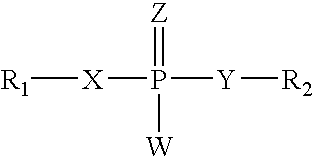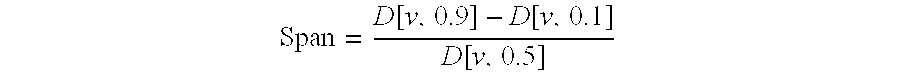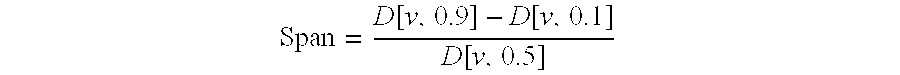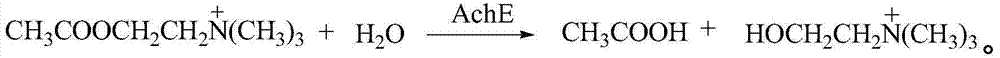Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
133 results about "Cholinergic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cholinergic agents are compounds which mimic the action of acetylcholine and/or butyrylcholine. In general, the word choline refers to the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation. Found in most animal tissues, choline is a primary component of the neurotransmitter acetylcholine and functions with inositol as a basic constituent of lecithin. Choline also prevents fat deposits in the liver and facilitates the movement of fats into cells. The richest nutritional sources of choline are liver, kidney, brain, wheat germ, brewer's yeast, and egg yolk. Neurologically, cholinergic is the abbreviated term referring to choline (the suffix -ergic means stimulating). The parasympathetic nervous system, which uses acetylcholine almost exclusively to send its messages, is said to be almost entirely cholinergic. Neuromuscular junctions, preganglionic neurons of the sympathetic nervous system, the basal forebrain, and brain stem complexes are also cholinergic. In addition, the receptor for the merocrine sweat glands are also cholinergic, since acetylcholine is released from postganglionic sympathetic neurons.
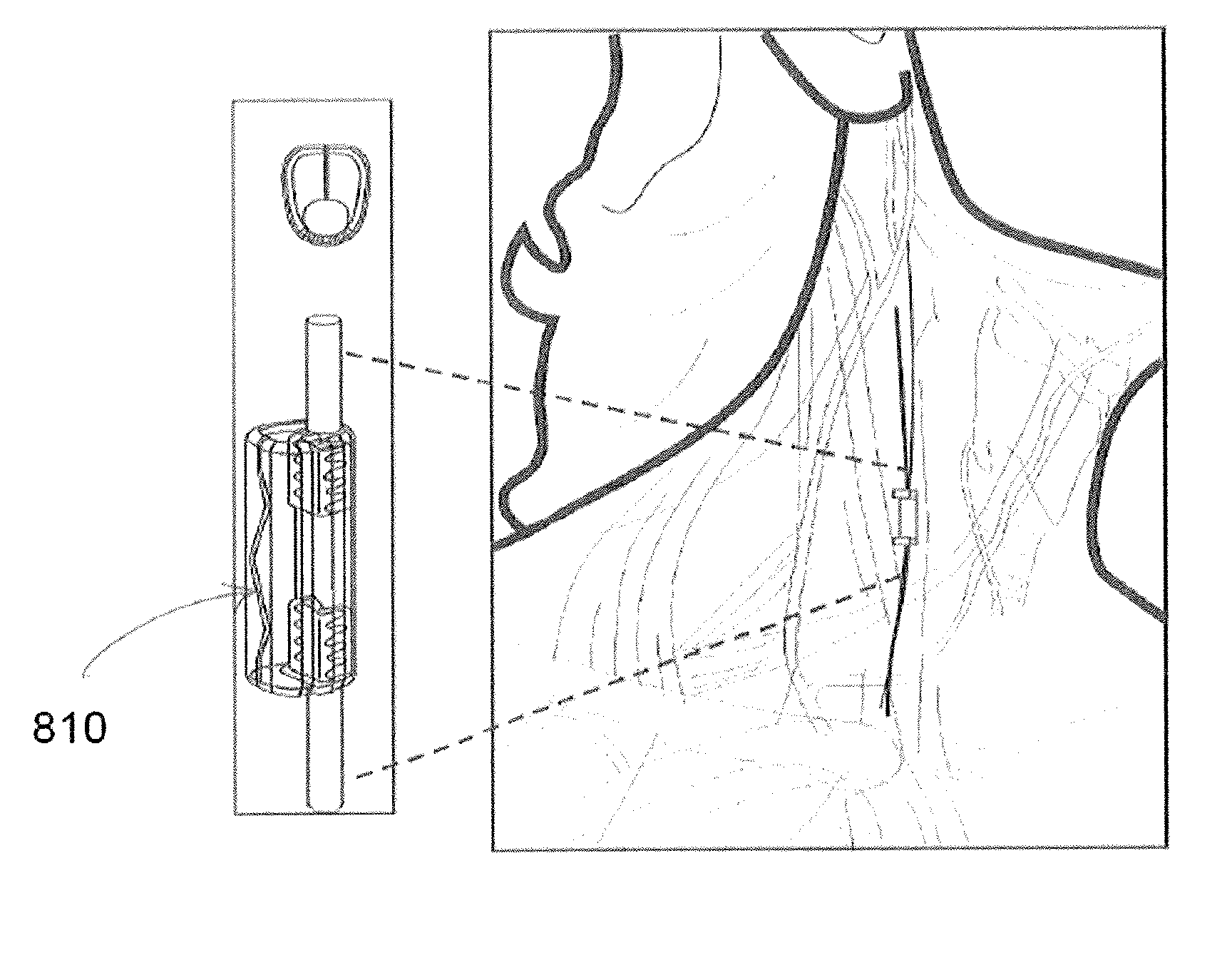
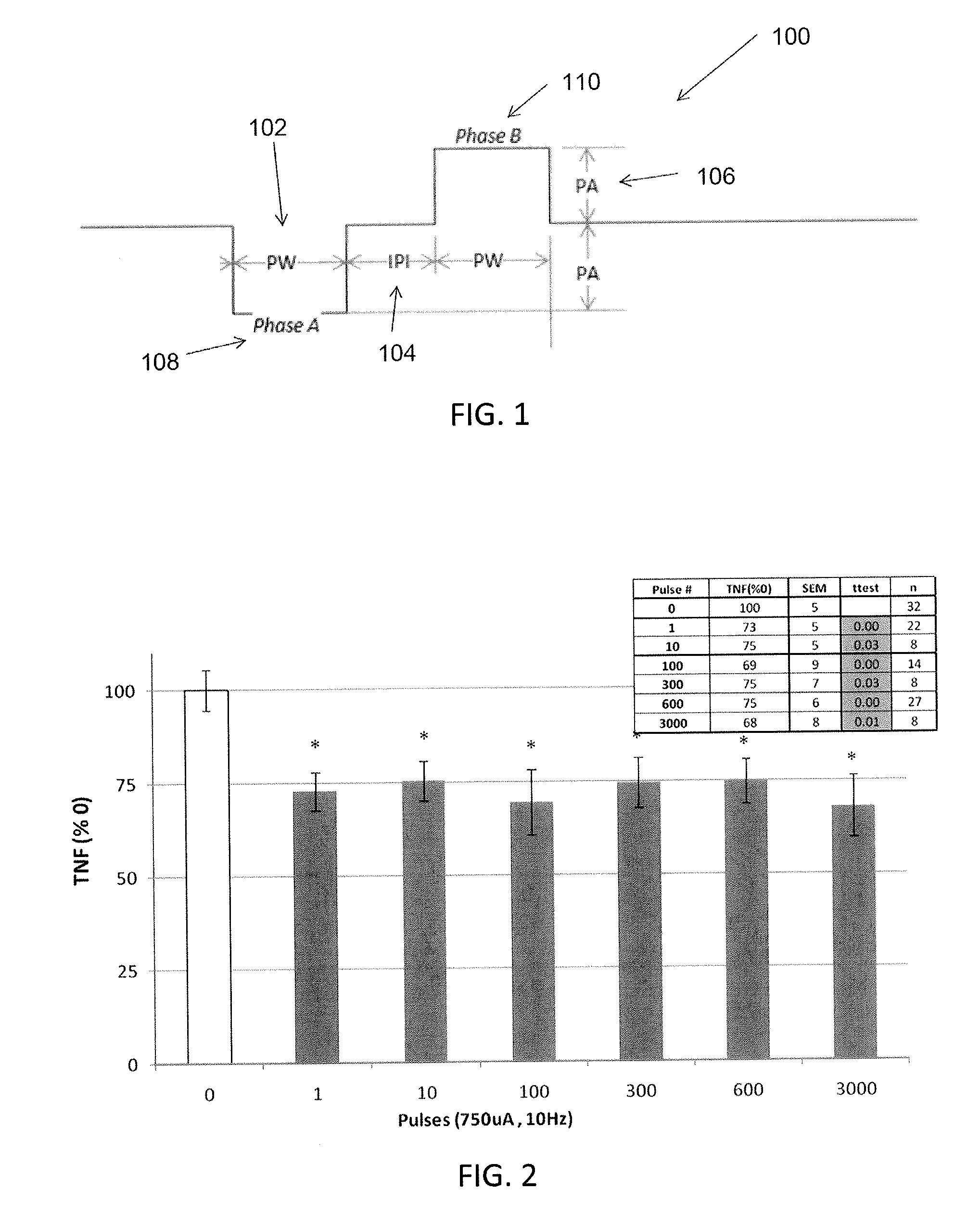
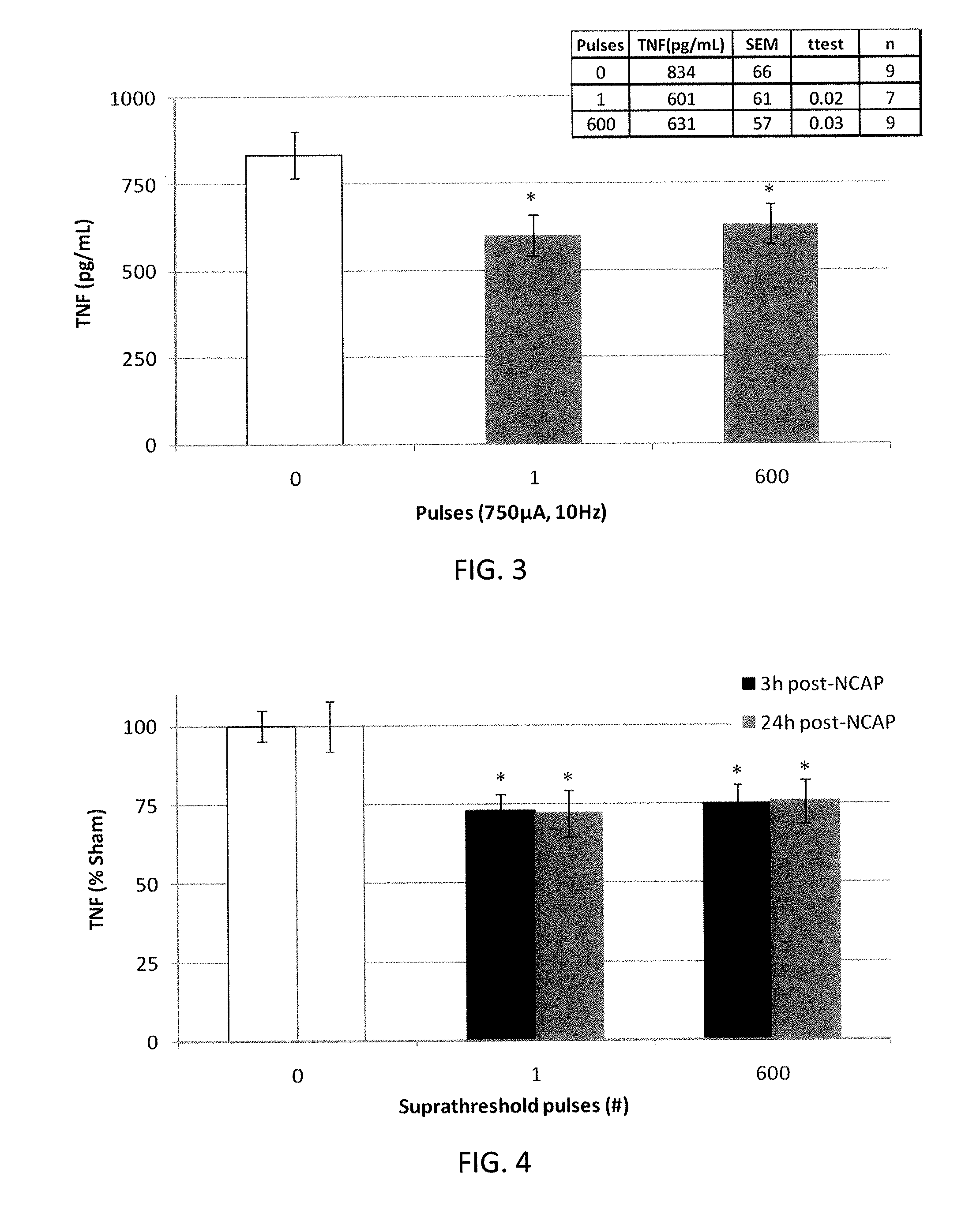
Extremely low duty-cycle activation of the cholinergic anti-inflammatory pathway to treat chronic inflammation
ActiveUS9211410B2Reduce power consumptionMaintain curative effectImplantable neurostimulatorsArtificial respirationInitial doseCholinergic anti-inflammatory pathway
Described herein are systems and methods for applying extremely low duty-cycle stimulation sufficient to treat chronic inflammation with progressively longer delays (off periods) from an initial stimulation. In particular, described herein are supra-threshold pulses of electrical stimulation sufficient to result in a long-lasting (e.g., >48 hours) inhibition of pro-inflammatory cytokines and / or effects of chronic inflammation; the delay between initial doses (which may be single-pulse doses) may be extended for subsequent doses, potentially dramatically enhancing battery and device longevity.
