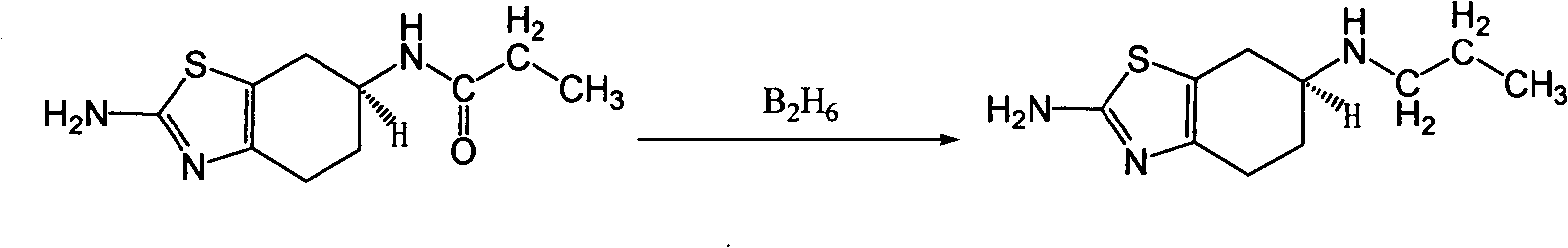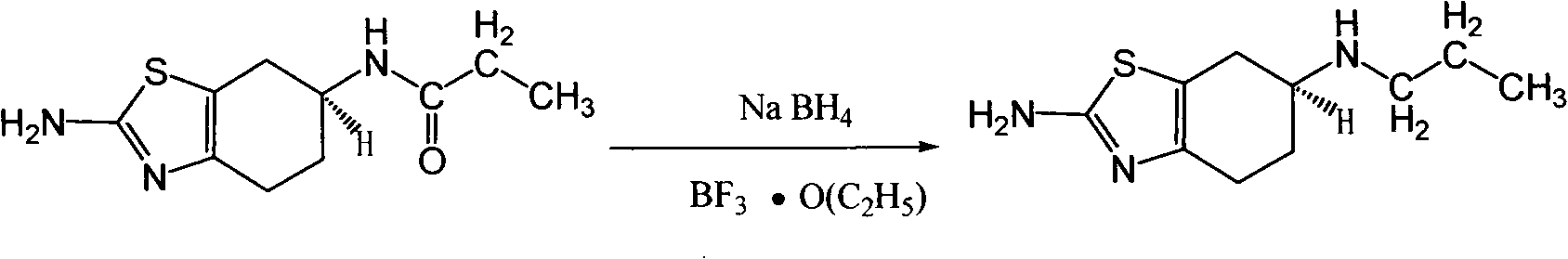Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
130 results about "Pramipexole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pramipexole is used alone or with other medications to treat Parkinson's disease.
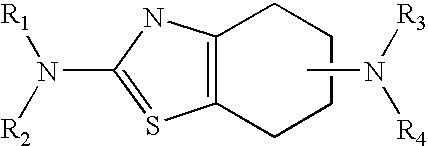
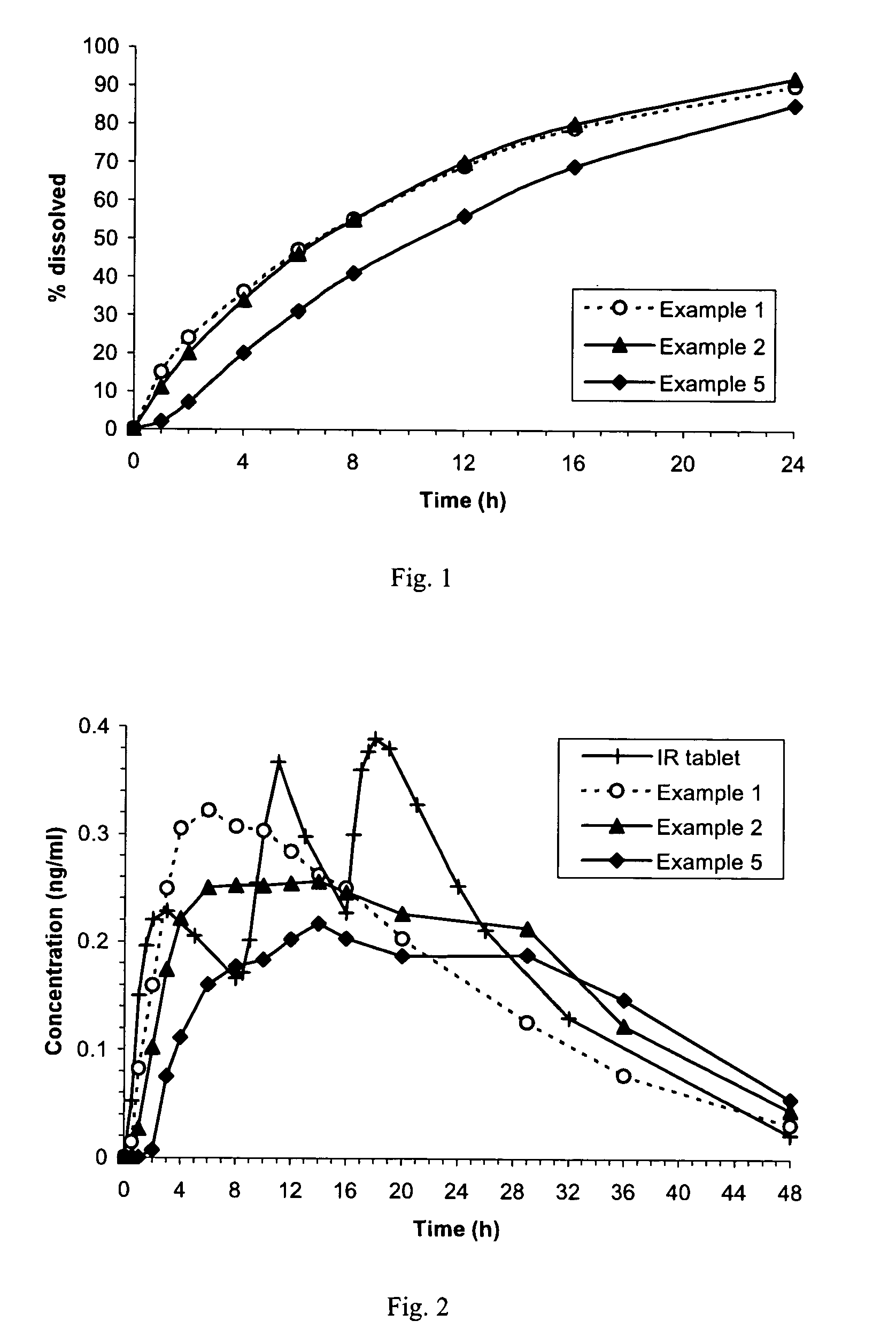
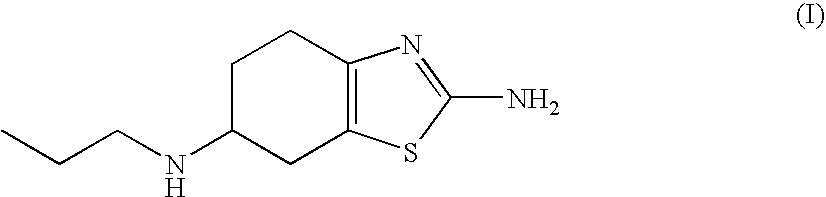
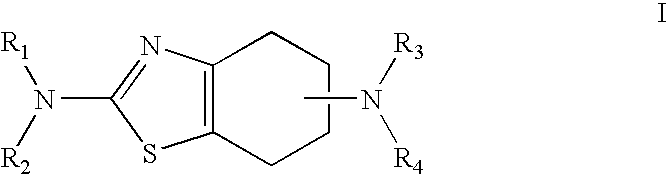
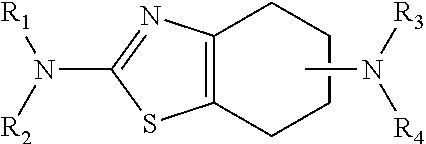
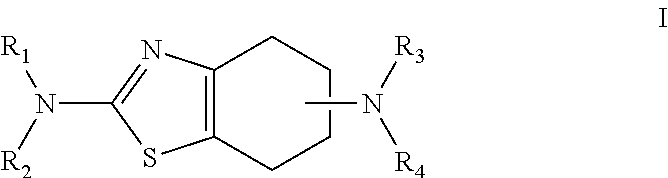
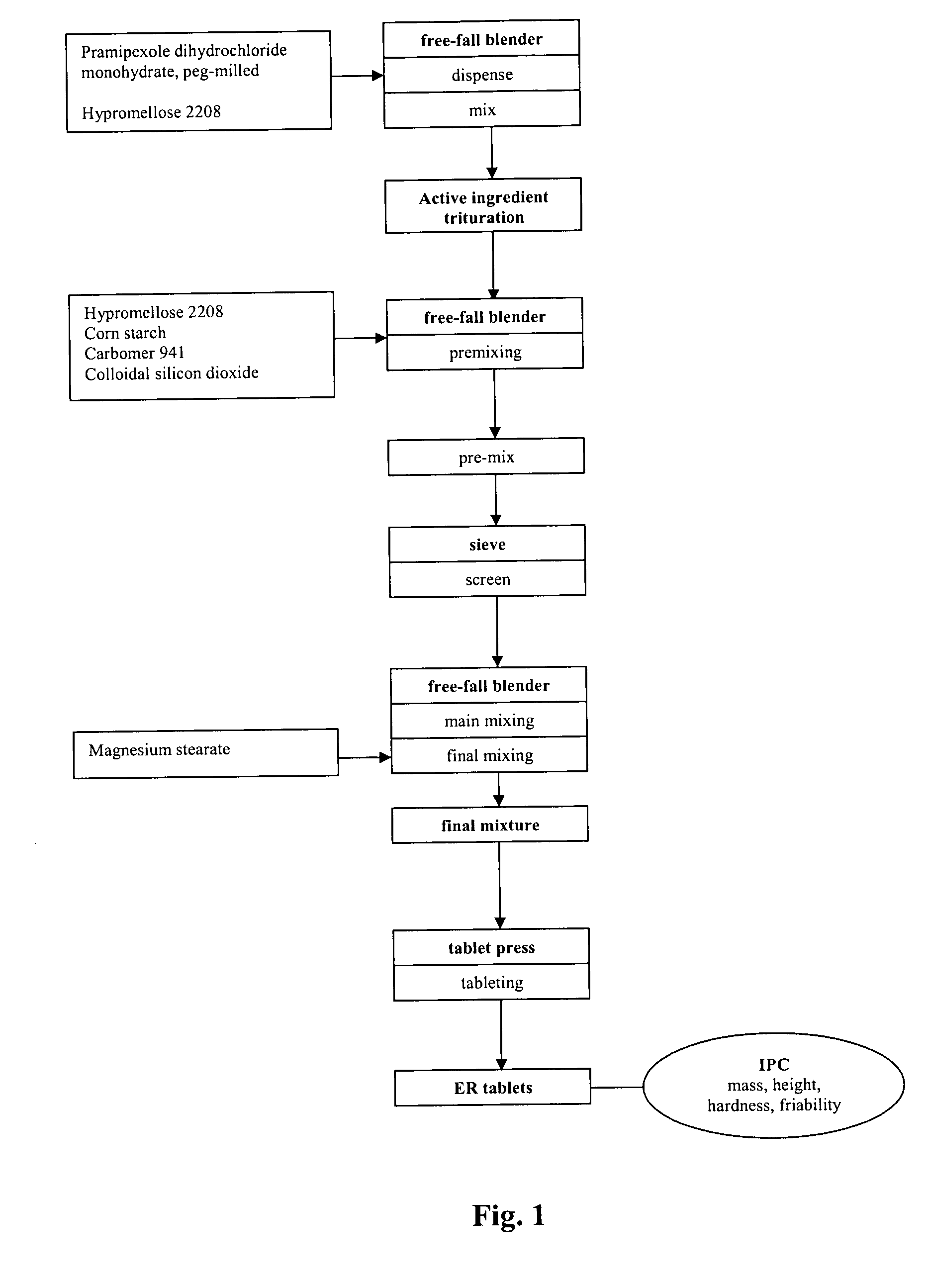
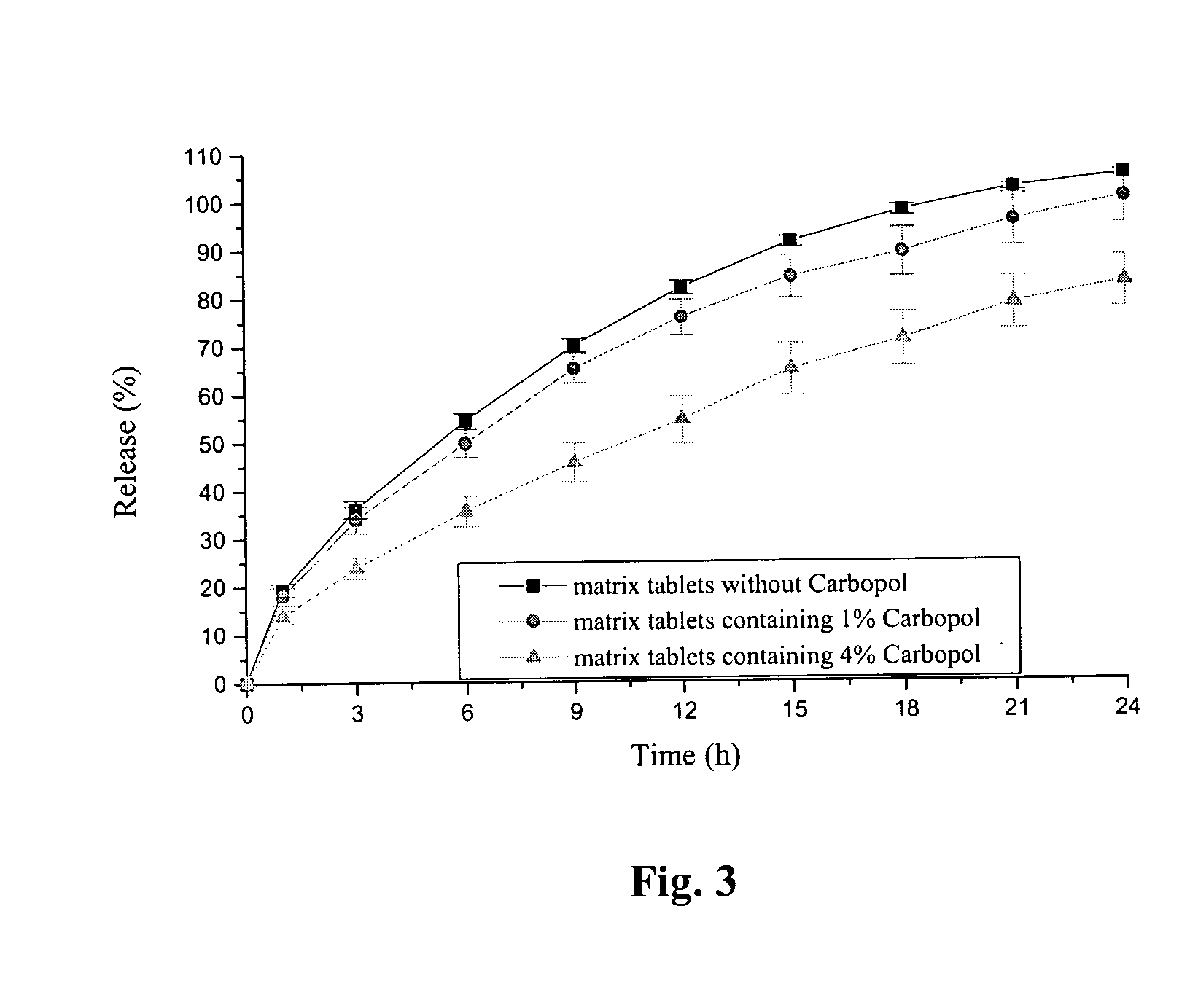
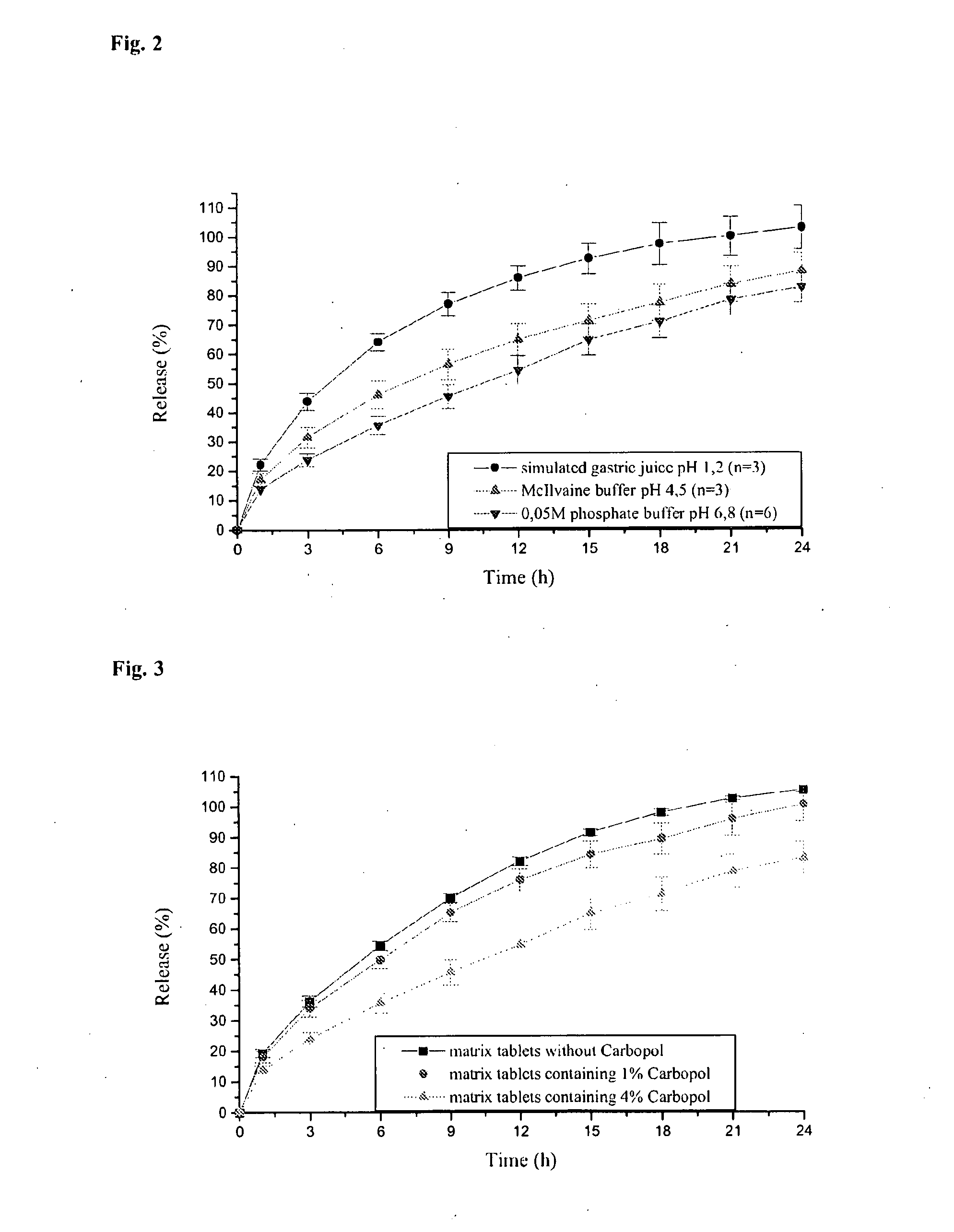
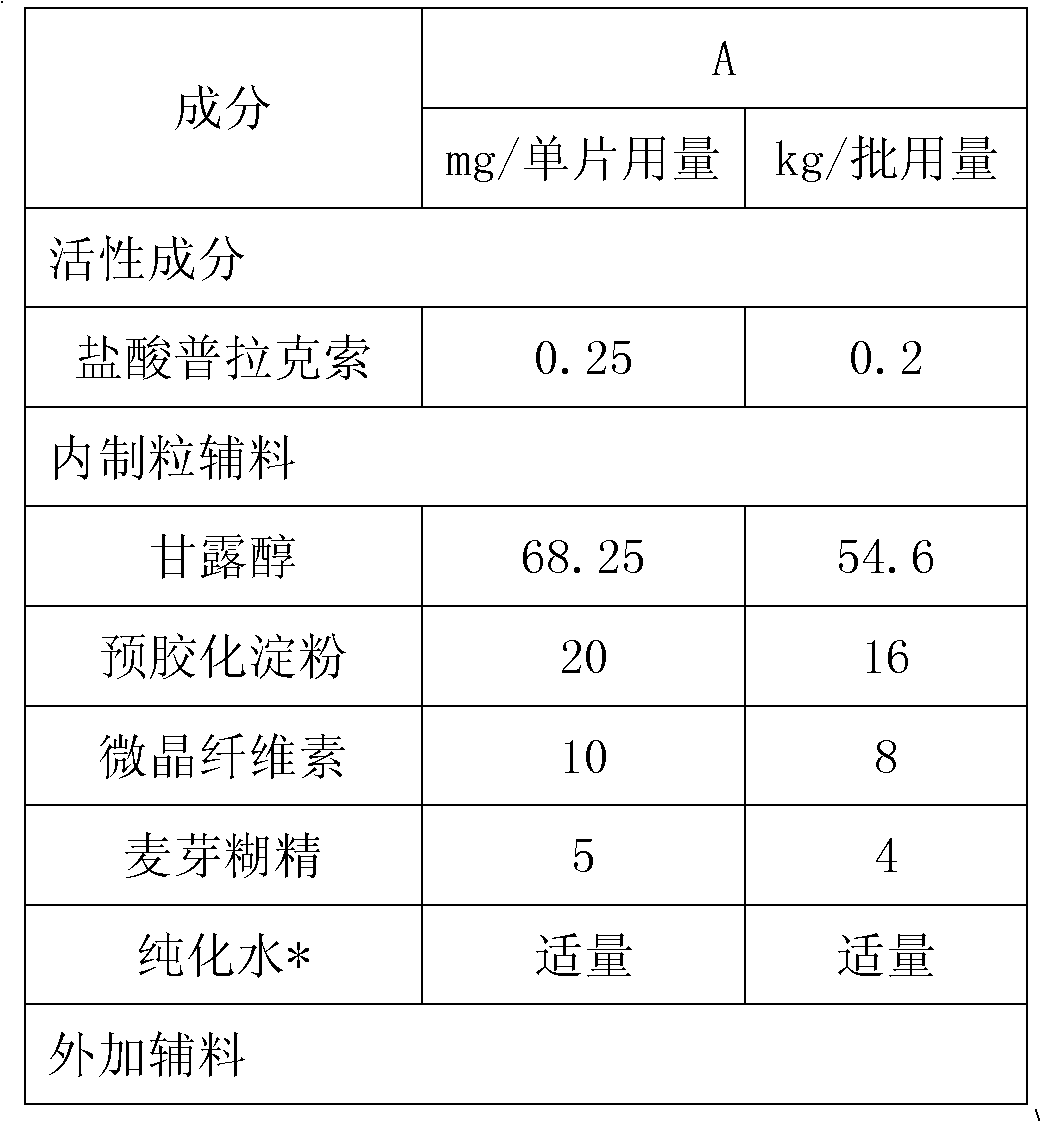
Sustained-release tablet composition of pramipexole
InactiveUS20050226926A1Organic active ingredientsNervous disorderSustained Release TabletHydrophilic polymers
