Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
734 results about "Intravenous Infusions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
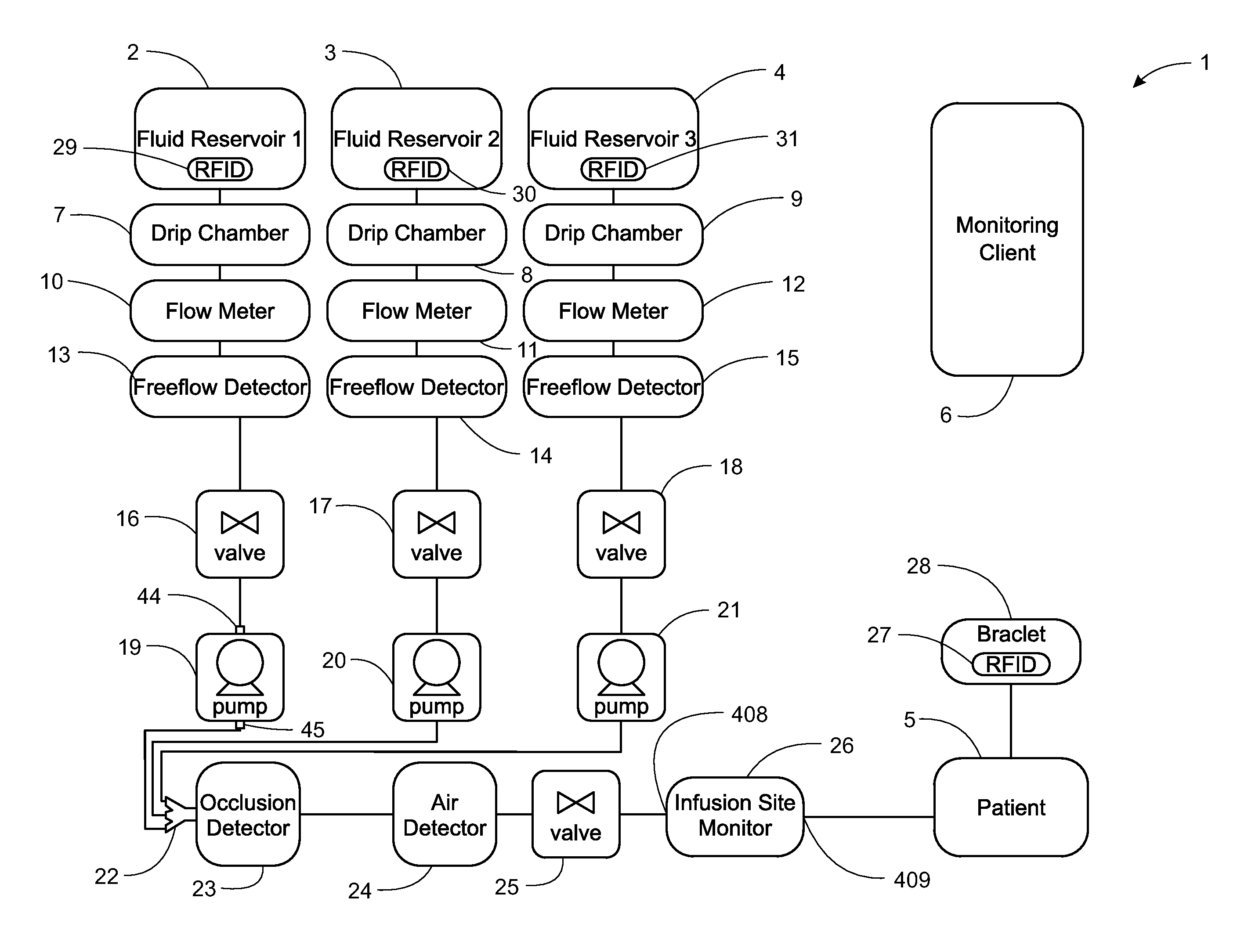
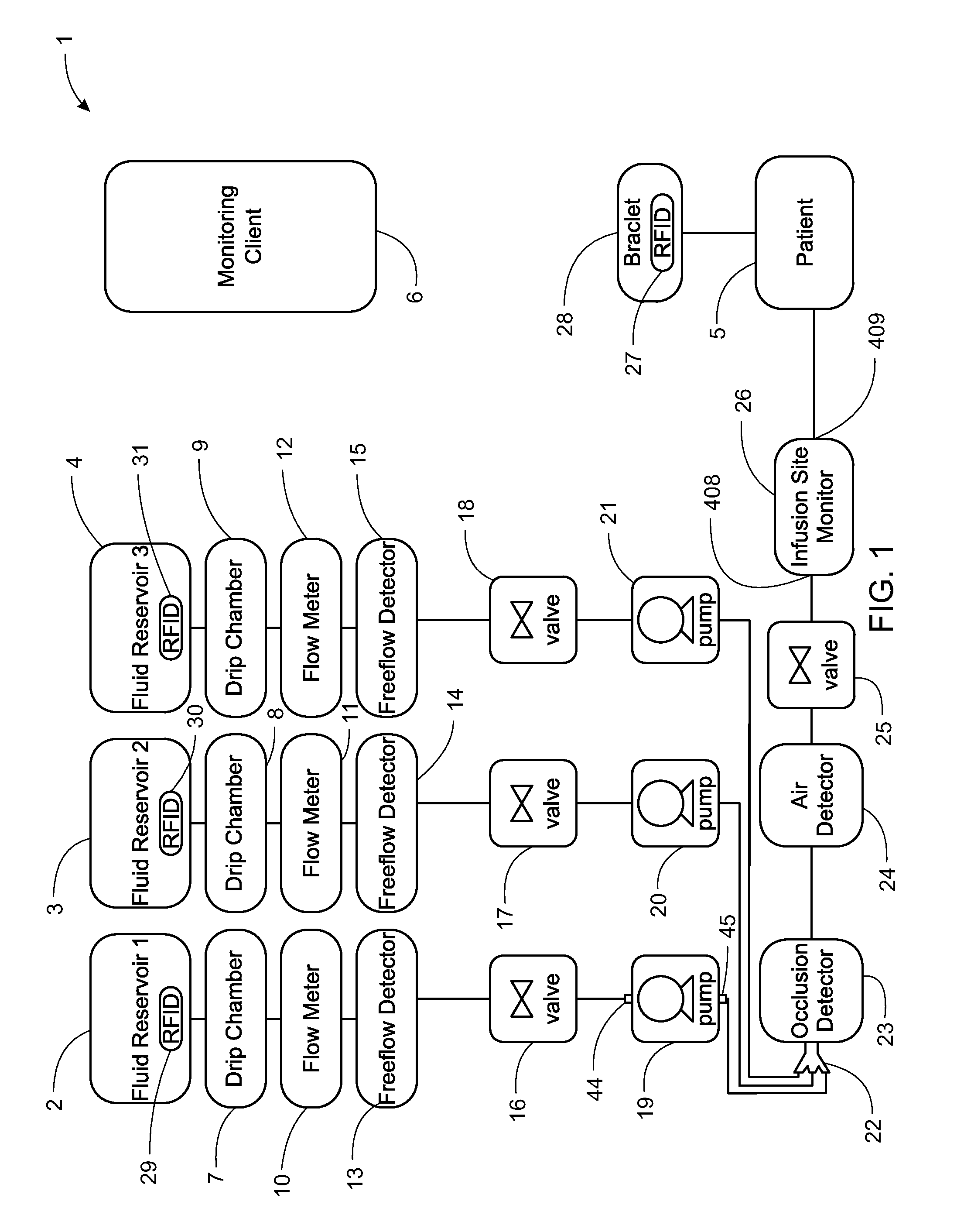
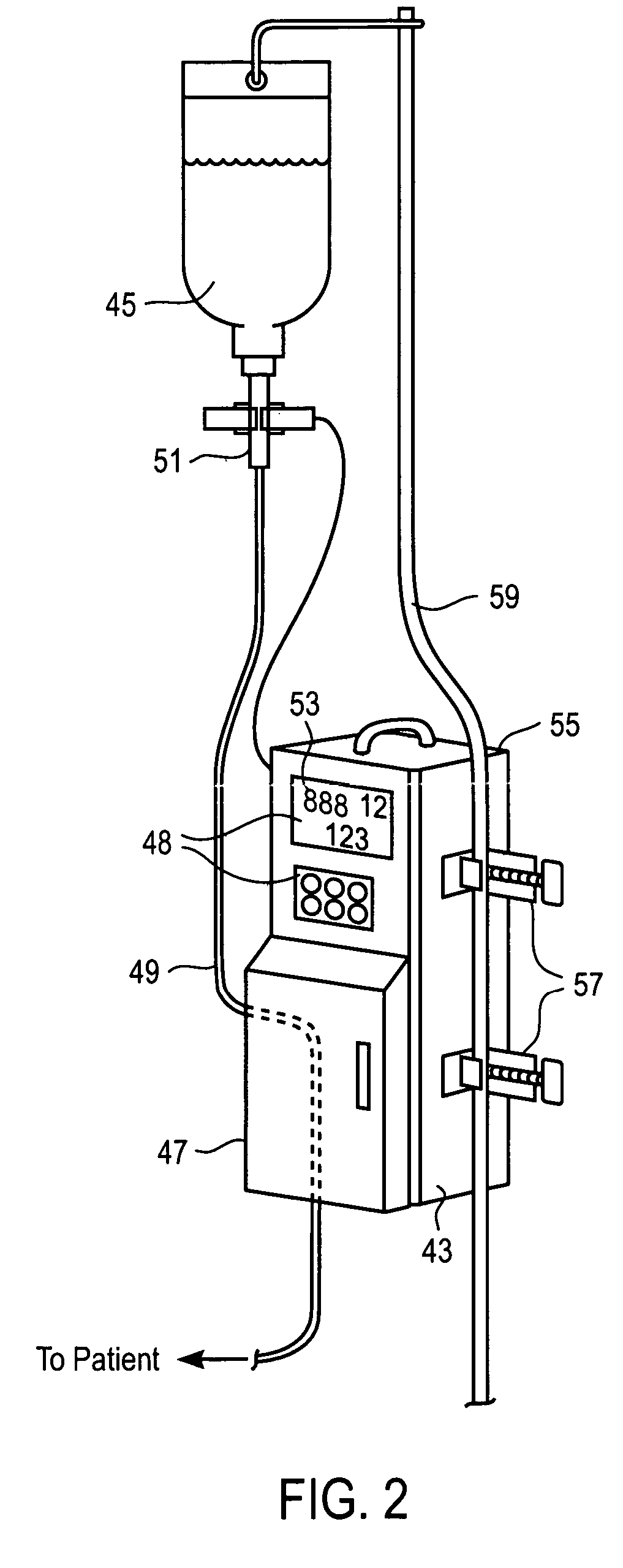
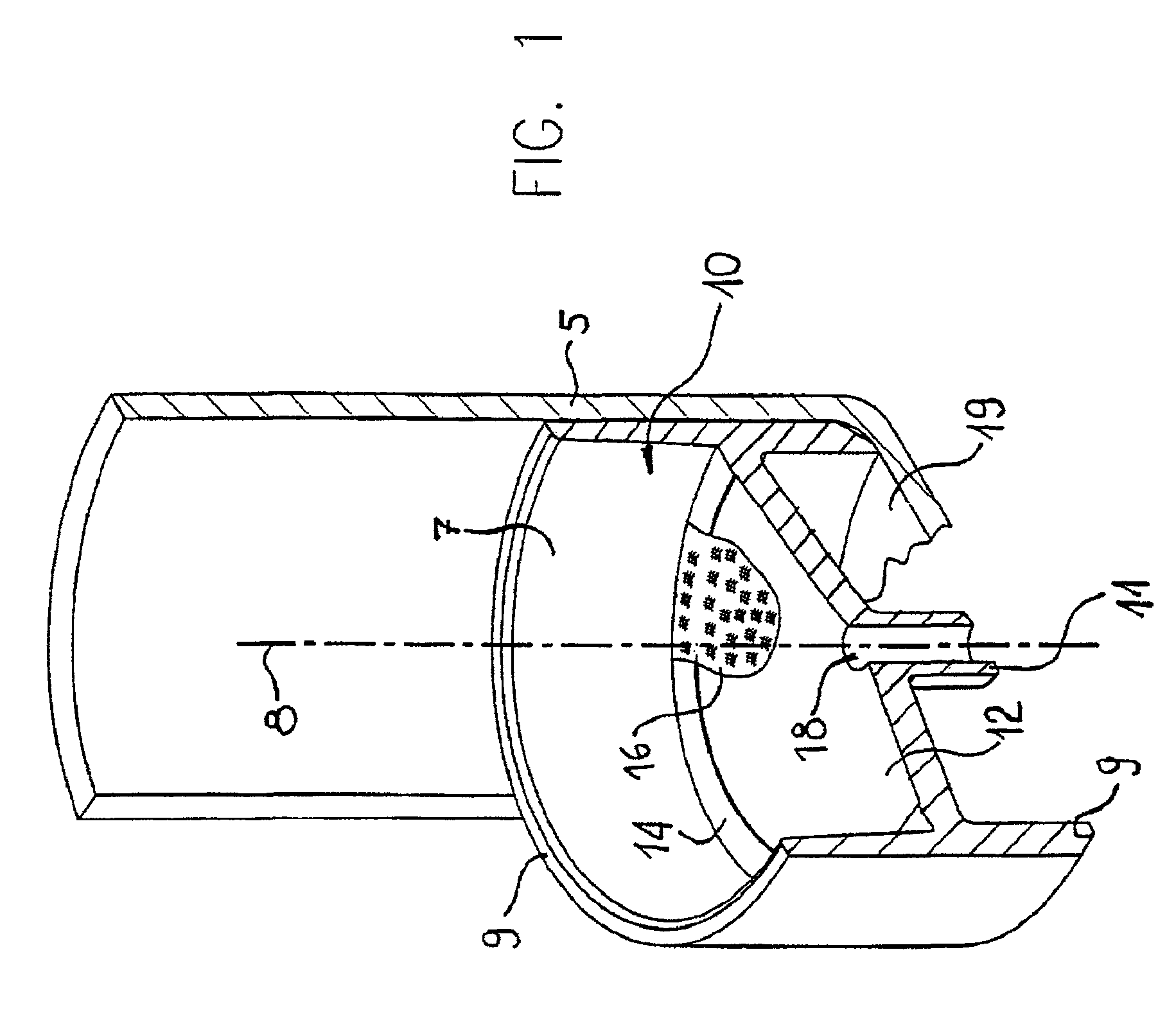
Intravenous infusion. 1 a solution administered into a vein through an infusion set that includes a plastic or glass vacuum bottle or bag containing the solution and tubing connecting the bottle to a catheter or a needle in the patient's vein.
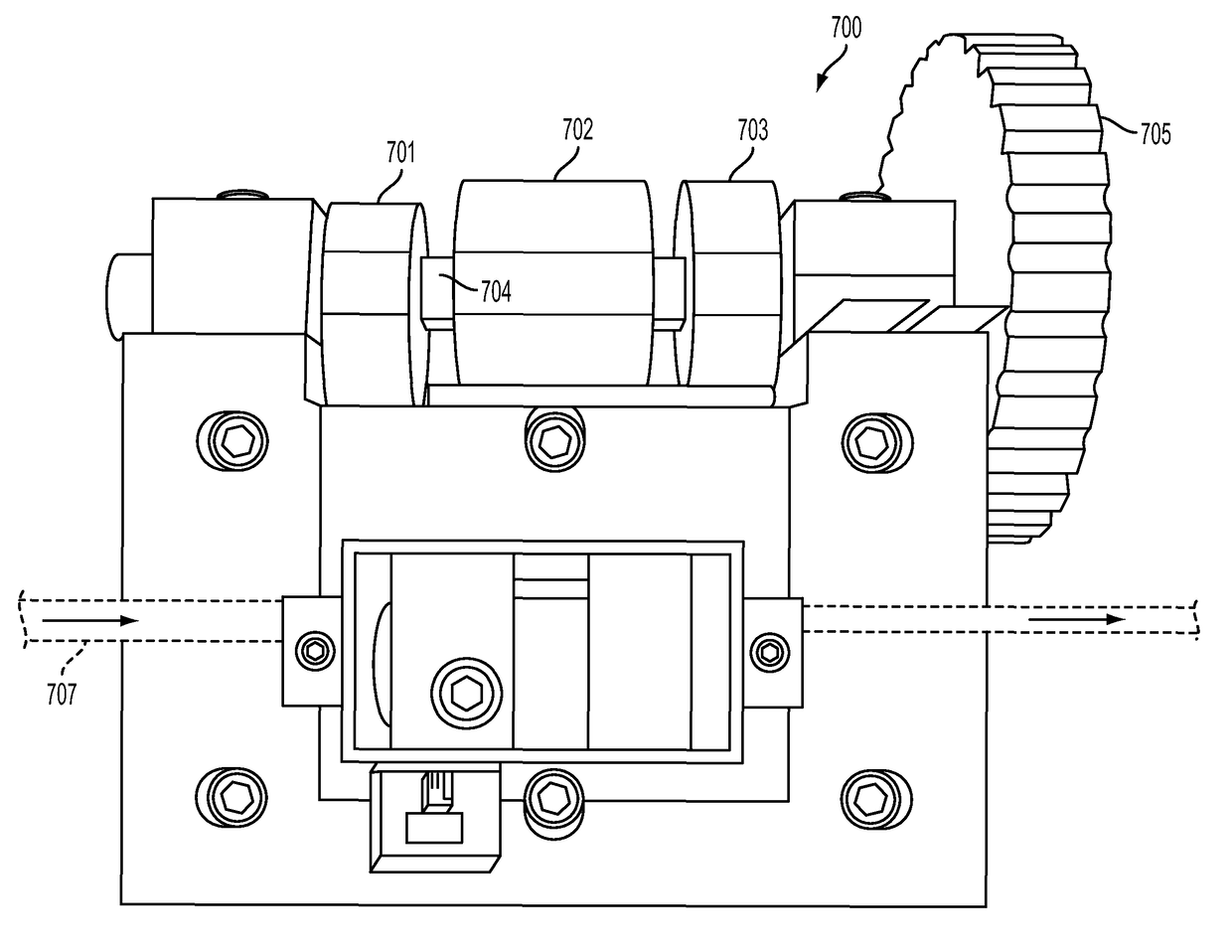
Apparatus for Infusing Fluid
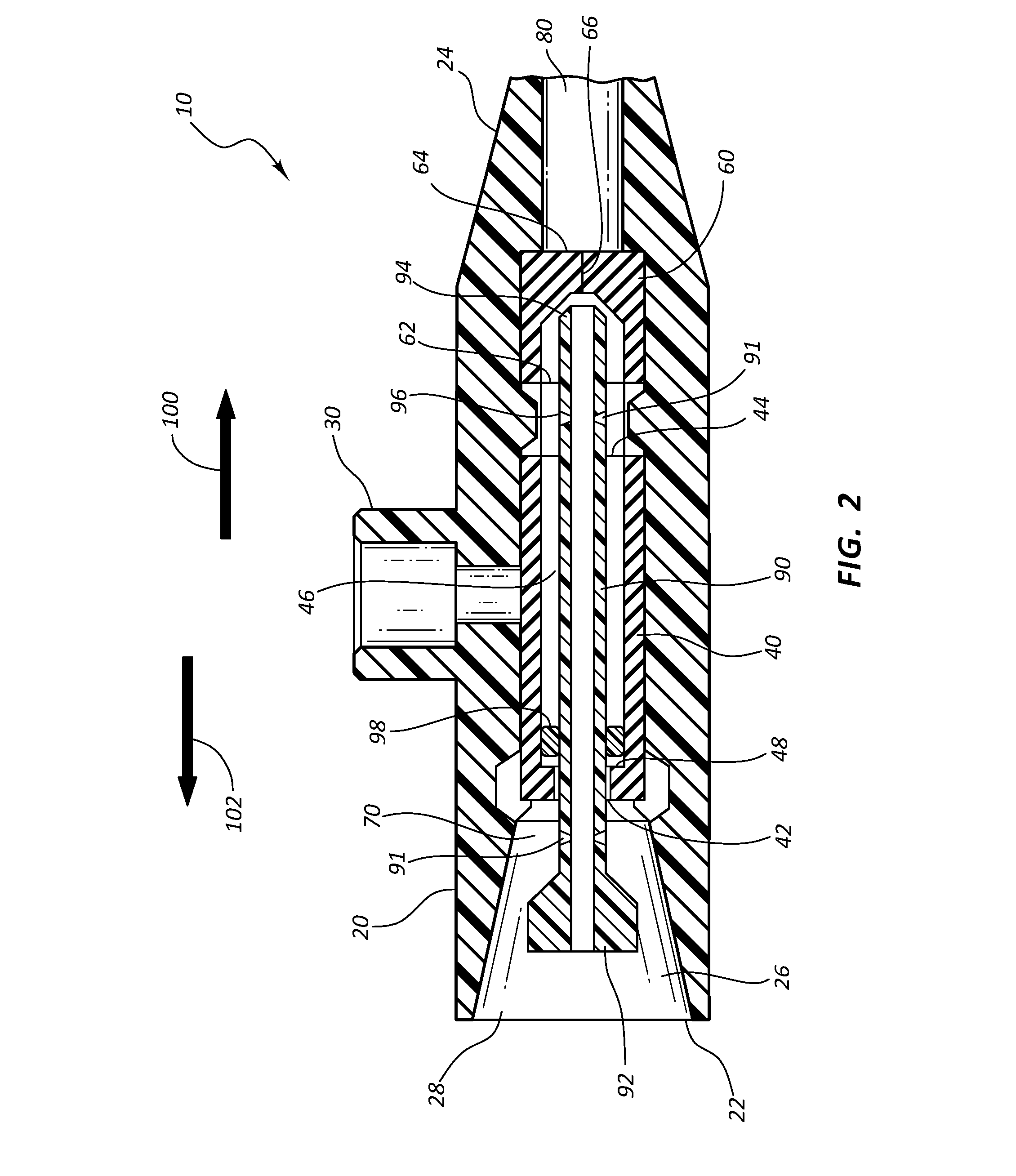
A pump for pumping fluid includes a tube platen, a plunger, a bias member, inlet and outlet valves, an actuator mechanism, a position sensor, and a processor. The plunger is configured for actuation toward and away from the infusion-tube when the tube platen is disposed opposite to the plunger. The tube platen can hold an intravenous infusion tube. The bias member is configured to urge the plunger toward the tube platen.
