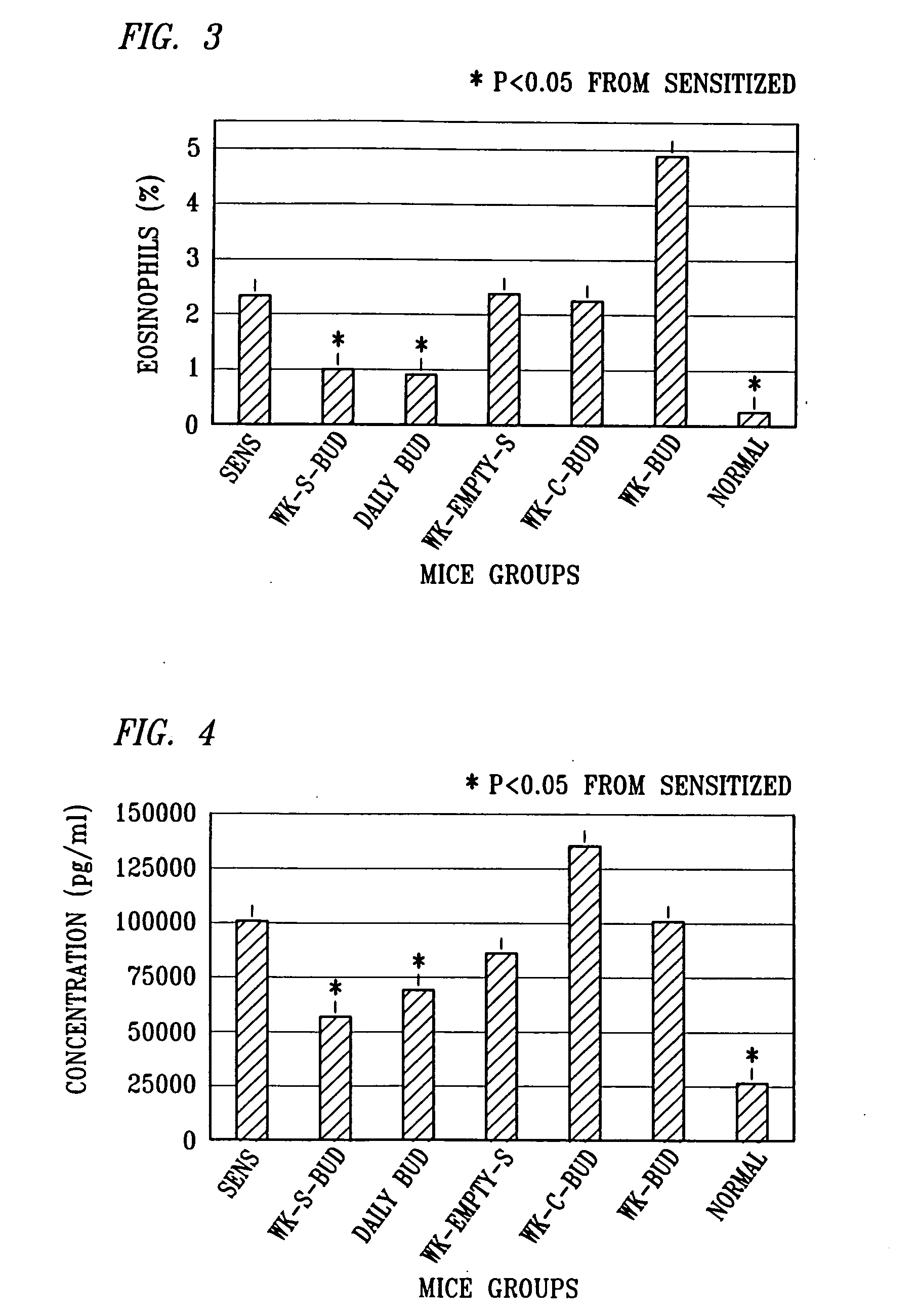
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Drug aerosol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aerosol Drug Administration. Definition. Aerosol drug administration is the administration of a drug via air particles delivered by an appropriate device that is inhaled and absorbed into the patient's body via the lungs.
