Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
182 results about "Glyceryl behenate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glyceryl behenate is a fat used in cosmetics, foods, and oral pharmaceutical formulations. In cosmetics, it is mainly used as a viscosity-increasing agent in emulsions. In pharmaceutical formulations, glyceryl behenate is mainly used as a tablet and capsule lubricant and as a lipidic coating excipient. It has been investigated for the encapsulation of various drugs such as retinoids. It has also been investigated for use in the preparation of sustained release tablets as a matrix-forming agent for the controlled release of water-soluble drugs and as a lubricant in oral solid dosage formulations. It can also be used as a hot-melt coating agent sprayed onto a powder.
Cleansing oil and preparation method thereof
ActiveCN107007492AGood effectDoes not cause allergiesCosmetic preparationsMake-upAntioxidantSkin irritation
The invention discloses a cleansing oil, which comprises the following components in parts by weight: 50-85 parts of grease, 5-30 parts of a polyglycerol emulsifying agent, 0.1-3 parts of phytosteryl / docosyl / octyldecanol lauryl glutamate, 0.1-4 parts of glyceryl behenate / eicosadioate, 0.1-5 parts of water, 0.01-0.2 part of an antioxidant, 0.01-0.2 part of a skin conditioner, 0.01-1.0 part of an essence, and 0.001-2 parts of a plant extractive solution. Through the addition of the special content of phytosteryl / docosyl / octyldecanol lauryl glutamate and the special content of glyceryl behenate / eicosadioate into plant-derived polyglycerol emulsifying agent and grease, the cleansing oil of the invention has excellent moisturizing and skin caring effects, but is not greasy, and does not cause skin allergy and irritation. Meanwhile, the cleansing oil has thixotropic property, so as to provide unique silk-like feeling during face skin massage.
Owner:HUAANTANG BIOTECH GRP CO LTD
Sirolimus formulation
InactiveUS8053444B2Composition is stableRelease rate is not affectedBiocidePowder deliveryPolymerGlyceryl behenate
The present invention relates to a stable pharmaceutical composition that includes sirolimus. The pharmaceutical composition includes sirolimus in the amorphous form, a fatty acid ester, such as glyceryl behenate, and a pharmaceutically acceptable polymer wherein the fatty acid ester is present at a concentration of less than 10% w / w compared to the total weight of the composition.
Owner:LEK PHARMA D D
Lip stick
InactiveUS20120308500A1Easy to spreadGood curative effectCosmetic preparationsMake-upJojoba esterDETANONOate
Disclosed is an anhydrous lipstick comprising a combination of active ingredients comprising Portulaca pilosa extract, sunflower oil, jojoba esters, mango butter, and tocopherol and a dermatologically acceptable carrier comprising glyceryl behenate / eicosadioate.
Owner:MARY KAY INC
Cefpodoxime proxetil suspension composition and preparation thereof
ActiveCN101278914AImprove bitternessEasy to acceptAntibacterial agentsPowder deliveryMedicineCefpodoxime Proxetil
The invention relates to a cefpodoxime proxetil dry suspension composition and a preparation method thereof. The invention provides the cefpodoxime proxetil dry suspension composition, containing 100 parts by weight of the cefpodoxime proxetil and 20 to 500 parts by weight of the glyceryl behenate. The invention also provides the preparation method of the dry suspension composition. The dry suspension composition of the invention is capable of reducing bitterness of the cefpodoxime proxetil remarkably and improving compliance of a clinical patient, thereby being suitable for a child to take a drug.
Owner:海南三叶美好制药有限公司
Oral prednisone time-selecting release preparation and preparation method thereof
InactiveCN103690545AGive full play to the therapeutic effectImprove balanceOrganic active ingredientsAntipyreticCelluloseFormulary
The invention discloses an oral prednisone time-selecting release preparation and a preparation method thereof. The oral prednisone time-selecting release preparation provided by the invention mainly consists of 0.3-5 parts of prednisone and derivatives thereof, 10-50 parts of glyceryl behenate and 3-30 parts of hydroxypropyl cellulose, and can further contain a disintegrating agent and other pharmaceutically acceptable excipients. The preparation method is as below: extruding tablet cores or granules containing the drug according to the formula by a tablet press or a dry granulator; and coating the tablet cores or particles containing the drug by a coating pan or a fluidized bed to attach the coating film to the tablet cores or particles containing the drug, so as to obtain the oral prednisone time-selecting release preparation. The oral prednisone time-selecting release preparation provided by the invention can achieve a good balance between the biological rhythm of the patients and the curative effects, and is safer, more convenient and effective compared with a traditional preparation. The oral prednisone time-selecting release preparation is prepared by an extrusion-coating process, which is simple for operation, and the obtained time-selecting release preparation has the advantages of drug stability and high reproducibility.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Waterproof liquid foundation formula and preparation method thereof
InactiveCN110051553ANon-irritatingHas the effect of isolating external damageCosmetic preparationsBody powdersSkin complexionGlycerol
Owner:广州蔓缇化妆品有限公司
Use of a matrix for orally administering sustained release magnesium, and composition containing said matrix
A tablet for oral administration comprises a matrix of progressive and continuous released magnesium. For the administration of 90 to 110 parts by weight of magnesium, the matrix comprises 180 to 190 parts by weight of hydroxypropylmethylcellulose, 19.8 to 22.2 parts by weight of glyceryl behenate, 10 to 12 parts by weight of lactose and 10 to 12 parts by weight of colloidal silica. A non-enteric protective coating that slows down the gastric dissolution of the magnesium may comprise 15 to 75 parts by weight of shellac, cellulose ether or a mixture thereof. The tablet may be administered to patients in need thereof.
Owner:JOANNY FABIENNE
Efficient seed germination promoting agent
InactiveCN105053056AIncrease productionReduce breedingBiocidePlant growth regulatorsPhosphatePurslane extract
An efficient seed germination promoting agent is characterized by being prepared from the following components in parts by weight: 90 to 110 parts of magnetized water, 1.5 to 2.5 parts of tea saponin, 1 to 2 parts of purple sweet potato anthocyanin, 1 to 2 parts of compound sodium nitrophenolate, 1.5 to 2.5 parts of purslane extract, 4 to 6 parts of alanine, 3 to 5 parts of potassium dihydrogen phosphate, 1 to 2 parts of borneol, 4 to 6 parts of tea water, 4 to 6 parts of 25% carbendazim wettable powder, 1.5 to 2.5 parts of ethanol, 1.5 to 2.5 parts of chelated iron, 0.4 to 0.6 part of glyceryl behenate, 1 to 2 parts of phosphoric buffer solution, 0.4 to 0.6 part of neutral protease, 3 to 5 parts of ammonia water, 1 to 2 parts of fulvic acid, 1.5 to 2.5 parts of gibberellin, 0.4 to 0.6 part of liquid paraffin, 1.5 to 2.5 parts of phoxim, 0.4 to 0.6 part of automobile anti-freezing liquid, 0.3 to 0.5 part of L-glutamic acid, 2 to 3 parts of activator, and 0.4 to 0.6 part of potassium sorbate. The provided seed germination promoting agent is nontoxic and harmless, has a sterilizing effect, and can promote the germination of paddy rice and increase the emergence rate. Moreover, there is no residue.
