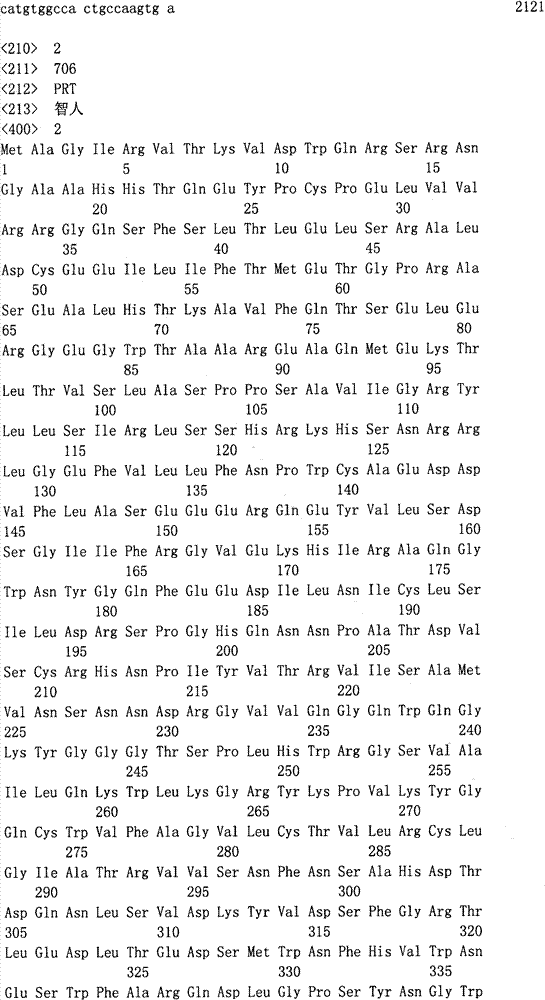Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Cerebellar ataxia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cerebellar ataxia is a form of ataxia originating in the cerebellum. Non-progressive congenital ataxia (NPCA) is a classical presentation of cerebral ataxias. Cerebellar ataxia can occur as a result of many diseases and may present with symptoms of an inability to coordinate balance, gait, extremity and eye movements. Lesions to the cerebellum can cause dyssynergia, dysmetria, dysdiadochokinesia, dysarthria and ataxia of stance and gait. Deficits are observed with movements on the same side of the body as the lesion (ipsilateral). Clinicians often use visual observation of people performing motor tasks in order to look for signs of ataxia.
Treatment of mitochondrial diseases
InactiveUS20050065099A1Limit prevent damageBiocideSenses disorderHuntingtons choreaCerebellar ataxia
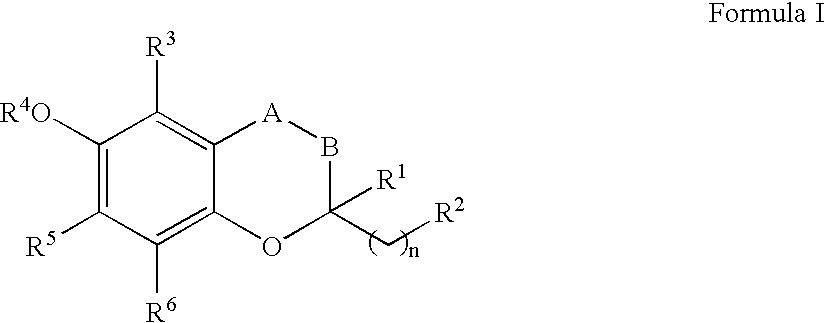
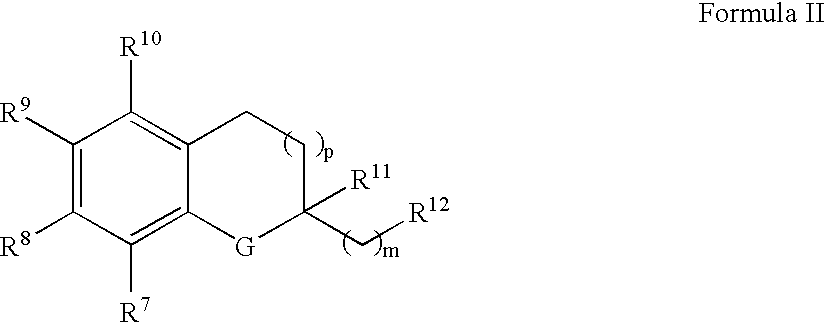
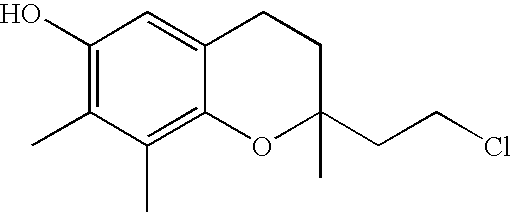
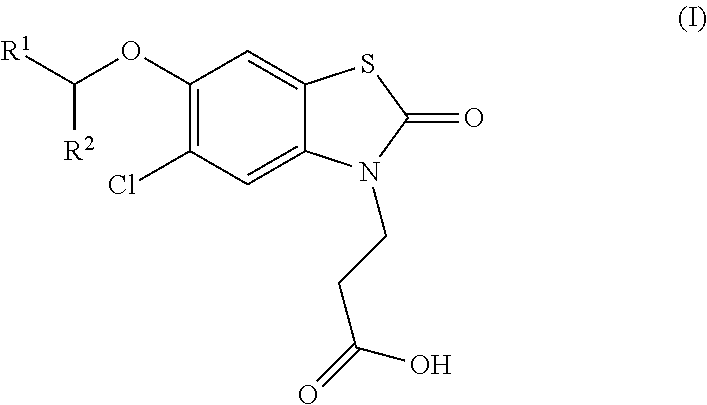
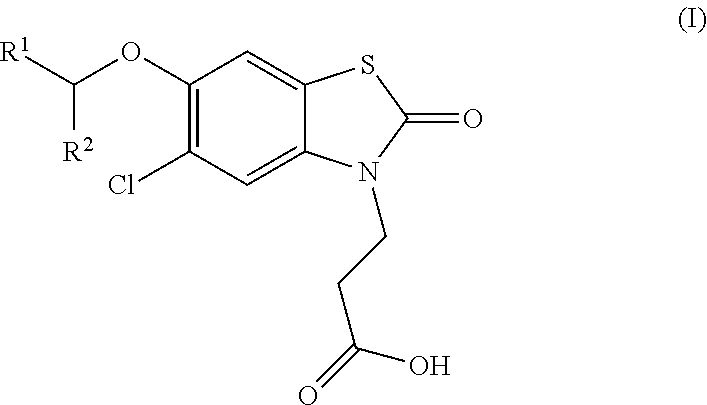
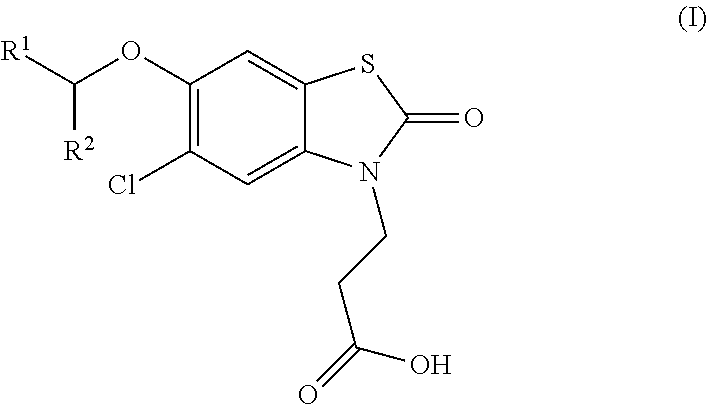
The invention relates the method of treatment or amelioration of mitochondrial disorders such as Alzheimer's disease, Parkinson's disease, Friedreich's ataxia (FRDA), cerebellar ataxias, Leber's hereditary optic neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Myoclonic Epilepsy with Ragged Red Fibers (MERFF), amyotrophic lateral sclerosis (ALS), motor neuron diseases, Huntington's disease, macular degeneration, and epilepsy, with chroman derivatives of Formula I or Formula II as described herein.
