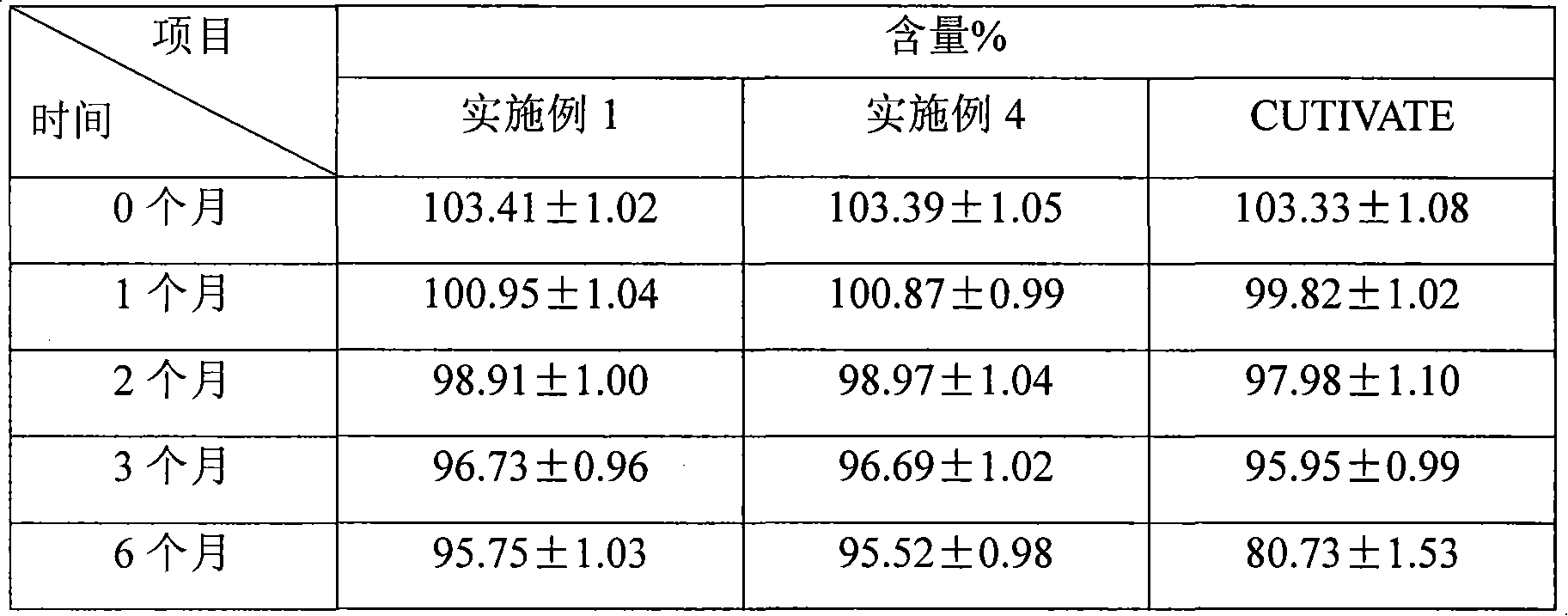
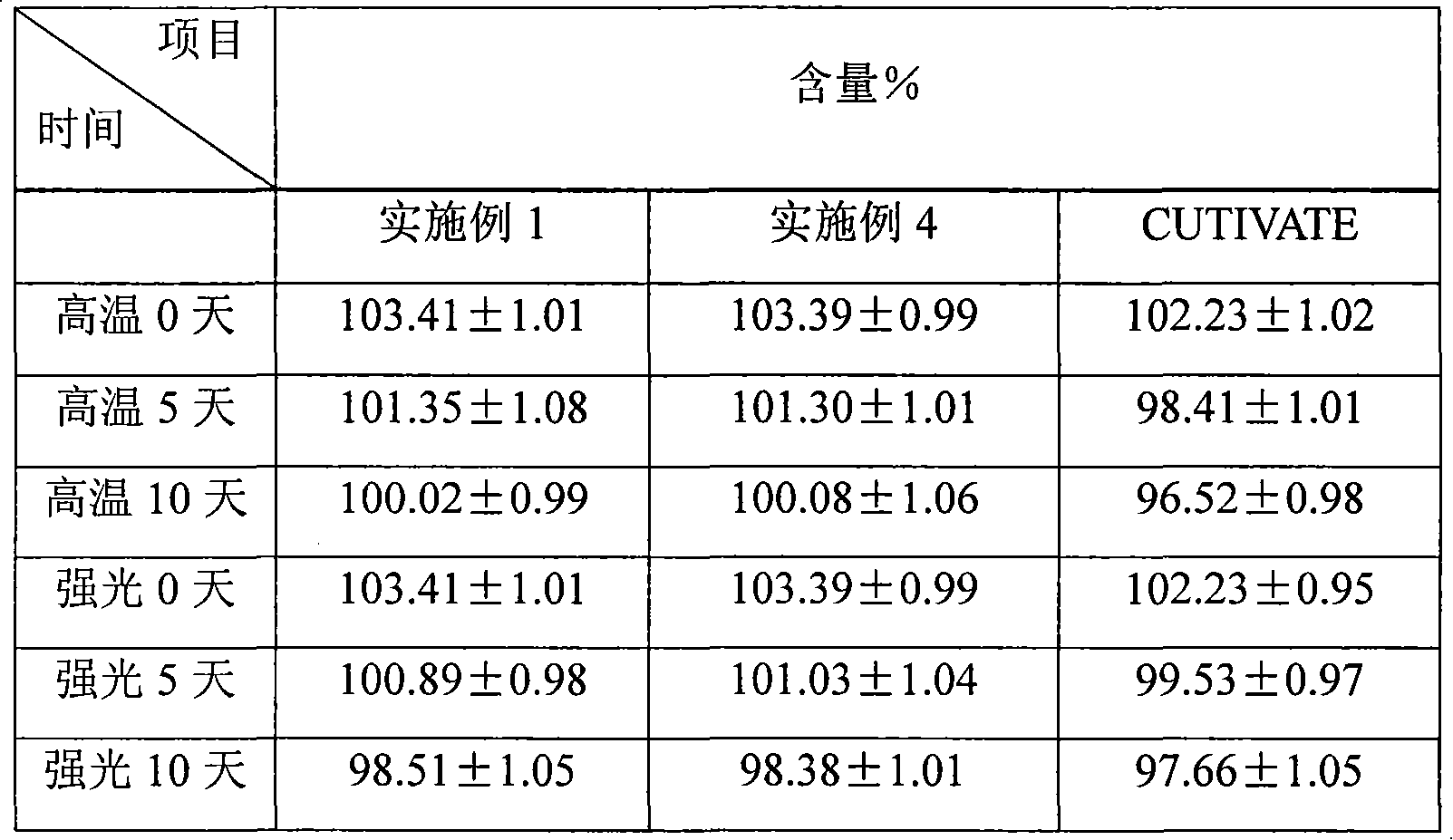
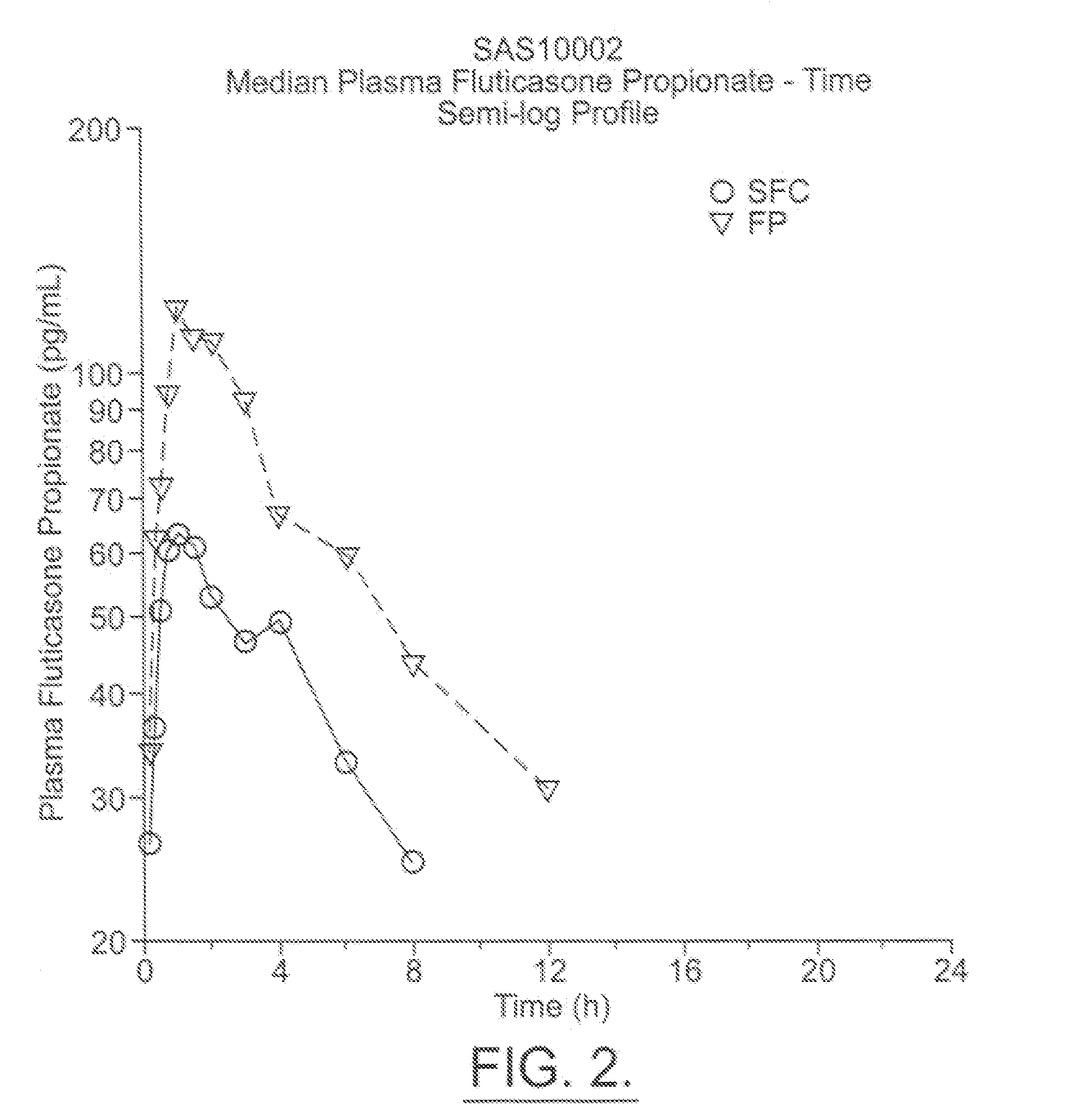
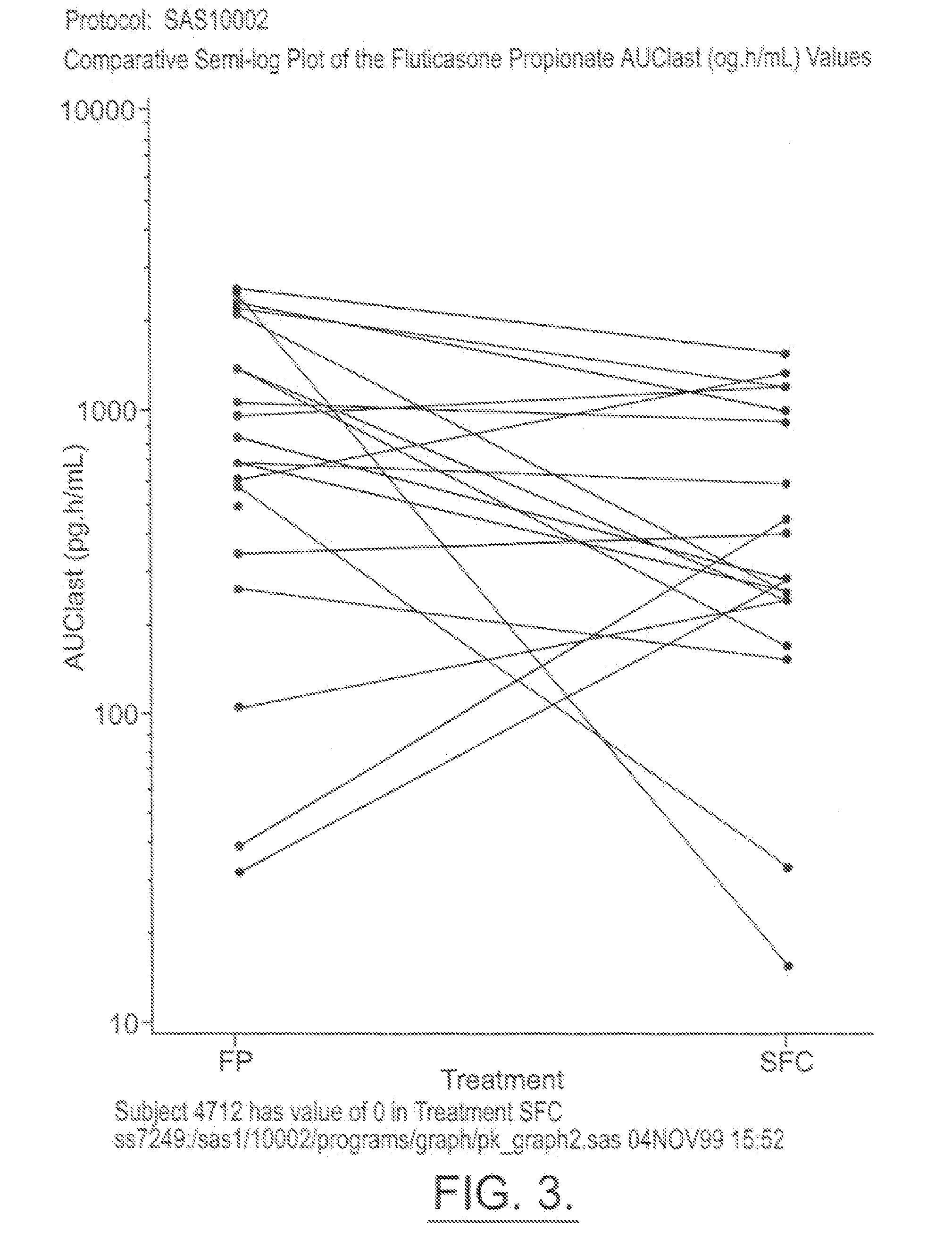
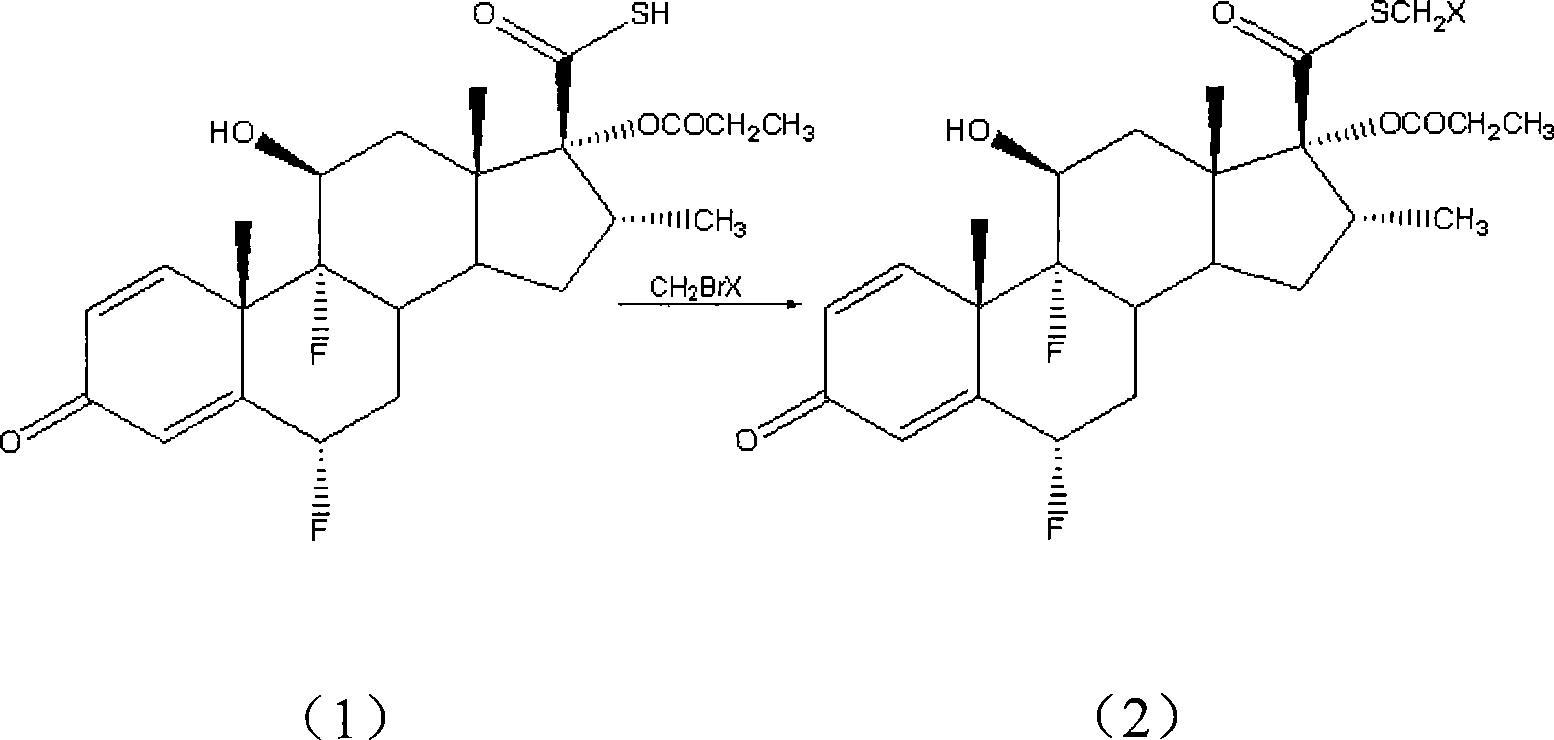
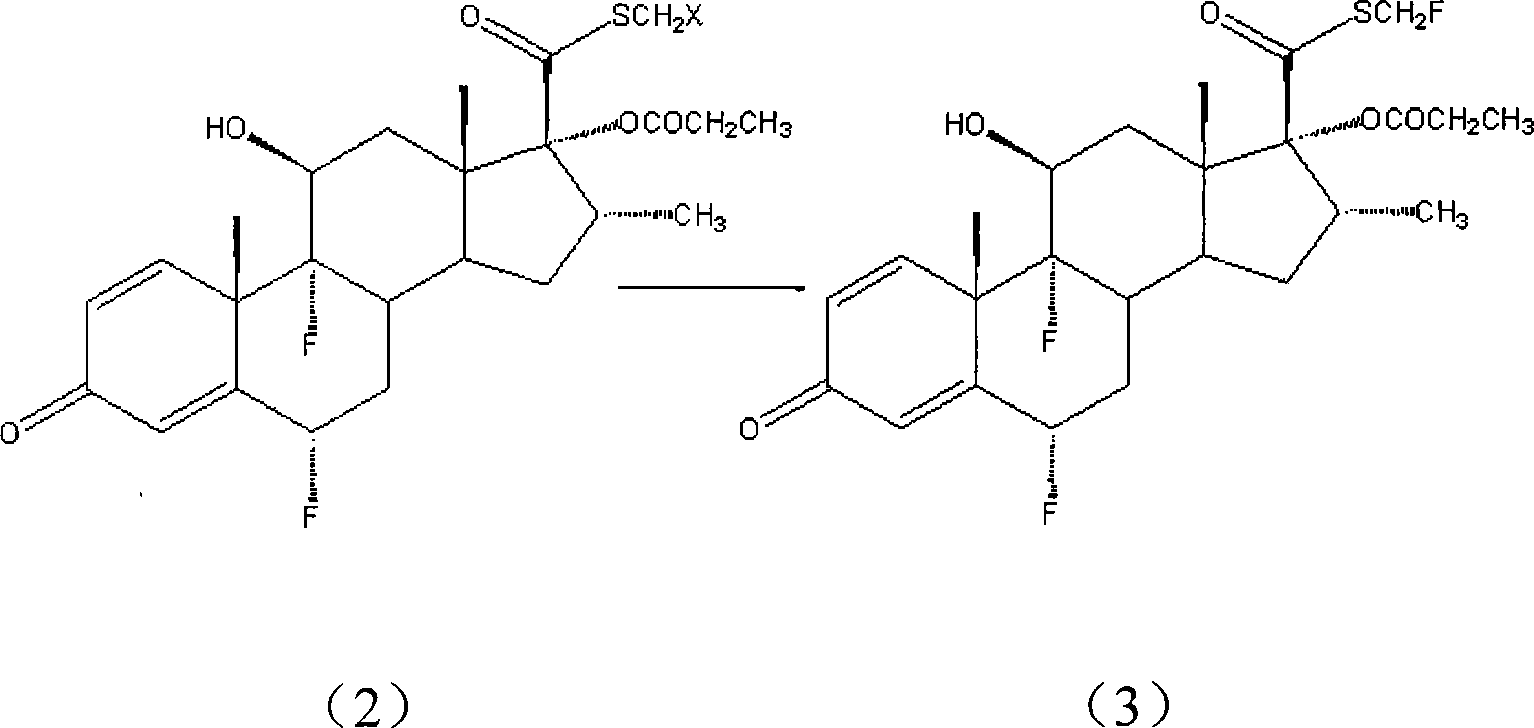
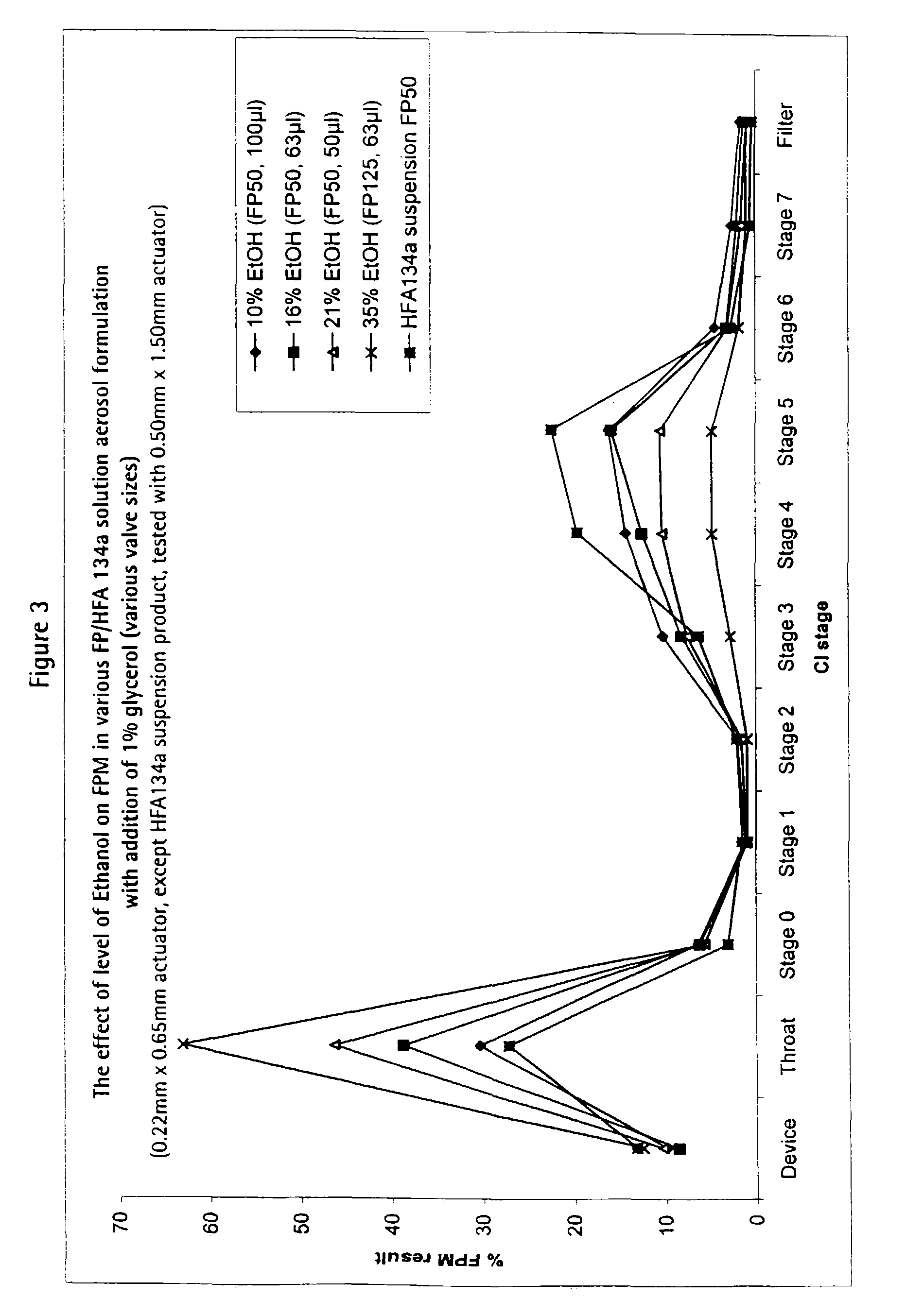
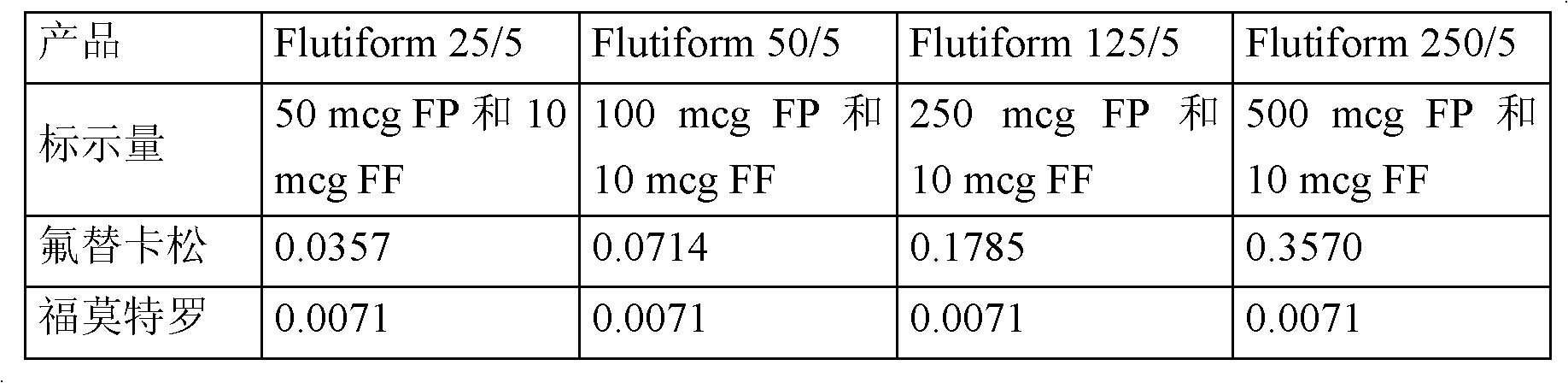
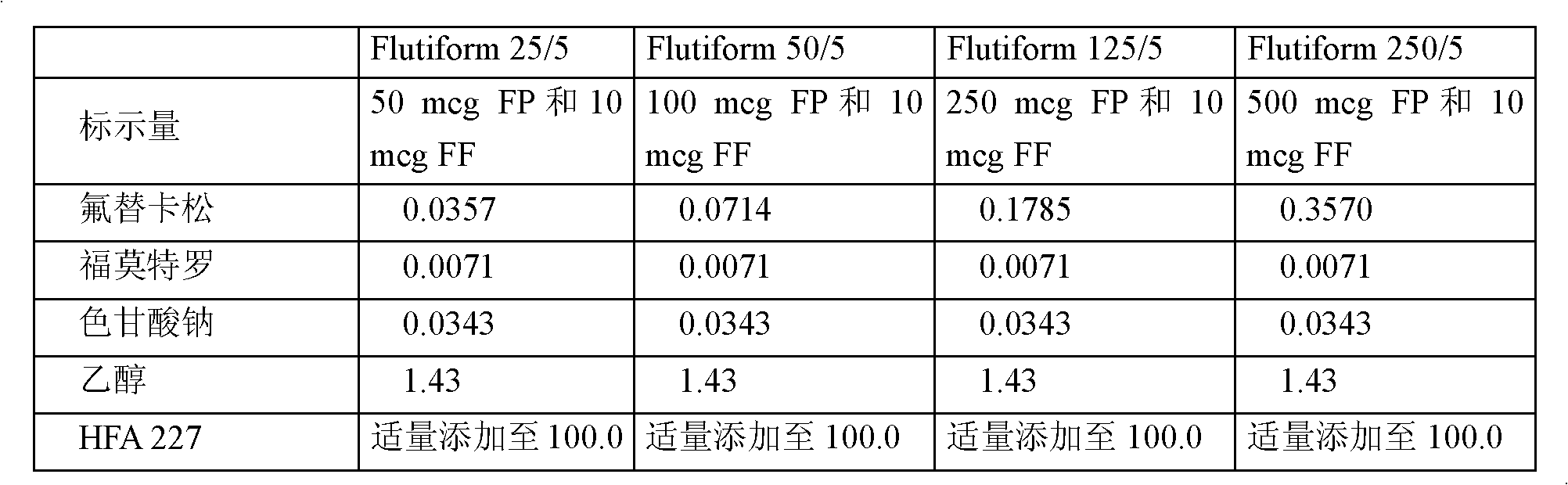
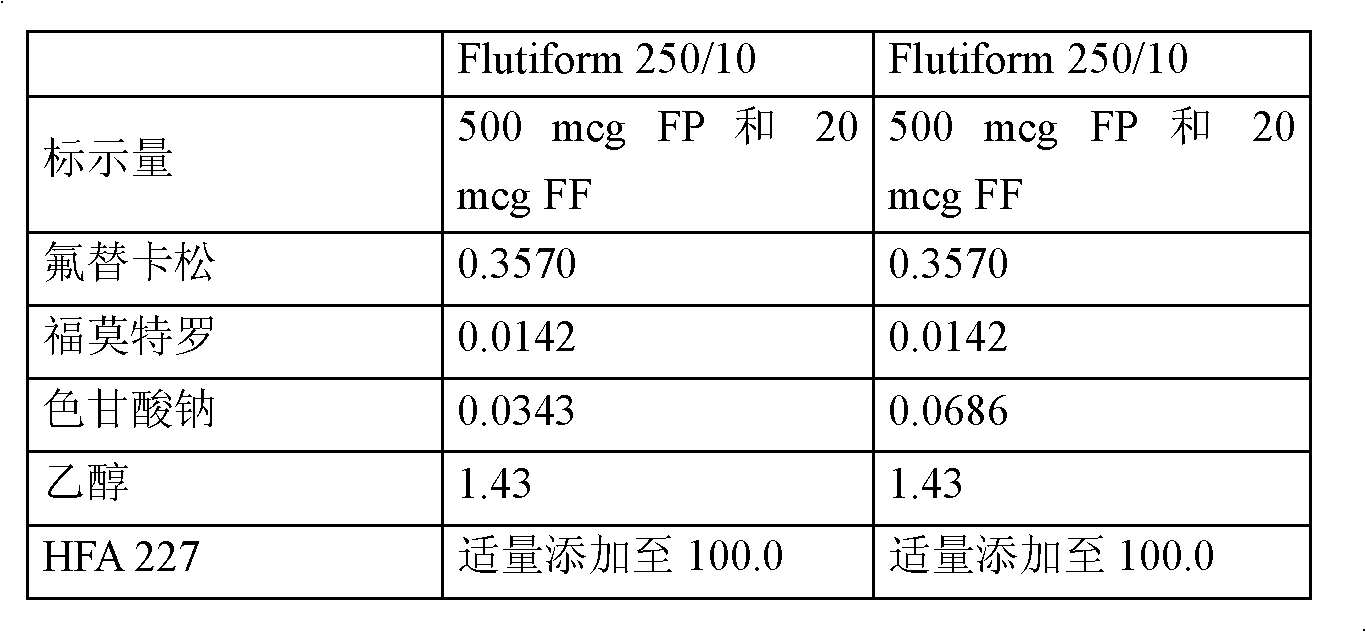
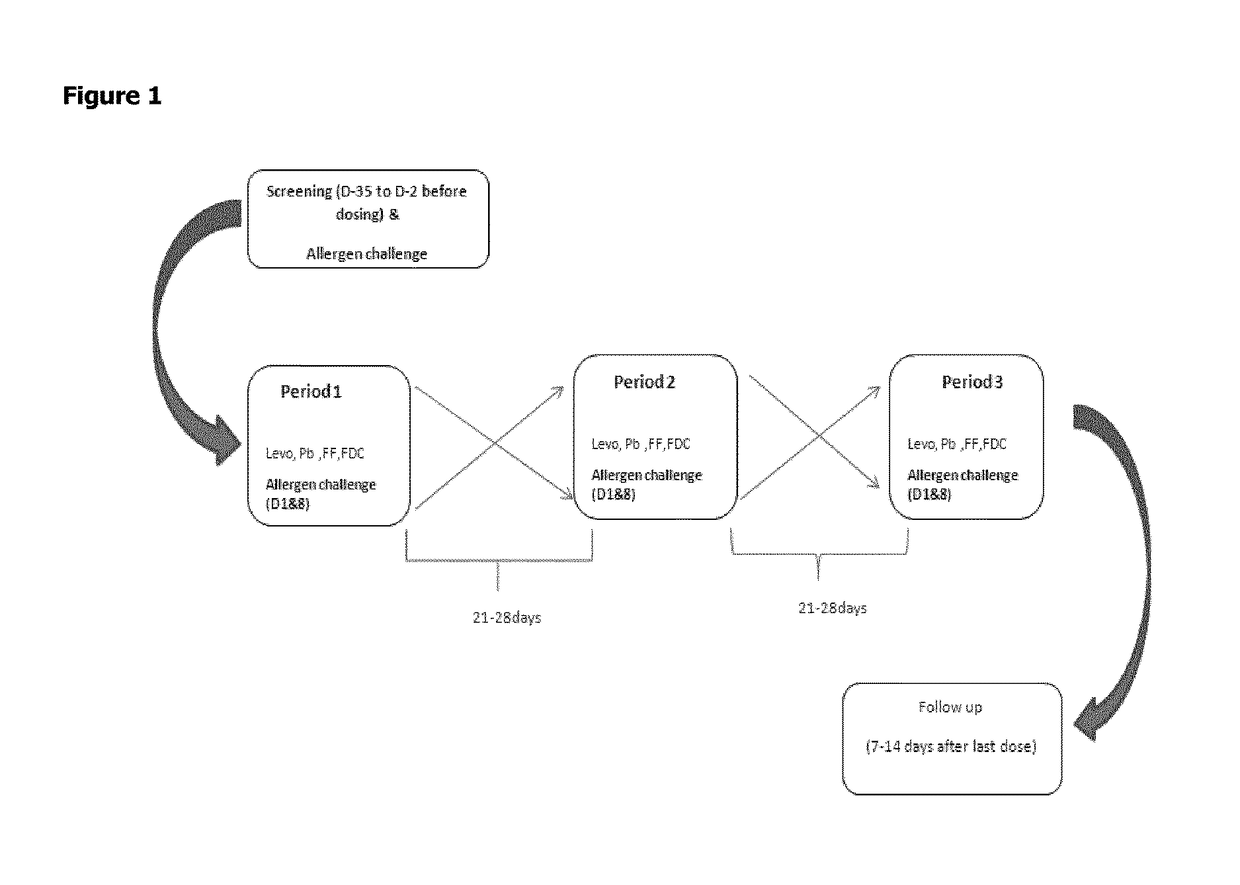
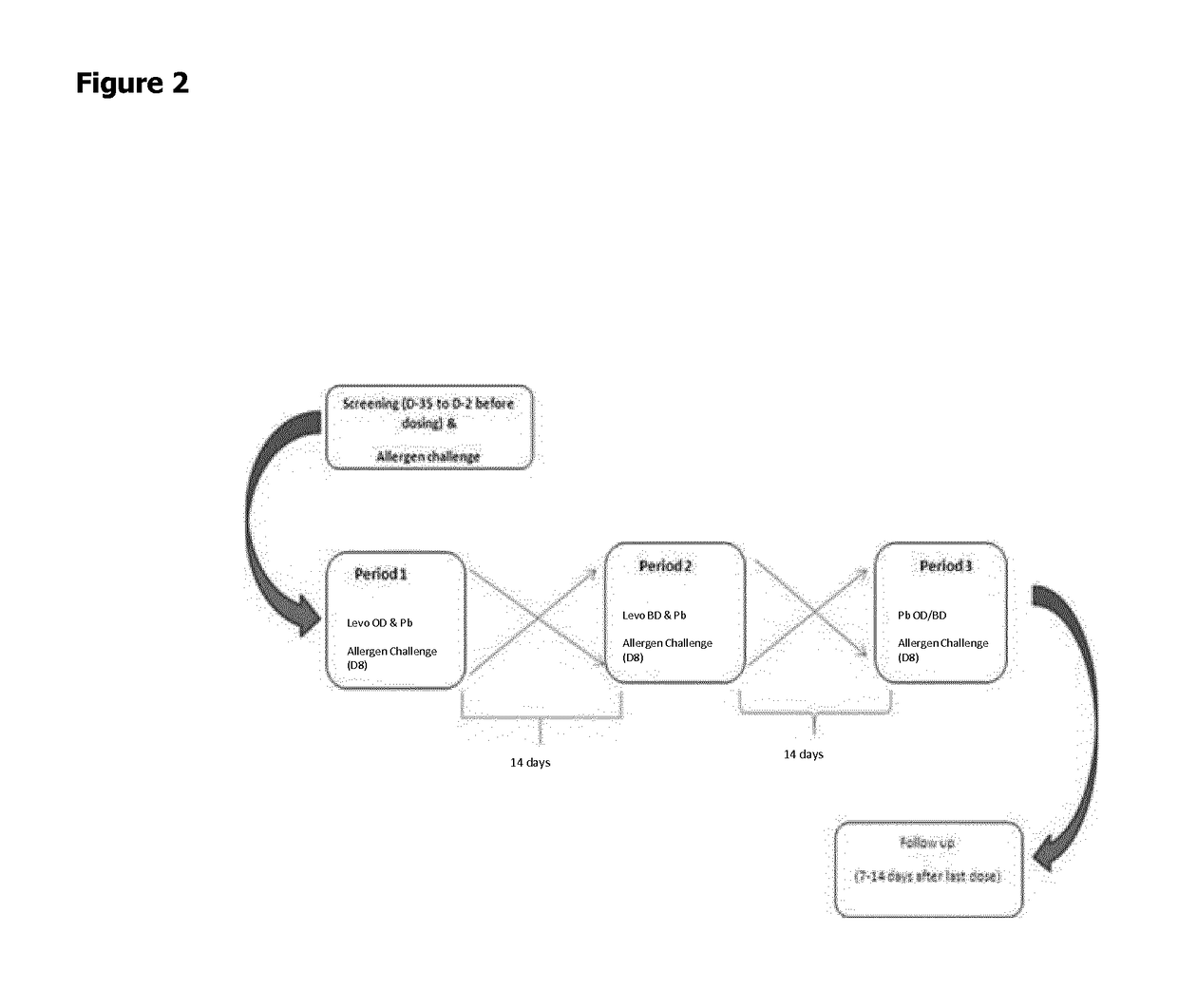
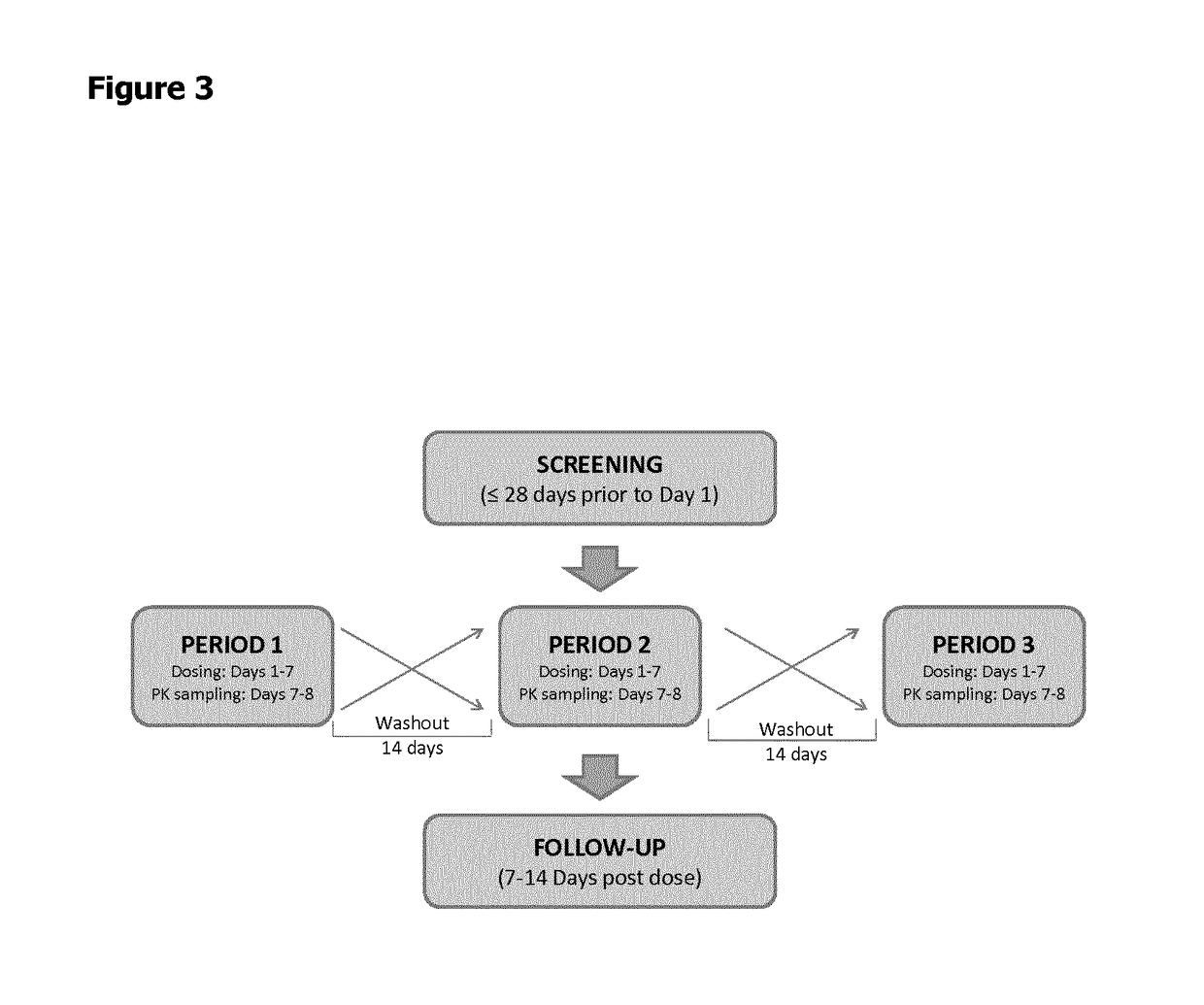