Owner:SETPOINT MEDICAL CORP
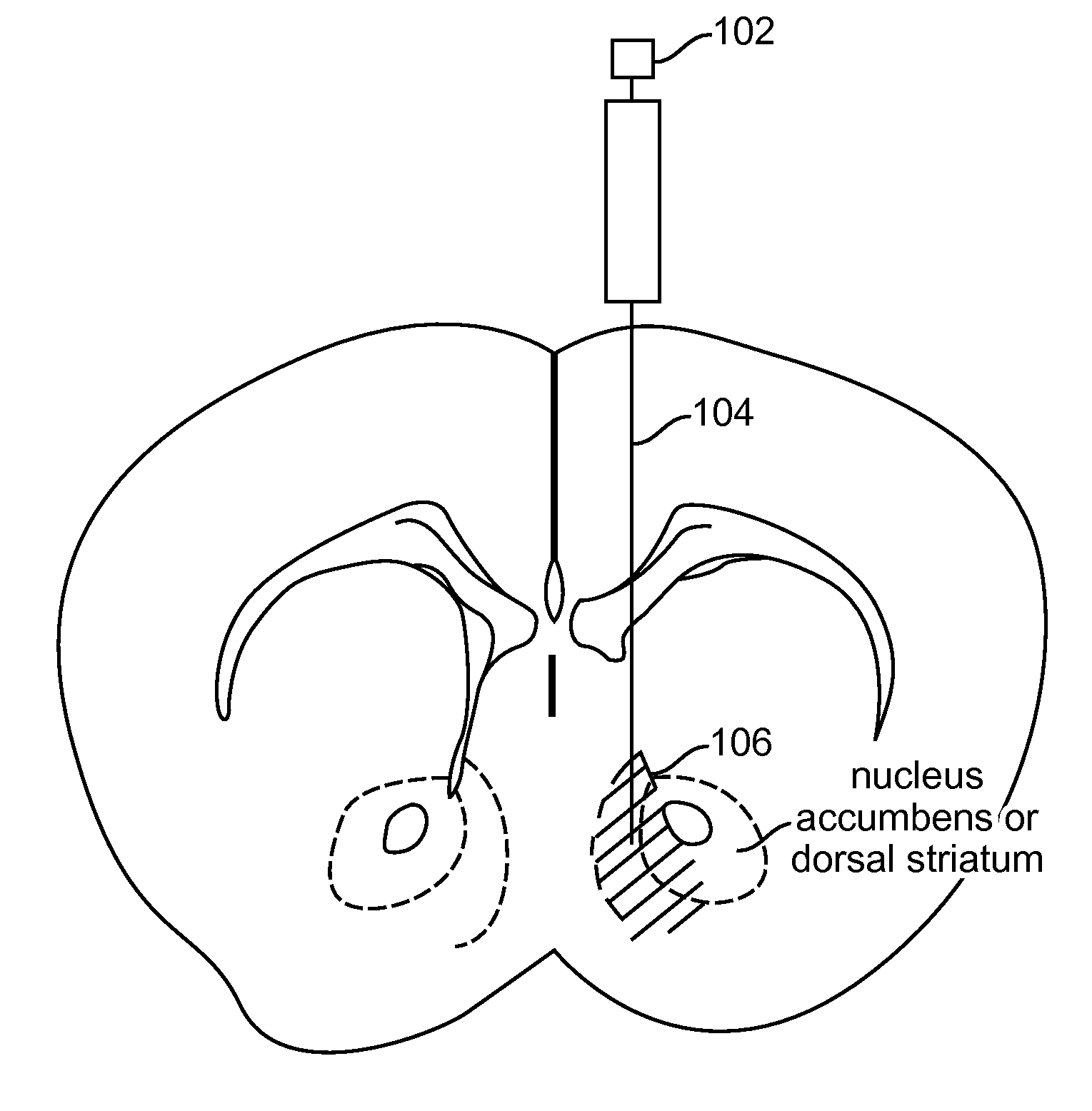
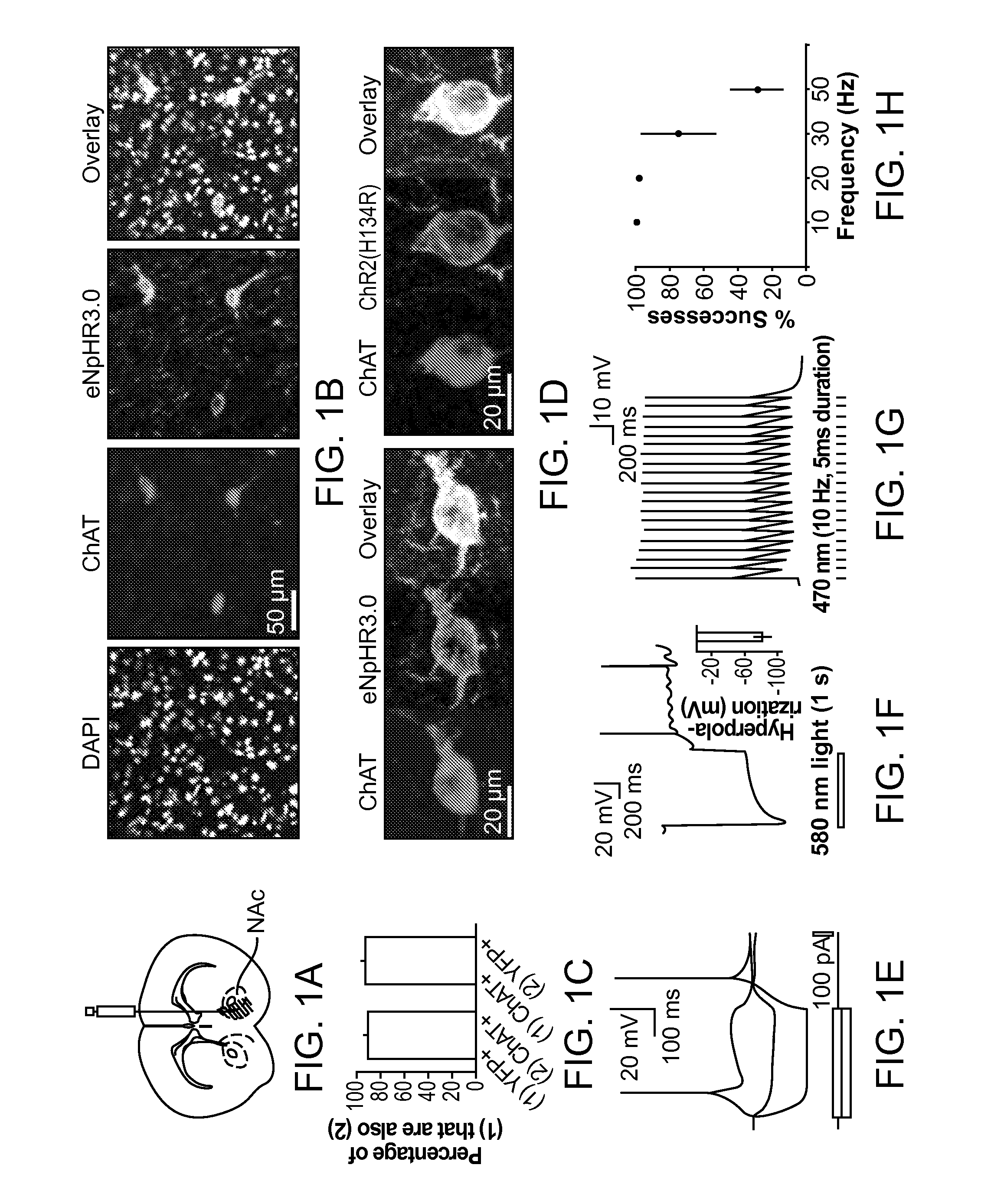
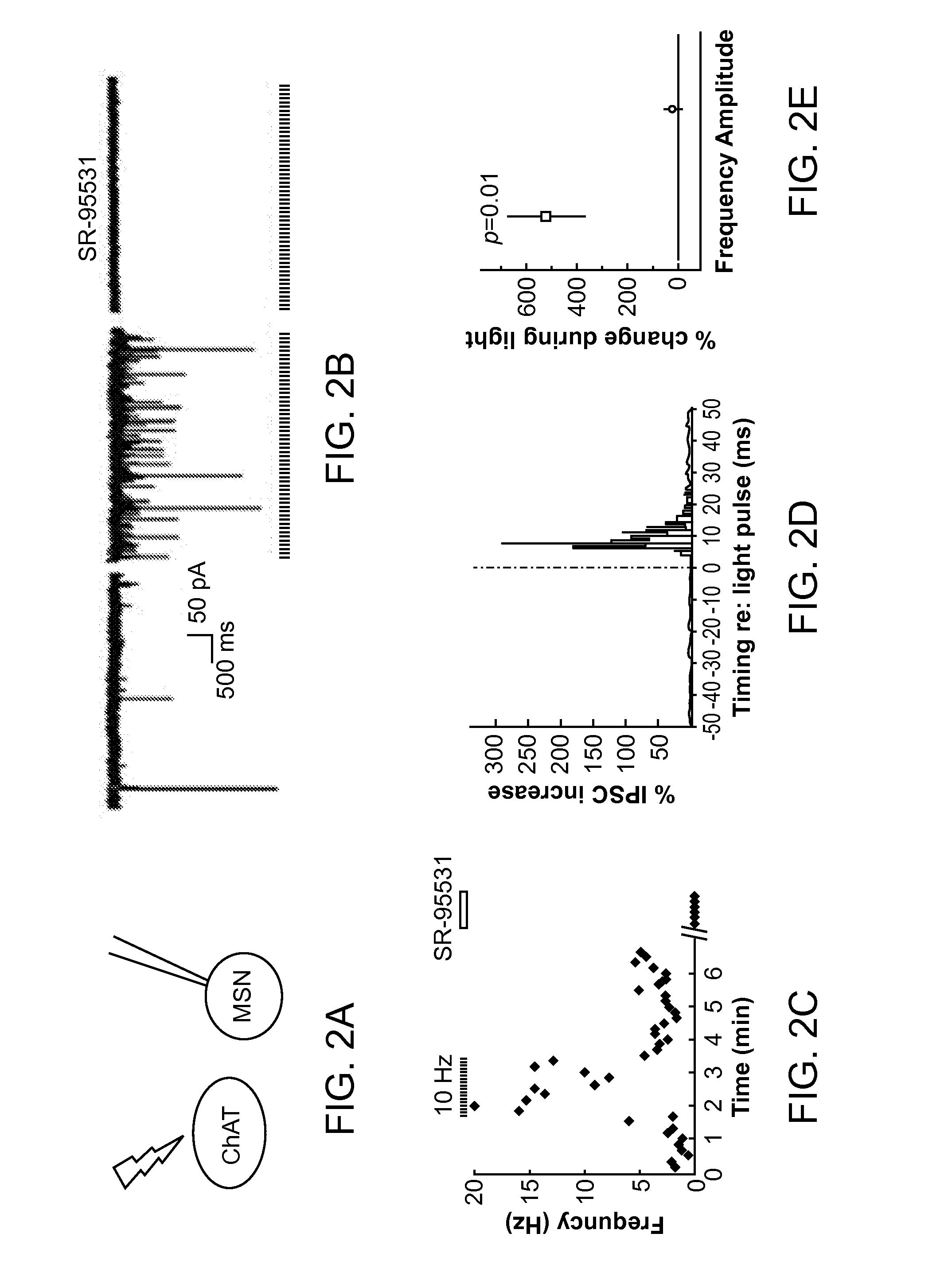
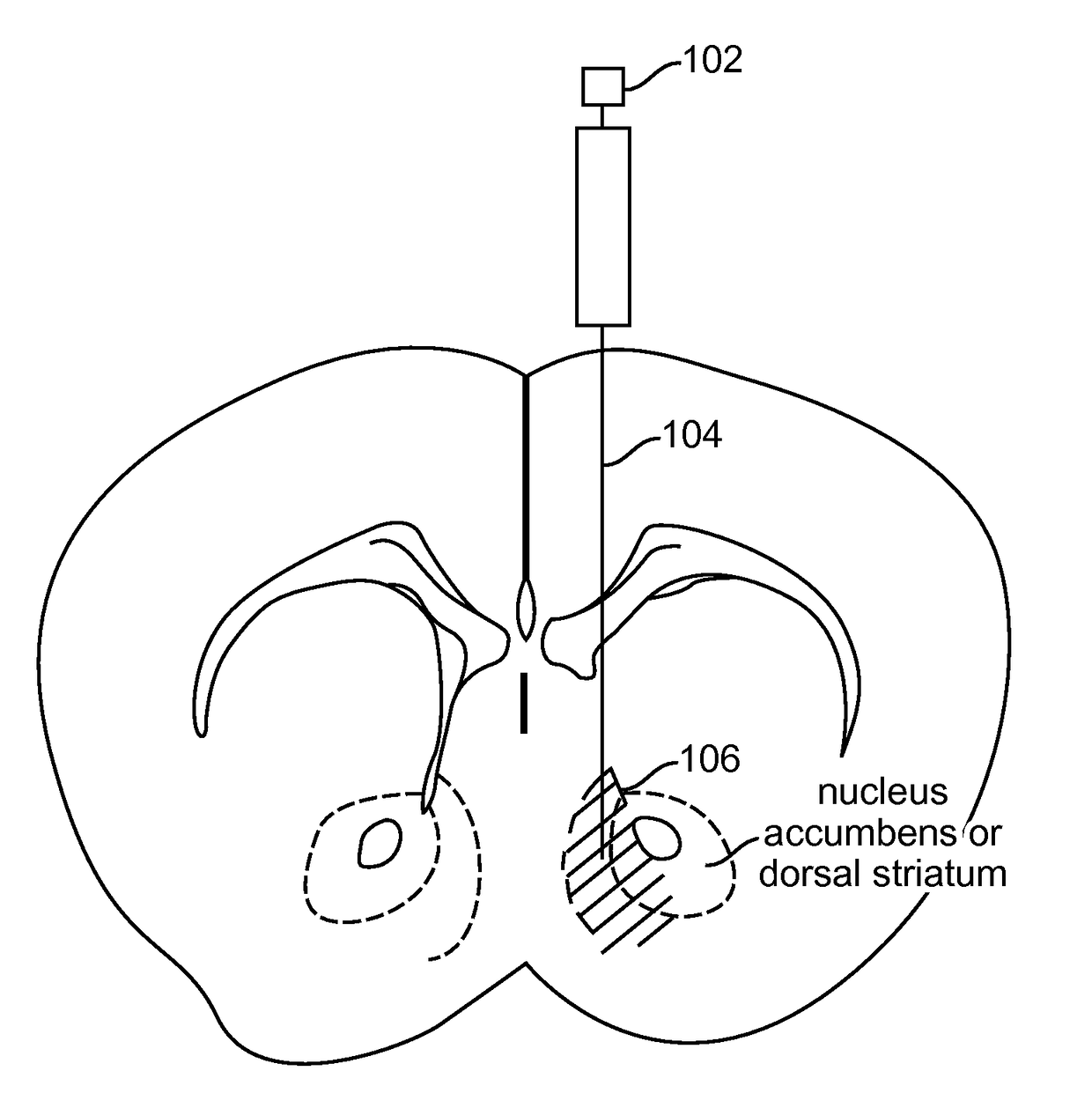
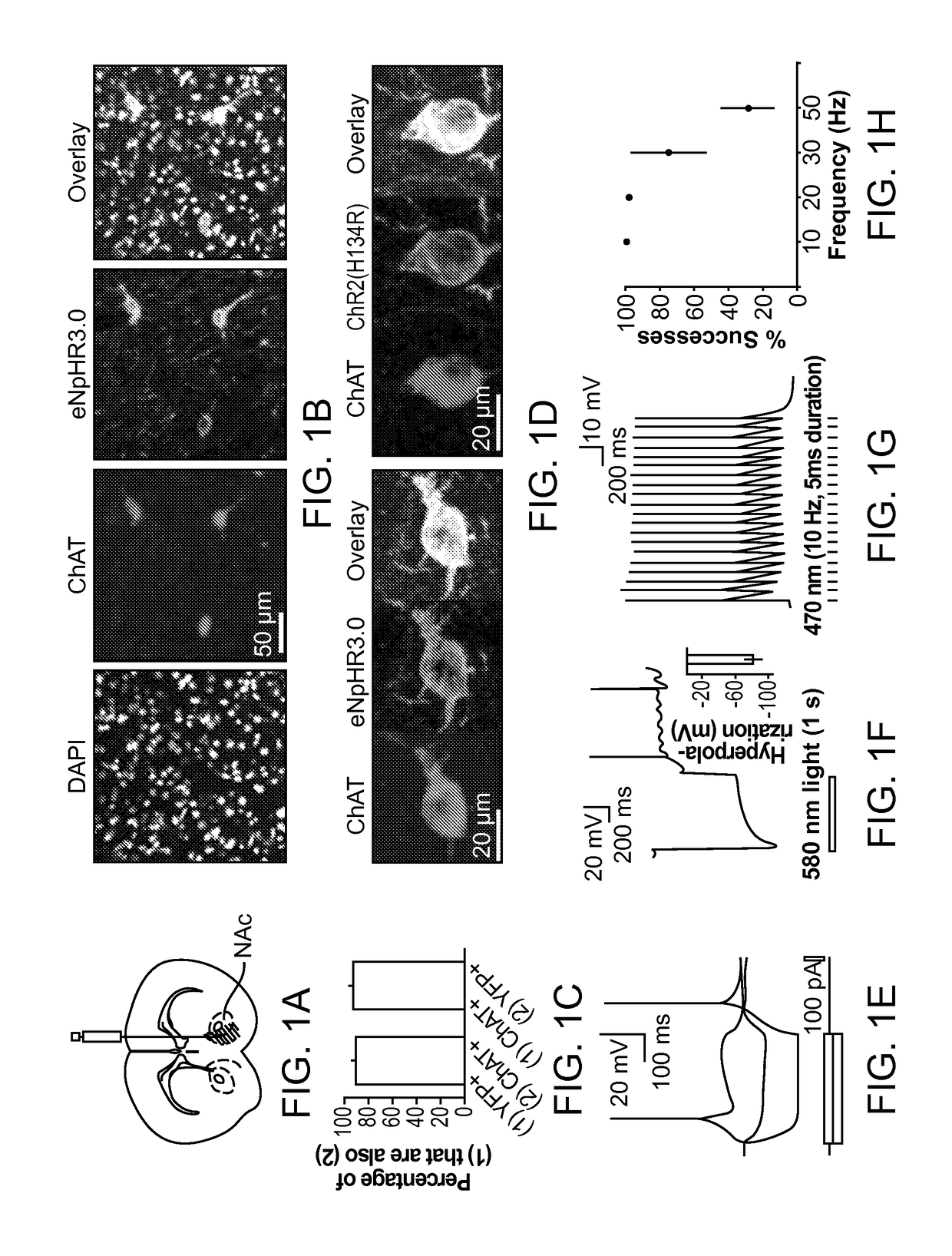
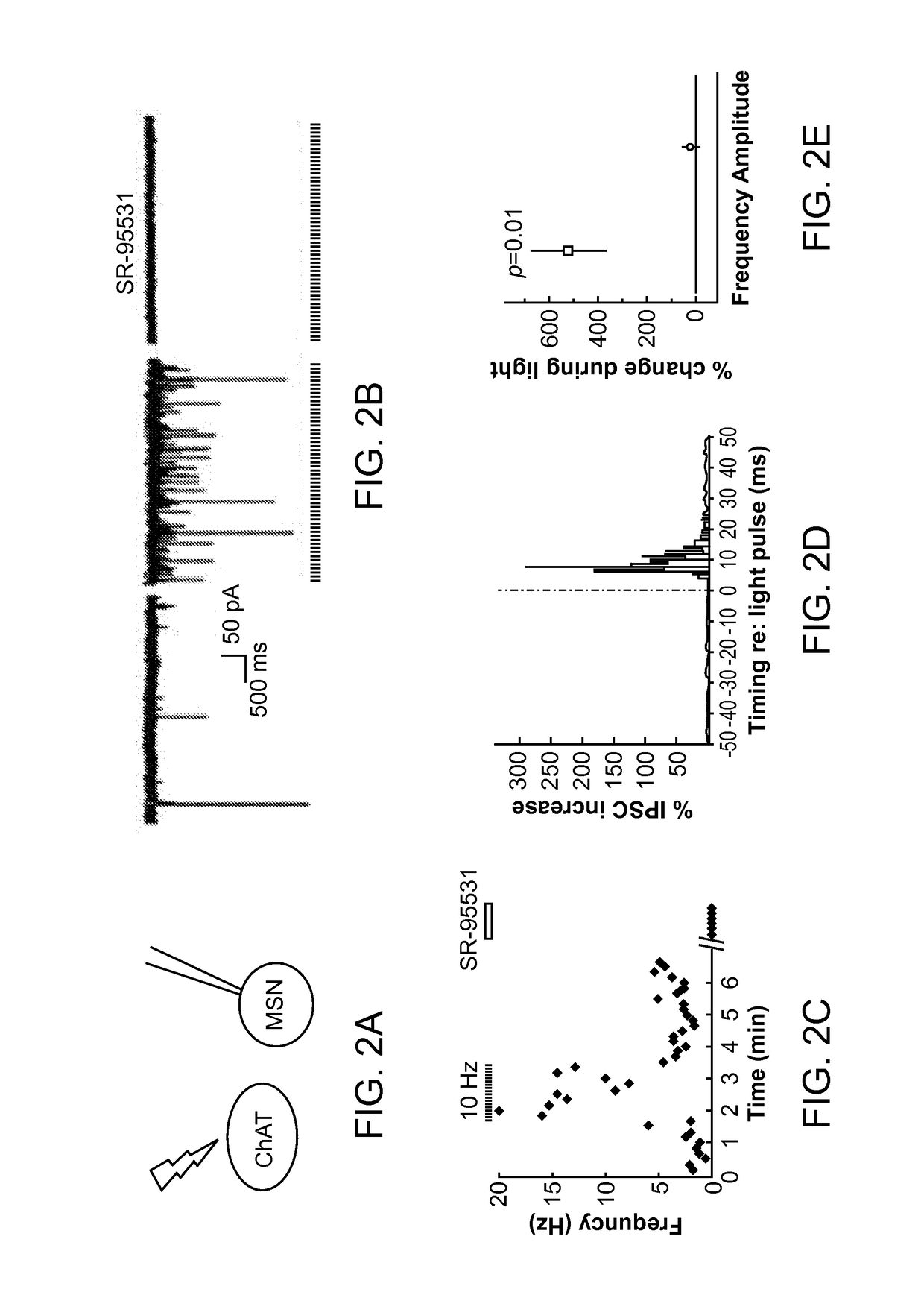
Optogenetic Control of Reward-Related Behaviors
ActiveUS20130317569A1Reduce and eliminate effectInhibition is effectiveNervous disorderPeptide/protein ingredientsOptogeneticsInterneuron
Provided herein are compositions and methods for disrupting at least one reward-related behavior in an individual through the use of light-responsive opsin proteins used to control the polarization state of the cholinergic interneurons of the nucleus accumbens or the striatum.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
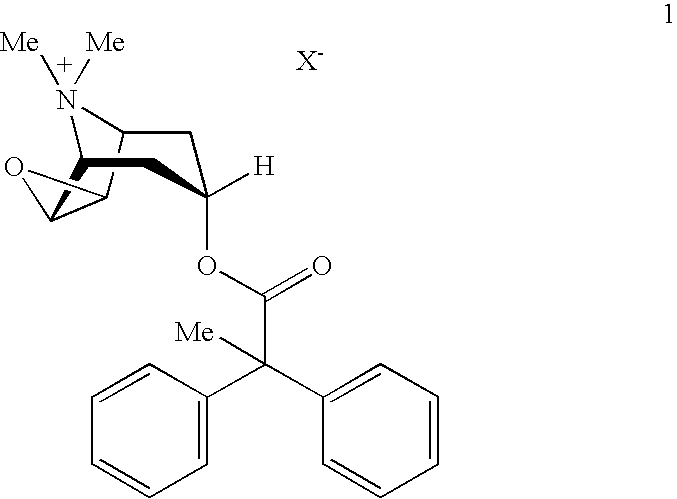
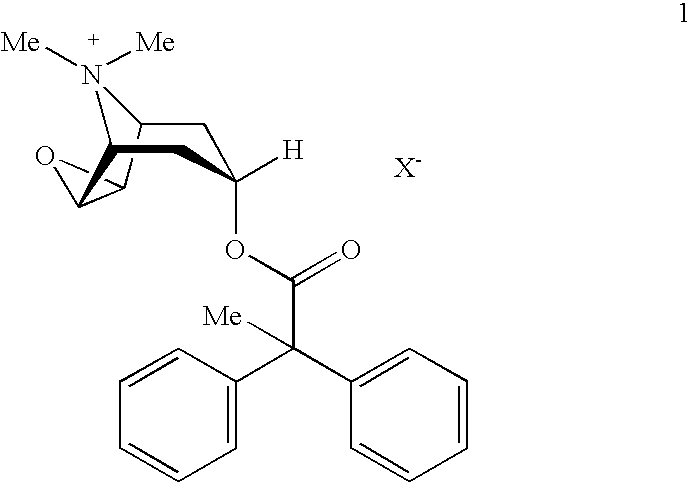
Anticholinergic powder formulations for inhalation
An inhalable powder comprising:(a) an active substance consisting essentially of a compound of formula 1 wherein X− is a pharmaceutically acceptable anion; and(b) a physiologically acceptable excipient having an average particle size of 10 μm to 50 μm,processes for preparing the inhalable powder, and methods of administration for the treatment of respiratory complaints, particularly for the treatment of chronic obstructive pulmonary disease (COPD) and asthma.
Owner:BOEHRINGER INGELHEIM INT GMBH
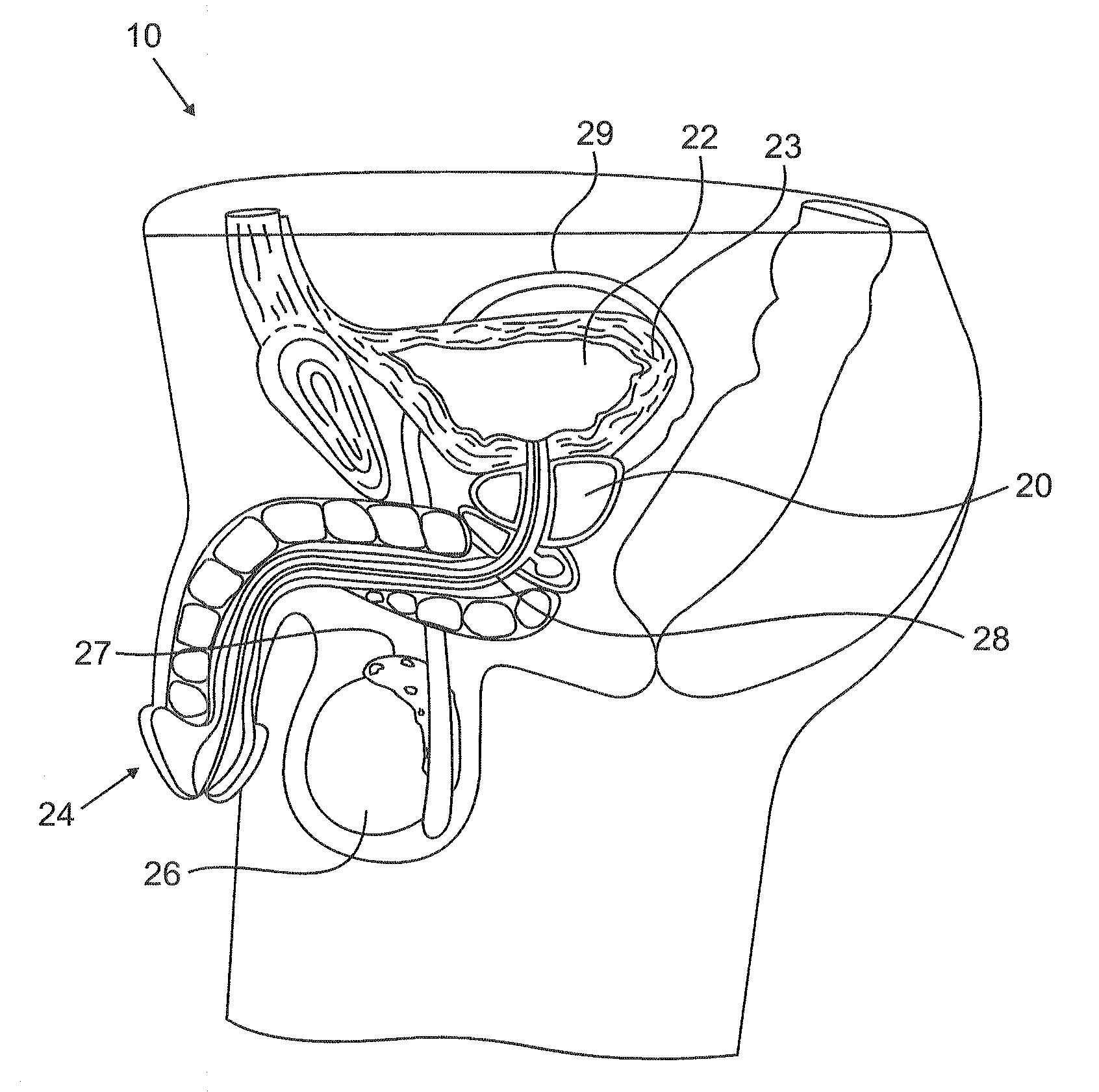
Urological medical devices for release of prostatically beneficial therapeutic agents
InactiveUS20080233167A1High quantity of drugAvoid the needAntibacterial agentsNervous disorderDiseaseGynecology
According to an aspect of the invention, urological medical devices are provided, which comprise a prostatically beneficial agent selected from alpha-adrenergic blockers, antispasmodic agents, anticholinergic / antimuscarinic agents, calcium channel blockers, anti-inflammatory agents, hormone-affecting