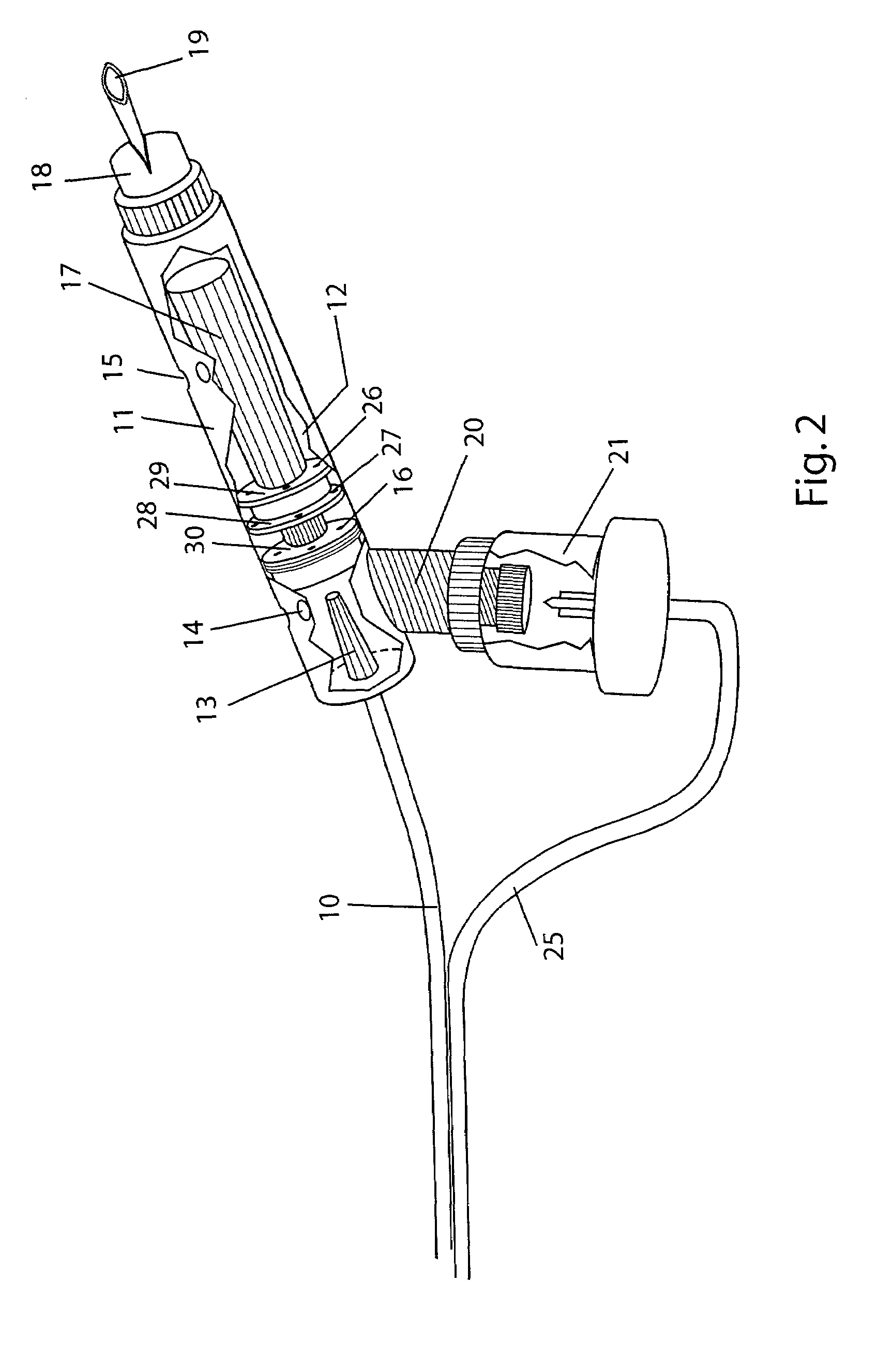
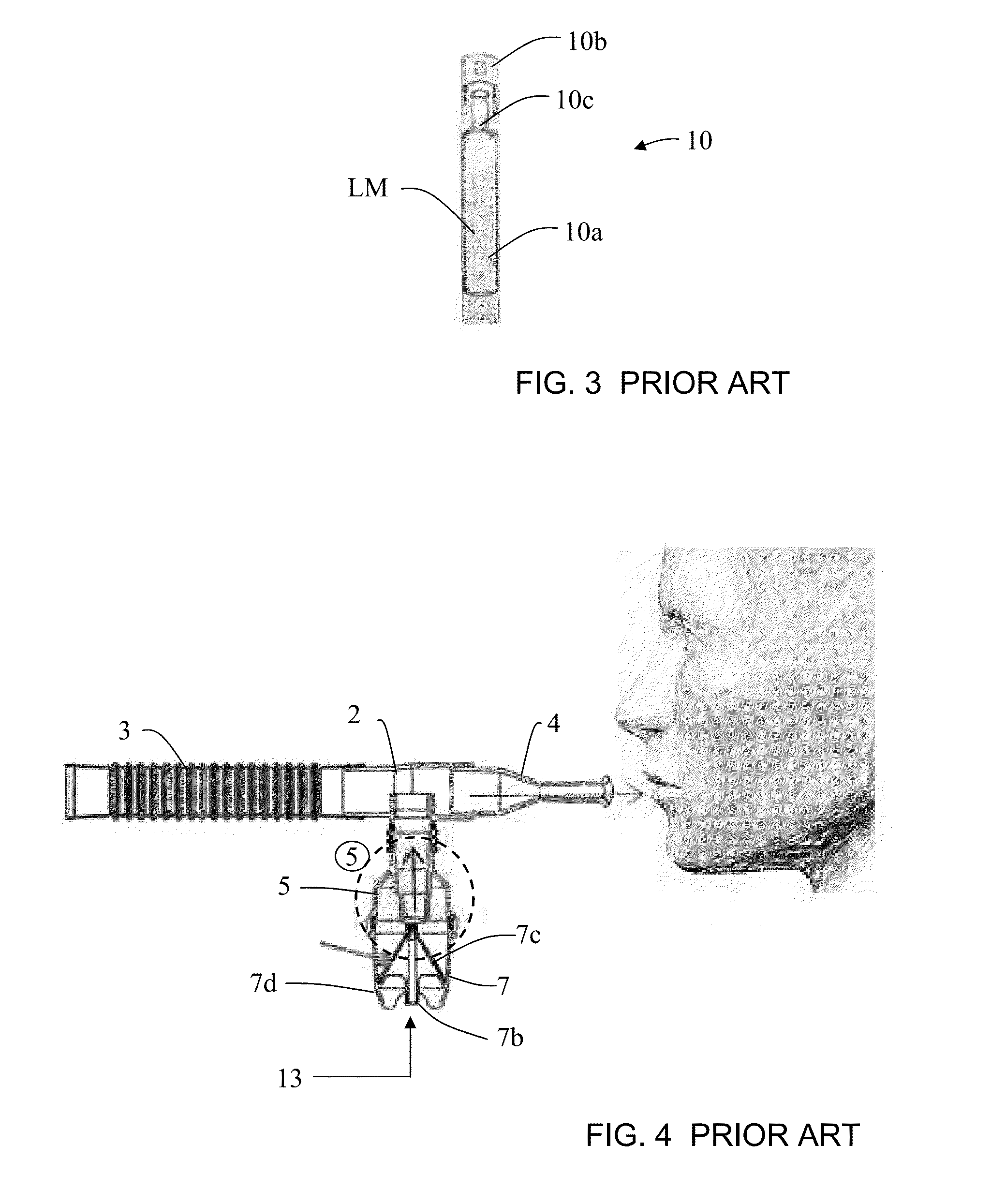
Drug delivery device and methods therefor
InactiveUS6578571B1Overcome fearOvercome mistrustRespiratory masksMedical devicesAnesthesiaVALVE PORT
An incentive inhaler device includes an inhalation device, at least one incentive toy coupled to the inhalation device, and at least one separator. The inhalation device has a respiration device and a connector that is linkable to a delivery device. The delivery device can deliver drugs, aerosols, powder and gas. The incentive toy is respiration driven and has at least one of a visible characteristic and an audible characteristic. The separator decouples the toy from at least one component of the inhalation device to ensure directional flow of respirational air which drives the toy. The separator can include a valve, a filter, or a baffle.
