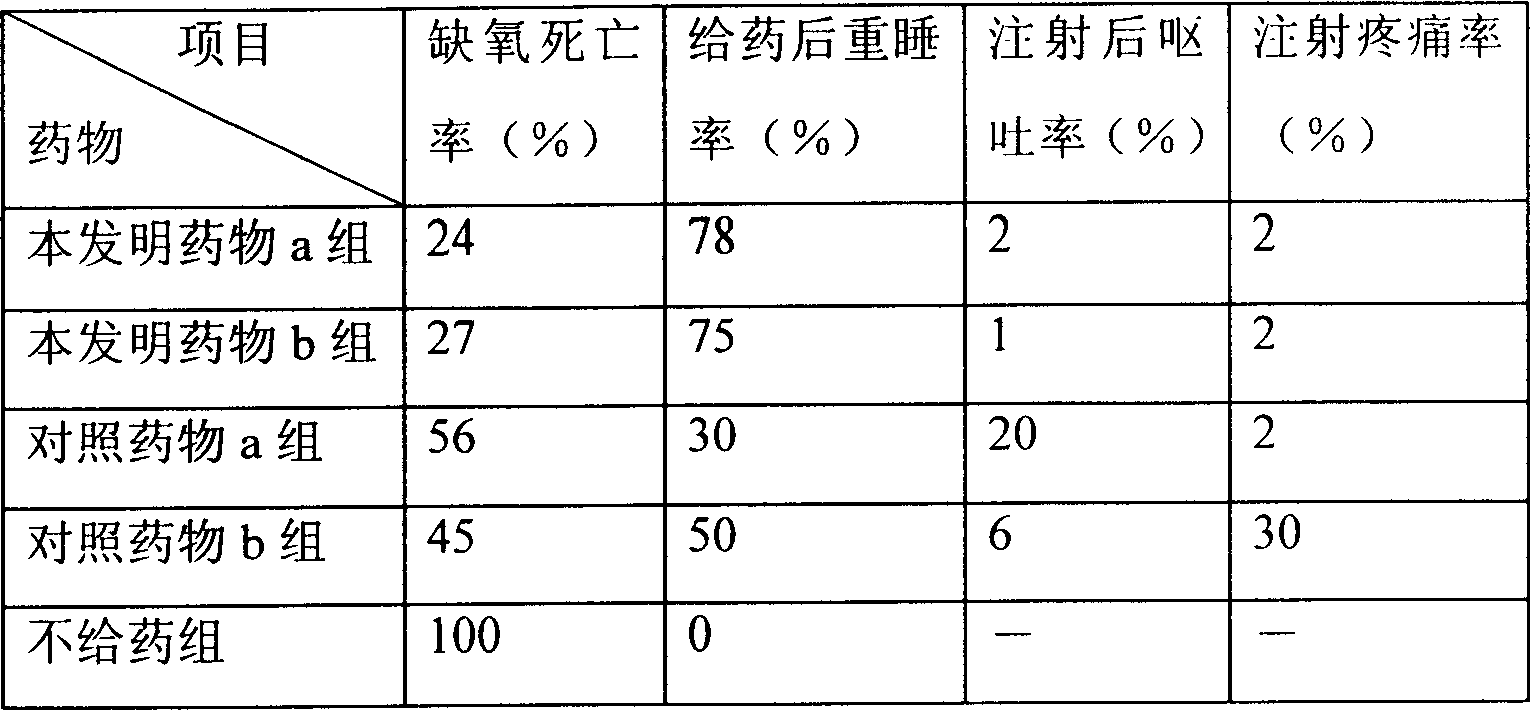
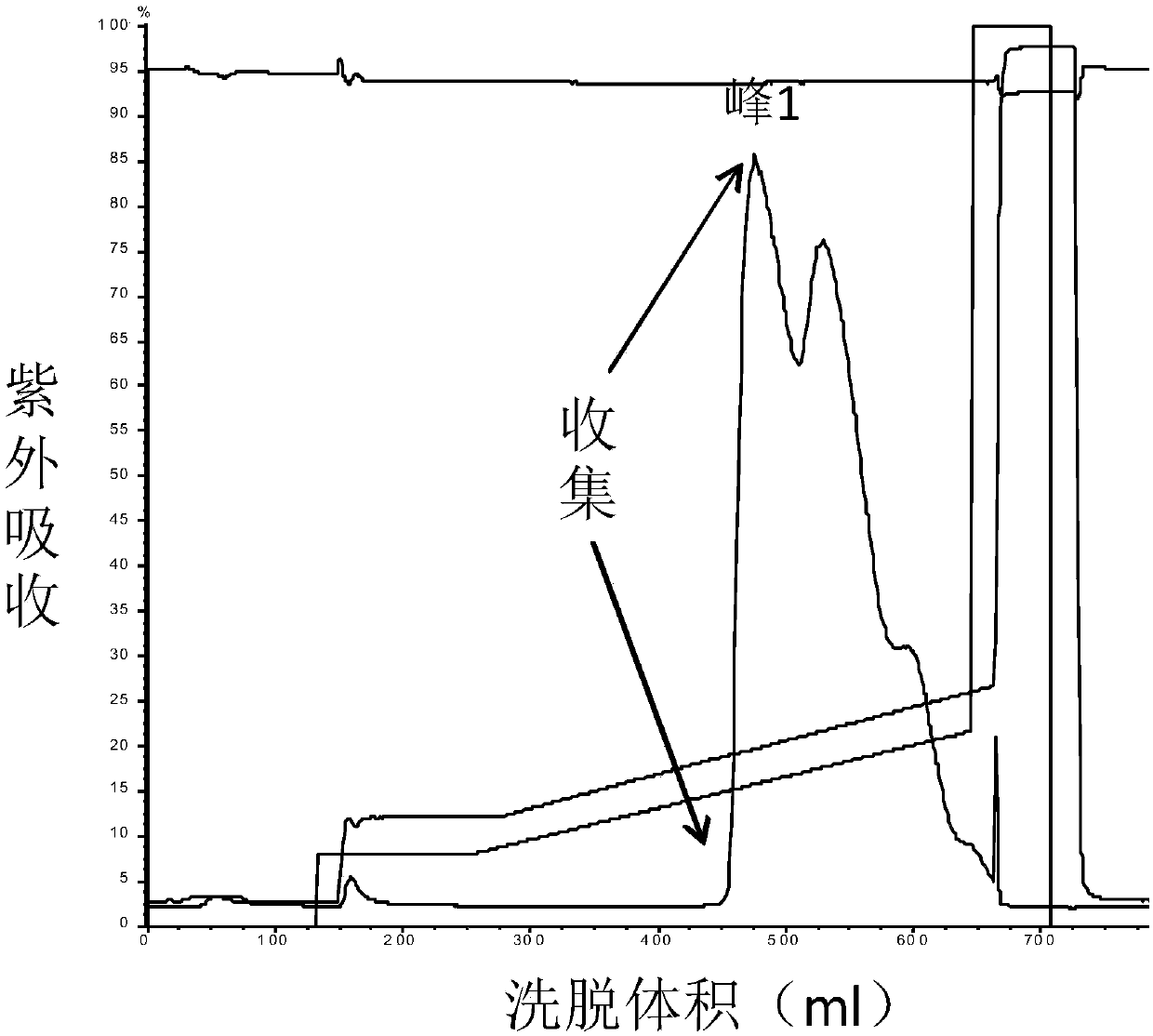
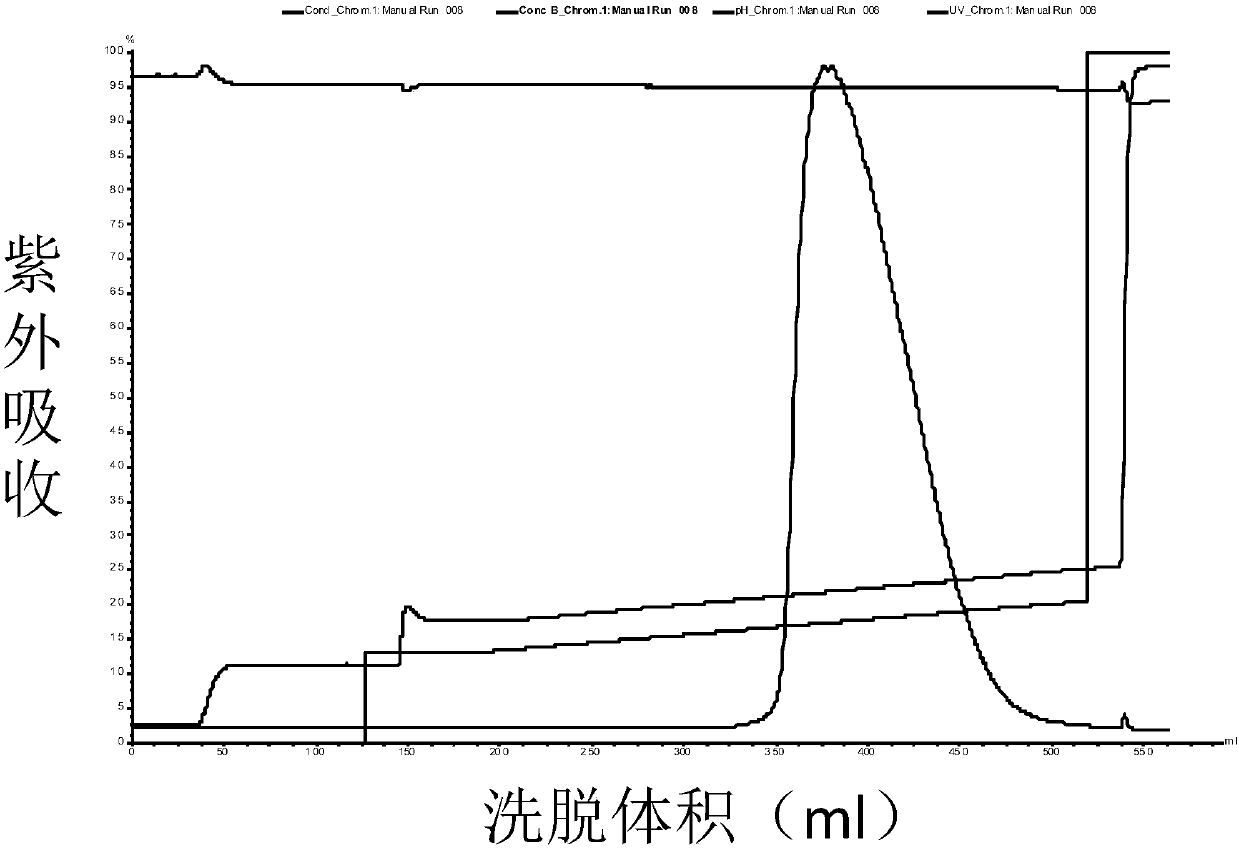
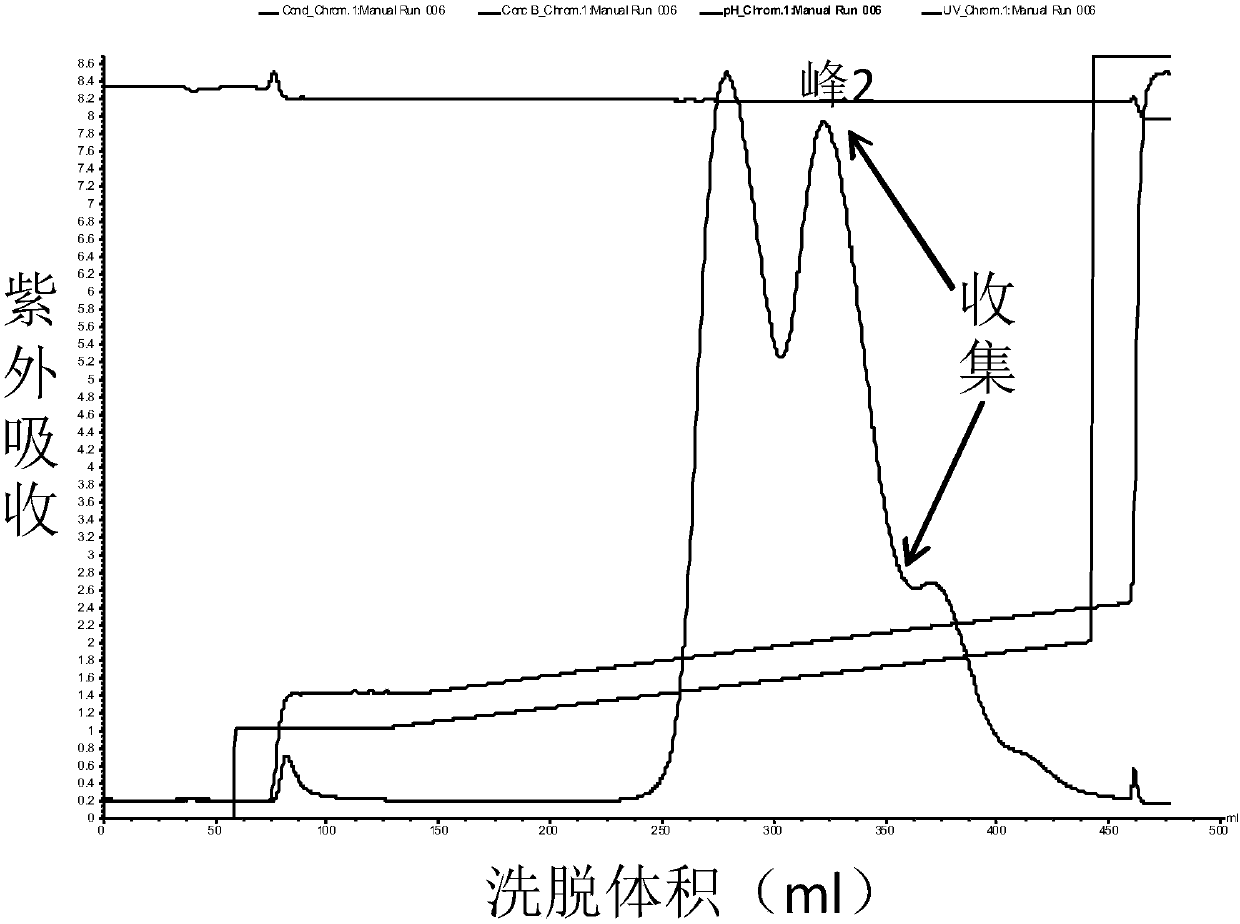
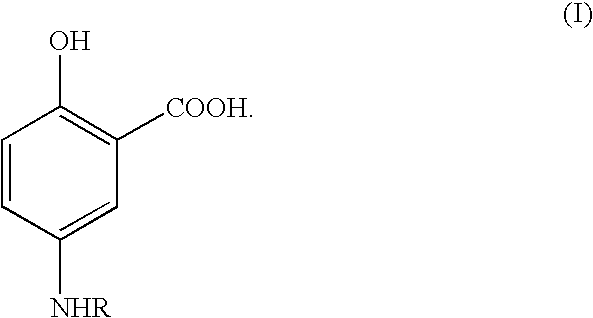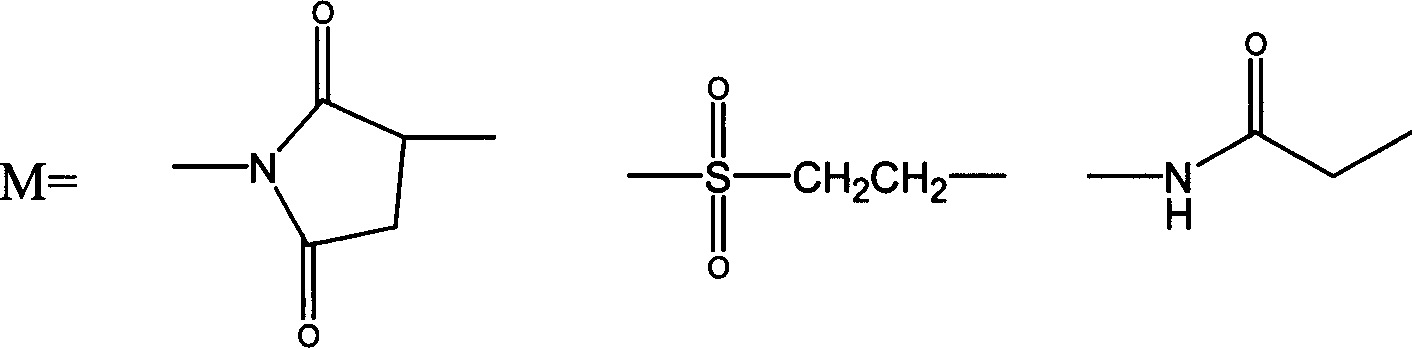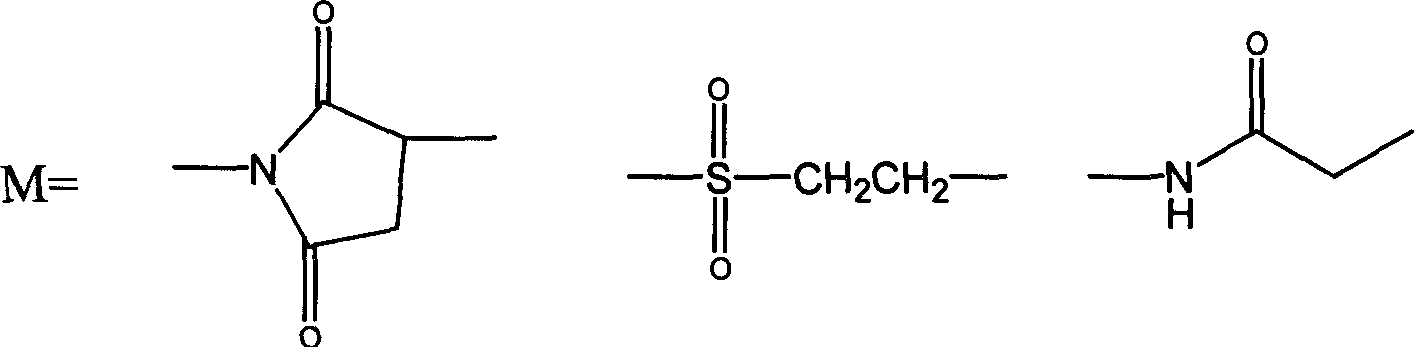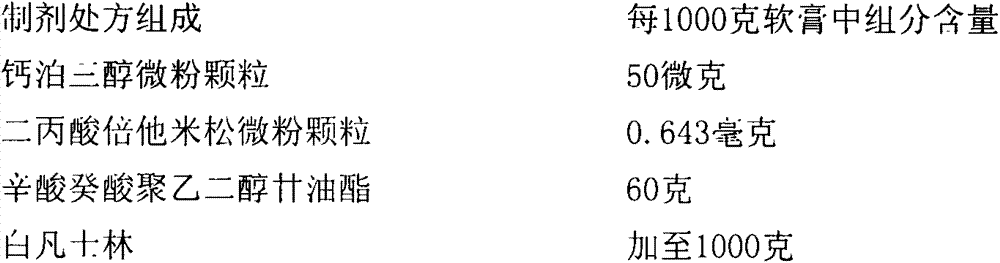Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
171 results about "Macrogol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Macrogol, also known as polyethylene glycol (PEG), is used as a medication to treat constipation in children and adults. It is also used to empty the bowels before a colonoscopy. It is taken by mouth. Benefits usually occur within three days. Generally it is only recommended for up to two weeks.
Reversible pegylated drugs
ActiveUS20060171920A1Prolonged Circulatory Half-LifeProvide benefitsAntibacterial agentsOrganic active ingredientsPhosphate9-fluorenylmethoxycarbonyl
