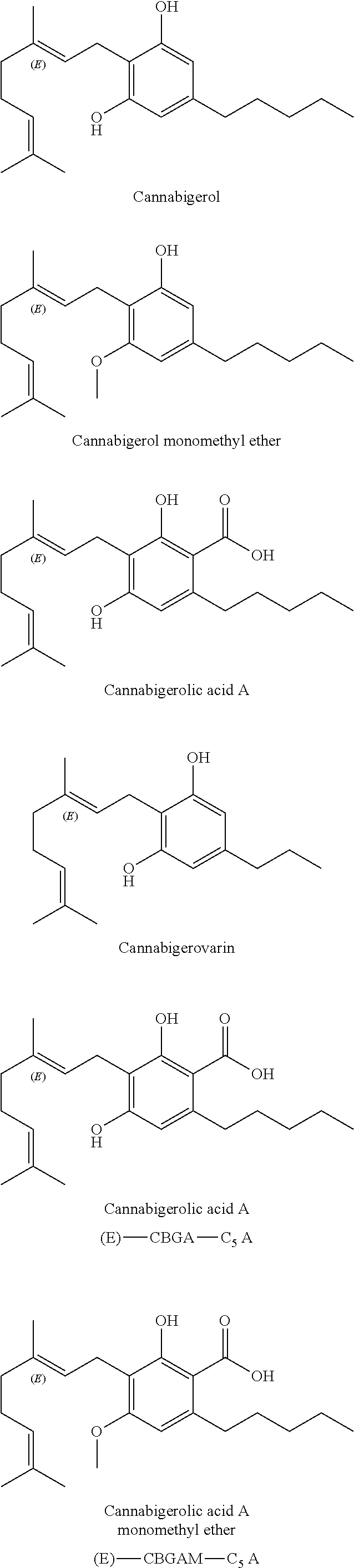
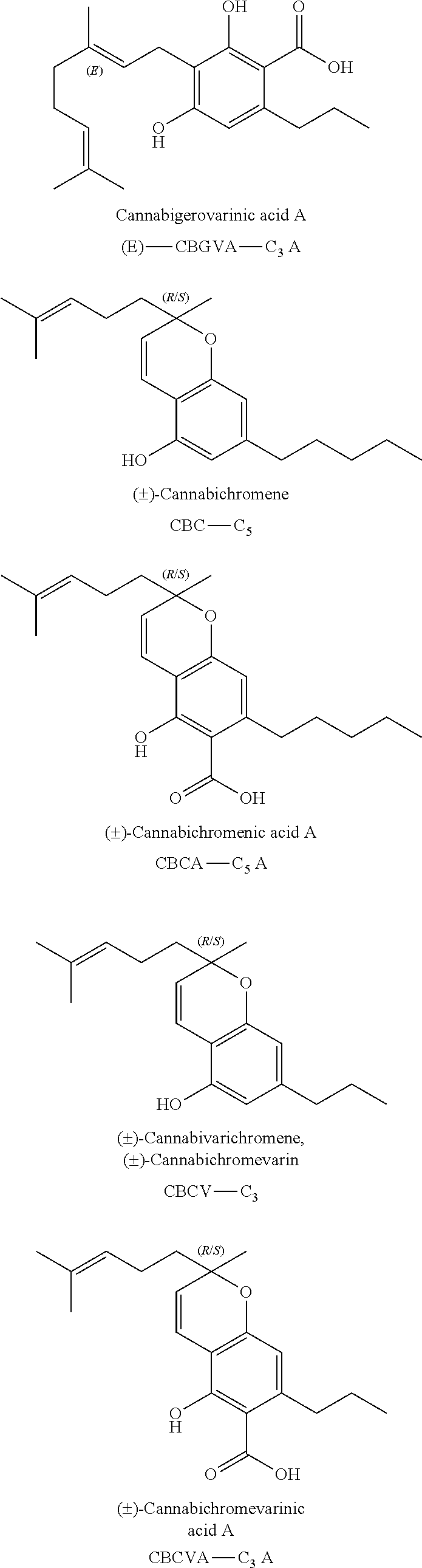
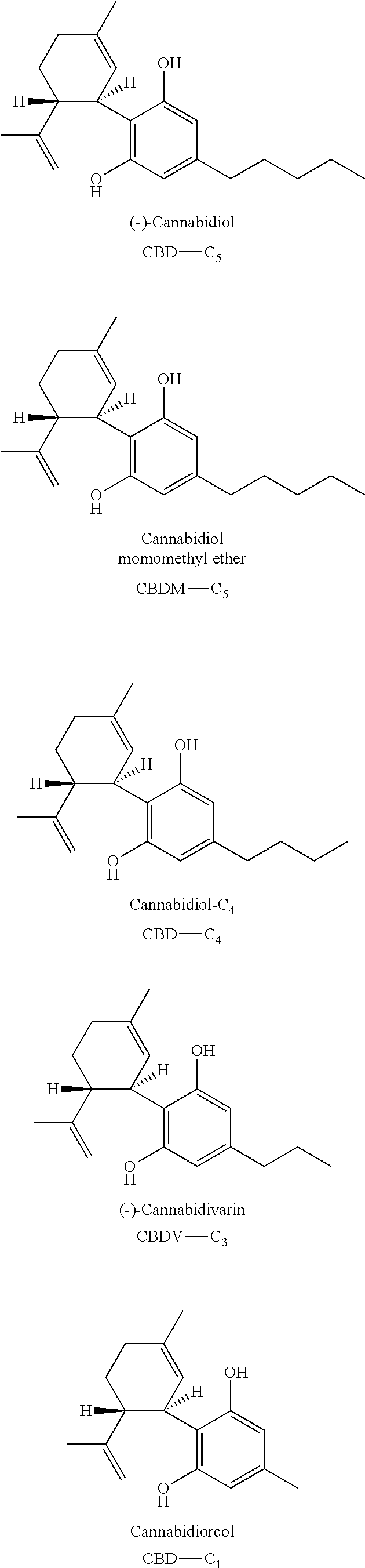
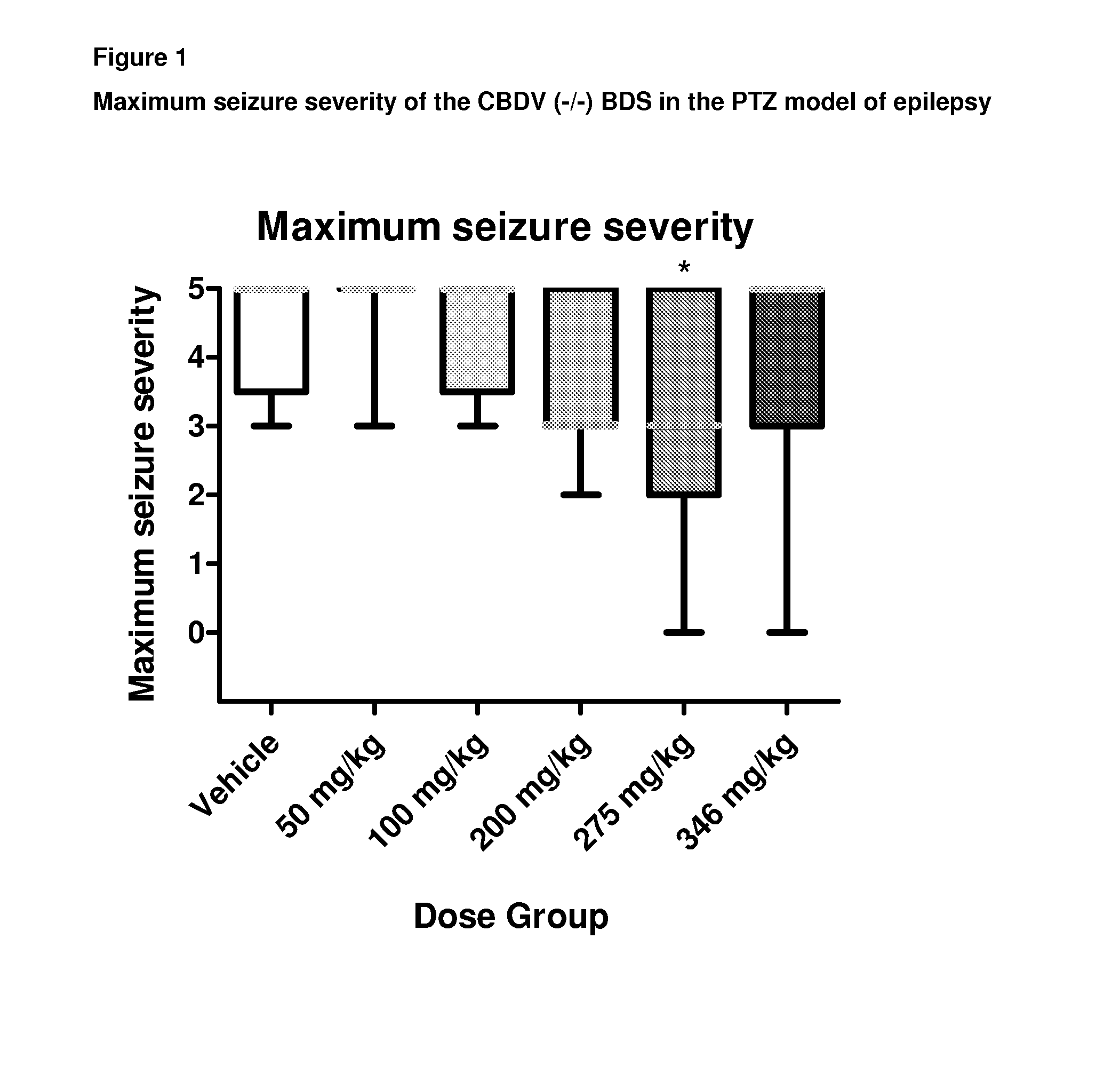
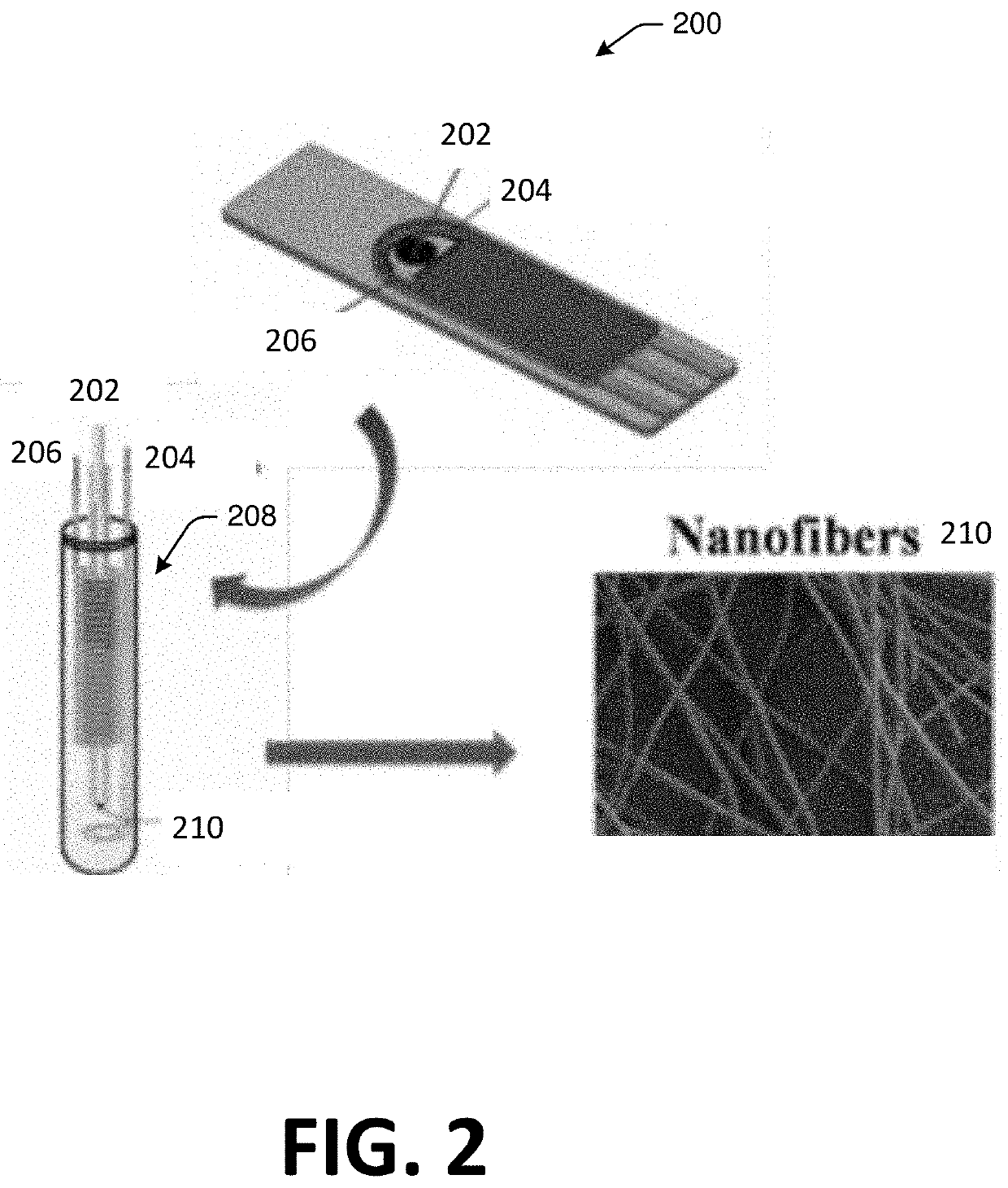
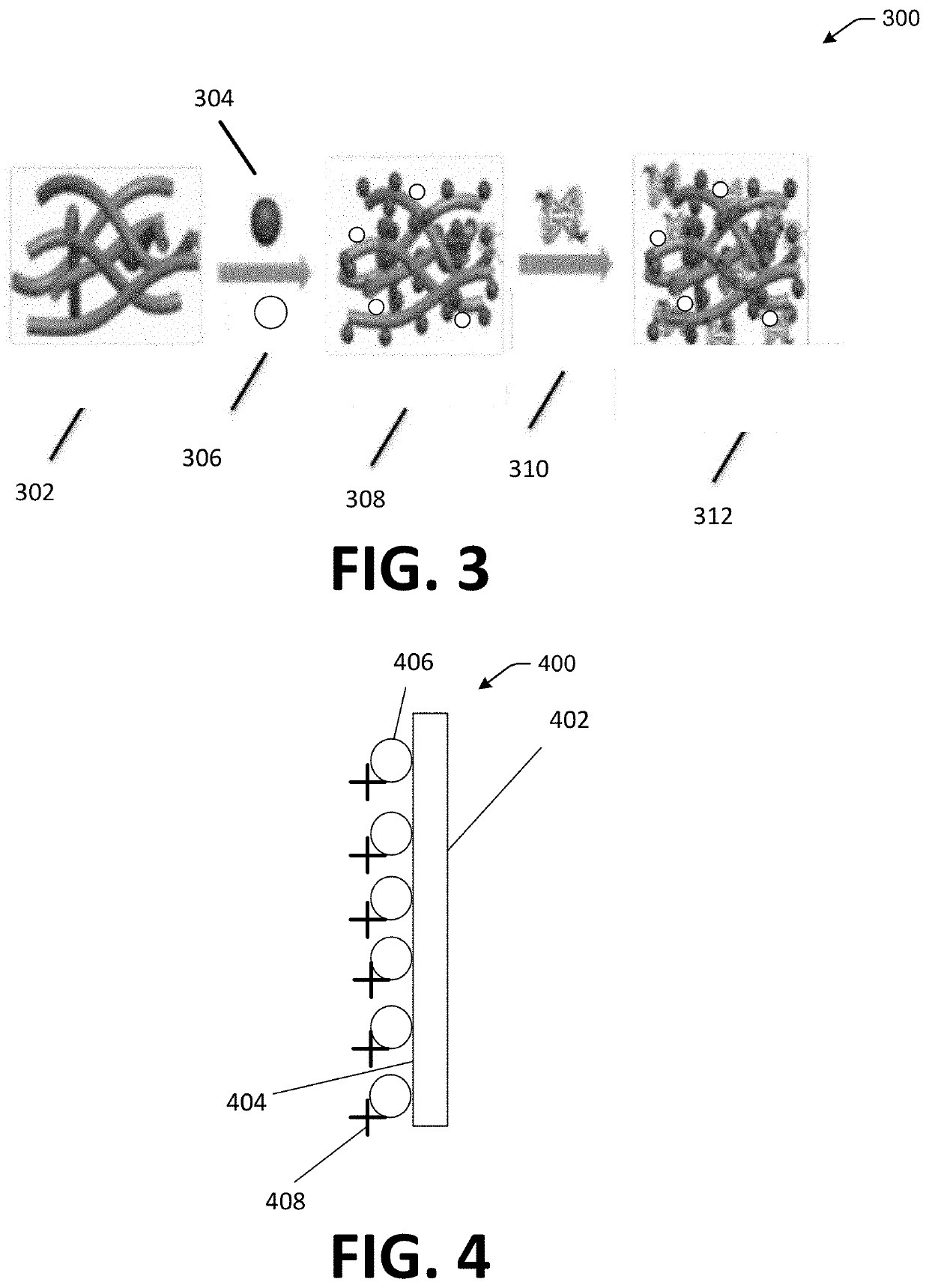
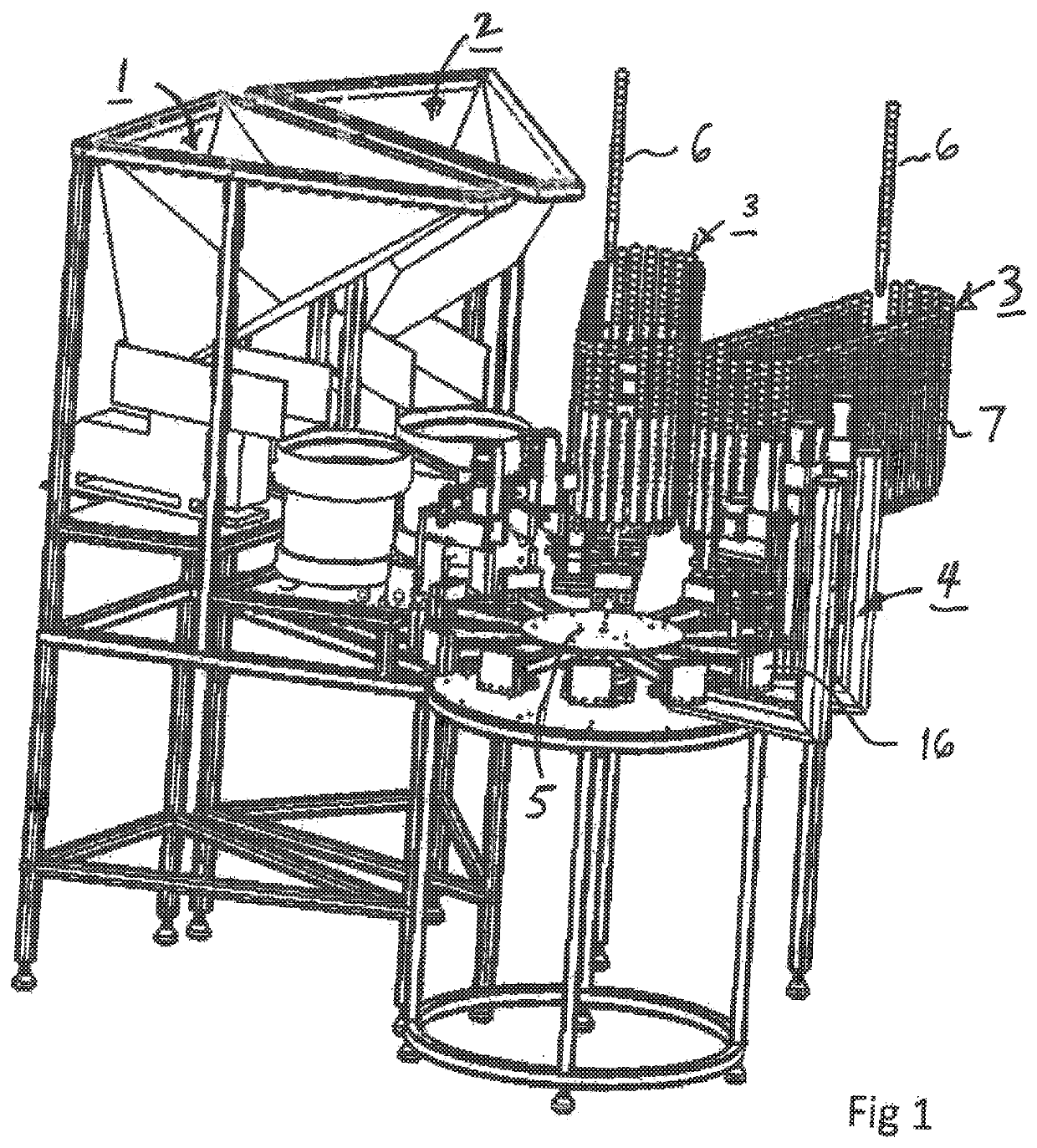
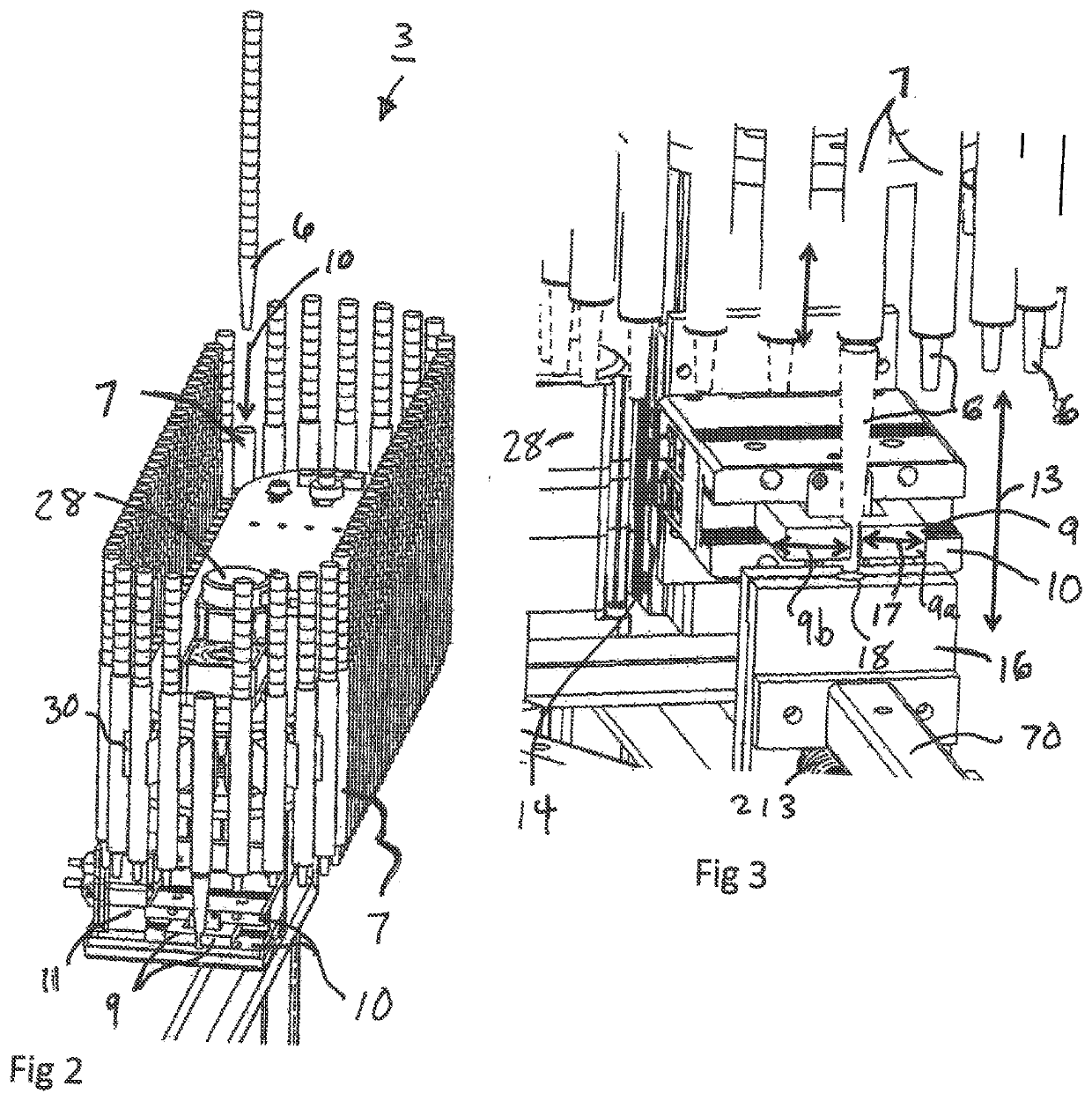
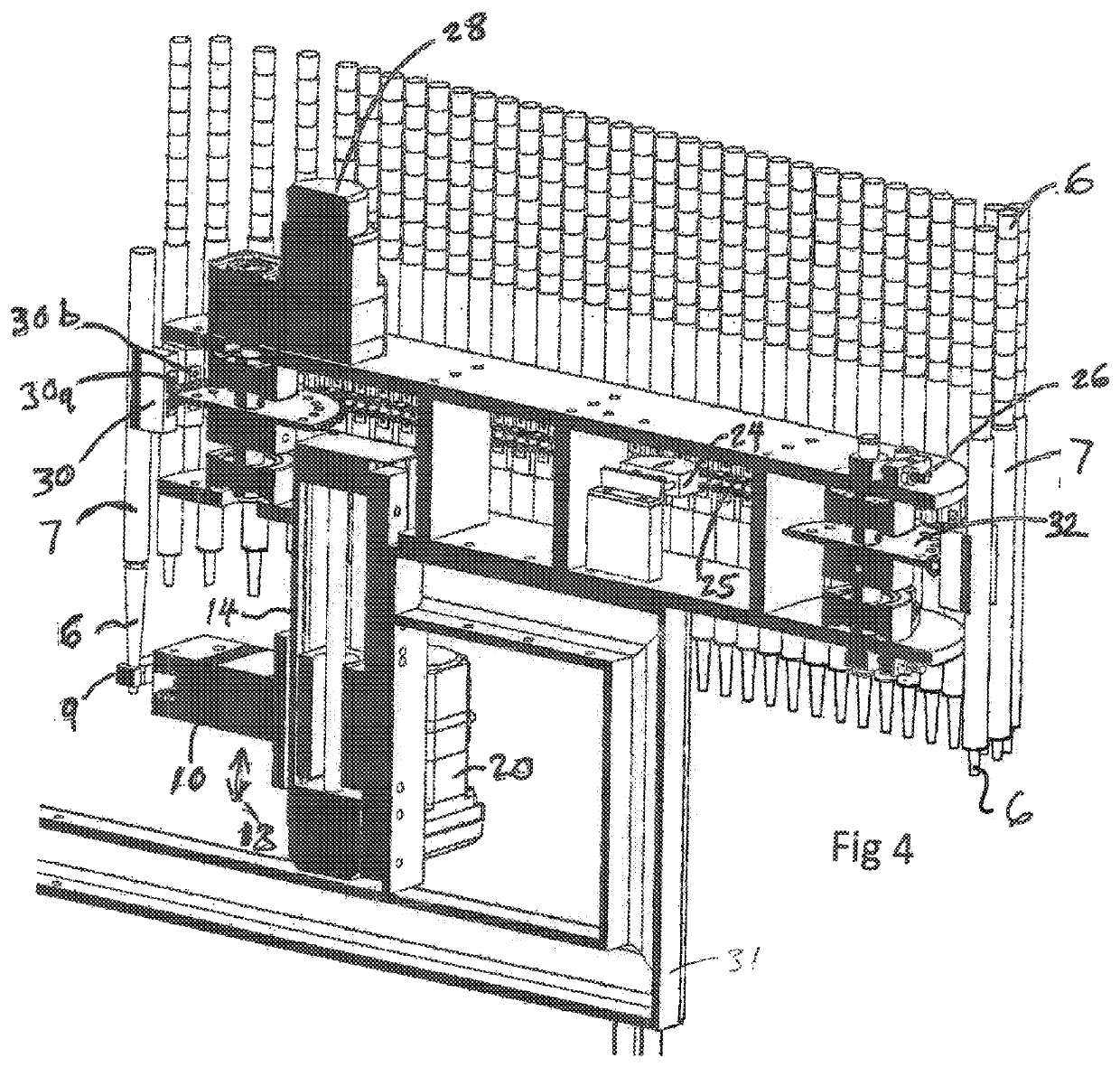
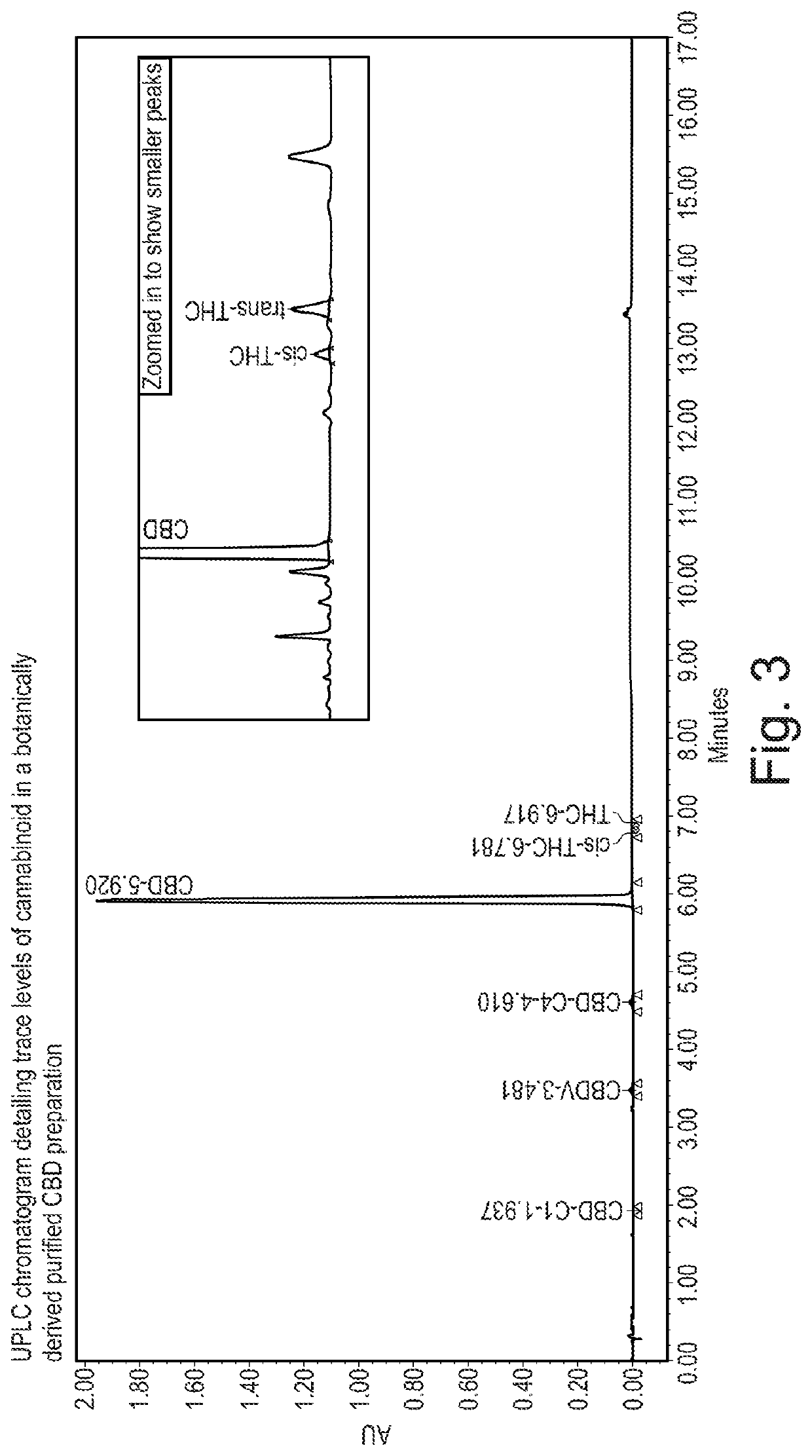
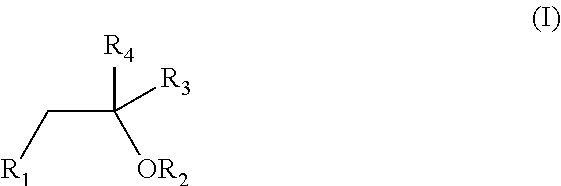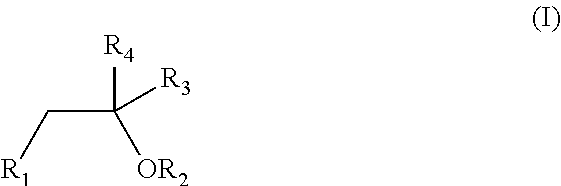Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
