Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
198 results about "Esterase inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Substance or agent which suppresses, prevents or opposes the action of esterase.
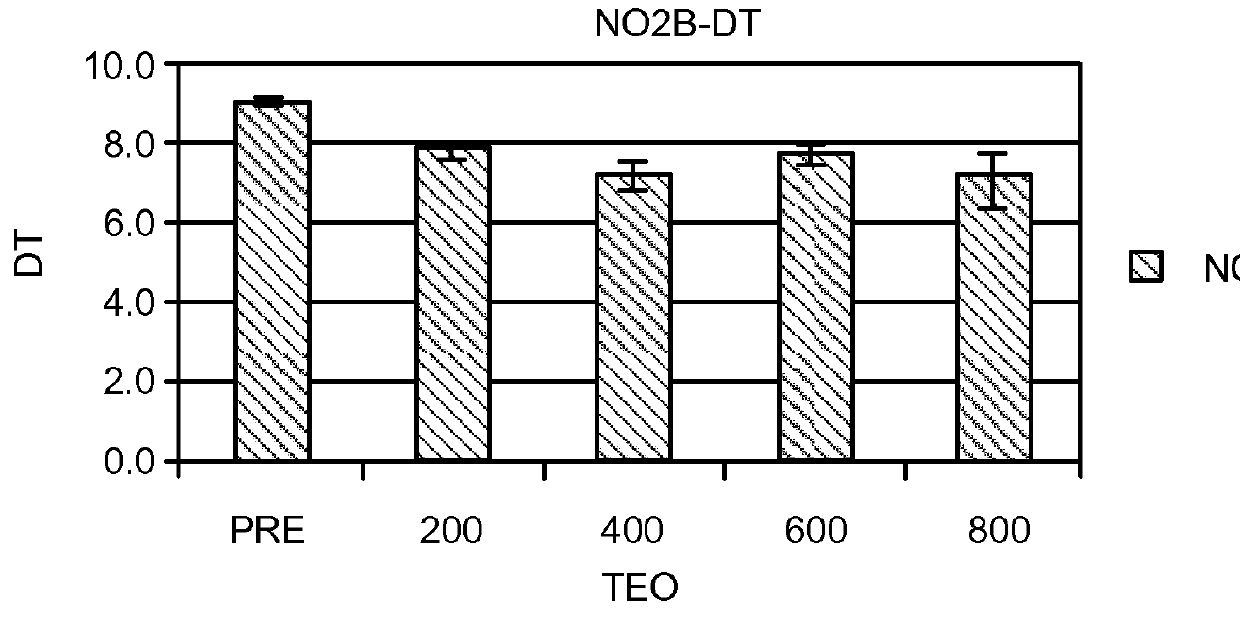
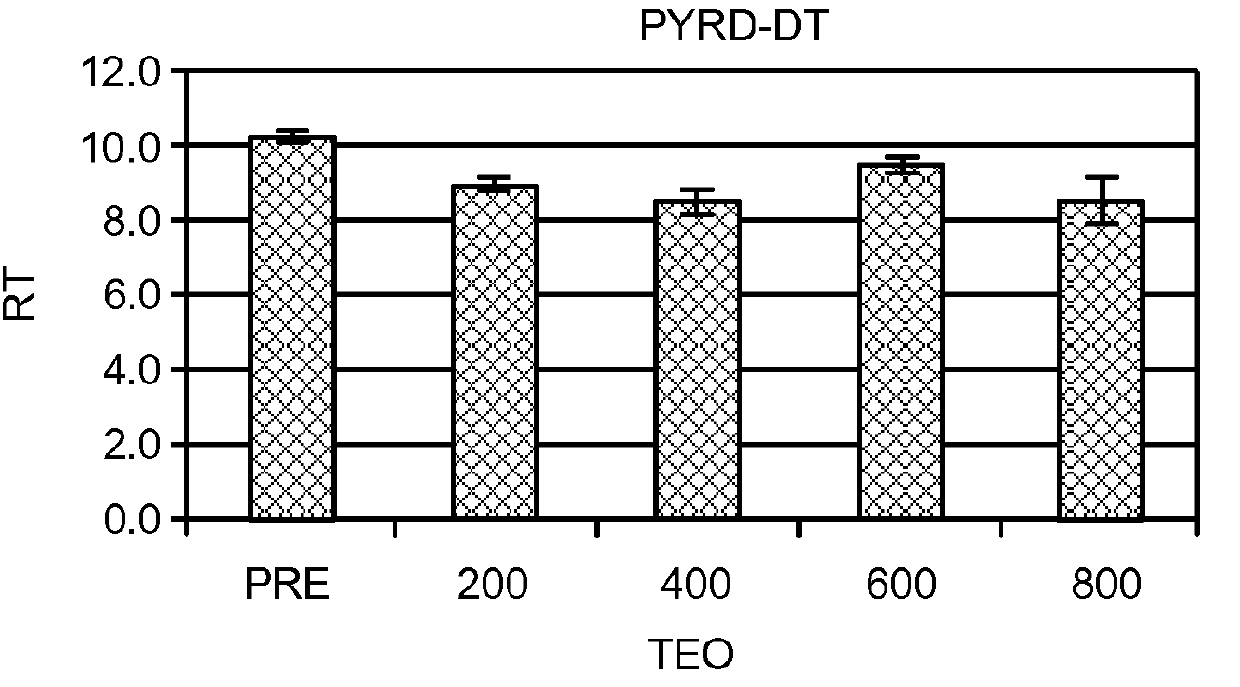
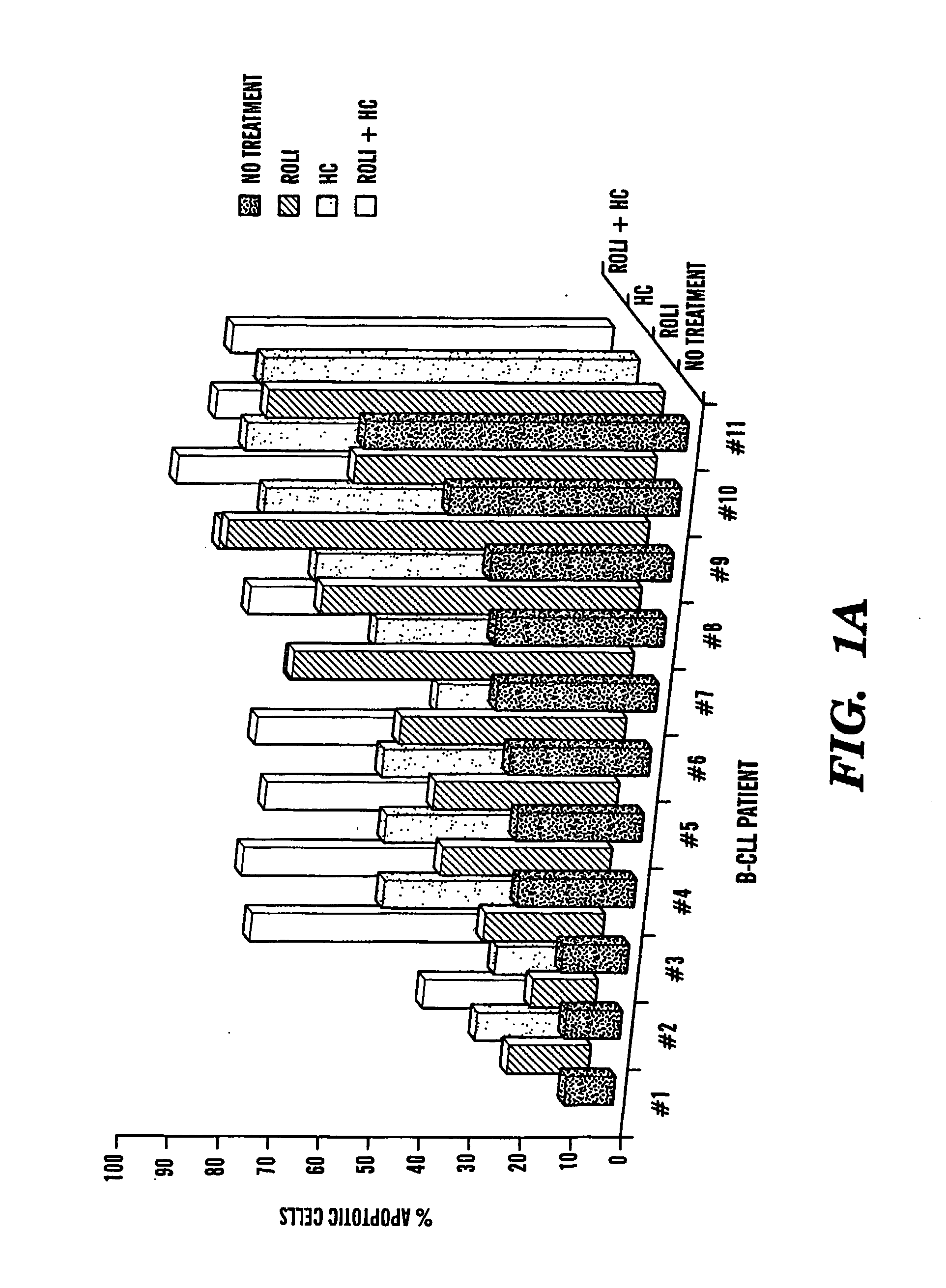
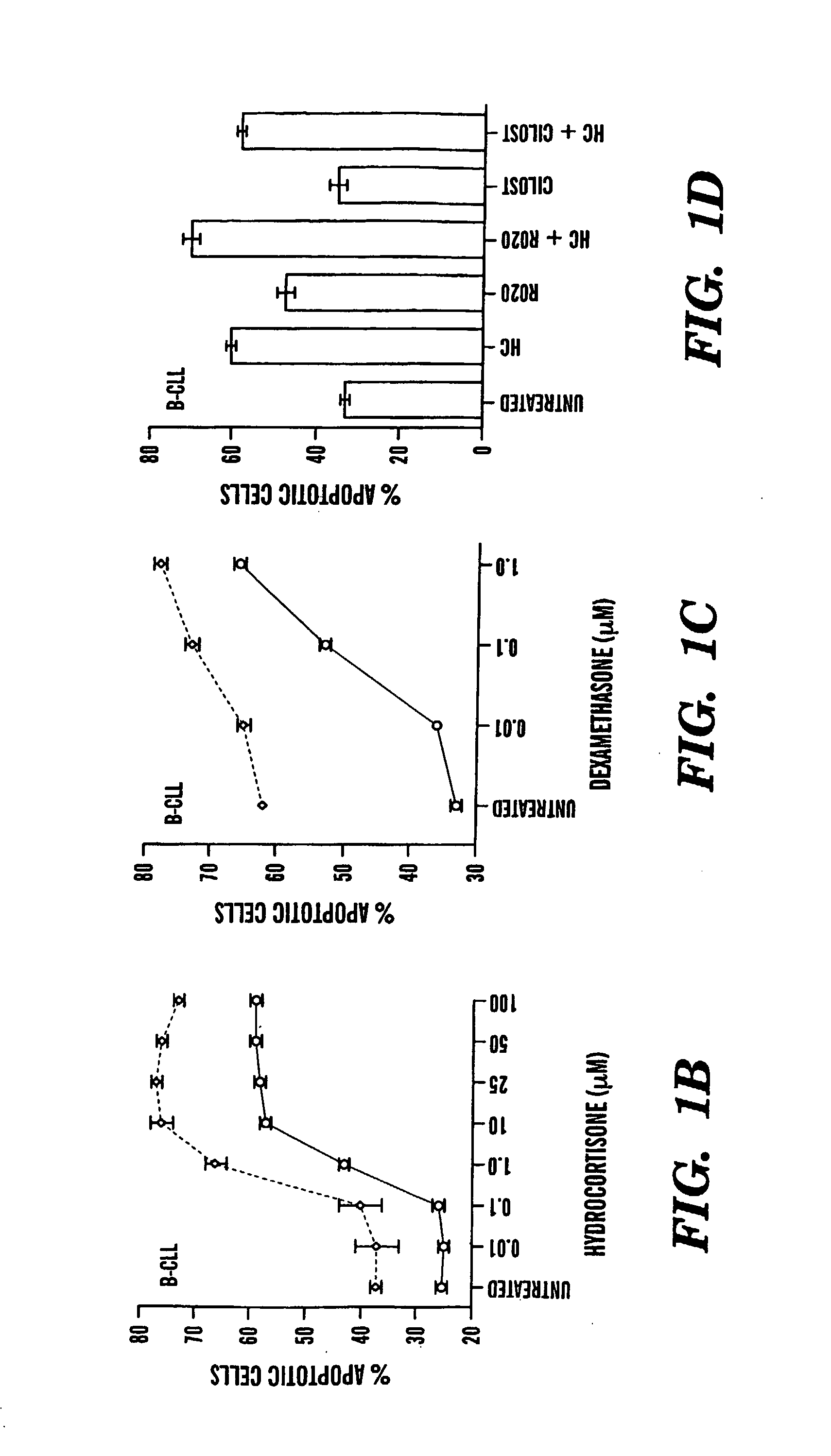
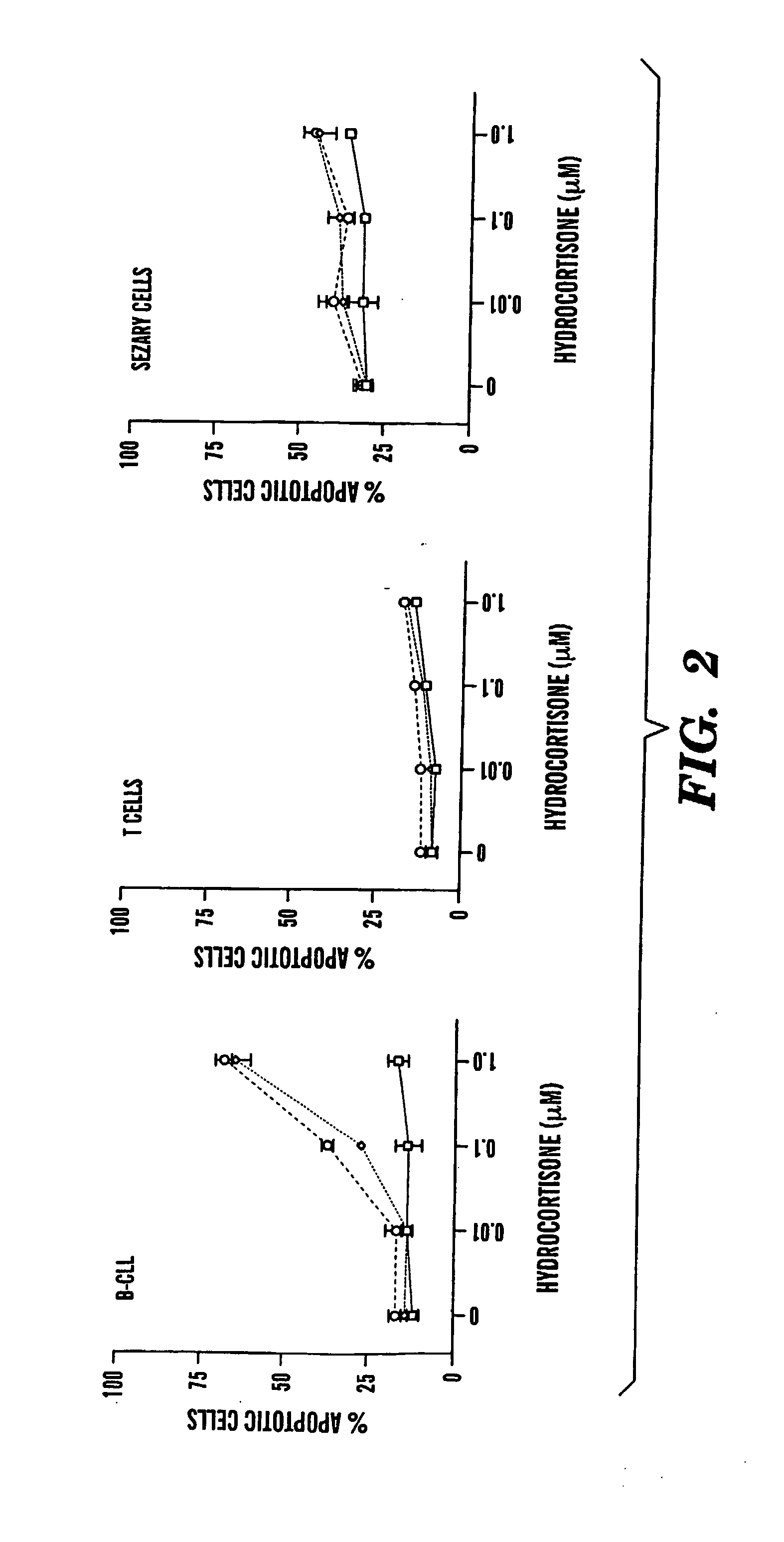
Compositions and Methods for the Treatment of Peripheral B-Cell Neoplasms
InactiveUS20080051379A1High level of apoptosisLevel of apoptosis inducedOrganic active ingredientsBiocidePDE4 InhibitorsAdenosine
The present invention is directed to the use of a PDE4 inhibitor and a glucocorticoid to treat peripheral B-cell neoplasms. In particular, the present invention provides a method of treating individuals (e.g. patients) diagnosed with peripheral B-cell leukemias by administering pharmaceutical compositions comprising Type 4 cyclic adenosine monophosphate phosphodiesterase inhibitors and a glucocorticoid. Preferably, the combination of the PDE4 inhibitor and the glucocorticoid has a synergistic effect on apoptosis such that the level of apoptosis induced is greater than the level that would be expected by simply adding a PDE4 inhibitor to a glucocorticoid.
