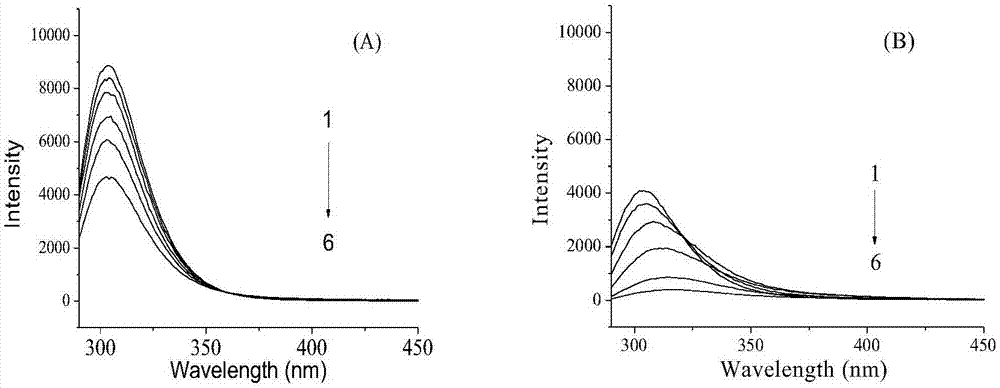
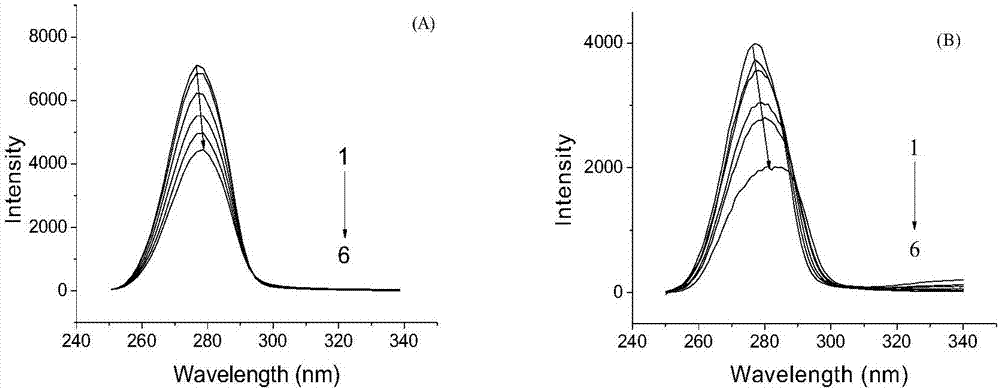
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Epithelial barrier" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
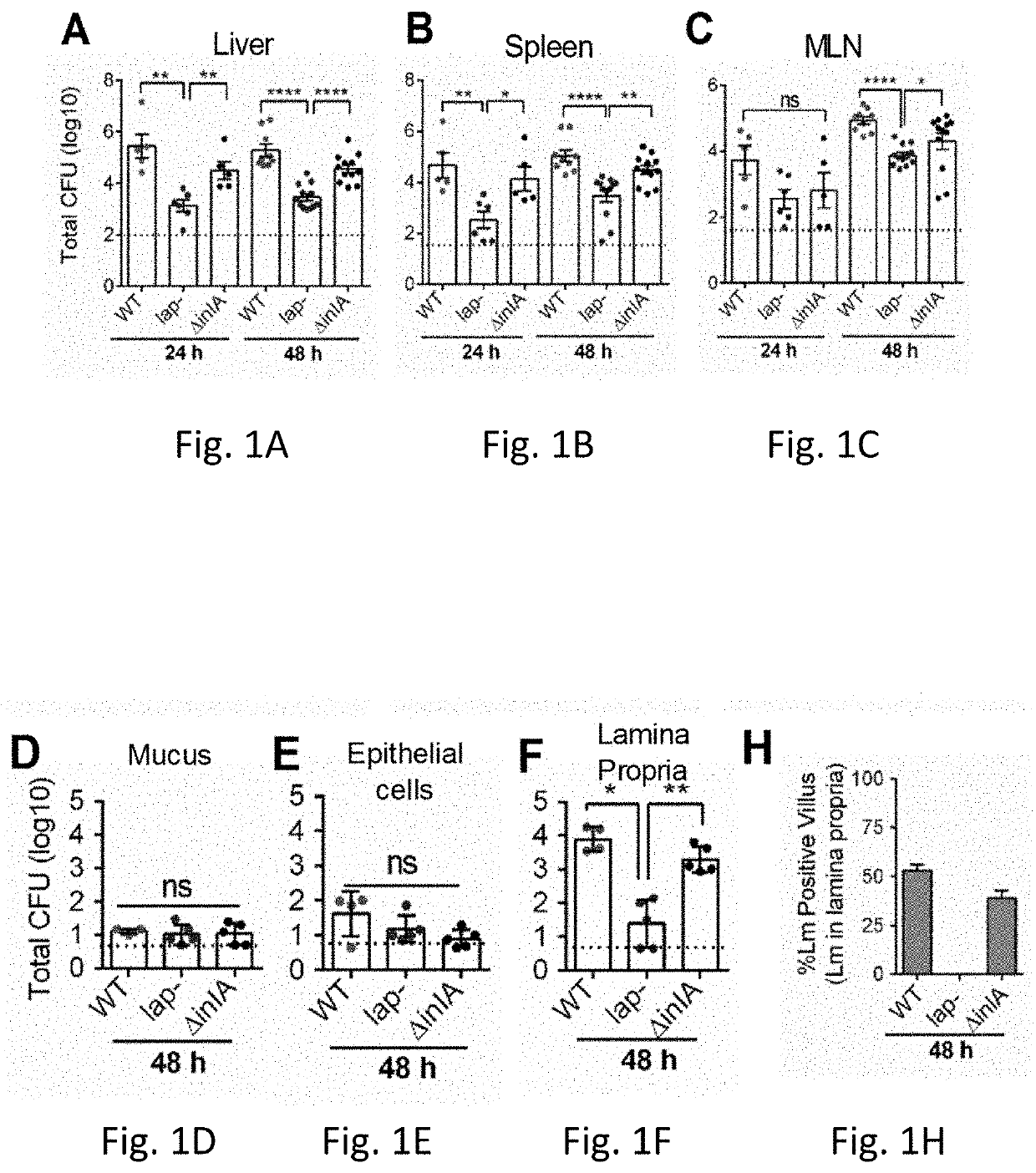
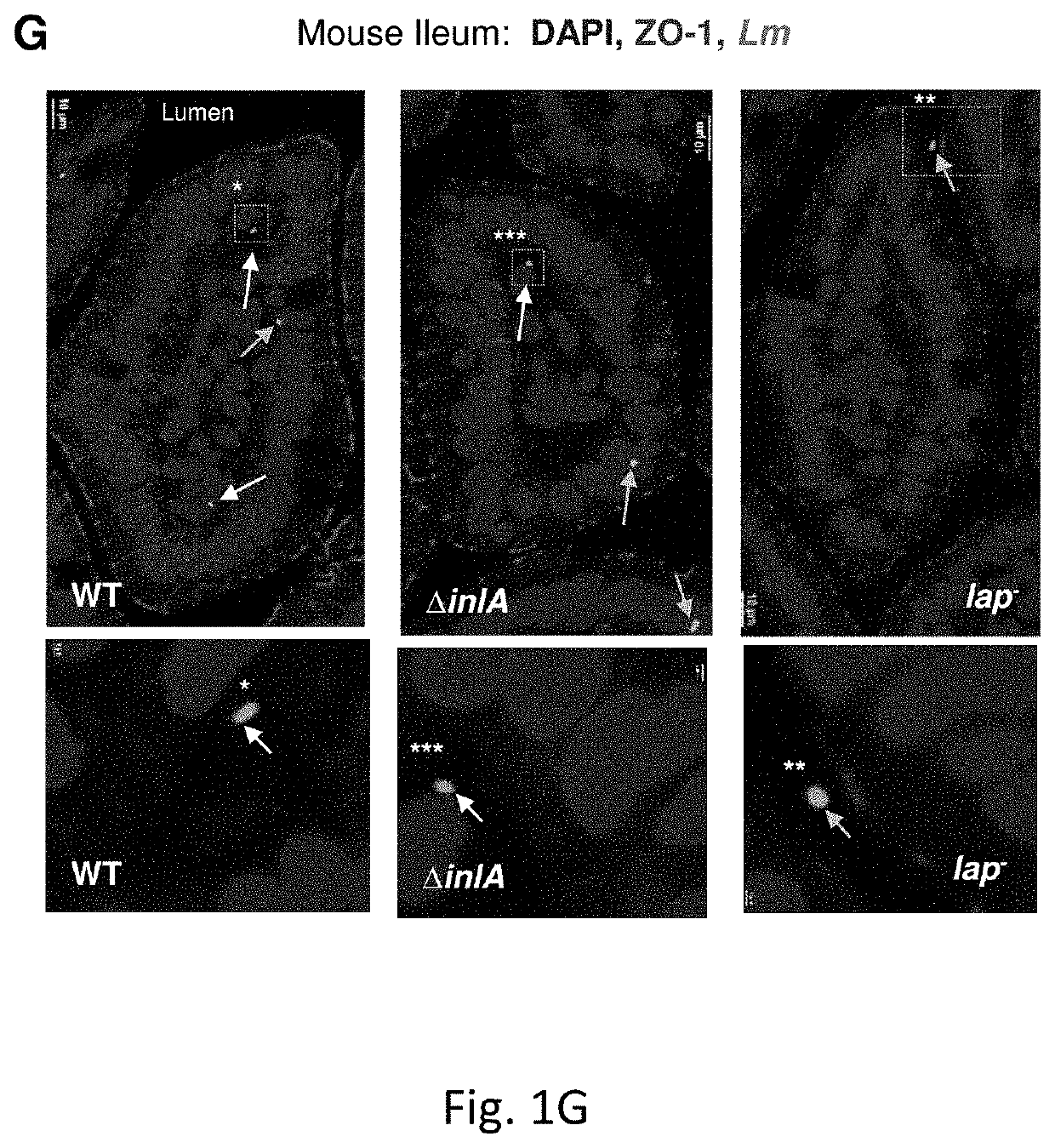
Epithelia form barriers that are essential to life. This is particularly true in the intestine, where the epithelial barrier supports nutrient and water transport while preventing microbial contamination of the interstitial tissues. Along with plasma membranes, the intercellular tight junction is the primary cellular determinant...
