Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Diagnostic substance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
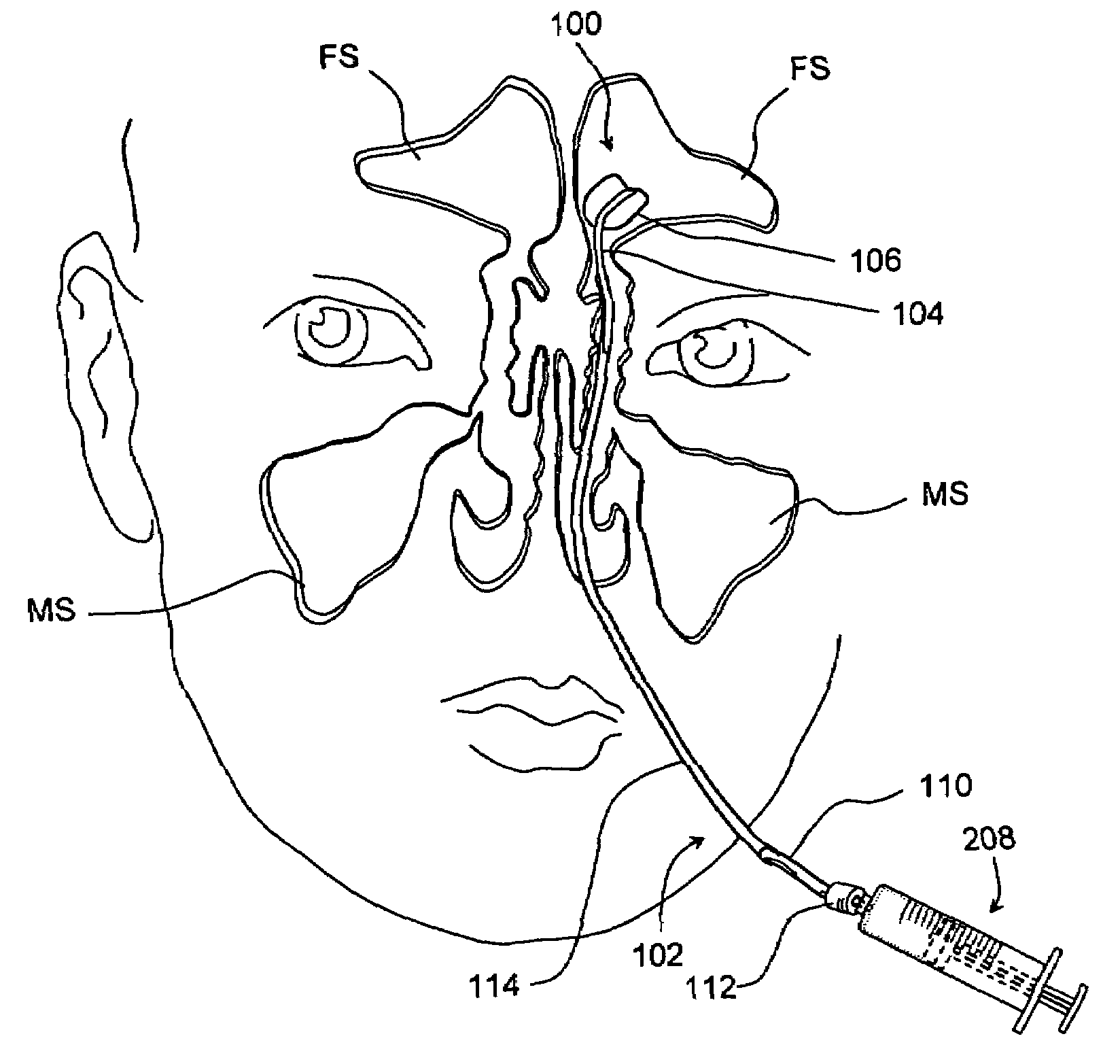
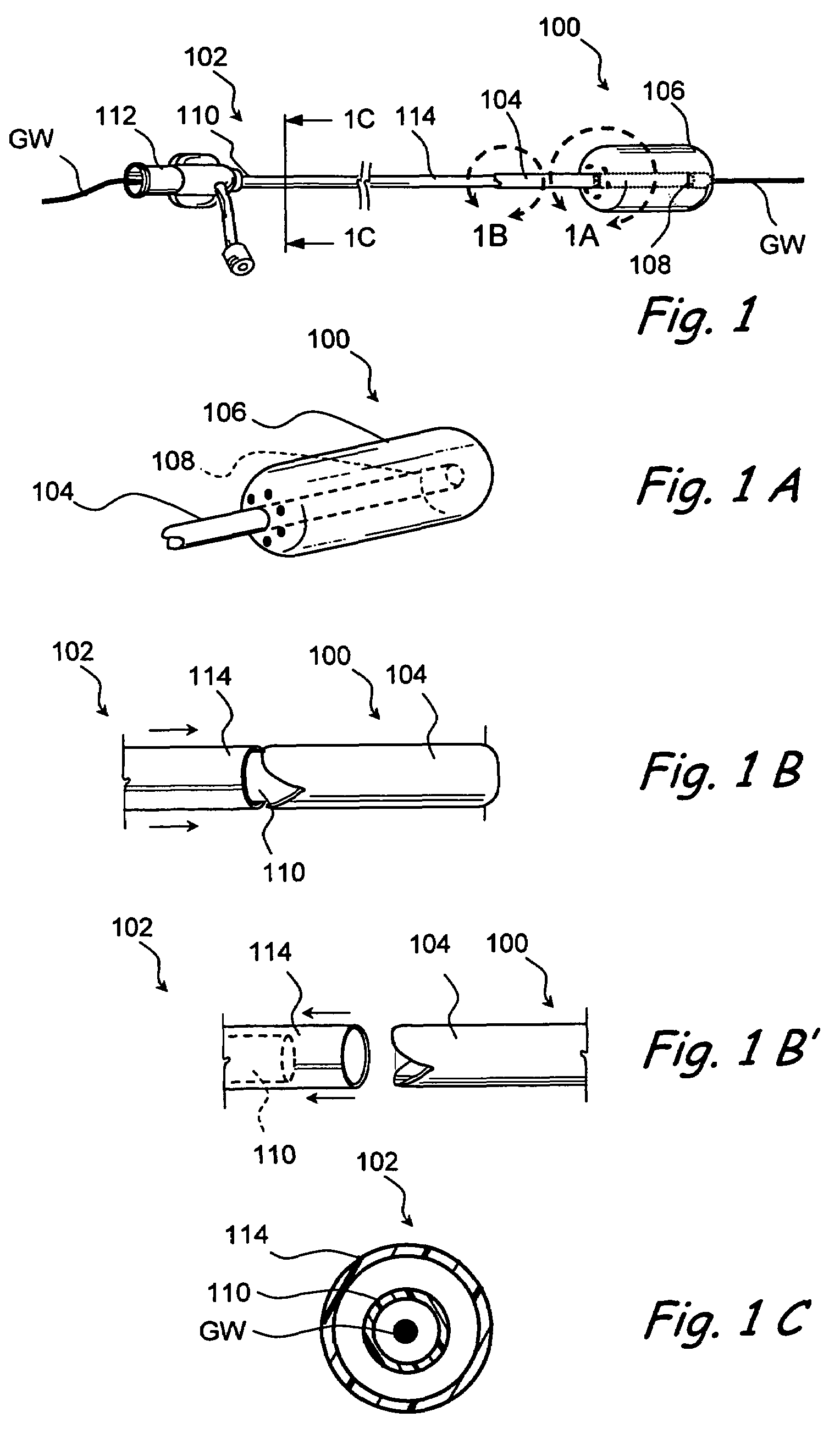
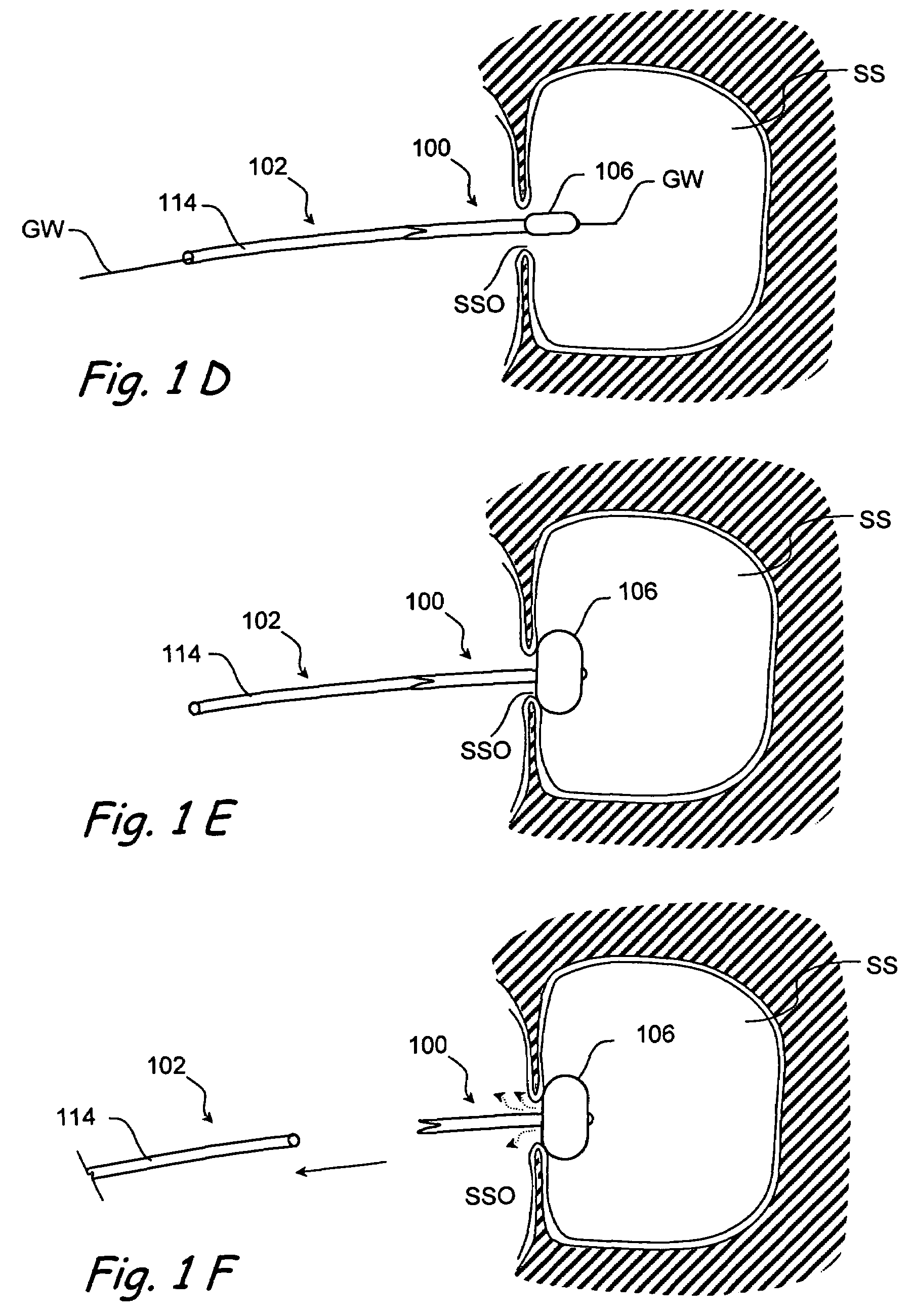
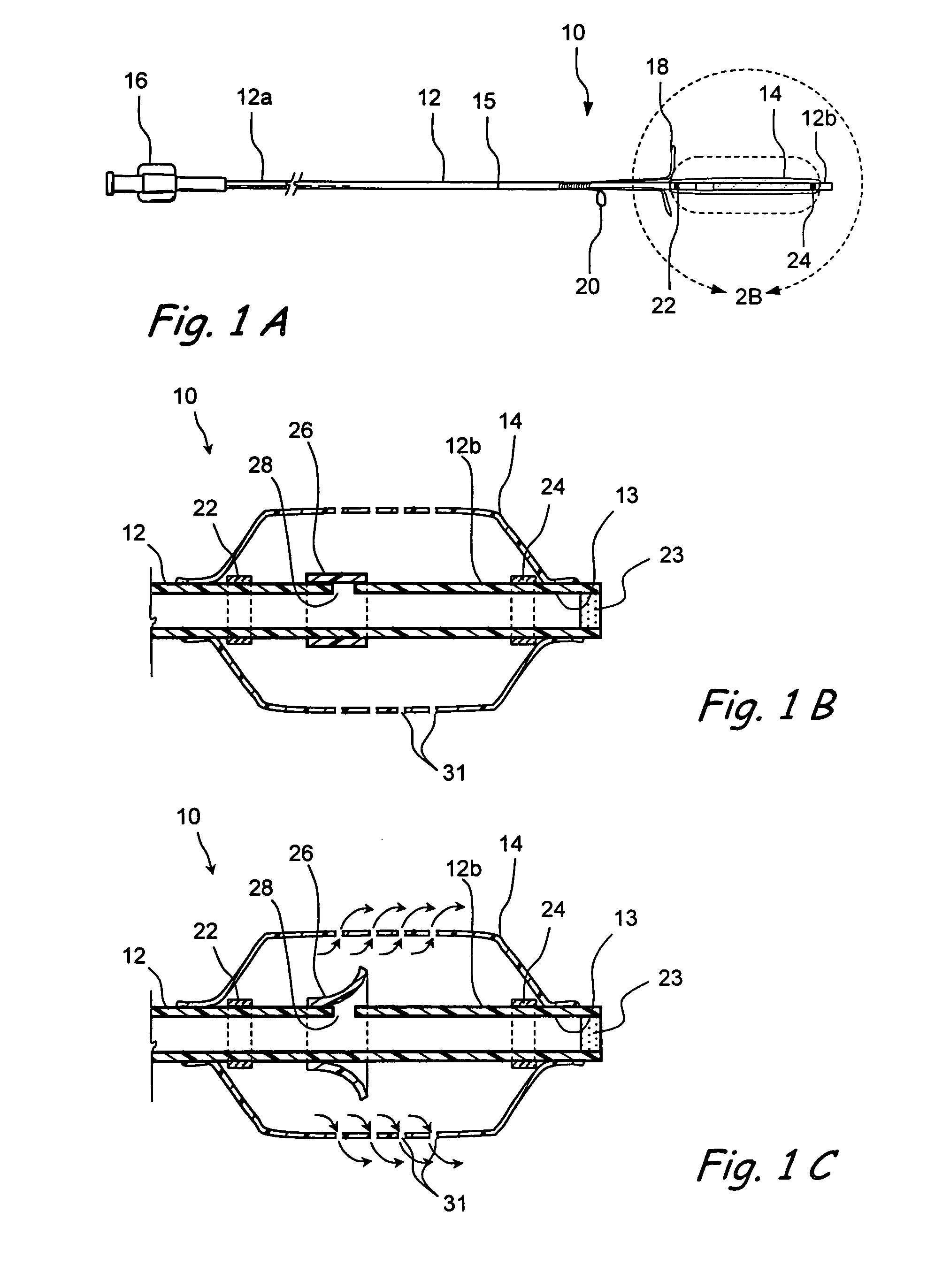
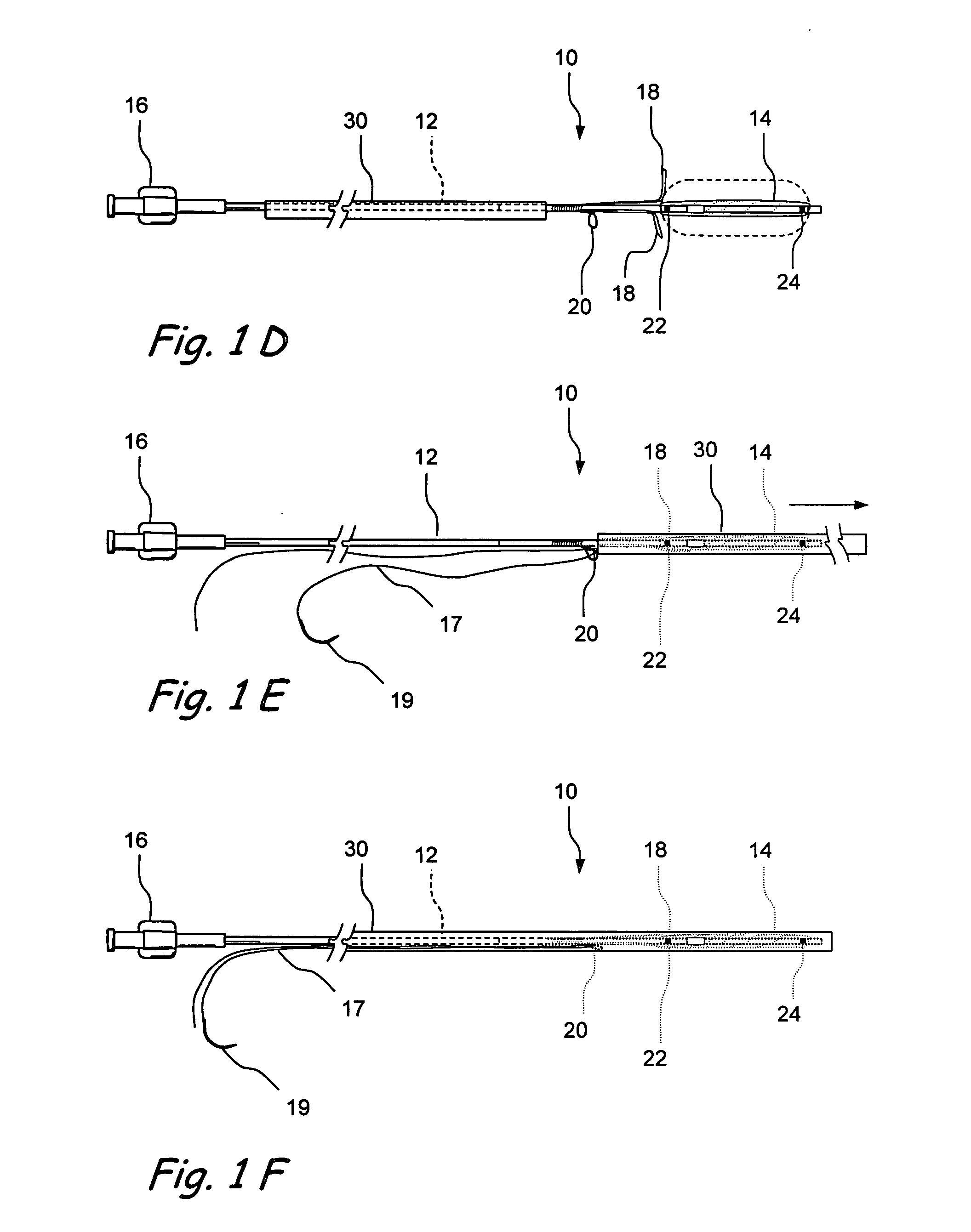
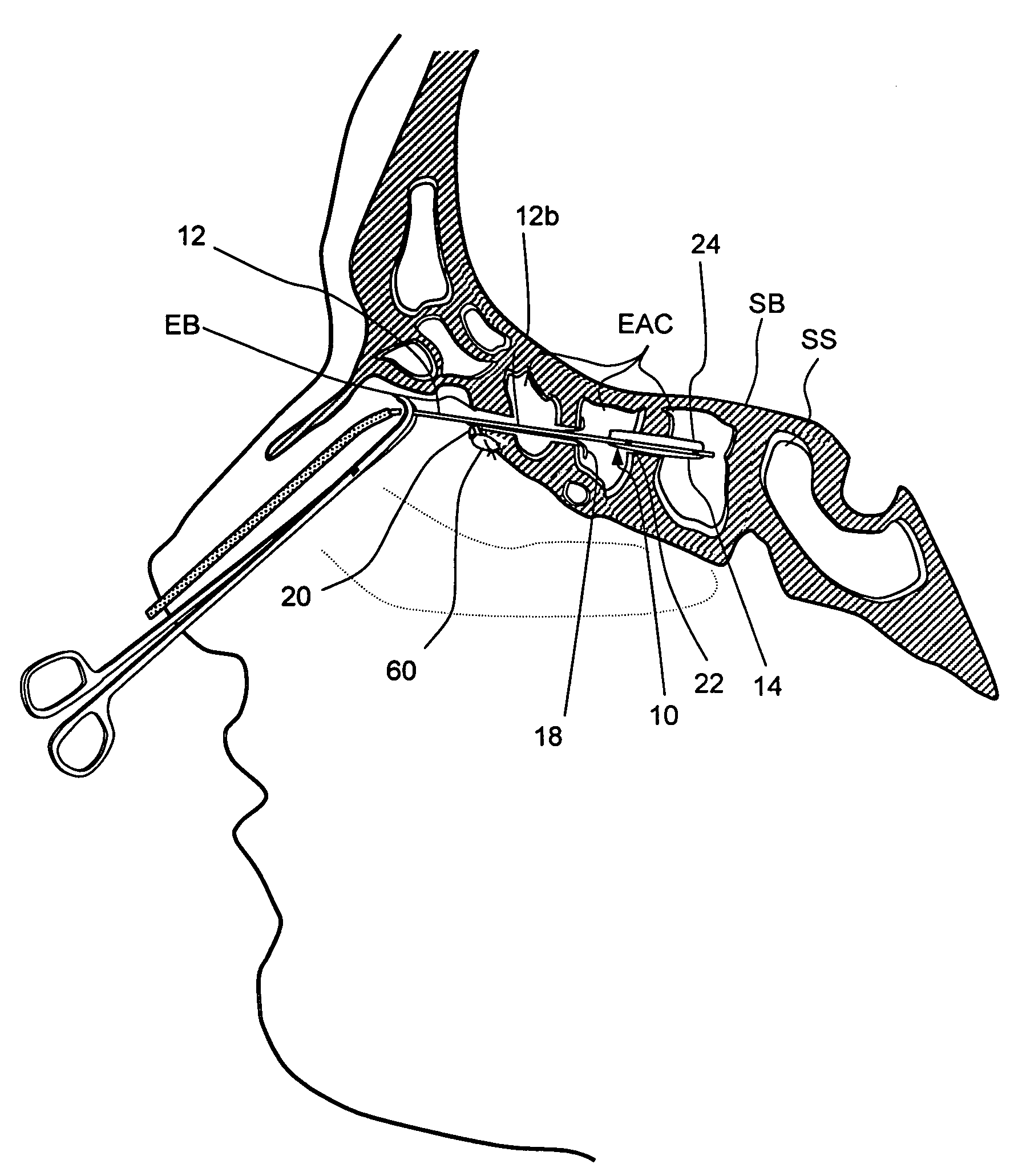
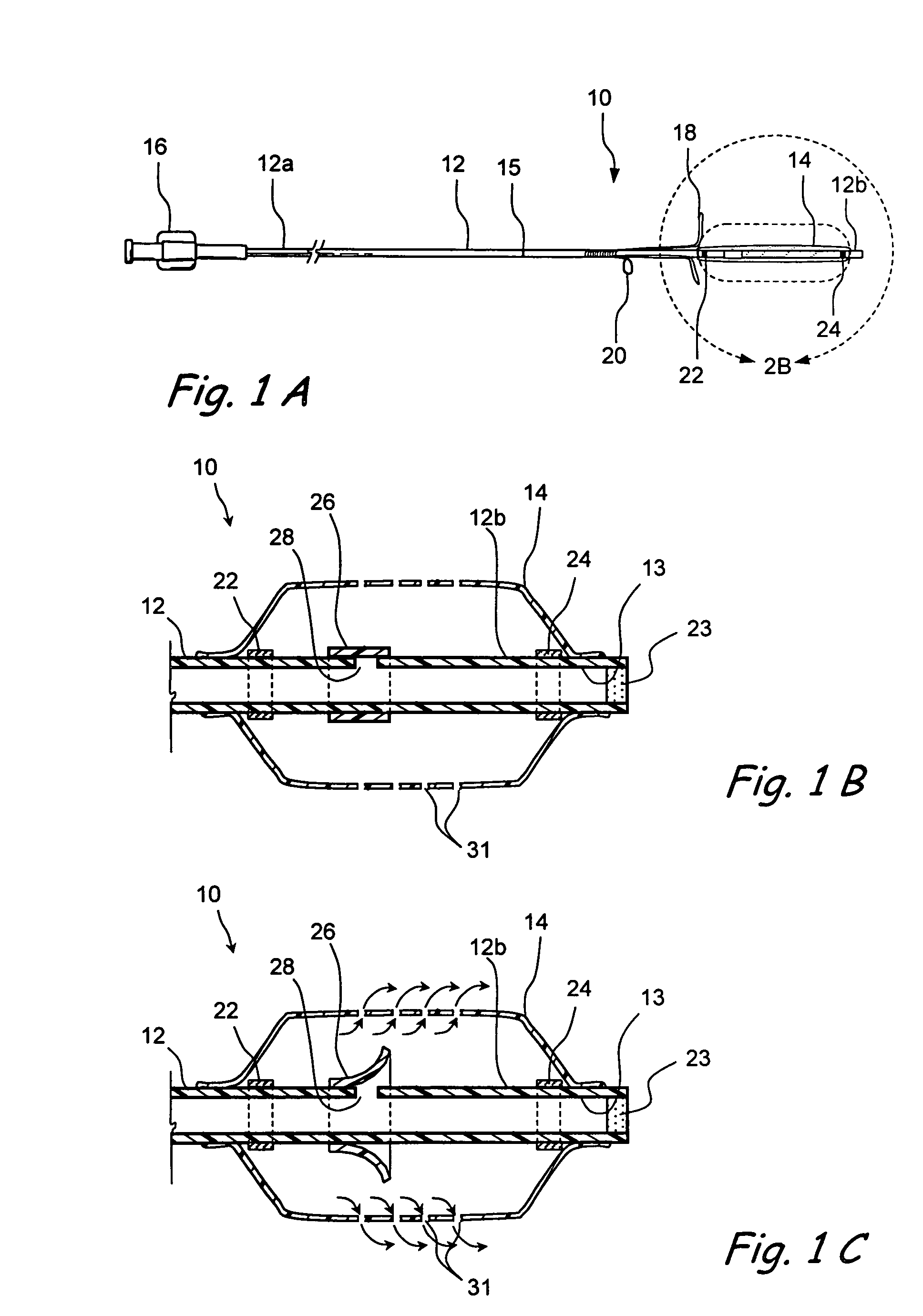
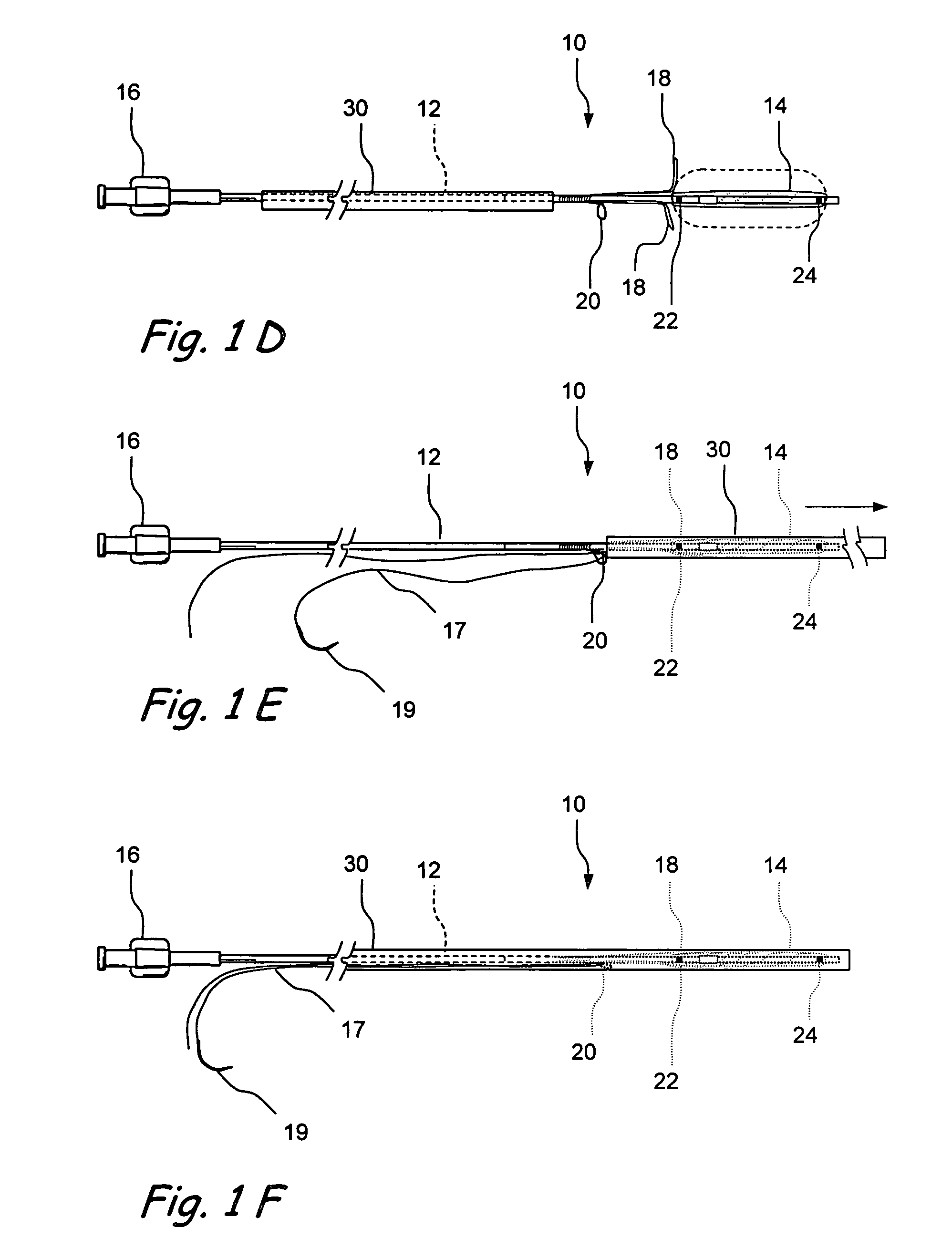
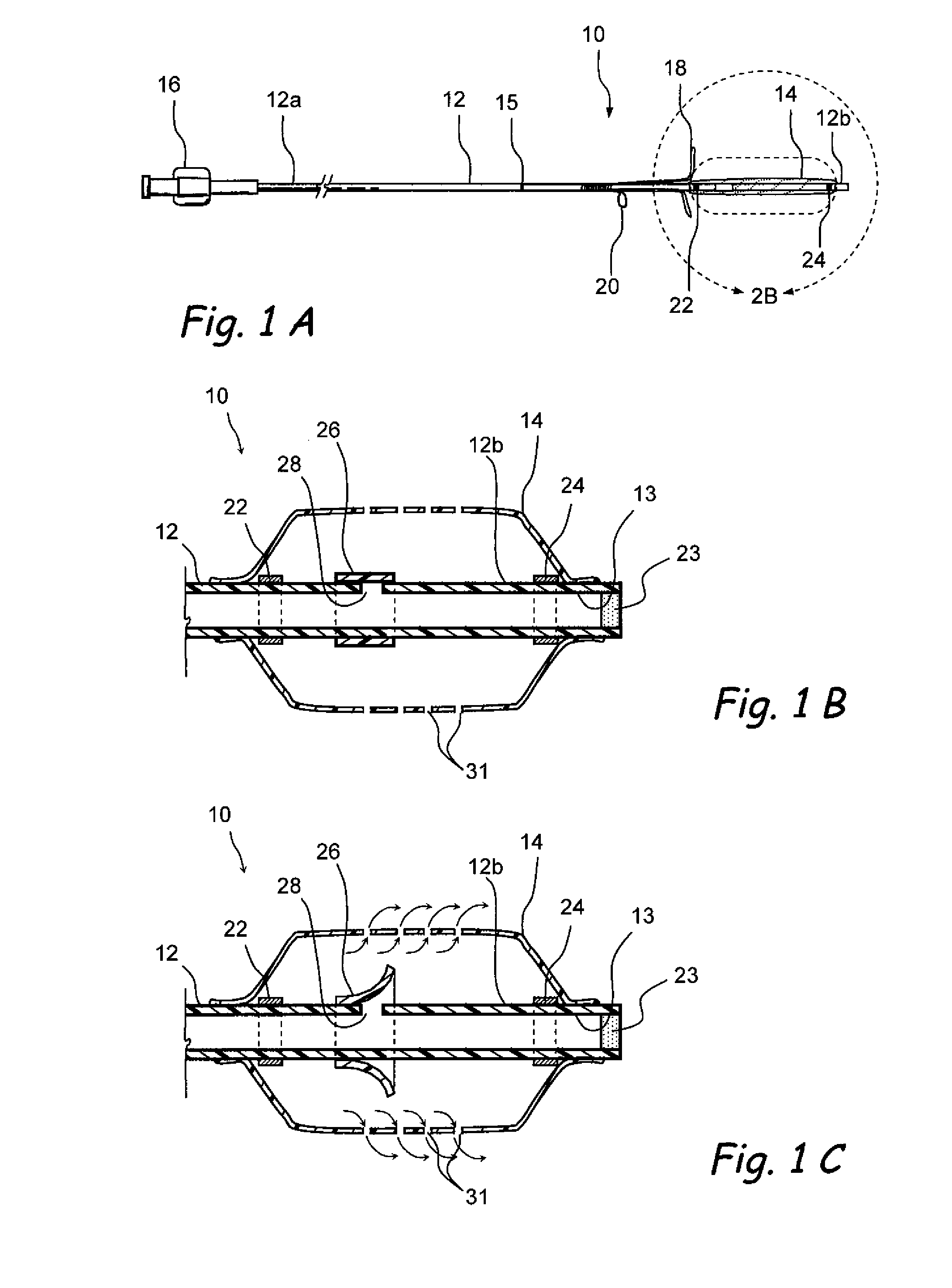
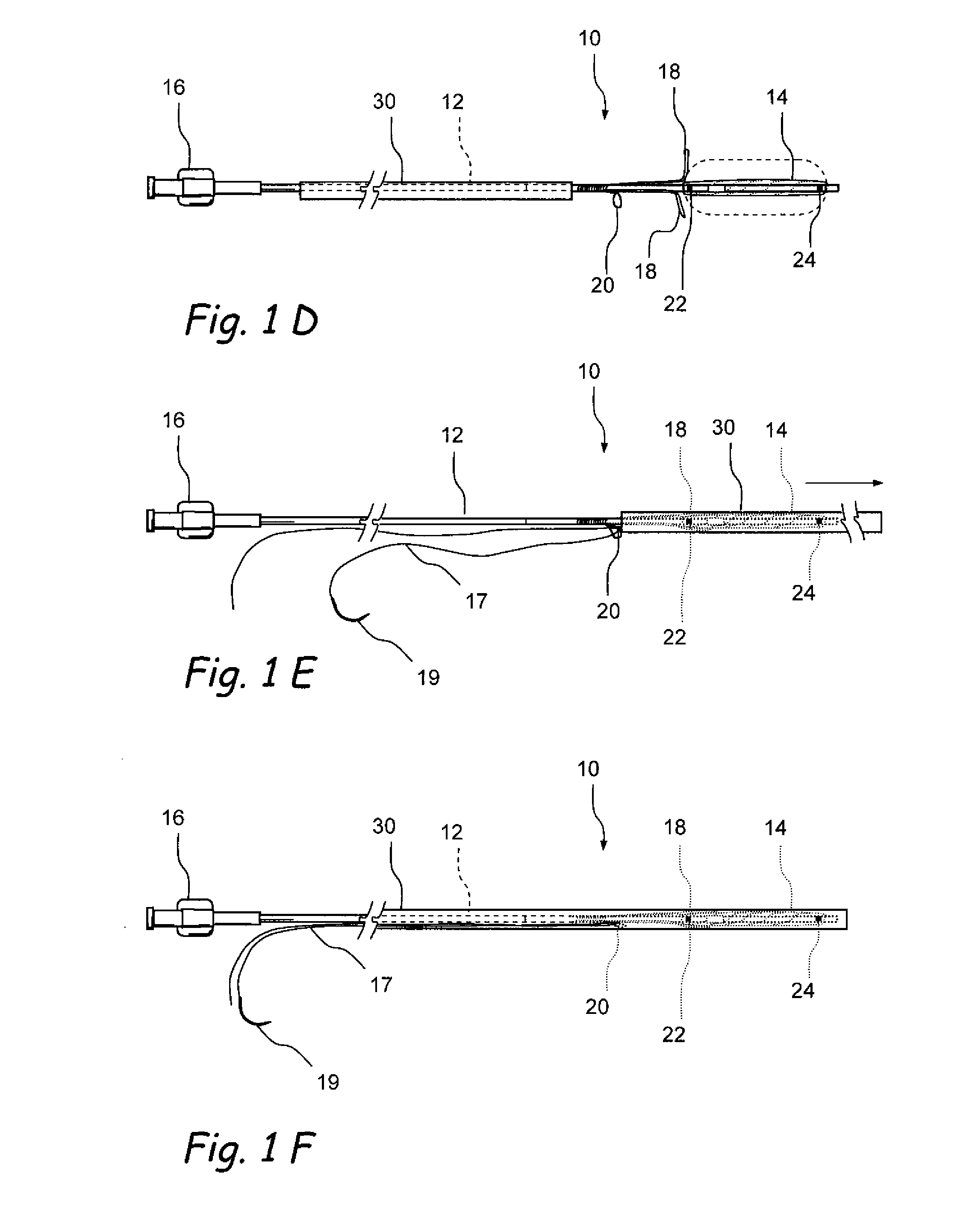
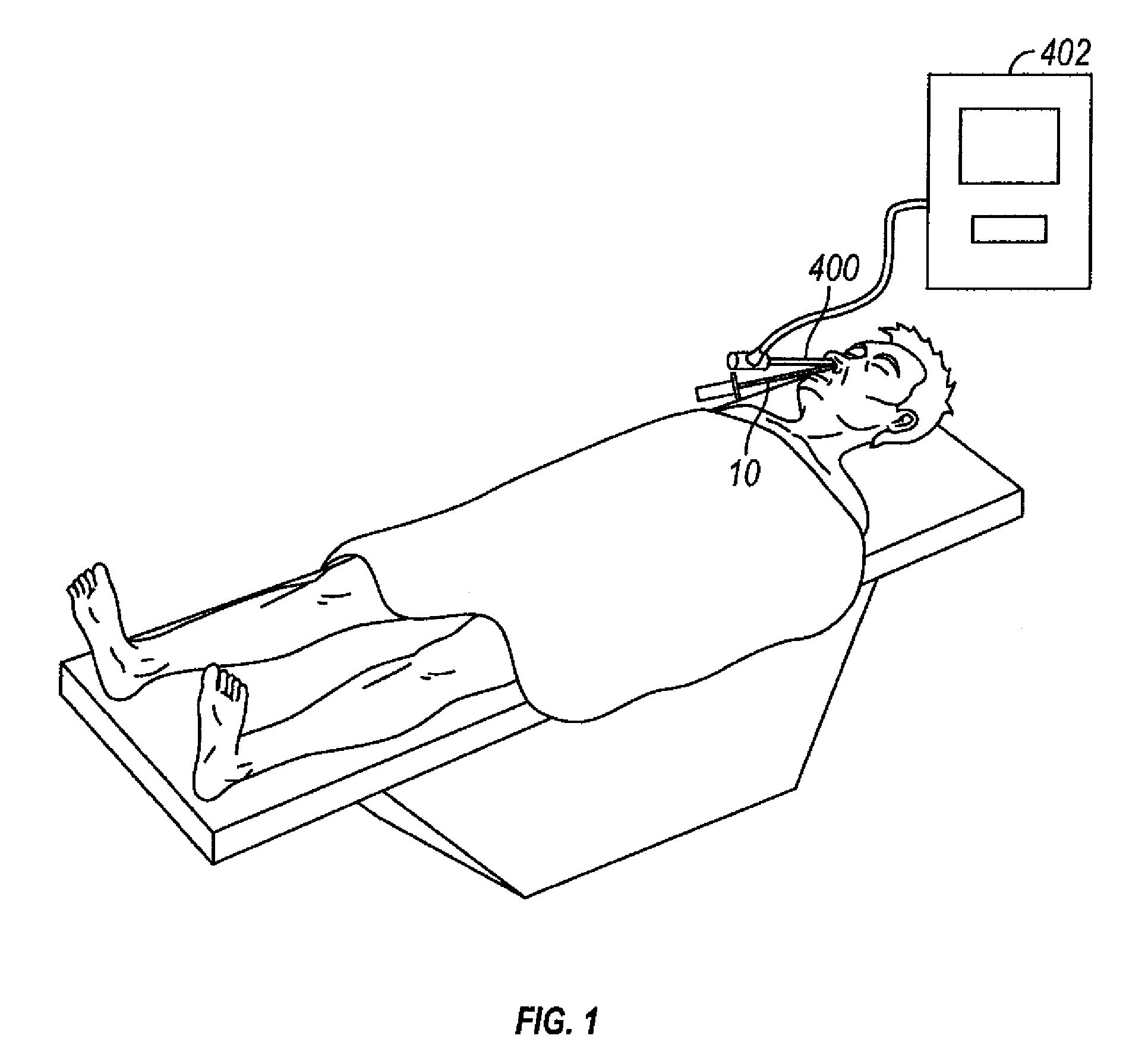
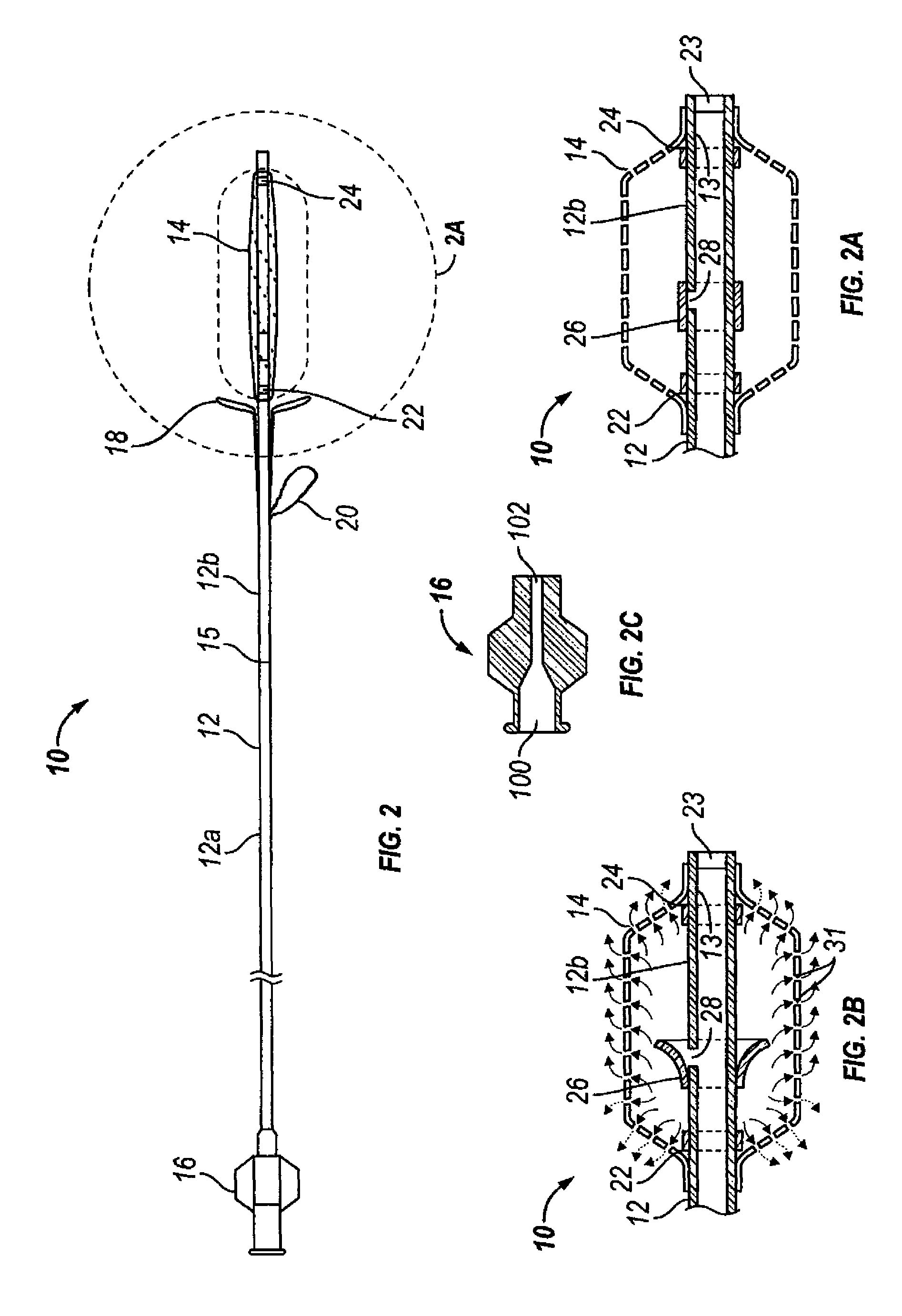
Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders
Devices and methods for delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects. An implantable delivery device comprising a reservoir is initianlly attached to a deliver catheter or delivery tool and is introduced into the body and positioned at a desired site. A therapeutic or diagnostic substance is then introduced into the reservoir and the delivery catheter or deliver tool is then removed, leaving the implantable delivery device implanted within the body. The substance is then delivered from the reservoir at a rate that causes the desire diagnostic or therapeutic effect. Also provided are substance eluting stents that elute substance from a selected surface of the stent (e.g., the outer surface) but not from another surface of the stent (e.g., the inner surface).