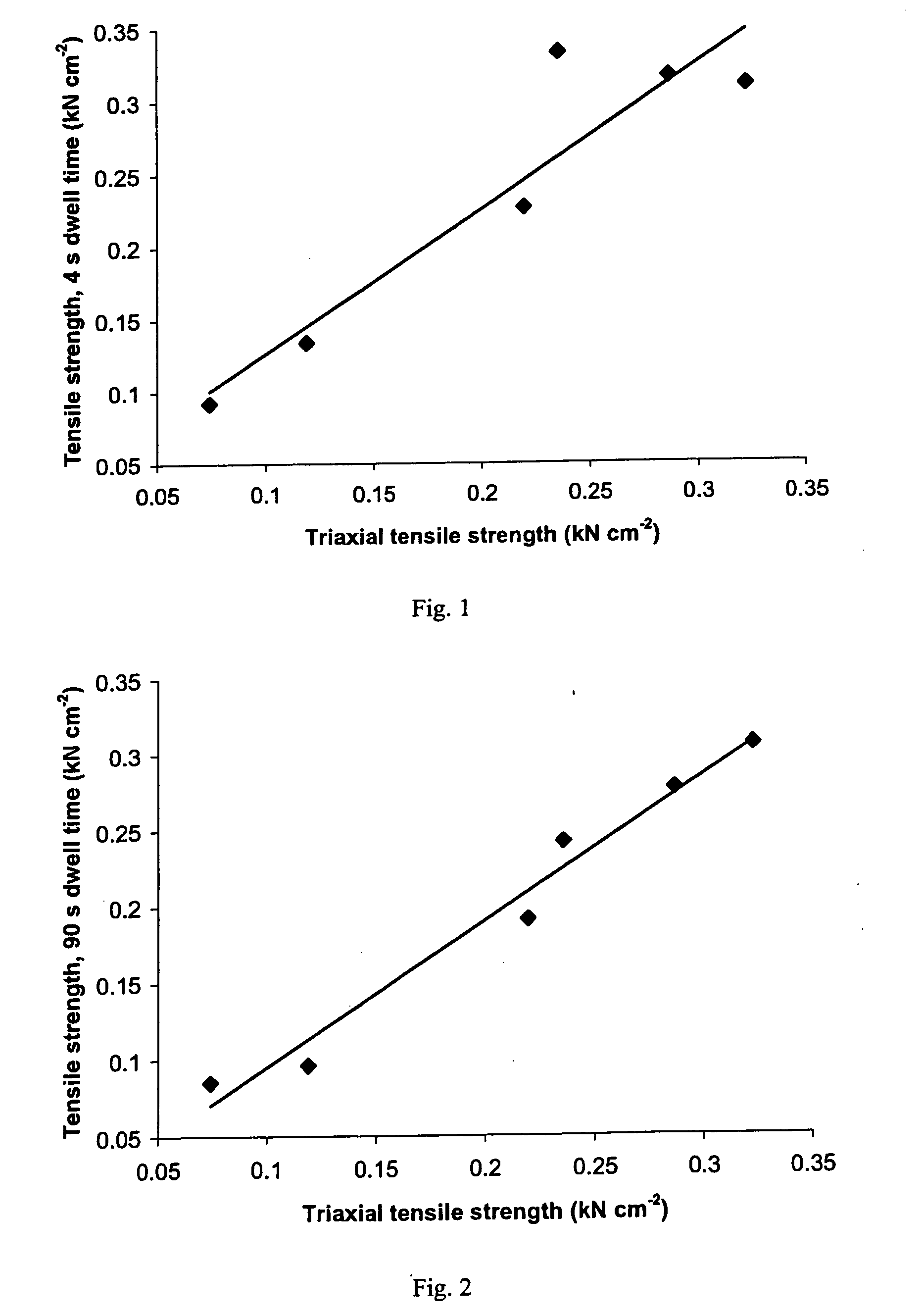
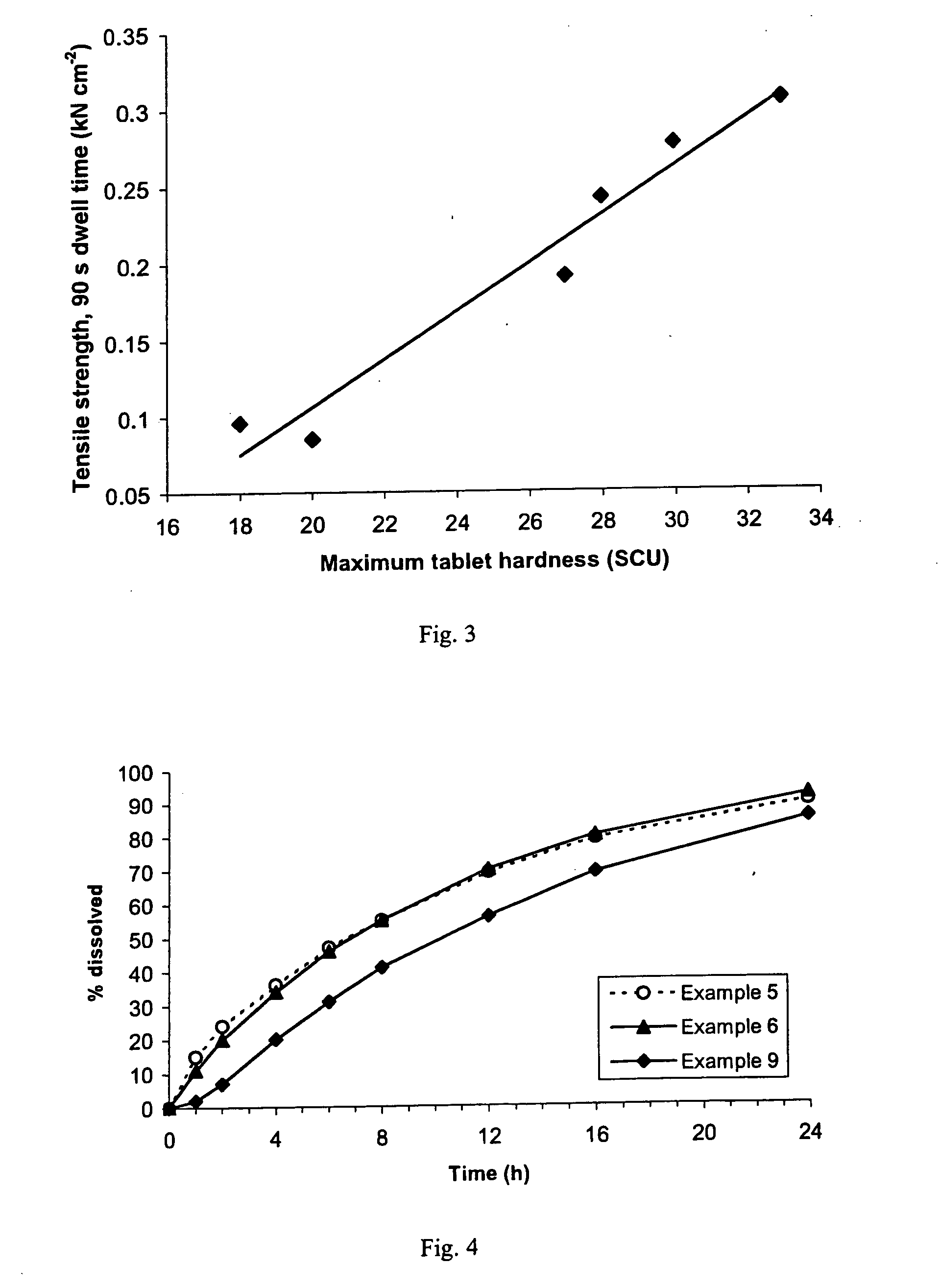
A sustained-release pharmaceutical composition in a form of an orally deliverable tablet comprises a water-soluble salt of pramipexole, dispersed in a matrix comprising a hydrophilic polymer and a starch having a tensile strength of at least about 0.15 kN cm−2 at a solid fraction representative of the tablet.
Owner:BOEHRINGER INGELHEIM INT GMBH
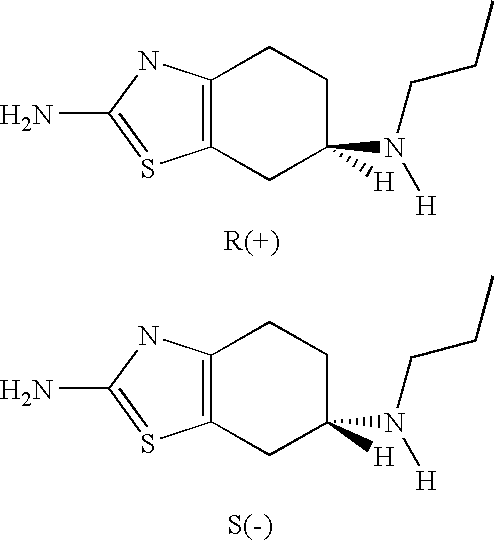
Compositions and methods of using (r)-pramipexole
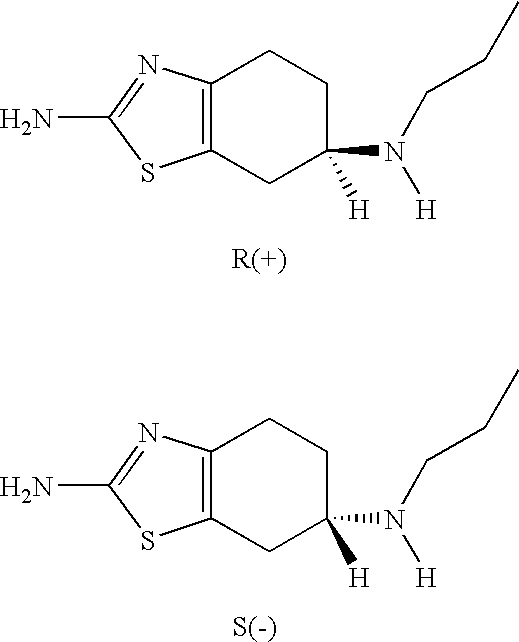
Pharmaceutical compositions of (R)-pramipexole and methods and kits of using such compositions for the treatment of neurodegenerative diseases, or those related to mitochondrial dysfunction or increased oxidative stress are disclosed.
