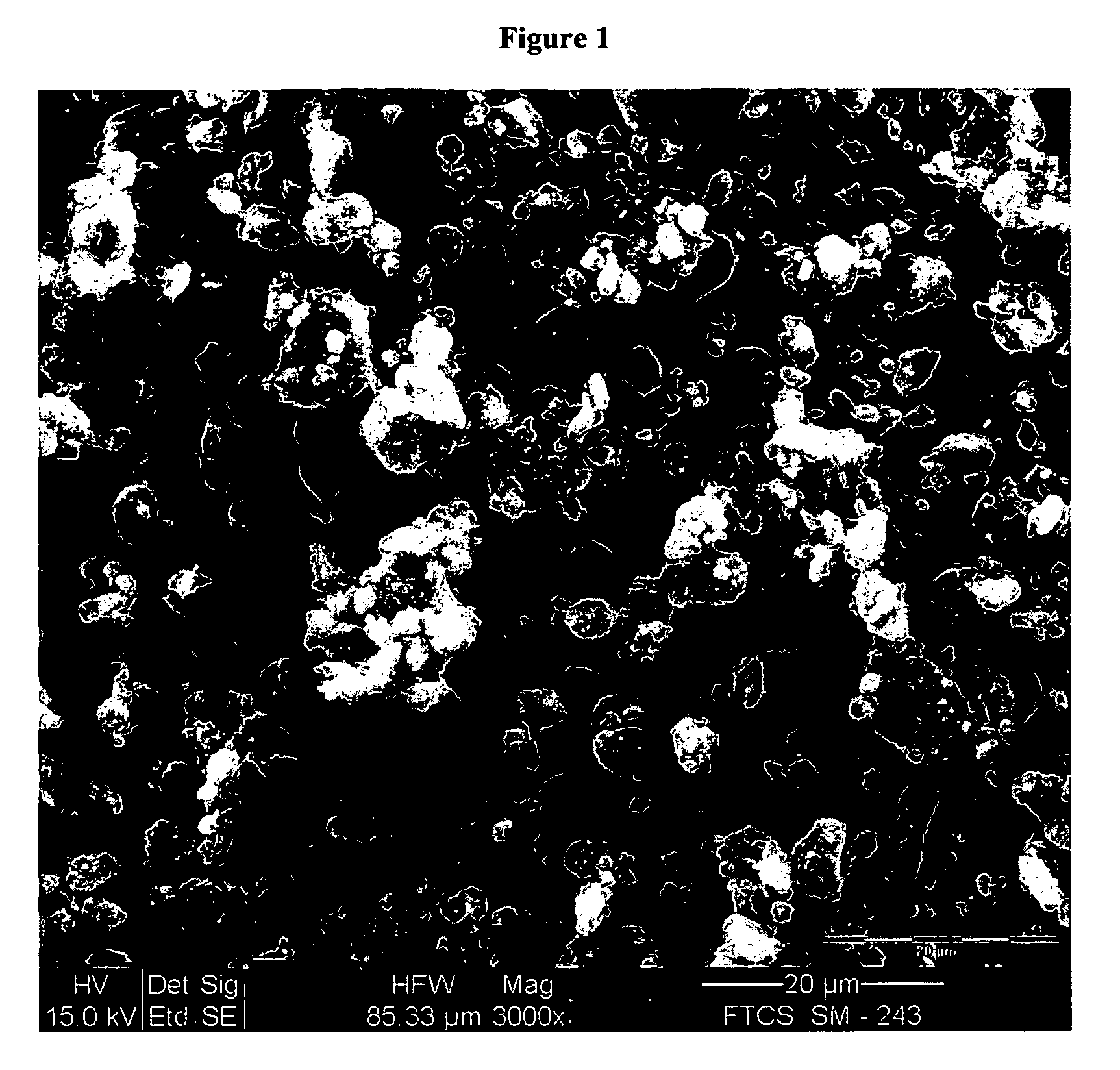
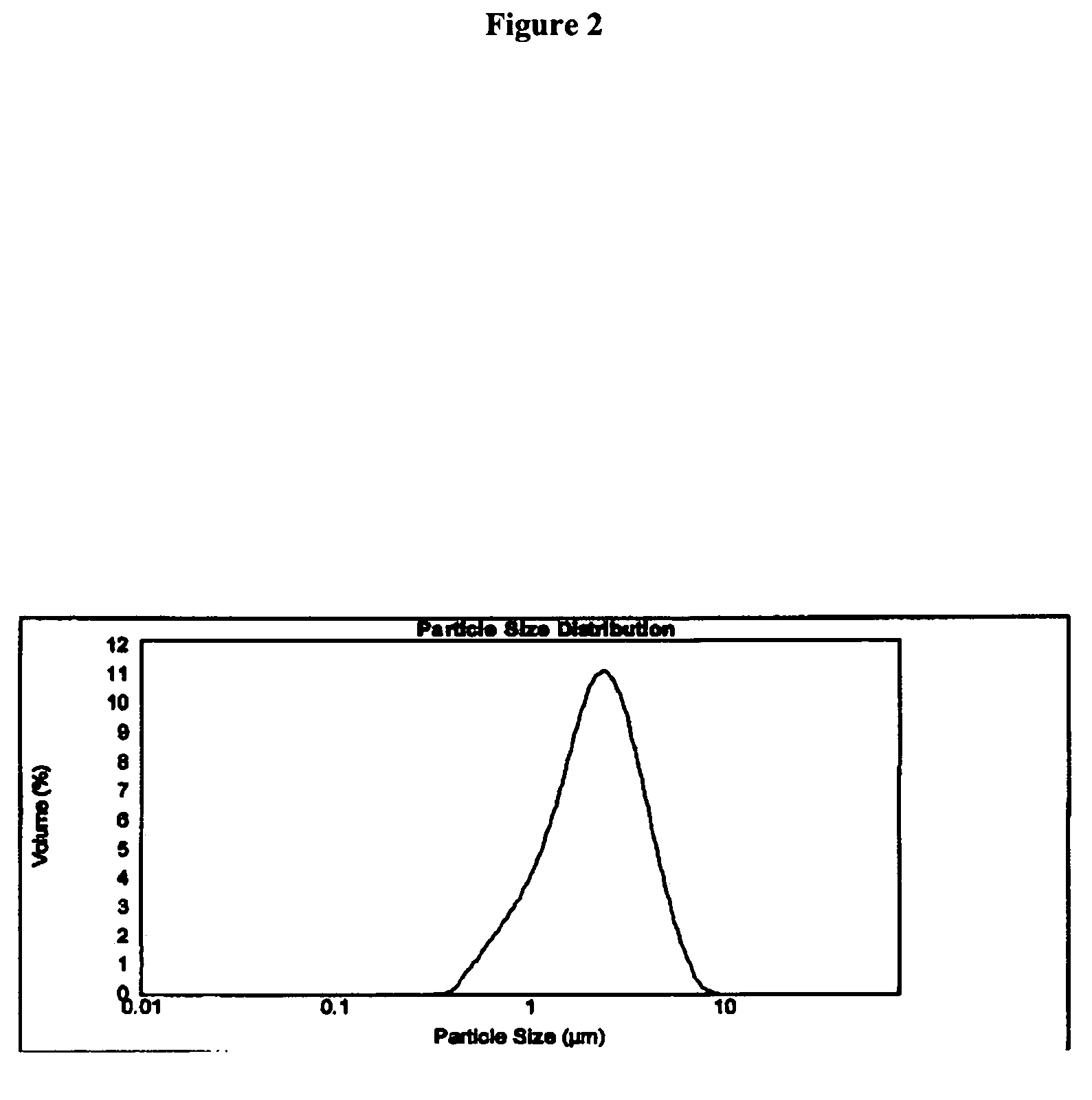
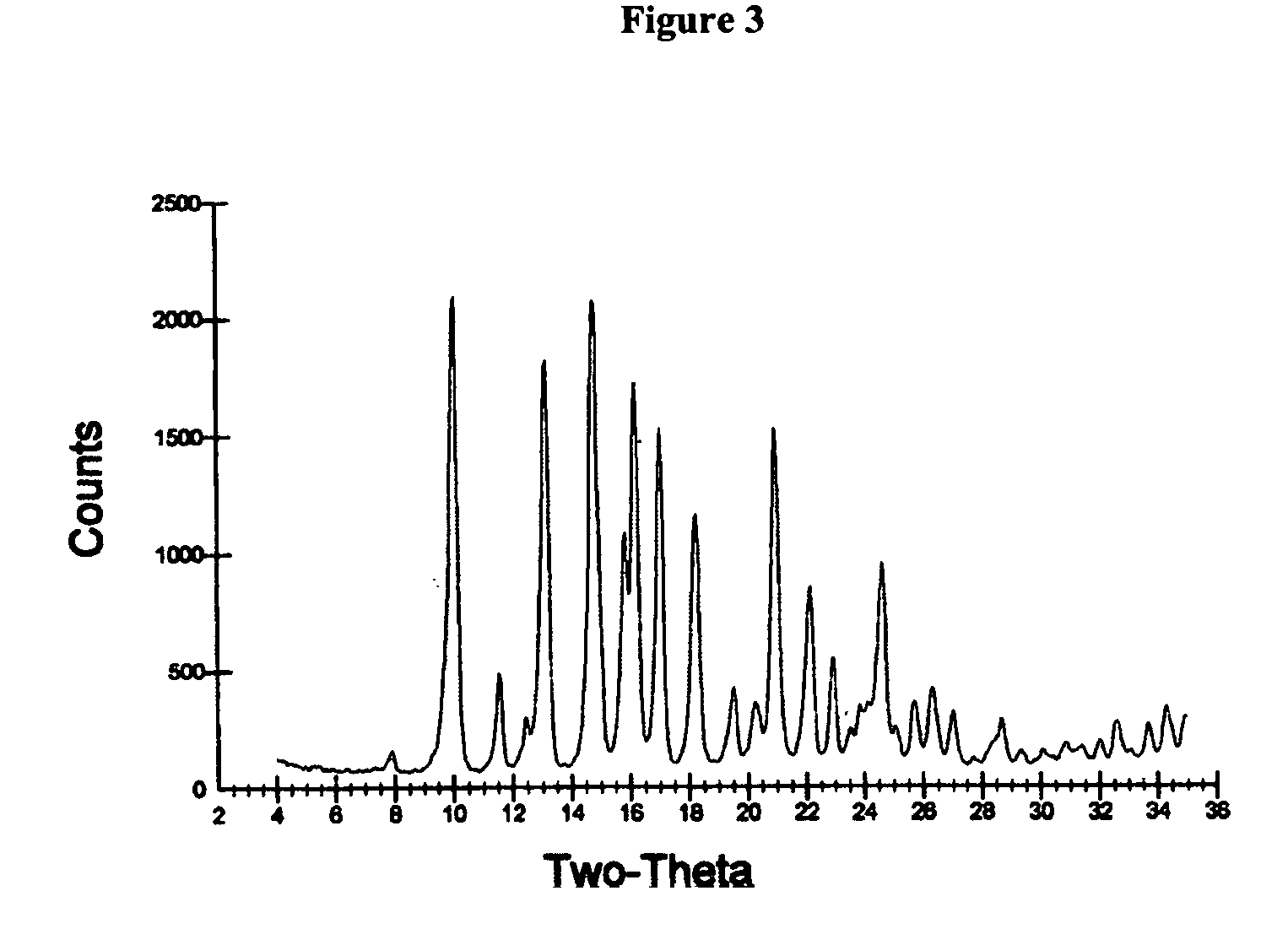
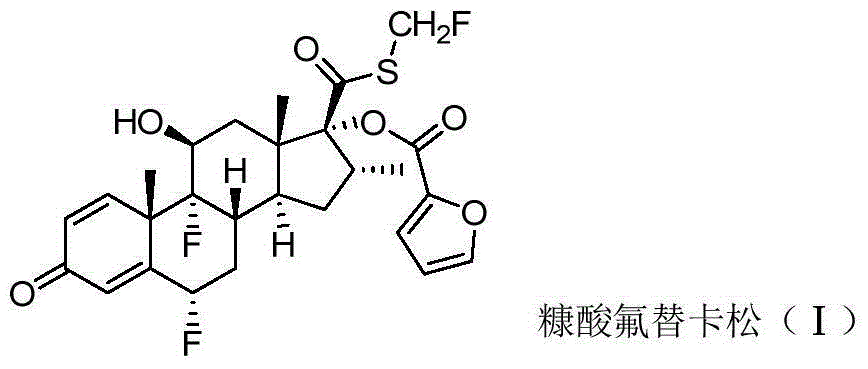
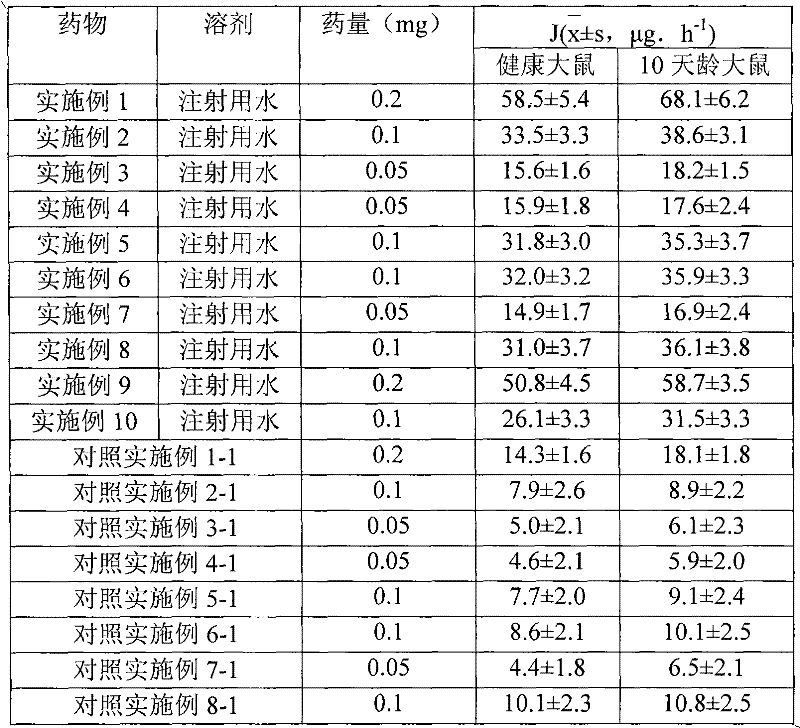
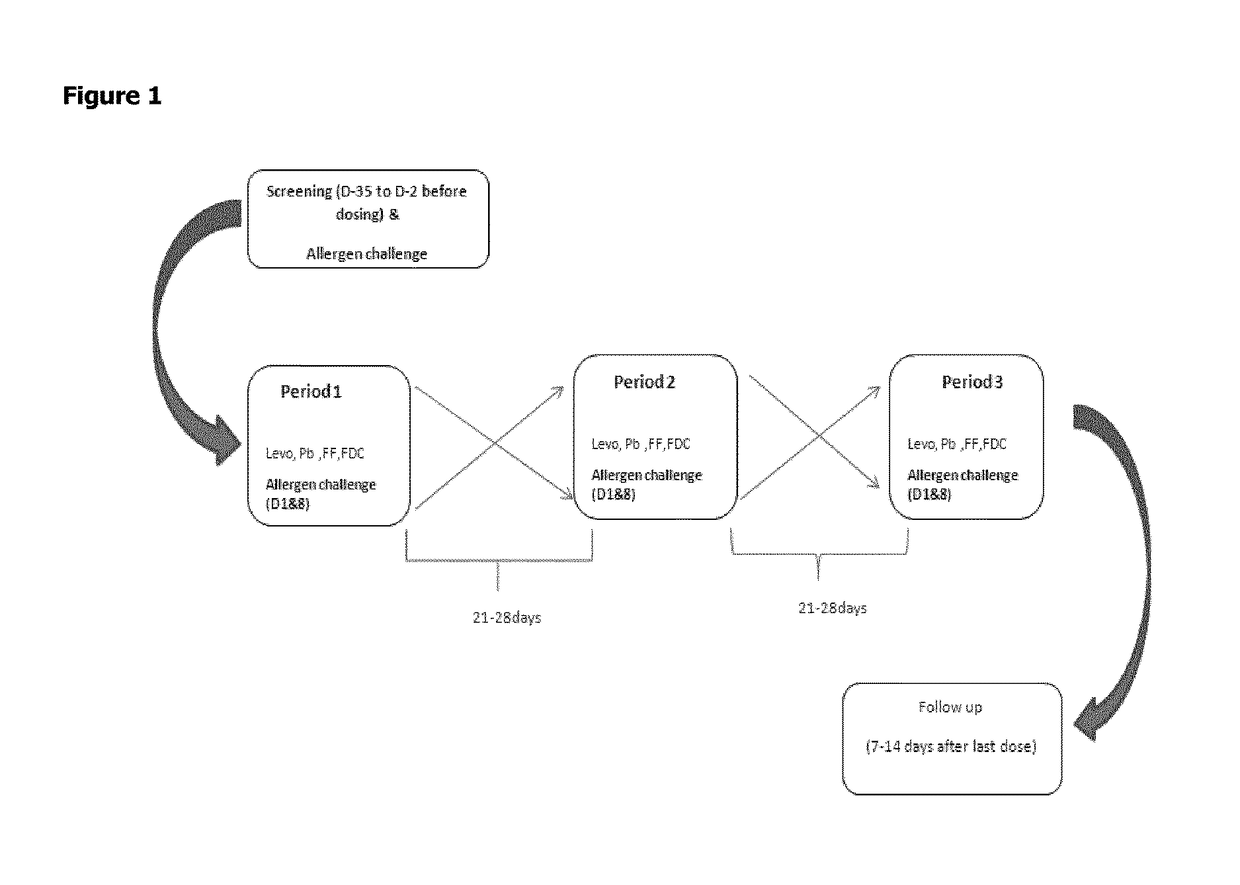
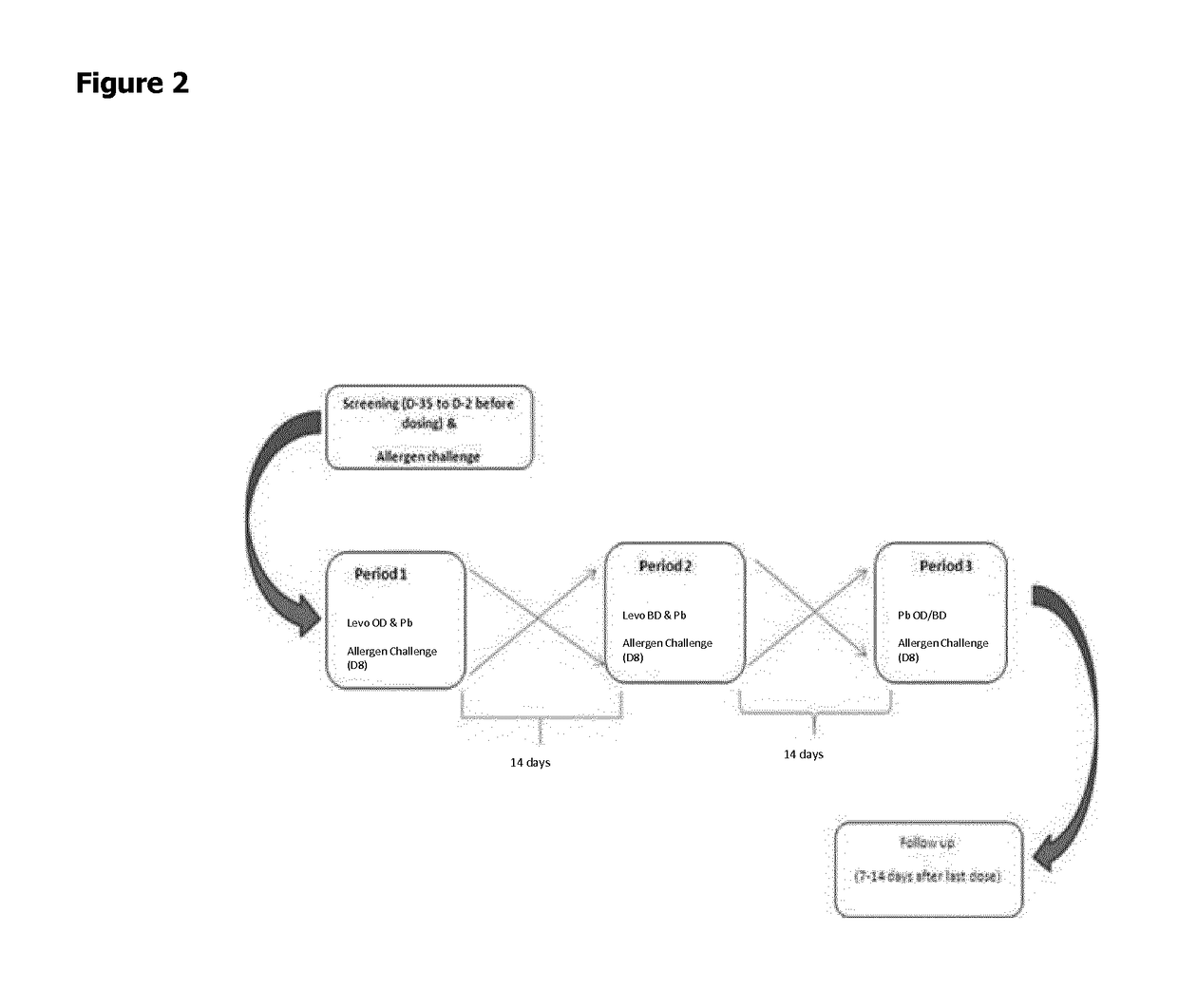
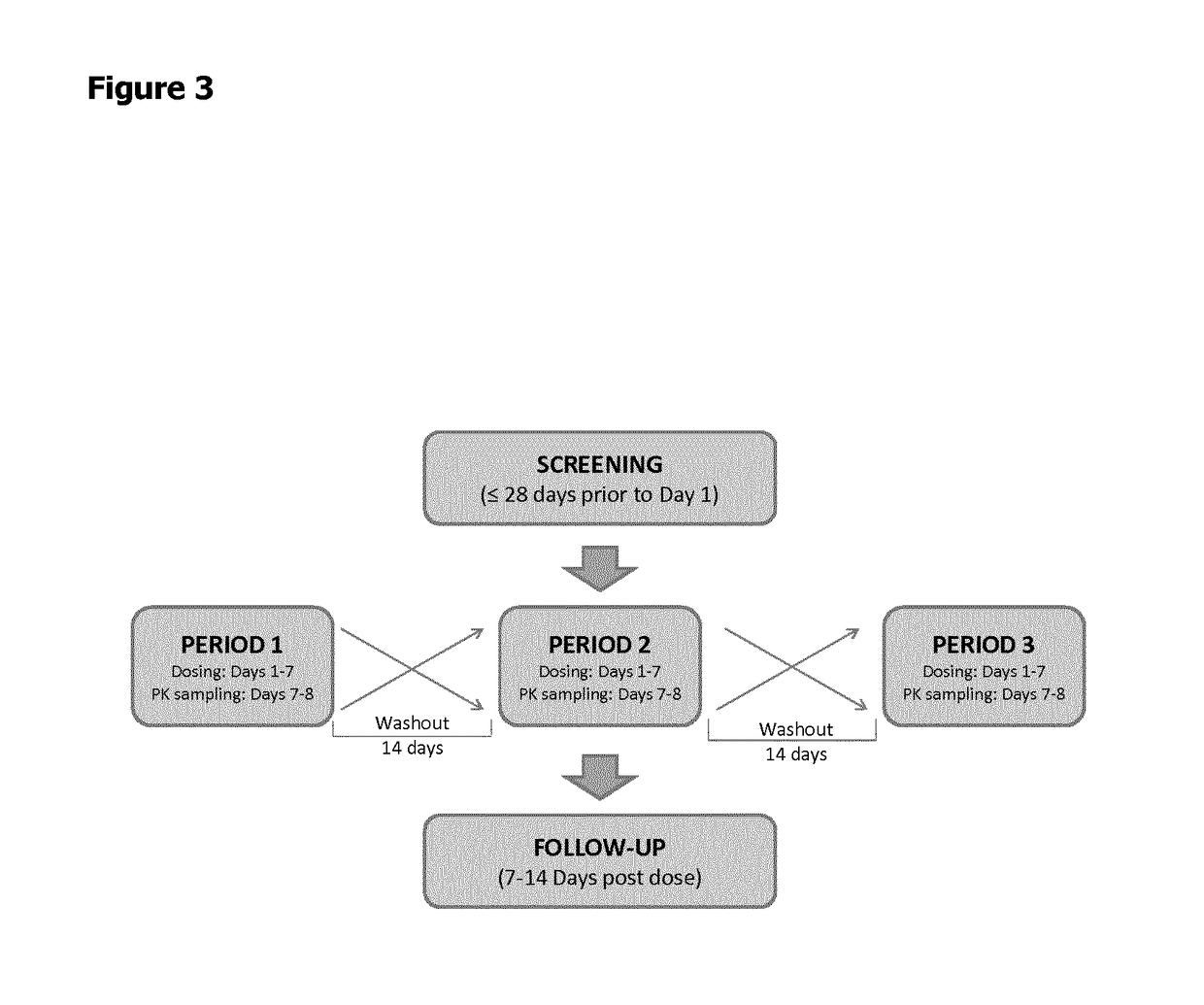
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Fluticasone furoate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluticasone is used to treat seasonal and year-round allergy symptoms such as stuffy/runny nose, itching, and sneezing.
Synthesis and powder preparation of fluticasone propionate
InactiveUS20060009435A1High yieldEfficient preparationPowder deliveryOrganic active ingredientsFluticasone propionateMedicine
An improved process for preparing fluticasone propionate, performed in the presence of water, is disclosed. Further disclosed is a process for preparing a fluticasone propionate that is highly suitable for administration by inhalation. Further disclosed are fluticasone propionate and a powdered fluticasone propionate prepared by these processes and pharmaceutical compositions for administration by inhalation containing same. A process of purifying a key intermediate in the synthesis of fluticasone propionate is also disclosed.
Owner:CHEMAGIS
Dosage forms containing fluticasone propionate for the treatment of inflammatory conditions of the esophagus
InactiveUS20160213681A1Reduce frequencyImprove clinical efficacyOrganic active ingredientsDispersion deliveryFluticasone propionateInflammation
The present invention describes novel and improved dosage forms containing fluticasone propionate for the treatment of conditions associated with inflammation of the esophagus.
Owner:EMS
Veramyst medicinal preparation and preparation method
InactiveCN101474192AImprove efficiencyGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismMedicineDosage form