Owner:ACCLARENT INC
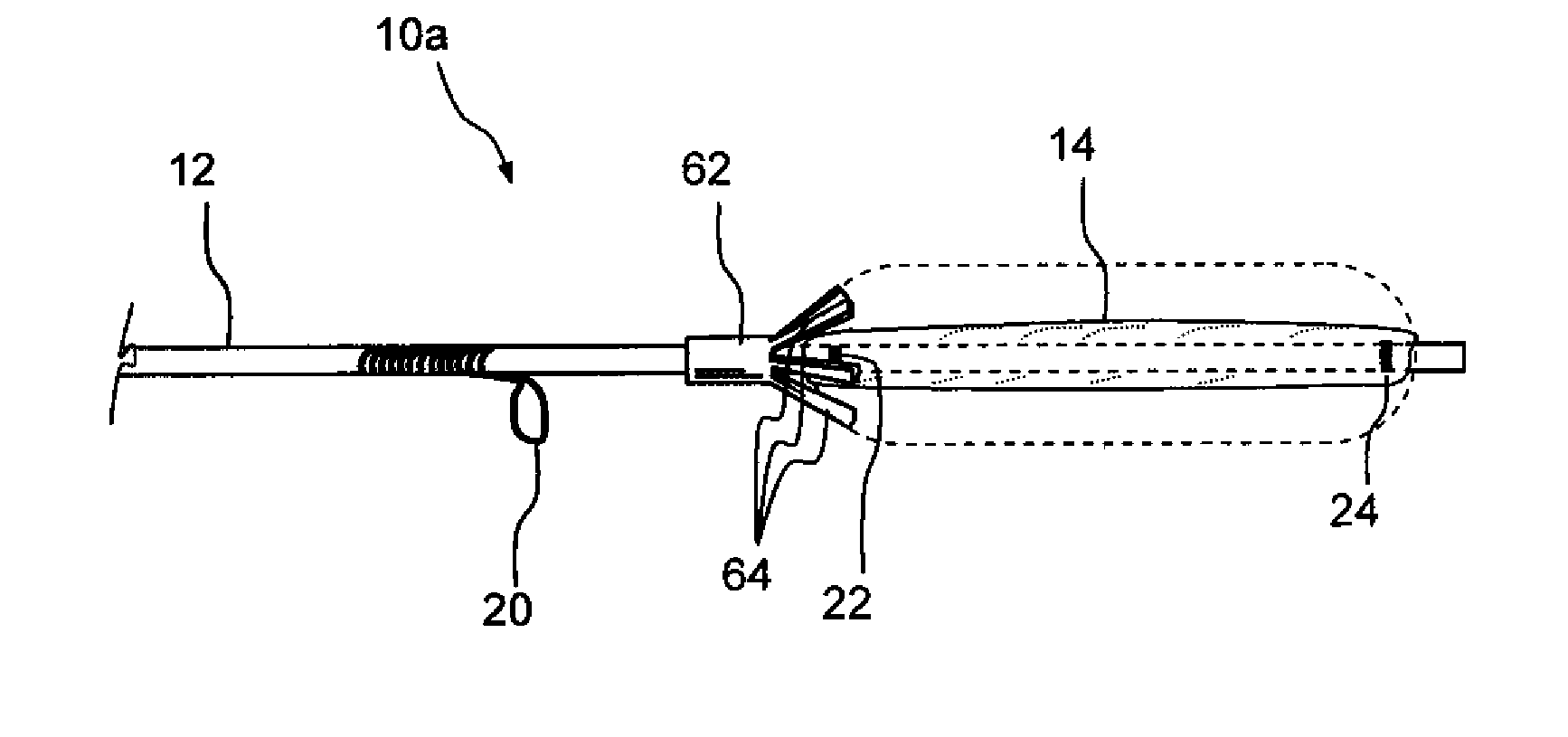
Implantable devices and methods for treating sinusitis and other disorders
ActiveUS20080015540A1Low profileMinimize traumaGuide needlesBalloon catheterParanasal sinusitisDisease
Devices, systems and methods for stenting, spacing, draining, ventilating and / or delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects, including methods and systems for treating paranasal sinusitis and ethmoid disease.
Owner:ACCLARENT INC
Methods for treating ethmoid disease
ActiveUS7419497B2Low profileMinimize traumaGuide needlesBalloon catheterParanasal sinusitisHuman animal
Devices, systems and methods for stenting, spacing, draining, ventilating and / or delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects, including methods and systems for treating paranasal sinusitis and ethmoid disease.
Owner:ACCLARENT INC
Implantable Devices and Methods for Treating Sinusitis and Other Disorders
ActiveUS20090028923A1Low profileMinimize traumaGuide needlesBalloon catheterParanasal sinusitisDisease
Owner:ACCLARENT INC
Bioabsorbable breast implant
InactiveUS6881226B2Reduce scarsOvercome deficienciesMammary implantsPharmaceutical delivery mechanismBreast implantBiomedical engineering
A breast implant has at least an outer shell which is composed of a resorbable material. The implant, which can be formed entirely of bioresorbable material such as a collagen foam, is sized and shaped to replace excised tissue. The implant supports surrounding tissue upon implantation, while allowing for in-growth of fibrous tissue to replace the implant. According to various alternative embodiments, the implant is elastically compressible, or can be formed from self-expanding foam or sponges, and can be implanted through a cannula or by injection, as well as by open procedures. The implant can carry therapeutic and diagnostic substances.
Owner:SENORX
Antisense oligonucleotides against VR1
InactiveUS7662948B2Reduce in quantityAvoid developmentOrganic active ingredientsFungiPain therapyNucleotide
Antisense oligodeoxynucleotides against VR1, corresponding nucleotide constructs, cells containing said nucleotide constructs, pharmaceutical and diagnostic substances, uses thereof in pain therapy, and methods for diagnosing symptoms related to VR1 and for identifying pain-modulating substances.
Owner:GRUNENTHAL GMBH
Tissue marking implant
InactiveUS7637948B2Reduce excised tissueReduce scarsMammary implantsCosmetic implantsSurgical departmentBiomedical engineering
A tissue marking implant includes a matrix material and a dye marker. The implant, which can be formed entirely of bioresorbable material such as a collagen foam, is sized and shaped to replace excised tissue. The implant supports surrounding tissue upon implantation, while allowing for in-growth of fibrous tissue to replace the implant. According to various alternative embodiments, the implant is elastically compressible, or can be formed from self-expanding foam or sponges, and can be implanted through a cannula or by injection, as well as by open procedures. The implant can carry therapeutic and diagnostic substances. The dye marker leaches from the implant such that a surgeon, upon subsequent surgical intervention, visibly recognizes the tissue marked by the dye marker.
Owner:SENORX
Biopsy cavity marking device and method
InactiveUS20050080338A1Mark accuratelyUltrasonic/sonic/infrasonic diagnosticsMammary implantsFiberBreast implant
These are breast implant devices and methods of use. The breast implants are made of a matrix of collagen material having a porous structure for supporting surrounding tissue of a breast. The implants are also configured to provide a framework for the in-growth of fibrous tissue into the matrix. The matrix can be resilient and / or self-expanding. The methods of use include the steps of forming a cavity having surrounding breast tissue and forming a resorbable implant made up of collagen that is sized to occupy the cavity. The implant is then implanted into the cavity, thereby supporting the surrounding tissue and allowing for in-growth of fibrous tissue into and replacing the resorbable material. The resorbable material may also be elastically compressible, such that the step of implanting includes the step of compressing the resorbable material. The implants may also contain a medicinal, therapeutic, or diagnostic substance.
Owner:DEVICOR MEDICAL PROD
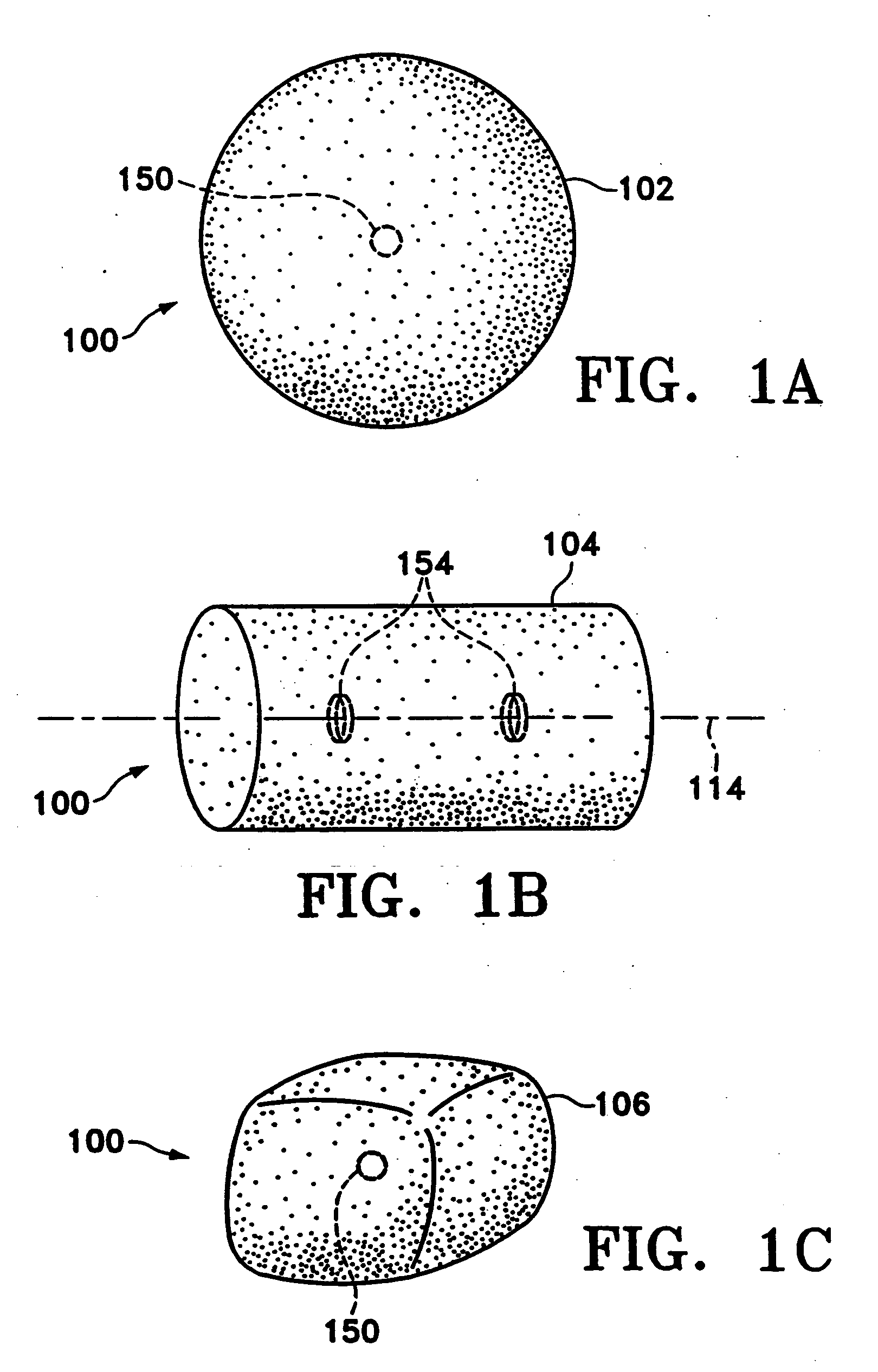
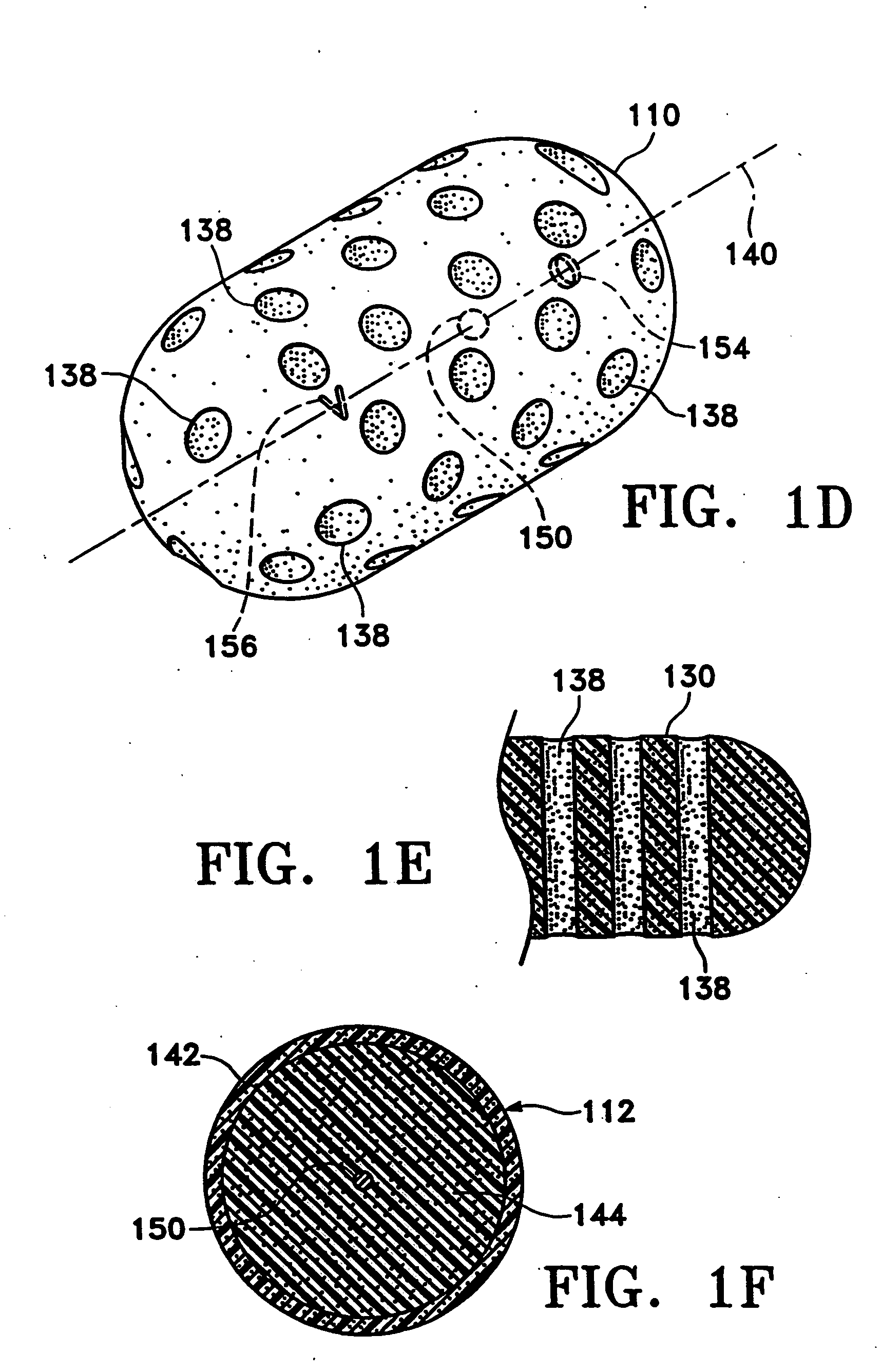
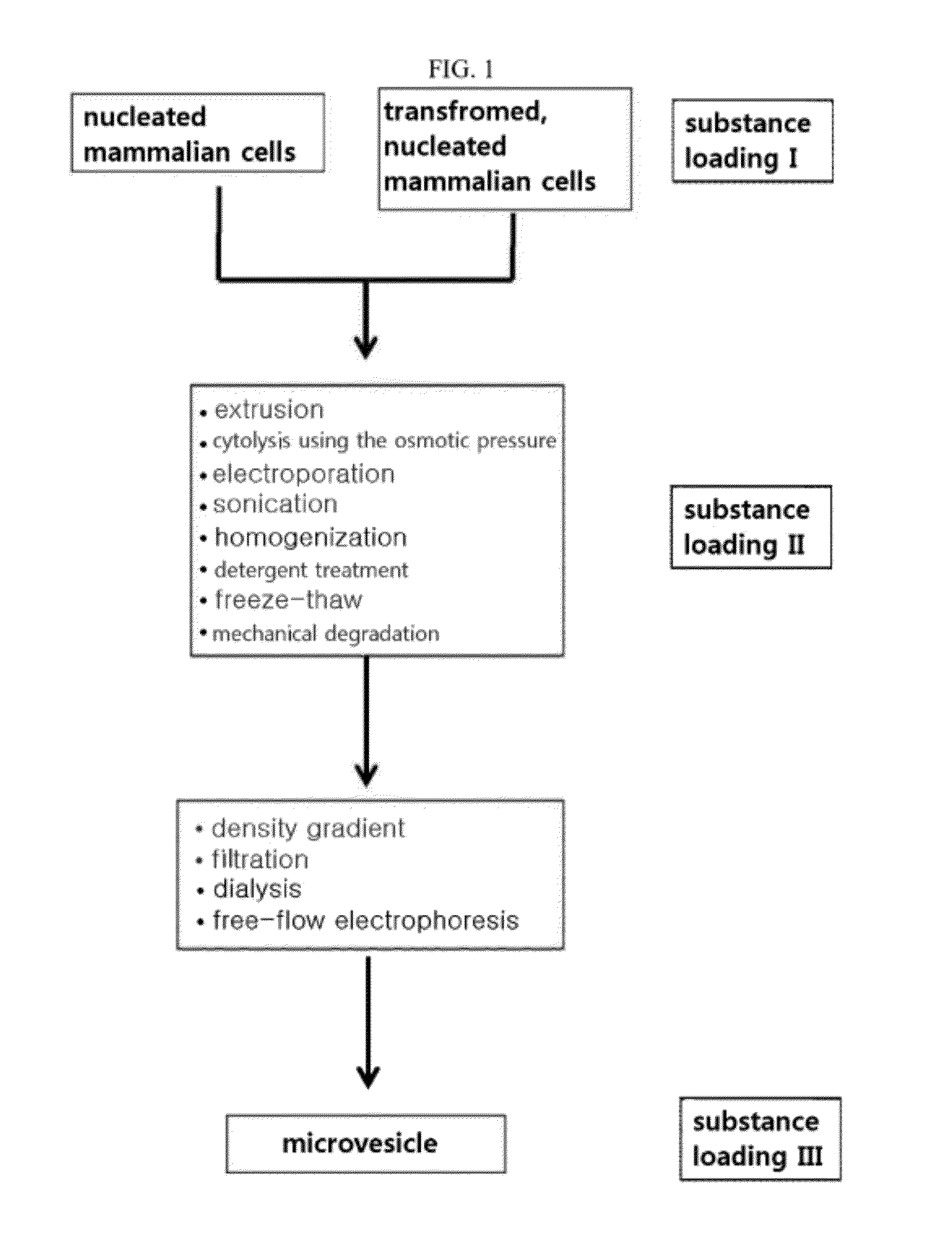
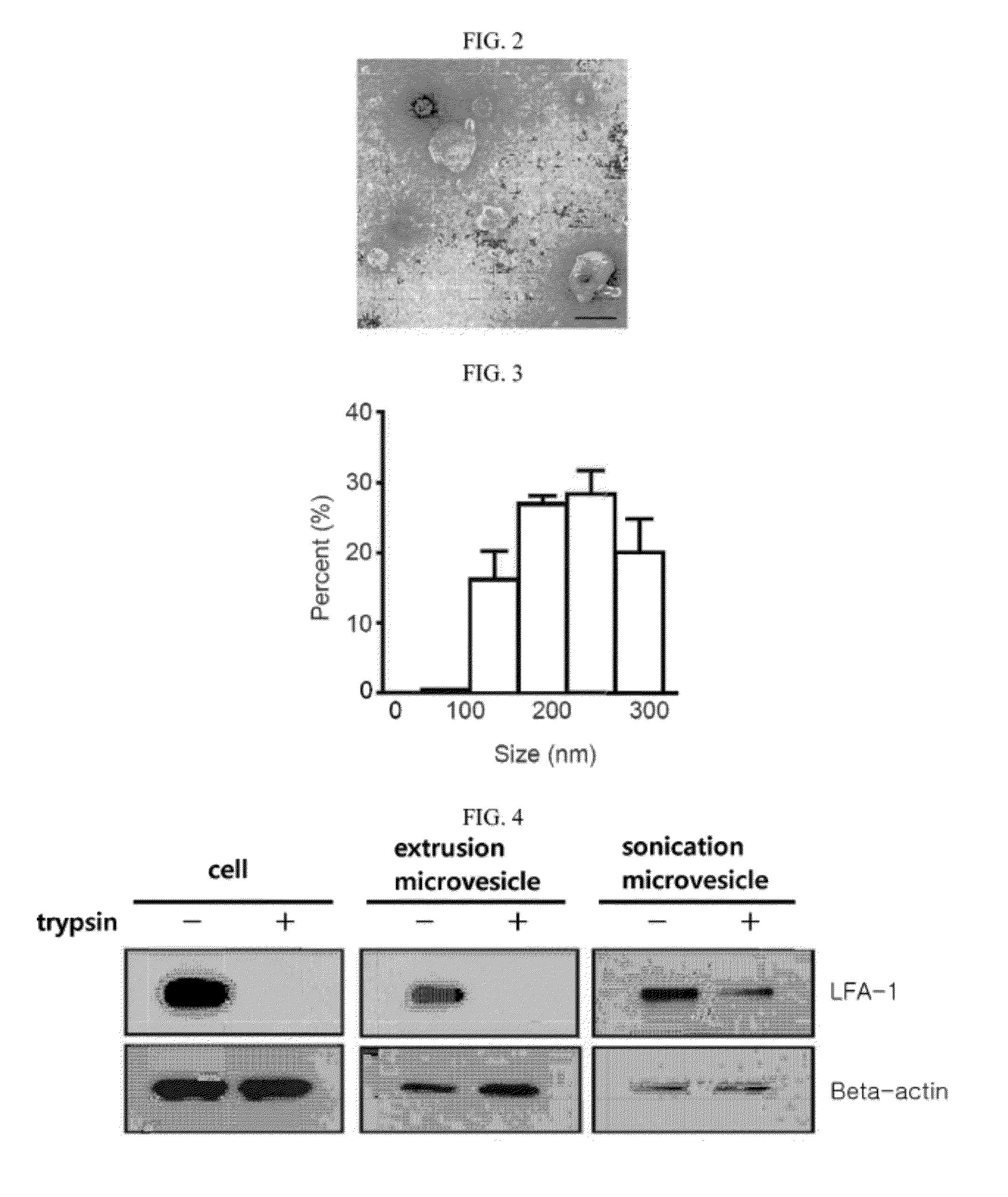
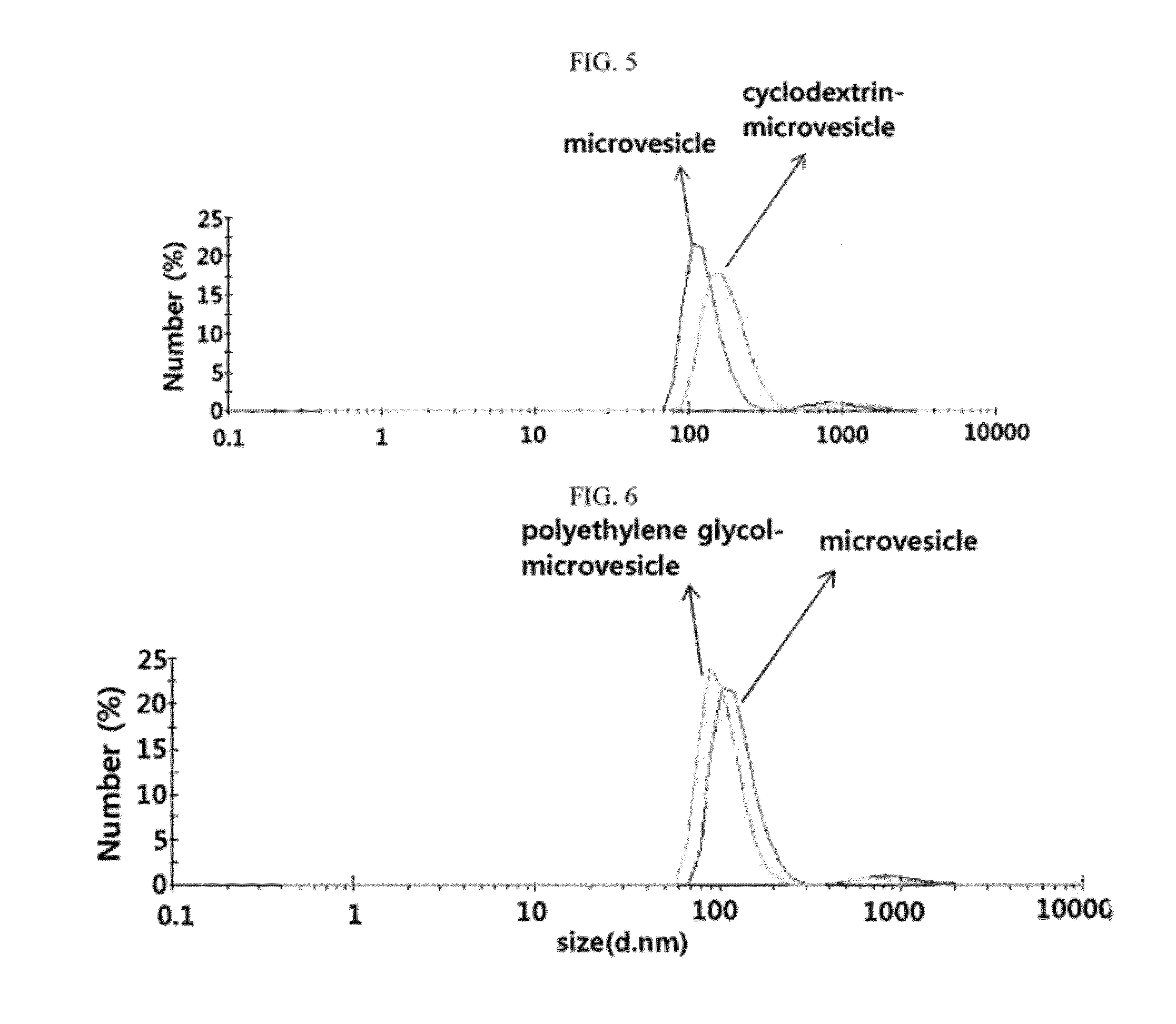
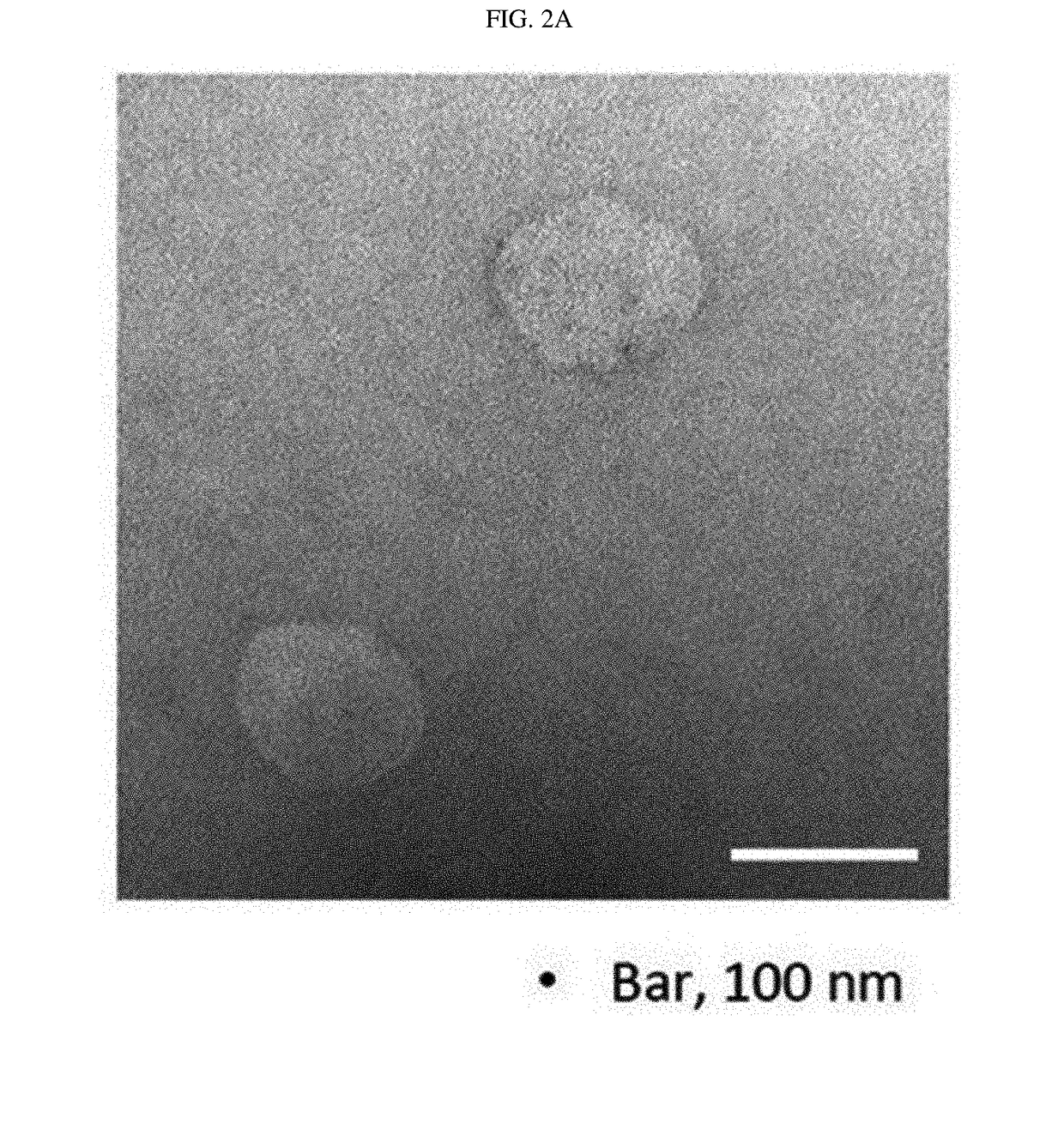
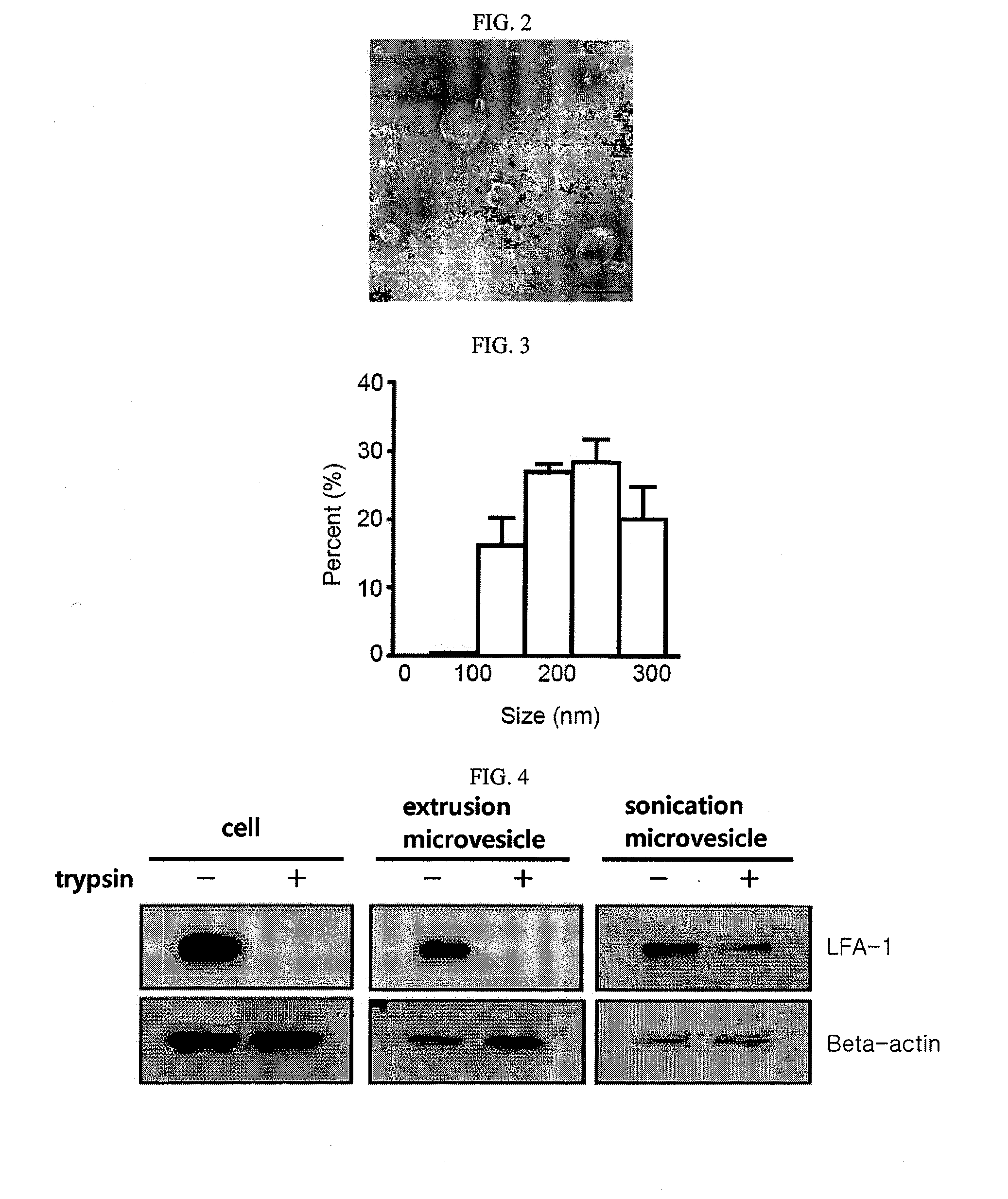
Microvesicles derived from nucleated, mammalian cells and use thereof
InactiveUS20120177574A1Reducing agonyReduce inconvenienceMammal material medical ingredientsIn-vivo testing preparationsDendritic cellMonocyte
