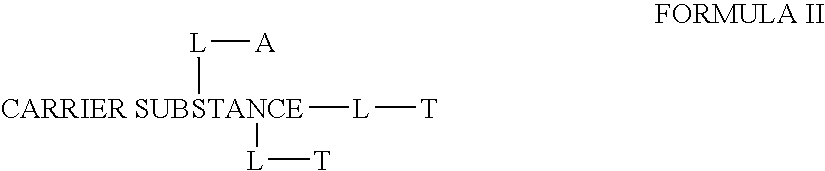Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1813 results about "Unexpected therapeutic effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapeutic effect refers to the responses(s) after a treatment of any kind, the results of which are judged to be desirable and beneficial. This is true whether the result was expected, unexpected, or even an unintended consequence. An adverse effect, including nocebo, on the other hand, refers to harmful or undesired responses(s).
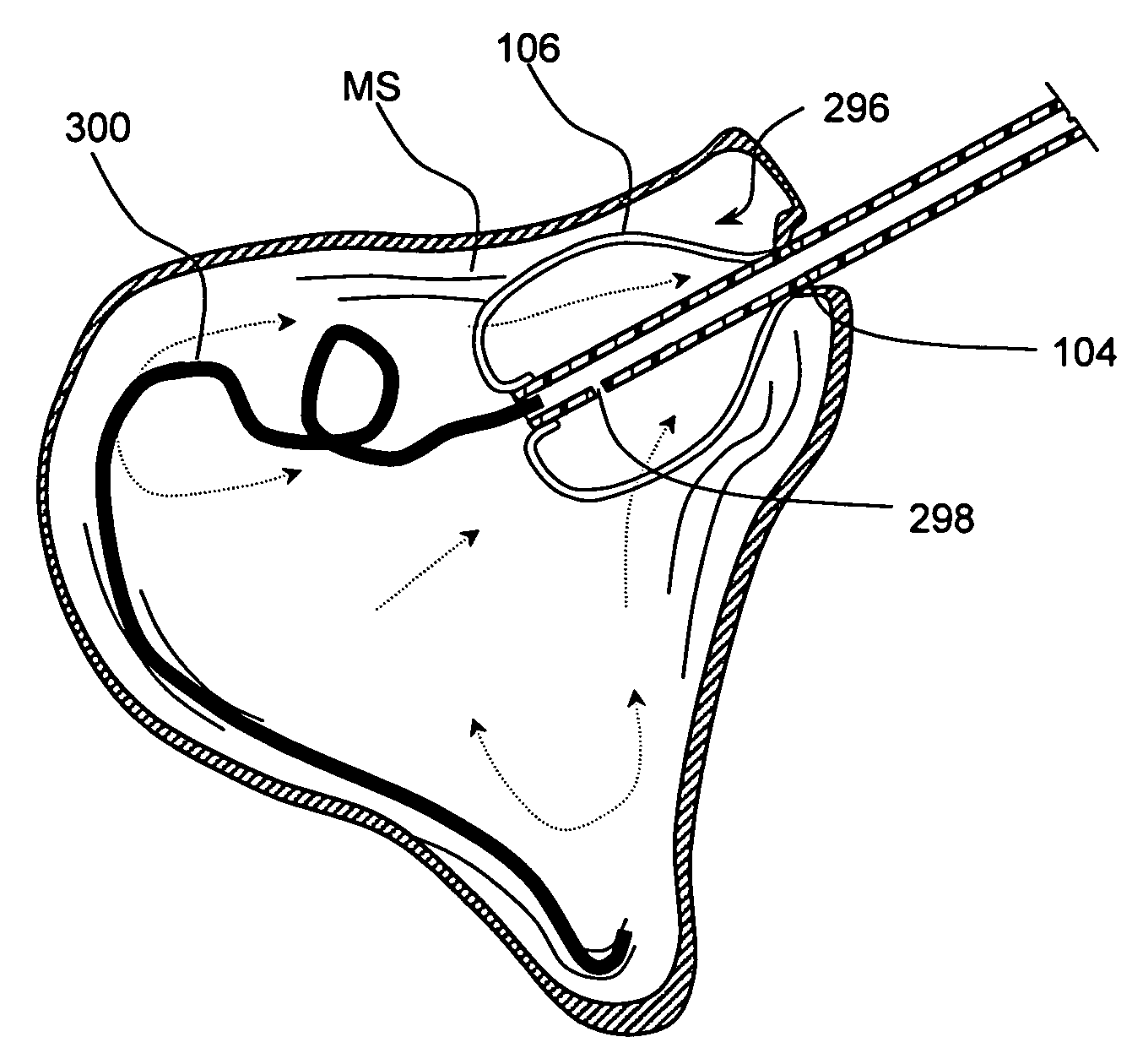
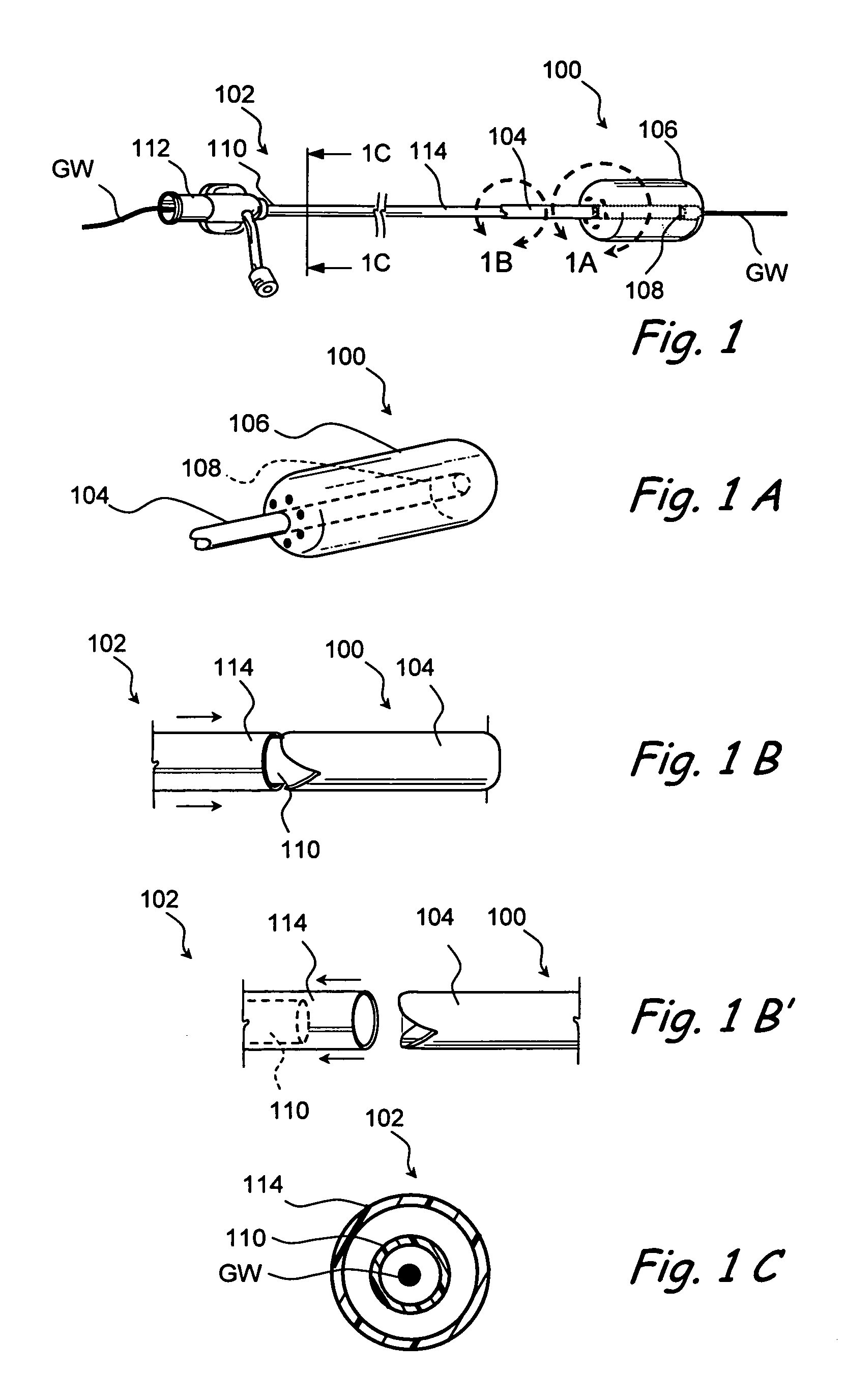
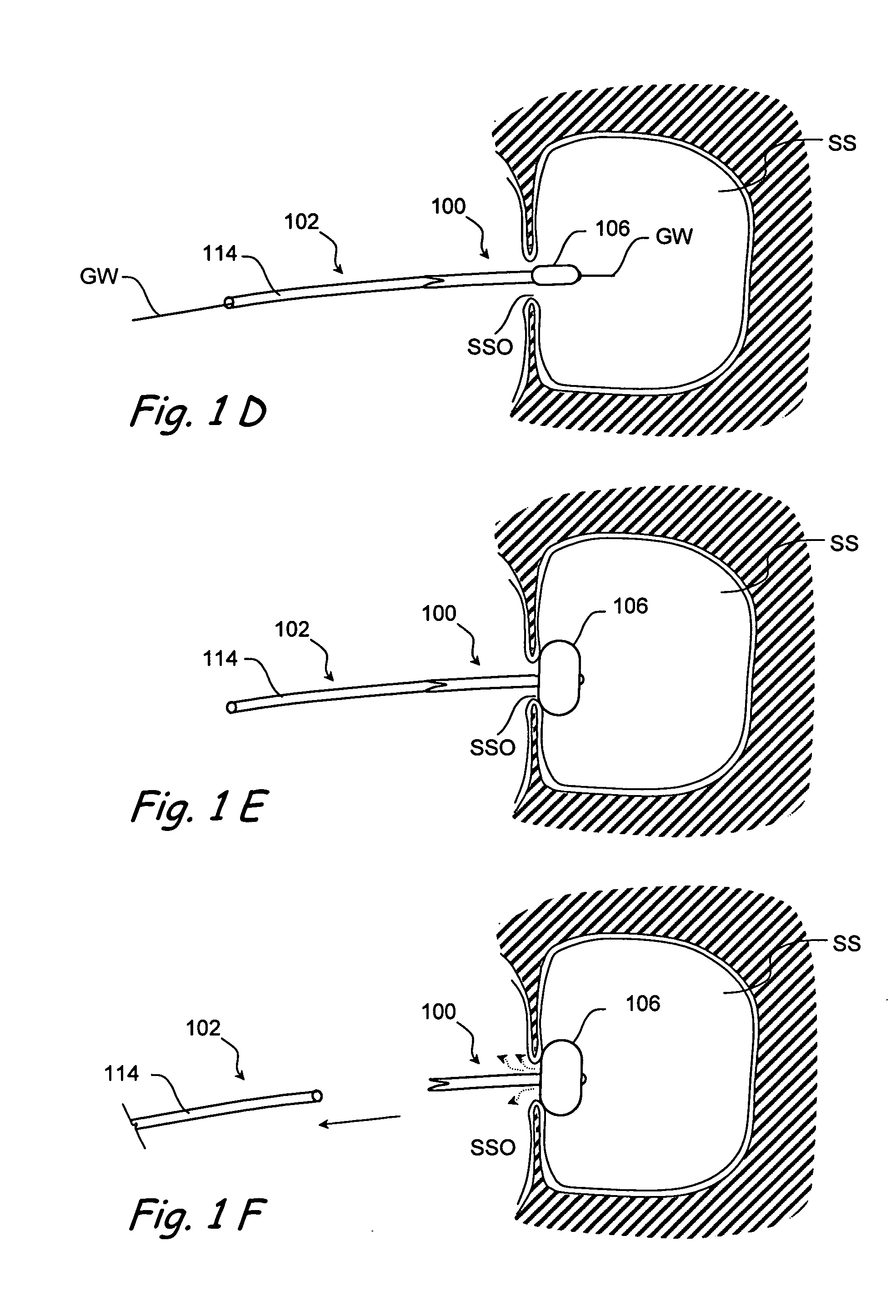
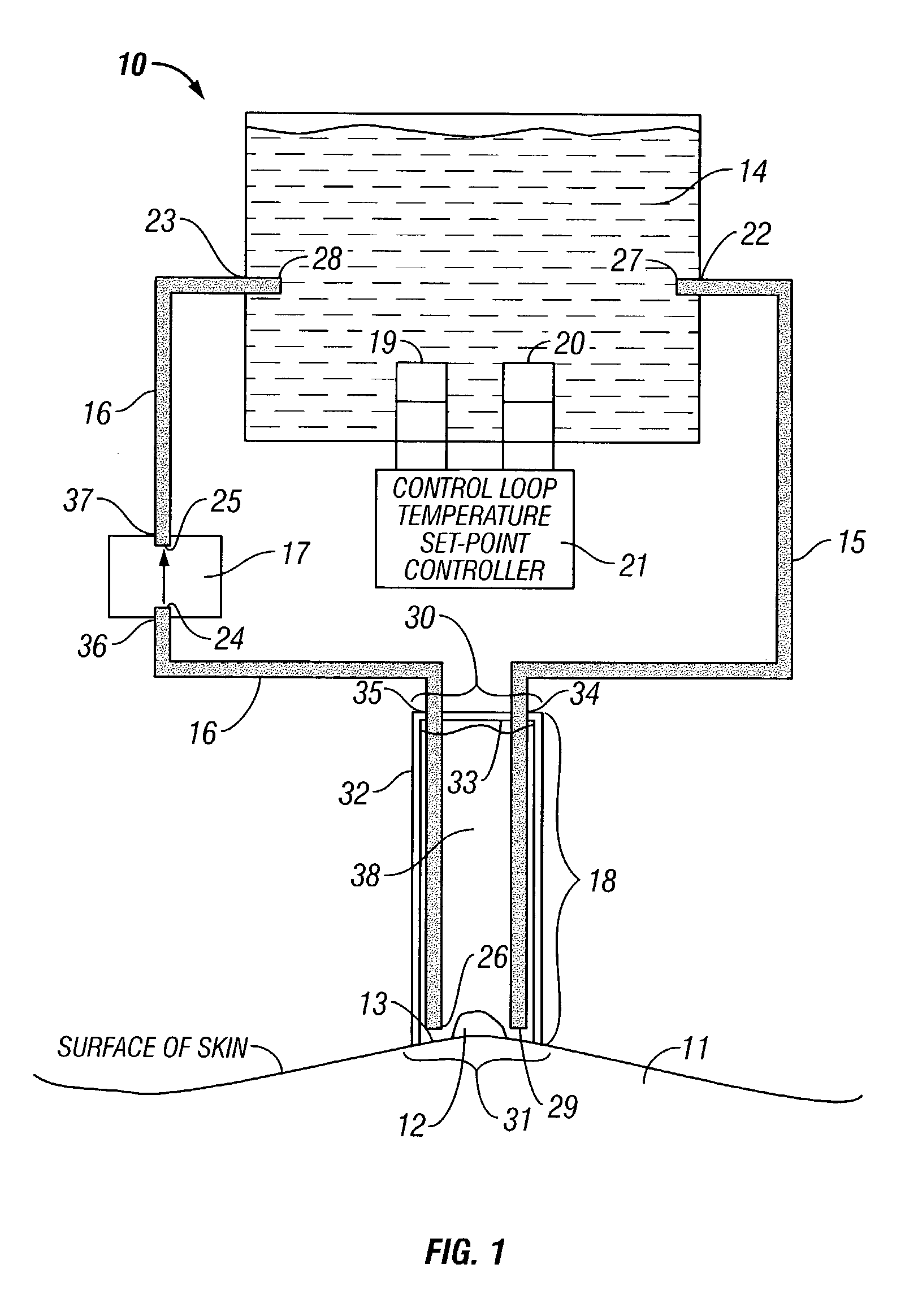
Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders
ActiveUS20060106361A1Surgical needlesPharmaceutical delivery mechanismDrugUnexpected therapeutic effect
Devices and methods for delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects. An implantable delivery device comprising a reservoir is initianlly attached to a deliver catheter or delivery tool and is introduced into the body and positioned at a desired site. A therapeutic or diagnostic substance is then introduced into the reservoir and the delivery catheter or deliver tool is then removed, leaving the implantable delivery device implanted within the body. The substance is then delivered from the reservoir at a rate that causes the desire diagnostic or therapeutic effect. Also provided are substance eluting stents that elute substance from a selected surface of the stent (e.g., the outer surface) but not from another surface of the stent (e.g., the inner surface).
Owner:ACCLARENT INC
Device and method for delivery of a medicament
The disclosure relates to a method of enhancing nicotine or other medicament concentrations in a gaseous carrier. The methods are adaptable to the delivery of nicotine or other medicaments for therapeutic effect in various diseases, in particular nicotine for tobacco product use cessation, substitution and / or harm reduction. The disclosure further relates various devices and device design principles for practicing these methods.
Owner:PHILIP MORRIS PROD SA
System and method of assessment of the efficacy of treatment of neurological disorders using the electroencephalogram
Disclosed is a system and method of assessing the efficacy of treatment of neurological or psychological disorders. The preferred embodiment uses at least two surface electrodes to acquire EEG signals from the surface of a patient's body, a processor for computing from the EEG signals various features and indices that are representative of the patient's neurological or psychological state. Changes in these parameters may be used to assess the efficacy of treatment and to modify the treatment to optimize the resultant patient state.
Owner:TYCO HEALTHCARE GRP LP
Osmotic pump drug delivery systems and methods
InactiveUS6471688B1Avoid mixingMedical devicesPharmaceutical delivery mechanismTreatment effectAnalgesics effects
Implantable osmotic pump devices and systems include multiple osmotic pumps and / or semipermeable membranes to extend the useful life cycle and functionality of the drug delivery system. Use of an implantable system including multiple implantable osmotic pumps allows different drugs to be administered from the same implanted system. One or more of the semipermeable membranes of the system may be initially sealed by an overlying impermeable membrane upon implantation of the system into the patient. When the patient develops a tolerance to a first drug or to a first dose of the first drug, the impermeable membrane may be breached, to expose the underlying semipermeable membrane to the osmotic pressure of the patient at the implant site. This causes the infusion rate to increase, thereby providing the patient with the needed relief and / or other desired therapeutic effect. In the case of a multiple pump system, breaching an impermeable membrane may cause the infusion of a second drug. The second drug may potentiate a therapeutic effect (such as an analgesic effect) of the first drug, as is the case with Sufentanil and Clonidine.
Owner:MICROSOLUTIONS
Treatment of cardiac arrhythmia utilizing ultrasound
InactiveUS20050080469A1Ultrasound therapyDiagnostic recording/measuringTreatment effectInvasive treatments
A noninvasive or minimally invasive treatment of cardiac arrhythmia such as supraventricular and ventricular arrhythmias, specifically atrial fibrillation and ventricular tachycardia, by treating the tissue with heat produced by ultrasound, (including High Intensity Focused Ultrasound or HIFU) intended to have a biological and / or therapeutic effect, so as to interrupt or remodel the electrical substrate in the tissue area that supports arrhythmia.
Owner:SONORHYTHM
Composition for therapy of diseases with ultrasonic and pharmaceutical liquid composition containing the same
InactiveUSRE36939E1Good effectWeaken energySonopheresisUltrasonic/sonic/infrasonic diagnosticsDiseaseTreatment effect
A booster comprising a plenty of microbubbles of a gas in a liquid, e.g. about 4x107 cells / ml of microbubbles of a gas having a diameter of 0.1 to 100 mu m in a 3 to 5% human serum albumin solution, and a pharmaceutical liquid composition comprising the booster as set forth above and a medicament, which are useful for the therapy of various diseases together with exposure of ultrasonic, where the therapeutic effects of the medicament is enhanced by the application of ultrasound in the presence of the booster.
Owner:EKOS CORP
Peptide oligonucleotide conjugates
ActiveUS20120289457A1Easy to transportImprove propertiesAntibacterial agentsOrganic active ingredientsDiseaseADAMTS Proteins
Oligonucleotide analogues conjugated to carrier peptides are provided. The disclosed compounds are useful for the treatment of various diseases, for example diseases where inhibition of protein expression or correction of aberrant mRNA splice products produces beneficial therapeutic effects.