Owner:KNOPP BIOSCIENCES LLC
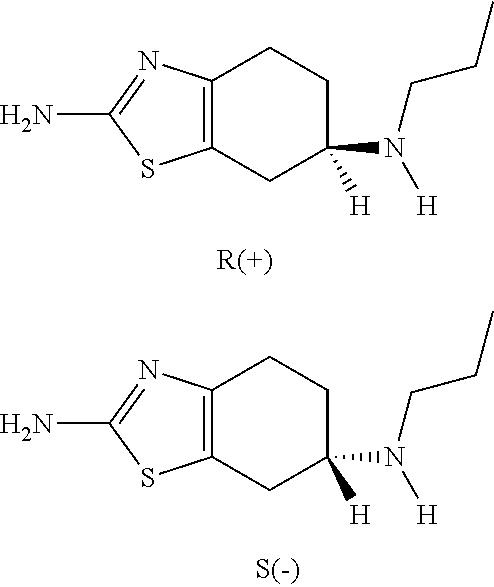
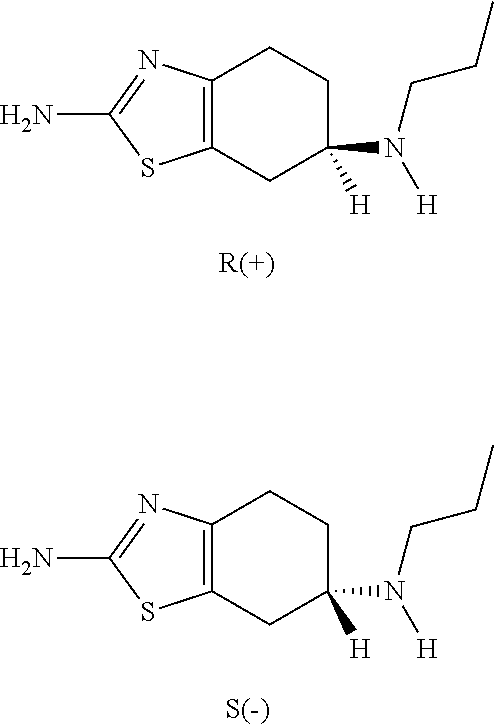
Neurorestoration with R(+) Pramipexole
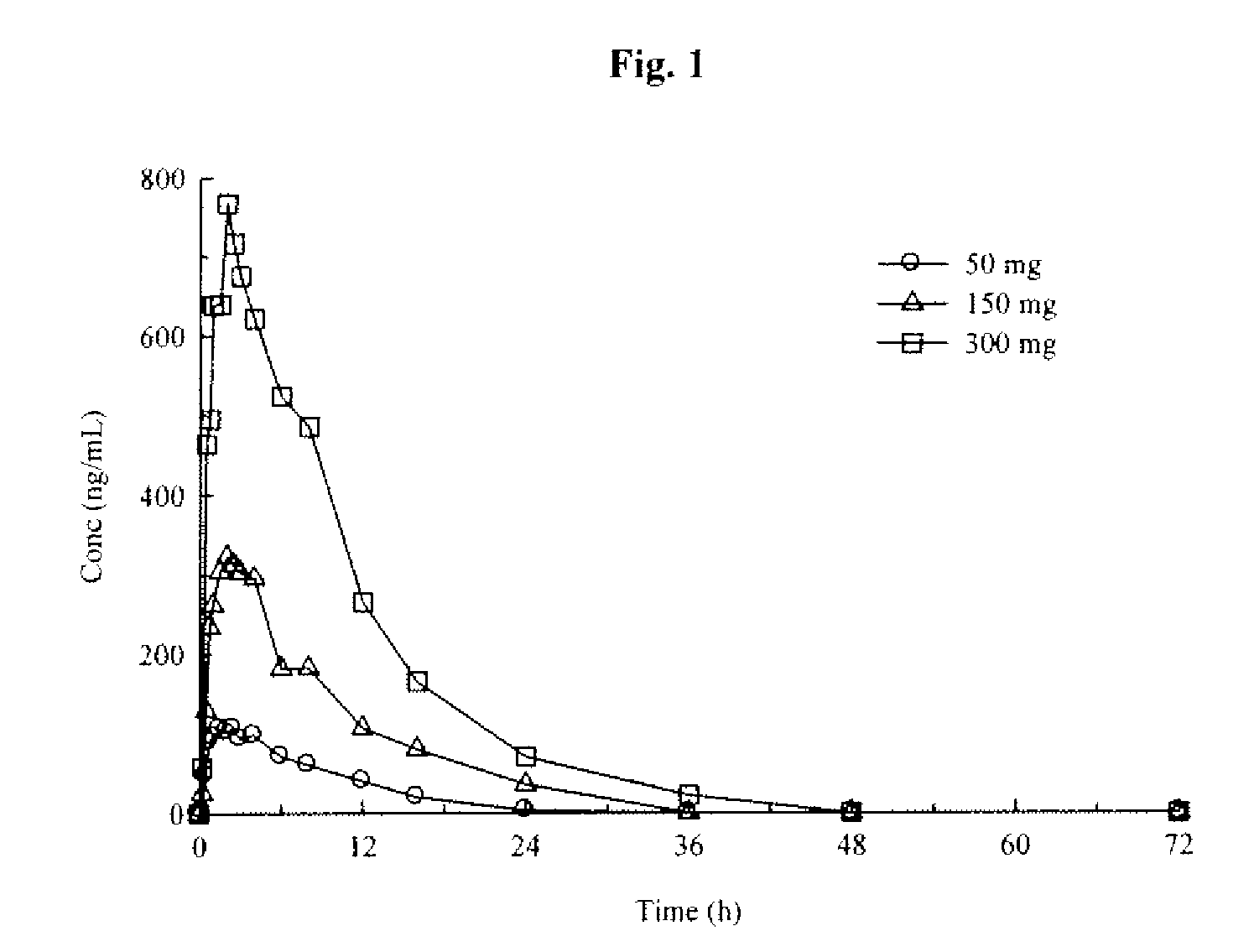
Formulations and methods of use thereof for restoring neuronal, muscular (cardiac and striated) and / or retinal tissue function in children and adults afflicted with chronic neurodegenerative diseases, such as neurodegenerative movement disorders and ataxias, seizure disorders, motor neuron diseases, and inflammatory demyelinating disorders, are described herein. Examples of disorders include Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). The method involves administering a pharmaceutical composition containing an effective amount of a tetrahydrobenzathiazole, preferably a formulation consisting substantially of the R(+) enantiomer of pramipexole. R(+) pramipexole is generally administered in doses ranging from 0.1-300 mg / kg / daily, preferably 0.5-50 mg / kg / daily, and most preferably 1-10 mg / kg / daily for oral administration. Daily total doses administered orally are typically between 10 mg and 500 mg. Alternatively, R(+) pramipexole can be administered parenterally to humans with acute brain injury in single doses between 10 mg and 100 mg and / or by continuous intravenous infusions between 10 mg / day and 500 mg / day.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
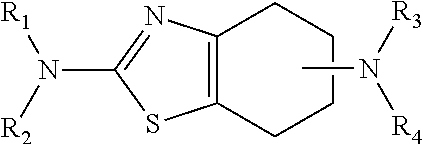
