Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
94 results about "Anticholinergic agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A drug used to relieve cramps or spasms of the stomach, intestines, and bladder
Therapy for the treatment of disease
ActiveUS20070053995A1Relieve constipationBiocideNervous disorderAnticholinergic agentsCompound (substance)
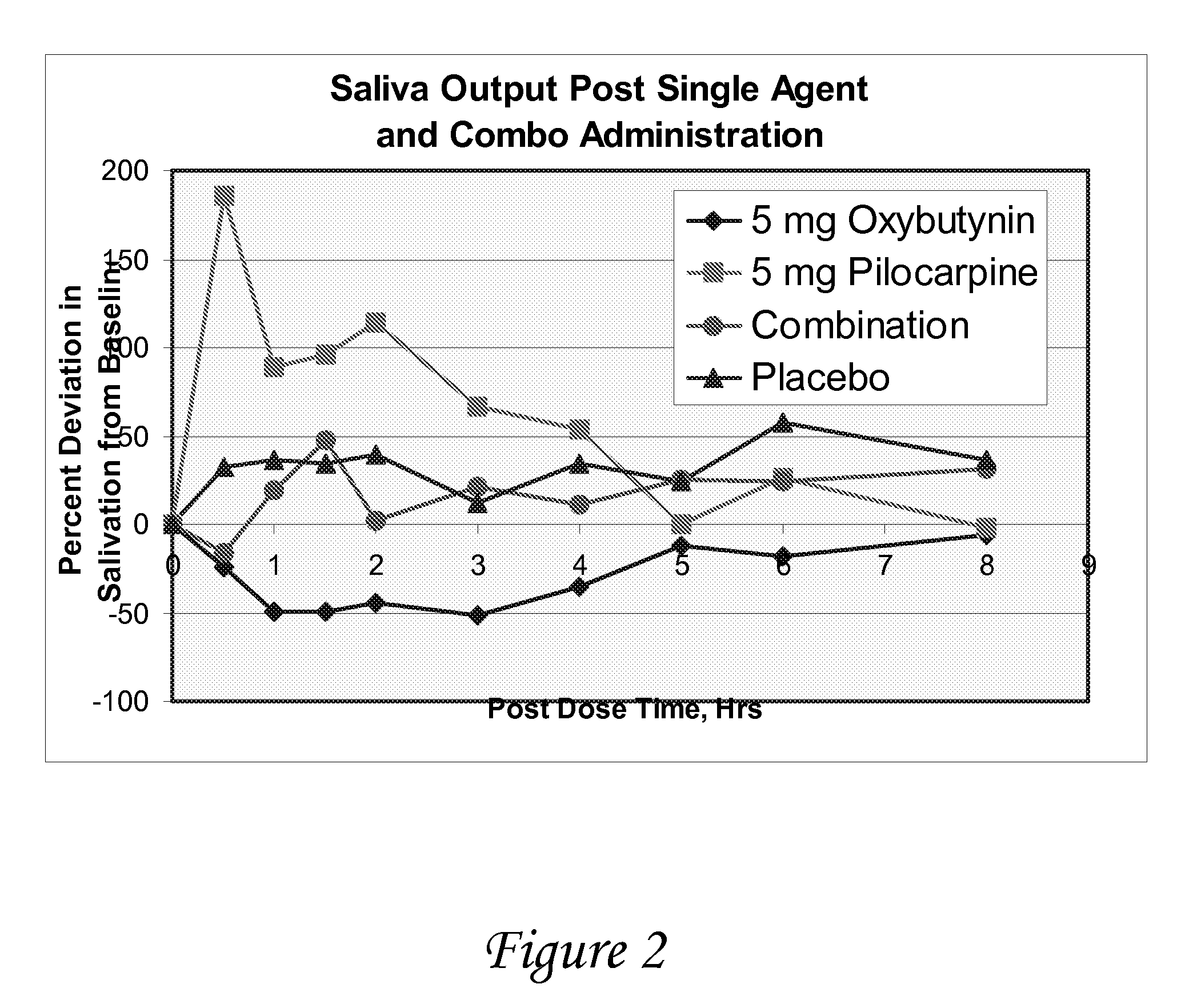
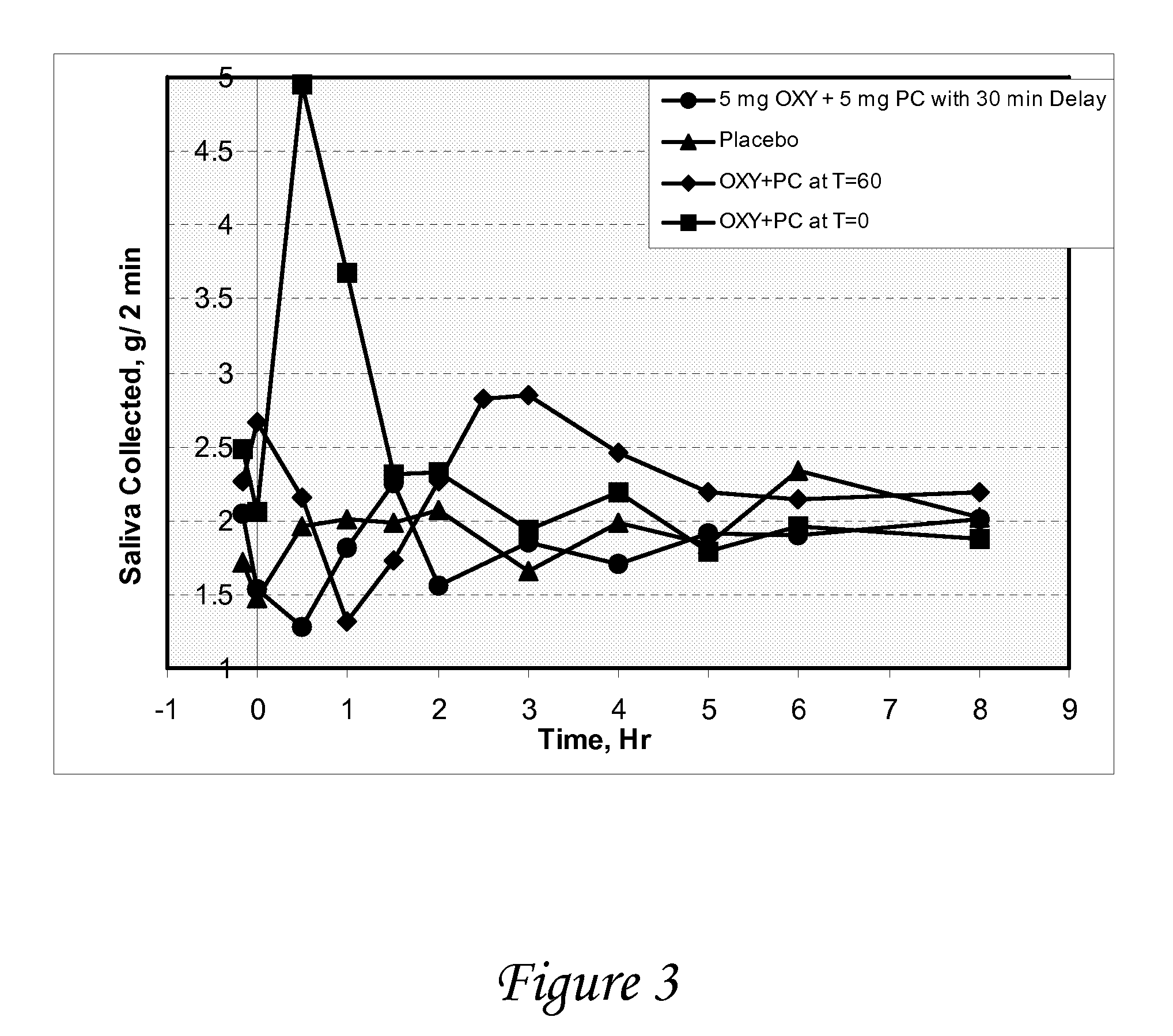
Disclosed herein are pharmaceutical compositions comprising various combinations of an antimuscarinic or an anticholinergic agent, a compound that causes stimulation of salivary glands, and a compound that relieves constipation. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
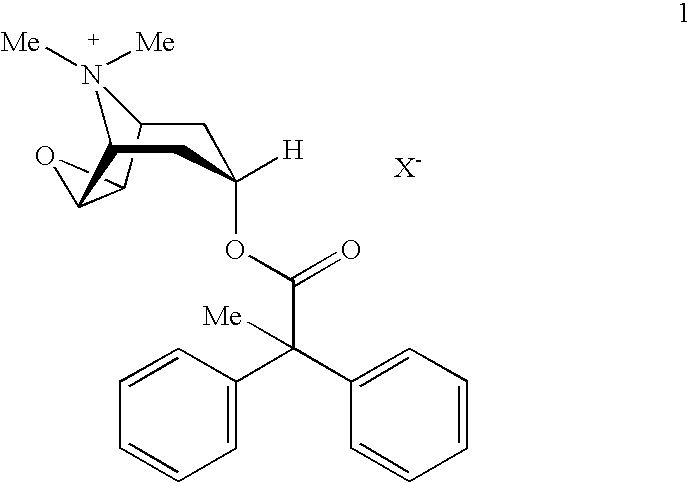
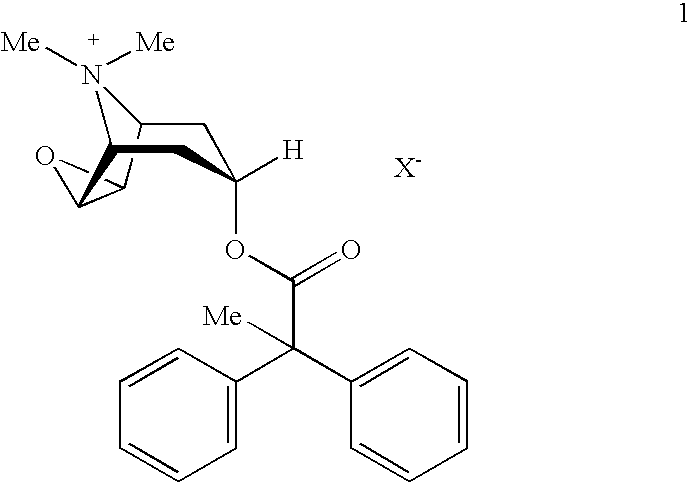
Anticholinergic powder formulations for inhalation
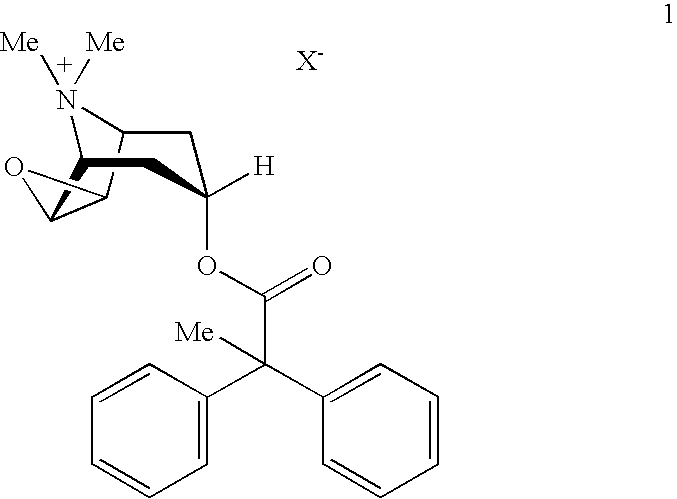
