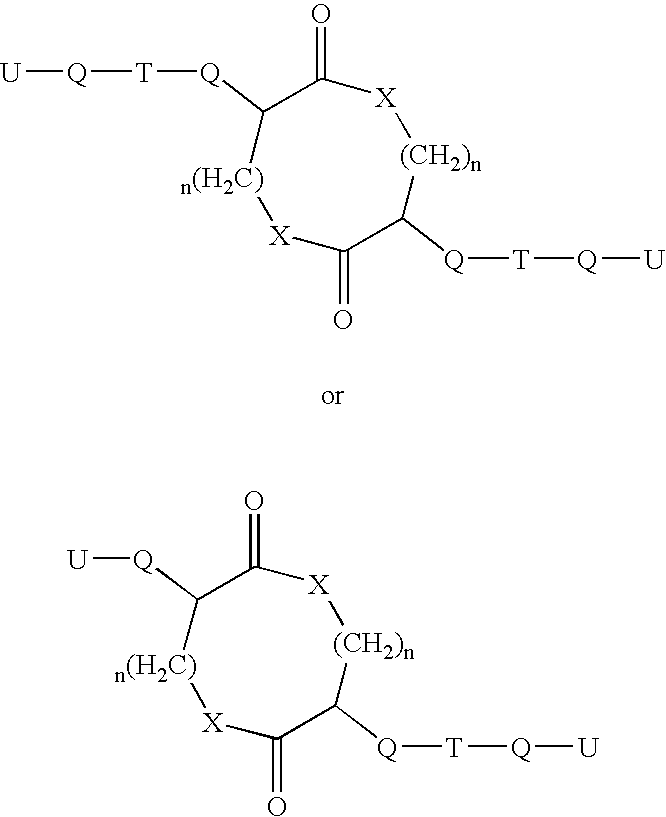
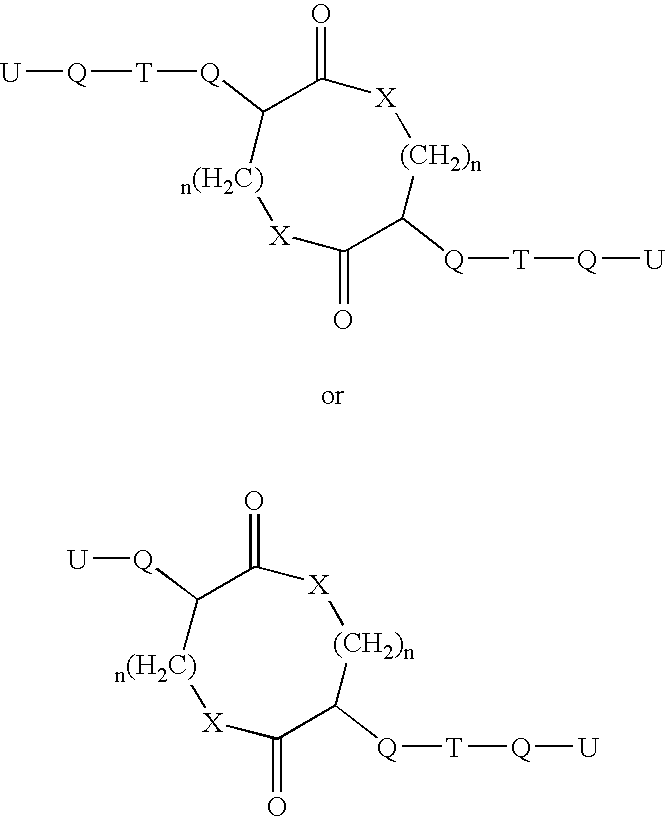
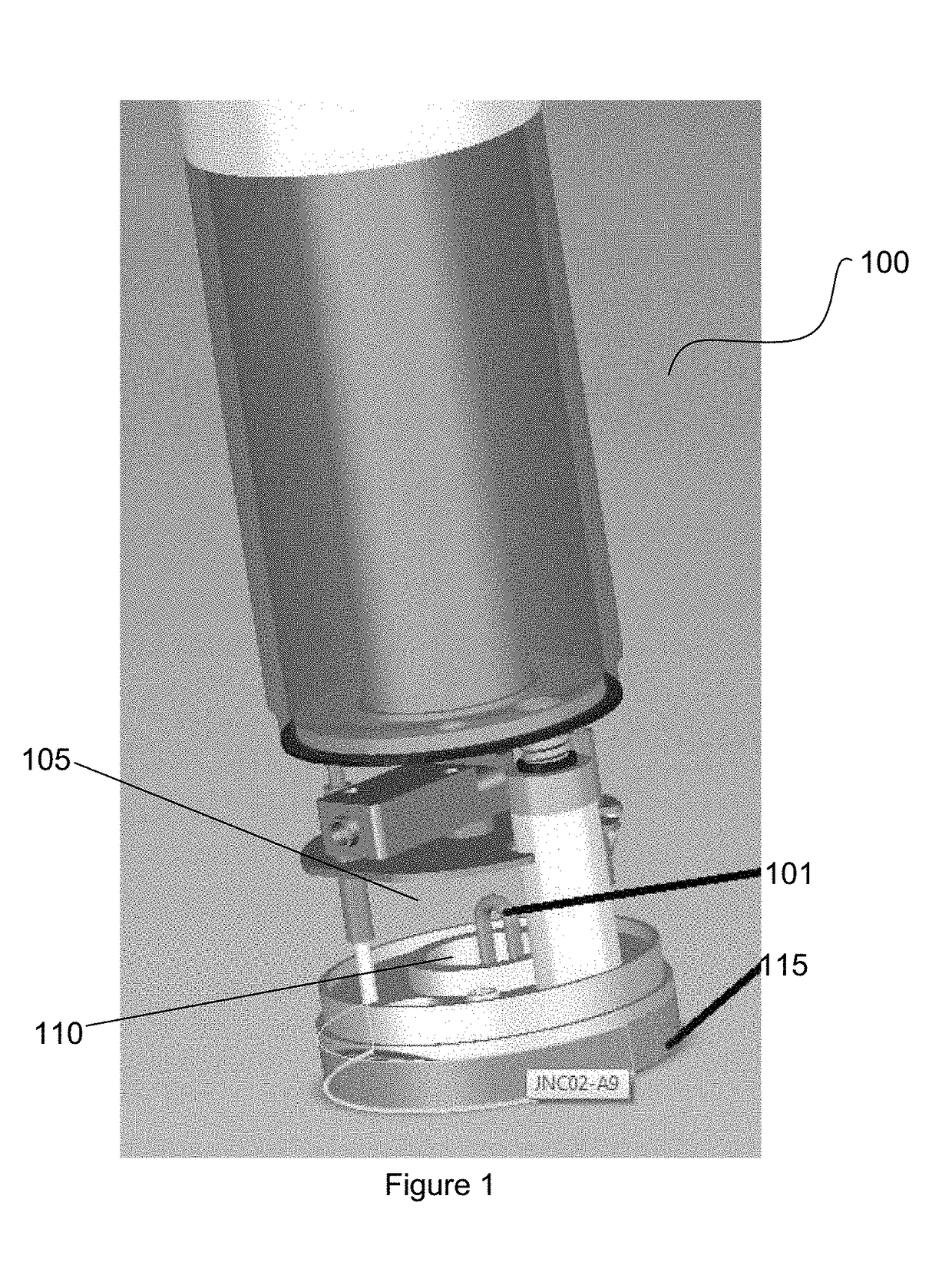
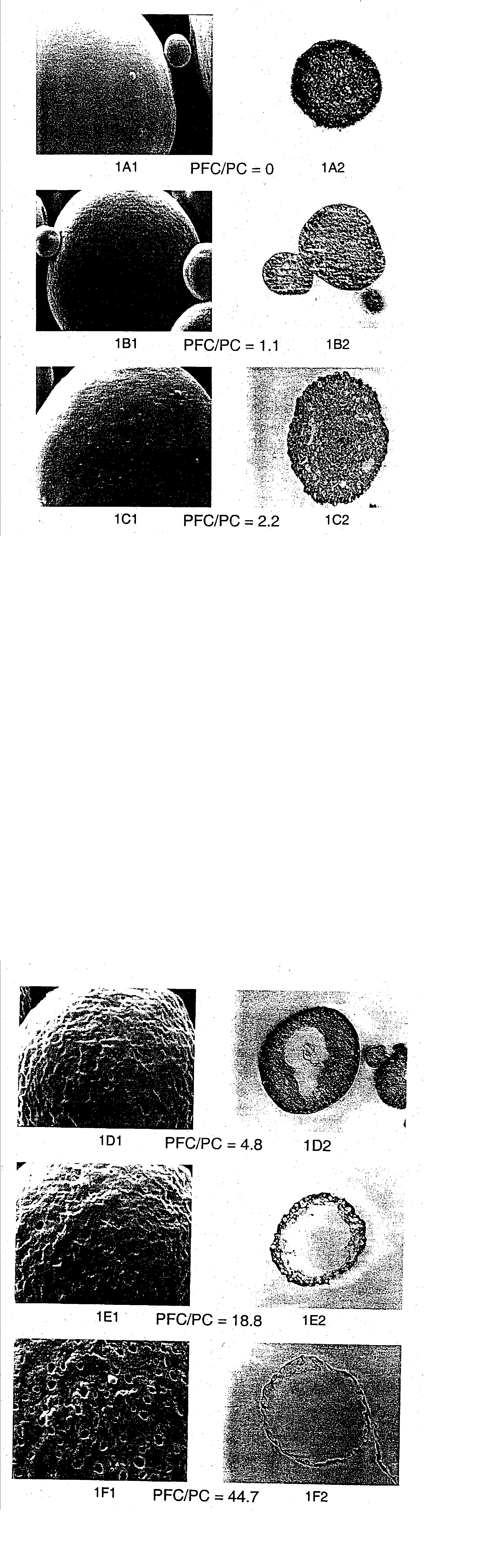
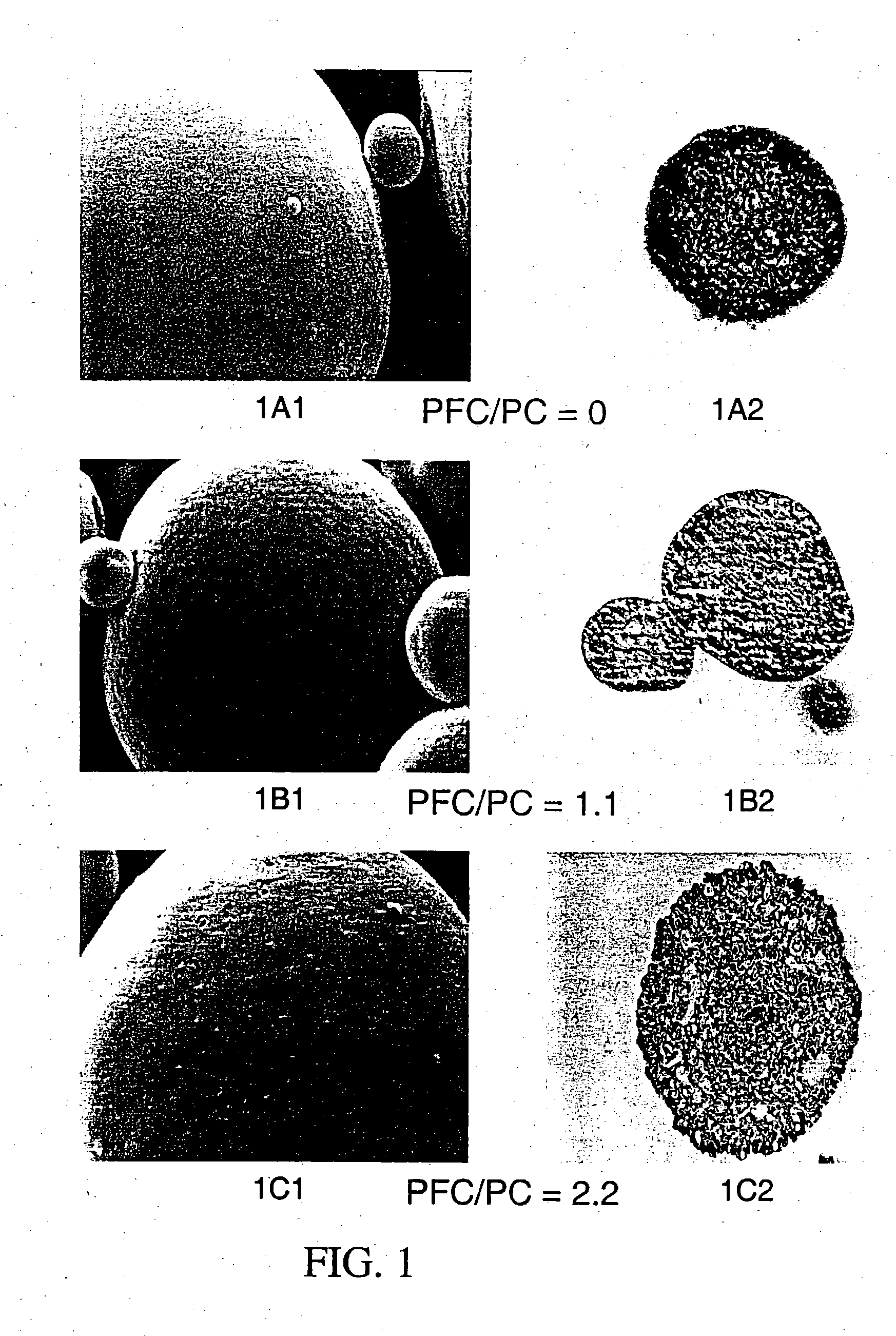
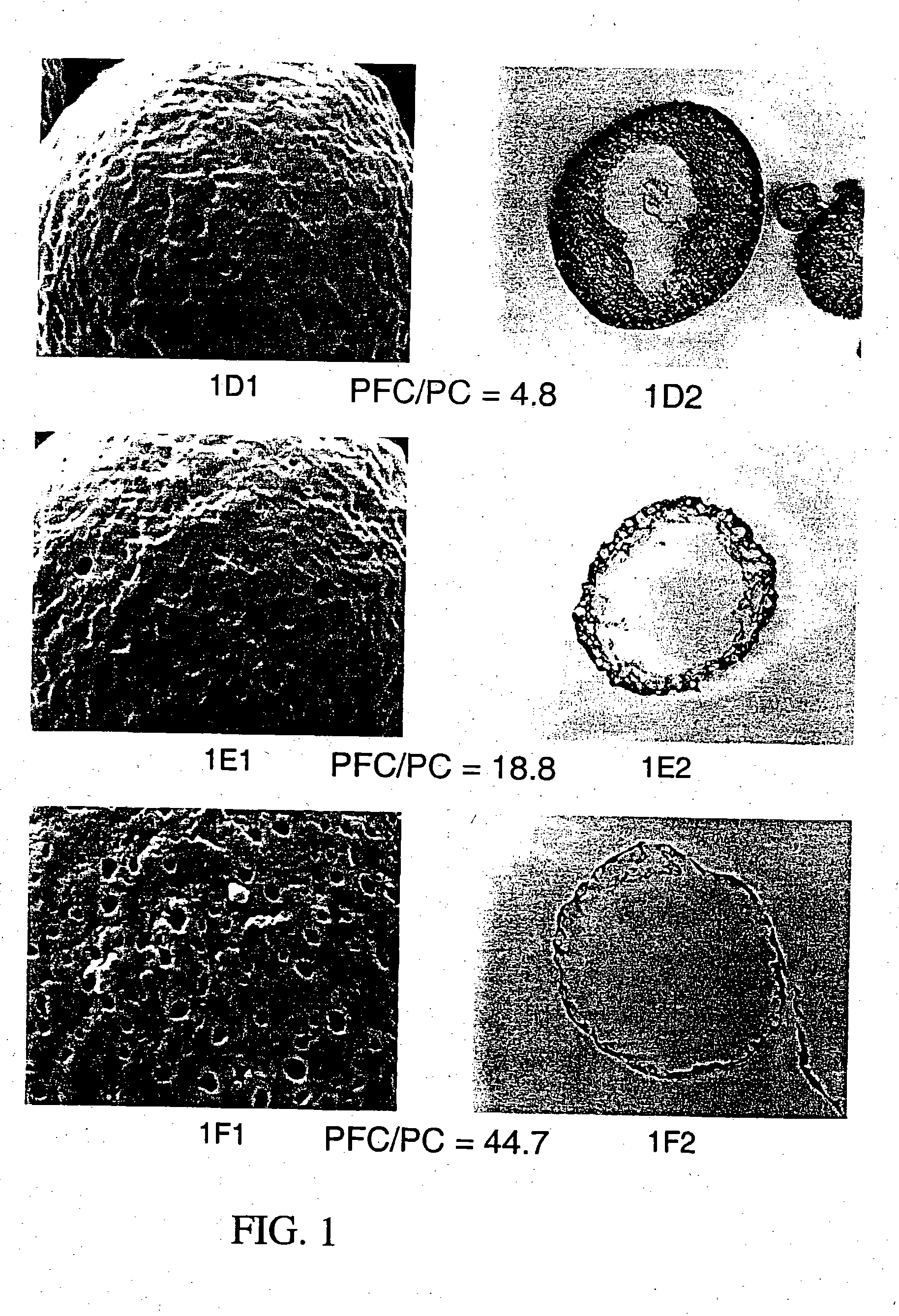
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
585 results about "Inhalation Devices" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
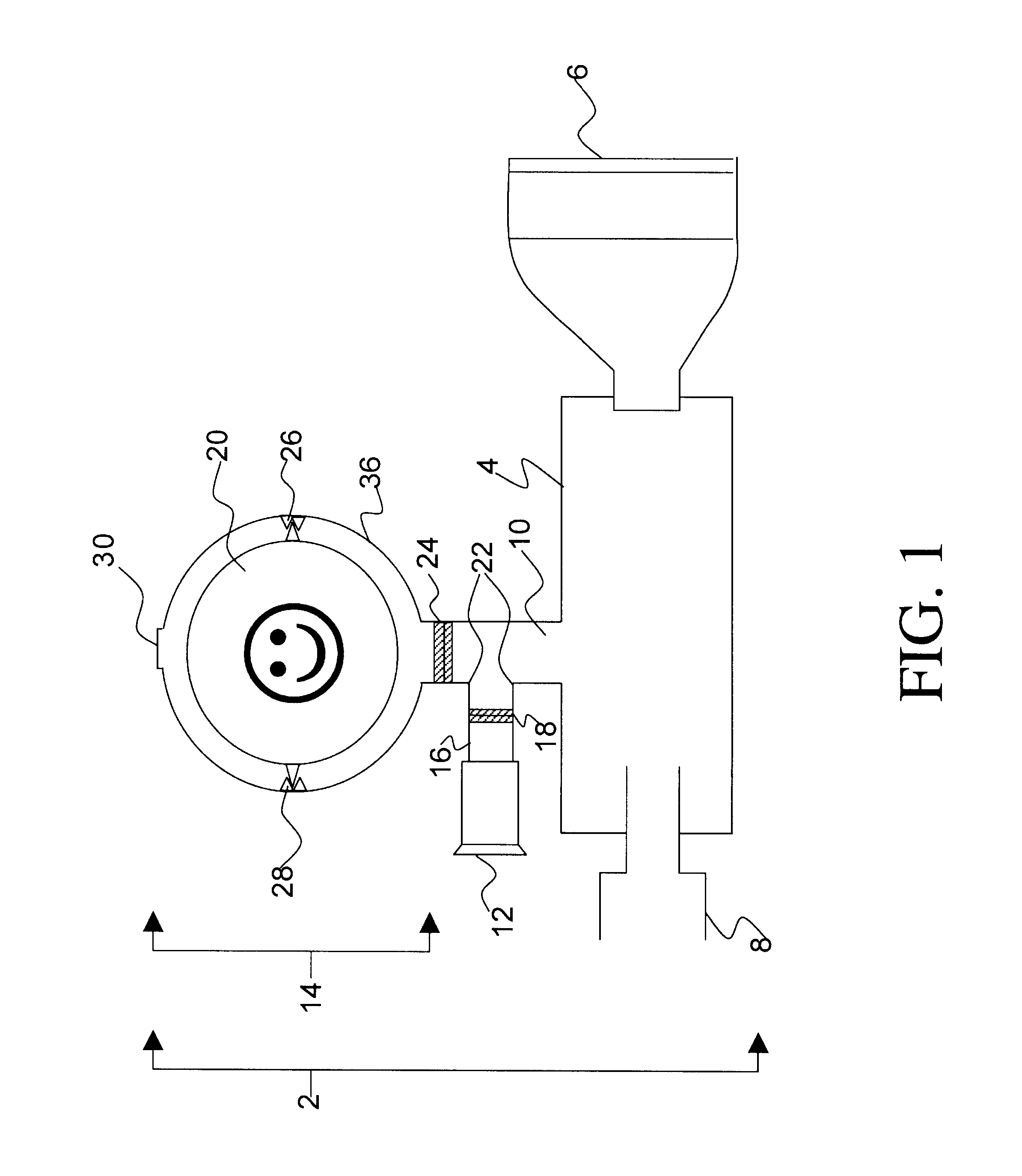
Introduction. Inhalation therapy has been used for thousands of years, albeit in a different form and use. Inhalation therapy was practiced by ancient civilizations in Egypt, Greece, India, and People’s Republic of China as evidenced by different artifacts displayed in museums, that may be considered the first used inhalation devices.1,2
Inhaler
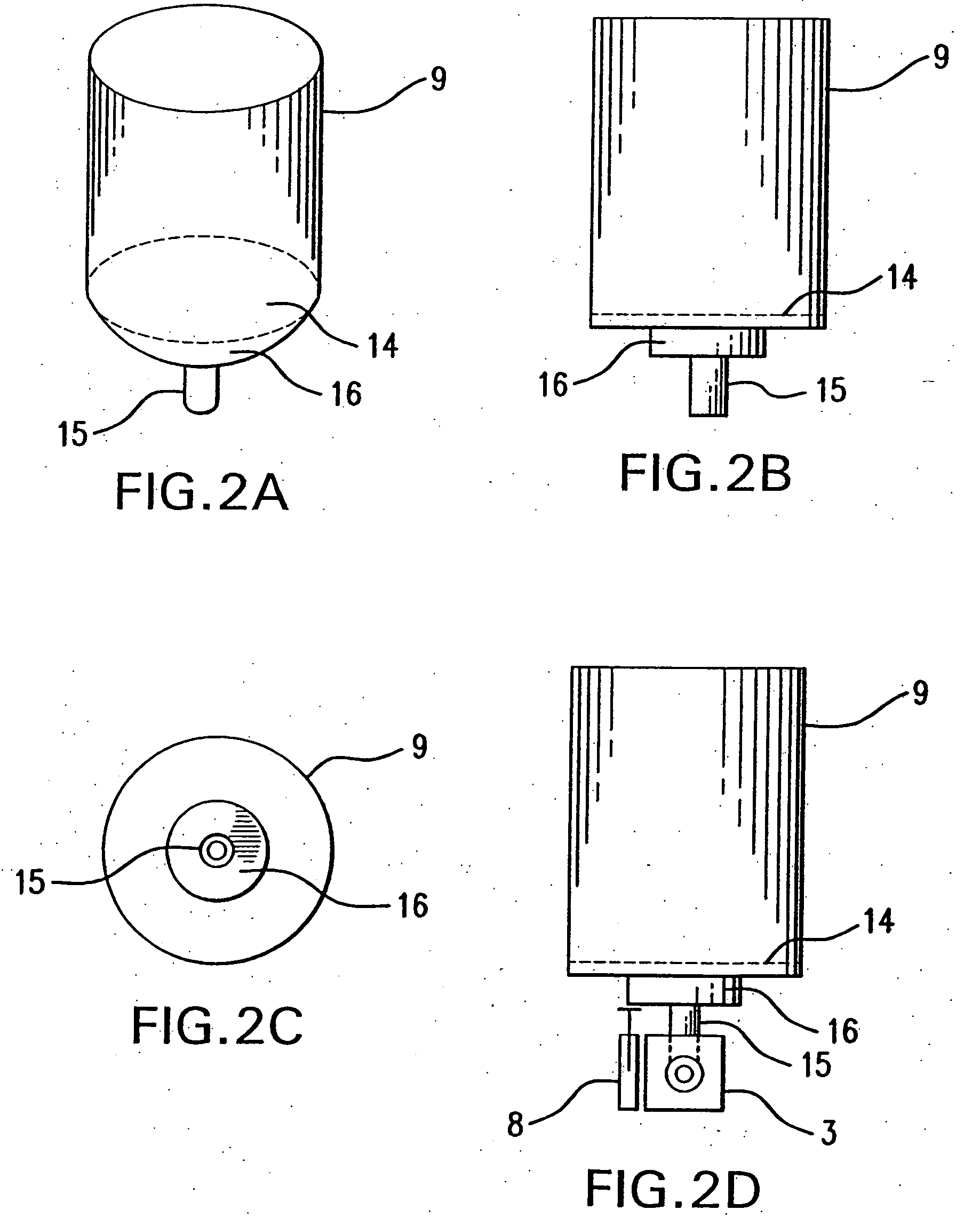
InactiveUS6880555B1Lifting efficiencyGood effectRespiratorsLiquid surface applicatorsInhalationMedicine
An inhaler for medicament in powder form with an opening intended for inhalation. The powder medicament is arranged in the inhaler in a number of enclosures, each enclosure including a specific dose of medicament. A member is provided for enabling access to the dose of medicament. The member is arranged and designed such that it is able to be inserted inside the enclosure and establish at least one outlet passage, between the interior of the enclosure and the inhalation opening, through which outlet passage the medicament is delivered to the patient upon inhalation.
Owner:SHL MEDICAL AG
Inhaler
InactiveUS6234169B1Reduce negative impactHigh simulationRespiratorsLiquid surface applicatorsParticulatesInhalation
An inhaler for use by an individual to inhale a particulate medicament from a reservoir comprises a chamber having a first end connectable to the reservoir to be in air flow communication therewith, a second end for delivering the medicament to the individual upon inhalation and a conduit defining an air flow path extending between the first end and the second end; and, an orifice in the chamber between the first end and the second end, the orifice utilizing the Coanda Effect when the reservoir is in air flow communication with the chamber and upon inhalation by the individual to draw medicament from the reservoir into the air flow path.
Owner:SANSA BARBADOS
Drug delivery device and methods therefor
InactiveUS6578571B1Overcome fearOvercome mistrustRespiratory masksMedical devicesAnesthesiaVALVE PORT