Owner:CHAOHU XINYU BREEDING FARMER PROFESSIONAL COOP
Light-emitting painted pottery glaze composition
The invention relates to a light-emitting painted pottery glaze composition which is characterized in that the glaze material is prepared from the components of, by weight, 45-55 parts of nano-silica, 25-35 parts of electronic-grade red lead, 0.4-0.6 part of graphene, 0.4-0.6 part of glyceryl behenate, 0.4-0.6 part of hydroxypropyl-beta-cyclodextrin, 0.4-0.6 part of modified corn starch, 4-6 parts of copper oxide, 1-2 parts of polyethylene wax, 0.1-0.3 part of iron oxide, 1-3 parts of potassium feldspar micro-powder, 0.4-0.6 part of silver nitrate, and 1-3 parts of sea mud. The painted pottery fired with the glaze composition has a crystal clear, bright and smooth glaze, and an excellent decorative effect. With the modified sea mud, the glaze never peels from a finished product, and the product can be preserved permanently.
Owner:柳培健
Stable cefaclor tablet composition and preparation method thereof
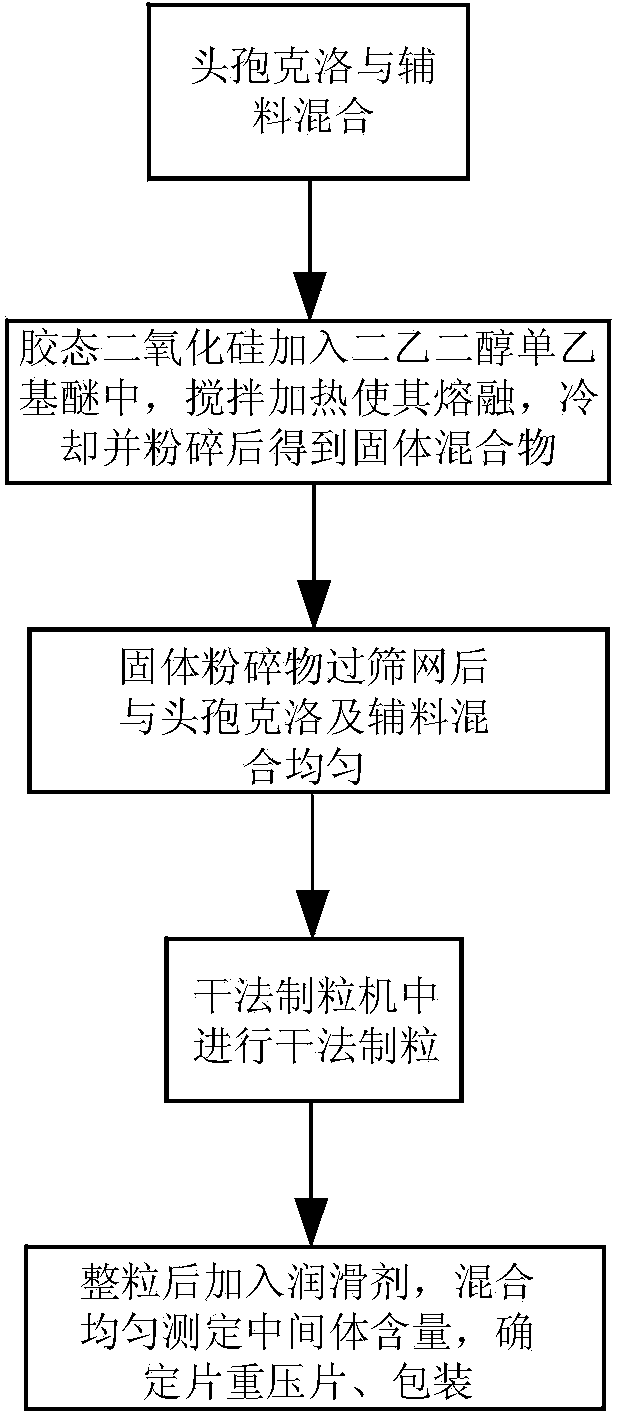
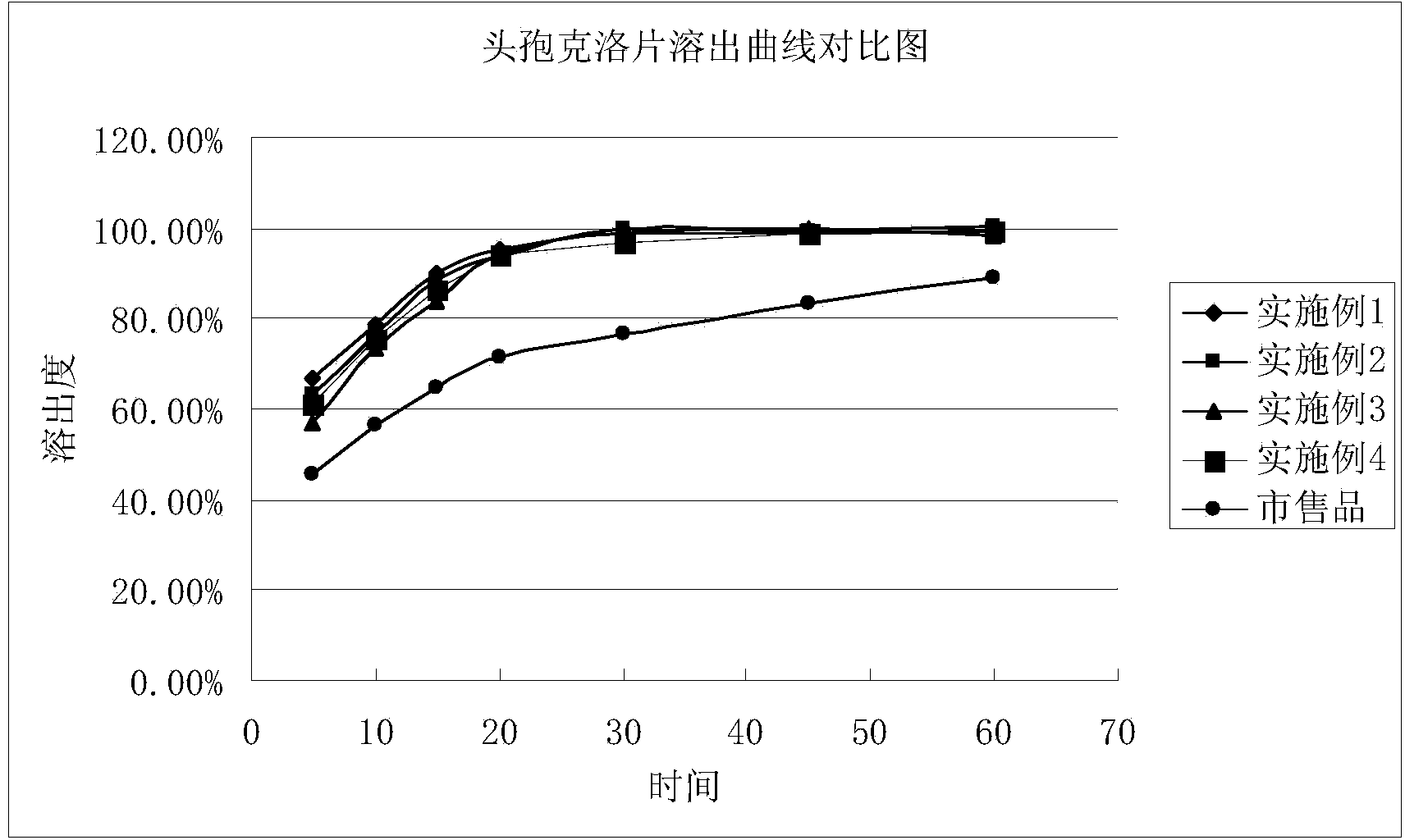
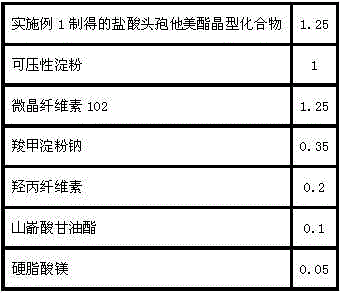
ActiveCN103623412AImprove solubilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsDiethylene glycol monoethyl etherBioavailability
The invention discloses a cefaclor tablet pharmaceutical tablet composition which comprises cefaclor, lactose, microcrystalline cellulose, crosslinked povidone, diethylene glycol monoethyl ether, colloidal silicon dioxide and glyceryl behenate or sodium stearyl fumarate. The preparation method comprises the following steps: evenly mixing the cefaclor with the pharmaceutical auxiliary materials; adding the colloidal silicon dioxide into the diethylene glycol monoethyl ether, heating while stirring for melting, and cooling to obtain a solid mixture; screening, and evenly mixing the cefaclor auxiliary materials; granulating by a dry process; and after finishing the granules, adding the lubricant, evenly mixing, measuring the intermediate content, determining the tablet weight, tabletting and packaging. The invention provides a legal and reasonable cefaclor table composition and a preparation method thereof, thereby obtaining the cefaclor tablet which has the advantages of stabler and more controllable quality, higher bioavailability and simpler technique.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Sustained release tablet for treating cardiovascular diseases and preparation method thereof
ActiveCN103845299AReduce usageAvoid potential harmOrganic active ingredientsPharmaceutical delivery mechanismVascular diseaseSustained Release Tablet
The invention discloses a nifedipine sustained release tablet for treating cardiovascular diseases and a preparation method thereof. Sustained release granules are prepared by adopting low-melting-point sustained release materials including glyceryl behenate, polyethylene glycol 4000 and nifedipine; use of an organic solvent is avoided; the nifedipine sustained release tablet disclosed by the invention is good for the environmental protection; potential hurt to patients caused by residual organic solvents is also avoided; the sustained release materials and active medicines form granules similar to solid dispersoid, so that sustained release of nifedipine is kept for a long time, and furthermore, rapid release of medicines in rapid release granules is not influenced. The nifedipine sustained release tablet disclosed by the invention is prepared by adopting the conventional granulating process; the complex production equipment and the production process are avoided; energy is saved; the production efficiency is greatly increased.
