Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Atypical antipsychotic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The atypical antipsychotics (AAP; also known as second generation antipsychotics (SGAs)) are a group of antipsychotic drugs (antipsychotic drugs in general are also known as major tranquilizers and neuroleptics, although the latter is usually reserved for the typical antipsychotics) largely introduced after the 1970s and used to treat psychiatric conditions. Some atypical antipsychotics have received regulatory approval (e.g. by the FDA of the US, the TGA of Australia, the MHRA of the UK) for schizophrenia, bipolar disorder, autism, and as an adjunct in major depressive disorder.
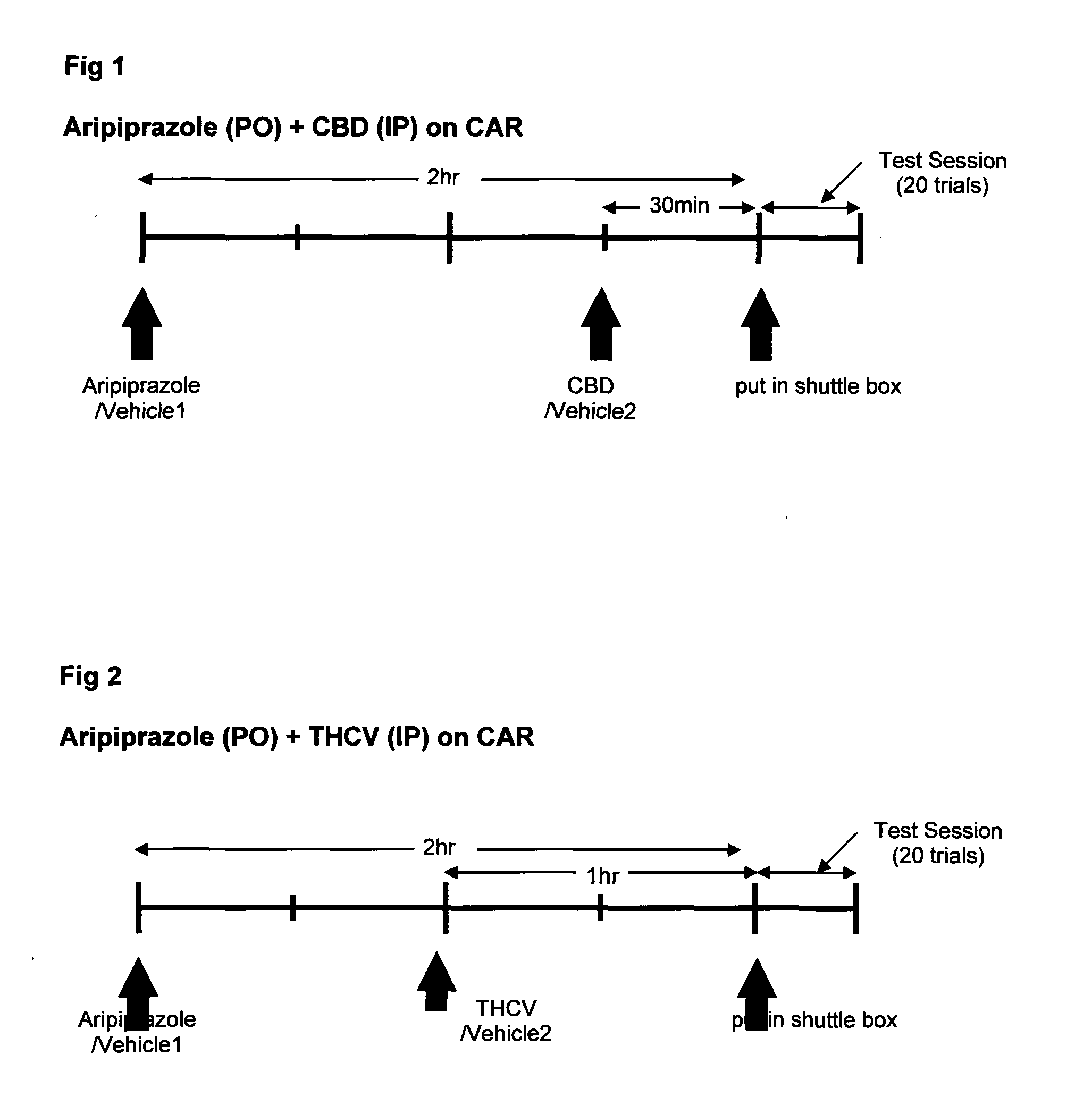
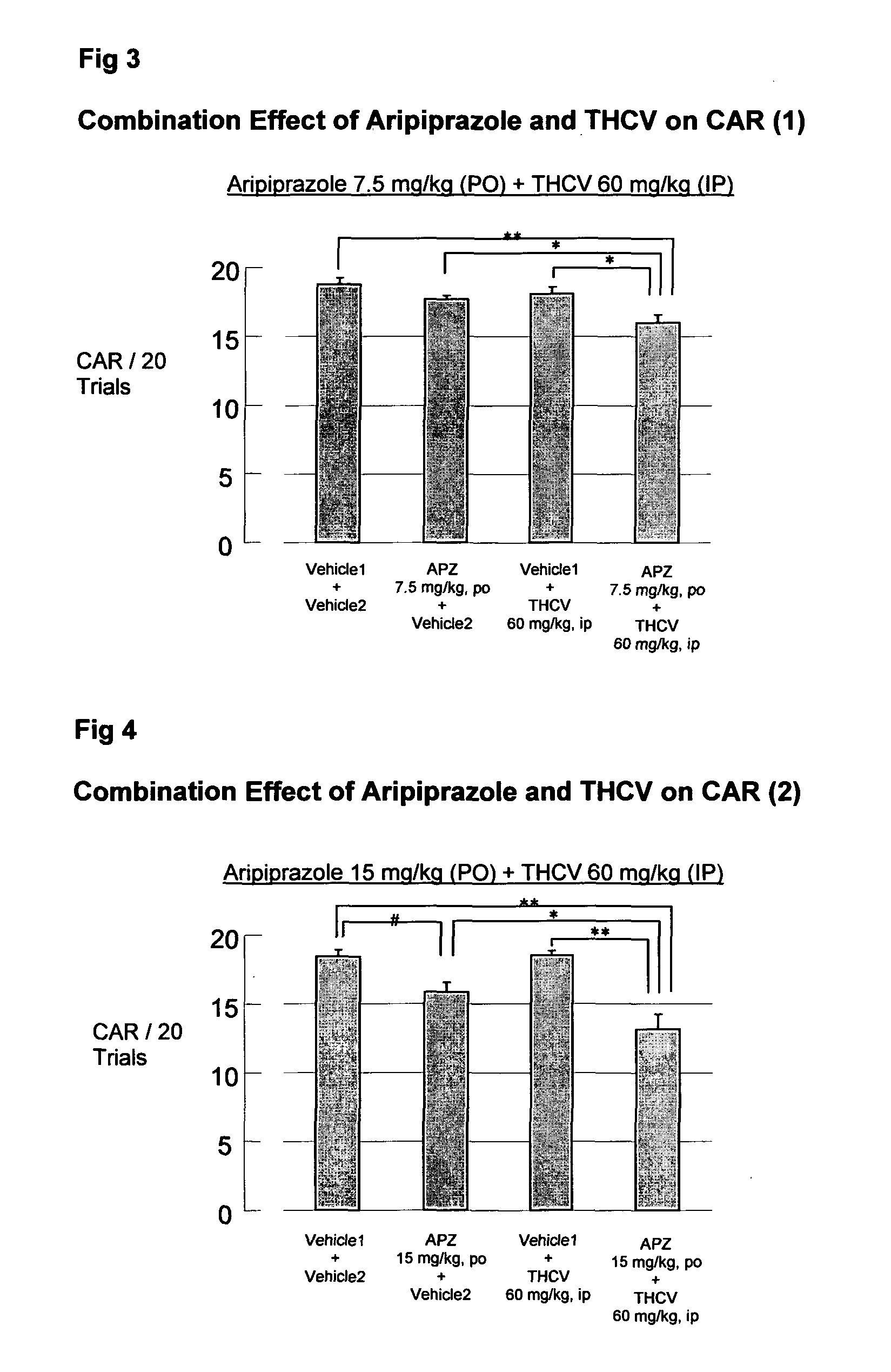
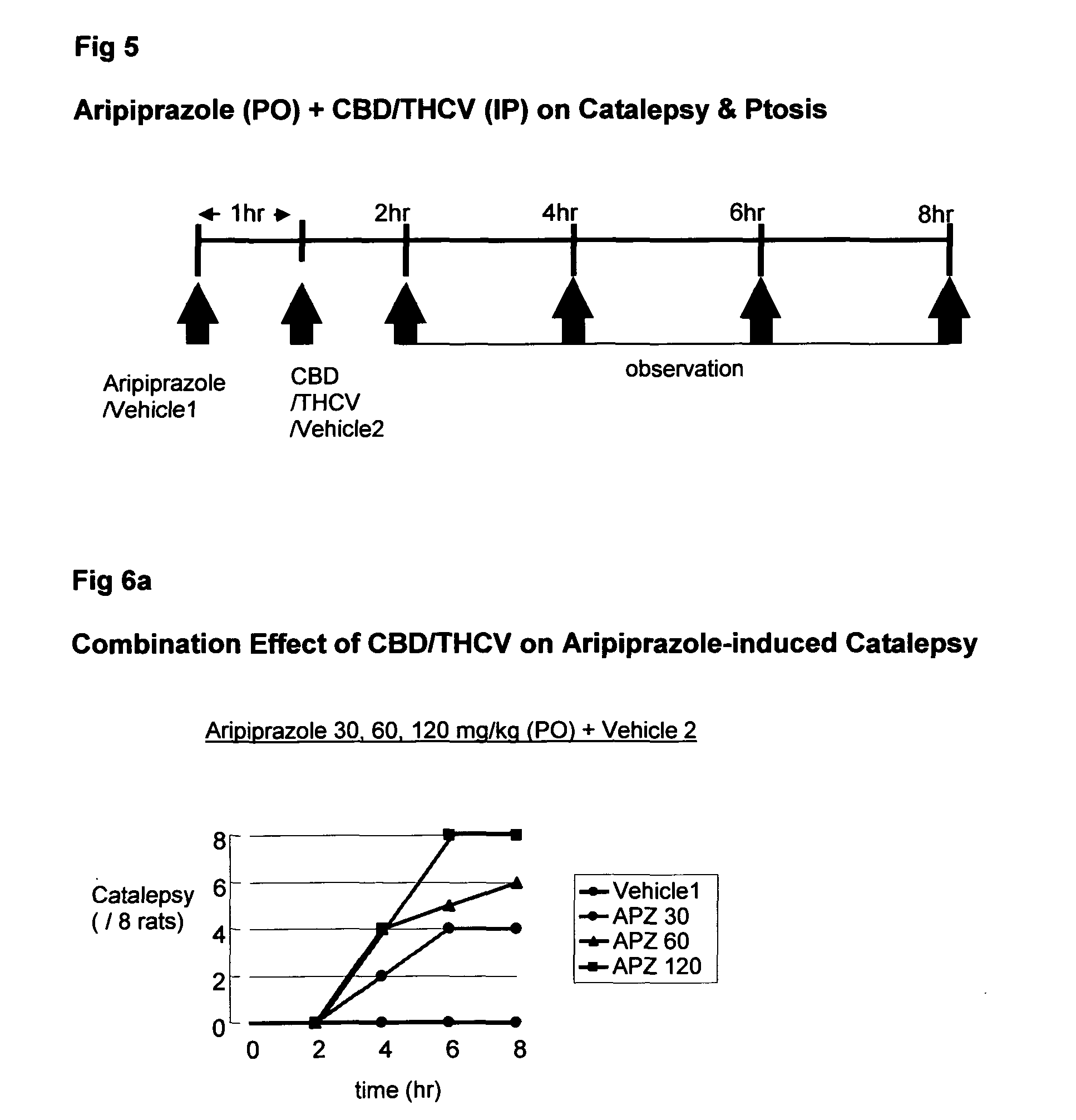
Use of cannabinoids in combination with an Anti-psychotic medicament
ActiveUS20110038958A1High activityLess degree of activityBiocideSenses disorderPsychosis drugTypical antipsychotic
The present invention relates to the use of one or more cannabinoids in combination with one or more anti-psychotic medicaments for use in the prevention or treatment of psychosis and psychotic disorders. Preferably the one or more cannabinoids are taken from the group: cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannbidivarin (THCV); tetrahydrocannbidivarinin acid (THCVA); cannabichromene (CBC); cannabichromenic acid (CBCA); cannabigerol (CBG) and cannabigerolic acid (CBGA). Preferably the anti-psychotic medication is an atypical anti-psychotic medication.
Owner:GW PHARMA LTD
Therapeutic combination for cognititon enhancement and psychotic disorders
InactiveUS20050215571A1Reduce the amount requiredGood effectBiocideNervous disorderAtypical antipsychoticPharmaceutical drug
This invention relates to combinations of an atypical antipsychotic, and a nicotinic receptor agonist or antagonist, kits containing such combinations, pharmaceutical compositions comprising such combinations, and methods of using such combinations to treat patients suffering from cognitive impairment disorders or psychotic disorders or conditions.
Owner:PFIZER INC
Combinations of GABA modulators and anticonvulsants, and atypical antipsychotics
InactiveUS20050004106A1Reduce the amount requiredGood effectBiocideNervous disorderBenzodiazepineAtypical antipsychotic
This invention relates to combinations of an atypical antipsychotic, and a GABA modulator, a benzodiazepine, and / or an anticonvulsant drug, kits containing such combinations, pharmaceutical compositions comprising such combinations, and methods of using such combinations to treat patients suffering from treatment-resistant anxiety disorders, psychotic disorders or conditions, or mood disorders or conditions.