Owner:MEDICAL DEV INT
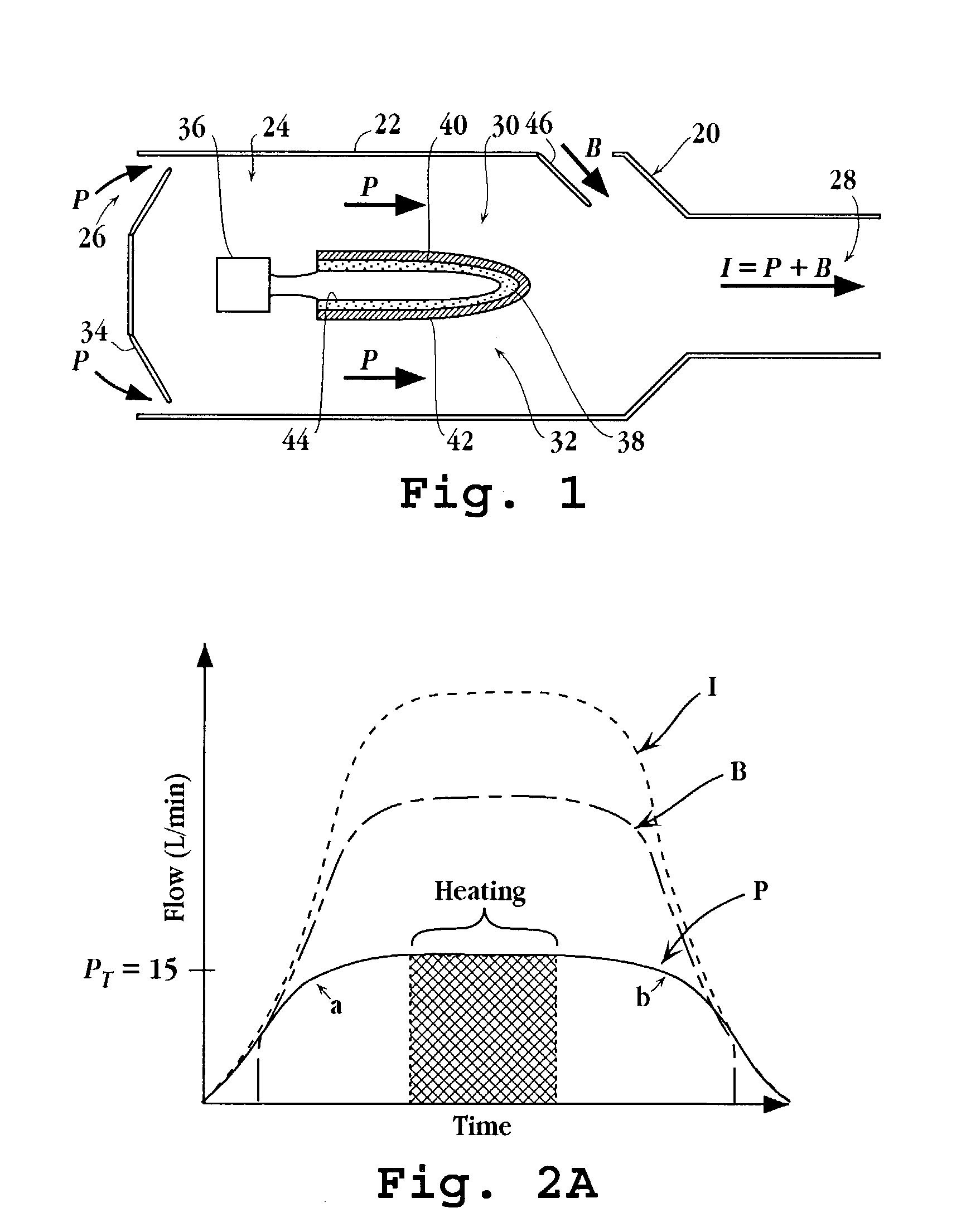
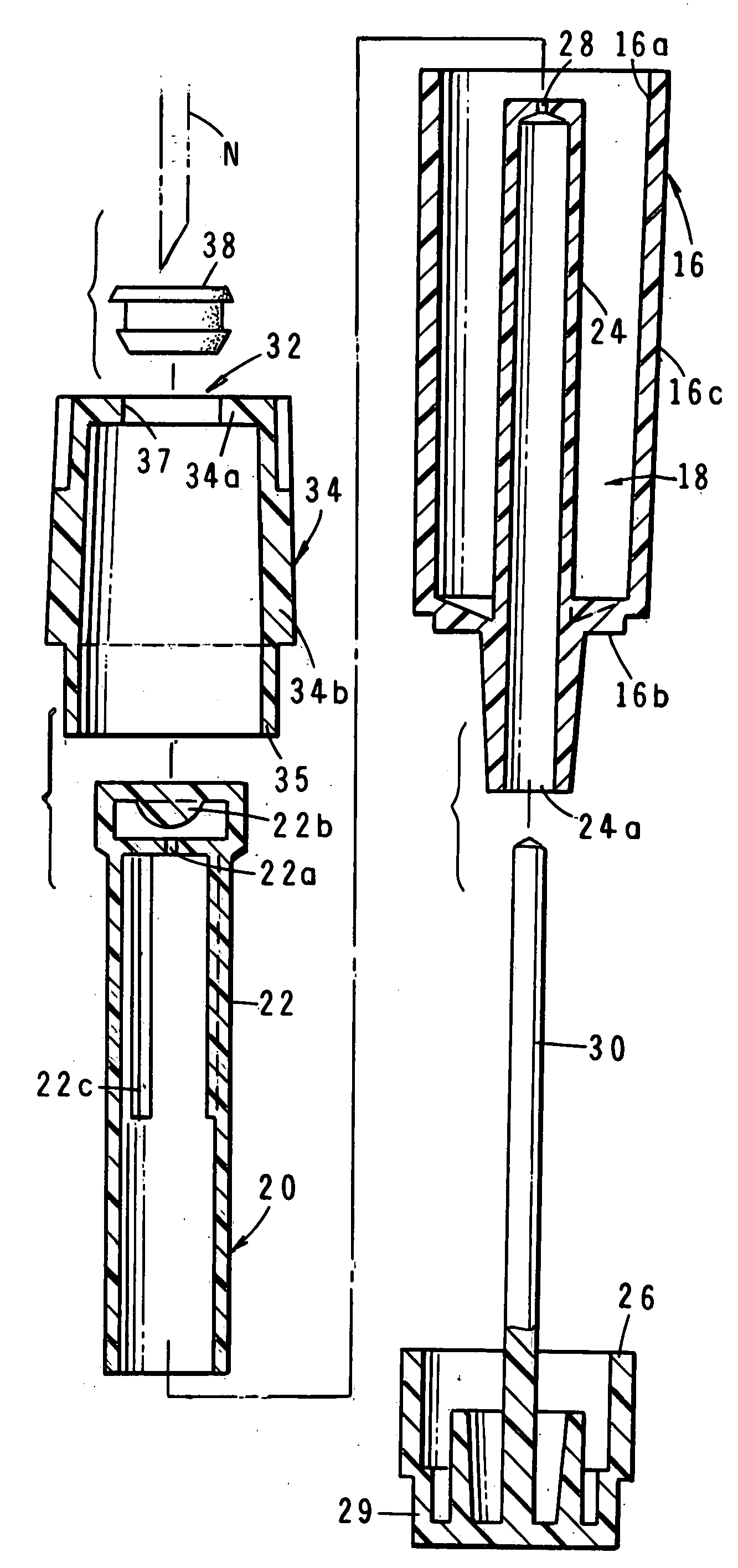
Inhalation device for producing a drug aerosol
A device for delivering a drug by inhalation is disclosed. The device includes a body defining an interior flow-through chamber having upstream and downstream chamber openings, and a drug supply unit contained within the chamber for producing, upon actuation, a heated drug vapor in a condensation region of the chamber. Gas drawn through the chamber region at a selected gas-flow rate is effective to form drug condensation particles from the drug vapor having a selected MMAD between 0.02 and 0.1 MMAD or between 1 and 3.5 μm. A gas-flow control valve disposed upstream of the unit functions to limit gas-flow rate through the condensation region to the selected gas-flow rate. An actuation switch in the device activates the drug-supply unit, such that the drug-supply unit can be controlled to produce vapor when the gas-flow rate through the chamber is at the selected flow rate.
Owner:ALEXZA PHARMA INC
Continuous high-frequency oscillation breathing treatment apparatus
ActiveUS7191780B2Assist in mucus secretionSimply and inexpensively manufacturingRespiratorsOperating means/releasing devices for valvesInhalationBreathing treatments
Owner:COMEDICA INC
Thin-film drug delivery article and method of use
InactiveUS20070031340A1Reduce the amount requiredFast evaporationRespiratorsPowder deliveryDrug aerosolMedicine
