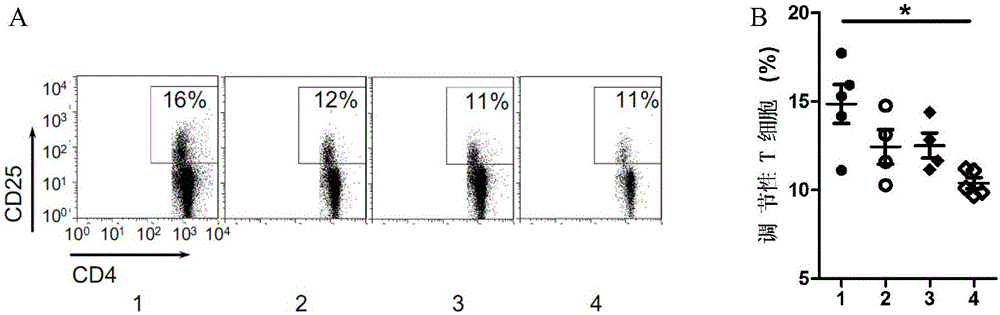
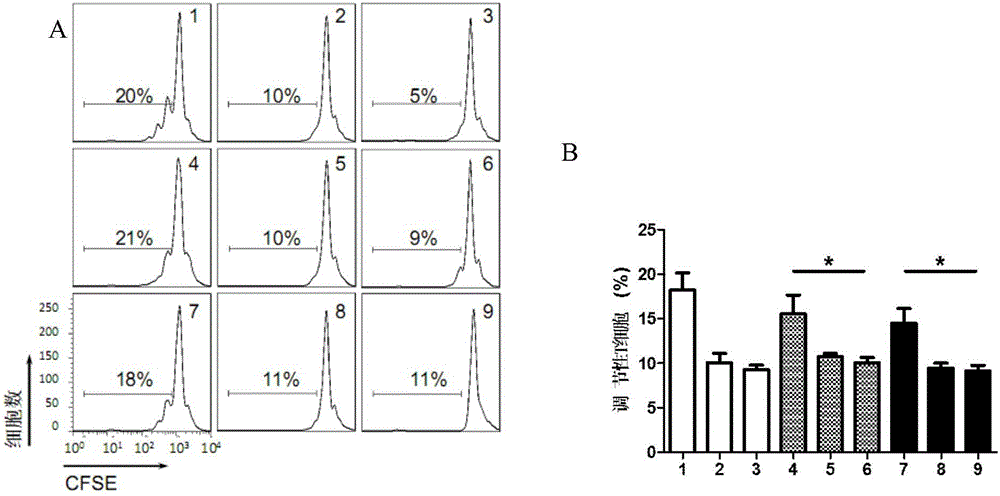
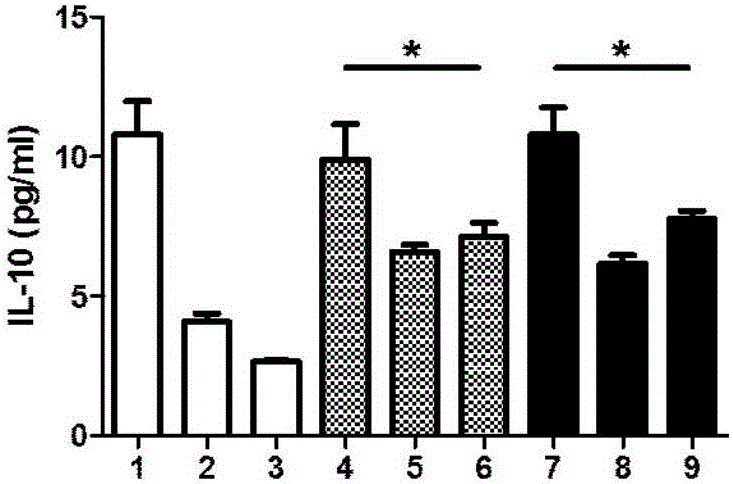
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82 results about "Prednisone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prednisone is used to treat conditions such as arthritis, blood disorders, breathing problems, severe allergies, skin diseases, cancer, eye problems, and immune system disorders.
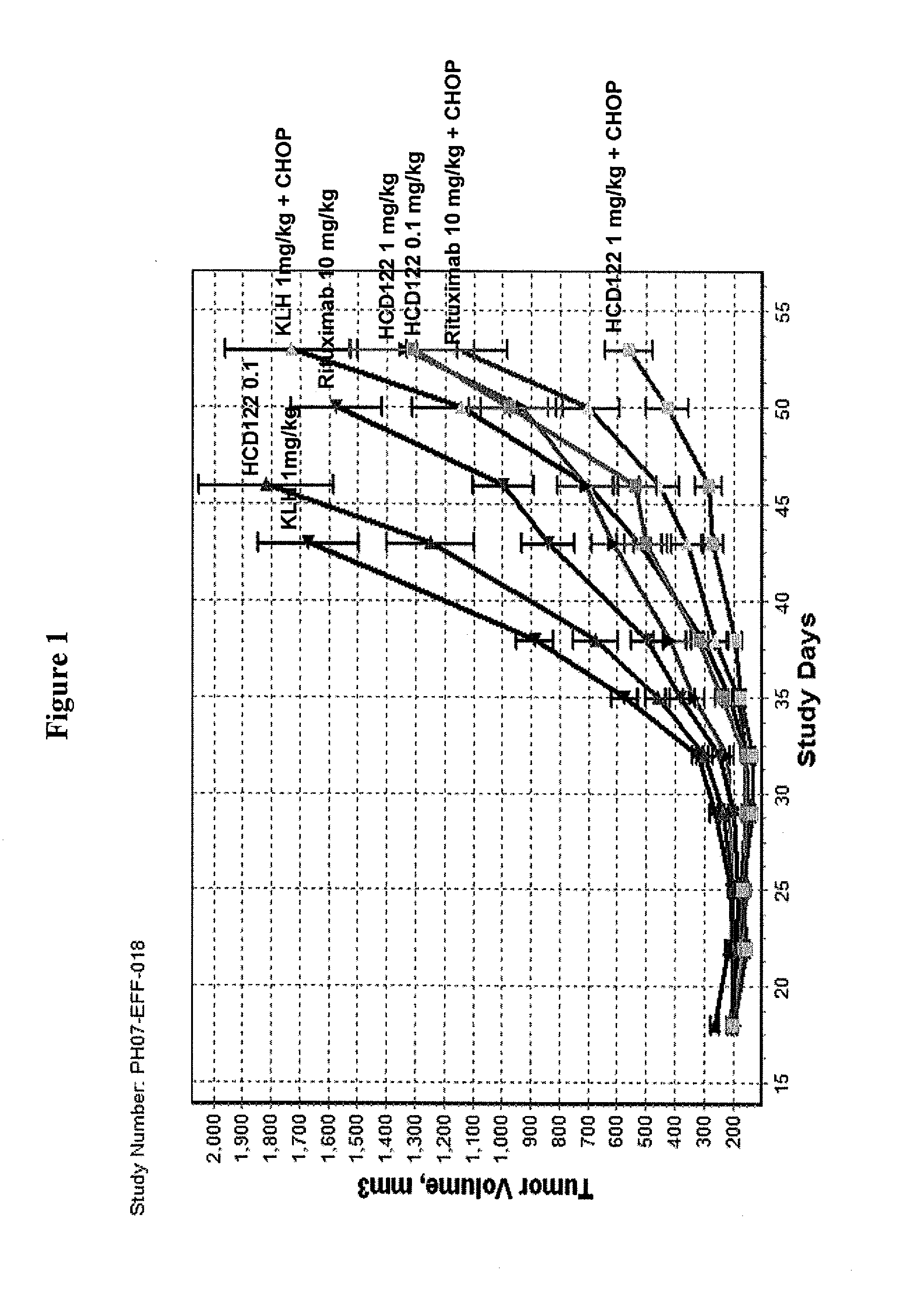
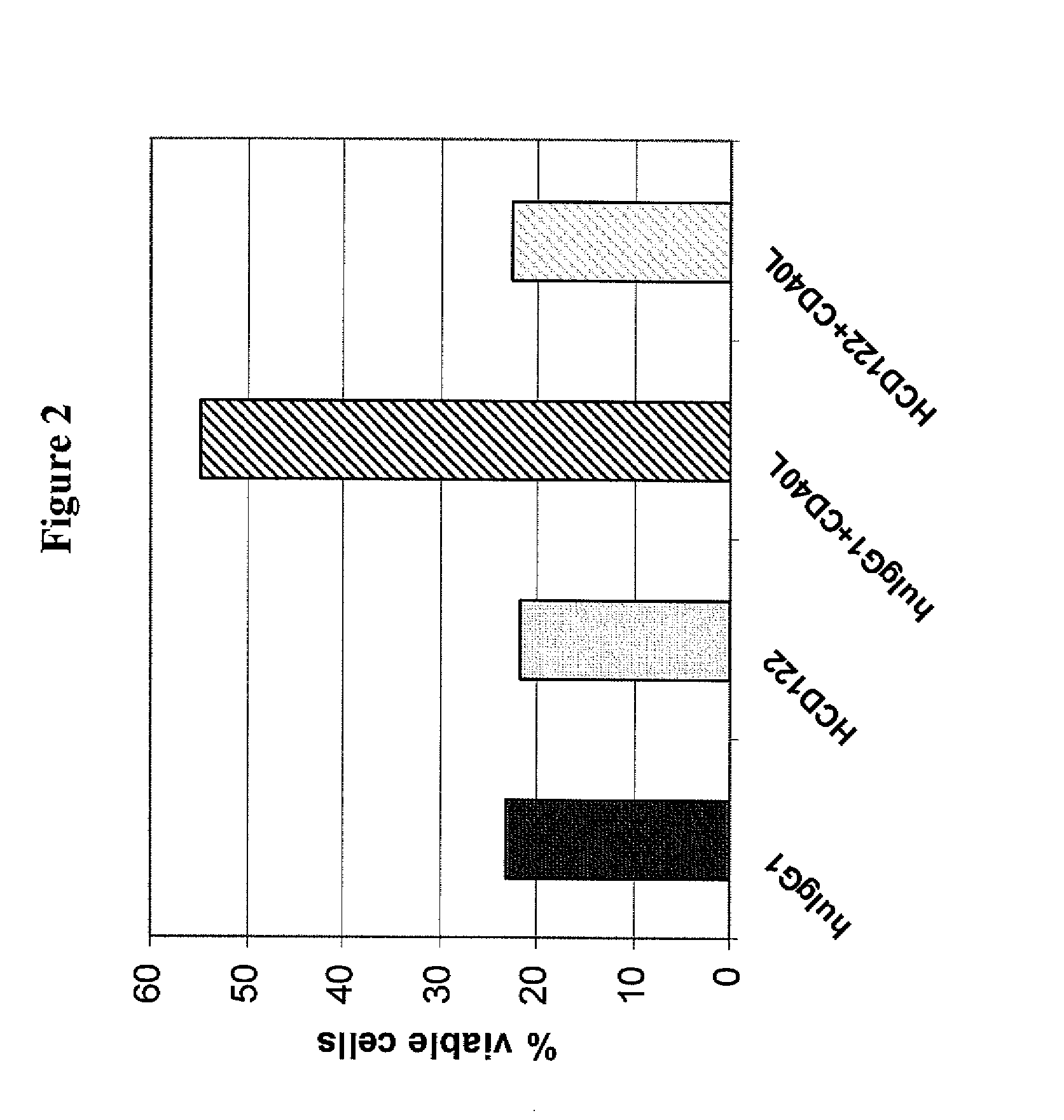
Uses of Anti-cd40 antibodies
This invention relates to new uses of anti-CD40 antibodies in the treatment of diseases or conditions associated with neoplastic B-cell growth in particular use of anti-CD40 antibodies in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP). The invention is particularly useful for the treatment of patients who have previously been administered (i) CHOP, (ii) the chimeric anti-CD20 monoclonal antibody rituximab, or (iii) combination therapy with CHOP and rituximab.
Owner:XOMA TECH LTD +1
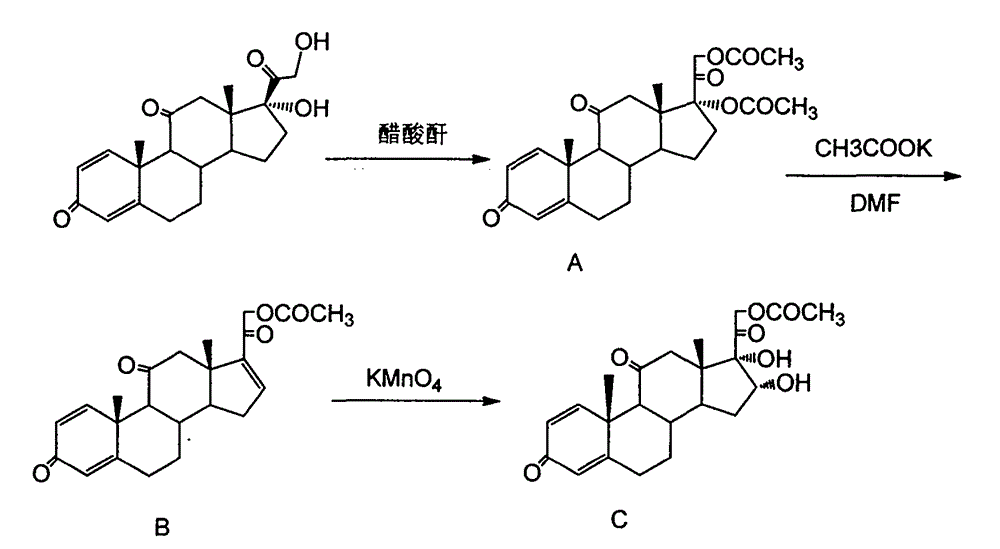
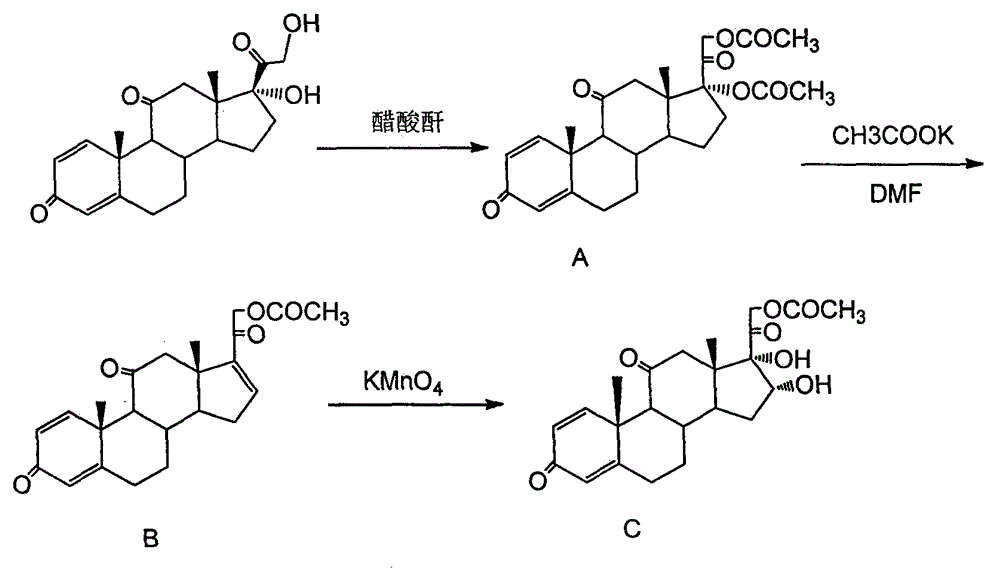
Method for synthesizing 16 alpha-hydroxy prednisolone
The invention relates to a method for synthesizing 16 alpha-hydroxy prednisolone, and belongs to the field of chemical technologies. In the method, prednisone is taken as an initiative raw material, and subjected to five-step reaction of elimination, oxidation, condensation, reduction and hydrolysis to form the 16 alpha-hydroxy prednisolone; the prednisone is taken as the raw material, organic weak base is taken as a retarder to inhibit the oxidation rate of potassium permanganate to reactants, a three-phase acid solvent is taken as a solvent, ketoxime is hydrolyzed by adding aqueous solution of nitrite, and refining is carried out by a multiphase separation crystallization technology. The method has the advantages of simple operation, mild reaction conditions, readily available raw material, low cost and light environmental pollution.
