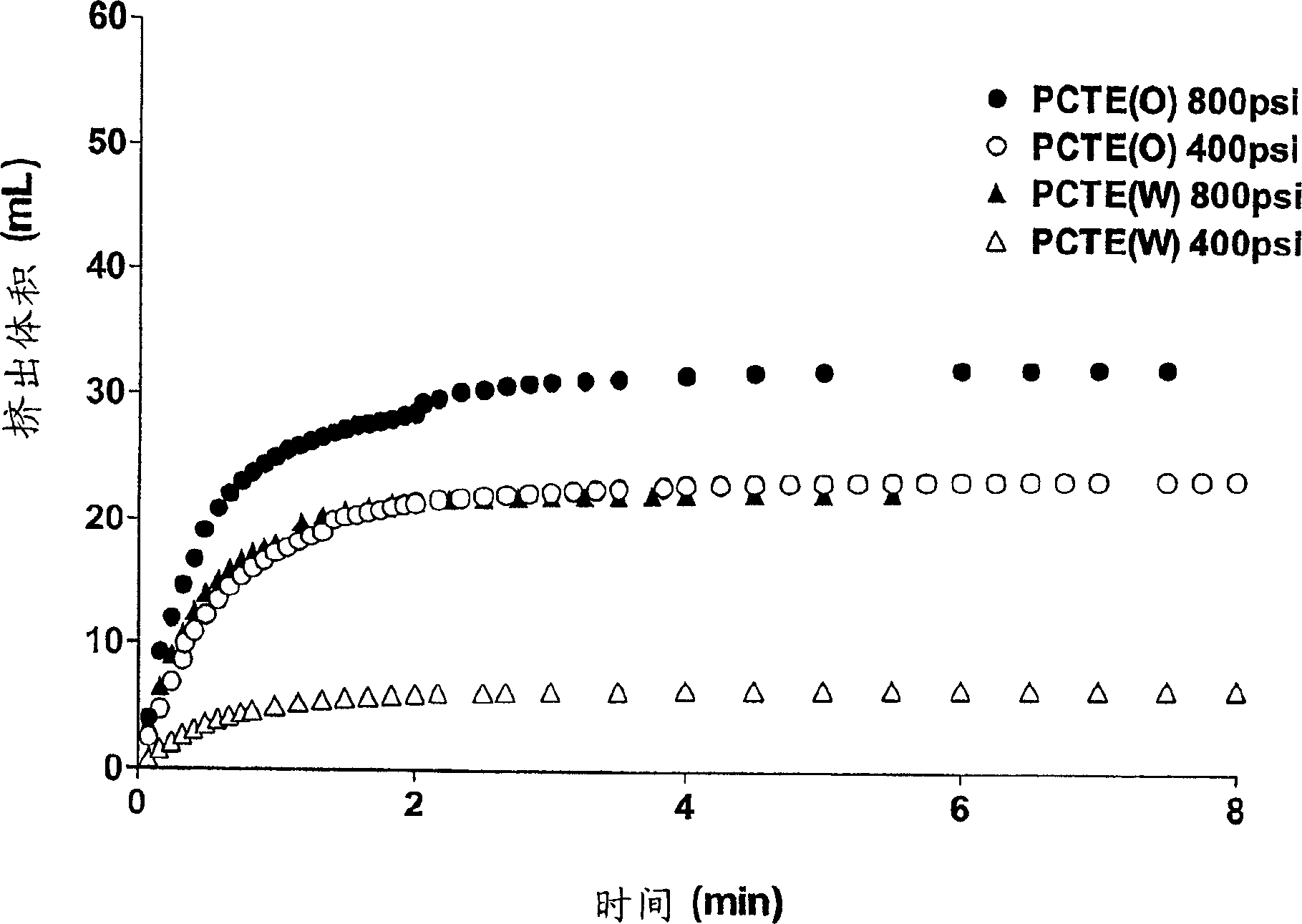
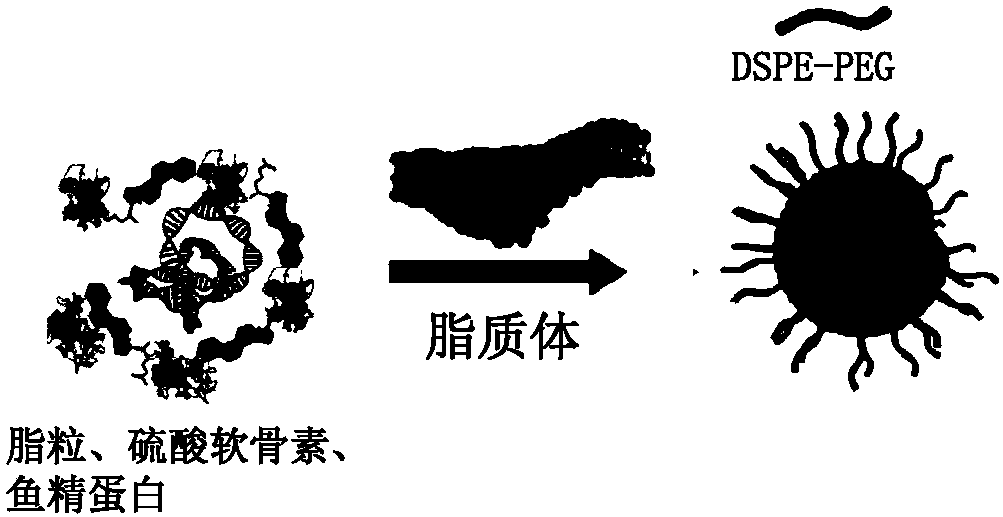
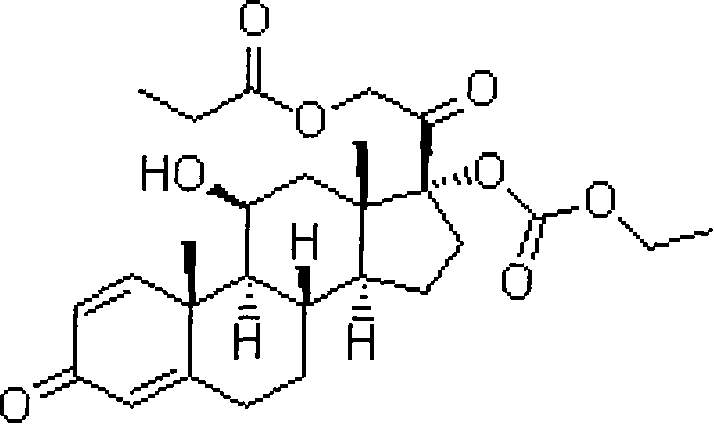
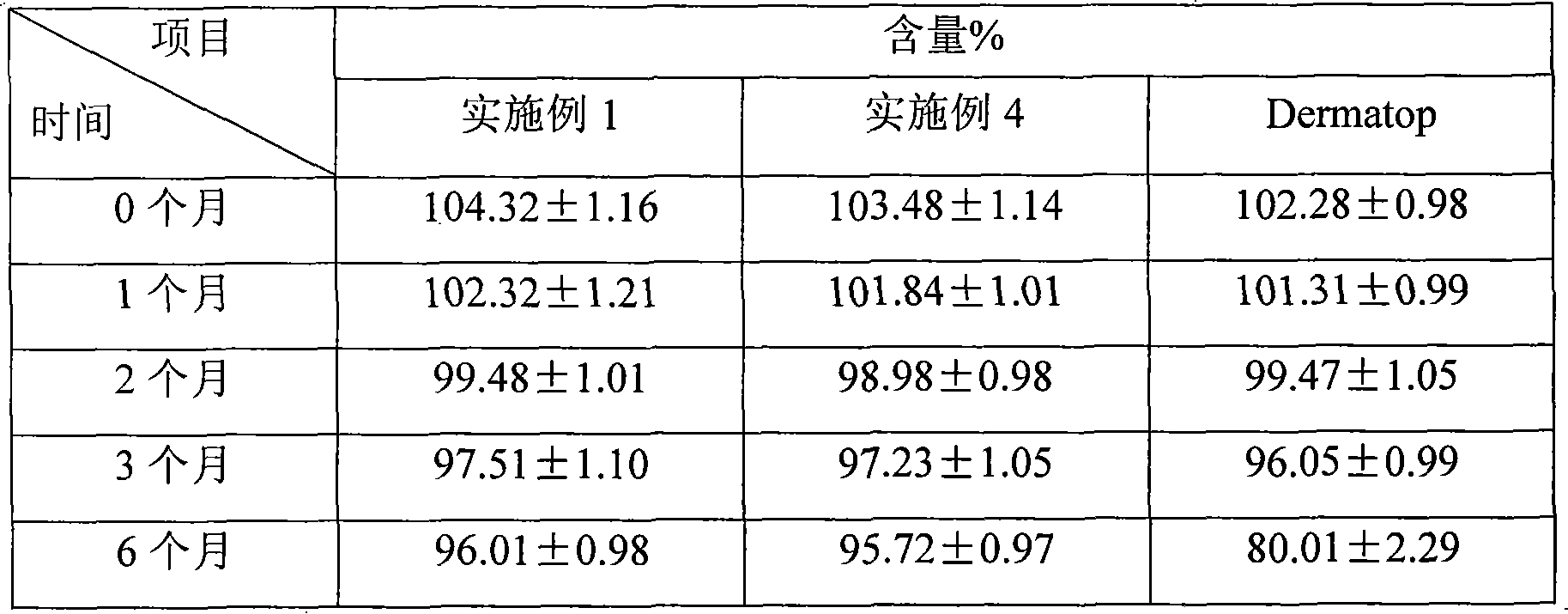
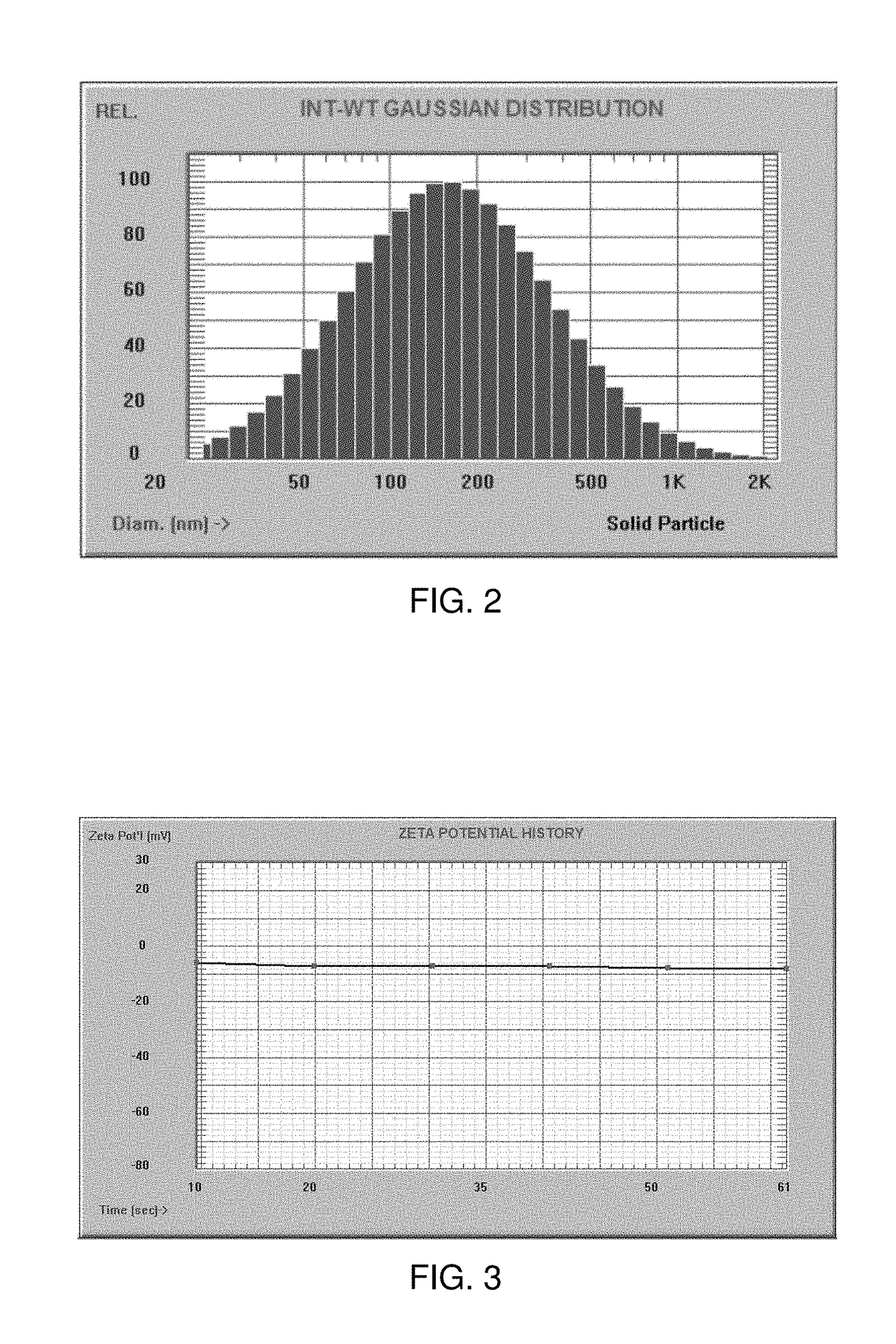
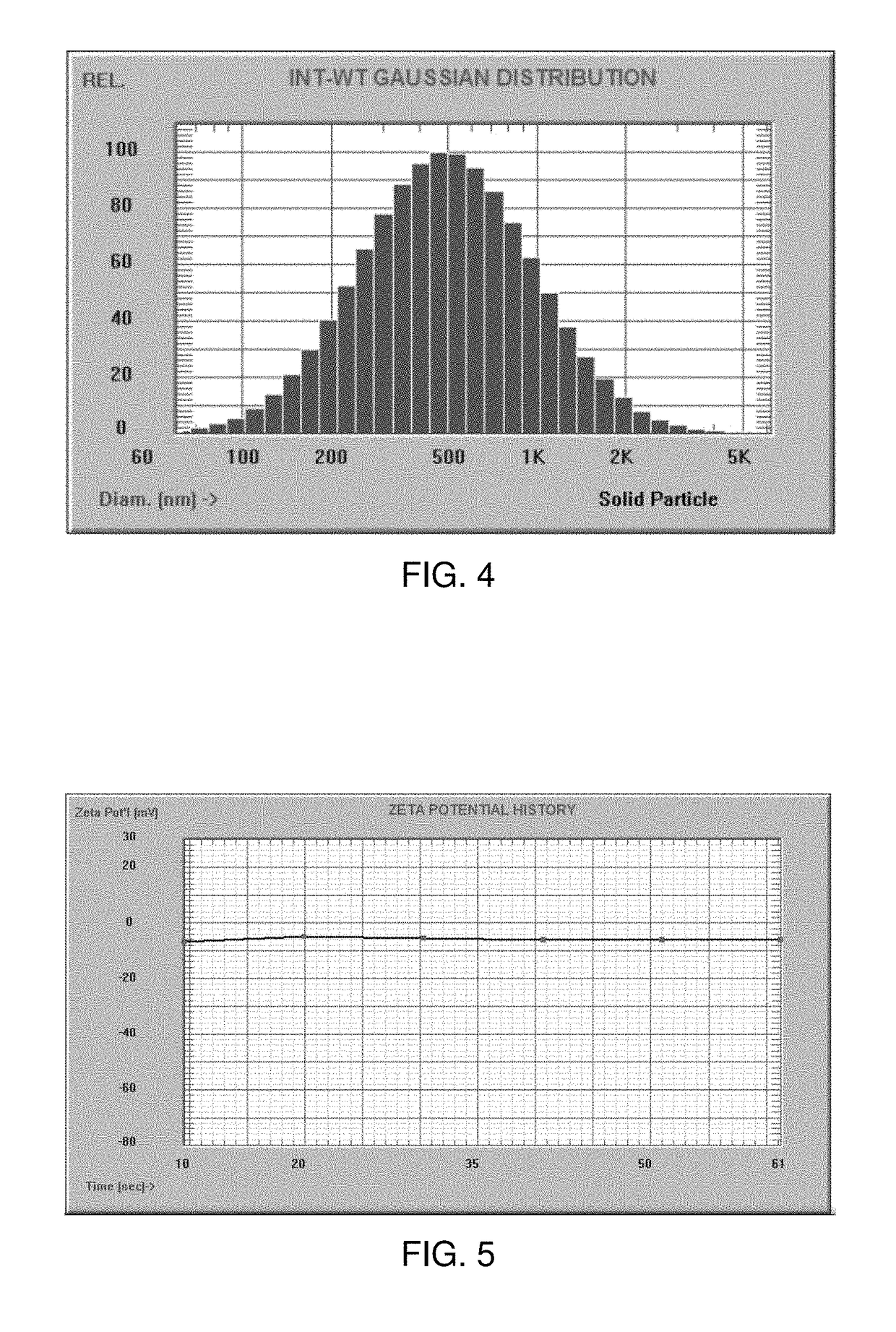
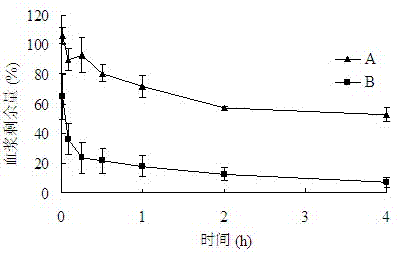
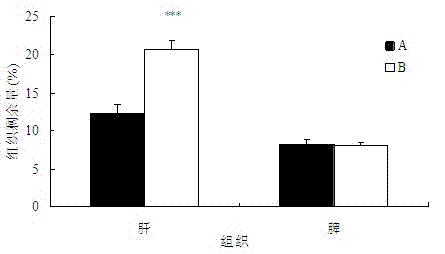
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Liposome Vesicle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vesicle | liposome |. is that vesicle is (cytology) a membrane-bound compartment found in a cell while liposome is (biochemistry) an aqueous compartment enclosed by a bimolecular phospholipid membrane; a lipid vesicle.
