Method for preparing steroide compound 17-alpha ester
A technology of steroidal compound and hydrocortisone, which is applied in the field of preparation of steroidal compound 17α-ester, can solve the problems such as unresolved reaction selectivity, and achieve the effects of improving hydrolysis conversion rate, mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
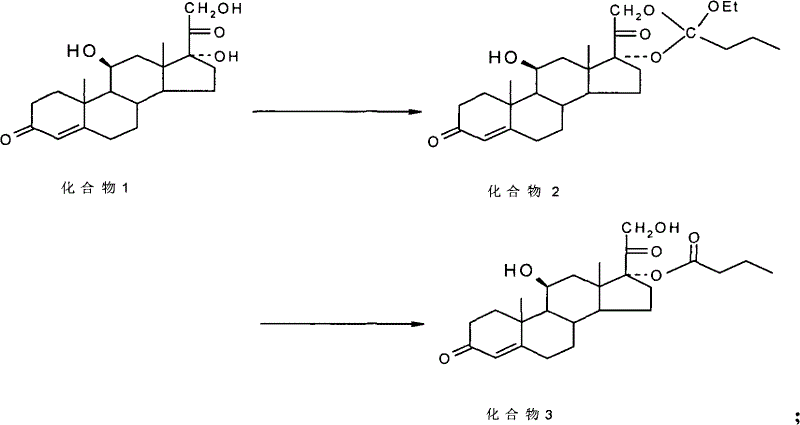
[0027] The preparation method of steroidal compound 17α-ester of the present invention, to have 17α, 21-dihydroxy steroid compound (as analogs such as hydrocortisone, prednisolone, prednisone, dexamethasone, betamethasone) As a raw material, dissolve the raw material in a cyclic ester solvent (such as dioxane), add triethyl orthobutyrate (or trimethyl orthobutyrate, or one of the original esters with seven carbons or less), and then add Catalyst (such as p-toluenesulfonic acid, naphthalenesulfonic acid, benzenesulfonic acid, or sulfo-salicylic acid, etc.) to react to form a cyclic ester; dissolve the produced cyclic ester in a hydrolysis solvent (such as methanol) , adding an orientation reagent (such as ammonium chloride aqueous solution), and then adding a hydrolysis reagent (such as aluminum trichloride aqueous solution), hydrolyzed into 17α-ester.
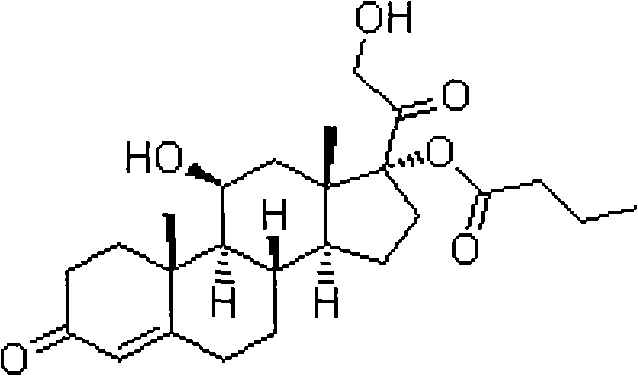
[0028] Adopt the present invention to prepare hydrocortisone butyrate, use hydrocortisone (compound 1) as raw material, obtai...
Embodiment 1
[0035] Put 40L dioxane and 4Kg hydrocortisone into a 200L cyclic ester reaction tank, stir and dissolve for 10 minutes, adjust the temperature to 20°C, add 4.4L triethyl orthobutyrate, then add 40.0gPTS, and stir for 2.5 hours. TLC showed the reaction was complete.
[0036] Heat up and concentrate under reduced pressure until nearly dry, add 20L of saturated sodium bicarbonate solution, stir for 10 minutes, then add 160L of ice water to fully stir and harden; when fine off-white solids are seen after dispersion, let stand for about 10 hours.
[0037] Centrifuged tap water was fully washed, and the weight of the wet product was 6.1Kg after drying. Put the obtained wet product into a 300L hydrolysis tank, add 180L of industrial methanol, adjust the temperature to about 20°C, stir to dissolve, add 20L of ammonium chloride saturated aqueous solution, and then add 50L of 0.28% aluminum trichloride aqueous solution, at 20 Stir for 1.5 hours at °C. TLC showed the reaction was compl...
Embodiment 2
[0040] Put 40L dioxane and 4Kg hydrocortisone into a 200L cyclic ester reaction tank, stir and dissolve for 10 minutes, adjust the temperature to 20°C, add 4.4L triethyl orthobutyrate, then add 40.0gPTS, and stir for 2.5 hours. TLC showed the reaction was complete.
[0041] Heat up and concentrate under reduced pressure until nearly dry, add 20L of saturated sodium bicarbonate solution, stir for 10 minutes, then add 160L of ice water to fully stir and harden; when fine off-white solids are seen after dispersion, let stand for about 10 hours.
[0042] Centrifuged tap water was fully washed, and the weight of the wet product was 6.2Kg after drying. Put the obtained wet product into a 300L hydrolysis tank, add 180L of industrial methanol, adjust the temperature to about 20°C, stir to dissolve, add 20L of ammonium chloride saturated aqueous solution, and then add 50L of 0.28% aluminum trichloride aqueous solution, at 20 Stir for 1.5 hours at °C. TLC showed the reaction was compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com