Owner:BOSTON MEDICAL CENTER INC
Carboxylesterase inhibitors
This disclosure relates to amides, aryl sulphonamides, aryl ureas, and α,β-diketones derivatives useful as carboxylesterase esterase inhibitors. The disclosure is also directed to the use of these compounds as selective human intestinal carboxylesterase inhibitors and insect carboxylesterase inhibitors. The disclosure is also directed to pharmaceutical compositions and pesticide formulations containing these compounds, and to methods for treating or ameliorating the toxic effects following administration of drugs such as cancer therapy drugs, treating or ameliorating the effects of a drug overdose, and to the use of the compounds for increasing the effectiveness of insecticides and pesticides.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Tablets immediately disintegrating in the oral cavity
The present invention provides an intraoral quickly disintegrating tablet containing a phosphodiesterase inhibitor having an effect of improving the erectile dysfunction and a method for manufacturing the tablet. The present invention also provides an intraoral quickly disintegrating tablet containing a slightly soluble pharmaceutical agent having an improved solubility and a method for manufacturing the tablet. That is, it is an intraoral quickly disintegrating tablet containing a cyclic GMP phosphodiesterase inhibitor and a saccharide, and a method for manufacturing the tablet. Further, it is a method for manufacturing an intraoral quickly disintegrating tablet, which comprises dissolving a slightly soluble pharmaceutical agent in an organic solvent or an aqueous organic solvent together with a surfactant and / or a water-soluble polymer, coating the solution on a filler or granulating it with a filler to obtain molded products, mixing a saccharide with them, adding an organic solvent, water or an aqueous organic solvent thereto, followed by kneading, and subjecting it to a compression-molding.
Owner:EISIA R&D MANAGEMENT CO LTD
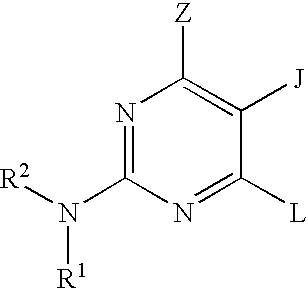
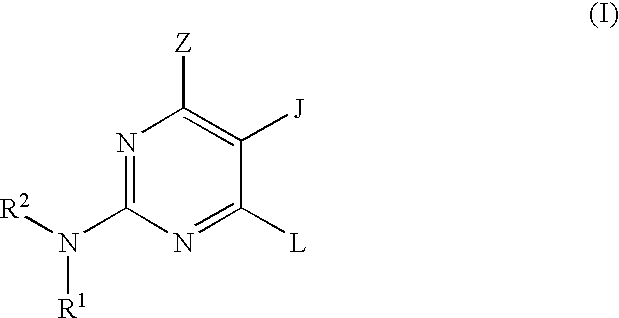
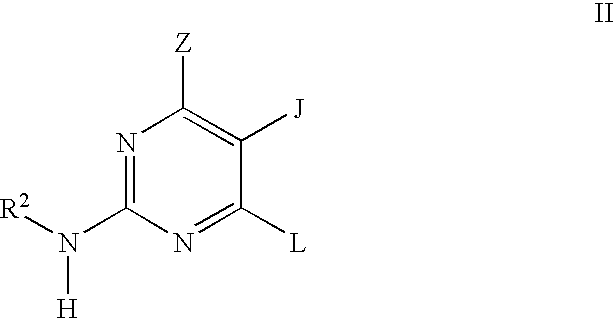
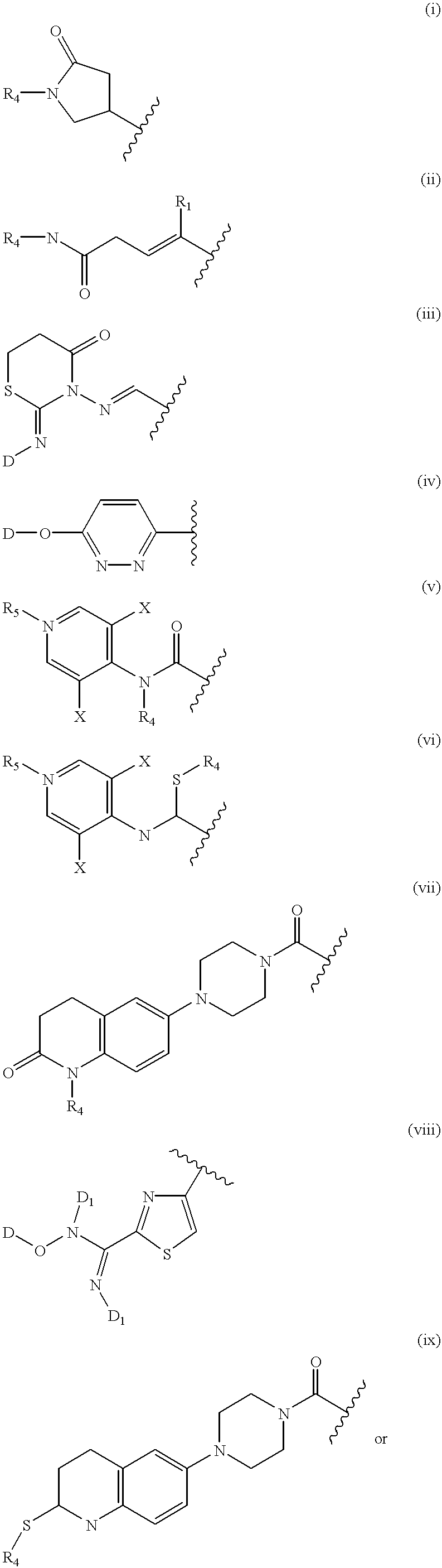
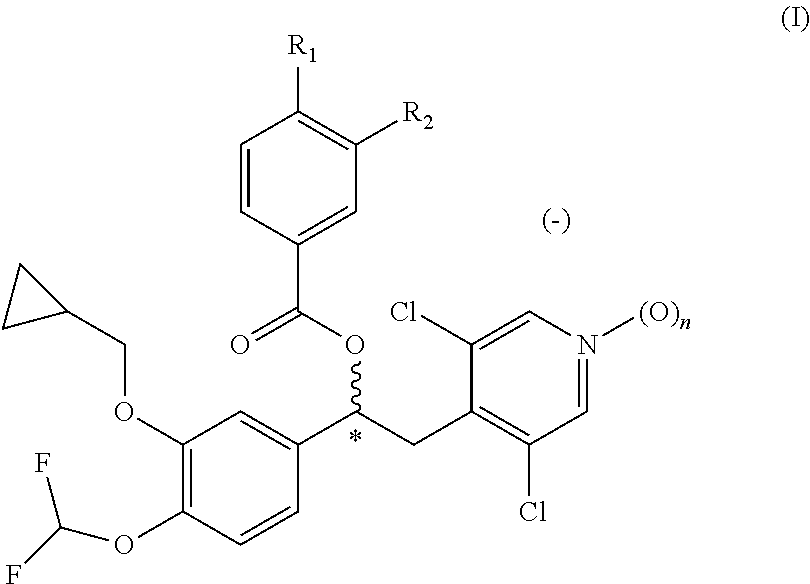
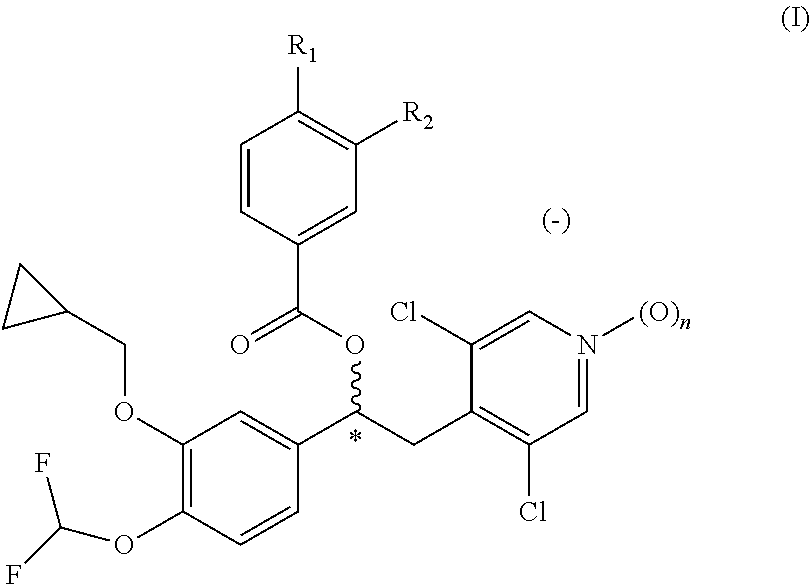
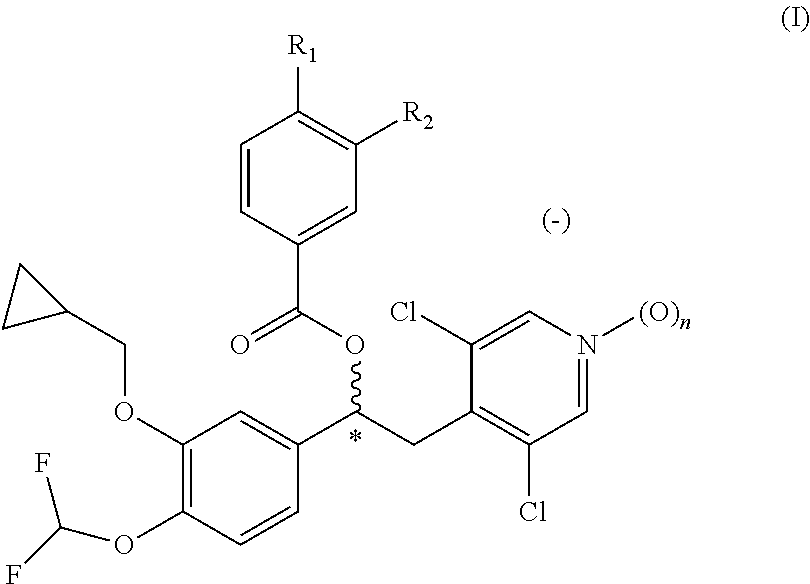