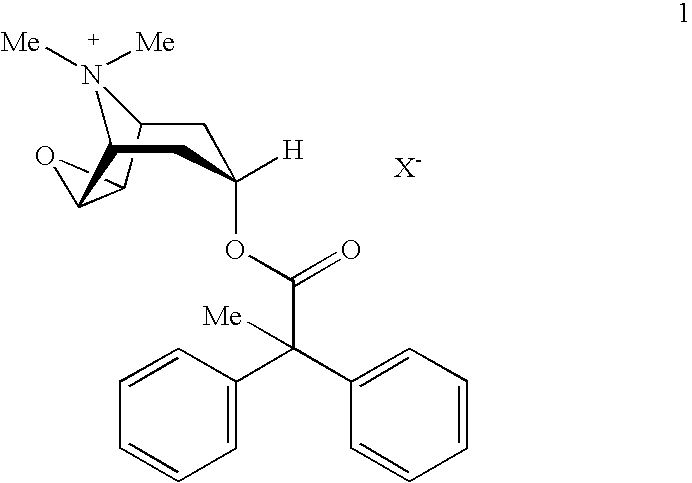
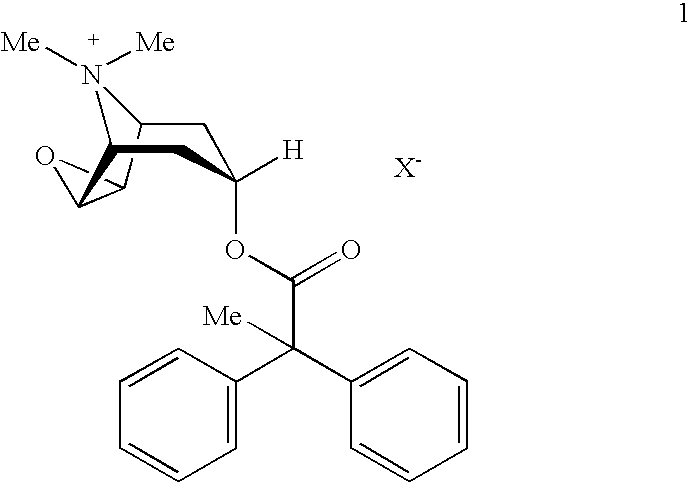
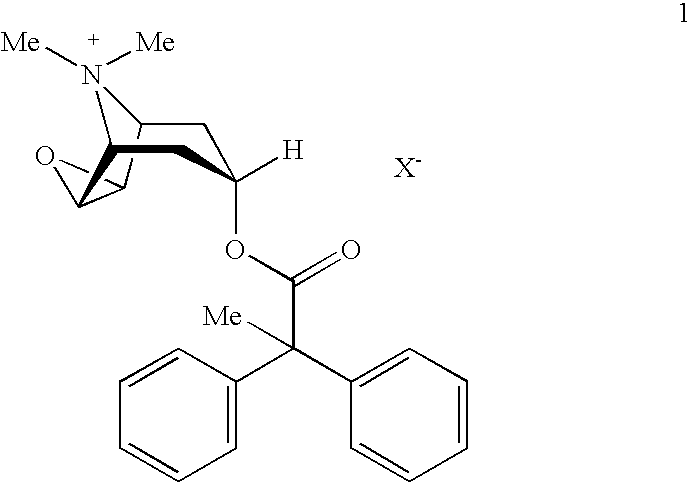
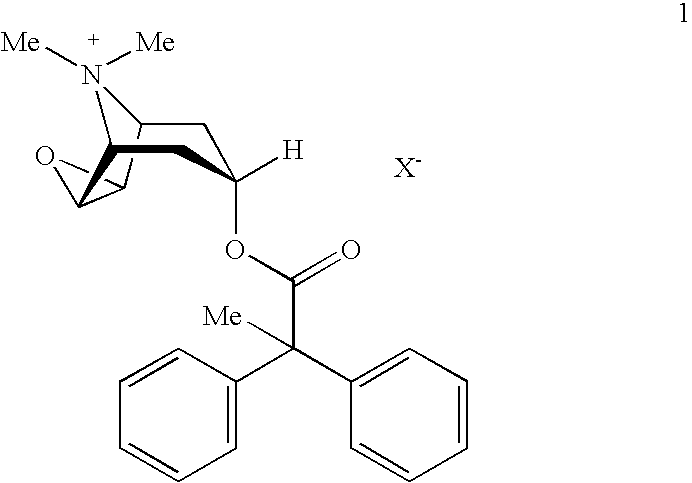
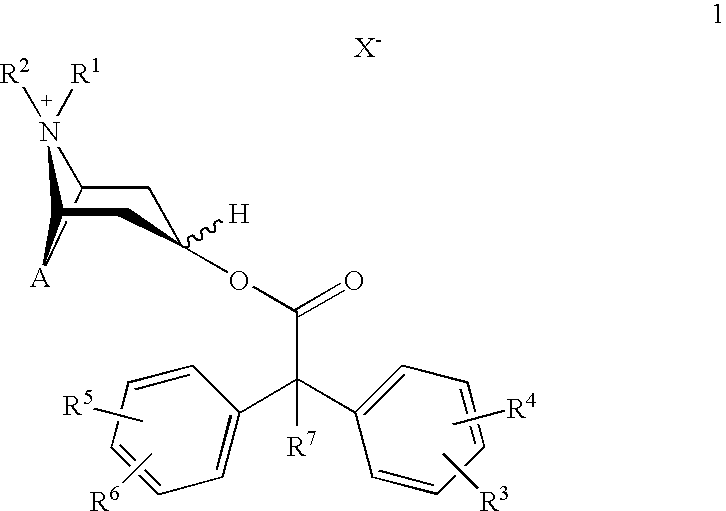
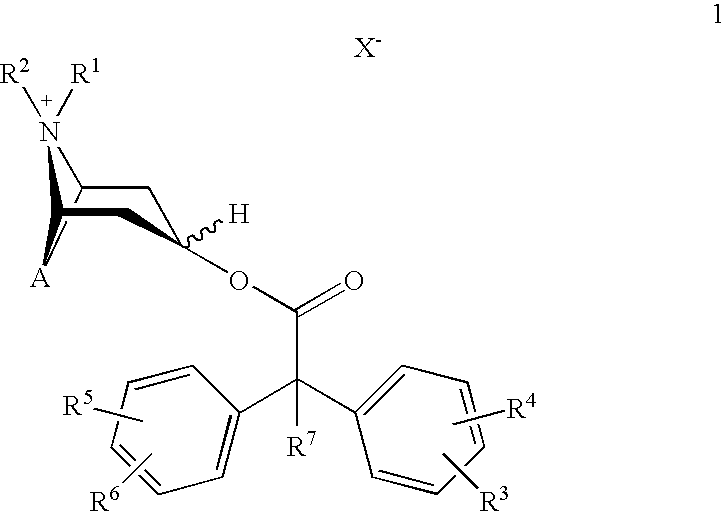
An inhalable powder comprising:(a) an active substance consisting essentially of a compound of formula 1 wherein X− is a pharmaceutically acceptable anion; and(b) a physiologically acceptable excipient having an average particle size of 10 μm to 50 μm,processes for preparing the inhalable powder, and methods of administration for the treatment of respiratory complaints, particularly for the treatment of chronic obstructive pulmonary disease (COPD) and asthma.
Owner:BOEHRINGER INGELHEIM INT GMBH
Compositions and methods for bowel care in individuals with chronic intestinal pseudo-obstruction
ActiveUS7635709B2Shorten the construction periodDifficult to administerBiocideAmine active ingredientsBowel careSide effect