Lipid particles and suspensions and uses thereof
The present invention relates to formulations and methods for the mucosal and parenteral administration of lipid particles an suspensions. The formulations of this invention are stable lipid particles useful for oral delivery of water-insoluble therapeutic agents, vaccines and diagnostics. The compositions of this invention promote the mucosal absorption of biologically active molecules across mucosal epithelial barriers. Stabilization of lipid particles is achieved by coating the hydrophobic central core with a polymer shell. The polymer shell can include bioadhesive agents, ligands, and absorption promoting agents. This invention relates to oral drug delivery systems for hydrophobic drugs, and in particular is concerned with improving the bioavailability of hydrophobic drugs from such systems. Using this system, anticancer drugs such as taxanes are orally effective.
Owner:CONSTANTINIDES PANAYIOTISP +2
Cell culture array system for automated assays and methods of operation and manufacture thereof
ActiveUS20090203126A1Elimination of and connectorBioreactor/fermenter combinationsBiological substance pretreatmentsEpithelial barrierMultiplexing
A number of novel improved microfluidic configurations and systems and methods of manufacture and operation. In one embodiment, three wells are used for independent cell culture systems in a cell culture array. In a second aspect, artificial sinusoids with artificial epithelial barriers are provided with just one (optionally shared or multiplexed) fluidic inlet and one (optionally shared or multiplexed) fluidic output, where the medium output also functions as a cellular input. A pneumatic cell loader combined with other components provides a fully automated cell culture system. Magnetic alignment of plate molds provides advantages and ease of molded manufacture.
Owner:MILLIPORE CORP
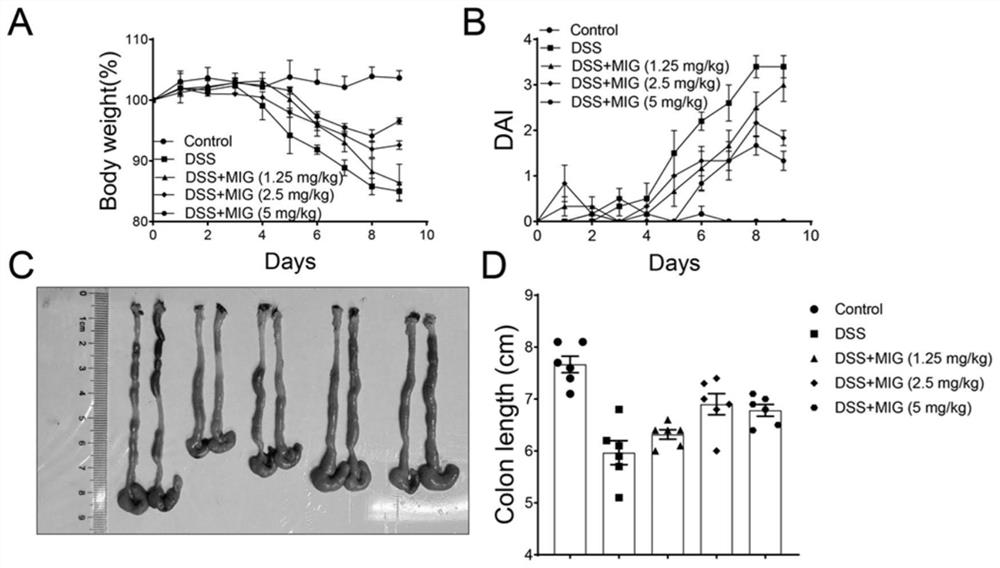
Excellent lactobacillus reuteri for preventing ulcerative colitis
InactiveCN110591945APrevent effectPromote infiltrationBacteriaDigestive systemUlcerative colitisDisease activity
The present invention provides lactobacillus reuteri for preventing ulcerative colitis. The strain is named as lactobacillus reuteri RAM0101 and deposited on May 27, 2019 at China General Microbiological Culture Collection Center and has a preservation number of CGMCC NO.17853. C57BL / 6N mice are used as test objects, dextran sodium sulfate (DSS) is used to induce the mice to form an ulcerative colitis model and the model is also used to investigate effectiveness of the strain in preventing the ulcerative colitis. The lactobacillus reuteri can relieve weight loss, diarrhea, hematochezia and disease activity index (DAI) score of the ulcerative colitis mice induced by the DSS, protects pathological damages of colon tissues of the mice induced by the DSS, can enhance colon tissue epithelial barrier completeness and is also free of toxin and harms.
Owner:JILIN UNIV
Resorption Enhancers As Additives To Improve The Oral Formulation Of Non-Anticoagulant Sulfated Polysaccharides
ActiveUS20130035288A1Enhancing coagulation of bloodOrganic active ingredientsBiocideEpithelial barrierSulfated polysaccharides
Aspects of the invention include methods for enhancing blood coagulation in a subject. In practicing methods according to certain embodiments, an amount of a non-anticoagulant sulfated polysaccharide (NASP) in combination with a gastrointestinal epithelial barrier permeation enhancer is orally administered to a subject in a manner sufficient to enhance blood coagulation in the subject. Compositions and kits for practicing methods of the invention are also described.
Owner:TAKEDA PHARMA CO LTD
Cell Culture Array System For Automated Assays And Methods Of Operation And Manufacture
ActiveUS20160289623A1Elimination of and connectorBioreactor/fermenter combinationsBiological substance pretreatmentsEpithelial barrierAssay
A number of novel improved microfluidic configurations and systems and methods of manufacture and operation. In one embodiment, three wells are used for independent cell culture systems in a cell culture array. In a second aspect, artificial sinusoids with artificial epithelial barriers are provided with just one (optionally shared or multiplexed) fluidic inlet and one (optionally shared or multiplexed) fluidic output, where the medium output also functions as a cellular input. A pneumatic cell loader combined with other components provides a fully automated cell culture system. Magnetic alignment of plate molds provides advantages and ease of molded manufacture.
Owner:EMD MILLIPORE CORP
Cell culture array system for automated assays and methods of operation and manufacture thereof
ActiveUS9376658B2Elimination of and connectorBioreactor/fermenter combinationsBiological substance pretreatmentsEpithelial barrierMultiplexing
A number of novel improved microfluidic configurations and systems and methods of manufacture and operation. In one embodiment, three wells are used for independent cell culture systems in a cell culture array. In a second aspect, artificial sinusoids with artificial epithelial barriers are provided with just one (optionally shared or multiplexed) fluidic inlet and one (optionally shared or multiplexed) fluidic output, where the medium output also functions as a cellular input. A pneumatic cell loader combined with other components provides a fully automated cell culture system. Magnetic alignment of plate molds provides advantages and ease of molded manufacture.
Owner:MILLIPORE CORP
Rat-pig intestinal epithelial cell integrated model established by lipopolysaccharide (LPS) stimulation
InactiveCN104031878AMitigation structureMitigation of structural changesMicrobiological testing/measurementVertebrate cellsRat intestineIn vivo
The invention discloses a rat-pig intestinal epithelial cell integrated model established by lipopolysaccharide (LPS) stimulation. Bacterial lipopolysaccharide (LPS) has a function in damaging intestinal epithelial barrier, and the integrated model disclosed by the invention aims at identifying whether an antimicrobial peptide Cathelicidin-BF (C-BF) derived from a snake has a protective effect on LPS-induced enteritidis or not. IPEC-J2 is an intestinal epithelial cell line isolated from the porcine jejunum segment and the porcine digestive system is very similar to the human digestive system. By in vivo rat model and in vitro porcine IPEC-J2 cell model, the protective effect of Cathelicidin-BF on impaired LPS-induced intestinal barrier function is tested. The test results show that the Cathelicidin-BF has protective effects on impaired LPS-induced intestinal barrier function and LPS-induced IPEC-J2 cell damage model.
Owner:ZHEJIANG UNIV
Eye drop for treating eye dryness
ActiveCN107049938AProtect epithelial barrier functionRecovery quantityOrganic active ingredientsSenses disorderConjunctivaEye dryness
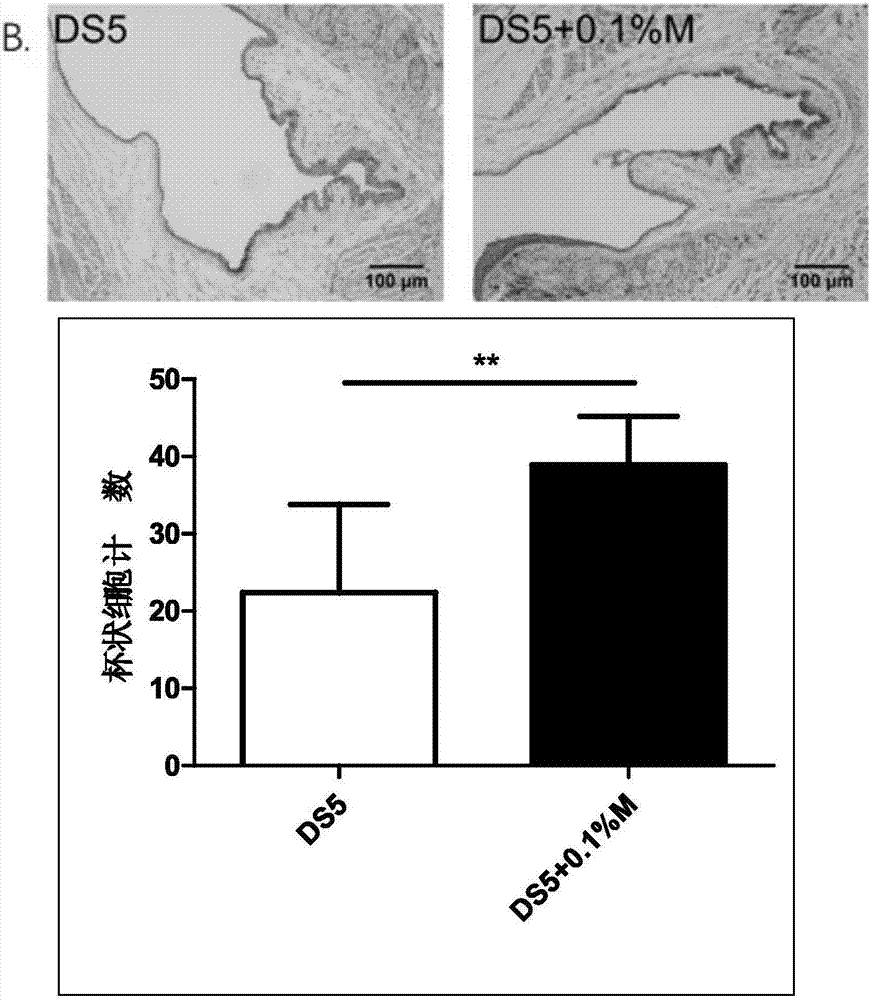
The invention discloses an eye drop for treating eye dryness. The pH value of the eye drop is 5.0-7.0, the eye drop comprises effective components of 5-hydroxy-1-beta-D-furanose-1H-imidazole-5-carboxamide, and specifically the eye drop consists of the following components in percentage by weight: 0.05-0.1% of 5-hydroxy-1-beta-D-furanose-1H-imidazole-5-carboxamide, a proper amount of a pH value adjusting agent, 0.01-3% of an iso-osmotic agent, 0.003-0.5% of a bacteriostatic agent, 0.001-0.5% of a stabilizer, 0.01-0.5% of a tackifier, 2-5% of a solubilizer and the balance of water. Through partial treatment of the eye drop, epithelial barrier functions of dry eye mouse cornea can be protected, the number of goblet cells of cornea can be recovered, and relatively good anti-inflammatory and immunity inhibition effects can be achieved.