Owner:SAREPTA THERAPEUTICS INC
Chloroquine coupled antibodies and other proteins with methods for their synthesis
InactiveUS20070166281A1Improve efficacyImprove transportBiocidePeptide/protein ingredientsDrug conjugationTreatment effect
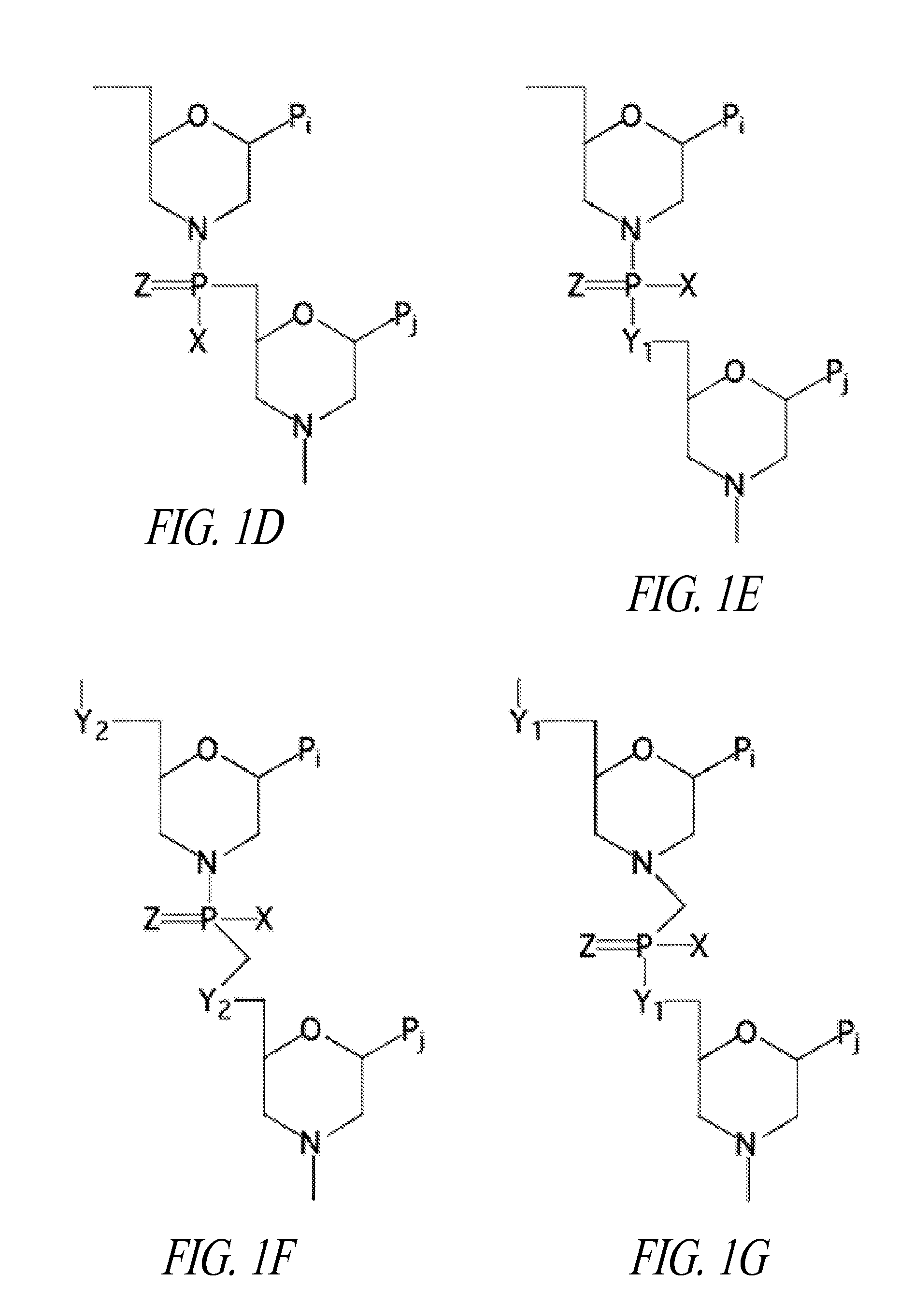
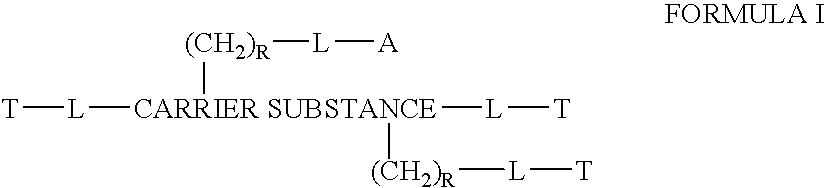
This invention discloses compositions of chloroquine-coupled active agents such as therapeutic antibodies or insulin, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the drug is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a drug directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing the drug for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and antibody or insulin to their site of action.
Owner:KOSAK KENNETH M
Localized liquid therapy and thermotherapy device
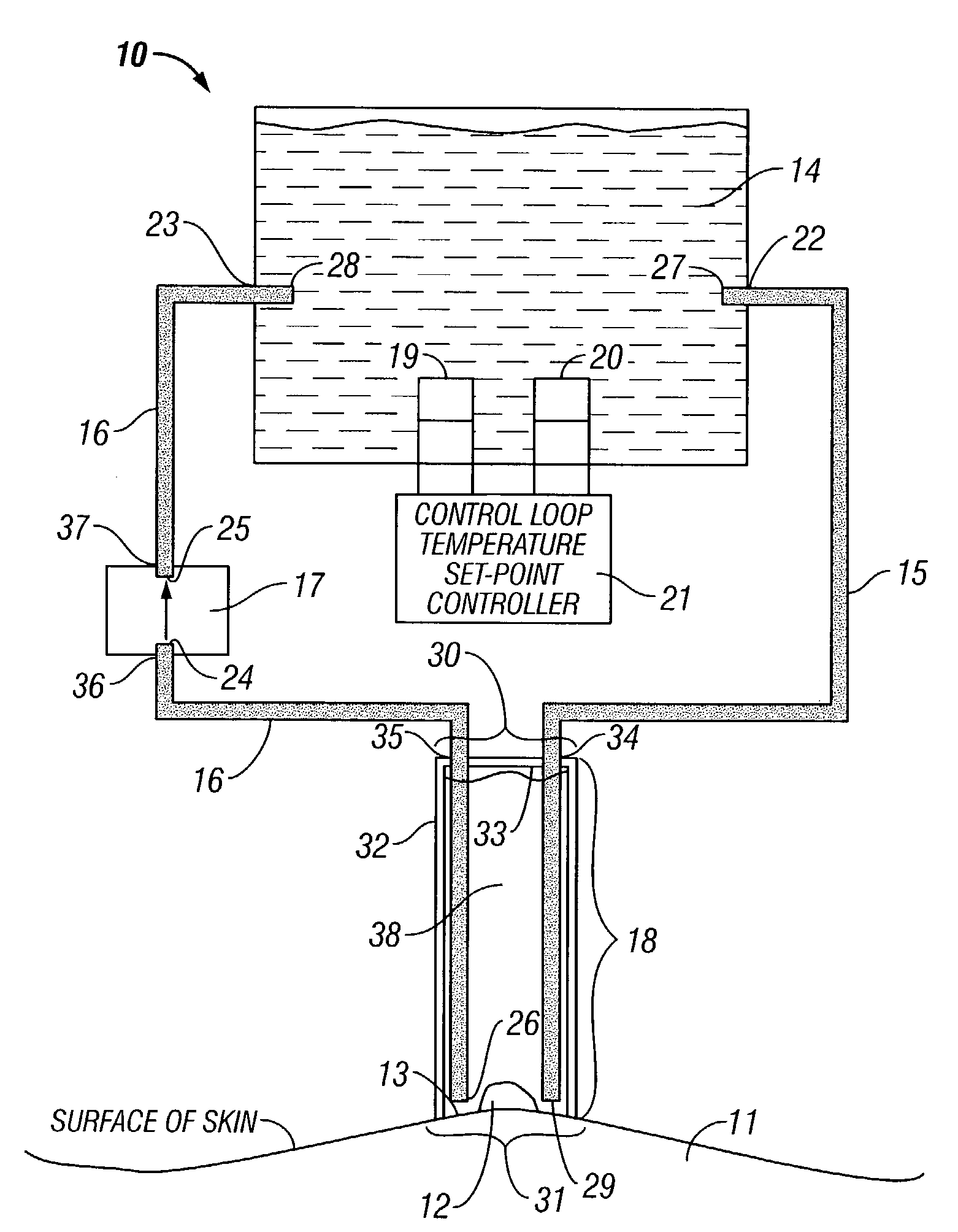
InactiveUS7422576B2Fast circulationGood treatment effectDiagnosticsSurgeryWater basedTherapeutic effect
A device for directly applying thermotherapeutic liquid to an area upon the surface of an afflicted patient, and methods of use thereof, are described. In particular a device for applying water-based liquid at a therapeutic temperature directly to an afflicted area in order to create a localized hyperthermia, is presented. The afflicted area may be either on the skin of the patient, or subcutaneous. The device is also effective for disinfection, irrigation, lavage, and the like, when employing a suitable solution. The liquid may also have a mild oxidizing effect, which, if greater upon afflicted than upon non-afflicted cells, would enhance the therapeutic effect in conjunction with the therapy herein described.
Owner:KCI LICENSING INC
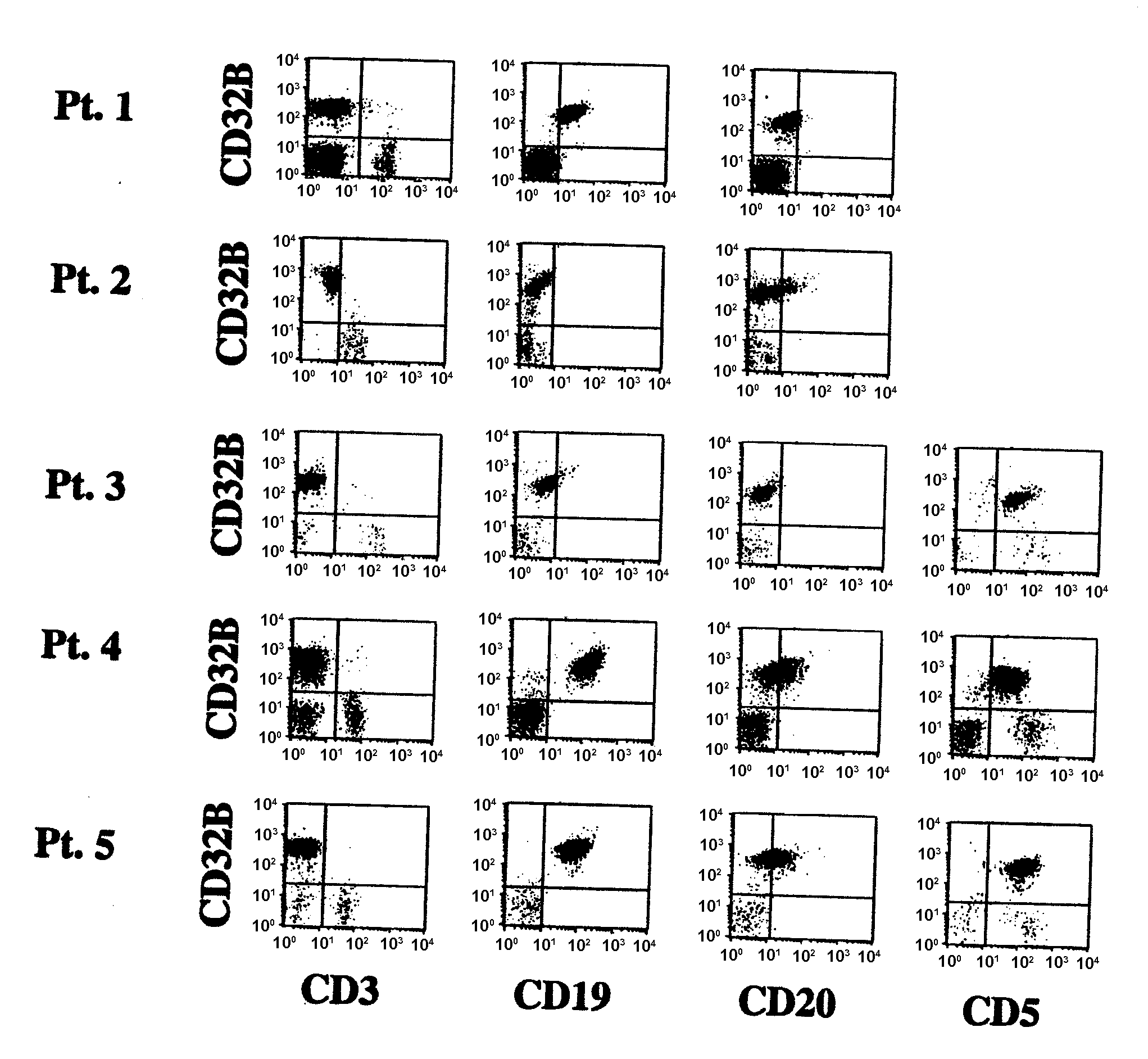
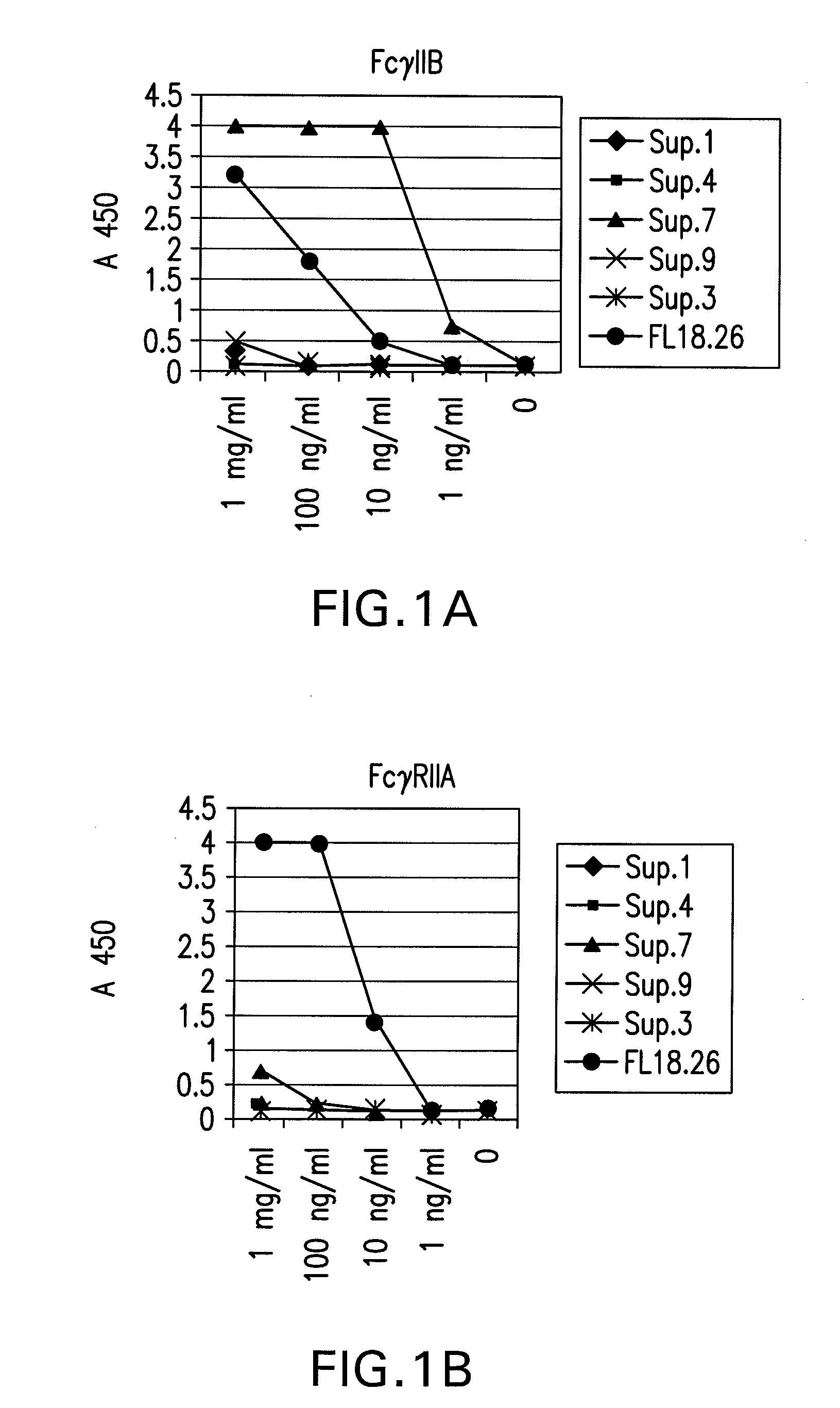
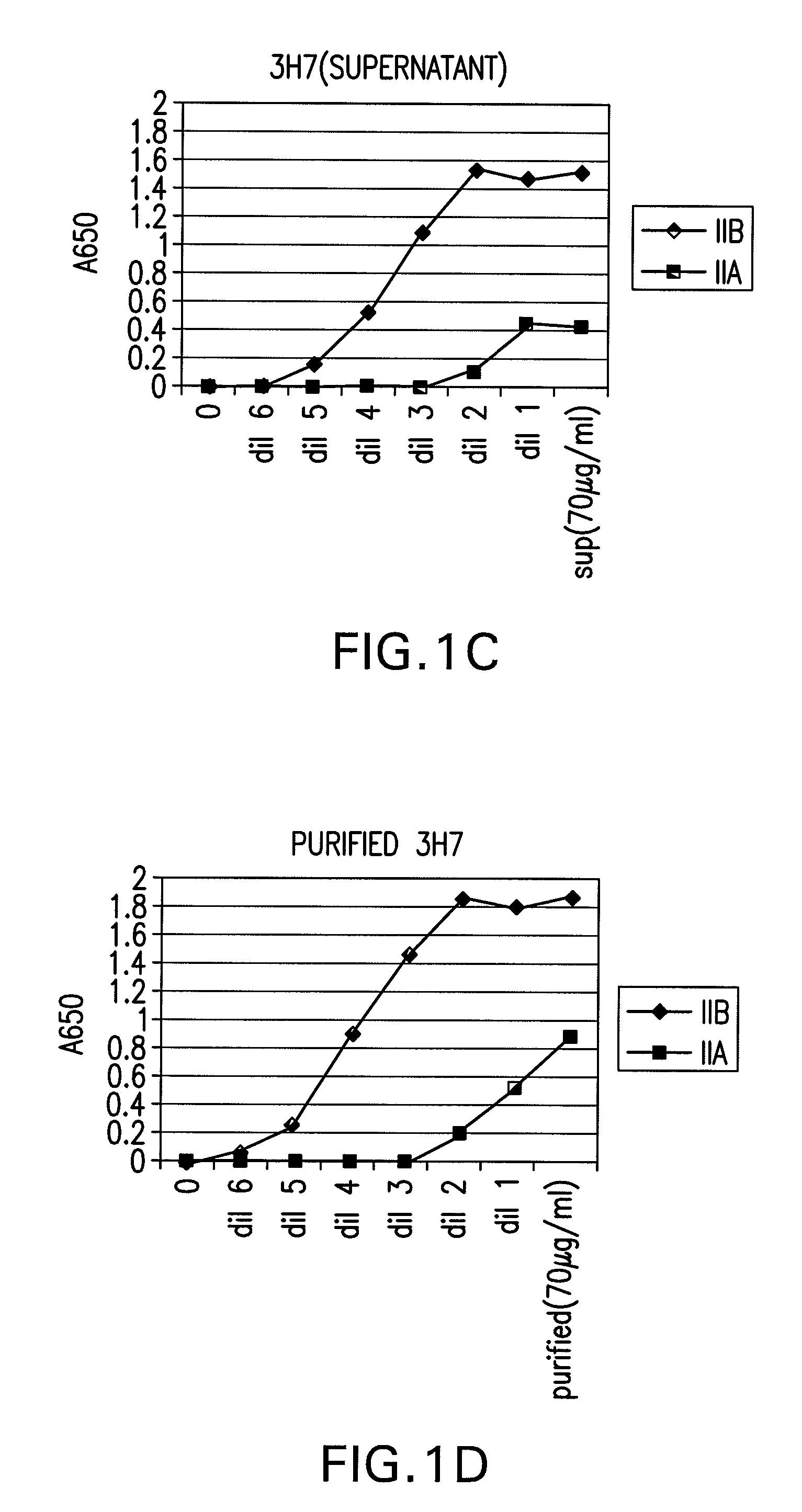
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090053218A1Good curative effectAvoid managementAntibody ingredientsImmunoglobulinsTreatment effectAutoimmune responses
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition.