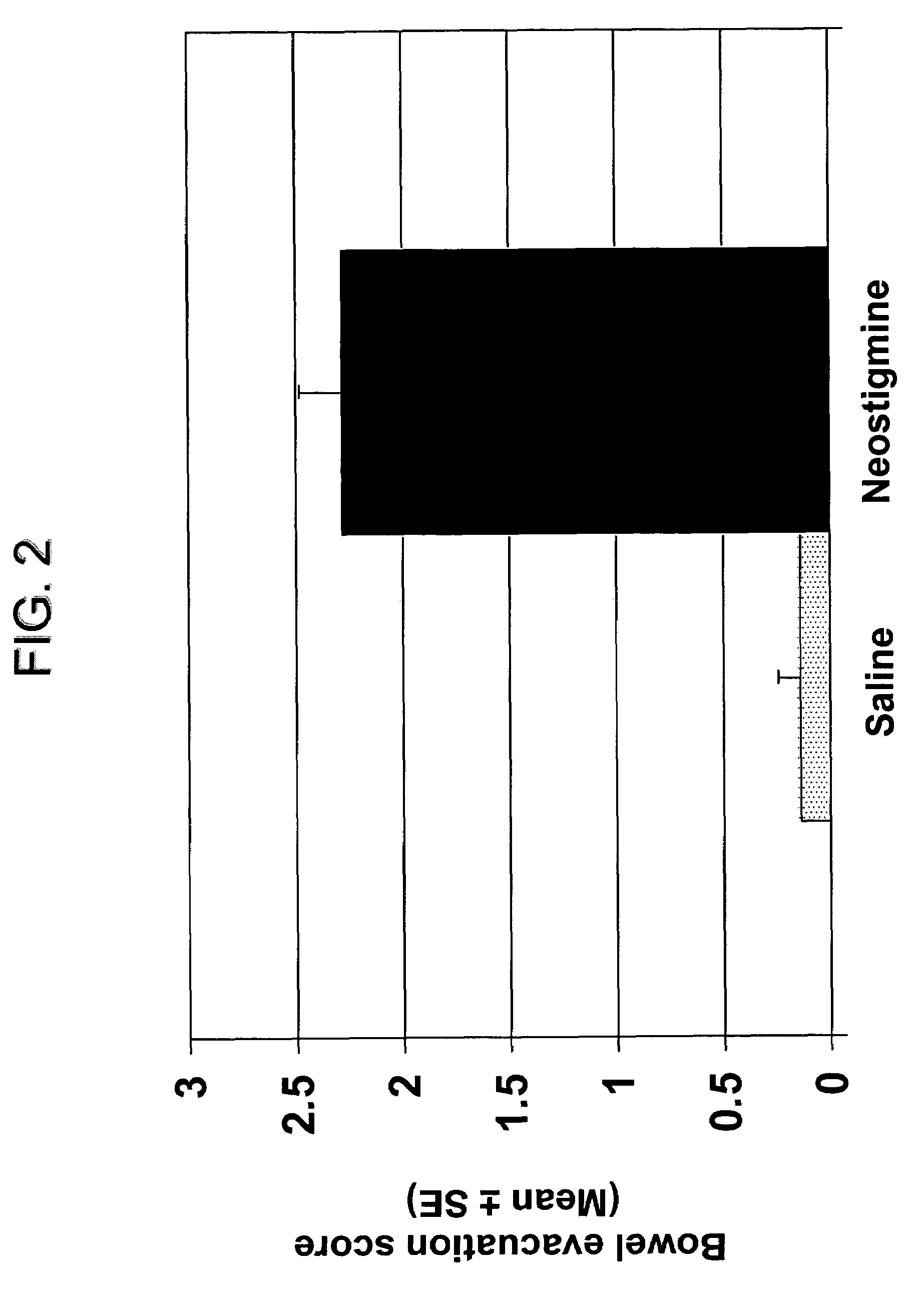
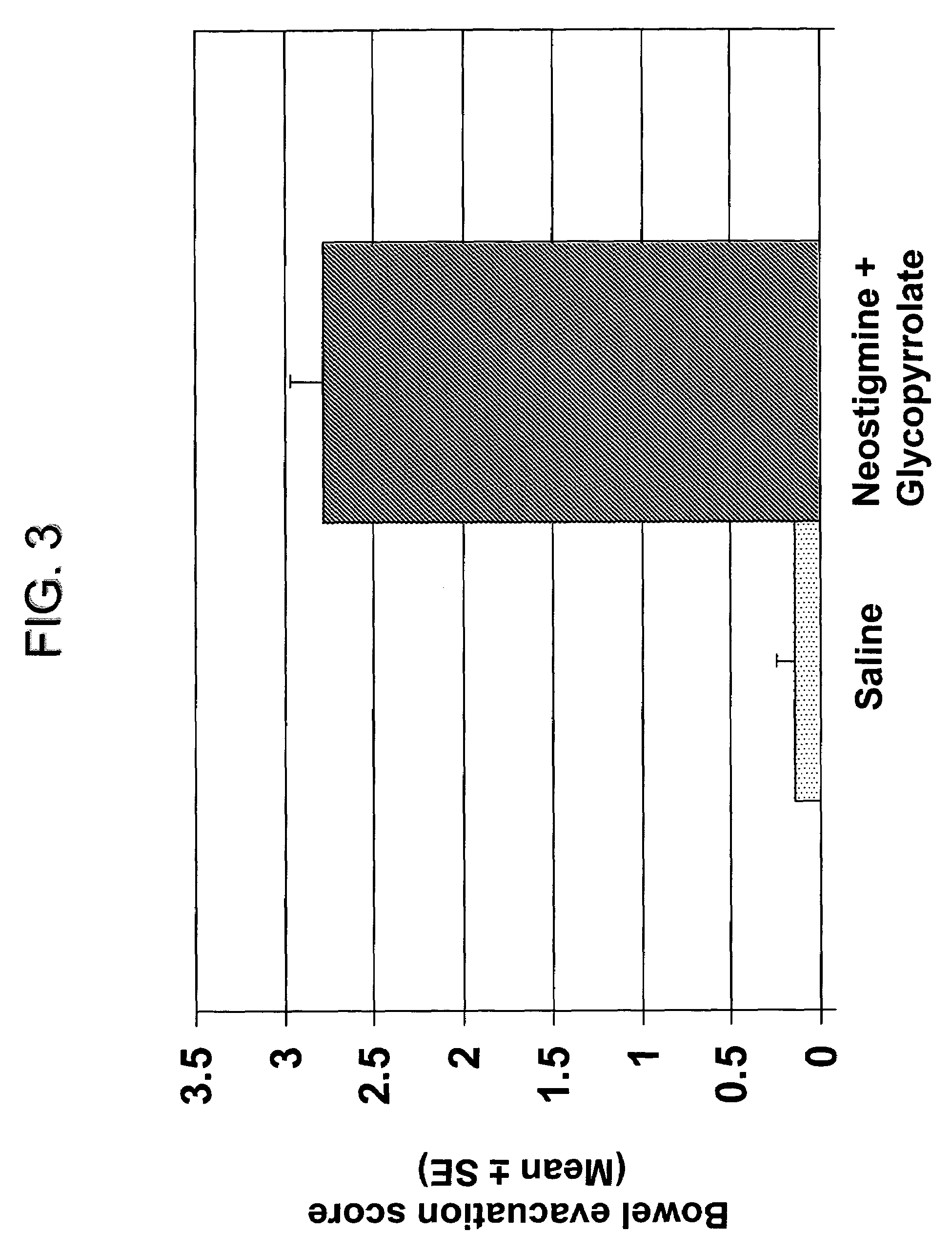
The present disclosure provides compositions and methods for on-going bowel care for persons with chronic intestinal pseudo-obstruction. The compositions and methods can be administered in a non-clinical setting. The compositions comprise acetylcholinesterase inhibitors for stimulating motility of the bowel in combination with anti-cholinergic agents to counteract the potentially dangerous cardiac side effects of the acetylcholinesterase inhibitor. In some examples, the acetylcholinesterase inhibitor, neostigmine, and the anti-cholinergic agent, glycopyrrolate, are combined in a pharmaceutical composition. Certain examples also provide the frequency and duration of administration of the disclosed drug combinations.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
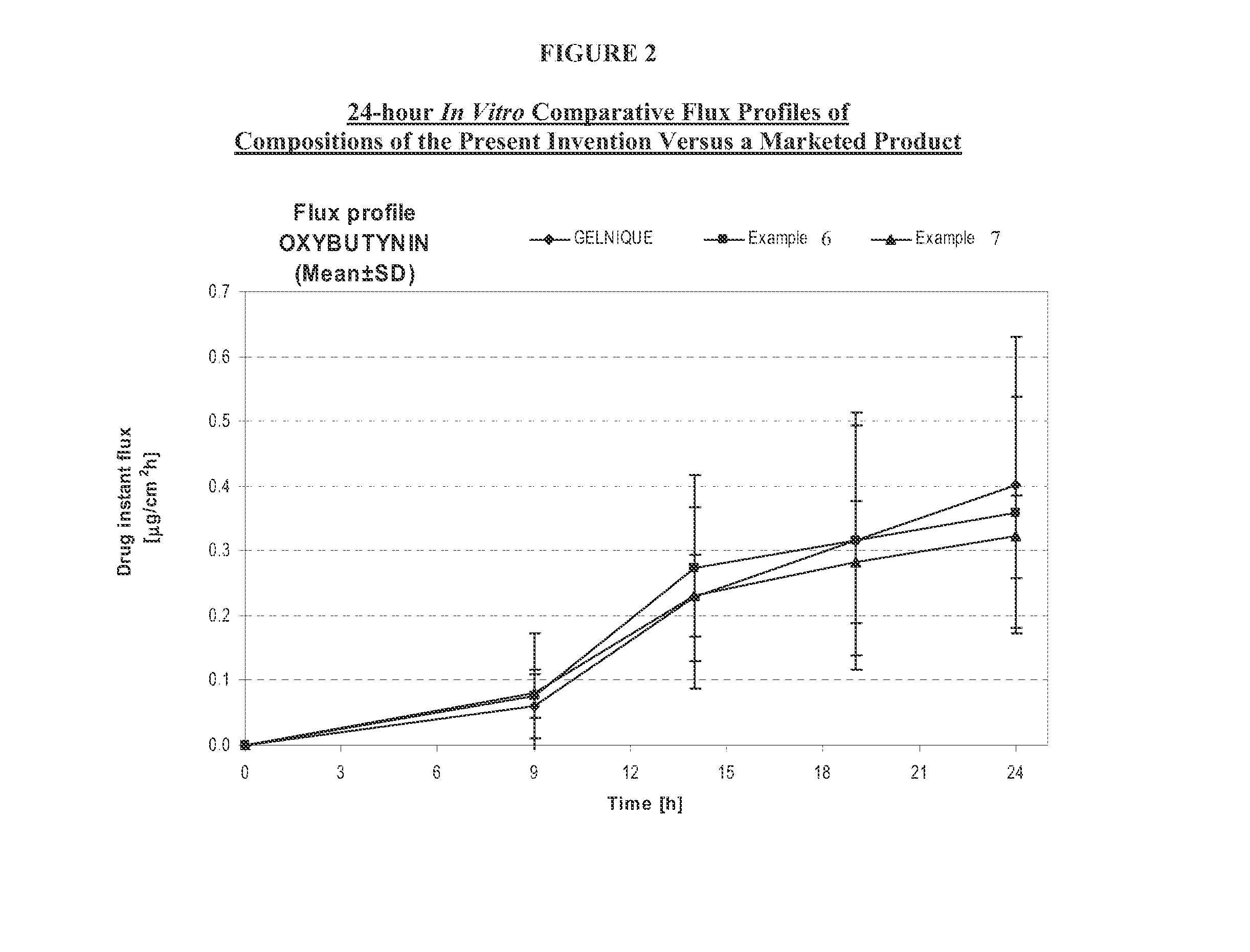