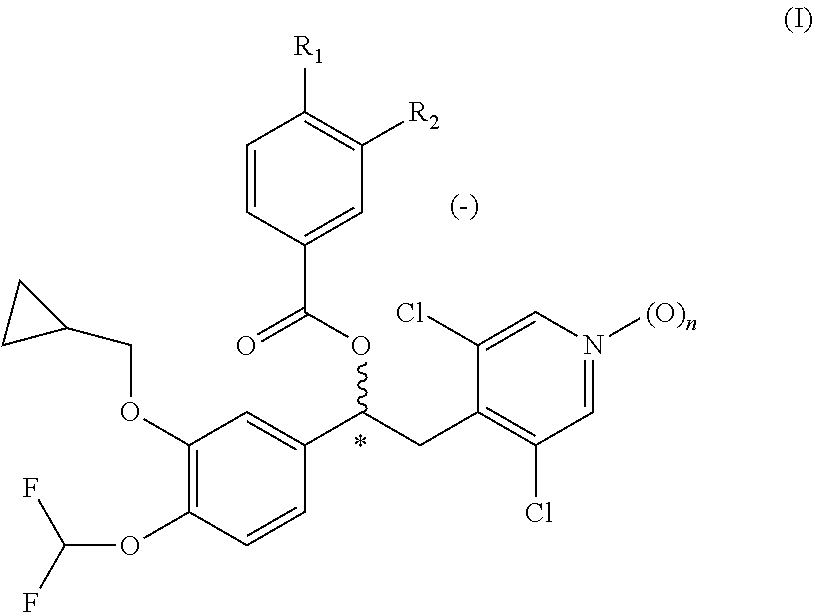
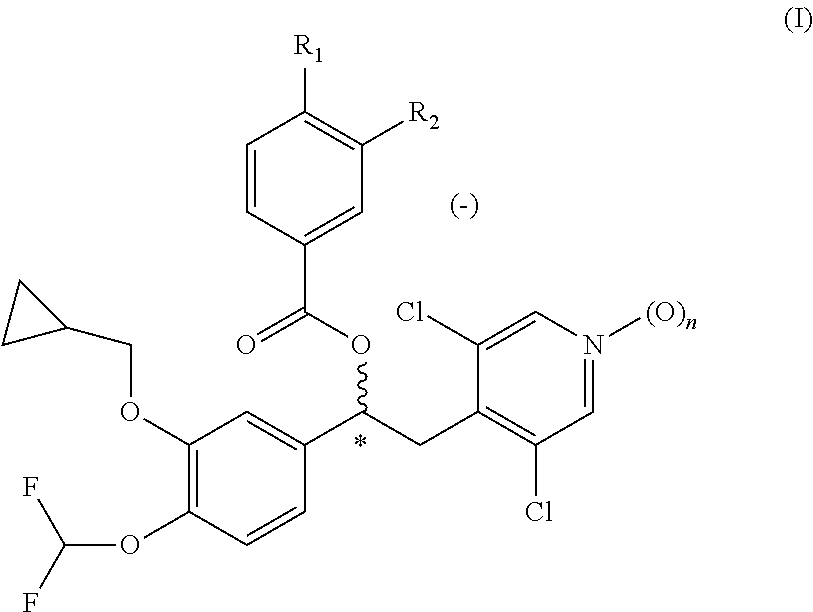
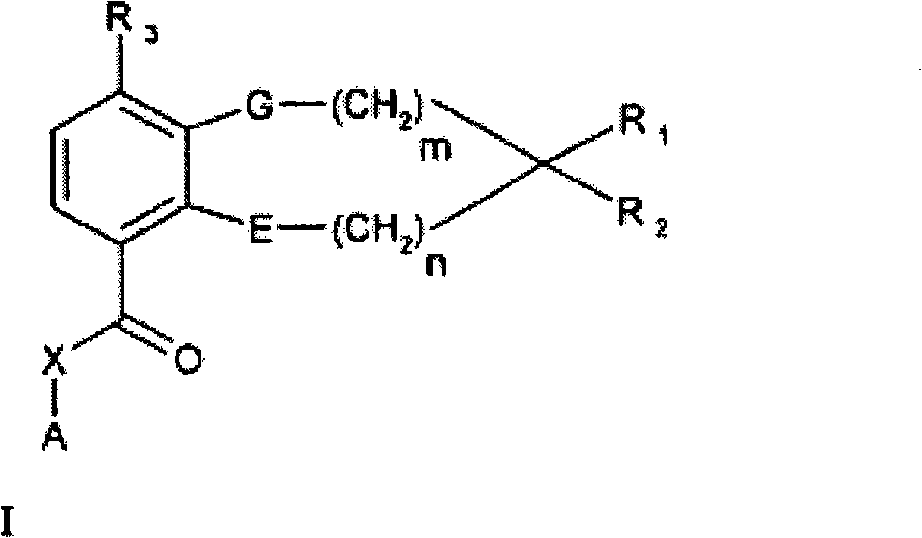
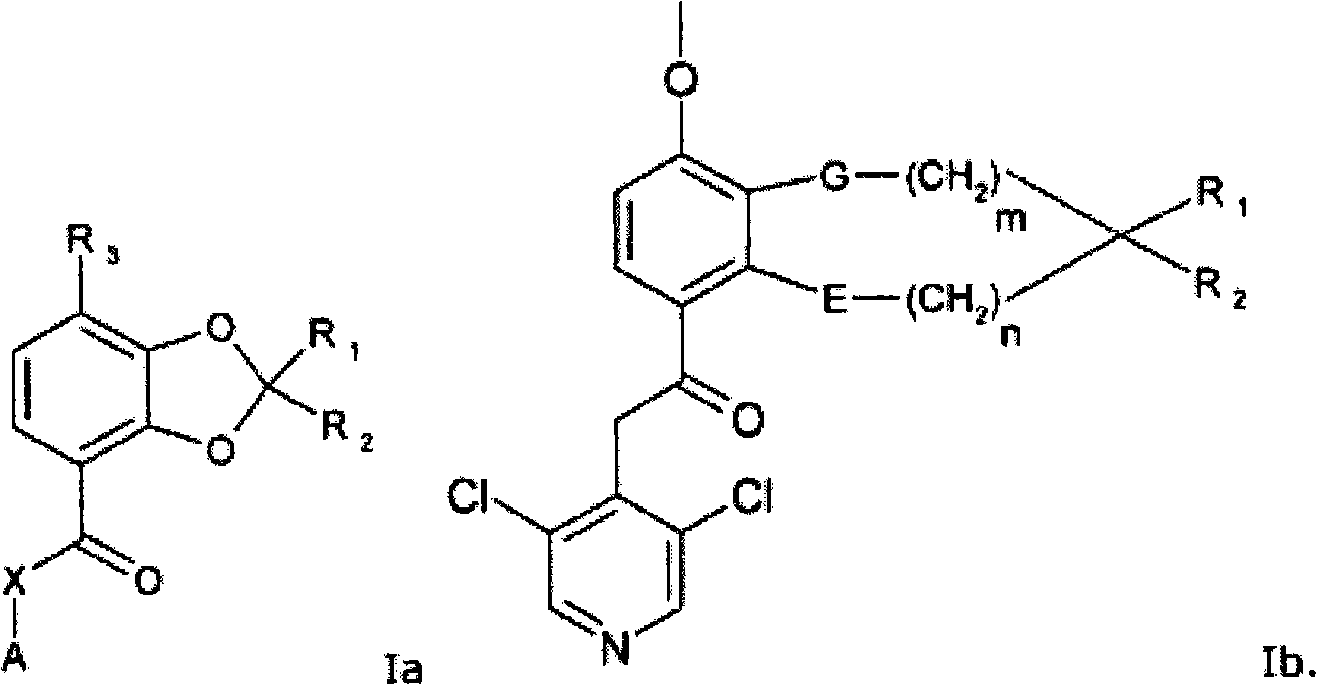
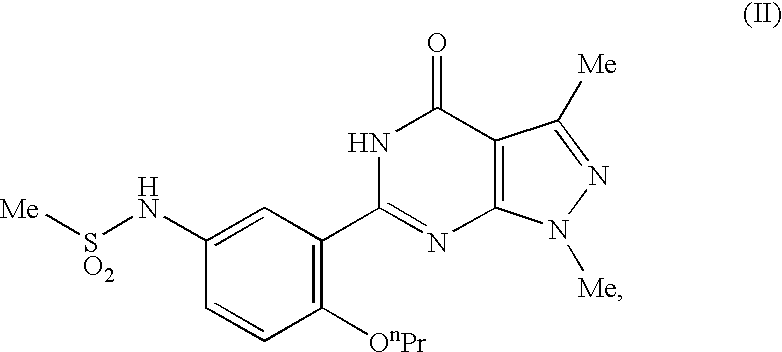
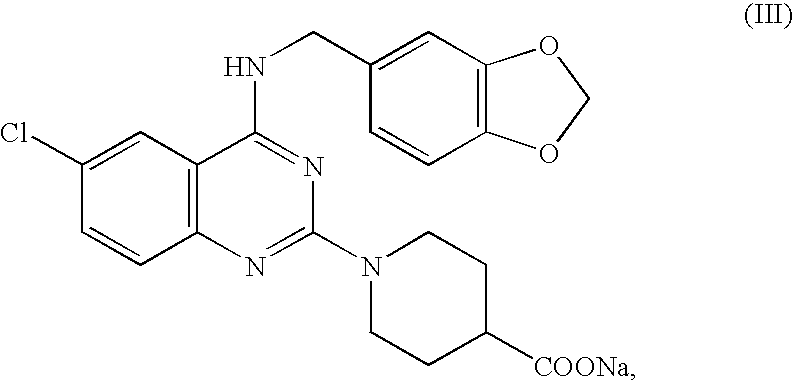
Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7
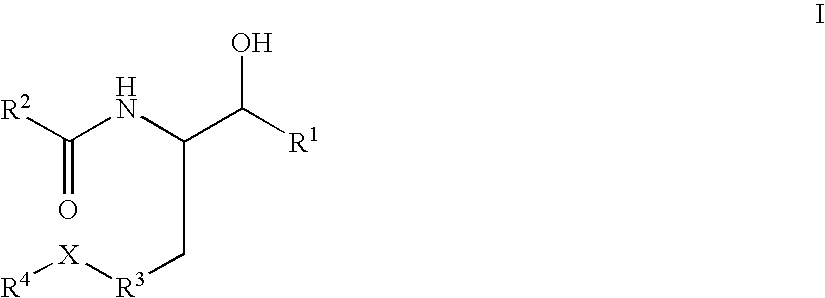
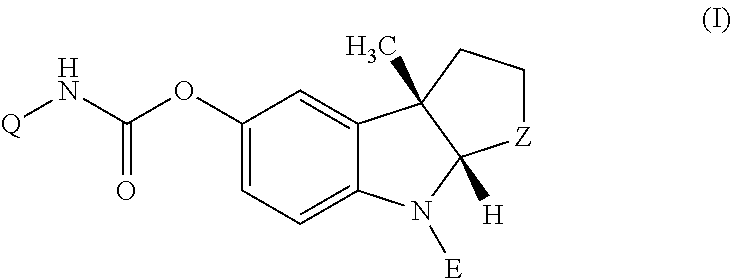
Quinazoline and pyrido[2,3-d]pyrimidine phosphodiesterase 7 (PDE 7) inhibitors of the following formula wherein R1, R2, L, Y1, Y2, Y3 and Z are as described herein, are provided which are useful in treating T-cell mediated diseases.
Owner:BRISTOL MYERS SQUIBB CO
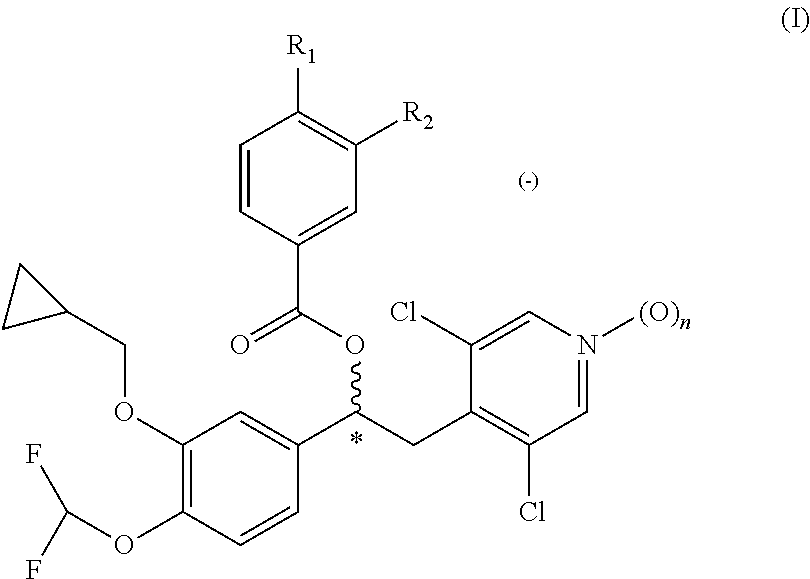
Pyrimidine inhibitors of phosphodiesterase (PDE) 7
Pyrimidine phosphodiesterase 7 (PDE 7) inhibitors of the following formulawherein R1, R2, Z, J and L are described herein, and analogs thereof are provided which are useful in treating T-cell mediated diseases.
Owner:BRISTOL MYERS SQUIBB CO
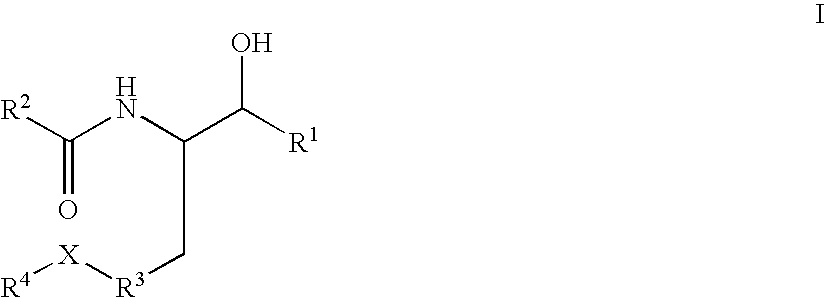
Substituted amide beta secretase inhibitors
Disclosed are novel compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein R1, R2, R3, R4 and X are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are methods of treating a cognitive or neurodegenerative disease comprising administering to a patient I need of such treatment a combination of at least one compound of formula I and at least one compound selected from the group consisting of β-secretase inhibitors other than those of formula I, HMG-CoA reductase inhibitors, gamma-secretase inhibitors, non-steroidal anti-inflammatory agents, N-methyl-D-aspartate receptor antagonists, cholinesterase inhibitors and anti-amyloid antibodies.
Owner:MERCK SHARP & DOHME LLC
Novel combination of drugs as antidepressant
A novel antidepressant composition of a cholinesterase inhibitor in combination with a selective serotonin reuptake inhibitor, milnacipran or duloxetine is disclosed, which has a significantly high therapeutic effect as compared with conventional antidepressants. The therapeutic method using a cholinesterase inhibitor in combination with a selective serotonin reuptake inhibitor, milnacipran or duloxetine is beneficial for the treatment of depression, in particular, refractory depression.