Owner:MACROGENICS INC
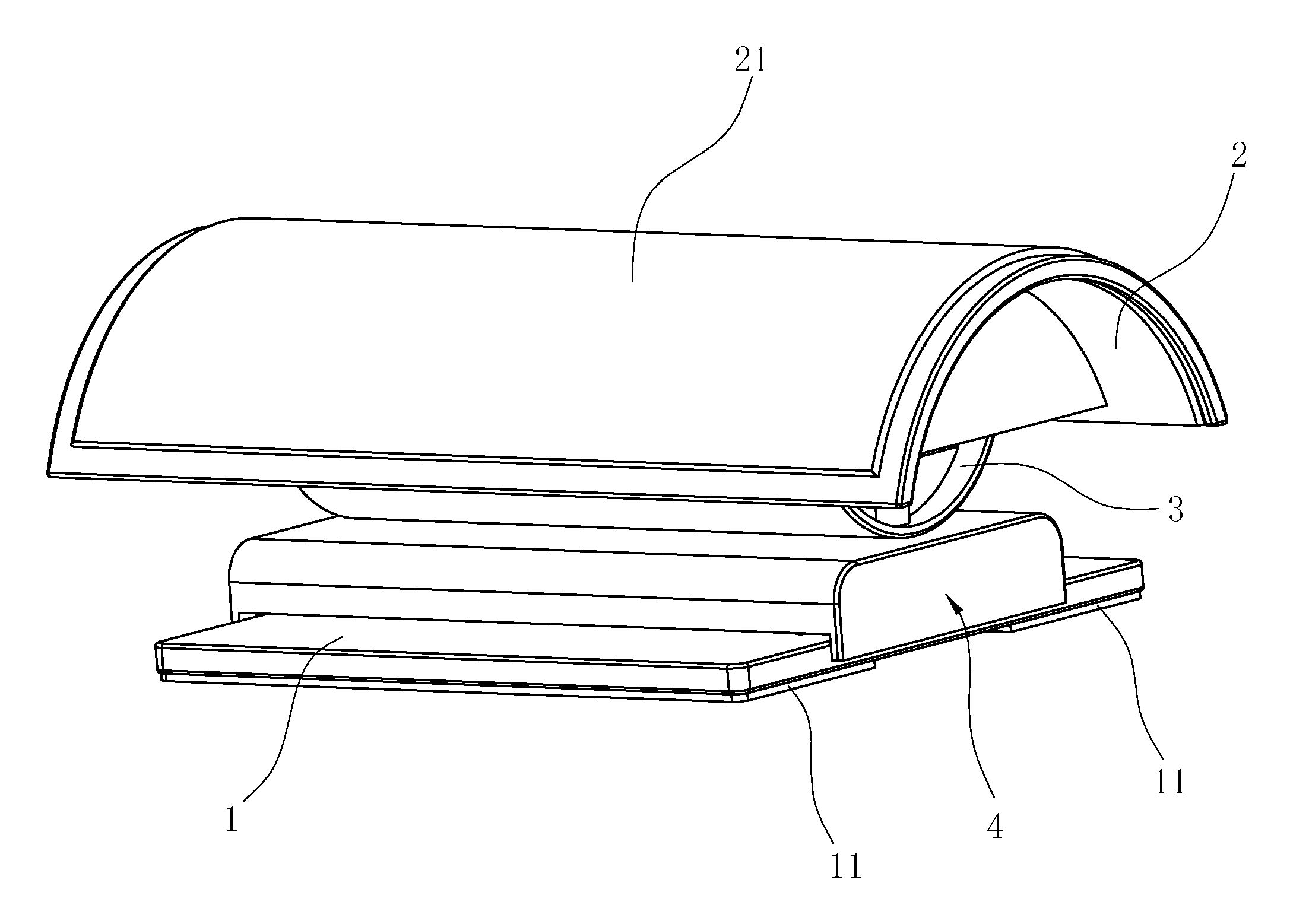
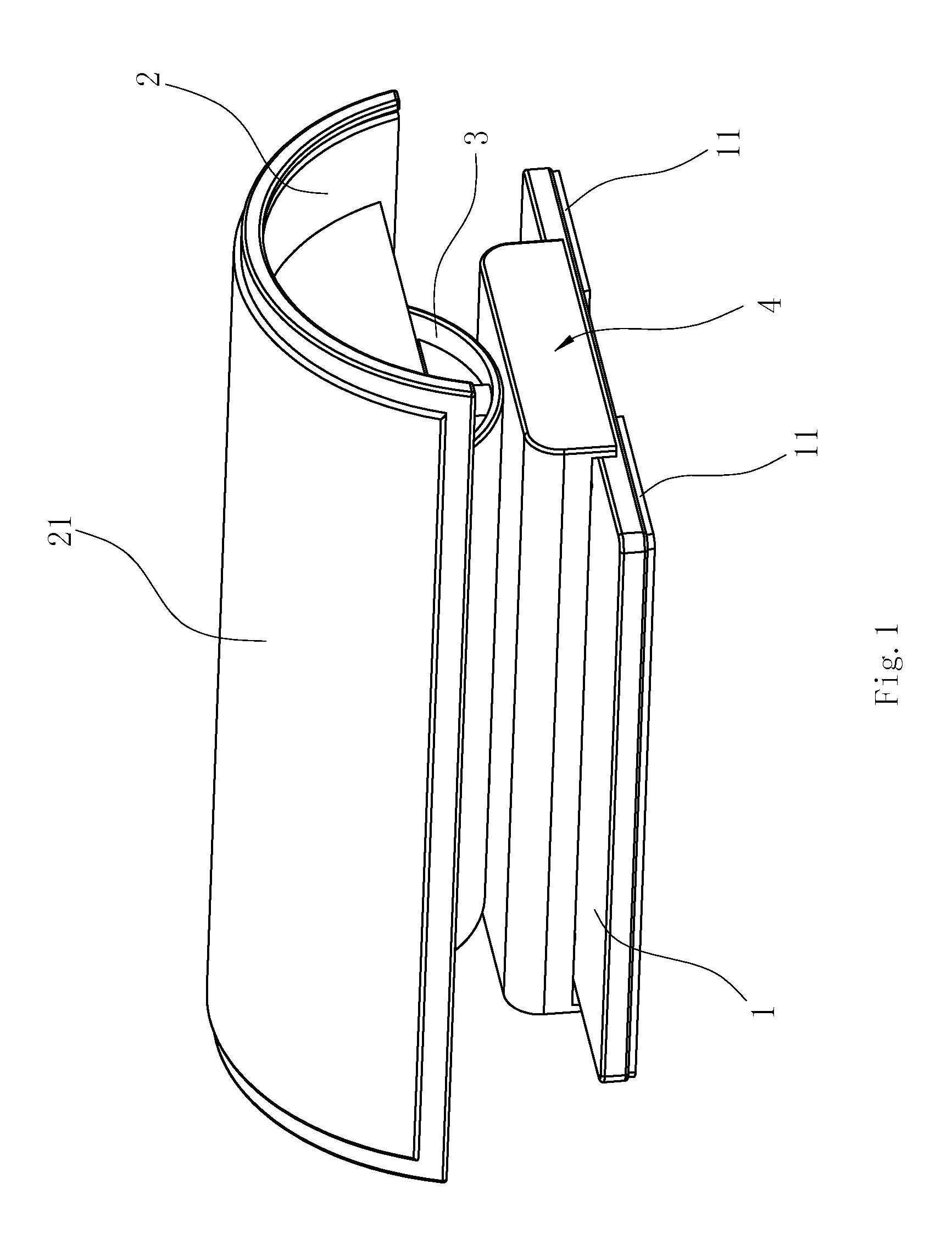
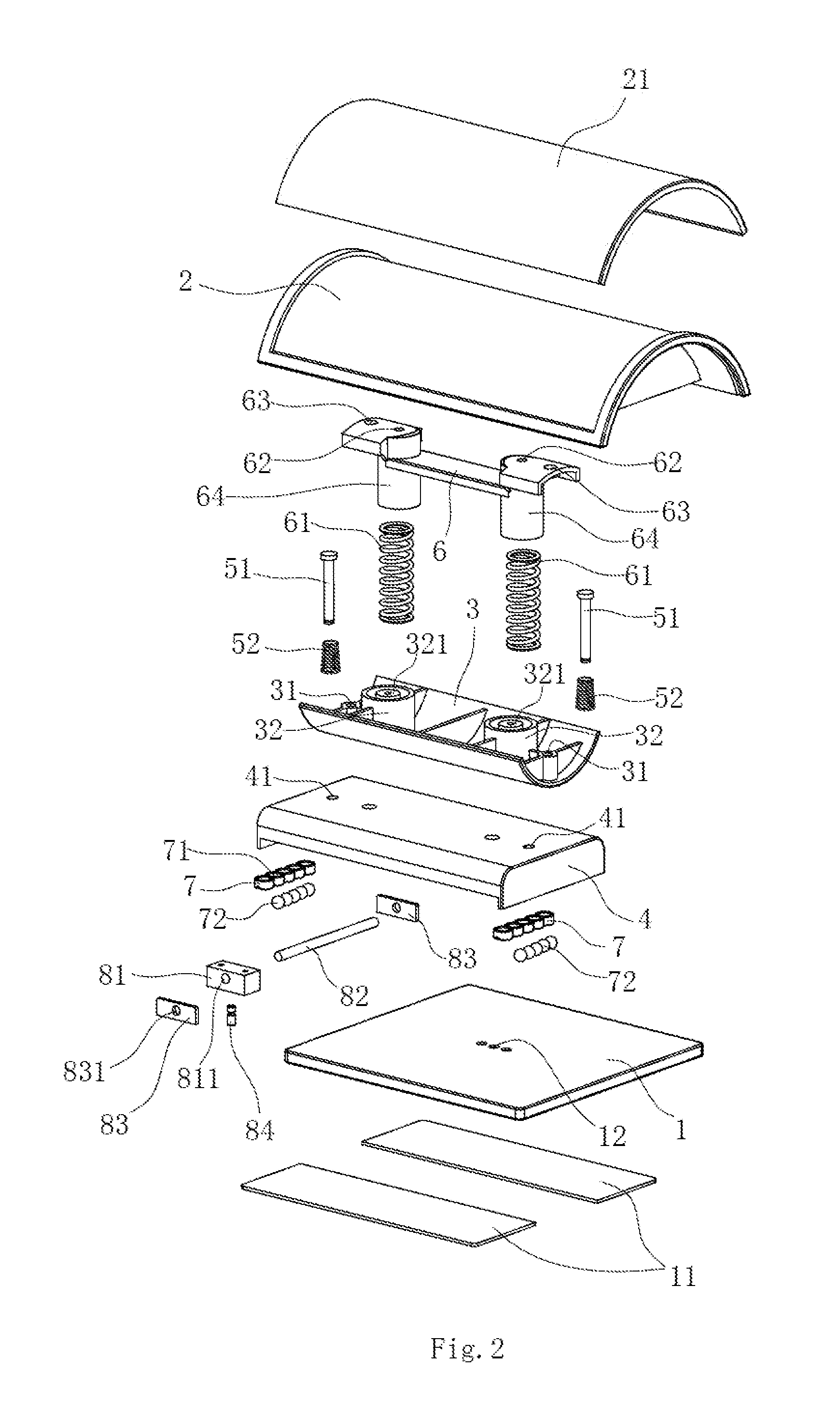
Spine Rehabilitation Exercise Device
ActiveUS20140378283A1Avoid injuryEasy to placeChiropractic devicesStiltsEngineeringTherapeutic effect
A spine rehabilitation exercise device comprises a soleplate; and an exercise mechanism disposed on the soleplate, wherein the exercise mechanism comprises a sliding mechanism movably disposed on the soleplate; a supporting base movably mounted on top of the sliding mechanism, the supporting base being capable of rocking on top of the sliding mechanism; a pillow mounted on the supporting base; and an elastic assembly disposed between the pillow and the supporting base. The whole device has simple structure, convenient assembly, small and compact volume and good mobility and portability; the device can not only have health care and therapeutic effects on spine, but also be used as a pillow on other occasions, thereby having high practicability and better flexibility in use; moreover, the device is not driven by a power supply, so that it is safer and more reliable to use.
Owner:QIU AIGUO
Method and apparatus for electrical stimulation of the lower esophageal sphincter
InactiveUS6901295B2Increasing esophageal sphincter tonePreventing TLESRElectrotherapyArtificial respirationTreatment effectSphincter
A method and apparatus for electrical stimulation of the lower esophageal sphincter (LES) is provided. Electrode sets are placed in the esophagus in an arrangement that induce contractions of the LES by electrical stimulation of the surrounding tissue and nerves. The electrical stimulus is applied by a pulse generator for periods of varying duration and varying frequency so as to produce the desired contractions. The treatment may be short-term or may continue throughout the life of the patient in order to achieve the desired therapeutic effect. The stimulating electrode sets can be used either alone or in conjunction with electrodes that sense esophageal peristalsis. The electrode sets can be placed endoscopically, surgically or radiologically.
Owner:PARAS HLDG LLC
Skin penetrating device and method for subcutaneous solid drug delivery
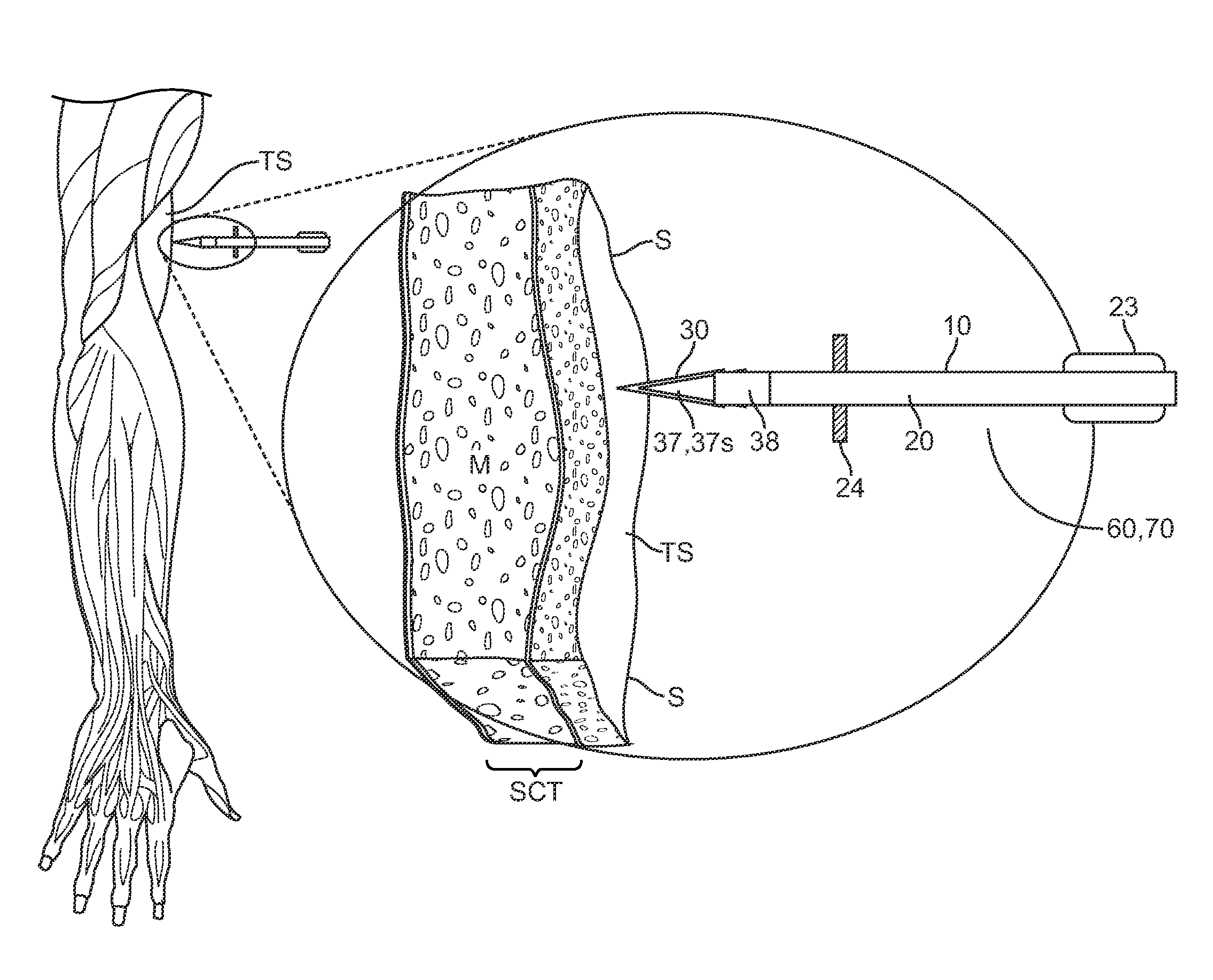
ActiveUS20100204678A1Easy to keepHigh hardnessInfusion syringesMedical devicesTreatment effectUnexpected therapeutic effect
Embodiments described herein provide a skin penetrating device and method for the subcutaneous delivery of therapeutic agents in solid form. One embodiment provides such a device comprising an elongated shaft having proximal and distal ends and a skin penetrating element detachably coupled to the shaft. At least a portion of the penetrating element is fabricated from a solid form therapeutic agent composition that dissolves in body tissue and is absorbed into the blood stream so as to produce a therapeutic effect. The penetrating element has shape for penetrating and lodging beneath the skin when inserted through the skin by force applied from the shaft. The penetrating element is configured to detach from the shaft when the shaft is pulled away from the skin so as to leave the element in place beneath the skin where it is absorbed by body tissue and the therapeutic agent is released.
