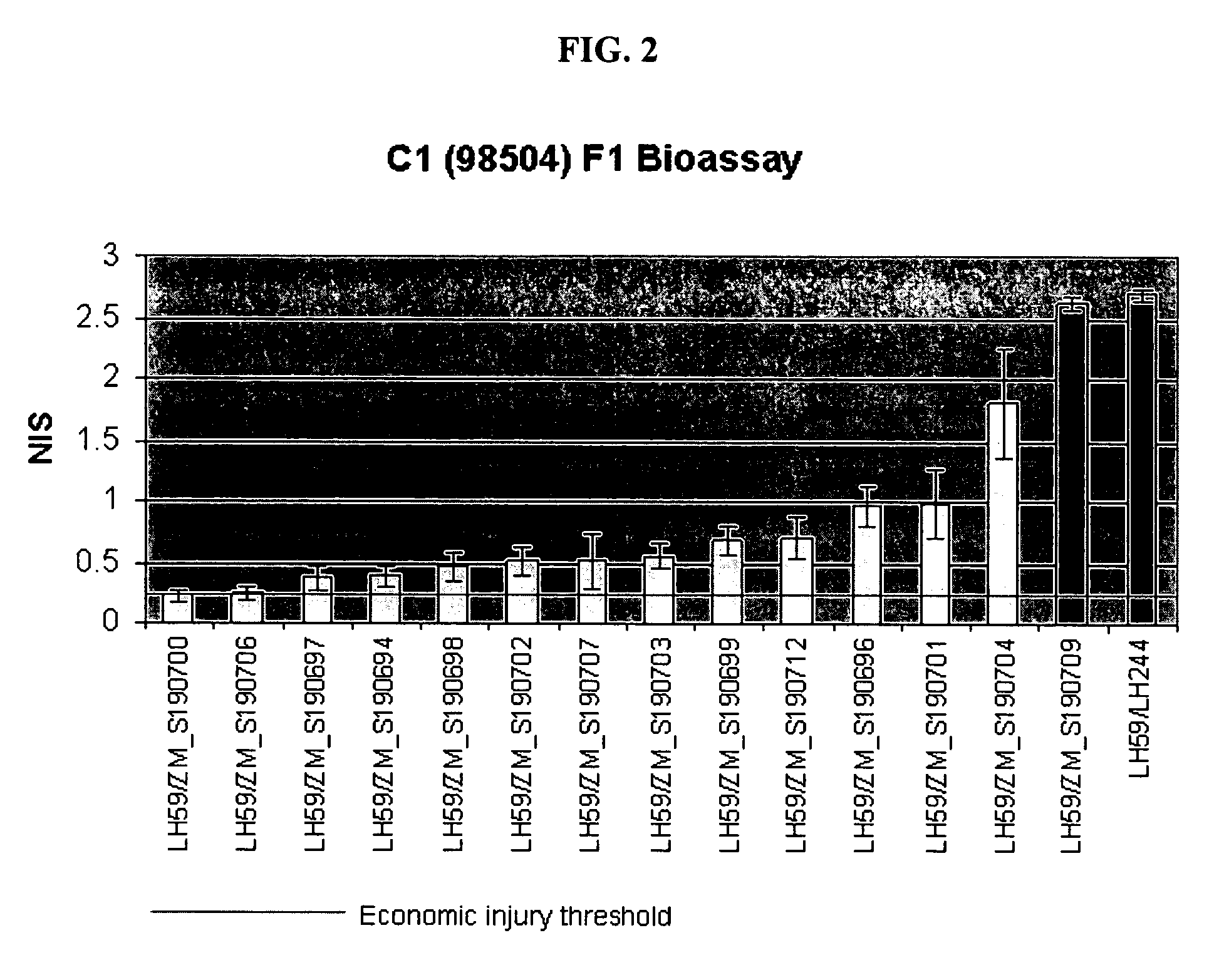
ActiveUS20070124836A1Inhibit expressionReduced expression levelSugar derivativesClimate change adaptationBiotechnologyDouble strand
The present invention relates to control of pest infestation by inhibiting one or more biological functions. The invention provides methods and compositions for such control. By feeding one or more recombinant double stranded RNA molecules provided by the invention to the pest, a reduction in pest infestation is obtained through suppression of gene expression. The invention is also directed to methods for making transgenic plants that express the double stranded RNA molecules, and to particular combinations of transgenic pesticidal agents for use in protecting plants from pest infestation.
Owner:MONSANTO TECH LLC
Methods for genetic control of insect infestations in plants and compositions thereof
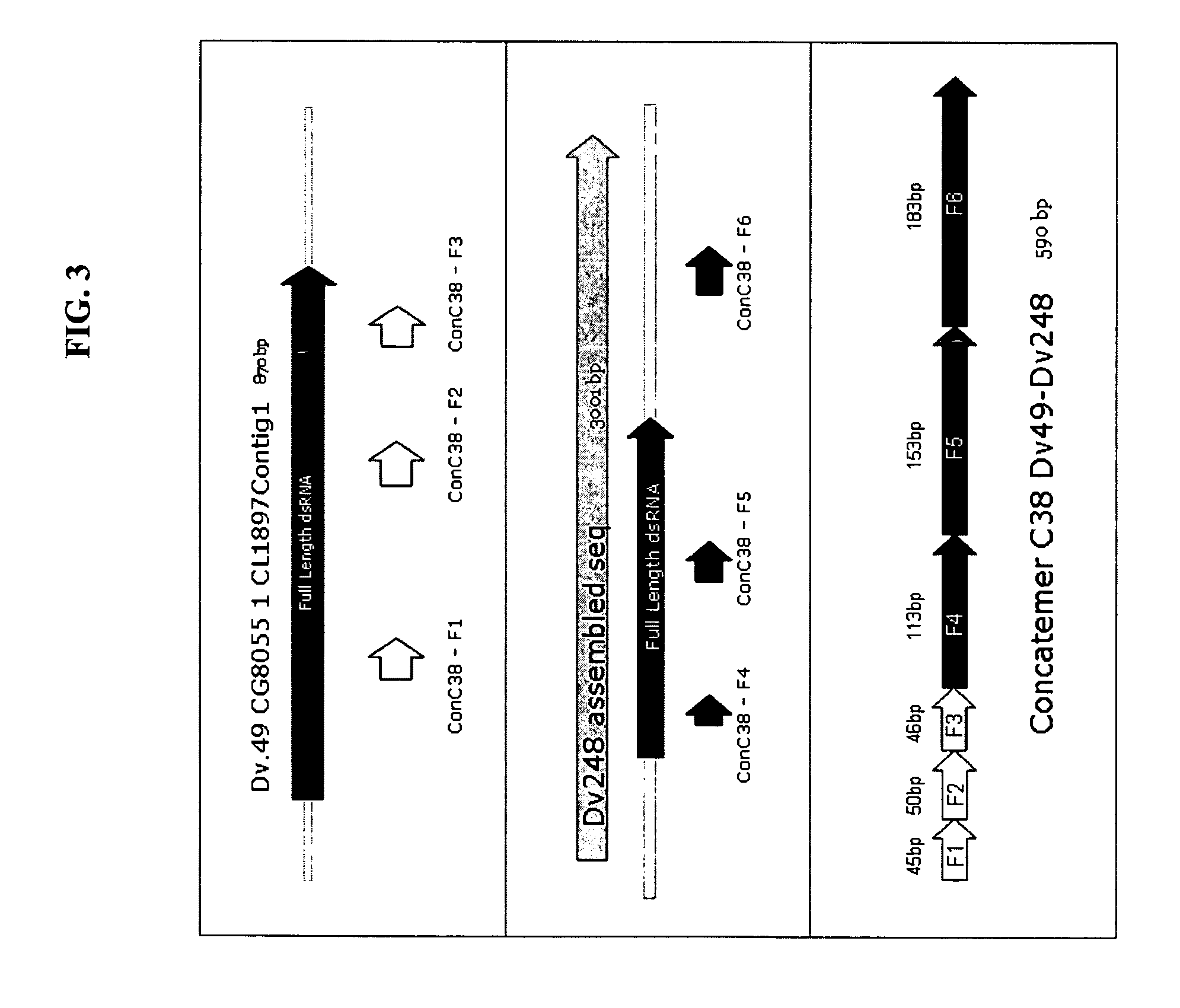
ActiveUS7943819B2Reduced expression levelLow effective doseSugar derivativesClimate change adaptationGMO PlantsDouble stranded rna
Owner:MONSANTO TECH LLC
Compositions for treating CNS disorders
InactiveUS20110262442A1Improve blood-brain barrier permeabilityGood effectBiocideNervous disorderDiseaseCombination therapy
The present invention provides combination therapies for treating a disease, disorder, or condition, and methods thereof.
Owner:ADENIOS
Composition and Method of Treatment of Bacterial Infections
InactiveUS20090061009A1Effective treatmentImprove antibacterial propertiesAntibacterial agentsBiocideNanoparticleAntibacterial drug
The invention is intended for a treatment of severe infections using an injectable drug-delivery system comprising nanoparticles of a biodegradable polymer with incorporated antibacterial drug.