Owner:EISIA R&D MANAGEMENT CO LTD
Inhibitors of cyclic amp phosphodiesterases
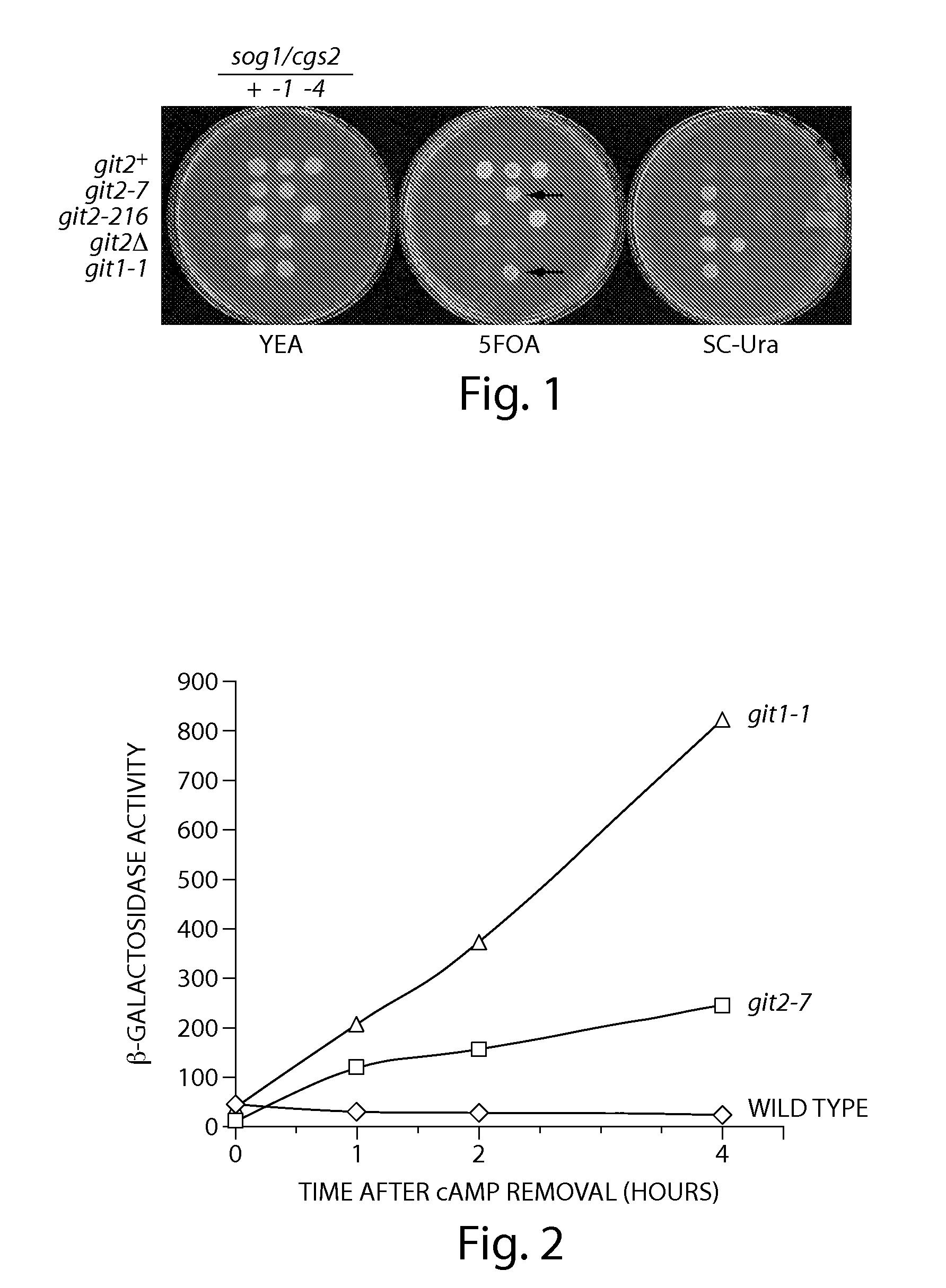
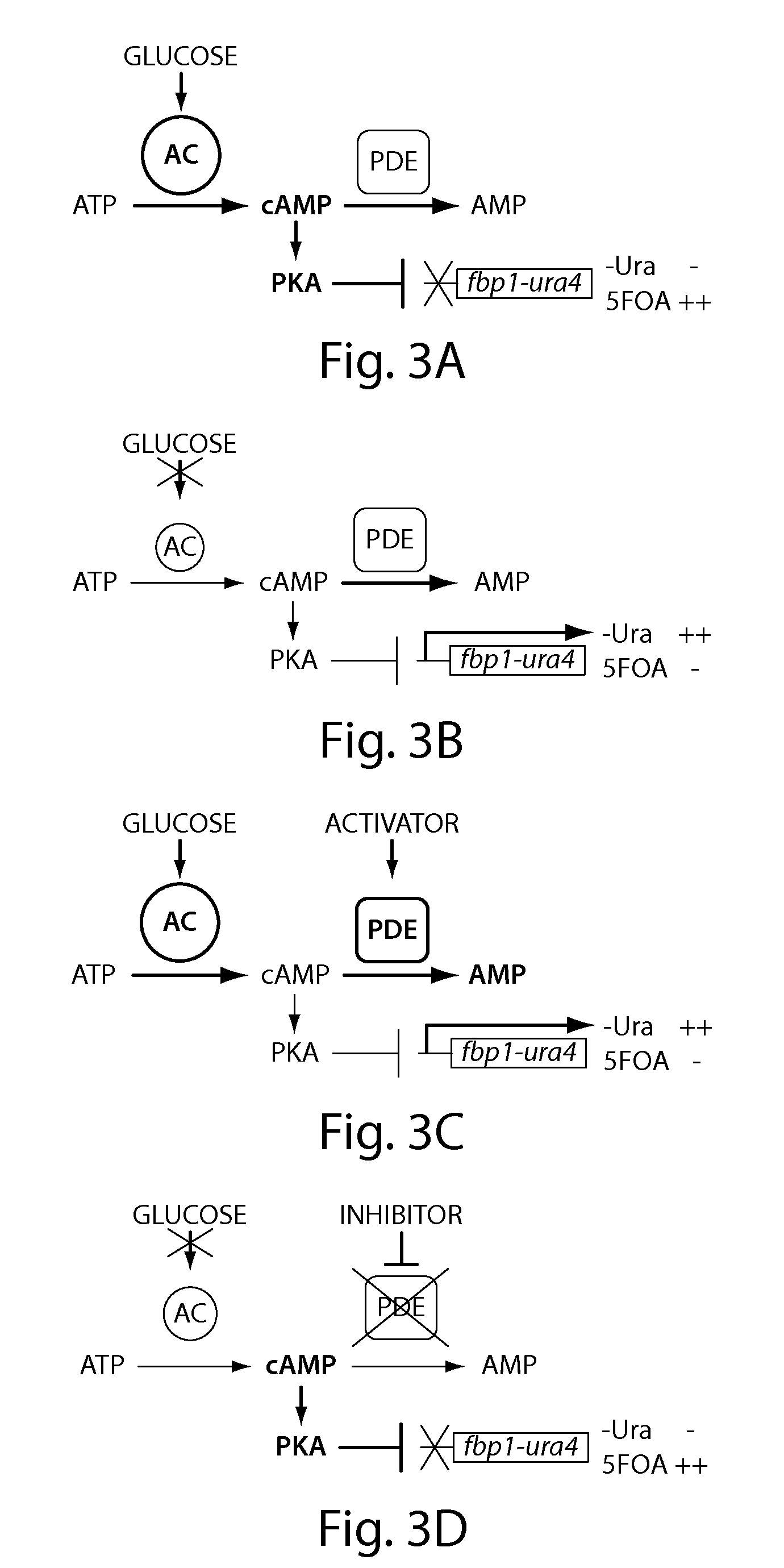
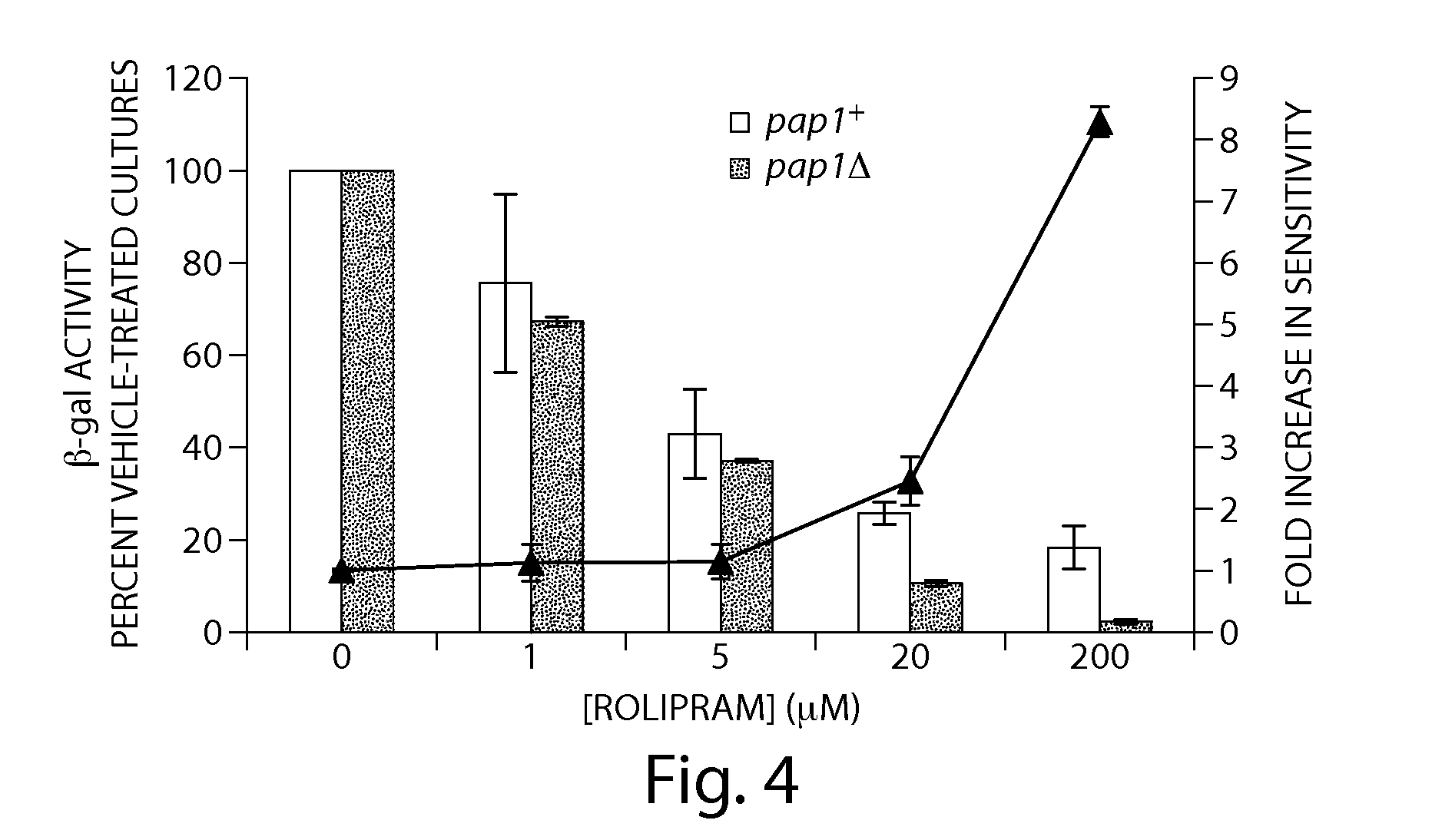
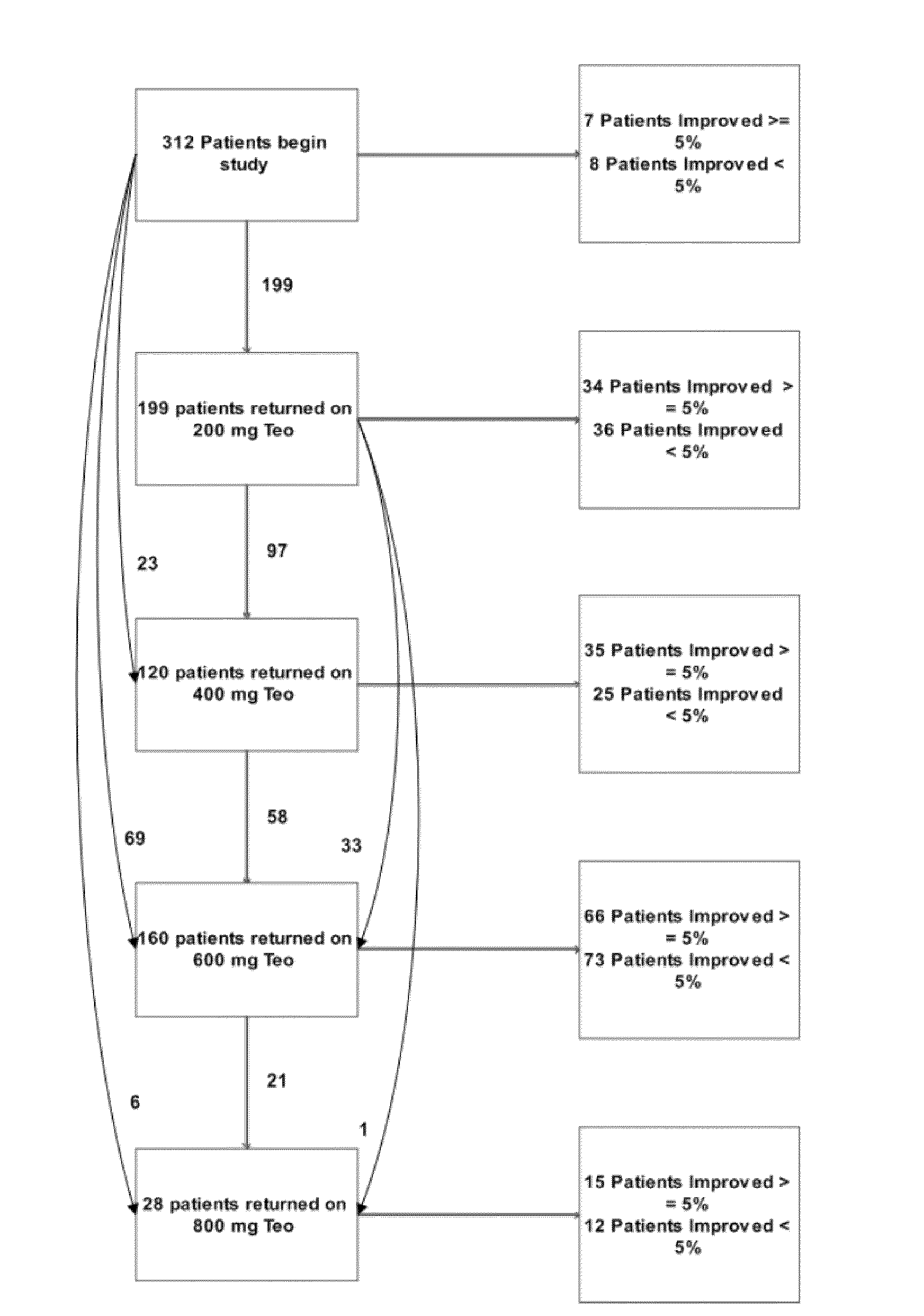
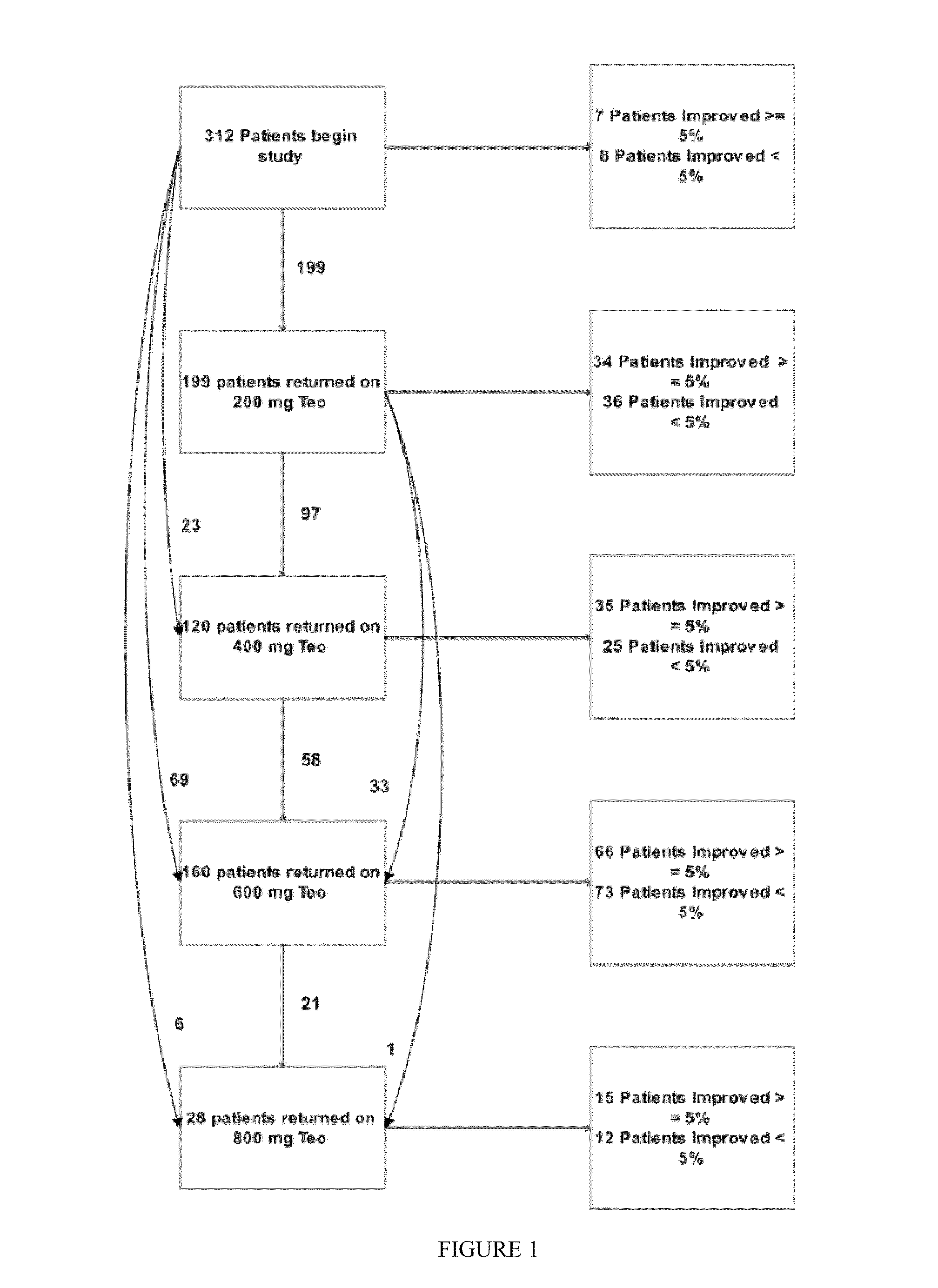
Recombinant fission yeast cells and methods of using them are described, which provide for identification of chemical and biological inhibitors or activators of a target exogenous phosphodiesterase (PDE). The invention provides, in some aspects, compounds that inhibit cAMP PDE activity and compositions that include such compounds. The invention, in part, also includes methods of using cAMP PDE-inhibiting compounds in the treatment of cAMP PDE-associated diseases and / or disorders.