The invention provides a single preparation and compound preparation using furoic acid fluticasone as main constituent. The weight ratio of furoic acid fluticasone in pharmaceuticals is 0.0001-50%. The pharmaceuticals can be made into jellies, supensoid agent, liquor and pulvis, and the like, which are administrated in a percutaneous way. The preparation is suitable for anaphylactic rhinitis, asthma and stubborn skin disease. The invention has the advantages of high healing rate, high safety and high promotional value.
Owner:崔晓廷
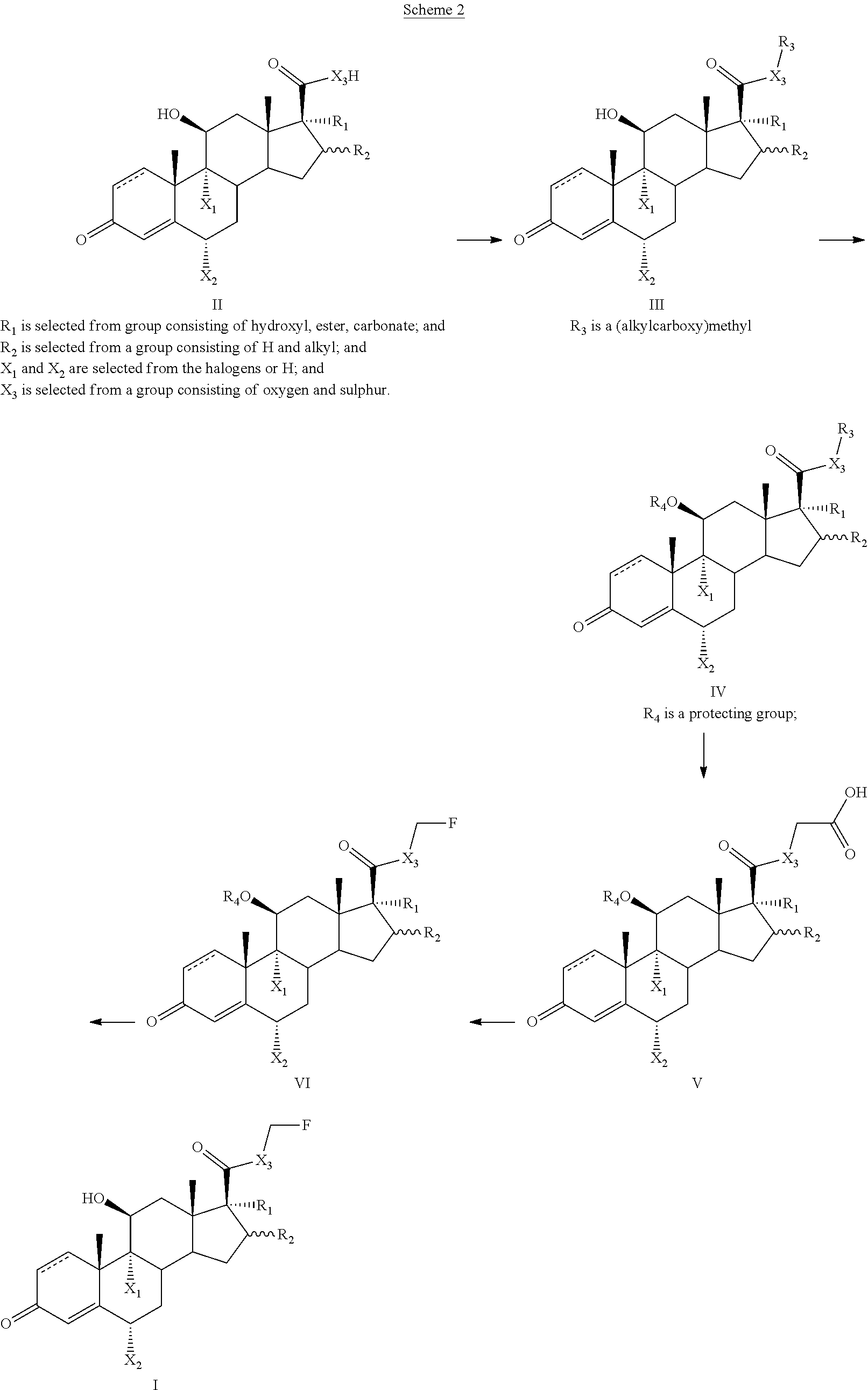
Preparation method of fluticasone furoate
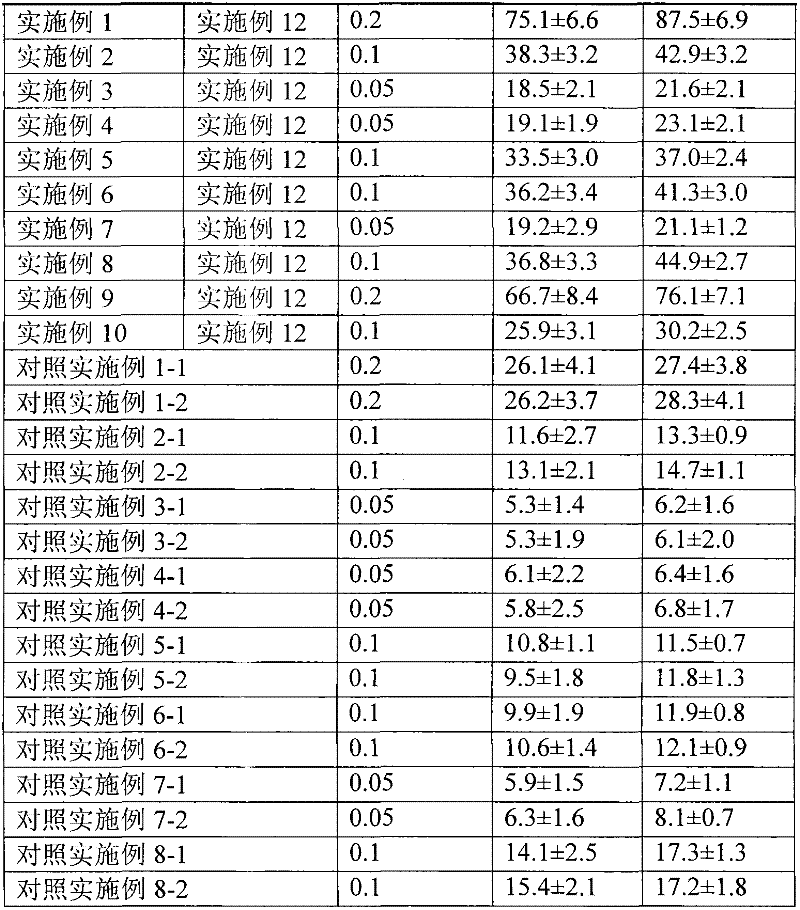
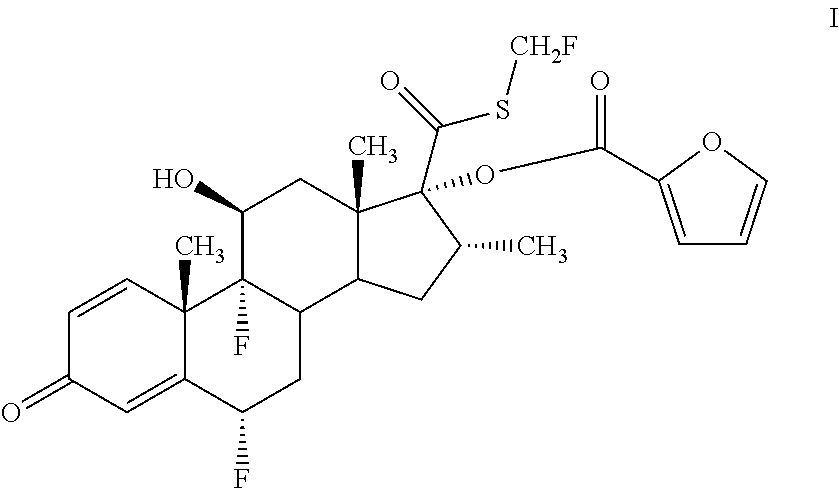
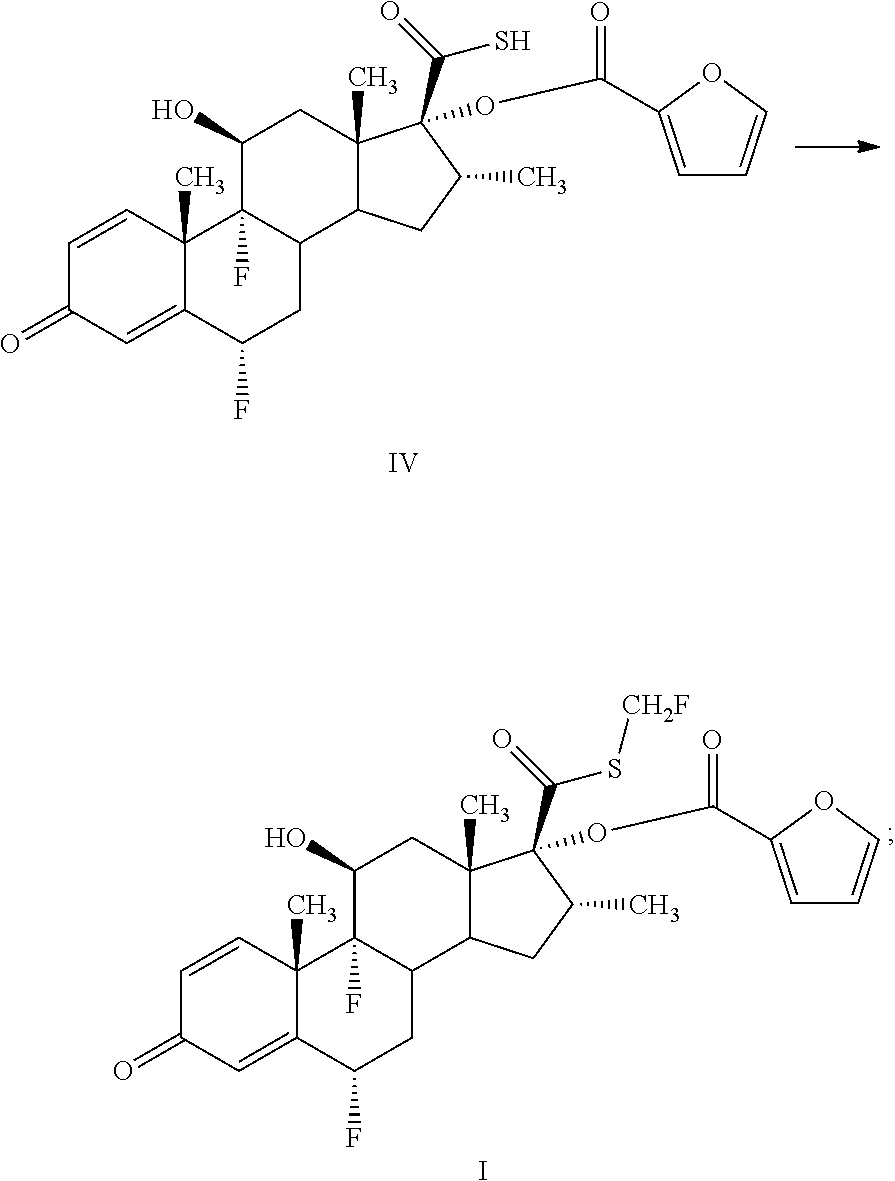
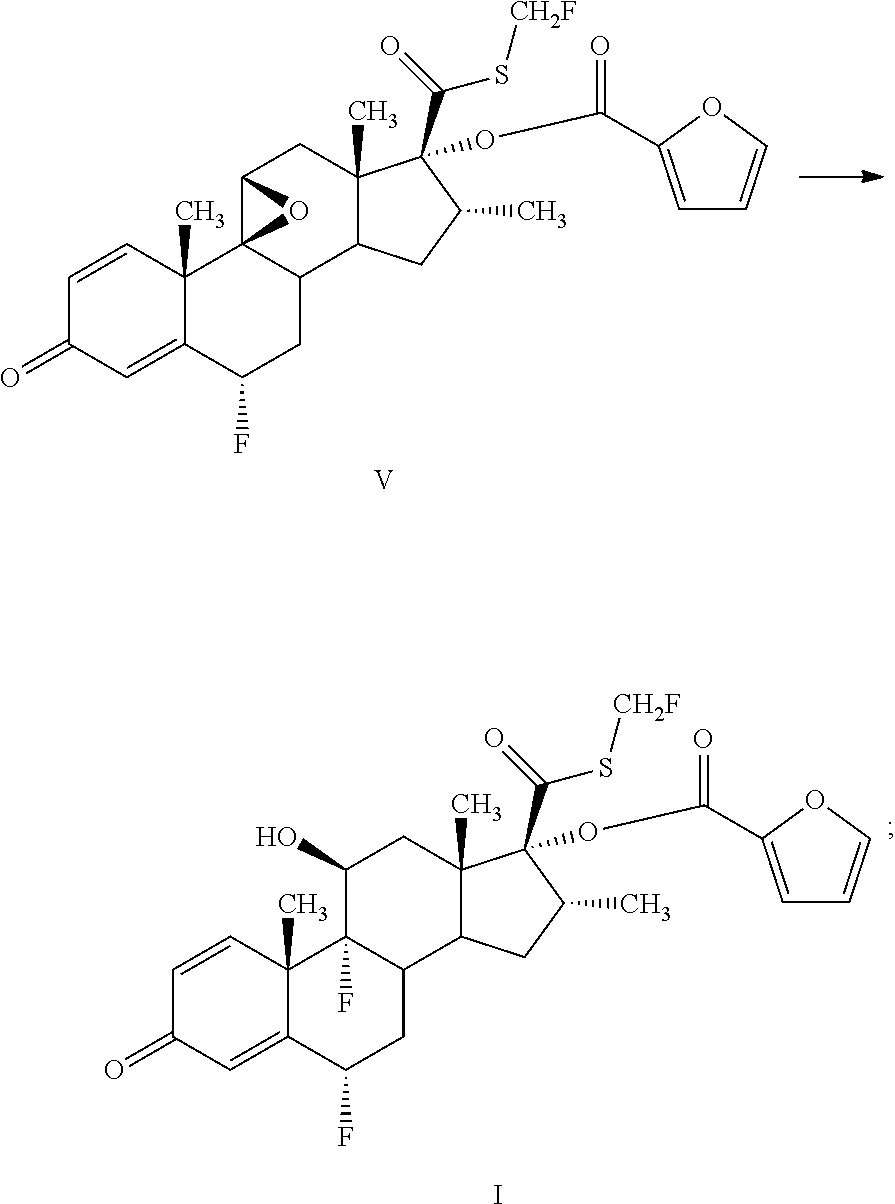
InactiveCN106279341AReduce the difficulty of refiningHigh puritySteroidsThiocarboxylic acidAndrostanes
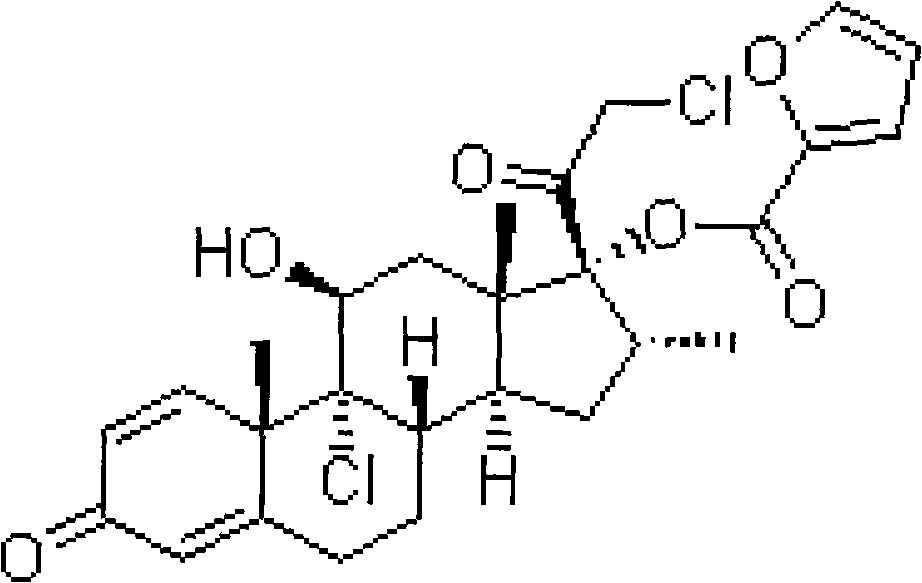
The invention relates to a preparation method of fluticasone furoate, especially preparation and purification of an intermediate 6alpha, 9alpha-difluoro-17alpha-[(2-furylcarbonyl)oxy]-11beta-hydroxy-16alpha-methyl-3-oxo-androstane-1,4-diene-17beta-thiocarboxylic acid. The preparation method comprises the following steps: in the presence of alkali and alcoholic solvent, a compound as shown in the formula VIII is converted into a mixture containing the compound of formula II; the mixture containing the compound of formula II is mixed with an aqueous solution of inorganic base and an ester solvent; a water phase is separated; pH value of the water phase is regulated by the use of acid until a solid is precipitated out; and the solid is separated. The invention provides a preparation method capable of remarkably raising purity of fluticasone furoate. Safety of drug application is guaranteed.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Dry powder inhaler