Owner:EYE MEDICAL XIAMEN BIOTECHNOLOGY CO LTD
Method for enhanced ocular drug penetration
InactiveUS20070117750A1Improve breathabilityImprove permeabilityBiocideSenses disorderEpithelial barrierCo administration
Provided is a method for enhanced intraocular drug penetration that comprises the co-administration of ocular therapeutics with agents that increases the permeability of ocular and periocular vessels and ocular epithelial barriers. Due to its unique and novel concept and additive nature, the method of the present invention can be used in combination with previous methods for enhancement of ocular drug penetration.
Owner:INSERM TRANSFERT
Preparation method of short amylase-insulin or short amylase-insulin-procyanidine nanocomposite
ActiveCN107213457AInhibit aggregationSmall particle sizePeptide/protein ingredientsMetabolism disorderAmylaseNanocomposite
The invention discloses a preparation method of short amylase-insulin or short amylase-insulin-procyanidine nanocomposite. The preparation method comprises the steps of mixing short amylase with insulin or mixing short amylase, insulin and procyanidine, and carrying out retrogradation, so as to obtain short amylase-insulin or short amylase-insulin-procyanidine nanocomposite. The short amylase-insulin or short amylase-insulin-procyanidine nanocomposite is relatively small in particle size and can tightly adhere to a membrane adhesion layer of a small intestine, so that the standing time in the stomach and intestine is prolonged, meanwhile, a mucous layer barrier, an enzyme barrier and an epithelial barrier existing in an alimentary system can be overcome, and the bioavailability of oral insulin is increased.
Owner:JIANGNAN UNIV
Construction method for human intestinal tract epithelial cell model
ActiveCN110628702AAccurate responseClear responseGastrointestinal cellsEpidermal cells/skin cellsHuman body3D cell culture
The invention discloses a construction method for a human intestinal tract epithelial cell model, and relates to the technical field of cell model construction. The human intestinal tract epithelial cell model is characterized in that a Caco-2 cell is taken as a model cell, and a DMEM (dulbecco's modified eagle medium) containing 20%FBS (fetal calf serum) is used for carrying out 3D culture on theCaco-2 cell in a micro-fluidic chip so as to construct the in vitro model of the human intestinal tract epithelial cell. On one hand, a human cell is adopted for culture, the problem that an experiment result is in accurate since an animal cell is adopted for culture is avoided, and on the other hand, the 3D cell culture enables intestinal tract cells to own a mechanical microenvironment which ismore similar to the human physiology, so that intestinal tract cells can be accelerated to differentiate. Therefore, the human intestinal tract epithelial cell model constructed by the construction method disclosed by the invention can more clearly and accurately reflect the characteristics of small intestine epithelial barriers.
Owner:WUHAN CHOPPER BIOLOGY
Targeting oxazole structures for therapy against inflammatory diseases
InactiveUS20190225683A1Prevent and inhibitImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisEpithelial barrierColitis
Described herein are novel compositions, targeted therapeutic methods, and assays for neutralizing and / or inhibiting the activity of “oxazole-containing (OxC) compounds,” to prevent or delay the onset of epithelial barrier dysfunction and chronic inflammation associated with various disorders, such as colitis.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Oral and injection dual-effect protein or polypeptide drug controlled-release vesicle and preparation method thereof