The present invention relates to a microvesicle that is derived from nucleated mammalian cells, which are smaller than the nucleated cells. The microvesicles of the present invention can be used in the delivery of a therapeutic or diagnostic substance to specific tissues or cells, and more particularly, relates to microvesicles derived from monocytes, macrophages, dendritic cells, stem cells or the like, which can be used to deliver specific therapeutic or diagnostic substances for treating and / or diagnosing tissue associated with cancer, diseased blood vessels, inflammation, or the like.
Owner:AEON MEDIX
Tissue marking implant
InactiveUS20050187624A1Reduce decreaseReduce scarsMammary implantsCosmetic implantsSurgical departmentFibrous tissue
A tissue marking implant includes a matrix material and a dye marker. The implant, which can be formed entirely of bioresorbable material such as a collagen foam, is sized and shaped to replace excised tissue. The implant supports surrounding tissue upon implantation, while allowing for in-growth of fibrous tissue to replace the implant. According to various alternative embodiments, the implant is elastically compressible, or can be formed from self-expanding foam or sponges, and can be implanted through a cannula or by injection, as well as by open procedures. The implant can carry therapeutic and diagnostic substances. The dye marker leaches from the implant such that a surgeon, upon subsequent surgical intervention, visibly recognizes the tissue marked by the dye marker.
Owner:SENORX
Ethmoidotomy system and implantable spacer devices having therapeutic substance delivery capability for treatment of paranasal sinusitis
InactiveUS8864787B2Effective treatmentGuide needlesBalloon catheterParanasal sinusitisAnatomical structures
Substance delivering spacer devices may comprise expandable reservoirs that are implantable in paranasal sinuses and other cavities, openings and passageways of the body to maintain patency and to provide sustained local delivery of a therapeutic or diagnostic substance. Also provided are sinus penetrator devices and systems for performing ethmoidotomy procedures or for creating other openings in the walls of paranasal sinuses or other anatomical structures.
Owner:ACCLARENT INC
Microbubble compositions and methods for oligonucleotide delivery
InactiveUS7115583B2Low effective doseHigh indexUltrasonic/sonic/infrasonic diagnosticsAntibacterial agentsMedicineBiodistribution
The invention relates to a new and improved pharmaceutical composition and method for delivery of therapeutic agents. The methods and composition of the invention can be used with several therapeutic agents and can achieve site specific delivery of a therapeutic or diagnostic substance. This can allow for lower doses and for improved efficacy with drugs which traditionally reach targeted sites and can result in improved utility for agents such as oligonucleotides and polynucleotides which are plagued with problems with biodistribution.
Owner:AVI BIOPHARMA
Tissue marking implant
InactiveUS20100121445A1Reduce excised tissueReduce scarsMammary implantsCosmetic implantsSurgical departmentBiomedical engineering
A tissue marking implant includes a matrix material and a dye marker. The implant, which can be formed entirely of bioresorbable material such as a collagen foam, is sized and shaped to replace excised tissue. The implant supports surrounding tissue upon implantation, while allowing for in-growth of fibrous tissue to replace the implant. According to various alternative embodiments, the implant is elastically compressible, or can be formed from self-expanding foam or sponges, and can be implanted through a cannula or by injection, as well as by open procedures. The implant can carry therapeutic and diagnostic substances. The dye marker leaches from the implant such that a surgeon, upon subsequent surgical intervention, visibly recognizes the tissue marked by the dye marker.
Owner:SENORX
Treatment of transformed or infected biological cells
InactiveUS20080069772A1High activityEasy to distinguishBiocidePeptide/protein ingredientsBiological cellOrganism
The present invention relates to a therapeutic or / and diagnostic substance. Furthermore it relates to an expression vector, to a composition comprising the afore-mentioned substance or / and the afore-mentioned expression vector, a method for diagnosing a tumor disease or / and an infectious disease in a living being, as well as to a method for the treatment of a tumor disease or / and of an infection in a living being.
Owner:UNIV TUBINGEN
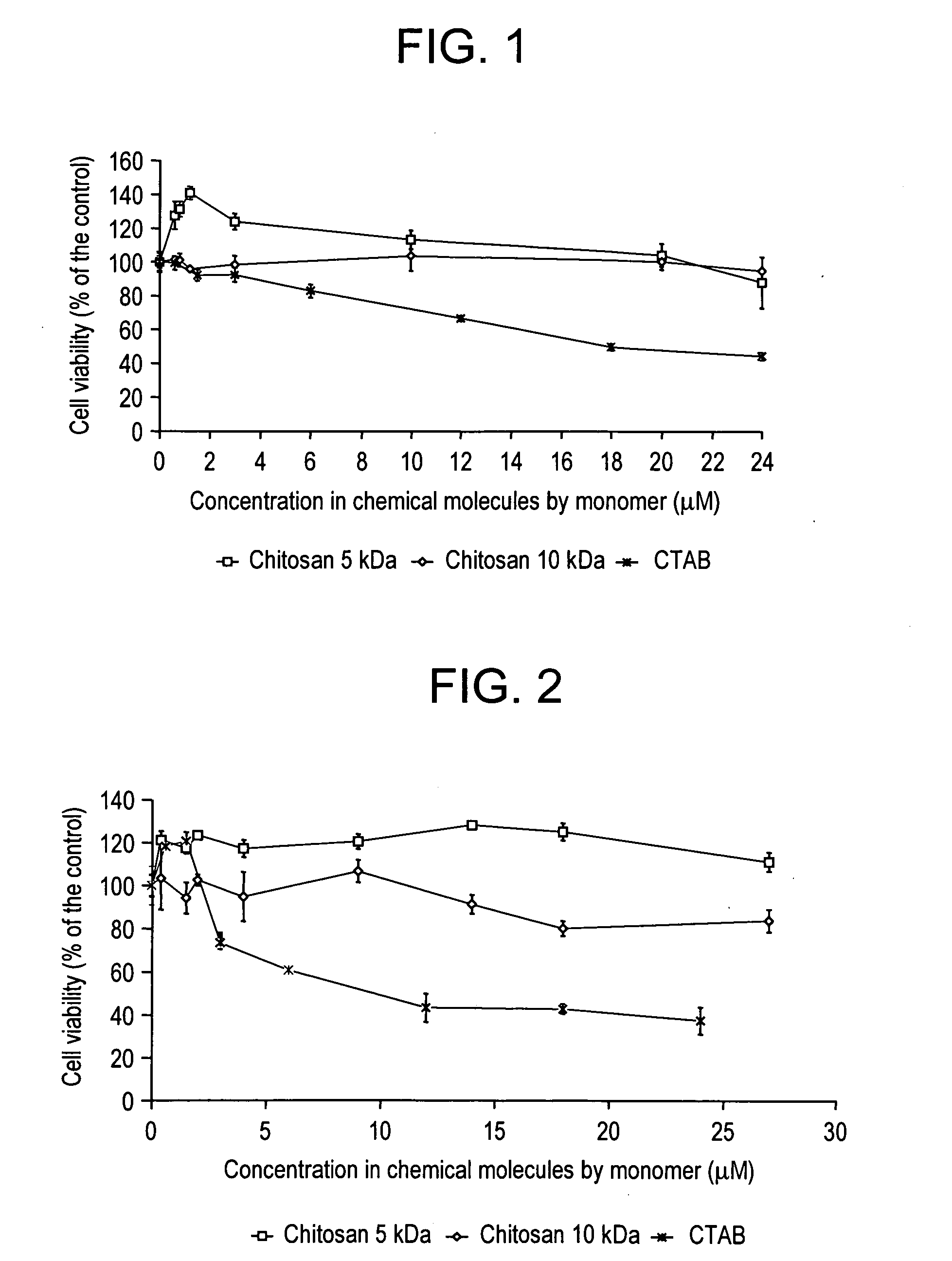
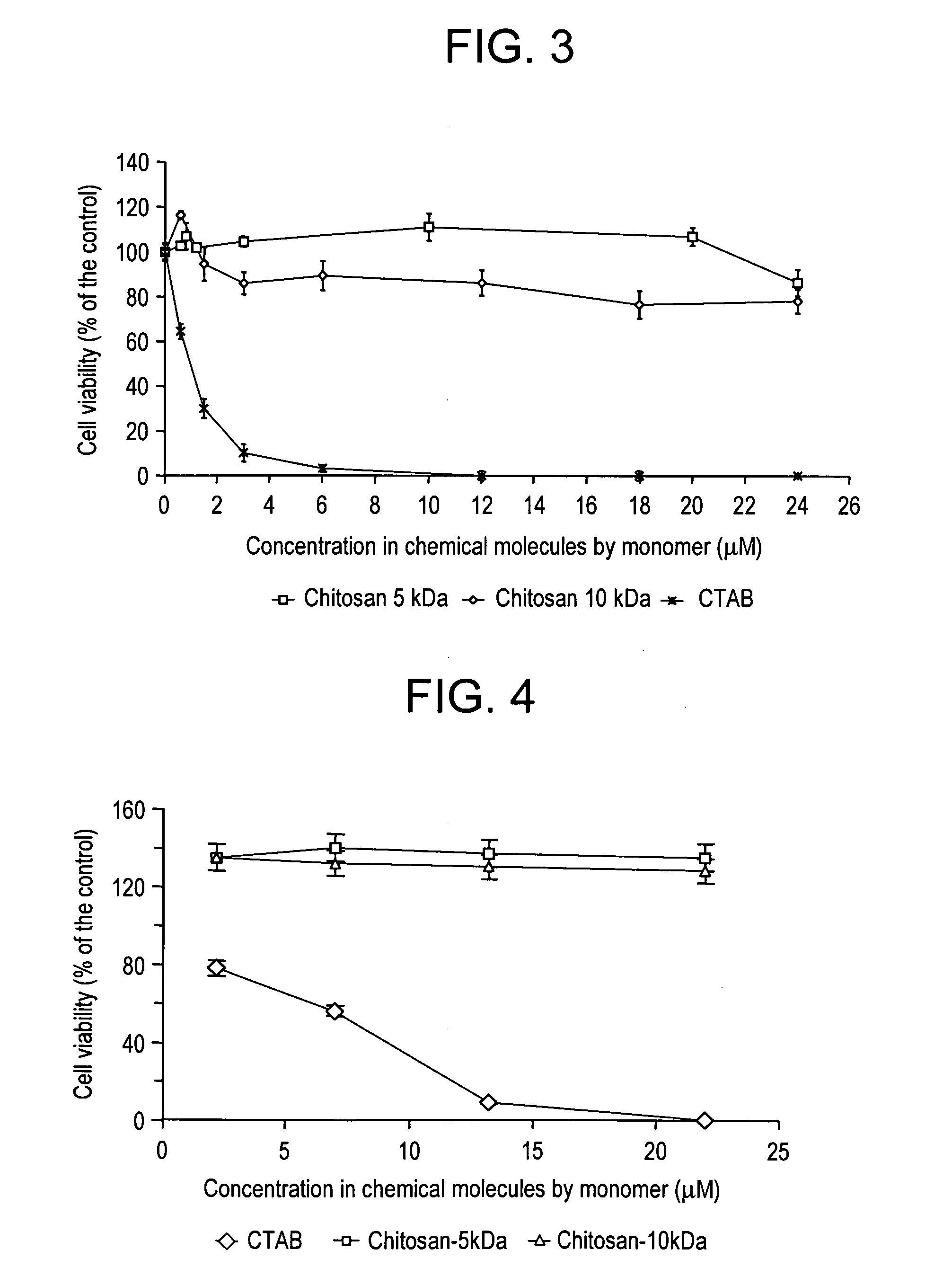
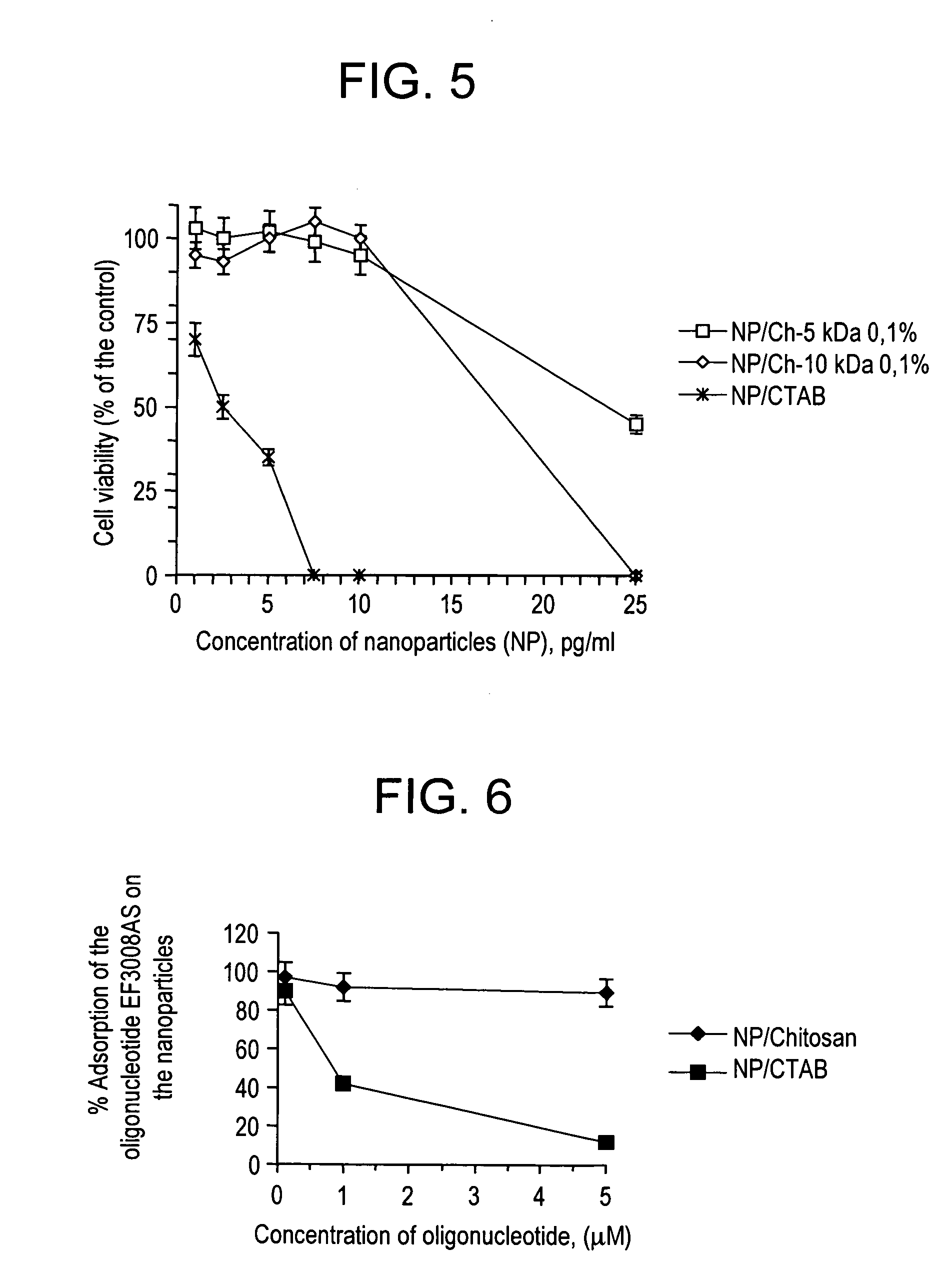
Vectorization system comprising nanoparticles of homogenous size of at least one polymer and at least one positively charged polysaccharide and method for the preparation thereof
A vectorization and delivery system of therapeutic or diagnostic substances including (i) at least one polymer and (ii) at least one nontoxic positively charged polysaccharide, the nanoparticles having a substantially homogeneous distribution of size.