This invention provides a dry powder inhaler comprising: a dry powder medicament comprising fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of fluticasone propionate per actuation is less than 100 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose. A method of treating a patient includes administering to a patient a dry powder medicament having fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of fluticasone propionate per actuation is less than 100 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose.
Owner:TEVA BRANDED PHARMA PROD R & D
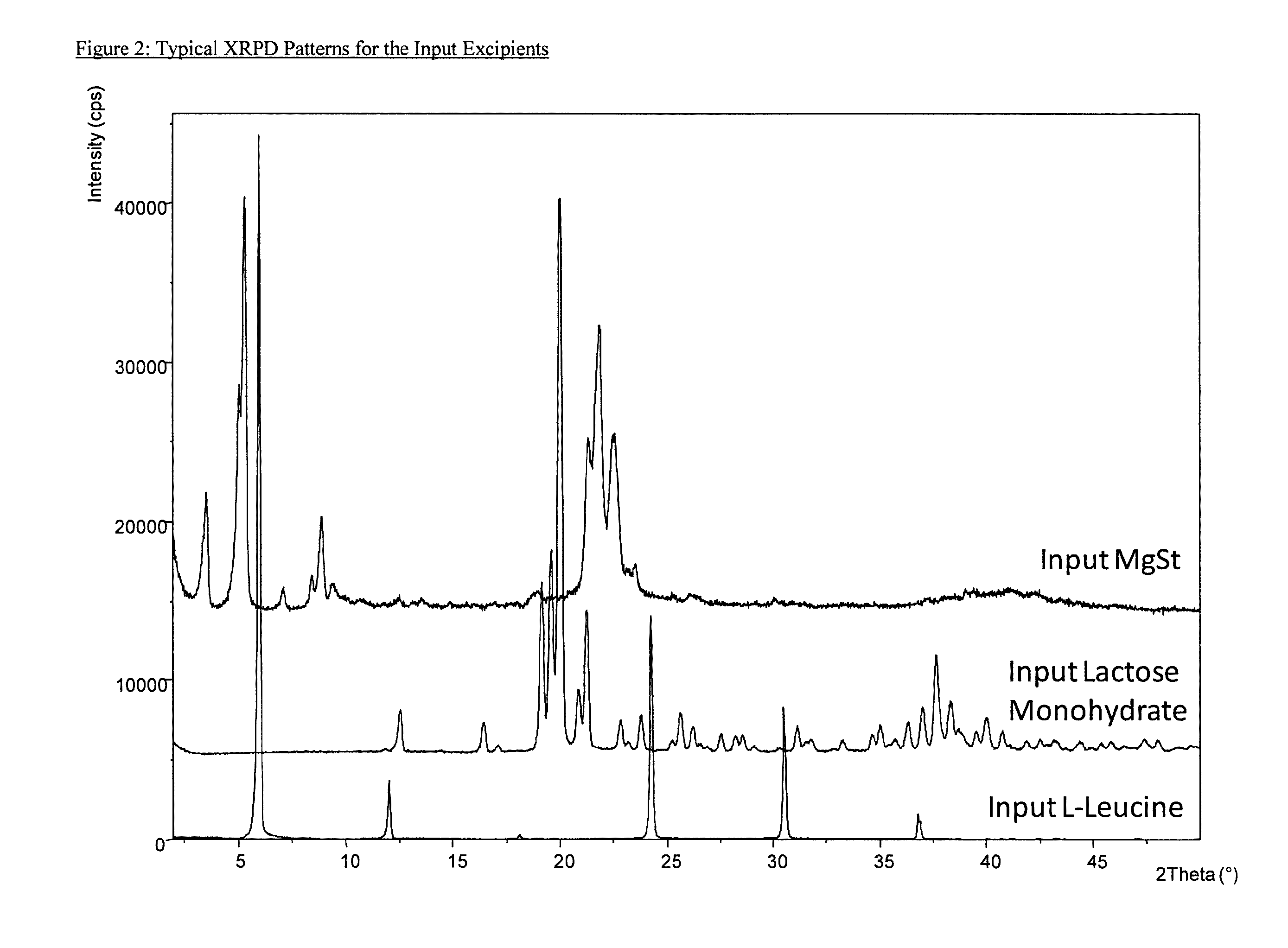
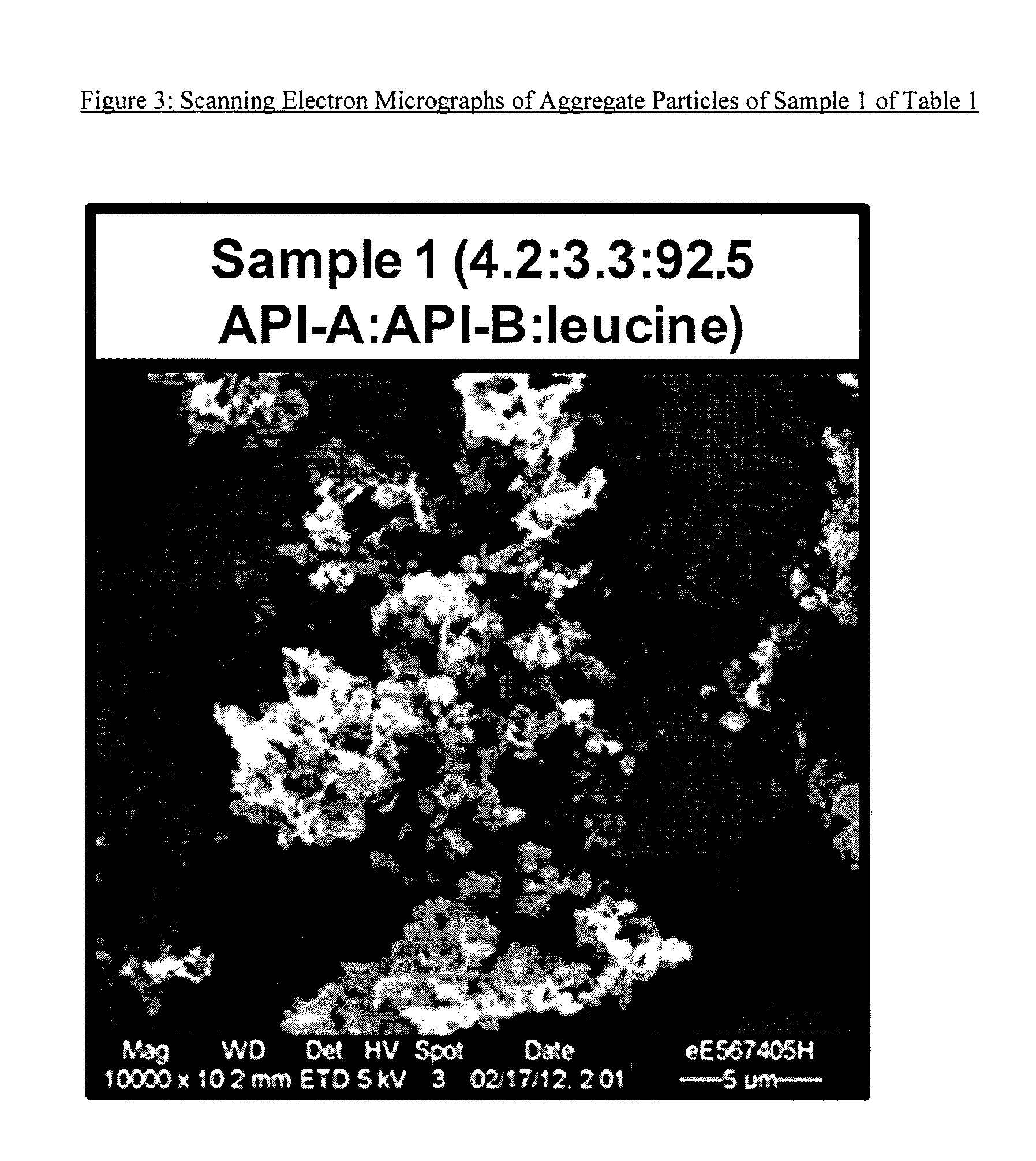
Aggregate particles
The present invention relates to aggregate particles comprising nanoparticulate drug particles. In particular, the present invention is directed to aggregate particles comprising nanoparticulate drug particles of umeclidinium bromide and optionally vilanterol trifenatate and / or fluticasone furoate. Aggregate particles of the present invention may further comprise nanoparticulate excipient particles and one or more binders. The invention also relates to powder compositions suitable for inhalation that comprise said aggregate particles, processes of producing said aggregate particles, and use of said powder compositions in the treatment of respiratory diseases, such as asthma and COPD.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Combination of umeclidinium, fluticasone propionate and salmeterol xinafoate for use in the treatment of inflammatory or respiratory tract diseases
ActiveUS20150313841A1BiocideOrganic active ingredientsRespiratory tract diseaseMuscarinic receptor site
The present invention is directed to pharmaceutical combination products comprising a muscarinic acetylcholine receptor antagonist, fluticasone propionate and salmeterol xinafoate, and to their use in the treatment of inflammatory or respiratory tract diseases.
