Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Clonidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used alone or with other medications to treat high blood pressure (hypertension).
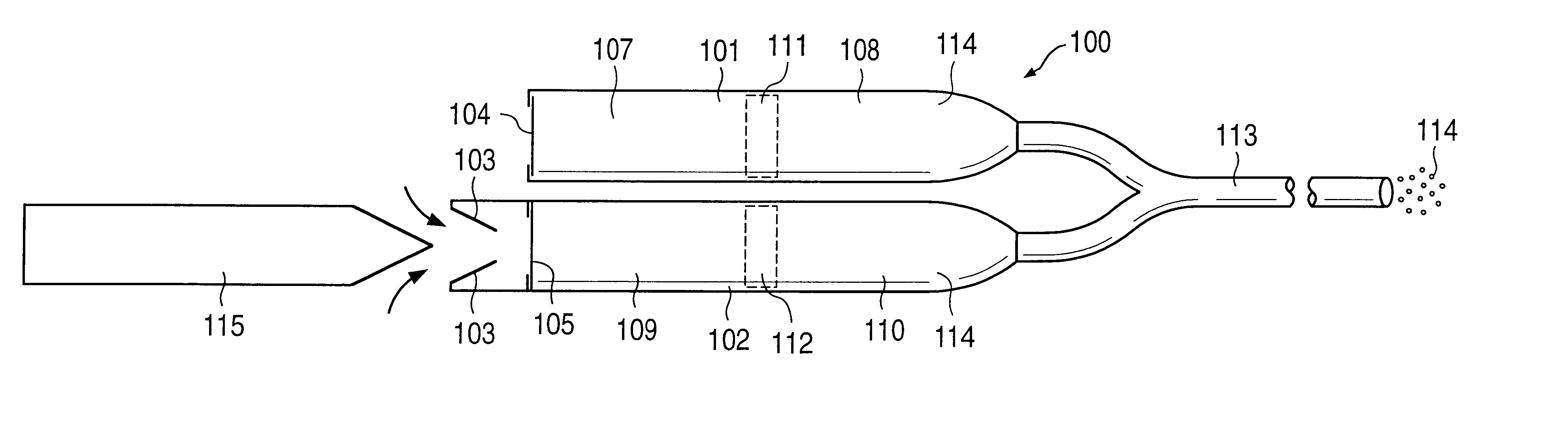
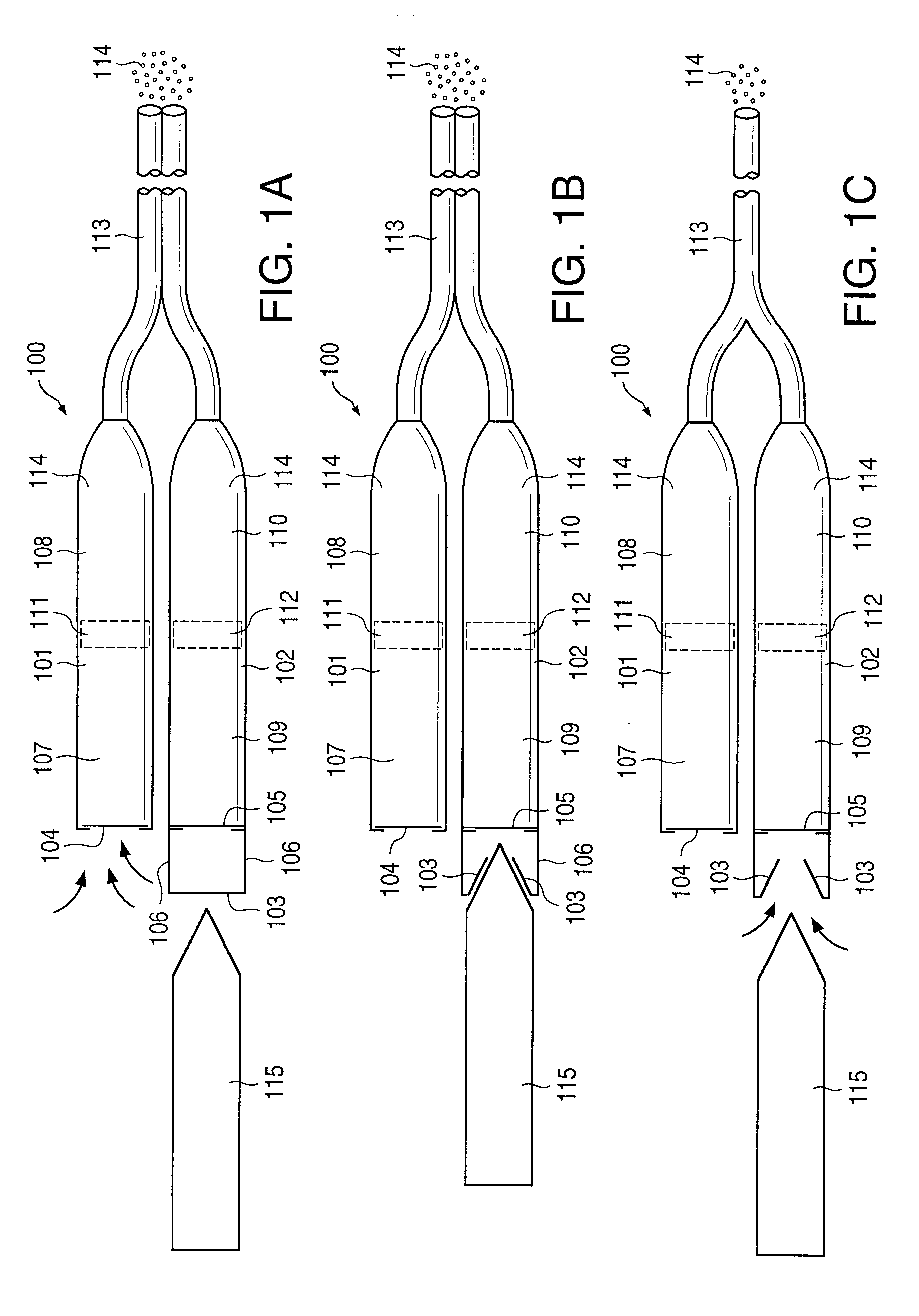
Osmotic pump drug delivery systems and methods
InactiveUS6471688B1Avoid mixingMedical devicesPharmaceutical delivery mechanismTreatment effectAnalgesics effects
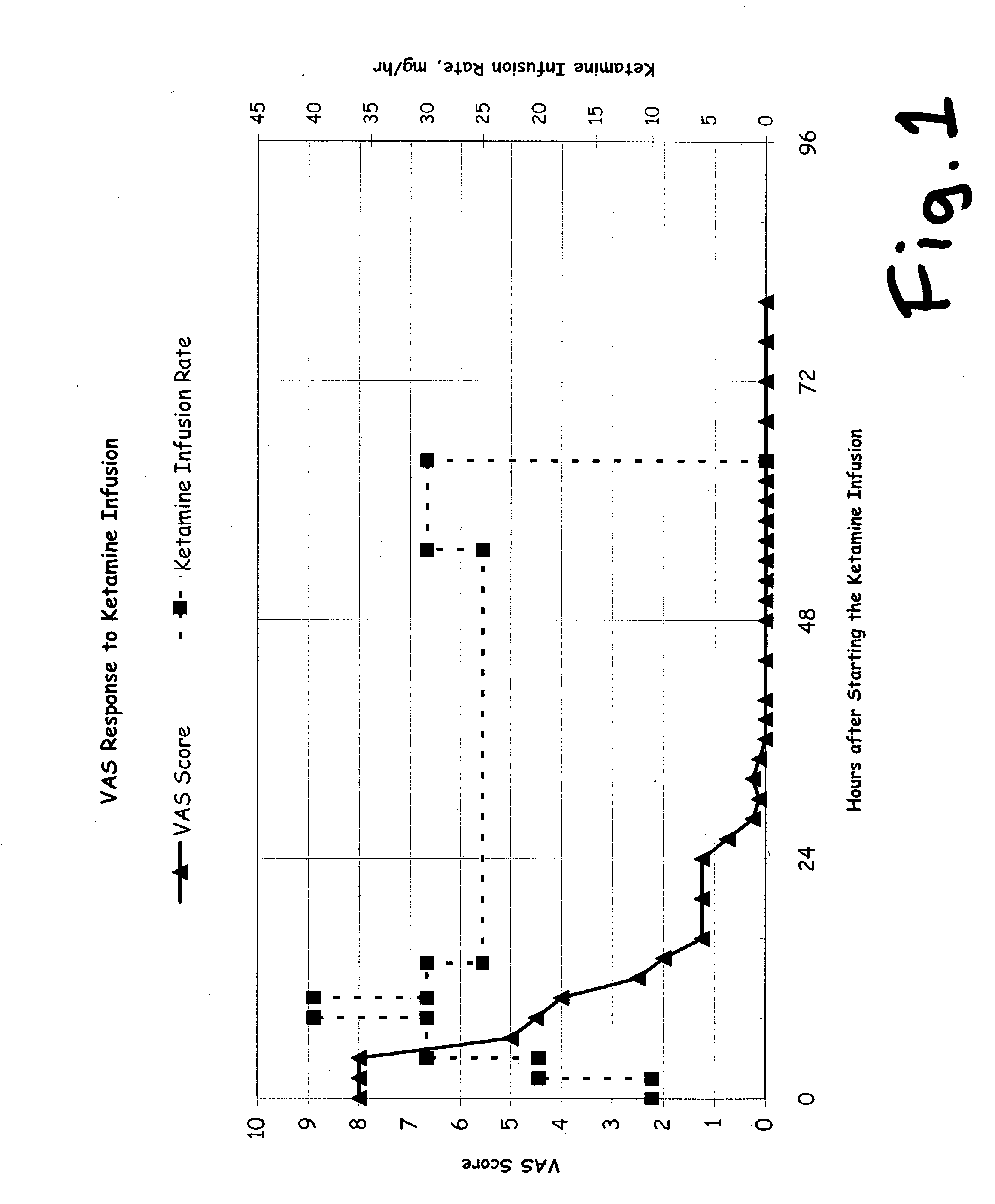
Implantable osmotic pump devices and systems include multiple osmotic pumps and / or semipermeable membranes to extend the useful life cycle and functionality of the drug delivery system. Use of an implantable system including multiple implantable osmotic pumps allows different drugs to be administered from the same implanted system. One or more of the semipermeable membranes of the system may be initially sealed by an overlying impermeable membrane upon implantation of the system into the patient. When the patient develops a tolerance to a first drug or to a first dose of the first drug, the impermeable membrane may be breached, to expose the underlying semipermeable membrane to the osmotic pressure of the patient at the implant site. This causes the infusion rate to increase, thereby providing the patient with the needed relief and / or other desired therapeutic effect. In the case of a multiple pump system, breaching an impermeable membrane may cause the infusion of a second drug. The second drug may potentiate a therapeutic effect (such as an analgesic effect) of the first drug, as is the case with Sufentanil and Clonidine.
Owner:MICROSOLUTIONS
Smoking cessation treatments using naltrexone and related compounds
InactiveUS6541478B1Reduce weight gainPrevent relapseBiocideNervous disorderOpioid antagonistAntianxiety Agent
Nicotine dependency is treated by administration of an opioid antagonist. In some embodiments, rapid or ultra rapid detoxification techniques include using a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone or naltrexone or a mixture of any one of these, and either clonidine or related compounds either while awake, or while under sedation or anesthesia, followed by continued administration of an effective amount of an opioid antagonist with or without agents that enhance nicotine dependency treatment. Persons are also treated for nicotine dependency with more gradual detoxification methods using administration of a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone, naltrexone, or a mixture of any of these, and an effective amount of agents used to treat nicotine withdrawal including nicotine, such as that delivered by a nicotine patch, nicotine chewing gum, nicotine inhaler or other methods for delivering nicotine, antidepressants and antianxiety agents, and / or clonidine and related compounds. Administration of an effective amount of an opioid antagonist to prevent relapse, attenuate craving, and reduce weight gain during and after treatment for nicotine dependency is continued in some embodiments.