Owner:DEKA PROD LLP
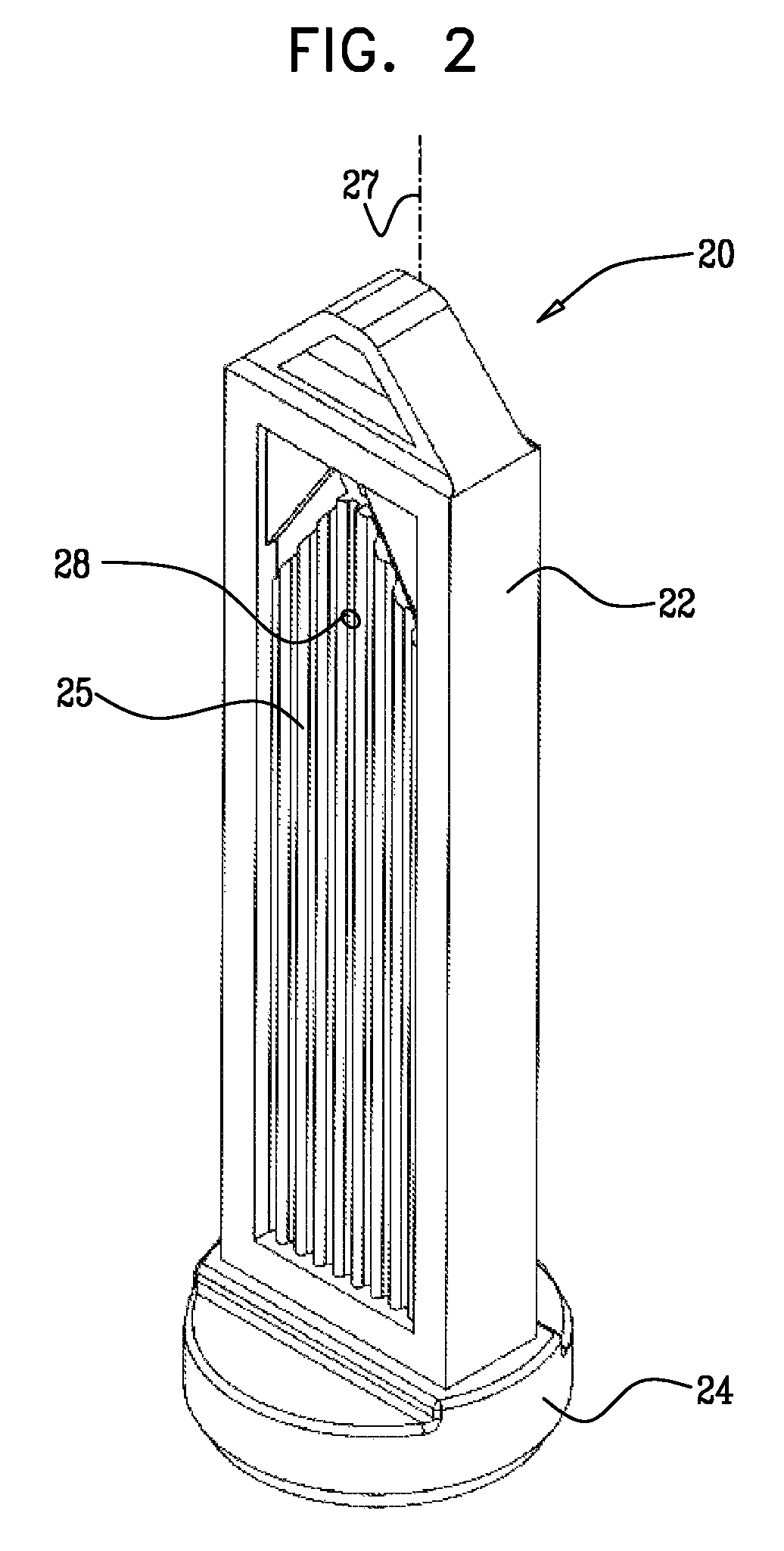
Apparatus for infusing fluid
Owner:DEKA PROD LLP
Safe intravenous infusion port injectors
We describe connectors for safely and conveniently injecting measured doses of sterile liquid medications into a patient via one or more infusion ports in intravenous access assemblies. Each connector comprises a tubular injector divided by a rigid septum which holds a hollow needle sharp on each end safely recessed in a leading and in a trailing chamber. The leading chamber snugly holds the trailing limb and penetrable cap of an inserted standard infusion port. The trailing recess snugly holds the leading end of a cartridge with a leading penetrable diaphragm, a bore containing liquid medication, a cartridge piston and trailing bore suitable for insertion of a separate cartridge plunger having markers for measuring doses delivered; or, alternatively, the pentrable cap and trailing limb of second similar infusion port attached by trailing tubing to a large measured volume infusion source. When assembled such that the trailing cap of infusion port is penetrated by the needle in the leading chamber and the leading diaphragm of the cartridge or, alternatively, the leading penetrable cap on a second infusion port is penetrated by the needle in the trailing chamber, such that flow can proceed through the needle, precisely measured doses of sterile liquid medication can be injected into the venous access assembly without possibilities for the user to touch, get stuck or finger-contaminate the needle or its contents. Unique features added to increase the efficiency of the system are a biased leading end on the tubular injector to conveniently and securely accommodate a Y-infusion port; an eccentric needle in the connector, such that rotation prevents the leading sharp end of the needle from passing through the infusion port cap via the same track; and an easily removed biased cap for keeping the cartridge diaphragm sterile.
Owner:WALKER JACK M +1
Liquid infusion apparatus
InactiveUS7553295B2Reduce materialEasy to controlDC motor speed/torque controlFlexible member pumpsPeristaltic pumpEngineering
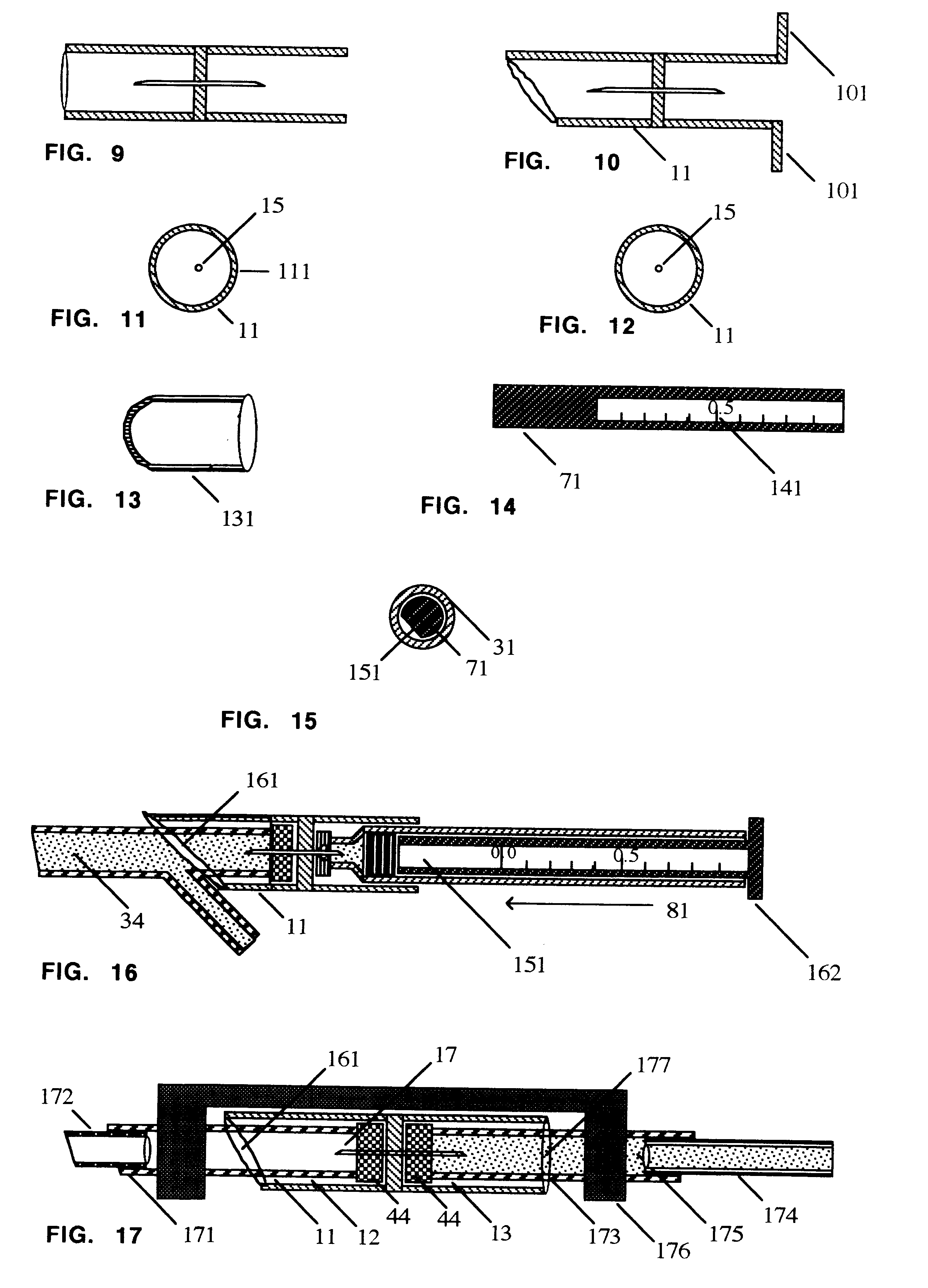
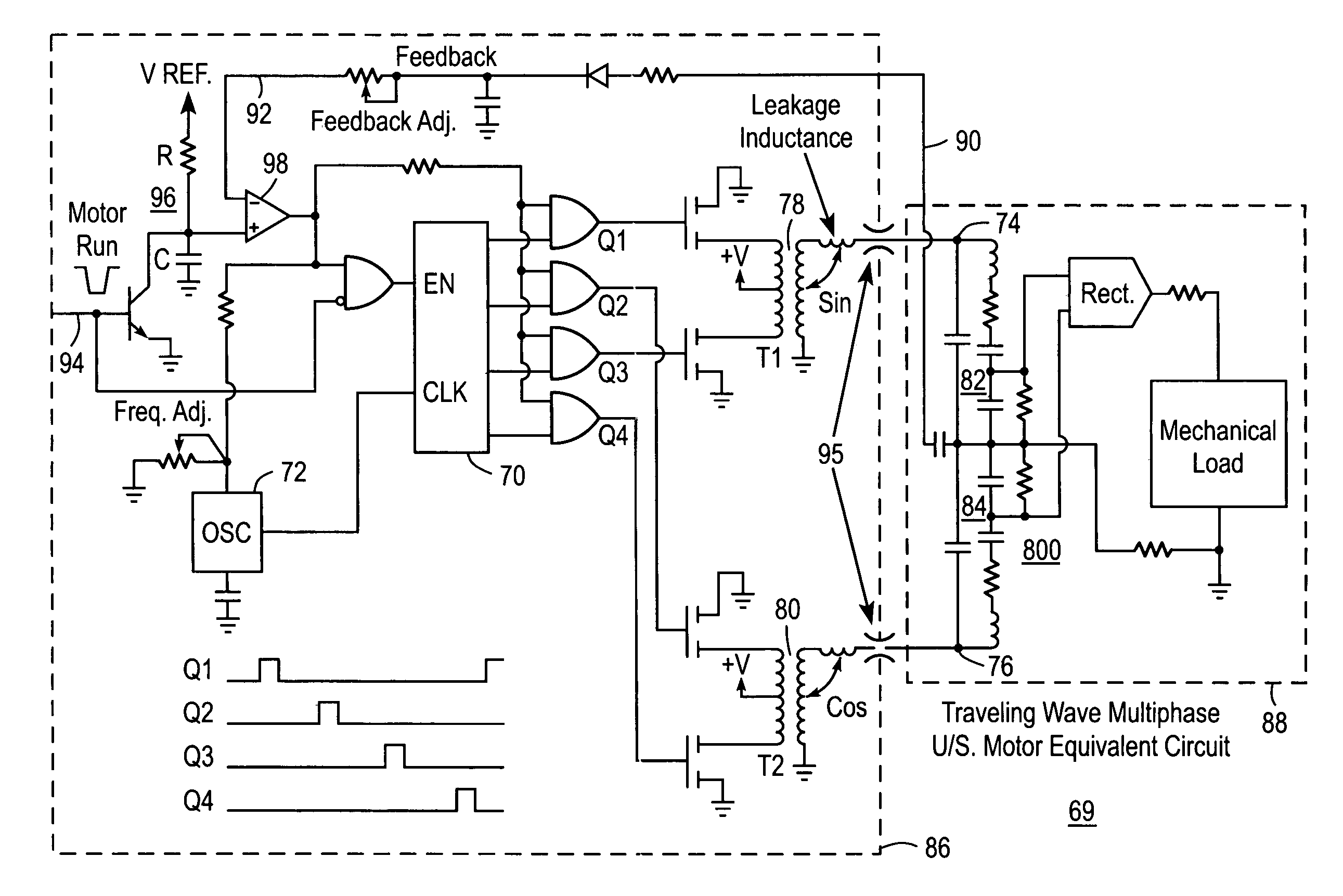
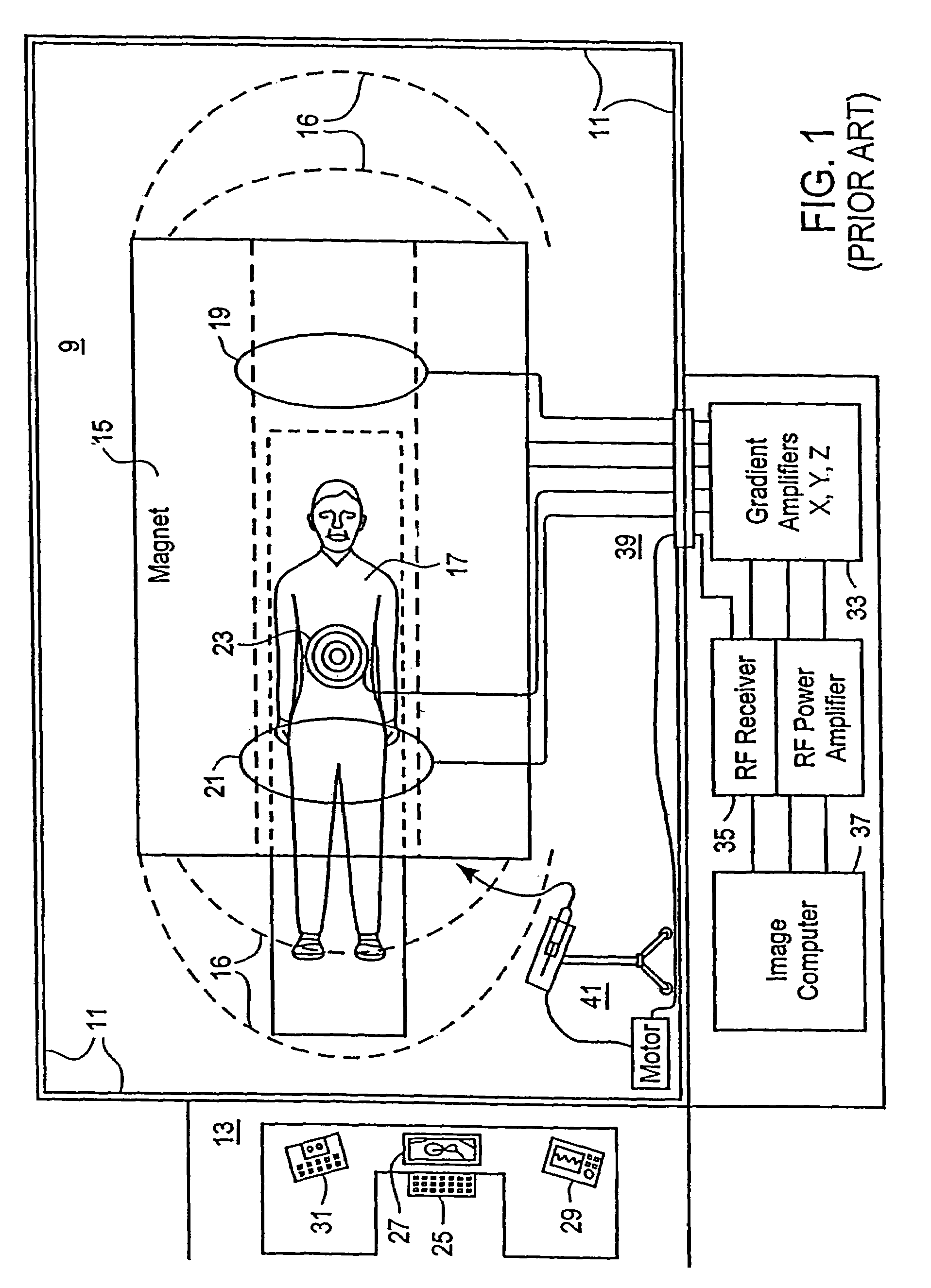
Liquid infusion apparatus includes non-magnetic materials in a pumping structure and ultrasonic drive motor therefor, and in a controller that supplies drive signals to the motor to facilitate convenient operation in intense magnetic fields without distorting the magnetic fields and without radiating objectionable radio-frequency interference. A non-MRI-compatible liquid infusion apparatus is temporarily replaced with MRI-compatible, non-magnetic liquid infusion apparatus without disconnecting a patient from an installed intravenous infusion set to continue infusing liquid within the MRI environment. The pumping apparatus operates on a segment of a liquid conduit that is mounted in tension between a linear peristaltic pump and platen, with associated safety interlocks to assure proper operation of infusing liquid into a patient compatibly with conditions in an MRI environment. Drive circuitry generates low-harmonic signals for operating the ultrasonic motor at variable speeds to compensate for flow rate discontinuities through the peristaltic pumping cycles.
