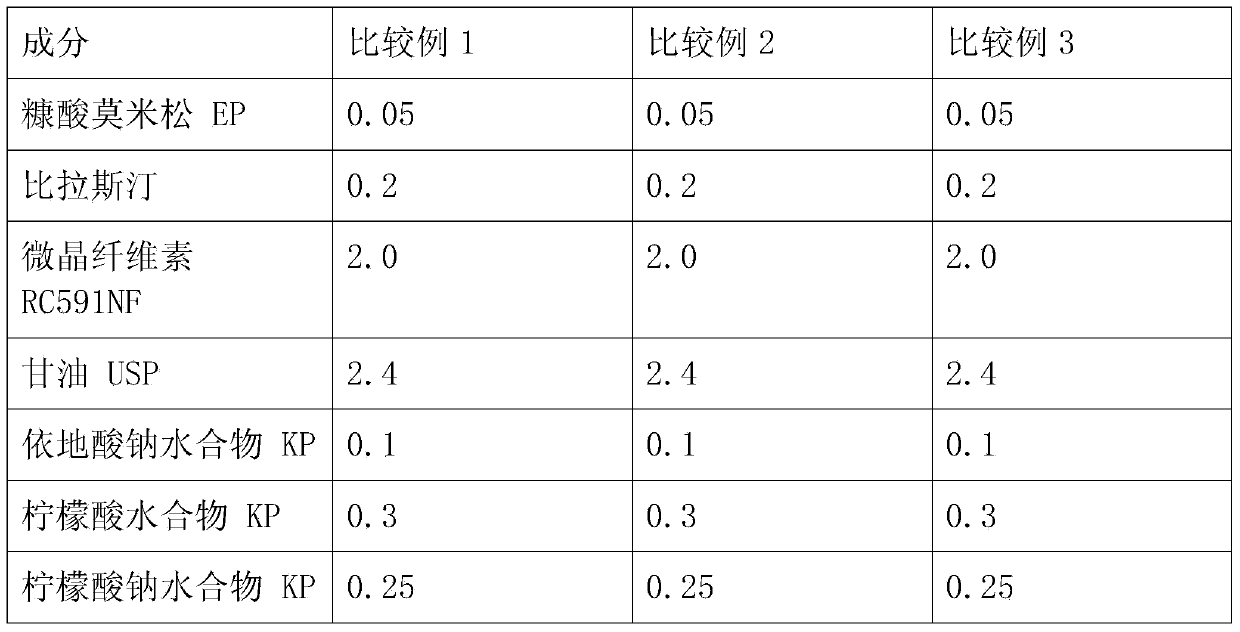
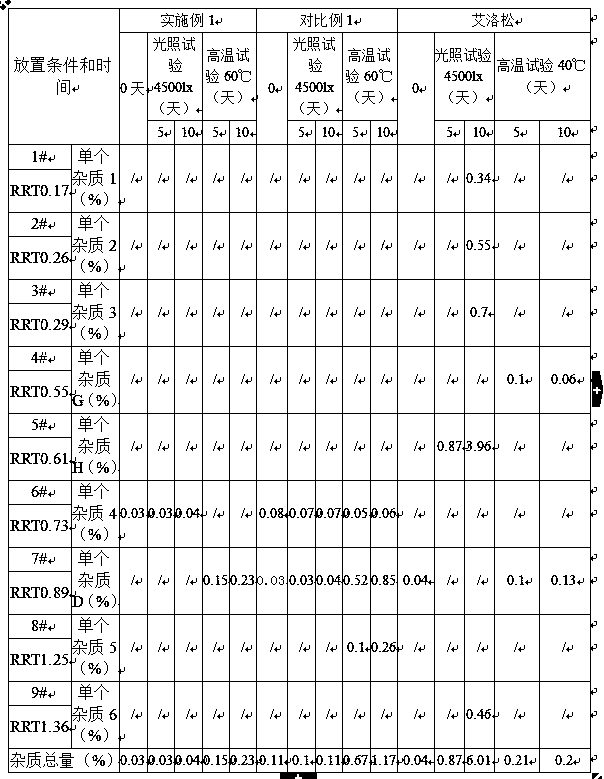
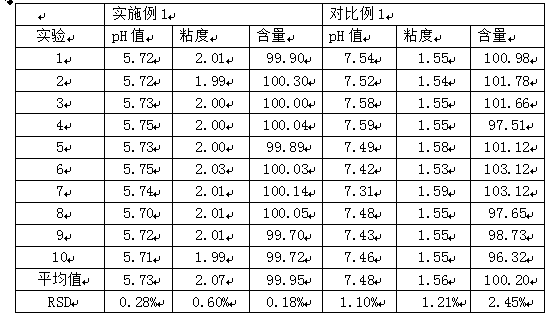
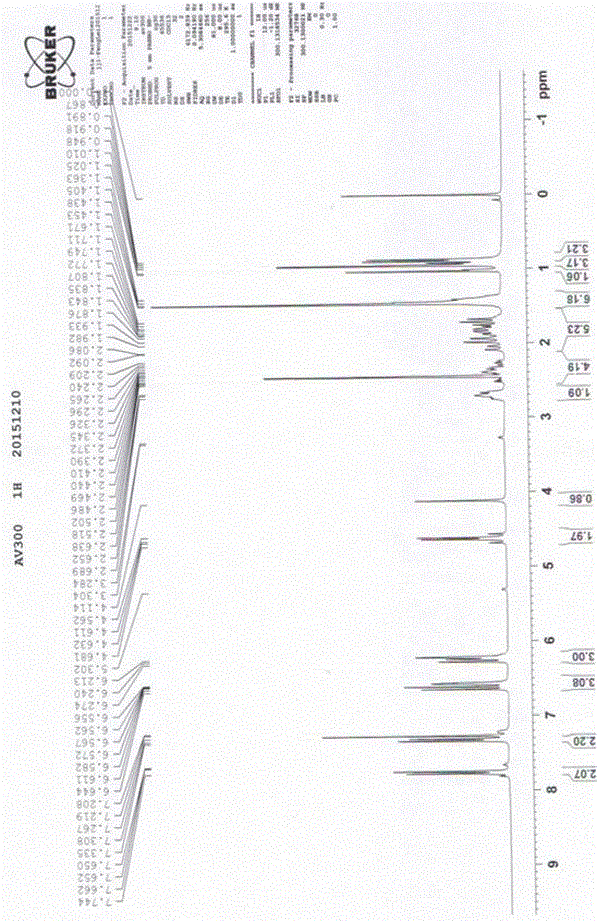
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
112 results about "Mometasone furoate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mometasone, also known as mometasone furoate, is a steroid medication used to treat certain skin conditions, hay fever, and asthma. Specifically it is used to prevent rather than treat asthma attacks. It can be applied to the skin, inhaled, or used in the nose.
