Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
219 results about "Mri compatible" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
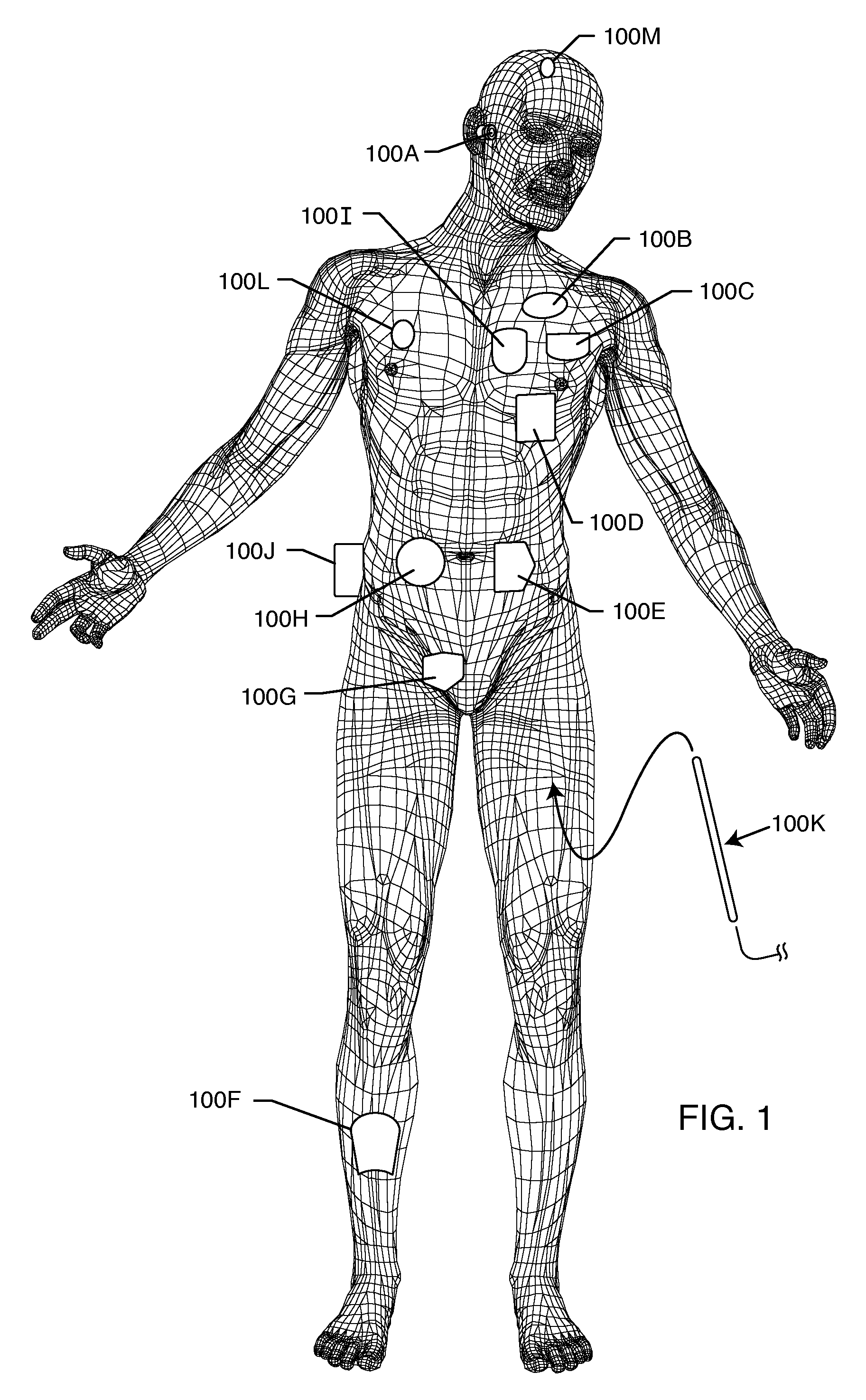
Mentor is occasionally asked if breast implants or tissue expanders are compatible with MRI (Magnetic Resonance Imaging). All MENTOR® Saline-Filled and Gel-Filled Breast Implants are MRI compatible. The implants are made from silicone elastomers and contain no metal or magnetic material.
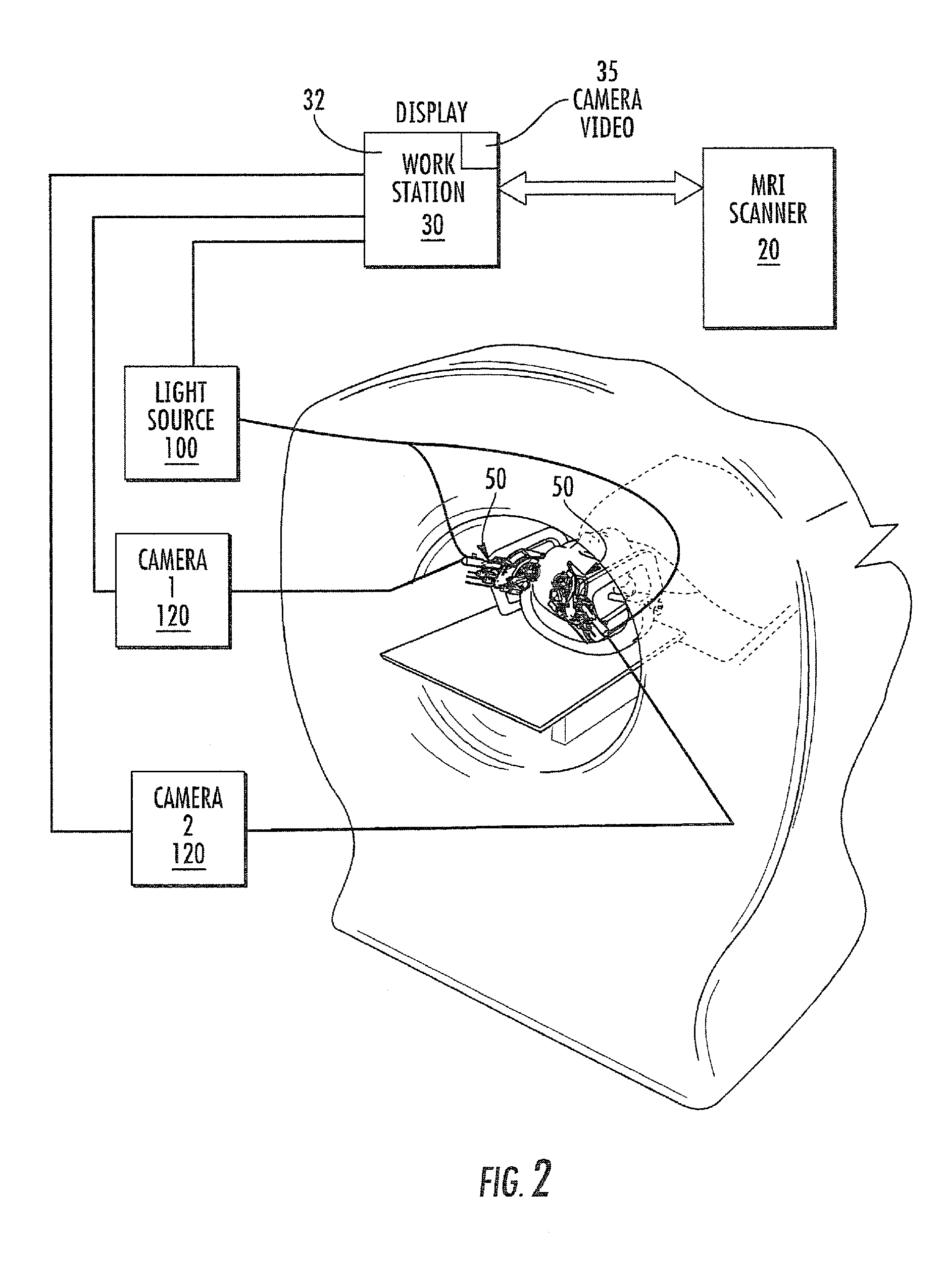
Microsurgical robot system
ActiveUS7155316B2Minimize patient riskComplete efficientlyProgramme-controlled manipulatorEndoscopesMicroscopic observationDisplay device
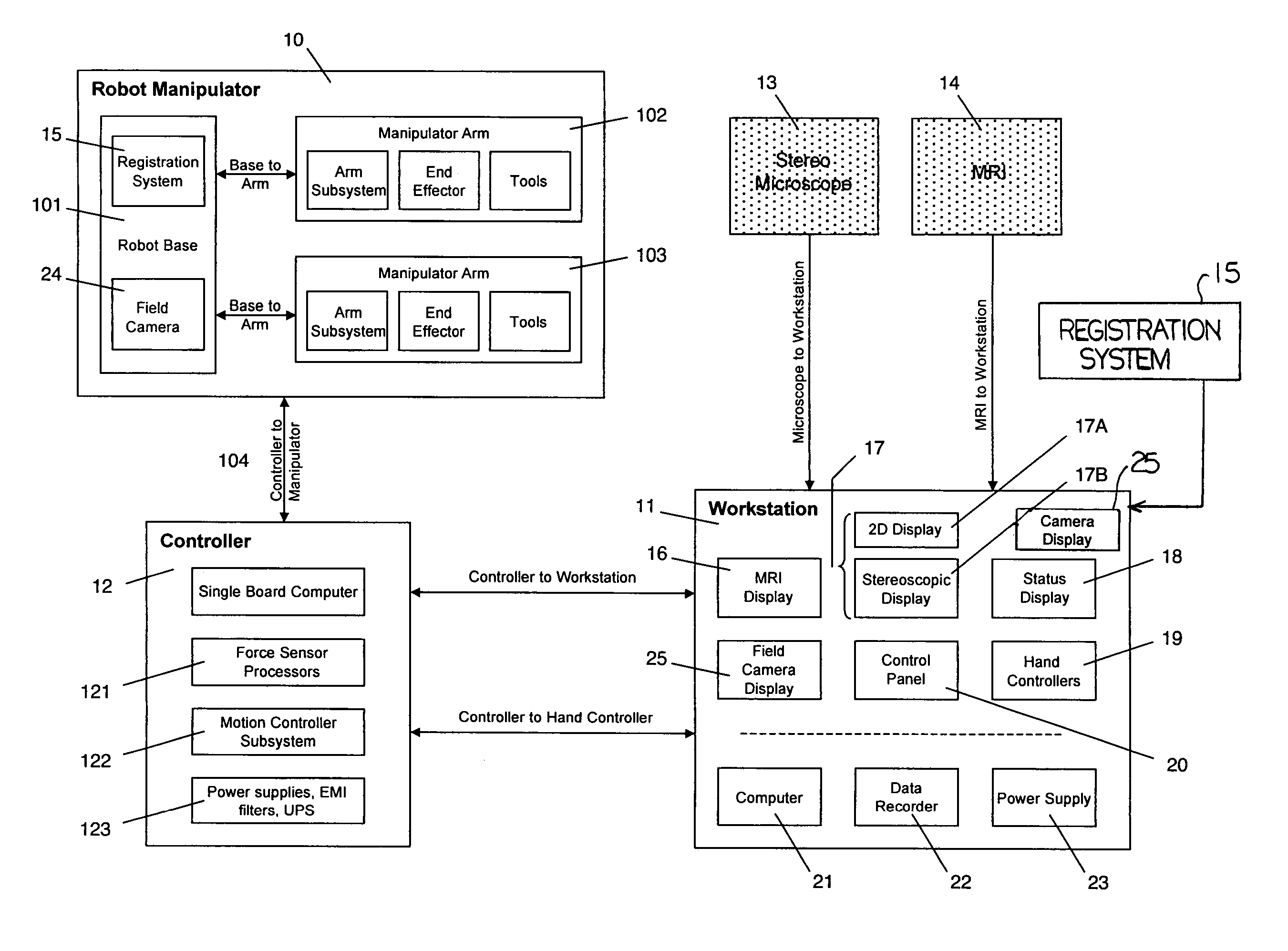
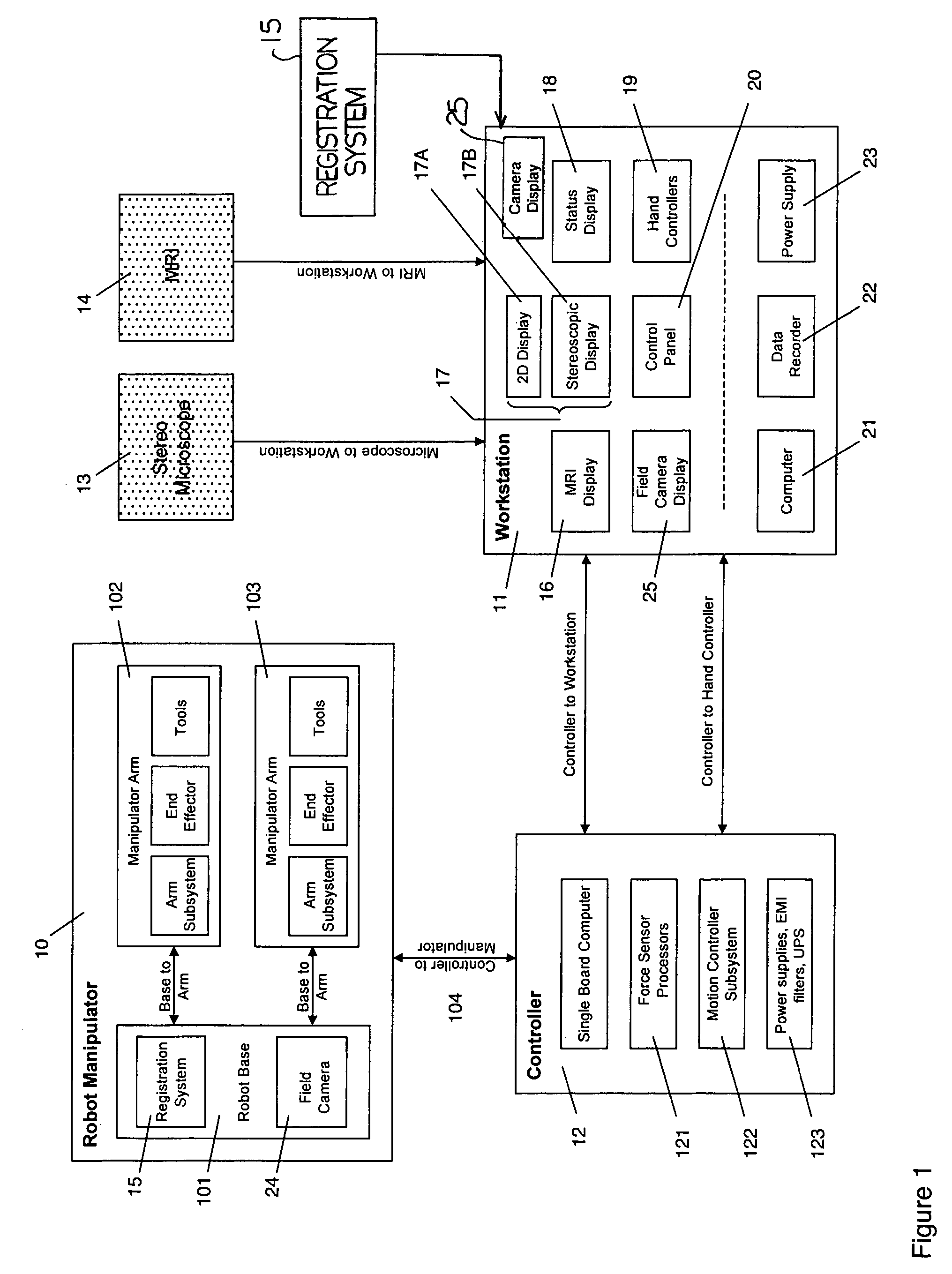
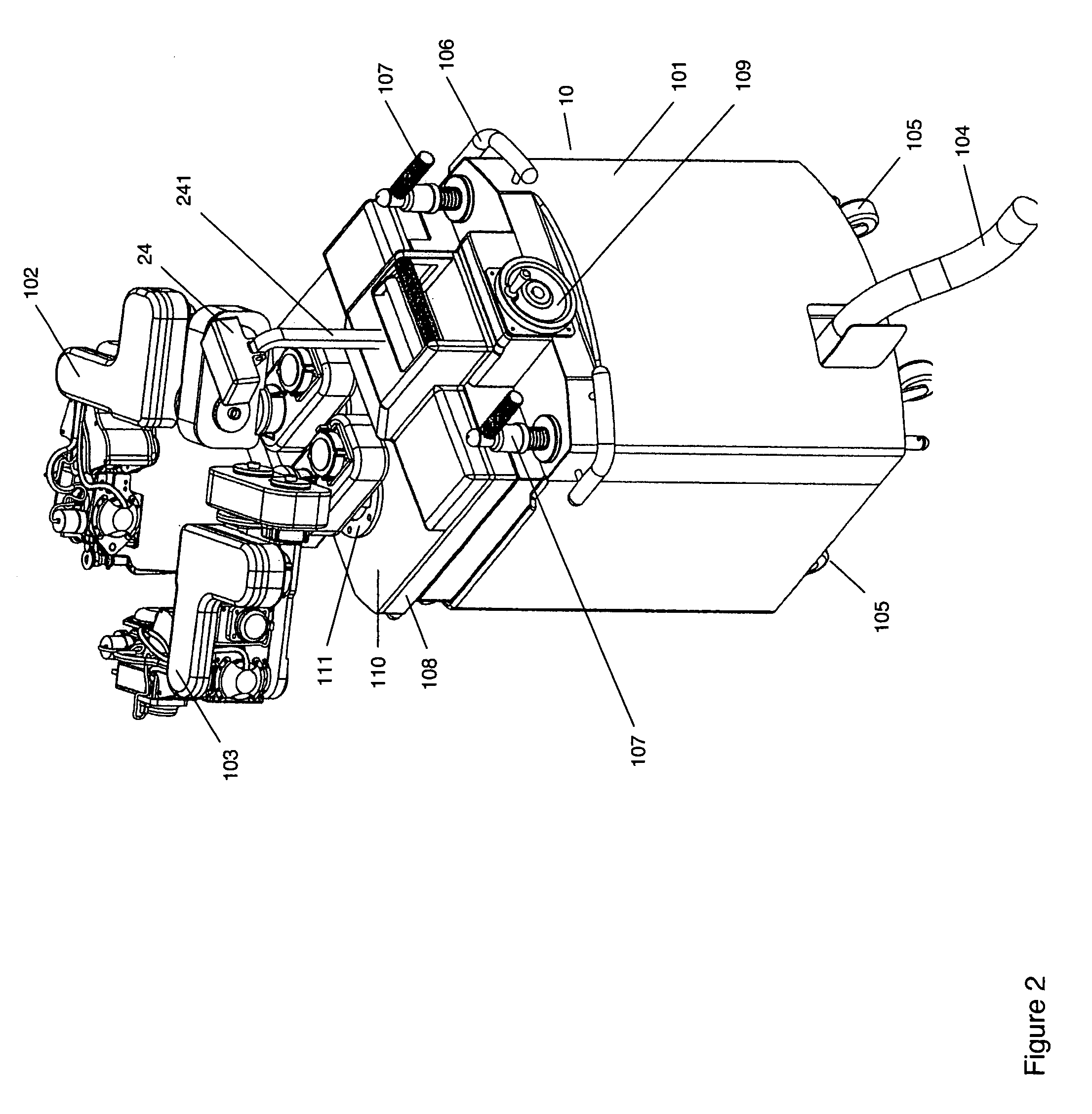
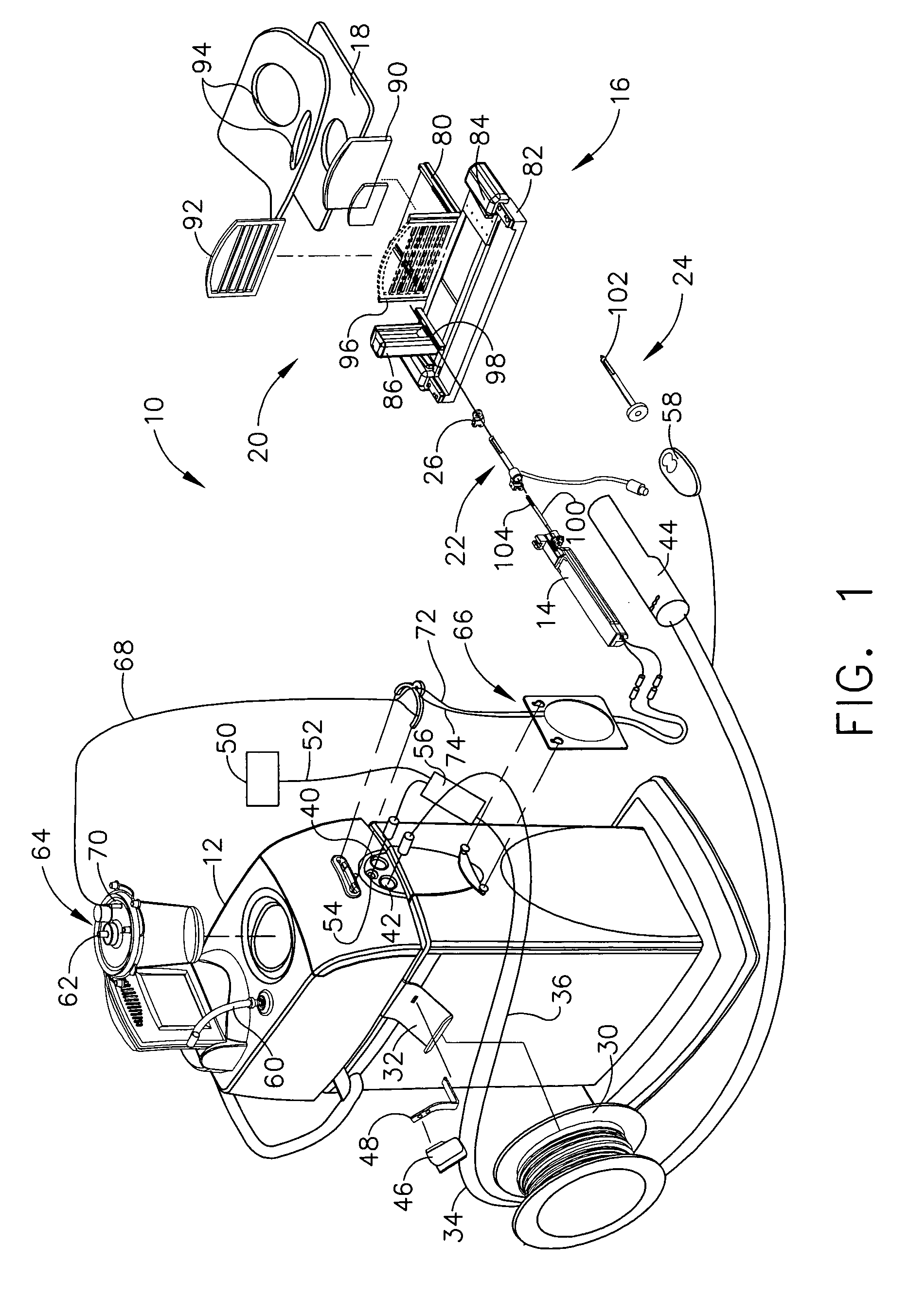
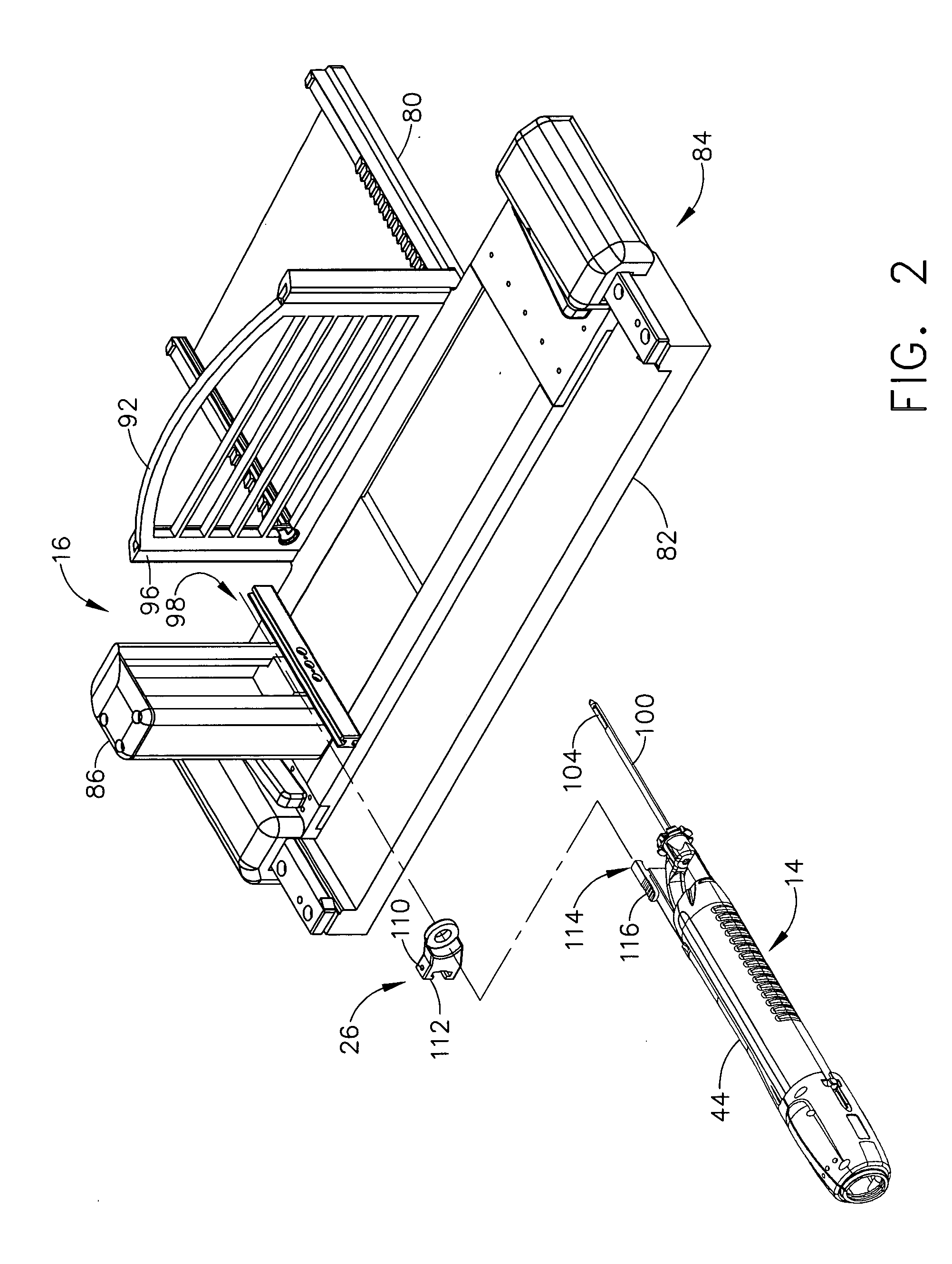
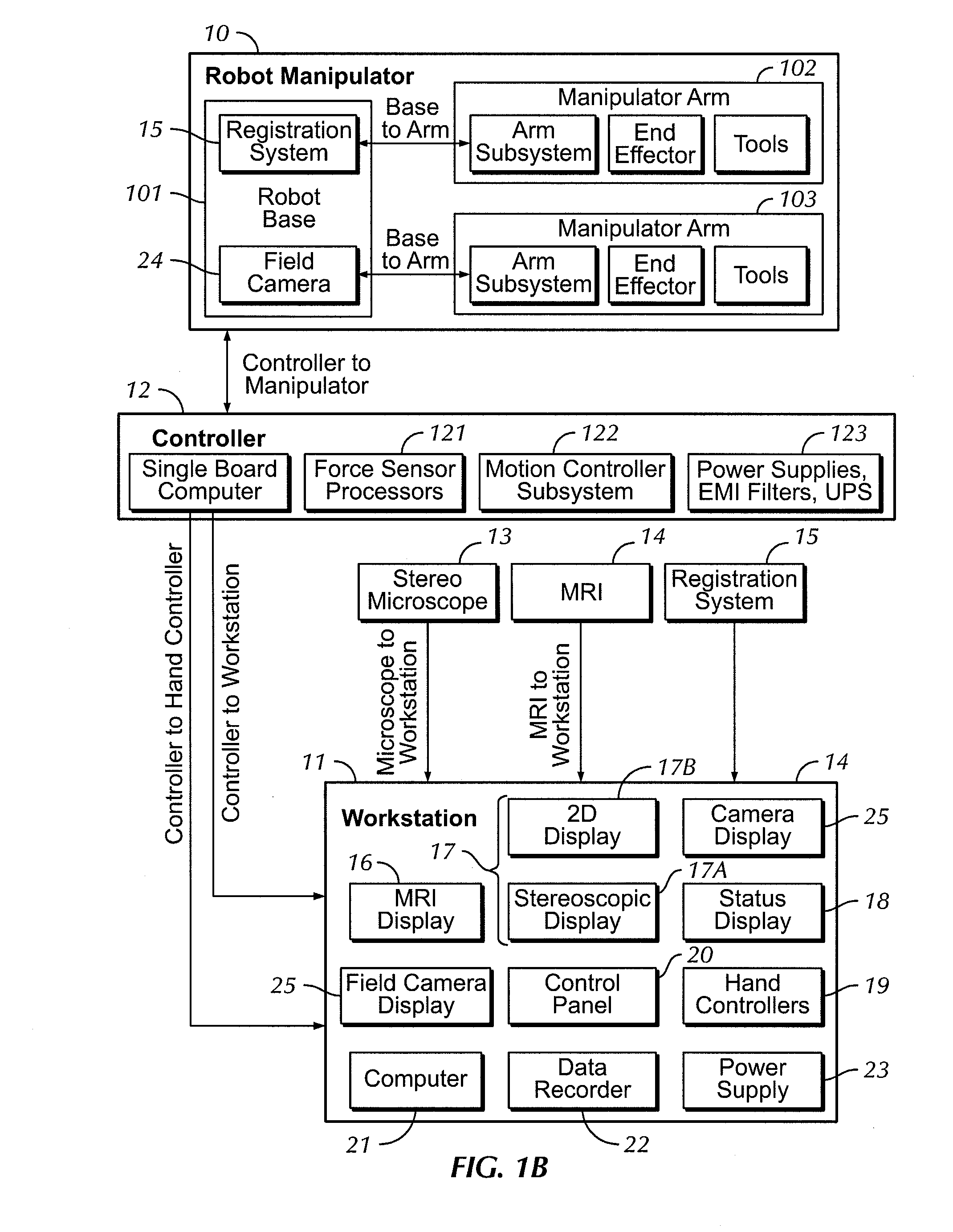
A robot system for use in surgical procedures has two movable arms each carried on a wheeled base with each arm having a six of degrees of freedom of movement and an end effector which can be rolled about its axis and an actuator which can slide along the axis for operating different tools adapted to be supported by the effector. Each end effector including optical force sensors for detecting forces applied to the tool by engagement with the part of the patient. A microscope is located at a position for viewing the part of the patient. The position of the tool tip can be digitized relative to fiducial markers visible in an MRI experiment. The workstation and control system has a pair of hand-controllers simultaneously manipulated by an operator to control movement of a respective one or both of the arms. The image from the microscope is displayed on a monitor in 2D and stereoscopically on a microscope viewer. A second MRI display shows an image of the part of the patient the real-time location of the tool. The robot is MRI compatible and can be configured to operate within a closed magnet bore. The arms are driven about vertical and horizontal axes by piezoelectric motors.