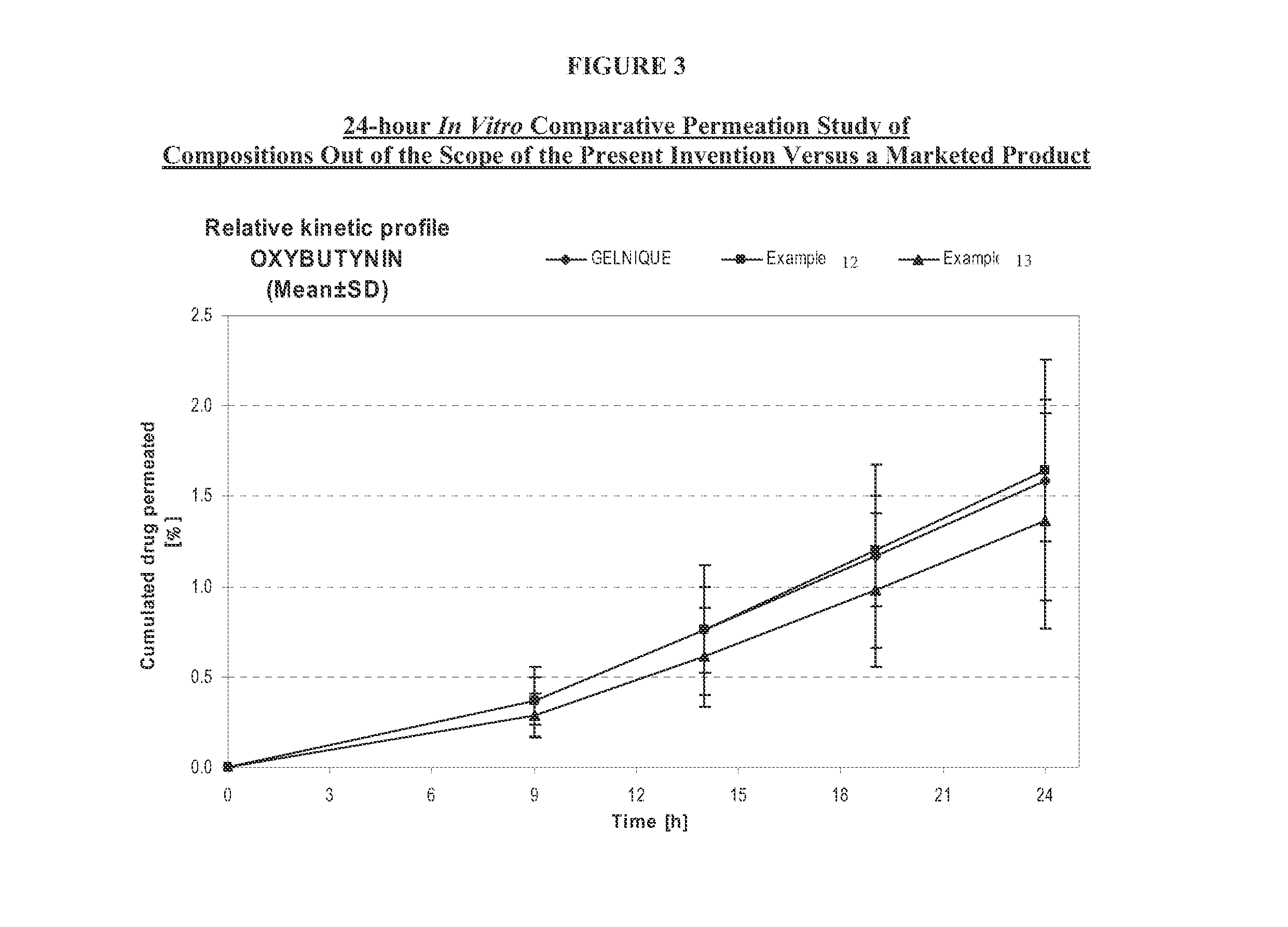
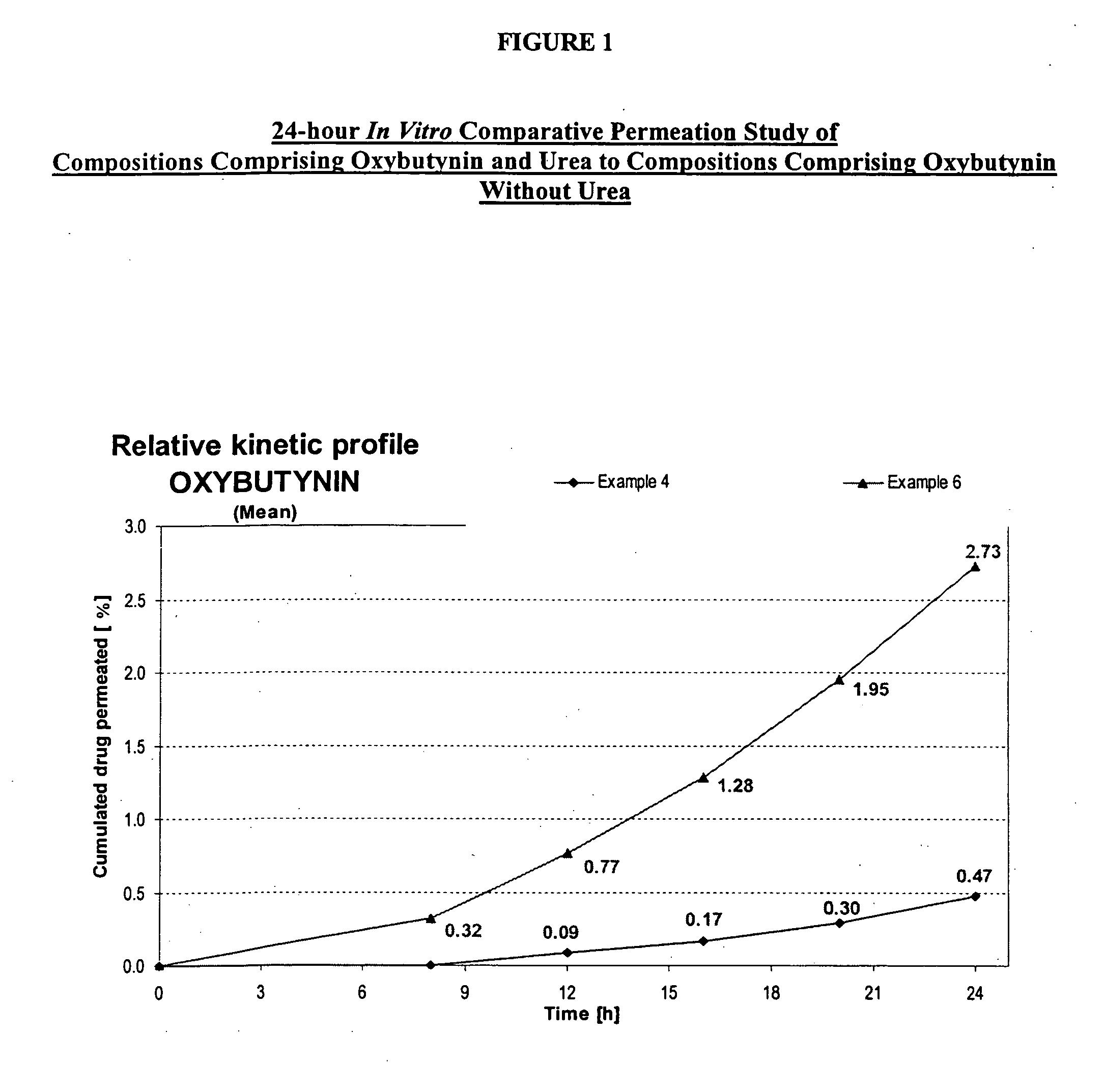
Transdermal compositions for Anti-cholinergic agents
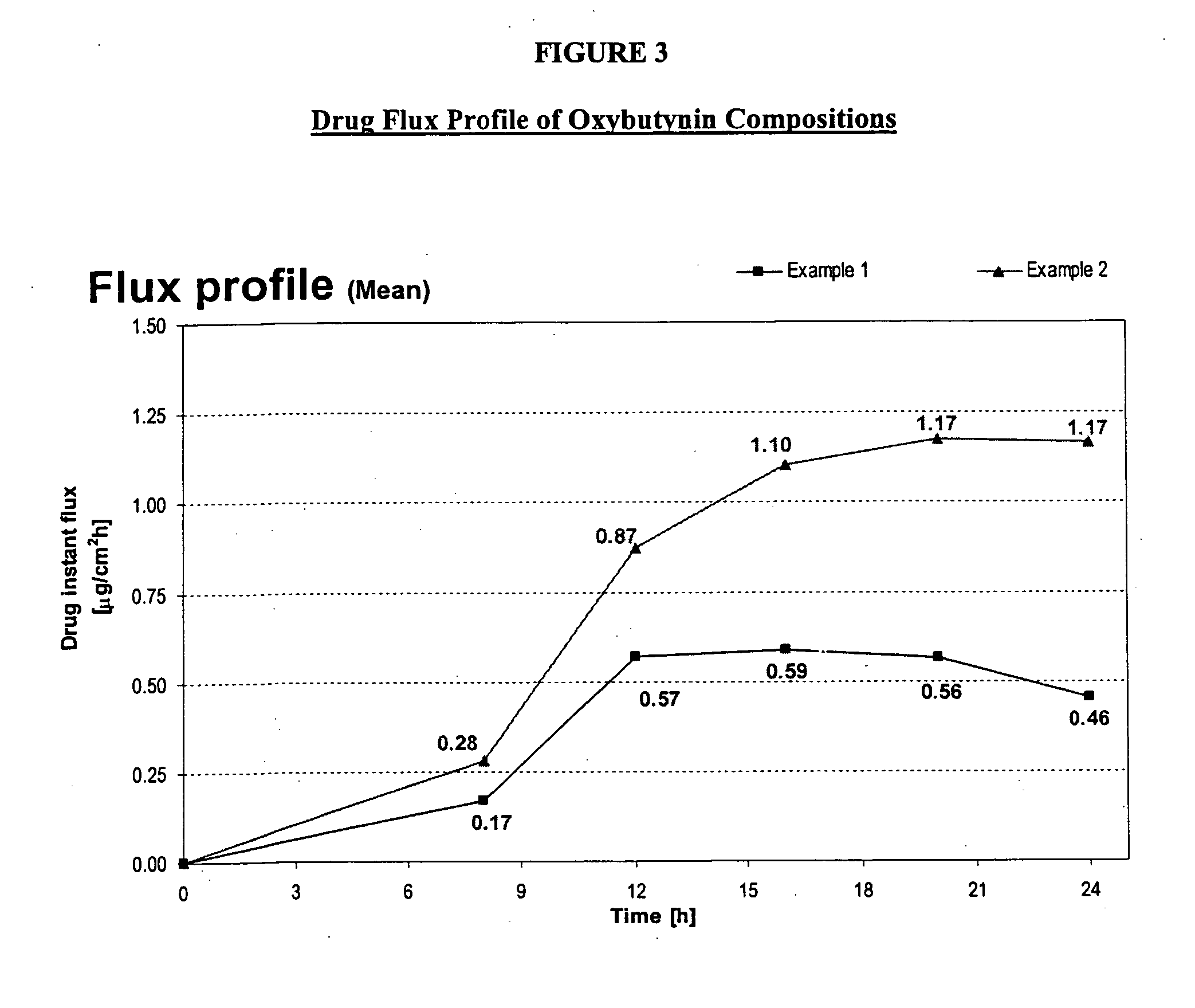
InactiveUS20140037713A1Reduce morbidityAvoids undesirable odor and irritation effectsCosmetic preparationsOrganic active ingredientsOxybutyninSide effect
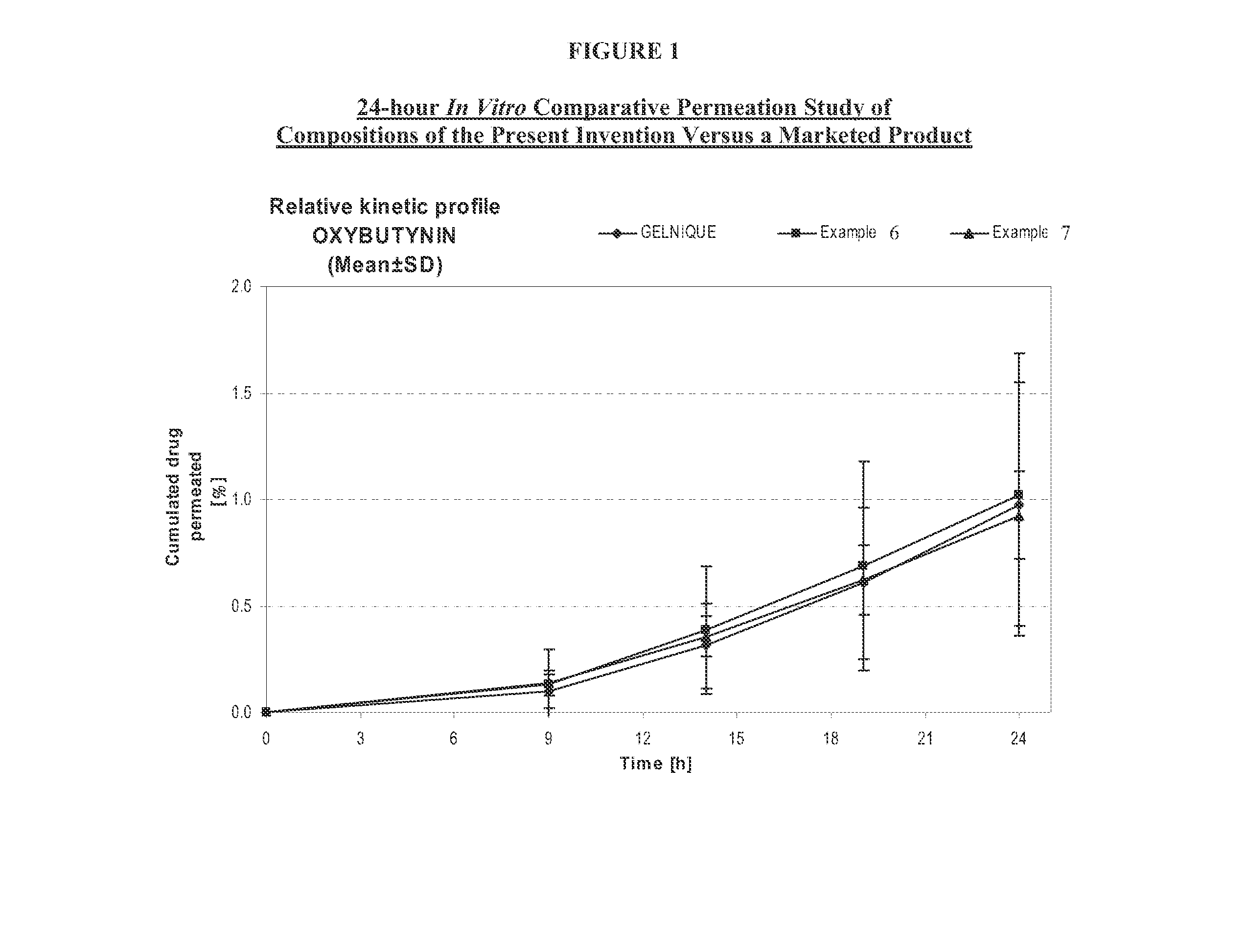
The present invention relates generally to compositions or formulations for transdermal or transmucosal administration of anti-cholinergic agents such as oxybutynin. The invention utilizes a novel delivery vehicle and is a substantially malodorous-free and irritation free transdermal formulation which is substantially live of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters. A method is disclosed for treating a subject for hyperhidrosis with these formulations while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anti-cholinergics.