Use of mometasone furoate for treating airway passage and lung diseases
InactiveUS6057307AMaximize treating said rhinitisMinimize absorptionPowder deliveryBiocideDiseaseAerosolize
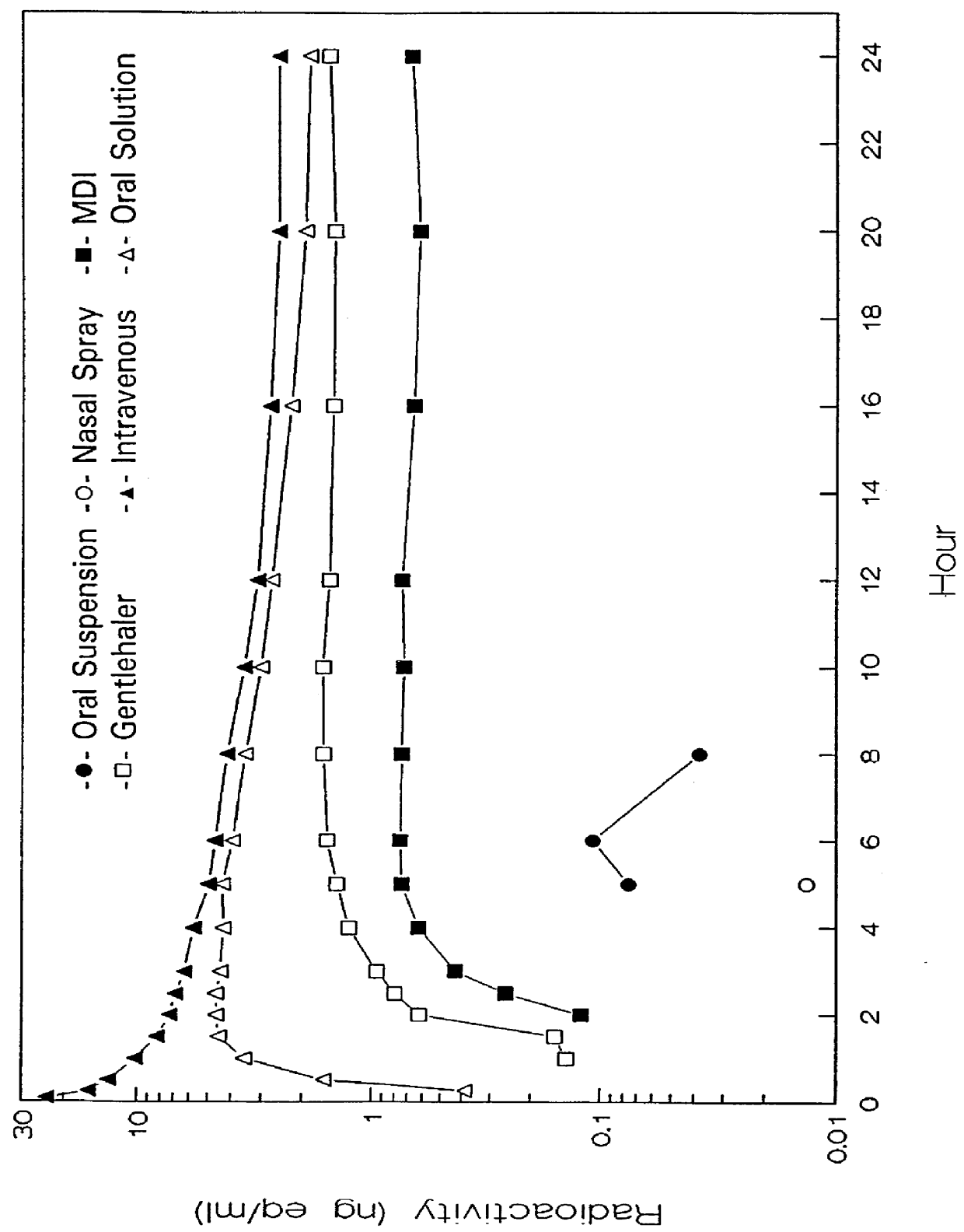
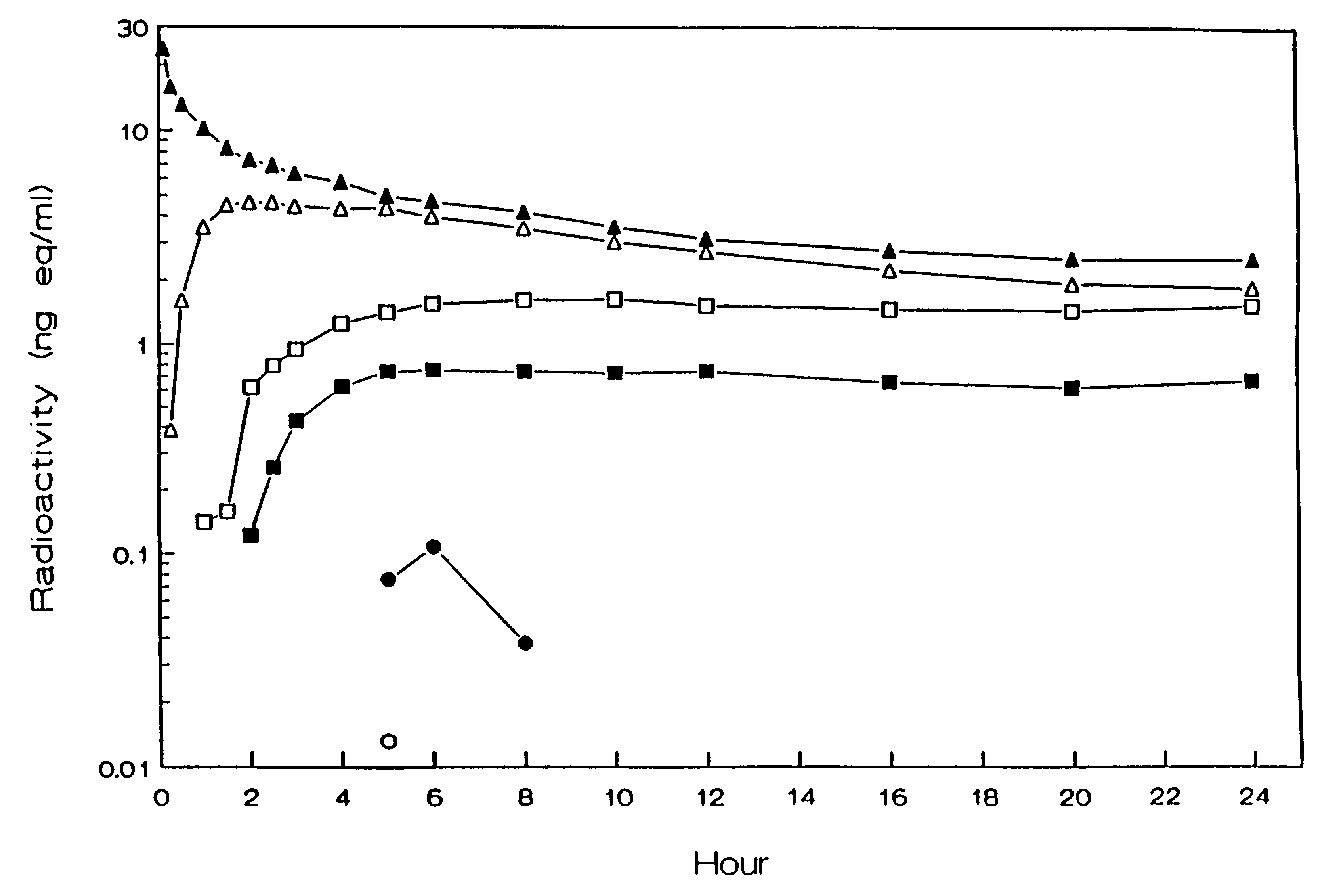
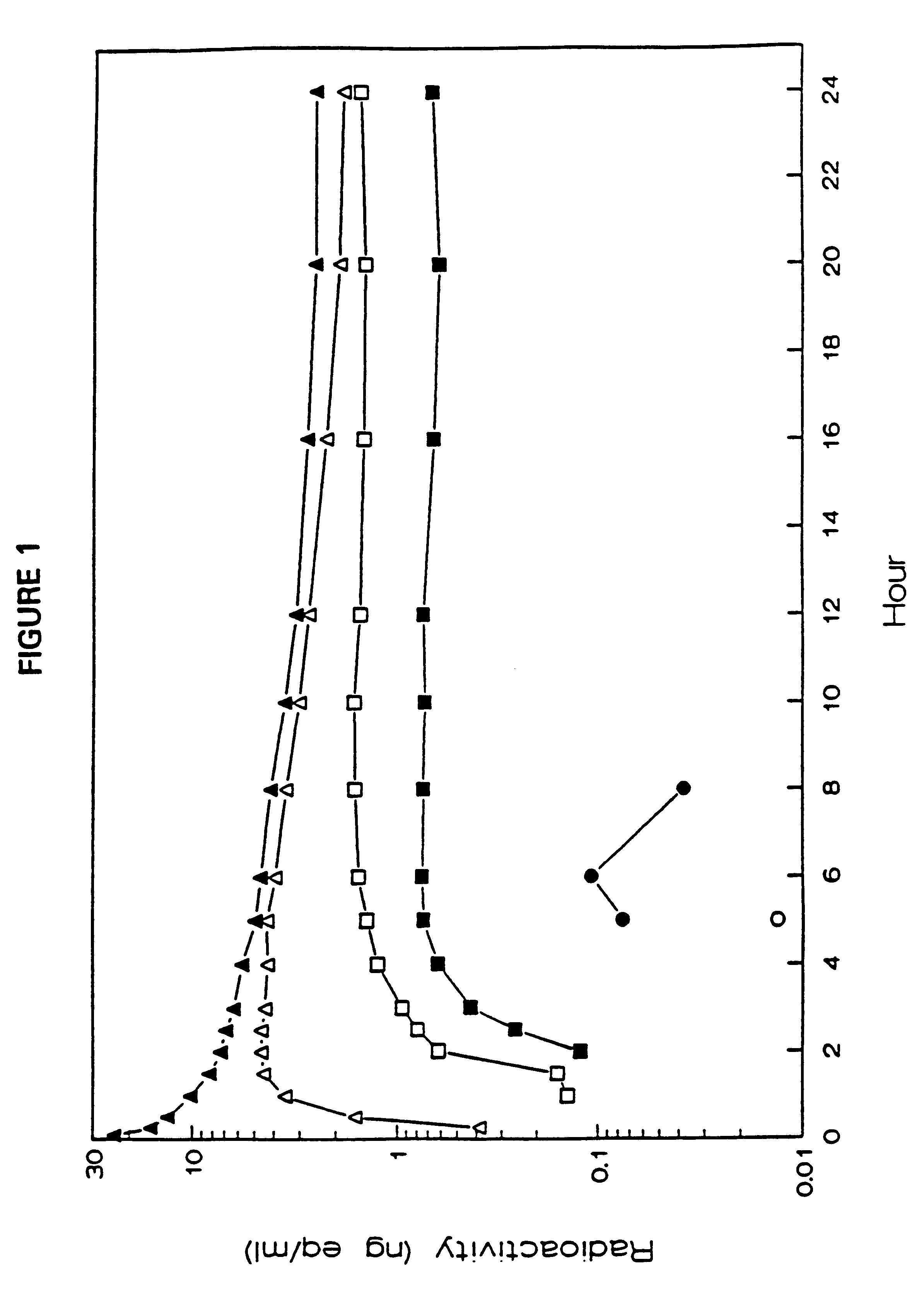
The administration of aerosolize particles of mometasone furoate in the form of dry powders, solutions, or aqueous suspension for treating corticosteroid-responsive diseases of the surfaces of upper and / or lower airway passages and / or lungs, e.g., allergic rhinitis and asthma is disclosed.
Owner:MERCK SHARP & DOHME CORP
Use of mometasone furoate for treating airway passage and lung diseases
InactiveUS6365581B1Maximize treating said rhinitisMinimize absorptionBiocideOrganic active ingredientsDiseaseAerosolize
Owner:MERCK SHARP & DOHME CORP
Chlorofluorocarbon-free mometasone furoate aerosol formulations
The invention relates to suspension aerosol formulations which exhibit stable particle sizes, containing mometasone furoate, about 1 to about 10 weight percent ethanol and 1,1,1,2,3,3,3-Heptafluoropropane as the propellant. A surfactant, such as oleic acid, can also be included. These formulations are suitable for use in metered dose inhalers.
Owner:MERCK SHARP & DOHME CORP
Pharmaceutical compositions for the treatment of asthma
InactiveUS20050147565A1Improve stabilityGood compatibilityOrganic active ingredientsDispersion deliveryMometasone furoateFormoterol Fumarate
Disclosed are aerosolized formulations for the treatment of asthma that contain mometasone furoate and formoterol fumarate and processes for preparing the same.
Owner:SCHERING CORP
Pharmaceutical composition including mometasone furoate and azelastine hydrochloride for nasal administration
InactiveUS8859531B2Reduce bitternessLess irritatingBiocidePharmaceutical delivery mechanismAzelastine hclNasal cavity
The present invention provides a pharmaceutical composition for nasal administration comprising mometasone furoate and azelastine hydrochloride, wherein the pharmaceutical composition comprises thaumatin as an agent for reducing bitterness and irritation.
