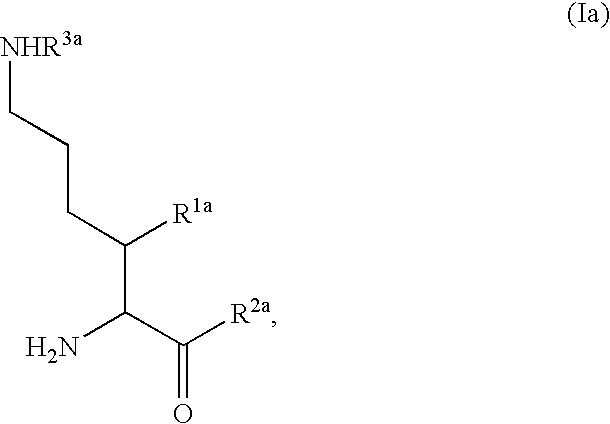
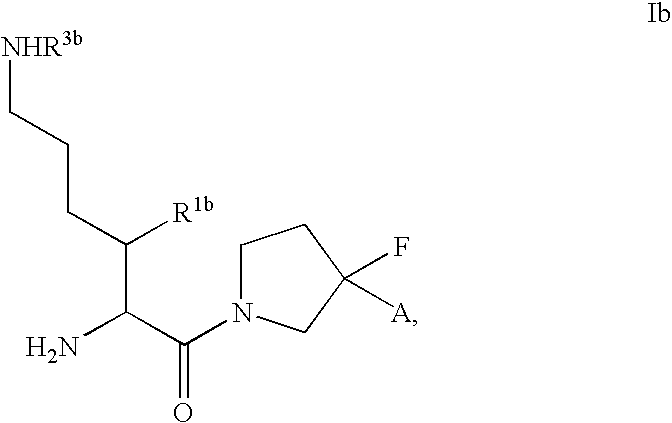
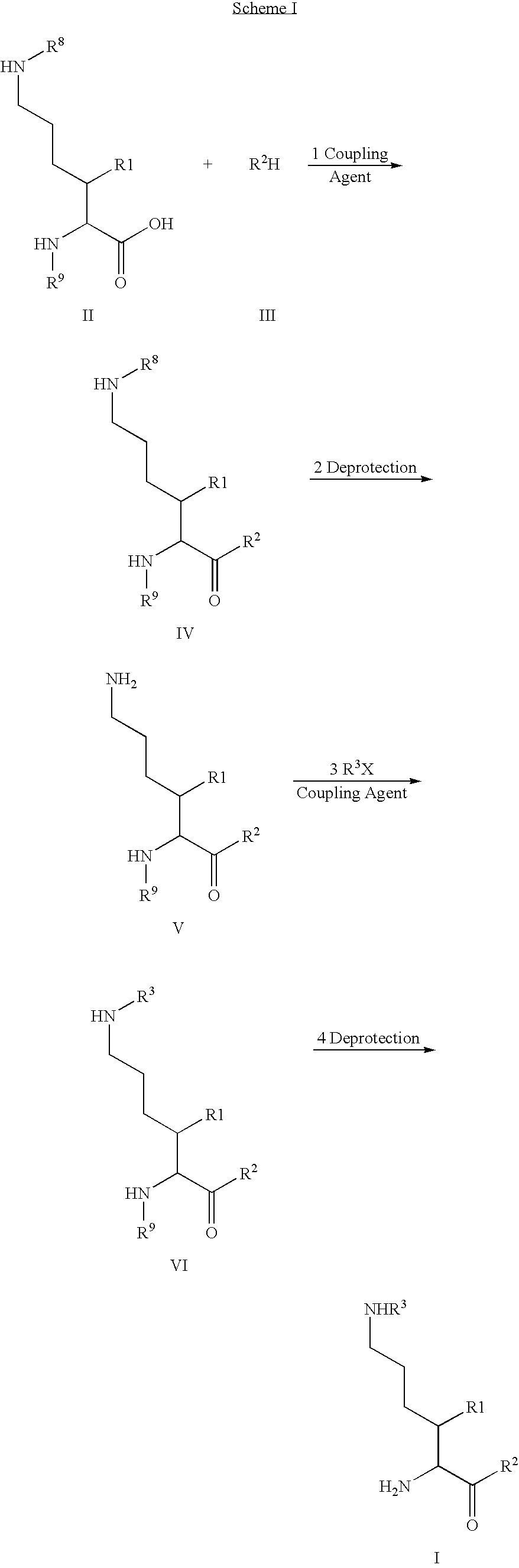
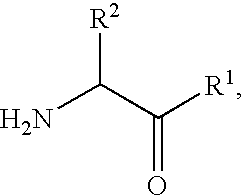
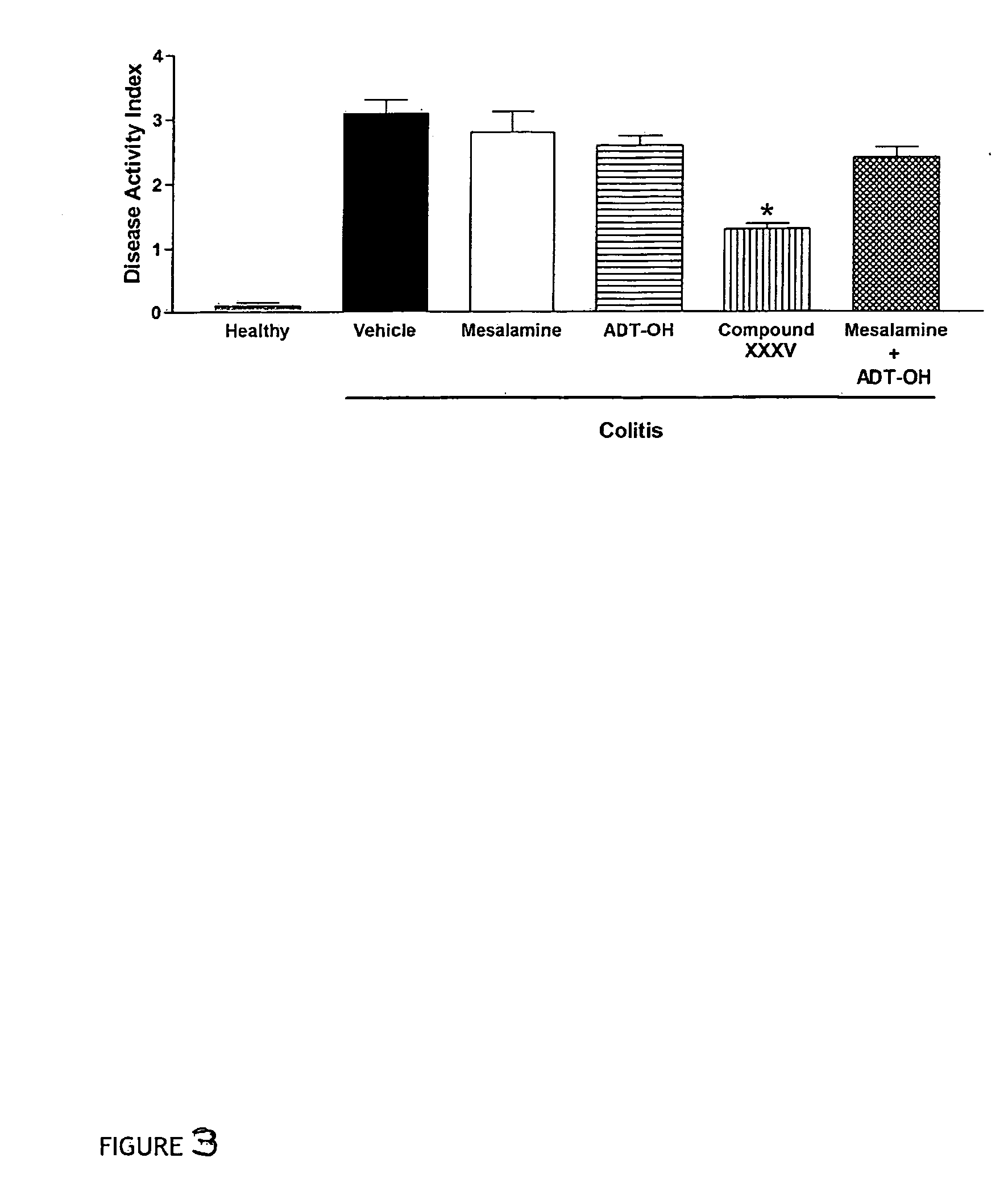
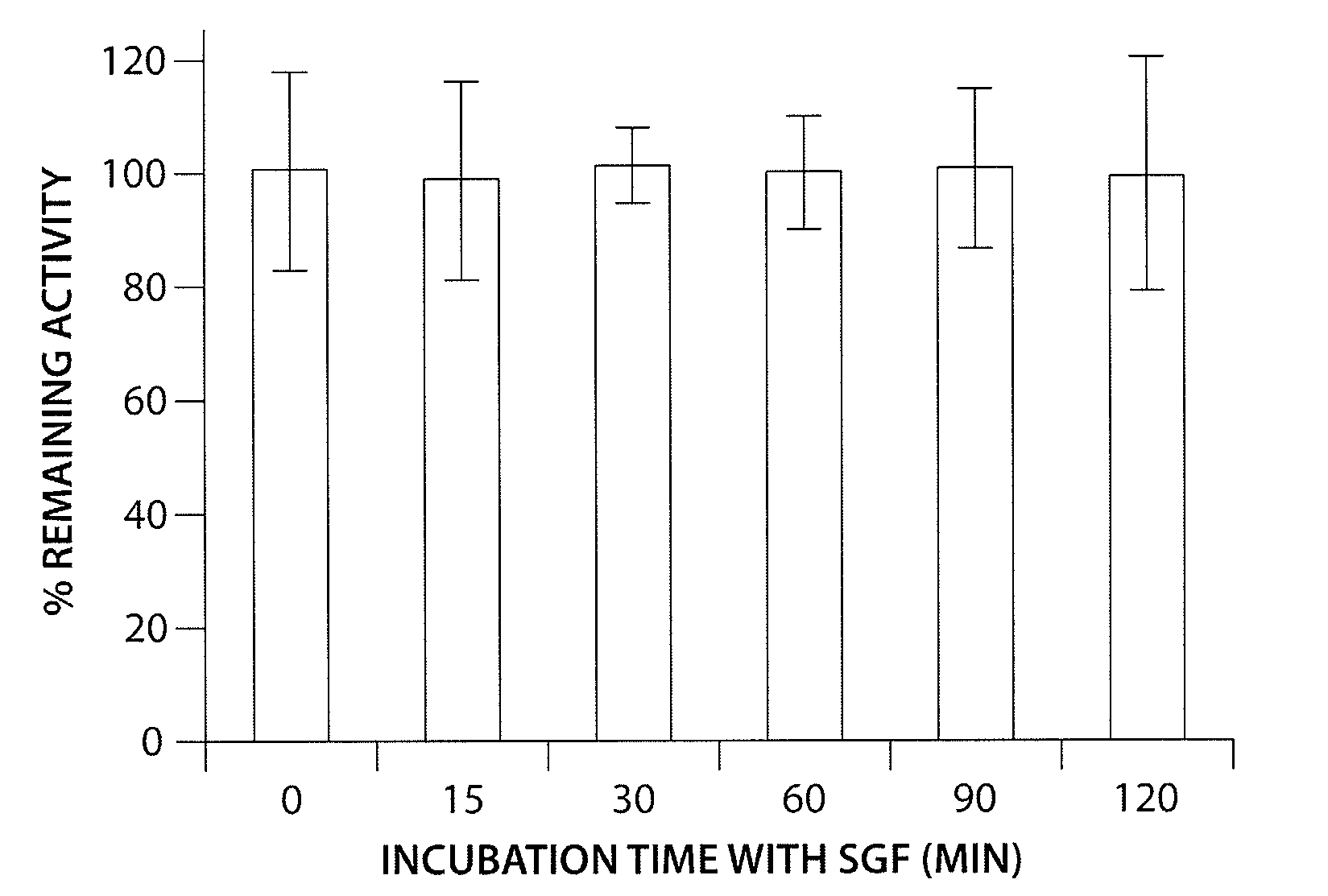
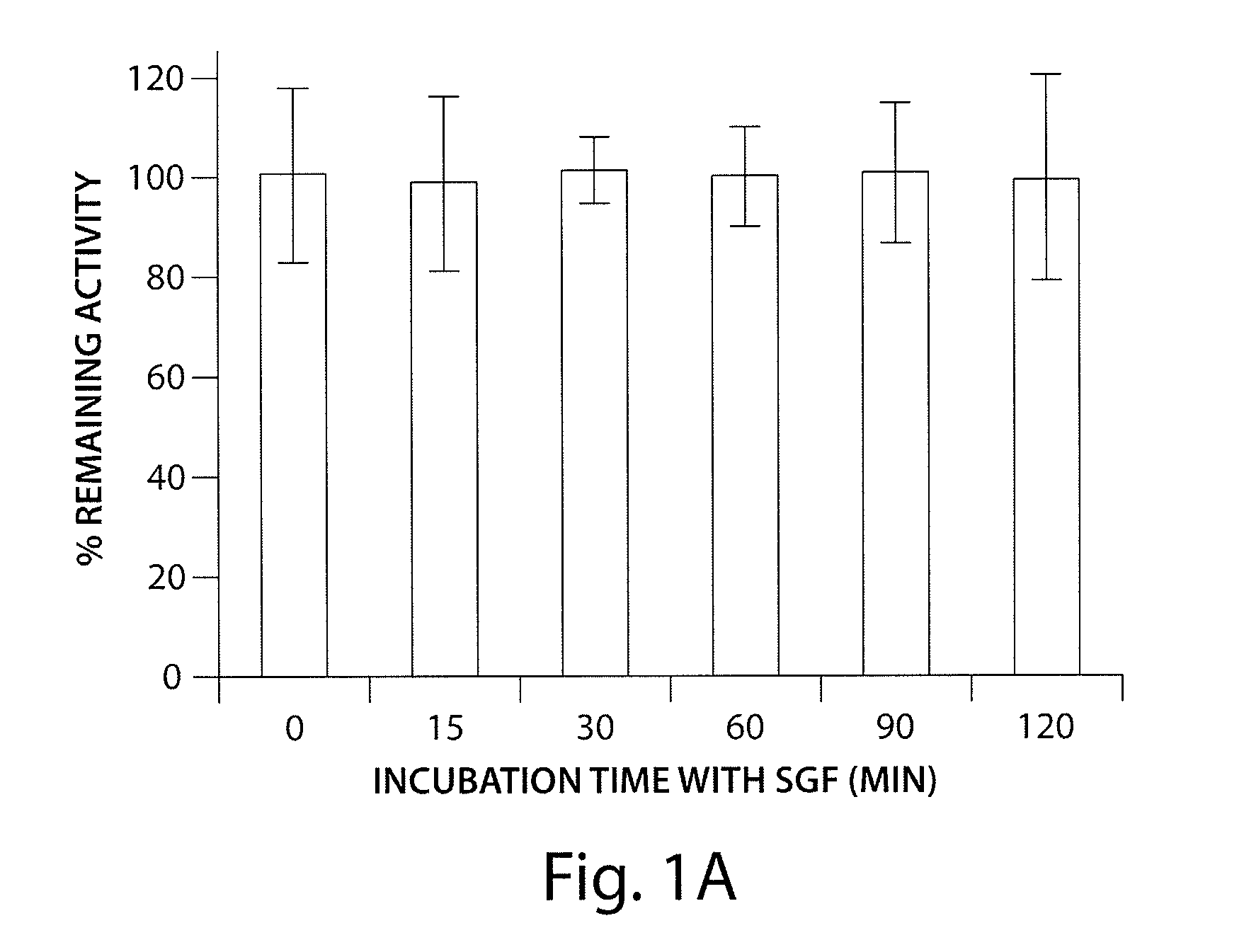
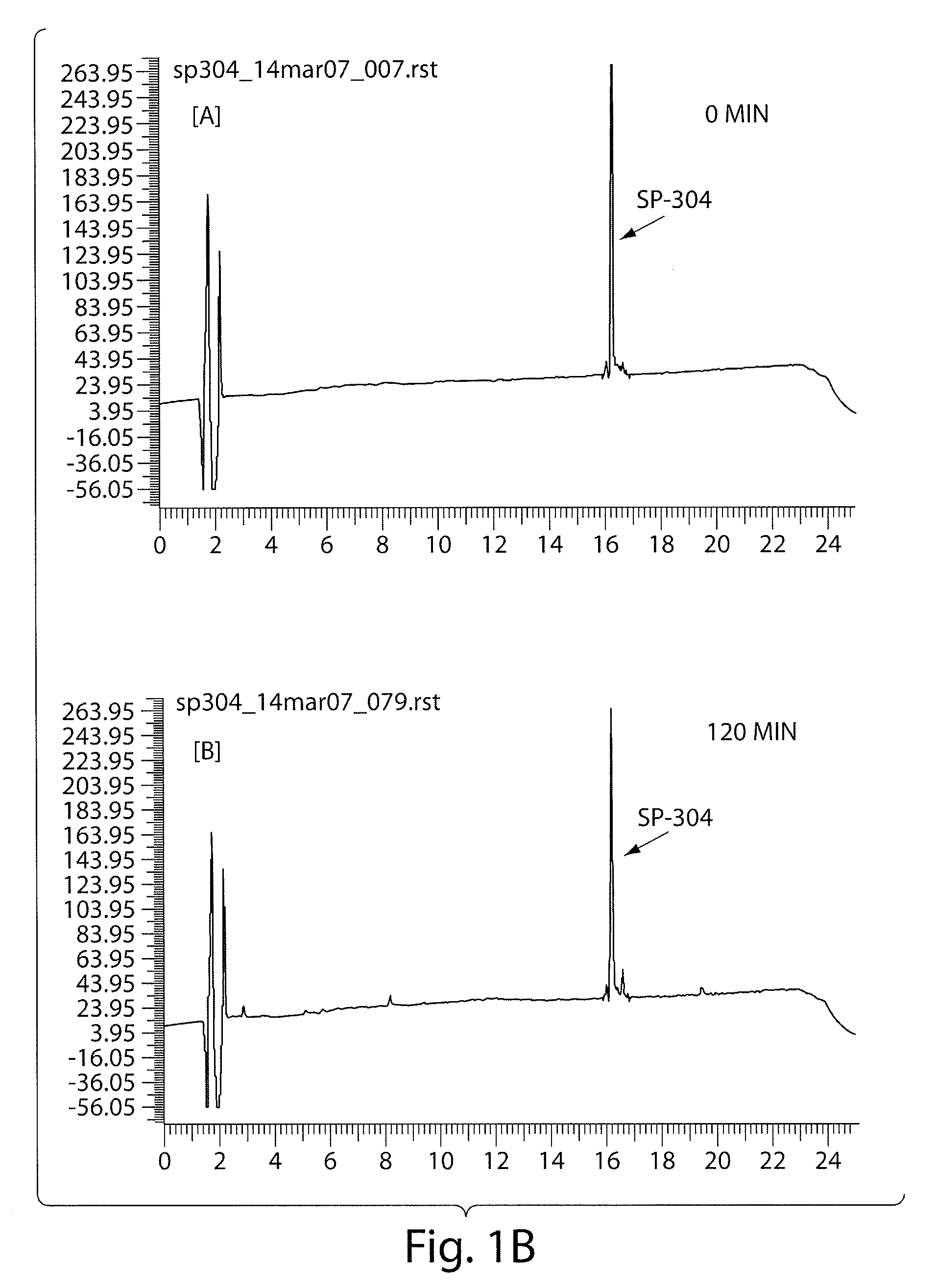
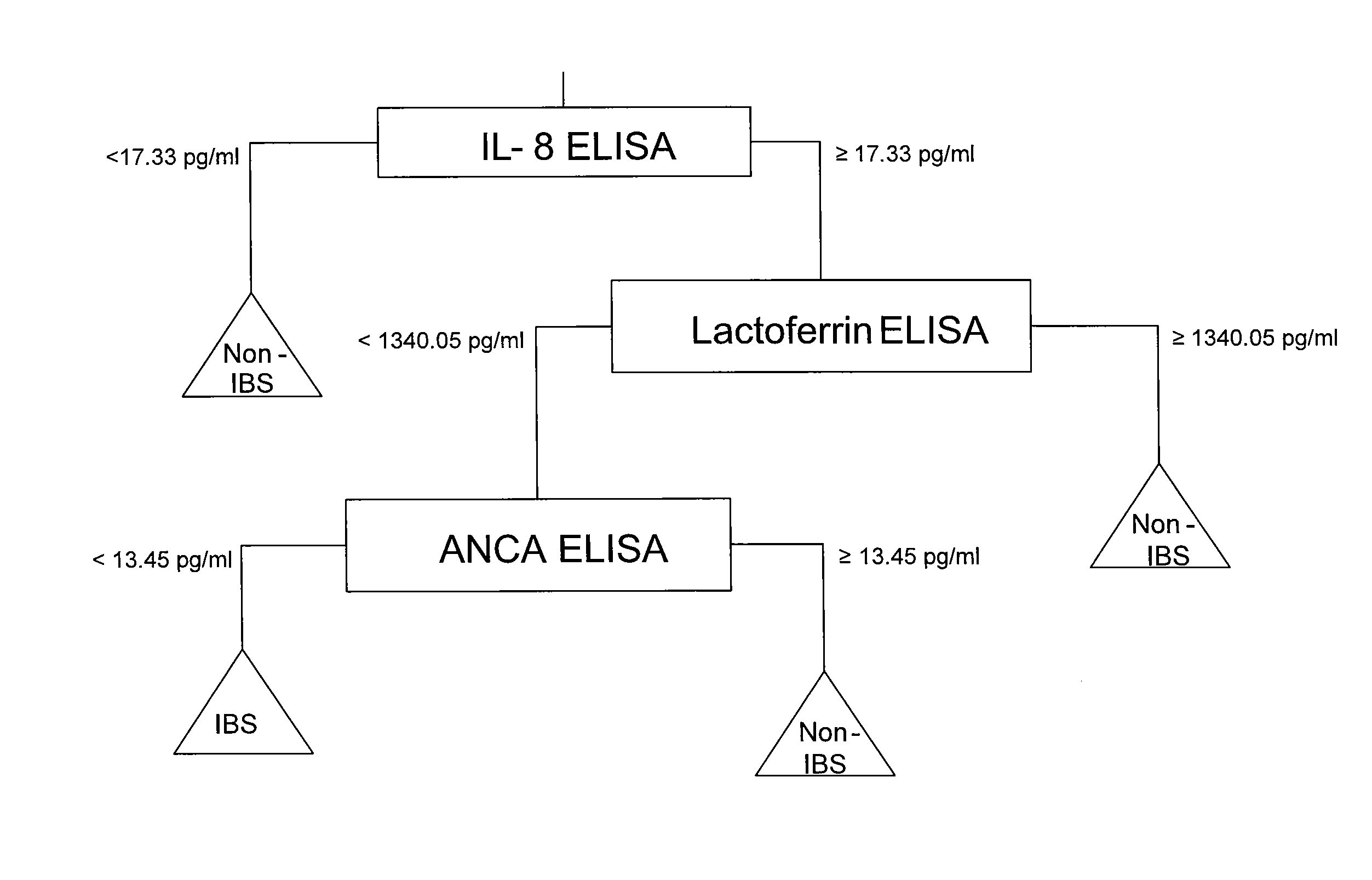
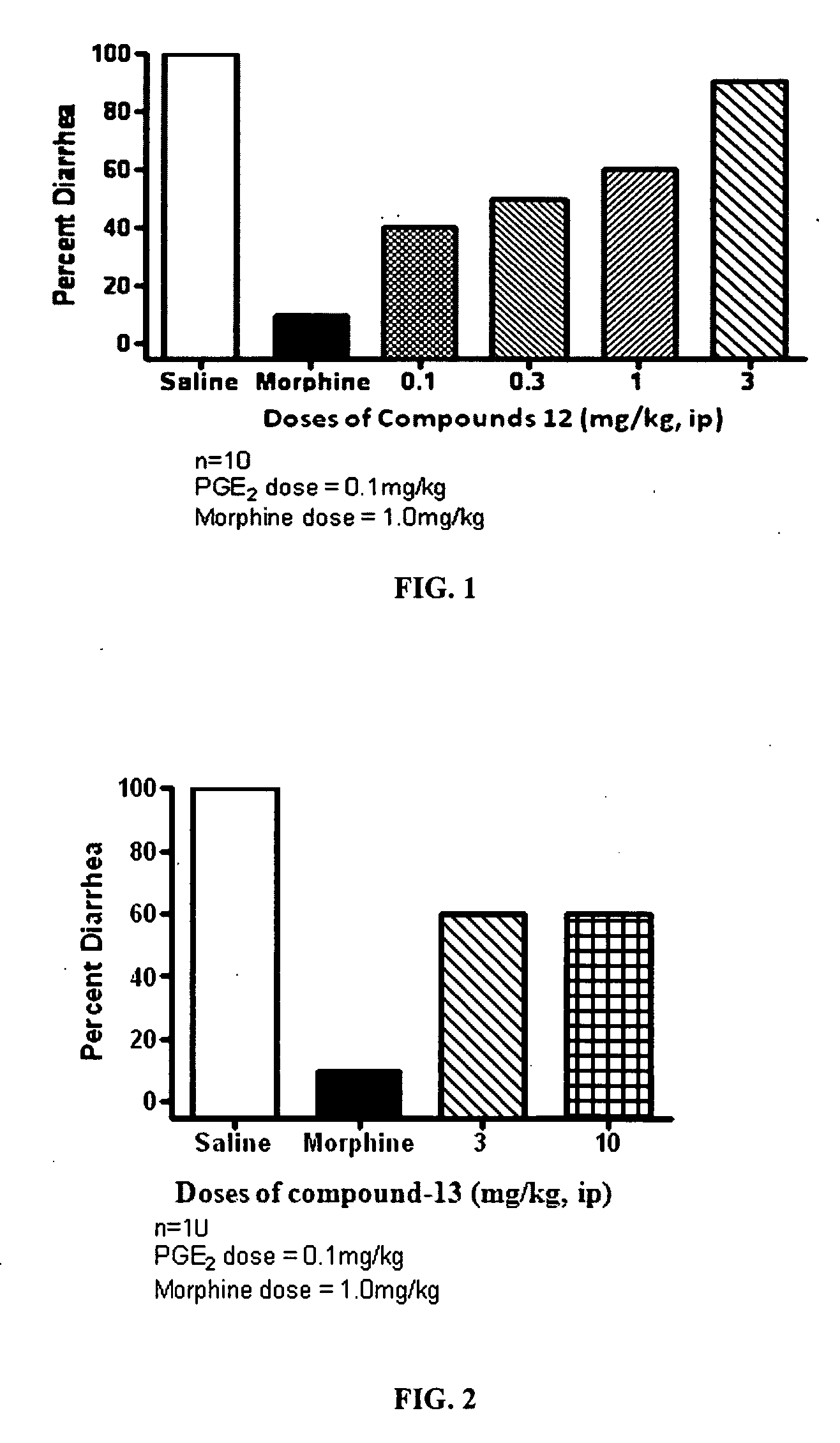