Process for the preparation of pramipexole base and/or its salts
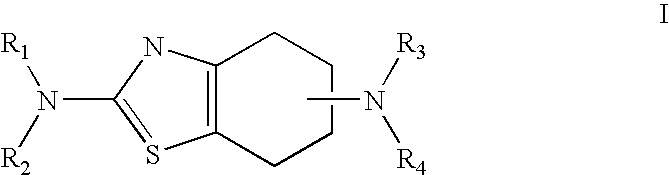
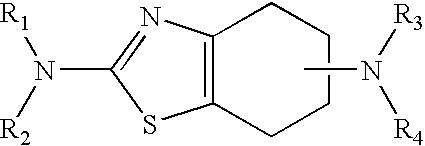
The process for the preparation of pramipexole base and / or its pharmaceutically acceptable salts, especially the hydrochloride salt, in the alkylation reaction of (S)-(−)2,6-diamino-4,5,6,7-tetrahydrobenzothiazole with an alkylating agent, wherein the reaction is carried out in the absence of a base, and in a solvent from which the resulting N-monoalkylated product selectively precipitates out as a salt. After isolation from the reaction mixture, the N-monoalkylated product is converted a) into the free pramipexole base upon treatment with an inorganic base and is then converted into another pharmaceutically acceptable pramipexole salt; or b) directly into another pharmaceutically acceptable pramipexole salt or the hydrate thereof.
Owner:INSTITUT FARMACEUTYCZNY
Process For Preparation Of Pramipexole By Chiral Chromatography
A novel process for the preparation of S(−)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole (pramipexole).
Owner:GENERICS UK LTD
Use of pramipexole to treat amyotrophic lateral sclerosis
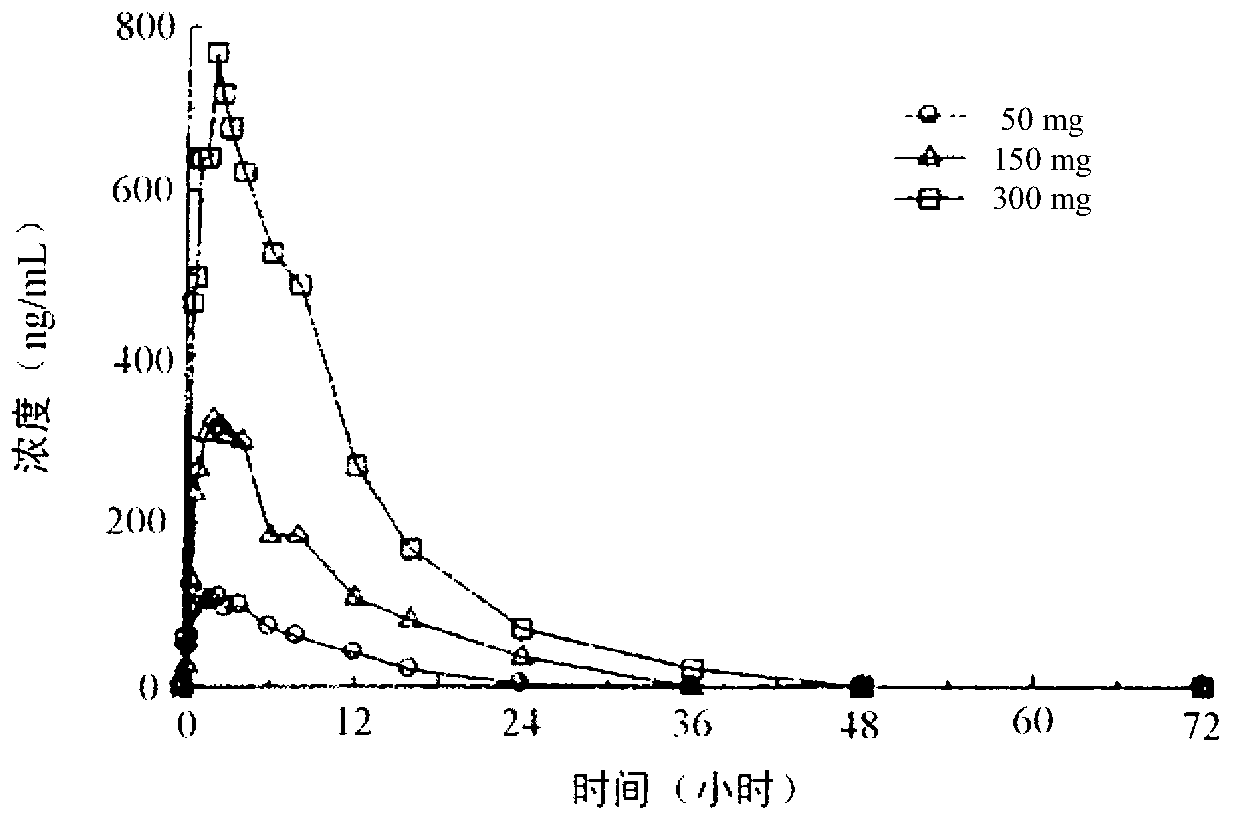
The present invention is directed to compositions comprising pramipexole and the use of such compositions to treat neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS). As shown in FIG. 6B the mean + / − SEM serum 2,3-DHBA levels for the 12 ALS participants decreased significantly after pramipexole treatment.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