Owner:YALE UNIV
Methods and compositions for treating post-operative pain comprising clonidine
ActiveUS20090264491A1Ease the pain of treatmentReduce deliveryOrganic active ingredientsBiocidePolymerPostoperative pain
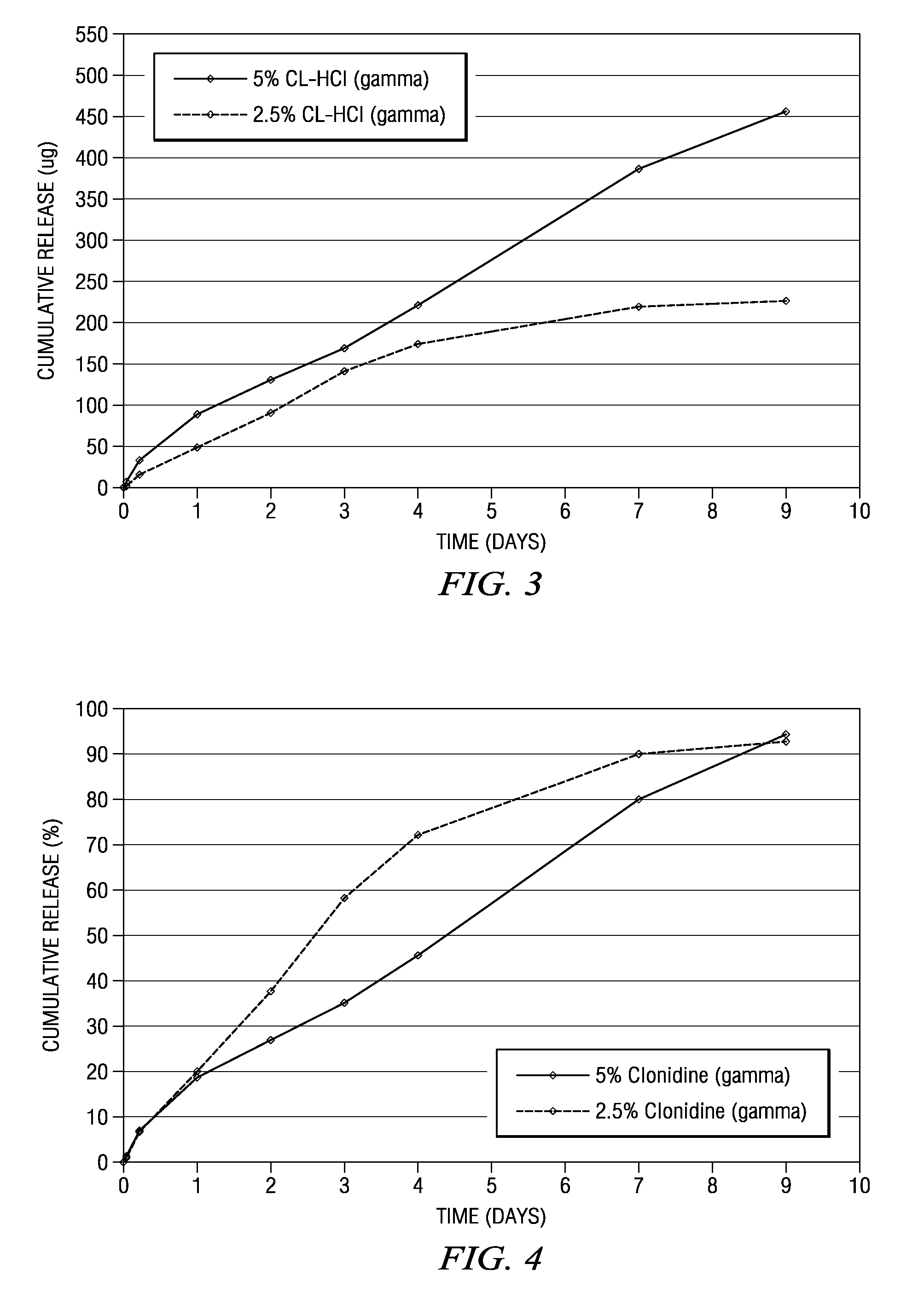
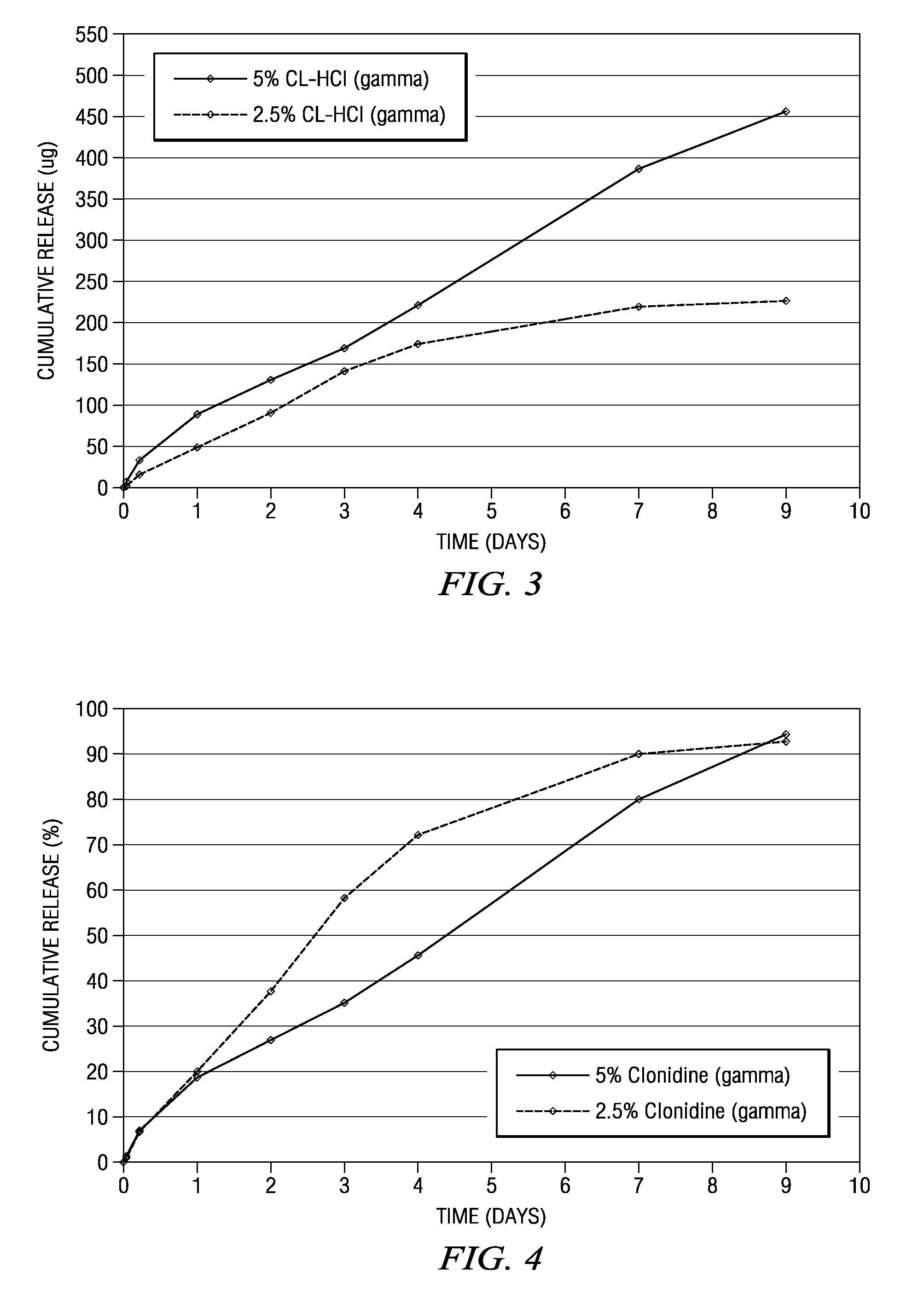
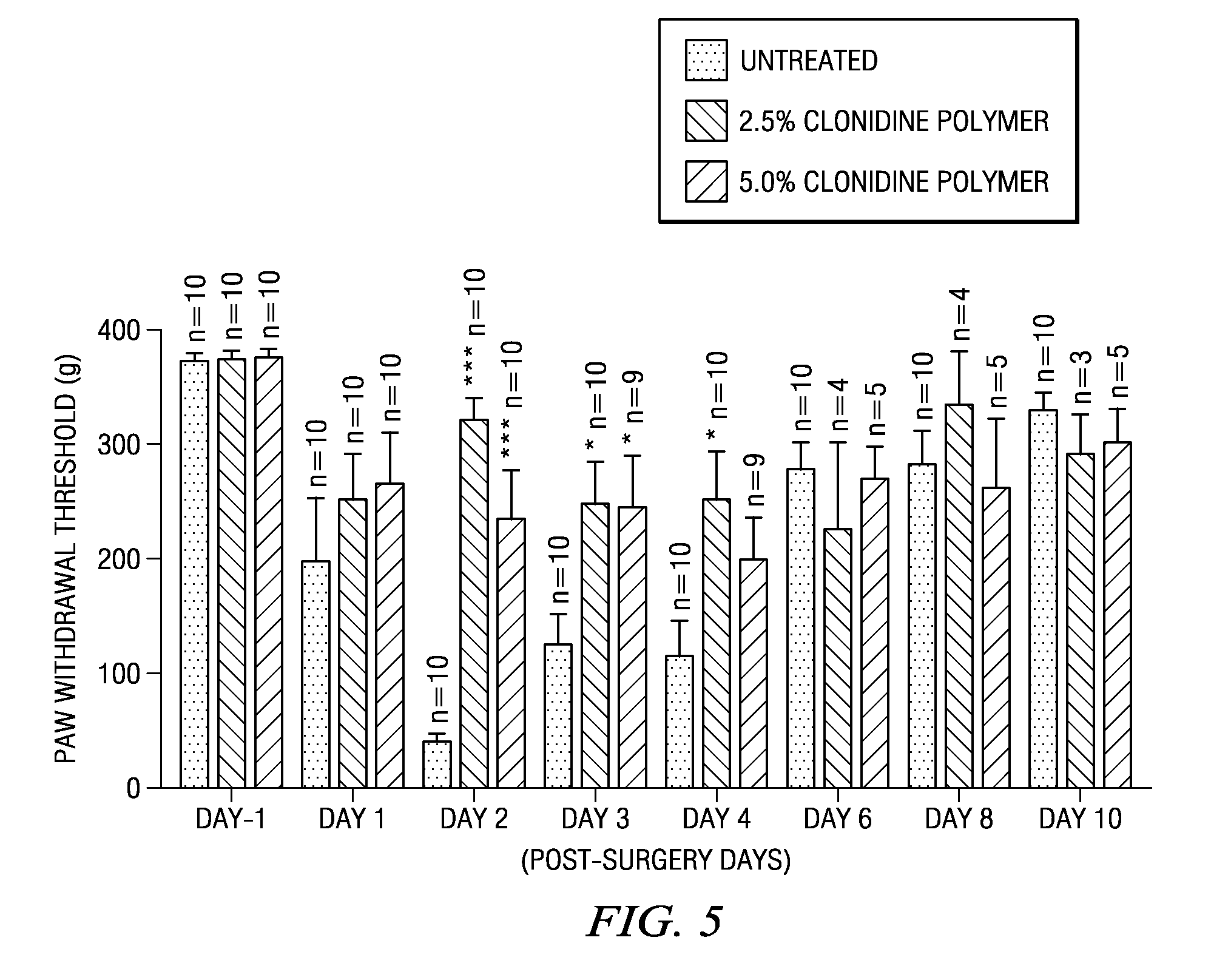
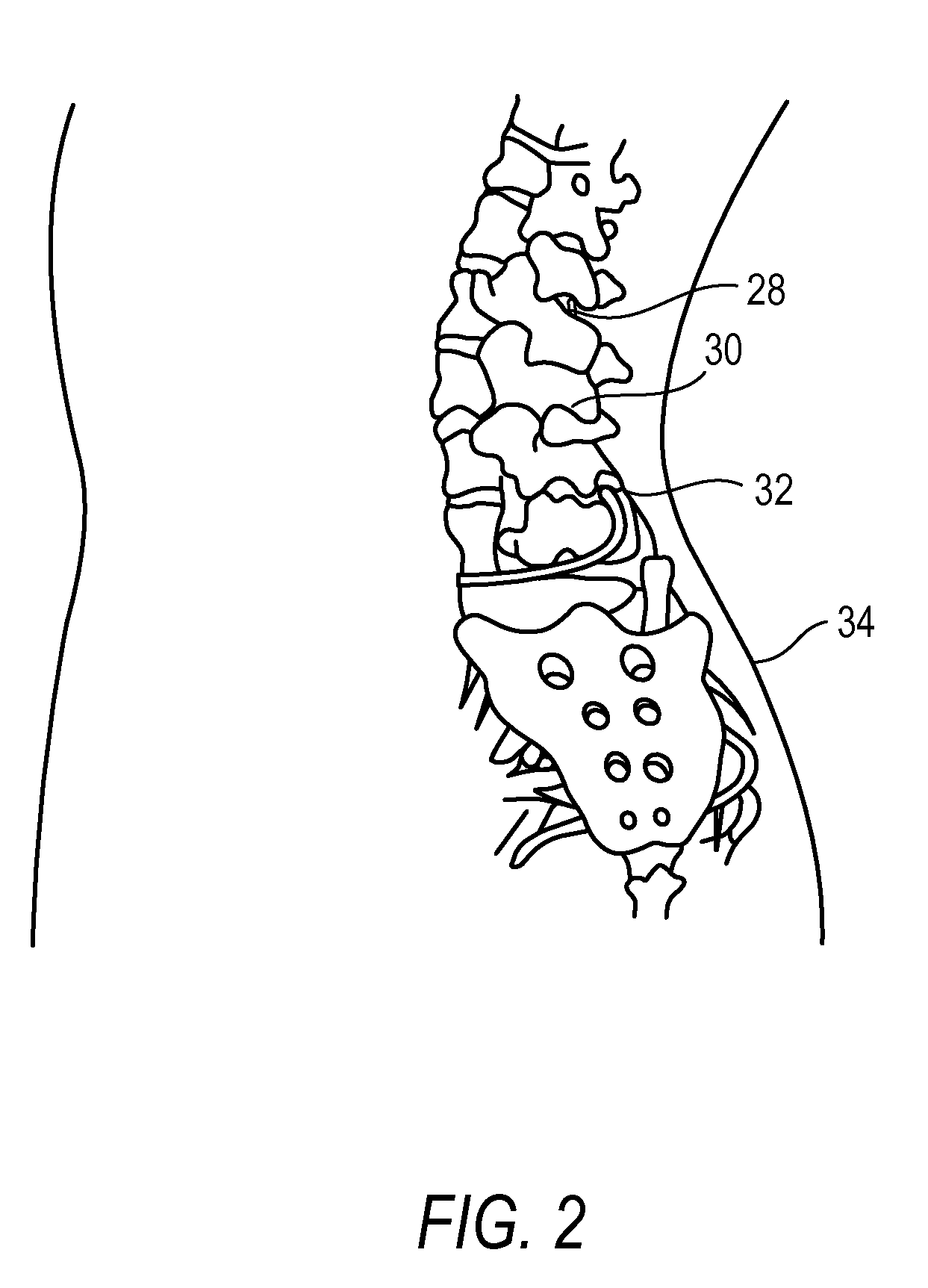
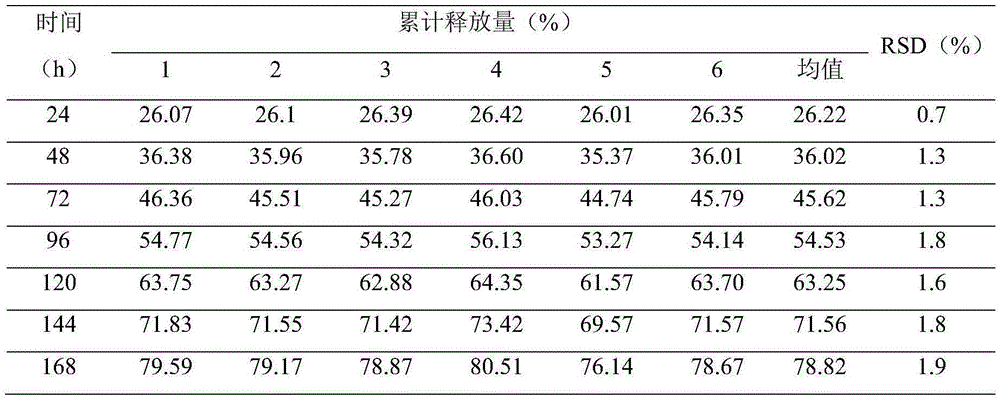
The present invention is directed to an implantable drug depot useful for reducing, preventing or treating post-operative pain in a patient in need of such treatment, the implantable drug depot comprising a therapeutically effective amount of clonidine or pharmaceutically acceptable salt thereof and a polymer; wherein the depot is implantable at a site beneath the skin to reduce, prevent or treat post-operative pain, and the depot is capable of releasing (i) about 5% to about 45% of the clonidine or pharmaceutically acceptable salt thereof relative to a total amount of the clonidine or pharmaceutically acceptable salt thereof loaded in the drug depot over a first period of up to 48 hours and (ii) about 55% to about 95% of the clonidine or pharmaceutically acceptable salt thereof relative to a total amount of the clonidine or pharmaceutically acceptable salt thereof loaded in the drug depot over a subsequent period of at least 3 days.