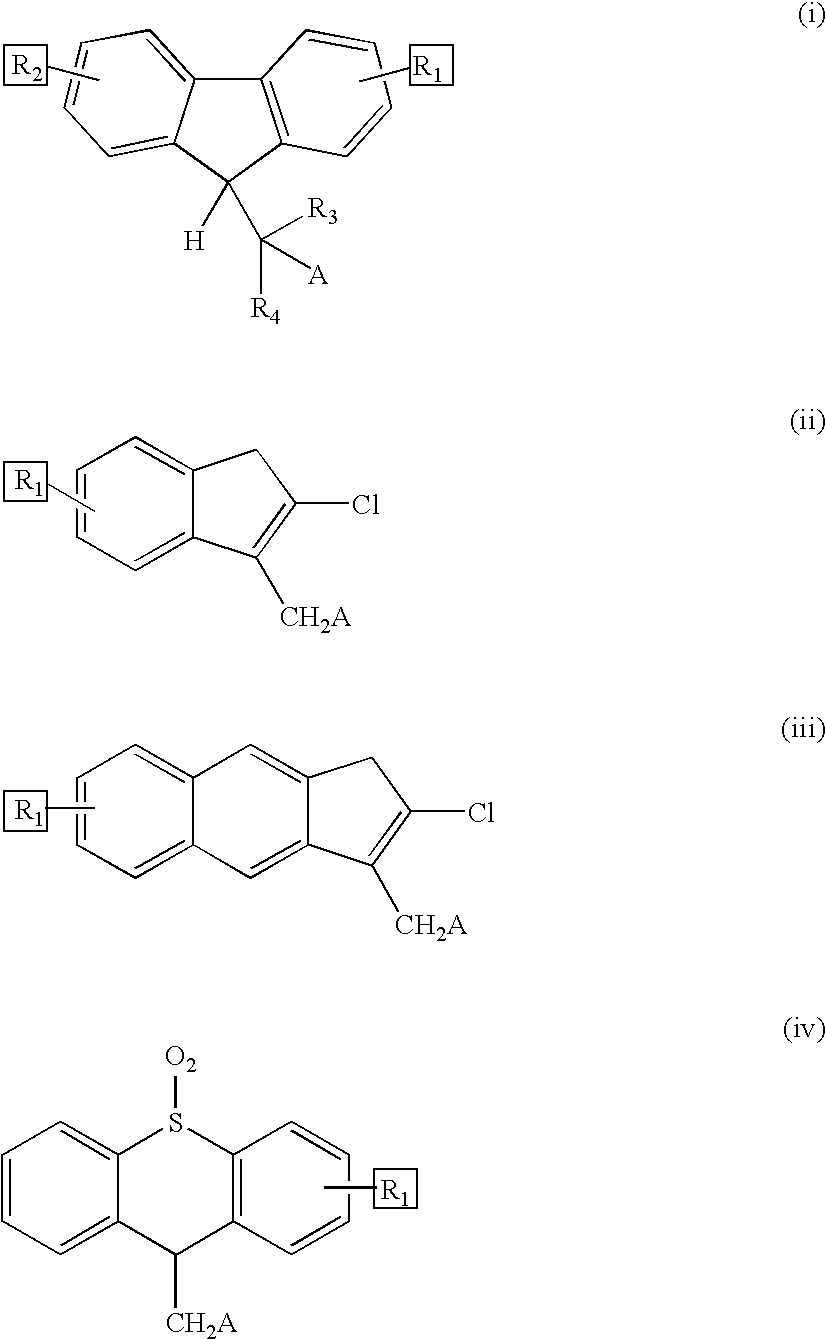
Reversible pegylated drugs are provided by derivatization of free functional groups of the drug selected from amino, hydroxyl, mercapto, phosphate and / or carboxyl with groups sensitive to mild basic conditions such as 9-fluorenylmethoxycarbonyl (Fmoc) or 2-sulfo-9-fluorenylmethoxycarbonyl (FMS), to which group a PEG moiety is attached. In these pegylated drugs, the PEG moiety and the drug residue are not linked directly to each other, but rather both residues are linked to different positions of the scaffold Fmoc or FMS structure that is highly sensitive to bases and is removable under physiological conditions. The drugs are preferably drugs containing an amino group, most preferably peptides and proteins of low or medium molecular weight. Similar molecules are provided wherein a protein carrier or another polymer carrier replaces the PEG moiety.