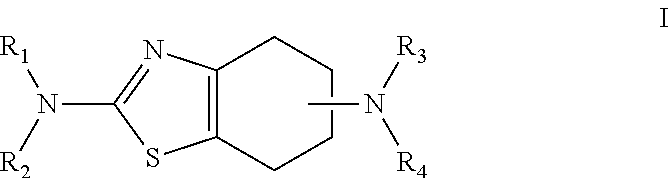
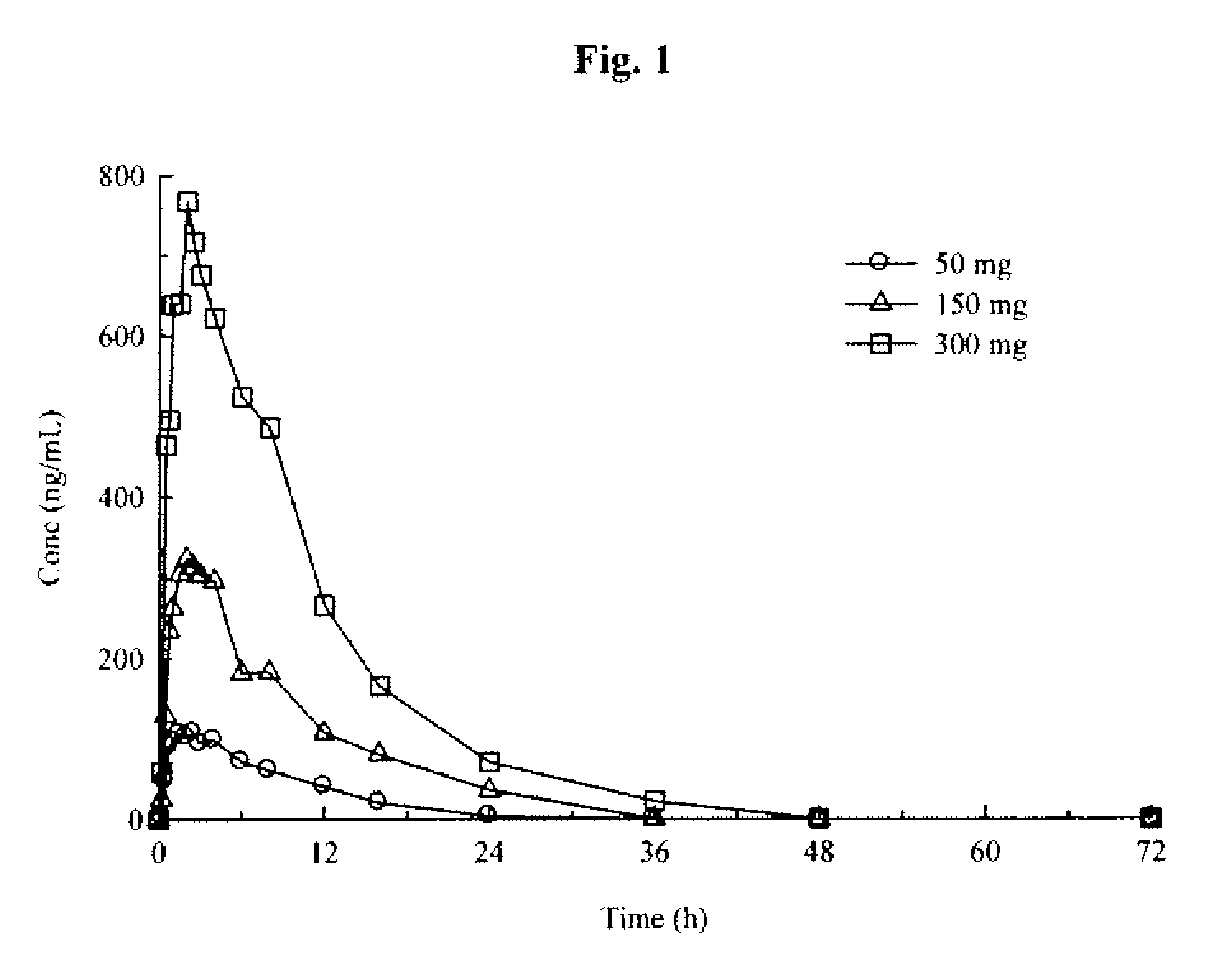
Pramipexole once-daily dosage form
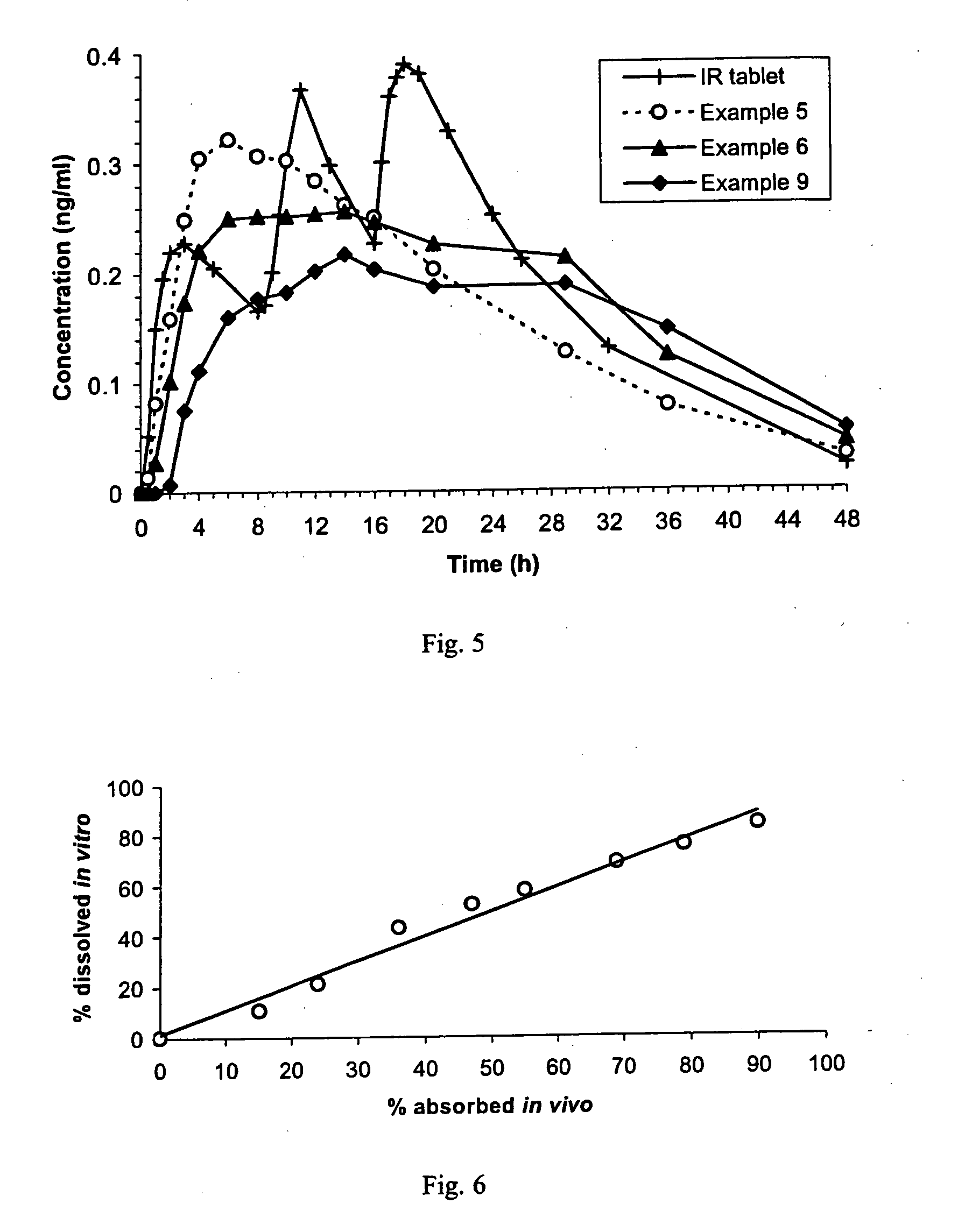
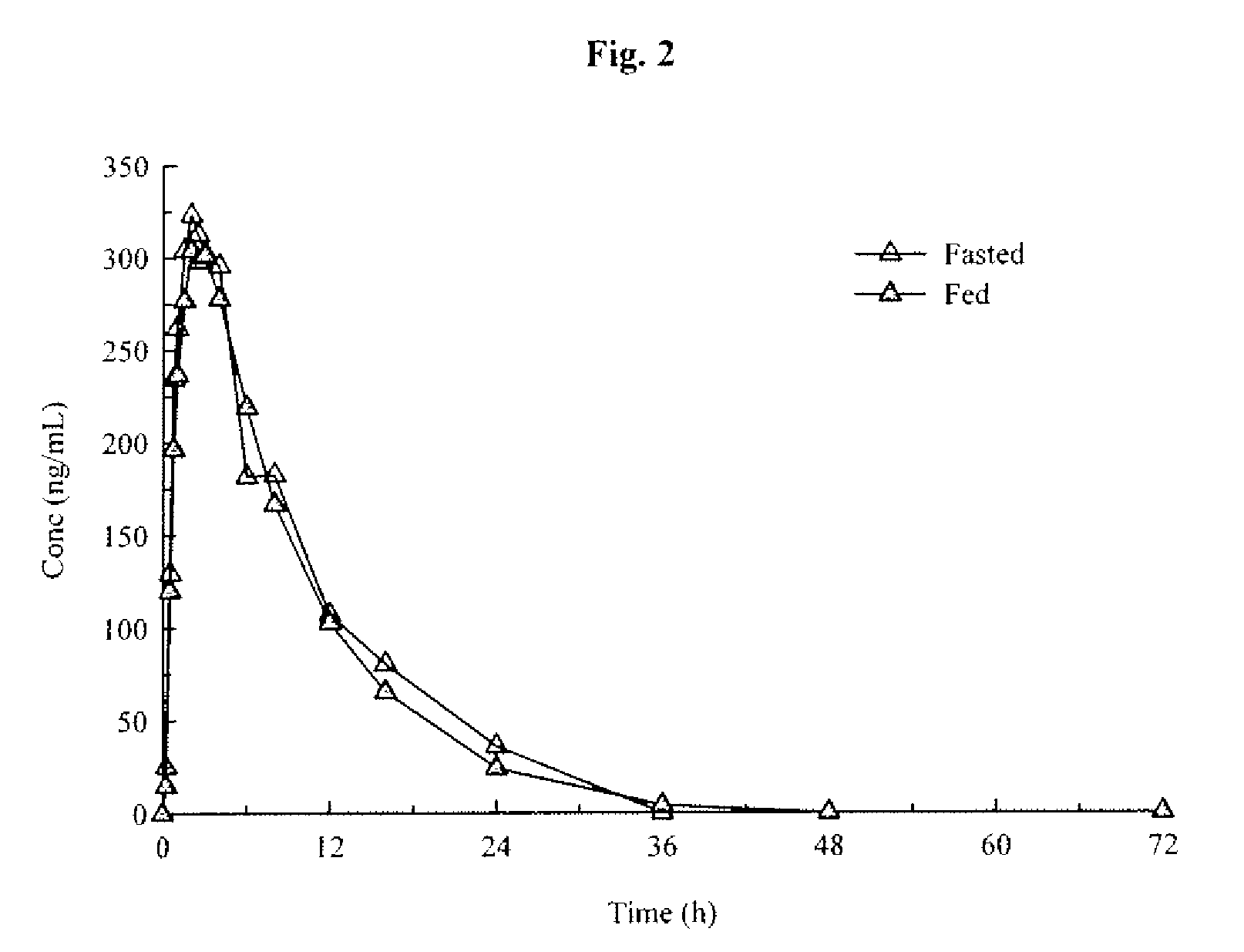
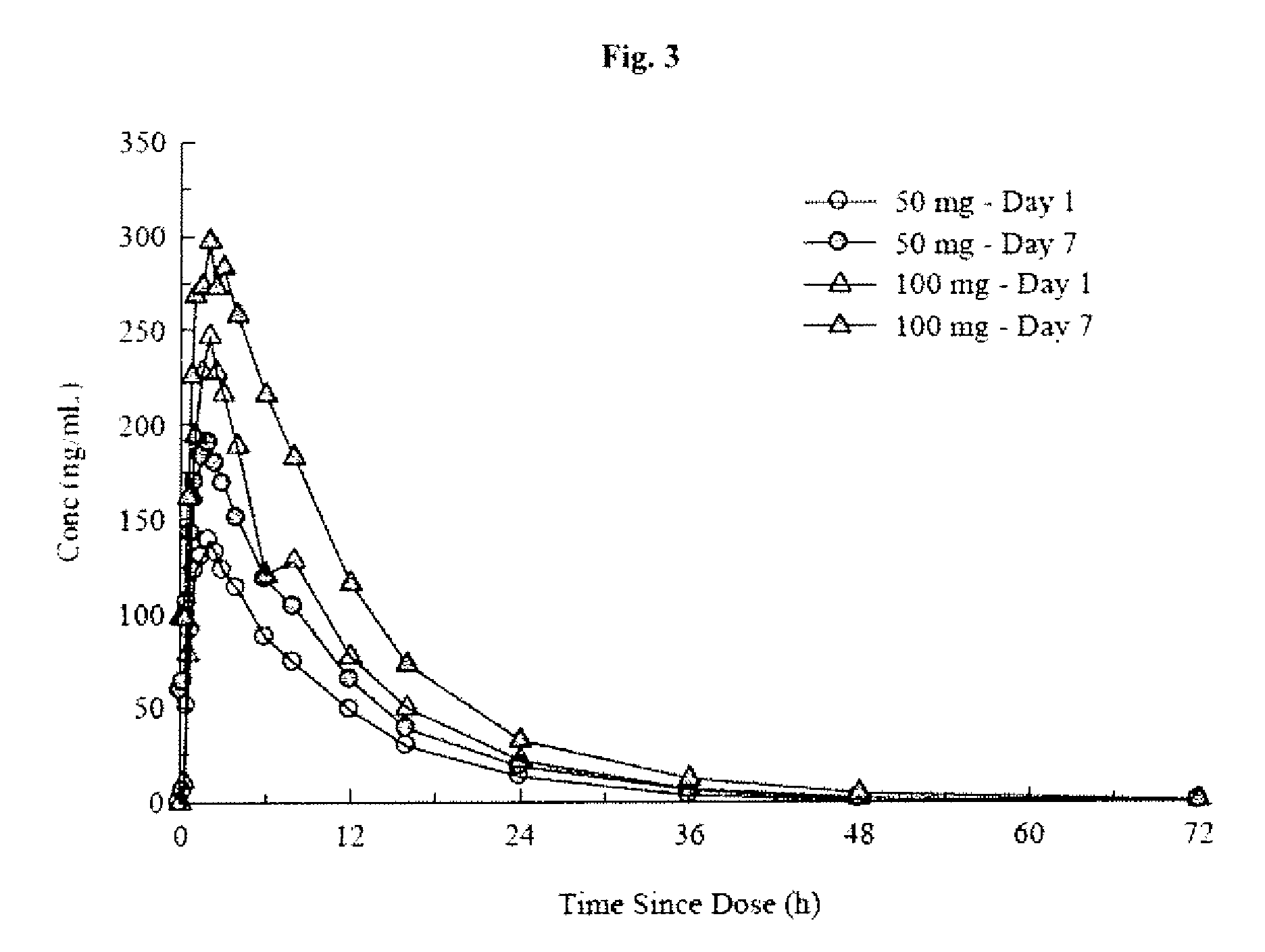
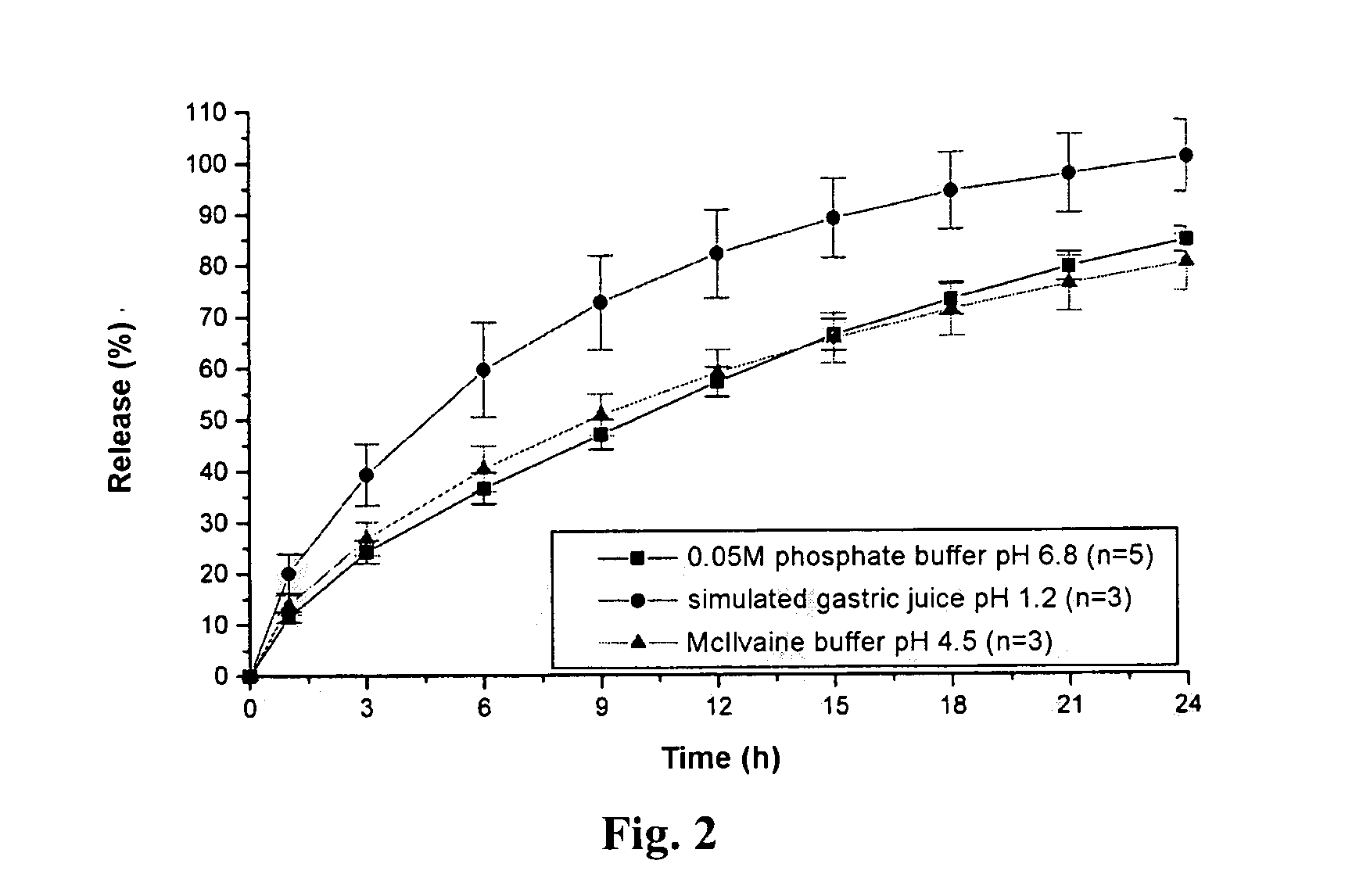
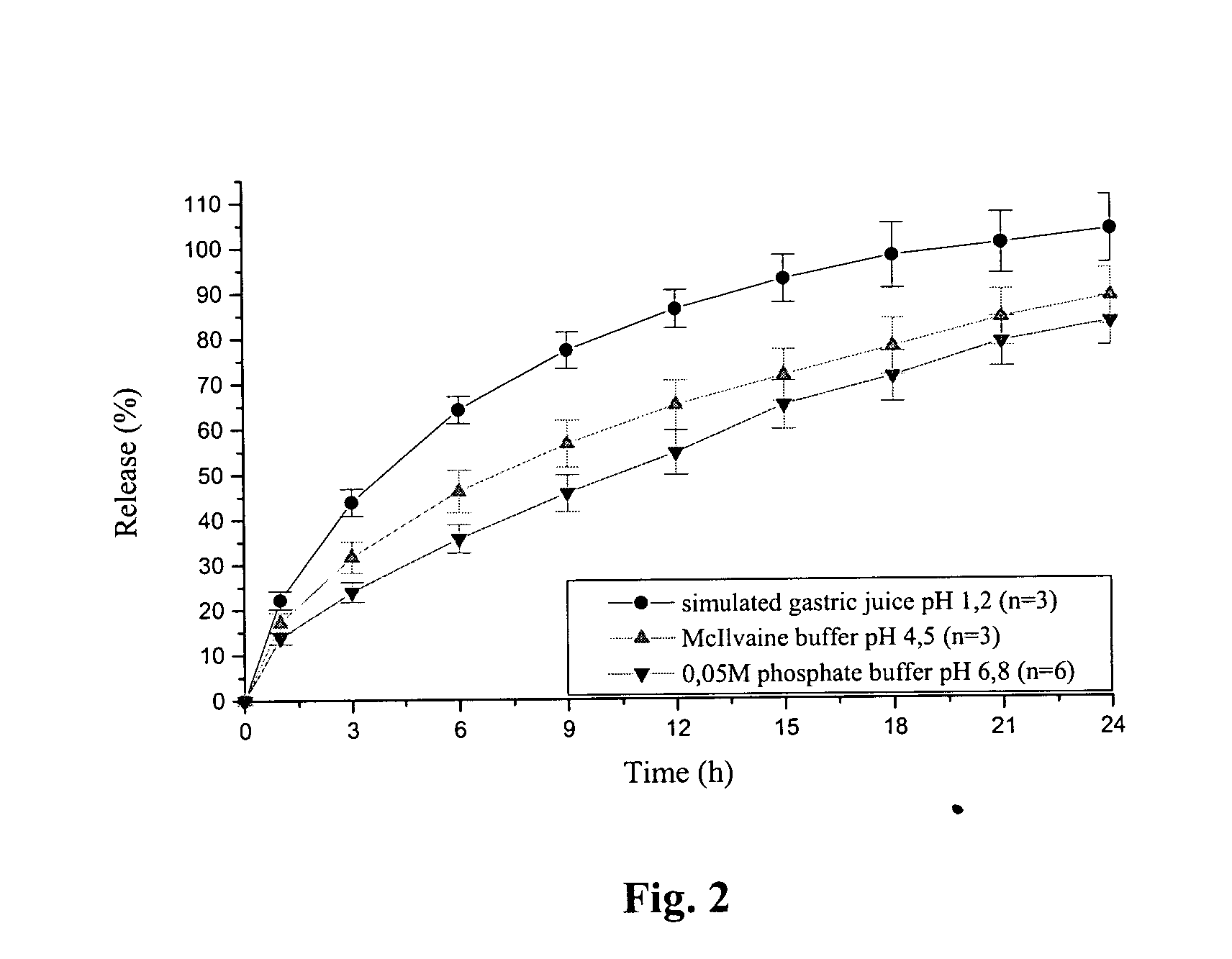
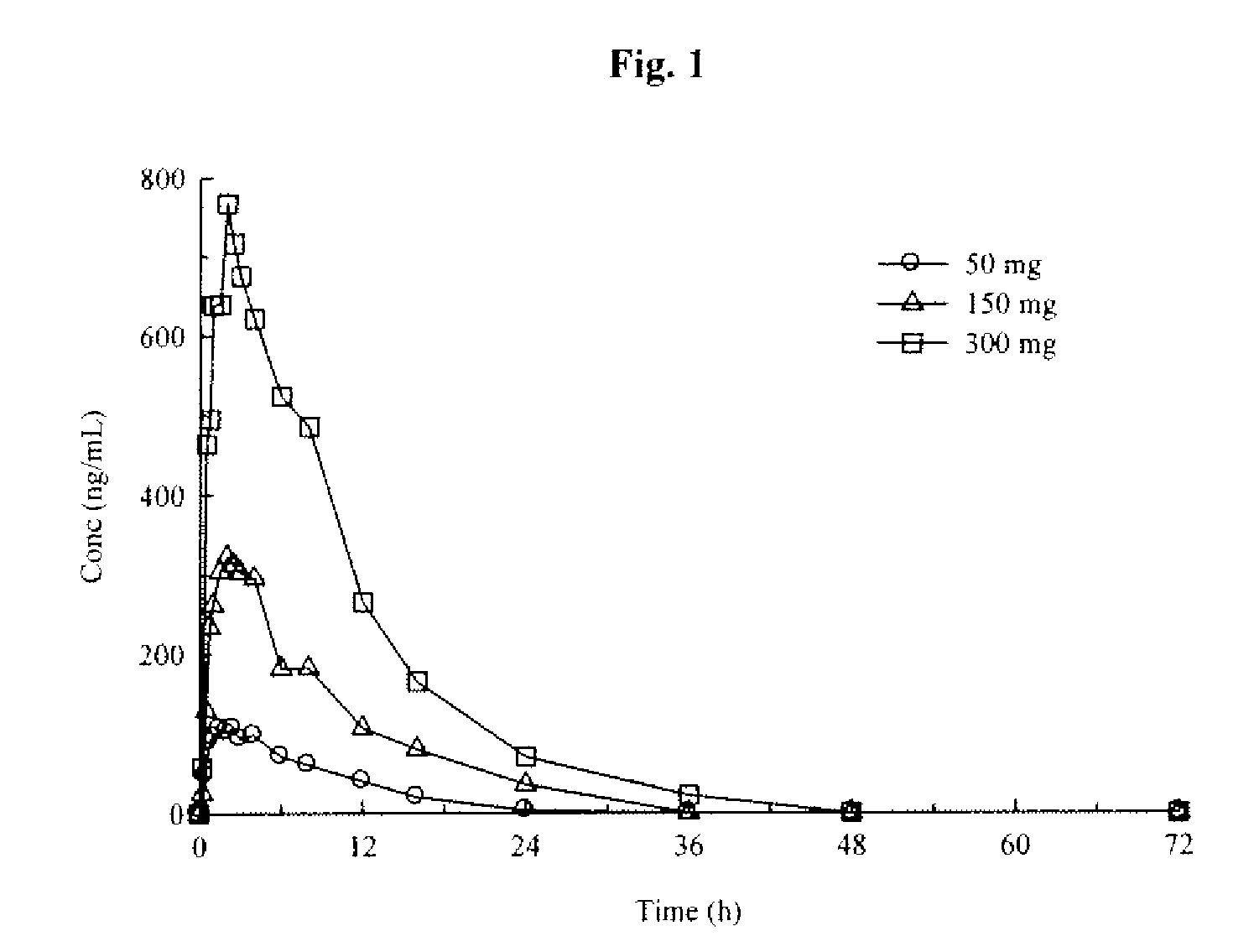
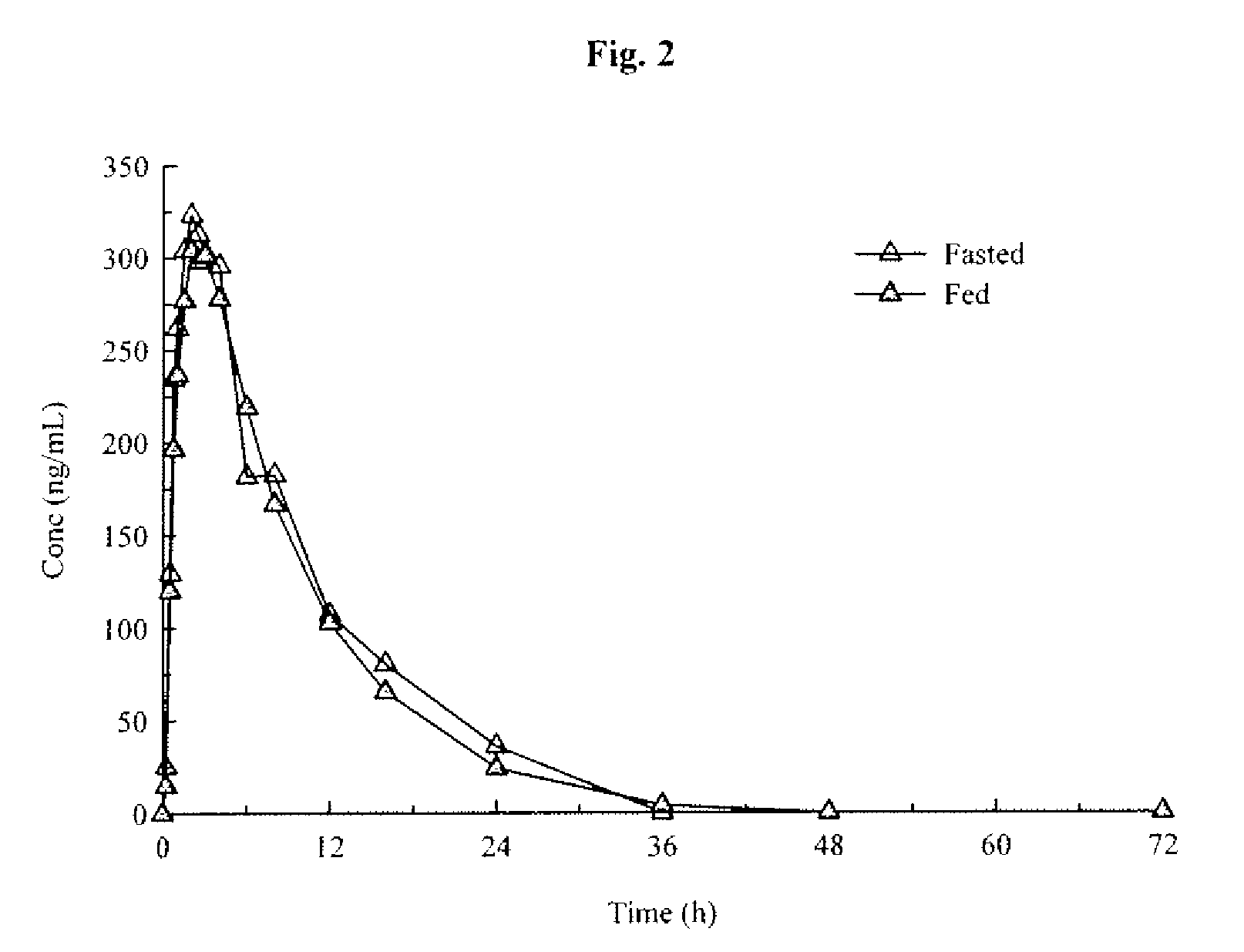
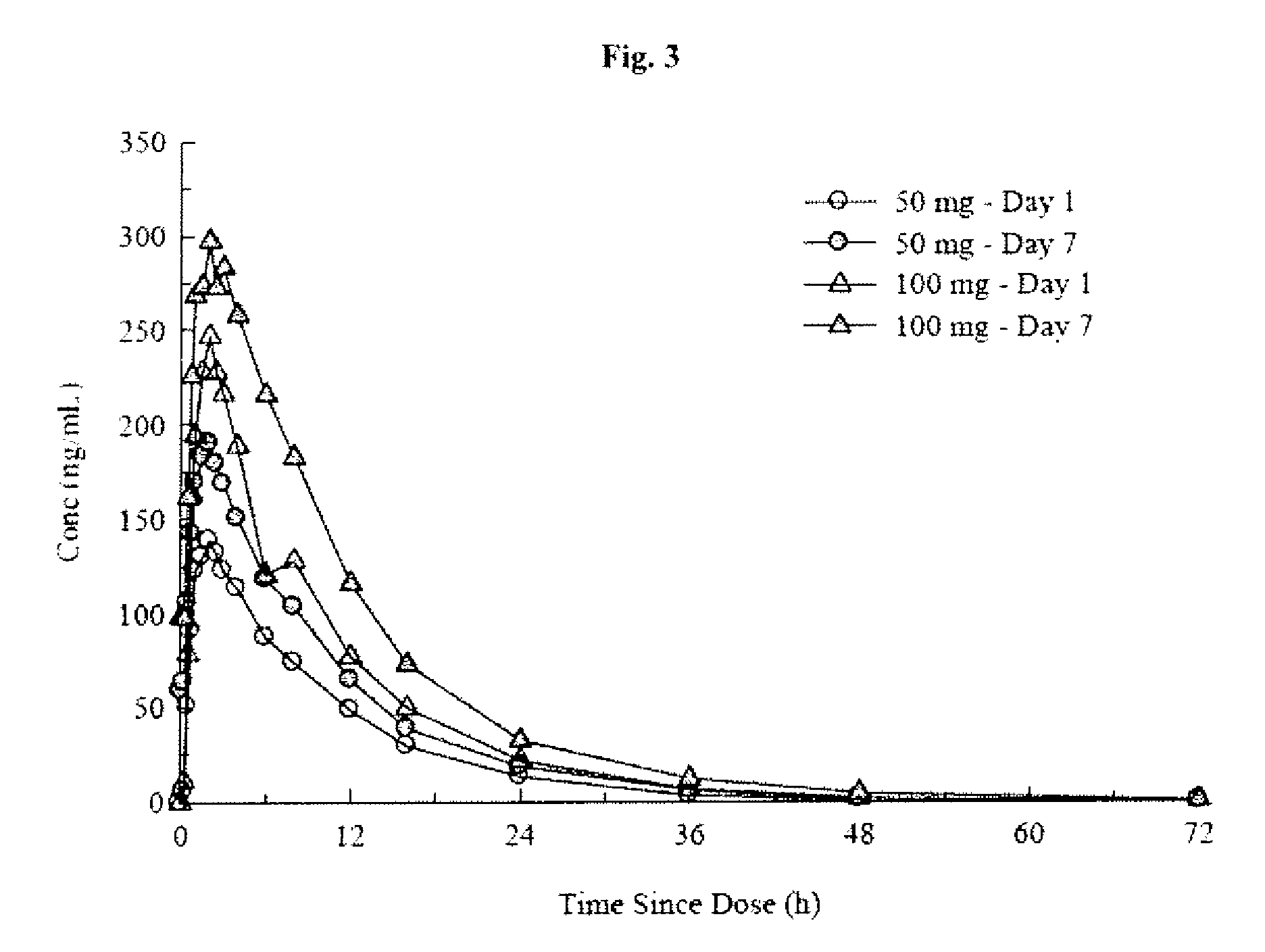
An orally deliverable pharmaceutical composition comprises a therapeutically effective amount of pramipexole or a pharmaceutically acceptable salt thereof and at least one pharmaceutically acceptable excipient, said composition exhibiting at least one of (a) an in vitro release profile wherein on average no more than about 20% of the pramipexole is dissolved within 2 hours after placement of the composition in a standard dissolution test; and (b) an in vivo pramipexole absorption profile following single dose administration to healthy adult humans wherein the time to reach a mean of 20% absorption is greater than about 2 hours and / or the time to reach a mean of 40% absorption is greater than about 4 hours. The composition is useful for oral administration, not more than once daily, to a subject having a condition or disorder for which a dopamine receptor agonist is indicated.