Owner:DEERFIELD IMAGING INC
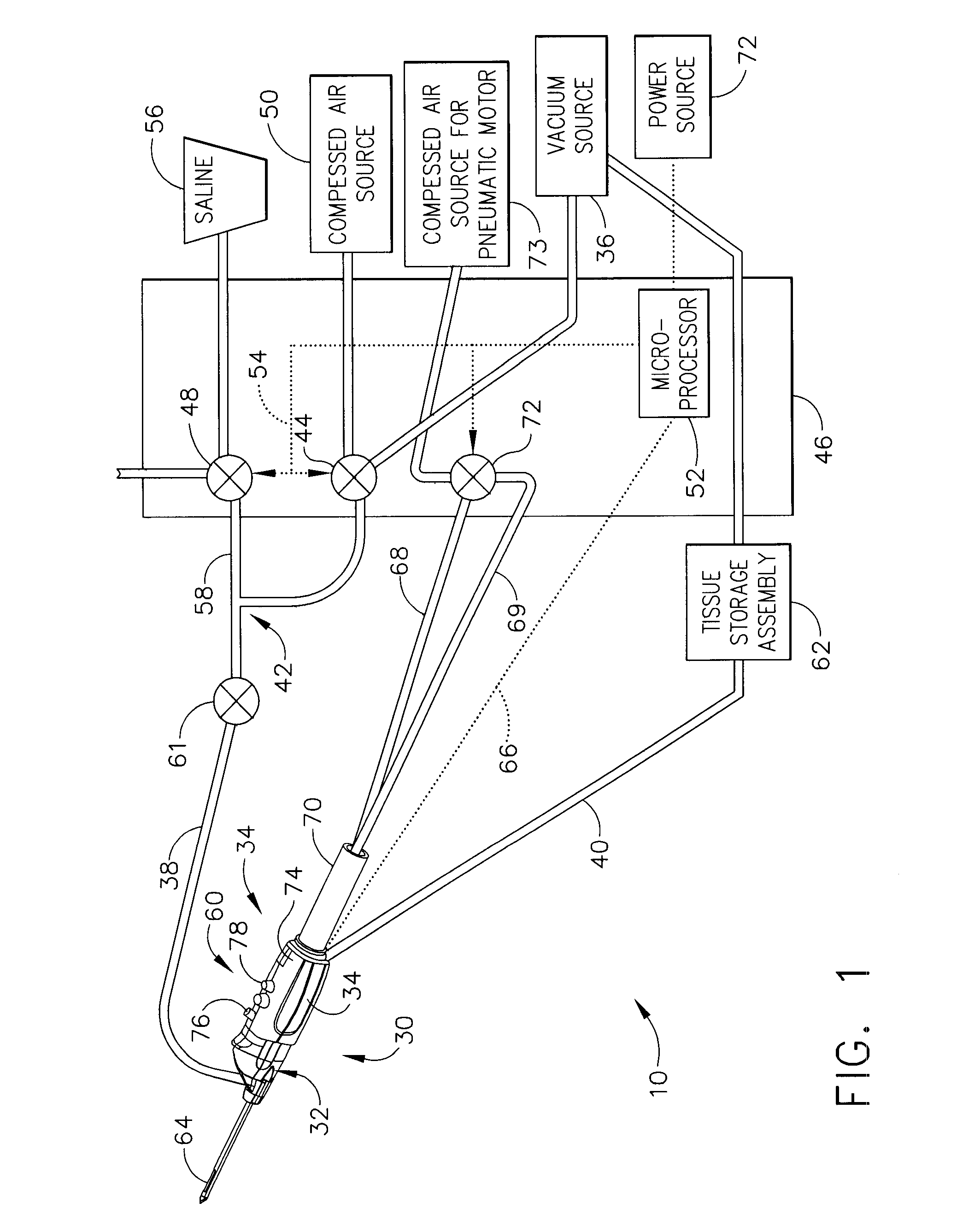
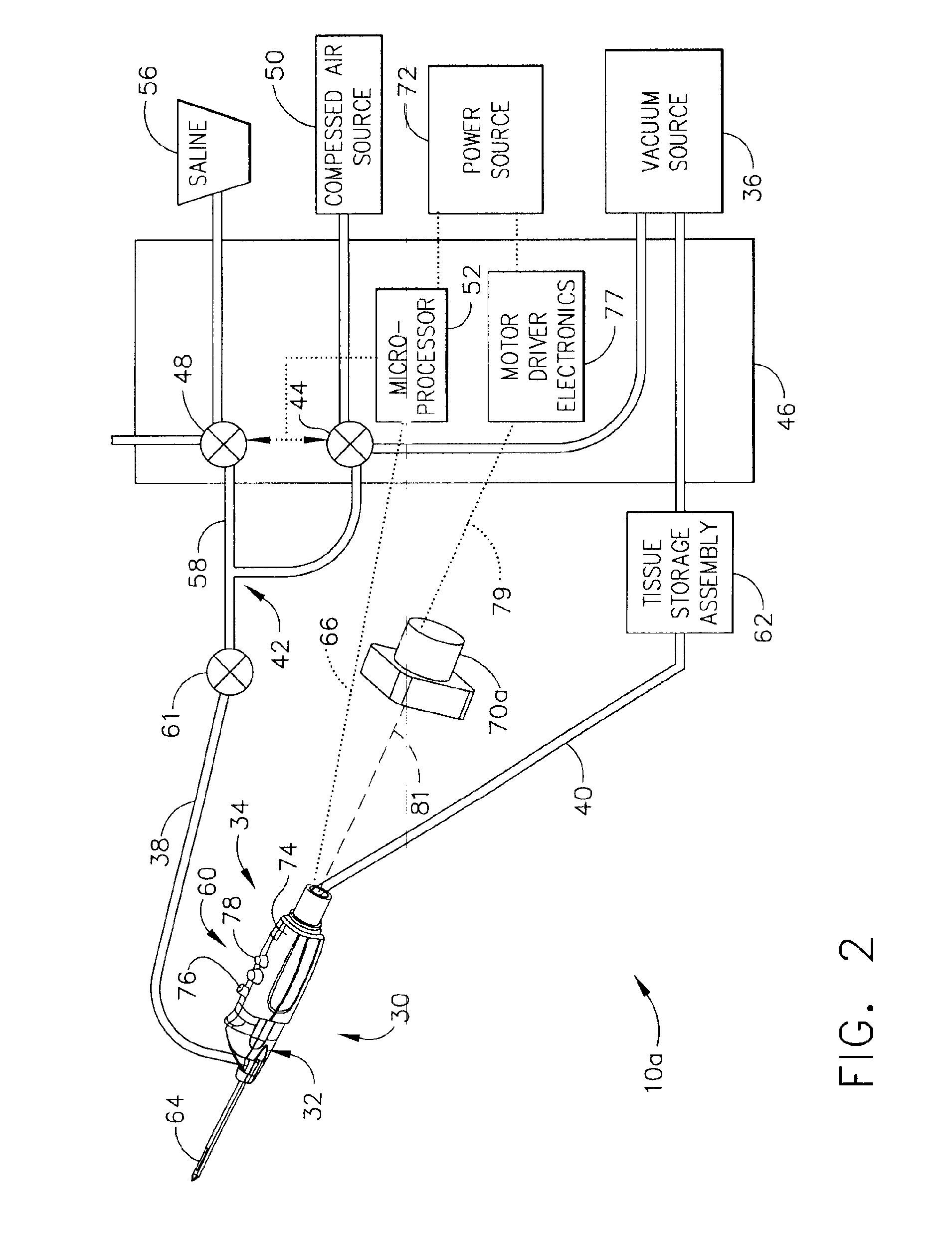
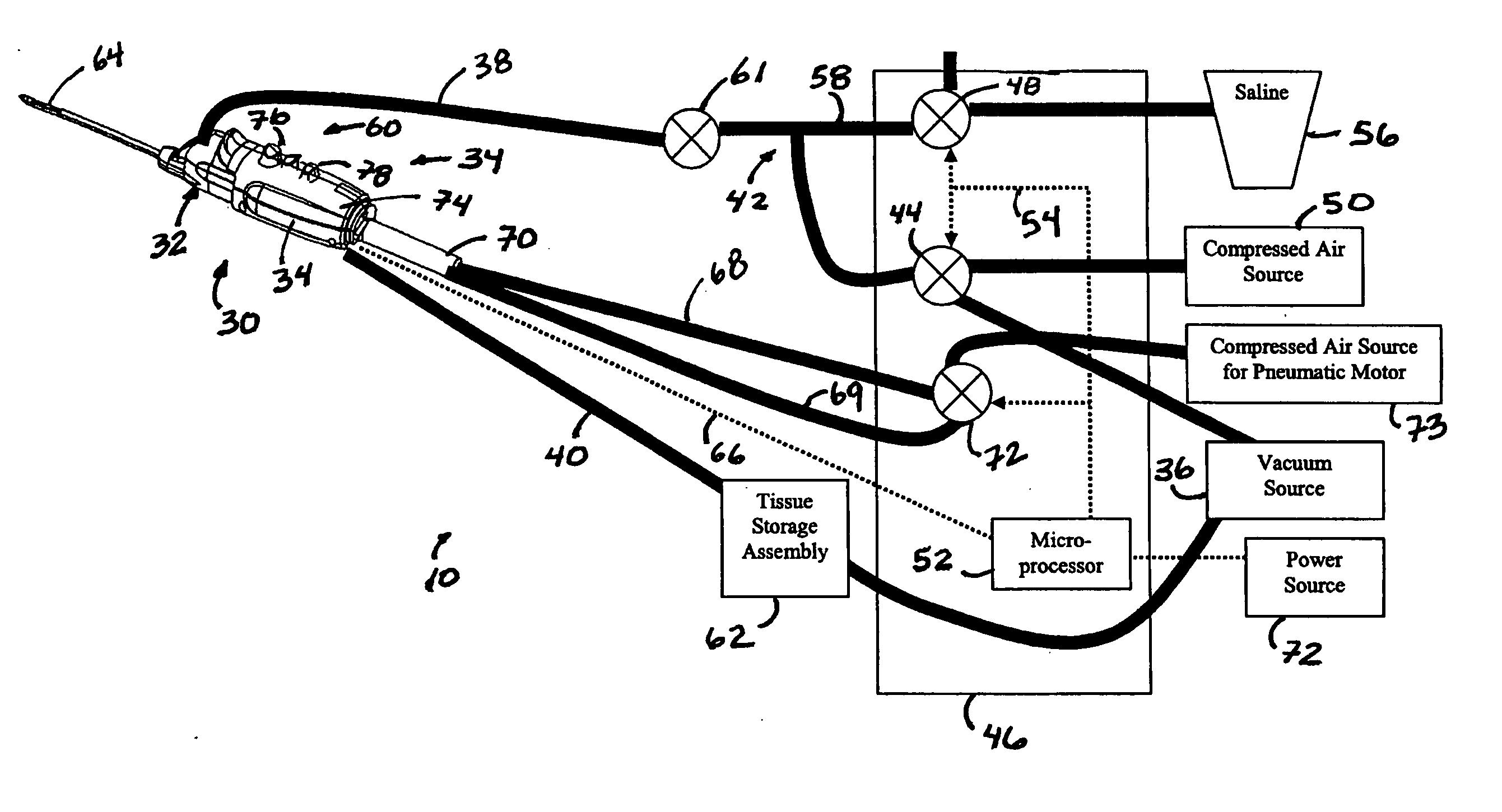
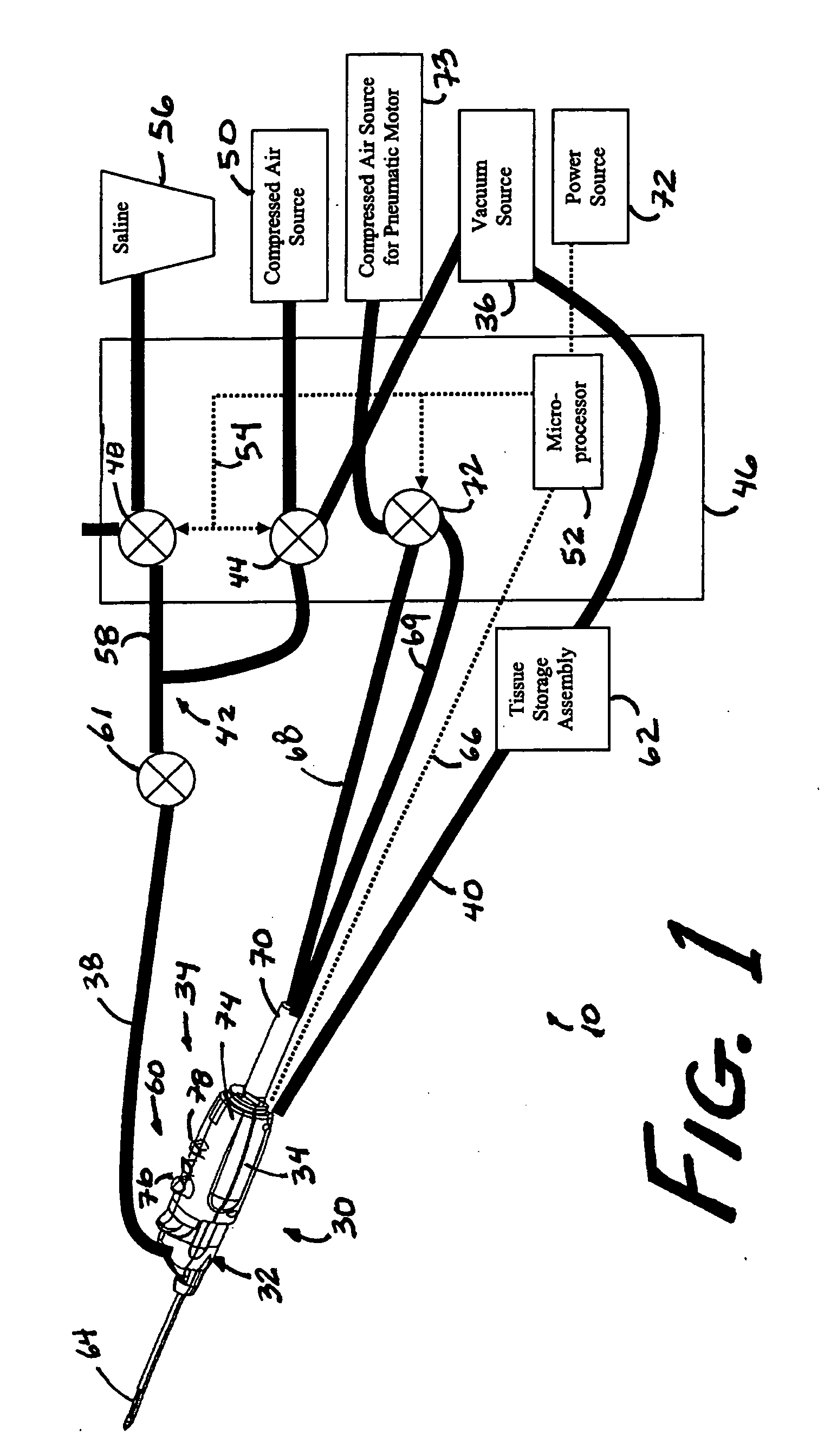
Core sampling biopsy device with short coupled MRI-compatible driver
A core sampling biopsy device is compatible with use in a Magnetic Resonance Imaging (MRI) environment by being driven by either a pneumatic rotary motor or a piezoelectric drive motor. The core sampling biopsy device obtains a tissue sample, such as a breast tissue biopsy sample, for diagnostic or therapeutic purposes. The biopsy device may include an outer cannula having a distal piercing tip, a cutter lumen, a side tissue port communicating with the cutter lumen, and at least one fluid passageway disposed distally of the side tissue port. The inner cutter may be advanced in the cutter lumen past the side tissue port to sever a tissue sample. A cutter drive assembly maintains a fixed gear ratio relationship between a cutter rotation speed and translation speed of the inner cutter regardless of the density of the tissue encountered to yield consistent sample size.
Owner:DEVICOR MEDICAL PROD
Core sampling biopsy device with short coupled MRI-compatible driver
InactiveUS20060149163A1Reduce speedTranslation speed is decreasedSurgeryVaccination/ovulation diagnosticsTissue sampleOuter Cannula
A core sampling biopsy device is compatible with use in a Magnetic Resonance Imaging (MRI) environment by being driven by either a pneumatic rotary motor or a piezoelectric drive motor. The core sampling biopsy device obtains a tissue sample, such as a breast tissue biopsy sample, for diagnostic or therapeutic purposes. The biopsy device may include an outer cannula having a distal piercing tip, a cutter lumen, a side tissue port communicating with the cutter lumen, and at least one fluid passageway disposed distally of the side tissue port. The inner cutter may be advanced in the cutter lumen past the side tissue port to sever a tissue sample.
