Oral and injection dual-effect protein or polypeptide drug controlled-release vesicle and preparation method thereof
A technology of polypeptides and proteins, which is applied in medical preparations of non-active ingredients, drug delivery, pharmaceutical formulations, etc., can solve problems such as human injury, achieve the effects of avoiding inconvenience and injury, facilitating long-term storage, and prolonging drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
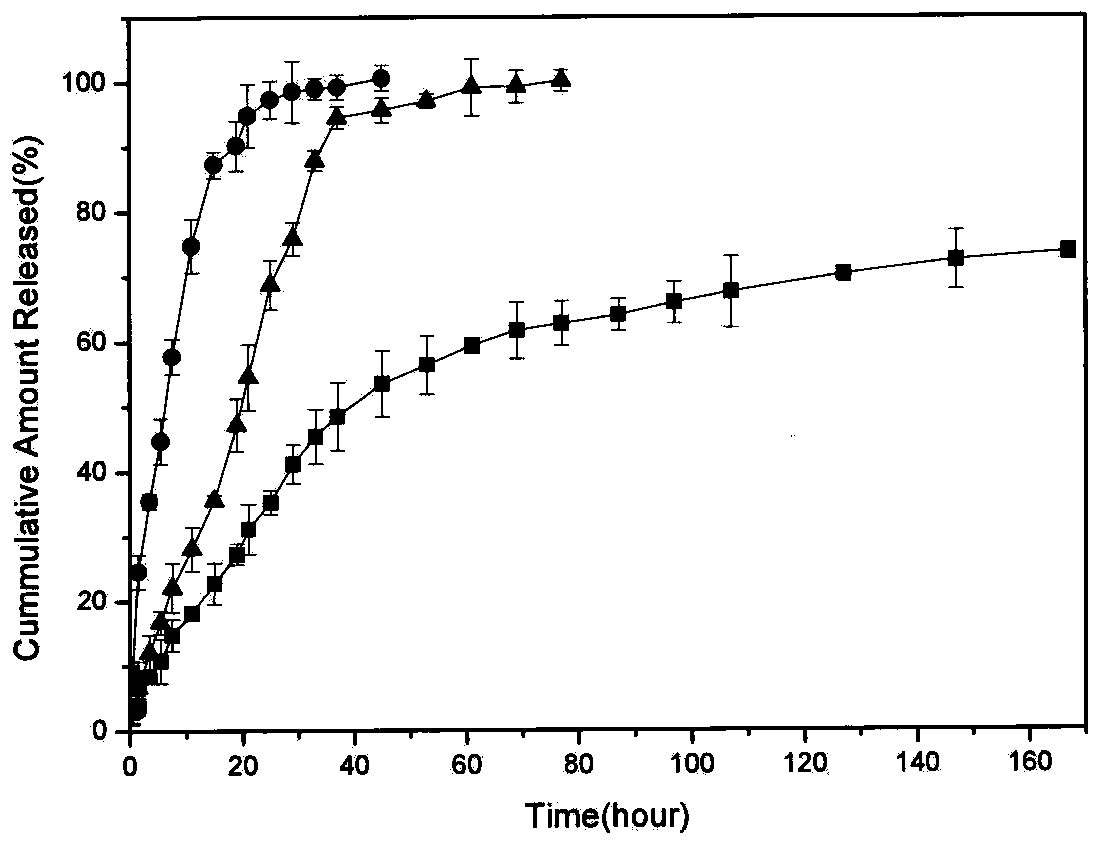
[0033]Accurately weigh 3 mg of the above-mentioned silicisome precursor lipid with an electronic balance, put it into a 25 mL round bottom flask, add 2 mL of chloroform, and then depressurize with a rotary evaporator under the conditions of 40 °C and 120 r / min The organic solvent, chloroform, was removed by rotary evaporation, and a lipid film was observed to form on the walls of the round-bottomed flask. Then add a certain amount of prepared insulin mother liquor and polyvinylpyrrolidone solution to the round bottom flask according to different drug-to-lipid ratios, make up to 4 mL with deionized water, and then place the round bottom flask in a water bath for 30 minutes at 40°C. , and then sonicated in a water bath for 5 minutes to form multilamellar vesicles. Finally, use a probe-type ultrasonic instrument to sonicate in an ice bath with 27W ultrasonic treatment for 3 minutes (over 3 seconds and stop for 3 seconds), so that it can be fully dispersed to form a stable system ...
specific Embodiment approach 2
[0034] This embodiment is different from Embodiment 1 in that before adding chloroform, after weighing the organic-inorganic composite lipid, a certain amount of acidic ethanol solution is added to acidify for 30 minutes. Others are the same as the first embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com