An incentive inhaler device includes an inhalation device, at least one incentive toy coupled to the inhalation device, and at least one separator. The inhalation device has a respiration device and a connector that is linkable to a delivery device. The delivery device can deliver drugs, aerosols, powder and gas. The incentive toy is respiration driven and has at least one of a visible characteristic and an audible characteristic. The separator decouples the toy from at least one component of the inhalation device to ensure directional flow of respirational air which drives the toy. The separator can include a valve, a filter, or a baffle.
Owner:MEDICAL DEV INT
Inhalation device for producing a drug aerosol
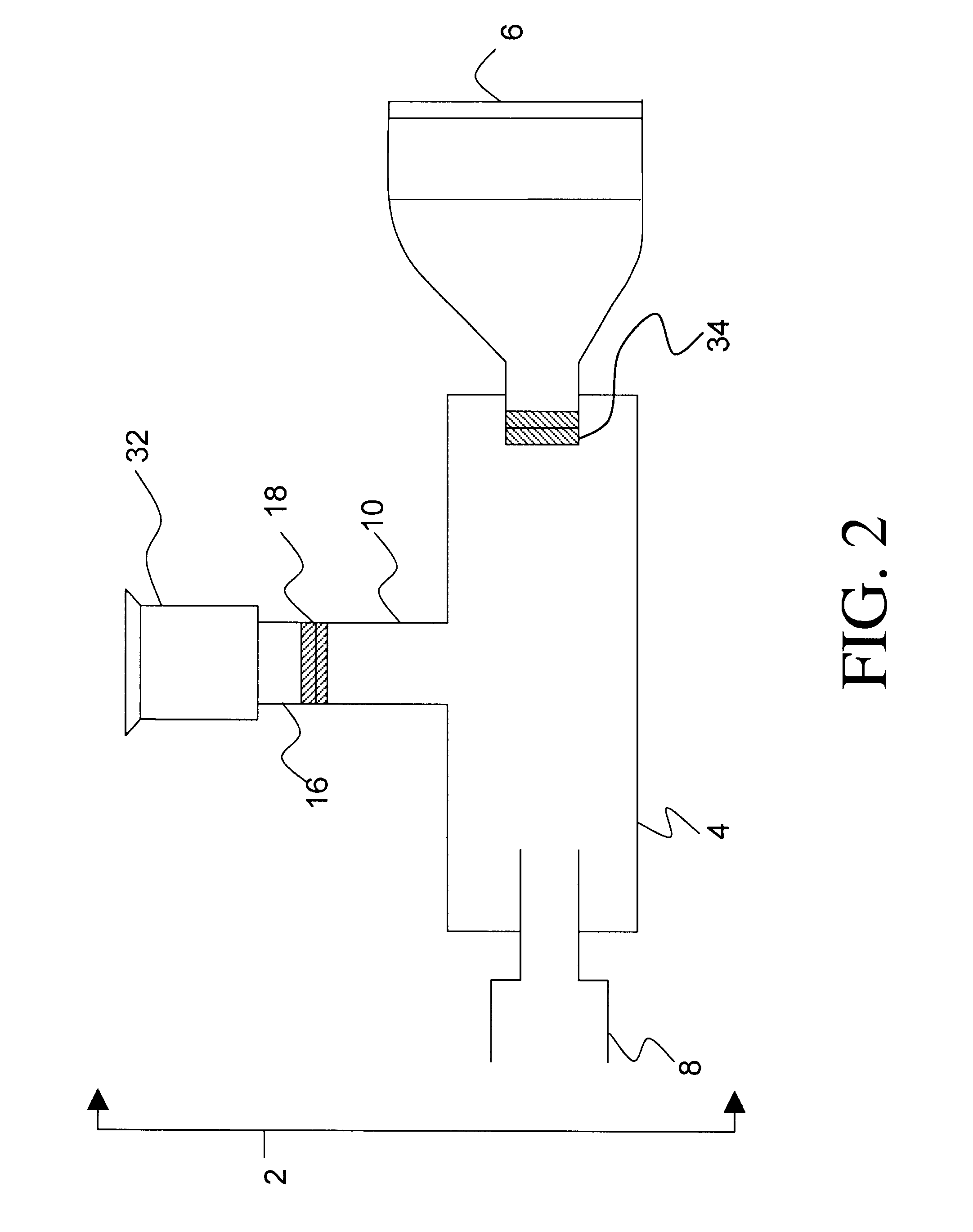
A device for delivering a drug by inhalation is disclosed. The device includes a body defining an interior flow-through chamber having upstream and downstream chamber openings, and a drug supply unit contained within the chamber for producing, upon actuation, a heated drug vapor in a condensation region of the chamber. Gas drawn through the chamber region at a selected gas-flow rate is effective to form drug condensation particles from the drug vapor having a selected MMAD between 0.02 and 0.1 MMAD or between 1 and 3.5 μm. A gas-flow control valve disposed upstream of the unit functions to limit gas-flow rate through the condensation region to the selected gas-flow rate. An actuation switch in the device activates the drug-supply unit, such that the drug-supply unit can be controlled to produce vapor when the gas-flow rate through the chamber is at the selected flow rate.
Owner:ALEXZA PHARMA INC
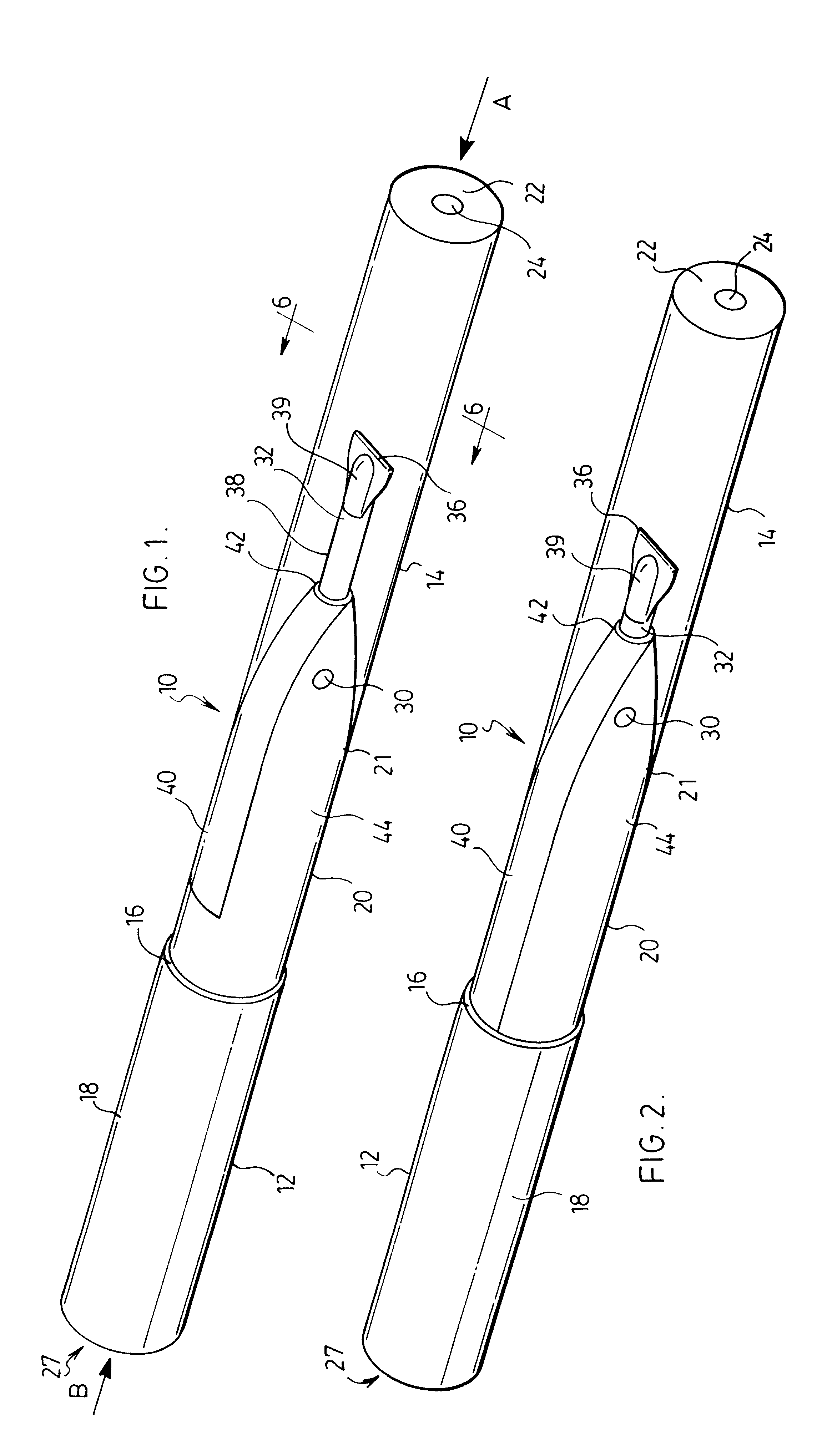
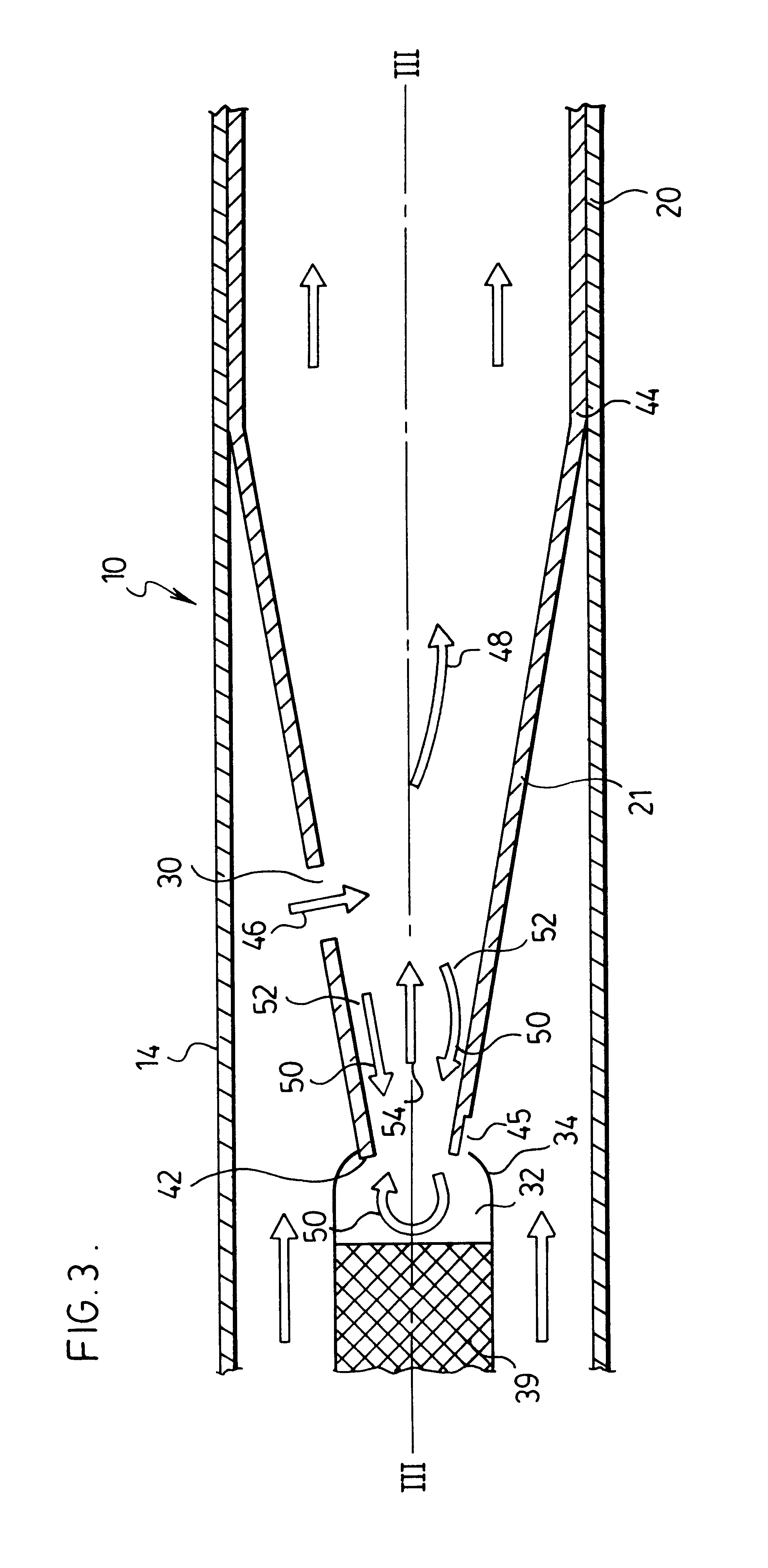
Inhalation device
ActiveUS20070151562A1Inhibit migrationRespiratorsLiquid surface applicatorsInhalation DevicesDelivery system
The present invention provides for the integration of drug dispersion methods into a drug or medicine delivery system. The drug dispersion methods used include shear (e.g., air across a drug, with or without a gas assist), capillary flow or a venturi effect, mechanical means such as spinning, vibration, or impaction, and turbulence (e.g., using mesh screens, or restrictions in the air path). These methods of drug dispersion allow for all of the drug in the system to be released, allowing control of the dosage size. These methods also provide for drug metering, fluidization, entrainment, deaggragation and deagglomeration. The present invention also provides for the integration of a drug sealing system into the device. The drug sealing system provides a way of blocking the migration of drug from one area of the package to another. The drug seal system can also provide a method of tightly containing the drug until the package is opened, of directing airflow through the package and of managing and containing the drug during the package / device manufacturing process.
