Compositions of (r)-pramipexole and methods of using the same
A technology of pramipexole and a composition, applied in the field of medicine, can solve the problems of limiting the timely development of neuroprotective potential, limiting the neuroprotective potential of enantiomers and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0307] Preparation of (R)- and (S)-pramipexole
[0308] The preparation of pramipexole is described in US Patent No. 4,843,086 and US Patent No. 4,886,812 to Griss et al., each of which is incorporated herein by reference in its entirety. The (R)-pramipexole of the present invention can be obtained by the co-pending March 14, 2007 submission, entitled "Methods of Synthesizing and Purifying R(+) and S(-)pramipexole and Purifying R(+)and(S)-promipexole)", U.S. Provisional Application No. 60 / 894,829", and filed on March 14, 2007, entitled "Methods of Enantiomerically Purifying Chiral Compounds Purifying Chiral Compounds), which is incorporated herein by reference in its entirety. Specifically, preparations of pramipexole that are chirally pure for the R(+) enantiomer can be produced using a bimolecular nucleophilic substitution reaction (SN2). In a one-pot synthetic scheme, a diamine of the formula 2,6-diamino-4,5,6,7-tetrahydro-benzothiazole is reacted with propyl sulfonate or...
Embodiment 1
[0363] Example 1 - Determination of dopamine receptor affinity for the R(+) and S(-) enantiomers of pramipexole.
[0364] The S(-) enantiomer of pramipexole has been characterized in the past as a high affinity dopamine receptor ligand at D2 (both S and L isomers), D3 and D4 receptors, although for The highest affinity was seen for the D3 receptor subtype. The dopamine receptor ligand affinities of S(-) pramipexole and R(+) pramipexole from journal publications have been tabulated (data reproduced in Table 10). Although each study or experiment was performed under slightly different conditions, and different radioligands were used, the data show comparable affinities for different dopamine receptors. Dopamine receptor affinity studies on pramipexole S(-) and R(+) enantiomers are also shown in Table 10. These data demonstrate an unexpectedly large difference in the affinity of the two enantiomers of pramipexole for all dopamine receptors. Table 10 shows that, contrary to exp...
Embodiment 2
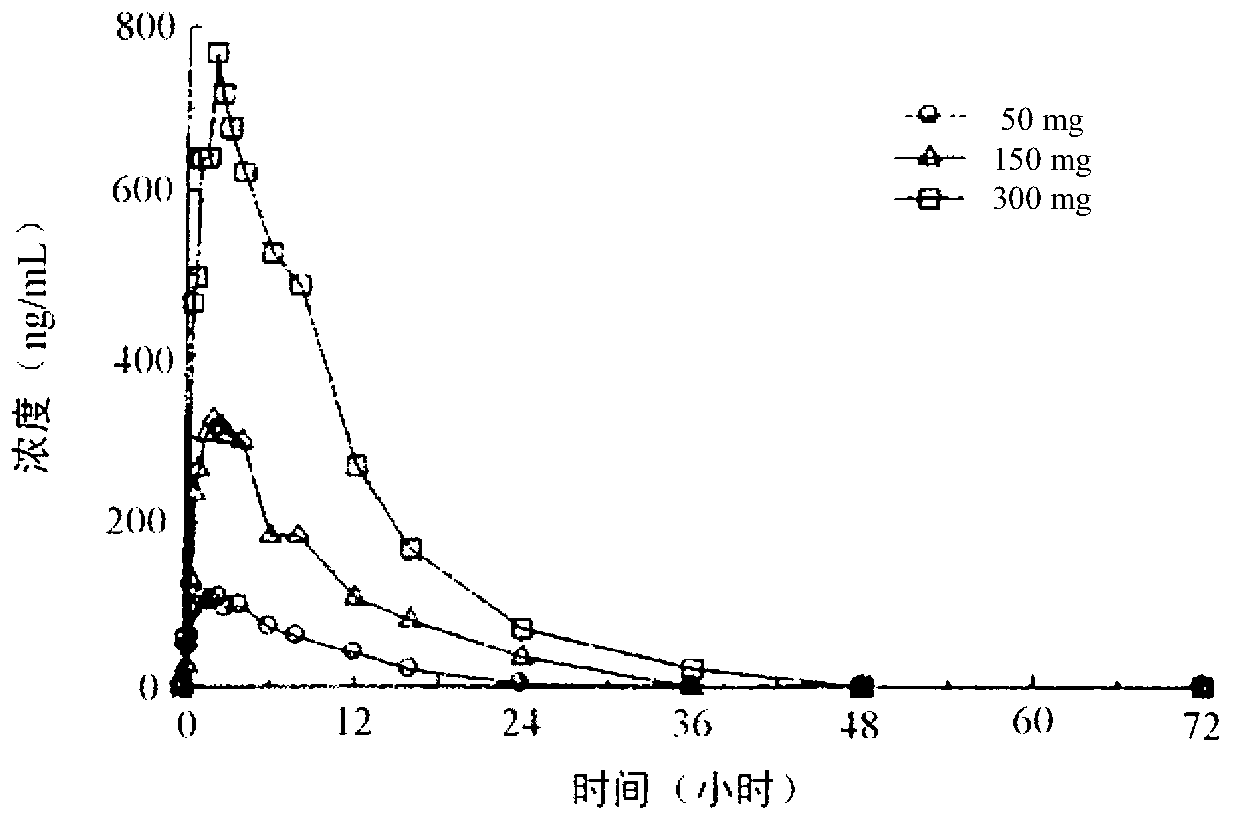
[0372] Example 2 - Determination in dogs for the R(+) and S(-) enantiomers of 100% pure pramipexole, and a preparation of a mixture (R99.5% / S0.5%) In vivo studies of the MTD and NOAEL described above. The form of (R)-pramipexole is (R)-pramipexole dihydrochloride monohydrate.
[0373] The following in vivo study in Beagle dogs was performed to test the hypothesis that the R(+) and S(-) enantiomers of pramipexole play an important role in the receptor affinity observed Large differences would translate into large observed differences between the two enantiomers at the observed maximum tolerated dose (MTD) and / or no observable adverse effect level (NOAEL). Dogs were administered preparations of each enantiomer (within limits of analytical detection limit) of each enantiomer prepared as highly purified compounds, or prepared with 0.5% of pramipexole's S Preparation of R (+) enantiomer contaminated with (-) enantiomer.
[0374] In this study, three groups of 4 treated Male Be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com