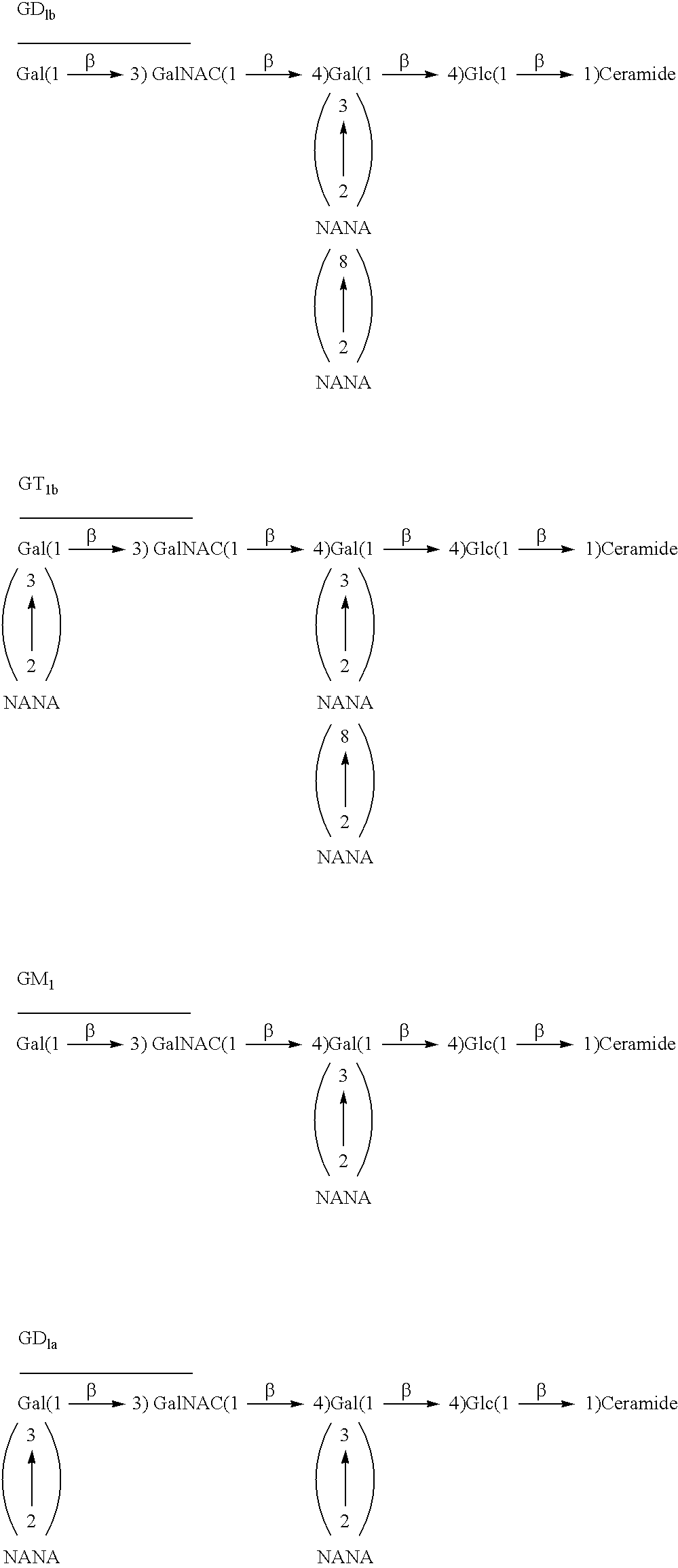Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
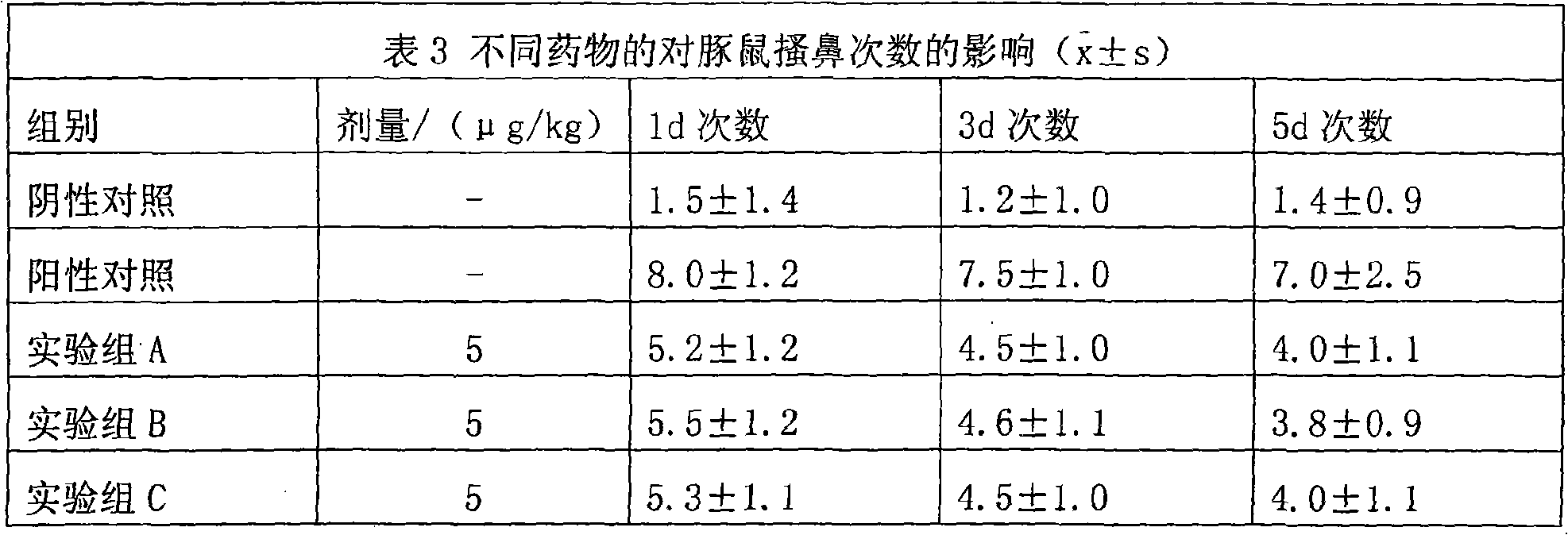
94 results about "Methylprednisolone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methylprednisolone is used to treat conditions such as arthritis, blood disorders, severe allergic reactions, certain cancers, eye conditions, skin/kidney/intestinal/lung diseases, and immune system disorders.
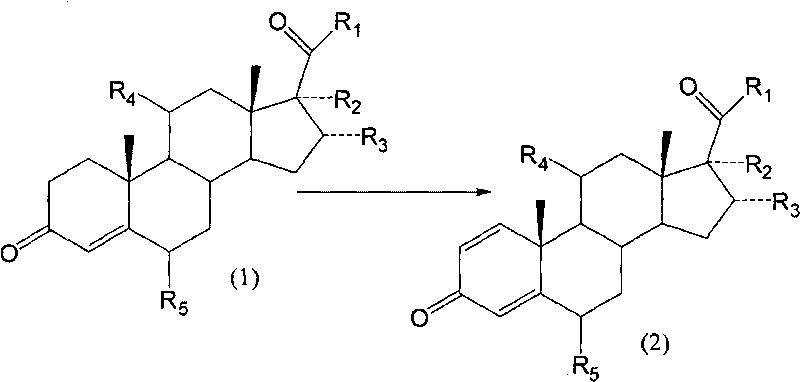
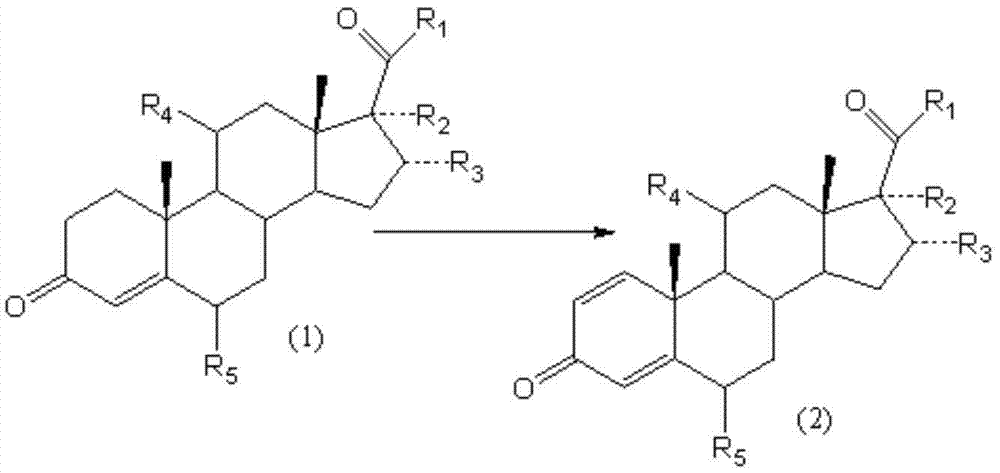
Biological dehydrogenation preparation method of 6 alpha-methylprednisolone intermediate
ActiveCN101760495AAvoid residueFlexible choiceMicroorganism based processesFermentationDehydrogenationMethylprednisolone
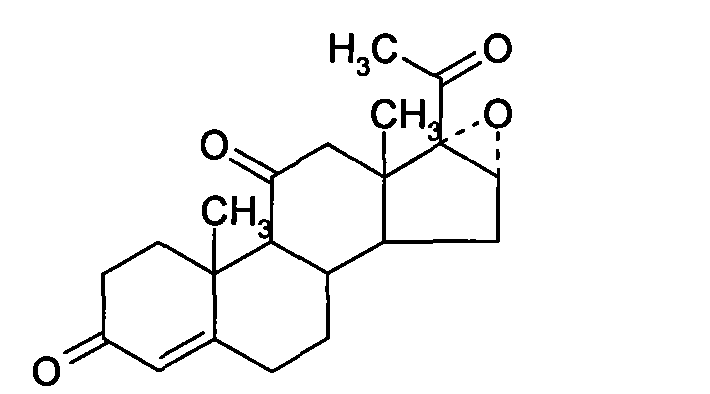
The invention relates to a biological dehydrogenation preparation method of a 6 alpha-methylprednisolone intermediate, which uses a compound of formula (1) as a substrate and obtains a compound of formula (2) by adopting a simple arthrobacterium biological dehydrogenation method. The process comprises the following steps of: crushing the formula (1) compound or dissolving the formula (1) compoundby using a solvent; adding to a fermentation tank with cultivated arthrobacterium to perform biotransformation; extracting, separating, and refining; drying, and then obtaining a dehydrogenation matter, i.e. the formula (2) compound. The invention can achieve the biotransformation rate of 70-90 percent.