Owner:ANTARES PHARMA IPL
HFC solution formulations containing an anticholinergic
An aerosol solution formulation comprising: (a) a salt of formula 1 wherein X− is an anion (b) an HFC propellant; (c) a cosolvent; and (d) an inorganic or an organic acid, wherein the concentration of the acid is in a range that corresponds with a pH range of 2.5 to 5.5 in aqueous solution.
Owner:BOEHRINGER INGELHEIM INT GMBH
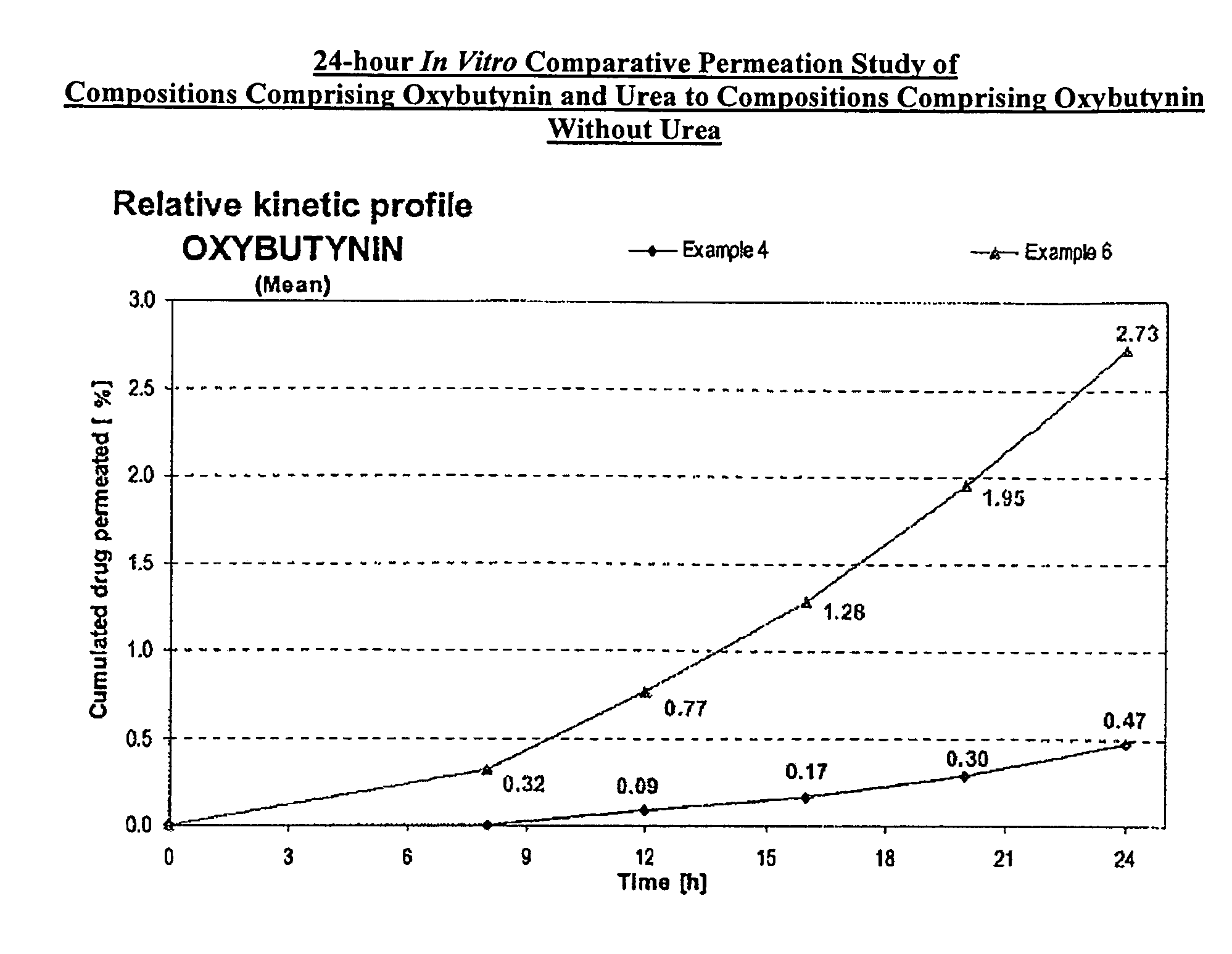
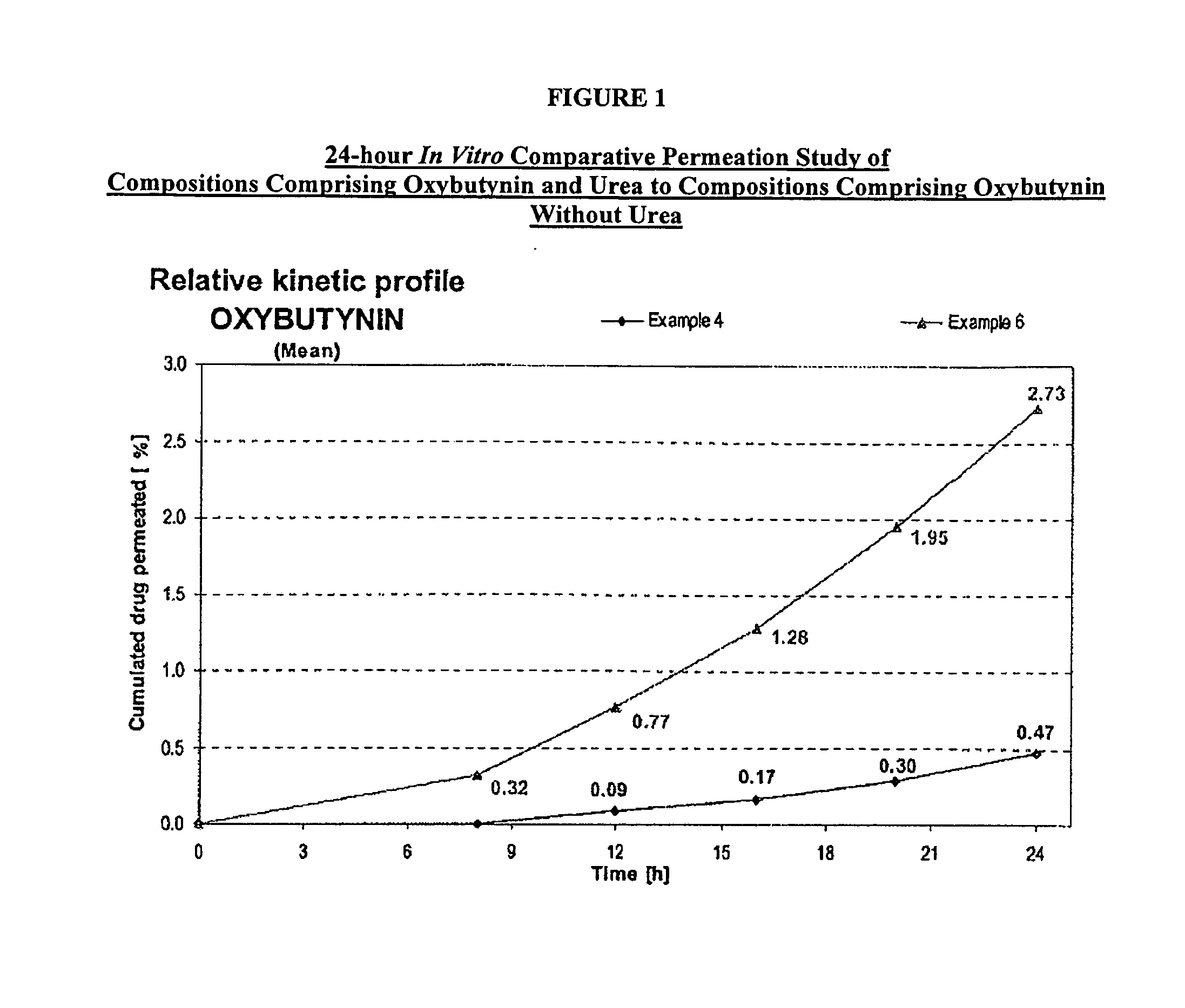
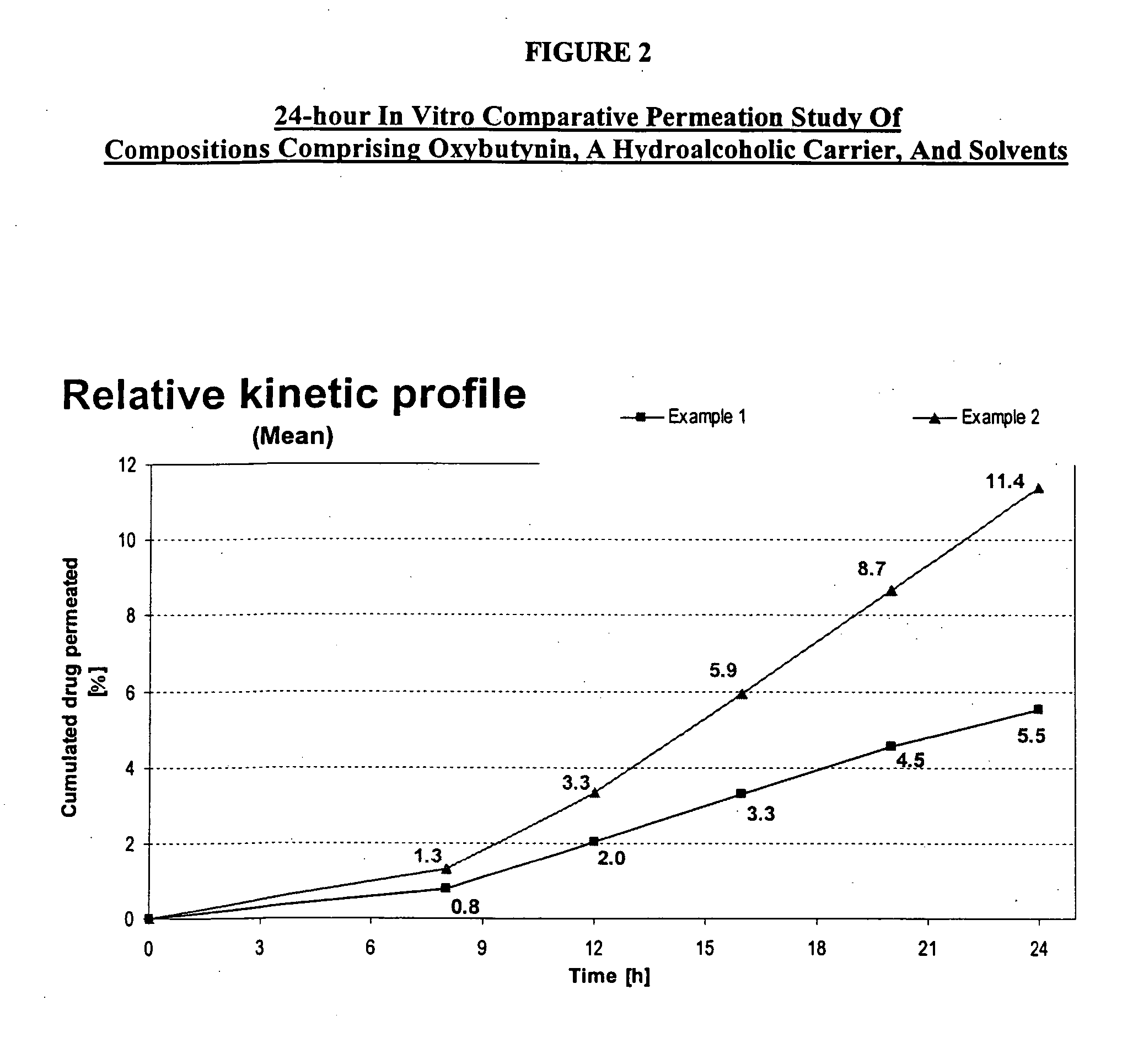
Permeation enhancing compositions for anticholinergic agents
InactiveUS7425340B2Improve permeabilityIncrease permeationBiocideNervous disorderOxybutyninSide effect
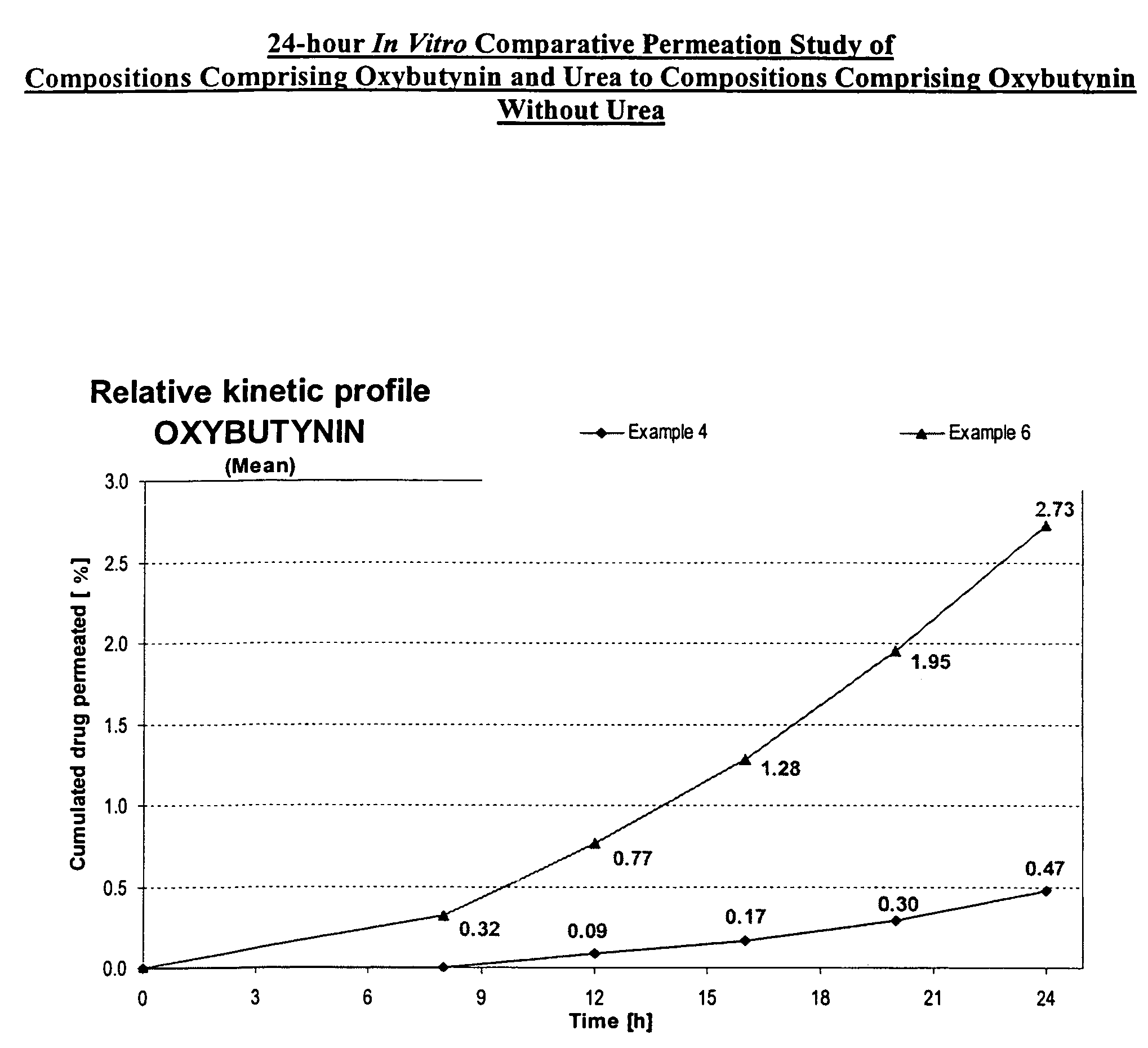
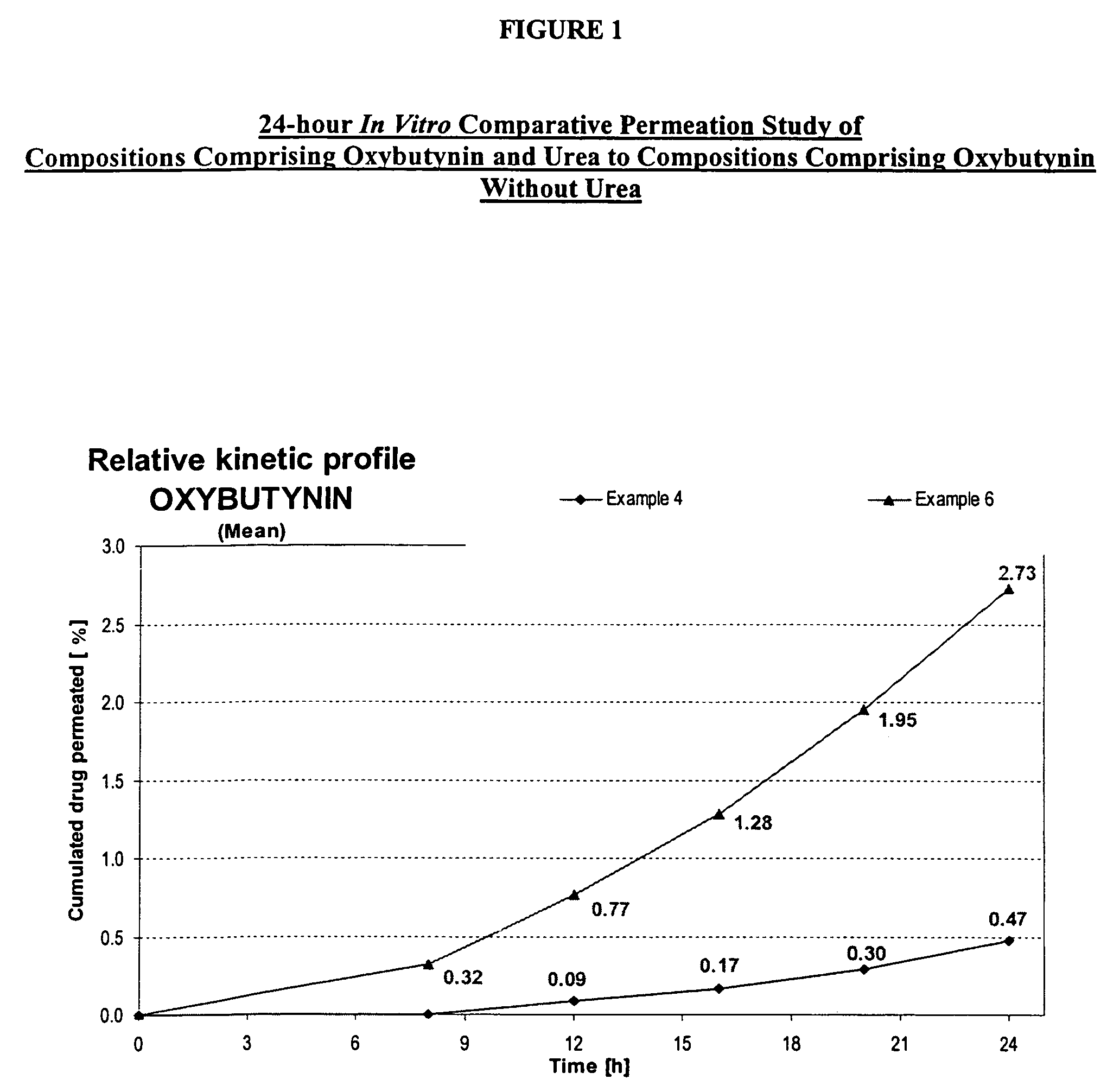
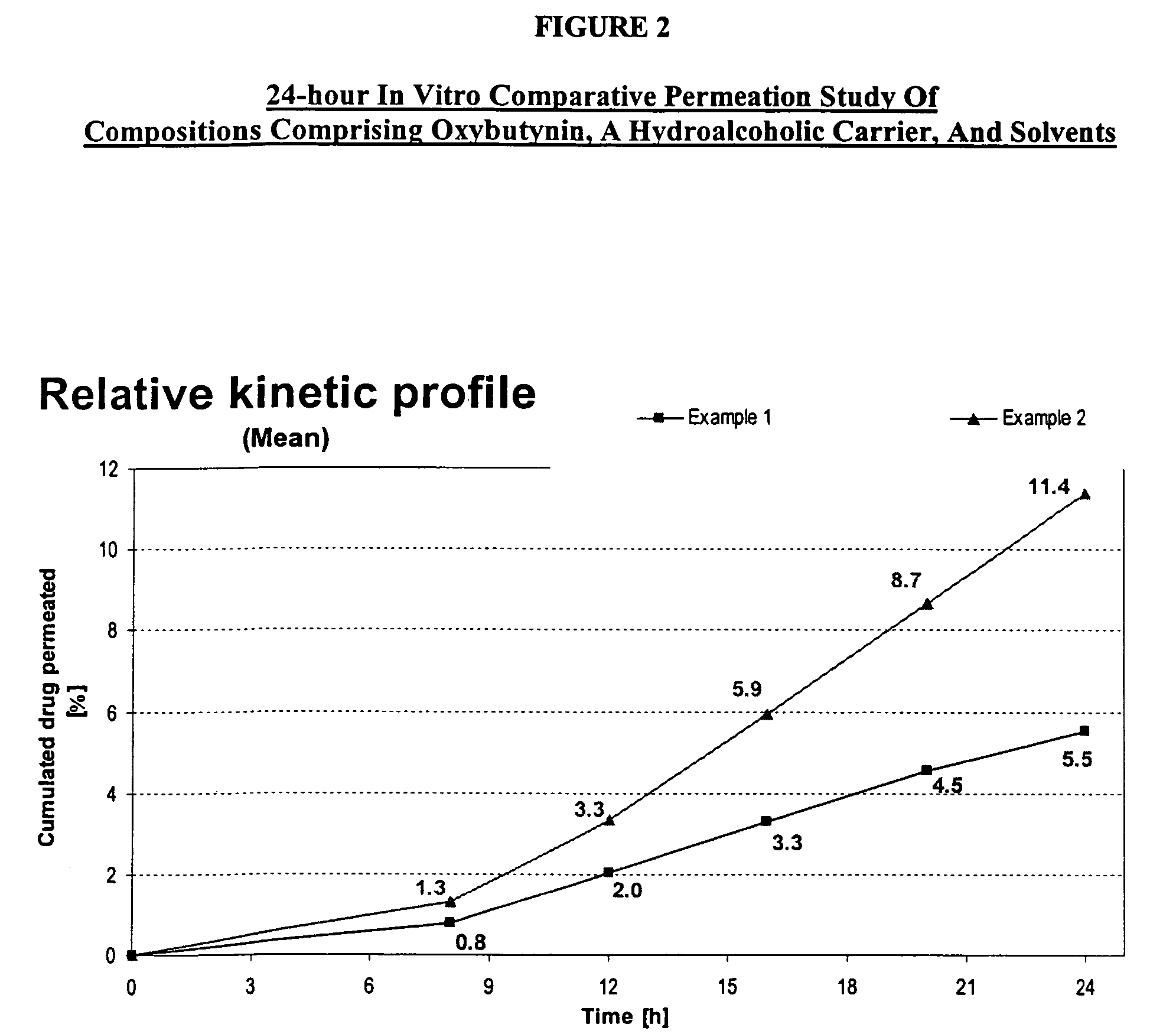
A transdermal or topical composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects.
Owner:ANTARES PHARMA IPL
Novel colored solutions of injectable drugs and their pharmaceutically acceptable salts
InactiveUS20090156562A1Reduce errorsAvoid confusionBiocideHydroxy compound active ingredientsDrugAnticholinergic agents
The invention is directed to pharmaceutical compositions comprising colored solutions, colored emulsions, or colored powders of injectable pharmaceuticals wherein said pharmaceuticals are selected from the group consisting of muscle relaxants, hypnotics, induction agents, and anticholinergics. The formulations of the present invention may all be colored using fluorescein. Different colors may be achieved by either varying the concentration of fluorescein, or by combining fluorescein with another dye. The invention is also directed to methods involving the use of said pharmaceutical compositions.
Owner:WINCH PETER D
Compositions and methods for tolerizing the immune system to allergens
InactiveUS20160263212A1Reduce development riskReduce riskHydroxy compound active ingredientsAllergen ingredientsIMMUNE SUPPRESSANTSBULK ACTIVE INGREDIENT
Compositions and methods can be used for tolerizing the immune system. The compositions can be physiologically acceptable and can include any of a wide variety of allergens that are designed to be administered in escalating doses to, for example, an infant. The compositions can include other active ingredients (e.g., one or more of a steroid, vitamin, mineral, vasodilator, hormone, decongestant, anticholinergic agent, leukotriene inhibitor, immunomodulator, mast cell stabilizer, expectorant, immune suppressant, anti-histamine, or anti-inflammatory agent) and / or a carrier.
Owner:STALLERGENES GREER PLC
Method and composition for treating alzheimer-type dementia
ActiveUS20110071135A1Extended durationFunction increaseBiocideNervous disorderTolerabilityH1 Receptor Antagonist
There is described a method for increasing the maximal tolerated close and thus the efficacy of an acetyl choline esterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetyl choline esterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetyl choline esterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholine esterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Therapy for the treatment of disease
Disclosed herein are pharmaceutical compositions comprising various combinations of an antimuscarinic or an anticholinergic agent, a compound that causes stimulation of salivary glands, and a compound that relieves constipation. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