Low-concentration polyethylene glycol-lipid (PEG-lipid) derivative and application thereof
InactiveCN102813929ARelieve pressureReduce the number of cyclesPharmaceutical non-active ingredientsLiposome VesicleLiposome
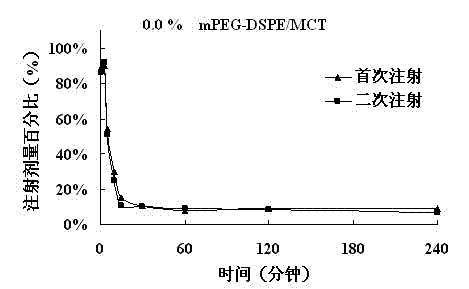
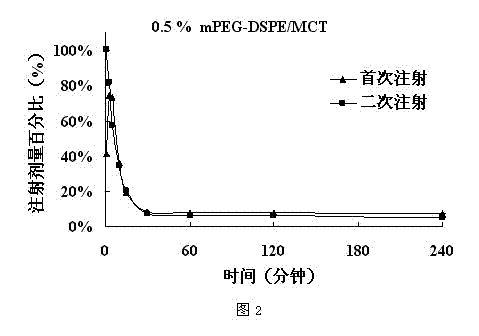
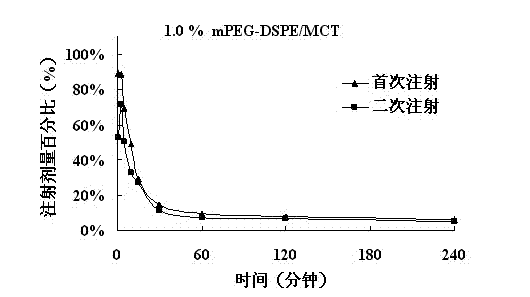
The invention belongs to the technical field of medicine, relates to a low-concentration PEG-lipid derivative and an application thereof, particularly to a prescription composition and an application thereof which can relieving or avoiding accelerated blood clearance (ABC) of PEGylation preparations. According to the low-concentration PEG-lipid derivative and the application thereof, the low-content PEG-lipid derivative is used for modifying liquid particle preparations such as emulsions, liposome, vesicles, micro emulsions, micelles and nano-particles, PEG and lipid chain segments are connected through ester bonds, amido bonds or ether bonds in the PEG-lipid derivative, and the molecular weight of the PEG is 200-20000 Dalton. Preparations prepared by the PEG-lipid derivative can only cause slight or cannot cause the ABC, namely, can reduce or avoid the ABC after repeated injection. The low-concentration PEG does not reduce in vitro anti-tumor activity of preparations, and stability of the preparation can be improved if the low-concentration PEG-lipid derivative is added, particularly shaking instability of antagonistic emulsions.
Owner:SHENYANG PHARMA UNIVERSITY
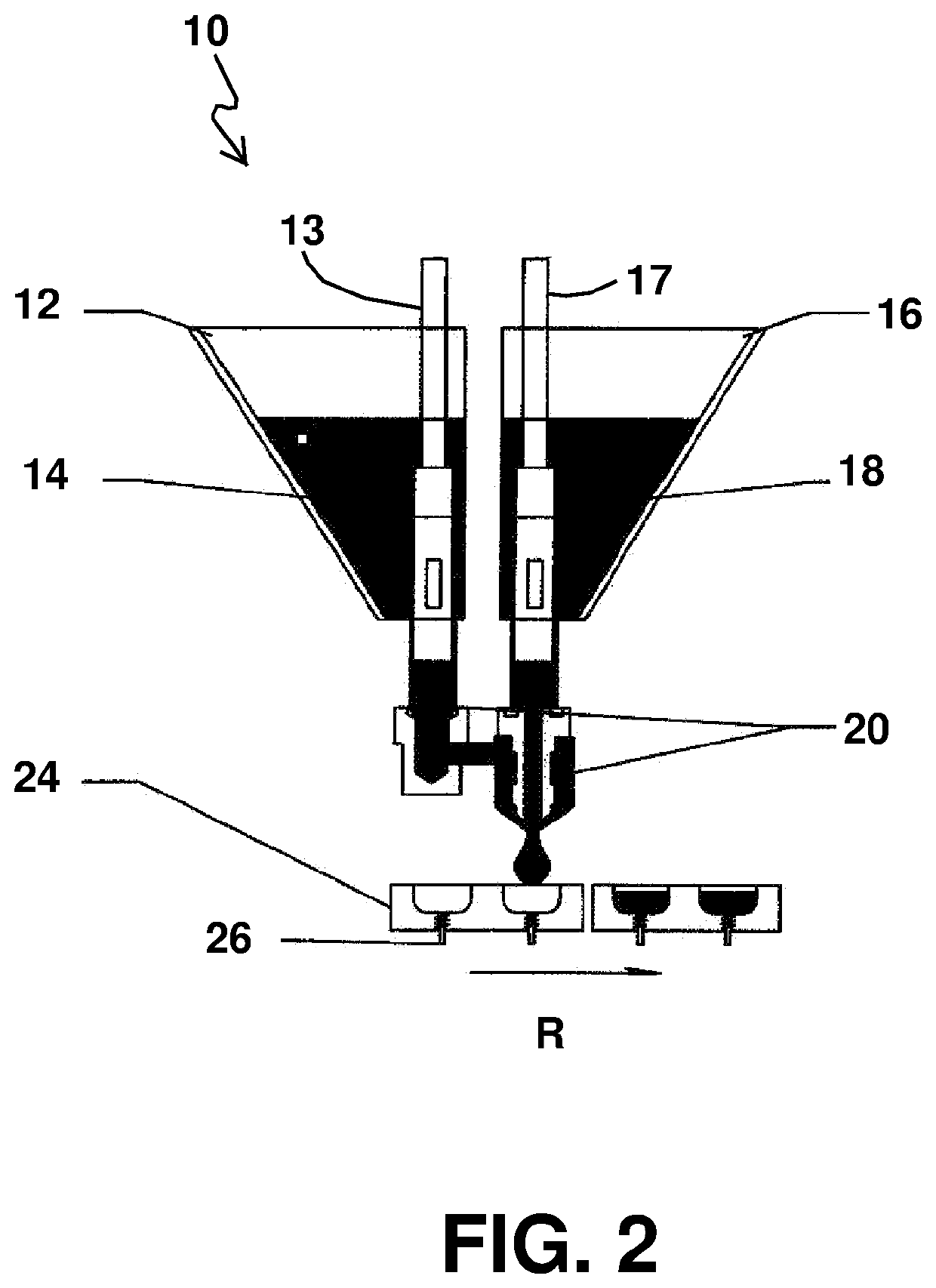
Automatic liquid injection system and method
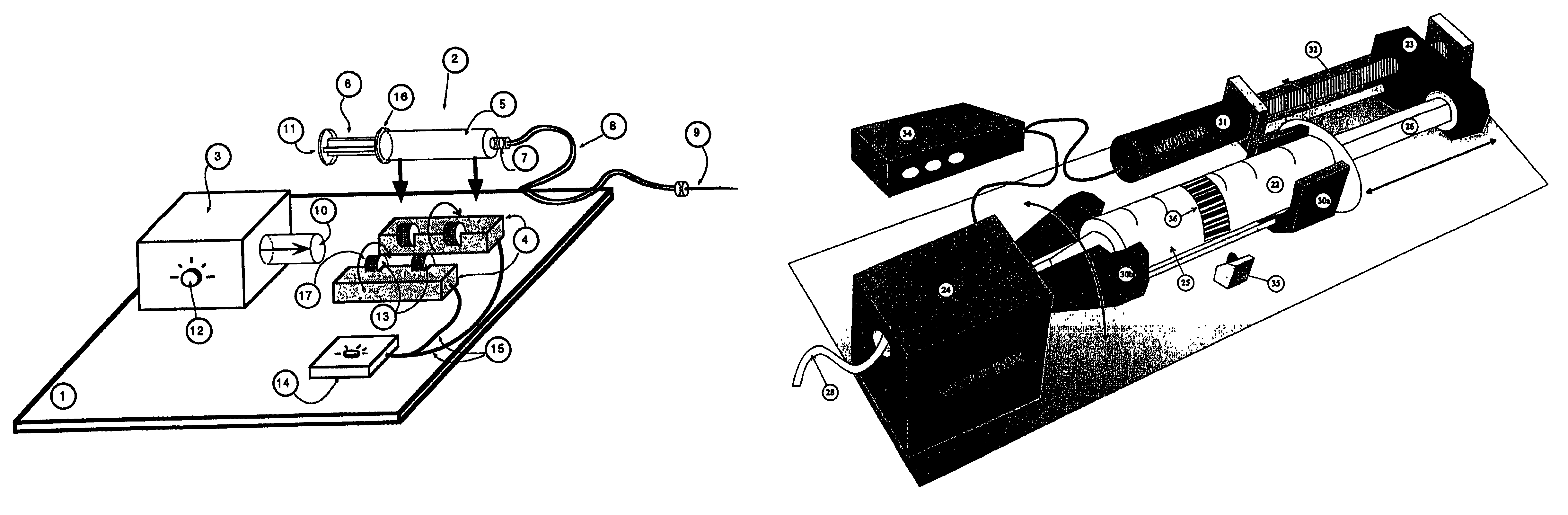
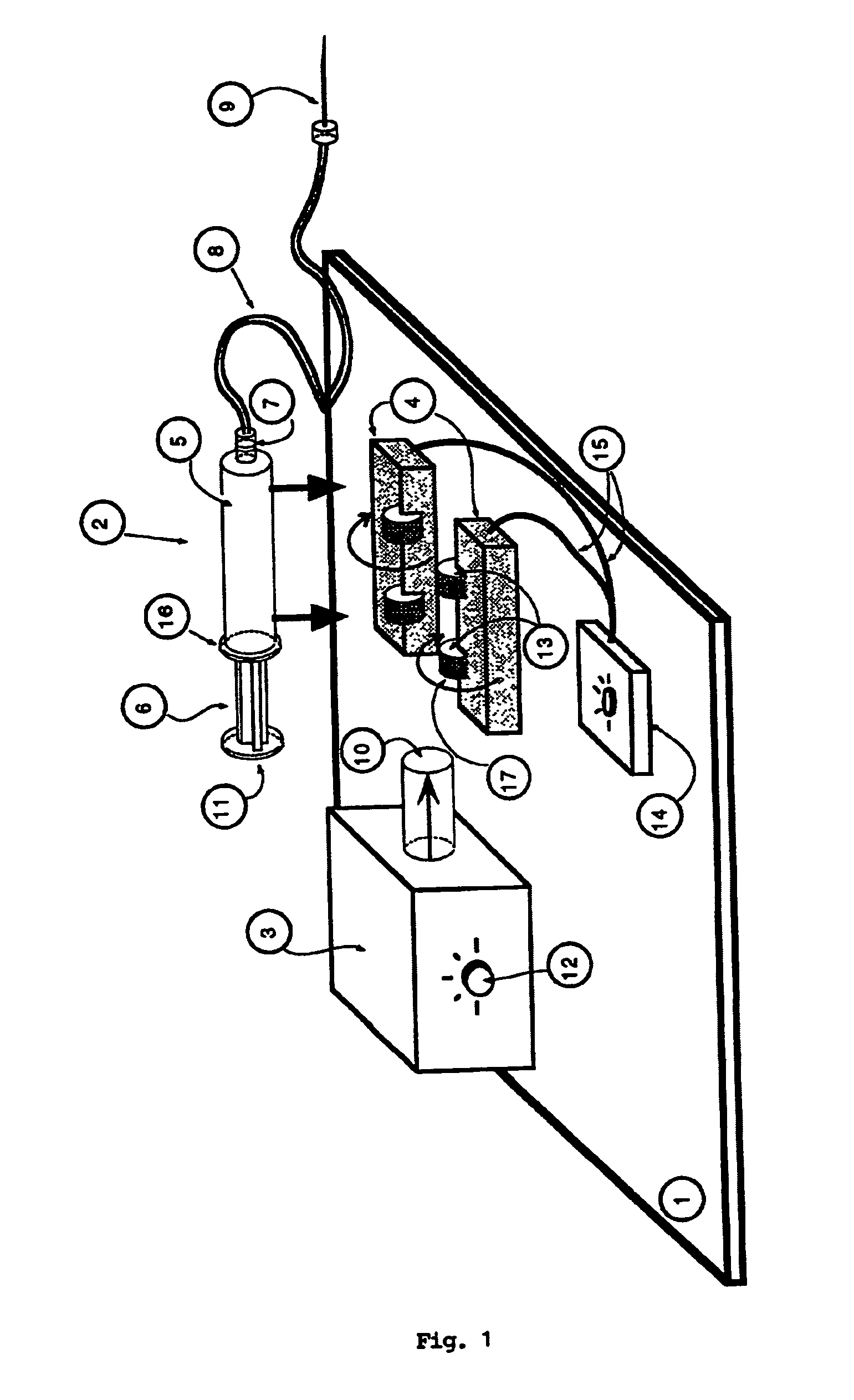
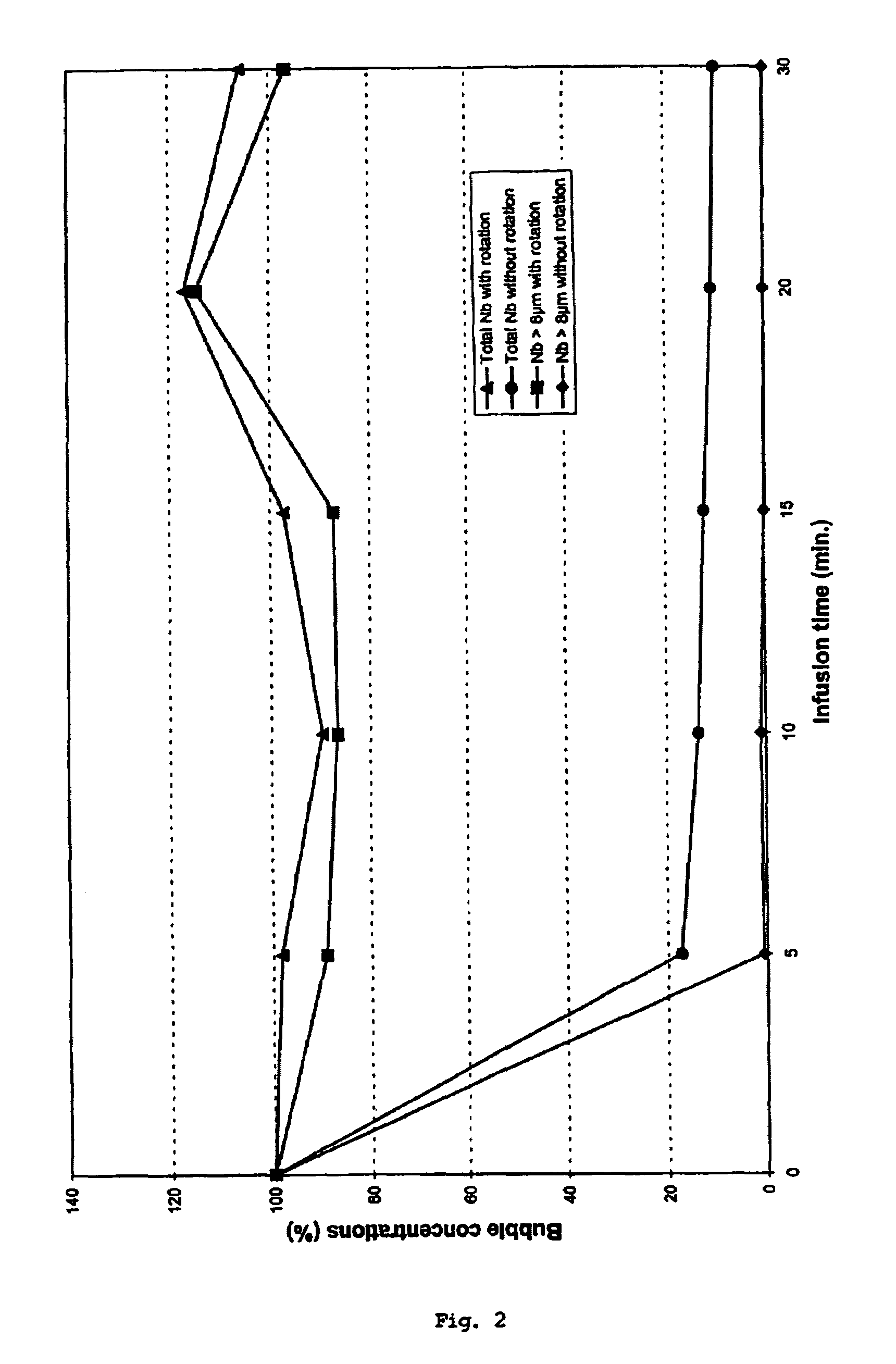
InactiveUS7534239B1Stay efficientRotating receptacle mixersShaking/oscillating/vibrating mixersLiposome VesicleActive agent
A power assisted method and injector device for controllably delivering to patients a dispersion medicament or diagnostically active agent, the homogeneity of which is preserved throughout delivery. Diagnostically active agents disclosed are gas microbubble suspensions useful in ultrasonic diagnostic imaging and liposomal formulations in which liposome vesicles are loaded with iodinated compounds.
Owner:BRACCO SUISSE SA
Application of cleavable polyethylene glycol (PEG) lipid derivative to preparation
InactiveCN102068701AEasy to prepareNo radioactive contaminationPowder deliveryPharmaceutical non-active ingredientsLiposome VesicleMethyl carbonate
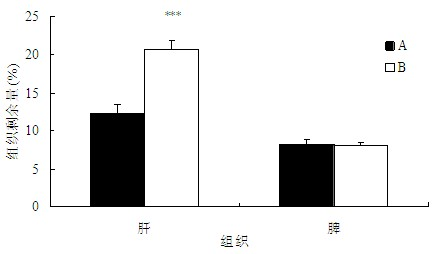
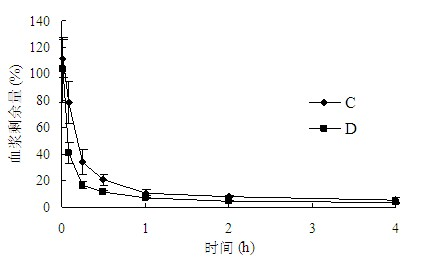
The invention belongs to the technical field of medicaments, and provides application of a cleavable polyethylene glycol (PEG) lipid derivative to preparation of a PEGylated preparation for relieving or avoiding accelerated blood clearance. In the application, liquid microparticle preparations such as liposome, vesicles, emulsions, microemulsion, micelles, nanoparticles and the like are modified by the cleavable PEG lipid derivative such as PEG-cholesteryl hemisuccinate, PEG-cholesteryl methyl carbonate, PEG-alpha tocopheryl hemisuccinate and the like; and the measurement of variation of preparation elimination in tissues such as animal blood plasma, liver, spleen and the like after a cleavable PEG lipid derivative-modified medicinal preparation is repeatedly injected proves that repeated injection of cleavable PEG lipid derivative-modified microparticle preparations only causes light accelerated blood clearance or avoids the accelerated blood clearance, namely the accelerated blood clearance can be relieved or avoided. The invention discloses new application of the cleavable PEG lipid derivative.