Owner:PFIZER INC
Treatment of psychosis associated with parkinson's disease and subcortical dementias using a combination of an atypical antipsychotic with a dopamine agonist
InactiveUS20070015763A1Opportunities decreaseSimple processBiocidePeptide/protein ingredientsAtypical antipsychoticZiprasidone
This invention relates to combinations of an atypical antipsychotic, for example ziprasidone, and a dopamine agonist, kits containing such combinations, pharmaceutical compositions comprising such combinations, and methods of using such combinations to treat patients suffering from psychosis and movement disorders associated with Parkinson's disease and subcortical dementias.
Owner:PFIZER INC +1
Lithium combinations, and uses related thereto
InactiveUS20050233010A1Prevent precipitating manic episodeLessening and preventing riskBiocideNervous disorderPsychoactive drugAdrenergic antagonist
The present invention relates to combinatorial therapies for treating anxiety, depression or psychotic conditions using a lithium salt and a psychoactive drug selected from the group consisting of serotonin reuptake inhibitor, a 5HT2 receptor antagonist, an anticonvulsant, a norepinephrine reuptake inhibitor, an α-adrenoreceptor antagonist, an NK-3 antagonist, an NK-1 receptor antagonist, a PDE4 inhibitor, an Neuropeptide Y5 Receptor Antagonists, a D4 receptor antagonist, a 5HT1A receptor antagonist, a 5HT1D receptor antagonist, a CRF antagonist, a monoamine oxidase inhibitor, a sedative-hypnotic drug, and an atypical antipsychotic.
Owner:NOVEN THERAPEUTICS
Memantine as adjunctive treatment to atypical antipsychotic in schizophrenia patients
The present invention provides a method for treating schizophrenia in a patient in need thereof, the method comprising administering to the patient a therapeutically effective amount of memantine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount an atypical antipsychotic. The method of the present invention embodies both the co-administration of memantine with an atypical antipsychotic, and the use of memantine as an adjunctive treatment to treatment with an atypical antipsychotic.
Owner:FOREST LAB HLDG LTD
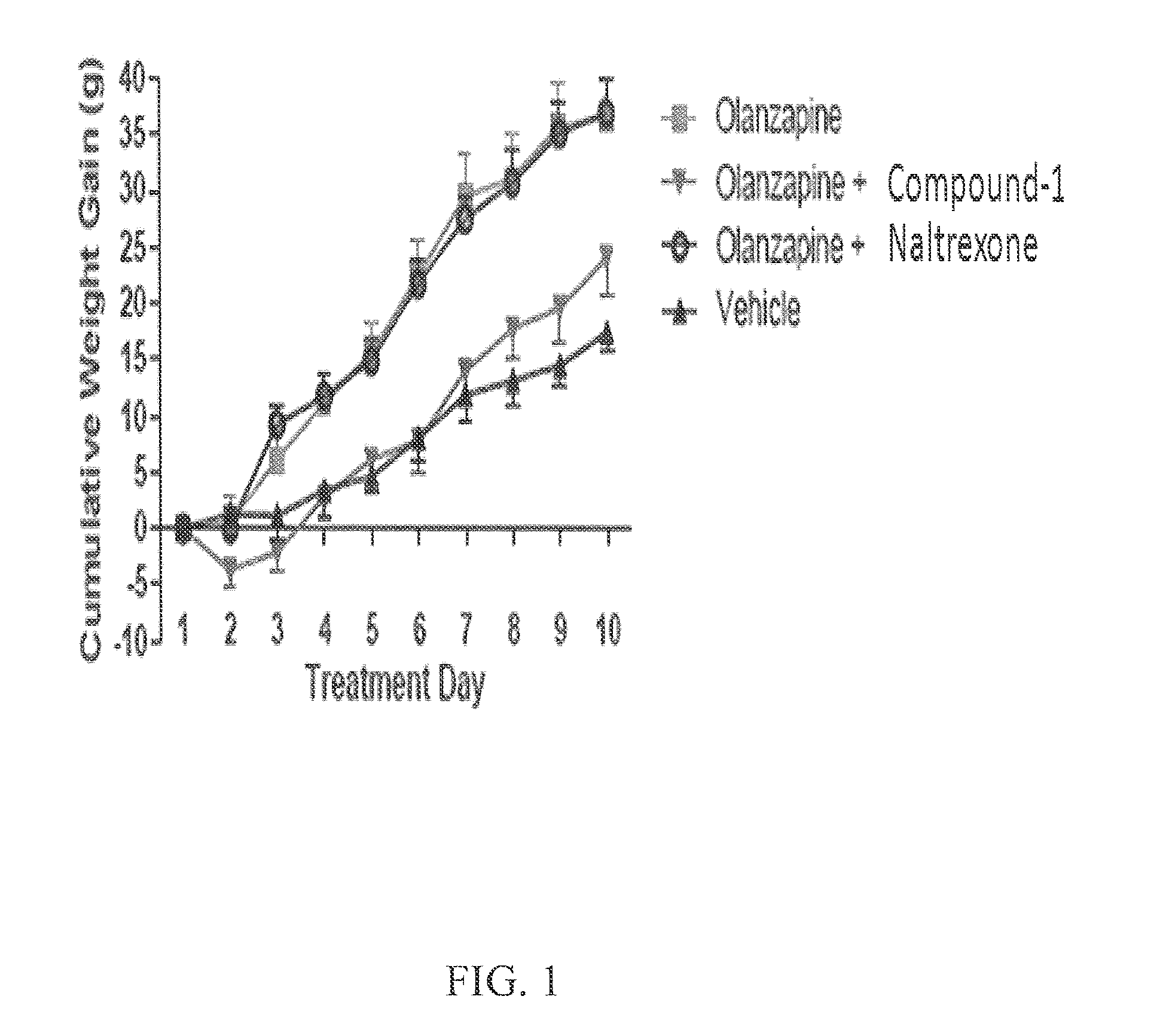
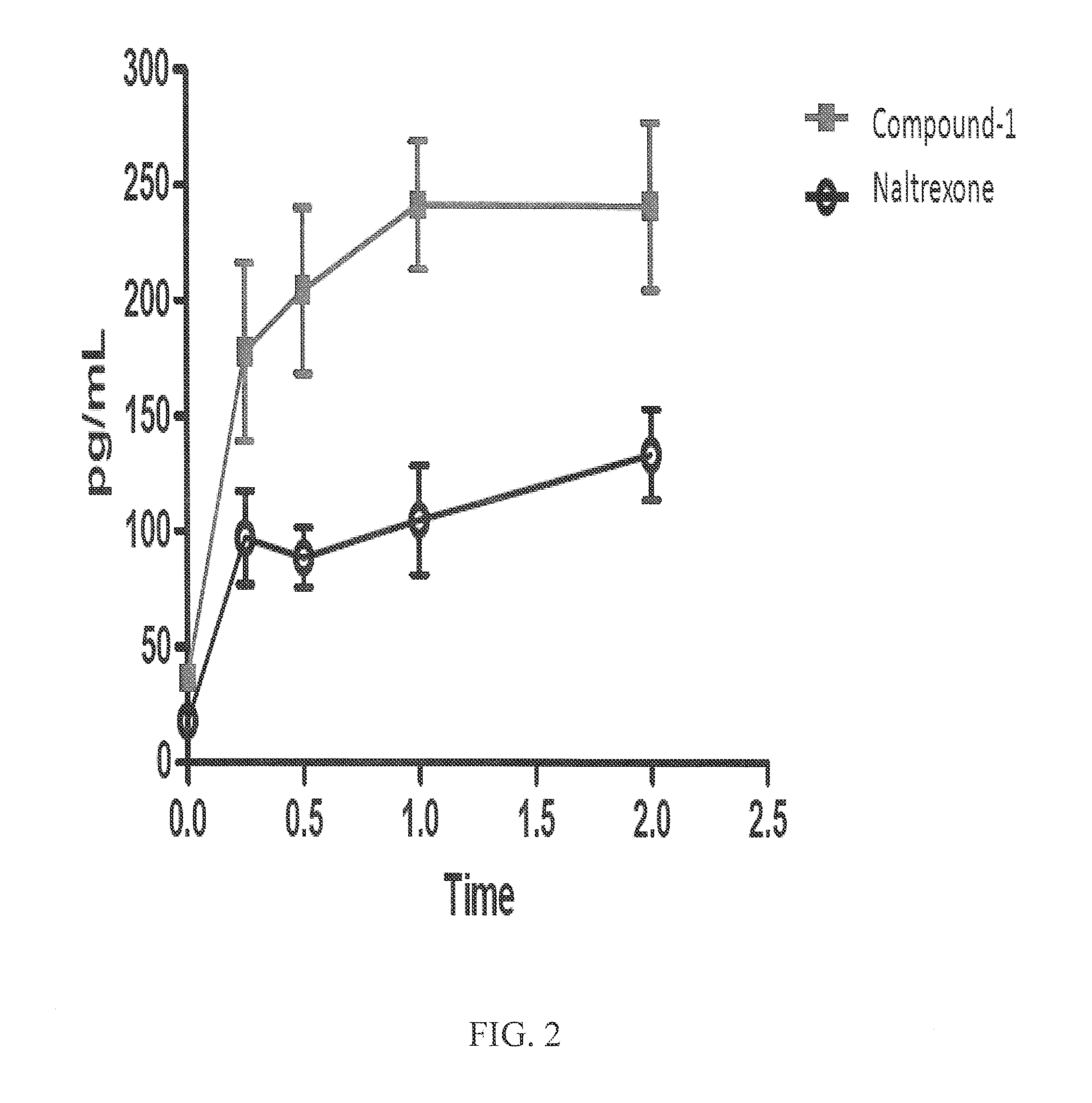
Methods for treating antipsychotic-induced weight gain
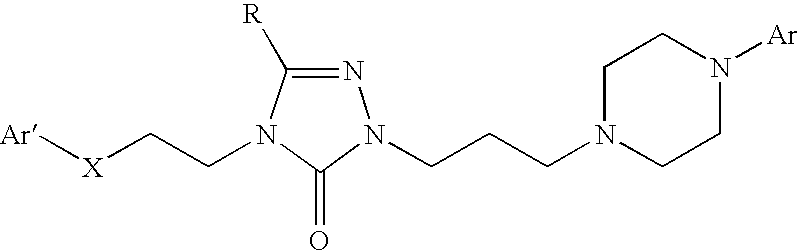
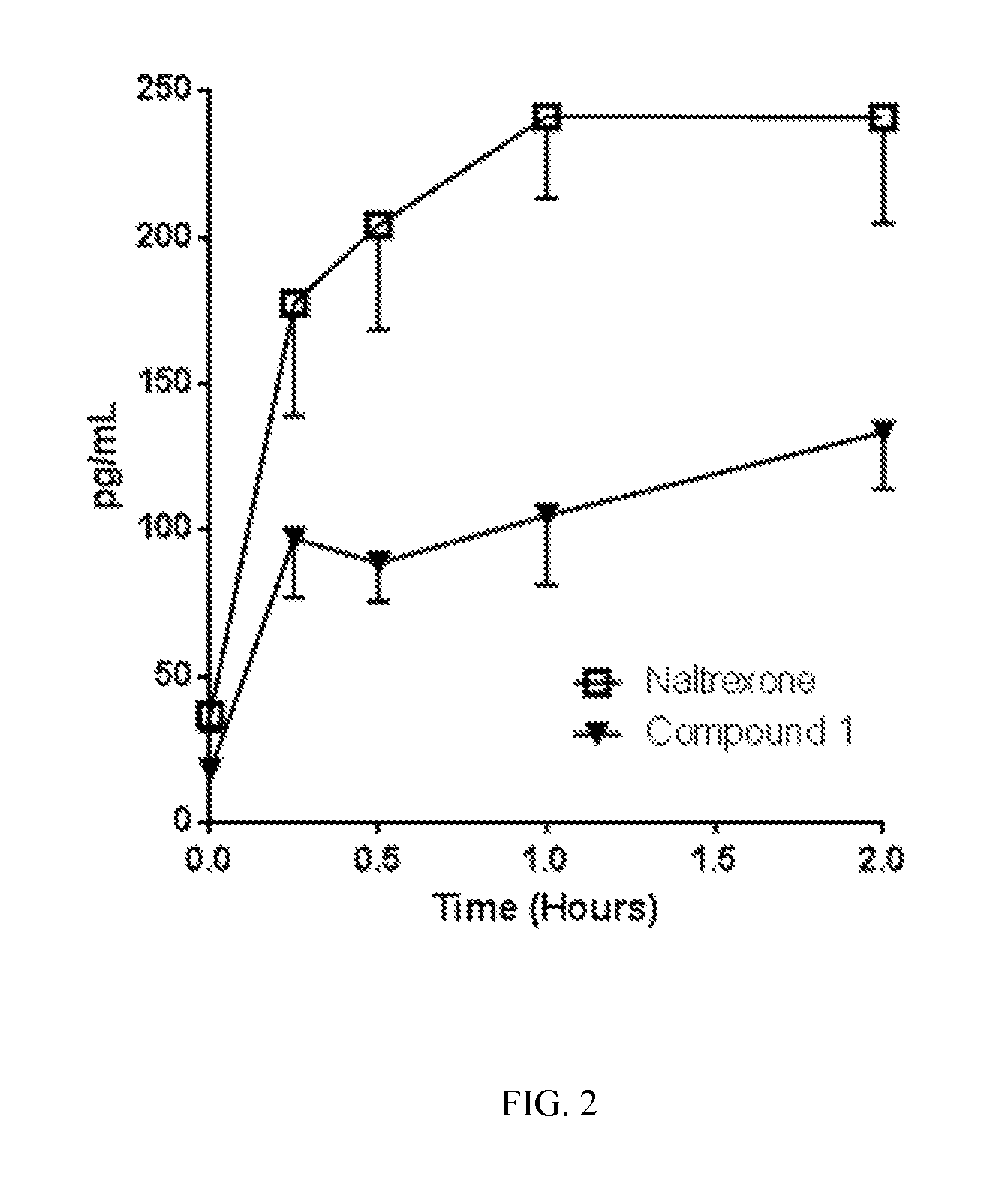
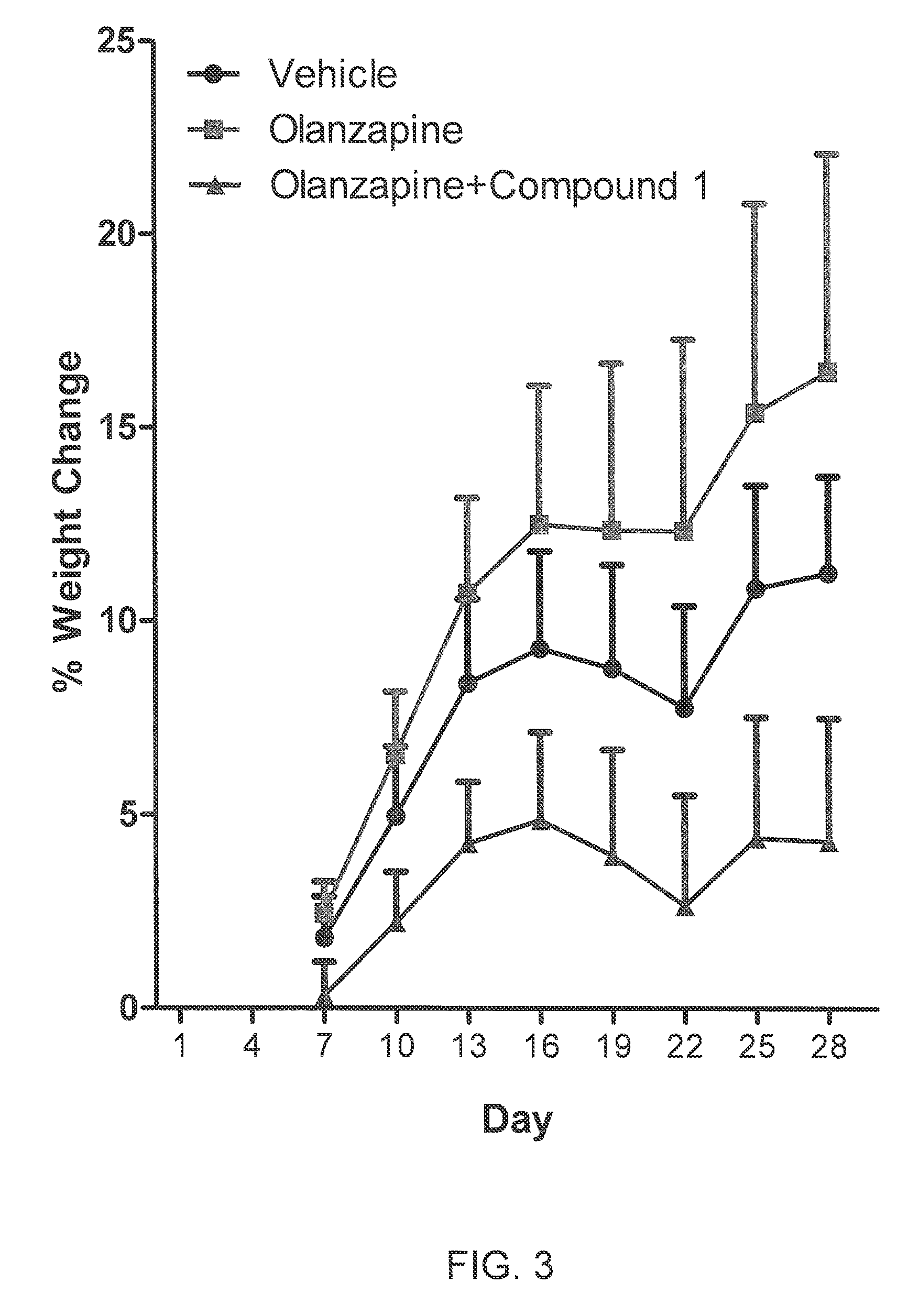
The present invention relates to the discovery of a novel opioid modulator effective in reducing pharmacologically induced weight gain associated with atypical antipsychotic use. The present invention provides methods of reducing antipsychotic induced weight gain, methods for suppressing food intake and reducing ghrelin levels induced by atypical antipsychotic medications in a patient.