Owner:PHARMACIA CORP
Neurorestoration With R(+) Pramipexole
Formulations and methods of use thereof for restoring neuronal tissue function in children and adults afflicted with chronic neurodegenerative diseases, such as neurodegenerative movement disorders and ataxias, seizure disorders, motor neuron diseases, and inflammatory demyelinating disorders. Examples of disorders include Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). The method involves administering a pharmaceutical composition containing an effective amount of a tetrahydrobenzathiazole, preferably a formulation consisting substantially of the R(+) enantiomer of pramipexole. R(+) pramipexole is generally administered in doses ranging from 0.1-300 mg / kg / daily, preferably 0.5-50 mg / kg / daily, and most preferably 1-10 mg / kg / daily for oral administration. Daily total doses administered orally are typically between 10 mg and 500 mg. Alternatively, R(+) pramipexole can be administered parenterally to humans with acute brain injury in single doses between 10 mg and 100 mg, and / or by continuous intravenous infusions between 10 mg / day and 500 mg / day.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Neurorestoration with r(+) pramipexole
Formulations and methods of use thereof for restoring neuronal, muscular (cardiac and striated) and / or retinal tissue function in children and adults afflicted with chronic neurodegenerative diseases, such as neurodegenerative movement disorders and ataxias, seizure disorders, motor neuron diseases, and inflammatory demyelinating disorders, are described herein. Examples of disorders include Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). The method involves administering a pharmaceutical composition containing an effective amount of a tetrahydrobenzathiazole, preferably a formulation consisting substantially of the R(+) enantiomer of pramipexole. R(+) pramipexole is generally administered in doses ranging from 0.1-300 mg / kg / daily, preferably 0.5-50 mg / kg / daily, and most preferably 1-10 mg / kg / daily for oral administration. Daily total doses administered orally are typically between 10 mg and 500 mg. Alternatively, R(+) pramipexole can be administered parenterally to humans with acute brain injury in single doses between 10 mg and 100 mg, and / or by continuous intravenous infusions between 10 mg / day and 500 mg / day.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Neurorestoration with r(+) pramipexole
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof
InactiveUS20060051417A1Improve complianceImprove conveniencePowder deliveryOrganic active ingredientsExtended release tabletsPramipexole
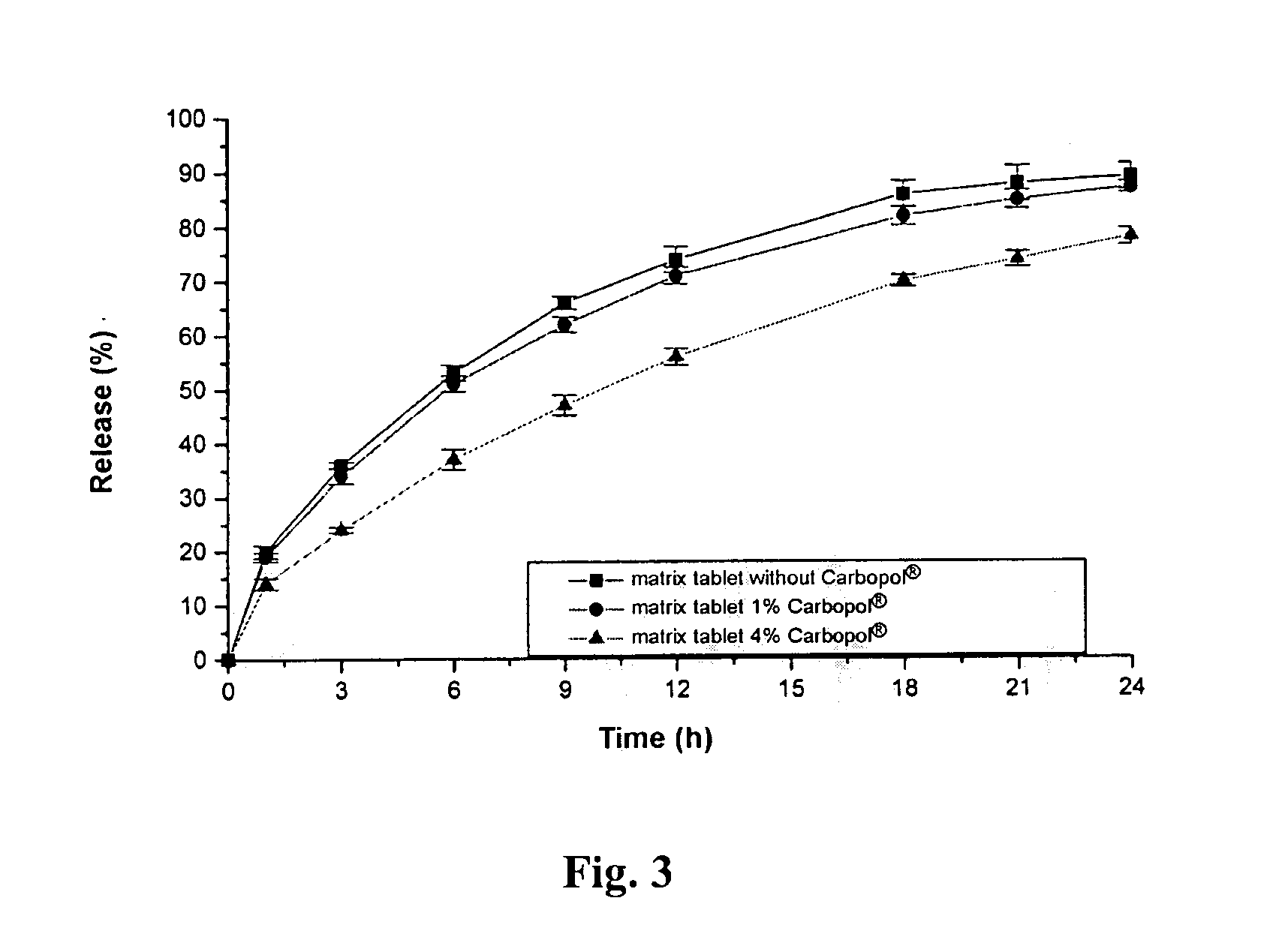
An extended release tablet formulation comprising pramipexole or a pharmaceutically acceptable salt thereof in a matrix, the matrix comprising at least two water swelling polymers, wherein one of the polymers is pregelatinized starch, and wherein another one of the polymers is an anionic polymer.
Owner:BOEHRINGER INGELHEIM INT GMBH
Implantable drug delivery compositions and methods of treatment thereof
Owner:BRAEBURN PHARMA INC
Pramipexole for the treatment of HIV dementia
InactiveUS20030166696A1Reduce formationReduce in quantityBiocideOrganic active ingredientsPramipexoleTherapy HIV
The invention relates to the use of pramipexole and the pharmacologically acceptable acid addition salts thereof as well as hydrates and solvates thereof, for preparing a pharmaceutical composition for the prevention and / or treatment of HIV encephalopathy.
Owner:BOEHRINGER INGELHEIM PHARM KG
Compositions of (r)-pramipexole and methods of using the same
InactiveCN102772404AUncovering Therapeutic PotentialOrganic active ingredientsNervous disorderPramipexolePharmacology
Compositions of predetermined amounts of R(+) pramipexole and S(-) pramipexole and methods of using the same, including for the treatment and prevention of Parkinson's disease, are provided.
Owner:KNOPP BIOSCIENCES LLC
Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof
ActiveUS20060198887A1Improve complianceImprove conveniencePowder deliveryOrganic active ingredientsExtended release tabletsPramipexole
Owner:BOEHRINGER INGELHEIM INT GMBH
Modified Release Formulation
InactiveUS20090041844A1Improve complianceImprove convenienceBiocideOrganic active ingredientsExtended release tabletsPramipexole
Owner:BOEHRINGER INGELHEIM INT GMBH
Preparation method of pramipexole
InactiveCN101676272AEasy to operateMild reaction conditionsNervous disorderOrganic chemistryEnantiomerPramipexole
The invention discloses a preparation process of pramipexole, namely enantiomers or racemates of 2-amino-6-acrylamido-4,5,6,7-tetrahydrobenzothiazole. The pramipexole is prepared from 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole as the raw material by the reduction reaction with a reducing agent. The process has easy operation, and mild reaction condition, is easy to control, and greatly improve the safety of production.
Owner:BEIJING D VENTUREPHARM TECH DEV
Compositions and methods of using (r)-pramipexole
Pharmaceutical compositions of (R)-pramipexole and methods and kits of using such compositions for the treatment of neurodegenerative diseases, or those related to mitochondrial dysfunction or increased oxidative stress are disclosed.
Owner:KNOPP BIOSCIENCES LLC
Extended Release Formulation
InactiveUS20090098202A1Steady state plasma concentrations of the drugEffective and tolerableBiocideOrganic active ingredientsPramipexolePharmacology
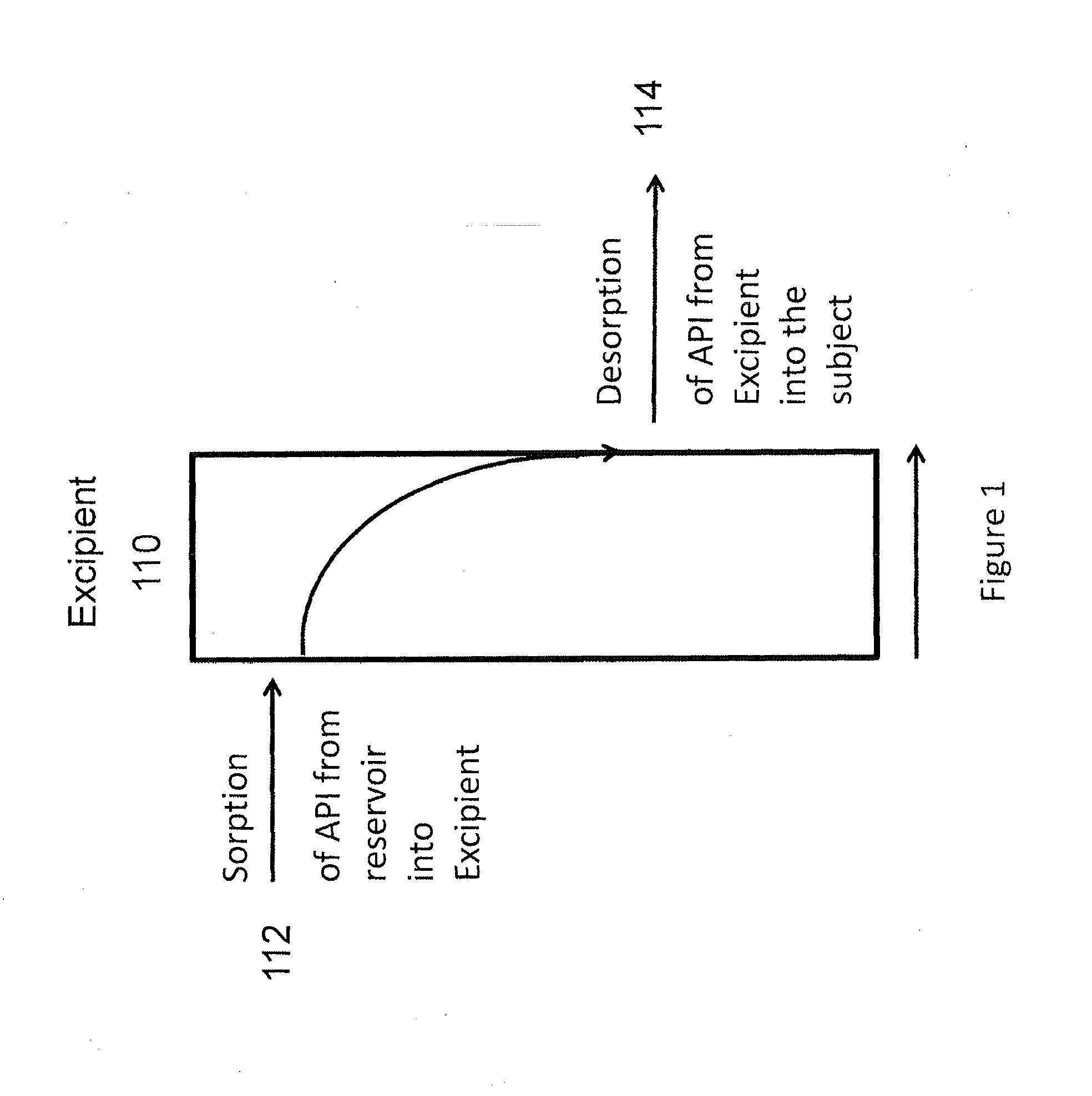
The invention is directed to an extended release formulation comprising pramipexole or a pharmaceutically acceptable salt thereof.
Owner:BOEHRINGER INGELHEIM INT GMBH
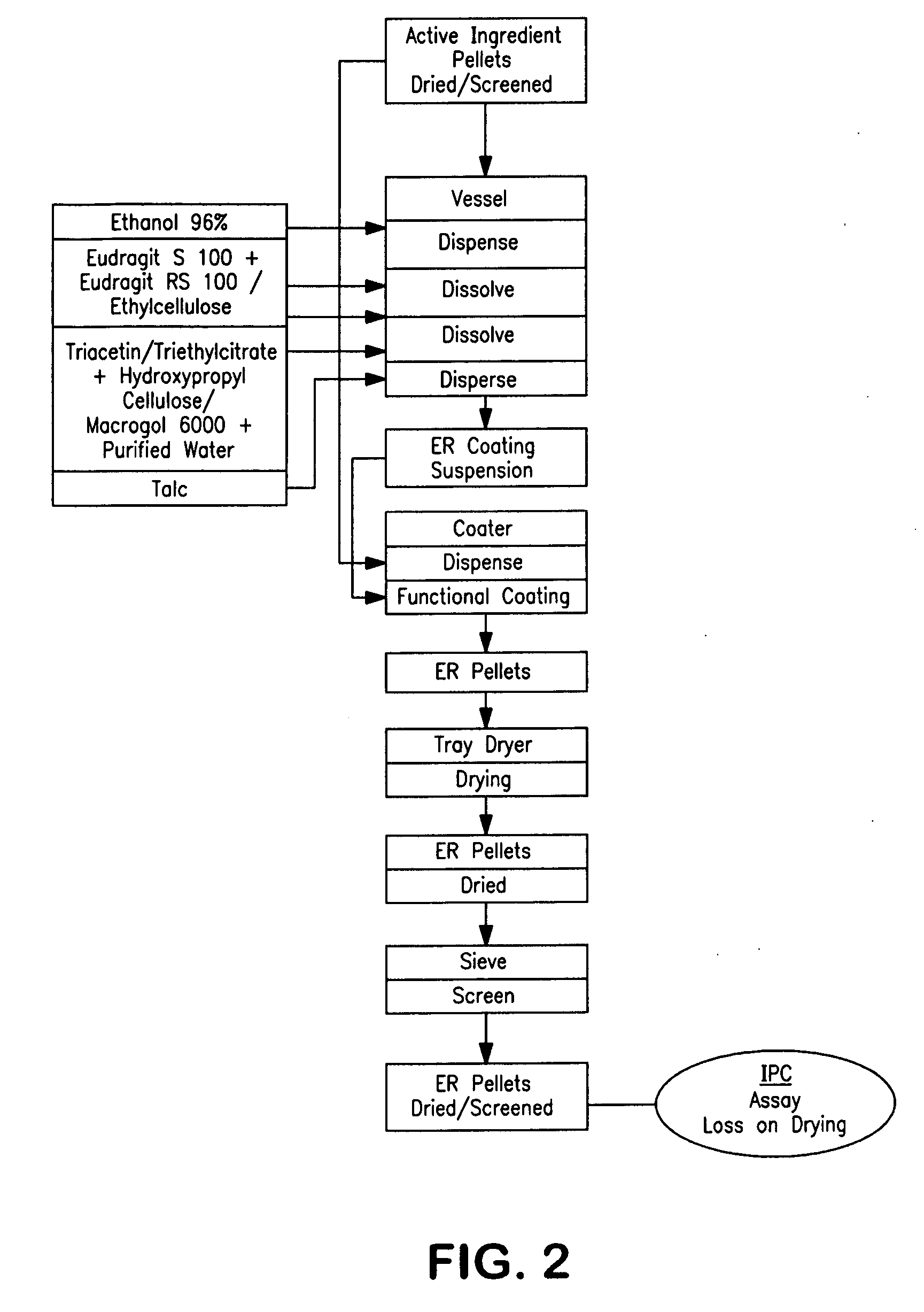
Extended release pellet formulation containing pramipexole or a pharmaceutically acceptable salt
InactiveUS20090130197A1Improve complianceImprove convenienceOrganic active ingredientsBiocidePramipexoleBULK ACTIVE INGREDIENT
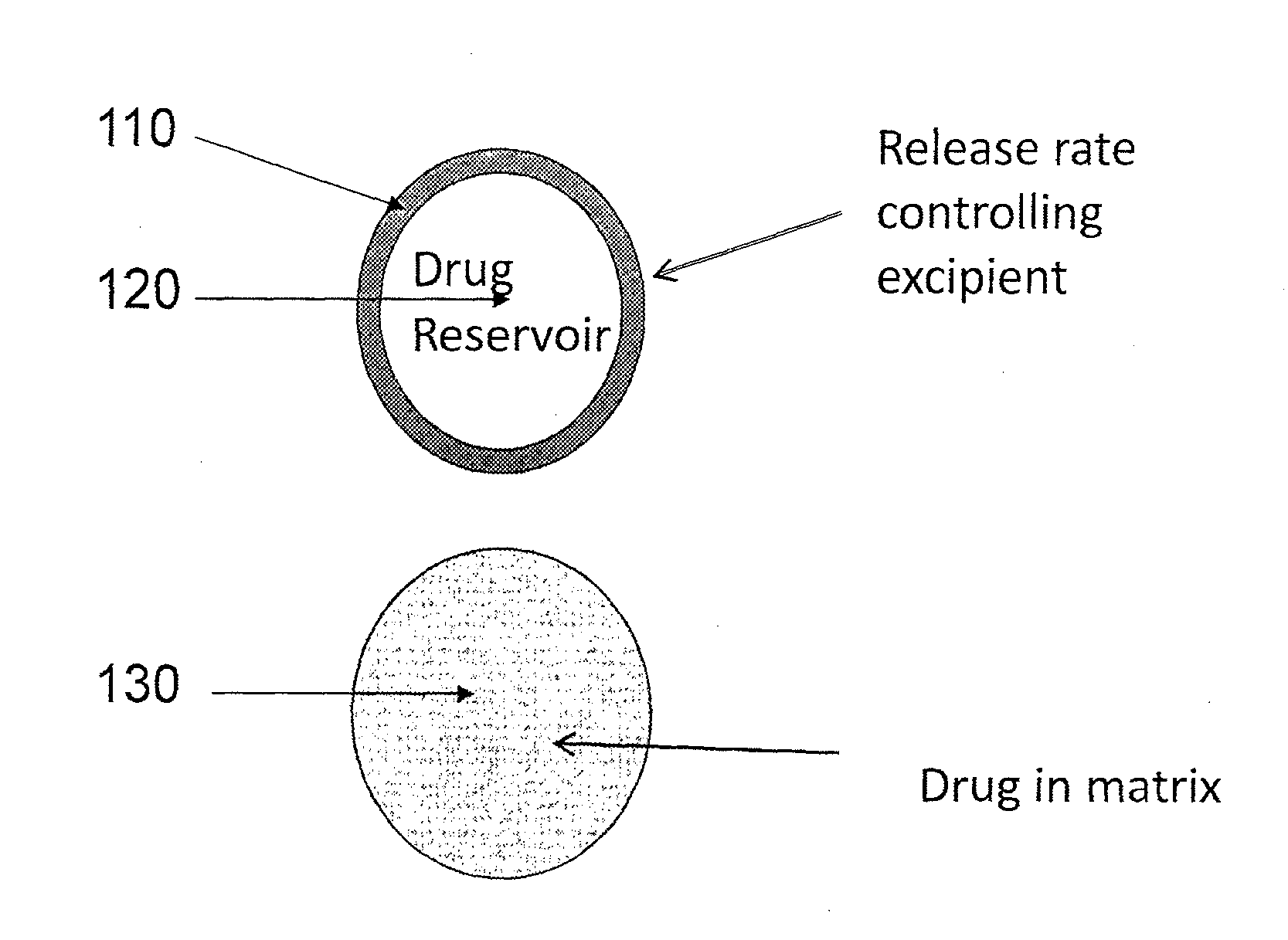
An extended release pellet comprising an active ingredient selected from pramipexole and the pharmaceutically acceptable salts thereof, and at least one release-modifying excipient.