Owner:BOSTON COLLEGE
Method and composition for treating alzheimer-type dementia
ActiveUS20110071135A1Extended durationFunction increaseBiocideNervous disorderTolerabilityH1 Receptor Antagonist
There is described a method for increasing the maximal tolerated close and thus the efficacy of an acetyl choline esterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetyl choline esterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetyl choline esterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholine esterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Phosphodiesterase inhibitor treatment
ActiveUS20100022563A1Great tasteImprove the level ofBiocideSenses disorderDiseasePhosphodiesterase inhibitor
Methods and compositions are disclosed for the treatment of diseases or conditions produced by or associated with low cyclic nucleotide levels. The compositions comprise phosphodiesterase inhibitors and are formulated for intranasal and pulmonary administration.
Owner:CYRANO THERAPEUTICS INC
Dihydropyridine compounds and compositions for headaches
The invention provides methods for treating and / or preventing headaches by administering to patients therapeutically effective amounts of 1,2-dihydropyridine compounds, and, optionally, cholinesterase inhibitors and / or anti-migraine agents. The headaches may be primary headaches, such as migraines, or secondary headaches. The invention also provides combinations, commercial packages, and pharmaceutical compositions comprising therapeutically effective amounts of 1,2-dihydropyridine compounds and, optionally, cholinesterase inhibitors and / or anti-migraine agents. The 1,2-dihydropyridine compound may be, for example, 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one. The cholinesterase inhibitor may be, for example, 1-benzyl-4-((5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine.
Owner:EISIA R&D MANAGEMENT CO LTD
Use and composition for treating dementia
ActiveUS8404701B2Maximize the effectSymptoms improvedBiocideNervous disorderMaximum tolerated doseAnti cholinergic
Owner:CHASE PHARMA CORP
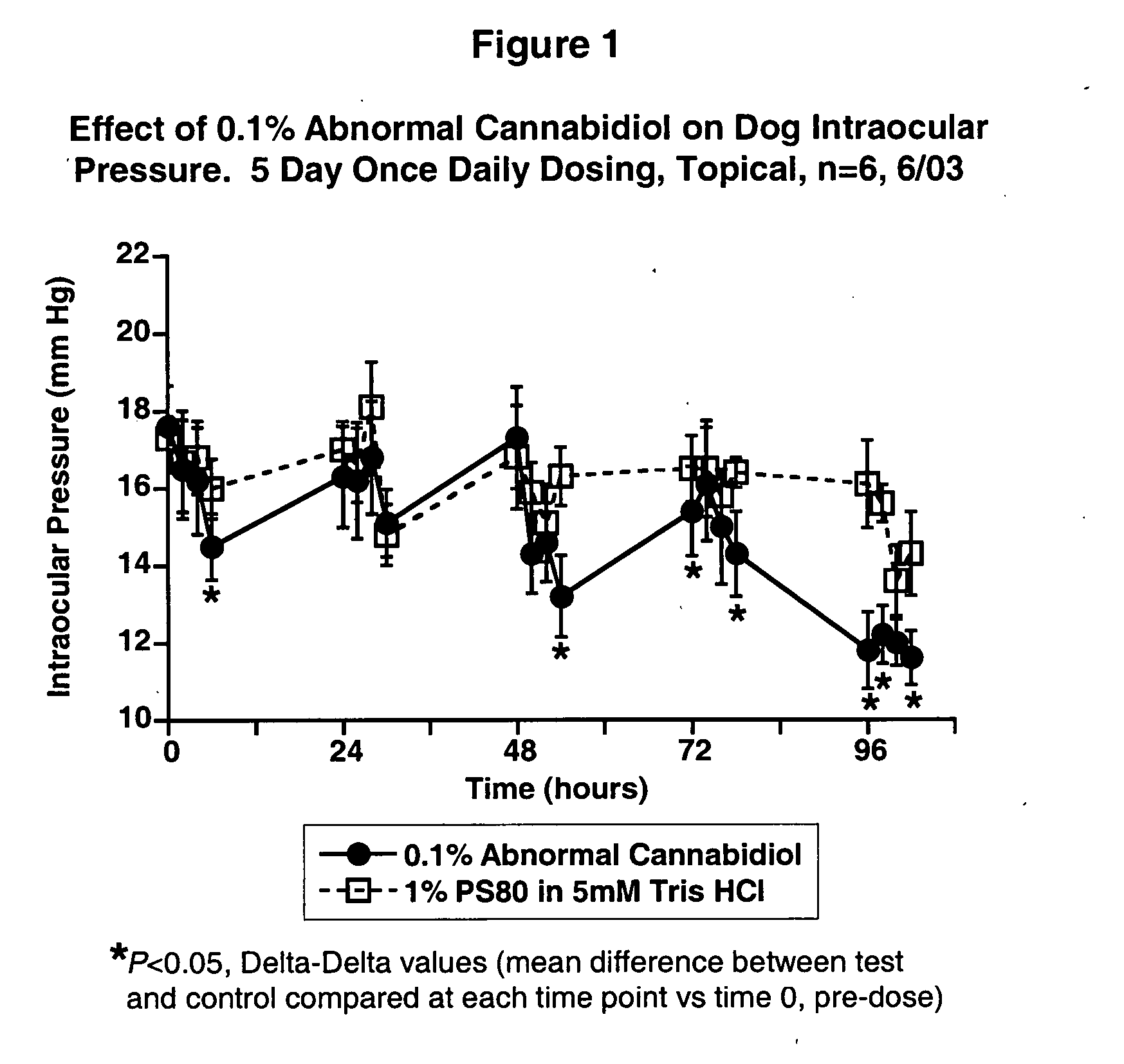
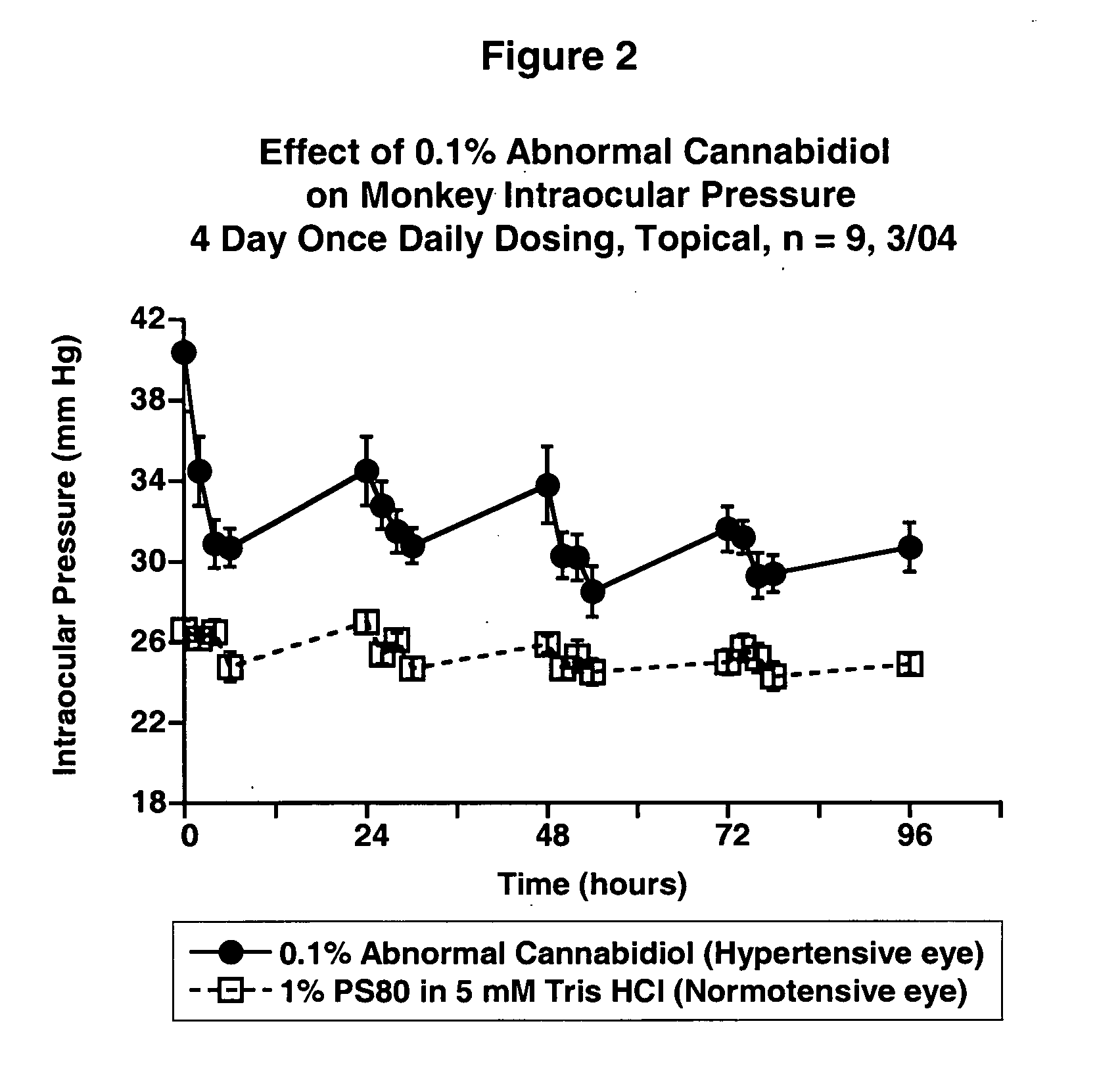
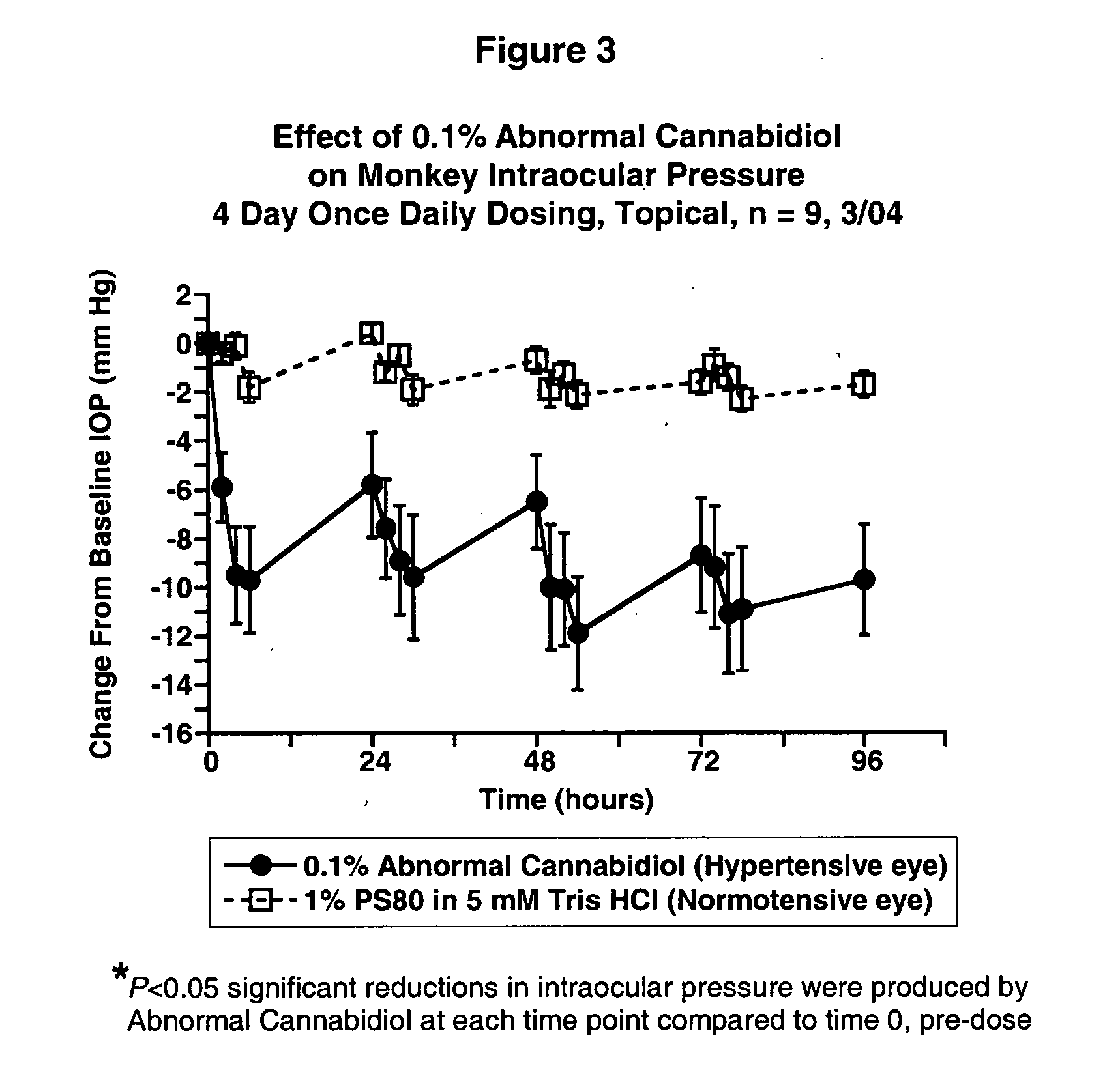
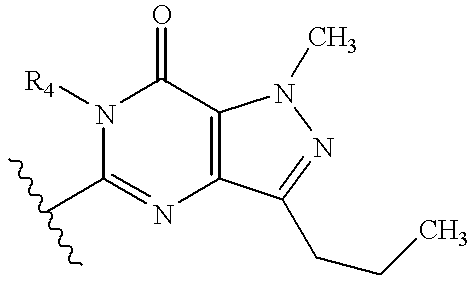
Abnormal cannabidiols as agents for lowering intraocular pressure
InactiveUS20050282902A1Lower intraocular pressureBiocideElcosanoid active ingredientsDrugAdrenergic agonist
The invention relates to the use of Abnormal Cannabidiols in a combination with a drug selected from the group consisting of β-blockers, adrenergic agonists, carbonic anhydrase inhibitors, cholinergic agonists, chlolinesterase inhibitors, glutamate antagonists, prostamides and prostaglandins and the like, or pharmaceutically acceptable salts or prodrugs thereof as potent ocular hypotensives. Said combinations are particularly suitable for the management of glaucoma. In particular said Abnoral Cannibidiols are represented by formula I or formula II or formula III
Owner:ALLERGAN INC
Nitrosated and nitrosylated phosphodiestrase inhibitor compounds, compositions and their uses
Disclosed are nitrosated and / or nitrosylated phosphodiesterase inhibitors having the formula NOn-PDE inhibitor where n is 1 or 2. The invention also provides compositions comprising such compounds in a pharmaceutically acceptable carrier. The invention also provides a composition comprising a therapeutically effective amount of an phosphodiesterase inhibitor (PDE inhibitor), which can optionally be substituted with at least one NO or NO2 moiety, and one to ten fold molar excess of a compound that donates, transfers or releases nitrogen monoxide as a charged species, i.e., nitrosonium (NO+) or nitroxyl (NO-), or as the neutral species, nitric oxide (NO.) or which stimulates endogenous EDRF production. The invention also provides compositions comprising such compounds in a pharmaceutically acceptable carrier. The invention also provides methods for treating sexual dysfunctions in males and females.