449 results about "Cannabis substance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
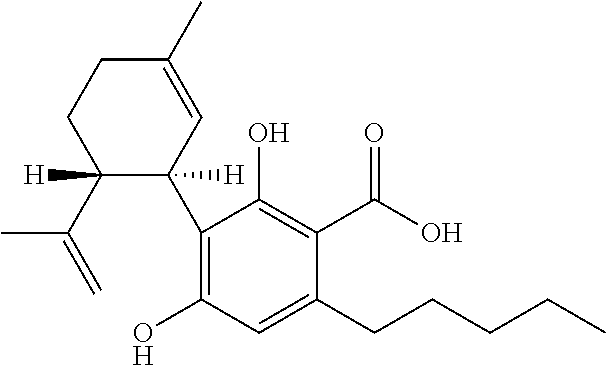
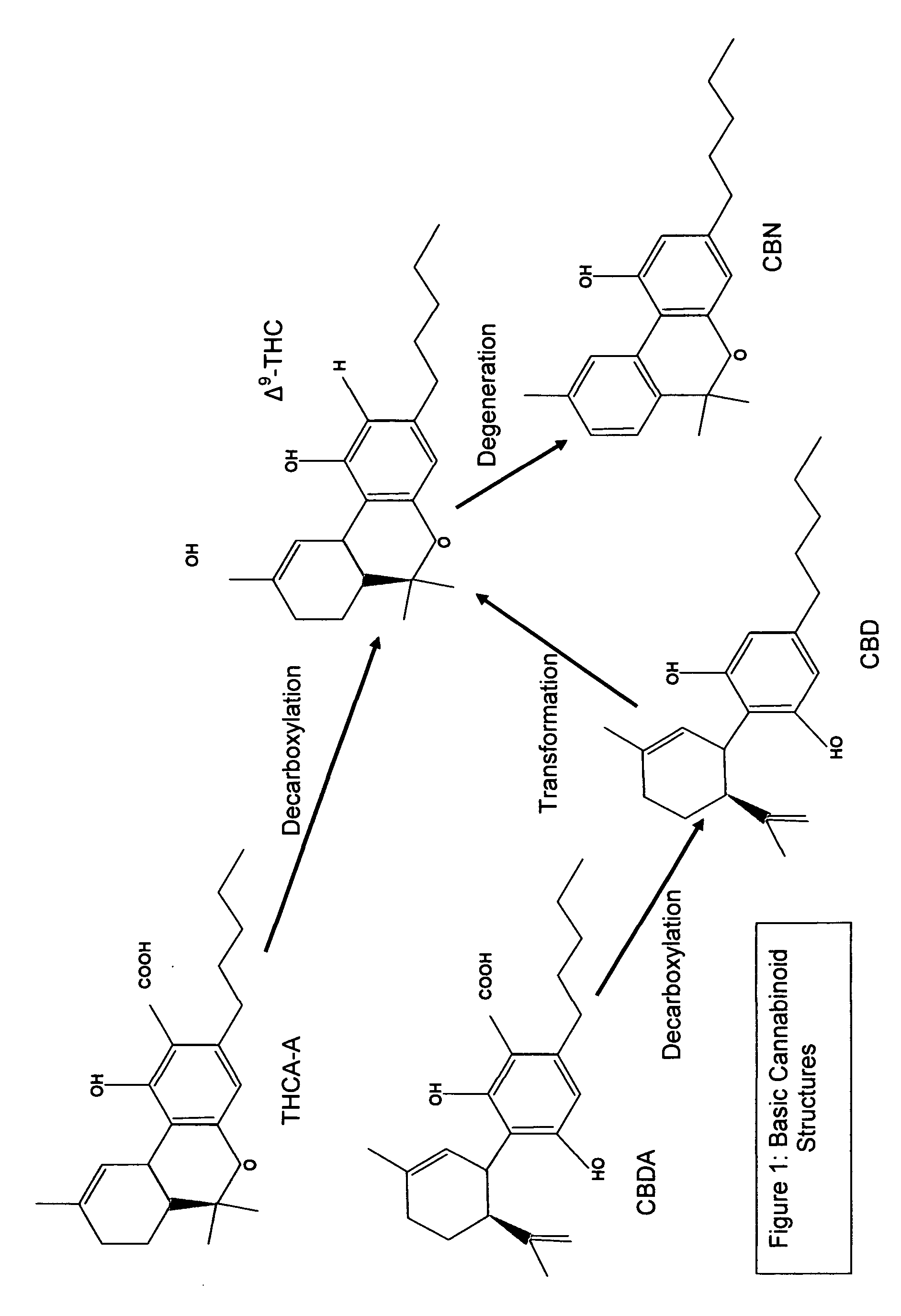
Cannabis, also known as marijuana among other names, is a psychoactive drug from the Cannabis plant intended for medical or recreational use. The main psychoactive part of cannabis is tetrahydrocannabinol (THC), one of 483 known compounds in the plant, including at least 65 other cannabinoids.