Owner:IRADIMED CORP
Membrane support for drip chamber
InactiveUS7892204B2Safe installationIncrease surface areaFiltering accessoriesMedical devicesIntravenous InfusionsBiomedical engineering
Owner:KRAUS GMBH
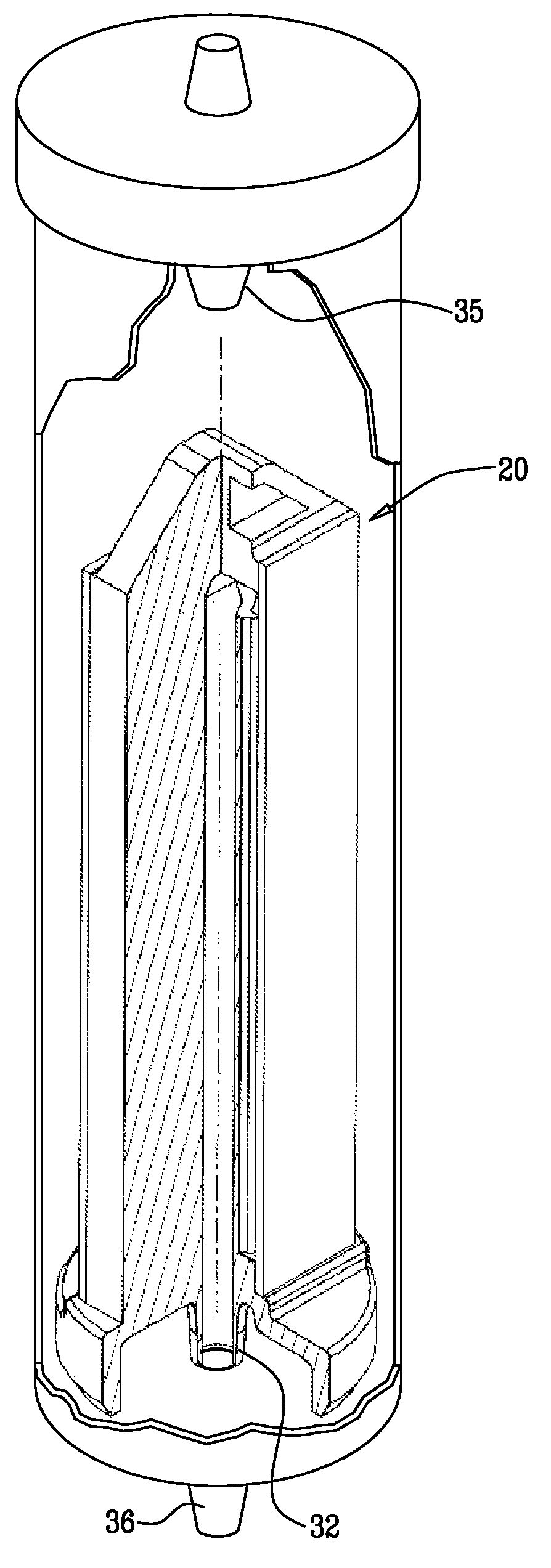
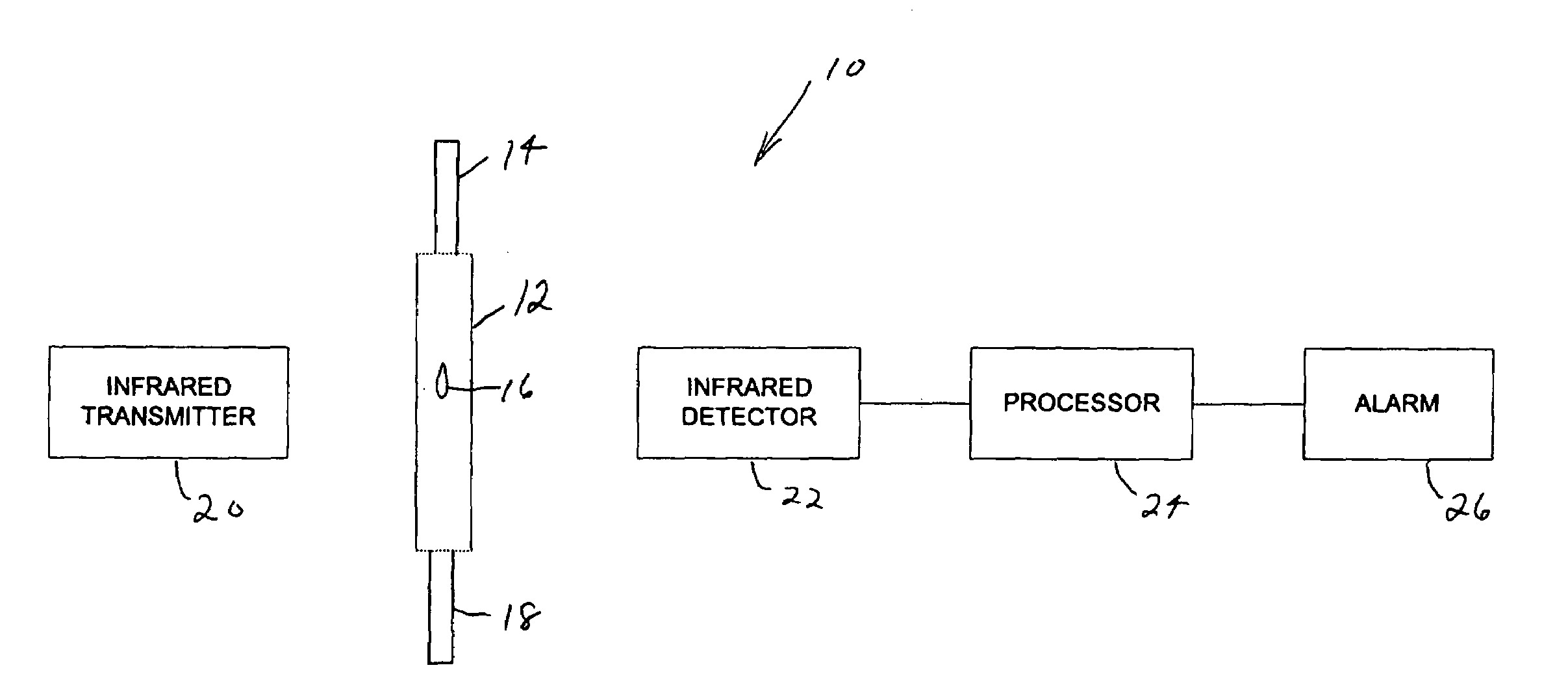
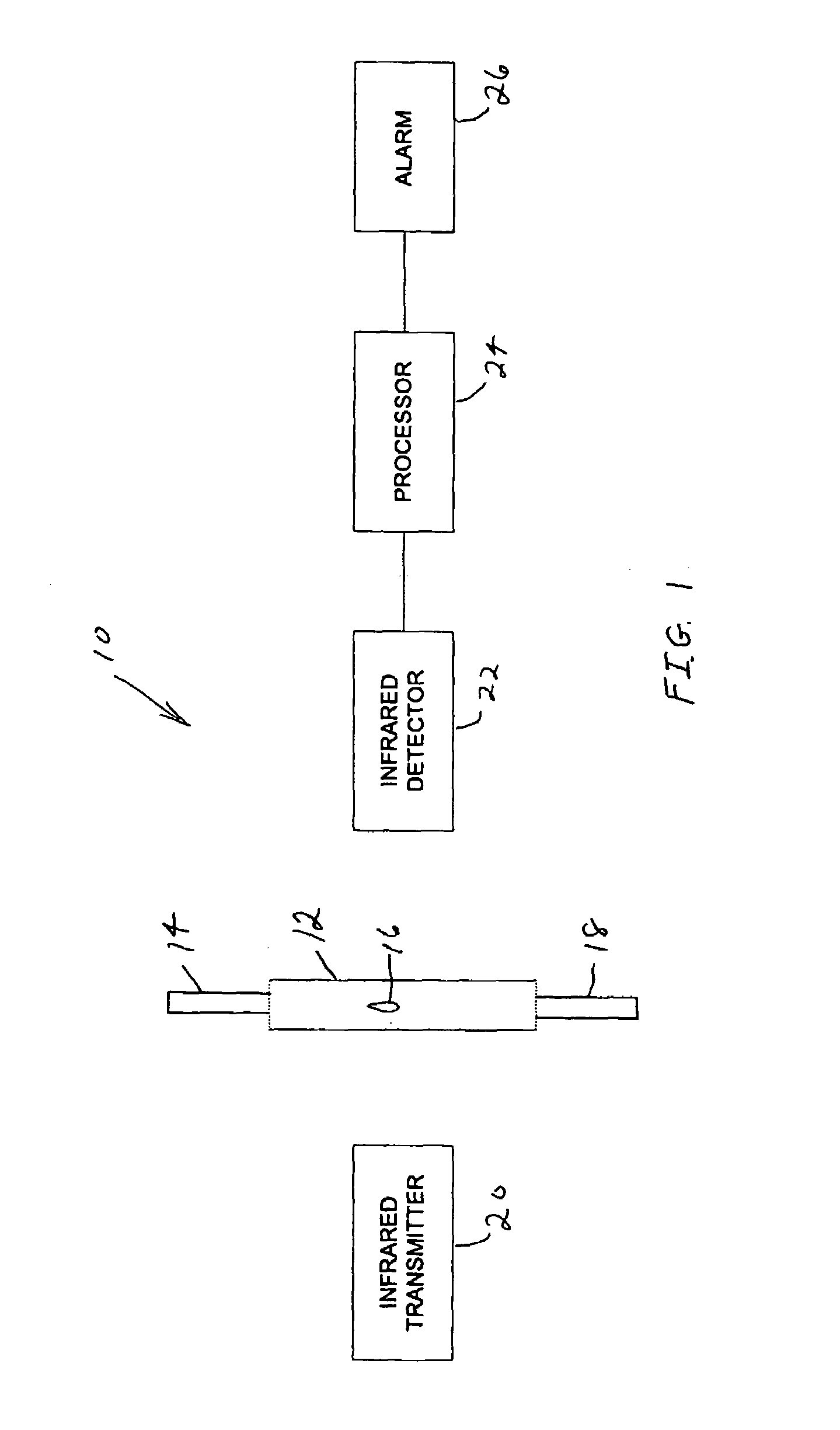
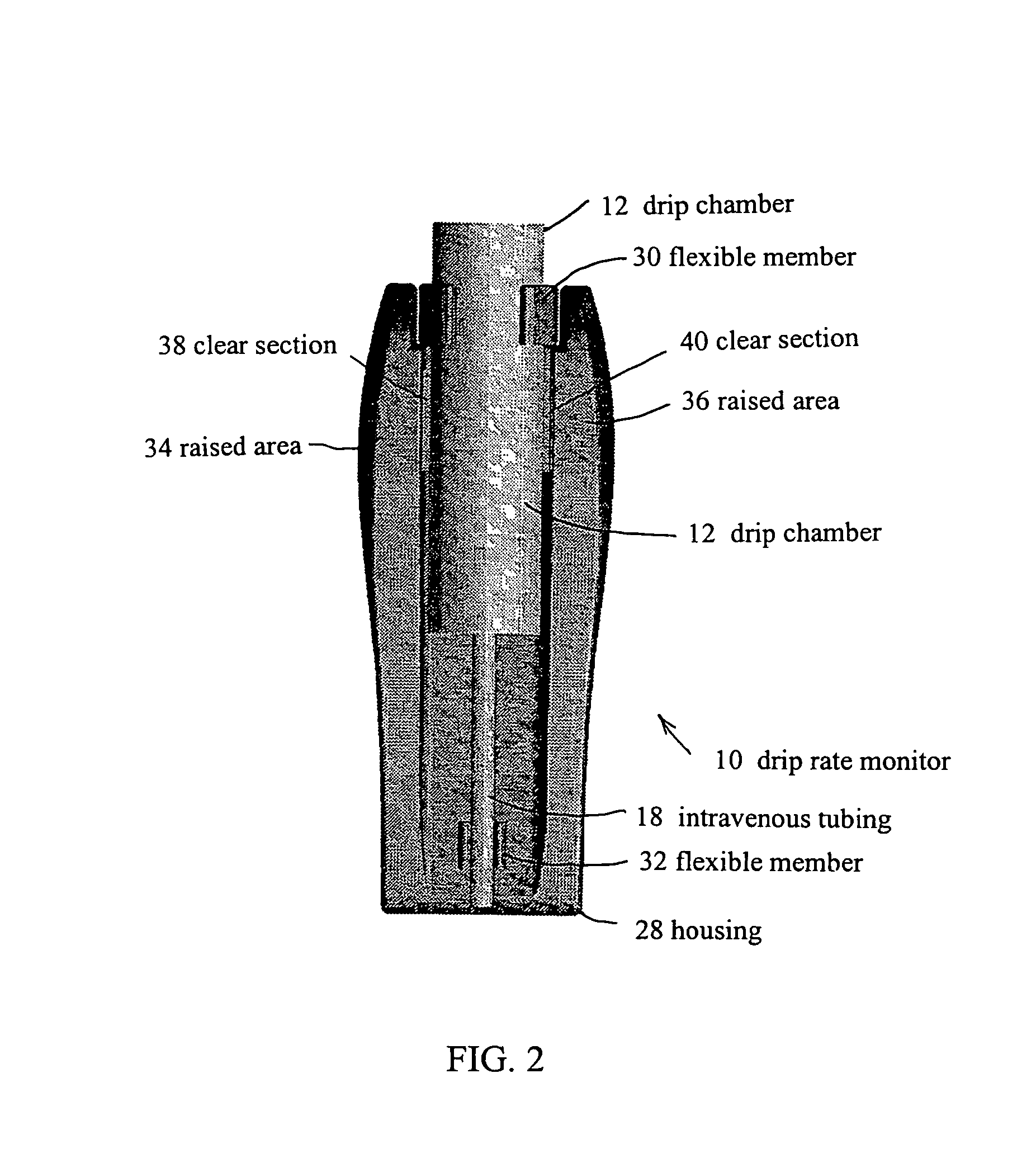
Drip rate monitor for intravenous infusion set
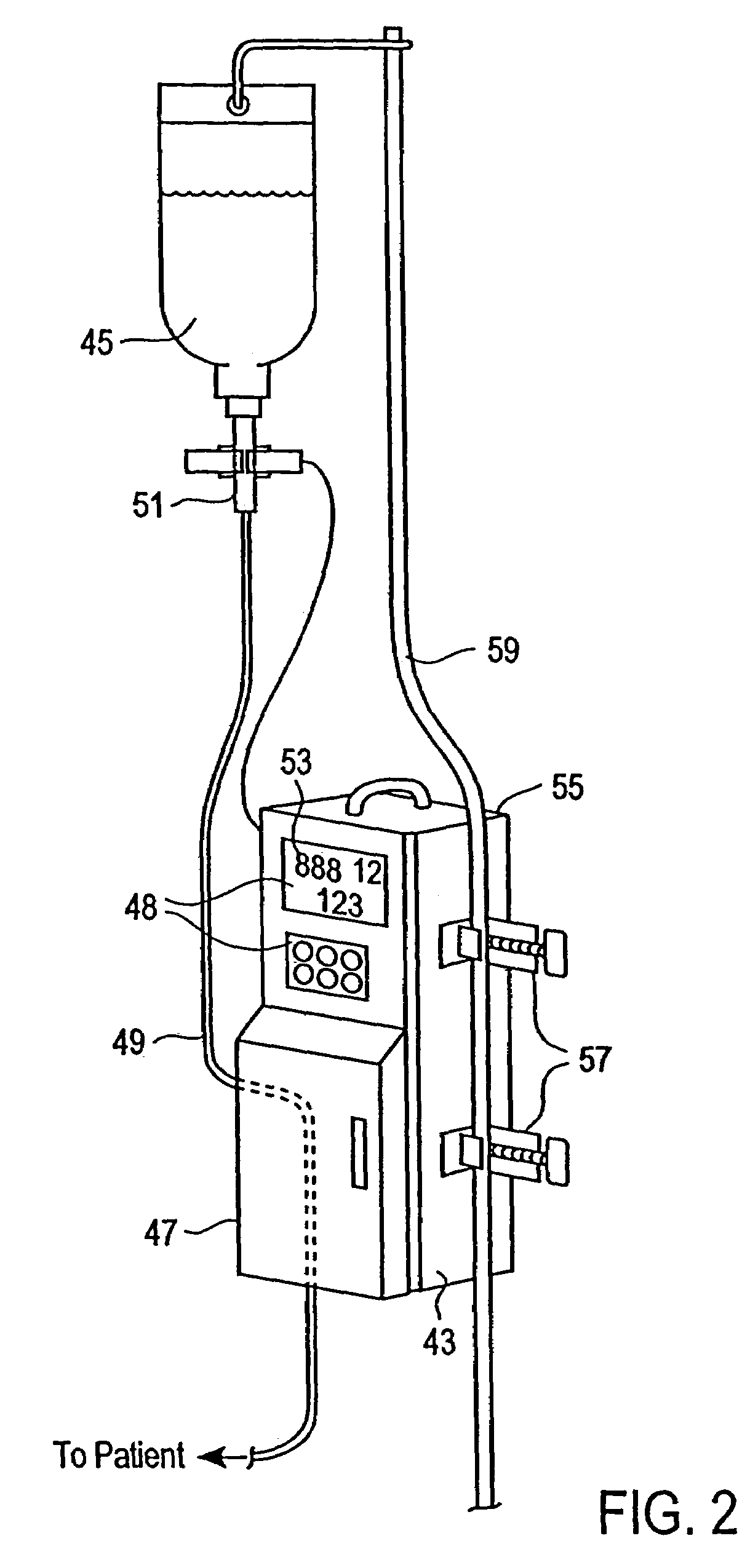
An apparatus for monitoring the drip rate of an infusion fluid being administered by an intravenous infusion set comprising a housing, drop sensor, alarm and processor. The housing is attachable to a drip chamber, and the drop sensor is positioned in the housing to detect drops of the infusion fluid being administered by the intravenous infusion set. The processor determines a first amount of time required for the drop sensor to detect a first set of drops of infusion fluid having a predetermined number of drops, determines a second amount of time required for the drop sensor to detect a second set of drops of infusion fluid having the predetermined number of drops, compares the second amount of time to a range of time having a minimum that is less than the first amount of time and a maximum that is greater than the first amount of time, and activates the alarm if the second period of time is not within the range of time.