Owner:YABAO PHARMA BEIJING
Medicinal cefetamet pivoxil hydrochloride composition for treating sensitive bacteria infectious diseases
ActiveCN104800221AReduce contentStrong antibacterial activityAntibacterial agentsOrganic active ingredientsBiotechnologyMagnesium stearate
The invention discloses a medicinal cefetamet pivoxil hydrochloride composition for treating sensitive bacteria infectious diseases, and belongs to the field of medical technology. The medicinal cefetamet pivoxil hydrochloride composition is prepared from cefetamet pivoxil hydrochloride, compressible starch, microcrystalline cellulose 102, carboxymethyl starch sodium, hydroxypropylcellulose, glyceryl behenate and magnesium stearate. Experiments discover that compared with the prior art, the tablet prepared by novel crystal type compound of the cefetamet pivoxil hydrochloride is relatively low in high-molecular polymer content and good in stability; the increasing of the content of the high-molecular polymer is quite few with the increasing of storage time; meanwhile, the composition has more remarkable antimicrobial activity on pneumococcus and hemophilus influenzae as well as has relatively strong antimicrobial activity on enterococcus and staphylococcus.
Owner:启东市和洪农副产品专业合作社
Pig raising manure deodorization preparation
InactiveCN105148307AGuaranteed freshGuaranteed living environmentBiocideDisinfectantsPagoda treePumpkin seed
The invention discloses a pig raising manure deodorization preparation. The pig raising manure deodorization preparation is characterized by comprising, by weight, 5 parts of tourmaline powder, 5 parts of glyceryl behenate, 10 parts of stearic acid, 3 parts of theophylline, 3 parts of curcumin, 3 parts of citric acid, 20 parts of lotus leaves, 10 parts of dried tangerine or orange peel, 5 parts of camphor, 10 parts of clove, 5 parts of pumpkin seeds, 5 parts of garland chrysanthemum, 10 parts of licorice, 5 parts of camellia, 5 parts of portulaca oleracea, 3 parts of coptis chinensis, 5 parts of bitter gourd, 3 parts of pagoda tree pod, 3 parts of peppermint roots, 10 parts of chili leaves, 5 parts of tilia bark and 500 parts of distilled water. The pig raising manure deodorization preparation has the advantages that effects of effectively removing smelly substances in pig raising manure can be realized by the pig raising manure deodorization preparation which is a deodorant, the pig raising manure deodorization preparation further has a sterilization function, accordingly, survival environments can be guaranteed to a certain extent while odor of the manure is removed, and the air freshness can be kept; the pig raising manure deodorization preparation is made of raw materials which are easily available, and is low in production and service costs.
