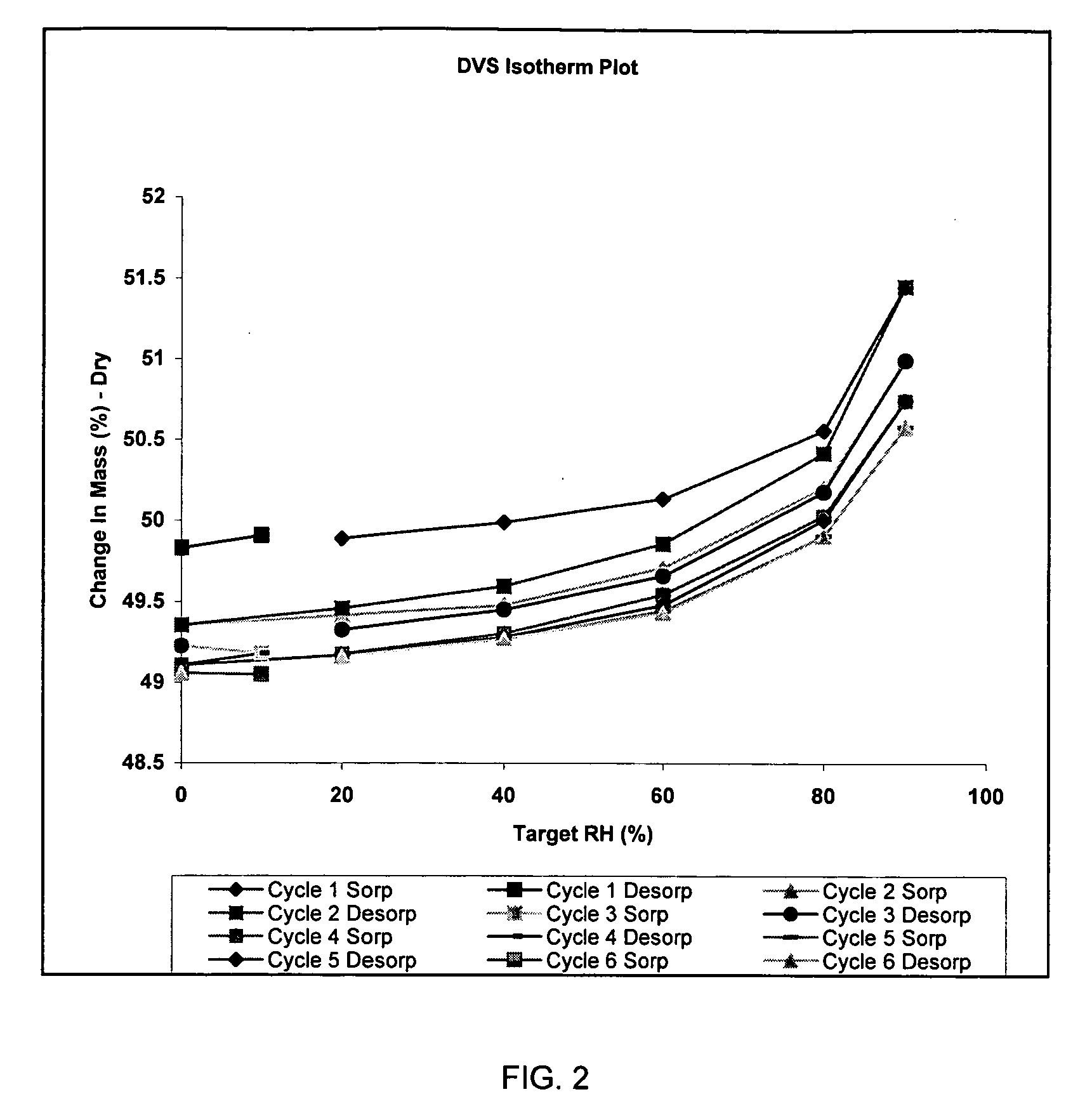
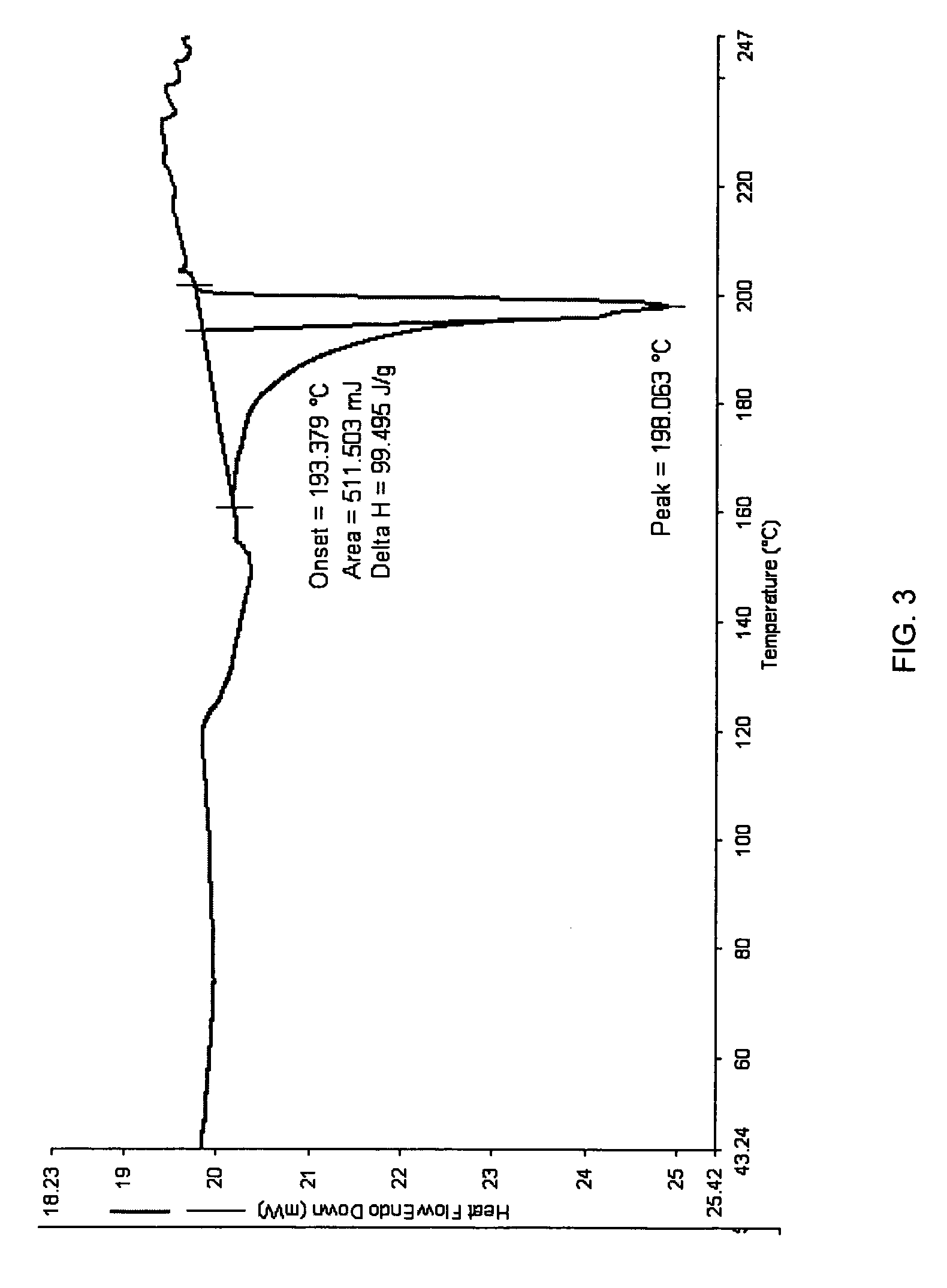
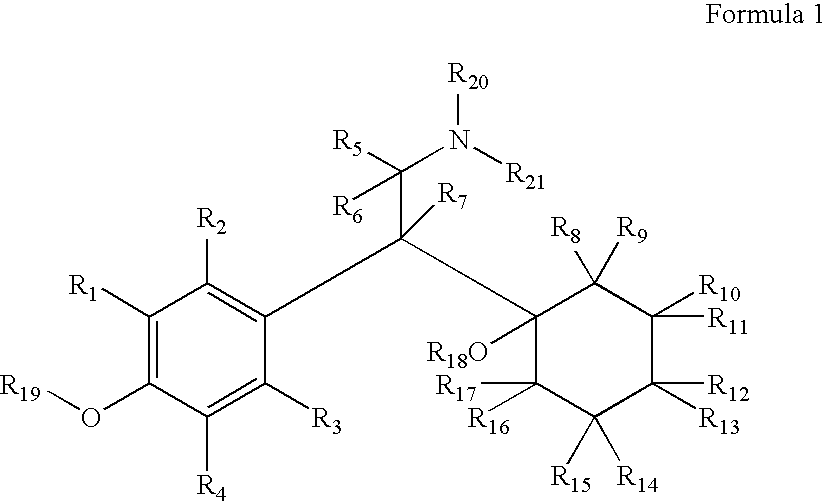
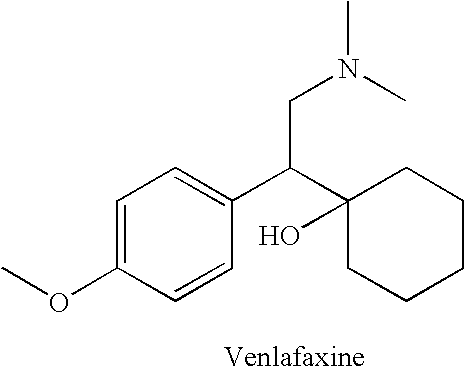
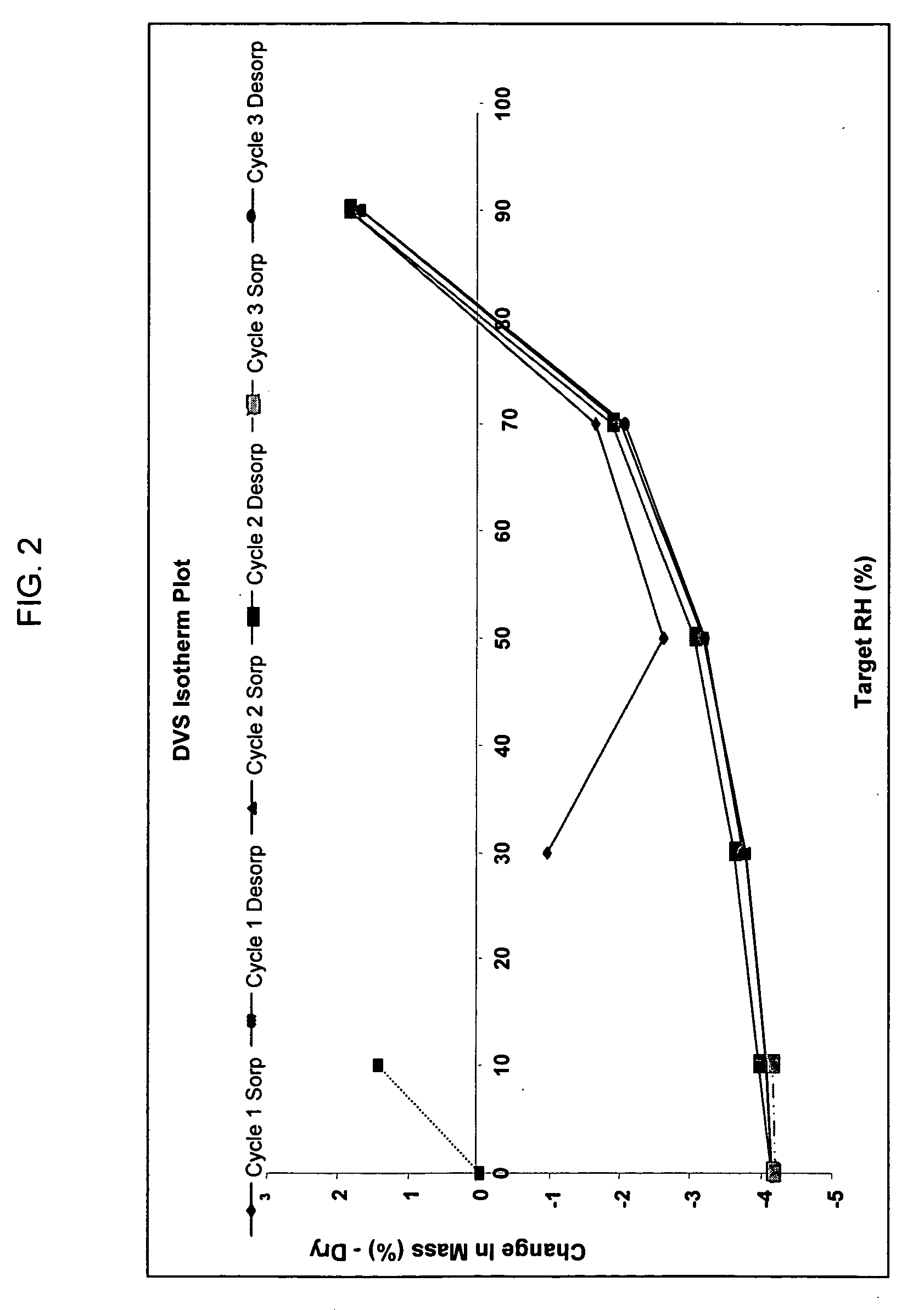
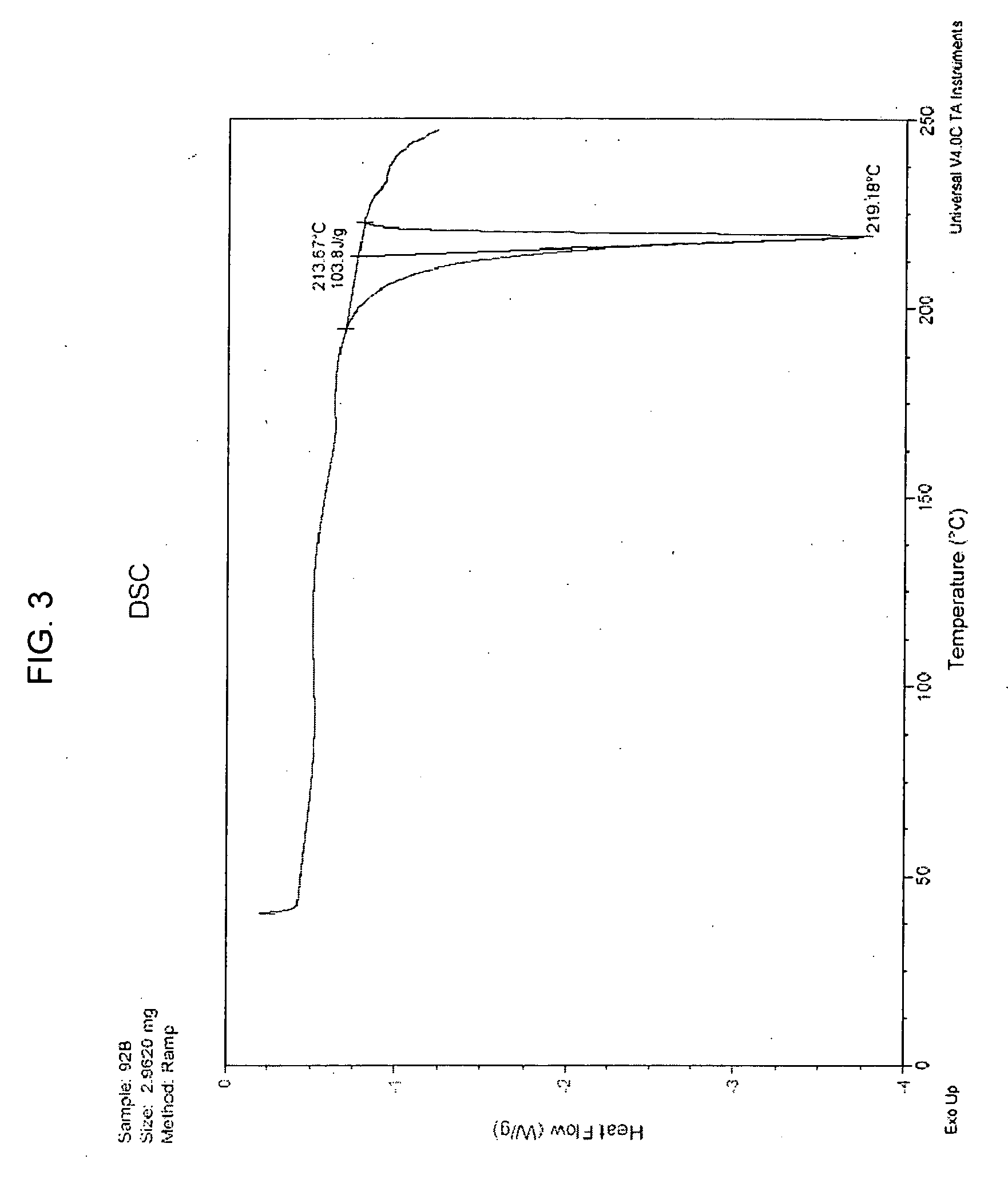
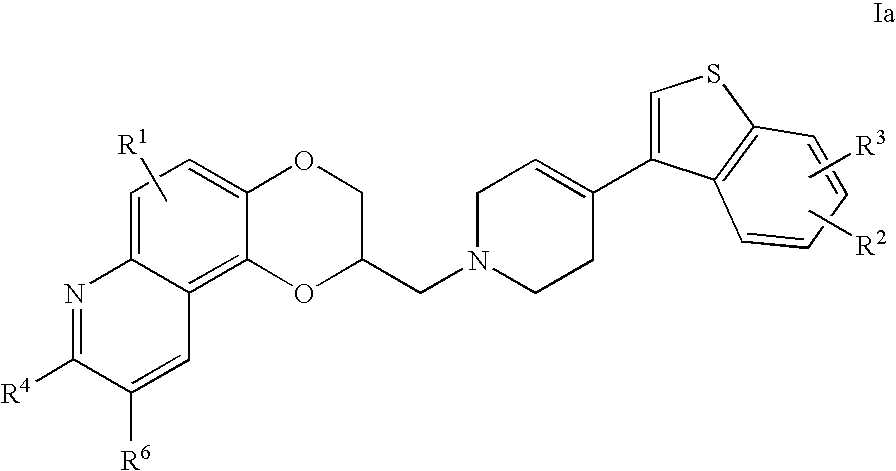
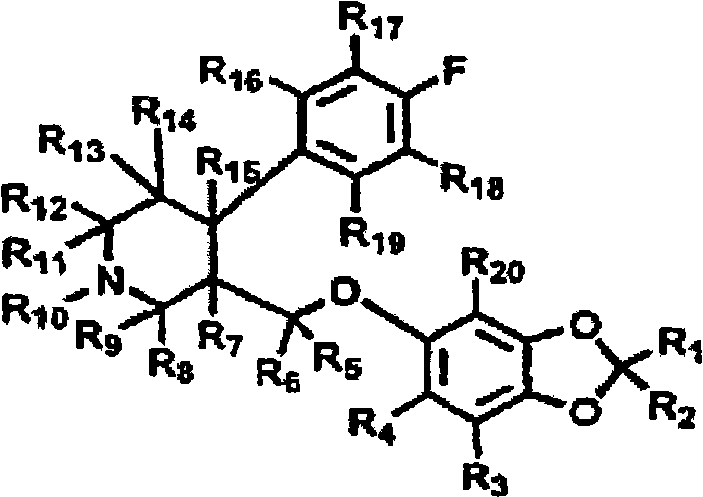
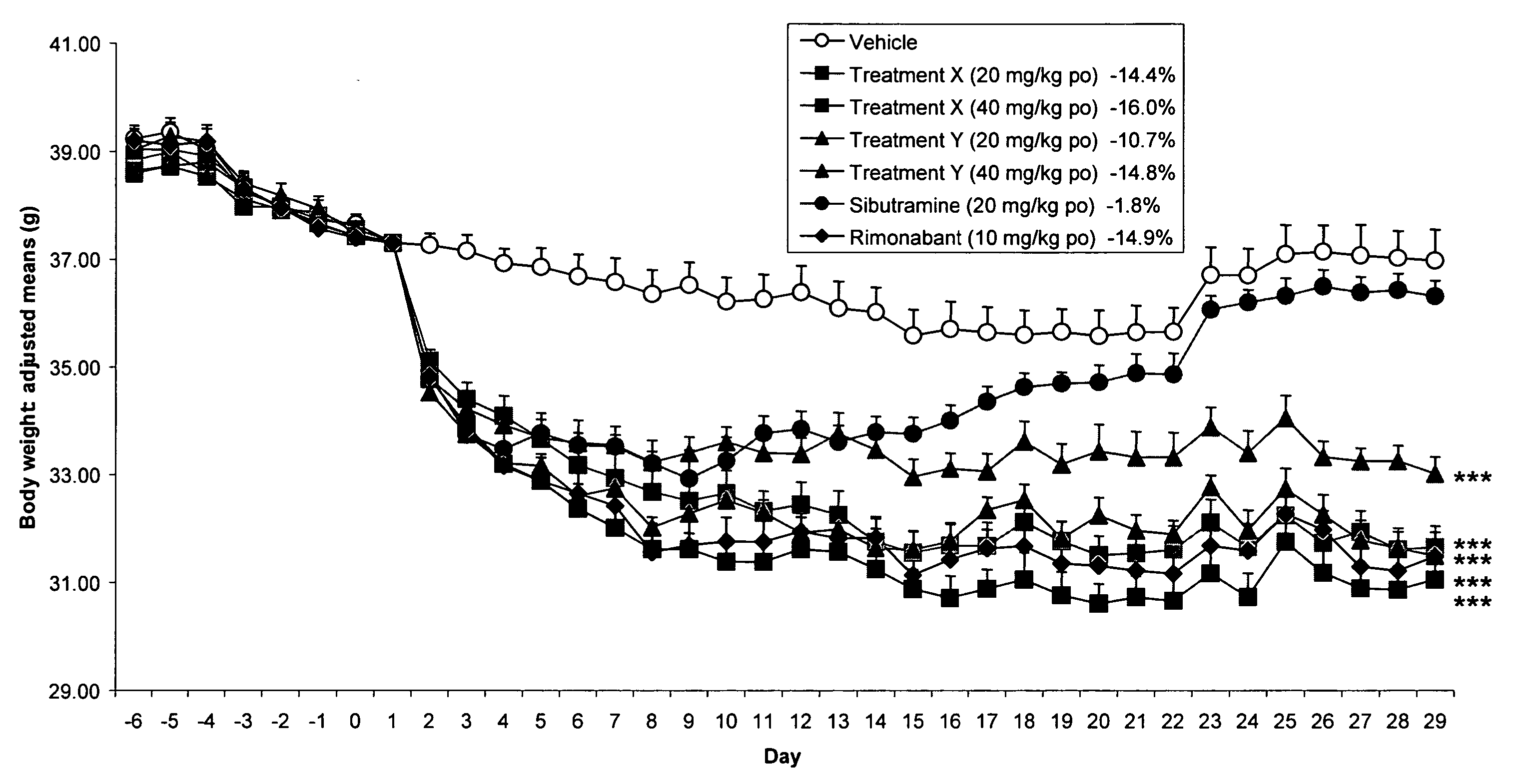