Owner:DEVICOR MEDICAL PROD
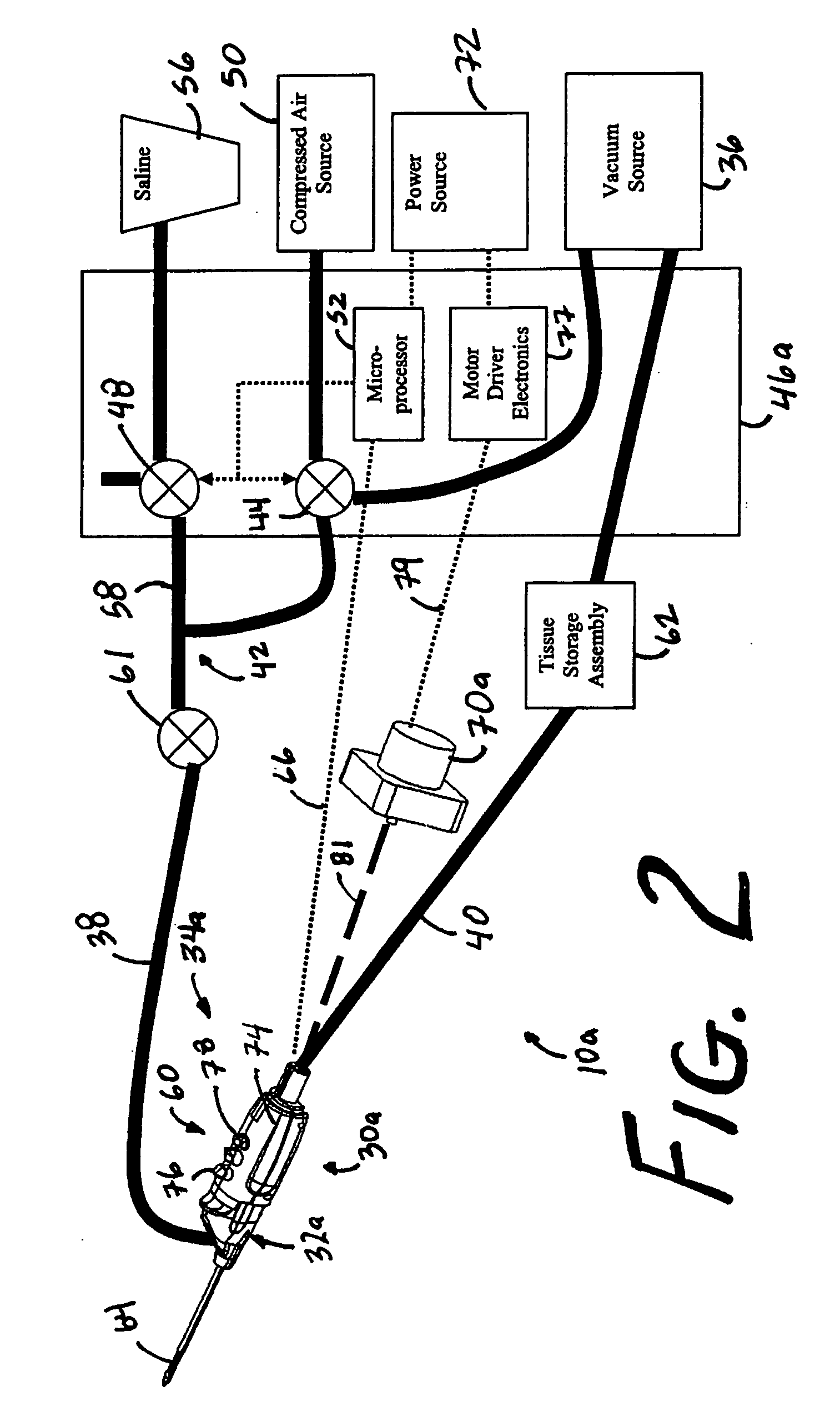
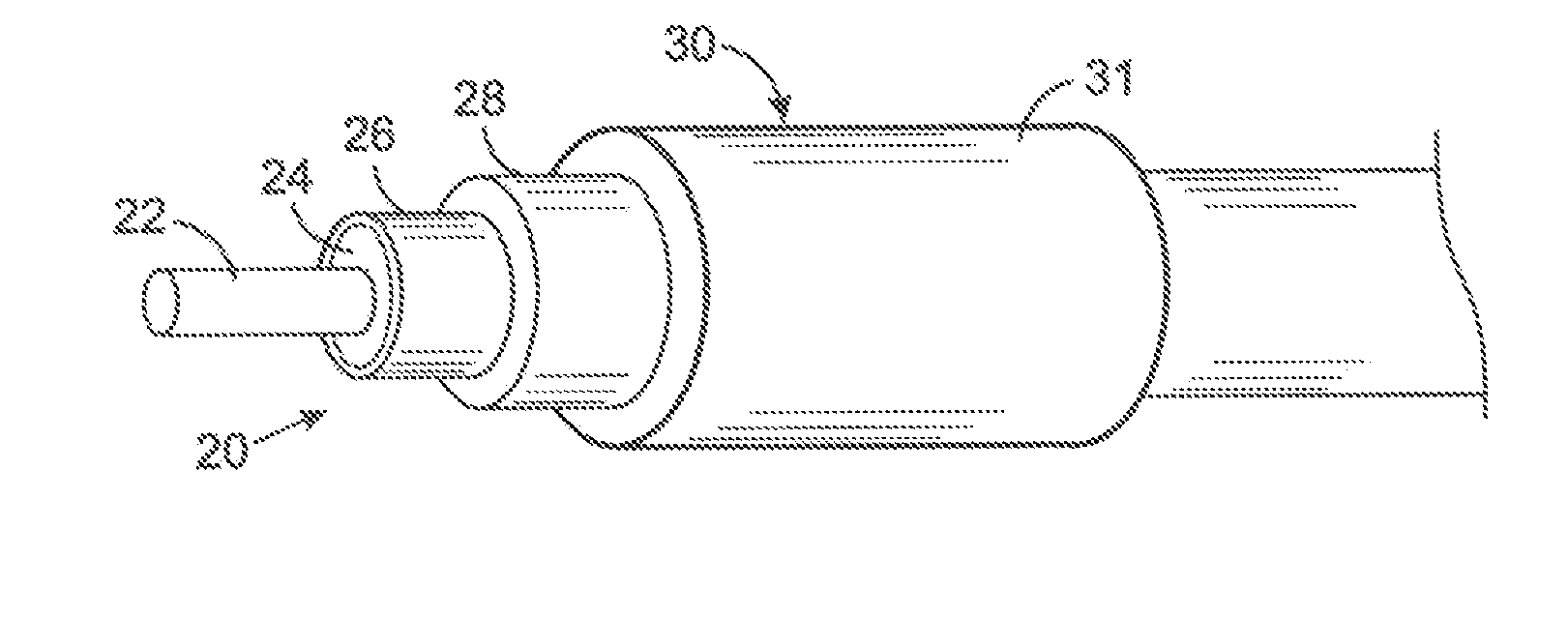
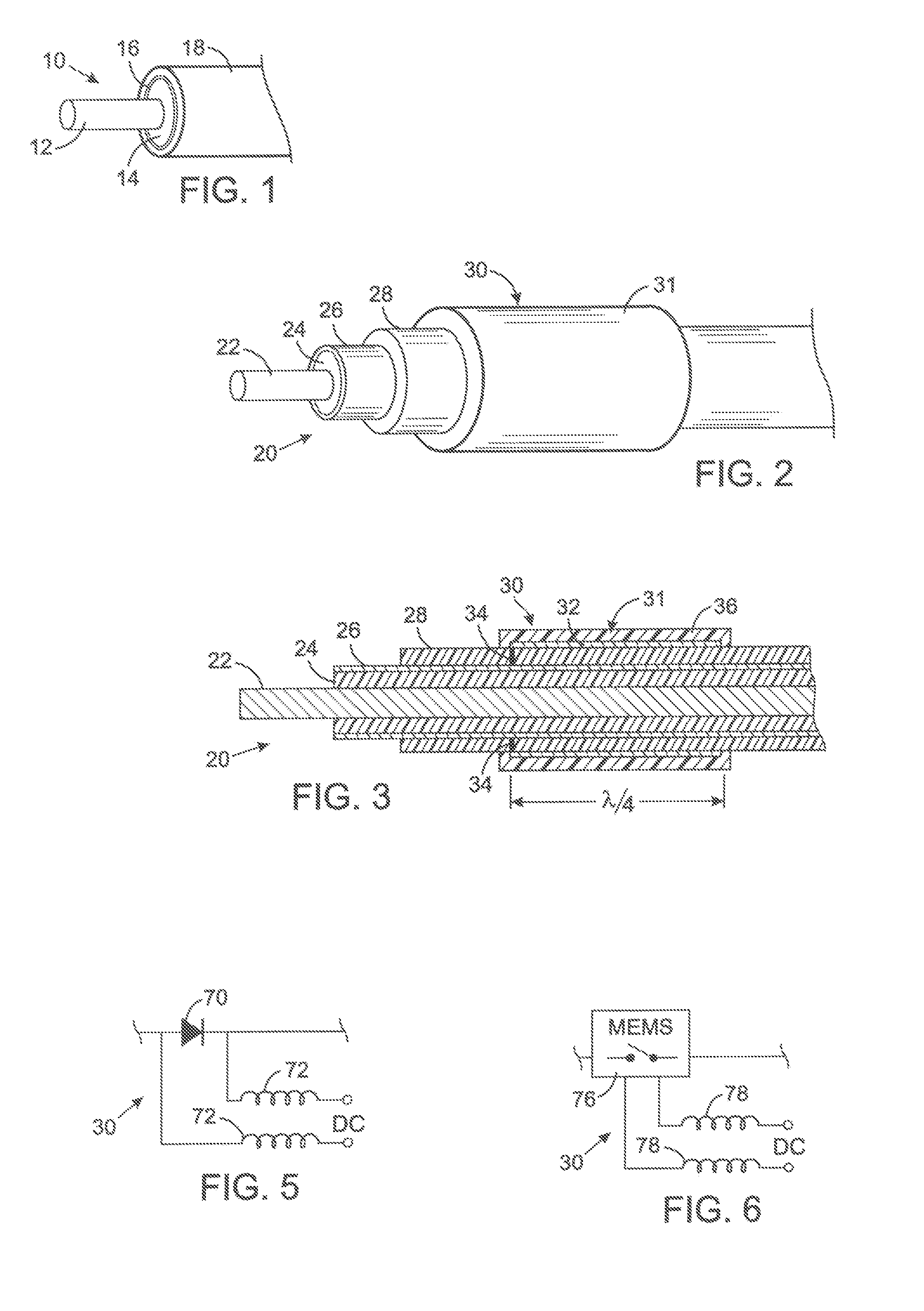
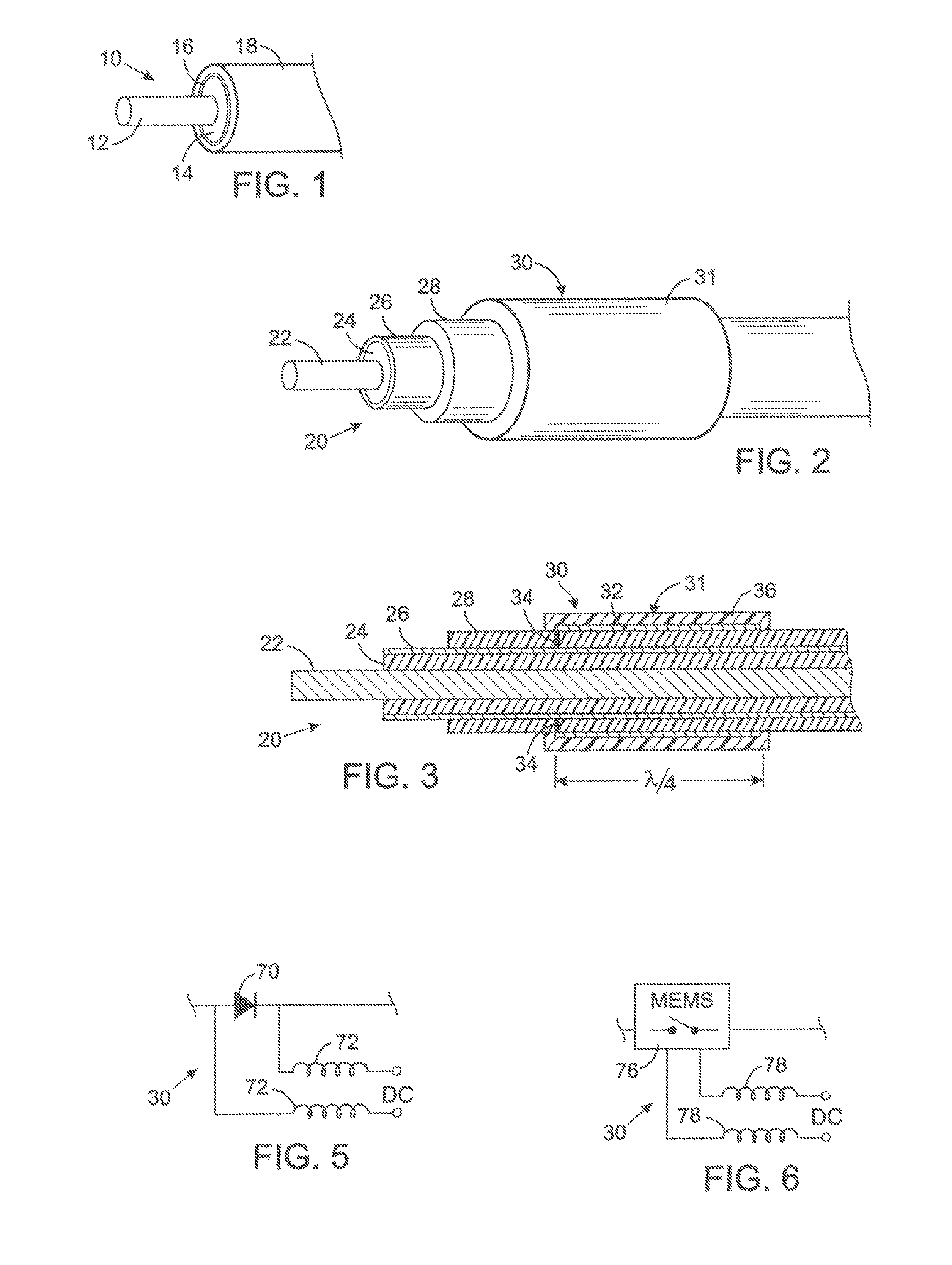
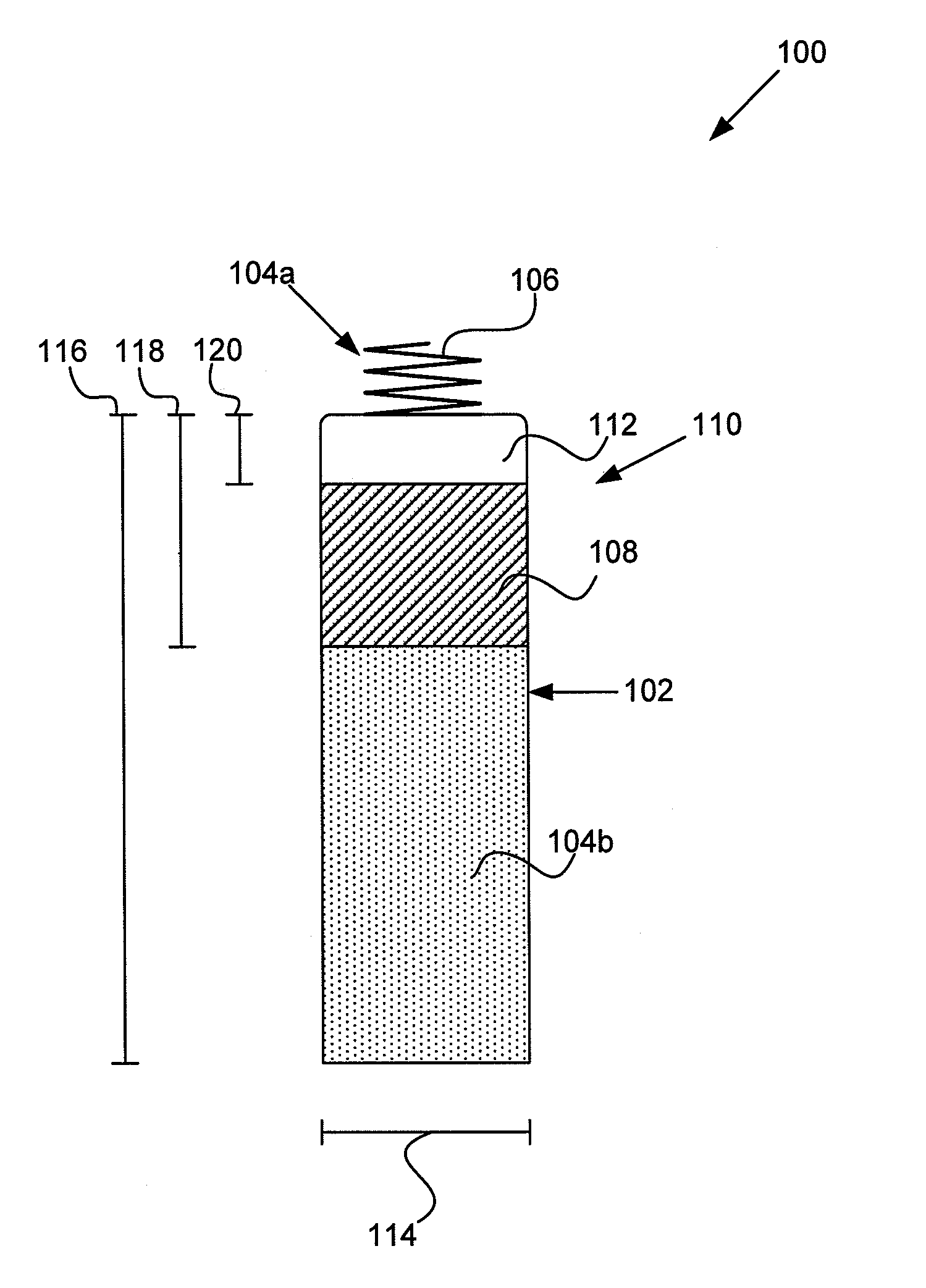
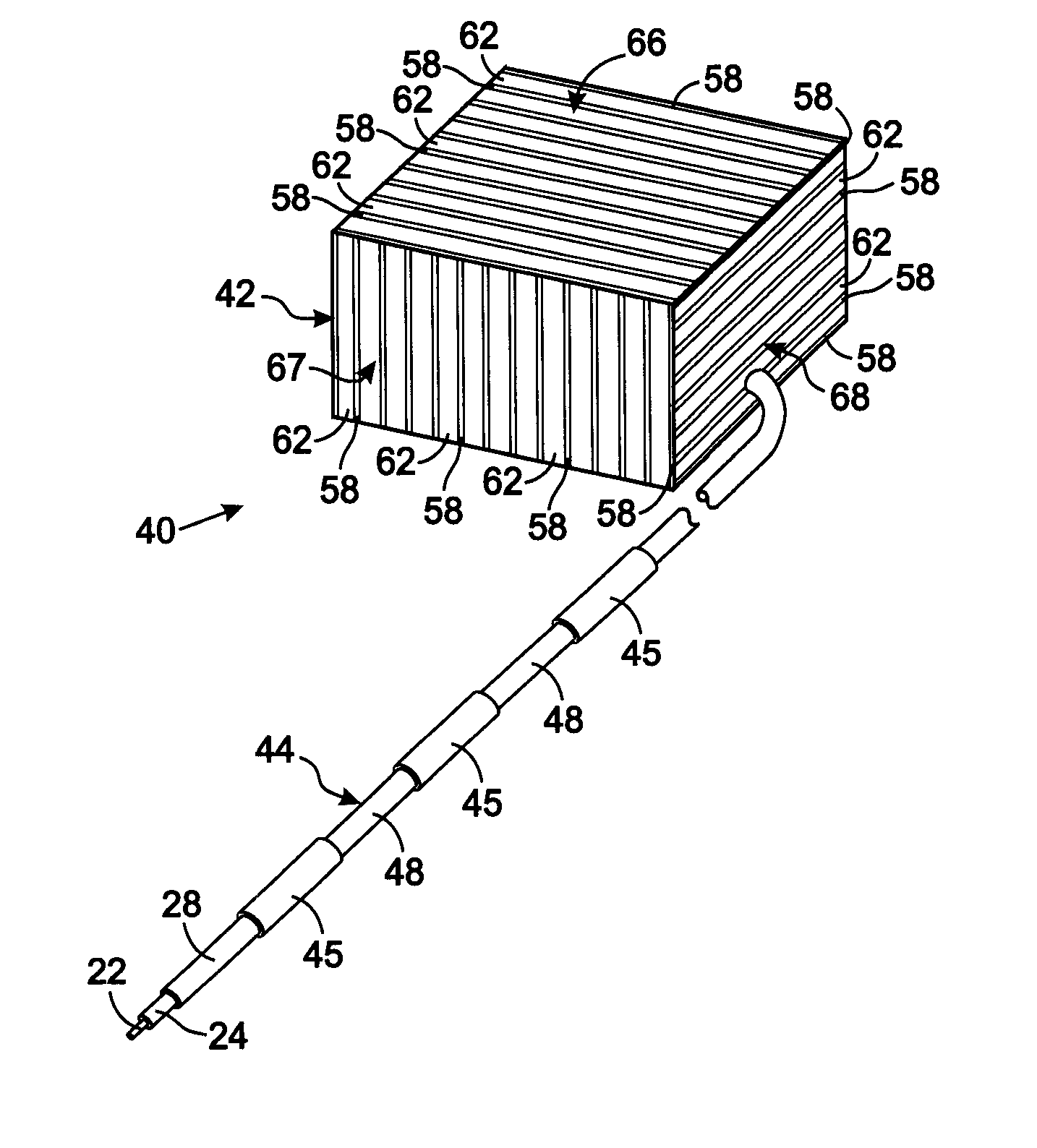
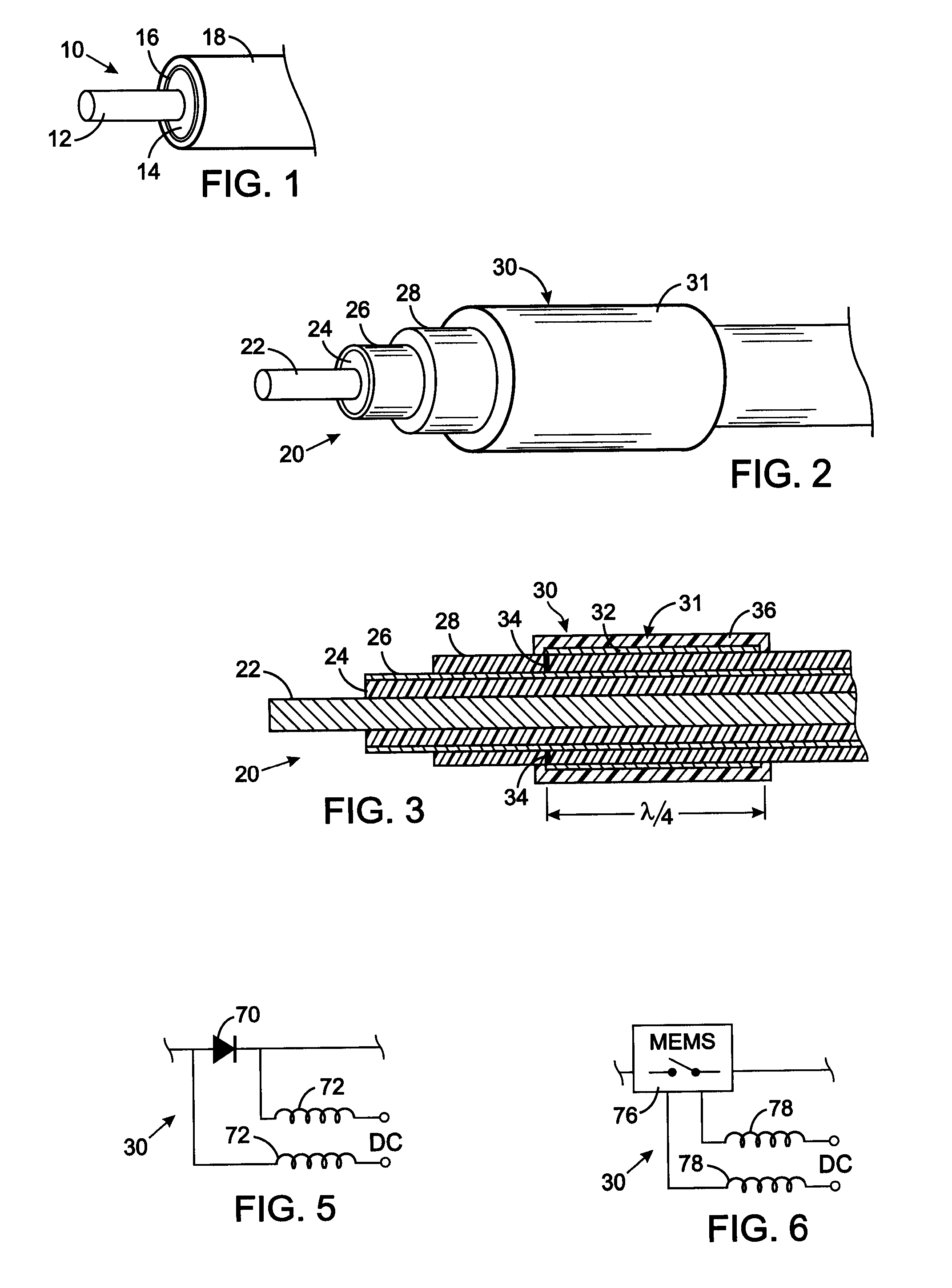
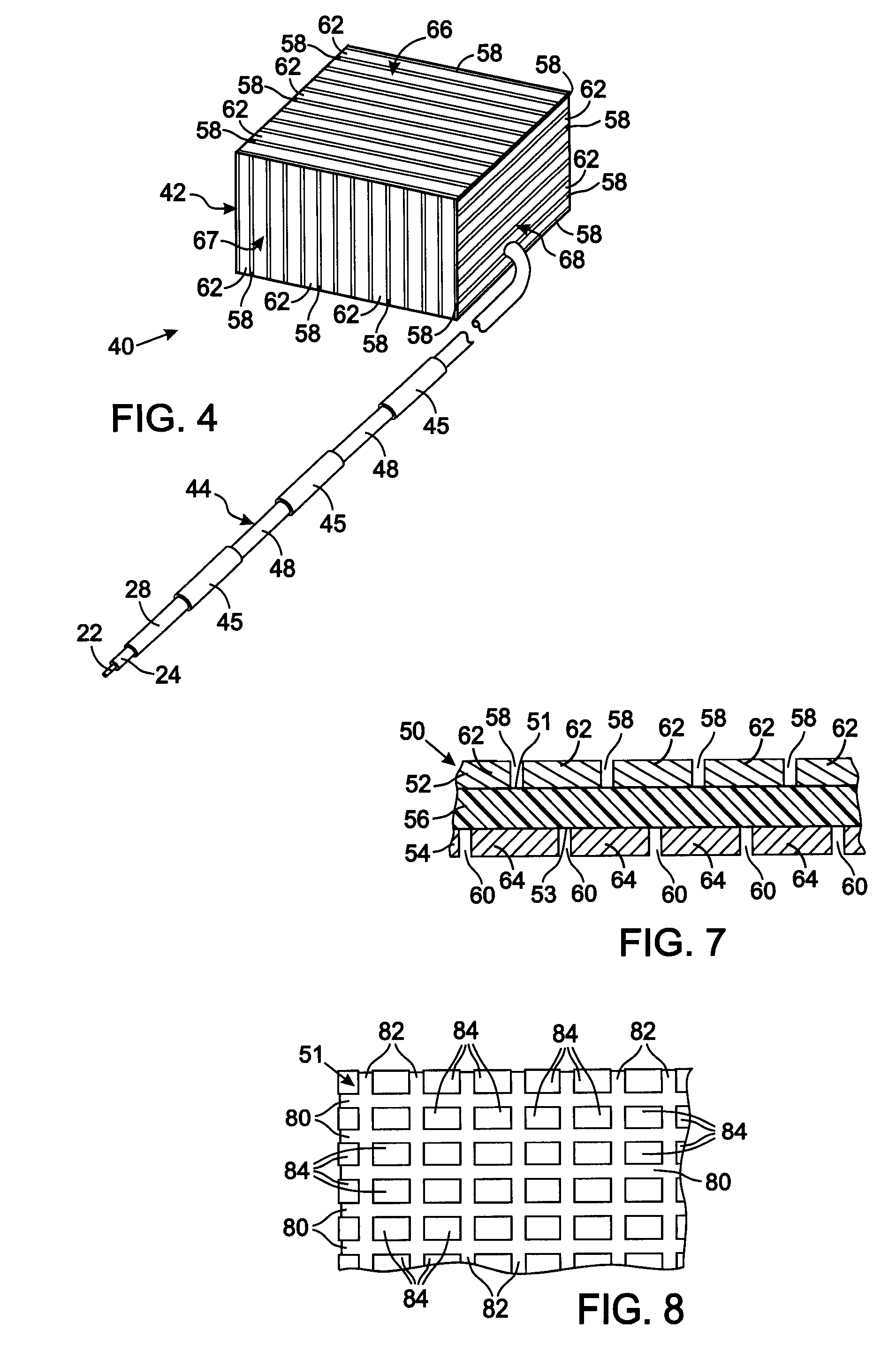
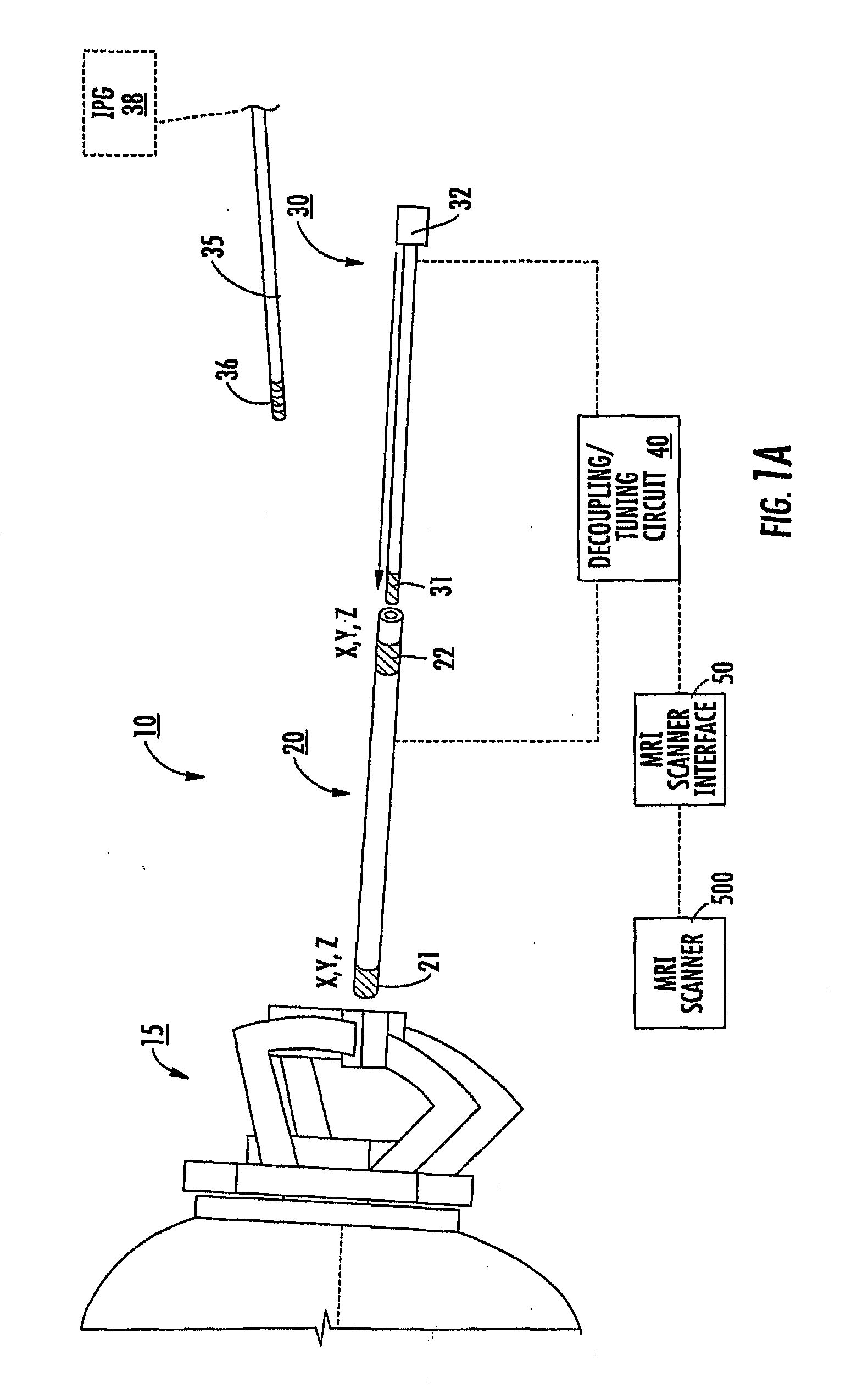
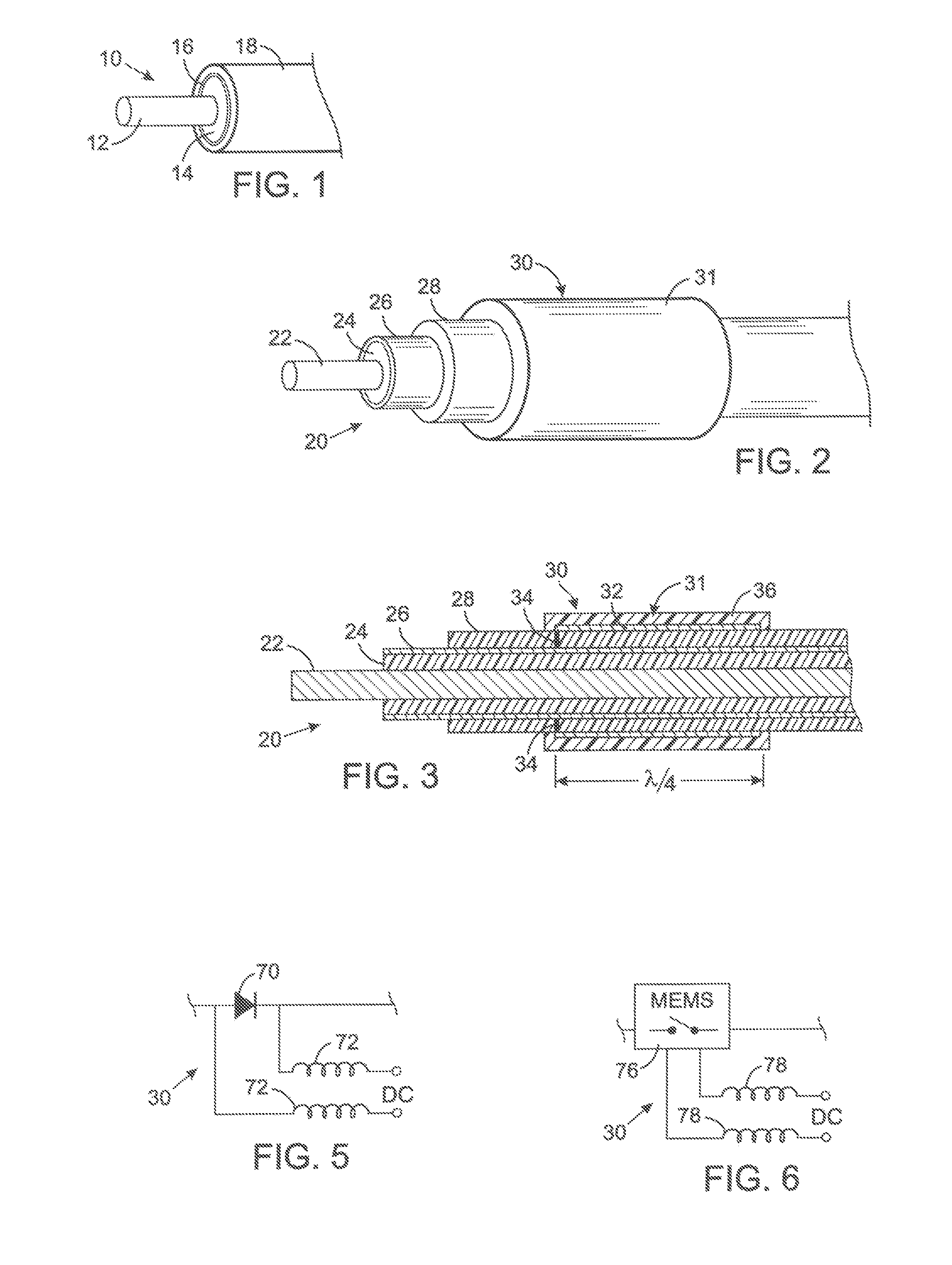
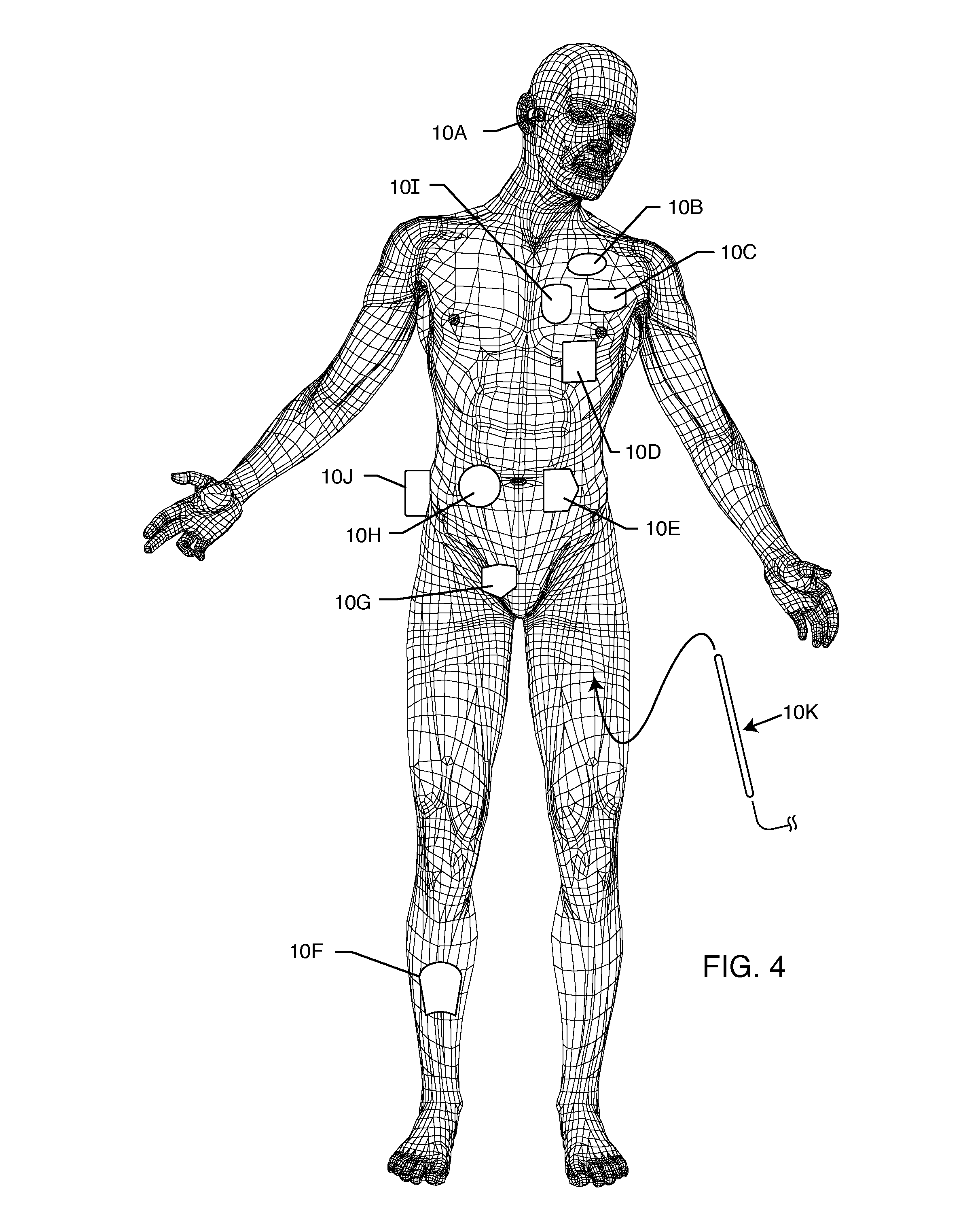
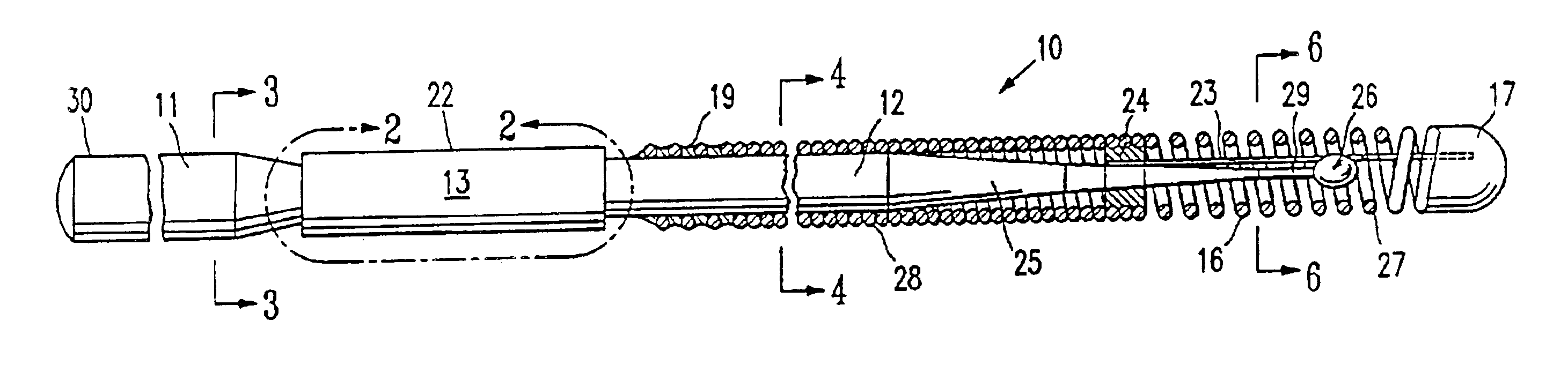
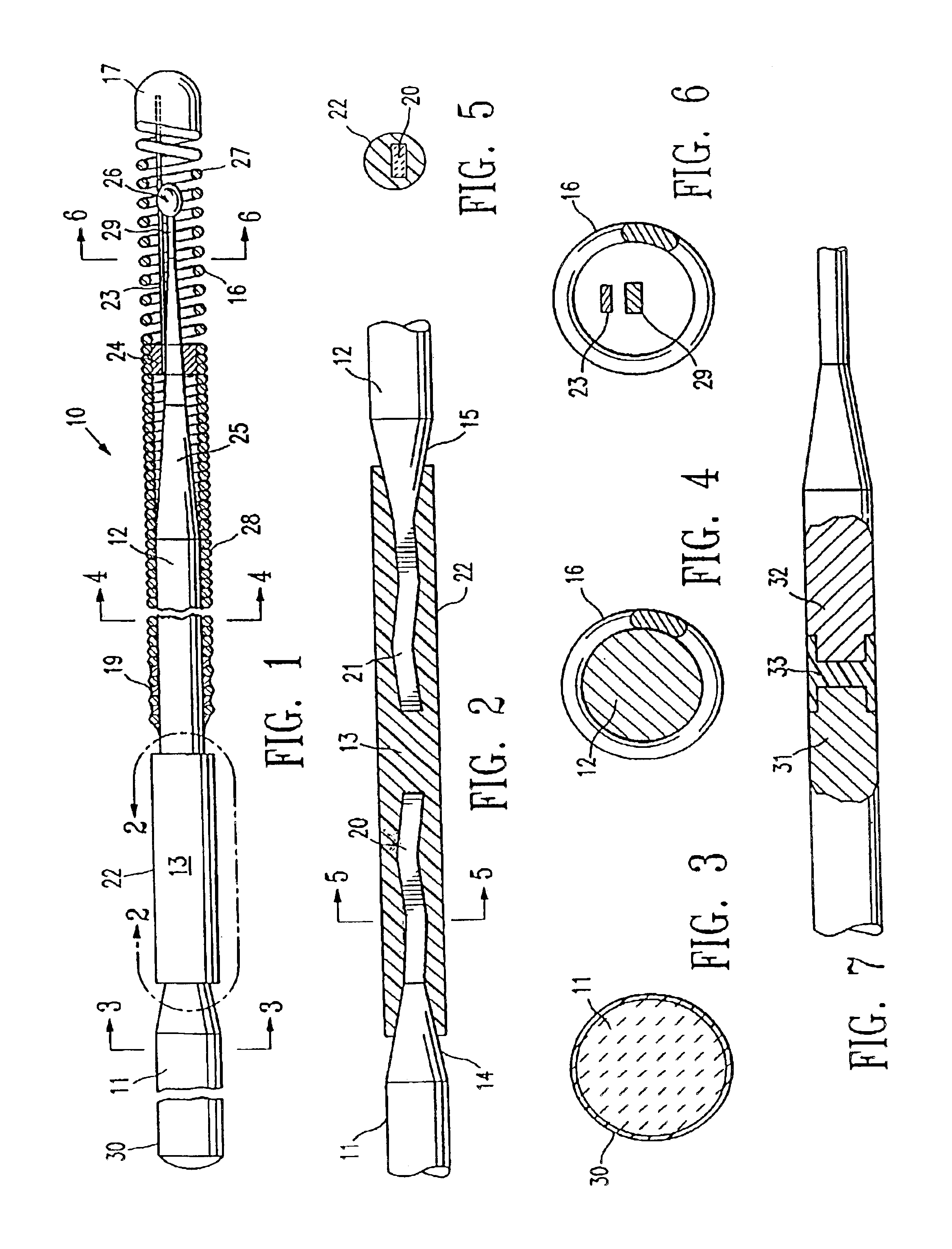
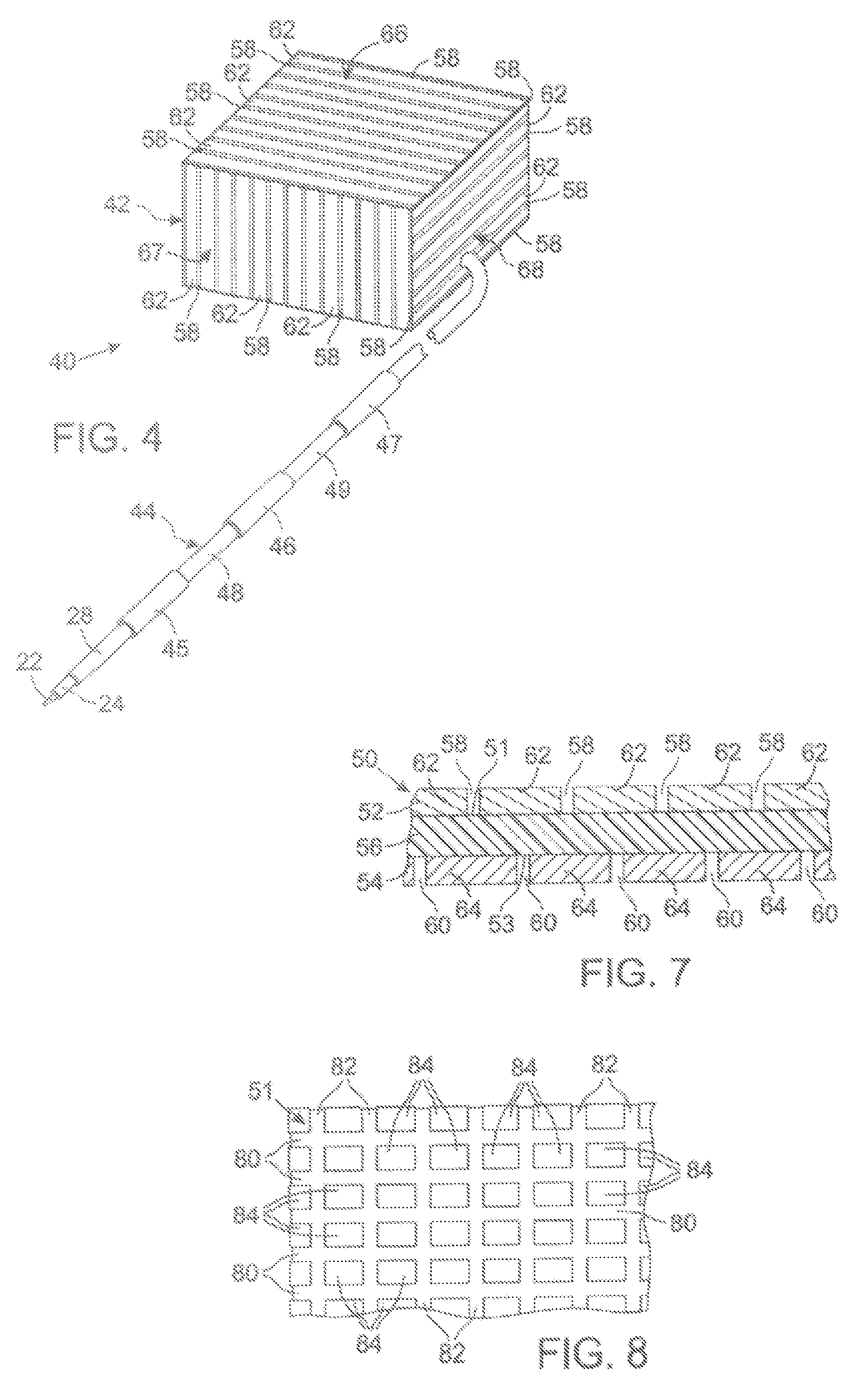
MRI and RF compatible leads and related methods of operating and fabricating leads
ActiveUS20080243218A1Prevent undesired heatingEasy to useLine/current collector detailsInternal electrodesElectricityCelsius Degree
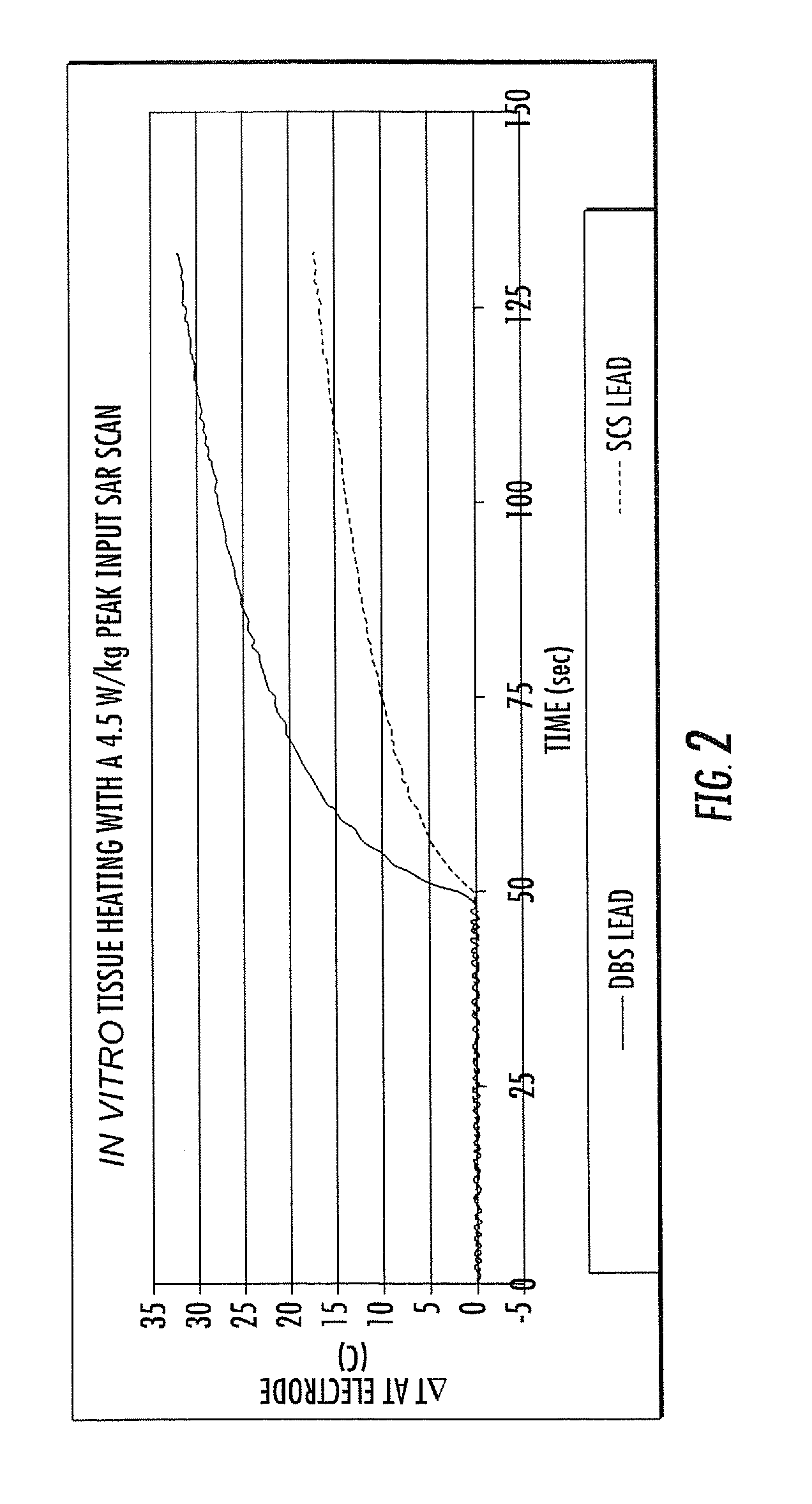
RF / MRI compatible leads include at least one conductor that turns back on itself at least twice in a lengthwise direction, and can turn back on itself at least twice at multiple locations along its length. The at least one electrical lead can be configured so that the lead heats local tissue less than about 10 degrees Celsius (typically about 5 degrees Celsius or less) or does not heat local tissue when a patient is exposed to target RF frequencies at a peak input SAR of at least about 4 W / kg and / or a whole body average SAR of at least about 2 W / kg. Related devices and methods of fabricating leads are also described.
Owner:MRI INTERVENTIONS INC +1
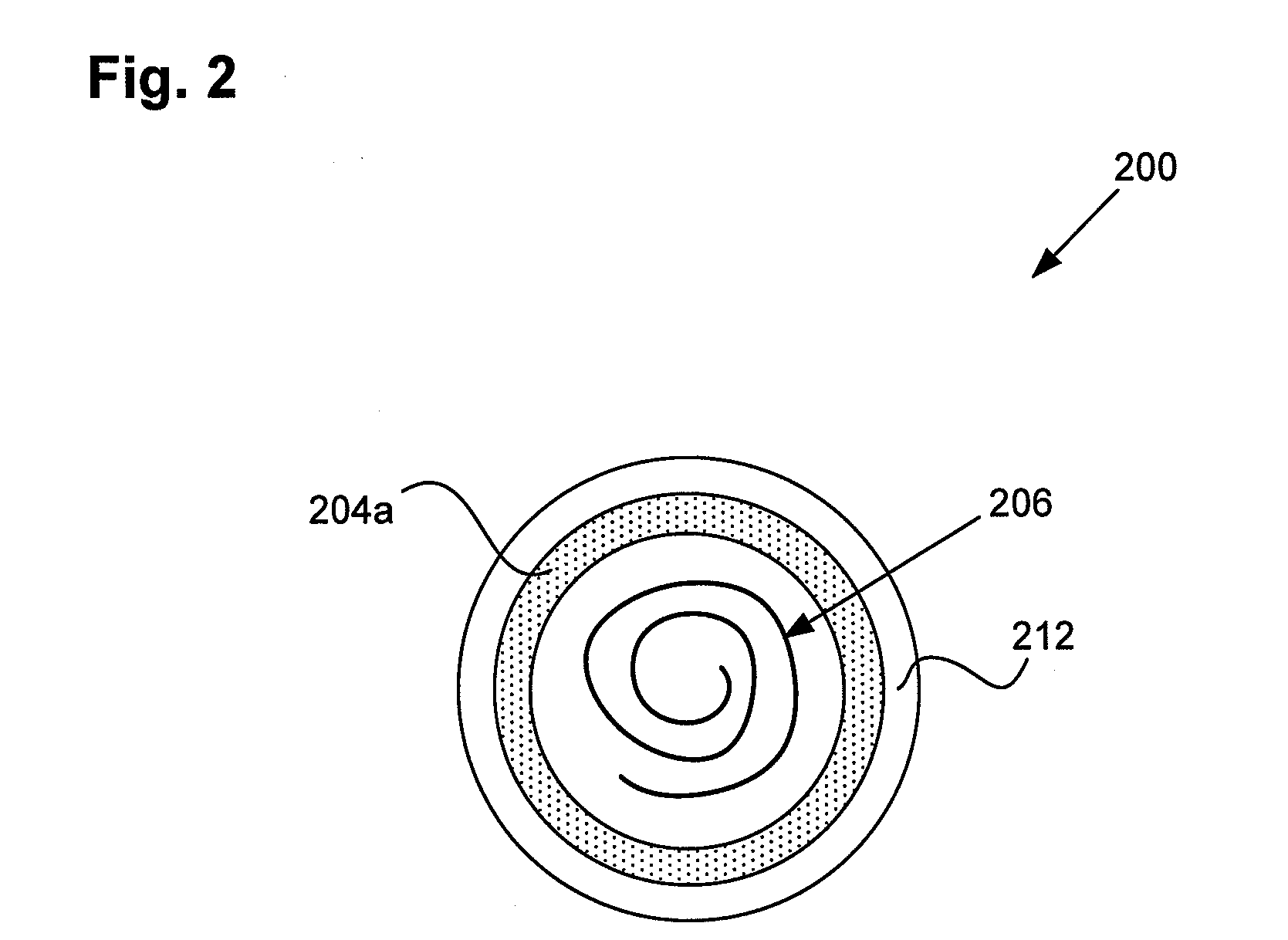
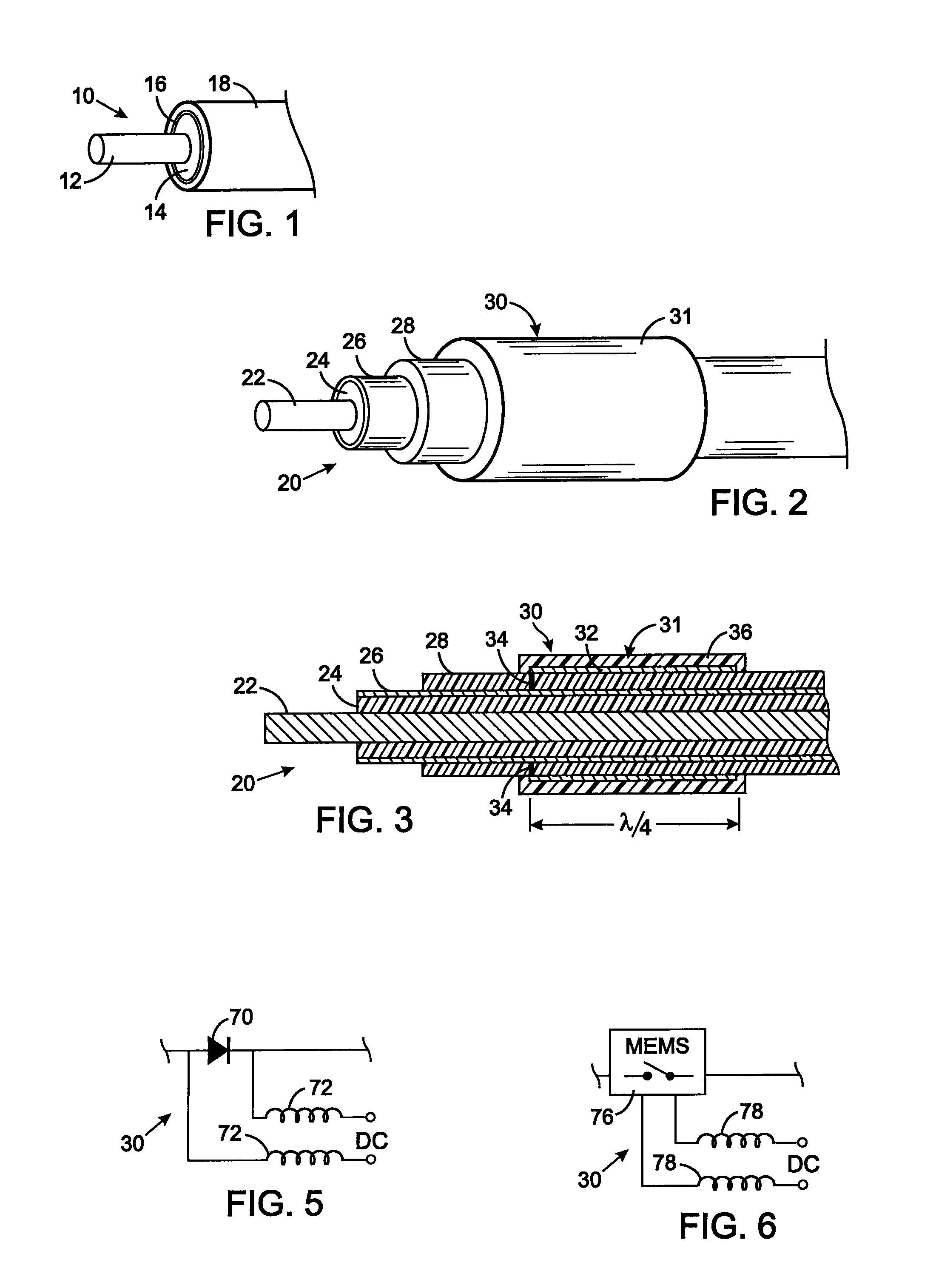
MRI compatible implanted electronic medical device and lead
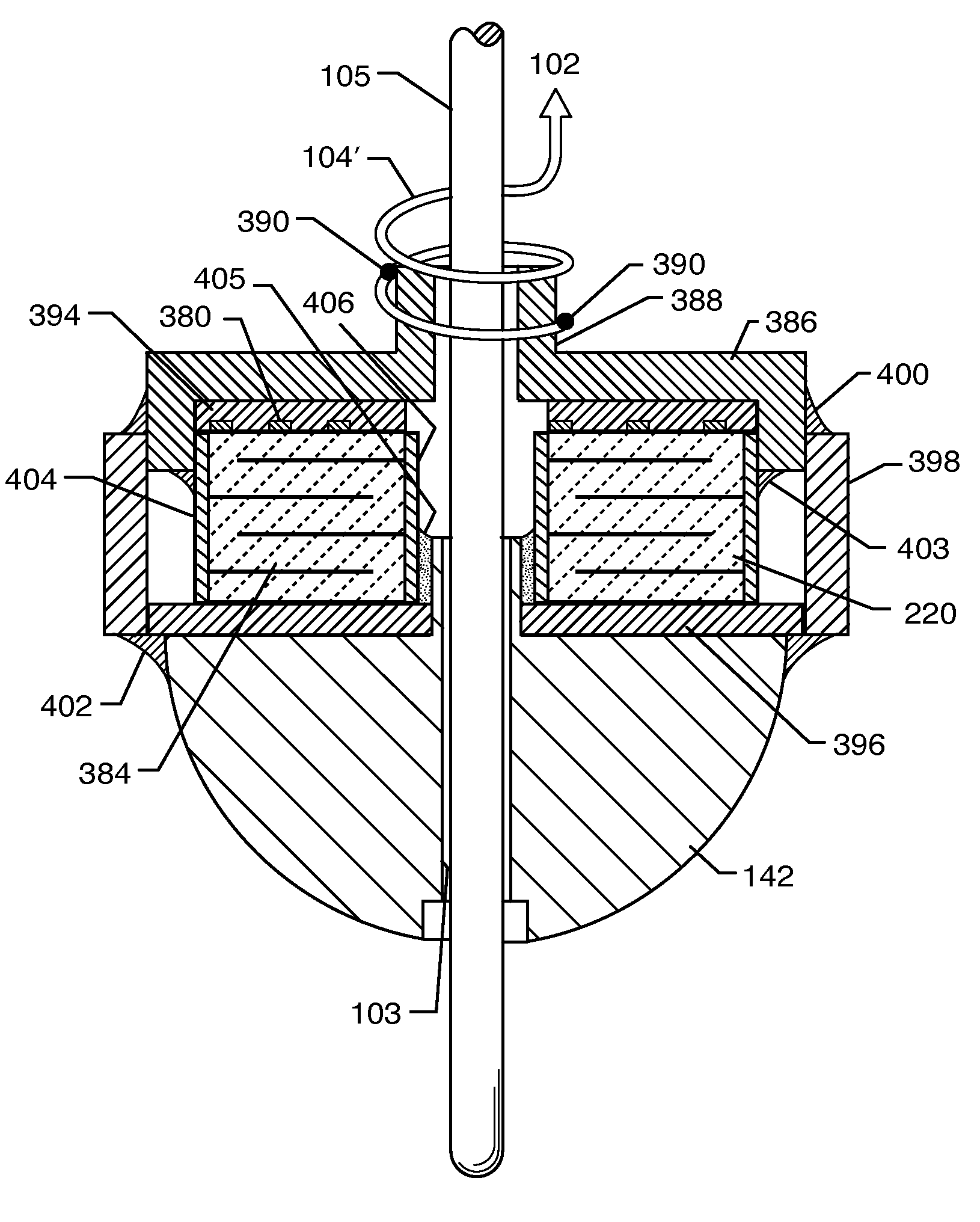