Owner:YEDA RES & DEV CO LTD
Controlled release pharmaceutical compositions for prolonged effect
InactiveUS20100239667A1Simple wayFew stepsOrganic active ingredientsAntipyreticEfferalganControl release
Layered pharmaceutical composition suitable for oral use in the treatment of diseases where absorption takes place over a large part of the gastrointestinal tract. The composition comprising A) a solid inner layer comprising i) an active substance, and ii) one or more disintegrants / exploding agents, one of more effervescent agents or a mixture thereof. the solid inner layer being sandwiched between two outer layers B1) and B2), each outer layer comprising iii) a substantially water soluble and / or crystalline polymer or a mixture of substantially water soluble and / or crystalline polymers, the polymer being a polyglycol in the form of one of a) a homopolymer having a MW of at least about 100,000 daltons, and b) a copolymer having a MW of at least about 2,000 daltons, or a mixture thereof, and iv) an active substance, which is the same as in said solid inner layer A), and layer A being different from layer B, the layered composition being coated with a coating C) that has at least one opening exposing at least one surface of said outer layer, the coating being substantially insoluble in and impermeable to fluids and comprising a polymer, and the composition having a cylindrical form optionally with one or more tapered ends, wherein the ratio between the surface area of one end surface of the cylinder and the length of the cylinder is in a range of from 0.02 to 45 mm.
Owner:EGALET LTD
Medicine for preventing and treating coronary heart disease and angina pectoris and its prepn and other use
InactiveCN1348815AGood curative effectLess frequent seizuresNervous disorderMetabolism disorderSalvia miltiorrhizaCoronary artery disease
The present invention relates to traditional Chinese medicine for treating coronary heart disease and stenocardia is made from (by wt.%) salvia root 50-97%, notoginseng 2-48% and borneol 0.2-3%, in which the salvia root and notoginseng and hot extracted with water, then filtering, concentrating, alcohol precipitating, standing still, recovering ethyl alcohol, concentrating, into extractum, further blended with borneol and adjuvant polyglycol-6000 to form into product.
Owner:TIANJIN TASLY PHARMA CO LTD
Pegylated glutenase polypeptides
InactiveUS20090304754A1Lower Level RequirementsHigh activityHydrolasesPeptide/protein ingredientsCrystallographyProlyl endopeptidase
Owner:IMMUNOGENICS LLC
Formulations and doses of pegylated uricase
PendingUS20170258927A1Improve efficacyReduce incidenceOrganic active ingredientsPeptide/protein ingredientsFlareHyperuricemia
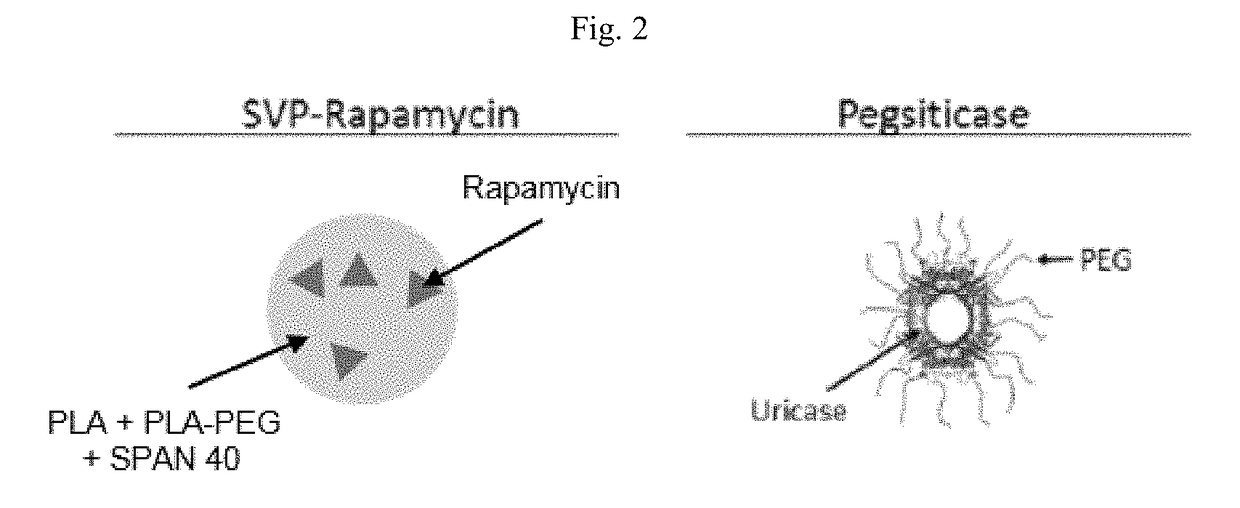
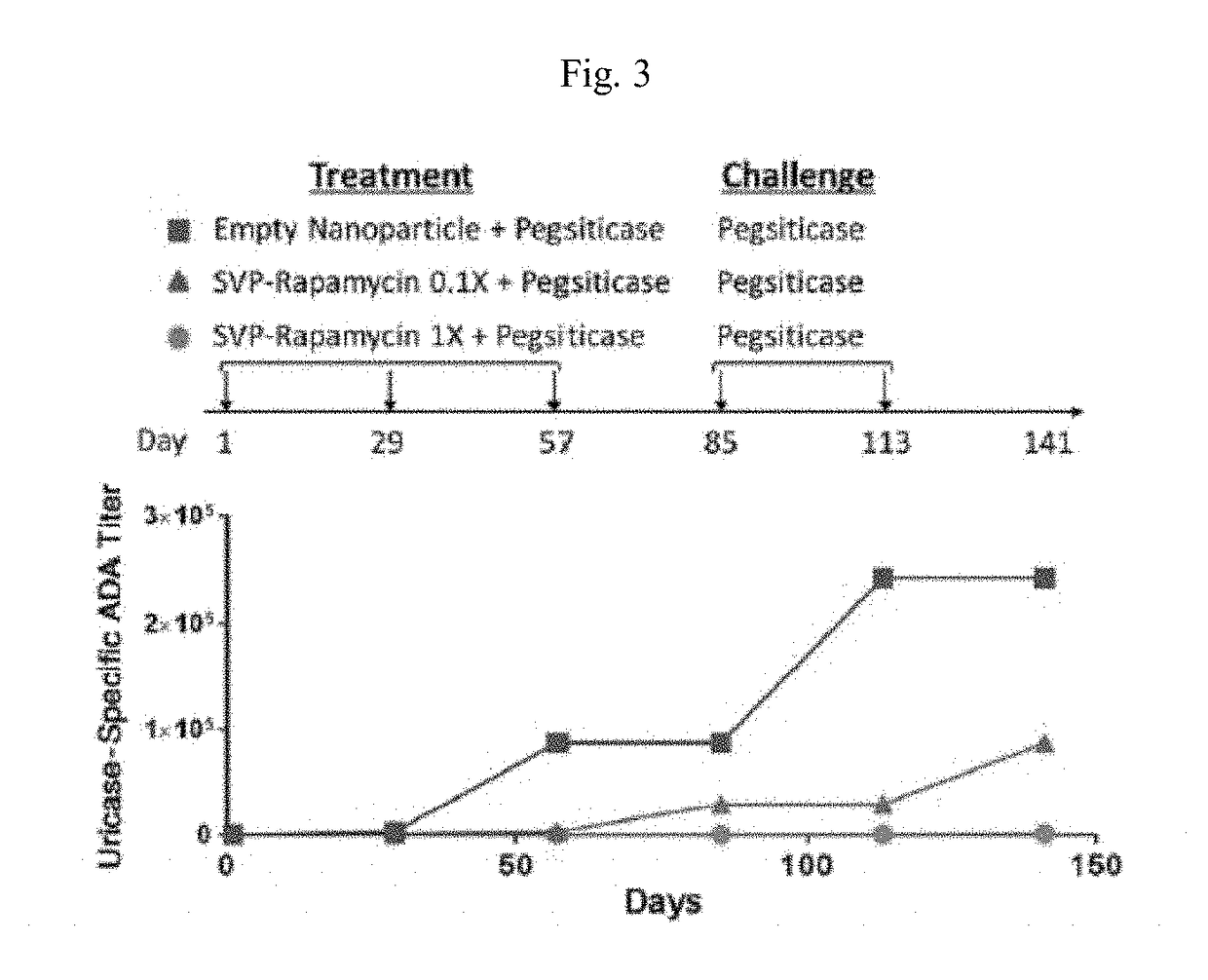
Provided herein are methods and compositions and kits related to uricase compositions and / or compositions comprising synthetic nanocarriers comprising an immunosuppressant. Also provided herein are methods and compositions and kits for the treatment of subjects, including subjects with hyperuricemia, gout or a condition associated with gout, and for preventing gout flare.