Owner:GLAXO GROUP LTD
Fluticasone furoate in the treatment of COPD
ActiveUS20170189424A1Reduce probabilityOrganic active ingredientsPowder deliveryFluticasone propionateEosinophil
The present invention relates to pharmaceutical products comprising fluticasone furoate for use in the treatment of COPD patients, particularly a subgroup of COPD patients that through analysis have been identified as possessing an eosinophil blood count of≧150 cells / W. The present invention is further directed to methods for treating a patient with COPD which methods include identifying a patient that will respond to treatment and administering a pharmaceutical product of the present invention comprising fluticasone furoate to said patient.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same
InactiveUS20150157566A1Good treatment complianceImprove convenienceBiocidePowder deliveryDiseaseRespiratory disease
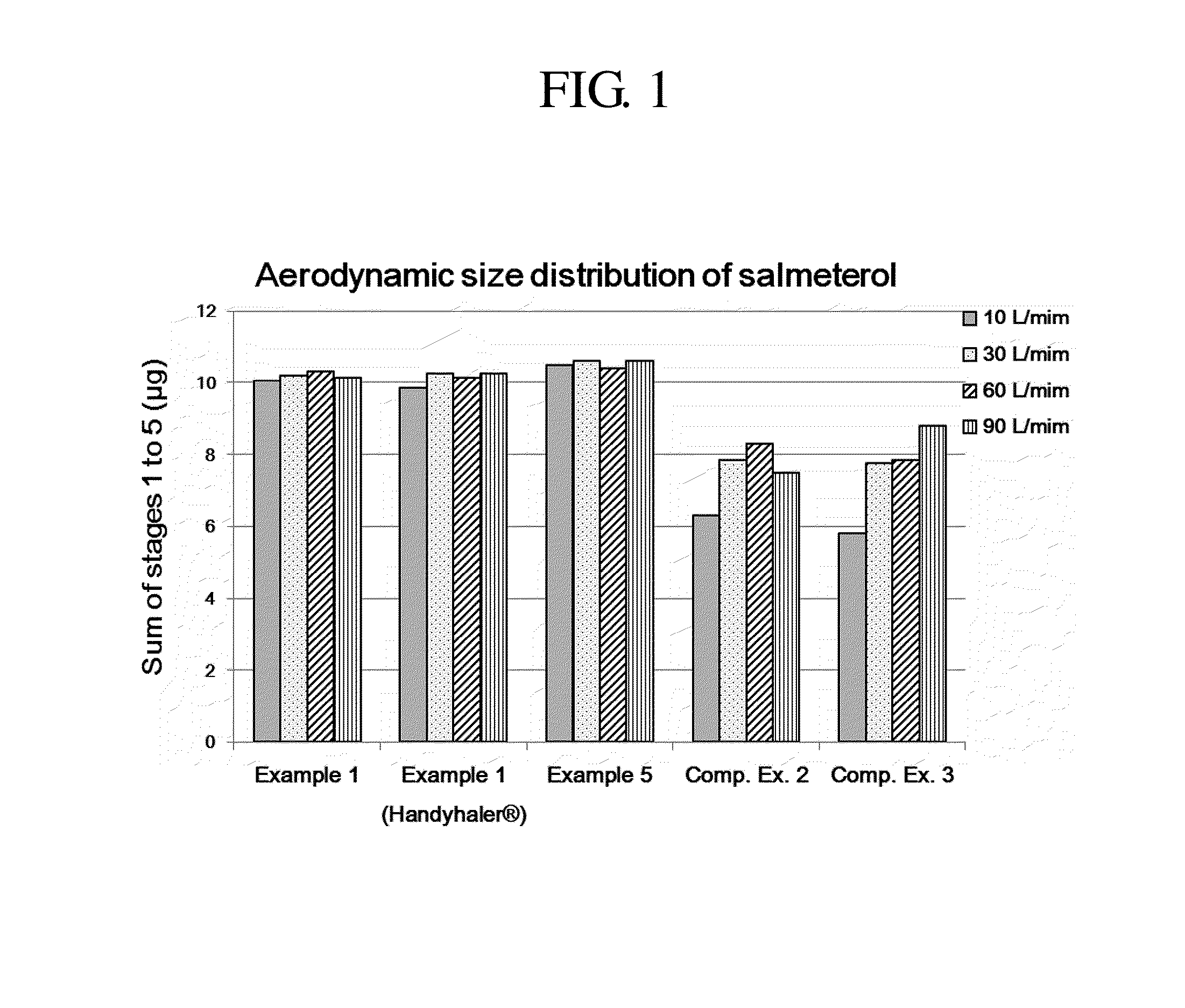
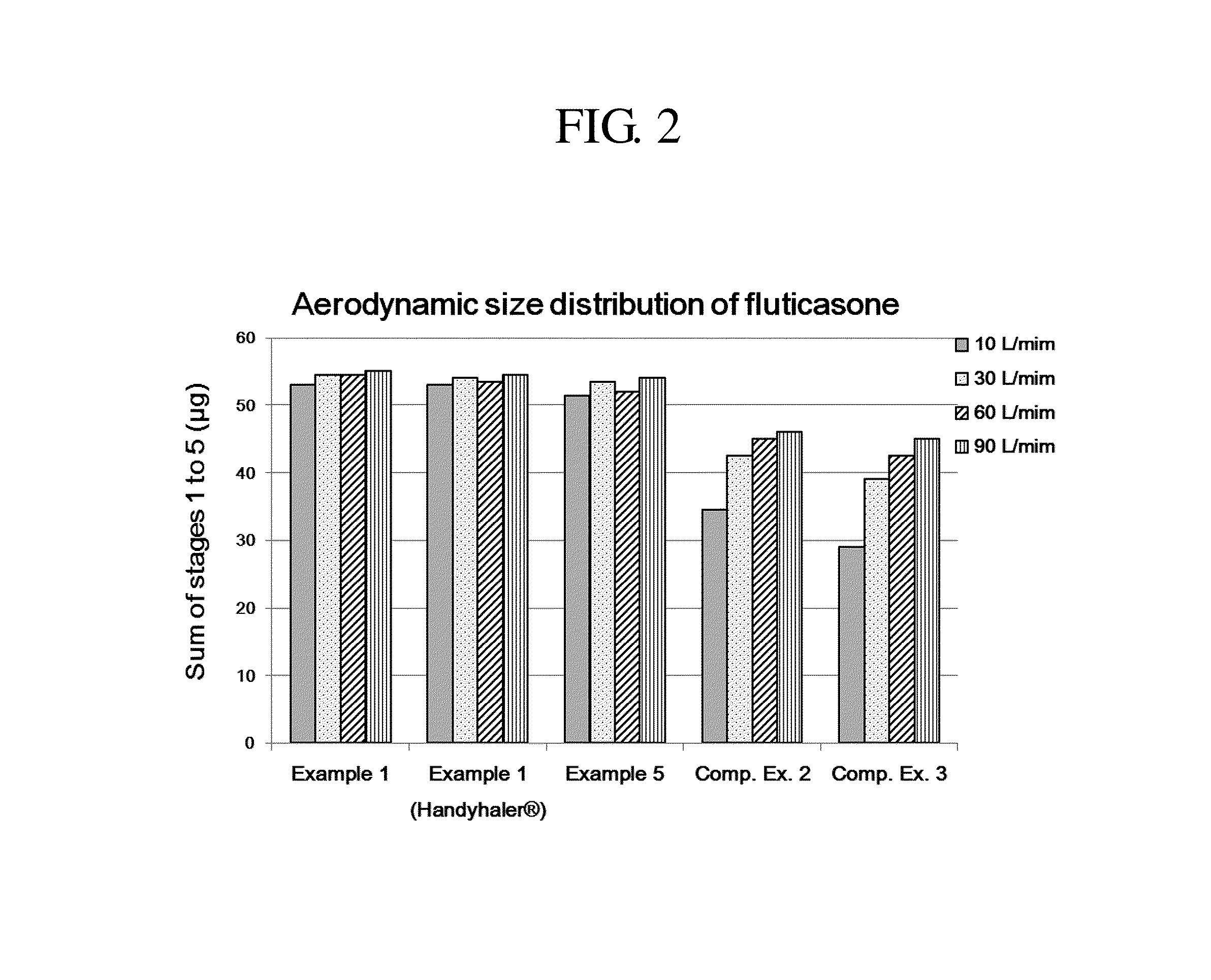
Provided is a dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, as pharmaceutically active ingredients, and a carrier, and an inhalation formulation comprising same. The inventive dry powder inhalation formulation having good content uniformity and showing small changes in the aerodynamic size distribution in accordance with the flow rate changes can effectively deliver said pharmaceutically active ingredients to a target site upon administration, and thus can be useful in the prevention or treatment of respiratory diseases, particularly asthma and COPD.
Owner:HANMI PHARMA
Separate type water suspension medicament of fluticasone propionate containing auxiliary materials for treating skin disease
InactiveCN102526066AReduce wasteEasy to useOrganic active ingredientsSolution deliveryDiseaseFluticasone propionate
The invention relates to a separate type water suspension medicament of fluticasone propionate containing auxiliary materials for treating skin disease, composed of separately packed fluticasone propionate and separately packed water, wherein the fluticasone propionate contains one or more kinds of pharmaceutical auxiliary materials for skin, is insoluble in water and has a D90 particle size of 0.1-10mu m.
Owner:TIANJIN JINYAO GRP
Combination of levocabastine and fluticasone furoate for the treatment of inflammatory and/or allergic conditions
InactiveUS9675624B2Improve solubilityReduce the amount requiredOrganic active ingredientsSenses disorderAllergic conditionFluticasone propionate
The present invention relates to pharmaceutical formulations comprising an anti-inflammatory glucocorticoid compound of the androstane series and levocabastine, an H1 antagonist / anti-allergic, and also relates to therapeutic uses thereof, particularly for the treatment of inflammatory and allergic conditions, specifically rhinitis.
Owner:GLAXO GROUP LTD
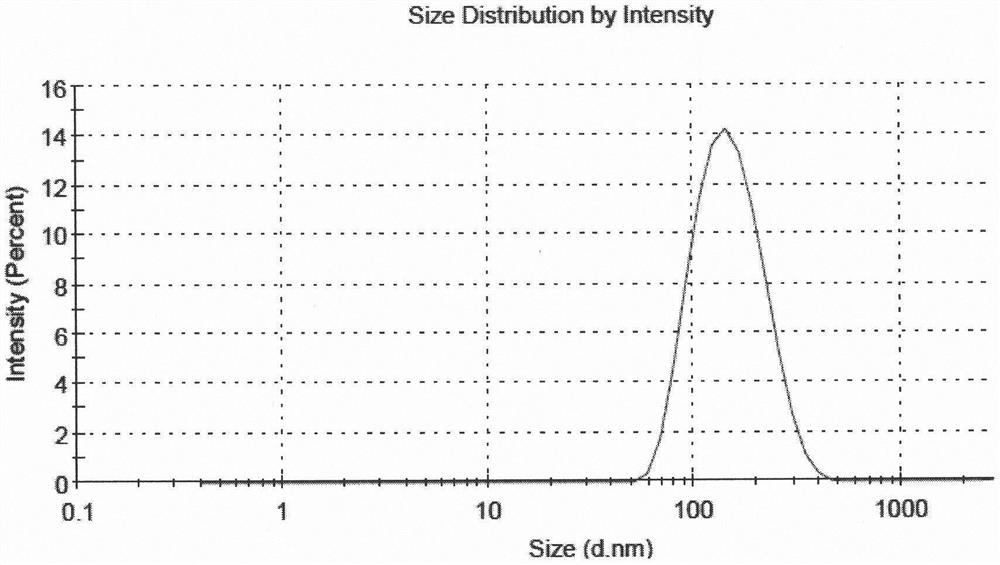
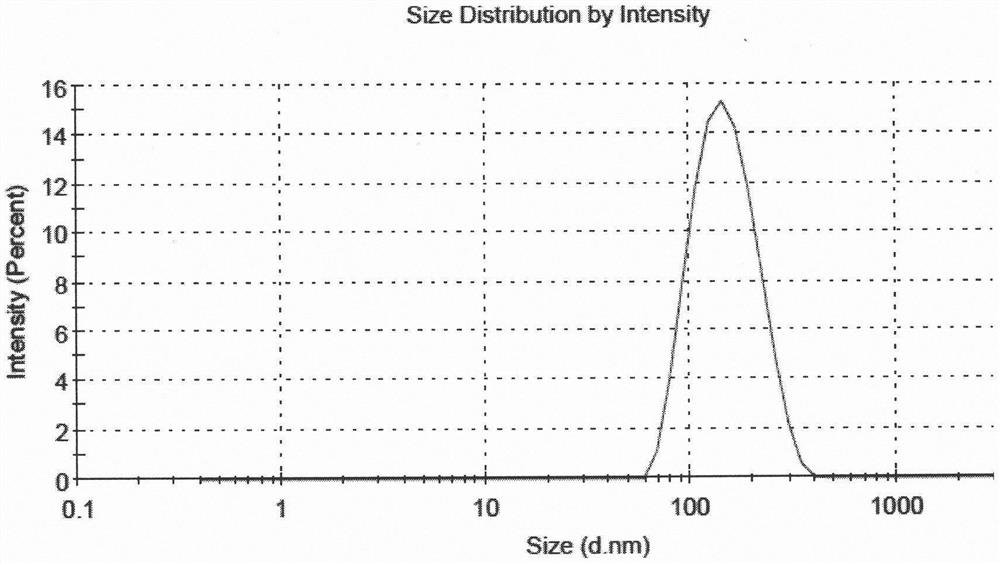
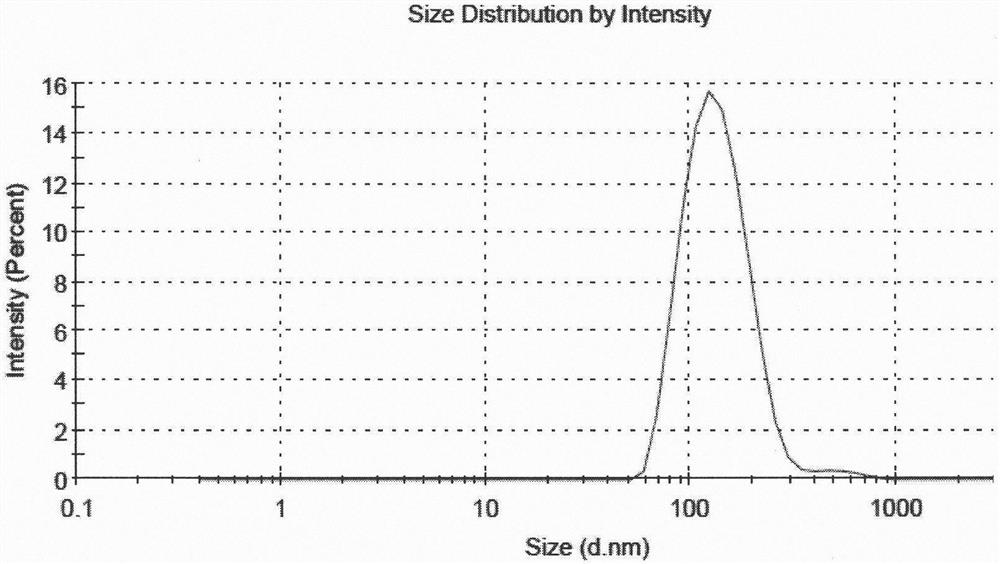
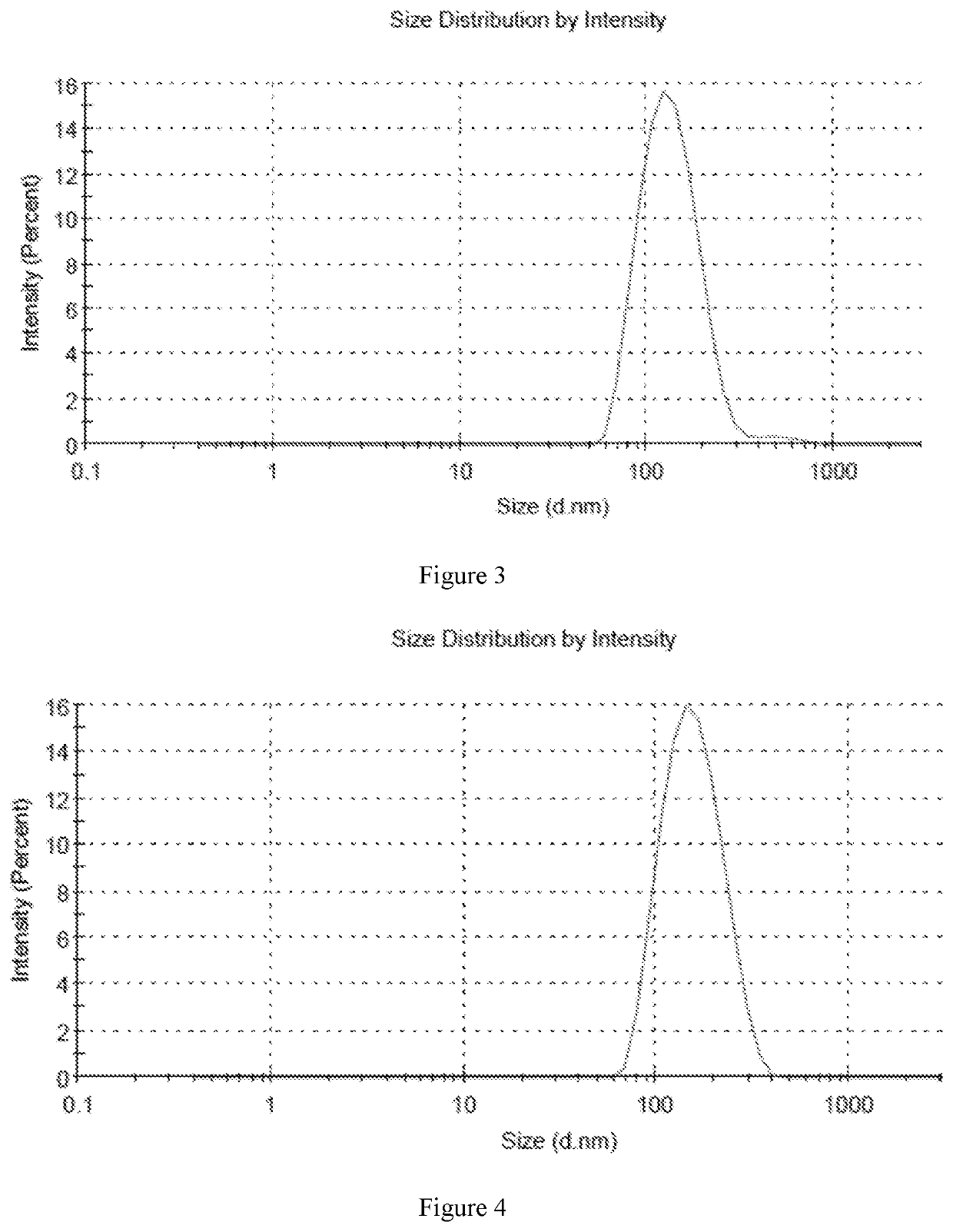
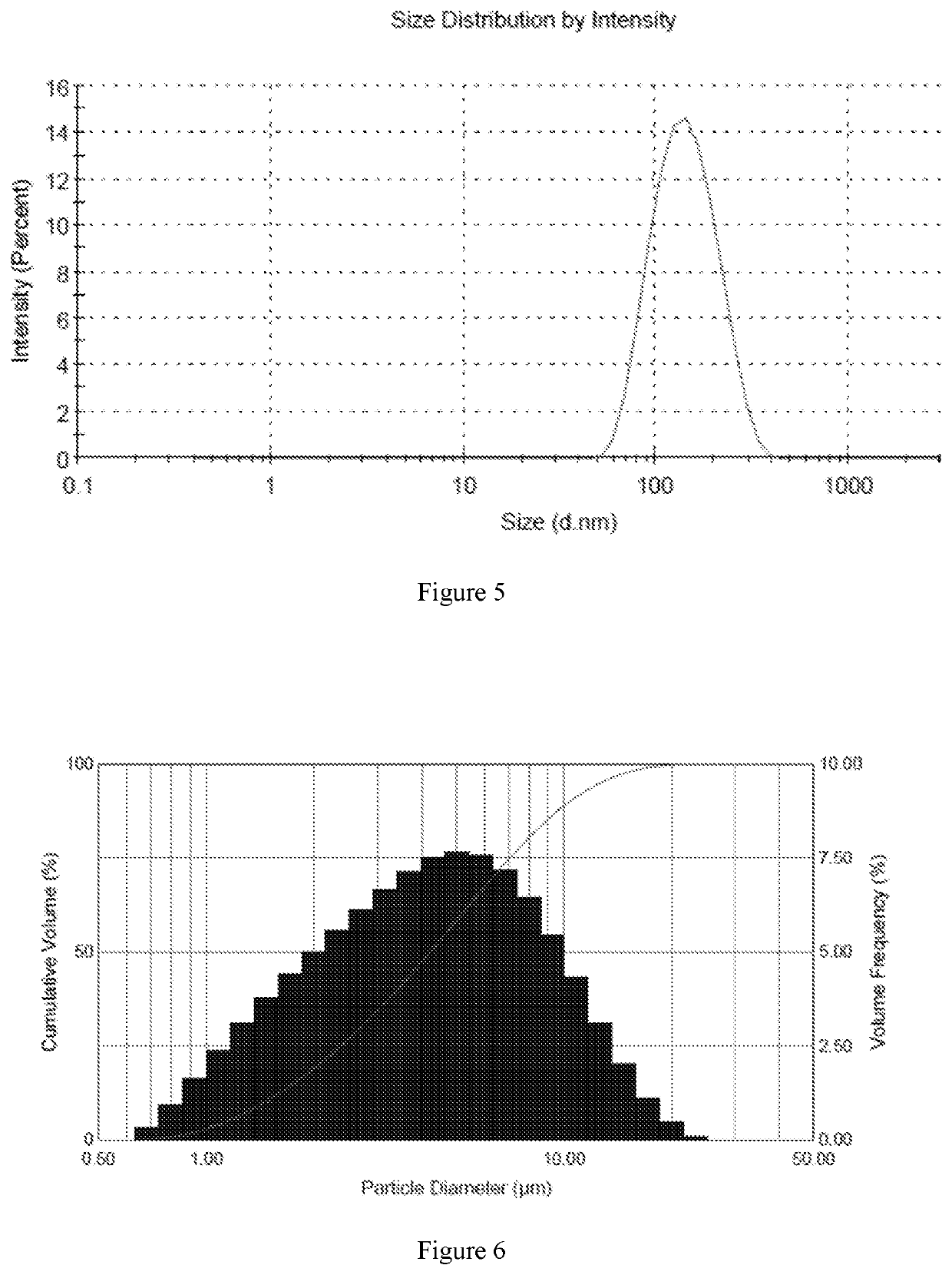
Fluticasone furoate liposome suspension and preparation method thereof
ActiveCN111789816AHigh encapsulation efficiencySimple production processOrganic active ingredientsDispersion deliverySterolPhospholipid
The invention provides a fluticasone furoate liposome and a preparation method thereof. The fluticasone furoate liposome comprises the components of phospholipid, sterol and fluticasone furoate, wherein the fluticasone furoate accounts for 0.01%-1%, the phospholipid accounts for 0.065%-0.196%, and the sterol accounts for 0.035%-0.104%. A preparation method of the fluticasone furoate liposome comprises the steps of (1) performing mixing and injecting; and (2) performing filtering and concentrating. The method is simple to operate, stable in technology and suitable for mass production.