Owner:HANLIM PHARMA CO LTD
Process for producing metered dose inhaler formulations
InactiveUS20080066744A1Organic active ingredientsPowder deliveryBULK ACTIVE INGREDIENTMometasone furoate
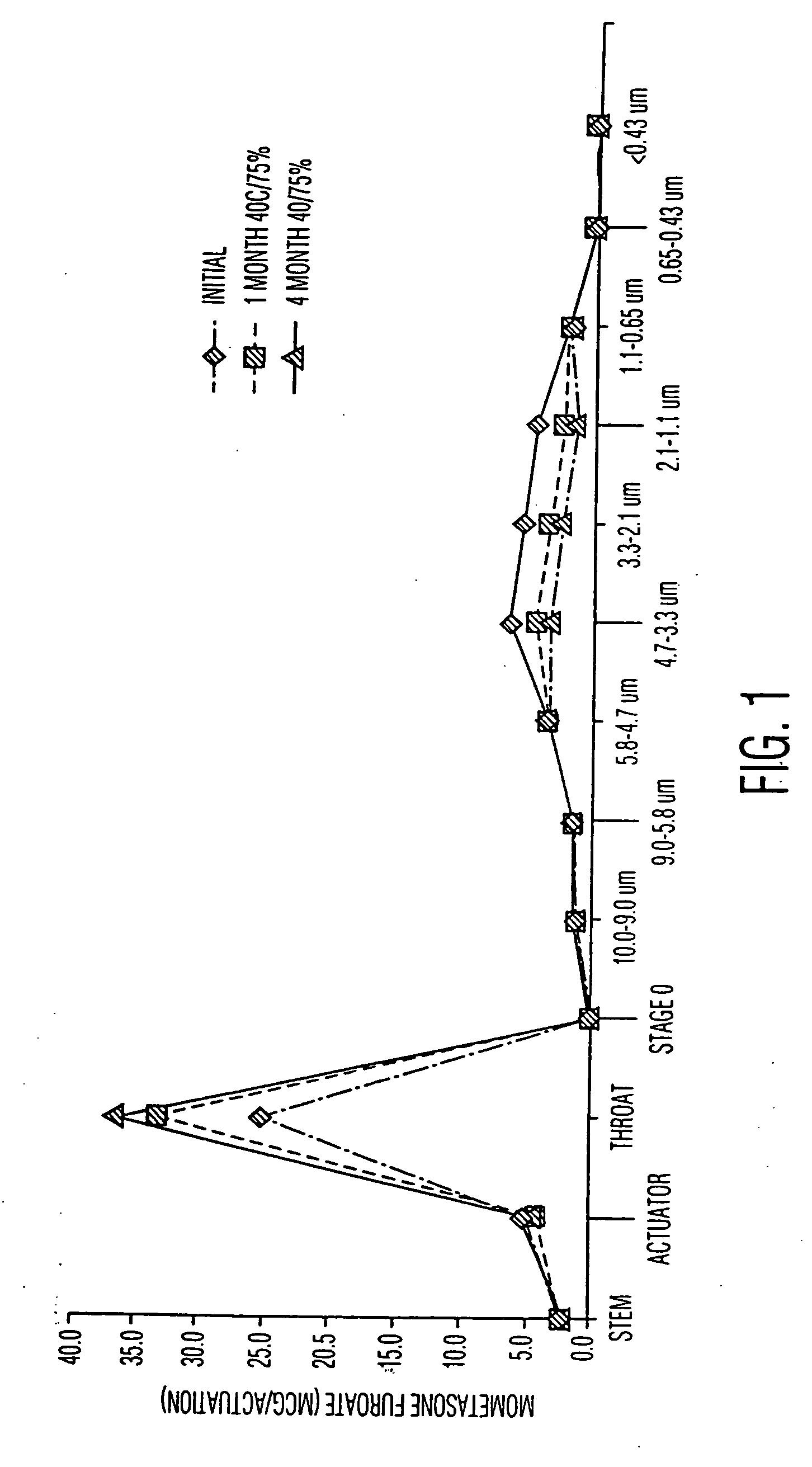
Disclosed are methods of introducing a suspension or solution of a medicament, preferably mometasone furoate anhydrous, into a metered dose inhaler container, said container having a valve attached thereto, said method comprising the steps of introducing mometasone furoate anhydrous, a surfactant and a chlorofluorocarbon free propellant into a vessel that is held under pressure to form a suspension or solution, circulating said suspension or solution from the vessel through a line which includes a filling head, bringing said filling head into communication with said metered dose inhaler container through said valve of said metered dose inhaler container, introducing a quantity of such suspension or solution into the container from the filling head of the line through said valve of said metered dose inhaler container, withdrawing said filling head from said metered dose inhaler container, and sealing said metered dose inhaler container, as well as the products produced thereby having an improved particle size distribution of the active ingredients in metered dose inhalers.
Owner:MERCK SHARP & DOHME CORP
Pulmonary disease treatment
The invention relates to treating chronic obstructive pulmonary disease, involving the administration by inhalation of mometasone furoate particles in daily doses where at least about 250 μg of the inhaled particles have sizes equal to or less than 6.5 μm.
Owner:SCHERING CORP
External use antifungal compound composition and its use
The present invention relates to antifungal composition with two or more active components and its application in medicine, cosmetics and sanitary articles. The externally applied antifungal composition butenafine, butenafine hydrochloride or ketoconazole in 0.1-10 wt%, and mometasone furoate in 0.001-10 wt%, except substrate. Animal test shows that the antifungal composition has obvious synergistic effect in resisting Trichophyton mentagrophytes infection.
Owner:DIHON PHARMA GROUP
Pharmaceutical composition including mometasone furoate and azelastine hydrochloride for nasal administration
InactiveUS20130252929A1Reduce bitternessLess irritatingBiocidePharmaceutical delivery mechanismAzelastine hclNasal cavity
The present invention provides a pharmaceutical composition for nasal administration comprising mometasone furoate and azelastine hydrochloride, wherein the pharmaceutical composition comprises thaumatin as an agent for reducing bitterness and irritation.
Owner:HANLIM PHARMA CO LTD
Method for synthesizing mometasone furoate
ActiveCN105481933ASolve the shortcomings of the original process route, such as long, complex reaction system, and long time-consumingSimple processSteroidsChlorideEsterification reaction
The invention belongs to synthetic methods of medicines, and in particular relates to a method for synthesizing mometasone furoate. According to the method, chlorination is carried out to obtain a compound 2 while an esterification reaction is carried out between a compound 1 and furoyl chloride, and ring opening operation is carried out on the compound 2 in the presence of hydrochloric acid to generate mometasone furoate. According to the method, the defects of long process route, complex reaction system, long consumed time and the like of the original process are effectively overcome. The method provided by the invention is simple in process, mild in reaction condition, high in yield, low in cost, high in quality, and low-price and easily-available in raw and auxiliary materials, and is suitable for industrial production.