Owner:TIANJIN JINYAO GRP
Chemical synthesis method of methylprednisolone
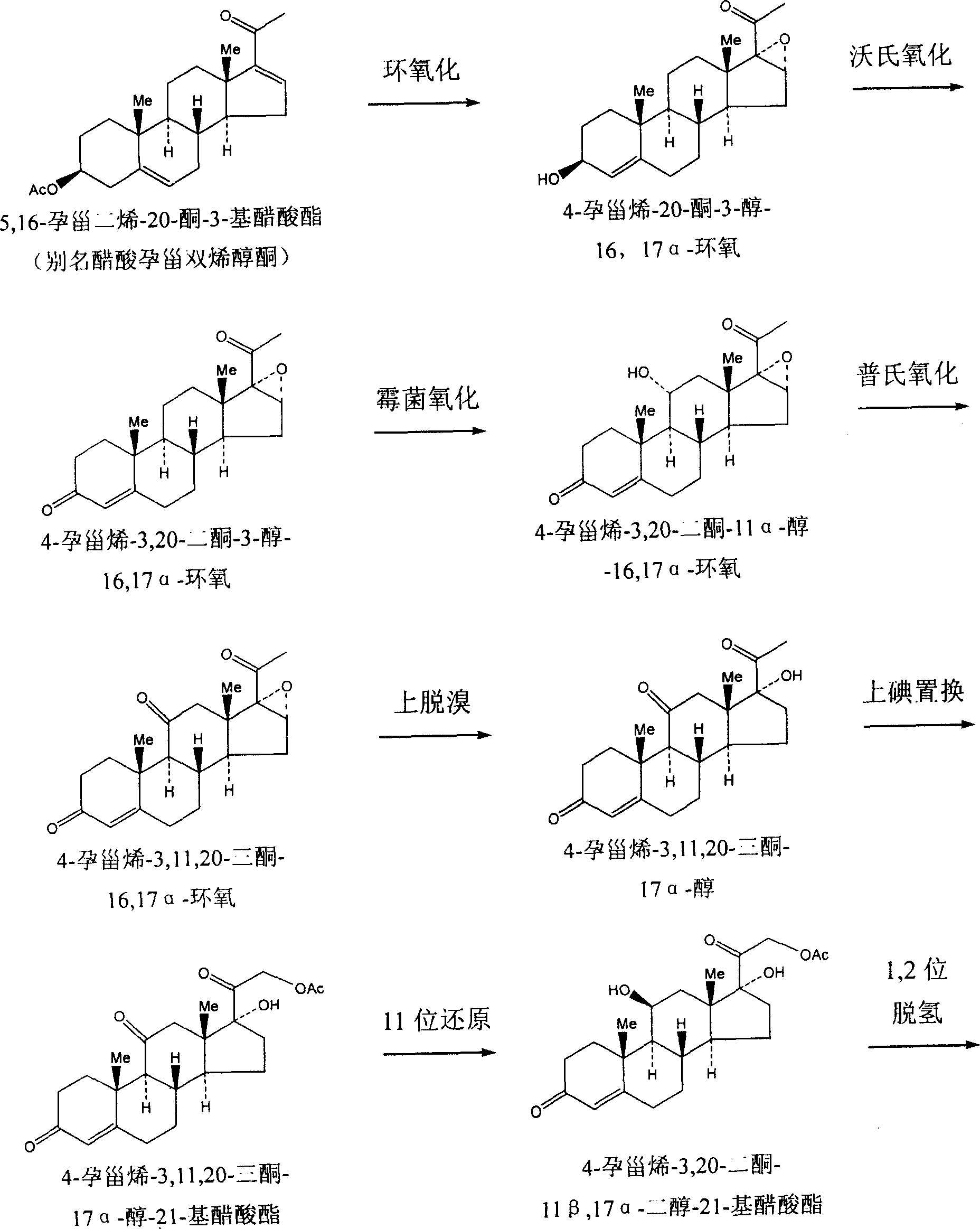
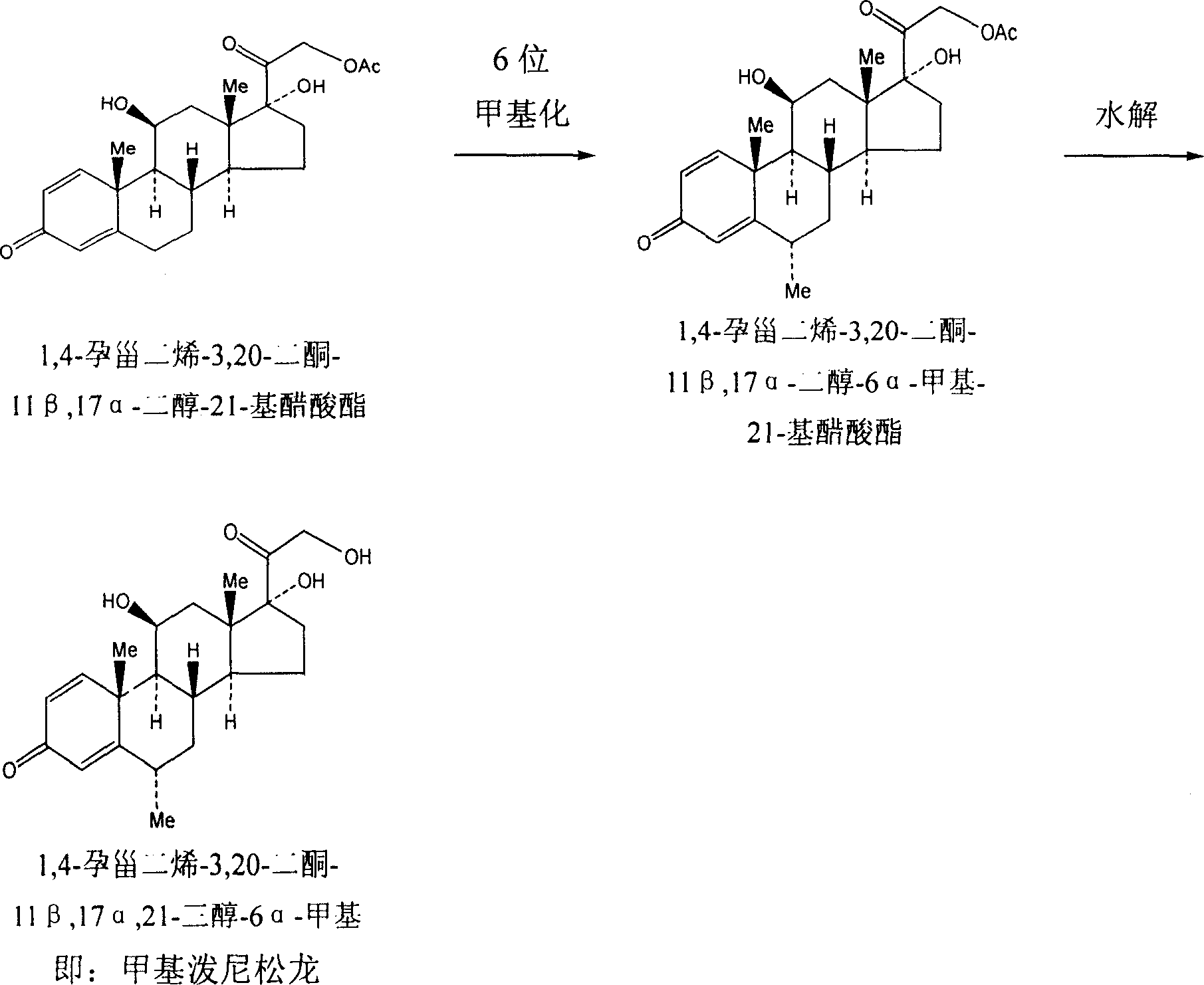
InactiveCN101230084AHigh yieldThe yield is improved compared with the one-step methylation reaction yield of the prior artSteroids preparationChemical synthesisSynthesis methods
The invention discloses a chemical synthesis method of methylpredmisolone. Fungi oxide is adopted as the initial material and methylpredmisolone is obtained after prevotella oxidation, bromine application, bromine removal, 6-bit methylenation, 6-bit methylation, ketal protection, 11- bit reduction, ketal hydrolization, 1 and 2 bit dehydrogenation, 21-bit iodine application, 21-bit permutation and 21-bit hydrolytic reaction. The flow of the synthesis method of the invention is reasonable, the yield rate and the purity of the intermediate products are high, the reaction condition is temperate, the by-products in the end product are few, the purity is high (reaching above 98 percent), the industrialization is easy to be realized, thereby the method has high application value.
Owner:TAIZHOU TAIFA PHARMA
Purine and Pyrimidine Cdk Inhitbitors and Their use for The Treatment of Autoimmune Diseases
InactiveUS20080125404A1Inhibition formationEase of preparation and detectabilityBiocideAntipyreticDiseaseImmunologic disorders
The present invention relates to the use of an inhibitor of CDK2 and / or CDK7 and / or CDK9, or a pharmaceutically acceptable salt thereof, in the preparation of a medicament for treating a disease associated with antinuclear antibodies, wherein the inhibitor of CDK2 and / or CDK7 and / or CDK9 or pharmaceutically acceptable salt thereof is administered in an amount sufficient to down-regulate the levels of antinuclear antibodies. A further aspect of the invention relates to a combination comprising an inhibitor of CDK2 and / or CDK7 and / or CDK9, or a pharmaceutically acceptable salt thereof, and methylprednisolone, and its use in the treatment of diseases associated with antinuclear antibodies, such as SLE.
Owner:CYCLACEL
Intravascular delivery of methylprednisolone
The present invention provides improved devices and methods for minimizing and / or inhibiting restenosis and hyperplasia after intravascular intervention. In particular, the present invention provides luminal prostheses which allow for programmed and controlled methylprednisolone delivery with increased efficacy to selected locations within a patient's vasculature to inhibit restenosis. An intraluminal delivery prosthesis may comprise an expansible structure and means on or within the structure for releasing methylprednisolone into the body lumen to inhibit smooth muscle cell proliferation.
Owner:ALTAI MEDICAL TECH
Methylprednisolone production method and production device
ActiveCN108912192AGuaranteed purityQuality assuranceDrying gas arrangementsSteroidsMethylprednisoloneElectric machinery
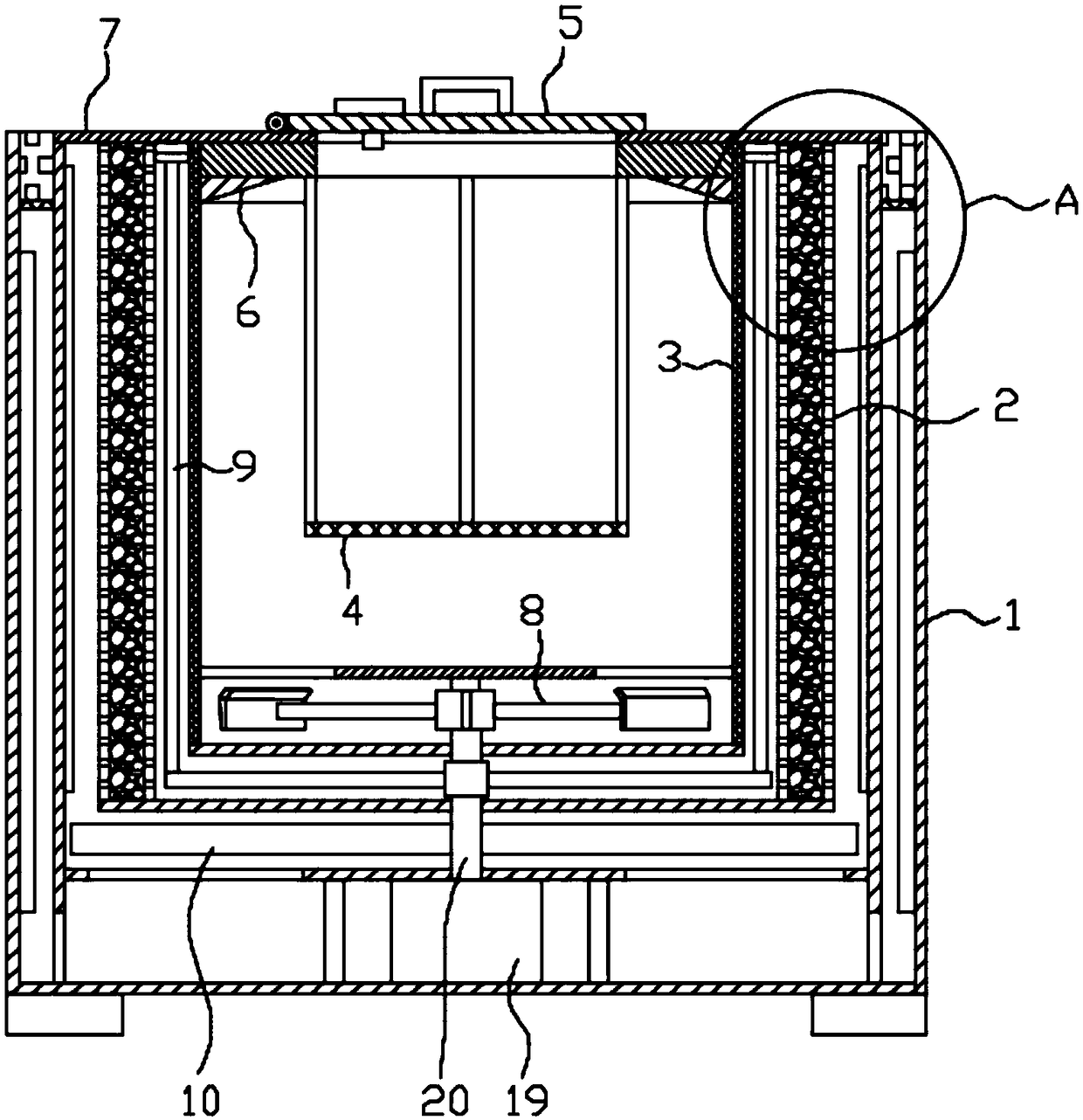
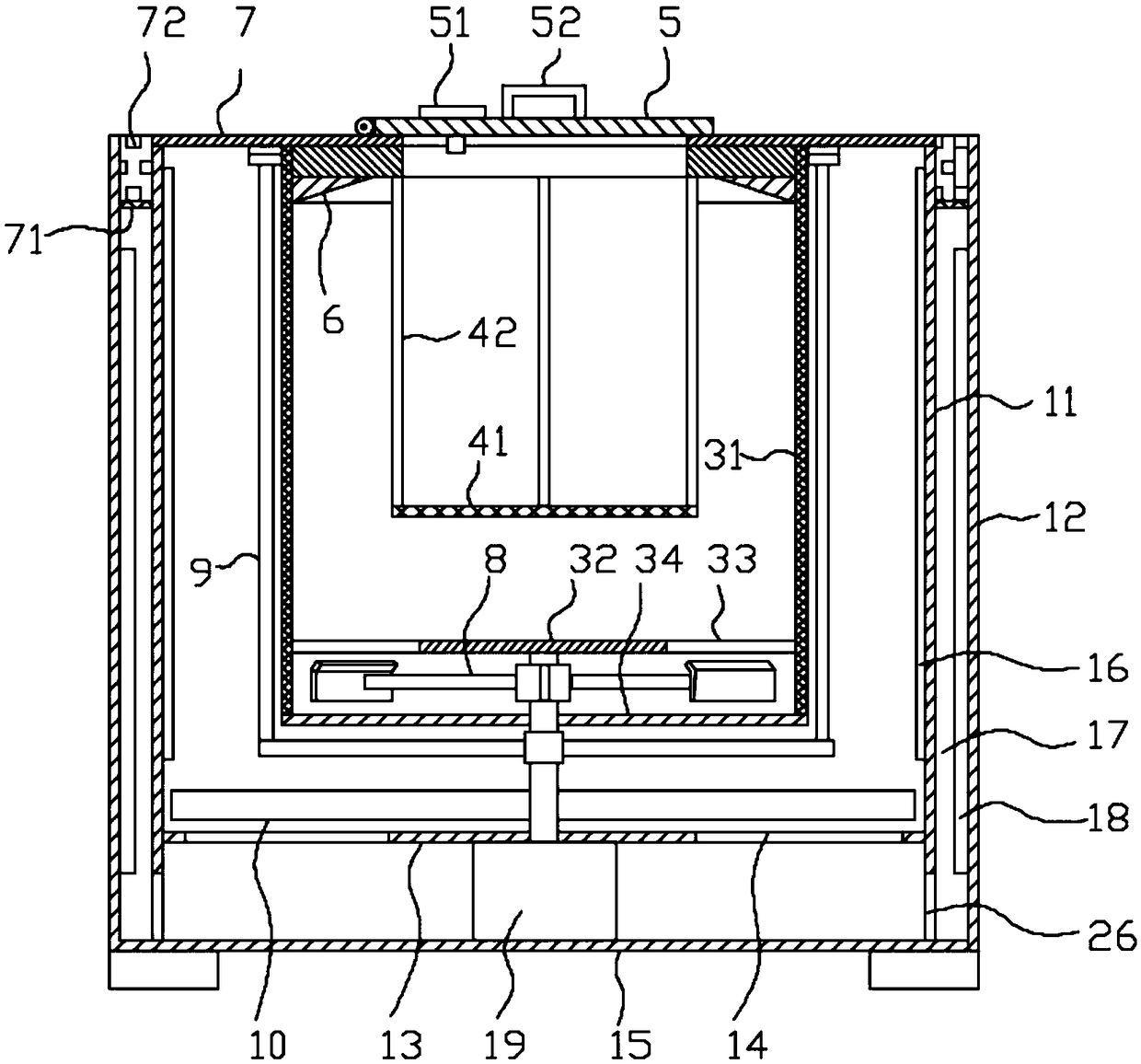
The invention discloses a methylprednisolone production device, which comprises a reaction kettle, an elutriation kettle, a centrifugal filtration device, a concentration kettle, a decoloration kettle, a biological fermentation tank and a drying device which are connected in sequence, wherein the drying device comprises an outer cylinder, a drying cylinder and an inner cylinder; a motor is arranged on the bottom surface of the outer cylinder; an exhaust fan blade, a cylinder type impeller and a vortex impeller are arranged on a motor rotating shaft; the exhaust fan blade is arranged between the drying cylinder and the bottom surface of the outer cylinder; the cylinder type impeller is arranged between the drying cylinder and the inner cylinder; the vortex impeller is arranged at the bottomof the inner cylinder; an infrared heating pipe is arranged on the top surface of the inner cylinder; a feeding port is formed in the center of the upper end of the inner cylinder; the side wall of the outer cylinder comprises an inner layer wall and an outer layer wall; an annular interlayer cavity is formed between the inner layer wall and the outer layer wall; an annular baffle is arranged atthe outlet of the upper end of the interlayer cavity, a vent is formed in the baffle, and a rotatable air purification ring is arranged at the upper end of the baffle. According to the method, the preparation time of intermediate products in the production process of the methylprednisolone can be reduced, and rapid and high-quality production of the methylprednisolone can be realized.