Owner:GOLDBERG BARRY A +1
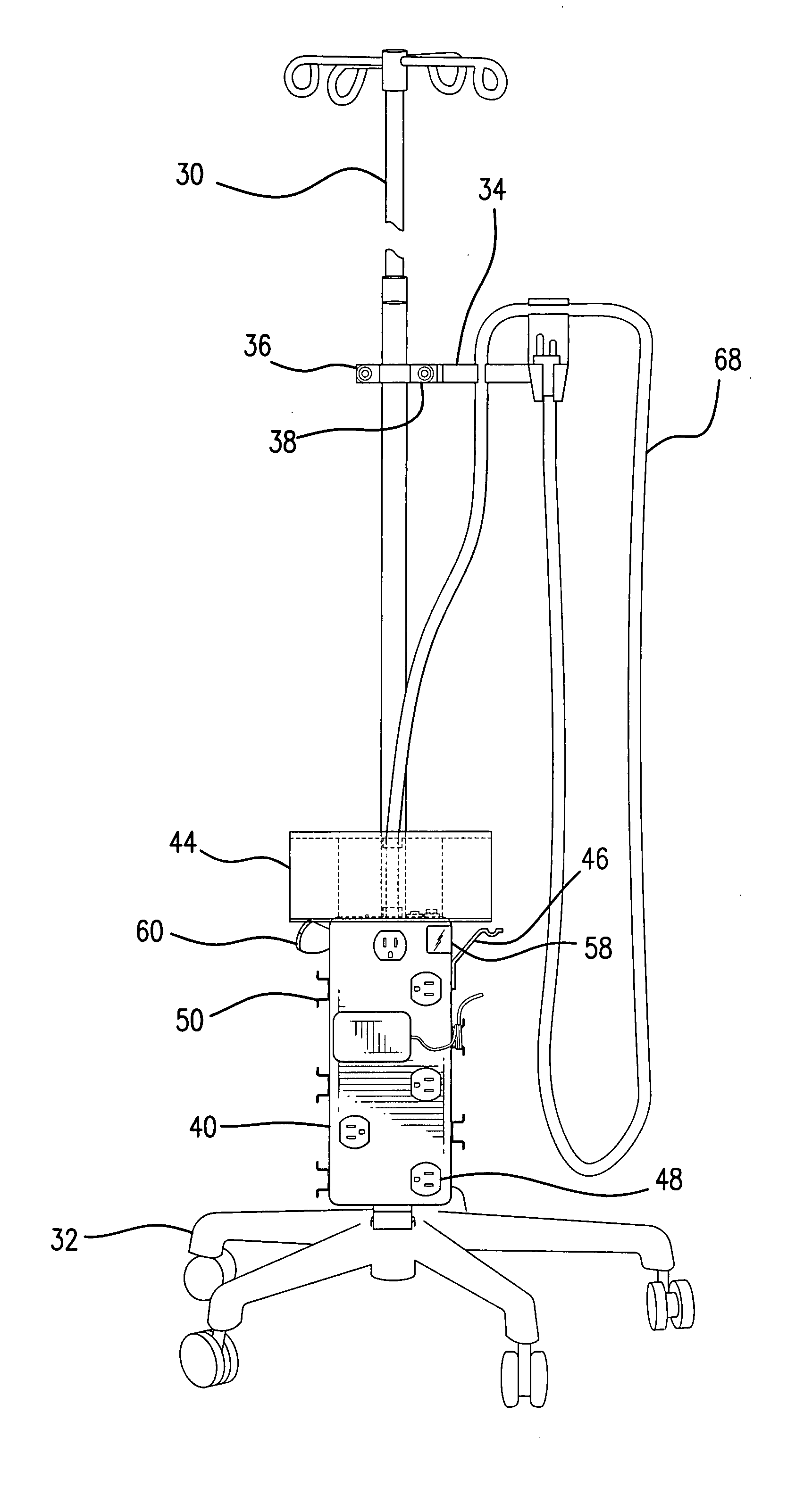
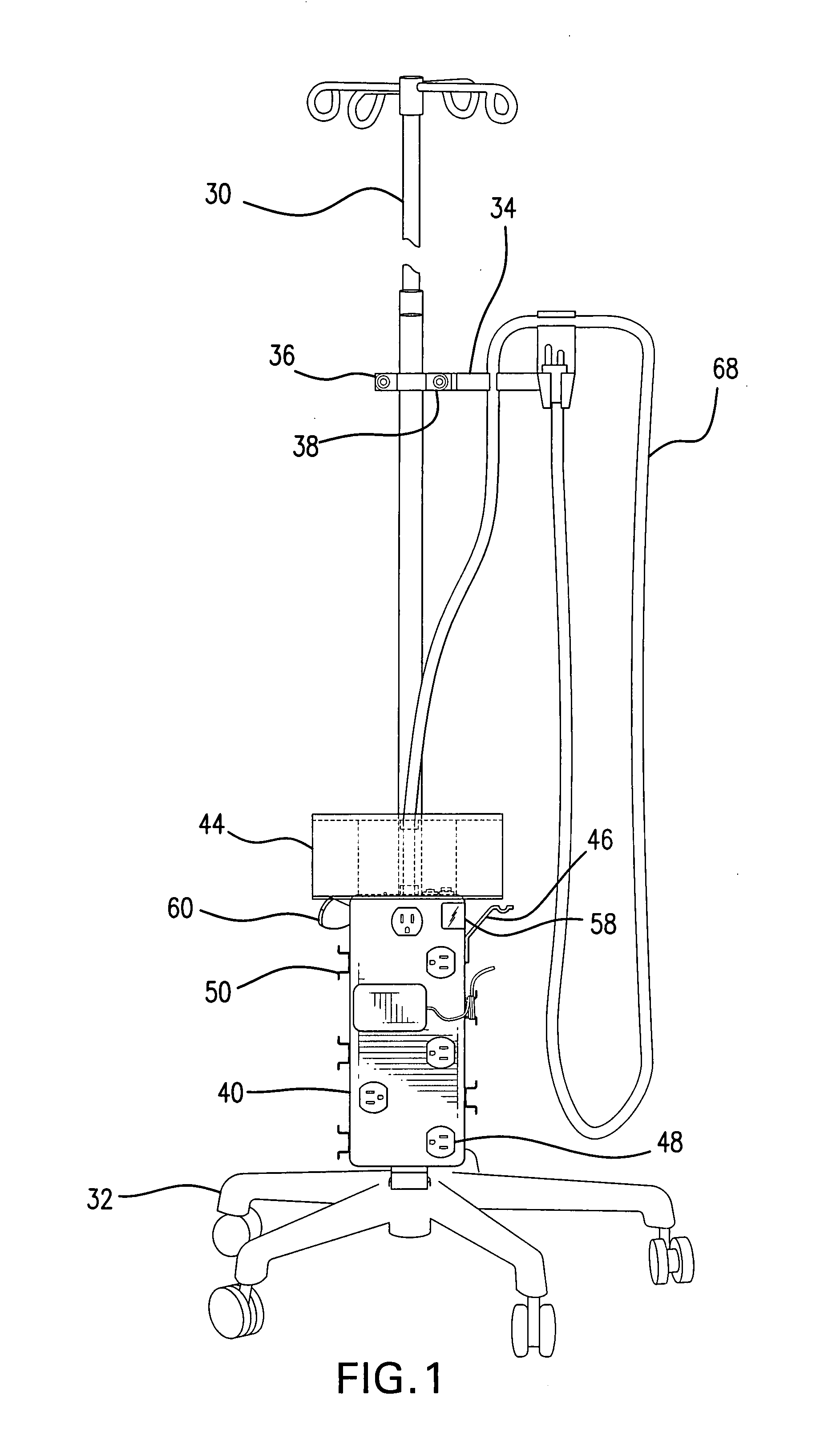
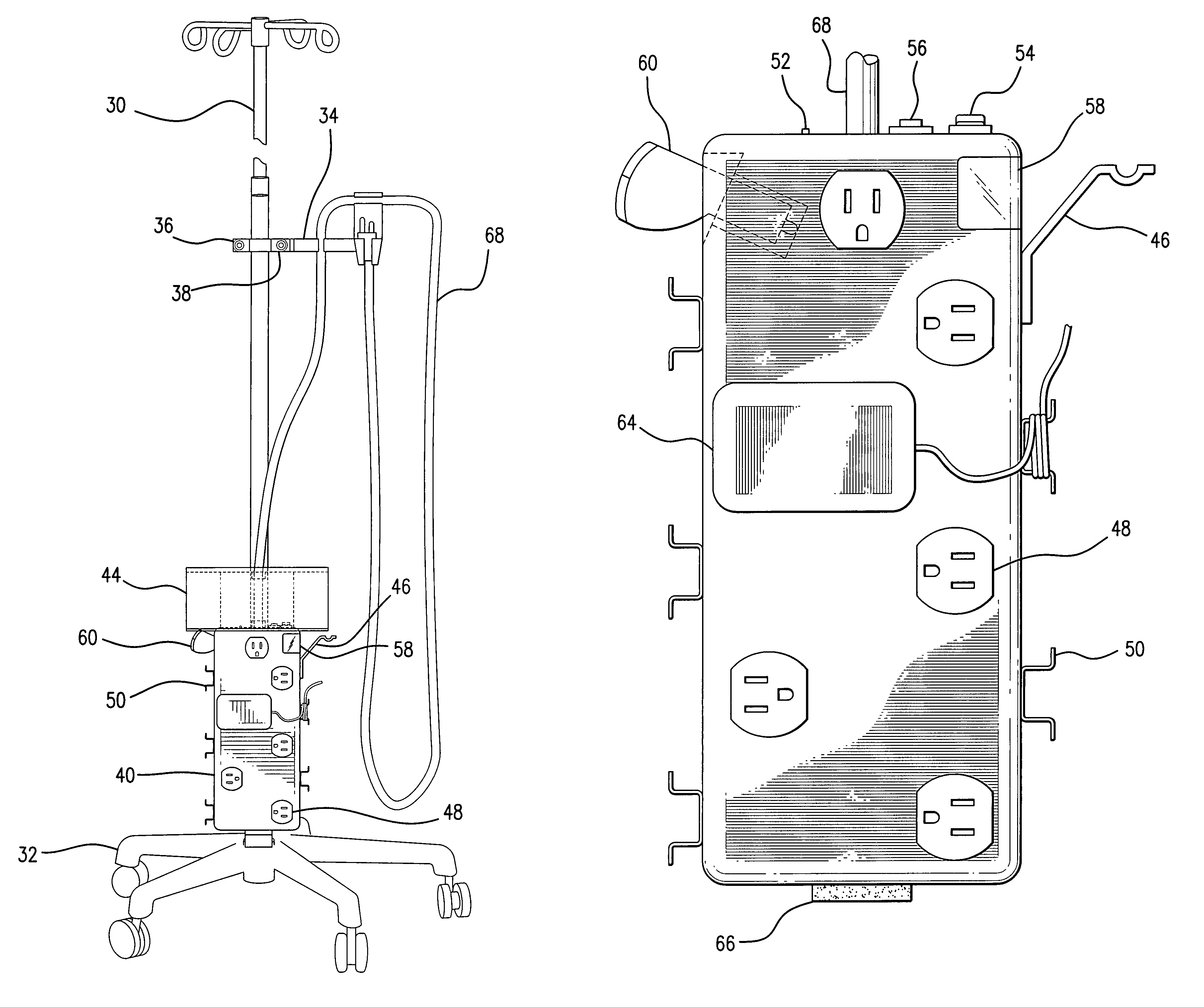
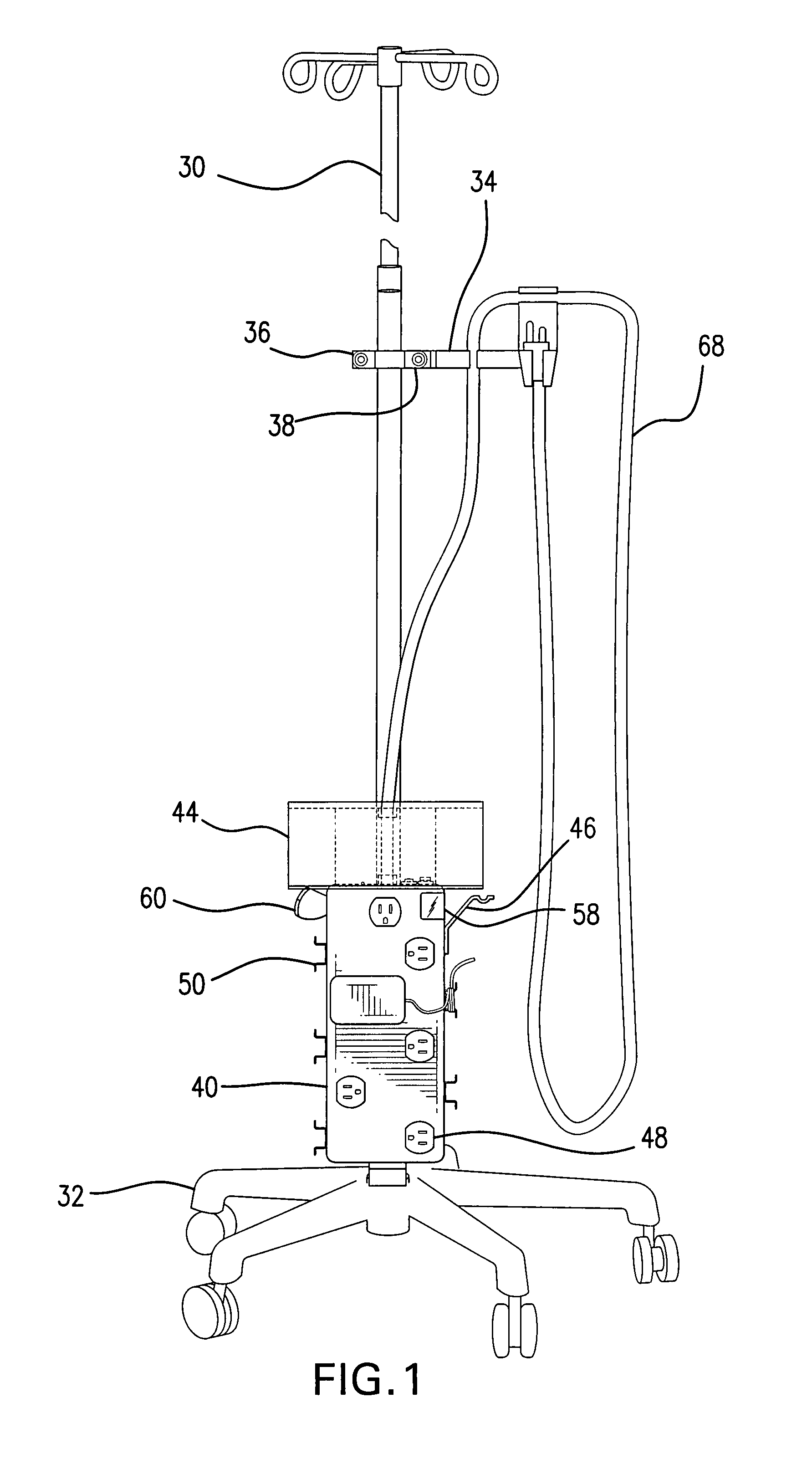
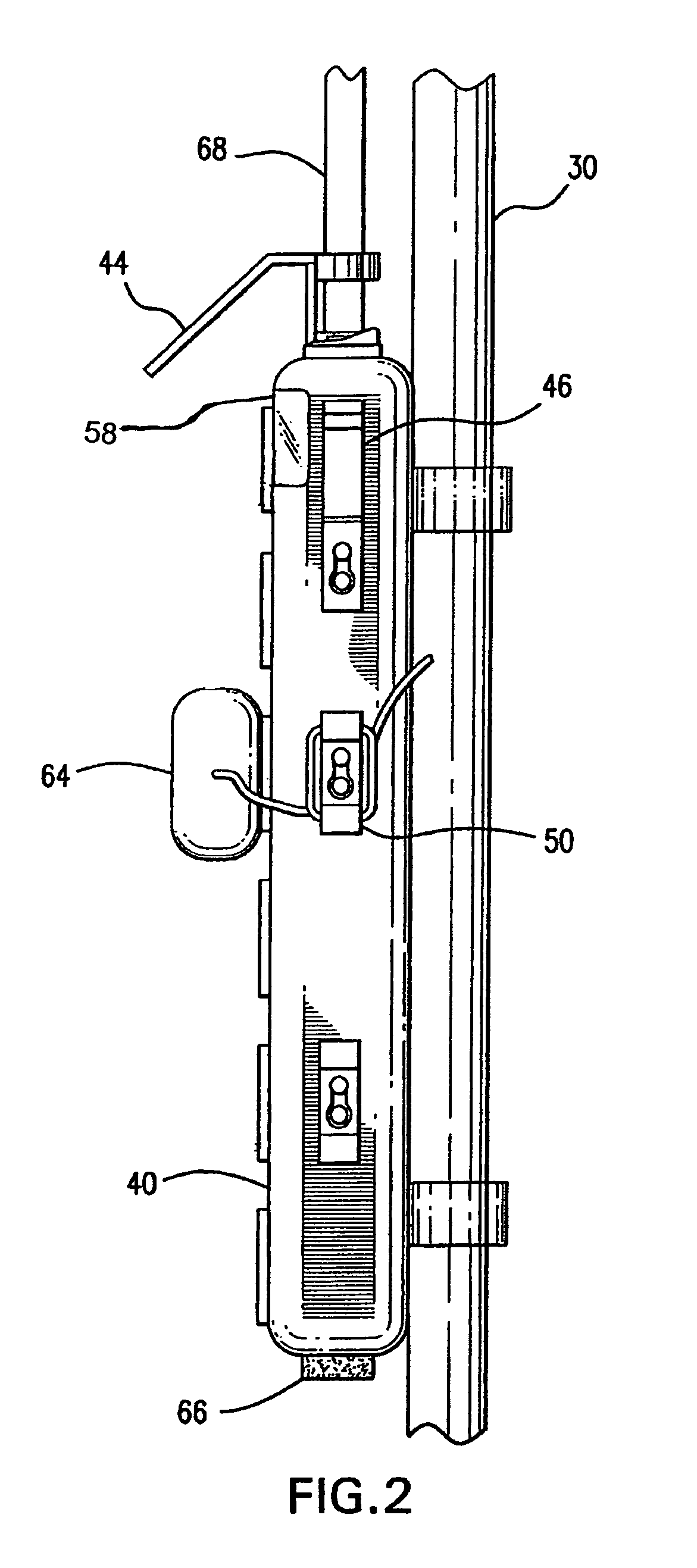
Intravenous pole power organizer (IVPPO)
ActiveUS20080116157A1Improve patient careImprove convenienceWash-standsMedical devicesPatient roomMedicine
The invention provides an apparatus for safely storing and retrieving power cords and electronic wiring associated with a mobile intravenous pole serving patients in intensive care settings wherein a plurality of devices are required and enabling the patient to be mobile without causing power cords and wiring to become tangled and dragging on patient room or hospital floors.