Owner:SELECTA BIOSCI
Aminosalicylate derivatives for treatment of inflammatory bowel disease
InactiveUS7157444B2High retention rateReduce absorptionSalicyclic acid active ingredientsBiocideIntestinal structureOral medication
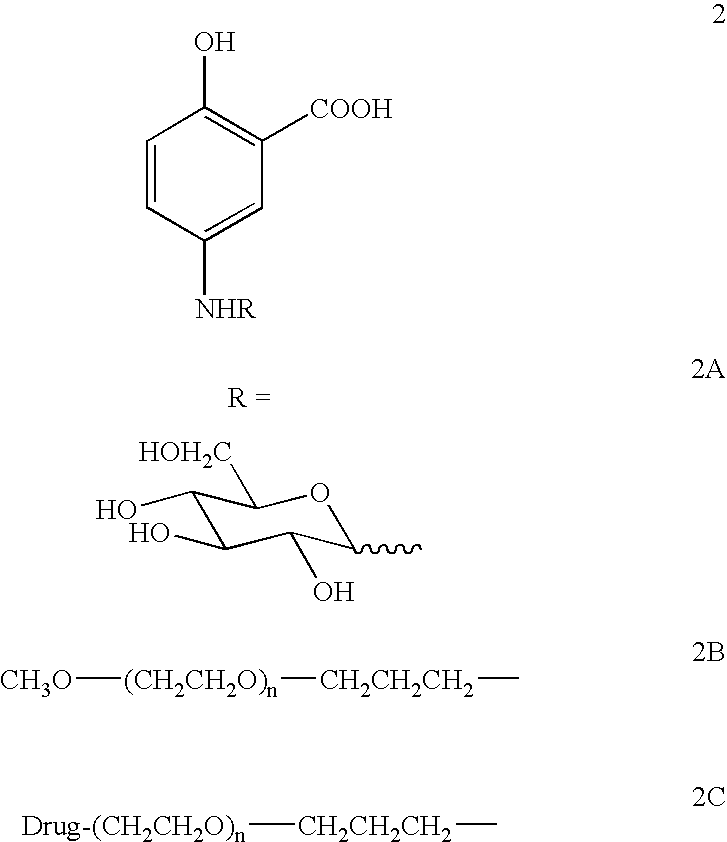
Therapeutic 5-aminosalicylic acid derivative compositions having general formula (I), wherein R is a 1-deoxy sugar residue or a poly(ethylene glycol) chain-containing residue, are provided. The compositions enable topical delivery of 5-aminosalicylic acid to the gastrointestinal tract following oral administration in pharmaceutical preparations. According to the invention, the compositions stabilize pharmaceutical compositions containing therapeutic 5-aminosalicylic acid derivatives in a manner that enhances the retention of said compositions in the intestine, decreases the cellular absorption thereof, and decreases the transfer of said compositions or the 5-aminosalicylic acid derived therefrom to the systemic circulation
Owner:NELSON DEANNA JEAN
Slow-releasing bFGF-PLA-PEG microball and its prepn and use
InactiveCN1398585AImproved particle size distributionGood conditionPeptide/protein ingredientsGranular deliveryDiseaseMicrosphere
In the present invention the microsphere has coated bFGF as medicine, and matrix of lactic acid-glycol copolymer (PLA-PEG). The slow-releasing bFGF microsphere is prepared by using PVA-PEG mixed liquid as dispersing medium and through evaporation and mechanical stirring. It has excellent slow-releasing performance of over two-week releasing period. Its freeze dried powder has good dispersivity and low organic solvent residue. The present invention can be used for local administration or vein administration of treating fracture, bone defect and other diseases.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
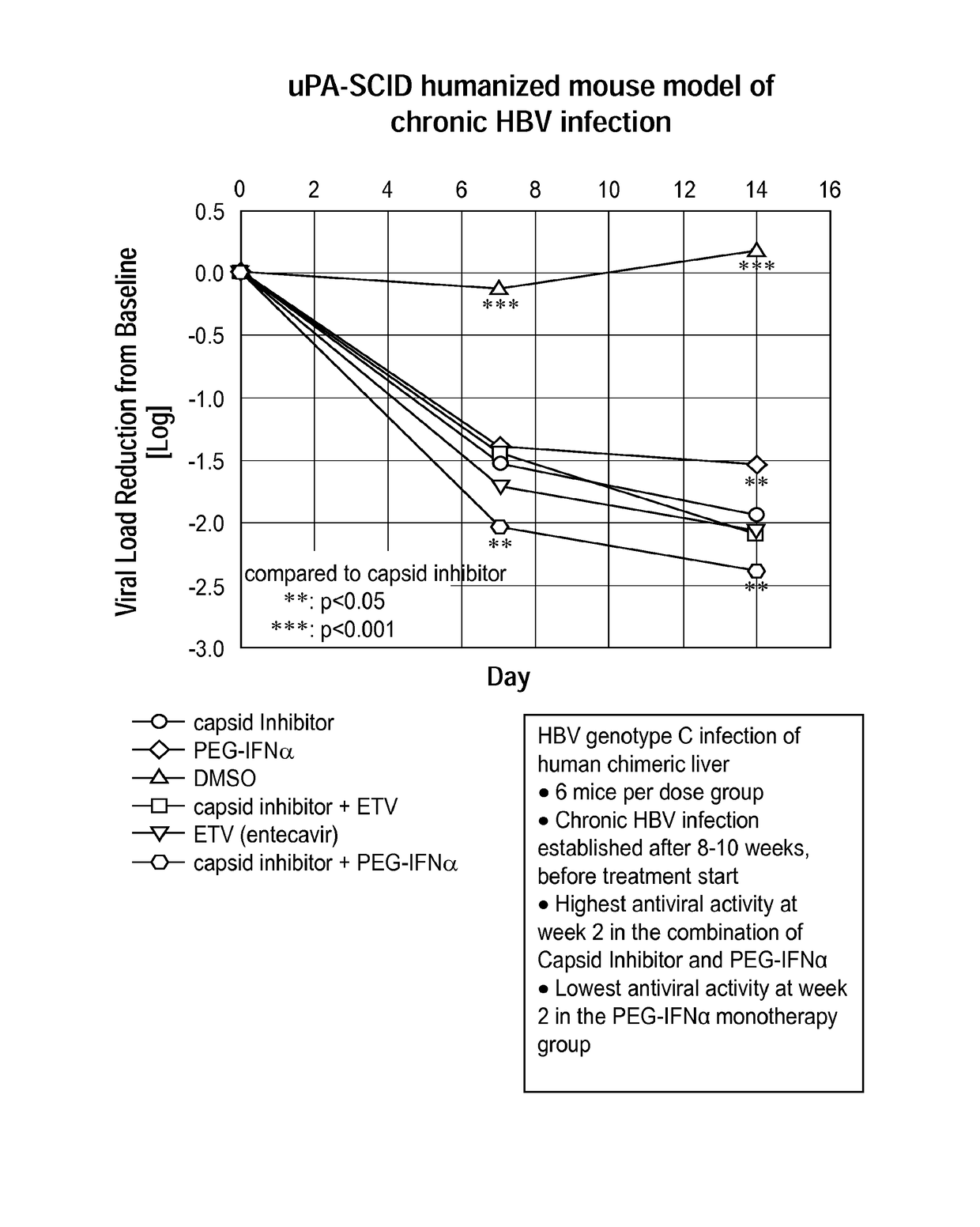
Combination therapy for treatment of hbv infections
InactiveUS20170182021A1Additional HBV virus replication suppression efficacyUseful in treatmentPeptide/protein ingredientsAntiviralsCombined Modality TherapyPeginterferon alfa-2a
Provided herein is a combination therapy comprising a compound of Formula I and peginterferon alfa-2a, or another interferon analog. The combination therapy is useful for the treatment of HBV infection. Also provided herein are compositions comprising a compound of Formula I and peginterferon alfa-2a, or another interferon analog.
Owner:NOVIRA THERAPEUTICS
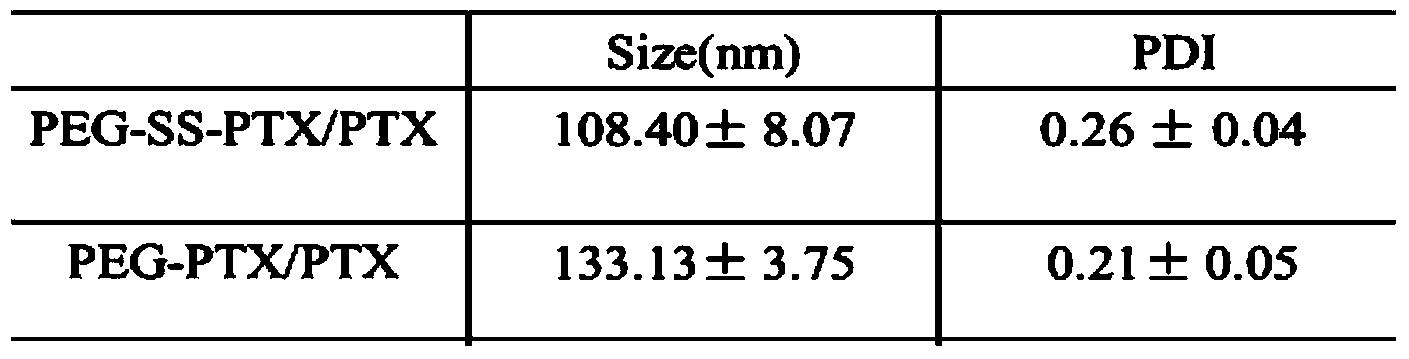
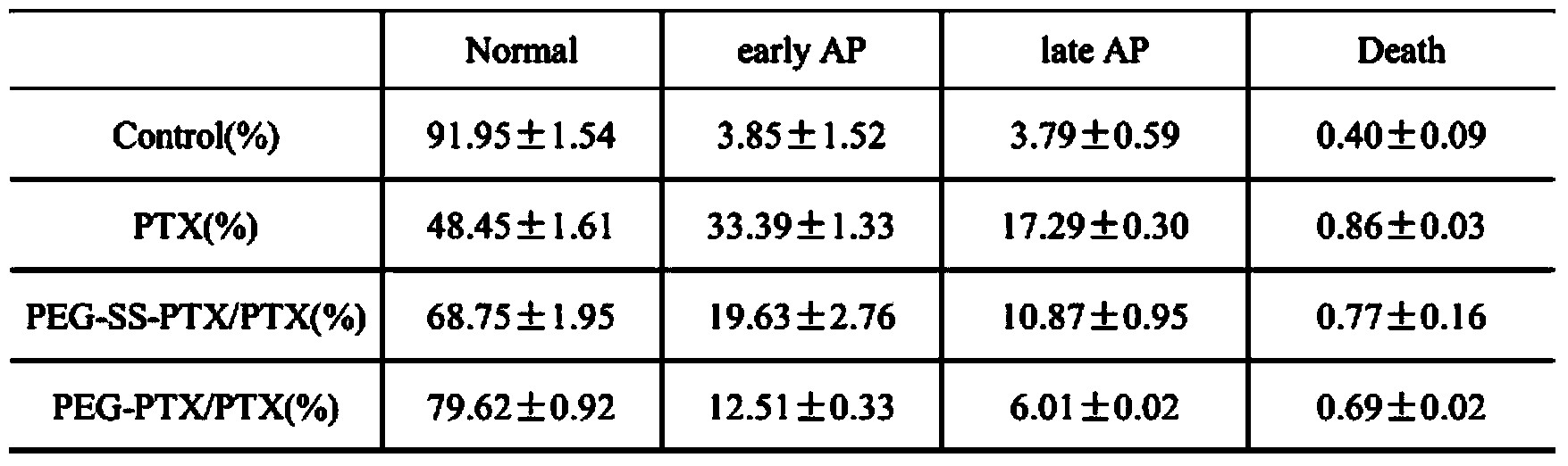
Preparation method and application of reduction-response-type pegylation (PEG) nanomedicine composition
ActiveCN103705943AImprove securityEliminate hidden dangers such as allergiesPowder deliveryOrganic active ingredientsDisulfide bondingDrug conjugation
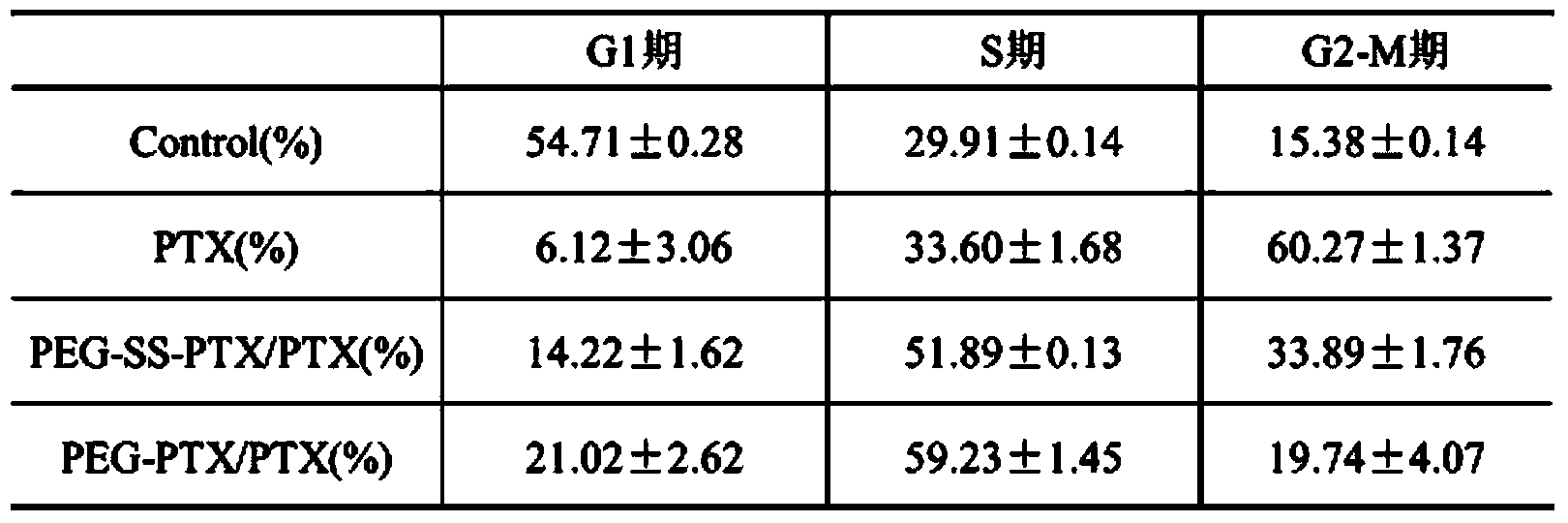
The invention relates to a preparation method and application of a reduction-response-type pegylation (PEG) nanomedicine composition. The nanomedicine composition is characterized by being prepared by coupling the PEG with a medicine via disulfide bonds which are sensitive in reduction. Thus, the water solubility of the medicine is improved; the in-vivo behavior of the medicine is also improved. Meanwhile, the full release and the activity of the medicine are ensured by utilizing the characteristic that the disulfide bonds in the PEG medicine, which are sensitive in reduction, can be specifically degraded in a tumor site. Thus, a good tumor treatment scheme is provided.