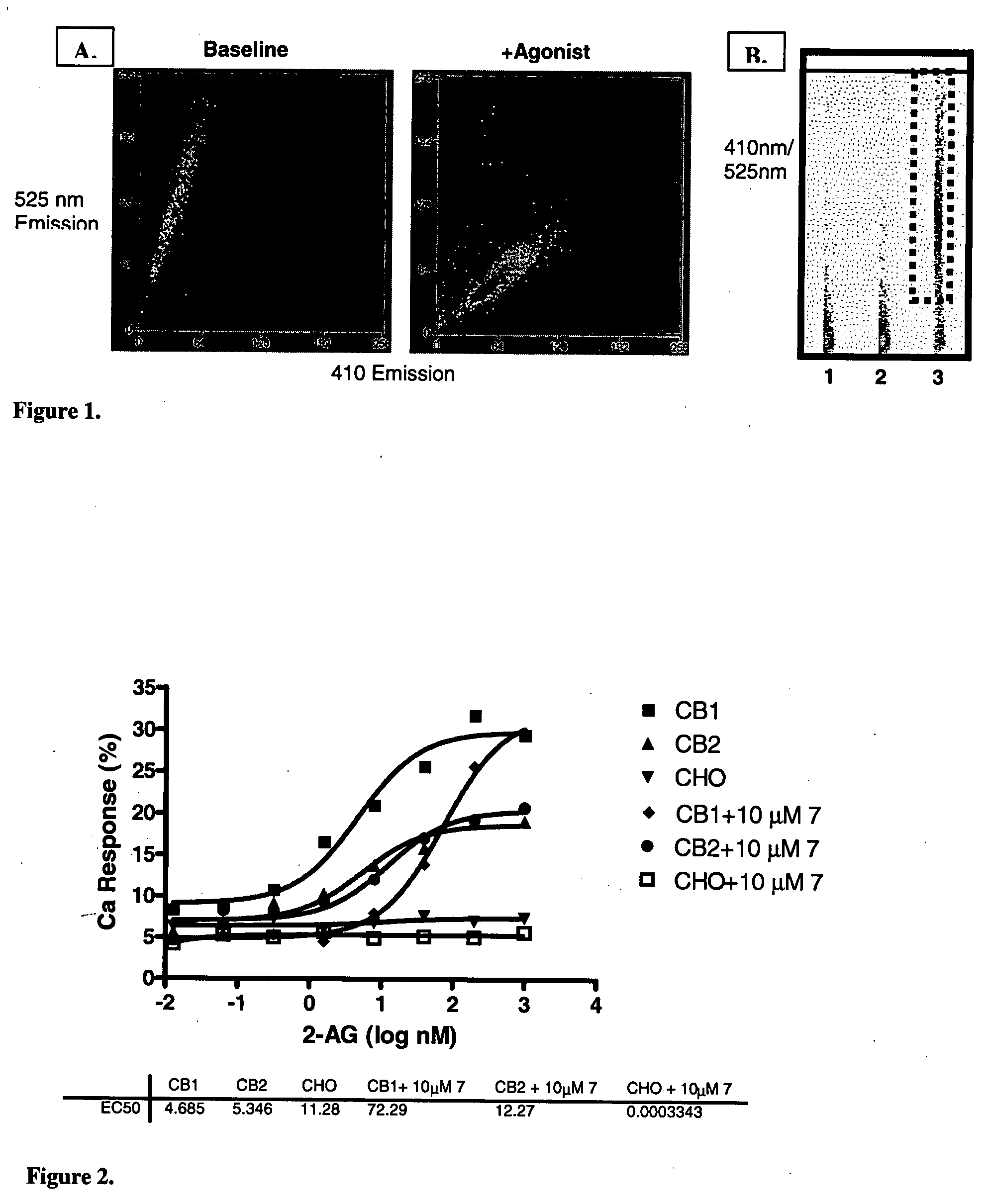
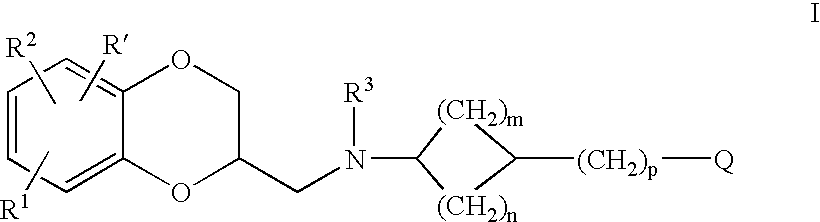
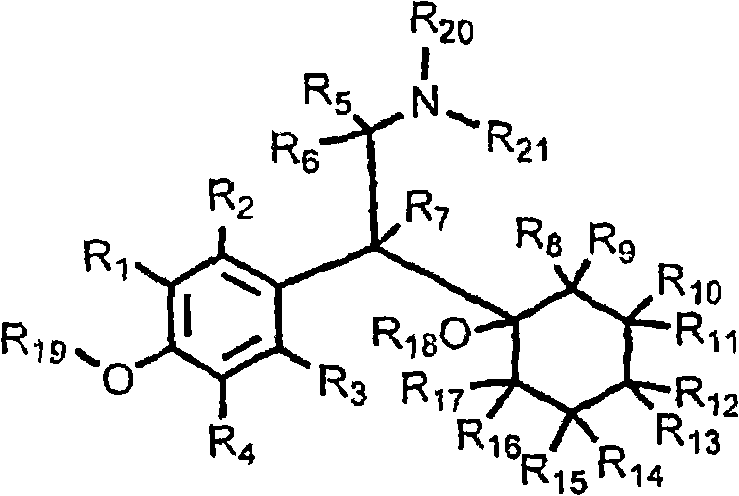
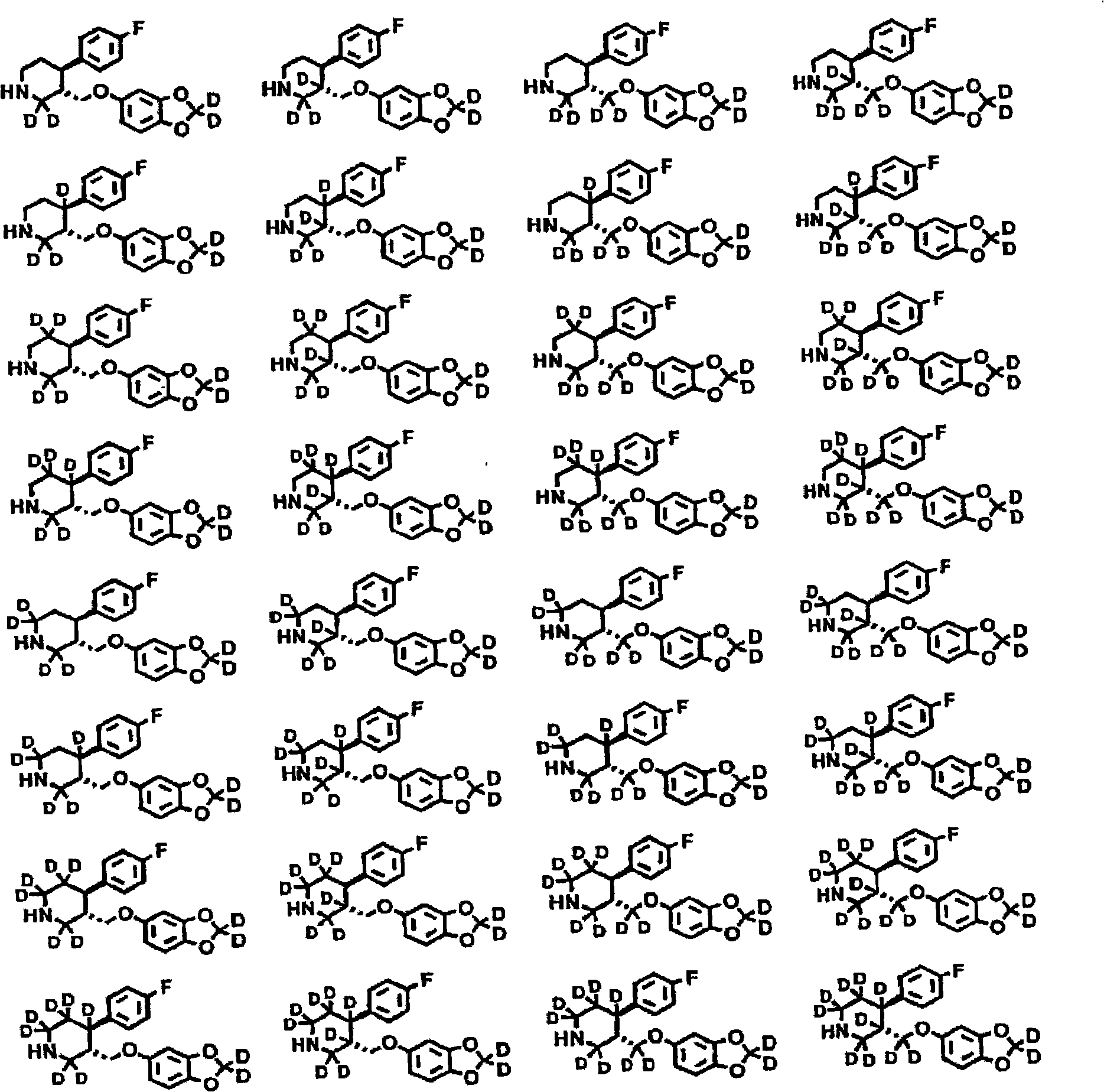Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Bulimia nervosa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Eating disorder characterized by binge eating, followed by methods to avoid weight gain.
Methods for treating irritable bowel syndrome using optically pure (+) norcisapride
Methods for the prevention, treatment, or management of apnea, apnea disorders, bulimia nervosa, irritable bowel syndrome, urinary incontinence, bradycardia, bradyarrhythnia, syncope, other disorders, or symptoms thereof using (+) norcisapride, or a pharmaceutically acceptable salt thereof, substantially free of its (-) stereoisomer.