Owner:INCUBE LABS
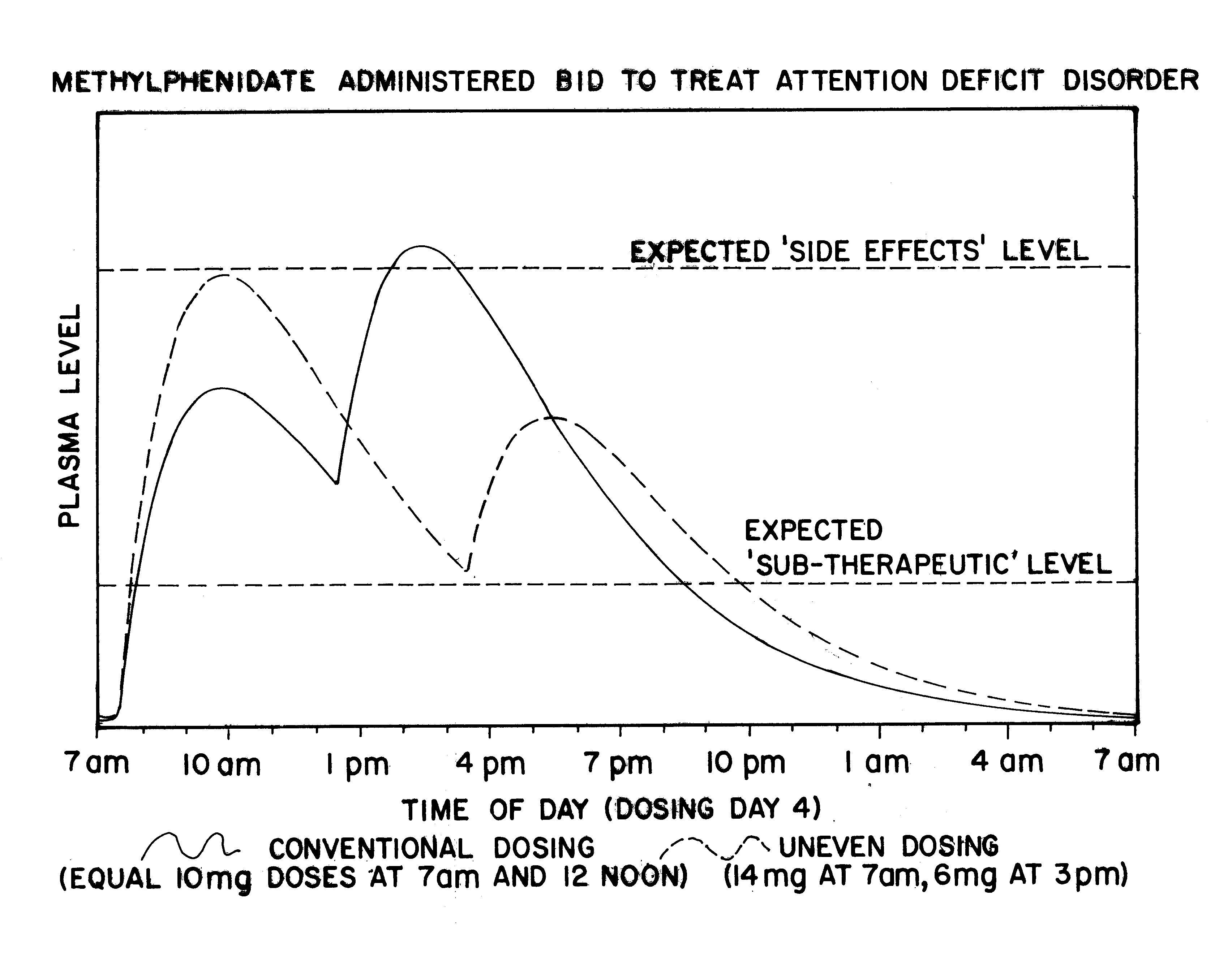
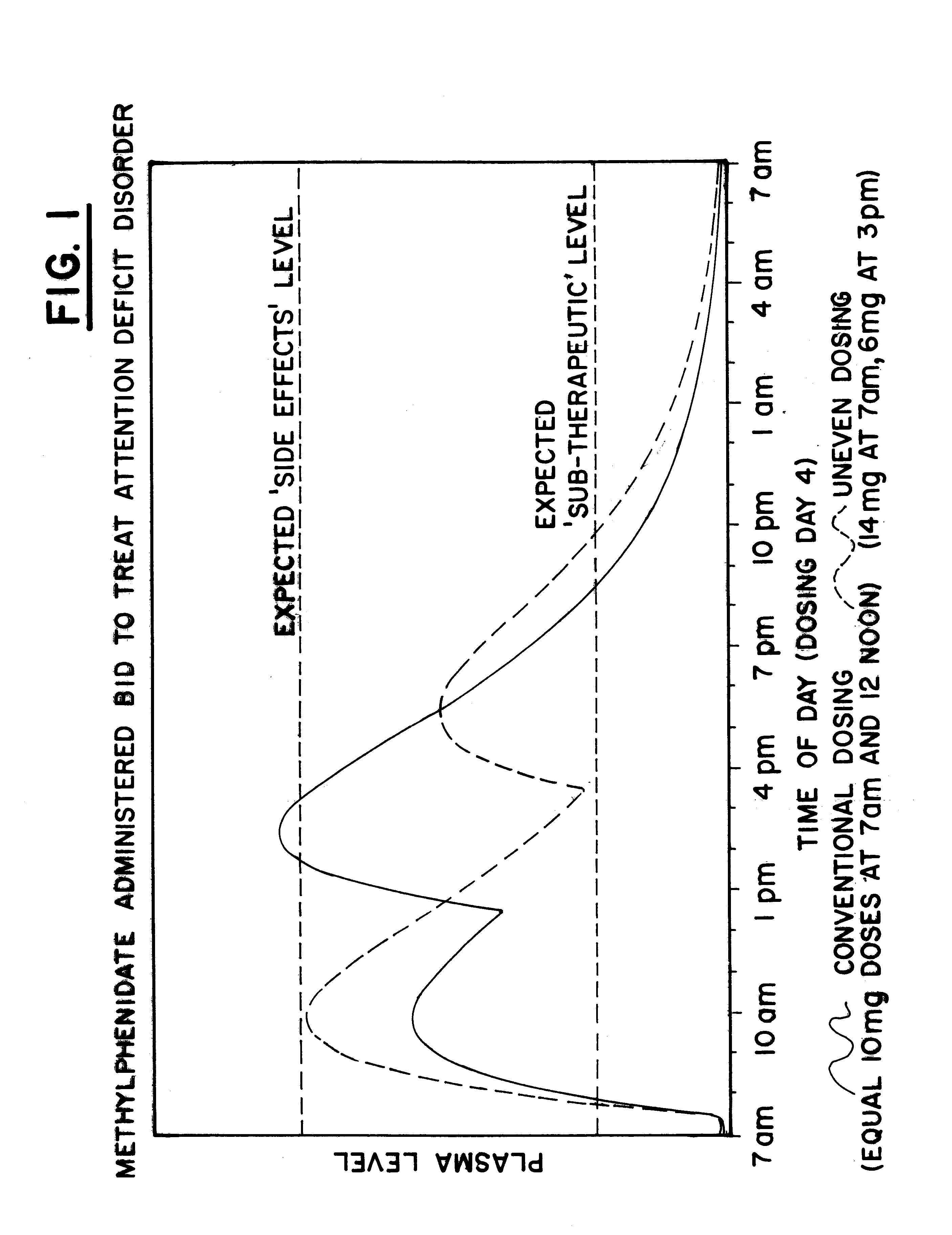
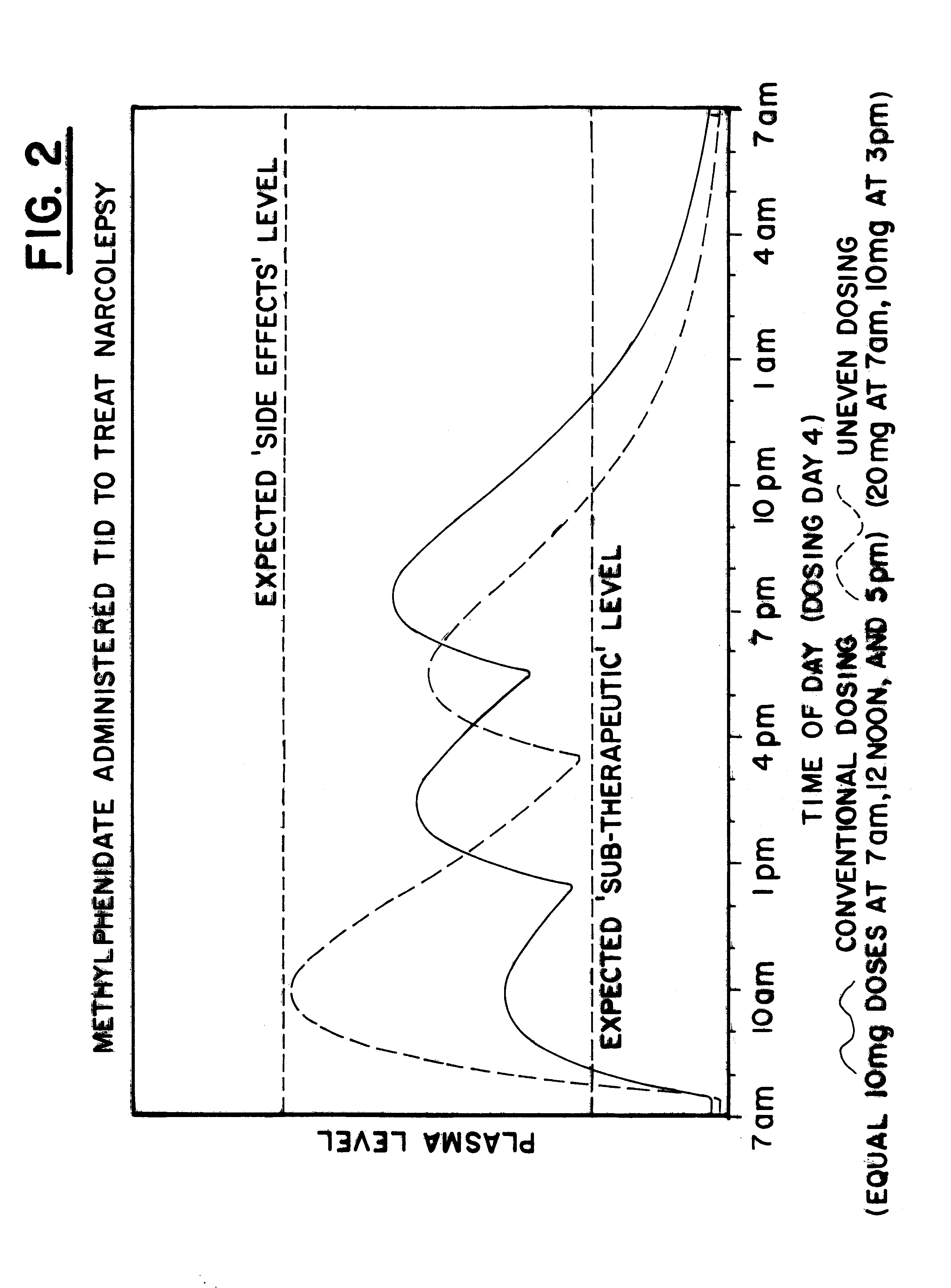
Maximizing effectiveness of substances used to improve health and well being
The present disclosure relates to novel dosage forms, drug delivery regimens, methods and pharmaceutical compositions which optimize the therapeutic effects of active therapeutic substances through the application of the concept of uneven dosing.
Owner:LUMARA HEALTH IP
Composition and method for reducing the risk or progression of cardiovascular, glaucoma, tardive dyskinesia and other diseases
InactiveUS20040087479A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided, the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, betacarotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such a grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
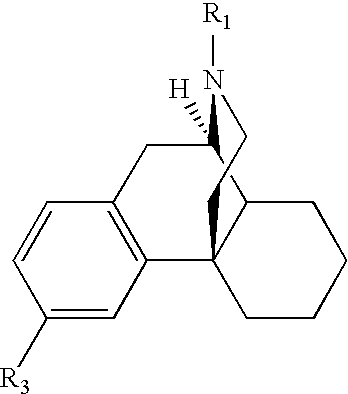
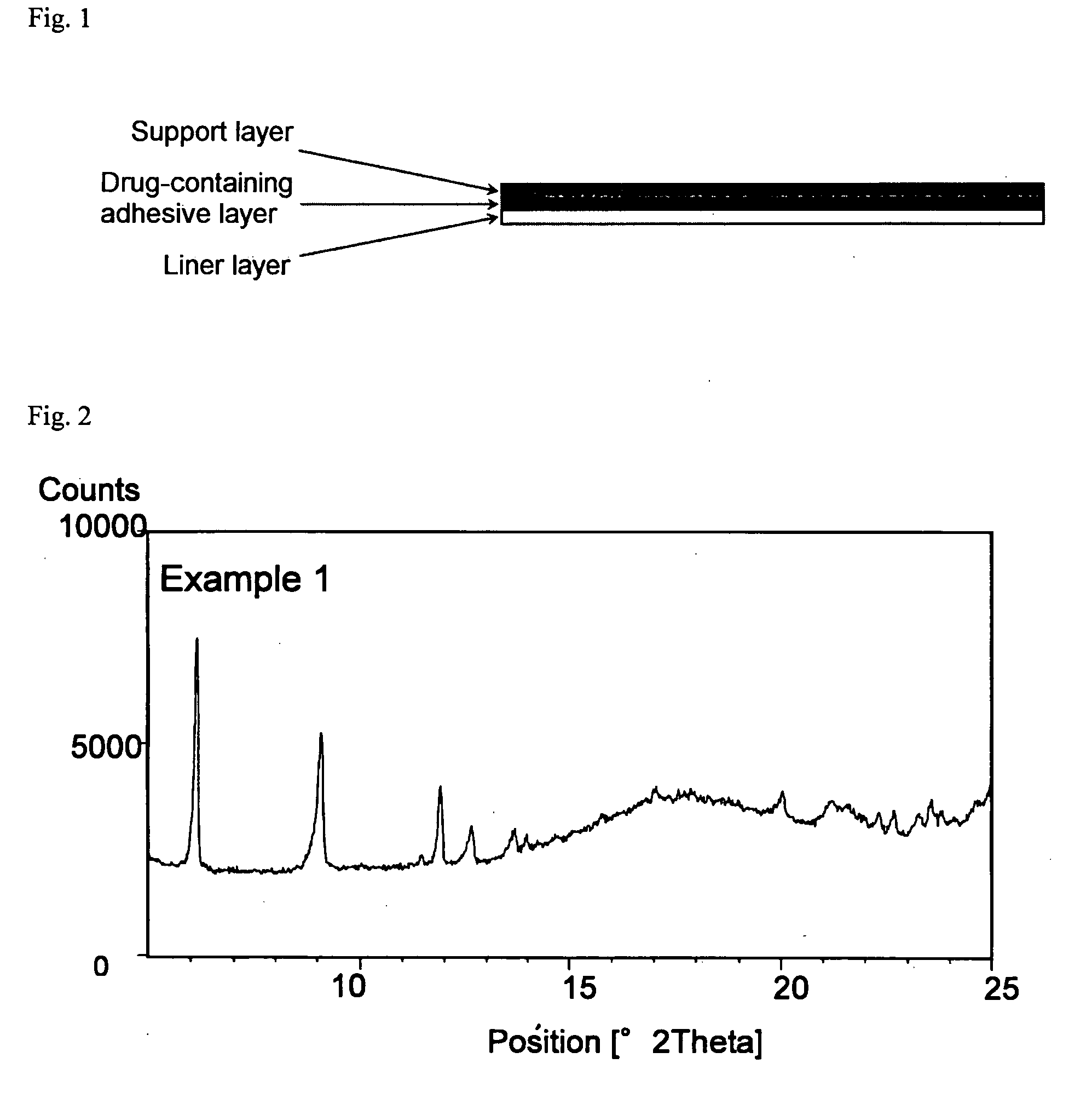
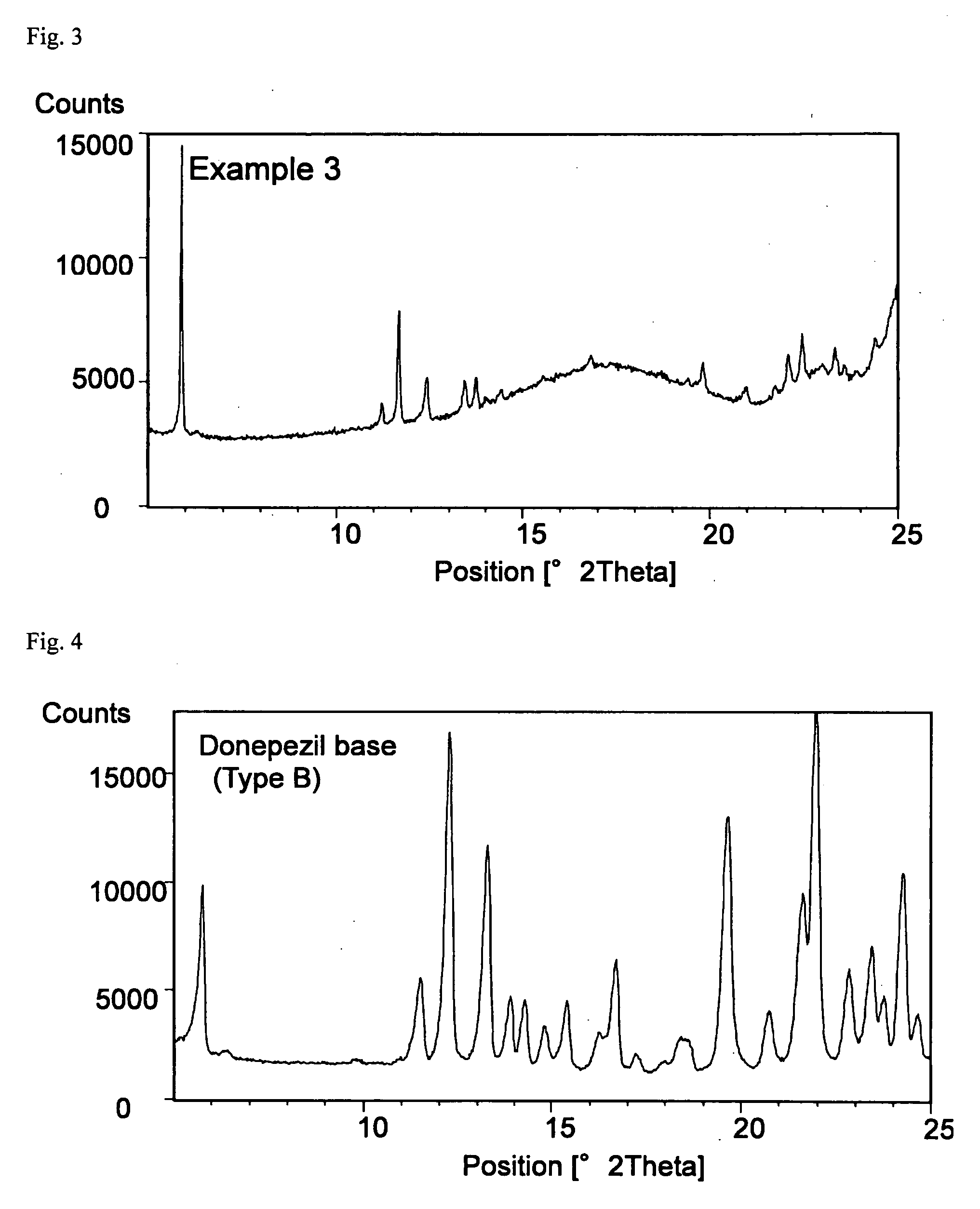
Transdermally absorbable Donepezil Preparation
InactiveUS20090175929A1Improve drug stabilityLess localized stimulationBiocideNervous disorderChemistryUnexpected therapeutic effect
Disclosed is a donepezil-containing transdermally absorbable preparation which develops reduced adverse side effects and shows a satisfactory level of therapeutic effect. The preparation comprises an adhesive and a donepezil component (containing crystalline donepezil having type-B crystal polymorphism) and / or a salt thereof, wherein the donepezil component or the salt thereof is contained in an amount of 9 to 50% by mass relative to the total weight of the adhesive. The preparation (particularly, one having a non-aqueous adhesive layer) shows an excellent penetration of donepezil and / or a salt thereof into the skin, retains good stability of the active ingredient therein, and is remarkably reduced in local stimulation and adverse side effects.
Owner:HISAMITSU PHARM CO INC
Stabilized biodegradable neurotoxin implants
InactiveUS20050232966A1Patient compliance is goodReduce complicationsAntibacterial agentsBacterial antigen ingredientsOligomerTherapeutic effect
Biodegradable neurotoxin implants and methods of making and using such implants are provided. Biodegradable neurotoxin implants include a neurotoxin, a biodegradable polymer component, and an acidity regulating component. The biodegradable polymer component is effective in controlling the release of the neurotoxin from the implant when the implant is located in a patient's body. The acidity regulating component is effective in maintaining a pH of the implant in a desired range that may be effective in stabilizing the neurotoxin as the implant biodegrades when the implant is located in a patient's body. In one embodiment, an implant includes a botulinum toxin, a biodegradable polymer, and either monomers from which a biodegradable polymer is derived or oligomers including monomeric units substantially identical to a monomer from which a biodegradable polymer is derived, or a combination of such monomers and oligomers. The oligomers and biodegradable polymer may be derived from a single type of monomer. The implants disclosed herein may be administered to a human or animal patient in which a therapeutic effect is desired for prolonged periods of time.