Owner:COMPANION SPINE LLC +1
Neuropathy cream
Owner:OZTURK BINNUR +1
Prolonged administration of NMDA antagonist drug and safener drug to create improved stable neural homeostasis
InactiveUS20050222270A1Stable and lasting improved neural homeostasisBiocideOrganic active ingredientsDiseaseNervous system
An NMDA antagonist (such as ketamine) is administered with a safener (such as clonidine) in patients suffering from neurologic disorders other than pain. The ketamine is adminsitered at a dosage that causes slurred speech, for a span of several days. This treatment enables a patient's nervous system to return to a healthy “set point”, also called an improved stable neural homeostasis, in a manner similar to a broken bone healing itself if protected from jostling and reinjury by a cast. In at least some patients, this treatment can ease problems such as addictions to illegal or pain-killing drugs, nicotine, or alcohol, compulsive or criminal behavioral problems, severe depression, obsessive-compulsive disorders, phobias, etc. It may also provide some relief in some patients for problems such as chronic fatigue, chemical sensitivities, allergies, autoimmune disorders, and diabetes.
Owner:OLNEY JOHN W +3
Osmotic pump drug delivery systems and methods
InactiveUS20030032947A1Avoid mixingMedical devicesPharmaceutical delivery mechanismTherapeutic effectAnalgesics effects
Owner:MICROSOLUTIONS
Prolonged administration of NMDA antagonist and safener drug to alter neuropathic pain condition
InactiveUS20050148673A1Reduce neurotoxic side effectInherent activityBiocideOrganic active ingredientsNR1 NMDA receptorSide effect
A drug that inhibits NMDA receptors (such as ketamine, a surgical anesthetic) is continuously administered to patients suffering from neuropathic pain. Unless the NMDA antagonist drug has inherent safening activity, this treatment requires a “safener” drug to prevent the neurotoxic side effects of NMDA antagonists. One class of safener drugs that increase the efficacy of the treatment include alpha-2 adrenergic agonists, such as clonidine. The treatment lasts for several days and nights, continuously. A maximum tolerated dosage is titered for each patient, such as by observing slurring of speech, and the patient does not lose consciousness except during normal sleep. Magnesium and / or drugs that inhibit ketamine-degrading enzymes can also be used. Patients who suffered for years from chronic intractable pain emerged from this treatment with apparently permanent relief, or with lasting reductions in their levels of pain.
Owner:HARBUT RONALD E +2
Method for reducing pain
ActiveUS20050192218A1Relieve painRetain potencyBiocideNervous disorderIntrathecal usePharmaceutical formulation
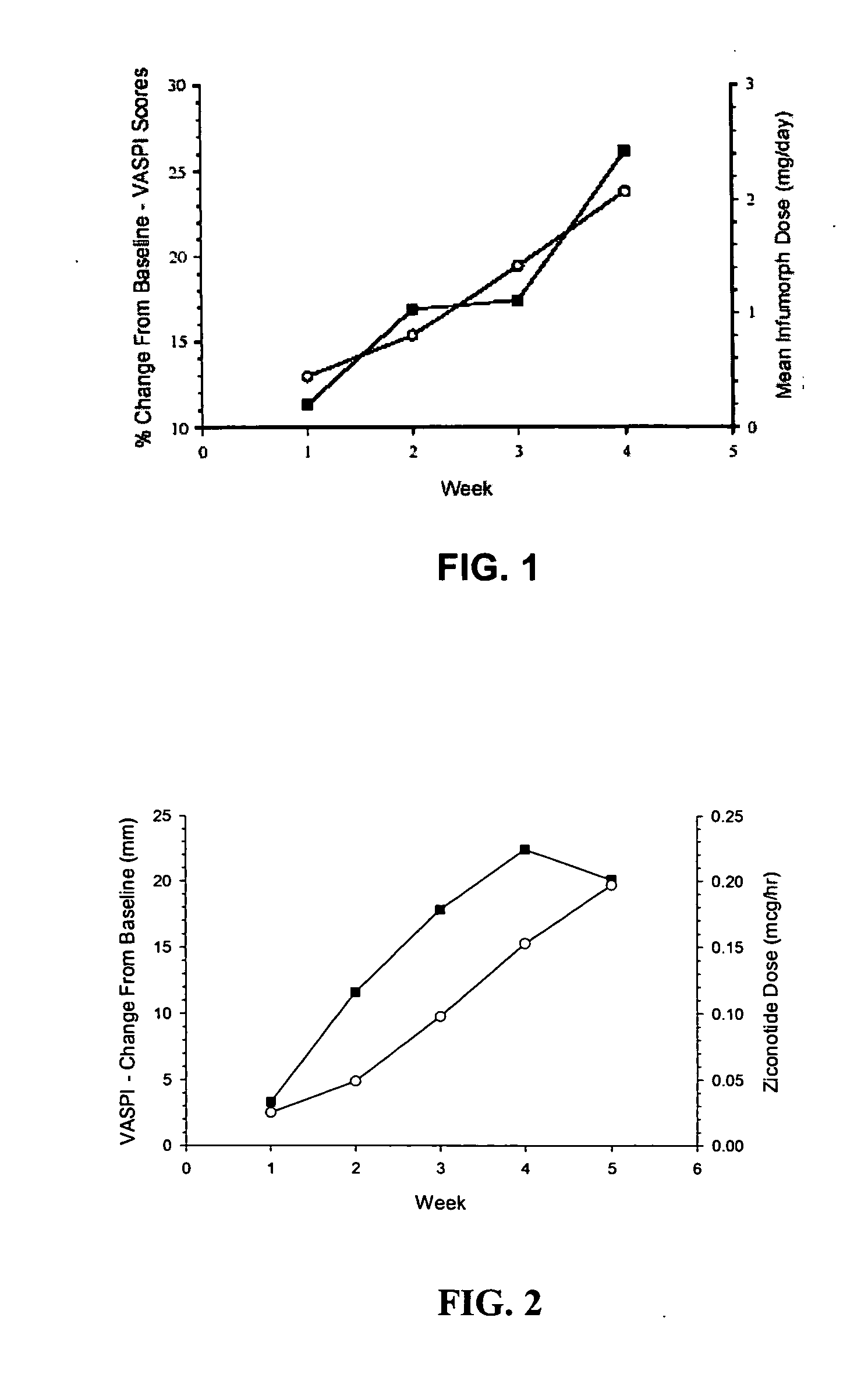
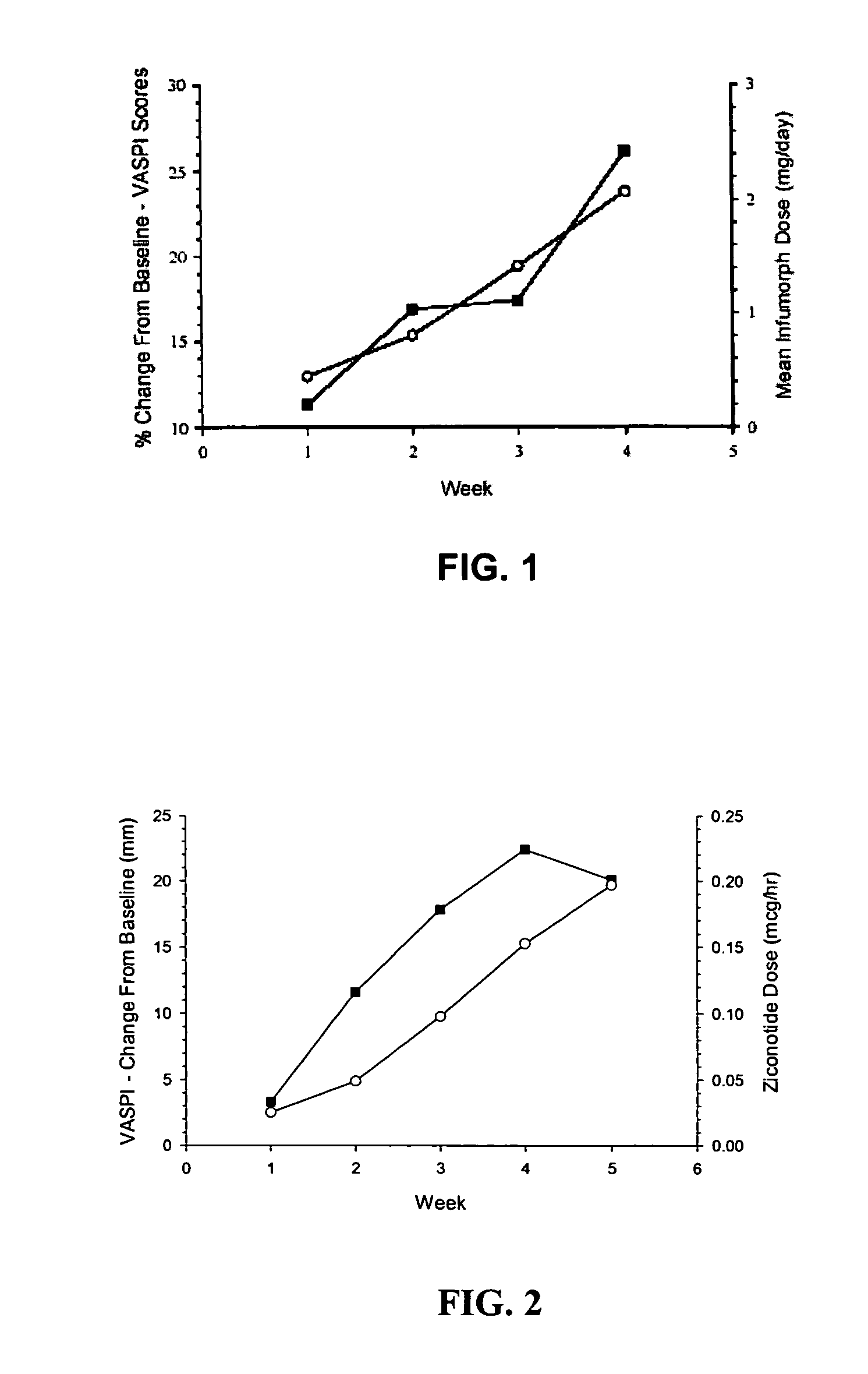
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Silicone-containing acrylic polymers for transdermal drug delivery compositions
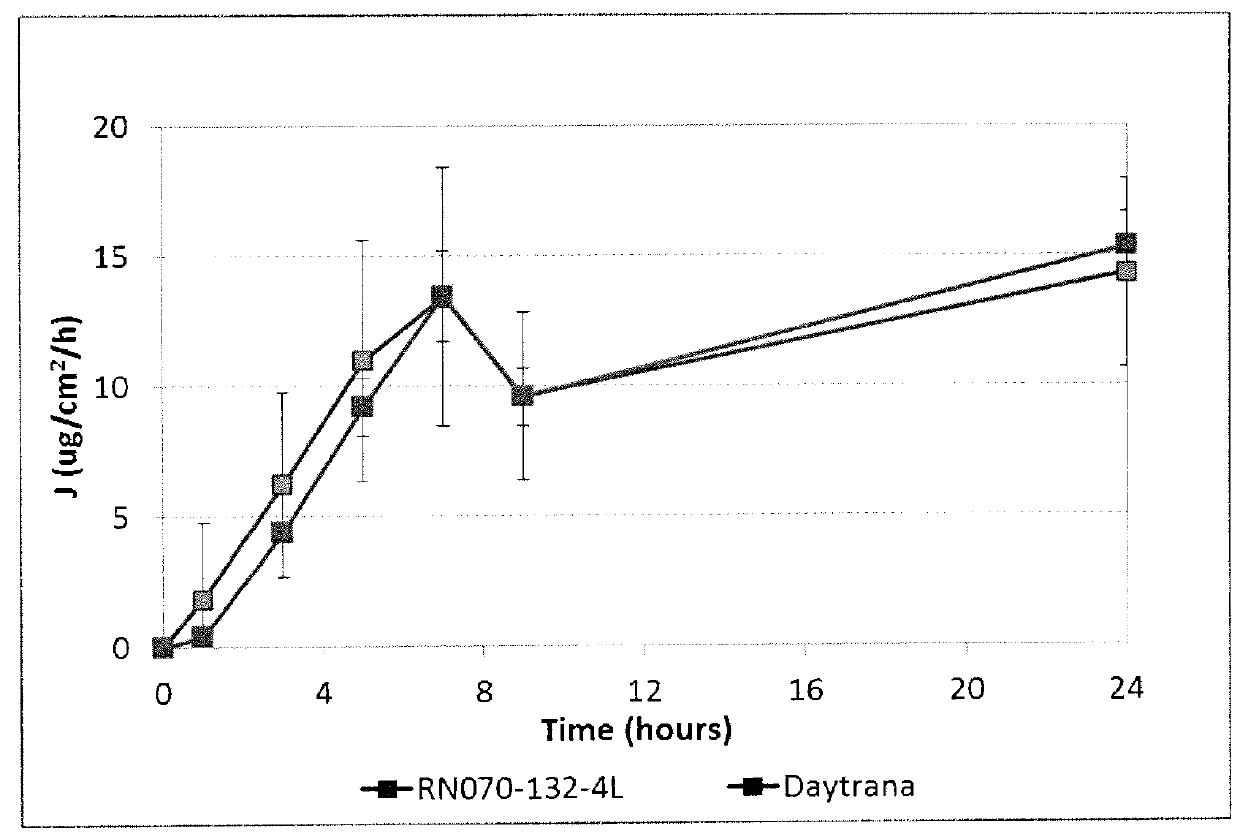
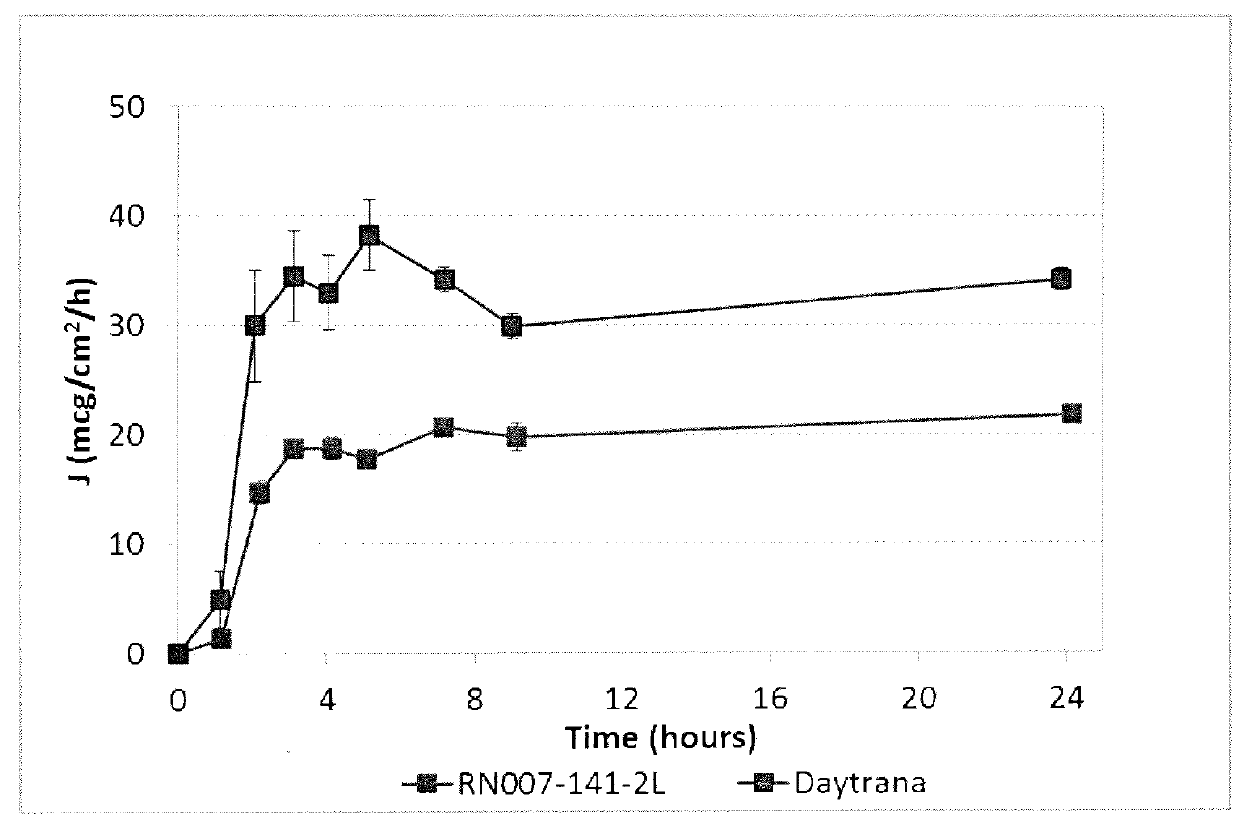
