Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Generalized anxiety disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Exaggerated tension, worrying, and nervousness about daily life events.
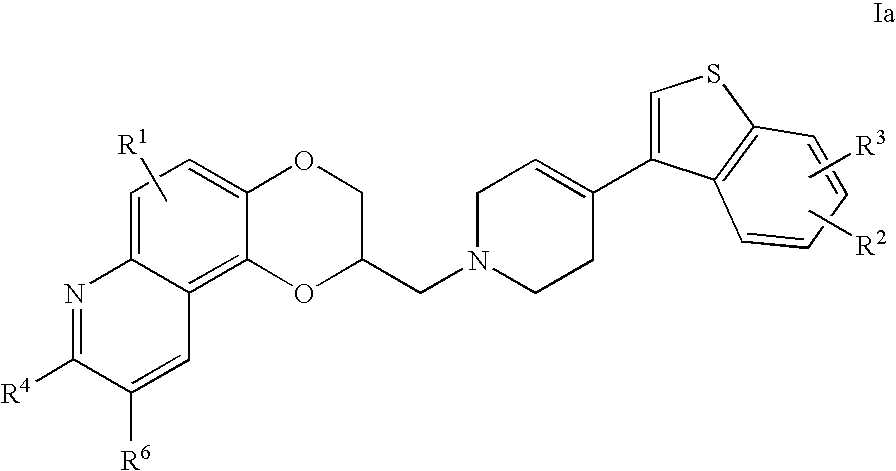
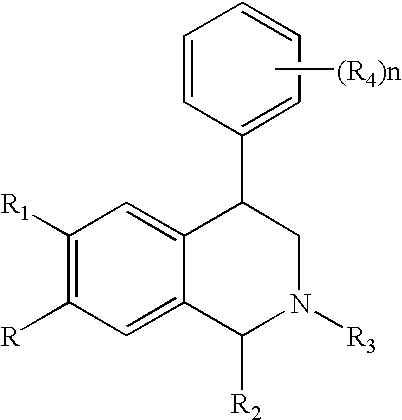
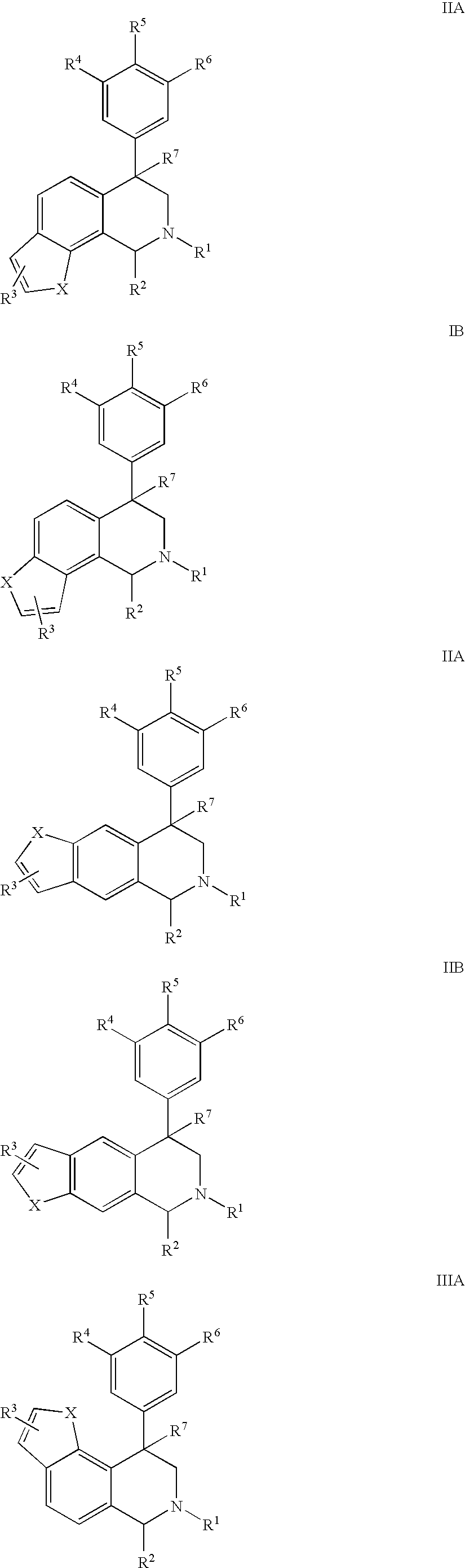
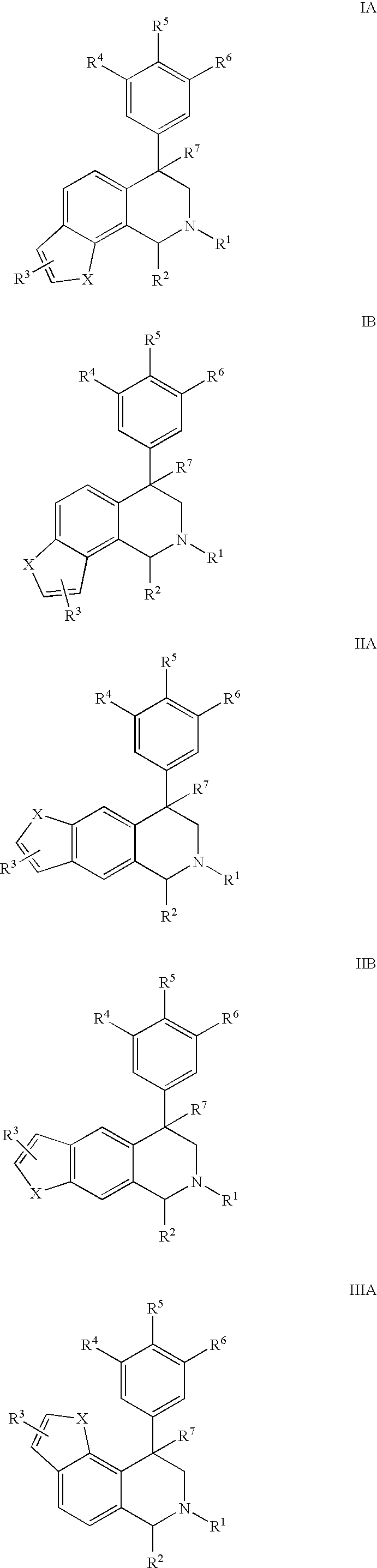
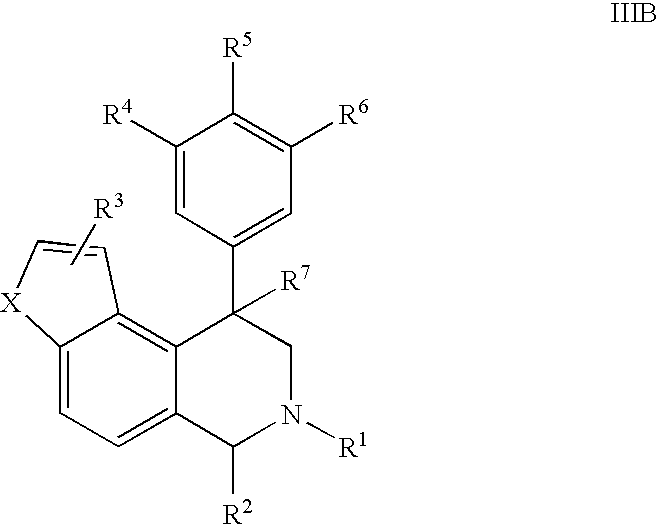
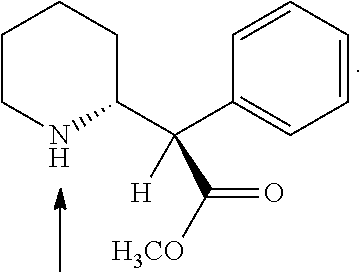
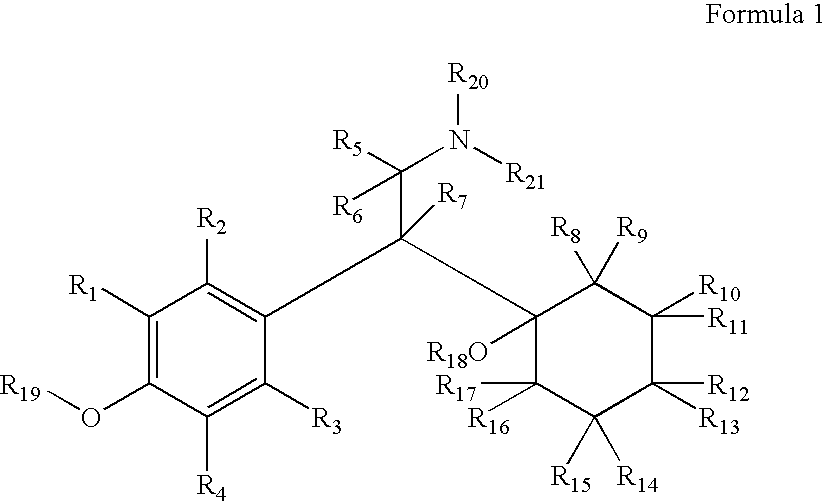