Owner:EDISON PHARMA
Combination of atypical antipsychotics and 5HT-1B receptor antagonists
InactiveUS20050256112A1Reduce morbidityDifferent recognizableNervous disorderMetabolism disorderDiseaseHeadaches
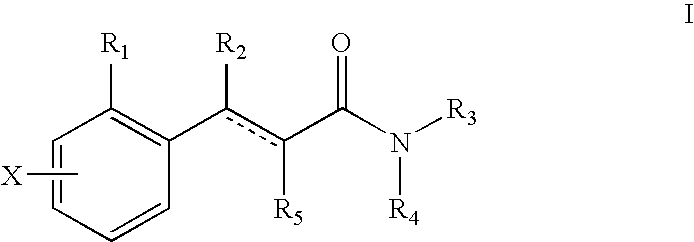
The present invention relates to a pharmaceutical composition for treating, for example, a disorder or condition selected from the group consisting of hypertension, depression, generalized anxiety disorder, phobias, posttraumatic stress disorder, avoidant personality disorder, sexual dysfunction, eating disorders, obesity, chemical dependencies, cluster headache, migraine, pain, Alzheimer's disease, obsessive-compulsive disorder, panic disorder, memory disorders, Parkinson's diseases, endocrine disorders, cerebellar ataxia, gastrointestinal tract disorders, negative symptoms of schizophrenia, premenstrual syndrome, Fibromyalgia Syndrome, stress incontinence, Tourette syndrome, trichotillomania, kleptomania, male impotence, cancer, chronic paroxysmal hemicrania and headache in a mammal, preferably a human, comprising (i) an atypical antipsychotic or a pharmaceutically acceptable salt thereof, (ii) a 5-HT1B receptor antagonist or a pharmaceutically acceptable salt thereof, wherein the 5-HT1B receptor antagonist is selected from the group consisting of (A) a compound of the formula I as described in the specification and (B) a compound of the formula II as described in the specification, and optionally (iii) a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Method for constructing disease model on basis of gene operation strategy and application
ActiveCN110100788AWide range of usesIncrease the speed of proliferationAnimal husbandryCerebellar ataxiaVascular endothelium
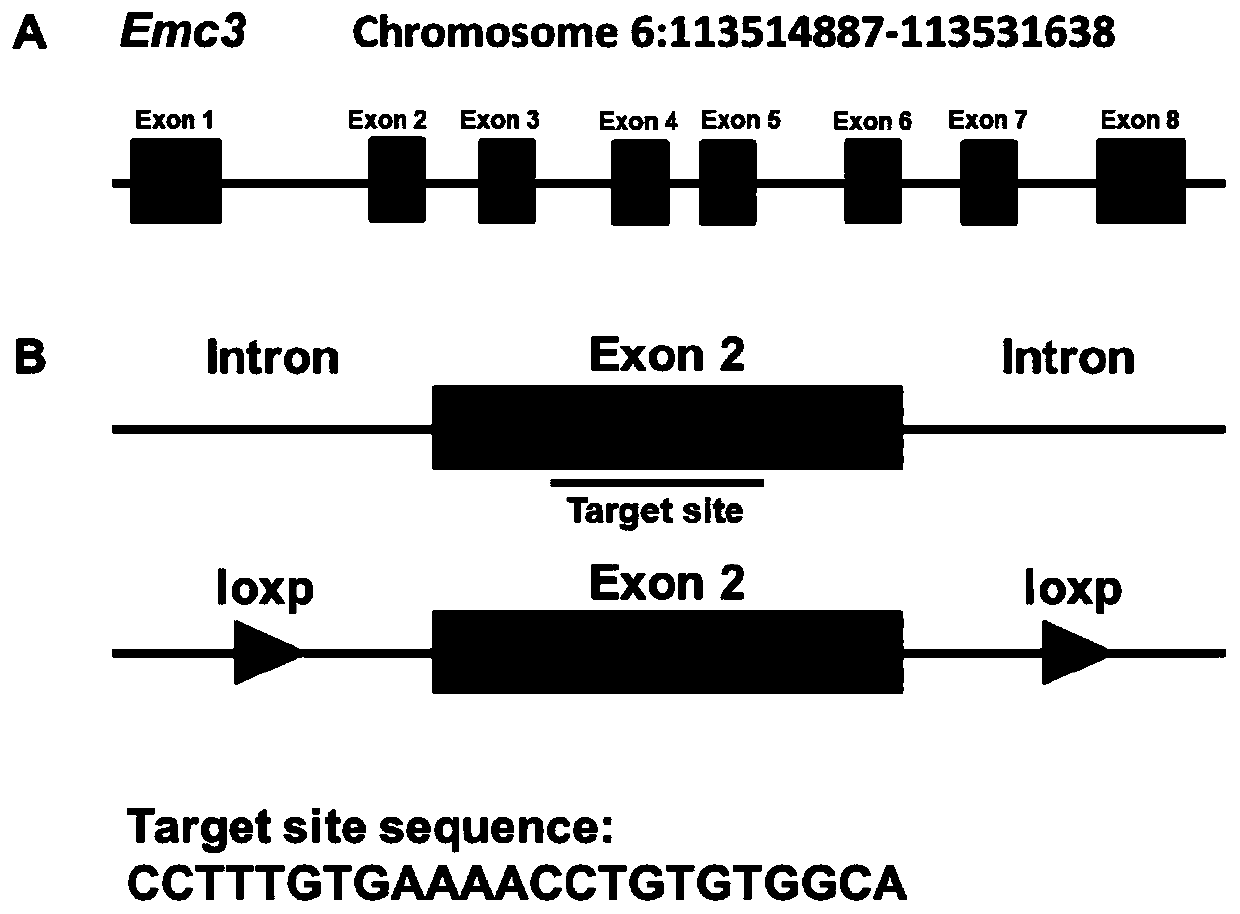
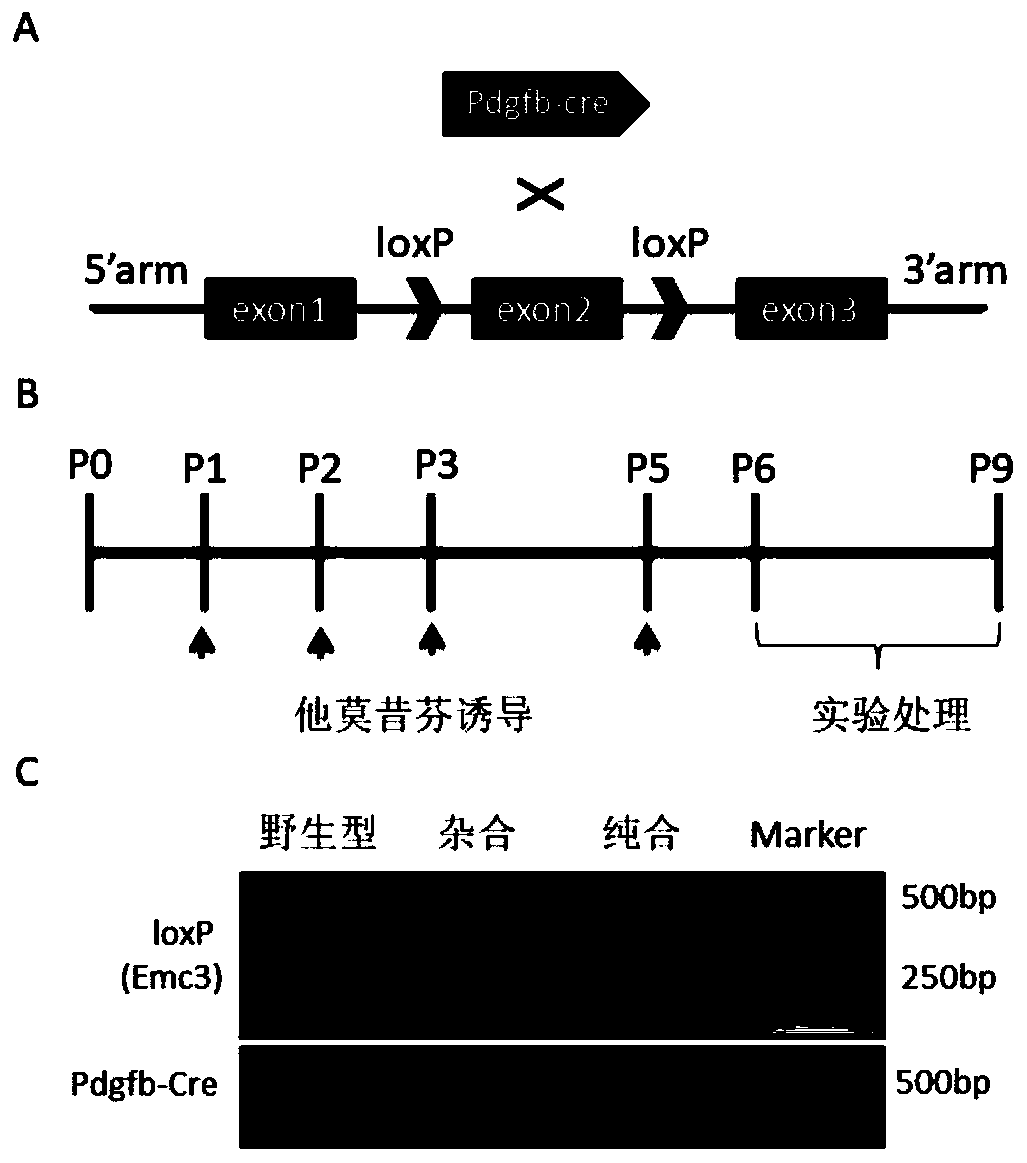
The invention discloses a method for constructing a disease model on the basis of a gene operation strategy and application, and relates to the technical field of biology. According to the method, anEmc3 gene is prevented from being expressed in vascular endothelial cells or cerebellum Purkinje cells of a target animal through a gene operation technology. By using the method, a disease animal model showing typical characteristics of retinal neovascularization diseases or cerebellar ataxia diseases can be obtained. The method provides a new thought or strategy for constructing a retinal neovascularization disease model or a cerebellar ataxia disease model. In addition, the disease model obtained by means of the method also provides a model basis for science researchers to study the retinalneovascularization diseases and screen medicines for treating the retinal neovascularization diseases or the cerebellar ataxia diseases.
Owner:SICHUAN ACADEMY OF MEDICAL SCI SICHUAN PROVINCIAL PEOPLES HOSPITAL
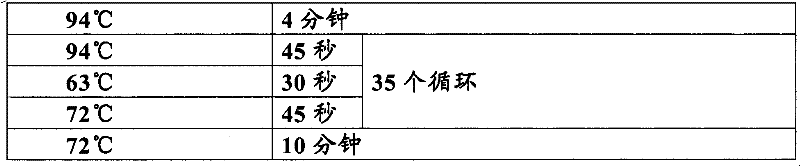
Primer for detecting dynamic mutation of CAG repetitive sequence of ATXN3 gene and PCR amplification method thereof
InactiveCN101654711AStrong specificityImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationCerebellar ataxiaDynamic equation
The invention relates to an improved primer for detecting the dynamic mutation of a CAG repetitive sequence of a spinocerebellar ataxia ATXN3 gene and a method thereof, characterized in that a specially designed specific primer is adopted to amplify the amount of a DNA fragment containing the CAG repetitive sequence in the ATXN3 gene to the observable degree through a special PCR procedure, and gel electrophoresis is utilized to observe the size of a PCR amplification product fragment and estimate the number of replication of the CAG dynamic mutation in the ATXN3 gene. The invention has the advantages that: 1. simplicity and economy, and no sequence detection, i.e. the method can be operated in common molecular biology laboratories or control laboratories, thereby establishing a simple detection method of the amplification CAG repetitive sequence; 2. high sensitivity and reliability: 25ng genome DNA is enough to detect the number of replication of the CAG dynamic mutation in the ATXN3gene, thereby a reliable basis is provided for clinically diagnosing spinocerebellar ataxia.
Owner:SUZHOU UNIV
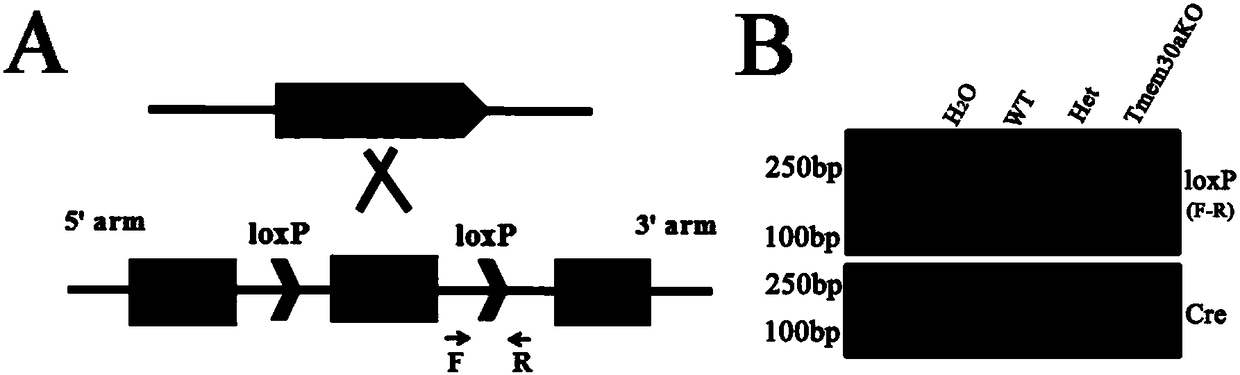
Construction method and application of incoordination animal model
ActiveCN107586791AUnsteady gaitStable introduction of DNAVector-based foreign material introductionCerebellar ataxiaDisease
The invention discloses a construction method and application of an incoordination animal model and relates to the technical field of medical engineering. The construction method of the incoordinationanimal model, disclosed by the invention, comprises the step of knocking out a target sequence on a genome in a cerebellar purkinje cell of a target animal, wherein the target sequence is a Tmem30a gene. According to the method disclosed by the invention, a Tmem30a gene sequence on the genome in the cerebellar purkinje cell of the target animal is specifically knocked out for the first time and acerebellum incoordination animal model can be constructed; the animal model has typical cerebellum incoordination characteristics: unstable treads, cerebellar atrophy, progressive apoptosis of the cerebellar purkinje cell and the like. The model can be used for researching cerebellum incoordination and provides a foundation for finding disease-causing gene of the cerebellum incoordination and exploring pathogenesis.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Compositions and methods for treating spinocerebellar ataxia
The present invention provides intravenous compositions of trehalose for the treatment of signs and symptoms of spinocerebellar ataxia (SCA).