An implantable biocompatible lead that is also compatible with a magnetic resonance imaging scanner for the purpose of diagnostic quality imaging is described. The implantable electrical lead comprises a plurality of coiled insulated conducting wires wound in a first direction forming a first structure of an outer layer of conductors of a first total length with a first number of turns per unit length and a plurality of coiled insulated conducting wires wound in a second direction forming a second structure of an inner layer of conductors of a second total length with a second number of turns per unit length. The first and the second structures are separated by a distance with a layer of dielectric material. The distance and dielectric material are chosen based on the field strength of the MRI scanner. The lead may further comprise a conducting layer formed by coating a material consisting of medium conducting particles in physical contact with each other and a mechanically flexible, biocompatible layer forming an external layer of lead and in contact with body tissue or body fluids.
Owner:KENERGY INC
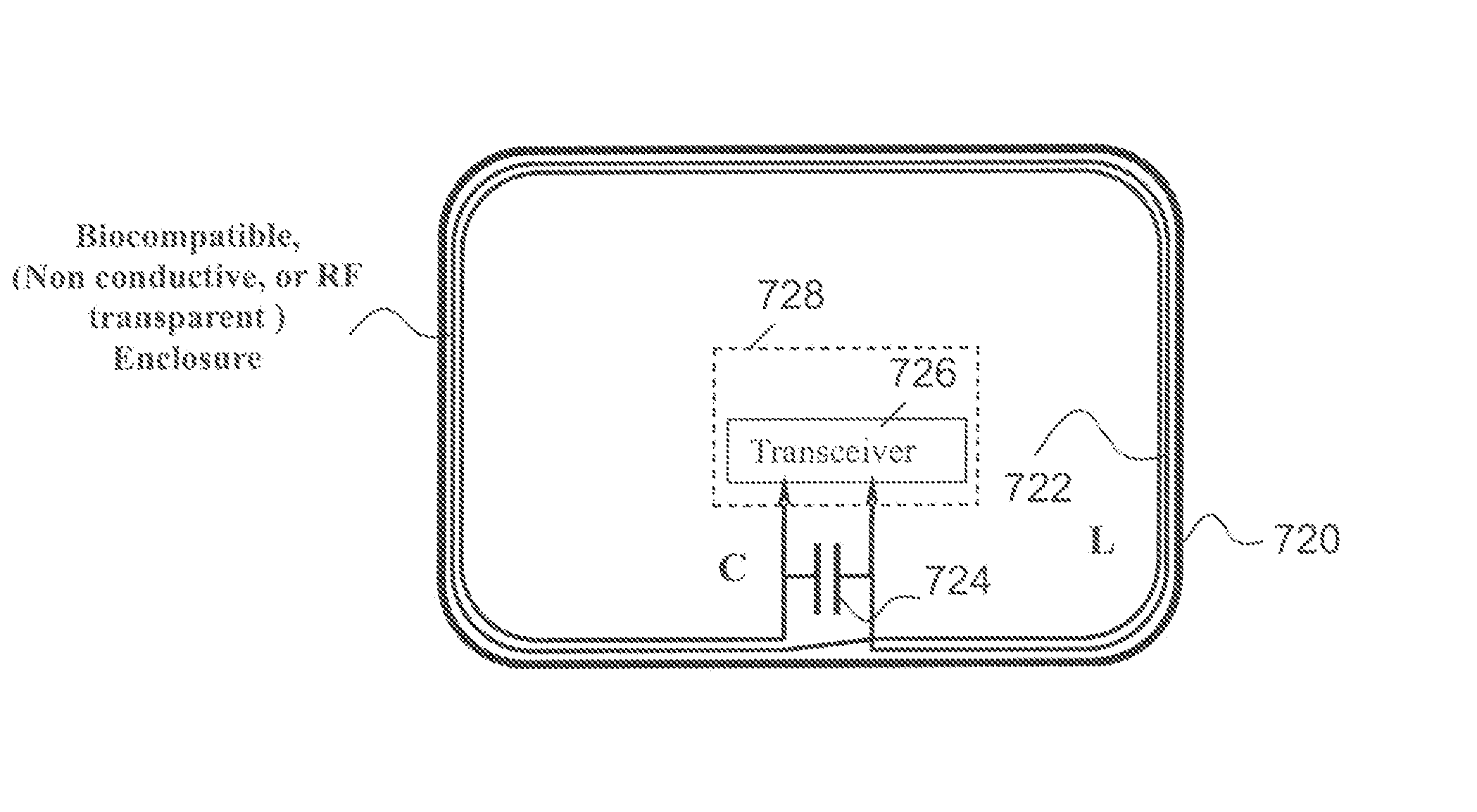
MRI compatible implanted electronic medical device with power and data communication capability
InactiveUS20080051854A1Minimizing electromagnetic interferenceInterference minimizationElectrotherapyElectromagnetic interferenceMagnetic Resonance Imaging Scan
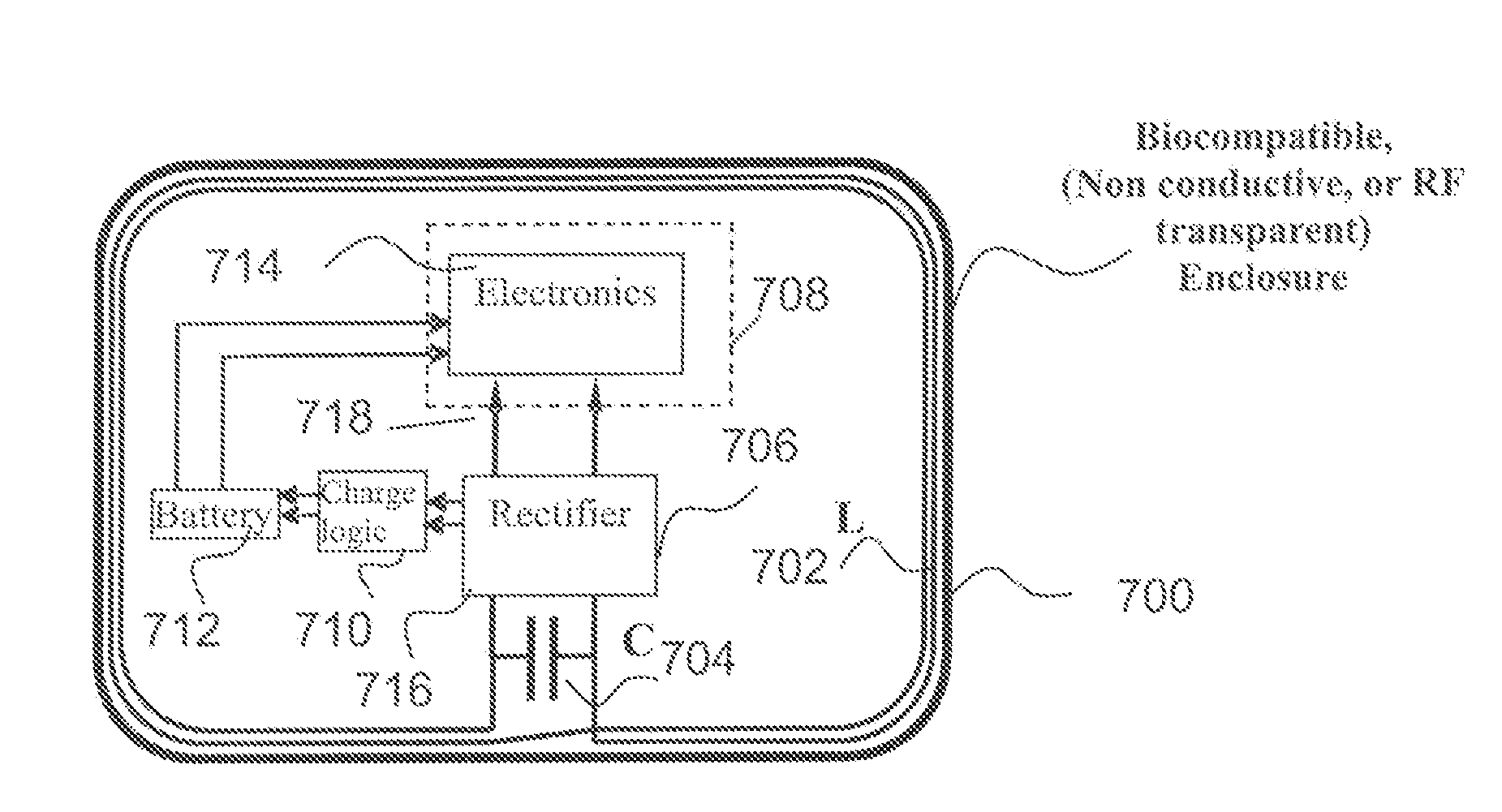
An antenna module, that is compatible with a magnetic resonance imaging scanner for the purpose of diagnostic quality imaging, is adapted to be implanted inside an animal. The antenna module comprises an electrically non-conducting, biocompatible, and electromagnetically transparent enclosure with inductive antenna wires looping around an inside surface. An electronic module is enclosed in an electromagnetic shield inside the enclosure to minimize the electromagnetic interference from the magnetic resonance imaging scanner.