Owner:SHANGHAI ANOVENT PHARMA CO LTD
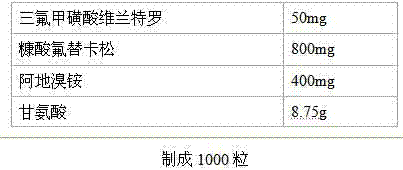
Vilanterol trifluoromethanesulfonate-containing pharmaceutical preparation
InactiveCN103933049AReduce the number of acute exacerbationsImprove lung functionOrganic active ingredientsAntipyreticAnticholinergic DrugsGlucocorticoid
The invention discloses a vilanterol trifluoromethanesulfonate-containing pharmaceutical preparation, which comprises the following main active ingredients: long-acting beta2 receptor agonist vilanterol trifluoromethanesulfonate, glucocorticoid drug fluticasone furoate and long-acting anticholinergic drug aclidinium bromide, or physiologically acceptable salts of the fluticasone furoate and aclidinium bromide, thus composing a compound preparation. The vilanterol trifluoromethanesulfonate has the effect of continuously dilating blood vessels for 24 hours; the fluticasone furoate is fast-acting potent glucocorticoid, and the duration time of the drug is longer than that of other glucocorticoids; the aclidinium bromide is long-acting cholinergic receptor antagonist, and combines with beta2 receptor agonist so as to play roles of synergism and reduction of adverse drug reaction. The vilanterol trifluoromethanesulfonate, the fluticasone furoate and the aclidinium bromide are combined for use to prepare an inhalable compound preparation which can be taken once daily due to 24-hour acting. The vilanterol trifluoromethanesulfonate-containing pharmaceutical preparation can enhance the curative effect, solves the compliance problem of patients with coexisting chronic obstructive pulmonary disease and asthma, thus being appreciable in application prospects.
Owner:SHANGHAI NEW ASIA PHARMA +1
Fluticasone propionate foaming agent composition
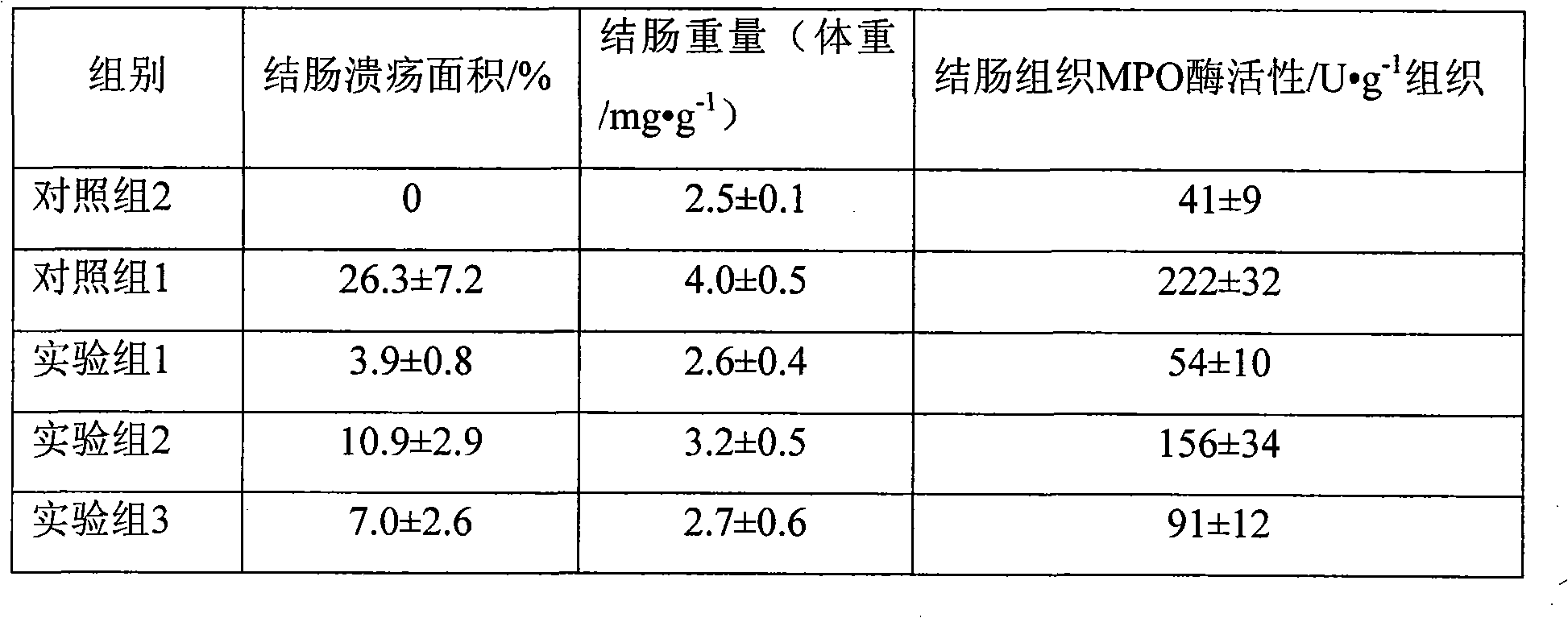
InactiveCN101926765AConvenient treatmentOvercomes the drawbacks of being unsuitable for the treatment of ulcerative colitisOrganic active ingredientsAerosol deliveryFoaming agentFluticasone propionate
The invention discloses a fluticasone propionate foaming agent composition, which contains fluticasone propionate serving as an active ingredient and one or more pharmaceutically acceptable auxiliary materials for a foaming agent, wherein the content of the fluticasone propionate serving as the active ingredient is 0.05 to 0.2 percent (weight / weight), and the volume expansion ratio of the foaming agent composition is 25 to 50.
Owner:TIANJIN JINYAO GRP
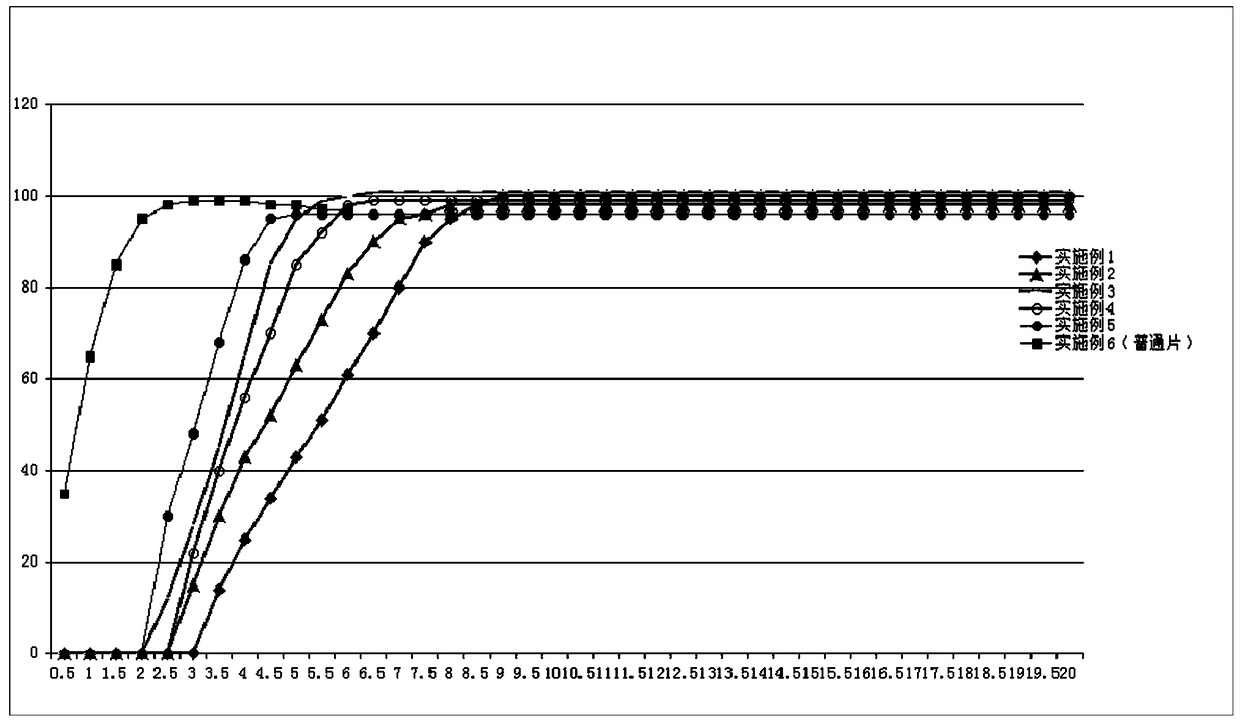
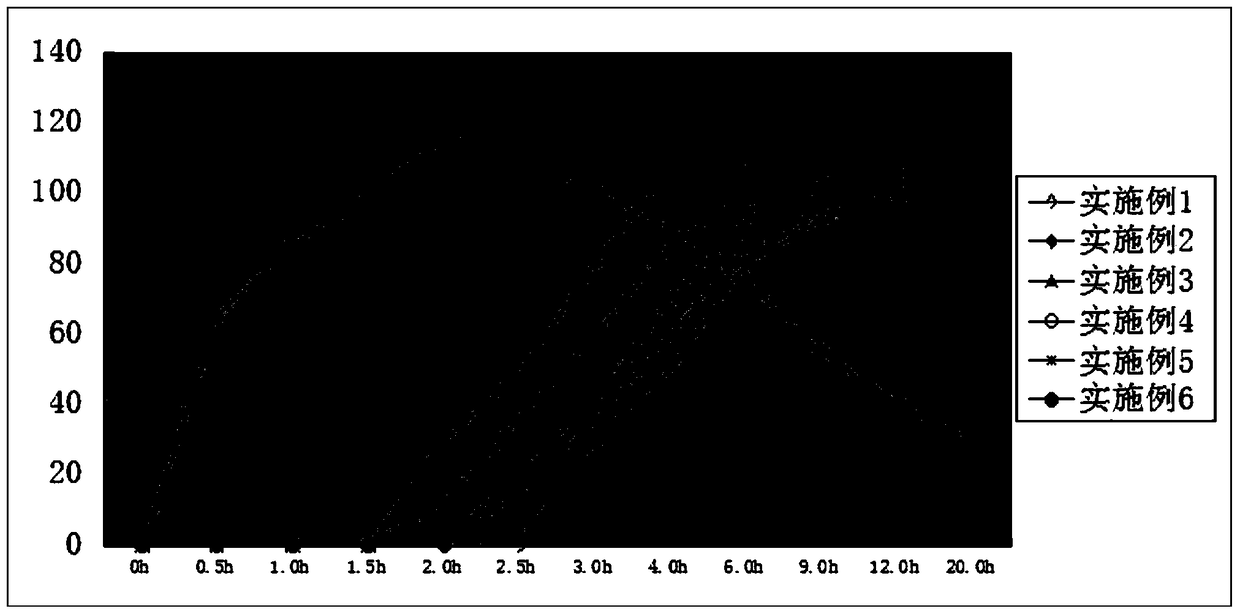
Pulse-controlled release tablet containing fluticasone furoate and vilanterol and preparation method thereof
InactiveCN105796522BSolve the problem of not being able to float and suspend in the stomach contentsAvoid untimely release drugsOrganic active ingredientsRespiratory disorderGastric fluidDissolution
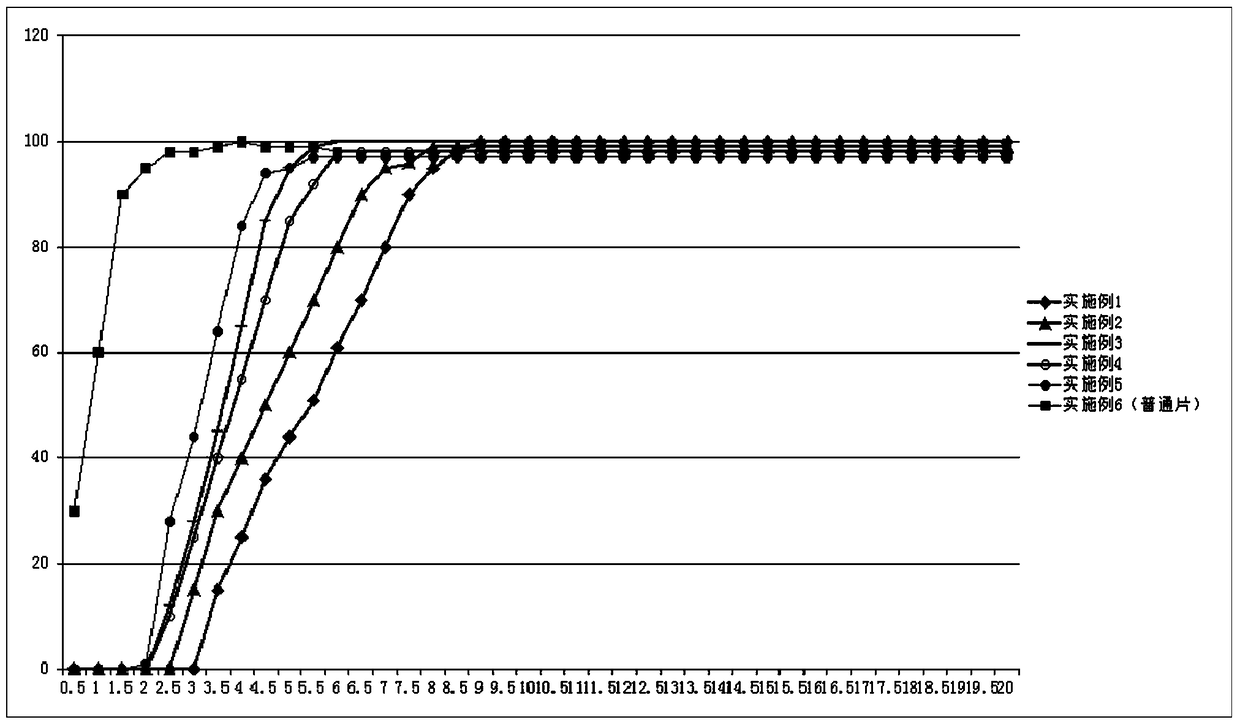
The invention provides a pulsatile controlled-release tablet containing fluticasone furoate and vilanterol. The pulsatile controlled-release tablet can reach plasma concentration at midnight and early morning, so the purpose of chrono-chemotherapy is achieved. The pulsatile controlled-release tablet comprises a floating layer and a tablet core. The floating layer uses hydrophilic high molecules and fatty alcohol as adjuvant materials, so the floating layer can retain for a certain period of time in the stomach; when the tablet contacts with gastric juice, the surface of the floating layer hydrates to form gel, so the size of the tablet becomes larger; when the gel is smaller than the concentration of the gastric juice, the tablet is allowed to float above contents in the stomach; and the tablet core appears along with dissolution of the skeleton of the floating layer, so drugs are released from the tablet core.
Owner:HYBIO PHARMA
Process for preparing fluticasone propionate/furoate
InactiveUS20140148593A1Improve product qualityReduce the possibilitySteroidsFluticasone propionatePerylene derivatives
The present invention relates to an improved process for the preparation of substituted Fluticasone derivatives. The invention also reveals the processes for the purification of Fluticasones and related intermediates to provide the highly pure product.