Owner:SEELOS THERAPEUTICS INC
Experimental animal rat cerebellar ataxia detection model
The invention discloses an experimental animal rat cerebellar ataxia detection model, which is characterized by detecting the changes of induced ataxia after cerebellar injury through an experimental animal rat cerebellar ataxia detector via the establishment of the experimental animal rat cerebellar ataxia detection model. The detector is formed by connecting a camera lucida, a camera obscura, a balance rod, a water pool, an electric stimulus pole piece, an adjustable transformer, a switch, a power supply and wires, wherein a plastic pot is arranged between the camera lucida and the camera obscura; the balance rod is arranged above the water pool and is connected with the camera lucida and the camera obscura; and the electric stimulus pole piece in the camera lucida is successively connected with the switch, the adjustable transformer and the power supply via the wires. The detection model is simple, easy to manufacture and operate, objective and sensitive, and has repeatability, scientificalness, reliability and practicability on orientated, quantitive and qualitative researches on rat ataxia.
Owner:成都军区昆明总医院
Acylphloroglucinol derivative as well as pharmaceutical composition and application thereof
ActiveCN111170967APotential clinical application valueSignificant and potent activating activityOrganic active ingredientsNervous disorderCerebellar ataxiaIonic Channels
The invention provides a dearylated isopentenyl acylphloroglucinol derivative, a preparation method thereof, a pharmaceutical composition for neurodegenerative diseases and an application of the pharmaceutical composition, and belongs to the technical field of medicines. The compound Hypatone A provided by the invention has a new rare cage structure, is the strongest Cav3.1 low-voltage gated calcium ion channel activator at present, and has potential medicinal value in Alzheimer's disease and spinal cerebellar ataxia 42.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Application of medicine for treating chronic neurodegenerative diseases
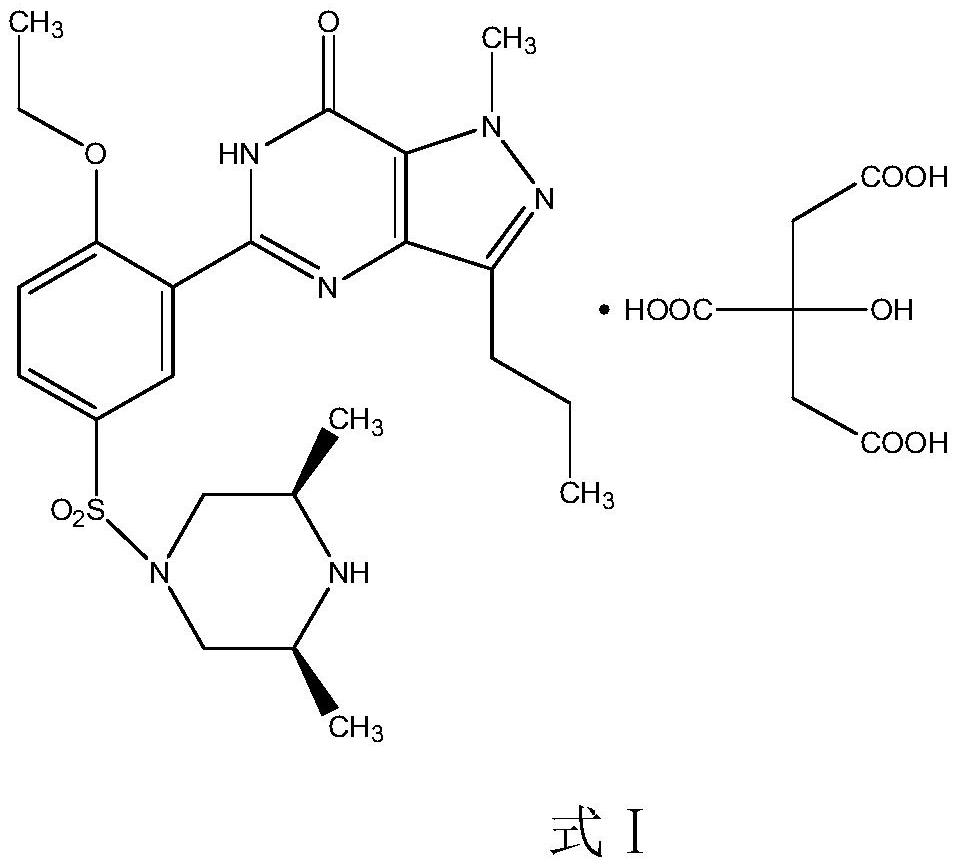
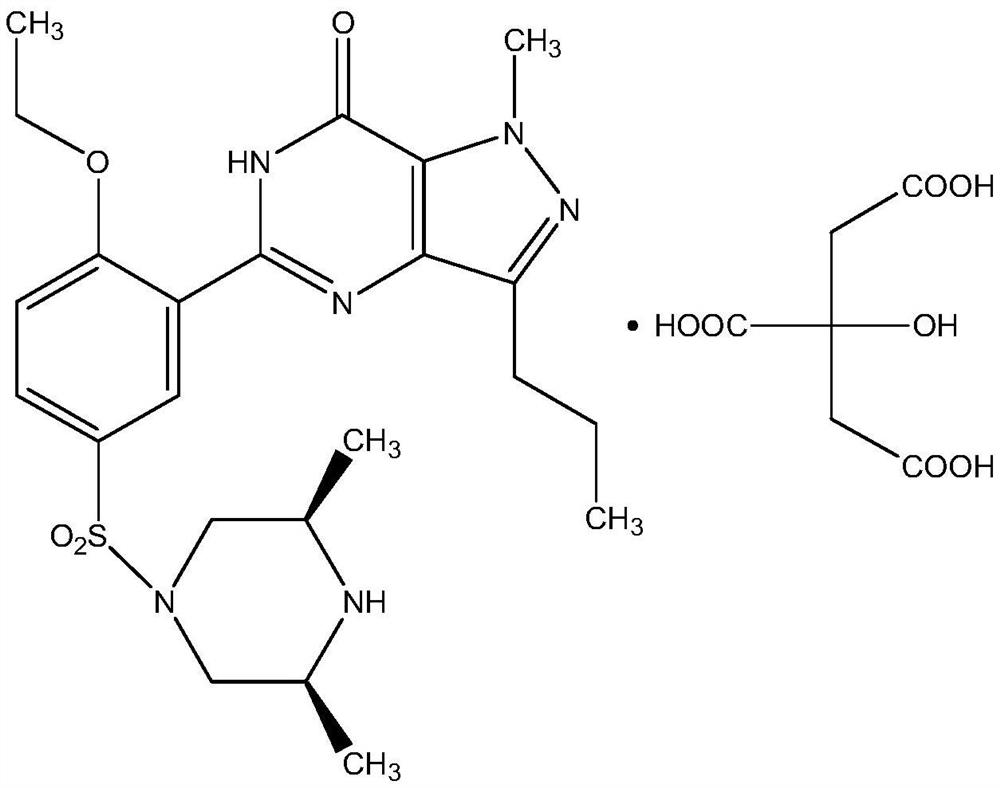
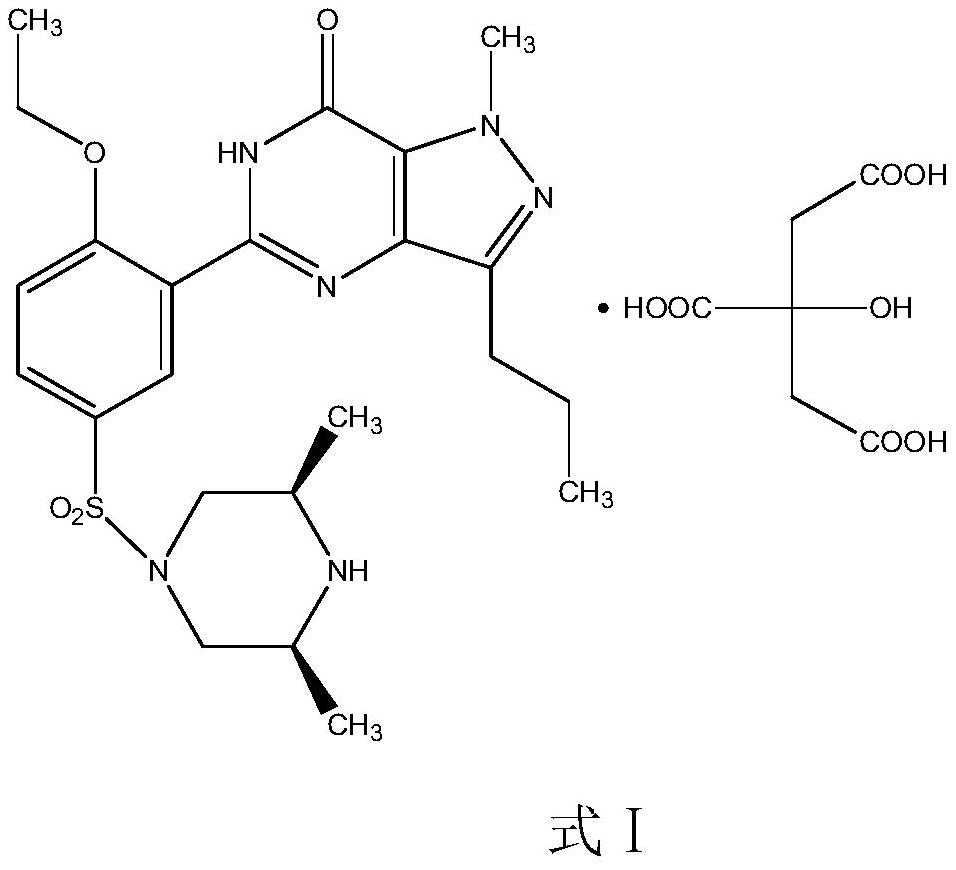
The invention relates to application of 1-[3-(6, 7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4, 3-d] pyrimidine-5-yl)-4-ethoxy benzenesulfonyl]-cis-3, 5-dimethylpiperazine citrate or Aildenafil citrate in preparation of a medicine for treating neurodegenerative diseases, wherein the treatment of chronic neurodegenerative diseases comprises the treatment of Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and different types of spinal cerebellar ataxia medicine diseases. The Aildenafil citrate has a wide application prospect in preparation of the medicine for treating the neurodegenerative diseases and treatment of the neurodegenerative diseases.
Owner:刘桂坤
1,7-naphthyridine derivatives
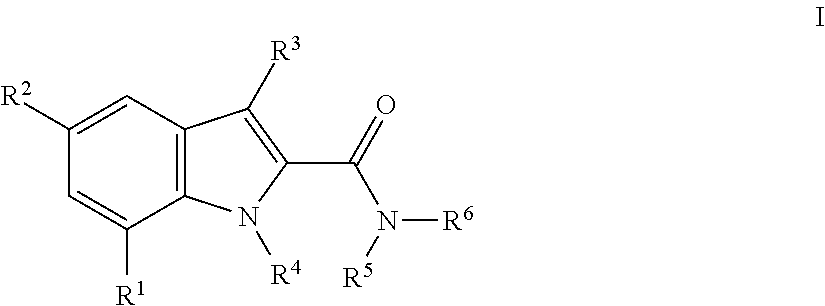
The present invention relates to compounds of general formula I wherein R1, R2, R3 and R4 are as defined herein which maybe used for the treatment of schizophrenia, obsessive-compulsivepersonality disorder, major depression, bipolar disorders, anxiety disorders, normal aging, epilepsy, retinal degeneration, traumatic brain injury, spinal cord injury, post-traumatic stress disorder, panic disorder, Parkinson's disease, dementia, Alzheimer's disease, cognitive impairment, chemotherapy-induced cognitive dysfunction (“chemobrain”), Down syndrome, autism spectrum disorders, hearing loss, tinnitus, spinocerebellar ataxia, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, stroke, and disturbances due to radiation therapy, chronic stress, optic neuropathy or macular degeneration, or abuse of neuro-active drugs, selected from alcohol, opiates, methamphetamine, phencyclidine and cocaine.