Owner:SHENYANG PHARMA UNIVERSITY
Methods of improving therapy of perfluorocarbons (PFC)
InactiveUS20100093873A1Extended stayEliminate side effectsHalogenated hydrocarbon active ingredientsBiocideEmulsionLiposome Vesicle
This invention describes a novel, two-step method for administering PFC. The first step is designed to block the RES by administration of empty, small, liposomal vesicles (ESV) that are rapidly and preferentially engulfed by macrophages, thereby inhibiting their phagocytosis of subsequently infused PFC emulsions. The second step is the subsequent injection of PFC. ESV are devoid of materials that interfere with the macrophage's metabolic processes and do not impair their ability to clear the circulation of pathogenic organisms. Inhibition of the removal of PFC from the blood stream by the RES will achieve increased circulating PFC, enhanced binding and transport of oxygen throughout the blood stream and consequential reduction of undesirable consequences such as organomegaly and cytokine toxicity.
Owner:GOLDFISCHER SIDNEY L
Submicron liposome suspensions obtained from preliposome lyophilizates
InactiveUS7238366B1Severe adverse effect on liposome integrityShorten hydration timePowder deliveryTetracycline active ingredientsLipid formationLiposome Vesicle
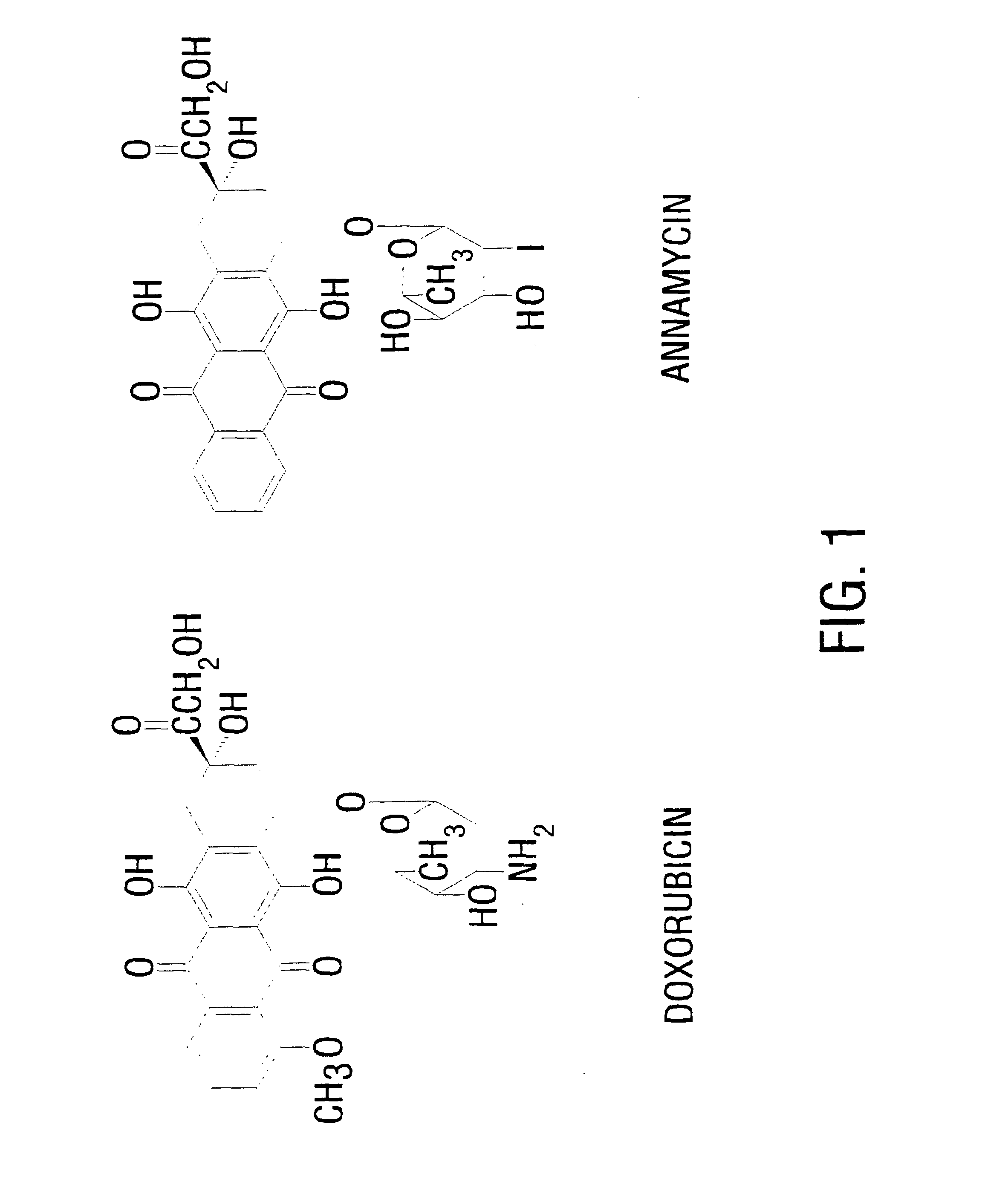
This invention provides an aqueous / t-butanol solvent-system, facile reconstitute, submicron-reconsitiute preliposome-lyophilaye and method of its preparation and use.In one embodiment this entails a modified method for the preparation of a submicron and stable liposome formulation of the non-cross-resistant anthracycline Annamycin is described. The optimal lipid composition was DMPC:DMPG at a 7:3 molar ratio and the optimal lipid:drug weight ratio 50:1. The selected formulation is a preliposome lyophilized powder that contains the phospholipids, Annamycin, and 1.7 mg Tween 20 per mg of Annamycin. The liposome suspension is obtained on the day of use by adding normal saline at 37° C. (1 ml per mg Annamycin) and hand-shaking for one minute. The presence of Tween 20 is essential in shortening the reconstitution step (from >2 hours to 1 minute), avoiding the early formation of free drug crystals, and reducing the median particle size (from 1.5 μm to 0.15-0.20 μm) without destruction of the liposome vesicles. The chemical stability of the preliposome powder at room temperature was >3 months and the chemical and physical stability of the liposome suspension at room temperature >24 hours. The in vitro cytotoxicity of the formulation was equivalent to that prepared by the standard evaporation method. The results of the study indicate that small amounts of surfactant may be used to enhance the reconstitution step and reduce the liposome size of lyophilized liposome formulations of lipophilic drugs.
Owner:BOARD OF REGENTS
Combination meningitidis B/C vaccines
InactiveUS8007815B1Antibacterial agentsBacterial antigen ingredientsLiposome VesicleNeisseria meningitidis
Combination vaccines for treating or preventing Neisseria meningitidis infection are described. The vaccines include Neisseria meningitidis serogroup B proteoliposomic vesicles and Neissera meningitidis serogroup C conjugated oligosaccharides.