Owner:FRIEDL THOMAS +1
Preparation method for 4-substituted acylamino cyclohexanone
InactiveCN102584618AReasonable routingMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationCyclohexanoneChemical synthesis
The invention relates to a method for preparing 4- substituted acylamino cyclohexanone through oxidizeing 4- substituted acylamino cyclohexanol. The 4- substituted acylamino cyclohexanone prepared by the method can be used for preparing Pramipexole. The method comprises the following steps: adding 4- substituted acylamino cyclohexanol as is shown in formula I, catalyst 2, 2, 6, 6- tetramethyl piperidine - nitrogen - oxide, NaBr, into organic solvent, then dropwise adding sodium hypochlorite water solution for reaction until oxidation reaction is complete, and obtaining the 4- substituted acylamino cyclohexanone shown in formula II after separation and purification to reaction solution; and the method provided by the invention mainly has the following benefits: a novel chemical synthesis method for producing key intermediate of medicine Pramipexole is found, the route is enabled to be more reasonable, the reaction conditions are milder, the yield is higher, and the method is environment-friendly and suitable for large scale industrial production.
Owner:ZHEJIANG UNIV OF TECH +2
Compositions and methods of using (R)-pramipexole
Pharmaceutical compositions of (R)-pramipexole and methods and kits of using such compositions for the treatment of neurodegenerative diseases, or those related to mitochondrial dysfunction or increased oxidative stress are disclosed.
Owner:KNOPP BIOSCIENCES LLC
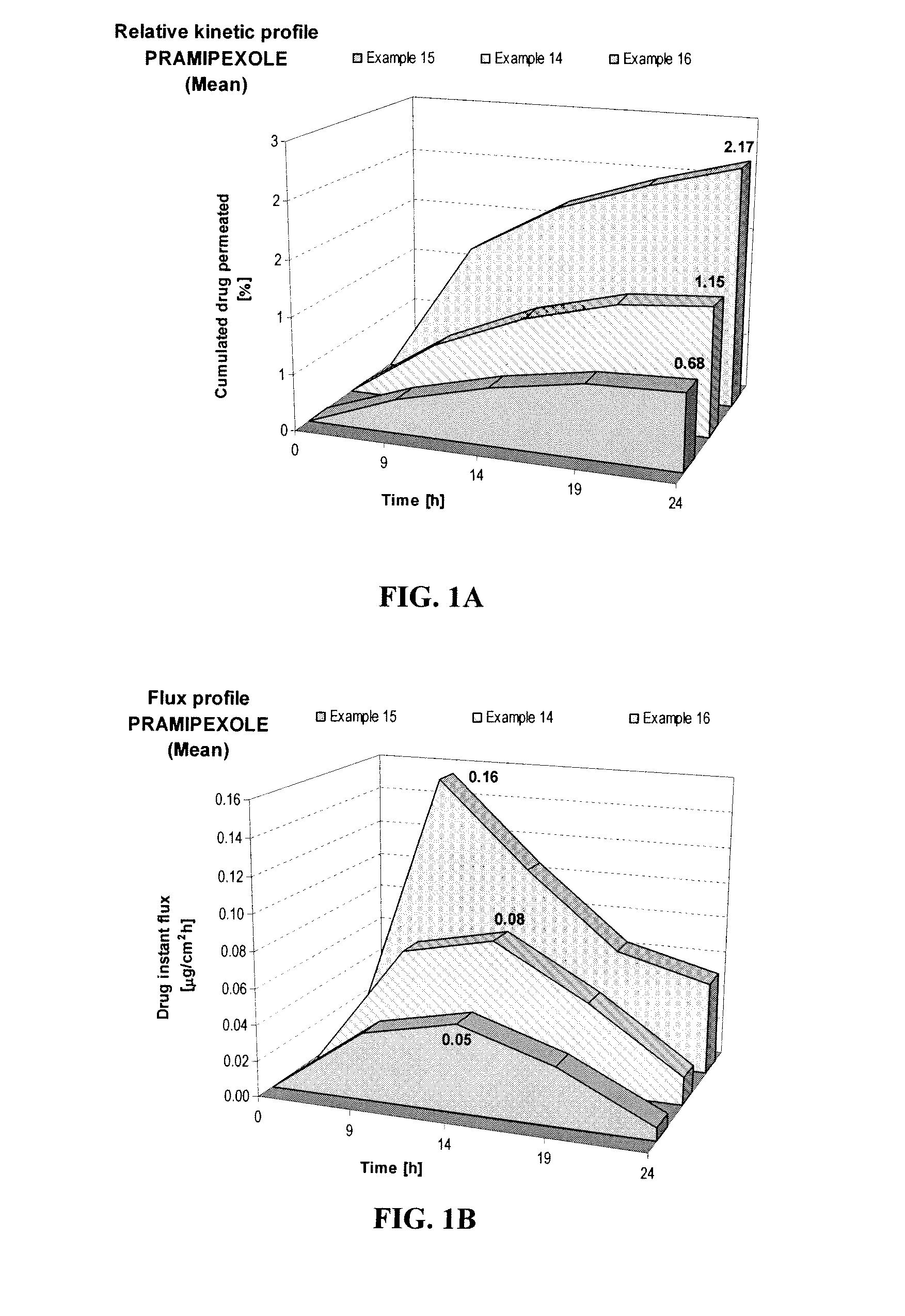
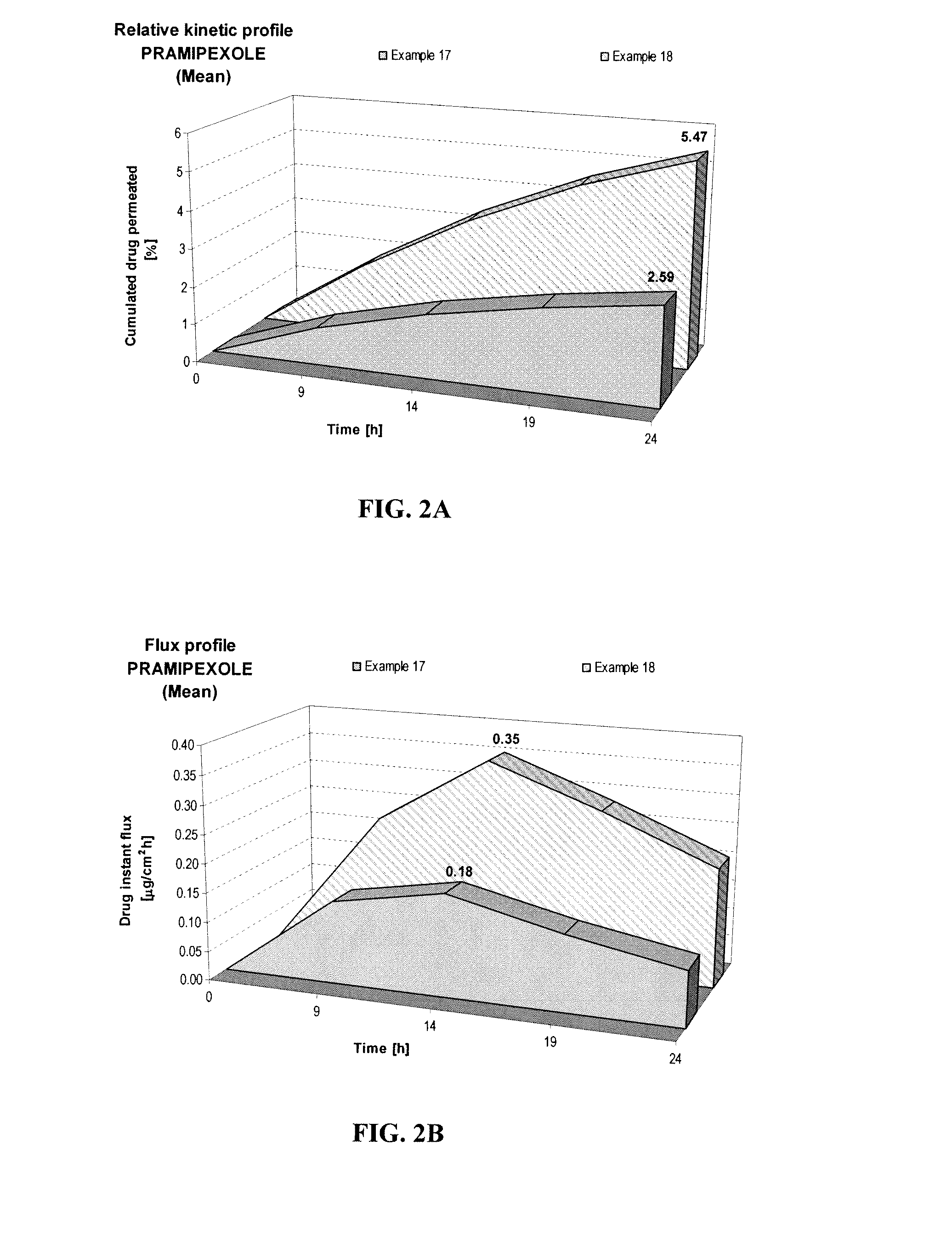
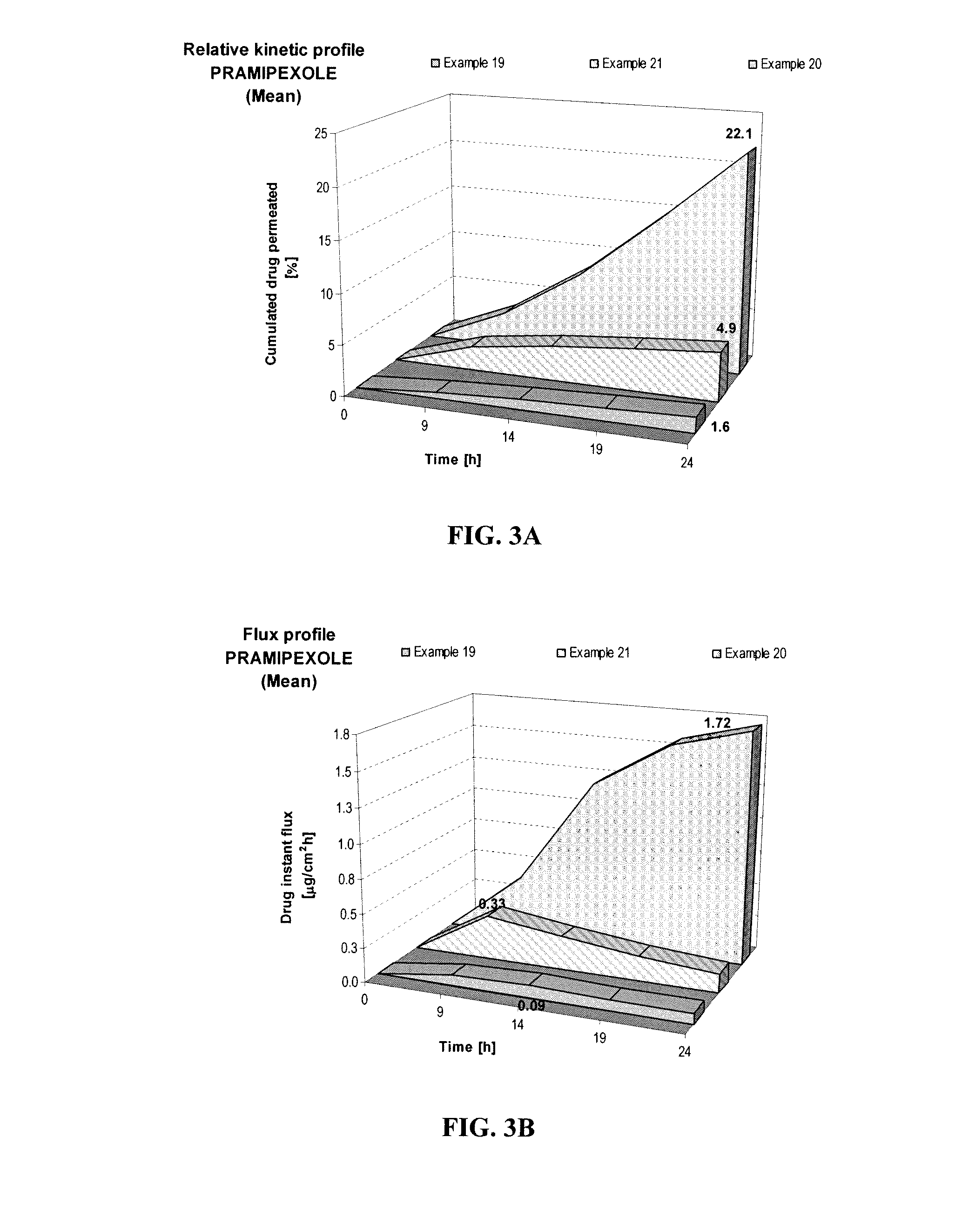
Transdermal compositions of pramipexole having enhanced permeation properties
A pharmaceutical composition for transdermal or transmucosal delivery of an active agent to treat a movement disorder such as Parkinson's disease. The composition provides enhanced transdermal or transmucosal delivery of the active agent by including an alkanolamine as a permeation enhancer with a carrier of water and at least one short-chain alcohol and with the composition having a neutral pH. The composition provides controlled and sustained release of the active agent suitable for daily administration.
Owner:ANTARES PHARMA IPL
Preparation method for pramipexole preparation
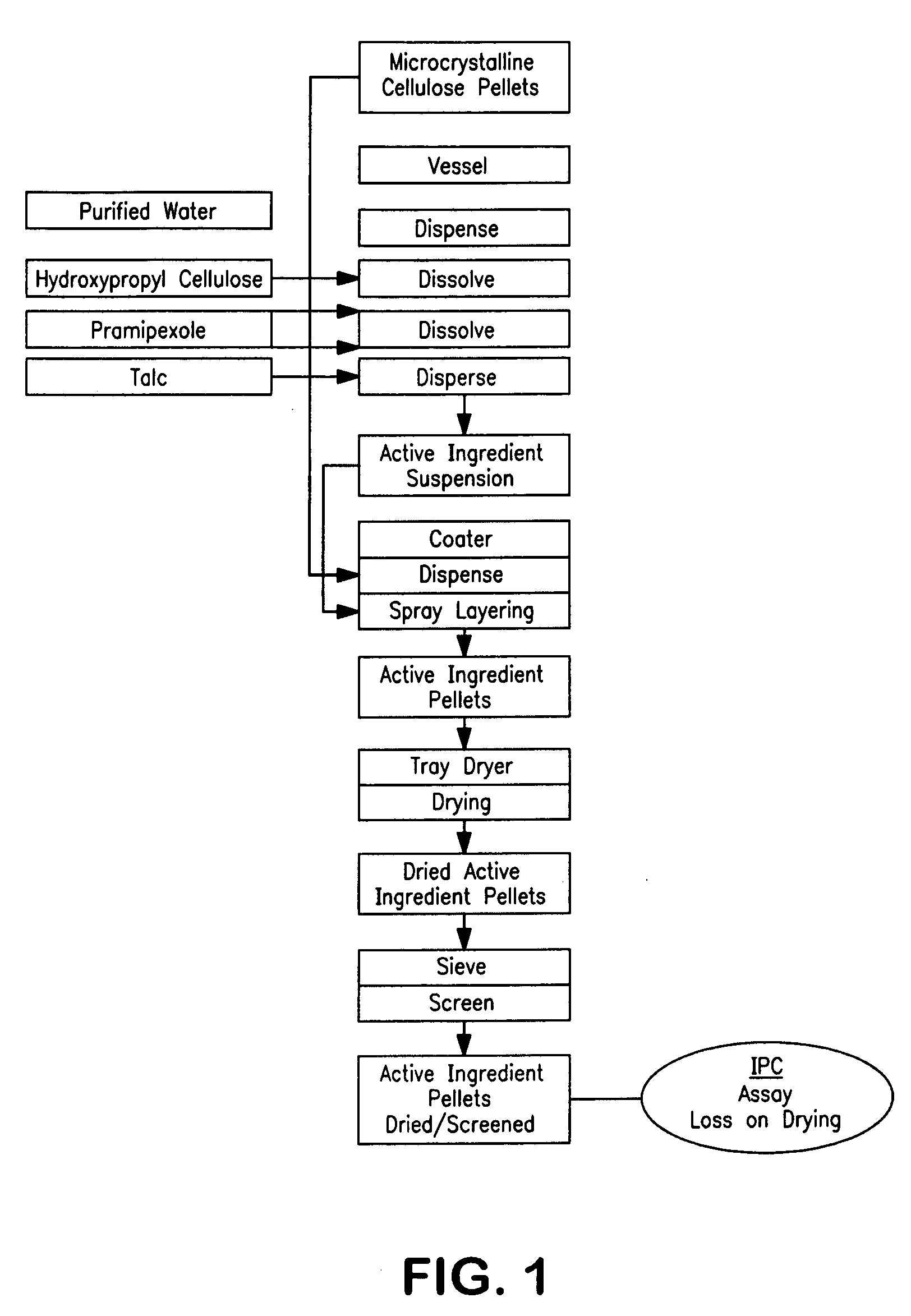
ActiveCN102772403AReduce liquidityGood compressibilityOrganic active ingredientsPharmaceutical non-active ingredientsPramipexoleActive component
The invention relates to a pramipexole preparation which comprises pramipexole or a medicinal salt thereof which accounts for 0.05 to 1.5% of the total weight of the preparation and is used as an active component, microcrystalline cellulose and pregelatinized starch, wherein a weight ratio of microcrystalline cellulose and pregelatinized starch is 1:1 to 1: 5. The invention also relates to a preparation method for the pramipexole preparation. The preparation prepared by using the preparation method has low hygroscopicity and is beneficial for storage; and the preparation method has good production feasibility and is suitable for commercial production.