Owner:MANTA DEVICES
Bioactive concentrates and uses thereof
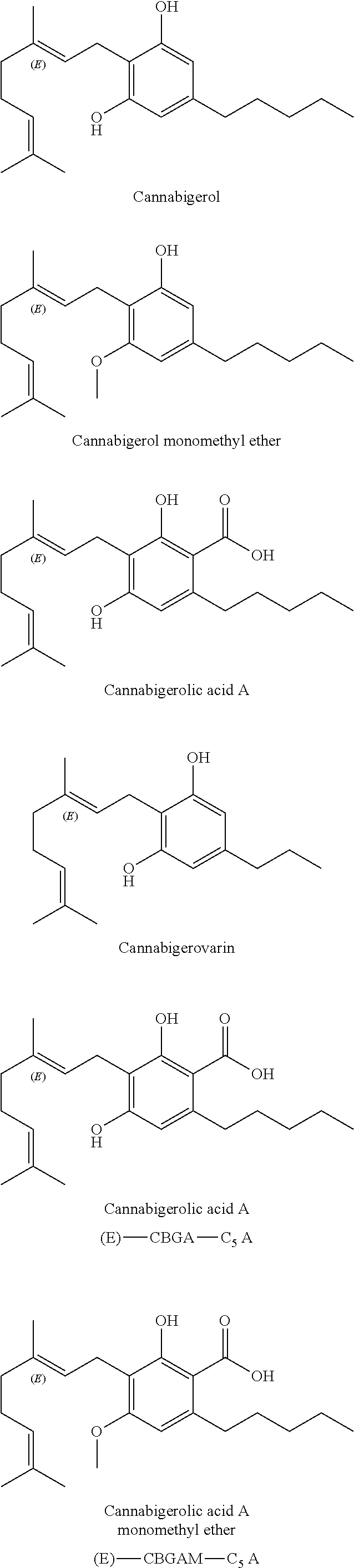
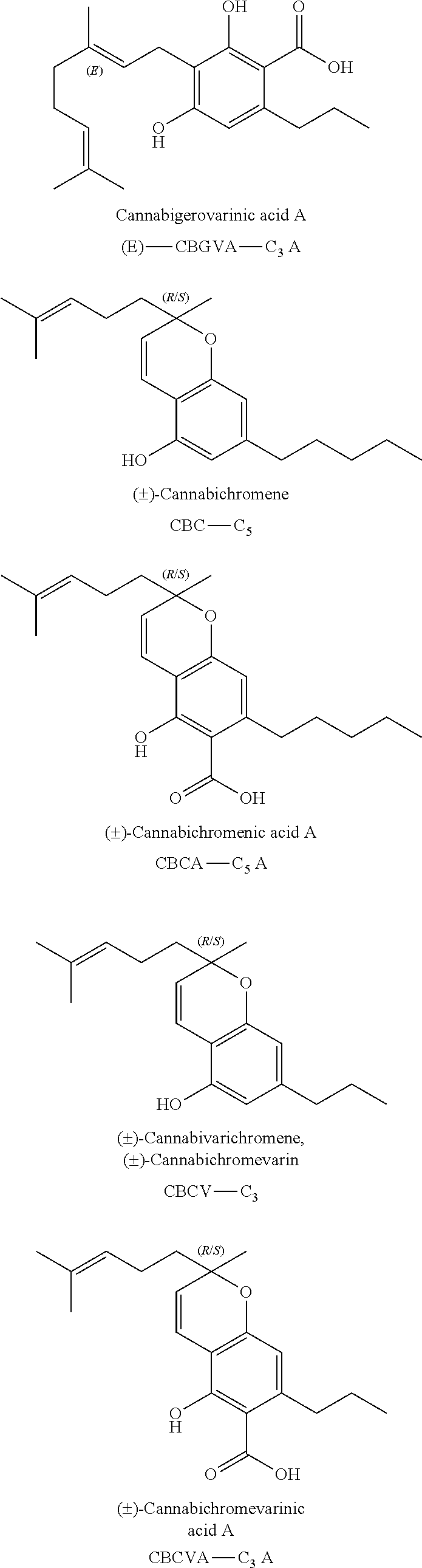
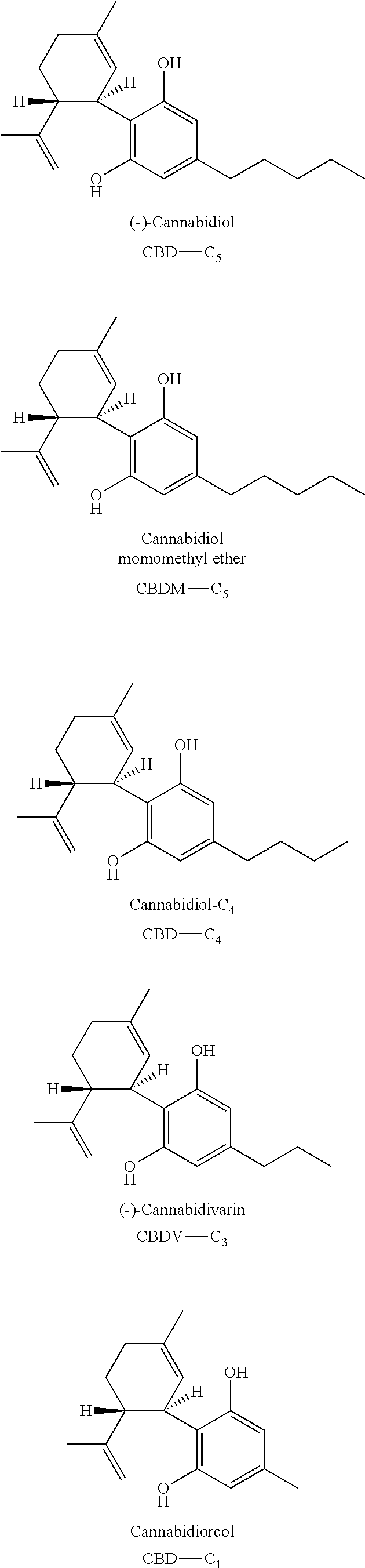
The present invention relates to concentrates obtained from extraction from Cannabis, preferably cannabinoid and / or terpene concentrates, and formulation of the concentrates, particularly for use for direct vaporization, infusion into edible matrices, in electronic inhalation devices, and as nutraceuticals.
Owner:PURPLE MUNDO INC
Engineered particles and methods of use
InactiveUS7306787B2Reduce deliveryLess attractivePowder deliveryOrganic active ingredientsNebulizerActive agent
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS AG
inhaler
ActiveCN102264420AReduce power densityEvaporation stableRespiratorsOrganic active ingredientsMedicineInhalation
The invention relates to an inhaler component for producing a steam / air mixture or / and condensation aerosol in an intermittent and inhalation- or pull-synchronous manner. The inhaler component comprises the following elements: a housing (3); a chamber (21) arranged in the housing (3); an air inlet opening (26) for the supply of air from the surroundings to the chamber (21); an electrical heating element for evaporating a portion of a liquid material(16), the steam produced mixing in the chamber (21) with the air supplied through the air inlet opening (26), thereby producing the steam / air mixture or / and condensation aerosol; and a wick having a capillary structure, which wick forms a composite structure (22) with the heating element and automatically supplies the heating element with fresh liquid material (16) after evaporation. In order to provide the high specific evaporative capacity required for an intermittent, inhalation- or pull-synchronous operation of the inhaler component (2) while guaranteeing a high efficiency of the evaporator, the composite structure (22) is a flat structure and at least one heated section of the composite structure (22) is arranged in the chamber (21) in a contact-free manner, and the capillary structure of the wick is substantially exposed in the section on at least one side (24) of the flat composite structure.
Owner:NICOVENTURES TRADING LTD
Aerosol drug formulations containing hydrofluoroalkanes and alkyl saccharides
InactiveUS6932962B1Function increaseGood dispersionPowder deliveryDispersion deliveryAerosol drugsActive agent
Aerosol formulations suitable for use in pressurised metered dose inhalers comprise a hydrofluoroalkane propellant, an medicament for inhalation and a surfactant which is a a C8–C16 fatty acid or salt thereof, a bile salt, a phospholipid, or an alkyl saccharide.
Owner:ASTRAZENECA AB
Disposable aerosol generator system and methods for administering the aerosol
InactiveUS6799572B2Avoid contaminationNegates needRespiratorsOther heat production devicesBiomedical engineeringAerosol generator
A disposable aerosol generator for use with an inhaler device which includes a heater adapted to volatilize fluid stored in the disposable aerosol generator and method of using the inhaler. The disposable body includes a sealed chamber and an outlet, the chamber being located between first and second layers of material. The chamber holds a predetermined volume of a fluid which is expelled through the outlet when the fluid in the chamber is volatilized by the heater. The disposable body can include a series of spaced apart aerosol generators, each of which can be advanced to a release position at which the heater can heat one of the fluid containing chambers. Prior to heating the fluid, the outlet can be formed by severing the first and / or second layer with a piercing element and the volatilized fluid can be expelled from the outlet into a passage of a dispensing member.
Owner:PHILIP MORRIS USA INC
Aerosol dispensing inhaler training device
An aerosol dispensing inhaler training device for determining whether a user is properly operating an aerosol dispensing device. The training device includes an aerosol dispensing device having a container with a valve stem extending longitudinally therefrom and movable between a closed position and an open position. The container dispenses a portion of the contents within the container when the valve stem is moved to the open position. The aerosol dispensing device includes a housing adapted to support the container reciprocally moveable within the housing along a longitudinal axis from a first position, the housing comprising a well adapted to receive the valve stem and an exhaust port comprising one end in fluid communication with the well and a second end in fluid communication with the ambient atmosphere, wherein the portion of the contents within the container is dispensed from the first end of the exhaust port to the second end of the exhaust port when the housing moves to an actuation position where the valve stem is actuated so that a portion of the contents within the container is dispensed through the second end of the exhaust port when the valve stem is moved to the open position. An actuation sensor generates a signal that indicates when the housing is moved to the actuation position and the valve stem is actuated. A shake sensor determines whether the contents within the container have been properly agitated for consumption by a user.
Owner:1263152 ONTARIO
Non-burning type flavor inhaler
A non-burning type flavor inhaler includes an aerosol source that generates an aerosol; an atomizer that atomizes the aerosol source without burning; a power source that supplies power to the atomizer; a light-emitting element; and a control unit that controls the light-emitting element. The control unit controls the light-emitting element in a first light-emitting mode in a puff state inhaling the aerosol, and controls the light-emitting element in a second light-emitting mode different from the first light-emitting mode in a non-puff state not inhaling the aerosol. The second light-emitting mode changes according to a number of puff actions for inhaling the aerosol.