Owner:BIOALLIANCE PHARM SA
Treatment of transformed or infected biological cells
InactiveUS20060046971A1Easy to prepareEasily mutatedBiocideOrganic active ingredientsDiseaseOncology
The present invention relates to a therapeutic or / and diagnostic substance. Furthermore it relates to an expression vector, to a composition comprising the afore-mentioned substance or / and the afore-mentioned expression vector, a method for diagnosing a tumor disease or / and an infectious disease in a living being, as well as to a method for the treatment of a tumor disease or / and of an infection in a living being.
Owner:UNIV TUBINGEN
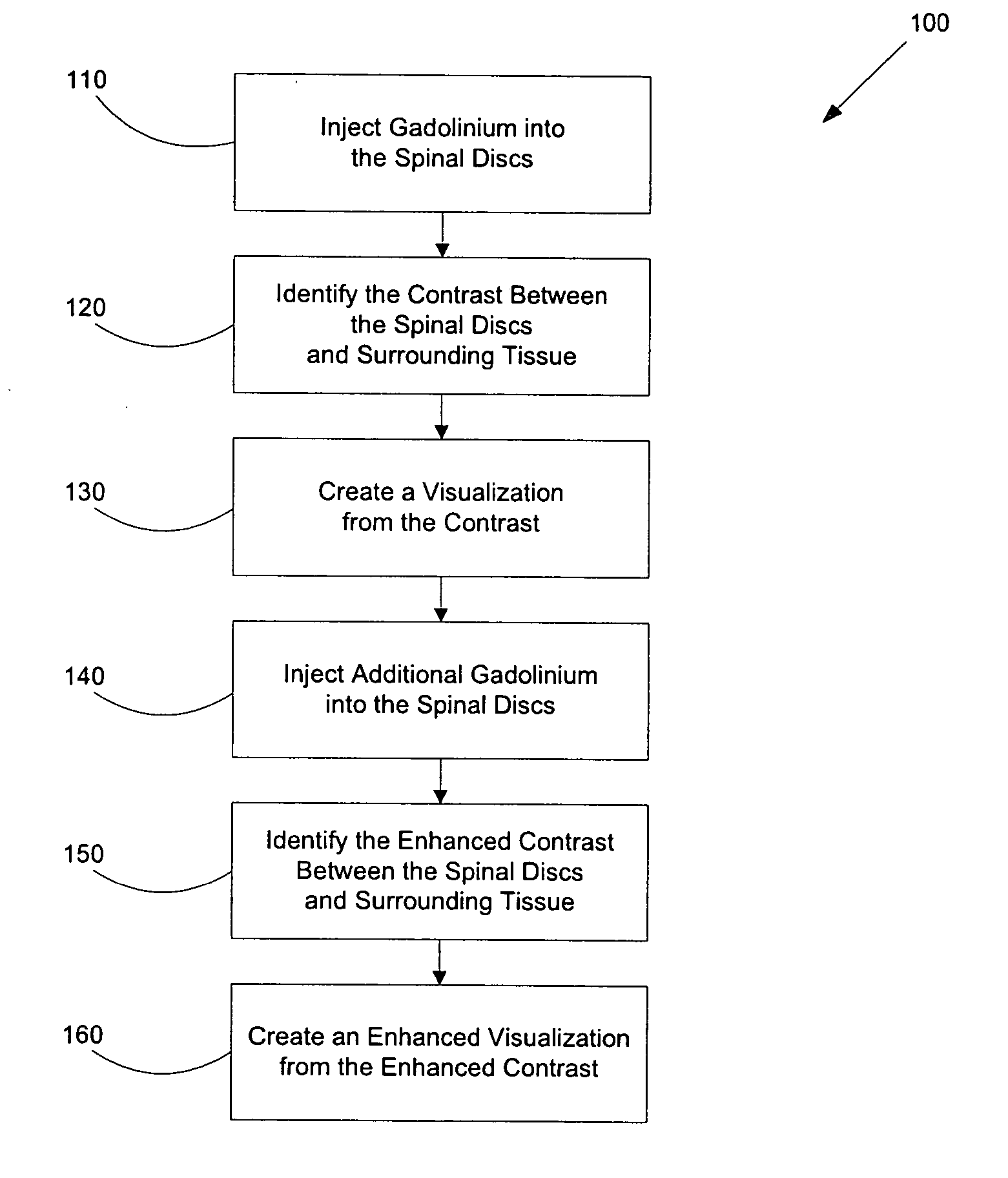
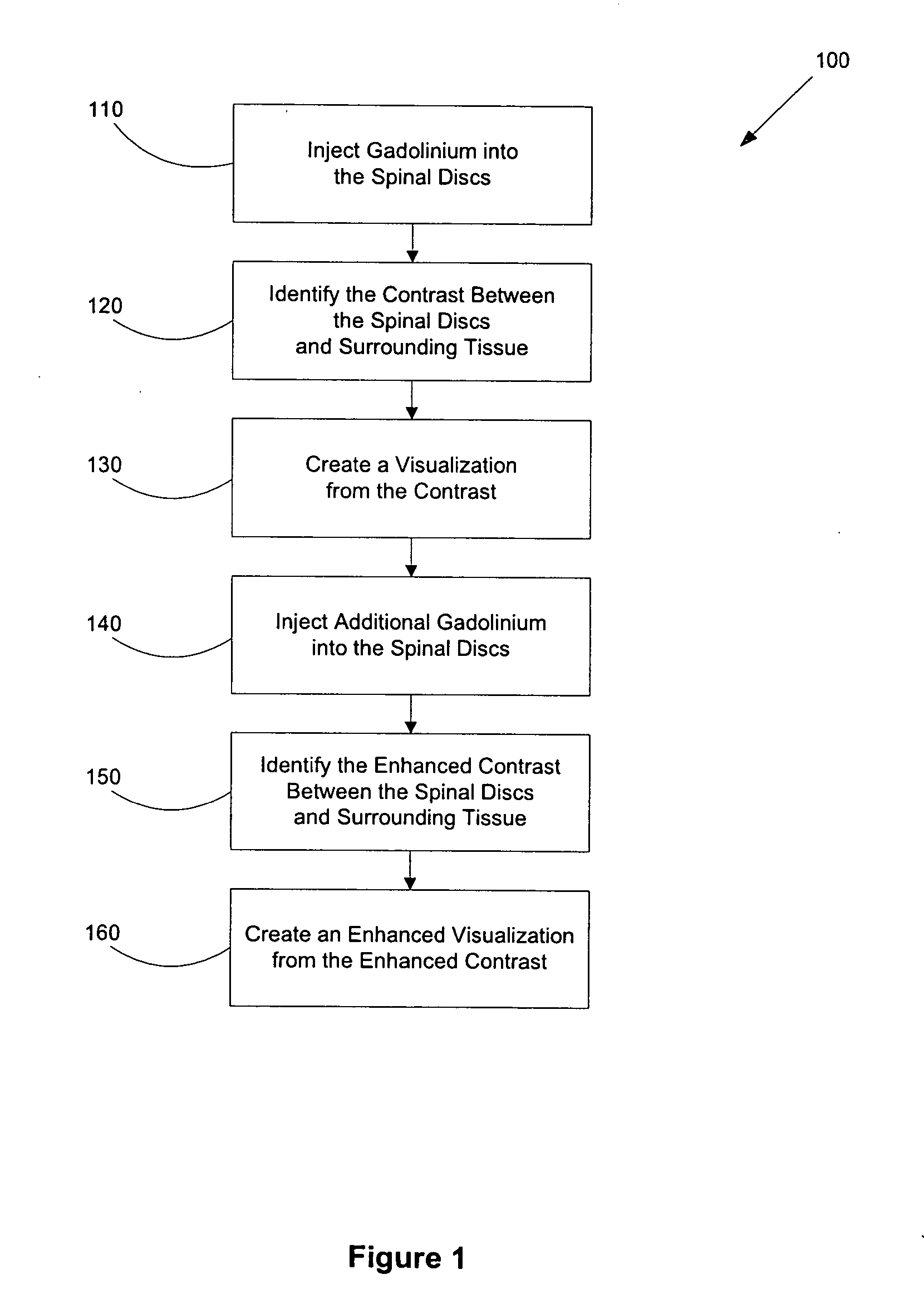
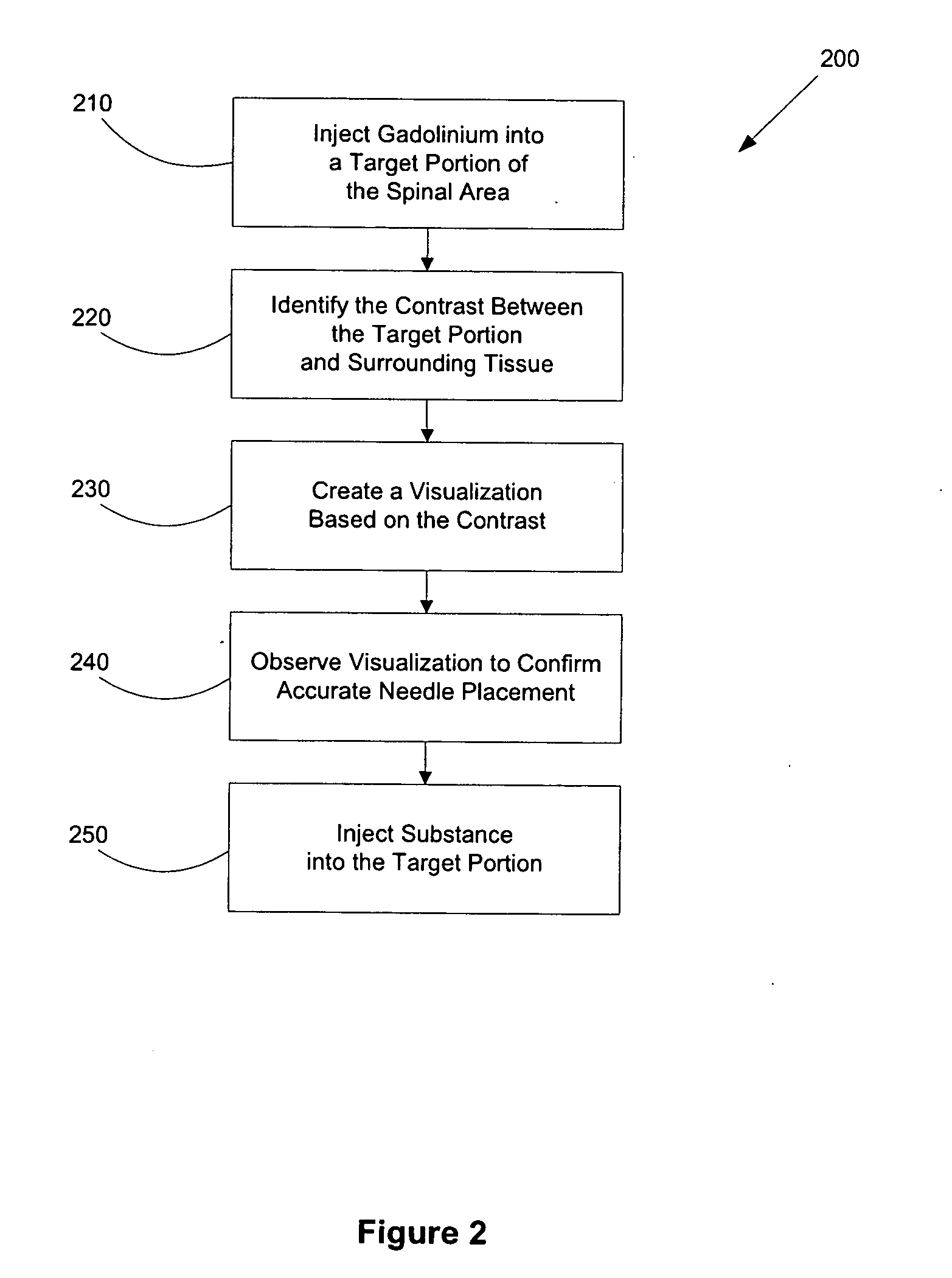
Methods for using gadolinium as a contrast media
InactiveUS20050100510A1Increase contrastImprove visualizationIn-vivo radioactive preparationsDiagnostic recording/measuringGadoliniumContrast medium
Disclosed herein are methods of using gadolinium as a contrast substance. Preferred embodiments of the method use gadolinium to localize placement of a needle for an injection of a substance. The preferred method includes injecting gadolinium into a target portion of a spinal area to create a contrast between the target portion and surrounding tissue and fluoroscopically creating a visualization of the contrast for guiding injection into the target portion of at least one of a diagnostic substance and a therapeutic substance. At least one of the diagnostic substance and the therapeutic substance is preferably then injecting into the target portion. In some aspects of the preferred embodiment, the placement of the needle is adjusted prior to injecting into the target portion at least one of the diagnostic substance and the therapeutic substance. Additional methods are disclosed herein, including for example, methods of using gadolinium to facilitate lumbar discography
Owner:FALCO FR
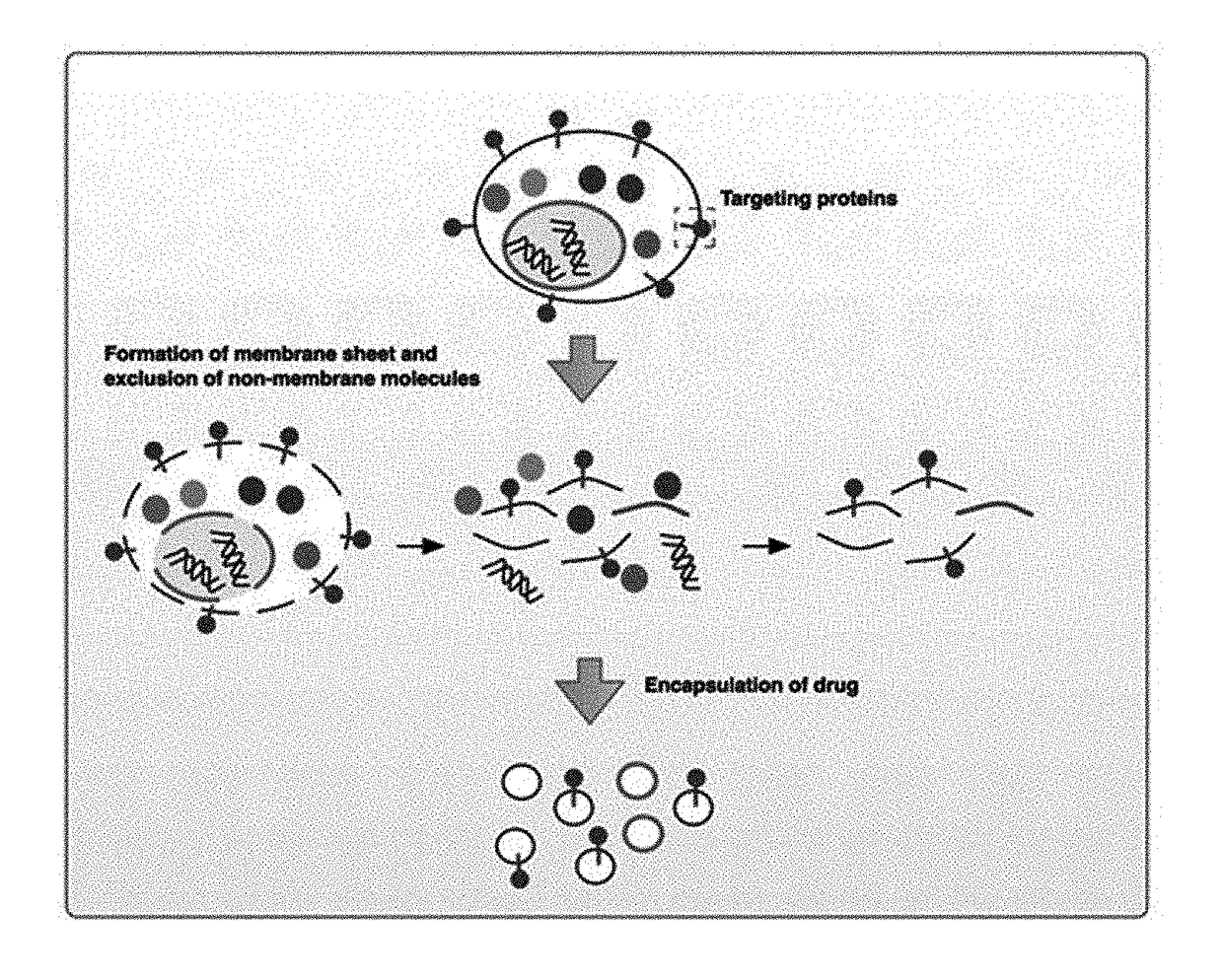
Cell membrane-derived nanovesicles and use thereof
ActiveUS20180036240A1Avoid it happening againGood curative effectHeavy metal active ingredientsOrganic active ingredientsDiseaseTreatment effect
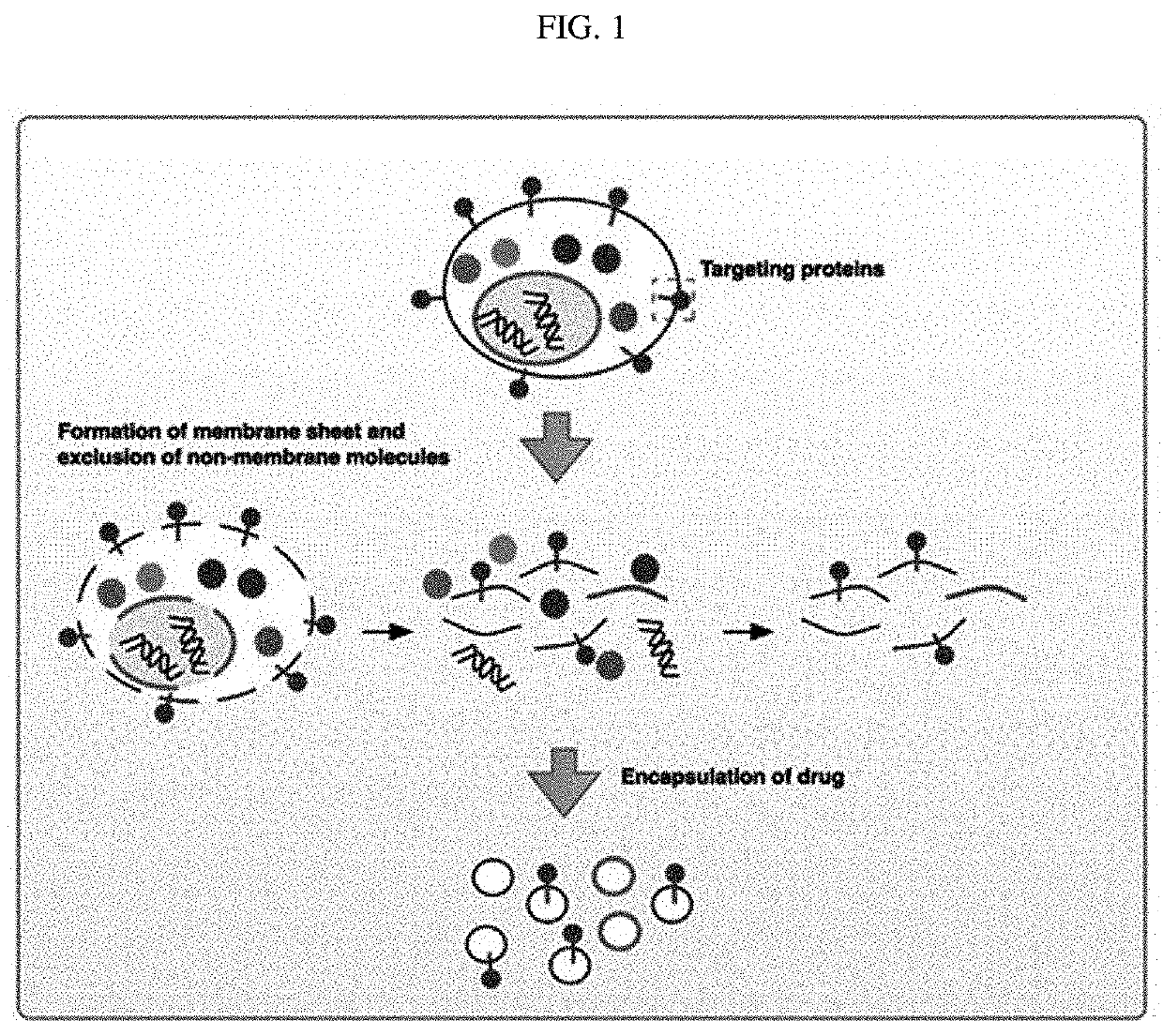
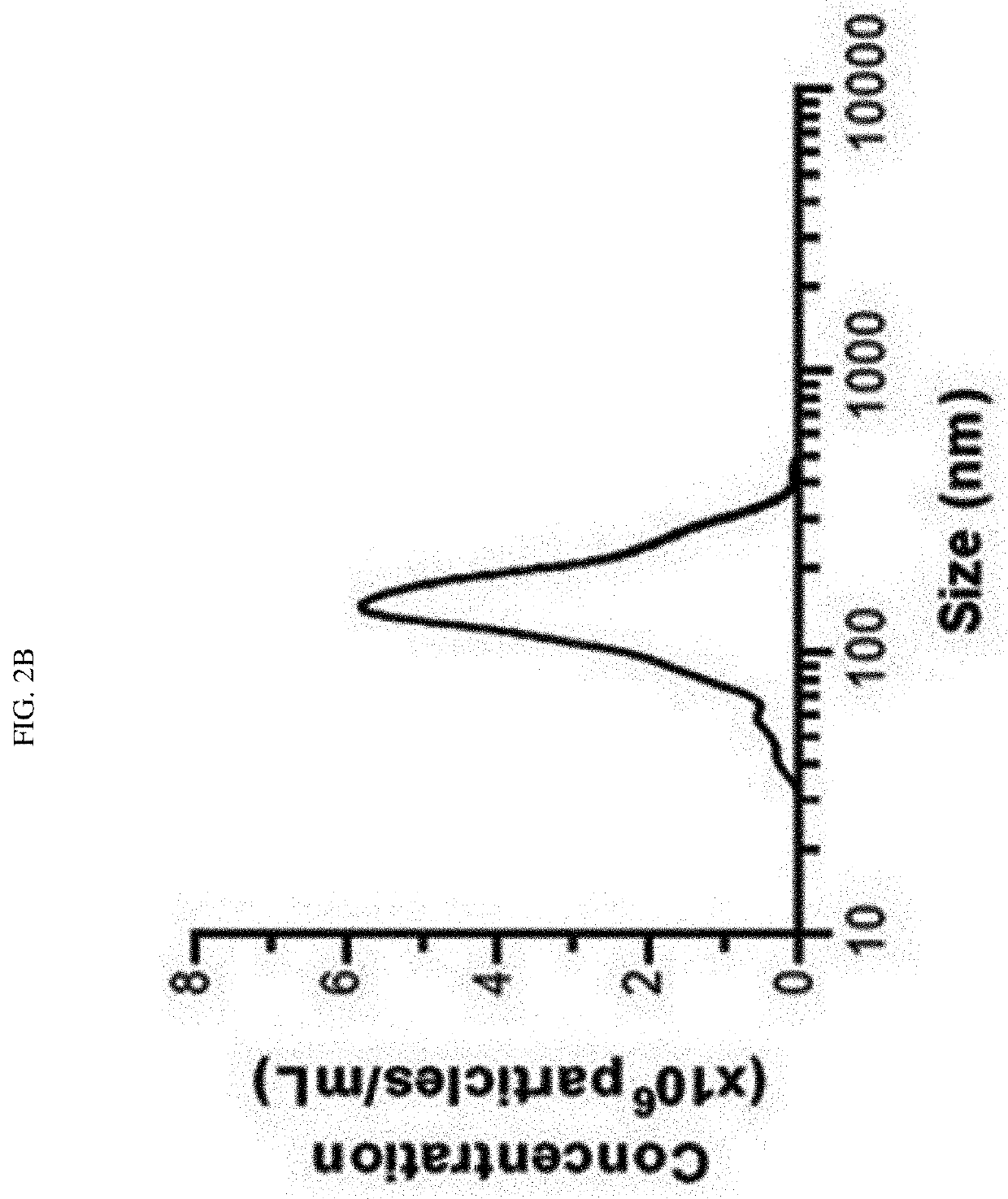
The present invention relates to cell membrane-derived nanovesicles, a method of preparing the same, and a pharmaceutical composition and a diagnostic kit using the nanovesicles. The cell membrane-derived nanovesicles according to the present invention may prevent the occurrence of potential side effects because intracellular materials (e.g., genetic materials and cytosolic proteins) unnecessary for delivery of therapeutic or diagnostic substances are removed from the nanovesicles. In addition, since the nanovesicles may be targeted to the specific types of cells or tissues, therapeutic or diagnostic substances may be predominantly delivered to the targeted cells or tissues while delivery of therapeutic or diagnostic substances to untargeted sites may be inhibited. Therefore, when the cell membrane-derived nanovesicles are applied to disease treatment, the side effects of therapeutic substances such as drugs may be reduced, so that suffering and inconvenience of patients may be alleviated during the course of treating diseases, and therapeutic efficacy may be improved. In addition, the cell membrane-derived nanovesicles of the present invention, in which substances for the treatment or diagnosis of diseases are loaded, and a method of preparing the nanovesicles may be used in vitro or in vivo for therapeutic or diagnostic purposes, or for experimental use.