Owner:山东锐顺药业有限公司
Topically applied composition containing povidone iodine and mometasone furoate
InactiveCN102078326AOvercoming Prejudice That Doesn't Stabilize ExistenceOvercoming contraindications not applicable to skin inflammation caused by fungal infectionAntipyreticAnalgesicsIodineMometasone furoate
The invention relates to a topically applied composition containing povidone iodine and mometasone furoate, in particular to a topically applied pharmaceutical composition which contains the micronized mometasone furoate, the povidone iodine and one or more physiologically acceptable excipients applicable to topical application; and in the pharmaceutical composition, the content of the povidone iodine is 1%-10%, and the content of the mometasone furoate is 0.01%-0.1%.
Owner:TIANJIN JINYAO GRP
Momestasone furoate cream and preparation method thereof
ActiveCN102000023ASmooth and delicate appearanceBroaden applicationOrganic active ingredientsAerosol deliveryParaffin waxGlyceryl monostearate
The invention relates to the technical field of an external preparation medicament, in particular to an external preparation medicament containing momestasone furoate. The external preparation medicament is prepared from the following raw materials in part by weight: 15 parts of the momestasone furoate, 45 parts of hexadecyl-octadecyl alcohol, 7.5 parts of glyceryl monostearate, 5 parts of beeswax, 8 parts of triethanolamine, 35 parts of light liquid paraffin, 2 parts of sodium lauryl sulfate, 8 parts of sodium lauryl sulfate, 25 parts of propylene glycol, 1 part of Hydroxy acetate, 2 parts of tween-80, and the balance of purified water which is added to ensure that the total weight is 500 parts. The cream has better moisture retention and stability because the humectant combination of the triethanolamine and the propylene glycol is adopted.
Owner:华润三九(南昌)药业有限公司
Functional preparation for adjuvant therapy of atopic dermatitis and preparation method
InactiveCN103006686ALittle side effectsImprove dry eczema symptomsOrganic active ingredientsAntipyreticEczematous rashAdjuvant therapy
The invention provides a functional preparation for adjuvant therapy of atopic dermatitis AD and a preparation method thereof. The functional preparation comprising raw material components in parts by weight: 0.2-1 part of sodium hyaluronate, 5-10 parts of emulsifying thickening agent, 5-10 parts of grease and the balance of de-ionized water which enables the total weight to be 100 parts. The functional preparation provided by the invention is the functional preparation containing sodium hyaluronate and is combined with mometasone furoate emulsifiable paste to obviously improve a xerotic eczema symptom, effectively recover a skin barrier function, and reduce the recurrence; the curative effect of the functional preparation combined with mometasone furoate emulsifiable paste is better than that of the single use of the mometasone furoate emulsifiable paste; and the functional preparation can be used for the adjuvant therapy of eczematous dermatitis diseases, has the good curative effect and safety, and reduces the reoccurrence.
Owner:何黎
Liranaftate and mometasone furoate containing locally applied compound pharmaceutical composition
ActiveCN102379879ASlowed transdermal absorption rateReduce absorption rateAntimycoticsAntisepticsArteriolar VasoconstrictionAntifungal drugs
The invention relates to the technical field of antimycotic medicaments, specifically to a liranaftate and mometasone furoate containing locally applied compound pharmaceutical composition, which contains one or more acceptable auxiliary materials for local application. The invention is characterized in that the weight ratio of liranaftate to mometasone furoate is 1:80-80:1; the balance contains matrix auxiliary materials; The pharmaceutical composition can be emulsifiable paste or ointment, gelata, a solution or an emulsion or a suspension, a coating agent, aerosol or a spraying agent or spray-film, a foaming agent and patch. With the application of the liranaftate and mometasone furoate compound pharmaceutical composition, as mometasone furoate has a vasoconstriction effect, the transdermal absorption rate of liranaftate slows down, local skin tissue concentration of liranaftate is increased, the medicament is minimized to penetrate through the skin into blood, and local skin concentration of the antimycotic medicament is maintained for a longer time.
Owner:南昌百济制药有限公司
Pharmaceutical compositions for the treatment of asthma
InactiveUS20100143269A1Improved stability and compatibilityEasy to manufacturePowder deliveryOrganic active ingredientsMometasone furoateFormoterol Fumarate
Owner:MERCK SHARP & DOHME CORP
Mometasone furoate cream and preparation method thereof
InactiveCN112402366ALow impurity contentHigh yieldOrganic active ingredientsAntipyreticPreservativeTopical medication
The invention relates to an external medicinal preparation, and particularly relates to an external medicine containing mometasone furoate and a preparation method thereof. In order to achieve the purpose, the invention provides mometasone furoate cream. The mometasone furoate cream is prepared from the following raw materials in parts by weight: 0.5 to 2 parts of mometasone furoate, 75 to 150 parts of an oil phase regulator, 30 to 50 parts of a consistency regulator, 60 to 100 parts of a humectant, 80 to 120 parts of a co-emulsifier, 60 to 90 parts of a co-solvent, 1 to 3 parts of a brightening agent, 30 to 50 parts of an emulsifier, 0.3 to 1 part of a preservative and 500 to 600 parts of water. The mometasone furoate cream prepared by the preparation method disclosed by the invention hasthe advantages of reduced impurity content, increased yield, advanced process, simple method and high yield.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Nasal in-situ gel containing mometasone furoate and H1 receptor antagonist
InactiveCN102078612AImprove bioavailabilityGood treatment effectOrganic active ingredientsPharmaceutical delivery mechanismNK1 receptor antagonistBULK ACTIVE INGREDIENT
The invention discloses nasal in-situ gel containing mometasone furoate and an H1 receptor antagonist. The nasal in-situ gel is prepared from mometasone furoate serving as an active ingredient and / or a hydrate of mometasone furoate, one or more H1 receptor antagonists, an environmentally-sensitive hydrophilic gel material, other pharmaceutically acceptable auxiliary materials and the balance of water.