An article for use in an aerosol device, for producing an aerosol of a drug composition is disclosed. The article includes a heat-conductive substrate having a surface with a selected surface area, and a drug composition film on the substrate surface having a selected film thickness of between 0.05 and 20 μm. The film thickness is such that an aerosol formed by vaporizing the drug composition by heating the substrate and condensing the vaporized compound contains 10% or less drug-degradation product and at least 50% of the total amount of drug composition contained in the film. The selected substrate surface area is such as to yield an effective human therapeutic dose of the drug aerosol. Also disclosed are methods of making and using the article.
Owner:ALEXZA PHARMA INC
System and method for delivering a substance to a body cavity
ActiveUS20050137529A1Efficient and safe and effective applicationSurgical needlesMedical devicesDrug aerosolPost operative
A system and method for creating a medicated atmosphere in an organ, or body cavity is disclosed. The system includes a flexible aerosolization catheter that can be manipulated during use, a device for the introduction of the aerosolization catheter, a medication delivery apparatus configured to control delivery of a medication to the catheter, a gas delivery apparatus in communication with the catheter, a gas pressure relief apparatus configured to relieve pressure in the organ or body cavity, and a central controller in communication with the medication delivery apparatus, gas delivery apparatus, and gas pressure relief apparatus control of the various means. The method includes providing insufflation gas and an aerosol of medication to an organ or body cavity while controlling overall pressure in the organ or cavity. The method may also include re-entering a patient through at least one port to apply gas and an aerosolized medicament, in either a post-operative procedure or in a chemotherapy context.