Owner:KENERGY INC
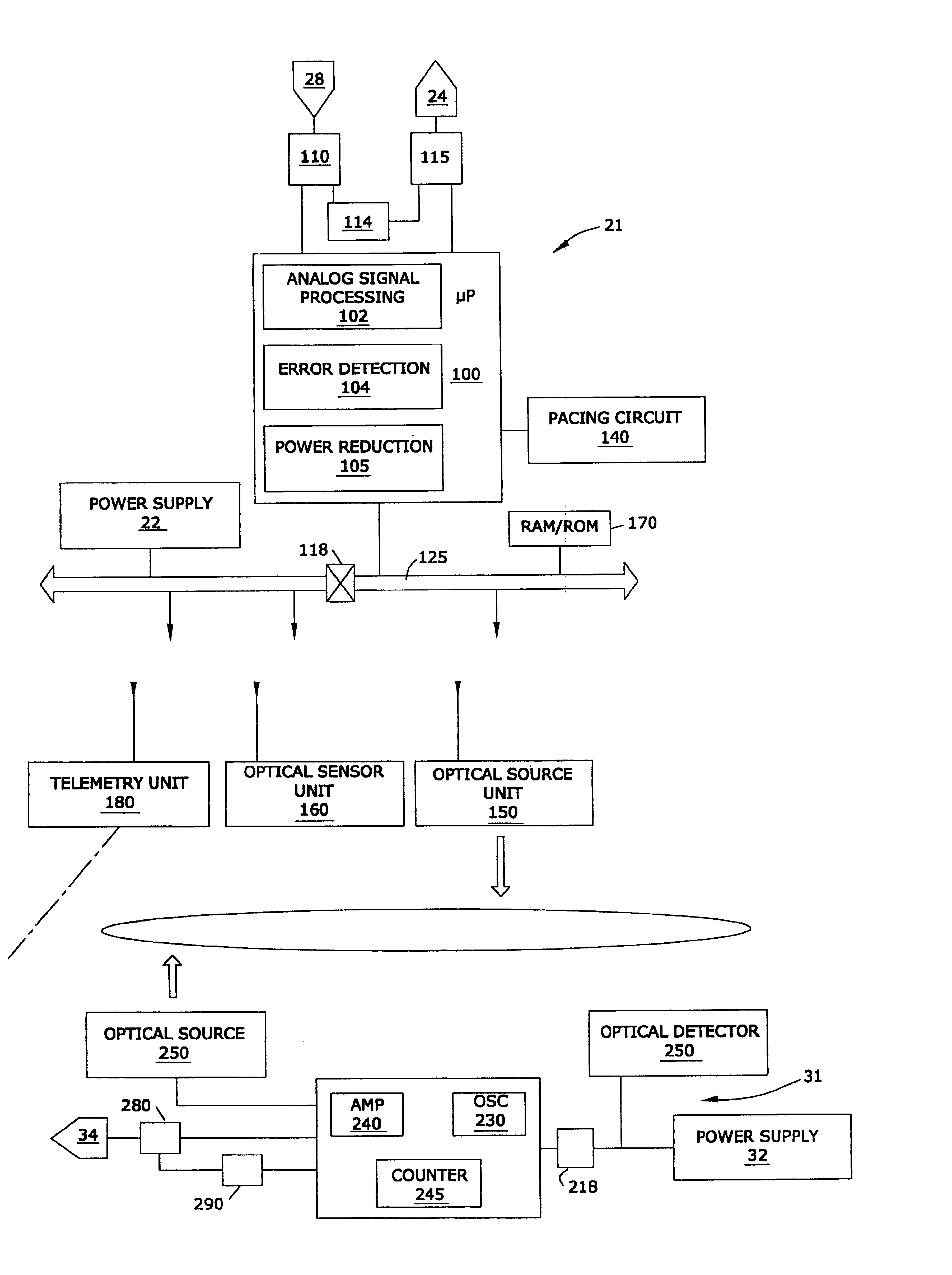
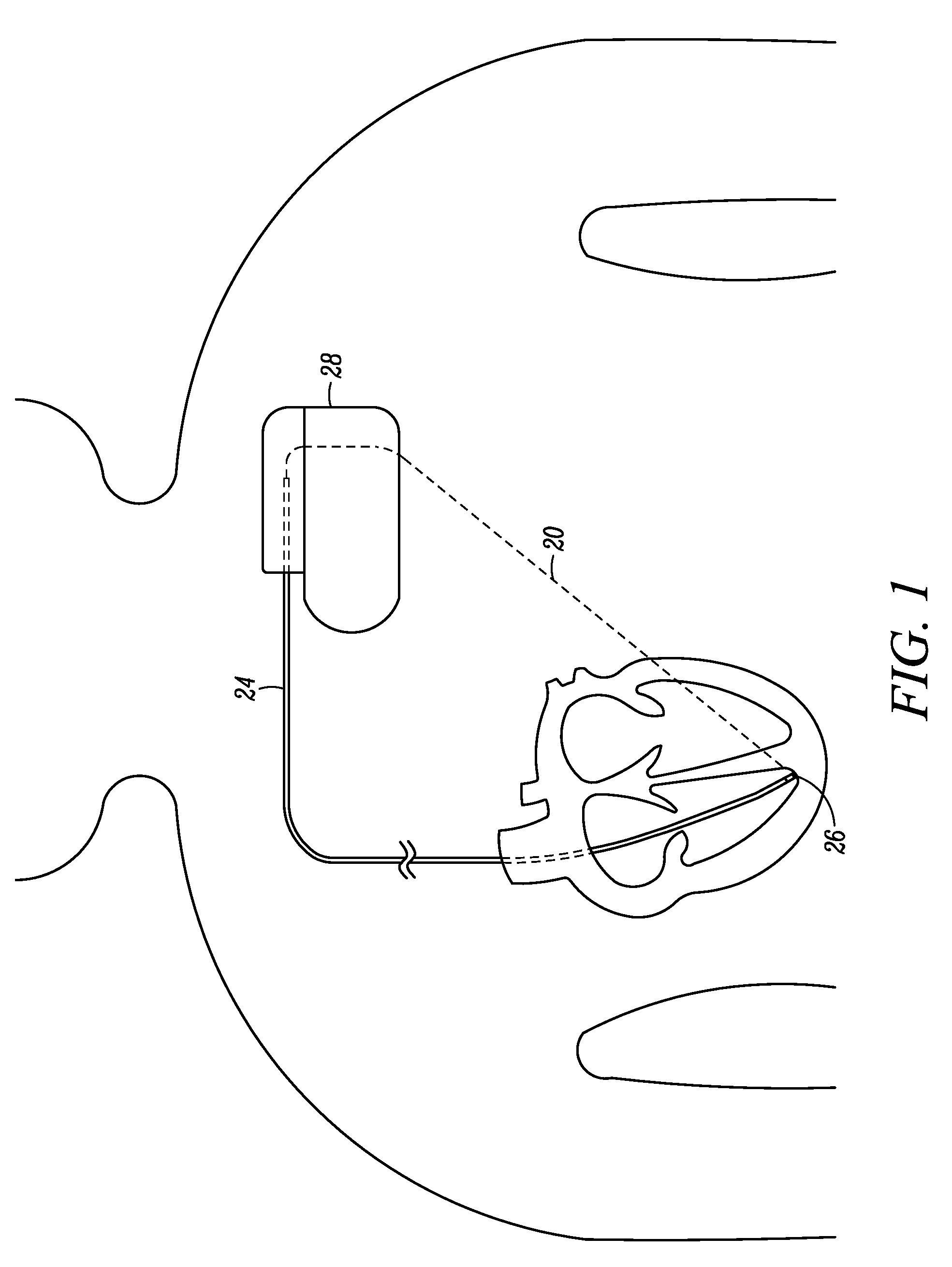
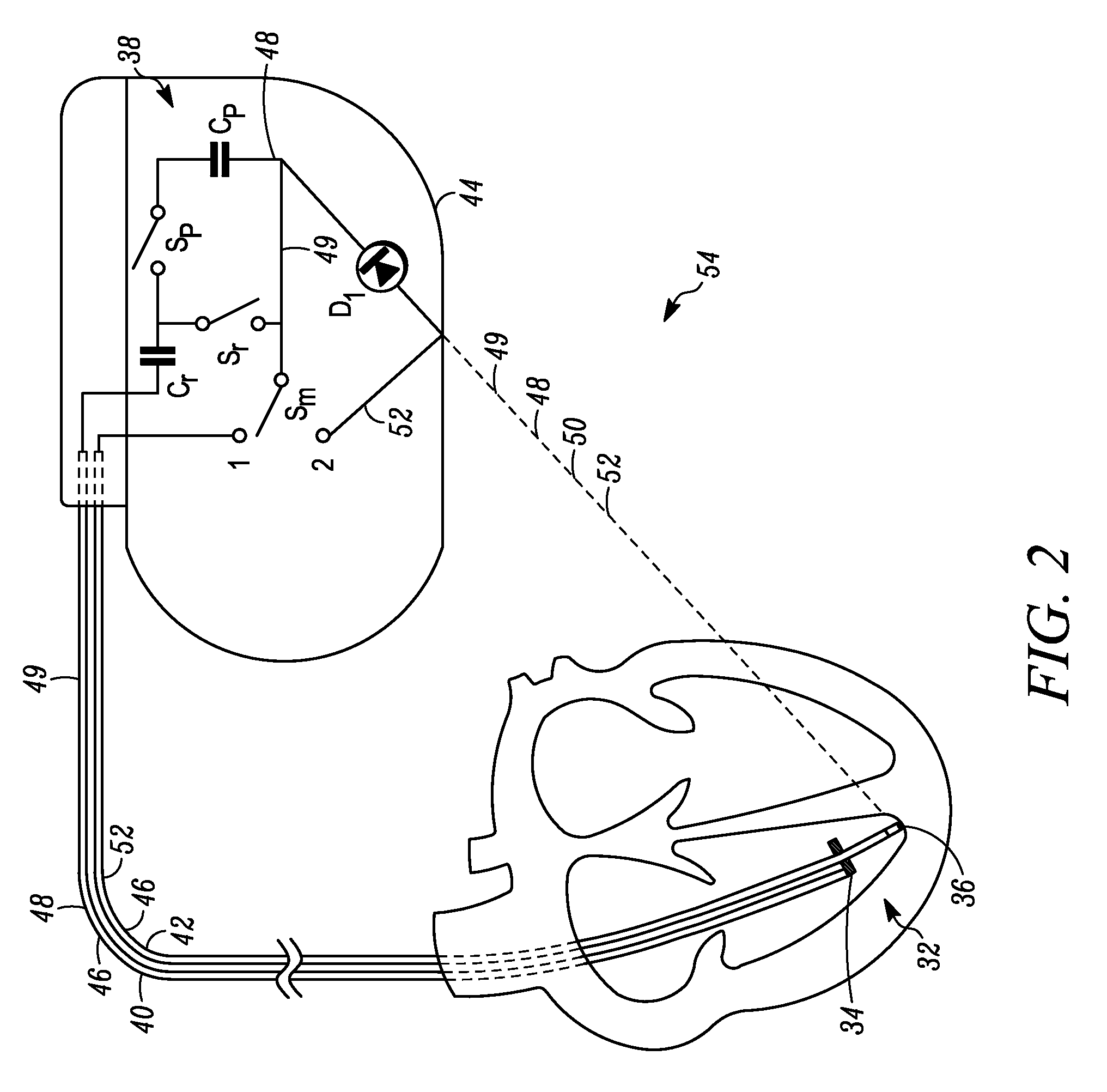
MRI-compatible implantable device
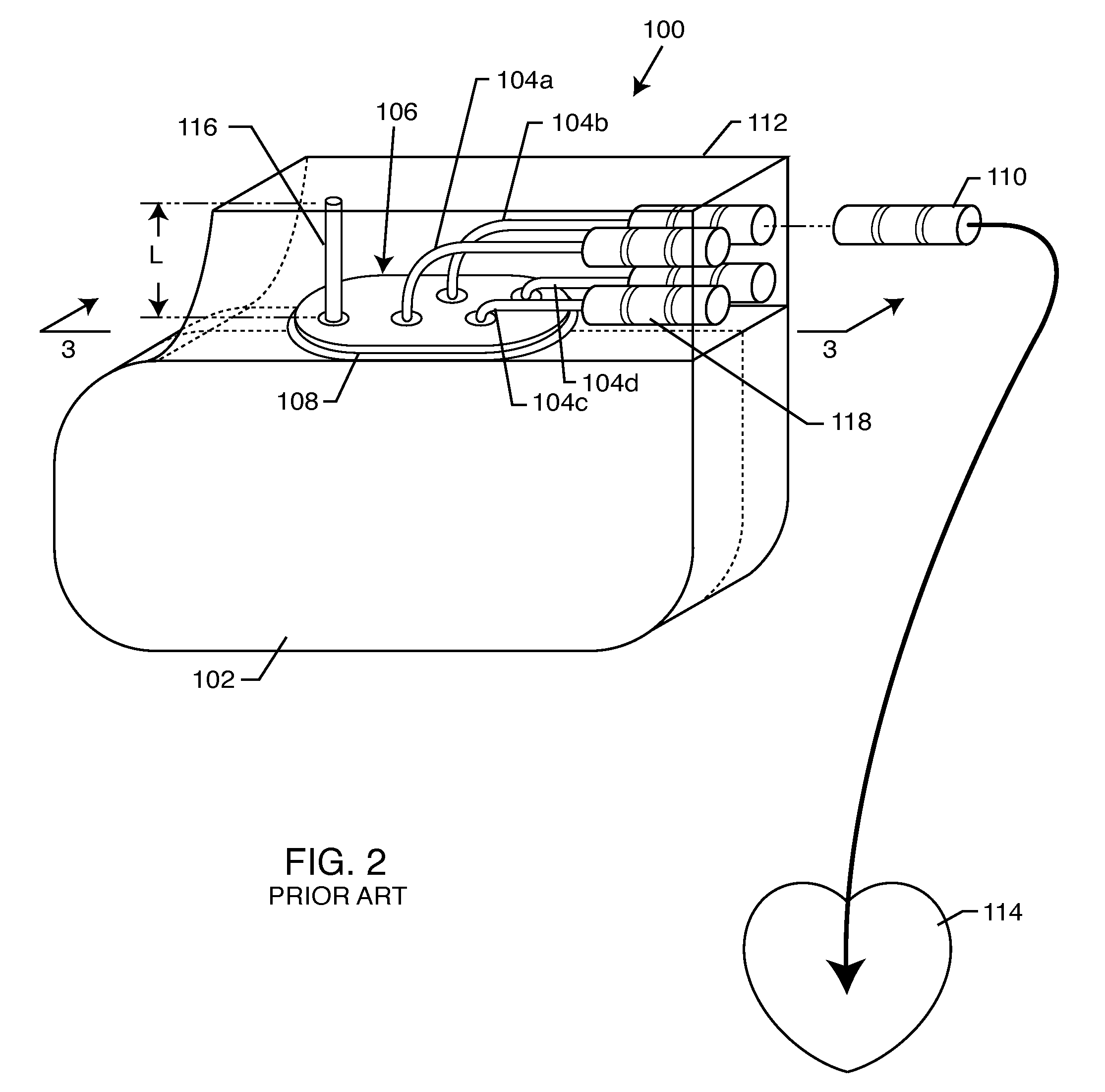
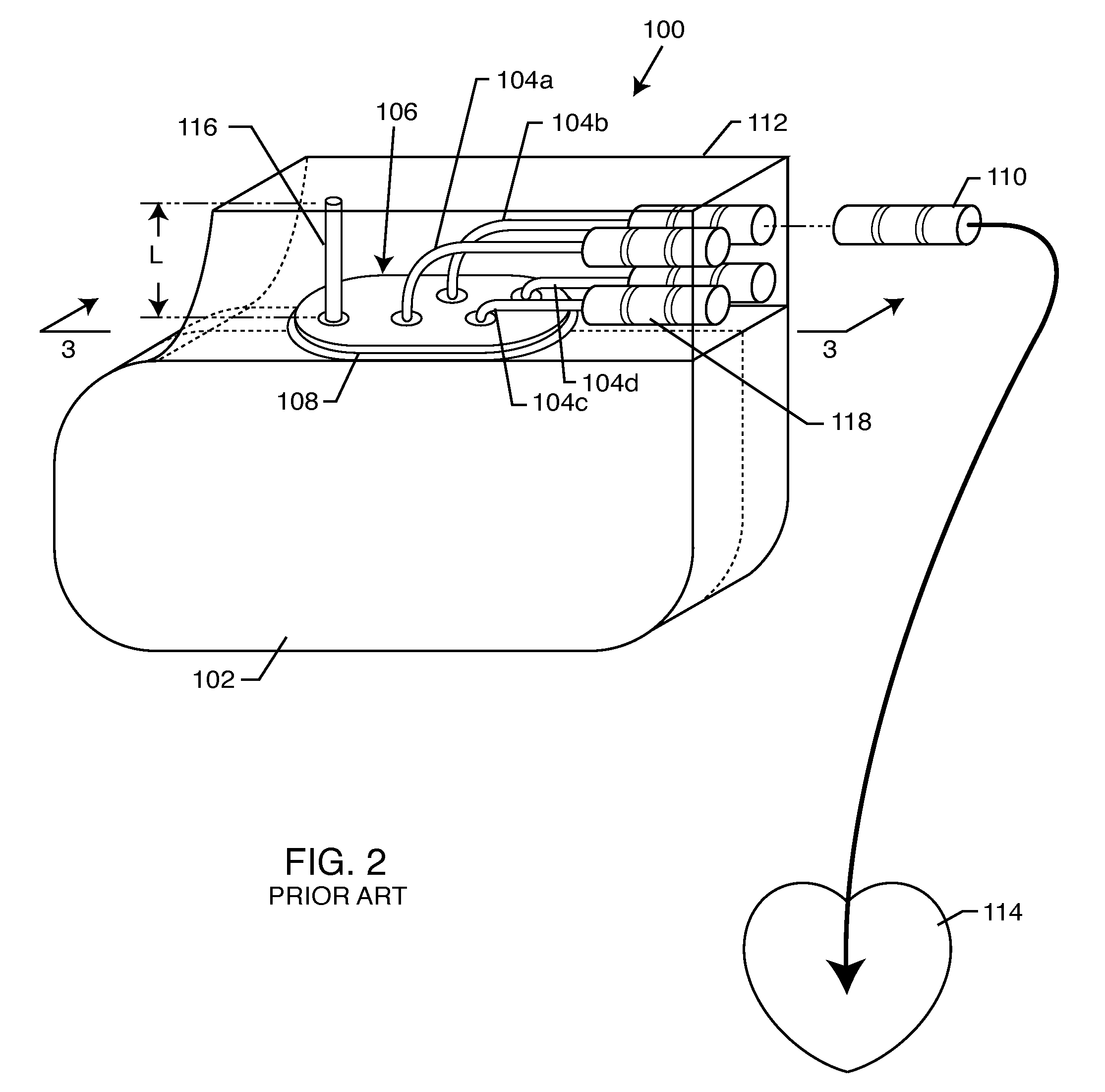
A cardiac assist device containing a device for connecting the cardiac assist device to a heart, for furnishing electrical impulses from the cardiac assist device to the heart, for ceasing the furnishing of electrical impulses to the heart, for receiving pulsed radio frequency fields, for transmitting and receiving optical signals, and for protecting the heart and the cardiac assist device from currents induced by the pulsed radio frequency fields. The cardiac assist device contains a control circuit comprised of a parallel resonant frequency circuit.
Owner:MEDTRONIC INC
MRI Compatible Leadless Cardiac Pacemaker
InactiveUS20110077708A1Maintain safe operationEpicardial electrodesHeart stimulatorsPower flowPath length
An implantable battery powered leadless pacemaker or biostimulator is provided that may include any of a number of features. One feature of the biostimulator is that it safely operates under a wide range of MRI conditions. One feature of the biostimulator is that it has a total volume small enough to avoid excessive image artifacts during a MRI procedure. Another feature of the biostimulator is that it has reduced path lengths between electrodes to minimize tissue heating at the site of the biostimulator. Yet another feature of the biostimulator is that a current loop area within the biostimulator is small enough to reduce an induced current and voltage in the biostimulator during MRI procedures. Methods associated with use of the biostimulator are also covered.
Owner:NANOSTIM
Mri biopsy apparatus incorporating a sleeve and multi-function obturator
ActiveUS20050277829A1Facilitate invasive procedureEasy to confirmCannulasSurgical needlesBiopsy procedureBiopsy instruments
A localization mechanism, or fixture, is used in conjunction with a breast coil for breast compression and for guiding a core biopsy instrument during prone biopsy procedures in both open and closed Magnetic Resonance Imaging (MRI) machines. The localization fixture includes a three-dimensional Cartesian positionable guide for supporting and orienting an MRI-compatible biopsy instrument, and in particular a sleeve, to a biopsy site of suspicious tissues or lesions. A depth stop enhances accurate insertion, and prevents over-insertion or inadvertent retraction of the sleeve. The sleeve receives a probe of the MRI-compatible biopsy instrument and may contain various features to enhance its imagability, to enhance vacuum and pressure assist therethrough, and marker deployment etc.