InactiveCN103720656ASimple preparation processMild preparation conditionsMacromolecular non-active ingredientsLiposomal deliveryIntraperitoneal routeMicrosphere
The invention discloses an oral and injection dual-effect polypeptide drug controlled-release preparation and a preparation method thereof. Moreover, the insulin controlled-release preparation can continuously display the medicinal effect in vivo for a long time in a stable manner. The oral and injection dual-effect polypeptide drug controlled-release preparation uses a non-toxic silica material with superstability as a carrier; polypeptide drug molecules are embedded into the carrier to form nanoscale microspheres; the nanoscale polypeptide drug microspheres have an average particle size of about 200nm. One layer of adhesion molecules is adsorbed to the surfaces of the nanoscale microspheres under the action of electrostatic adsorption, so that the drug nanoscale microspheres can be easily adhered to the wall of the small intestine and detention time is prolonged, so that more polypeptide drug molecule nanoscale microspheres permeate the intestinal mucosa and enter the blood circulation and the lymphatic system by intra-intestinal cells through an epithelial barrier; in the carrier manner, no matter intraperitoneal injection or oral administration, the oral and injection dual-effect polypeptide drug controlled-release preparation can take a good treatment effect so as to display high bioavailability and a continuous medicinal effect.
Owner:PEKING UNIV
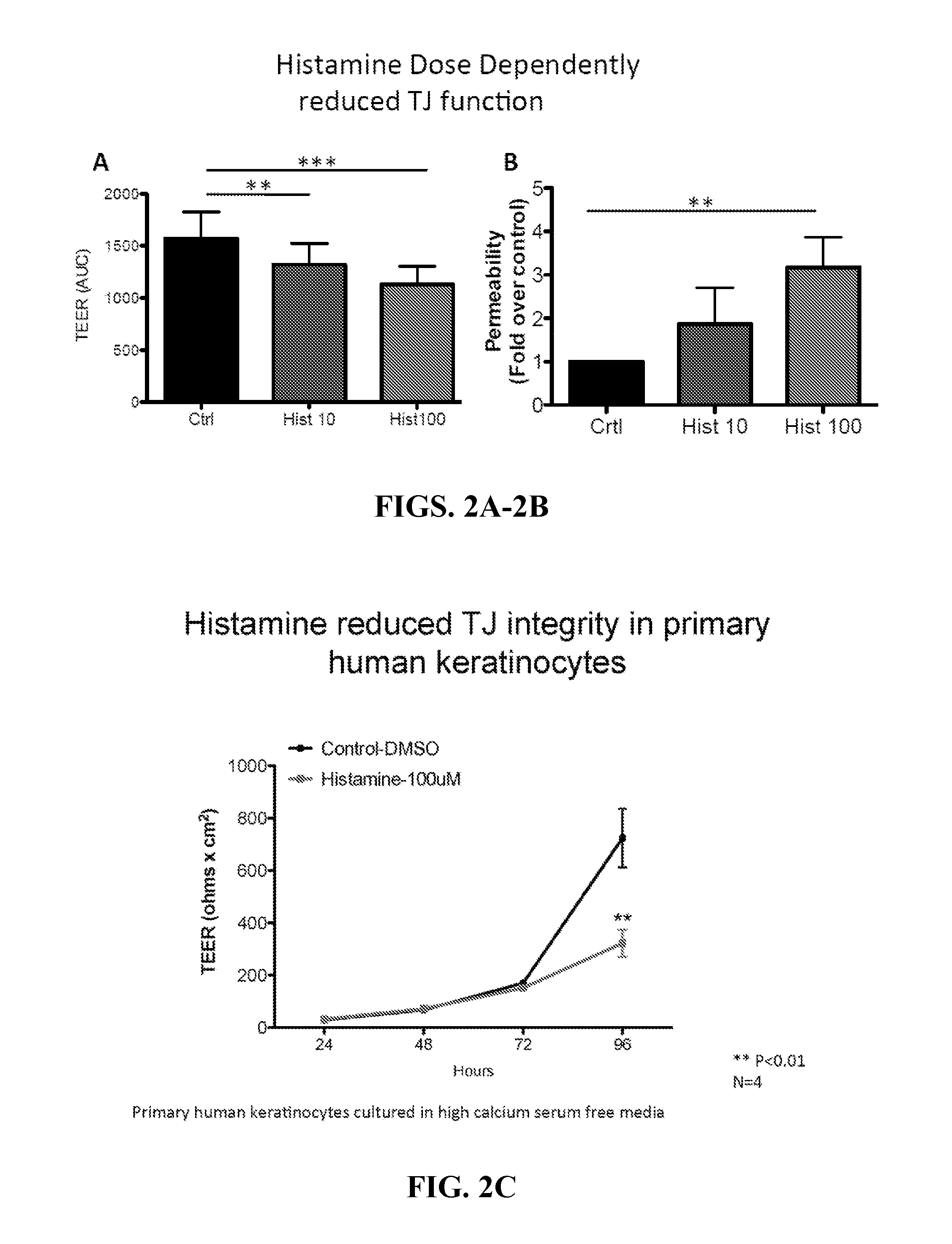
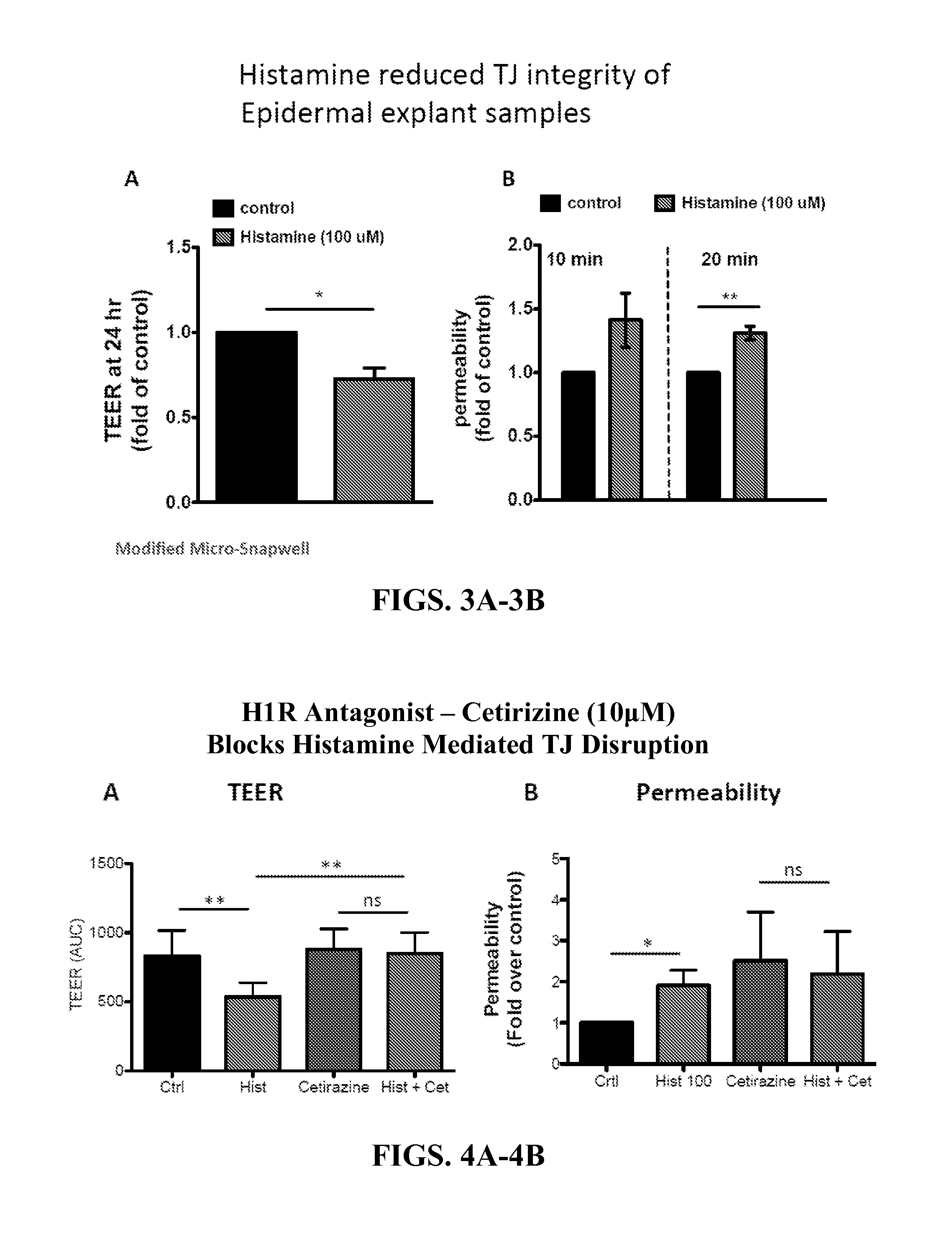
Methods of using histamine receptor agonists and antagonists
InactiveUS20160000777A1Promoting epithelial functionEnhances tight junction formationOrganic active ingredientsBiocideAntigenEpithelial barrier
This invention relates to a transdermal drug formulation that includes a pharmaceutically suitable carrier; an effective amount of a therapeutic agent; and a histamine type 4 receptor (“H4R”) agonist, as well as a transdermal vaccine formulation that includes a pharmaceutically suitable carrier; an effective amount of an antigen or antigen-encoding nucleic acid molecule present in the carrier, and optionally one or more adjuvants; and an H4R agonist. The present invention also relates to transdermal delivery device including such formulations and methods of administering such formulations. The present invention also relates to methods of enhancing epithelial barrier formation in a patient involving administering to the patient at a site of epithelial disruption an amount of a formulation that comprises an H4R antagonist. The present invention also relates to a method of inhibiting pathogen infection or local spread of infection in the epithelia using an H4R antagonist, an H1R antagonist, or a combination thereof.
Owner:UNIVERSITY OF ROCHESTER
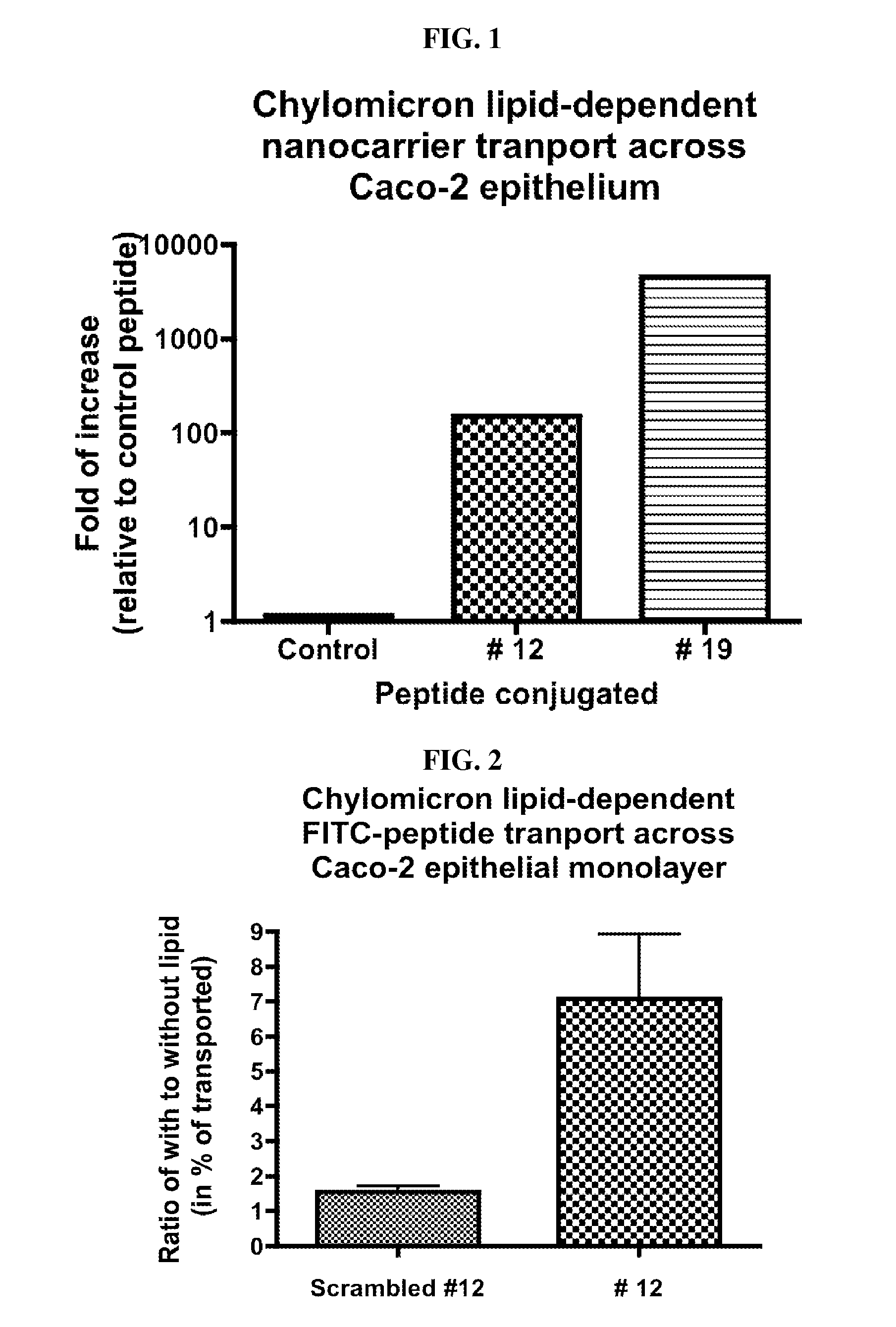
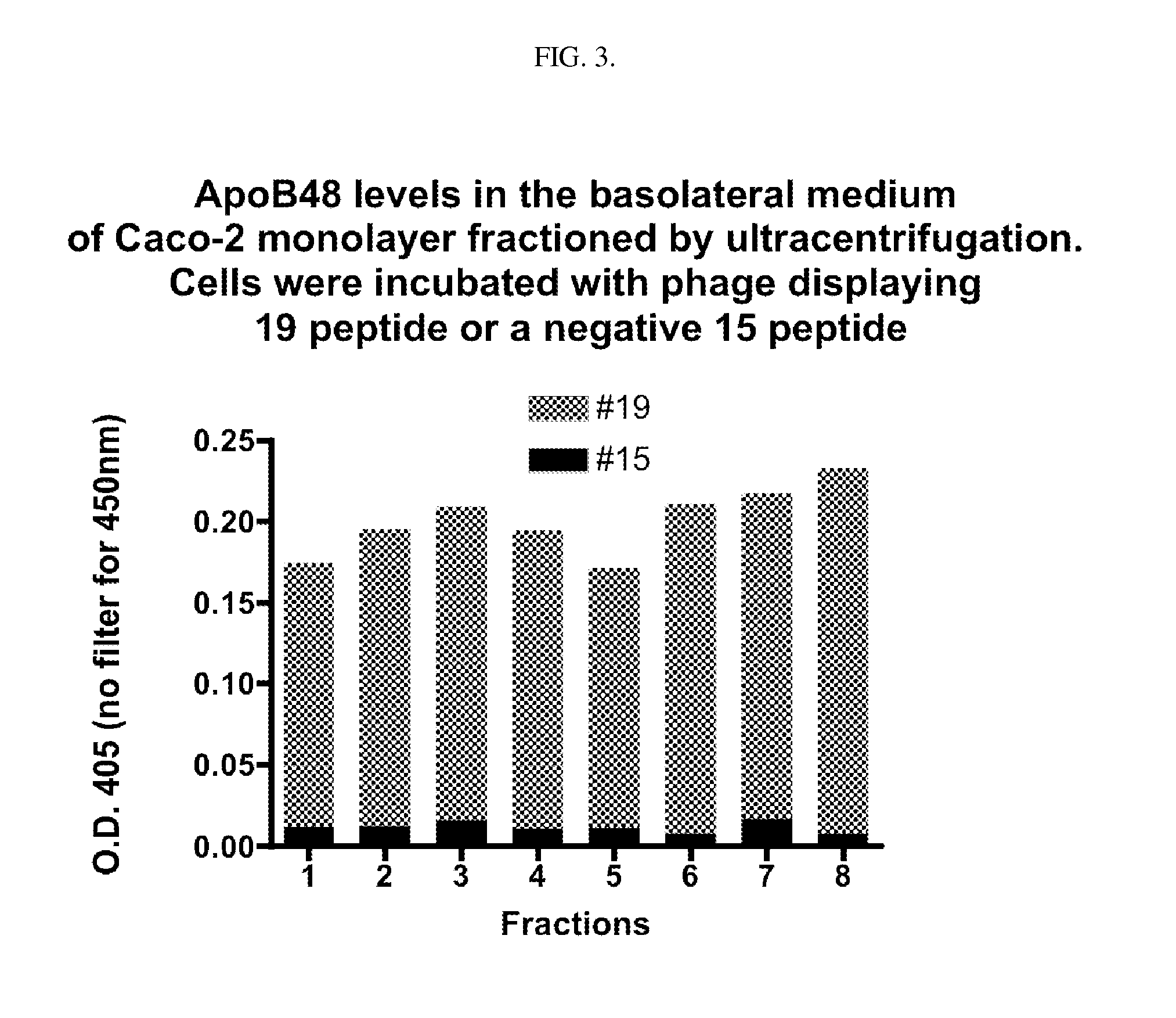
Intestinal peptide targeting ligands
Peptide ligands for transporting therapeutic agents across the intestinal epithelial barrier that ordinarily are inadequately absorbed and must be delivered by alternative means, which contain an isolated amino acid sequence wherein at least one pair of amino acids are of an opposite charge and the pair members are separated by a spacer of 1-12 amino acid residues including at least one hydrophobic amino acid, and wherein the length of the amino acid sequence is greater than 5 and less than 20 amino acids. Pharmaceutical compositions for gastro-intestinal delivery and methods for the gastrointestinal delivery of poorly absorbed therapeutic agents are also disclosed.