Owner:YUEYANG HUANYU PHARMA +6
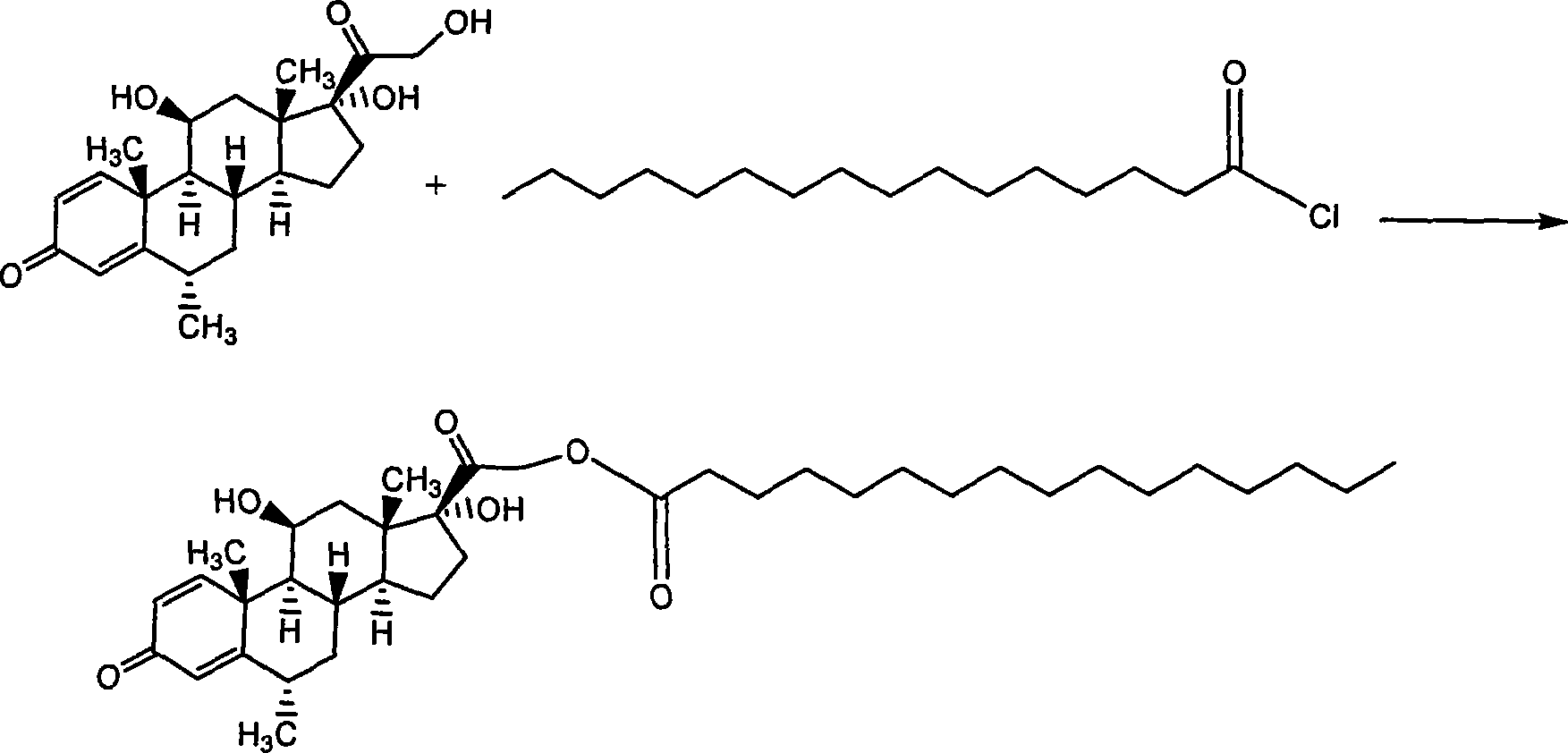
Composition with methylprednisolone palmitate as active component for treating local inflammation
InactiveCN101412741AEnhanced inhibitory effectOrganic active ingredientsAntipyreticActive componentMethylprednisolone
The invention relates to a drug composition for treating local inflammation, which uses methylprednisolone palmitate as an active composition. The drug composition consists of the methylprednisolone palmitate as the active composition and an inactive composition suitable for local injection, and is used for treating local inflammation of people or mammal through local injection.
Owner:TIANJIN JINYAO GRP
Method for preparing 6 beta-methylprednisolone
InactiveCN107602652AHigh purityImprove pharmacological activitySteroidsDehydrogenationMethylprednisolone
The invention discloses a method for preparing 6 beta-methylprednisolone. The method comprises the following steps: taking 6-methyl epoxides as raw materials, after Grignard reaction, hydrolysis, elimination, generating 6 beta-methyl elimination, and then after dehydrogenation by fermentation, iodine putting, replacement, hydrolysis, preparation of liquid phase purification, obtaining 6 beta-methylprednisolone. A reaction formula is as follows: the formula is shown in the description, the 6 beta-methylprednisolone of the invention is a major synthetic impurity in methylprednisolone, separation and purification of the 6 beta-methylprednisolone have great significance on impurity analysis.
Owner:ZHEJIANG XIANJU PHARMA
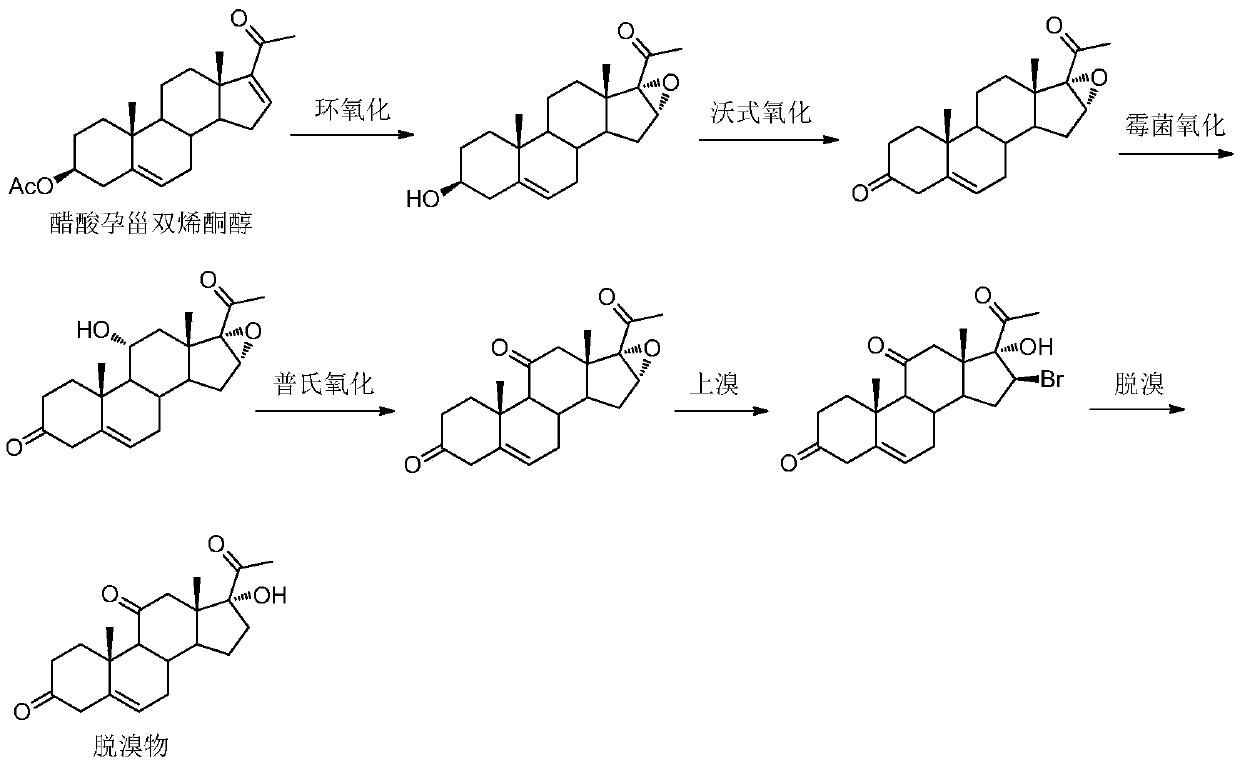
Methylprednisolone chemical synthesis method
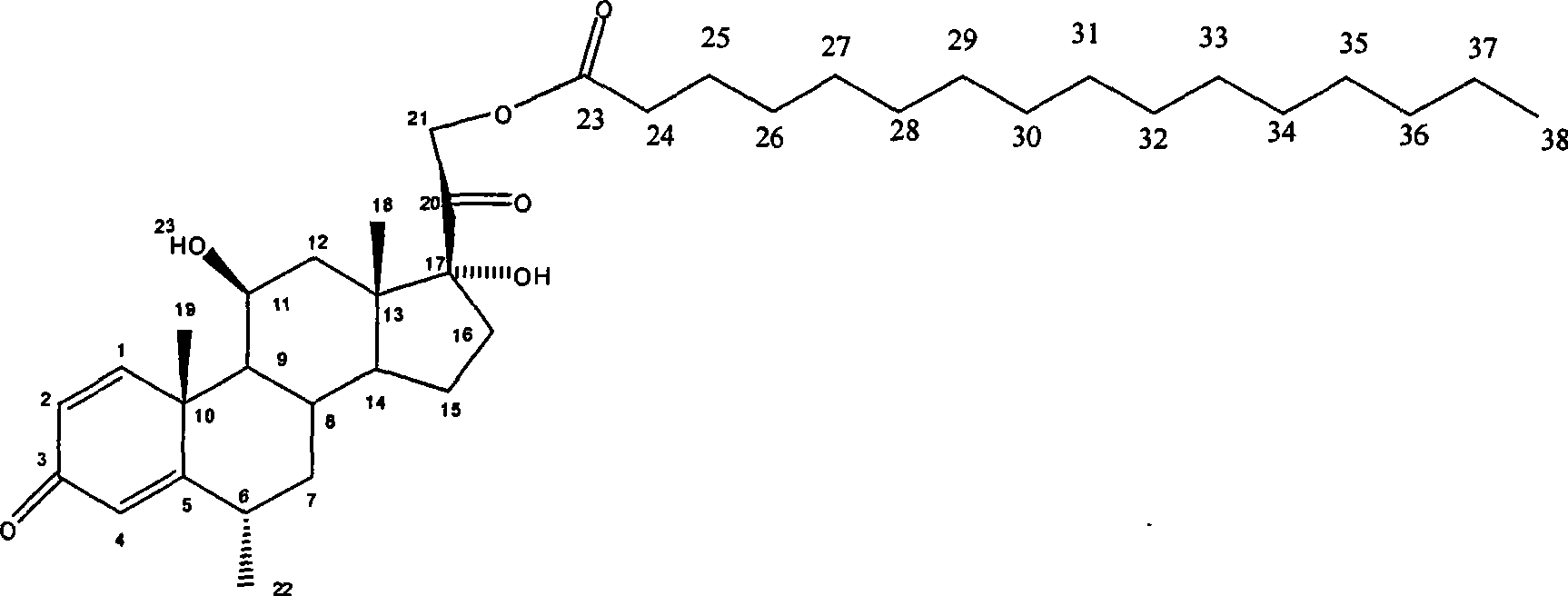
The present invention discloses the chemical synthesis process of methyl prednisoline. By using pregnenolone acetate as initial material, the present invention prepares methyl prednisoline through the steps of: epoxidation, mildew oxidation, place-11 reduction, place I and II dehydrogenation, place VI methylation and other reaction steps. The process of the present invention has mild reaction condition, high product purity, stable product quality, high product yield and low production cost, and is suitable for industrial production of methyl prednisoline.