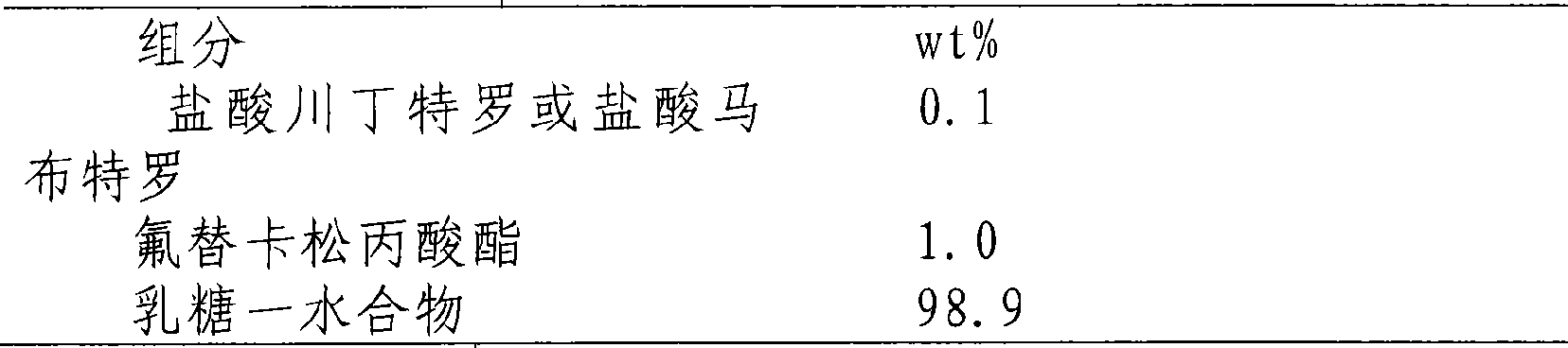
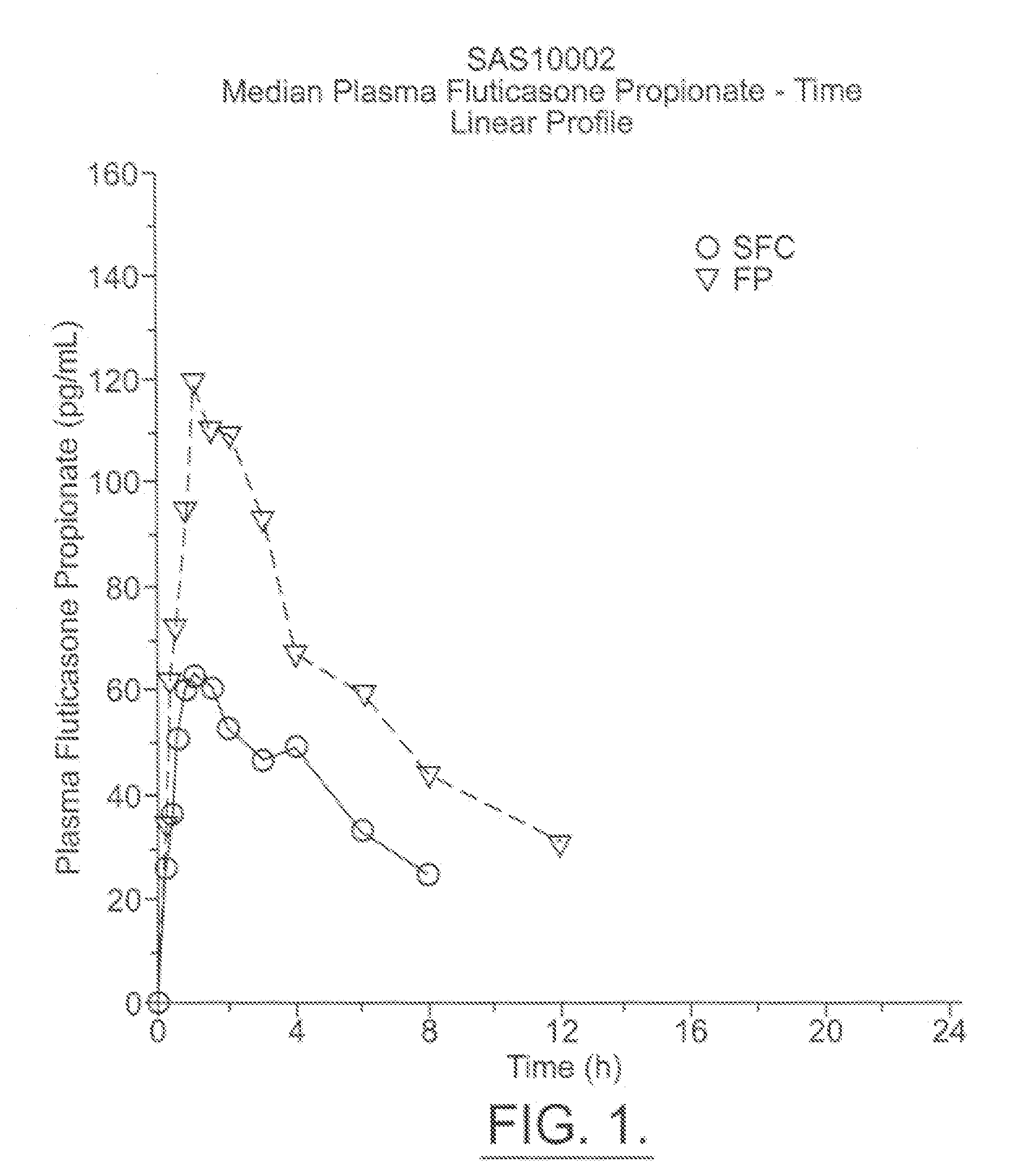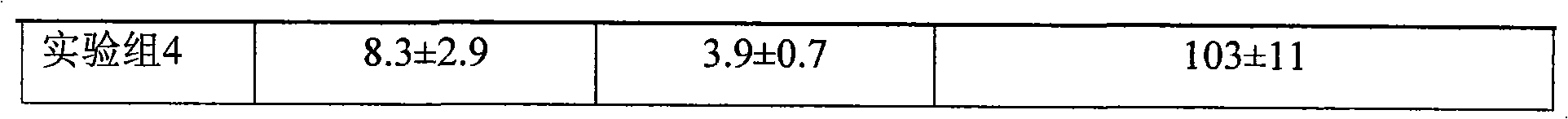Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Fluticasone propionate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
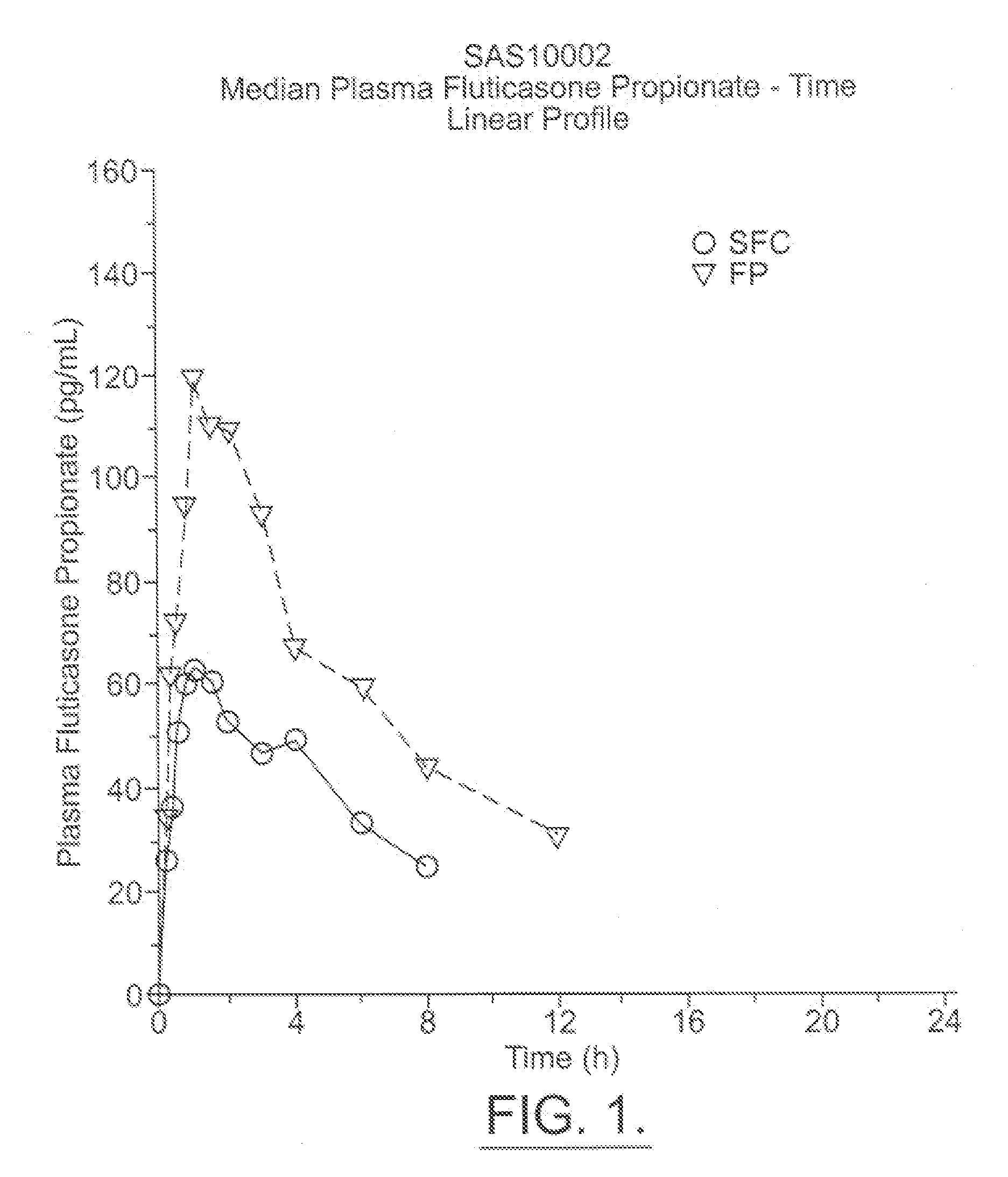
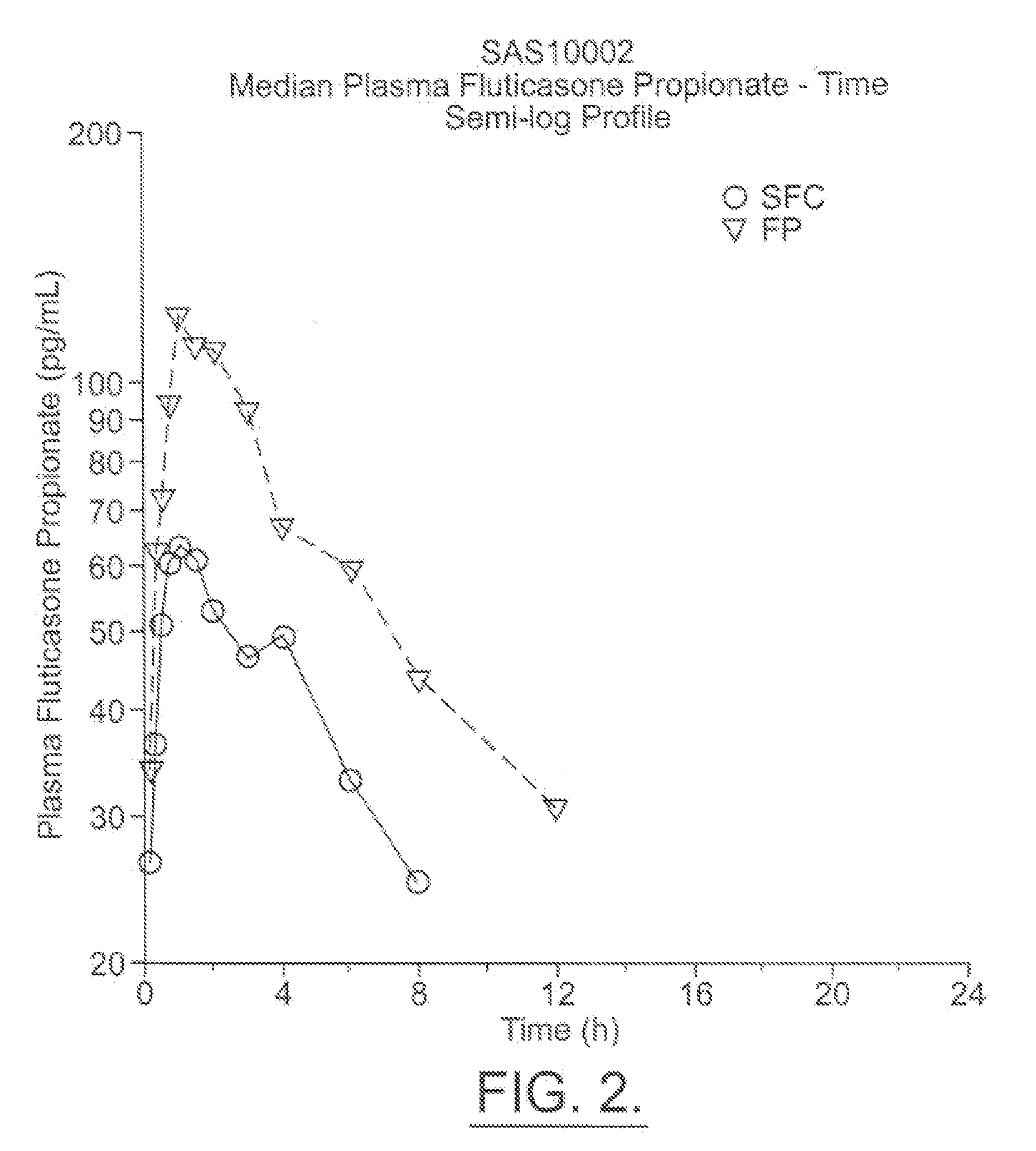
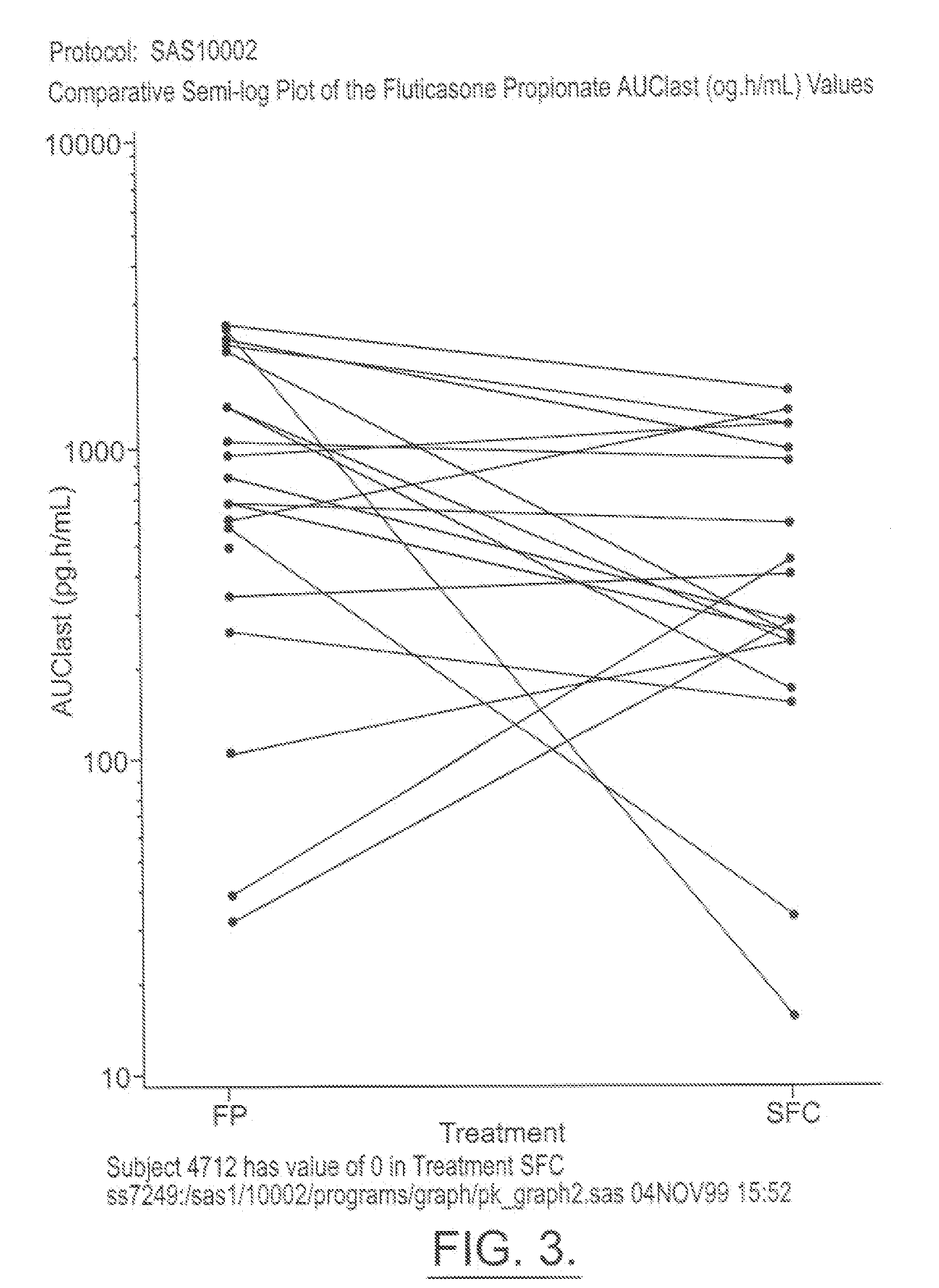
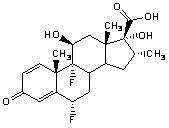
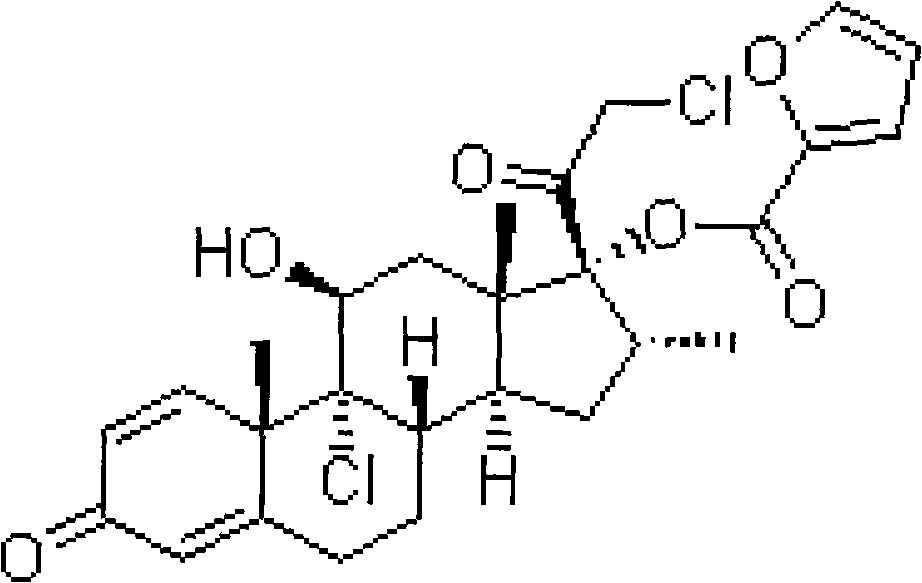
Fluticasone propionate, sold under the brand names Flovent and Flonase among others, is a steroid medication. When