Owner:PEKING UNIV
Formulations of amlodipine maleate
InactiveUS20050019395A1Reduce productionCertain stabilityBiocidePill deliveryMedicineAmlodipine Maleate
The present invention provides improved, more stable formulations of amlodipine maleate where the formulations comprise from none to a minimal amount of magnesium. Such stable formulations show decreased production of the impurity amlodipine aspartate. Accordingly, the present invention provides formulations of amlodipine maleate comprising lubricants such as sodium stearyl fumarate, dimeticone, macrogol 6000, hydrogenated castor oil, and stearic acid. Methods of making and using the improved formulations are also provided.
Owner:TEVA PHARM USA INC
Thymosin alpha 1 active segment cyclicpeptide analogue and its poly glycol derivative
The present invention relates to a kind of cyclopeptide derivative containing natural or artificial amino acid substituted active thymosin alpha-1 segemnt, and its preparation process, medicine composition and their medicines for treating or preventing diseases related to immune deficiency, hypoimmunity, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
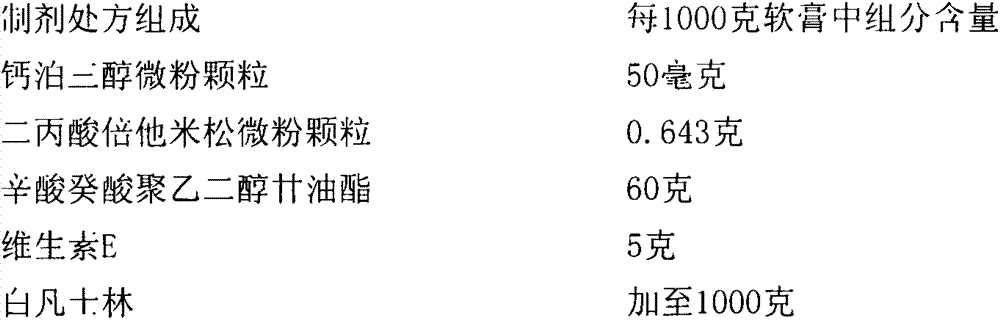
Calcipotriol betamethasone ointment and preparation method thereof
InactiveCN103110648AOrganic active ingredientsAerosol deliveryBetamethasone propionatePropanoic acid
The invention a calcipotriol betamethasone ointment prepared from caprylocaproyl macrogolglycerides. Under the conditions of high speed cutting and a homogeneous state, calcipotriol micronized particles with particle size range being 5-110 microns and the micronized particles of betamethasone dipropionate are uniformly dispersed to caprylocaproyl macrogolglycerides, affinities of caprylocaproyl macrogolglycerides and a commonly used ointment matrix are utilized to obtain the uniform ointment with uniformly distributed particle size. Furthermore, the stability of resisting high temperature (50 DEG C) is better, and is beneficial to improving the quality and the stability of a medicine.
Owner:JIANGSU SEMPOLL PHARMA
Quality standard of throat smoothing dropping pills
InactiveCN101152241AImprove quality levelHydroxy compound active ingredientsComponent separationCholic acidLicorice acid