Owner:郎溪县凌达养猪专业合作社
A sildenafil citrate taste-masking preparation
A sildenafil citrate taste-masking preparation and a preparing method thereof are disclosed. The sildenafil citrate taste-masking preparation at least comprises, based on the total weight of tablets, 15-30% of sildenafil citrate, 30-65% of hydroxypropyl cellulose and 15-20% of glyceryl behenate. The preparing method includes: mixing a formula dosage of the sildenafil citrate and a formula dosage of the hydroxypropyl cellulose to prepare melt dispersion, cutting the melt dispersion into particles, grinding the particles together with the glyceryl behenate, fully mixing with other auxiliary materials, and tabletting into tablets for oral administration.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Mesalazine enteric-coated sustained-release pellet and preparation method thereof
The invention provides a mesalazine enteric-coated sustained-release pellet, which consists of a slow-release pellet core and an enteric-coated dressing. The slow-release pellet core contains 40-45 wt.% of mesalazine, 45-50 wt.% of a matrix sustained-release composite, 5-10% of a chaotropic agent and 0-5 wt.% of other additives; the matrix sustained-release composite consists of glyceryl behenate and microcrystalline cellulose in the weight ratio of 2-4:1. The invention also provides a preparation method of the mesalazine enteric-coated sustained-release pellet. The method provided by the invention employs a skeleton controlled-release technology to control drug release, reaches ideal drug release rate and good inter-batch reproducibility in preparation, does not have high requirement on equipment, and is in favor of ndustrial production.
Owner:广东暨大基因药物工程研究中心有限公司
Gliclazide tablets (II) and preparation method thereof
PendingCN110585155AEffective controlled releaseAvoid sudden releaseMetabolism disorderSulfonylurea active ingredientsMedicineAdhesive
The invention provides gliclazide tablets (II) and a preparation method thereof, and belongs to the field of pharmaceutical preparations. According to the invention, glyceryl behenate is used as a lipid sustained-release matrix, is used as a sustained-release skeleton at the same time, and is subjected to fluidization granulation, so that solid bridges are formed among the raw materials, raw material powder is bonded, sustained-release particles are formed, the release of gliclazide can be effectively controlled, the phenomenon of sudden release of active ingredients is avoided, and the medication safety is guaranteed. Meanwhile, the glyceryl behenate is subjected to the fluidization granulation, no explosive solvent such as ethanol is required to be used for preparing an adhesive, no residual solvent is generated, and industrial mass production is facilitated.
Owner:SHANDONG LUKANG PHARMA
Moisture-proof coating method for Chinese medicine granule
ActiveCN1857328AReduce dosageImprove stabilityPharmaceutical non-active ingredientsGranular deliveryPrillSilica gel
The present invention relates to moisture-proof coating method for Chinese medicine granule, and belongs to the field of moisture-proof technology for Chinese medicine granule. The method includes mixing Chinese medicine granule in 100 weight portions, glyceryl behenate in 1-5 weight portions and fine silica gel powder in 0.1-1 weight portion through stirring in a homogenizer at 75-80 deg.c for 15-20 min to prepare the moisture-proof coated Chinese medicine granule. The glyceryl behenate has low smelting point and is favorable to taking the coated Chinese medicine granule together with hot water, the fine silica gel powder can increase the flowability of the granule and promote the disintegration of granule, and the combination of glyceryl behenate and fine silica gel powder forms excellent Chinese medicine granule coating material.
Owner:JIANGYIN TIANJIANG PHARMA
Seed treating agent capable of shortening germination time of insect-resistant cotton seeds
InactiveCN105076266AImprove the bactericidal effectSoften fastBiocidePlant growth regulatorsNeutral proteasePolydextrose
The invention provides a seed treating agent capable of shortening germination time of insect-resistant cotton seeds. The seed treating agent is prepared from, by weight, 90-110 parts of spring water, 1.5-2.5 parts of tea saponin, 1-2 parts of purple sweet potato anthocyanin, 1-2 parts of compound sodium nitrophenolate, 1.5-2.5 parts of table salt, 4-6 parts of alanine, 3-5 parts of monopotassium phosphate, 1.5-2.5 parts of highland barley wine, 1.5-2.5 parts of alga micro-powder, 1-2 parts of portulaca oleracea extract, 1.5-2.5 parts of armoise commune ash, 0.4-0.6 part of polydextrose, 0.4-0.6 part of glyceryl behenate, 1-2 parts of witch hazel extract, 0.4-0.6 part of neutral protease, 1.5-2.5 parts of amylodextrin, 1-2 parts of garlic extract, 1.5-2.5 parts of gibberellin, 0.4-0.6 part of potassium permanganate and 0.4-0.6 part of potassium sorbate. A method is simple, actual operation is convenient, the treating fluid capable of shortening germination time has the effects of quick sterilization and seed softening within short time, the influences of bacteria and the like on seed germination are avoided, and meanwhile the seeds can be stimulated to quickly germinate in proper environments.
Owner:CHAOHU XINYU BREEDING FARMER PROFESSIONAL COOP
Novel prescription composition and preparation method of paroxetine hydrochloride enteric controlled release tablet
The invention relates to a novel prescription and a preparation method of an oral paroxetine hydrochloride enteric controlled release tablet. The paroxetine hydrochloride enteric controlled release tablet consists of a double-layer core and an enteric coating, wherein the enteric coating enables active pharmaceutical ingredients to release in a fixed position at the lower end of the small intestine; the double-layer core controls the constant release speed of a medicine; the core consists of two layers, wherein one layer is a corroded blockage layer free of the active ingredients and is used for limiting the liquid permeation on the surface of a matrix, and another layer is a medicine-containing layer of a hydrophilic matrix; and when an aqueous medium enters a hydrophilic layer containing the active ingredients, a polymer can be hydrated and gelled, and thus a screen is constructed for the active ingredients to release and disperse from a preparation. The oral paroxetine hydrochloride enteric controlled release tablet is high in medicine release performance and completely meets the requirement on zero-order release. A controlled release material of a corroded layer is a mixture of docosanoic acid glycerol ester and / or ethyecellulose based on the mass ratio of 5: 1 to 1: 5; and the material adopted in the hydrophilic matrix layer is HPMC (Hydroxy Propyl Methyl Cellulose) series, and K4M is preferable.
Owner:王进京
Gliclazide sustained-release tablets
ActiveCN107375224AStable release rateImprove stabilityMetabolism disorderSulfonylurea active ingredientsExtended release tabletsMedicine
The invention discloses gliclazide sustained-release tablets, which are prepared by evenly mixing drug-loading particles with pharmaceutically acceptable adjuvant materials and tabletting the obtained mixture; a preparation method of the drug-loading particles comprises the following steps: heating glyceryl behenate up to 65-70 DEG C for melting, then adding meglumine and evenly stirring in a heat preservation process, adding gliclazide and stirring until the mixture is molten, cooling for solidifying, and pelletizing, wherein the mass ratio of the gliclazide to the glyceryl behenate to the meglumine is equal to 1 to (1.5-4.5) to (0.1-0.3). The gliclazide sustained-release tablets are stable in drug release speed, better in drug stability, high in bioavailability, simple in preparation technology and suitable for industrial mass production.