Owner:NORTHGATE TECH
Continuous high-frequency oscillation breathing treatment apparatus
ActiveUS20050061318A1Assist in mucus secretionSimply and inexpensively manufacturingRespiratorsOperating means/releasing devices for valvesBreathing treatmentsDuring expiration
A continuous high-frequency oscillation breathing device delivers therapy during both inhalation and exhalation in order to assist in clearing secretions from the lungs. A fixed shrouded-venturi patient interface circuit is combined with medicated aerosol to deliver continuous high-frequency oscillation therapy. Fixed open apertures in the patient interface circuit allow ingress and egress of flow, and are calibrated to allow exhalation and prevent stacking of successive breaths.
Owner:COMEDICA INC
Nebulised Antibiotics for Inhalation Therapy
The present invention provides pharmaceutical aerosols which are useful for the prevention or treatment of infectious diseases of the airways, such as the lungs, the bronchi, or the sinunasal cavities. The aerosols comprise an active agent selected from the group of quinolone antibiotics. Also disclosed are liquid and solid compositions suitable for being converted into the aerosols, and kits comprising such compositions.
Owner:PARI PHARMA GMBH
Thin-film drug delivery article and method of use
InactiveUS7585493B2Improve the level ofReduce the amount requiredRespiratorsPowder deliveryDrug aerosolMedicine
An article for use in an aerosol device, for producing an aerosol of a drug composition is disclosed. The article includes a heat-conductive substrate having a surface with a selected surface area, and a drug composition film on the substrate surface having a selected film thickness of between 0.05 and 20 μm. The film thickness is such that an aerosol formed by vaporizing the drug composition by heating the substrate and condensing the vaporized compound contains 10% or less drug-degradation product and at least 50% of the total amount of drug composition contained in the film. The selected substrate surface area is such as to yield an effective human therapeutic dose of the drug aerosol. Also disclosed are methods of making and using the article.
Owner:ALEXZA PHARMA INC
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
Nebulizer with flow-based fluidic control and related methods
ActiveUS20070227535A1Reduce trafficFaster and/or more consistent delivery of medication to the patientRespiratorsSpray nozzlesNebulizerDrug aerosol
Various embodiments of a breath-activated nebulizer with flow-based fluidic control and related methods of using such a nebulizer are disclosed. The nebulizer may include a body comprising a reservoir for holding medication, a nozzle for emitting a jet of pressurized gas, and a fluid conduit in communication with the reservoir for delivery of the medication proximate the jet to produce an aerosol of medication. The nebulizer may also include a nebulizer outlet in communication with the body for delivery of the aerosol to a patient, an entrainment passage for providing entrainment flow from atmosphere during inhalation by the patient, and a control conduit in fluid communication with the fluid conduit for delivery of a control gas to the fluid conduit to prevent the delivery of the medication proximate the jet. In some exemplary embodiments, the control conduit may comprise a gas passage proximate the entrainment passage to allow the control gas to flow across the entrainment passage. During the inhalation by the patient, the entrainment flow through the entrainment passage may substantially prevent the control gas from flowing across the entrainment passage so as to interrupt the delivery of the control gas to the fluid conduit.
Owner:SUNMED GRP HLDG LLC
Mixed drug aerosol compositions
InactiveUS20080299048A1Overcome Manufacturing ComplexityOvercomes shelf-life stability issuePowder deliveryNervous disorderControlled drugsDrug aerosol
The present invention pertains to aerosols which comprise a first compound which is physiologically active and a second compound which is different from the first compound. Such aerosols may be produced “on demand” and can be used to control drug release, to improve vaporizability, or to reduce, modify or eliminate undesirable taste associated with a drug aerosol. The present invention also pertains to methods for producing such aerosols.
Owner:ALEXZA PHARMA INC
Nebulizer with pressure-based fluidic control and related methods
ActiveUS20070227536A1Reduce stressFaster and/or more consistent delivery of medication to the patientCircuit elementsSpray nozzlesNebulizerAerosol spray
Various embodiments of a breath-activated nebulizer with fluidic control and related methods of using such a nebulizer are disclosed. The nebulizer may include a body comprising a reservoir for holding medication, a nozzle for emitting a jet of pressurized gas, and a fluid conduit in communication with the reservoir for delivery of the medication proximate the jet to produce an aerosol of medication. The nebulizer may also include a nebulizer outlet in communication with an interior of the body for delivery of the aerosol to a patient, a control conduit in fluid communication with the fluid conduit for delivery of a control gas to the fluid conduit to prevent the delivery of the medication proximate the jet, and a fluidic amplifier configured to control the delivery of the control gas to the control conduit.
Owner:SUNMED GRP HLDG LLC
Formoterol and mometasone aerosol formulations
ActiveUS20050255049A1Improve homogeneityHigh physical stabilityOrganic active ingredientsBiocideDrug aerosolMometasone
A pharmaceutical aerosol formulation comprising particles of (a) formoterol or a pharmaceutically acceptable salt, solvate or physiologically functional derivative thereof and (b) mometasone or a pharmaceutically acceptable salt, solvate, or physiologically functional derivative, thereof dispersed in a propellant selected from the group consisting of 1,1,1,2-tetrafluoroethane, 1,1,1,2,3,3,3-heptafluoropropane and a mixture thereof, and a bulking agent having a mass median diameter of less than one micron.
Owner:KINDEVA DRUG DELIVERY LP
Sterically stabilized carrier for aerosol therapeutics, compositions and methods for treating diseases of the respiratory tract of a mammal
InactiveUS20060251711A1Extend effective lifeBiocideDispersion deliveryDrug aerosolEffective treatment
This invention relates to a sterically stabilized liposome carrier effective for the delivery of treatment to a mammal in a composition comprising a sterically stabilized liposome and a drug effective for the treatment of a mammal with the composition as an aerosol with the composition providing effective treatment for a period of time at least 1.5 times as long as the effective time for aerosol treatment with the drug alone. This invention also relates to a composition comprising the sterically stabilized liposome and the drug, as well as a method for treating a mammal for respiratory tract and lung disorders.