Owner:FULBROOK JASON D +2
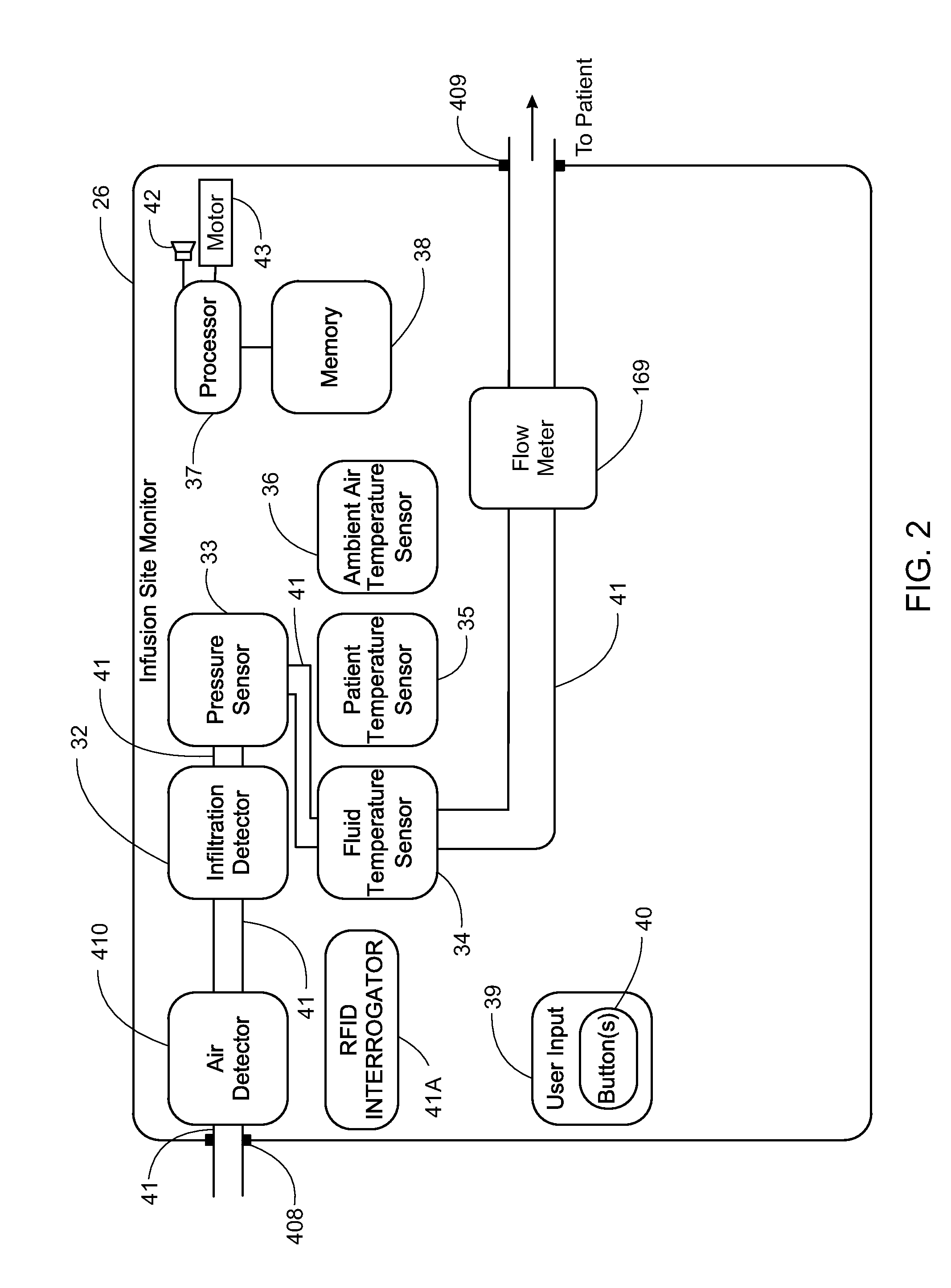
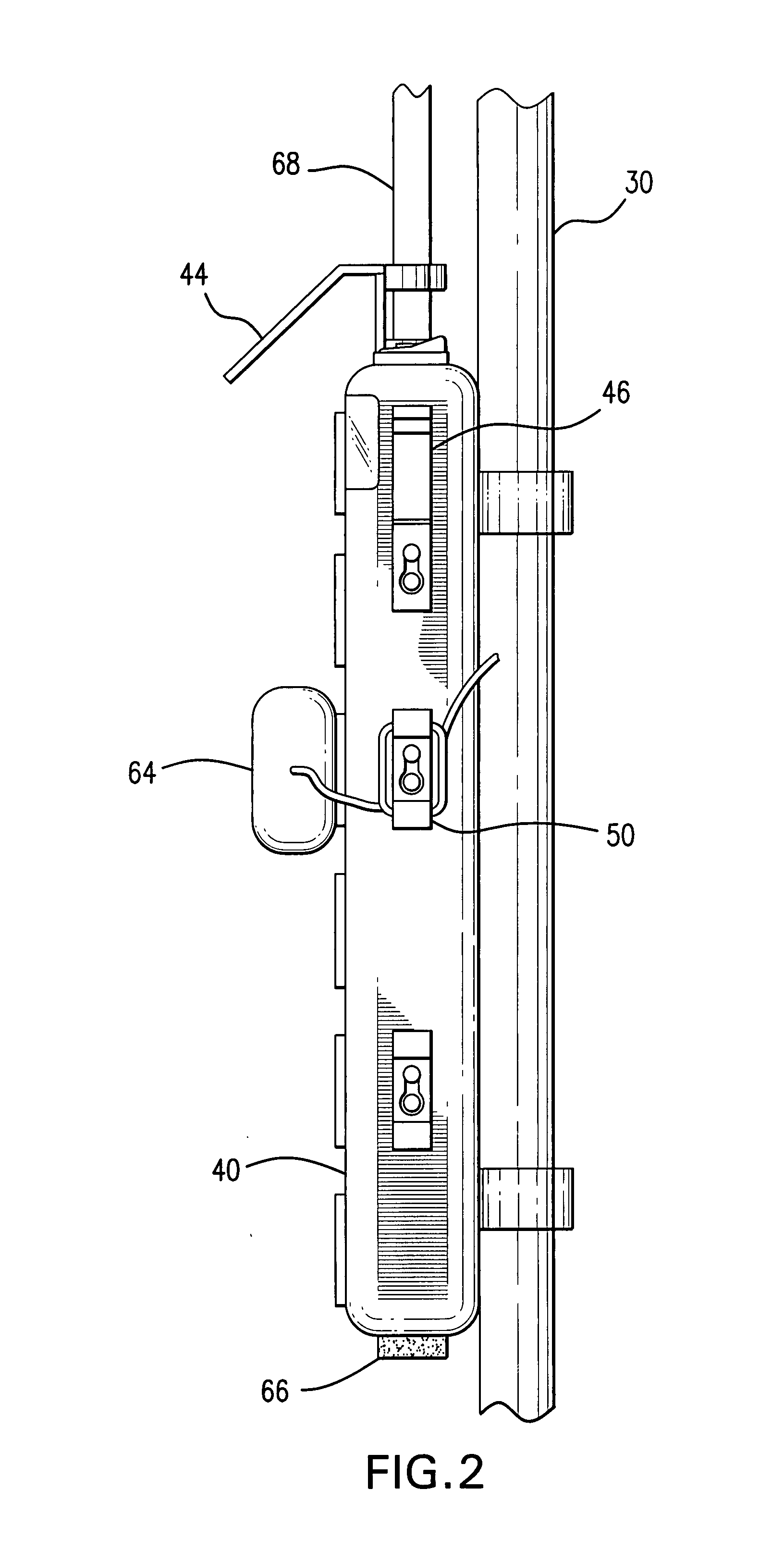
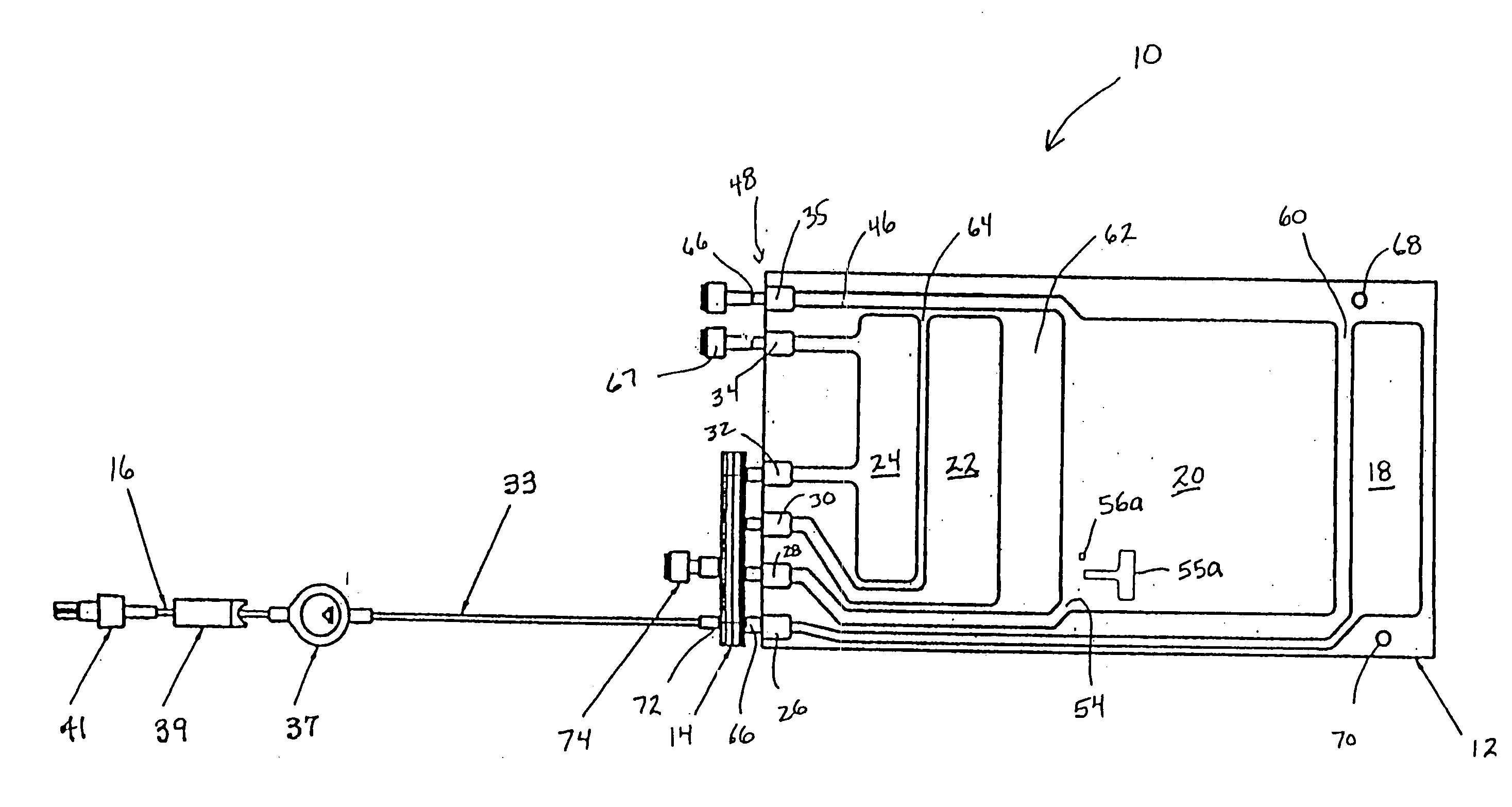
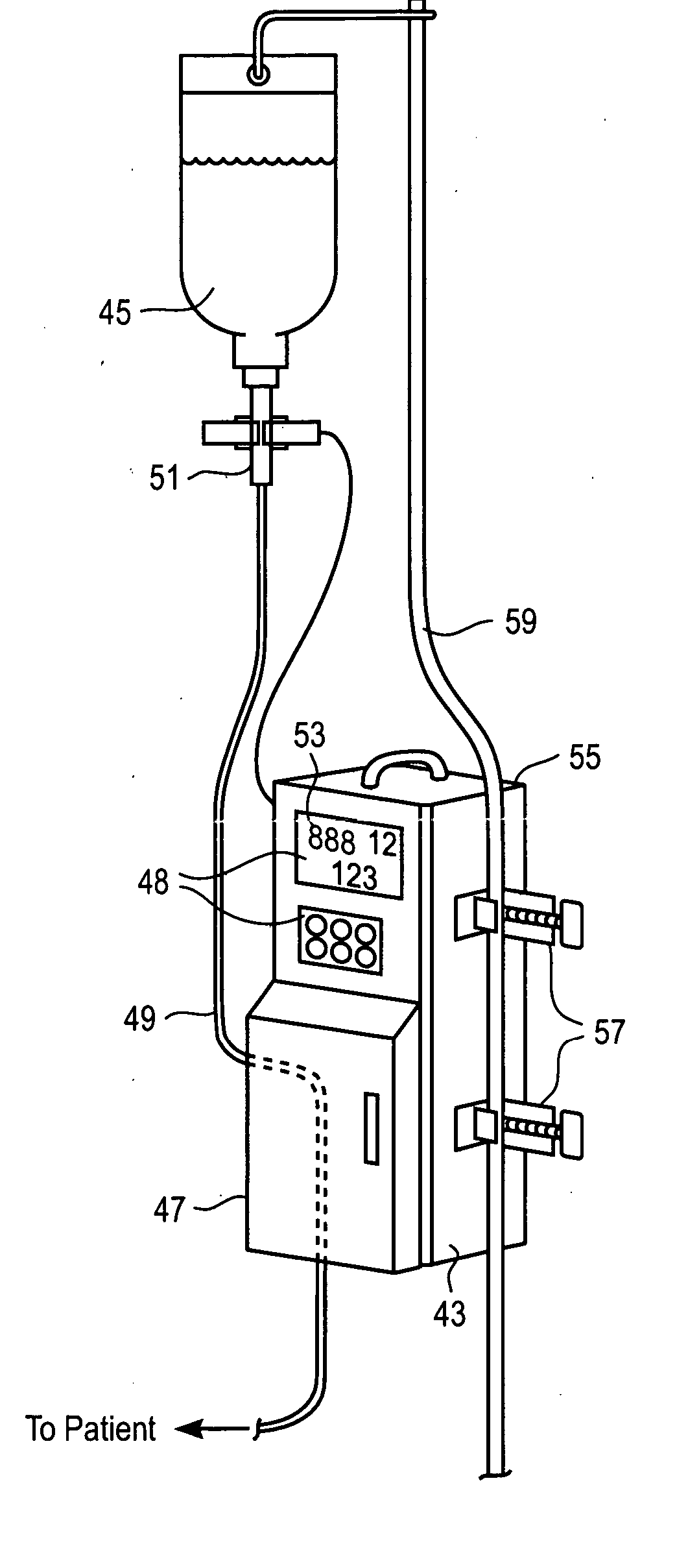
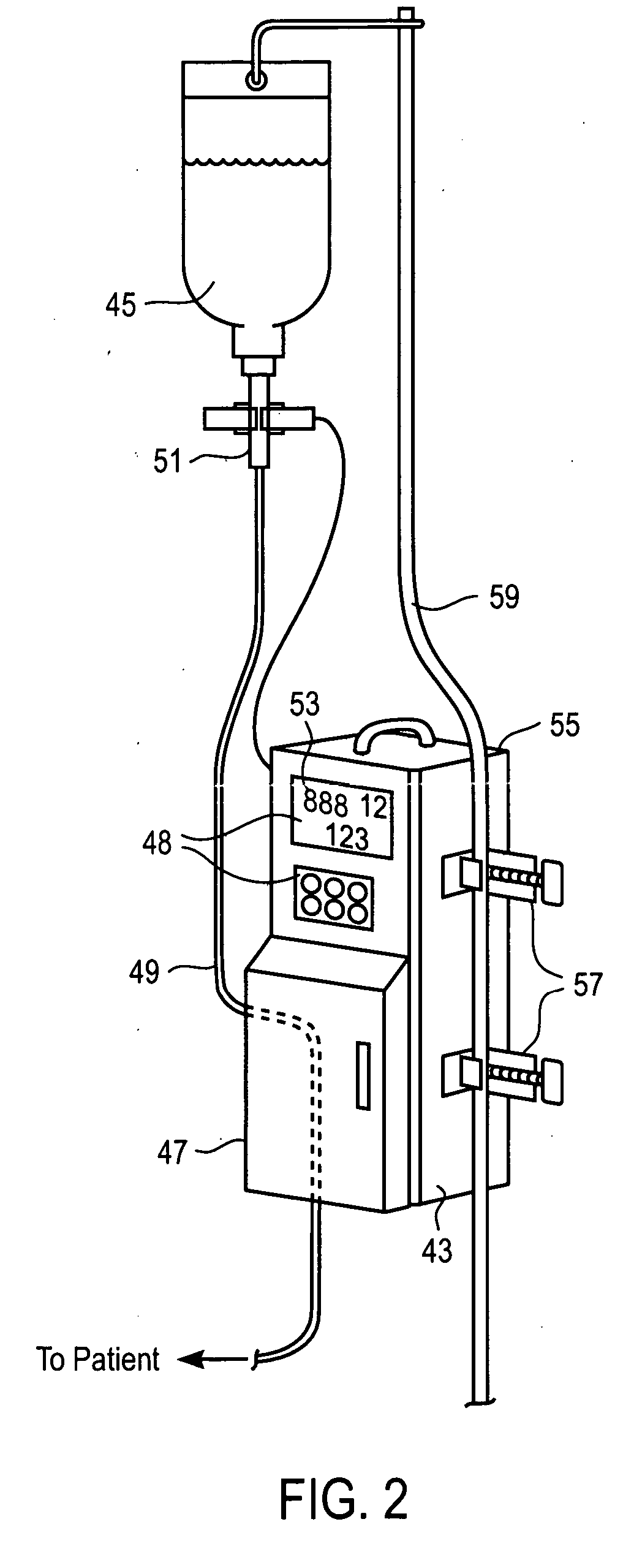
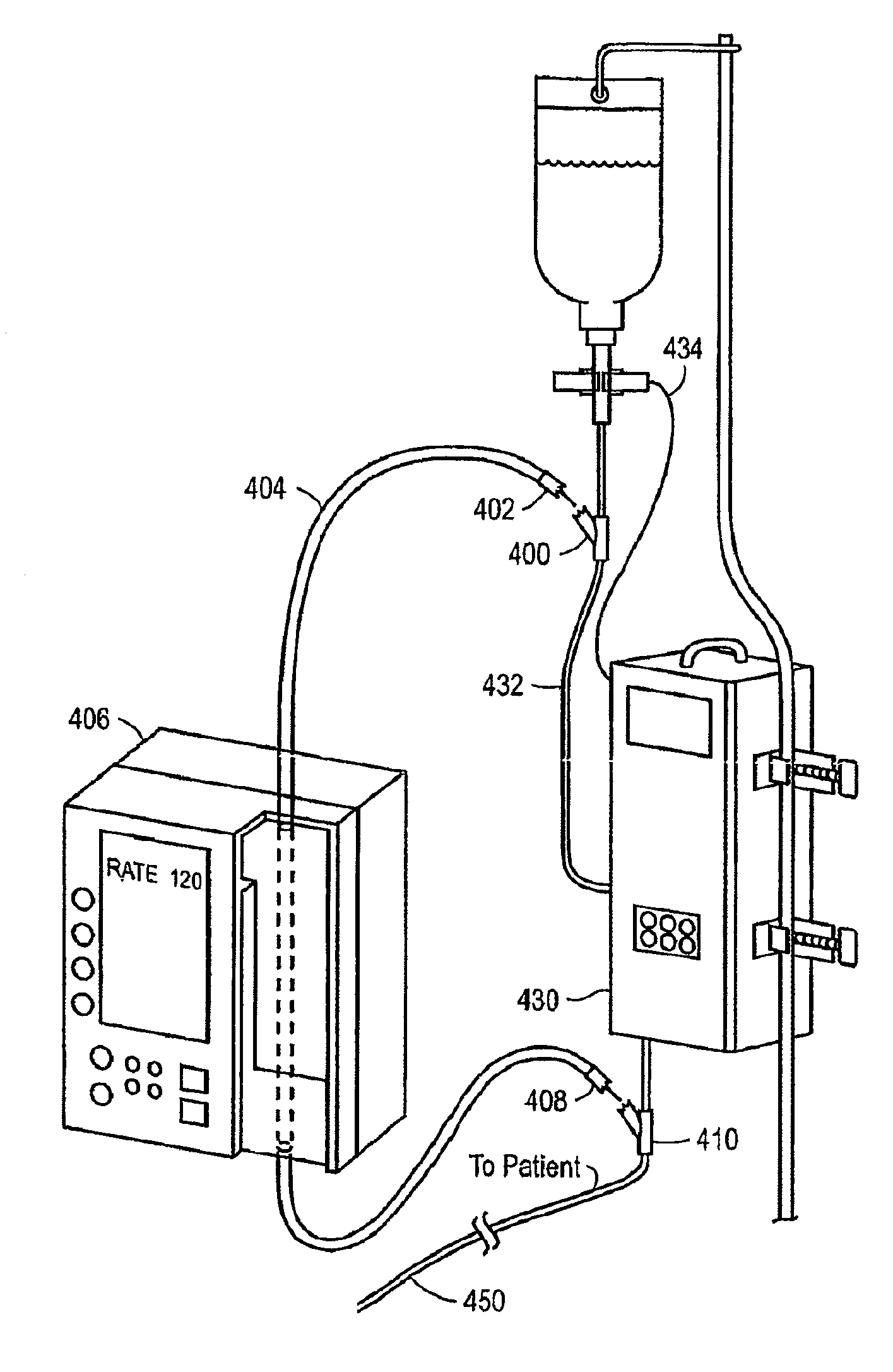
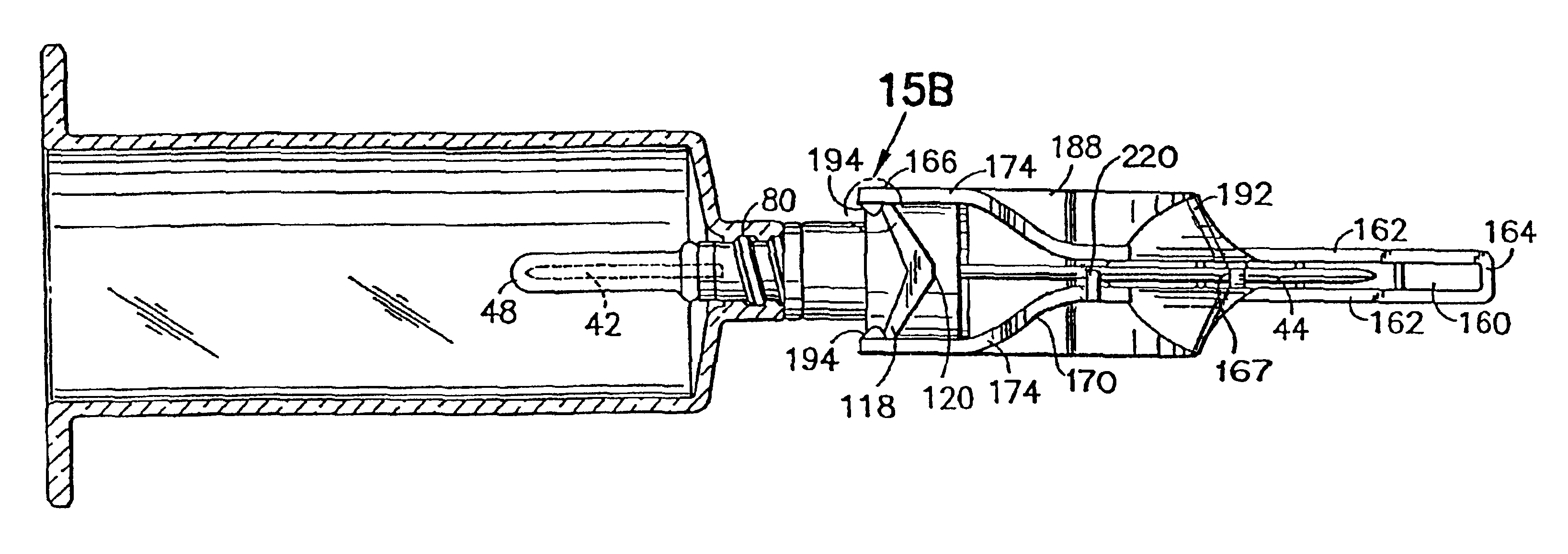
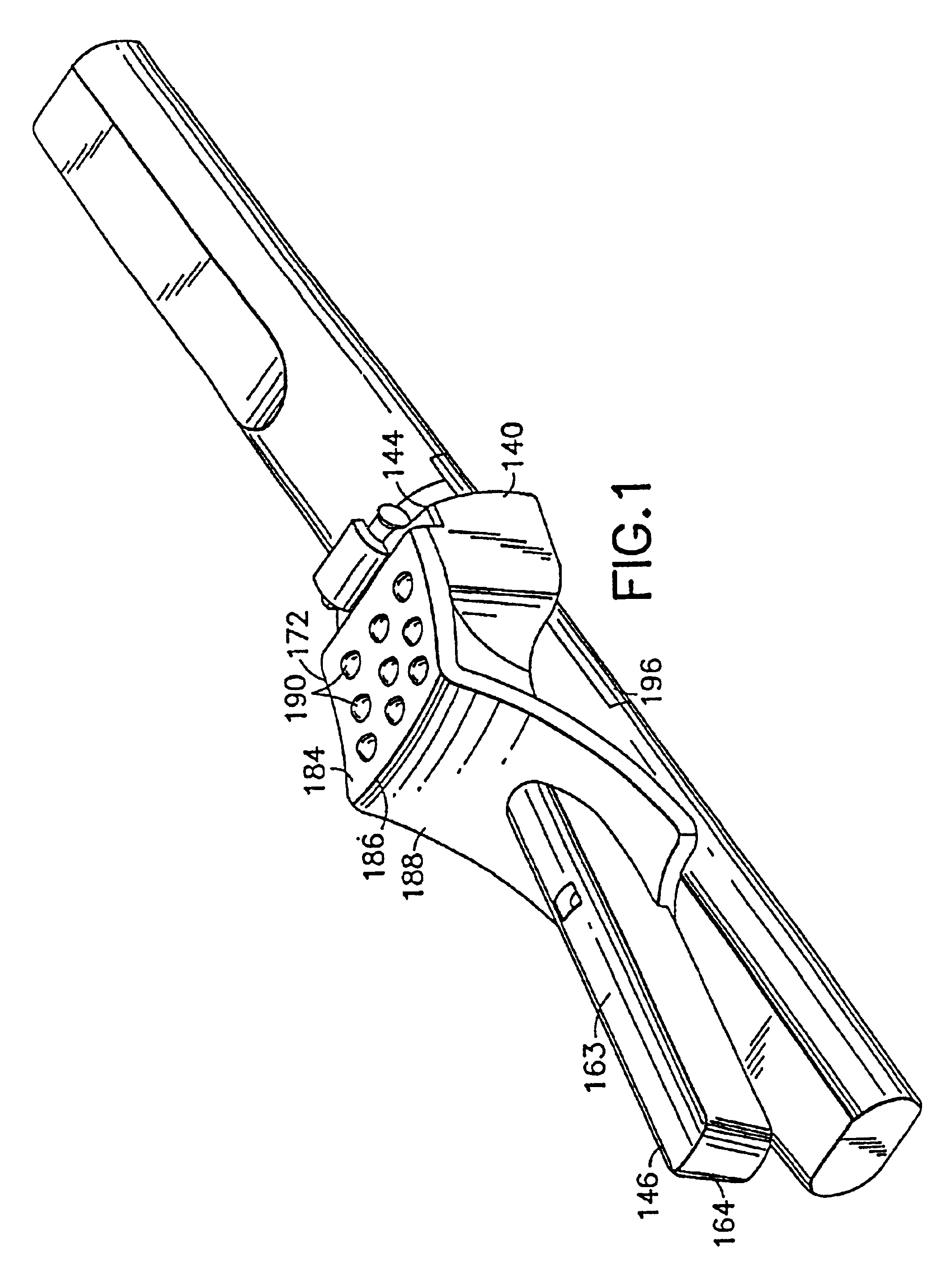
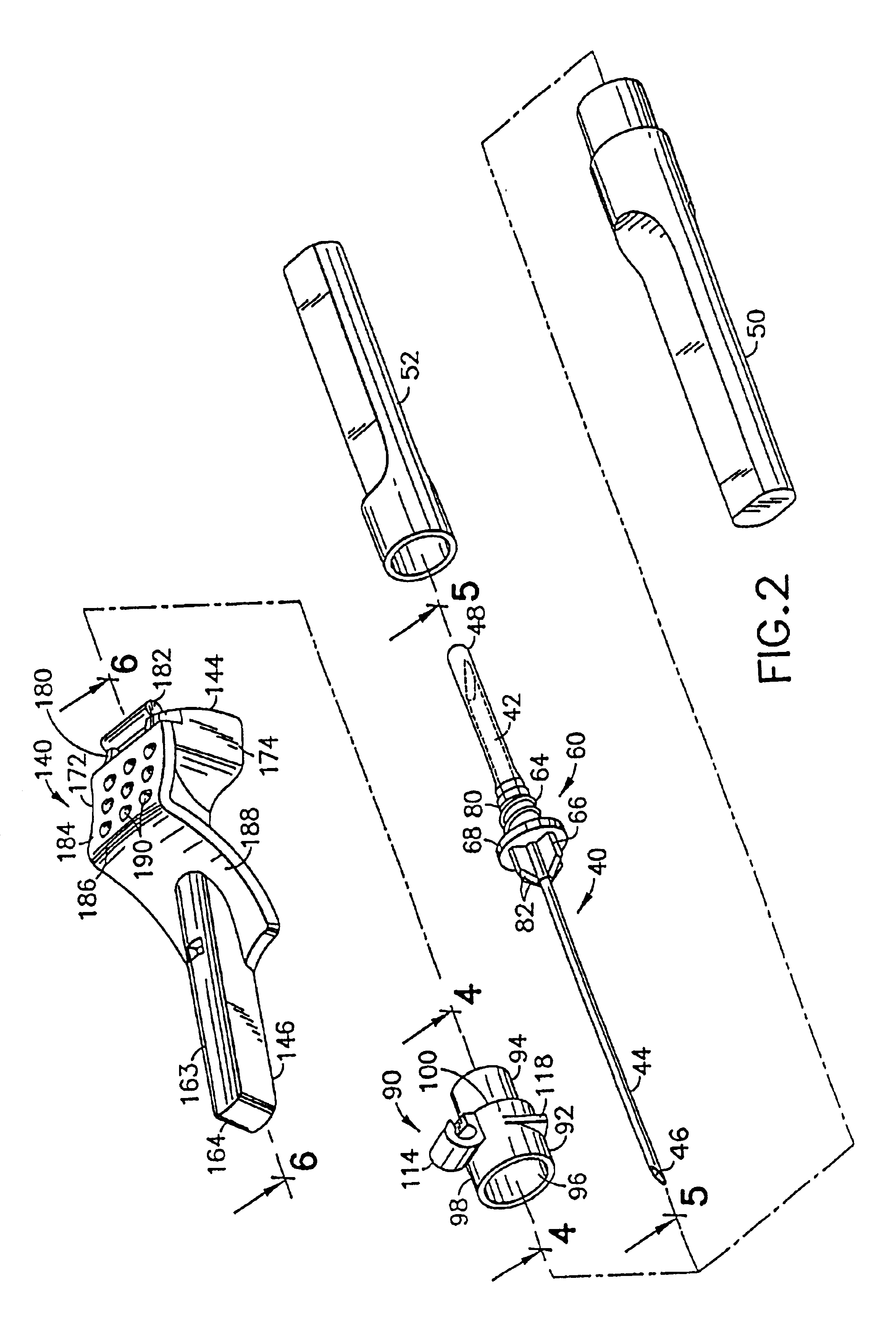
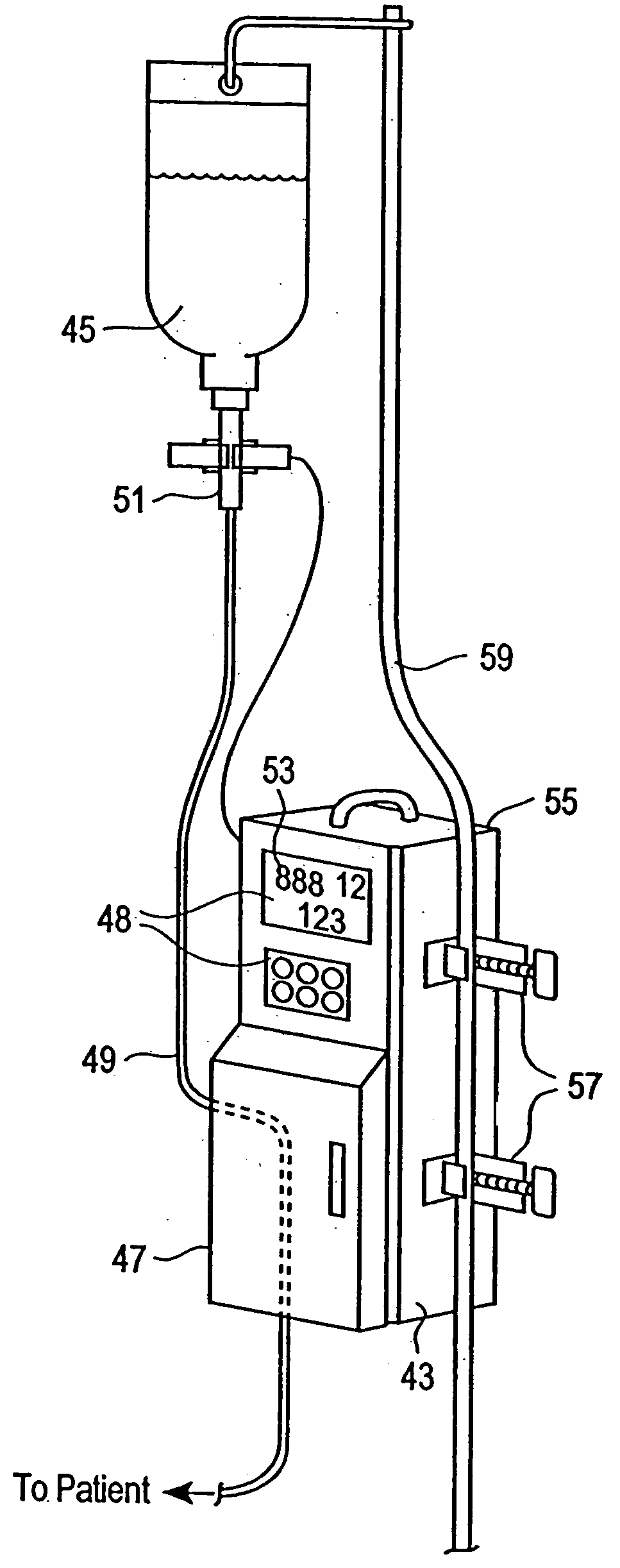
Medication delivery apparatus and methods for intravenous infusions
InactiveUS20060100578A1Reduce complicationsReduce errorsPharmaceutical containersMedical devicesVeinIntravenous Infusions
The present invention relates to delivery containers designed to deliver fluids for infusion to patients in a predetermined sequence, and methods for their construction and use. The containers described herein integrally comprise a plurality of non-fluidly connected chambers. The containers may be configured to deliver a volume of each medication of an infusion therapy in a predetermined sequence, duration, and / or interval from these chambers; alternatively, a container may be part of a larger device that provides the necessary hardware to perform such predetermined delivery. The container provides improved infusion therapy administration by reducing opportunities for error, infection, adverse drug interactions, or other complications.
Owner:TANDEM MEDICAL
Liquid infusion apparatus
InactiveUS20060173412A1Reduction of magnetic materialEliminate the problemDC motor speed/torque controlFlexible member pumpsPeristaltic pumpEngineering