Owner:浙江东晖药业有限公司
Oral prednisone time-selecting release preparation and preparation method thereof
InactiveCN103690545AGive full play to the therapeutic effectImprove balanceOrganic active ingredientsAntipyreticCelluloseFormulary
The invention discloses an oral prednisone time-selecting release preparation and a preparation method thereof. The oral prednisone time-selecting release preparation provided by the invention mainly consists of 0.3-5 parts of prednisone and derivatives thereof, 10-50 parts of glyceryl behenate and 3-30 parts of hydroxypropyl cellulose, and can further contain a disintegrating agent and other pharmaceutically acceptable excipients. The preparation method is as below: extruding tablet cores or granules containing the drug according to the formula by a tablet press or a dry granulator; and coating the tablet cores or particles containing the drug by a coating pan or a fluidized bed to attach the coating film to the tablet cores or particles containing the drug, so as to obtain the oral prednisone time-selecting release preparation. The oral prednisone time-selecting release preparation provided by the invention can achieve a good balance between the biological rhythm of the patients and the curative effects, and is safer, more convenient and effective compared with a traditional preparation. The oral prednisone time-selecting release preparation is prepared by an extrusion-coating process, which is simple for operation, and the obtained time-selecting release preparation has the advantages of drug stability and high reproducibility.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
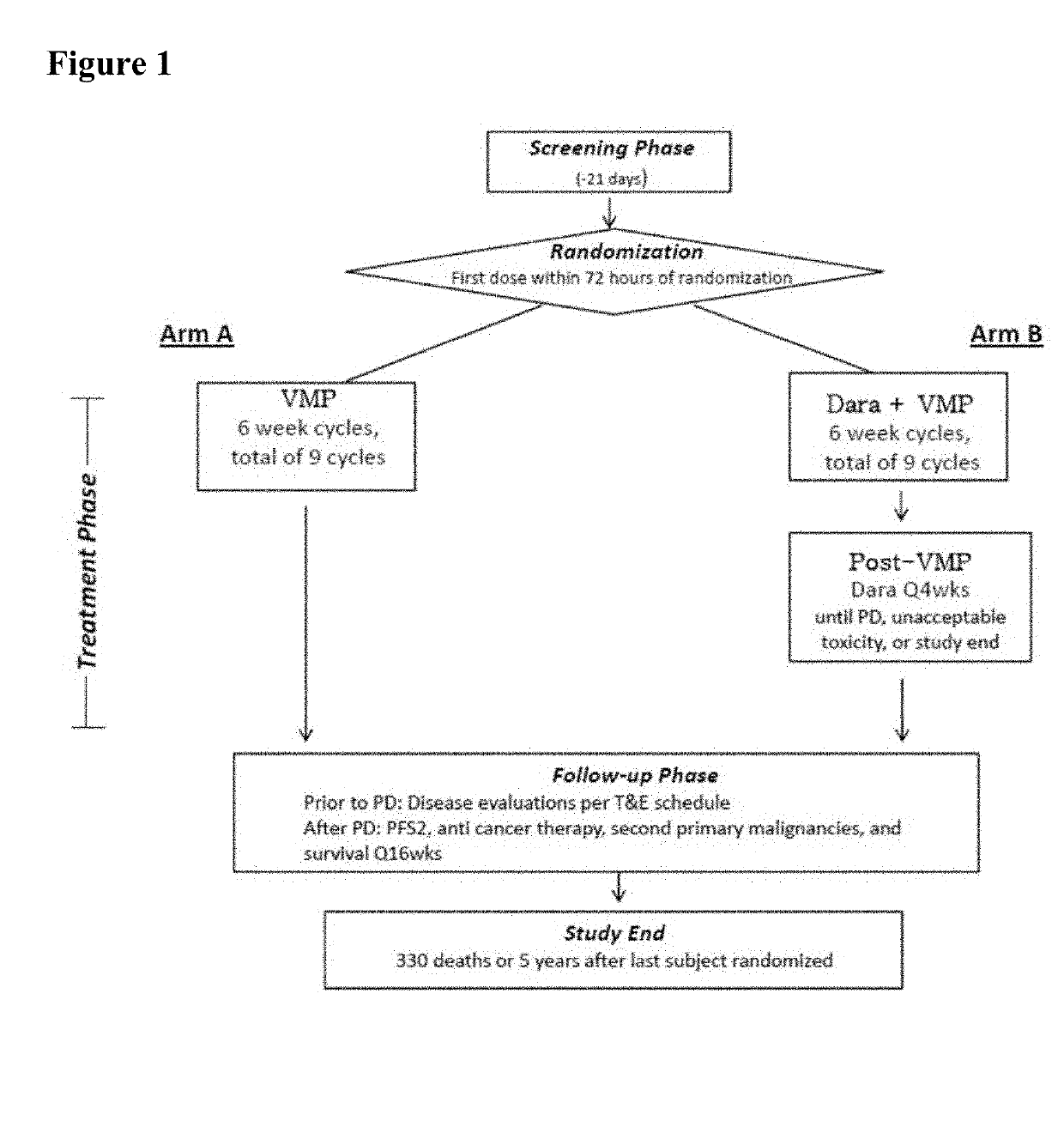
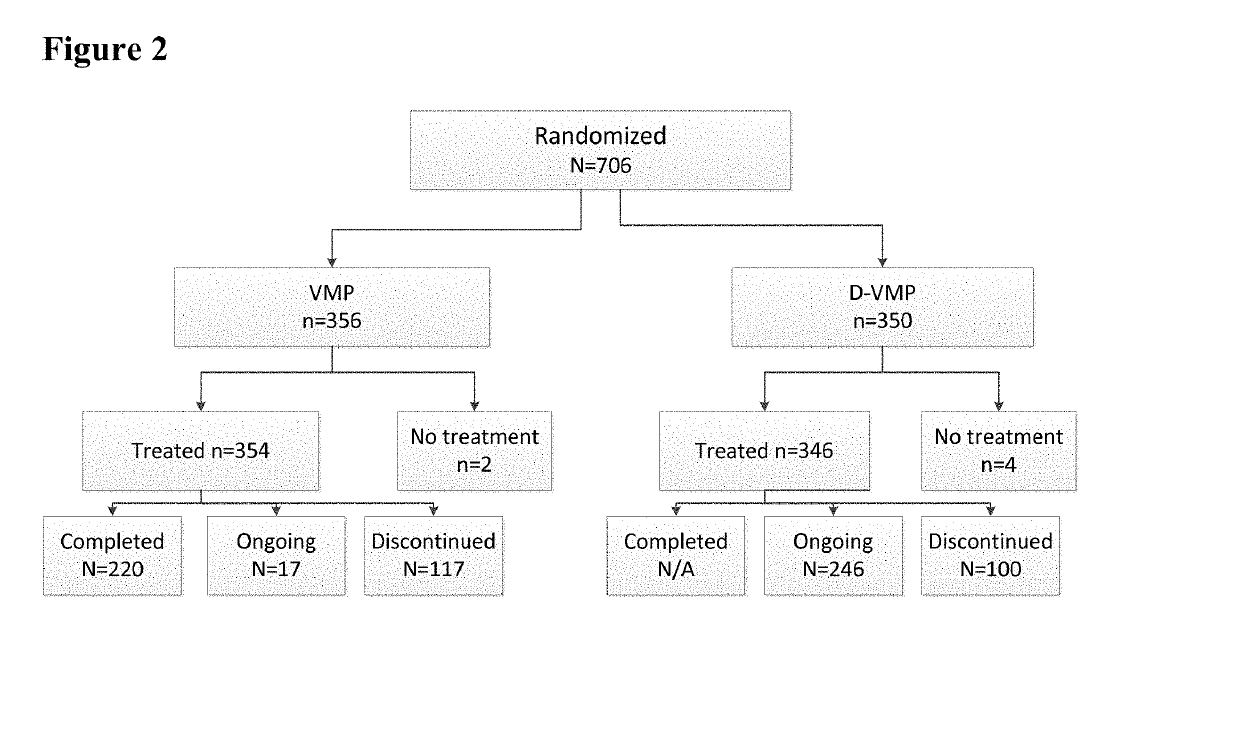
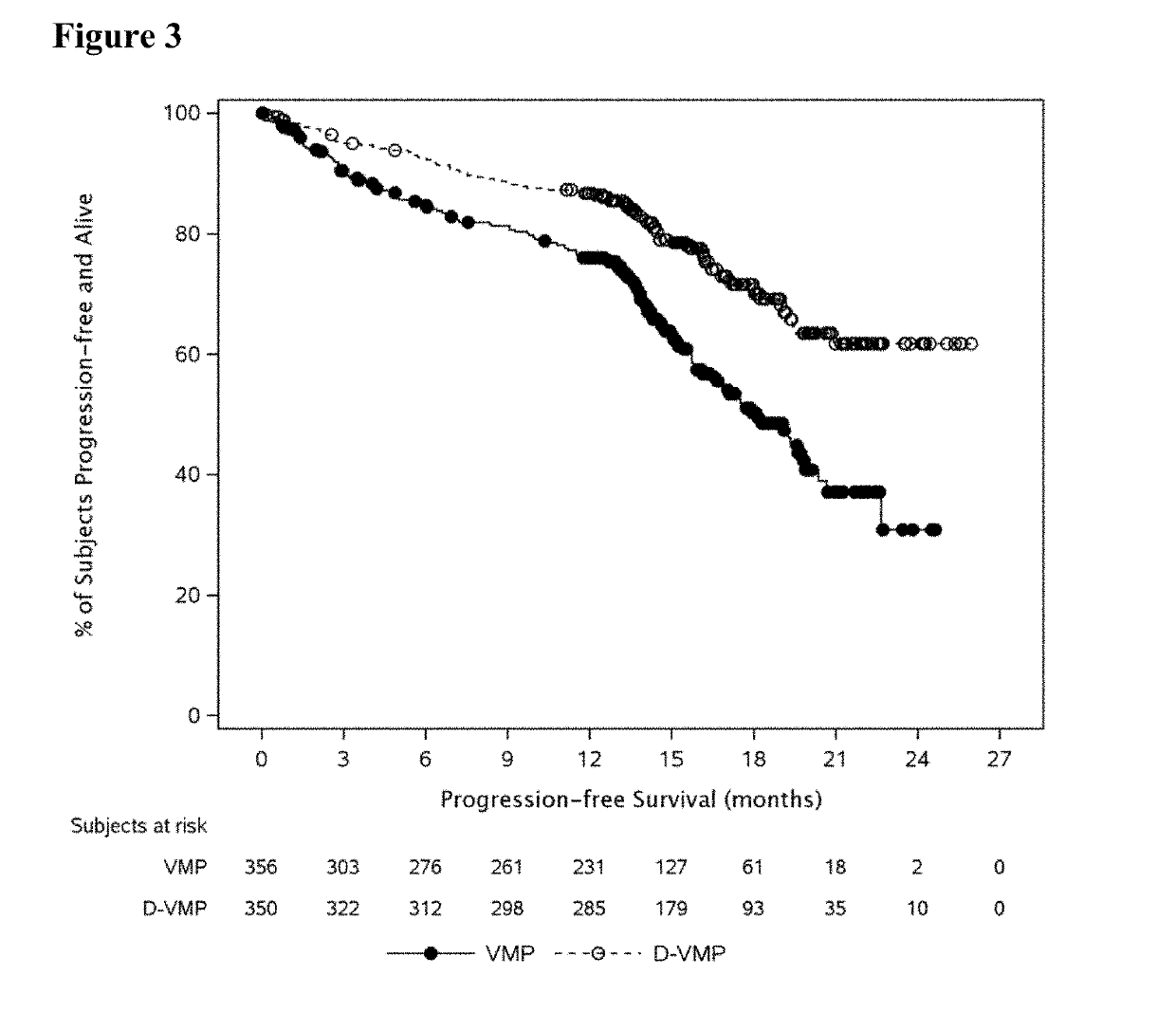
Methods of Treating Multiple Myeloma
PendingUS20220062415A1Conducive to survivalReduce riskInorganic non-active ingredientsBoron compound active ingredientsAntiendomysial antibodiesPharmaceutical drug
Described herein are methods of treating multiple myeloma with clinically proven safe and effective amounts of an antibody that specifically recognizes CD38 with bortezomib, melphalan, and prednisone. Also described are methods of selling or offering for sale an antibody that specifically recognizes CD38 or pharmaceutical compositions thereof with bortezomib, melphalan, and prednisone.