Owner:RUTGERS THE STATE UNIV
Excellent clostridiumbutyricum for preventing ulcerative colitis
InactiveCN110591944APromote infiltrationControl weight lossBacteriaAntipyreticNon toxicityDisease activity
The invention provides a strain of clostridiumbutyricum for preventing ulcerative colitis. The strain is preserved in CGMCC (China General Microbiological Culture Collection Center) on May 27, 2019, and the preservation number is CGMCC NO.17854. A C57BL / 6N mouse is used as an experiment object; DSS (Dextran Sulfate Sodium) is used for inducing the mouse to form an ulcerative colitis model; and themodel is used for investigating the effectiveness of the strain of the invention for preventing the ulcerative colitis. The clostridiumbutyricum provided by the invention can be used for effectivelyrelieving the body weight loss, diarrhea and hematochezia of the mouse with the ulcerative colitis induced by the DSS and effectively improving the DAI (Disease Activity Index) grade; the mouse colontissue pathologic lesion of the mouse induced by DSS can be protected; the epithelial barrier completeness of the colon tissues can be enhanced; and non-toxicity and harmlessness are realized.
Owner:JILIN UNIV
Intestinal peptide targeting ligands
ActiveUS8791234B2Easy to transportPoor gastrointestinal absorbtionPeptide/protein ingredientsPeptide preparation methodsIntestinal structureEpithelial barrier
Peptide ligands for transporting therapeutic agents across the intestinal epithelial barrier that ordinarily are inadequately absorbed and must be delivered by alternative means, which contain an isolated amino acid sequence wherein at least one pair of amino acids are of an opposite charge and the pair members are separated by a spacer of 1-12 amino acid residues including at least one hydrophobic amino acid, and wherein the length of the amino acid sequence is greater than 5 and less than 20 amino acids. Pharmaceutical compositions for gastro-intestinal delivery and methods for the gastrointestinal delivery of poorly absorbed therapeutic agents are also disclosed.
Owner:RUTGERS THE STATE UNIV
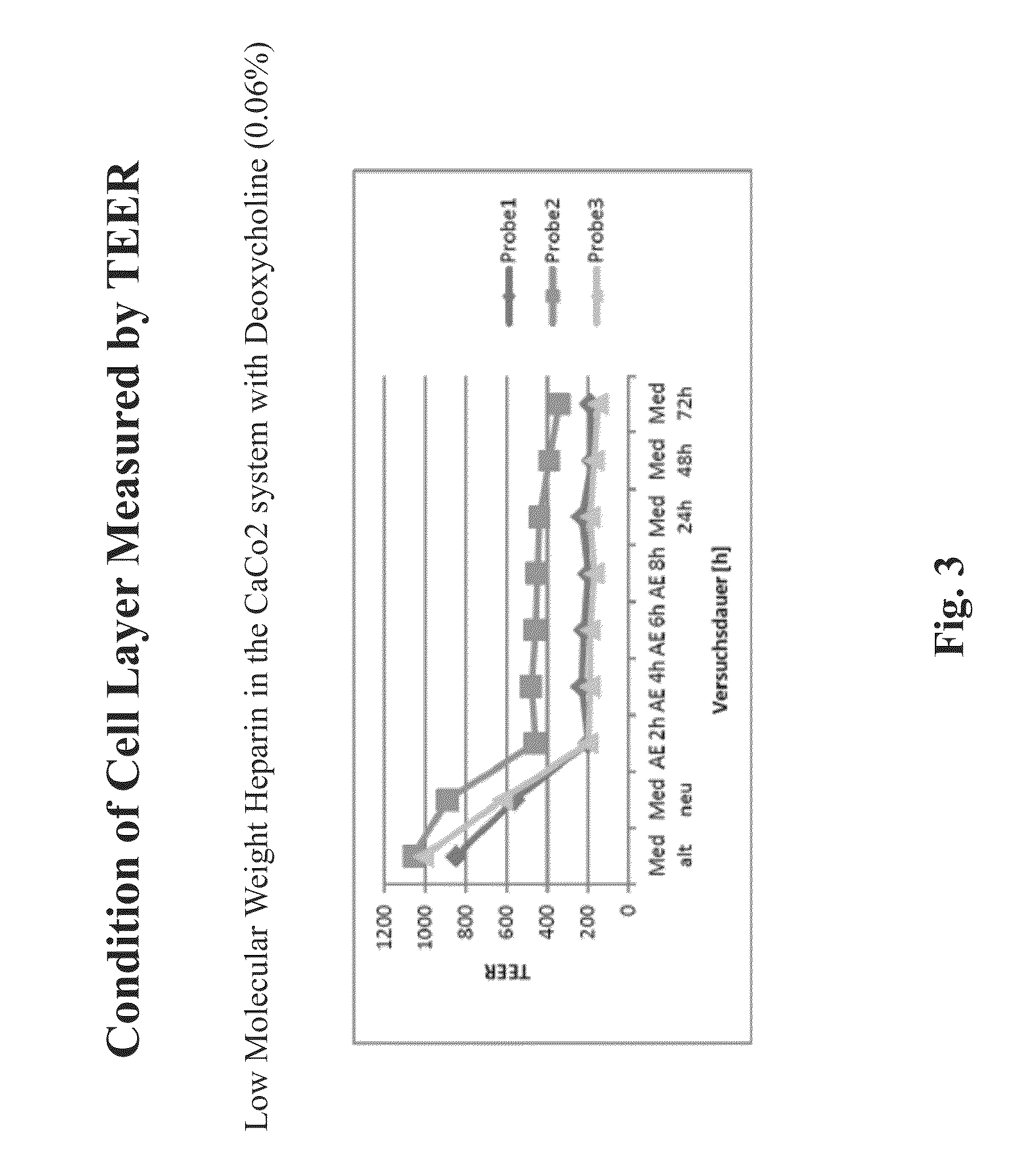
Resorption enhancers as additives to improve the oral formulation of low molecular weight heparins
ActiveUS20140271608A1Improve permeabilityOrganic active ingredientsPeptide/protein ingredientsEpithelial barrierPermeation
Aspects of the invention include methods for treating or preventing a thromboembolic disease in a subject. In practicing methods according to certain embodiments, an amount of a low molecular weight heparin (LMWH) and a synergistically effective combination of two or more gastrointestinal epithelial barrier permeation enhancers is orally administered to a subject in a manner sufficient to treat the thromboembolic disease in the subject. Compositions and kits for practicing methods of the invention are also described.