Transdermal compositions for anticholinergic agents
InactiveUS20100216880A1Avoiding undesirable peak in drug concentrationReduce morbidityAntibacterial agentsBiocideOxybutyninLong chain fatty acid
The present invention relates generally to compositions or formulations for transdermal or transmucosal administration of anticholinergic agents such as oxybutynin. The invention utilizes a novel delivery vehicle and is a substantially malodorous-free and irritation free transdermal formulation which is substantially free of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters. A method is disclosed for treating a subject for urinary incontinence with these formulations while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anticholinergics.
Owner:ANTARES PHARMA IPL
Use of autonomic nervous system neurotransmitters inhibition and atrial parasympathetic fibers ablation for the treatment of atrial arrhythmias and to preserve drug effects
InactiveUSRE42961E1Increased occurrence of initiation of atrial flutterShorten the construction periodDiagnosticsHeart defibrillatorsNervous systemRight atrium
Atrial arrhythmias, a major contributor to cardiovascular morbidity, are believed to be influenced by autonomic nervous system tone. The main purpose of this invention was to highlight new findings that have emerged in the study of effects of autonomic nervous system tone on atrial arrhythmias, and its interaction with class III antiarrhythmic drug effects. This invention evaluates the significance of sympathetic and parasympathetic activation by determining the effects of autonomic nervous system using a vagal and stellar ganglions stimulation, and by using autonomic nervous system neurotransmitters infusion (norepinephrine, acetylcholine). This invention evaluates the autonomic nervous system effects on the atrial effective refractory period duration and dispersion, atrial conduction velocity, atrial wavelength duration, excitable gap duration during a stable circuit (such atrial flutter circuit around an anatomical obstacle), and on the susceptibility of occurrence (initiation, maintenance and termination) of atrial re-entrant arrhythmias in canine. This invention also evaluates whether autonomic nervous system activation effects via a local neurotransimitters infusion into the right atria can alter those of class III antiarrhythmic drug, sotalol, during a sustained right atrial flutter. This invention represents an emergent need to set-up and develop a new class of anti-cholinergic drug therapy for the treatment of atrial arrhythmias and to combine this new anti-cholinergic class to antiarrhythmic drugs. Furthermore, this invention also highlights the importance of a local application of parasympathetic neurotransmitters / blockers and a catheter ablation of the area of right atrium with the highest density of parasympathetic fibers innervation. This may significantly reduce the occurrence of atrial arrhythmias and may preserve the antiarrhythmic effects of any drugs used for the treatment of atrial re-entrant arrhythmias.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Solid pharmaceutical dosage unit for alleviating symptoms of rhinorrhea
InactiveUS20080292699A1Reducing and eliminating over-dryingBiocidePill deliveryAnticholinergic agentsDecongestant