Owner:ALPHARX
Compositions and Methods for Targeted in Vitro and in Vivo Drug Delivery to Mammalian Cells Via Bacterially Derived Intact Minicells
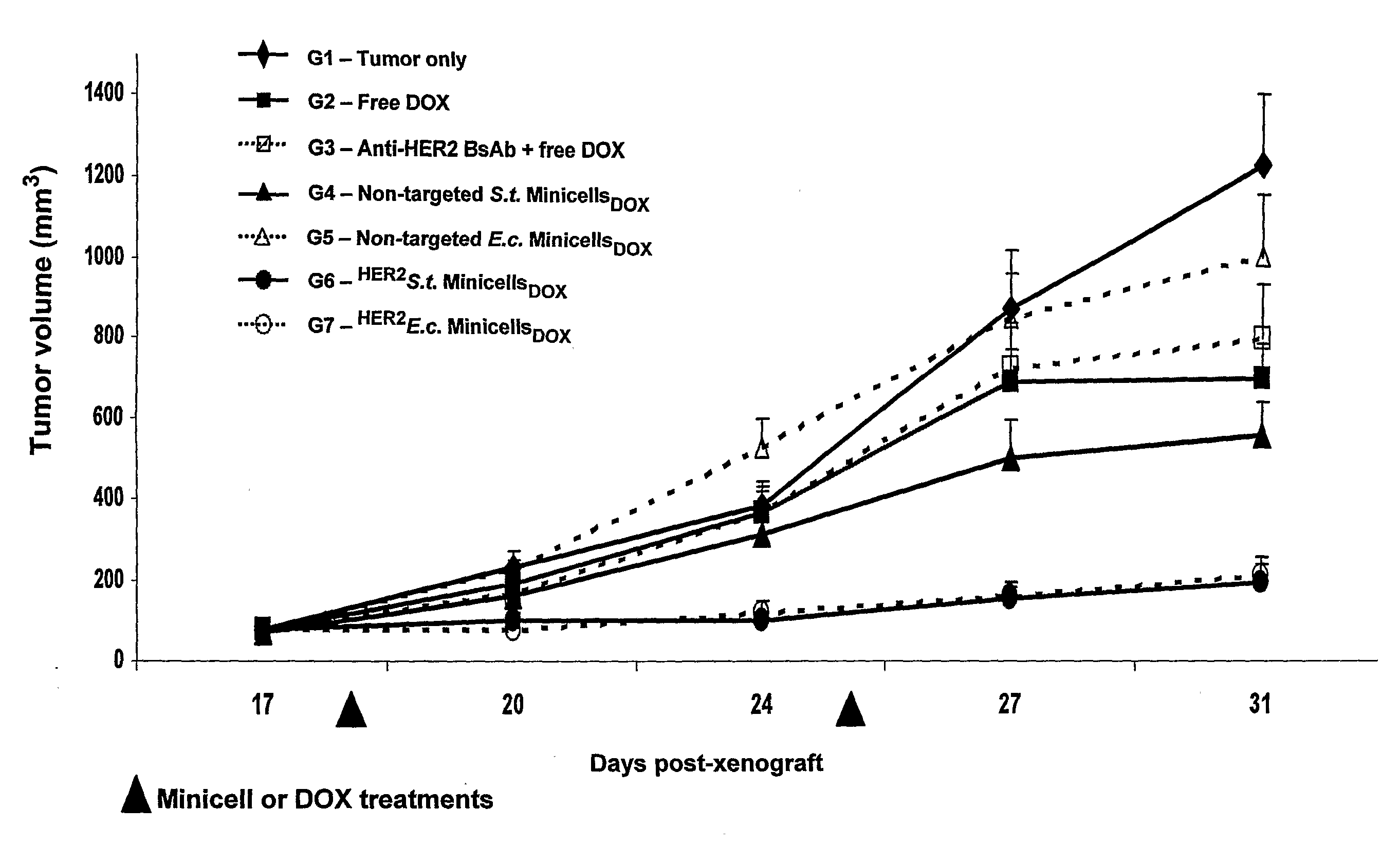
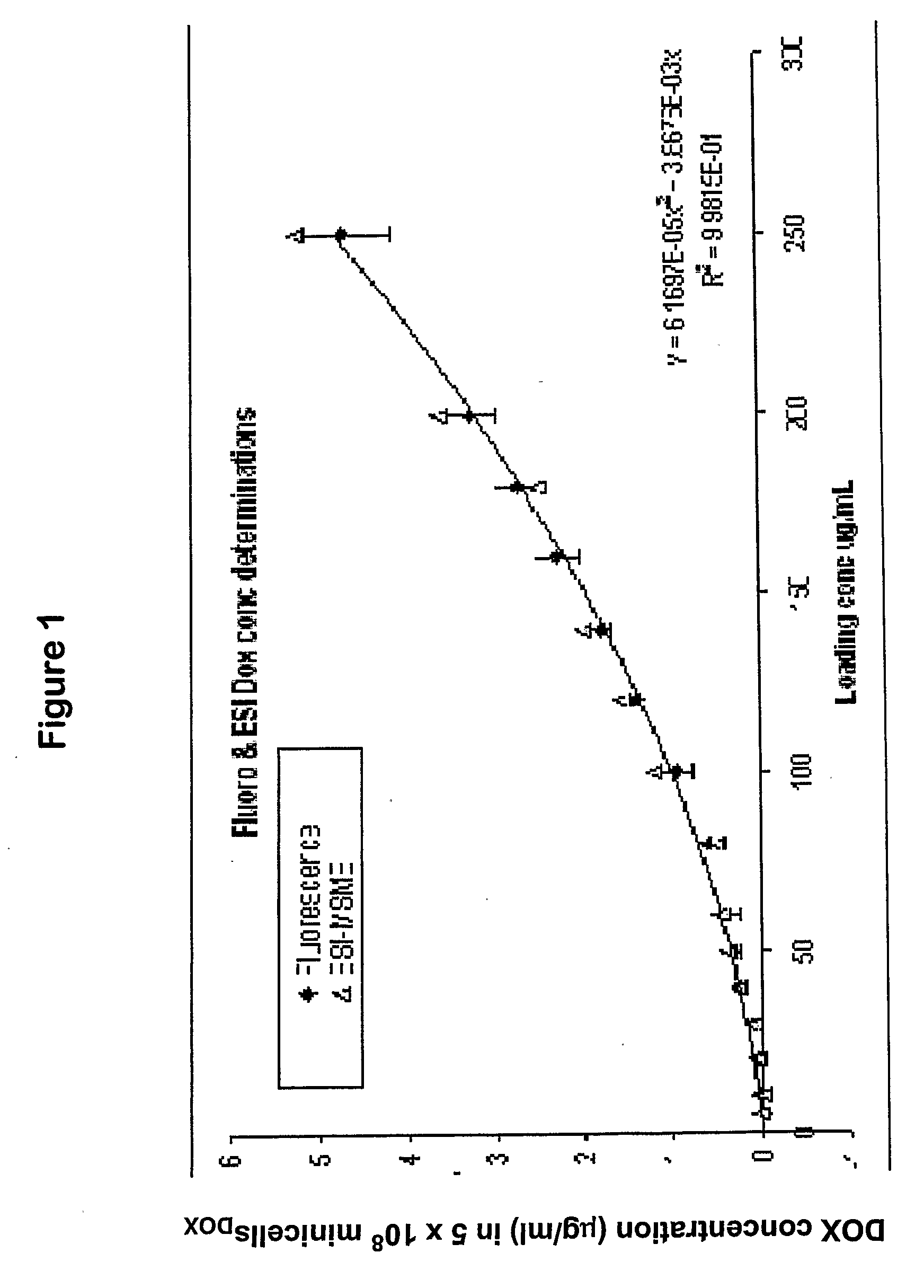
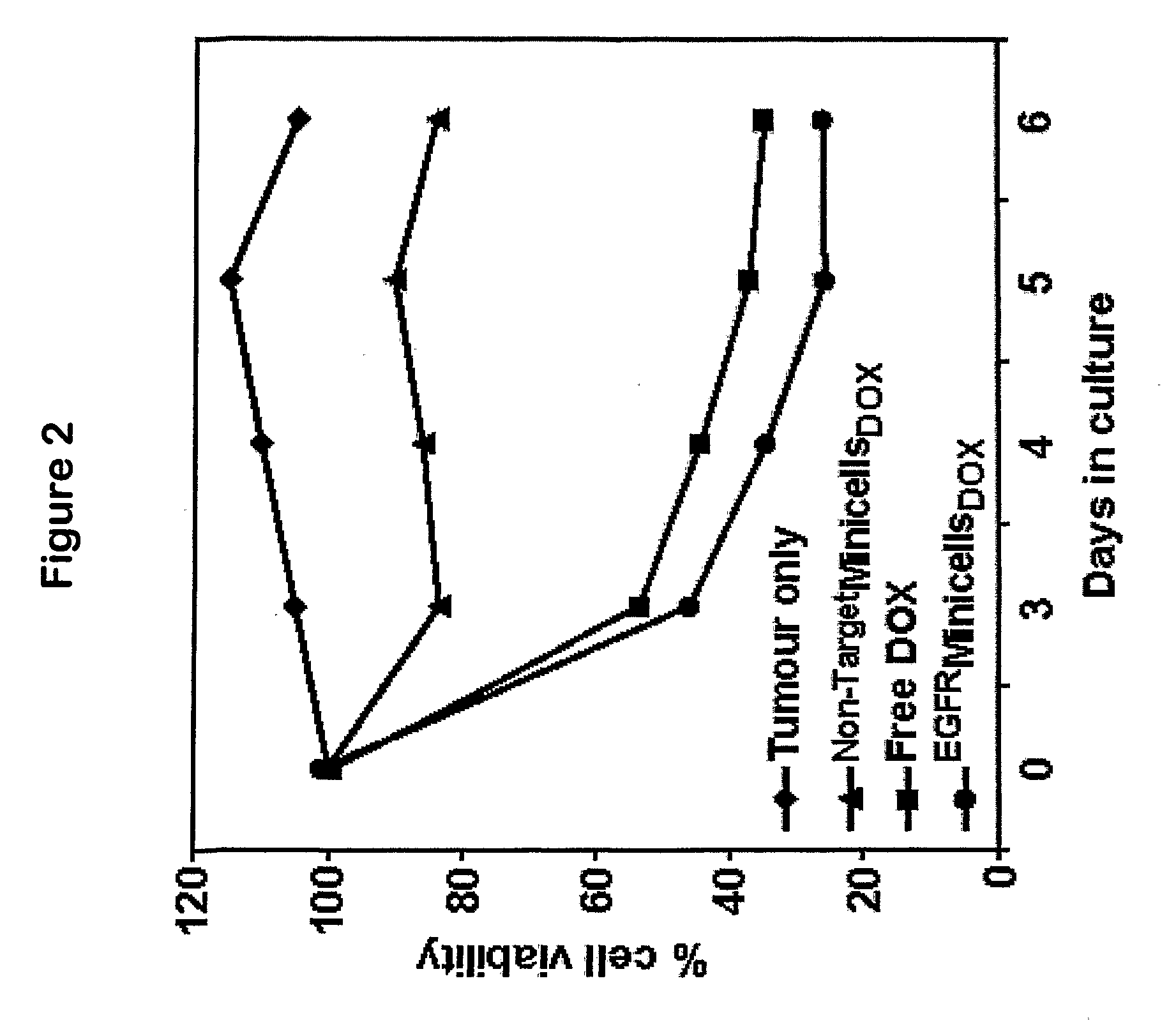
ActiveUS20080051469A1Low toxicityEliminate side effectsOrganic active ingredientsBiocideDrug targettingIn vivo
A composition comprising intact minicells that contain a drug molecule is useful for targeted drug delivery. One targeted drug delivery method employs bispecific ligands, comprising a first arm that carries specificity for a bacterially derived minicell surface structure and a second arm that carries specificity for a mammalian cell surface receptor, to target drug-loaded minicells to specific mammalian cells and to cause endocytosis of the minicells by the mammalian cells. Another drug delivery method exploits the natural ability of phagocytic mammalian cells to engulf minicells without the use of bispecific ligands.
Owner:ENGENEIC MOLECULAR DELIVERY PTY LTD
Compositions and Methods for Control of Insect Infestations in Plants
ActiveUS20080214443A1Inhibit expressionLower Level RequirementsBiocideOrganic active ingredientsDouble strandDouble stranded rna
The present invention is directed to controlling pest infestation by inhibiting one or more biological functions in an invertebrate pest. The invention discloses methods and compositions for use in controlling pest infestation by feeding one or more different recombinant double stranded RNA molecules to the pest in order to achieve a reduction in pest infestation through suppression of gene expression. The invention is also directed to methods for making transgenic plants that express the double stranded RNA molecules, and to particular combinations of transgenic pesticidal agents for use in protecting plants from pest infestation.
Owner:MONSANTO TECH LLC
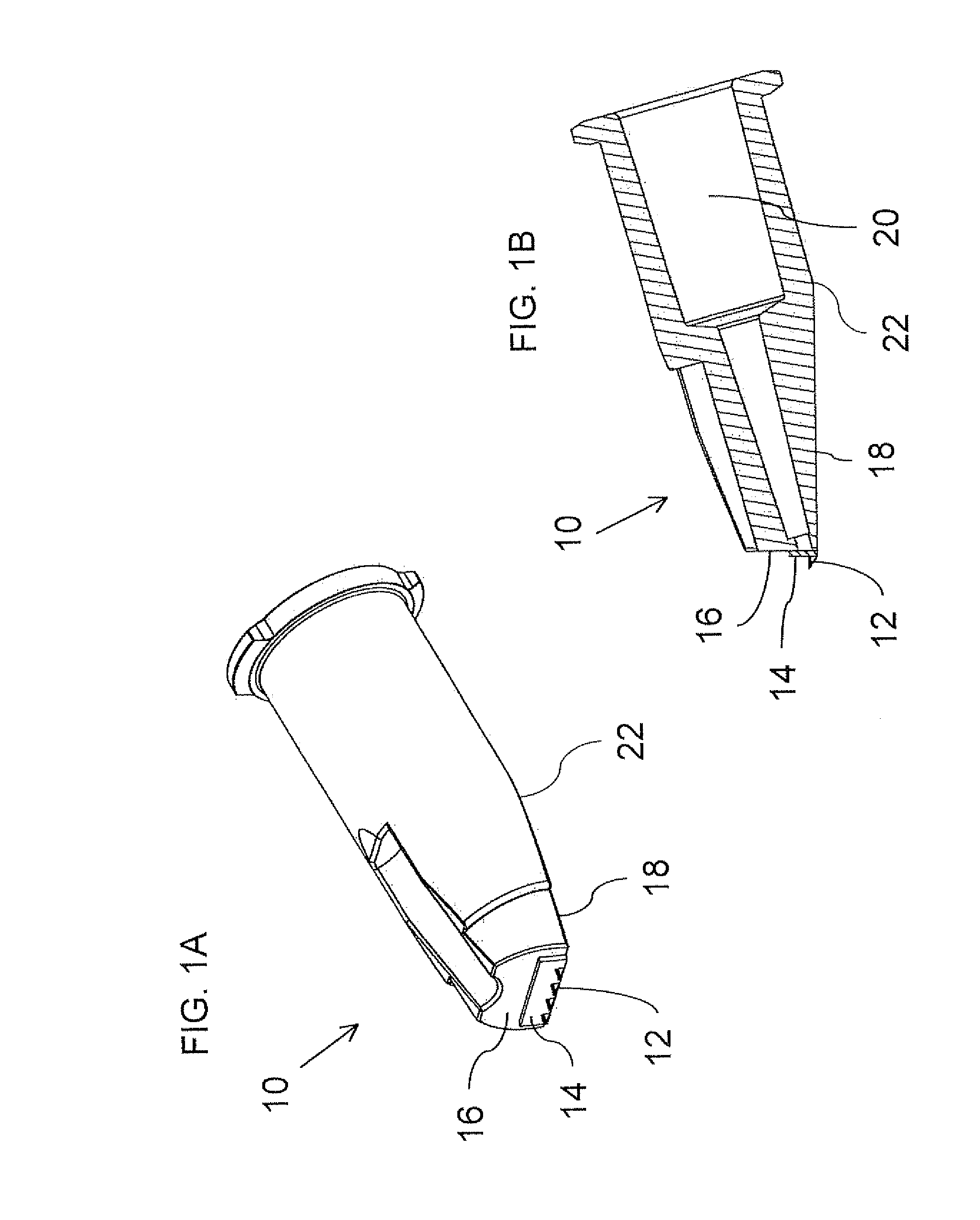
Intradermal delivery of biological agents
InactiveUS20090012494A1Significant comprehensive benefitsMinimal expertiseBiocidePeptide/protein ingredientsActive agentWhole body
The present invention relates to methods for intradermally delivering one or more biologically active agents such as vaccines and therapeutic agents into the dermis layer of the skin of a subject to obtain systemic delivery or an immune response using a microneedle drug delivery device containing the agent to be delivered. The methods employ a microneedle device with a row of hollow microneedles. The microneedles penetrate the skin of the subject and assume an anchored state in which the microneedles are anchored in the skin and project laterally from the device. A pivotal motion is then performed with the device so that the skin in which the microneedles are engaged is lifted above the initial plane of the surface of the skin while the biologically active agent is delivered. The methods of the invention elicit increased humoral and / or cellular response as compared to conventional vaccine delivery routes, facilitating dose sparing.