Liquid infusion apparatus includes non-magnetic materials in a pumping structure and ultrasonic drive motor therefor, and in a controller that supplies drive signals to the motor to facilitate convenient operation in intense magnetic fields without distorting the magnetic fields and without radiating objectionable radio-frequency interference. A non-MRI-compatible liquid infusion apparatus is temporarily replaced with MRI-compatible, non-magnetic liquid infusion apparatus without disconnecting a patient from an installed intravenous infusion set to continue infusing liquid within the MRI environment. The pumping apparatus operates on a segment of a liquid conduit that is mounted in tension between a linear peristaltic pump and platen, with associated safety interlocks to assure proper operation of infusing liquid into a patient compatibly with conditions in an MRI environment. Drive circuitry generates low-harmonic signals for operating the ultrasonic motor at variable speeds to compensate for flow rate discontinuities through the peristaltic pumping cycles.
Owner:IRADIMED CORP
Non-magnetic medical infusion device
ActiveUS7404809B2Reduce materialEasy to controlFlexible member pumpsMedical devicesEngineeringDrive motor
Liquid infusion apparatus includes non-magnetic materials in a pumping structure and drive motor therefor, and in a controller that supplies drive signals to the motor to facilitate convenient operation in intense magnetic fields without distorting the magnetic fields and without radiating objectionable radio-frequency interference. A non-MRI-compatible liquid infusion apparatus is temporarily replaced with MRI-compatible, non-magnetic liquid infusion apparatus without disconnecting patient from an installed intravenous infusion set to continue infusing liquid within the MRI environment.