Owner:JAPAN TOBACCO INC
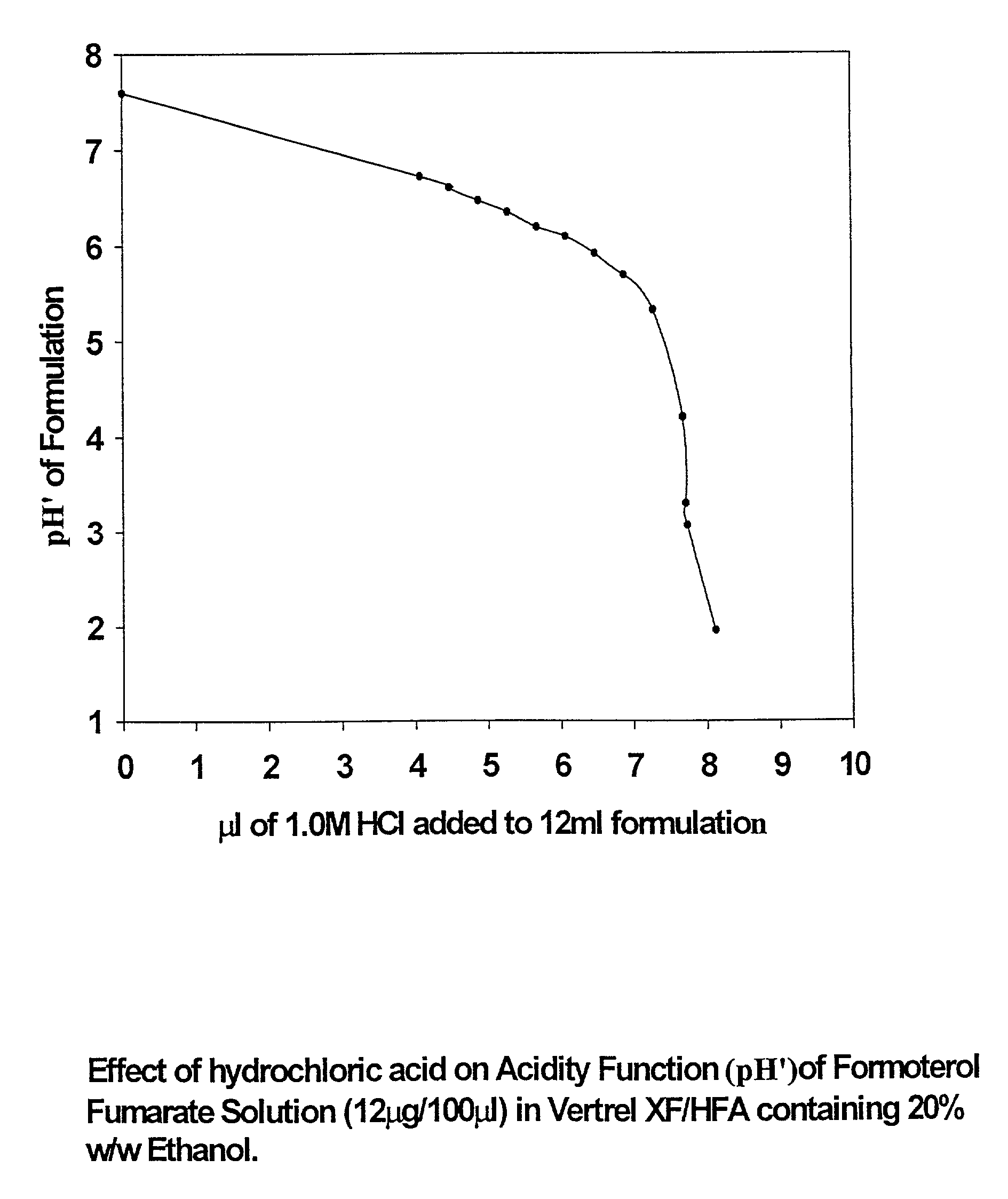
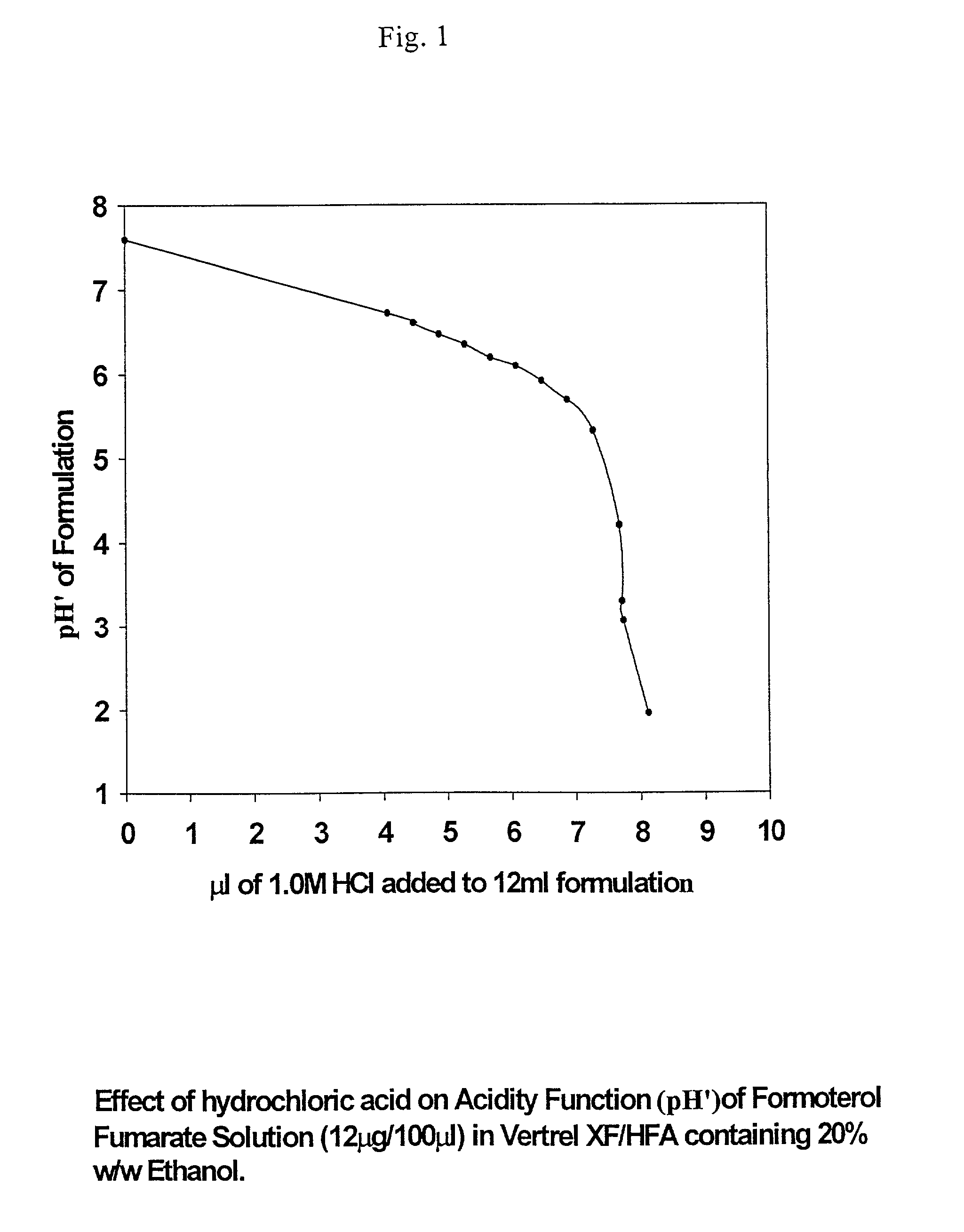
Stable pharmaceutical solution formulations for pressurised metered dose inhalers
An aerosol solution composition for use in an aerosol inhaler comprises an active material, a propellant containing a hydrofluoroalkane, a cosolvent and optionally a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler. The composition is stabilized by using a small amount of mineral acid and a suitable can having part or all of its internal metallic surfaces made of stainless steel, anodized aluminium or lined with an inert organic coating.
Owner:CHIESI FARM SPA
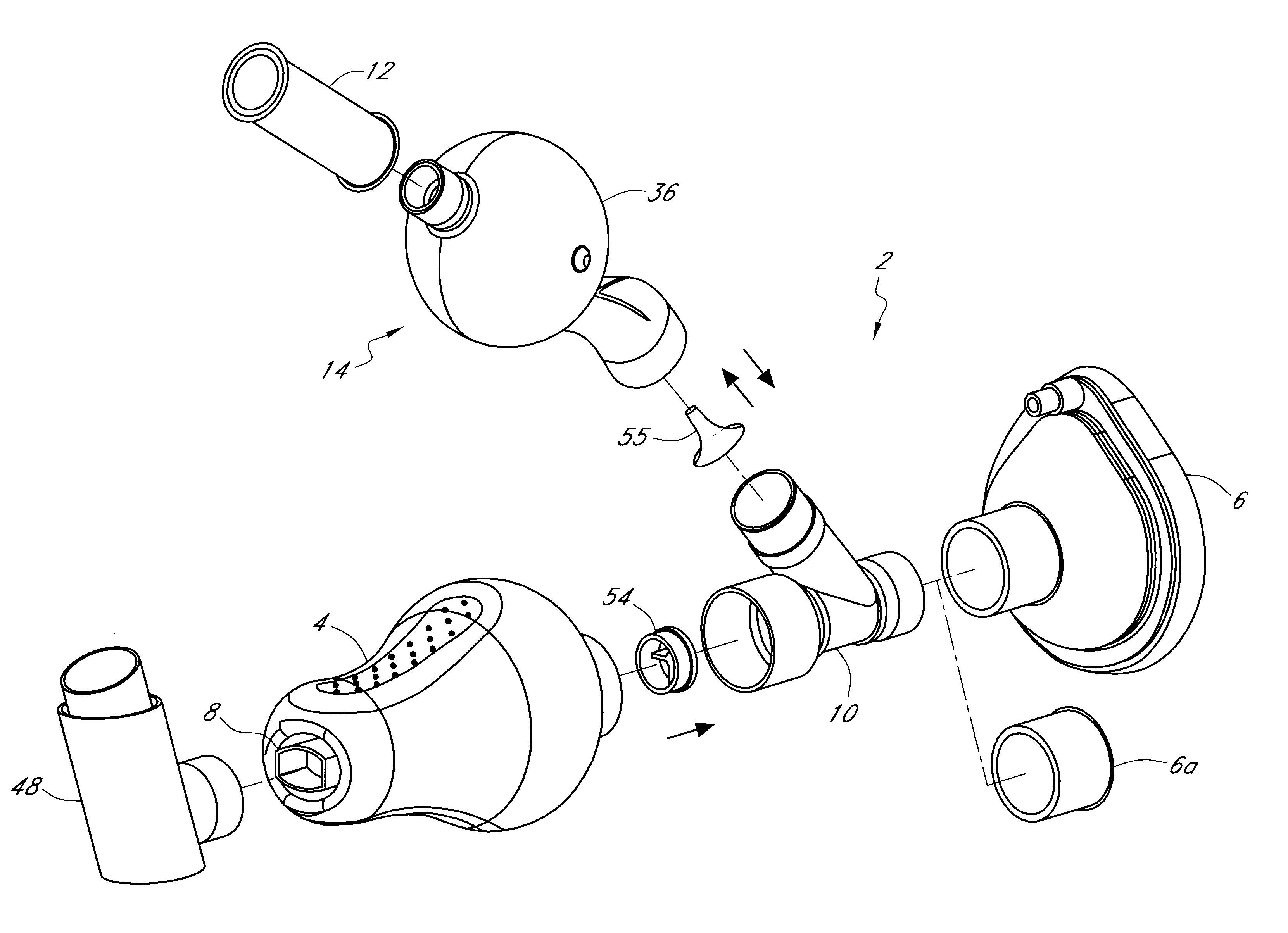
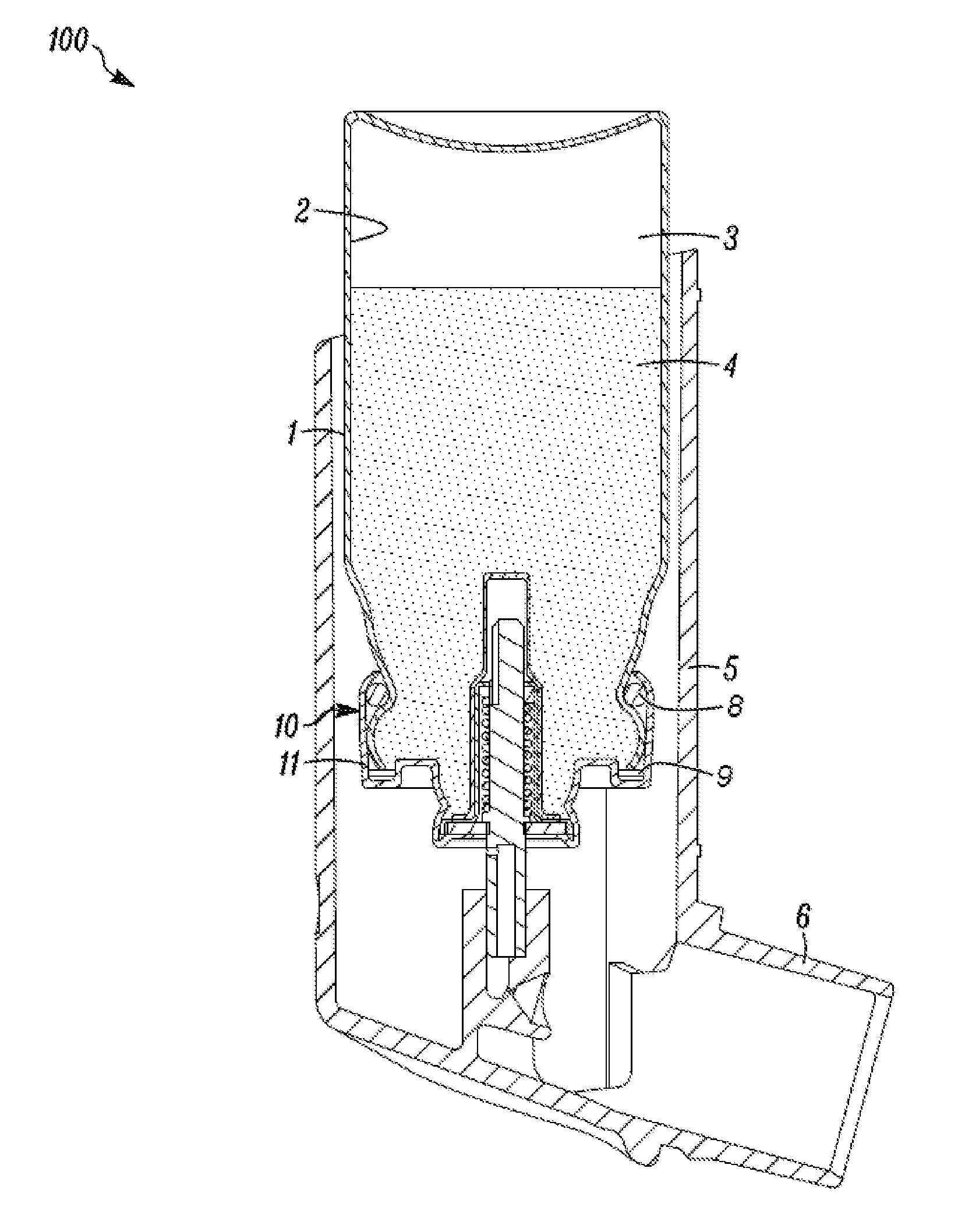
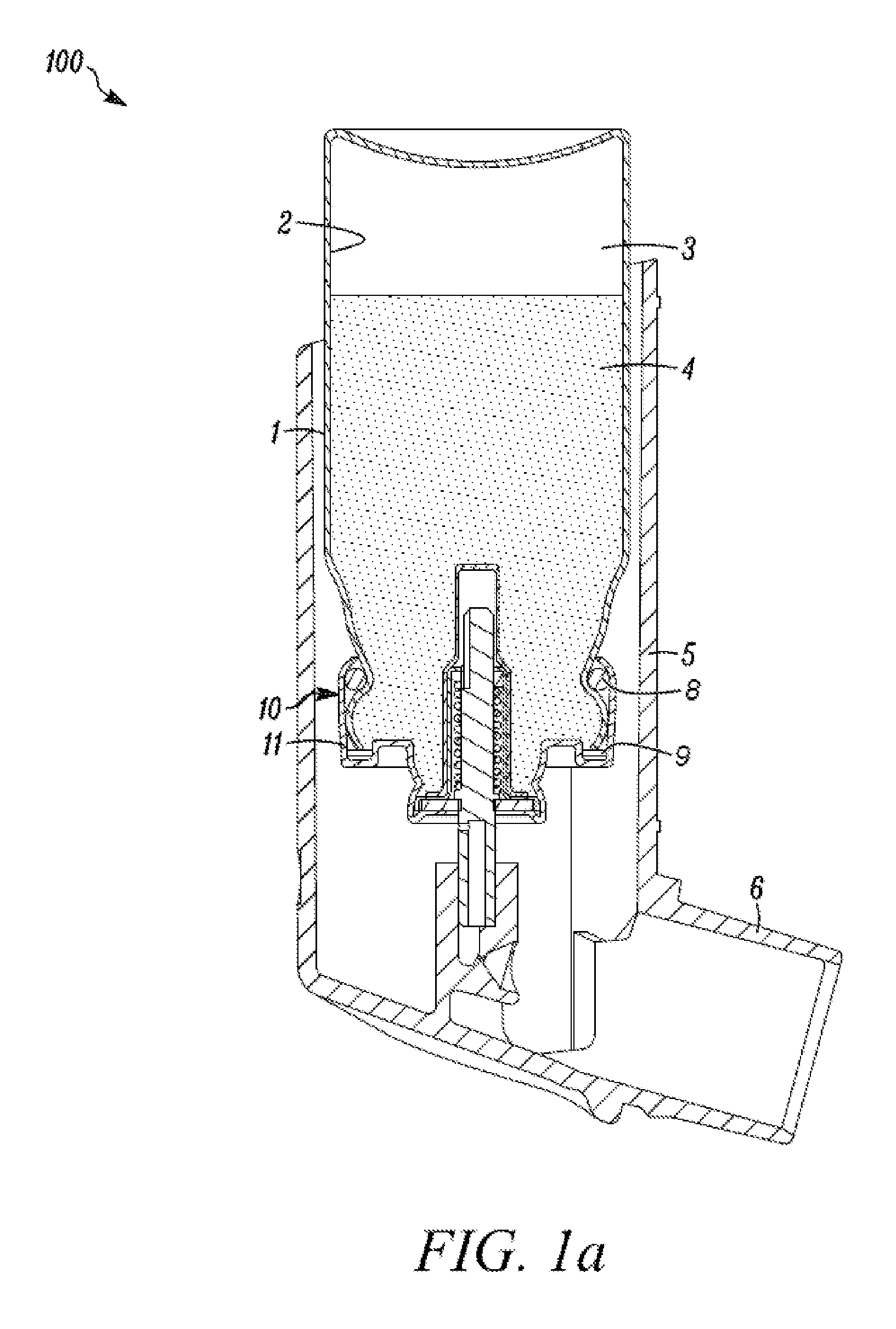
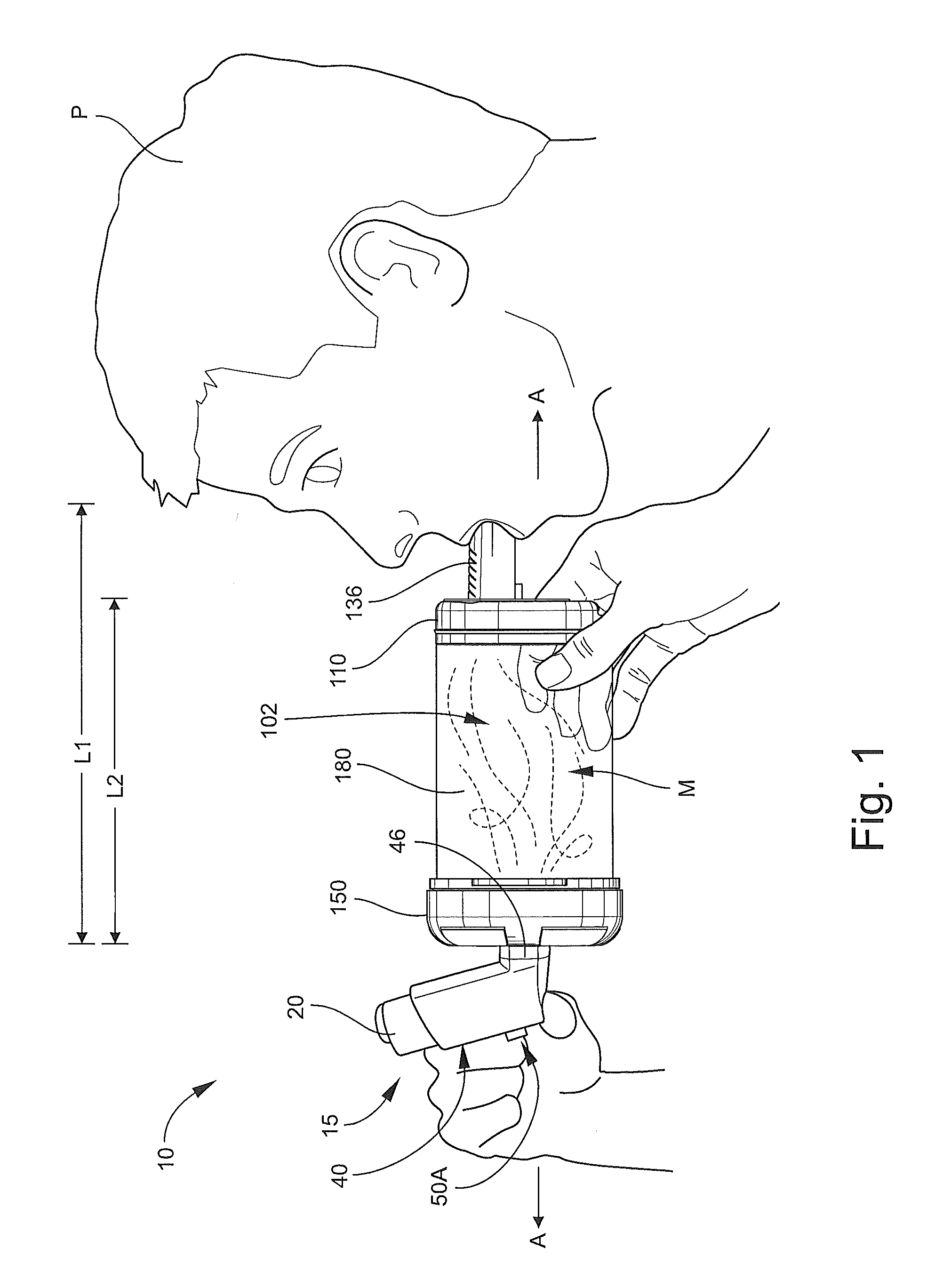
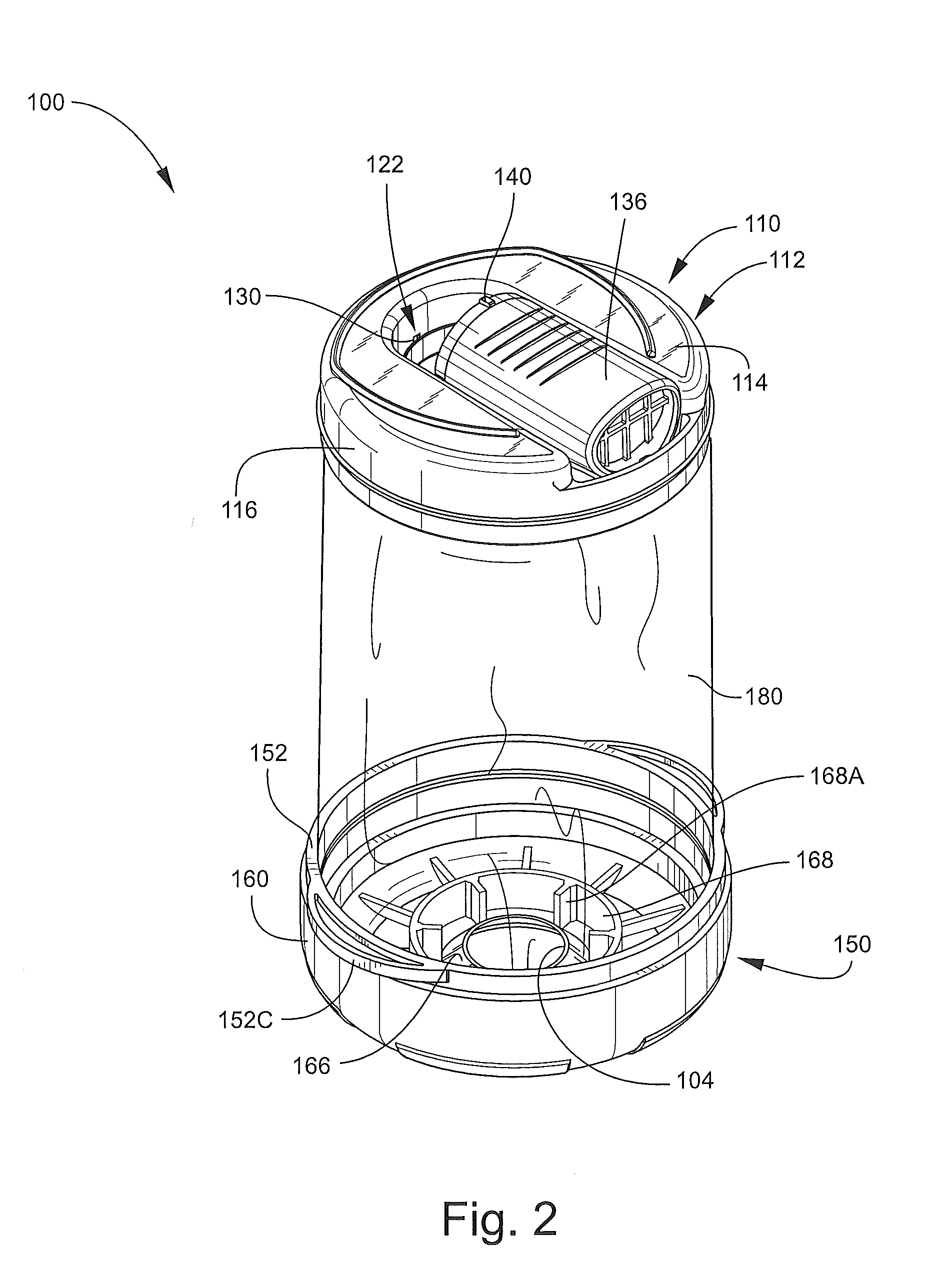
Inhalation device for drugs in powder form
InactiveUS20100012120A1Great degree of economyEasy doseRespiratorsLiquid surface applicatorsInhalationInhalation Devices
To provide an inhalation device which has improved use properties, particularly advanced moisture protection while in use, an inhalation device (1) for powder drugs is proposed comprising at least one storage chamber (13) for accommodating a plurality of drug powder doses and a dosing device which includes at least one dosing slider (15) which is movable approximately with a translatory movement in a dosing slider passage (16) at least from a filling position into an emptying position, wherein the inhalation device (1) further includes a device for inhalation-triggered automatic movement of the dosing slider (15) from its filling position into the emptying position and a return device for automatic movement of the dosing slider (15) back into the filling position.