Owner:POSTECH ACAD IND FOUND
Microvesicles derived from nucleated, mammalian cells and use thereof
InactiveUS20140044647A1Reducing agony and inconvenienceEasy and accurate diagnosisBiocidePeptide/protein ingredientsDendritic cellMonocyte
The present invention relates to a microvesicle that is derived from nucleated mammalian cells, which are smaller than the nucleated cells. The microvesicles of the present invention can be used in the delivery of a therapeutic or diagnostic substance to specific tissues or cells, and more particularly, relates to microvesicles derived from monocytes, macrophages, dendritic cells, stem cells or the like, which can be used to deliver specific therapeutic or diagnostic substances for treating and / or diagnosing tissue associated with cancer, diseased blood vessels, inflammation, or the like.
Owner:MDIMUNE INC
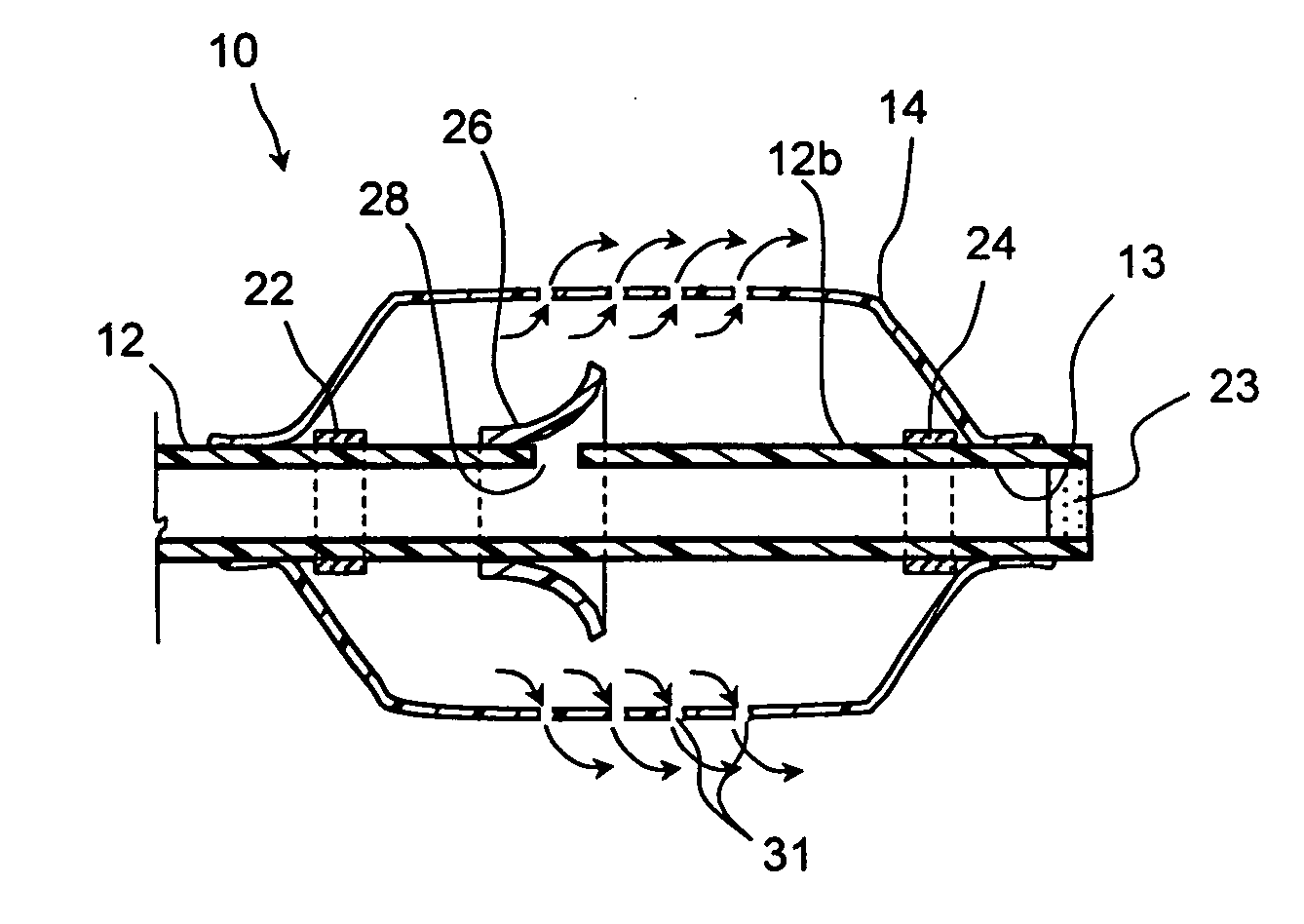
Frontal sinus spacer
Substance delivering spacer devices may comprise expandable reservoirs that are implantable in paranasal sinuses and other cavities, openings and passageways of the body to maintain patency and to provide sustained local delivery of a therapeutic or diagnostic substance. Delivery apparatus including elongate tubular members as well as shapeable distal portions and atraumatic tips are provided. Also provided are sinus penetrator devices and systems for performing ethmoidotomy procedures or for creating other openings in the walls of paranasal sinuses or other anatomical structures.
Owner:ACCLARENT INC
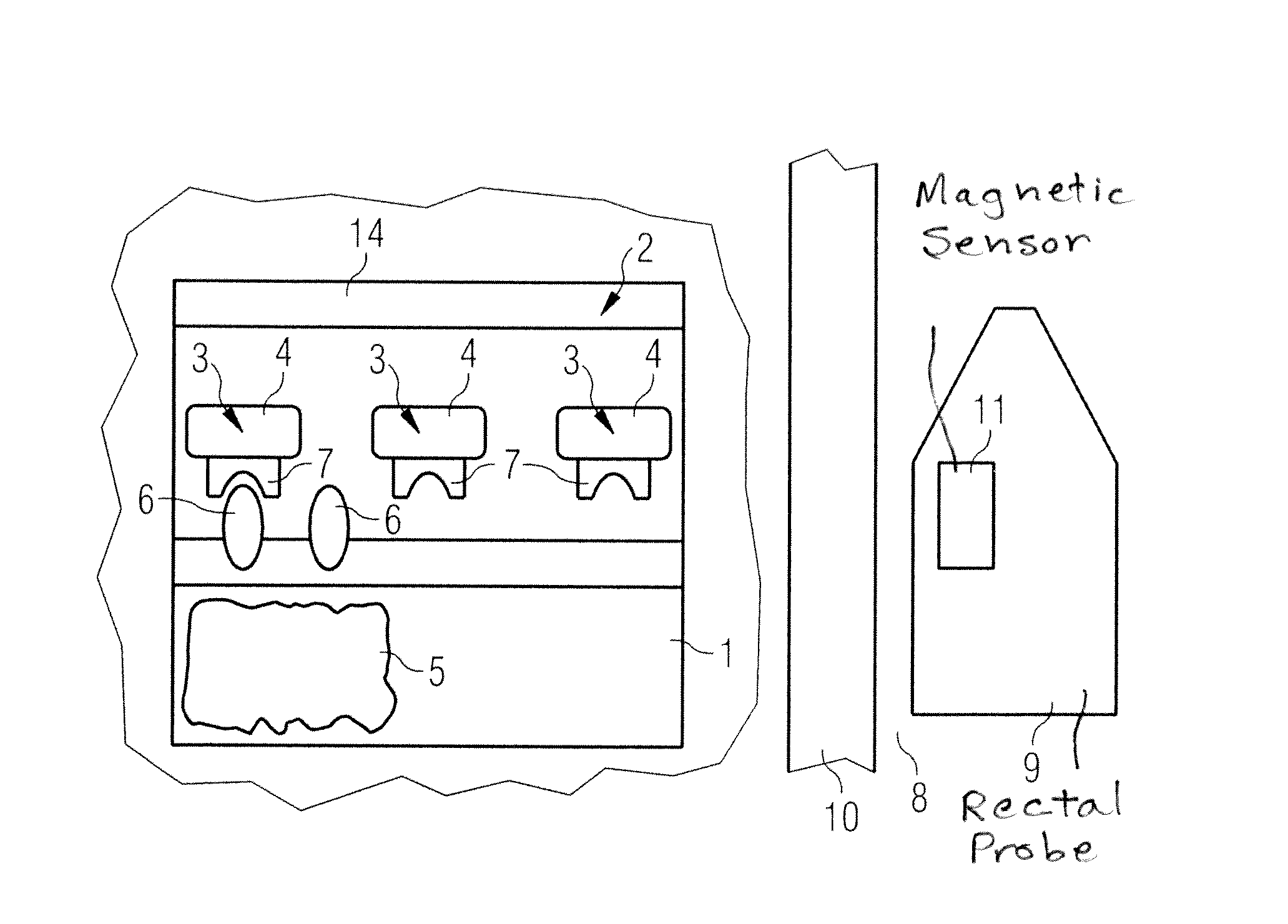
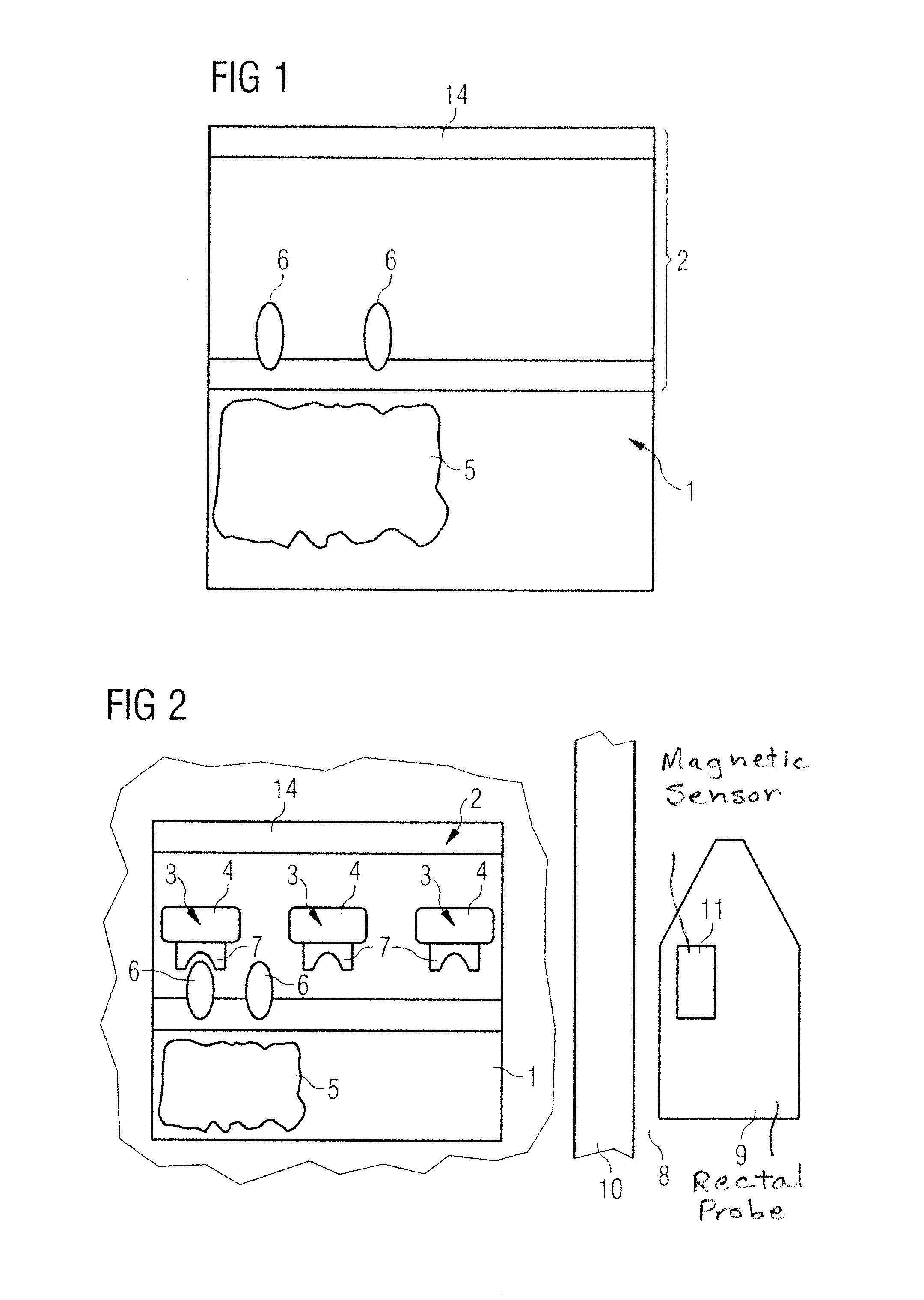
Screening test for recognizing prostate diseases and apparatus and diagnosis substance for carrying out the test
InactiveUS8386012B2Compounds screening/testingDiagnostic recording/measuringProstate gland formationBlood stream
In a screening test method, and a device for implementing the screening test method, a diagnostic substance is provided that contains at least one biomarker connected with at least one ferromagnetic particle, the biomarker binding specifically to a target molecule that is formed by specific pathological prostrate tissue. The diagnostic substance is administered to the blood stream of a patient. A magnetometer is used to detect enrichment of the ferromagnetic particle in the prostrate, as an indicator of a level of the specific pathological prostrate tissue.