Owner:ALKERMES PHARMA IRELAND LTD
Combination of atypical antipsychotics and 5HT-1B receptor antagonists
InactiveUS20050256112A1Reduce morbidityDifferent recognizableNervous disorderMetabolism disorderDiseaseHeadaches
The present invention relates to a pharmaceutical composition for treating, for example, a disorder or condition selected from the group consisting of hypertension, depression, generalized anxiety disorder, phobias, posttraumatic stress disorder, avoidant personality disorder, sexual dysfunction, eating disorders, obesity, chemical dependencies, cluster headache, migraine, pain, Alzheimer's disease, obsessive-compulsive disorder, panic disorder, memory disorders, Parkinson's diseases, endocrine disorders, cerebellar ataxia, gastrointestinal tract disorders, negative symptoms of schizophrenia, premenstrual syndrome, Fibromyalgia Syndrome, stress incontinence, Tourette syndrome, trichotillomania, kleptomania, male impotence, cancer, chronic paroxysmal hemicrania and headache in a mammal, preferably a human, comprising (i) an atypical antipsychotic or a pharmaceutically acceptable salt thereof, (ii) a 5-HT1B receptor antagonist or a pharmaceutically acceptable salt thereof, wherein the 5-HT1B receptor antagonist is selected from the group consisting of (A) a compound of the formula I as described in the specification and (B) a compound of the formula II as described in the specification, and optionally (iii) a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Compositions, Synthesis, and Methods of Using Quinolinone Based Atypical Antipsychotic Agents
ActiveUS20080293736A1Reduce adverse side effectsEffective treatmentOrganic active ingredientsNervous disorderBipolar mood disorderAtypical antipsychotic
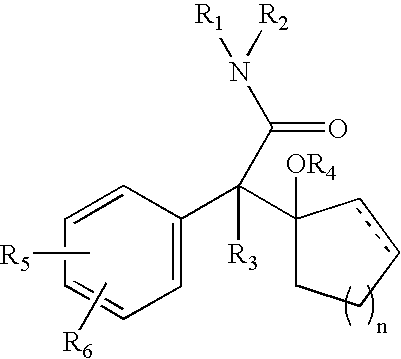
The present invention provides novel quinolinone derivatives which can be advantageously used for treating schizophrenia and related psychoses such as acute manic, bipolar disorder, autistic disorder, and depression.
Owner:REVIVA PHARMA INC
Combination of glyt1 compound with antipsychotics
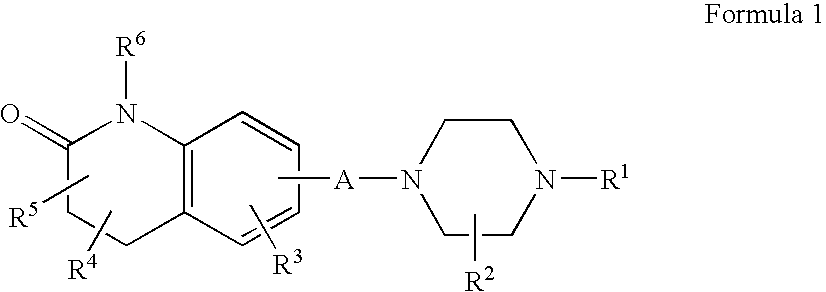
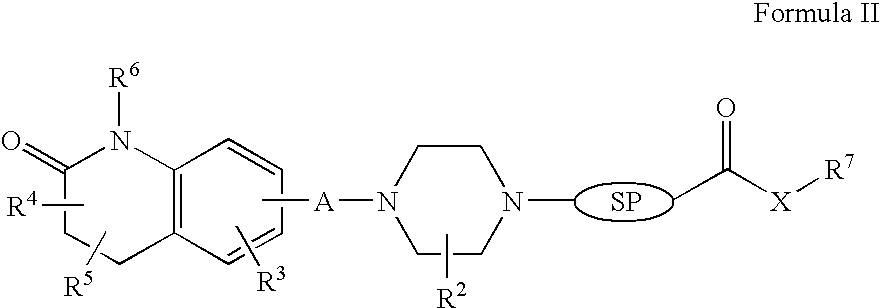
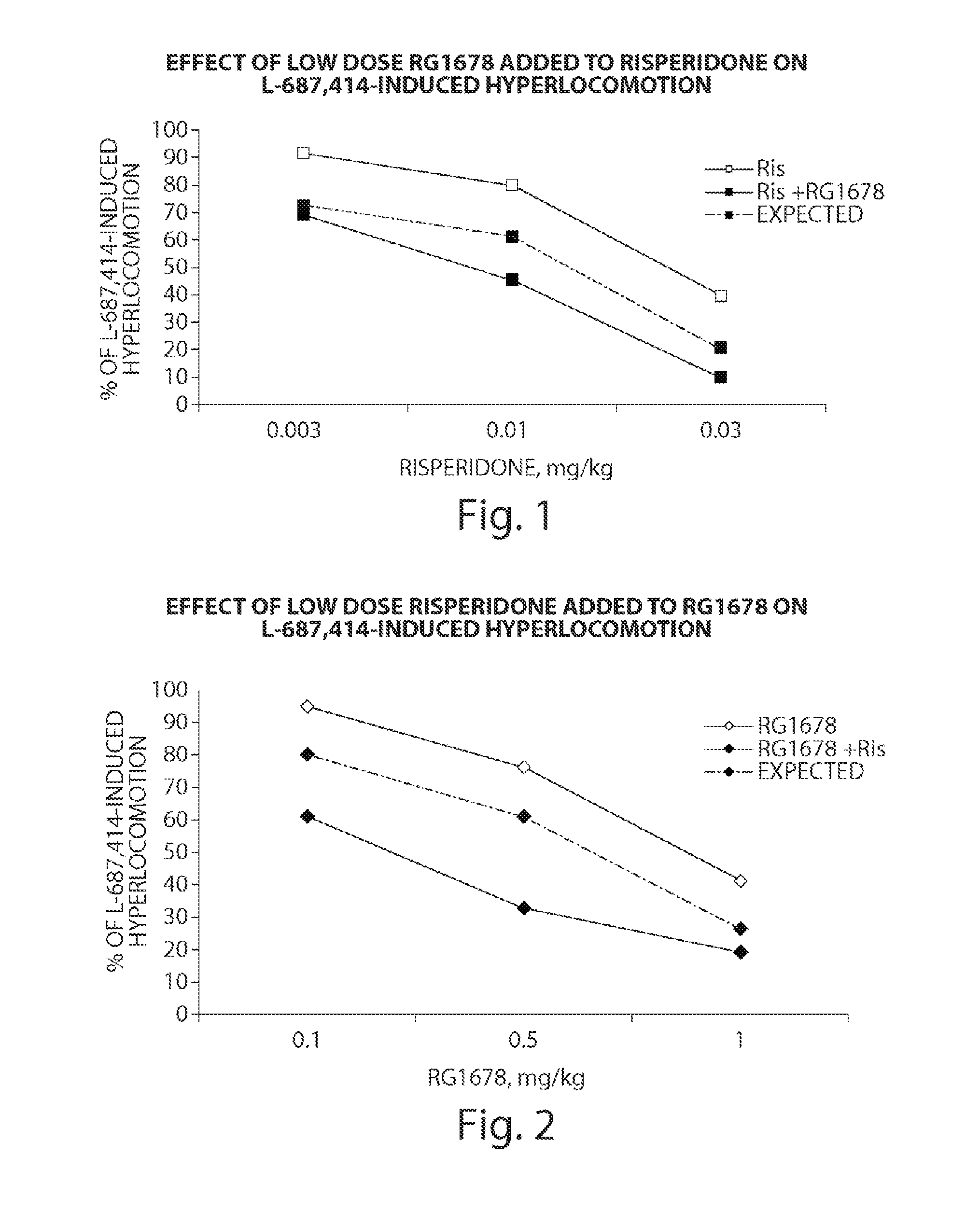
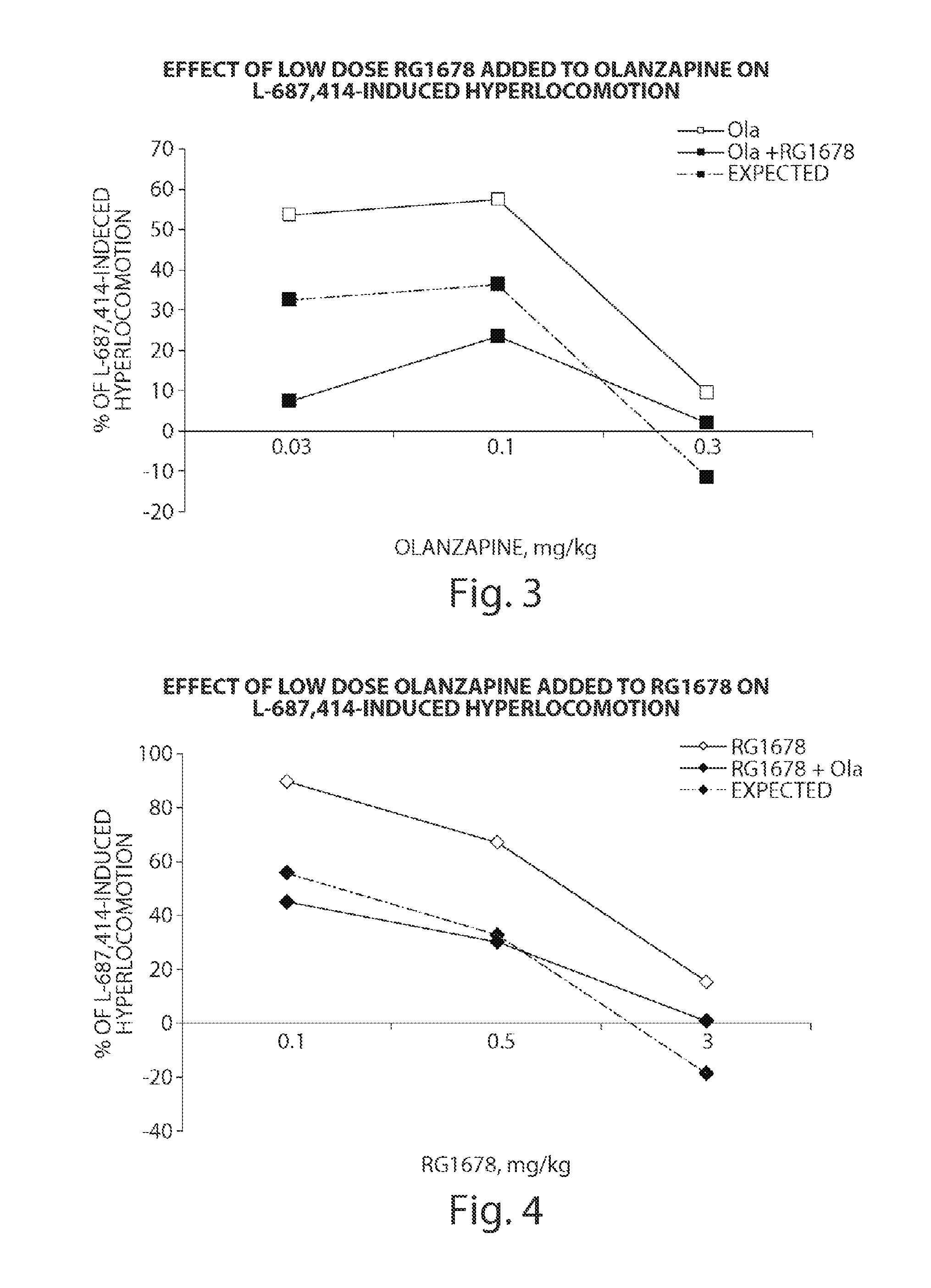
InactiveUS20120035156A1Affecting/increasing side-effect profileBiocideNervous disorderNegative symptomAtypical antipsychotic
The present invention relates to a pharmaceutical combination of a glycine transporter inhibitor (GlyT1) and an atypical antipsychotic drug which may be used for the treatment of positive and negative symptoms of schizophrenia.
