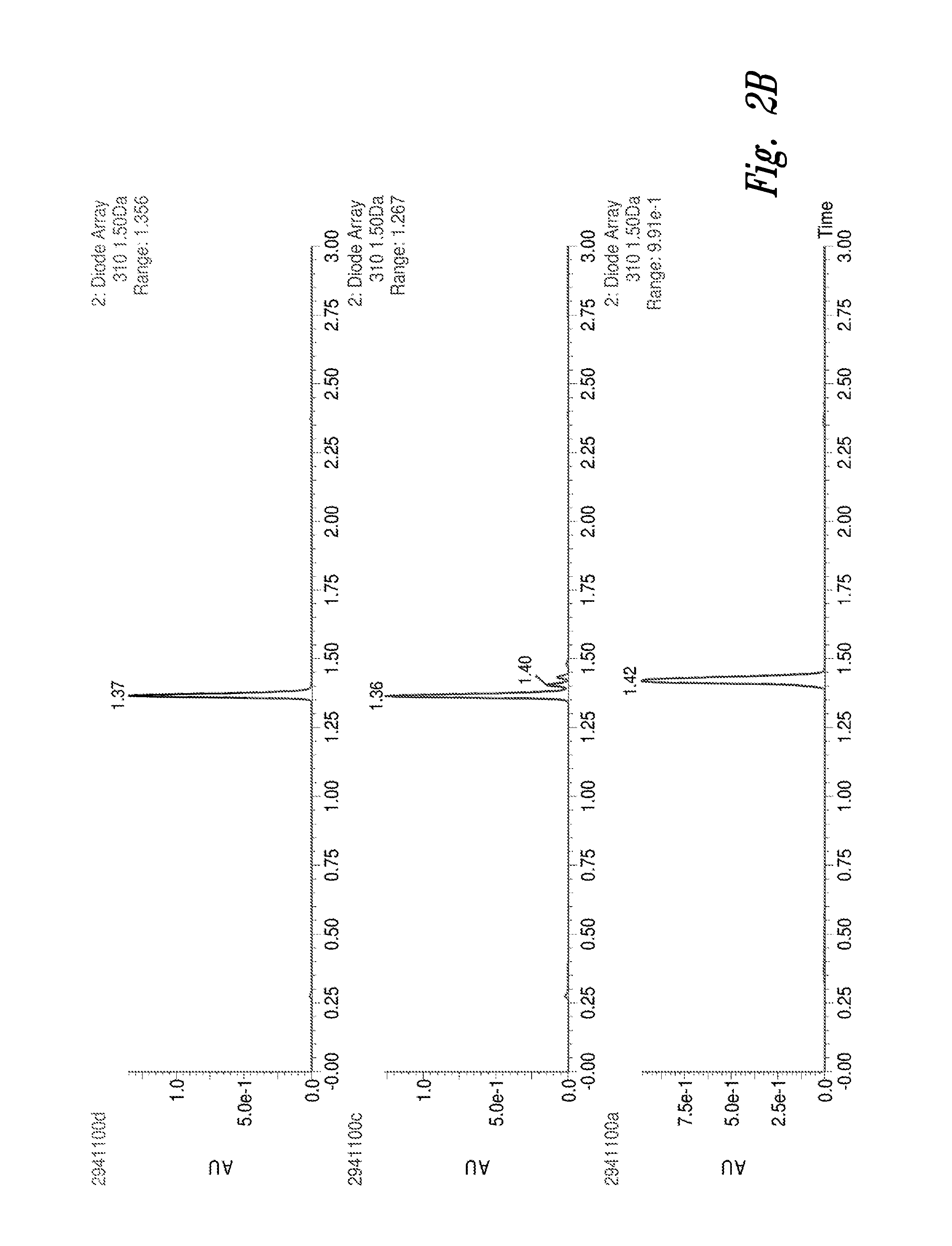
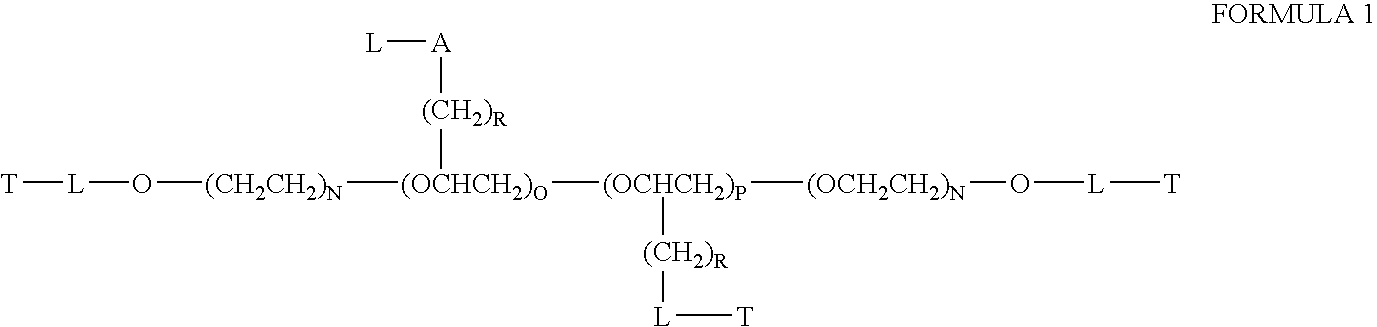
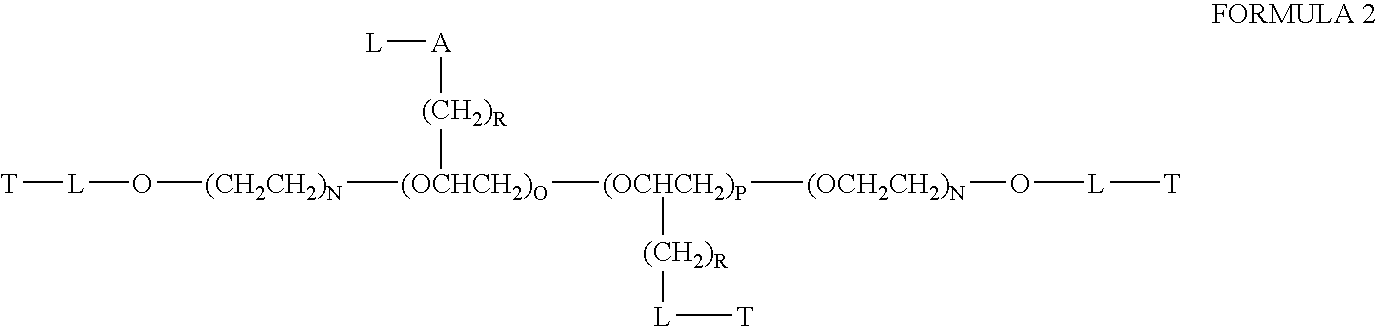
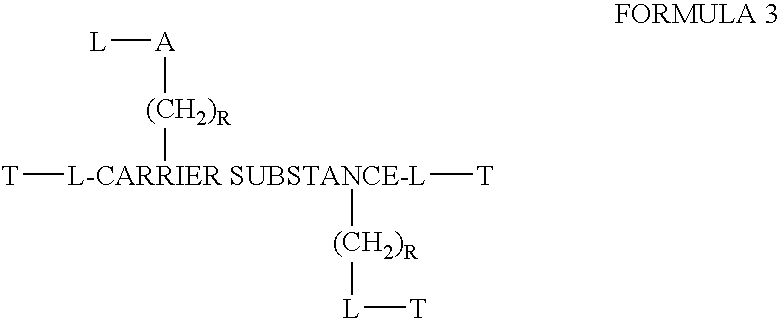
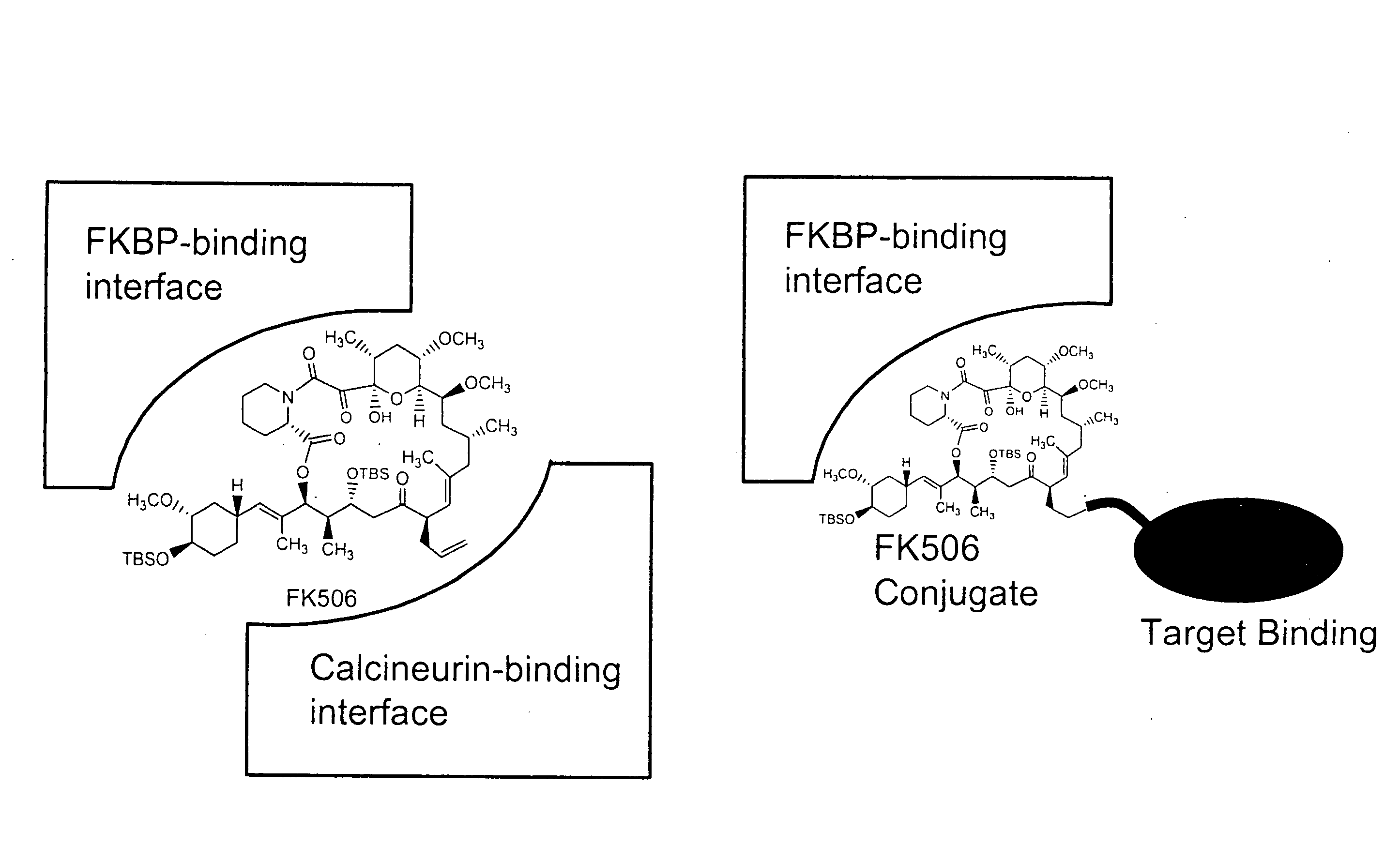
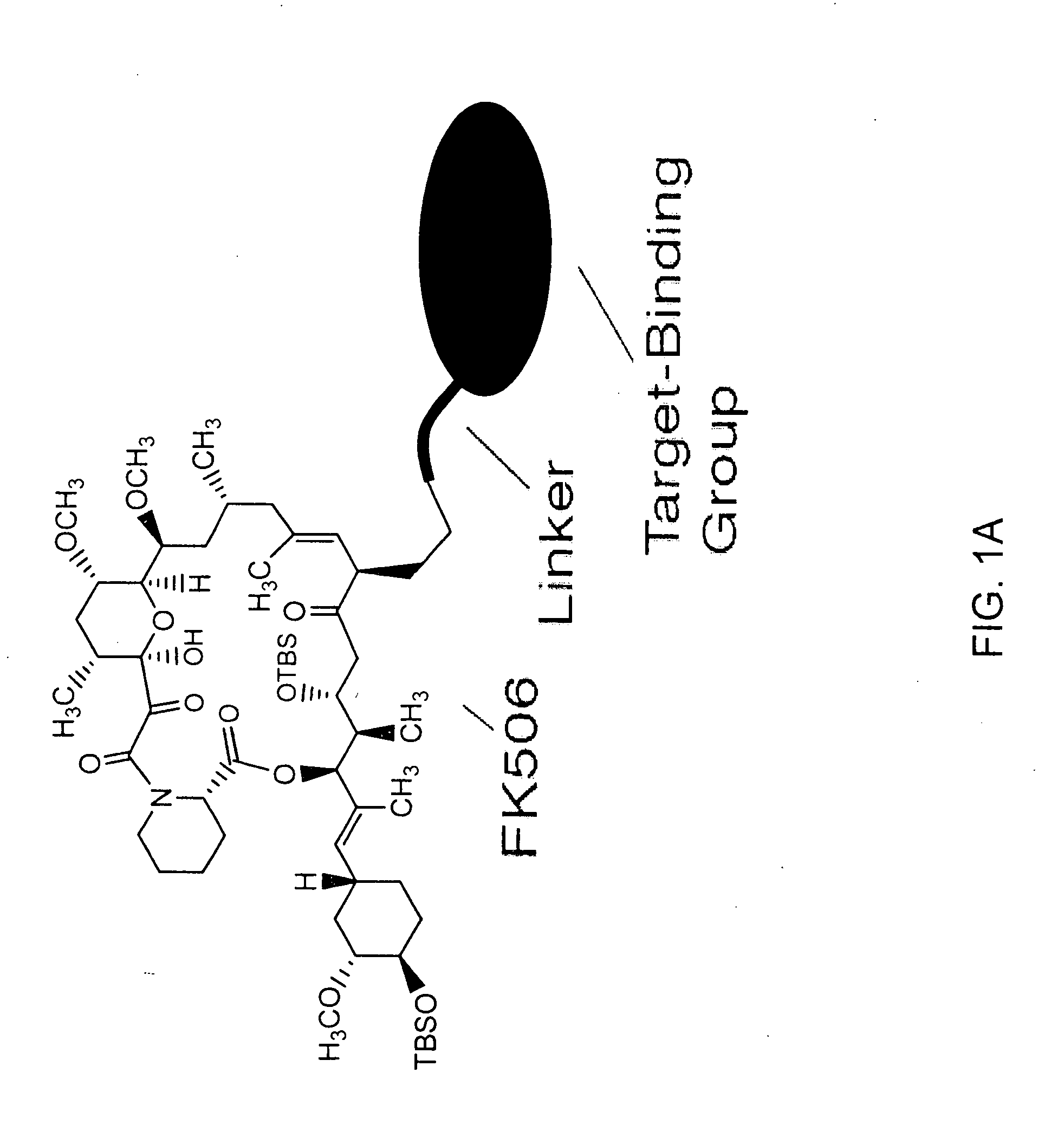
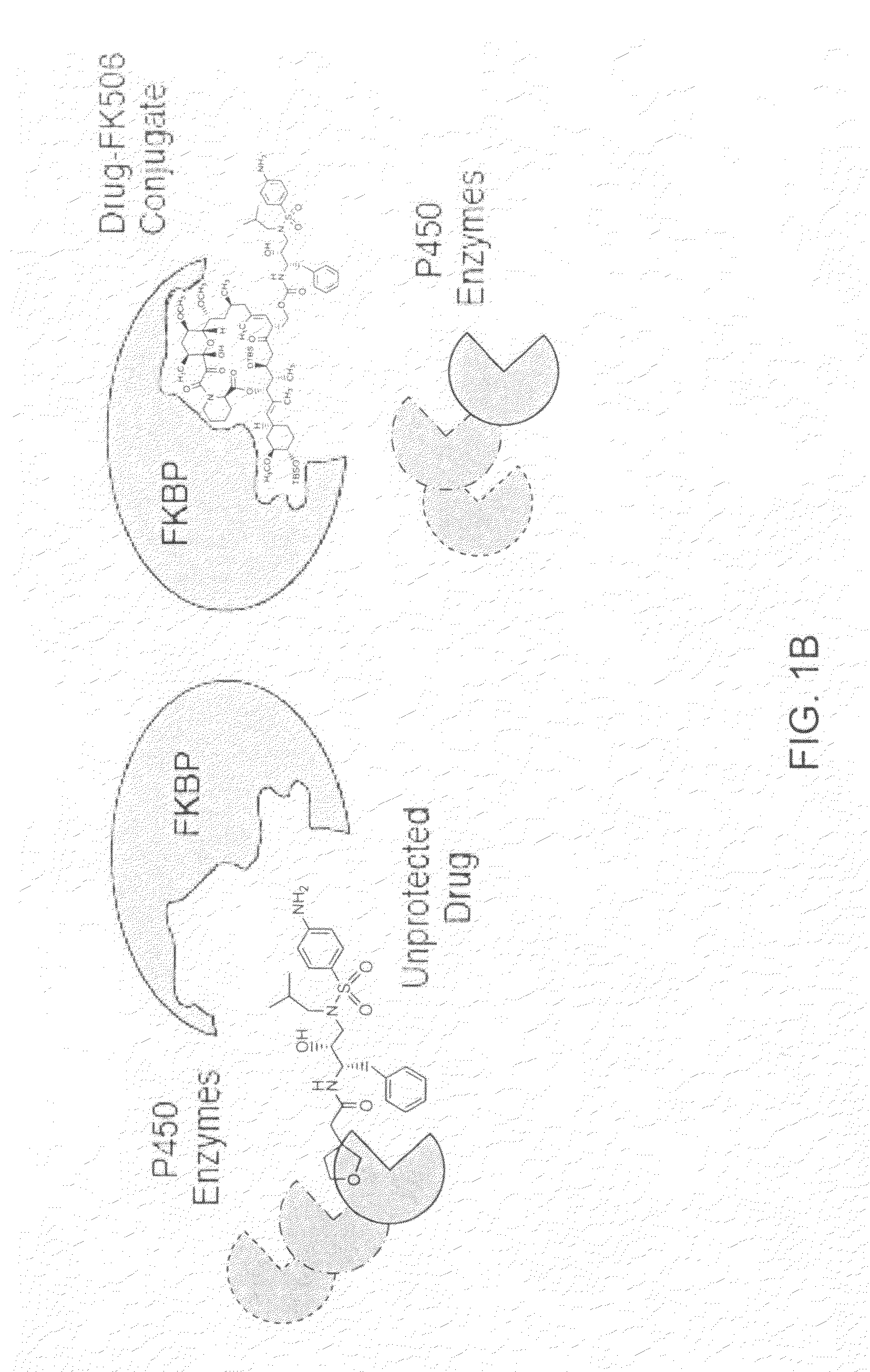
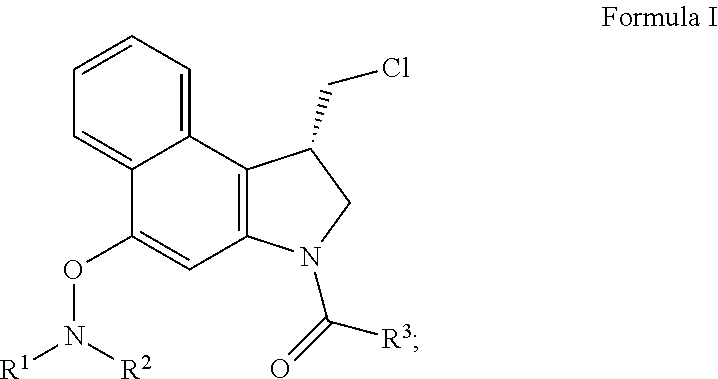
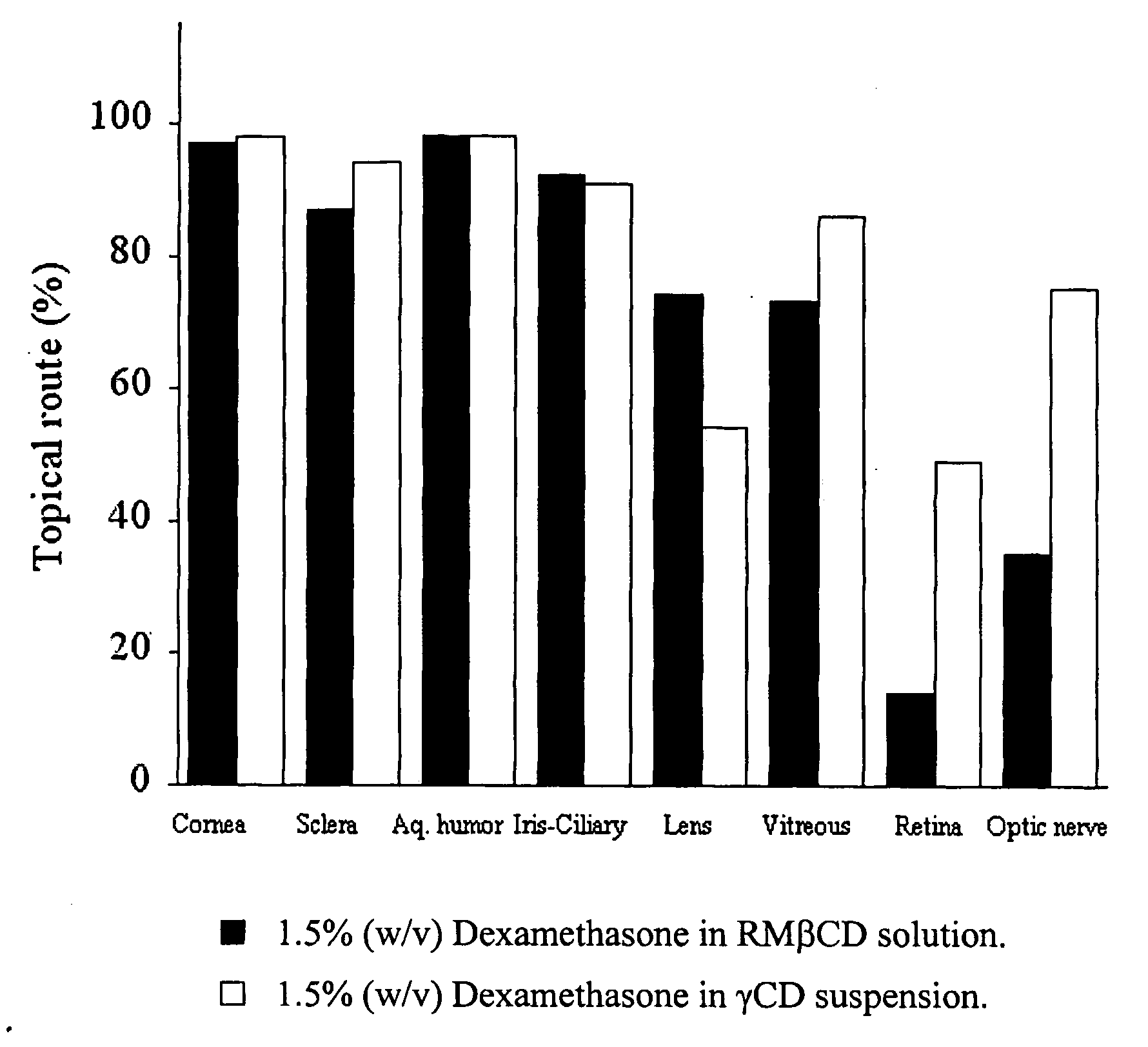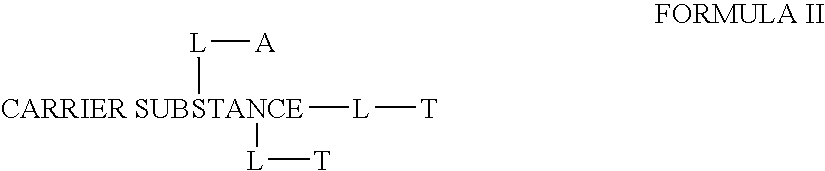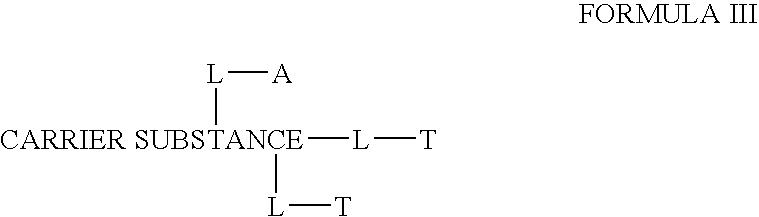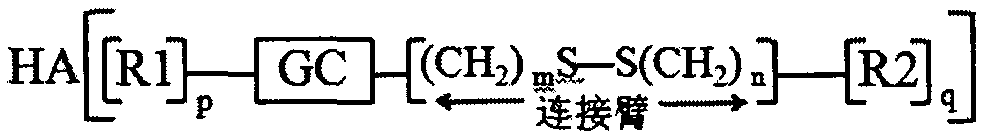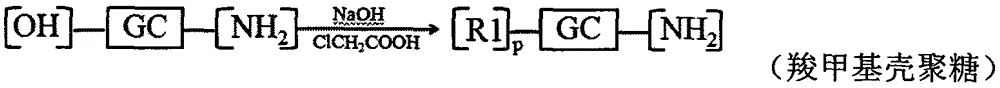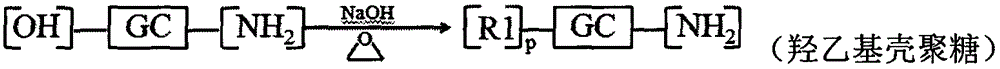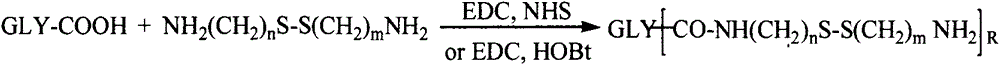Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
157 results about "Free drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclodextrin nanotechnology for ophthalmic drug delivery
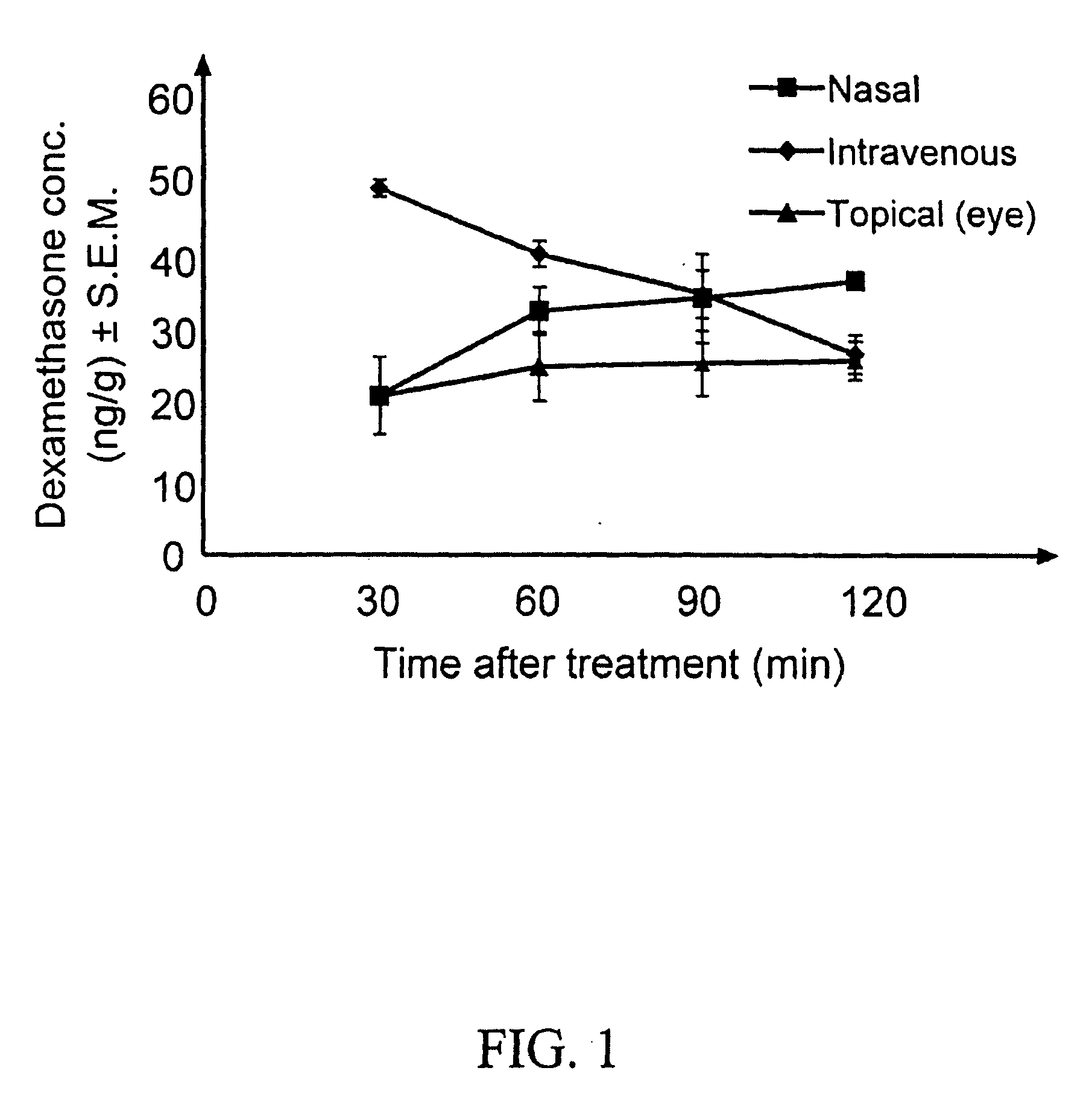
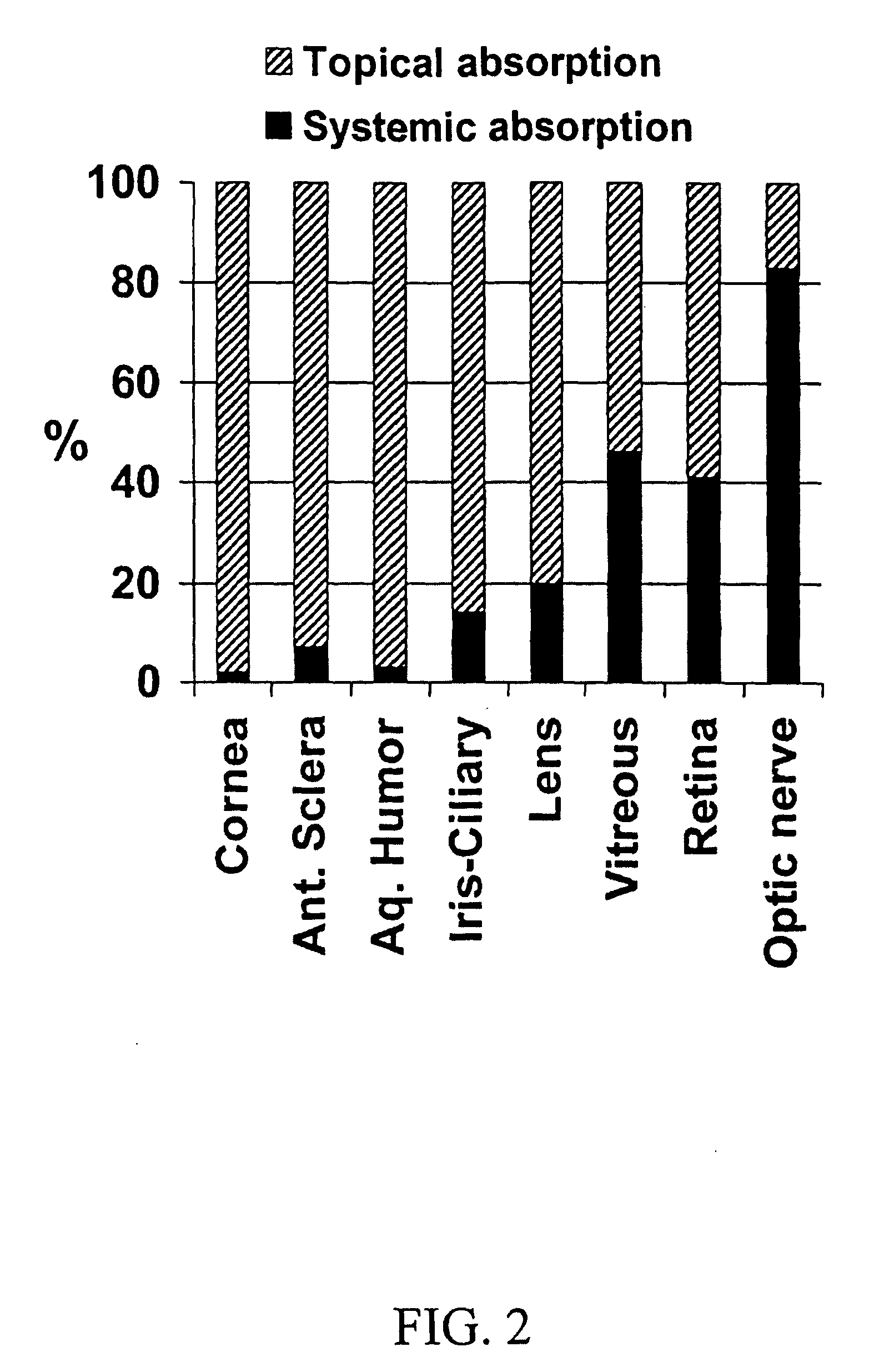
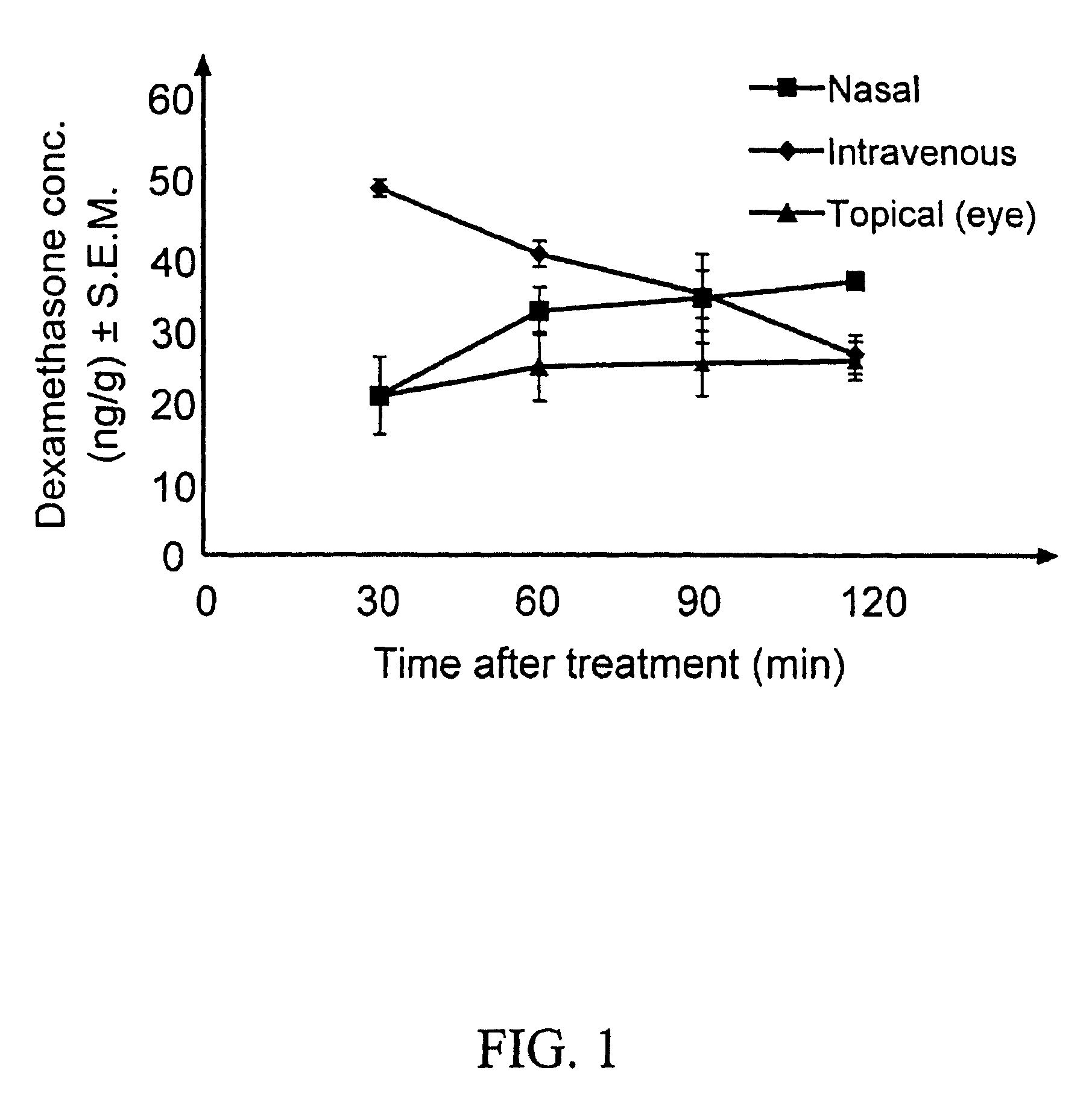
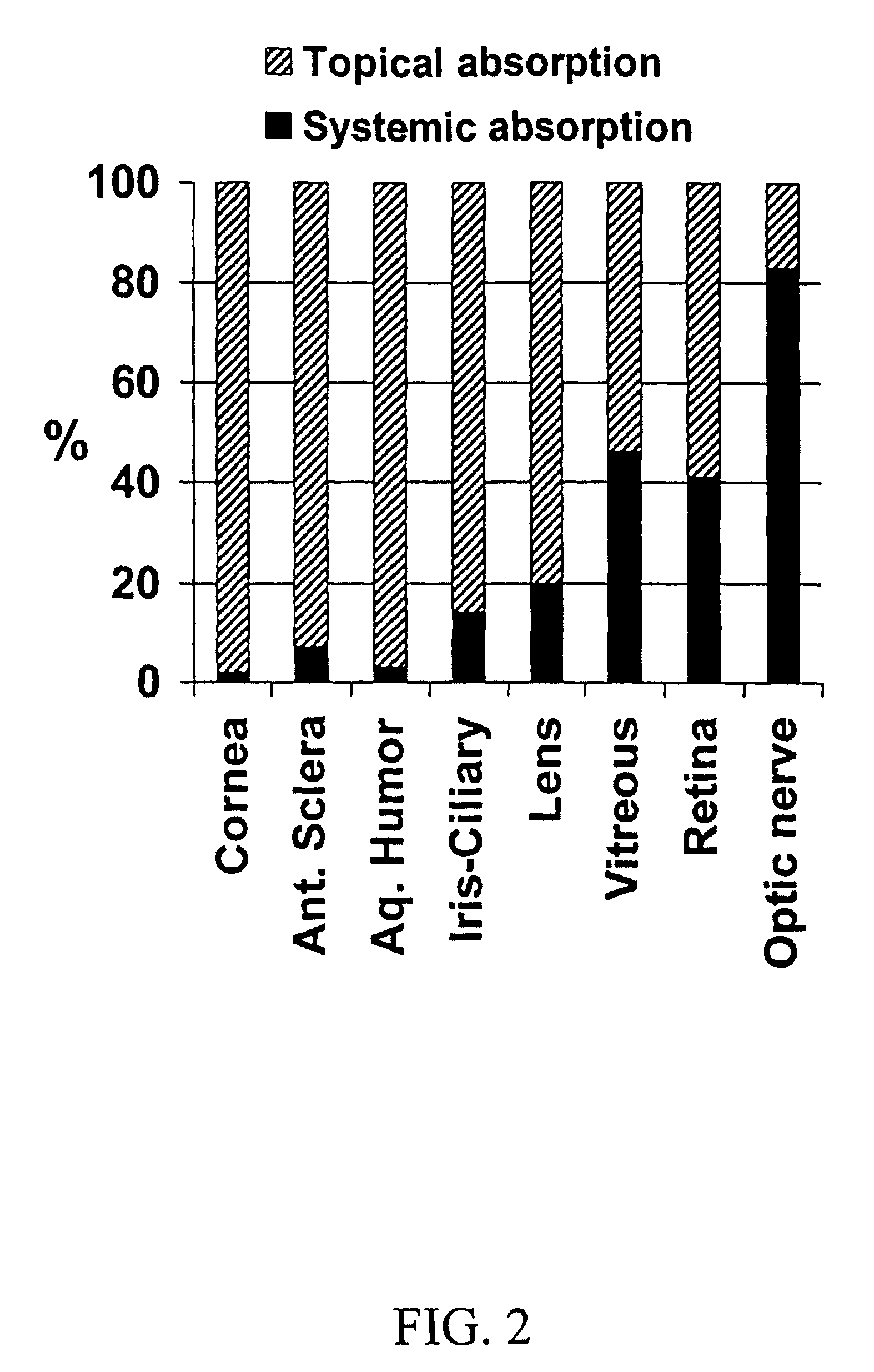
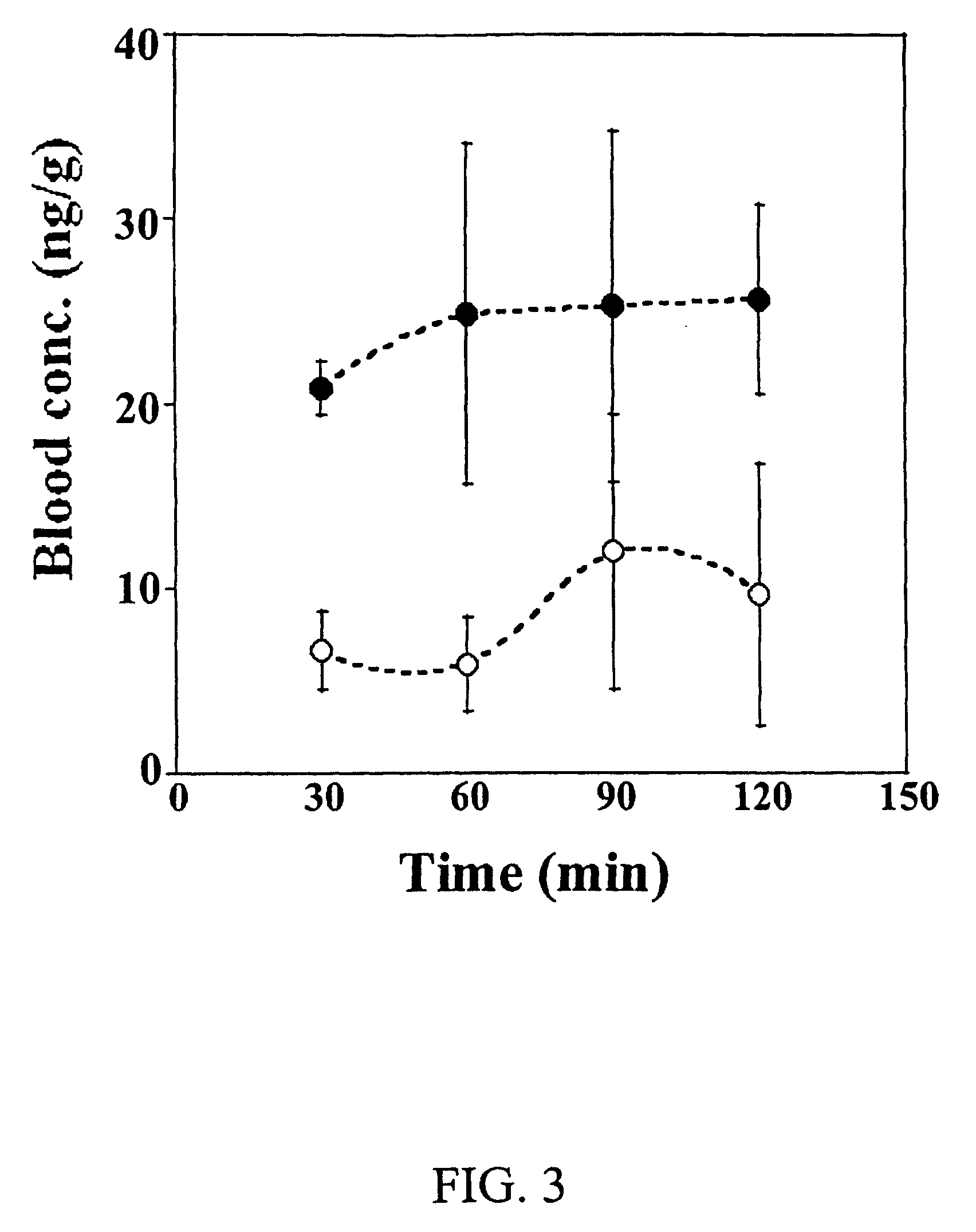
The invention provides an ophthalmic composition which is an aqueous suspension comprising drug, cyclodextrin and water, the composition having an aqueous phase of from about 0.1% (w / v) to about 90% (w / v) of the drug in solution, as dissolved free drug and as dissolved drug / cyclodextrin complex(es), and a solid phase of from about 10% (w / v) to about 99.9% (w / v) of the drug as solid drug / cyclodextrin particles, suspended in the aqueous phase; the size of the solid particles being from about 10 nm to about 1 mm, the drug / cyclodextrin particles being capable of dissolving in aqueous tear fluid within 24 hours of application to the eye surface. The aqueous eye suspension can be in the form of eye drops, eye gel or eye mist. Further, the invention provides a method for treating a condition of the posterior segment and / or anterior segment of the eye comprising applying to the eye surface, in an amount which delivers to said segment or segments a therapeutically effective amount of a drug suitable for treating said condition, an ophthalmic composition which is as defined above. Nasal compositions and methods and ophthalmic and nasal compositions in powder form are also provided.