Owner:台州百大药业有限公司
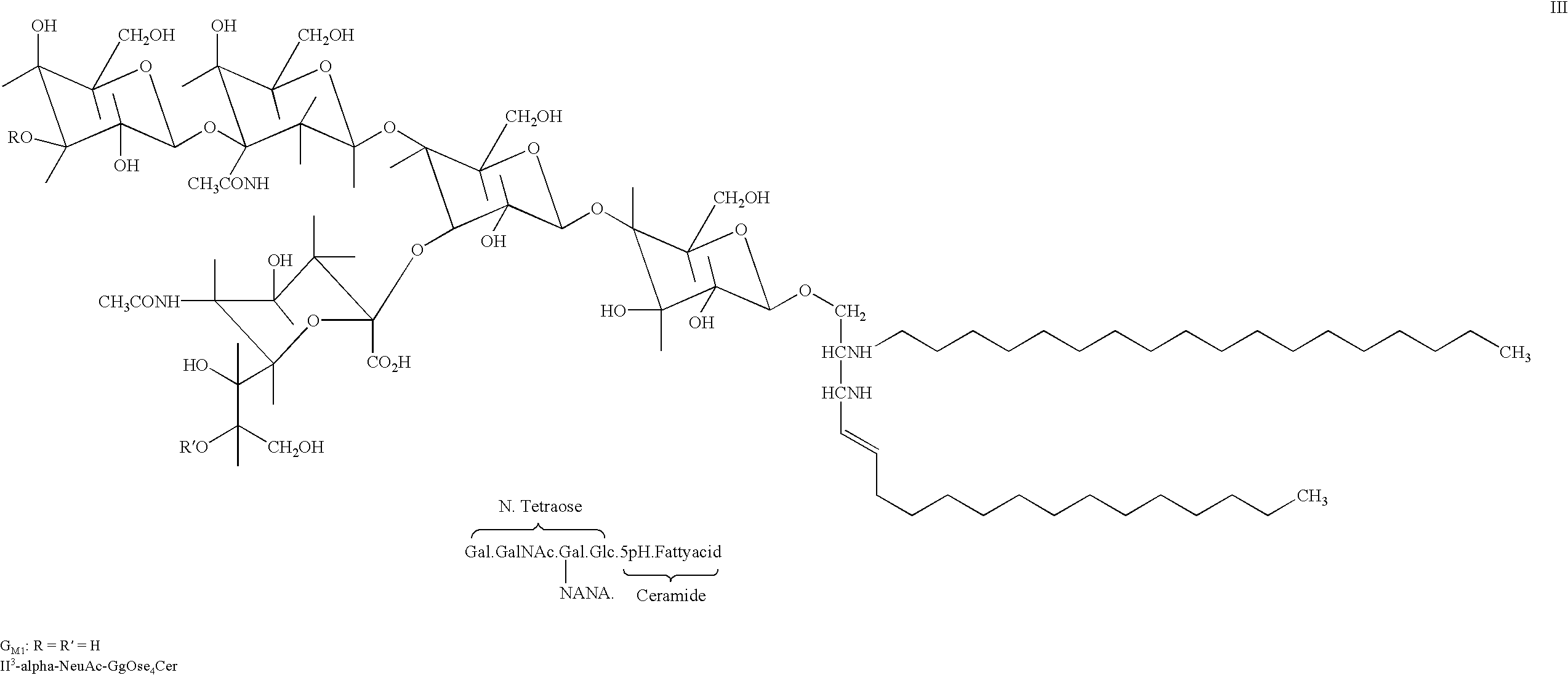
Therapeutic use of ganglioside GM1 in the treatment of spinal cord injury
The invention relates to a method for amelioration of neurological outcome in humans with spinal cord damage by administration of ganglioside GM1. Another object of the present invention is to provide combination therapies for the treatment of spinal cord damage comprised of the administration of the ganglioside GM1 and other drugs which have therapeutical benefit in patients with spinal cord damage, preferably methylprednisolone.
Owner:FIDIA FARM SPA
Preparation method and application of release speed-controllable type silk fibroin nanometer microsphere
InactiveCN106729741AGood curative effectSmall toxicityOrganic active ingredientsNervous disorderDexamethasoneHalf-life
The invention relates to a release speed-controllable type silk fibroin nanometer microsphere loading methylprednisolone and dexamethasone. The release speed-controllable type silk fibroin nanometer microsphere comprises silk fibroin with lower and higher release speeds; after the silk fibroin with lower and higher release speeds are dissolved, the molecular weight cutoff of the silk fibroin with lower drug release speed after dialysis is 12000 to 14000; the molecular weight cutoff of the silk fibroin with higher drug release speed is 100 to 500; the average particle size of the silk fibroin nanometer microsphere loading methylprednisolone and dexamethasone is 35 to 125nm. The release speed-controllable type silk fibroin nanometer microsphere loading methylprednisolone and dexamethasone has the advantages that the action times of methylprednisolone and dexamethasone at certain dosages can be effectively controlled according to the administration time after injury, the half-life period is controlled, the medicine effect is effectively realized under the condition of joint administration, the therapy effect is improved, and the toxic or side effect and poor reaction during single administration is reduced.
Owner:NANTONG UNIVERSITY
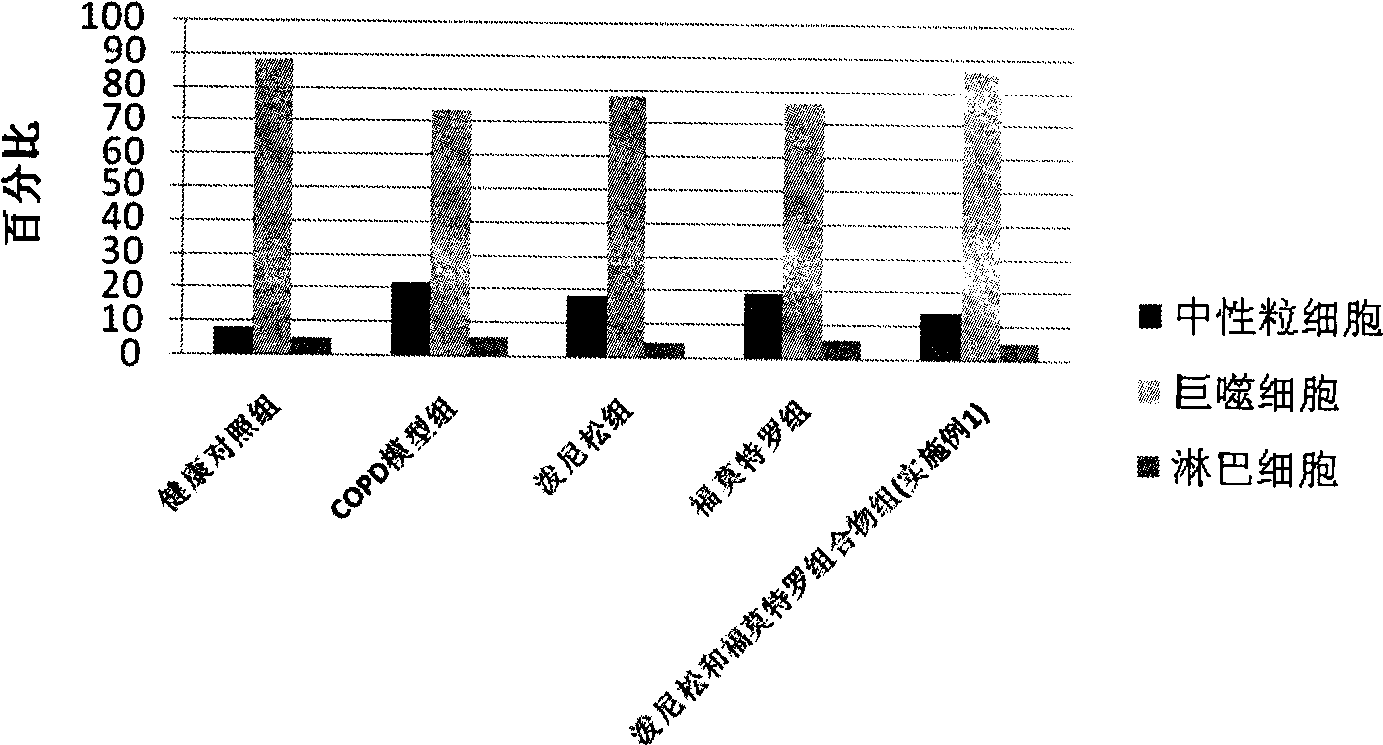
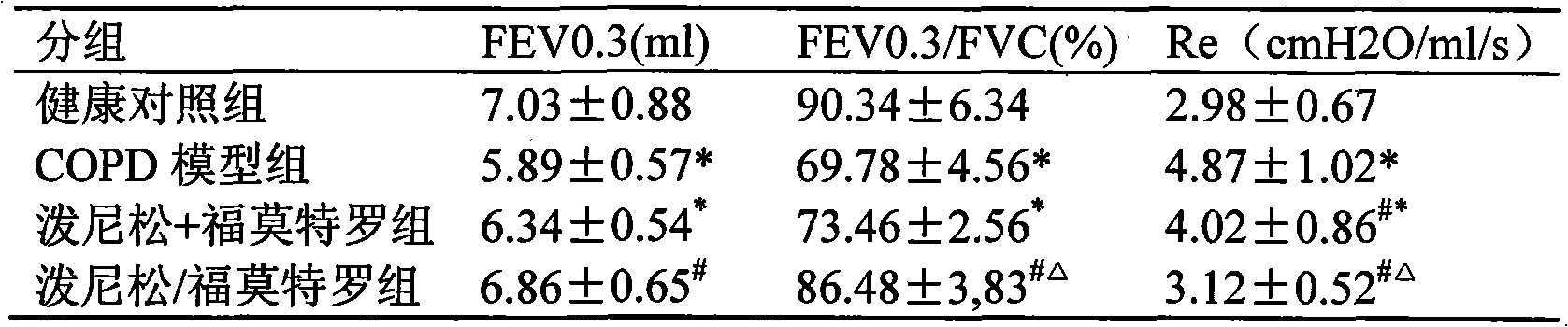
Pharmaceutical composition for the treatment of chronic obstructive pulmonary disease and bronchial asthma
InactiveCN101530618ALower doseReduce or avoid side effectsRespiratory disorderHeterocyclic compound active ingredientsDiseaseAdditive ingredient
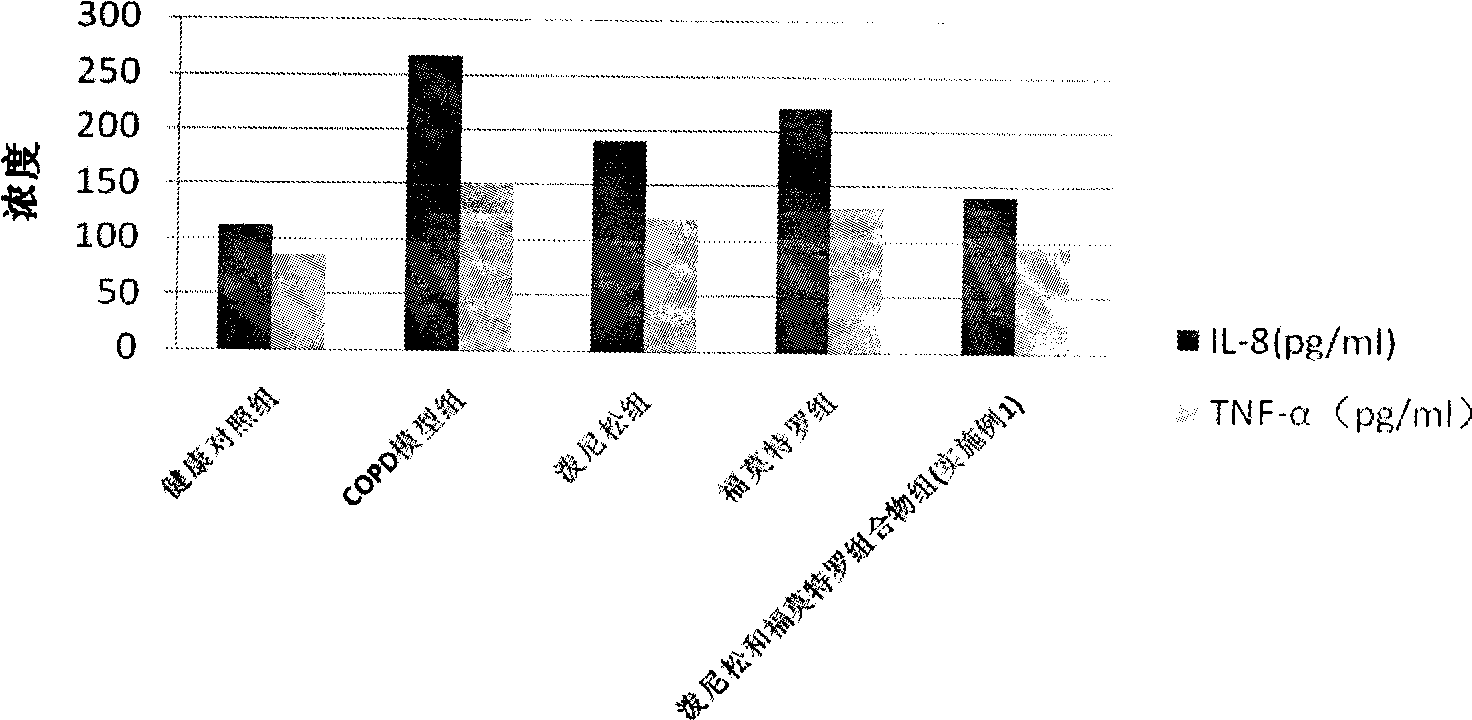
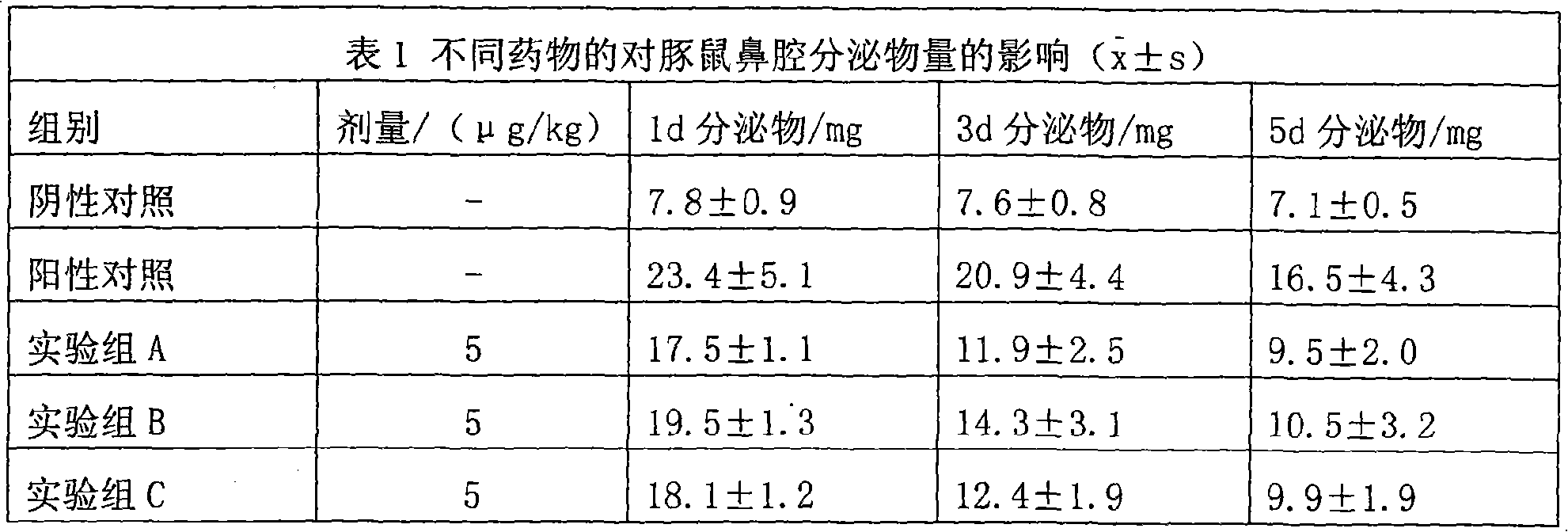
The invention discloses a pharmaceutical composition for the treatment of chronic obstructive pulmonary disease (COPD) and bronchial asthma, which is composed of a glucocorticoid, a bronchodilator and a pharmaceutically acceptable auxiliary material or carrier; the composition is a preparation for oral use. The glucocorticoid in the inventive pharmaceutical composition is selected from prednisone, prednisolone, methylprednisolone, betamethasone, decamethasone or hydrocortisone; the bronchodilator is selected from formoterol, clenbuterol, procaterol or theophylline. The inventive composition has better therapeutic effect on the COPD and the bronchial asthma than independent administration of two ingredients, and has synergistic effect. The composition has easily-available raw materials, inexpensive price and increased medicine taking compliance as well as plays a significant role in preventing and treating the COPD and the bronchial asthma of patients in vast rural areas and patients in low-income class of the city in China.