Owner:TIANJIN JINYAO GRP
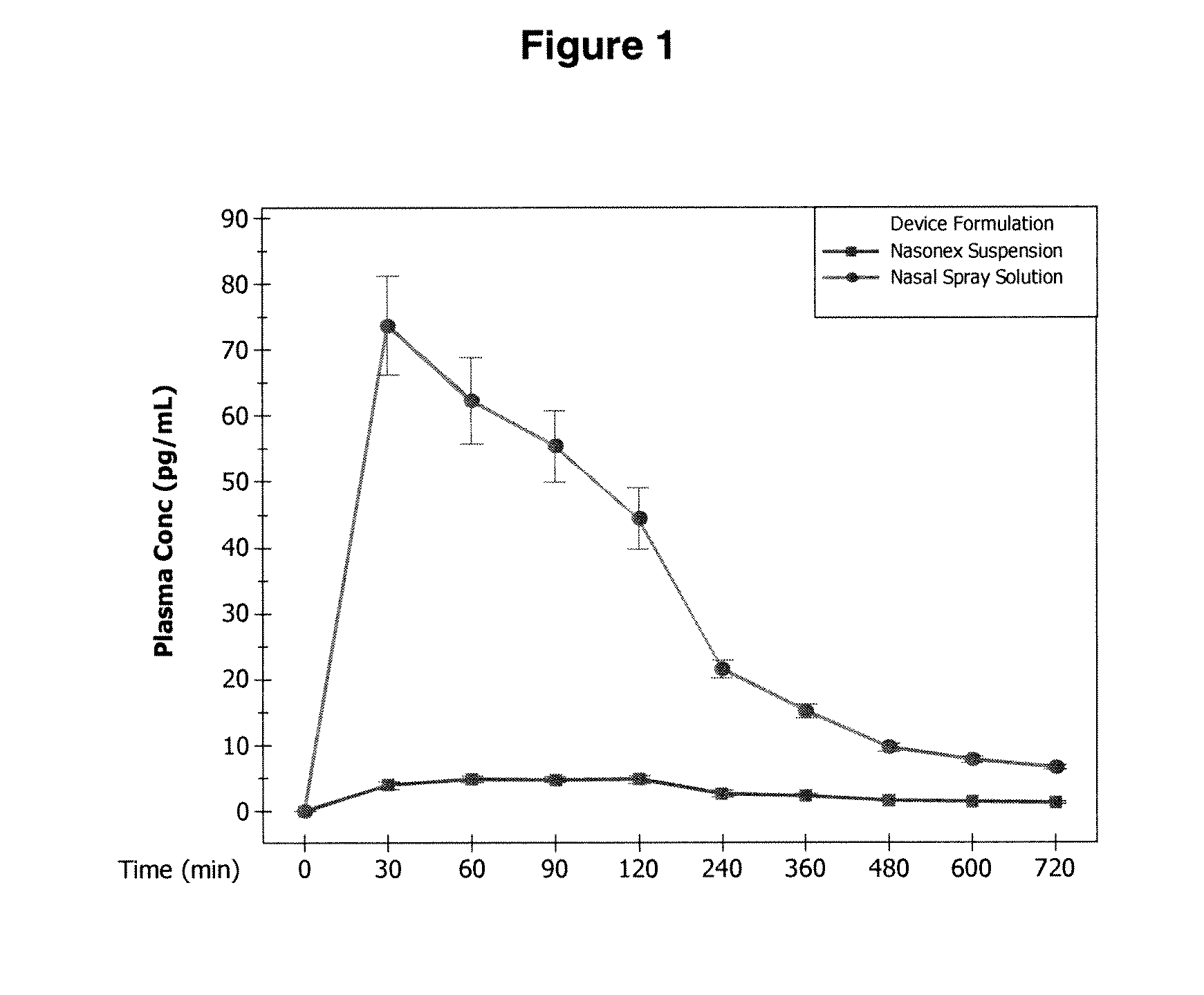
Mometasone furoate suspension nasal spray composition
The invention discloses mometasone furoate suspension nasal spray composition containing a mometasone furoate crystal form M and a pharmaceutically acceptable carrier. The X-ray powder diffractionof the mometasone furoate crystal form M has characteristic peaks at the diffraction angles 2 theta equal to8.1+ / -0.1 degrees, 9.8+ / -0.1 degrees, 12.0+ / -0.1 degrees, 14.6+ / -0.1 degrees, 15.0+ / -0.1 degrees, 16.4+ / -0.1 degrees, 16.7+ / -0.1 degrees, 17.3+ / -0.1 degrees, 17.9+ / -0.1 degrees, 19.7+ / -0.1 degrees and 24.8+ / -0.1 degrees.
Owner:TIANJIN JINYAO GRP
Intranasal medicinal composition containing bilastine and mometasone furoate
InactiveCN103736098ARelieve bitternessRelieve irritationOrganic active ingredientsAerosol deliveryThaumatinMedicine
The invention provides an intranasal medicinal composition. The intranasal medicinal composition contains bilastine, mometasone furoate, and thaumatin serving as a bitterness and irritation alleviator.
Owner:于运红
Mometasone furoate gelatin and preparation method thereof
InactiveCN109602693AImprove stabilityStable efficacyOrganic active ingredientsAerosol deliveryTreatment effectGlycerol
The invention provides a preparation method of mometasone furoate gelatin. The mometasone furoate gelatin is prepared from the following raw materials and auxiliary materials in proportion by weight of 1.0g of mometasone furoate, 8.0-10.0g of carbomer, 4.0-6.0g of triethanolamine, 50.0g of medical ethanol, 150.0g of glycerine, and 0-1.5g of ethylparaben, and purified water is added until the totalweight is 1000g, wherein the pH value of the mometasone furoate gelatin is 5.5-6.5, and the mass ratio of the triethanolamine to the carbomer is 0.40-0.55; single unknown impurities of the mometasonefuroate gelatin do not exceed 0.2%; the total impurities are not greater than 1.0%, and the mometasone furoate gelatin is stable under the high-temperature illumination condition. Besides, radix angelicae pubescentis, herba selaginellae, Chinese arborvitae twigs and leaves, hedyotis diffusa, a pharmaceutic adjuvant, azone and rice milk, which are added, have favorable treatment effect on treatment of foot fracture.
Owner:华润三九(南昌)药业有限公司
Nasal nano-preparation mometasone furoate liquid crystal gel nanoparticle and preparation method thereof
ActiveCN108283621ASmall particle sizeAchieve sustained releaseAntibacterial agentsPowder deliveryTherapeutic effectSolvent
The invention discloses a nasal nano-preparation mometasone furoate liquid crystal gel nanoparticle and a preparation method thereof. The nanoparticle consists of 0.06-0.09wt% of mometasone furoate, 30-50wt% of a liquid crystal material, 20-30wt% of phosphatidylcholine, 5-15wt% of a surfactant, 5-15wt% of a solvent and 0-40wt% of de-ionized water. The preparation method of the liquid crystal gel nanoparticle comprises the following steps: firstly, uniformly mixing all raw materials except for the solvent and the de-ionized water, and adding the solvent and promoting dispersion; and then, adding the water, and implementing further dispersion via a high shear homogenizer or ultrasonic wave, so that a finished product (the liquid crystal gel nanoparticle) is obtained. The prepared mometasonefuroate liquid crystal gel nanoparticle is small in particle size, high in encapsulation efficiency, good in medicine absorption rate, good in sustained release effect and good in therapeutic effect;and the liquid crystal gel nanoparticle is obvious curative effect on allergic rhinitis and bacterial rhinitis.
Owner:武汉百纳礼康生物制药有限公司
Pharmaceutical compositions
InactiveUS20100095963A1Uniform drug contentOrganic active ingredientsPowder deliveryMedicineMometasone furoate
Disclosed are aerosolized formulations for the treatment of asthma that contain mometasone furoate and processes for preparing the same.