Owner:F HOFFMANN LA ROCHE & CO AG
A construction method and application of an ataxia animal model
ActiveCN107586791BUnsteady gaitStable introduction of DNAVector-based foreign material introductionDiseaseCerebellar ataxia
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
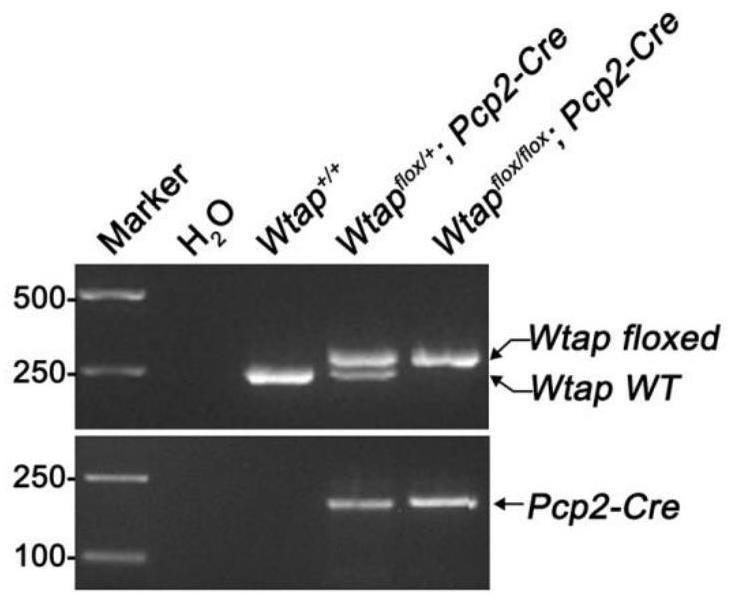
Construction method and application of cerebellar ataxia disease model
The invention discloses a construction method and application of a cerebellar ataxia disease model, and relates to the technical field of biology. The construction method comprises the step that a Wtap gene in a target animal genome is not expressed or expression is inhibited, and therefore the cerebellar ataxia disease model is obtained. The model can show the cerebellar ataxia disease characteristics, can be used for researching cerebellar ataxia diseases, can help to clarify the HA morbidity process and mechanism, and provides a new target for treating or preventing the diseases. In addition, the invention further provides application of the cerebellar ataxia disease model in researching the cerebellar ataxia disease morbidity mechanism or screening drugs for preventing or treating the cerebellar ataxia diseases.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Method for Inhibiting Spinocerebellar Ataxia
InactiveUS20140249095A1Promote absorptionReduce deliveryBiocideNervous disorderCerebellar ataxiaMedicine
A method for inhibiting spinocerebellar ataxia is disclosed, which comprises: administering an extract of Paeonia lactiflora to a subject in need; wherein a concentration of the extract of Paeonia lactiflora is in the range from 1 μg / mL to 80 μg / mL.
Owner:NATIONAL TAIWAN NORMAL UNIVERSITY
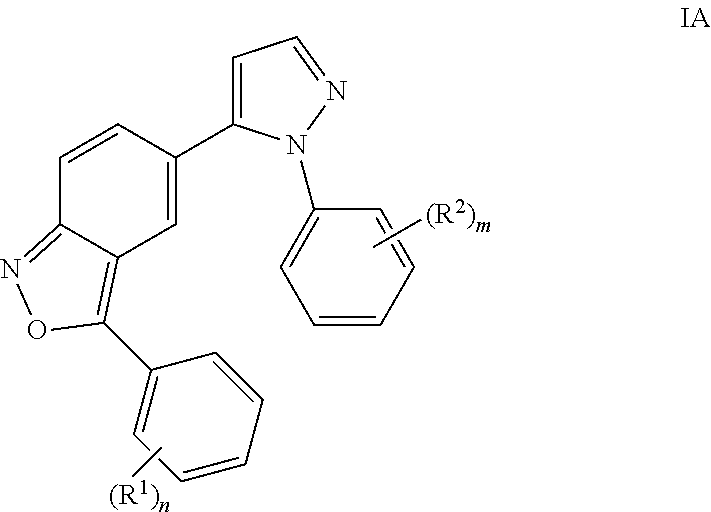
Benzisoxazoles
The present invention relates to compounds of general formulawhereinAr1 / Ar2 are phenyl or a 5 or 6-membered heteroaryl;R1 / R2 is hydrogen, halogen, lower alkyl, CF3 or lower alkoxy; n,m are 1 or 2;or to a pharmaceutically acceptable acid addition salt, to a racemic mixture or to its corresponding enantiomer and / or optical isomers thereof, with the exception of the compound 2,1-benzisoxazole, 3-(4-chlorophenyl)-5-(1-phenyl-1H-pyrazol-5 -yl)-.The compounds may be used for the treatment of schizophrenia, obsessive-compulsive personality disorder, major depression, bipolar disorders, anxiety disorders, normal aging, epilepsy, retinal degeneration, traumatic brain injury, spinal cord injury, post-traumatic stress disorder, panic disorder, Parkinson's disease, dementia, Alzheimer's disease, mild cognitive impairment, chemotherapy-induced cognitive dysfunction, Down syndrome, autism spectrum disorders, hearing loss, tinnitus, spinocerebellar ataxia, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, stroke, radiation therapy, chronic stress, abuse of neuro-active drugs, such as alcohol, opiates, methamphetamine, phencyclidine and cocaine.
Owner:F HOFFMANN LA ROCHE INC
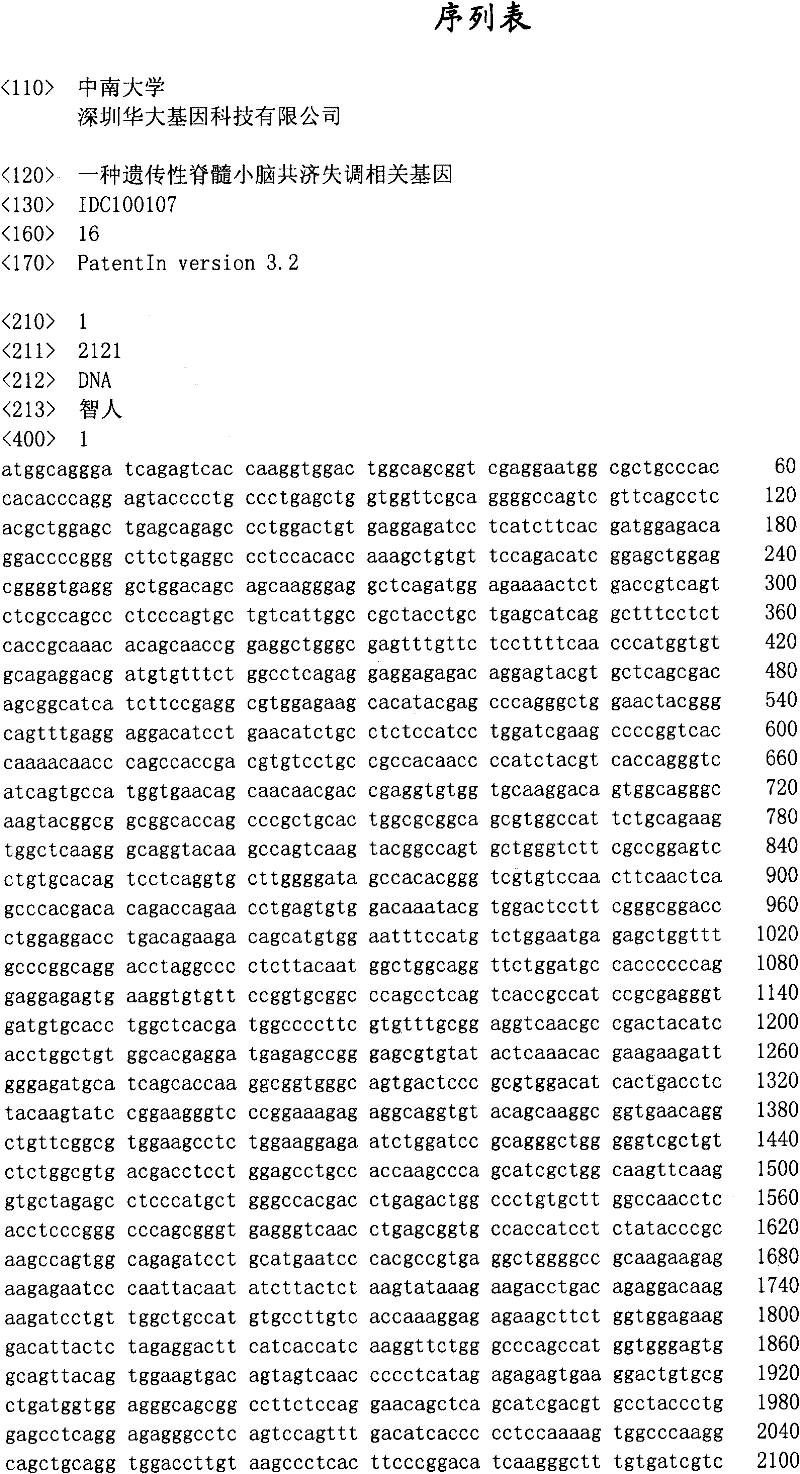
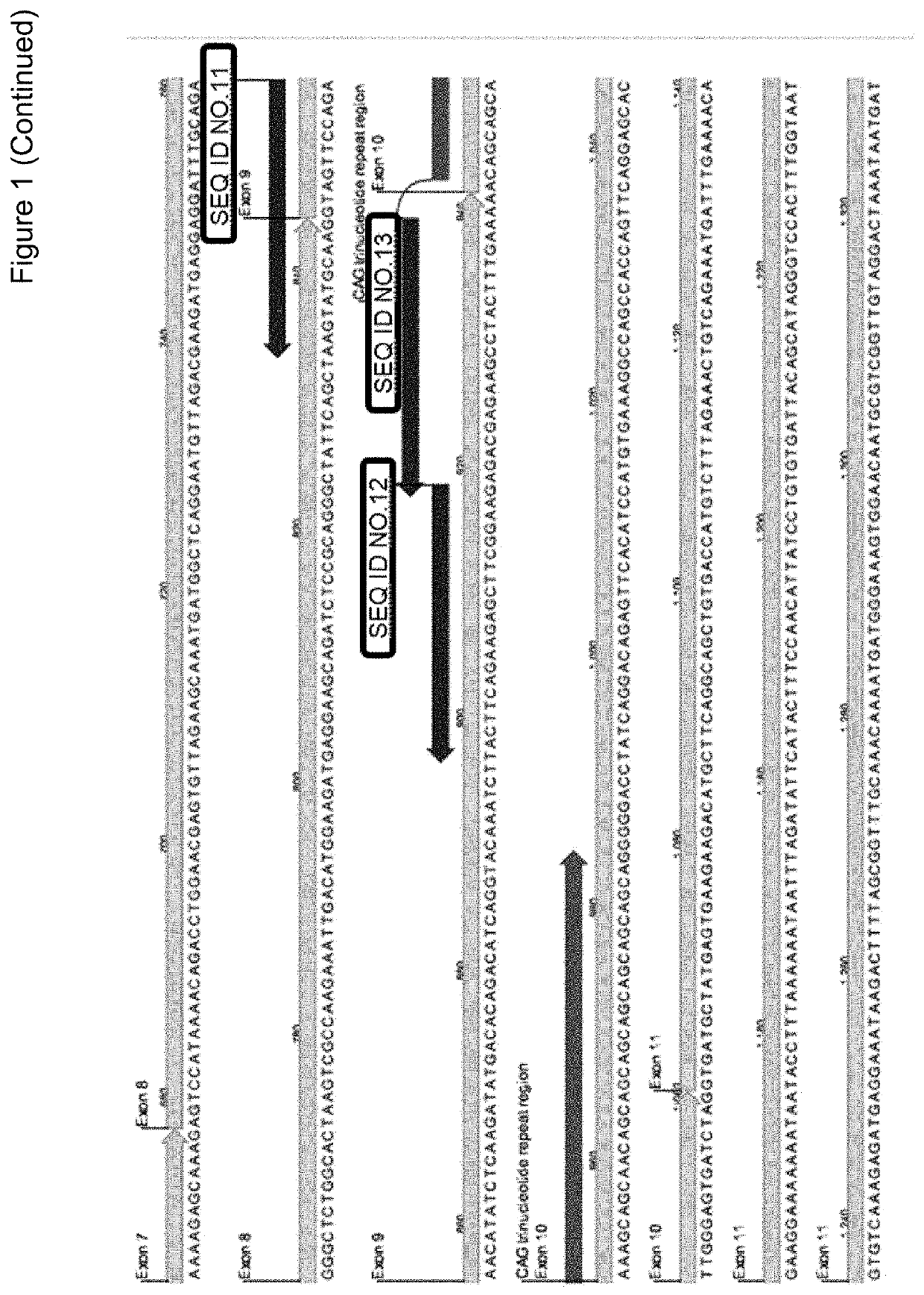
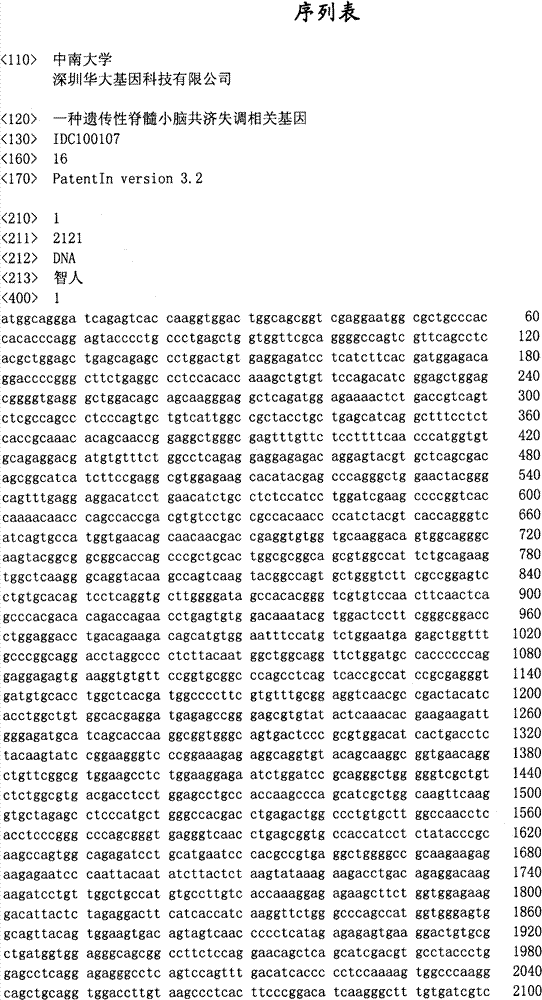