inhaled it is used for the long term management of asthma and COPD. In the nose it is used for hay fever and nasal polyps.
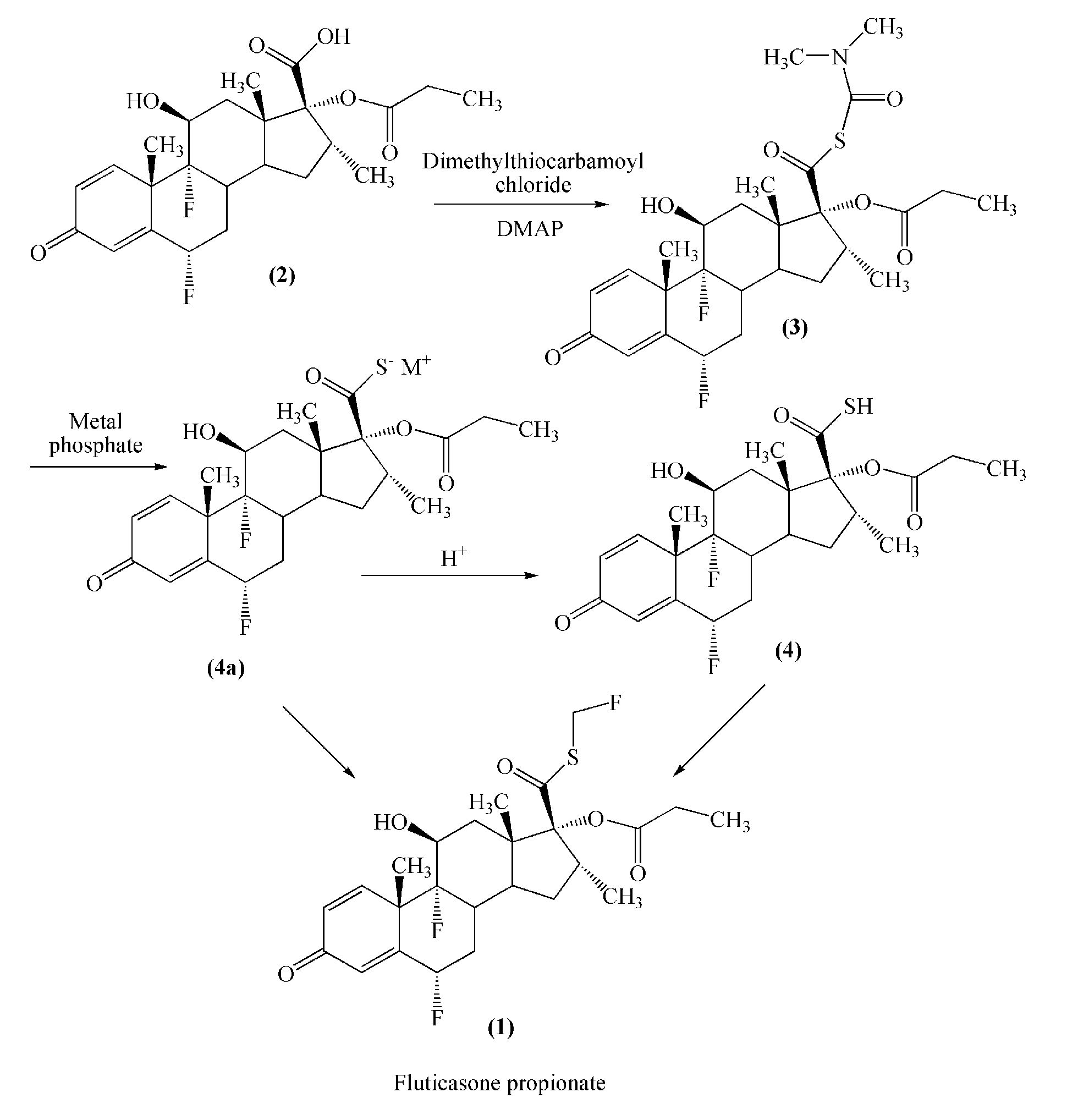
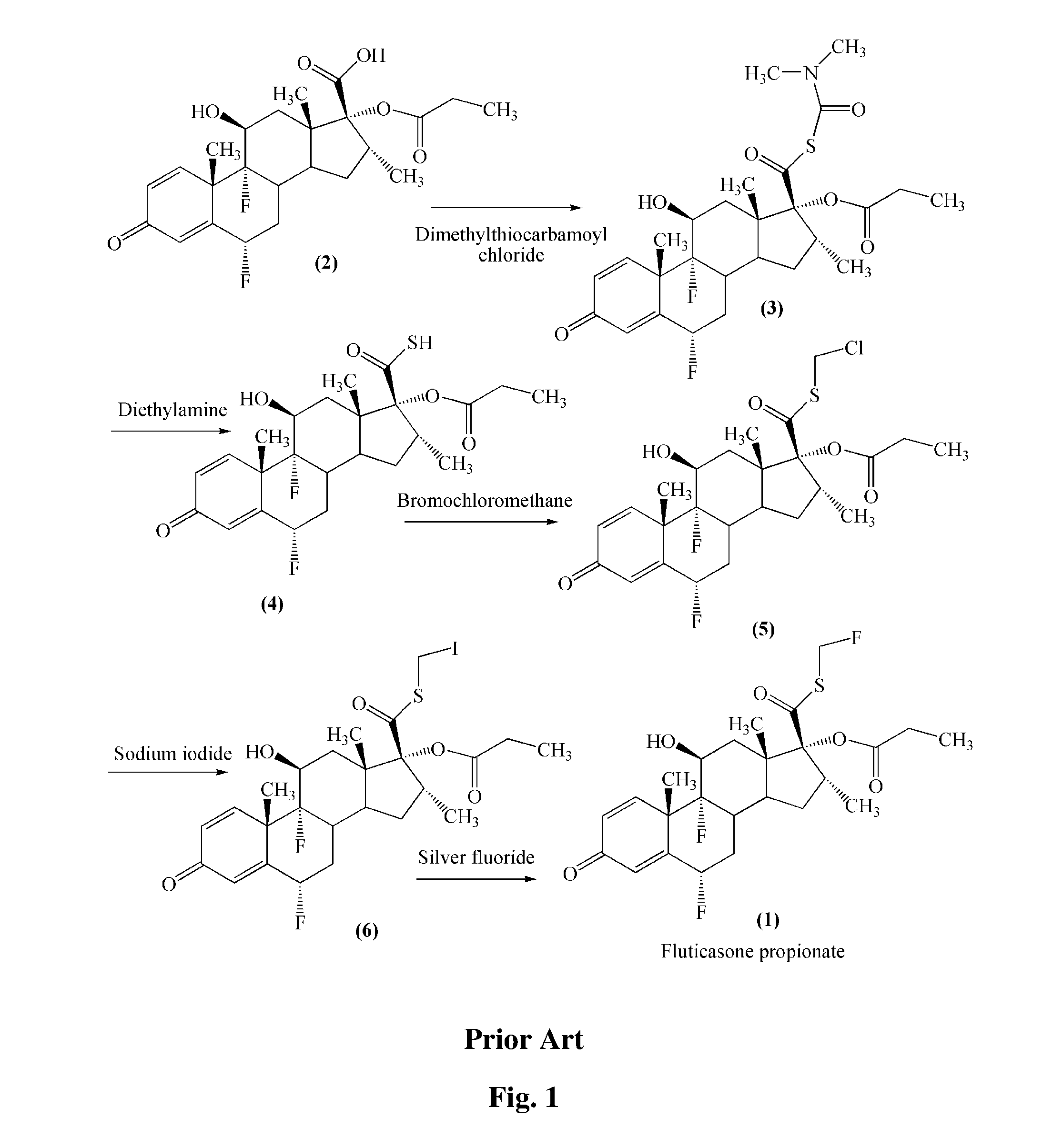
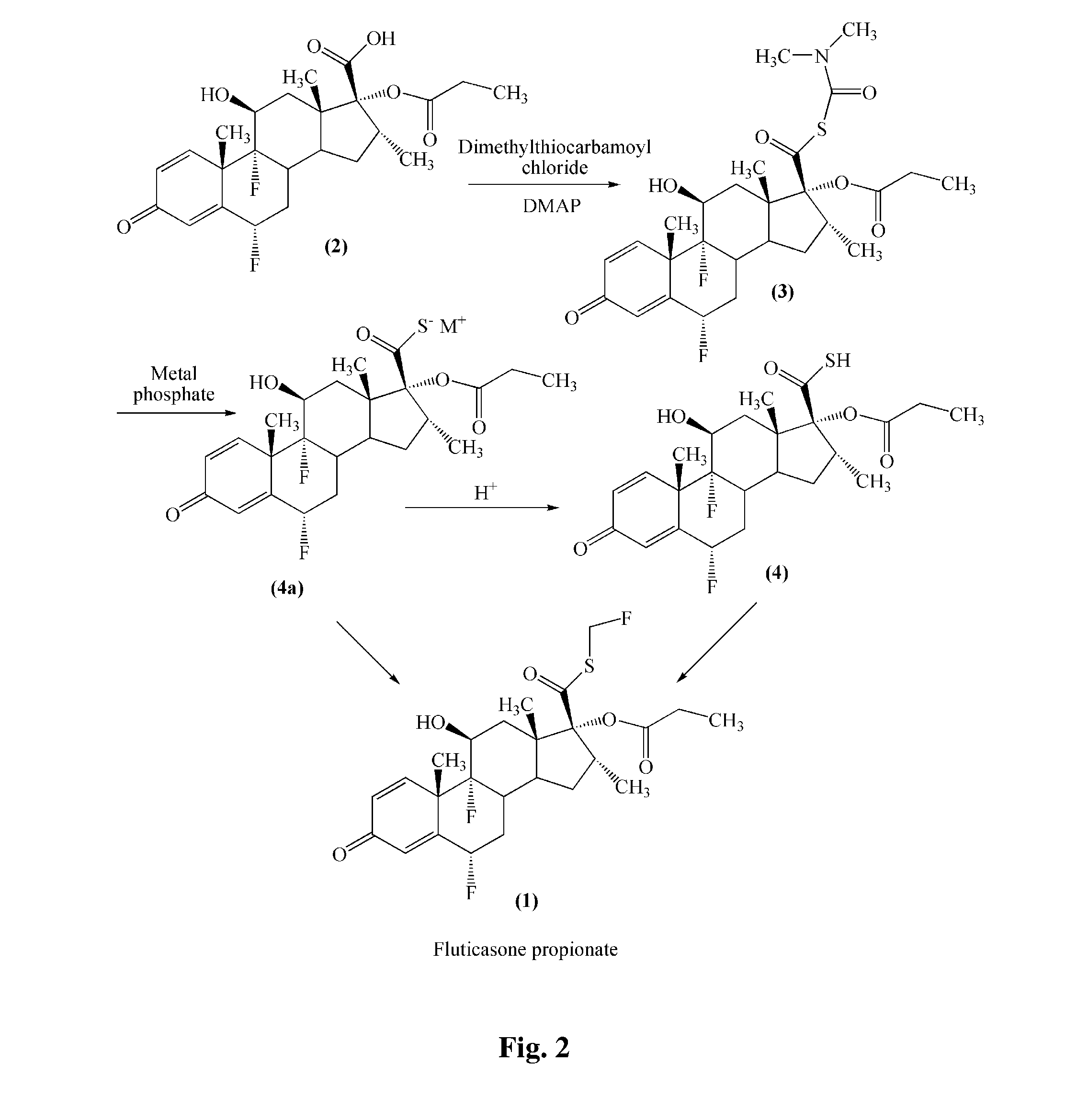
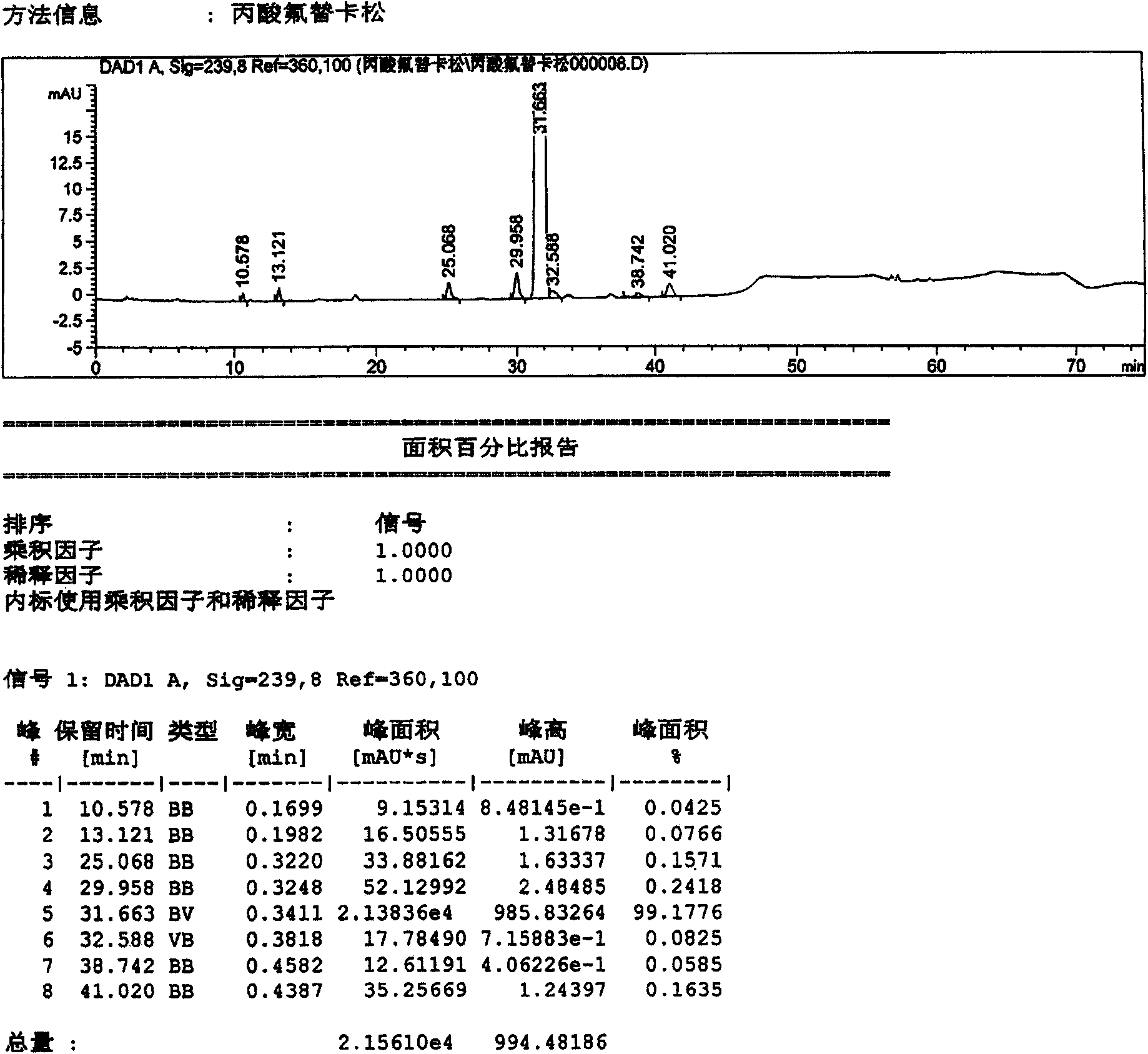
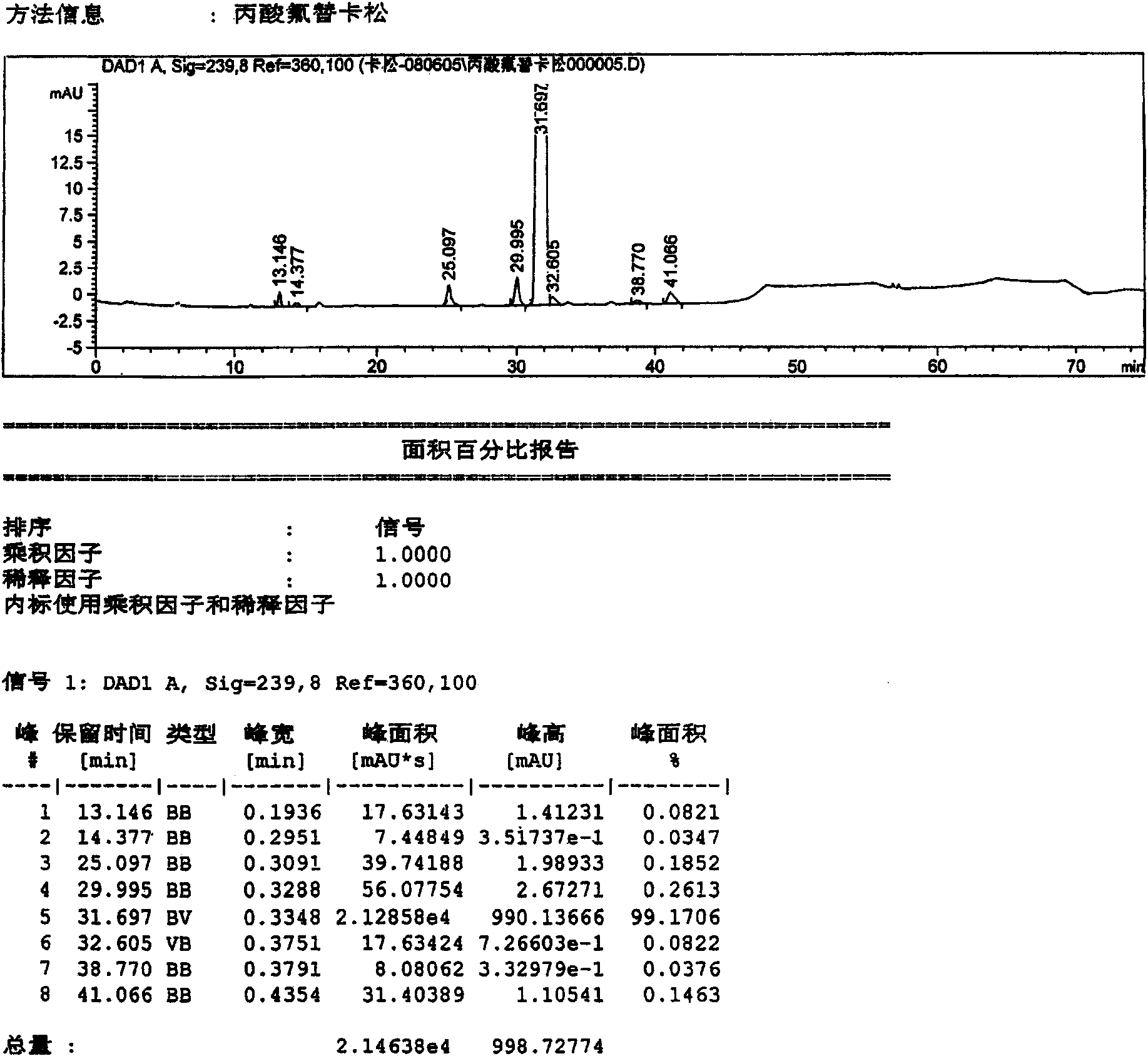
Method for preparation of fluticasone propionate
InactiveUS20080125407A1Simple and efficient and economical methodSimple and efficient and economicalOrganic active ingredientsSteroidsPresent methodFluticasone propionate