Owner:DEVICOR MEDICAL PROD
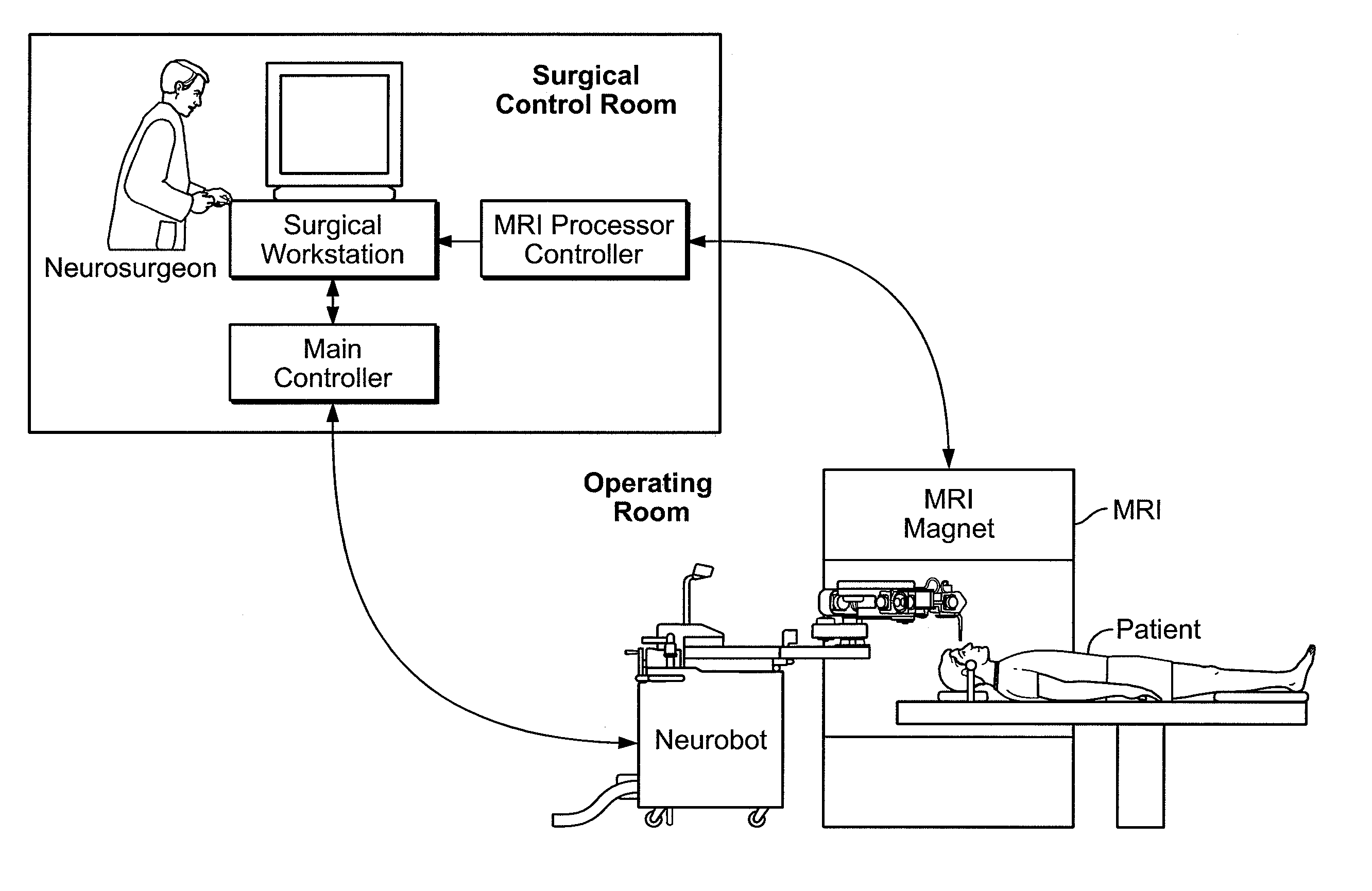
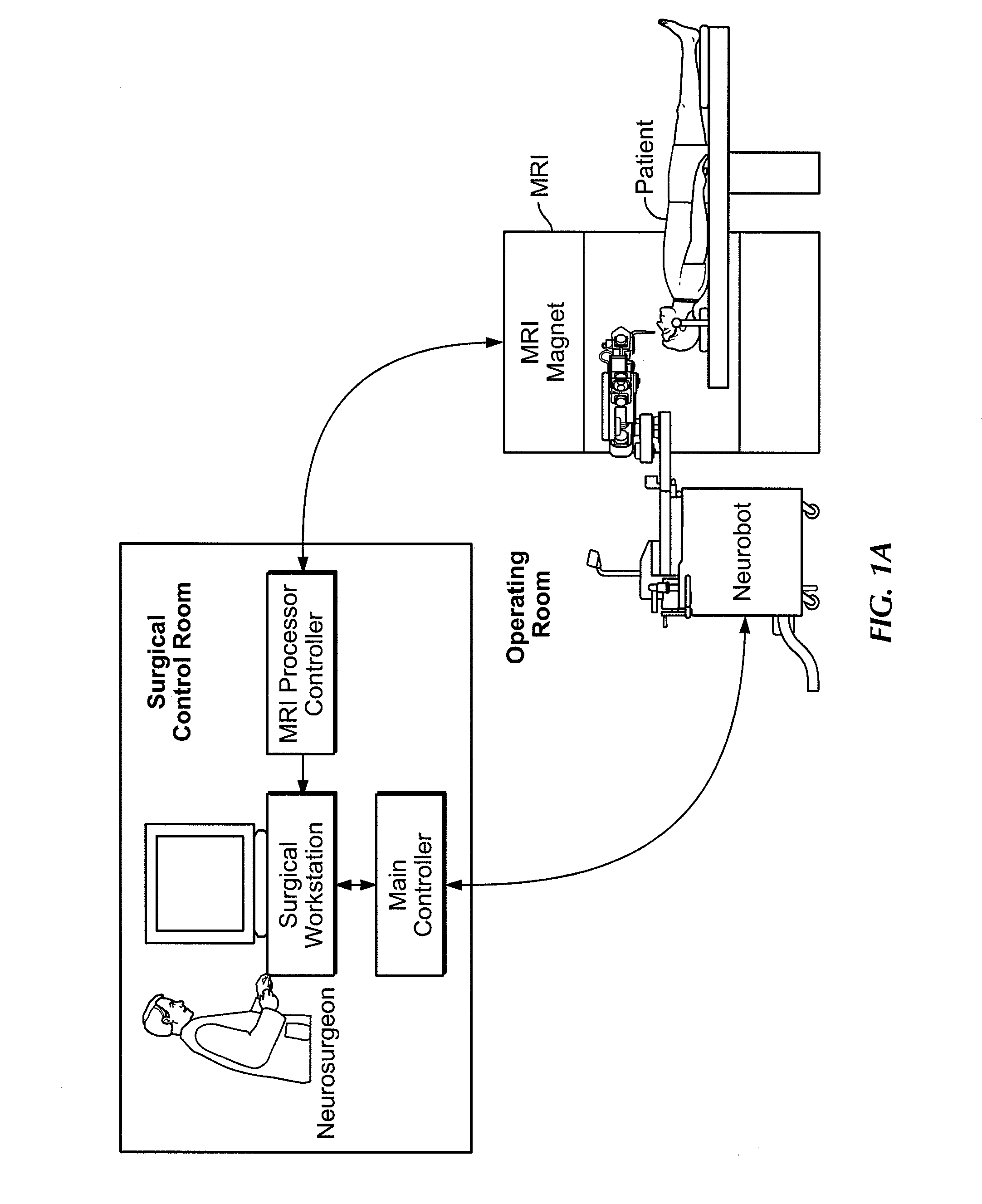
Microsurgical robot system
InactiveUS20070032906A1Enhanced Situational AwarenessReduce loadProgramme-controlled manipulatorEndoscopesMicroscopic observationHorizontal axis
Owner:DEERFIELD IMAGING INC
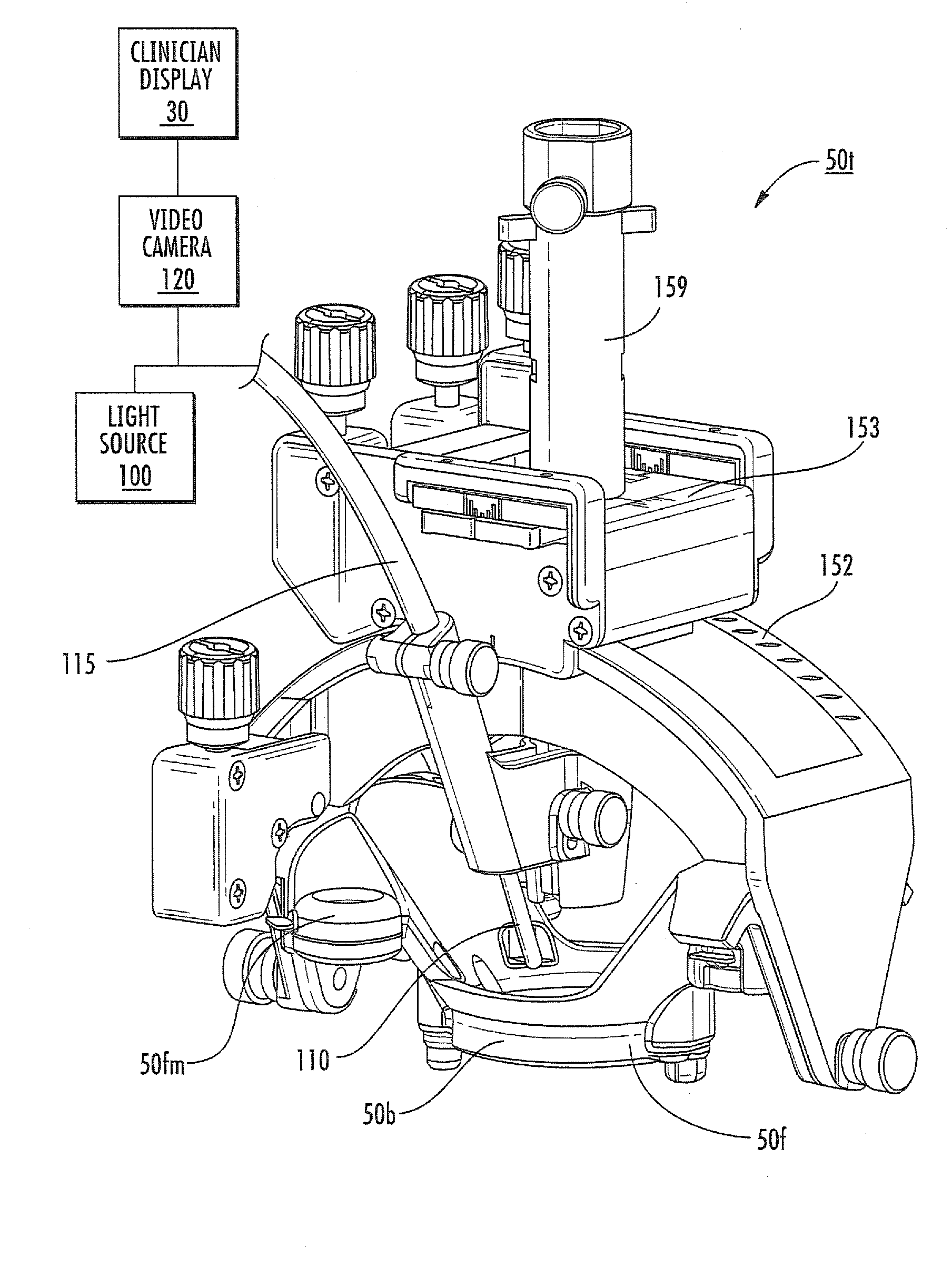
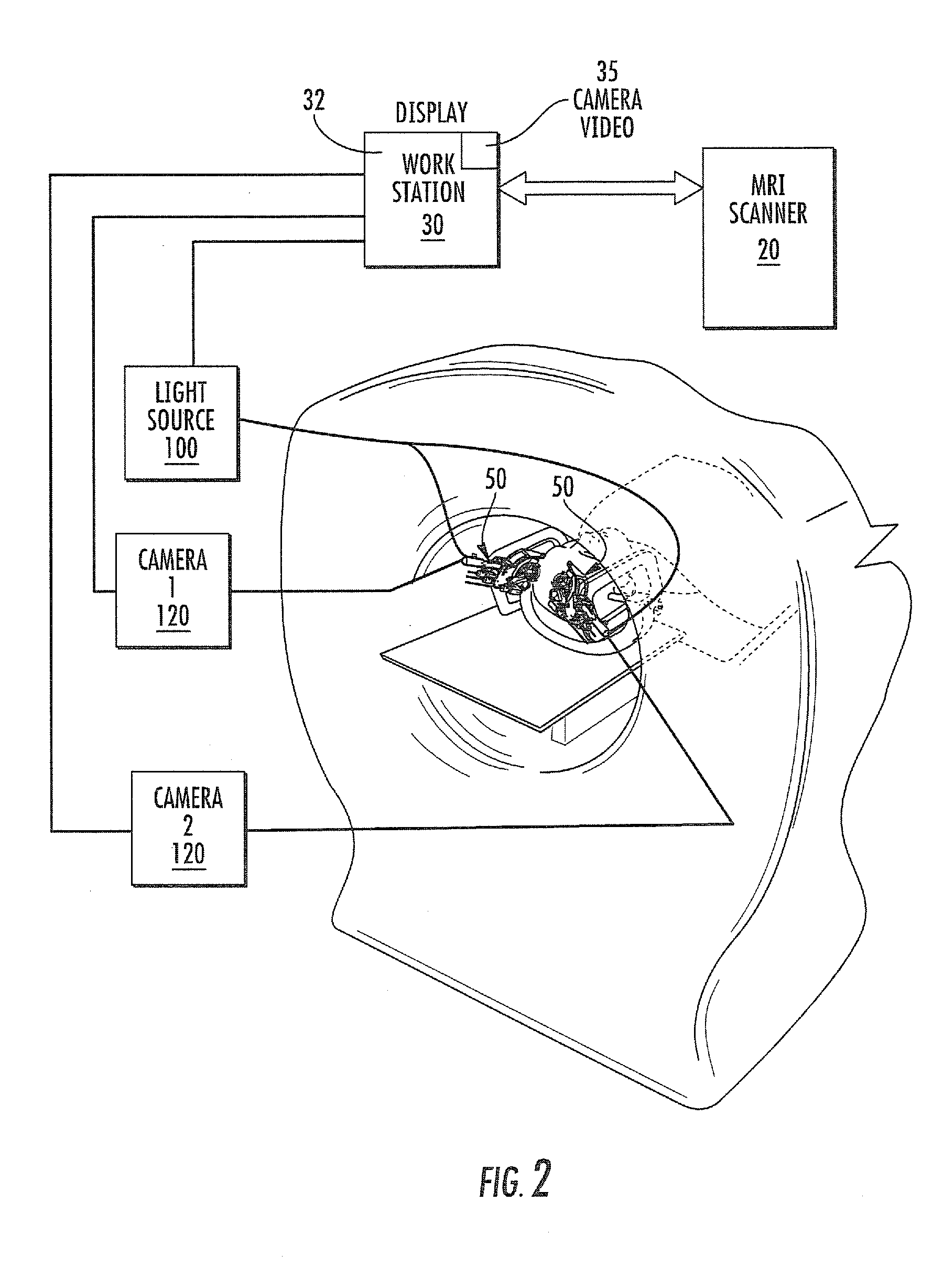
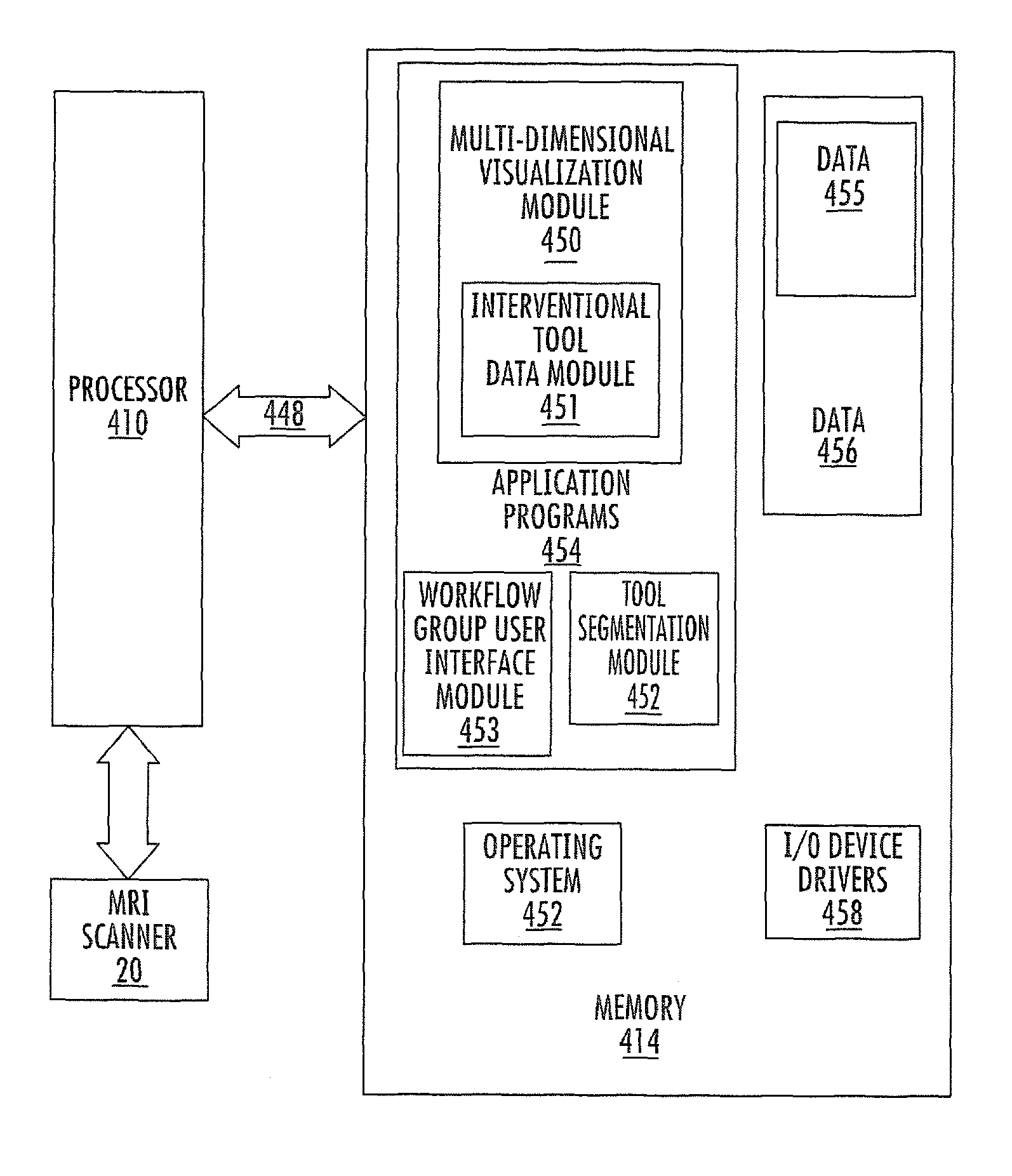
MRI surgical systems for real-time visualizations using MRI image data and predefined data of surgical tools
ActiveUS20090171184A1Increase speedImprove reliabilityMagnetic measurementsSurgical needlesMri guidedDisplay device
MRI-Surgical systems include: (a) at least one MRI-compatible surgical tool; (b) a circuit adapted to communicate with an MRI scanner; and (c) at least one display in communication with the circuit. The circuit electronically recognizes predefined physical characteristics of the at least one tool to automatically segment MR image data provided by the MRI scanner whereby the at least one tool constitutes a point of interface with the system. The circuit is configured to provide a User Interface that defines workflow progression for an MRI-guided surgical procedure and allows a user to select steps in the workflow, and wherein the circuit is configured to generate multi-dimensional visualizations using the predefined data of the at least one tool and data from MRI images of the patient in substantially real time during the surgical procedure.
Owner:CLEARPOINT NEURO INC
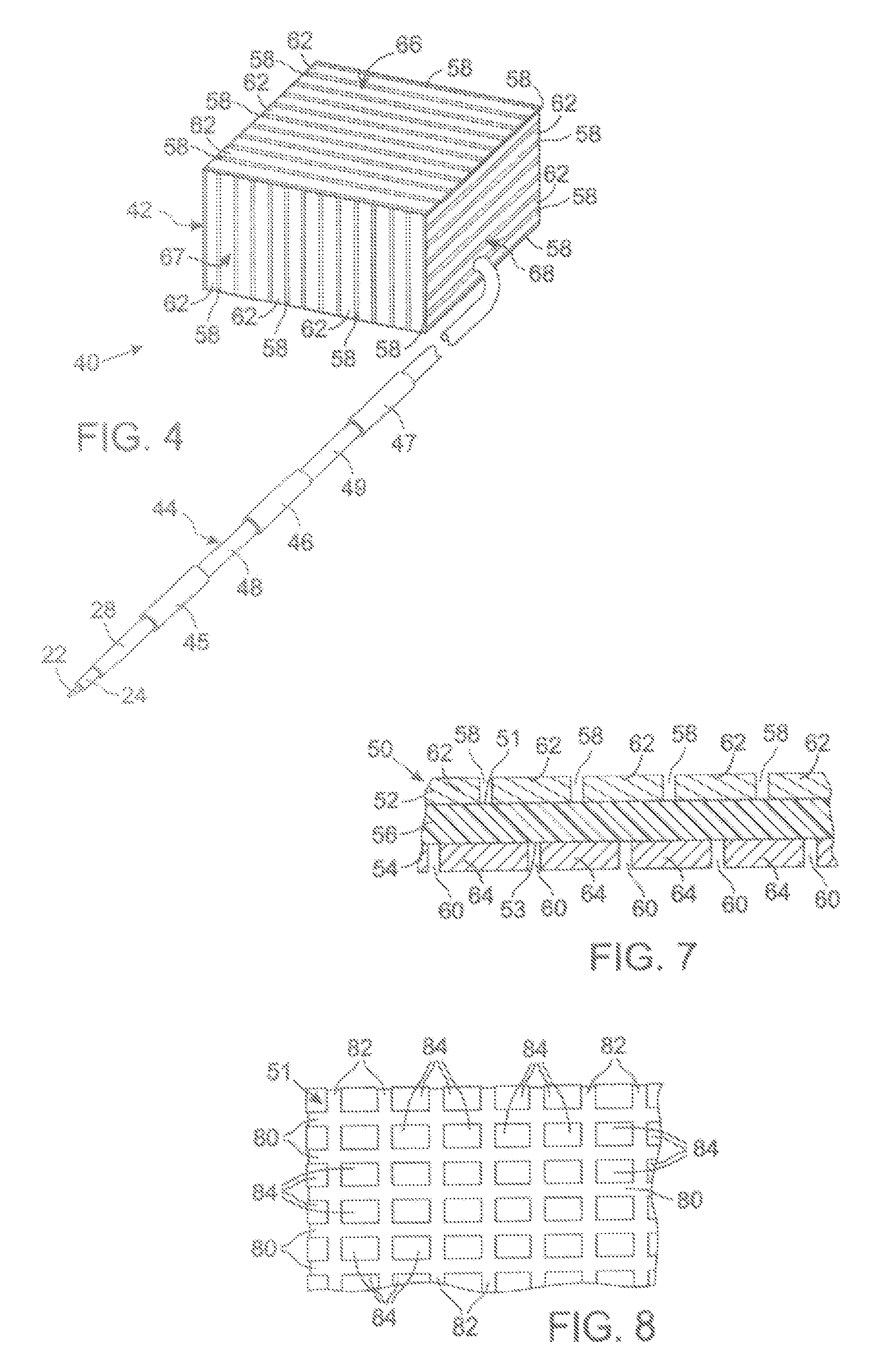
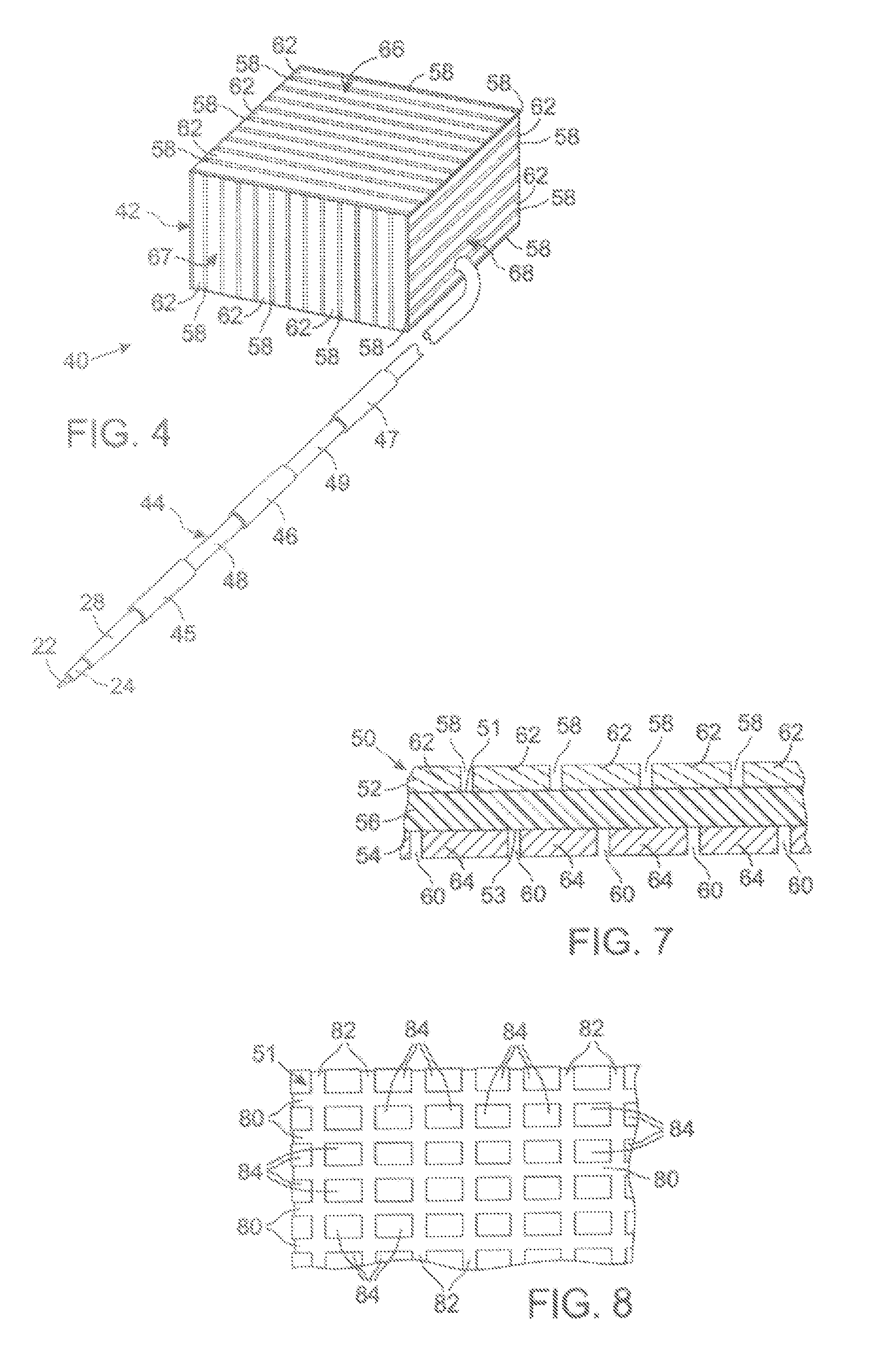
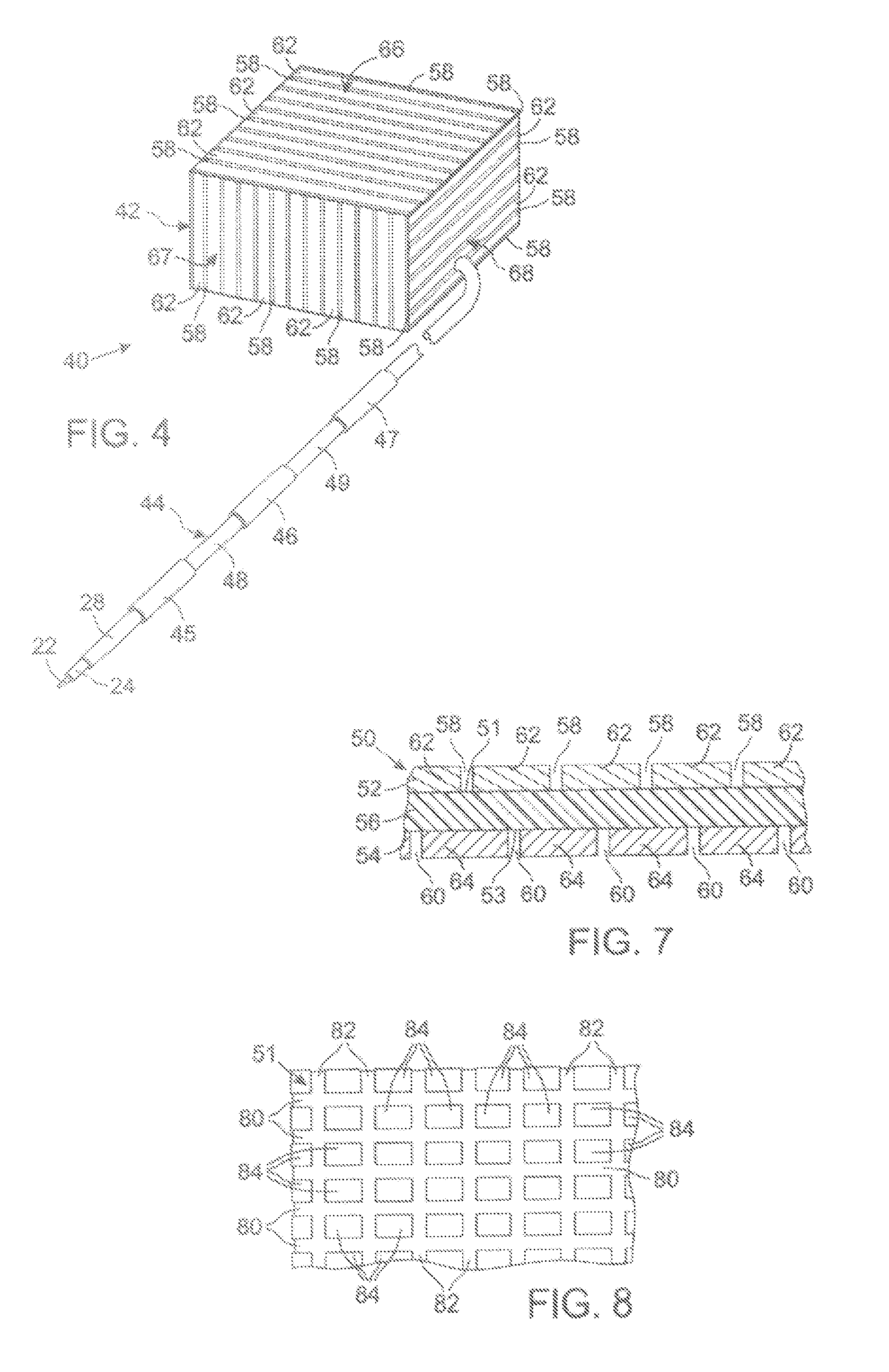
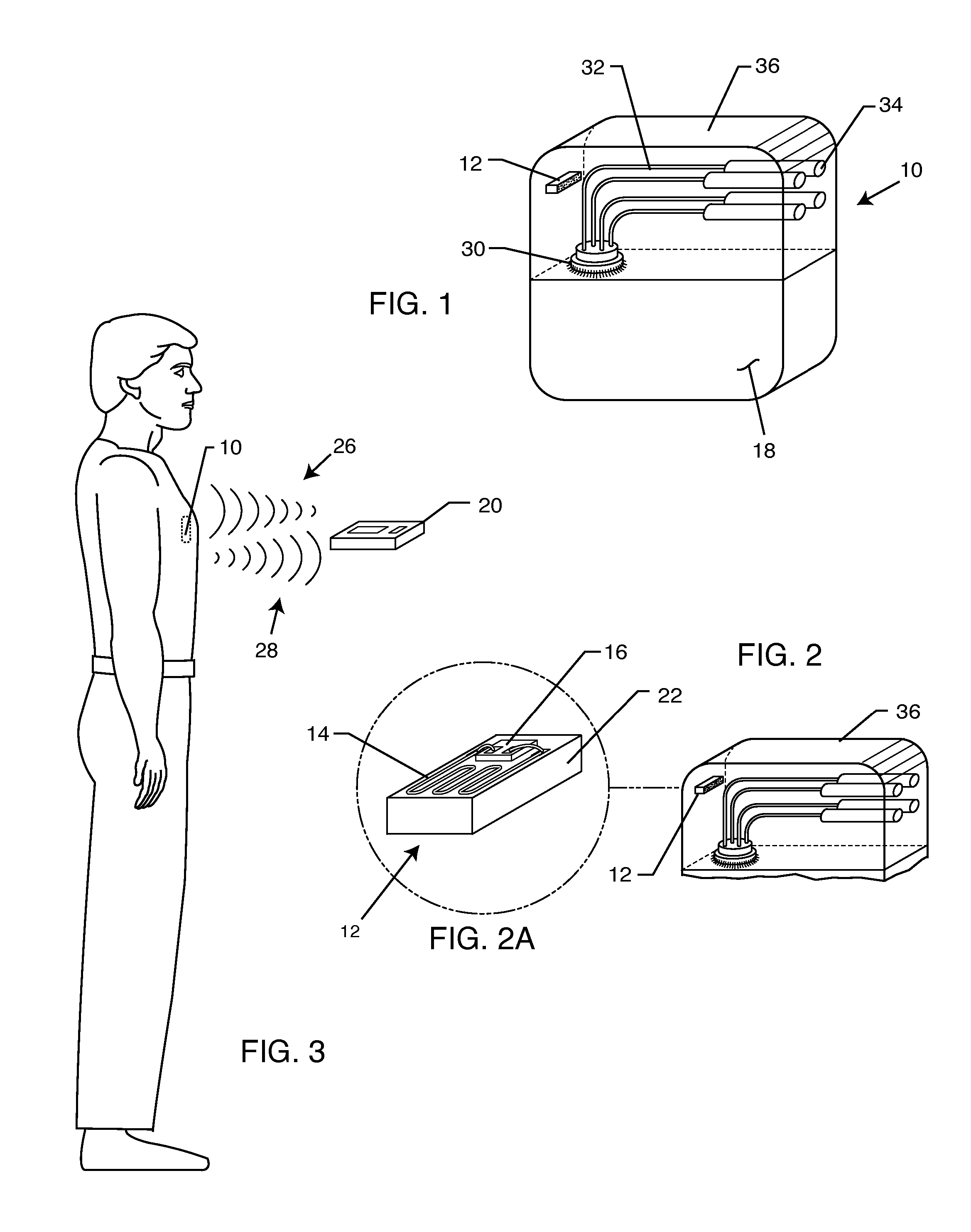
MRI Compatible Implanted Electronic Medical Device
An implantable electronic medical device is compatible with a magnetic resonance imaging (MRI) scanner. The device has a housing with exterior walls, each formed by a dielectric substrate with electrically conductive layers on interior and exterior surfaces. A series of slots divide each layer into segments. Segmenting the layers provides high impedance to eddy currents produced by fields of the MRI scanner, while capacitive coupling of the segments provides radio frequency shielding for components inside the housing. Electrical leads extending from the housing have a pair of coaxially arranged conductors and traps that attenuate currents induced in the conductors by the fields of the MRI scanner.