Hereditary medullispinal cerebellar ataxia related gene
The invention belongs to the field of molecular pathology, and relates to a hereditary medullispinal cerebellar ataxia related gene (mutant), particularly a hereditary medullispinal cerebellar ataxia related gene of which the amino acid sequence is disclosed as SEQ ID NO:7 or SEQ ID NO:9, and more particularly a hereditary medullispinal cerebellar ataxia related gene of which the nucleic acid sequence is disclosed as SEQ ID NO:6 or SEQ ID NO:8. The invention also relates to a recombinant vector containing the medullispinal cerebellar ataxia related gene, a recombinant cell containing the recombinant vector, and a coding protein of the medullispinal cerebellar ataxia related gene. Besides, the invention also relates to an exon of the hereditary medullispinal cerebellar ataxia related gene and a coding protein thereof. The invention provides references for auxiliary molecular diagnosis, molecular typing, prenatal diagnosis, drug target selection and clinical treatment of the hereditary medullispinal cerebellar ataxia.
Owner:CENT SOUTH UNIV +1
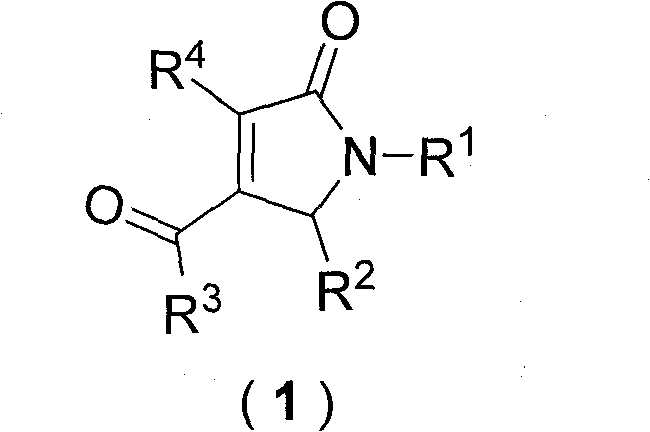
Dihydropyrrolones derivative as Caspase-3 inhibitor
Owner:SOUTH CHINA UNIV OF TECH +1
Chemical ifg-i composition for the treatment and prevention of neurodgenerative diseases
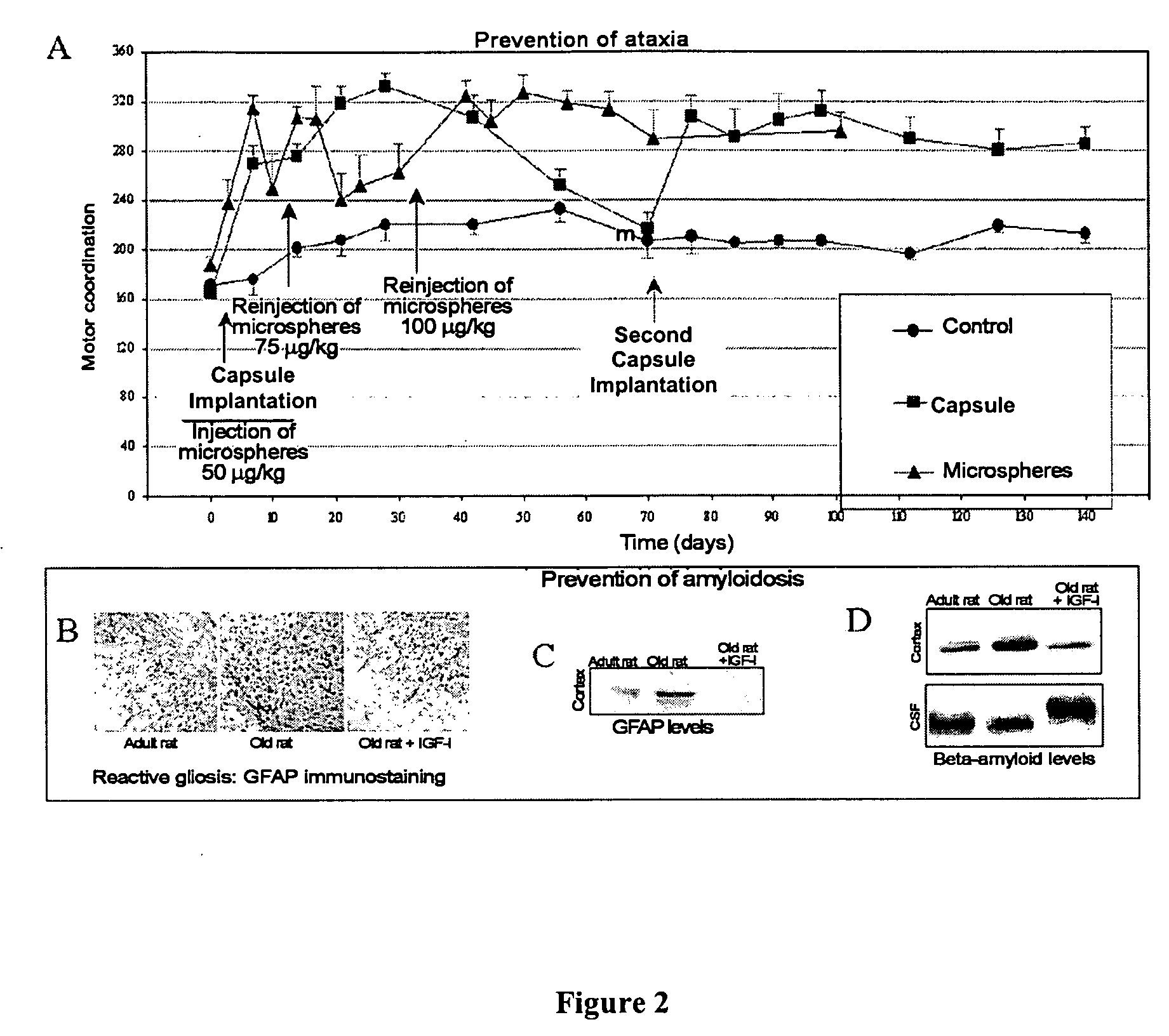
InactiveUS20050208126A1Prevent disease of nervous systemAvoid problemsPowder deliveryNervous disorderCerebellar ataxiaMicrosphere
The invention relates to novel, sustained-release IGF-I therapeutic compositions, a preparation and production method thereof and the use of same in the production of medicaments for the treatment and prevention of neurodegenerative diseases such as, among others, Alzheimer's disease or cerebellar ataxia. The inventive compositions take the form of microspheres having a size of less than 5 micrometres, among other characteristics, and subcutaneous implantation capsules.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
stub1 gene mutant and its application
ActiveCN104513822BBioreactor/fermenter combinationsBiological substance pretreatmentsCerebellar ataxiaCombined method
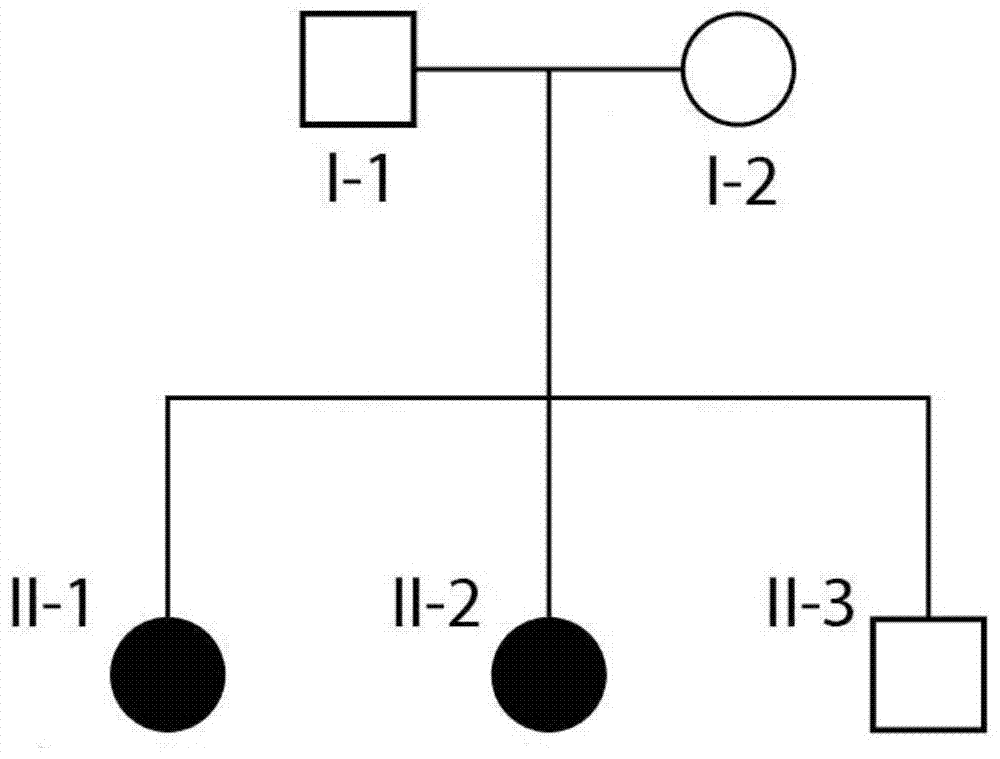
The invention discloses STUB1 gene mutants and applications thereof, in particular to isolated nucleic acids encoding STUB1 mutants, isolated polypeptides, methods for screening biological samples susceptible to gonadal hypoplasia combined with cerebellar ataxia, and screening for susceptible gonad development A system for biological samples of hypogonadism with cerebellar ataxia, and a kit for screening biological samples for predisposition to gonadal hypogenesis with cerebellar ataxia. Wherein, compared with SEQ ID NO: 1, the isolated nucleic acid encoding the STUB1 mutant has a c.737C>T mutation. By detecting whether the new mutant exists in the biological sample, it is possible to effectively detect whether the biological sample is susceptible to gonadal hypogenesis combined with cerebellar ataxia.