Owner:NOVARTIS AG +2
Gold nanocluster-liposome composite nanoparticles, preparation method and applications thereof
ActiveCN108498460AImprove transfection efficiencyEfficient deliveryPeptide/protein ingredientsGenetic material ingredientsTreatment effectLiposome Vesicle
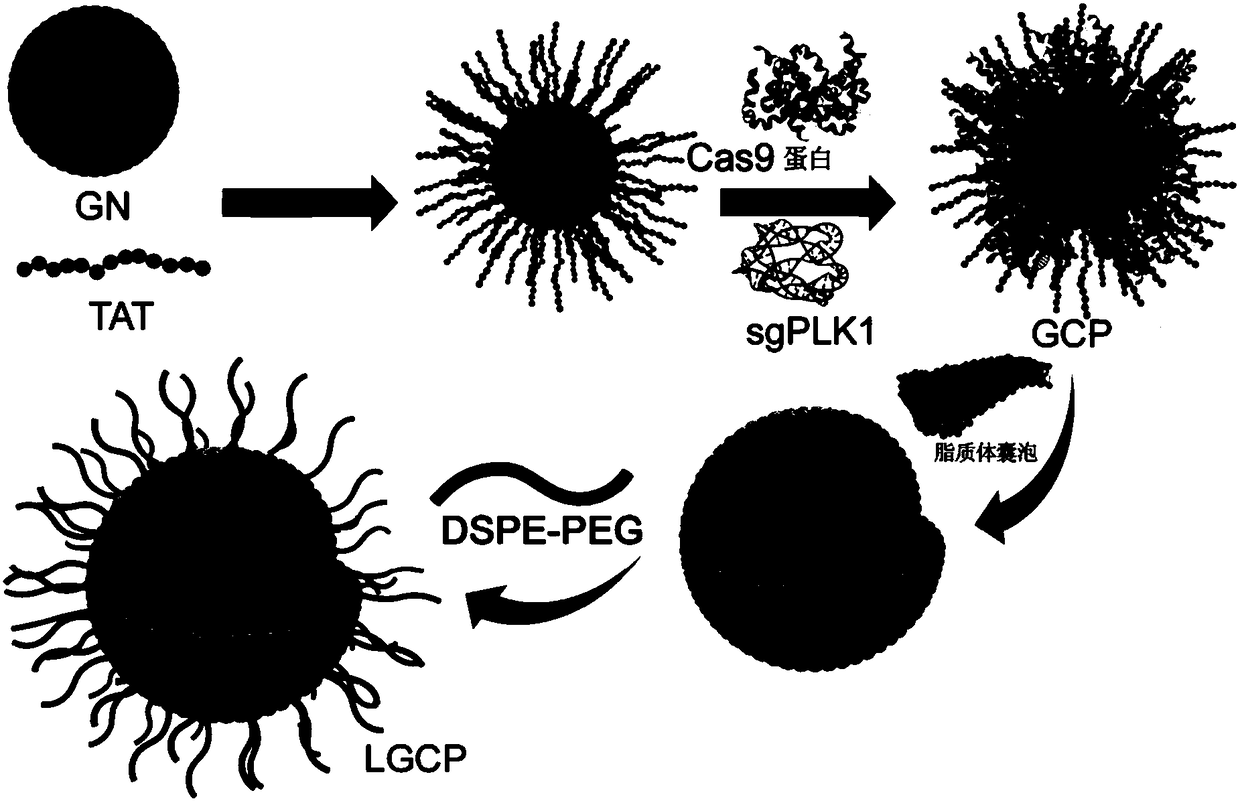
The present invention provides gold nanocluster-liposome composite nanoparticles, which are a gold nanocluster encapsulated with a liposome vesicle, wherein the surface of the liposome vesicle is modified with distearoyl phosphatidylethanolamine-polyethylene glycol, the surface of the gold particle in the gold nanocluster is modified with a penetrating peptide and is bound to Cas9 protein and guiding ribonucleic acid, and the liposome vesicle comprises dioleoyl phosphoethanolamine, 2,3-dioleyloxypropyltrimethyl ammonium chloride and cholesterol. The invention further provides a preparation method and applications of the gold nanocluster-liposome composite nanoparticles. According to the present invention, by using the gold nanocluster-liposome composite nanoparticles, the CRISPR / Cas9 system can be efficiently delivered into cells without any other transfection reagents so as to improve the cell intake rate and the transfection efficiency, such that the gene treatment effects on a variety of tumors can be achieved, and the tracing effect can be achieved.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
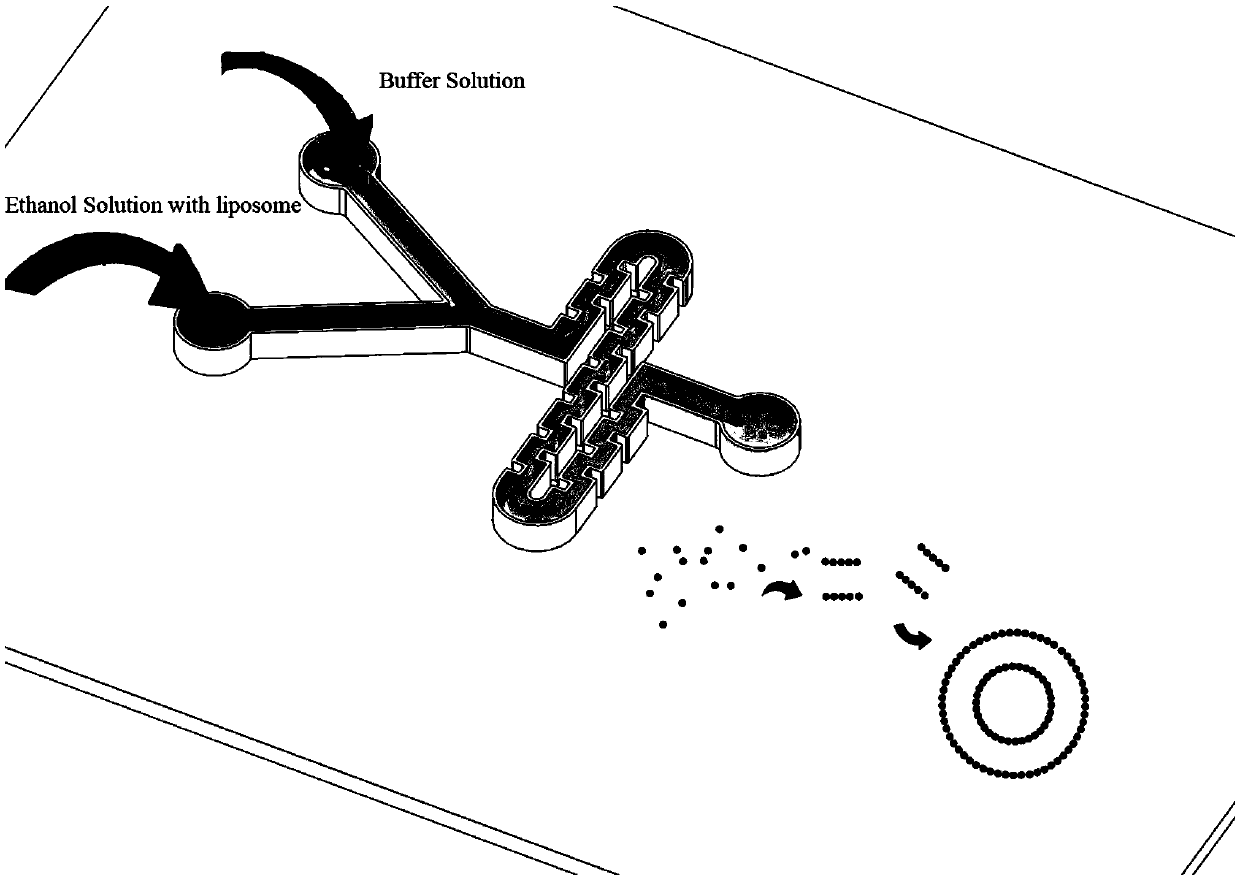
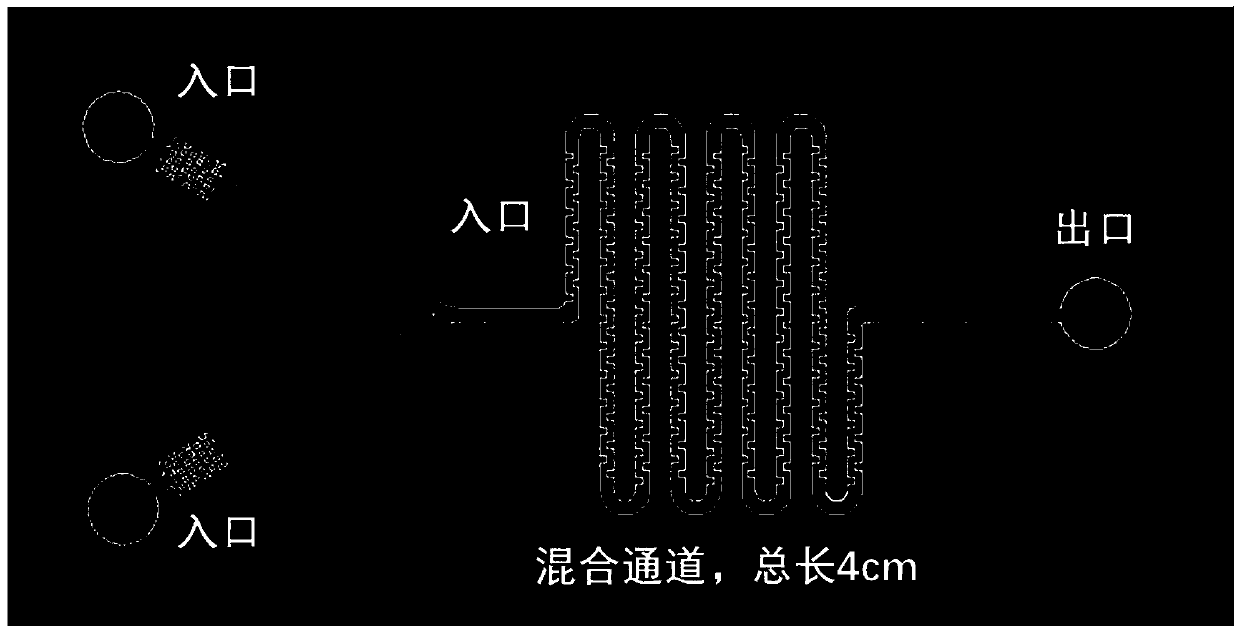
Controllable preparation method of liposome vesicle based on microfluidic device
InactiveCN109603930ALow costPrecisely control the amount addedLaboratory glasswaresPharmaceutical non-active ingredientsLiposome VesicleFlow ratio
The invention provides a preparation method of a liposome vesicle based on a microfluidic device. The preparation method comprises the steps of: preparing a microfluidic chip by a soft lithography method; preparing a precursor solution for preparing the liposome vesicle; and using a constant-pressure injection pump to inject the precursor solution into a ''Y''-shaped chip through different injection ports, setting a total flow rate, adjusting the flow ratio of a buffer solution to a phospholipid molecular alcohol solution, and collecting a product at an outlet, wherein the product is the prepared liposome vesicle. The microfluidic chip selected by the invention is a ''Y''-shaped microfluidic chip with a serpentine mixing channel of a square structure, and the chip is prepared by the soft lithography method. By using the chip, based on the principle of self-assembly on an interface, by adjusting the total flow rate and the flow rate ratio of the aqueous buffer solution to the phospholipid molecule ethanol solution, regulation of the size of the liposome vesicle and control on the dimensional uniformity are achieved, and an efficient low-cost method is provided for preparation of artificial cells with uniform properties as well as drug carriers.
Owner:SHANGHAI UNIV
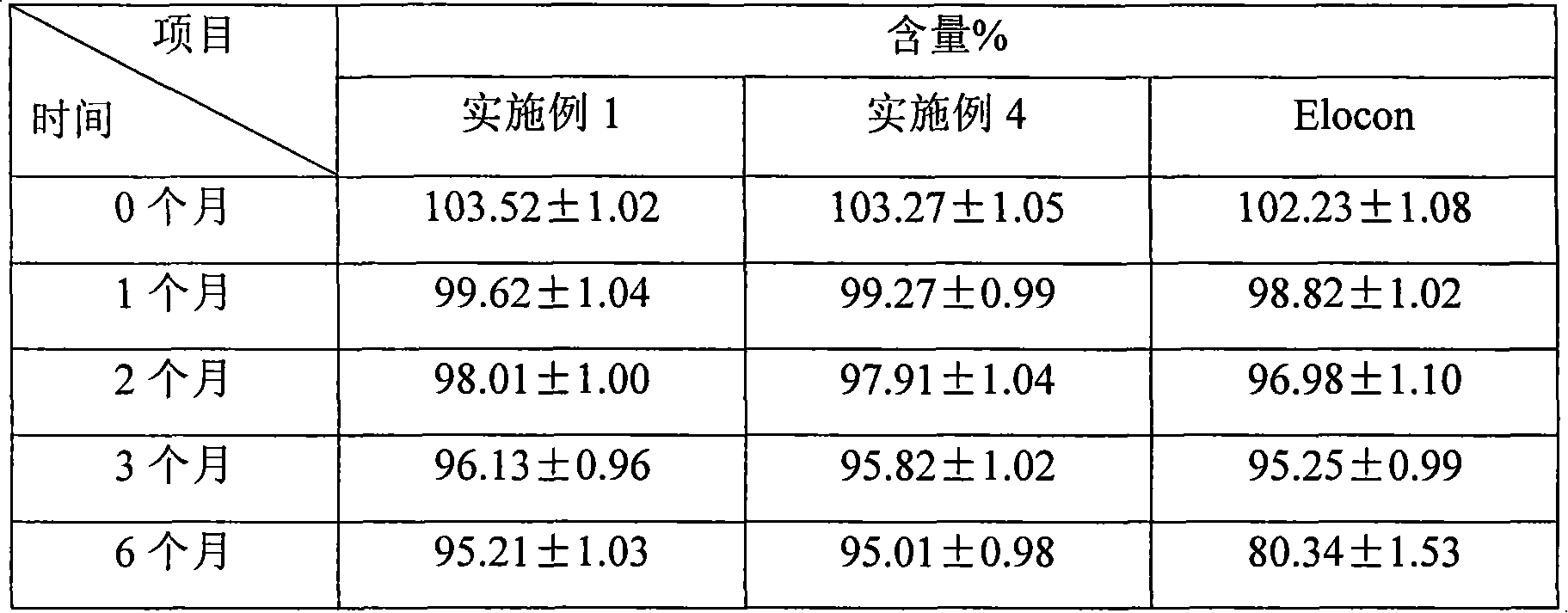
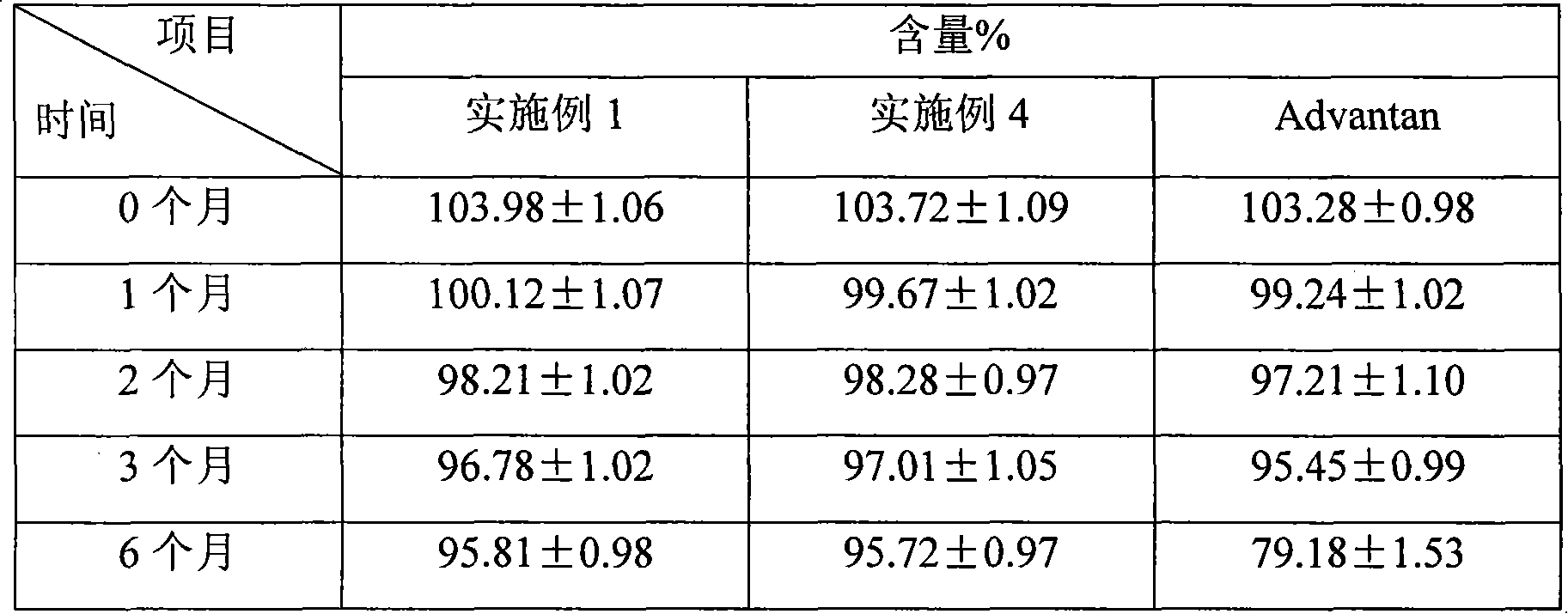
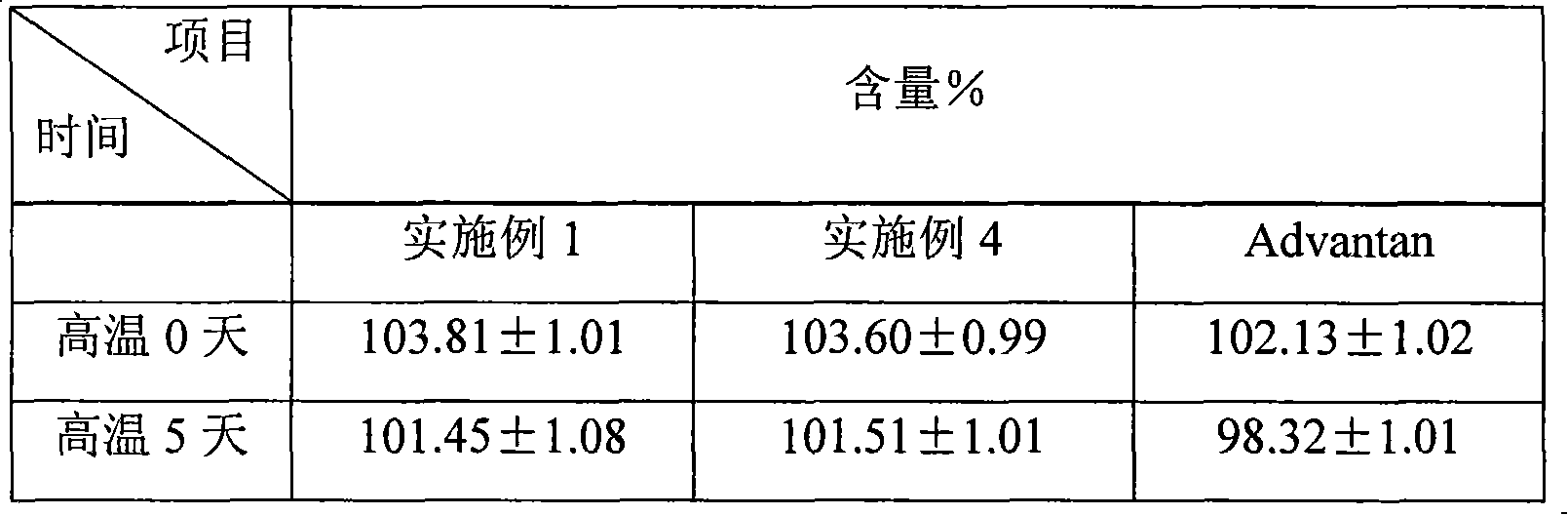
Fluticasone propionate lipidosome cream
InactiveCN101601650AHigh encapsulation efficiencyEncapsulationOrganic active ingredientsAerosol deliveryDiseaseFluticasone propionate
The invention relates to a lipidosome medical composition which uses fluticasone propionate as an active component, and a cyst diameter of lipidosome is smaller than 800 nm. The composition comprises 0.025 percent to 0.2 percent of fluticasone propionate as the active component, 0.5 percent to 6 percent of phospholipid, 0 percent to 1 percent of lipophilic additive, 0.01 percent to 1 percent of antioxidant which is used for preserving the medical composition, a pH buffering agent which is used for retaining the pH value from 5 to 7.5, 3 percent to 15 percent of humectant, 20 percent to 30 percent of oil phrase component, 0.01 percent to 0.1 percent of antimicrobial preservative and the balanced of water. The fluticasone propionate lipidosome cream is used for treating skin diseases of human beings or animals.