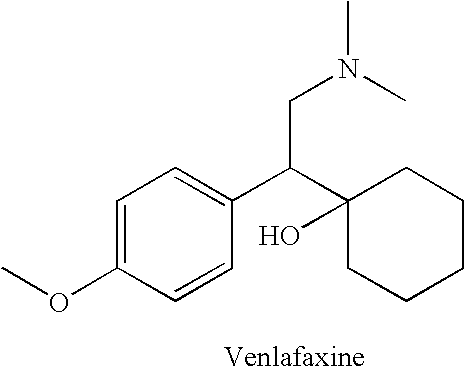
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
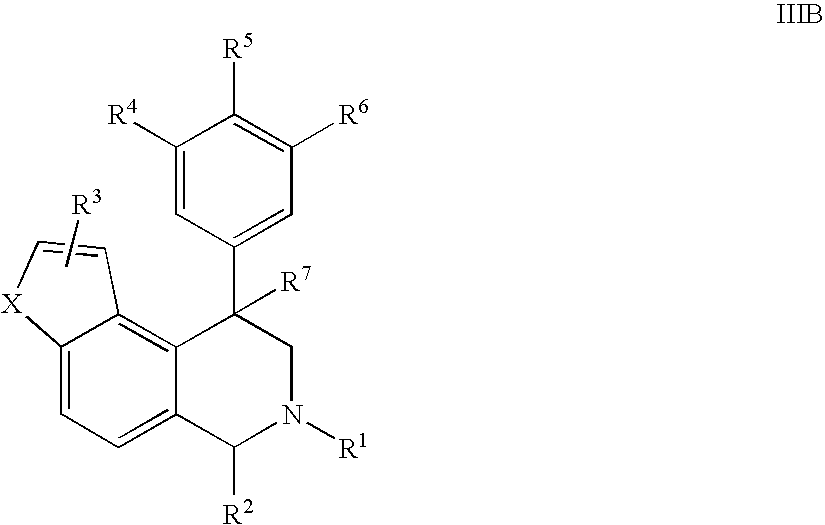
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
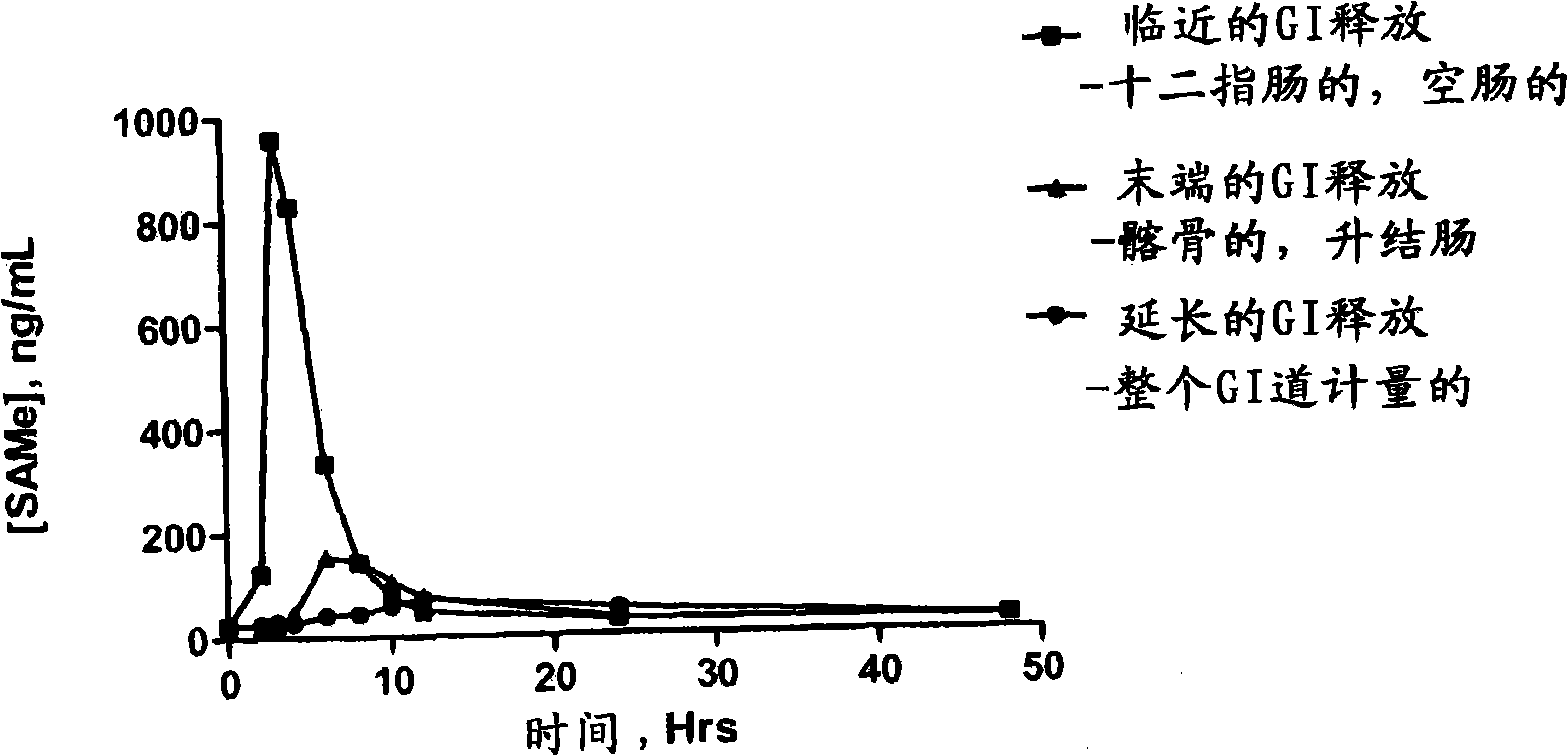
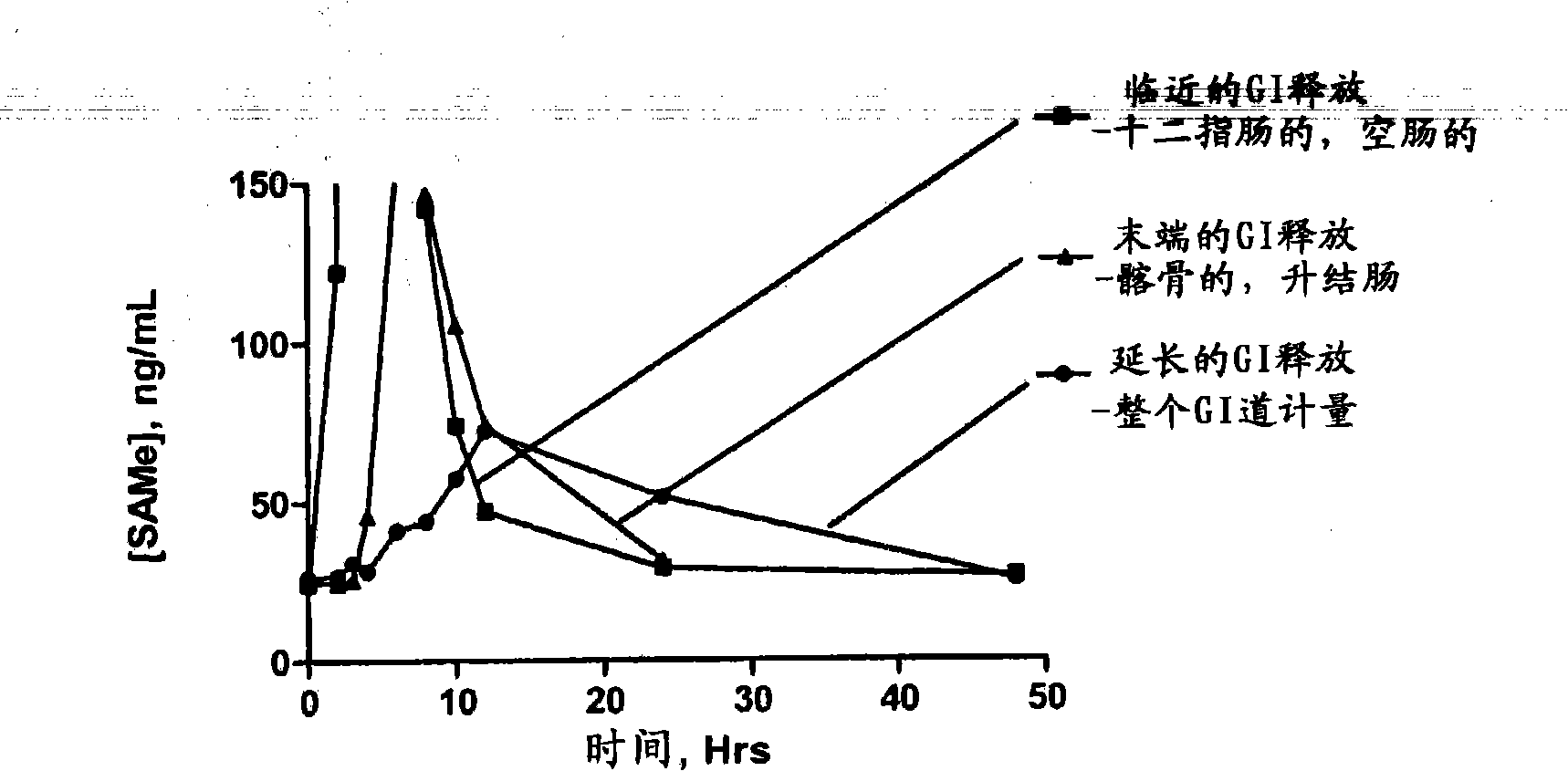
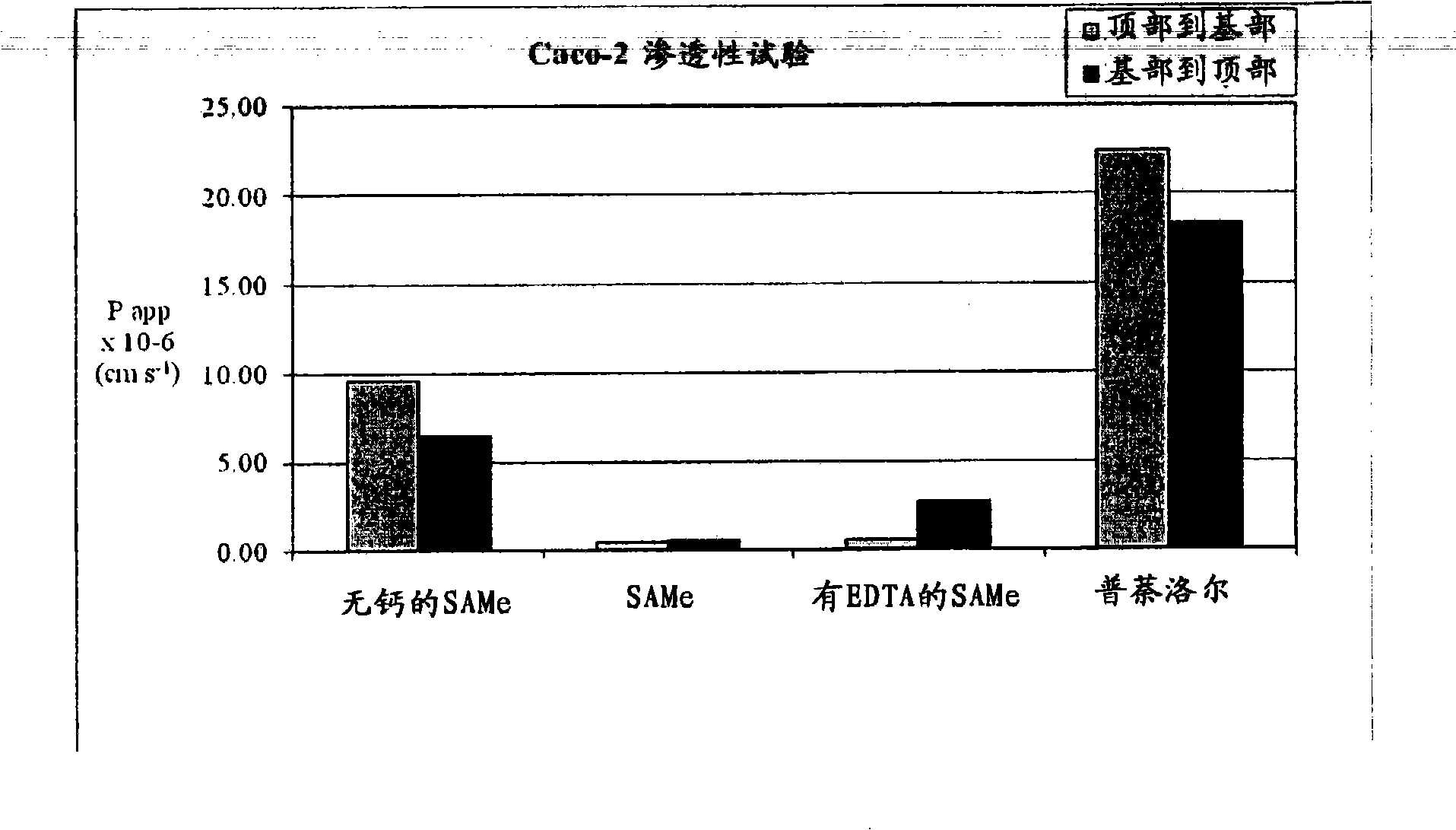
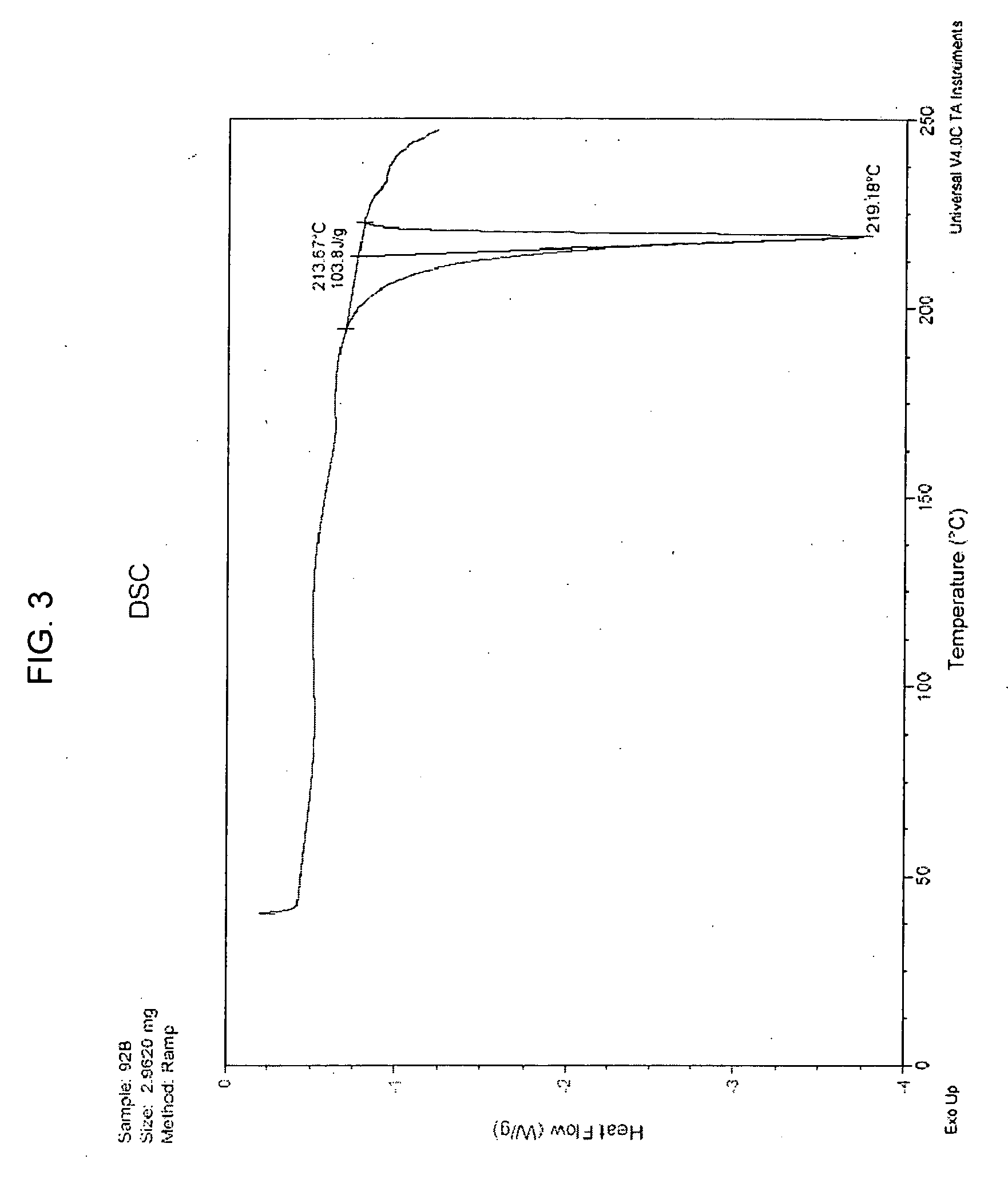
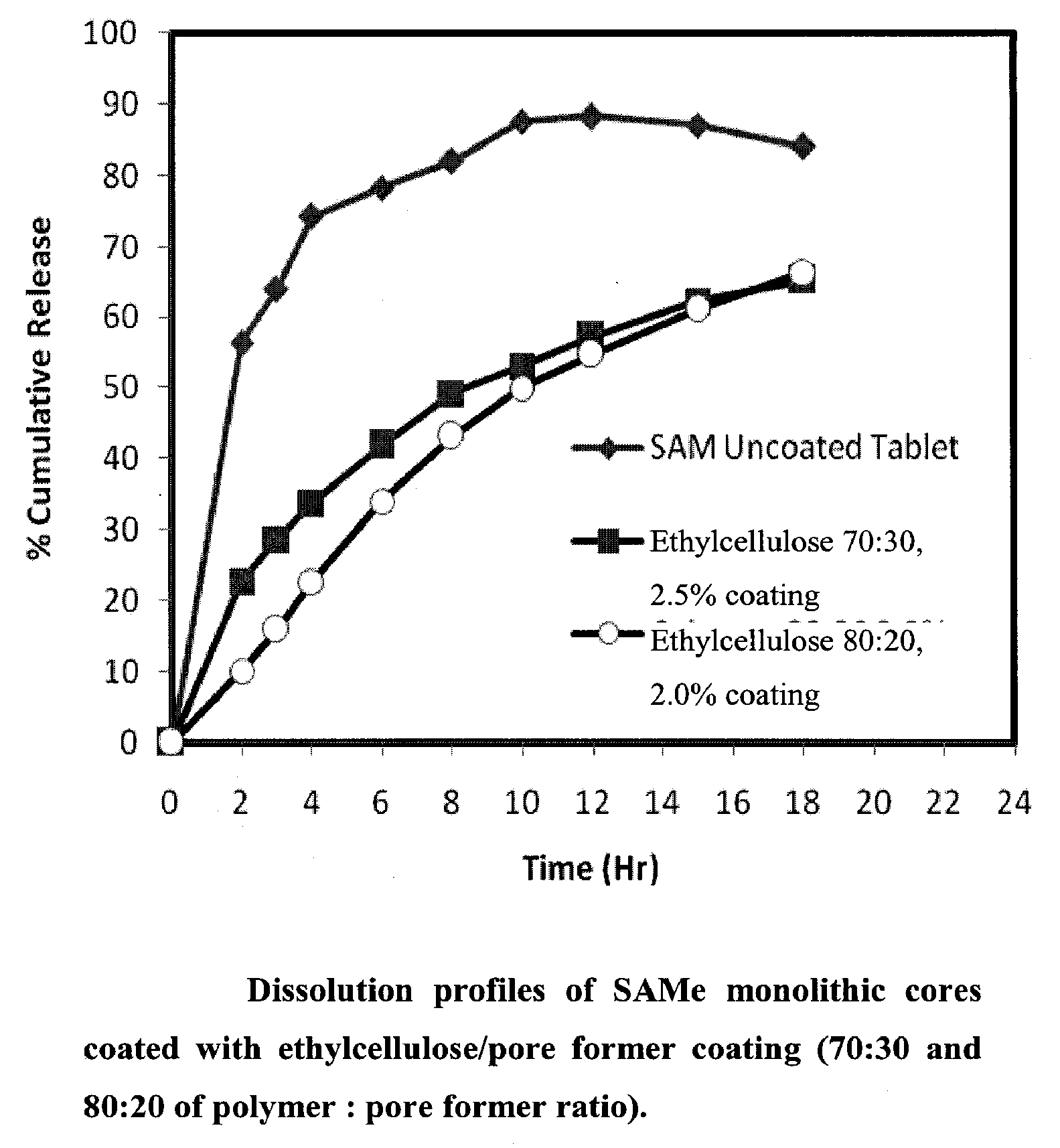
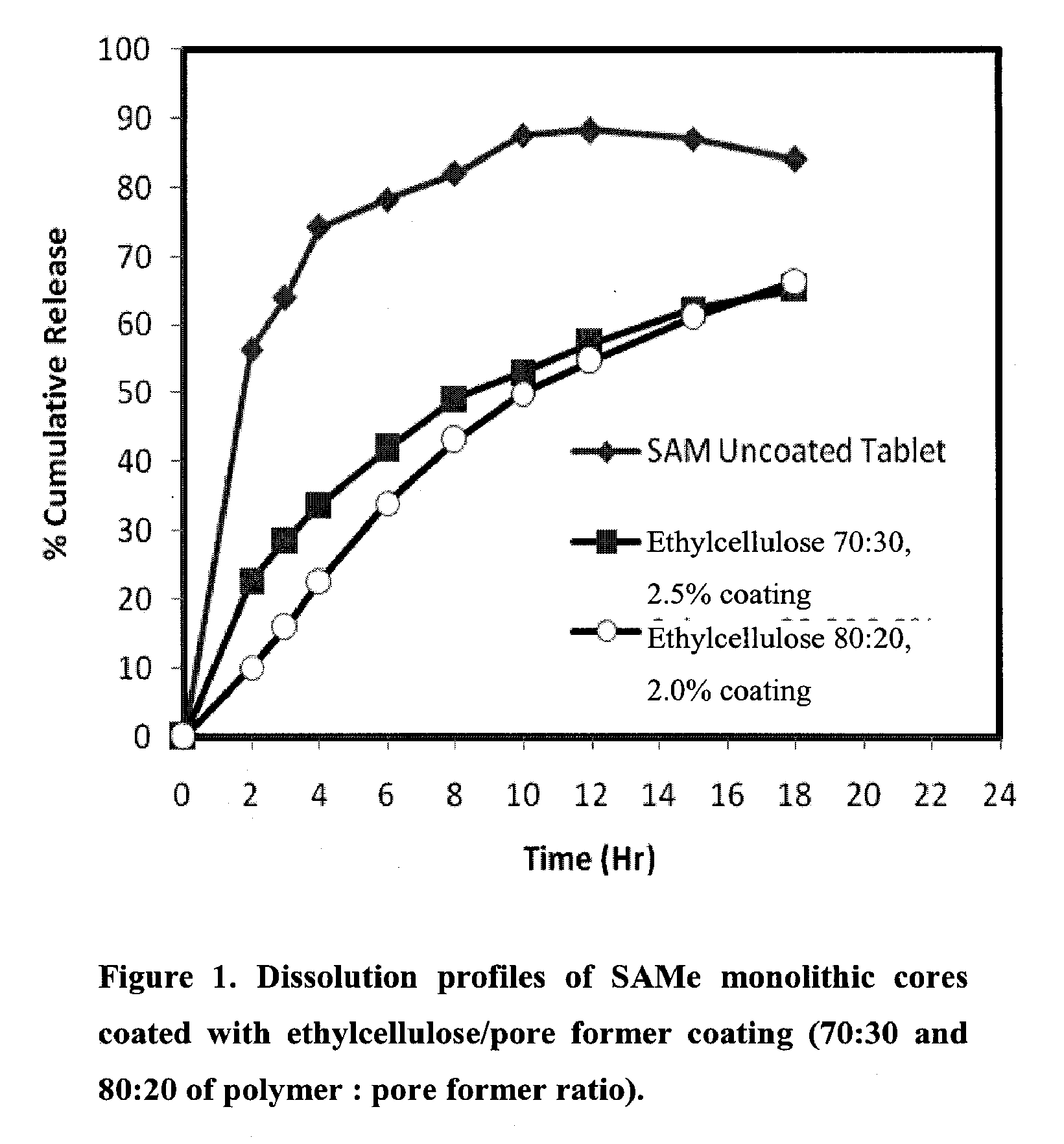
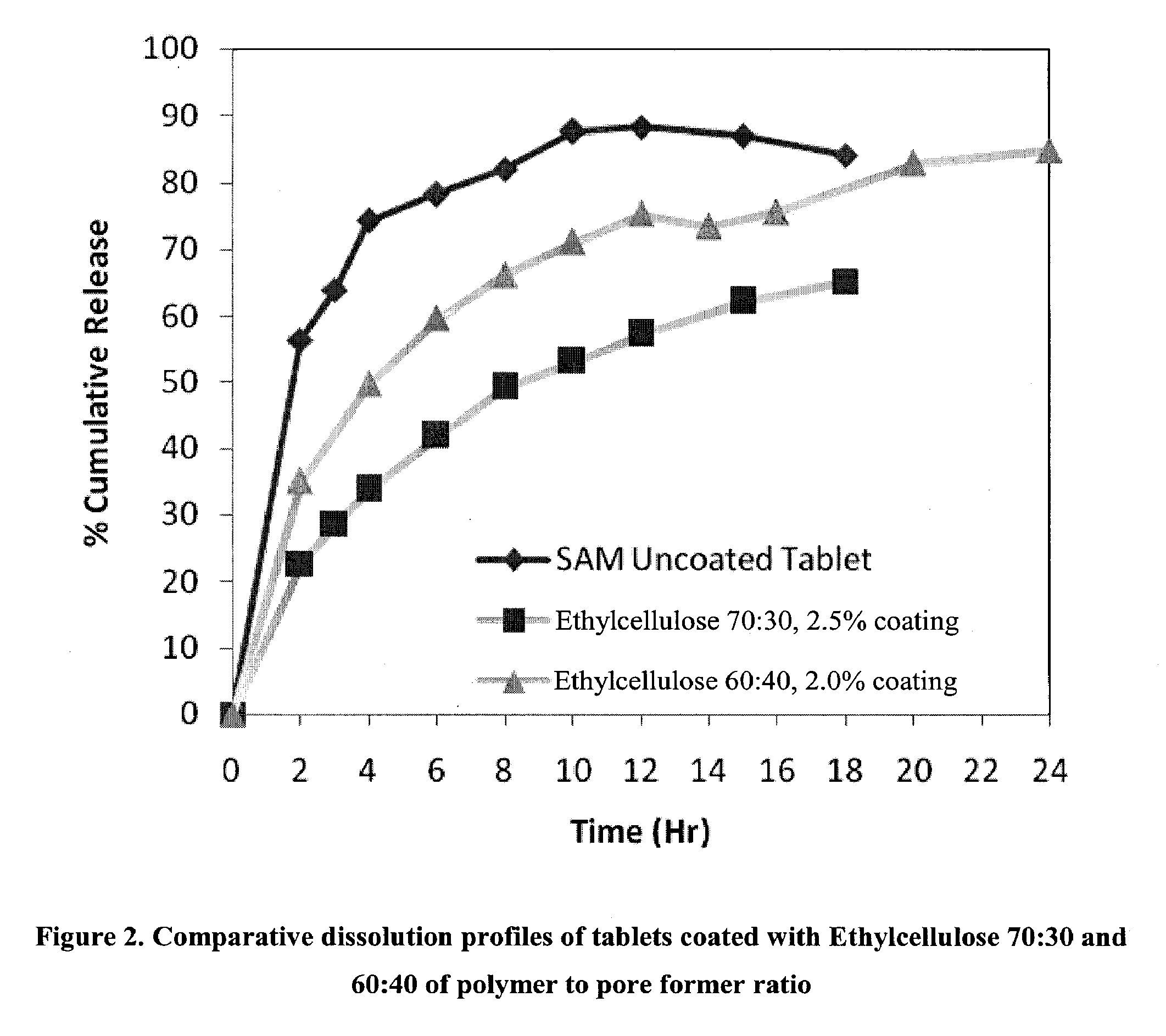
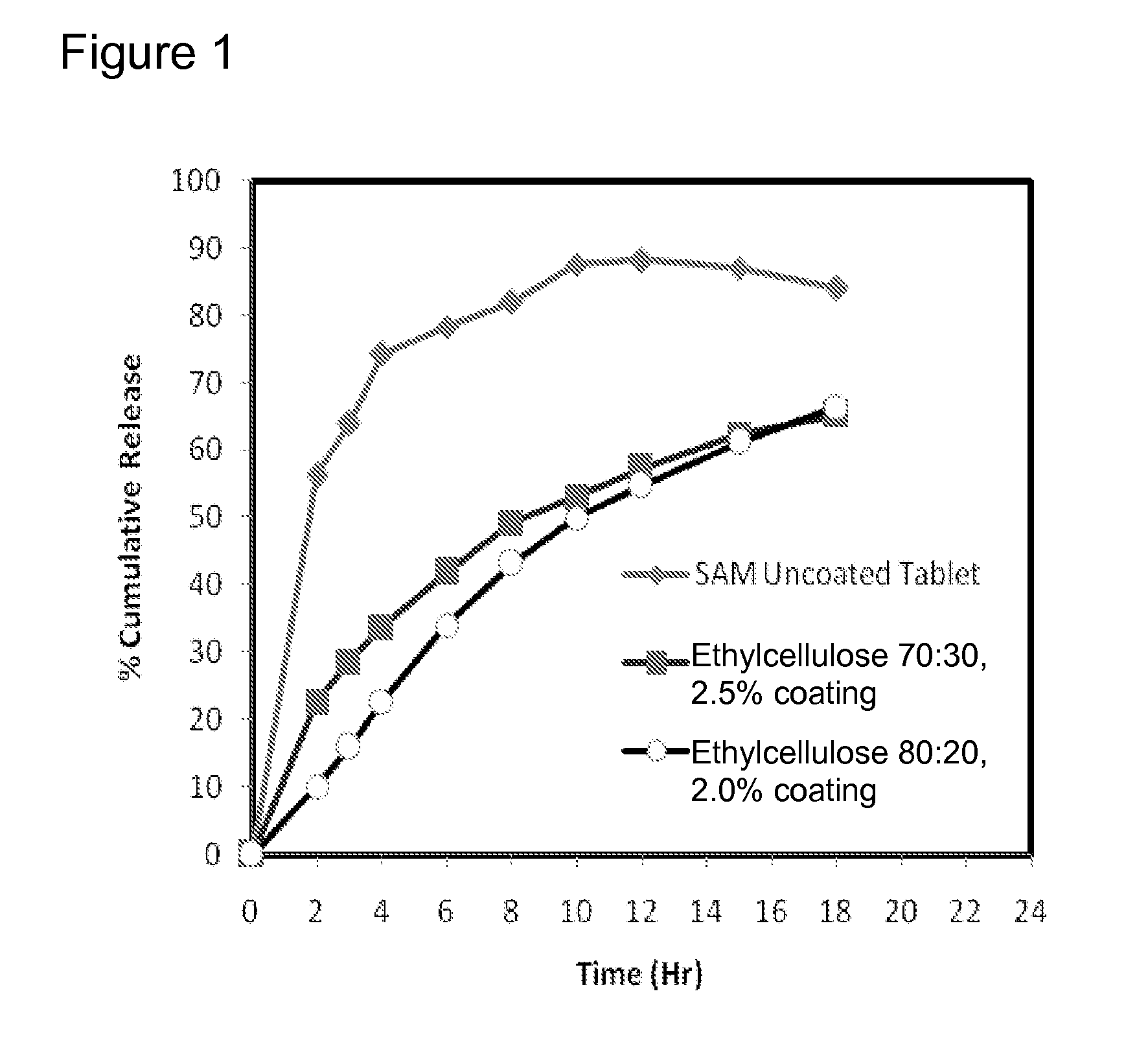