Owner:浙江康德药业集团股份有限公司
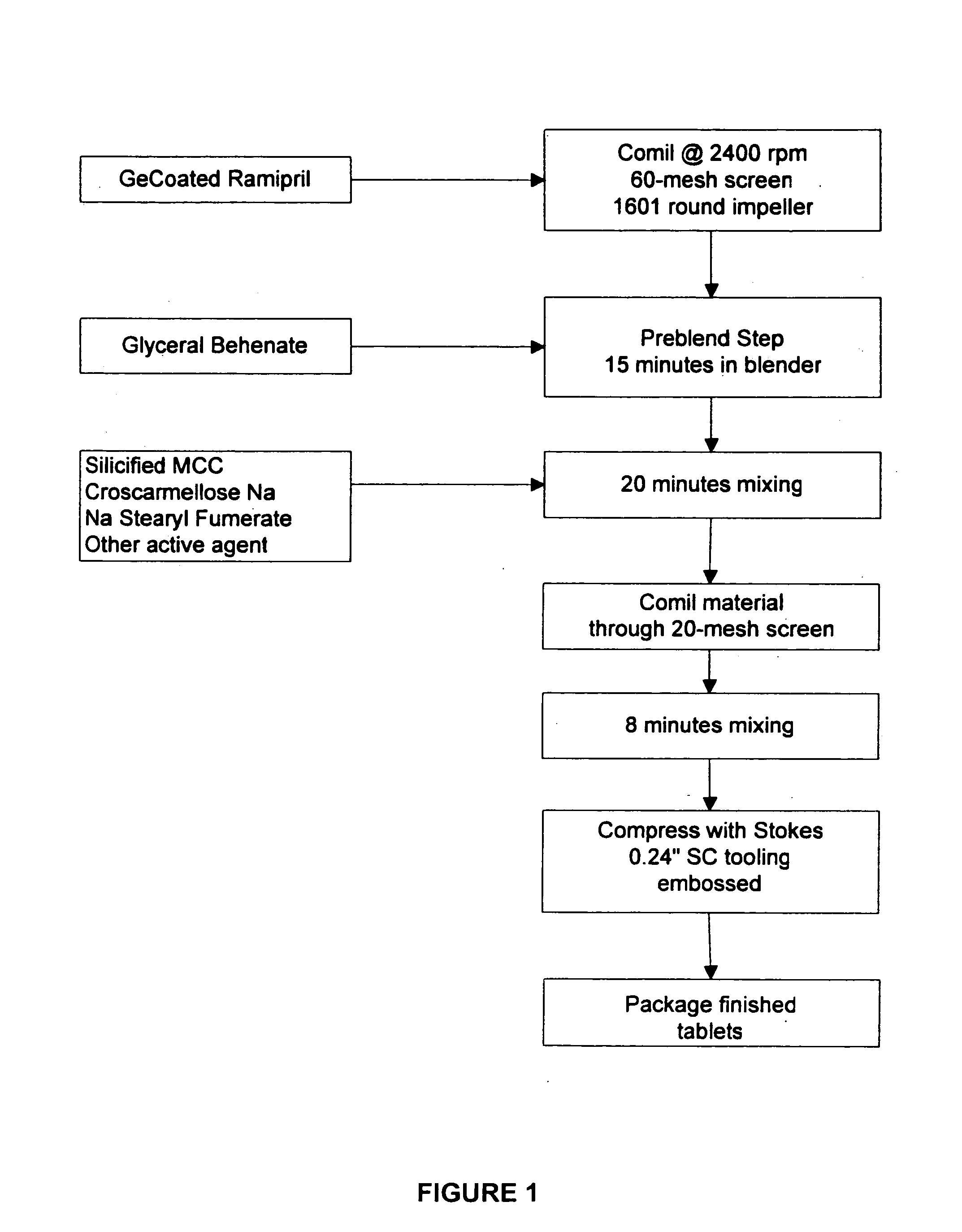
Compositions of stabilized ramipril in combination with another active agent
A pharmaceutical composition comprising ramipril, another active agent, and a blending agent, wherein in the ramipril is coated by the blending agent, and wherein the blending agent is glyceryl behenate, glyceryl stearate, stearyl alcohol, macrogol stearate ether, palmitosearate, ethylene glycol, polyethylene glycol, stearic acid, cetyl alcohol, lauryl alcohol, amylopectin, poloxymer or combinations thereof.
Owner:KING PHARMA RES & DEV
Trimetazidine hydrochloride sustained release tablet taking glyceryl behenate as framework material and preparation method of trimetazidine hydrochloride sustained release tablet
InactiveCN104274419ASimple processEase of industrial productionOrganic active ingredientsSenses disorderTrimetazidine DihydrochlorideSustained Release Tablet
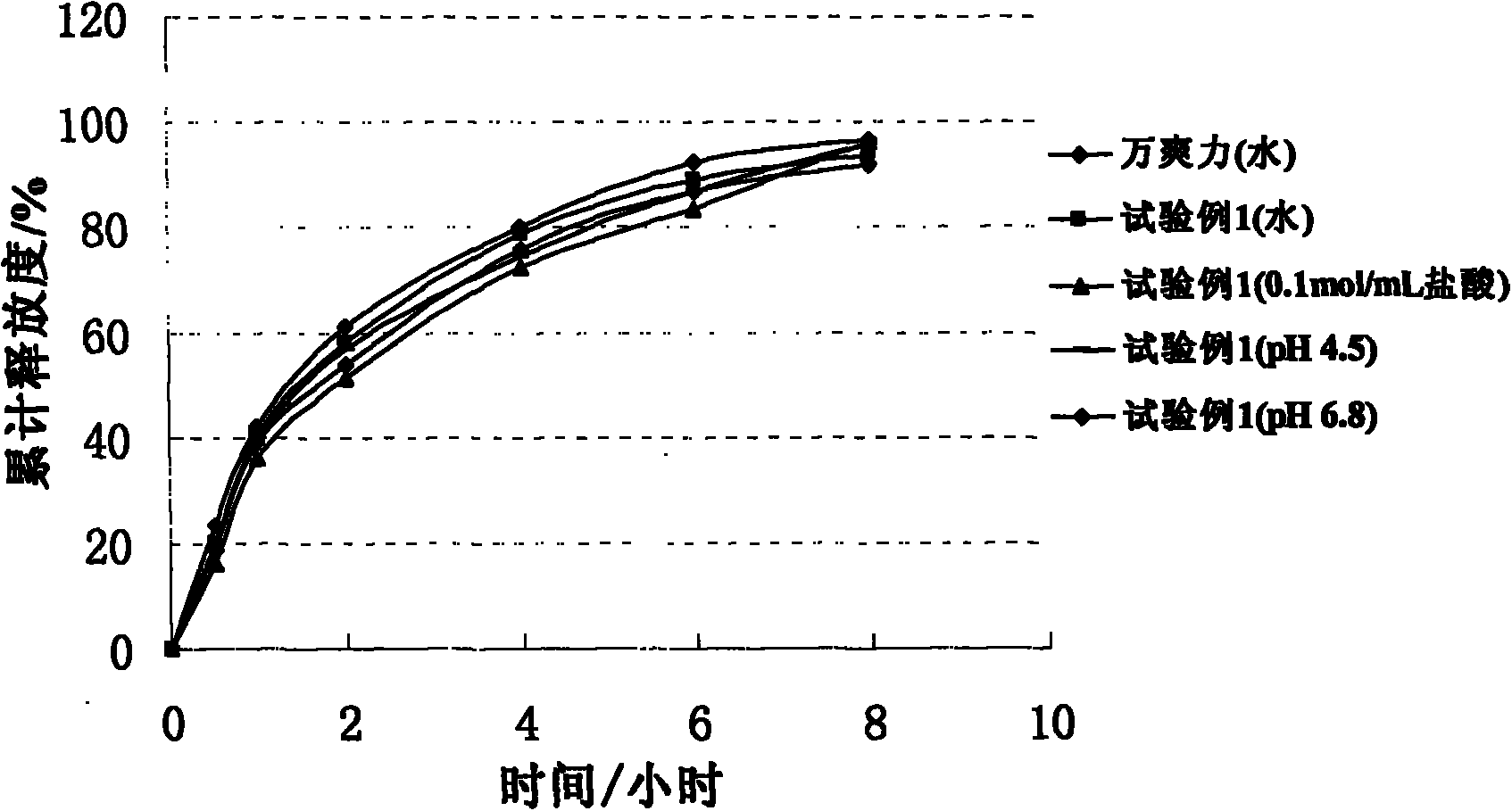
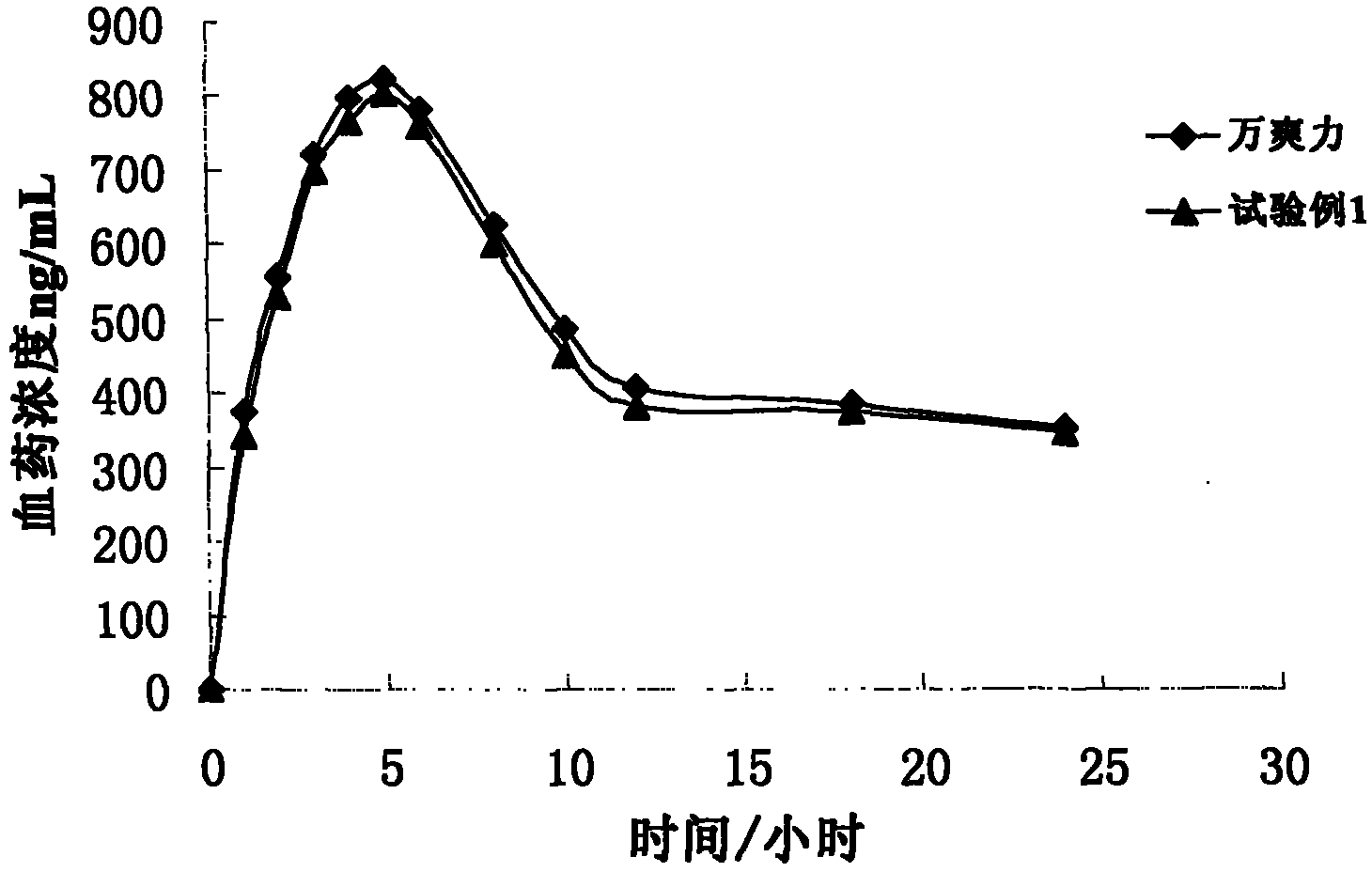
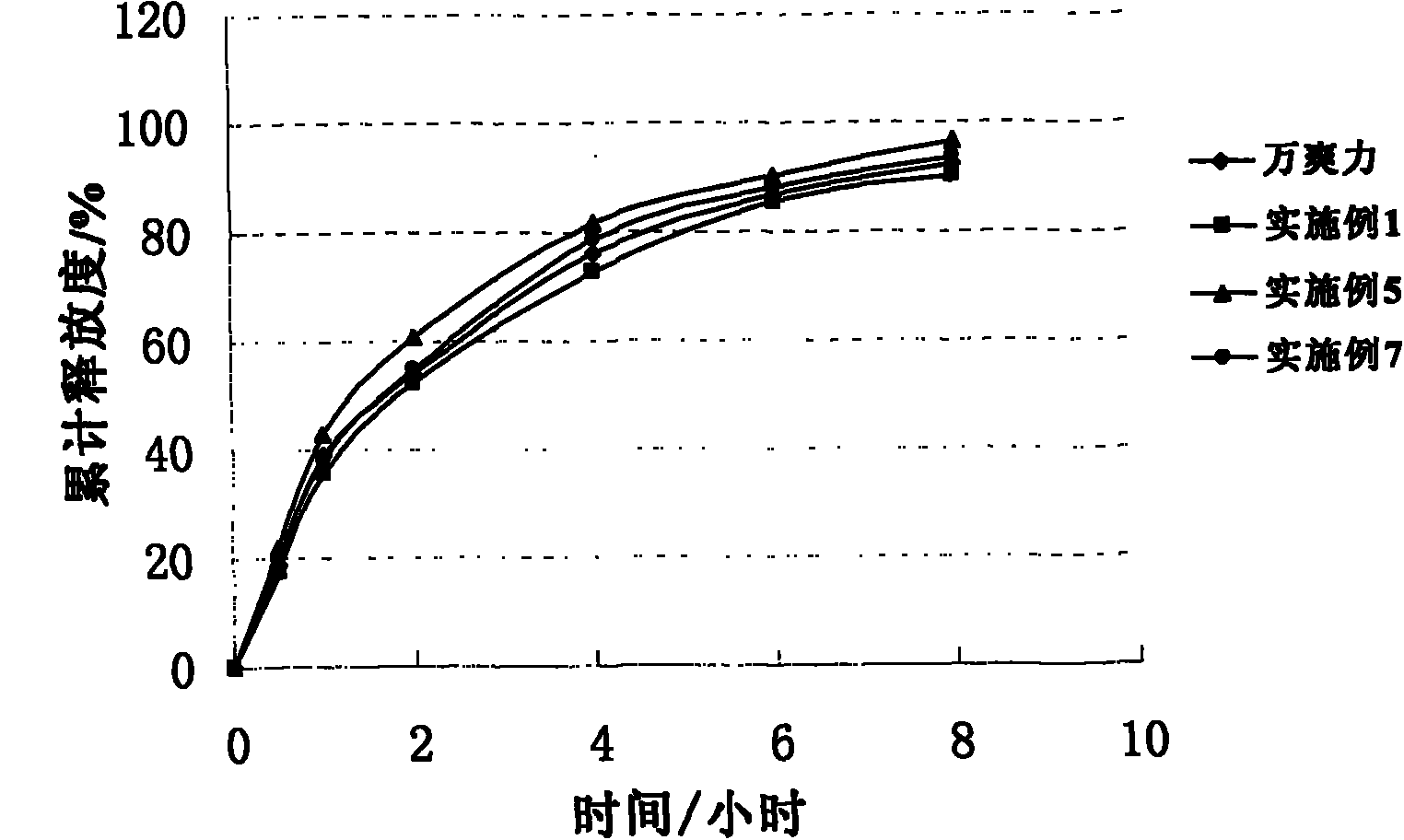
The invention belongs to the field of sustained-release drug preparations, and particularly relates to a trimetazidine hydrochloride sustained release tablet taking glyceryl behenate as a framework material and a preparation method of the trimetazidine hydrochloride sustained release tablet. According to the main technical scheme disclosed by the invention, a sustained-release preparation is prepared from trimetazidine hydrochloride as an effective component, only glyceryl behenate as a sustained-release framework material and auxiliary materials such as a small amount of release speed regulator. According to the trimetazidine hydrochloride sustained release tablet provided by the invention, an in vitro