Owner:SIEMENS HEALTHCARE GMBH
Compositions and methods for preparing an injectable medium for administration into the central nervous system
InactiveUS20170151286A1Promote cell survivalEasy to manageOrganic active ingredientsPharmaceutical delivery mechanismMedicineCell survival
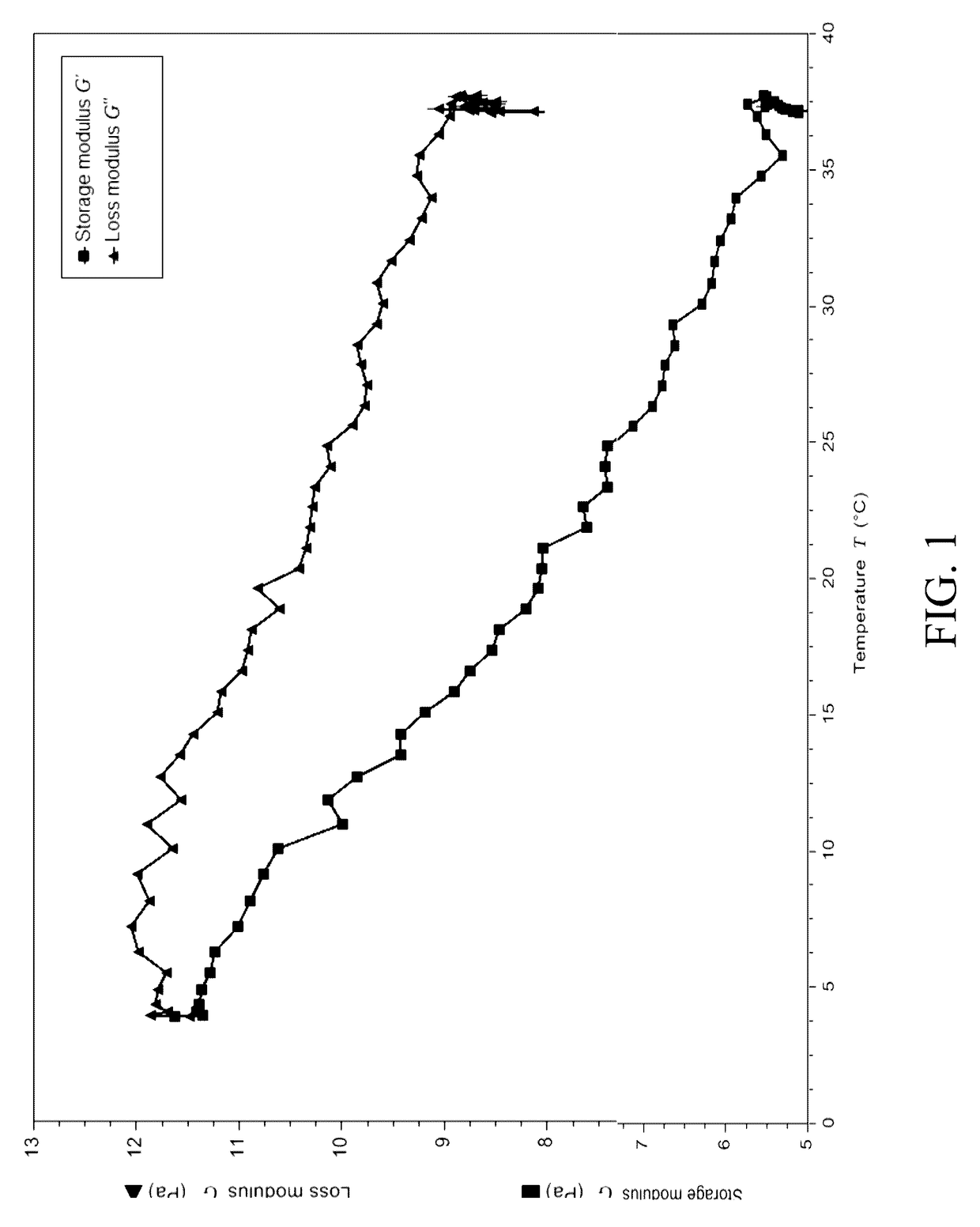
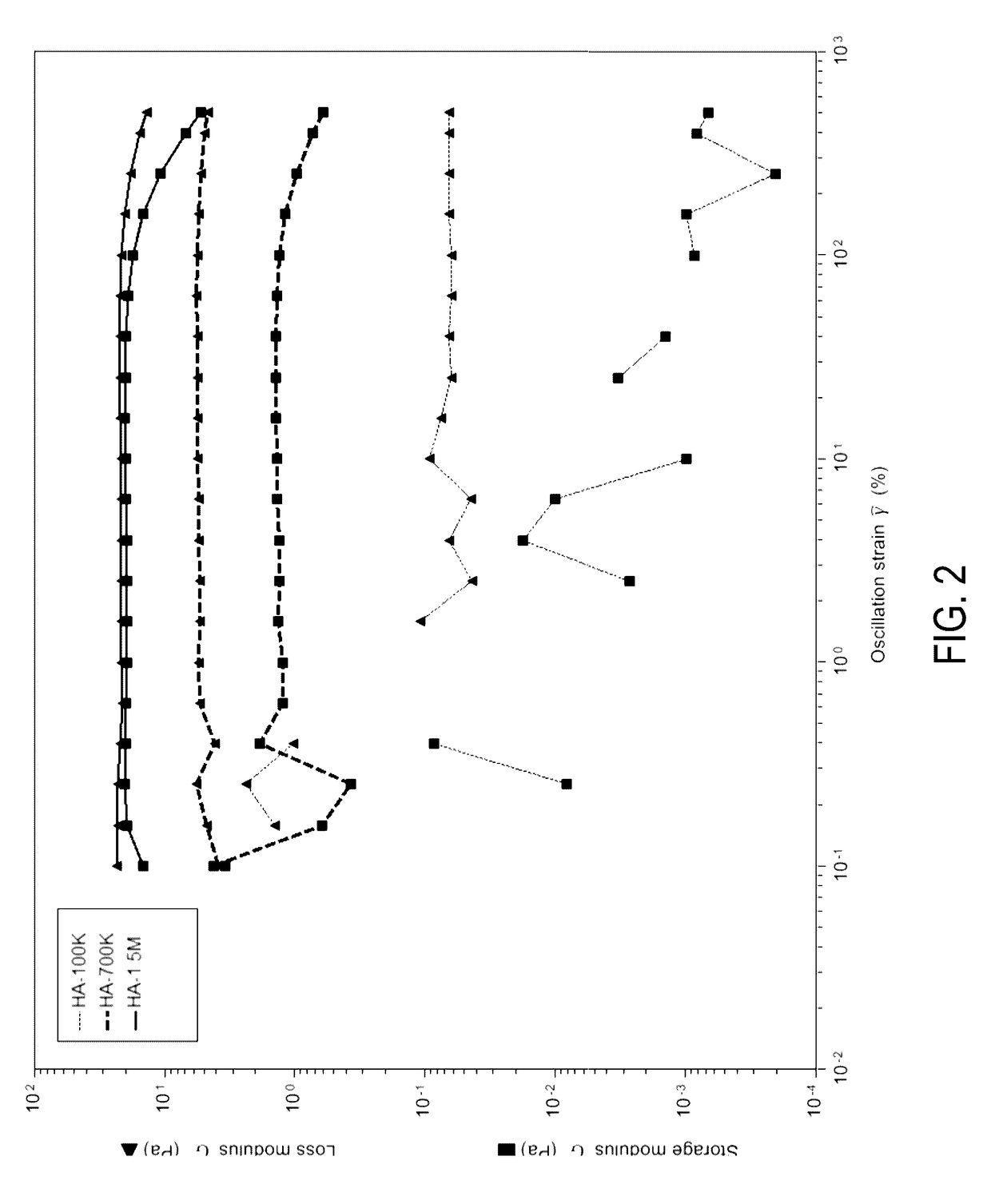
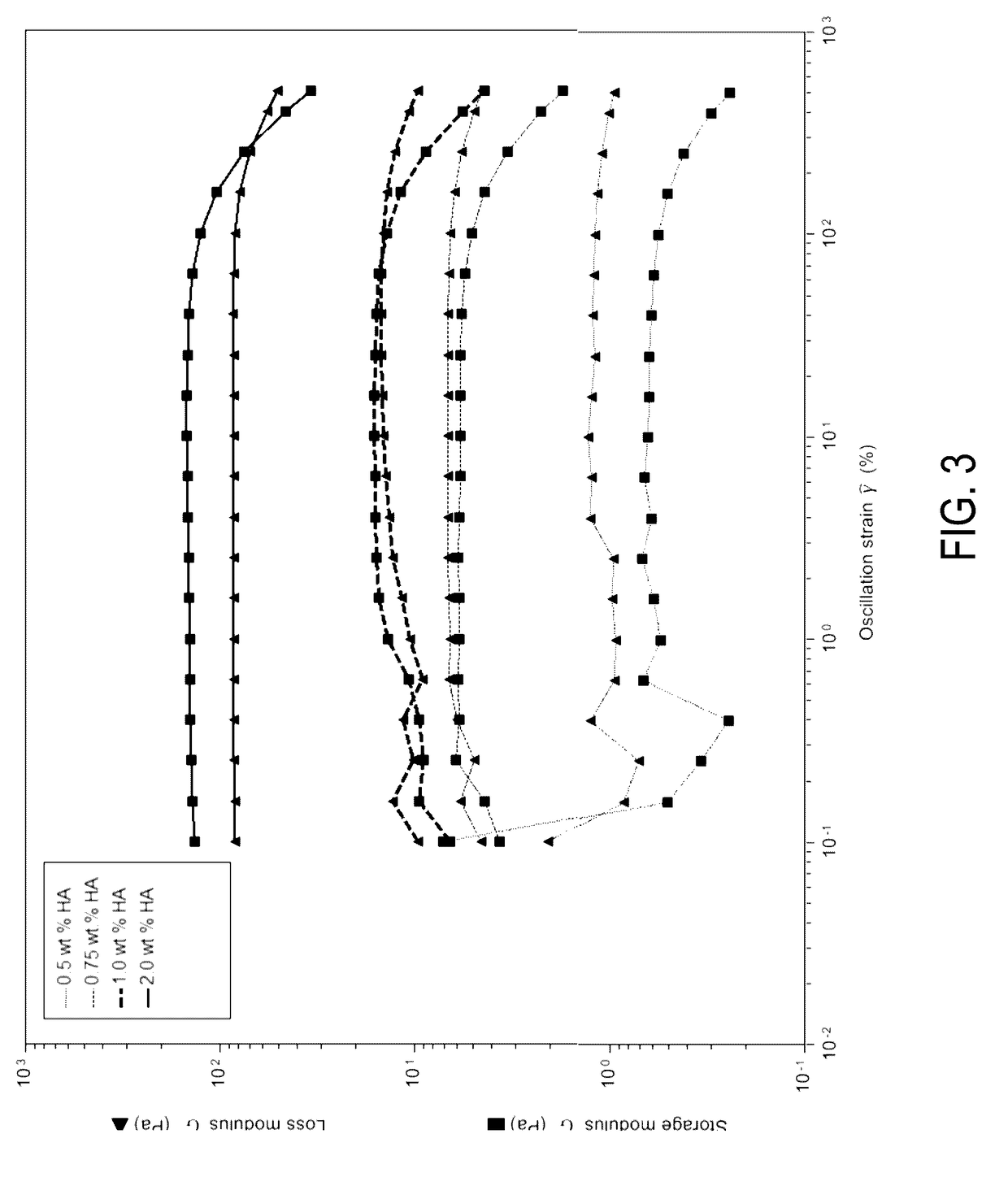
Injectable mediums and methods for preparing and administering an injectable medium comprising therapeutic cells, and optionally one or more therapeutic or diagnostic substance, suitable for injection into an anatomical space of a human or animal subject, comprising hyaluronic acid in concentrations of 0.5 weight percent to 1.0 weight percent having a molecular weight of about ≧700 kDa to about 1,900 kDa and a storage modulus within the range of 5-25 Pa, which injectable mediums and methods prevent cell settling during transportation and storage of such injectable mediums comprising therapeutic cells, and optionally therapeutic or diagnostic substances; promote cell survival; facilitate administration of homogeneous injectable mediums comprising therapeutic cells, in particular NSCs; and enable rapid clearance by the body following injection, so as not to interfere with cellular integration with surrounding tissue.
Owner:INVIVO THERAPEUTICS CORP
Compositions and methods for altering the biodistribution of biological agents
InactiveUS7198949B2Low effective doseHigh indexAntibacterial agentsAntimycoticsMedicineBiodistribution
Owner:BOARD OF RGT UNIV OF NEBRASKA
Antisense Oligonucleotides Against VR1
Antisense oligodeoxynucleotides against VR1, corresponding nucleotide constructs, cells containing said nucleotide constructs, pharmaceutical and diagnostic substances, uses thereof in pain therapy, and methods for diagnosing symptoms related to VR1 and for identifying pain-modulating substances.
Owner:GRUNENTHAL GMBH
Treatment of transformed or infected biological cells
InactiveUS7939266B2High activityEasy to distinguishOrganic active ingredientsBiocideBiological cellWilms' tumor
The present invention relates to a therapeutic or / and diagnostic substance. Furthermore it relates to an expression vector, to a composition comprising the afore-mentioned substance or / and the afore-mentioned expression vector, a method for diagnosing a tumor disease or / and an infectious disease in a living being, as well as to a method for the treatment of a tumor disease or / and of an infection in a living being.
Owner:UNIV TUBINGEN
Nanovesicles derived from cell membrane, and use thereof
PendingUS20210212948A1Avoid it happening againEliminate the effects ofOrganic active ingredientsHeavy metal active ingredientsSide effectEfficacy
Disclosed are cell membrane-derived nanovesicles, a method of preparing the nanovesicles, and a pharmaceutical composition and a diagnostic kit using the nanovesicles. The cell membrane-derived nanovesicles may prevent potential adverse effects because intracellular materials (e.g., genetic materials and cytosolic proteins) unnecessary for delivering therapeutic or diagnostic substances are removed from the nanovesicles. In addition, as the nanovesicles may be targeted to specific cells or tissues, therapeutic or diagnostic substances may be predominantly delivered to the targeted cells or tissues, while delivery of the substances may be inhibited. Therefore, the nanovesicles may alleviate suffering and inconvenience of patients by reducing adverse effects of therapeutic substances and by improving efficacy of the substances. In addition, the cell membrane-derived nanovesicles loaded with therapeutic or diagnostic substances and a method of preparing the nanovesicles may be used in vitro or in vivo for therapeutic or diagnostic purposes, or for experimental use.
Owner:SLBIGEN INC