Owner:ALLERGAN INC
Methods for the identification of polypeptide antigens associated with disorders involving aberrant cell proliferation and compositions useful for the treatment of such disorders
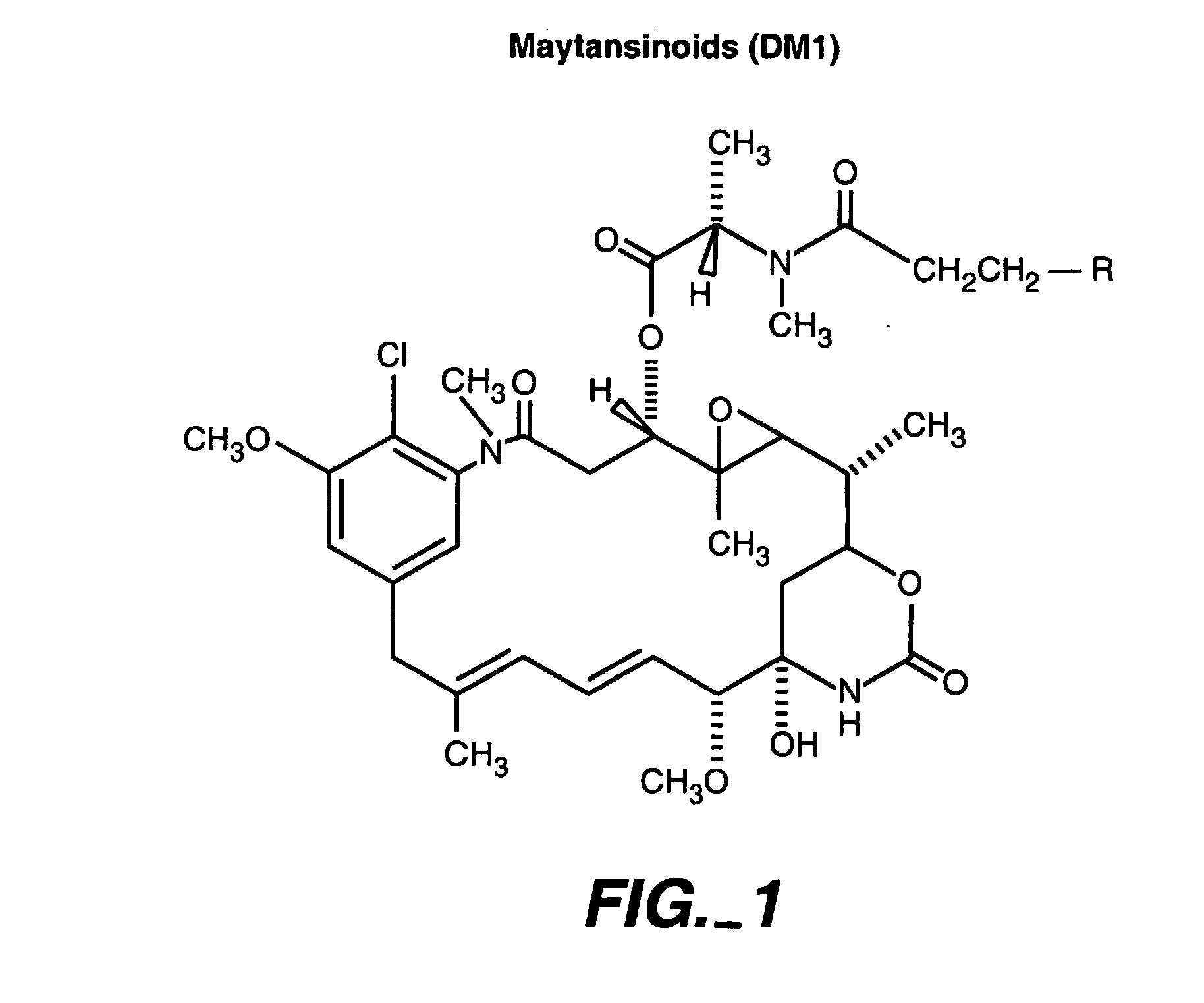
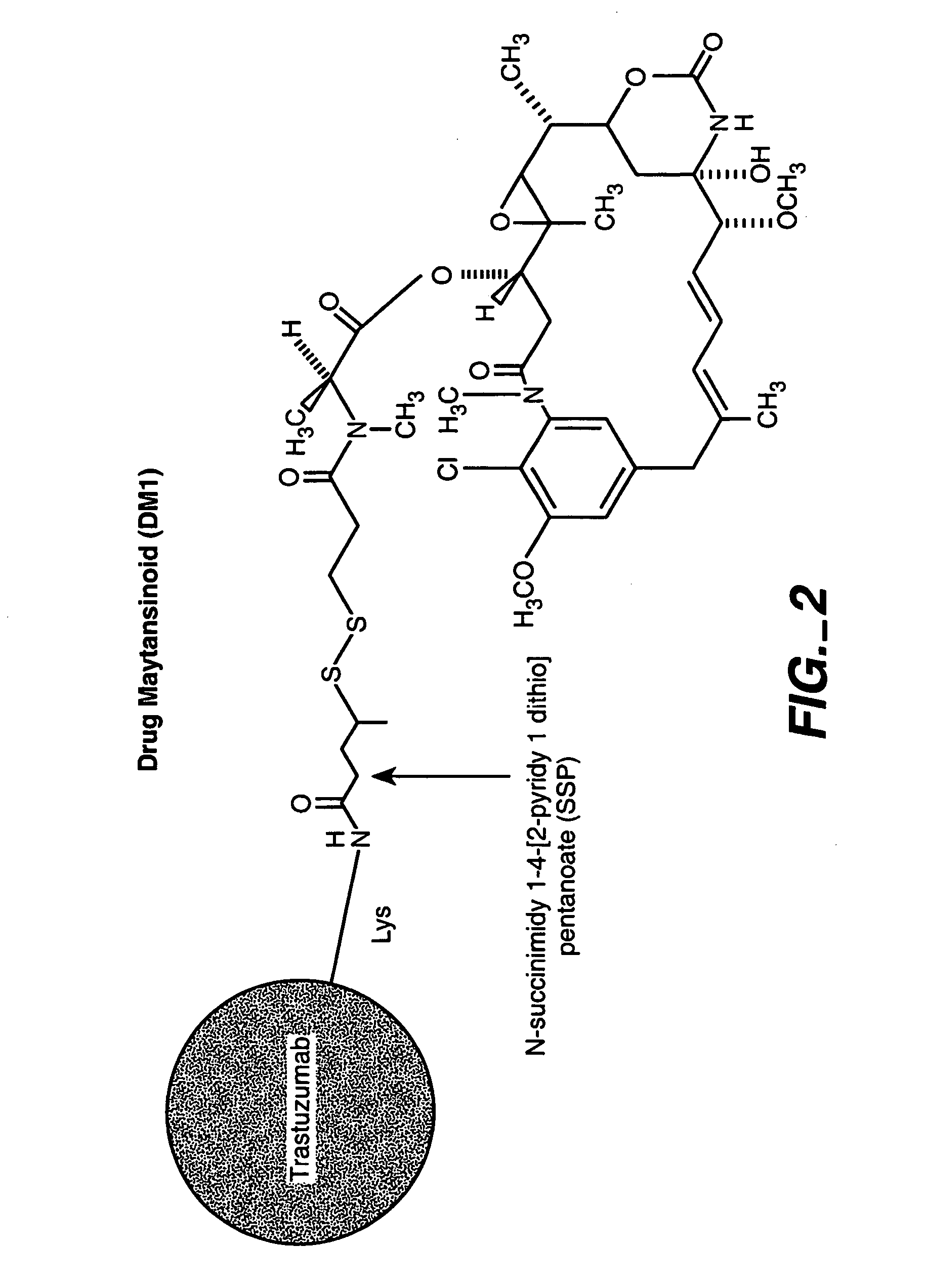
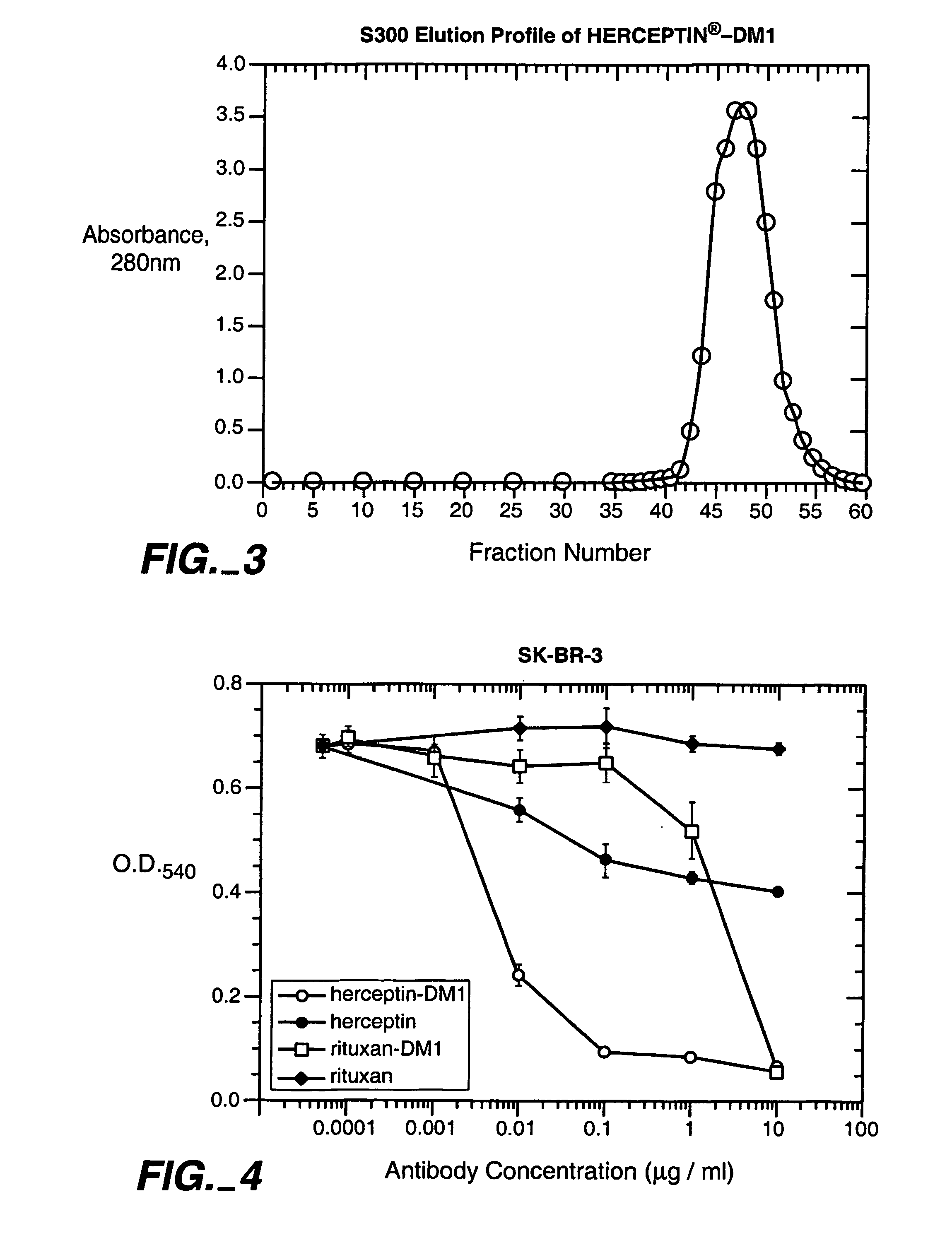
Methods and compositions for the development of effective cancer therapies using mitotic inhibitors which have limited general toxicity to normal, non-cancerous cells and tissues are provided. The methods and compositions utilize cytotoxic compounds comprised of a cell-binding agent (e.g., antibodies) conjugated to an anti-mitotic compound (e.g., maytansinoids). The invention further provides antibodies which are substantially incapable of inducing antibody-dependent cell-mediated cytotoxicity (ADCC) and / or complement dependent cytotoxicity (CDC), thereby ensuring that the therapeutic effect is mediated primarily by the anti-mitotic component of the cytotoxic compound, rather than by indirect cell killing via ADCC and / or CDC. The antibodies of the invention further are capable of differentiating between polypeptide antigens which are more highly expressed on proliferating cancer cells as compared to proliferating non-cancer cells.
Owner:GENENTECH INC
Microneedle array chip, device and patch for transdermal drug delivery utilizing the same, and preparation method therof
InactiveUS20110237925A1Easy to punctureEasy to adjustElectrolysis componentsDecorative surface effectsAcute angleTherapeutic effect
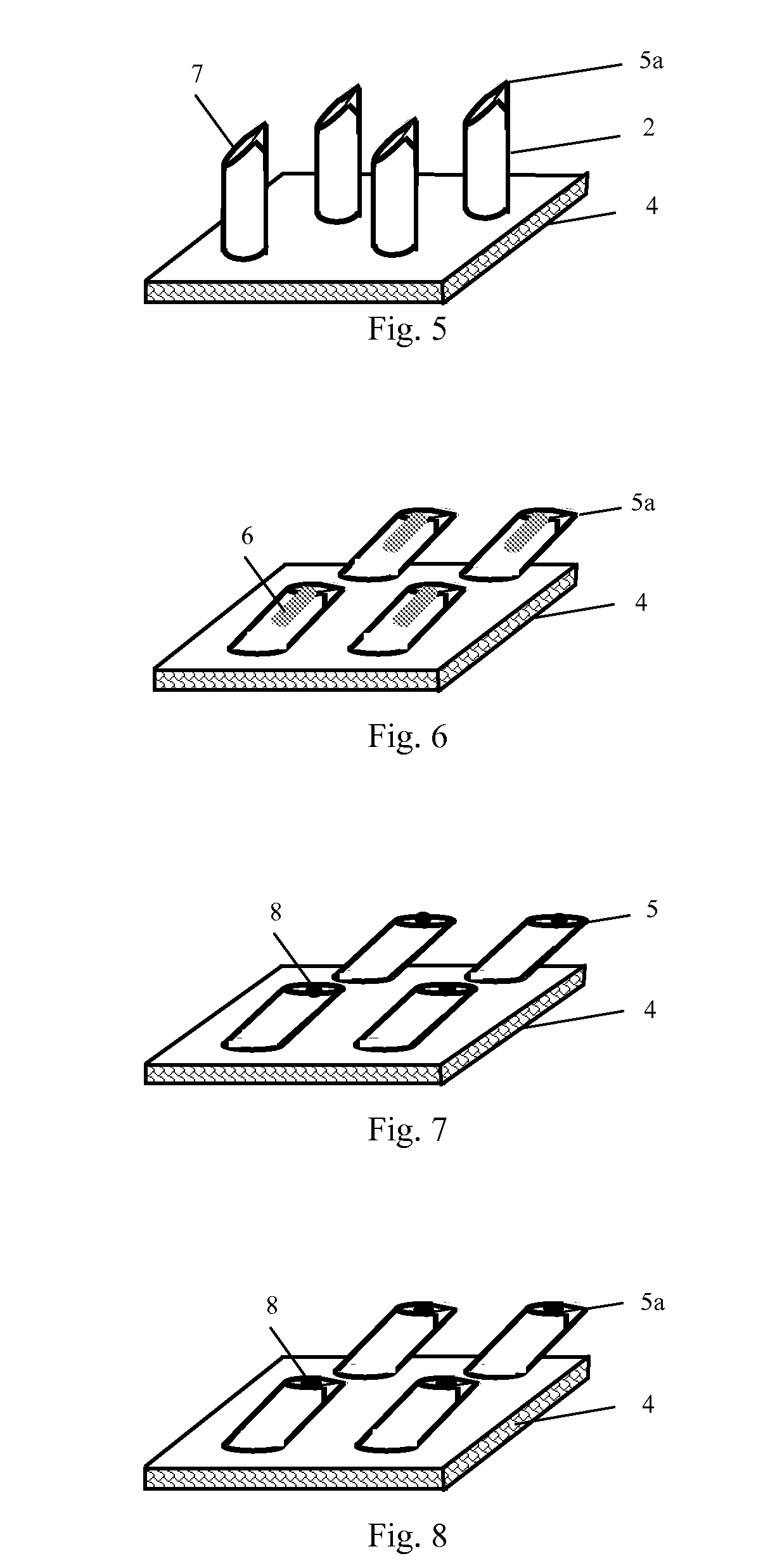
The invention discloses a microneedle array chip comprising metal microneedles and a substrate, wherein the microneedle consists of a needle head with a tip at its top, a needle bar and a needle seat, and is fixed onto the substrate via the needle seat; and the needle bar of the metal microneedle, having a cylindrical or conical shape, is inclined toward the substrate at a preset angle, and the needle head has a conical shape, or the upper surface of the tip is an oval plane or oval ring plane parallel to the substrate or inclined toward it at a preset acute angle. The metal microneedles in the microneedle array chip of the invention have firm structures to avoid fracture, and have sharp tips to facilitate puncturing. The maximal puncturing depth of the microneedles is easy to adjust and control. The microneedles in the array have good uniformity, and are safe and reliable to use. The hollow microneedles, like conventional syringe needles, have lateral openings, and thus can effectively avoid the blockage of the infusion poles by skin, thereby facilitating rapid diffusion and absorption of drugs, and resulting in significant therapeutic effects.
Owner:TSINGHUA UNIV
Concomitant drugs
InactiveUS20050197376A1Good treatment effectReduce the amount requiredBiocideMetabolism disorderConcomitant drugGlycosidase inhibitor
This invention provides a pharmaceutical agent containing, in combination, a sulfonamide compound and other therapeutic agent, preferably, at least one compound represented by the formula (I): R1—SO2—NH—CO-A1-CH2—R2 [each symbol is as defined in the specification] or a pharmaceutically acceptable salt thereof, and at least one pharmaceutical agent selected from the group consisting of an α-glucosidase inhibitor, an insulin secretagogue, a sulfonylurea and a biguanide, which has a superior therapeutic effect.
Owner:ASTELLAS PHARMA INC
Ultrasound moxibustion method and device
InactiveUS20090198157A1Finish quicklyRapid meanUltrasound therapySurgeryEnergy transferTreatment effect
The present invention is directed towards a device and methods for the use of ultrasonic energy for providing therapeutic effects by heating and stimulating bioactive points (BAPs) in the body of a patient. The device includes an ultrasound generator driving an ultrasound transducer. The ultrasound transducer generates ultrasound waves which are focused to a focal point through a lens which is attached to the ultrasound generator. A coupling medium is used displace any air between the lens and the patient's skin surface to allow ultrasonic energy transfer into the patient. The device is pressed against the patient's skin surface and the unit is energized using a foot or hand activated switch.