Owner:KENERGY INC
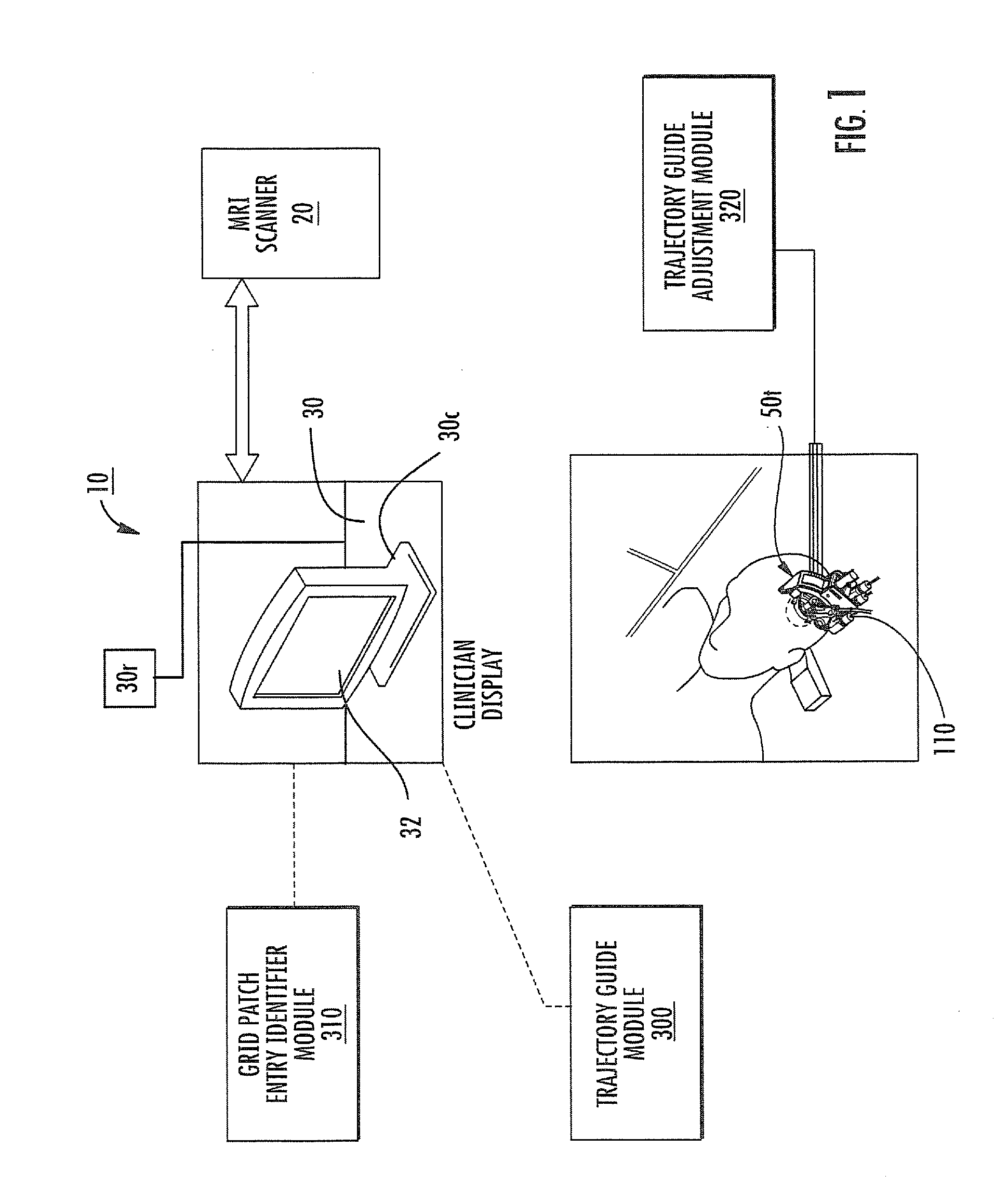
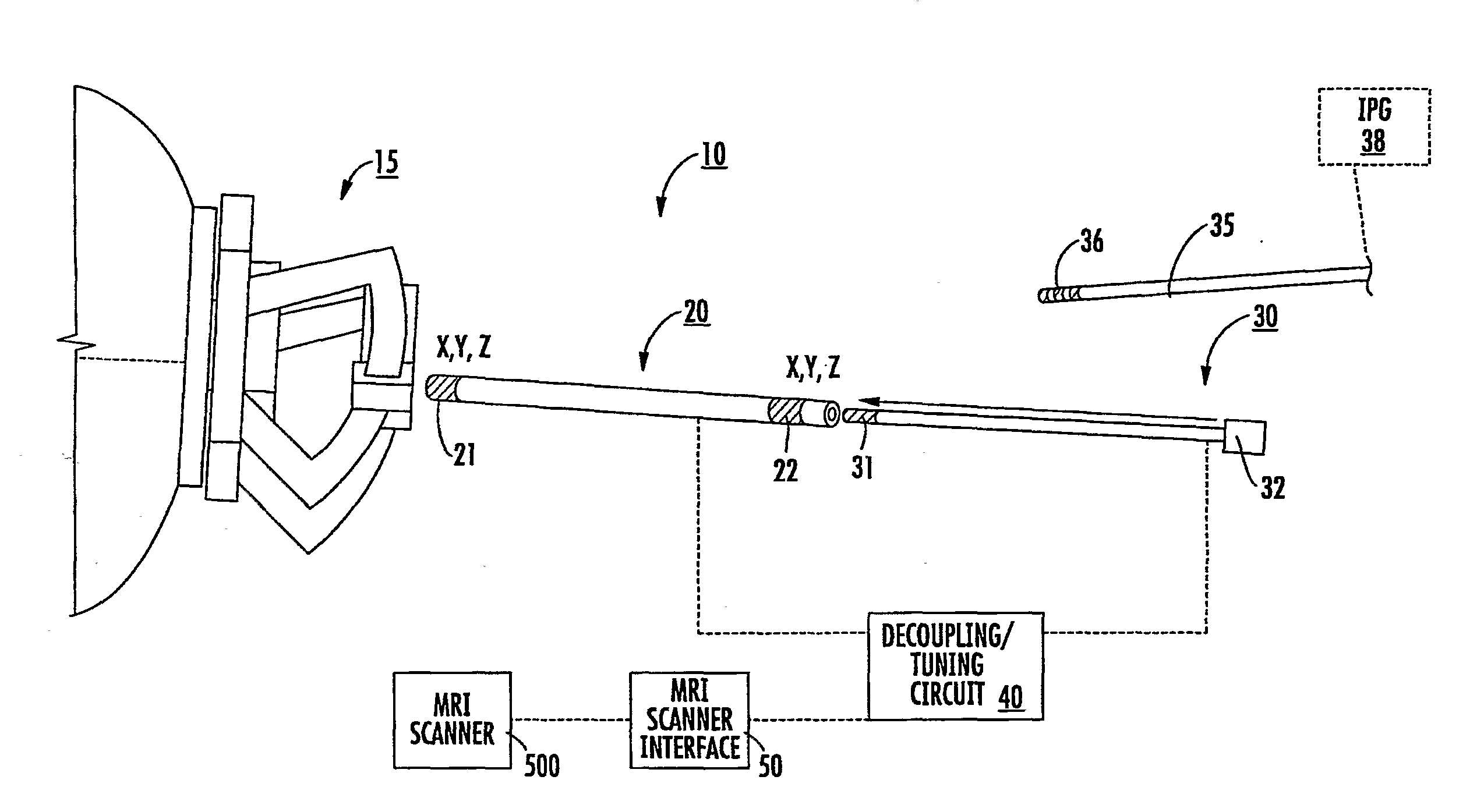
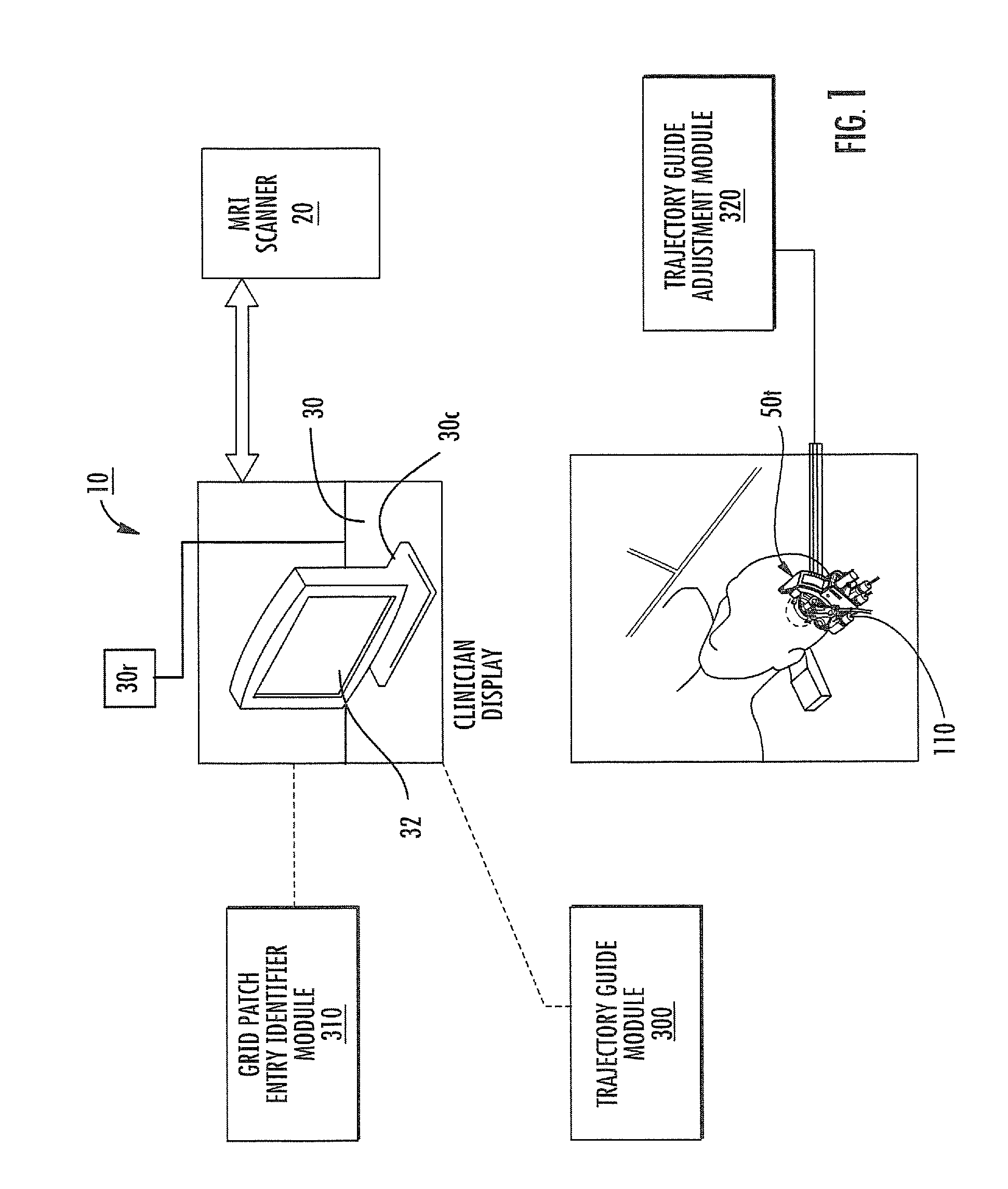
Mri-guided localization and/or lead placement systems, related methods, devices and computer program products
MRI compatible localization and / or guidance systems for facilitating placement of an interventional therapy and / or device in vivo include: (a) a mount adapted for fixation to a patient; (b) a targeting cannula with a lumen configured to attach to the mount so as to be able to controllably translate in at least three dimensions; and (c) an elongate probe configured to snugly slidably advance and retract in the targeting cannula lumen, the elongate probe comprising at least one of a stimulation or recording electrode. In operation, the targeting cannula can be aligned with a first trajectory and positionally adjusted to provide a desired internal access path to a target location with a corresponding trajectory for the elongate probe. Automated systems for determining an MR scan plane associated with a trajectory and for determining mount adjustments are also described.
Owner:CLEARPOINT NEURO INC
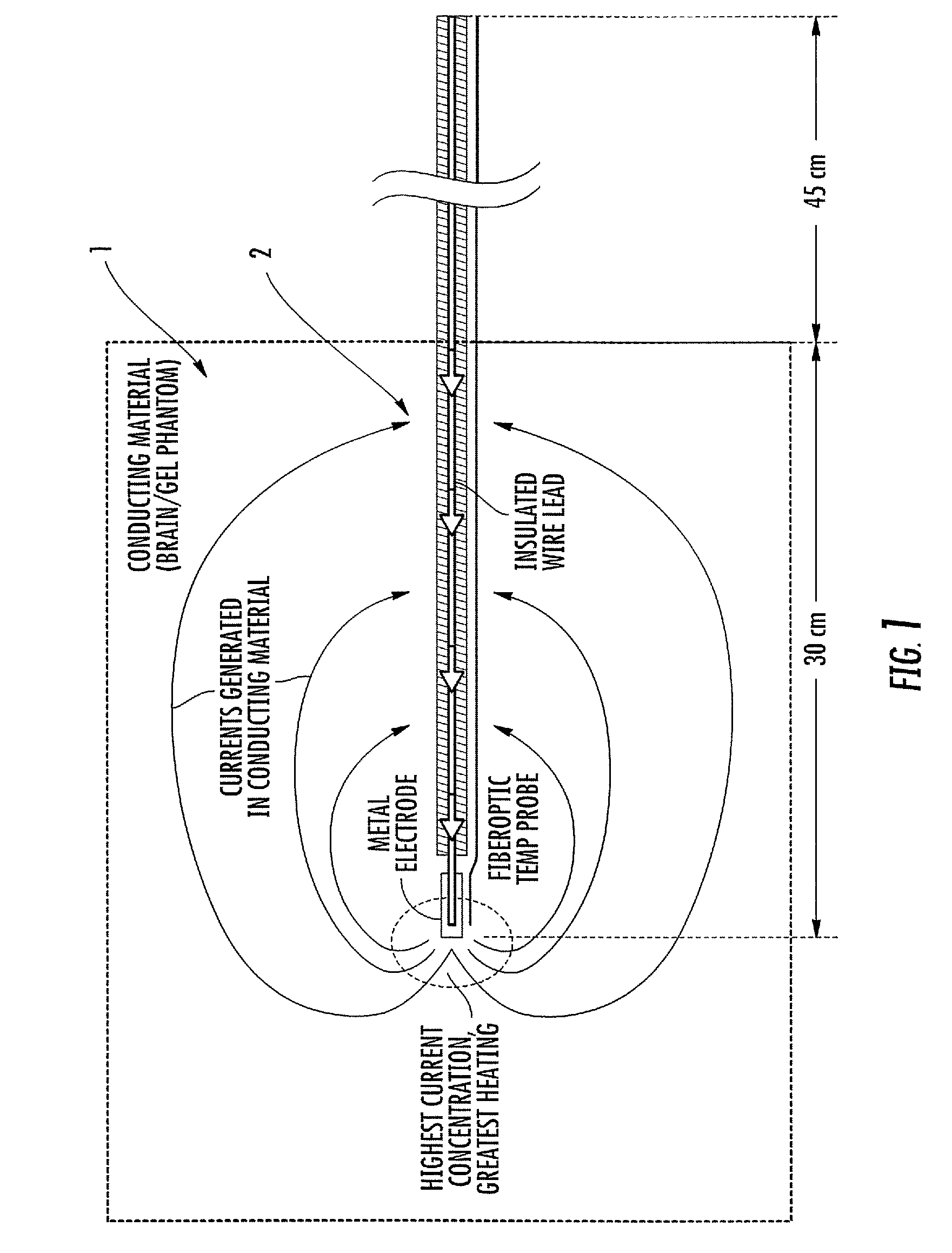
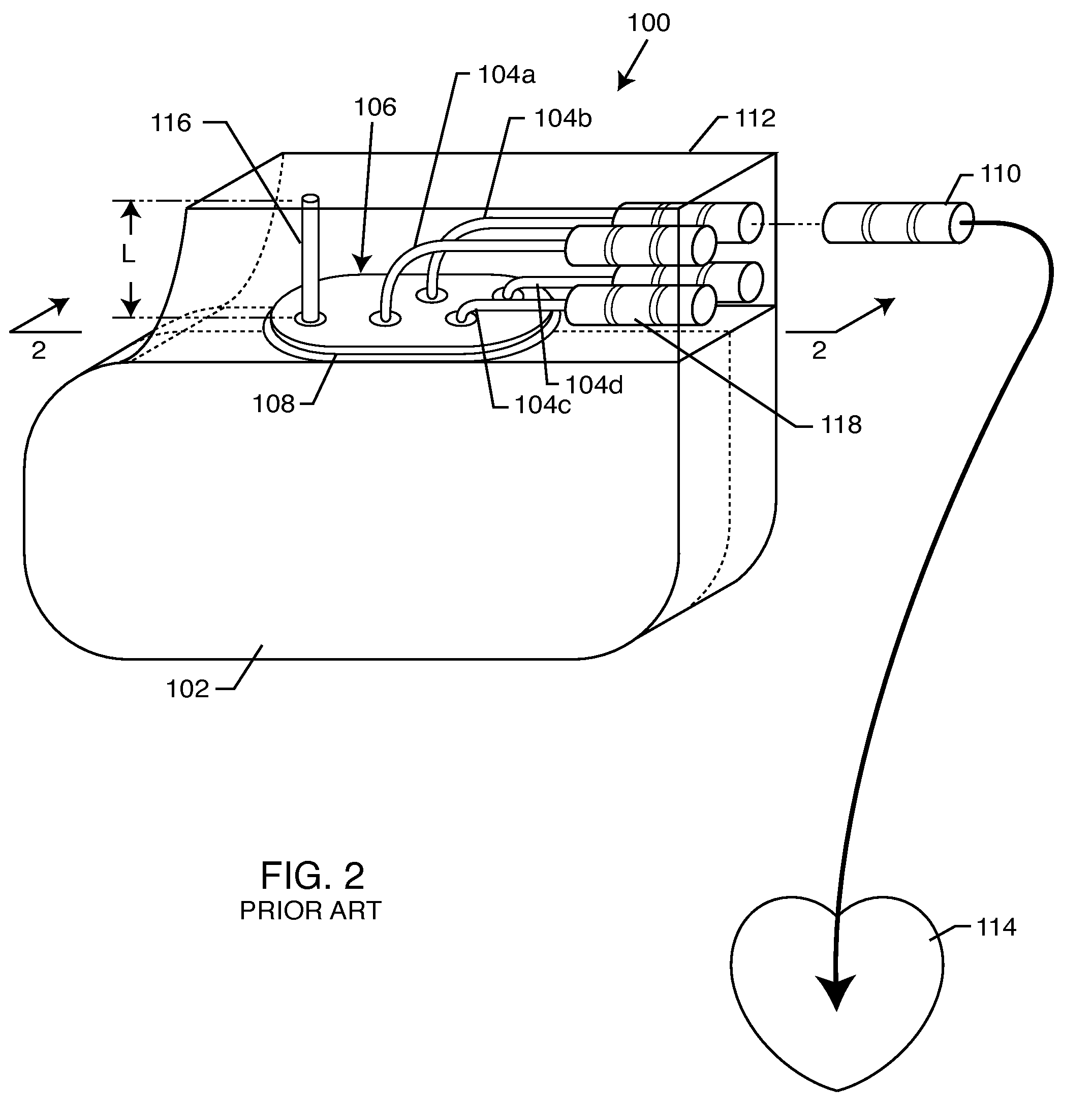
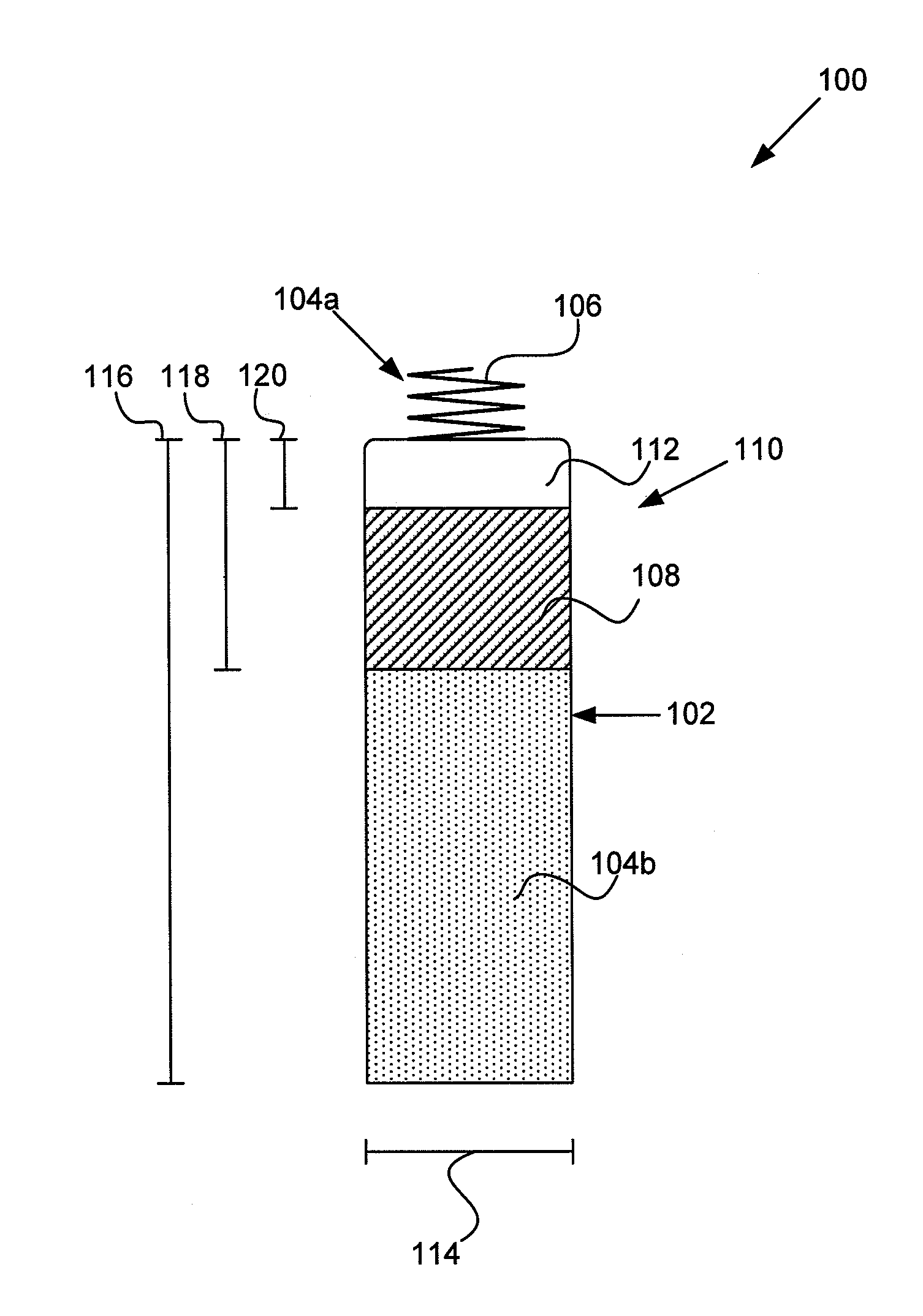
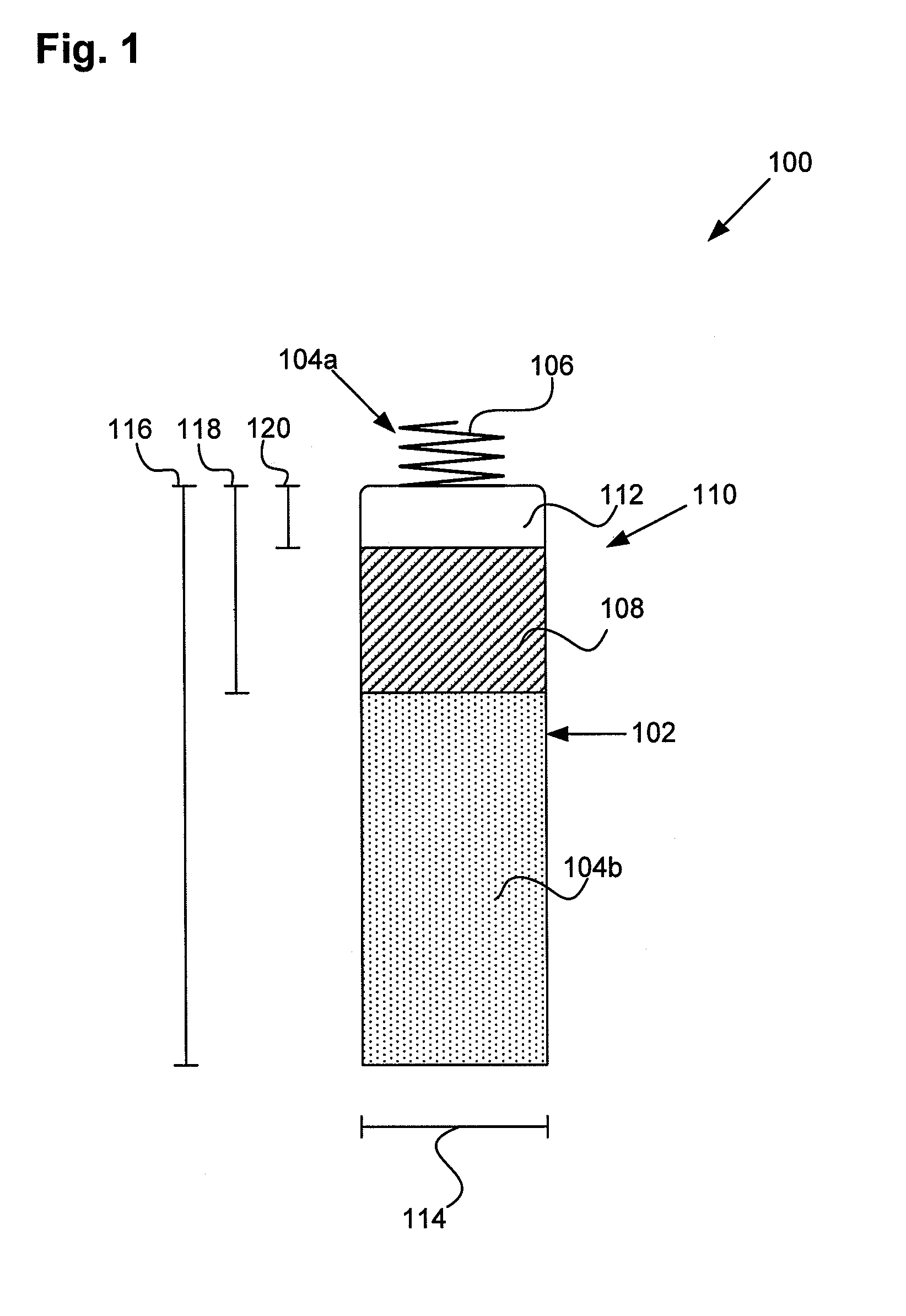
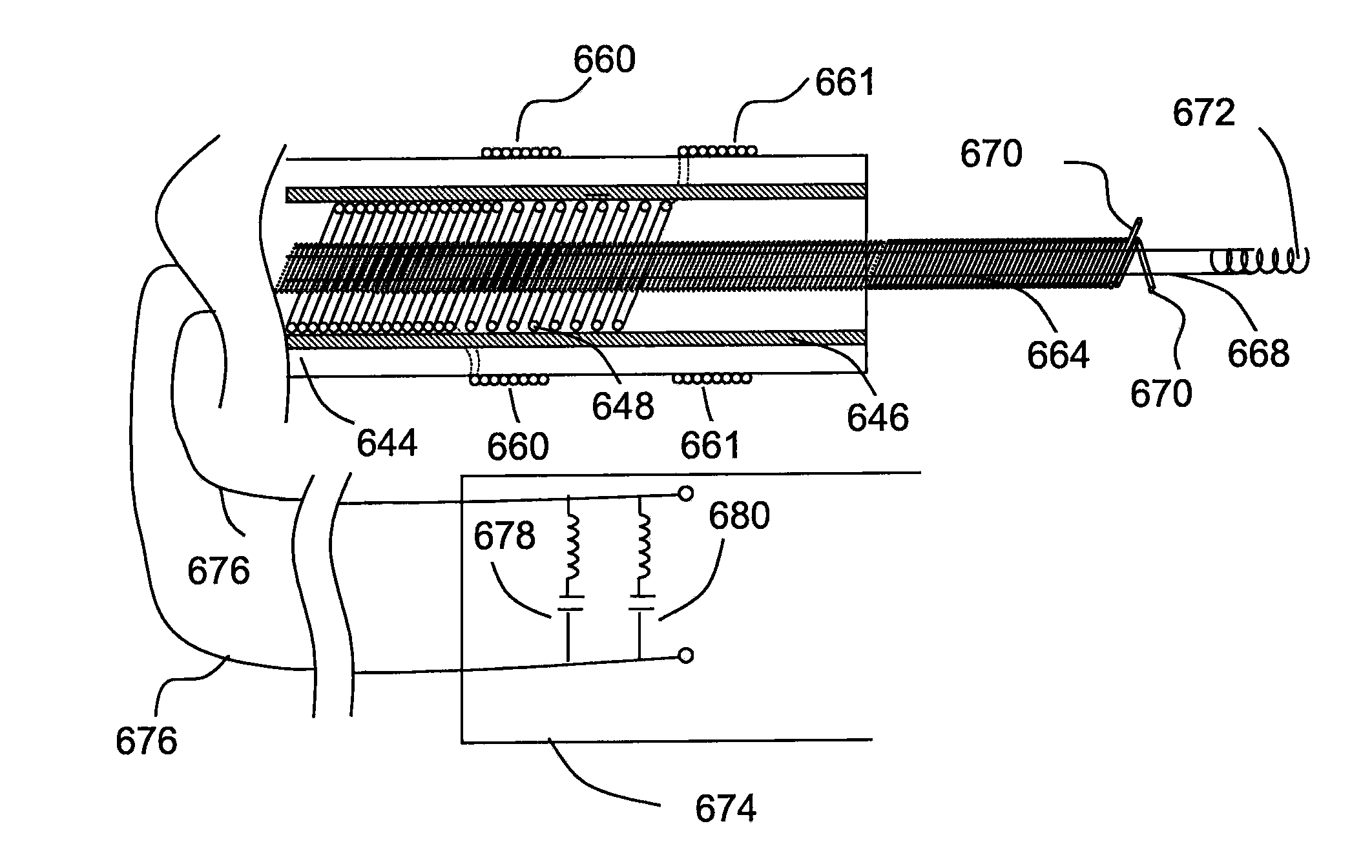
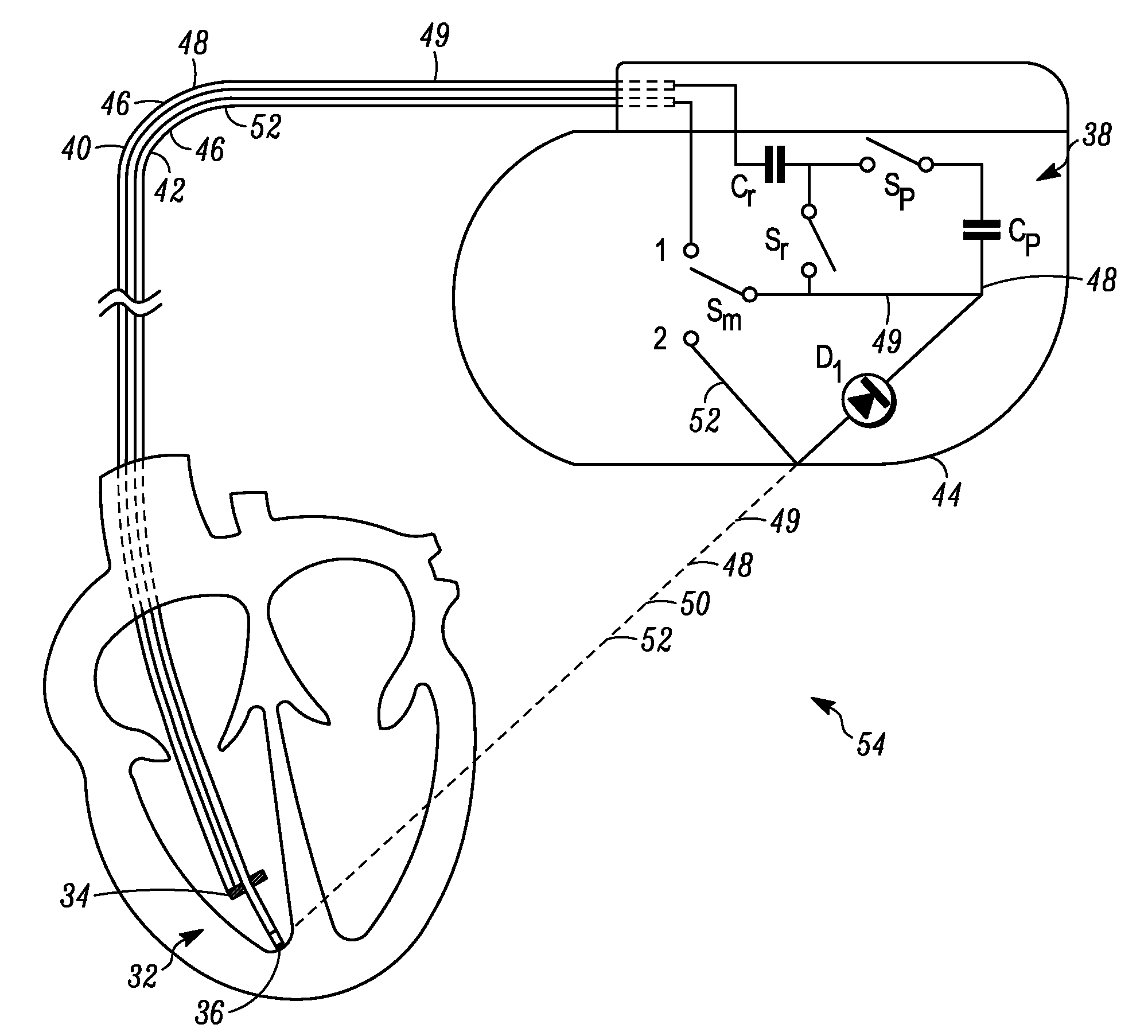
Tank filters adaptable for placement with a guide wire, in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
InactiveUS20080161886A1Reduce sensitivityReduce heatMultiple-port networksAnti-noise capacitorsEngineeringInductor
A tank filter is provided for a lead wire of an active medical device (AMD). The tank filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the tank filter is resonant at a selected frequency. A passageway through the tank filter permits selective slidable passage of a guide wire therethrough for locating the lead wire in an implantable position. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the tank filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the tank filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
MRI-compatible implantable device
A medical device containing a device for connecting the medical device to a substrate, for furnishing electrical impulses from the medical device to the substrate, for ceasing the furnishing of electrical impulses to the substrate, for receiving pulsed radio frequency fields, for transmitting and receiving optical signals, and for protecting the substrate and the medical device from currents induced by the pulsed radio frequency fields. The medical device contains a control circuit comprised of a parallel resonant frequency circuit.
Owner:MEDTRONIC INC
Metal alloy for medical devices and implants
InactiveUS20080312740A1Maintain good propertiesImprove uniformityStentsHeart valvesNiobiumBiocompatibility Testing
Owner:ABBOTT IRELAND
Tank filters adaptable for placement with a guide wire, in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
InactiveUS7702387B2Reduce sensitivityReduce heatMultiple-port networksAnti-noise capacitorsCapacitanceEngineering
A tank filter is provided for a lead wire of an active medical device (AMD). The tank filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the tank filter is resonant at a selected frequency. A passageway through the tank filter permits selective slidable passage of a guide wire therethrough for locating the lead wire in an implantable position. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the tank filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the tank filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
Implantable lead bandstop filter employing an inductive coil with parasitic capacitance to enhance MRI compatibility of active medical devices
InactiveUS8145324B1Maximized (or minimizedMinimize resistance lossMultiple-port networksSpinal electrodesParasitic capacitanceEngineering
A medical lead system includes at least one bandstop filter for attenuating current flow through the lead across a range of frequencies. The bandstop filter has an overall circuit Q wherein the resultant 3 dB bandwidth is at least 10 kHz. The values of capacitance and inductance of the bandstop filter are selected such that the bandstop filter is resonant at a selected center frequency or range of frequencies. Preferably, the bandstop filter has an overall circuit Q wherein the resultant 10 dB bandwidth is at least 10 kHz. Such bandstop filters are backwards compatible with known implantable deployment systems and extraction systems.