Owner:F HOFFMANN LA ROCHE & CO AG
Lithium combinations, and uses related thereto
InactiveUS20080107756A1Prevent precipitating manic episodeLessening and preventing riskBiocideNervous disorderNorepinephrine reuptake inhibitorPsychoactive drug
The present invention relates to combinatorial therapies for treating anxiety, depression or psychotic conditions using a lithium salt and a psychoactive drug selected from the group consisting of serotonin reuptake inhibitor, a 5HT2 receptor antagonist, an anticonvulsant, a norepinephrine reuptake inhibitor, an α-adrenoreceptor antagonist, an NK-3 antagonist, an NK-1 receptor antagonist, a PDE4 inhibitor, an Neuropeptide Y5 Receptor Antagonists, a D4 receptor antagonist, a 5HT1A receptor antagonist, a 5HT1D receptor antagonist, a CRF antagonist, a monoamine oxidase inhibitor, a sedative-hypnotic drug, and an atypical antipsychotic.
Owner:NOVEN THERAPEUTICS
Method for preparing lurasidone
The invention relates to a method for preparing lurasidone and preparation of atypical antipsychotic compound lurasidone, and in particular relates to a method for preparing single salt silicate. The method comprises the following steps of: halogenating hydroxy by using a compound I as an initial raw material to prepare an intermediate compound II; reacting the intermediate compound II with a compound V under an alkalinity condition to generate an intermediate compound VI; and reacting the intermediate compound VI with a compound III under the alkalinity condition to prepare a target product of lurasidone. The method is short in process routine, easy to control and convenient to operate; and the generation of impurities is avoided, the reaction yield is obviously improved, the raw materials are cheap and easy to acquire, and the large-scale production is facilitated.
Owner:BEIJING MEDISAN TECH +1
Stabilized Atypical Antipsychotic Formulation
A pharmaceutical composition that contains an atypical antipsychotic drug and succinic acid, fumaric acid or a mixture of succinic acid and fumaric acid.
Owner:HANDA PHARM LLC
Pharmaceutical compositions of Lurasidone and Process for preparation thereof
Pharmaceutical compositions comprising an atypical antipsychotic as an active agent, process of preparation thereof and method of using the same are provided. Particularly the present invention relates to pharmaceutical compositions comprising lurasidone, process of preparation thereof and method to treat various psychotic disorders such as schizophrenia, positive and negative symptoms of schizophrenia, memory or learning dysfunctions caused by schizophrenia, senile dementia, attention deficit / hyperactivity disorder (ADHD), central nervous system (CNS) disorder responsive to modulation of glutamate levels, major depressive episodes associated with bipolar I disorder and other associated CNS disorders.
Owner:AUROBINDO PHARMA LTD
Imaging neuroleptic compounds
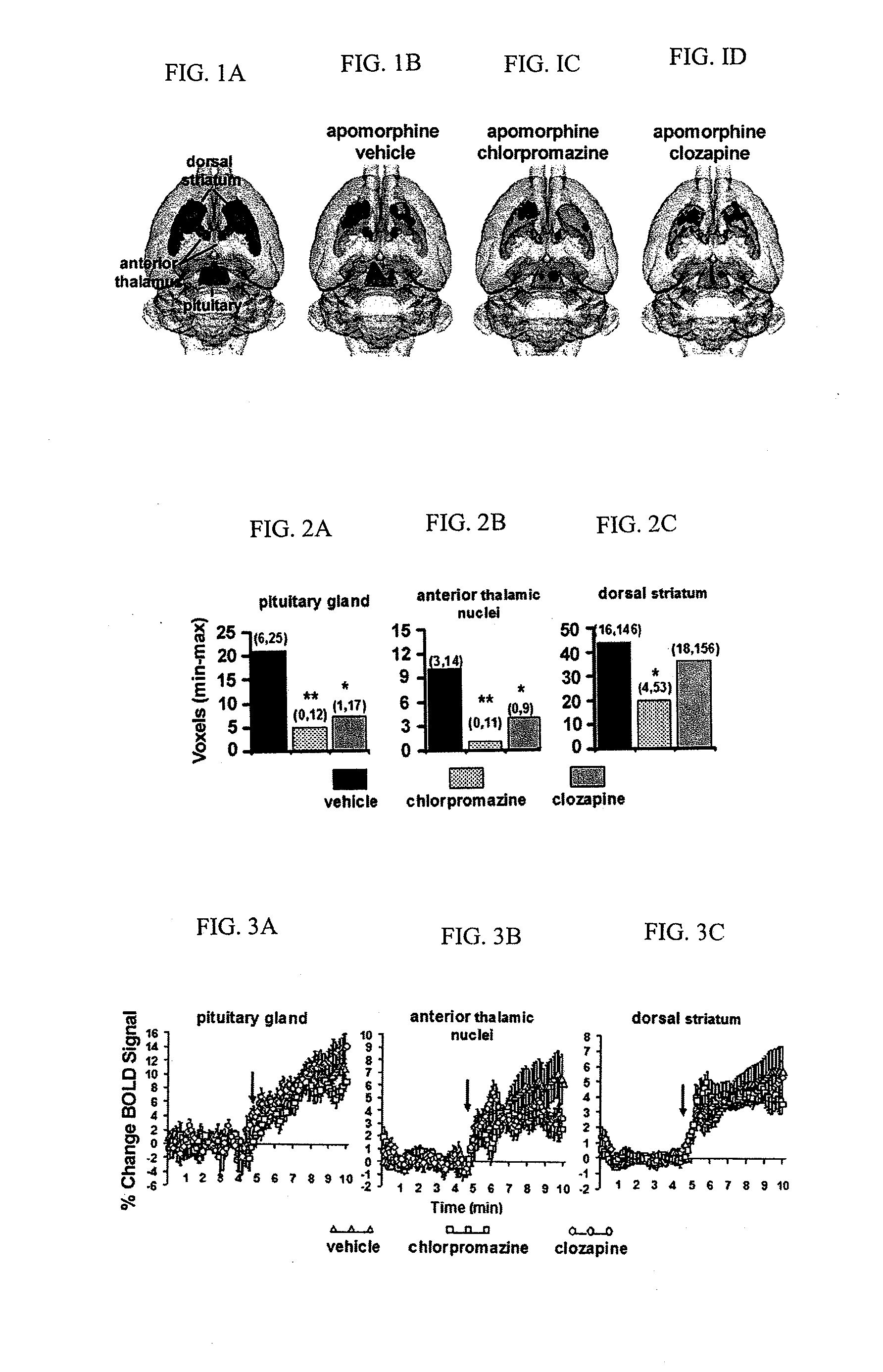
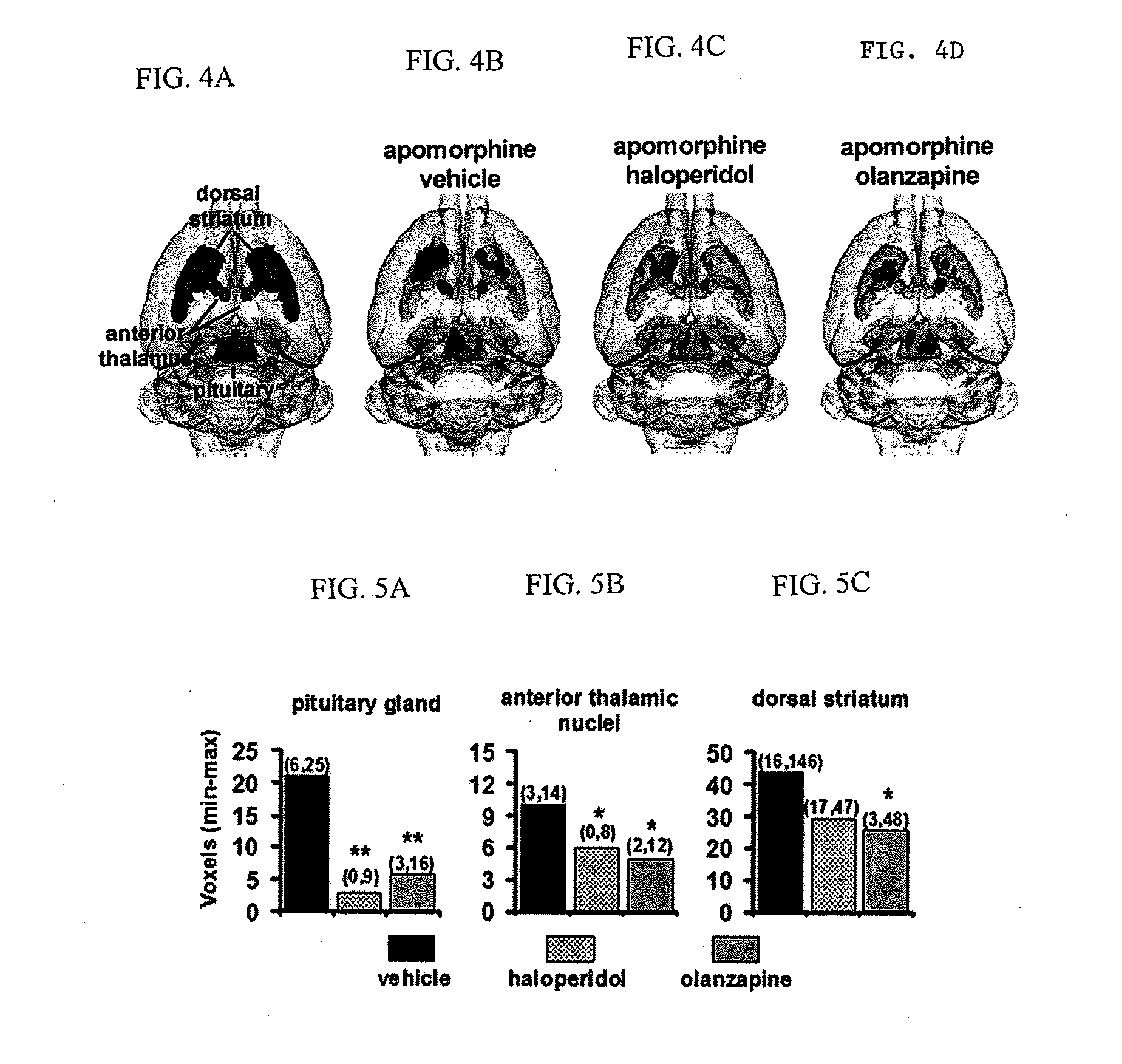