Owner:BABAEV EILAZ
Method and apparatus for application of a neural stimulus
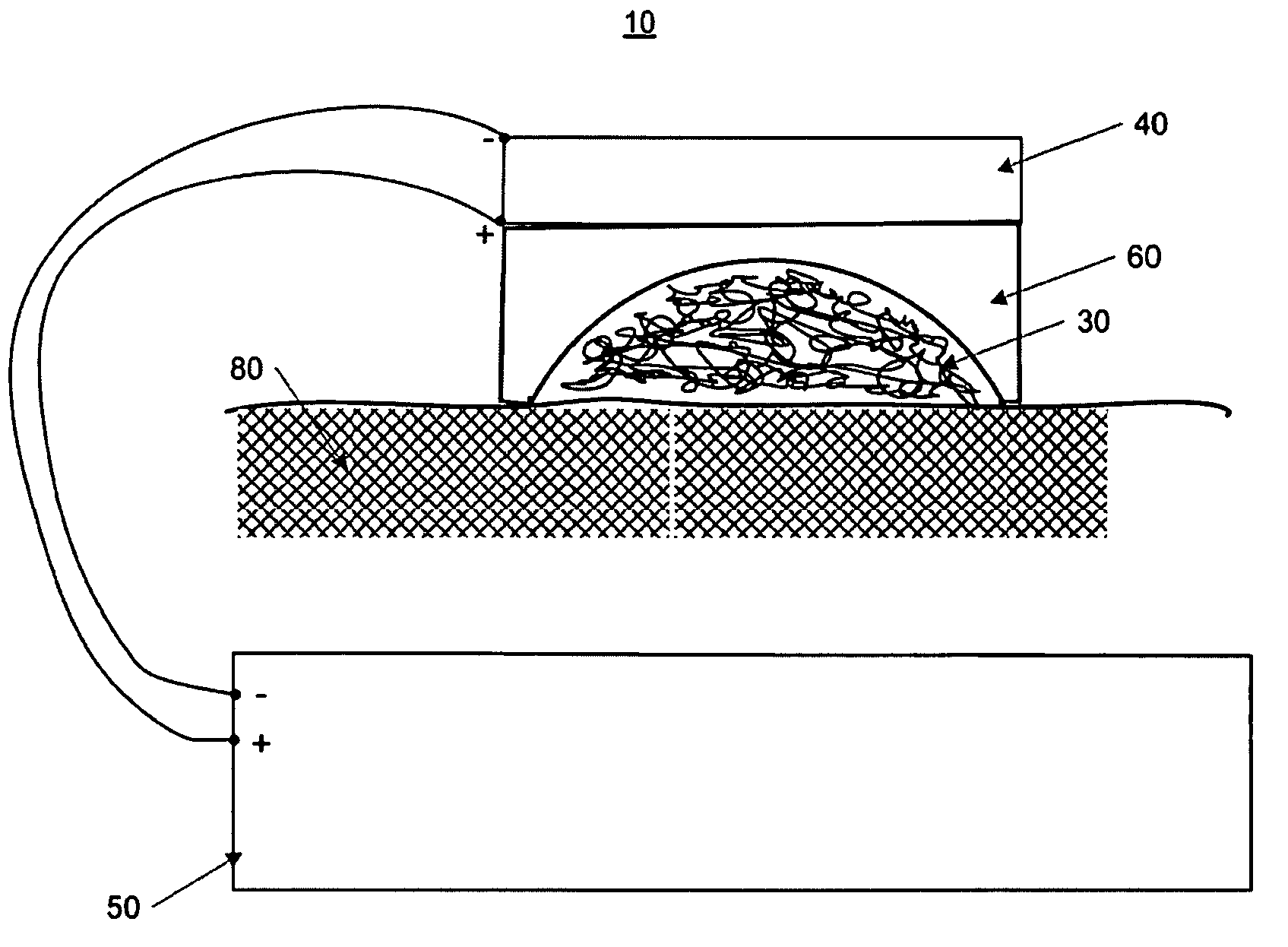
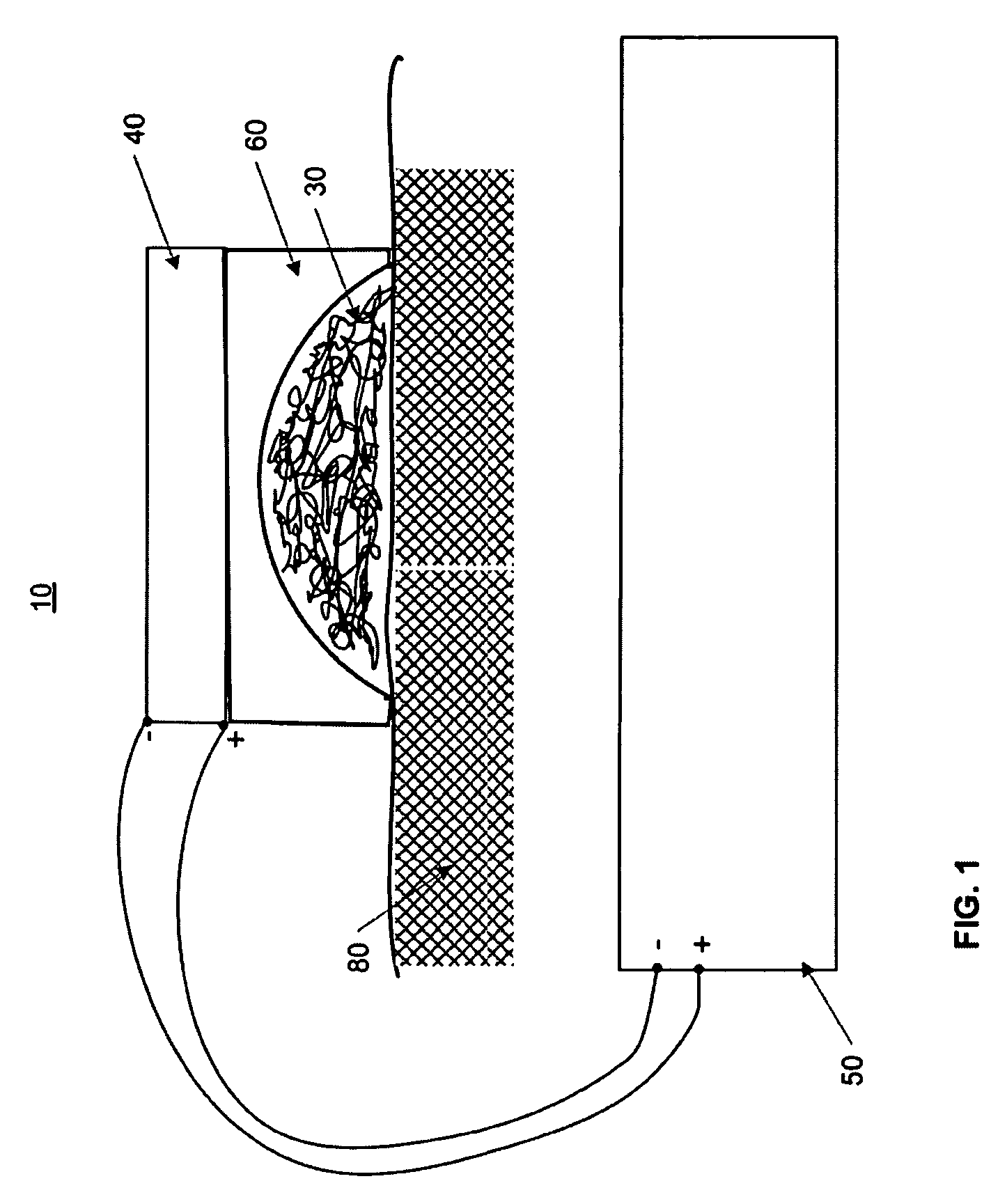
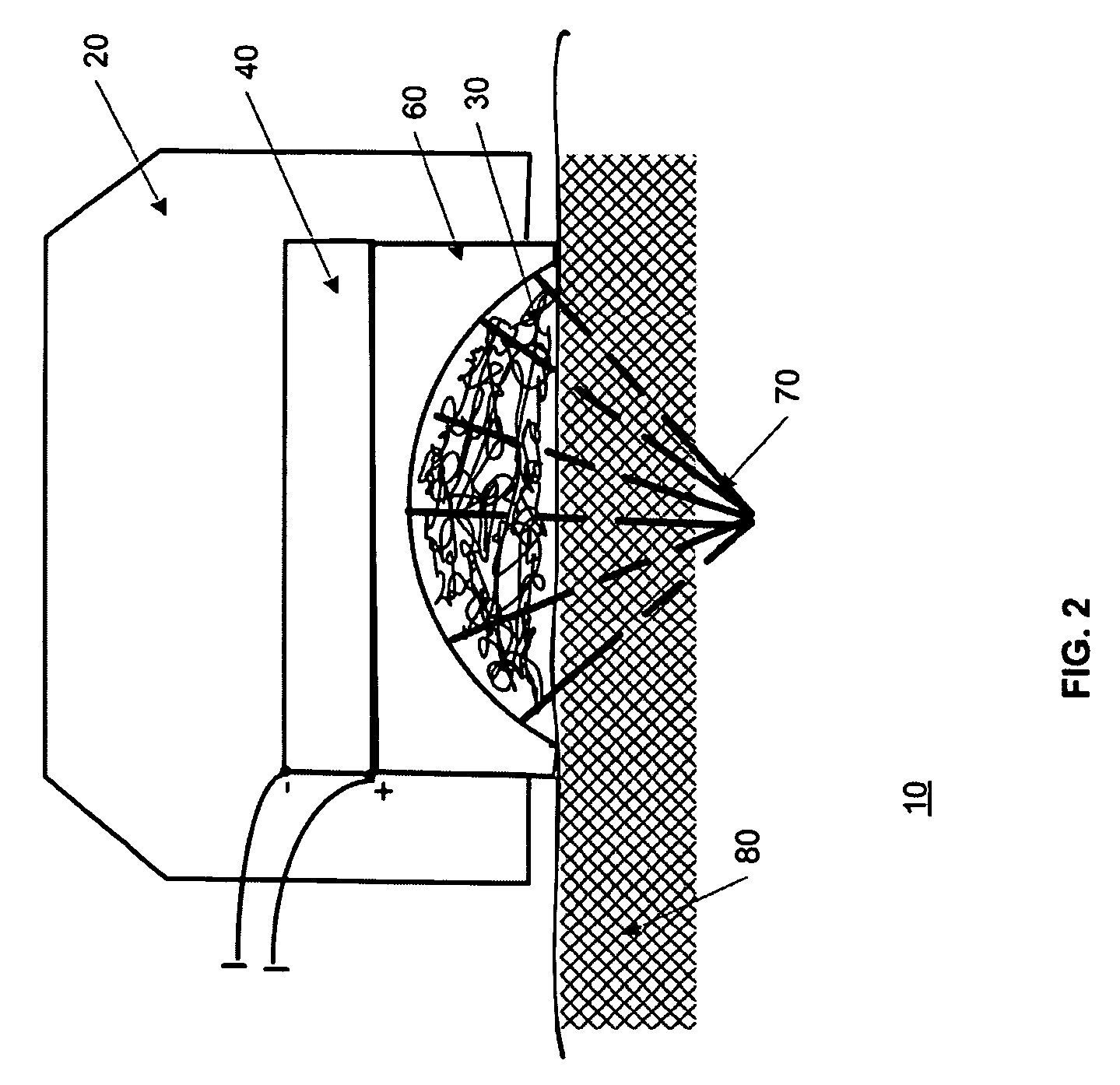
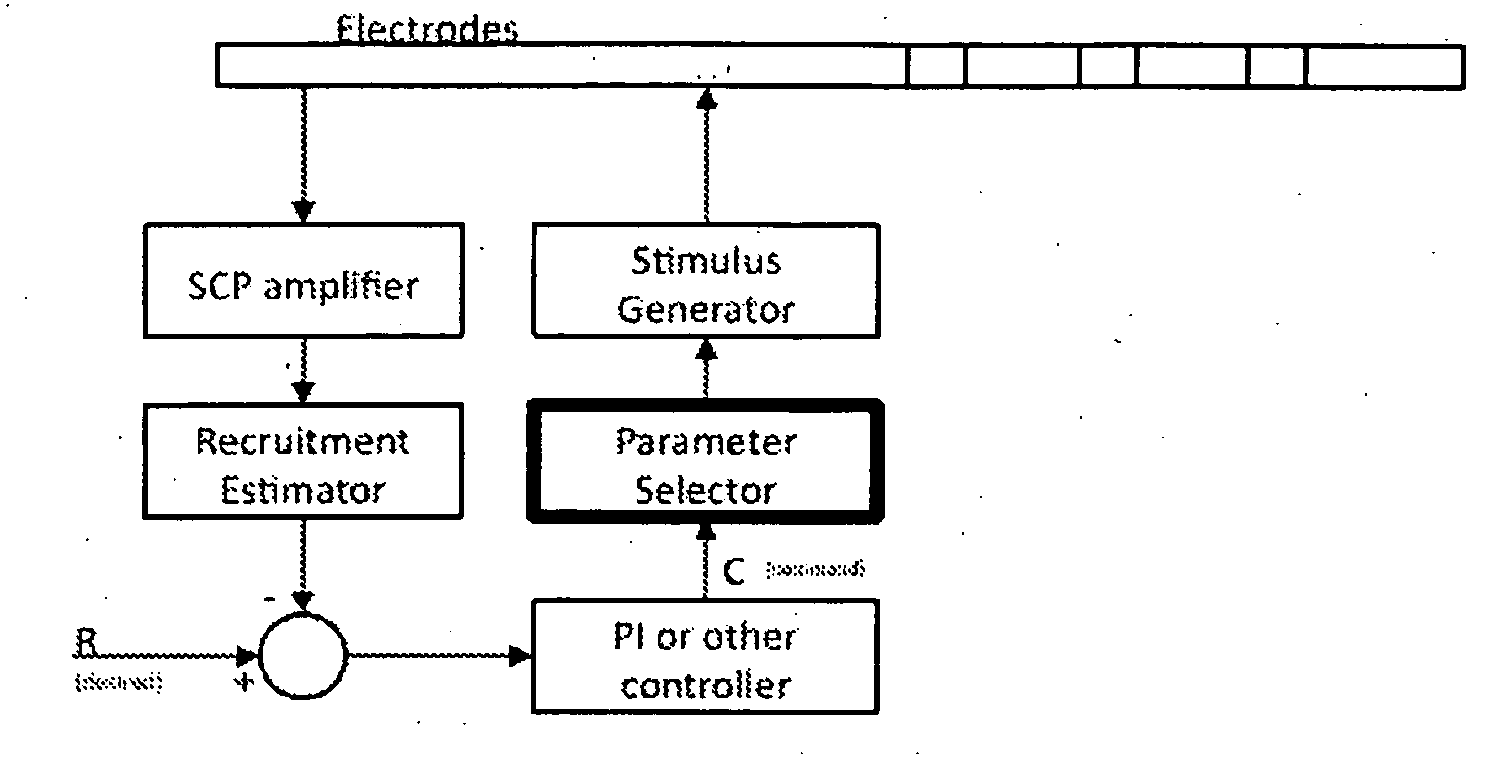
A method of applying a neural stimulus with an implanted electrode array involves applying a sequence of stimuli configured to yield a therapeutic effect while suppressing psychophysical side effects. The stimuli sequence is configured such that a first stimulus recruits a portion of the fibre population, and a second stimulus is delivered within the refractory period following the first stimulus and the second stimulus being configured to recruit a further portion of the fibre population. Using an electrode array and suitable relative timing of the stimuli, ascending or descending volleys of evoked responses can be selectively synchronised or desynchronised to give directional control over responses evoked.
Owner:SALUDA MEDICAL PTY LTD
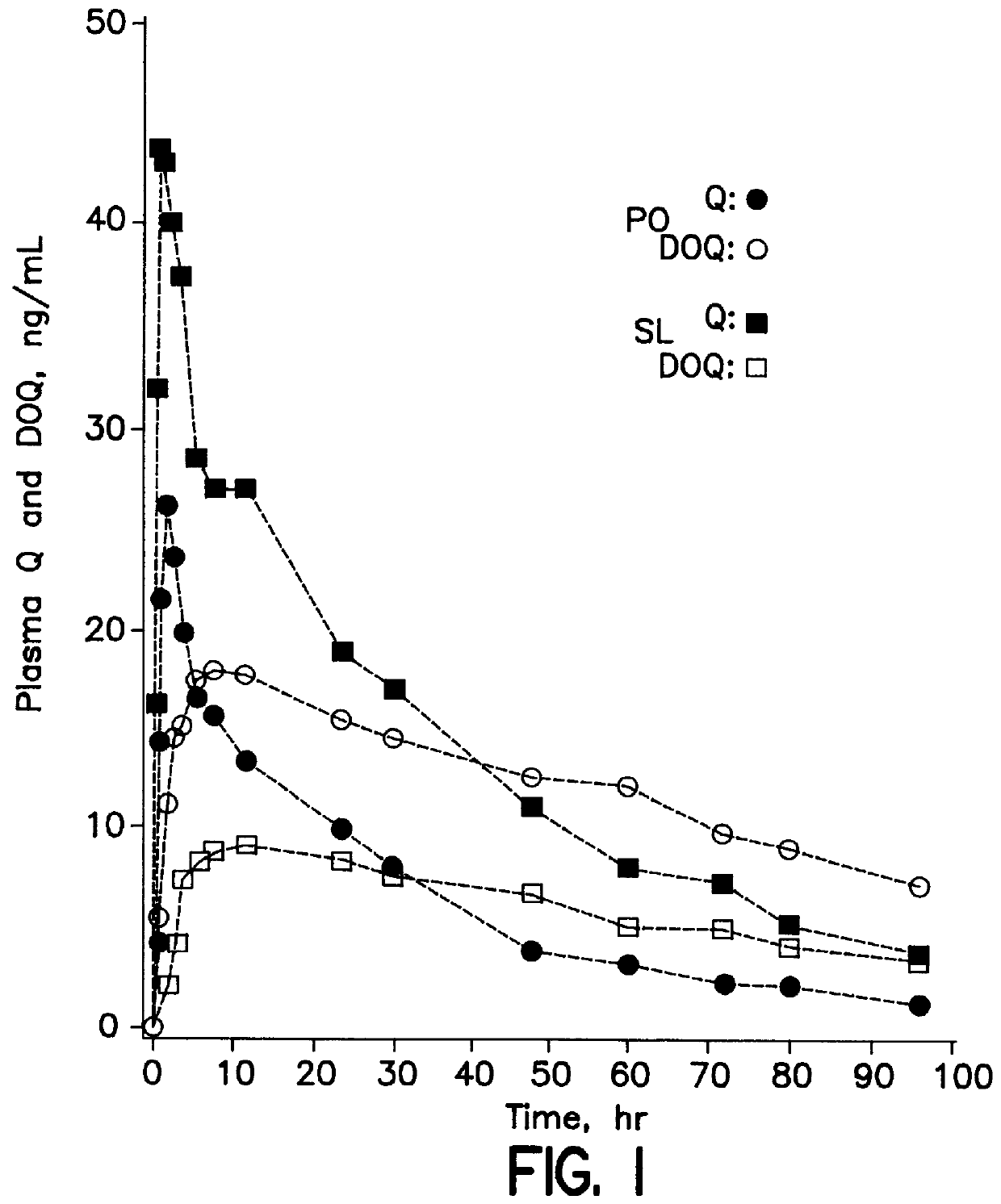
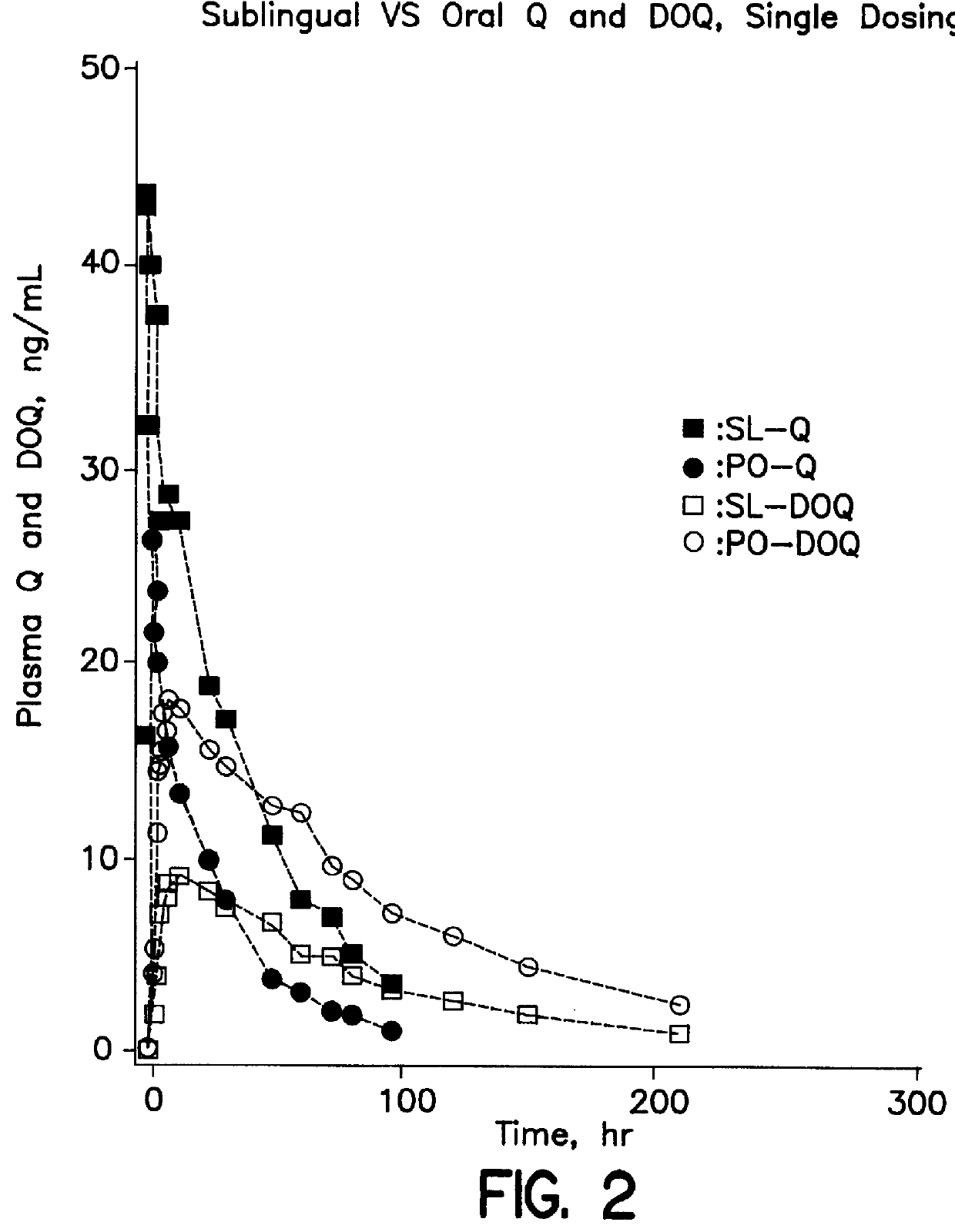
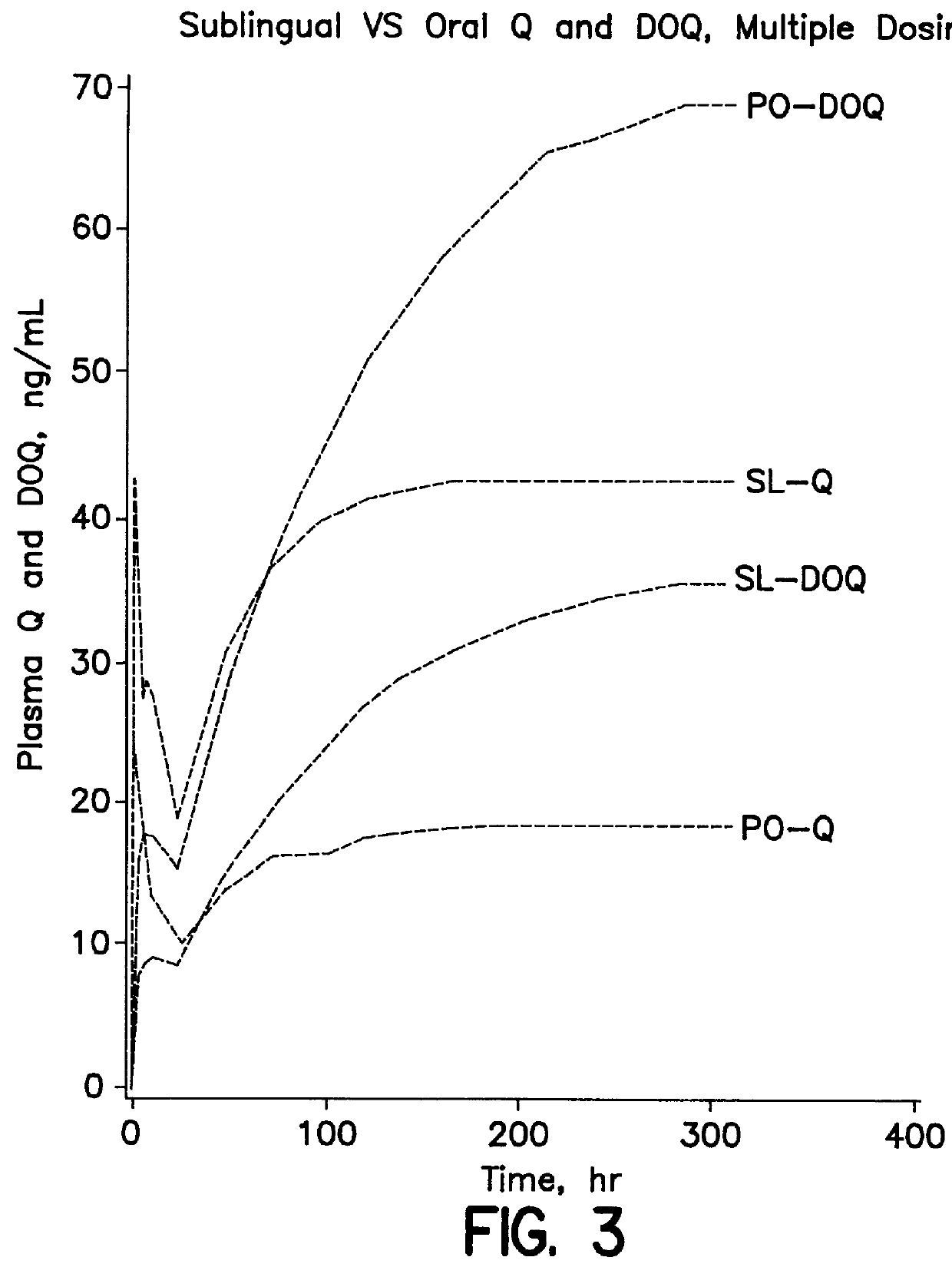
Dosing method of administering medicaments via inhalation administration or skin administration
InactiveUS6140323ALower metabolismReducing DOQ levelBiocideAnimal repellantsCo administrationSide effect
A method of therapeutically administering certain medicaments in order to maximize the desired effects and minimize the unwanted metabolite effects on the human body, including the central nervous system, in order to maximize therapeutic effects, such as anti-anxiety, anticonvulsant and hypnotic effects, and minimize unwanted side effects, such as ataxic and incoordination effects, of the medicament. Also, a method of inhalation administration or skin administration of certain medicaments in order to decrease metabolism of the medicaments to unwanted metabolites.