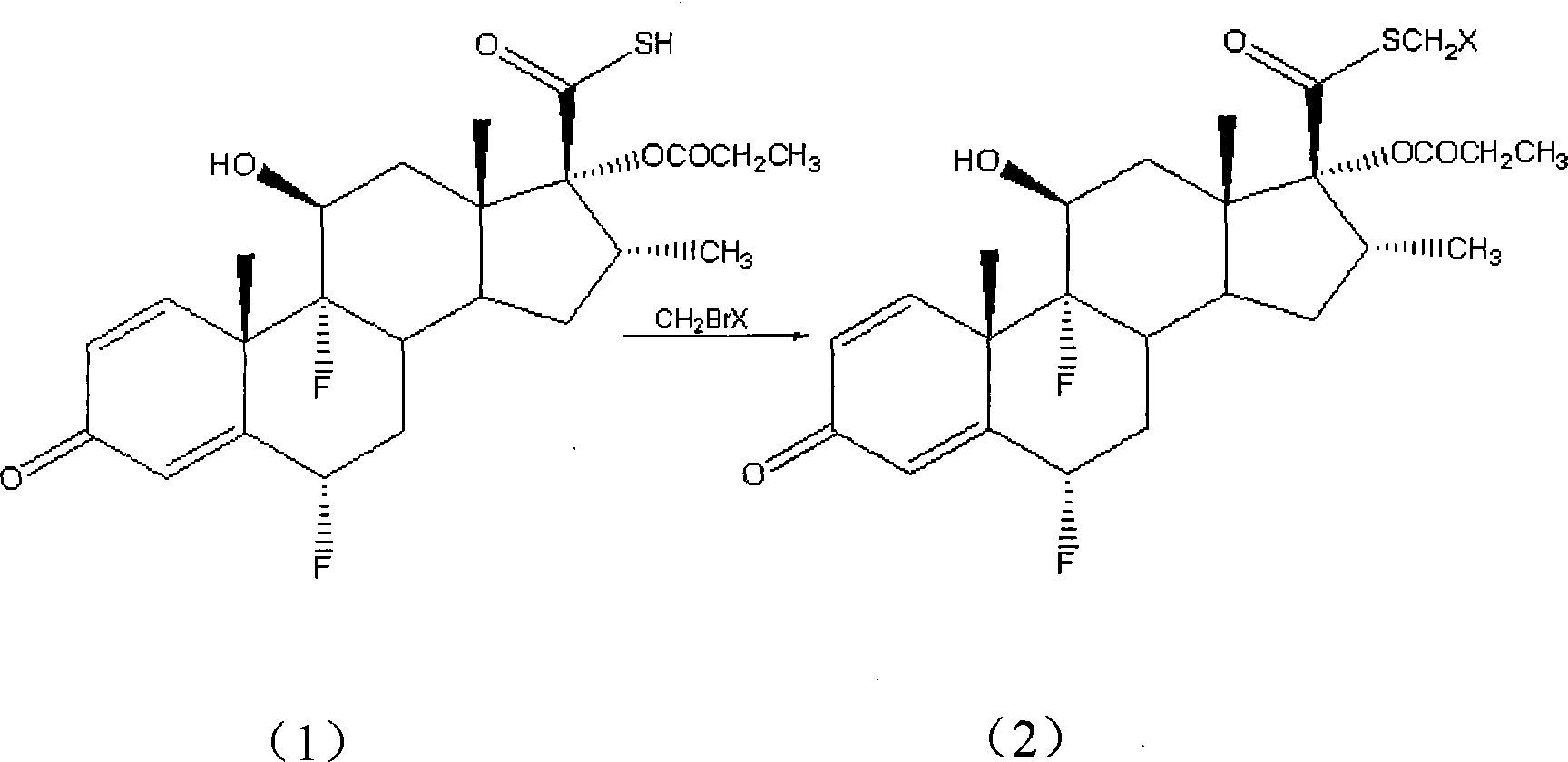
Taught is a method for preparing S-fluoromethyl-6α,9α-difluoro-11β-hydroxy-16α-methyl-17α-propionyloxy-3-oxoandrosta-1,4-diene-17β-carbothioate (fluticasone propionate). The present method is simple, convenient, and mild, yields highly pure product, and is suitable for use commercially on a large scale.
Owner:SHANGHAI AURISCO INT TRADING
Synthesis and powder preparation of fluticasone propionate
InactiveUS20060009435A1High yieldEfficient preparationPowder deliveryOrganic active ingredientsFluticasone propionateMedicine
An improved process for preparing fluticasone propionate, performed in the presence of water, is disclosed. Further disclosed is a process for preparing a fluticasone propionate that is highly suitable for administration by inhalation. Further disclosed are fluticasone propionate and a powdered fluticasone propionate prepared by these processes and pharmaceutical compositions for administration by inhalation containing same. A process of purifying a key intermediate in the synthesis of fluticasone propionate is also disclosed.