The invention relates to the technical field of the traditional Chinese medicine and in particular to the quality standard of a pharynx-clearing dropping pill which is made from the raw materials of a natural indigo, liquorice, myrobalam, menthol, borneol, artificial cow-bezoar, polyglycol 6000. The item of physicochemical identification in the former quality standard is deleted; the thin layer identification of the borneol and the menthol, the thin layer identification of indigotin or indirubin and cholic acid or deoxycholic acid and the item of mensuration of bilirubin content are revised; the thin layer identification of the myrobalam, the layer identification of the liquorice and the item of the mensuration and detection of glycyrrhizic acid content are added, and the quantitative index of the liquorice content in each pill can not be less than 32 microgrammes, which is calculated according to the glycyrrhizic acid (C42H62O16). The invention improves the controllability of the quality standard of the pharynx-clearing dropping pill and further ensures the internal quality of the product, and the invention has great meaning for promoting the product sale and guaranteeing the medication safeness for the patient.
Owner:津药达仁堂集团股份有限公司第六中药厂
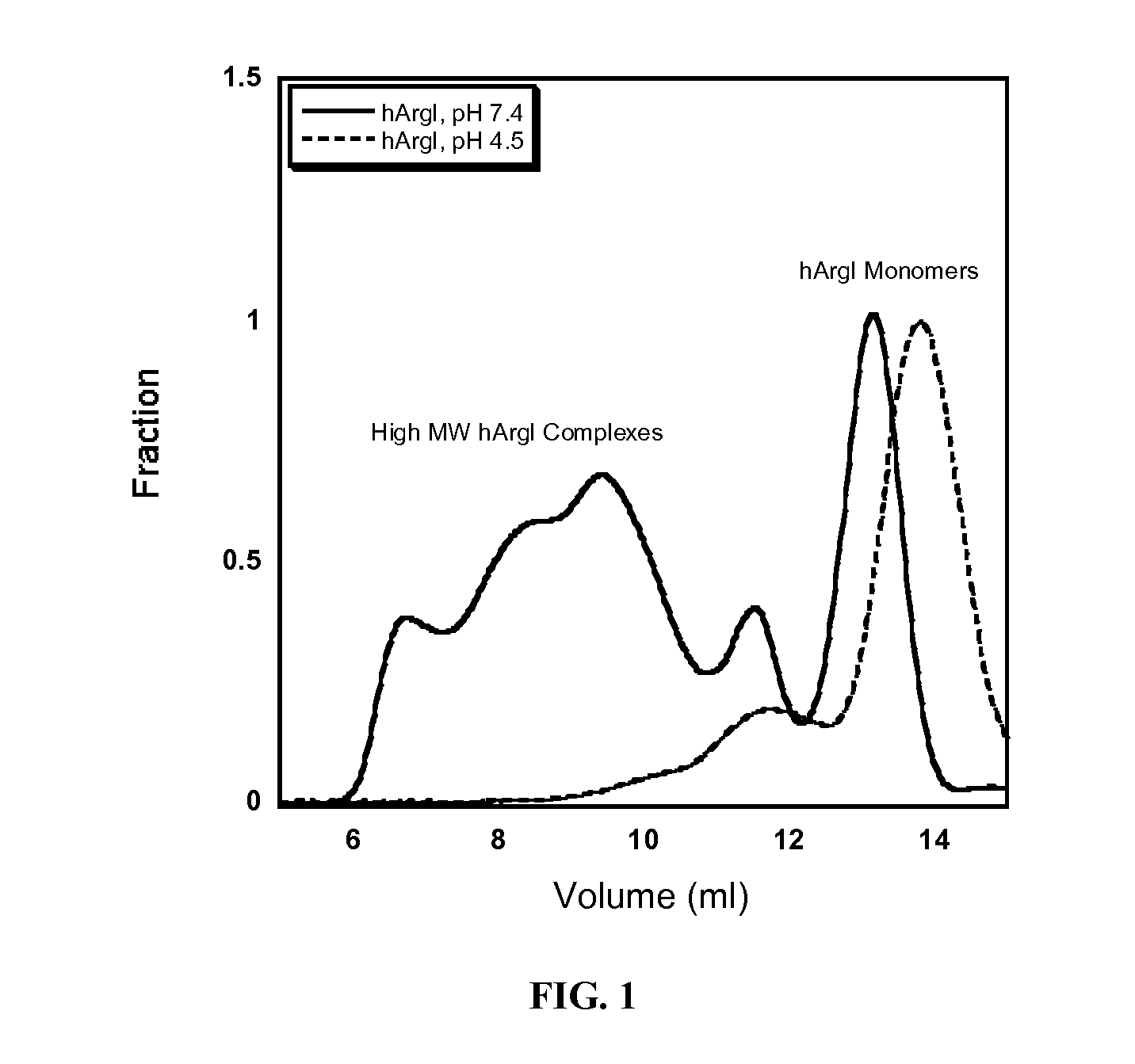
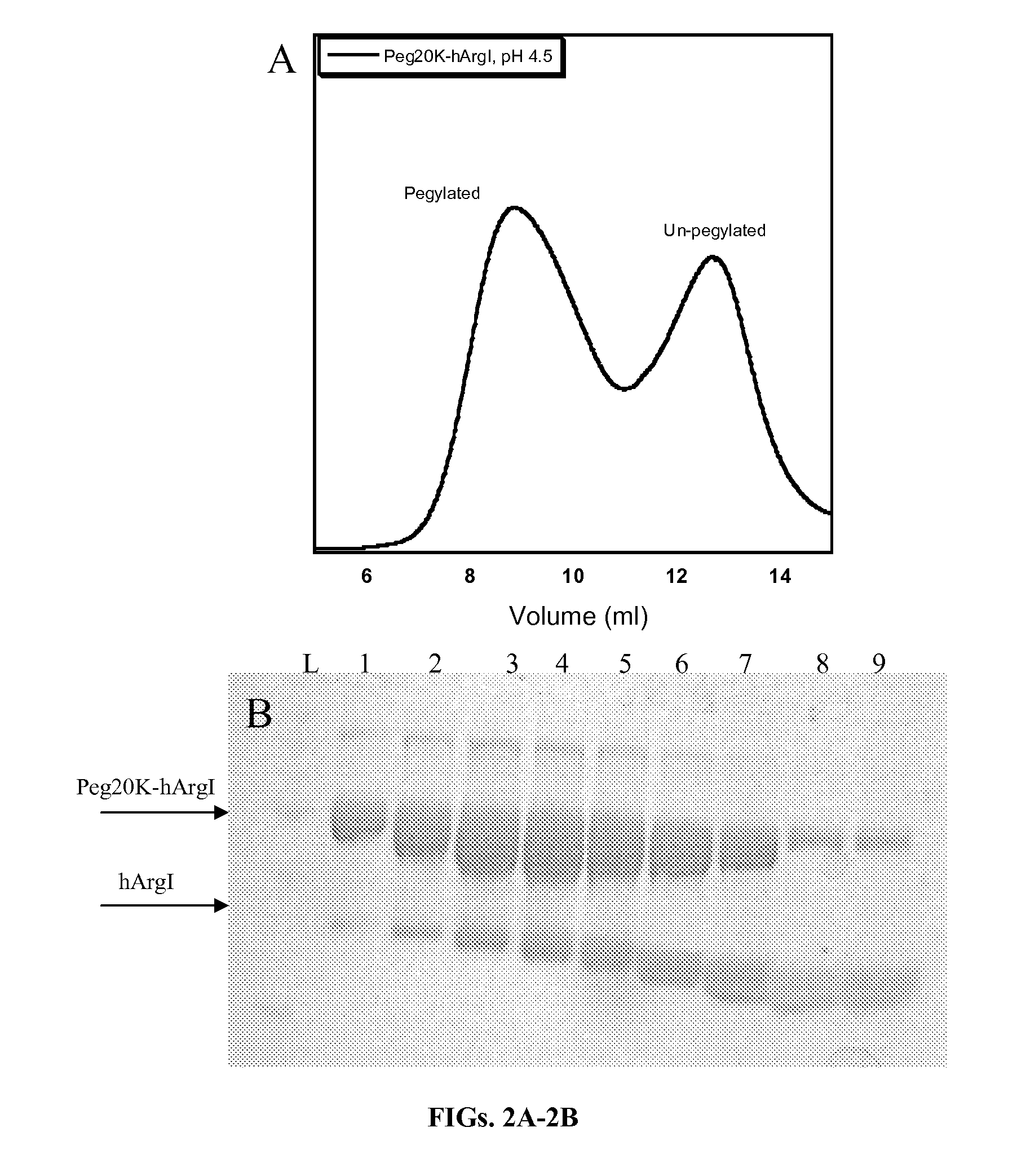
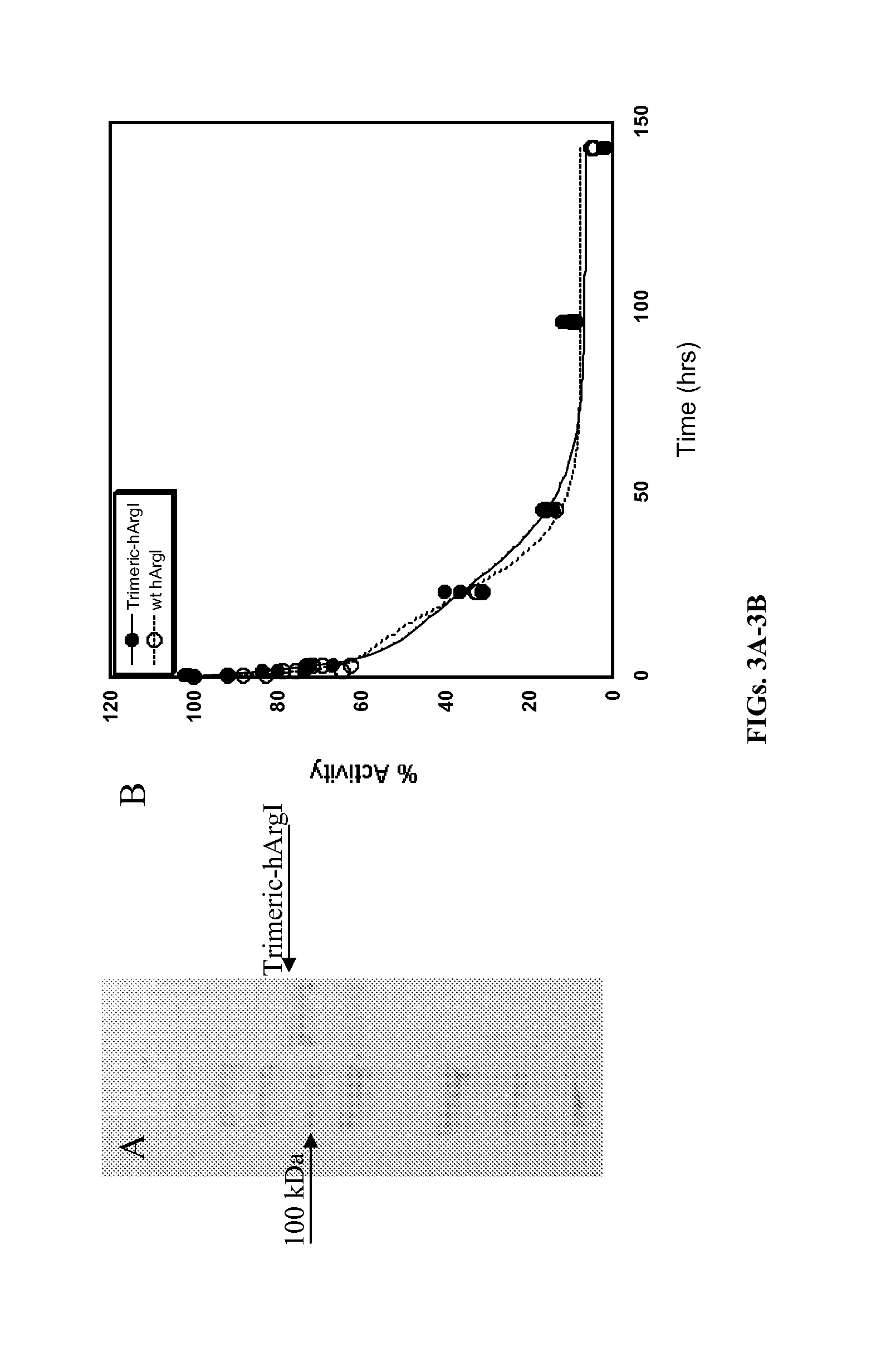
Arginase formulations and methods
ActiveUS20120177628A1High degree of homogeneityHigh molecular weightPeptide/protein ingredientsHydrolasesArginasePEGylation
Methods and composition for generation of arginase variants with high serum persistence are provided. For example, in certain aspects methods for purifying pegylated arginase are described. Furthermore, the invention provides stabilized arginase multimers or pharmaceutical composition thereof.
Owner:AERASE
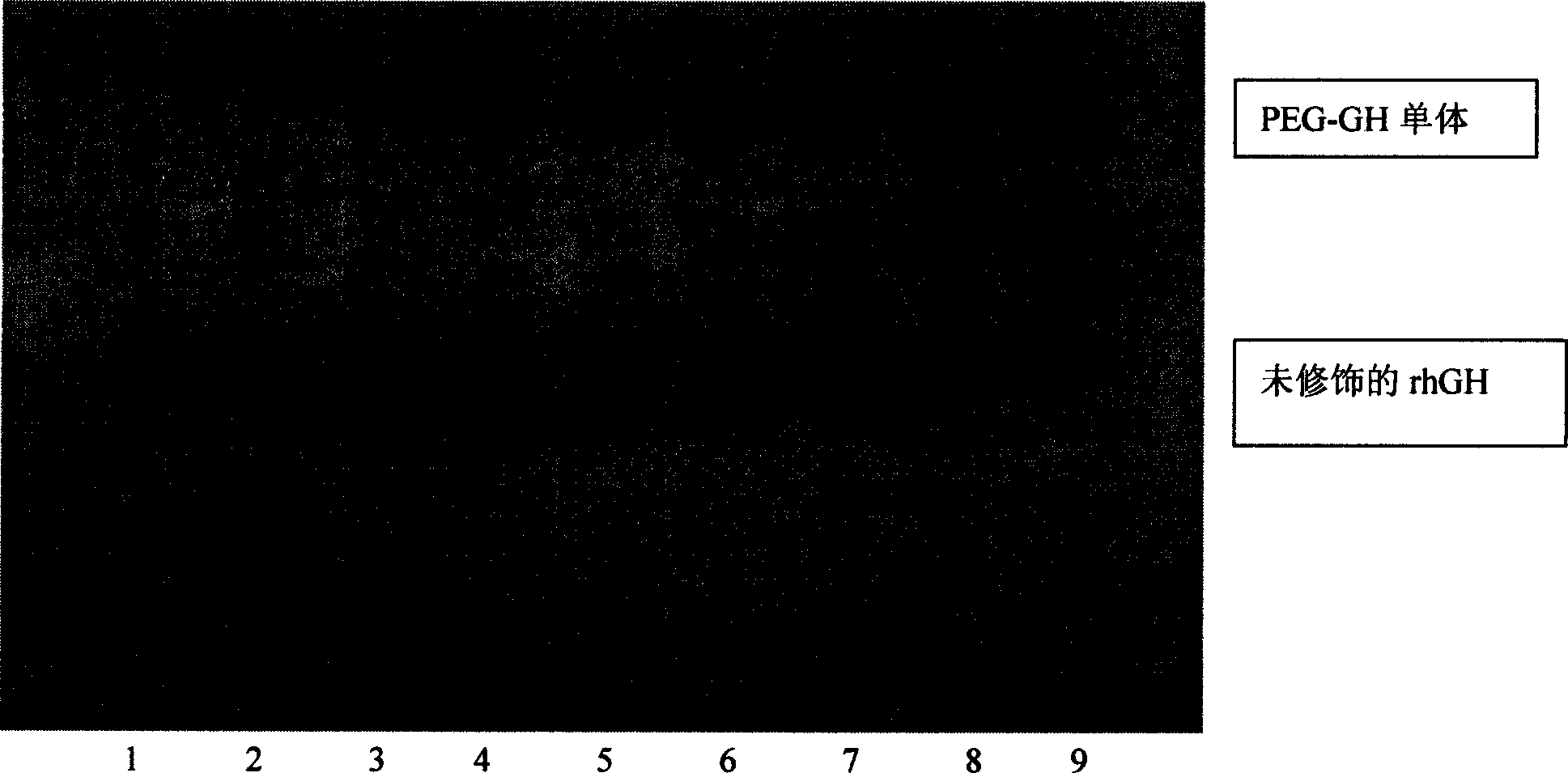
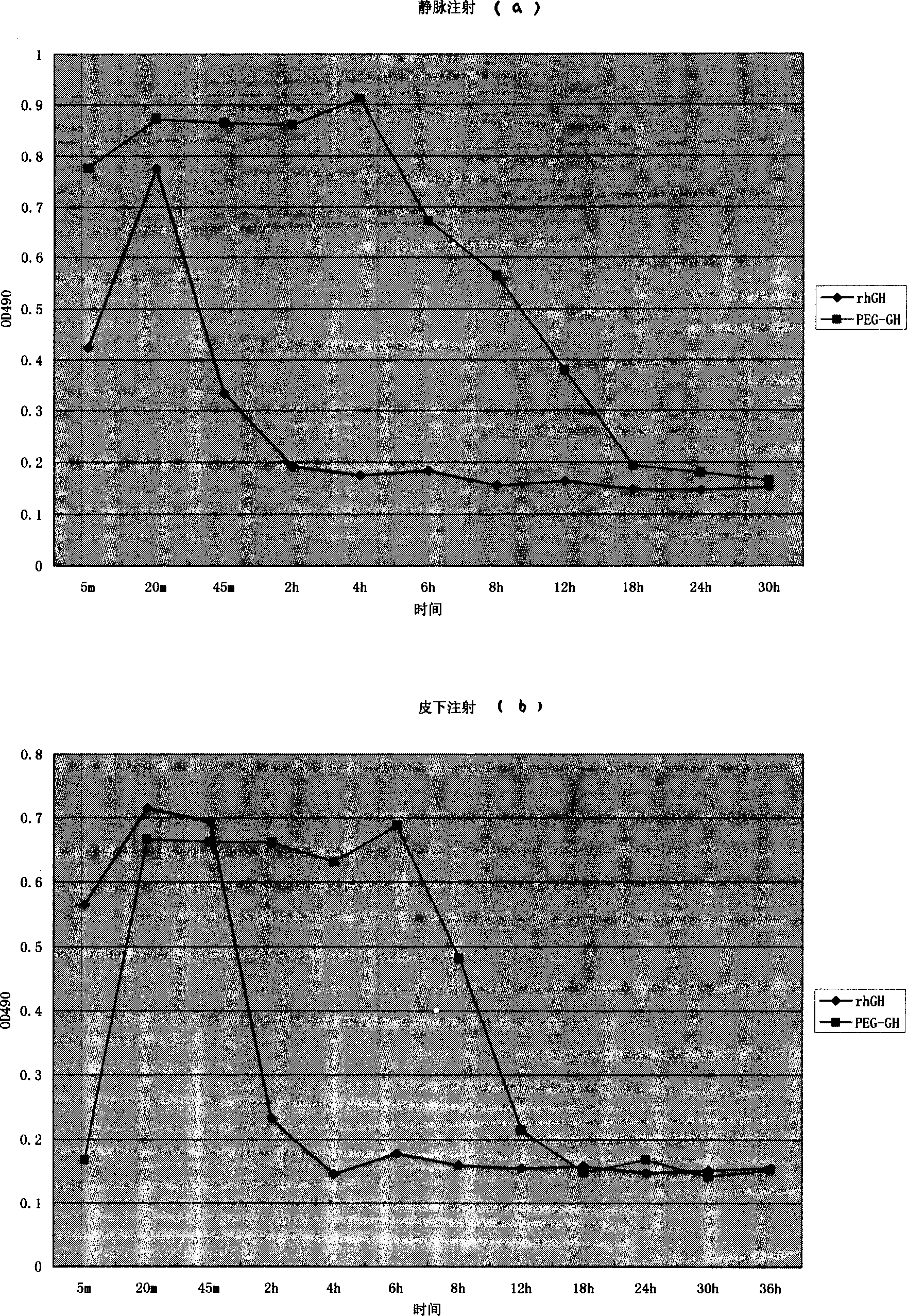
Carbowaxing recombiant human growth hormone medicine and its preparation process
InactiveCN1565624AHighlight substantiveSignificant technological progressPeptide/protein ingredientsGrowth hormonesHuman growth hormoneMedicine
The invention relates to a clinical therapeutic medicine for recombined human growth hormone (rhGH) deficiency disease. Carbowax rhGH medicine is made up of carbowax and rhGH by conjuncting The manufacturing process mainly includes providing a buffer system of PH4.0-9.0, enabling rhGH and carbowax react 2-48 hours in the buffer system, obtaining purification through separating and purifying process, the temperature should be controlled in 4-37 DEG C. The invention has advantages of easy purification, long effect time and be convenient for industrial production.