Owner:SEPACOR INC
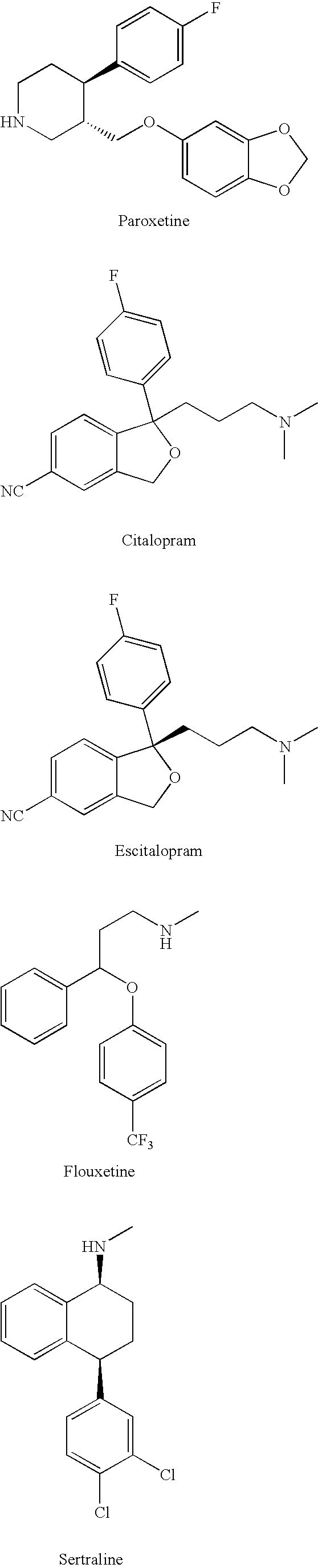
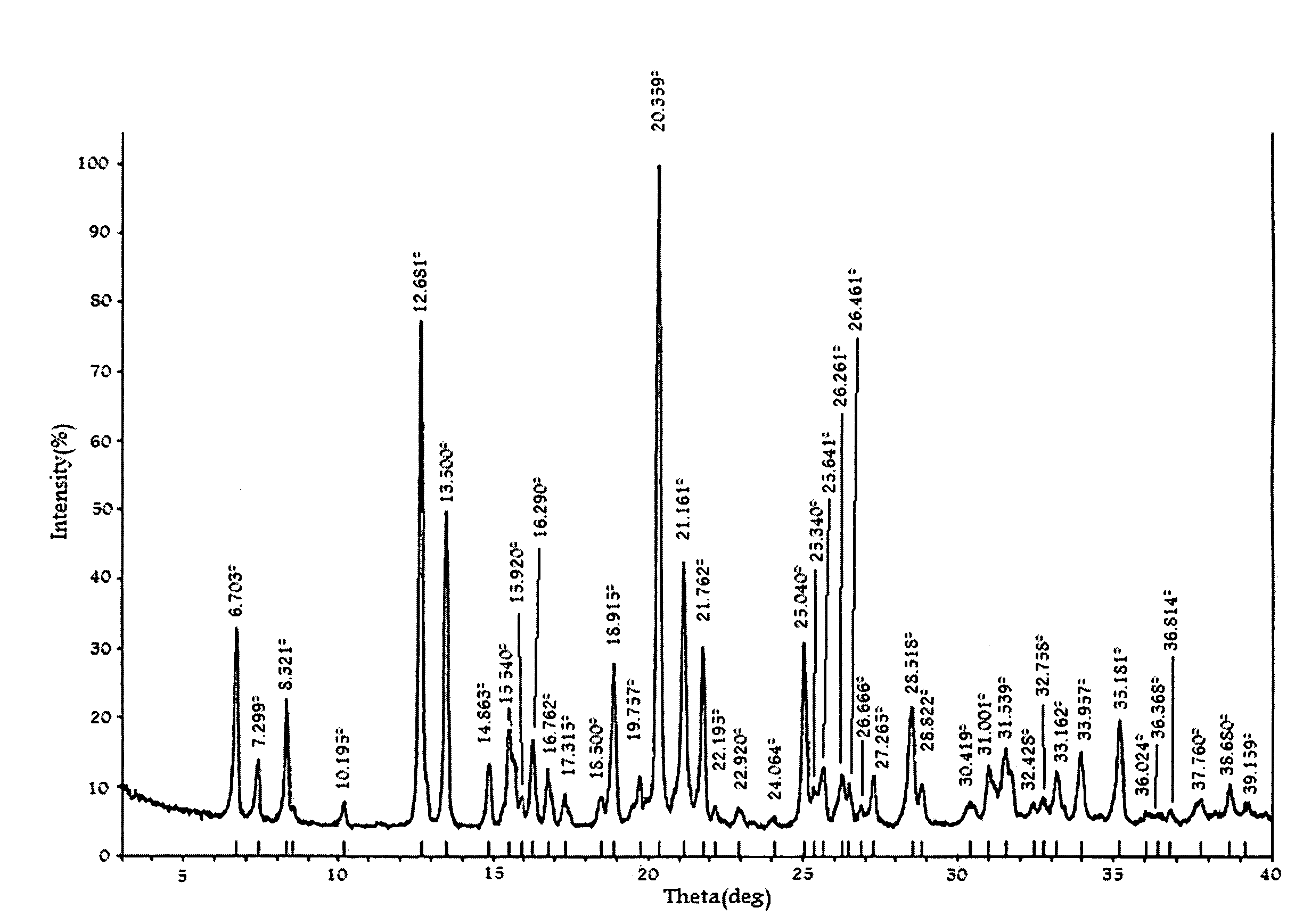
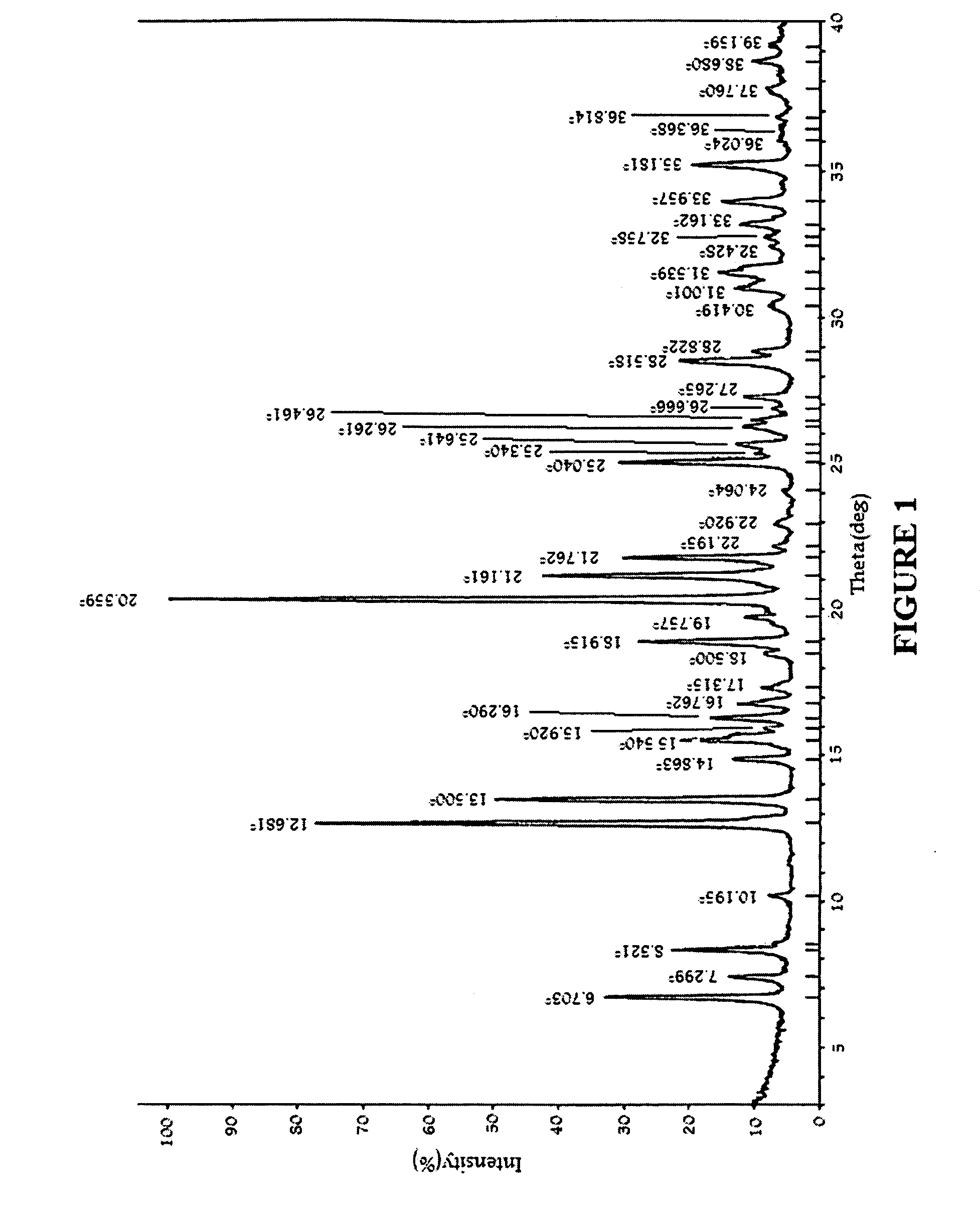
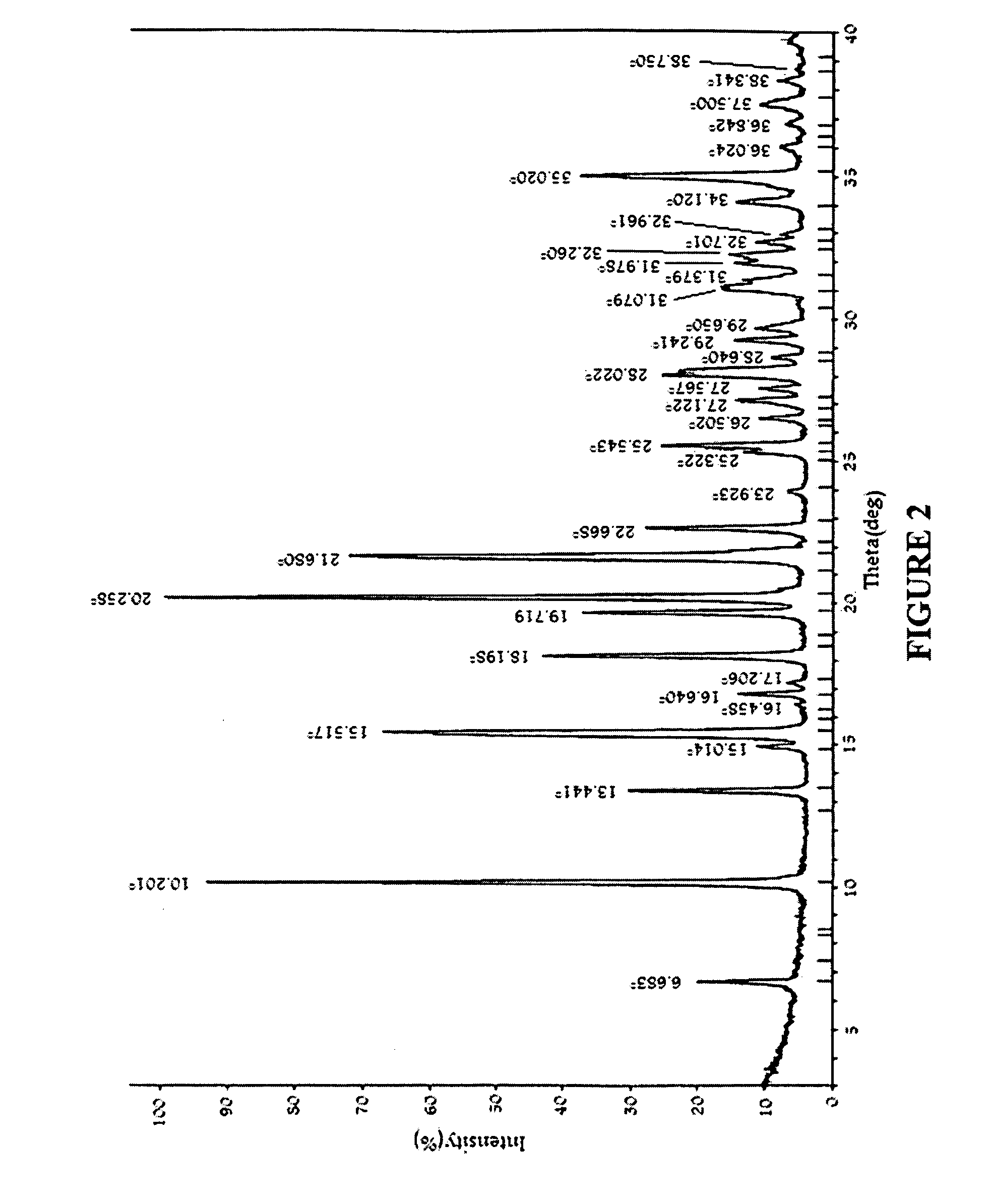
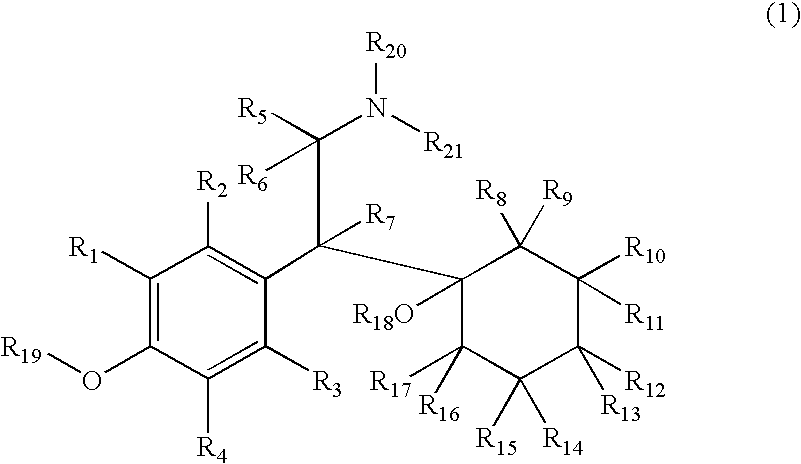
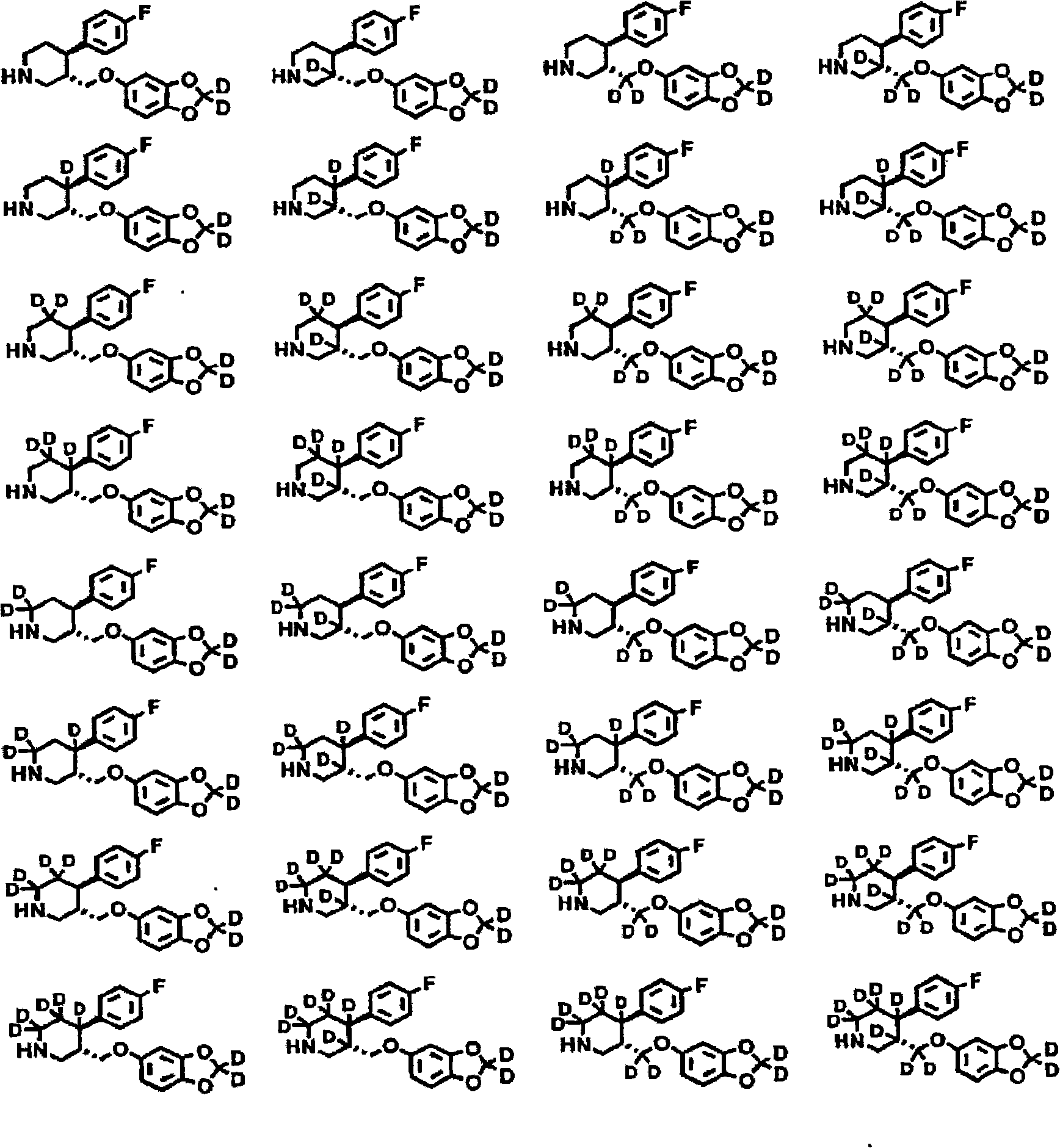
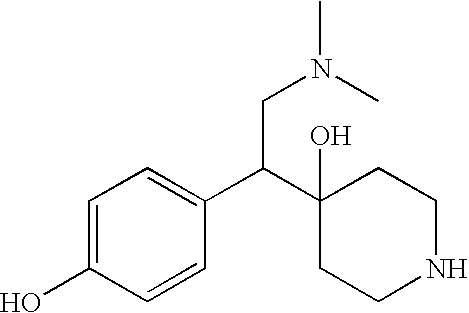
Substituted phenylpiperidines with serotoninergic activity and enhanced therapeutic properties
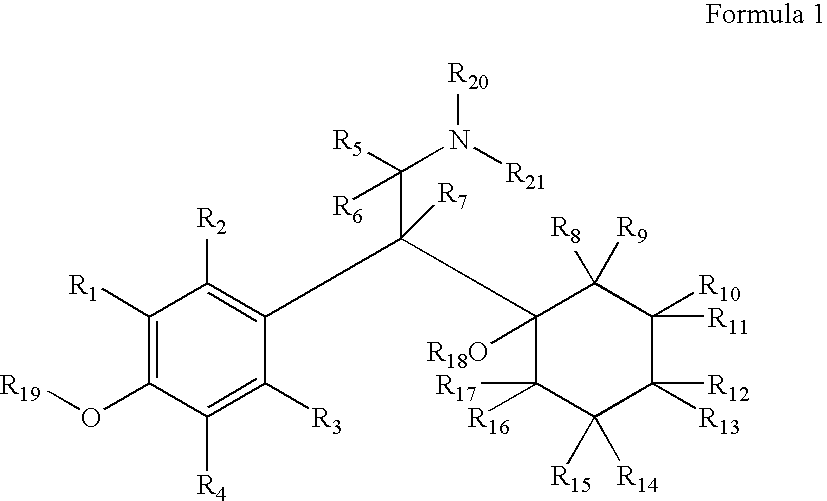
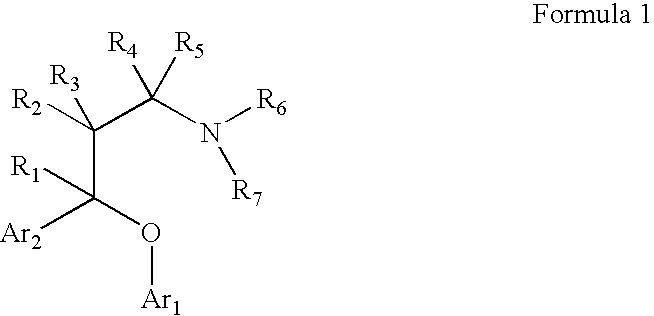
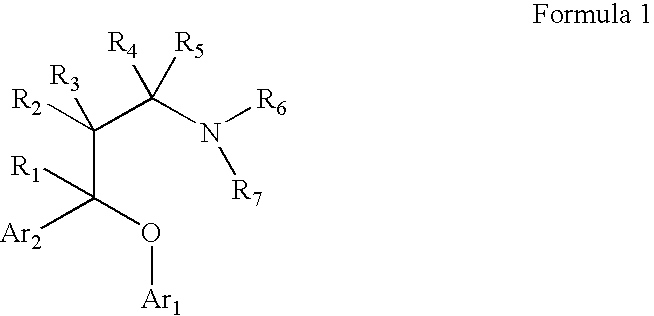
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
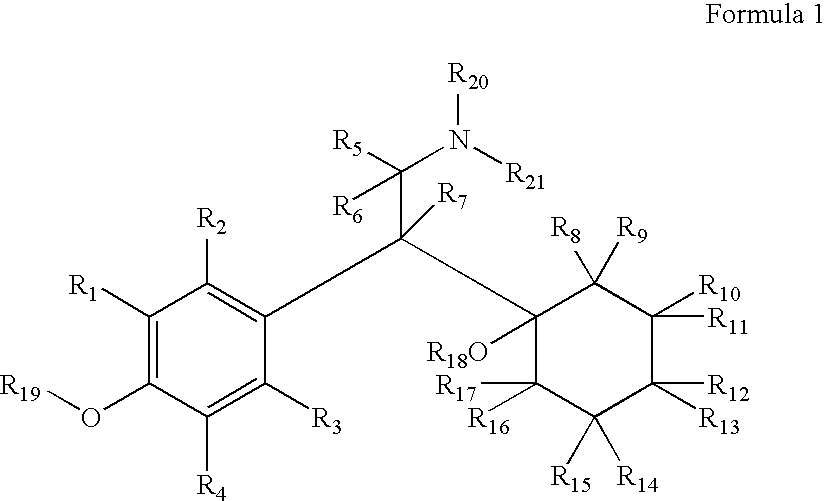
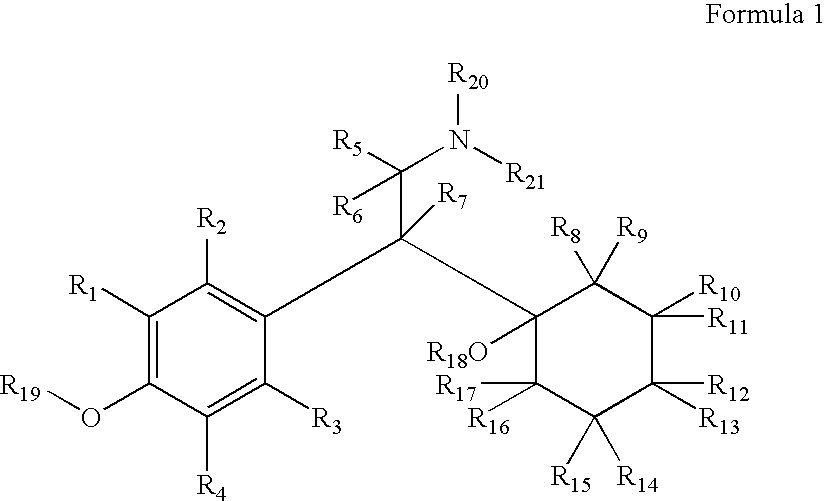
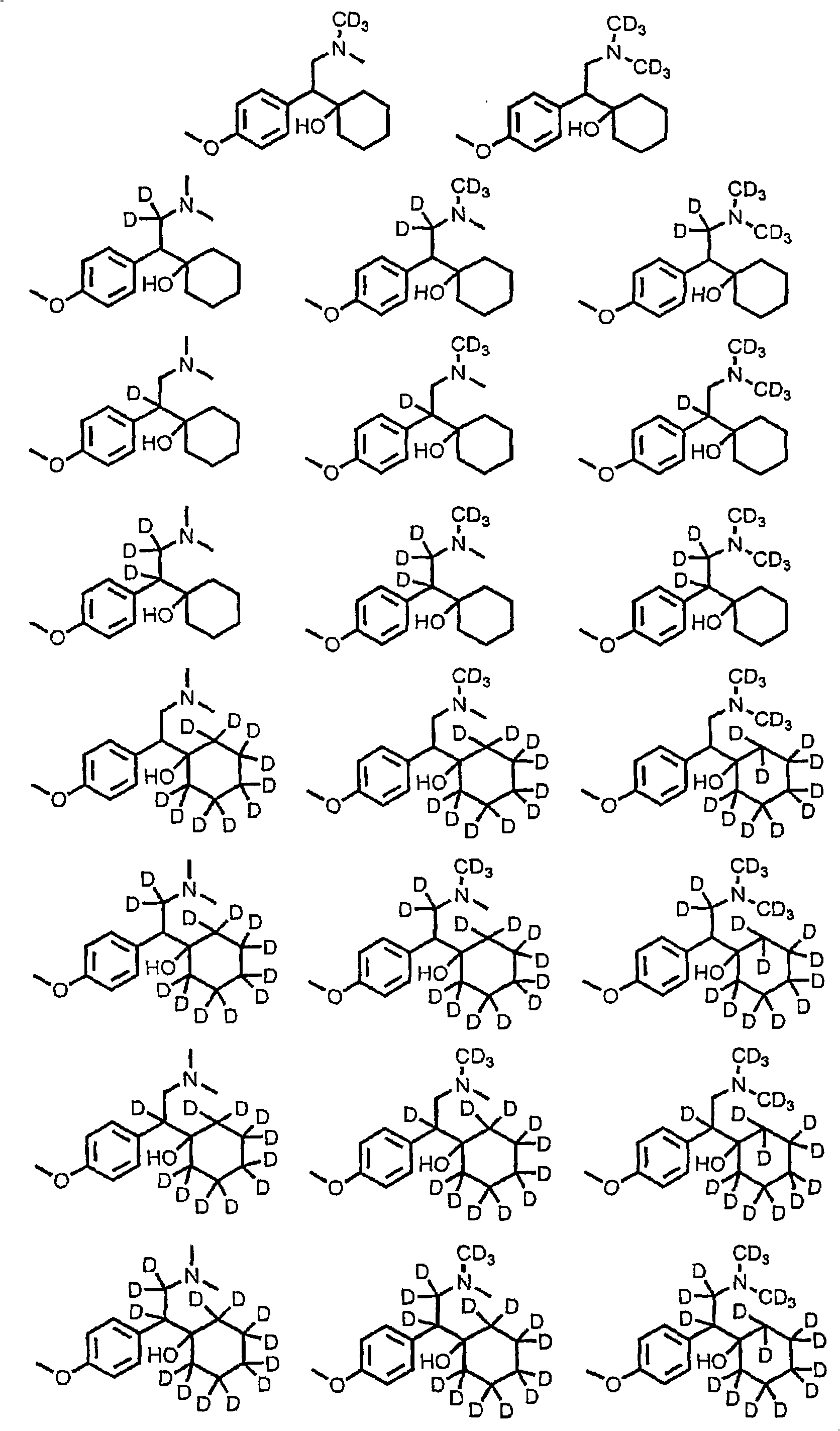
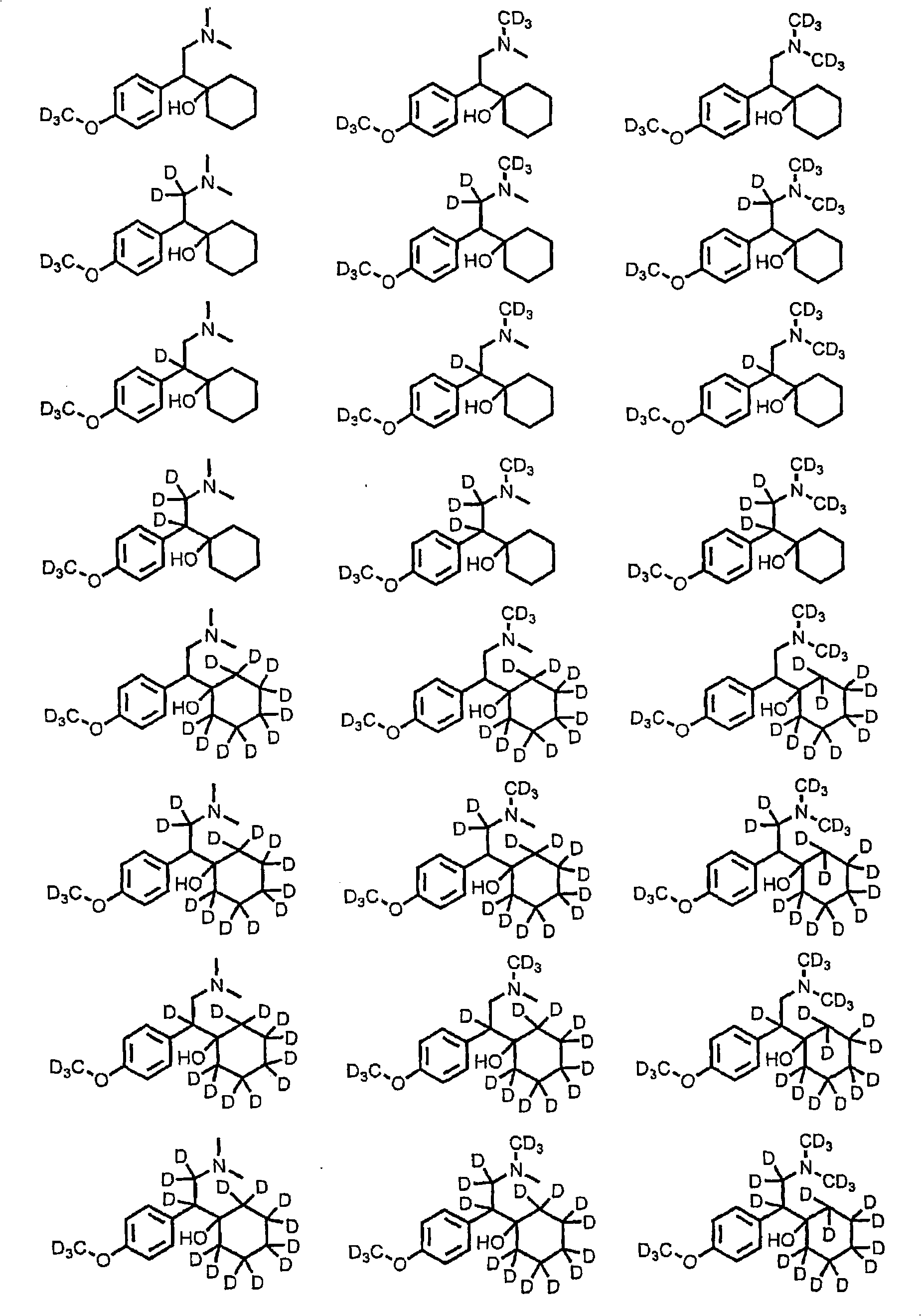
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
InactiveUS20080234257A1High crystallinityThe result is moreBiocideNervous disorderDiabetic retinopathyChemical synthesis