agents, anti-cancer agents, and combinations thereof, among others. The urological medical devices are adapted for implantation or insertion into a subject's urinary tract, whereupon at least a portion of the prostatically beneficial agent is released into the subject's prostatic urethra. The release profile of the prostatically beneficial agent is effective to treat a prostatic disorder, for example, benign prostate hypertrophy, prostate cancer or prostatitis, among others. Other aspects of the invention are directed to treating prostatic disorders.
Owner:BOSTON SCI SCIMED INC
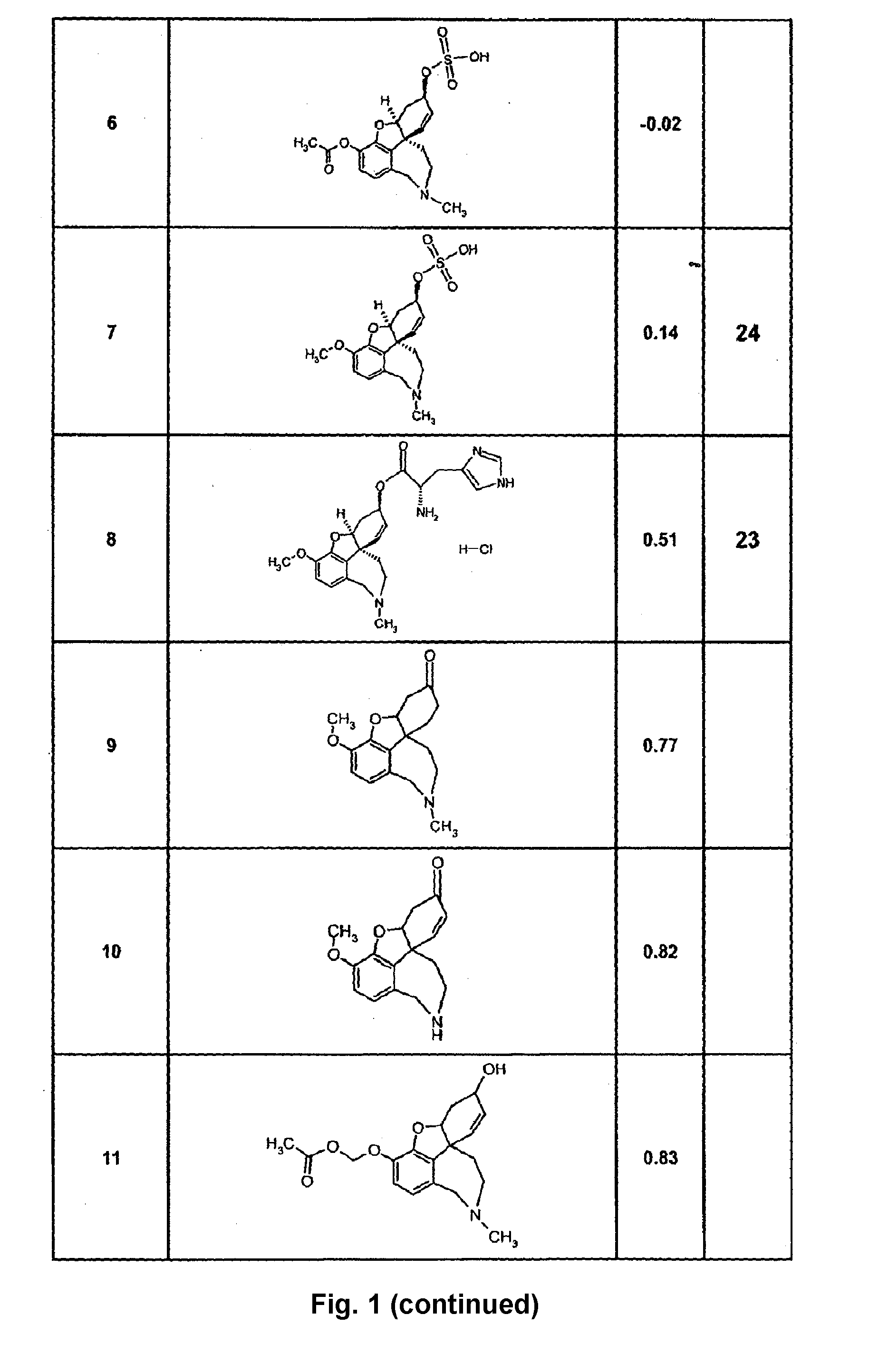
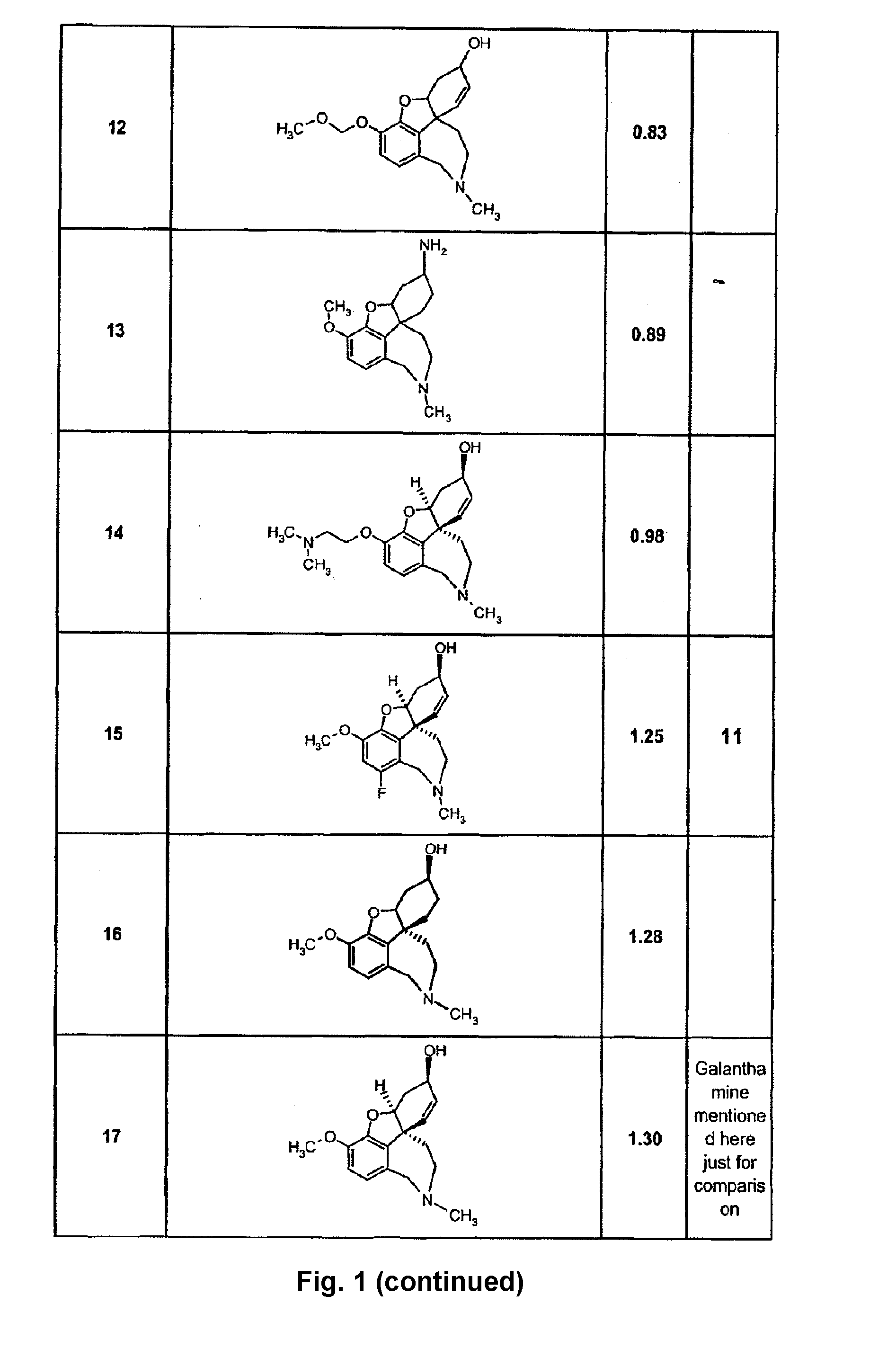
Cholinergic enhancers with improved blood-brain barrier permeability for the treatment of diseases accompanied by cognitive impairment
InactiveUS20090253654A1Improve efficacyLow peripheral side effectBiocideGroup 5/15 element organic compoundsChemical structureChemical synthesis
The present invention refers to compounds that, in addition to enhancing the sensitivity to acetylcholine and choline, and their exogenous agonists, of neuronal cholinergic receptors and / or acting as cholinesterase inhibitors and / or neuroprotective agents, have enhanced blood-brain barrier permeability in comparison to their parent compounds. The compounds are derived (either formally by their chemical structure or directly by chemical synthesis) from natural compounds belonging to the class of amaryllidaceae alkaloids e.g., galantamine, narwedine and lycoramine, or from metabolites of said compounds. The compounds of the present invention can either interact as such with their target molecules, or they can act as “pro-drugs”, in the sense that after reaching their target regions in the body they are converted by hydrolysis or enzymatic attack to the original parent compound and react as such with their target molecules, or both. The compounds of this invention may be used as medicaments.