Owner:ASTRAZENECA AB
Inhaler
InactiveUS6866037B1Quality improvementRespiratorsIntravenous devicesInhalationBiomedical engineering
Device for use with an inhaler, the inhaler having a body, an aerosol canister arranged in the body containing medicament, including a metered dose chamber and able to dispense a metered dose of the medicament, a nozzle in fluid communication with the canister, an opening for dispensing of the medicament in fluid communication with the nozzle. The device includes elements for activating the canister to open and dispense the medicament in response to an airflow in the inhaler caused by inhalation of a user through the opening, return elements for deactivating the canister to close it, characterized in that the return elements deactivate the canister when the airflow drops below a certain threshold value.
Owner:SHL GRP AB
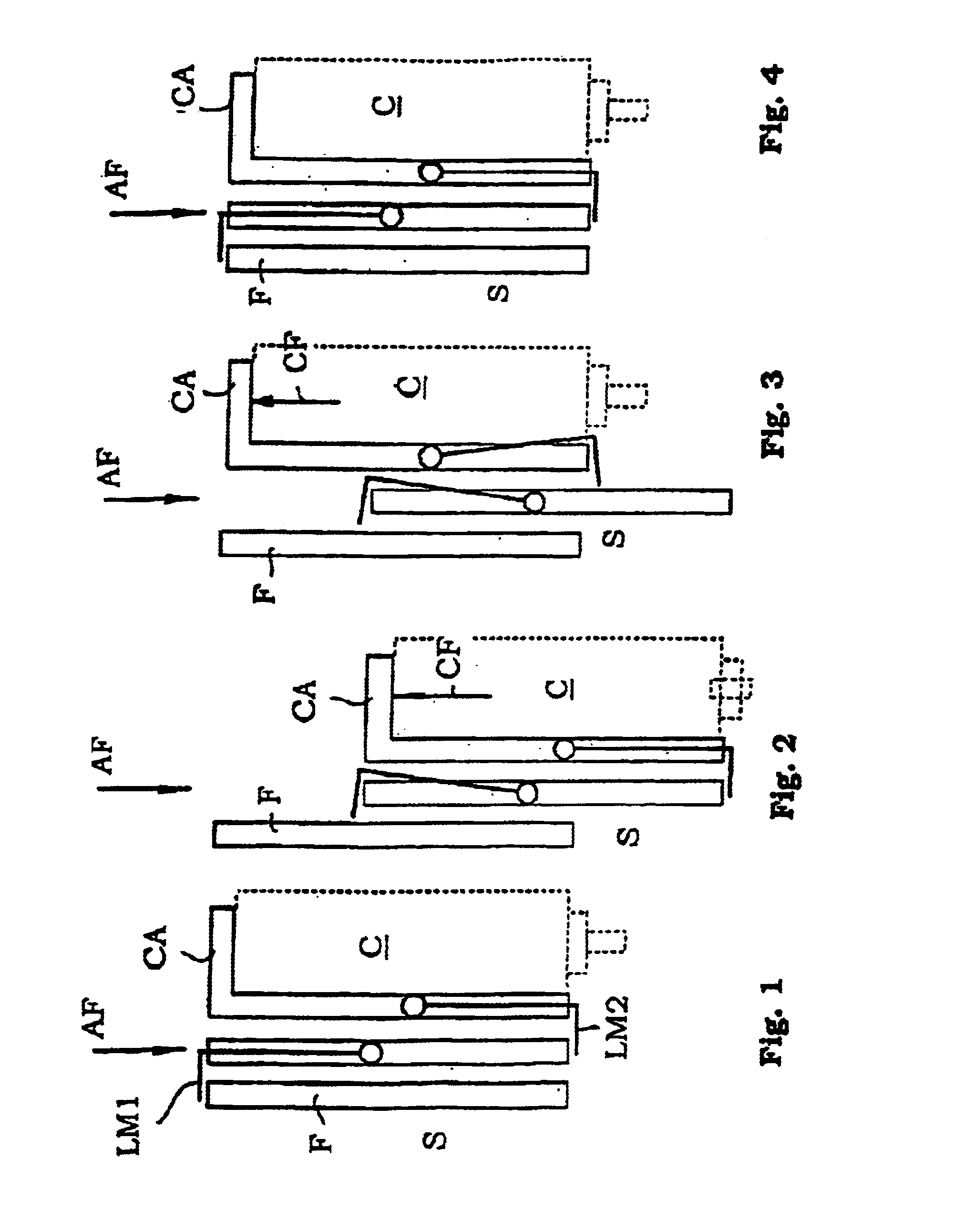
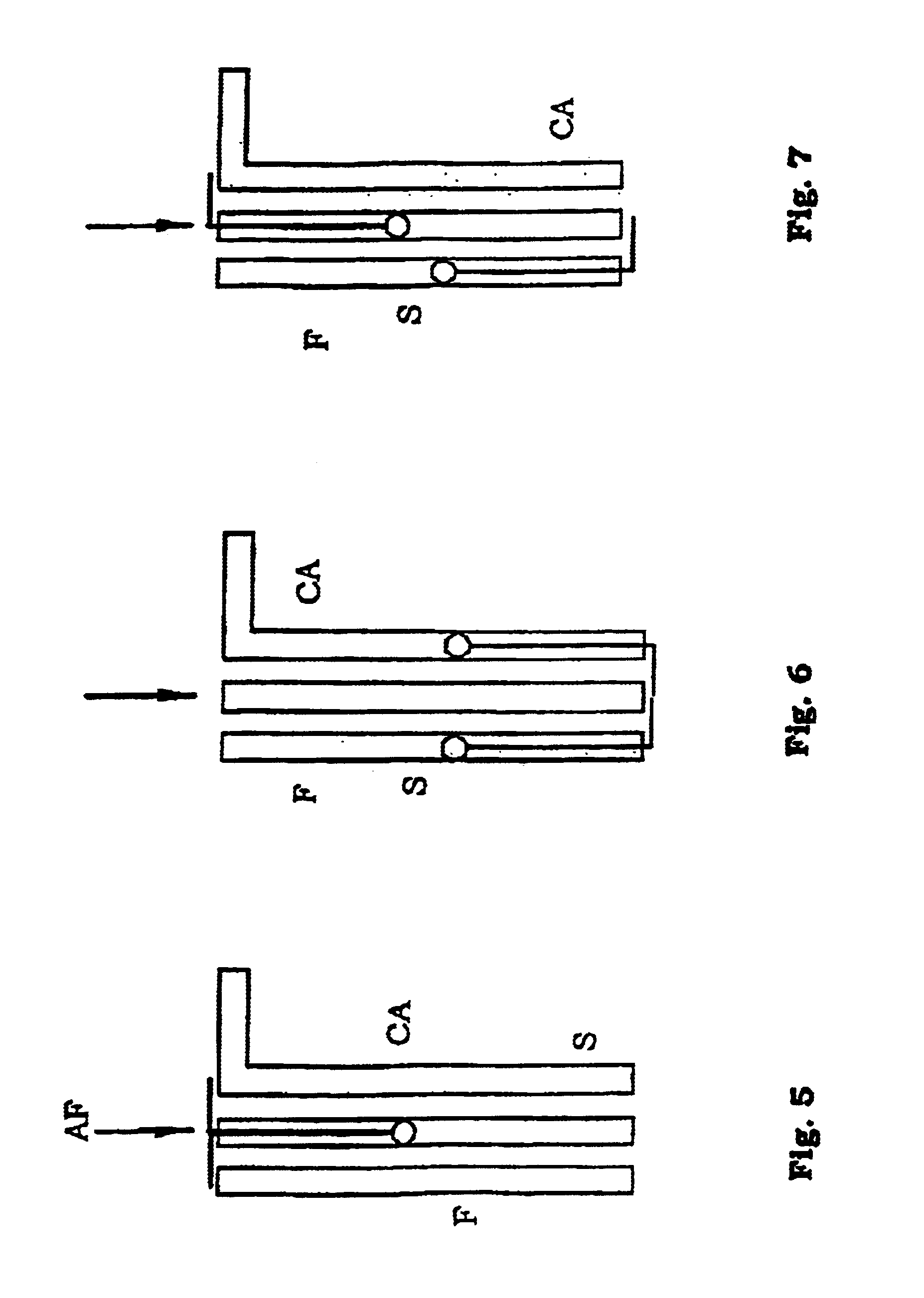
Inhalation device and method
Inhalation device and associated method for facilitating inhalation by a patient of powder medicaments contained in a receptacle. The inhalation device has a chamber for receiving the receptacle. A ring is circumferentially coupled to an inner surface of the chamber to achieve a higher reproducible emitted dose of medicament from the receptacle. The inhalation device also includes an improved implement for puncturing the receptacle, requiring less force and experiencing fewer failures.
Owner:CIVITAS THERAPEUTICS
Inhalation device
InactiveUS20100163042A1Avoids wastage and double dosingRespiratorsLiquid surface applicatorsInhalationInhalation Devices
The present invention relates to an inhalation-activatable device for administration of medicament in powder form to the respiratory system of a patient.