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, attention deficit hyperactivity disorder, fibromyalgia, irritable bowel syndrome, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Owner:ACADIA PHARMA INC
Highly selective serotonin and norepinephrine dual reuptake inhibitor and use thereof
InactiveUS20070015828A1Eliminate side effectsBiocideNervous disorderNorepinephrine reuptake inhibitorVisceral pain
Highly selective dual serotonin and norepinephrine reuptake inhibitors are provided. These compounds have a lower side-effect profile and are useful in compositions and products for use in treatment of a variety of conditions including depression, fibromyalgia, anxiety, panic disorder, agorophobia, post traumatic stress disorder, premenstrual dysphoric disorder, attention deficit disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, autism, schizophrenia, obesity, anorexia nervosa, bulimia nervosa, Gilles de la Tourette Syndrome, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction, borderline personality disorder, fibromyalgia syndrome, diabetic neuropathic pain, chronic fatigue syndrome, pain, visceral pain, Shy Drager syndrome, Raynaud's syndrome, Parkinson's Disease, and epilepsy.
Owner:WYETH
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Substituted aryloxypropylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Methods for treating apnea, apnea disorders, bulimia, and other disorders using optically pure (+) norcisapride
InactiveUS6048879ARelieve symptomsReducing and avoiding adverse effectBiocideNervous disorderDiseaseNorcisapride
Methods for the prevention, treatment or management of apnea, apnea disorders, bulimia nervosa, irritable bowel syndrome, urinary incontinence, bradycardia, bradyarrhythmia, syncope, other disorders, or symptoms thereof using (+) norcisapride, or a pharmaceutically acceptable salt thereof, substantially free of its (-) stereoisomer.