Owner:OCULIS EHF
Chloroquine coupled antibodies and other proteins with methods for their synthesis
InactiveUS20070166281A1Improve efficacyImprove transportBiocidePeptide/protein ingredientsDrug conjugationTreatment effect
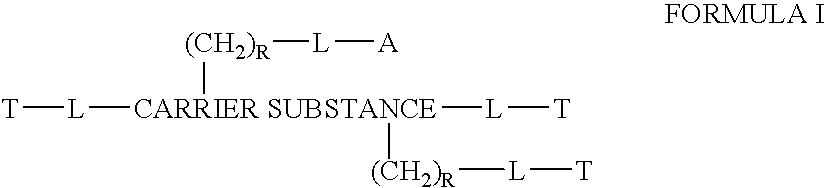
This invention discloses compositions of chloroquine-coupled active agents such as therapeutic antibodies or insulin, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the drug is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a drug directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing the drug for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and antibody or insulin to their site of action.
Owner:KOSAK KENNETH M
Cyclodextrin nanotechnology for ophthalmic drug delivery
The invention provides an ophthalmic composition which is an aqueous suspension comprising drug, cyclodextrin and water, the composition having an aqueous phase of from about 0.1% (w / v) to about 90% (w / v) of the drug in solution, as dissolved free drug and as dissolved drug / cyclodextrin complex(es), and a solid phase of from about 10% (w / v) to about 99.9% (w / v) of the drug as solid drug / cyclodextrin particles, suspended in the aqueous phase; the size of the solid particles being from about 10 nm to about 1 mm, the drug / cyclodextrin particles being capable of dissolving in aqueous tear fluid within 24 hours of application to the eye surface. The aqueous eye suspension can be in the form of eye drops, eye gel or eye mist. Further, the invention provides a method for treating a condition of the posterior segment and / or anterior segment of the eye comprising applying to the eye surface, in an amount which delivers to said segment or segments a therapeutically effective amount of a drug suitable for treating said condition, an ophthalmic composition which is as defined above. Nasal compositions and methods and ophthalmic and nasal compositions in powder form are also provided.
Owner:OCULIS EHF
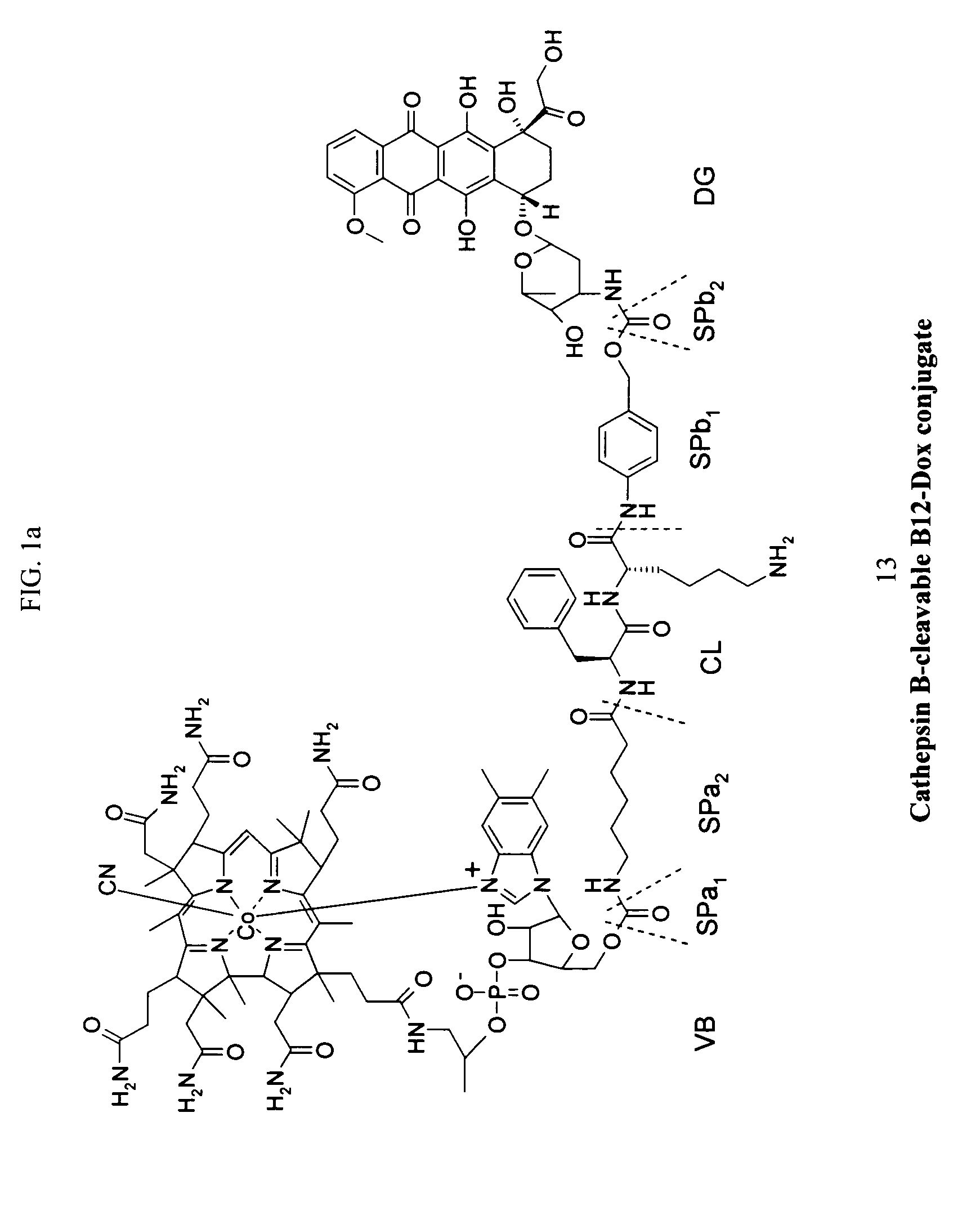
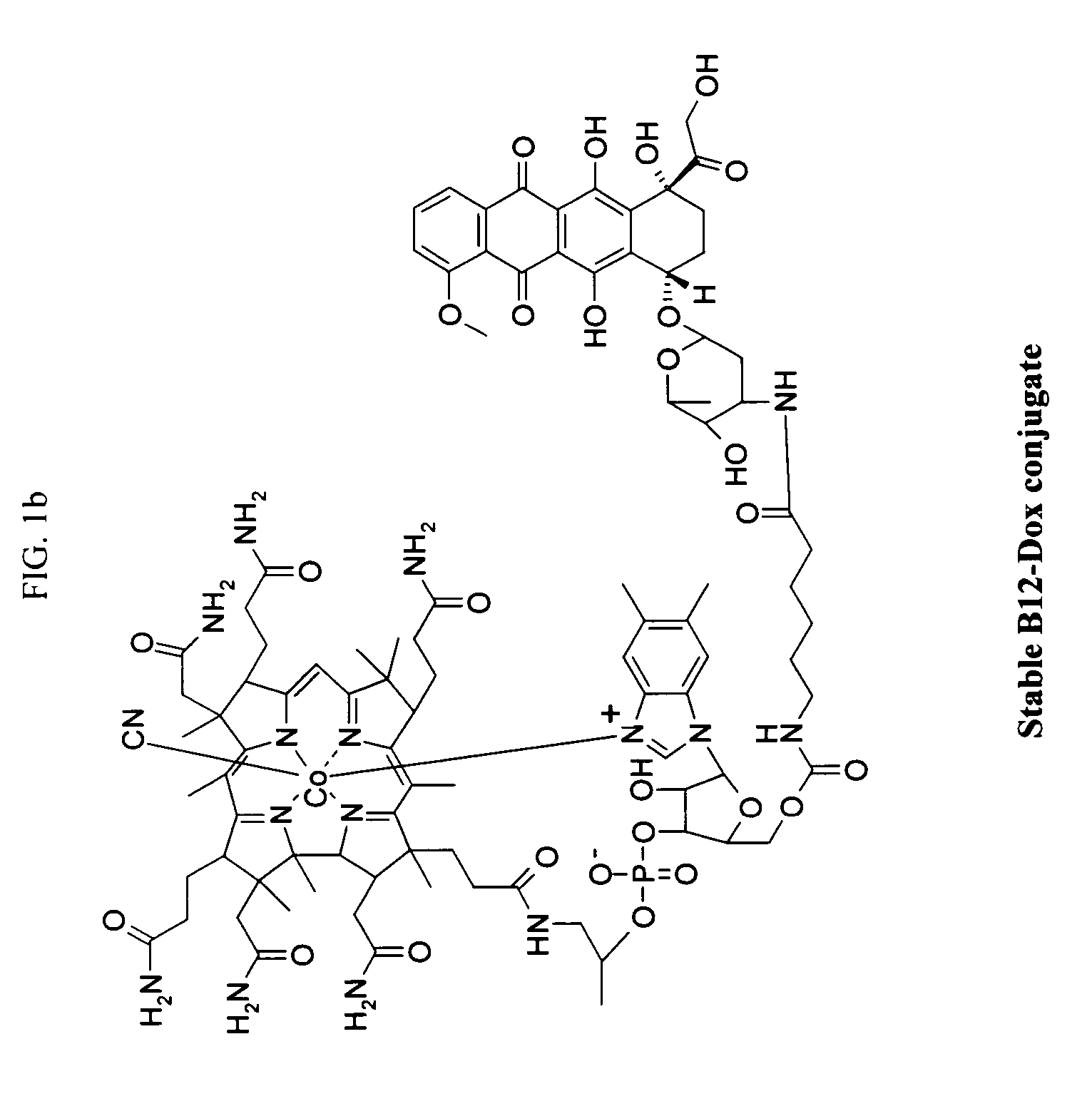
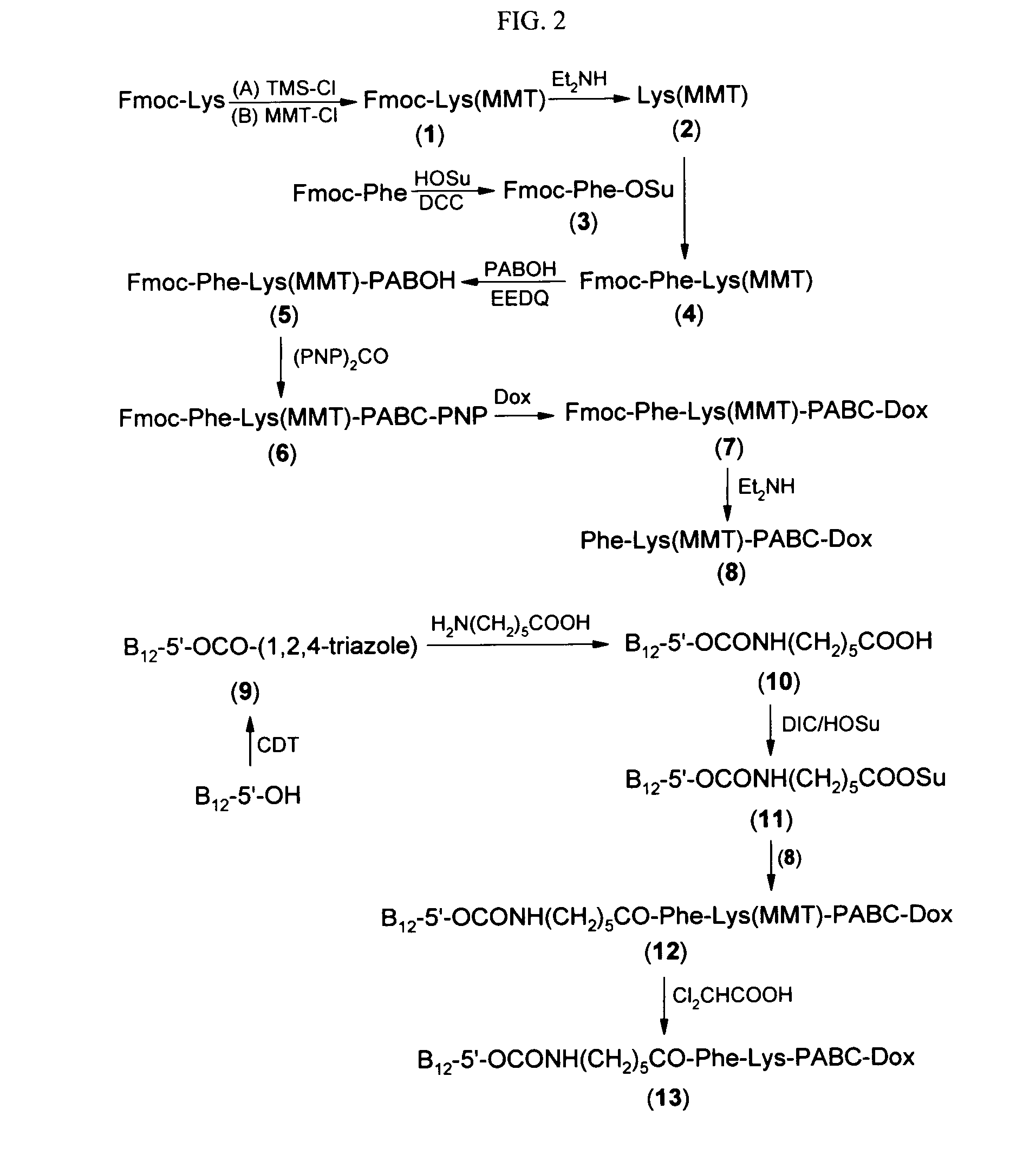
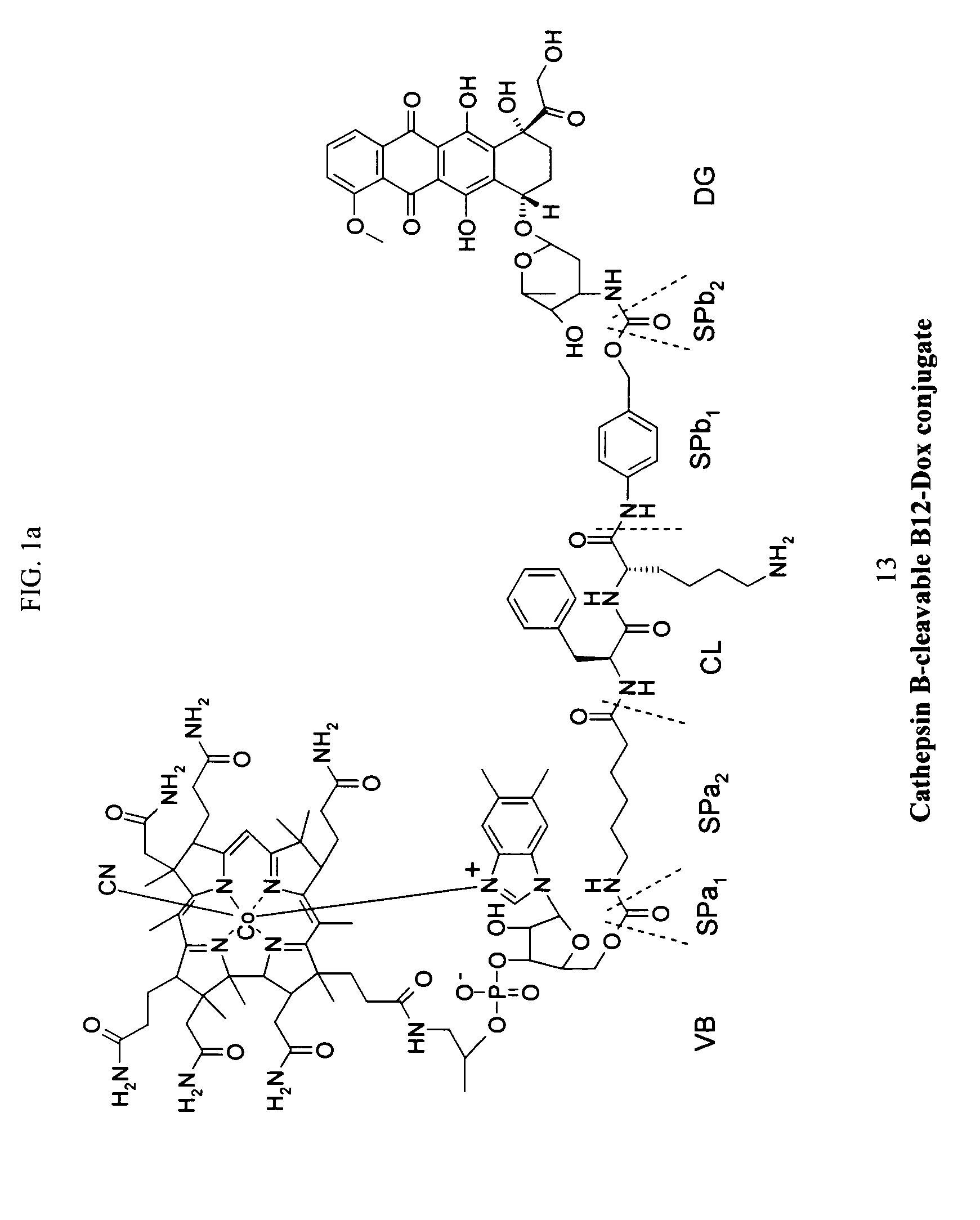
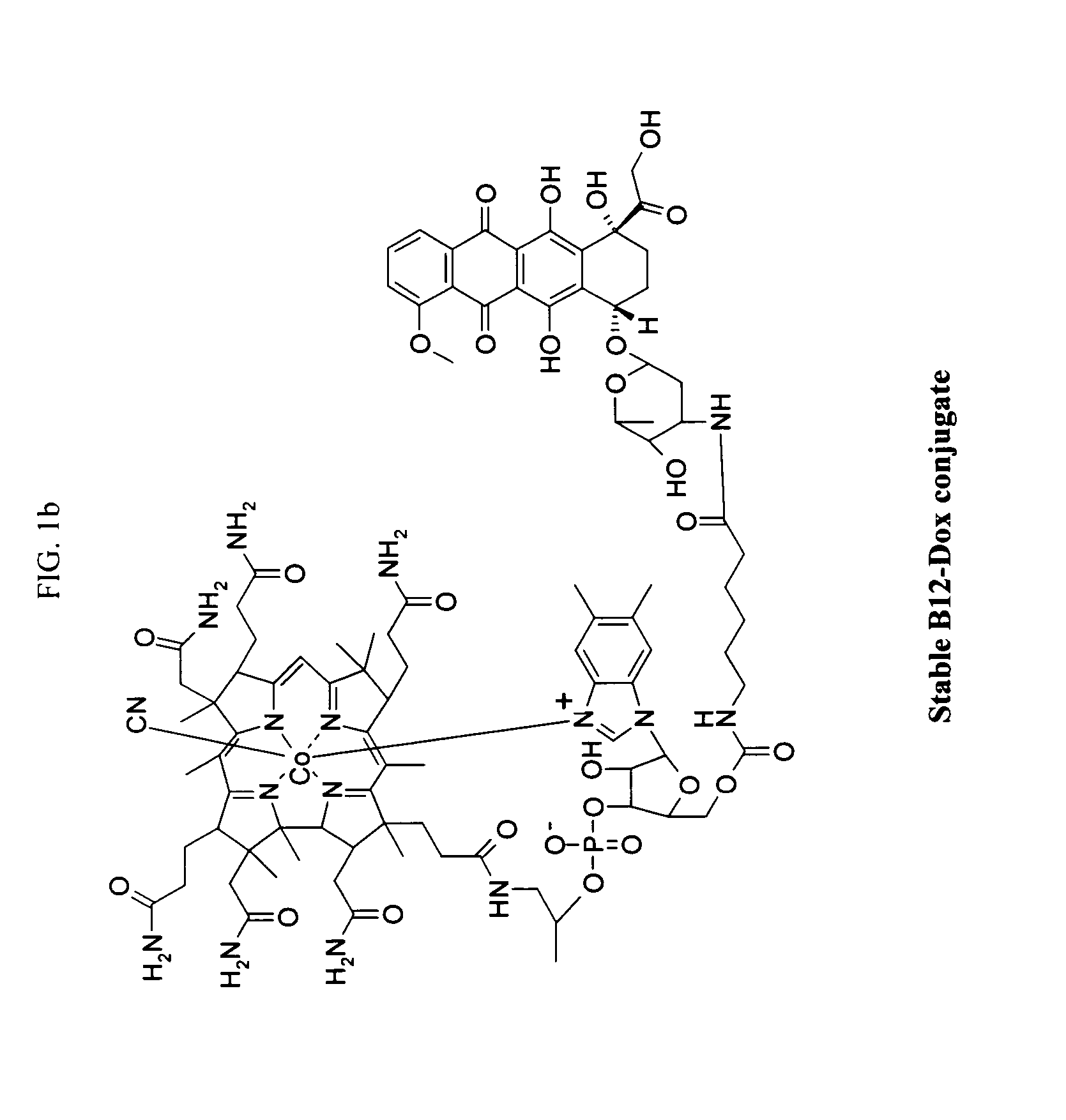
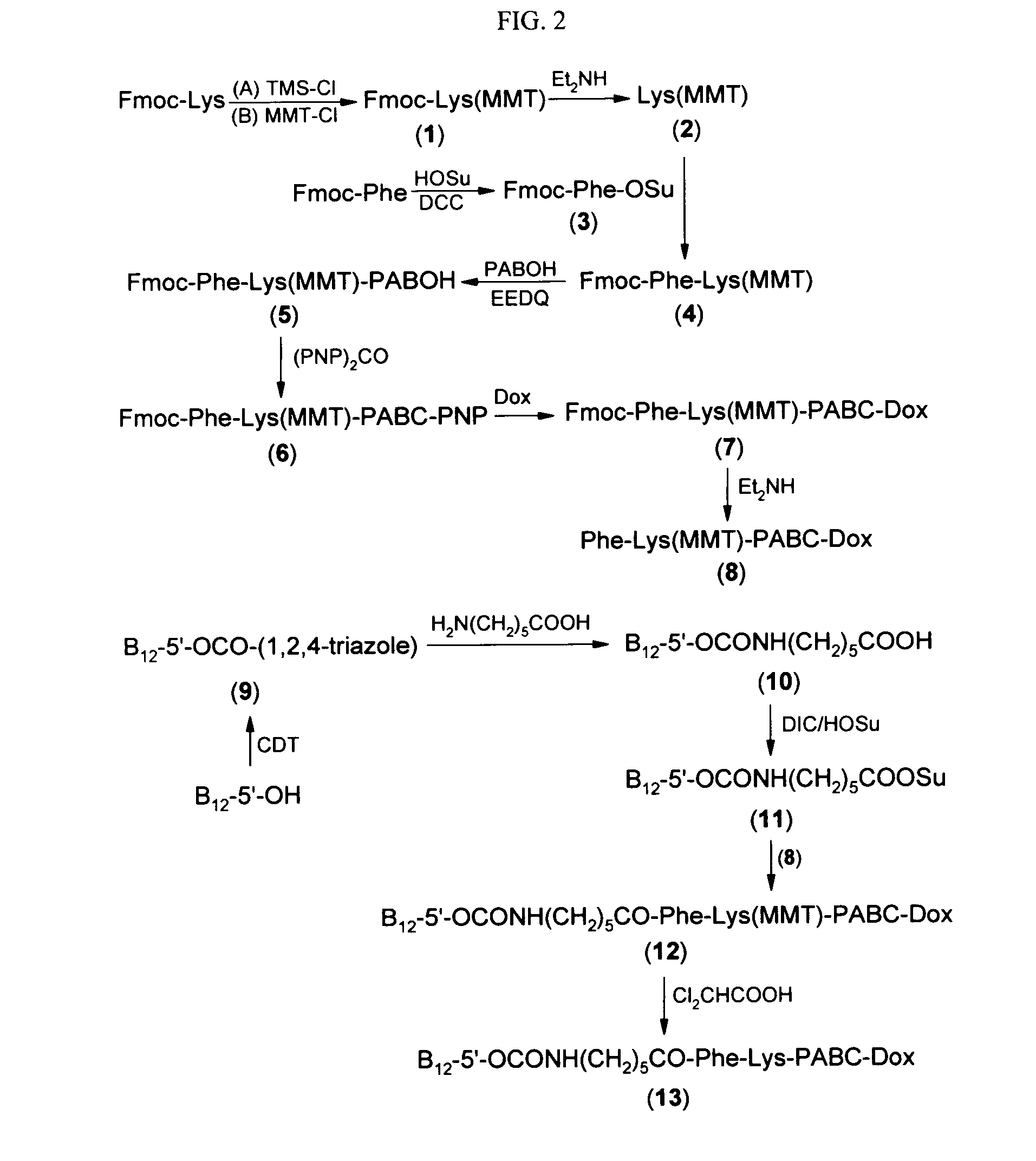
Cobalamin conjugates for anti-tumor therapy
The present invention provides a cobalamin-drug conjugate suitable for the treatment of tumor related diseases. Cobalamin is indirectly covalently bound to an anti-tumor drug via a cleavable linker and one or more optional spacers. Cobalamin is covalently bound to a first spacer or the cleavable linker via the 5′-OH of the cobalamin ribose ring. The drug is bound to a second spacer of the cleavable linker via an existing or added functional group on the drug. After administration, the conjugate forms a complex with transcobalamin (any of its isoforms). The complex then binds to a receptor on a cell membrane and is taken up into the cell. Once in the cell, an intracellular enzyme cleaves the conjugate thereby releasing the drug. Depending upon the structure of the conjugate, a particular class or type of intracellular enzyme affects the cleavage. Due to the high demand for cobalamin in growing cells, tumor cells typically take up a higher percentage of the conjugate than do normal non-growing cells. The conjugate of the invention advantageously provides a reduced systemic toxicity and enhanced efficacy as compared to a corresponding free drug.