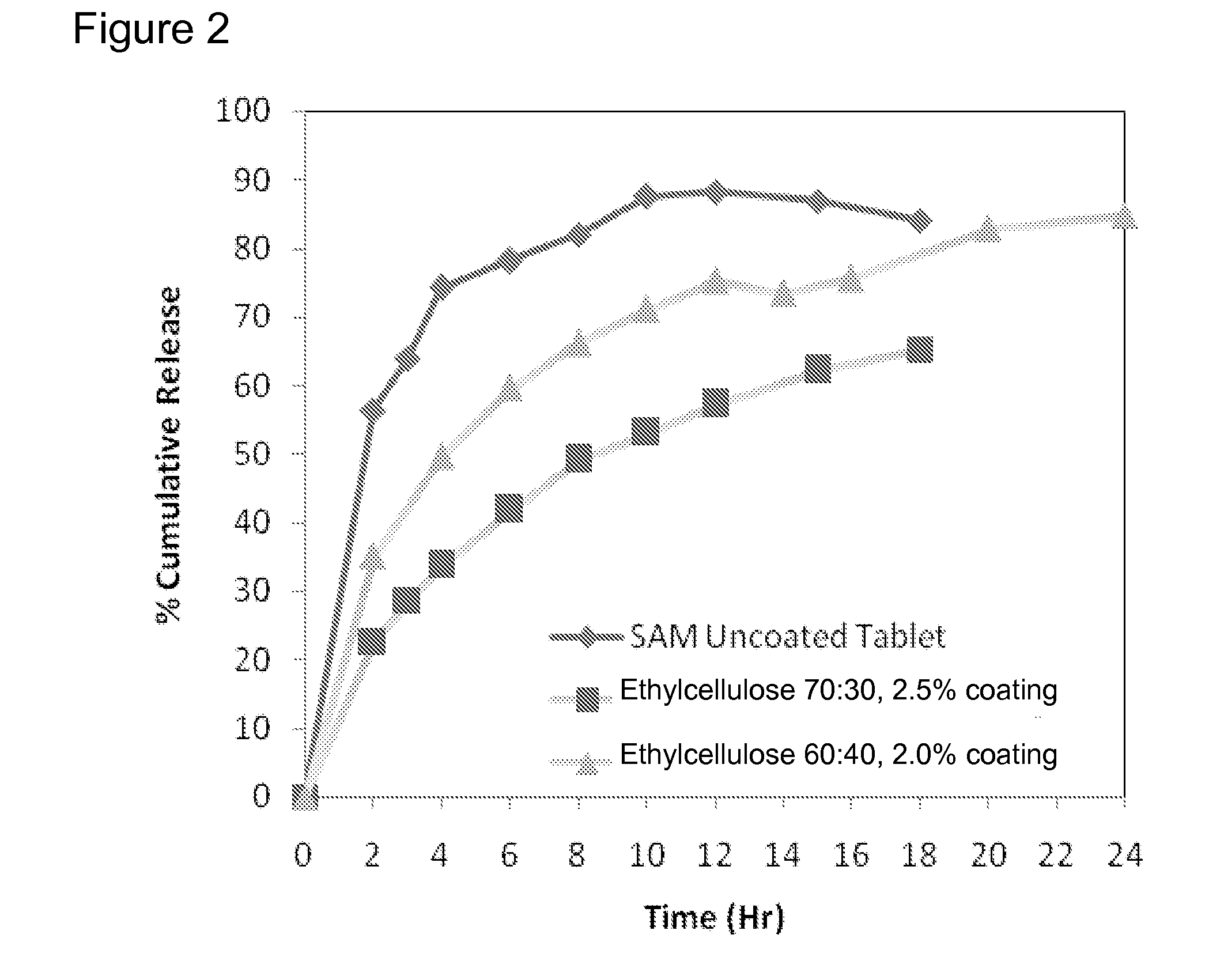
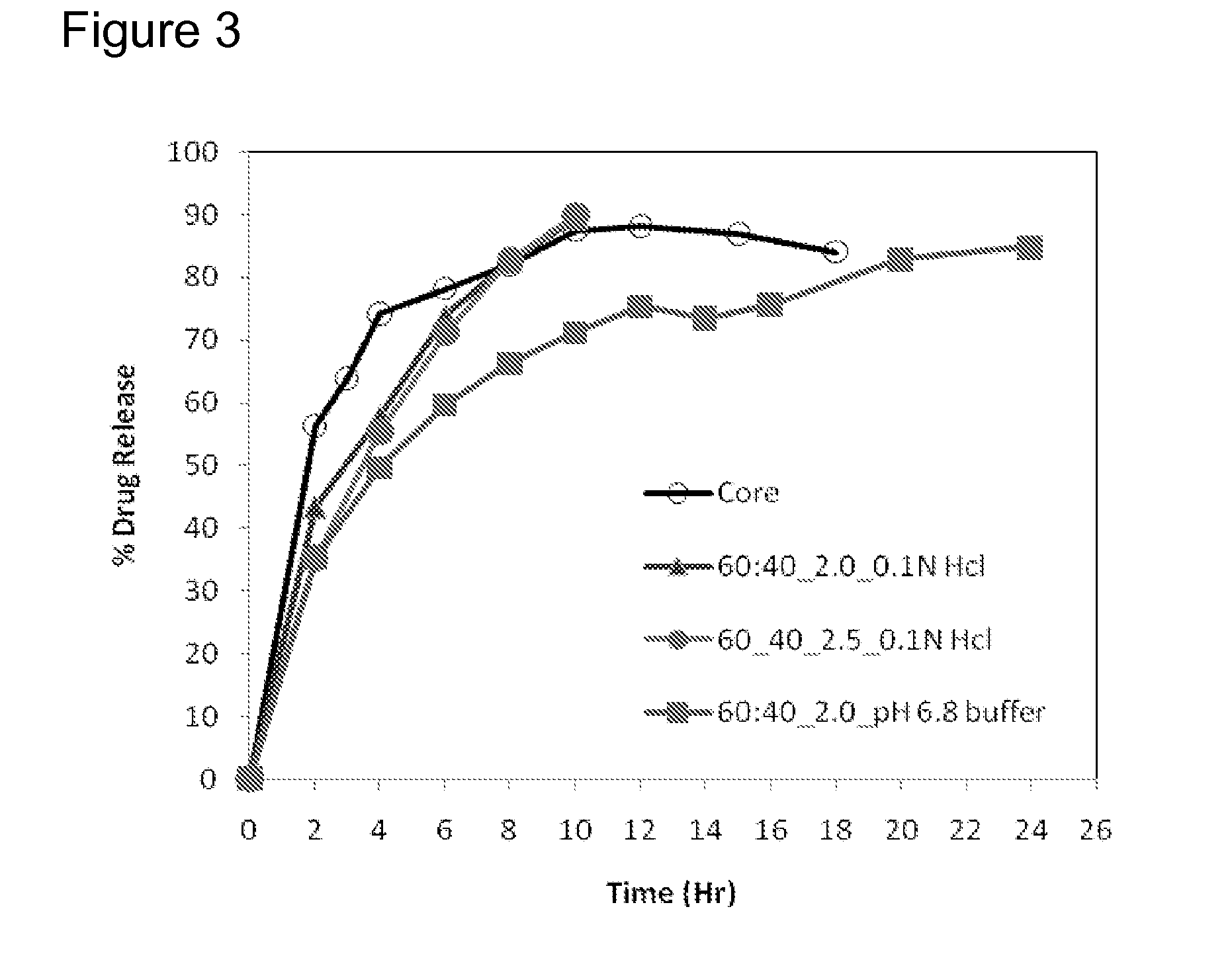
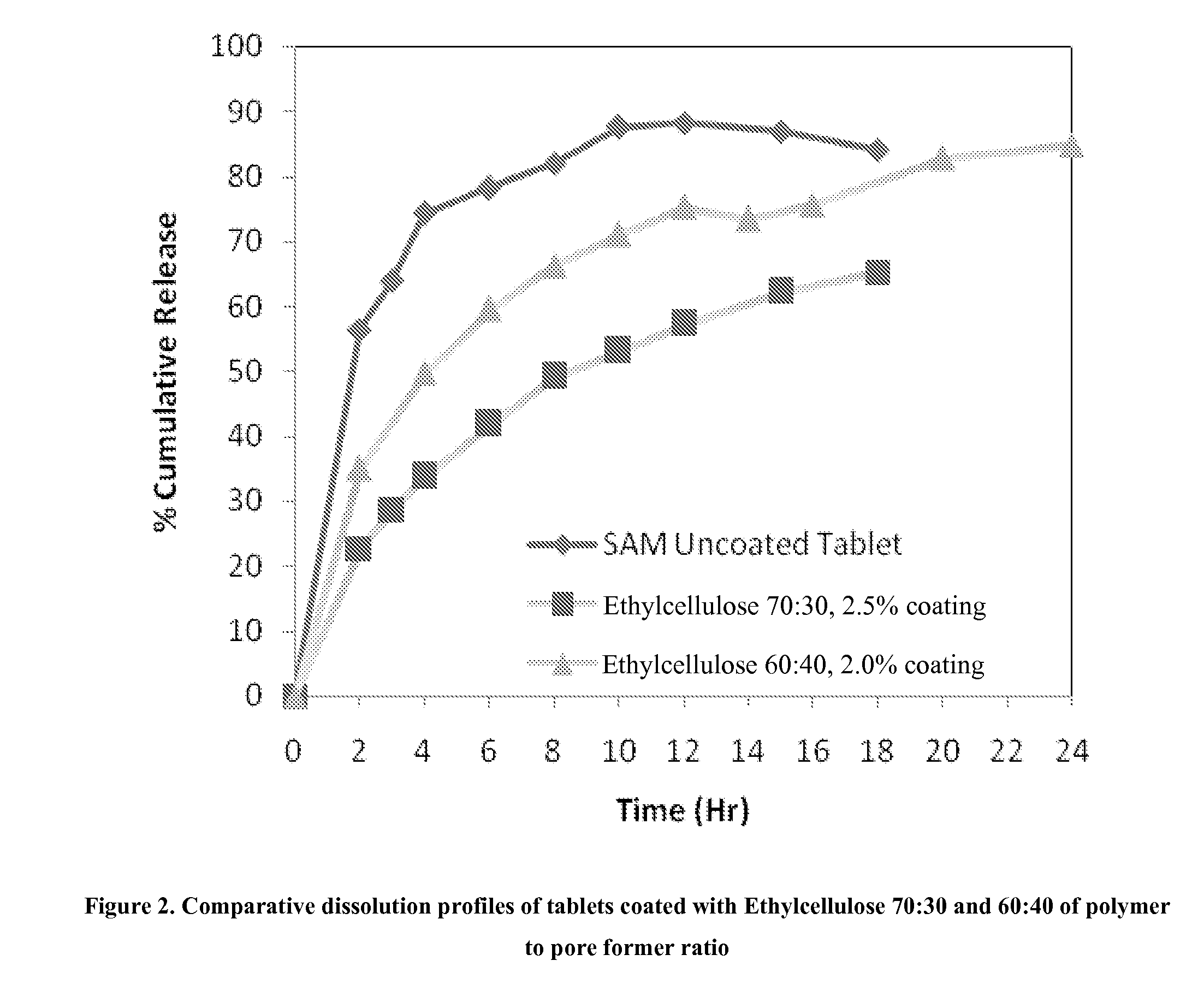
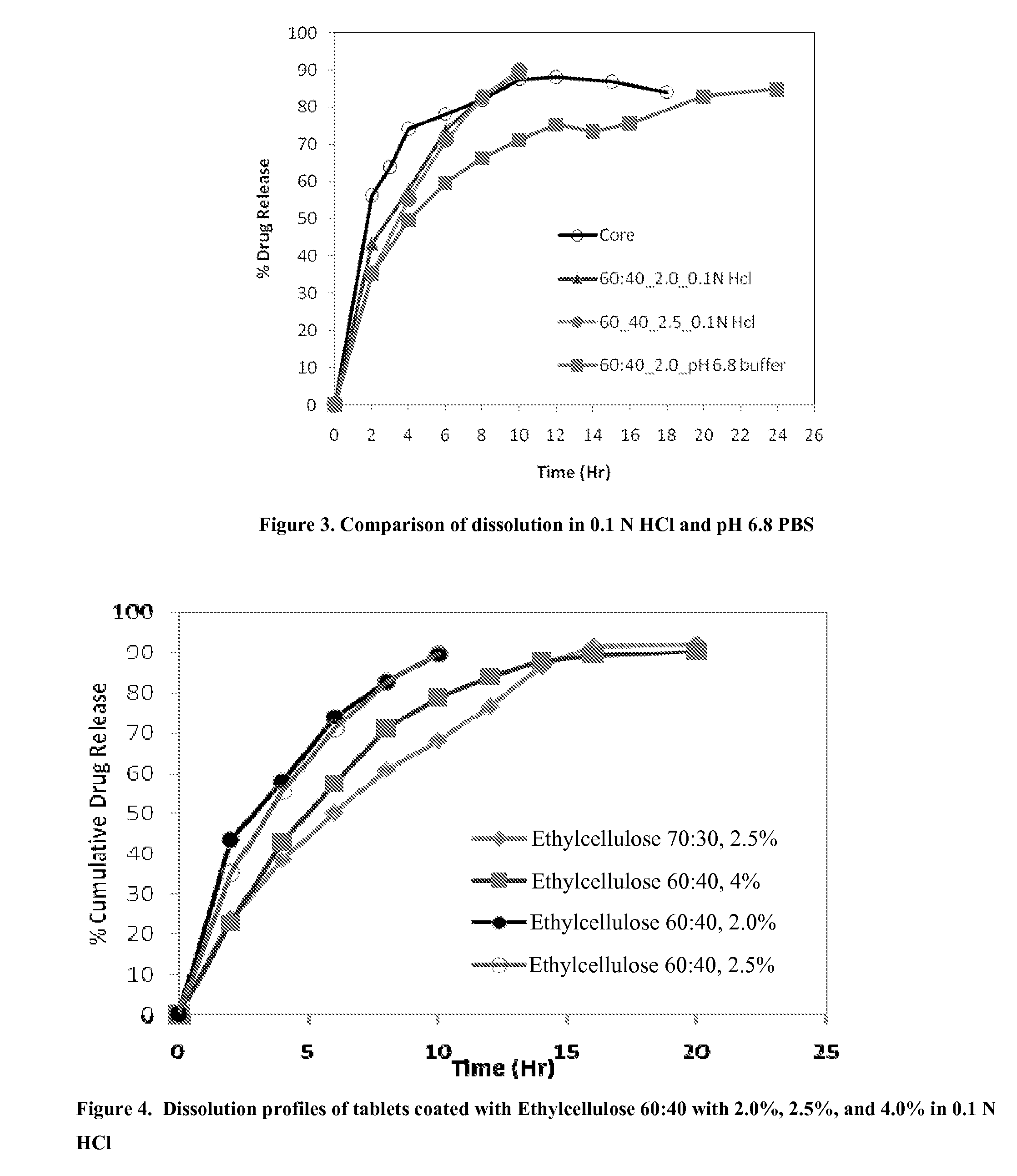
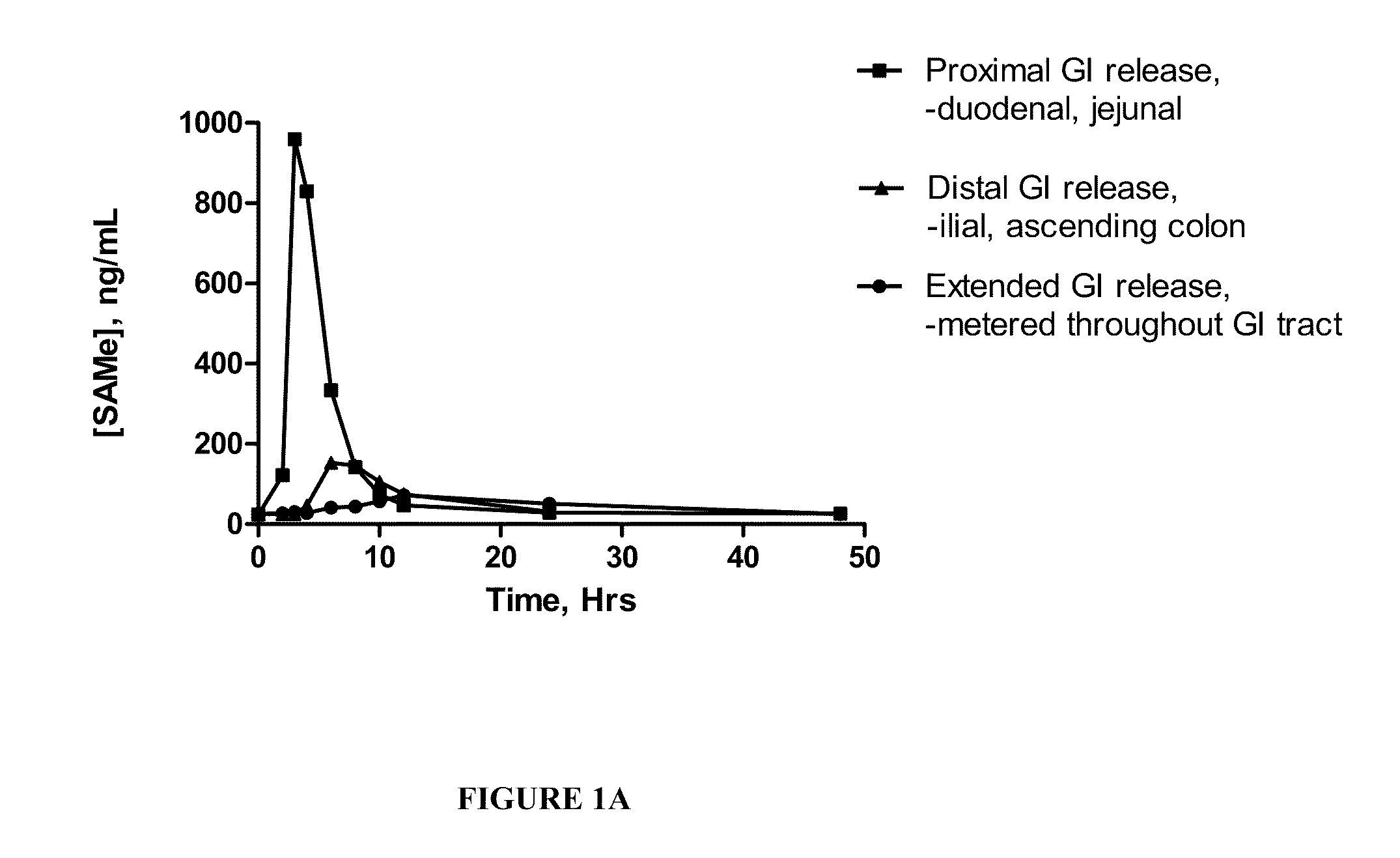
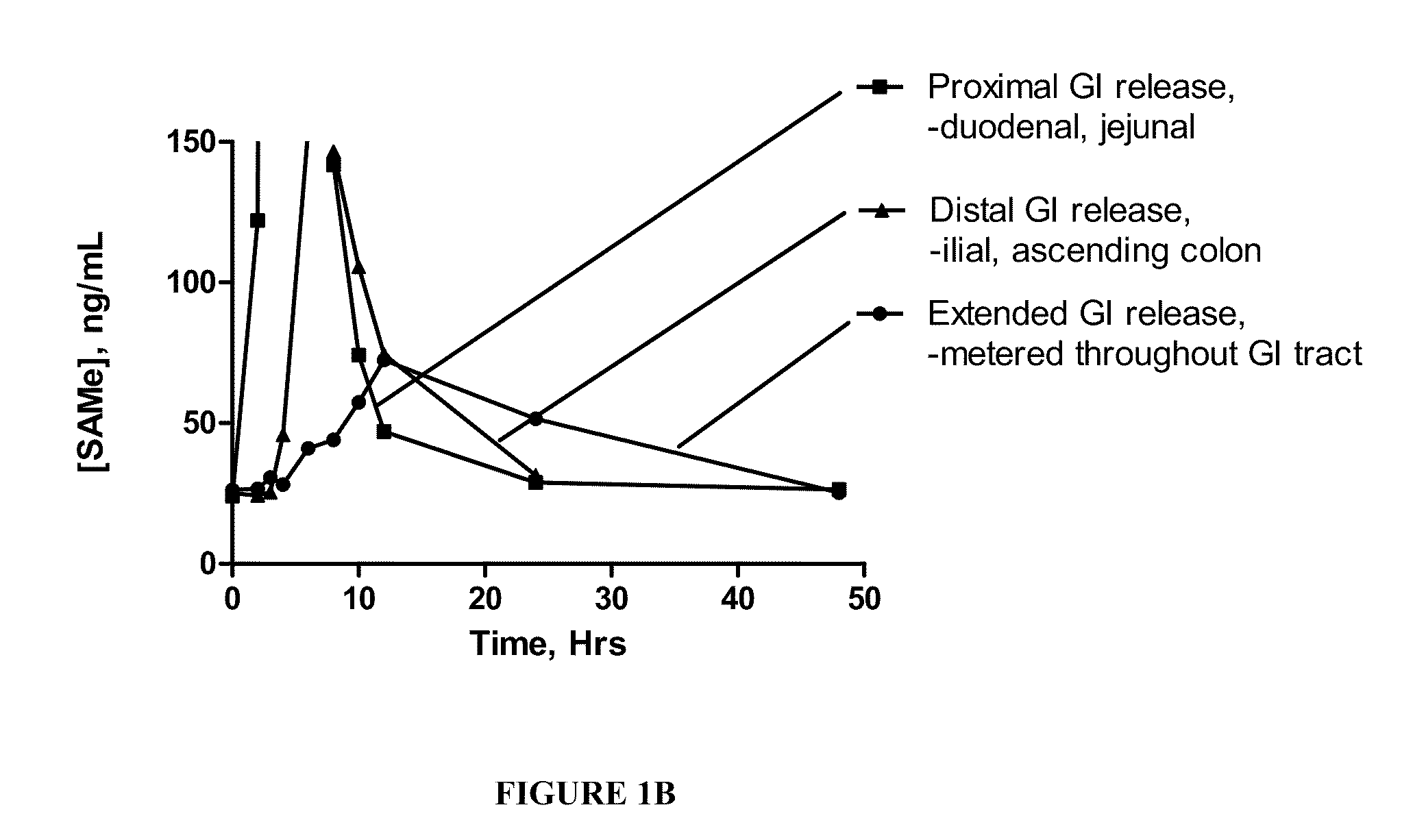
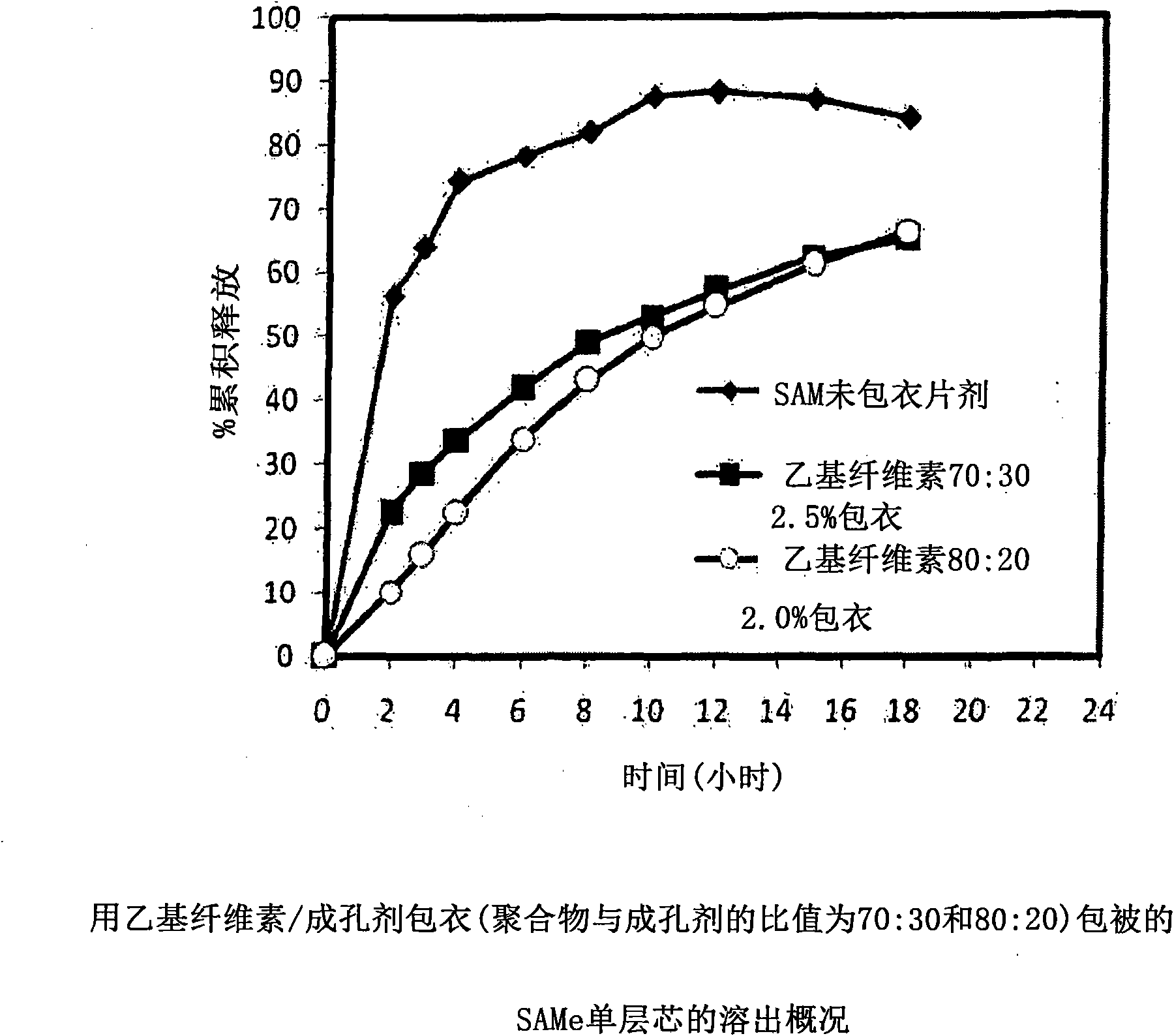
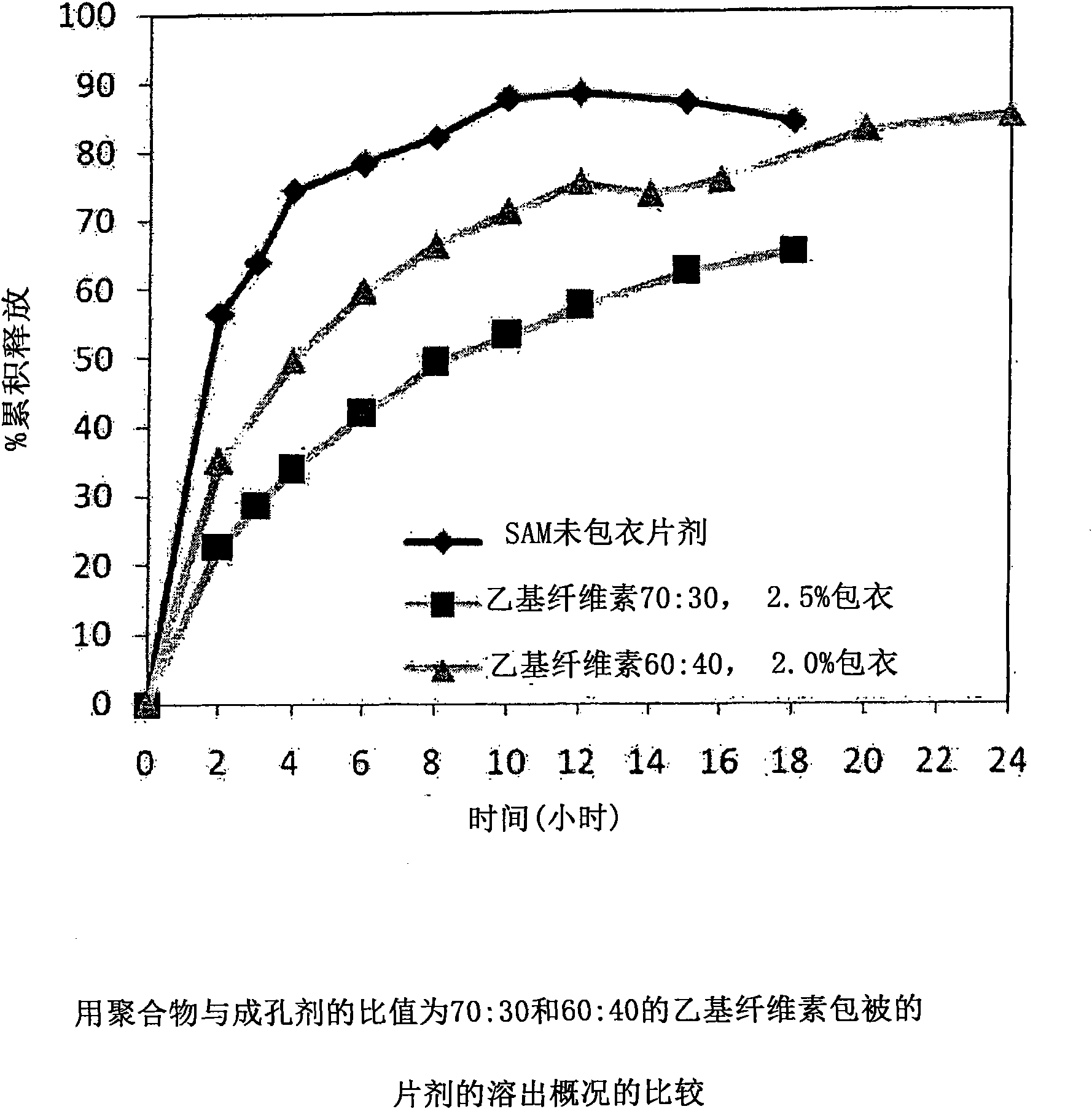
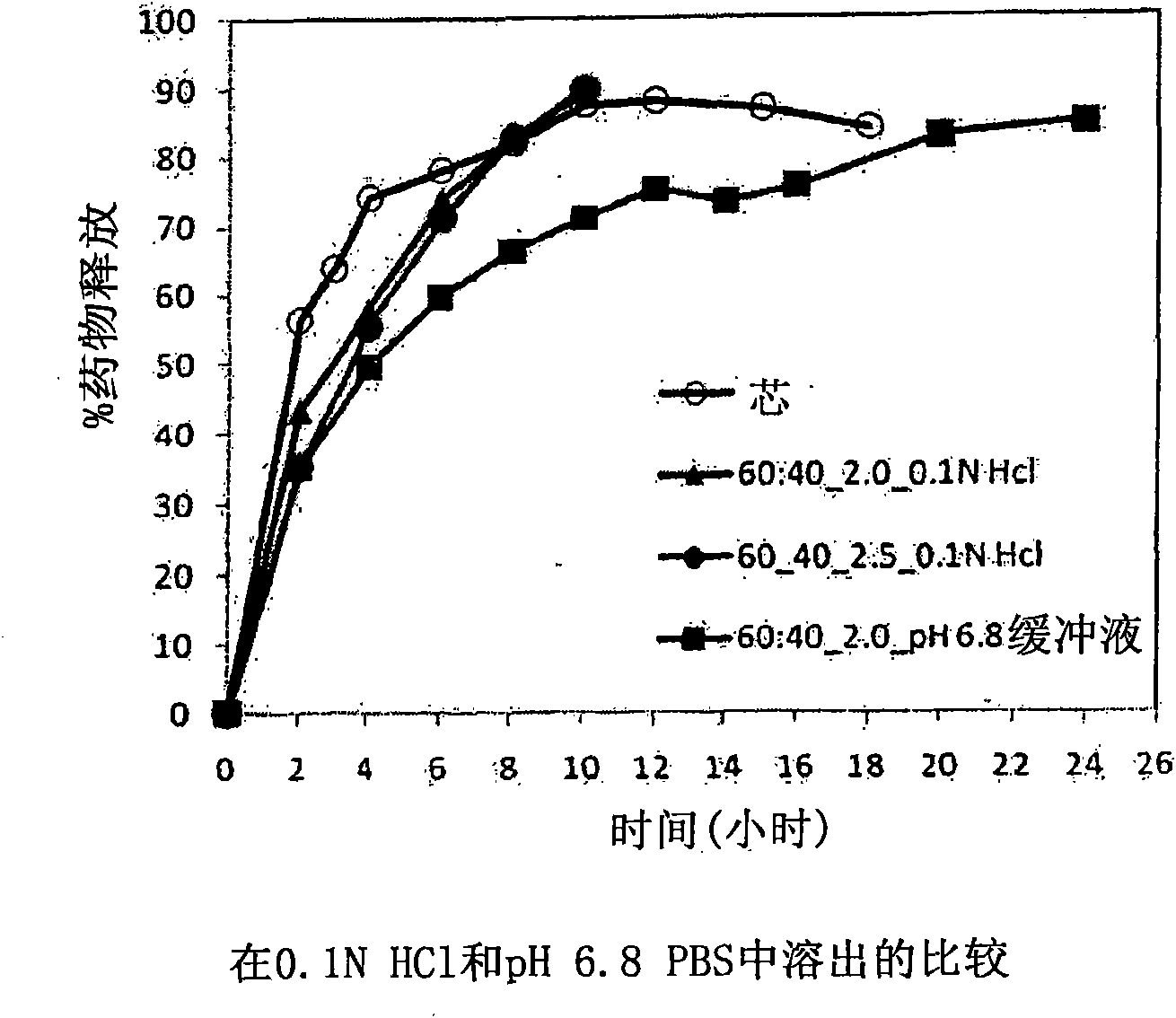
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090088404A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