Owner:GALANTOS PHARMA
Treatment of diseases modulated by a h4 receptor agonist
The invention provides a method for the treatment of H4R modulated diseases and / or conditions comprising administering to the subject an effective amount of a H4R agonist. The invention also provides a method for treating COPD comprising administering to the subject an effective amount of a H4R agonist, a H1R antagonist and an anticholinergic drug. Further, the invention provides a pharmaceutical formulation comprising a H4R agonist, a second active agent and a pharmaceutically acceptable carrier.
Owner:NICOLAOU MICHALIS +3
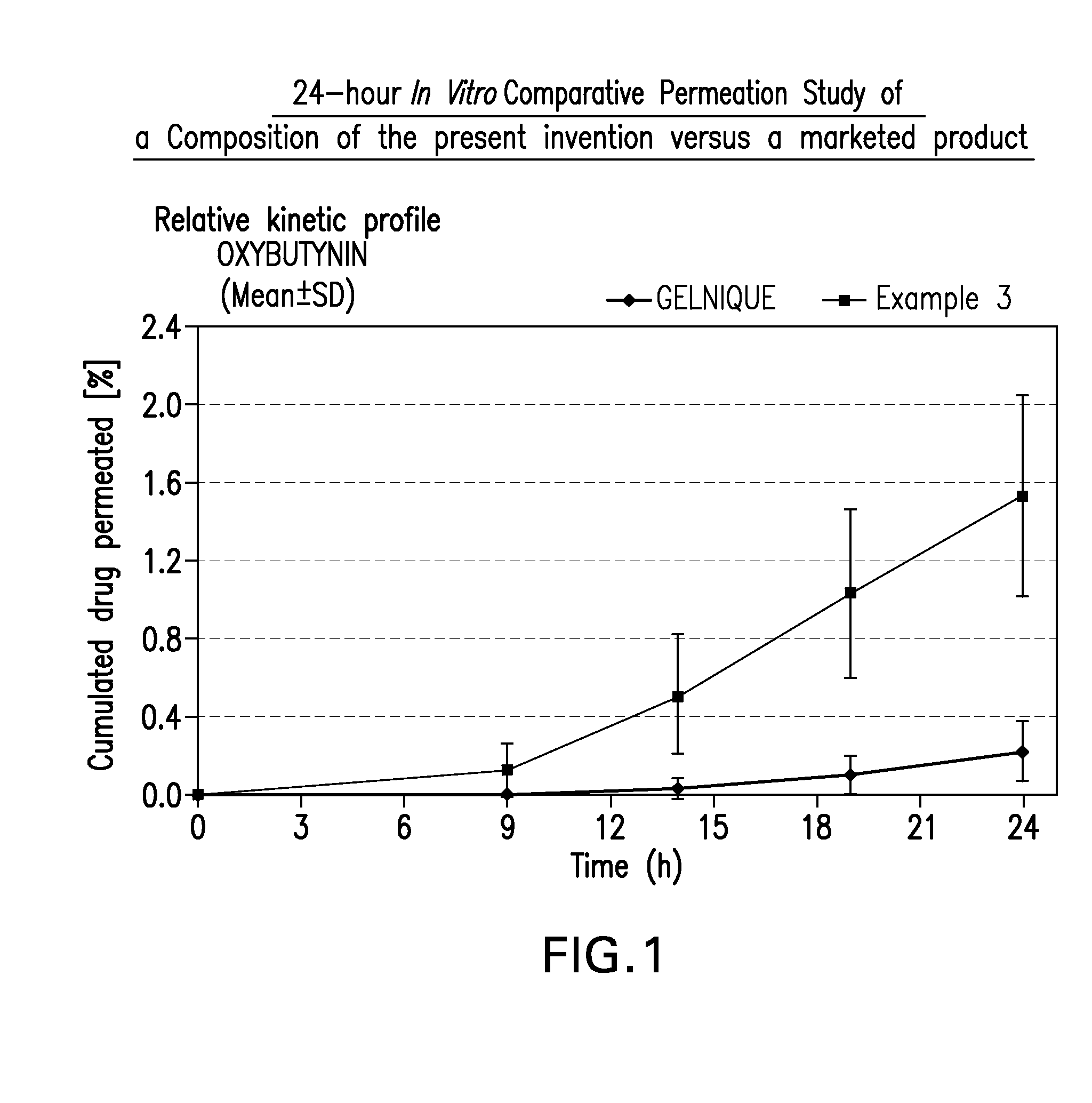
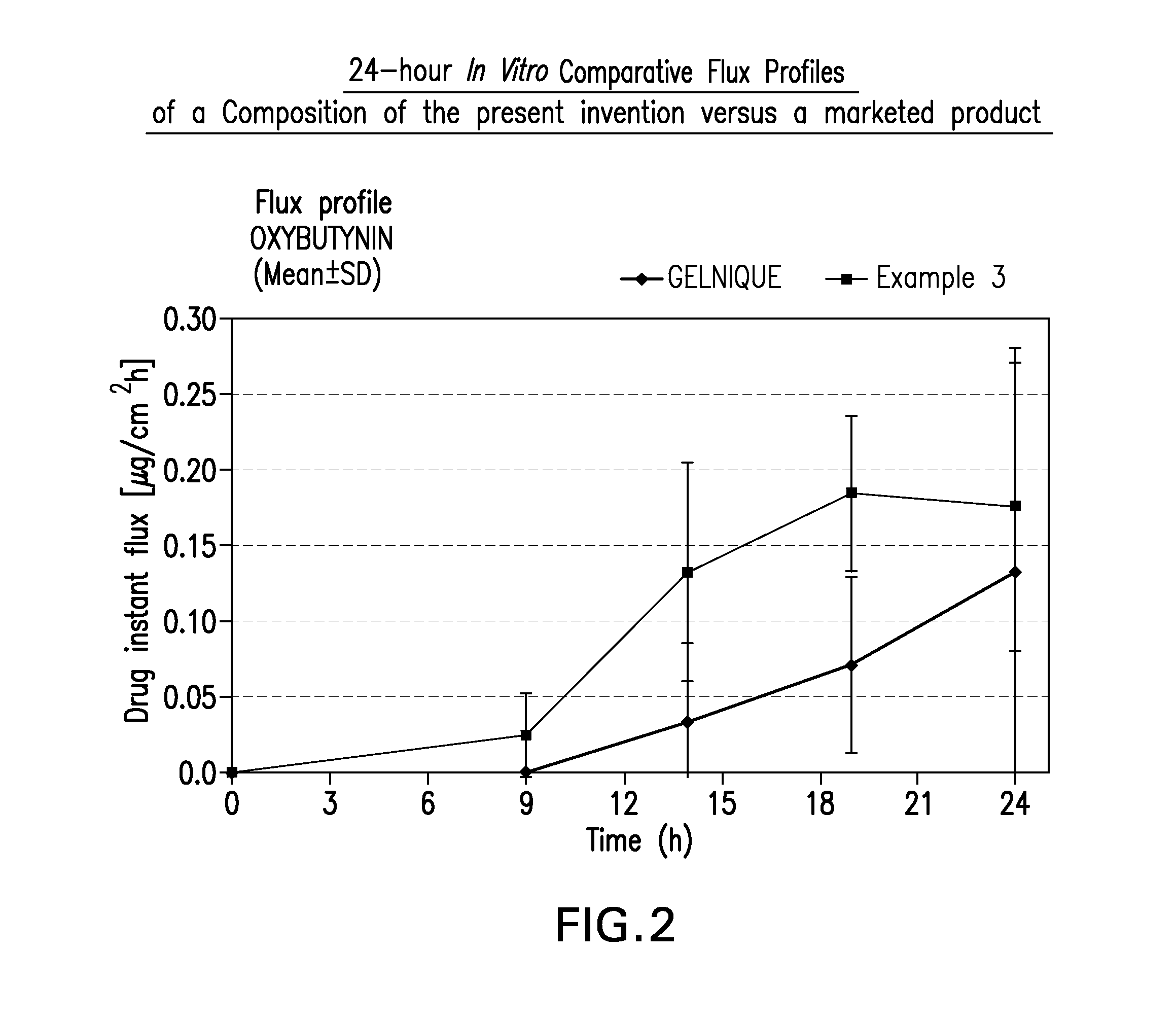
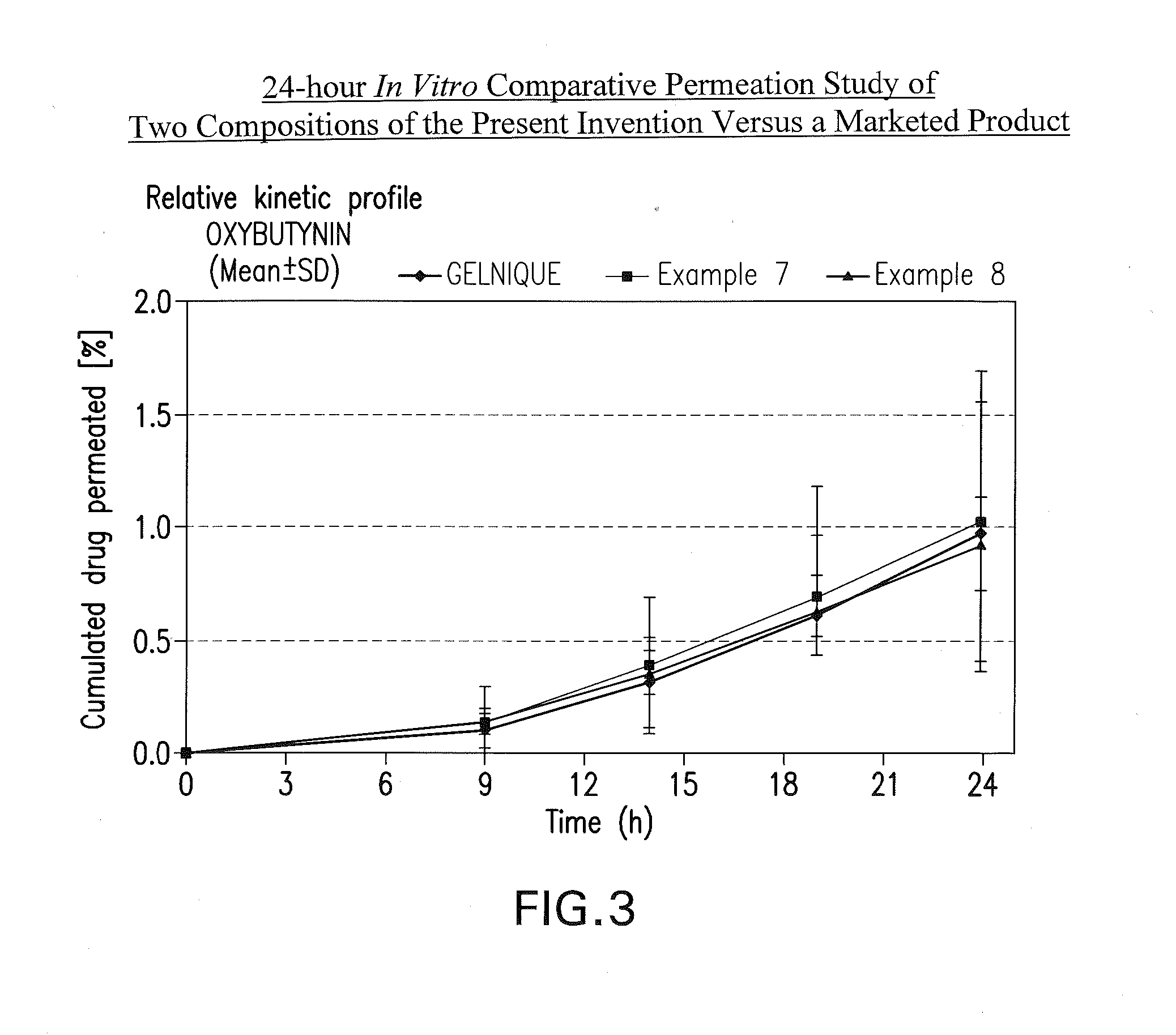
Transdermal compositions for anticholinergic agents
InactiveUS20100216880A1Avoiding undesirable peak in drug concentrationReduce morbidityAntibacterial agentsBiocideOxybutyninLong chain fatty acid
The present invention relates generally to compositions or formulations for transdermal or transmucosal administration of anticholinergic agents such as oxybutynin. The invention utilizes a novel delivery vehicle and is a substantially malodorous-free and irritation free transdermal formulation which is substantially free of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters. A method is disclosed for treating a subject for urinary incontinence with these formulations while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anticholinergics.
Owner:ANTARES PHARMA IPL
RNA interference mediated inhibition of cholinergic muscarinic receptor (CHRM3) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050176664A1Improve bioavailabilityMinimize the possibilityCompounds screening/testingNervous disorderMuscarinic receptor siteCholinergic receptor muscarinic 3
This invention relates to compounds, compositions, and methods useful for modulating cholinergic muscarinic receptor gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of cholinergic muscarinic receptor gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of cholinergic muscarinic receptor genes, such as M3 muscarinic acetylcholine receptor or cholinergic receptor muscarinic 3 (CHRM3).