Owner:SUN PHARMA INDS
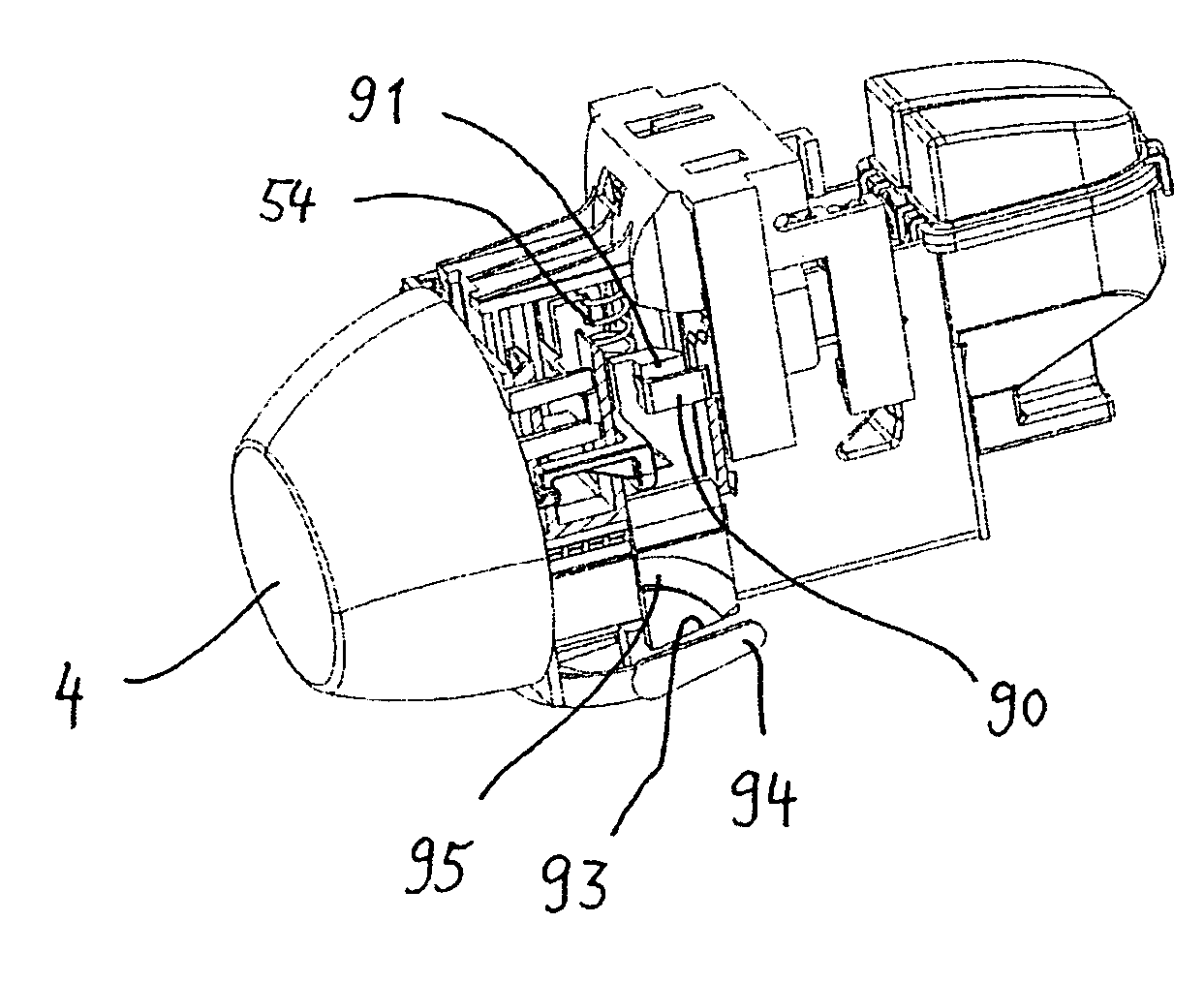
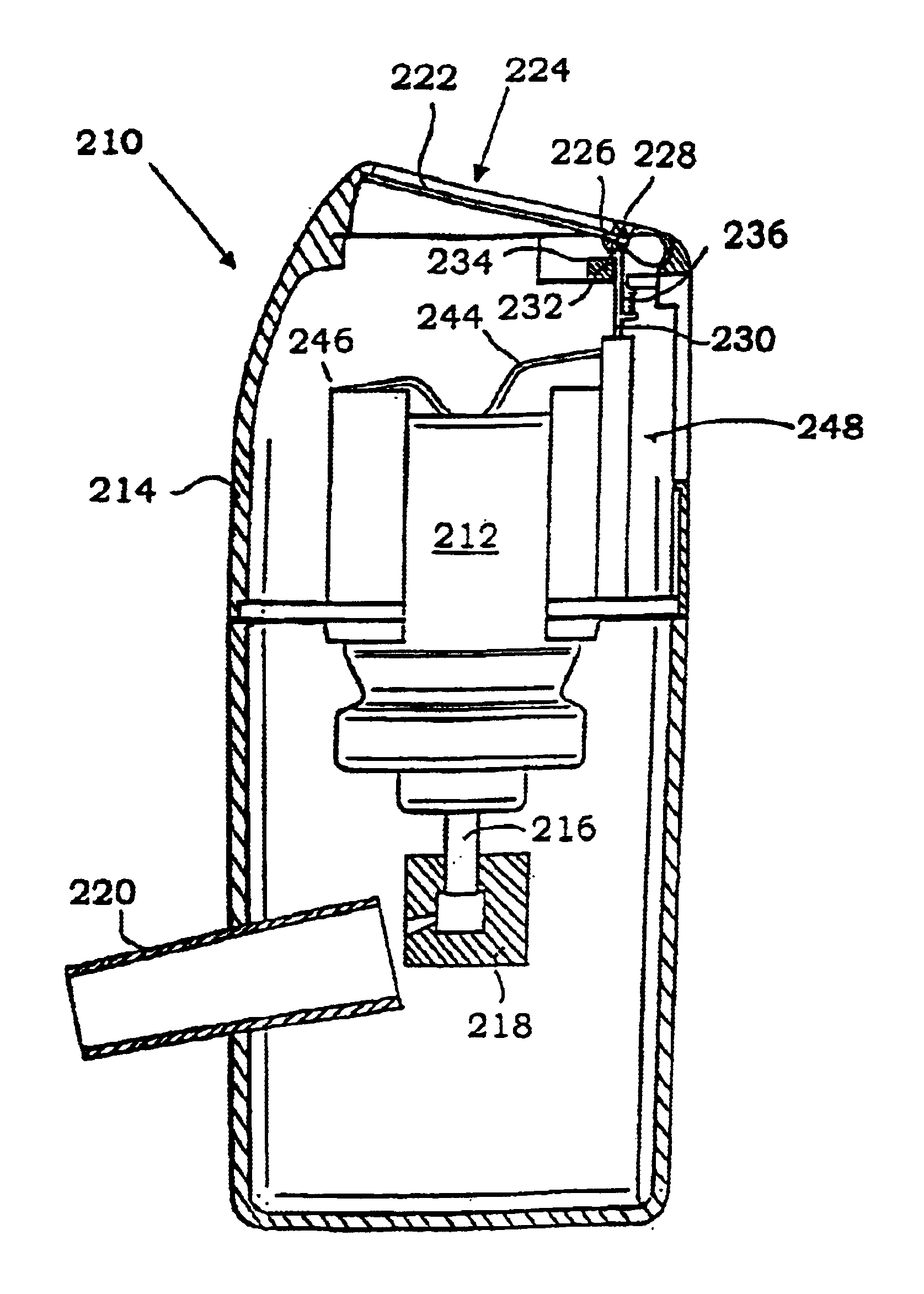
Inhalation device and heating unit therefor
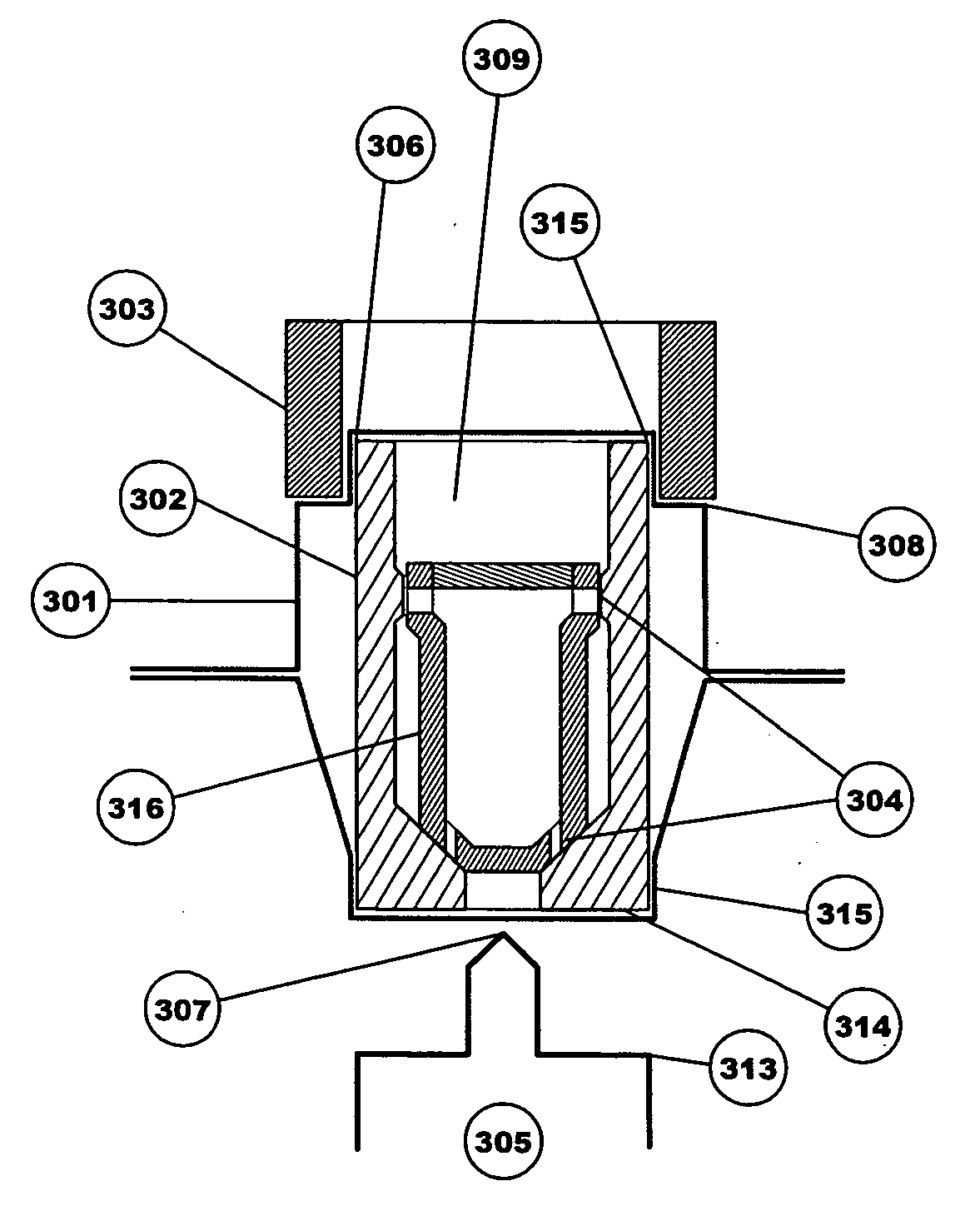
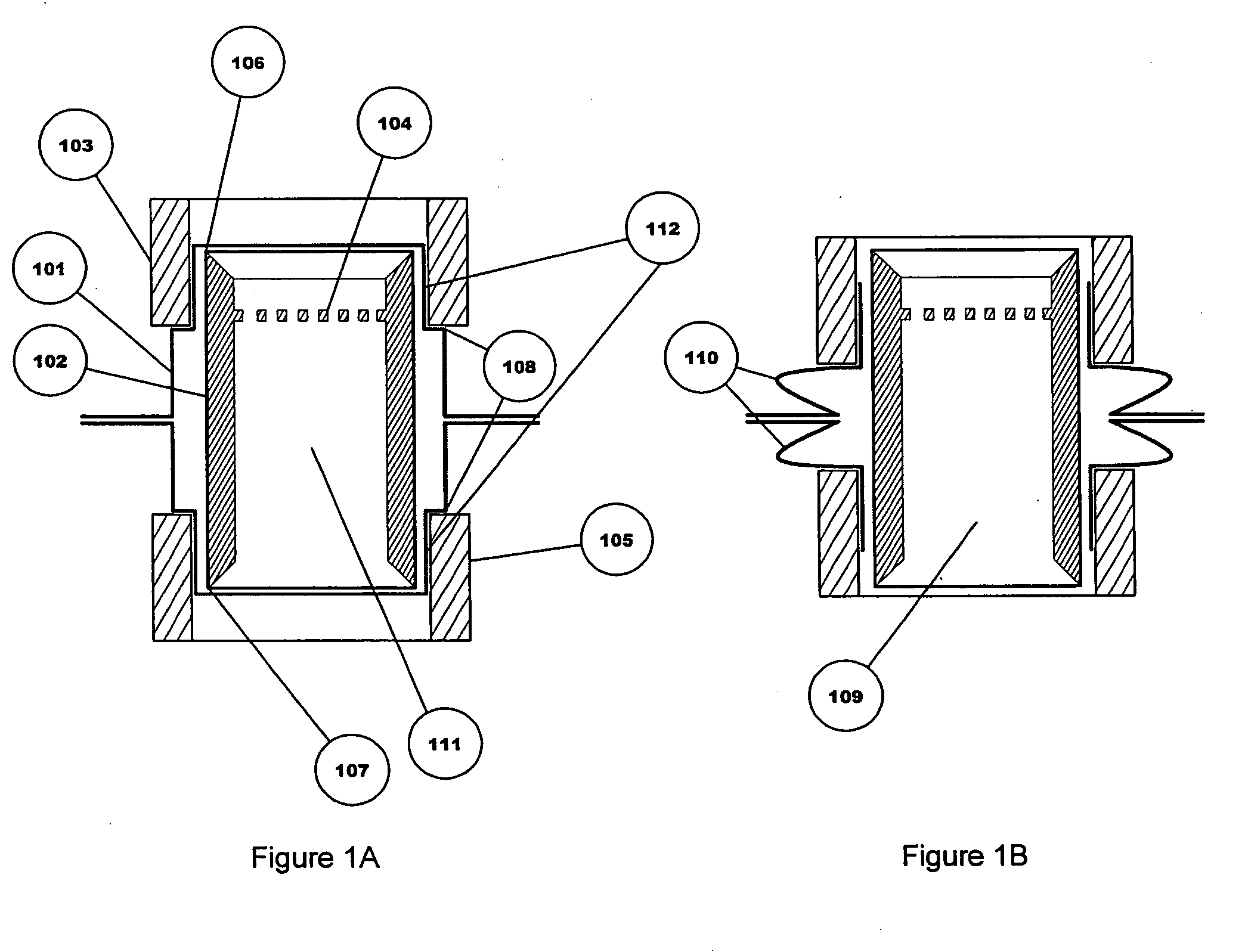
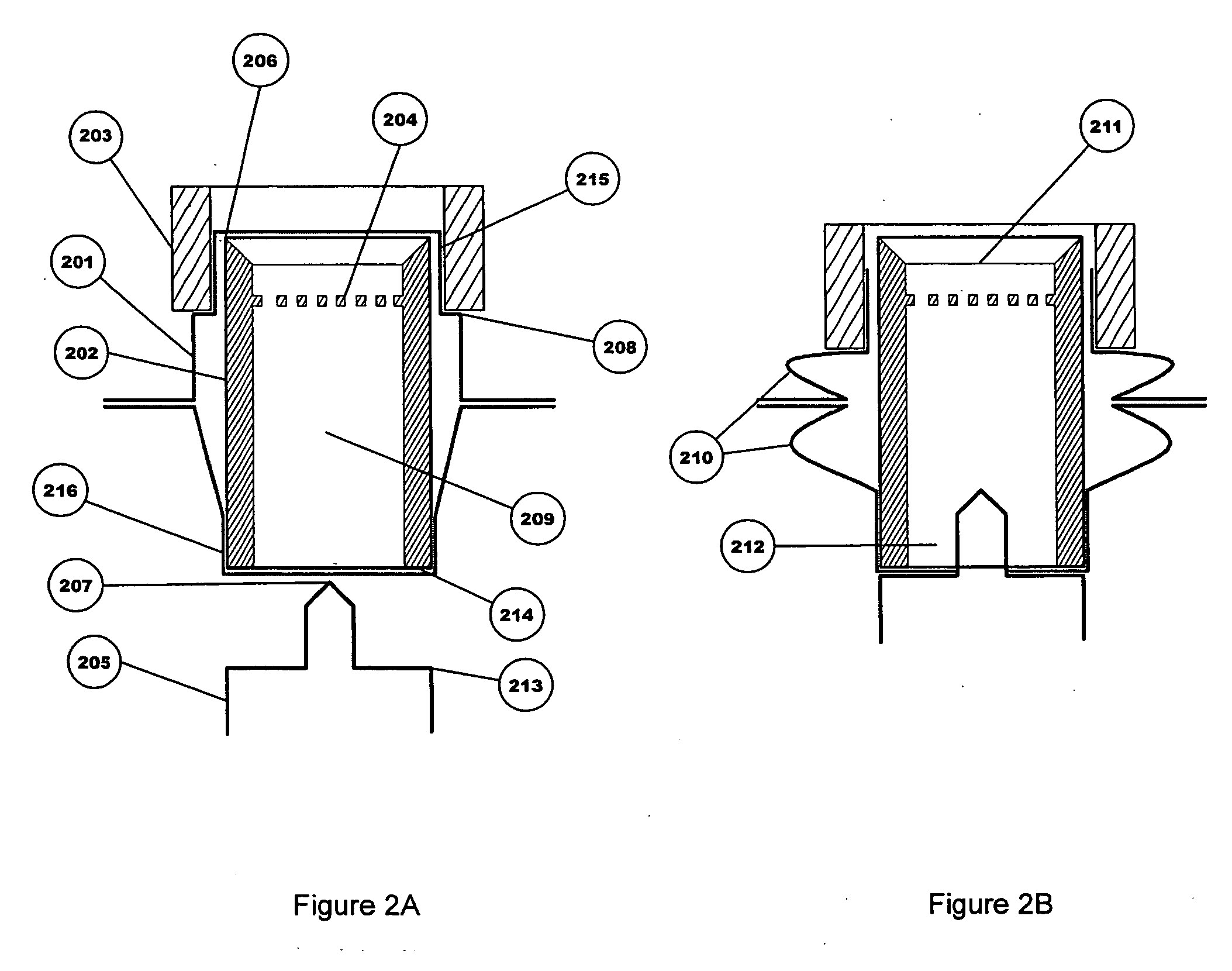
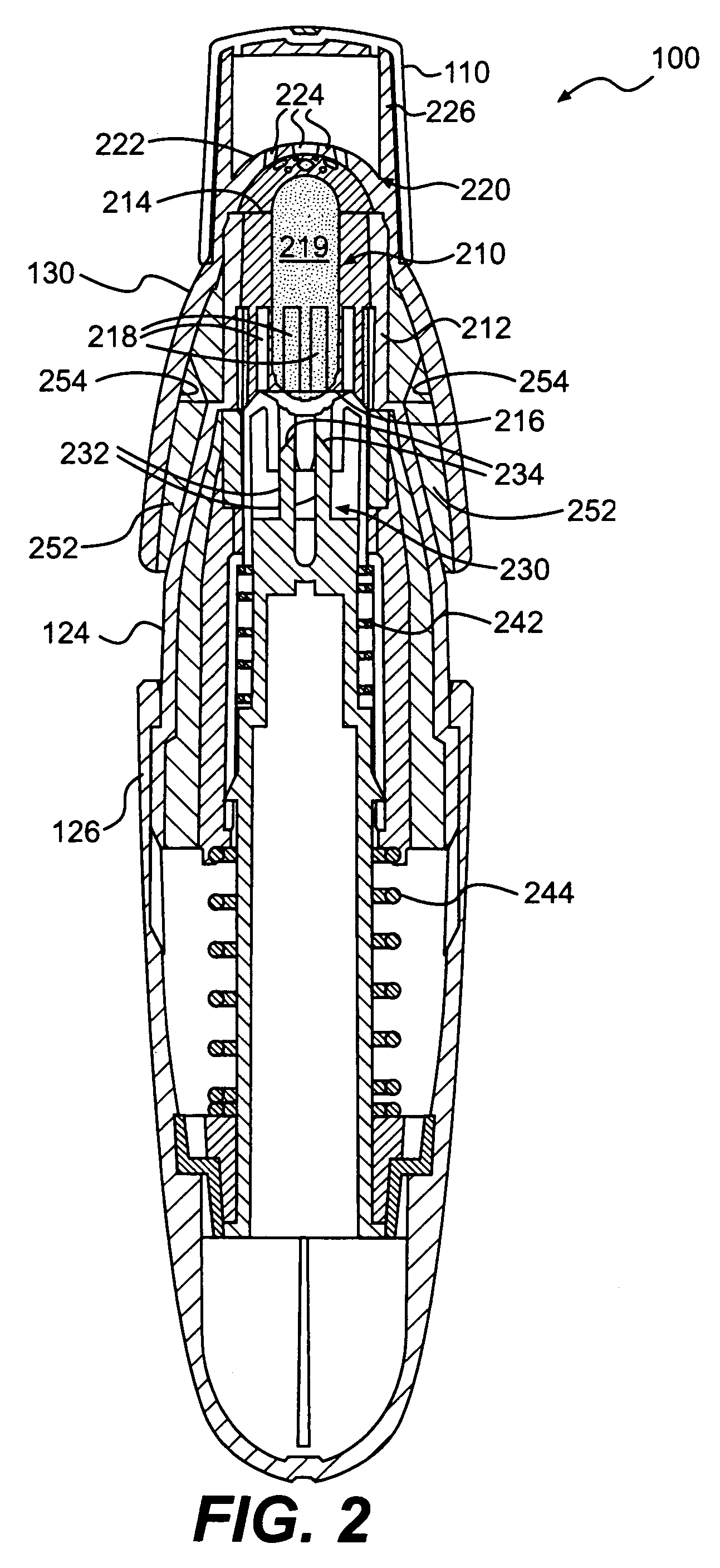
The invention relates to a heating unit for an inhalation device for the inhalation administration of an inhalation mixture of air and at least one additive material, having a fuel storage (252) which is filled or can be filled with a thermally combustible solid or liquid fuel (258), and with a combustion chamber (256) for the combustion of the fuel (258), which is essentially sealed from the surroundings by a combustion chamber wall (222). The invention further relates to an inhalation device (210) with such a heating unit.According to the invention, the combustion chamber (256) is designed for forming a flame, and the combustion chamber wall (222) has at least some micro openings (224). The micro openings are designed in such a way that the sum of the outer side lengths of all micro openings (224) is at least 140 mm, and the sum of the outer side lengths of the micro openings (224) per surface in the area of the combustion chamber wall (222) averages at least 80 mm / cm2.Application as cigarette substitute or as aid for nicotine withdrawal.