Owner:JANSSEN BIOTECH INC
Glucocorticosteroid and chemotherapy medicament carried by anticancer sustained-release agent
InactiveCN101502484AInhibition formationImprove permeabilitySolution deliveryPharmaceutical non-active ingredientsGlucocorticoidPolyethylene glycol
The invention provides an anti-cancer sustained-release agent co-carrying glucocorticoid and chemotherapeutic drugs and belongs to sustained-release injections. The anti-cancer sustained-release agent comprises sustained-release microspheres and a solvent, wherein, the sustained-release microspheres comprise anti-cancer active components and sustained-release auxiliary materials; and the solvent is a particular solvent containing a suspending agent. The glucocorticoid is selected from prednisolone, methylprednisolone, dexamethasone, betemethasone, triamcinolone acetonide or triamcinolone acetonide; the chemotherapeutic drugs are selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogues and the like; the sustained-release auxiliary materials are biocompatible high-polymers, such as polylactic acid and the copolymers thereof, polyethylene glycol, carboxyl-terminated polylactic acid copolymers, polyfatty acid dimer-sebacic acid copolymers, poly(erucic acid dimer-sebacic acid), poly(fumaric-co-sebacic acid), polifeprosan and the like; and the suspending agent with the viscosity being 100cp to 3,000cp (at the temperature of 20 to 30 DEG C) is selected from sodium carboxymethyl cellulose and the like. The anti-cancer active components and the sustained-release microspheres can further be prepared into sustained-release implants which can effectively inhibit the growth of tumors, alleviate edema and improve the curative effects of radiotherapy and chemotherapy by intra-tumor or peri-tumor injection or placement.
Owner:SHANDONG LANJIN PHARMA
Heparin derivative-poloxamer temperature sensitive hydrogel and preparation method thereof
InactiveCN108904522AReduce the number of dressing changesImprove complianceOrganic active ingredientsAerosol deliveryRoom temperatureBottle
The invention discloses a heparin derivative-poloxamer temperature sensitive hydrogel and a preparation method thereof. The poloxamer is used as a framework material, and N-Acetylated low molecular weight heparin and prednisone are compounded to obtain the hydrogel. The mass ratio of the N-Acetylated low molecular weight heparin to the poloxamer is 15-90:100-300. The preparation method comprises the following steps that step 1, the N-Acetylated low molecular weight heparin is weighed and placed in a serum bottle, and a PBS buffer solution is added; step 2, a prednisone drug PBS buffer solutionwith mass concentration of 5% is added, stirring at the room temperature is conducted and lasts for 12-36 hours; and step3, the poloxamer is weighed, and the solution in the step 2 is added, solutionstirring is conducted at 4-10 DEG C and last for 12-36 hours. The heparin derivative-poloxamer temperature sensitive hydrogel has the excellent effect, is long in antibacterial time, and has simple procedures; and industrialization can be achieved easily, and popularization and application are facilitated.
Owner:JIANGNAN UNIV
Clinically Proven Subcutaneous Pharmaceutical Compositions Comprising Anti-CD38 Antibodies and Their Uses in Combination with Bortezomib, Mephalan and Prednisone
InactiveUS20200330593A1Peptide/protein ingredientsPharmaceutical delivery mechanismAntiendomysial antibodiesPharmaceutical drug
The present invention relates to clinically proven subcutaneous pharmaceutical compositions comprising anti-CD38 antibodies and methods of their uses in combination with bortezomib, melphalan and prednisone.
Owner:JANSSEN BIOTECH INC
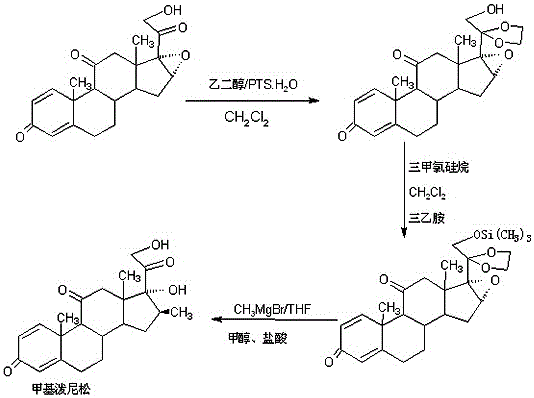
Preparation method of methylprednisone
ActiveCN106518944AWide variety of sourcesProcess economy and environmental protectionSteroidsGrignard reagentStrong acids
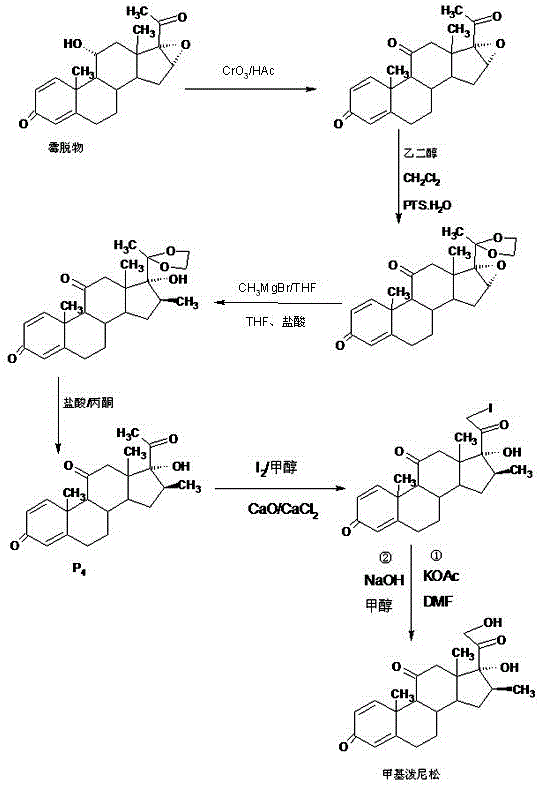
The invention relates to a preparation method of methylprednisone. The method comprises the following steps: carrying out acid catalyzed reaction on 16(17)a-epoxy prednisone prepared from 4-androstenedione (4AD for short) and ethanediol in an organic solvent at 10-50 DEG C to obtain a ketal substance 20-ketal-16(17)a-epoxy prednisone; carrying out alkali catalyzed reaction on the ketal substance and trimethylchlorosilane in an organic solvent to obtain a silyl ether substance 21-methylsilyl-ether-20-ketal-16(17)a-epoxy prednisone; and carrying out Grignard reaction on the silyl ether substance and a 2M methyl grignard reagent in an organic solvent, hydrolyzing the Grignard substance in strong acid to obtain the methylprednisone. The detection indicates that the HPLC (high performance liquid chromatography) content is 99.0% or above, the melting point is 228-237 DEG C, and the synthetic weight total yield is 80-85%. When being used for producing methylprednisone, the method has the advantages of wide raw material sources, economical and environment-friendly technique, simple production operation, short synthesis route, high synthesis yield and lower production cost (than the traditional method by 30-40%). The method is convenient for industrial production.
Owner:HUNAN KEREY BIOTECH
External-use ointment for treatment of dermatopathy
InactiveCN106668864AWide range of treatmentHigh cure rateHydroxy compound active ingredientsAerosol deliveryDiseaseTherapeutic effect
The invention relates to an external-use ointment for treatment of dermatopathy. The ointment is prepared by mixing and blending the following medicines (by weight): 6-9 parts of sulfamethoxazole, 5-8 parts of chloramphenicol, 5-8 parts of prednisone, 3-5 parts of borneol, 0-8 parts of sulfur flour, 5-8 parts of vitamin E, 4-8 parts of vitamin complex B, 6-10 parts of VC, 5-7 parts of B1, 0.01-0.04 part of triamcinolone acetonide, 10 parts of urea ointment, 30 parts of a Chinese herbal antibacterial paste, 8-10 parts of chlorpheniramine, 4-8 parts of chloramphenicol raw powder, 6-10 parts of metronidazole, 10 parts of B6 ointment, 0-15 parts of terbinafine hydrochloride emulsifiable paste and 0-10 parts of ketoconazole emulsifiable paste. The ointment of the invention is used for treatment of skin diseases such as dermatitis, eczema, acne, yellow fluid ulcers, manus and tinea pedis, psoriasis, etc., can be used for realizing antibiosis, anti-inflammation, itching-relieving and antiallergic functions and the function of boosting body immunity, reduces exudation and inflammatory response and promotes inflammatory resorption of wounds and growth of granulation tissues, and has a good comprehensive treatment effect.
Owner:毕明华
Method for establishing mouse lung cytomegalovirus latent reactivation infection model
InactiveCN111955415AImprove reproductive abilityImprove economyOrganic active ingredientsAnimal husbandryBasic researchViral infection
The invention discloses a method for establishing a mouse lung cytomegalovirus latent reactivation infection model. The method comprises the following steps: injecting MCMV into the abdominal cavity of a mouse to establish mouse lung latent infection cytomegalovirus, selecting latent MCMV-infected mouse, orally taking a medicine prednisone to induce reactivation of the lung latent cytomegalovirus,and identifying reactivation of the lung latent cytomegalovirus. Lung latent cytomegalovirus infection is induced to be reactivated by orally taking the medicine prednisone, the mouse lung is subjected to cytomegalovirus latent reactivation infection, and an animal model with cytomegalovirus latent reactivation can be constructed. The mouse model shows the typical characteristic of lung cytomegalovirus latent reactivation that: a virus expression-related protein appears in lung tissues. The model can be used for fundamental research in the related fields of cytomegalovirus infection, and laysan experimental animal foundation for exploring the pathogenesis of latent reactivation of cytomegalovirus.
Owner:张志辉
Drug-release type gel for treating fundus macular degeneration and preparation method thereof
InactiveCN109966243AAchieve sustained releaseLittle side effectsSenses disorderAerosol deliveryPeroxydisulfateNon invasive
The invention discloses drug-release type gel for treating fundus macular degeneration and a preparation method thereof. The drug-release type gel comprises an anti-angiogenesis drug, an inducing compound and gel and is characterized in that the inducing compound is used for increasing the permeation of the drug, and the gel attaches to the cornea during use and serves as the carrier of the drug;the anti-angiogenesis drug is selected from Lucentis or Compaq; the inducing compound is selected from optional one of dexamethasone, prednisone and cortisone or the combination of two of dexamethasone, prednisone and cortisone; the gel serving as the drug carrier is prepared by preparing a solution containing polydopamine and methacrylate gelatin, adding crosslinking agent N,N-methylene bisacrylamide and initiator potassium peroxydisulfate into the solution, and performing fixed-die forming to obtain the gel which does not contain the drug. The drug-release type gel has the advantages that non-invasive drug delivery is adopted, sustained drug release is achieved after the gel attaches to the cornea, and the anti-VEGF drug is allowed to act on the vitreous body by utilizing the permeability of the inducing compound.