Owner:INFLABLOC PHARMA
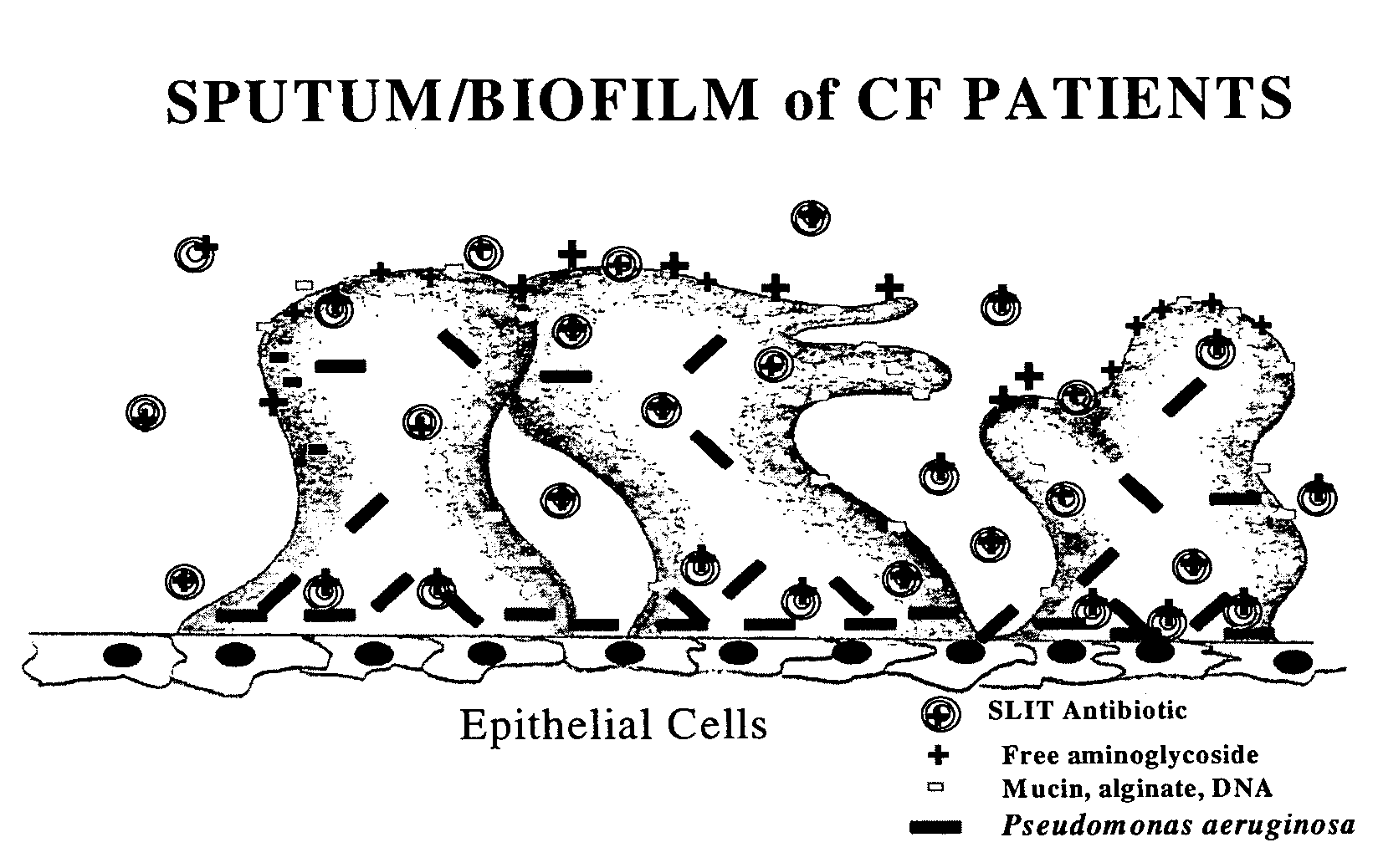
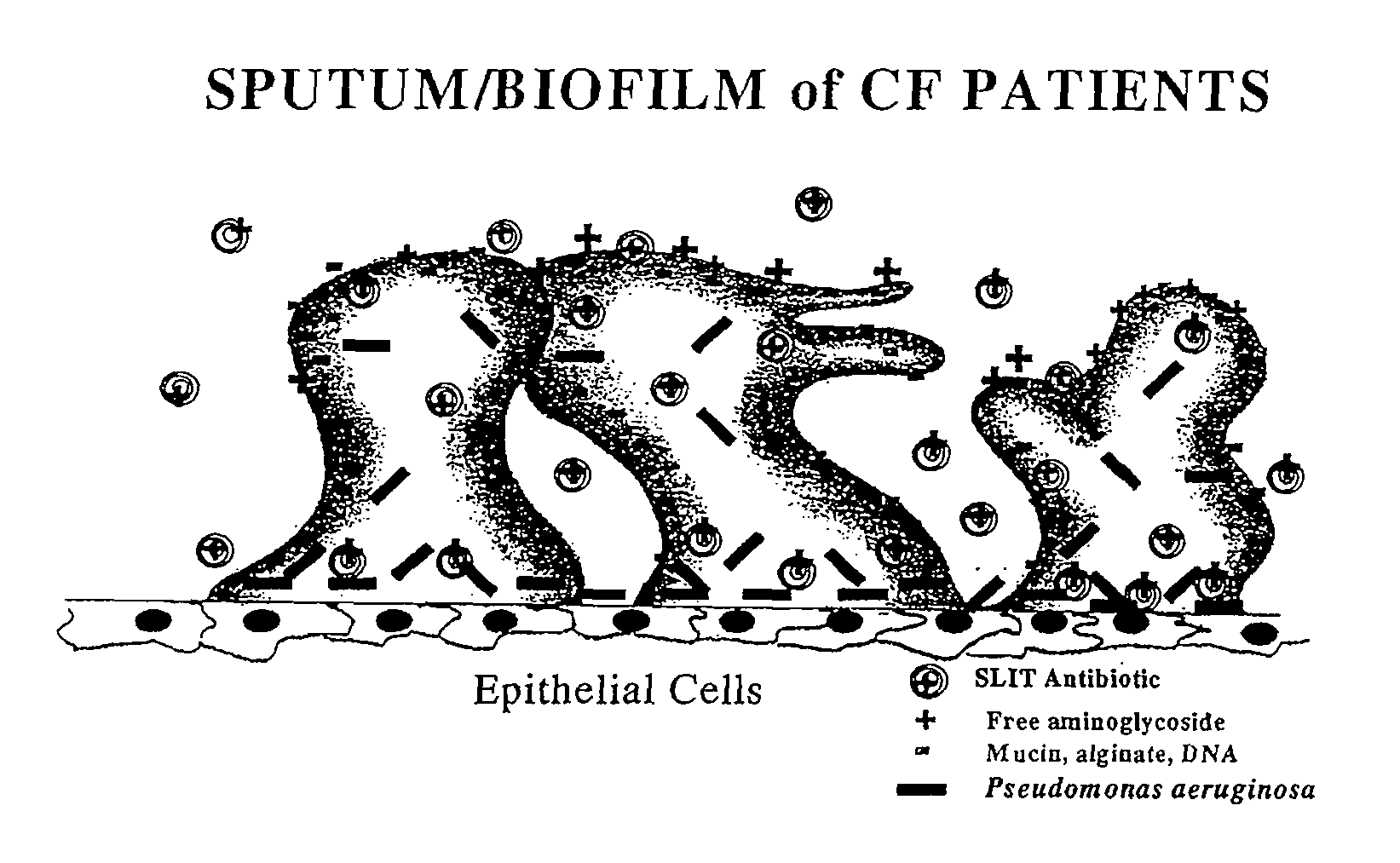
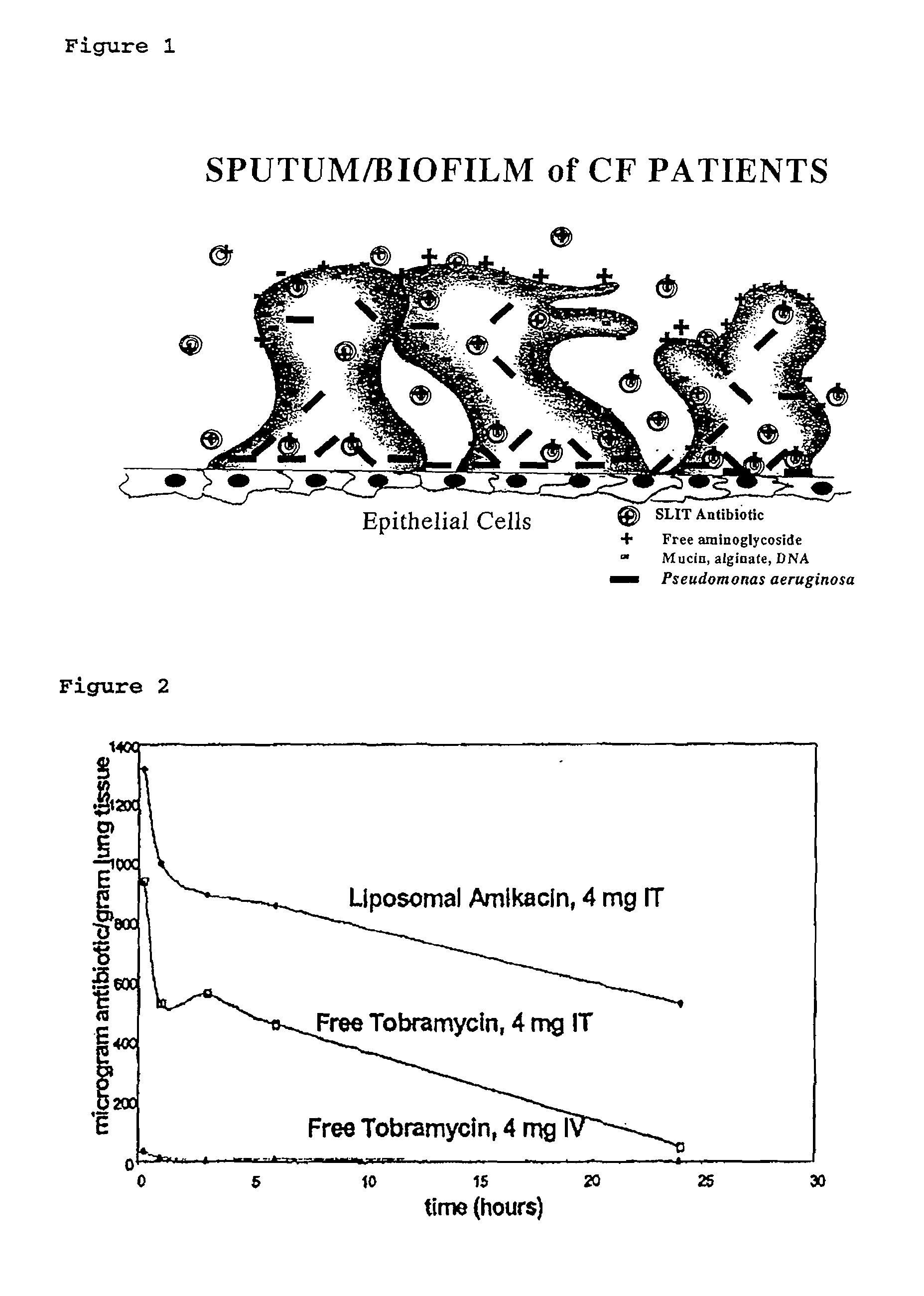
Sustained release of antiinfectives
ActiveUS7544369B2Reduce maintenanceEffective targetingAntibacterial agentsPowder deliveryPulmonary infectionCystic fibrosis lungs
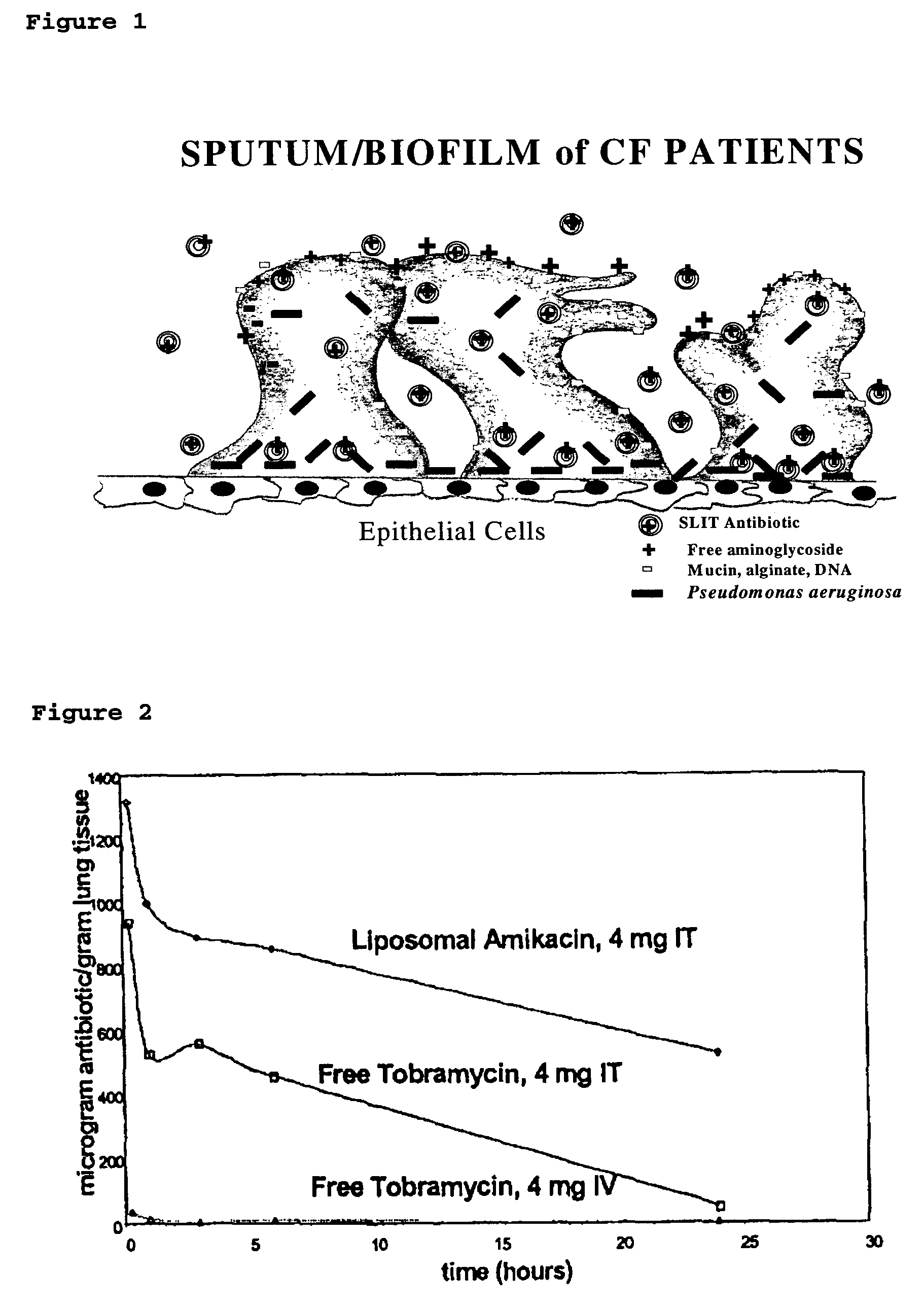
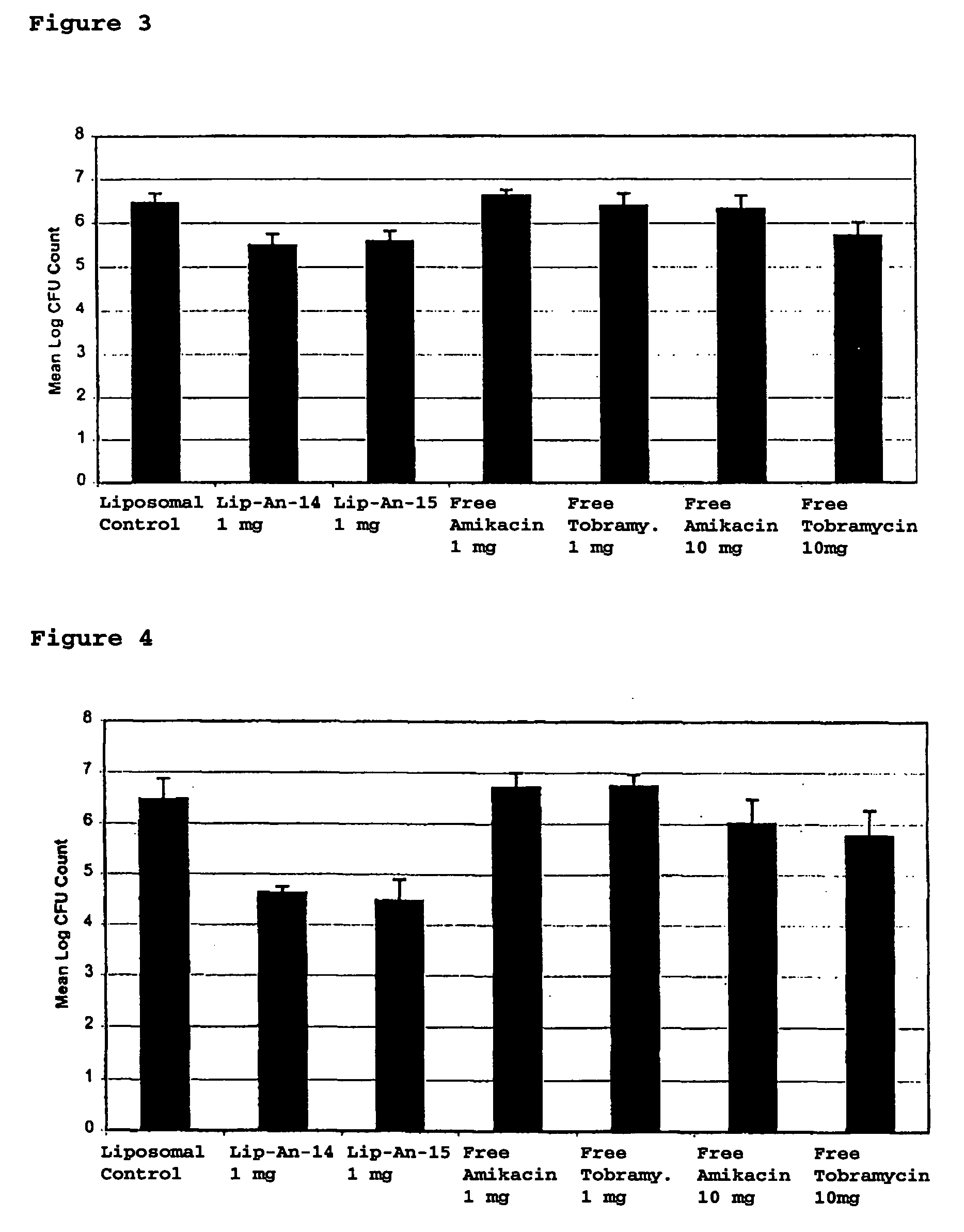
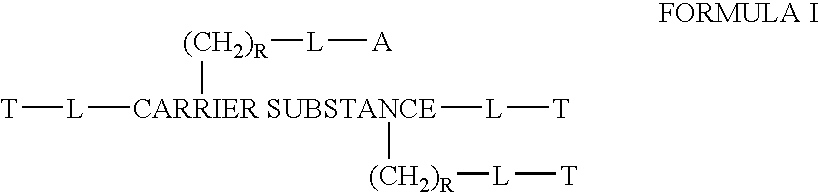
Provided, among other things, is a method of treating or ameliorating pulmonary infection in a cystic fibrosis patient comprising pulmonary administration of an effective amount of a liposomal / complexed antiinfective to the patient, wherein the (i) administrated amount is 50% or less of the comparative free drug amount, or (ii) the dosing is once a day or less, or (iii) both.