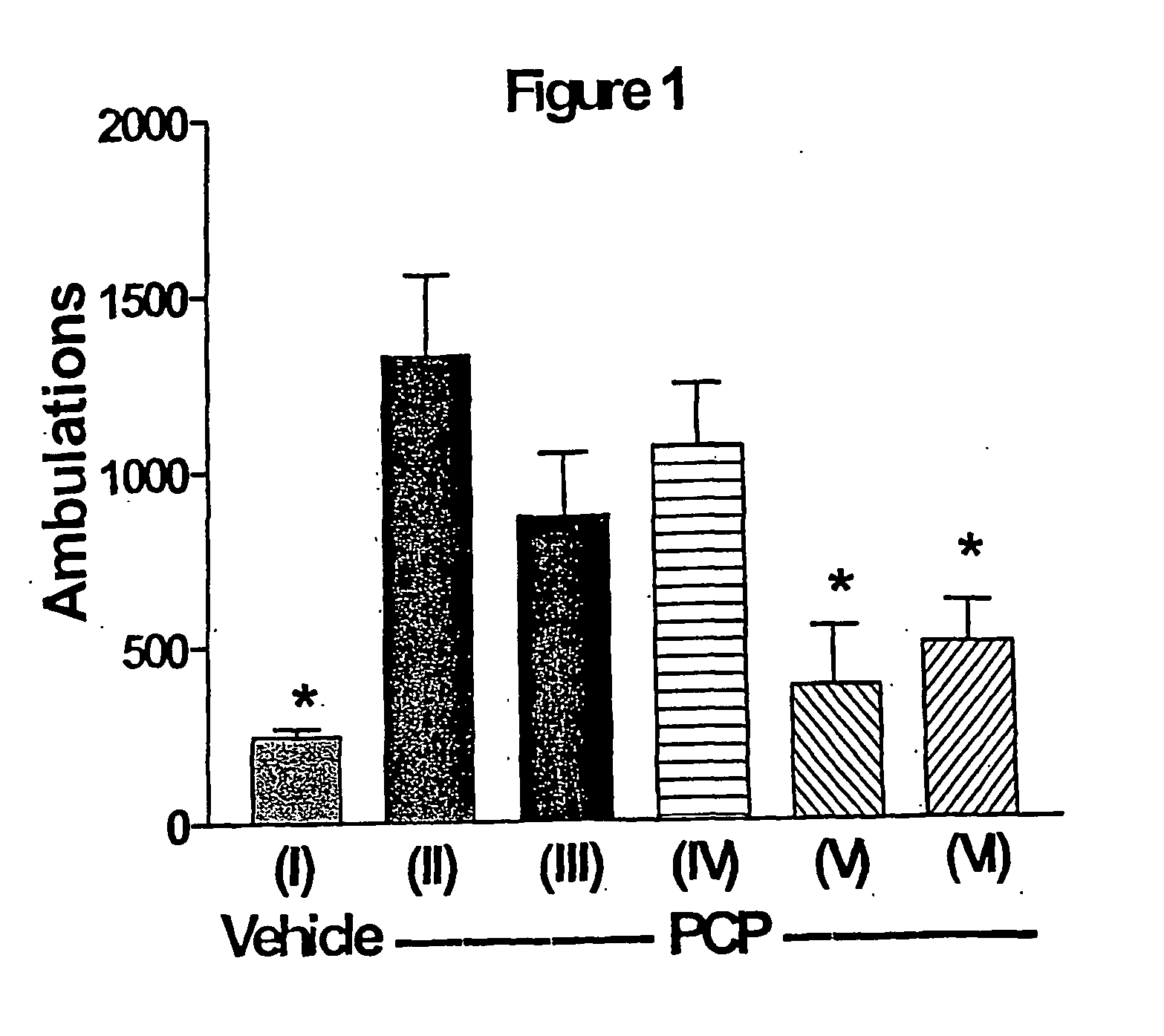
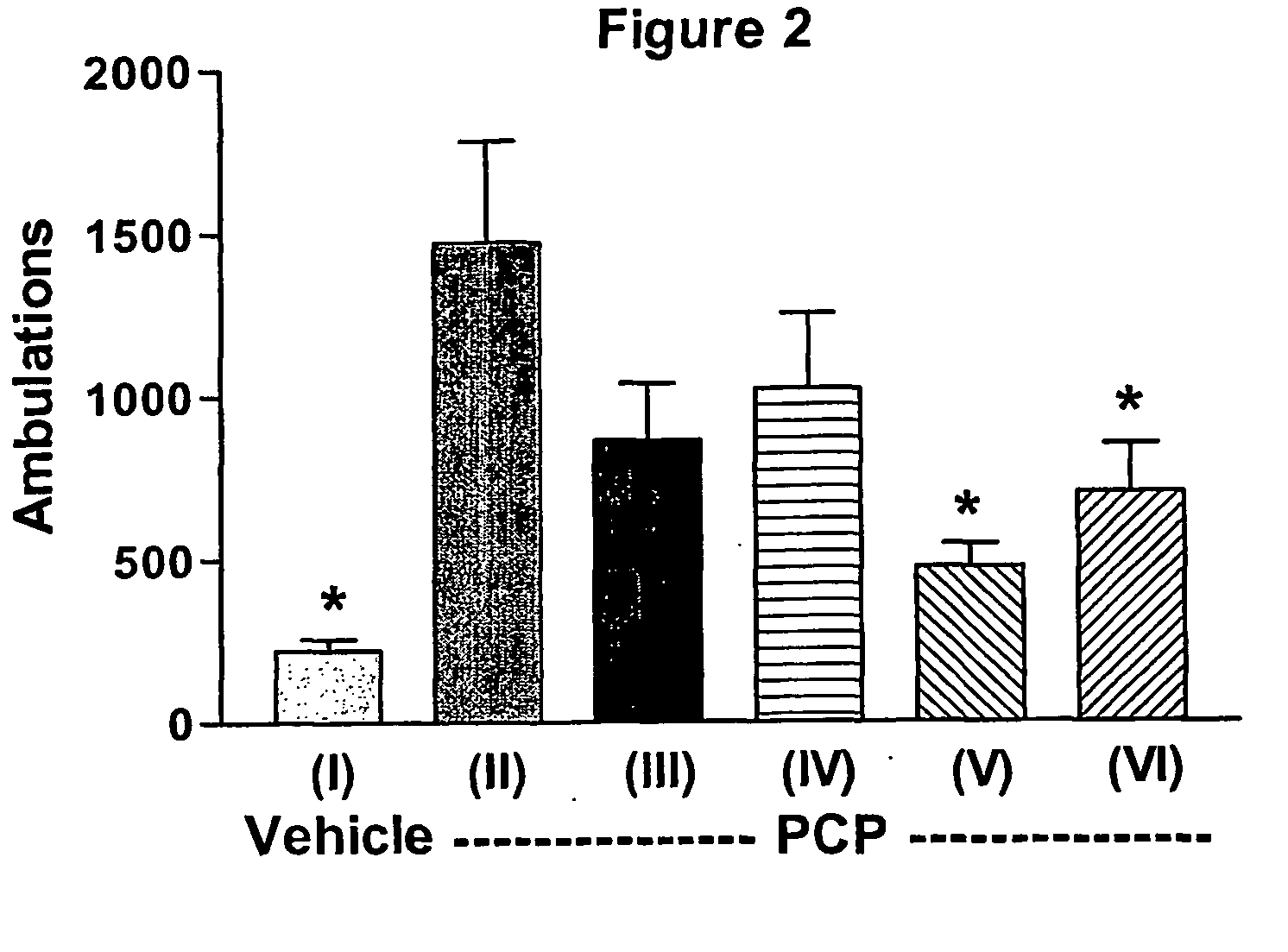
InactiveUS20110217240A1Increase and decrease brain activity in brainAlter measurable subject behaviorOrganic active ingredientsDisease diagnosisCompound aDopaminergic neurotransmission
A method for identifying typical and atypical antipsychotics based on their ability to reduce neuronal / glial activity in specific brain regions upon dopaminergic neurotransmission is disclosed.
Owner:NORTHEASTERN UNIV
Therapy for psychoses combining an atypical antipsychotic and an mglu2/3 receptor agonist
The present invention provides for a pharmaceutical composition and methods for treating psychosis comprising the combination or a first component which is an atypical antipsychotic with a second component which is a mGlu2 / 3 receptor agonist. The present invention also provides for a pharmaceutical composition and method of treating a psychiatric disorder comprising the combination of a first component which is an atypical antipsychotic with a second component which is a compound which allosterically enhances receptor activity for mGlu2 and / or mGlu3.
Owner:JOHNSON BRYAN GLENN +1
Compositions, synthesis, and methods of using quinolinone based atypical antipsychotic agents
ActiveUS8247420B2Reduce adverse side effectsEffective treatmentOrganic active ingredientsNervous disorderAtypical antipsychoticPsychosis
The present invention provides novel quinolinone derivatives which can be advantageously used for treating schizophrenia and related psychoses such as acute manic, bipolar disorder, autistic disorder, and depression.
Owner:REVIVA PHARMA INC
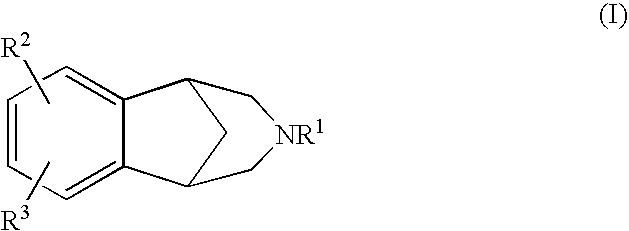
Certain tricyclic substituted diazabicyclo (3.2.1) octane derivatives
This invention encompasses compounds of the formula: where either R1 or R2 represents and the other represents hydrogen or straight or branched chain lower alkyl having 1-6 carbon atoms; and X is oxygen, methylene, or NH; Y is represents various inorganic and organic substituents; Z is hydrogen, amino or NHR6 where R6 is lowere alkyl having 1-6 carbon atoms; T is hydrogen, halogen, hydroxy, or lower alkoxy having 1-6 carbon atoms; and A is methylene, carbonyl or CHOH. These compounds are selective partial agonists or antagonists at brain monoamine receptor subtypes or prodrugs thereof and are useful in the diagnosis and treatment of affective disorders such as schizophrenia and depression as well as certain movement disorders such as Parkinsonism. Furthermore compounds of this invention may be useful in treating the extrapyramidal side effects associated with the use of conventional neurolepticagents. These compounds show unexpectedly atypical antipsychotic profiles (clozapine-like) in the animal models described in this patent.
Owner:NEUROGEN
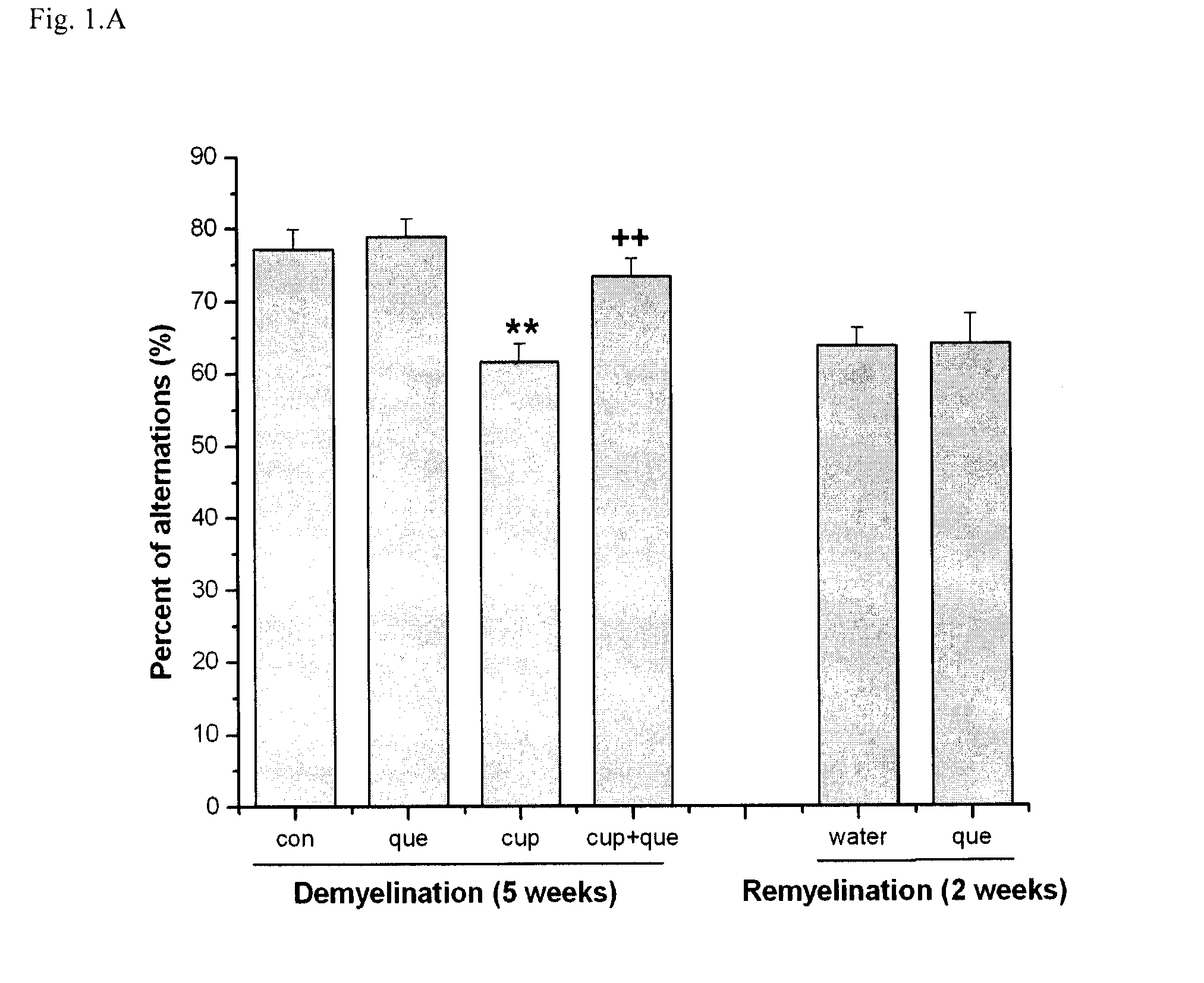
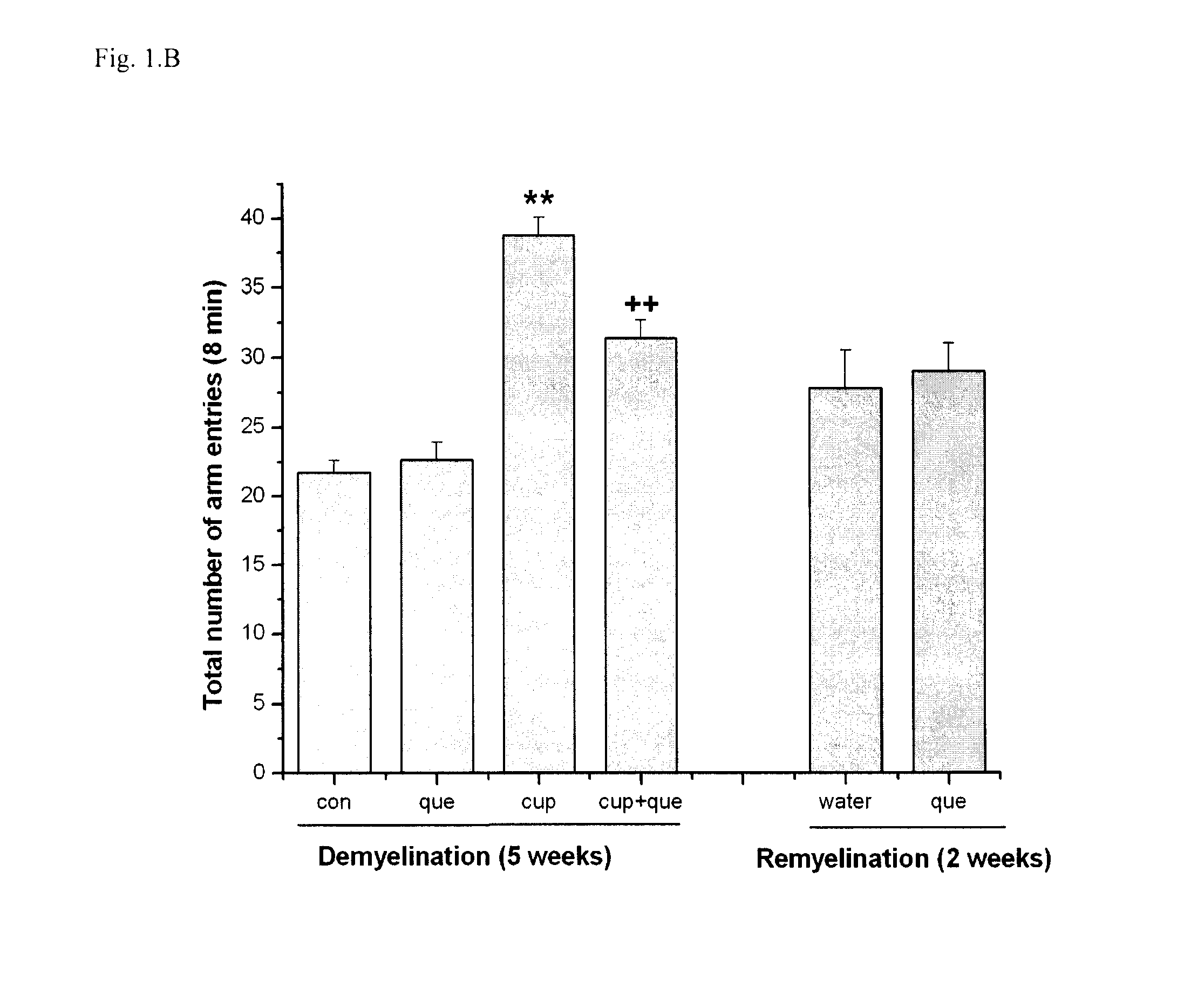
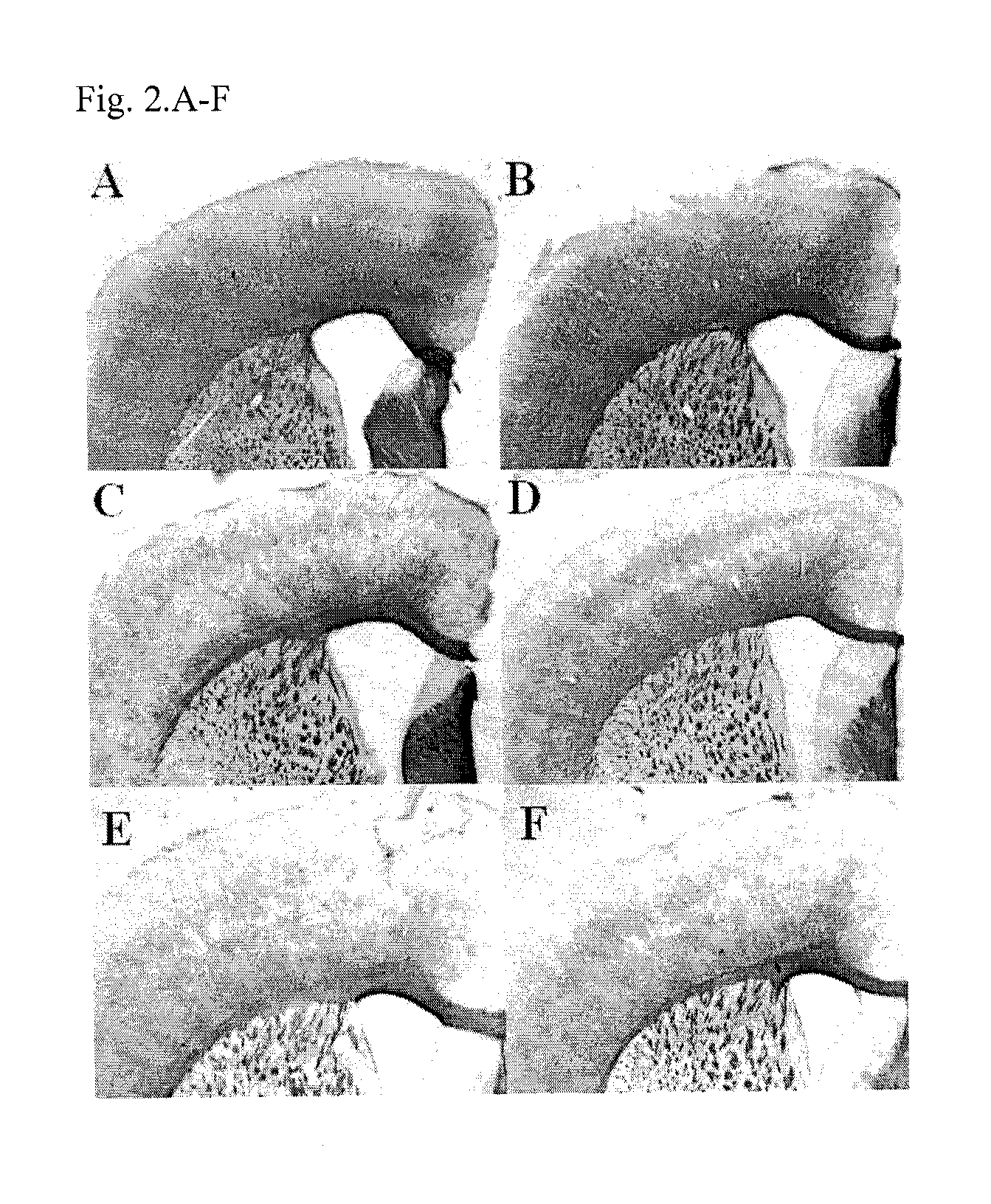
Method of treating demyelination diseases