Owner:BGI GENOMICS CO LTD
Kit, method and device for detecting dynamic mutation of spinal cerebellar ataxia
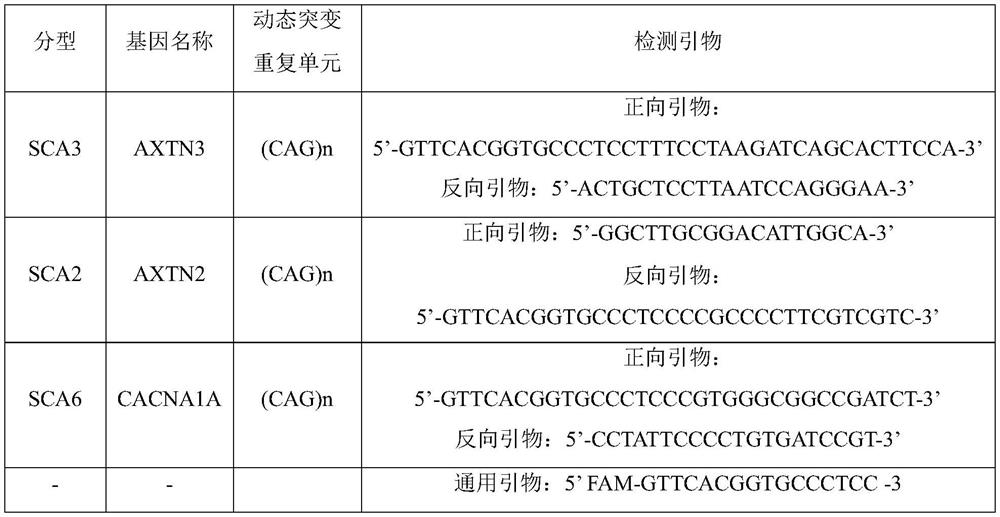
PendingCN114317719AEnsure consistencyImprove detection efficiencyMicrobiological testing/measurementProteomicsCerebellar ataxiaMedicine
The invention relates to a kit, a detection method and a device for detecting dynamic mutation of spinal cerebellar ataxia, and the kit comprises a first specific primer for amplifying an AXTN3 gene, a second specific primer for amplifying an AXTN2 gene, a third specific primer for amplifying a CACNA1A gene and a universal primer, the specific primer of each gene is connected with a preset fixed fragment with the same sequence as the universal primer, and the universal primer is connected with a fluorophore. According to the kit disclosed by the invention, multiple specific primers and universal primers are creatively combined for use, so that the repeated copy number of CAG in an AXTN3 gene, an AXTN2 gene or a CACNA1A gene can be detected at the same time, and the detection efficiency of dynamic mutation of spinal cerebellar ataxia is improved.
Owner:SUZHOU BASECARE MEDICAL DEVICE CO LTD
Butylphthalide Derivatives and Their Application in Preparation of Medicines for Protecting Nerve Cells
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
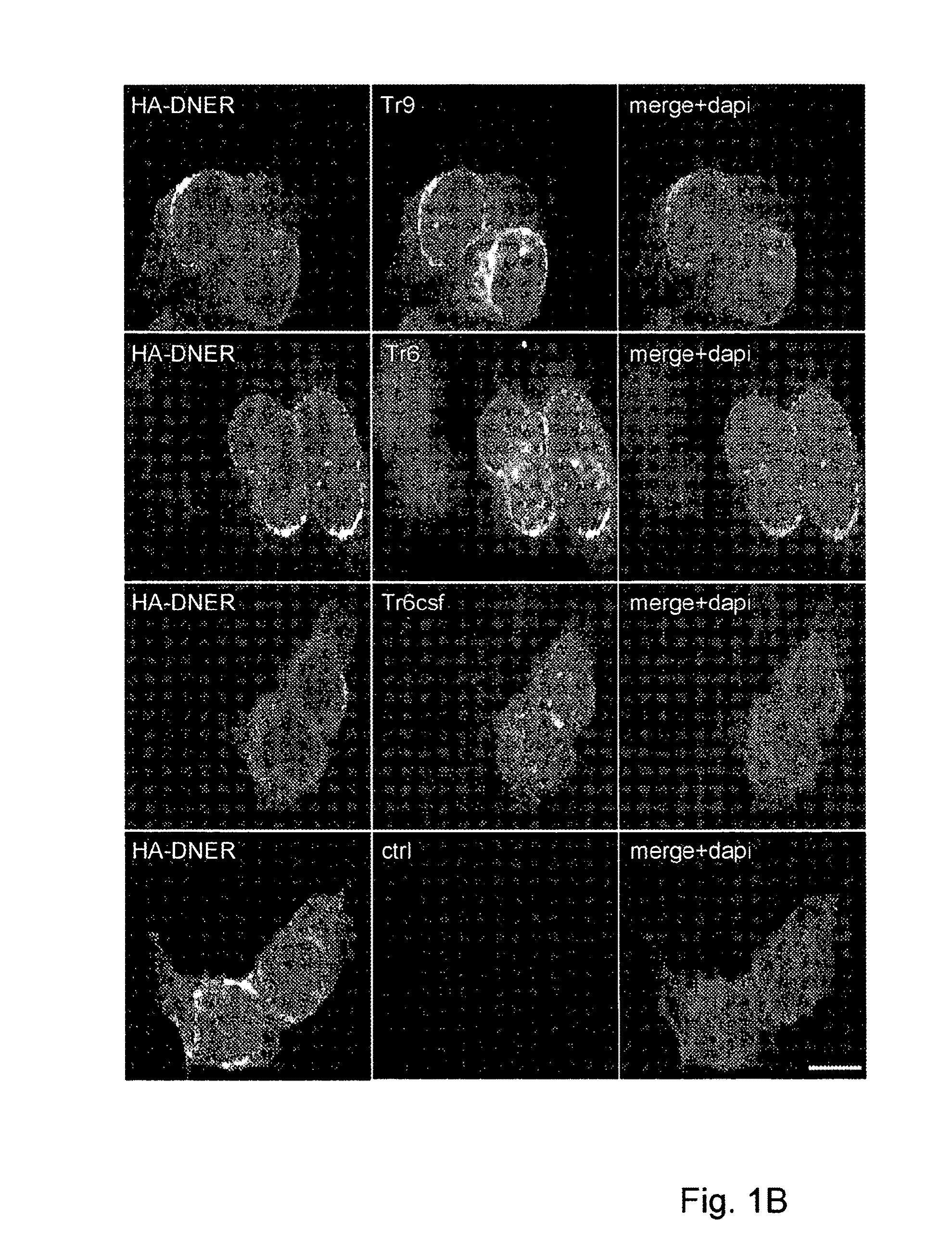
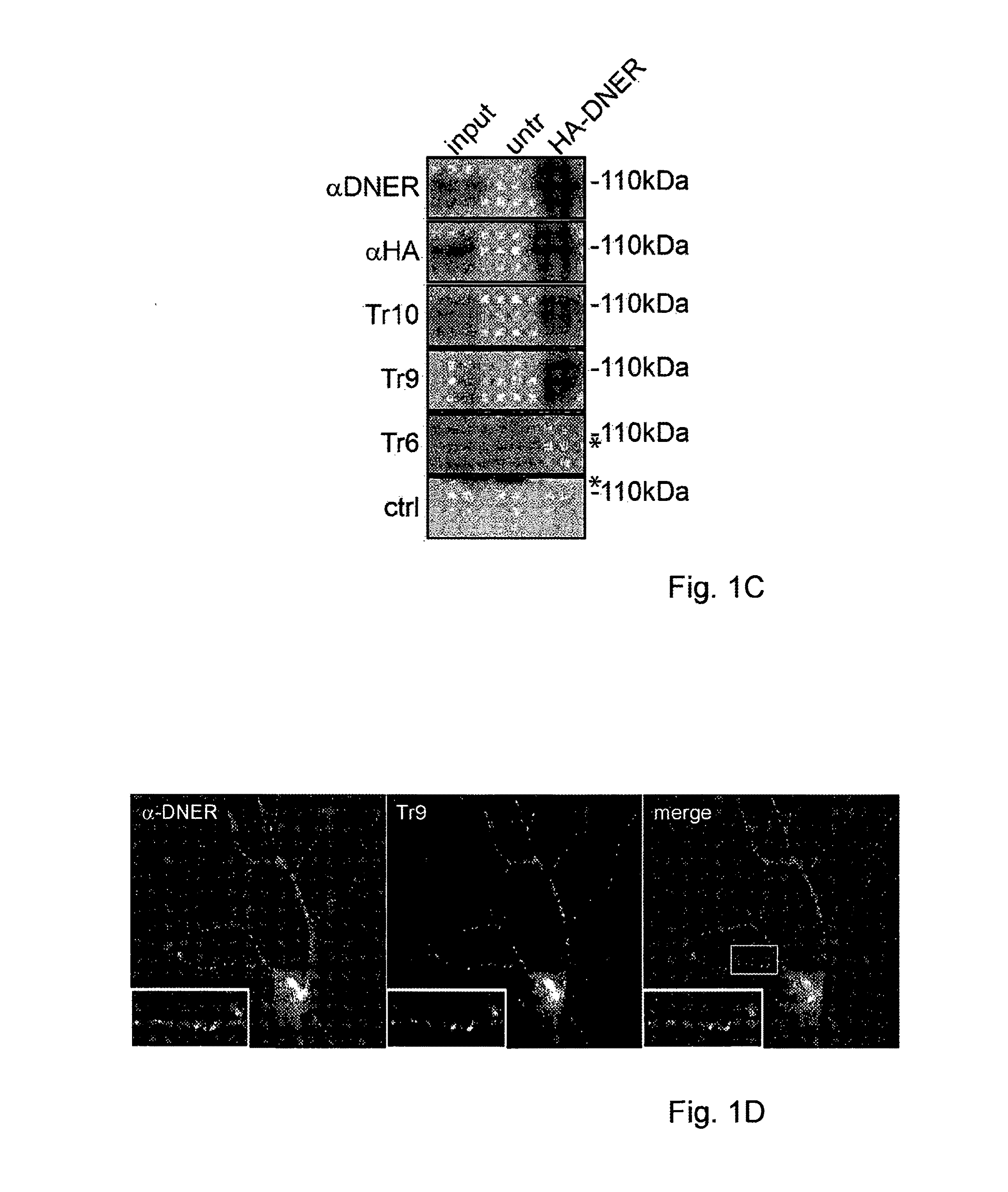
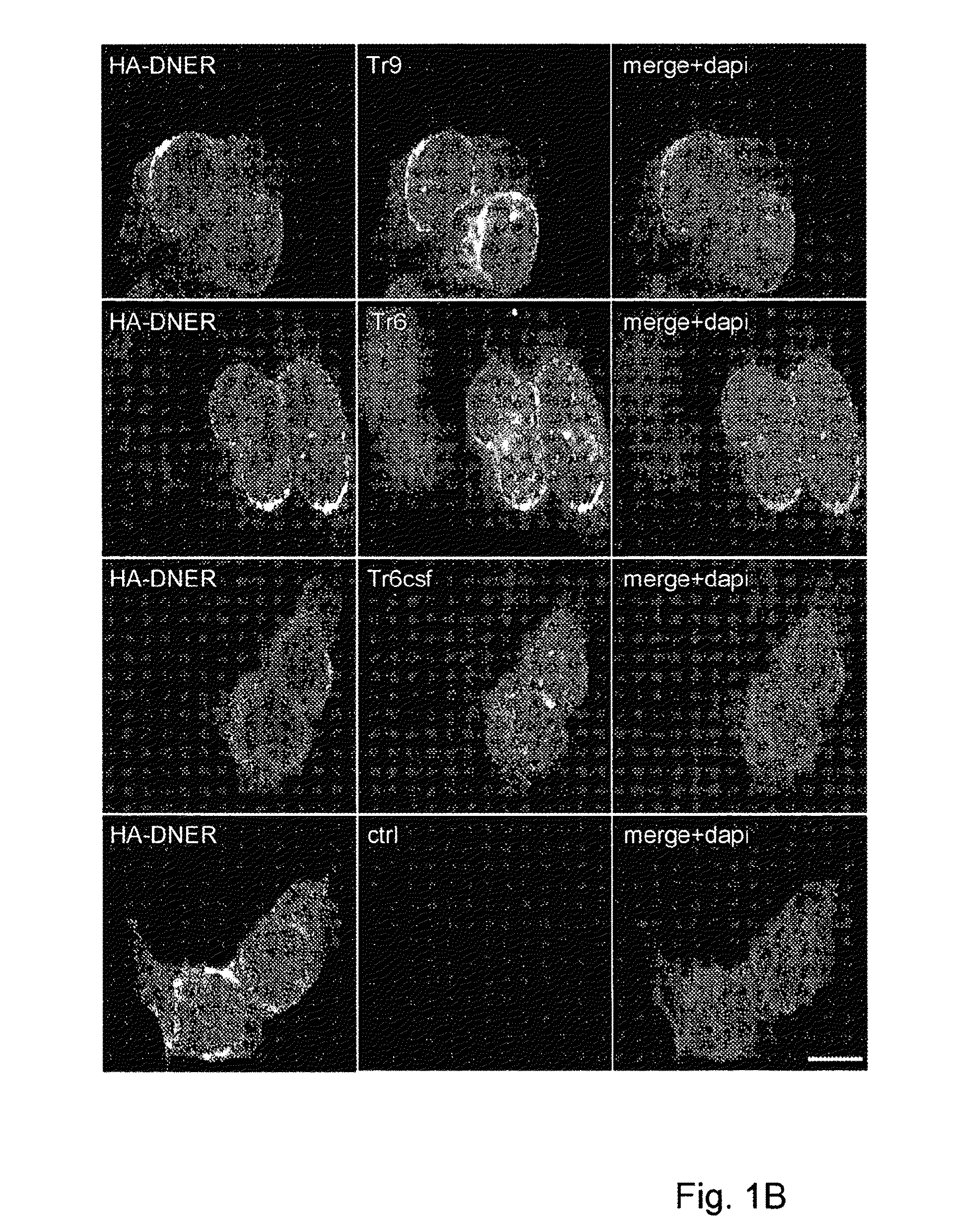
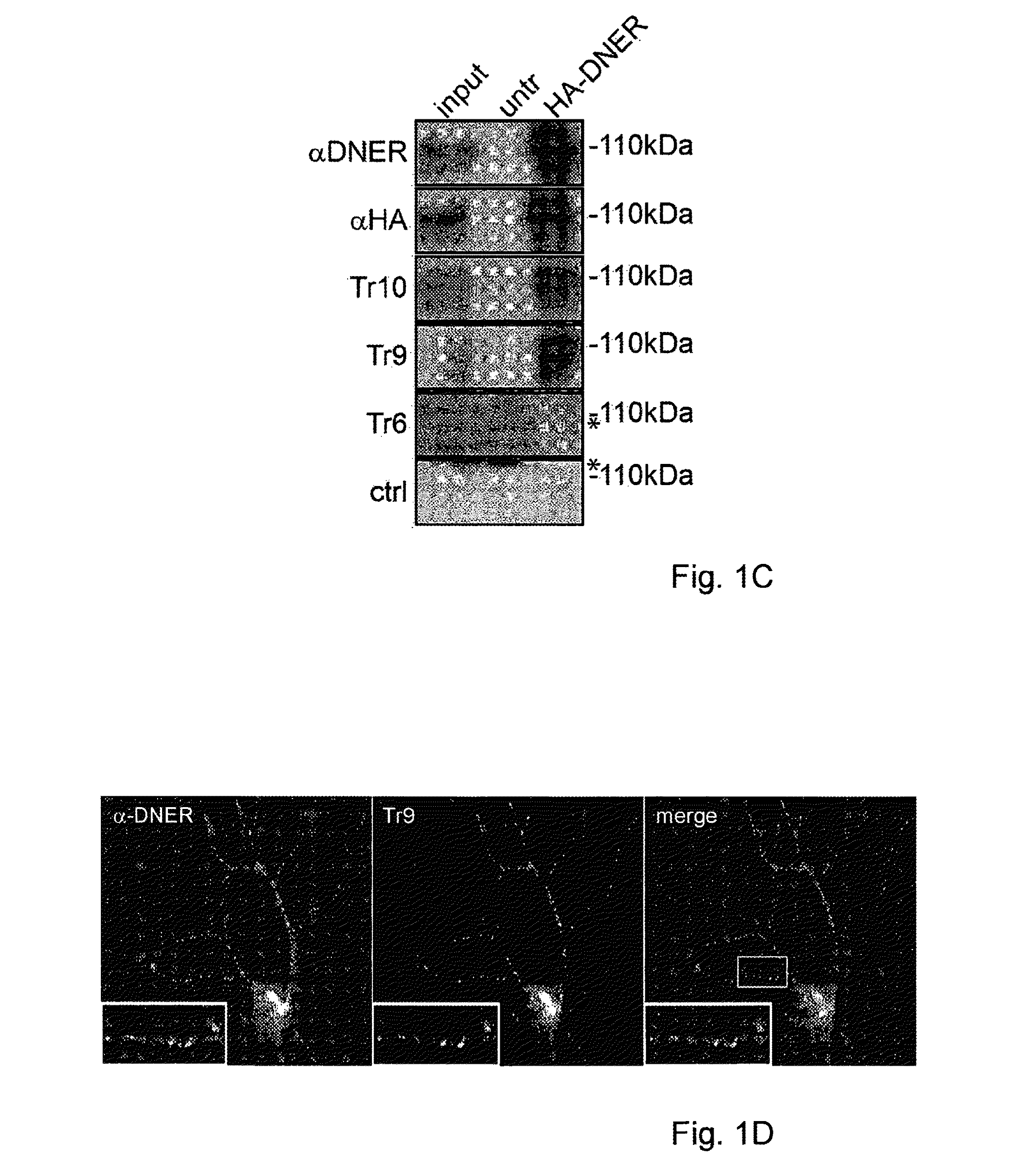
Assay for Anti-tr antibodies
The present invention relates to a method for determining the presence of anti-Tr antibodies in a subject comprising the steps of obtaining a sample from said subject testing the presence of said antibodies in said sample by addition of DNER protein or an antigenic part thereof and checking whether said DNER protein is bound by any antibodies in said sample Such an assay is useful for the diagnosis of paraneoplastic cerebellar degeneration that is associated with Hodgkin lymphoma, or, more generally, to type patients suffering from cerebellar ataxia. Also comprised in the invention is a kit for performing such an assay.