Owner:INSMED INC
Novel improved compositions for cancer therapy
InactiveUS20100166872A1Reduced chemotherapy-induced side-effectsOrganic active ingredientsBiocideDocetaxel-PNPSide effect
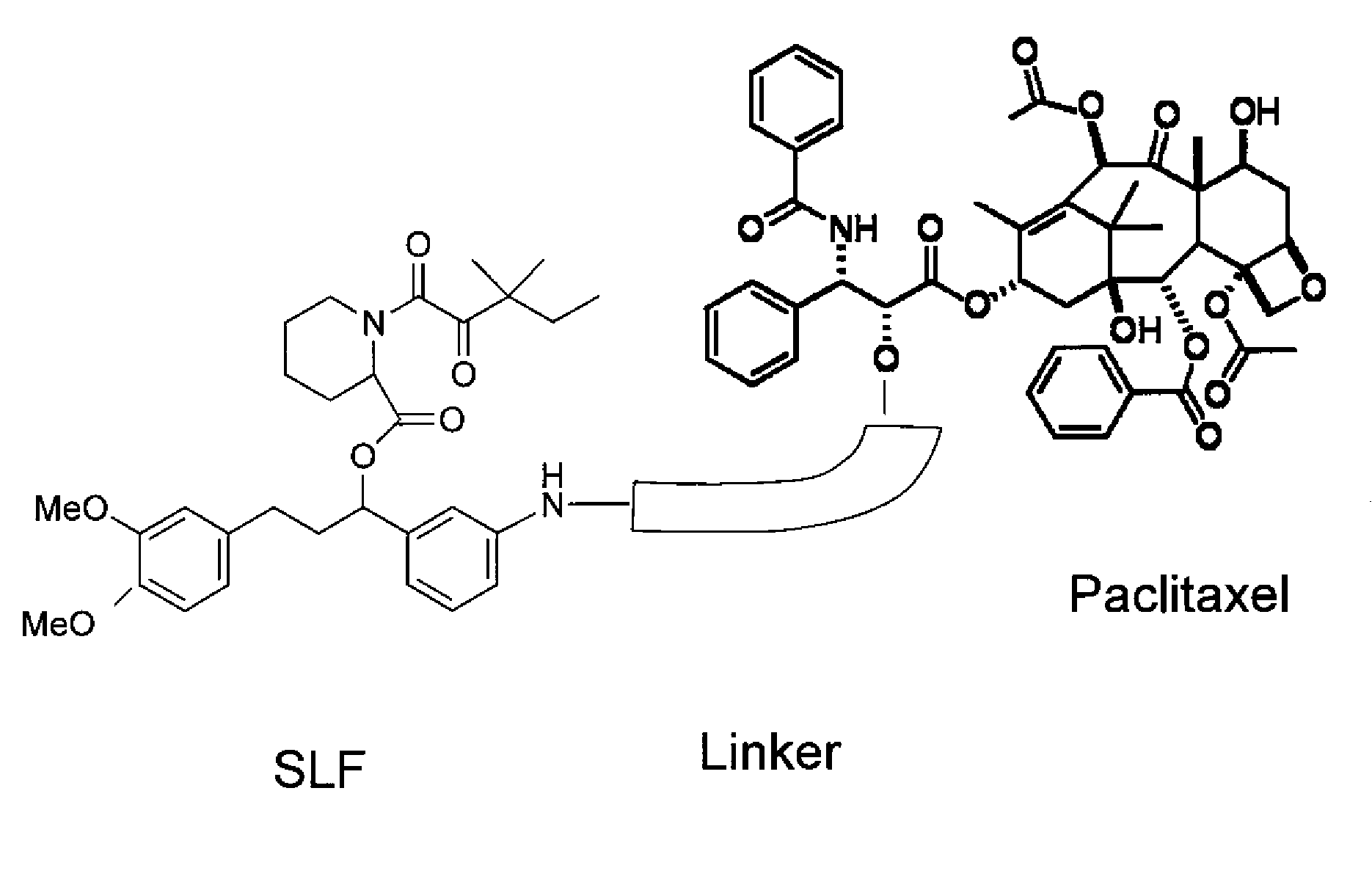
The present invention relates to novel and improved compositions of anticancer drugs, preferably taxanes, such as paclitaxel and docetaxel, their derivatives or their analogues, methods of manufacturing these compositions and methods of fractionating the particles in particular size range and methods of treating cancer patients with these compositions, which provide reduced chemotherapy-induced side-effects especially reduced chemotherapy-induced-alopecia. The composition is such that there is substantially no free drug in the said composition.
Owner:PANACEA BIOTEC
Chloroquine combination drugs and methods for their synthesis
InactiveUS20070060499A1Facilitated releaseReduce needBiocideGenetic material ingredientsActive agentSynthesis methods
This invention discloses compositions of chloroquine-coupled active agents, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the active agent is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to an active agent directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing active agents for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and active agents to their site of action.
Owner:KOSAK KENNETH M
Combined Active and Passive Targeting of Biologically Active Agents
InactiveUS20070287680A1Good curative effectLow in DOPolypeptide with localisation/targeting motifOrganic active ingredientsActive agentEfficacy
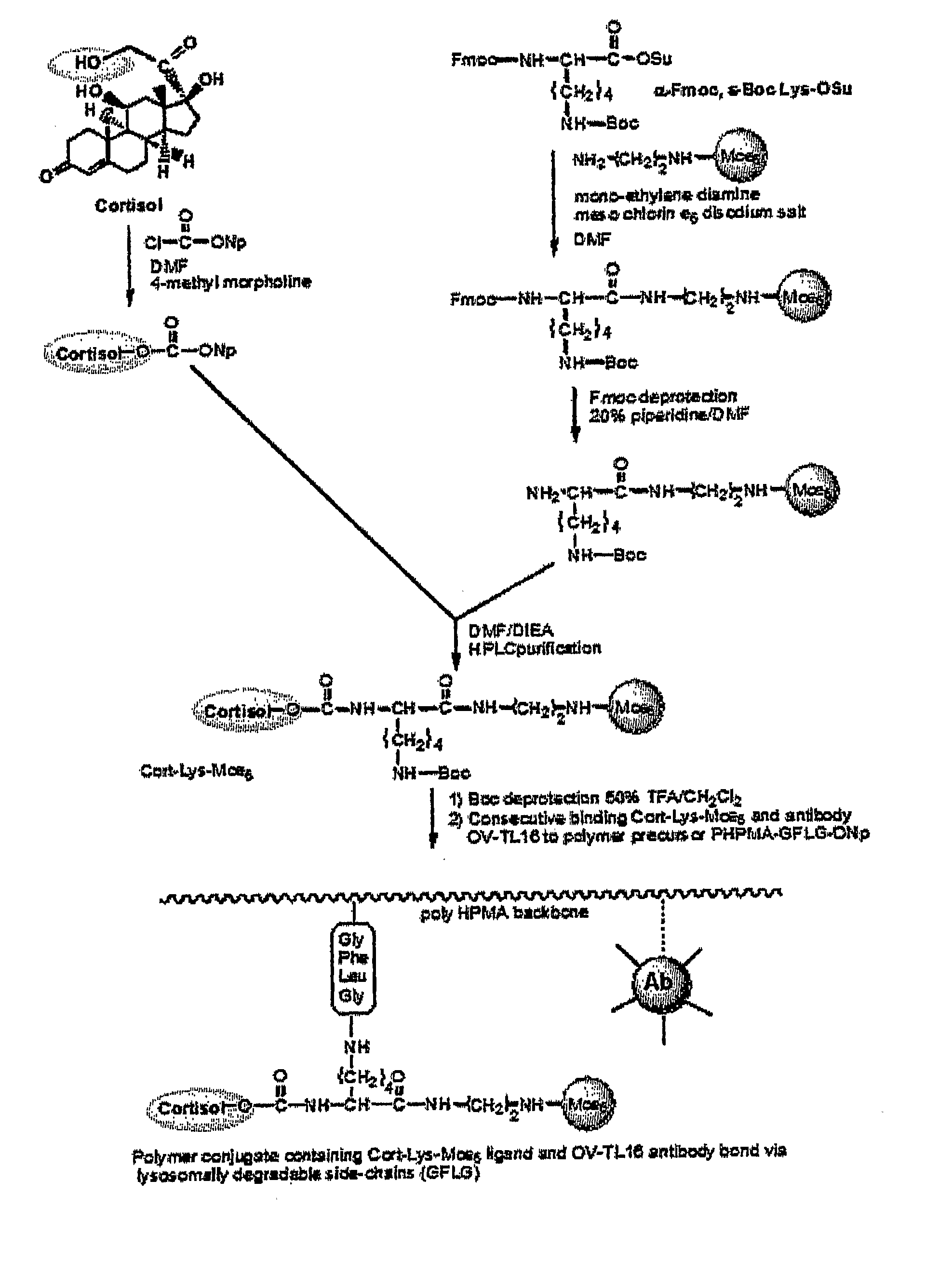
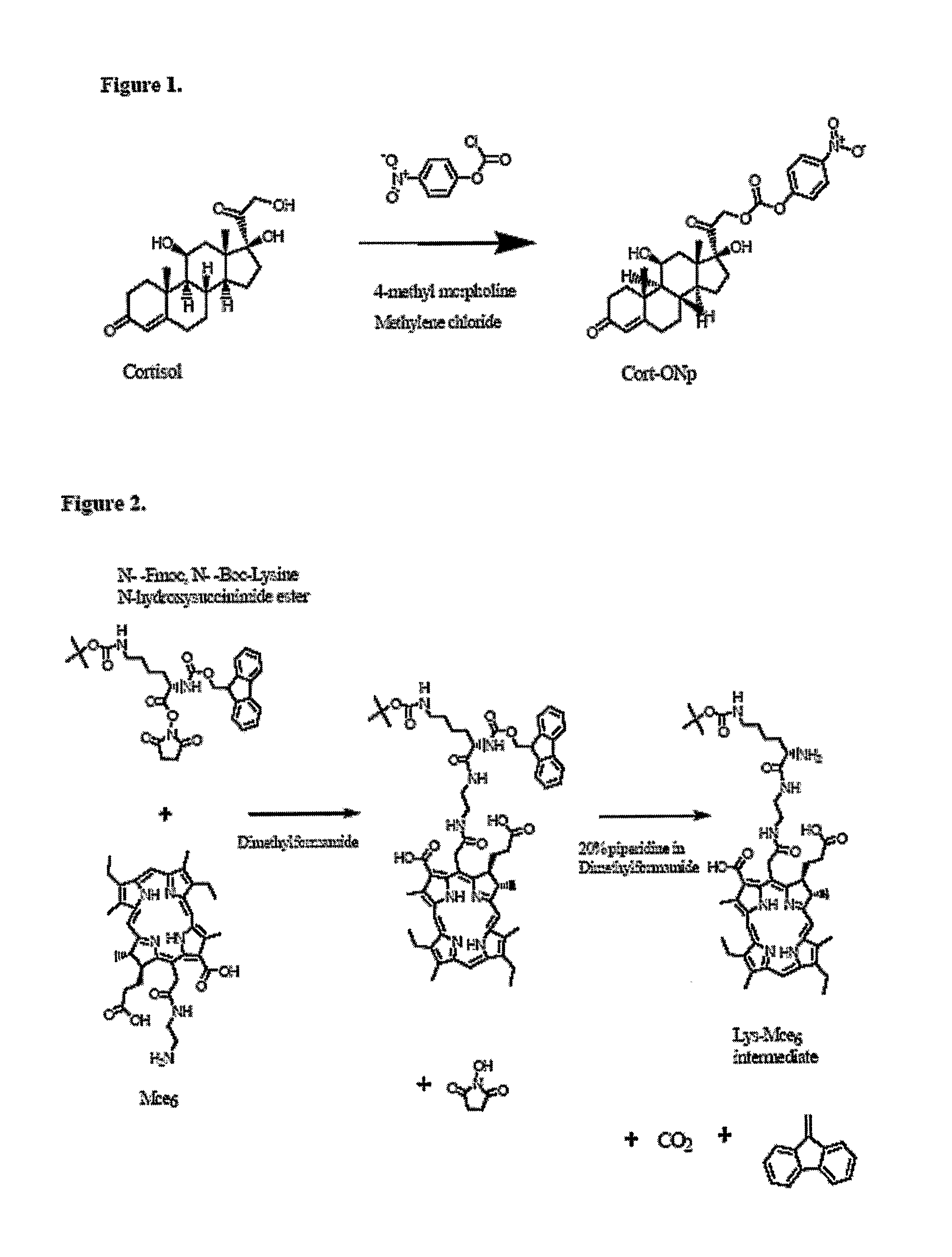
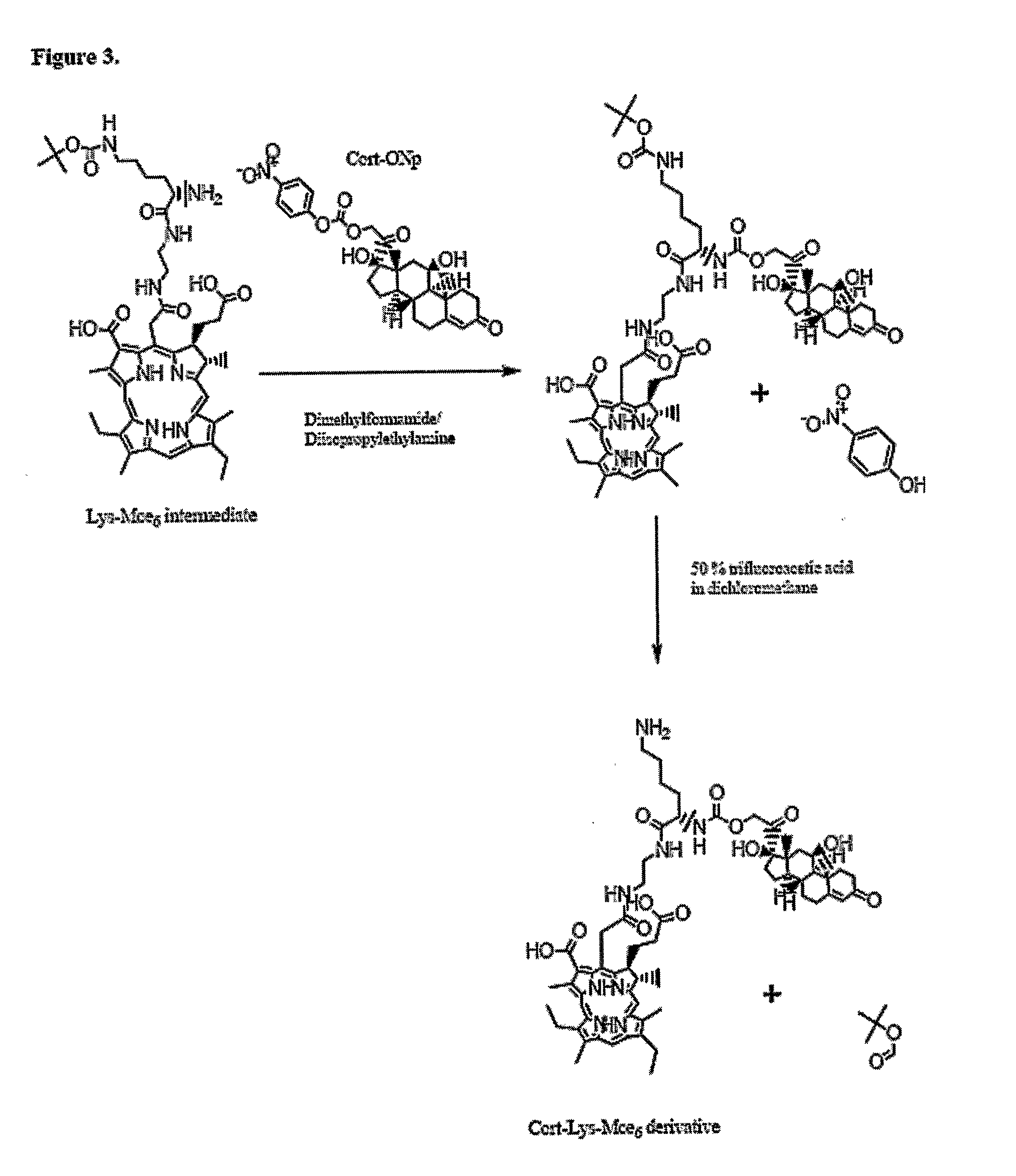
Disclosed is a conjugate comprising a biologically active agent (drug) linked to a subcellular targeting moiety that targets a drug specifically to the nucleus. Targeting is achieved by attaching a steroid hormone (or an analog) to the drug. The steroid hormone attached to the drug binds its corresponding receptor, the formation of the receptor-ligand complex results in the internalization of the complex into the nucleus, thus resulting in nuclear translocation of the drug. Also disclosed is a conjugate (comprising the complex of the drug and the steroid hormone) bound to a polymer by spacers allowing for concurrent passive targeting to the tumor cell (afforded by attachment to the polymer by the EPR effect) and nuclear targeting of the conjugate (due to the presence of the steroid). Using a suitable degradable spacer allows for the release of free drug in the tumor and enhances nuclear targeting efficacy. The polymer can be further linked to a cellular targeting molecule, where the targeting molecule directs the polymer to specific cells. One may thus be able to effectively target drugs to the nucleus of tumor cells. With little or modifications, several therapeutic agents can be targeted using the invention.
Owner:UNIV OF UTAH RES FOUND
Sustained Release of Antiinfectives
InactiveUS20100068257A1Reduce maintenanceEffective targetingPowder deliveryBiocidePulmonary infectionCystic fibrosis lungs
Provided, among other things, is a method of treating or ameliorating pulmonary infection in a cystic fibrosis patient comprising pulmonary administration of an effective amount of a liposomal / complexed antiinfective to the patient, wherein the (i) administrated amount is 50% or less of the comparative free drug amount, or (ii) the dosing is once a day or less, or (iii) both.
Owner:INSMED INC
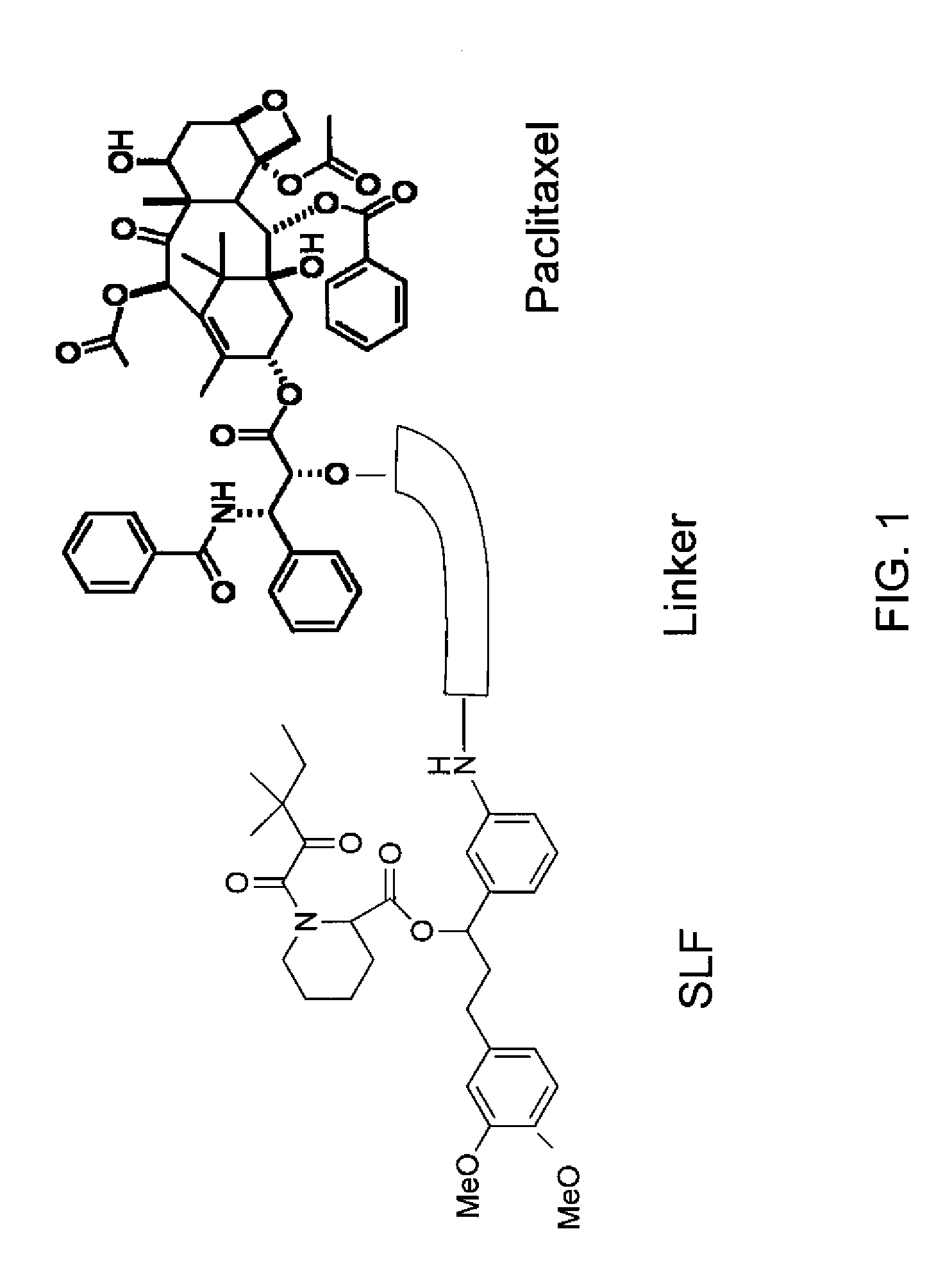
Methylene carbamate linkers for use with targeted-drug conjugates
ActiveUS20160303254A1Pharmaceutical non-active ingredientsImmunological disordersCarbamateTargeting ligands
The present invention provides Lig-and-Drug Conjugates and Drug-Linker Compounds comprising a methylene carbamate unit. The invention provides inter alia, Ligand-Drug Conjugates, wherein the Ligand-Drug Conjugate is comprised of a Self-immolative Assembly Unit having a methylene carbamate unit for conjugation of a drug to a targeting ligand, methods of preparing and using them, and intermediates thereof. The Ligand-Drug Conjugates of the present invention are stable in circulation, yet capable of inflicting cell death once free drug is released from a Conjugate in the vicinity or within tumor cells.
Owner:SEAGEN INC
Preparation and application of hyaluronic acid-modified amphipathic chitosan derivative carrier with tumor microenvironment specificity drug release effect
InactiveCN106581686AEvade captureImprove stabilityPharmaceutical non-active ingredientsEmulsion deliveryTreatment effectDrug release
The invention relates to a hyaluronic acid-modified amphipathic chitosan derivative carrier with tumor microenvironment specificity drug release effect. The hyaluronic acid-modified amphipathic chitosan derivative carrier is characterized in that firstly, a hydrophilic group is introduced into a chitosan skeleton; then, a hydrophobic group is introduced into a specifically degradable link arm containing disulfide bonds, so as to realize the amphipathic function; the amphipathic derivative is assembled into nanomicelle by self in a waterborne medium, and is further modified by a charge adsorbing principle to target the molecular hyaluronic acid; the nanomicelle can effectively load an anti-tumor drug, and the hyaluronic acid is targeted to the tumor microstructure and then is degraded by hyaluronic acid enzyme in focus cells, so that the drug can be quickly released from the nanomicelle to act on the focal part, thereby obviously improving the concentration, therapy effect and biological utilization degree of free drug on the focal part. The hyaluronic acid-modified amphipathic chitosan derivative carrier has the advantages that the carrier which carries pharmaceutical activity or pharmacological activity molecules can be applied to internal injection of blood vessels or muscles or oral administration, so as to obviously improve the anti-tumor activity of drug; the preparation method is simple, the technology is matured, and the preparation method is suitable for large-scale production.