Owner:莫始平
Uses of methylprednisolone and derivatives thereof in preparing medicament for treating allergic rhinitis
ActiveCN101347436ADecreased systemic effectsLow therapeutic doseOrganic active ingredientsAerosol deliveryNasal cavityAdditive ingredient
A pharmaceutical composition for treating allergic rhinitis comprises one or a plurality of kinds from methylprednisolone taken as active ingredient or pharmaceutically acceptable salt thereof or esterified matters thereof, and a pharmaceutical composition consisting of one or a plurality of inactive ingredients applicable to local action inside nasal cavity.
Owner:天津药业集团有限公司
Glucocorticosteroid and chemotherapy medicament carried by anticancer sustained-release agent
InactiveCN101502484AInhibition formationImprove permeabilitySolution deliveryPharmaceutical non-active ingredientsGlucocorticoidPolyethylene glycol
The invention provides an anti-cancer sustained-release agent co-carrying glucocorticoid and chemotherapeutic drugs and belongs to sustained-release injections. The anti-cancer sustained-release agent comprises sustained-release microspheres and a solvent, wherein, the sustained-release microspheres comprise anti-cancer active components and sustained-release auxiliary materials; and the solvent is a particular solvent containing a suspending agent. The glucocorticoid is selected from prednisolone, methylprednisolone, dexamethasone, betemethasone, triamcinolone acetonide or triamcinolone acetonide; the chemotherapeutic drugs are selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogues and the like; the sustained-release auxiliary materials are biocompatible high-polymers, such as polylactic acid and the copolymers thereof, polyethylene glycol, carboxyl-terminated polylactic acid copolymers, polyfatty acid dimer-sebacic acid copolymers, poly(erucic acid dimer-sebacic acid), poly(fumaric-co-sebacic acid), polifeprosan and the like; and the suspending agent with the viscosity being 100cp to 3,000cp (at the temperature of 20 to 30 DEG C) is selected from sodium carboxymethyl cellulose and the like. The anti-cancer active components and the sustained-release microspheres can further be prepared into sustained-release implants which can effectively inhibit the growth of tumors, alleviate edema and improve the curative effects of radiotherapy and chemotherapy by intra-tumor or peri-tumor injection or placement.
Owner:SHANDONG LANJIN PHARMA
Preparation method for 9,11-de-esterification of metllylpredllisolone series products
ActiveCN1907999AAvoid damage to the structureQuality improvementSteroidsTemperature controlDansyl chloride
The invention discloses a manufacturing method of 9, 11-deestering material, which comprises the following steps: adopting intermediate dehydrogenation as raw material; proceeding sulfur-esterifying reaction through dansyl chloride; diluting; filtering; obtaining the wet product; heating to reflux completely; complementing temperature-control water; adjusting reflux reaction at 109+-2 deg.c; diluting; filtering; drying to obtain methylprednisolone 9, 11- deestering material.
Owner:TIANJIN TIANYAO PHARM CO LTD
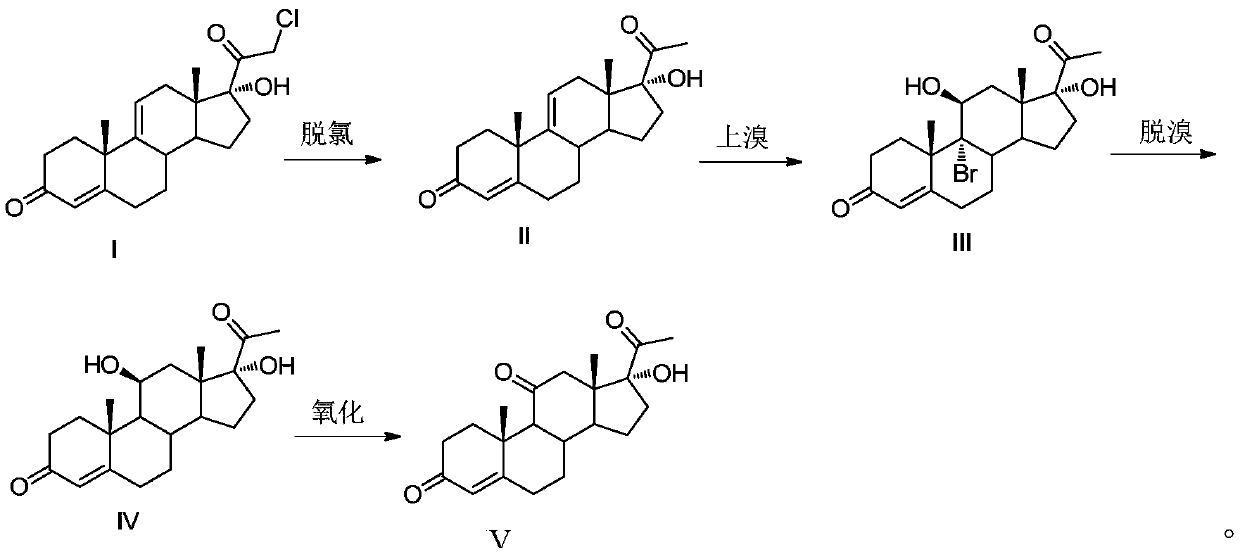
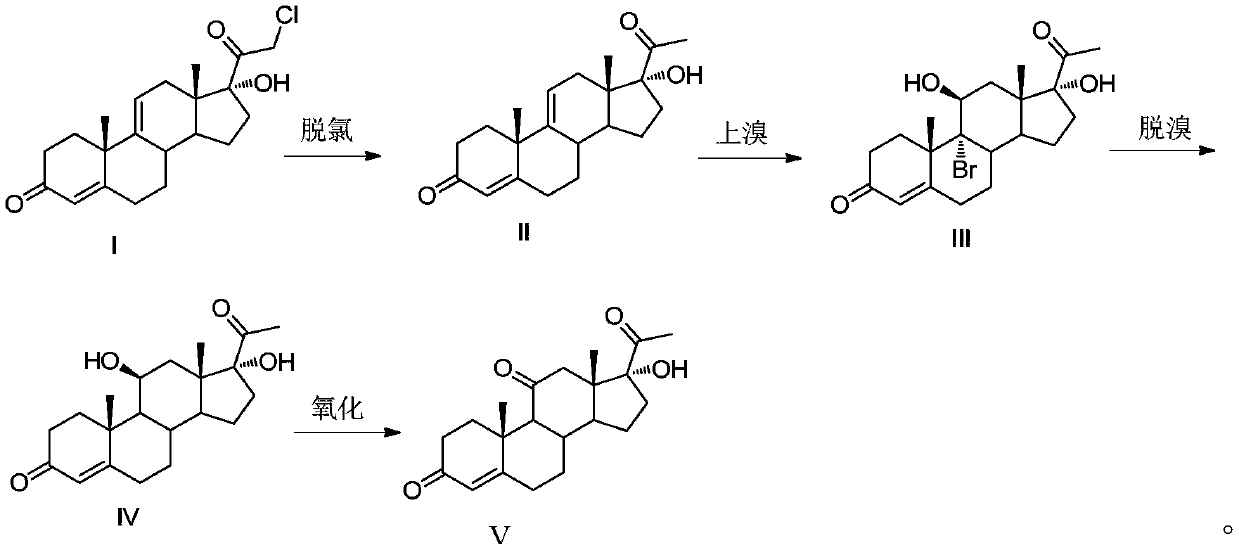
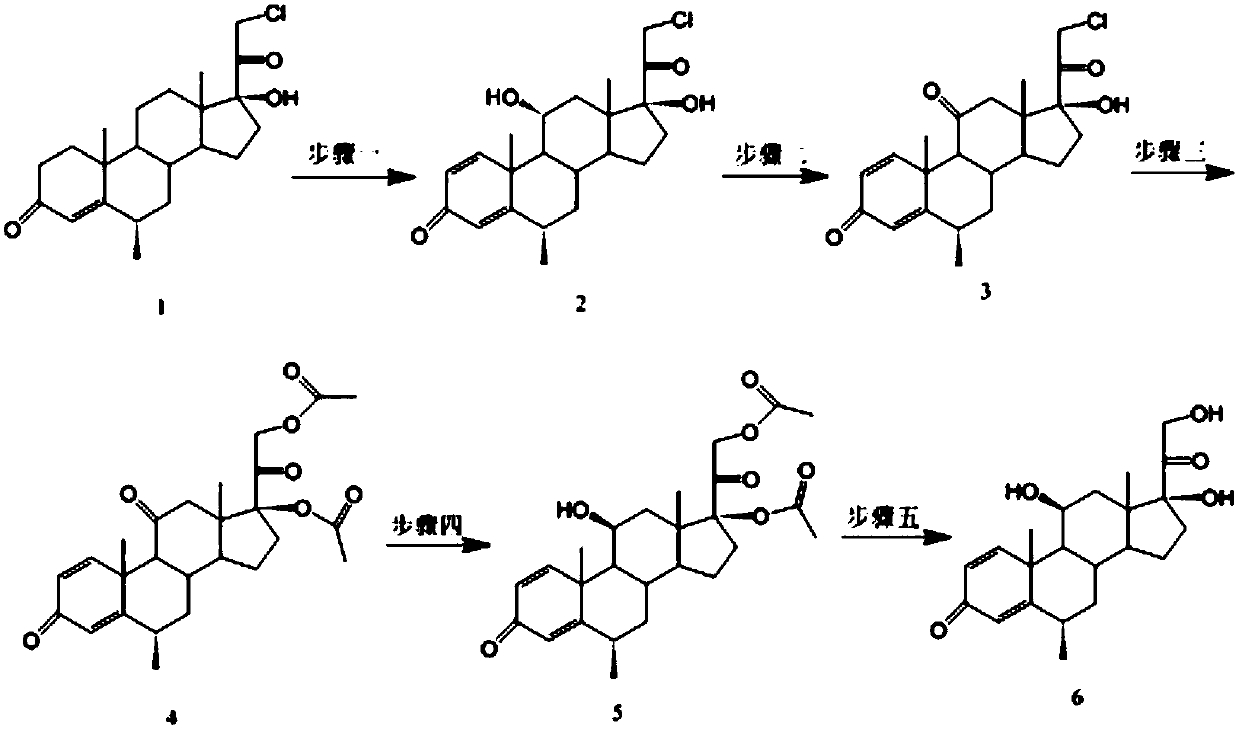
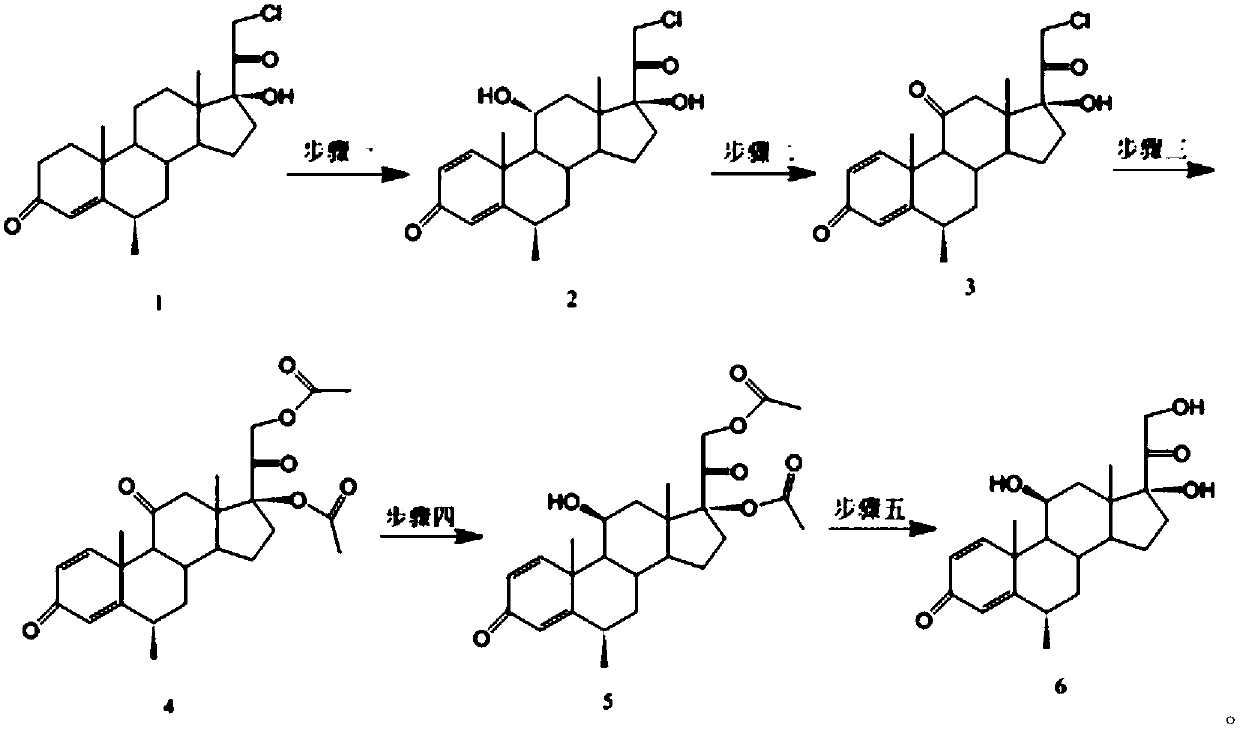
Methylprednisolone intermediate debrominated product and preparation method thereof
ActiveCN110698528AShort synthetic routeHigh yieldSteroidsBulk chemical productionPrednisoloneBiochemical engineering
The invention discloses a methylprednisolone intermediate debrominated product and a preparation method thereof. According to the preparation method, a compound shown as a formula I is used as a raw material, and a dechlorination reaction, a bromination reaction, a debromination reaction and an oxidation reaction are sequentially performed to prepare a compound (debrominated substance) shown as aformula V. The preparation method has the advantages of short synthetic route, high yield, low cost and easily available raw materials, is suitable for industrial production, and has very high industrial value.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Method for preparing methylprednisolone
InactiveCN107338281ALow costCost competitiveMicroorganism based processesFermentationMethylprednisoloneHydrolysis
The invention discloses a method for preparing methylprednisolone. Hydrocortisone serves as the raw material, a hydroxyl group is protected through ketalation, six methyl groups are introduced through methylene reaction and catalytic hydrogenation, 6 alpha-methyl hydrocortisone is obtained through hydrolysis deprotection, and methylprednisolone is obtained through biodehydrogenation, wherein the equation is shown in the description. Hydrocortisone serves as the initial raw material, through five-step reaction, methylprednisolone is obtained with the total weight yield of 60% or above, reaction is simple, selectivity is good, and methylprednisolone is suitable for industrial production.