Owner:NANOPASS TECH LTD
Combination therapy for effecting weight loss and treating obesity
InactiveUS7056890B2Efficient and effective treatmentReduce effectBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSDrug
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss.The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
Treatment of HIV and other viral infections using combinatorial therapy
InactiveUS6475491B1Good curative effectLow toxicityBiocidePeptide/protein ingredientsAntiviral drugNon toxicity
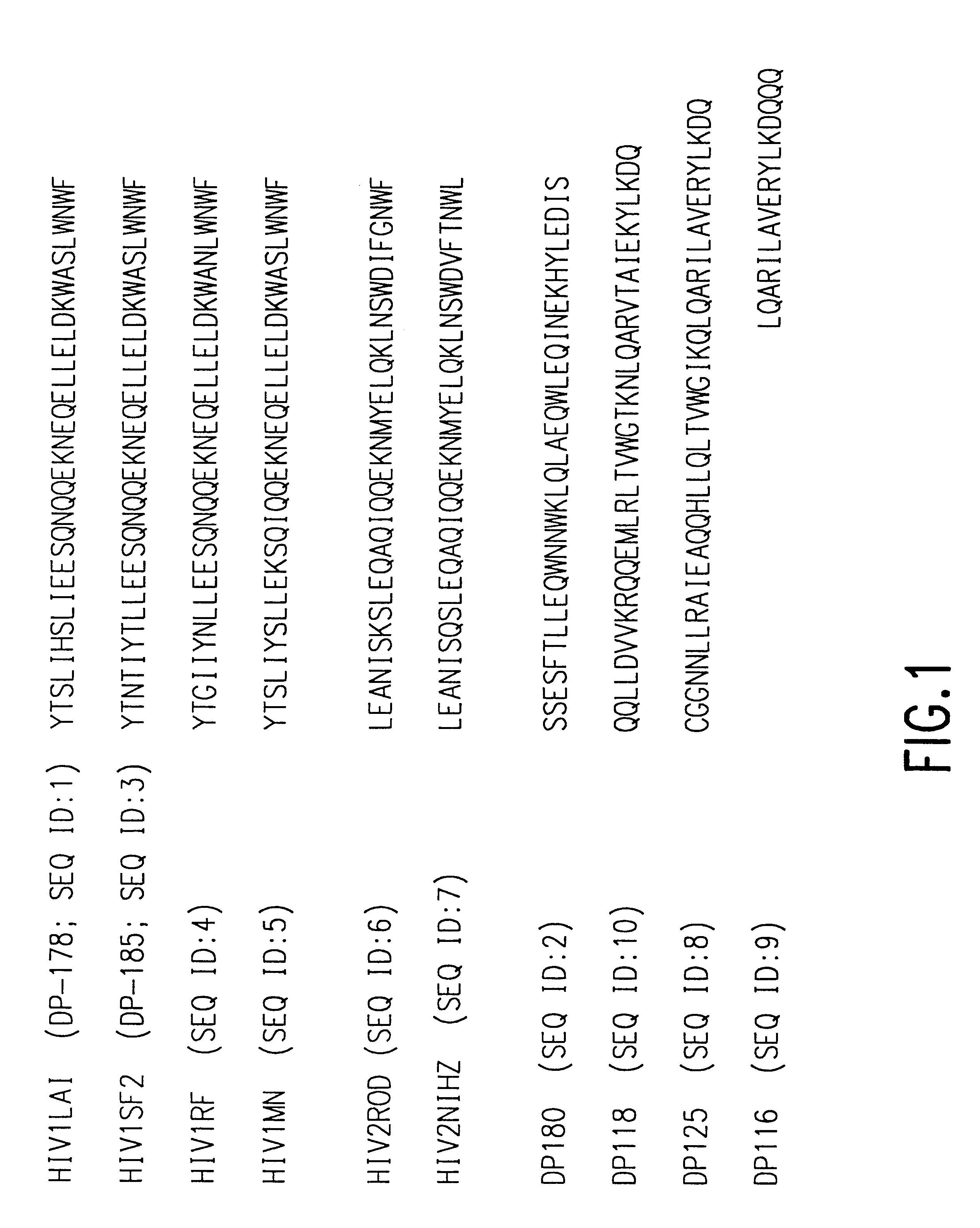
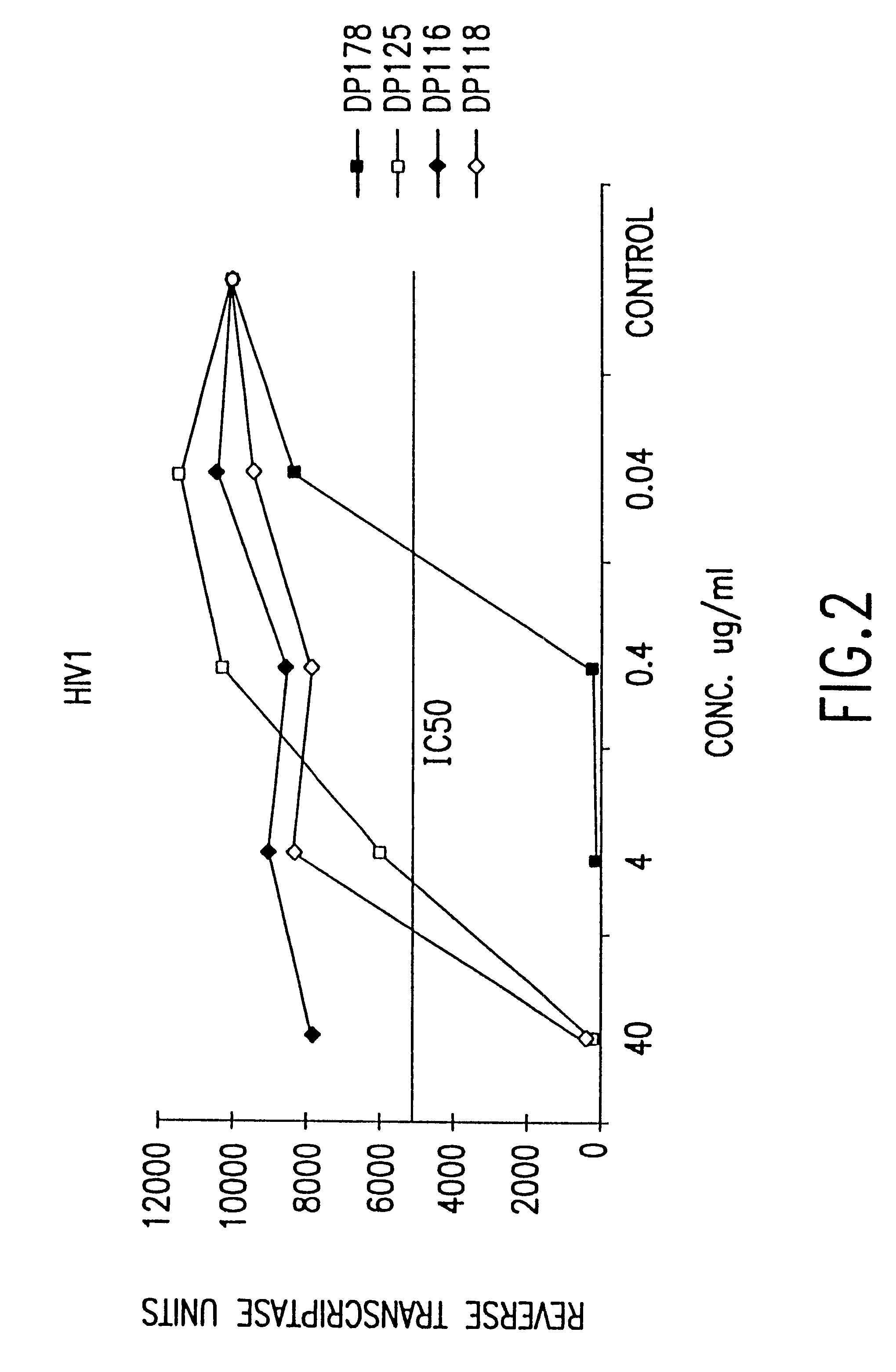
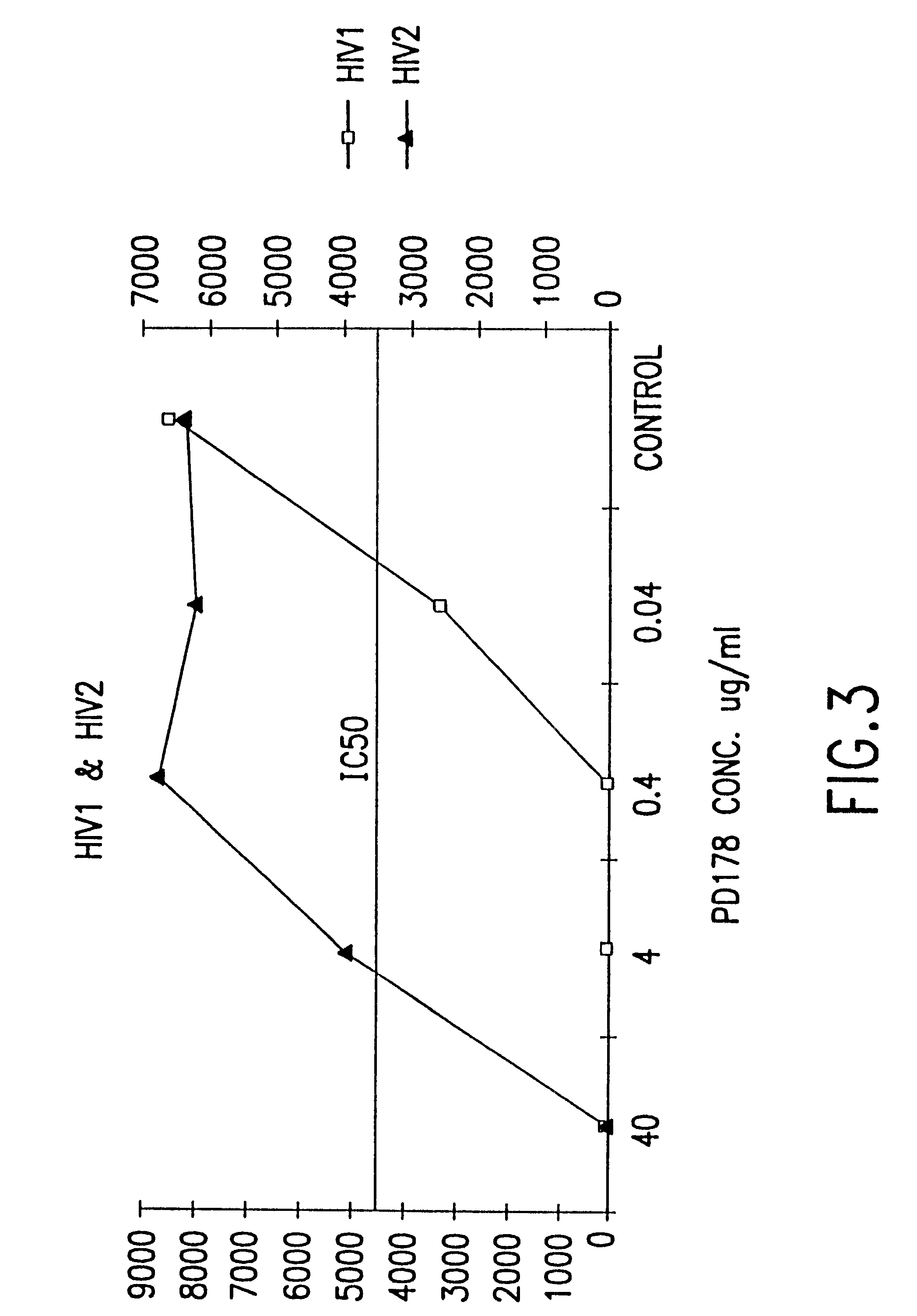
Novel antiviral combinations for the treatment or prevention of viral infections, in particular, HIV, are disclosed. This new antiviral therapy employs either DP-178 or DP-107, viral fusion inhibitors, in combination with at least one other antiviral therapeutic agent. The combinations of the invention are better than single therapies alone, and in certain cases are synergistic. The use of DP-178 or DP-107 is an ideal therapy to combine with another antiviral, given both the novel mechanism which this therapeutic blocks HIV transmission and the non-toxicity of the therapeutic.