Owner:CHINA PHARM UNIV
Chloroquine coupled nucleic acids and methods for their synthesis
InactiveUS20060040879A1Overcome limitationsFacilitated releaseSugar derivativesGenetic therapy composition manufactureTherapeutic effectPharmaceutical Substances
This invention discloses compositions and methods for preparing chloroquine-coupled nucleic acid compositions. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the nucleic acid needs to be released, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a nucleic acid directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing nucleic acids for therapeutic or other medical uses. The invention also discloses nucleic acid carrier compositions that are coupled to targeting molecules for targeting the delivery of nucleic acids to their site of action.
Owner:KOSAK KENNETH
Pharmacokinetics of protease inhibitors and other drugs
A method for modulating at least one pharmacokinetic property of a protease inhibitor upon administration to a host is provided. One administers to the host an effective amount of a bifunctional compound of less than about 5000 daltons comprising the protease inhibitor or an active derivative thereof and a pharmacokinetic modulating moiety. The pharmacokinetic modulating moiety binds to at least one intracellular protein. The bifunctional compound has at least one modulated pharmacokinetic property upon administration to the host as compared to a free drug control that comprises the protease inhibitor.
Owner:AMPLYX PHARMA INC
Ductless medicine fluid infusion device
ActiveCN104784777ALow costPositive displacement pump componentsMedical devicesFluid infusionElectrical connection
A catheter-free drug fluid infusion device, comprising a controller (11) and a pump (12) combined with the controller (11). The controller (11) comprises a first housing having a first built-in circuit. The first housing is provided with a first engagement portion and a first insertion portion electrically connected to the first built-in circuit. The pump (12) comprises a second housing having a second built-in circuit, a drug storage cylinder, a piston, a push rod, a driving means and a battery. The second housing is provided with a second engagement portion correspondingly engaged with the first engagement portion and a second insertion portion electrically connected to the second built-in circuit. The second insertion portion is correspondingly inserted in the first insertion portion to realize electrical connection between the first built-in circuit and the second built-in circuit. The catheter-free drug fluid infusion device employs a detachable structural design to reduce the cost, so as to solve the problem that a drug fluid infusion device in the prior art has complex operation, large size and high cost.
Owner:MEDTRUM TECH
Drug delivery devices and methods for drug delivery
ActiveUS20160008271A1Organic active ingredientsMedical devicesControlled releasePharmaceutical formulation
An orifice-free drug delivery device is provided. In an embodiment, the device includes a body having at least one water-permeable wall bounding a reservoir defined within the body. A drug formulation is disposed within the reservoir. The body has an elastic portion and at least one restraining plug closing off an opening of the body. The opening is in fluid communication with the reservoir, and the restraining plug contacts the elastic portion of the body and controls release of the drug from the device by the transient formation of one or more microchannels between the elastic portion of the body and the at least one restraining plug. The elastic portion may define an opening having an inner diameter, which is exceeded by the outer diameter of the restraining plug by at least 3%.
Owner:TARIS BIOMEDICAL
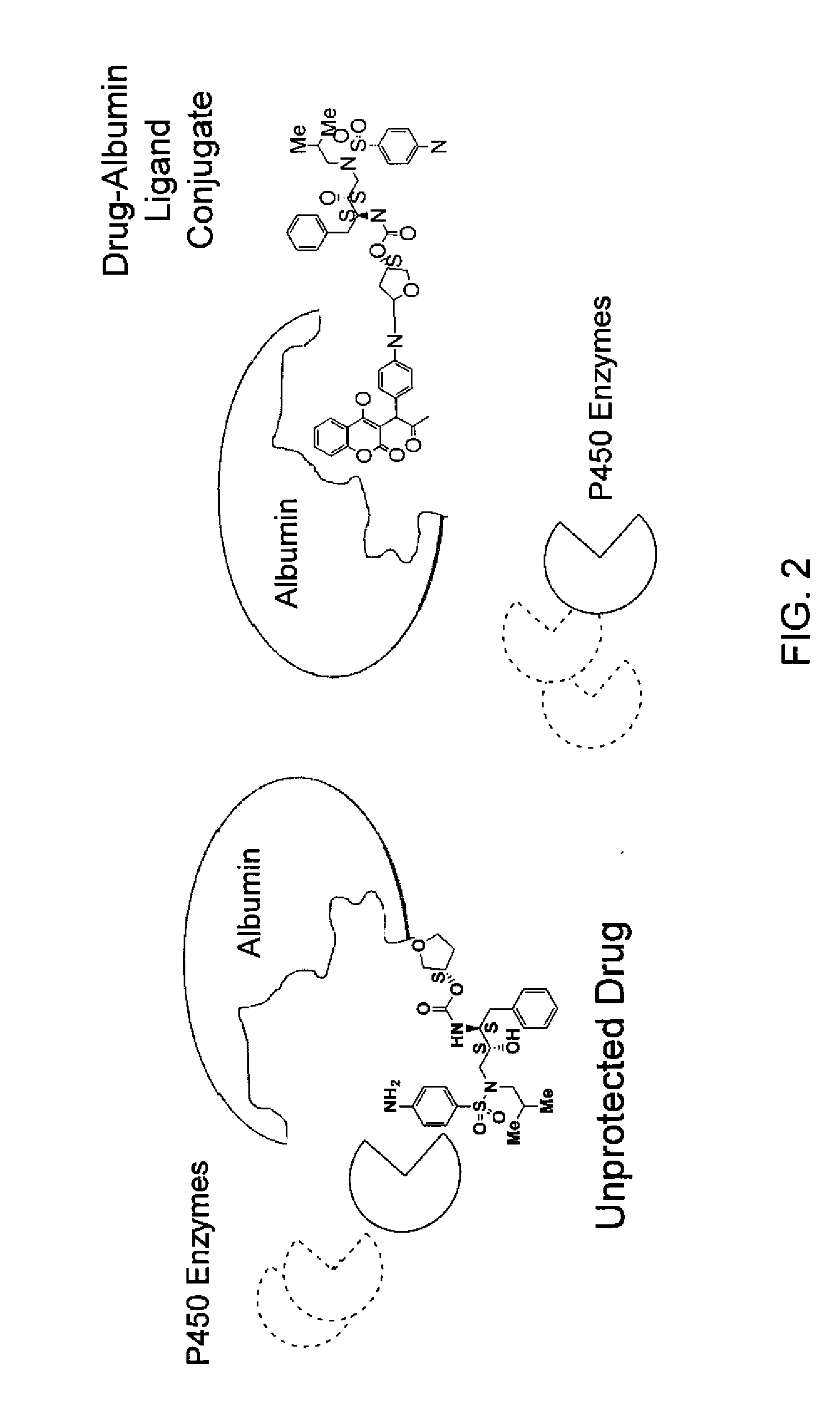
Methods and compositions for optimizing blood and tissue stability of camptothecin and other albumin-binding therapeutic compounds
InactiveUS20100240602A1Improve stabilityHigh affinitySalicyclic acid active ingredientsBiocideNitrocamptothecinHIV positives
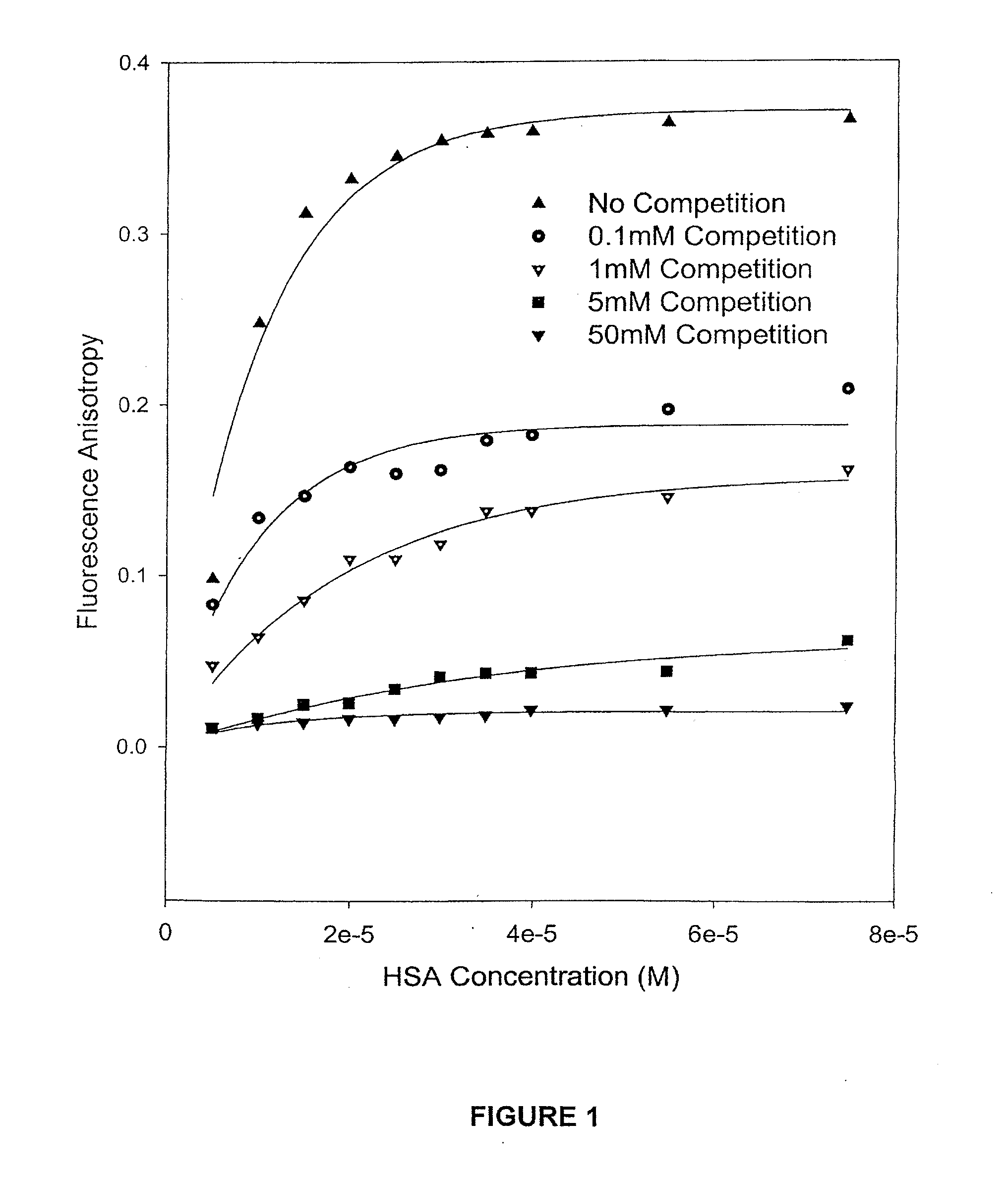
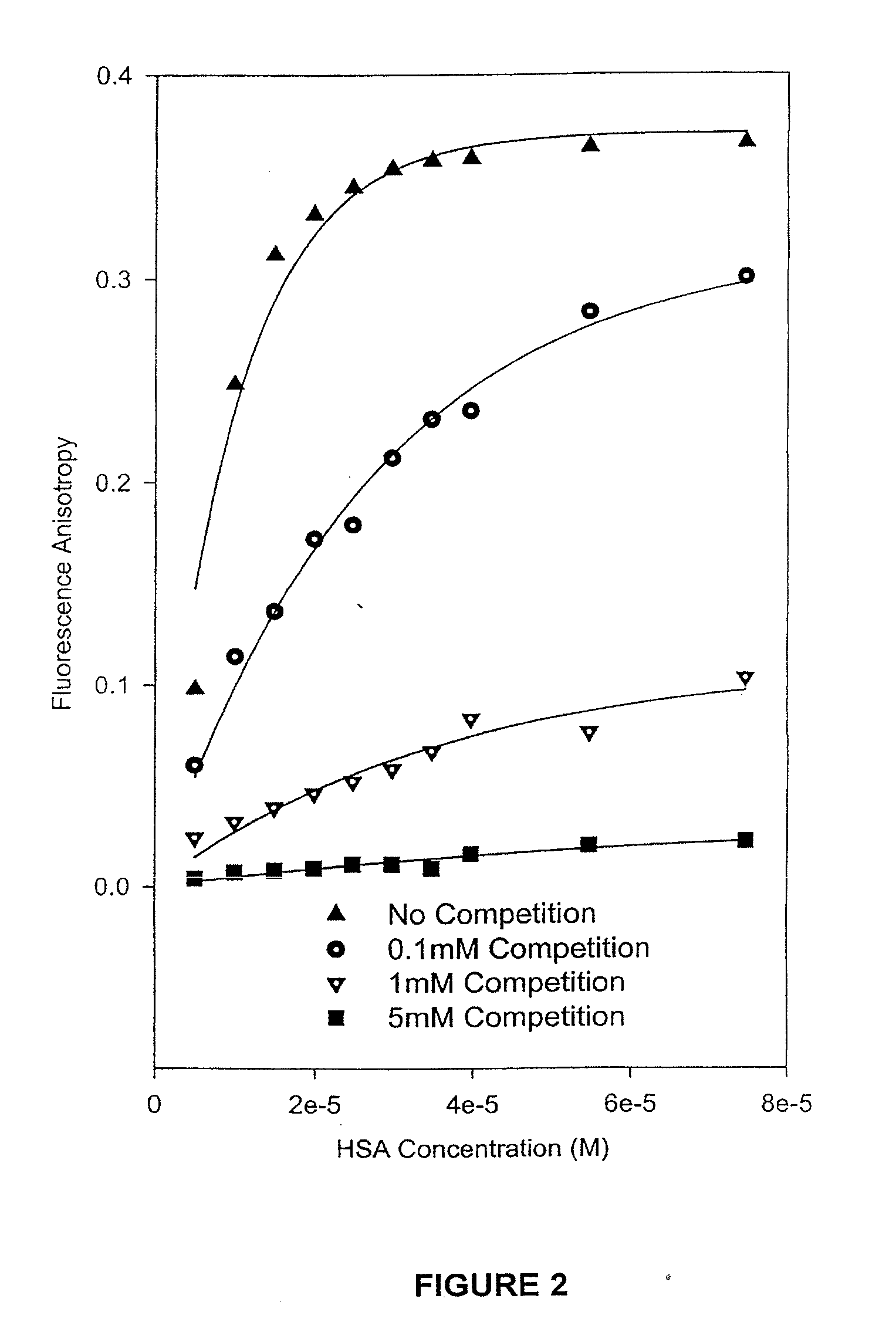
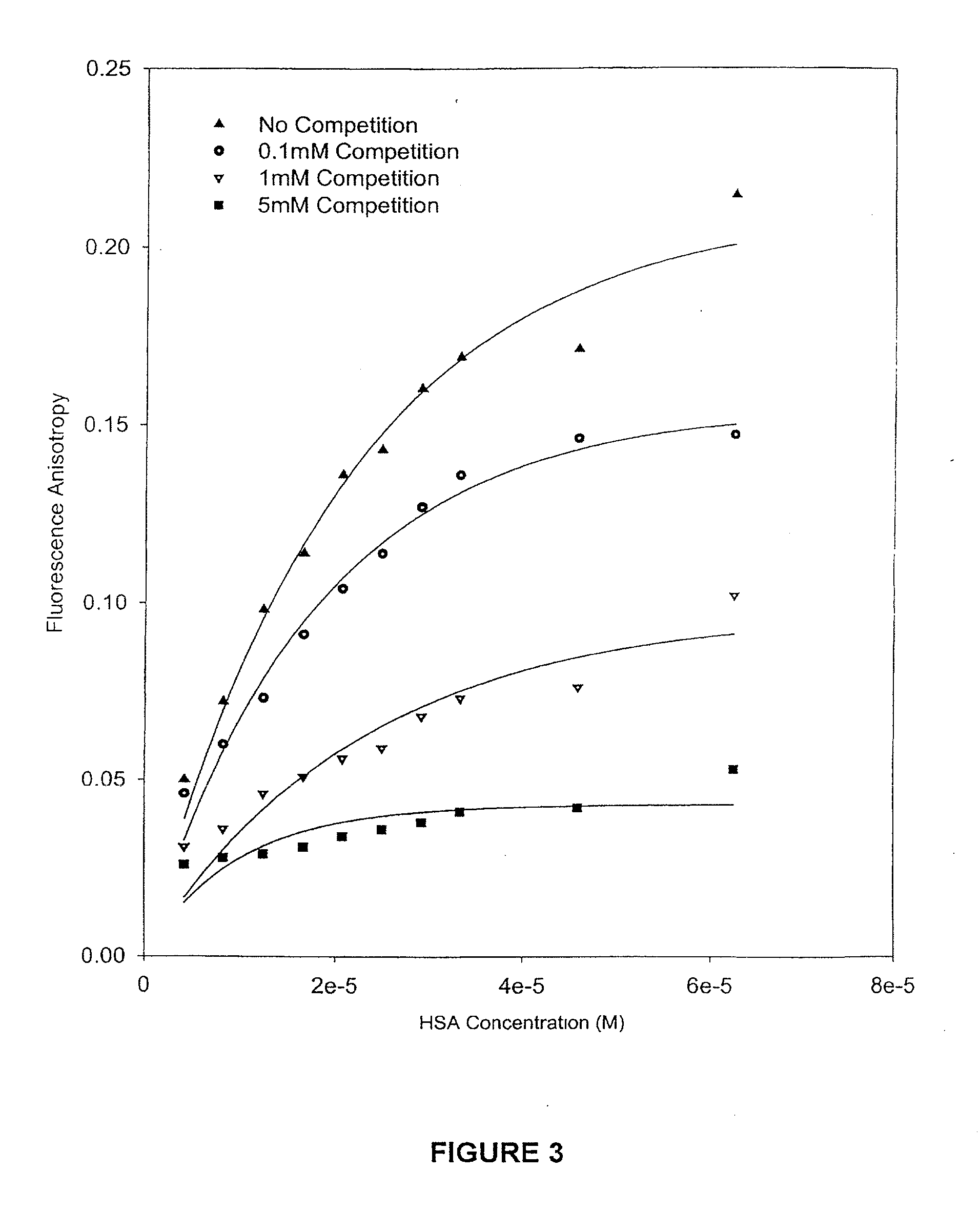
The present invention provides methods and formulations for optimizing the anti-cancer and anti-HIV activities of a camptothecin drug, including camptothecin and its related analogs including 9-aminocamptothecin and 9-nitrocamptothecin. The invention involves methodologies and formulations that limit human serum albumin-mediated reduction of the anti-cancer and anti-HIV effects of the camptothecins, and the methods and formulations provide combination therapies in which binding of the camptothecin agent to human serum albumin can be modulated by the administration of a competing agent such as ibuprofen, clofibrate or clofibric acid that also binds human serum albumin. Reduced camptothecin drug binding to human serum albumin can result in elevated camptothecin free drug levels and thus improve the effectiveness of treatment regimens involving these drugs. Further agents such as methotrexate and AZT can also be used in cancer and HIV-positive patients employing camptothecin drugs.
Owner:BURKE THOMAS G +1
Combinatorial improvement of bifunctional drug properties
InactiveUS20090054334A1High affinityImprove propertiesBiocidePeptide/protein ingredientsAvidityChemistry
A method is provided for improving at least one pharmacokinetic property and maintaining or improving affinity of a therapeutic upon administration to a host. In the method, one administers to the host an effective amount of a bifunctional compound of less than about 5000 Daltons comprising the therapeutic or an active derivative, fragment or analog thereof and a recruiter ligand moiety. The recruiter ligand moiety binds to at least one biomoiety. The bifunctional compound has at least one modulated pharmacokinetic property upon administration to the host and equivalent or greater affinity for a target of the therapeutic as compared to a free drug control that comprises the therapeutic. In addition, the overall drug efficacy is improved by the steric bulk of the bifunctional complexed with the recruited biomoiety.
Owner:AMPLYX PHARMA INC
Submicron liposome suspensions obtained from preliposome lyophilizates
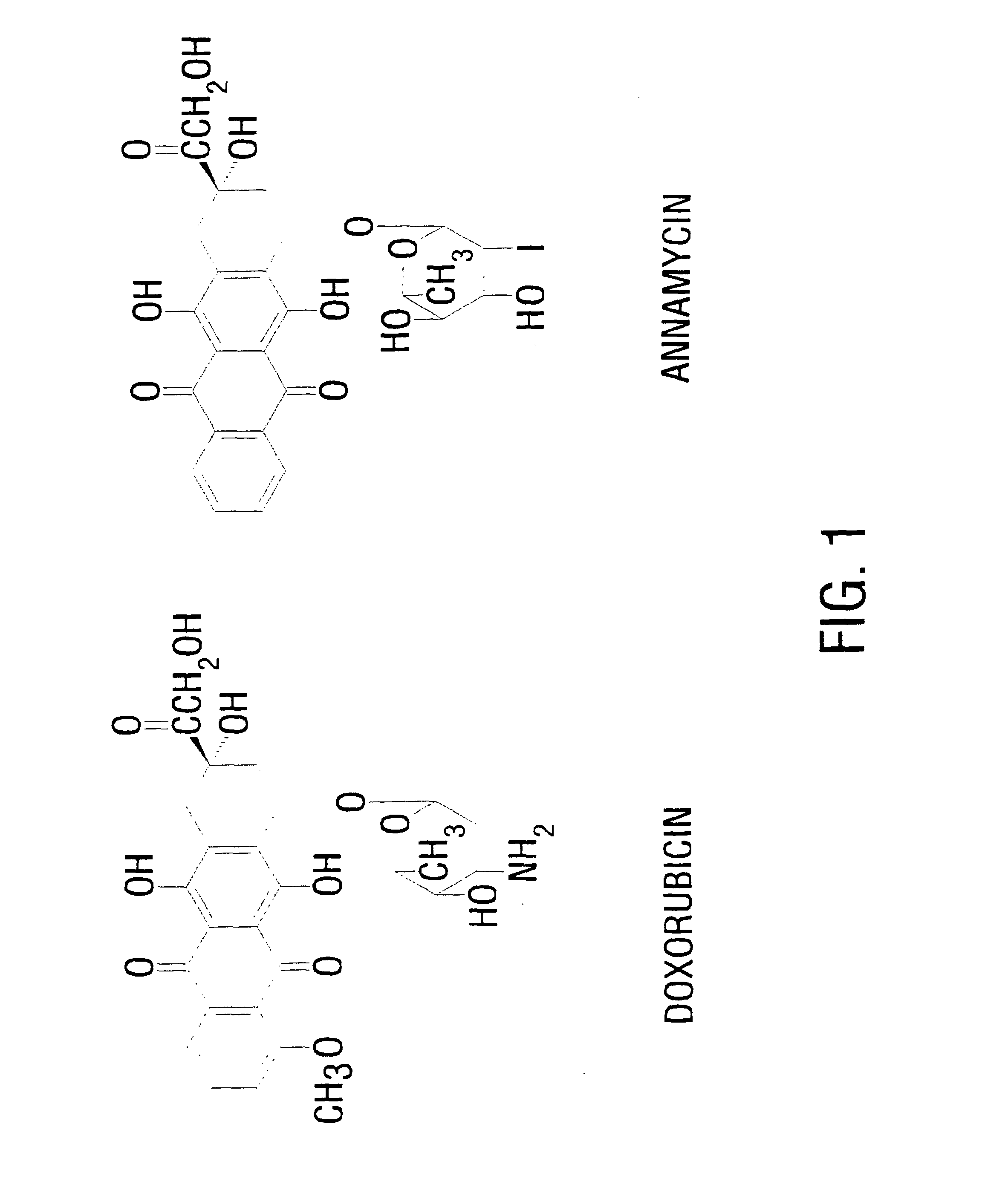
InactiveUS7238366B1Severe adverse effect on liposome integrityShorten hydration timePowder deliveryTetracycline active ingredientsLipid formationLiposome Vesicle
This invention provides an aqueous / t-butanol solvent-system, facile reconstitute, submicron-reconsitiute preliposome-lyophilaye and method of its preparation and use.In one embodiment this entails a modified method for the preparation of a submicron and stable liposome formulation of the non-cross-resistant anthracycline Annamycin is described. The optimal lipid composition was DMPC:DMPG at a 7:3 molar ratio and the optimal lipid:drug weight ratio 50:1. The selected formulation is a preliposome lyophilized powder that contains the phospholipids, Annamycin, and 1.7 mg Tween 20 per mg of Annamycin. The liposome suspension is obtained on the day of use by adding normal saline at 37° C. (1 ml per mg Annamycin) and hand-shaking for one minute. The presence of Tween 20 is essential in shortening the reconstitution step (from >2 hours to 1 minute), avoiding the early formation of free drug crystals, and reducing the median particle size (from 1.5 μm to 0.15-0.20 μm) without destruction of the liposome vesicles. The chemical stability of the preliposome powder at room temperature was >3 months and the chemical and physical stability of the liposome suspension at room temperature >24 hours. The in vitro cytotoxicity of the formulation was equivalent to that prepared by the standard evaporation method. The results of the study indicate that small amounts of surfactant may be used to enhance the reconstitution step and reduce the liposome size of lyophilized liposome formulations of lipophilic drugs.