Described herein are silicone-containing acrylic polymers useful, for example, in transdermal drug delivery compositions, to methods of making and using them, to transdermal drug delivery compositions comprising them, and to methods of making and using such transdermal drug delivery compositions. The polymers are particular suitable for formulating amine drugs, such as amphetamine, methylphenidate, rivastigmine, paroxetine and clonidine.
Owner:NOVEN PHARMA
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
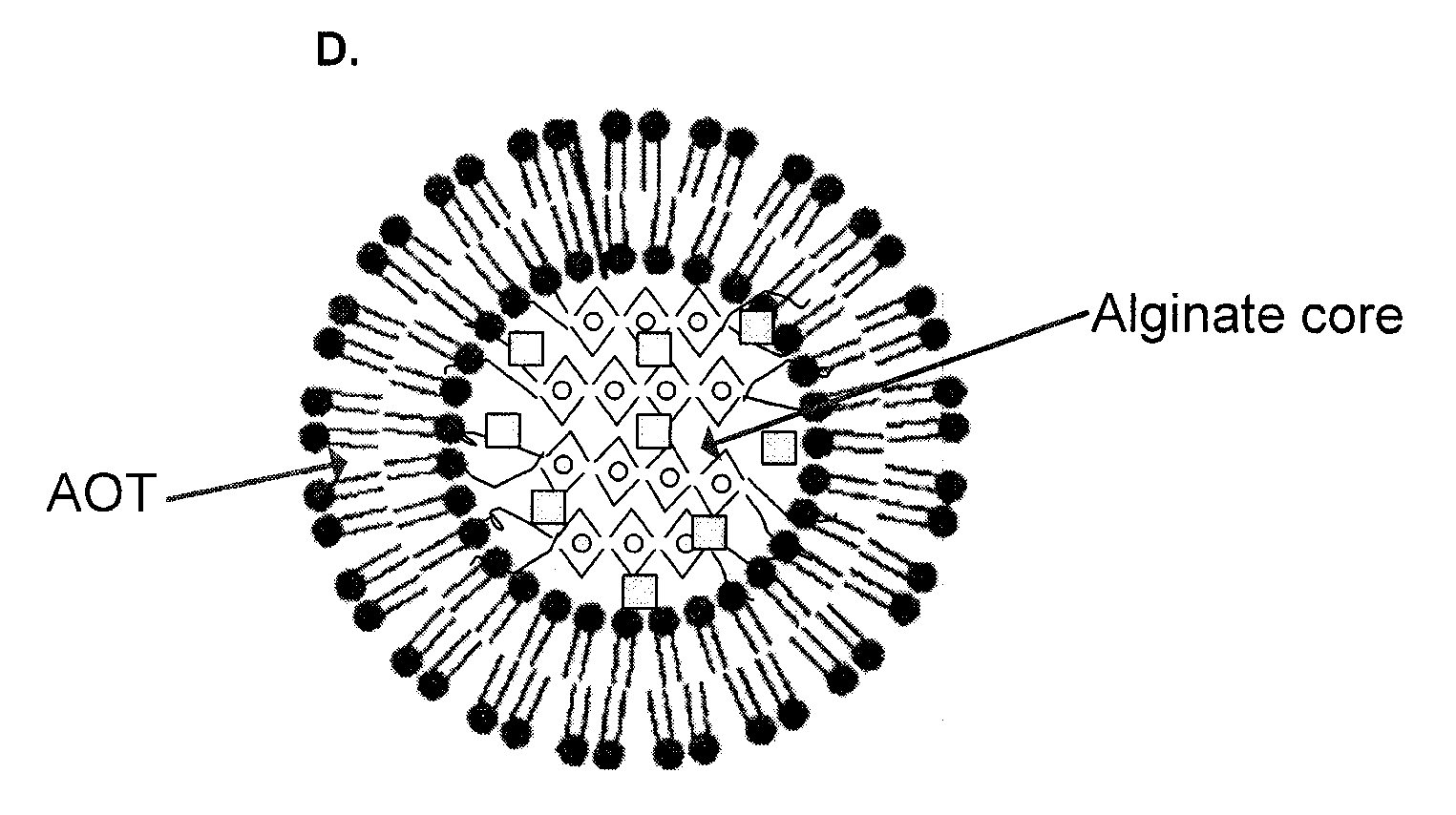
Polymer-surfactant nanoparticles for sustained release of compounds
A polymer-surfactant nanoparticle formulation, using the anionic surfactant aerosol OT (AOT) and polysaccharide polymer alginate, is used for sustained release of water-soluble drugs. The AOT-alginate nanoparticles are suitable for encapsulating doxorubicin, verapamil and clonidine, as well as therapeutic agents effective against dermal conditions such as psoriasis. The nanoparticles are also suitable for encapsulating photo-activated compounds such as methylene blue for use in photo-dynamic therapy of cancer and other diseases, and for treating tumor cells that exhibit resistance to at least one chemotherapeutic drug.
Owner:WAYNE STATE UNIV
Cotton breeding matrix
InactiveCN106588411APromote growthImprove disease resistanceExcrement fertilisersBioloigcal waste fertilisersPotamogeton crispusAdditive ingredient
The invention mainly relates to the field of cultivation and discloses a cotton breeding matrix. The cotton breeding matrix is prepared from cotton straws, corn stalks, wheat straws, biogas residues, sweet potato residues, Potamogeton crispus, cow dung, edible fungus wastes, EM bacteria, traditional Chinese medicines, clonidine, rose flower extract, river sand, vermiculite and medical stone. The cotton breeding matrix is prepared from abundant raw materials, has balanced nutrition, can provide the necessary nutrients for cotton growth, has antibacterial and insecticidal effects, enhances the disease resistance of cotton, and realizes an emergence rate of cotton of 89%, a survival rate of 94%, a disease and pest rate of 4% and economic efficiency of 9.6%. Cotton straw as a raw material is fully used and application approaches of clonidine and rose flower extract are increased. Through strain culture fermentation and use of medical stone rich in small molecule beneficial ingredients, the growth of cotton is promoted, a planting period is shortened by 15 to 20 days and planting efficiency is accelerated. Through cooperation of medical stone and a variety of Chinese herbal medicines, the cotton breeding matrix inhibits occurrence of pests and diseases, reduces an incidence rate of pests and diseases and saves a planting cost.
Owner:国营全椒县棉花原种总场
Method for treating restless leg syndrome using pramipexole and clonidine
InactiveUS20020010201A1High response rateHigh activityOrganic active ingredientsBiocidePramipexolePharmacology
The invention relates to an active substance combination consisting of clonidine and pramipexole for treating Restless Leg Syndrome.