Owner:TIANJIN JINYAO GRP
Preparation method of multifunctional liposome vesicle
InactiveCN103877024AAchieve targeted deliveryRealize functionPharmaceutical non-active ingredientsIn-vivo testing preparationsLiposome VesicleCholesterol
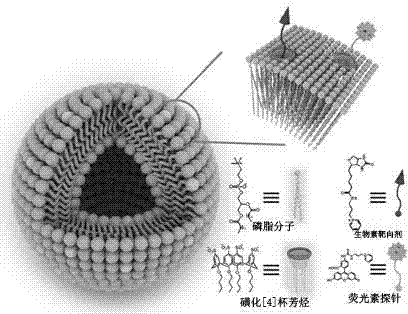
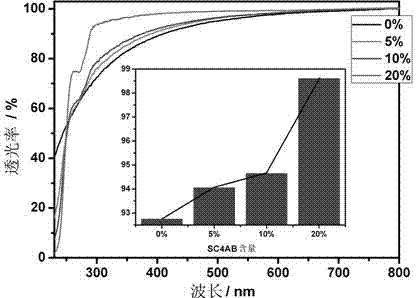
The invention belongs to the technical field of nanometer supermolecular materials. A liposome vesicle which is non-covalently bonded, good in biocompatibility and high in stability and can realize the functions of targeted transmission and monitoring tracking is prepared by adding amphiphilic p-sulfonated calix [4] arene in proper proportion on the basis that the liposome vesicle is prepared through the traditional phospholipid molecule and cholesterol. The preparation method disclosed by the invention has the advantages of easiness, convenience, small usage amount of main and object raw materials and wide application prospect in the field of targeted transportation of cancer medicines.
Owner:NANKAI UNIV
Automatic Liquid Injection System and Method
InactiveUS20090076477A1Stay efficientRotating receptacle mixersShaking/oscillating/vibrating mixersLiposome VesicleActive agent
A power assisted method and injector device for controllably delivering to patients a dispersion medicament or diagnostically active agent, the homogeneity of which is preserved throughout delivery. Diagnostically active agents disclosed are gas microbubble suspensions useful in ultrasonic diagnostic imaging and liposomal formulations in which liposome vesicles are loaded with iodinated compounds.
Owner:BRACCO RES USA
Momestasone furoate lipidosome cream
InactiveCN101601652ASmall diameterHigh encapsulation efficiencyOrganic active ingredientsAerosol deliveryDiseaseLiposome Vesicle
The invention relates to a lipidosome medical composition which uses momestasone furoate as an active component, and a cyst diameter of lipidosome is smaller than 800nm. The composition comprises 0.025 percent to 0.2 percent of momestasone furoate as the active component, 0.5 percent to 6 percent of phospholipid, 0 percent to 1 percent of lipophilic additive, 0.01 percent to 1 percent of antioxidant which is used for preserving the medical composition, a pH buffering agent which is used for retaining a pH value from 5 to 7.5, 3 percent to 15 percent of humectant, 20 percent to 30 percent of oil phrase component, 0.01 percent to 0.1 percent of antimicrobial preservative and the balanced of water. The momestasone furoate lipidosome cream is used for treating skin diseases of human beings or animals.
Owner:TIANJIN JINYAO GRP
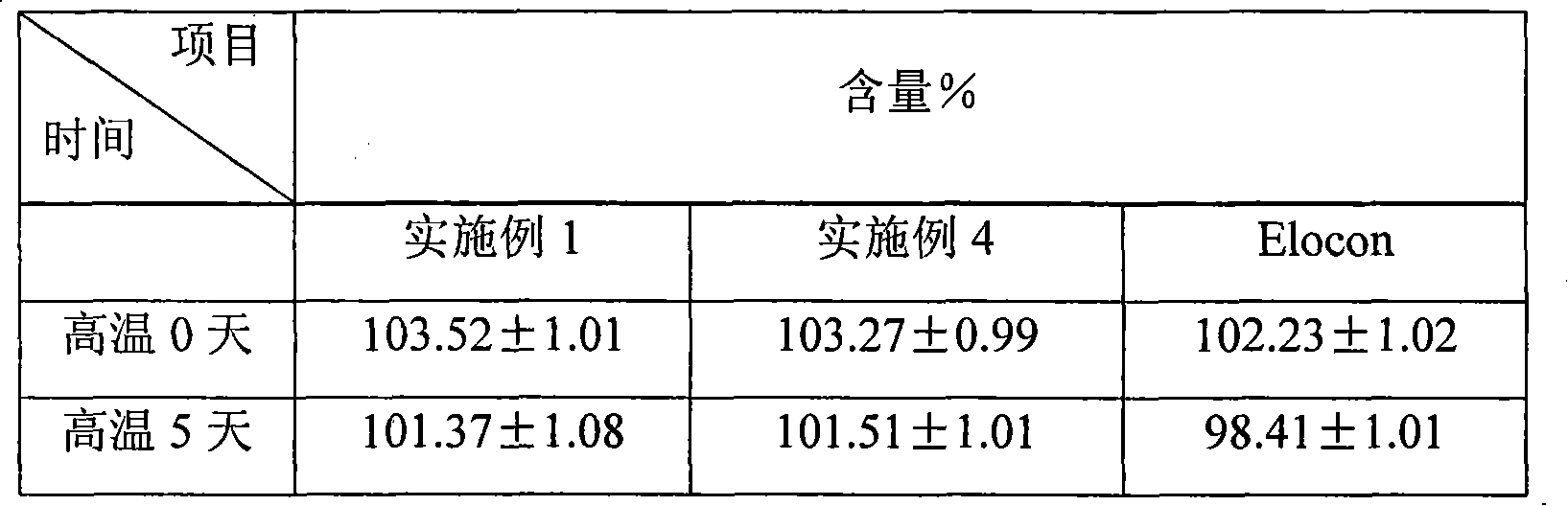
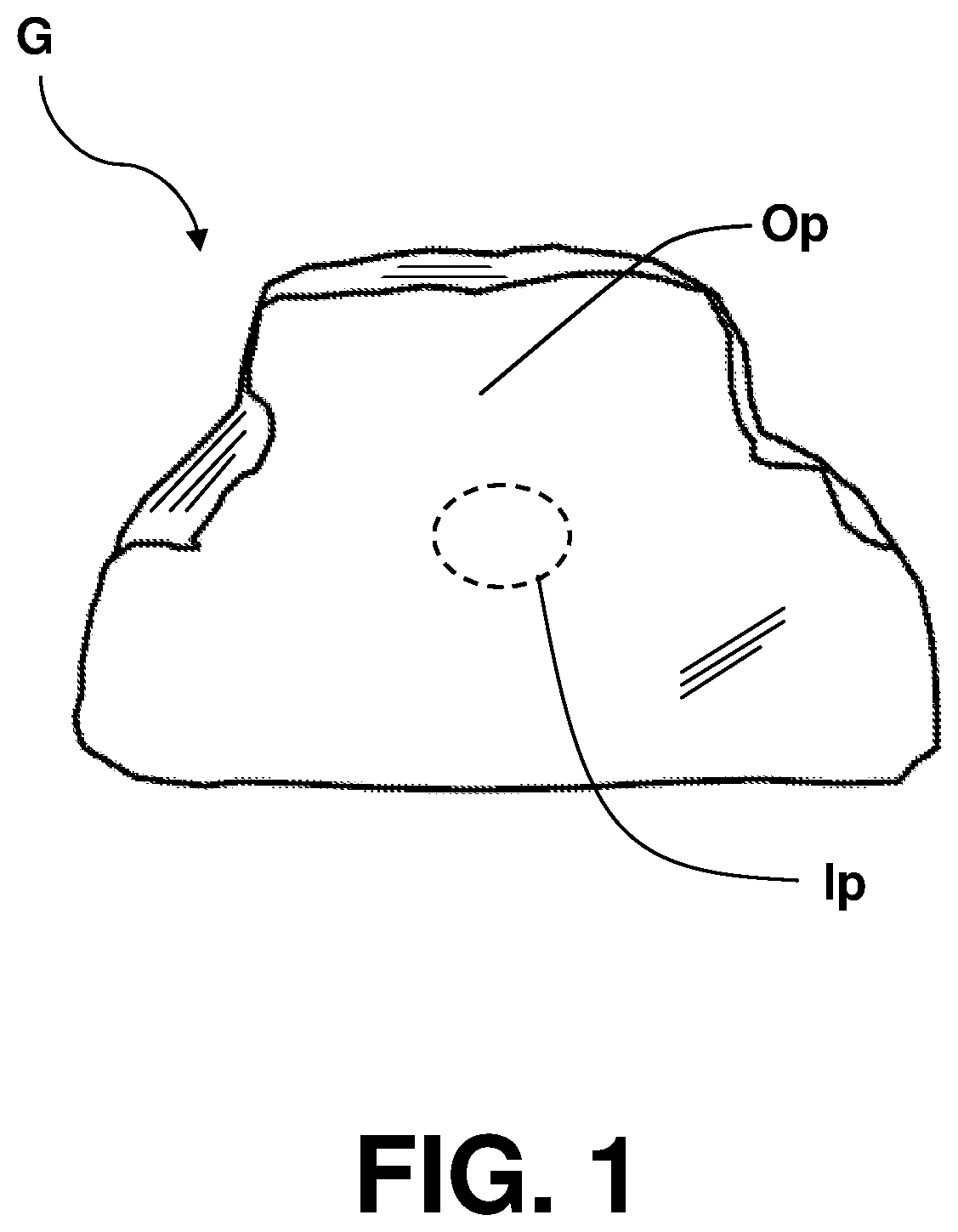
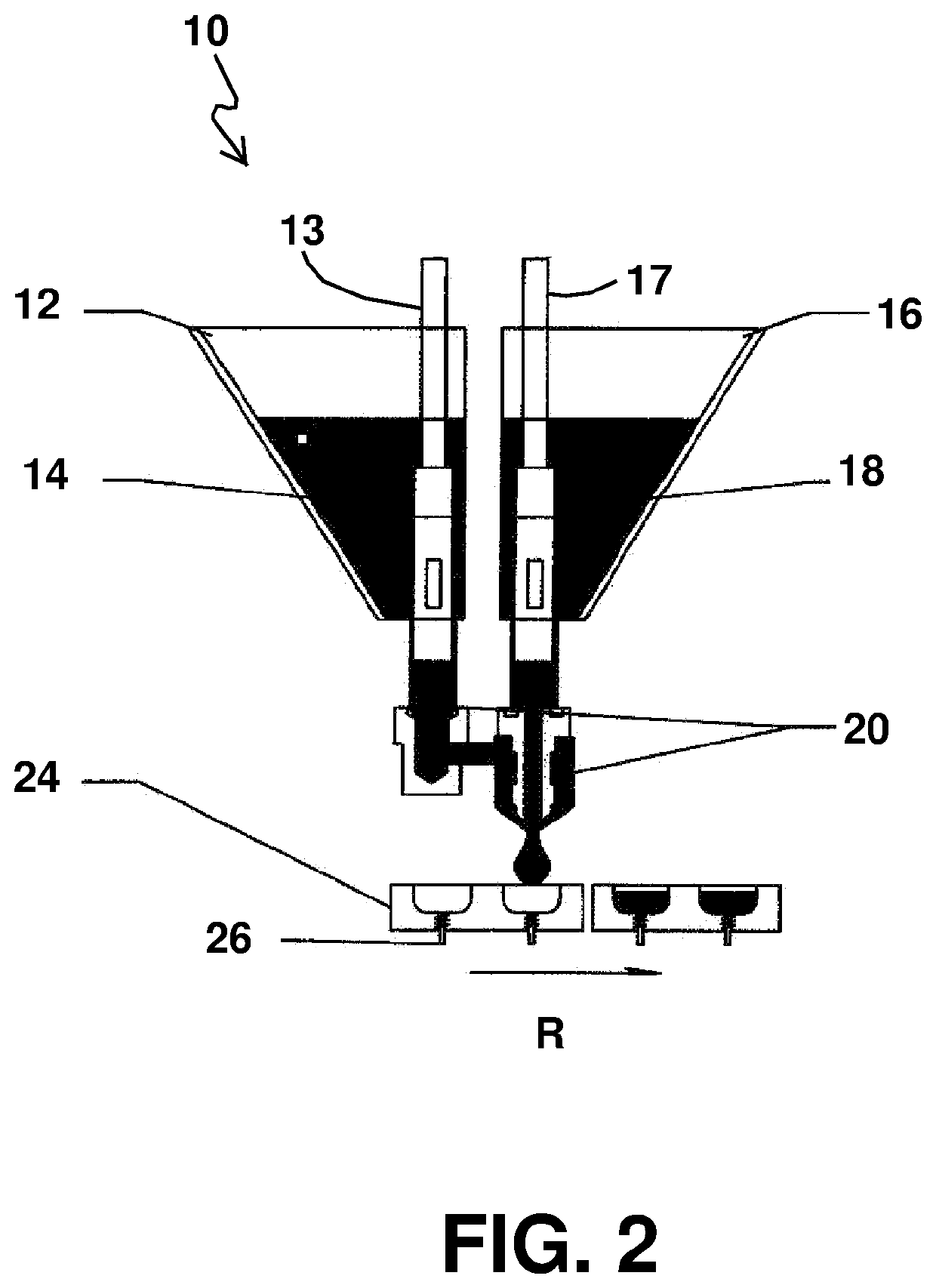
Gummies containing formulations with enhanced delivery matrix, and methods of making same
A gummy includes an inner portion, a formulation having a dispersion including a plurality of liposomal vesicles contained in the inner portion; and an outer portion which surrounds the inner portion. The formulation includes an active ingredient including cannabidiol (CBD), phospholipid contained in the liposomal vesicles, and a optionally added coating material, the CBD being incorporated within the liposomal vesicles. A method of making such gummy having the CBD formulation in the inner portion thereof includes setting up a central filling apparatus having a shell syrup hopper, a central filling hopper, and a manifold branch and nozzle mechanism; filling the shell syrup hopper with the base material; filling the central filling hopper with the CBD formulation; dispensing the base material and CBD formulation in the manifold branch and nozzle mechanism, and further into the gummy mold; and extracting the gummy from the gummy mold.