Owner:SEPACOR INC
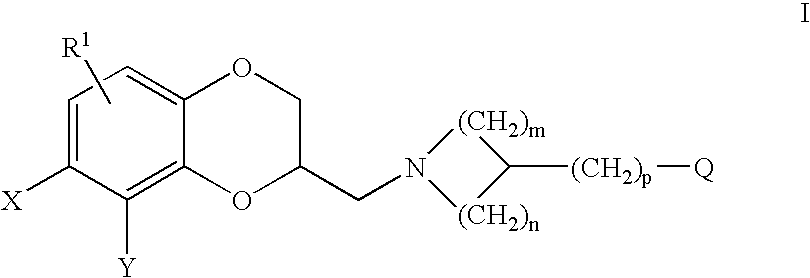
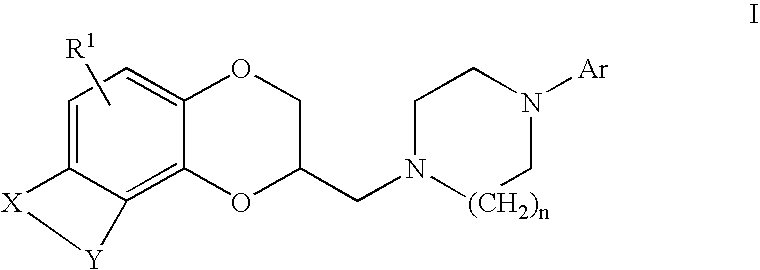
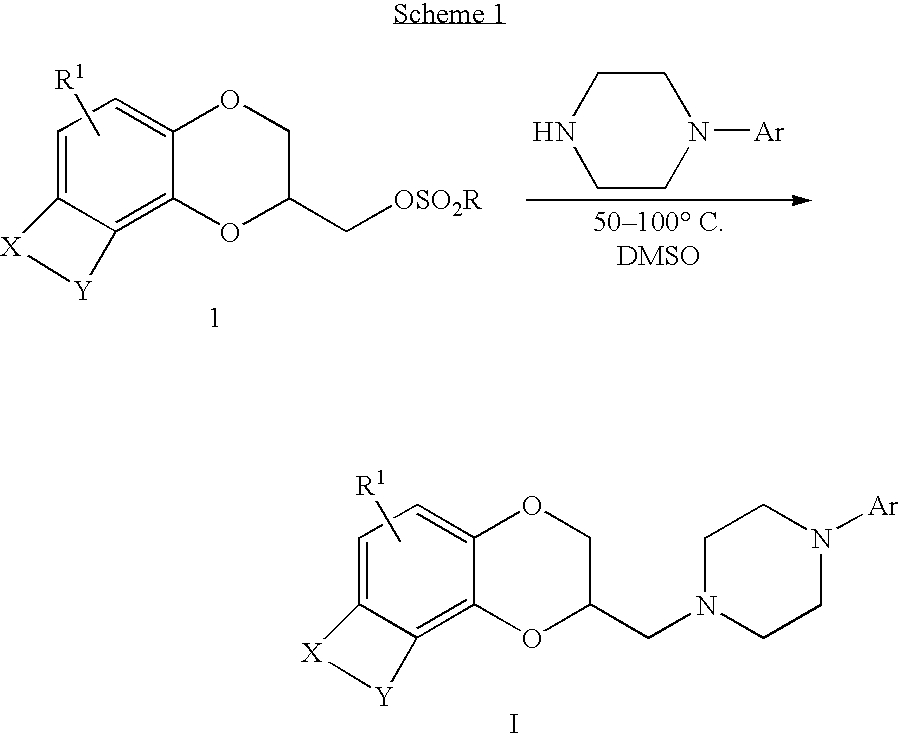
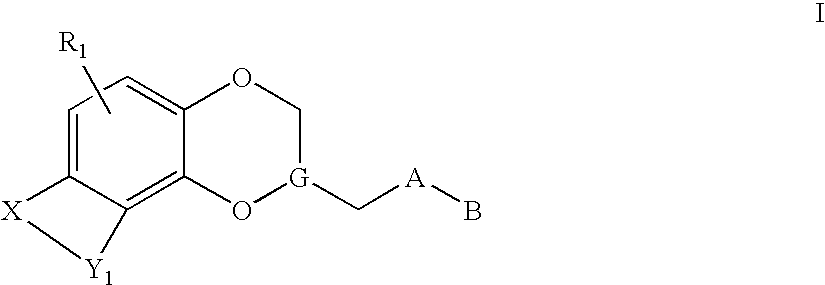
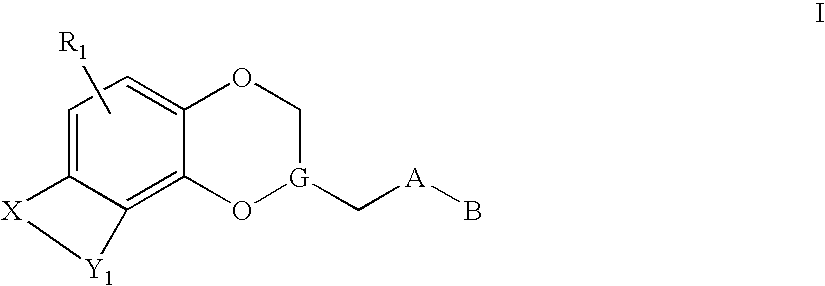
Antidepressant azaheterocyclylmethyl derivatives of heterocycle-fused benzodioxans
Compounds of the Formula:are useful for the treatment of depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as pre-menstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa and bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH LLC
Serotonin and norepinephrine reuptake inhibitor and uses thereof
InactiveUS20070015824A1Low level of undesirable side-effectsBiocideOrganic chemistryNorepinephrine reuptake inhibitorDepressant
Selective dual serotonin and norepinephrine reuptake inhibitors are provided. These compounds have a lower side-effect profile and are useful in compositions and products for use in treatment of a variety of conditions including depression, fibromyalgia, anxiety, panic disorder, agoraphobia, post traumatic stress disorder, premenstrual dysphoric disorder, attention deficit disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, autism, schizophrenia, obesity, anorexia nervosa, bulimia nervosa, Gilles de la Tourette Syndrome, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction, borderline personality disorder, fibromyalgia syndrome, diabetic neuropathic pain, chronic fatigue syndrome, pain, Shy Drager syndrome, Raynaud's syndrome, Parkinson's Disease, and epilepsy.
Owner:WYETH
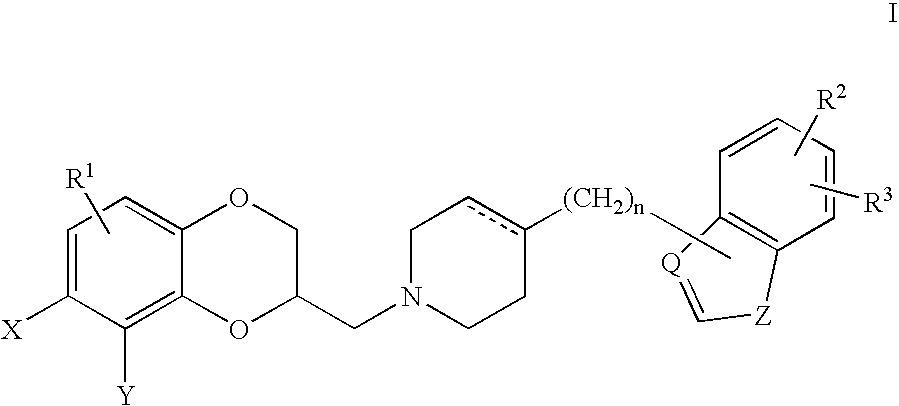
Antidepressant piperidine derivatives of heterocycle-fused benzodioxans
Compounds of the Formula: are useful for the treatment of depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as premenstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa and bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH LLC
Substituted phenylpiperidines with serotoninergic activity and enhanced therapeutic properties
InactiveCN101360742AOrganic active ingredientsNervous disorderCompulsive disordersDiabetic retinopathy
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive- compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
Serotonin and norepinephrine reuptake inhibitors and uses thereof
InactiveUS20070015791A1Reduce adverse side effectsBiocideOrganic chemistryNorepinephrine reuptake inhibitorS syndrome
Selective dual serotonin and norepinephrine reuptake inhibitors are provided. These compounds have a lower side-effect profile and are useful in compositions and products for use in treatment of a variety of conditions including depression, fibromyalgia, anxiety, panic disorder, agorophobia, post traumatic stress disorder, premenstrual dysphoric disorder, attention deficit disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, autism, schizophrenia, obesity, anorexia nervosa, bulimia nervosa, Gilles de la Tourette Syndrome, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction, borderline personality disorder, fibromyalgia syndrome, diabetic neuropathic pain, chronic fatigue syndrome, pain, Shy Drager syndrome, Raynaud's syndrome, Parkinson's Disease, and epilepsy.
Owner:WYETH LLC
Methods for treating apnea, apnea disorders, and other disorders using optically pure (-) norcisapride
InactiveUS20030158229A1Relieve symptomsReducing and avoiding adverse effectBiocideNervous disorderDiseaseNorcisapride
Methods for the prevention, treatment, or management of apnea, apnea disorders, bulimia nervosa, irritable bowel syndrome, urinary incontinence, bradycardia, bradyarrhythmia, syncope, other disorders, or symptoms thereof using (-) norcisapride, or a pharmaceutically acceptable salt thereof, substantially free of its (+) stereoisomer.
Owner:SEPACOR INC
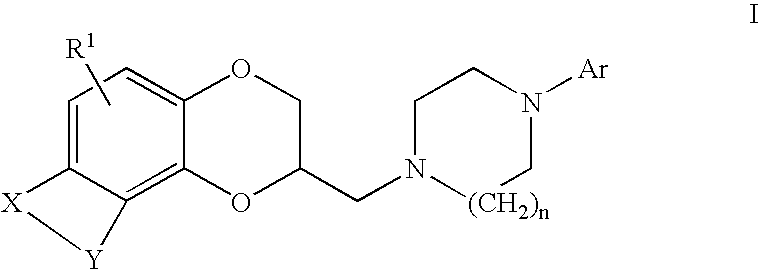
Antidepressant arylpiperazine derivatives of hetrocycle-fused benzodioxans
Compounds of the formula I:are useful for the treatment of depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as pre-menstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive-compulsive disorder, social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa and bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH LLC
Antidepressant azaheterocyclyl methyl derivatives of 7,8-dihydro-6H-5-oxa-1-aza-phenanthrene
Compounds of the formula useful for the treatment of such as depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as pre-menstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder (including trichotillomania), social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa, bulimia nervosa, vasomotor flushing, cocaine and alcohol addition, sexual dysfunction (including premature ejaculation), and related illnesses.
Owner:WYETH LLC
CB1 antagonists and inverse agonists
InactiveUS20080027087A1Minimizing metabolic risk factorEfficient dosingBiocideOrganic chemistryMetabolic syndromeAN - Anorexia nervosa
The present invention relates to methods of treating obesity, anorexia nervosa, or bulimia nervosa comprising administering a compound of the invention. The present invention further relates to the treatment of metabolic syndrome comprising administering a compound of the invention.