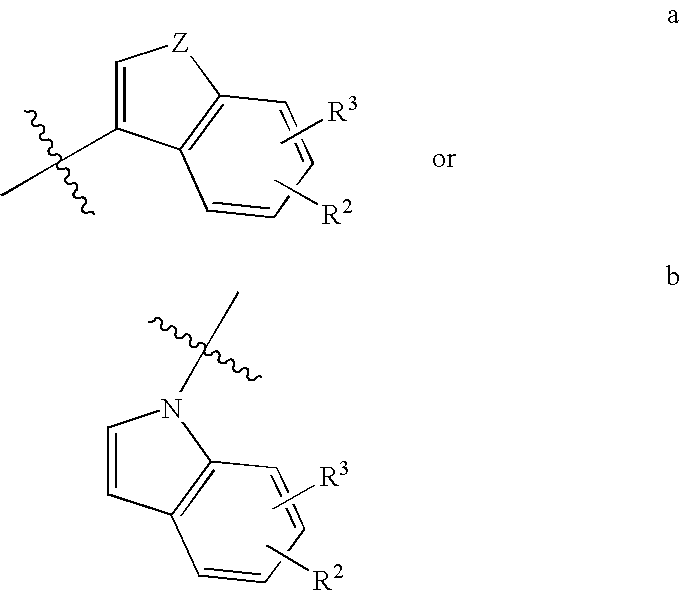
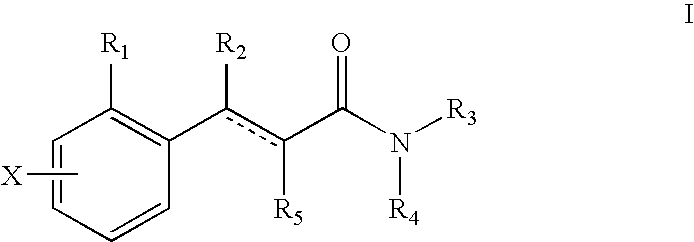
Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives
This invention provides compounds of the formula: 1 wherein R.sub.1 and R.sub.2 are each, independently, H, alkyl, cycloalkyl, --CH.sub.2-cycloalkyl, alkoxy, halogen, fluorinated alkyl, --CN, --NH--SO.sub.2-alkyl, --SO.sub.2--NH-alkyl, alkyl amide, amino, alkylamino, dialkylamino, fluorinated alkoxy, acyl, or phenoyl or thiophenoyl; R.sub.3 and R.sub.4 are each, independently, H, alkyl or cycloalkyl; R.sub.5 is H or alkyl; R.sub.6 is H or; and the dashed line indicates an optional double bond; or a pharmaceutically acceptable salt thereof, and pharmaceutical compositions and methods utilizing these compounds for the treatment or prevention of disorders including obsessive-compulsive disorder, depression, anxiety, generalized anxiety disorder, schizophrenia, migraine, sleep disorders, eating disorders, obesity, epilepsy, and spinal cord injury.