Bioactive concentrates and uses thereof
The present invention relates to concentrates obtained from extraction from Cannabis, preferably cannabinoid and / or terpene concentrates, and formulation of the concentrates, particularly for use for direct vaporization, infusion into edible matrices, in electronic inhalation devices, and as nutraceuticals.
Owner:PURPLE MUNDO INC
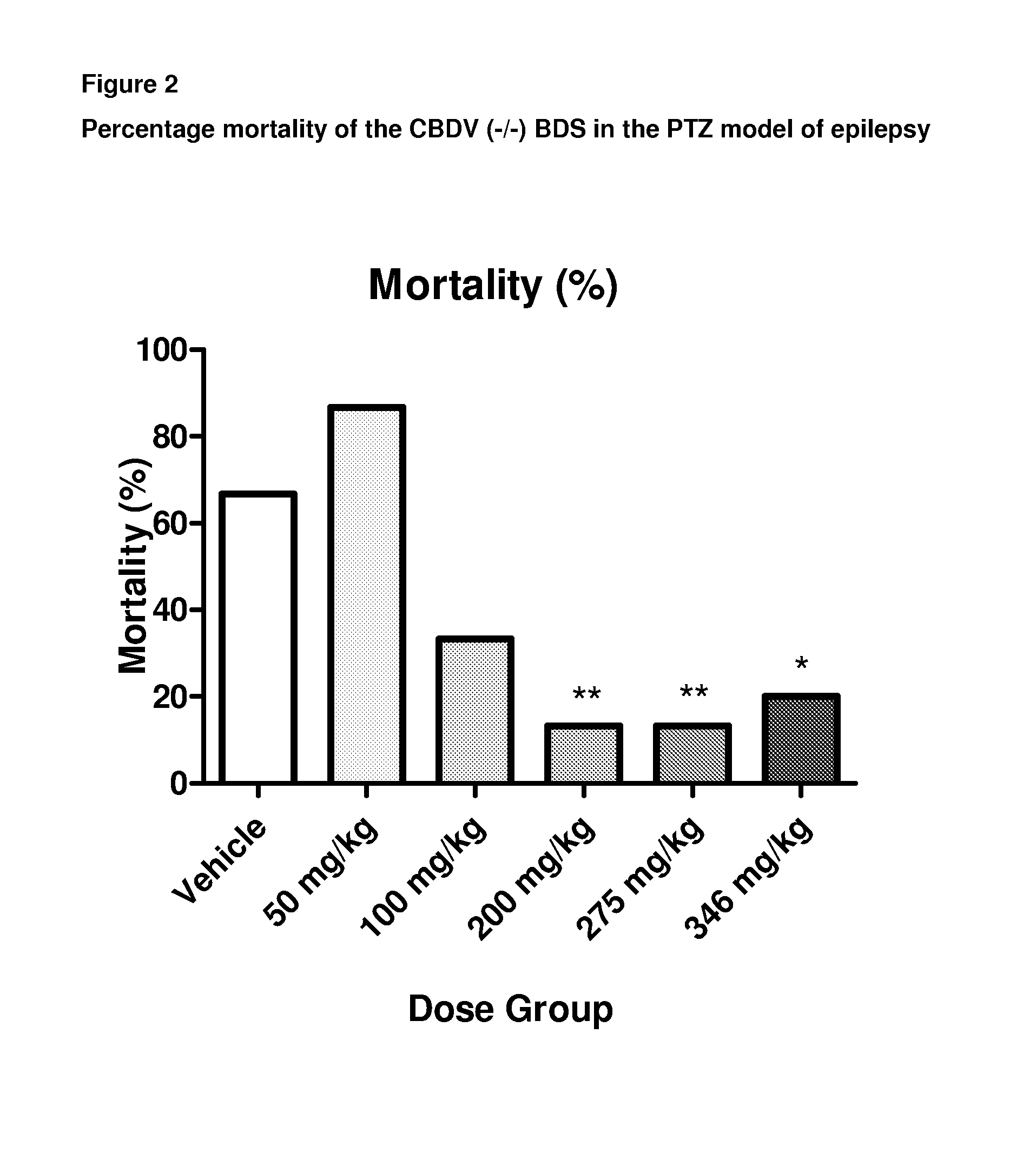
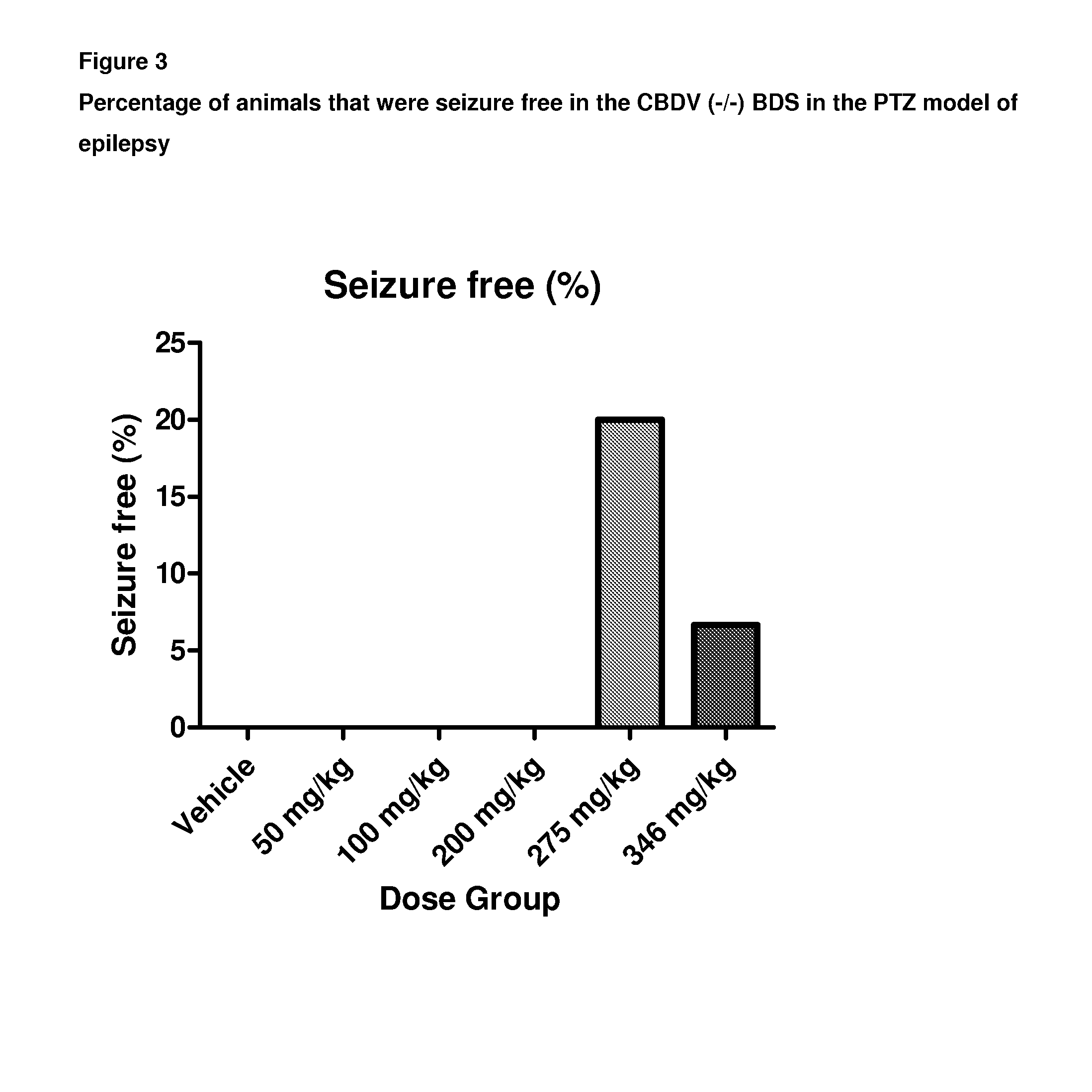
A pharmaceutical composition comprising the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD)
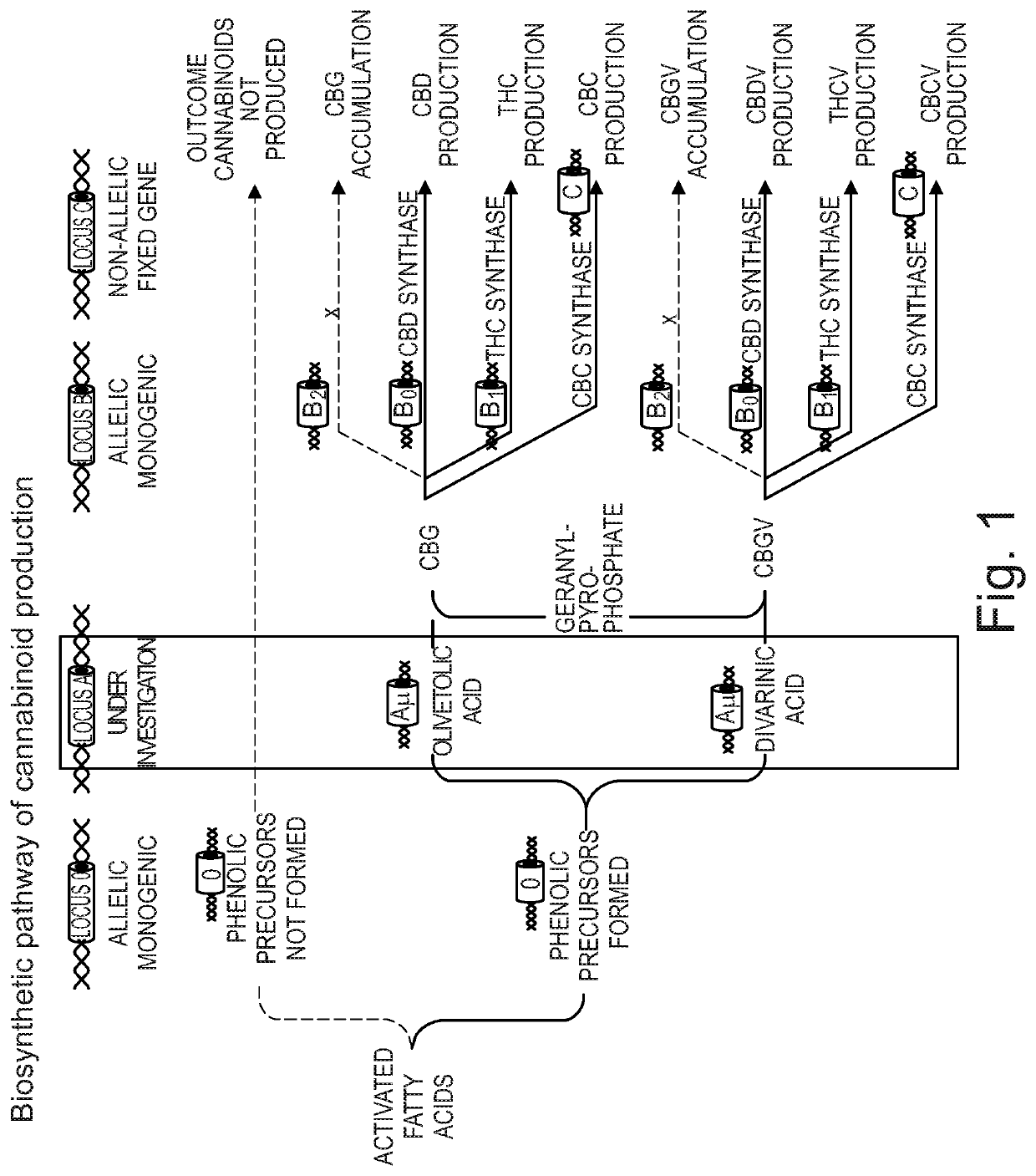
This invention relates to a pharmaceutical composition comprising or consisting essentially of the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD). The composition is particularly safe and efficacious for use in the treatment of neurological conditions, characterized by hyper-excitability of the central nervous system, convulsions or seizures such as occur in epilepsy. Preferably the CBDV and the CBD are present with at least one non-cannabinoid component of cannabis such as one or more terpenes or a terpene fraction. More particularly the composition further comprises one or more cannabichromene type compounds. Particularly cannabichromene propyl variant (CBCV) and / or cannabichromene (CBC). More particularly still the composition is absent or substantially absent of other cannabinoids, including in particular tetrahydrocannabinol (THC) and tetrahydrocannabivarin (THCV), which would normally be present in significant amounts in cannabis chemotypes bred to contain a significant amount of CBDV and / or CBD.
Owner:GW PHARMA LTD
Enhanced Delivery of Nicotine, THC, Tobacco, Cannabidiol or Base Alkaloid from an Electronic Cigarette or Other Vapor or Smoke Producing Device Through Use of an Absorption Conditioning Unit
A method for the administration of nicotine, THC, tobacco, cannabidiol or a base alkaloid includes administering in the oral or nasal cavity an absorption conditioning unit having at least two agents selected from the group consisting of (a) a buffer agent, (b) a capturing agent, (c) a penetration agent, and (d) a thermal agent, to the mammal, and then administering by inhalation a bioactive agent selected from the group consisting of nicotine, THC, cannabidiol and a base alkaloid. The absorption conditioning unit may be in a dosage form not containing a drug. The absorption conditioning unit may create a pH in the oral cavity or nasal cavity of 7.8-10 for a period of ten minutes or more after administration, the dosage form not containing an acid and not containing a drug.