Owner:SIRNA THERAPEUTICS INC
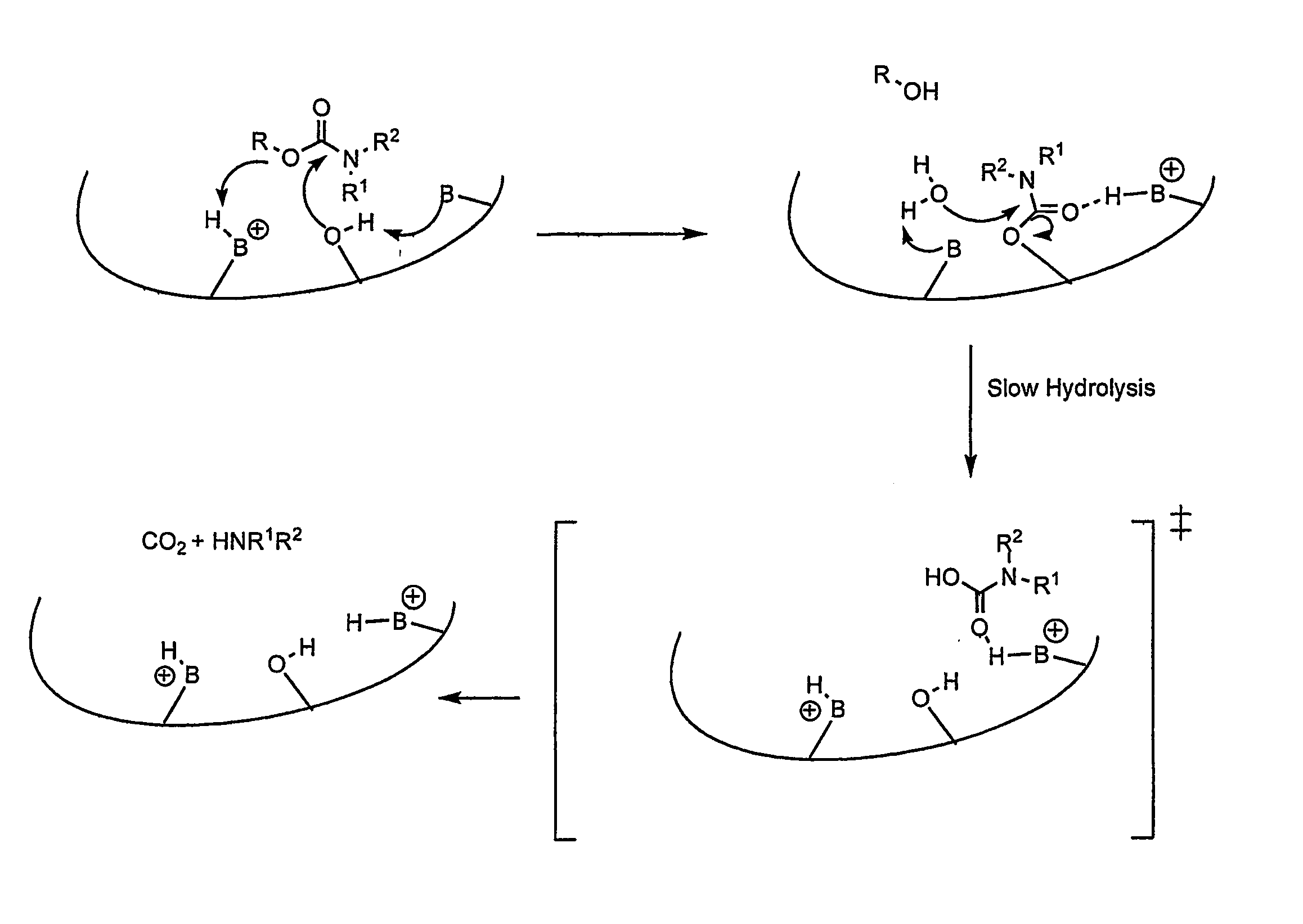
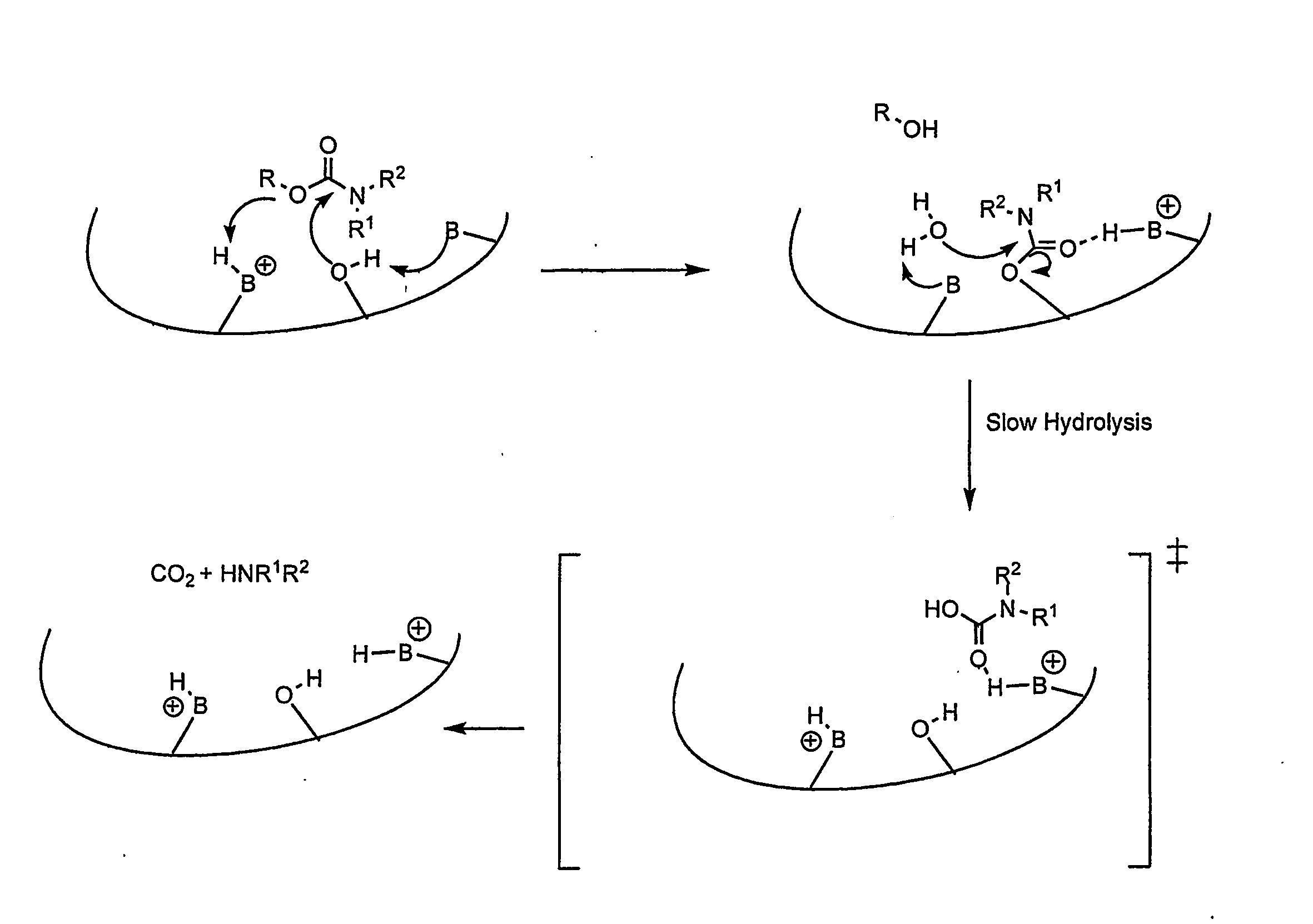
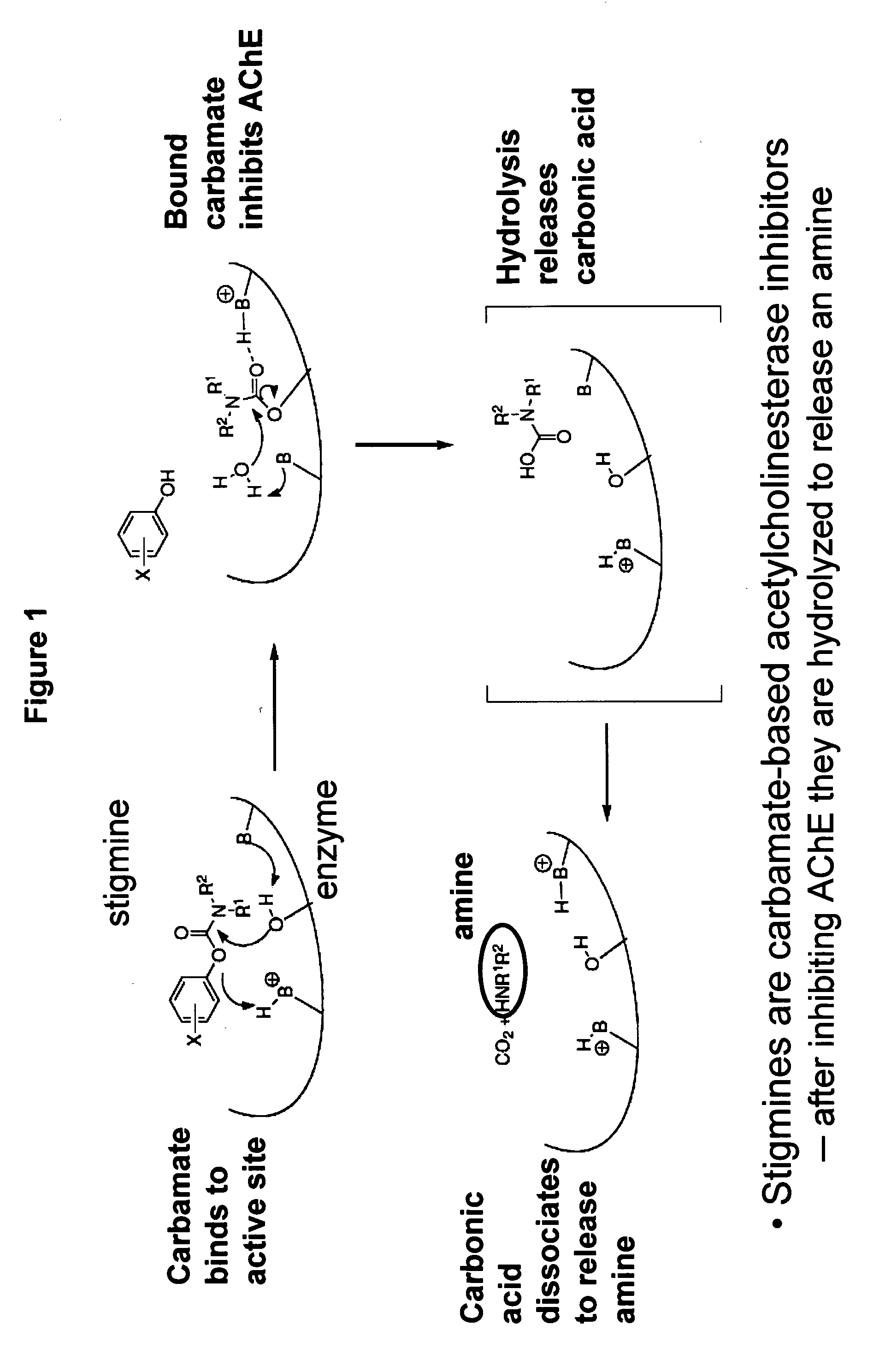
Carbamoyl Esters That Inhibit Cholinesterase And Release Pharmacologically Active Agents
Carbamoyl esters inhibit cholinesterase activity and, upon hydrolysis release a pharmacologically active agent. In one embodiment, the carbamoyl ester has the following structure: Formula (I) wherein A is selected from the group consisting of an unsubstituted aryl, a substituted aryl, an unsubstituted heteroaryl and a substituted heteroaryl. The carbamoyl esters are employed in methods to treat an individual. The pharmacologically active agent obtained by hydrolysis of the carbamoyl esters can treat, for example, a nervous system condition, a cholinergic deficiency and conditions or diseases associated with a deficiency in a pharmacologically active agent, such as acetylcholine.
Owner:COLUCID PHARM INC
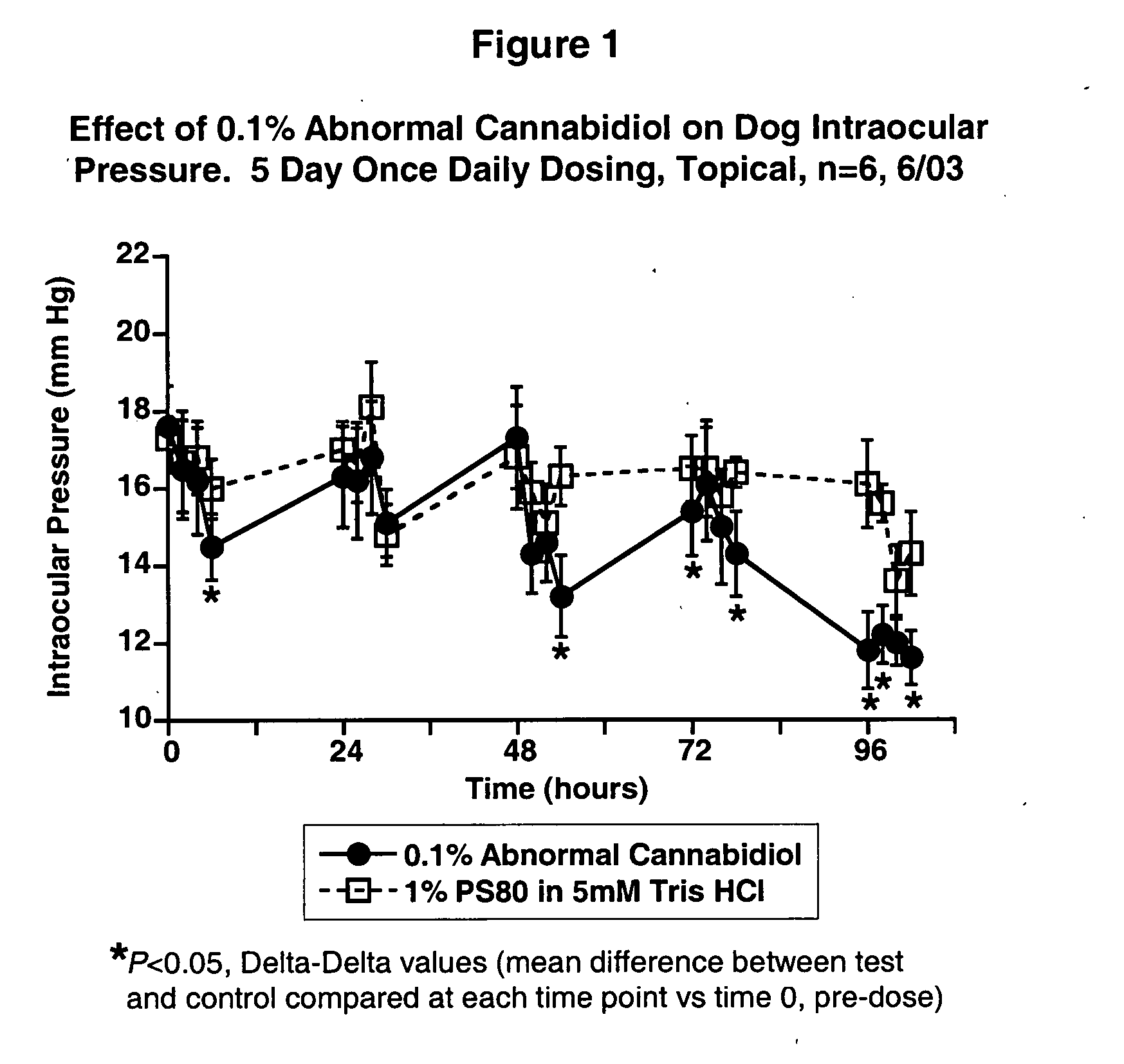
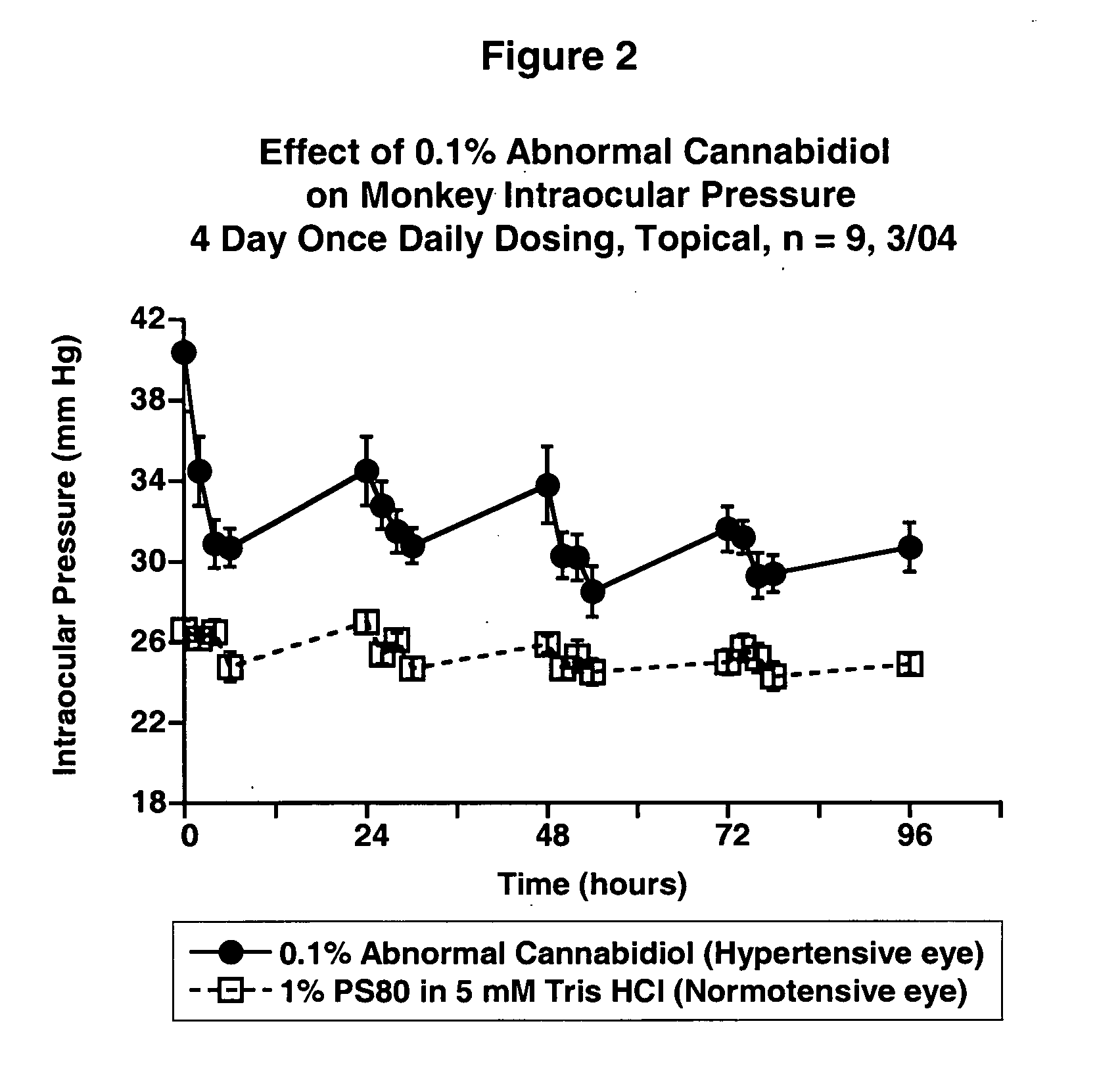
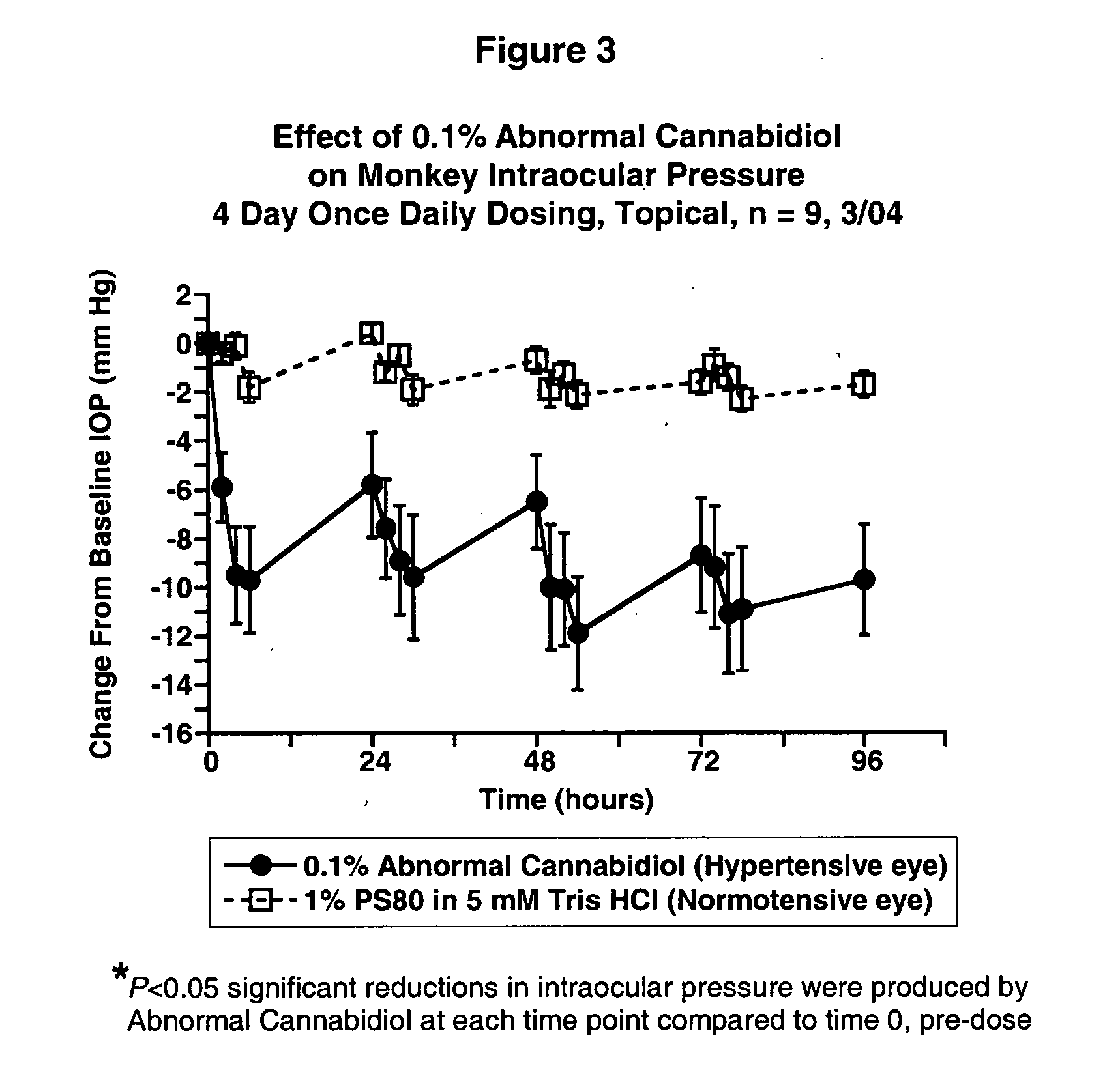
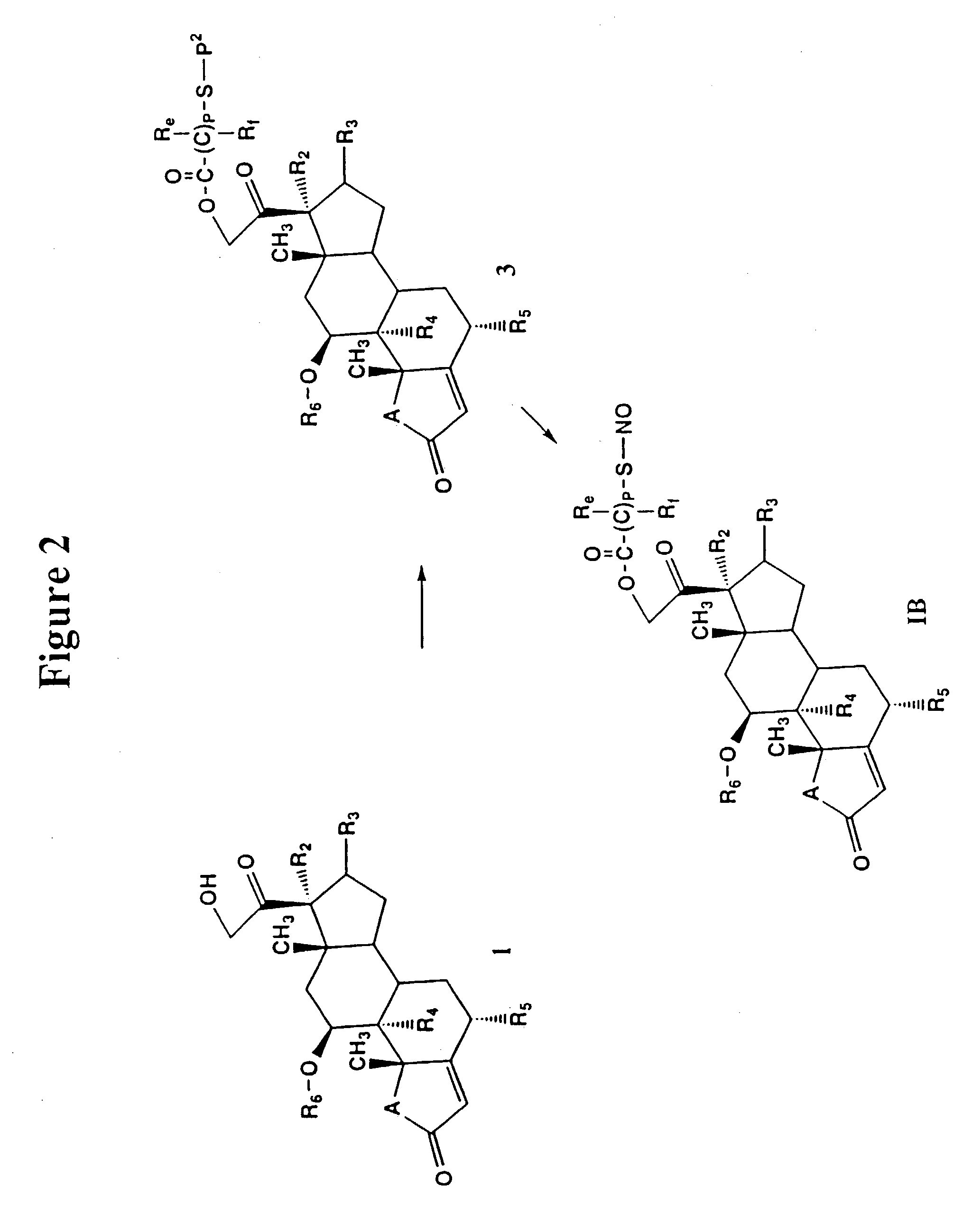
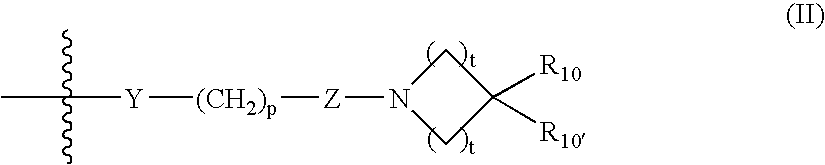
Abnormal cannabidiols as agents for lowering intraocular pressure
InactiveUS20050282902A1Lower intraocular pressureBiocideElcosanoid active ingredientsDrugAdrenergic agonist
The invention relates to the use of Abnormal Cannabidiols in a combination with a drug selected from the group consisting of β-blockers, adrenergic agonists, carbonic anhydrase inhibitors, cholinergic agonists, chlolinesterase inhibitors, glutamate antagonists, prostamides and prostaglandins and the like, or pharmaceutically acceptable salts or prodrugs thereof as potent ocular hypotensives. Said combinations are particularly suitable for the management of glaucoma. In particular said Abnoral Cannibidiols are represented by formula I or formula II or formula III
Owner:ALLERGAN INC
Compounds co-inducing cholinergic up-regulation and inflammation down-regulation and uses thereof
InactiveUS20020160988A1High pharmacological activity and Therapeutic IndexEfficiently cross blood brain barrierBiocideSalicyclic acid active ingredientsDiseaseNon steroid anti inflammatory drug
Chimeric compounds are disclosed which are covalent conjugates of reversible or irreversible cholinergic up-regulators and non-steroidal anti-inflammatory drugs (NSAIDs), methods for their synthesis and use thereof for treatment and / or prevention of central nervous system (CNS) disorders and diseases.
Owner:STATE OF ISRAEL MINIST OF AGRI & RURAL DEV AGRI RES ORG (A R O) (VOLCANI CENT) +1
Nitrosated and nitrosylated compounds and compositions and their use for treating respiratory disorders
Disclosed are (i) compounds of a steroid, a β-agonist, an anticholinergic, a mast cell stabilizer and a phosphodiesterase (PDE) inhibitor directly or indirectly linked to a NO or NO2 group or a group which stimulates endogenous production of NO or EDRF in vivo; (ii) compositions of steroids, β-agonists, anticholinergics, mast cell stabilizers and PDE inhibitors, which can optionally be substituted with at least one NO or NO2 moiety or a group which stimulates endogenous production of NO or EDRF in vivo, and a compound that donates, transfers or releases nitric oxide as a charged species, i.e., nitrosonium (NO+) or nitroxyl (NO−), or as the neutral species, nitric oxide (NO.) or that stimulates endogenous production of NO or EDRF in vivo; and (iii) uses for them in preventing and / or treating respiratory disorders.