Owner:SHANGHAI TONGREN HOSPITAL
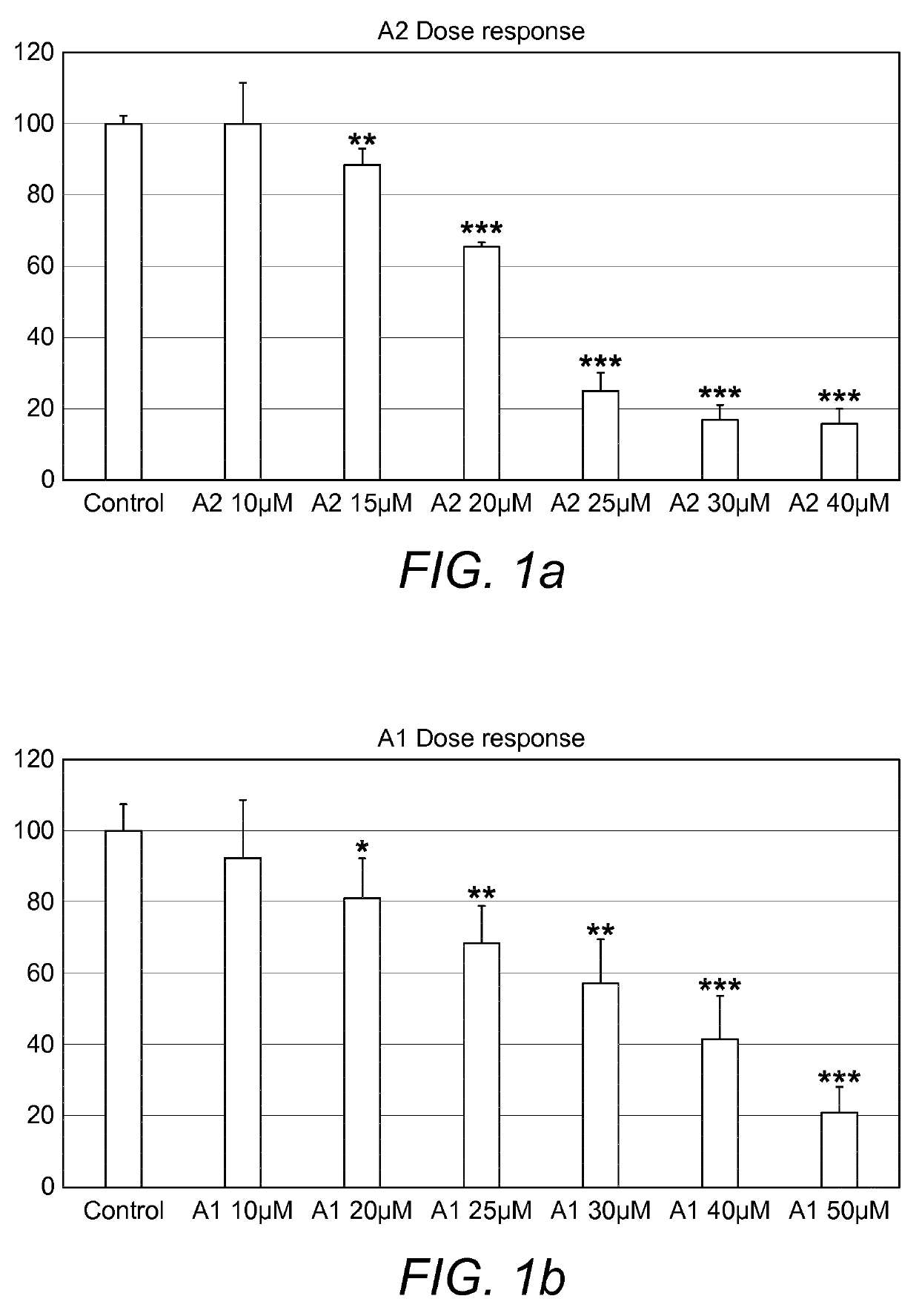
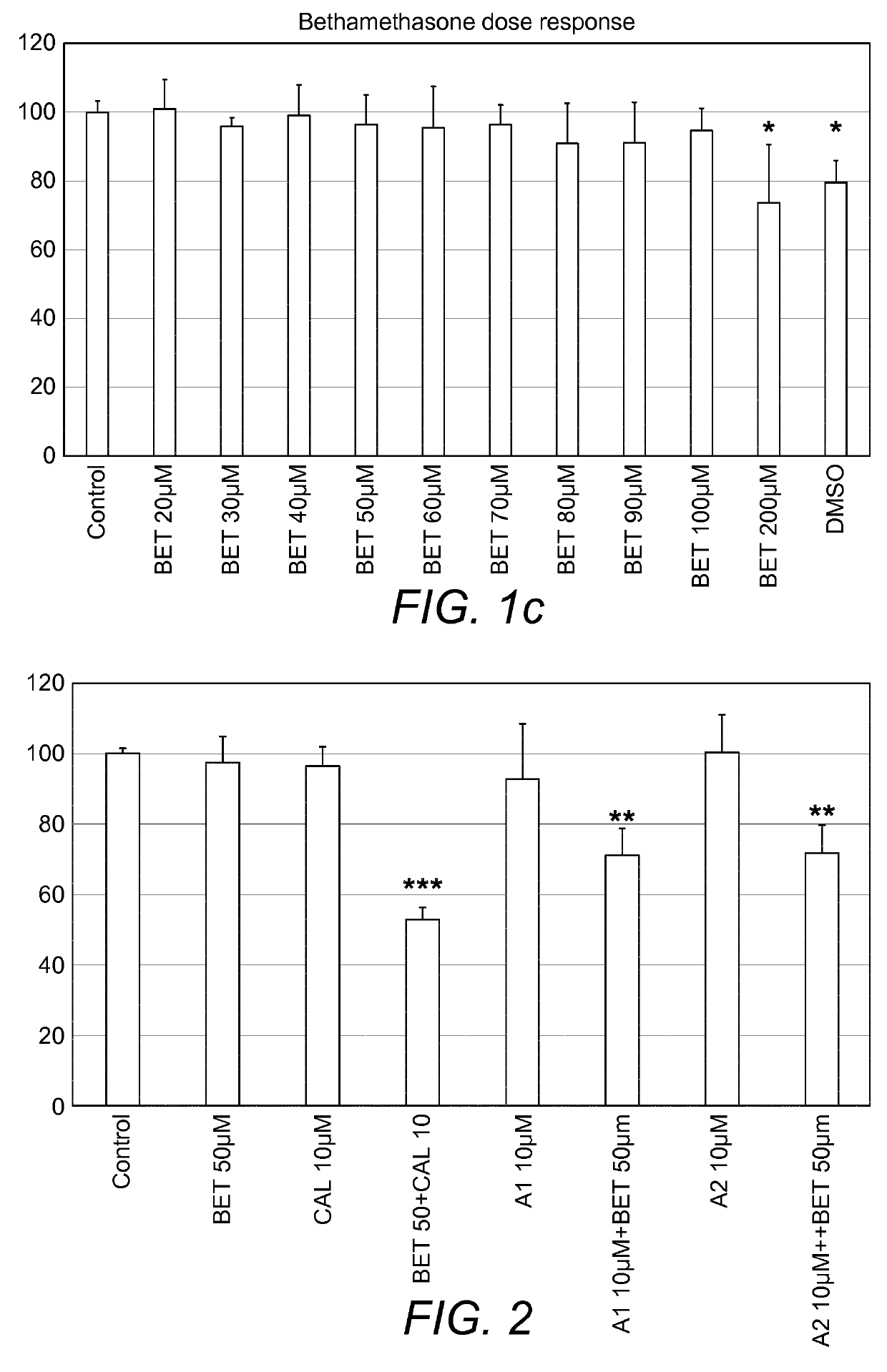
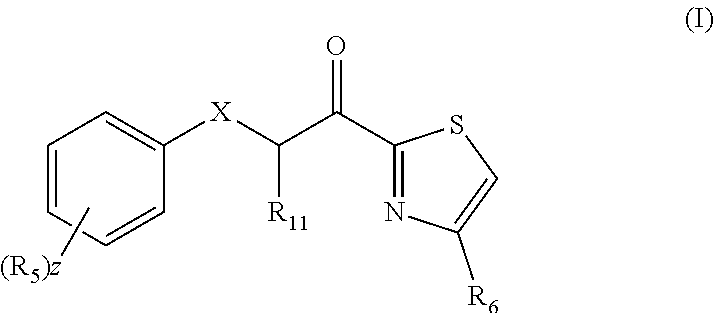
Combination therapy comprising a thiazole and a corticosteroid to treat skin conditions
InactiveUS20190255023A1Preferable effectReduce inflammation and itchinessOrganic active ingredientsAerosol deliveryFluocinoloneFluocinonide
A pharmaceutical composition comprising: (A) at least one compound of formula (I): wherein X is O or S, preferably O R6 is H, C1-6alkyl, —(CH2)pCOOH, —(CH2)pCOOC1-6alkyl, —(CH2)pCONH2, —(CH2)pCONHC1-6alkyl, —(CH2)pCON(C1-6alkyl)2, R11 is H or C1-6 alkyl; each R5 is —OC1-10alkyl, —SC1-10alkyl, —C1-12alkyl, or OAr2; wherein Ar2 is phenyl, optionally substituted with one or more halo; each p is 0 to 3; each z is 1 to 2; or a pharmaceutically acceptable salt, or a hydrate or solvate thereof; and (B) one or more corticosteroid partners, preferably selected from the group consisting of betamethasone, clobetasol, halometasone, dexamethasone, fluocortolone, desoximetasone, diflorasone, fluocinonide, flurandrenolide, halobetasol, amcinonide, halocinonide, triamcinolone, hydrocortisone, aclometasone, fluticasone, mometasone, clocortolone, fluocinolone, desonide, prednisone, prednisolone, and prednicarbate or a pharmaceutically acceptable salt, or a hydrate or solvate thereof, especially betamethasone or a pharmaceutically acceptable salt, or a hydrate or solvate thereof.
Owner:AVEXXIN
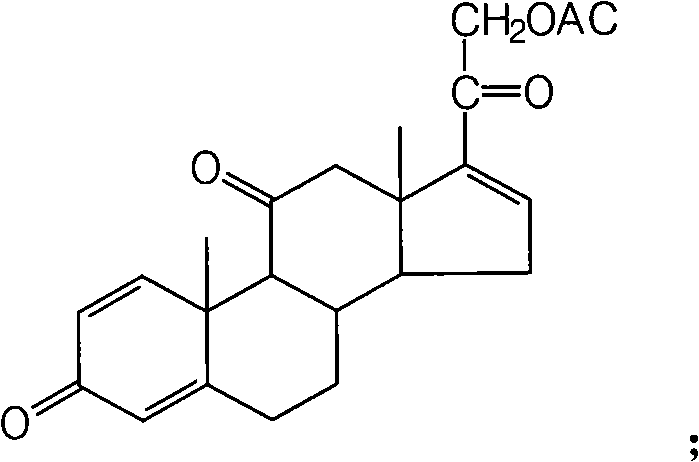
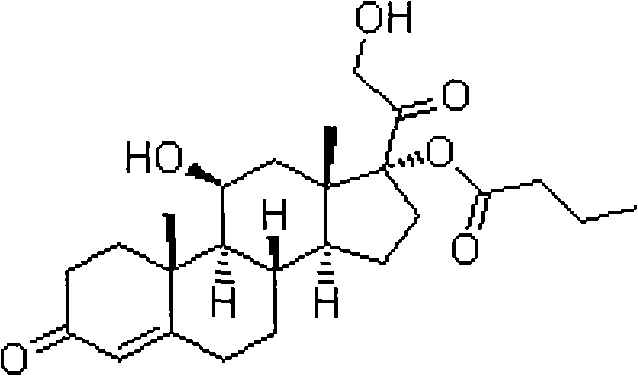
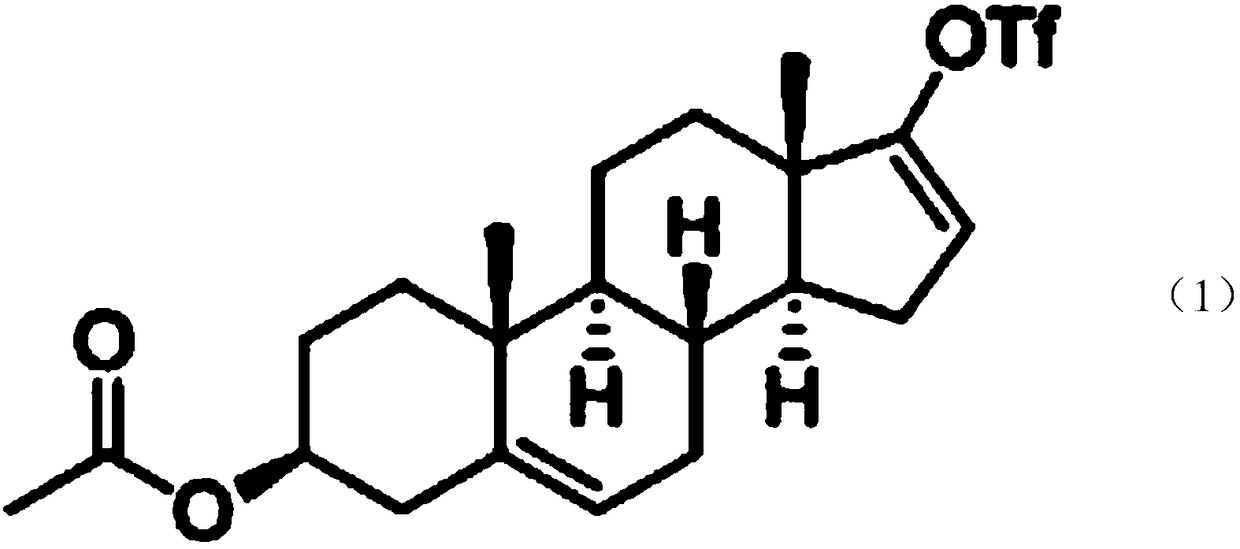
Preparation method of 16a,17a-dyhydroxyl-21-acetoxyl-1,4-pregnene diene-3,11,20-triketone
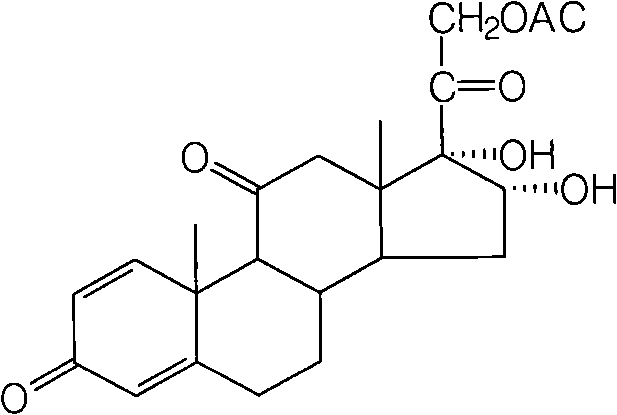
The invention provides a preparation method of 16a,17a-dyhydroxyl-21-acetoxyl-1,4-pregnene diene-3,11,20-triketone. The preparation method comprises the following steps: step 1, by taking prednisone as a raw material, allowing the prednisone to have an esterification reaction with acetic oxide; step 2, performing a degreasing reaction by use of alkali; and step 3, oxidizing by use of potassium permanganate under an acidic condition. The method has the advantages of adopting raw materials which are low in cost and easy to obtain, simplifying the production process route and being simple to operate and suitable for large-scale industrial production.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Lactococcus lactis subsp.lactis HFY14 and application thereof
PendingCN114686402AImprove immune organ indexImprove kidney functionBacteriaMicroorganism based processesBiotechnologyStaphylococcus lactis
The invention discloses a lactococcus lactis subsp. Lactis HFY14 and application thereof, and belongs to the technical field of biology, the lactococcus lactis subsp. Lactis is named as HFY14, the preservation number is CGMCC No.16647, the strain is separated from naturally fermented yoghurt, and the strain has the advantages that the strain can be used for preparing the lactococcus lactis subsp. Lactis HFY14 and the application of the lactococcus lactis subsp. Lactis HFY14; animal experiments, histopathologic observation and other experiments prove that the renal dysfunction of lupus nephritis mice induced by hypophytane through intragastric administration of viable bacterial liquid of the strain can be obviously improved, and the effect is close to that of a medicine prednisone. The invention provides a theoretical basis for the subsequent development and utilization of the lactococcus lactis subsp. Lactis HFY14 and the application of the lactococcus lactis subsp. Lactis HFY14 as a probiotic to improve the renal function of nephropathy, and the strain has the application potential of performing long-term intervention on lupus nephritis to improve the renal function.
Owner:善恩康生物科技(苏州)有限公司
Nursing preparation for wound surface of skin and preparing method thereof
InactiveCN107596171ACosmetic RestorationGood antibacterial effectOrganic active ingredientsAnthropod material medical ingredientsChlorhexidine AcetateTreatment period
The invention discloses a nursing preparation for a wound surface of a skin. The nursing preparation is prepared from the following raw materials in parts by weight: 5 to 15 parts of rubus corchorifolius extract, 4 to 11 parts of pumpkin peel, 5 to 10 parts of cortex phellodendri chinensis, 4 to 8 parts of folium eriobotryae, 5 to 10 parts of fructus momordicae, 2 to 4 parts of periostracum cicadae, 1 to 3 parts of pearl powder, 1 to 2 parts of prednisone, 1 to 2 parts of chlorhexidine acetate, 2 to 6 parts of moringa oleifera leaf, 10 to 20 parts of camellia oleosa seed oil, 1 to 3 parts of stigma maydis, 1 to 3 parts of ophicalcitum, 2 to 5 parts of tea leaf residue, 1 to 3 parts of snail powder, 1 to 2 parts of honeycomb, 2 to 5 parts of dried earthworm and 30 to 55 parts of vaseline. The nursing preparation for the wound surface of the skin, which is provided by the invention, can be used for effectively nursing the wound surface of the skin, is good in antibacterial effect and bacteriostatic effect, short in treatment period and quick in wound healing speed; further, after cure, a scar cannot be formed in the surface of the skin; meanwhile, the nursing preparation for the wound surface of the skin can be also used for effectively repairing the scar and has favorable economic value and social value, and the value added of the nursing preparation is increased.