Owner:WYETH LLC
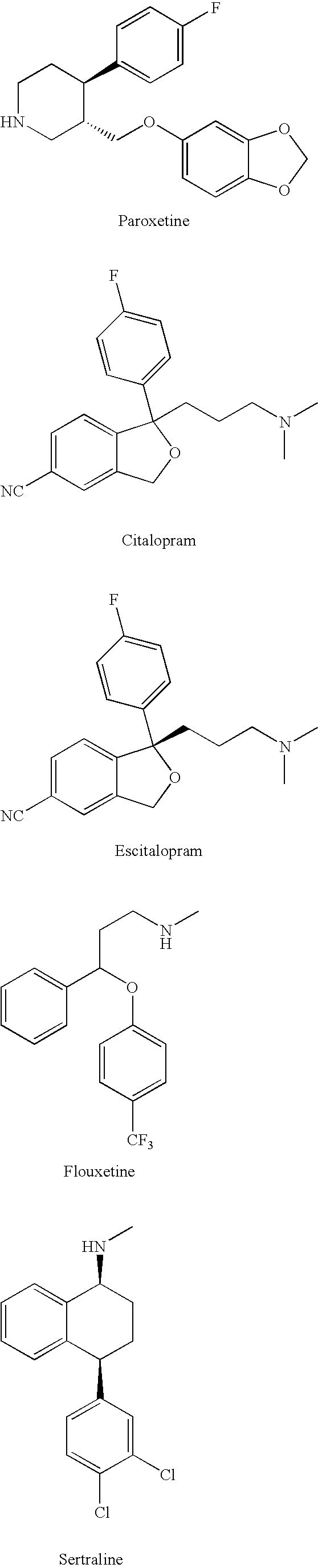
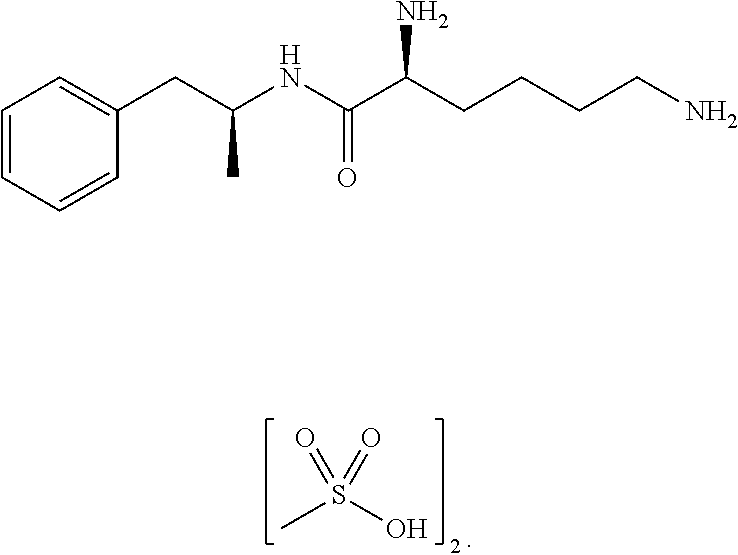
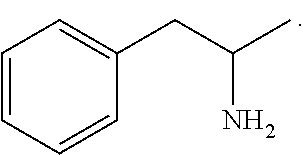
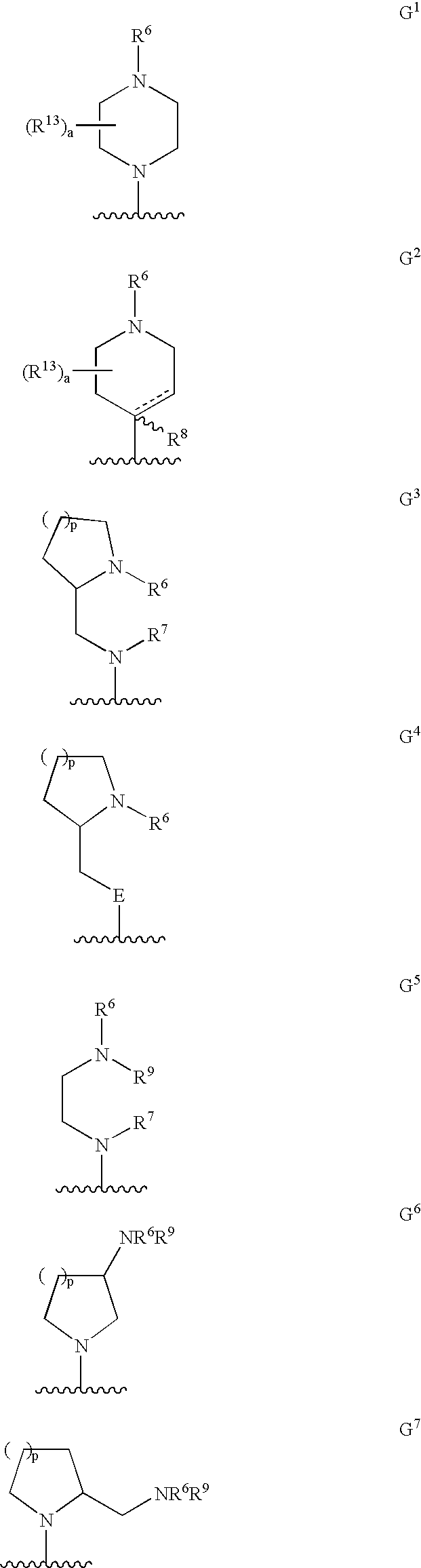
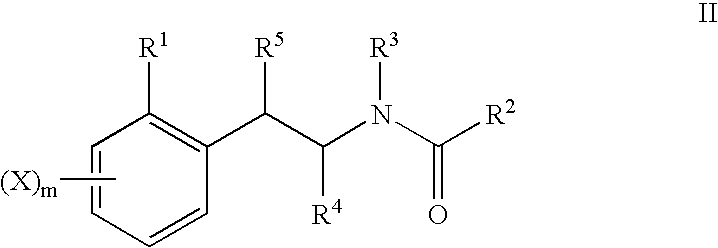
Substituted phenylpiperidines with serotoninergic activity and enhanced therapeutic properties
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
InactiveUS20080234257A1High crystallinityThe result is moreBiocideNervous disorderDiabetic retinopathyChemical synthesis
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, attention deficit hyperactivity disorder, fibromyalgia, irritable bowel syndrome, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Method of treating binge eating disorder and obesity resulting from binge eating behavior
Owner:LUCERNE BIOSCI
Combination of atypical antipsychotics and 5HT-1B receptor antagonists
InactiveUS20050256112A1Reduce morbidityDifferent recognizableNervous disorderMetabolism disorderDiseaseHeadaches
The present invention relates to a pharmaceutical composition for treating, for example, a disorder or condition selected from the group consisting of hypertension, depression, generalized anxiety disorder, phobias, posttraumatic stress disorder, avoidant personality disorder, sexual dysfunction, eating disorders, obesity, chemical dependencies, cluster headache, migraine, pain, Alzheimer's disease, obsessive-compulsive disorder, panic disorder, memory disorders, Parkinson's diseases, endocrine disorders, cerebellar ataxia, gastrointestinal tract disorders, negative symptoms of schizophrenia, premenstrual syndrome, Fibromyalgia Syndrome, stress incontinence, Tourette syndrome, trichotillomania, kleptomania, male impotence, cancer, chronic paroxysmal hemicrania and headache in a mammal, preferably a human, comprising (i) an atypical antipsychotic or a pharmaceutically acceptable salt thereof, (ii) a 5-HT1B receptor antagonist or a pharmaceutically acceptable salt thereof, wherein the 5-HT1B receptor antagonist is selected from the group consisting of (A) a compound of the formula I as described in the specification and (B) a compound of the formula II as described in the specification, and optionally (iii) a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Owner:ACADIA PHARMA INC
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090197824A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Highly selective serotonin and norepinephrine dual reuptake inhibitor and use thereof
InactiveUS20070015828A1Eliminate side effectsBiocideNervous disorderNorepinephrine reuptake inhibitorVisceral pain
Highly selective dual serotonin and norepinephrine reuptake inhibitors are provided. These compounds have a lower side-effect profile and are useful in compositions and products for use in treatment of a variety of conditions including depression, fibromyalgia, anxiety, panic disorder, agorophobia, post traumatic stress disorder, premenstrual dysphoric disorder, attention deficit disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, autism, schizophrenia, obesity, anorexia nervosa, bulimia nervosa, Gilles de la Tourette Syndrome, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction, borderline personality disorder, fibromyalgia syndrome, diabetic neuropathic pain, chronic fatigue syndrome, pain, visceral pain, Shy Drager syndrome, Raynaud's syndrome, Parkinson's Disease, and epilepsy.
Owner:WYETH
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Antidepressant indoletetrahydropyridine derivatives of 2,3-dihydro-7H-[1,4]dioxino[2,3-e]indole
Compounds of the formula are useful in the treatment of central nervous system disorders including depression, obsessive compulsive disorder, panic attacks, generalized anxiety disorder, sexual dysfunction, eating disorders and addictive disorders caused by ethanol or cocaine abuse.
Owner:WYETH LLC
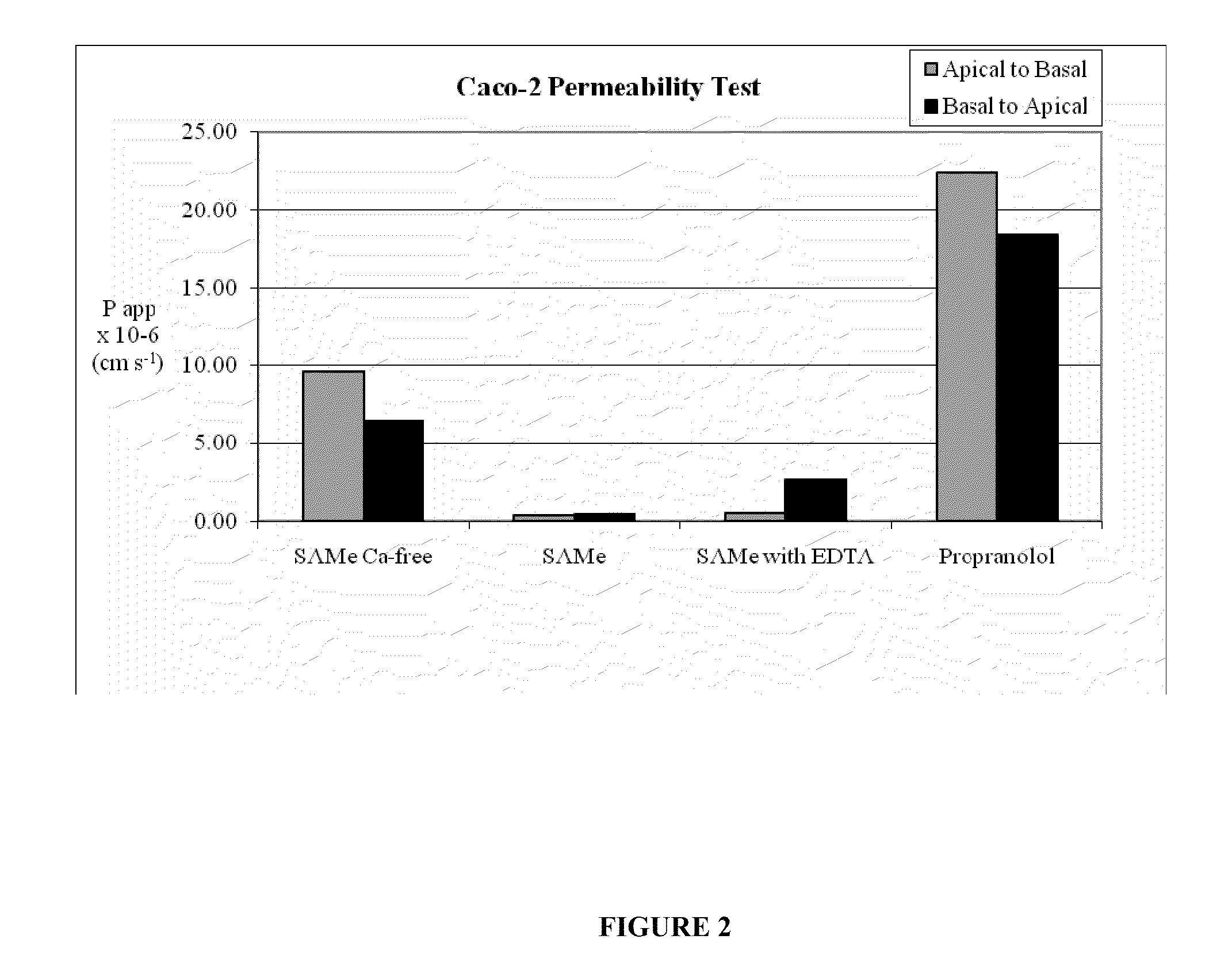
S-adenosylmethionine formulations with enhanced bioavailability
InactiveUS20110027342A1Reduce penetrationPromote absorptionBiocideNervous disorderImmunologic disordersS-Adenosyl-l-methionine
The invention relates to compositions and methods to enhance the absorption of S-adenosylmethionine (SAMe) and to methods of treating various disorders or diseases using non-parenteral SAMe formulations with enhanced-absorption and improved bioavailability. The enhanced bioavailability formulations may be used to treat a variety of diseases or disorders, such as for example, psychiatric disorders including, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, depressive disorders (e.g. major clinical depression) and dysthymia; as well as treating liver disorders, cancer, autoimmune disorders, inflammatory disorders, joint disorders, gastrointestinal disorders and cardiovascular disease.
Owner:MSI METHYLATION SCI
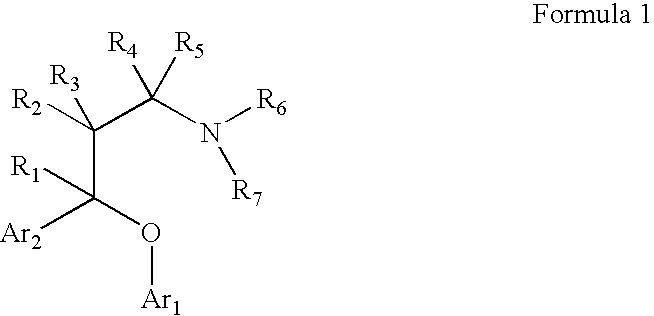
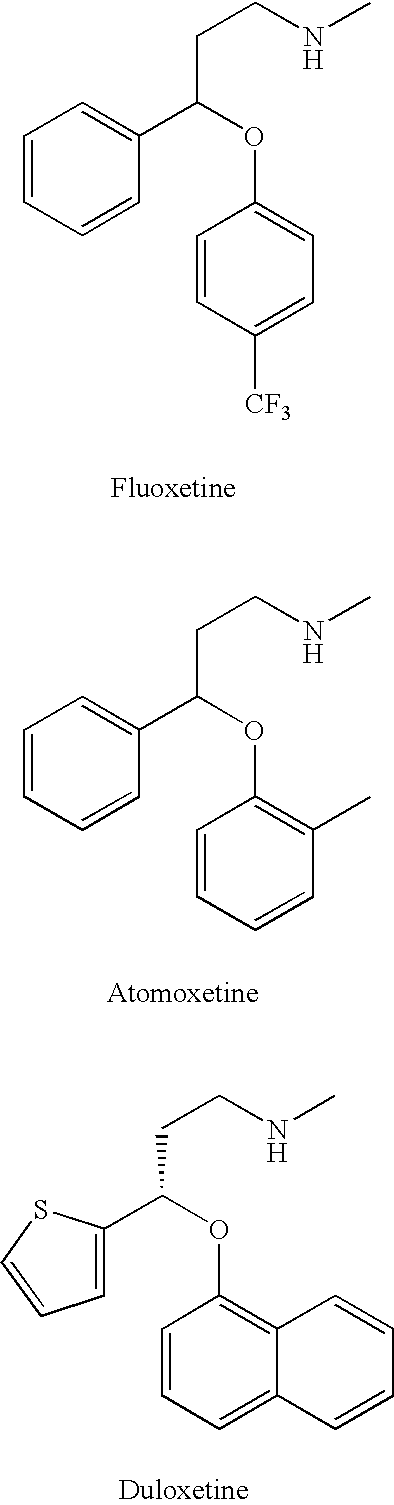
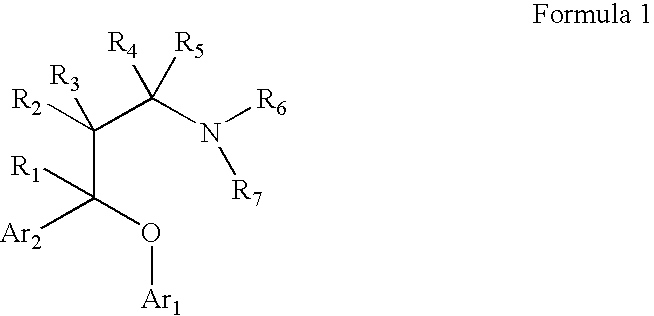
Substituted aryloxypropylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Cyclohepta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives
This invention provides compounds of the formula: wherein R1 and R2 are each, independently, H, alkyl, cycloalkyl, —CH2-cycloalkyl, alkoxy, halogen, fluorinated alkyl, —CN, —NH—SO2-alkyl, —SO2—NH-alkyl, alkyl amide, amino, alkylamino, dialkylamino, fluorinated alkoxy, acyl, or phenoyl or thiophenoyl; R3 and R4 are each, independently, H, alkyl or cycloalkyl; R5 is H or alkyl; R6 is H or; and the dashed line indicates an optional double bond; or a pharmaceutically acceptable salt thereof, and pharmaceutical compositions and methods utilizing these compounds for the treatment or prevention of disorders including obsessive-compulsive disorder, depression, anxiety, generalized anxiety disorder, schizophrenia, migraine, sleep disorders, eating disorders, obesity, epilepsy, and spinal cord injury.
Owner:WYETH LLC
Extended release pharmaceutical formulations of s-adenosylmethionine
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. majorclinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Cycloocta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives
This invention provides compounds of formula I having the structurewherein: R1 and R2 are H, alkyl, cycloalkyl, alkoxy, halogen, fluorinated alkyl, —CN, —NH—SO2-alkyl, —SO2—NH-alkyl, alkyl amide, amino, alkylamino, dialkylamino, fluorinated, acyl, or aroyl; R3, R4 are H, alkyl, cycloalkyl or —CH2-cycloalkyl; R5 is H or alkyl; R6 is H or alkyl; and wherein the dashed line indicates an optional double bond; or a pharmaceutically acceptable salt thereof, as well as methods for using these compounds to treat central nervous system disorders, including obsessive-compulsive disorder, depression, anxiety, generalized anxiety disorder, schizophrenia, migraine, sleep disorders, eating disorders, obesity, epilepsy, and spinal cord injury.
Owner:WYETH LLC
Cyclopenta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives
This invention provides compounds of the formulae:wherein:R1 is hydrogen, —C(O)CH3 or alkyl of 1-6 carbon atoms;R2 and R3 are each, independently, hydrogen, alkyl of 1-6 carbon atoms, cycloalkyl, alkoxy of 1-6 carbon atoms, —CH2OH, fluoroalkyl, alkyl sulfonamide of 1-6 carbon atoms, alkyl amide of 1-6 carbon atoms, amino, alkylamino of 1-6 carbon atoms, dialkylamino of 1-6 carbon atoms per alkyl moiety, fluoroalkoxy of 1-6 carbon atoms, acyl of 2-7 carbon atoms, aryl, or aroyl;R4 and R5 are each, independently, hydrogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, halogen, fluoroalkyl, —CN, alkyl sulfonamide of 1-6 carbon atoms, alkyl amide of 1-6 carbon atoms, amino, alkylamino of 1-6 carbon atoms, dialkylamino of 1-6 carbon atoms per alkyl moiety, fluoroalkoxy of 1-6 carbon atoms, acyl of 2-7 carbon atoms, or aroyl;R6 and R7 are each independently hydrogen, C1-C6 alkyl or cycloalkyl;or a pharmaceutically acceptable salt thereof, as well as pharmaceutical compositions containing these compounds and methods for their use, including treatment of obsessive-compulsive disorder, panic disorder, depression, anxiety, generalized anxiety disorder, schizophrenia, migraine, sleep disorders, eating disorders, obesity, epilepsy, and spinal cord injury.