Owner:ZHEJIANG JINGXIN PHARMA
Modified release formulations of (6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine and methods of using the same
Modified release pharmaceutical compositions (controlled release, sustained release, and / or extended release) of the R-(+) enantiomer of pramipexole (RPPX) and methods of using such compositions for the treatment of neurodegenerative diseases, or those related to mitochondrial dysfunction or increased oxidative stress are disclosed.
Owner:KNOPP BIOSCIENCES LLC
Method for treating restless leg syndrome using pramipexole and clonidine
InactiveUS20020010201A1High response rateHigh activityOrganic active ingredientsBiocidePramipexolePharmacology
The invention relates to an active substance combination consisting of clonidine and pramipexole for treating Restless Leg Syndrome.
Owner:BRECHT HANS MICHAEL
Pramipexole sustained-release preparation for injection and preparation method thereof
ActiveCN108685858AExcellent temperature sensitive performanceImprove complianceOrganic active ingredientsPowder deliveryOrganic solventOil emulsion
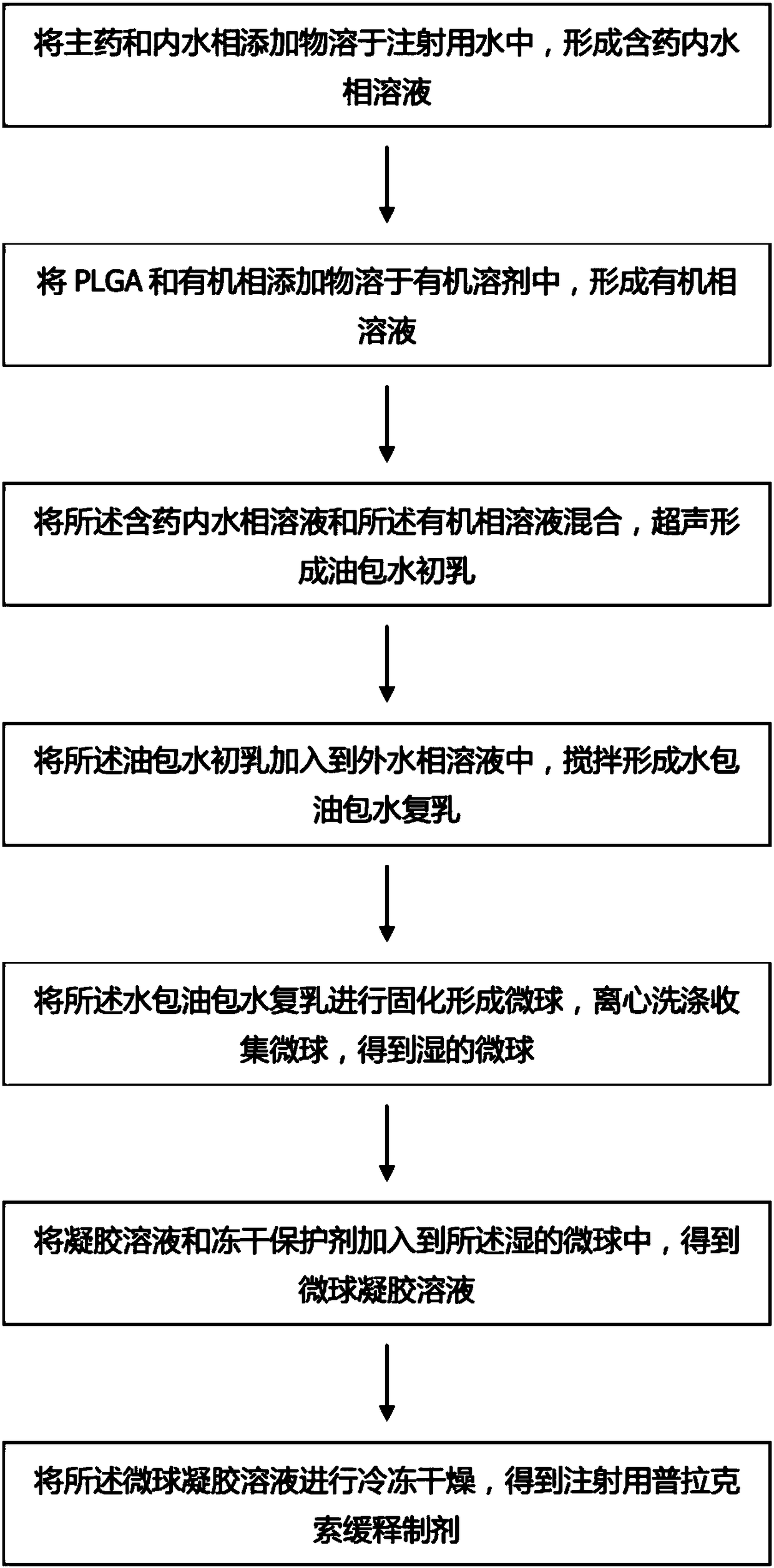
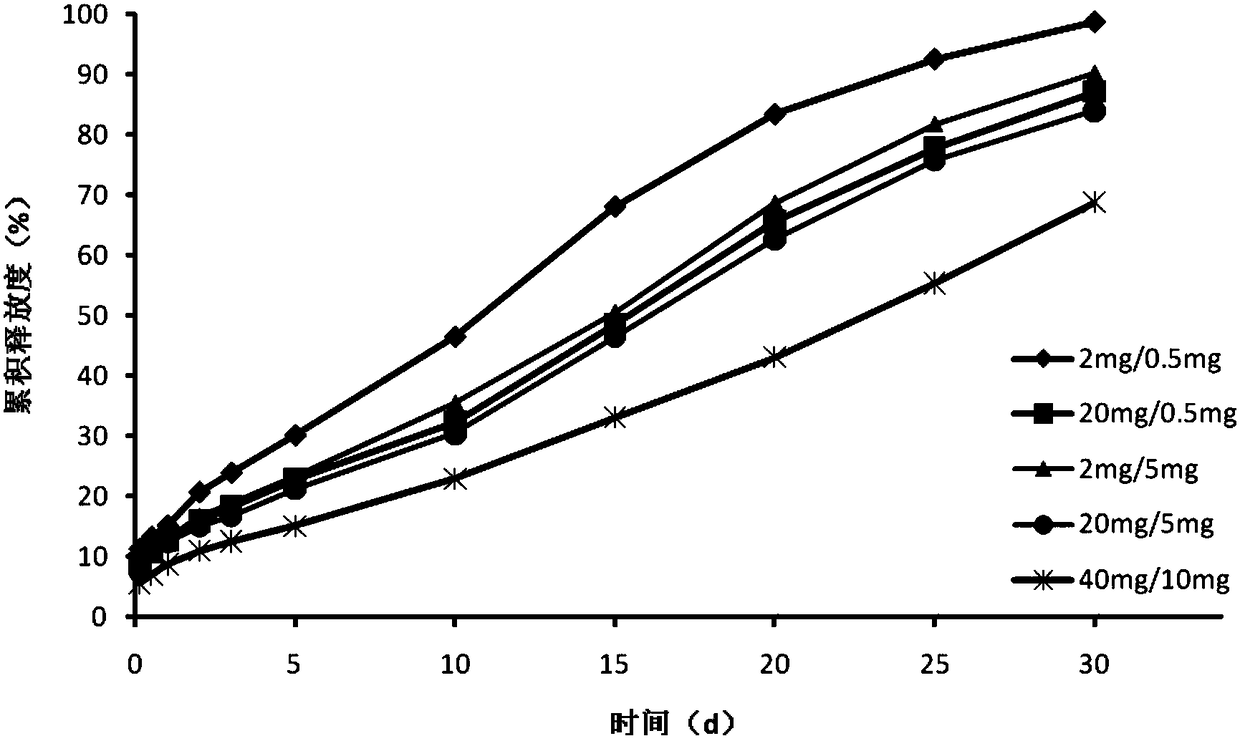
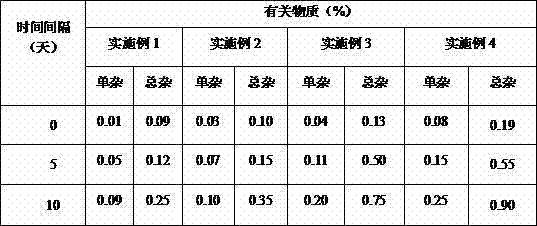
The invention relates to a pramipexole sustained-release preparation for injection and a preparation method thereof, belonging to the technical field of medicines. The preparation method comprises thefollowing steps: dissolving a main drug and an internal water phase additive into water for injection so as to form a main drug-containing internal water phase solution; dissolving PLGA and an organic phase additive into an organic solvent so as to form an organic phase solution; mixing the main drug-containing internal water phase solution with the organic phase solution and carrying out ultrasonic treatment so as to form a primary water-in-oil emulsion; adding the primary water-in-oil emulsion into an external water phase solution, and carrying out stirring so as to form a water-in-oil-in-water multiple emulsion; solidifying the water-in-oil-in-water multiple emulsion so as to form microspheres, carrying out centrifugal washing, and collecting the microspheres so as to obtain wet microspheres; adding a gel solution and a freeze-drying protective agent into the wet microspheres so as to obtain a microsphere gel solution; and subjecting the microsphere gel solution to freeze-drying. The pramipexole sustained-release preparation for injection provided by the invention has high drug loading capacity and good temperature sensitivity and biological compatibility, sustainably and stably releases drugs, can effectively reduce the occurrence of motor complications, and improves the compliance of a patient.
Owner:SHENYANG PHARMA UNIVERSITY
A transdermal patch containing pramipexole
ActiveCN103432104BImprove uniformityImprove solubilityOrganic active ingredientsNervous disorderHigh concentrationTransdermal patch
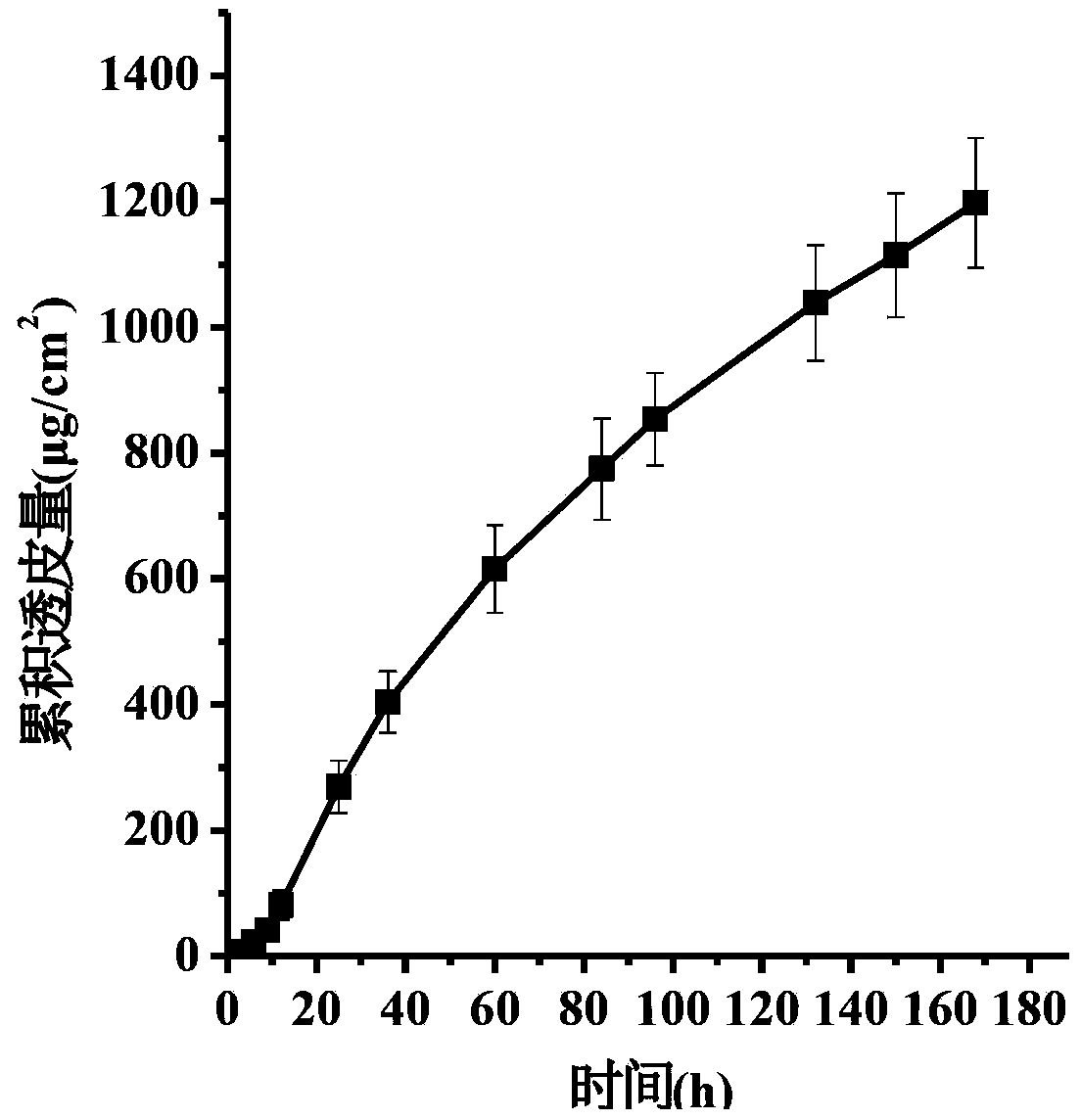
The invention discloses a transdermal patch containing pramipexole. The transdermal patch comprises a medicine carrying pressure-sensitive adhesive layer, the pramipexole, solvent and penetration enhancer, wherein the medicine carrying pressure-sensitive adhesive layer comprises acrylate pressure-sensitive adhesive containing carboxyl base groups and acrylate pressure-sensitive adhesive containing hydroxyl base groups, which form mixed pressure-sensitive adhesive; the pramipexole is dissolved in the acrylate mixed pressure-sensitive adhesive, with the content being 10 to 30 weight percent; the content of the mixed pressure-sensitive adhesive is 50 to 80 weight percent; the content of the solvent is 5 to 20 weight percent; the content of the penetration enhancer is 2 to 15 weight percent. The pramipexole can be dissolved in a mixed pressure-sensitive adhesive patch in a high concentration way and is not crystallized, the medicine availability is high, the pramipexole has good stability in pressure-sensitive adhesive matrix, and can be continuously administrated for 5 to 7 days in a transdermal way at a relatively stable permeation rate of being larger than 5.0 micrograms / cm2 / h, and the application area of a pramipexole patch is smaller than 40cm2.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Pramipexole oral liquid and preparation method thereof
The present invention belongs to the technical field of medicine, and provides a pramipexole oral liquid and a preparation method thereof. Pramipexole is a dopamine D2 receptor agonist for treatment of Parkinson's disease; early symptoms of the Parkinson's disease comprise static tremor, myotonia, bradykinesia, and abnormal posture and gait; and moderate and advanced symptoms of the Parkinson's disease patients often comprise difficult chewing and swallowing. With the pramipexole oral liquid of the present invention, medication compliance of patients is easily improved, and a therapeutic effect is improved.
Owner:BEIJING WANQUAN SUNSHINE MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com