Owner:孙振惠
Traditional Chinese and western medicine formula for treating hemorrhoid
InactiveCN102225104AQuick liftQuick resultsHydroxy compound active ingredientsTetracycline active ingredientsDiseaseTherapeutic effect
The invention relates to a traditional Chinese and western medicine formula for treating hemorrhoid, which is characterized by comprising traditional Chinese medicines and western medicines, wherein the traditional Chinese medicines comprise 1-10 parts of catechu, 1-10 parts of goldthread and 1-10 parts of borneol and the western medicines comprise a pill of chlorpheniramine maleate and a pill of prednisone, wherein the traditional Chinese medicines such as catechu, goldthread and borneol and the western medicines such as chlorpheniramine maleate and prednisone are grinded into powder together; and the powder is uniformly mixed with chlorotetracycline eye ointment for standby. In the formula provided by the invention, the traditional Chinese medicines and the western medicines are used specifically for treating the root causes and symptoms of diseases, has the functions of diminishing inflammation, repelling swelling, relieving itching and relieving pain, can rapidly relieve all the symptoms of external hemorrhoid, when used for treating the hemorrhoid, particularly the external hemorrhoid, has the unique effects of fast effect acting, high recovery rate, long stabile period and the like and shows the obvious and unique treatment effects as compared with other medicines.
Owner:李辉
Specific dermatophytosis cream
The invention discloses a specific dermatophytosis cream medicament, which is paste for external application. The paste comprises main elements including chloromycetin, prednisone and hexadecadrol (in a ratio of 5: 1: 1) and auxiliary materials (which are incredible in formula theory, but hasve a very good treatment effect) including glycerin and a proper amount of transdermal agent. According to an action principle-based formula, sterilizing anti-infectious elements in the formula together with the glycerin and the transdermal agent in the auxiliary materials quickly seep into skin tissues to sterilize germs, moisturize skin, promote the union and recovery of ulcer skin and restore immunity. The specific dermatophytosis cream medicament is applied to the following symptoms of: dermatophytosis (athlete's foot) peeling and pus, blisters, foot tickle and foot odor. The using method of the specific dermatophytosis cream medicament comprises the following steps of: washing feet with hot water, drying feet, and coating the paste on infected parts for 3 to 5 times a day. Serious patients can restore health in 2 to 3 days. The specific dermatophytosis cream medicament has the advantages that: the ulcer face can restore to health completely in a week and is thoroughly cured without recrudescence; and the specific dermatophytosis cream medicament has the same curing effect on re-infection.
Owner:刘卫峰
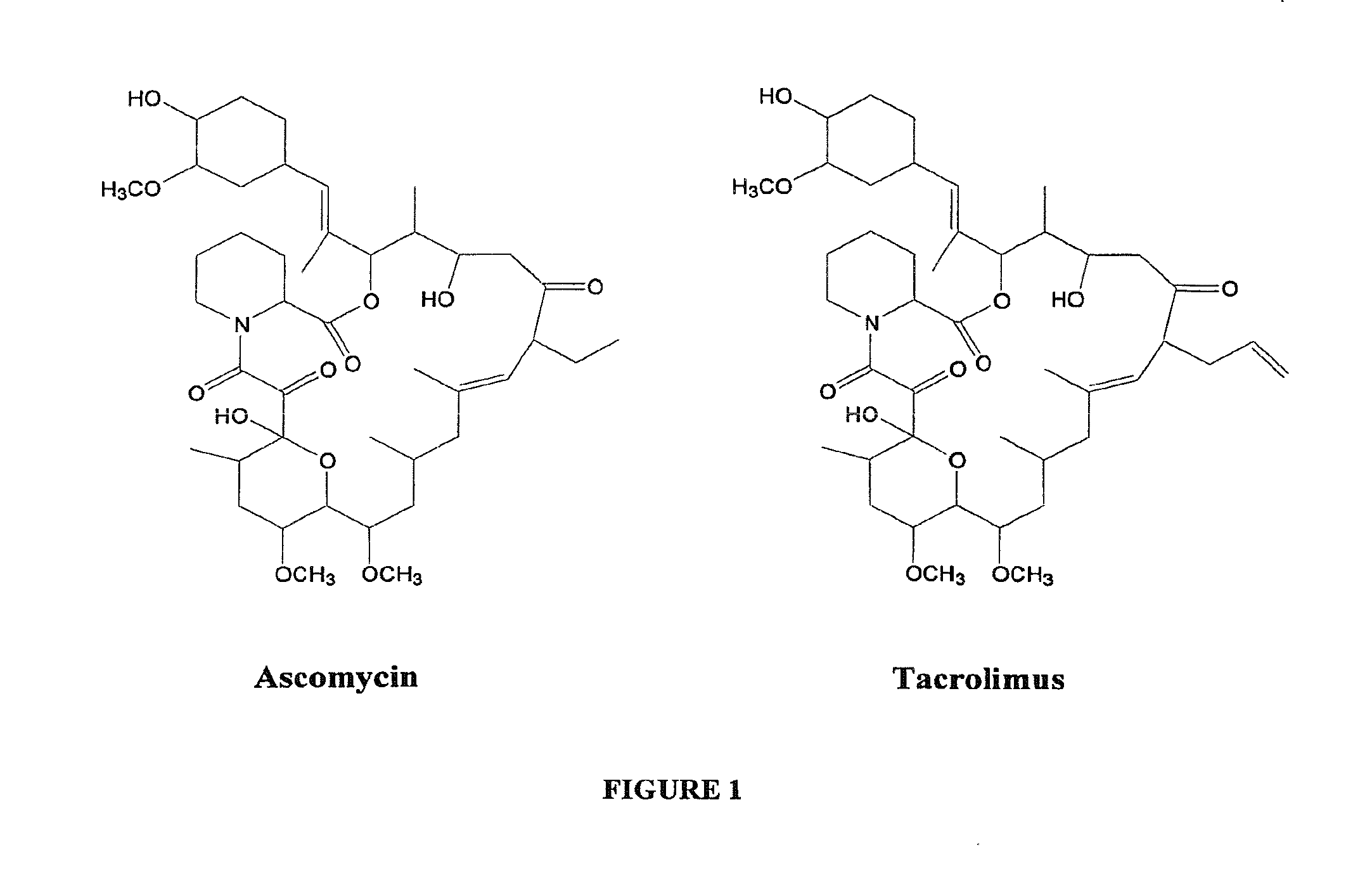
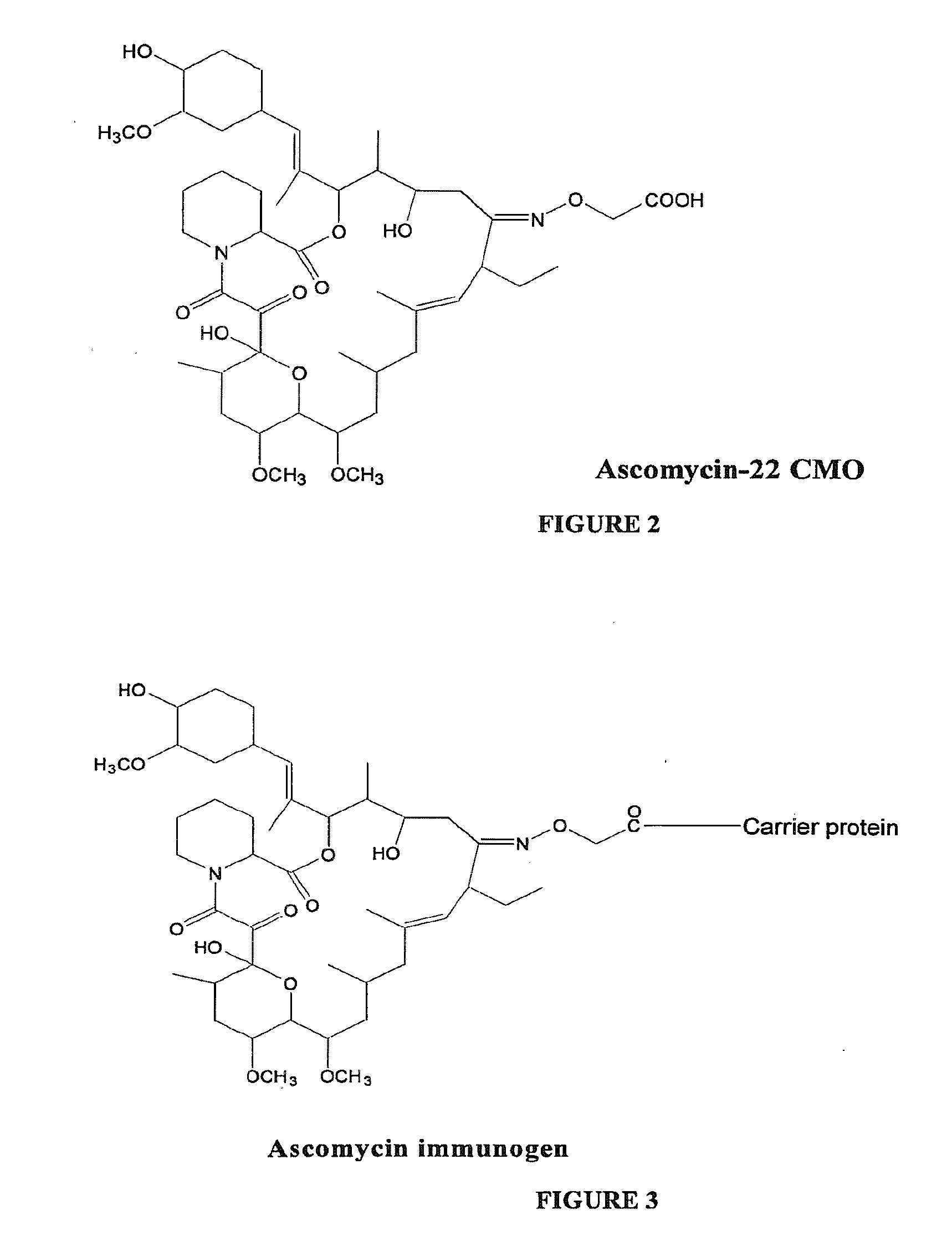
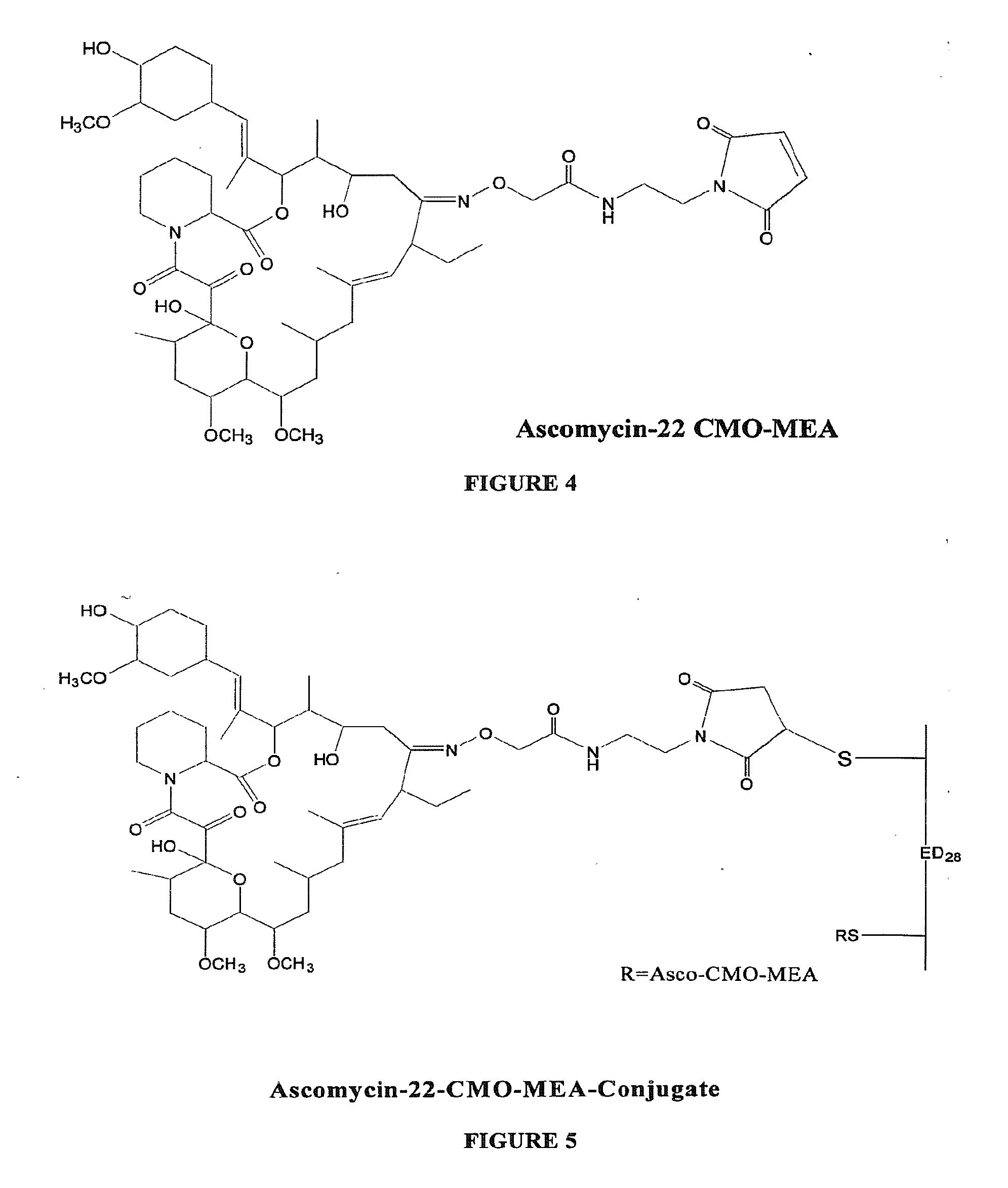
Hapten, Immunogens and Derivatives of Ascomycin Useful for Preparation of Antibodies and Immunoassays
The invention teaches derivatives of ascomycin and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of tacrolimus in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to tacrolimus with cross-reactivity of no more than 5% with each of 15-O-demethyl tacrolimus, 31-O-demethyl tacrolimus, and 13,31-O-didemethyl tacrolimus, less than 40% with 13-O-demethyl tacrolimus, and less than 1% with cyclosporin, rapamycin, mycophenolic acid, prednisone, hydrocortisol, and prednisolone are described. Further, immunoassays for measuring the concentration of tacrolimus using such antibodies are taught.