Owner:ANHUI ANKE BIOTECHNOLOGY (GRP) CO LTD
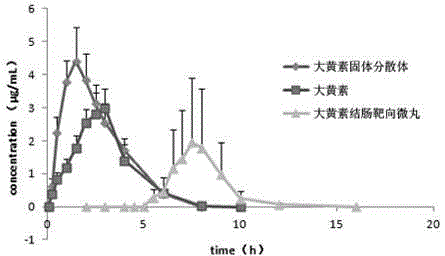
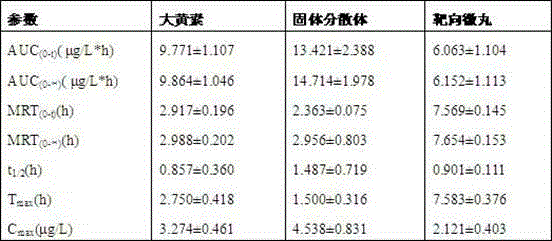
Emodin solid dispersion, drug-containing pellet core, colonic targeted micropill, and applications of three
InactiveCN103550158AImprove solubilityImprove bioavailabilityOrganic active ingredientsPowder deliveryPharmaceutical SubstancesOrganic chemistry
The invention relates to emodin solid dispersion, a drug-containing pellet core, a colonic targeted micropill, and applications of the three. The emodin solid dispersion is prepared from emodin and a carrier material, wherein the carrier material is one or several selected from poloxamer 188, poloxamer 407, Kollidon 12 PF, Kollidon VA 64, Kollicoat IR or Soluplus, and the weight ratio of emodin to the carrier material is 1:2-1:15. The drug-containing pellet core and the colonic targeted micropill both contain the emodin solid dispersion. The emodin solid dispersion is substantially improved in solubility of indissolvable drug emodin; and the targeted micropill helps to realize colonic positioning release in vivo / vitro of emodin, and has substantial protective effect on intestinal barrier of a rat severe acute pancreatitis pancreatitis model.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV +1
Somatostation a aqua prepn and its prepn process and application
ActiveCN100336557CSimple production processHigh activityMetabolism disorderAerosol deliveryPediatricsDisease
The present invention relates to one kind of somatostation aqua preparation and its preparation process and application in preparing medicine for preventing and treating serious acute esophagus varicose hemorrhage, acute gastric and duodenal ulcer hemorrhage, acute erosive gastritis, acute hemorrhagic gastritis, etc. The preparation consists of somatostation in 0.1-1000 mg each 0.1-500 ml, medicinal supplementary material, which may be mannitol, polyglycol, cyclodextrin, etc., and water. The preparation process includes compounding, sterilizing, packing, and other steps. The somatostation aqua preparation has high stability, easy preparation and other features, and has wide application foreground in preparing medicine for preventing and treating serious acute esophagus varicose hemorrhage, acute gastric and duodenal ulcer hemorrhage, acute erosive gastritis, acute hemorrhagic gastritis, etc.
Owner:BEIJING SL PHARMA
Transdermal delivery of cannabinoids
ActiveUS20090291128A1Avoid gastrointestinal (first-pass) metabolismEliminate side effectsBiocideNervous disorderDiseaseSide effect
The present invention overcomes the problems associated with existing drug delivery systems by delivering cannabinoids transdermally. Preferably, the cannabinoids are delivered via an occlusive body (i.e., a patch) to alleviate harmful side effects and avoid gastrointestinal (first-pass) metabolism of the drug by the patient. A first aspect of the invention provides a method for relieving symptoms associated with illness or associated with the treatment of illness in a mammalian subject, comprising the steps of selecting at least one cannabinoid from the group consisting of cannabinol, cannabidiol, nabilone, levonantradol, (−)-HU-210, (+)-HU-210, 11-hydroxy-Δ9-THC, Δ8-THC-11-oic acid, CP 55,940, and R(+)-WIN 55,212-2, selecting at least one permeation enhancer from the group consisting of propylene glycol monolaurate, diethylene glycol monoethyl ether, an oleoyl macrogolglyceride, a caprylocaproyl macrogolglyceride, and an oleyl alcohol, and delivering the selected cannabinoid and permeation enhancer transdermally to treat an illness.
Owner:KENTUCKY ECONOMIC DEV FINANCE AUTHORITY
Methods of treatment with arginine deiminase
ActiveUS20150132278A1Stable diseaseInhibit angiogenesisHeavy metal active ingredientsOrganic active ingredientsPegylated arginine deiminaseCancer
Owner:POLARIS GROUP
Medicine composition and method for treating hepatitis with arginase
InactiveCN1745847ALower arginine levelsPeptide/protein ingredientsDigestive systemPharmaceutical drugHepatitis
Owner:BIO CANCER TREATMENT INT
Nano micro granules, their preparation and medicinal uses of campotothecin derivative
InactiveCN1582934AGood dispersionWater insoluble problem solvedOrganic active ingredientsPowder deliveryNanoparticlePolyethylene glycol
A nanoparticle of camptothecin derivative for preparing the antineoplastic medicines is prepared from gycol-poly-gamme-benzylglutamic acide block copolymer and camptothecin, and is composed of the hydrophobic core and the hydrophilic shell.
Owner:王安训
Chinese medicine drippling pill preparation for promoting blood circulation and removing blood stasis, promoting Qi circulation and rilieving pain
The present invention relates to a kind of Chinese medicine dripping pill preparation, named Xinkeshu dripping pill. The dripping pill preparation is prepared through the following technological steps: a. extracting haw, kudzu vine root and notoginseng with alcohol and filtering to obtain filtrate; b. extracting red sage with alcohol or water and filtering to obtain filtrate; c. merging the filtrate in the foregoing steps, concentration, extracting the concentrate with ethyl acetate with or without alcohol, concentration to obtain dense paste or drying to obtain powder; d. crushing aucklandia root, diacolation with alcohol and concentration to obtain dense paste; mixing the dense paste or powder obtained in the step c., the dense paste obtained in the step d. and polyglycol to obtain medicine liquid, and making dripping pill in a dripping pill machine.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Ginkgo ketone ester dropping pill and its prepn process
InactiveCN1931160AIncrease surface areaImprove absorption rateOrganic active ingredientsPill deliveryMedicineCoronary heart disease
The present invention relates to one kind of ginkgo ketone ester dripping pill and its preparation process, and belongs to the field of Chinese medicine technology. The ginkgo ketone ester dripping pill is prepared mainly with ginkgo ketone ester and medicinal matrix, which is preferably the mixture of polyglycol-4000 and polyglycol-6000. The ginkgo ketone ester dripping pill preparation has ideal treating effect on apoplexy and coronary heart disease caused by blood stasis, and possesses the advantages of quick absorption, high bioavailability, small dosage, several taking modes, low production cost, determined curative effect, etc.
Owner:北京汉典中西药研究开发中心
Composition of liposome, and preparation method
InactiveCN1915222AInhibit transferRegulate immunityOrganic active ingredientsNervous disorderYolkDisease
A liposome composition used to prepare the medicines for treating tumor, cardiovascular and cerebrovascular disease, altitude ischemia and insomnia is prepared from the mixture of aspirin, prostaglandin E1, antioxidant, 2-hydroxypropyl-beta-dextrin and gamma-dextrin, the mixture of hydrogenated soybean lecithin and yolk lecithin, and the mixture of soybean sterol, polyethanediol-2000, VC and glycine. Its preparing process is also disclosed.
Owner:江苏仲德医药科技有限公司
PEG-modified medicinal kininogenase and preparation method and application thereof
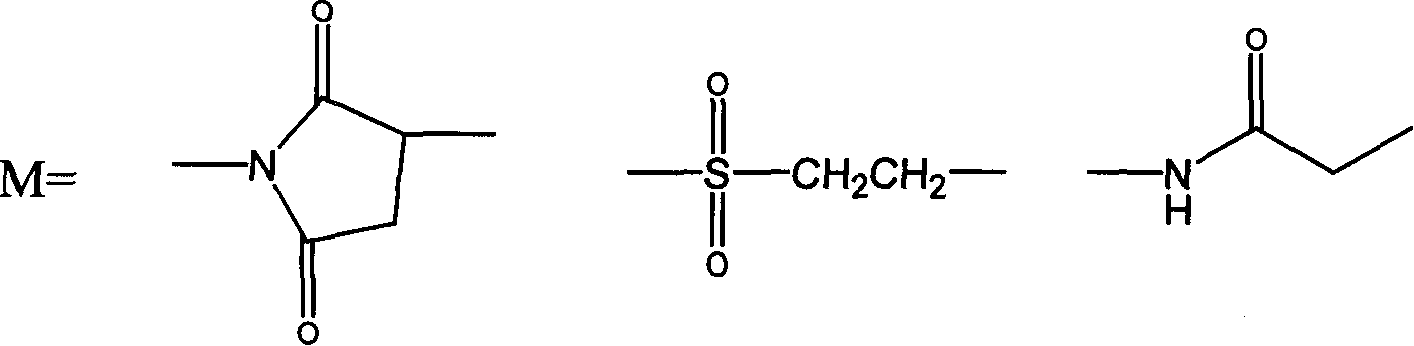
ActiveCN107760661AHigh purityImprove stabilityPeptide/protein ingredientsHydrolasesSide effectHalf-life
The invention relates to PEG-modified medicinal kininogenase and a preparation method and application thereof. The kininogenase does not contain lowly-glycosylated KLK1, and the lowly-glycosylated KLK1 is a stripe with the lowest molecular weight in three stripes during SDS-PAGE protein electrophoresis of porcine pancreas-derived KLK1; PEG adopts a structural general formula shown as a formula (1)or a formula (2). According to the pegylated kininogenase, on one hand, component nonuniformity caused by different glycosylation modifications of the kininogenase is eliminated, so that the purity,the stability, the bioactivity and the medicinal efficacy are improved; on the other hand, after PEG modification of the kininogenase, the half-life period is significantly prolonged, the immunogenicity is significantly reduced, and side effects of a raw medicine are effectively reduced.