Owner:VGSK TECH
Nebulizer with flow-based fluidic control and related methods
ActiveUS7841335B2Faster and/or more consistent delivery of medication to the patientReduce trafficRespiratorsSpray nozzlesDrug aerosolNebulizer
Various embodiments of a breath-activated nebulizer with flow-based fluidic control and related methods of using such a nebulizer are disclosed. The nebulizer may include a body comprising a reservoir for holding medication, a nozzle for emitting a jet of pressurized gas, and a fluid conduit in communication with the reservoir for delivery of the medication proximate the jet to produce an aerosol of medication. The nebulizer may also include a nebulizer outlet in communication with the body for delivery of the aerosol to a patient, an entrainment passage for providing entrainment flow from atmosphere during inhalation by the patient, and a control conduit in fluid communication with the fluid conduit for delivery of a control gas to the fluid conduit to prevent the delivery of the medication proximate the jet. In some exemplary embodiments, the control conduit may comprise a gas passage proximate the entrainment passage to allow the control gas to flow across the entrainment passage. During the inhalation by the patient, the entrainment flow through the entrainment passage may substantially prevent the control gas from flowing across the entrainment passage so as to interrupt the delivery of the control gas to the fluid conduit.
Owner:SUNMED GRP HLDG LLC
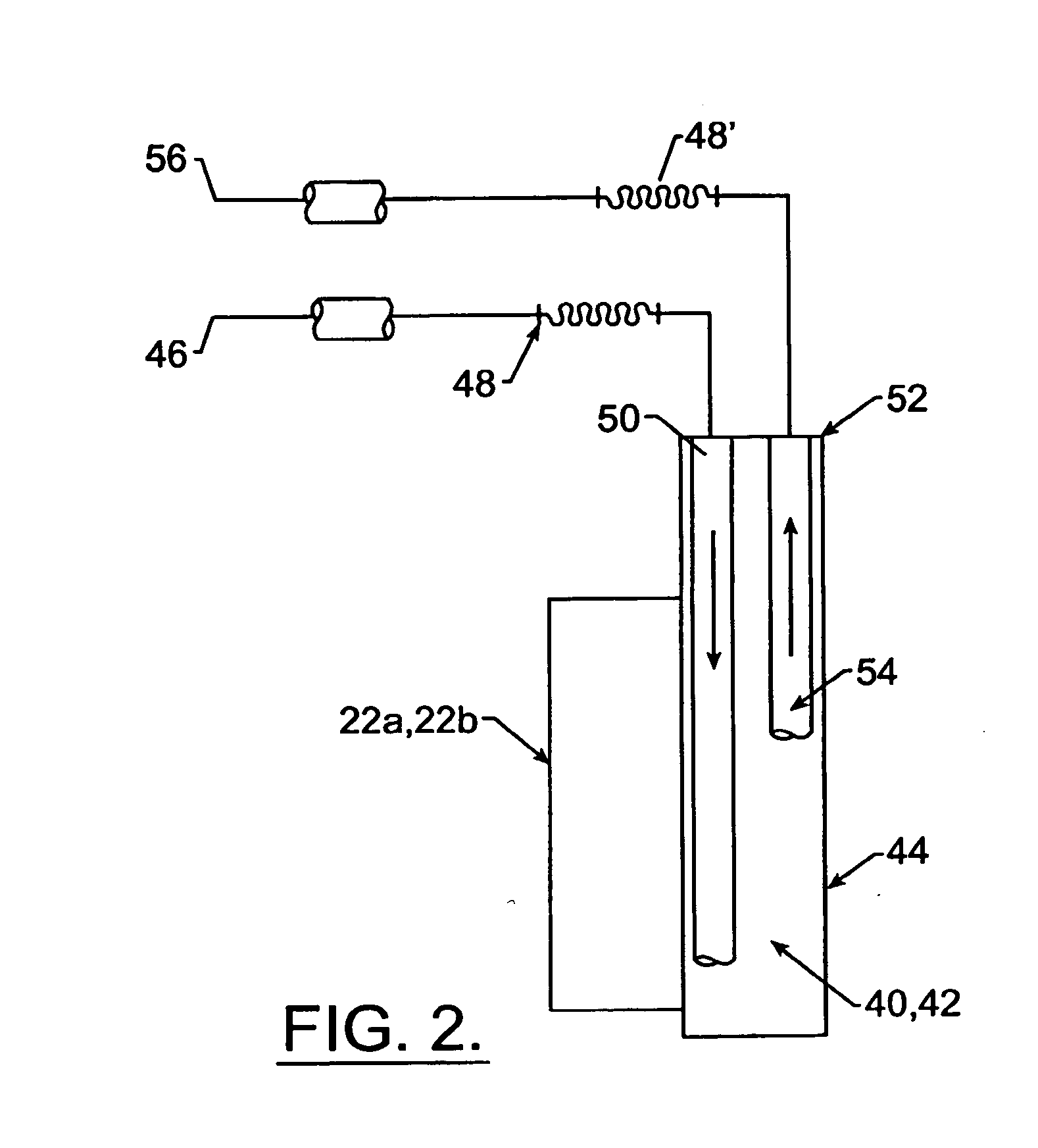
Method and apparatus to stress test medicament inhalation aerosol device by inductive heating
The invention relates to a method and apparatus for heat stress testing medicament aerosol inhalation devices, such as metered dose inhaler. An electrical induction work coil (24, 26, 28) is used to heat the inhalers to 55° C.±5 C.°. Heated inhalers are subsequently weighed to detect and reject nonconforming inhalers where a nonconforming amount of propellant has leaked.