Owner:CHEMAGIS
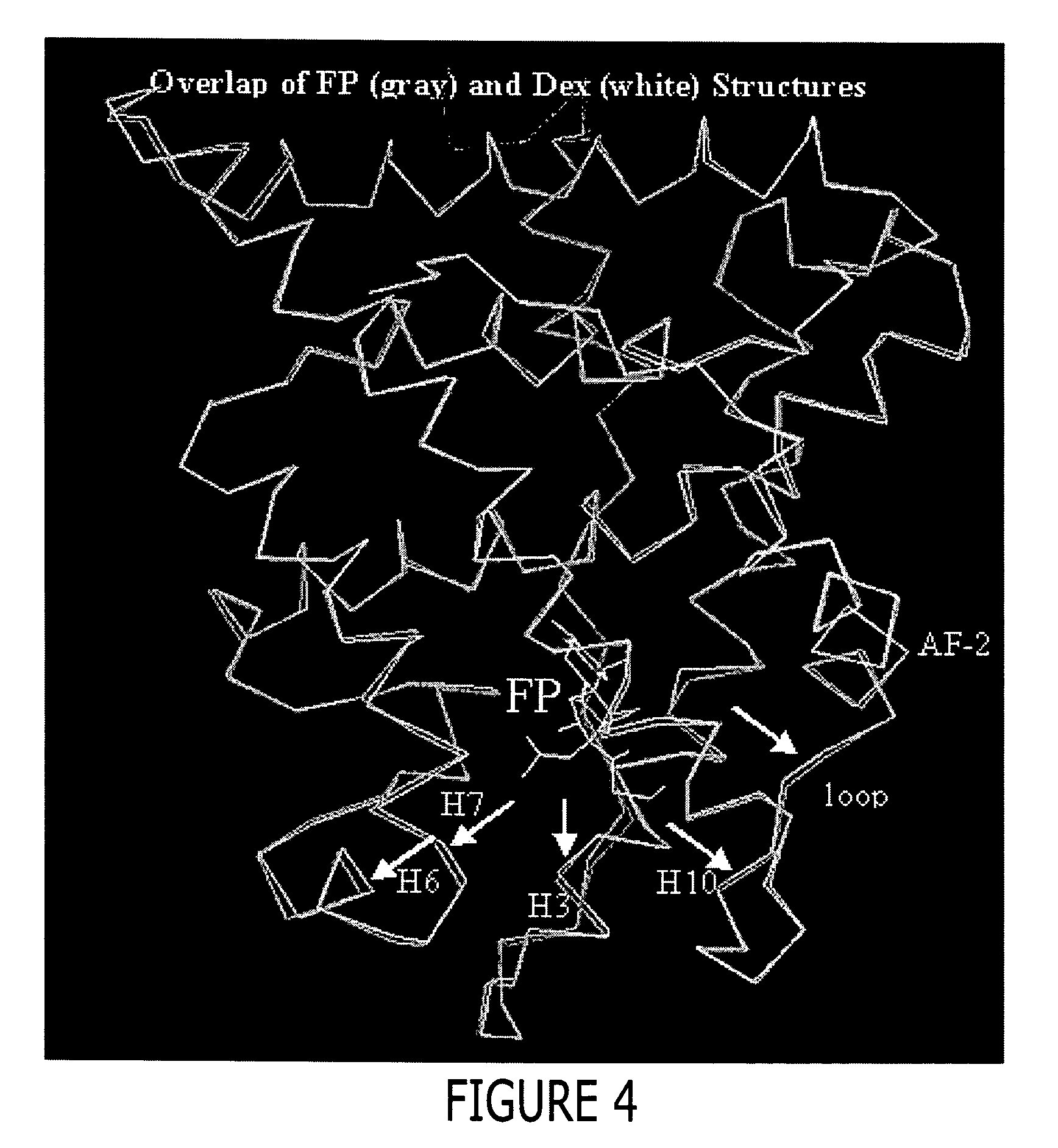
Structure of a glucocorticoid receptor ligand binding domain comprising an expanded binding pocket and methods employing same
InactiveUS20070020684A1Peptide/protein ingredientsBiological material analysisFluticasone propionatePR - Progesterone receptor
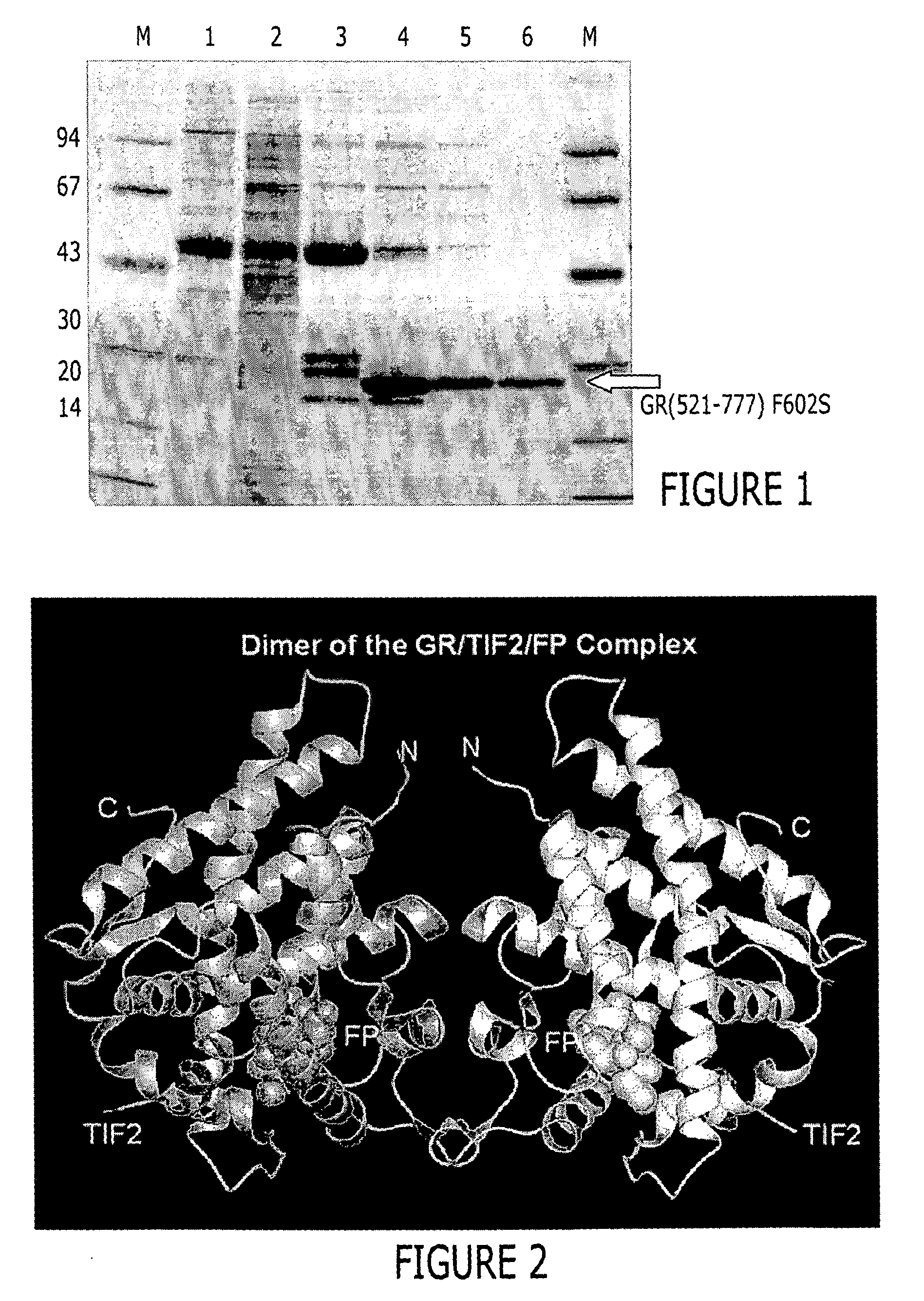
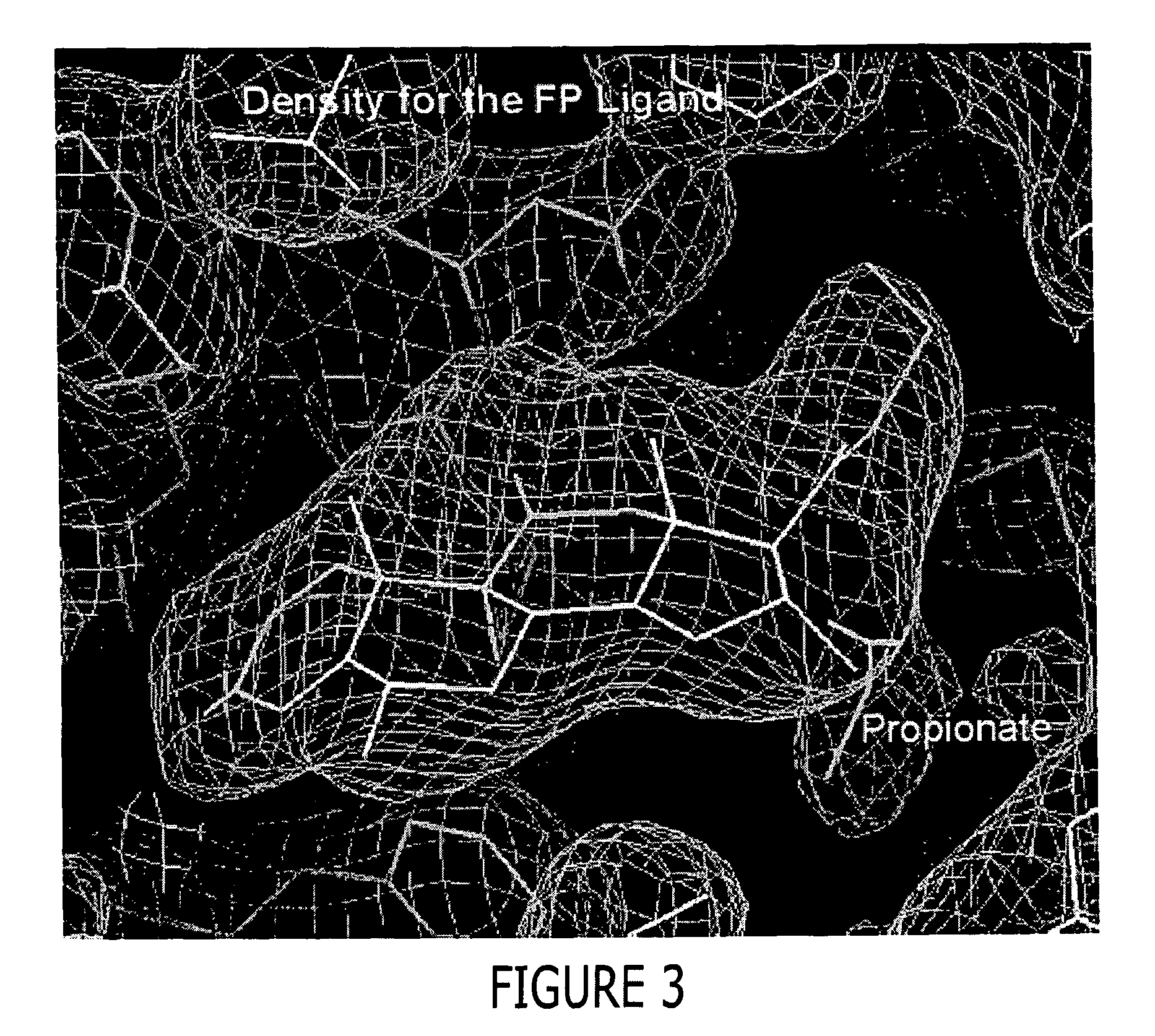
A solved three-dimensional crystal structure of a glucocorticord receptor (GR) α ligand binding domain polypeptide is disclosed, in the form of a crystalline glucocorticord receptor α ligand binding domain polypeptide in complex with the ligand fluticasone propionate (FP) and a peptide derived from the co-activator TIF2. The GR / FP / TIF2 structure includes an expanded binding pocket not seen in other GR structures. Methods of designing steroid and non-steroid modulators of the biological activity of GR and other nuclear receptors (NRs) are also disclosed. In another aspect of the present invention homology models of androgen receptor (AR), progesterone receptor (PR) and mineralcorticoid receptor (MR) are disclosed, as well as methods of forming homology models for other NRs. Methods of forming a soluble GR / FP / TIF2 complex are also disclosed.
Owner:SMITHKLINE BECKMAN CORP
Nasal pharmaceutical formulations and methods of using the same
InactiveUS20060051299A1Improve bioavailabilityGood curative effectPowder deliveryOrganic active ingredientsFluticasone propionateCurative effect
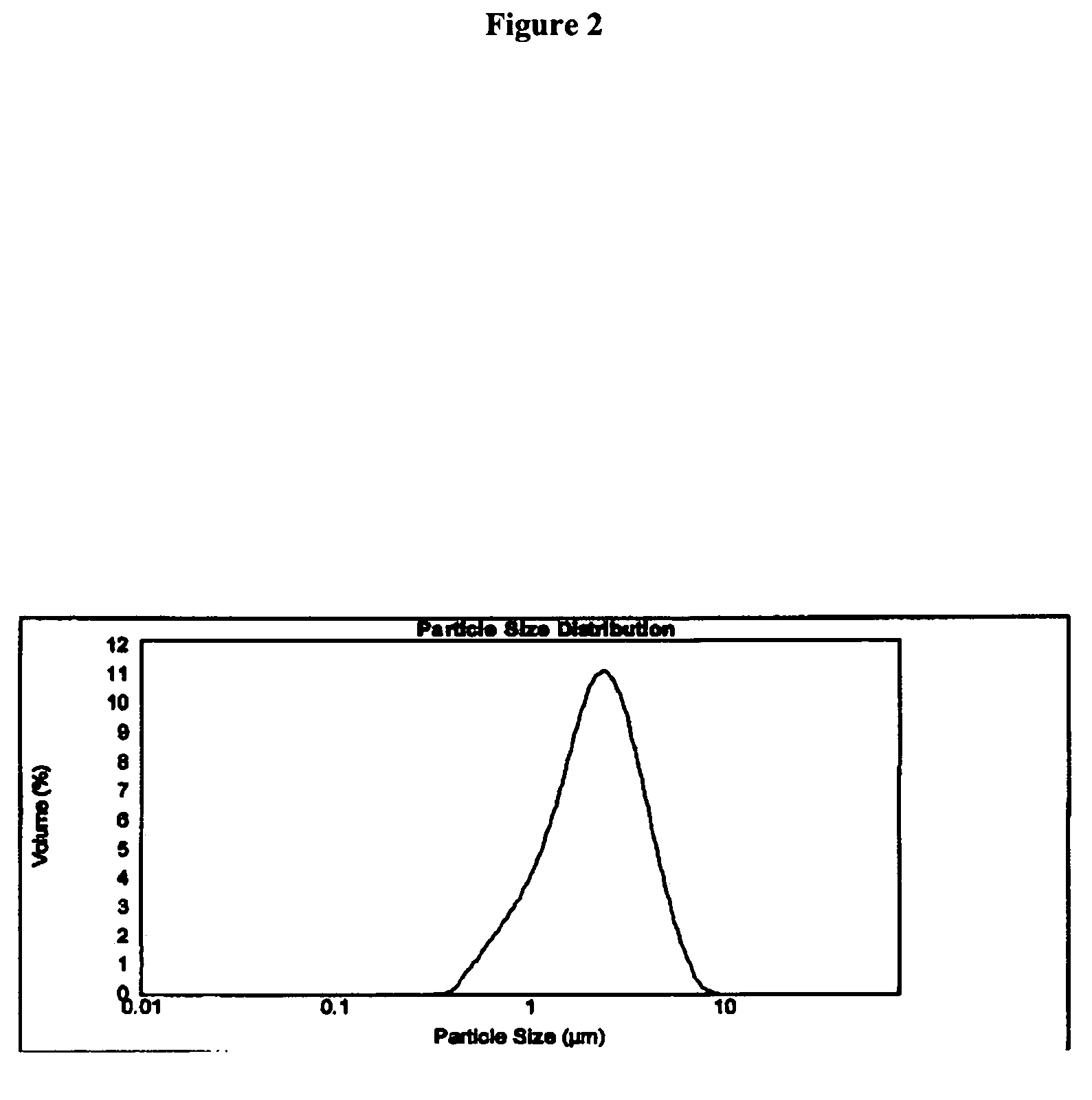
Nasal pharmaceutical formulations comprising a drug substance having a specific particle size distribution profile are disclosed herein. Such profile provides increased bioavailability, increased efficacy or prolonged therapeutic effect of the drug substance when administered intranasally. The formulations of the present invention may comprise one or more corticosteroids having a specific particle size distribution profile. In a preferred embodiment, the corticosteroid is fluticasone or a pharmaceutically acceptable derivative thereof for the treatment of one or more symptoms of rhinitis. Preferably, the drug substance is fluticasone propionate. The formulations herein may be provided as an aqueous suspension suitable for inhalation via the intranasal route.
Owner:MYLAN SPECIALTY
Fluticasone lotion having improved vasoconstrictor activity
InactiveUS7300669B2Cosmetic preparationsOrganic active ingredientsFluticasone propionateSafety profile
A fluticasone lotion having improved vasoconstrictor and anti-inflammatory activity and higher than expected potency. The fluticasone lotion contains 0.05 weight percent fluticasone propionate and an oil-in-water vehicle that includes excipients. The fluticasone lotion is unexpectedly efficacious while exhibiting an improved safety profile.
Owner:FOUGERA PHARM INC
Inhalation Drug Combinations
InactiveUS20070122351A1Less systemic exposureEliminate side effectsPowder deliveryBiocideDiseaseFluticasone propionate
A method for treating respiratory disorders by administrating by inhalation an effective amount of a β2-receptor agonist, an acceptable amount of a corticosteriod, and HFA 134a, to a patient in need thereof, is disclosed. Preferably, the β2-receptor agonist is salmeterol or a physiologically acceptable salt thereof, and the corticosteriod is fluticasone propionate or a solvate thereof. The combination of salmeterol, fluticasone proprionate, and HFA 134a may lower the risk of cardiac arrhythmias, sudden death, or hypercorticism that are sometimes associated with the simultaneous administration of a β2-receptor agonist and an anti-inflammatory corticosteroid.
Owner:GLAXO GROUP LTD
Formulations and methods for treating rhinosinusitis
ActiveUS20050180925A1Lower Level RequirementsInhibit deteriorationAntibacterial agentsPowder deliverySinusitisFluticasone propionate
The invention involves methods and formulations for treating or preventing rhinosinusitis, including but not limited to, bacterial-induced, viral-induced and / or fungus-induced rhinosinusitis in mammals, and / or rhinosinusitis not induced by an infective agent, such as bacteria, fungus or virus. In one embodiment, the formulation of the present invention comprises an anti-inflammatory agent (e.g. fluticasone propionate) having a specific particle size distribution profile. The formulation may also comprise an antifungal agent, antibiotic or antiviral agent.
Owner:MYLAN SPECIALTY
Medicinal Cream Made Using Fluticasone Propionate And Chitosan And A Process To Make The Same
InactiveUS20120028943A1Antibacterial agentsOrganic active ingredientsFluticasone propionateBiopolymer
The present invention relates to a composition for treating skin inflammation, along with skin rejuvenation. More particularly, the present invention relates to a pharmaceutical cream comprising a biopolymer, and a corticosteroid. It discloses a composition for treating skin inflammation, along with skin rejuvenation containing a) a biopolymer in the form of chitosan, b) an active pharmaceutical ingredient (API) composition in the form of fluticasone propionate, used in treating skin inflammation c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The active ingredients, namely chitosan, and a corticosteroid in the form of fluticasone propionate, are incorporated in cream base for use in treating skin inflammation due to allergy & itching & wounds on human skin involving contacting human skin with the above identified composition.
Owner:APEX LAB PRIVATE LTD
Fluticasone propionate lipidosome cream
InactiveCN101601650AHigh encapsulation efficiencyEncapsulationOrganic active ingredientsAerosol deliveryDiseaseFluticasone propionate
The invention relates to a lipidosome medical composition which uses fluticasone propionate as an active component, and a cyst diameter of lipidosome is smaller than 800 nm. The composition comprises 0.025 percent to 0.2 percent of fluticasone propionate as the active component, 0.5 percent to 6 percent of phospholipid, 0 percent to 1 percent of lipophilic additive, 0.01 percent to 1 percent of antioxidant which is used for preserving the medical composition, a pH buffering agent which is used for retaining the pH value from 5 to 7.5, 3 percent to 15 percent of humectant, 20 percent to 30 percent of oil phrase component, 0.01 percent to 0.1 percent of antimicrobial preservative and the balanced of water. The fluticasone propionate lipidosome cream is used for treating skin diseases of human beings or animals.
Owner:TIANJIN JINYAO GRP
Dosage forms containing fluticasone propionate for the treatment of inflammatory conditions of the esophagus
InactiveUS20160213681A1Reduce frequencyImprove clinical efficacyOrganic active ingredientsDispersion deliveryFluticasone propionateInflammation
The present invention describes novel and improved dosage forms containing fluticasone propionate for the treatment of conditions associated with inflammation of the esophagus.
Owner:EMS
Compound pharmaceutical composition of iodine polymer and glucocorticosteroid for treating dermatitis
InactiveCN102379893AGood treatment effectLower doseAntipyreticAnalgesicsFluticasone propionateDrug effect
The invention relates to the field of medicinal preparation and relates to a compound pharmaceutical composition of iodine polymer and glucocorticosteroid for treating dermatitis. The pharmaceutical composition of the present invention comprises 0.01-5% of polyvinylpyrrolidone iodine, 0.01-10% of glucocorticosteroid, 0.001-0.5% of potassium iodate and the balance of pharmaceutically accepted carrier, wherein the glucocorticosteroid is fluticasone propionate or halometasone. Drug effect test has proved that the compound medicament can treat dermatitis by synergism; furtherly, addition of a certain proportion of potassium iodate overcomes unfavorable influences of drug effect decrease or auxiliary effect caused by medicament instability.
Owner:JIANGSU DEDA PHARMA
Inhalation Drug Combinations
InactiveUS20070122352A1Good curative effectReduce systemic side effectsHalogenated hydrocarbon active ingredientsBiocideDiseaseFluticasone propionate
A method for treating respiratory disorders by administrating by inhalation an effective amount of a β2-receptor agonist, an acceptable amount of a corticosteroid, and HFA 134a, to a patient in need thereof, is disclosed. Preferably, the β2-receptor agonist is salmeterol or a physiologically acceptable salt thereof, and the corticosteroid is fluticasone propionate or a solvate thereor. The combination of salmeterol, fluticasone proprionate, and HFA 134a may lower the risk of cardiac arrhythmias, sudden death, or hypercorticism that are sometimes associated with the simultaneous administration of a β2-receptor agonist and an anti-inflammatory corticosteroid.