Owner:TRIMERIS
Positive modulator of bone morphogenic protein-2
InactiveUS20050196425A1Improve biological activityMaximize bioactivityOrganic active ingredientsPeptide/protein ingredientsDiseaseBone formation
Compounds of the present invention of formula I and formula II are disclosed in the specification and wherein the compounds are modulators of Bone Morphogenic Protein activity. Compounds are synthetic peptides having a non-growth factor heparin binding region, a linker, and sequences that bind specifically to a receptor for Bone Morphogenic Protein. Uses of compounds of the present invention in the treatment of bone lesions, degenerative joint disease and to enhance bone formation are disclosed.
Owner:BROOKHAVEN SCI ASSOCS +1
Simplified and improved method for preparing an antibody or an antibody fragment targeted immunoliposome for systemic administration of a therapeutic or diagnostic agent
InactiveUS7780882B2Low effective doseLessening severe side effectVectorsPeptide/protein ingredientsDiagnostic agentAntibody fragments
A method of preparing an antibody- or antibody fragment-targeted cationic immunoliposome or polymer complex comprises the steps of (a) preparing an antibody or antibody fragment; (b) mixing said antibody or antibody fragment with a cationic liposome to form a cationic immunoliposome or with a cationic polymer to form a polyplex; and (c) mixing said cationic immunoliposome or said polyplex with a therapeutic or diagnostic agent to form said antibody- or antibody fragment-targeted cationic immunoliposome or polymer complex.
Owner:GEORGETOWN UNIV
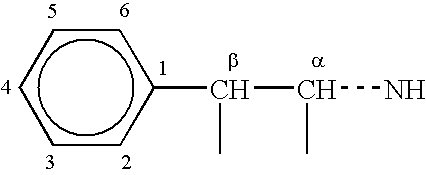
ACE-2 inhibiting compounds and methods of use thereof
InactiveUS6632830B1Inhibit functioningReduce riskOrganic active ingredientsBiocideBlood pressureKidney disorder
ACE-2 inhibiting compounds are disclosed. These compounds include compounds of formula (IV):wherein the variables are as described in the specification. Pharmaceutical compositions containing the compounds are also discussed. The pharmaceutical compositions may contain an effective amount of a compound of the invention to treat ACE-2 associated disorders such as a blood pressure related disease or disorder, cell proliferation disorder, kidney disorder, kinetensin associated disorder, inflammation associated disorder, or an allergic disorder.< / PTEXT>
Owner:MILLENNIUM PHARMA INC
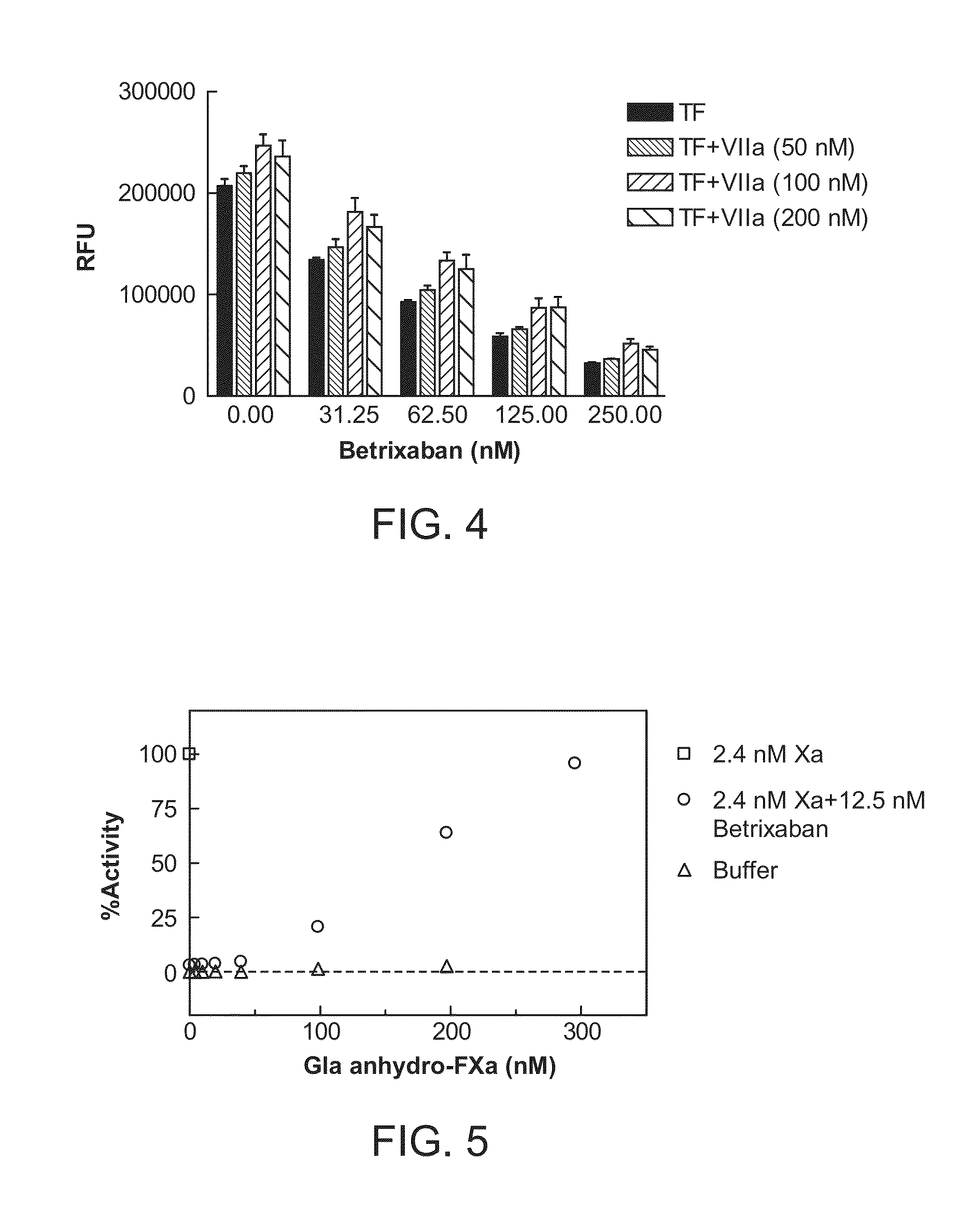
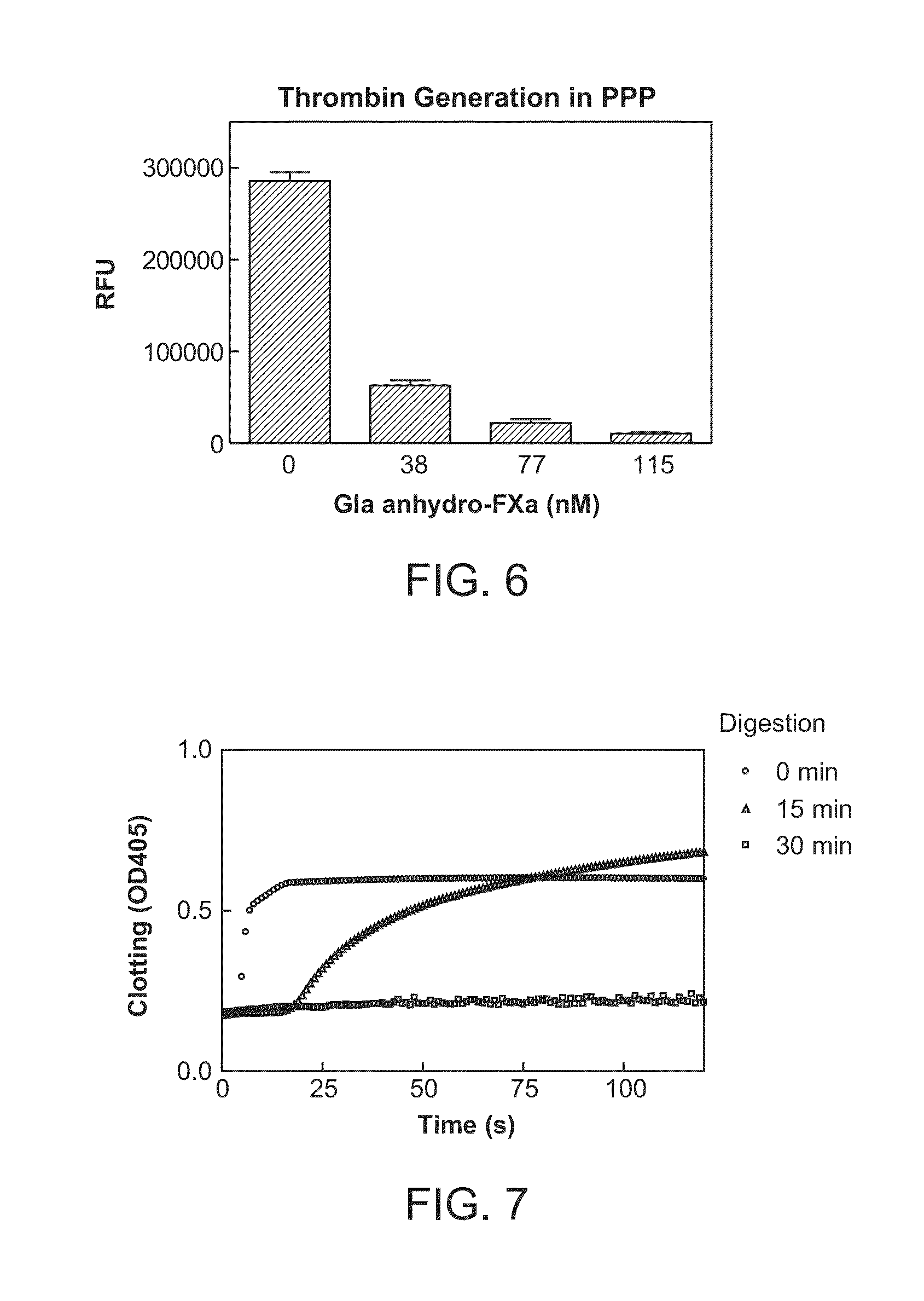
Antidotes for factor Xa inhibitors and methods of using the same in combination with blood coagulating agents
ActiveUS8455439B2Low effective doseReduce potential side effectsPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates to antidotes of anticoagulants targeting factor Xa which antidotes are used in combination with blood coagulating agents or other heparin antidotes to prevent or reduce bleeding in a subject. The antidotes described herein have reduced or no intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is or will be undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Method of preparing hydroxyapatite based drug delivery implant for infection and cancer treatment
InactiveUS20070190102A1Sustained releaseLow effective dosePharmaceutical containersPretreated surfacesDiseaseAnticarcinogen
A bioresorbable material is incorporated with bioactive agents to form an implant used for treatment against hard tissue or soft tissue defects and diseases. Antibiotics or anti-cancer agents are incorporated to treat hard or soft tissue infections or cancers. Sustained release of the bioactive agents or drug molecules may be achieved after implantation at the targeted sites. The dosage of the active agents or molecules, the microstructure, morphology, and composition of the bioresorbable material allow control of the release profile. The invented implant may be used for drug delivery, chemotherapy, or gene therapy. Various microstructure and the morphologies of the implants are injectable like putty or shaped with multilayers.