Owner:FUISZ RICHARD C +1
Cannabis extracts and methods of preparing and using same
The invention relates to the extraction of pharmaceutically active components from plant materials, and more particularly to the preparation of a botanical drug substance (BDS) for incorporation in to a medicament. It also relates to a BDS, for use in pharmaceutical formulations. In particular it relates to BDS comprising cannabinoids obtained by extraction from cannabis
Owner:BLACKMON EARNEST VINSON MR
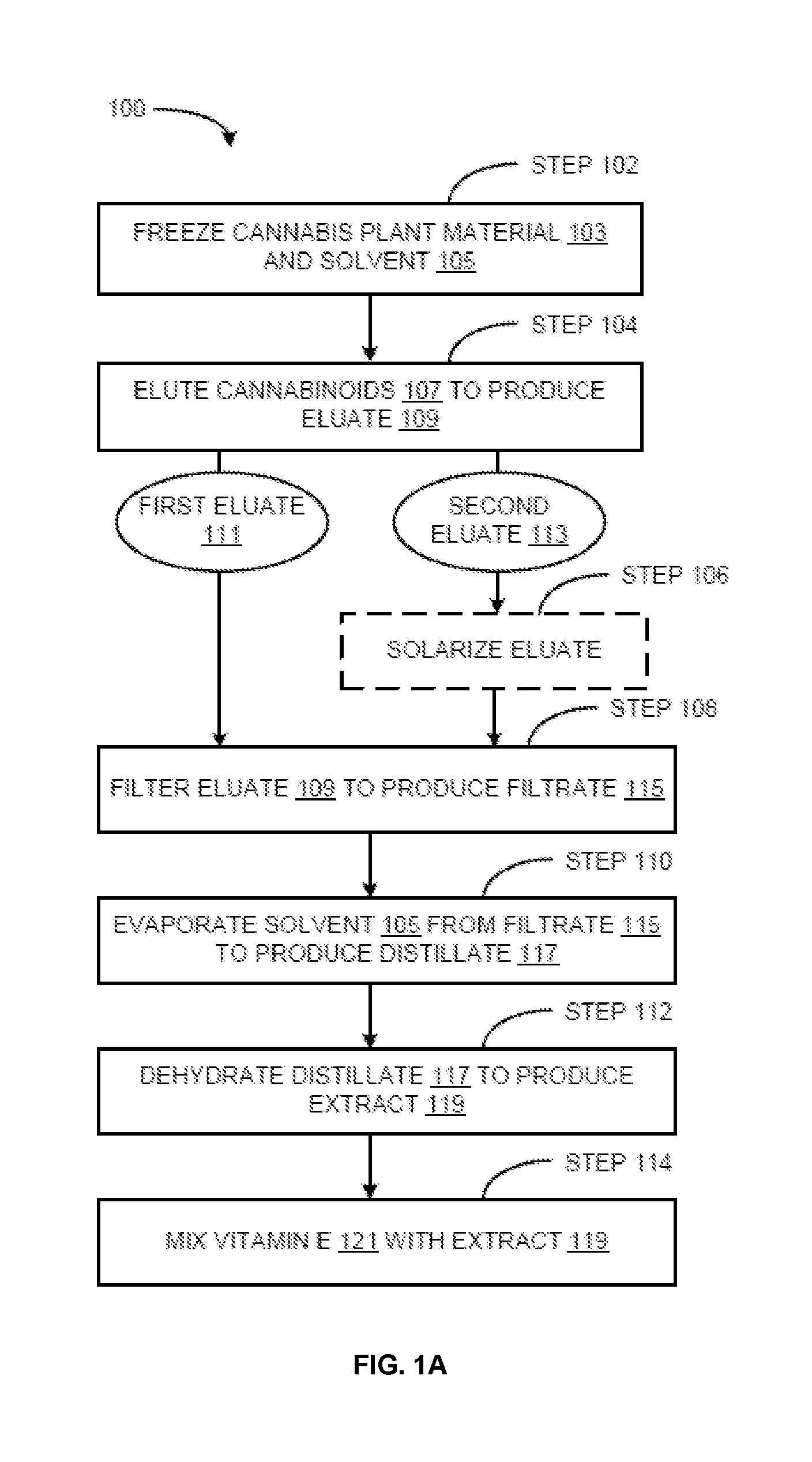
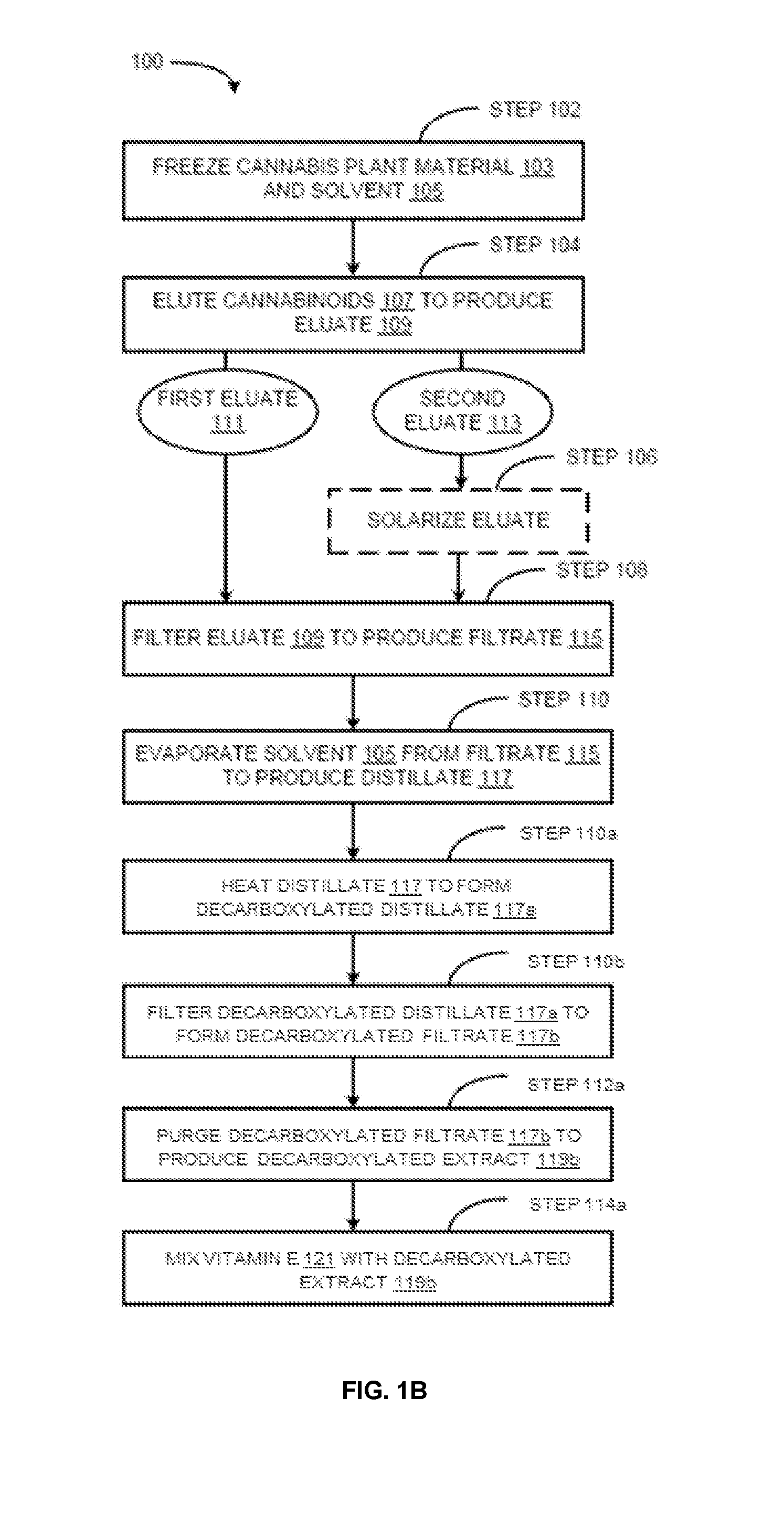
Methods for preparation of cannabis oil extracts and compositions
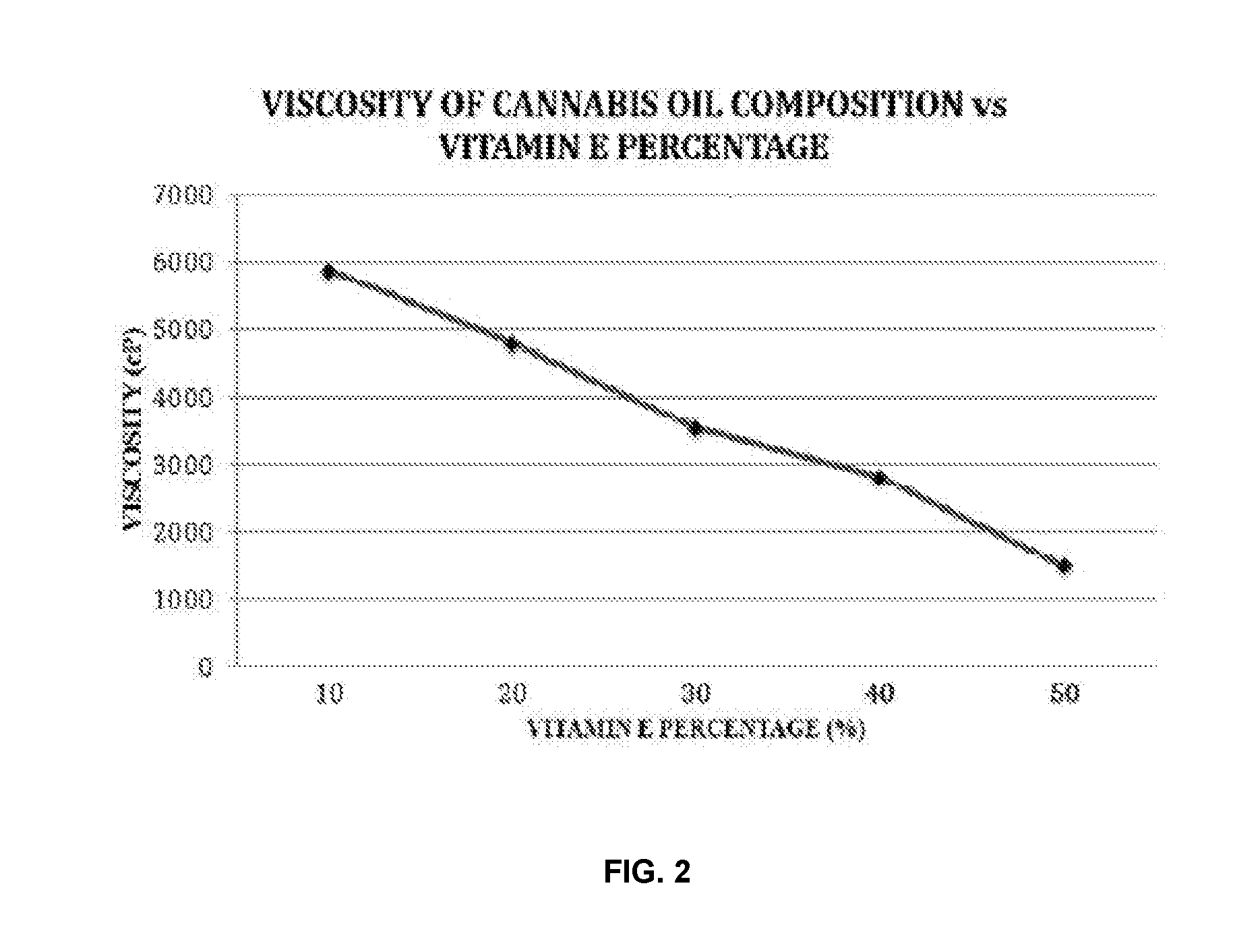
The present invention provides cannabis oil extracts and compositions thereof, including cannabis oil compositions containing vitamin E, and methods for preparing the extracts and compositions. In some embodiments, the present invention provides a method for preparing a cannabis oil extract comprising eluting cannabinoids from cannabis plant material with a solvent to produce an eluate, filtering the eluate with a filter to produce a filtrate, evaporating the solvent from the filtrate with a distiller to produce a distillate, and purging the distillate under conditions sufficient to remove residual solvent, thereby preparing the extract. In some embodiments, the method further includes mixing a quantity of vitamin E with the extract to produce a cannabis oil composition.
Owner:CONSTANCE THERAPEUTICS INC
Process for the Rapid Extraction of Active Ingredients from Herbal Materials
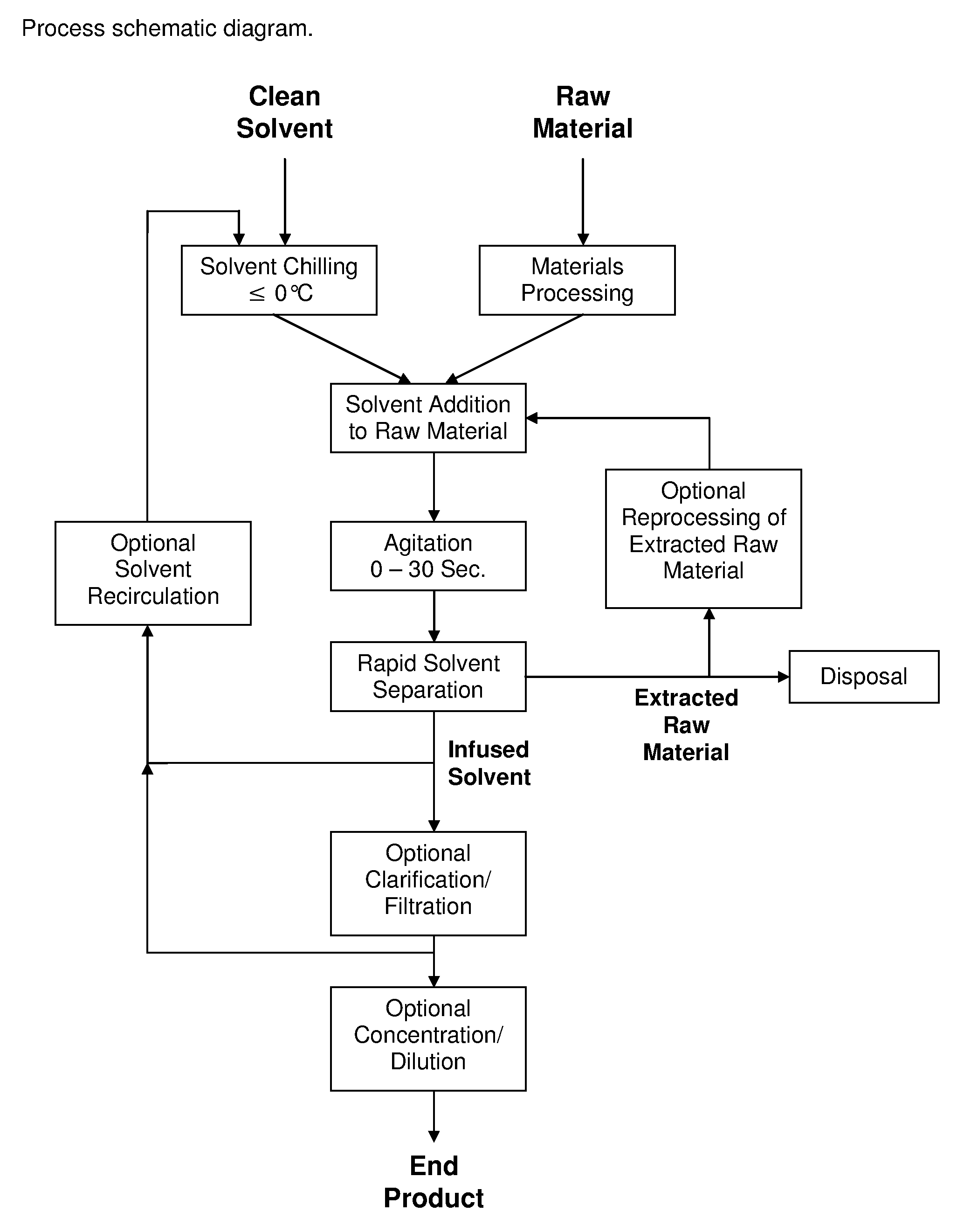
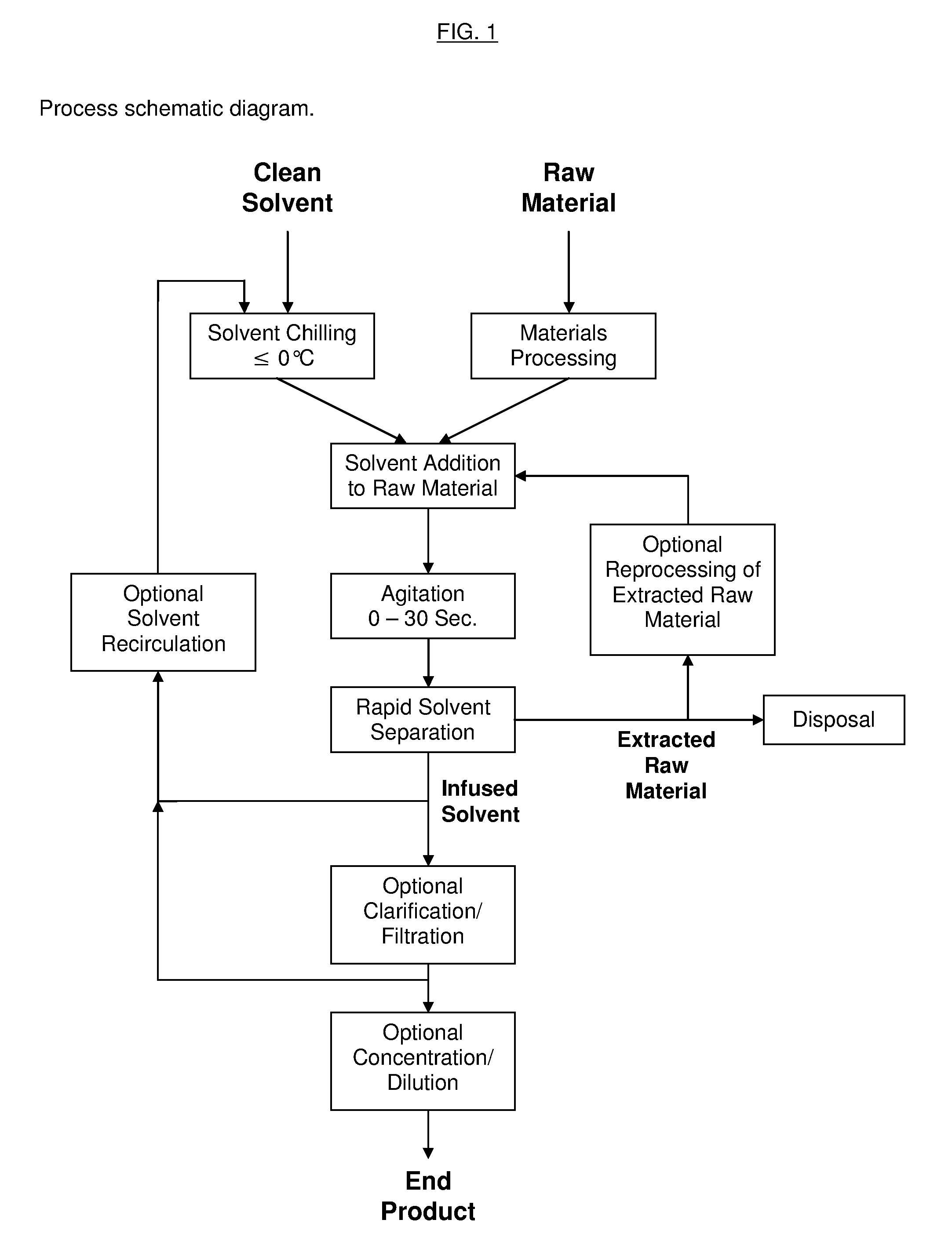
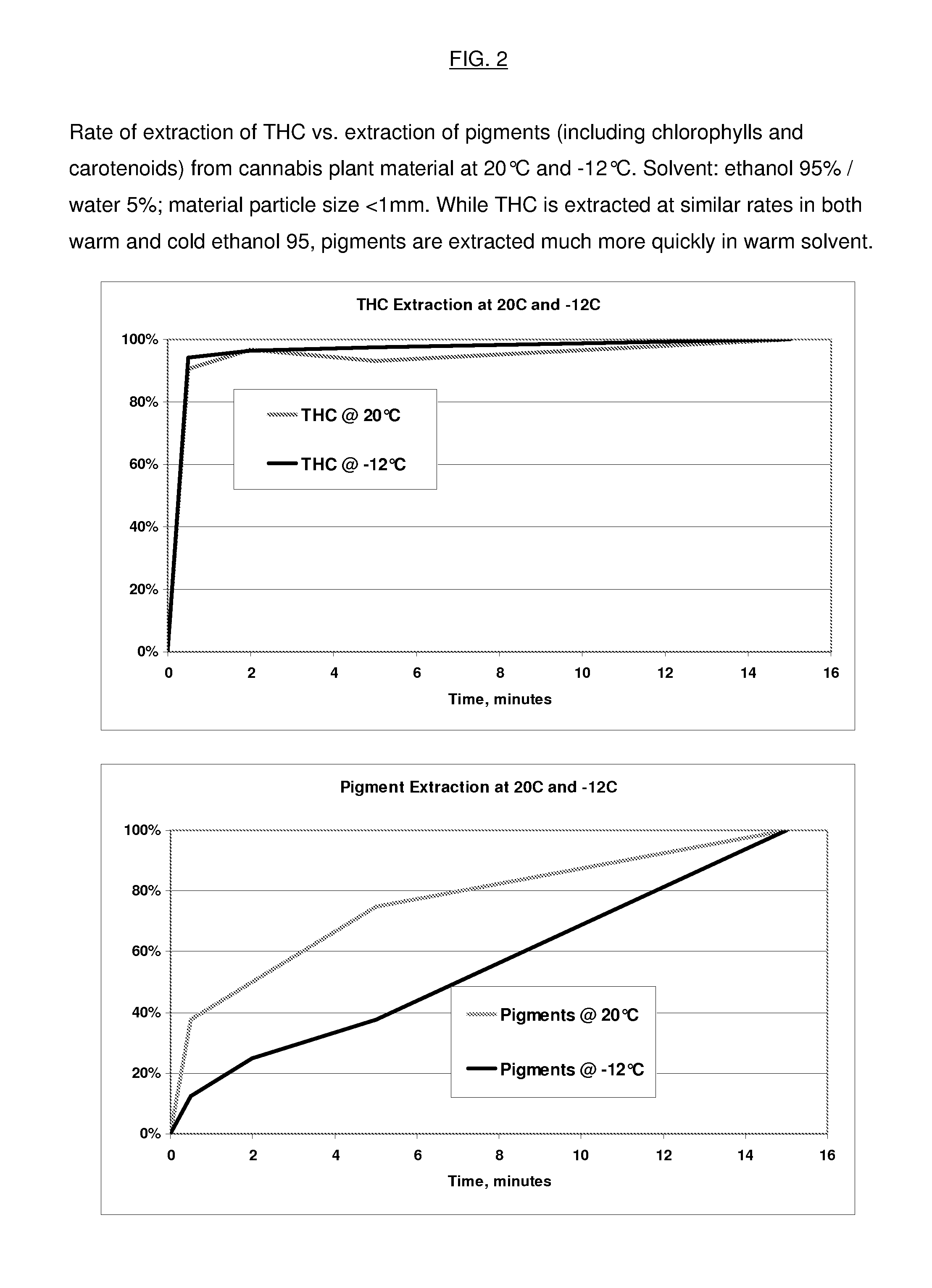
InactiveUS20130079531A1Reduce solubilityRapid extractionOrganic chemistryLiquid solutions solvent extractionSolventCannabis
The invention is a process for the rapid extraction of active ingredients from herbal materials using a cold solvent and a very short mixing period in order to yield commercially desirable extracts. In particular, the process can be applied to the rapid extraction of cannabinoids from cannabis. The claimed invention also includes any equipment or machine, or assemblage of equipments or machines, designed or employed to utilize this process.