Owner:BRECHT HANS MICHAEL
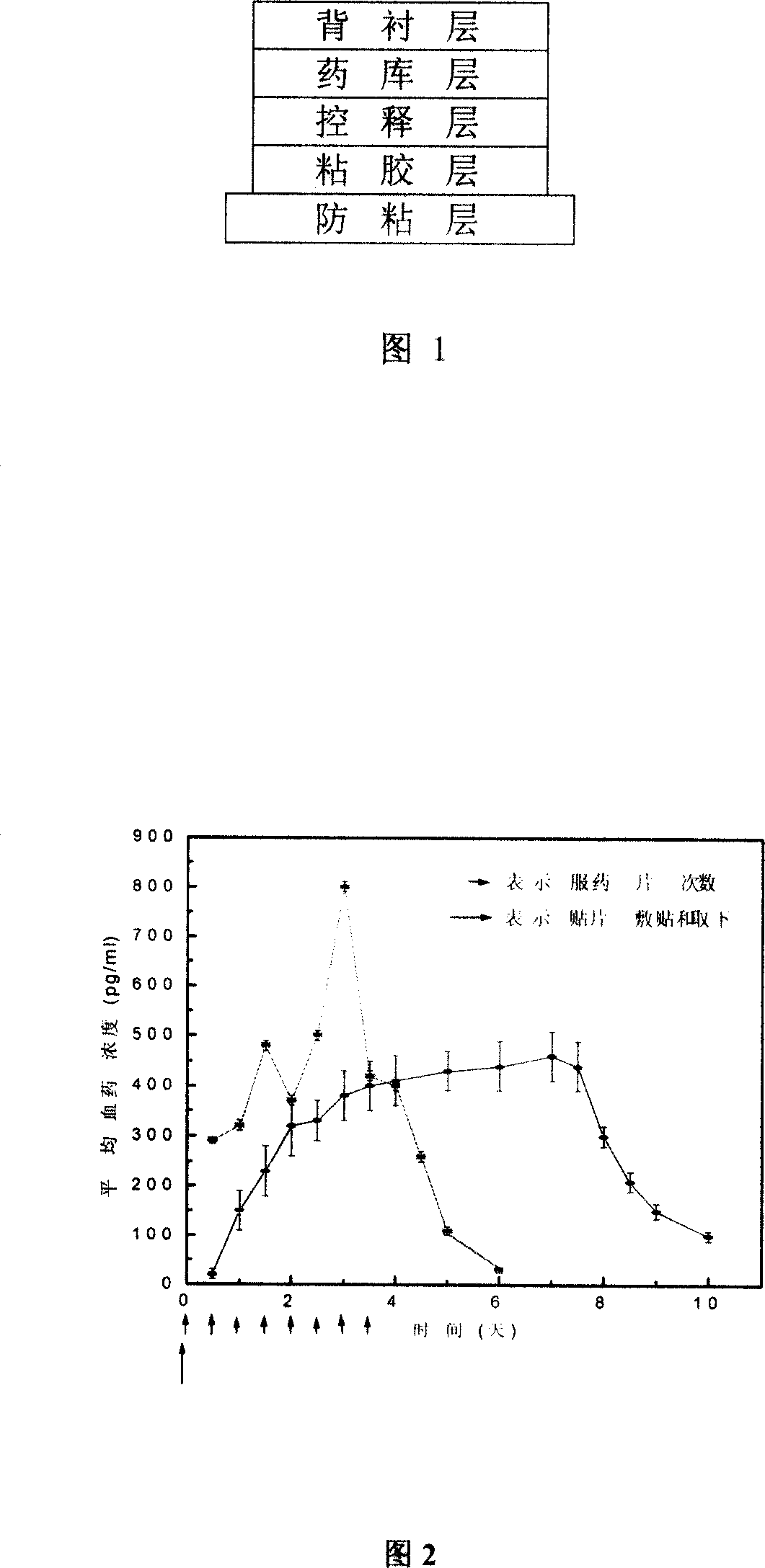
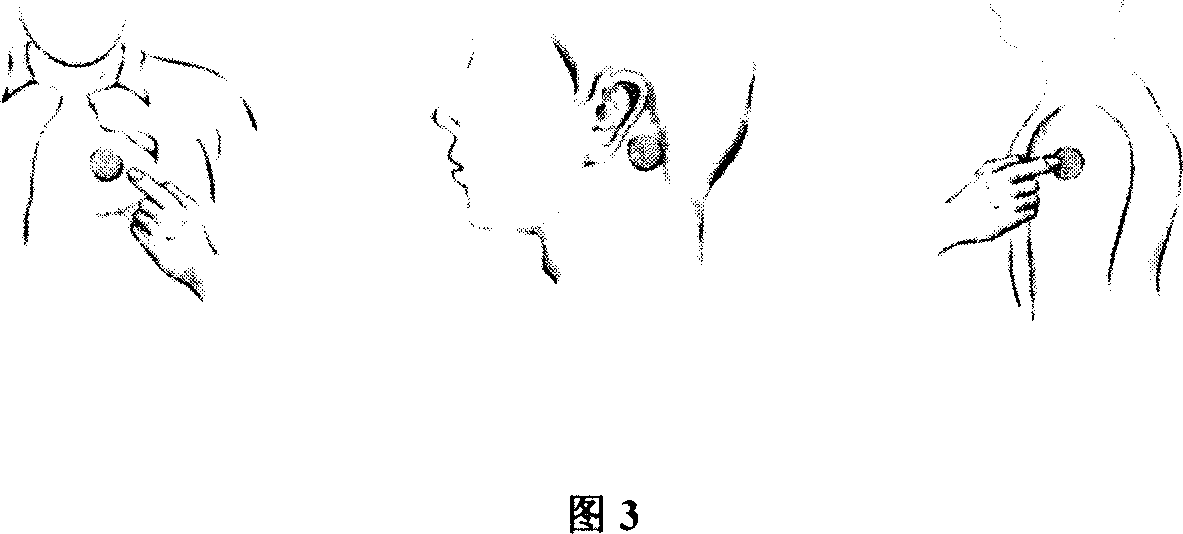
Clonidine paster for treating children's hyperkinetic symptom, twitch symptom and its preparation method
InactiveCN101011391AImprove bioavailabilityAvoid peaks and valleys in blood concentrationOrganic active ingredientsNervous disorderDrug contentControl layer
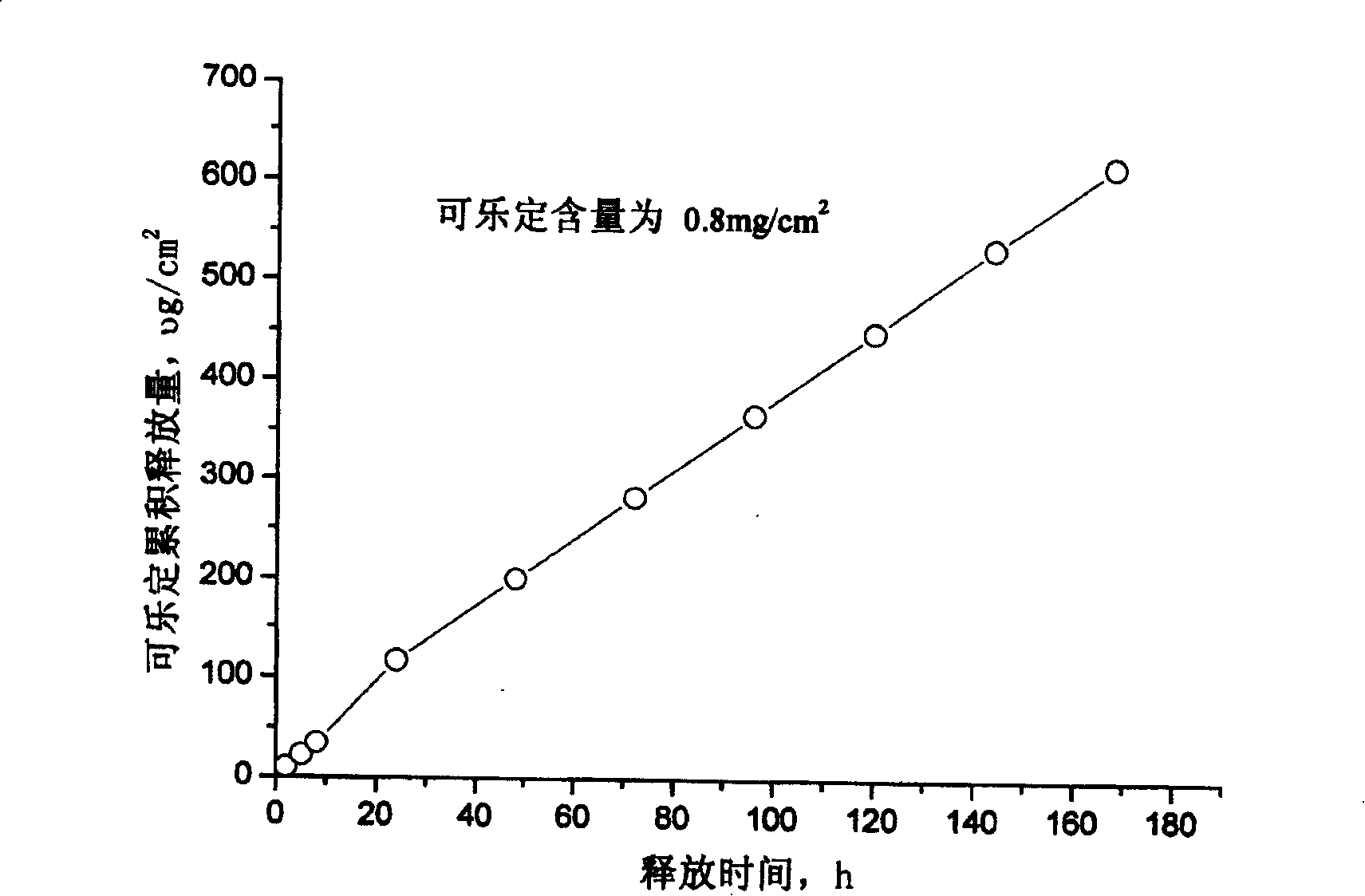
The invention relates to a cola stable paster which can treat hyperkinetic symptom of children, which comprises back liner, drug layer, release-control layer, paste layer, and anti-paste layer. The invention is characterized in that the back liner is aluminized film; the drug layer is cola stable drug and polyacrylacid ester pressure-sensitive gel; the release-control layer is modified EVA film or nuclear track porous film; the paste layer is cola stable drug and polyacrylacid ester pressure-sensitive gel; the cola stable drug content of drug layer is higher than the cola stable drug content of paste layer. The invention can avoid oral taking, with high biological utilization, stable effect, continuous release, and high safety. The invention also provides relative production.
Owner:北京克莱斯瑞控释药业有限公司
Clonidine, weekly-effect, skin-permeable and control-released plaster
InactiveCN1911222AIncrease stickinessApplicable internal forceOrganic active ingredientsNervous disorderControlled releaseMedicine
A release controlled percutaneous adhesive of clonidine with long curative period (7 days) is prepared from clonidine, the pressure-sensitive adhesive prepared from solvent(cyclohexane)-type acrylate-vinyl acetate copolymer, and release controlling membrane prepared from modified ethene-vinyl acetate copolymer.
Owner:LKF GUANGDONG MEDICAL
Methods and compositions for treating post-operative pain comprising clonidine
ActiveUS8629172B2Easily allow accurate and precise implantation of a drug depotMinimal physical and psychological trauma to a patientOrganic active ingredientsBiocidePharmaceutical drugEngineering
Owner:COMPANION SPINE LLC +1
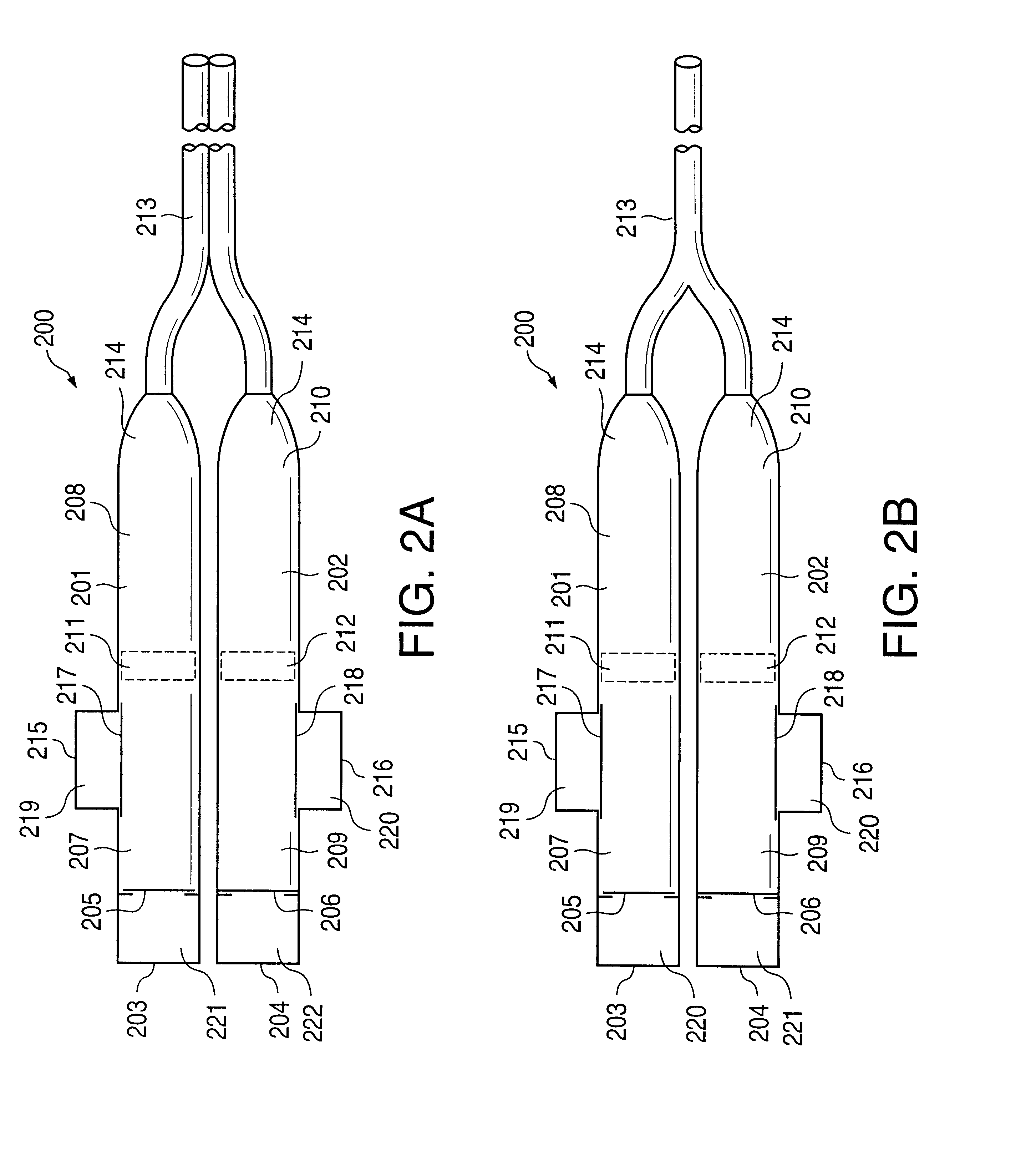
Methods of administering drugs in an implantable multi-chamber pump
InactiveUS20140296830A1Relieve painReducing severe and chronic painMedical devicesPressure infusionNeuropathic painZiconotide
One embodiment of the present invention is a method for reducing pain using a multi chamber pump to separately administer multiple drugs. For example, one chamber may contain an omega conopeptide, such as ziconotide, and the other chamber or chambers may contain one or more other drugs, which may include of morphine, hydromorphone, fentanyl, sufentanil, bupivacaine, baclofen, clonidine, and buprenorphine, or their pharmaceutically acceptable salts thereof. Other applications of the present invention include methods for treating severe chronic pain due to cancer, failed back syndrome, CRPS, neuropathic pain, mixed neuropathic and nociceptive pain.