Owner:BOARD OF REGENTS
Pharmaceutical composition comprising at least one anticancer drug and at least one polymer
InactiveCN101495148AReduce leakageLittle side effectsAntineoplastic agentsPharmaceutical active ingredientsSide effectDocetaxel
The present invention relates to novel and improved compositions of anticancer drugs, preferably taxanes, such as paclitaxel and docetaxel, their derivatives or their analogues, methods of manufacturing these compositions and methods of fractionating the particles in particular size range and methods of treating cancer patients with these compositions, which provide reduced chemotherapy-induced side-effects especially reduced chemotherapy-induced- alopecia. The composition is such that there is substantially no free drug in the said composition.
Owner:PANACEA BIOTEC
Methods for detecting substances in biological samples
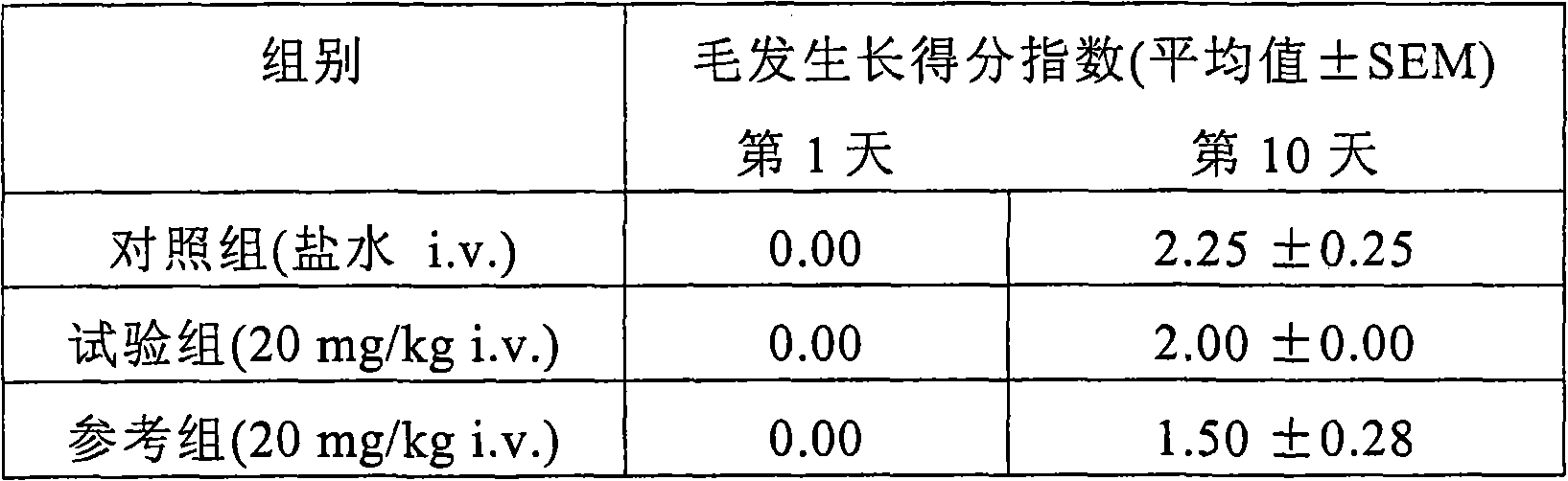
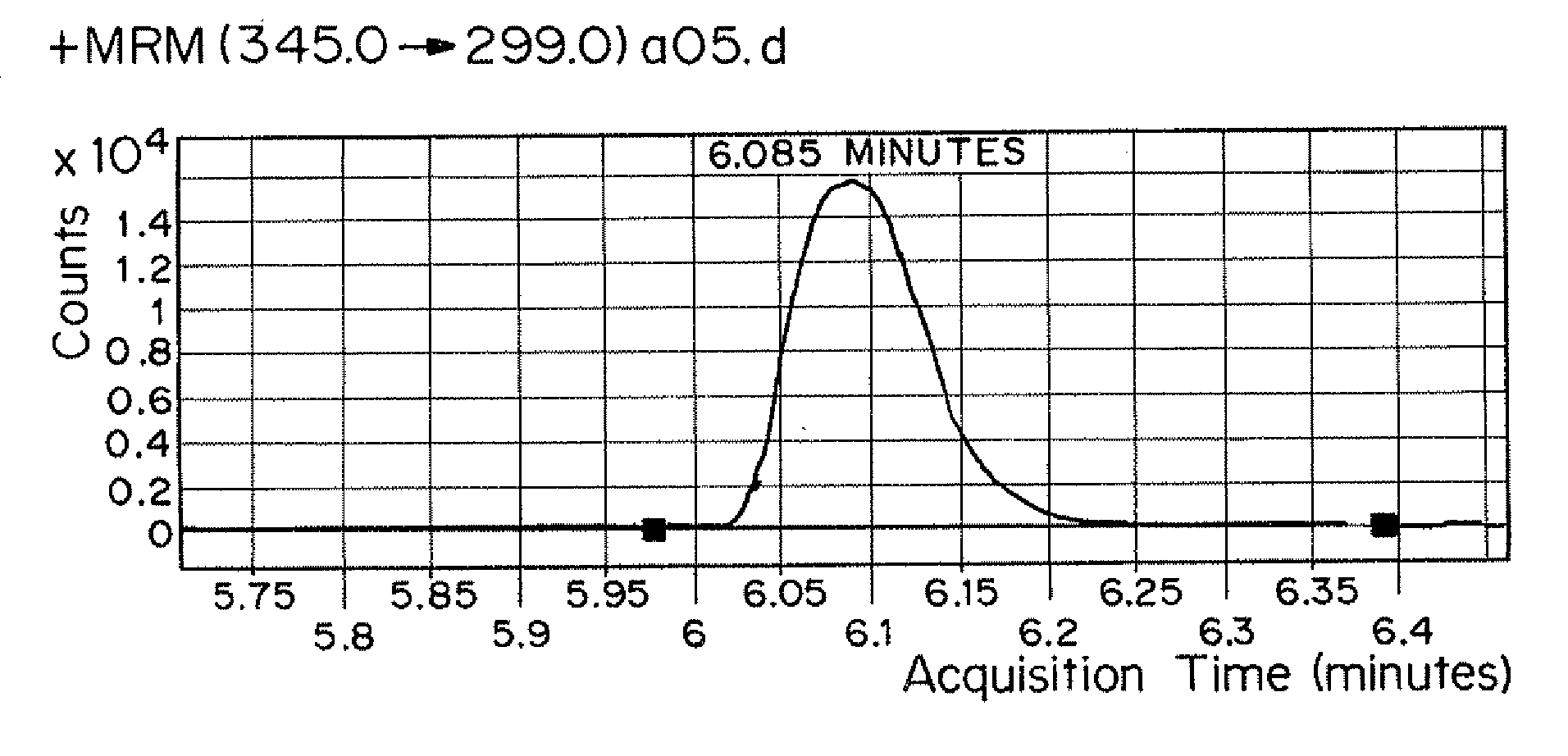
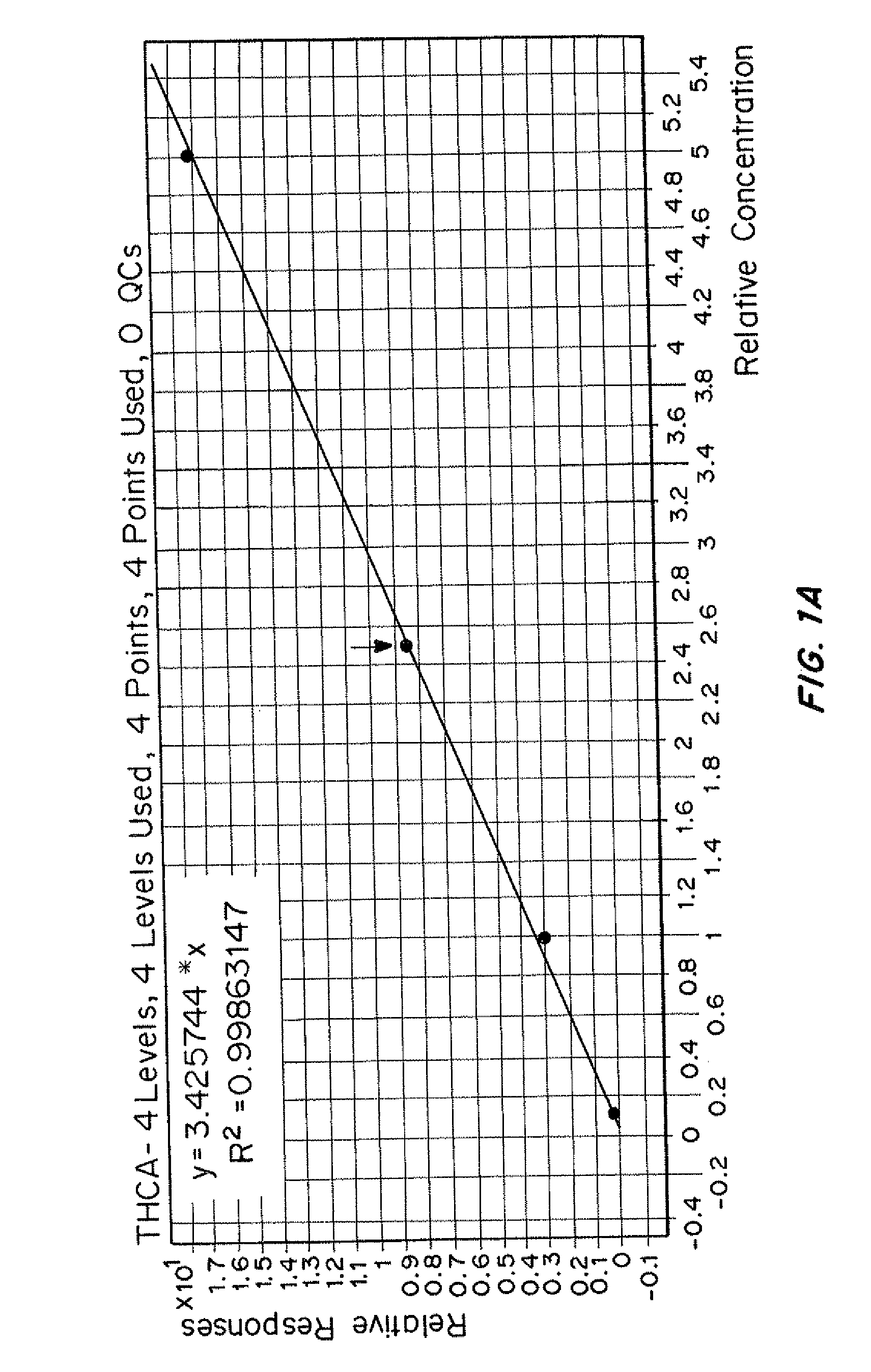
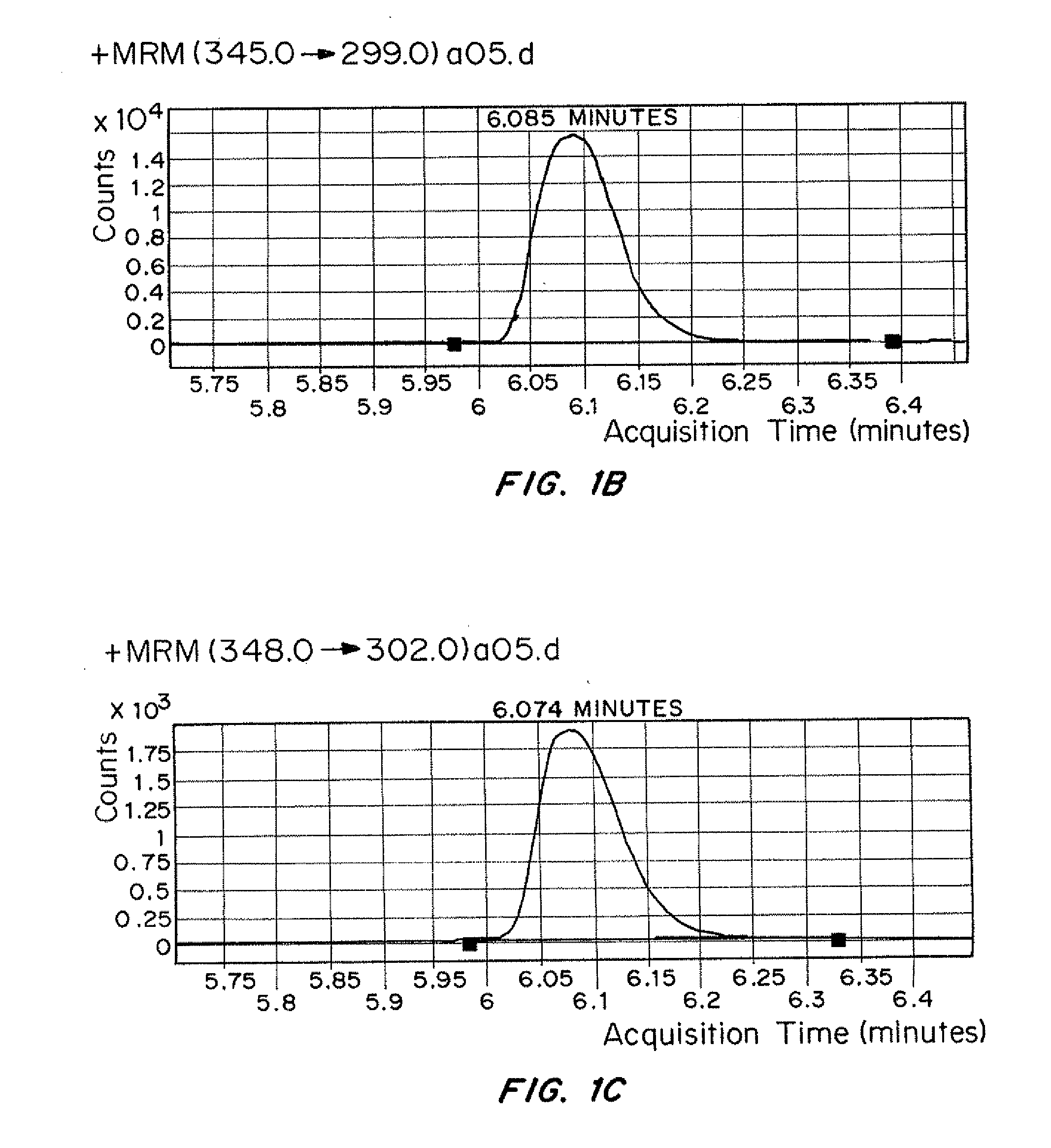
InactiveUS20120153138A1Low costAccurate detectionIsotope separationMass spectrometersMedicineTriple quadrupole mass spectrometry
Methods for simultaneously detecting multiple drug classes in a biological sample are described herein. In one embodiment, the biological sample is taken from an animal, such as a human. Suitable classes of drugs include drugs prone to abuse and prescription medications. In one embodiment, the biological sample is enzymatically hydrolyzed to liberate free drug in the sample. Once the biological sample has been prepared, the sample is extracted using solid-phase extraction (SPE). Following solid phase extraction (SPE), the resulting eluate containing the compounds to be detected is diluted and injected into a liquid chromatograph coupled to a triple quadrupole mass spectrometer. Multiple classes of drugs can be analyzed simultaneously in less than about 13 minutes, preferably less than about 11 minutes, more preferably less than about 10 minutes, more preferably less than about 9 minutes, most preferably less than about 8 minutes.
Owner:ELAB CONSULTING SERVICES
Cbi derivatives subject to reductive activation
ActiveUS20110112163A1Improve readabilityMinimize seeming duplicationBiocideOrganic chemistryCytotoxicityBiological activation
A unique class of N-acyl O-amino phenol prodrugs of CBI-TMI and CBI-indole2 were synthesized and shown to be prodrugs, subject to reductive activation by nucleophilic cleavage of a weak N—O bond, effectively releasing the free drug in functional cellular assays for cytotoxic activity approaching or matching the activity of the free drug, yet remain essentially stable to ex vivo DNA alkylation conditions. Most impressively, assessment of the in vivo antitumor activity of a representative O-(acylamino) prodrug, 8, indicate that they approach the potency and exceed the efficacy of the free drug itself (CBI-indole2), indicating that the inactive prodrugs not only effectively release the free drug in vivo, but that they offer additional advantages related to a controlled or targeted release in vivo.
Owner:THE SCRIPPS RES INST
Complimentary drug delivery sheath for an implantable medical device
A complementary drug delivery sheath for implantable medical devices. The complementary drug delivery sheath is covered, impregnated or otherwise carries one or more drugs. The complementary drug-delivery sheaths are manufactured separately from to the implantable device and are operationally combined with the device subsequent to the device's manufacture and / or sterilization. For example, embodiments of the complementary drug-delivery sheaths may be configured, for example, to attain an implanted position adjacent to one or more surfaces of an implantable medical device. In certain embodiments, the sheath is configured in the form of a glove, pocket, pouch, or the like to receive and to partially or completely wrap around or envelop an implantable medical device. Embodiments of the complementary drug delivery sheath may be implanted into the recipient prior to, concurrently with, or subsequent to the implantation of the implantable medical device.
Owner:COCHLEAR LIMITED
Throwable duct-free drug delivery system
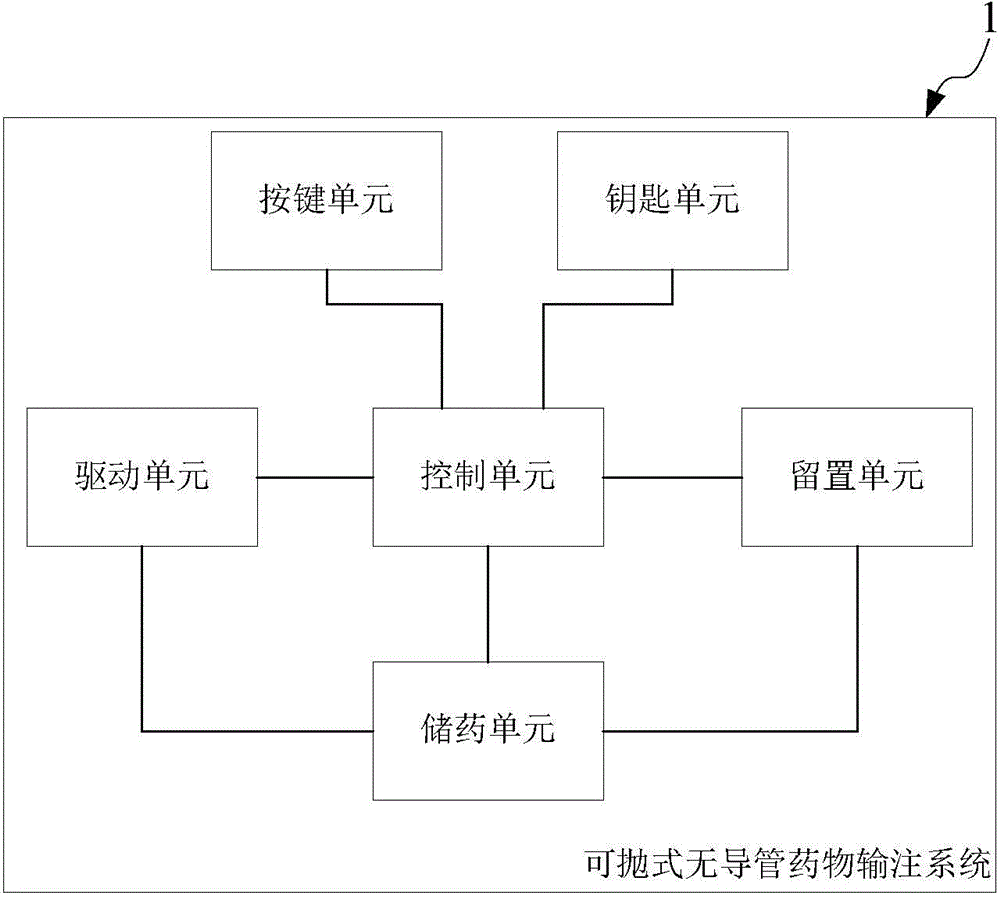
ActiveCN105311702ATo achieve the purpose of treatmentImprove convenienceMedical devicesPressure infusionWireless controlRemote control
The invention provides a throwable duct-free drug delivery system, comprising a key unit for receiving a user's key command; a key unit for outputting a selection command carrying at least one operating mode, the operating modes including a basal rate delivery rate, a programmable basal rate delivery mode, a delivery pause mode, a locking system mode and a wireless control mode; a drug storage unit for storing a drug liquid; a dwelling unit for delivering the drug liquid to the patient while being implanted subcutaneously in the patient; a drive unit for pushing the drug liquid subcutaneously in the patient while receiving a delivery command; a control unit for outputting the delivery command matching with the key command and / or operating modes to the drive unit so that the drive unit executes or pauses the delivery of the drug liquid while receiving the key command and / or operating mode selection command. A pump and a wireless remote control device are integrated for the purposes of small size, low cost and portability.