A solid pharmaceutical dosage unit for alleviating the symptoms of rhinorrhea. The dosage unit comprises an anticholinergic agent and an antihistamine and, optionally, a decongestant and, when placed in a basket in 500 ml of 0.01 N HCl of 37° C. which is stirred at 100 rpm, releases at least about 75% of the at least one anticholinergic agent within 45 minutes and releases the at least one antihistamine at a rate of from about 20% to about 60% after 2 hours, from about 45% to about 80% after 4 hours and at least about 75% after 8 hours. This Abstract is not intended to define the invention disclosed in the specification, nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Compositions for treatment of common cold
InactiveUS7652030B2Efficient dosingReducing the runny noseBiocideAerosol deliveryCommon coldAnticholinergic Drugs
New stable compositions comprising the combination of a topically active vasoconstrictor and a topically active anticholinergic drug are disclosed. Preferably, the composition comprises ipratropium or a salt thereof in combination with xylometazoline hydrochloride and a salt thereof. Upon topically administering such compositions to a nasal mucosa in individuals suffering from the common cold the symptoms of rhinorrhea are significantly reduced.
Owner:TAKEDA PHARMA AS
Use and composition for treating dementia
ActiveUS8404701B2Maximize the effectSymptoms improvedBiocideNervous disorderMaximum tolerated doseAnti cholinergic
Owner:CHASE PHARMA CORP
Treatment of chronic obstructive pulmonary disease with nebulized beta 2-agonist or combined nebulized beta 2-agonist and anticholinergic administration
InactiveUS20110132355A1Good curative effectExtended durationDispersion deliverySolution deliveryMuscarinic antagonistAnticholinergic agents
Inhalation solutions for administration of beta 2-agonists or combinations of muscarinic antagonists and beta 2-agonists for the treatment of breathing disorders, such as COPD, are provided. The inhalation solutions are administered by nebulization, particularly with a high efficiency nebulizer.
Owner:SUNOVION RESPIRATORY DEV
Crystalline anti-cholinergic tiotropium crystal
ActiveCN1634921ANo significant difference in formulation qualityOrganic active ingredientsOrganic chemistryAnticholinergic agentsTiotropium bromide
The invention relates to crystal unhydrous (1R,2R,4S,5S,7S)-7-[2-hydroxy-2,2-bis(2-thienyl) acetoxy]-9,9-dimethyl-3-oxa-9-azo cation tricyclo octane [3.3.1.02.4] nonane bromide, clinic use of tiotropium bromide anhydrous crystal as anti-cholinergic medicines, and preparation process of tiotropium bromide anhydrous crystal.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Nitrosated and nitrosylated compounds and compositions and their use for treating respiratory disorders
Disclosed are (i) compounds of a steroid, a β-agonist, an anticholinergic, a mast cell stabilizer and a phosphodiesterase (PDE) inhibitor directly or indirectly linked to a NO or NO2 group or a group which stimulates endogenous production of NO or EDRF in vivo; (ii) compositions of steroids, β-agonists, anticholinergics, mast cell stabilizers and PDE inhibitors, which can optionally be substituted with at least one NO or NO2 moiety or a group which stimulates endogenous production of NO or EDRF in vivo, and a compound that donates, transfers or releases nitric oxide as a charged species, i.e., nitrosonium (NO+) or nitroxyl (NO−), or as the neutral species, nitric oxide (NO.) or that stimulates endogenous production of NO or EDRF in vivo; and (iii) uses for them in preventing and / or treating respiratory disorders.