release rate experiment shows that the drug release is not affected by the pH environment, compared with commercially available 'vasorel' (trimetazidine dihydrochloride tablet), the trimetazidine hydrochloride sustained release tablet has good similarity (f2 is greater than 65); and the Beagle pharmacokinetic experiment in dogs shows that the trimetazidine hydrochloride sustained release tablet has bioequivalence in comparison with 'vasorel'. One trimetazidine hydrochloride sustained release tablet provided by the invention is taken twice a day, and the trimetazidine hydrochloride sustained release tablet is convenient to take, has good medicine compliance in patients, and is capable of keeping steady state plasma concentration for a long period of time. The preparation method disclosed by the invention is simple and stable in process, and is easily put into volume production.
Owner:广东省中药研究所
Compound amoxicillin and clavulanate potassium tablet and preparation method thereof
ActiveCN103340855ASlow down disintegrationHigh hardnessAntibacterial agentsPill deliveryAdhesiveSilicon dioxide
The invention relates to a compound amoxicillin and clavulanate potassium tablet and a preparation method thereof. The tablet consists of the following components in percentage by weight: 15 to 30 percent of amoxicillin, 5 to 15 percent of a clavulanate potassium mixture, 30 to 65 percent of a filler, 1.5 to 8 percent of crosslinked povidone, 0.6 to 3 percent of povidone K30, 1 to 3 percent of silica and 1 to 5 percent of glyceryl behenate. The preparation method comprises the following steps: adding the amoxicillin, the filler and the crosslinked povidone into a pelletizer, mixing and adding an adhesive in a mixing process; and adding