Pramipexole sustained-release preparation for injection and preparation method thereof
A technology of pramipexole and sustained-release preparations, applied in the field of medicine, can solve problems such as difficult to control exercise-related symptoms, easily lead to exercise complications, and reduce patient compliance, so as to improve compliance, improve biocompatibility, and sustain The effect of steady drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
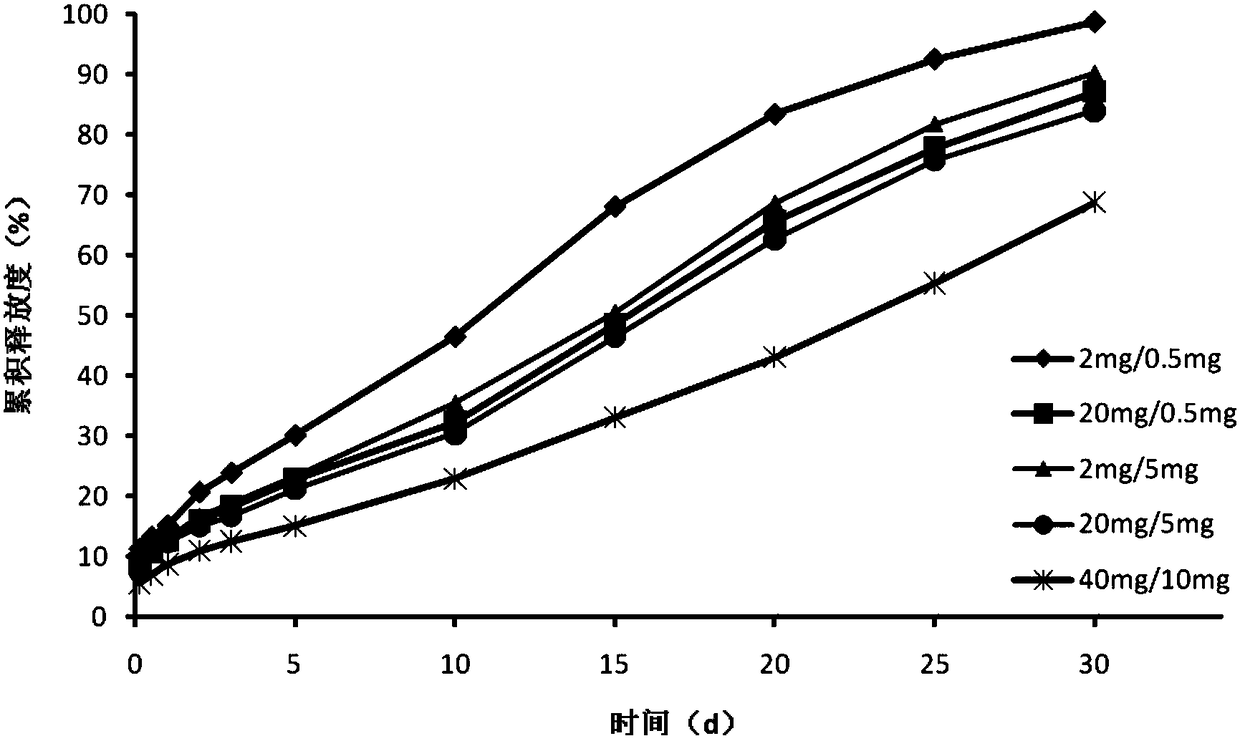
[0060] This example provides a pramipexole sustained-release preparation for injection, which is composed of the following raw materials: pramipexole hydrochloride 150mg, internal aqueous phase additive 25mg, PLGA 0.75g, organic phase additive 0.25g, poloxa 200mg / ml Mu 188 and 20mg / ml chitosan gel solution 10ml, mannitol 100mg.
[0061] The pramipexole hydrochloride is a dopamine receptor agonist, which is highly selective and specific in binding to the D2 subfamily of dopamine receptors, has preferential affinity for the D2 receptors, and has complete intrinsic activity. Dopamine receptors in the striatum alleviate motor impairment in Parkinson's disease. The half-life of the pramipexole hydrochloride is short, being 8-12 hours.
[0062] Described poloxamer (Poloxamer) is polyoxyethylene-polyoxypropylene block copolymer, trade name is Pluronic (Pluronic), according to the proportioning of ethylene oxide and propylene oxide in polymerization, poloxamer There are a series of ...
Embodiment 2
[0125] This embodiment provides a pramipexole sustained-release preparation for injection, which consists of the following raw materials: 150 mg of pramipexole hydrochloride, 25 mg of internal aqueous phase additives, 1.0 g of PLGA, 0.25 g of organic phase additives, 10 ml of gel solution, manna Alcohol 100mg.
[0126] The internal water phase additive is a mixture of poloxamer and chitosan, wherein the mass ratio of poloxamer shell to polysaccharide is 20mg / 5mg.
[0127] The molar ratio of lactide and glycolide in the PLGA is 75 / 25, and the molecular weight of the PLGA is 50kDa.
[0128] The organic phase additive is a mixture of poloxamer 407 and chitosan, wherein the mass ratio of poloxamer 407 to chitosan is 0.2g / 0.05g.