Owner:TAKEDA PHARMA CO LTD
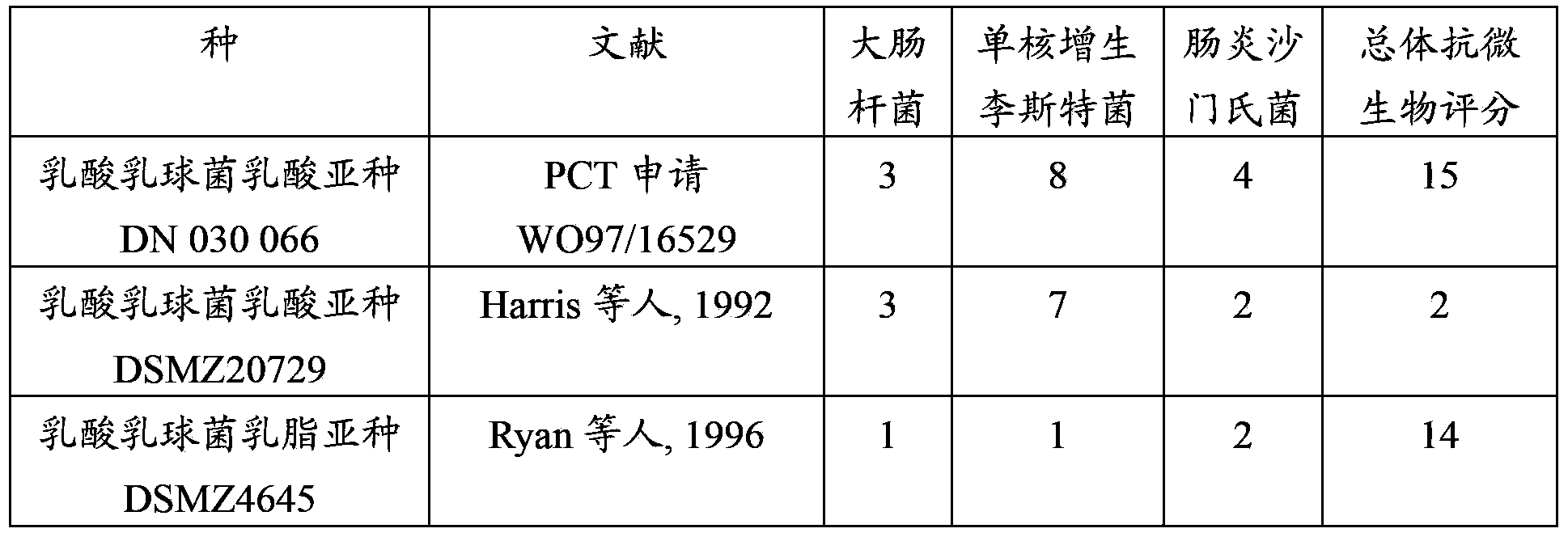
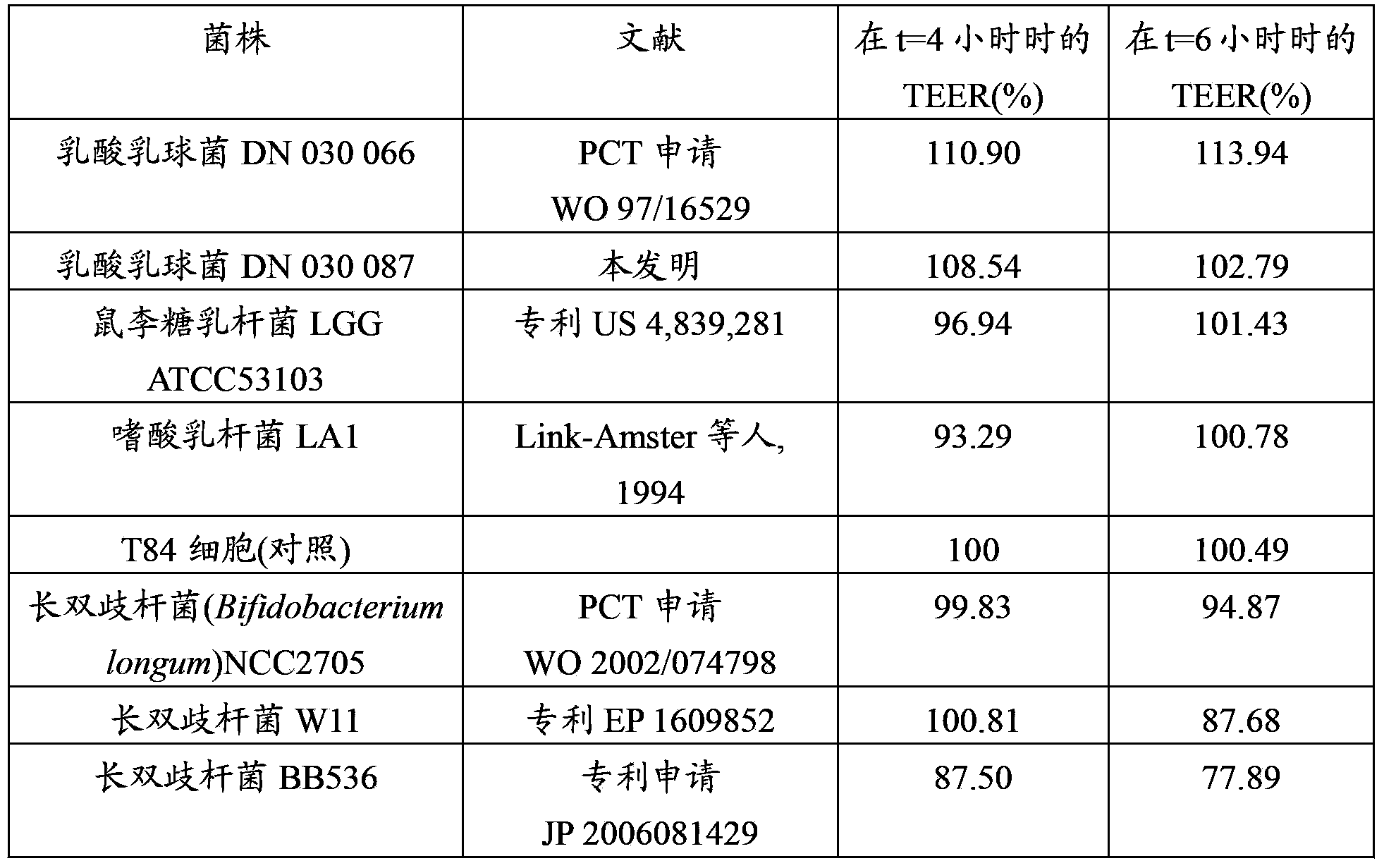
Lactococcus lactis strains for use in improving digestive condition
The present invention concerns strains of Lactococcus lactis capable of inhibiting the growth of a pathogenic microorganism and / or improving the intestinal epithelial barrier integrity. These strains are suitable for use in the treatment or prevention of a digestive disorder.
Owner:DANONE
Construction method for dry eye model in vitro
ActiveCN101508974AEasy to makeGood repeatabilityVertebrate cellsArtificial cell constructsConjunctival EpitheliumMetaplasia
A method for constructing a xerophthalmia model in vitro relates to a xerophthalmia model. A method for constructing a xerophthalmia model in vitro with easy operation, good repeatability, lower cost than animal model and a plurality of similar histopathological characteristics similar to xerophthalmia and application thereof in simulating, studying and preventing xerophthalmia are provided. The conjunctiva tissue containing conjunctival epithelium and conjunctival submucosa is cut and placed in culture media; the conjunctiva tissue is trimmed into a conjunctiva tissue block which is placed on a culture vessel inserter coated by type I collagen, with conjunctival epithelium being upward; the culture media are added into the culture vessel outside the inserter to cause the gas-liquid interface of the culture media to be at the junction of the conjunctival epithelium and conjunctival submucosa of the conjunctiva tissue block; and the conjunctiva tissue block is placed into a culture tank to be cultured. The xerophthalmia model in vitro can be used for such researches as xerophthalmia squamous epithelium metaplasia, ocular surface epithelial barrier breakdown, ocular surface epithelial mucoprotein change, and function of the participation and atopsis of inflammatory mediator in xerophthalmia diseases in the generation and development of xerophthalmia.
Owner:福建和泽生物科技有限公司
Application of magnesium isoglycyrrhizinate to preparation of medicine for treating colitis
InactiveCN112043718AConvenient for clinical operationEasy to prepareOrganic active ingredientsAntipyreticInflammatory factorsDisease
The invention belongs to the technical field of pharmacy, and relates to an application of magnesium isoglycyrrhizinate to preparation of a medicine for treating colitis. In a disease model of mouse acute and chronic enteritis induced by dextran sodium sulfate DSS, the magnesium isoglycyrrhizinate can effectively improve the conditions of weight loss, diarrhea, hemafecia and colonic shortening ofmice, reduce inflammatory cell infiltration and the expression level of inflammatory factors in the colons of the mice, maintain the intestinal epithelial barrier integrity, increase connexins Zo-1, occludin and E-caderin, reduce the Claudin-2 expression level, and reduce fibrosis caused by colitis. It is found for the first time that the magnesium isoglycyrrhizinate can treat colitis, clinical application of the magnesium isoglycyrrhizinate is increased, and remarkable popularization value is achieved.
Owner:南京澳特飞吉生物科技有限公司
Microelement-loaded yeast micro-nano robot sugar pill and preparation method thereof
ActiveCN114404386AEfficient drug deliveryImprove drug delivery efficiencyMetabolism disorderPharmaceutical non-active ingredientsBiotechnologyEpithelial barrier
The invention provides a trace element-loaded yeast micro-nano robot sugar pill, which is characterized by comprising an external polysaccharide coat and at least one internal yeast micro-nano robot, the yeast micro-nano biological robot is composed of yeast cells, biological enzyme and trace elements, wherein the yeast cells are half-covered with the biological enzyme, and the trace elements are loaded in the yeast cells. The polysaccharide coat of the yeast micro-nano robot sugar pill can be degraded in the intestinal tract to release a yeast micro-nano robot carrying trace elements, and the yeast micro-nano robot can utilize glucose in the intestinal tract as power to autonomously move, so that the yeast micro-nano robot can penetrate through mucus barriers, epithelial barriers and other barriers to reach the intestinal wall, and absorption of the organism to the trace elements is efficiently promoted.
Owner:SHENZHEN INST OF ADVANCED TECH
Choroid plexus organoids and methods for production thereof
PendingUS20220106571A1Same selectivityDrug screeningNervous system cellsEpithelial barrierEpithelium
Methods and materials relating to cultured choroid plexus organoids comprising: (a) an epithelium comprising a tight epithelial barrier; and / or (b) one or more cysts surrounded by an epithelium, plus other authentic features and markers.