Owner:RM3 LABS
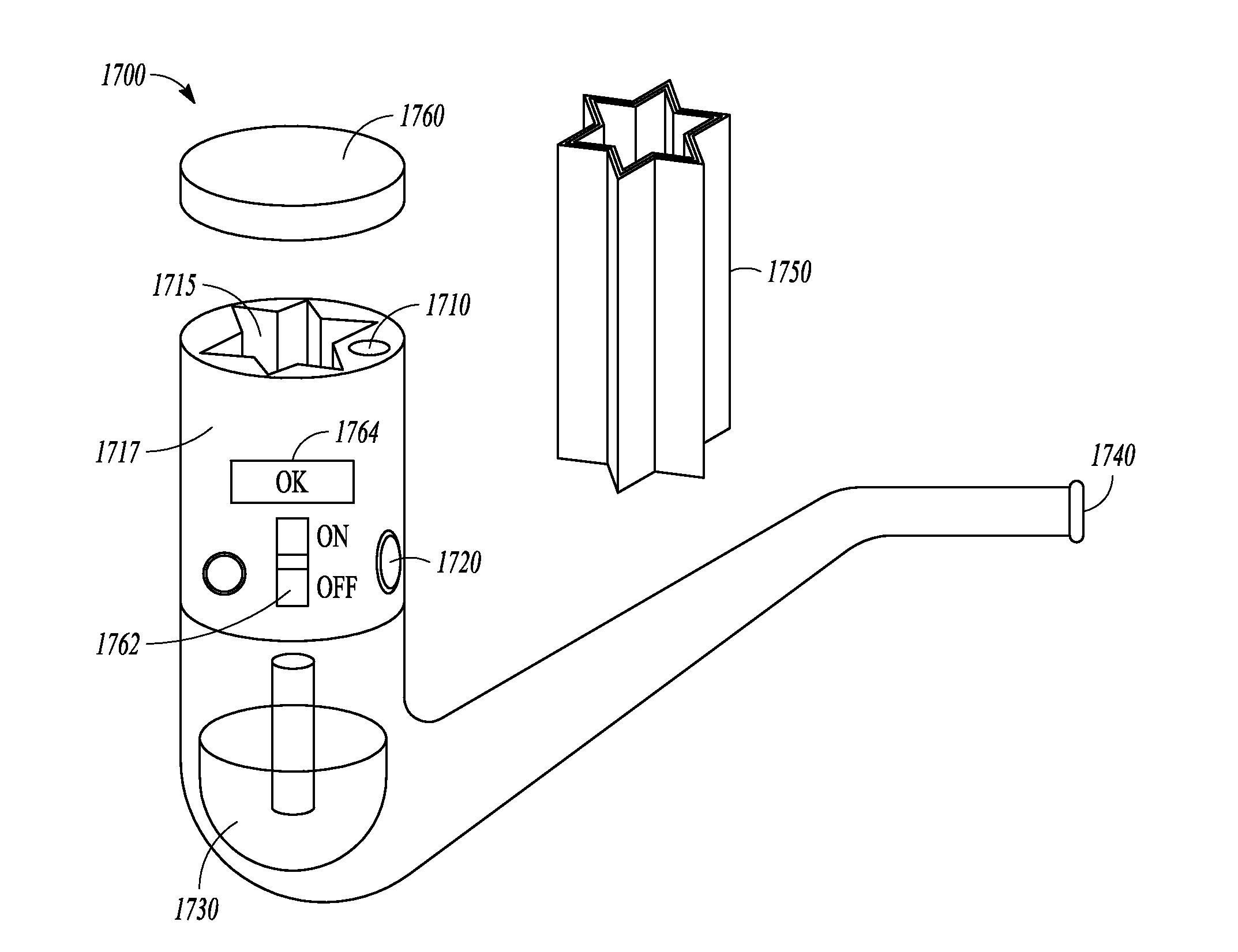
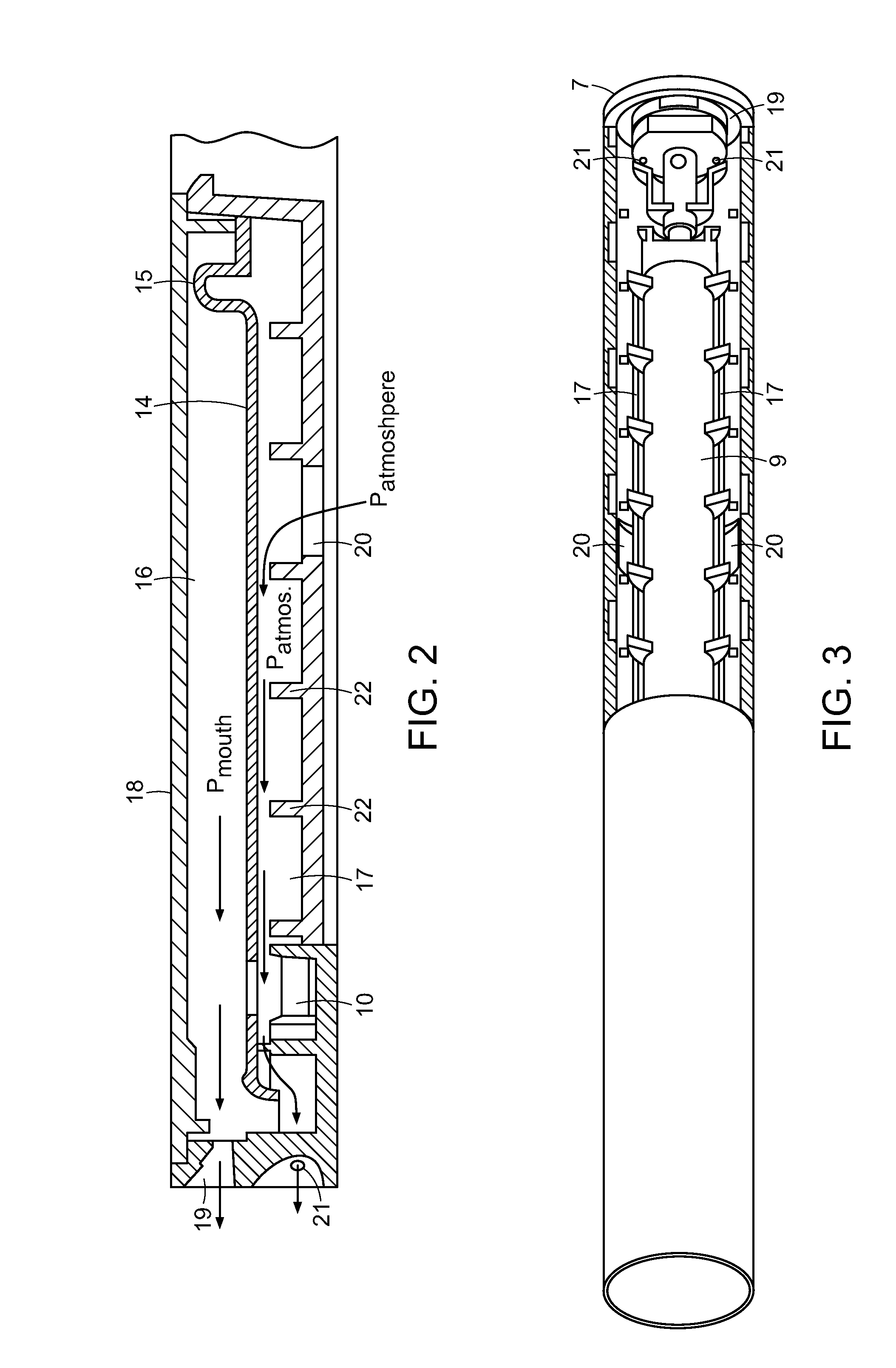
Methods and devices using cannabis vapors
A method of purifying at least one of THC and CBD from a cannabis-containing composition includes heating the cannabis-containing composition to a temperature sufficient to volatilize at least one of THC and CBD into a vapor and condensing the vapor on a substrate. A drug delivery cartridge includes a substrate coated with at least one of THC and CBD and configured to allow for passage of air through the cartridge to volatilize at least one of THC and CBD for inhalation by a user to induce a medicinal or therapeutic effect to the user.
Owner:VAPOR CARTRIDGE TECH LLC
Enhanced Delivery of Nicotine, THC, Tobacco, Cannabidiol or Base Alkaloid from an Electronic Cigarette or Other Vapor Producing Device Through Use of an Absorption Conditioning Unit
A method for the administration of nicotine, THC, tobacco, cannabidiol or a base alkaloid includes administering an absorption conditioning unit having at least two agents selected from the group consisting of (a) a buffer agent, (b) a capturing agent, (c) a penetration agent, and (d) a thermal agent, to the mammal, and then administering by inhalation a bioactive agent selected from the group consisting of nicotine, THC, cannabidiol and a base alkaloid. The absorption conditioning unit may be in a dosage form not containing a drug. The absorption conditioning unit may create a pH in the oral cavity of 7.8-10 for a period of ten minutes or more after administration, the dosage form not containing an acid and not containing a drug.
Owner:FUISZ RICHARD C +1
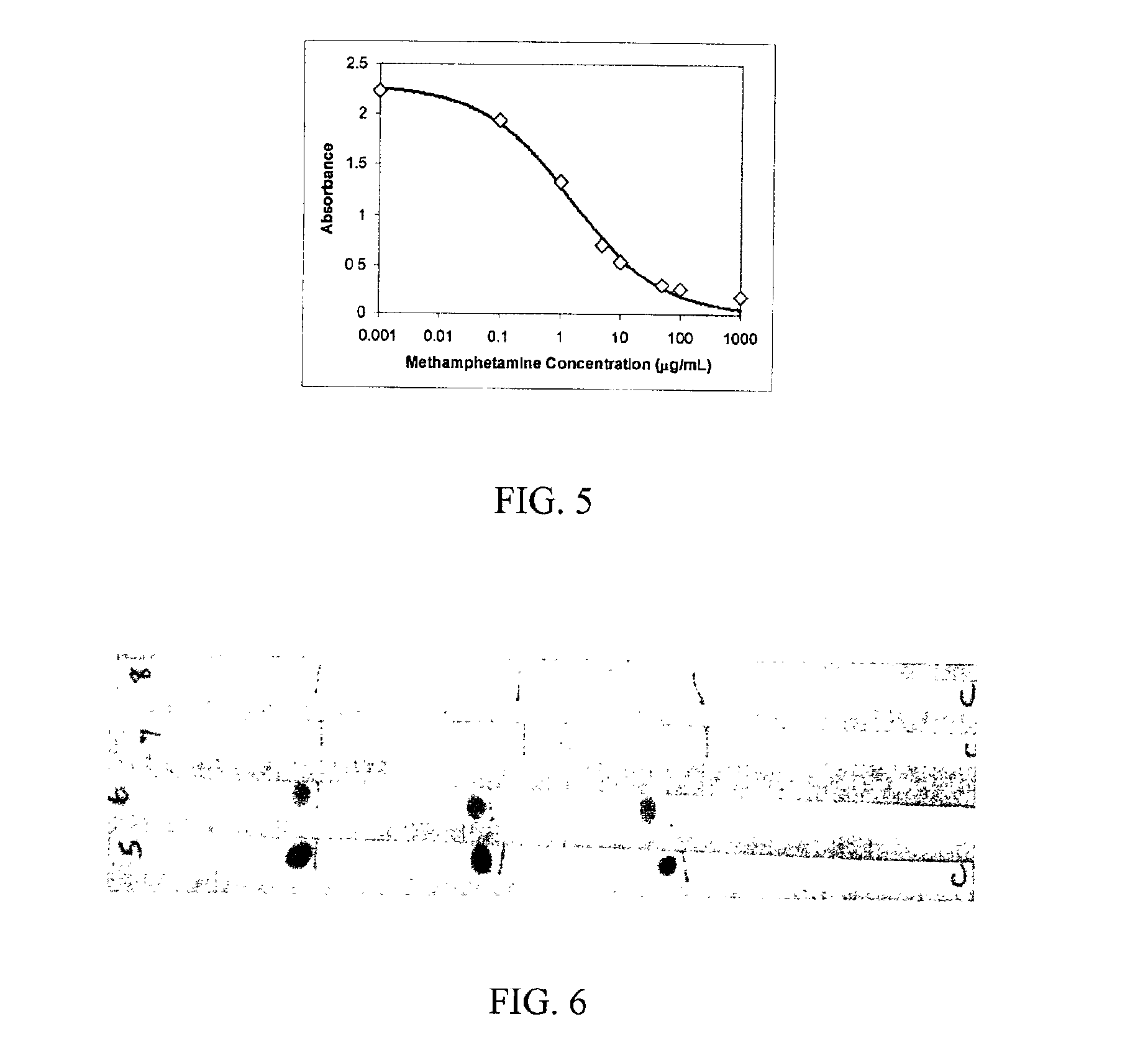
Rapid classification of biological components
InactiveUS6989276B2Analysis using chemical indicatorsMicrobiological testing/measurementAnalyteImmune complex deposition
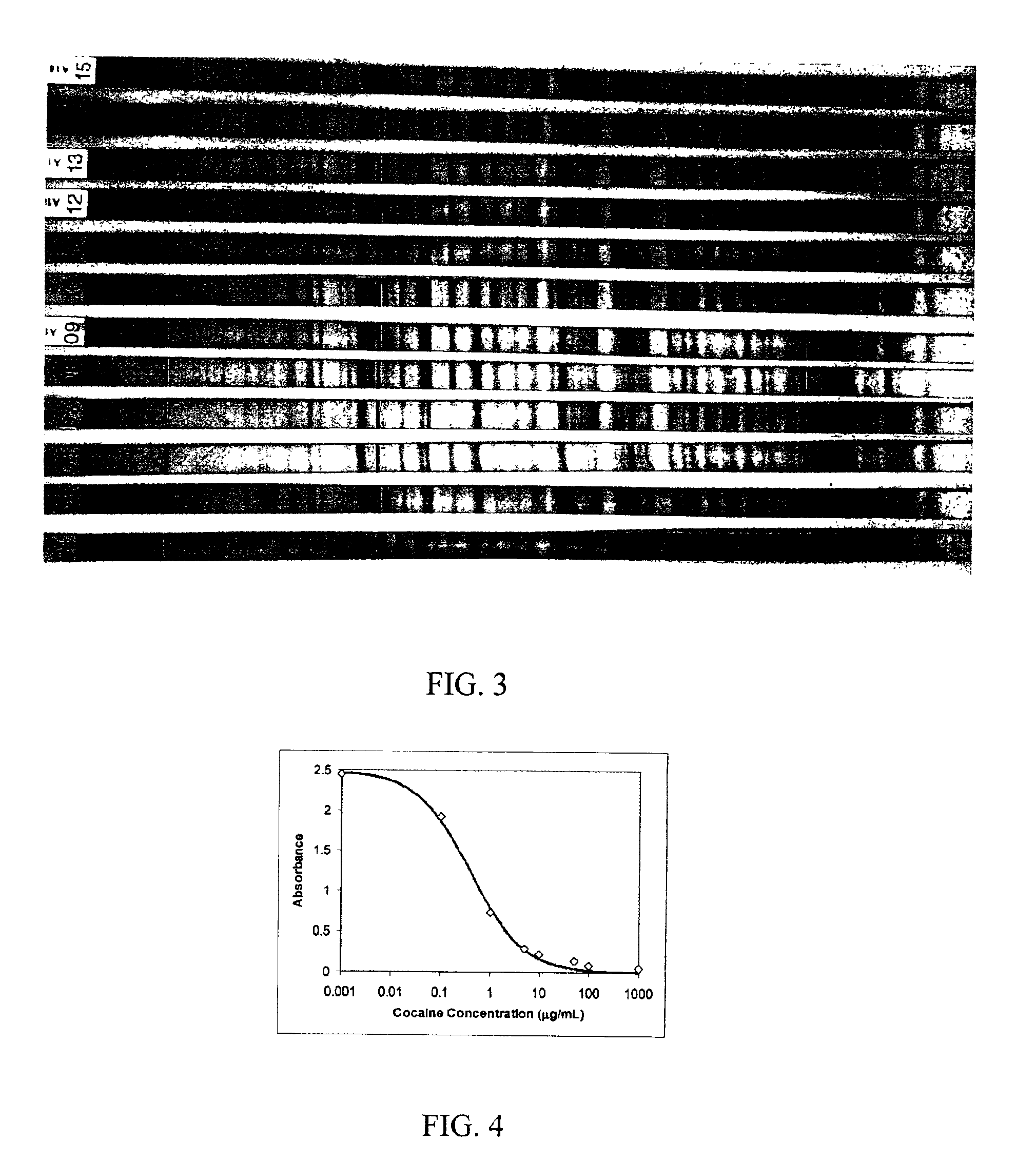
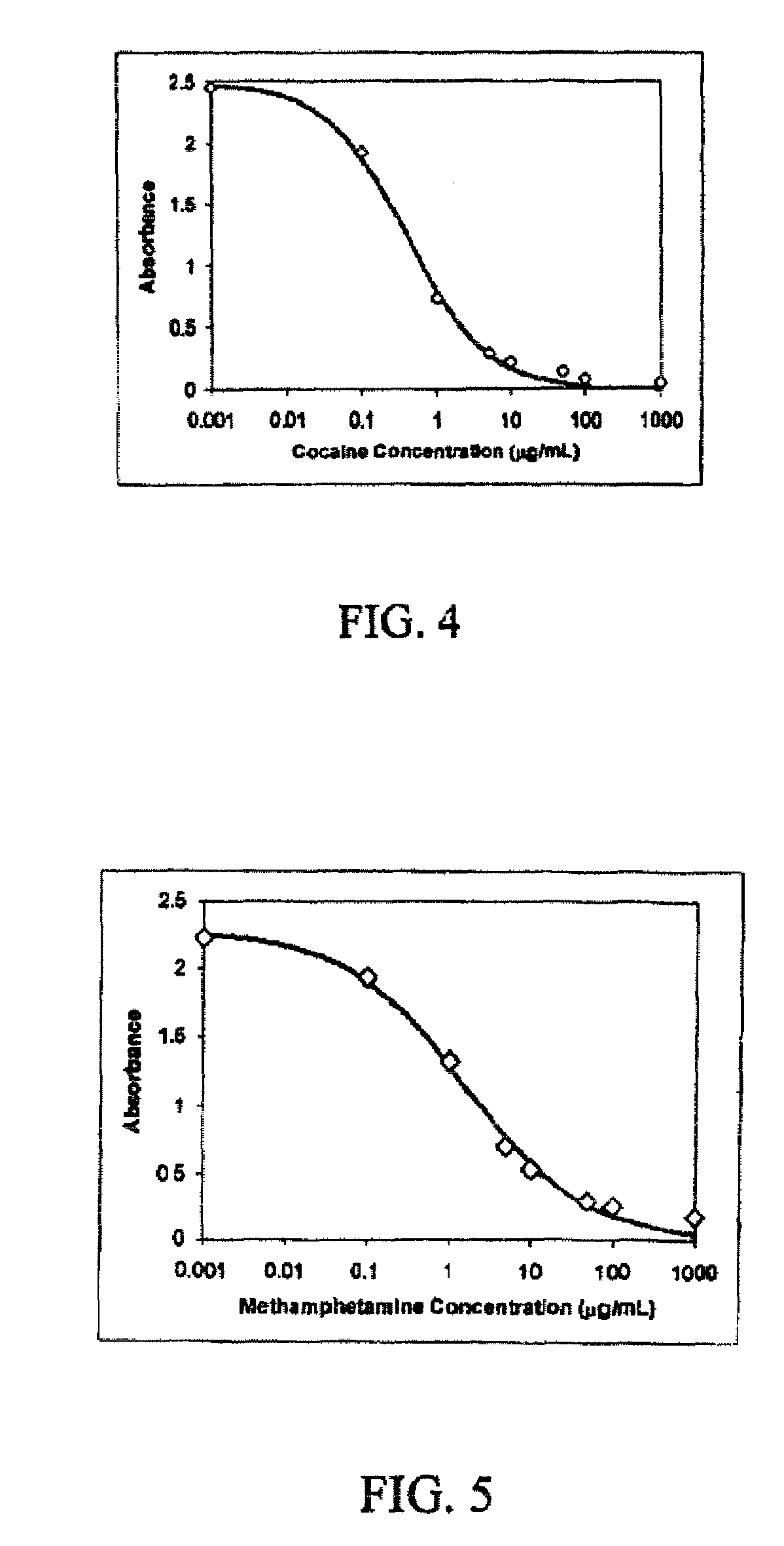
A method is disclosed for analyzing a biological sample by antibody profiling for identifying forensic samples or for detecting the presence of an analyte. In an illustrative embodiment of the invention, the analyte is a drug, such as marijuana, cocaine, methamphetamine, methyltestosterone, or mesterolone. The method involves attaching antigens to the surface of a solid support in a preselected pattern to form an array wherein the locations of the antigens are known; contacting the array with the biological sample such that a portion of antibodies in the sample reacts with and binds to antigens in the array, thereby forming immune complexes; washing away antibodies that do form immune complexes; and detecting the immune complexes, thereby forming an antibody profile. Forensic samples are identified by comparing a sample from an unknown source with a sample from a known source. Further, an assay, such as a test for illegal drug use, can be coupled to a test for identity such that the results of the assay can be positively correlated to the subject's identity.