[0129] The gel solution was formed from 150 mg / ml poloxamer 188 and 75 mg / ml chitosan.
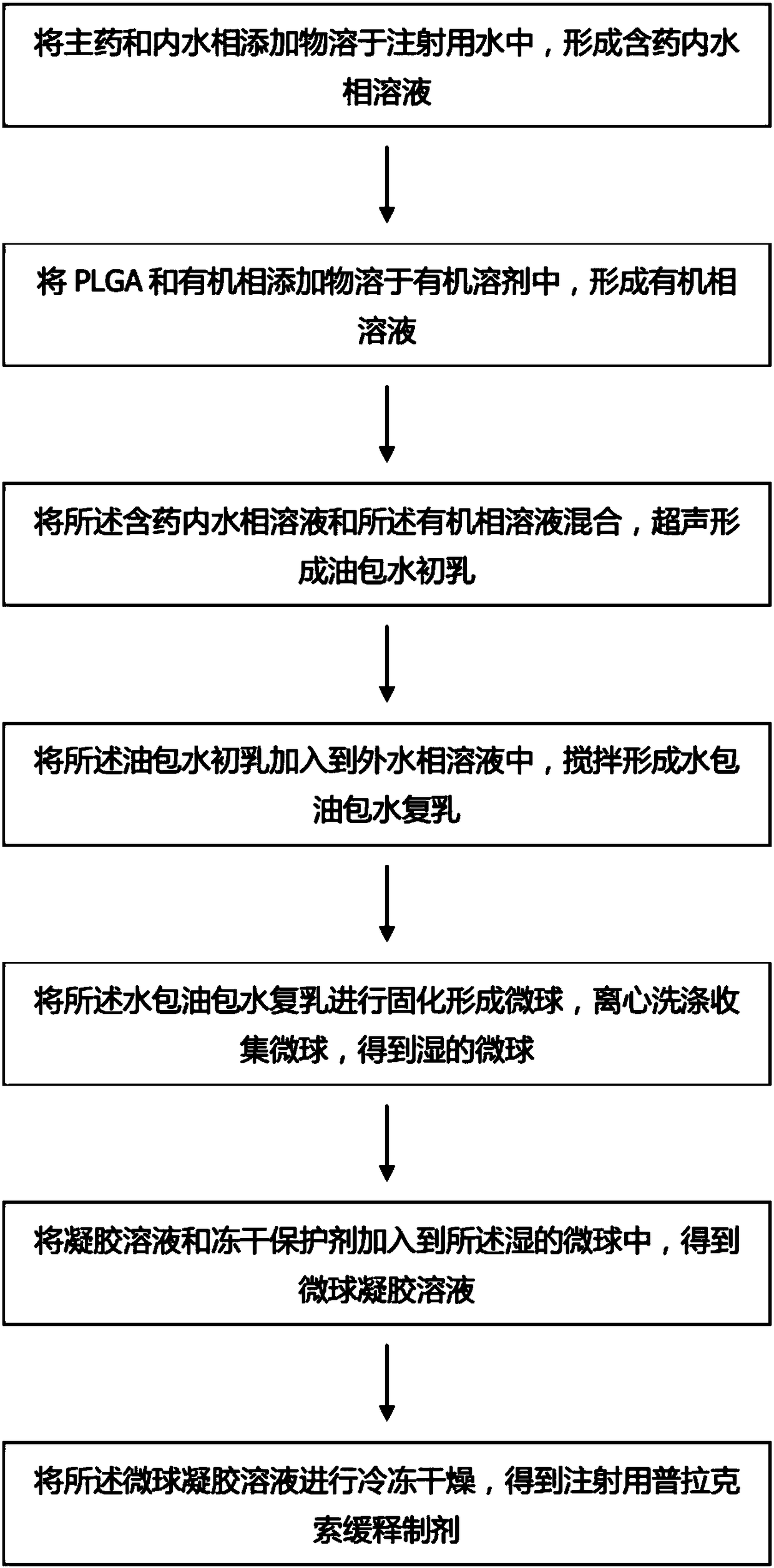
[0130] This embodiment also provides a preparation method of the pramipexole sustained-release preparation for injection, comprising the following steps:
[0131] ...
Embodiment 3
[0144] This embodiment provides a pramipexole sustained-release preparation for injection, which consists of the following raw materials: 150 mg of pramipexole hydrochloride, 25 mg of internal aqueous phase additives, 1.0 g of PLGA, 0.25 g of organic phase additives, 10 ml of gel solution, manna Alcohol 100mg.
[0145] The internal water phase additive is a mixture of poloxamer and chitosan, wherein the mass ratio of poloxamer to chitosan is 20mg / 5mg.
[0146] The molar ratio of lactide and glycolide in the PLGA is 50 / 50, and the molecular weight of the PLGA is 60kDa.
[0147] The organic phase additive is a mixture of poloxamer 407 and chitosan, wherein the mass ratio of poloxamer 407 to chitosan is 0.2g / 0.05g.
[0148] The gel solution was formed from 50 mg / ml chitosan and 200 mg / ml poloxamer 188.
[0149] This embodiment also provides a preparation method of the pramipexole sustained-release preparation for therapeutic injection, comprising the following steps:
[0150] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com