Owner:VITASOME LABS INC
Liposome-containing radiographic contrast medium and preparation method thereof
InactiveUS20060018828A1Improve efficiencyHigh selectivityIn-vivo radioactive preparationsDispersion deliverySterolLiposome Vesicle
A method of pereparing a rediographic contrast medium containing liposomes is disclosed, comprising (a) dissolving a phospholipid and a sterol in a supercritical or subcritical carbon dioxide in the presence of a compound containing a hydroxyl group or a polyalkyleneoxide group, (b) adding thereto an aqueous solution containing an iodine compound to form micelles and (c) discharging the carbon dioxide to form liposomal vesicles enclosing the iodine compound.
Owner:KONICA MINOLTA MEDICAL & GRAPHICS INC
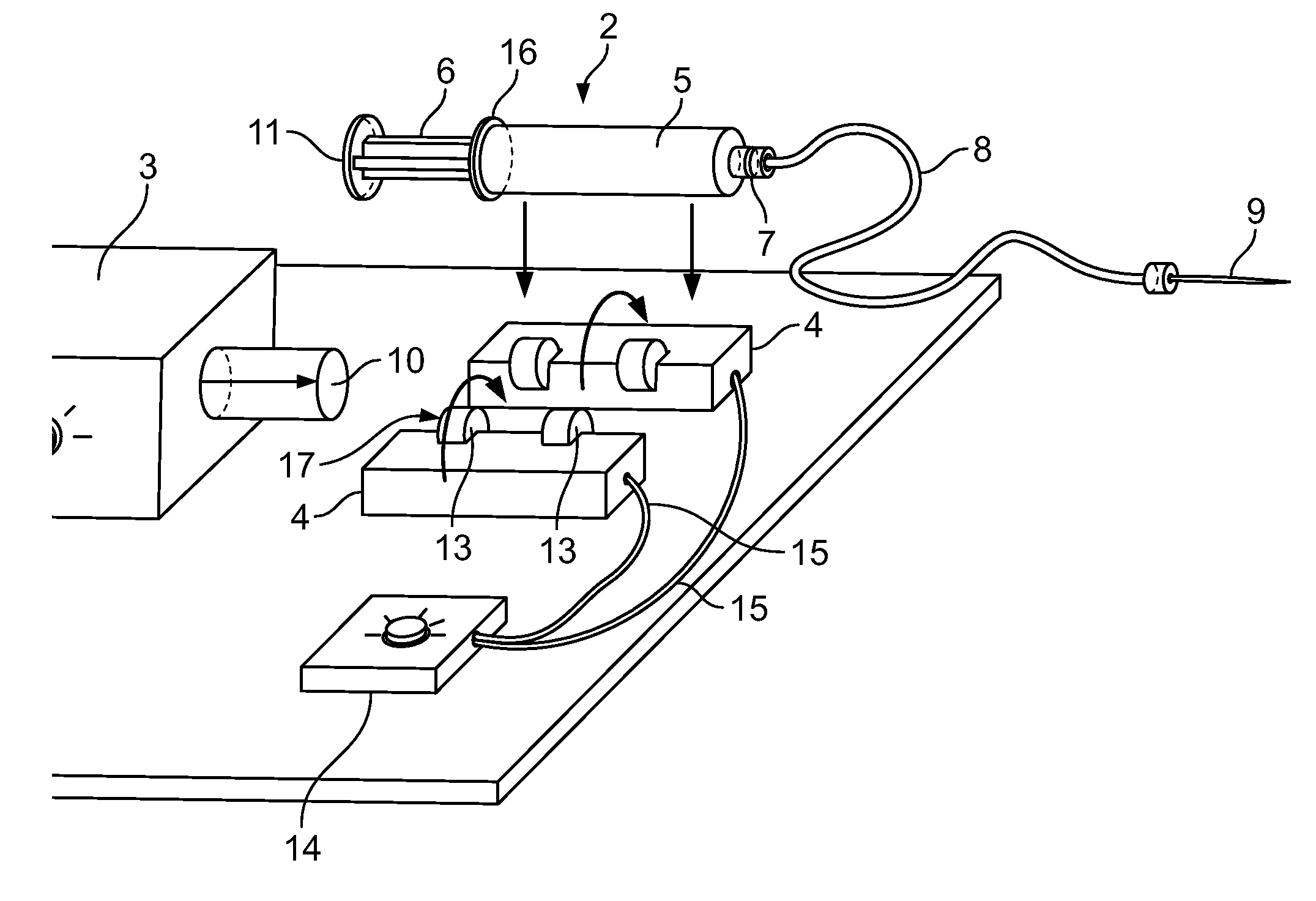
Methods and apparatus for extrusion of vesicles at high pressure
InactiveCN1635873ASolution deliveryPharmaceutical non-active ingredientsLiposome VesicleHigh pressure
The present invention relates generally to methods and devices for the preparation of vesicles, including micelles, particularly liposomes, the method comprising extruding a solution containing a substance capable of forming vesicles through a mesh membrane under high pressure.
Owner:ESPERION THERAPEUTICS
PLNPs as well as pharmaceutical composition and application thereof
InactiveCN108096189AImprove transfection efficiencyGood treatment effectMacromolecular non-active ingredientsAntineoplastic agentsLipid formationLipid particle
The invention provides PLNPs. The PLNPs are nanoparticles wrapped by liposome vesicles, and the surfaces of the liposome vesicles are modified with distearoyl phosphatidylethanolamine-PEG; the nanoparticles are prepared from lipid particles, chondroitin sulfate and protamine; the liposome vesicles are prepared from dioleoyl phosphatidylethanolamine, N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethylammonium chloride and cholesterol. The invention further provides a preparation method of the PLNPs and pharmaceutical composition containing the PLNPs. The PLNPs as a carrier have higher transfection effect. The pharmaceutical composition has better treatment effect on various tumors.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Prednicarbate lipidosome cream
InactiveCN101601653ASmall diameterHigh encapsulation efficiencyOrganic active ingredientsAerosol deliveryDiseaseLiposome Vesicle
The invention relates to a lipidosome medical composition which uses prednicarbate as an active component, and a cyst diameter of lipidosome is smaller than 800 nm. The composition comprises 0.025 percent to 0.2 percent of prednicarbate as the active component, 0.5 percent to 6 percent of phospholipid, 0 percent to 1 percent of lipophilic additive, 0.01 percent to 1 percent of antioxidant which is used for preserving the medical composition, a pH buffering agent which is used for retaining the pH value from 5 to 7.5, 3 percent to 15 percent of humectant, 20 percent to 30 percent of oil phrase components, 0.01 percent to 0.1 percent of antimicrobial preservative and the balanced of water. The prednicarbate lipidosome cream is used for treating skin diseases of human beings or animals.
Owner:TIANJIN JINYAO GRP
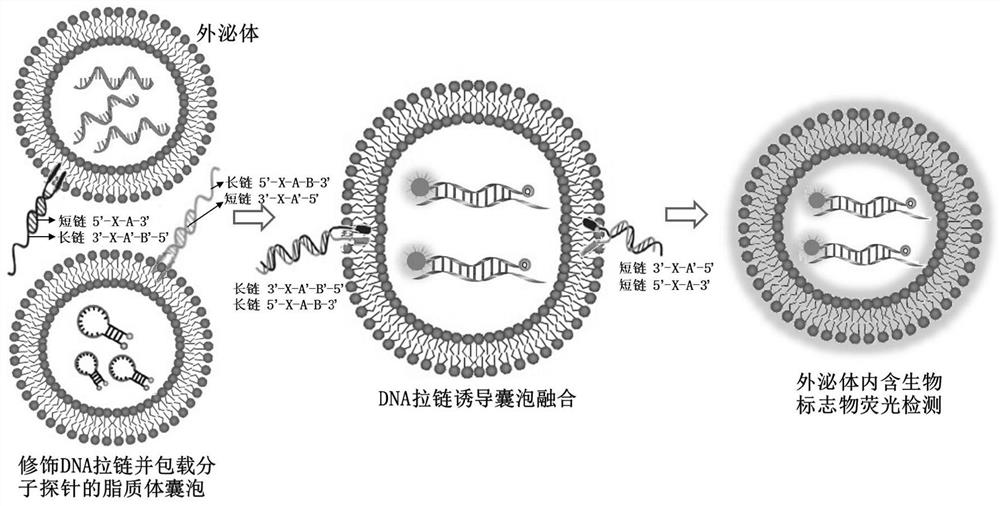
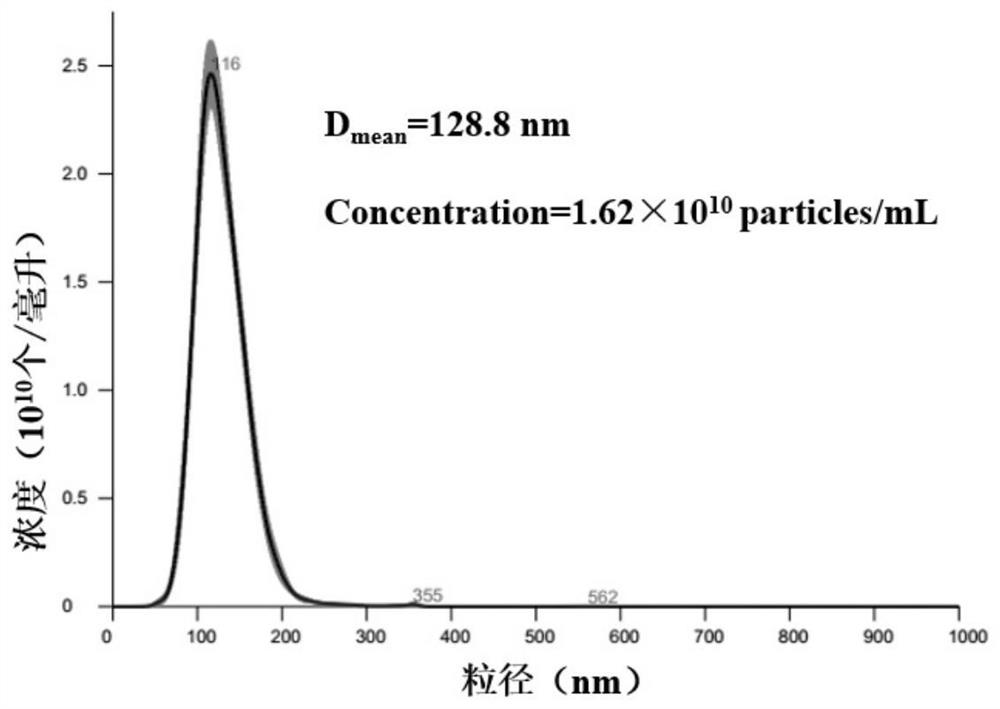
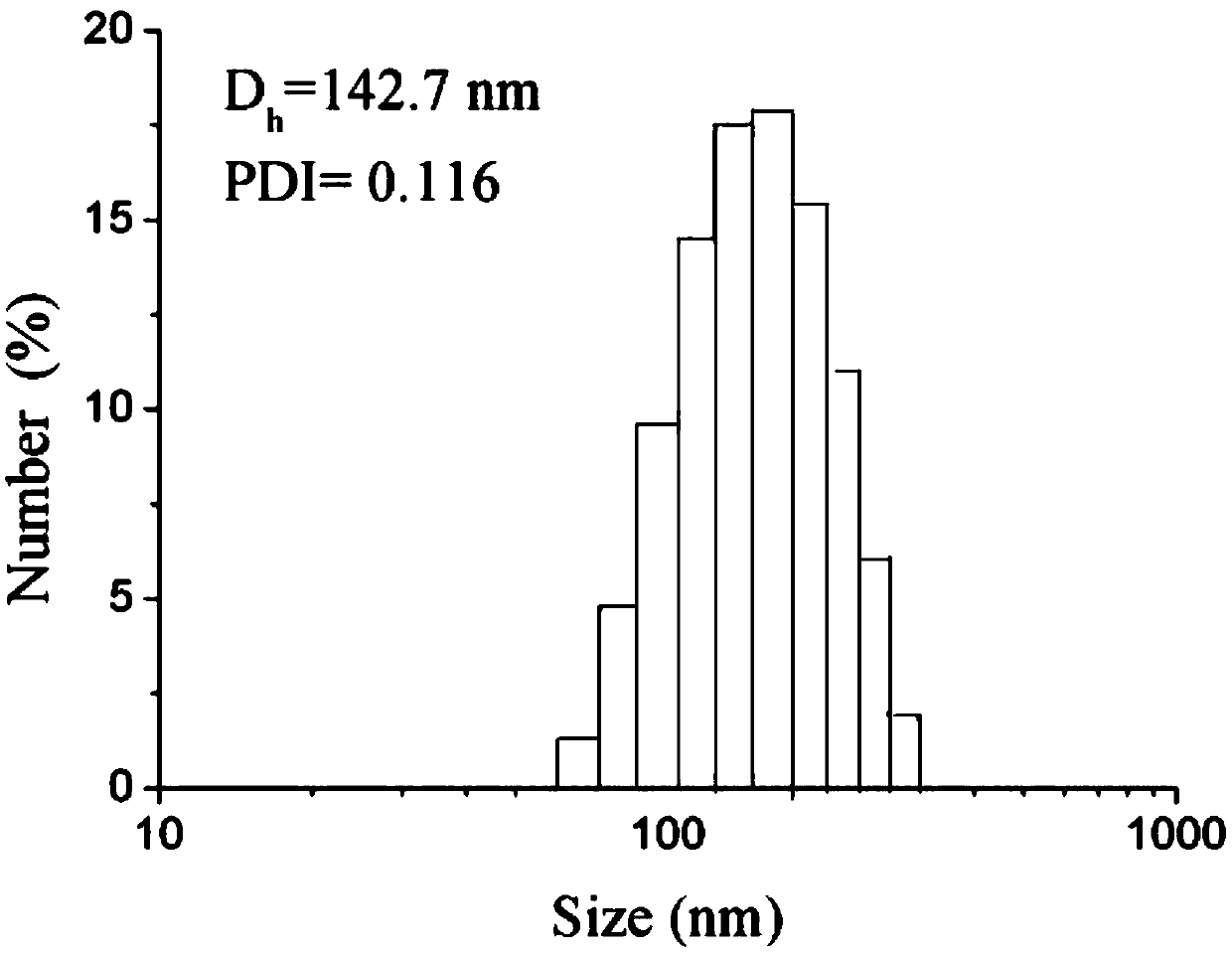
DNA zipper molecule modified lipidosome vesicle and preparation method and application thereof
PendingCN112852920AAchieve in situAvoid degradationMicrobiological testing/measurementLipofectamineLiposome Vesicle
The invention provides a DNA zipper molecule modified lipidosome vesicle and a preparation method and application thereof. The lipidosome vesicle is composed of a DNA zipper molecule modified bimolecular layer membrane and a molecular probe wrapped in the bimolecular layer membrane, wherein DNA zipper molecules have a DNA zipper sequence promoting lipidosome fusion, the lipidosome vesicle is rapidly subjected to membrane fusion with an exosome modified by the DNA zipper molecules under the action of a DNA zipper, so that the molecular probe contained in the vesicle is sent into the exosome, and the in-situ, accurate and rapid detection of a nucleic acid-containing biomarker in the exosome is realized. The preparation method of the liposome vesicle comprises the steps: mixing the bimolecular layer membrane and the molecular probe, extruding to obtain the liposome vesicle, and incubating the liposome vesicle with the DNA zipper molecule modified with a lipophilic group. The DNA zipper molecule modified on the surface of the liposome vesicle is actively fused with the exosome, so that the efficiency is higher, the nucleic acid marker to be detected does not need to be separated from the exosome in advance, and the detection effect is better.