Owner:ARBOR PHARMA LLC
Muscarinic agonists
Compounds and methods are provided for the treatment of disease conditions in which modification of cholinergic, especially muscarinic m1, m4, or both m1 and m4, receptor activity has a beneficial effect. In the method, an effective amount of a compound is administered to a patient in need of such treatment.
Owner:ACADIA PHARMA INC
Method and system for the treatment of chronic obstructive pulmonary disease with nebulized anticholinergic administrations
Inhalation solutions for administration of muscarinic antagonists for the treatment of breathing disorders, such as COPD, are provided.
Owner:SUNOVION RESPIRATORY DEV
Compounds that inhibit cholinesterase
InactiveUS20080261950A1Improve cognitive functionEnhancing cholinergic functionAntibacterial agentsBiocideDiseaseCholinesterase
Compounds that inhibit cholinesterase activity and, upon hydrolysis release a pharmacologically active agent. The compounds of the invention are employed in methods to treat an individual. The pharmacologically active agent obtained by hydrolysis of the compound can treat, for example, a nervous system condition, a cholinergic deficiency and conditions or diseases associated with a deficiency in a pharmacologically active agent, such as acetylcholine.
Owner:COLUCID PHARM INC
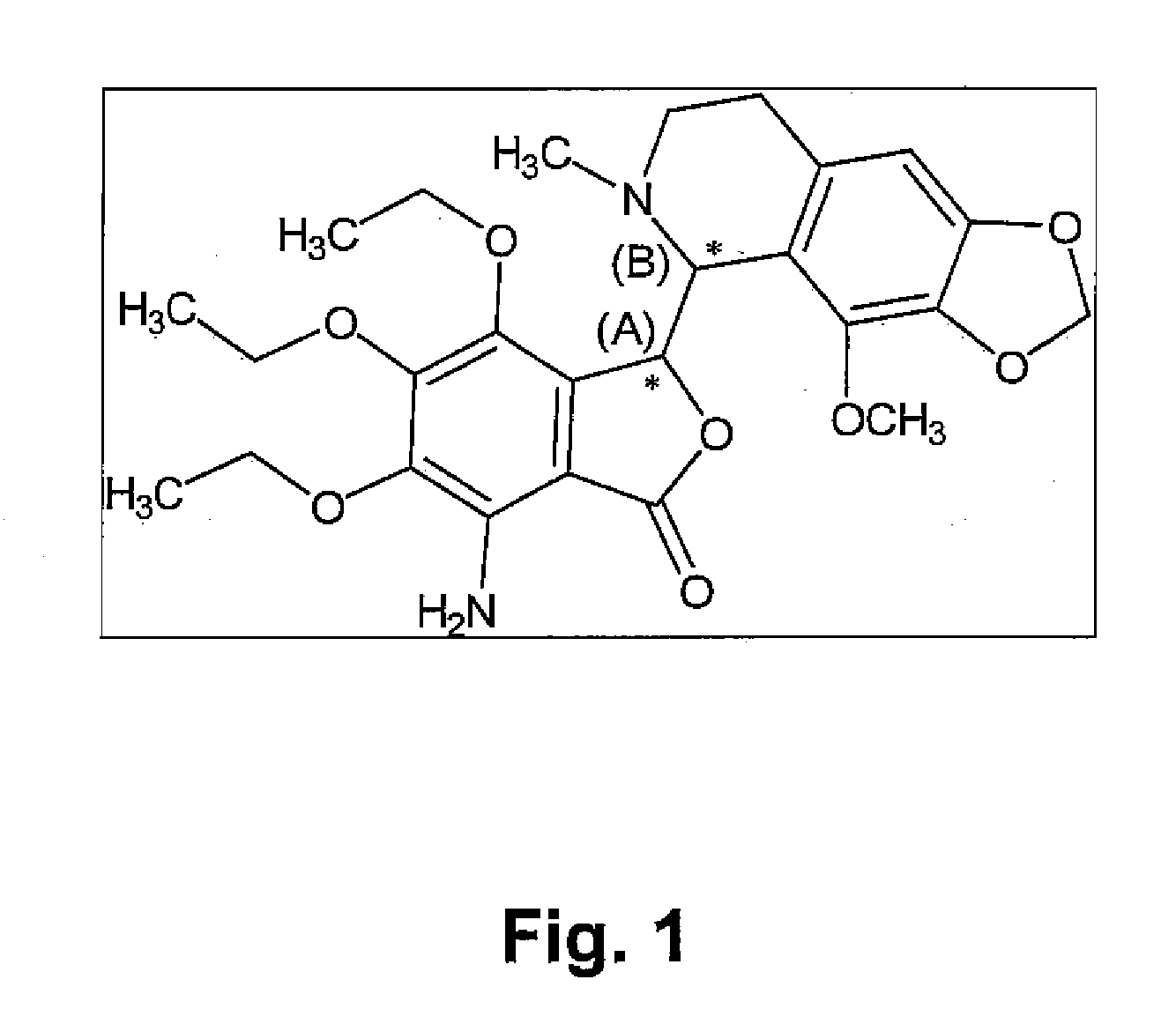
Preparation method and application of glycopyrronium bromide chiral antipode
The invention belongs to the technical field of medicine, and discloses a preparation method of (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) four type chiral monomers of muscarine receptor antagonist racemic medicine glycopyrronium bromide. The method comprises the following steps: resolving racemic alpha-cyclopentylmandelic acid by a chemical resolution method by using L-Tyrosine methyl ester and (R)-alpha-phenylethylamine as resolution reagents to respectively prepare (S)-alpha-cyclopentylmandelic acid and (R)-alpha-cyclopentylmandelic acid; and carrying out esterification reaction to respectively obtain chiral intermediates (S) / (R)-alpha-cyclopentylmethyl mandelate. L / D-malic acid used as the raw material is subjected to four reaction steps, including condensation, carbonyl reduction, catalytic hydrogenation or transfer hydrogenation reduction debenzylation, and reduction alkylation or alkylogen alkylation, in a chiral synthesis mode to obtain another important chiral intermediate (S) / (R)-N-methyl-3-hydroxypyrrolidine. The chiral intermediate is subjected to ester exchange and quaterisation to respectively obtain the four (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) type glycopyrronium bromide chiral monomers. The result indicates that the (3R,2'S)-glycopyrronium bromide has the strongest cholinergic antagonistic action.