Owner:WYETH LLC
Novel pharmaceutical compositions and methods for anxiety, depression and other psychiatric disorders
PendingUS20200323876A1Organic active ingredientsPharmaceutical delivery mechanismSeasonal Affective DisordersCompulsive disorders
Pharmaceutical compositions comprising azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating patients suffering from one or more psychiatric disorders such as major depressive disorder, generalized anxiety disorder, panic disorder, agitation, social anxiety disorder, mild chronic depression, obsessive-compulsive disorder, premenstrual dysphoric disorder, seasonal affective disorder, dysthymia, childhood enuresis, bipolar disorder, posttraumatic stress disorder, sleep disorder related to anxiety, are also disclosed.
Owner:LA PHARMATECH INC
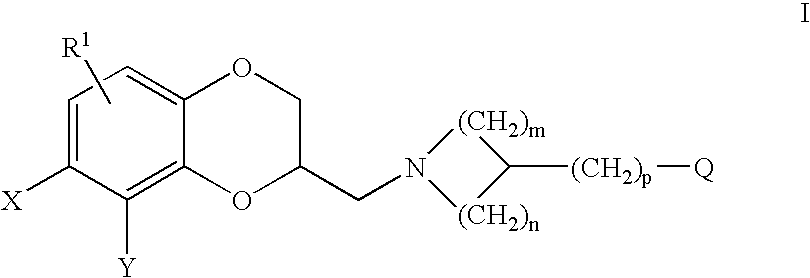
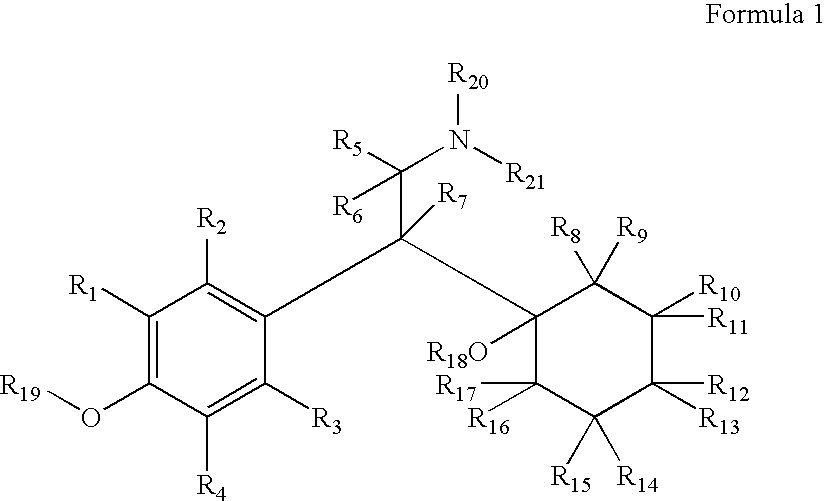
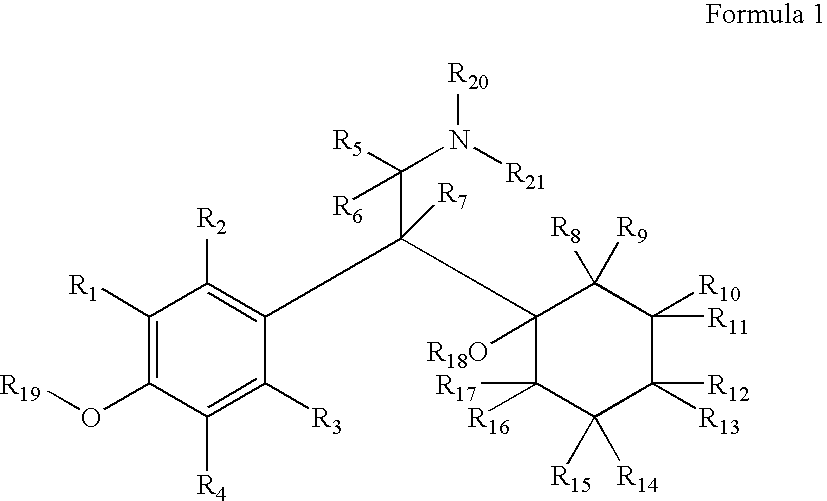
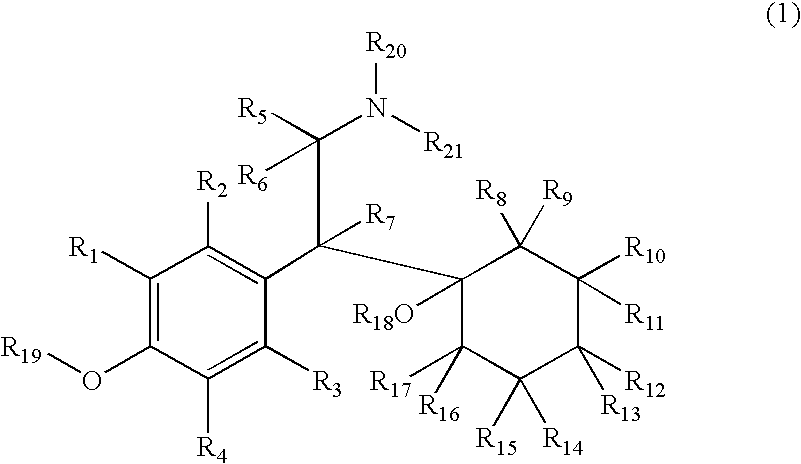
Therapeutic Pyrrolidines
InactiveUS20090215857A1Effective conditioningBiocideNervous disorderGeneralized anxiety disorderChronic Widespread Pain
The present invention provides for compounds of Formula (I),and pharmaceutically acceptable salts thereof, wherein A, J, Z, and R20 have any of the values defined therefore in the specification, and pharmaceutically acceptable salts thereof, that are useful as agents in the treatment of disorders and conditions including attention deficit hyperactivity disorder, neuropathic pain, urinary incontinence, generalized anxiety disorder, depression, schizophrenia, and fibromyalgia. Also provided are pharmaceutical compositions comprising one or more compounds of Formula (I) or pharmaceutically acceptable salts thereof.
Owner:PFIZER PROD INC
Antidepressant azaheterocyclylmethyl derivatives of heterocycle-fused benzodioxans
Compounds of the Formula:are useful for the treatment of depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as pre-menstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa and bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH LLC
S-adenosylmethionine formulations with enhanced bioavailability
InactiveCN102695514AGood obedienceImprove side effect performanceOrganic active ingredientsNervous disorderImmunologic disordersS-Adenosyl-l-methionine
The invention relates to compositions and methods to enhance the absorption of S- adenosylmethionine (SAMe) and to methods of treating various disorders or diseases using non-parenteral SAMe formulations with enhanced-absorption and improved bioavailability. The enhanced bioavailability formulations may be used to treat a variety of diseases or disorders, such as for example, psychiatric disorders including, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, depressive disorders (e.g. major clinical depression) and dysthymia; as well as treating liver disorders, cancer, autoimmune disorders, inflammatory disorders, joint disorders, gastrointestinal disorders and cardiovascular disease.
Owner:MSI METHYLATION SCI
Treatment of neurotic disorders
Use of escitalopram (the S-(+)-enantiomer of citalopram) or a pharmaceutically acceptable salt thereof for the preparation of a medicament useful in the treatment of neurotic disorders is provided, including anxiety states, in particular generalised anxiety disorder and social anxiety disorder, post traumatic stress disorder, obsessive compulsive disorder and panic attacks.
Owner:H LUNDBECK AS
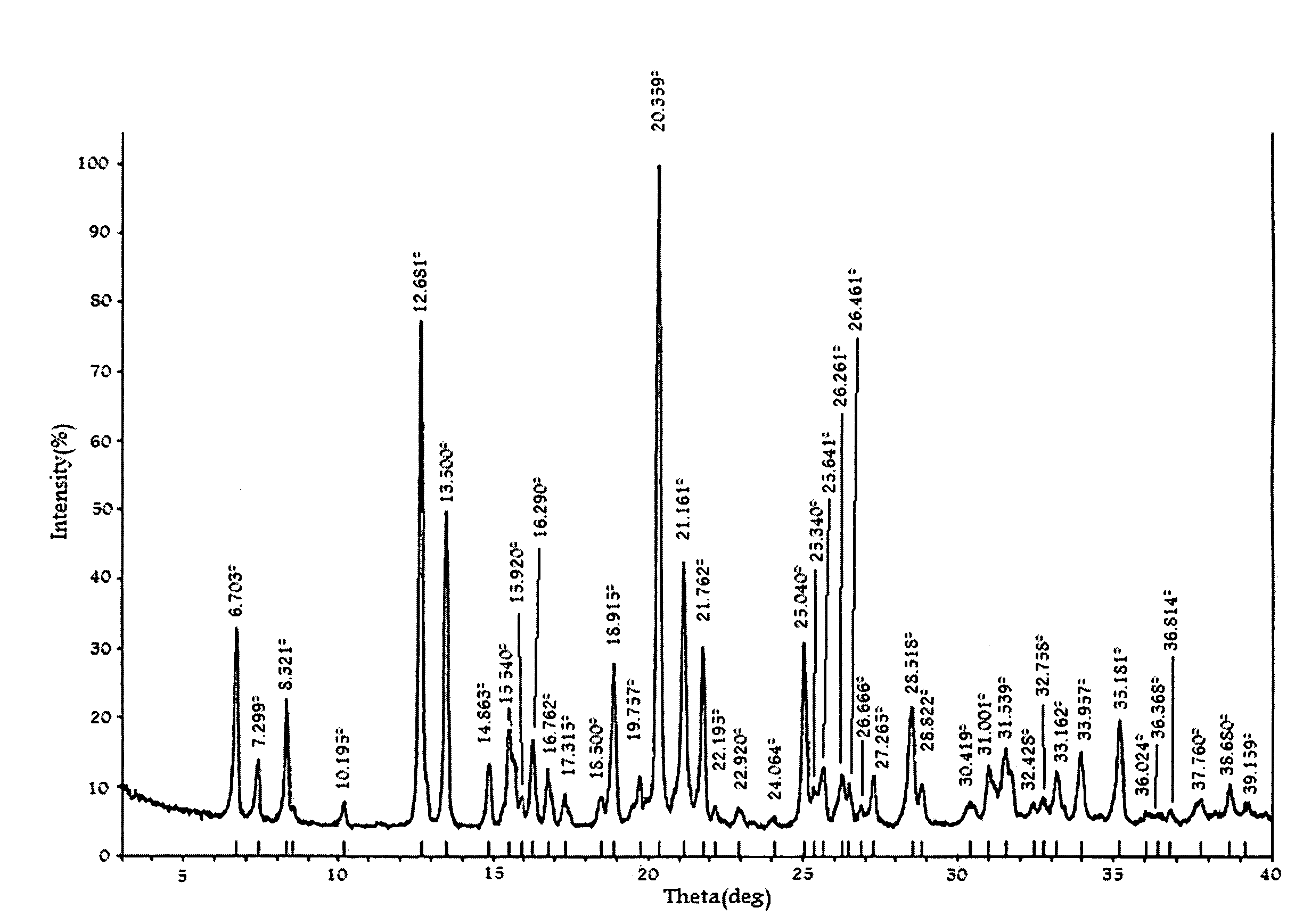
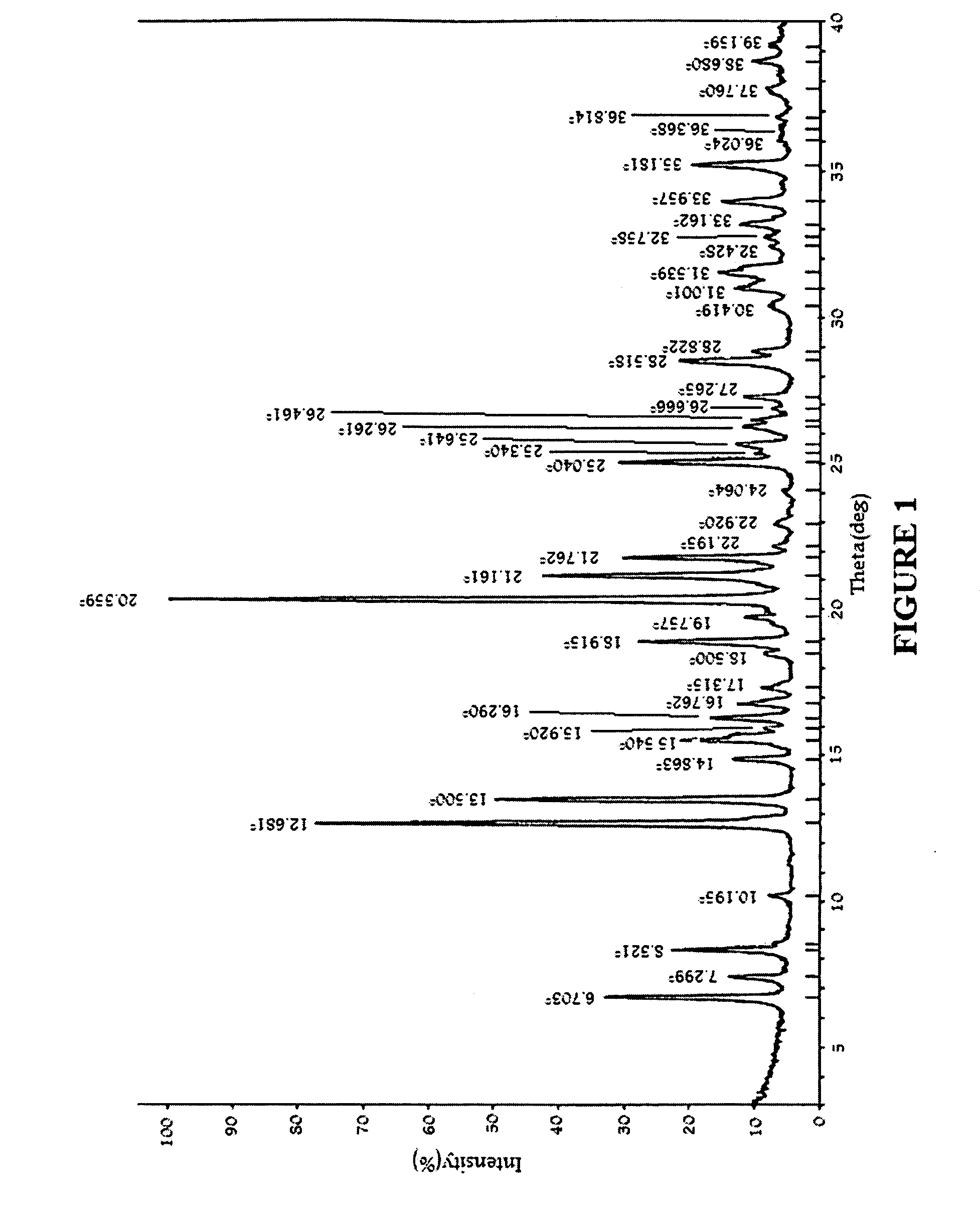
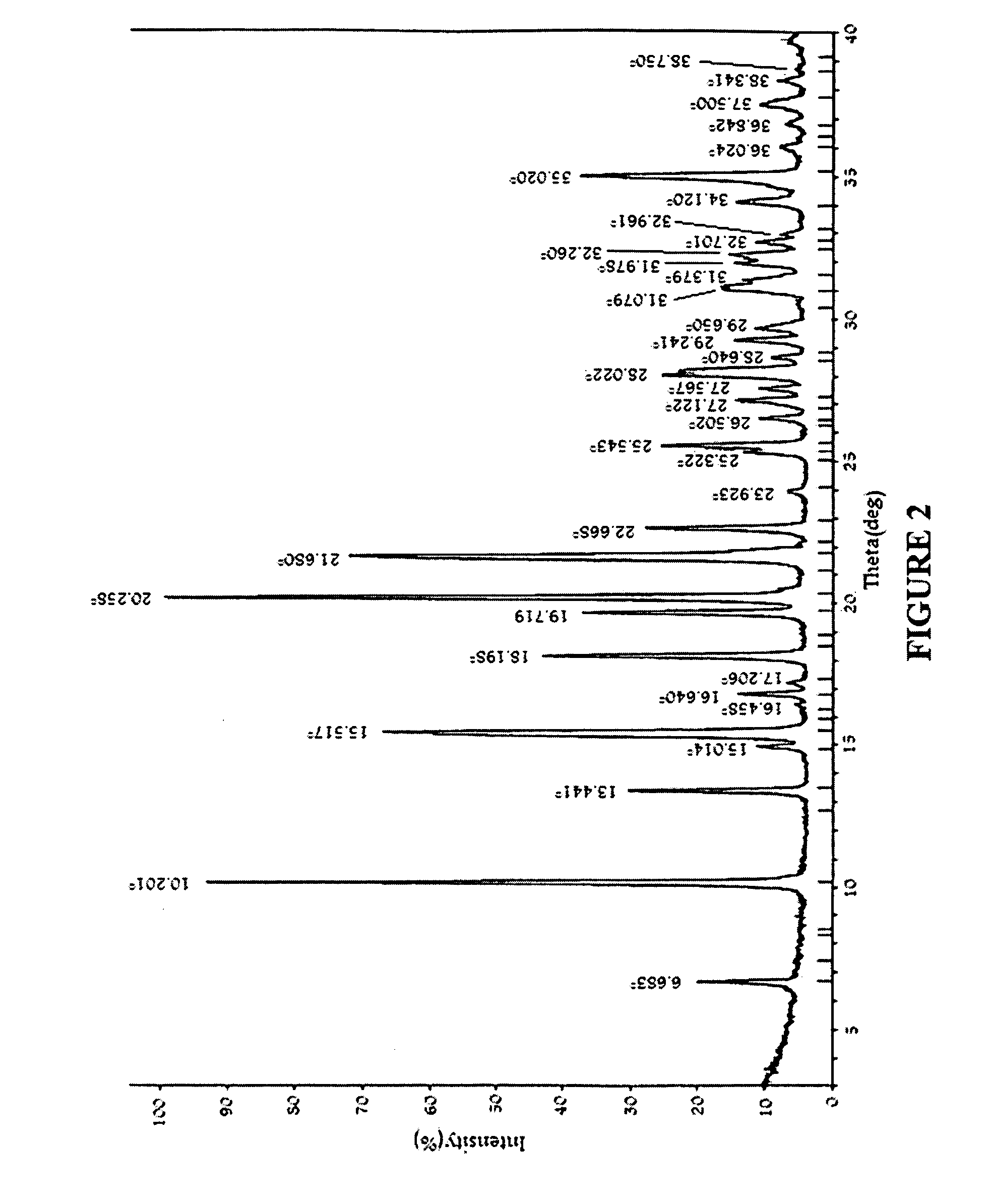
An amorphous vortioxetine and salts thereof
The present invention relates to an amorphous vortioxetine and salts thereof. In particular, the invention relates to a process for the preparation of an amorphous vortioxetine hydrobromide. Further, the invention also relates to a process for preparation of amorphous vortioxetine free base. The invention also relates to pharmaceutical compositions comprising an amorphous vortioxetine or hydrobromide salt thereof for oral administration for treatment of major depressive disorder (MDD) and generalized anxiety disorder (GAD).