Owner:ARBOR PHARMA LLC
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
InactiveUS20070293460A1Adequate levelShortness of breathRespiratorsBiocideObstructive Pulmonary DiseasesCombination therapy
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and an aqueous solution comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium. A pharmaceutical composition is also described for the treatment of respiratory conditions and diseases comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer.
Owner:CMPD LICENSING
Permeation enhancing compositions for anticholinergic agents
InactiveUS20050287194A1Improve permeabilityIncrease permeationBiocideNervous disorderOxybutyninSide effect
A transdermal or topical composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects.
Owner:ANTARES PHARMA IPL
Methods of improving quality of sleep
InactiveUS20110245294A1Improve sleep qualityBiocideNervous disorderAnticholinergic agentsOveractive bladder
Disclosed herein are methods of treating a patient suffering from overactive bladder (OAB) comprising administering to the patient a combination of antimuscarinic or anticholinergic agent and muscarinic agonist for the treatment of poor quality of sleep in the OAB patient.
Owner:THERAVIDA INC
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and a fluid comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic in a pharmaceutically acceptable vehicle, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium in a an aqueous solution, suspension or emulsion suitable for administration with the nebulizer.
Owner:RICHIES PHARMACY & MEDICAL SUPPLY
Method and composition for treating alzheimer-type dementia
ActiveUS8877768B2Maximize the effectSymptoms improvedBiocideNervous disorderTolerabilityH1 Receptor Antagonist
There is described a method for increasing the maximal tolerated close and thus the efficacy of an acetyl choline esterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetyl choline esterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetyl choline esterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholine esterase inhibitor are also described.
Owner:CHASE PHARMA CORP
HFC solution formulations containing an anticholinergic
An aerosol solution formulation comprising:(a) a salt of formula 1wherein X− is an anion(b) an HFC propellant;(c) a cosolvent; and(d) an inorganic or an organic acid, wherein the concentration of the acid is in a range that corresponds with a pH range of 2.5 to 5.5 in aqueous solution.
Owner:BOEHRINGER INGELHEIM INT GMBH
Anticholinergics which may be used as medicaments as well as processes for preparing them
InactiveUS7569581B2High activityEasy to controlBiocideOrganic chemistryAnticholinergic agentsMedicinal chemistry
Owner:BOEHRINGER INGELHEIM PHARMA KG
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
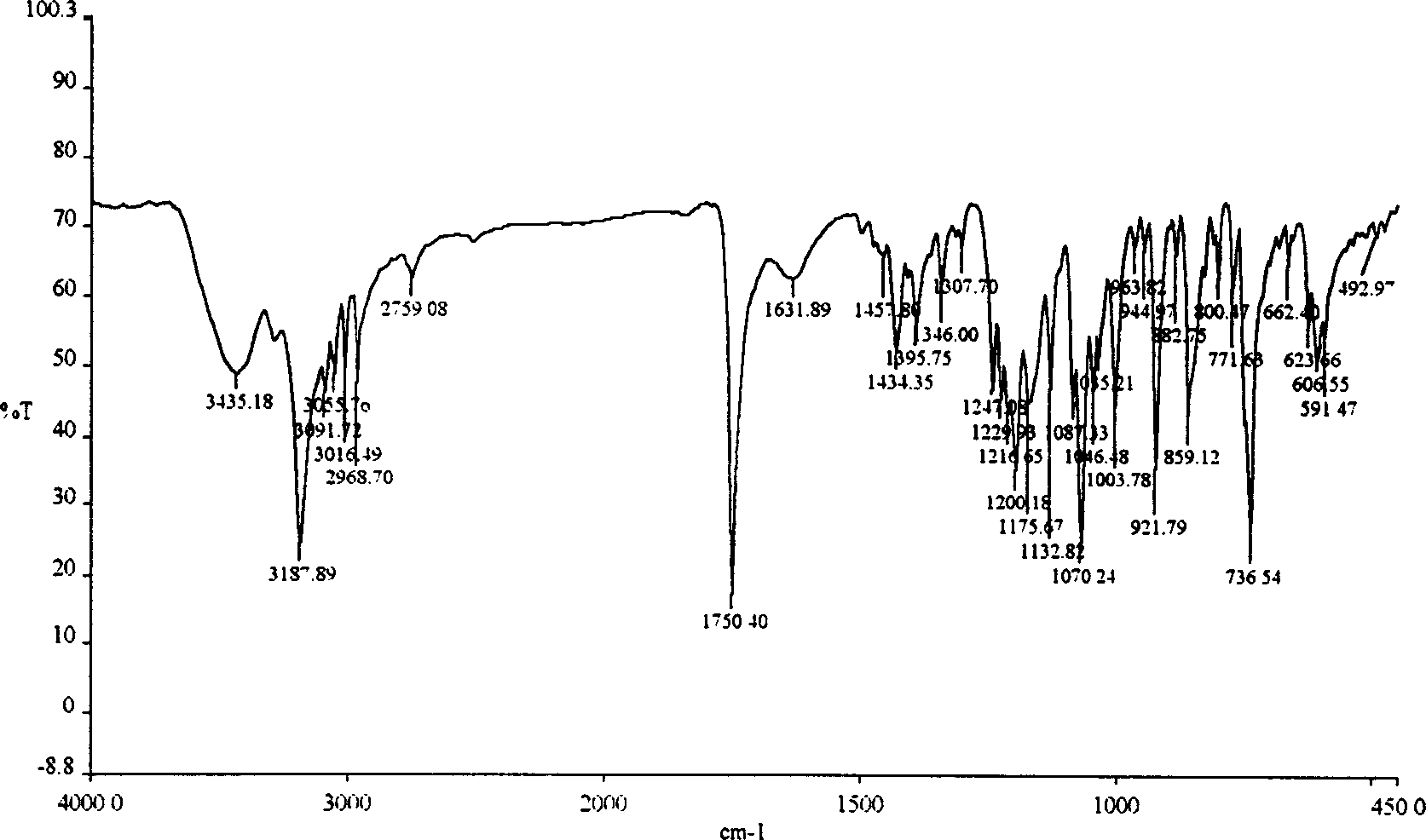
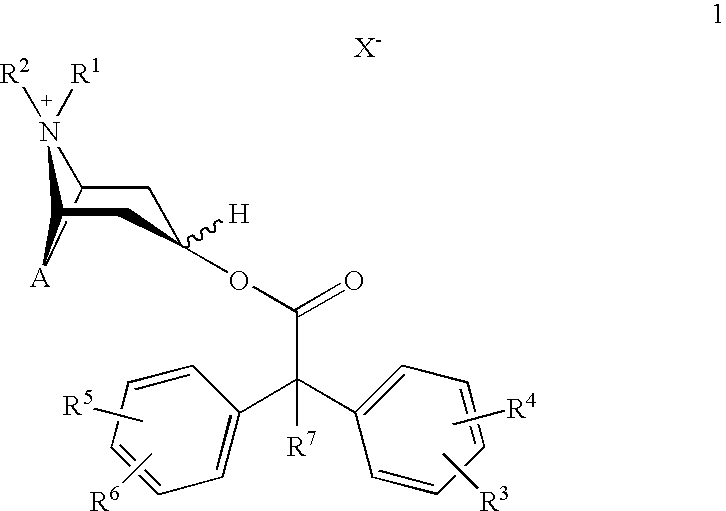
Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester
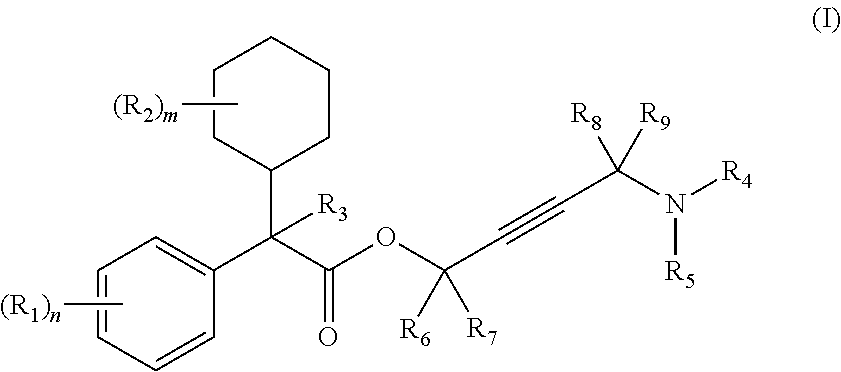
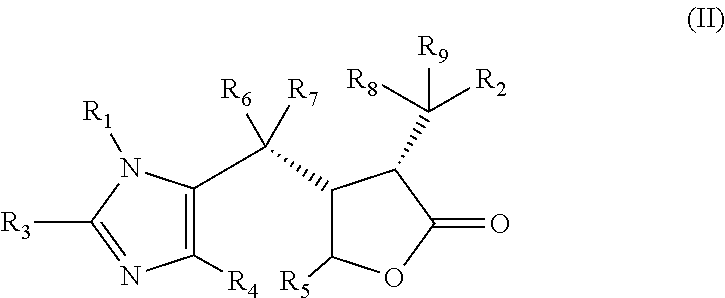
The invention discloses a method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester which is a key intermediate of trospium chloride of an anticholinergic agent. The method comprises the following steps that: firstly, 2-hydroxyl-2,2-diphenyl acetic acid serving as a raw material is mixed with carbonyl imidazole for activation of acyl radicals; secondly, theresulting product reacts with tropine alcohol to form 2-hydroxyl-2,2-diphenyl acetic acid tropine ester; and finally, the resulting product of the previous step undergoes N formylation and acidulation reaction to obtain the 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester. The method has the advantages of mild reaction conditions, little pollution and easy realizationof industrialized production.
Owner:北京迈劲医药科技有限公司
Permeation enhancing compositions for anticholinergic agents
InactiveUS20080260842A1Avoiding undesirable peak in drug concentrationReduce morbidityBiocideAerosol deliveryOxybutyninAnticholinergic agents
A transdermal or topical skin-friendly composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anticholinergics.
Owner:ANTARES PHARMA IPL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com








![Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/294f2403-8724-4bb6-a08b-c9b9c3645f57/A20081014732600031.PNG)
![Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester Method for preparing 2-hydroxyl-2,2-diphenyl acetic acid-3alpha-(8-azabicyclo[3,2,1]-3-octyl ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/294f2403-8724-4bb6-a08b-c9b9c3645f57/A20081014732600051.PNG)