Owner:LUO PING
American goldenrod herb total flavone extract and its preparing method and use
InactiveCN1899341AEfficient enrichmentHigh in flavonoidsSugar derivativesSugar derivatives preparationDiseaseChlorogenic acid
The present invention relates to American goldenrod herb total flavone extract and its preparation process and use. The American goldenrod herb total flavone extract contains quercetin, quercetin-3-O-beta-D-heteroside, kaempferol-3-O-alpha-L-rhamnoside, chlorogenic acid, etc. capable of preventing and treating senile dementia, various inflammatory respiratory diseases, esophagus cancer, etc. The preparation process is simple, high extracting rate, and the extract has high pharmacological effect, stable property, low toxicity and controllable quality.
Owner:LINSAIJIAO BIOLOGICAL SCI & TECH DEV SHANGHAI
Microbubble compositions and methods for oligonucleotide delivery
InactiveUS7115583B2Low effective doseHigh indexUltrasonic/sonic/infrasonic diagnosticsAntibacterial agentsMedicineBiodistribution
The invention relates to a new and improved pharmaceutical composition and method for delivery of therapeutic agents. The methods and composition of the invention can be used with several therapeutic agents and can achieve site specific delivery of a therapeutic or diagnostic substance. This can allow for lower doses and for improved efficacy with drugs which traditionally reach targeted sites and can result in improved utility for agents such as oligonucleotides and polynucleotides which are plagued with problems with biodistribution.
Owner:AVI BIOPHARMA
Combination therapy
ActiveUS8633219B2Low effective doseEffective treatmentBiocideOrganic chemistrySide effectCombined Modality Therapy
Owner:JAPAN TOBACCO INC
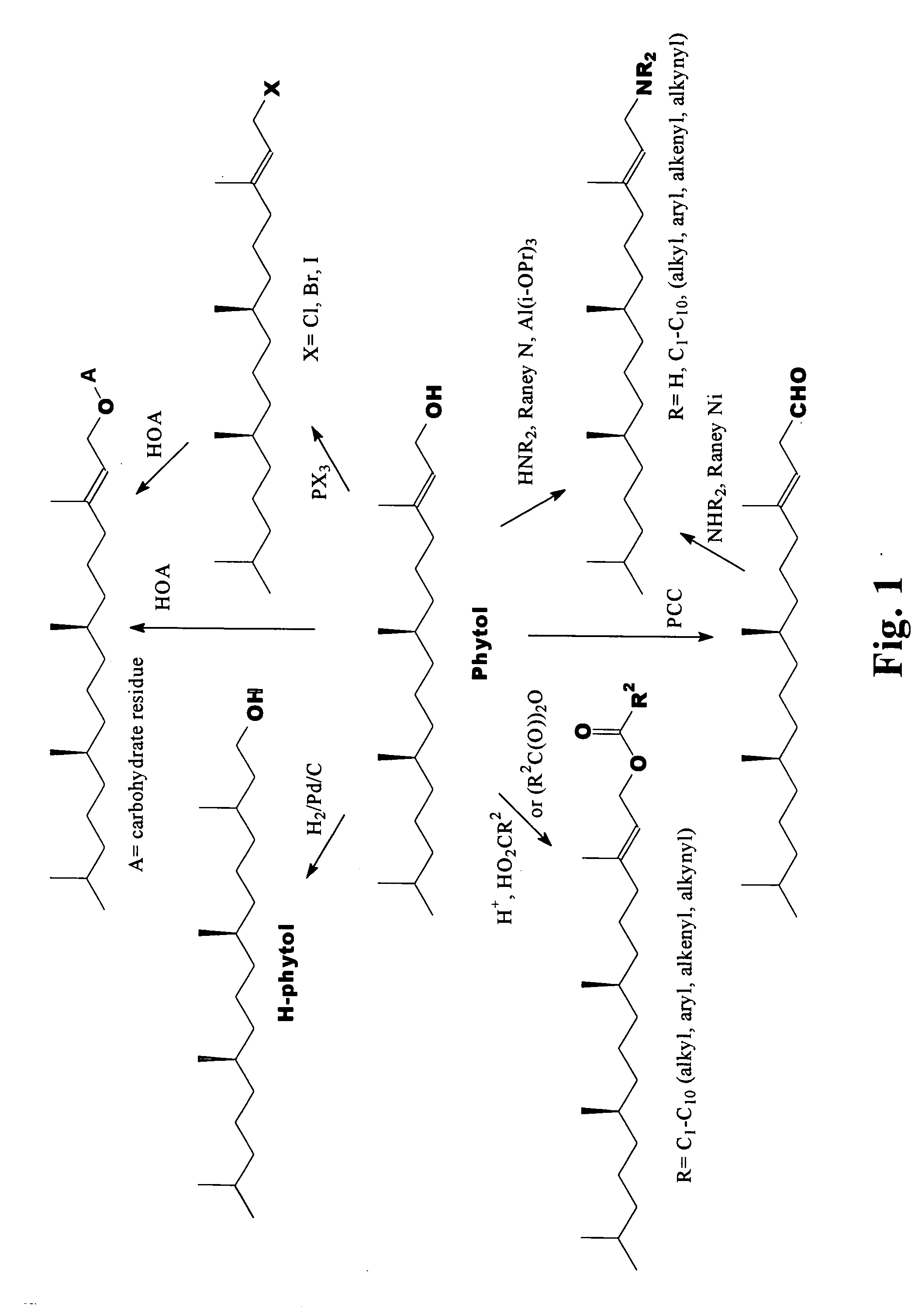
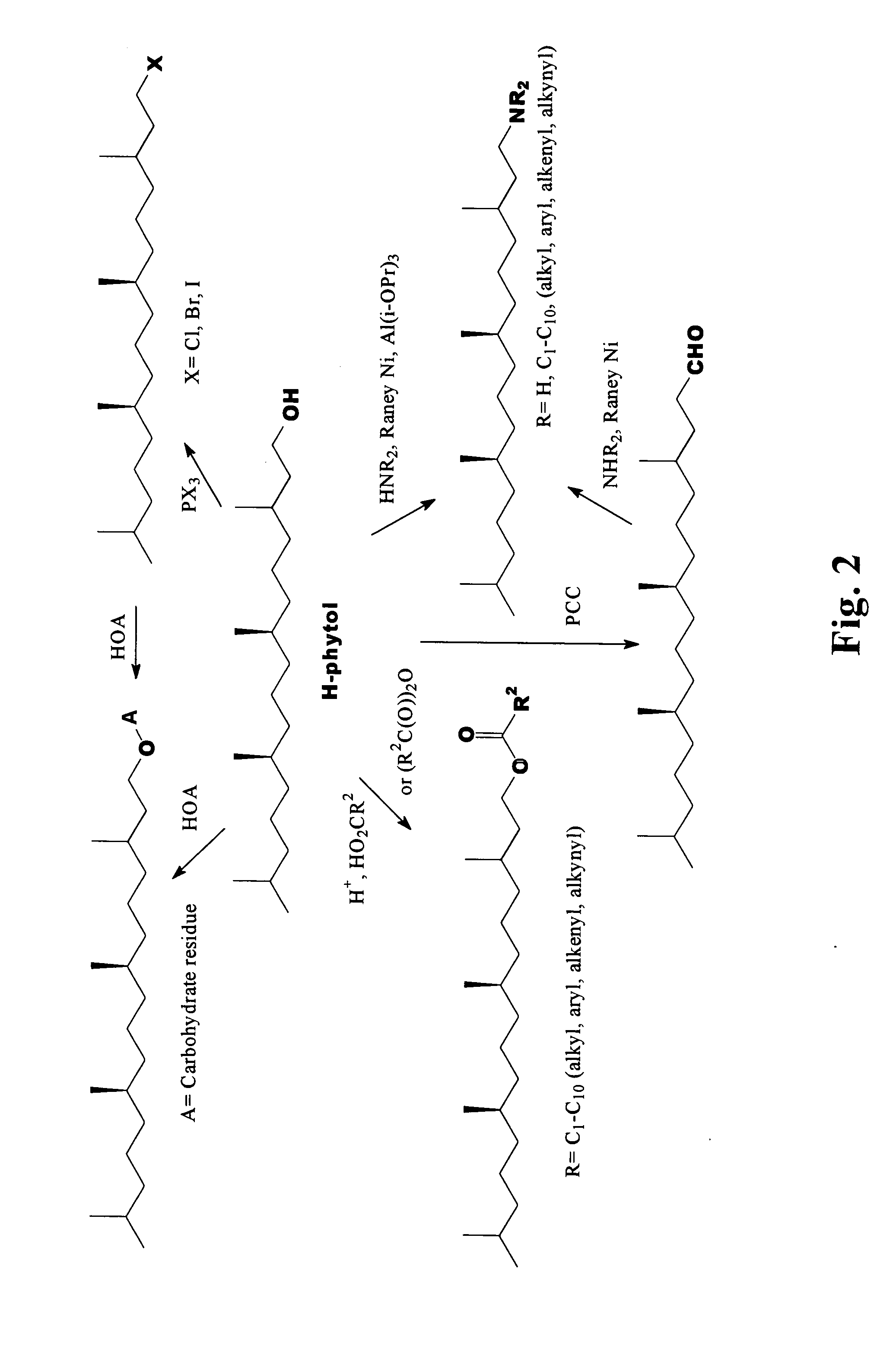
Novel phytol derived immunoadjuvants and their use in vaccine formulations
InactiveUS20050158329A1Improving immunogenicityInduce immunogenic responseBiocideHydroxy compound active ingredientsSide effectParticulate antigen
This invention relates to a novel immunoadjuvant, an adjuvant component, and vaccines containing the adjuvant component. The adjuvant includes phytol or a phytol derivative. The adjuvant component, when combined with a soluble or particulate antigen, provides a vaccine with an enhanced ability to induce both humoral and cytotoxic immune responses while displaying reduced toxicity and / or adverse side effects over vaccines that include the antigen but without the benefit of this adjuvant component.
Owner:GHOSH SWAPAN K
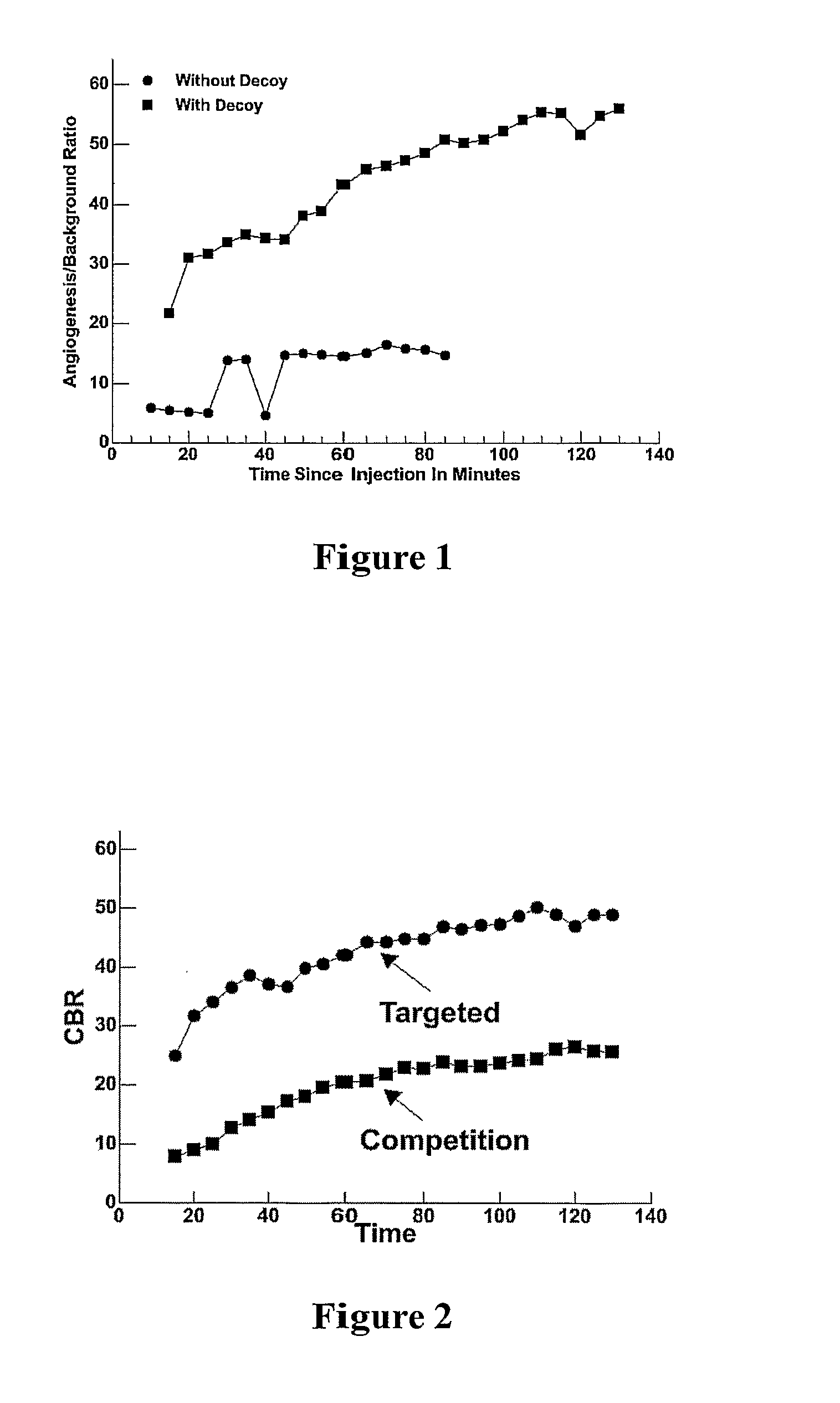
Efficacy and Safety of Targeted Particulate Agents with Decoy Systems
InactiveUS20080193372A1Sharp contrastIncrease contrastUltrasonic/sonic/infrasonic diagnosticsPowder deliveryParticulatesMedicine
A decoy inactive carrier composition is administered simultaneously with a targeted composition containing vehicles for delivering a desired agent to a biological target. This simultaneous administration enhances the delivery of the targeted composition to the desired location in a subject.