InactiveUS8623861B2Reducing demyelinationPromotes resettlementBiocideNervous disorderAtypical antipsychoticQuetiapine
The present invention relates to a method of treating demyelination diseases, such as multiple sclerosis, comprising administration of an atypical antipsychotic drug, such as quetiapine or an analog thereof, to a subject in need thereof.
Owner:LI XIN MIN
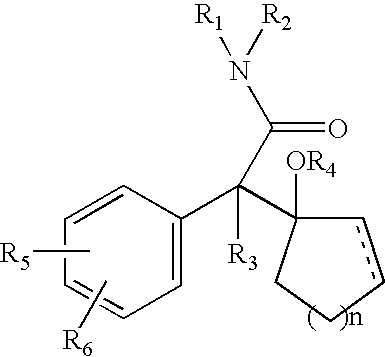
Atypical antipsychotic agents having low affinity for the D2 receptor
InactiveUS6890919B2Diminished and without side effectGood effectOrganic active ingredientsBiocideLow affinityAtypical antipsychotic
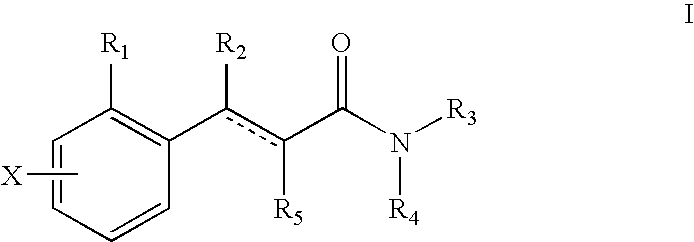
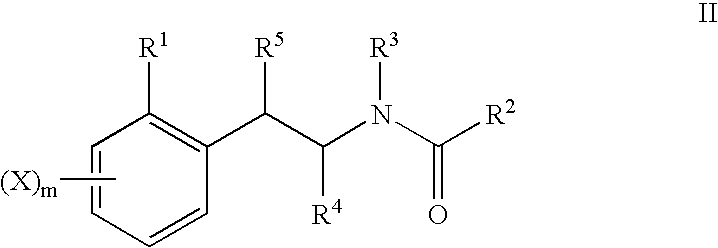
The present invention provides novel compounds of Formula I: The invention further relates to pharmaceutical compositions comprising compounds of Formula I and to methods of using compounds of Formula I to treat neuropsychiatric disorders (e.g., psychosis, depression, schizophrenia).
Owner:NEUROMOLECULAR INC
Methods for Treating Antipsychotic-Induced Weight Gain
The present invention relates to the discovery of a novel opioid modulator effective in reducing pharmacologically induced weight gain associated with atypical antipsychotic use. The present invention provides methods of reducing antipsychotic induced weight gain, methods for suppressing food intake and reducing ghrelin levels induced by atypical antipsychotic medications in a patient.
Owner:ALKERMES PHARMA IRELAND LTD
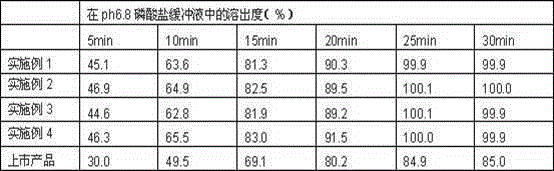
Asenapine tablets and preparation method thereof
InactiveCN106667929AGood dissolution effectOrganic active ingredientsNervous disorderFiller ExcipientAtypical antipsychotic
The invention provides Asenapine tablets and a preparation method thereof. The tablets comprise the following components in percentages by weight: 10-50% of Asenapine, 30-80% of a filler, 3-20% of a disintegrating agent, 0.5-1% of lubricant, and 0.2-2.5% of a flavouring. The preparation method comprises the following steps: micronization is carried out for Asenapine and the filler, and the particle size is controlled at 0.5-20[mu]m; the fine powder obtained in the last step and partial disintegrating agent are prepared into a soft material with an ethanol water, and granulation is carried out with 24 mesh sieve; prepared fine granules are dried at 50-70 DEG C, and granulation is carried out with 20 mesh sieve; the residual disintegrating agent, the flavouring and the lubricant are added into dried particles in order, total mixing is carried out twice, one mixing is carried out before the lubricant is added, the other mixing is carried out after the lubricant is added, till intermediate detection is qualified, the tablets are compressed, and the product is prepared. Asenapine tablets are common tablets with coating or without coating, the product is a novel atypical antipsychotic drug; the drug has the advantages of fast drug absorption, high bioavailability, and usage convenience.