Owner:CADILA HEALTHCARE LTD
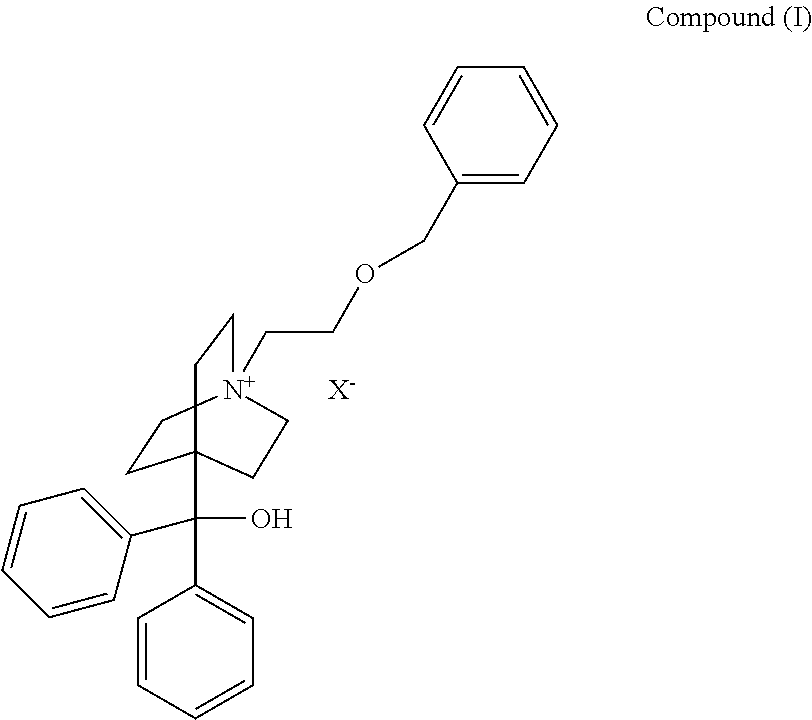
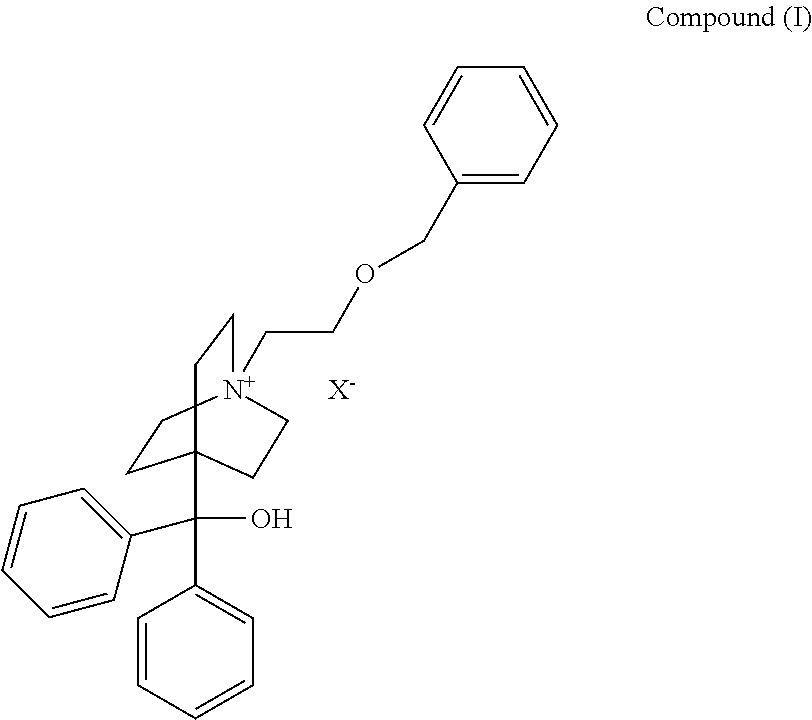
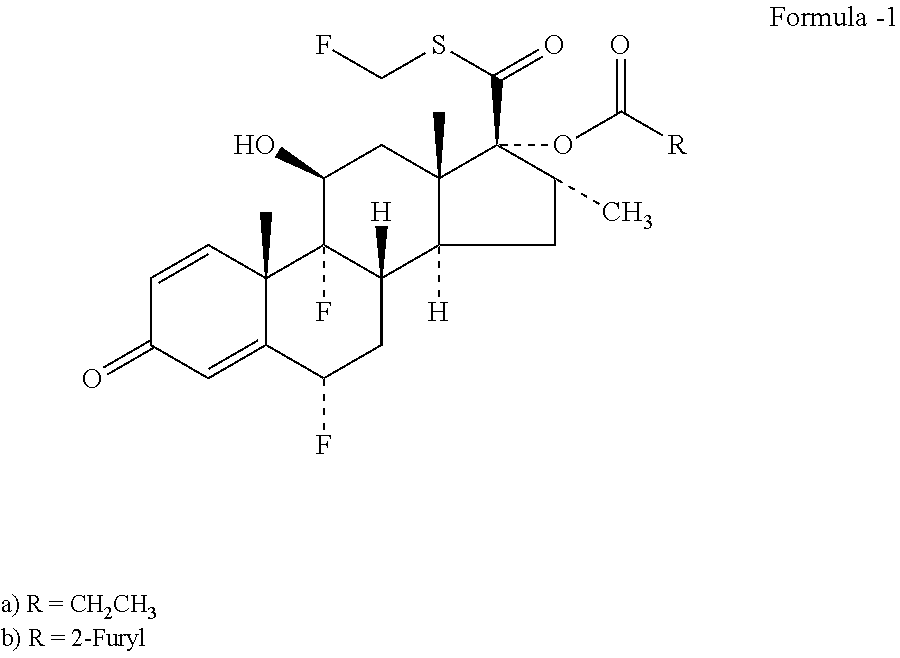
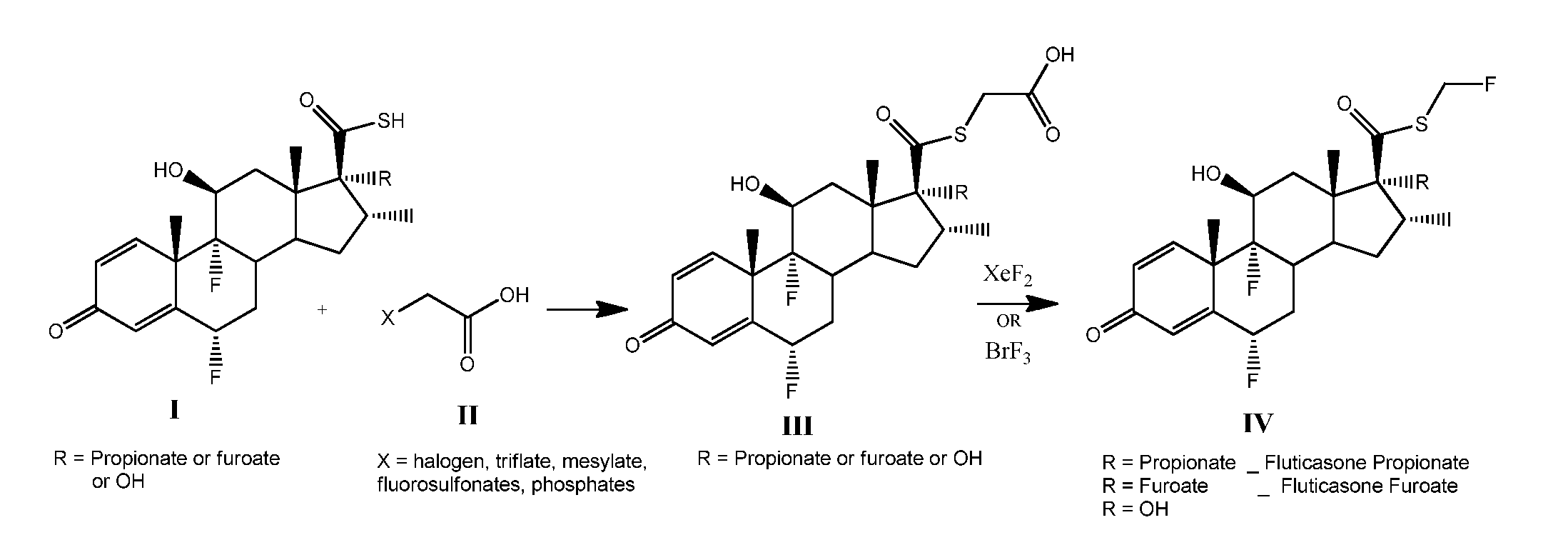
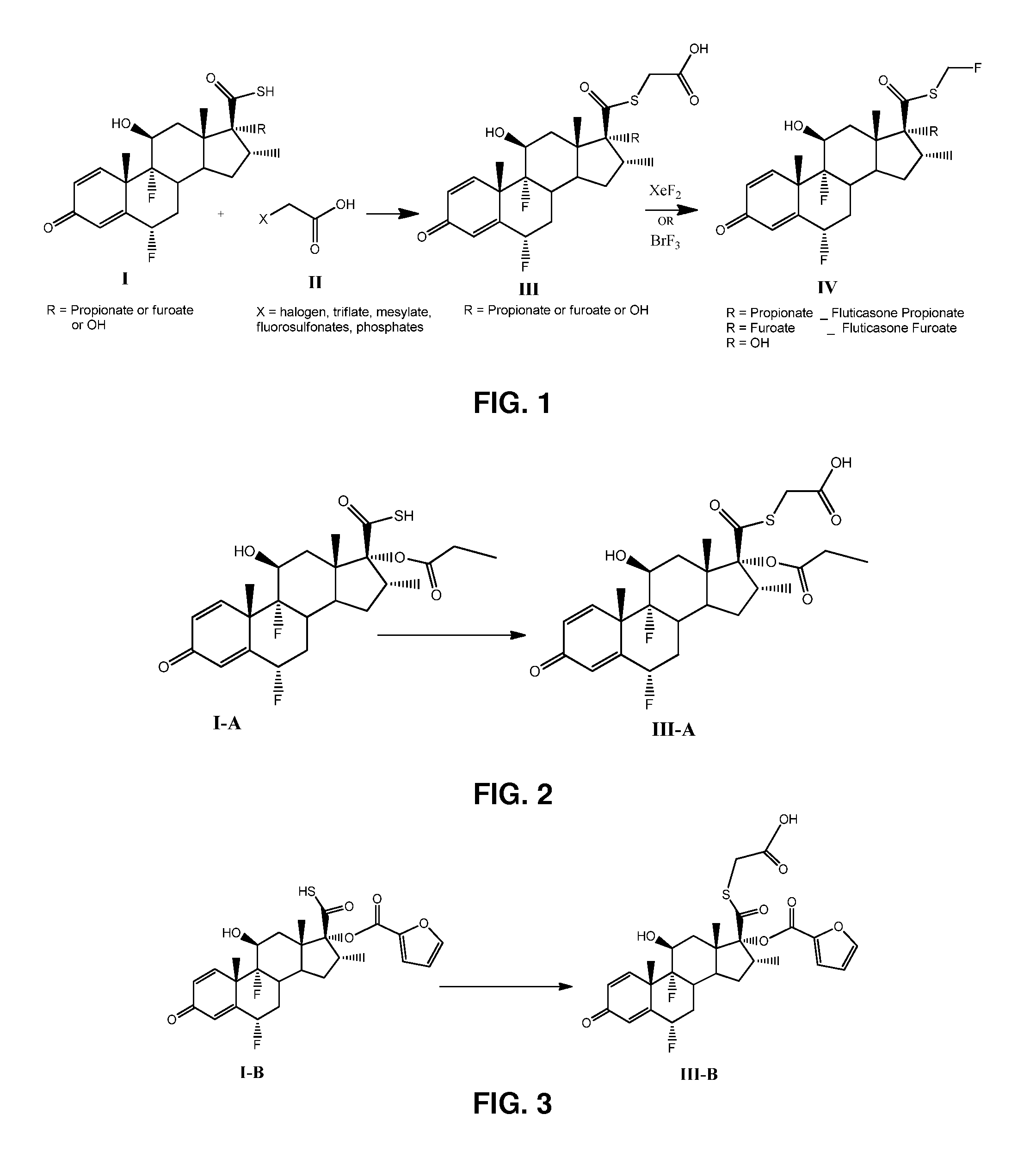
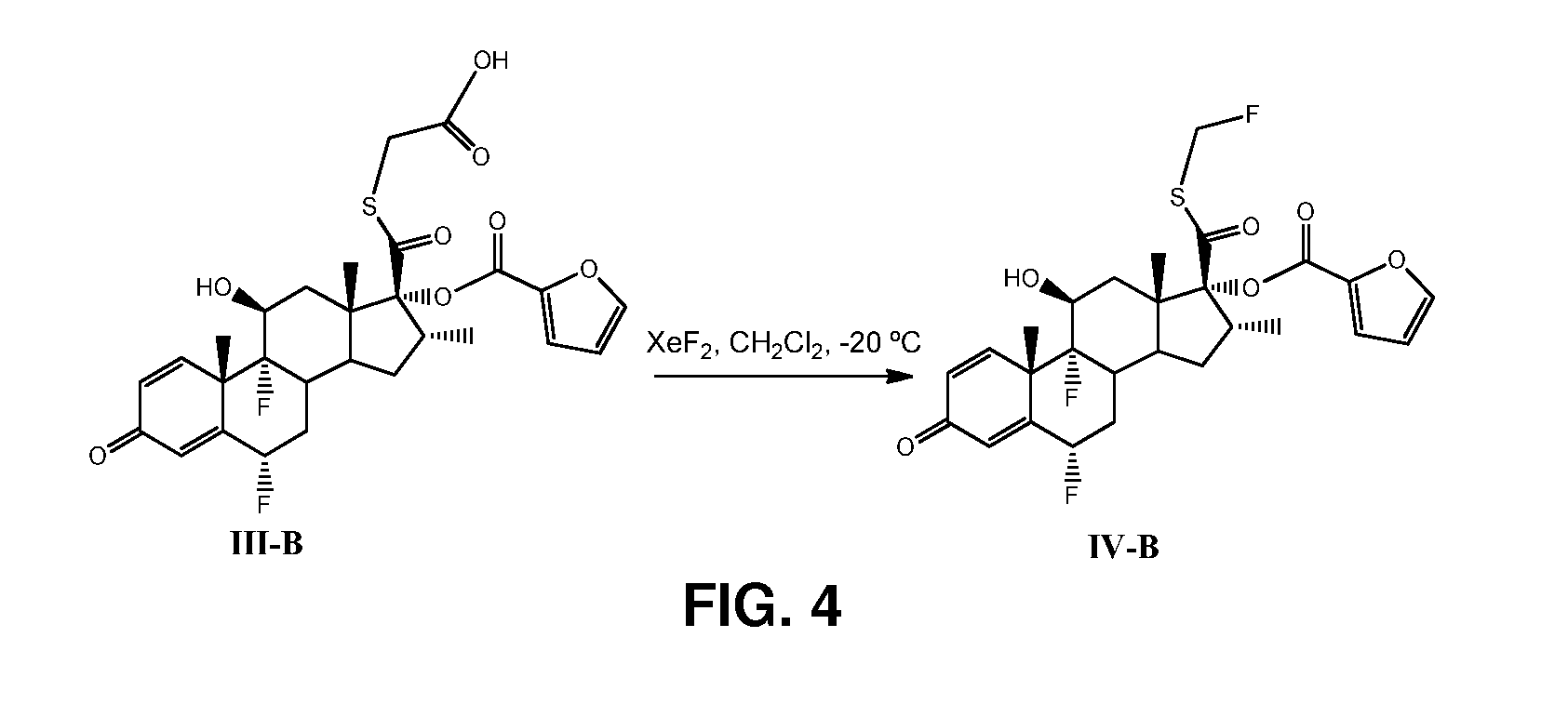
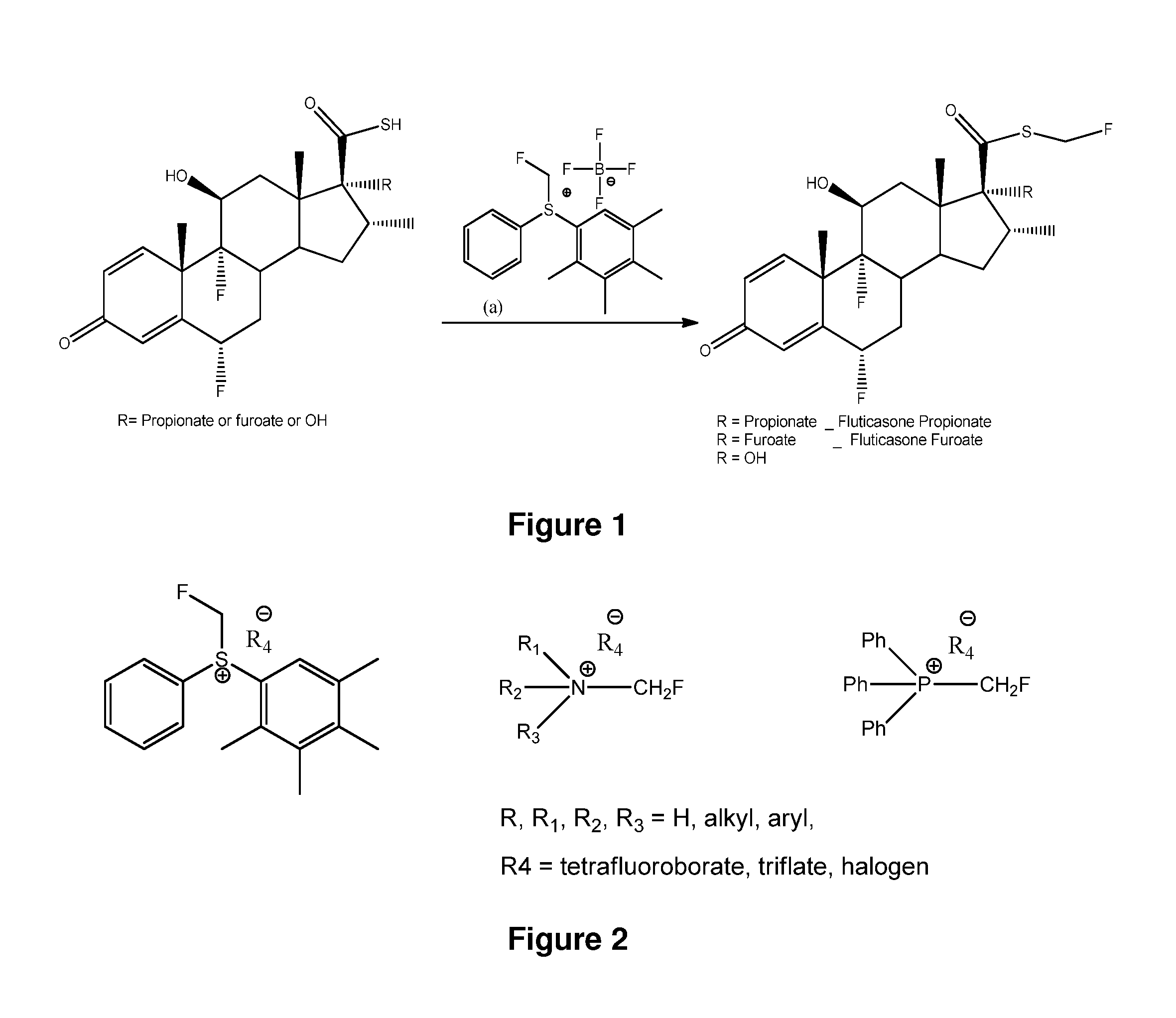
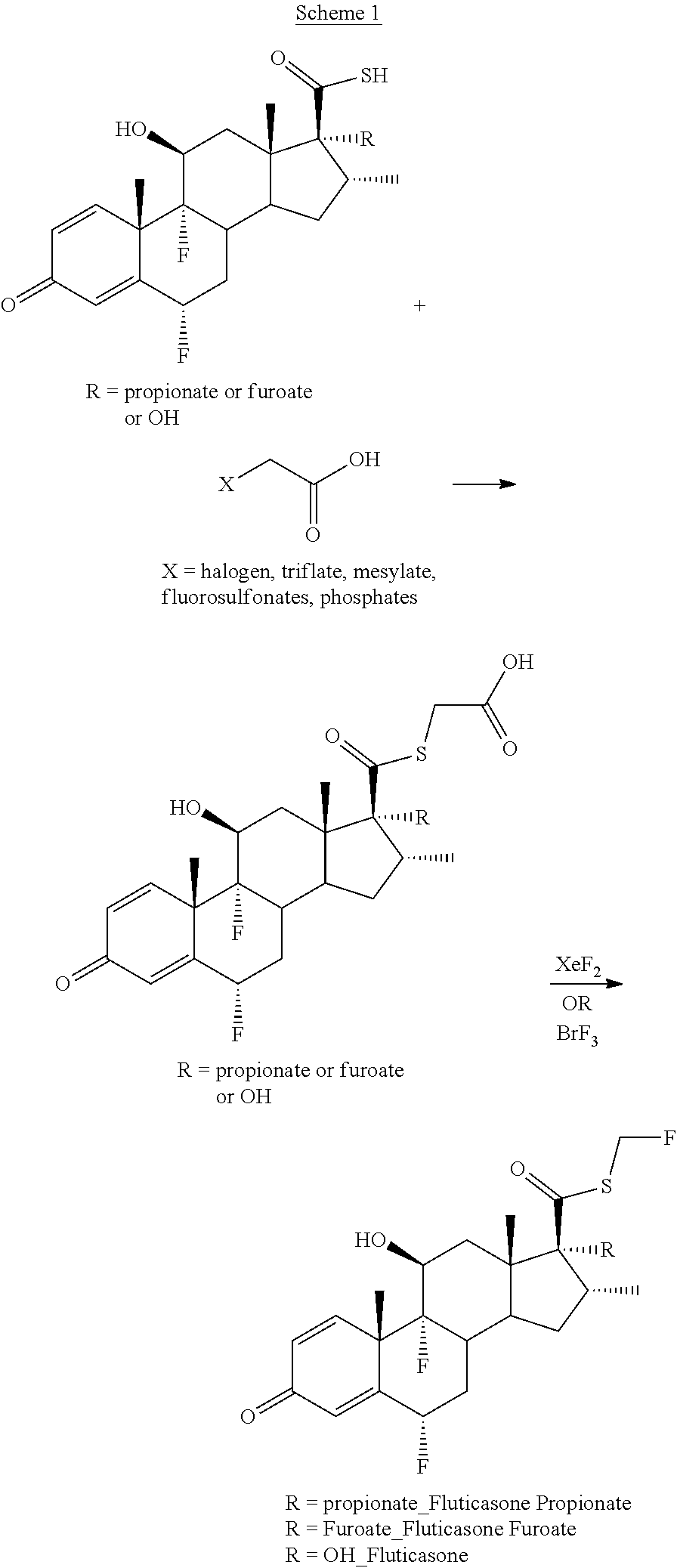
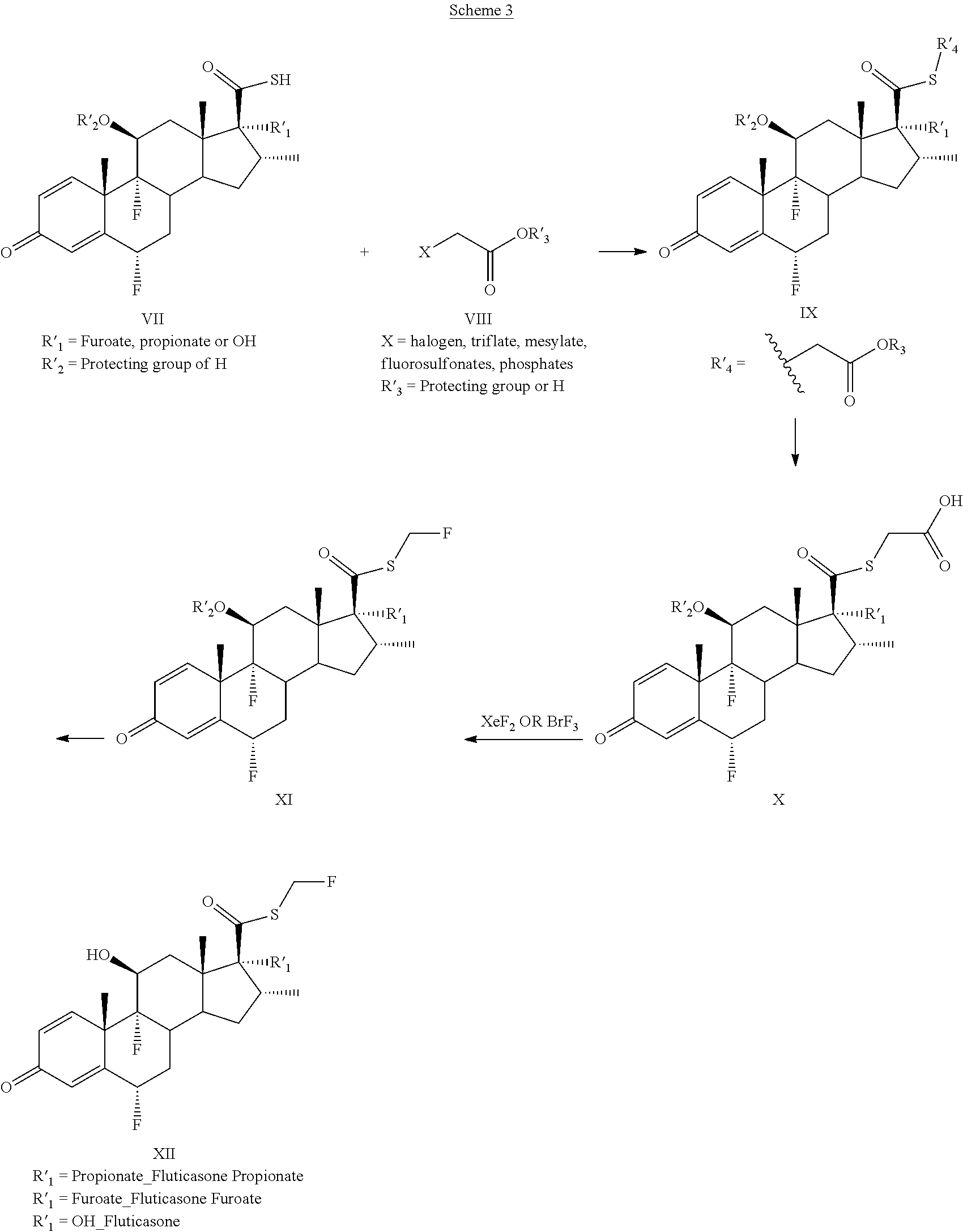
Method and Compounds for the Preparation of Monofluoromethylated Biologically Active Organic Compounds
Described are processes for the preparation of monofluoromethylated organic biologically active compounds, such as Fluticasone Propionate and Fluticasone Furoate, in the presence of fluorodecarboxylating reagents such as XeF2 and BrF3.
Owner:HOVIONE INTER
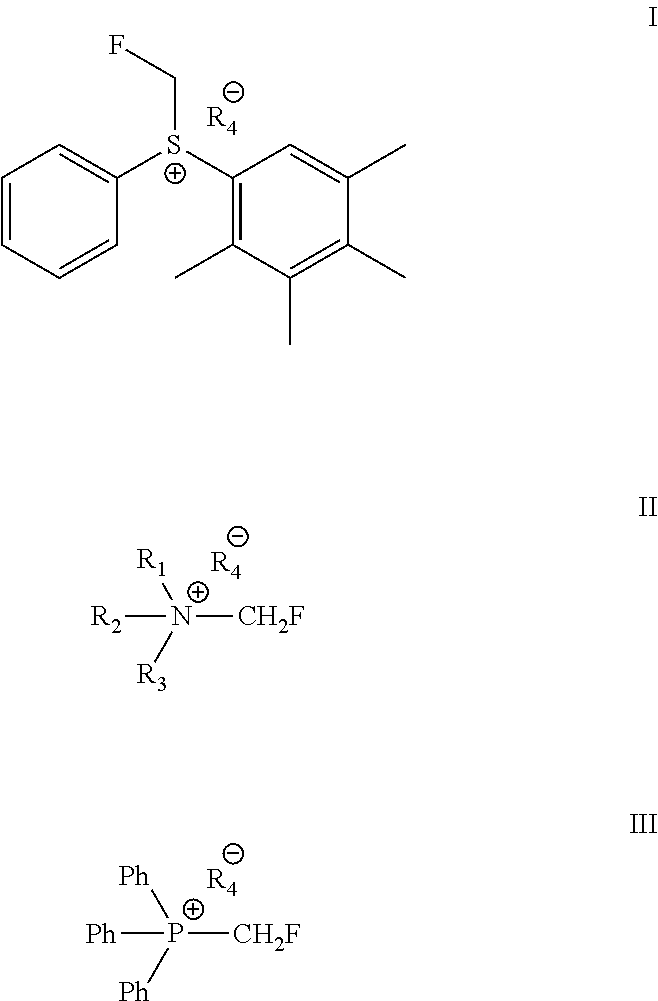
Method for Monofluoromethylation of Organic Substrates to Prepare Biologically Active Organic Compounds
Described is a process for the preparation of monofluoromethylated organic biologically active compounds using monofluoromethylated reagents. Fluticasone Propionate and Fluticasone Furoate can be prepared using, for example, S-monofluoromethyl-S-phenyl-2,3,4,5-tetramethylphenylsulfonium tetrafluoroborate as monofluoromethylating reagent instead of bromofluoromethane.
Owner:HOVIONE INTER
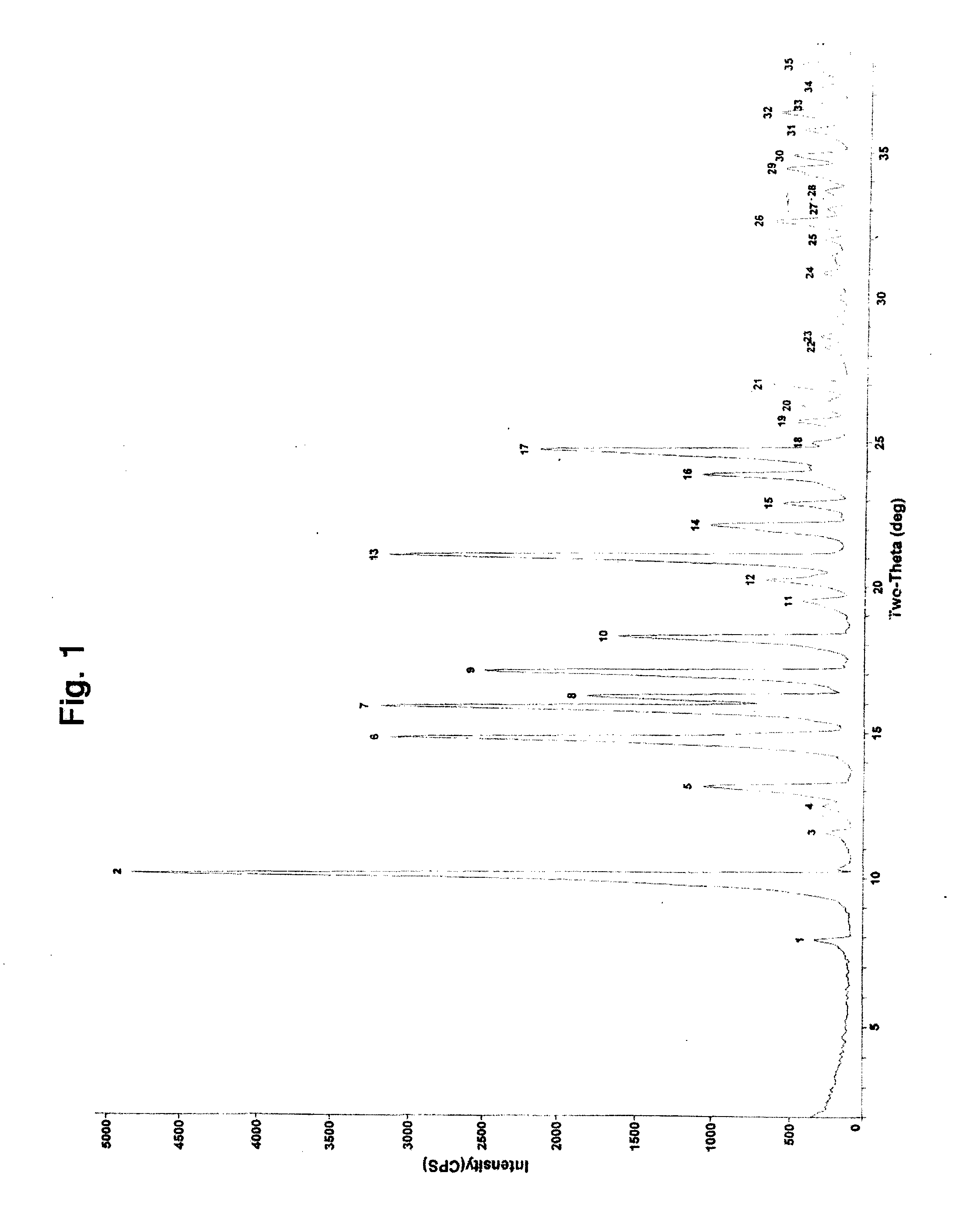
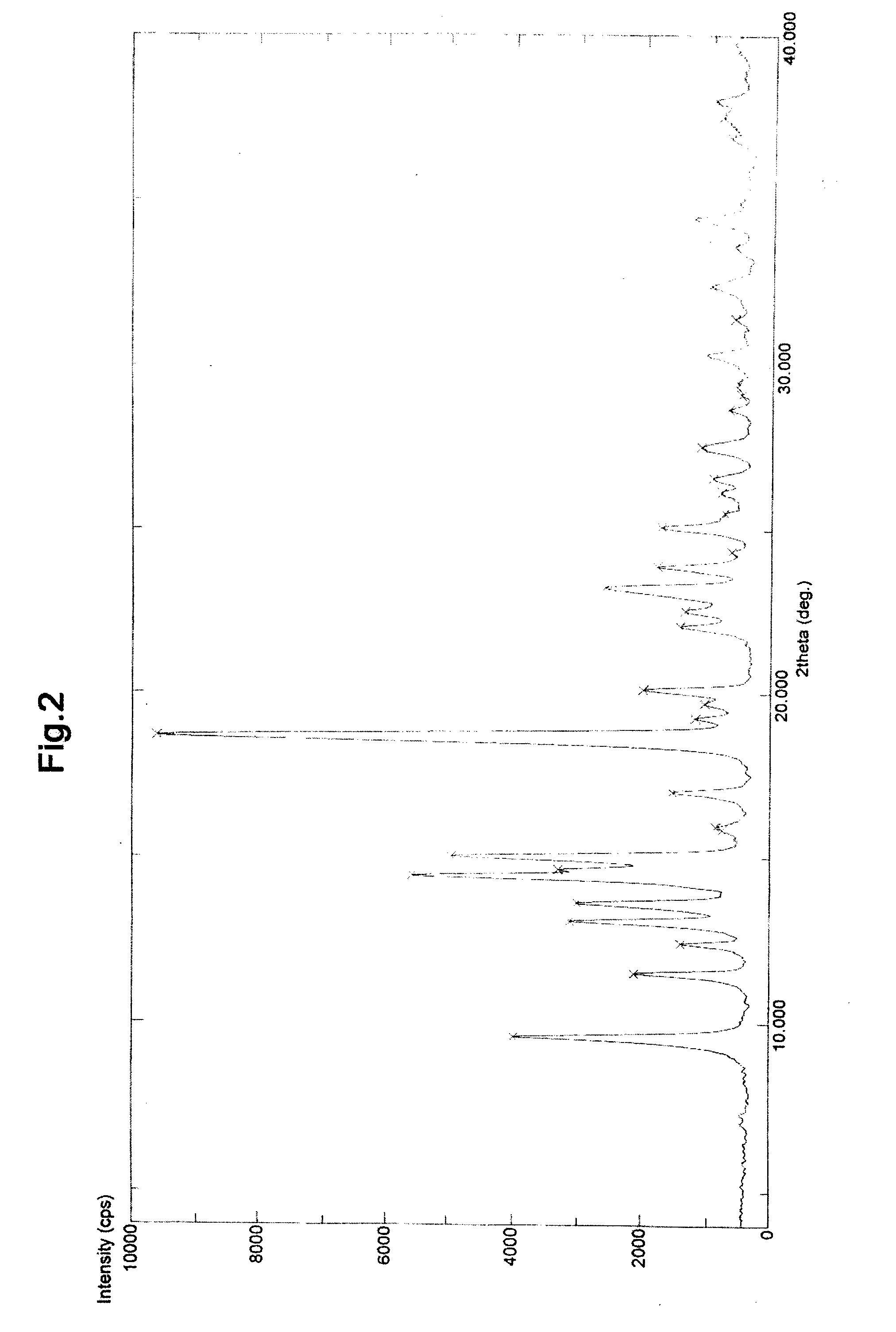
Preparation method of high-purity fluticasone furoate
The invention relates to a preparation method of high-purity fluticasone furoate. The method comprises the steps as follows: adding a compound in formula III, an alcohol solvent and alkali to a reaction flask for an alcoholysis reaction to prepare a mixture of a compound in formula II, and performing after-treatment to prepare a solution of the compound in formula II; adding the solution of the compound in formula II, organic alkali and a fluoro-halogen methylation reagent to a reaction flask for a substitution reaction to prepare a crude product of