Owner:GLAXO GROUP LTD
Method for preparing fluticasone propionate
ActiveCN101125875AImprove stabilityAvoid purification difficultiesSteroidsThiocarboxylic acidFluticasone propionate
Owner:湖南玉新药业有限公司
Method for separating and detecting six cortical hormones in skin-care cosmetic
The invention provides a method for separating and detecting six cortical hormones in a skin-care cosmetic. The six cortical hormones, i.e. fluticasone propionate, triamcinolone acetonide, cortisone acetate, dexamethasone, hydrocortisone and prednisone, in the cosmetic are separated and detected by a reverse micro emulsion electrokinetic chromatography (MEEKC) with ionic liquid and beta-cyclodextrin as additives. Compared with a conventional MEEKC separation and detection method, the method has higher separation degree. An experiment result shows that the method is simple in sample treatment, low in using amount, economic, high in speed, and high in separation degree and sensitivity. Capillary electrophoretic detection on the cortical hormones in the cosmetic is realized by the method.
Owner:JIANGNAN UNIV
Pharmaceutical formulation of fluticasone propionate
InactiveUS7220403B2Stability advantageMitigate suchBiocideOrganic active ingredientsFluticasone propionateDrug aerosol
There is provided according to the invention a pharmaceutical aerosol formulation which comprises:(i) fluticasone propionate and(ii) a hydrofluoroalkane (HFA) propellant,characterised in that the fluticasone propionate is completely dissolved in the formulation. The invention also provided canisters containing the formulation and uses thereof.
Owner:GLAXO GROUP LTD
Improved formulations
ActiveCN102573791AEnsure consistencyHigh Aerodynamic Particle Size DistributionDispersion deliveryHydroxy compound active ingredientsFluticasone propionateMedicine
In a metered dose inhaler, comprising a canister and metering valve, containing a suspension aerosol formulation comprising particles of formoterol fumarate dihydrate and fluticasone propionate suspended in an HFA propellant, a method of reducing deposition of particles on the surfaces of the canister and the metering valve, the method comprising the step of adding a wetting agent to the formulation.
Owner:JAGOTEC AG
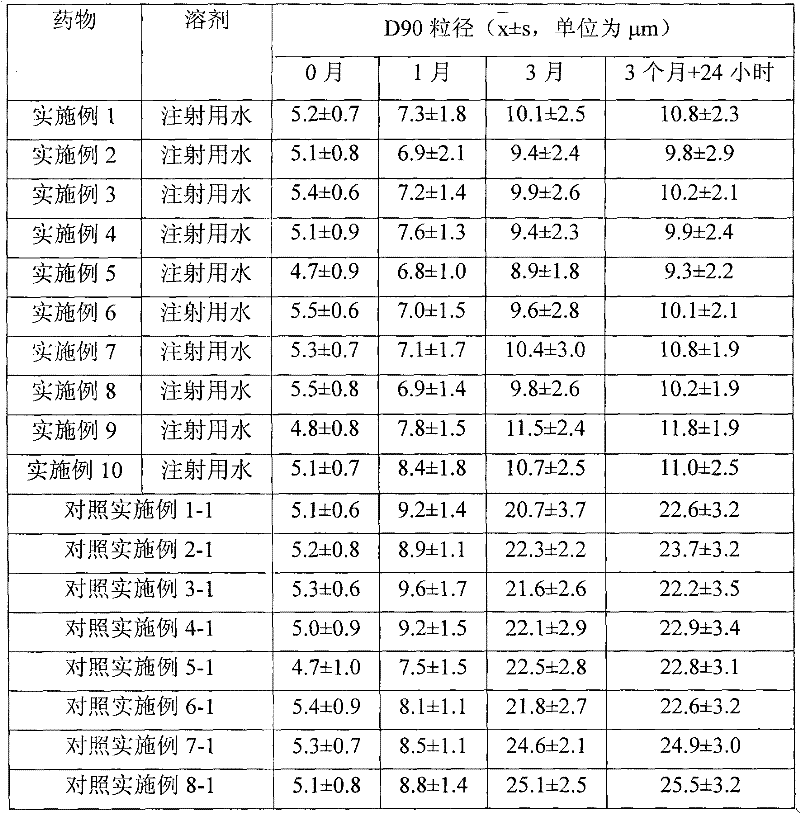
Preparation method of fluticasone propionate particles and applications of the particle
ActiveCN103588846AReduce manufacturing costReduce energy consumptionOrganic active ingredientsAerosol deliveryFluticasone propionateMicroparticle
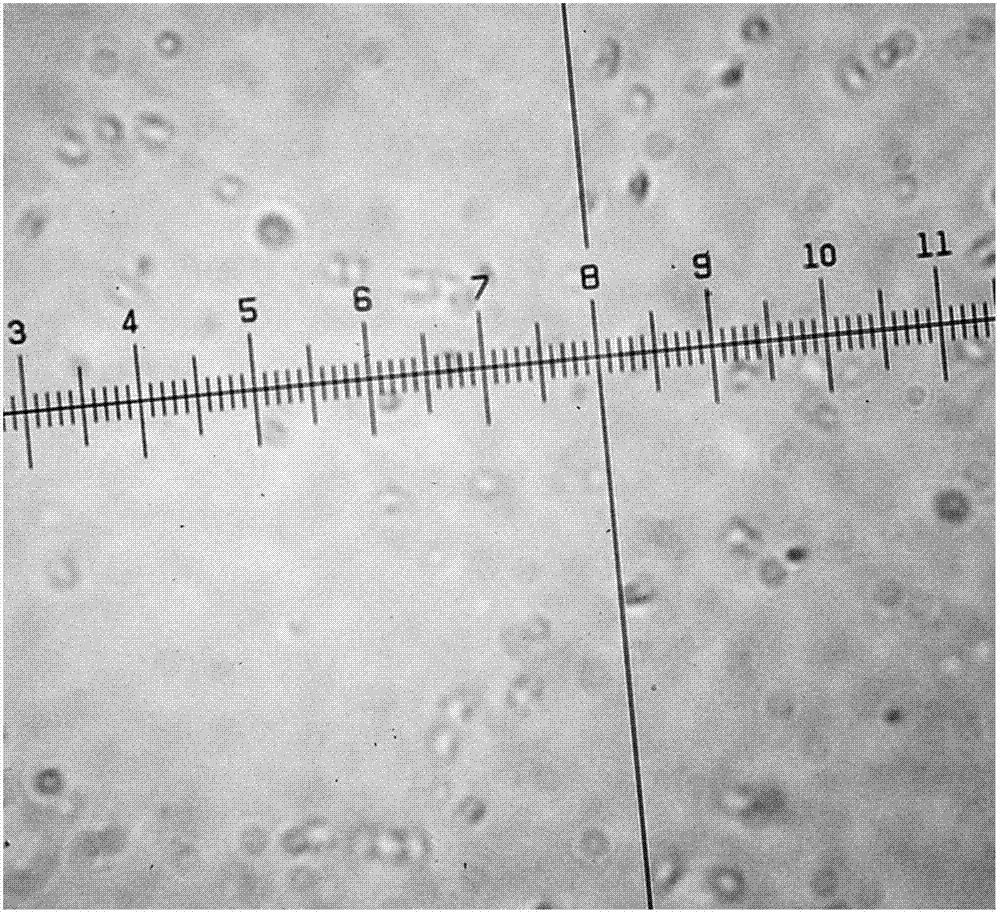
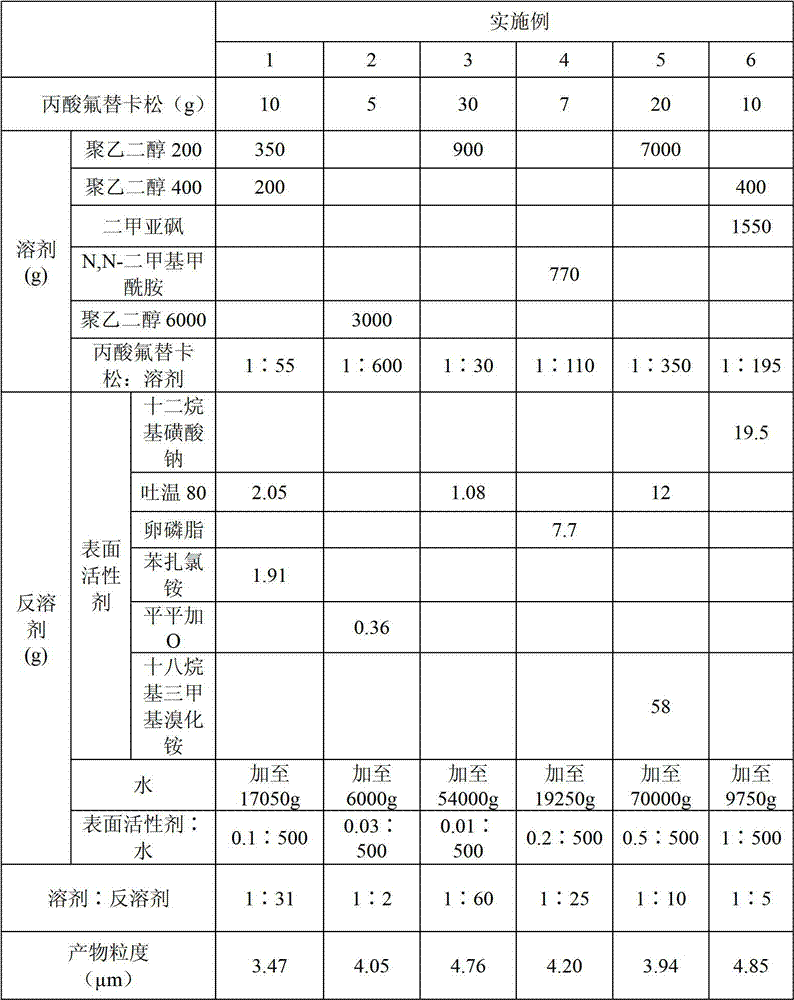
The invention discloses a preparation method of fluticasone propionate particles having a D98 particle size smaller than 5 [mu]m. By utilization of a recrystallization method, a fluticasone propionate raw material is dissolved in a solvent to prepare a solution, and the solution is added into water containing a surfactant to precipitate the fluticasone propionate particles having the needed particle size. Compared with traditional methods, the preparation method provided by the invention has high yield, low production cost, no dependence on special equipment, and low energy consumption. The preparation method provided by the invention is free from duct generation. Reagents used in the preparation method are nontoxic, harmless and environmental friendly. Products have small particle sizes, narrow particle size distribution, high roundness and good fluidity, and are particularly suitable to be used as raw materials for medicinal preparations.
Owner:CHONGQING HUAPONT PHARMA
Highly pure fluticasone propionate preparation method
InactiveCN104004044AHigh purityThe number of impurities is smallSteroidsFluticasone propionateMethyl group
The invention discloses a highly pure S-fluoromethyl-6alpha,9alpha-difluoro-11beta-hydroxy-16alpha-methyl-17alpha-propionooxo-3-androsterone-1,4-diene-17beta-benzothiodiazole (fluticasone propionate) preparation method. The method is characterized in that various highly-pure intermediates of fluticasone propionate are prepared in order to obtain highly pure fluticasone propionate; and post-treatment and purification of the intermediates obtained in each step are carried out in order to obtain the highly pure fluticasone propionate. The method has the advantages of simple operation, high purity and low quantity of impurities, common and cheap reagents, and suitableness for the large-scale industrial production.
Owner:WUHAN NUOAN PHARMACY
Novel crystalline forms of 6alpha, 9alpha -difluoro-11beta-hydroxy-16alpha-methyl-3-oxo-17alpha-propionyloxy-androsta-1,4-diene 17beta-carboxylic acid and processes for preparation thereof
InactiveUS20060019937A1Maintain stabilitySuitable for useOrganic active ingredientsAntipyreticFluticasone propionateCarboxylic acid
Novel crystalline forms II, III, IV, V, VI, VII and VIII of 6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carboxylic acid, a chemical intermediate useful in the preparation of fluticasone propionate, and novel methods of making these forms, substantially free of water, are disclosed.
Owner:CHEMAGIS
Fluticasone furoate in the treatment of COPD
ActiveUS20170189424A1Reduce probabilityOrganic active ingredientsPowder deliveryFluticasone propionateEosinophil
The present invention relates to pharmaceutical products comprising fluticasone furoate for use in the treatment of COPD patients, particularly a subgroup of COPD patients that through analysis have been identified as possessing an eosinophil blood count of≧150 cells / W. The present invention is further directed to methods for treating a patient with COPD which methods include identifying a patient that will respond to treatment and administering a pharmaceutical product of the present invention comprising fluticasone furoate to said patient.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Fluticasone propionate spraying agent with improved stability
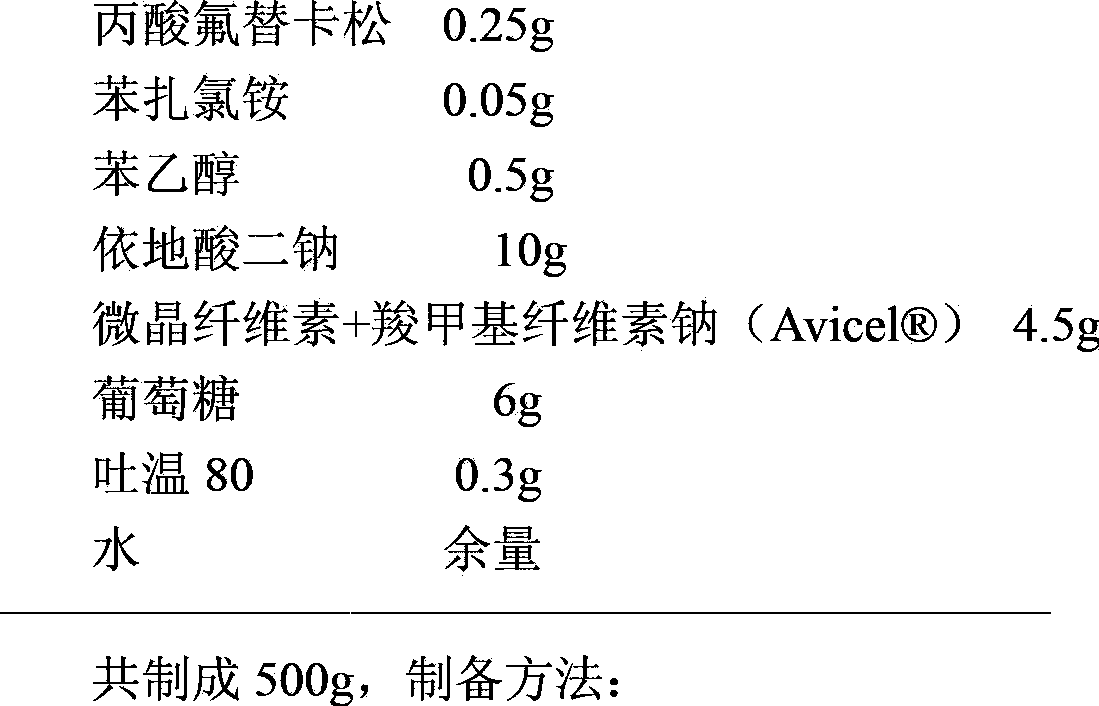
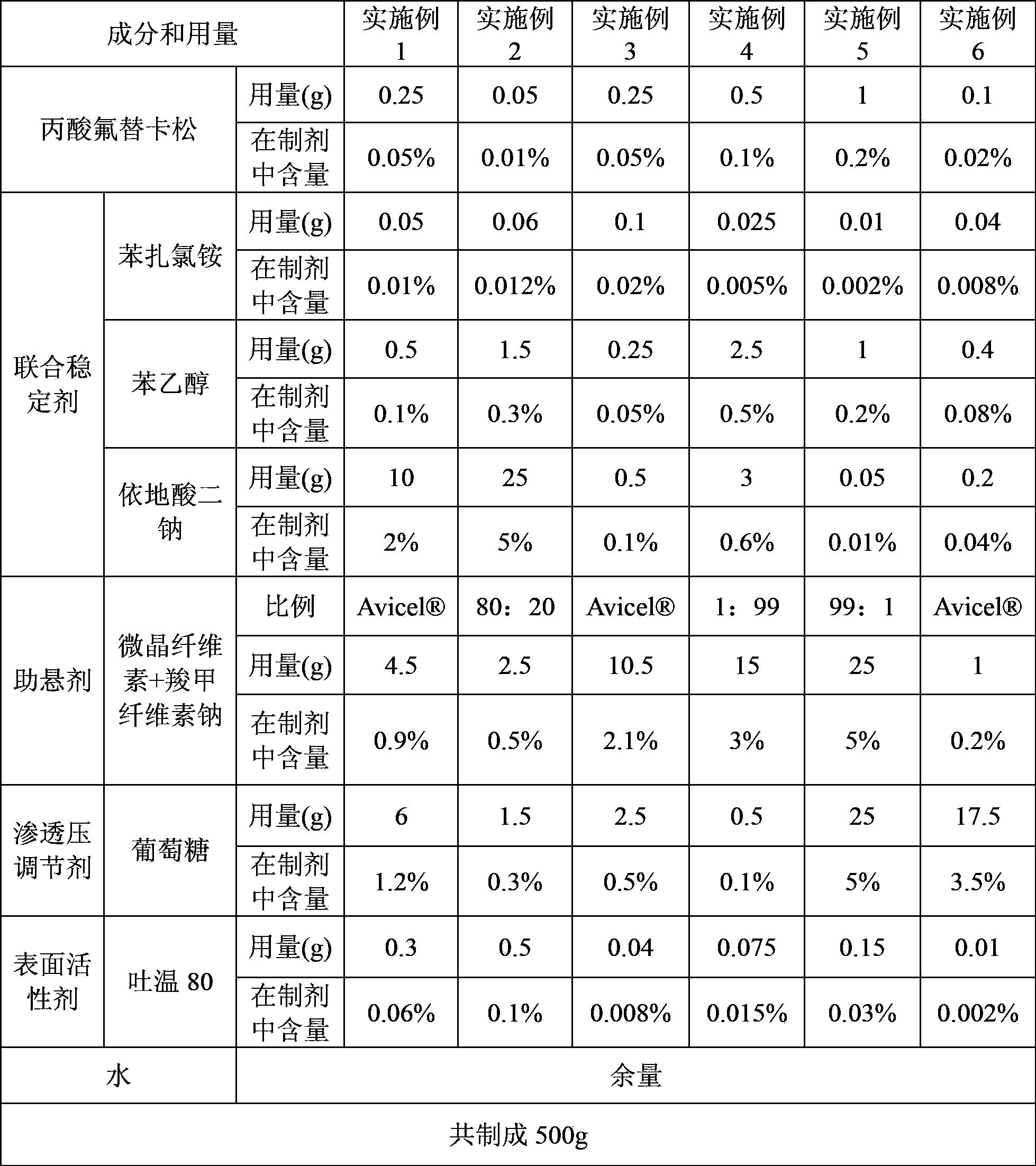
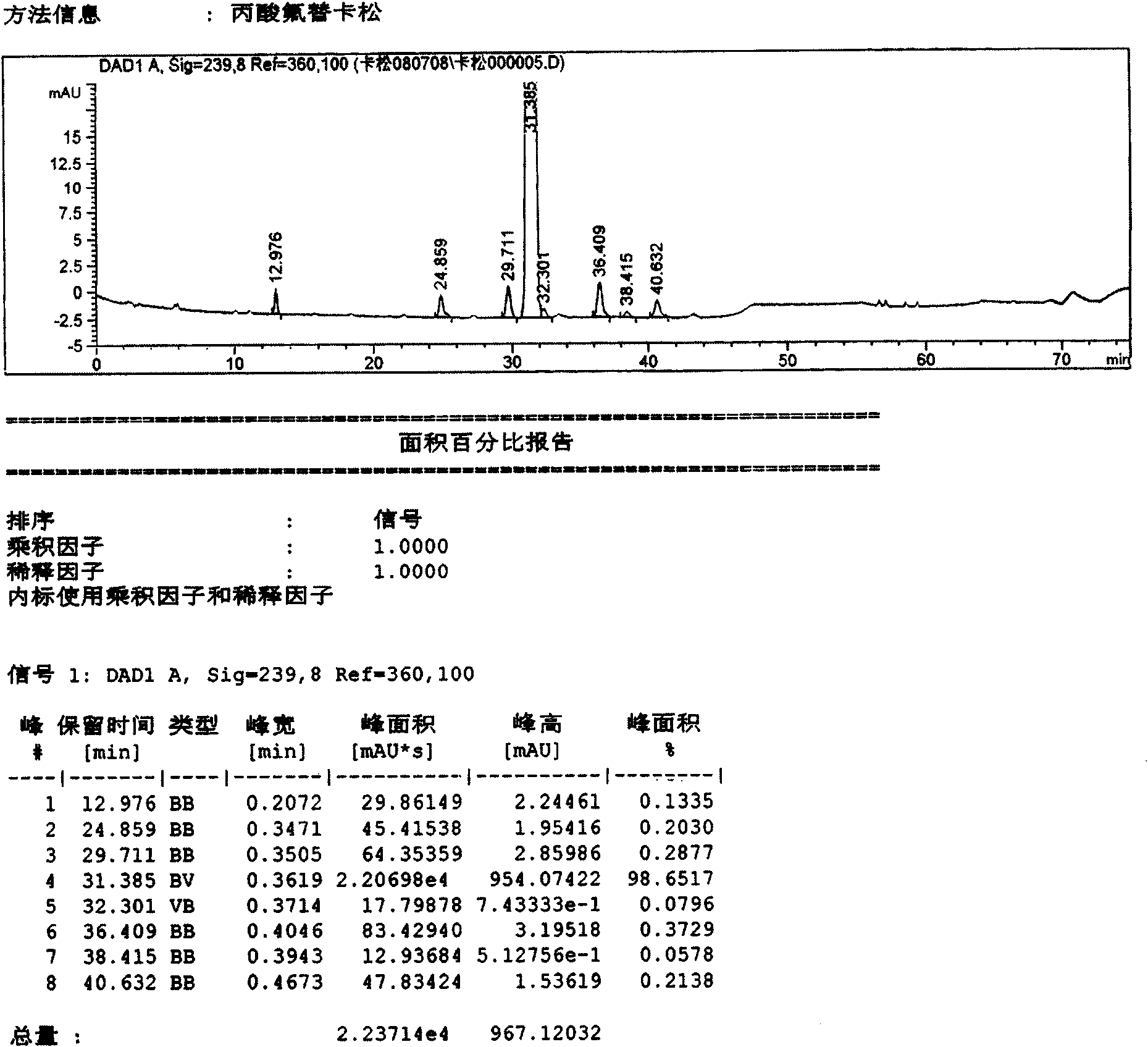
InactiveCN103893120AStable pHSpray pattern is stableOrganic active ingredientsAerosol deliveryFluticasone propionatePreservative
The invention provides a fluticasone propionate spraying agent with improved stability. According to the invention, it is found for the first time that a combined stabilizing agent can be formed by adding disodium edetate into conventional antiseptics--benzalkonium chloride and phenylethyl alcohol, which enables the fluticasone propionate spraying agent to have better stability compared with traditional preparations. After long-term storage, the spraying mode and a droplet distribution scope of the fluticasone propionate spraying agent basically maintain unchanged; the pH value of the preparation is maintained in a proper range without usage of a pH value adjusting agent, and the pH value basically maintains unchanged after long-term storage. The invention further provides a preparation method for the spraying agent.