Owner:TIANJIN HANRUI PHARMA
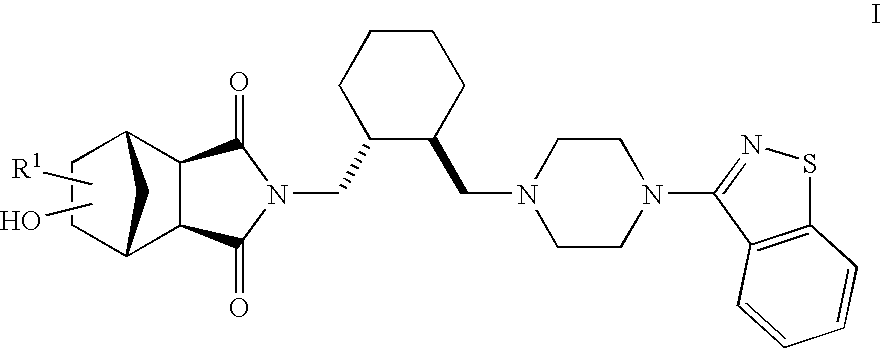
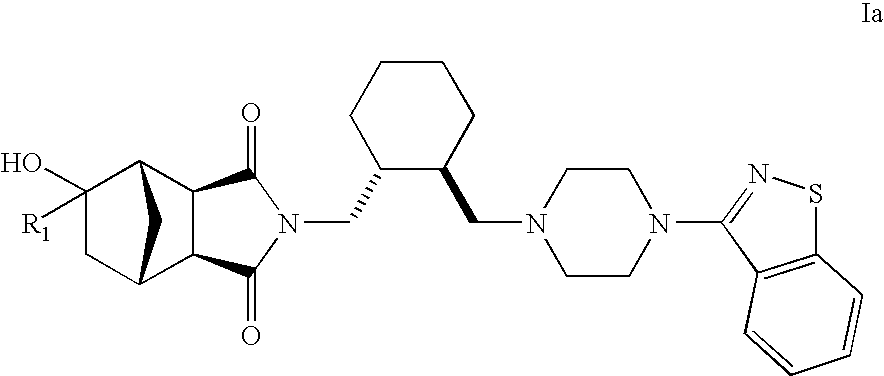
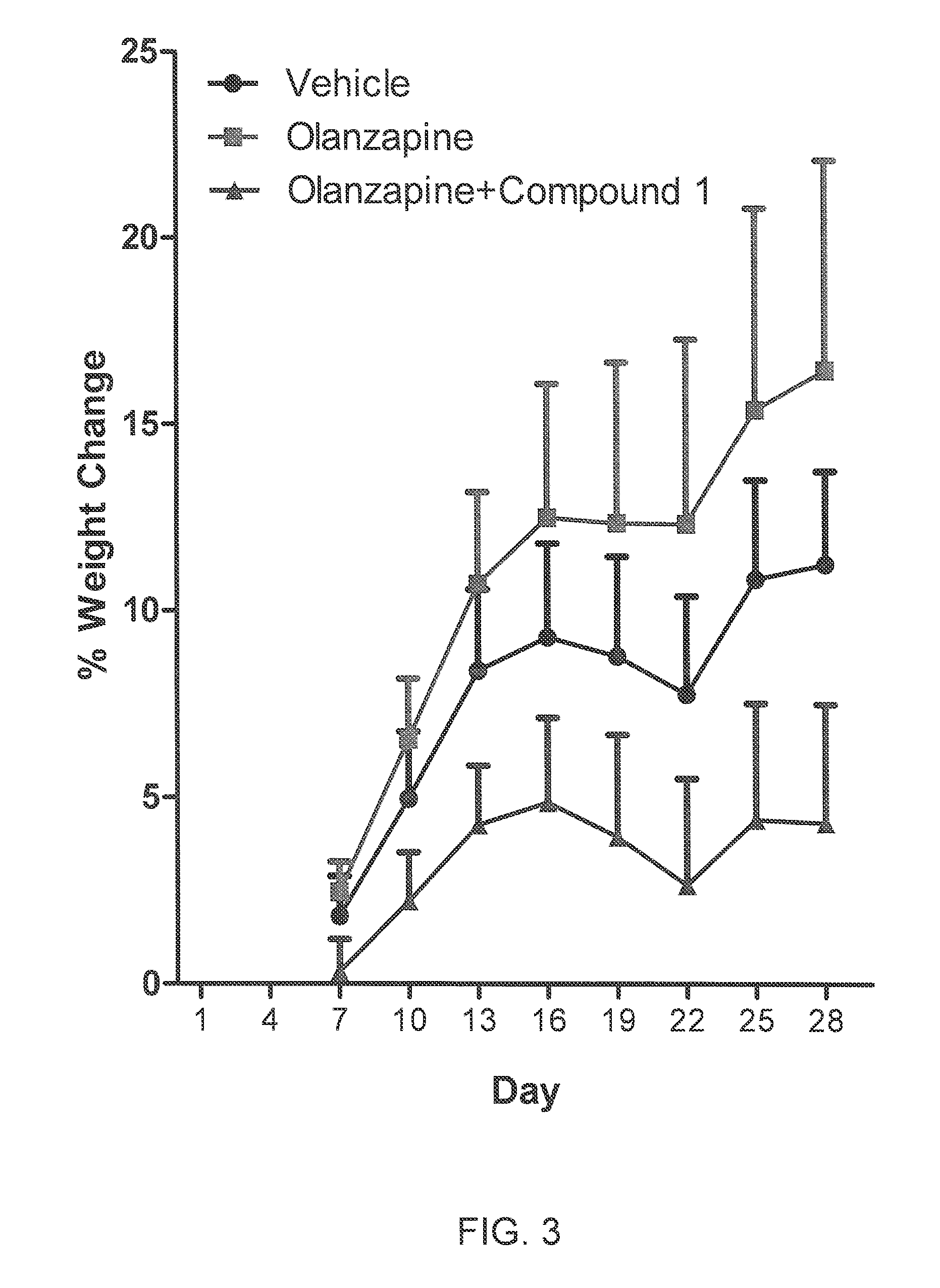
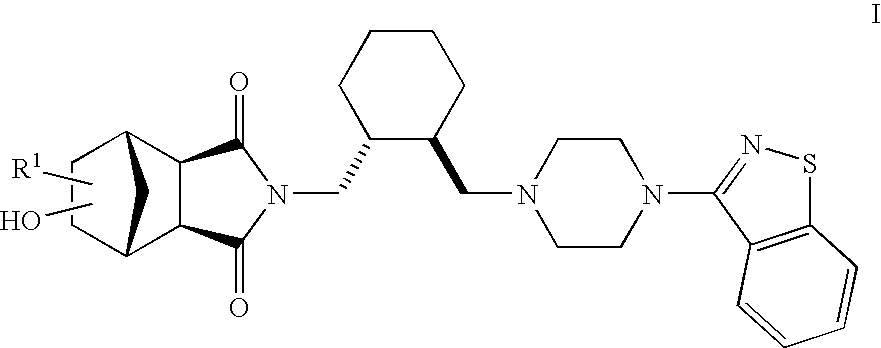
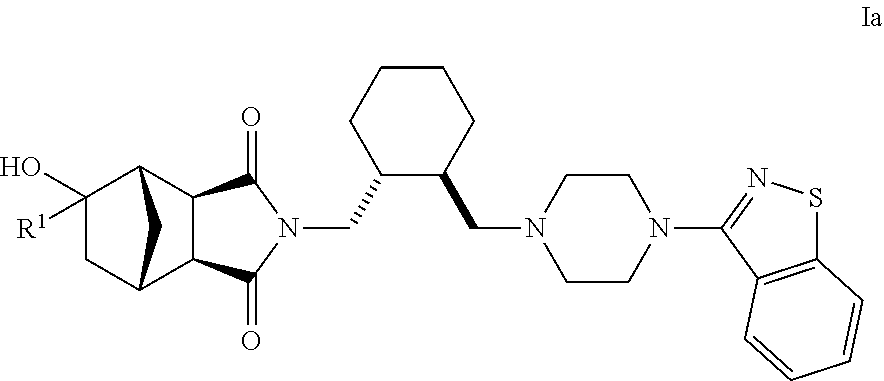
Hexahydro-1H-4,7-methanoisoindole-1,3-dione compounds
The present invention is directed to therapeutic agents of the formula (I) which are atypical antipsychotics and which are useful in the treatment of neurological and psychiatric disorders associated with dopamine D2 and serotonin 5-HT2A neurotransmission dysfunction. wherein; R1 is C1-6alkyl, which is unsubstituted or substituted with 1-6 fluoro, wherein R1 and the hydroxyl group on the ring are attached to the same carbon atom; or a pharmaceutically acceptable salt thereof.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD +1
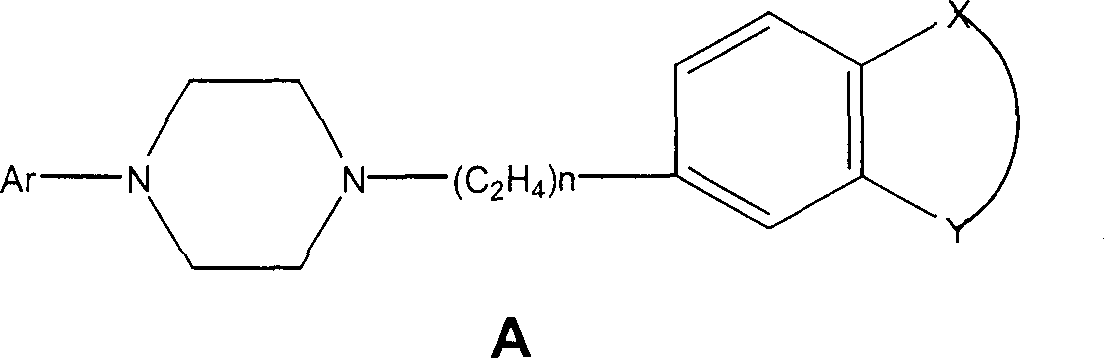
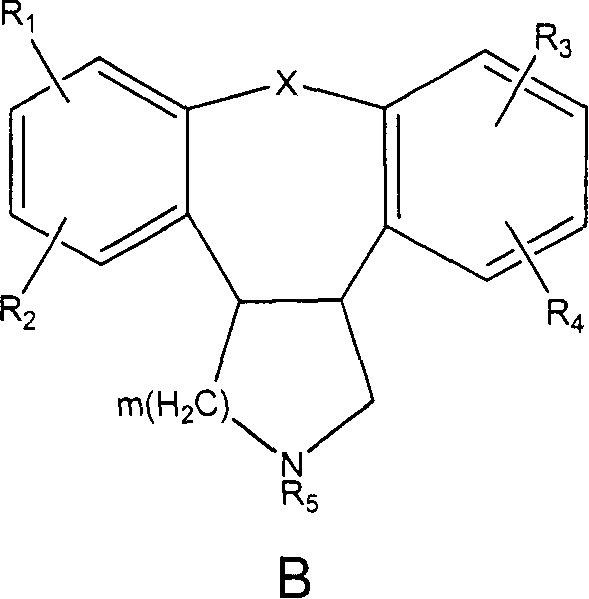
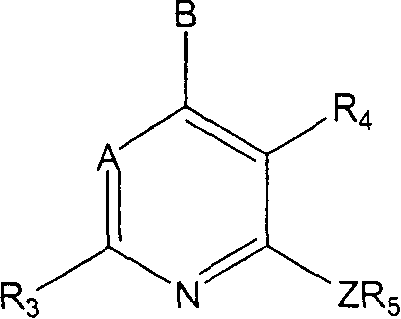
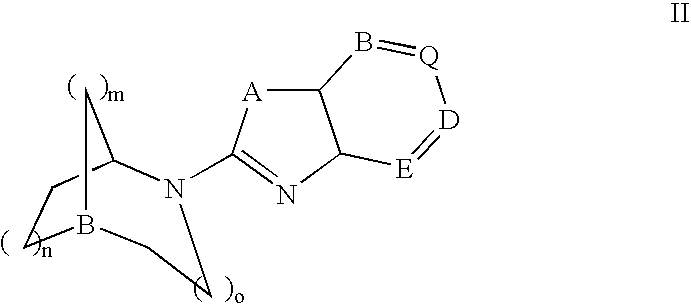
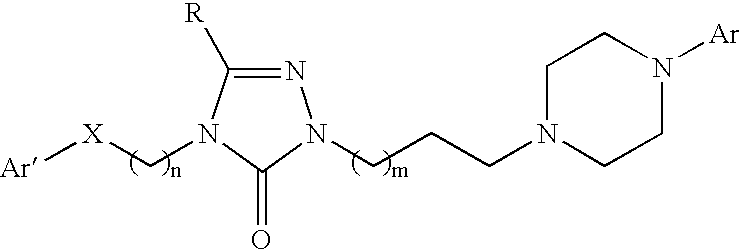
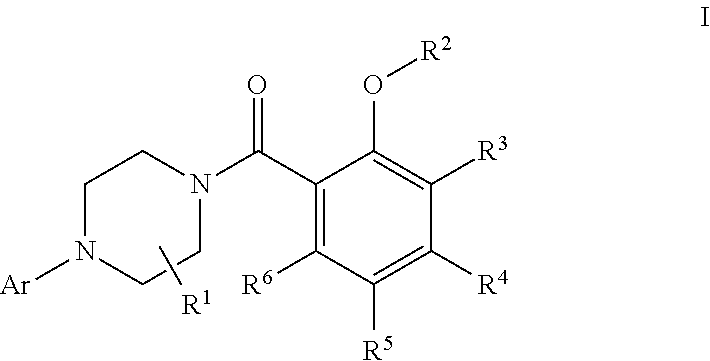
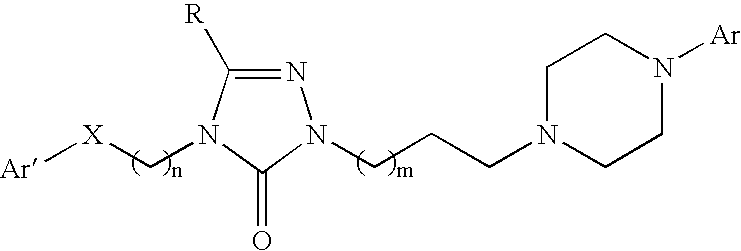
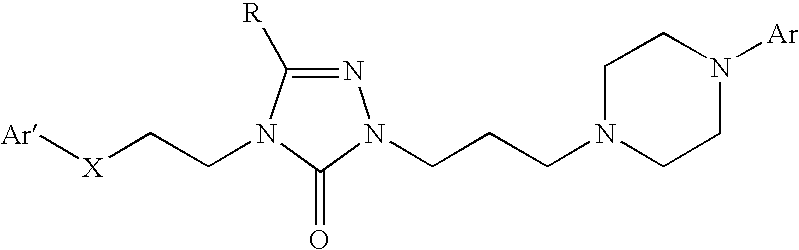
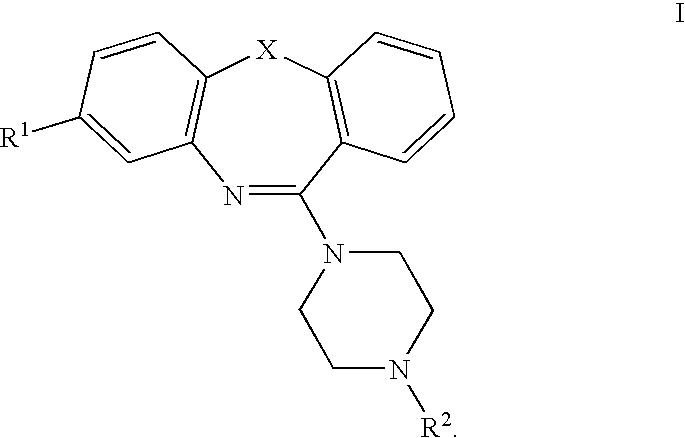
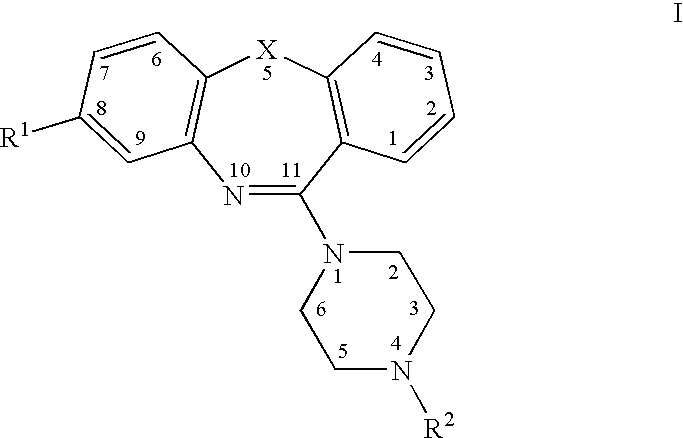
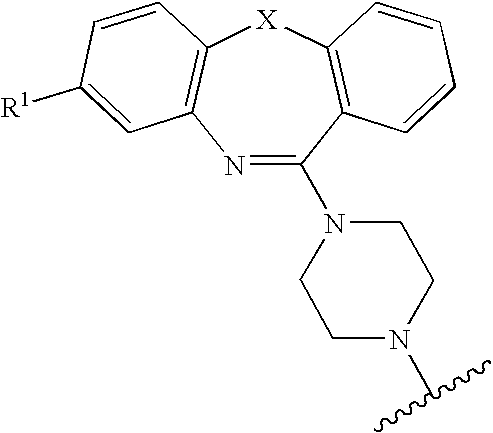
Pyrrolo [2,1-b][1,3]benzothiazepines with atypical antipsychotic activity
InactiveUS6391870B2High yieldReduce doseBiocideNervous disorderAtypical antipsychoticAntipsychotic Agent
Polycondensated heterocycles with a pyrrole[2,1-b][1,3]benzothiazepine structure of the following formula (I)where the groups are defined as in the description are disclosed. As compared to known antipsychotic agents, these compounds present substantial activity associated with a simultaneous reduction in unwanted extrapyramidal symptoms. These compounds can be formulated in pharmaceutical compositions for the treatment of psychoses such as, for example, schizophrenia.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
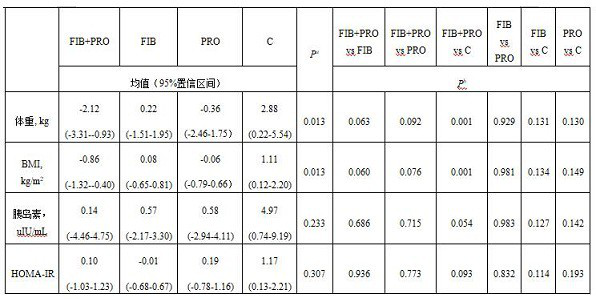
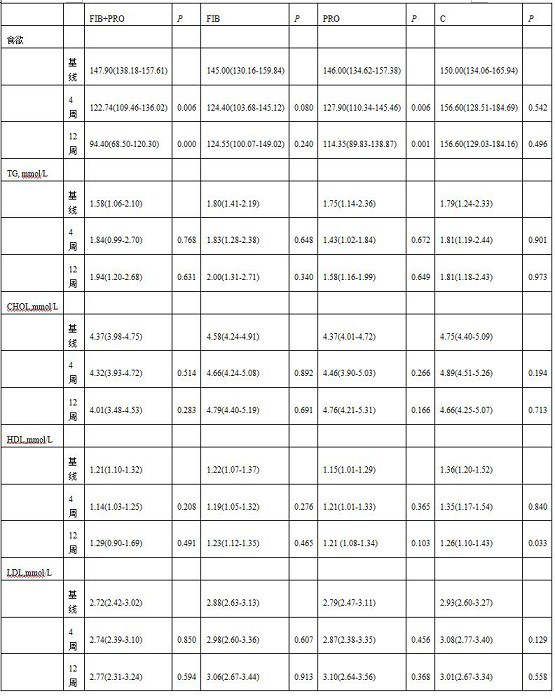
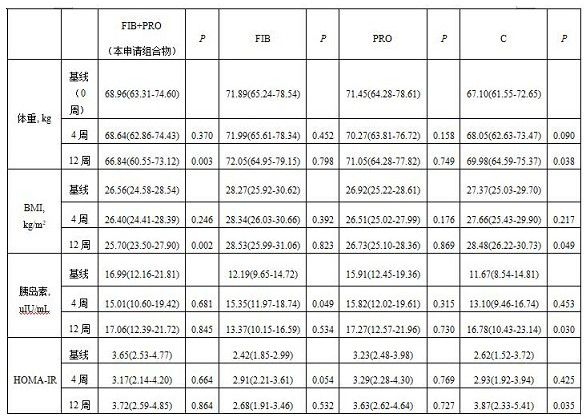
Composite probiotic dietary fiber composition as well as application and health food thereof
PendingCN113854568AReduce appetiteIncrease body mass indexFood ingredient functionsBiotechnologyWeight gaining
The invention provides a composite probiotic dietary fiber composition, which is used for relieving weight gain caused by atypical antipsychotic drugs, the composition is composed of dietary fibers and probiotics, the mass ratio of the probiotics to the dietary fibers is 1: (30-40), the dietary fibers comprise soluble dietary fibers and insoluble dietary fibers, the mass ratio of the soluble dietary fibers to the insoluble dietary fibers is (8-10): 1; the viable count of the probiotics is not less than 5.0*10 <7> CFU / g. By using the composite probiotic dietary fiber composition, weight gain caused by atypical antipsychotics can be effectively relieved.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Therapeutic combinations of atypical antipsychotics with corticotropin releasing factor antagonists
InactiveCN1917882ANervous disorderHeterocyclic compound active ingredientsCorticotropinsAtypical antipsychotic
Owner:PFIZER PRODS ETAT DE CONNECTICUT