the prepared amoxicillin granules and clavulanate potassium mixture, the silica and the glyceryl behenate into a mixing machine, mixing, discharging and tabletting. Although the compound amoxicillin and clavulanate potassium tablet adopts a half-wet method to pelletize, the clavulanate potassium is unlikely to degrade, so that the stability of a medicine is guaranteed effectively.
Owner:上海汉维生物医药科技有限公司
Preparation method of slow-release solid microspheres with anti-wrinkle effect
ActiveCN107982095AImprove efficiencyGood storage stabilityCosmetic preparationsToilet preparationsMethacrylateMicrosphere
The invention relates to a preparation method of slow-release solid microspheres with an anti-wrinkle effect. The preparation method is characterized by comprising the steps as follows: A, vitamin A and isododecane are mixed and dissolved; B, vitamin A is adsorbed with polymethyl methacrylate; C, polymethyl methacrylate powder with vitamin A adsorbed is dried and crushed; D, tert-butylhydroquinone, a mixed solution of diethylhexyl syringylidene malonate and octanoic / capric triglyceride, a mixed solution of glyceryl behenate and polyglycerol-6 octastearate, behenyl alcohol, hydrogenated polyisobutene and polydimethylsiloxane are weighed in mass ratio and mixed, and an oil-wax mixed solution is prepared; E, solid particles, the oil-wax mixed solution, deionized water and an ammonium acryloyldimethyltaurate / beheneth-25 methacrylate crosslinked polymer are mixed, and an aqueous solution containing solid microspheres is prepared; F, washing and sieving are performed, and the slow-release solid microspheres with the anti-wrinkle effect are obtained. The solid microspheres have the slow vitamin A release and anti-wrinkle effects.