Owner:NITROMED
Therapeutic antisense phosphodiesterase inhibitors
InactiveUS20030045490A1Improve bindingShorten the counting processBiocideSenses disorderPhosphodiesteraseDisease
This patent describes the invention of a series of novel therapeutic oligonucleotides targeted at inhibiting expression of genes coding for Phosphodiesterase 4. They are useful as analytical tools in the study of individual PDE isoforms and in the therapeutic treatment of depression, thrombosis, cystic fibrosis, gastric lesions, pulmonary hypertension, glaucoma, multiple sclerosis, atopic dermatitis, asthma and other allergic disorders as well as other illnesses in which an increase of cyclic AMP or a decrease in phosphodiesterase levels is useful.
Owner:LAKEWOOD AMEDEX
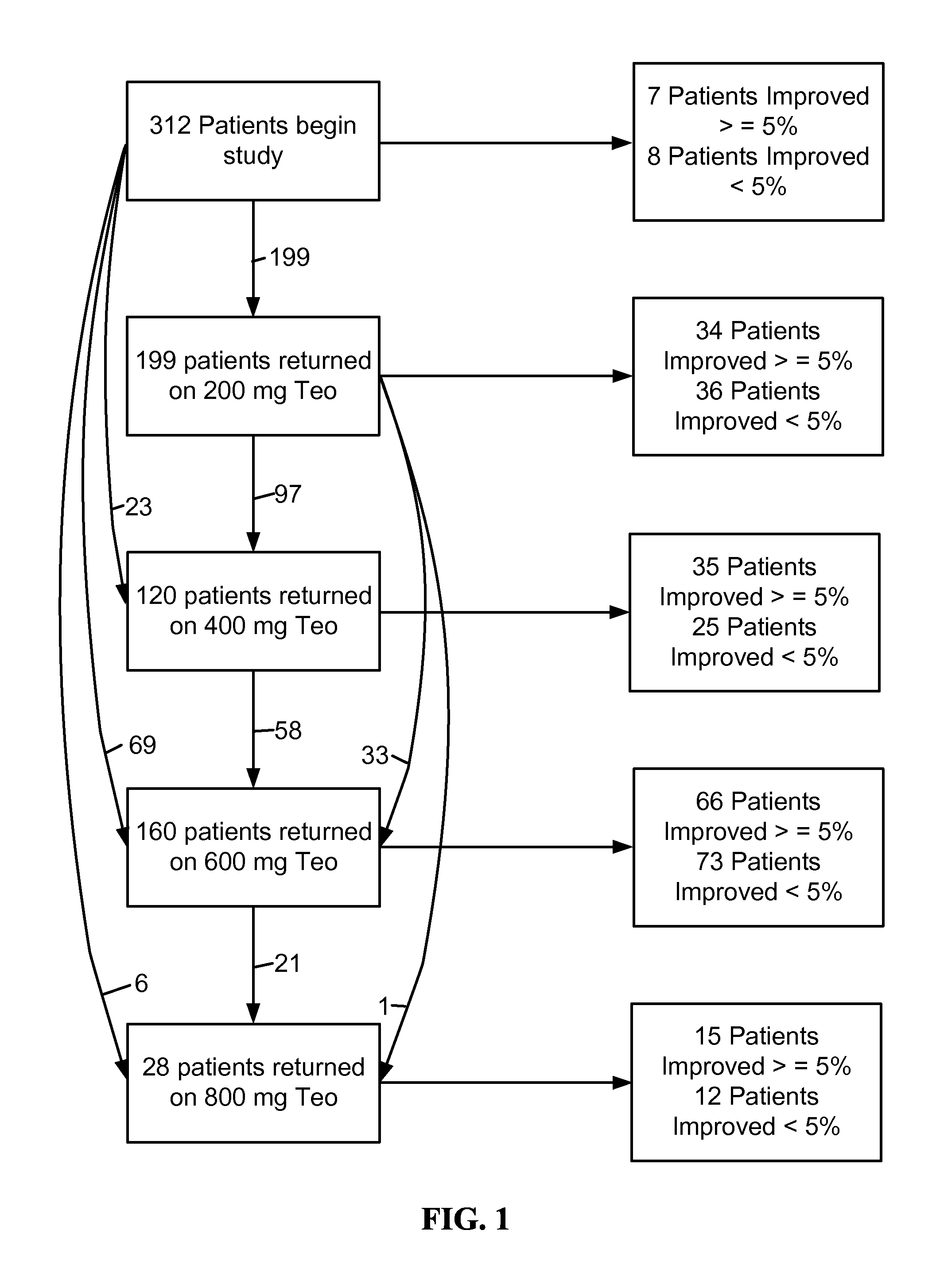
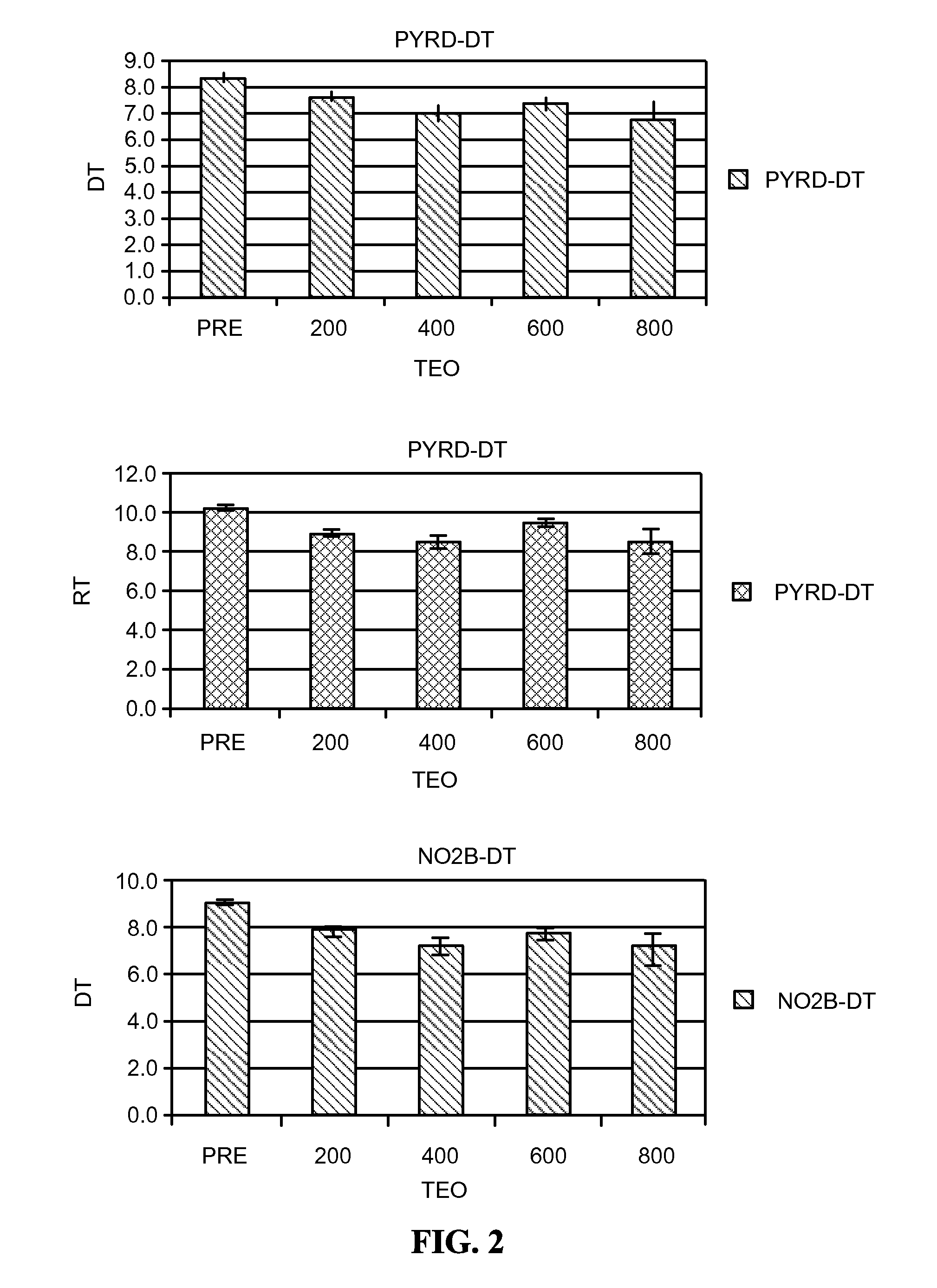
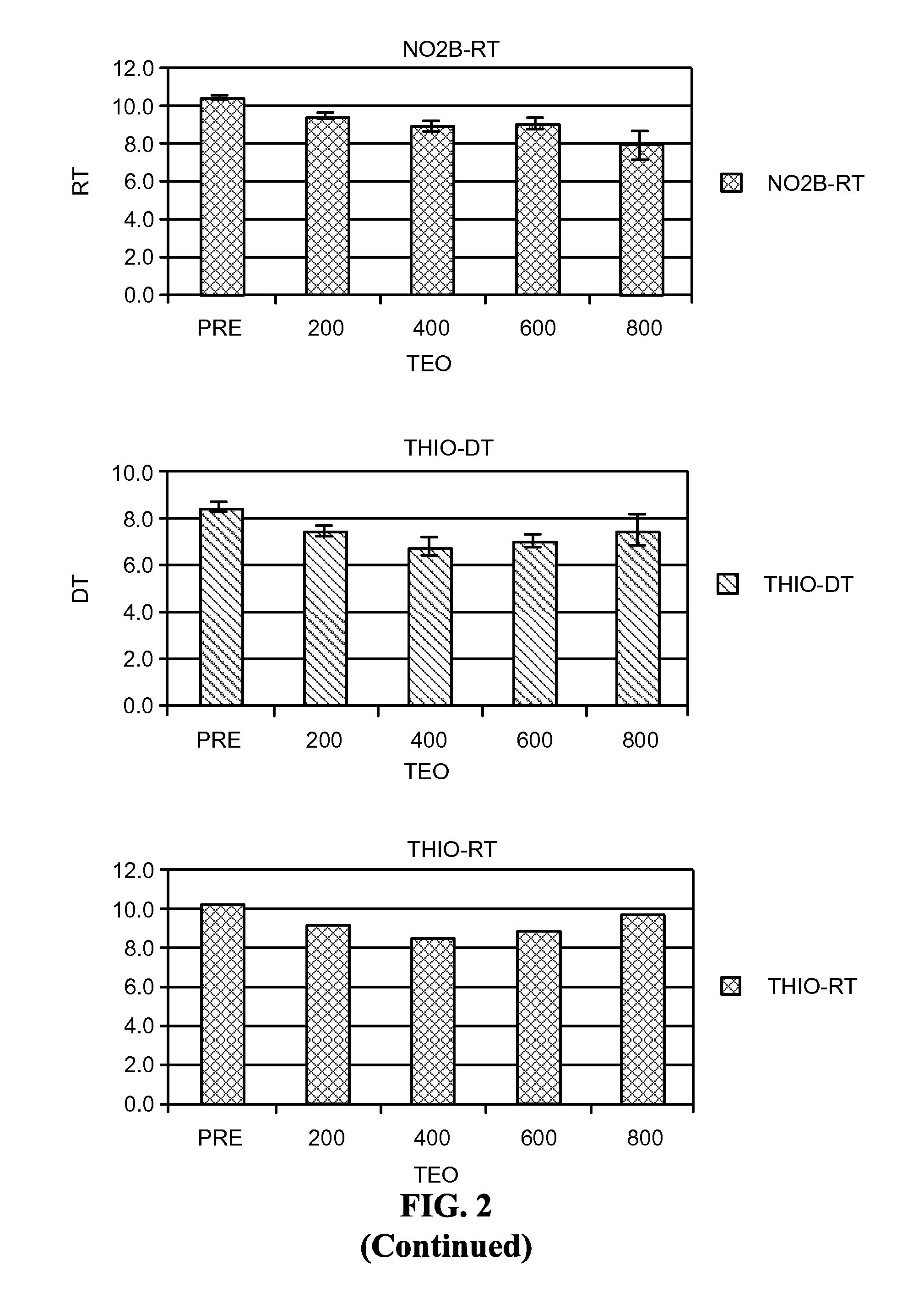
Reducing Side Effects of Tramadol
InactiveUS20080261991A1Reduce morbidityOrganic active ingredientsNervous disorderSexual functionSide effect
The invention provides methods of reducing the side effects of tramadol. Accordingly, in one embodiment, the invention provides a method of reducing the incidence of newly-discovered side effects related to sexual function in human males taking a tramadol material. The method comprises administering a phosphodiesterase inhibitor to a male taking the tramadol material. The invention also provides pharmaceutical compositions. In one embodiment, the composition comprises a tramadol material and a phosphodiesterase inhibitor. The invention further provides kits. In one embodiment, the kit comprises a tramadol material and a phosphodiesterase inhibitor.