Owner:SMITHKLINE BECKMAN CORP
Pre-filled, single-use, disposable small volume medication nebulizer
InactiveUS20090050141A1Easy to useEffectively mitigates against possibilityMedical devicesSpray nozzlesMechanical ventilatorsNebulizer
A miniaturized pre-filled, single-use, disposable, small volume medication nebulizer unit for medicinal use that delivers a mist of properly sized aerosol particles of medicament to the patient with a very high level of efficiency. The nebulizer can be effectively used in conjunction with conventional tee and mouthpiece patient interface devices as well as with more sophisticated interface devices such as dosimetric / reservoir systems, or mechanical ventilator systems.
Owner:KING RUSSELL WAYNE +1
System and method for delivering a substance to a body cavity
ActiveUS20100268153A1Efficient and safe and effective applicationSurgical needlesMedical devicesDrug aerosolPost operative
A system and method for creating a medicated atmosphere in an organ, or body cavity is disclosed. The system includes a flexible aerosolization catheter that can be manipulated during use, a device for the introduction of the aerosolization catheter, a medication delivery apparatus configured to control delivery of a medication to the catheter, a gas delivery apparatus in communication with the catheter, a gas pressure relief apparatus configured to relieve pressure in the organ or body cavity, and a central controller in communication with the medication delivery apparatus, gas delivery apparatus, and gas pressure relief apparatus control of the various means. The method includes providing insufflation gas and an aerosol of medication to an organ or body cavity while controlling overall pressure in the organ or cavity. The method may also include re-entering a patient through at least one port to apply gas and an aerosolized medicament, in either a post-operative procedure or in a chemotherapy context.
Owner:NORTHGATE TECH
Aerosols for sinunasal drug delivery
InactiveUS8852557B2Convenient treatmentReduce gapOrganic active ingredientsBiocideDiseaseNasal cavity
Owner:PARI PHARMA GMBH
System and method for delivering a substance to a body cavity
ActiveUS7704223B2Efficient and safe and effective applicationSurgical needlesMedical devicesDrug aerosolPost operative
A system and method for creating a medicated atmosphere in an organ, or body cavity is disclosed. The system includes a flexible aerosolization catheter, a device for introducing of the aerosolization catheter, a medication delivery apparatus configured to control delivery of a medication to the catheter, a gas delivery apparatus in communication with the catheter, a gas pressure relief apparatus configured to relieve pressure in the organ or body cavity, and a central controller in communication with the medication delivery apparatus, gas delivery apparatus, and gas pressure relief apparatus. The method includes providing insufflation gas and an aerosol of medication to an organ or body cavity while controlling overall pressure in the organ or cavity. The method may also include re-entering a patient through at least one port to apply gas and an aerosolized medicament, in either a post-operative procedure or in a chemotherapy context.
Owner:NORTHGATE TECH
Nebulizer with pressure-based fluidic control and related methods
ActiveUS7841336B2Faster and/or more consistent delivery of medication to the patientReduce stressCircuit elementsSpray nozzlesAerosol sprayDrug aerosol
Various embodiments of a breath-activated nebulizer with fluidic control and related methods of using such a nebulizer are disclosed. The nebulizer may include a body comprising a reservoir for holding medication, a nozzle for emitting a jet of pressurized gas, and a fluid conduit in communication with the reservoir for delivery of the medication proximate the jet to produce an aerosol of medication. The nebulizer may also include a nebulizer outlet in communication with an interior of the body for delivery of the aerosol to a patient, a control conduit in fluid communication with the fluid conduit for delivery of a control gas to the fluid conduit to prevent the delivery of the medication proximate the jet, and a fluidic amplifier configured to control the delivery of the control gas to the control conduit.
Owner:SUNMED GRP HLDG LLC
Cigarette for rehabilitation and preparation thereof
InactiveCN101297867AEasy to prepareEasy to implementTobacco preparationOrganic active ingredientsDrug aerosolAdditive ingredient
The invention discloses a cigarette for detoxification, wherein, tobacco shreds are composed of tobacco and lotus leaves absorbing ingredients of a detoxification drug, and the tobacco accounts for 10 to 50 percent of the total mass of the tobacco shreds. The invention further discloses a preparation method of the cigarette for detoxification. The cigarette for detoxification of the invention can allow drug aerosol to be absorbed by the human inner mucosa in the ionic state with the help of a smoking mode of the cigarette by the people, thus having significant efficacy, rapid effectiveness and facilitating the fine adjustment and control of the dose of the psychotropic drug; furthermore, the preparation method is simple and easy to realize, thus having broad market prospect.
Owner:肖明
Pre-filled disposable nebulizer chamber
InactiveUS20140373831A1Patient compliance is goodImprove efficiencyDiagnosticsSurgical needlesNebulizerDrug aerosol
A pre-filled, single-use, disposable medication chamber for use in a medical nebulizer system that delivers a mist of properly sized aerosol particles of medicament to the patient. The nebulizer can be effectively used in conjunction with conventional nebulizer cap, tee and mouthpiece devices that interface with the patient.