Owner:ELLINWOOD JR EVERETT H +1
Sentinel node identification using fluorescent nanoparticles
ActiveUS20080255459A1Ultrasonic/sonic/infrasonic diagnosticsPowder deliverySentinel nodeTreatment effect
Various compositions, methods, and devices are provided that use fluorescent nanoparticles, which can function as markers, indicators, and light sources. The fluorescent nanoparticles can be formed from a fluorophore core surrounded by a biocompatible shell, such as a silica shell. In one embodiment, the fluorescent nanoparticles can be delivered to tissue to mark the tissue, enable identification and location of the tissue, and / or illuminate an area surrounding the tissue. In another embodiment, the fluorescent nanoparticles can be used on a device or implant to locate the device or implant in the body, indicate an orientation of the device or implant, and / or illuminate an area surrounding the device or implant. The fluorescent nanoparticles can also be used to provide a therapeutic effect.
Owner:ETHICON ENDO SURGERY INC
Modified release analgesic suspensions
A pharmaceutical dosage form comprising non-steroidal-anti-inflammatory drugs, in particular propionic acid derivatives such as ibuprofen, along with a second active ingredient having a shorter therapeutically effective plasma concentration duration, such as phenylephrine, and methods of administering the same are provided. This method provides improved therapeutic effect, in particular pain relief along with decongestant relief, over extended time periods.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
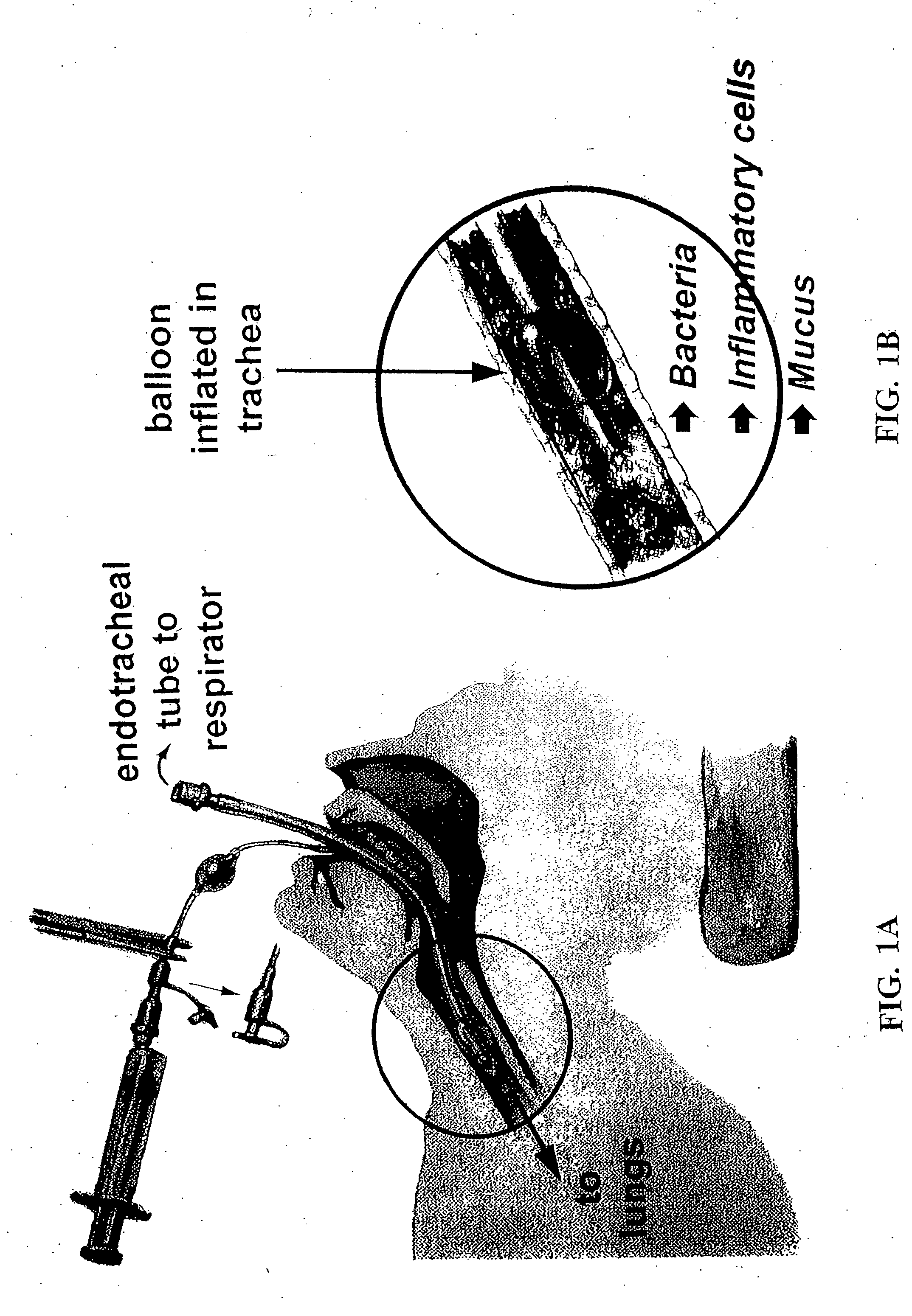
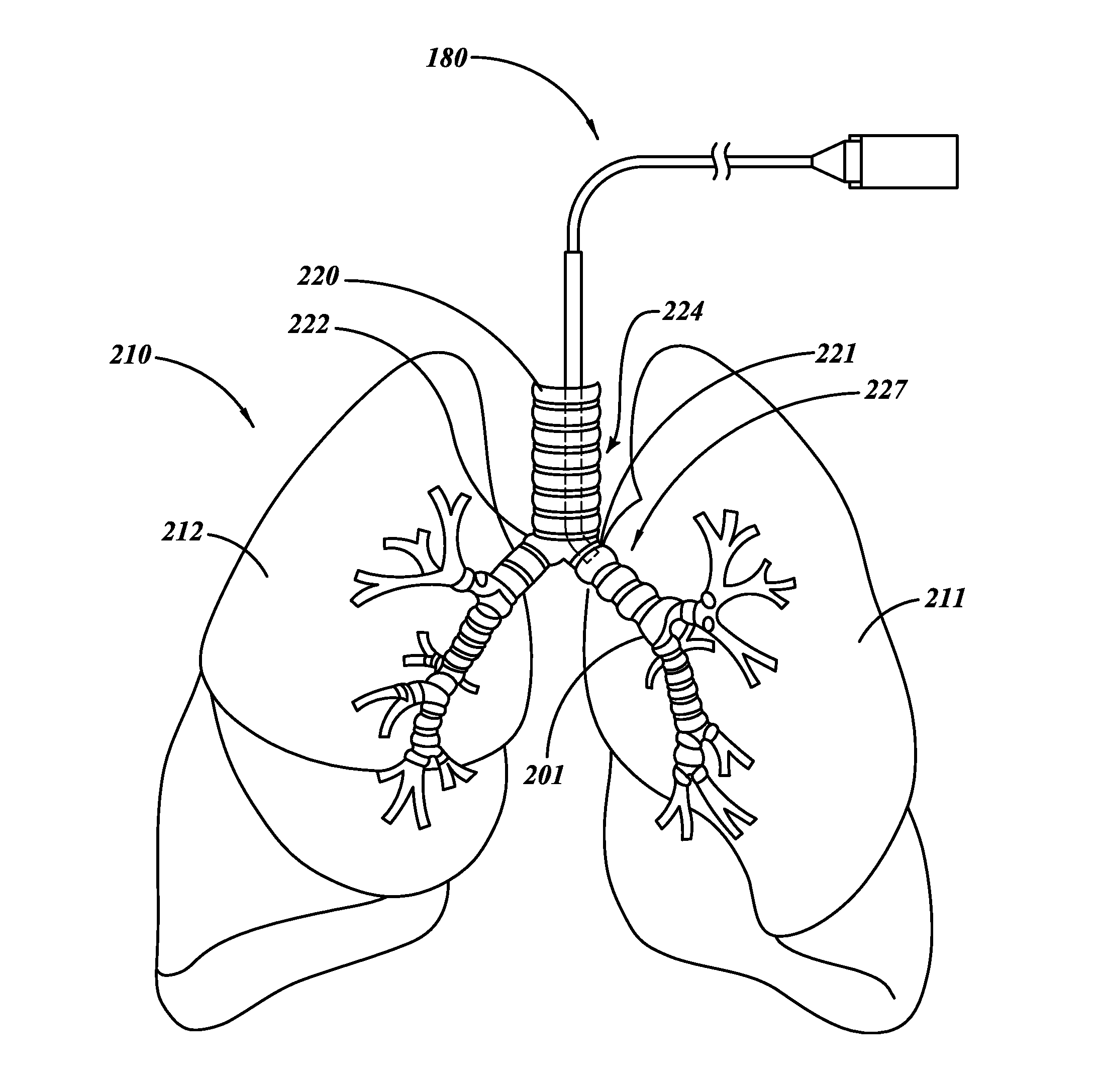
Methods, devices and formulations for targeted endobronchial therapy
ActiveUS20050211253A1Mortality rate is decreasedReduce morbidityAntibacterial agentsTracheal tubesTracheobronchitisTreatment effect
The present invention provides an improved means of treating tracheobronchitis, bronchiectasis and pneumonia in the nosocomial patient, preferably with aerosolized anti gram-positive and anti-gram negative antibiotics administered in combination or in seriatim in reliably sufficient amounts for therapeutic effect. In one aspect, the invention assures this result when aerosol is delivered into the ventilator circuit. In one embodiment the result is achieved mechanically. In another embodiment, the result is achieved by aerosol formulation. In another aspect, the invention assures the result when aerosol is delivered directly to the airnvays distal of the ventilator circuit. The treatment means eliminates the dosage variability that ventilator systems engender when aerosols are introduced via the ventilator circuit. The treatment means also concentrates the therapeutic agent specifically at affected sites in the lung such that therapeutic levels of administrated drug are achieved without significant systemic exposure of the patient to the drug. The invention further provides a dose control device to govern this specialized regimen.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Inhibitor which is deactivatable by a reagent produced by a target cell
ActiveUS8809504B2Undesirable effectModulating responseNGF/TNF-superfamilyAntibody ingredientsActive agentWhole body
The invention relates to molecules inhibiting biologically active compounds and further comprising moieties specifically cleavable by a reagent produced by a target cell. The invention relates to inhibitors that bind, inhibit, suppress, neutralize, or decrease activity of a biologically active agent. Inhibitors comprise at least one moiety that bind, inhibit, suppress, neutralize, or decrease activity of a biologically active agent and at least one moiety that can be cleaved specifically by a reagent produced by target cells. The cleavage deactivates the inhibitor. Following cleavage, the active agent is liberated into the local environment. Administration of the inhibitor alone or together with the active agent suppress the compound's activity until it reaches the proximity of a target cell. Targeted specific release enables the agent concentration in specific site to reach levels that have desired therapeutic effects without systemic toxicity.
Owner:VYTACERA BIO LLC
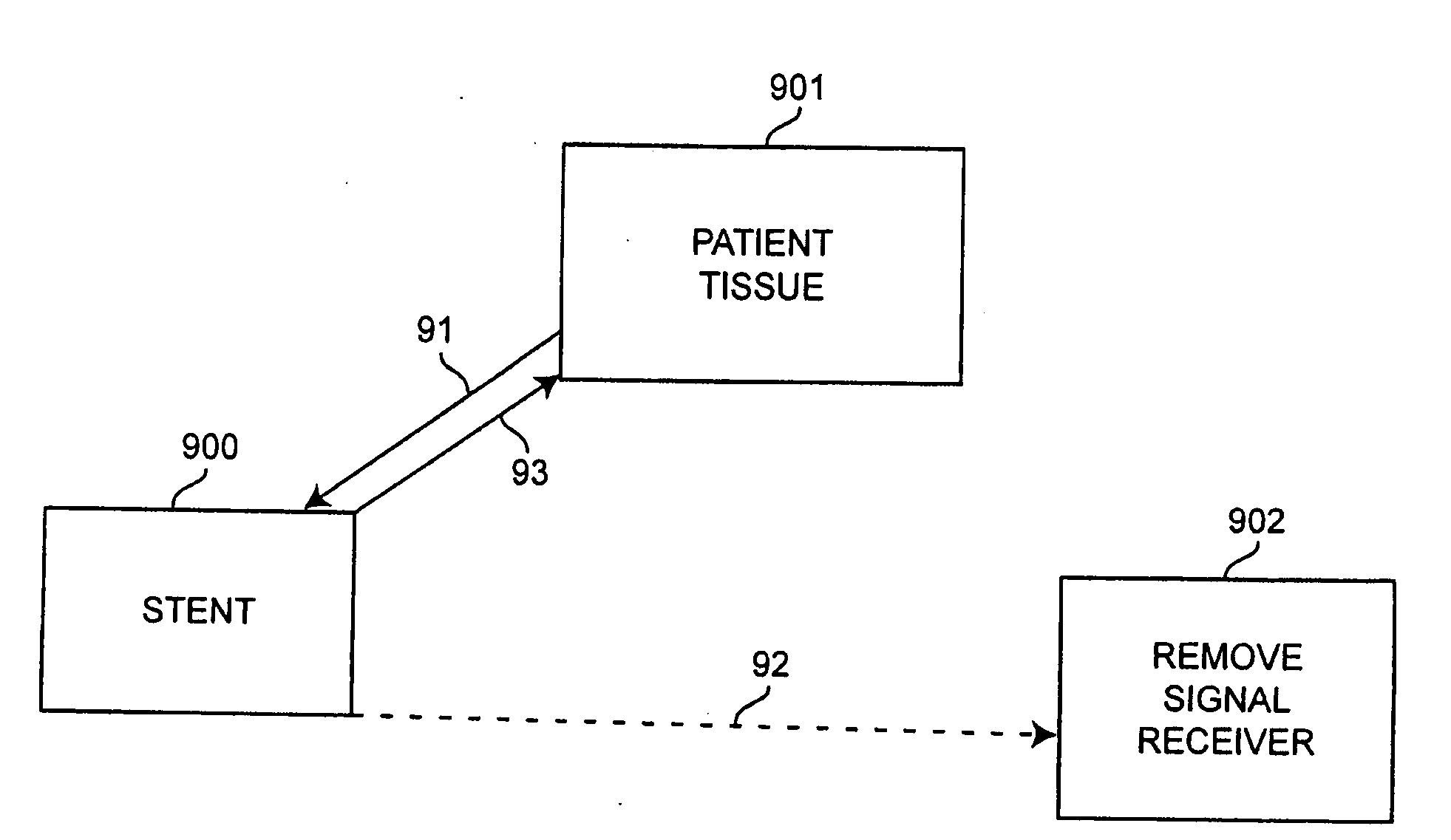
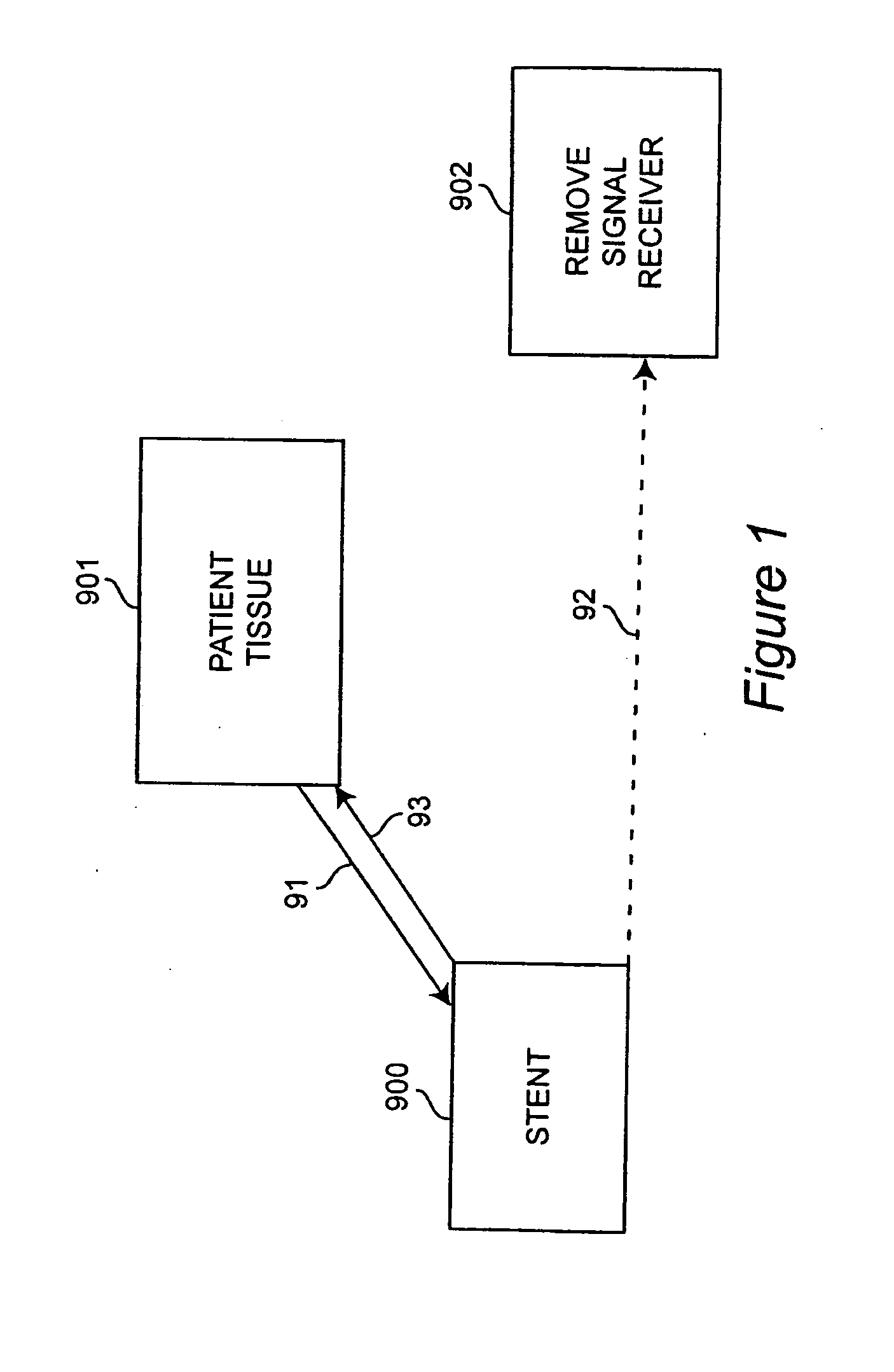
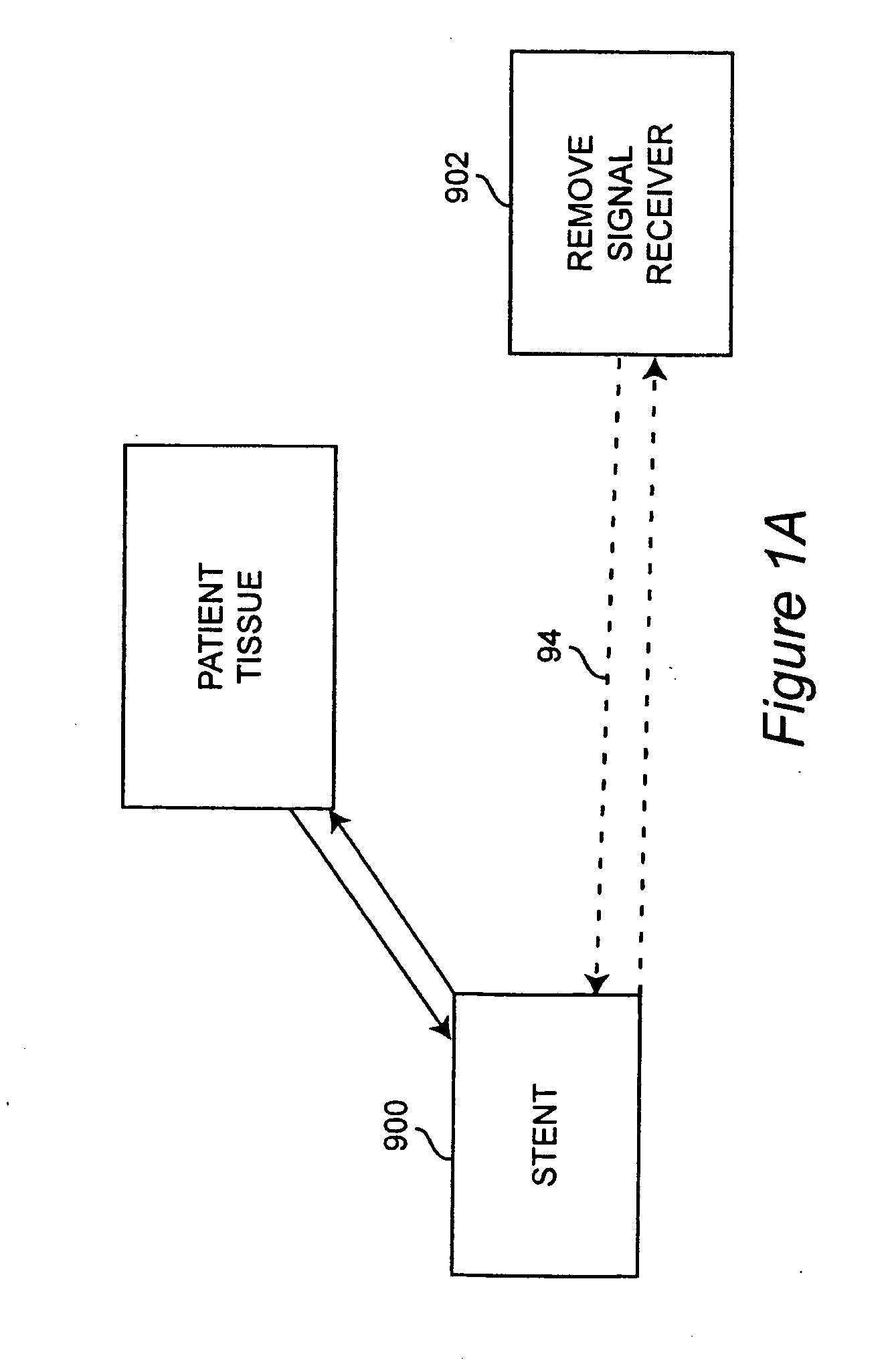
Self-sensing stents, smart materials-based stents, drug delivery systems, other medical devices, and medical uses for piezo-electric materials
InactiveUS20090036975A1Advanced technologyProvide solutionStentsElectrotherapyTreatment effectSelf sensing
A medically implantable stent comprising at least one piezo-electric material may be active, such as by one or more of: delivering an anti-coagulant or other therapeutic effect to a patient in which it is implanted; powering itself; and / or sending an outbound electronic signal to a remote device. When a stent can send such an outbound signal, a physician may non-invasively ascertain the condition of the tissue near the stent.