a compound in formula I, and performing recrystallization to obtain a refined product of the compound in formula I. The target product is prepared by improving the after-tretament method of the compound in formula II and improving preparation methods of the compound in formula II to the compound in formula I, and the method is simple to operate, can effectively control methyl esters and oxidation impurities, is high in yield, high in purityand lower in cost, and can better meet the requirement of medicinal materials and the requirement of industrial enlargement.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD +1
Dermal medicine composition containing fluticasone propionate and nitric oxide synthase (NOS) inhibitor
InactiveCN103127132AEliminate side effectsDoes not increase systemic absorptionOrganic active ingredientsAntipyreticFluticasone propionateBULK ACTIVE INGREDIENT
A dermal medicine composition containing fluticasone propionate and a nitric oxide synthase (NOS) inhibitor comprises the fluticasone propionate used as an active ingredient, an amino acid derivative used as the NOS inhibitor, one type or a plurality of types of medicine auxiliary materials suitable for skins and the balance water.
Owner:TIANJIN JINYAO GRP
Inhalation preparation containing calcitriol and fluticasone propionate and preparation method thereof
InactiveCN102247384ALower serum IgEPrevents loss of bone densityOrganic active ingredientsPharmaceutical delivery mechanismFluticasone propionateActive component
The invention relates to an inhalation preparation containing calcitriol and fluticasone propionate and a preparation method thereof. The inhalation preparation comprises calcitriol, and fluticasone propionate as active components, and one or more of pharmaceutic adjuvants which are suitable for inhalation administration, a mass ratio of ciclesonide to calcitriol is 100-800:1, and the inhalation preparation is used for treating airway diseases of humans or mammals.