Owner:UK RES & INNOVATION LTD
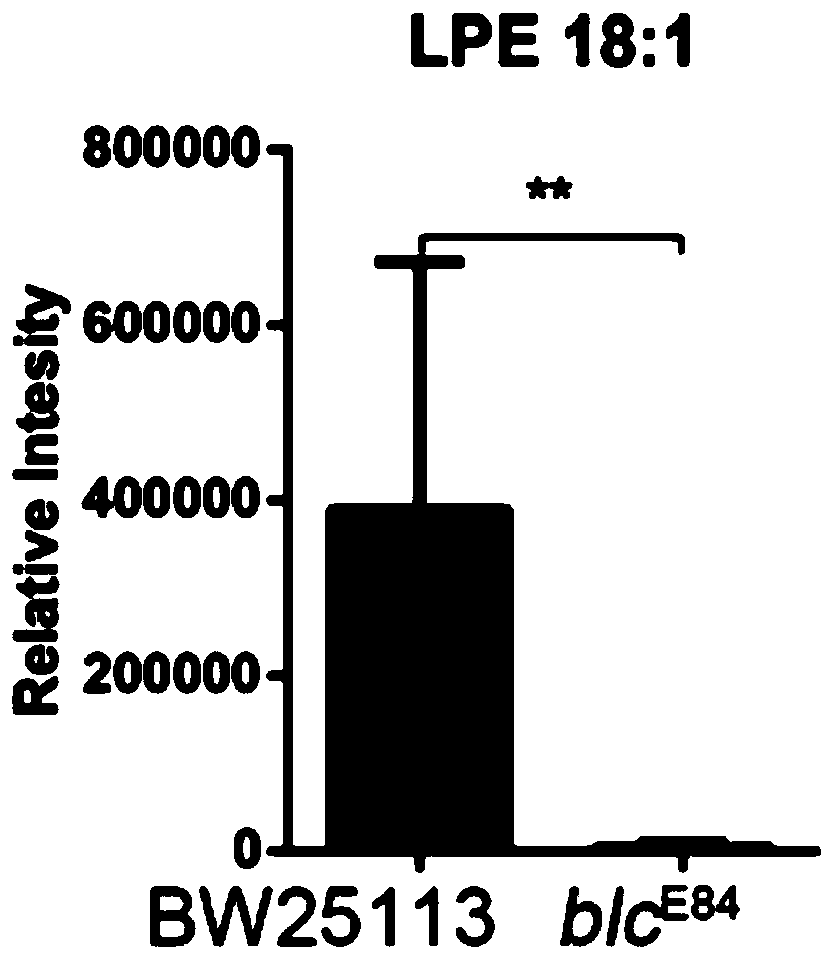
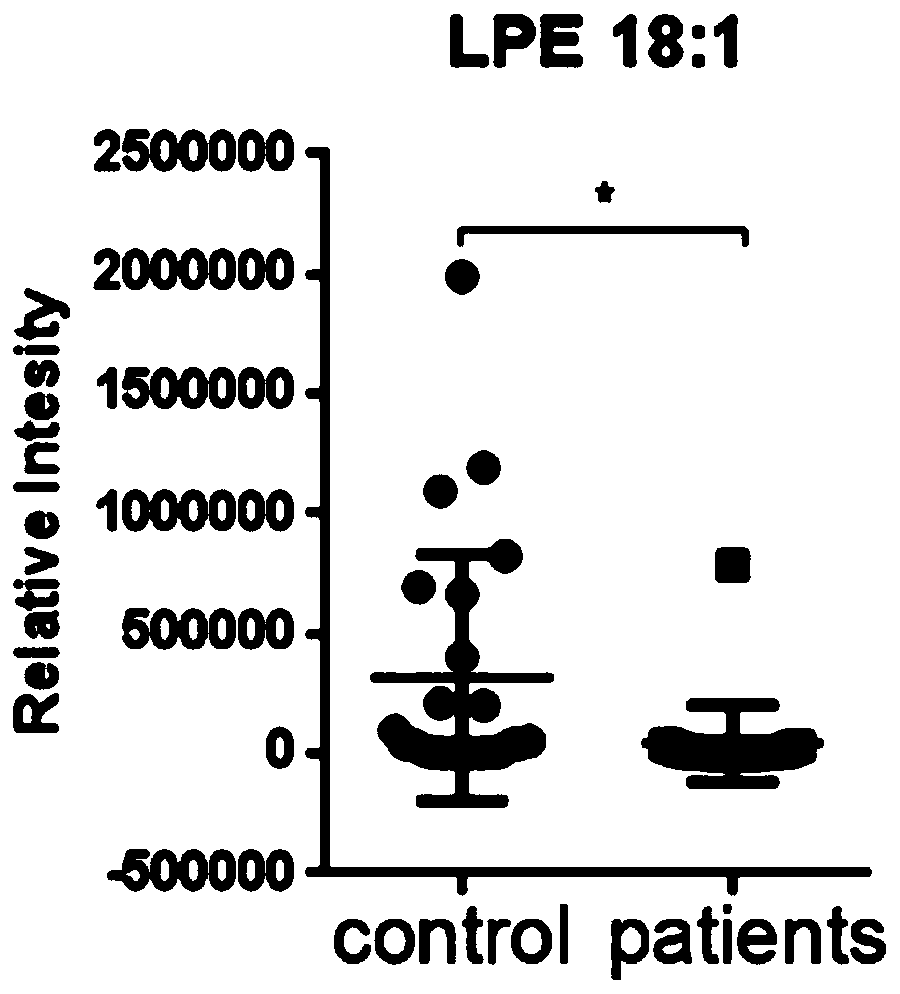
Application of lysophosphatidylethanolamine 18: 1 in preparing drug for relieving and treating inflammatory bowel diseases
InactiveCN110840901AOrganic active ingredientsAntipyreticEpithelial barrierLysophosphatidylethanolamine
The invention belongs to the field of medicine and relates to an application of lysophosphatidylethanolamine 18: 1 in preparing a drug for relieving and treating inflammatory bowel diseases. The invention discloses that lysophosphatidylethanolamine 18: 1 alleviates or treats enteritis and inflammatory bowel diseases by increasing the stability of intestinal epithelial barrier. The lysophosphatidylethanolamine 18: 1 is an effective drug for the treatment of inflammatory bowel diseases.
Owner:NANJING UNIV
Compositions and methods for treating or preventing hyperglycemia, insulin resistance, and associated organ damage
InactiveUS20210069286A1Reduced dysfunctionImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderRegimenPancreatic hormone
The present invention provides compositions and methods for treating hyperglycemia, insulin resistance, and associated organ damage, including in some embodiments diabetes mellitus (type 1 or 2), metabolic syndrome, obesity, fatty liver diseases, or kidney disease. In various embodiments, the invention involves administering a regimen of larazotide (or a derivative of larazotide) to a subject. In various embodiments, the regimen reduces dysfunction of the gastrointestinal epithelial barrier, thereby improving glycemic control. In various embodiments, the regimen of larazotide improves the effectiveness of conventional pharmaceutical interventions for hyperglycemia or diabetes mellitus.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +1
Peptide-mediated drug delivery across epithelial barrier
ActiveUS10632208B2Organic active ingredientsPeptide/protein ingredientsEpithelial barrierPharmaceutical formulation
Owner:PURDUE RES FOUND INC
Adult and neonatal stem cell therapy to treat diabetes through the repair of the gastrointestinal tract
ActiveUS9101570B1Increase glucose levelsRegeneration of the insulin producing capacity of the pancreasPharmaceutical delivery mechanismMammal material medical ingredientsIntestinal structureMetabolite
The anatomic and functional arrangement of the gastrointestinal tract suggests an important function of this organ is its ability to regulate the trafficking of metabolites as well as control the equilibrium between tolerance and immunity through gut-associated lymphoid tissue, the neuroendocrine network, and the intestinal epithelial barrier. Combining nucleated cells from various tissues and introducing them directly into the small intestine will have a positive effect on diabetes.
Owner:ENDOCELLUTIONS
Construction method for dry eye model in vitro
ActiveCN101508974BEasy to makeGood repeatabilityArtificial cell constructsVertebrate cellsConjunctival EpitheliumDisease
A method for constructing a xerophthalmia model in vitro relates to a xerophthalmia model. A method for constructing a xerophthalmia model in vitro with easy operation, good repeatability, lower cost than animal model and a plurality of similar histopathological characteristics similar to xerophthalmia and application thereof in simulating, studying and preventing xerophthalmia are provided. The conjunctiva tissue containing conjunctival epithelium and conjunctival submucosa is cut and placed in culture media; the conjunctiva tissue is trimmed into a conjunctiva tissue block which is placed on a culture vessel inserter coated by type I collagen, with conjunctival epithelium being upward; the culture media are added into the culture vessel outside the inserter to cause the gas-liquid interface of the culture media to be at the junction of the conjunctival epithelium and conjunctival submucosa of the conjunctiva tissue block; and the conjunctiva tissue block is placed into a culture tank to be cultured. The xerophthalmia model in vitro can be used for such researches as xerophthalmia squamous epithelium metaplasia, ocular surface epithelial barrier breakdown, ocular surface epithelial mucoprotein change, and function of the participation and atopsis of inflammatory mediator in xerophthalmia diseases in the generation and development of xerophthalmia.