Owner:SHANGHAI JIAO TONG UNIV
Sorafenib solid lipid nanoparticles as well as preparation method and application thereof
InactiveCN109091672AIncreased toxicityImprove solubilityEnergy modified materialsDigestive systemSolubilityLiposome Vesicle
The invention provides sorafenib solid lipid nanoparticles and a preparation method thereof. A phospholipid film is used as a shell, sorafenib and indocyanine green are taken as cores, wherein liposoluble sorafenib is encapsulated in a hydrophobic region of the phospholipid bilayer, and hydrophilic indocyanine green is located in liposome vesicles formed by the phospholipid bilayer. The preparation method comprises the following steps: weighing sorafenib and phospholipid, adding an organic solvent for dissolution to obtain an organic phase, and then removing an organic solvent to obtain the phospholipid film containing sorafenib; weighing indocyanine green, and dissolving indocyanine green in deionized water to obtain a water phase; adding the obtained water phase to the obtained phospholipid film, stirring and mixing the solution uniformly, and performing squeezing and filtering to obtain the final product. According to the sorafenib solid lipid nanoparticles, sorafenib and indocyanine green are loaded into a phospholipid film nano carrier together, so that toxicity and solubility of sorafenib can be improved, and light resistance and tumor aggregation resistance of indocyanine green can be enhanced.
Owner:HUBEI UNIV +1
Liposome-containing radiographic contrast medium and preparation method thereof
InactiveUS7588751B2Improve efficiencyHigh selectivityIn-vivo radioactive preparationsDispersion deliveryLiposome VesicleSterol
A method of pereparing a rediographic contrast medium containing liposomes is disclosed, comprising (a) dissolving a phospholipid and a sterol in a supercritical or subcritical carbon dioxide in the presence of a compound containing a hydroxyl group or a polyalkyleneoxide group, (b) adding thereto an aqueous solution containing an iodine compound to form micelles and (c) discharging the carbon dioxide to form liposomal vesicles enclosing the iodine compound.
Owner:KONICA MINOLTA MEDICAL & GRAPHICS INC
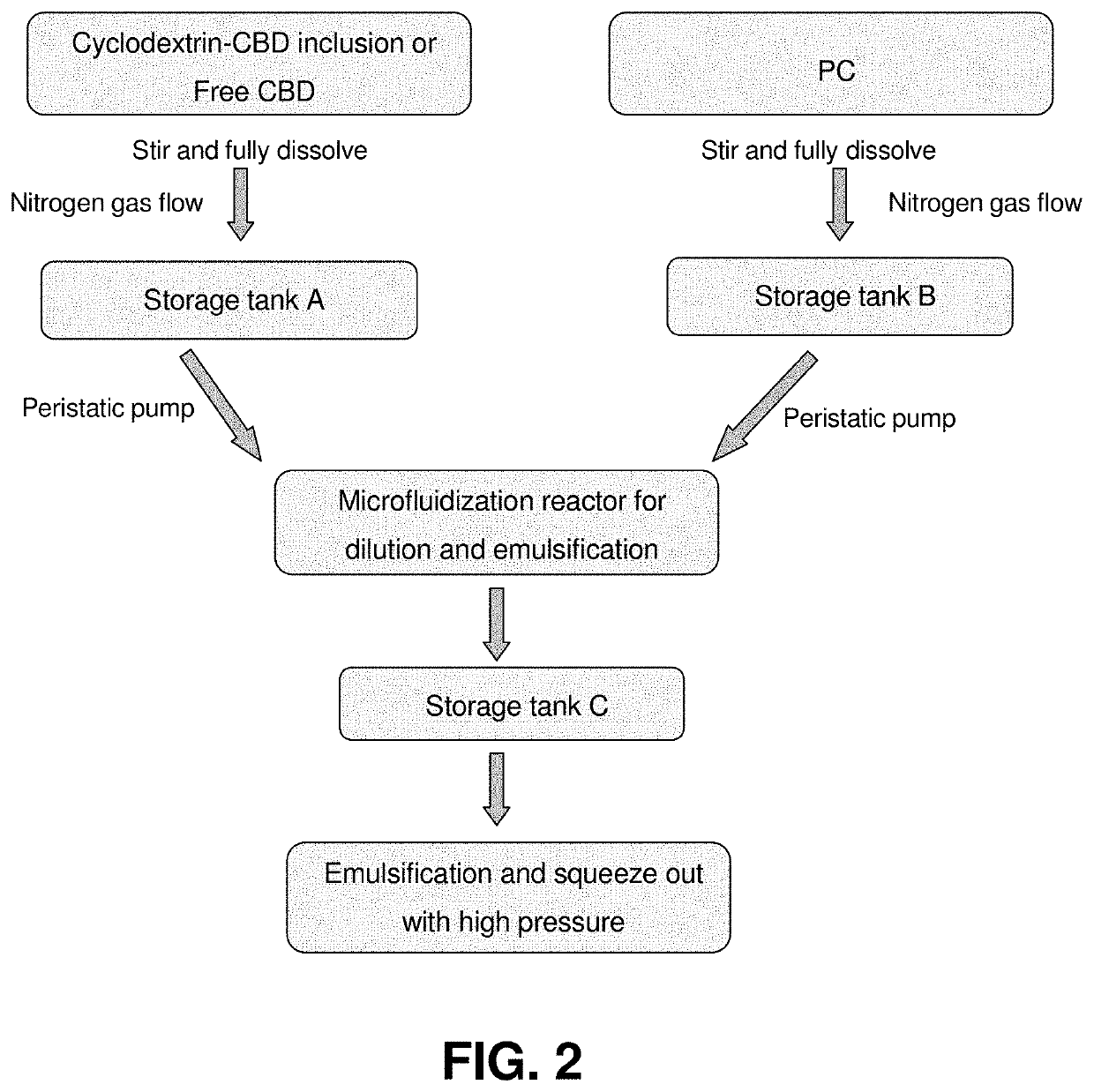
Dietary supplement compositions including cannabidiol formulations having enhanced bioavailability with sustained time release and enhanced organoleptics, and methods of making same
ActiveUS11252985B1Improve sensory propertiesExtended shelf lifeHydroxy compound active ingredientsNatural extract food ingredientsLiposome VesicleDietary supplement
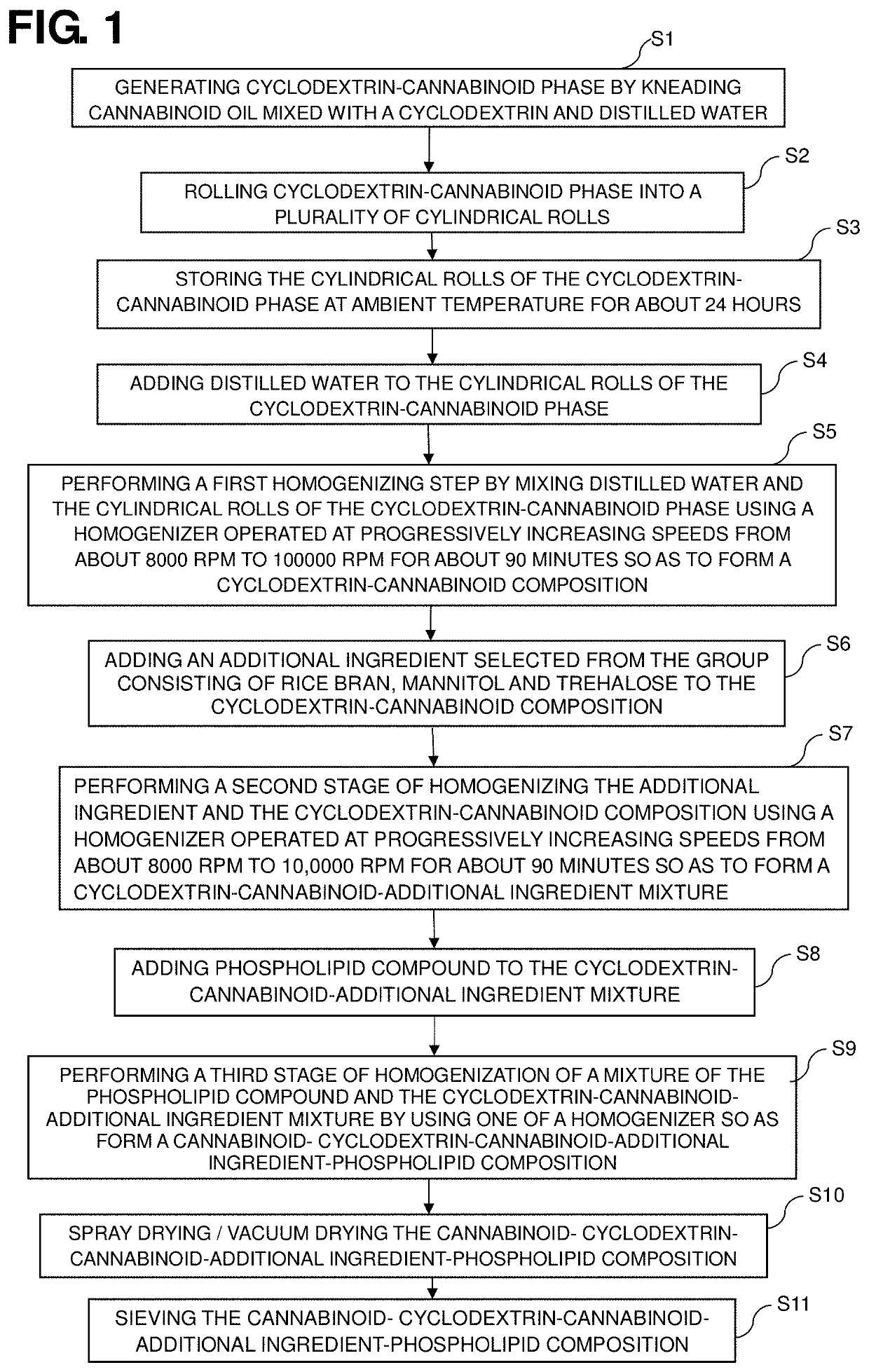
A dietary supplement composition has a dispersion including a plurality of liposomal vesicles. The dietary supplement composition includes an active ingredient including one or more hydrophobic nutrient, such as cannabinoid or cannabinoid compound (cannabinoid), and phospholipid contained in the liposomal vesicles. The cannabinoid, which may be combined with cyclodextrin, is incorporated within the liposomal vesicles. The dietary supplement composition may further include one or more of rice bran extract, mannitol and trehalose. A method of preparing such dietary supplement composition includes generating a cannabinoid phase, generating an phospholipid phase, adding the cannabinoid phase and the phospholipid phase in vessel; performing a homogenizing step by mixing the cannabinoid phase and the phospholipid phase so as to form a cannabinoid-phospholipid composition having a plurality of liposomal vesicles having the cannabinoid incorporated therein, wherein the concentration of cannabinoid in the cannabinoid-phospholipid composition is about 10 to 25 mass %.
Owner:CANNASPHERE BIOTECH LLC
Methylprednisolone aceponate lipidosome cream
InactiveCN101601651AImprove stabilityPromote absorptionOrganic active ingredientsOrganic non-active ingredientsLiposome VesiclePh buffering
The invention relates to a lipidosome medical composition which uses methylprednisolone aceponate as an active component, and a cyst diameter of lipidosome is smaller than 800 nm. The composition comprises 0.025 percent to 0.2 percent of methylprednisolone aceponate as the active component, 0.5 percent to 6 percent of phospholipid, 0 percent to 1 percent of lipophilic additive, 0.01 percent to 1 percent of antioxidant which is used for preserving the medical composition, a pH buffering agent which is used for retaining the pH value from 5 to 7.5, 3 percent to 15 percent of humectant, 20 percent to 30 percent of oil phrase component, 0.01 percent to 0.1 percent of antimicrobial preservative and the balanced of water. The methylprednisolone aceponate lipidosome cream is used for treating skin diseases of human beings or animals.