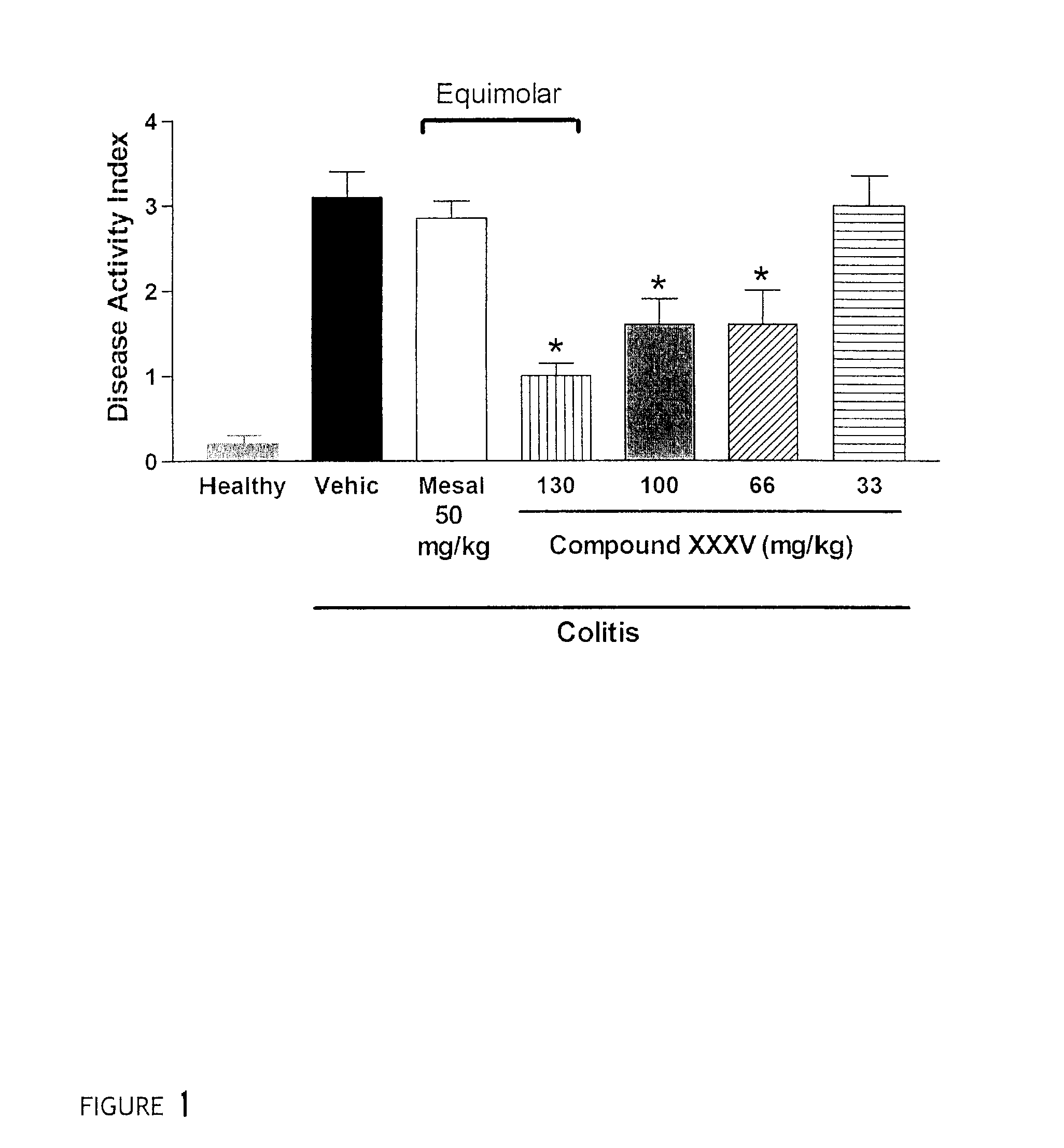
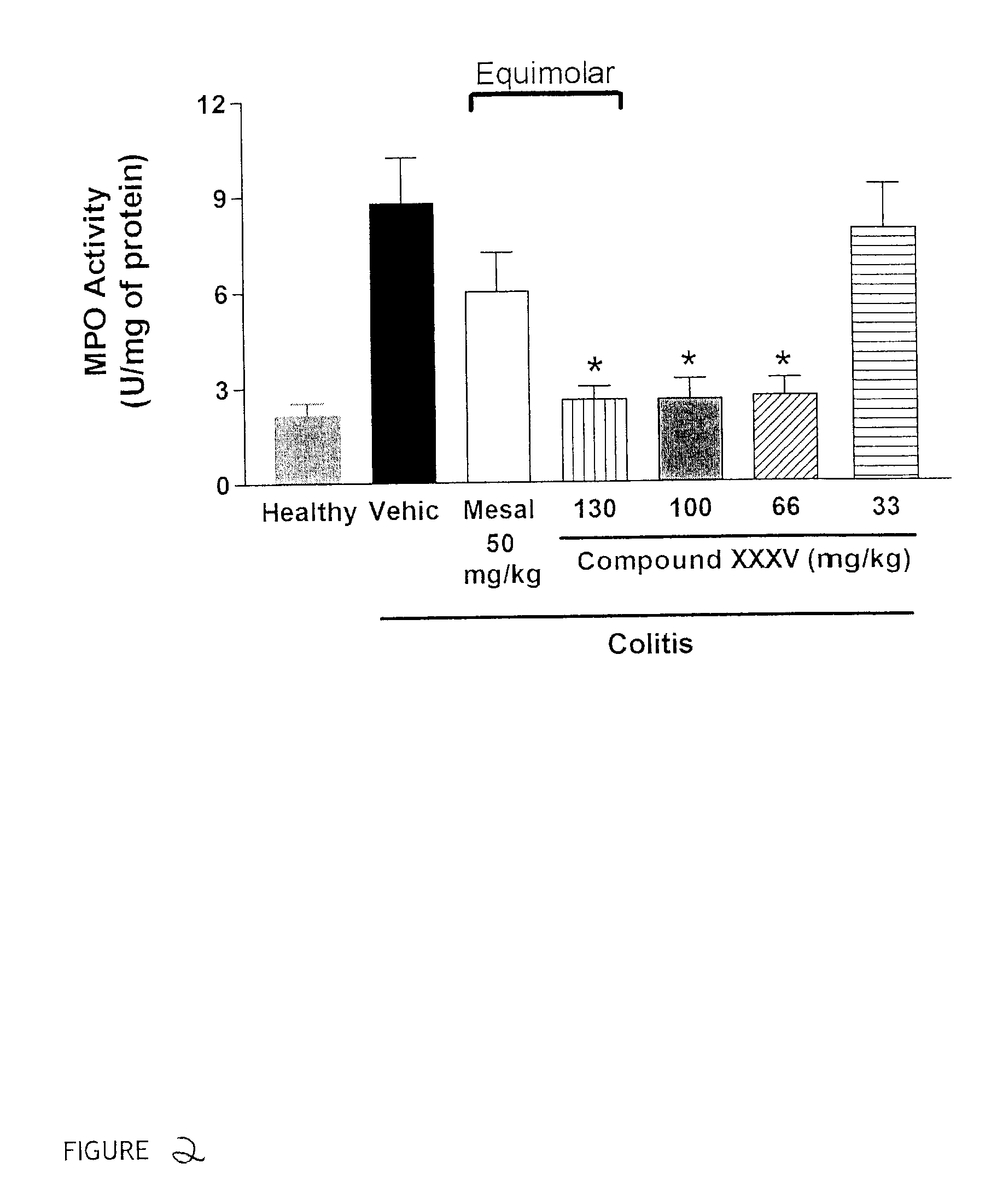
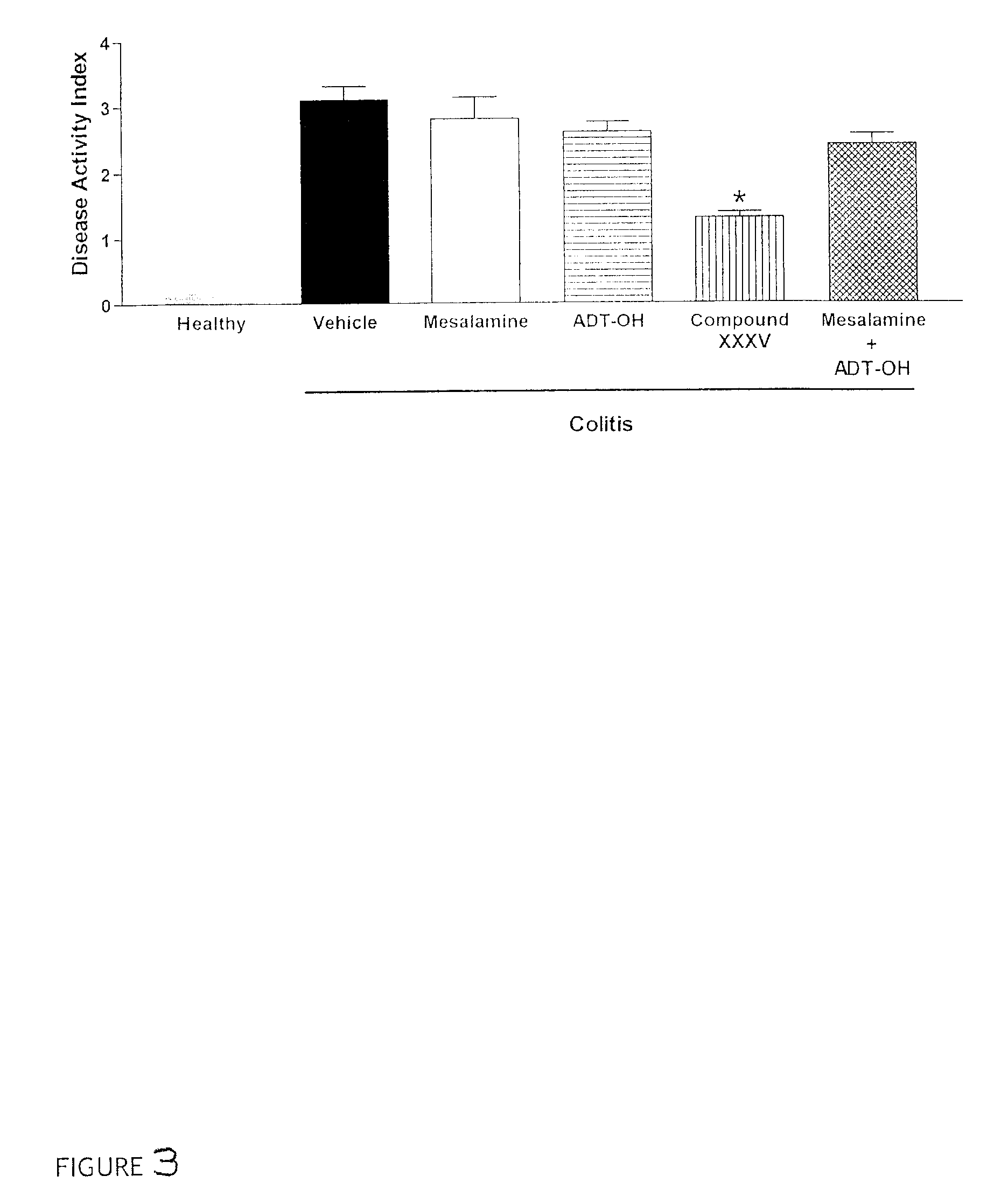
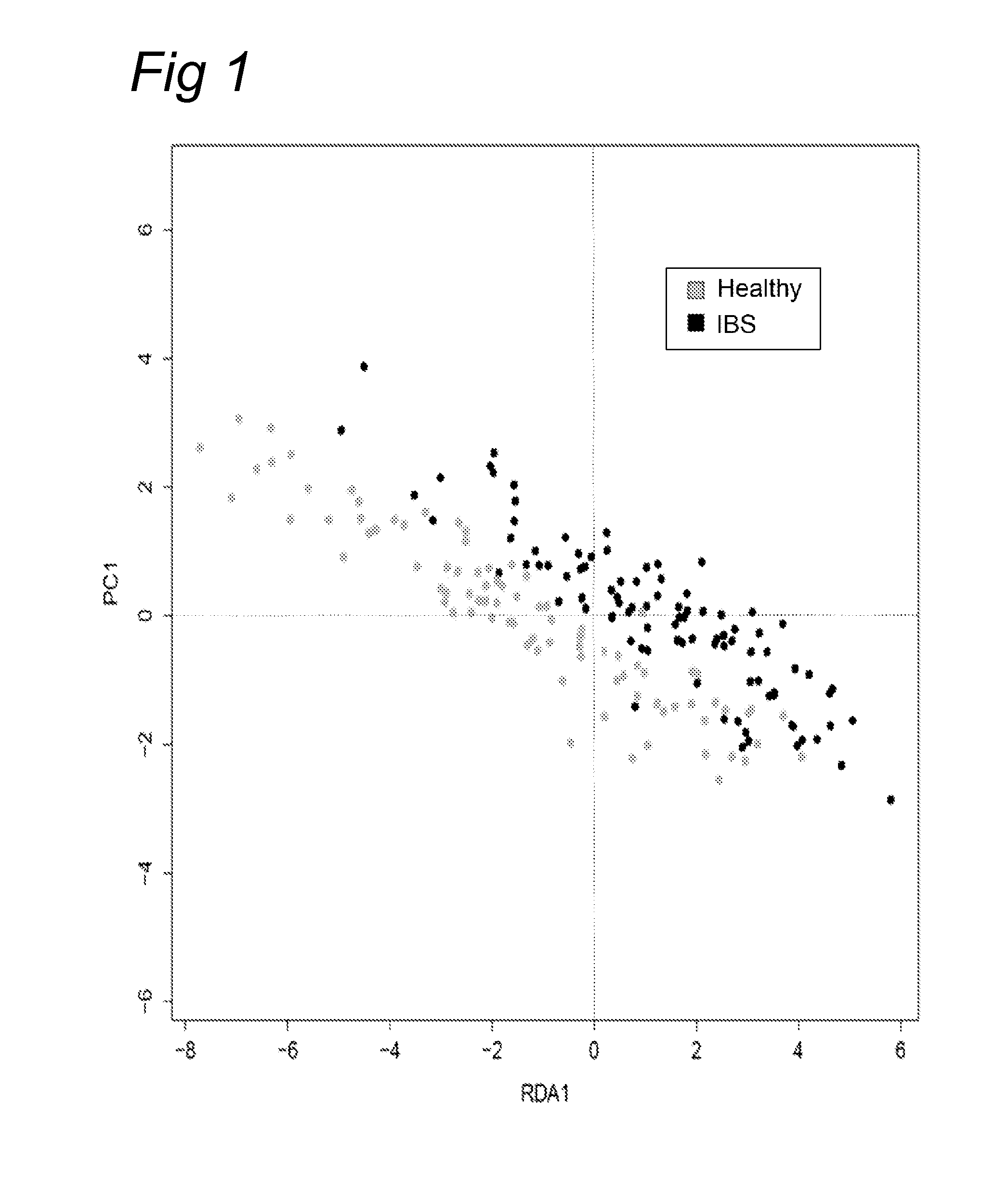
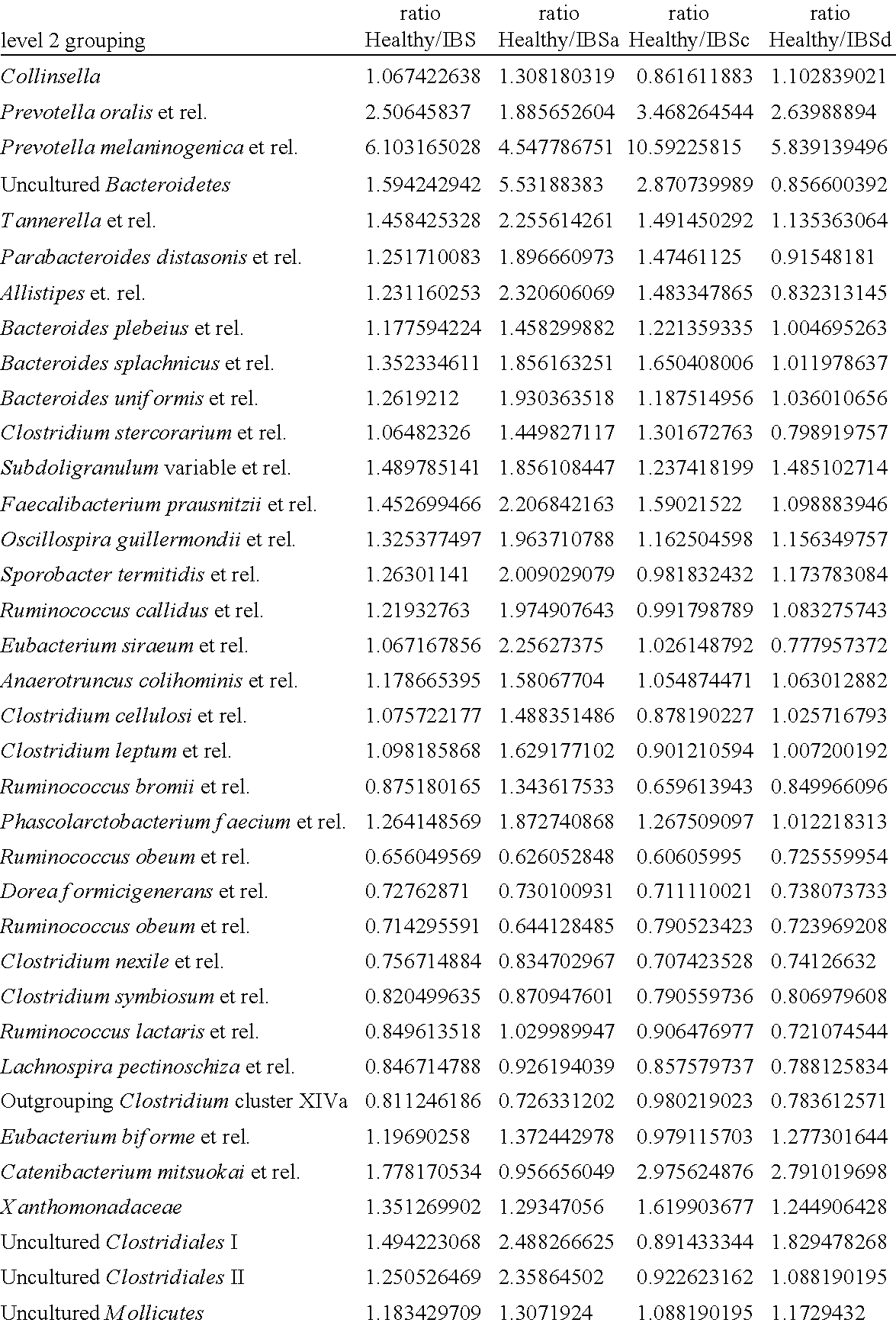
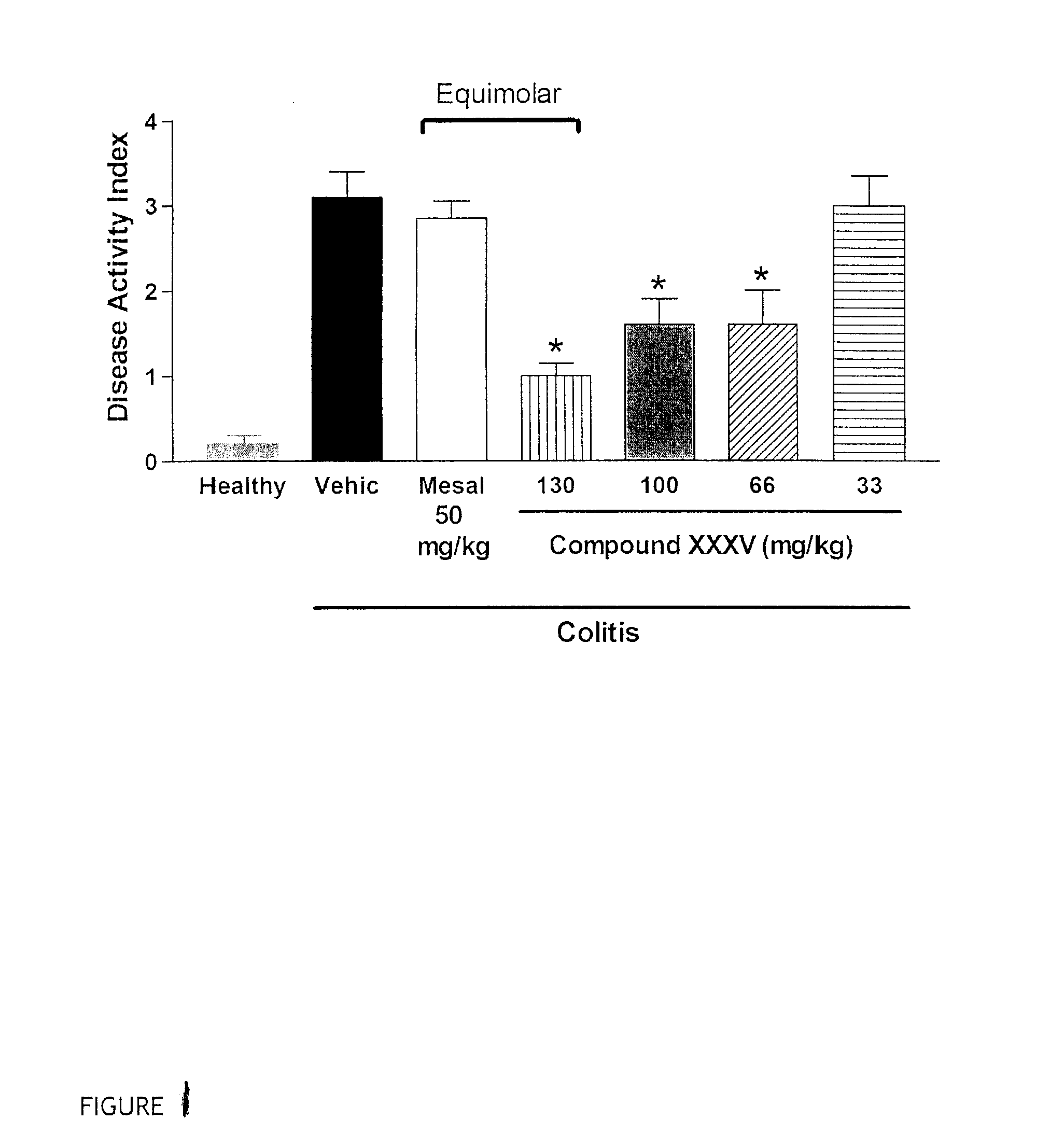
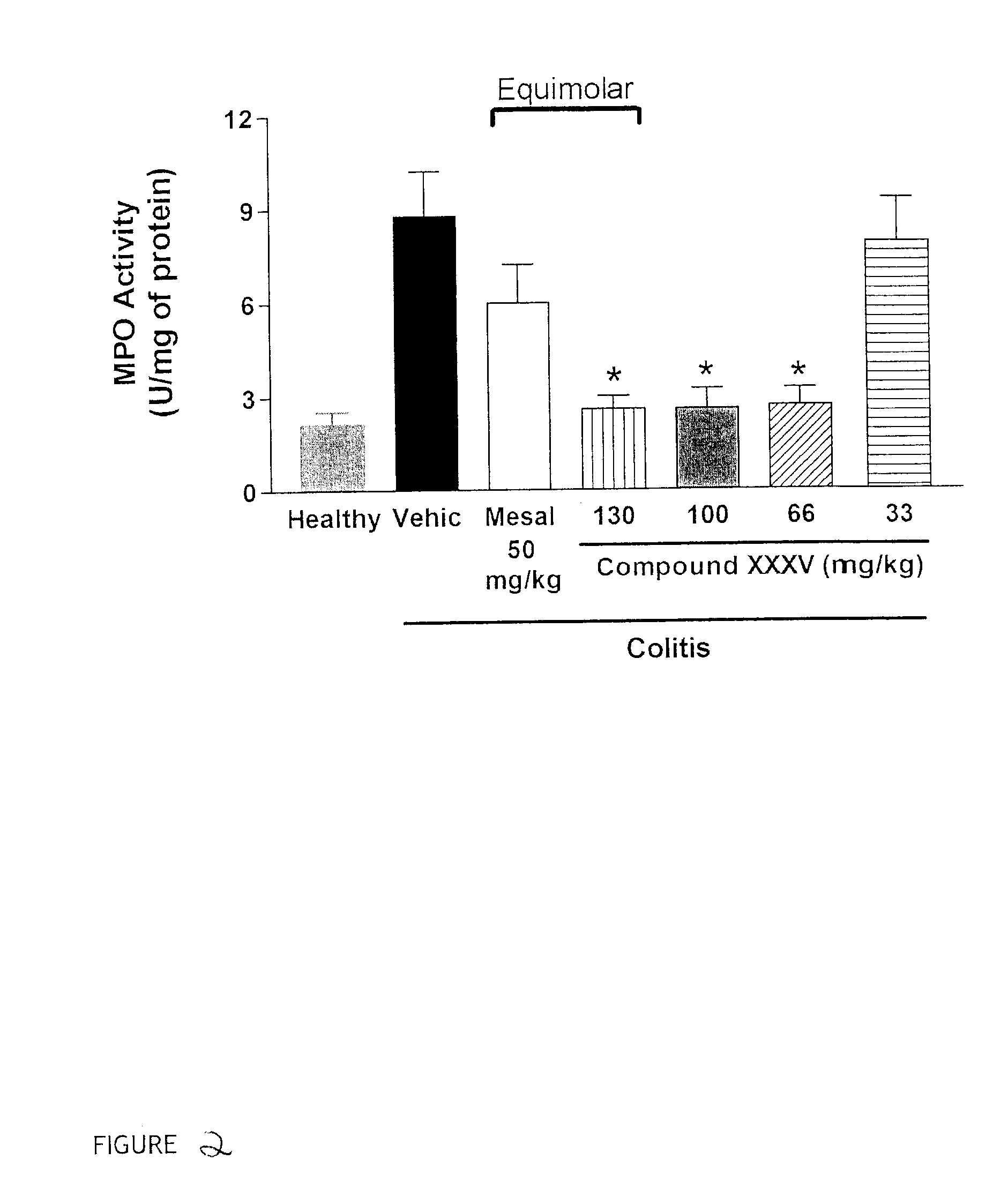
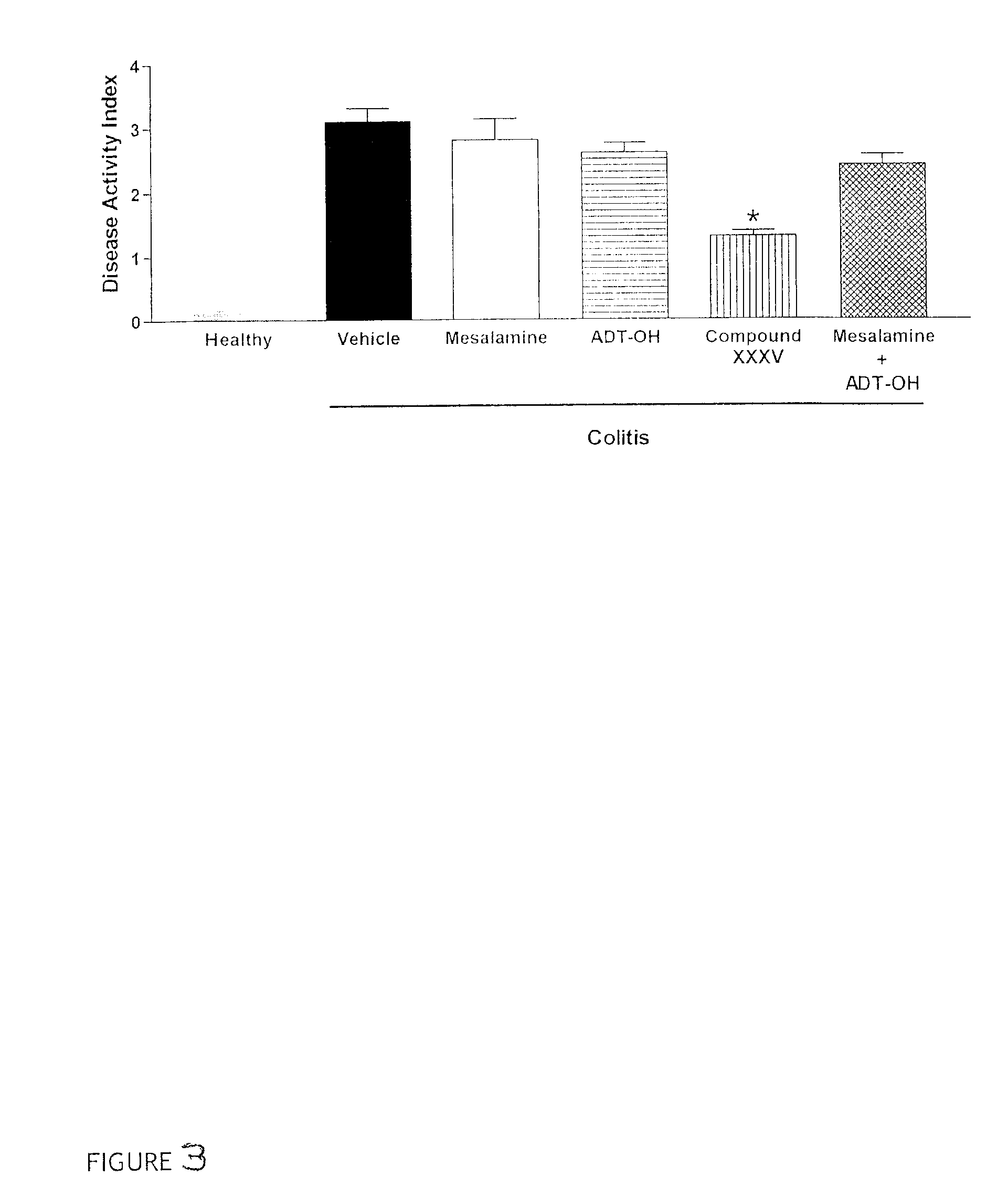
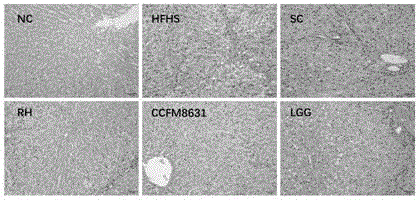
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
701 results about "Irritable bowel syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chronic gastrointestinal disorder that affects the large intestine causing diarrhea, abdominal pain, cramps, bloating and gas.
Methods of using and compositions comprising (+) sibutramine optionally in combination with other pharmacologically active compounds
This invention encompasses methods for the treatment and prevention of disorders that include, but are not limited to, eating disorders; weight gain; obesity; irritable bowel syndrome; obsessive-compulsive disorders; platelet adhesion; apnea; affective disorders such as attention deficit disorders, depression, and anxiety; male and female sexual function disorders; restless leg syndrome; osteoarthritis; substance abuse including nicotine and cocaine addiction; narcolepsy; pain such as neuropathic pain, diabetic neuropathy, and chronic pain; migraines; cerebral function disorders; chronic disorders such as premenstrual syndrome; and incontinence. The invention further encompasses pharmaceutical compositions and dosage forms which comprise optically pure (+) sibutramine, optionally in combination with a phosphodiesterase inhibitor or a lipase inhibitor.