Owner:SCHERING CORP
8DM derivative, and method for synthesizing mometasone furoate from 8DM derivative
ActiveCN105566437ASolve the shortcomings of the original process route, such as long, complex reaction system, and long time-consumingSimple processSteroidsChlorideEsterification reaction
The invention relates to a 8DM derivative, and a method for synthesizing mometasone furoate from the 8DM derivative. The method comprises the following steps: carrying out an esterification reaction on a compound 1 and furoyl chloride to generate a compound 2 which is the 8DM derivative, and simultaneously carrying out a ring opening reaction and a 21th position chlorination reaction on the compound 2 in the presence of concentrated hydrochloric acid to generate a compound 3 which is mometasone furoate. The disadvantages of long route, complex reaction system and long time of original technologies are effectively solved in the invention. The method has the advantages of simple process, mild reaction conditions, high yield, low cost, high quality, cheap and easily available raw and auxiliary materials, and suitableness for industrial production.
Owner:YANGTAI PHARMA SHANDONG
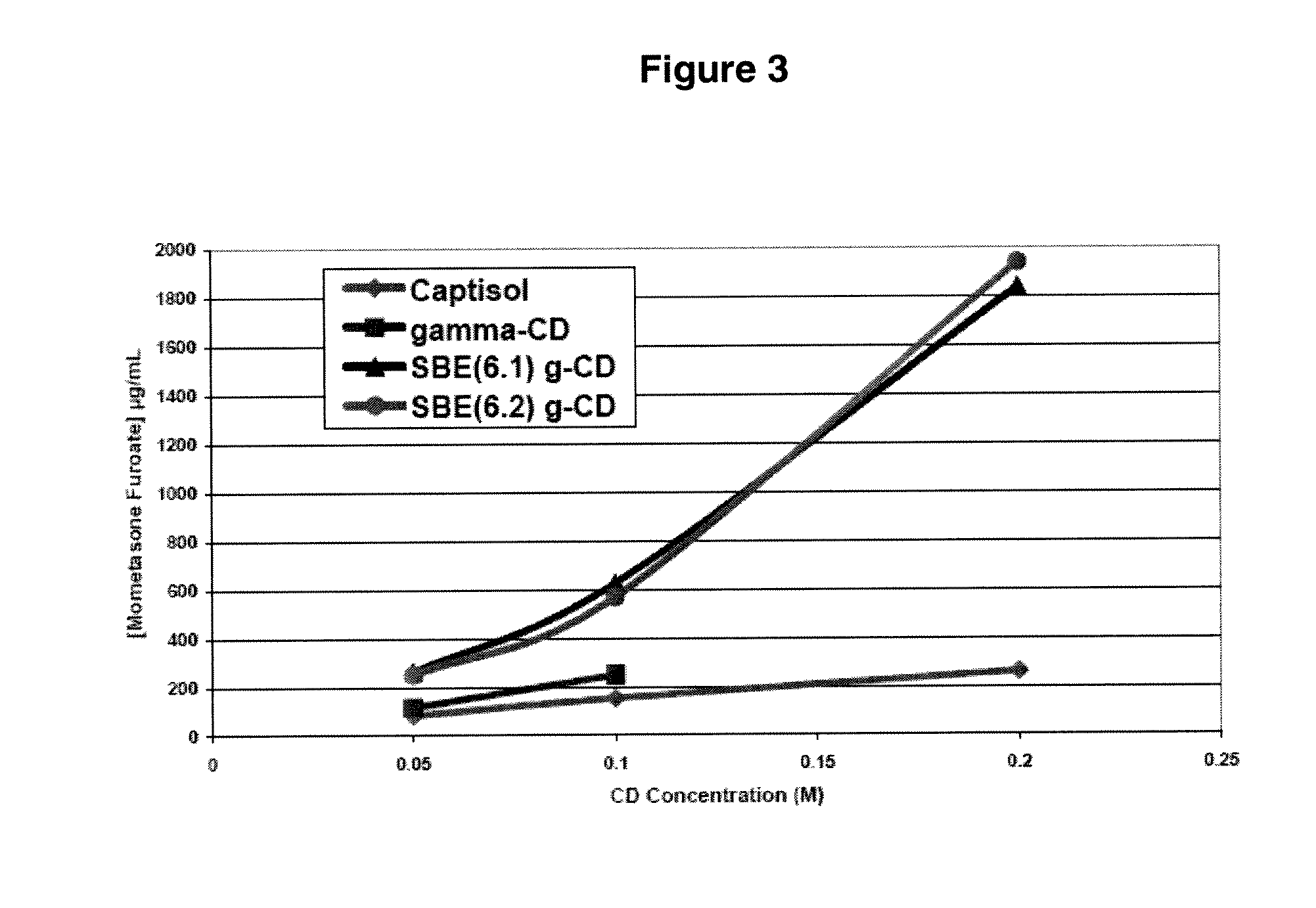
Corticosteroid compositions and methods of treatments thereof
This invention relates to steroidal solutions for the preparation of medicaments and drug products useful for treating diseases of the upper and lower airway passages. Various embodiments of the present invention provide compositions, compositions and dosage forms with mometasone furoate in a dissolved state that are suitable for inhalation and can be used for the treatment of diseases of the upper and / or lower airway passages.