Owner:MICROGENICS CORP
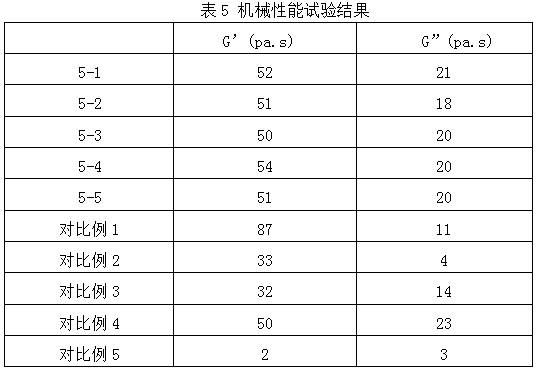
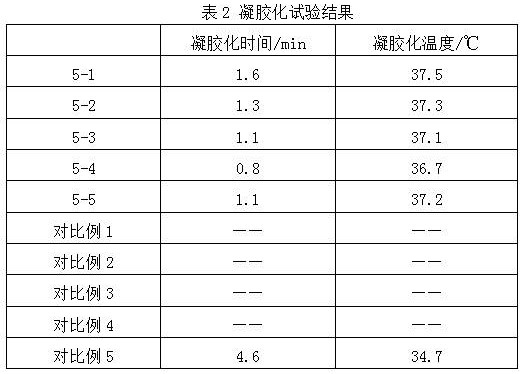
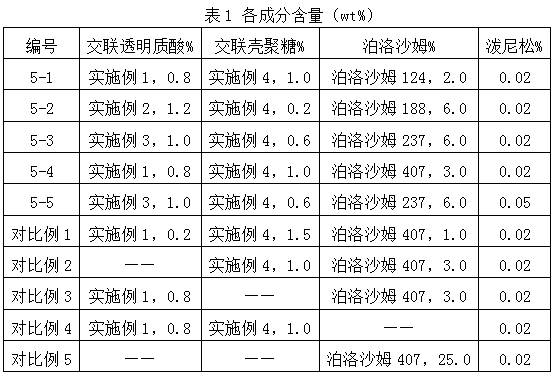
Hyaluronic acid-chitosan thermosensitive hydrogel loaded with prednisone and preparation method thereof
ActiveCN112516075AImprove biodegradation rateReduced release rateOrganic active ingredientsAntipyreticHuman bodyJoint cavity
The invention discloses hyaluronic acid-chitosan thermosensitive hydrogel loaded with prednisone and a preparation method thereof. The thermosensitive hydrogel is prepared from 0.8%-1.2% of cross-linked hyaluronic acid, 0.2%-1.0% of cross-linked chitosan, 0.01%-0.05% of prednisone and 2.0%-6.0% of poloxamer. The cross-linked hyaluronic acid and the cross-linked chitosan are used as main hydrogel matrixes, an articular cavity can be lubricated, the filling and supporting effects are achieved, the biodegradation rate and the release rate of the anti-inflammatory drug prednisone are decreased, and symptoms such as knee joint pain can be relieved and treated for a long time. According to the thermosensitive hydrogel, the dosage of the poloxamer is reduced, the gelation time of the obtained hydrogel is short, the gelation temperature is close to the body temperature of a human body, and articular cavity injection is easier to carry out.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Anti-cancer sustained release agent loaded with glucocorticoid and chemical curing medicine
InactiveCN101450036AInhibition formationImprove permeabilitySolution deliveryPharmaceutical non-active ingredientsPolyesterAdditive ingredient
A anti-cancer slow-release agent co-loaded with glucocorticosteroid and chemotherapy medicament is a slow-release injection agent composed of slow-release microsphere and solvent, wherein, the slow-release microsphere comprises anti-cancer effective ingredient and slow-release auxiliary materials, the solvent is the special solvent containing suspending agent. The glucocorticosteroid is selected from prednisolone, methylprednisolone, dexamethasone, betamethasone, omcilon or triamcinoloneAcetonide, the chemotherapy medicament is selected from phosphoinositide 3-kinase restrainer and pyrimidine analogue or the like; the slow-release auxiliary materials are polylactic acid and copolymer thereof, polyethyleneglycol, polylactide-COOH copolymer, 2-aliphatic acid, sebacic acid polyester, poly(erucic acid dimmer-sebacic acid), poly(fumaric acid-sebacic acid), polyphenyl and polylactic acid or the like biocompatibility high molecules; the suspending agent viscosity is 100cp-3000cp (20 DEG C-30DEG C) and the suspending agent is selected from sodium carboxymethyl cellulose. The anti-cancer effective compositin and the slow-release microsphere can be made into a slow-release implantation agent, by intra-tumor injection or tumor circumference injection or arrangement, the tumor growth can be effectively inhibited, the edema can be alleviated, and the curative effects of the chemotherapy and the radiation therapy can be reinforced.
Owner:JINAN KANGQUAN PHARMA TECH +1
Traditional Chinese medicinal preparation for treating nephritis
InactiveCN102552716ASignificant effectGood for nourishing qi and nourishing yinOrganic active ingredientsAnthropod material medical ingredientsAchyranthes bidentataNephrotic syndrome
The invention relates to a traditional Chinese medicinal preparation for treating nephritis and is characterized in that the traditional Chinese medicinal preparation is prepared from the following raw materials of: by weight, pseudostellaria root, heydyotis, achyranthes bidentata bl, lily, Chinese yam, nequim, Alisma Orientalis, tortoiseshell, anemarrhena, Cortex moutan, phellodendron, cicada slough, lumbricus, muscardine silkworm, couchgrass root, broom cypress fruit, silver flower, Japanese honeysuckle stem, pseudo-ginseng, pyrrosia leaf, a pair of Gekko gecko and 200 Prednisone tablets. The traditional Chinese medicinal preparation is prepared by the following steps of: uniformly mixing the above dried Chinese herbal medicines, crushing, grinding the Prednisone tablets into a powder, mixing the Chinese herbal medicinal powders with the Prednisone powder, sieving through a sieve of 180 meshes, and packaging into capsules. The traditional Chinese medicinal preparation for treating nephritis has good efficacy of supplementing qi and nourishing yin and stimulating blood circulation to end stasis and diuresis, and has good curative effects of treating nephritis, edema of nephrotic syndrome, proteinuria and the like. At present, the traditional Chinese medicinal preparation has been used to treat a thousand of patients with nephritis and nephrotic syndrome during the recovery period. The effective rate reaches 95.2% and the cure rate reaches 90%.
Owner:张成林
Medicination for relieving cough and asthma
InactiveCN1562317ARestoration of adaptive functionAdaptation eliminationUnknown materialsRespiratory disorderDiseaseTracheitis
A medicine for treating cough, asthma, branchial asthma, tracheitis, pneumonectasis and pneumocardial disease is prepared from 7 Chinese-medicinal materials including ephedra, almond, tangerine peel, etc, to clase, prednisone, etc.
Owner:李文章
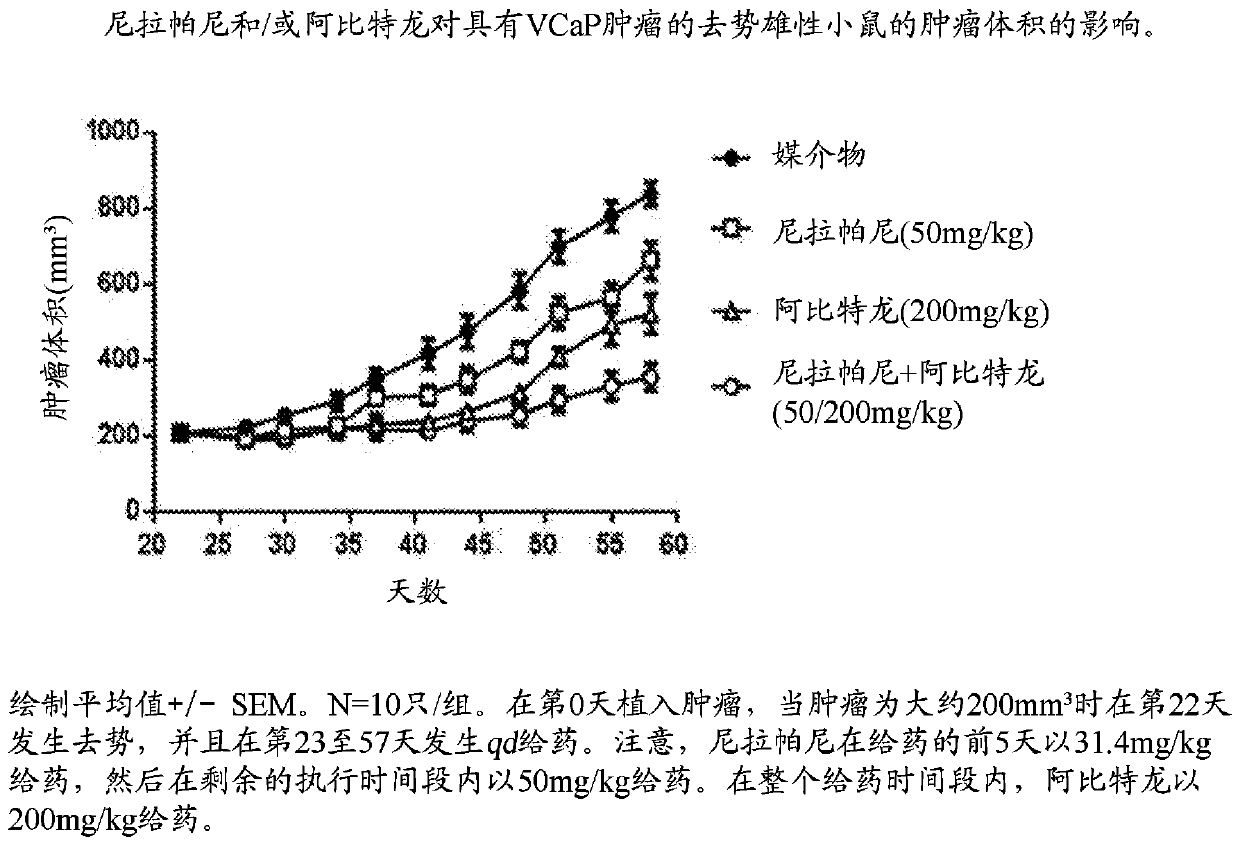
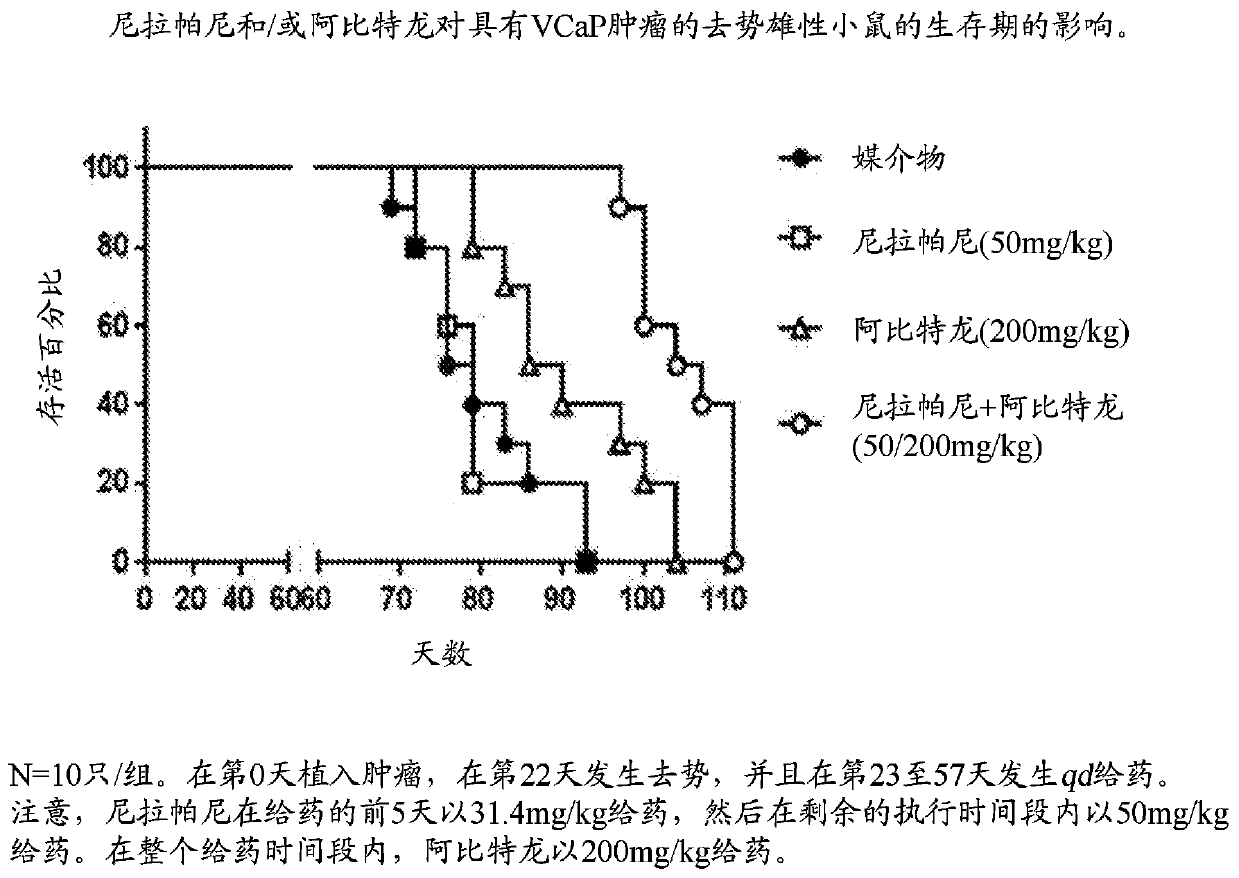
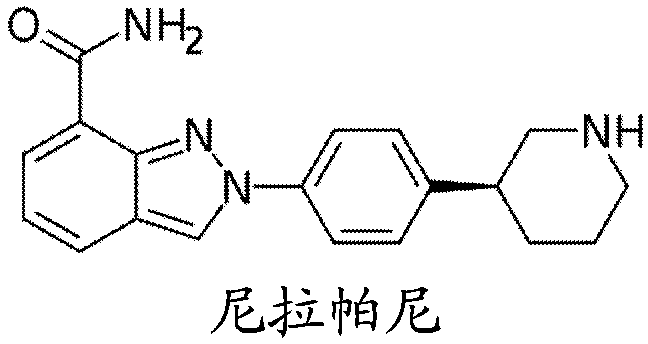
Combination therapy for prostate cancer
Provided are methods and compositions, for treating prostate cancer by administering to a patient in need thereof a therapeutically effective amount of a PARP inhibitor, e.g., niraparib; a therapeutically effective amount of a CYP17 inhibitor, e.g., abiraterone acetate, and a therapeutically effective amount of a glucocorticoid, e.g., prednisone.