Owner:CADILA HEALTHCARE LTD
Serotonin and norepinephrine reuptake inhibitor and uses thereof
InactiveUS20070015824A1Low level of undesirable side-effectsBiocideOrganic chemistryNorepinephrine reuptake inhibitorDepressant
Selective dual serotonin and norepinephrine reuptake inhibitors are provided. These compounds have a lower side-effect profile and are useful in compositions and products for use in treatment of a variety of conditions including depression, fibromyalgia, anxiety, panic disorder, agoraphobia, post traumatic stress disorder, premenstrual dysphoric disorder, attention deficit disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, autism, schizophrenia, obesity, anorexia nervosa, bulimia nervosa, Gilles de la Tourette Syndrome, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction, borderline personality disorder, fibromyalgia syndrome, diabetic neuropathic pain, chronic fatigue syndrome, pain, Shy Drager syndrome, Raynaud's syndrome, Parkinson's Disease, and epilepsy.
Owner:WYETH
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20080206333A1Improved pharmacokinetic propertiesAct quicklyBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Methods for the treatment of depression and anxiety
ActiveUS9814755B2Cosmetic preparationsOrganic active ingredientsGeneralized anxiety disorderClinical psychology
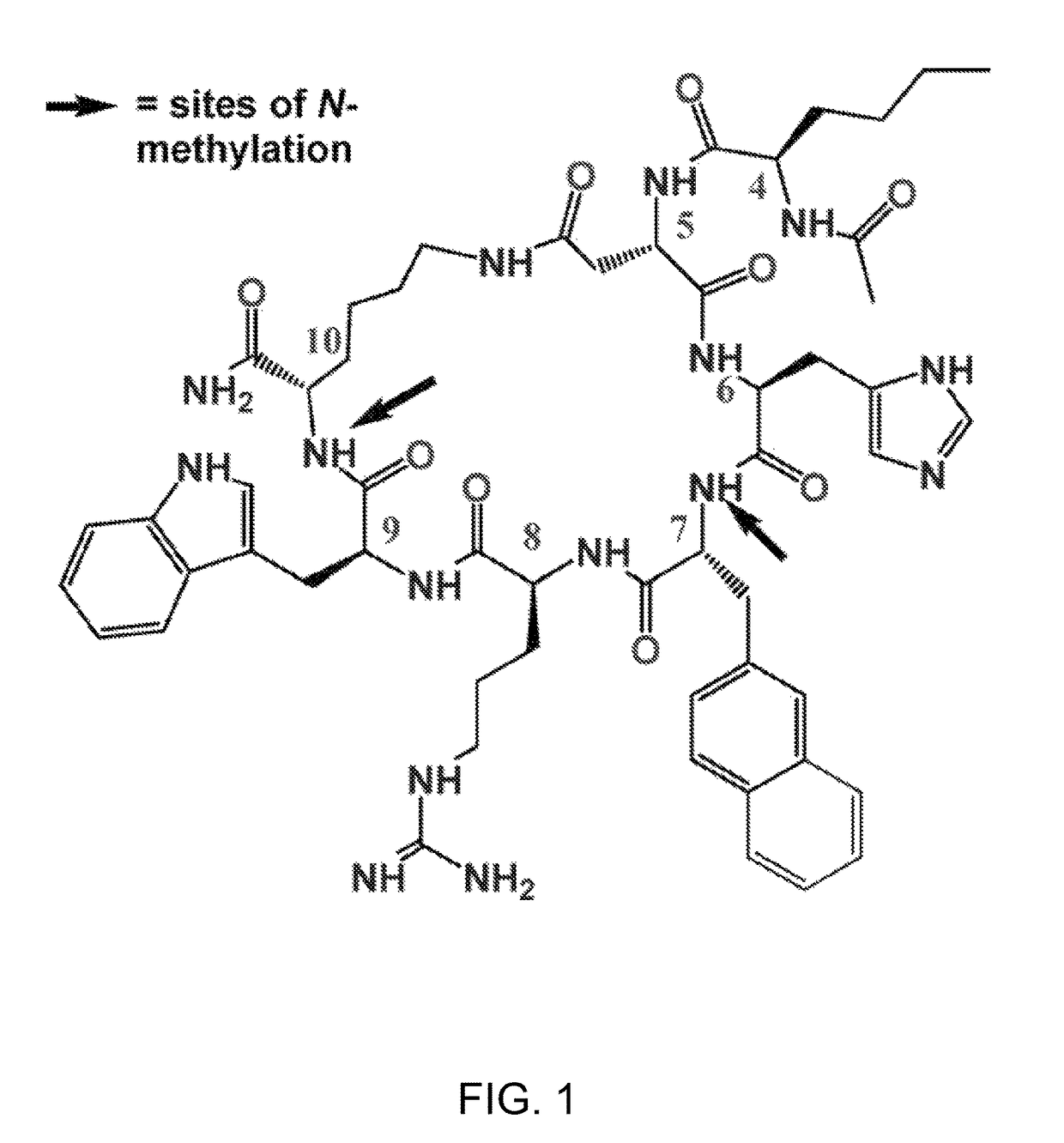
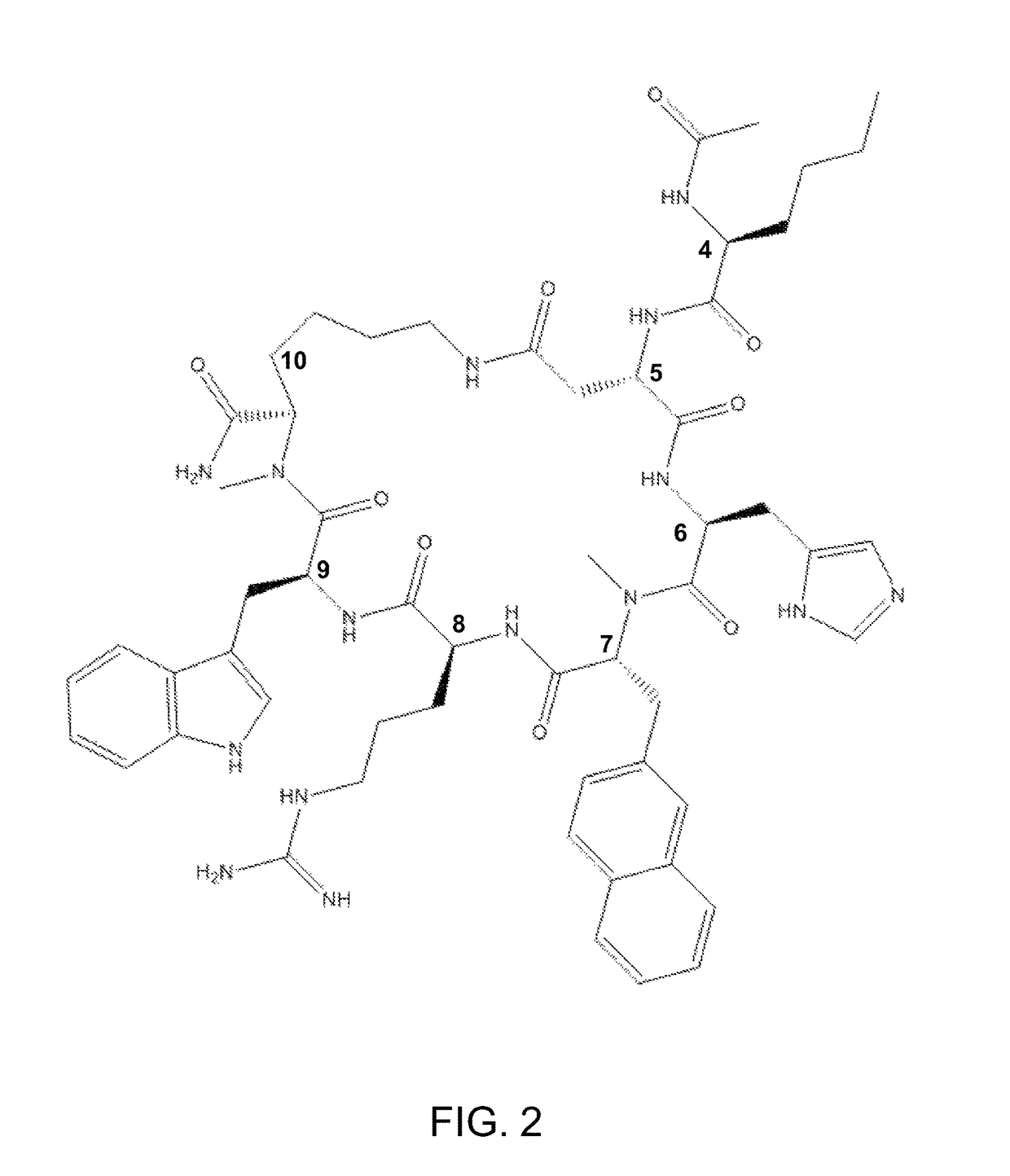
Methods of treating a depressive disorder or an anxiety disorder in a subject in need of such treatment are described. A therapeutically effective amount of a composition comprising Ac-Nle4-c[Asp5-His6-(NMe)D-Nal(2′)7-Arg8-Trp9-(NMe)Lys10]-NH2 (PEPTIDE 9), in a pharmaceutically acceptable carrier is administered to the subject. PEPTIDE 9 is a selective MC5R antagonist, in which administration thereof to the subject can treat the depressive or generalized anxiety disorder with clinical improvement observed in a relatively short time.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Antidepressant piperidine derivatives of heterocycle-fused benzodioxans
Compounds of the Formula: are useful for the treatment of depression (including but not limited to major depressive disorder, childhood depression and dysthymia), anxiety, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder (also known as premenstrual syndrome), attention deficit disorder (with and without hyperactivity), obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, obesity, eating disorders such as anorexia nervosa and bulimia nervosa, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction and related illnesses.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18b7c78f-e97e-45ca-abd5-187dfef2a747/US20020128261A1-20020912-C00001.png)
![Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18b7c78f-e97e-45ca-abd5-187dfef2a747/US20020128261A1-20020912-C00002.png)
![Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives Cyclohepta [b] [1,4] diazepino [6,7, 1-hi] indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18b7c78f-e97e-45ca-abd5-187dfef2a747/US20020128261A1-20020912-C00003.png)



![Antidepressant indoletetrahydropyridine derivatives of 2,3-dihydro-7H-[1,4]dioxino[2,3-e]indole Antidepressant indoletetrahydropyridine derivatives of 2,3-dihydro-7H-[1,4]dioxino[2,3-e]indole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fa638479-6562-4a9b-9860-80212c0a54d3/US20020183352A1-20021205-C00001.png)
![Antidepressant indoletetrahydropyridine derivatives of 2,3-dihydro-7H-[1,4]dioxino[2,3-e]indole Antidepressant indoletetrahydropyridine derivatives of 2,3-dihydro-7H-[1,4]dioxino[2,3-e]indole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fa638479-6562-4a9b-9860-80212c0a54d3/US20020183352A1-20021205-C00002.png)
![Antidepressant indoletetrahydropyridine derivatives of 2,3-dihydro-7H-[1,4]dioxino[2,3-e]indole Antidepressant indoletetrahydropyridine derivatives of 2,3-dihydro-7H-[1,4]dioxino[2,3-e]indole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fa638479-6562-4a9b-9860-80212c0a54d3/US20020183352A1-20021205-C00003.png)


![Cyclohepta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cyclohepta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b64c92b5-bf7b-4b0c-abe7-3bb93fd02b7b/US06858604-20050222-C00001.png)
![Cyclohepta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cyclohepta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b64c92b5-bf7b-4b0c-abe7-3bb93fd02b7b/US06858604-20050222-C00002.png)
![Cyclohepta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cyclohepta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b64c92b5-bf7b-4b0c-abe7-3bb93fd02b7b/US06858604-20050222-C00003.png)
![Cycloocta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cycloocta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8f9a203-a5d9-426e-92ce-fdf934fd0a4a/US07271162-20070918-C00001.png)
![Cycloocta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cycloocta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8f9a203-a5d9-426e-92ce-fdf934fd0a4a/US07271162-20070918-C00002.png)
![Cycloocta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cycloocta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8f9a203-a5d9-426e-92ce-fdf934fd0a4a/US07271162-20070918-C00003.png)
![Cyclopenta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cyclopenta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/429a9f3f-bfa4-449f-a95e-69850e31cb4b/US07271163-20070918-C00001.png)
![Cyclopenta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cyclopenta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/429a9f3f-bfa4-449f-a95e-69850e31cb4b/US07271163-20070918-C00002.png)
![Cyclopenta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives Cyclopenta[b][1,4]diazepino[6,7,1-hi]indoles and derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/429a9f3f-bfa4-449f-a95e-69850e31cb4b/US07271163-20070918-C00003.png)