Owner:CHONGQING HUAPONT PHARMA
Method for recrystallizing and refining fluticasone propionate
ActiveCN101659688ASolve the problem of impuritiesSolve the impurity problemSteroidsRespiratory disorderFluticasone propionateOrganic solvent
The invention provides a method for recrystallizing fluticasone propionate. The method is characterized in that the fluticasone propionate is recrystallized in one or more solvents of alkylene oxide organic solvents and amide organic solvents to solve the impurity problem of the fluticasone propionate, so that related substances of the fluticasone propionate completely accord with the requirementof European Pharmacopoeia 2008 Edition EP6, namely total related substances are 1.2 percent maximally, impurities D and G are 0.3 percent maximally, impurities A, B, C, E, F, H and I are 0.2 percent maximally, and other unknown impurities are 0.1 percent maximally.
Owner:TIANJIN JINYAO GRP
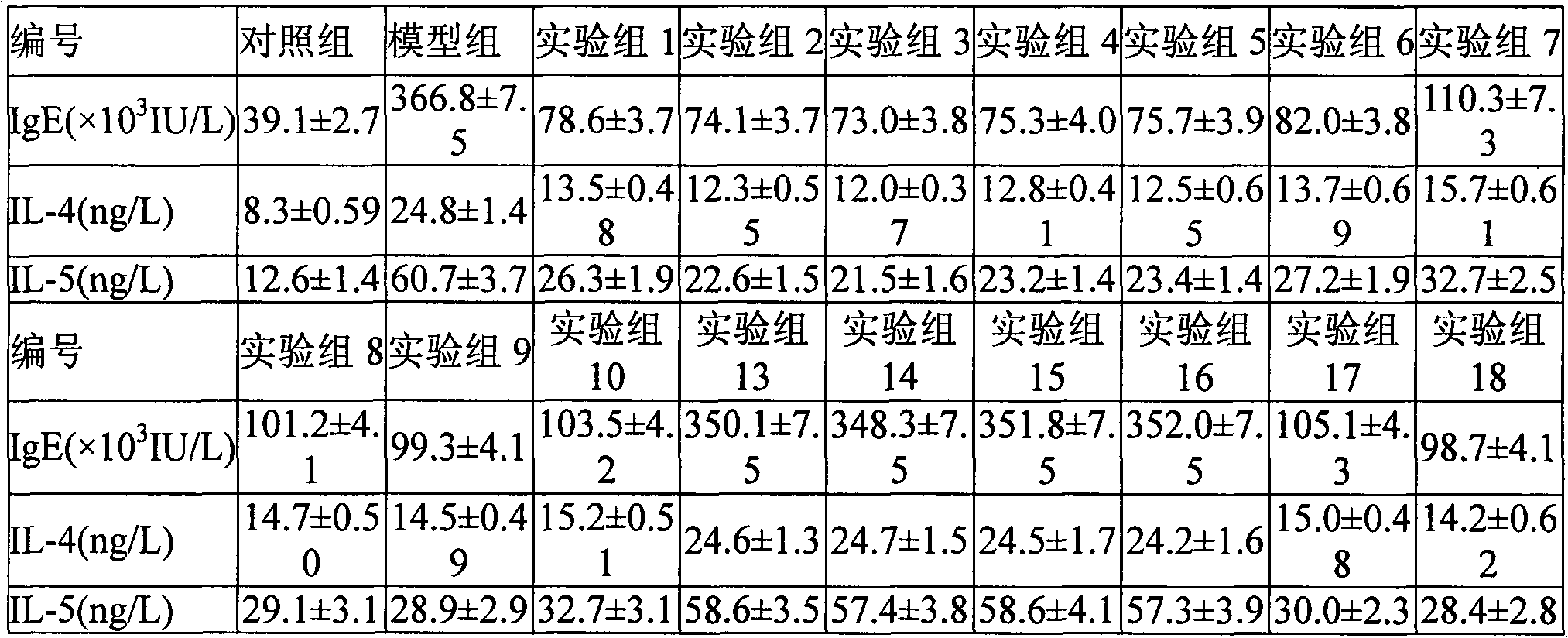
Inhalation drug composition with fluticasone propionate and nitric oxide synthase (NOS) inhibitor
InactiveCN103127133ALower serum IgE, IL-4Reduce IL-5 contentOrganic active ingredientsPharmaceutical delivery mechanismFluticasone propionateMedicine
An inhalation drug composition with fluticasone propionate and a nitric oxide synthase (NOS) inhibitor comprises the fluticasone propionate which serves as an active ingredient, an amino acid derivative which serves as the NOS inhibitor and one or more drug auxiliary materials which suit to be inhaled.
Owner:TIANJIN JINYAO GRP
Separate type water suspension medicament of fluticasone propionate containing auxiliary materials for treating skin disease
InactiveCN102526066AReduce wasteEasy to useOrganic active ingredientsSolution deliveryDiseaseFluticasone propionate
The invention relates to a separate type water suspension medicament of fluticasone propionate containing auxiliary materials for treating skin disease, composed of separately packed fluticasone propionate and separately packed water, wherein the fluticasone propionate contains one or more kinds of pharmaceutical auxiliary materials for skin, is insoluble in water and has a D90 particle size of 0.1-10mu m.
Owner:TIANJIN JINYAO GRP
Method for preparing fluticasone propionate suspension for inhalation
InactiveCN103505412ALow costOrganic active ingredientsSolution deliveryFluticasone propionateUrology
The invention discloses a method for preparing fluticasone propionate suspension for inhalation. Sterile fluticasone propionate suspension is prepared in a way of treating fluticasone propionate and accessories respectively. A solution is prepared from the accessories, and is filtered and sterilized through a microporous filter membrane, and microbial load control over each accessory is not required, so that the cost is lowered.
Owner:SHANGHAI CHENPON PHARMA TECH
Methods of preparing intermediate of fluticasone propionate
A method of preparing a thioic acid intermediate of fluticasone propionate includes: treating a 17beta-[(N,N-dimethyl carbamoyl)thio]carbonyl compound in a solution including an alcohol and an alkali metal hydroxide, an alkaline-earth metal hydroxide, or a mixture thereof to cleave an amide from the 17beta-[(N,N-dimethyl carbamoyl)thio]carbonyl compound; treating the solution to separate an aqueous portion; and adding an acid to the aqueous portion to obtain the thioic acid intermediate of fluticasone propionate. A method of preparing fluticasone propionate includes preparing the thioic acid intermediate of fluticasone propionate, and alkylating the thioic acid intermediate of fluticasone propionate to prepare the fluticasone propionate.
Owner:AMPHASTAR NANJING PHARMA
Combination of levocabastine and fluticasone furoate for the treatment of inflammatory and/or allergic conditions
InactiveUS9675624B2Improve solubilityReduce the amount requiredOrganic active ingredientsSenses disorderAllergic conditionFluticasone propionate
The present invention relates to pharmaceutical formulations comprising an anti-inflammatory glucocorticoid compound of the androstane series and levocabastine, an H1 antagonist / anti-allergic, and also relates to therapeutic uses thereof, particularly for the treatment of inflammatory and allergic conditions, specifically rhinitis.
Owner:GLAXO GROUP LTD
Preparation method of high-performance acrylic resin
InactiveCN106146743AImprove wear resistanceImprove yellowing defectsCoatingsFluticasone propionateAcrylic resin
The invention relates to a preparation method of high-performance acrylic resin. According to the method, water, sodium dodecyl benzene sulfonate, fatty alcohol-polyoxyethylene ether and 2-acrylamide-2-methylpropanesulfonic acid are added; the temperature is raised; stirring is performed; monomers A are added; the emulsification is performed for 40min; when the temperature is raised to 70 DEG C, backflow water is introduced; when the temperature is raised to 85 DEG C, a potassium sulphate solution is added; the reaction is performed for 2h; then, fluticasone propionate is added; stirring and reaction are performed to obtain core layer emulsion; monomers B and a potassium peroxodisulfate solution are simultaneously dripped into the core layer emulsion; after the dripping is completed, stirring and reaction are performed; the temperature is lowered to 50 DEG C; the fatty alcohol-polyoxyethylene ether and the fatty alcohol-polyoxyethylene ether are added; the reaction time is 70 minutes; the reaction is performed for 1h at 70 DEG C; ammonia water is added for regulating the pH value to 7 to 8 to obtain the acrylic resin. The preparation method has the advantages that the abrasive resistance of the acrylic resin is greatly improved; the defect that a film formed by the acrylic resin emulsion becomes yellow is overcome.
Owner:司红康
Pharmaceutical composition containing beta2 agonists and steroids, and uses thereof
InactiveCN101433541AImprove lung functionEasy to controlOrganic active ingredientsAerosol deliveryDiseaseFluticasone propionate
The invention belongs to the medicine technical field, and relates to a pharmaceutical composition of a beta2 receptor agonist and steroid and application thereof, in particular to a pharmaceutical composition of tranditerol or salt thereof, or mabuterol or salt thereof, and fluticasone propionate and application thereof, wherein the mixture ratio of the tranditerol or the salt thereof to the mabuterol or the salt thereof to the fluticasone propionate is 10 to 1-1 to 10,000; and the composition can be a composition which can be atomized in respirable mode, and can be used for preparing an aerosol inhalant and an aerosol. The composition can be prepared into capsules and other formulations with other auxiliary materials after the composition is prepared into dried powder with pharmaceutically acceptable carriers. The composition can significantly improve pulmonary function, and can control obstructive or phlogistic airway diseases or slow down depravation of the diseases. The composition can be used for preparing medicaments for salving and treating the obstructive or phlogistic airway diseases.
Owner:SHENYANG PHARMA UNIVERSITY
Fluticasone propionate foaming agent composition
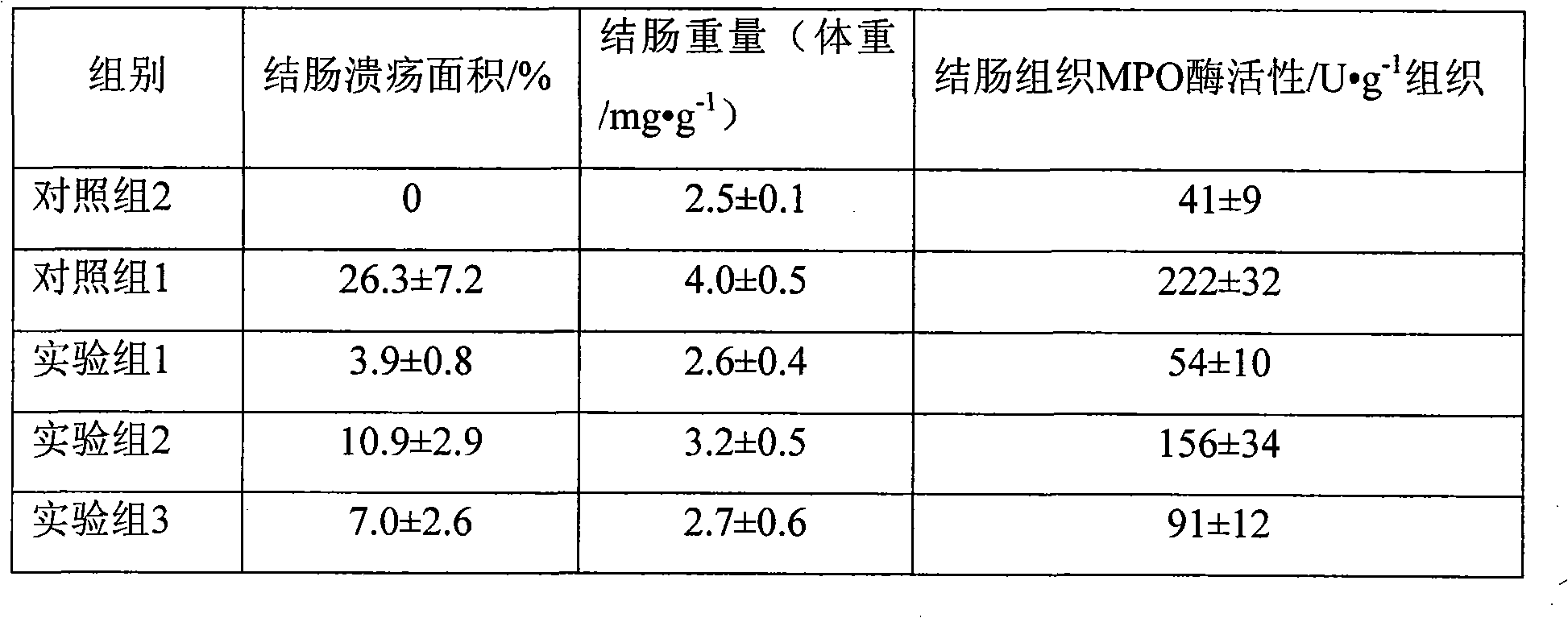
InactiveCN101926765AConvenient treatmentOvercomes the drawbacks of being unsuitable for the treatment of ulcerative colitisOrganic active ingredientsAerosol deliveryFoaming agentFluticasone propionate
The invention discloses a fluticasone propionate foaming agent composition, which contains fluticasone propionate serving as an active ingredient and one or more pharmaceutically acceptable auxiliary materials for a foaming agent, wherein the content of the fluticasone propionate serving as the active ingredient is 0.05 to 0.2 percent (weight / weight), and the volume expansion ratio of the foaming agent composition is 25 to 50.
Owner:TIANJIN JINYAO GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com