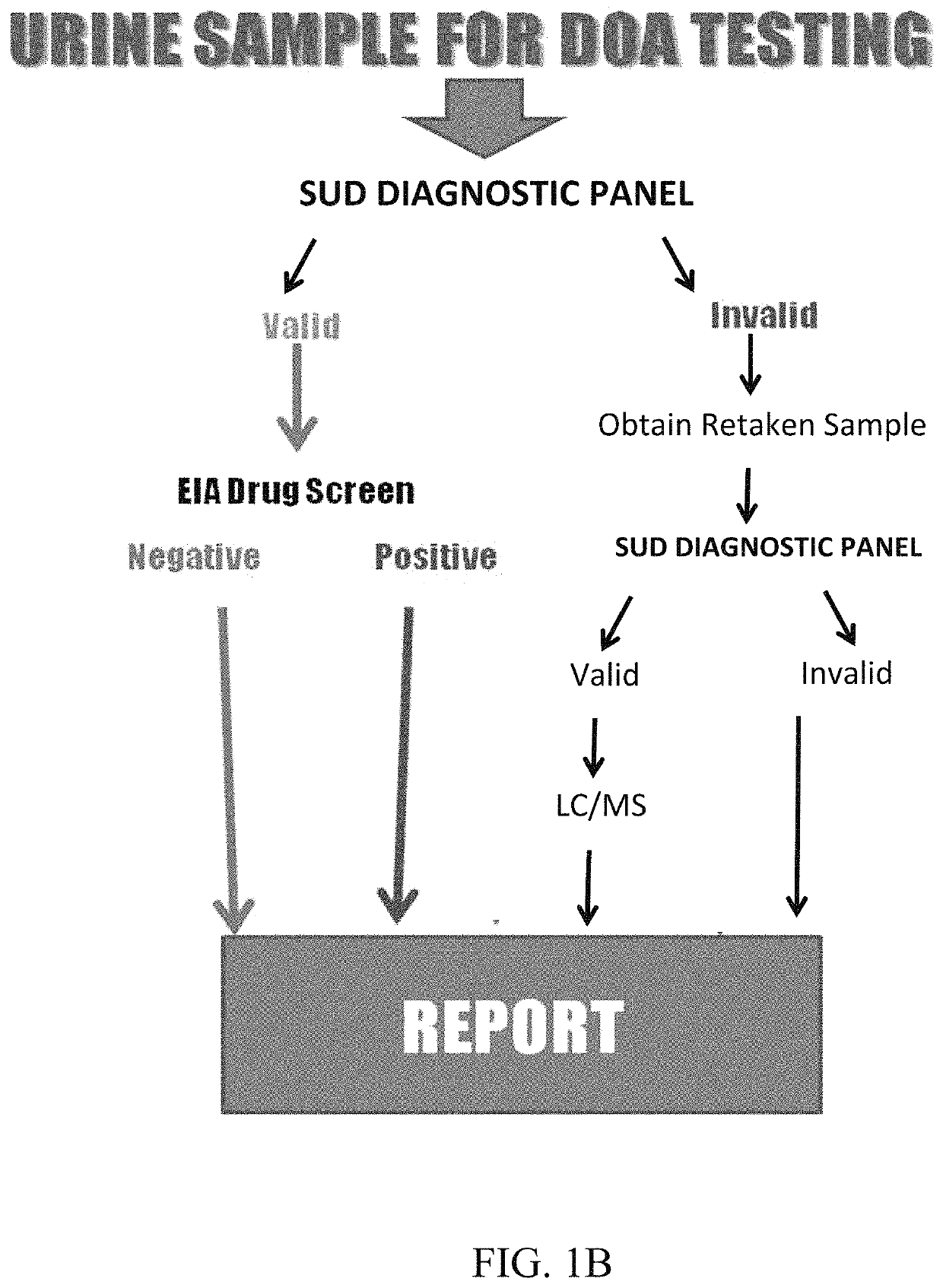
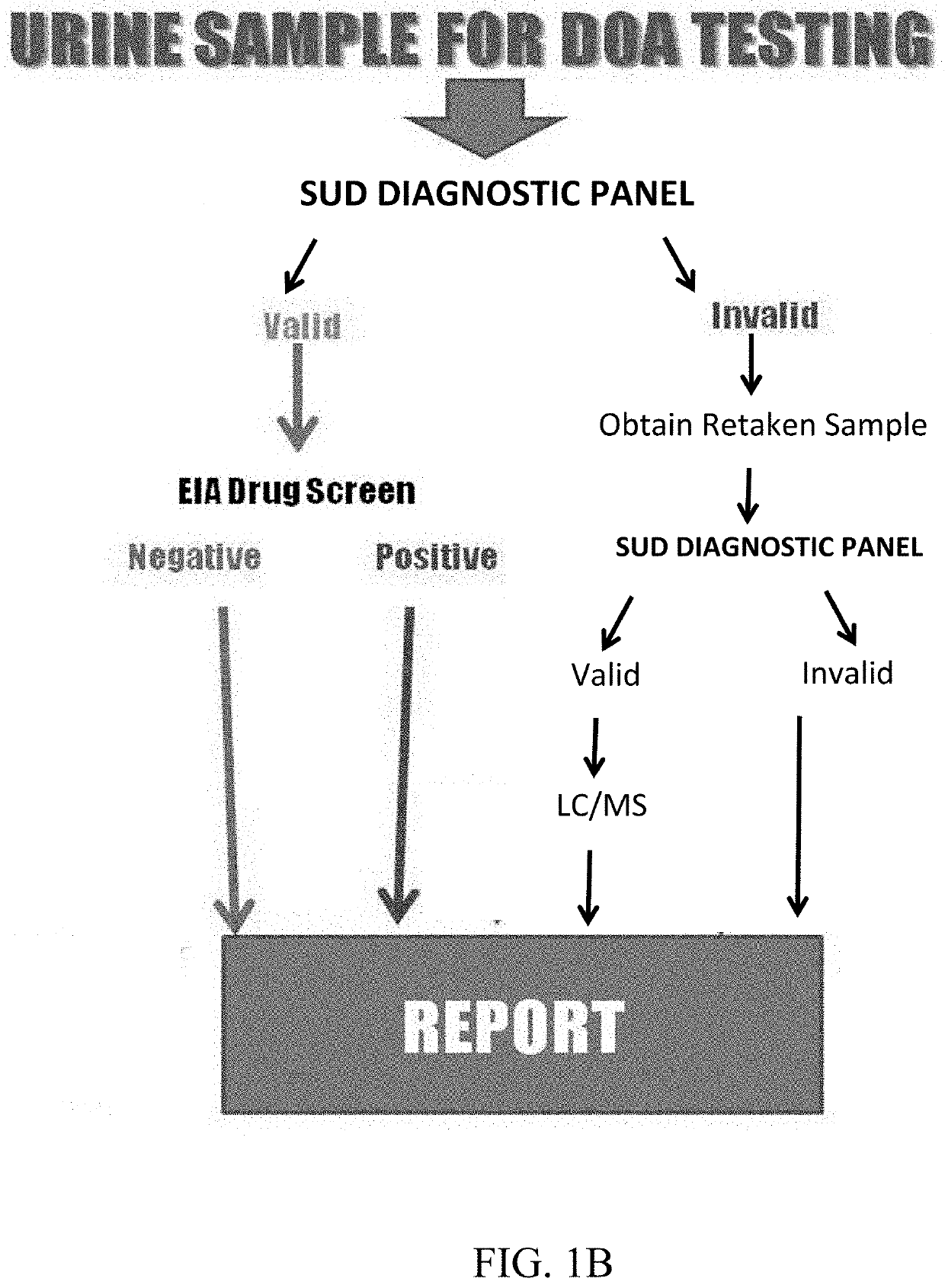
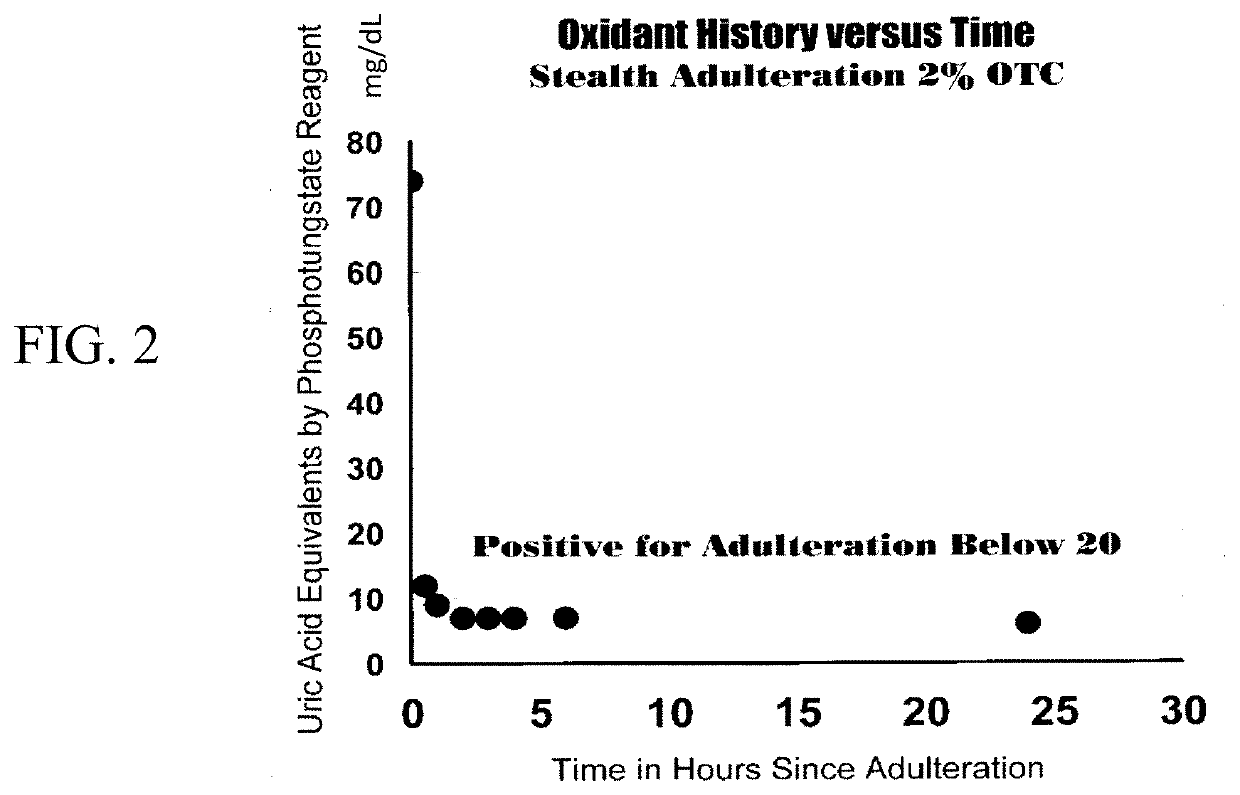
Assays and methods for diagnosing substance use disorder
ActiveUS11493498B2Inexpensive, and highly effective improvementsPromote resultsMass spectrometric analysisDisease diagnosisSubstance useAssay
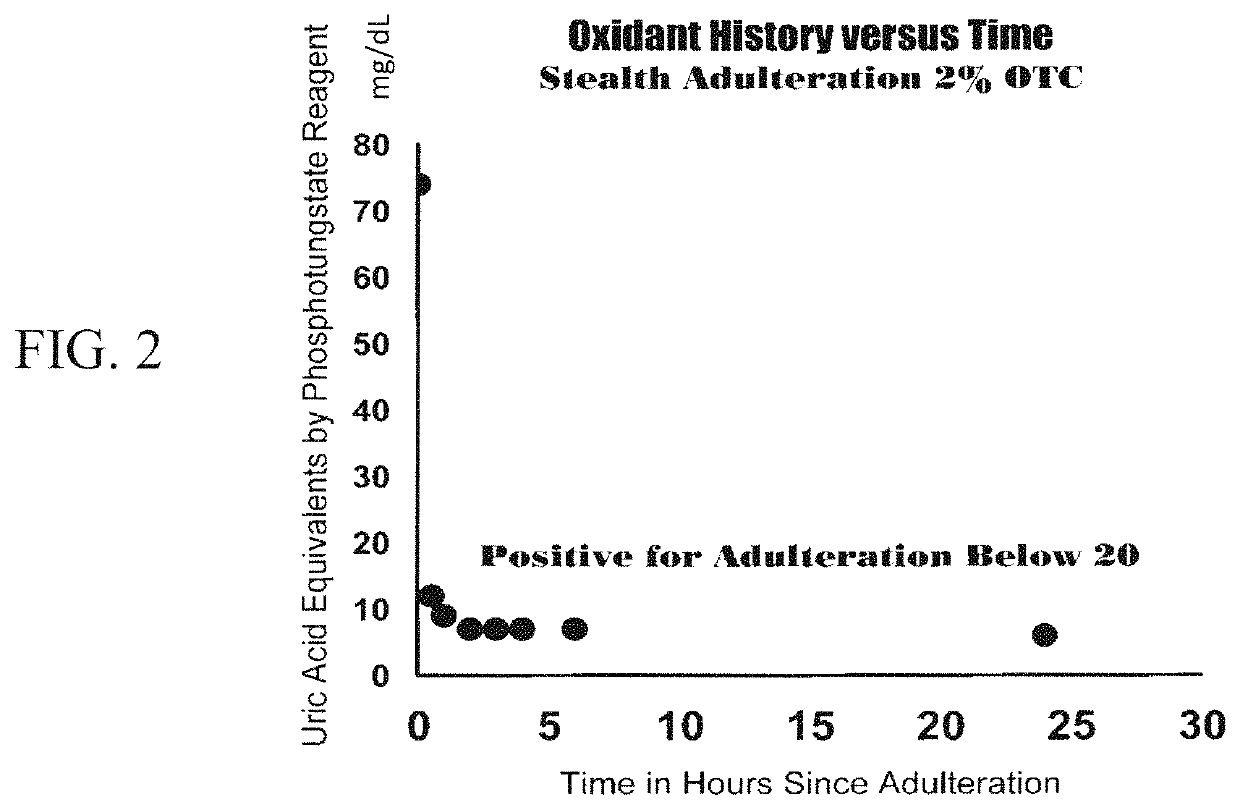
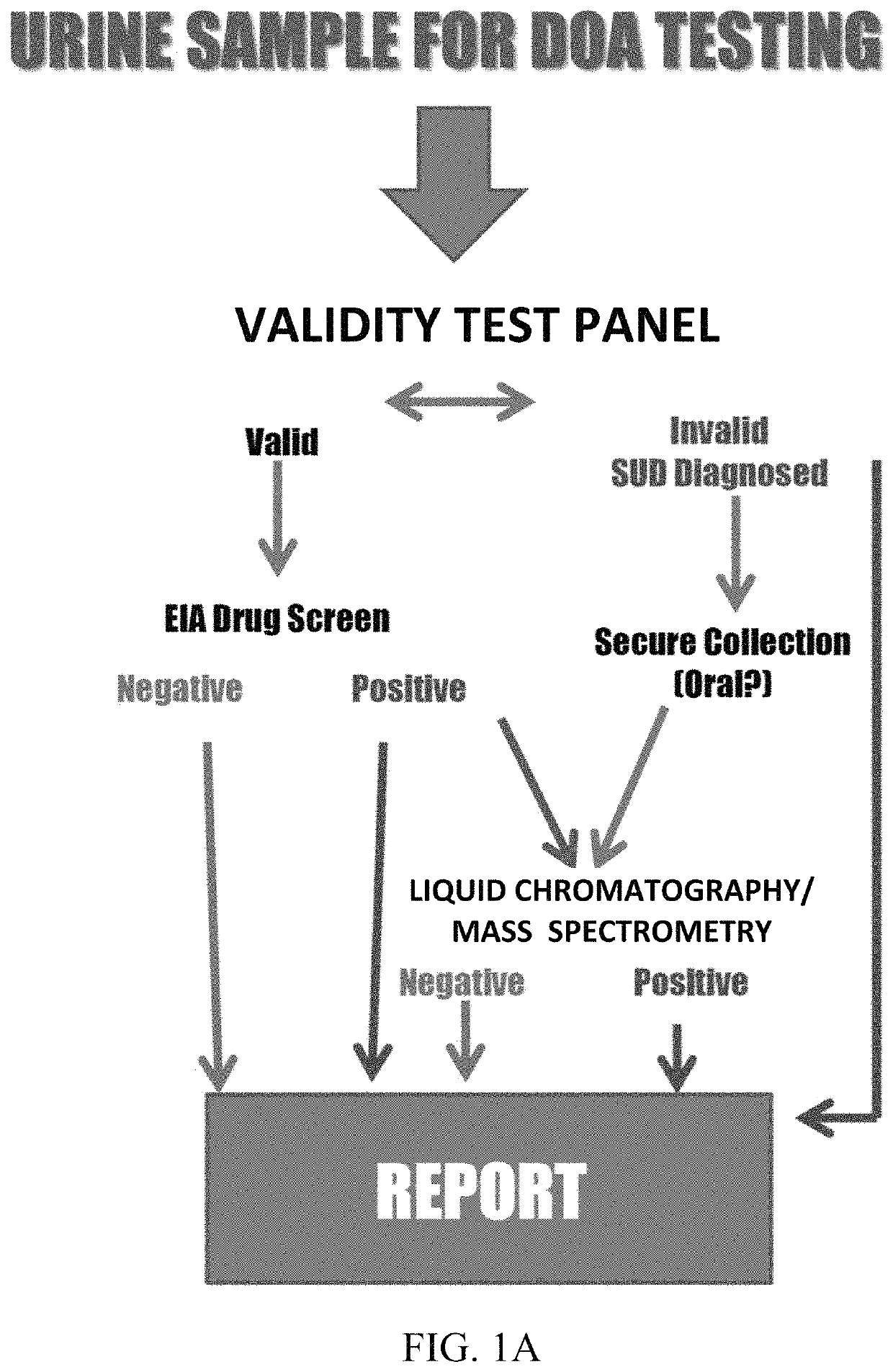
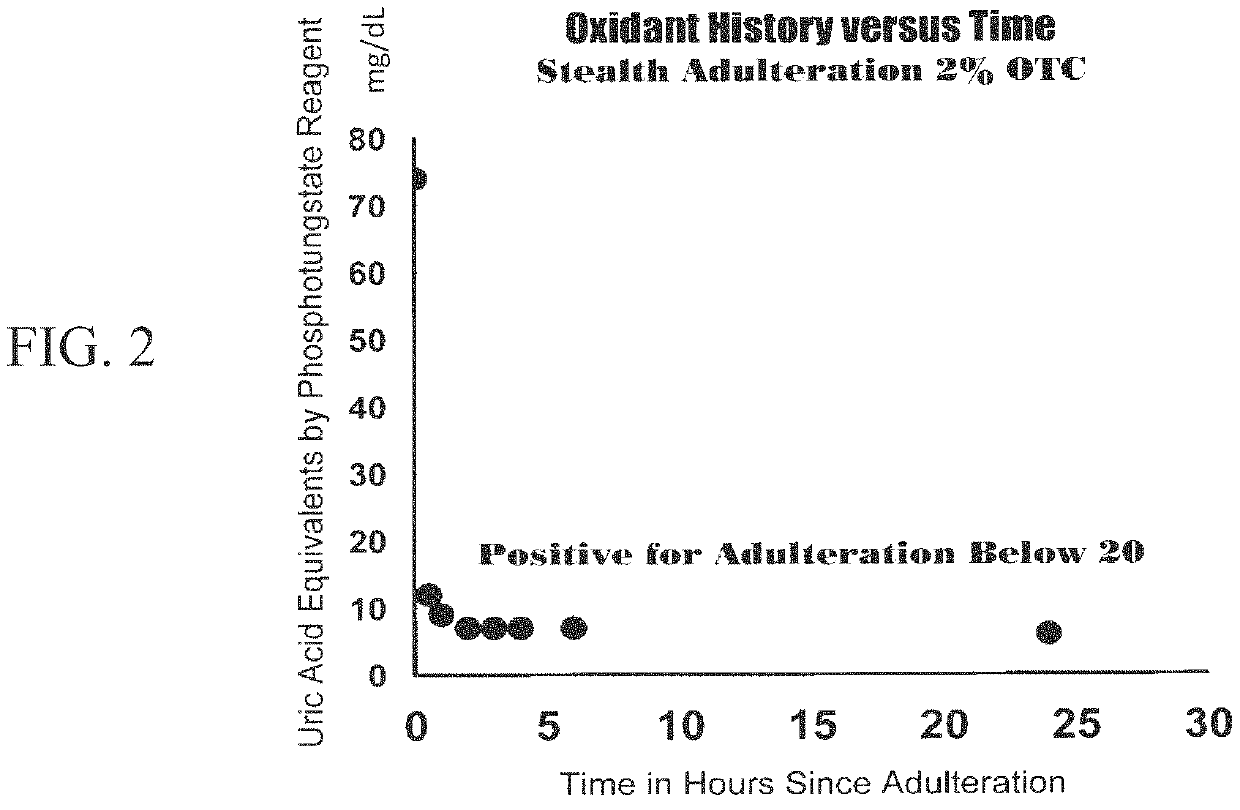
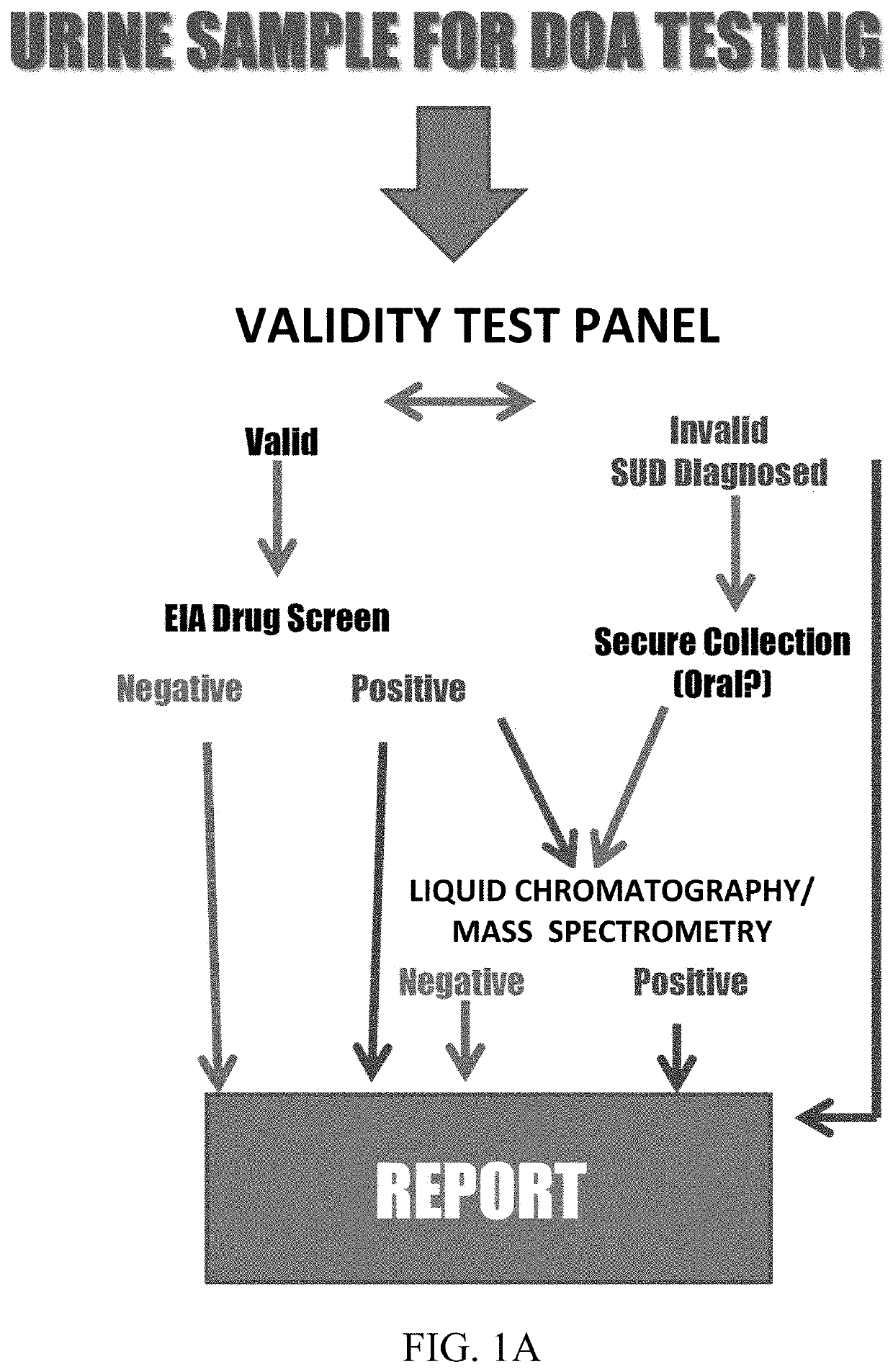
Assays and methods for verifying the validity of a urine sample submitted for Drugs of Abuse (DOA) testing. Embodiments include a SUD Diagnostic Panel that includes six assays: specific gravity index assay, long-duration counterfeit urine assay, short-duration counterfeit urine assay, oxidant history assay, pH assay, and creatinine assay. The SUD Diagnostic Panel detects twelve principle classes of adulteration. Detection of adulteration of one or more urine samples from a patient indicates an attempt to subvert test results and provides an objective indication in one instance and an object diagnosis in another instance of SUD.
Owner:VISION DIAGNOSTICS INC
Assays and methods for diagnosing substance use disorder
ActiveUS11493497B1Inexpensive, and highly effective improvementsPromote resultsMass spectrometric analysisDisease diagnosisSubstance useAssay
Assays and methods for verifying the validity of a urine sample submitted for Drugs of Abuse (DOA) testing. Embodiments include a SUD Diagnostic Panel that includes six assays: specific gravity index assay, long-duration counterfeit urine assay, short-duration counterfeit urine assay, oxidant history assay, pH assay, and creatinine assay. The SUD Diagnostic Panel detects twelve principle classes of adulteration. Detection of adulteration of one or more urine samples from a patient indicates an attempt to subvert test results and provides an objective indication in one instance and an object diagnosis in another instance of SUD.
Owner:VISION DIAGNOSTICS INC
Assays and methods for diagnosing substance use disorder
ActiveUS20220128540A1Inexpensive, and highly effective improvementsPromote resultsMass spectrometric analysisDisease diagnosisSubstance useAssay
Assays and methods for verifying the validity of a urine sample submitted for Drugs of Abuse (DOA) testing. Embodiments include a SUD Diagnostic Panel that includes six assays: specific gravity index assay, long-duration counterfeit urine assay, short-duration counterfeit urine assay, oxidant history assay, pH assay, and creatinine assay. The SUD Diagnostic Panel detects twelve principle classes of adulteration. Detection of adulteration of one or more urine samples from a patient indicates an attempt to subvert test results and provides an objective indication in one instance and an object diagnosis in another instance of SUD.
Owner:VISION DIAGNOSTICS INC
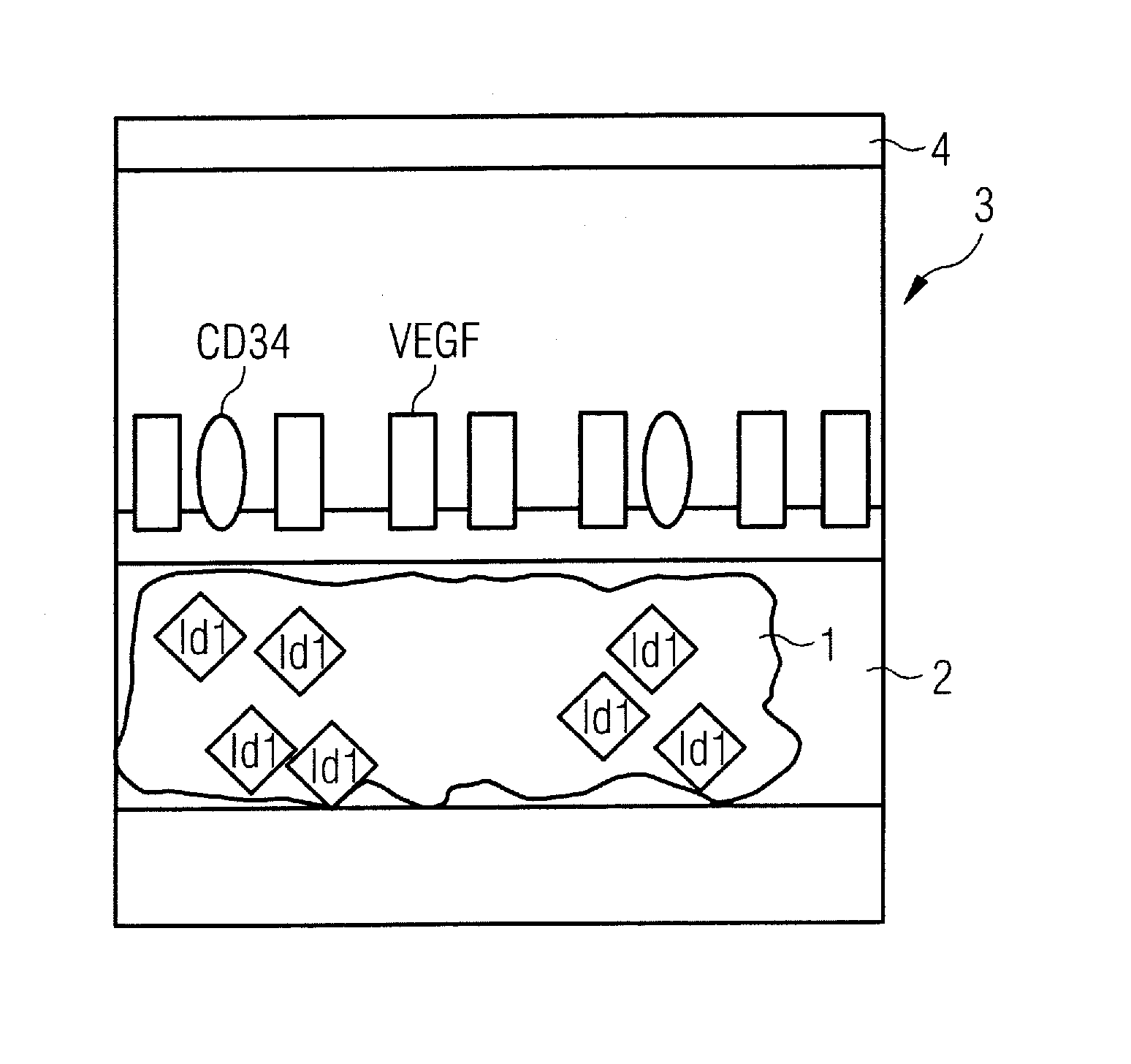
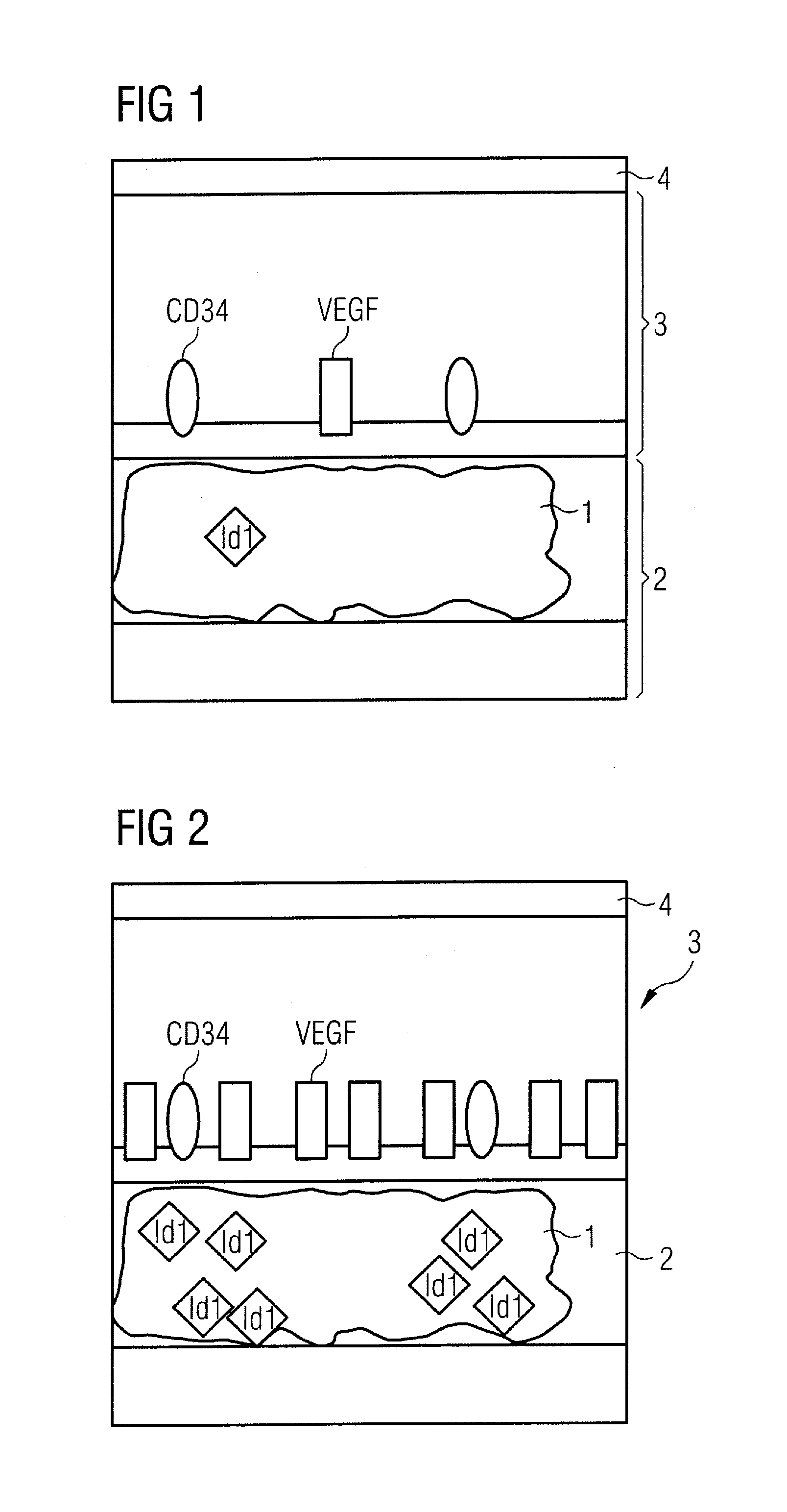
Diagnostic substance for use in a method for determining the aggressiveness of a prostate tumor and diagnostic method
InactiveUS20110223109A1Compounds screening/testingUltrasonic/sonic/infrasonic diagnosticsHealthy tissueBlood vessel
In order to determine the aggressiveness of a prostate tumor, a diagnostic substance is administered to a patient that includes a biomarker provided with a first label that is detectable with a detection device and that specifically binds to a VEGF molecule, and that contains a biomarker that binds specifically to a target molecule that occurs uniformly in the endothelium of blood vessels of healthy tissue and the blood vessels of a prostate tumor, and that is provided with a second label that is detectable with the detection device independently of the first label.
Owner:SIEMENS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com