Owner:SEPACOR INC
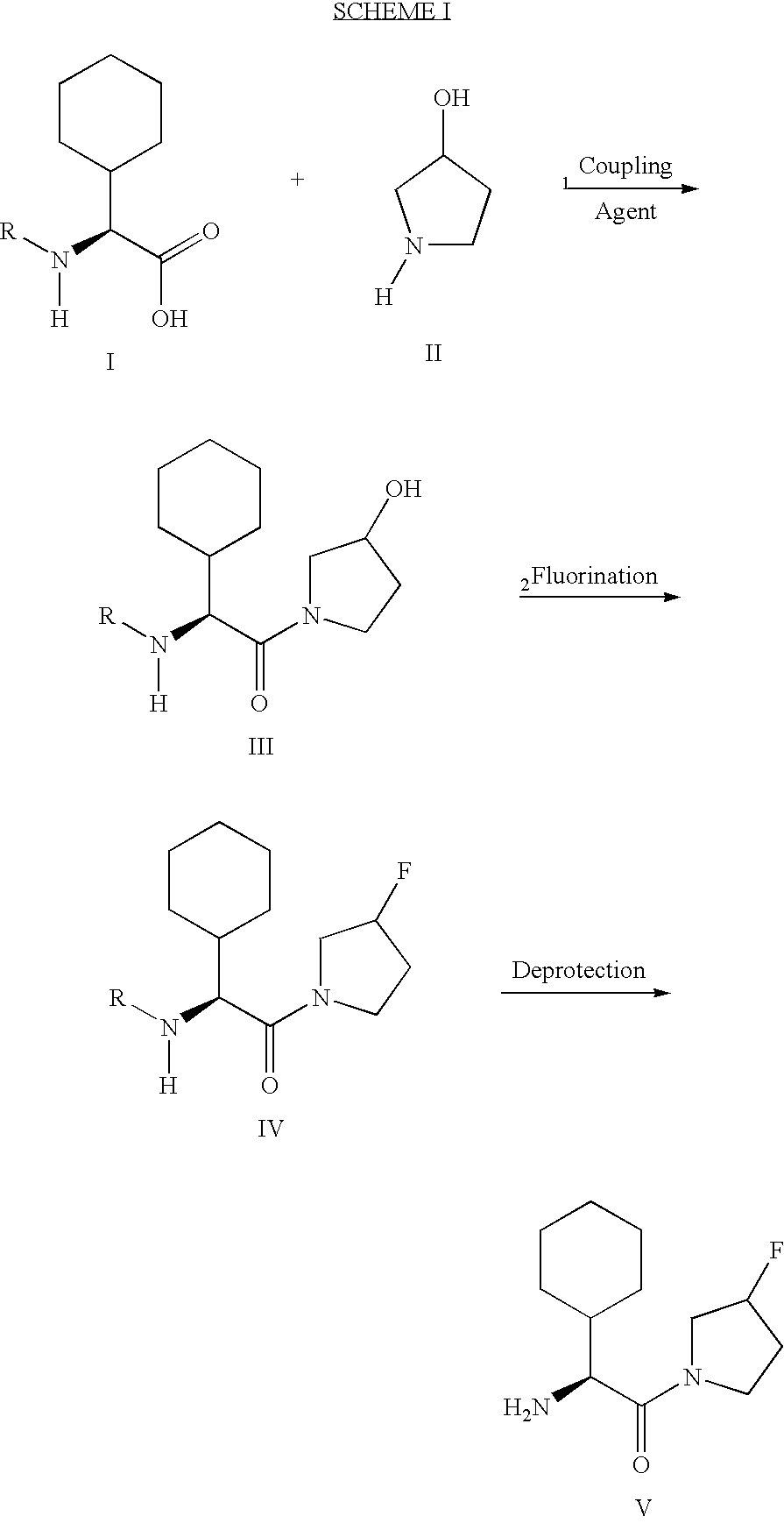
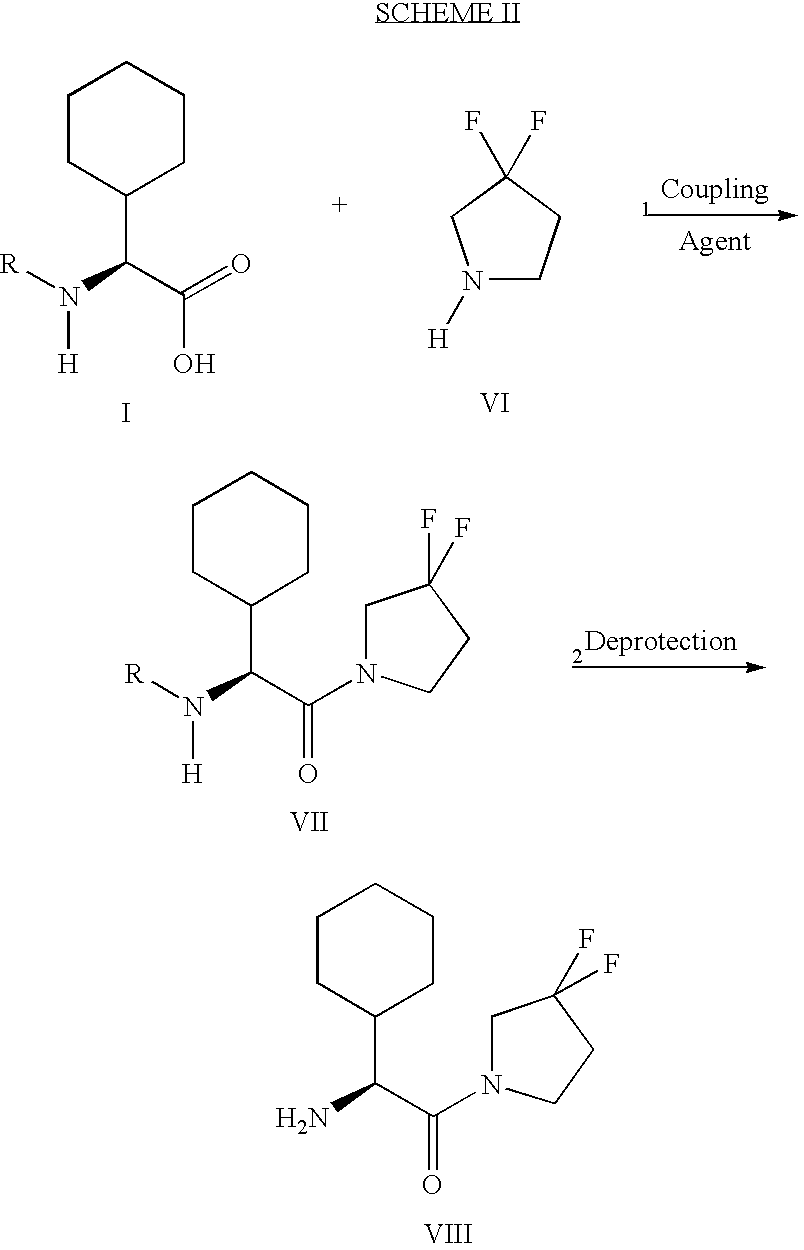
Fluorinated lysine derivatives as dipeptidyl peptidase IV inhibitors
InactiveUS20050043292A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDiabetic retinopathyArthritis
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV (“DPP-IV”), pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes, metabolic syndrome (syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Use of methylnaltrexone to treat irritable bowel syndrome
Methods of treating irritable bowel syndrome with peripheral opioid antagonists, such as methylnaltrexone, are provided. Formulations comprising peripheral opioid antagonists, such as methylnaltrexone, and irritable bowel syndrome therapeutic agents are also provided.
Owner:PROGENICS PHARMA INC
Dipeptidyl peptidase IV inhibiting fluorinated cyclic amides
InactiveUS20040110817A1Ease of preparation and detectabilityGood metabolic stabilityBiocideSenses disorderDiabetic retinopathyDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Derivatives of 4- or 5-aminosalicylic acid
InactiveUS20060270635A1Less readily absorbedEasily reach colonBiocidePhosphorous compound active ingredientsThioester synthesisSalicylic acid
The present invention provides new derivatives of 4- or 5-aminosalicylic acid, and a pharmaceutical composition containing these derivatives of 4- or 5-aminosalicylic acid as active ingredients, useful for the treatment of intestinal diseases such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) and for the prevention / treatment of colon cancer. More particularly, these derivatives comprise a hydrogen sulfide releasing moiety linked via an azo, an ester, an anhydride, a thioester or an amide linkage to a molecule of 4- or 5-aminosalicylic acid. Furthermore, the present invention provides a process for preparing these compounds and their use for treating IBD and IBS and the prevention / treatment of colon cancer.
Owner:ANTIBE THERAPEUTICS INC
Treatment of irritable bowel syndrome using probiotic composition
A method of treating irritable bowel syndrome using a probiotic composition including the bacilli (1) Bacillus subtilis, (2) Bacillus coagulans, and (3) Enterococcus faecium. The composition may further include a carrier medium, such as fructo-oligo-saccharides (FOS), as incorporated in a dose form such as a pill, capsule, powder or sachet. The compositions of the invention may be usefully employed as health or nutritional supplements, food additives, or therapeutic agents for combating a wide variety of physiological disorders.
Owner:COBB & ASSOCS
Treatment of disease states and adverse physiological conditions utilizing anti-fungal compositions
InactiveUS20060177424A1Effectively ameliorateEffective regulationBiocideBacteria material medical ingredientsSinusitisVaginal Yeast Infections
A method for treatment or prophylaxis of a disease state or other physiological condition, e.g., autism, delayed development, acid reflux disease, vaginal yeast infections, impaired hearing, chronic ear infections, seasonal allergies, Fibromyalgia syndrome, Crohn's disease, colitis, irritable bowel syndrome, interstitial cystitis, acne, sinusitis, rheumatoid arthritis, chronic fatigue syndrome, asthma, attention deficit disorder, attention deficit / hyperactivity disorder, rosacea, multiple sclerosis, hyperglycemia, or Ménière's disease, by administration of an anti-fungal composition that includes at least one of the bacilli (1) Bacillus subtilis, (2) Lactobacillus sporogenes, and (3) Streptococcus faecalis.
Owner:COBB AND CO
Aerosol and injectable formulations of nanoparticulate benzodiazepine
InactiveUS20060198896A1Easy doseReduce injection volumeBiocidePowder deliveryBenzodiazepinePolyethylene glycol
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Owner:ELAN PHRMA INT LTD
Pharmaceutical compositions containing pediococcus and methods for reducing the symptoms of gastroenterological syndromes
The invention provides a method and composition for ameliorating or reducing the symptoms, signs, and markers and for the treatment of irritable bowel syndrome, inflammatory bowel disease or gastritis in a mammal in need thereof, said method comprising administering effective amounts of a pharmaceutically acceptable composition containing at least one probiotic microorganism strain comprising Pediococcus for a time sufficient to ameliorate, reduce or treat at least one symptom, sign, or marker of irritable bowel syndrome, inflammatory bowel disease or gastritis.
Owner:PROTHERA
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Pharmaceutical composition and method for the transdermal delivery of magnesium
InactiveUS20050196434A1Reduce disadvantagesBiocideAerosol deliveryAutonomic bladder dysfunctionMagnesium salt
The present invention relates to a method and transdermal pharmaceutical composition for preventing magnesium deficiency or imbalances associated with magnesium deficiency including diabetes, hypertension, high cholesterol, cardiac arrhythmias, acute myocardial infarction, arteriosclerosis, atherosclerosis, preeclampsia, dysautonomia, mitral valve prolapse, asthma, constipation, irritable bowel syndrome, migraines, muscle spasms and cramping, premenstrual syndrome, osteoporosis, kidney stones, chronic fatigue syndrome, and fibromyalgia. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of magnesium and a pharmaceutically acceptable carrier. A therapeutically effective amount of a pharmaceutically acceptable salt of zinc a vitamin such as B-complex vitamin, a carotenoid, a mineral, or a combination thereof may also be included in the transdermal pharmaceutical composition. A therapeutically effective amount of progesterone may also be included in the transdermal pharmaceutical composition. The transdermal pharmaceutical composition may be topically administered to prevent magnesium deficiency or imbalances caused by magnesium deficiency.