Owner:TIANJIN JINYAO GRP
Nasal medicine composition enclosing fluticasone propionate by cyclodextrin
InactiveCN101757634AGood effectQuick effectOrganic active ingredientsRespiratory disorderFluticasone propionateMedicine
The invention relates to a nasal medicine composition enclosing fluticasone propionate by cyclodextrin, which contains the fluticasone propionate and one or more auxiliary material medicine compositions acceptable in pharmacy. The nasal medicine composition is characterized that the fluticasone propionate contained in the medicine composition and used as an active component is enclosed by the cyclodextrin and / or derivatives thereof. The medicine composition is used for treating the allergic rhinitis of human or mammals.
Owner:TIANJIN JINYAO GRP
Transdermally-absorbed medicament used for skin and consisting of adjuvant-containing fluticasone propionate and adjuvant-containing water
InactiveCN102657659AGuaranteed stabilityReduce wasteOrganic active ingredientsSolution deliveryAdjuvantFluticasone propionate
A transdermally-absorbed medicament used for skin and consisting of adjuvant-containing fluticasone propionate and adjuvant-containing water comprises: fluticasone propionate which is single packaged and water-insoluable, has a D90 particle size of 0.1 to 10 [mu]m, and contains one or a plurality of solid pharmaceutic adjuvants; and water which is single packaged and contains one or a plurality of water-soluable pharmaceutic adjuvants.
Owner:TIANJIN JINYAO GRP
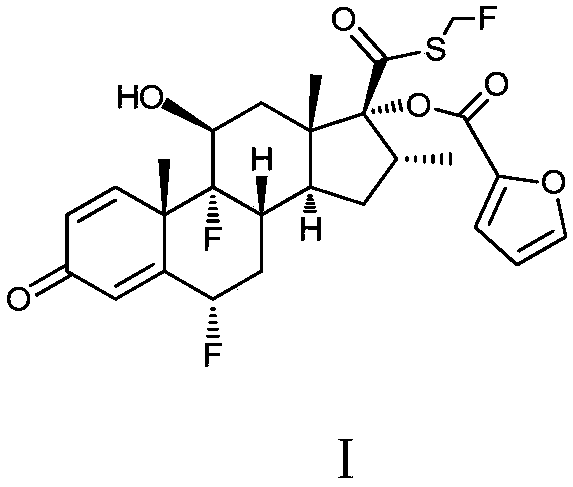
Method for preparing fluticasone furoate
Method for preparing fluticasone furoate (6α,9α-difluoro-11β-hydroxy-16α-methyl-17α-[(2-furoyl)oxy]-3-oxo-androsta-1,4-diene-17β-carbothioic acid S-fluoromethyl ester) by directly subjecting a compound of Formula III and a complex of a fluoromethylating reagent in presence of an organic base to a replacement reaction to obtain the target compound. Generation of impurities in a process via Compound IV is avoided; the method is simple with mild reaction conditions, suitable for industrial production, and yields products with purity of 98% by HPLC.
Owner:AURISCO PHARMACEUTICAL CO LTD
Pulsatile controlled-release tablet containing fluticasone furoate and vilanterol and preparation method thereof
InactiveCN105796522ASolve the problem of not being able to float and suspend in the stomach contentsAvoid untimely release drugsOrganic active ingredientsRespiratory disorderDissolutionControlled Release Tablet
The invention provides a pulsatile controlled-release tablet containing fluticasone furoate and vilanterol. The pulsatile controlled-release tablet can reach plasma concentration at midnight and early morning, so the purpose of chrono-chemotherapy is achieved. The pulsatile controlled-release tablet comprises a floating layer and a tablet core. The floating layer uses hydrophilic high molecules and fatty alcohol as adjuvant materials, so the floating layer can retain for a certain period of time in the stomach; when the tablet contacts with gastric juice, the surface of the floating layer hydrates to form gel, so the size of the tablet becomes larger; when the gel is smaller than the concentration of the gastric juice, the tablet is allowed to float above contents in the stomach; and the tablet core appears along with dissolution of the skeleton of the floating layer, so drugs are released from the tablet core.
Owner:HYBIO PHARMA
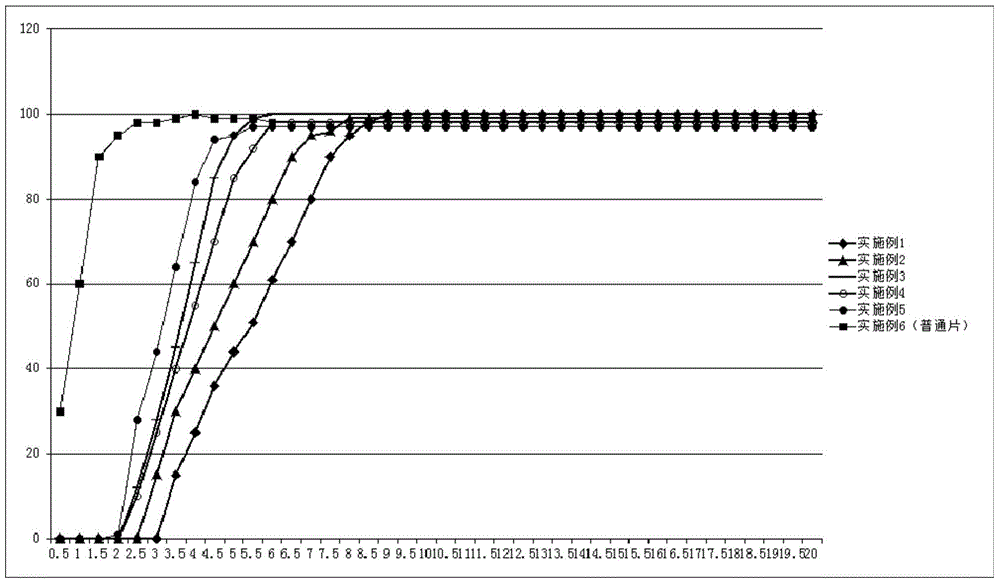
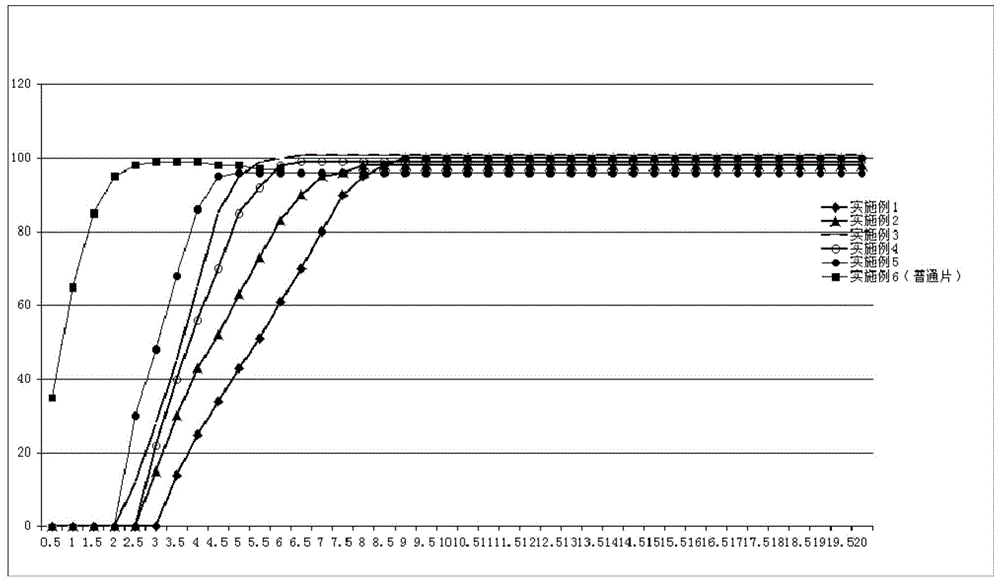
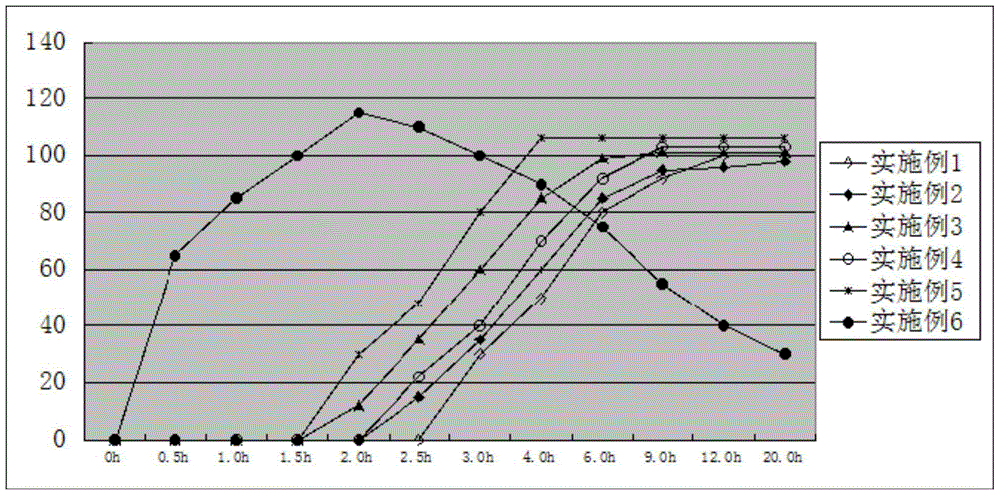
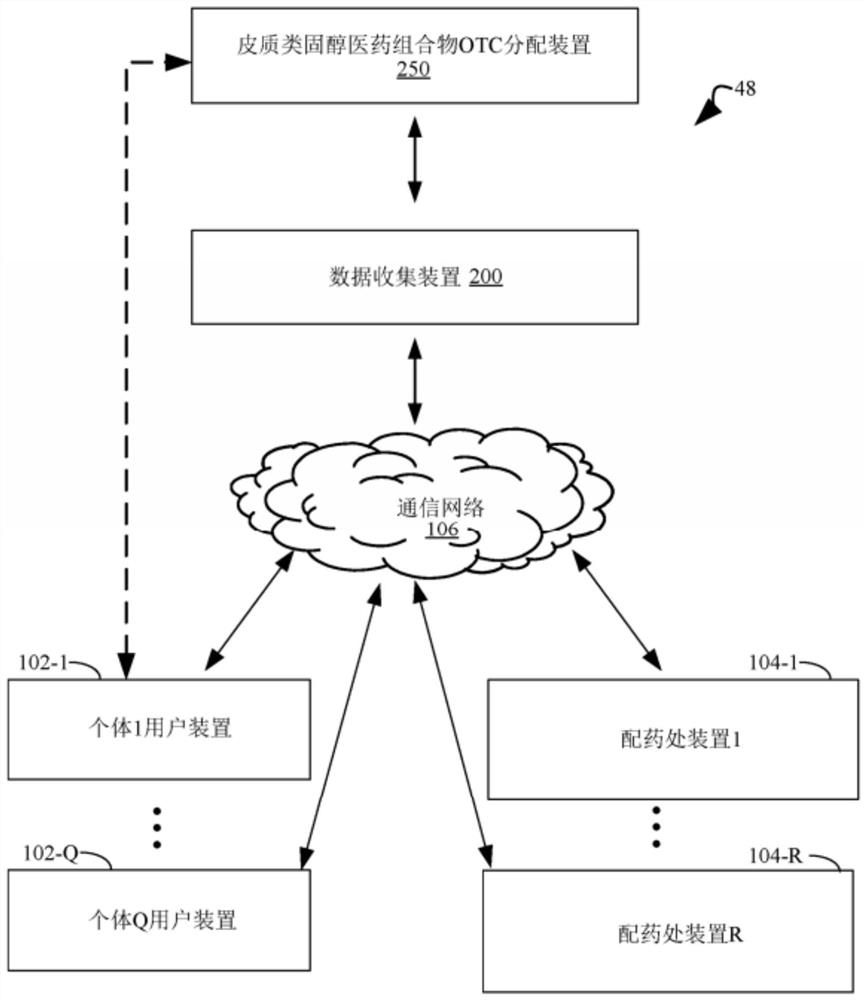
Methods for treating and preventing symptoms of asthma with a corticosteroid pharmaceutical composition
PendingCN112997256AOrganic active ingredientsDigital data information retrievalFluticasone propionateMometasone furoate
A method is provided for treating or preventing symptoms of asthma in a subject in need thereof by administering a corticosteroid pharmaceutical composition to a subject qualified for over-the-counter access to the corticosteroid pharmaceutical composition. In some embodiments, the corticosteroid pharmaceutical composition includes a class B corticosteroid, a glucocorticosteroid, budesonide, ciclesonide, fluticasone furoate, mometasone furoate, fluticasone propionate, or beclomethasone dipropionate.
Method for the Production of Fluoromethyl Esters of Androstan-17 beta Carboxylic Acids
Described herein are processes for the preparation of monofluoromethylated organic biologically active compounds, starting from protected intermediates and / or reagents to obtain compounds such as fluticasone propionate and fluticasone furoate, in presence of decarboxylating reagents XeF2 and BrF3, or using FCH2SH as a reagent.
Owner:HOVIONE INTER
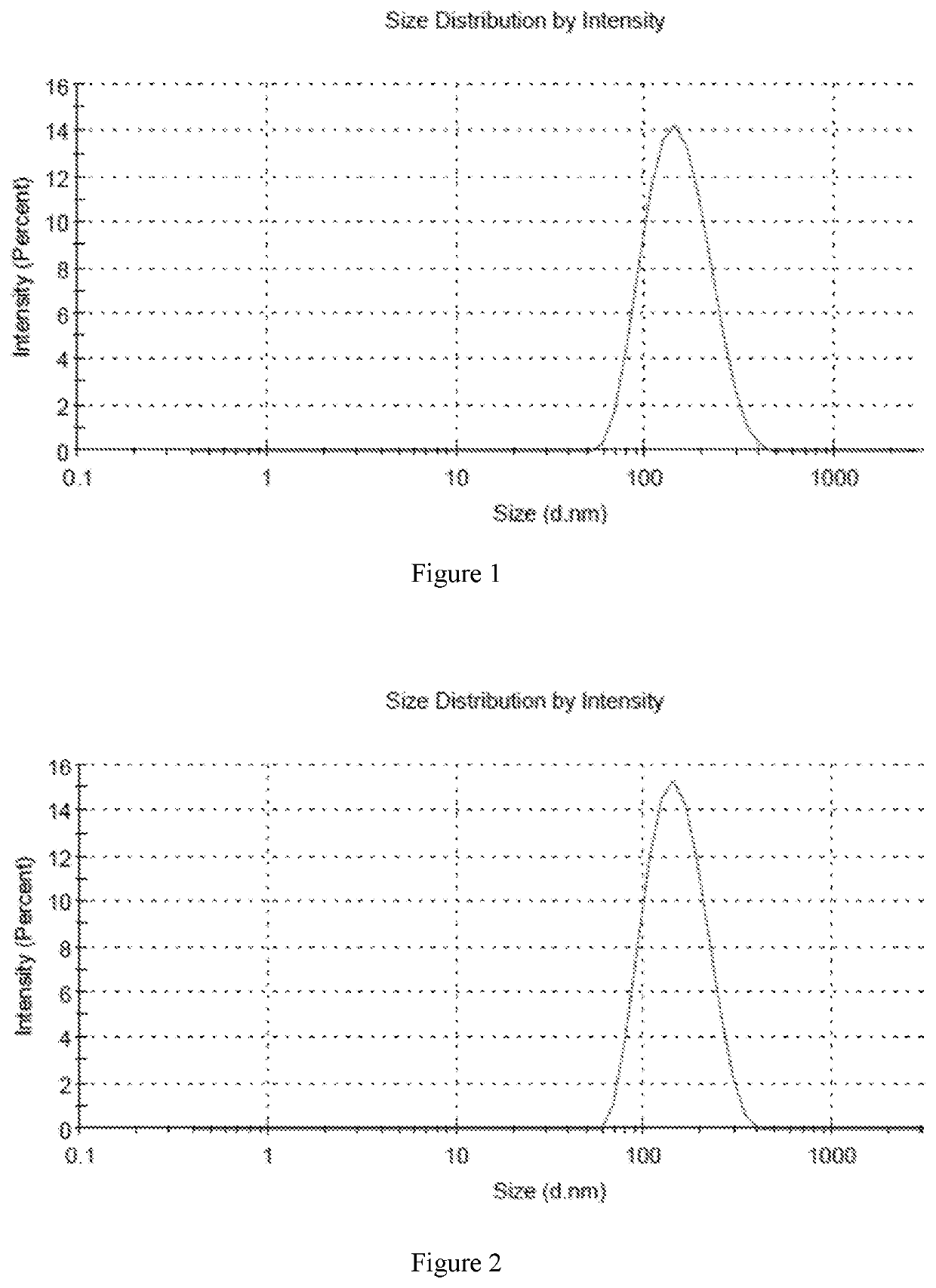
Liposome Formulation of Fluticasone Furoate and Method of Preparation
ActiveUS20210059937A1Improve uniformityMinimize side effectsOrganic active ingredientsDispersion deliverySterolSaline solutions
The present invention is directed to a liposomal formulation having a lipid ingredient encapsulating fluticasone furoate, and a method for preparing the liposomal formulation. The liposome formulation comprises a lipid and a sterol. The method of preparing the liposomes comprises the steps of (1) mixing fluticasone furoate with lipid ingredients comprising a lipid and a sterol, (2) injecting the mixture into normal saline solution, and (3) ultrafiltering and concentrating the resulting solution. This preparation method can produce a liposome formulation having desirable properties and compositions, for example, the ratio of the lipid ingredient, the drug to lipid ratio, and the pH value, which is suitable for nebulization inhalation.
Owner:ANOVENT PHARM U S LLC
Dosage forms containing fluticasone propionate for the treatment of inflammatory conditions of the esophagus
InactiveUS20170266202A1Increase contact timeHigh viscosityOrganic active ingredientsDispersion deliveryFluticasone propionateMedicine
Owner:EMS SA
A kind of fluticasone furoate liposome suspension and preparation method thereof
ActiveCN111789816BHigh encapsulation efficiencySimple production processOrganic active ingredientsDispersion deliverySterolPhospholipid
The invention provides a fluticasone furoate liposome and a preparation method thereof. The fluticasone furoate liposome comprises phospholipids, sterols and fluticasone furoate, wherein the amount of fluticasone furoate is 0.01% to 1%, and the amount of phospholipids is 0.065% %~0.196%, the amount of sterol is 0.035%~0.104%, the preparation method of the fluticasone furoate liposome comprises: (1) mixing and injecting (2) diafiltration concentration steps, the method of the present invention is simple to operate, and the process is stable , suitable for mass production.
Owner:SHANGHAI ANOVENT PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com