Owner:BATTELLE ENERGY ALLIANCE LLC
Use of cannabinoids in the treatment of epilepsy
ActiveUS20190083418A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsSturge–Weber syndromeMedicine
The present invention relates to the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Sturge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Medicinal cannabis added in food
The invention is a product and a process wherein cannabinoids such as Medicinal Δ9-THC and / or other substances associated with medicinal cannabis, including yet not necessarily limited to cannbidiols, cannabigerol are added to a foodstuff where the medicinal cannabis is not evenly distributed throughout the foodstuff where the food stuff contains a known weight of medicinal cannabis. Another provision of the invention is providing controlled amounts or ratios of Δ9-THC as compared to CBD in or on a foodstuff.
Owner:HOSPODOR ANDREW DAVID
Medicinal cannabis fatty foodstuff
InactiveUS20120043242A1Organic active ingredientsNervous disorderDelta-9-tetrahydrocannabinolMedicine
The invention is a product and a process wherein Medicinal Delta-9 tetrahydrocannabinol (Δ9-THC) and potentially other cannabinoids (medicinal cannabis substances) associated with decarboxylated cannabis, including yet not necessarily limited to cannbidiols, and cannabigerol are rendered into a fatty foodstuff and then molded into a mold that also acts as a package. The best mode of the invention is a blister pack containing a plurality of voids or receptacles of desired sizes. A product that is characterized by a controlled amount of medicinal cannabis per unit volume of a fatty foodstuff base material is inserted into the mold, then cooled, and finally sealed. Each void or receptacle contains a known amount of medicinal cannabis that are independently dispensable.
Owner:HOSPODOR ANDREW DAVID
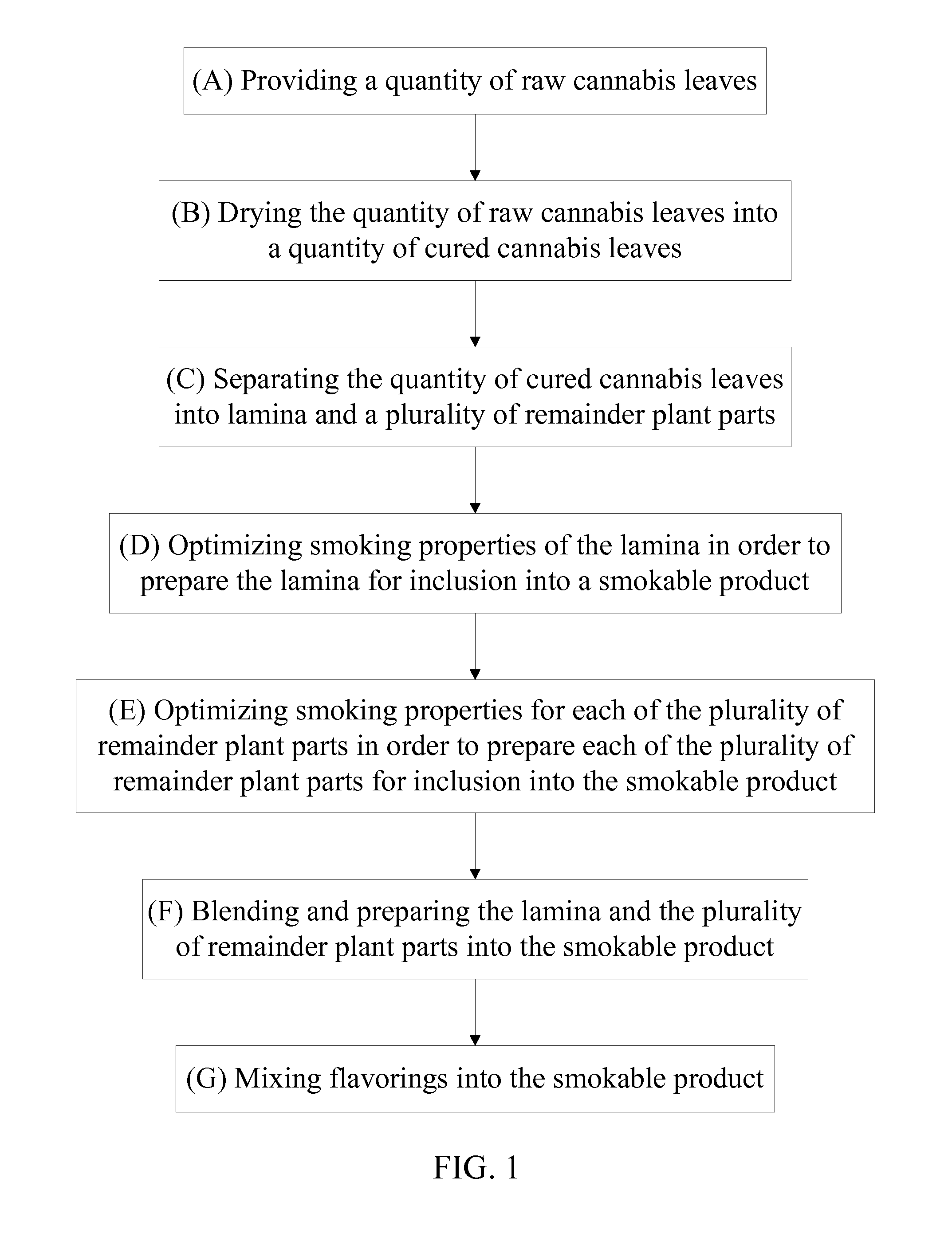
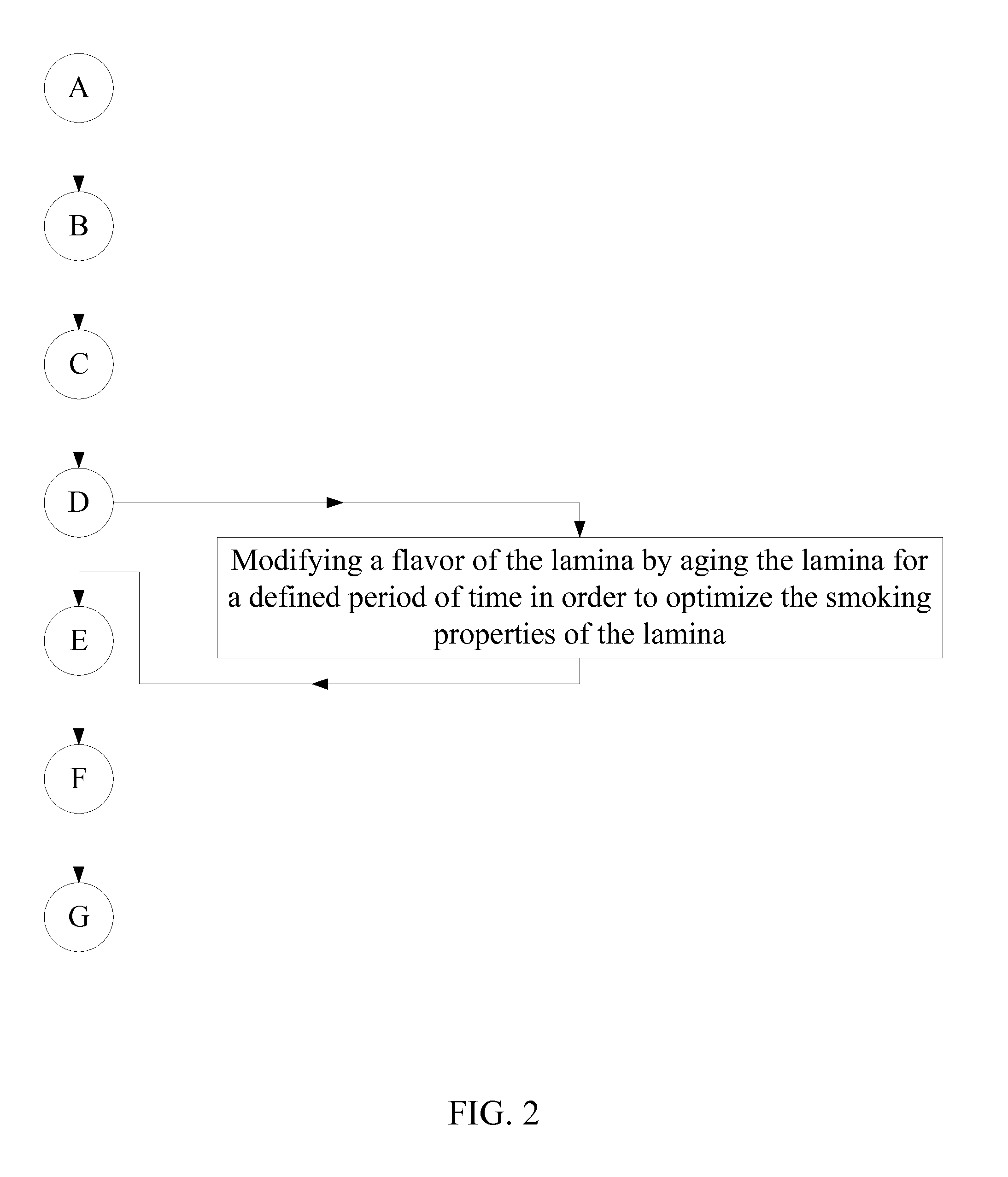
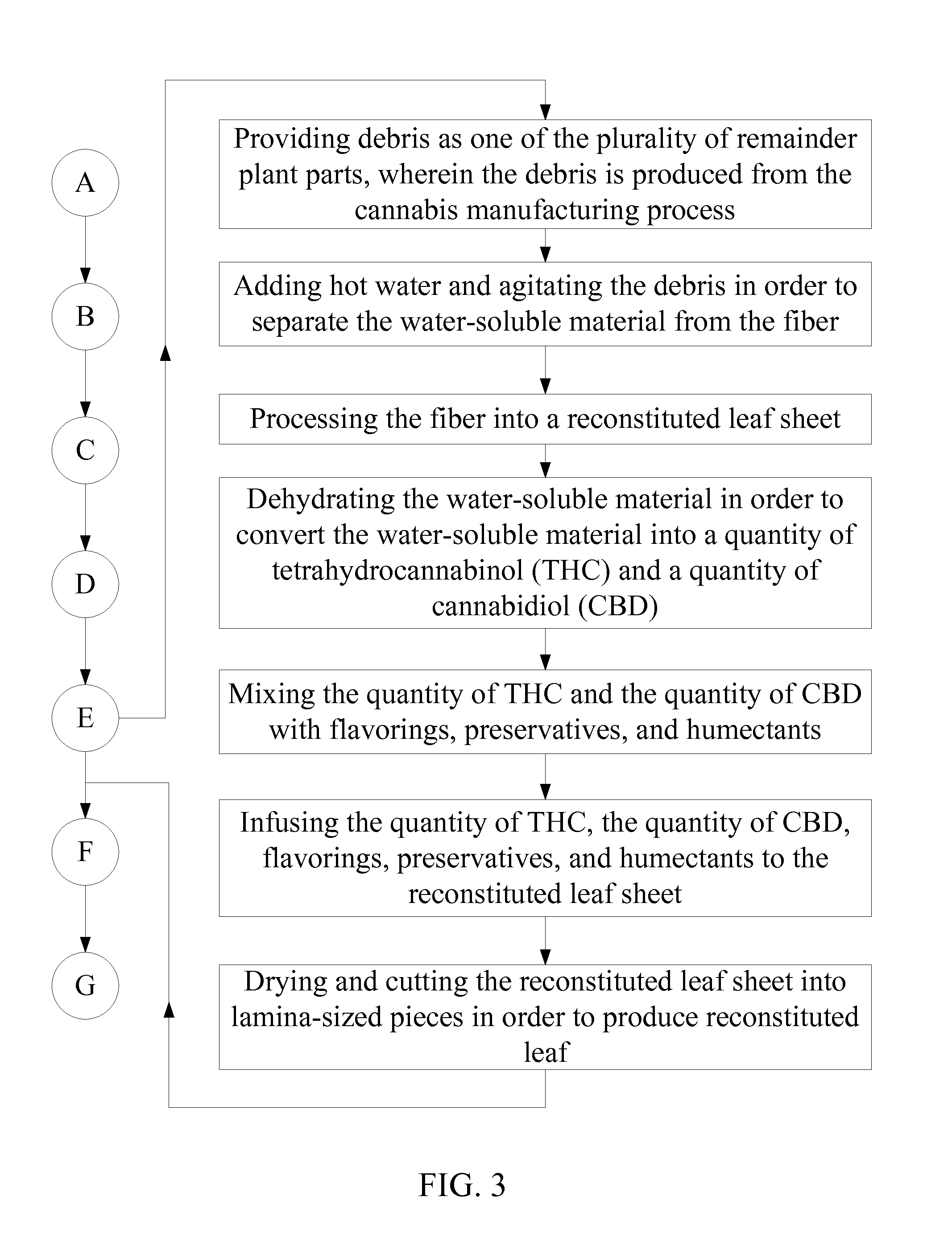
Method of Manufacturing a Smokable Cannabis Product
A method of manufacturing a smokable cannabis product allows for the thorough processing of a quantity of raw cannabis leaves into a quantity of cured cannabis leaves. The quantity of cured cannabis leaves is then separated into lamina and a plurality of remainder plant parts. The smoking properties of the lamina and each of the plurality of remainder plant parts are optimized prior to inclusion into the smokable product. The lamina and the plurality of remainder plant parts are then blended and prepared into the smokable product. The composition of the smokable product is adjusted by selectively incorporating the lamina and the plurality of remainder plant parts into the final blend. Flavorings are then mixed into the smokable product in order to achieve the desired flavor of the cannabis product. Tobacco may be incorporated into the cannabis product as well.