Owner:IRADIMED CORP
Automatic dispensing robot system and method
ActiveCN103006436AAvoid pollutionAvoid damageMixing methodsPharmaceutical containersHazardous substanceEngineering
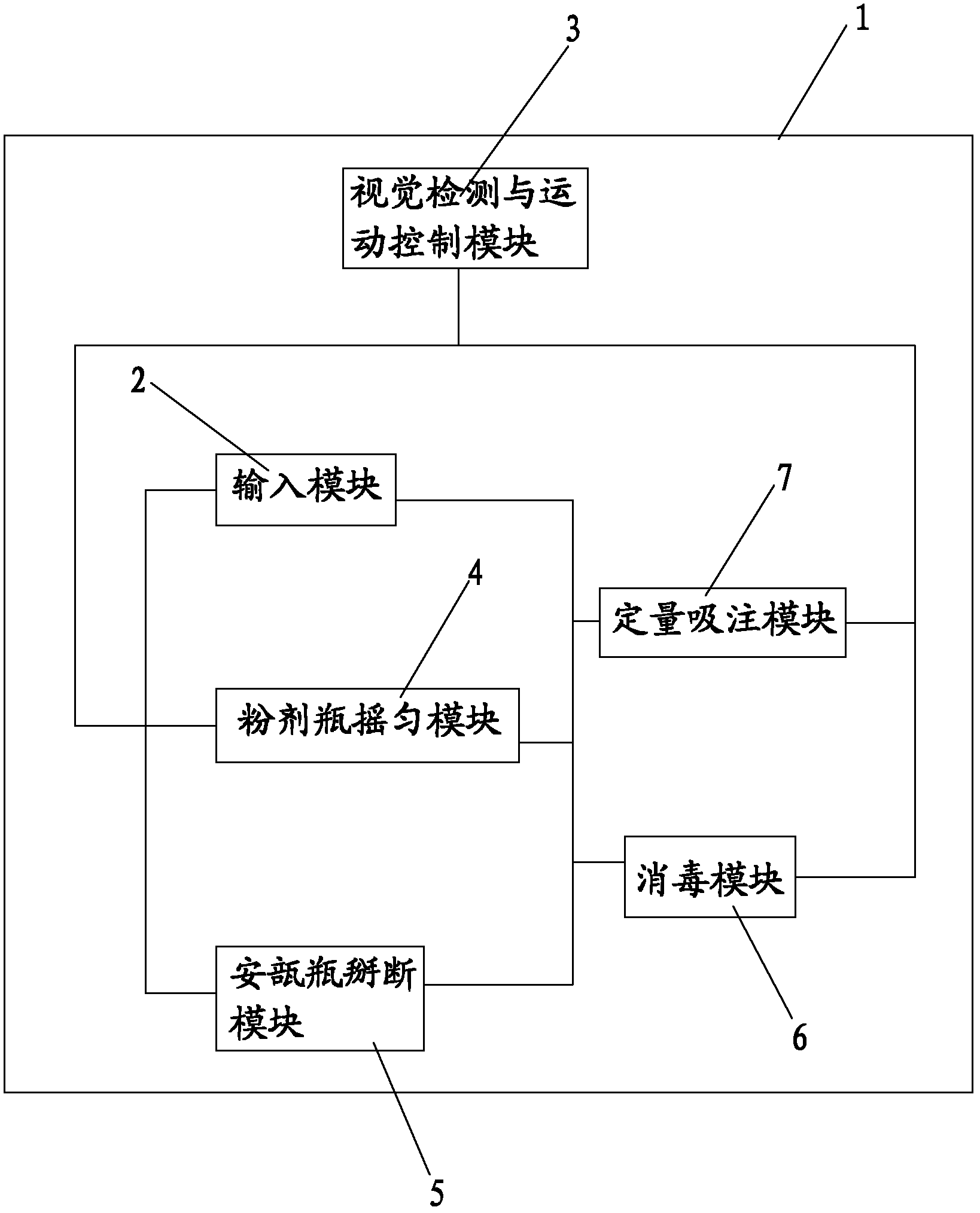
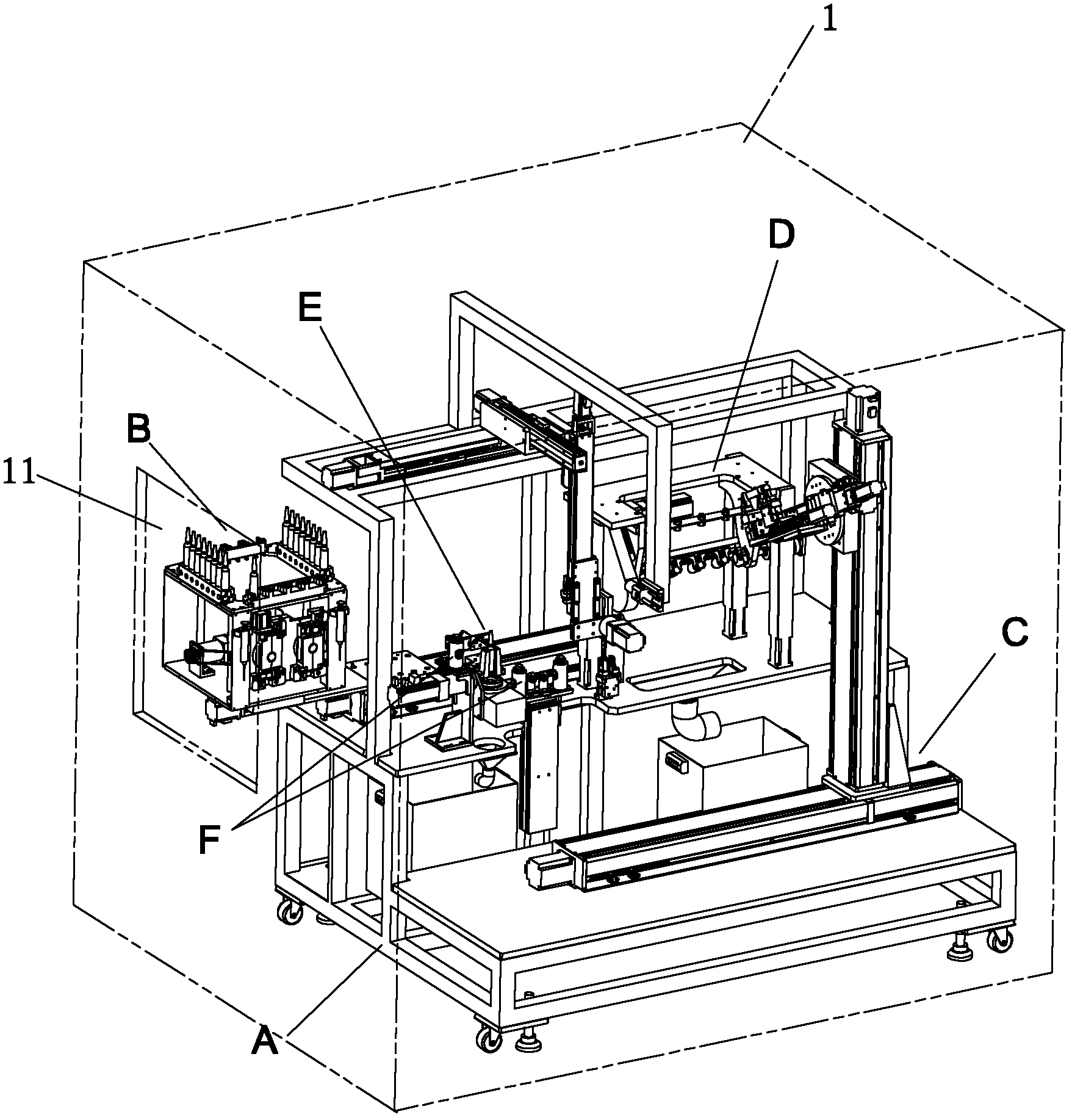
The invention discloses an automatic dispensing robot system and an automatic dispensing method, which are used for preparing an intravenous infusion solution. The system comprises a closed cavity, wherein the closed cavity can be opened, and an input module, a powder bottle shaking module, an ampoule bottle breaking module, a disinfection module, a quantitative injection module and a visual detection and motion control module are arranged in the closed cavity. According to the automatic dispensing robot system, the breaking, disinfection and quantitative suction of an ampoule bottle, the disinfection and quantitative suction of a powder bottle and the mixing with a mother solution can be automatically realized, and the solution is prevented from being polluted; additionally, nursing personnel are prevented from being radiated and hurt by harmful substances; the probability of misoperation is reduced; the operating standard of intravenous infusion dispensing is standardized; and the dispensing efficiency is increased.
Owner:SHENZHEN CITY WEIBANG TECH
Safety shield assembly
InactiveUS7223258B2Easy containmentEasily disposableInfusion syringesMedical devicesEngineeringIntravenous Infusions
The present invention is a safety shield assembly having a shield and a collar for connecting the shield to a fluid handling device whereby the shield may be pivoted with respect to the collar. Preferably, the safety shield assembly may be used with a needle assembly, an intravenous infusion set a syringe, a catheter or other fluid handling devices or assemblies that contain piercing elements.
Owner:BECTON DICKINSON & CO
Safety shield assembly
InactiveUS6780169B2Easy containmentEasily disposableGuide needlesGuide wiresEngineeringIntravenous Infusions
Owner:BECTON DICKINSON & CO
Non-magnetic medical infusion device
ActiveUS20060079758A1Reduce materialEasy to controlFlexible member pumpsMedical devicesDrive motorNon magnetic
Liquid infusion apparatus includes non-magnetic materials in a pumping structure and drive motor therefor, and in a controller that supplies drive signals to the motor to facilitate convenient operation in intense magnetic fields without distorting the magnetic fields and without radiating objectionable radio-frequency interference. A non-MRI-compatible liquid infusion apparatus is temporarily replaced with MRI-compatible, non-magnetic liquid infusion apparatus without disconnecting patient from an installed intravenous infusion set to continue infusing liquid within the MRI environment.
Owner:IRADIMED CORP
Automatic control-type, portable intravenous infusion apparatus and jacket therefor
InactiveUS6558346B1Constant dropping speed of an infusion liquid safely and surelyDrop in speedJet injection syringesMedical devicesAutomatic controlAir pump
Owner:MEDICOS HIRATA
Method for making a safety shield assembly and related combinations thereof
InactiveUS20050148942A1Easy containmentEasily disposableInfusion syringesMedical devicesEngineeringIntravenous Infusions
The present invention is a method for assembling a safety shield assembly and more particularly a method for assembling a safety shield assembly with a fluid handling device. Preferably, the safety shield assembly may be assembled with a needle assembly, an intravenous infusion set a syringe, a catheter or other fluid handling devices or assemblies that contain piercing elements.
Owner:BECTON DICKINSON & CO
Preparation of indissoluble medicament nano granule
InactiveCN101322682AIncrease Saturation SolubilityPassive targetingPowder deliveryPharmaceutical non-active ingredientsSide effectNanocrystal
The invention relates to the technical field of medicine, in particular to a preparation method of nano-particles of insoluble drugs. The method includes the following steps: (1) dissolving the drugs in a first solvent (good solvent) to form solution, (2) blending the solution with a second solvent (poor solvent) to form premixed suspension, (3) applying energy on the premixed suspension to form the nano-particles, the average effective grain diameter of which is less than 2Mum. By adopting the technology combining micro-deposition and homogenization, the invention suspends the drugs in poor solvent (usually water) in a form of pure nano-crystal, thus solving the problem that solution is difficult to be prepared since the drugs are difficult to be dissolved in water and oil; compared with the corresponding injection for intravenous infusion and the oral preparations such as tablets, capsules, and the like, the preparation method of the invention can lower adverse reaction, reduce toxic and side effect, improve bioavailability, has sustained-release effect and is convenient to be used by patients.
Owner:KANGYA OF NINGXIA PHARMA +1
Telescopic safety arteriovenous fistula needle
A telescopic intravenous infusion set and / or blood collection assembly is provided with a safety feature for covering the used needle. The safety feature is a telescopic device including a shield with handling wings, which when placed in cooperating relationship, allows accommodation of a conventional unmodified blood collection needle affixed to a hub, and a sleeve with a locking cap. After use, the hub with the needle are pulled rearwardly into positive locking position which prevent the hub and needle from moving out of the shield thereafter. The telescopic nature of the device then allows the locked needle / hub to move rearward in relation to the shield until the shield is unreleasably locked to the sleeve. The shield provides for passage of the needle and hub from a releasable locked use position to a shielded and unreleasable locked protected position.
Owner:JMS SINGAPORE PTE
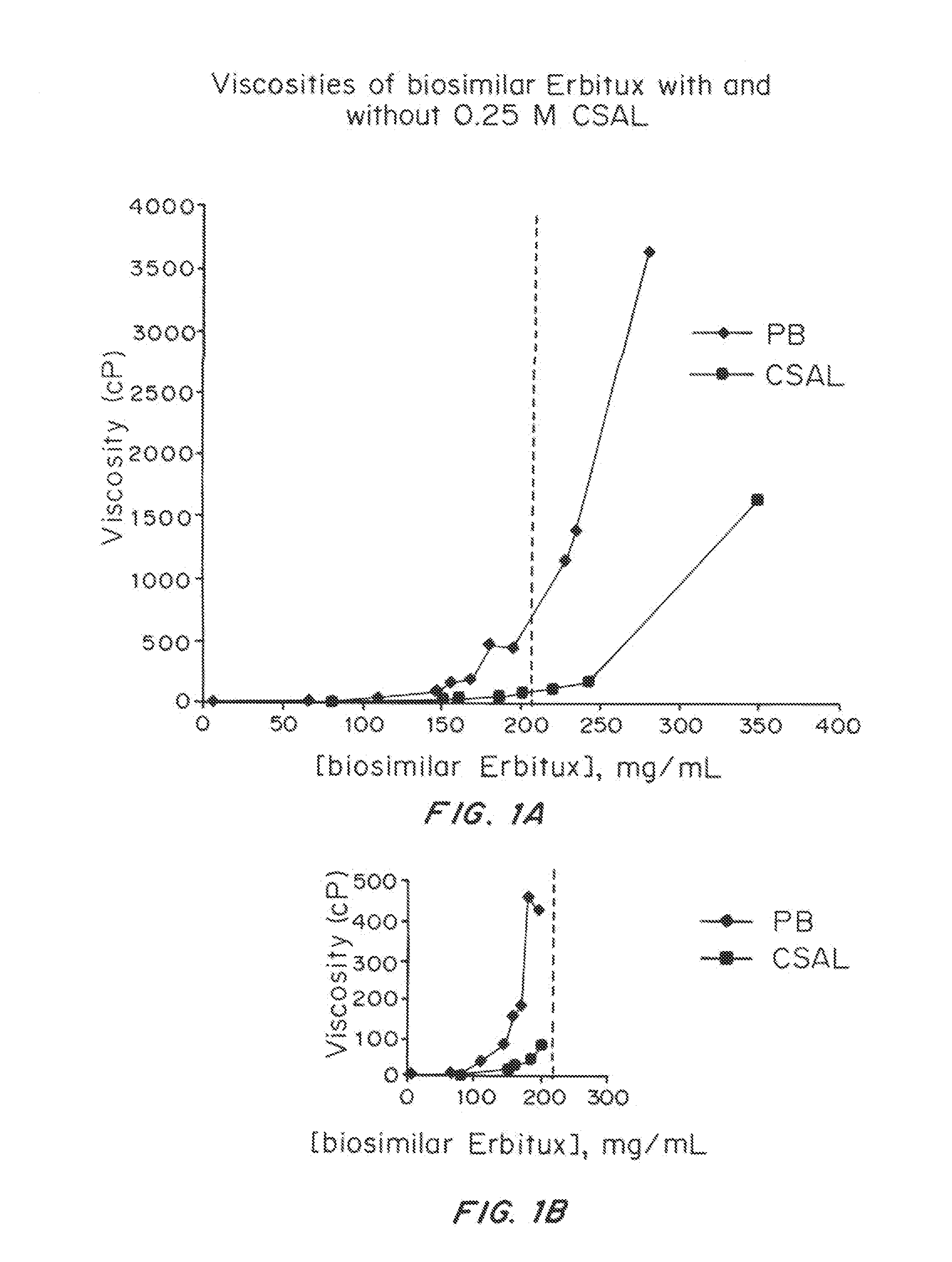
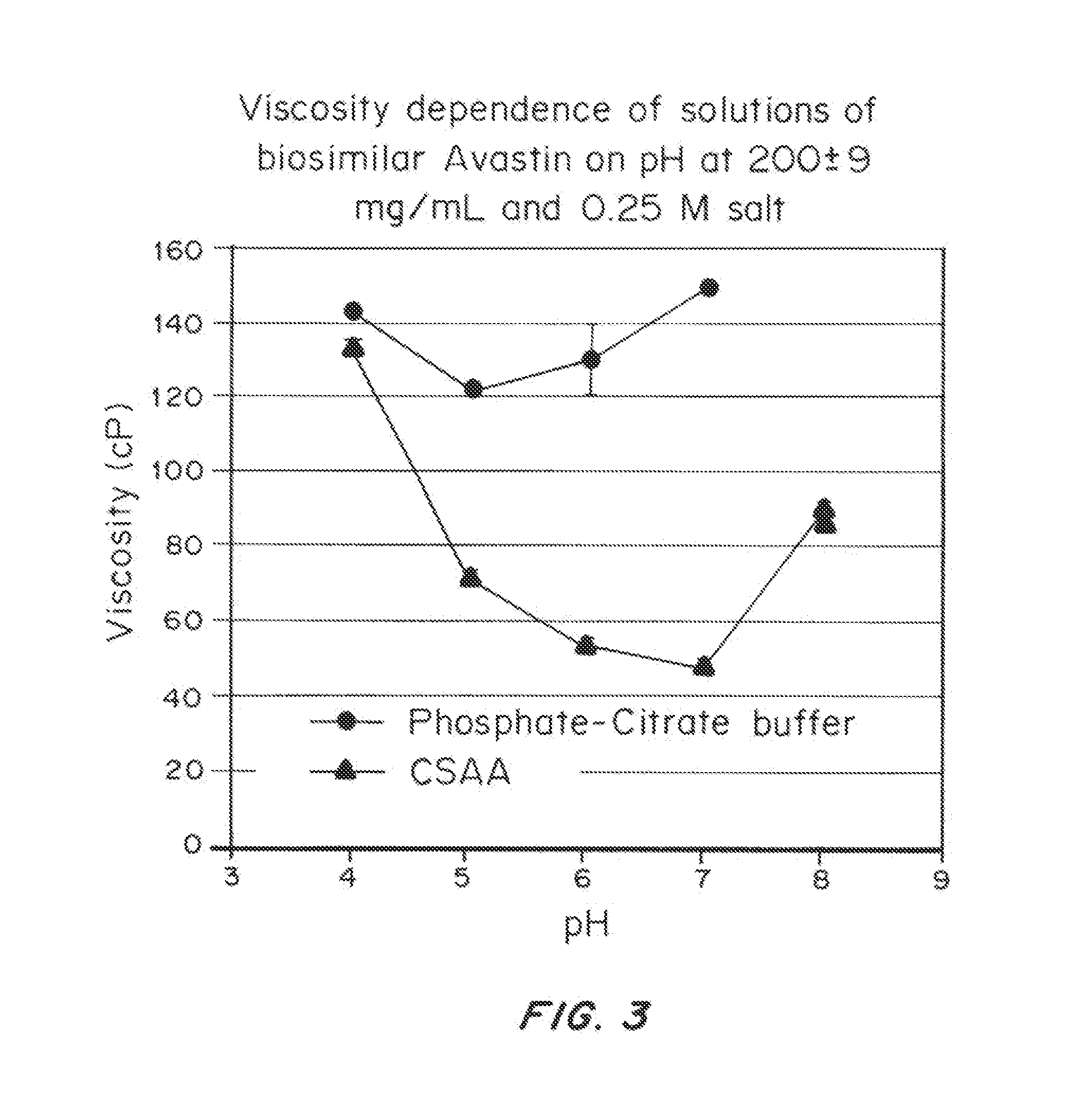
Liquid protein formulations containing viscosity-lowering agents
ActiveUS20150071925A1Facilitates and accelerates reconstitutionEasy to processNervous disorderPeptide/protein ingredientsIntramuscular injectionAdditive ingredient
Concentrated, low-viscosity, low-volume liquid pharmaceutical formulations of proteins have been developed. Such formulations can be rapidly and conveniently administered by subcutaneous or intramuscular injection, rather than by lengthy intravenous infusion. These formulations include low-molecular-weight and / or high-molecular-weight proteins, such as mAbs, and viscosity-lowering agents that are typically bulky polar organic compounds, such as many of the GRAS (US Food and Drug Administration List of compounds generally regarded as safe) and inactive injectable ingredients and FDA approved therapeutics.