Owner:SHENYANG PHARMA UNIVERSITY +1
Compositions and Methods for Treating Social Anxiety
InactiveUS20110218215A1Preventing social phobiaStop formationBiocideNervous disorderAtropine sulfateMedicine
The disclosure provides a pharmaceutical composition for treating social anxiety, performance anxiety, and social phobia comprising a therapeutic amount for die treatment of a patient of a β-adrenergic receptor antagonist, an anti-diarrheal compound, and an optional anticholinergic compound. The β-adrenergic receptor antagonist may be the lipophilic β-blocker propranolol HCl, the anti-diarrheal compound may be the opioid diphenoxylate HCl, and the optional anticholinergic compound may be atropine sulfate. The composition for treating performance anxiety and social phobia can further include a pharmaceutically acceptable carrier. A method of preventing or treating social anxiety, performance anxiety, and social phobia in a patient is also provided, comprising administering a composition of the disclosure to a patient in need of such treatment. The composition administered in the present method comprises a therapeutic amount of a β-adrenergic receptor antagonist, an anti-diarrheal compound, and an optional anticholinergic compound.
Owner:HOLLY BENJAMIN D
Method and composition for treating alzheimer-type dementia
InactiveUS20110201597A1Extended durationFunction increaseBiocideNervous disorderNK1 receptor antagonistMaximum tolerated dose
There is described a method for increasing the maximal tolerated dose and thus the efficacy of an acetylcholinesterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetylcholinesterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetylcholinesterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholinesterase inhibitor are also described.
Owner:CHASE PHARMA CORP
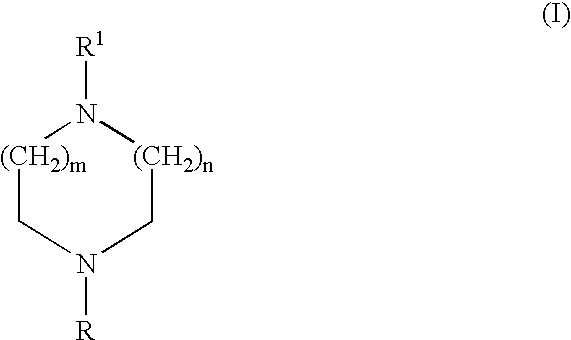
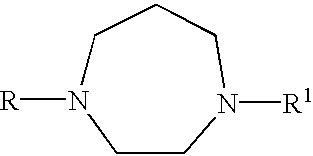
Certain heteroaryl diazacycloalkanes as cholinergic ligands at nicotinic acetylcholine receptors
Owner:DANPET
Transgenic non-human mammals producing a cholinesterase in their milk
InactiveUS6987211B1Increased neuromuscular transmissionGreat muscular strengthHydrolasesMicrobiological testing/measurementOrganophosphorous compoundsPhysiology
The present invention relates to novel alternative forms of human acetylcholinesterase (AChE) and nucleotide sequences encoding the same. The genes encoding the novel forms of human AChE have been identified in various malignant tumor cells. In a further aspect, the invention relates to a transgenic animal assay system for evaluating efficacy of drugs against cholinergic proteins, prior to or in the course of therapeutic treatment. Transgenic animals, preferably developing tadpole of Xenopus or mice which express human AChE, are used. The transgenic animal assay system is also useful for evaluating the toxicity of substances which potentially block human AChE (e.g. organophosphorous compounds).
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
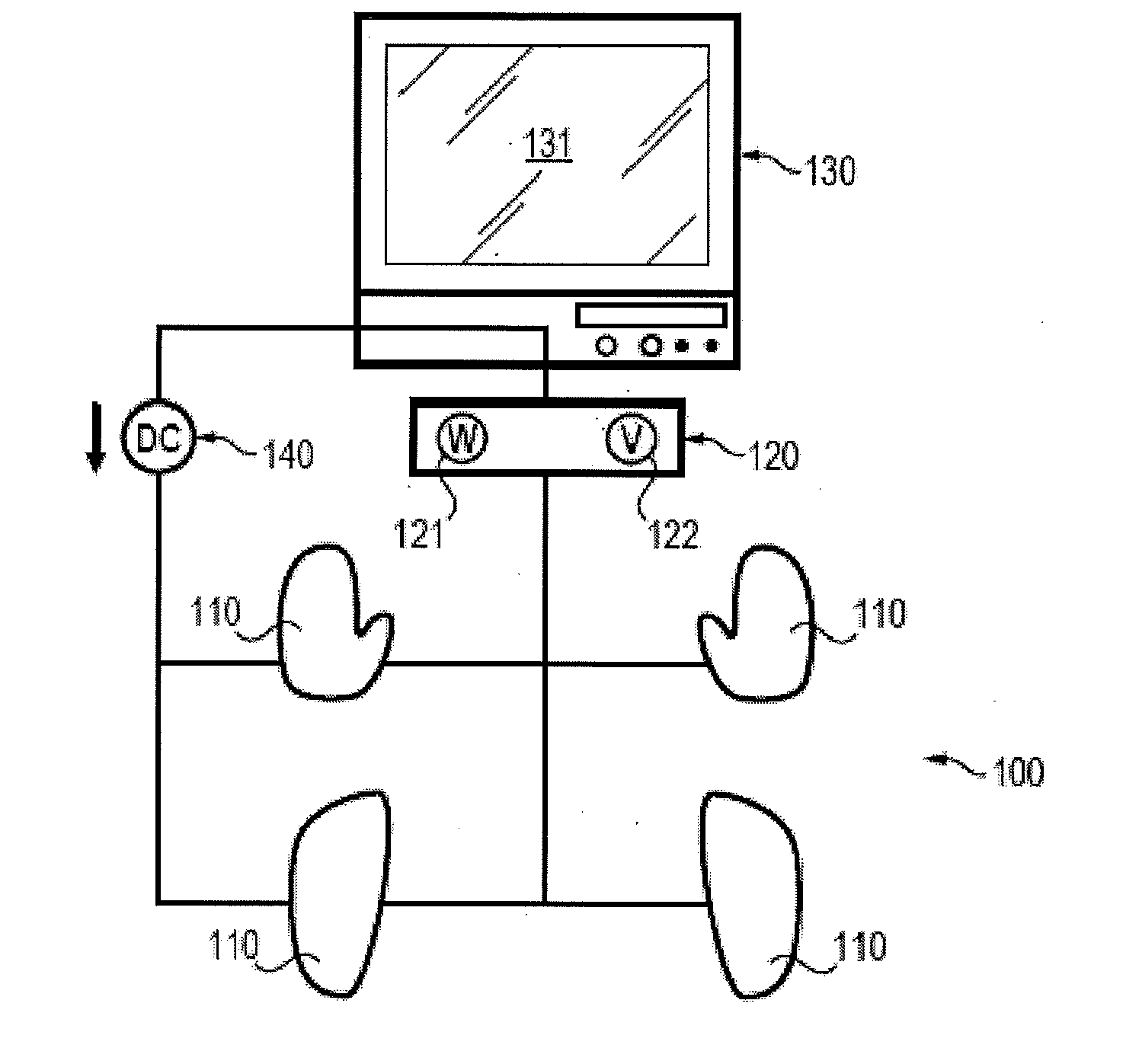
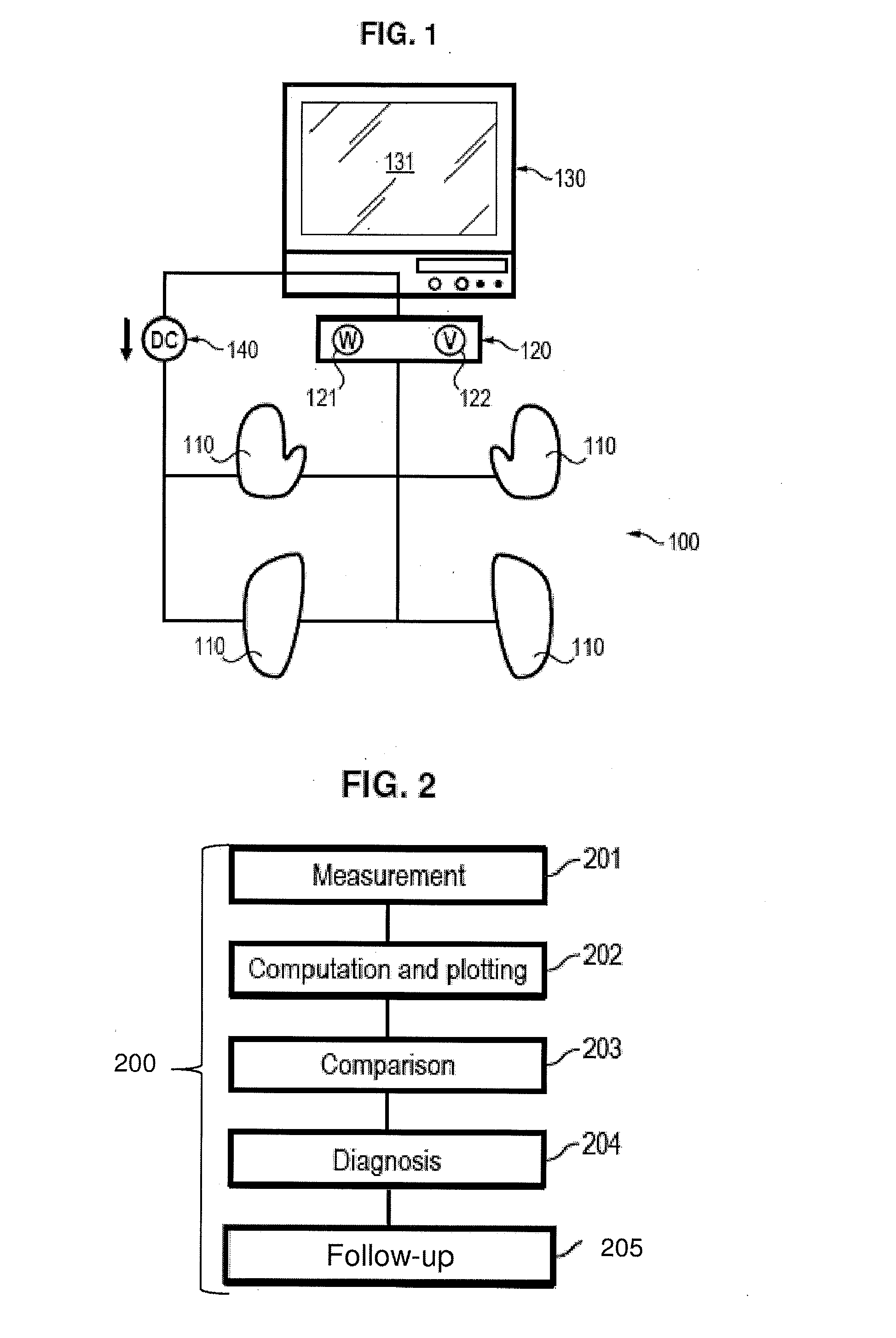
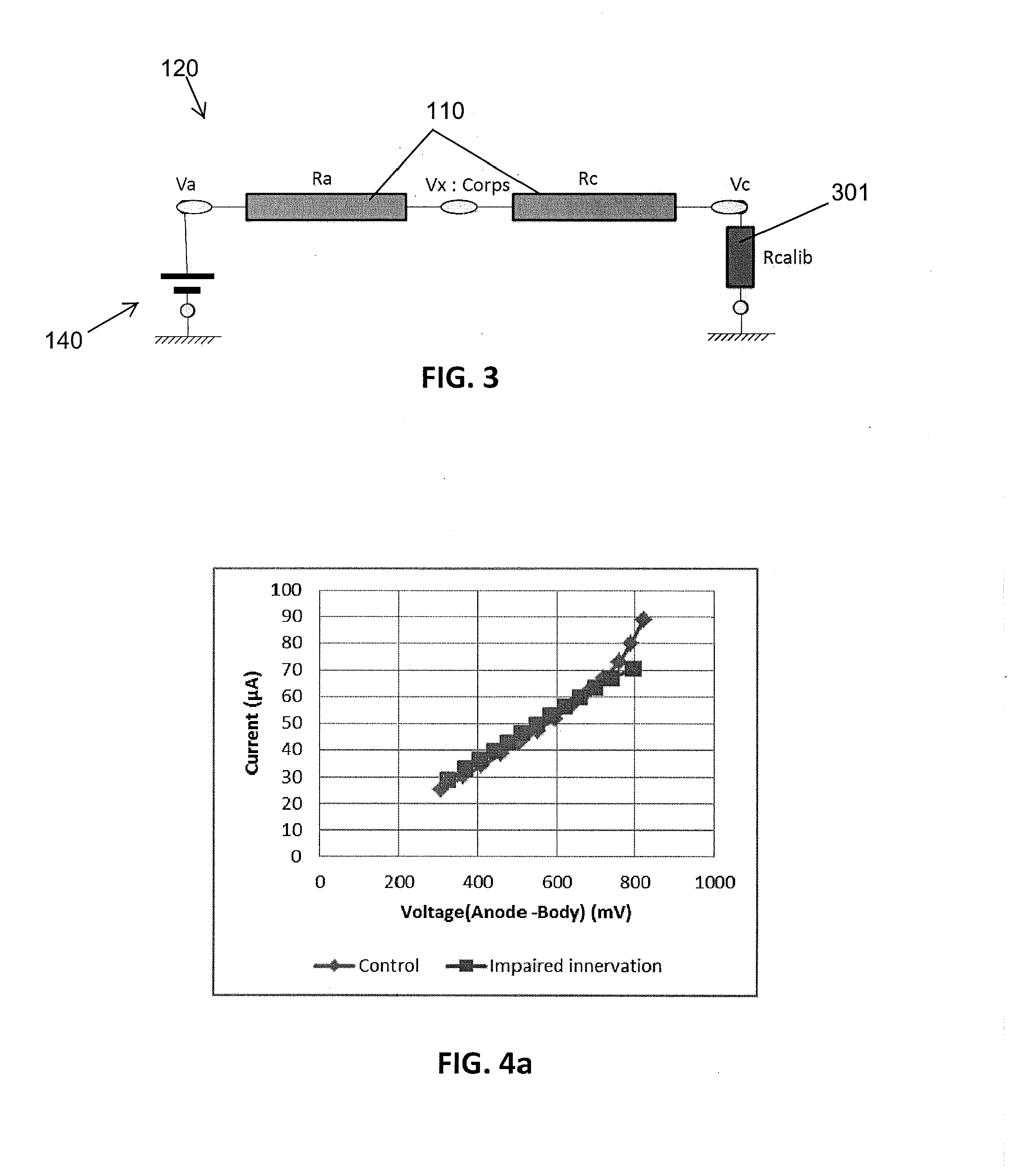
Assessment of relative proportions of adrenergic and cholinergic nervous receptors with non-invasive tests
InactiveUS20140350432A1High impedanceEarly detectionSensorsNervous system evaluationCholinergic NervesSweat gland
A system and method for assessing relative proportions of cholinergic and adrenergic nervous receptors in a patient is disclosed. The system includes: an anode, a cathode, and passive electrode for placement on different regions of the patient body. The method generally includes: applying DC voltage pulses of varying voltage values to stress sweat glands of the patient, collecting data representing the current between the anode and the cathode and the potential of the anode, the cathode, and the passive electrode for each of the different DC voltage, and computing data representing the electrochemical skin conductance of the patient. The computed data representing the electromechanical skin conductance of the patient is reconciled with reference data from control patients having known relative proportions of cholinergic and adrenergic nervous receptors. Thus, the relative proportions of cholinergic and adrenergic nervous receptors in the patient can be determined.