Owner:MEDTRUM TECH
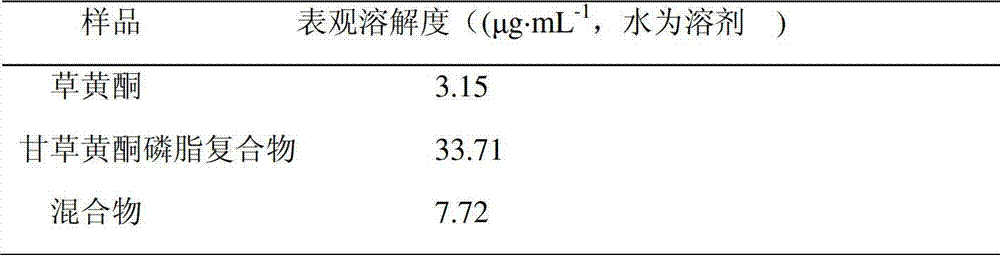
Licoflavone phospholipid complex and preparation method and application thereof
InactiveCN102949440ASimple preparation processGood reproducibilityAntiviralsAntineoplastic agentsOrganic solventFreeze-drying
The invention relates to a preparation method of a complex, in particular to a licoflavone phospholipid complex and a preparation method and the application of the licoflavone phospholipid complex; the licoflavone phospholipid complex comprises licoflavone and phospholipid by mass ratio of 1: 0.1-10; and the method comprises the steps of: according to the proportion, adding the licoflavone and the phospholipid into organic solvent, controlling the temperature to be within the range of 10-90 DEG C and carrying out reaction for 20min-8h until the reaction liquid is clear; carrying out pressure reduction or freeze drying to remove the organic solvent; after that, adding inert solvent to separate free drug which is not compounded; and removing the inert solvent and drying to obtain the licoflavone phospholipid complex. The invention is simple in preparation technology and good in reproducibility, and can improves the physicochemical properties including hydrophilic property and oleophylic property of the licoflavone by embedding the licoflavone by the phospholipid, so that the dissolving property of the licoflavone can be improved, the bioavailability of the licoflavone is increased, the problem that the licoflavone is not easily absorbed can be solved, the oxidation resistance of the licoflavone in different environments can be maintained or enhanced.
Owner:NINGXIA MEDICAL UNIV
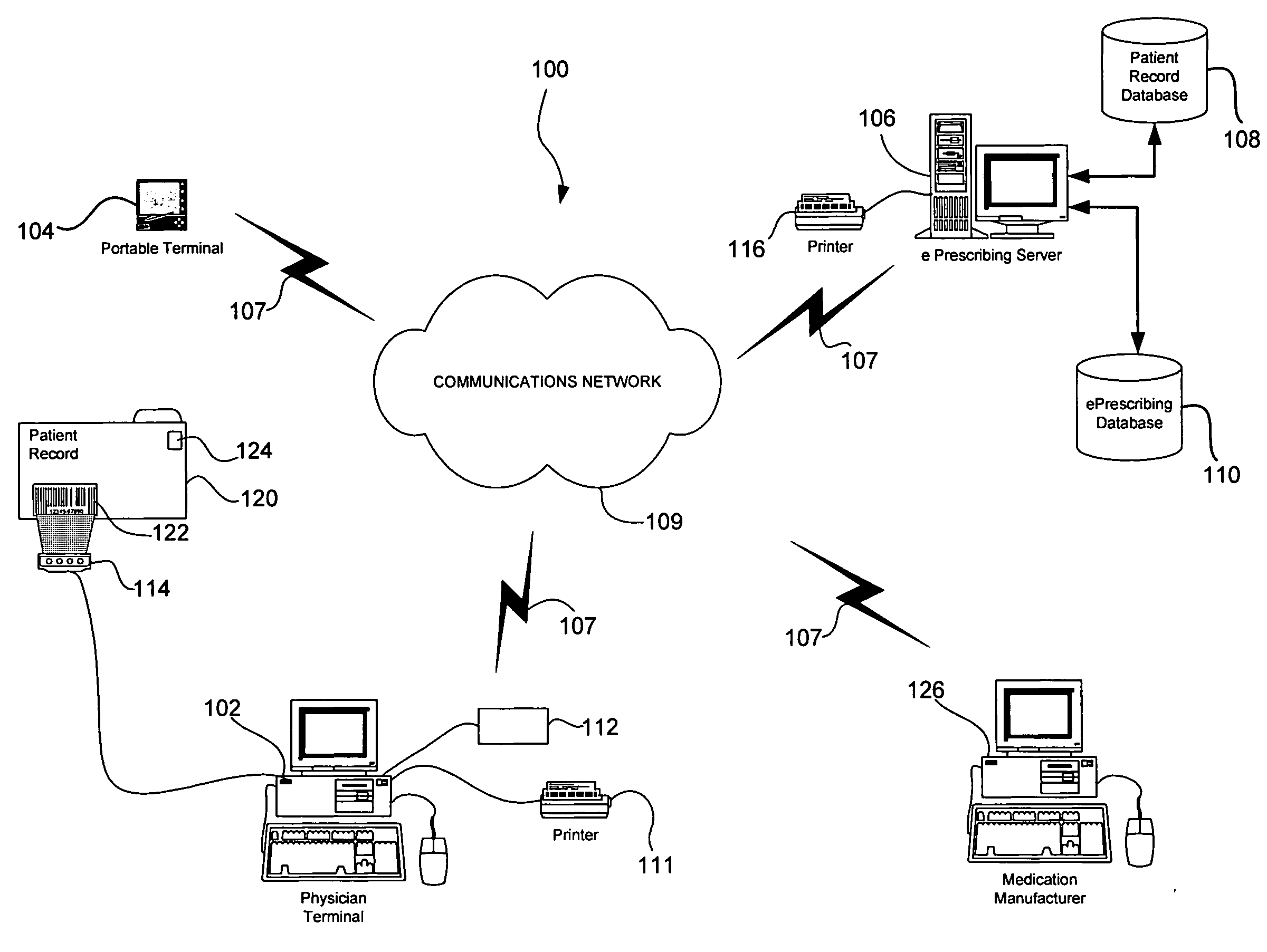
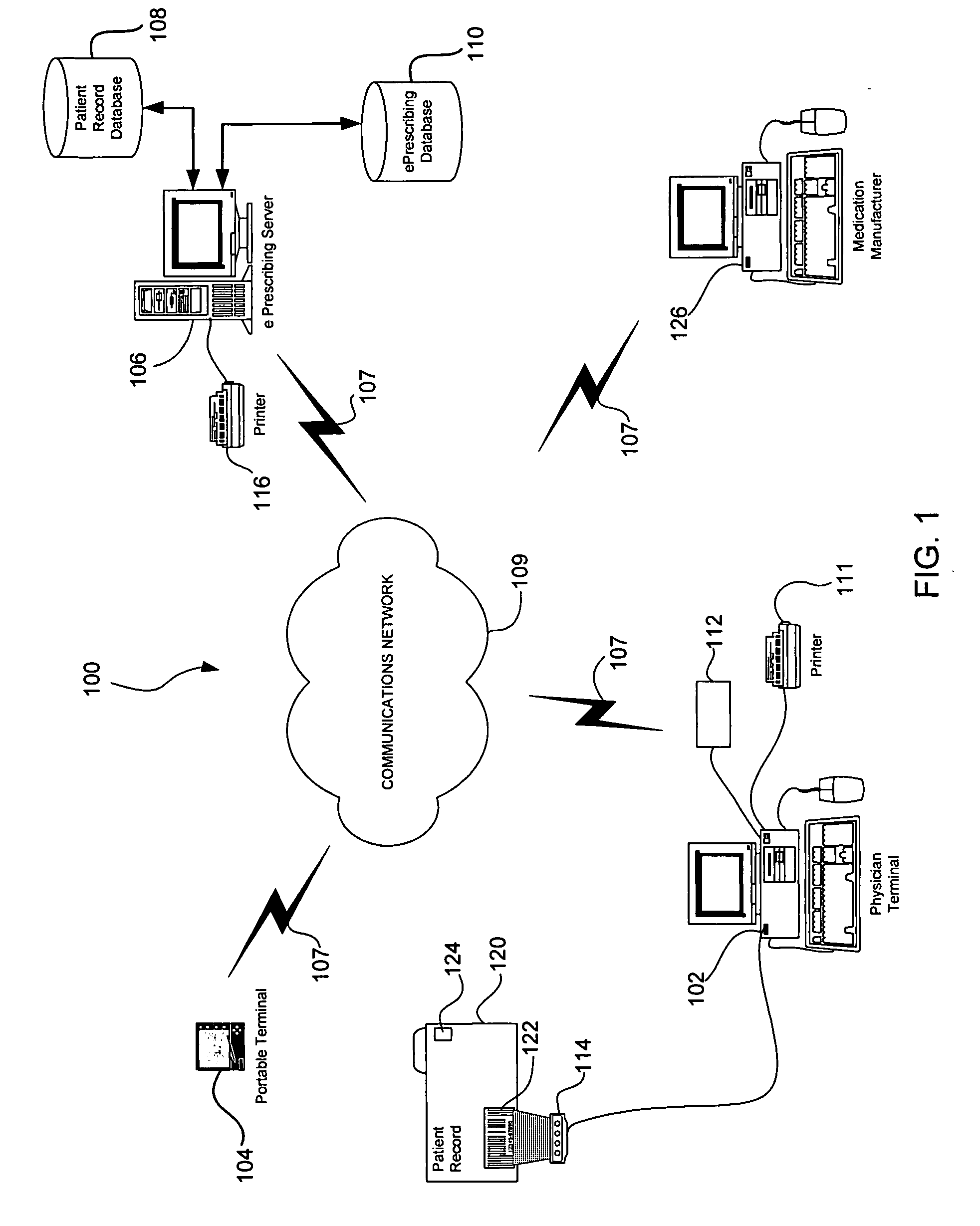
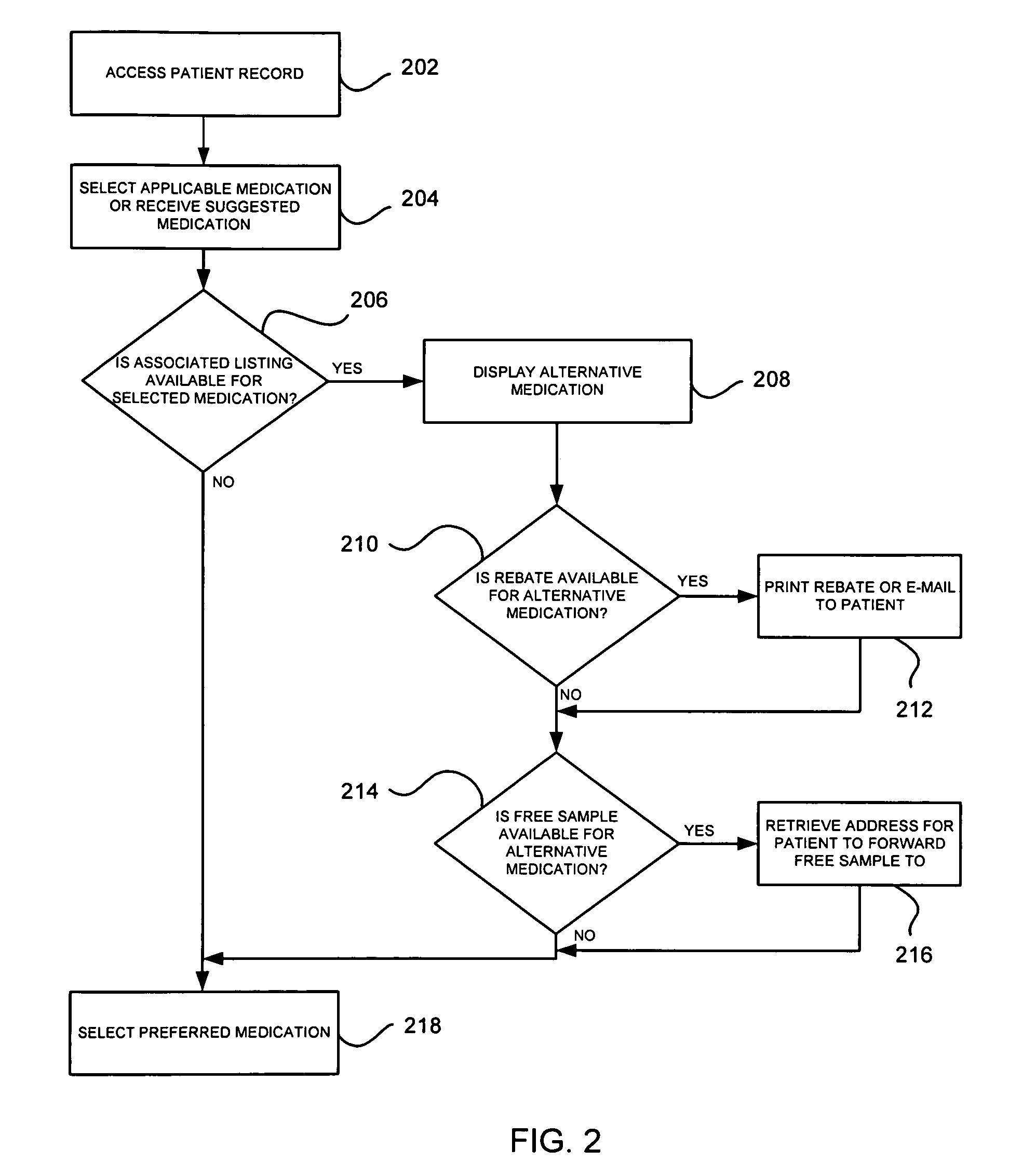
System and method of prescribing alternative medications
A system and method is provided to allow a physician to consider alternative medications while he is electronically prescribing medications to the patient, and to also allow the physician to be aware of and provide free medications and / or rebates to the patient.
Owner:SRR PATENT HLDG LLC
Polysaccharide modified reduction-sensitive graphene oxide carrier with organism lesion site triggered drug release and preparation and application of pharmaceutical composition thereof
ActiveCN104826128AHigh drug loadingEvade capturePharmaceutical non-active ingredientsSolubilityHigh concentration
The invention relates to a polysaccharide modified reduction-sensitive graphene oxide carrier with organism lesion site triggered drug release. The carrier introduces polysaccharide modification into graphene oxide through a specifically degradable linking arm containing disulfide bond, so that the polysaccharide modified graphene oxide loads the drug through non-covalent acting forces and gains reduction-sensitive release characteristics. The polysaccharide modified reduction-sensitive graphene oxide loaded drug reaches the lesion site, and the linking arm of the disulfide bond can be degraded by high concentration reductive substance glutathione in the lesion cells; shedding of hydrophilic polysaccharide leads to rapid release of drug from the surface of the graphene oxide, so as to significantly improve the concentration of, efficacy and bioavailability of the free drug in lesion site. The preparation process of the present invention is simple, and the carrier has excellent biocompatibility, water solubility and lesion triggered release, and is innovative in drug delivery systems.
Owner:CHINA PHARM UNIV
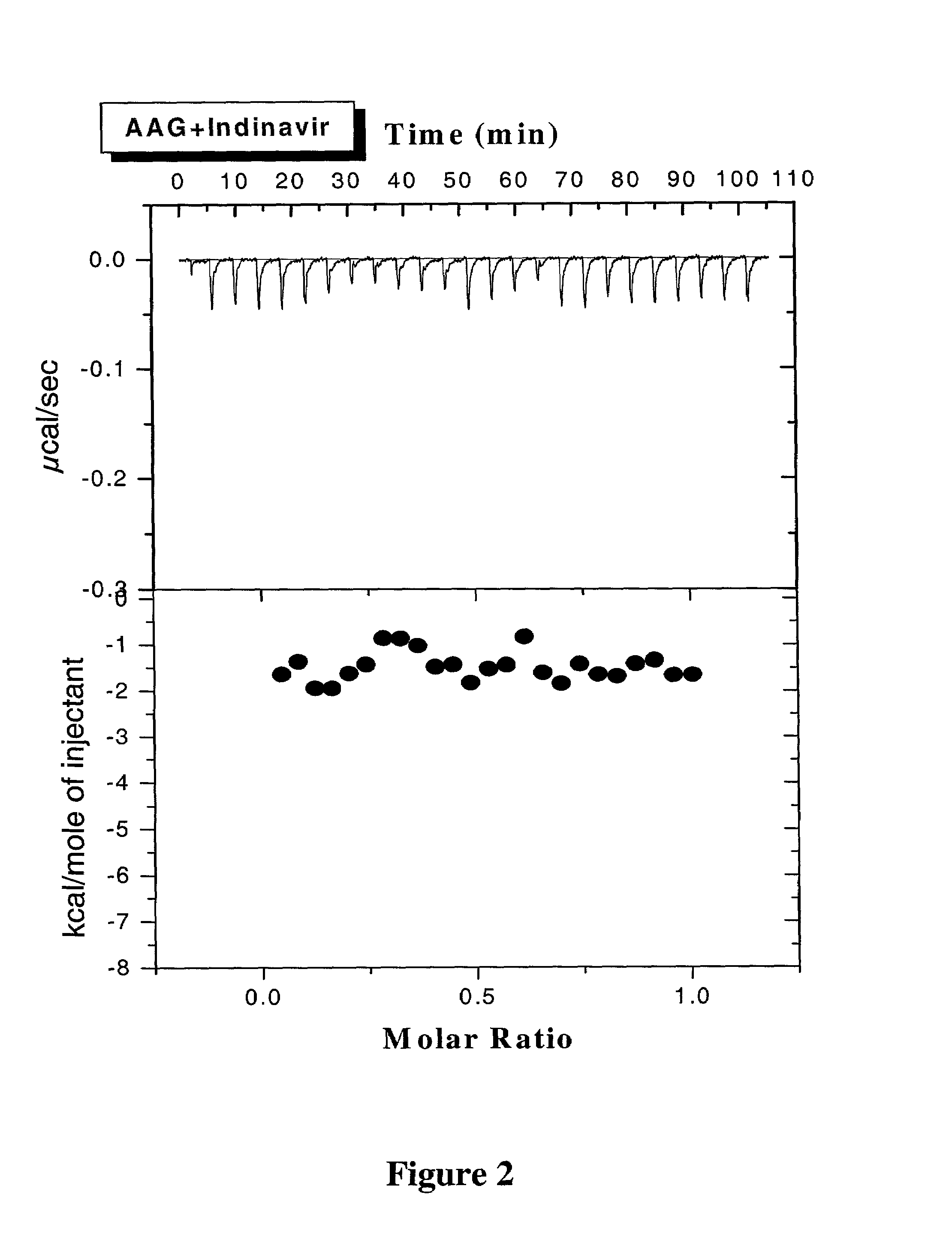
Methods for determining plasma free drug concentration by direct measurement of binding affinity of protease inhibitors to plasma proteins
InactiveUS7087373B2Evaluate the in vivo anti-HIV efficacy of PIsCompound screeningApoptosis detectionRegimenIn vivo
Methods for isothermal titration calorimetry analysis of the binding affinity of protease inhibitors to plasma proteins. A method that can quantitatively calculate free drug concentrations of protease inhibitors in human plasma, as well as a method to calculate therapeutic amounts and dosage regimens. Furthermore, the present invention provides a method that can calculate the effect of plasma proteins on the antiviral activity (EC50 values) of protease inhibitors from their binding affinities to plasma proteins. The present invention provides as well a method that can evaluate the in vivo anti-HIV efficacy of PIs in human plasma.
Owner:TIBOTEC PHARMA
Multiple sustained release vascular embolization drug-loading composition
ActiveCN109833509ASolve the sudden release problemSurgical adhesivesVascular embolizationPore distribution
The present invention discloses a multiple sustained release vascular embolization drug-loading composition. Release time of drugs from the composition is 1-360 d, and the drug-loading composition comprises a drug-loading microsphere made of a degradable material and / or a non-degradable material and a three-dimensional porous scaffold made of a degradable material and / or a non-degradable material.The three-dimensional porous scaffold has a uniform pore distribution and besides a pore diameter of pores is larger than a diameter of the drug-loading microsphere; the drug-containing drug-loadingmicrospheres pass through hydrogels or autologous coagulated blood blocks loaded in the three-dimensional porous scaffold, so that the drugs in the drug-loading microspheres are released from the drug-loading microspheres to the hydrogels or autologous coagulated blood blocks, then released into the three-dimensional porous scaffold and finally released outside the three-dimensional porous scaffold; or drug-free drug-loading microspheres are loaded into the three-dimensional porous scaffold to form a complex, then the drugs are loaded into the complex, and the drugs are released from the complex to the outside of the three-dimensional porous scaffold. The multiple sustained release vascular embolization drug-loading composition effectively solves a problem of drug burst release of the drug-loading microspheres.
Owner:太阳雨林(厦门)生物医药有限公司
Intelligent sewage treatment device and method
PendingCN107089757ARealize remote maintenanceRealize data monitoringWater treatment parameter controlSpecific water treatment objectivesOperational costsUltrafiltration
The invention provides an intelligent sewage treatment device and method. The device comprises a container, as well as a mixing tank, a supermagnetic sewage purification box, a first water tank, an activated carbon filter device, a second water tank, a nano ultrafiltration device, a third water tank, a PAM addition device, a PAC addition device, an ammonia-nitrogen-free drug addition device, an activated carbon cleaning device, a disinfectant addition device, an ultrafiltration cleaning device, a water suction pump, a magnetic seed recovery device, a desliming device and an electric control cabinet which are located in the container. By adopting a supermagnetic separation and purification technology and container installation, the floor area is saved and transportation is convenient; remote maintenance and data monitoring of the intelligent sewage treatment device are achieved through a cloud server; wiring is not needed, a distance limit is avoided, only a mobile phone signal is needed, and a master-slave limit is avoided; data exchange of various modes, such as a one-to-many mode, a many-to-one mode and a many-to-many mode is achieved; and fault maintenance of maintenance personnel can be achieved at any place, and thus the operation cost is reduced.
Owner:BEIJING AREOSTANARD NEW TECH +1
Cobalamin conjugates for anti-tumor therapy
The present invention provides a cobalamin-drug conjugate suitable for the treatment of tumor related diseases. Cobalamin is indirectly covalently bound to an anti-tumor drug via a cleavable linker and one or more optional spacers. Cobalamin is covalently bound to a first spacer or the cleavable linker via the 5′-OH of the cobalamin ribose ring. The drug is bound to a second spacer of the cleavable linker via an existing or added functional group on the drug. After administration, the conjugate forms a complex with transcobalamin (any of its isoforms). The complex then binds to a receptor on a cell membrane and is taken up into the cell. Once in the cell, an intracellular enzyme cleaves the conjugate thereby releasing the drug. Depending upon the structure of the conjugate, a particular class or type of intracellular enzyme affects the cleavage. Due to the high demand for cobalamin in growing cells, tumor cells typically take up a higher percentage of the conjugate than do normal non-growing cells. The conjugate of the invention advantageously provides a reduced systemic toxicity and enhanced efficacy as compared to a corresponding free drug.
Owner:INFLABLOC PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com