Owner:MERCK SHARP & DOHME CORP
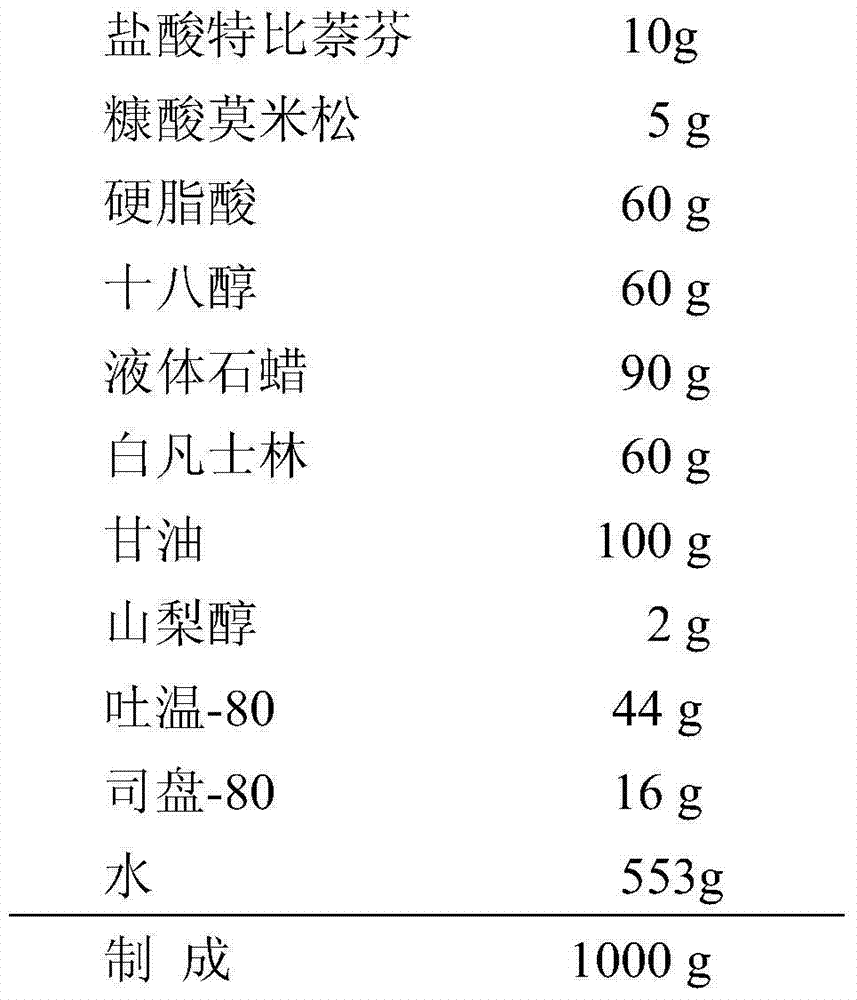
Compound terbinafine preparation and applications thereof
InactiveCN104771405AQuick effectSignificant relief of symptomsOrganic active ingredientsAntimycoticsSide effectCure rate
The invention belongs to the technical field of medicines, and provides a compound terbinafine preparation. The active component of the preparation is mainly composed of terbinafine hydrochloride and mometasone furoate. The provided compound terbinafine preparation is suitable for treating superficial fungal infection, and has the advantages of high curative effect, quick effect, safety, and little side effect of hormone. In the treatments of tinea of feet and hands, the clinical cure rate is 71.7%, the total effective rate is 96.7%, and the fungi eliminating rate is 100%. In the treatments of tinea corporis and cruris, the clinical cure rate is 72.4%, the total effective rate is 97.1%, and the fungus eliminating rate is 100%. The preparation does not cause prominent allergic reactions and hormone side effects.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
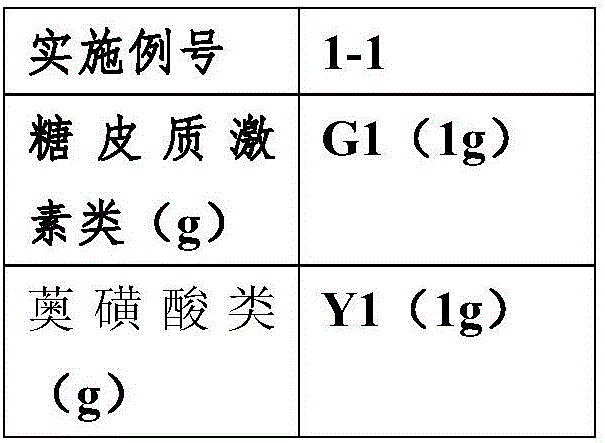
Skin pharmaceutical composition taking mometasone furoate as active component
The invention relates to a pharmaceutical composition which is prepared from an active component and drug auxiliary materials and is directly acted on skin. The pharmaceutical composition is characterized in that the active component contains one of mometasone furoate and azulene sulfonic acid or salt thereof.
Owner:TIANJIN JINYAO GRP
Mometasone furoate emulsifiable paste and preparation method thereof
ActiveCN112754990AAdvanced technologySimple methodOrganic active ingredientsAntipyreticWhitening AgentsMedicine
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Topical pharmaceutical compositions
InactiveUS20140200203A1Easily applied to skinEasy to spreadOrganic active ingredientsAntipyreticDiseaseAtopic dermatitis
Topical pharmaceutical compositions are described comprising, based on the total weight of the composition: a) 0.01 to 0.2 wt. % of mometasone furoate, b) 5 to 18 wt. % of hexylene glycol, c) 20 to 40 wt. % of water, and d) 25 to 70 wt. % of an oil phase. Said compositions are stable and can be safely and easily applied over large surface areas of the skin in an acceptable way by the general patient population for the treatment or prevention of psoriasis, atopic dermatitis (atopic eczema) and other skin disorders or diseases.
Owner:ALMIRALL
Mometasone furoate cream and preparation method thereof
ActiveCN106138066AReduce permeationImprove stabilityOrganic active ingredientsAerosol deliverySkin penetrationMometasone furoate
The invention discloses mometasone furoate cream and a preparation method thereof. The mometasone furoate cream is prepared through the technology method comprising the steps that mometasone furoate is mixed with a mixture of Tween-80 and anhydrous citric acid, and then the obtained mixture is added into an aqueous propylene glycol solution. The mometasone furoate cream has the better stability, the appropriate retention amount in the skin and the lower skin penetration amount.
Owner:REGENEX PHARMA LTD
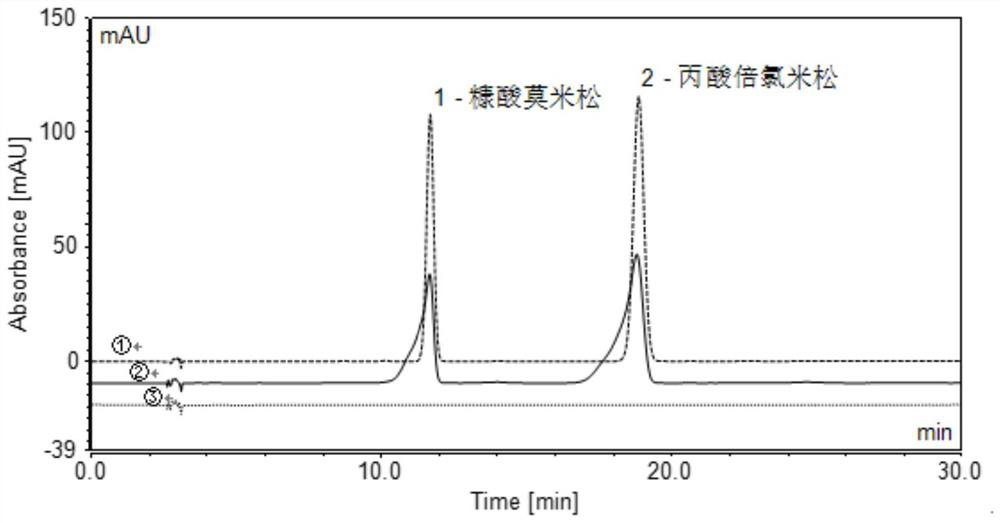
Content determination method of mometasone furoate cream
PendingCN113063886ASolve the problem of the front of the main peak and poor symmetryHigh sensitivityOrganic active ingredientsComponent separationPropanoic acidMometasone furoate
The invention provides a content determination method of mometasone furoate cream, and relates to the technical field of chemical detection. The content determination method comprises the following steps: preparing a beclomethasone propionate internal standard solution; preparing a mometasone furoate reference substance solution; preparing a mometasone furoate cream test solution; and measuring the content by an internal standard method. By improving the previous detection standard and resetting the detection conditions, the problems of leading peak pattern and poor symmetry of the main peak in the existing standard content determination method are effectively solved, so that the content detection of the mometasone furoate cream is more accurate and reliable.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com