Owner:JAZZ PHARMA
Patch of abstention containing cola stationary medicament and preparing method thereof
InactiveCN101199495AAvoid interferenceAvoid degradationOrganic active ingredientsNervous disorderAdhesiveNonwoven fabric
The invention discloses a detoxification plaster with clonidine and the related preparation method used for curing opioid and tobacco-dependentance. The detoxification plaster is structurally composed of a protective layer, an adhesive layer, a control release membrane layer, a medicine storehouse layer and a back lining layer. The ingredients of the preparation are that: the medicine storehouse layer contains 10 to 40 percent of clonidine and 0 to 20 percent of transdermal enhancer and the rest are soluble or non-soluble substrates; the adhesive layer contains 0 to 30 percent of clonidine and 2 to 20 percent of transdermal enhancer and the rest is pressure-sensitive adhesive; the back lining layer is a PET membrane or a non-woven fabric; the protective layer is a PET film processed with antisticking treatment; the control release membrane is an EVA polymer membrane. The area of each plaster is 1-5square centimeters; each plaster contains 1mg to 5mg of clonidine. The invention is not addictive but fast in effect, high in cure rate and safe and convenient. The external release of the invention reaches zero-degree rate process.
Owner:TIANJIN ZHONGBAO PHARMACY
Methods for treating conditions such as dystonia and post-stroke spasticity with clonidine
ActiveUS8722079B2Good sustained releaseRelieve post-stroke spasticity and/or dystoniaOrganic active ingredientsPill deliveryEffective treatmentPost stroke spasticity
Effective treatments of dystonia and / or post-stroke spasticity for extended periods of time are provided. Through the administration of an effective amount of clonidine at or near a target site, one can relieve dystonia and / or post-stroke spasticity caused by diverse sources. When appropriate formulations are provided within biodegradable polymers, this relief can be continued for at least five days. In some embodiments, the relief can be for at least twenty-five days, at least fifty days, at least one hundred days, at least one hundred and thirty-five days or at least one hundred and eighty days.
Owner:WARSAW ORTHOPEDIC INC
Combination of adrenergic agonist and tricyclo-alkylamine for relieving chronic pain without adverse side effects
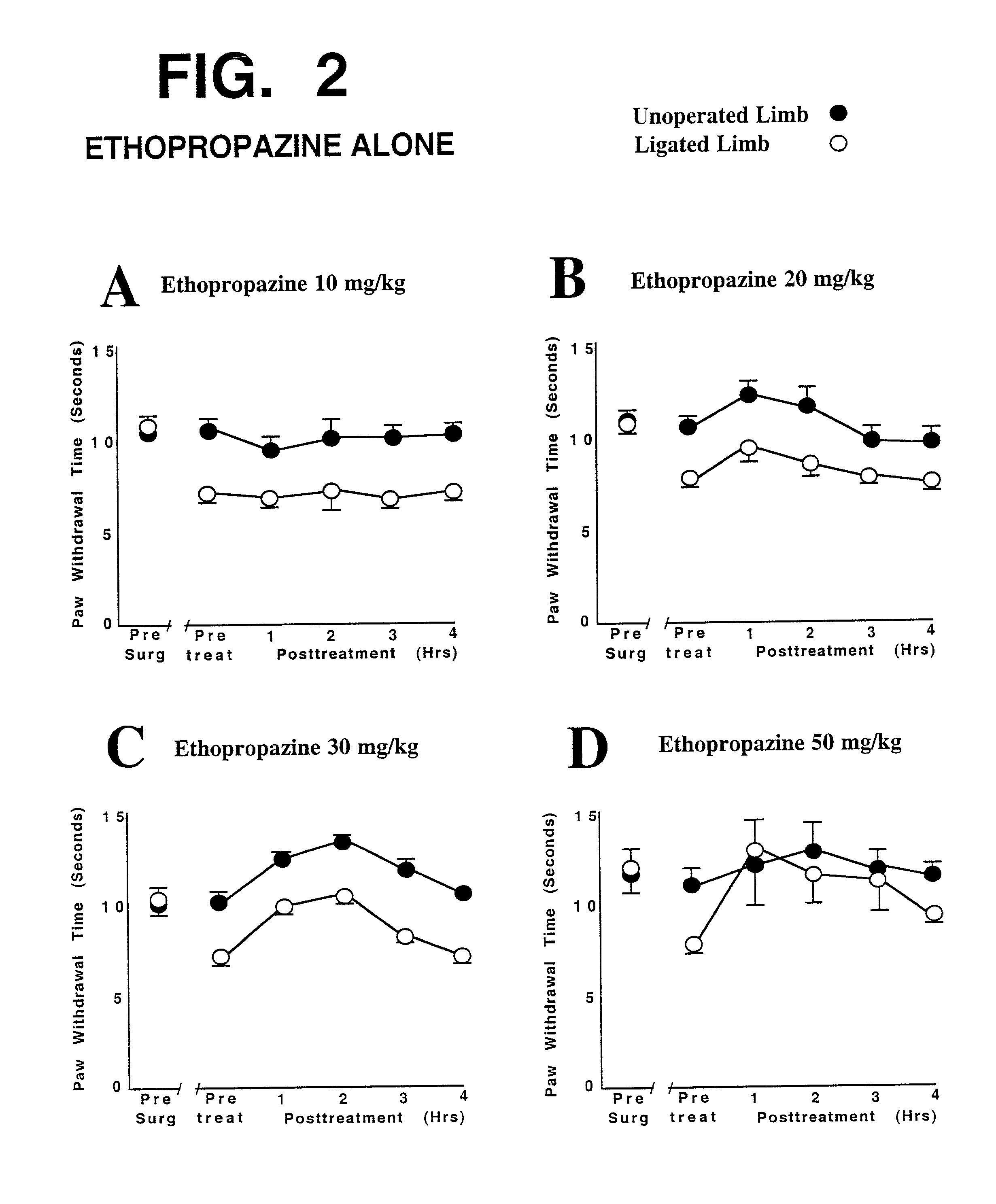
This invention discloses that a combination of two drugs, from two different and previously unrelated categories, provides effective and long-lasting relief from neuropathic pain. Both drugs can be taken orally, in a convenient, painless, non-invasive manner that does not require injections. One drug in this combination is an alpha2 adrenergic agonist, exemplified by clonidine. The other drug in the pain-relieving combination has a tri-cyclo-alkyl-amine (TCAA) structure. At least some TCAA drugs have antagonist (receptor-blocking) activity at two entirely different classes of neuronal receptors: the muscarinic subclass of acetylcholine (ACh) receptors, and the NMDA subclass of glutamate receptors. Such drugs include ethopropazine, normally used as an anti-cholinergic drug, and desipramine, normally used as an anti-depressant. Tests by the Applicants have shown that at least some TCAA drugs can relieve neuropathic pain to a limited extent, but at the doses required to relieve pain, they cause adverse side effects, and any pain relief is relatively brief and short-lived. However, when a TCAA drug such as ethopropazine is administered together with an alpha2 adrenergic agonist such as clonidine, these drugs mutually potentiate one another's neuropathic pain-relieving action, and provide potent and sustained neuropathic pain relief, even when each agent is administered at a low dosage that is below its threshold for causing adverse side effects. Accordingly, this drug combination can provide safe and effective relief of neuropathic pain and possibly other types of chronic and / or intractable pain, at dosages which are so low that they do not pose serious risks of adverse side effects.
Owner:OLNEY JOHN W +2
Clonidine multivesicular liposome and preparation method thereof
InactiveCN101756902AReduce releaseReduce wasteOrganic active ingredientsNervous disorderLipid formationMultivesicular liposomes
The invention discloses a clonidine multivesicular liposome and a preparation method thereof. The clonidine multivesicular liposome comprises clonidine serving as a medicinal active ingredient and an empty multivesicular liposome serving as a carrier. The empty multivesicular liposome comprises the following components in part by mass: 1 part of lipid component and 0.1 to 50 parts of ion gradient regulator, wherein the lipid component comprises amphiphilic lipids and neutral lipids in a molar ratio of 5-36:1. The clonidine multivesicular liposome is obtained by adopting the empty multivesicular liposome enveloped with the gradient regulator and using a transmembrane gradient active loading method to enable the clonidine to enter the aqueous phase of the empty multivesicular liposome through a phospholipid membrane. The clonidine multivesicular liposome has high utilization rate of raw materials and high loading concentation, shows good slow release effect in in vivo and in vitro experiments, and has better analgesic effect.
Owner:SHANGHAI INST OF PHARMA IND
Methods for Treating Conditions Such as Dystonia and Post-Stroke Spasticity with Clonidine
ActiveUS20090263462A1Good sustained releaseRelieve post-stroke spasticity and/or dystoniaOrganic active ingredientsPill deliveryMuscle DystoniaPost stroke spasticity
Effective treatments of dystonia and / or post-stroke spasticity for extended periods of time are provided. Through the administration of an effective amount of clonidine at or near a target site, one can relieve dystonia and / or post-stroke spasticity caused by diverse sources. When appropriate formulations are provided within biodegradable polymers, this relief can be continued for at least five days. In some embodiments, the relief can be for at least twenty-five days, at least fifty days, at least one hundred days, at least one hundred and thirty-five days or at least one hundred and eighty days.
Owner:WARSAW ORTHOPEDIC INC
Clonidine adhesive patch and preparation method thereof
ActiveCN105362255ASmooth releaseTo achieve the effect of long-acting controlled releaseOrganic active ingredientsPharmaceutical non-active ingredientsControlled releaseComposite film
The invention provides a clonidine adhesive patch capable of stably lowering blood pressure in long term, made from clonidine. A drug carrier is made of medicinal pressure-sensitive adhesive, a controlled release film, a lined film, and a release agent composite film, wherein a weight ratio of the clonidine to the pressure-sensitive adhesive is 1:6-1:12; by unit dose of clonidine 4g, all the controlled release film, the lined film and the release agent composite film are 0.4 m<2>. The pressure-sensitive adhesive has long-term action and controlled release, good tolerance, no foreign body sensation but breathability and rarity of dropping, effective components can be released at constant speed through the skin within 7 days, and in an application course of 7 days, the skin has no foreign body sensation but good sensation.
Owner:哈尔滨瀚钧现代制药有限公司
Instant gel rubber of clonidine hydrochloric for eyes
InactiveCN101342171AVarious dosage formsExtended stayOrganic active ingredientsSenses disorderGramPreservative
The invention discloses an immediate type ophthalmic gelatin of hydrochloric acid clonidine and a preparation method thereof. 100 milliliters of the immediate type ophthalmic gelatin of hydrochloric acid clonidine contains 1 to 5 grams of the hydrochloric acid clonidine, 0.05 to 1 gram of preservative, 1.0 to 7.5 grams of permeation pressure conditioning agent and 30 to 150 grams of gelatin substrate. Remaining components are acid-base buffer regent and water for rejection. The invention enriches the dosage forms of the hydrochloric acid clonidine by optimizing auxiliary materials and improving technology so that lag time in eyes is greatly prolonged and curative effect is improved without adverse stimulus reaction.