Owner:BARNES JEWISH HOSPITAL
Breakthrough pain management
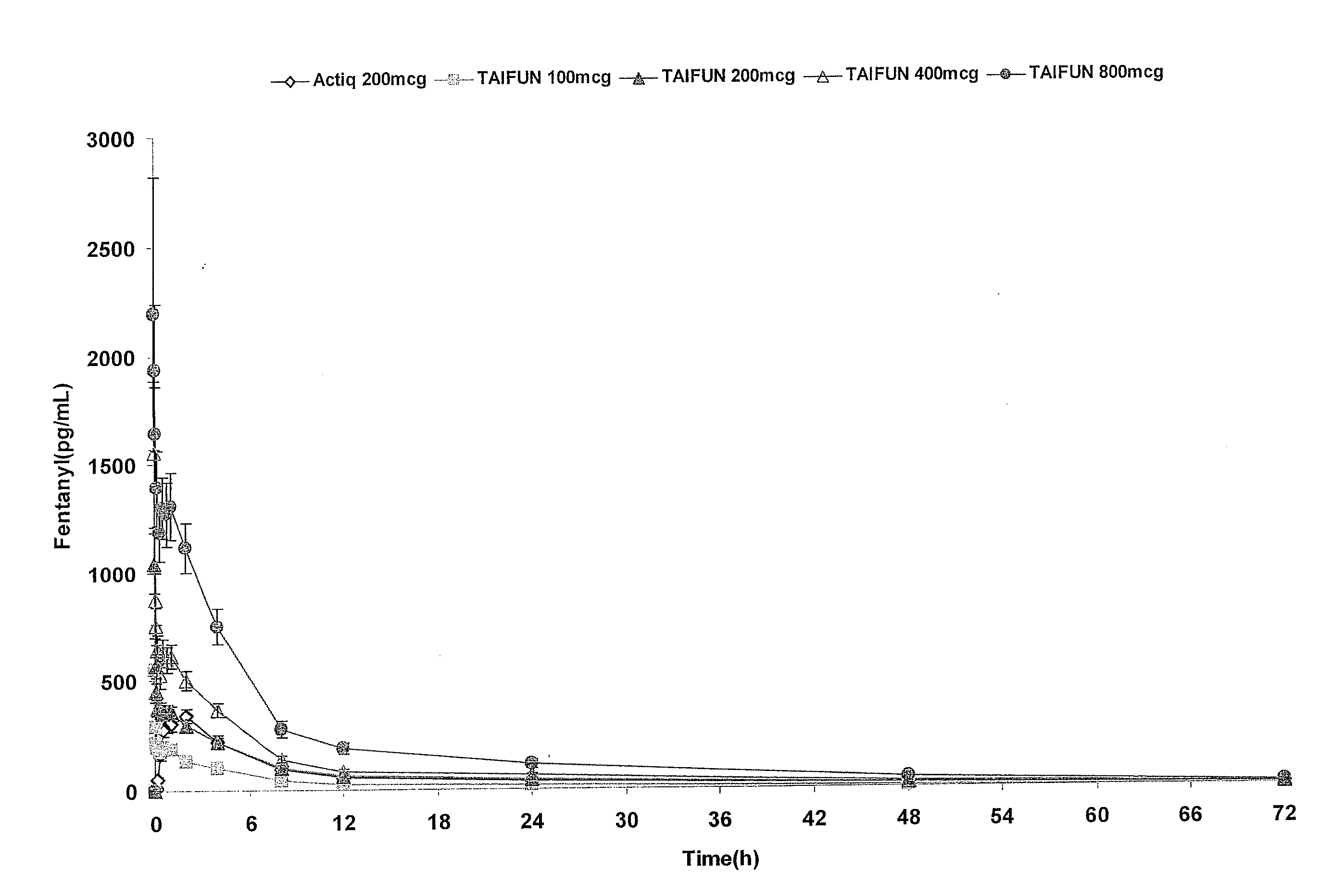
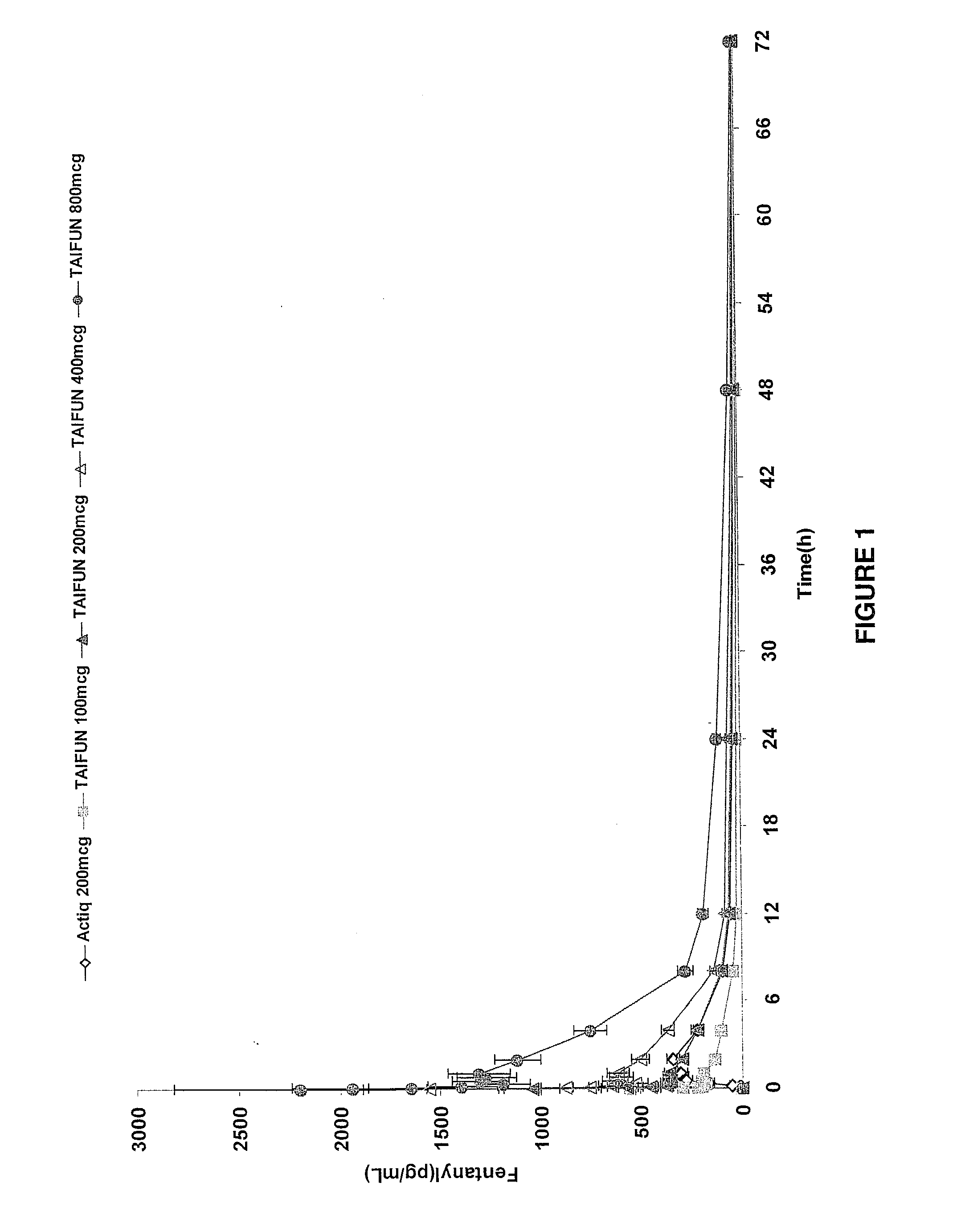
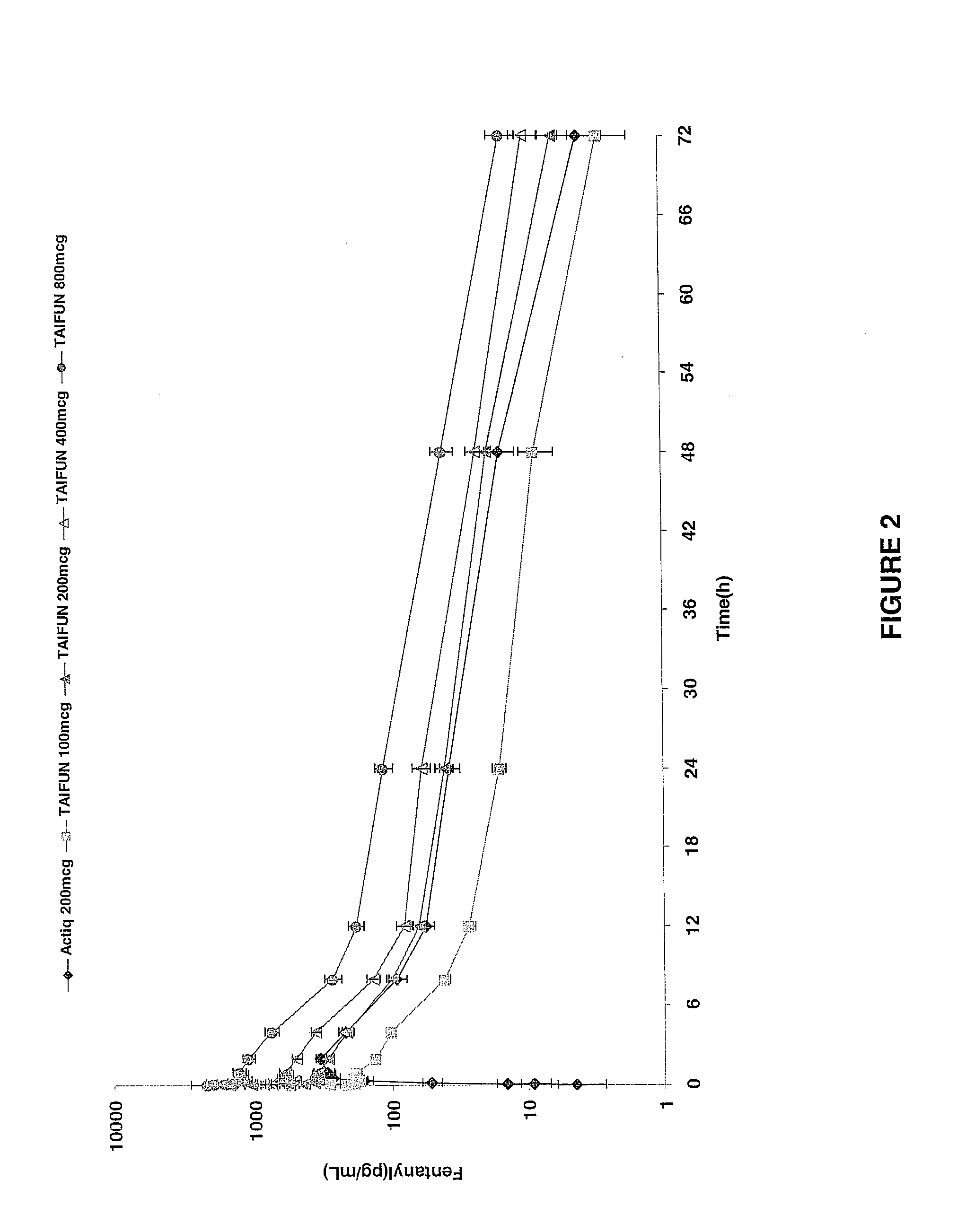
InactiveUS20090011030A1Treatment and alleviation of painAct quicklyBiocidePowder deliveryPulmonary inhalationFentanyl
The present invention is directed to a powdered formulation comprising an analgesic, preferably fentanyl, for use in pulmonary inhalation administration for the rapid analgesic titration of pain, in particular breakthrough pain. Upon administration, the powdered formulation is able to provide a narrower titration range in patients suffering from pain, as well as effective analgesic amounts of fentanyl in a shorter time and at lower dose levels of administered fentanyl when compared to fentanyl administered by an oral transmucosal route.