Owner:AYTU BIOSCI
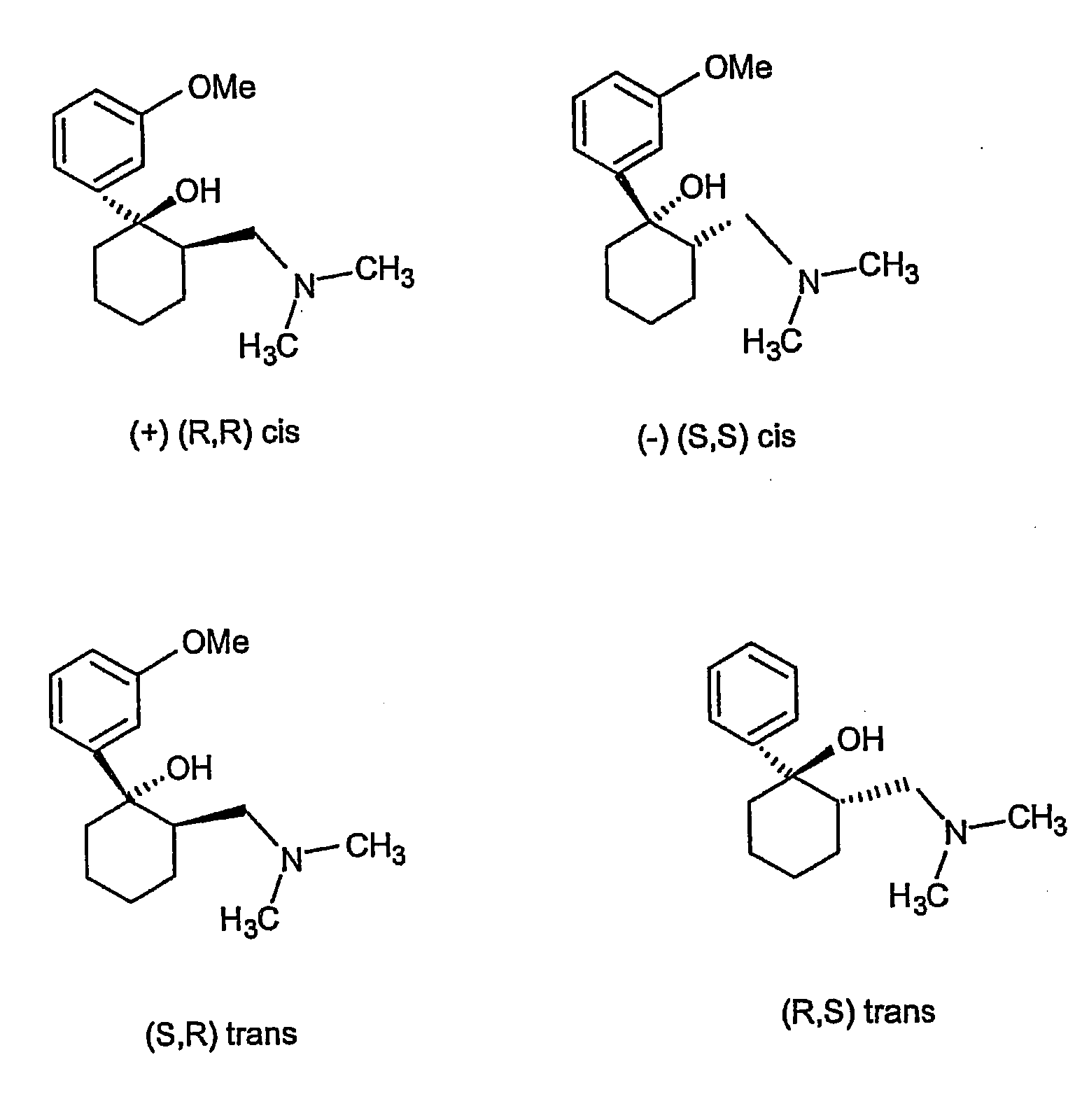
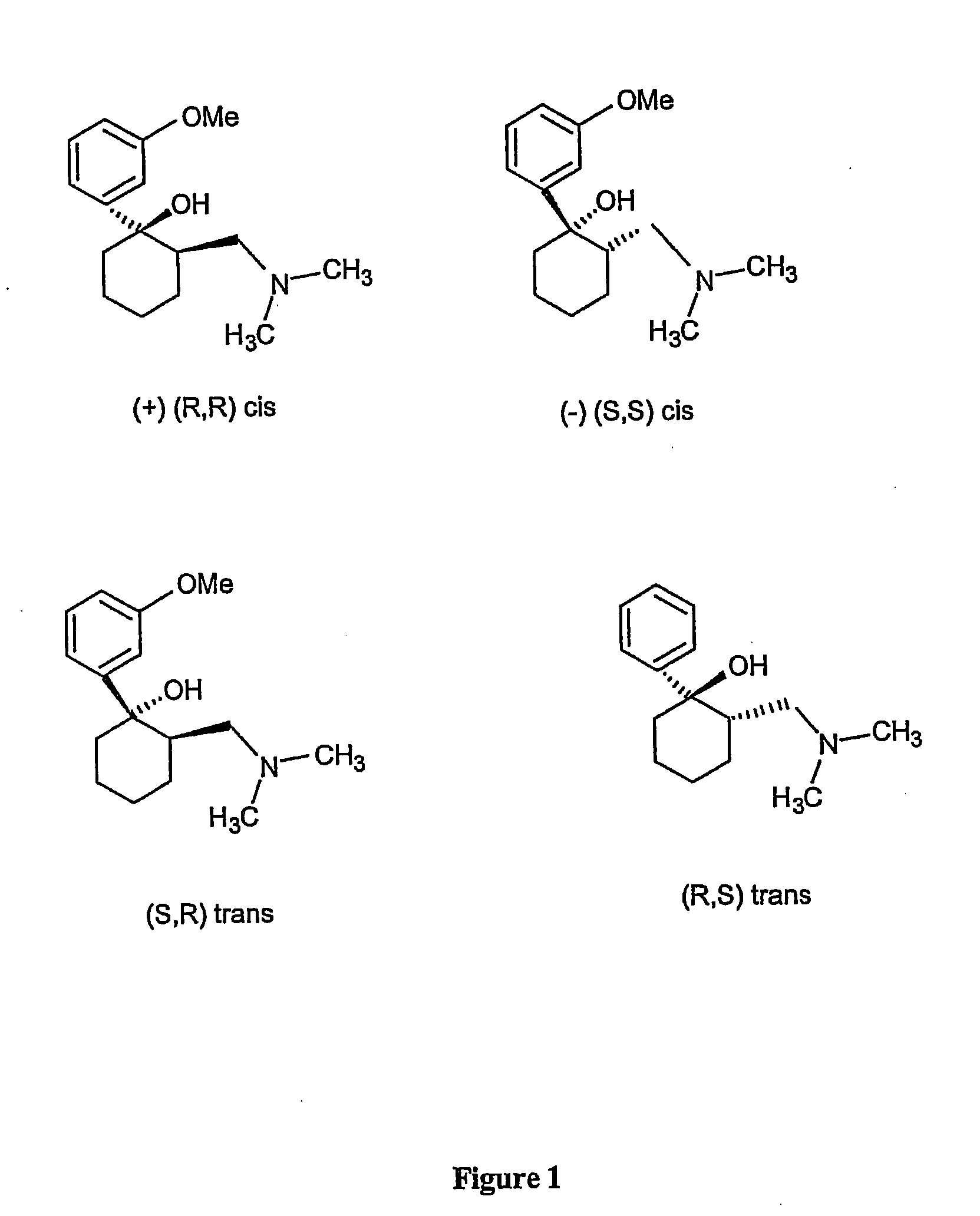
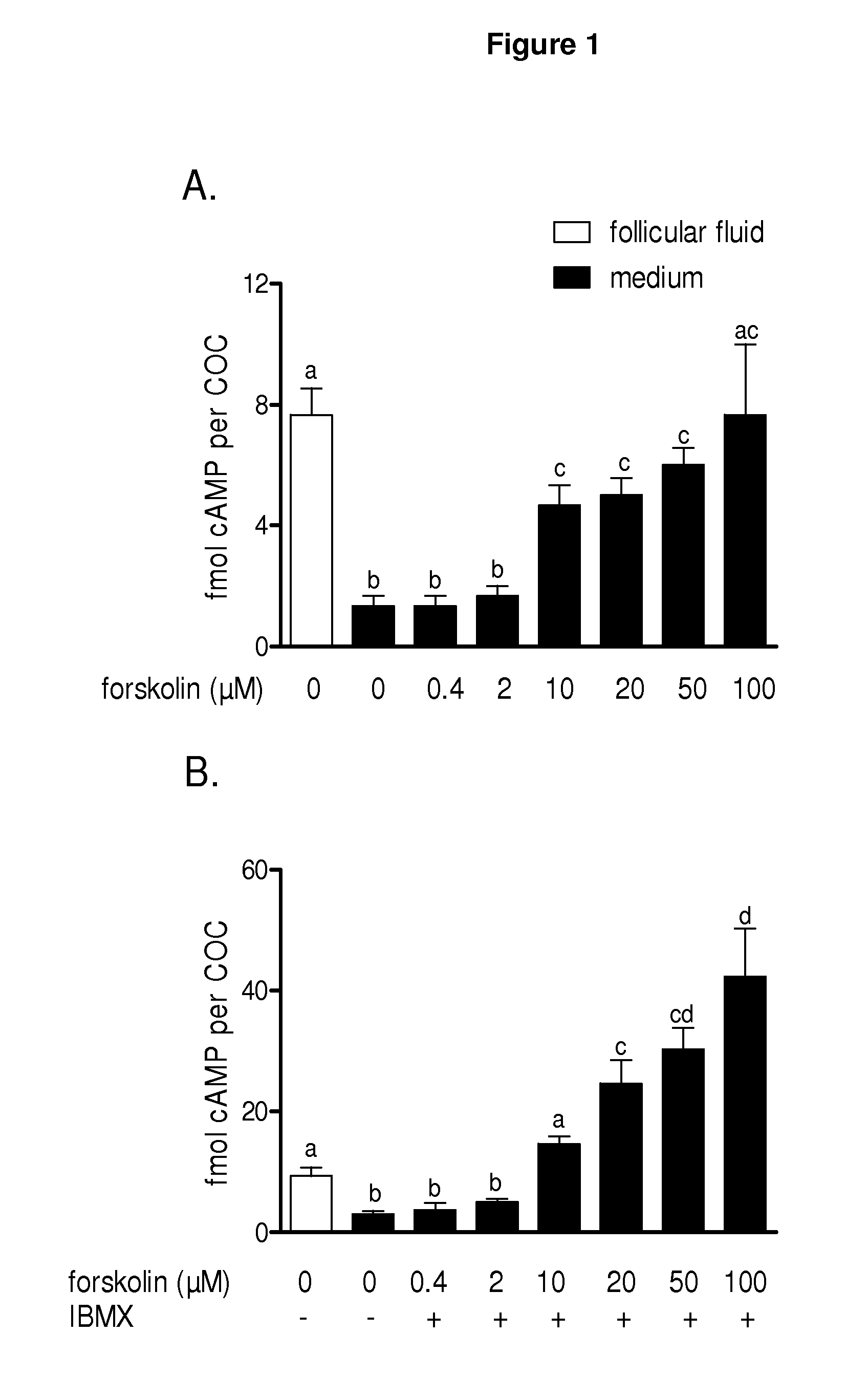
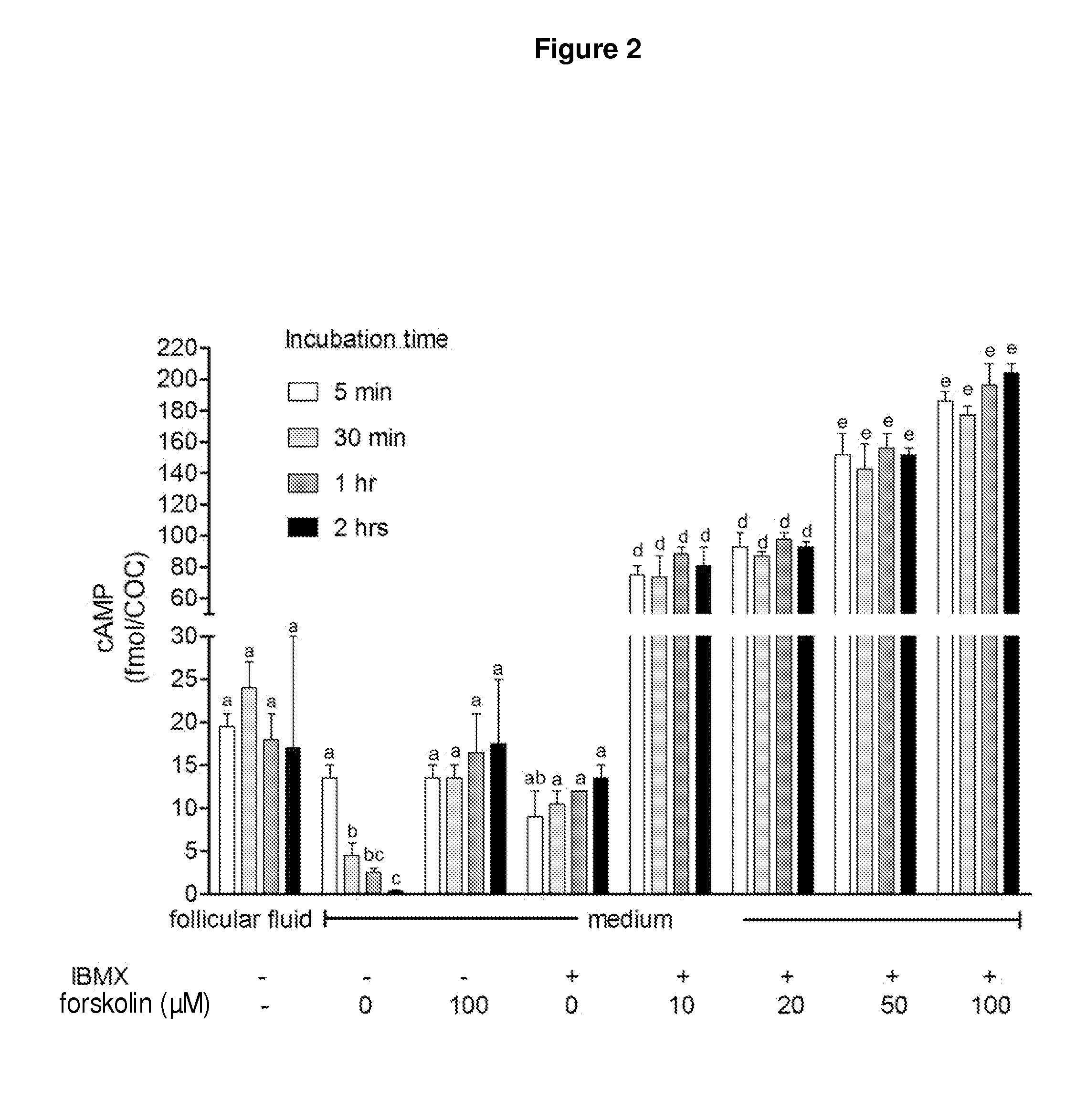
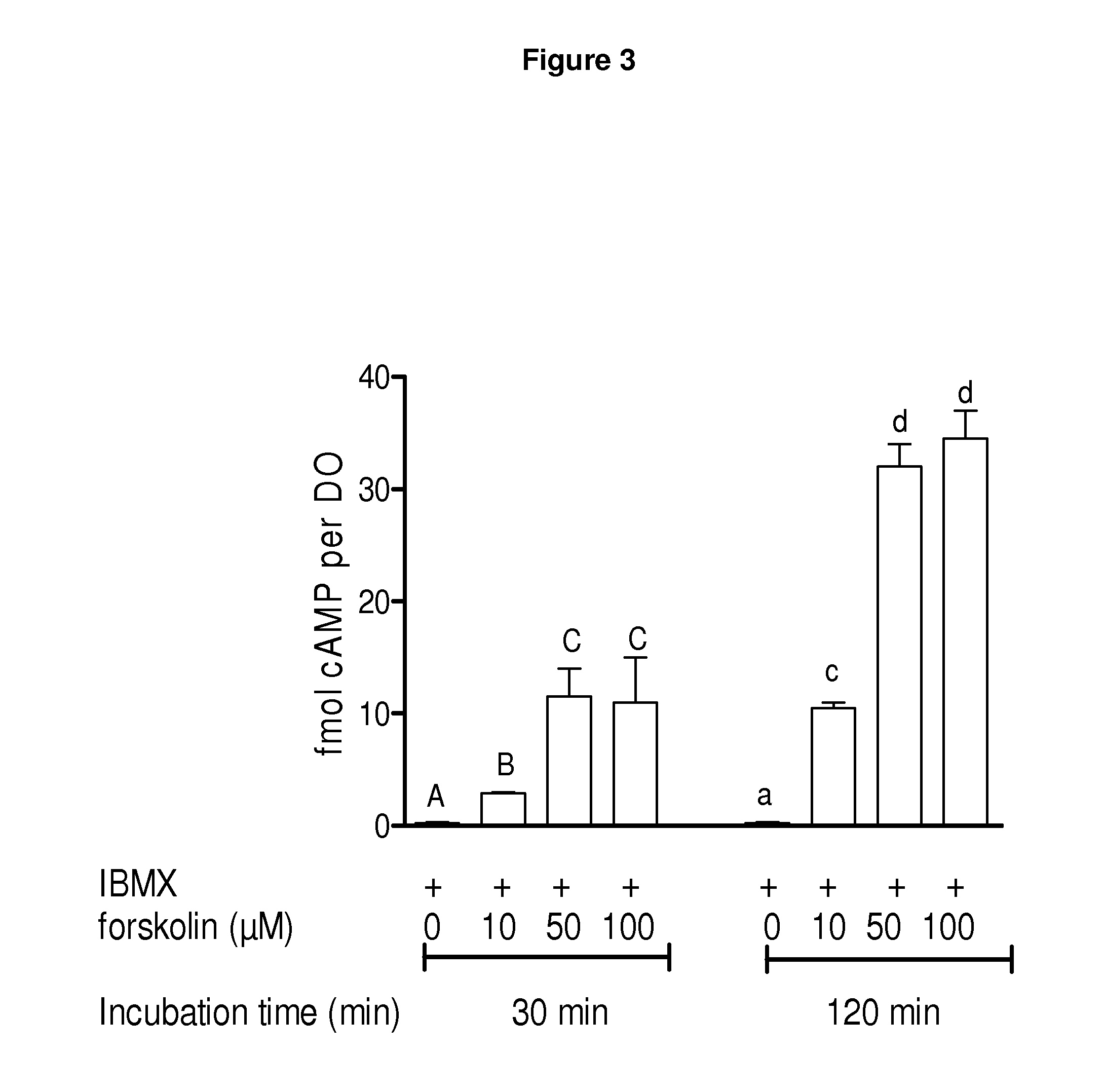
Methods for the collection and maturation of oocytes
InactiveUS20120252119A1New breed animal cellsPeptide/protein ingredientsPhosphodiesterase inhibitorOocyte Collection
The present invention relates to a method of producing an embryo from an oocyte by an assisted reproduction technology. The method includes (a) collecting an oocyte from an ovary of a subject in a collection medium comprising a first phosphodiesterase inhibitor and an agent that increases intracellular cAMP concentration in the oocyte, (b) culturing the oocyte in a maturation medium comprising a second phosphodiesterase inhibitor, and (c) producing an embryo from the oocyte by an assisted reproduction technology. The present invention also relates to methods of inducing oocyte maturation. For example a method of in vitro maturation of an oocyte is described which comprises steps (a) and (b) above. The present invention also relates to an oocyte maturation medium comprising a phosphodiesterase inhibitor and a ligand for inducing maturation of the oocyte. A combination product comprising an oocyte collection and maturation medium referred to above is also described.