Owner:AMPLA PHARMA
Antidepressant cycloalkylamine derivatives of 2,3-dihydro-1,4-benzodioxan
Compounds of the Formula I: are useful for the treatment of depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as pre-menstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa and bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
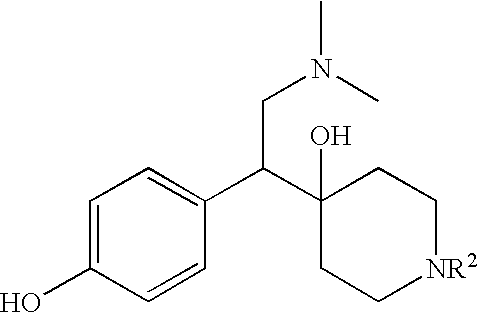
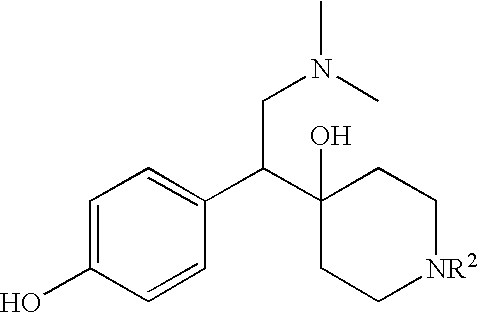
Treating method and medicine kit containing somatotrophic hormone secretory agent
The present invention relates to methods of treating bulimia nervosa, male erectile dysfunction, female sexual dysfunction, thyroid cancer, breast cancer, or ameliorating ischemic nerve or muscle damage. The present invention also relates to kits that can be used in the treatment of bulimia nervosa, male erectile dysfunction, female sexual dysfunction, thyroid cancer, breast cancer, or ameliorating ischemic nerve or muscle damage. The present invention further relates to increasing gastrointestinal motility after surgery and increasing gastrointestinal motility in patients who have been administered an agent that decreases gastrointestinal motility.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
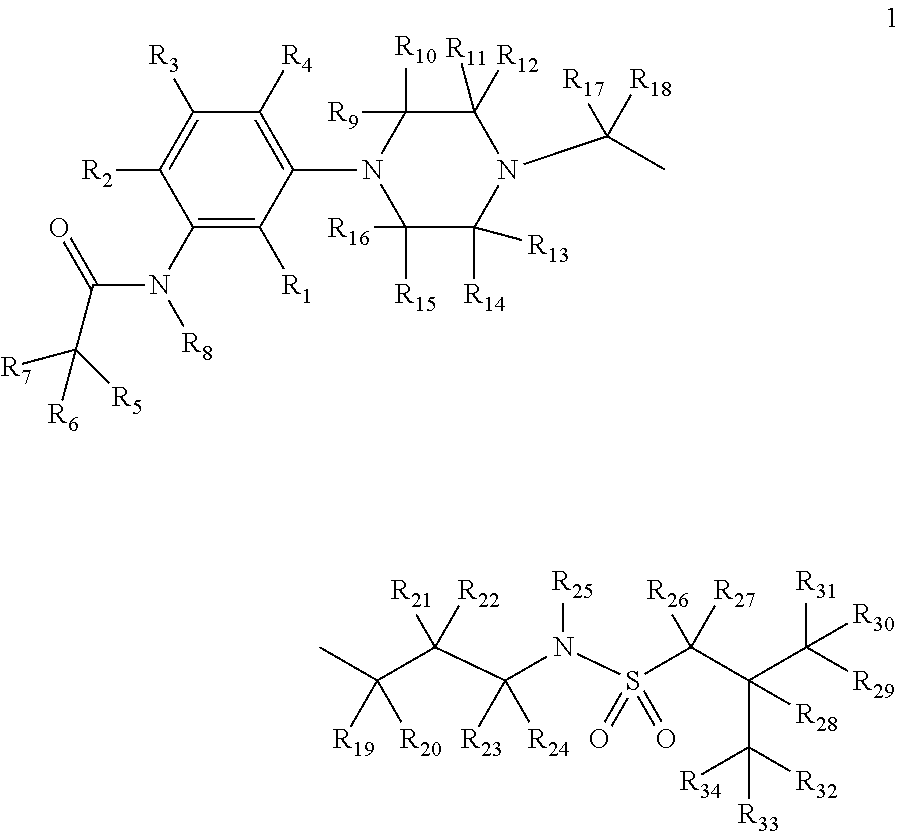
Deuterium-enriched alkyl sulfonamides and uses thereof
ActiveUS8927553B2Prevent curingOrganic active ingredientsOrganic chemistryRecurrent major depressive episodesFibromyalgia
The present invention is concerned with deuterium-enriched sulfonamides of formula 1, their pharmaceutically acceptable salts and methods of use thereof for the treatment of anxiety disorders including, General Anxiety Disorder (GAD), Panic Disorder (PD), Post-Traumatic Stress Disorder (PTSD), Social Phobia (SP), Health Anxiety (Hypochondriasis), depression, major depressive disorders, unipolar depression, bipolar I depression disorder, bipolar II depression disorder, treatment-resistant depression, single episodic and recurrent major depressive disorders, depression in the medically ill, attention deficit hyperactivity disorder (ADHD), attention deficit disorder (ADD), Obsessive-Compulsive Disorder (OCD), Obsessive-Compulsive Personality Disorder (OCPD), Autism Spectrum Disorder (ASD), schizophrenia, psychosis, epilepsy, seizures, hot flashes due to menopause, age-related macular degeneration (AMD), premature ejaculation, male erectile dysfunction, sexual dysfunction, obesity, eating disorders, bulimia nervosa, anorexia nervosa, angina, migraine, pain, nociception, sleep disorders, insomnia, fibromyalgia, alcohol withdrawal, autism, Rett's syndrome, cyclothymic disorder, neural injury, neurodegenerative diseases, Parkinson's disease, Parkinson's disease psychosis, Huntington disease, Alzheimer's disease, frontotemporal dementia, cognitive impairment associated with age-related dementia, Alzheimer's disease, schizophrenia, psychosis, depression, pain or discomfort associated with surgery and pain or discomfort associated with medical illness.
Owner:DHANOA DALJIT SINGH
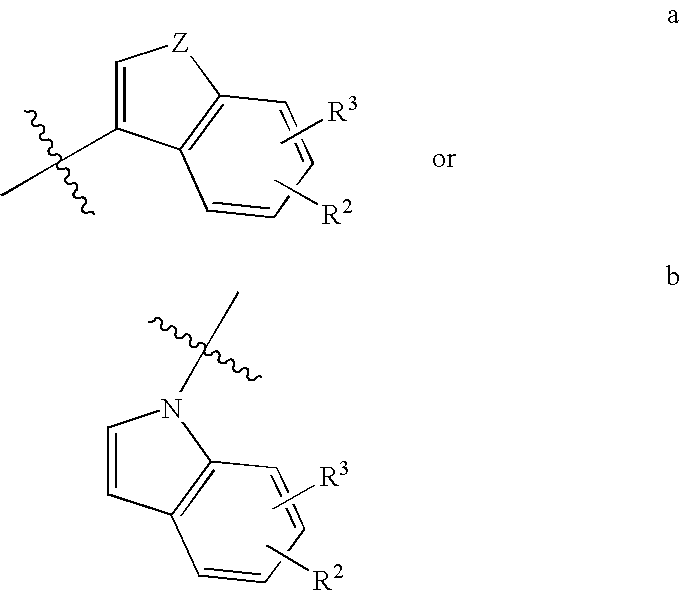
Antidepressant heteroaryl derivatives of heterocycle-fused benzodioxans
InactiveUS20090042874A1Shorten the incubation periodIncreased serotonin levelBiocideNervous disorderDiseaseAttention deficits
The invention provides compounds of the Formula:that are useful for the treatment of depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as pre-menstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa, bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH LLC
CB1 antagonists and inverse agonists
InactiveUS20090036426A1Minimizing metabolic risk factorMinimize risk factorBiocideMetabolism disorderMetabolic syndromeAN - Anorexia nervosa
The present invention relates to methods of treating obesity, anorexia nervosa, or bulimia nervosa comprising administering a compound of the invention conjointly with zonisamide, naltrexone, or a pharmaceutically acceptable salt thereof. The present invention further relates to the treatment of metabolic syndrome comprising administering a compound of the invention conjointly with zonisamide, naltrexone, or a pharmaceutically acceptable salt thereof.
Owner:AMPLA PHARMA
Antidepressant piperidine derivatives of heterocyclefused benzodioxans
Compounds of the Formula: are useful for the treatment of depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as pre-menstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa and bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH LLC
Antidepressant azaheterocyclylmethyl derivatives of 1,4,5-trioxa-phenanthrene
Compounds of the formula useful for the treatment of diseases such as depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder (including trichotillomania), social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa, bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH LLC
Traditional Chinese medicine for treating bulimia nervosa
InactiveCN105616887ADefinite curative effectNo side effectsNervous disorderAnthropod material medical ingredientsSide effectElaeagnus angustifolia
The invention discloses a traditional Chinese medicine for treating bulimia nervosa. The traditional Chinese medicine comprises exocarpium, seeds of champion bauhinia, root of Crinite Uraria, longstamen onion bulb, Chinese wampee fruits, fructus perillae, common vladimiria root, aspongopus, radish, trifoliate orange, citron, flos mume, reddish orange, morningstar lily bulb, amber, Elaeagnus angustifolia, pearl and south Sinkiang ziziphora herb. The traditional Chinese medicine utilizes pure natural Chinese herbal medicines as raw materials, realizes simultaneous treatment of principal and subordinate symptoms, has definite therapeutic effects and no toxic or side effect and has a low recurrence rate.
Owner:孙玉娥
Method for the detection and/or diagnosis of eating disorders and malnutrition using X-ray diffraction
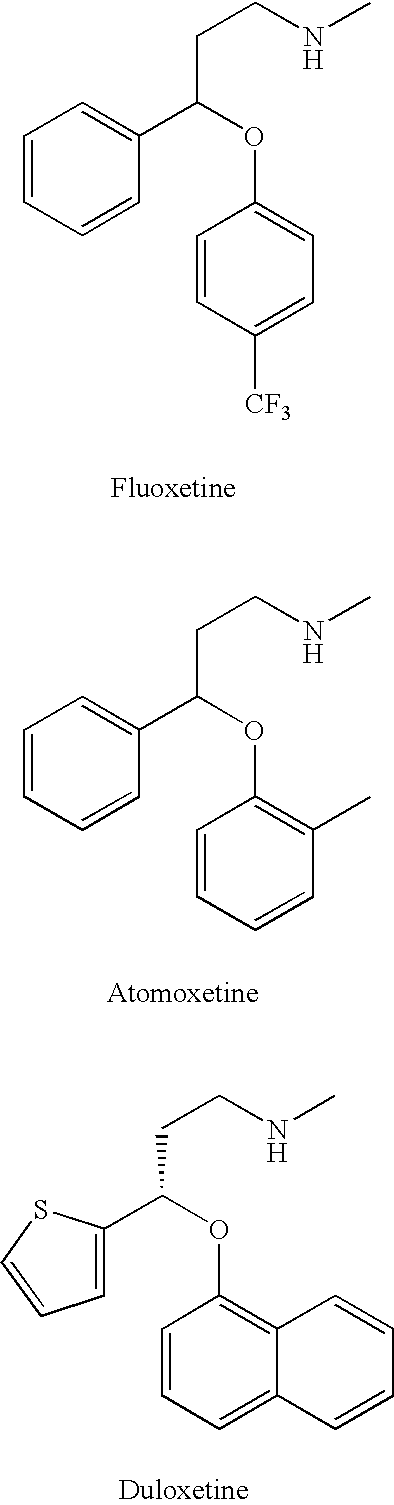
Alternatives described herein relate to methods for detecting, identifying, and / or diagnosing eating disorders, nutritional deficiencies, and / or malnutrition, including conditions such as bulimia nervosa and / or anorexia nervosa, by using X-ray diffraction on a sample of a tested subject's hair. In some alternatives, once an eating disorder, nutritional deficiency, or malnutrition is detected, identified, or diagnosed using the X-ray diffraction approaches set forth herein, a subject identified as having an eating disorder, nutritional deficiency, or malnutrition is provided counseling for the disorder and / or a medicament to treat, inhibit, or ameliorate said eating disorder or malnutrition, including Prozac, ami trip tyline, fluoxe tine, imipramine, Nardil, Tofranil, desipramine, Sarafem, Norpramin, and / or phenelzine.
Owner:STRUCTURE ASE
Antidepressant azaheterocyclylmethyl derivatives of 7,8-dihydro-6H-5-oxa-1-aza-phenanthrene
Compounds of the formula useful for the treatment of such as depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as premenstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder (including trichotillomania), social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa, bulimia nervosa, vasomotor flushing, cocaine and alcohol addition, sexual dysfunction (including premature ejaculation), and related illnesses.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com