Owner:LAB INT
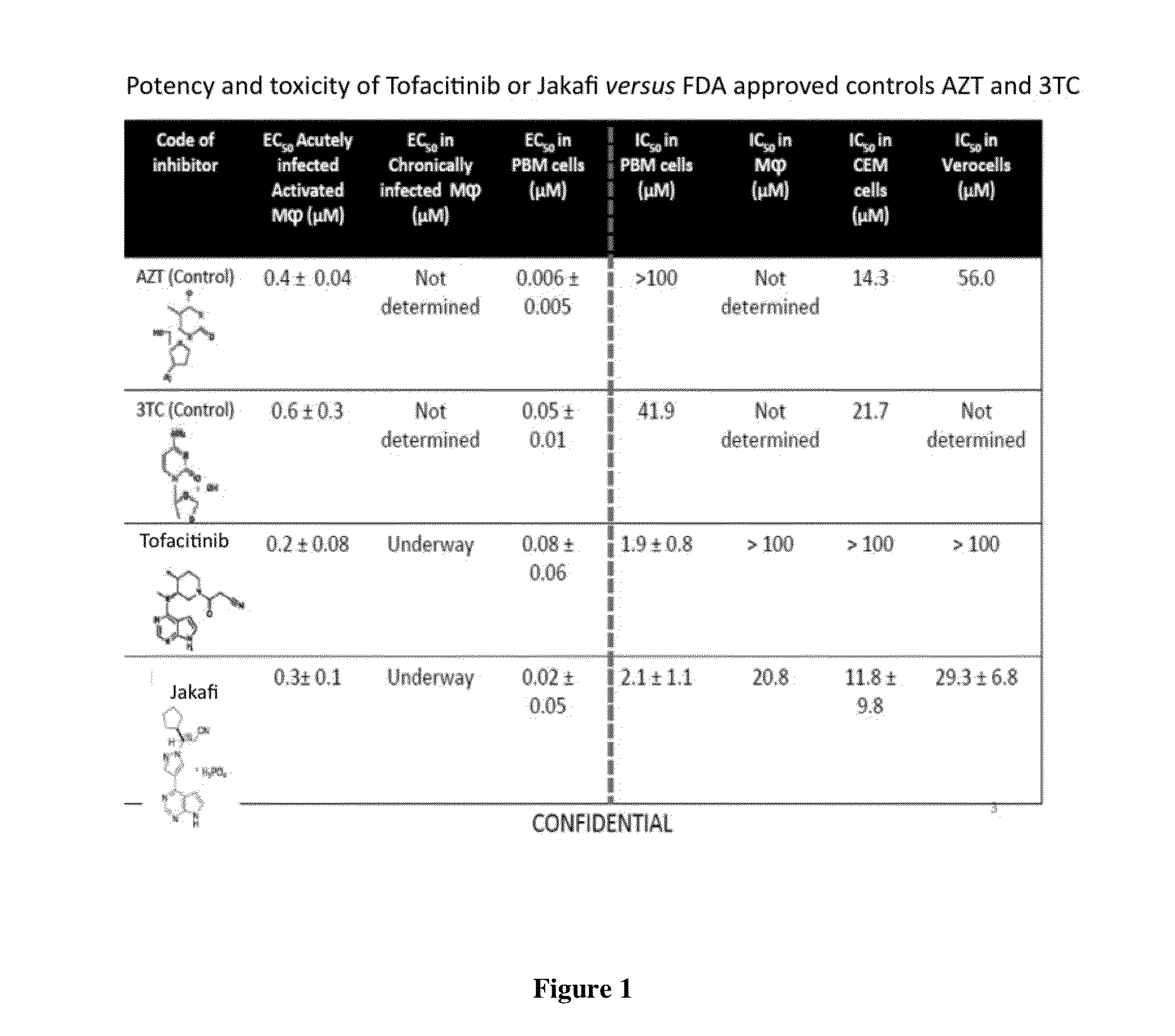
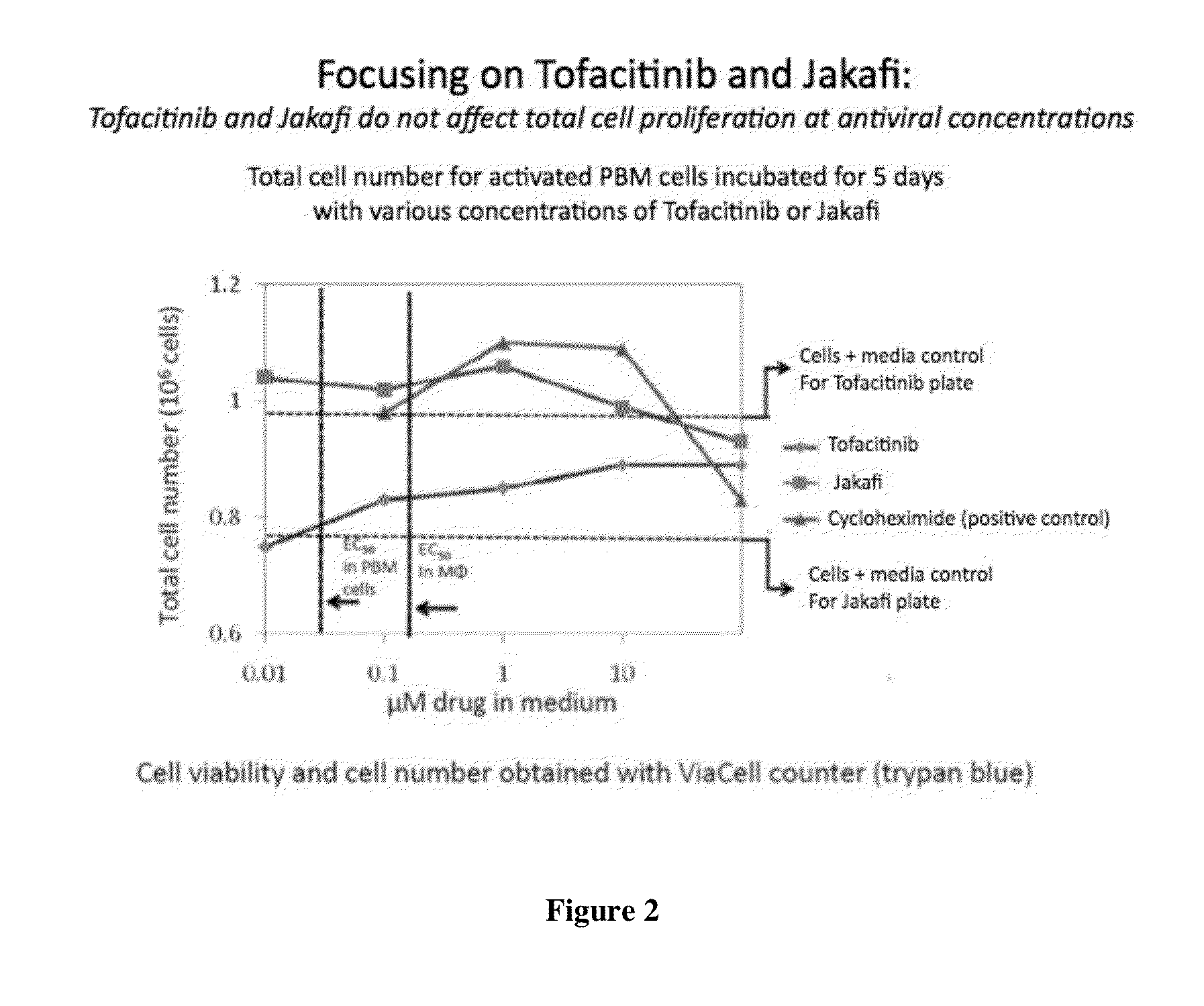
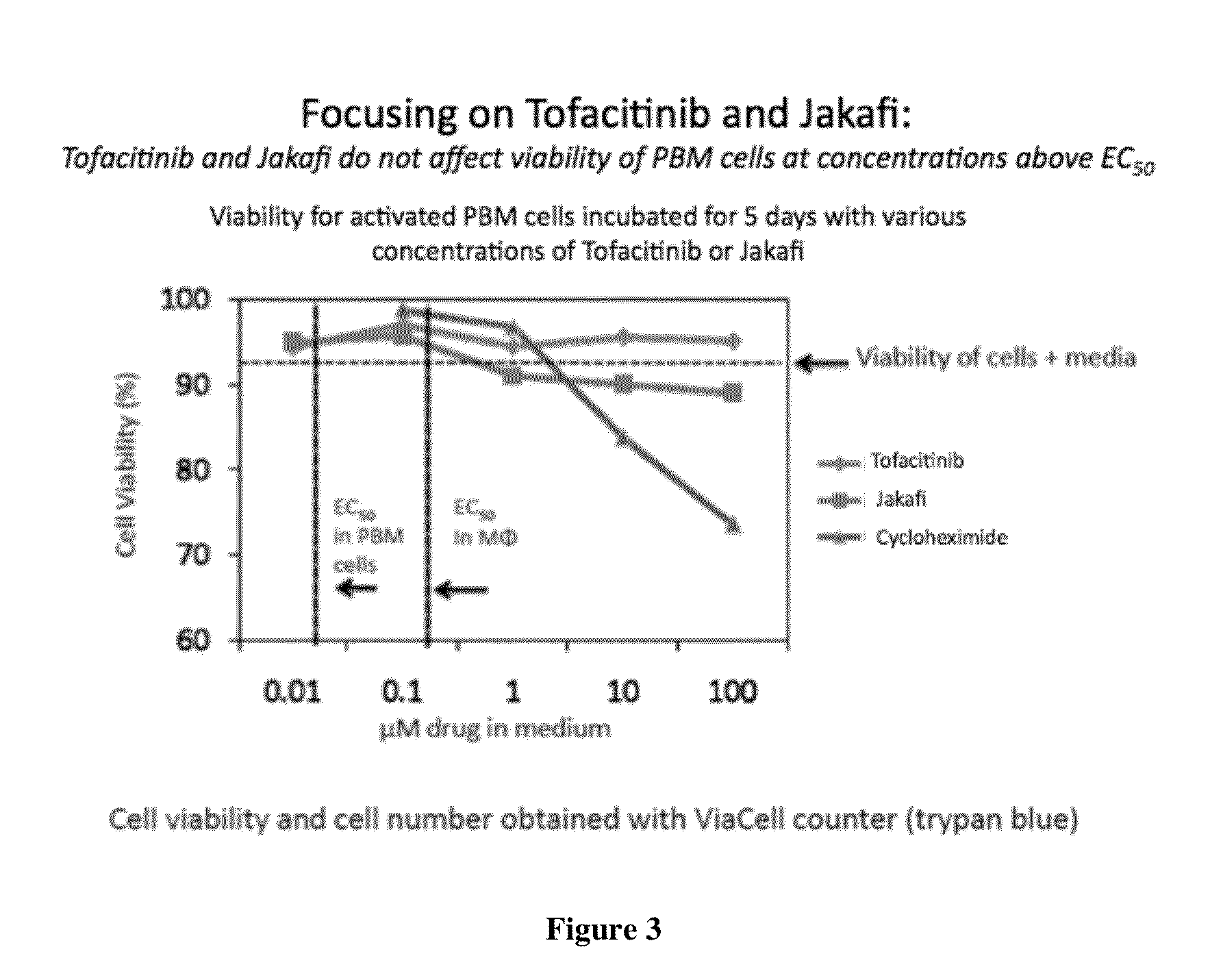
Antiviral jak inhibitors useful in treating or preventing retroviral and other viral infections
ActiveUS20140328793A1Improve their absolute antiviral effectLow toxicityBiocidePeptide/protein ingredientsProteinase inhibitorThymidine
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Ginkgo biloba leaf total terpene lactone extract, and its preparing method, medicinal composition and use
InactiveCN1977868ASignificant antidepressant effectClear antidepressant effectNervous disorderPill deliveryBilobalidesGinkgo nut
The present invention relates to a new-type high-effective safety medicine-ginkgo leaf total lactone for curing depression. It is a terpene lactone extracted from ginkgo biloba. L or other portions of said plant, such as ginkgo nut, ginkgo root bark and ginkgo twing, in which five terpene lactone compounds of bilobalide A, bilobalide B, bilobalide C, bilobalide J and bilobalide are mainly contained.