Owner:SILLER FRIEDRICH
Inhalation therapy assembly and method
InactiveUS20020069870A1Conveniently and easily allowChemical protectionLighting and heating apparatusDose deliveryInhalation
The inhalation therapy assembly and method of use described herein increases the efficiency of metered dose inhalers by allowing delivery of the doses to a collapsible reservoir which can be manually pumped, ensuring that medicants contained therein are properly and completely delivered to the patient. Terminal and proximal valves of the one-way diaphragm type allow flow of the aerosol medicants while preventing improper expulsion. An exhalation valve is adjustable to ensure the patient exhales suitably to permit proper medicant absorption. A conventional metered dosage inhaler having an approved FDA canister provides proper dosage to the patient and is joined to the collapsible reservoir by a connector having a plurality of apertures for receiving the MDI and an accessory T-fitting.
Owner:FARMER MEDICAL
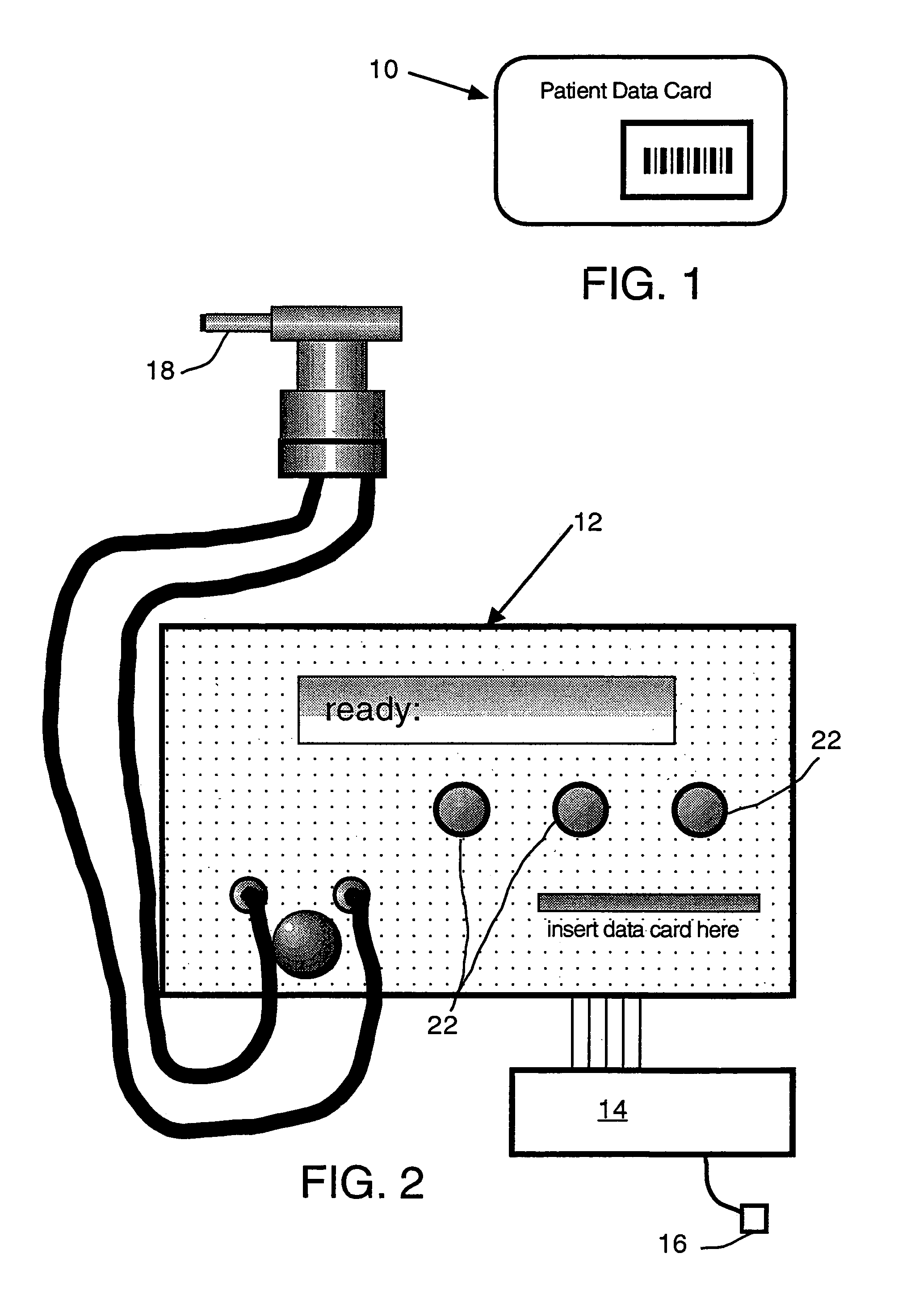
Inhalation device and system for the remote monitoring of drug administration
The present invention is directed to a device for monitoring the usage of inhaled drugs by a patient. The device includes an inhaler, a use sensor, a microprocessor, a wireless transmitter and a battery compartment. These components allow information concerning drug usage to be transmitted to health care personnel that can evaluate the data to determine whether there are changes in drug usage characteristics that are indicative of an impending acute attack. The invention includes not only the device, but also the systems and methods in which the device is employed.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Medicinal inhalation devices and components thereof
InactiveUS20120097159A1Improve performanceDesirable surface characteristicRespiratorsFibre treatmentMedicineInhalation Devices
A composition for modifying a surface of a substrate, the composition comprising: (a) a first polyfluoropolyether silane of the Formula Ia: CF3CF2CF2O(CF(CF3)CF2O)pCF(CF3)—C(O)NH(CH2)3Si(Y)3 wherein each Y is independently a hydrolyzable group and wherein p is 3 to 50; and (b) a second polyfluoropolyether silane of the Formula IIa: (Y′)3Si(CH2)3NHC(O)—CF2O(CF2O)m(C2F4O)qCF2-C(O)NH(CH2)3Si(Y′)3 wherein each Y′ is independently a hydrolyzable group and wherein m is 1 to 50 and q is 3 to 40. A method of making a medicinal inhalation device or a component of a medicinal inhalation device comprising a step of applying to at least a portion of a surface of the device or the component, respectively, the composition.
Owner:3M INNOVATIVE PROPERTIES CO
Compositions for treatment or prevention of bioterrorism
InactiveUS6991779B2Improved onsetRapid disseminationPowder deliveryBacterial antigen ingredientsDiseaseAntigen
Compositions containing biologically active molecules encapsulated in self-assembling, diketopiperazine microspheres (TECHNOSPHEREs™) and methods for making and administering such compositions are described herein. The compositions can be used to immunize individuals against agents of biological warfare. The biologically active molecules include atropine, antibodies, antigens, and antibiotics. The compositions can be placed in an inhalation device for self-administration. Pulmonary delivery of TECHNOSPHERE™ encapsulated atropine, antibodies, vaccines, and antibiotics provides an accelerated onset of immunity to the targeted disease. Furthermore, the TECHNOSPHERE™ encapsulated atropine, antibodies, vaccines, and antibiotics are stable formulations, suitable for stockpiling, rapid dissemination and mass treatment.
Owner:MANNKIND CORP
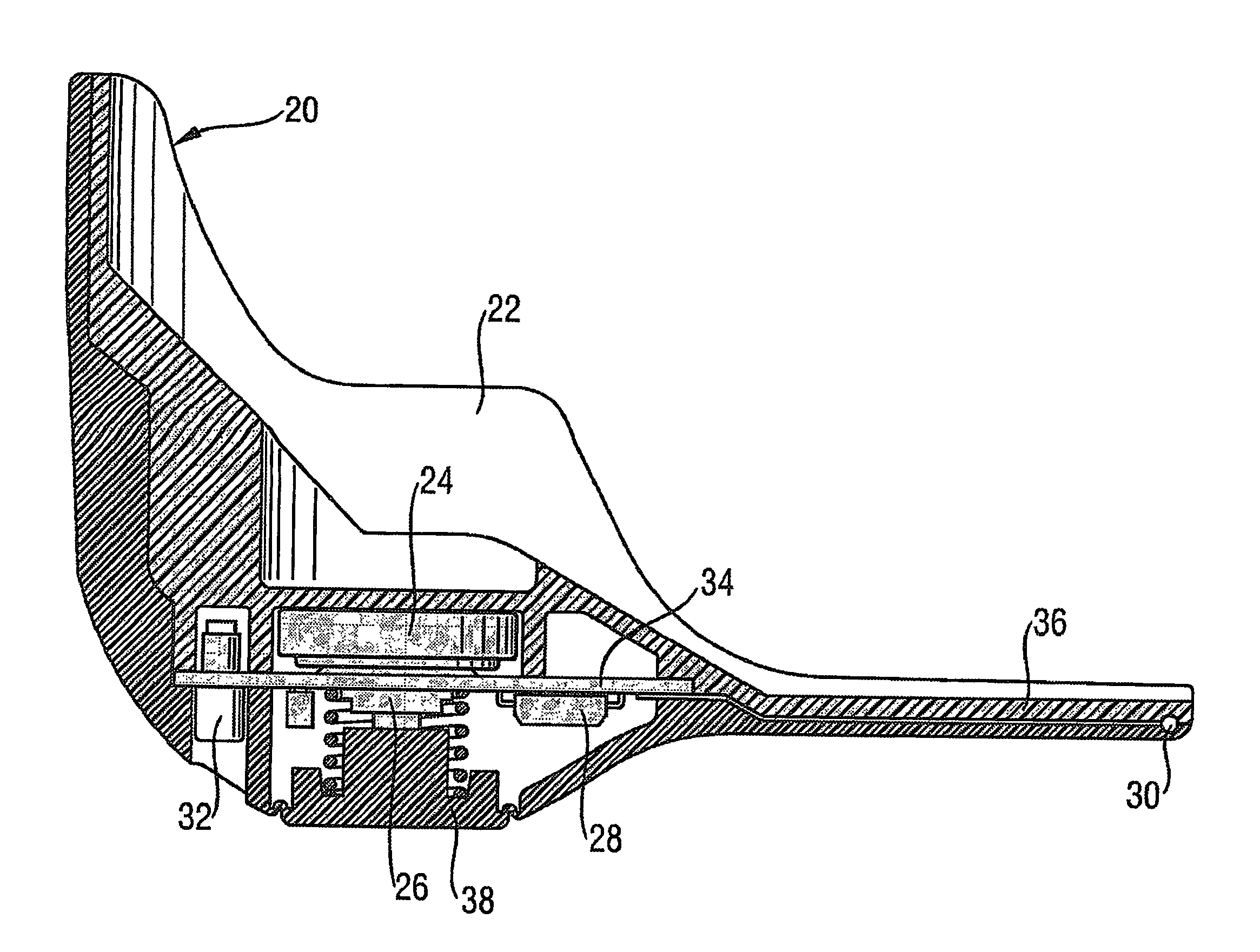
Compliance monitor and method
ActiveUS8464707B2Easily, or automatically, actuatedRespiratorsRespiratory device testingBiomedical engineeringDrug delivery
A compliance monitor (20) is attachable to or forms part of a drug delivery device, such as an inhaler (2). The monitor comprises a flexible portion (38) to enable a switch which is actuated by a user when delivering a dose of medicament. The monitor further comprises a sensor for sensing whether the device is properly positioned in contact with or relative to the user's body for administration of the medicament. For example, where the device is an inhaler and the sensor a temperature sensor, temperature variations caused by insertion of an inhaler mouthpiece into the user's mouth indicate whether the dose has been delivered into the patient's mouth. A memory in the compliance monitor stores a compliance record indicating whether or not the device was properly positioned each time a dose was delivered.
Owner:COVIS PHARM GMBH
Apparatus for dispensing pressurized contents
InactiveUS7832394B2Liquid surface applicatorsPowdered material dispensingMarine engineeringVALVE PORT
Containers for incrementally dispensing pressurized contents are described. The containers comprise a vessel suited to contain pressurized contents, a port integral with the vessel and through which pressurized contents contained in the vessel can be released from the vessel, preferably incrementally in approximately equal amounts, and a measuring device disposed in the vessel. The measuring device senses ambient conditions in the vessel and, directly or with other components, indicates, for example, the amount of pressurized contents remaining in the vessel and displaying it to an observer; methods for comparing the amount of contents actually released from the vessel compared to a theoretical constant; and methods for time logging (alone or in conjunction with the logging of other data) the actuation of the dispensing valve for comparison to a prescribed method. Devices (including metered dose inhalers) for incrementally dispensing pressurized contents from such containers are also described.