Owner:IMPETO MEDICAL
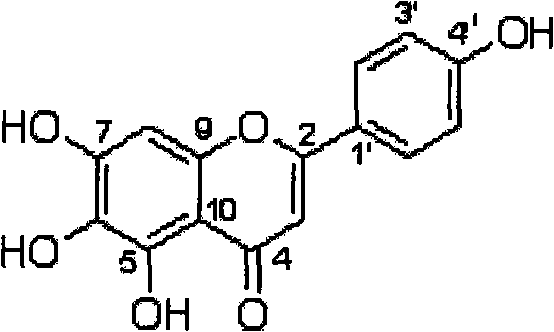
Method for preparing 5,6,7,4'-tetrahydroxyflavone and application of 5,6,7,4'-tetrahydroxyflavone in medicaments
ActiveCN101591321AFull close contactImprove solubilityOrganic active ingredientsNervous disorderArthritisCholinergic
The invention provides a method for preparing a 5,6,7,4'-tetrahydroxyflavone compound, which is characterized by comprising the following steps: taking breviscapinun as a raw material, and performing heating reflux reaction between the breviscapinun and alcohol under the catalysis of acid, namely adding 200 to 500 ml of alcohol substance and 1 to 3 ml of 4 to 5 percent acid into 12 to 20 g of the breviscapinun for the heating reflux reaction; and filtering a product, and drying to a constant weight to obtain 5,6,7,4'-tetrahydroxyflavone shown in a formula I. Experiments prove that the 5,6,7,4'-tetrahydroxyflavone shows obvious strengthening effect on acquisition, consolidation and representation of brain memory of a mice model of alzheimer disease, has obvious improving effect on learning memory of the mice model with memory loss caused by anisodine of an M-cholinergic inhibitor, and can obviously improve the recent memory and long-term memory capacities of mice. The experiments also prove that the 5,6,7,4'-tetrahydroxyflavone has the effect of treating arthritis, and also has good treating effects on diabetes mellitus and osteoporosis.
Owner:KPC PHARM INC
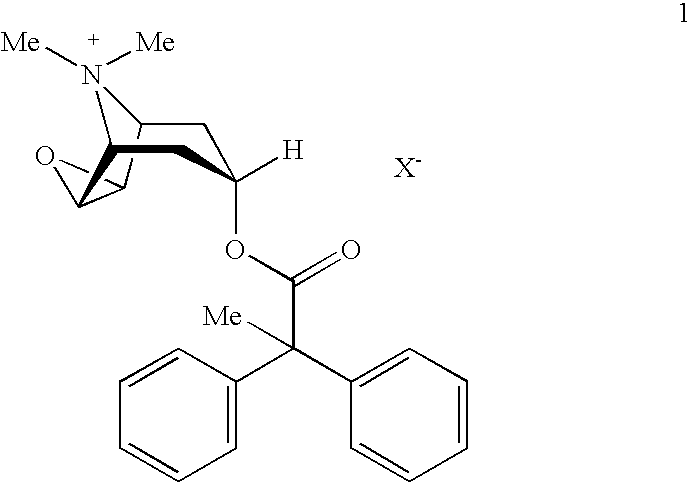
HFC solution formulations containing an anticholinergic
An aerosol solution formulation comprising:(a) a salt of formula 1wherein X− is an anion(b) an HFC propellant;(c) a cosolvent; and(d) an inorganic or an organic acid, wherein the concentration of the acid is in a range that corresponds with a pH range of 2.5 to 5.5 in aqueous solution.
Owner:BOEHRINGER INGELHEIM INT GMBH
Optogenetic control of reward-related behaviors
InactiveUS20170157269A1Reduce and eliminate effectBioreactor/fermenter combinationsNervous disorderOptogeneticsInterneuron
Provided herein are compositions and methods for disrupting at least one reward-related behavior in an individual through the use of light-responsive opsin proteins used to control the polarization state of the cholinergic intemeurons of the nucleus accumbens or the striatum.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
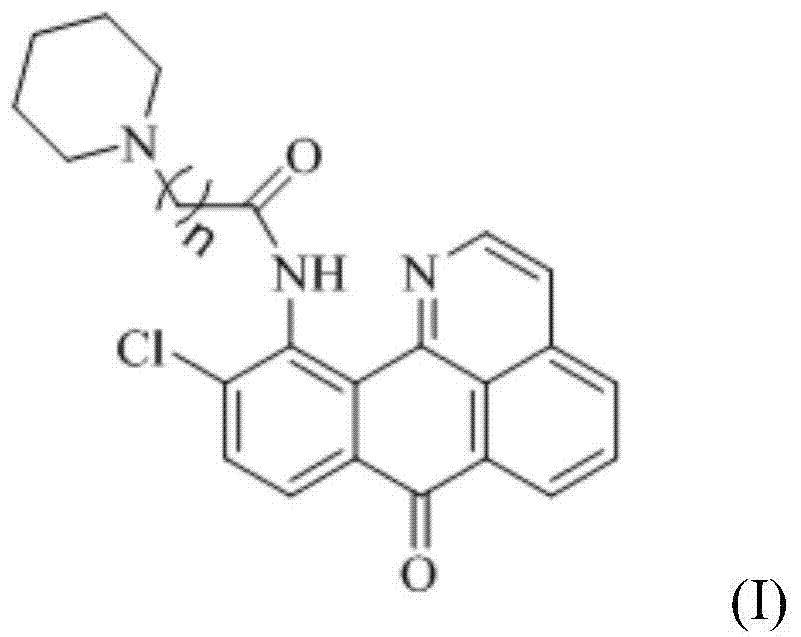
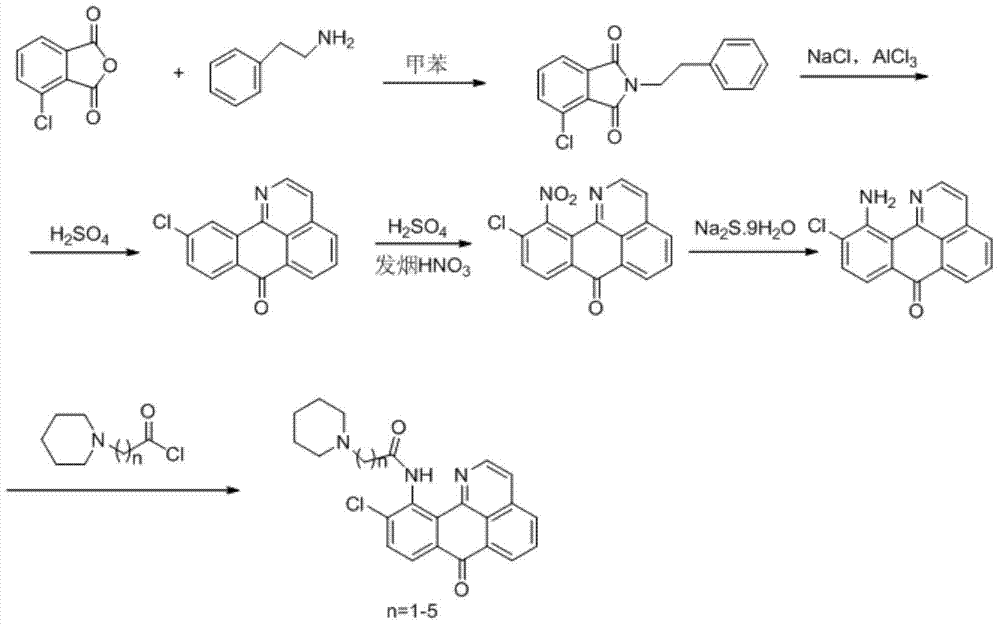
11-replaced oxoisoaporphine derivatives as well as synthetic method and application thereof
InactiveCN103923010AStrong inhibitory activityGood potential medicinal valueOrganic active ingredientsSenses disorderKetoneStructural formula
The invention discloses a series of 11-replaced oxoisoaporphine derivatives as well as a synthetic method and an application thereof. The synthetic method comprises the following steps: (1) carrying out ring closing reaction on 3-chlorophthalic anhydride and phenylethylamine as raw materials so as to construct a 10-Cl-1-azabenzanthrone parent body; (2) nitrating the parent body compound so as to obtain a 11-site nitrated product, and reducing the 11-site nitrated product so as to obtain 11-amino-10-chlorine-7H-dibenzoquinoline-7-ketone; and (3) reacting the 11-amino-10-chlorine-7H-dibenzoquinoline-7-ketone with an acyl chloride compound connected with piperidine so as to obtain a corresponding target product. Through study, the applicant finds that the series of derivatives have very strong inhibitory activity on acetylcholin esterase and are expected to be used for treating AD (Alzheimer Disease), cerebrovascular dementia and related diseases caused by cholinergic neurotransmitter reduction. The structural formula of the 11-replaced oxoisoaporphine derivatives is shown in descriptions.
Owner:GUANGXI NORMAL UNIV
Methods and implantable devices for treating supraventricular arrhythmias
InactiveUS20060079941A1Successfully addressBiocideTransvascular endocardial electrodesRoom treatmentAtrial flutter
A medication, method and device for cardiac treatment are provided, in particular, for treating supraventricular arrhythmias. Specifically, a method is provided for treating supraventricular arrhythmias, using a therapeutically effective amount of a cholinergic receptor agonist, for example, acetylcholine. This device maybe part of universal device which provides pacing and defibrillation. In particular, the present invention can be used to treat atrial fibrillation, atrial flutter and atrial tachycardia by a bolus injection of a rapidly hydrolysable cholinergic receptor agonist such as acetylcholine.
Owner:CLOSED LOOP THERAPIES
Muscarinic agonists
Owner:ACADIA PHARMA INC
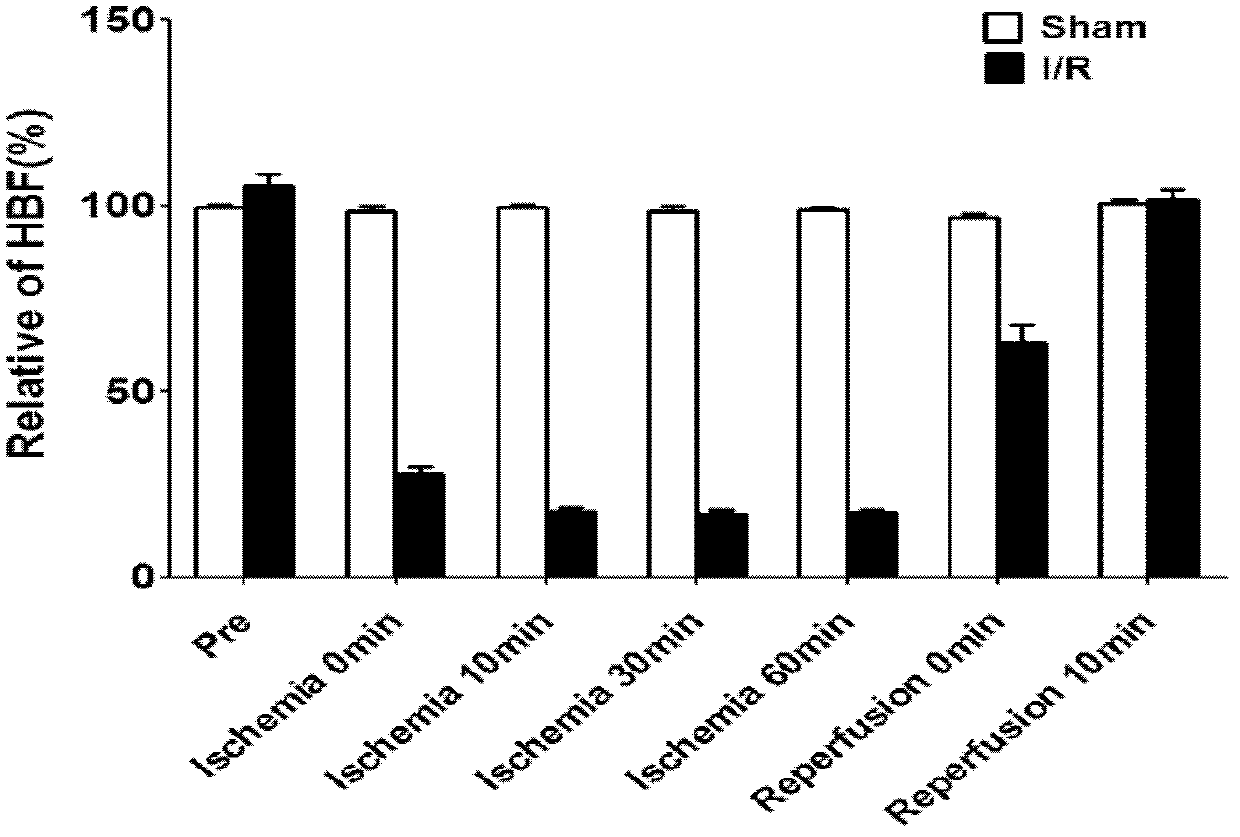
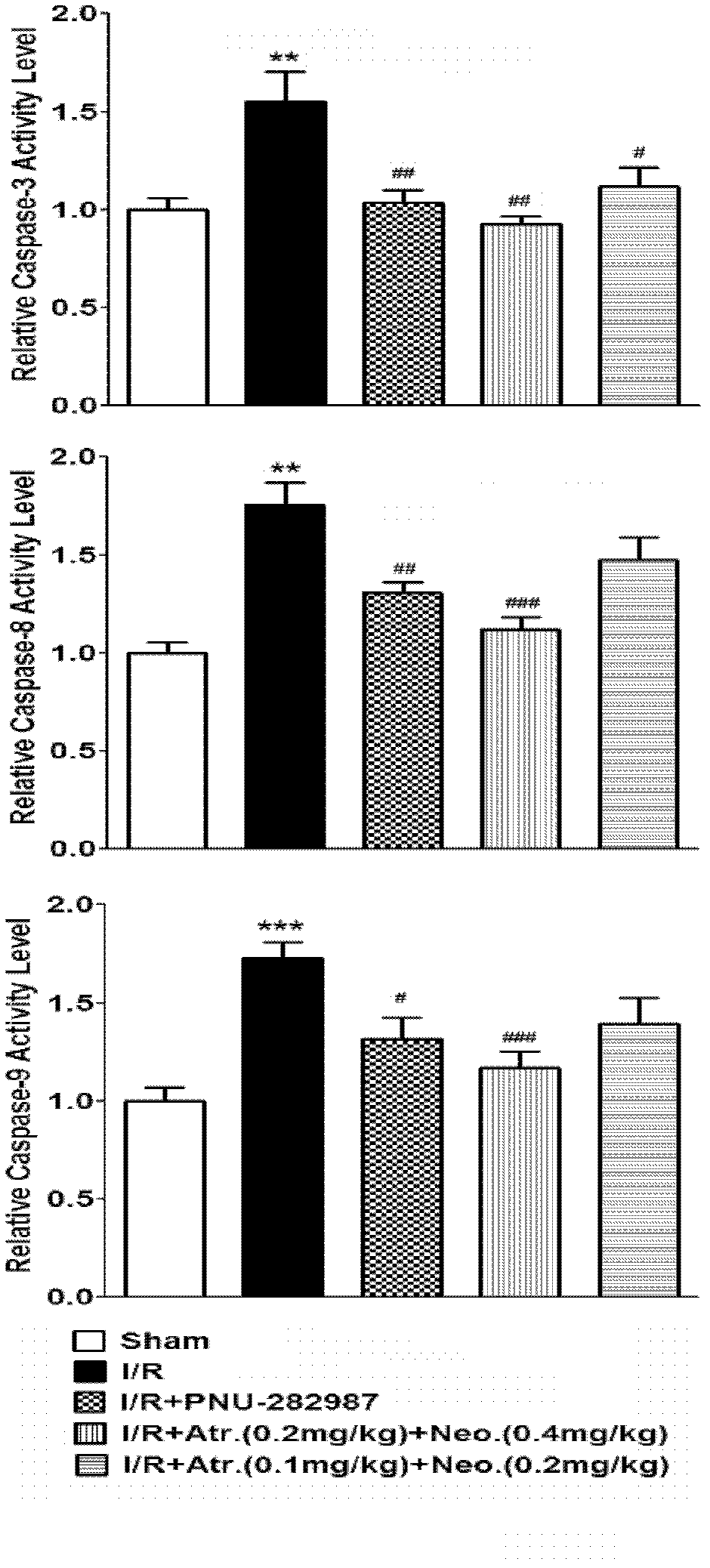
Composition for resisting ischemia reperfusion injury and preparation method and application thereof
InactiveCN102580099APracticalSimple preparation processDigestive systemEster active ingredientsSocial benefitsSide effect
The invention relates to a medicinal composition for treating an ischemia reperfusion injury, particularly a hepatic ischemia reperfusion injury, and a preparation method and application thereof. The composition comprises an M-cholinergic receptor blocker and a cholinesterase inhibitor, has a simple and convenient preparation process, is safe to use, has an obvious curative effect, can be used for obviously improving the cell apoptosis caused by the ischemia reperfusion injury and overcomes the side effects caused by existing common medicaments. The invention expands novel medical application of an existing muscarinic receptor blocker and also provides a novel medicine intervention means for preventing and treating the ischemia reperfusion injury. The medicinal composition is suitable for large-scale production and commercial application in the industries of medicine, reagents and the like, has excellent application prospect and has obvious social benefits and economic benefits.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com