Owner:PROYA COSMETICS
Andrographolide dry suspension prepared by fusion spraying and preparation method thereof
ActiveCN105581985ANo grittinessBitter tasteAntibacterial agentsOrganic active ingredientsOlder peoplePolyvinyl alcohol
The invention discloses an andrographolide dry suspension which is particles prepared from andrographolide and accessories by mixing and spraying, wherein the accessories have relatively low melting points and are any two of hexadecanol, octadecanol, glyceryl monostearate, glyceryl behenate, stearic acid, beewax, polyvinyl alcohol and polyethylene glycol; the total mass of the accessories with relatively low melting points is 0.1-10 times that of andrographolide; and the accessories also include at least one other accessory. The andrographolide dry suspension disclosed by the invention is convenient to take simply by adding a little water; and with little bitterness, the andrographolide dry suspension realizes a good taste modifying effect on andrographolide and is suitable for children, old people and patients suffering dysphagia.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Ointment combination matrix for sustained-release drug delivery
ActiveCN104338147APorogenActs as an adhesiveAerosol deliveryOintment deliveryActive agentSurface-active agents
The invention discloses an ointment combination matrix for sustained-release drug delivery, and belongs to the technical field of a medicine preparation. The ointment combination matrix contains a hydrophilic surfactant, a cellulose derivative, glyceryl behenate and vaseline, wherein the matrix is ground and mixed with the medicine to prepare the ointment; the ointment is swelled under in vitro release conditions of 37 DEG C and 100rpm; the medicine is kept to release at zero level or similar zero level for a long period of time; vaseline is good in adaptability to body tissues, and has the effect of promoting wound healing; the hydrophilic surfactant absorbs body liquid, so that the combination matrix is swelled and a drug release channel can be formed; the glyceryl behenate is good in biocompatibility; the thermal stability is increased; and matrix dissolution is avoided and release of the drug is promoted due to an adhesive effect of the cellulose derivative. The ointment is suitable for dosing normal or damaged tissues, pollution and cleaning of corroded matters are avoided, the drug frequency is reduced, the medication compliance of a patient is improved, and the ointment is a sustained-release ointment preparation applied to skin, oral cavities, rectums, vaginas and the like.
Owner:DALIAN UNIV OF TECH
Bactericide for Chinese gooseberry flower blight
InactiveCN105475396AGuaranteed qualityFormulation ScienceBiocideFungicidesSophocarpidineBenzoic acid
A bactericide for Chinese gooseberry flower blight is prepared from components by weight as follows: 400-600 g of deionized water, 4-6 g of an emulsifier, 4-6 g of 65% zineb wettable powder, 4-6 g of cis-form cypermethrin, 3-5 g of washing powder, 7-9 g of validamycin, 3-5 g of compound sodium nitrophenolate, 4-6 g of ethanol, 2-4 g of a golden thread extract, 2-4 g of a fresh ginger extract, 2-4 g of a pagodatree pod extract, 2-4 g of a cicada slough extract, 4-6 g of sophocarpidine, 2-4 g of glyceryl behenate, 2-4 g of benzoic acid, 2-4 g of brassinolide, 2-4 g of saponin, 2-4 g of naphthalene acetic acid, 2-4 g of coumarin, 2-4 g of purple sweet potato anthocyanin and 2-4 g of ethylparaben. During preparation, the deionized water is heated to 50-65 DEG C, other remaining components are added, the mixture is continuously stirred for about 10 minutes and then cooled to the room temperature, and the bactericide is obtained. The bactericide can be adopted to effectively control Chinese gooseberry flower blight, can kill bacteria and has little influence on natural environment and other living things due to the fact that no bactericide is left in plants under a normal use technology condition.
Owner:GUANGDE YUANYE FRUIT GROWING FAMILY FARM
Stable compositions of fesoterodine
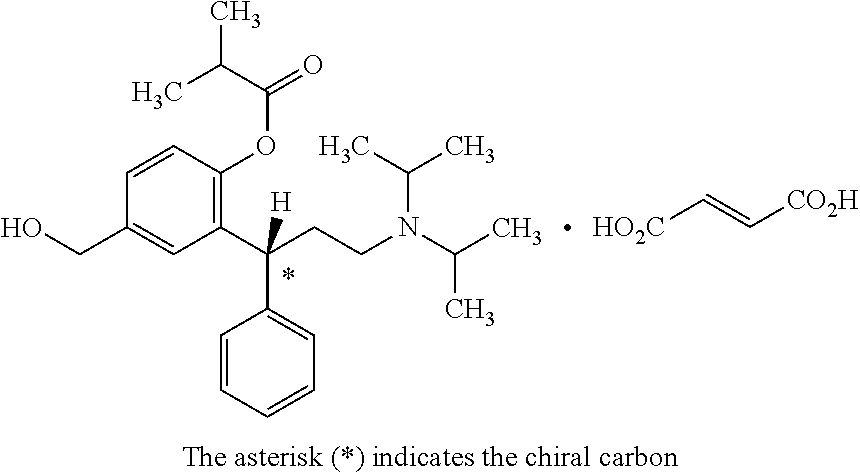
Stable pharmaceutical compositions of fesoterodine or its pharmaceutically acceptable salt thereof and process for preparing the same. In a first embodiment, a stable pharmaceutical composition is provided comprising fesoterodine fumarate, glyceryl behenate and a stabilizer. The stable pharmaceutical tablet composition may further comprise i) fesoterodine fumarate in an 5 amount of 1% to 5% by weight, ii) glyceryl behenate in an amount of 1% to 8% by weight, iii) pregelatinized starch in an amount of 30% to 50% by weight and iv) a stabilizer in an amount of 0.1% to 10% by weight based on total weight of the composition.
Owner:HETERO RES FOUND
Polyester resin for high leveling powder coating and preparation method and application thereof
ActiveCN109206599ALow viscosityLow end carboxyl activityPowdery paintsEpoxy resin coatingsHybrid typeTerephthalic acid
The invention belongs to the technical field of powder coatings, in particular to polyester resin for a high leveling powder coating, and further discloses a preparation method thereof and applicationthereof in preparing a 60 / 40 mixed powder coating. The polyester resin for the high leveling powder coating is prepped by polymerization of terephthalic acid, 4-oxo-1,4-dihydro-2,6-pyridine dicarboxylic acid, hydroquinone-O, O'-diacetic acid, D-Panthenol, neopentyl glycol, Glyceryl behenate, 3-glycidoxypropyl triethoxysilane and Carbic anhydride as raw materials. It is determined that the obtained polyester resin has a low melt viscosity at high temperature (200 DEG C), good fluidity, and long gelation time, ensures sufficient leveling time and contributes to obtaining a high leveling grade coated plate surface.
Owner:安徽恒隆新材料有限公司
Composition for improving stability of compound ibuprofen preparations and preparation method of composition
InactiveCN104758935AImprove stabilityReduce riskOrganic active ingredientsPowder deliveryTabletingOrganic chemistry
The invention discloses a composition for improving stability of compound ibuprofen preparations. The composition comprises the following components in parts by weight: 200 parts of ibuprofen, 10 parts of phenylephrine hydrochloride, 4 parts of chlorpheniramine maleate, 14-56 parts of glyceryl behenate and 50-800 parts of additives. The compound ibuprofen preparation composition can be directly used for preparing compound ibuprofen solid preparations such as tablets, capsules or granules or can be mixed with a proper quantity of additives to prepare the compound ibuprofen solid preparations such as tablets, capsules or granules by virtue of processes of tabletting, filling capsules or split charging. The compound ibuprofen preparation composition can be used for greatly improving the stability of ibuprofen, phenylephrine hydrochloride and chlorpheniramine maleate; the quality of products can be improved; the effective periods of the products can be prolonged; the risk of clinical medication is reduced.
Owner:铂镁医学临床研究(上海)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com