Owner:UREN MARK B
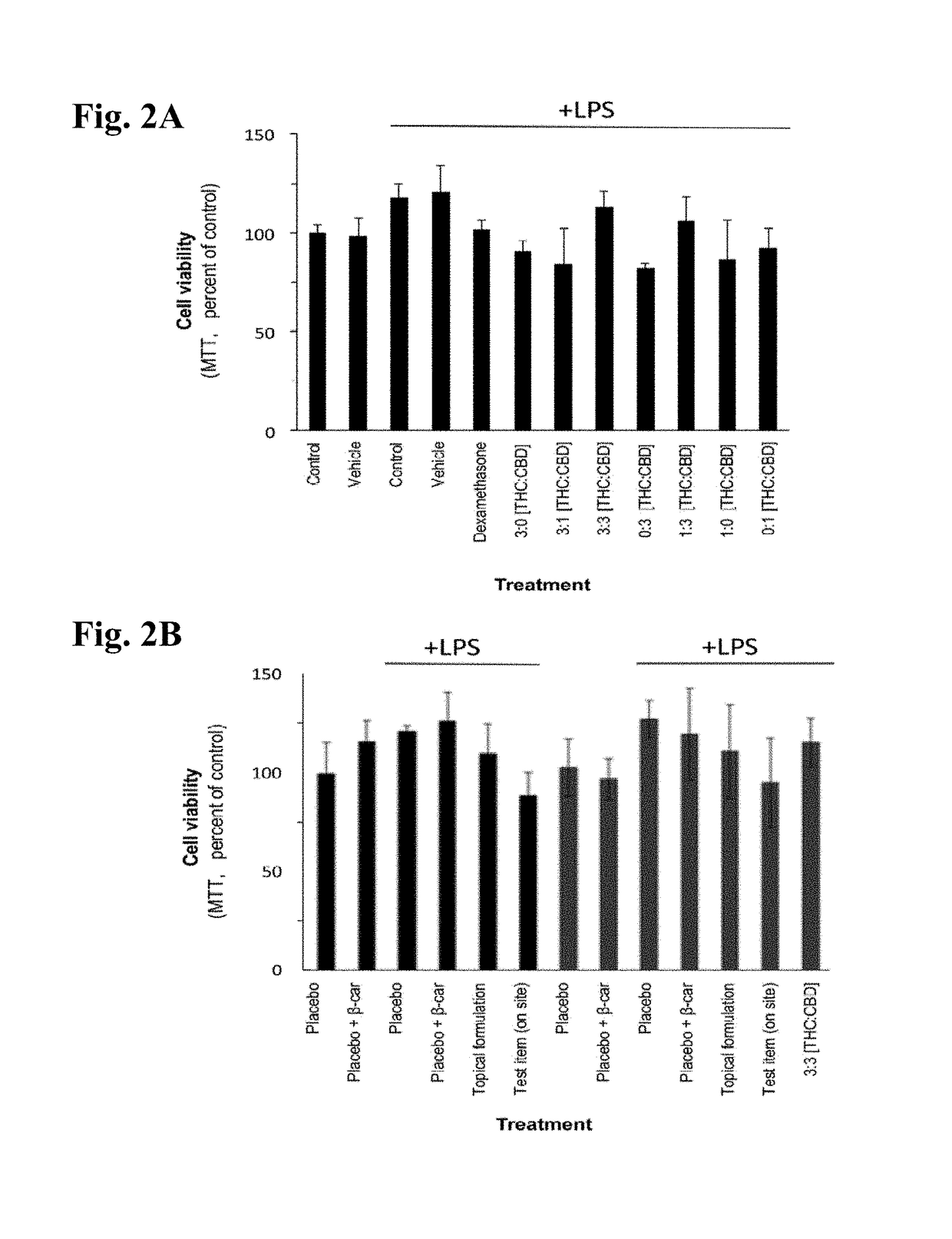
Cannabis-based extracts and topical formulations for use in skin disorders
The present invention discloses a pharmaceutical topical composition comprising cannabidiol (CBD) or a derivative thereof and Tetrahydrocannabinol (THC) or a derivative thereof in an about 1:1 ratio, useful for treatment or prevention of inflammatory skin disorders, and treatment methods thereof.
Owner:ONE WORLD CANNABIS LTD
Decarboxylated cannabis resins, uses thereof and methods of making same
The disclosure relates to decarboxylated cannabis resins and methods of making the decarboxylated cannabis resins by extraction and decarboxylation of cannabinoids from Cannabis species using microwaves and solvents. The disclosure also relates to use of the decarboxylated cannabis resins for making pharmaceutical products comprising same.
Owner:CANNSCI INNOVATIONS INC
Methods for Extraction and Isolation of Isoprenoid and Terpene Compounds from Biological Extracts
ActiveUS20180344785A1Rapidly cost-effectively extractedOrganic active ingredientsMagnoliophyta medical ingredientsCannabisPrenylation
A method for the extraction and isolation of the terpene and isoprenoid compounds from plant material, followed by a centrifugal force induced selective crystallization of isoprenoids resulting in a separation of terpene and isoprenoid fractions. This this method is suitable for the extraction of cannabinoids from Cannabis and the enrichment tetrahydrocannabinolic acid and reduction of tetrahydrocannabinol in an extract.
Owner:CONCENTRATED CONSULTING GRP LLC
Methodology and Formulation for Creating a Powder of an Encapsulated Cannabis-Based Component Embedded in a Polymer Matrix
InactiveUS20200054702A1Improve bioavailabilityNervous disorderDispersion deliveryCannabis sativa plantPolymer science
Provided are compositions and methods of forming a particulate material derived from a cannabis plant. The method includes introducing a component including at least one of: (i) a cannabinoid, and (ii) a terpene, to a polymer to produce a polymeric mixture. The component is dispersed in the polymeric mixture, which is then at least partially dehydrated to encapsulate the component within a polymeric material derived from the polymer. The polymeric material is water soluble. The dehydrated polymeric mixture is processed to form particulates comprising the component encapsulated within shells formed from the polymeric material.
Owner:HELLER MICHAEL +1
Cannabidiol preparations and its uses
Owner:GW RES LTD
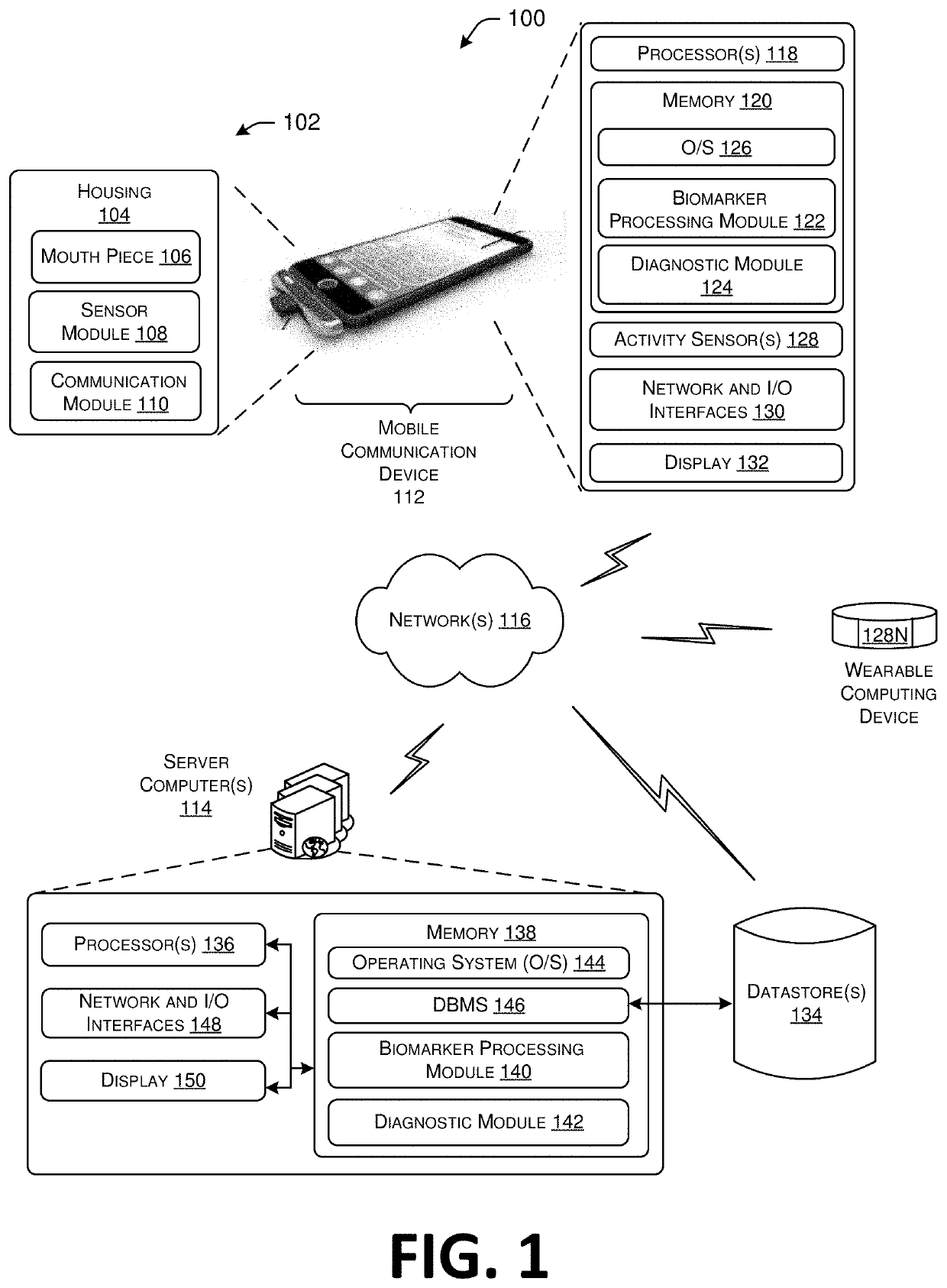
Biomarkers for systems, methods, and devices for detecting and identifying substances in a subject's breath, and diagnosing and treating health conditions
Embodiments of the disclosure can include biomarkers for systems, methods, and devices for detecting and identifying certain substances, such as chemicals, volatile organic compounds (VOCs), volatile gases (VGs), ketones, cannabis, controlled substances, pharmaceuticals, or anesthetics, in the exhaled breath of a subject or person in real-time; and further diagnosing, treating, and addressing one or more associated health conditions or diseases, including a virus, such as COVID-19, tuberculosis (TB), lung cancer, or chronic obstructive lung disease (COPD).
Owner:CANARY HEALTH TECH INC
Cone loading, weighing, filling, and twisting apparatus and method for mass production of smokable cannabis or hemp products
PendingUS20210022388A1Overcome disadvantagesAchieve mass productionCigarette manufactureEngineeringMechanical engineering
An apparatus for mass producing smokable products filled with cannabis, hemp, and / or other smokable materials includes a cone loading station that separates individual paper cones from stacks of cones, a cone weighing and filling station for dispensing precisely weighed amounts of powdered biomass into the cones, and a cone twisting station for twisting ends of the cones to complete the smokable products. A transport mechanism is provided to transfer the cones between stations and hold the cones during filling and twisting. The invention also provides a method of manufacturing smokable products that enables production steps to be performed simultaneously while also providing for capacity expansion within a limited production facility footprint.
Owner:SULLIVAN JOHN TIMOTHY
Use of cannabinoids in the treatment of epilepsy
InactiveUS20200138738A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsSturge–Weber syndromeEpilepsy seizure
The present invention relates in the use of cannabidiol (CBD) in the treatment of Sturge Weber syndrome. CBD appears particularly effective in reducing all types of seizures and non-seizure symptoms in patients suffering with Surge Weber syndrome. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Oral pharmaceutical formulation comprising cannabinoids and poloxamer
PendingUS20210059976A1Improve bioavailabilityTotalPowder deliveryNervous disorderPharmaceutical formulationCannabidiol
The present invention relates to a novel cannabinoid oral pharmaceutical dosage form, based on a Type IV or Type IV-like formulation, as classified using the Lipid Formulation Classification System. The formulation comprises a combination of at least two cannabinoids. The first cannabinoid is selected from the group consisting of tetrahydrocannabinol (THC) and analogues thereof; and the second cannabinoid is selected from the group consisting of cannabidiol (CBD) and analogues thereof.