Owner:JANSSEN PHARMA NV
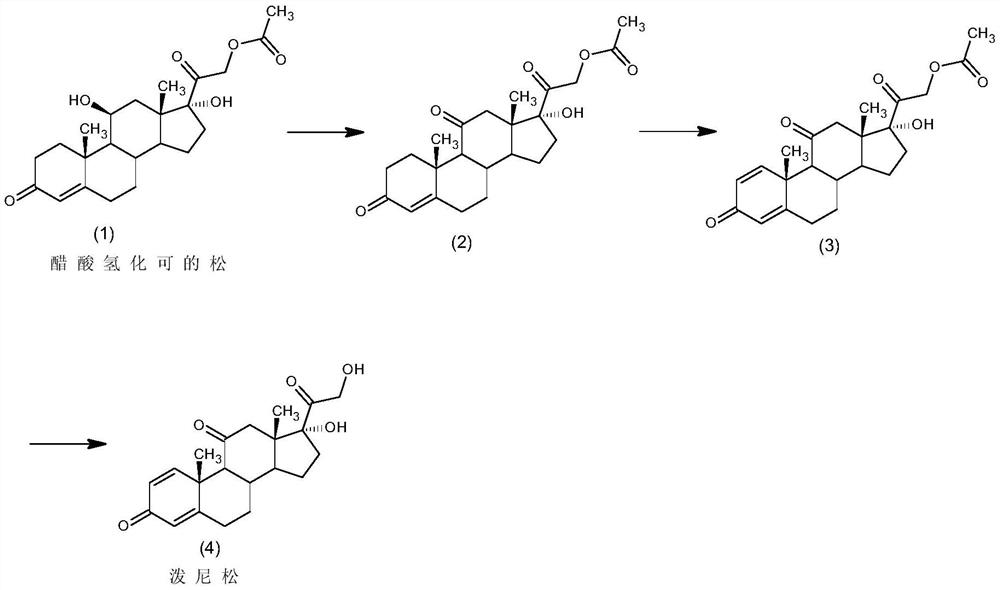
Preparation method of prednisone
ActiveCN111777654ALower requirementLow running costMicroorganism based processesSteroidsBiotechnologyDehydrogenation
The invention discloses a preparation method of prednisone, and belongs to the technical field of preparation and processing of medicines. According to the method, hydrocortisone acetate is used as aninitial raw material, and the prednisone is prepared through three steps of oxidation, biological fermentation dehydrogenation and hydrolysis. According to the preparation method of prednisone, the defects of a traditional process are overcome, the target product is high in purity, good in quality stability, high in yield, low in production cost and mild in reaction condition, a highly toxic cyanide reagent is prevented from being used, and the method is easy and convenient to operate, suitable for industrial production and wide in market prospect.
Owner:ZHEJIANG SHENZHOU PHARMA
Natural method for eliminating HIV to be employed by type 1 and 2 HIV sufferers
A defined natural procedure that lists a series of steps that will effectively eliminate HIV from the victim's body in 2 months. The procedure starts with prayer to the God of Abraham for healing which continues throughout the process. It includes treating the yeast infection with a physician prescribed antifungal product and prednisone 7-day step down regimen (if needed). The elimination of refined sugar products and the inclusion of daily cardiovascular and weight bearing exercise will cut off the HIV's food supply. The final steps involve performing daily internal cleanse to detoxify the body, abstaining from sexual intimacy, and consuming a combination of foods in the form of a salad for breakfast with the remaining meals calorie restricted. With the HIV virus eliminated from the victim's body, the immune system will rebuild / replenish itself to the point where it can effectively combat opportunistic diseases.
Owner:LUCKETT DENESSA ROCHELLE
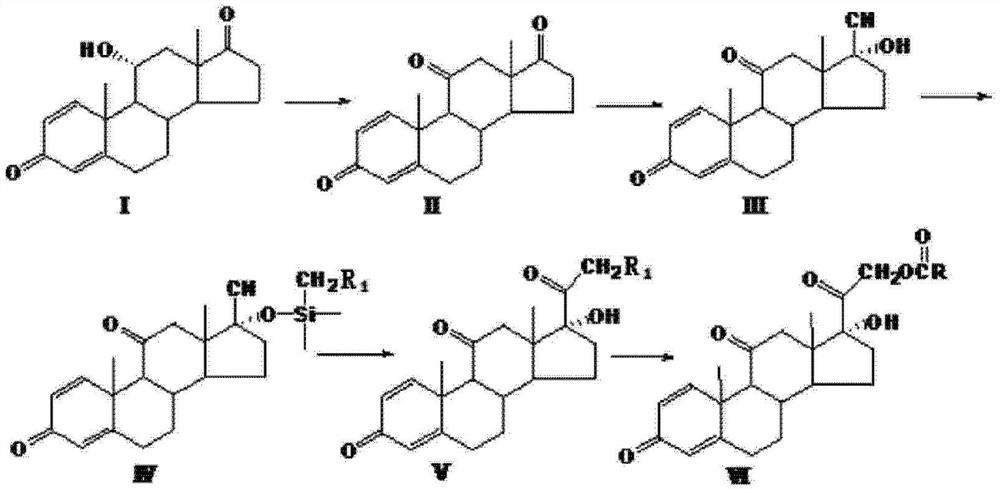
Method for preparing steroide compound 17-alpha ester
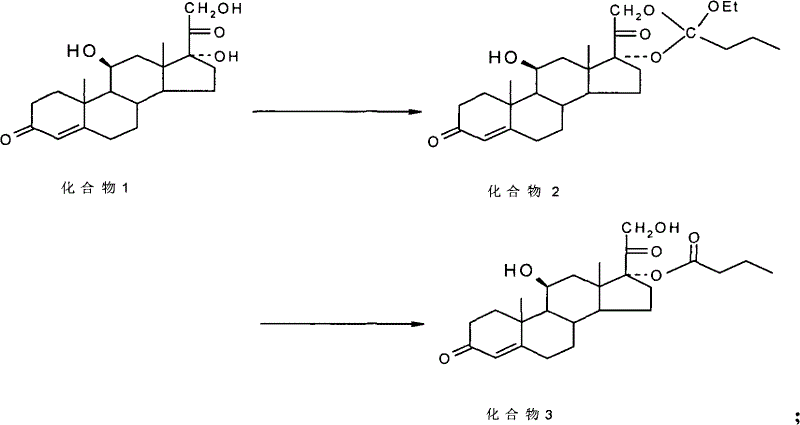
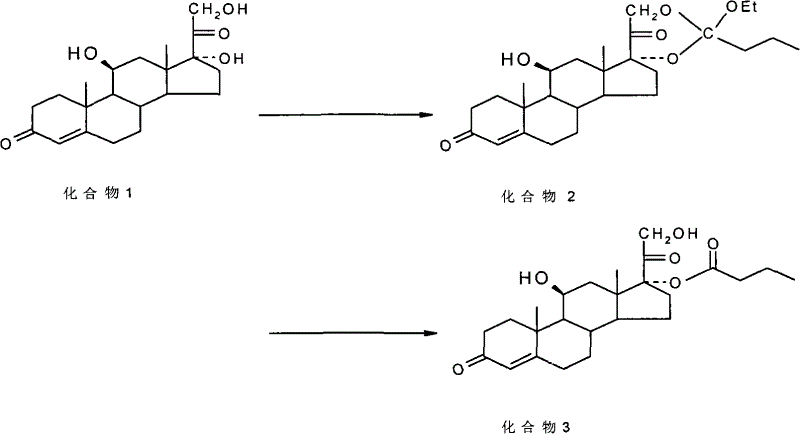
ActiveCN101891797BMake up for the impactPromote hydrolysisSteroids preparationCompound 17Prednisolone
The invention discloses a method for preparing a sterides compound 17-alpha ester. The method comprises the following steps of: dissolving 17 alpha, 21-dihydroxyl sterides compound serving as a raw material in a cyclic ester solvent; adding an ester and a catalyst into the mixture and reacting the mixture to prepare a cyclic ester; and dissolving the prepared cyclic ester in a hydrolysis solvent,adding an orientation reagent and a hydrolysis reagent into the mixed solution and hydrolyzing the cyclic ester into the 17-alpha ester. The sterides compound is hydrocortisone, prednisolone, prednisone, hexadecadrol or betamethasone. The method has selective hydrolysis effect,, and can effectively enhance the conversion rate of a product, greatly increase product yield and greatly promote the preparation capability of a sterides medicament.
Owner:ZHEJIANG XIANJU PHARMA
Abiraterone acetate tablets and preparation method thereof
InactiveCN108096253ALess impuritiesReduce allergic reactionsOrganic active ingredientsPharmaceutical non-active ingredientsCross-linkDocetaxel
The invention belongs to the technical field of biological pharmacy and discloses abiraterone acetate tablets and a preparation method thereof. The abiraterone acetate tablets are combined with prednisone to treat metastatic castration resistant prostate cancer patients who receive docetaxel-combined chemotherapy in the past. The abiraterone acetate tablets are prepared from dehydroepiandrosteroneacetate as a raw material and auxiliary materials including 3-pyridyllithium, 3-pyridineboronic acid, hydroxypropylcellulose, sodium lauryl sulfate, starch, microcrystalline cellulose, cross-linked povidone, magnesium stearate and silica. The prepared abiraterone acetate tablets contain fewer impurities and are purer, allergic reactions caused by impurity doping in the preparation process are reduced, and receivers are increased.