Owner:福建和泽生物科技有限公司
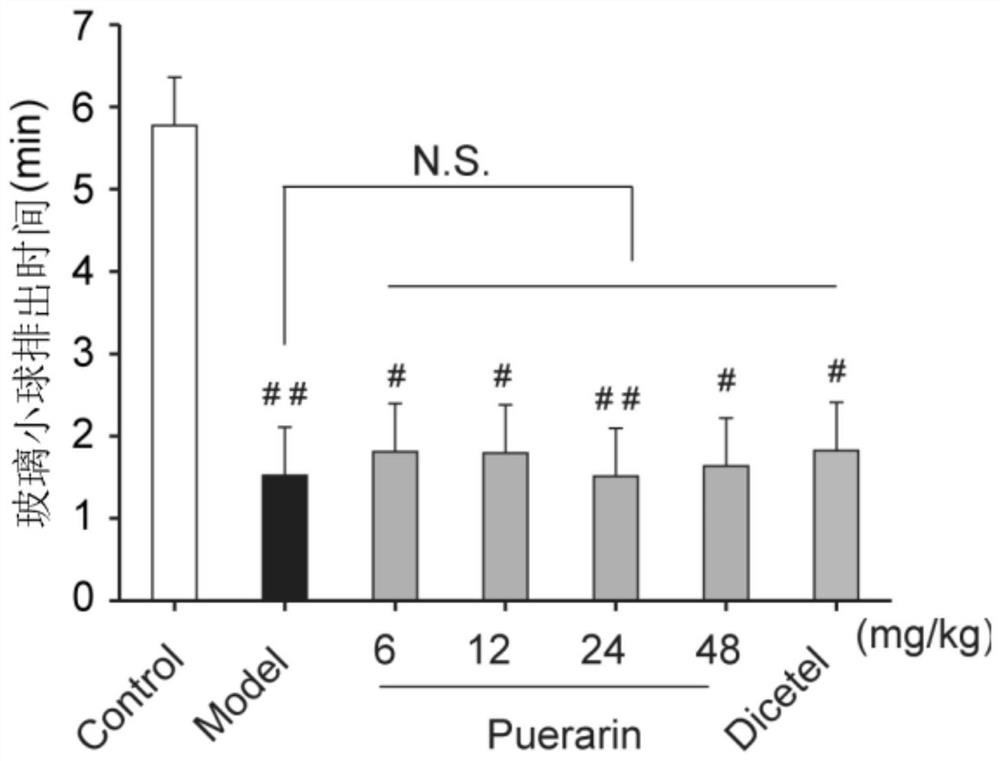
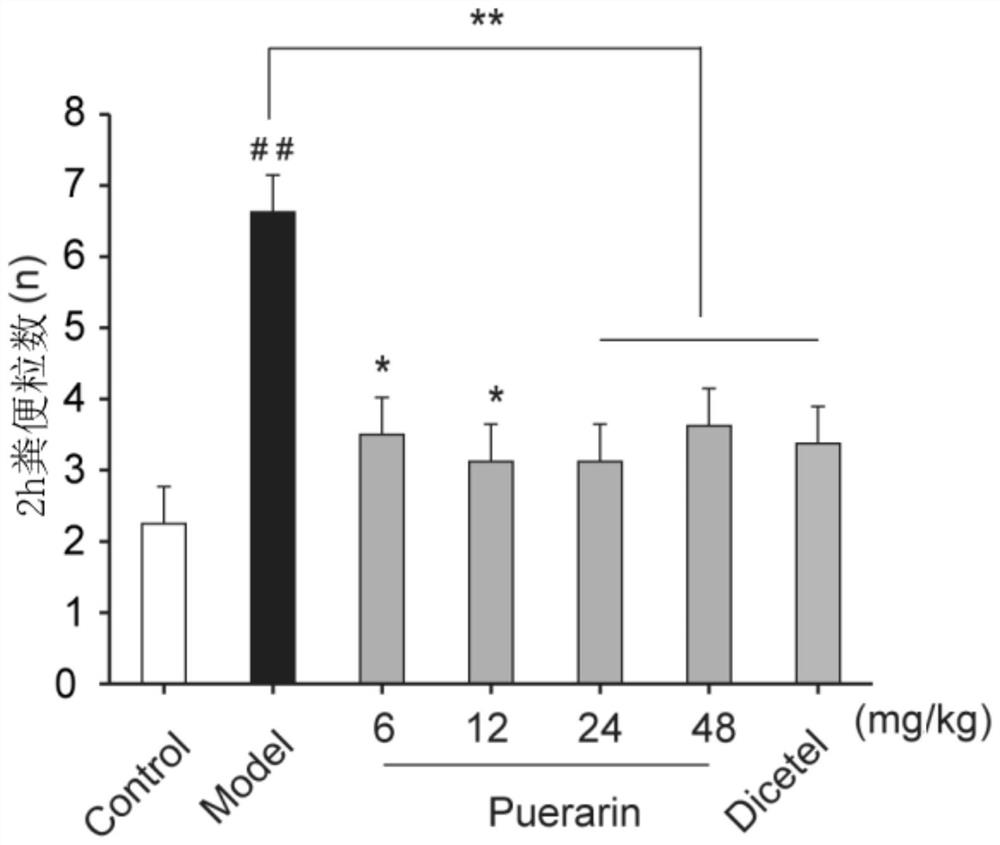
Application of puerarin in preparation of medicines for preventing and/or treating irritable bowel syndrome
ActiveCN113368097AReduce bowel movementsExtend discharge timeOrganic active ingredientsDigestive systemSide effectPhosphorylation
The invention discloses an application of puerarin for preventing, treating and / or relieving irritable bowel syndrome. The puerarin can restore retarded cell cycles and promote the proliferation of intestinal epithelial cells by up-regulating the level of phosphorylated extracellular regulatory protein kinase (p-ERK). The puerarin can participate in the repair of the intestinal epithelial barrier by up-regulating expression of tight junction protein (occulting), and inhibit the hyperfunction of the hypothalamic-pituitary-adrenal axis (HPA axis) by down-regulating expression of hypothalamic corticotropin release factor (CRF1) receptor protein, and improve the gastrointestinal motility and visceral hypersensitivity. Tests prove that puerarin has a remarkable curative effect on the irritable bowel syndrome, takes effect quickly and has small side effects. The puerarin is an efficient, safe, stable medicine with extensive sources for treating the irritable bowel syndrome. The puerarin of the invention provides a new medicine source for preventing and treating the irritable bowel syndrome and complications thereof.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
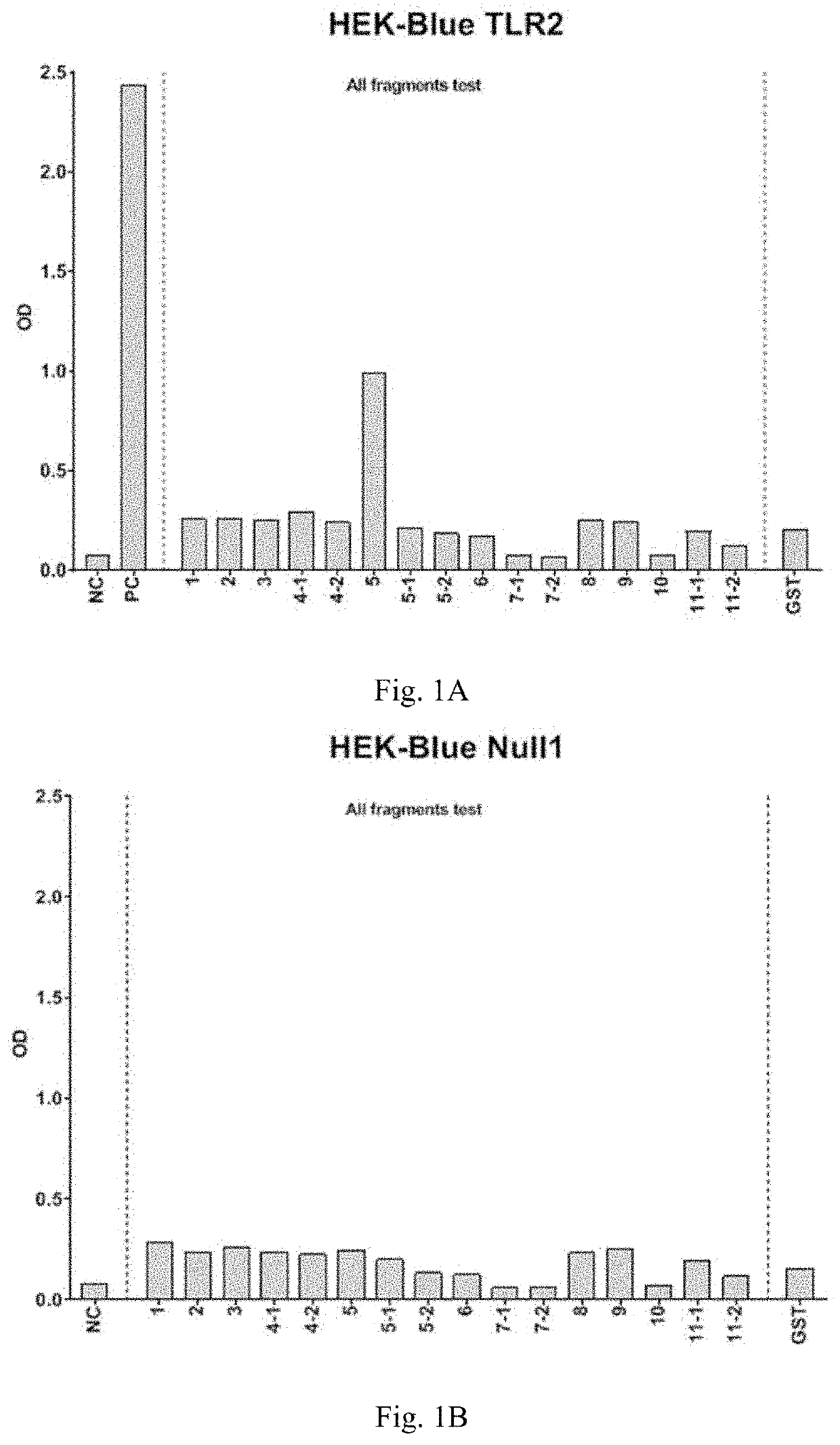
Peptide interacting with toll-like receptor 2 and the composition comprising the same
ActiveUS20210213097A1Stimulate immune responsePreventing intestinal cancerBacterial antigen ingredientsPeptide/protein ingredientsDiseaseIntestinal Cancer
Disclosed herein are peptide fragments of Amuc_1100* and the use thereof. The peptide is shown to be a ligand to activate TLR2 and is used to treat obesity and the related disease or condition. The peptide of the present invention is further used in the treatment of intestinal cancer, promoting immune response, and intestinal epithelial barrier dysfunction.
Owner:NAT TAIPEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com