Owner:ACELA BIOMEDICAL INC
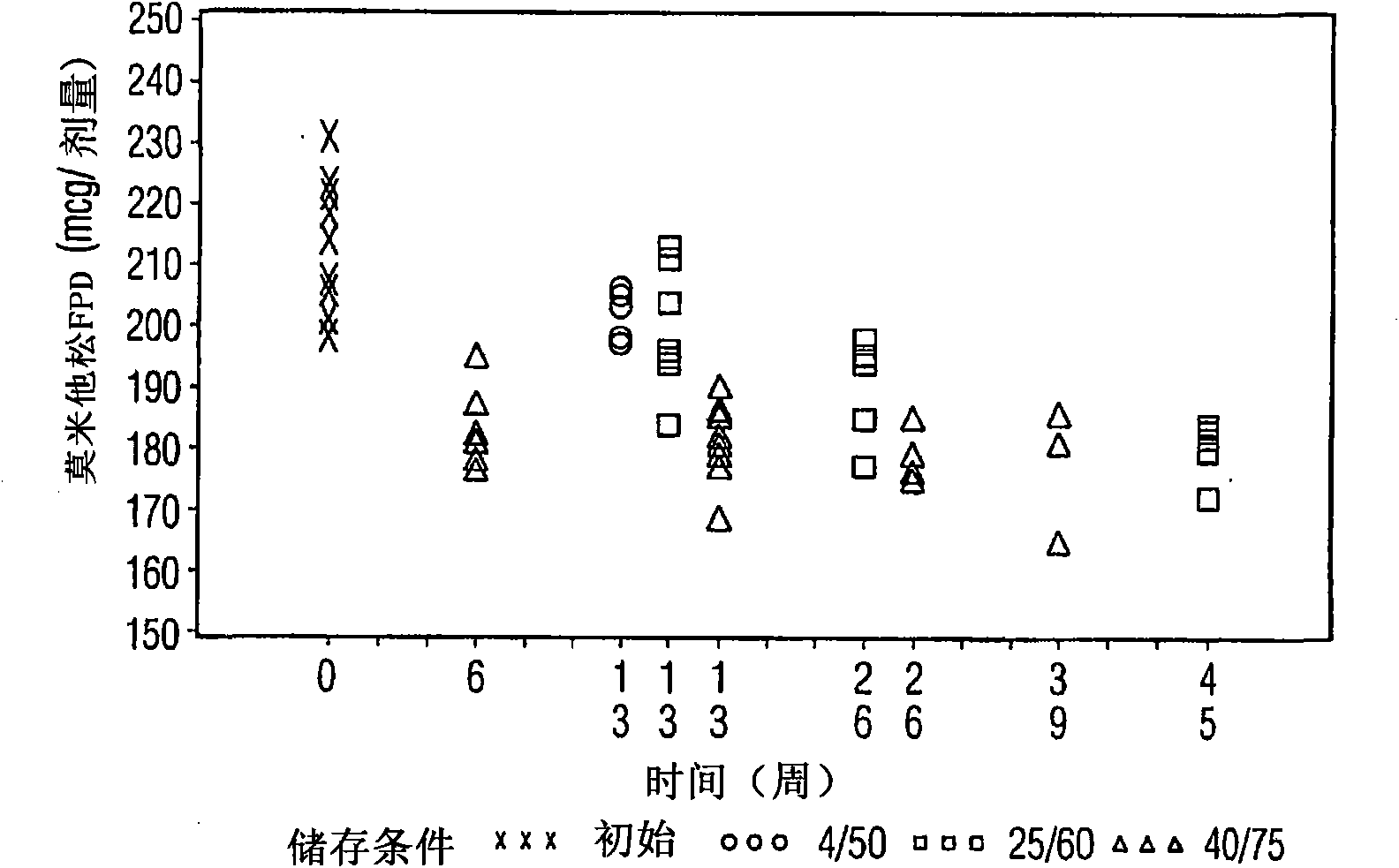
Stable pharmaceutical drug aerosols
Various aspects of the present invention provide for methods of manufacturing a pharmaceutical drug product, which include storing a container at a temperature greater than ambient conditions for at least about seven days and conducting release testing on the container after storing. Products manufactured by this method have a more consistent fine particle size distribution (FSD) and fine particlefraction (FPF) at ambient conditions and at accelerated stability conditions over the life of the drug product. Advantageously, such products may more reliably and regularly pass testing requirementsas required for an approved drug product by regulatory authorities such as the United States Food and Drug Administration (USFDA).
Owner:SCHERING AG
Laser diffraction method for particle size distribution measurements in pharmaceutical aerosols
InactiveUS20050142665A1Improve robustnessClose conformanceWithdrawing sample devicesParticle size analysisDrug aerosolParticle-size distribution
Disclosed are methods for measuring the particle size distribution of a pharmaceutical aerosol by the use of laser diffraction.
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical formulation of fluticasone propionate
InactiveUS7220403B2Stability advantageMitigate suchBiocideOrganic active ingredientsFluticasone propionateDrug aerosol
There is provided according to the invention a pharmaceutical aerosol formulation which comprises:(i) fluticasone propionate and(ii) a hydrofluoroalkane (HFA) propellant,characterised in that the fluticasone propionate is completely dissolved in the formulation. The invention also provided canisters containing the formulation and uses thereof.
Owner:GLAXO GROUP LTD
Medicine for treating injury and preparing method
InactiveCN101024003ASignificant effectGood for new woundsAntipyreticAerosol deliveryCure rateDrug aerosol
The present invention discloses a Chinese medicine aerosol for curing trauma and injury. Said Chinese medicine aerosol is made up by using (by weight portion) 5-120 portions of aconite main tuber, 5-100 portions of wild aconite tuber, 3-60 portions of carthamus flower and 1-30 portions of gardenia fruit through a certain preparation method. Its effective rate is 100% and its cure rate is 91.8%.
Owner:河南仲景药业股份有限公司
Metered dose inhaler product
The present invention relates to the provision and use of pressurised metered dose inhalers (MDIs) for the effective administration of pharmaceutical aerosol formulations. Such formulations comprise a drug, a propellant comprising one of either 1,1,1,2-tetrafluoroethane (HFA 134a) or 1,1,1,2,3,3,3-heptafluoropropane (HFA227) or a mixture thereof, a cosolvent having a higher polarity than HFA 134a or HFA227, and a surfactant in an amount at least 0.01% by weight of said formulation. Such MDIs comprise a canister.
Owner:KARIB KEMI PHARM
Pressurized metered dose inhalers and pharmaceutical aerosol formulations
Owner:BAKER NORTON PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com