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Rnai induced reduction of ataxin-3 for the treatment of spinocerebellar ataxia type 3
PendingUS20220010314A1Highly efficient loweringEfficient knock-down of diseaseOrganic active ingredientsNervous disorderCerebellar ataxiaNeuron
Owner:UNIQURE IP BV
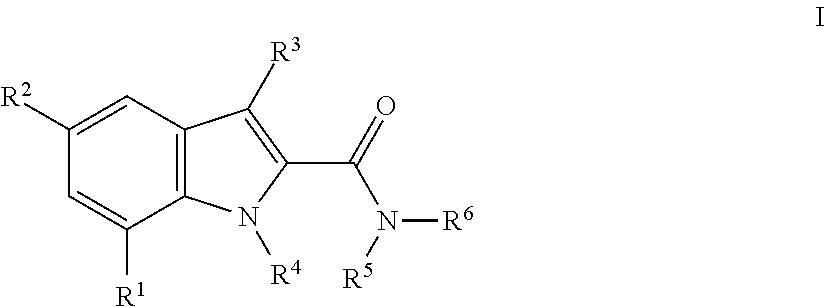
Indole-carboxamides
The present invention relates to compounds of general formulawhereinR1 is aryl or heteroaryl, which are optionally substituted by one, two or three substituents, selected from lower alkyl, halogen, lower alkyl substituted by halogen, hydroxyl, lower alkoxy, lower alkoxy substituted by halogen, cyano or nitro;R2 is halogen, lower alkyl or cyano;R3 is hydrogen or lower alkyl;R4 is hydrogen or lower alkyl;R5, R6 are hydrogen, lower alkyl, or may form together with the N-atom to which they are attached a heterocycloalkyl ring;or to a pharmaceutically acceptable acid addition salt, to a racemic mixture or to its corresponding enantiomer and / or optical isomers thereof.The compounds may be used for the treatment of schizophrenia, obsessive-compulsive personality disorder, major depression, bipolar disorders, anxiety disorders, normal aging, epilepsy, retinal degeneration, traumatic brain injury, spinal cord injury, post-traumatic stress disorder, panic disorder, Parkinson's disease, dementia, Alzheimer's disease, cognitive impairment, chemotherapy-induced cognitive dysfunction (“chemobrain”), Down syndrome, autism spectrum disorders, hearing loss, tinnitus, spinocerebellar ataxia, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, stroke, and disturbances due to radiation therapy, chronic stress, or abuse of neuro-active drugs, selected from alcohol, opiates, methamphetamine, phencyclidine and cocaine.