Owner:TIANJIN JINYAO GRP
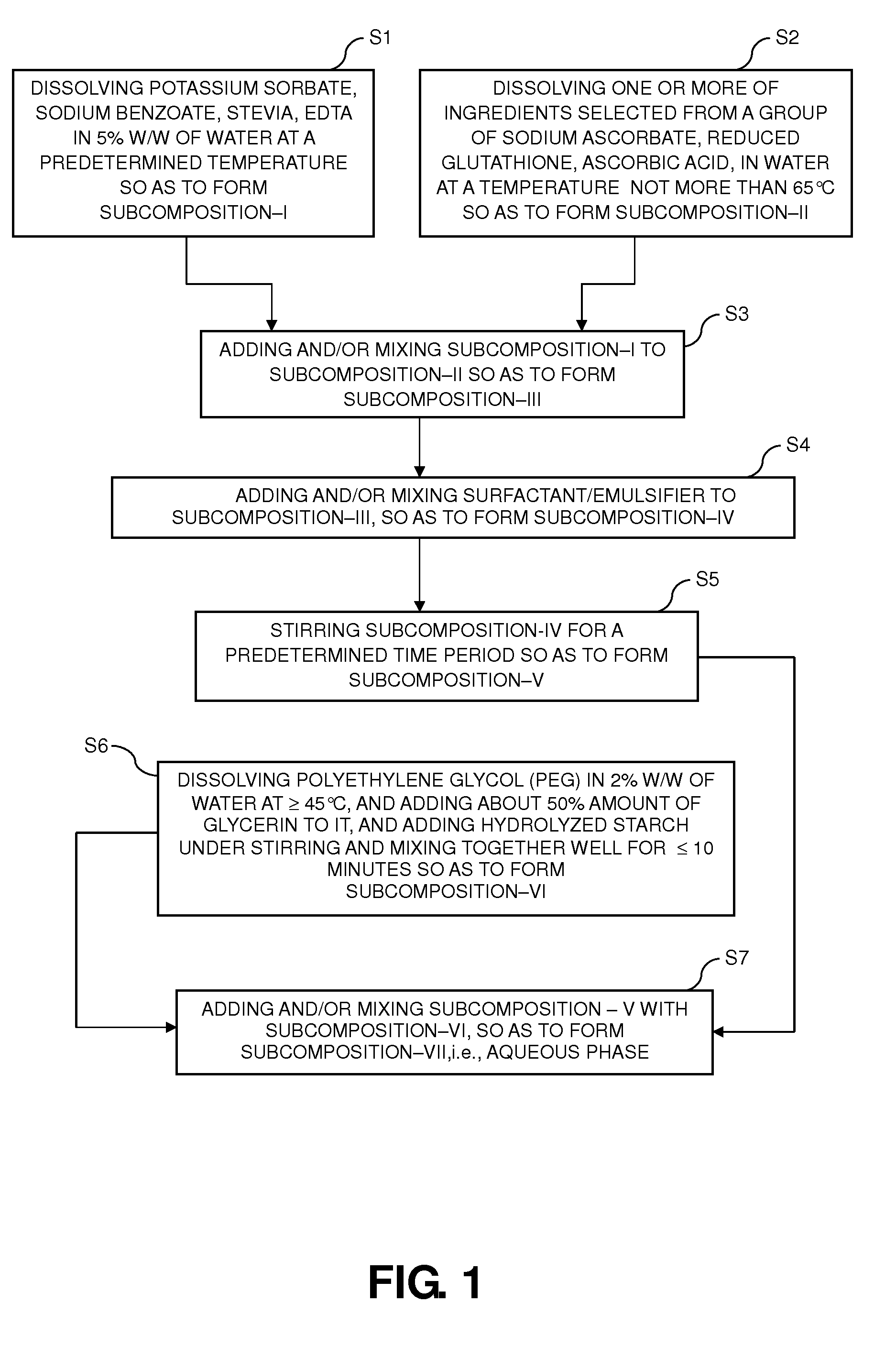
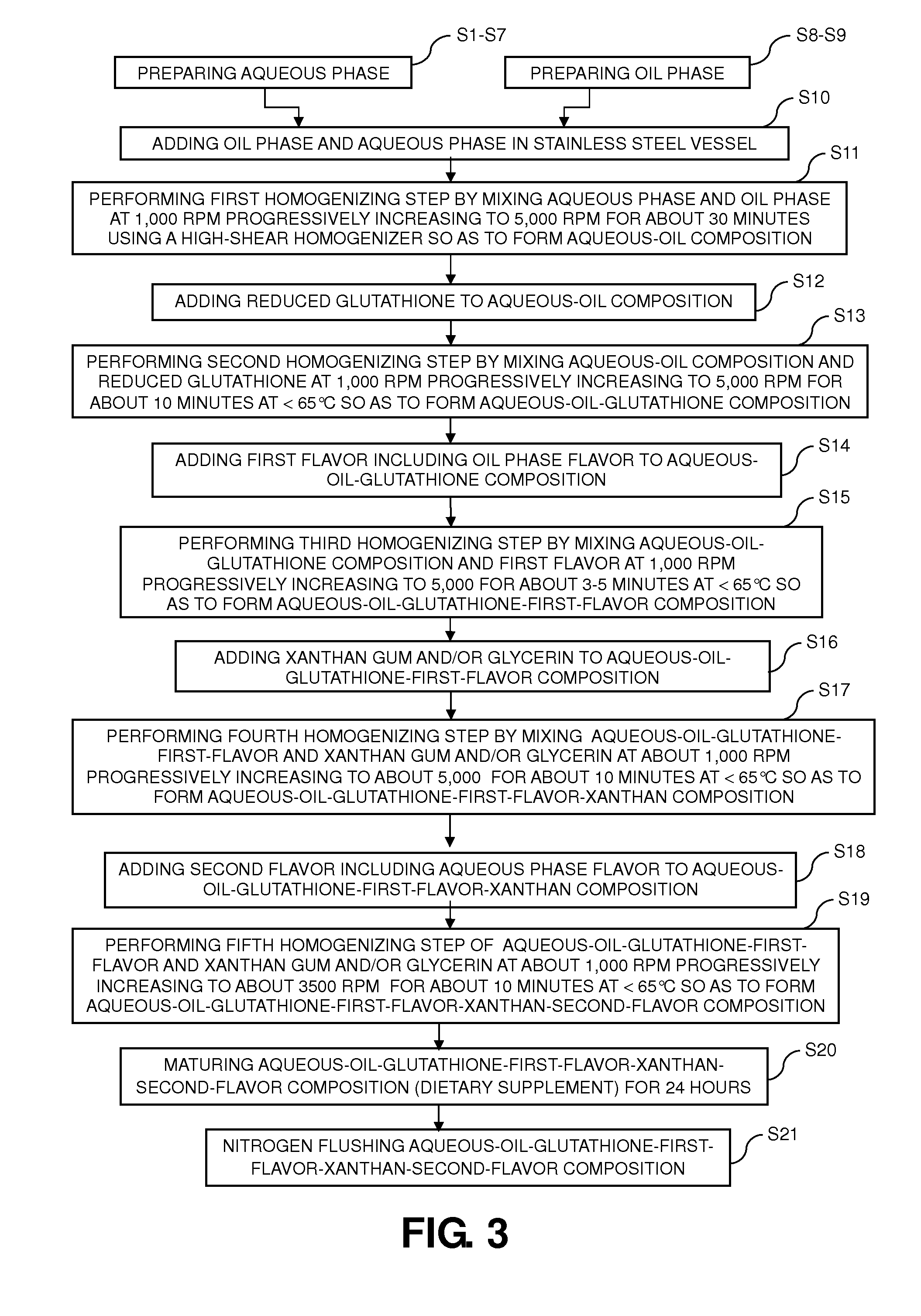
Dietary supplement compositions with enhanced delivery matrix, and methods of making the same
A method of making dietary supplement compositions includes generating an aqueous phase, generating an oil phase, performing a first homogenizing step by mixing the aqueous and oil phases, adding reduced glutathione to the aqueous-oil composition and performing a second homogenizing step by mixing the aqueous-oil composition and reduced glutathione, adding a first flavor to the aqueous-oil-glutathione composition, performing a third homogenizing step by mixing the aqueous-oil-glutathione composition and the first flavor, adding xanthan gum dispersed with glycerin to the aqueous-oil-glutathione-first-flavor composition and performing a fourth homogenizing step by mixing the aqueous-oil-glutathione-first-flavor composition and xanthan gum; adding a second flavor to the aqueous-oil-glutathione-first-flavor-xanthan composition and performing a fifth homogenizing step by mixing the aqueous-oil-glutathione-first-flavor-xanthan composition and the second flavor so as to form a dietary supplement composition. Such method provides a dietary supplement as a dispersion including active ingredients incorporated in liposomal vesicles having a barrier coating of polyethylene glycol.
Owner:VITASOME LABS INC
ARTIFICIAL beta-CELLS AND METHODS OF USE THEREOF
PendingCN111356448AAvoid hypoglycemiaPeptide/protein ingredientsNitro compound active ingredientsLiposome VesicleBiophysics
Disclosed herein is a particle containing an inner liposomal vesicle (ILV) encapsulating a therapeutic agent; an outer liposomal vesicle (OLV) encapsulating the ILV; a membrane fusion-promoting agent;and a pH-altering agent. Also disclosed are methods of delivering a therapeutic agent to a subject comprising: a) providing a herein disclosed particle b) triggering ILV and OLV fusion; and c) releasing the therapeutic agent outside of the OLV. Also disclosed are methods for treating a disease in a subject in need thereof comprising: administering to a subject a herein disclosed particle. Also disclosed are methods to release insulin to an environment comprising increased glucose levels, the method comprising exposing to the environment a herein disclosed particle.
Owner:NORTH CAROLINA STATE UNIV
Composition, preparation and application of a magnetic liposome vesicle
ActiveCN107998978BGood biocompatibilityThe size is easy to controlWax physical treatmentTransportation and packagingLiposome VesicleBiocompatibility Testing
The invention discloses the composition, preparation and application of a magnetic liposome vesicle, and belongs to the technical field of emulsifier preparation. The present invention adopts magnetic Fe 3 O 4 Nanoparticles induce self-assembly of conjugated linoleic acid (CLA) into magnetic vesicles, and then make CLA@Fe 3 O 4 Self-crosslinking of CLA molecules in bilayers yields stable SCLA@Fe 3 O 4 (Self-cross-linked conjugated linoleic acid) magnetic vesicles. The particle size of the prepared magnetic liposome vesicles does not exceed 25 nm, and can be used to prepare pH / magnetic dual-responsive Pickering emulsions, and the internal phase volume can be as high as 92%. The material used in the invention has good biocompatibility, simple process, easy-to-obtain raw materials, and easy control of particle size and shape.
Owner:JIANGNAN UNIV
Positively charged liposomes as lipophilic molecule carriers
A method of producing positively charged liposome vesicles for use as carriers of lipophilic molecules. A mixture of hydrogenated phospholipids, a cationic excipient and a lipophilic molecule are dissolved in a solvent to form a composition. The composition is dried to remove the solvent. The dried composition is hydrated to form liposome vesicles and optionally the liposome vesicles are homogenized to form smaller vesicles. The vesicles are useful for delivery lipophilic molecules, such as, but limited to, lutein and zeaxanthin, to ocular tissues using iontophoresis.
Owner:KEMIN IND INC
Application of cleavable polyethylene glycol (PEG) lipid derivative in preparation
InactiveCN102068701BEasy to prepareNo radioactive contaminationPowder deliveryPharmaceutical non-active ingredientsLiposome VesicleMethyl carbonate
The invention belongs to the technical field of medicaments, and provides application of a cleavable polyethylene glycol (PEG) lipid derivative to preparation of a PEGylated preparation for relieving or avoiding accelerated blood clearance. In the application, liquid microparticle preparations such as liposome, vesicles, emulsions, microemulsion, micelles, nanoparticles and the like are modified by the cleavable PEG lipid derivative such as PEG-cholesteryl hemisuccinate, PEG-cholesteryl methyl carbonate, PEG-alpha tocopheryl hemisuccinate and the like; and the measurement of variation of preparation elimination in tissues such as animal blood plasma, liver, spleen and the like after a cleavable PEG lipid derivative-modified medicinal preparation is repeatedly injected proves that repeatedinjection of cleavable PEG lipid derivative-modified microparticle preparations only causes light accelerated blood clearance or avoids the accelerated blood clearance, namely the accelerated blood clearance can be relieved or avoided. The invention discloses new application of the cleavable PEG lipid derivative.
Owner:SHENYANG PHARMA UNIVERSITY
Icaritin liposome oral preparation, and preparation method thereof
InactiveCN106983724AImprove bioavailabilityPromote absorptionOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityLiposome Vesicle
The invention discloses an icaritin liposome oral preparation, and a preparation method thereof. According to the preparation method, the icaritin liposome oral preparation is prepared via thin film dispersion combined ice bath ultrasonic treatment or high pressure homogenization emulsification treatment. 100ml of the icaritin liposome oral preparation contains, 0.2 to 2.4% of icaritin, 0.4 to 12% of lecithin, 0.1 to 1.2% of a film structure conditioning agent, and 0.2% of a nonionic surfactant. The preparation method is capable of solving problems in the prior art via conventional liposome preparation technology that the dissolvability of icaritin in water phase systems is poor, and degradation is easily caused under influences of the external environment; and stability of icaritin in gastrointestinal digestion process is increased. The obtained icaritin liposome oral preparation is regular spherical vesicles; embedding rate is 70% or higher; particle size ranges from 100 to 300nm; particle size distribution is uniform; the preparation method is simple; liposome quality is increased greatly; and the preparation method is suitable for large-scale industrialized production.
Owner:CHINA AGRI UNIV
Momestasone furoate lipidosome cream
InactiveCN101601652BSmall diameterHigh encapsulation efficiencyOrganic active ingredientsAerosol deliveryDiseaseLiposome Vesicle
The invention relates to a lipidosome medical composition which uses momestasone furoate as an active component, and a cyst diameter of lipidosome is smaller than 800nm. The composition comprises 0.025 percent to 0.2 percent of momestasone furoate as the active component, 0.5 percent to 6 percent of phospholipid, 0 percent to 1 percent of lipophilic additive, 0.01 percent to 1 percent of antioxidant which is used for preserving the medical composition, a pH buffering agent which is used for retaining a pH value from 5 to 7.5, 3 percent to 15 percent of humectant, 20 percent to 30 percent of oil phrase component, 0.01 percent to 0.1 percent of antimicrobial preservative and the balanced of water. The momestasone furoate lipidosome cream is used for treating skin diseases of human beings oranimals.
Owner:TIANJIN JINYAO GRP
Gummies containing formulations with enhanced delivery matrix, and methods of making same
Owner:VITASOME LABS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com