Owner:ZHEJIANG XIANJU PHARMA
Application of triptolide and triptolide derivative to preparation of medicine for treating and/or preventing lung injury diseases
InactiveCN106994129APrevent proliferationInhibition of replicationOrganic active ingredientsAntiviralsDiseaseMethylprednisolone
The invention provides a novel purpose of triptolide and a triptolide derivative. The triptolide can obviously inhibit the GFP fluorescent protein and P24 antigen rise effect in a phorbol ester activated lymphocyte model. When the concentration of the lymphocyte concentration is higher, the GFP positive cell percentage and the P24 antigen concentration are lower; the negative dosage-effect relationship exists. Even when the concentration of the methylprednisolone is as high as 400 uM, the inhibition effect cannot reach the inhibition effect of the triptolide with the concentration being 0.02 uM; the cell apoptosis proportion obviously exceeds the triptolide with the concentration being 0.02 uM. According to the principle, the triptolide inhibits the G0 / G1 period cell proportion rise and S period cell proportion descending due to PMA; the cell period is promoted to be stopped in the unactivated state; the triptolide achieves the effect of inhibiting the lymphocyte cell proliferation and activation through regulating the cell period; meanwhile, the effect of inhibiting the virus replication is also achieved. The triptolide and the triptolide derivative can replace glucocorticoid analogues or can be combined with the glucocorticoid analogues to be used, and are used for treating and / or preventing lung injury diseases.
Owner:王晓辉
Use of liposomal glucocorticoids for treating inflammatory states
InactiveUS7744920B2Ameliorating symptoms of rheumatoid arthritisOrganic active ingredientsBiocideDiseaseGlucocorticoid
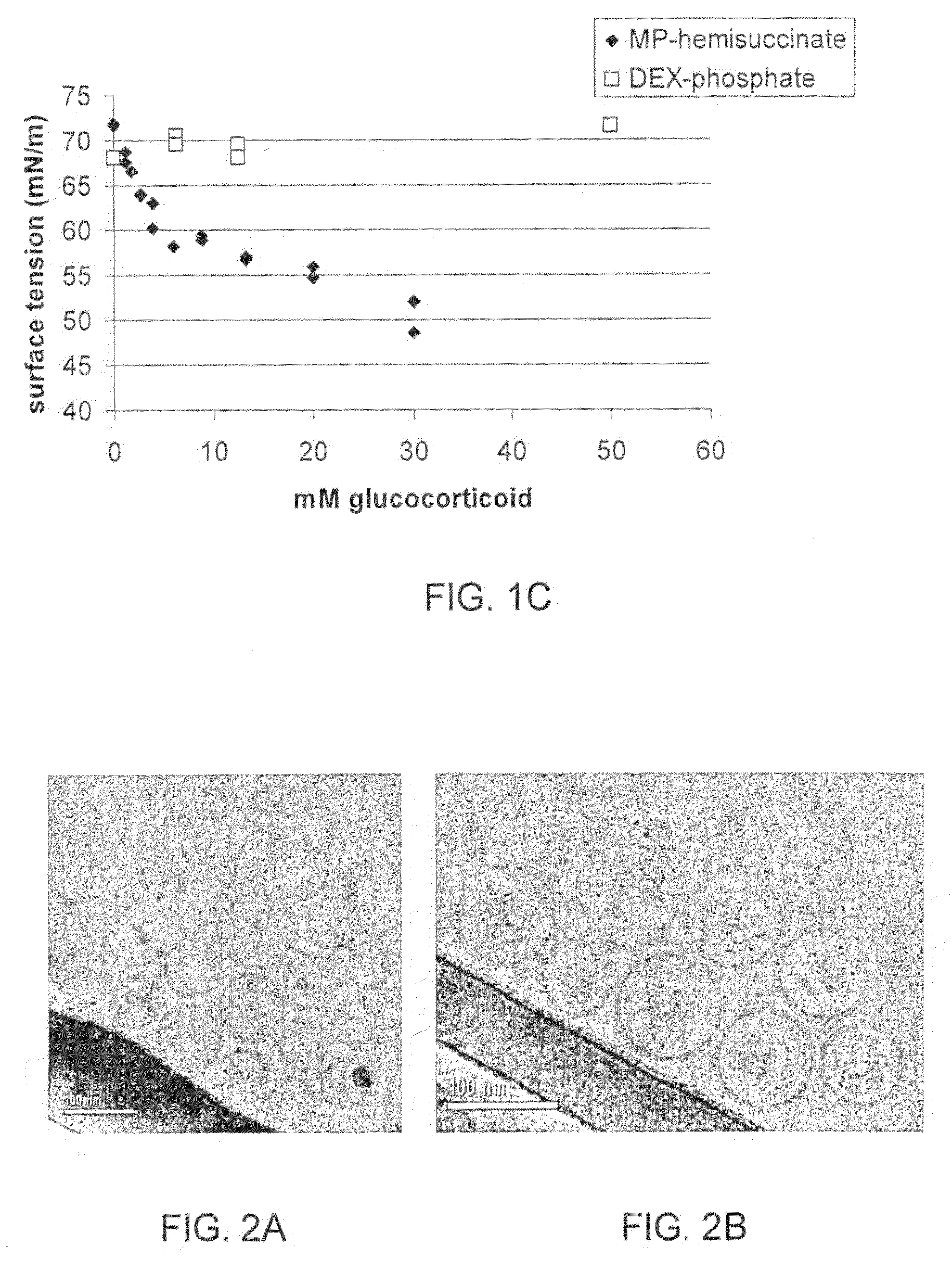
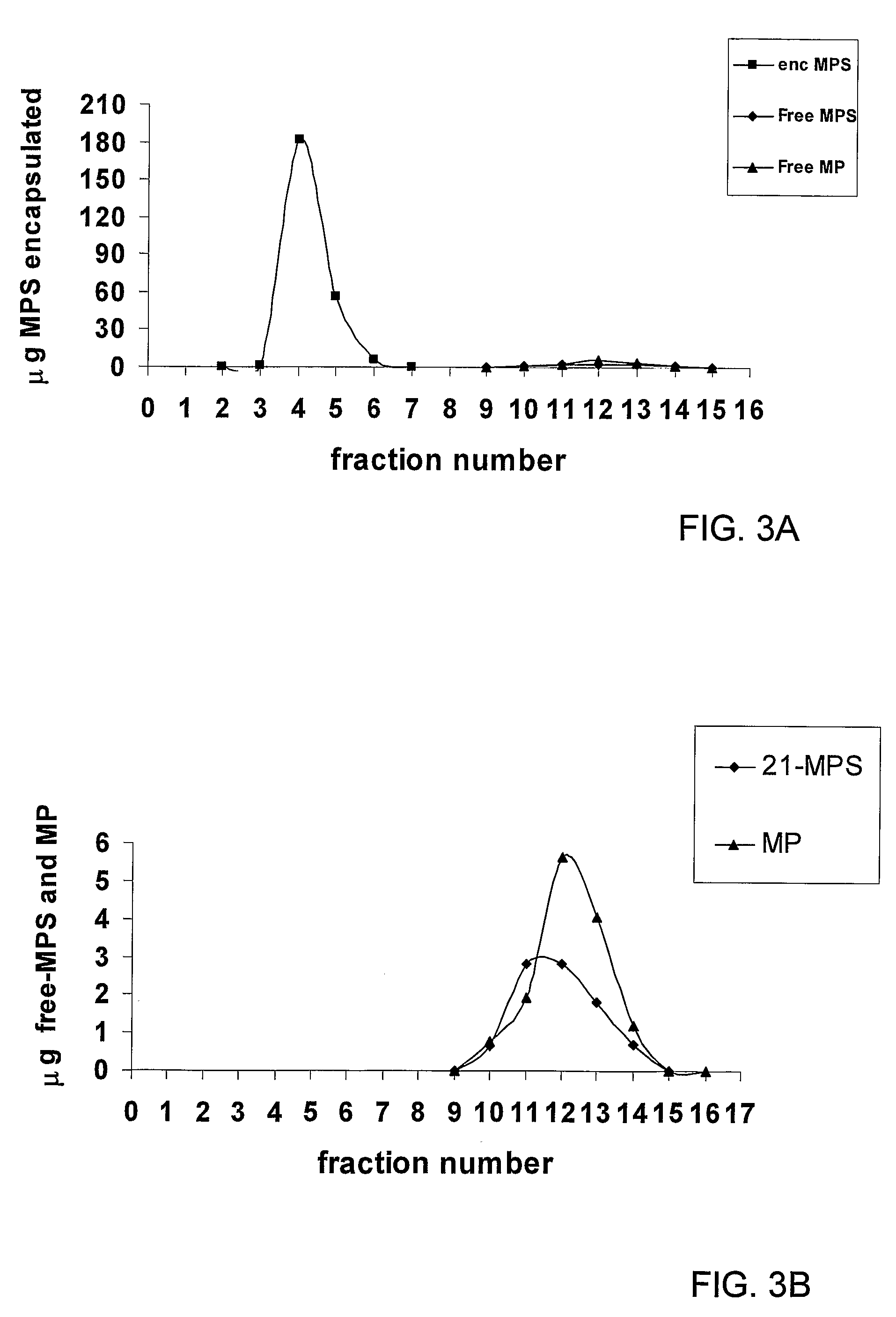
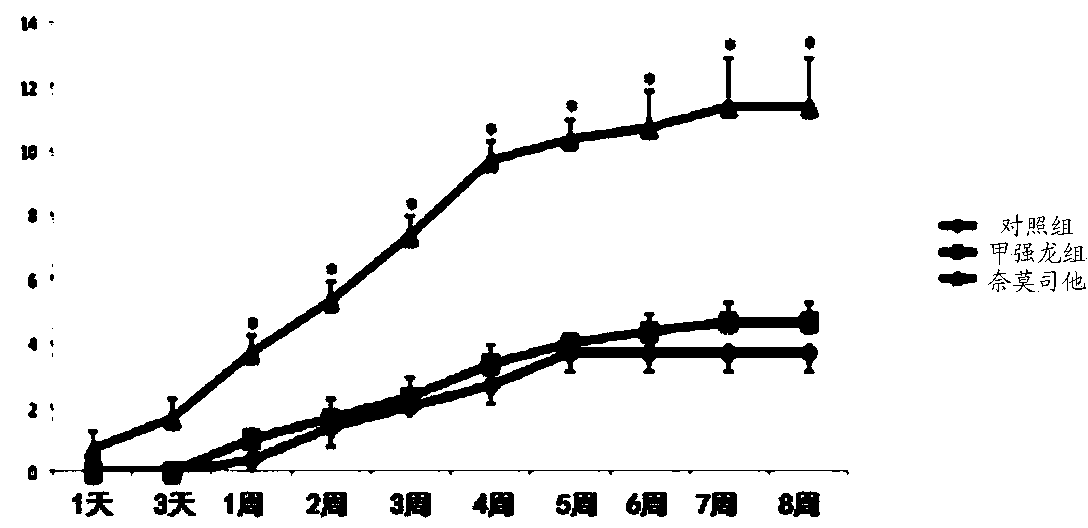
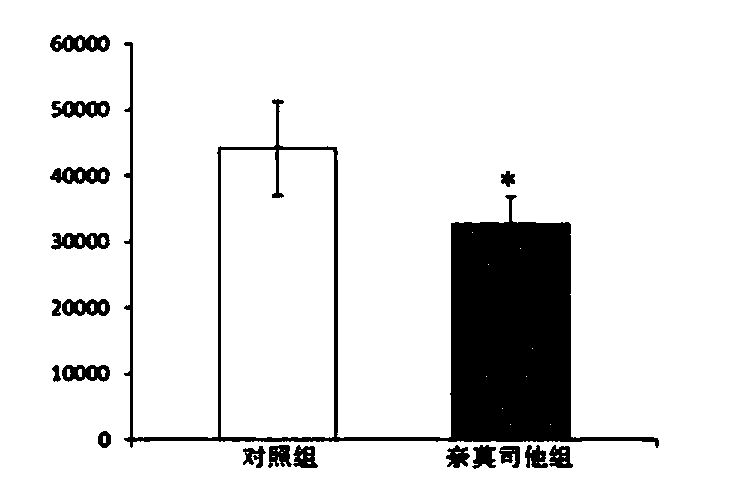
The present invention provides the use of a glucocorticoid (GC) or of a GC derivative encapsulated in a liposome for the preparation of a pharmaceutical composition for the treatment of an inflammatory associated condition in a subject, provided that said condition is not associated with a neurodegenerative disease or disorder. A specific use concerns a liposomal formulation comprising methylprednisolone sodium hemisuccinate (MPS) for the treatment of rheumatoid arthritis.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
Oral cavity pasting tablets using Methylprednisolone and derivatives thereof as active components
ActiveCN101371824AAvoid potential hazardsAvoid harmOrganic active ingredientsDigestive systemAdditive ingredientWater insoluble
The invention discloses an oral sticking tablet used for treating canker sore of humans or mammals. The oral sticking tablet comprises a sticking layer containing an active ingredient and a water insoluble protective layer, wherein, a non-active ingredient contained in the sticking layer consists of a filling agent, an adhesive sustained-release agent and a lubricant which are applicable to tablets; the water insoluble protective layer is composed of polyacrylic acid resin and / or ethyl cellulose which can be used as protective coating, and a plasticizer; in addition, the active ingredient is methylprednisolone, or one or a combination of pharmaceutically acceptable esters.