Owner:肖正连
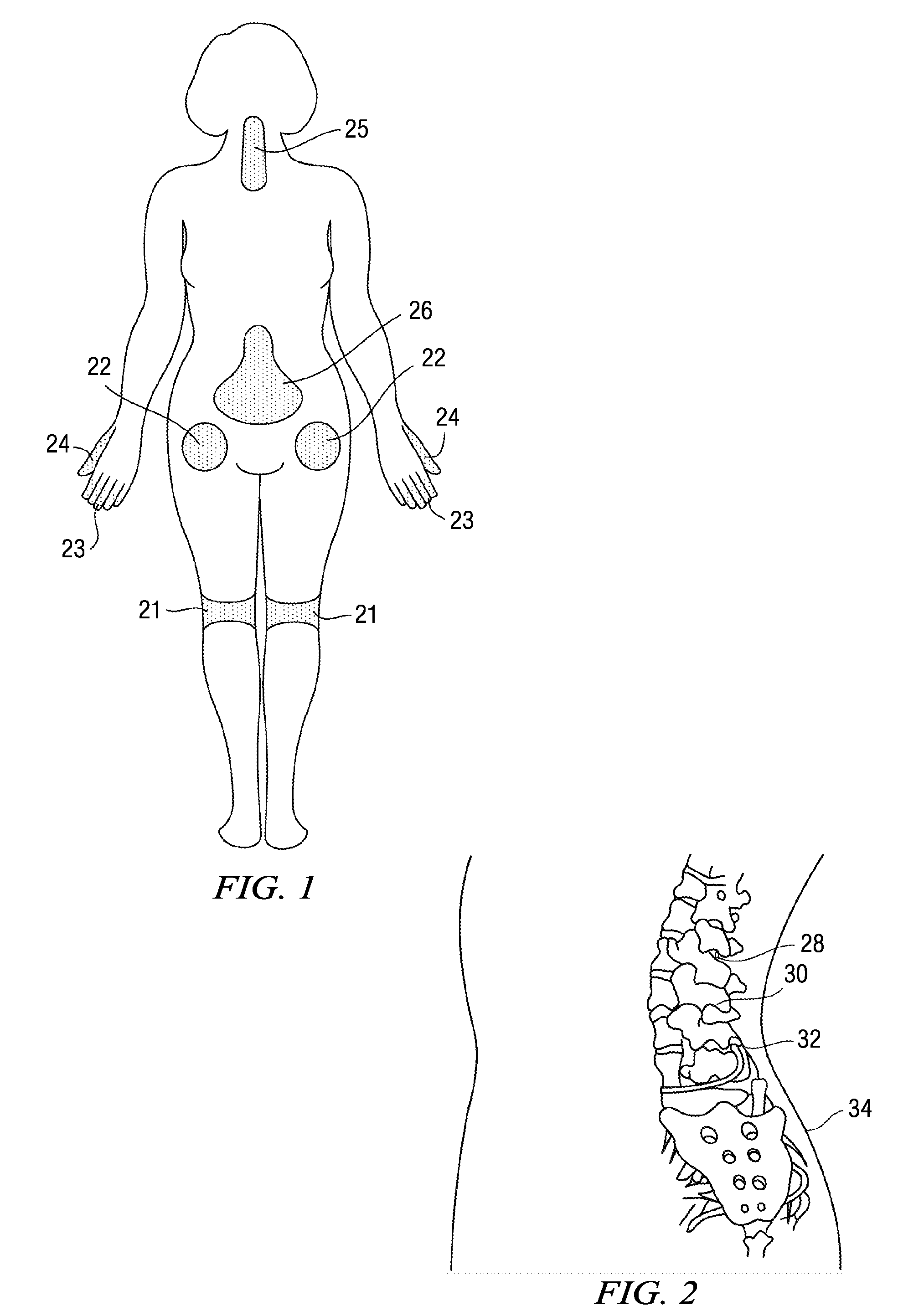
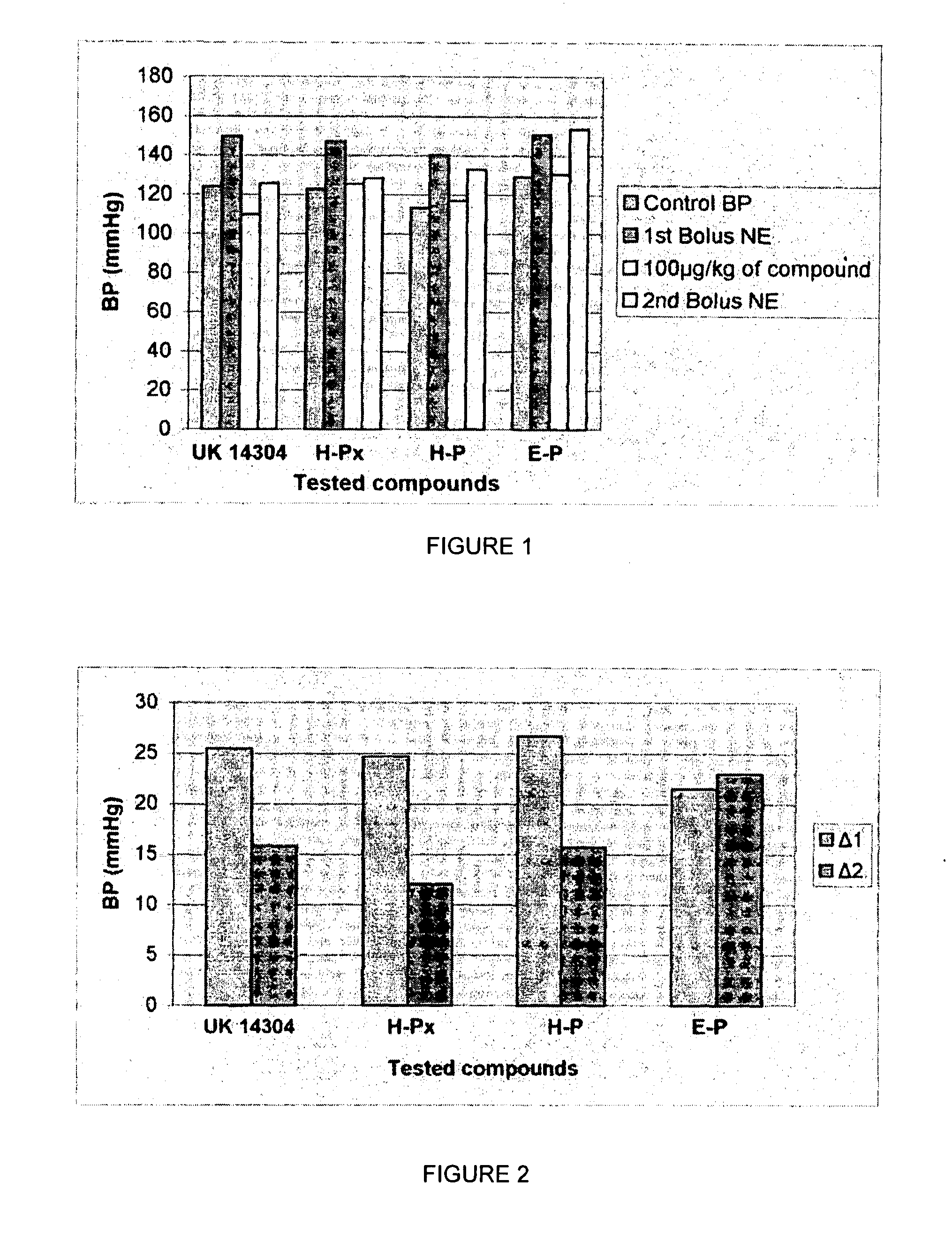
1,3 and 1,3,5 substituted imidazoles as antihypertensives
InactiveUS20100216854A1High affinityEnhanced inhibitory effectBiocideOrganic chemistryLosartanLipophilicity
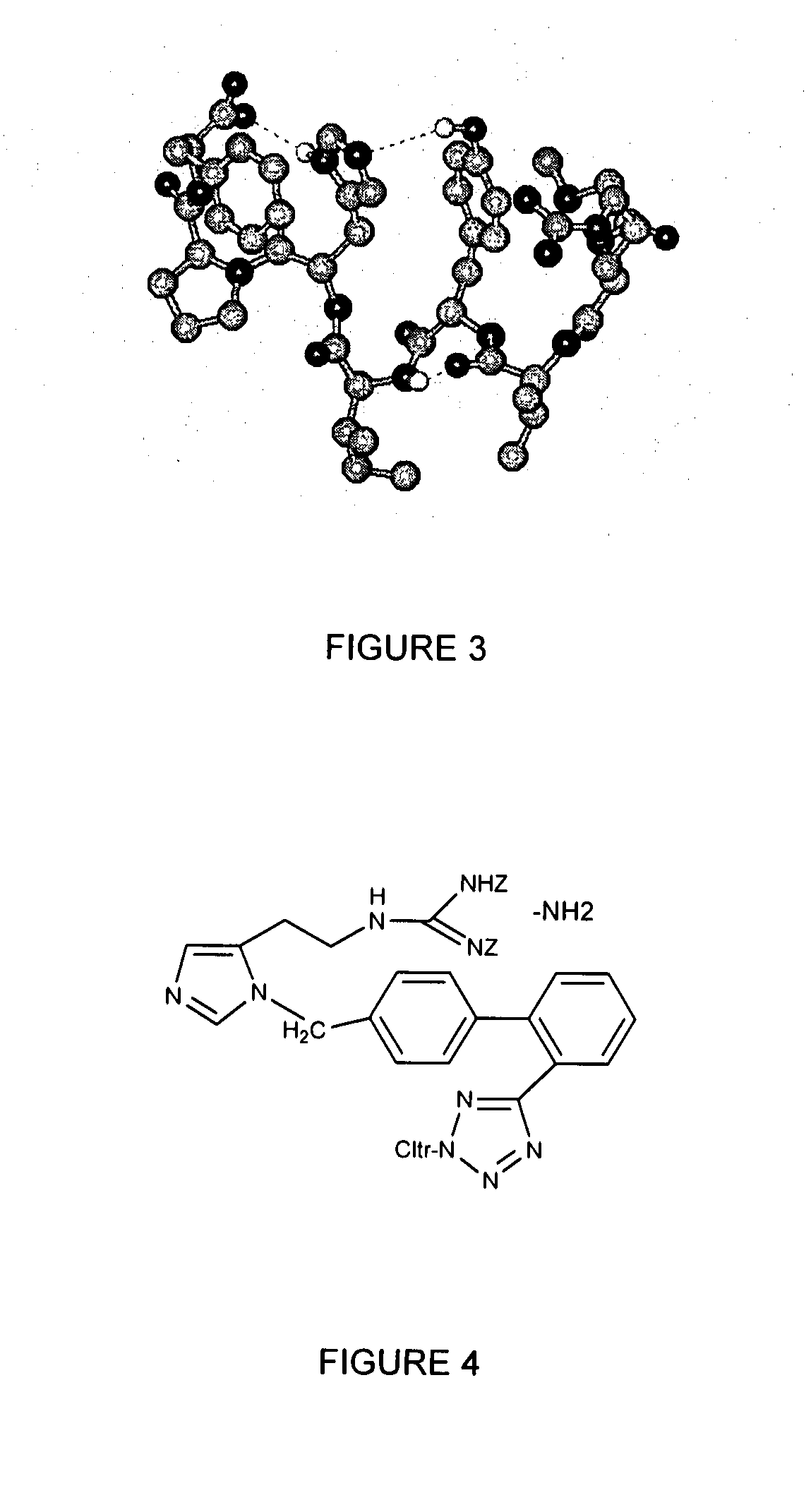
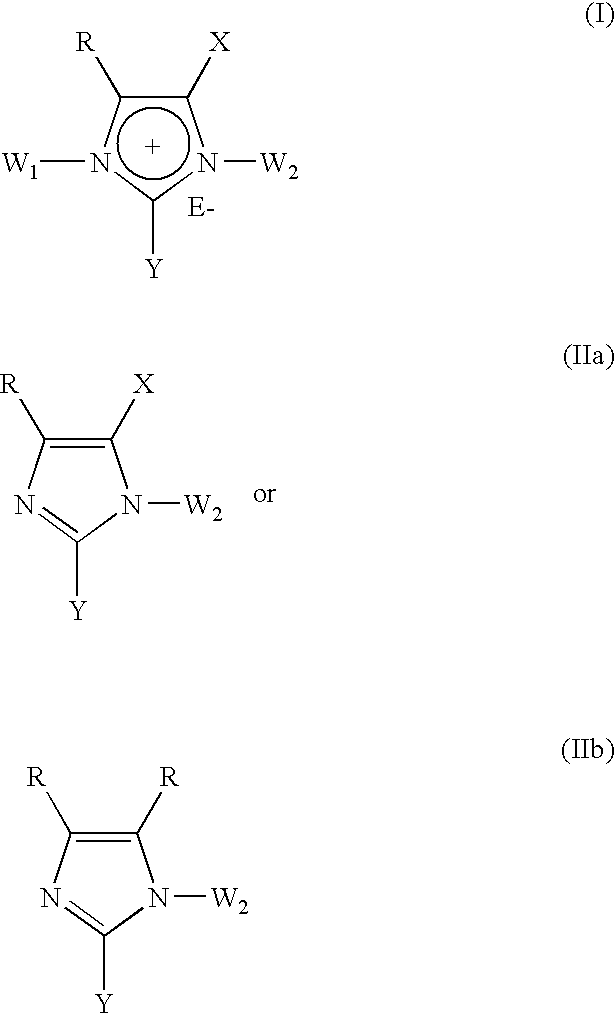
The present invention provides novel 1,5 and 1,3,5-substituted imidazole compounds of formulas (I), (IIa), (IIIb) in hydrophilic or lipophilic form, which are useful as angiotensin II AT1 receptor antagonists with sympathetic suppressant properties. In particular, the invention provides pharmaceutical compositions containing the pharmacophoric groups of Losartan and Clonidine as well compounds, processes and intermediates for preparing compounds and their use in methods of treating hypertension and cardiovascular diseases through Renin Angiotensin System (RAS) and Sympathetic System (SS). Alkylated histamine based double action Saltans are lipophilic and can act transdermally.
Owner:ELDRUG SA
Patch
InactiveCN103313710AGood storage stabilityImprove cohesionOrganic active ingredientsOrganic non-active ingredientsViscosityPolymer chemistry
A patch with stable transdermal absorbability, regardless of temperature changes during storage, in a crystallized formulation of clonidine is provided. This patch is characterized by including a backing, and a plaster layer integrally laminated on one surface of the backing, said plaster layer containing 5 to 30 wt% of clonidine containing crystallized clonidine, 25 to 90 wt% of a polymer base (A) having a viscosity-average molecular weight of at least 800,000, and 5 to 60 wt% of a liquid additive capable of dissolving the clonidine. Moreover, the weight ratio of the liquid additive to the polymer base (A) [liquid additive / polymer base (A)] is 0.1 to 2.0.