Owner:F HOFFMANN LA ROCHE INC
Hereditary medullispinal cerebellar ataxia related gene
The invention belongs to the field of molecular pathology, and relates to a hereditary medullispinal cerebellar ataxia related gene (mutant), particularly a hereditary medullispinal cerebellar ataxia related gene of which the amino acid sequence is disclosed as SEQ ID NO:7 or SEQ ID NO:9, and more particularly a hereditary medullispinal cerebellar ataxia related gene of which the nucleic acid sequence is disclosed as SEQ ID NO:6 or SEQ ID NO:8. The invention also relates to a recombinant vector containing the medullispinal cerebellar ataxia related gene, a recombinant cell containing the recombinant vector, and a coding protein of the medullispinal cerebellar ataxia related gene. Besides, the invention also relates to an exon of the hereditary medullispinal cerebellar ataxia related gene and a coding protein thereof. The invention provides references for auxiliary molecular diagnosis, molecular typing, prenatal diagnosis, drug target selection and clinical treatment of the hereditary medullispinal cerebellar ataxia.
Owner:CENT SOUTH UNIV +1
Compounds
InactiveUS20180127405A1Useful in treatmentAntibacterial agentsOrganic active ingredientsAIDS dementiaEthyl group
Compounds of formula (I)wherein:R1 is heteroaryl optionally substituted by methyl, ethyl, halo or =0; andR2 is H, methyl or ethyl.and salts thereof are KMO inhibitors and may be useful in the treatment of various disorders, for example acute pancreatitis, chronic kidney disease, acute kidney disease, acute kidney injury, other conditions associated with systemic inflammatory response syndrome (SIRS), Huntington's disease, Alzheimer's disease, spinocerebellar ataxias, Parkinson's disease, AIDS-dementia complex, HIV infection, amylotrophic lateral sclerosis (ALS), depression, schizophrenia, sepsis, cardiovascular shock, severe trauma, acute lung injury, acute respiratory distress syndrome, acute cholecystitis, severe burns, pneumonia, extensive surgical procedures, ischemic bowel disease, severe acute hepatic disease, severe acute hepatic encephalopathy or acute renal failure.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Compounds used to stabilize ryanodine receptors against abnormal levels of calcium release
Disclosed herein are compositions and methods comprising compounds capable of normalizing imbalances in neuronal calcium homeostasis. Also disclosed are methods involving the use of these compounds for the treatment of neuronal or neurological disorders including: Alzheimer's disease, Parkinson's disease, Huntington's disease, frontotemporal dementia, Pick's disease, chronic traumatic encephalopathy, traumatic brain injury, stroke, cerebellar ataxia, multiple sclerosis, Down's syndrome, and age-related CNS disorders.
Owner:ROSALIND FRANKLIN UNIVERSITY OF MEDICINE AND SCIENCE
1,7-naphthyridine derivatives
The present invention relates to compounds of general formula I wherein R1, R2, R3 and R4 are as defined herein which may be used for the treatment of schizophrenia, obsessive-compulsivepersonality disorder, major depression, bipolar disorders, anxiety disorders, normal aging, epilepsy, retinal degeneration, traumatic brain injury, spinal cord injury, post-traumatic stress disorder, panic disorder, Parkinson's disease, dementia, Alzheimer's disease, cognitive impairment, chemotherapy-induced cognitive dysfunction (“chemobrain”), Down syndrome, autism spectrum disorders, hearing loss, tinnitus, spinocerebellar ataxia, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, stroke, and disturbances due to radiation therapy, chronic stress, optic neuropathy or macular degeneration, or abuse of neuro-active drugs, selected from alcohol, opiates, methamphetamine, phencyclidine and cocaine.
Owner:F HOFFMANN LA ROCHE INC
Primer for detecting dynamic mutation of CAG repetitive sequence of ATXN3 gene and PCR amplification method thereof
InactiveCN101654711BStrong specificityImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationCerebellar ataxiaDynamic mutation
Owner:SUZHOU UNIV
Assay for anti-TR antibodies
The present invention relates to a method for determining the presence of anti-Tr antibodies in a subject comprising the steps of obtaining a sample from said subject testing the presence of said antibodies in said sample by addition of DNER protein or an antigenic part thereof and checking whether said DNER protein is bound by any antibodies in said sample Such an assay is useful for the diagnosis of paraneoplastic cerebellar degeneration that is associated with Hodgkin lymphoma, or, more generally, to type patients suffering from cerebellar ataxia. Also comprised in the invention is a kit for performing such an assay.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
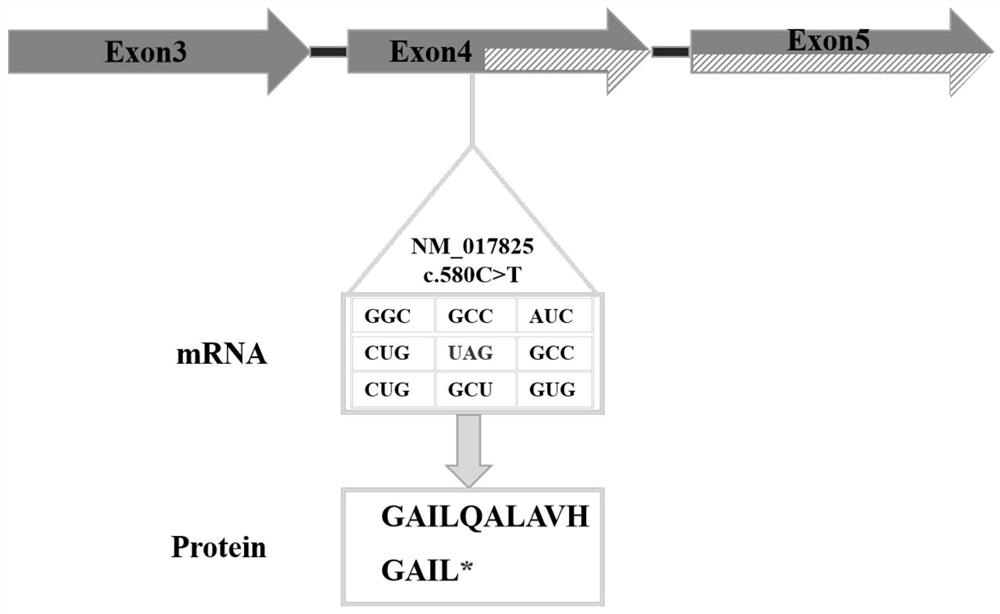
Rare neurodegenerative disease genetic screening kit, application and screening system
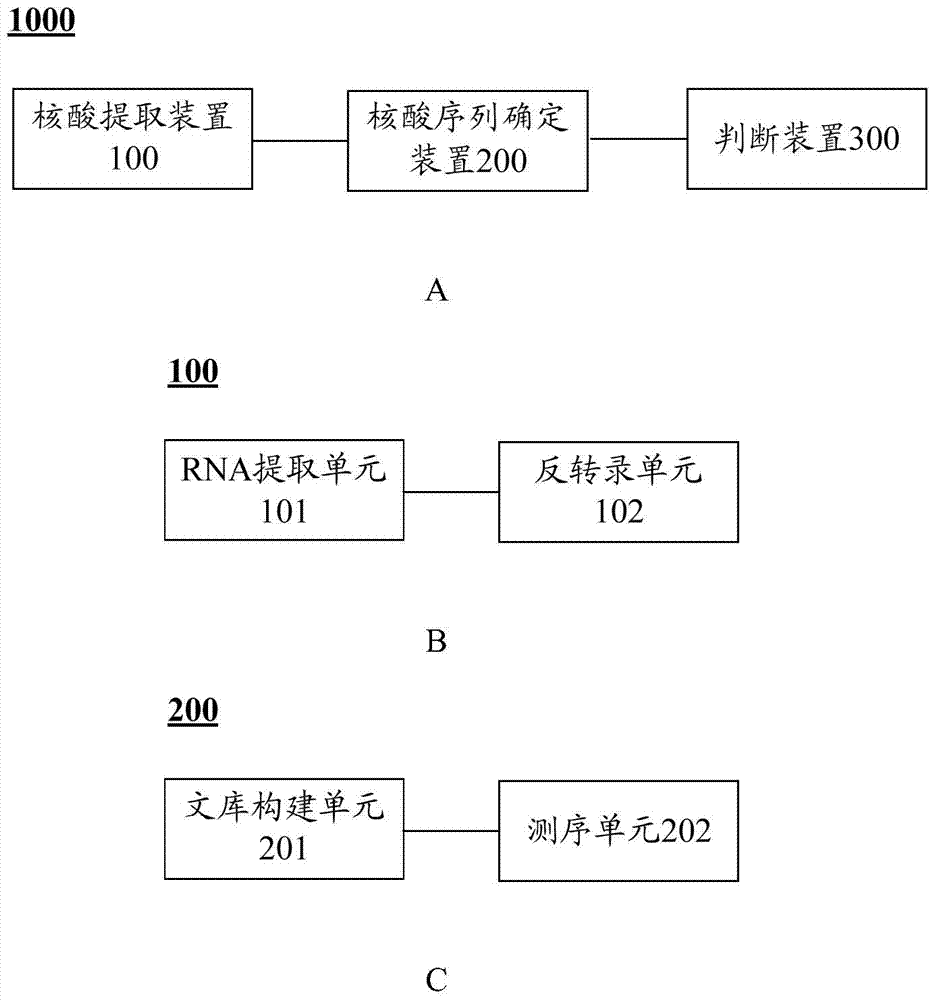
The invention discloses a genetic screening kit for rare neurodegenerative diseases, application of the genetic screening kit and a screening system. The genetic screening kit for the rare neurodegenerative diseases contains a mutation site screening reagent at the c.580 and / or c.803-1 position of the ADPRS gene. The compound is applied to preparation of a neurodegenerative disease screening reagent, and is preferably applied to preparation of a hereditary child epilepsy cerebellar ataxia screening reagent. The screening system comprises a gene sequence acquisition module and a judgment module, the gene sequence acquisition module is used for acquiring data of single nucleotide polymorphism containing ADPRS alleles c.580 and c.803-1; the judging module is used for judging whether the ADPRS gene contains the composite heterozygous variation c.580Cgt or not; t, and / or c.803-1 Ggt; and judging the genetic risk of the hereditary child epilepsy cerebellar ataxia disease according to the A variation. It is proved that the complex heterozygote is c.580Cgt; t and c.803-1 Ggt; the ADPRS gene variant of A causes a rare neurodegenerative disease, which indicates that the screening reagent provided by the invention has screening significance on the disease.
Owner:WUHAN CHILDRENS HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com