Owner:WILSON GREATBATCH LTD
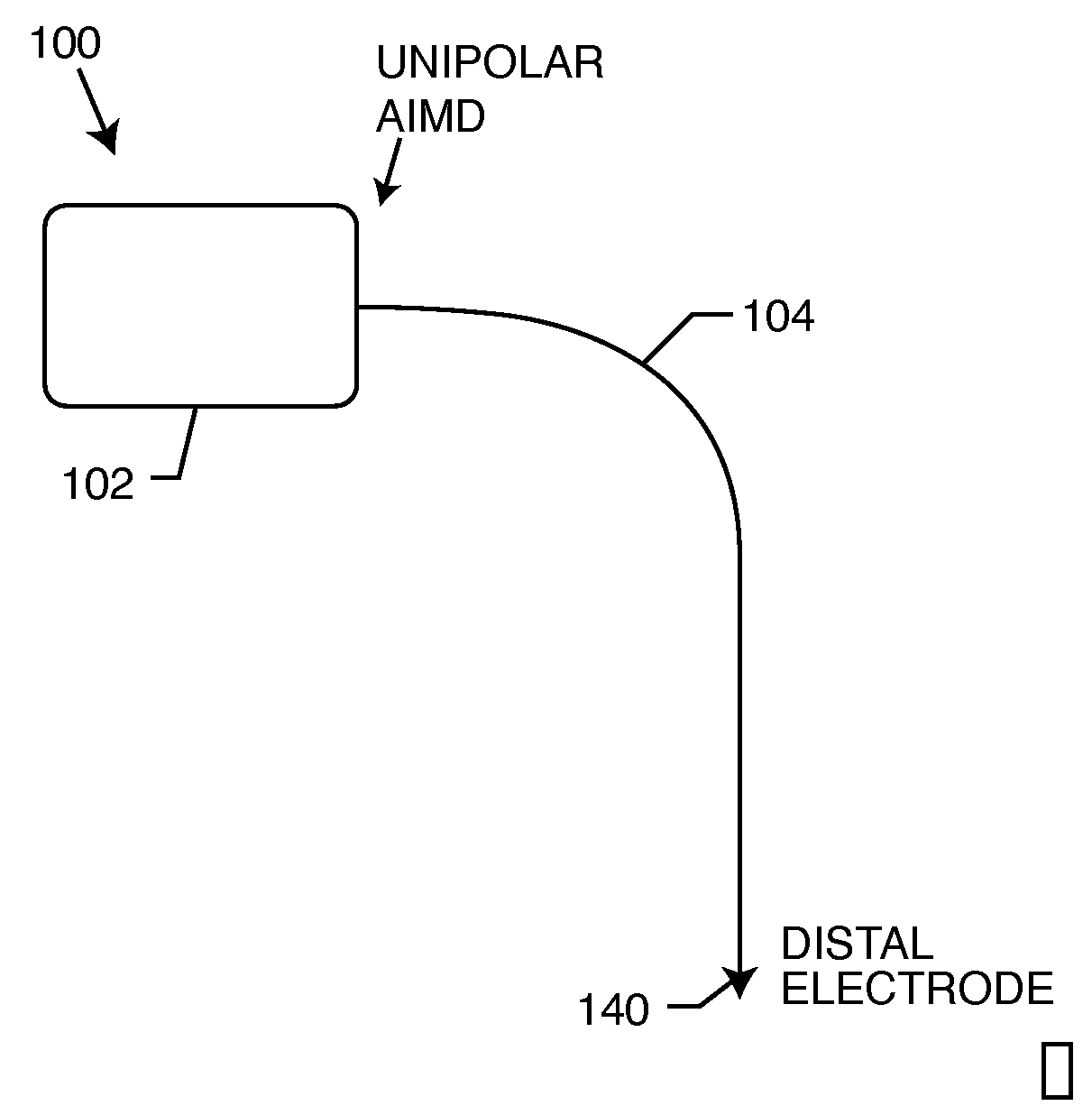
Switch for turning off therapy delivery of an active implantable medical device during MRI scans
InactiveUS20090163980A1Enhanced magnetic forceAvoid detectionElectrotherapyStatic fieldRadio frequency
An MRI-compatible electronic medical therapy system is provided for temporarily preventing current flow through an implanted lead wire in the presence of an induced radio frequency, magnetic, or static field. One or more normally closed switches are disposed in series between the AIMD and the one or more distal electrodes. The switch may be incorporated in the AIMD, lead wire, or within or adjacent to the electrode. The switch remains closed during normal AIMD-related therapy, but temporarily opens in the presence of an induced radio frequency, magnetic, or static field so as to prevent current flow through the electrode and lead wire. The switches prevent current from circulating that could be induced by a medical therapeutic diagnostic device, which can cause overheating of lead wires, excessive currents or temperatures and tissue damage.
Owner:WILSON GREATBATCH LTD
MRI Compatible Leadless Cardiac Pacemaker
InactiveUS20130274847A1Maintain safe operationEpicardial electrodesExternal electrodesTissue heatingPath length
An implantable battery powered leadless pacemaker or biostimulator is provided that may include any of a number of features. One feature of the biostimulator is that it safely operates under a wide range of MRI conditions. One feature of the biostimulator is that it has a total volume small enough to avoid excessive image artifacts during a MRI procedure. Another feature of the biostimulator is that it has reduced path lengths between electrodes to minimize tissue heating at the site of the biostimulator. Yet another feature of the biostimulator is that a current loop area within the biostimulator is small enough to reduce an induced current and voltage in the biostimulator during MRI procedures. Methods associated with use of the biostimulator are also covered.
Owner:PACESETTER INC
MRI biopsy device
InactiveUS20080015429A1Facilitate invasive procedureEasy to confirmCannulasSurgical needlesBiopsy procedureBiopsy instruments
A localization mechanism, or fixture, is used in conjunction with a breast coil for breast compression and for guiding a core biopsy instrument during prone biopsy procedures in both open and closed Magnetic Resonance Imaging (MRI) machines. The localization fixture includes a three-dimensional Cartesian positionable guide for supporting and orienting an MRI-compatible biopsy instrument, and in particular a sleeve, to a biopsy site of suspicious tissues or lesions. A depth stop enhances accurate insertion, and prevents over-insertion or inadvertent retraction of the sleeve. The sleeve receives a probe of the MRI-compatible biopsy instrument and may contain various features to enhance its imagability, to enhance vacuum and pressure assist therethrough, and marker deployment etc.
Owner:DEVICOR MEDICAL PROD
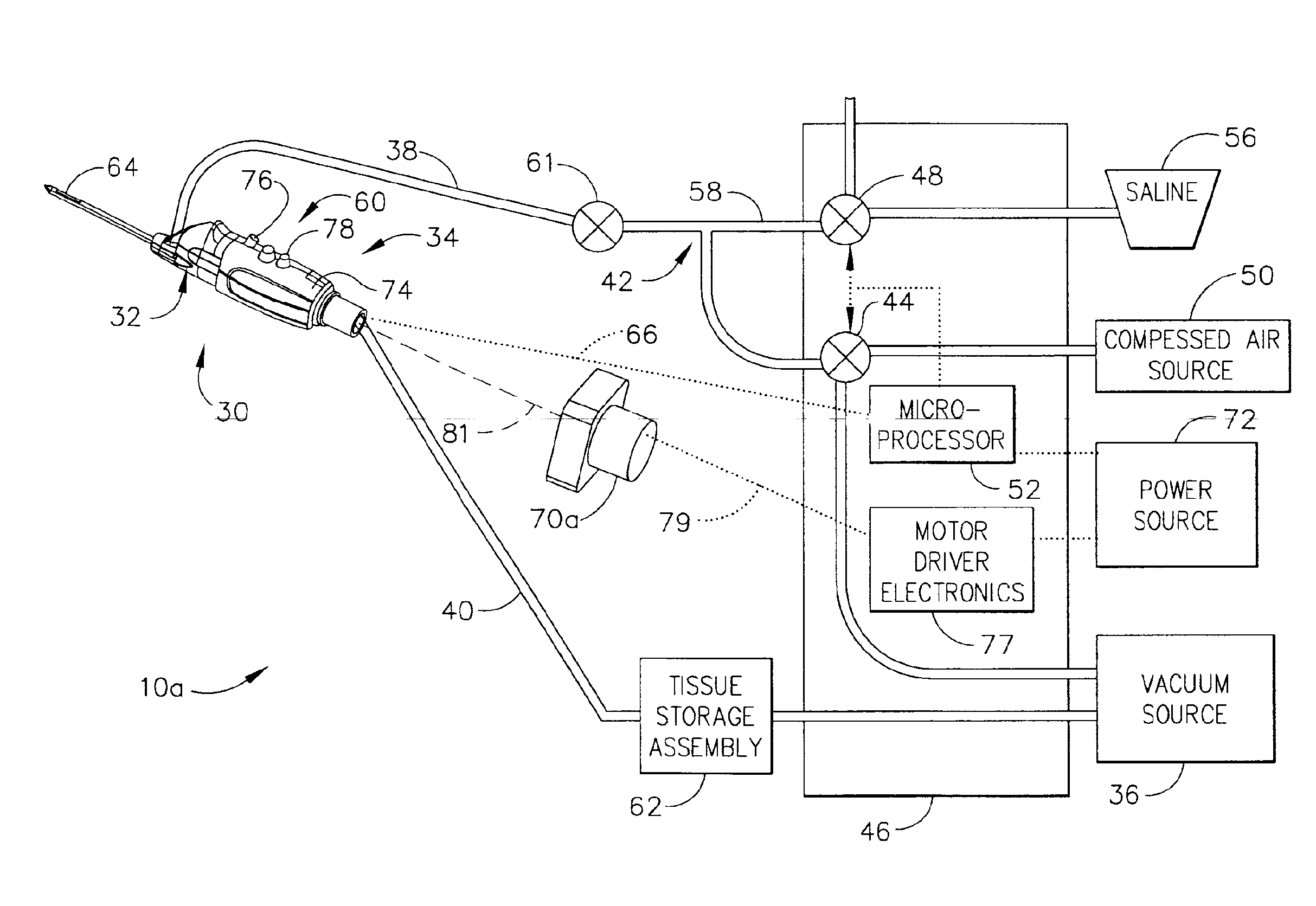
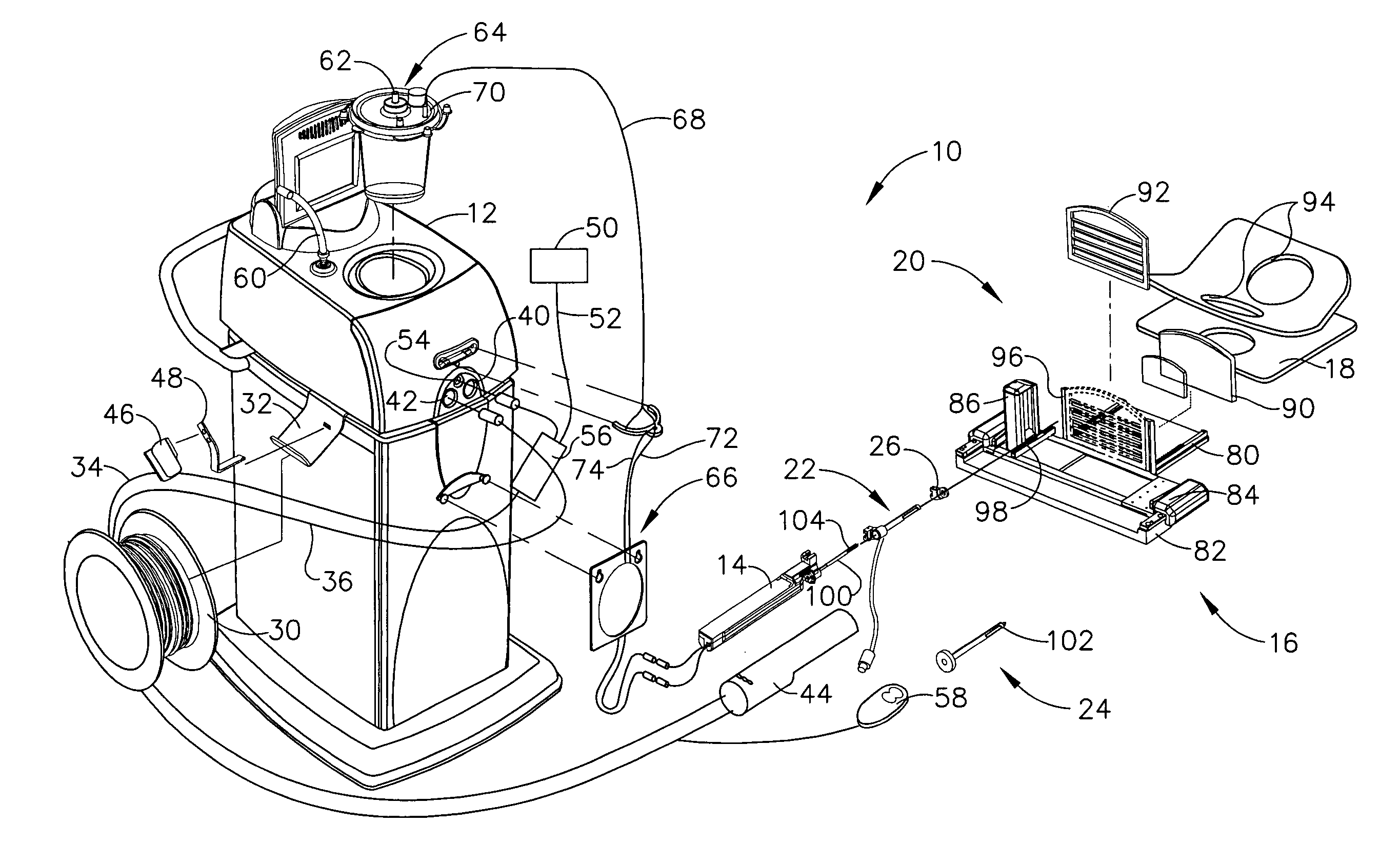
Liquid infusion apparatus
InactiveUS7553295B2Reduce materialEasy to controlDC motor speed/torque controlFlexible member pumpsPeristaltic pumpEngineering
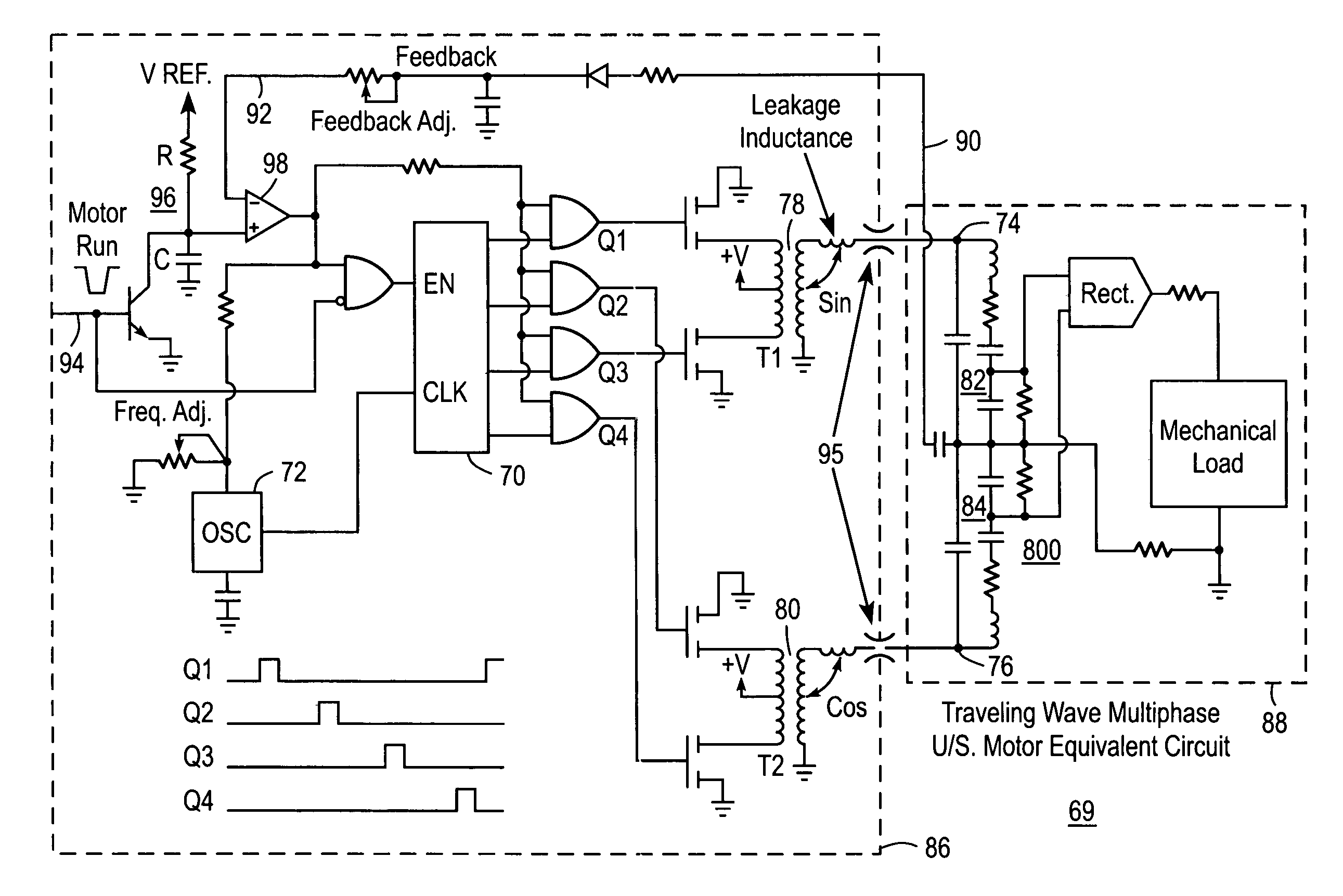
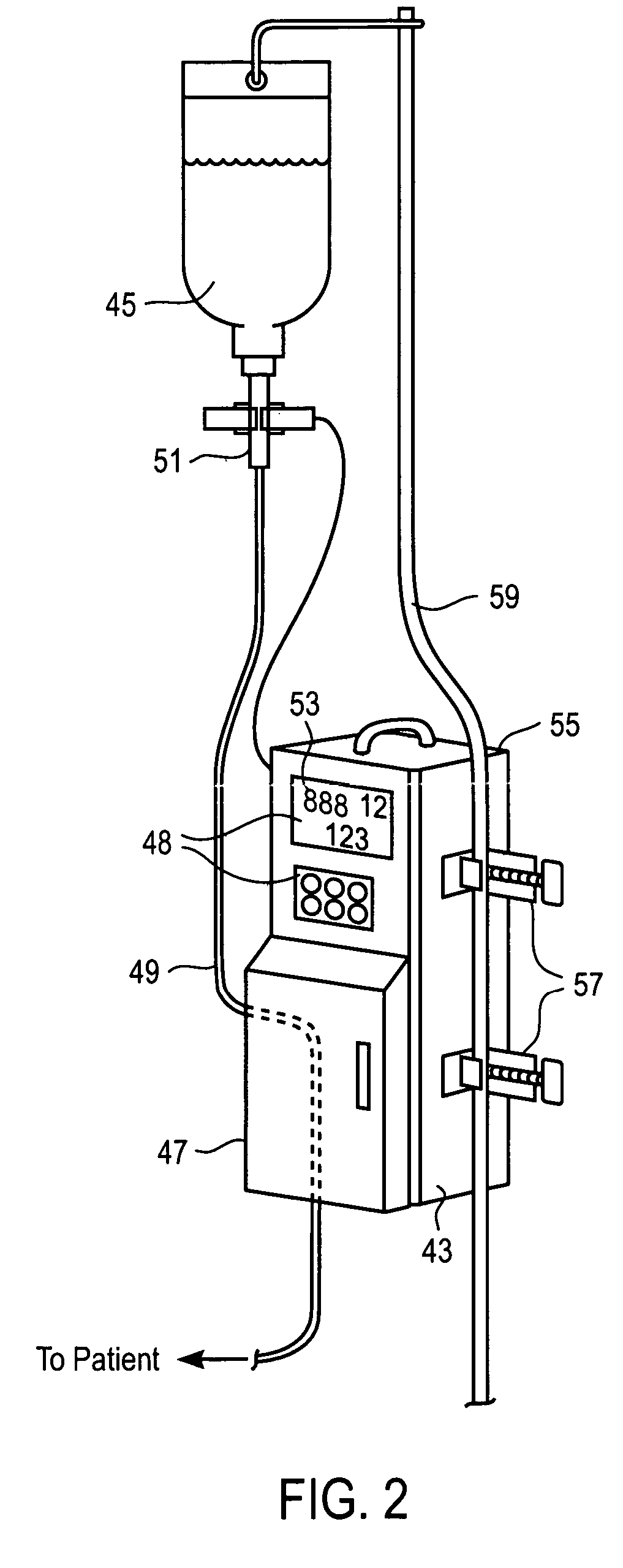
Liquid infusion apparatus includes non-magnetic materials in a pumping structure and ultrasonic drive motor therefor, and in a controller that supplies drive signals to the motor to facilitate convenient operation in intense magnetic fields without distorting the magnetic fields and without radiating objectionable radio-frequency interference. A non-MRI-compatible liquid infusion apparatus is temporarily replaced with MRI-compatible, non-magnetic liquid infusion apparatus without disconnecting a patient from an installed intravenous infusion set to continue infusing liquid within the MRI environment. The pumping apparatus operates on a segment of a liquid conduit that is mounted in tension between a linear peristaltic pump and platen, with associated safety interlocks to assure proper operation of infusing liquid into a patient compatibly with conditions in an MRI environment. Drive circuitry generates low-harmonic signals for operating the ultrasonic motor at variable speeds to compensate for flow rate discontinuities through the peristaltic pumping cycles.
Owner:IRADIMED CORP
MRI compatible implanted electronic medical device with power and data communication capability
InactiveUS8233985B2Interference minimizationElectrotherapyComputer moduleElectromagnetic interference
An antenna module, that is compatible with a magnetic resonance imaging scanner for the purpose of diagnostic quality imaging, is adapted to be implanted inside an animal. The antenna module comprises an electrically non-conducting, biocompatible, and electromagnetically transparent enclosure with inductive antenna wires looping around an inside surface. An electronic module is enclosed in an electromagnetic shield inside the enclosure to minimize the electromagnetic interference from the magnetic resonance imaging scanner.
Owner:KENERGY INC
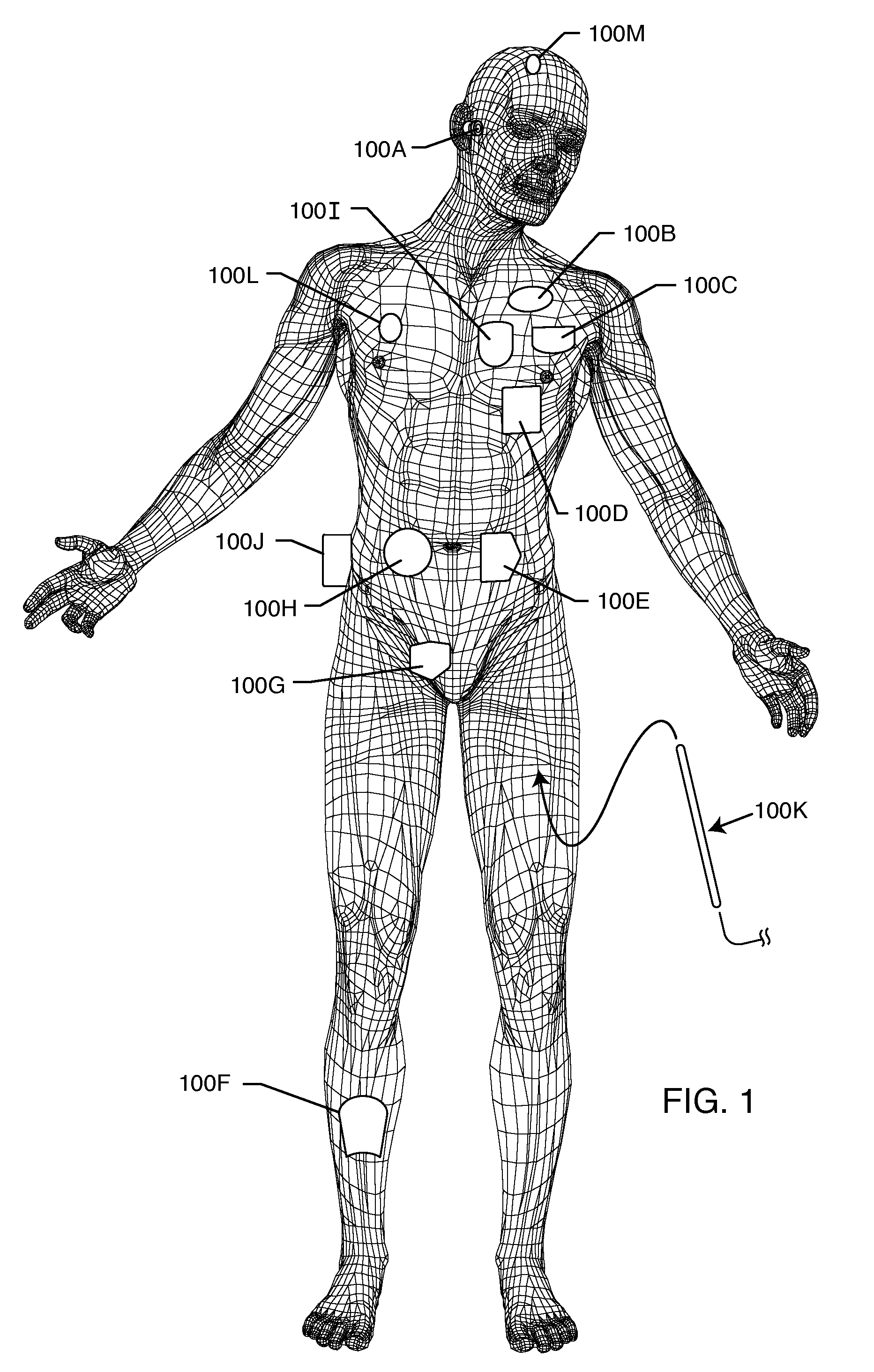
RFID detection and identification system for implantable medical lead systems
A system for identifying active implantable medical devices (AIMD) and lead systems implanted in a patient using a radio frequency identification (RFID) tag having retrievable information relating to the AIMD, lead system and / or patient. The RFID tag may store information about the AIMD manufacturer, model number, serial number; lead wire system placement information and manufacturer information; MRI compatibility due to the incorporation of bandstop filters; patient information, and physician and / or hospital information and other relevant information. The RFID tag may be affixed or disposed within the AIMD or lead wires of the lead system, or surgically implanted within a patient adjacent to the AIMD or lead wire system.
Owner:WILSON GREATBATCH LTD
MRI compatible electrical lead for an implanted electronic medical device
InactiveUS20100174348A1Reduce signalingReduce frequencyInternal electrodesExternal electrodesElectricityCapacitance
An electrical lead, for implantation in an animal, is compatible with an MRI scanner. The electrical lead has a first plurality of coiled insulated wires forming an outer layer of conductors that has a first inductance and a first capacitance, which act as a first parallel resonator tuned to a Larmor frequency of tissue in the animal. The lead may have a second plurality of coiled insulated wires forming an inner layer of conductors within the outer layer of conductors. The second plurality of coiled insulated wires has a second inductance and a second capacitance that act as a second parallel resonator tuned to the Larmor frequency. Those parallel resonators mitigate signals at the Larmor frequency from traveling along the respective coil. An electrically conductive layer extends around the inner and / or outer layer of conductors, and a layer of a biologically compatible material forms the electrical lead's exterior surface.
Owner:KENERGY INC
MRI compatible guide wire
InactiveUS6845259B2Easy to observeEasy to seeGuide wiresDiagnostic recording/measuringNon magneticGuide wires
An improved intracorporeal device such as a guide wire or other guiding member for use within a patient's body that is at least in part visible under magnetic resonance imaging (MRI) but is not detrimentally affected by the imaging is disclosed. The intercorporeal device has a non-conductive proximal core section, an essentially non-magnetic metallic distal core section that is preferably more flexible than the proximal core section, and that has an MRI visible member or coil in the distal section.
Owner:ABBOTT CARDIOVASCULAR
MRI compatible implanted electronic medical lead
An implantable biocompatible lead that is also compatible with a magnetic resonance imaging scanner for the purpose of diagnostic quality imaging is described. The implantable electrical lead comprises a plurality of coiled insulated conducting wires wound in a first direction forming a first structure of an outer layer of conductors of a first total length with a first number of turns per unit length and a plurality of coiled insulated conducting wires wound in a second direction forming a second structure of an inner layer of conductors of a second total length with a second number of turns per unit length. The first and the second structures are separated by a distance with a layer of dielectric material. The distance and dielectric material are chosen based on the field strength of the MRI scanner. The lead may further comprise a conducting layer formed by coating a material consisting of medium conducting particles in physical contact with each other and a mechanically flexible, biocompatible layer forming an external layer of lead and in contact with body tissue or body fluids.
Owner:KENERGY INC
Stent with variable properties
InactiveUS7226475B2Increase flexibilityExcellent fluoroscopic property propertyStentsMedical devicesVariable thicknessMedicine
A stent having variable thickness with improved radiopacity, MRI compatibility, radial stiffness, and flexibility and to the method of making such stents.
Owner:BOSTON SCI SCIMED INC
MRI surgical systems for real-time visualizations using MRI image data and predefined data of surgical tools
ActiveUS8315689B2Increase speedImprove reliabilityMagnetic measurementsSurgical needlesMri guidedDisplay device
MRI-Surgical systems include: (a) at least one MRI-compatible surgical tool; (b) a circuit adapted to communicate with an MRI scanner; and (c) at least one display in communication with the circuit. The circuit electronically recognizes predefined physical characteristics of the at least one tool to automatically segment MR image data provided by the MRI scanner whereby the at least one tool constitutes a point of interface with the system. The circuit is configured to provide a User Interface that defines workflow progression for an MRI-guided surgical procedure and allows a user to select steps in the workflow, and wherein the circuit is configured to generate multi-dimensional visualizations using the predefined data of the at least one tool and data from MRI images of the patient in substantially real time during the surgical procedure.
Owner:CLEARPOINT NEURO INC
MRI Compatible Implantable Medical Devices and Methods
An implantable medical device configured to be compatible with the environment inside an MRI machine. The implantable medical device includes a housing constructed of an electrically conductive material and pulse generation circuitry within the housing for generating electrical voltage pulses. The implantable medical device further includes a first conductor that is configured to transmit the electrical voltage pulses from the pulse generation circuitry to a patient's cardiac tissue and a second conductor that is configured to provide an electrically conductive path from the patient's cardiac tissue back to the pulse generation circuitry. The implantable medical device further includes a selectively interruptible electrically conductive path connecting the pulse generation circuitry with the housing.
Owner:CARDIAC PACEMAKERS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com