Owner:EAGLE BIOLOGICS INC
Safety shield assembly
InactiveUS20020151853A1Easy containmentEasily disposableGuide needlesInfusion syringesEngineeringIntravenous Infusions
The present invention is a safety shield assembly having a shield and a collar for connecting the shield to a fluid handling device whereby the shield may be pivoted with respect to the collar. Preferably, the safety shield assembly may be used with a needle assembly, an intravenous infusion set a syringe, a catheter or other fluid handling devices or assemblies that contain piercing elements.
Owner:BECTON DICKINSON & CO
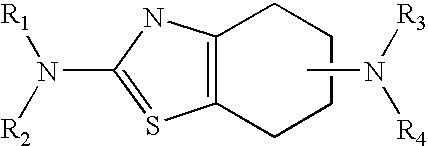
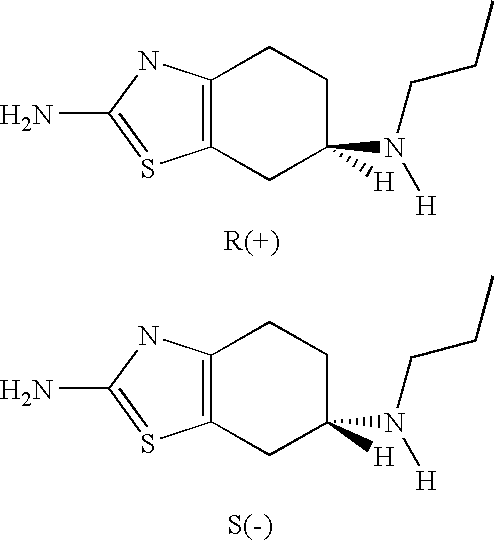
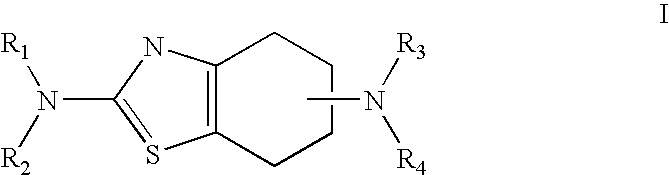
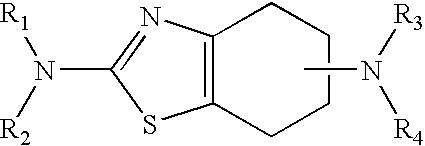
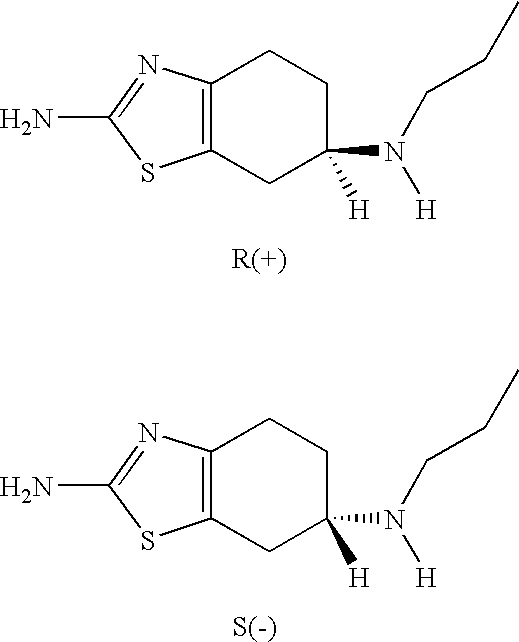
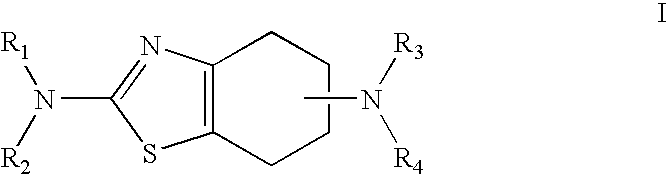
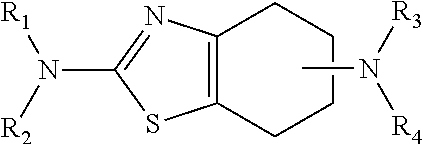
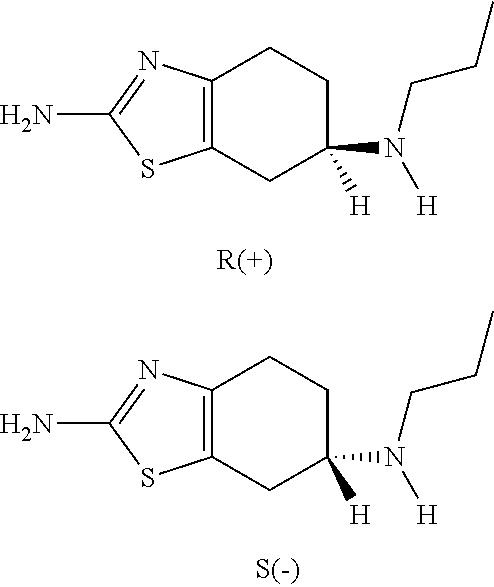
Neurorestoration with R(+) Pramipexole
Formulations and methods of use thereof for restoring neuronal, muscular (cardiac and striated) and / or retinal tissue function in children and adults afflicted with chronic neurodegenerative diseases, such as neurodegenerative movement disorders and ataxias, seizure disorders, motor neuron diseases, and inflammatory demyelinating disorders, are described herein. Examples of disorders include Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). The method involves administering a pharmaceutical composition containing an effective amount of a tetrahydrobenzathiazole, preferably a formulation consisting substantially of the R(+) enantiomer of pramipexole. R(+) pramipexole is generally administered in doses ranging from 0.1-300 mg / kg / daily, preferably 0.5-50 mg / kg / daily, and most preferably 1-10 mg / kg / daily for oral administration. Daily total doses administered orally are typically between 10 mg and 500 mg. Alternatively, R(+) pramipexole can be administered parenterally to humans with acute brain injury in single doses between 10 mg and 100 mg and / or by continuous intravenous infusions between 10 mg / day and 500 mg / day.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Safety shield assembly
InactiveUS20050245879A9Easy containmentEasily disposableInfusion syringesMedical devicesEngineeringIntravenous Infusions
Owner:BECTON DICKINSON & CO
Prostacyclin compounds, compositions and methods of use thereof
InactiveUS20160318844A1Increase the areaConvenient timeOrganic chemistryOrganic compound preparationDipeptideCarbamate
Prostacyclin compounds and compositions comprising the same are provided herein. Specifically, prostacyclin compounds comprising treprostinil covalently linked to a linear C2-C18 alkyl, branched C3-C18 alkyl, linear C2-C18 alkenyl, branched C3-C18 alkenyl, aryl, aryl-C1-C18 alkyl or an amino acid or a peptide (e.g., dipeptide, tripeptide, tetrapeptide) are described, for example, for administration via subcutaneous or intravenous infusion to a patient in need of pulmonary hypertension treatment. The linkage, in one embodiment, is via a carbamate, amide or ester bond. Prostacyclin compounds provided herein can also include at least one hydrogen atom substituted with at least one deuterium atom.
Owner:INSMED INC
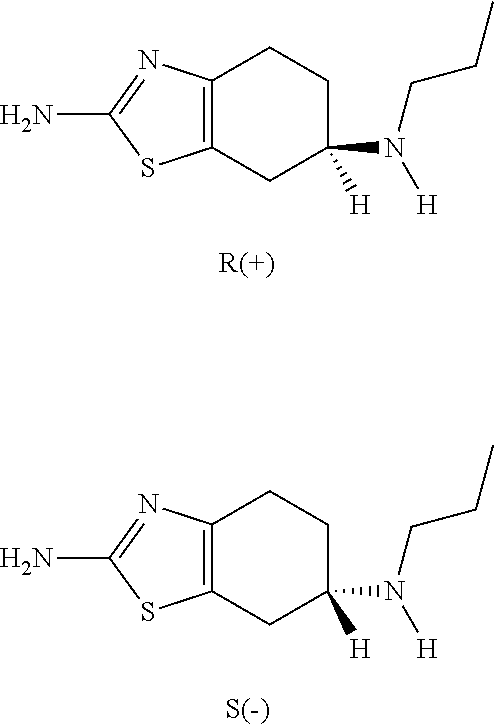
Neurorestoration With R(+) Pramipexole
Formulations and methods of use thereof for restoring neuronal tissue function in children and adults afflicted with chronic neurodegenerative diseases, such as neurodegenerative movement disorders and ataxias, seizure disorders, motor neuron diseases, and inflammatory demyelinating disorders. Examples of disorders include Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). The method involves administering a pharmaceutical composition containing an effective amount of a tetrahydrobenzathiazole, preferably a formulation consisting substantially of the R(+) enantiomer of pramipexole. R(+) pramipexole is generally administered in doses ranging from 0.1-300 mg / kg / daily, preferably 0.5-50 mg / kg / daily, and most preferably 1-10 mg / kg / daily for oral administration. Daily total doses administered orally are typically between 10 mg and 500 mg. Alternatively, R(+) pramipexole can be administered parenterally to humans with acute brain injury in single doses between 10 mg and 100 mg, and / or by continuous intravenous infusions between 10 mg / day and 500 mg / day.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Neurorestoration with r(+) pramipexole
Formulations and methods of use thereof for restoring neuronal, muscular (cardiac and striated) and / or retinal tissue function in children and adults afflicted with chronic neurodegenerative diseases, such as neurodegenerative movement disorders and ataxias, seizure disorders, motor neuron diseases, and inflammatory demyelinating disorders, are described herein. Examples of disorders include Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). The method involves administering a pharmaceutical composition containing an effective amount of a tetrahydrobenzathiazole, preferably a formulation consisting substantially of the R(+) enantiomer of pramipexole. R(+) pramipexole is generally administered in doses ranging from 0.1-300 mg / kg / daily, preferably 0.5-50 mg / kg / daily, and most preferably 1-10 mg / kg / daily for oral administration. Daily total doses administered orally are typically between 10 mg and 500 mg. Alternatively, R(+) pramipexole can be administered parenterally to humans with acute brain injury in single doses between 10 mg and 100 mg, and / or by continuous intravenous infusions between 10 mg / day and 500 mg / day.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Neurorestoration with r(+) pramipexole
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Liquid protein formulations containing water soluble organic dyes
ActiveUS20150071920A1Facilitates and accelerates reconstitutionEasy to processNervous disorderPeptide/protein ingredientsIntramuscular injectionMedicine
Concentrated, low-viscosity, low-volume liquid pharmaceutical formulations of proteins have been developed. Such formulations can be rapidly and conveniently administered by subcutaneous or intramuscular injection, rather than by lengthy intravenous infusion. These formulations include low-molecular-weight and / or high-molecular-weight proteins, such as mAbs, and viscosity-lowering water soluble organic dyes.
Owner:EAGLE BIOLOGICS INC
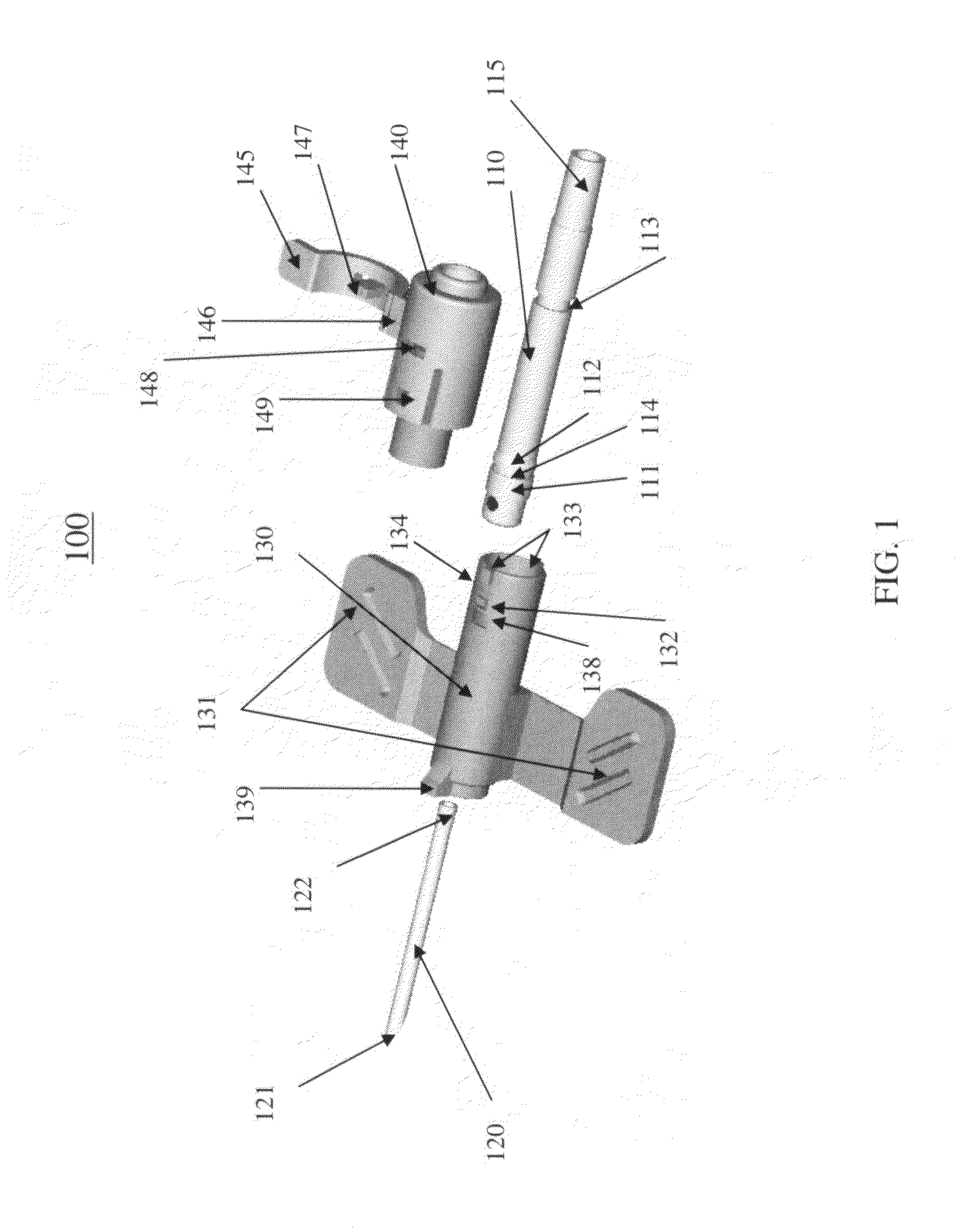
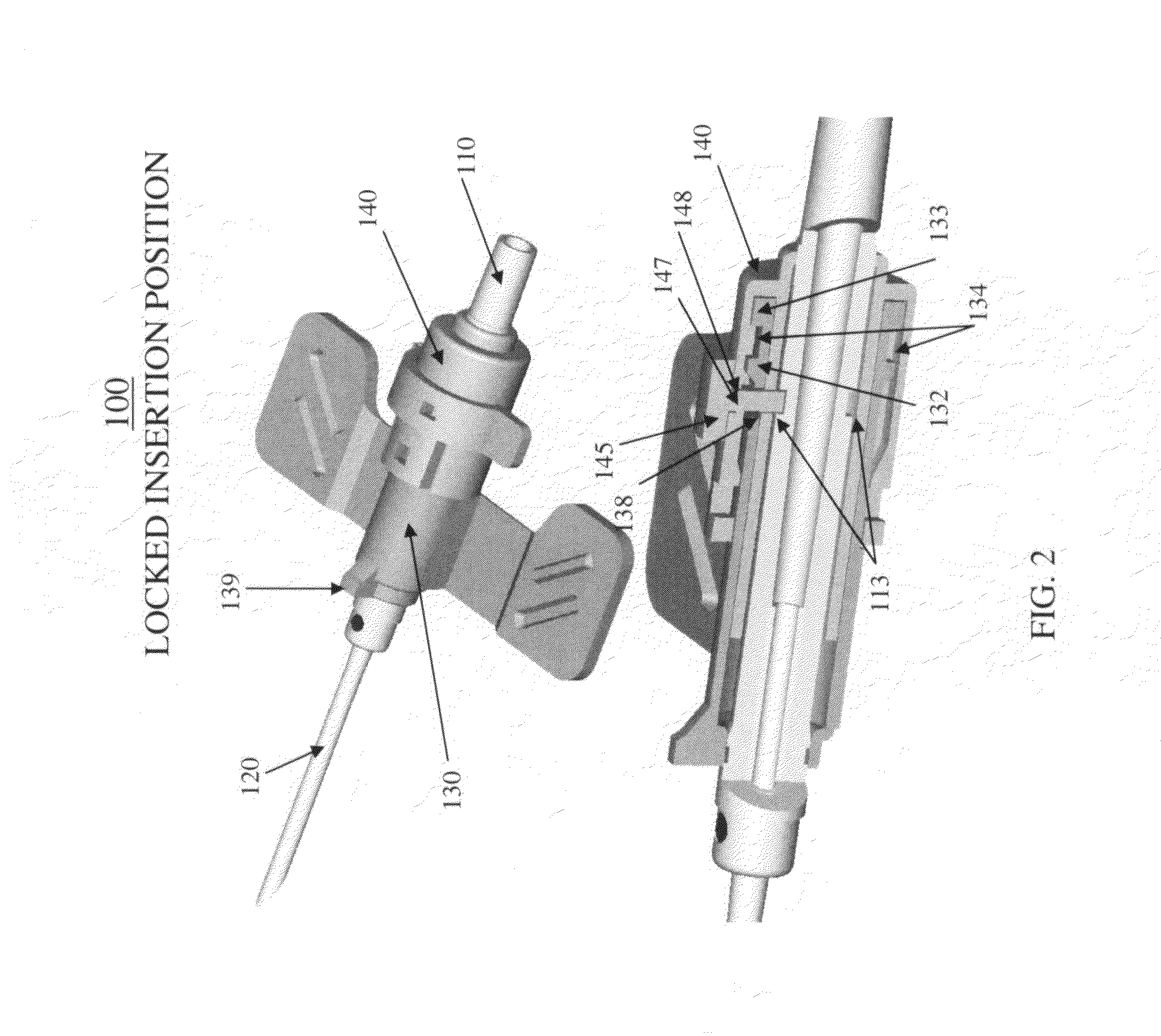
Ported catheter adapter with integrated septum actuator retention
ActiveUS20150202422A1Prevent displacement and dislodgingInhibition retentionGuide needlesInfusion syringesVeinCATHETER ADAPTER
A ported catheter adapter and septum actuator having various features to prevent displacement and dislodging of the septum actuator when accessing the patient's vasculature via the inserted infusion device. In particular, the systems and methods of the present invention provide an intravenous infusion device incorporating a septum actuator with a retention tab that interacts with a retention ring that is incorporated into the valve of a side port. This interaction retains the septum actuator within the lumen of the catheter adapter, thereby allowing for subsequent access to the patient's vasculature.
Owner:BECTON DICKINSON & CO
Intravenous pole power organizer (IVPPO)
ActiveUS7874410B2Improve efficiency and organizationEffective medicalWash-standsMedical devicesPatient roomMedicine
The invention provides an apparatus for safely storing and retrieving power cords and electronic wiring associated with a mobile intravenous pole serving patients in intensive care settings wherein a plurality of devices are required and enabling the patient to be mobile without causing power cords and wiring to become tangled and dragging on patient room or hospital floors.
Owner:FULBROOK JASON D +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com