Owner:VIRGINIA COMMONWEALTH UNIV
Methods and systems for screening subjects
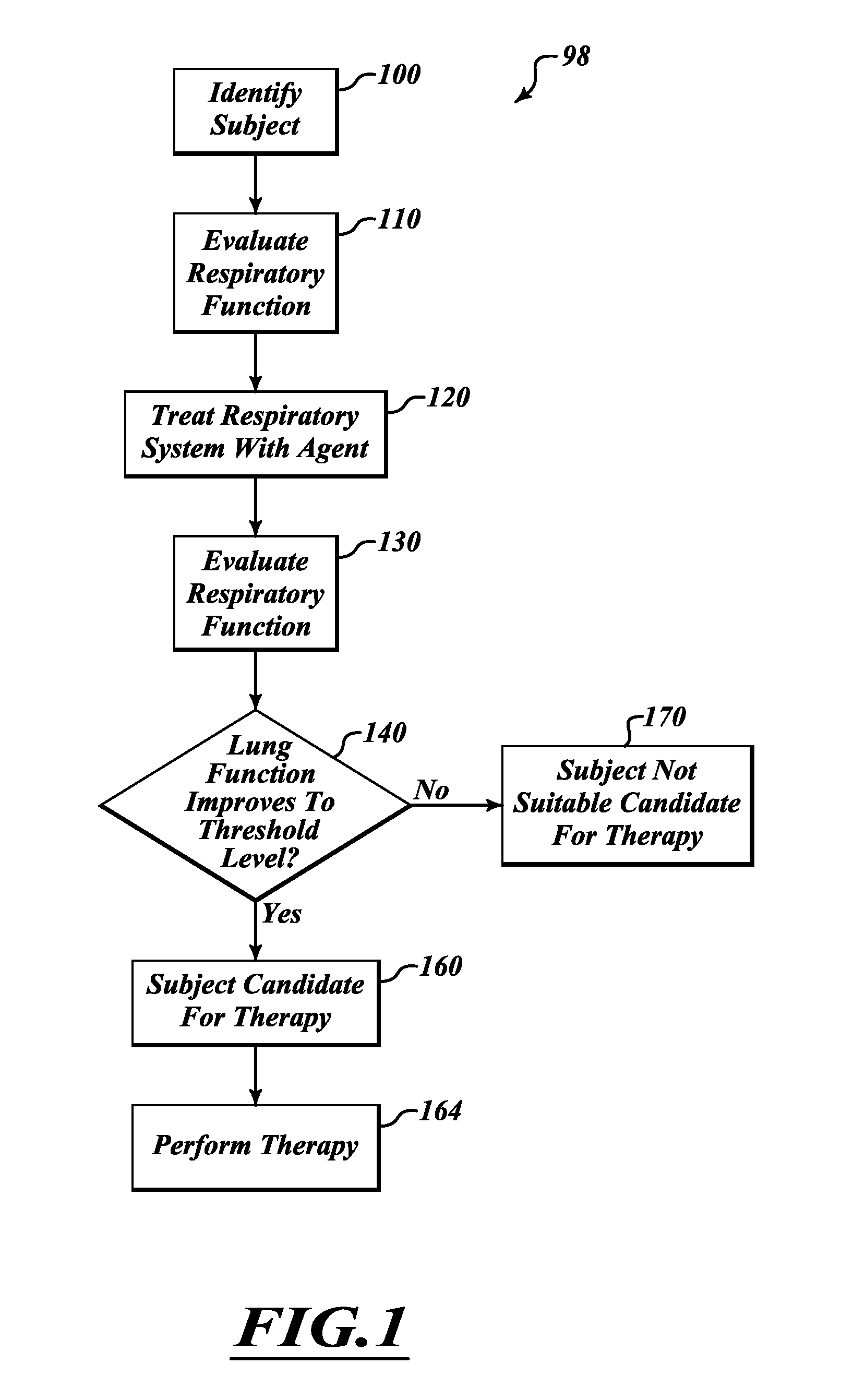
InactiveUS20120302909A1Improve exercise capacityImprove function capacityRespiratory organ evaluationSensorsTest agentScreening method
A screening method can be used to determine whether a subject is a suitable candidate for interventional therapy. The method can be used to determine the likelihood the subject will receive a therapeutic effect from denervation therapy. The determination is based, at least in part, on lung information obtained by performing lung function tests with and without treating the subject's lungs with a test agent. Based on the response to a test agent, the subject's response to a therapy is predicted.
Owner:NUVAIRA INC
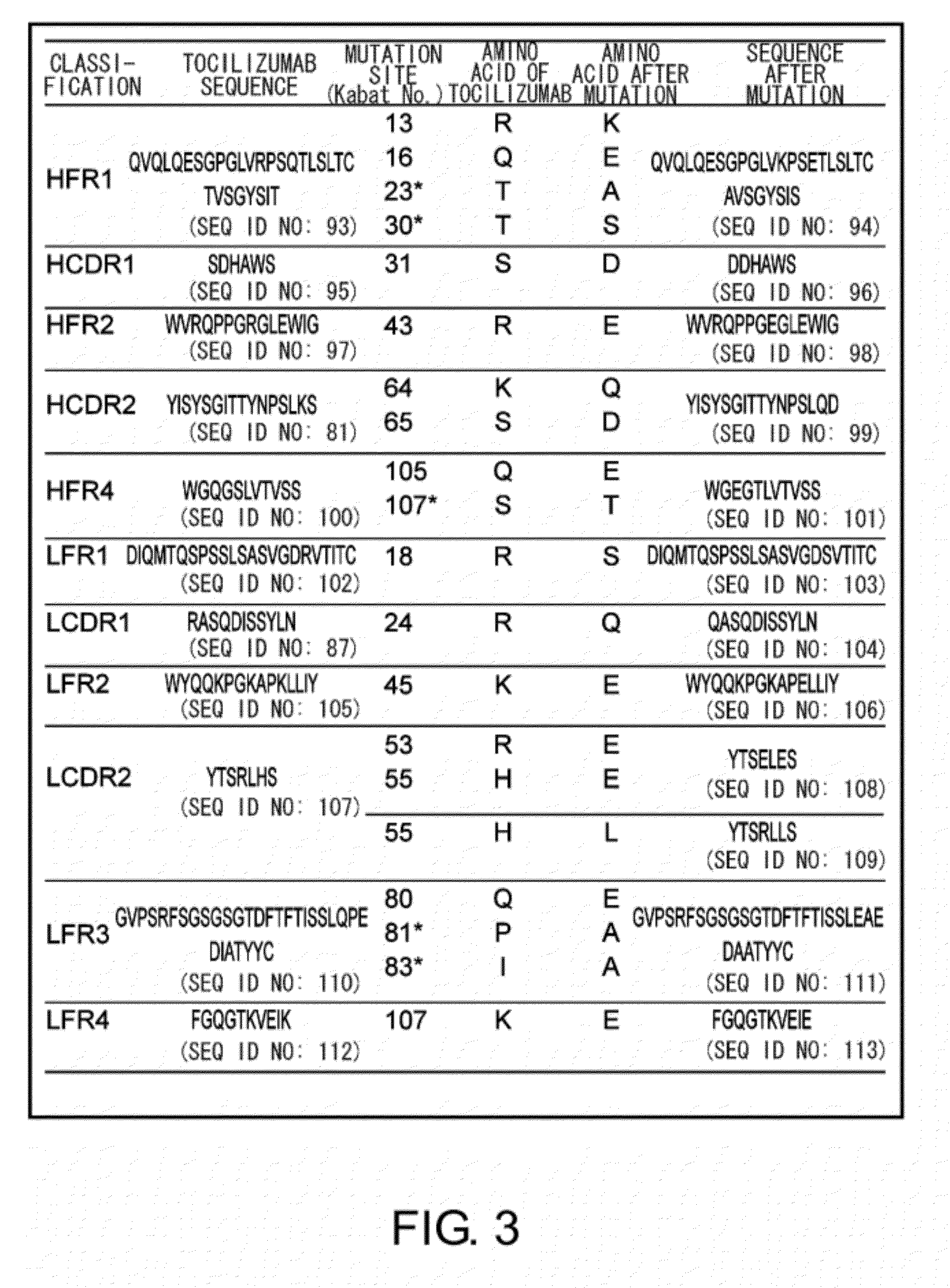
Antibody molecules that bind to il-6 receptor
InactiveUS20120253016A1Good curative effectImprove pharmacokineticsFungiSenses disorderTherapeutic effectIL-2 receptor
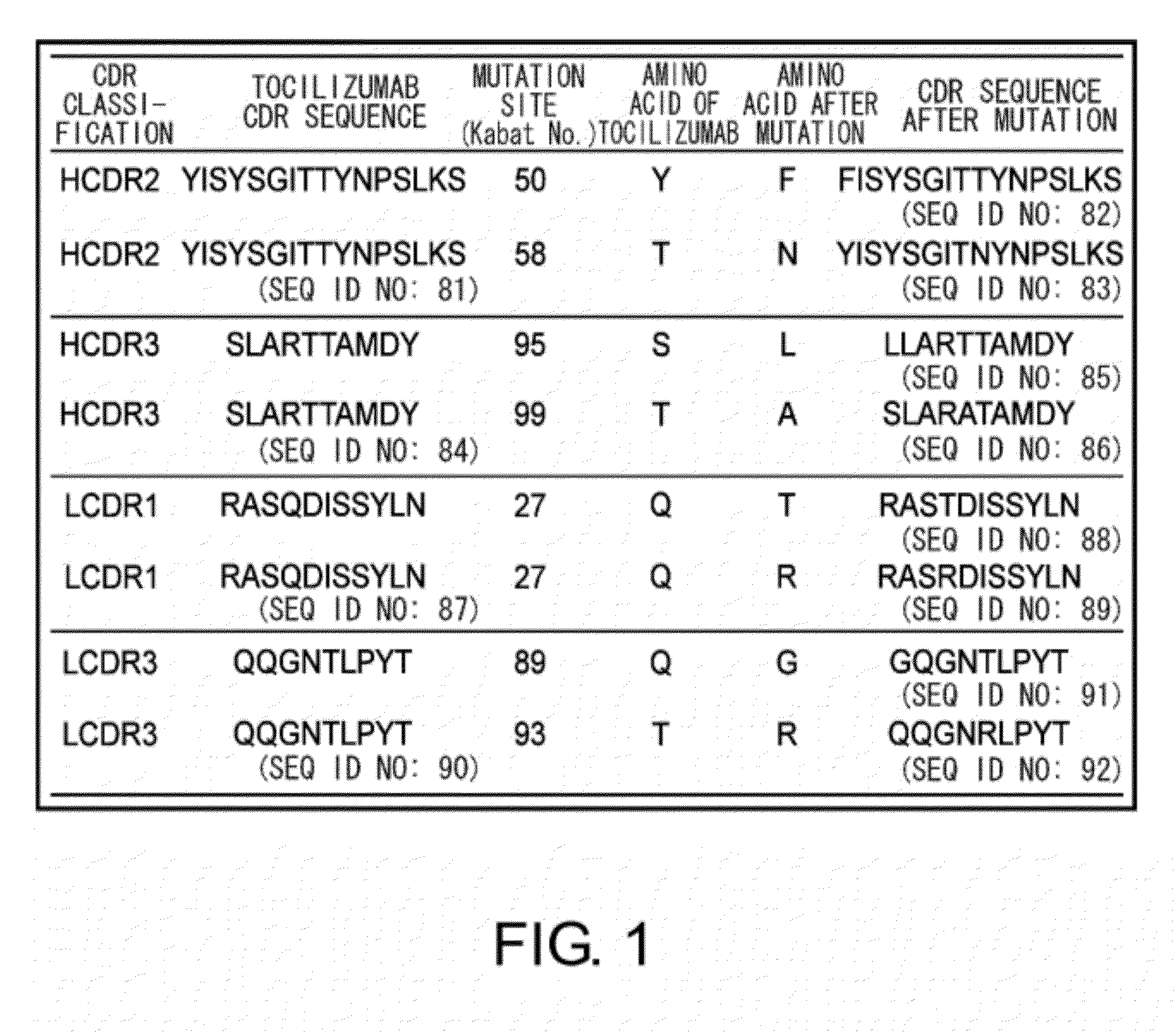
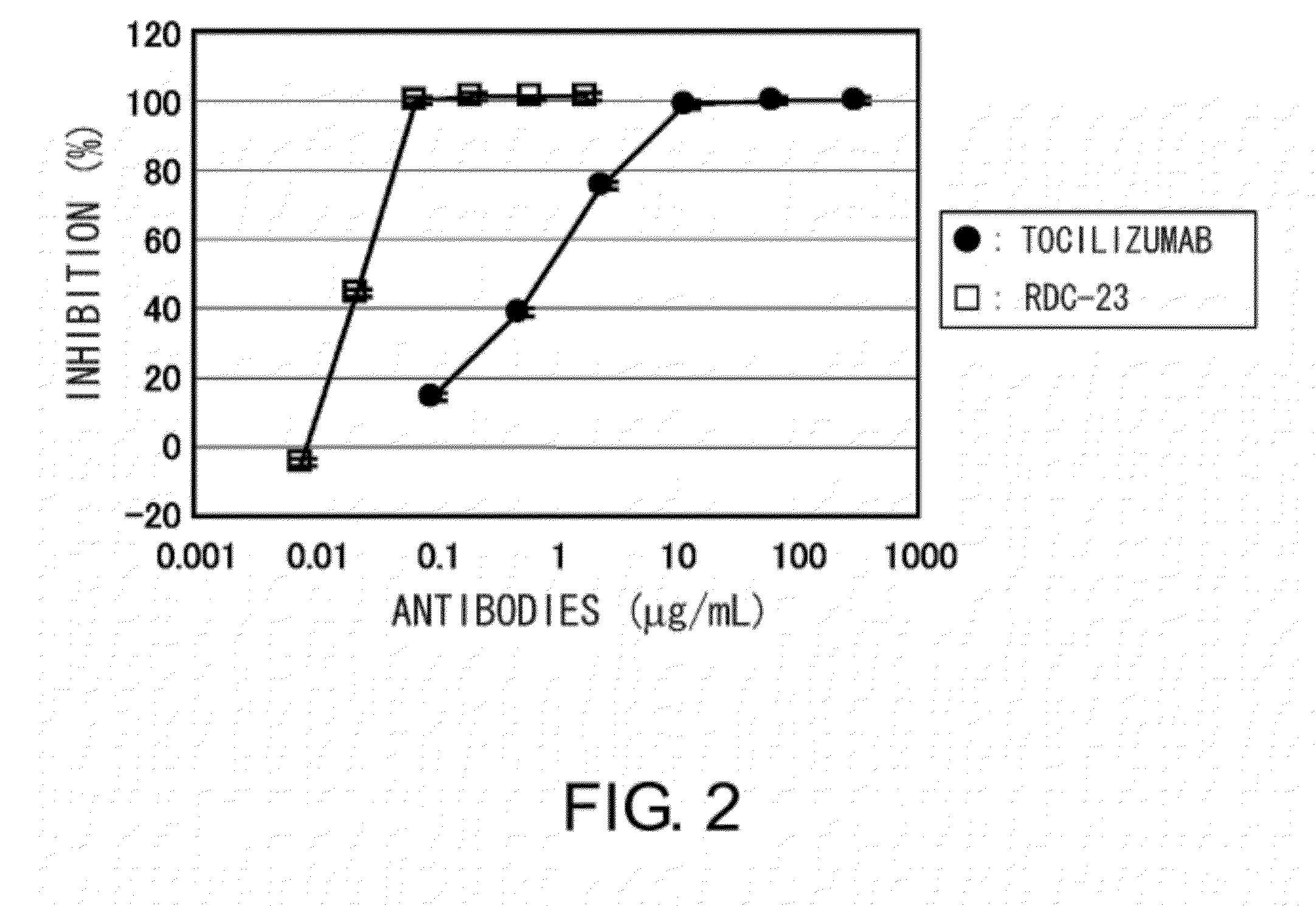
The present invention provides pharmaceutical compositions comprising second-generation molecules that are superior than TOCILIZUMAB, by altering the amino acid sequences of the variable and constant regions of TOCILIZUMAB, which is a humanized anti-IL-6 receptor IgG1 antibody, to enhance the antigen-neutralizing ability and increase the pharmacokinetics, so that the therapeutic effect is exerted with a less frequency of administration, and the immunogenicity, safety and physicochemical properties (stability and homogeneity) are improved. The present invention also provides methods for producing these pharmaceutical compositions. The present inventors have successfully generated second-generation molecules that are superior to TOCILIZUMAB by appropriately combining amino acid sequence alterations in the CDR domains, variable regions, and constant regions.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com