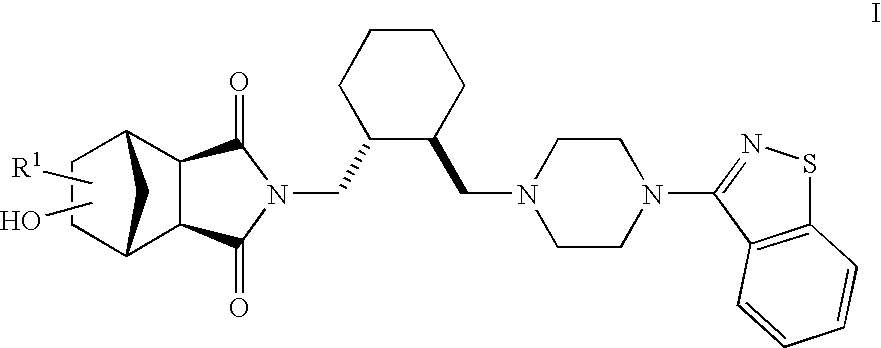
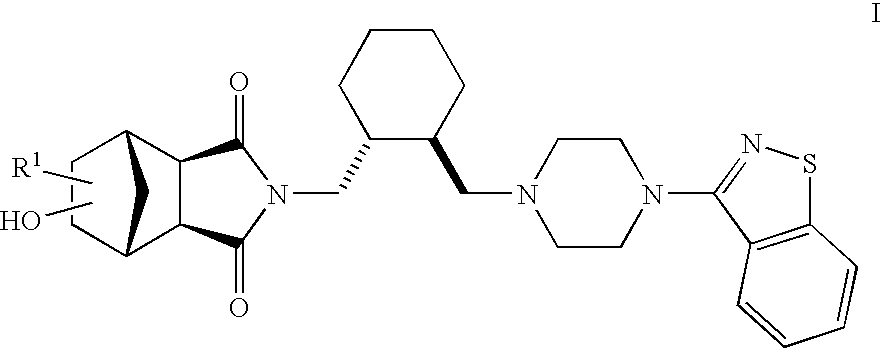
Therapeutic agents
The present invention is directed to therapeutic agents of the formula (I) which are atypical antipsychotics and which are useful in the treatment of neurological and psychiatric disorders associated with dopamine D2 and serotonin 5-HT2A neurotransmission dysfunction. wherein; R1 is C1-6alkyl, which is unsubstituted or substituted with 1-6 fluoro, wherein R1 and the hydroxyl group on the ring are attached to the same carbon atom; or a pharmaceutically acceptable salt thereof.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD +1
Stabilized atypical antipsychotic formulation
Owner:HANDA PHARM LLC
Methods and compositions for retarding weight gain associated with use of atypical antipsychotic drugs
InactiveUS20100311717A1Worsening of symptomPreventing and reducing serious side effectBiocideNervous disorderDiseaseQuetiapine
Methods and compositions for preventing or reducing weight gain and associated metabolic syndrome in patients receiving atypical antipsychotic drugs for treatment of mental illnesses are described. The invention comprises administering to a patient in need of treatment an effective amount of a dopamine agonist in conjunction with an effective amount of an atypical antipsyochotic drug. In one embodiment of the invention, the dopamine agonist is pramipexole. The dopamine agonist may be administered in a low dose, such as less than 1 mg per day of pramipexole. Examples of atypical antipsychotic drugs which may be administered in conjunction with the dopamine agonist include clozapine, olanzapine, quetiapine and risperadone.
Owner:MCINTOSH DIANE +1
Memantine as adjunctive treatment to atypical antipsychotic in schizophrenia patients
The present invention provides a method for treating schizophrenia in a patient in need thereof, the method comprising administering to the patient a therapeutically effective amount of memantine, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount an atypical antipsychotic. The method of the present invention embodies both the co-administration of memantine with an atypical antipsychotic, and the use of memantine as an adjunctive treatment to treatment with an atypical antipsychotic.
Owner:FOREST LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
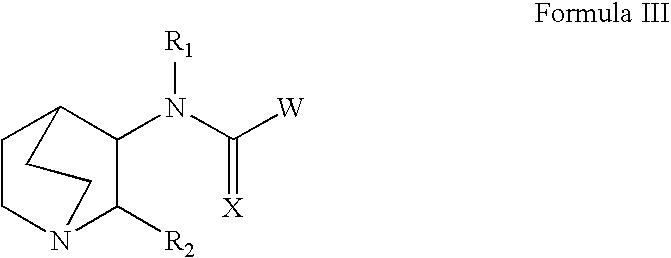
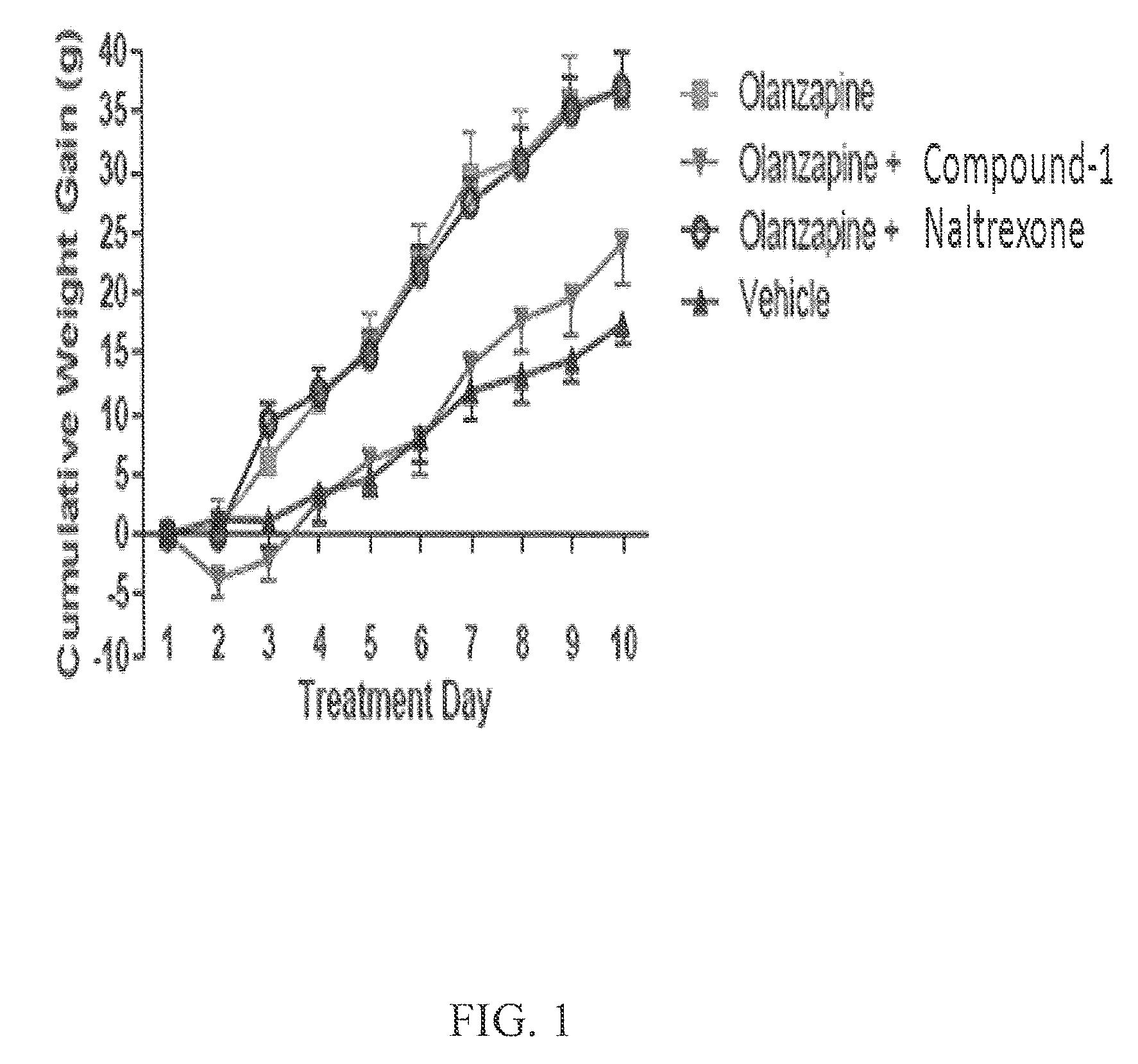


![Pyrrolo [2,1-b][1,3]benzothiazepines with atypical antipsychotic activity Pyrrolo [2,1-b][1,3]benzothiazepines with atypical antipsychotic activity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7a49d776-db2d-40f9-b31e-3d45f9f0d6fd/US06391870-20020521-C00001.png)
![Pyrrolo [2,1-b][1,3]benzothiazepines with atypical antipsychotic activity Pyrrolo [2,1-b][1,3]benzothiazepines with atypical antipsychotic activity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7a49d776-db2d-40f9-b31e-3d45f9f0d6fd/US06391870-20020521-C00002.png)
![Pyrrolo [2,1-b][1,3]benzothiazepines with atypical antipsychotic activity Pyrrolo [2,1-b][1,3]benzothiazepines with atypical antipsychotic activity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7a49d776-db2d-40f9-b31e-3d45f9f0d6fd/US06391870-20020521-C00003.png)