Owner:BRIERRE BARBARA T
Method of treating mast cell activation-induced diseases with a proteoglycan
InactiveUS6689748B1Decrease in urinaryBiocidePeptide/protein ingredientsInflammatory Bowel DiseasesInterstitial cystitis
The invention provides a method for preventing and treating the harmful biological effects of biochemicals secreted from activated mast cells in the organism of warm blooded animals and more especially human beings, said effects being associated with allergy (including but not limited to allergic conjunctivitis, allergic rhinitis, allergic otitis, asthma, allergic uticaria, food allergy and atopic dermatitis), hyperproliferative diseases such as leukemia and systemic mastocytosis, interstitial cystitis, inflammatory bowel disease, irritable bowel syndrome, osteoporosis and scleroderma. The method consists in administering to said animals and especially to human beings an effective amount of a proteoglycan such as chondroitin sulfate with mast cell secretion inhibitory activity, alone or in combination with one or more synergistic adjuvants such those belonging to the class of flavonoids or compounds with histamine-1 receptor antagonist activity.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
Methods for diagnosing irritable bowel syndrome
InactiveUS20080085524A1Accurate classificationAccurate predictionAnalogue computers for chemical processesDisease diagnosisSAPS IIStatistical algorithm
The present invention provides methods, systems, and code for accurately classifying whether a sample from an individual is associated with irritable bowel syndrome (IBS). In particular, the present invention is useful for classifying a sample from an individual as an IBS sample using a statistical algorithm and / or empirical data. The present invention is also useful for ruling out one or more diseases or disorders that present with IBS-like symptoms and ruling in IBS using a combination of statistical algorithms and / or empirical data. Thus, the present invention provides an accurate diagnostic prediction of IBS and prognostic information useful for guiding treatment decisions.
Owner:NESTEC SA
Selective opioid compounds
ActiveUS20090209569A1Reducing lipid permeability of drugReduce penetrationAntibacterial agentsBiocideDiseaseInterstitial cystitis
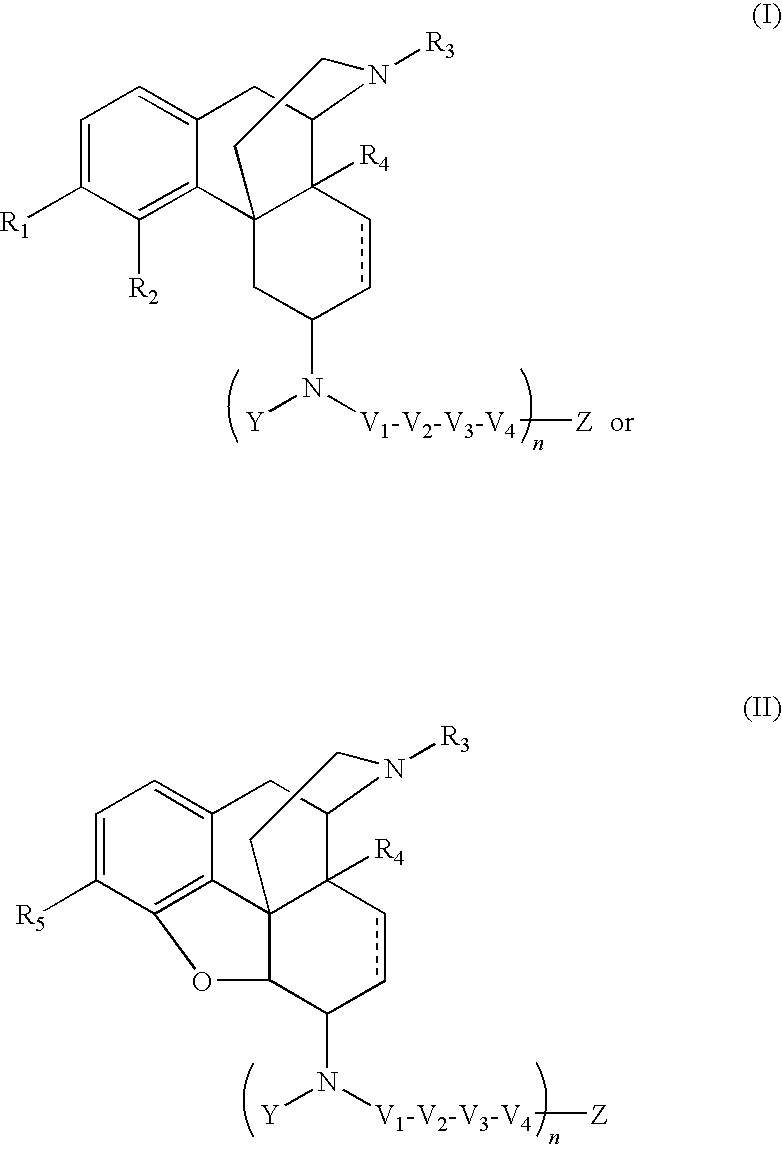
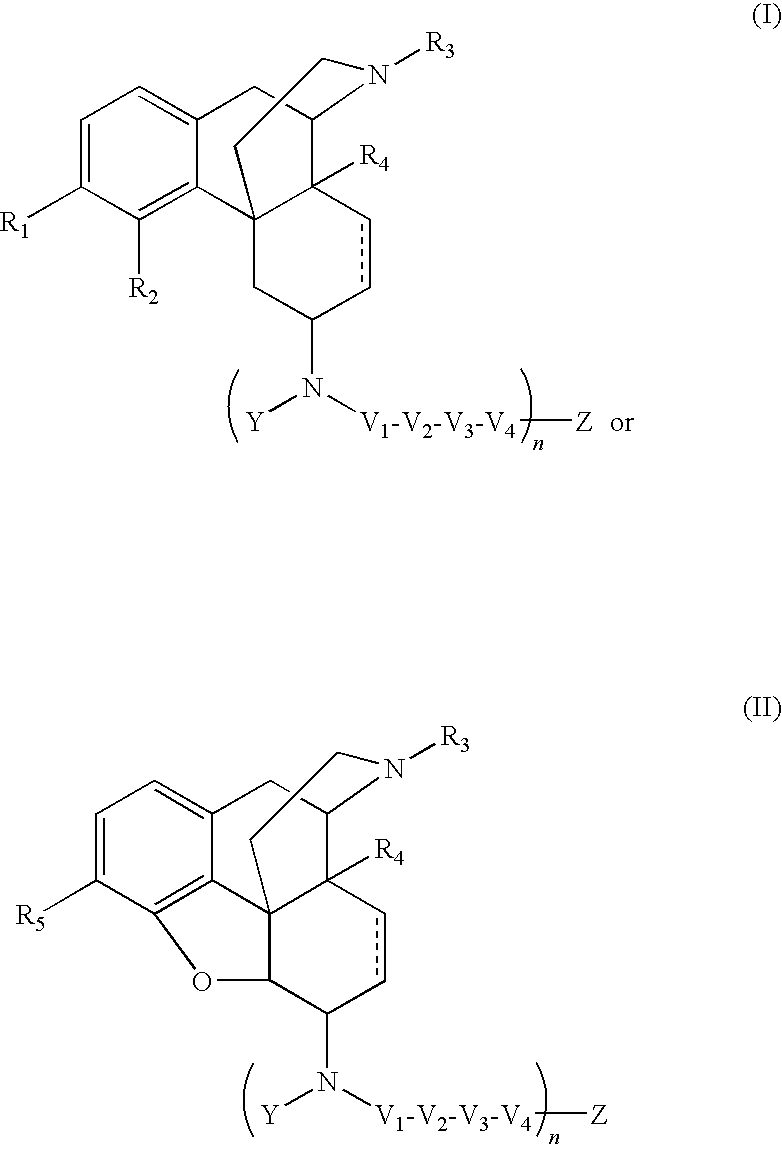
The present invention relates to compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which relates to morphinan compounds useful as μ, δ, and / or κ receptor opioid compounds and pharmaceuticals containing same that may be useful for mediating analgesia, combating drug addiction, alcohol addiction, drug overdose, mental illness, bladder dysfunctions, neurogenic bladder, interstitial cystitis, urinary incontinence, premature ejaculation, inflammatory pain, peripherally mediated and neuropathic pain, cough, lung edema, diarrhea, cardiac disorders, cardioprotection, depression, and cognitive, respiratory, diarrhea, irritable bowel syndrome and gastro-intestinal disorders, immunomodulation, and anti-tumor agents.
Owner:ALKERMES INC
Cellular Constituents From Bacteroides, Compositions Thereof, and Therapeutic Methods Employing Bacteroides or Cellular Constituents Thereof
A cellular constituent is lysed from, produced by and / or isolated from one or more bacteria from the genus Bacteroides, and the cellular constituent, a derivative thereof, and / or one or more bacteria from the genus Bacteroides, or a modified form thereof, is employed in compositions and methods for modulating an inflammatory response. Such methods include methods of treating, delaying the onset of or reducing the symptoms of one or more inflammatory conditions / diseases, including corporal or gastrointestinal inflammation, for example, Irritable Bowel Syndrome, Crohn's Disease, or colitis, and / or associated diseases such diabetes, asthma, multiple sclerosis, cancer, rheumatoid arthritis, gingivitis, atopic diseases, for example, hay fever, food allergies, eczema, rhinitis, dermatitis, conjunctivitis, atopic syndrome and keratosis pelaris, ocular inflammatory disease, strokes, cardiovascular disease, depression, atherosclerosis and hypertension, and comprise administering a composition comprising one or more natural and / or modified bacteria of the genus Bacteroides, and / or a cellular constituent lysed from, produced by, or isolated from one or more natural and / or modified bacteria from the genus Bacteroides, or a derivative thereof.