Owner:GW RES LTD
Cannabidiol preparations and its uses
ActiveUS20210015789A1Low back painGood blood pressurePowder deliveryNervous disorderCannabis sativa plantDisease
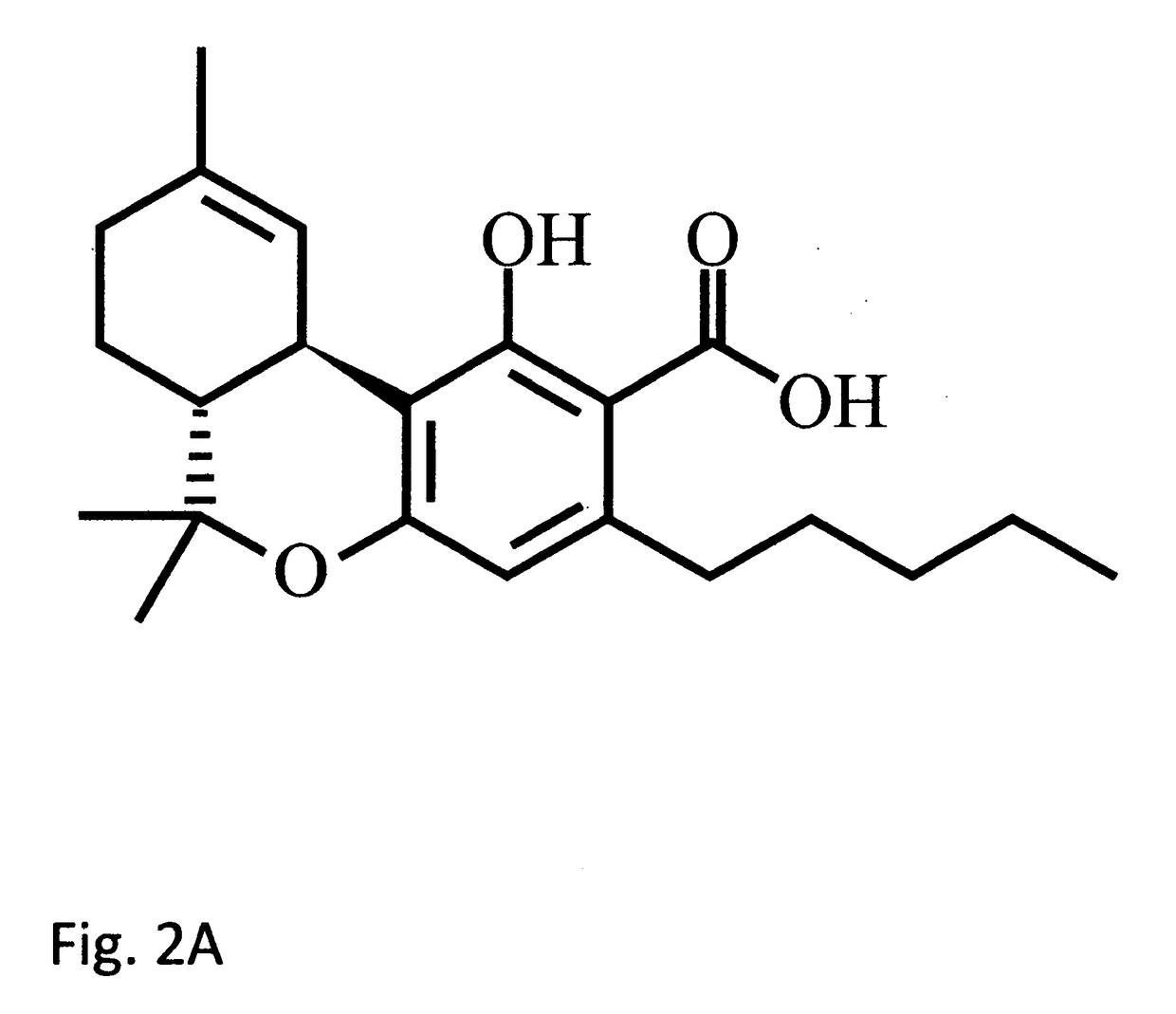
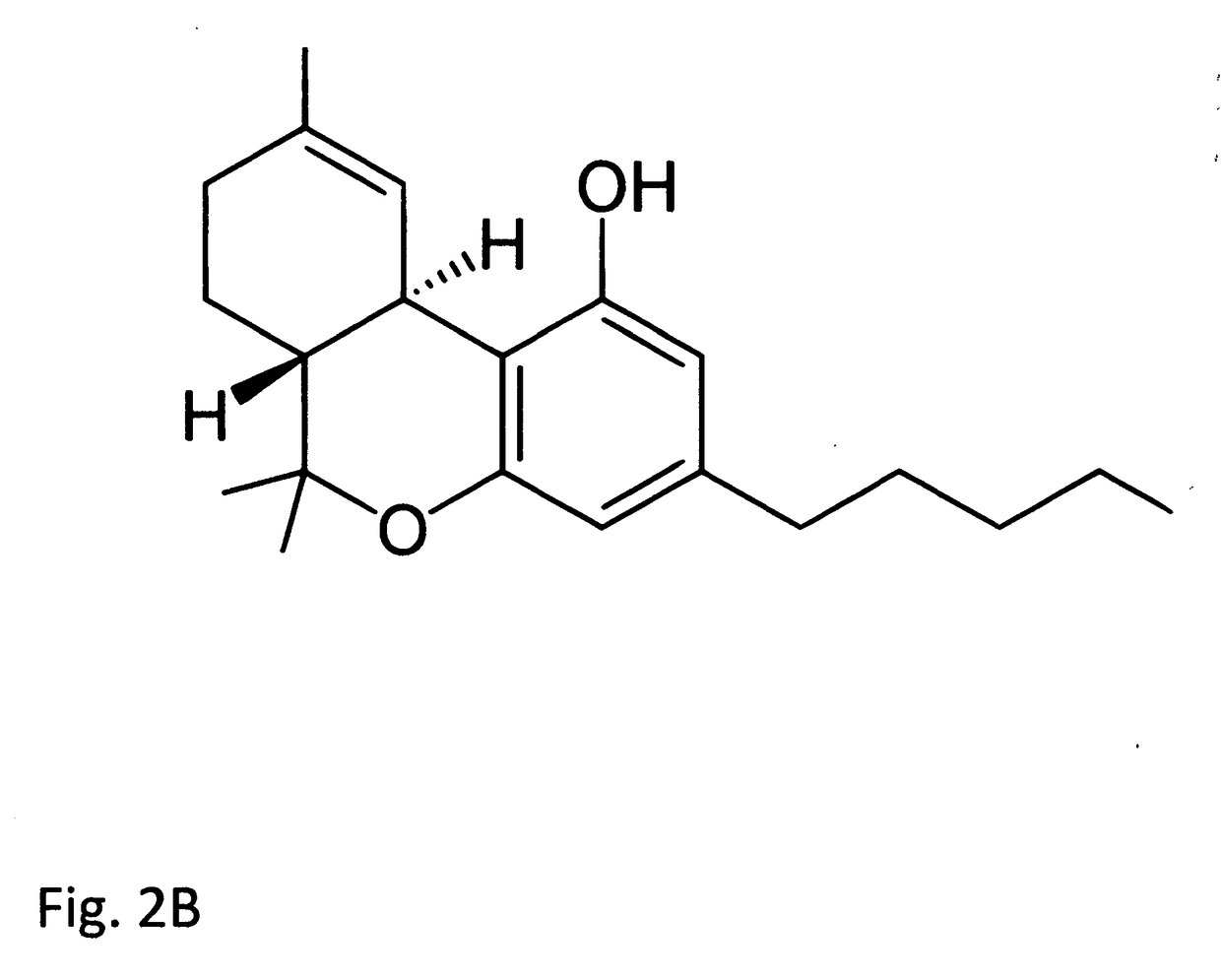
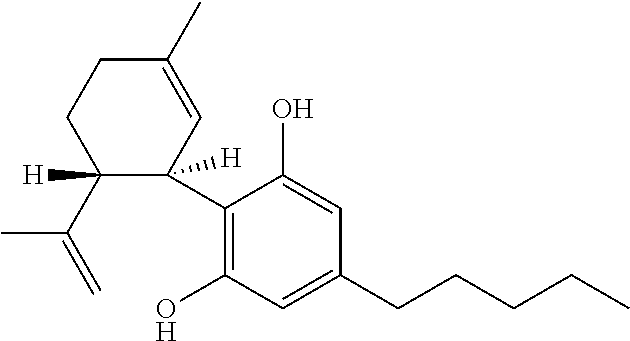
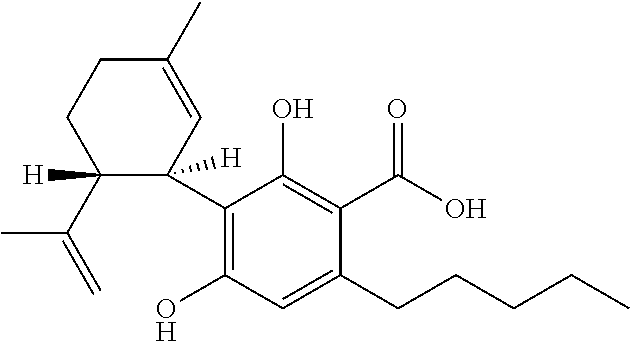
Cannabidiol (CBD) is a cannabinoid designated chemically as 2-[(1R,6R)-3-Methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol. Its empirical formula is C21H30O2 and its molecular weight is 314.46. CBD is a cannabinoid that naturally occurs in the Cannabis sativa L. plant. CBD is a white to pale yellow crystalline solid which is insoluble in water and soluble in organic solvents. The present invention encompasses the surprising recognition that certain CBD preparations which are prepared from a botanical origin are more effective in treating diseases or disorders than preparations of CBD which are synthetic or purified to the extent no other impurities in the form of other cannabinoids are present. Prior CBD compositions have been prepared such that no psychoactive components, e.g., tetrahydrocannabinol (THC), remain in the final CBD preparation. Surprisingly, the absence of such minor impurities reduces the efficacy of CBD treatment. Such CBD preparations are characterized by chemical components and / or funtional properties that distinguish them from prior CBD compositions. One or more components of the preparations described herein provide an unexpectedly synergistic effect when utilized in combination.
Owner:GW RES LTD
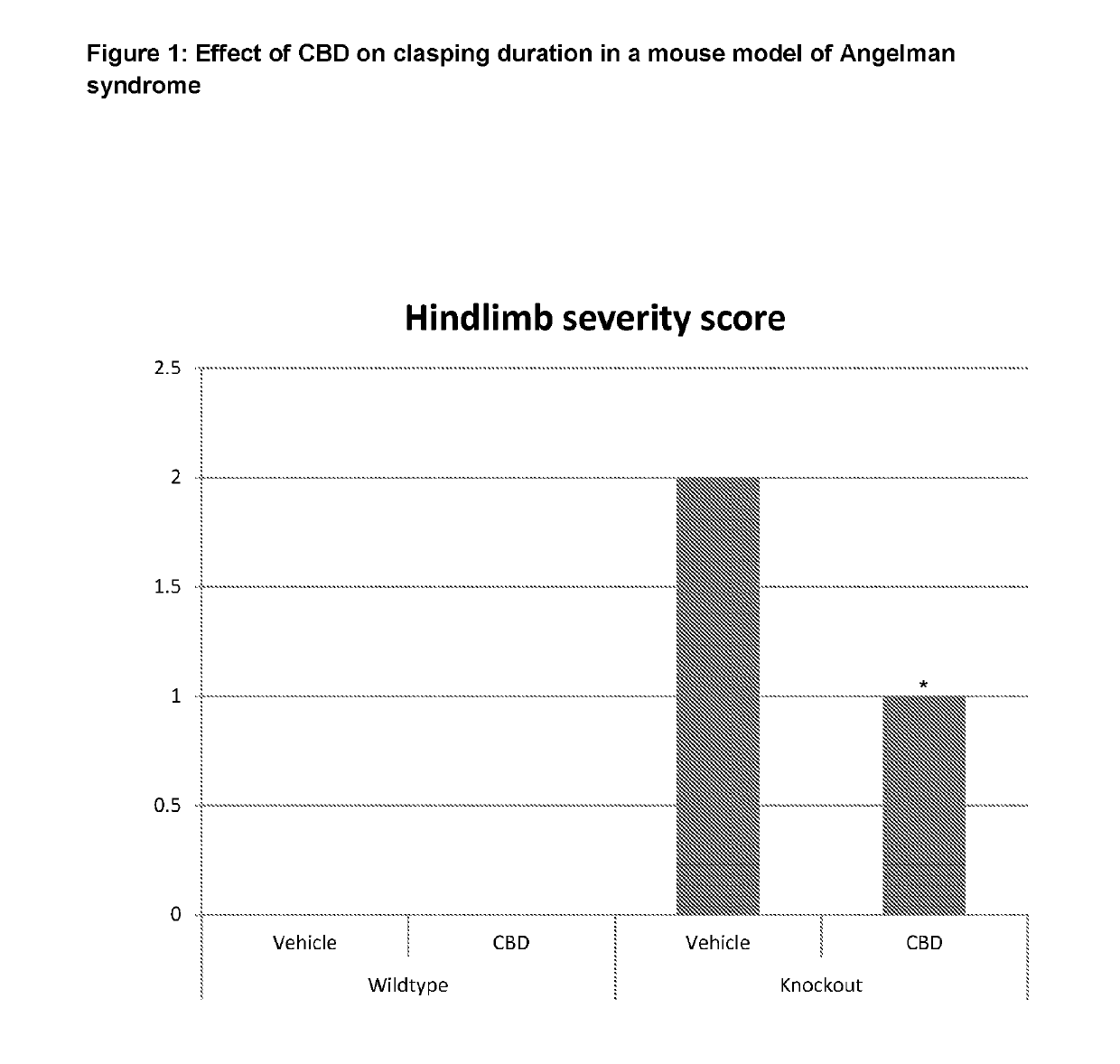
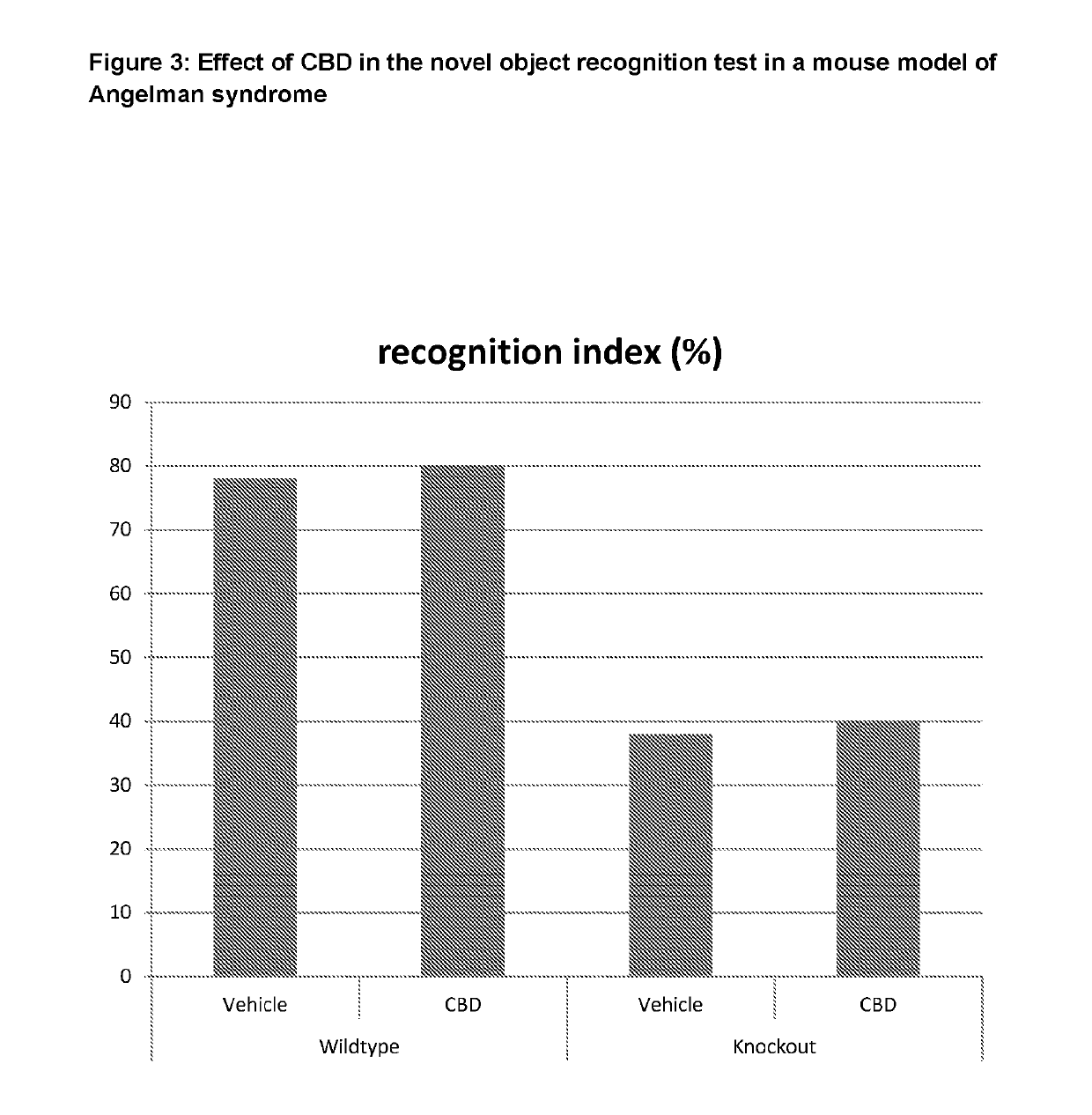
Use of cannabinoids in the treatment of angelman syndrome
ActiveUS20190321307A1Symptoms improvedNervous disorderHydroxy compound active ingredientsRodent modelAnxiety
The present invention relates to the use of cannabidiol (CBD) in the treatment of Angelman syndrome (AS). CBD has It been shown to be particularly effective in improving anxiety in rodent models of AS. The CBD is preferably substantially pure. It may take the form of a highly purified extract of Cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. Alternatively, the CBD is synthetically produced. Alternatively, the CBD may be used as a botanical drug substance (BDS) from a Cannabis plant in which CBD is the predominant cannabinoid. The CBD may also be present in combination with other cannabinoids and non-cannabinoid components such as terpenes. In use the CBD may be used concomitantly with one or more other medicaments. Alternatively, the CBD may be formulated for administration separately, sequentially or simultaneously with one or more medicaments or the combination may be provided in a single dosage form. Where the CBD is formulated for administration separately, sequentially or simultaneously it may be provided as a kit or together with instructions to administer the one or more components in the manner indicated. It may also be used as the sole medication, i.e. as a monotherapy.
Owner:GW RES LTD
Compositions and Methods for Oral Administration of Cannabinoids and Terpenoids
ActiveUS20190060225A1Precise and reliable dosageAct quicklyOrganic active ingredientsPowder deliveryDelivery vehicleCannabis Preparation
The present invention relates to oral administration of water soluble cannabinoid and terpenoid concentrates on a flavored fibrous carrier. The compositions described herein provide an alternative to smoked cannabis that also avoid the slow action and unpredictable dosing of edible cannabis preparations. Water soluble concentrates of cannabinoids and terpenoids may be produced by derivatization reactions which are known in the art. Such water soluble concentrates enjoy significantly increased absorption by mucosal membranes of the mouth and may therefore be experienced by the consumer more quickly. Water soluble cannabinoid and terpenoid concentrates may be used in combination with each other and in combination with non-derivatized molecules which provides the user with fast and slow acting components in a single delivery vehicle.
Owner:TRINIDAD CONSULTING LLC
Use of cannabinoids in the treatment of epilepsy
InactiveUS20200352878A1Reduce seizuresPoor response rateNervous disorderHydroxy compound active ingredientsAntiepileptic AgentsStiripentol
The present invention relates to the use of cannabidiol (CBD) in the treatment of patients with childhood-onset epilepsy who are concurrently taking one or more antiepileptic drugs that works via GABA receptor agonism. Preferably the AED is stiripentol. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Cannabinoid Inhaler and Composition Therefor
The present invention provides a cannabinoid inhaler, compositions, and methods for delivering a cannabinoid composition to a subject. The cannabinoid composition is delivered in the form of inhaled droplets of respirable size via pulmonary administration. The invention may be used in the treatment of a condition or disorder selected from the group consisting of neuropathic pain, cannabis addiction, nausea, motion sickness, arthritis, and neurodegenerative disease.
Owner:KIND CONSUMER LIMITED
Antibody profiling sensitivity through increased reporter antibody layering
InactiveUS7695919B2Bioreactor/fermenter combinationsBiological substance pretreatmentsImmune complex formationImmune complex deposition
A method for analyzing a biological sample by antibody profiling for identifying forensic samples or for detecting the presence of an analyte. In an embodiment of the invention, the analyte is a drug, such as marijuana, Cocaine (crystalline tropane alkaloid), methamphetamine, methyltestosterone, or mesterolone. The method comprises attaching antigens to a surface of a solid support in a preselected pattern to form an array wherein locations of the antigens are known; contacting the array with the biological sample such that a portion of antibodies in the sample reacts with and binds to the antigens in the array to form immune complexes; washing away antibodies that do form immune complexes; and detecting the immune complexes, to form an antibody profile. Forensic samples are identified by comparing a sample from an unknown source with a sample from a known source. Further, an assay, such as a test for illegal drug use, can be coupled to a test for identity such that the results of the assay can be positively correlated to the subject's identity.
Owner:BATTELLE ENERGY ALLIANCE LLC
Integrated grinding and storage system for optimizing and enhancing plant performance of plant-based medical therapies and related cannabis usage
InactiveUS20170368554A1Easy and convenient accessReduce the possibilityCoffee millsSpice millsCannabisEngineering
An integrated grinding and storage system for optimizing and enhancing plant performance for plant-based medical therapies and related uses of cannabis including an apparatus for grinding that includes a head operable to be removably attached to a receptacle or container. The head includes a magnet, an upper, inner grinding element, a lower grinding element, a shaft, and a neck sleeve. The upper, inner grinding element is in communication with the lower grinding element within the head. The grinding elements are operable to rotate relative to each other. The shaft extends between the upper and lower sections and magnetically couples with the grinding elements and orients them in relation to one another. The head may be manually or electrically activated.
Owner:NICHOLS WILLIAM
Film-shaped mucoadhesive administration form for administering cannabis active ingredients
InactiveCN1658840AImprove complianceOrganic active ingredientsSenses disorderActive agentBULK ACTIVE INGREDIENT
A film-shaped, mucoadhesive administration form having a content of at least one active agent. The active agent is a cannabis agent.
Owner:LTS LOHMANN THERAPIE-SYST AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com