Owner:SEKISUI MEDICAL CO LTD
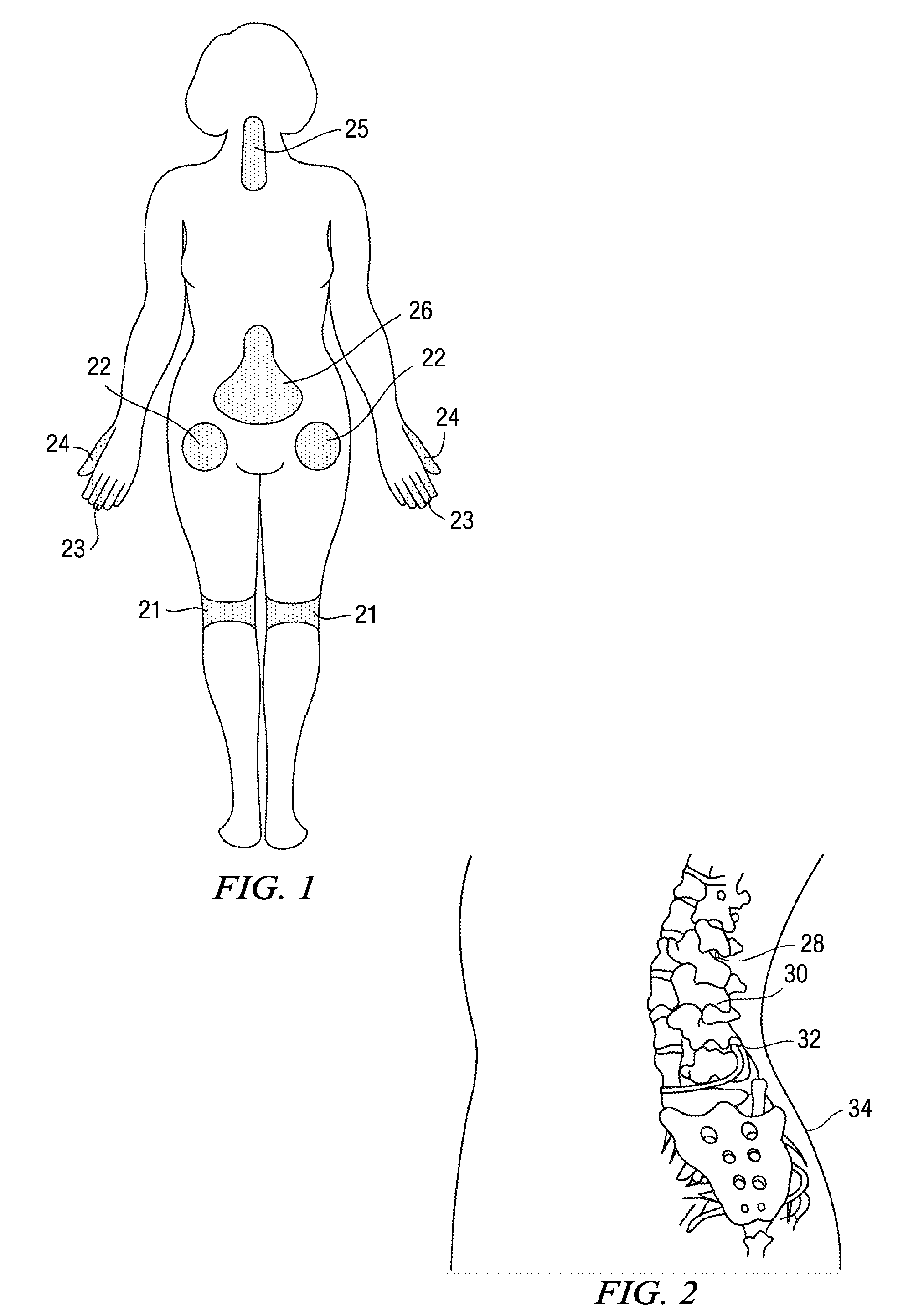
Treatment of length dependent neuropathy
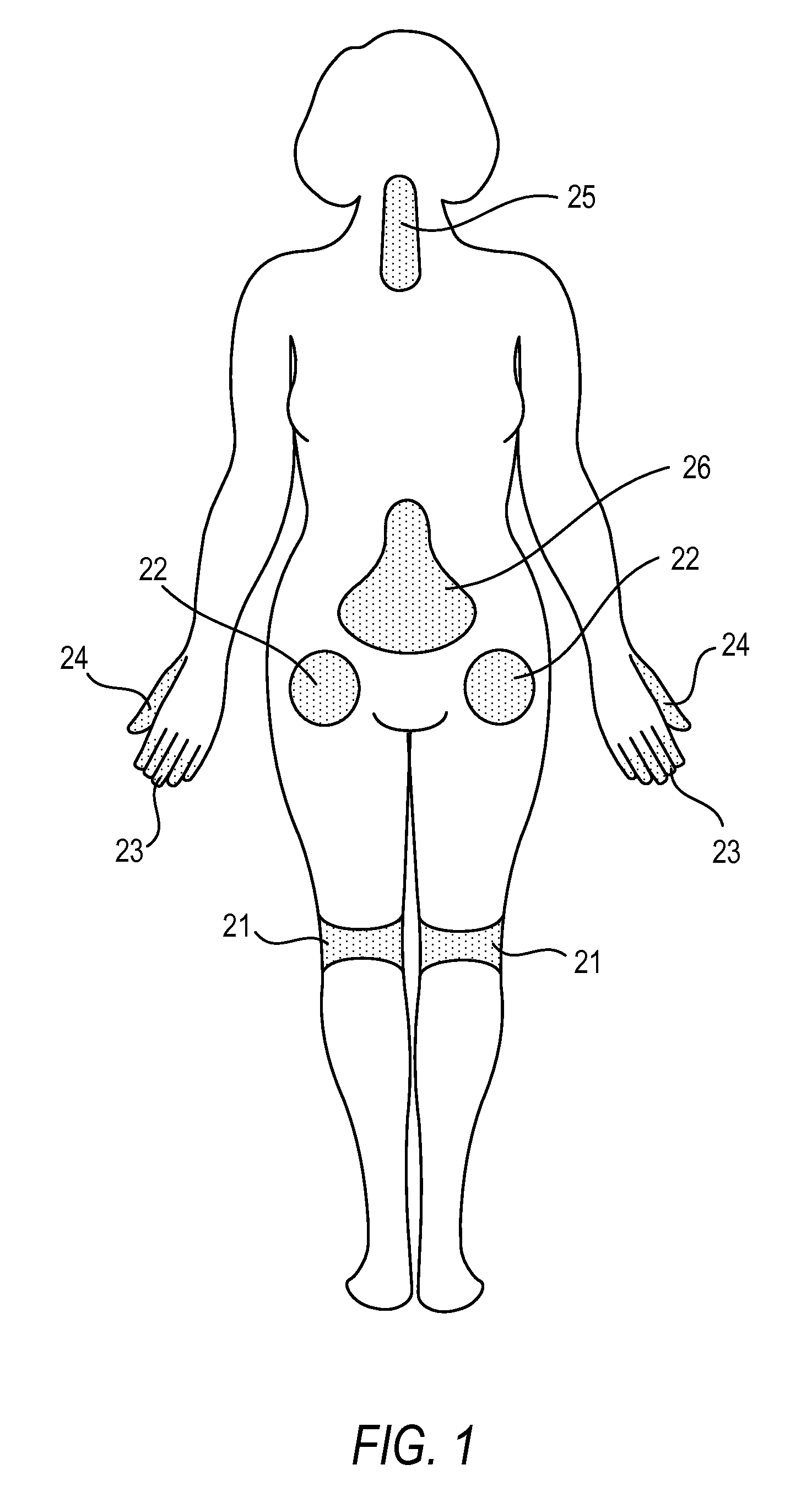
Compositions, and methods of use thereof, are provided for the treatment of painful neuropathy by local or topical delivery of compounds that interact with α-adrenergic receptors, especially an alpha2 adrenergic agonist such as clonidine, to the entire painful area such that the need for systemic dosing is minimized. The compounds are delivered to or adjacent to painful areas in patients with painful length dependent neuropathy, and other neuropathies that affect the pain signaling fibers in the skin. A preferred compound for the treatment of patients with length dependent neuropathy is clonidine applied in a transdermal patch, gel, ointment, lotion, liposomal formulation, cream, or emulsion, wherein the concentration is sufficient to provide an effective dose in the painful area or immediately adjacent areas.
Owner:CAMPBELL JAMES N
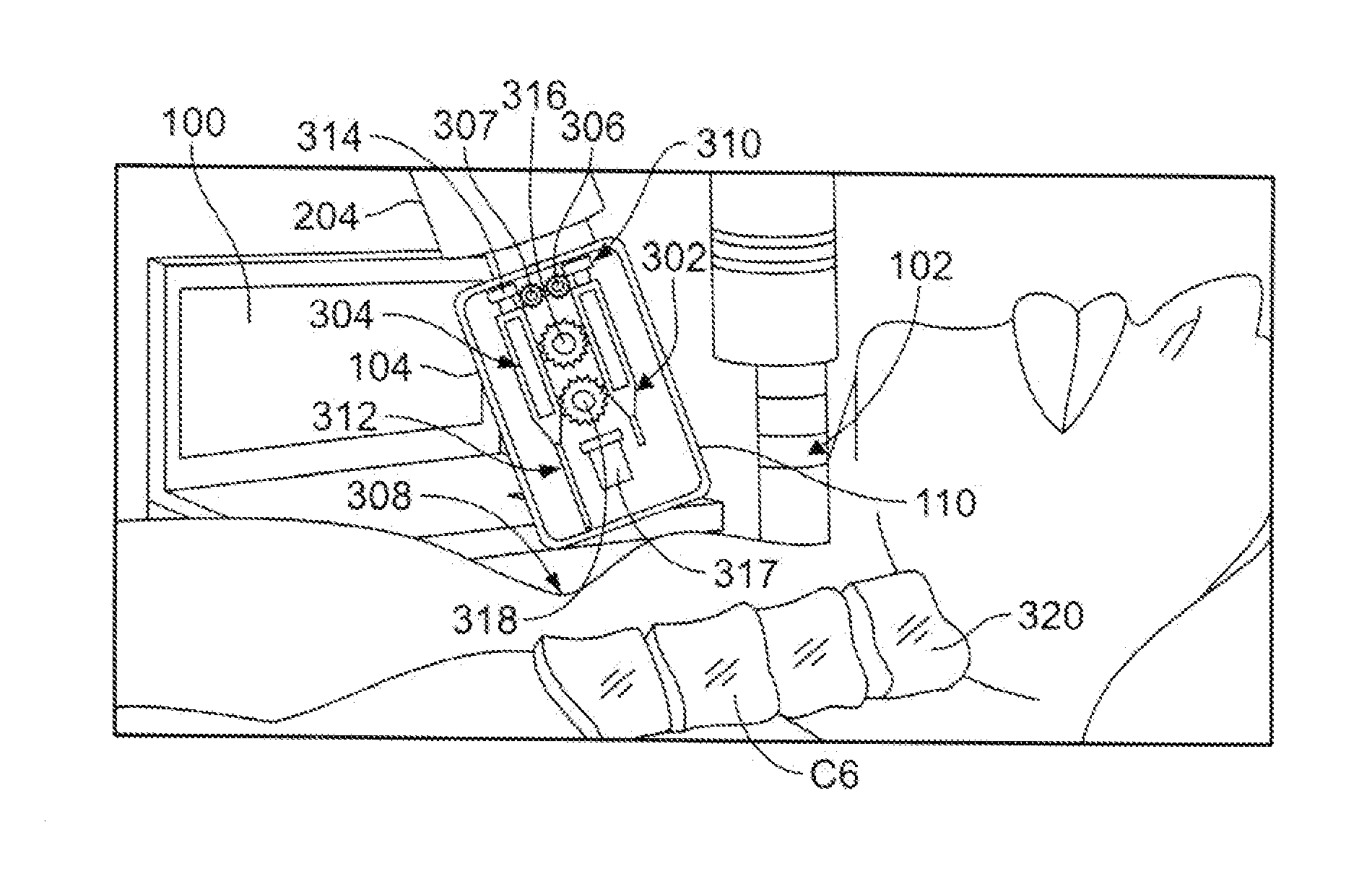
System and Device for A Fully Automated Approach to Facilitate Stellate Ganglion Block or Pulsed Radiofrequency Procedure for Treating PTSD
A system and method to facilitate a stellate ganglion block or a pulsed radiofrequency procedure to treat post-traumatic stress disorder. The method can be fully automated and guided by a computer or the process can be guided by the surgeon. A computer including a central processing unit (CPU) is connected to a body scanning device and an injection mechanism. The body scanning device is used to read images of a patient's body. The information collected from the images is transmitted to the CPU. A housing for the injection mechanism encloses syringes for delivery of an anesthetic and a clonidine mixture. After the anesthetic is injected into the patient's skin from a first syringe, the housing is relocated by a connecting arm and a second syringe moves a needle into contact with the patient's bone. An electric eye scans for blood before the clonidine mixture is injected by the second syringe.
Owner:CAD MEDICAL SOLUTIONS LLC
Liquid pharmaceutical composition of clonidine
The present invention relates to liquid pharmaceutical composition suitable for oral administration comprising clonidine or pharmaceutically acceptable salts thereof with at least one buffer and at least one preservative. The present invention also relates to process for preparing the said liquid pharmaceutical composition.
Owner:SYRI
Okra leaf smoking quitting tea and its production technology
InactiveCN103933108ASweet tasteImprove staminaNervous disorderTea substituesBiotechnologyNortriptyline
The invention discloses okra lea smoking quitting tea and its production technology, relates to the okra lea smoking quitting tea capable of quitting smoking addiction and its production technology, and mainly solves the problems of being ineffective and producing of discomforts caused by smoking addiction quitting of smoking quitting products, currently on the market, such as ''smoking quitting sugar'', and smoking quitting drugs of clonidine, nortriptyline and the like. The okra lea smoking quitting tea comprises 5-10 parts of fresh okra leaf and 1-5 parts of fresh eucommia leaf. The okra lea smoking quitting tea prepared by using the okra leaf as a main raw material tastes sweet, refreshes the throat, and can eliminate bad breath, theanine contained in the tea can rapidly decompose nicotine and acetylcholine, can clear deposited toxins in lung, and is easy to quit smoking, quit smoking addiction, and a preliminary experiment shows that the smoking quitting rate can reach above 90%, and the time is short, the effect is quick, and the smoking quitting can be achieved in about 20 days; the okra lea smoking quitting tea has smoking quitting effect, also can regulate blood lipid, reduce blood pressure, reduce blood fat, and reduce weight.
Owner:马国云
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com