Owner:XUZHOU COLLEGE OF INDAL TECH
Methods of Treating Multiple Myeloma
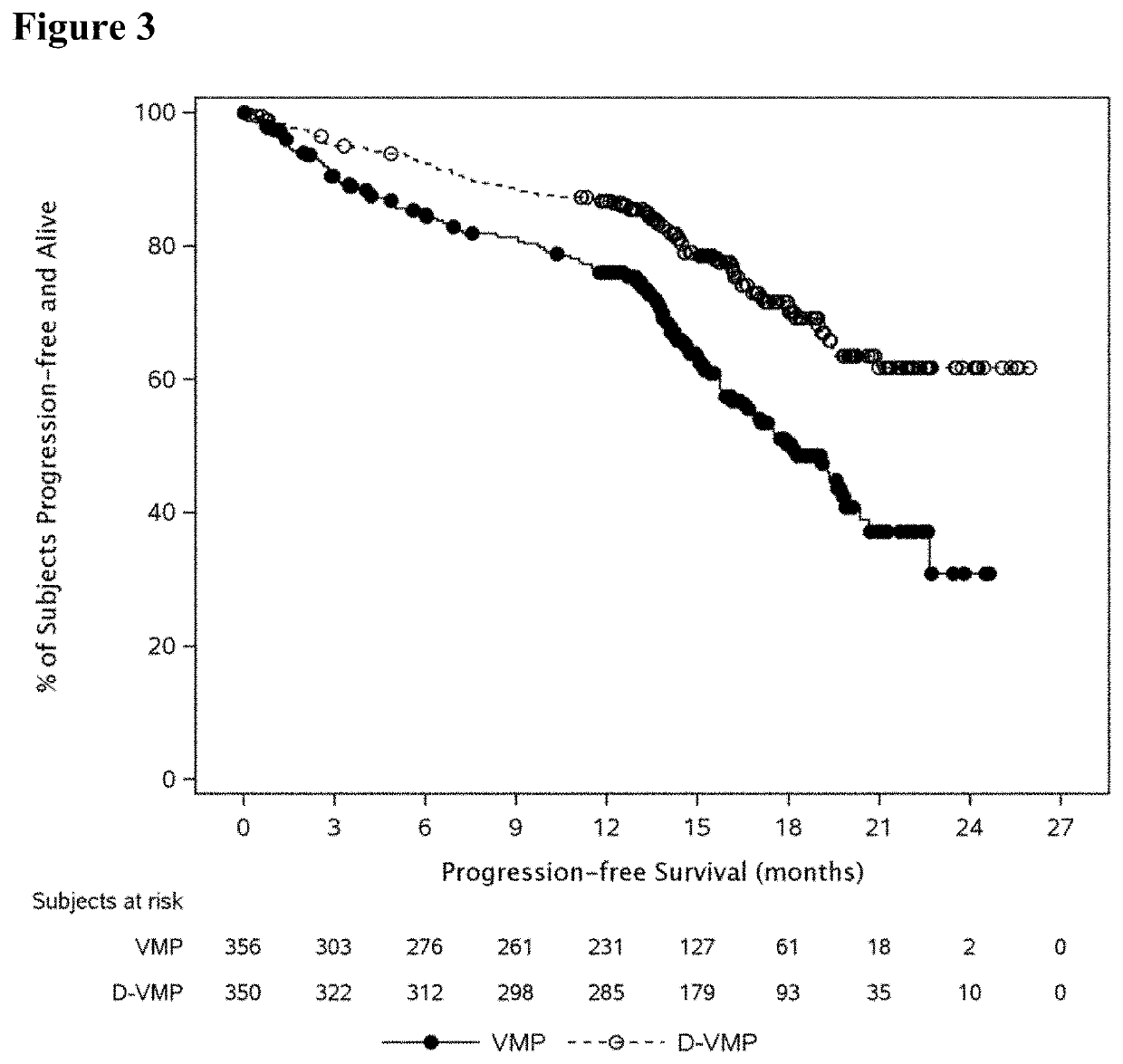
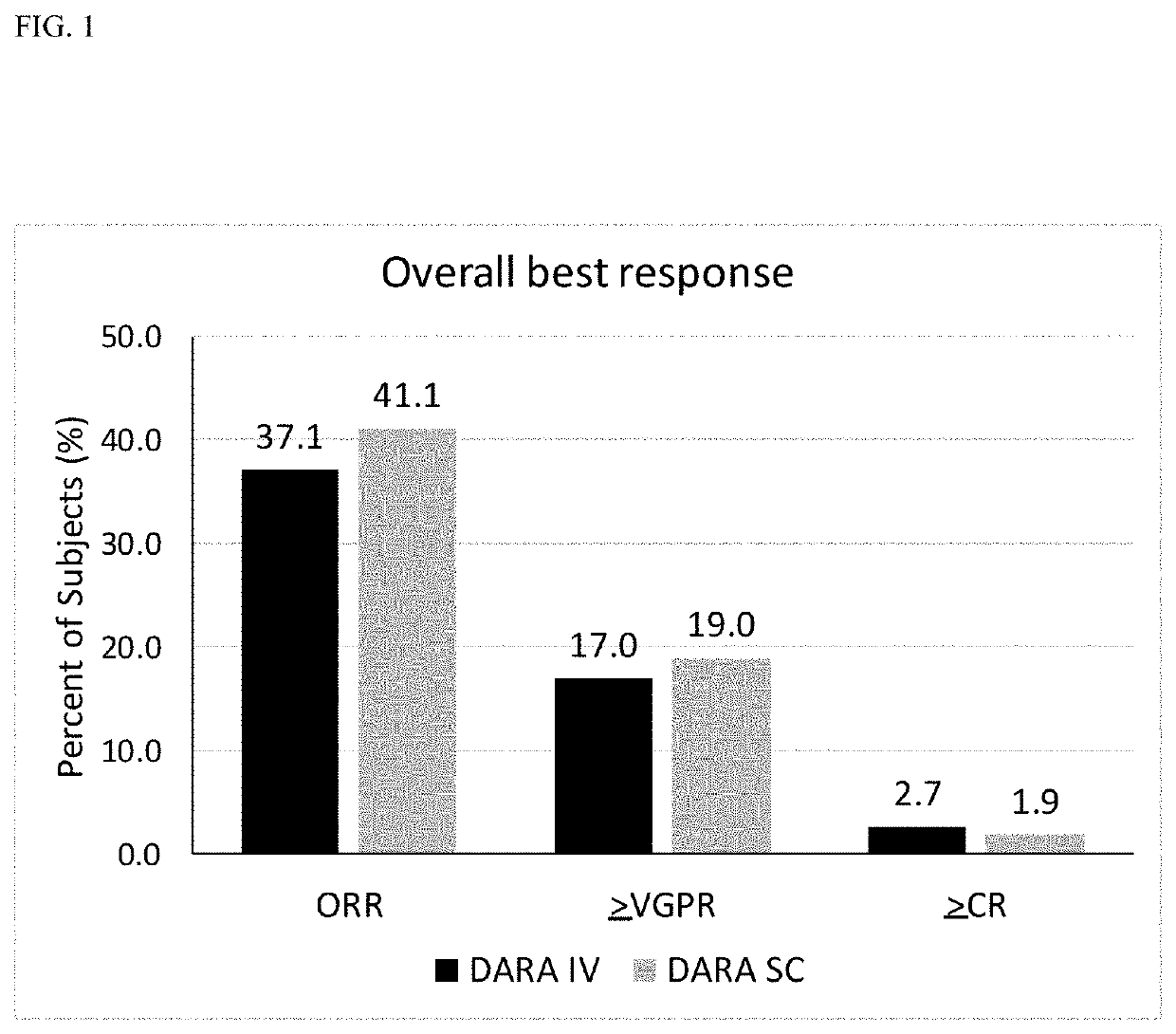
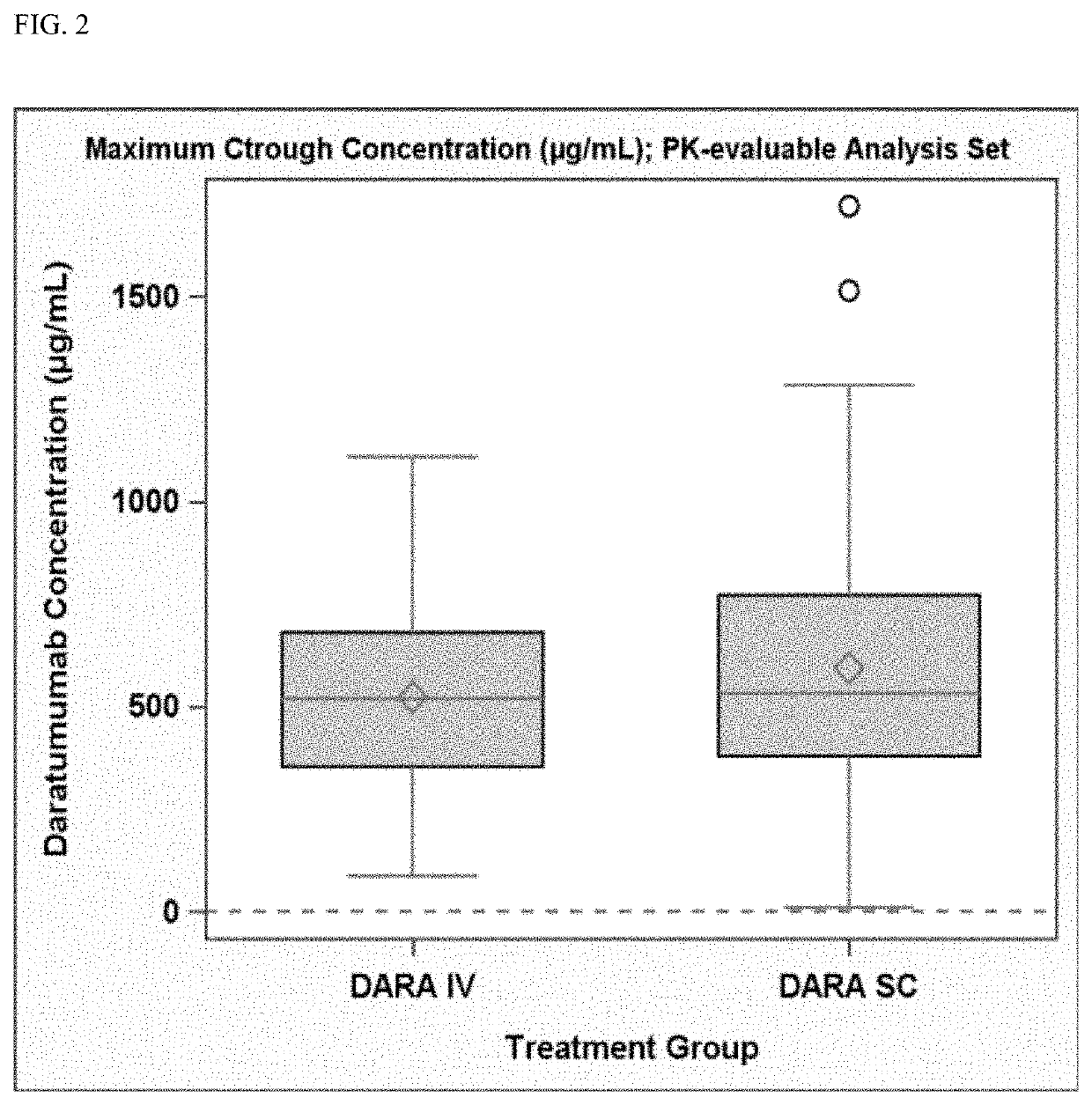
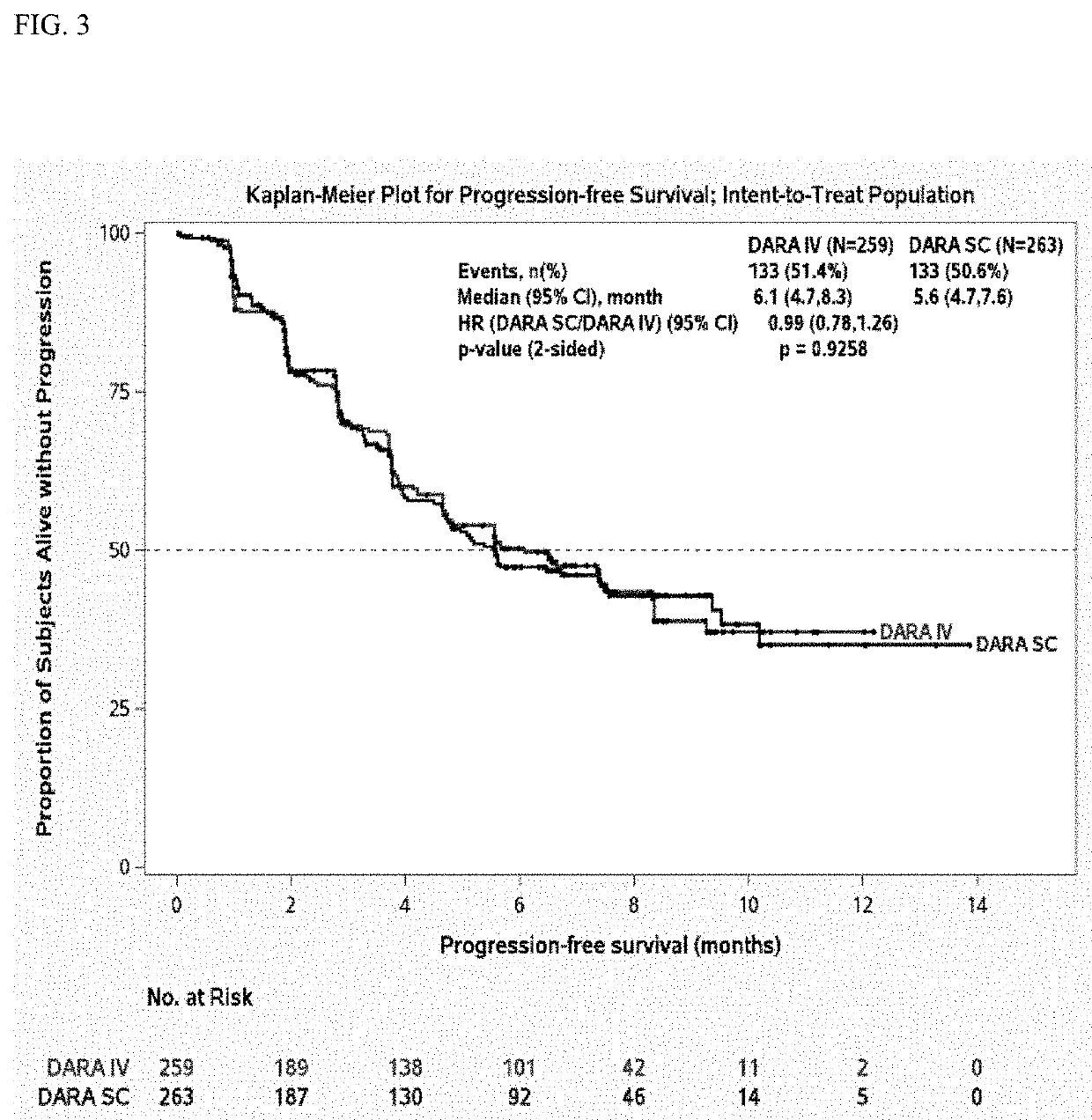
InactiveUS20190298827A1Prolonged progression-free survivalHigh response rateInorganic non-active ingredientsBoron compound active ingredientsCancer researchPrednisone
Described herein are methods of treating multiple myeloma with clinically proven safe and effective amounts of an antibody that specifically recognizes CD38 with bortezomib, melphalan, and prednisone. Also described are methods of selling or offering for sale an antibody that specifically recognizes CD38 or pharmaceutical compositions thereof with bortezomib, melphalan, and prednisone.
Owner:JANSSEN BIOTECH INC
Vaccine for treating and/or preventing type I diabetes and application thereof
ActiveCN106668852AEffective treatmentRaise the ratioMetabolism disorderAntibody ingredientsIMMUNE SUPPRESSANTSCD8
The invention discloses a vaccine for treating and / or preventing type I diabetes and an application thereof. The active ingredients of the vaccine are as follows: 1) or 2) or 3): 1) proteantigen of type I diabetes and an immunosuppressor; 2) an epitope peptide of the proteantigen of type I diabetes and the immunosuppressor; and 3) the proteantigen of type I diabetes, the epitope peptide of the proteantigen of type I diabetes and the immunosuppressor, wherein the proteantigen of type I diabetes is at least one of insulin, glutamate decarboxylase and islet amyloid polypeptide; and the immunosuppressor is at least one of dexamethasone, cyclosporin A, tacrolimus, mycophenolate mofetil, azathioprine, prednisone, early-group prednisolone, anti-CD4 monoclonal antibody and anti-C3 monoclonal antibody. The composition can promote Treg proliferation, accelerate secretion of IL-10 and TGF-beta, control the blood glucose level, inhibit the killing action of autoimmune responsive CD8 T cells, inhibit DC cell maturation, and induce immunosuppression so as to effectively treat type I diabetes.
Owner:ADVACCINE SUZHOU BIOPHARMACEUTICALS CO LTD
Usage of semen ziziphi spinosae oil in the treatment of medicinal insomnia
The present invention involves the application of Semen Ziziphi Spinosae oil in the preparation of drugs for treating medicinal insomnia. Semen Ziziphi Spinosae oil has a therapeutic effect on insomnia caused by ephedrine, prednisone and other drugs, and Semen Ziziphi Spinosae oil that is prepared within the pressing temperature of 80 to 100° C. has the best efficacy for treating medicinal insomnia.
Owner:SHIJIAZHUANG YILING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com