Owner:SCHECHTER ALAN M +1
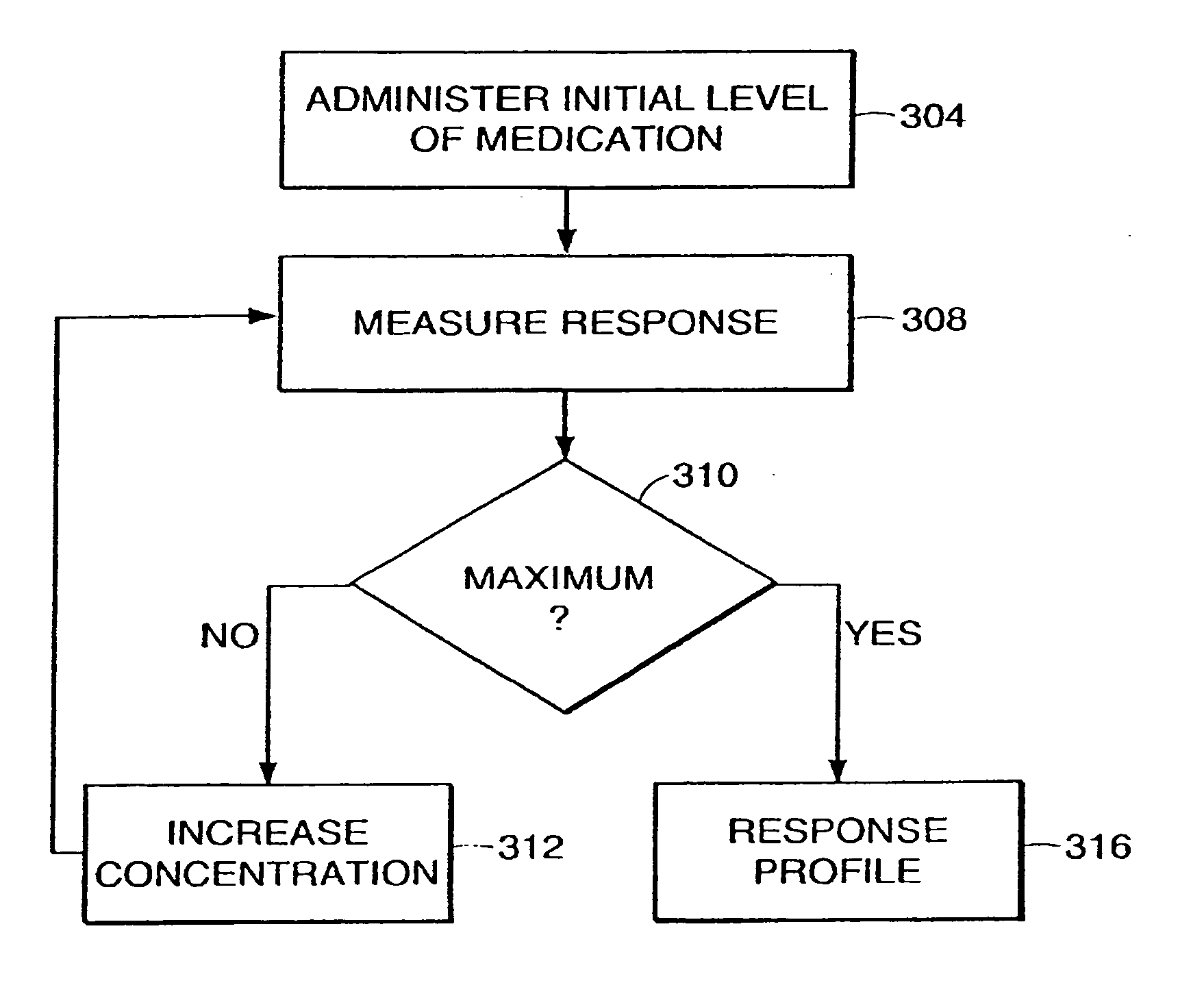
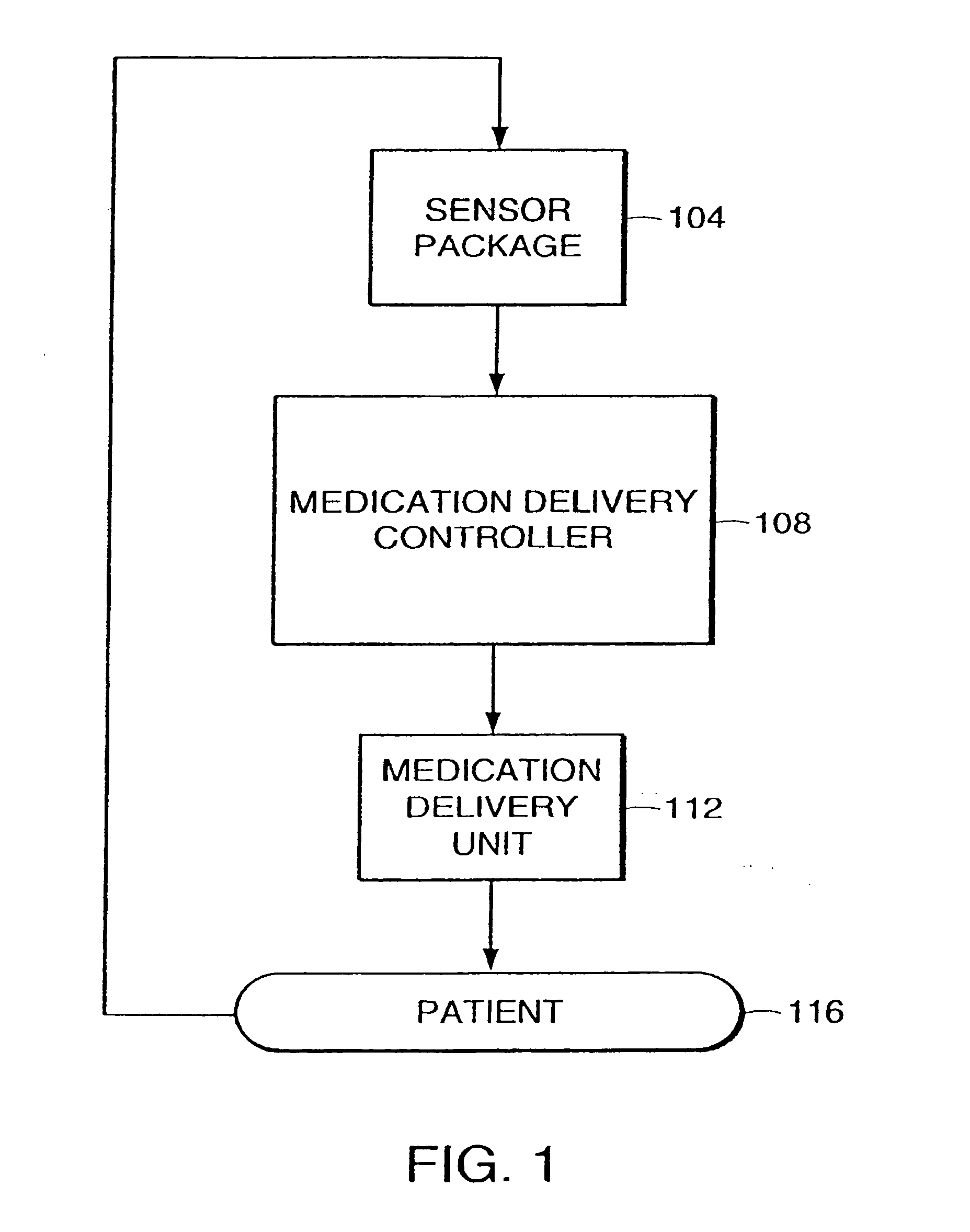
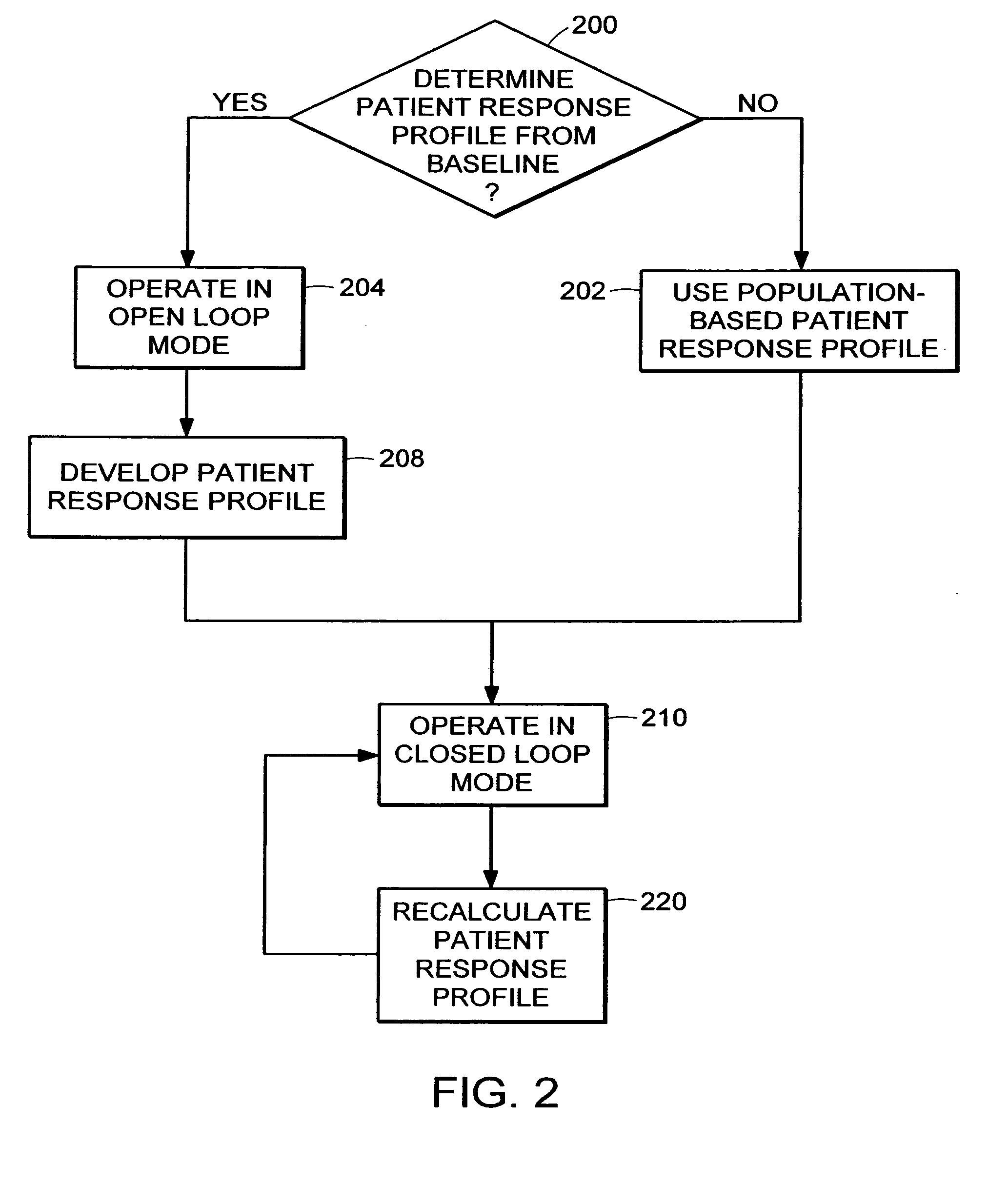
System and method for adaptive drug delivery
InactiveUS20060167722A1ChangeDesired effectElectroencephalographyData processing applicationsAnalytical expressionsInhalation Devices
The present invention provides a system and method for determining and maintaining a concentration level of medication in a patient sufficient to achieve and maintain a desired effect on that patient. Generally speaking, in accordance with one embodiment of the invention, a medication delivery controller uses a patient response profile to determine a concentration of medication in the patient that will achieve the desired effect on the patient. The patient response profile is a graphical, tabular or analytical expression of the relationship between the concentration of a medication and the effect of the medication at the specific concentration. Using this information, the medication delivery controller provides instructions to a medication delivery unit such as, for example, an infusion pump or inhalation device, to deliver the medication to the patient at a rate that will achieve the desired concentration level of the medication in the patient.
Owner:FRESENIUS VIAL
Inhalation device with heating, stirring and leak preventing components
A device including a water chamber for filtering smoke or vapor prior to inhalation of the smoke or vapor. The device including a vaporization chamber that contains a substance to be heated or burned. The device including an air flow chamber, herein the air flow chamber connects the vaporization chamber, wherein the air flow chamber is configured to provide a tunnel between an upper edge and a lower edge of the air flow chamber, for passage of air / vapor from an air / vapor entry port to a filtered air exit port for air / vapor at the water chamber, in a snake like manner; wherein the air / vapor entry port is located at a point where the vaporization chamber is joined to the water chamber. The air flow chamber is configured to prevent leakage of water from the water chamber to the vaporization chamber
Owner:HUANG YAO TIEN RICHARD
Engineered particles and methods of use
InactiveUS20050074498A1Reduce deliveryLess attractivePowder deliveryOrganic active ingredientsNebulizerActive agent
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS FARMA
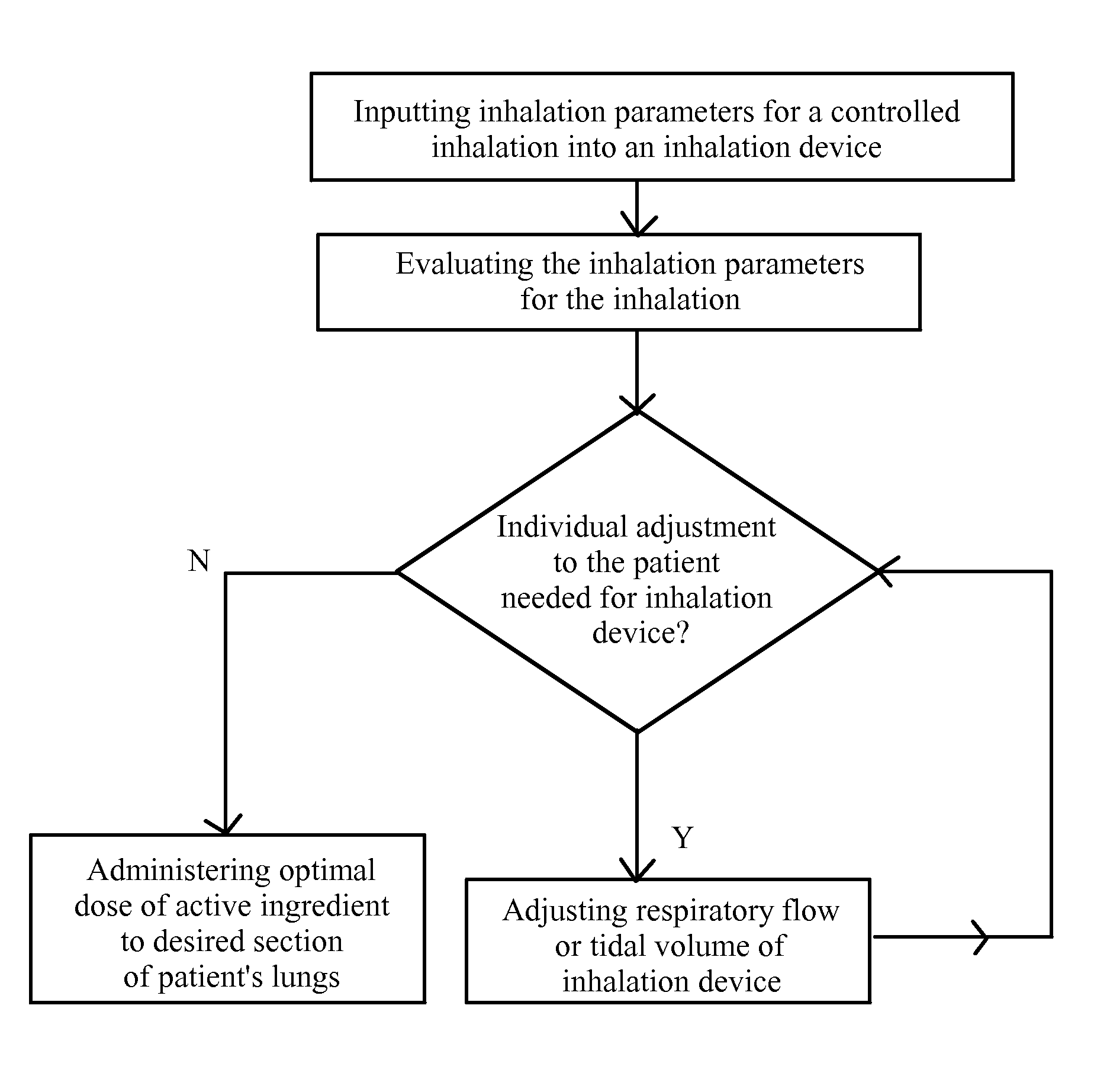
Device for the controlled inhalation of therapeutic aerosols
A device for the controlled inhalation of therapeutic aerosols comprises means providing individual patient parameters and / or aerosol parameters for the inhalation. The aerosol doses, such as the tidal volume and the respiratory flow, are individually adjusted on the basis of these individual parameters. Thus, the inhalation device may be individually adjusted to the patient to be treated.
Owner:VECTURA LTD
Inhalation Devices and Systems and Methods Including the Same
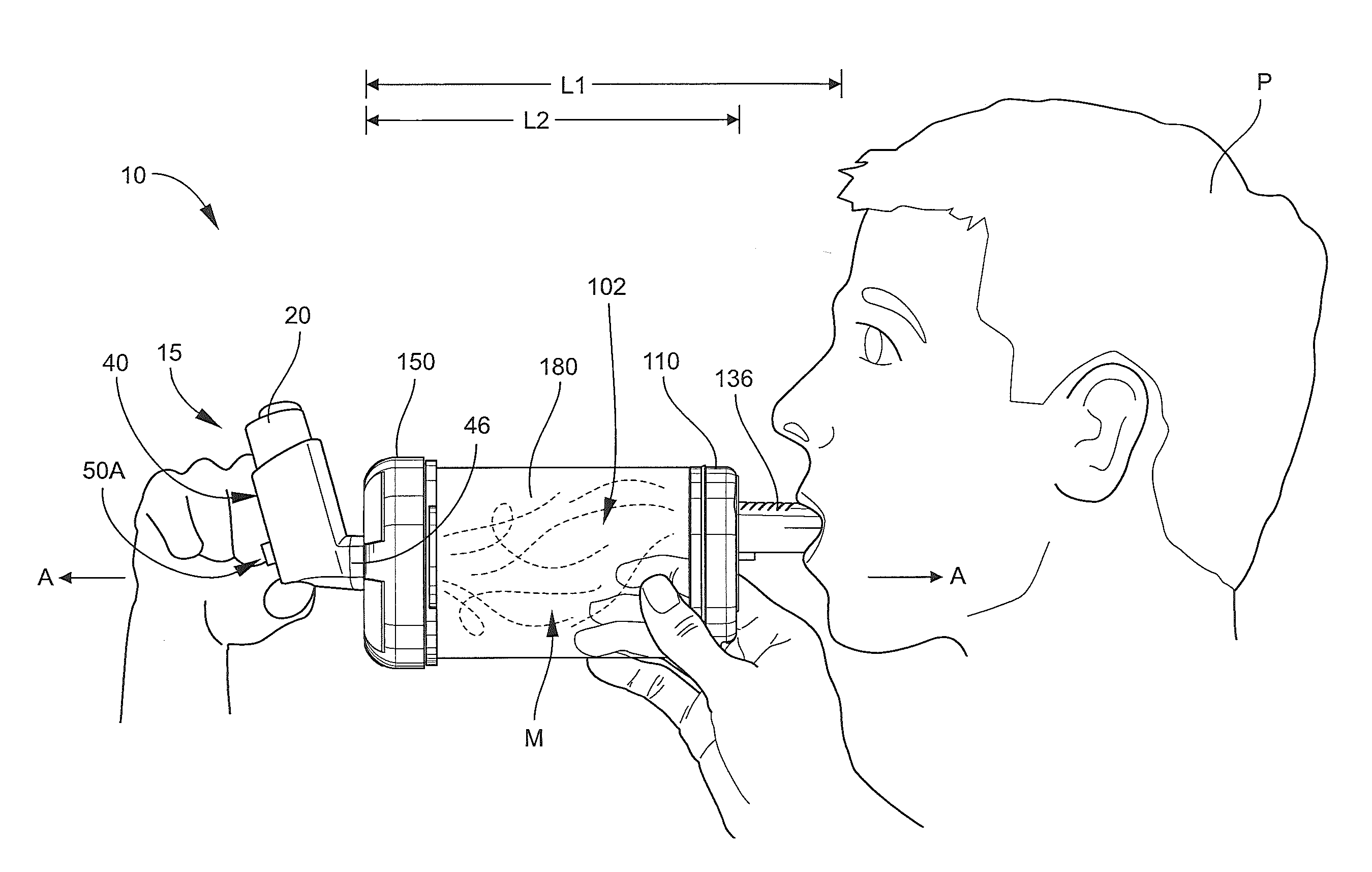
A collapsible inhalation device for use with a metered dose inhaler (MDI) dispenser includes an outlet end member, an inlet end member and a tubular, pliable, collapsible sleeve member attached at either end to the inlet and outlet members. The outlet end member includes a mouthpiece. The inlet end member includes an inlet port and an MDI dispenser mount structure configured to receive and engage the MDI dispenser. The inhalation device is positionable in each of an open position, wherein the sleeve member defines a chamber, and a closed position, wherein the sleeve member is collapsed and enveloped by the outlet end member and the inlet end member. When the inhalation device is in the open position with the MDI dispenser mounted in the MDI dispenser mount structure, a dose of the medication can be dispensed from the MDI dispenser into the chamber through the inlet port to mix with air in the chamber and thereby form a mixture of the air and the dose of the medication that can be inhaled by a patient from the chamber through the mouthpiece.
Owner:CERECOR INC
Dry powder inhaler
InactiveUS7318435B2Easy to manufactureEasy to operateRespiratorsLiquid surface applicatorsMedicineInhalation
An inhalation device for the uptake of medicaments that are in the form of dry powder contained in the blisters of specially designed single dose blister strips. The device is comprised of a mouthpiece (A), a strip support surface area (B). and one or more storage areas (C). Furthermore, the single dose blister strip is described. It is comprised of two sheets (17, 20) that are fixed in such a manner so that when they get separated the powder becomes available for inhalation.
Owner:PENTAFRAGAS DIMITRIOS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com