Owner:GILCHRIST ROBERT BRUCE +2
Pharmaceutical formulation comprising a phosphodiesterase inhibitor
ActiveUS20120034172A1BiocideDispersion deliveryObstructive airway diseasePhosphodiesterase inhibitor
Owner:CHIESI FARM SPA
Dry powder formulation comprising a phosphodiesterase inhibitor
ActiveUS20120031403A1Improve liquidityGood chemical stabilityRespiratorsBiocideCOPDPhosphodiesterase inhibitor
Pharmaceutical formulations in the form of inhalable dry powder comprising particles of a phosphodiesterase-4 inhibitor as active ingredient are useful for the prevention and / or treatment of respiratory diseases, such as asthma and COPD.
Owner:CHIESI FARM SPA
Method and composition for treating alzheimer-type dementia
ActiveUS8877768B2Maximize the effectSymptoms improvedBiocideNervous disorderTolerabilityH1 Receptor Antagonist
There is described a method for increasing the maximal tolerated close and thus the efficacy of an acetyl choline esterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetyl choline esterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetyl choline esterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholine esterase inhibitor are also described.
Owner:CHASE PHARMA CORP
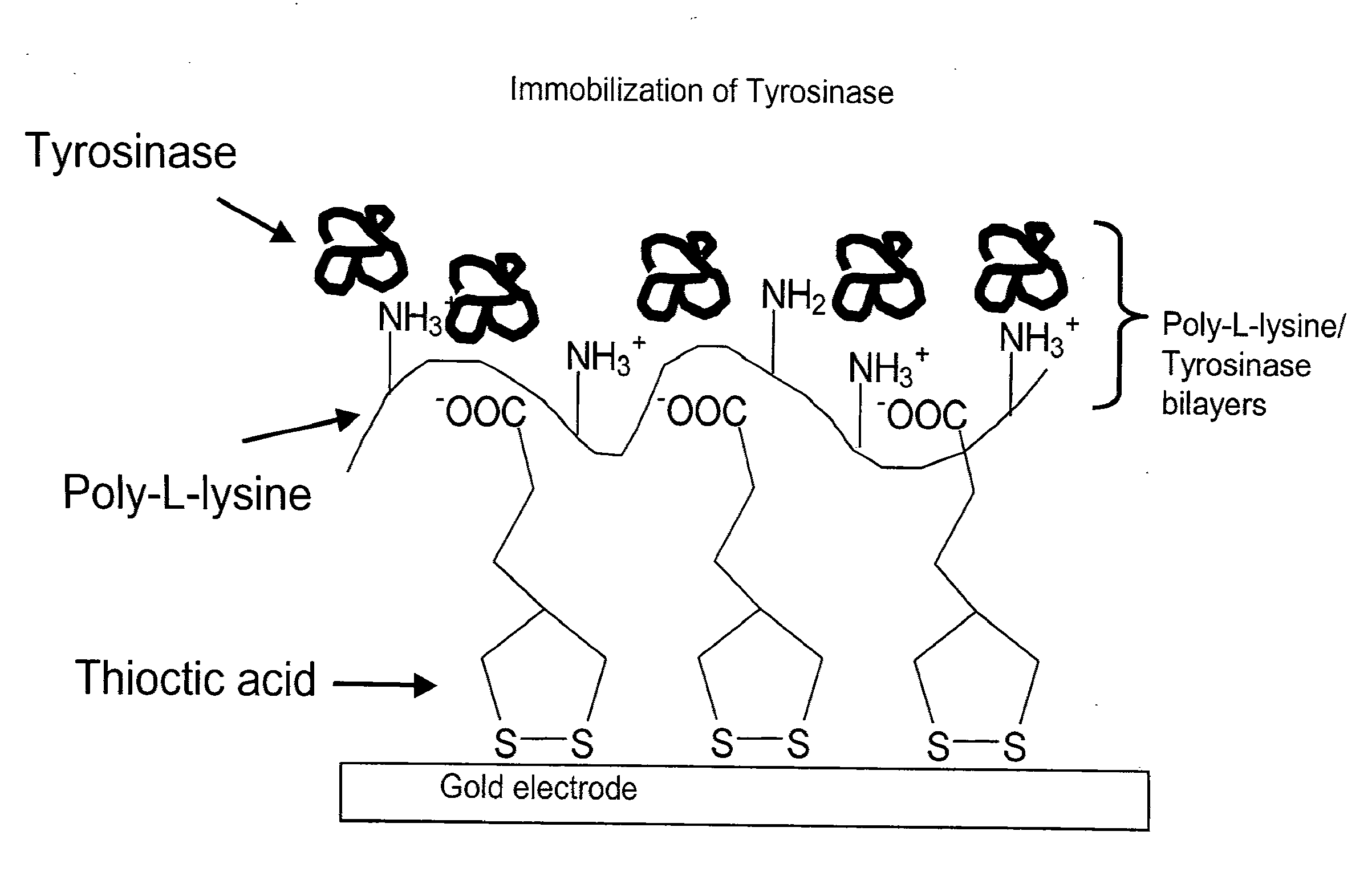
Nanostructured Biosensor Containing Neuropathy Target Esterase Activity
InactiveUS20120160708A1High sensitivityContinuous measurementImmobilised enzymesBioreactor/fermenter combinationsNeuropathy target esteraseHomeland security
The present invention provides compositions, devices and methods for detecting esterase activity. The present invention also provides devices and methods of detecting esterase inhibitors, for example, organophosphates. In particular, the present invention provides a biosensor comprising Neuropathy Target Esterase (NTE) polypeptides. Further, the present invention relates to medicine, industrial chemistry, agriculture, and homeland security.
Owner:MICHIGAN STATE UNIV +1
Phosphodiesterase inhibitor treatment
Methods and compositions are disclosed for the treatment of taste and smell disorders. The compositions comprise phosphodiesterase inhibitors and are formulated for intranasal administration.
Owner:CYRANO THERAPEUTICS INC
Dihydropyridine compounds and compositions for headaches
InactiveUS20060270709A1Slow onsetBiocideNervous disorderHuntingtons choreaAmytrophic lateral sclerosis
The invention provides methods for treating and / or preventing cognitive impairments, dementia, or neurodegenerative diseases and disorders (e.g., Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis) in patients by administering therapeutically effective amounts of an AMPA receptor antagonist (e.g., 1,2-dihydropyridine compounds) and therapeutically effective amounts of nootropics (e.g., cholinesterase inhibitors) to patients. The invention also provides combinations, commercial packages, and pharmaceutical compositions comprising therapeutically effective amounts of AMPA receptor antagonists (e.g., 1,2-dihydropyridine compounds) and therapeutically effective amounts nootropics (e.g., cholinesterase inhibitors). The 1,2-dihydropyridine compound may be, for example, 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one. The cholinesterase inhibitor may be, for example, donepezil.
Owner:EISAI CO LTD
Anticholinergic neuroprotective composition and methods
The present invention relates to a pharmaceutical composition comprising propiverine, trospium or glycopyrrolate; and a non-anticholinergic antiemetic agent. It is also related to a pharmaceutical composition comprising a high dose of solifenacin or a pharmaceutically acceptable salts thereof; and a non-anticholinergic antiemetic agent. Pharmaceutical compositions containing high dose of nsPAChA for use for increasing the AChEI blood concentrations and for combating neurodegeneration are also described. The invention also relates to a method for inducing neuroprotection and combating neurodegeneration in a patient suffering from Alzheimer type dementia as well as to a method for increasing the blood levels of an acetyl choline esterase inhibitor (AChEI) in a human subject treated with an AChEI dose.
Owner:CHASE PHARMA CORP
Self-emulsifying agglomerant of oral polypeptide medicine
InactiveCN1456351APromote absorptionHas enzyme inhibitory effectPeptide/protein ingredientsPharmaceutical non-active ingredientsAdhesiveMicroemulsion
An oral-applied self-emulsifying polypeptide medicine is prepared from polypeptide, gel adhesive, surfactant, co-surfactant, oil enzyme inhibitor, and diluent. Its preparing process is also disclosed. It features that after it comes in intestinal tract, it is emulsified by itself to become nano-class microemulsion, which can penetrate through intestinal mucosa and epithelial barrier into lymph and blood, and be attached on intestinal inner surface to promote its absorption.
Owner:SHANGHAI INST OF PHARMA IND
Nitrosated and nitrosylated phosphodiesterase inhibitors, compositions and methods of use
The present invention describes novel nitrosated and / or nitrosylated phosphodiesterase inhibitors, and novel compositions containing at least one nitrosated and / or nitrosylated phosphodiesterase inhibitor, and, optionally, one or more compounds that donate, transfer or release nitric oxide, elevate endogenous levels of endothelium-derived relaxing factor, stimulate endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase and / or one or more vasoactive agents. The present invention also provides novel compositions containing at least one phosphodiesterase inhibitor, and one or more compounds that donate, transfer or release nitric oxide, elevate endogenous levels of endothelium-derived relaxing factor, stimulate endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase and / or one or more vasoactive agents. The present invention also provides methods for treating or preventing sexual dysfunctions in males and females, for enhancing sexual responses in males and females, and for treating or preventing diseases induced by the increased metabolism of cyclic guanosine 3′,5′-monophosphate (cGMP), such as hypertension, pulmonary hypertension, congestive heart failure, renal failure, myocardial infraction, stable, unstable and variant (Prinzmetal) angina, atherosclerosis, cardiac edema, renal insufficiency, nephrotic edema, hepatic edema, stroke, asthma, bronchitis, chronic obstructive pulmonary disease (COPD), cystic fibrosis, dementia, immunodeficiency, premature labor, dysmenorrhoea, benign prostatic hyperplasis (BPH), bladder outlet obstruction, incontinence, conditions of reduced blood vessel patency, e.g., postpercutaneous transluminal coronary angioplasty (post-PTCA), peripheral vascular disease, allergic rhinitis, glucoma, and diseases characterized by disorders of gut motility, e.g., irritable bowel syndrome (IBS).
Owner:NITROMED
Novel phosphodi esterase inhibitors
Owner:UNION THERAPEUTICS AS
Phosphodiesterase inhibitor treatment
ActiveUS20120178768A1Improve acuityGreat tasteBiocideSenses disorderDiseasePhosphodiesterase inhibitor
Methods and compositions are disclosed for the treatment of diseases or conditions produced by or associated with low cyclic nucleotide levels. The compositions comprise phosphodiesterase inhibitors and are formulated for intranasal and pulmonary administration.
Owner:CYRANO THERAPEUTICS INC
Dry powder formulation comprising a phosphodiesterase inhibitor
ActiveUS20150342936A1Improve liquidityImprove stabilityPowder deliveryBiocideCOPDPhosphodiesterase inhibitor
Pharmaceutical formulations in the form of inhalable dry powder comprising particles of a phosphodiesterase-4 inhibitor as active ingredient are useful for the prevention and / or treatment of respiratory diseases, such as asthma and COPD.
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00001.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00002.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00003.png)