Owner:MOORE RES ENTERPRISES
Agonists of Guanylate Cyclase UseFul For The Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100093635A1Reduce inflammationPositive therapeutic effectAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS20160022592A1Improve imbalanceAntibacterial agentsBiocideAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS7879802B2Excellent propertyIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
Owner:BAUSCH HEALTH IRELAND LTD
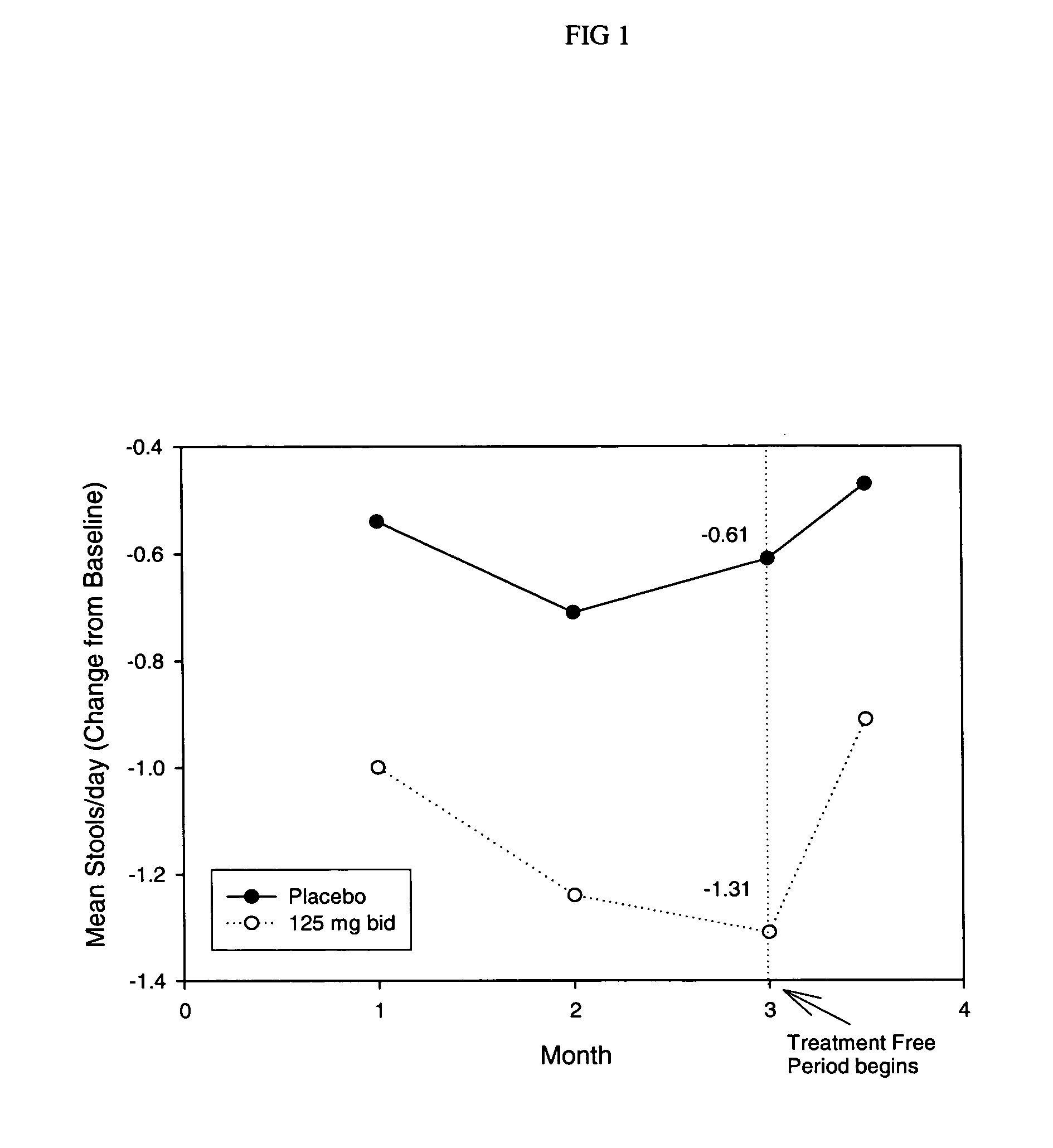
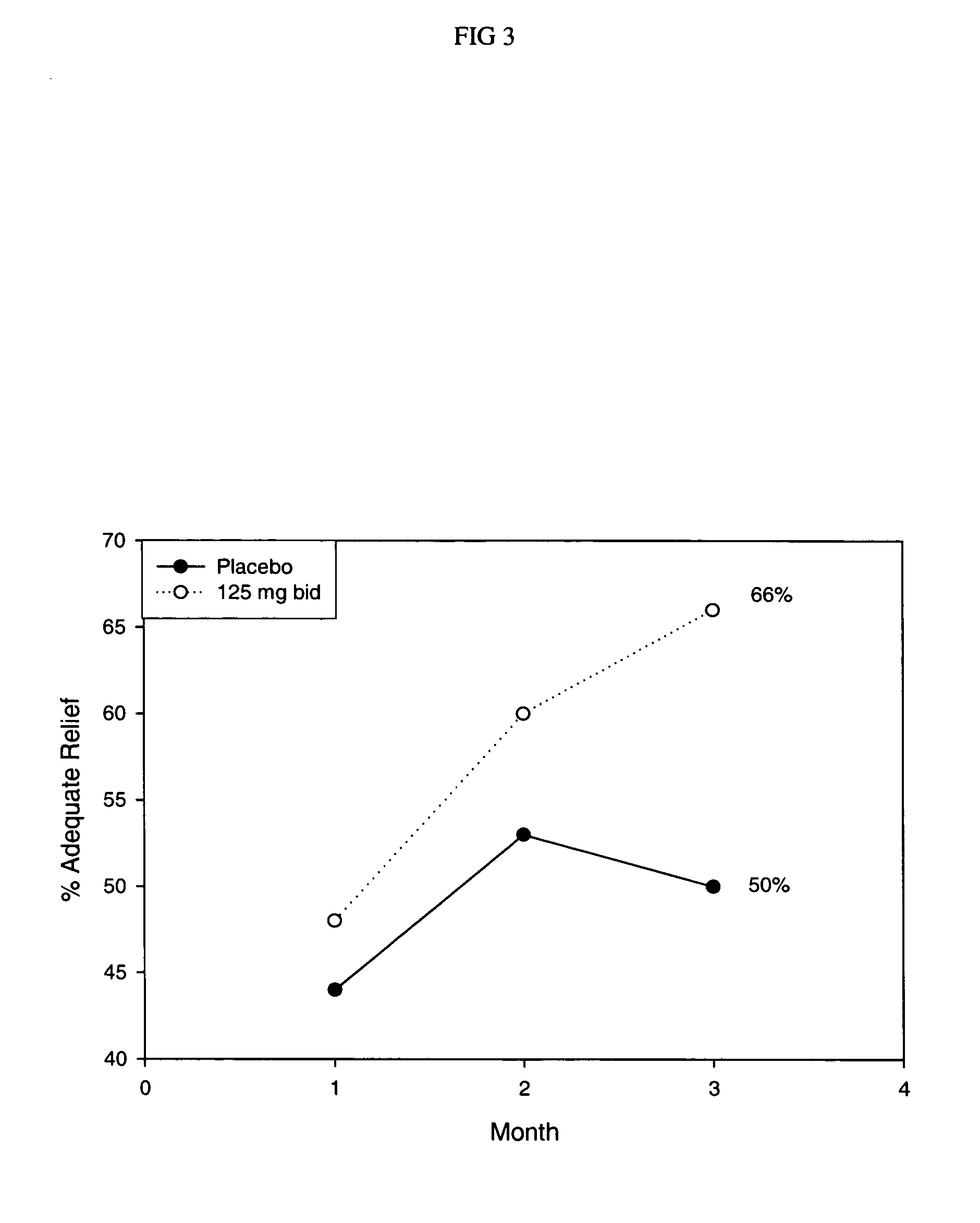
Methods for treating irritable bowel syndrome using optically pure (+) norcisapride
Methods for the prevention, treatment, or management of apnea, apnea disorders, bulimia nervosa, irritable bowel syndrome, urinary incontinence, bradycardia, bradyarrhythnia, syncope, other disorders, or symptoms thereof using (+) norcisapride, or a pharmaceutically acceptable salt thereof, substantially free of its (-) stereoisomer.
Owner:SEPACOR INC
Derivatives of 4- or 5-aminosalicylic acid
InactiveUS7910568B2Easily reach colonPromote absorptionBiocideAntipyreticSalicylic acidBULK ACTIVE INGREDIENT
Owner:ANTIBE THERAPEUTICS INC
Methods for diagnosing irritable bowel syndrome
InactiveUS20120238468A1Improve the level ofNucleotide librariesMicrobiological testing/measurementTest sampleBacilli
The present invention discloses a method for diagnosing Irritable Bowel Syndrome (IBS) in a test sample by determining the level of several bacterial taxa in the test sample, comparing this level with the levels of those bacterial taxa in a control sample, and relating the level to a diagnosis of IBS. Additionally, the present invention provides a method for treatment of IBS based on said diagnosis. Also, the invention provides a method for subtyping IBS in a test sample.
Owner:AAK PATENT
Chinese herbal medicine combination for treating disease of disorder of bowels's function and its product
A Chinese medicine for treating irritable bowel syndrome (IBS) is prepared from 6 Chinese-medicinal materials including bupleurum root, white peony root, Chuan-xiong rhizome, liquorice root, etc. It may be particles, capsules, tablets, oral liquid injection, etc.
Owner:NANJING NORMAL UNIVERSITY
Derivaitves of 4-Or 5-Aminosalicylic Acid
InactiveUS20080207564A1Less readily absorbedEasily reach colonBiocideAntipyreticThioester synthesisSalicylic acid
The present invention provides new derivatives of 4- or 5-aminosalicylic acid, and a pharmaceutical composition containing these derivatives of 4- or 5-aminosalicylic acid as active ingredients, useful for the treatment of intestinal diseases such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) and for the prevention / treatment of colon cancer. More particularly, these derivatives comprise a hydrogen sulfide releasing moiety linked via an azo, an ester, an anhydride, a thioester or an amide linkage to a molecule of 4- or 5-aminosalicylic acid. Furthermore, the present invention provides a process for preparing these compounds and their use for treating IBD and IBS and the prevention / treatment of colon cancer.
Owner:ANTIBE THERAPEUTICS INC
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS8034782B2Excellent propertyIncrease resistanceAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Novel compounds, isomer thereof, or pharmaceutically acceptable salts thereof as vanilloid receptor antagonist; and pharmaceutical compositions containing the same
This present invention relates to novel compounds, isomer thereof or pharmaceutically acceptable salts thereof as vanilloid receptor (Vanilloid Receotor 1; VR1; TRPV1 )antagonist; and a pharmaceutical composition containing the same. The present invention provides a pharmaceutical composition for preventing or treating a disease such as pain, migraine, arthralgia, neuralgia, neuropathies, nerve injury, skin disorder, urinary bladder hypersensitiveness, irritable bowel syndrome, fecal urgency, a respiratory disorder, irritation of skin, eye or mucous membrane, stomach-duodenal ulcer, inflammatory diseases, ear disease, and heart disease.
Owner:AMOREPACIFIC CORP
Therapy for enteric infections
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
Lactobacillus reuteri and its application
ActiveCN107523526AImprove the level ofImprove triglyceridesMilk preparationNervous disorderDiseaseGut flora
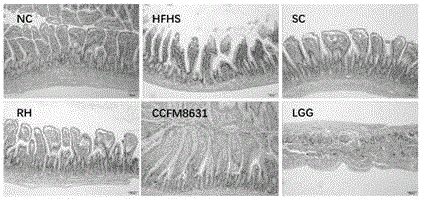
The invention relates to the technical field of microorganism, and discloses a Lactobacillus reuteri and its application. The preservation number of the Lactobacillus reuteri CCFM8631 is CGMCC No.14394; the level of mice peripheral neurotransmitter 5-hydroxytryptamine can be significantlyimproved; the rise of the mice peripheral blood testonsterone level and the abundant abnormity of Blautia, Turicibacter, Oscillospira and Bifidobacterium in the intestinal flora by high glucose and high fatty diets are recovered; the tolerated simulative gastrointestinal fluid is rapidly planted in an intestinal tract, so as to significantly improve the pathological injury of metabolic syndrome mice liver and duodenum and rise of triglyceride and total cholesterol content in the serum by high glucose and high fatty diets are significantly improved; the Lactobacillus reuteri can be used for preventing, delaying or treating metabolic disorder such as metabolic syndrome, irritable bowel syndrome, and anxiety, depression and other metal diseases related to irritable bowel syndrome.
Owner:INFINITUS (CHINA) CO LTD
Method for treatment of diarrhea-predominant irritable bowel syndrome
InactiveUS20070254050A1Inhibition of secretionAdequate doseBiocideNervous disorderGynecologyCrofelemer
The present invention provides methods for treating diarrhea-predominant irritable bowel syndrome comprising administering to a patient in need thereof, an inhibitor of chloride-ion transport in an amount sufficient to treat diarrhea-predominant irritable bowel syndrome (d-IBS). Treatment of d-IBS includes the treatment of the diarrhea component of d-IBS as well as the pain, abdominal discomfort and other symptoms associated with d-IBS. In one embodiment, the inhibitor of chloride-ion transport is crofelemer.
Owner:NAPO PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com