Owner:ZHEJIANG HISUN PHARMA CO LTD +1
Compounding traditional Chinese medicine, and its use
InactiveCN101002906ADefinite curative effectLow effective doseMetabolism disorderDigestive systemNephrosisCurative effect
A Chinese medicine for treating diabetes and nephrosis is prepared from 4 Chinese-medicinal materials including astragalus root, rhubarb, etc.
Owner:QINGFENGGE MEDICINE TECH SHANGHAI
Use of casticin in preparation of medicine against premenstrual syndrome
InactiveCN101028261AGood preventive effectEasy to adjustOrganic active ingredientsBiological testingDiseaseChinese drug
An application of the casticin extracted from shrub chastetree fruit in preparing the medicines for preventing, diagnosing and treating premenstrual syndrome is disclosed.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hybird molecules having factor VII/VIIa activity
InactiveUS20060258851A1Same and increased activityIncreased serum half-lifeFibrinogenPeptide/protein ingredientsSemi syntheticFactor VII
The present invention relates to novel human coagulation Factor VII / VIIa proteins having coagulant potential / activity as well as pharmaceutical compositions comprising the polypeptides, uses and methods of treatment. In particular, the present invention relates to novel, semi synthetic analogues of human coagulation Factor VII and VIIa (FVII and FVIIa) as well as to a method of their production.
Owner:NOVO NORDISK AS
Traditional Chinese medicine extraction for preventing and curing osteoporosis and its preparation method
InactiveCN101085091AEasy to adjustGood preventive effectSkeletal disorderFood preparationDiseaseLife quality
The invention relates to a Chinese traditional medicine extract for preventing and treating osteoporosis and its preparation method and application. The extract mainly comprises rhizoma atractylodis volatile oil, total alkaloid of Phellodendron amurense, and total saponins of radix achyranthis bidentatae and total sterone of radix achyranthis bidentatae; can be used for preventing and / or treating osteoporosis and relevant disorder, which has gradually become important pathogenesis influencing life-quality. The extract has easily-accessible raw materials, simple preparation, high extraction efficiency, strong pharmacological action, stable quality, high safety, low toxicity, and controllable quality. The invention comprises three Chinese traditional medicines in classical prescription, come to optima activity for resisting osteoporosis, reduces adverse side effect, and provides a new medicament source for preventing, diagnostic ting, detecting, protecting, treating and researching these diseases and their relevant diseases.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Alpha-MSH analog for curing sexual disorder and preparation method
InactiveCN1563076AImprove biological activityImprove stabilityPeptide/protein ingredientsPeptidesDiseaseEndocrinology
The invention discloses an alpha-MSH analogue AC-Nle-cyclo(-Asp-His-D-Phe-Arg-Trg-Lys)-OH(SEQ ID No:1) polypeptide and derivative (SEQ ID No:2) whose C end is umidated for curing sexual dysfunction, and discloses a method for synthesizing the above-mentioned polypeptide, and discloses a medicine composite containing the above-mentioned polypeptide for curing sexual dysfunction and its preparation method, and application of said medicine composite for curing sexual dysfunction diseases, including male sexual dysfunction, for example, erectile dysfunction and female sexual dysfunction.
Owner:西南生物工程产业化中试基地有限公司
Compositions and methods to control bleeding
InactiveUS20030050225A1Reduce needReducing cost and efficaciousnessFactor VIIPeptide/protein ingredientsMammalBlood coagulations
Disclosed are compositions for treating blood coagulation disorders and allows for manipulation of the blood coagulation cascade. More particularly the invention, relates to compositions for altering bleeding that include a mixture of at least one blood coagulation factor in a low dose and phospholipid vesicles. The invention has a variety of important uses including controlling bleeding in a mammal that has or is suspected of having a potentially life-threatening blood coagulation disorder.
Owner:UNIVERSITY OF VERMONT
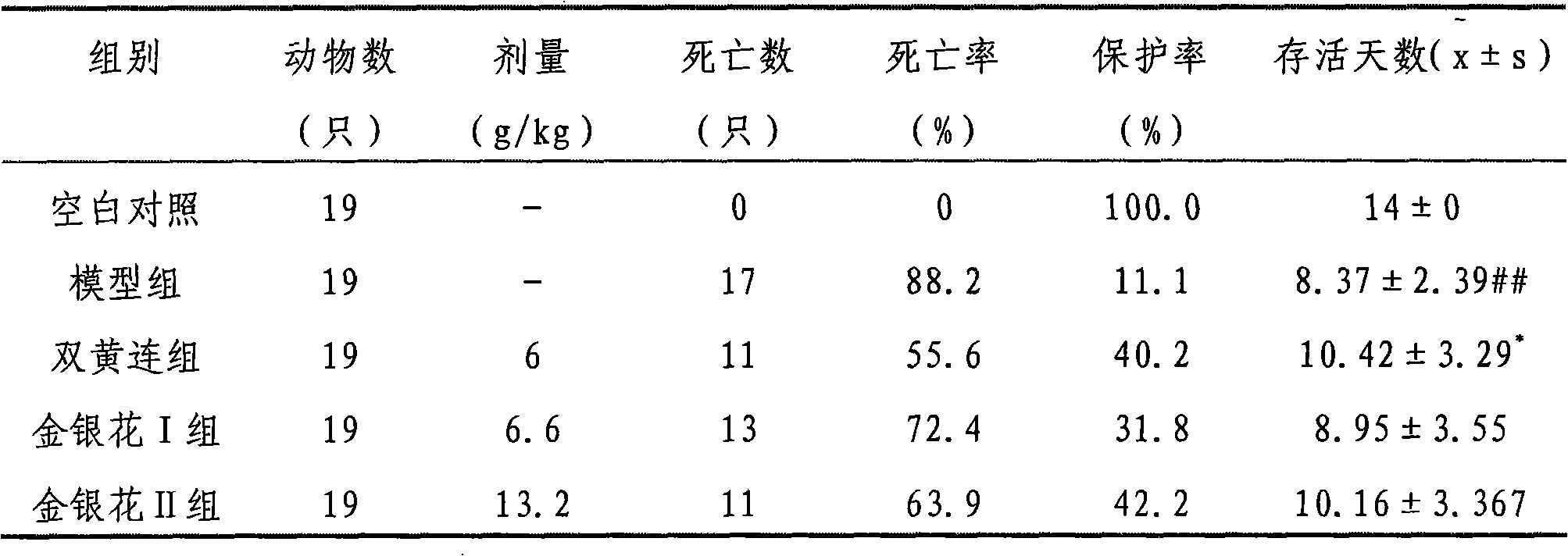
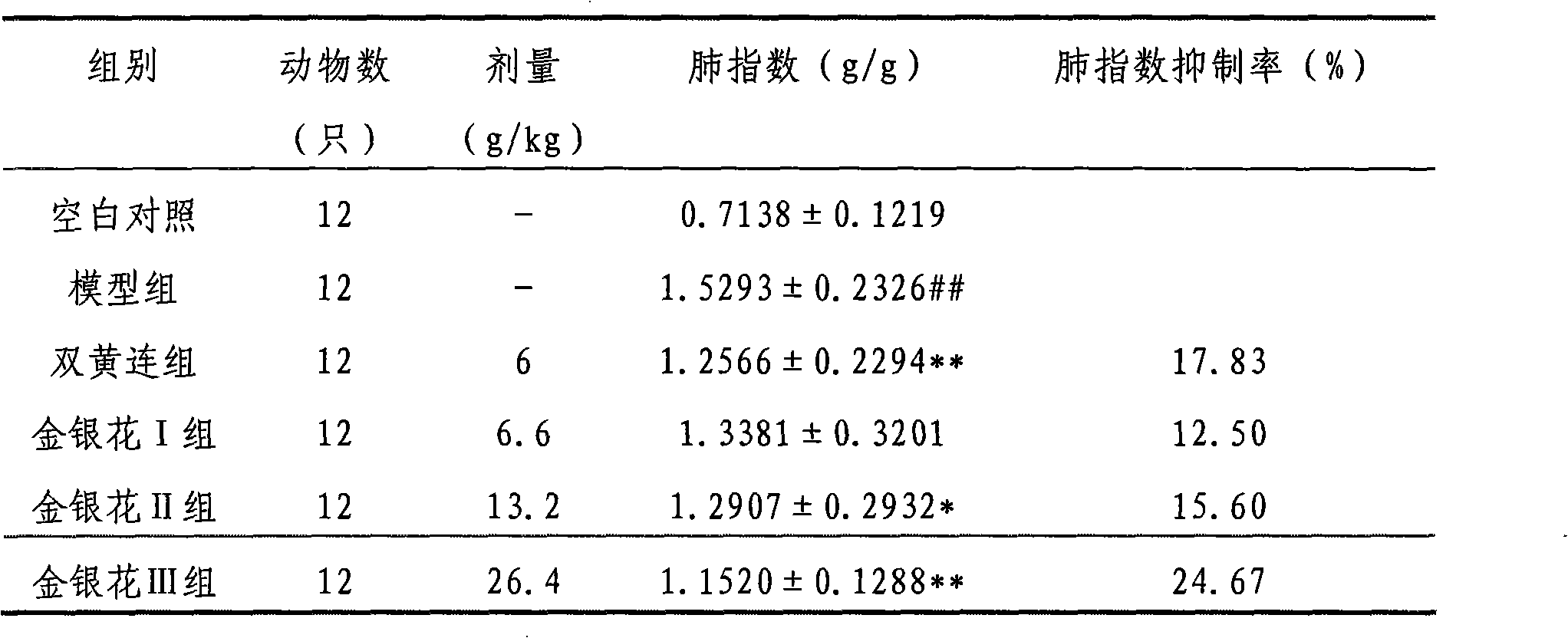
Preparation method for extracting chlorogenci acid from honeysuckle and application of honeysuckle extract
InactiveCN102134192AGood adsorption and separation performanceGood effectOrganic active ingredientsOrganic compound preparationChlorogenic acidSide effect
The invention provides a honeysuckle extract and a preparation method for extracting chlorogenci acid. The method has the advantages of good repeatability and good stability; by using the method, the content of an active component can be improved, a large amount of impurities are removed, raw materials are high in yield and low in cost; and the method is suitable for industrial production. The percentage of the main active component chlorogenci acid in the honeysuckle extract is 30%. The preparation process of the honeysuckle extract comprises the following steps: adding water the volume of which is 8-20 times as large as that of taken honeysuckle medicinal material in the taken honeysuckle medicinal to extract for 2-4 times for 0.5-1.5 hours each time; regulating the pH value of the extract to 1-6; and on a large pore absorption resin column, eluting impurities with water firstly, then eluting with 10-70% ethanol, colleting ethanol eluent, recovering the ethanol, concentrating, regulating the pH value of a concentrated liquid to 1-6, adding ethyl acetate for extraction, recovering the ethyl acetate, concentrating and drying. By using the method, the content of the active component in the extract is improved, the curative effect is improved, a large amount of impurities are removed, the problem of large moisture absorption commonly occurring in Chinese patent medicines is solved, and side effects are reduced.
Owner:重庆市集银中药材有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com