Owner:TIANJIN PHARMA GROUP CORP
Novel nanometer drug carrier loaded with methyllprednisolone and preparation method of novel nanometer drug carrier
ActiveCN105727306AExtended half-lifeGood slow release functionOrganic active ingredientsNervous disorderMicrosphereHalf-life
The invention discloses a novel nanometer drug carrier loaded with methyllprednisolone. The novel nanometer drug carrier comprises methyllprednisolone and a carrier, wherein the carrier is an ibuprofen modified dextran nanometer microsphere, the particle diameter of the novel nanometer drug carrier loaded with methyllprednisolone is 120 to 160 nanometers, the preparation method of the novel nanometer drug carrier loaded with methyllprednisolone comprises two steps of synthesizing the ibuprofen modified dextran nanometer microsphere and preparing novel nanoparticles loaded with methyllprednisolone. The novel nanometer drug carrier loaded with methyllprednisolone disclosed by the invention has the advantages that the nanometer microsphere synthesized by ibuprofen modified dextran is taken as the carrier, so that methyllprednisolone can be effectively and targetedly delivered to injured parts, the half-life of methylprednisolone drugs can be effectively prolonged, and methyllprednisolone and ibuprofen can play a synergistic role on promotion of central nervous system injury repair.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
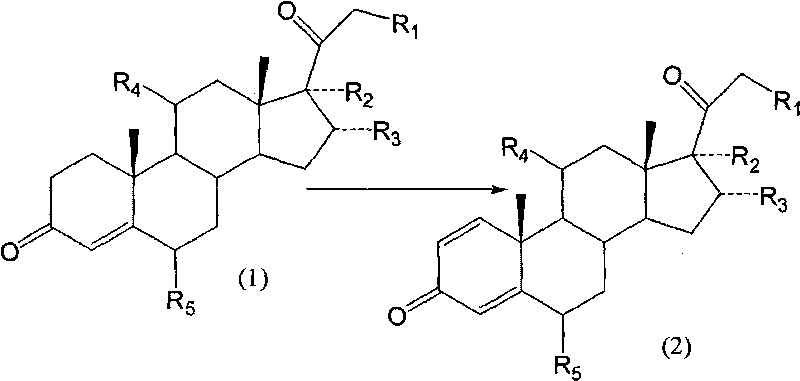
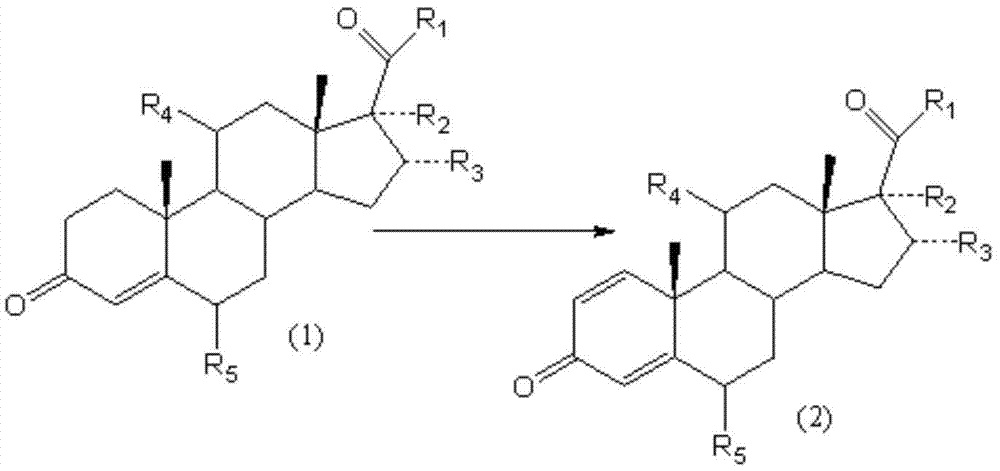
Method of producing methylprednisolone dehydrogenation article
InactiveCN101153313AAvoid residueHigh yieldMicroorganism based processesFermentationMethylprednisoloneDehydrogenation
The invention discloses a method for preparing dehydrogenated methyl-prednisone which adopts the medium product of the methyl-prednisone as the fermentation substrate and carries out biological dehydrogenation with the arthrobacter. Compared with the prior art, the invention adopts the medium product of the methyl-prednisone as the fermentation substrate, and substitutes the biological dehydrogenation with arthrobacter for the chemical dehydrogenation technique in the prior art, thereby increasing the harvest rate from 55 to 70-85 percent, decreasing greatly the cost, and improving the purity of the product to more than 97 percent, accomplishing the industrialized production of methyl-prednisone, and completely avoiding the residue of the deadly poison of SeO2 through the green technique.
Owner:ZHEJIANG XIANJU PHARMA
Pharmaceutical application of nafamostat
ActiveCN103479612AImprove BBB ratingReduce apoptosisOrganic active ingredientsNervous disorderApoptosisMethylprednisolone
The invention provides application of nafamostat in preparation of a medicament for treating spinal cord injury. The nafamostat is applied to therapeutic intervention of the spinal cord injury, and experiments show that the nafamostat can reduce apoptosis of cells, promote survival of neurons, inhibit formation of glial scars and remarkably improve the BBB score of a treated animal. Compared with a methylprednisolone medicament applied clinically at present, the nafamostat has the advantages of wider administration window, more lasting treatment effect and better long-term effect.
Owner:冯世庆
Experimental method for medicine used for treating spinal cord injury (SCI)
InactiveCN106665496AEffective treatmentCompounds screening/testingOrganic active ingredientsSpinal nerveMethylprednisolone
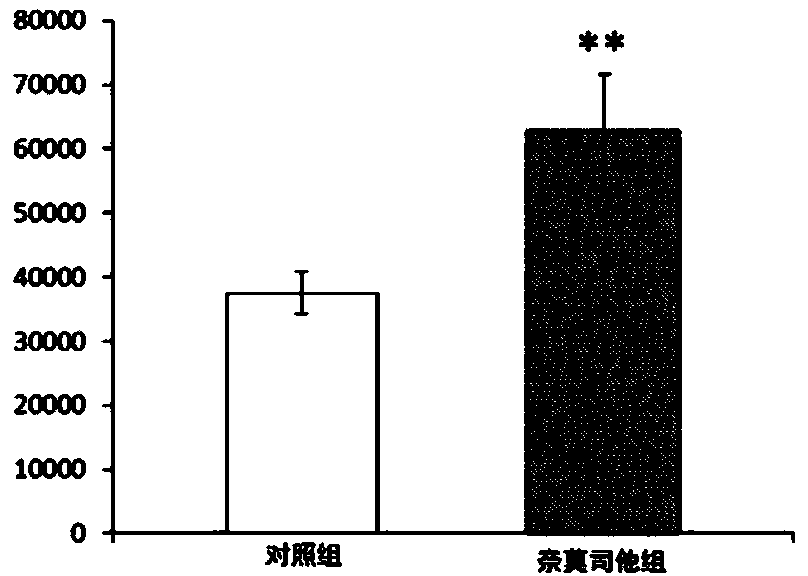
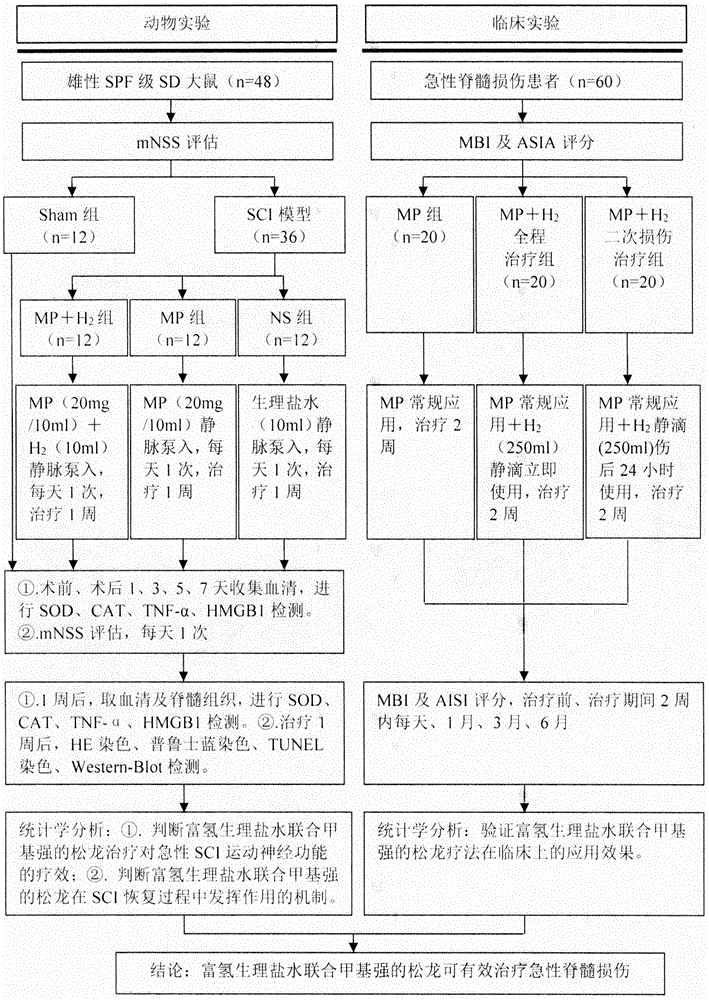
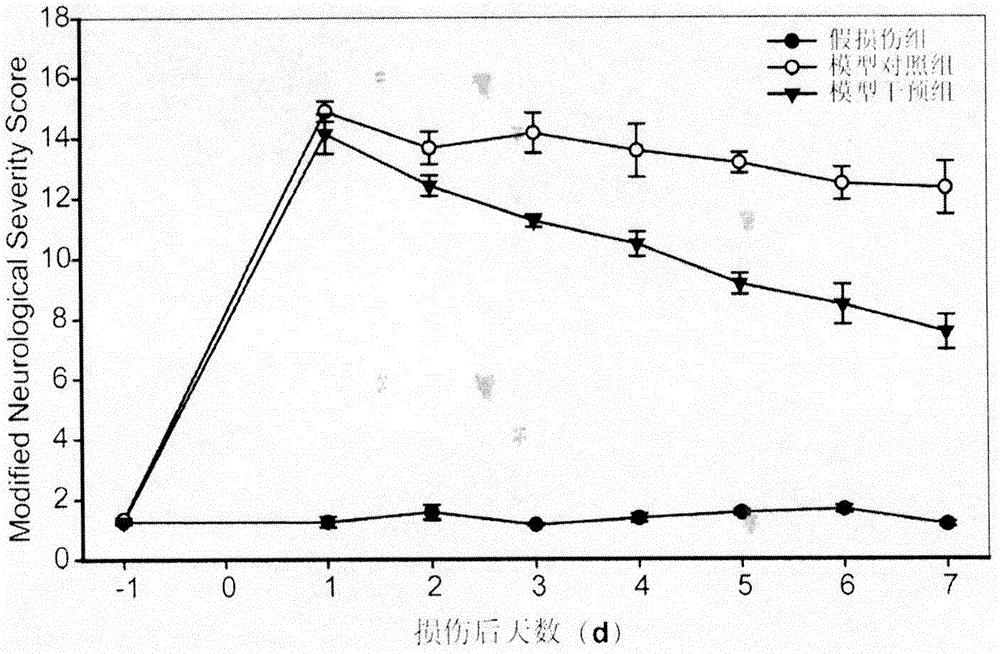
The invention discloses an experimental method for a medicine used for treating spinal cord injury (SCI). According to the method, SCI is treated by adopting hydrogen-rich normal saline and methylprednisolone; by comparing rat neural functional recovery, observing the morphological change of injured spinal nerve cells and the cell apoptosis condition, and exploring the effectiveness of the combined treatment, the therapeutic method of combination of hydrogen-rich normal saline and methylprednisolone is taken as a new method for treating SCI. MBI and ASIA score are recorded and analyzed; the clinic application effect of the therapeutic method is verified by adopting hydrogen-rich normal saline combined with methylprednisolone. According to the experimental method, evidence is acquired for the practical application of the therapeutic method by adopting hydrogen-rich normal saline combined with methylprednisolone in treating spinal cord injury. A more efficient therapeutic method is supplied for the spinal cord injury.
Owner:连云港市东方医院
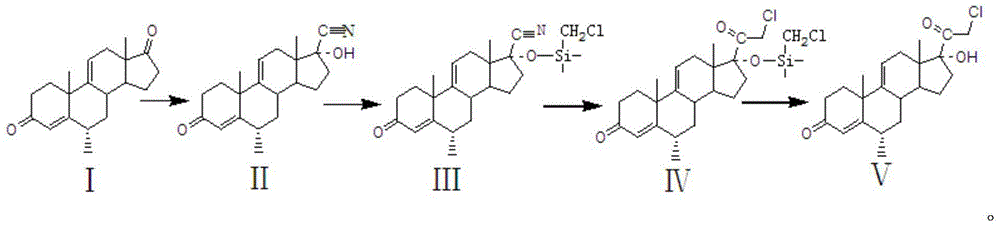
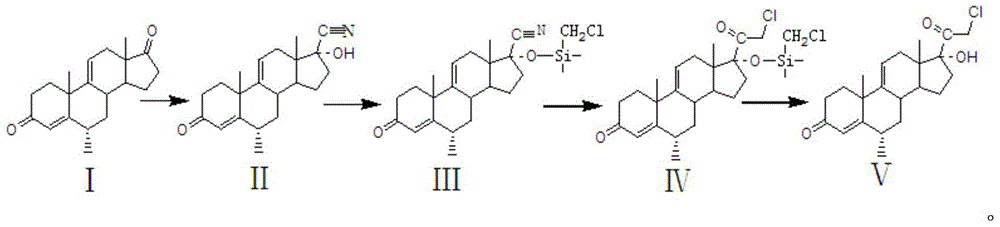
Preparation method of methylprednisolone key intermediate
The invention relates to a preparation method of a methylprednisolone key intermediate. According to the method, compound I serving as reactant is subjected to cyanohydrination reaction, protective reaction, low-temperature reaction and hydrolysis reaction to prepare the methylprednisolone key intermediate, namely, the compound V. The preparation method is low in production cost and high in yield, and the reaction line is as shown in the specification.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Medicine composition containing oral glucocorticoid and oral bronchodilator
InactiveCN101797258AReduce dosageEliminate side effectsRespiratory disorderHeterocyclic compound active ingredientsDiseaseAdditive ingredient
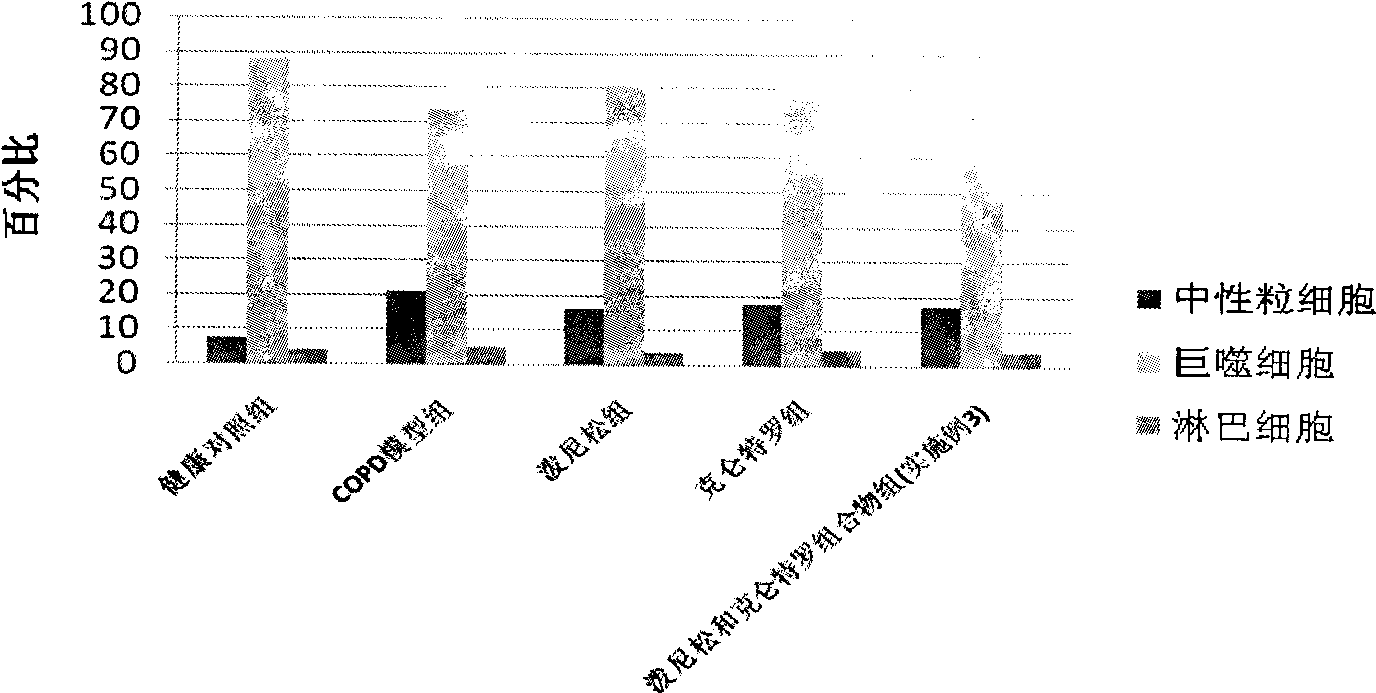
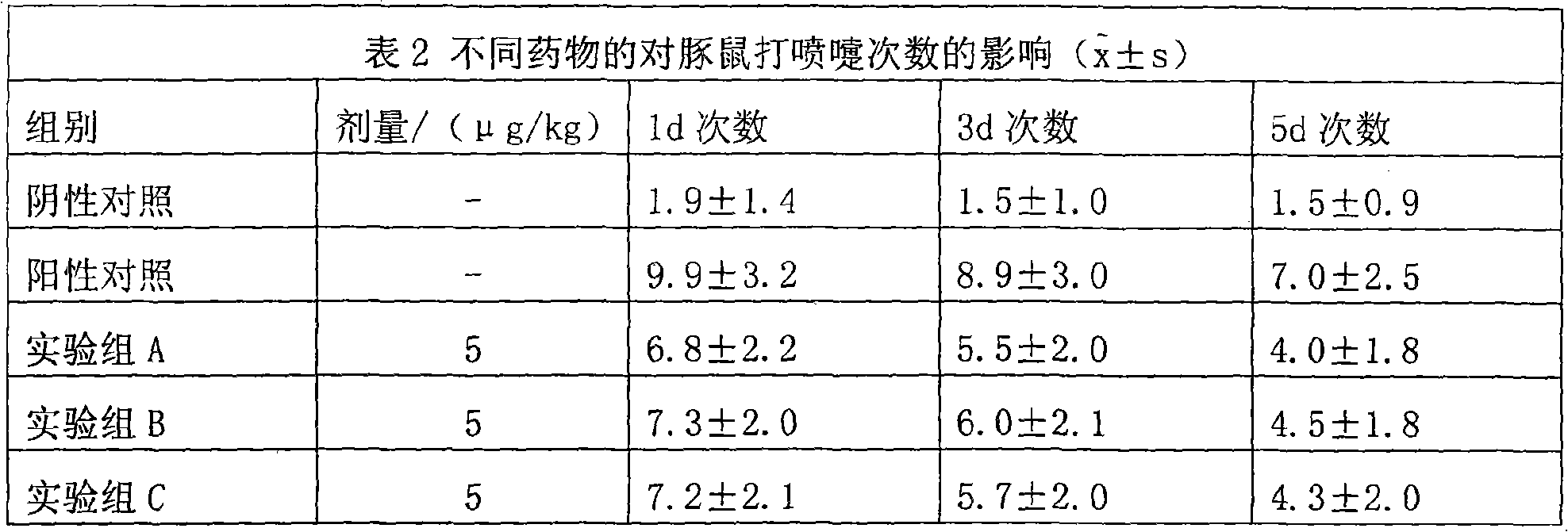
The invention discloses a medicine composition mainly containing oral glucocorticoid and an oral bronchodilator, as well as application thereof in preparing medicines for treating chronic obstructive pulmonary diseases (COPD) and bronchial asthma, which belongs to the technical field of medicine preparations. The oral glucocorticoid contained in the medicine composition is prednisone, prednisolone or methylprednisolone, and the oral bronchodilator is formoterol, clenbuterol or euphylline. The curative effect of the composition for treating the COPD or the bronchial asthma is better than that of separately using the two composition ingredients one after another, and the composition has the characteristics of synergistic action and low cost. Thus, the combination preparation of the oral glucocorticoid and the oral bronchodilator is of great significance to the treatment and the prevention of the COPD and the bronchial asthma of patients of lower income groups in wide rural and urban areas in China.
Owner:莫始平
Methylprednisolone preparation method
InactiveCN107840865AStarting materials are readily availableSimple lineSteroidsFermentationMethylprednisoloneDrug biotransformation
The invention discloses a methylprednisolone preparation method, wherein a compound represented by a formula 1 is used as a starting material, and biotransformation, oxidation, esterification, reduction and hydrolysis are sequentially performed to obtain the methylprednisolone.
Owner:TIANJIN JINYAO GRP
Biological dehydrogenation preparation method of 6 alpha-methylprednisolone intermediate
ActiveCN101760495BAvoid residueFlexible choiceMicroorganism based processesFermentationMethylprednisoloneDehydrogenation
The invention relates to a biological dehydrogenation preparation method of a 6 alpha-methylprednisolone intermediate, which uses a compound of formula (1) as a substrate and obtains a compound of formula (2) by adopting a simple arthrobacterium biological dehydrogenation method. The process comprises the following steps of: crushing the formula (1) compound or dissolving the formula (1) compound by using a solvent; adding to a fermentation tank with cultivated arthrobacterium to perform biotransformation; extracting, separating, and refining; drying, and then obtaining a dehydrogenation matter, i.e. the formula (2) compound. The invention can achieve the biotransformation rate of 70-90 percent.
Owner:TIANJIN JINYAO GRP
Methylprednisolone-loading nanoparticles as well as preparation method and application thereof
InactiveCN103705470AExtended half-lifeEasily damagedPowder deliveryOrganic active ingredientsSide effectNanoparticle
The invention discloses methylprednisolone-loading nanoparticles which consist of methylprednisolone and a carrier at a weight ratio of 1:(2-20), wherein the carrier is ibuprofen-modified ibuprofen and the grain size of the methylprednisolone-loading nanoparticles is 70-400nm. The invention further discloses a preparation method and application of the nanoparticles. The methylprednisolone-loading nanoparticles disclosed by the invention can be used for effectively transferring methylprednisolone to the injured part in a targeting manner, and have a very strong methylprednisolone slow-release function, so that side effects caused by high dosage and toxicity of methylprednisolone are avoided.
Owner:NANTONG UNIVERSITY
Production method of methylprednisolone dehydrogenate
ActiveCN112608970ACause toxic effectsQuality impactMicroorganism based processesFermentationBiotechnologyMicroorganism
The invention provides a production method of a methylprednisolone dehydrogenate. The production method of the methylprednisolone dehydrogenate comprises the following steps: A) adding water into a methylprednisolone Grignard substance, and pulping to obtain slurry; and B) biologically converting the methylprednisolone Grignard substance in the slurry into a methylprednisolone dehydrogenized substance by using arthrobacter simplex. According to the production method, no organic solvent is used, and the toxic effect on microorganisms is small during biotransformation; according to the method, the methylprednisolone Grignard substance is added with water and pulped into slurry, so that the feeding concentration and the substrate conversion rate of the methylprednisolone Grignard substance are greatly improved, the yield of a methylprednisolone dehydrogenized substance product is high, and the method is particularly beneficial to industrial production of the methylprednisolone dehydrogenized substance.
Owner:HENAN LIHUA PHARMA
Glucocorticoid drug tablet and preparation method thereof
InactiveCN109925288AControl impurity growthAvoid contactOrganic active ingredientsAntipyreticMedicineGlucocorticoid
The invention belongs to the field of pharmaceutical preparations, and relates to a glucocorticoid drug tablet and a preparation method thereof. The raw material medicine of the tablet is methylprednisolone having a particle size of D90 30-40 [mu]m, and the preparation method comprises the following steps: the raw material medicine is pulverized by air flow, and then uniformly mixed with a filler,a disintegrating agent and a lubricant, and then a mixture compressed to obtain a finished product. The glucocorticoid drug tablet prepared by the method has the advantages of high dissolution rate and high stability, and the preparation process improves preparation efficiency, reduces equipment energy consumption, and is suitable for industrial large-scale production.
Owner:JIANGSU SKYRUN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com