Owner:ZONHON BIOPHARMA INST +1
Danofloxacin mesylate microsphere formulation for livestock and poultry and preparation method thereof
ActiveCN101411689ALong elimination half-lifeHigh affinityAntibacterial agentsOrganic active ingredientsCross-linkIntramuscular injection
The invention relates to a danofloxacin mesylate microsphere preparation, which is prepared by the following steps: adding gelatin, or gelatin and polyglycol, or gelatin and polyglycol as well as xanthan gum into water, and adding danofloxacin mesylate for dissolution to obtain a water phase; adding an emulsifying agent into liquid petrolatum to mix evenly to obtain an oil phase; and adding the water phase into the oil phase, emulsifying, cross linking and solidifying the mixed phases and removing supernatant to obtain the danofloxacin mesylate microsphere preparation for livestock and birds. The preparation adopts an emulsifying and condensing method to wrap the danofloxacin mesylate in microspheres, can release slowly after using the microspheres for intramuscular injection, and prolong elimination half-life of medicament in vivo. The preparation technology has the advantages of simple operation, low requirements on preparation equipment and conditions, round shape of the obtained microspheres, and good dispersing property in water solution; and can prolong effective acting time of the medicament in vivo after intramuscular injection for pigs.
Owner:武汉回盛生物科技股份有限公司
Preparation method and an application of multi-vesicle type dihydromyricetin liposome
PendingCN111991353ASolution to low degree of hydrolysisSolve the short biological half-lifeAntibacterial agentsOrganic active ingredientsCholesterolCentrifugation
The invention relates to the technical field of dihydromyricetin, in particular to a preparation method and an application of a multi-vesicle type dihydromyricetin liposome. The preparation method comprises the following steps of (1) collecting vine tea stems and leaves to ampelopsis grossedentata powder, and performing water bath treatment, concentrating, filtering and cooling to dihydromyricetinextracted ingredients; (2) weighing the extracted ingredients, performing dissolving to obtain a dihydromyricetin solution, and detecting purity; (3) preparing an aqueous phase solution from Tween 80and PEG-4000, preparing an oil phase solution from the dihydromyricetin, cholesterol and egg yolk lecithin, and dropwise adding the oil phase solution to the aqueous phase solution, to prepare the multi-vesicle type dihydromyricetin liposome; and (4) performing centrifugation on the taken multi-vesicle type dihydromyricetin liposome solution, taking supernatant, and detecting absorbance. For extraction of the dihydromyricetin, the egg yolk lecithin is used as a liposome formwork, and macrogol 4000 is used as a modification wall material to prepare the multi-vesicle type dihydromyricetin liposome, so that the problems that the degree of hydrolysis of the dihydromyricetin is low, the biological half-life is short, and the film penetrating force is poor, are solved.
Owner:ZHONGKAI UNIV OF AGRI & ENG
Panax notoginseng floral leaf effervescent tablets and method for preparing the same
InactiveCN101095709ANovel dosage formPromote absorptionNervous disorderPill deliverySodium acid carbonateAqueous extract
The invention relates to a kind of medicine that takes plant extract as medical effective component and the method for preparing the same. The comprised components in effervescence and their proportions (by weight)are as follows: pseudo-ginseng flower and leaves and stem extract 10-40%, the left is medical findings; said findings comprises: urinary citric acid 6-20%, sodium acid carbonate 10-30%, macrogol 6000 2-10%, lactose 20-50%, stevia rebaudiava 2-10%, hydroxypropyl starch 5-15%, silicon micronized quartz powder 0.2-1%, and dolomol 0.5-1.5%. The method comprises following steps: disintegrating and screening pseudo-ginseng flower and leaves and stem extract, urinary citric acid, stevia rebaudiava, hydroxypropyl starch, silicon micronized quartz powder with 80 order screen, producing particle with 70-95% ethanol, drying at 60-80 Deg. C, getting particle, adding macrogol 6000 into sodium acid carbonate, stirring evenly, cooling and disintegrating, mixing with said particle and dolomol, sheeting and packing with complex memebrane.
Owner:YUNNAN BAIYAO GROUP
Double-sustained-release drug-loaded hydrogel dressing with semi-interpenetrating network entrapped double-layer microspheres as well as preparation method and application thereof
ActiveCN113648455AGood biocompatibilityHigh mechanical strengthMicrocapsulesBandagesMeth-Antimicrobial drug
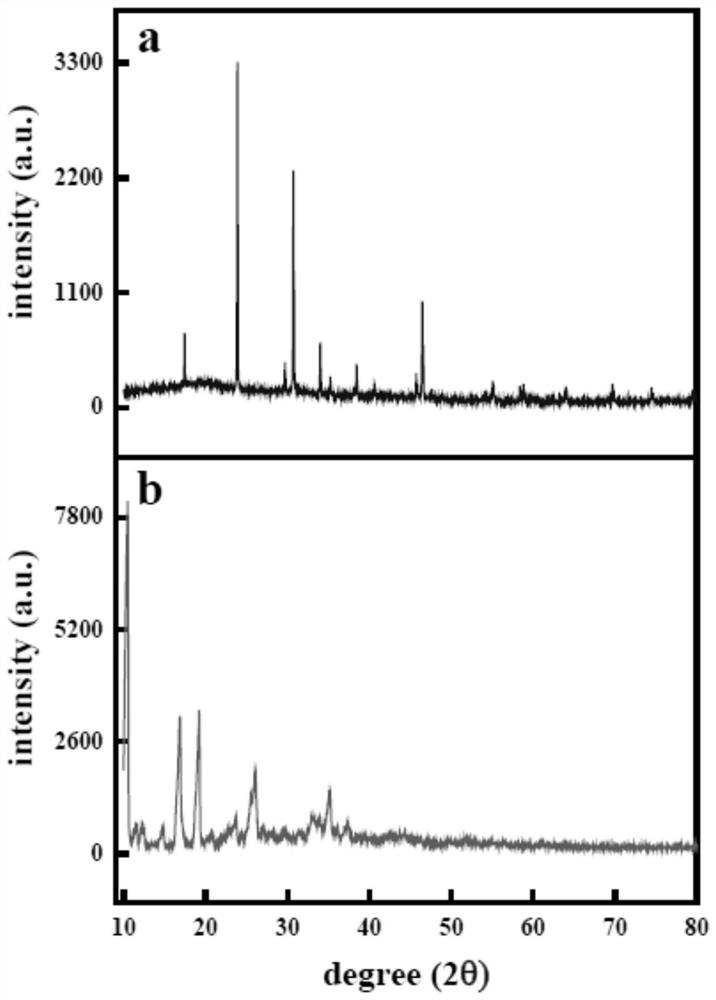
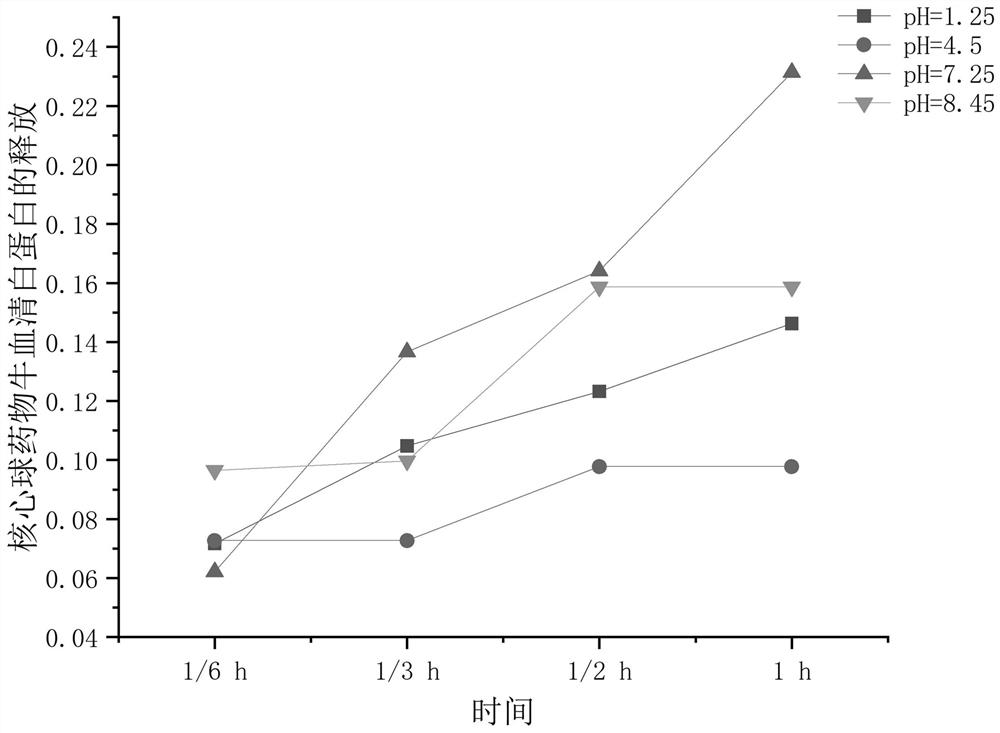
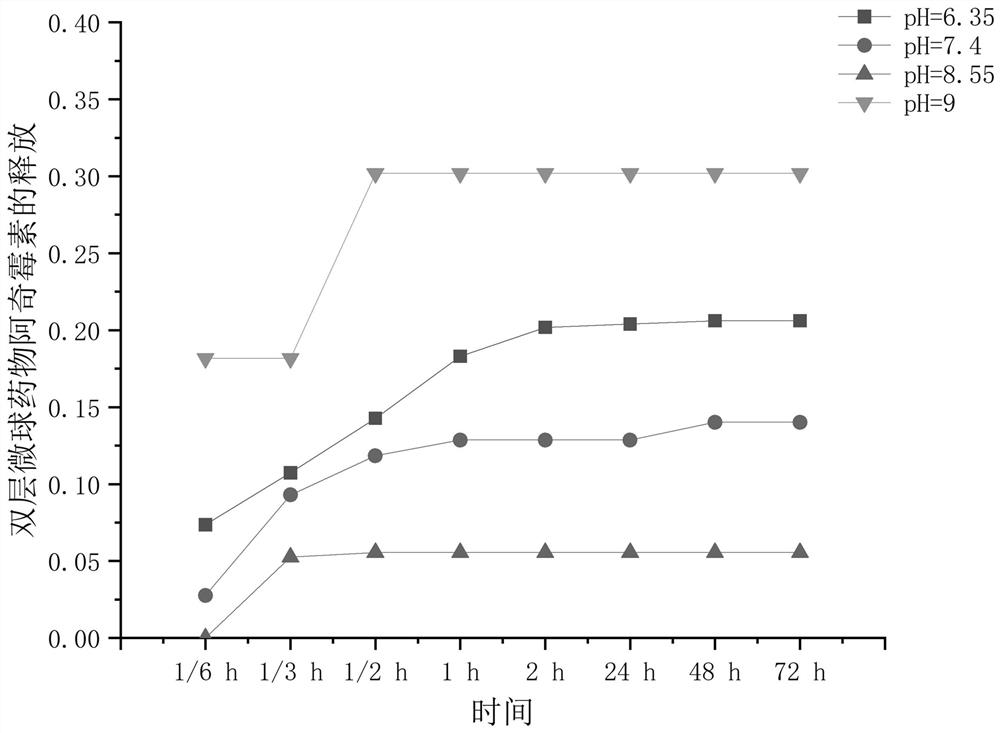
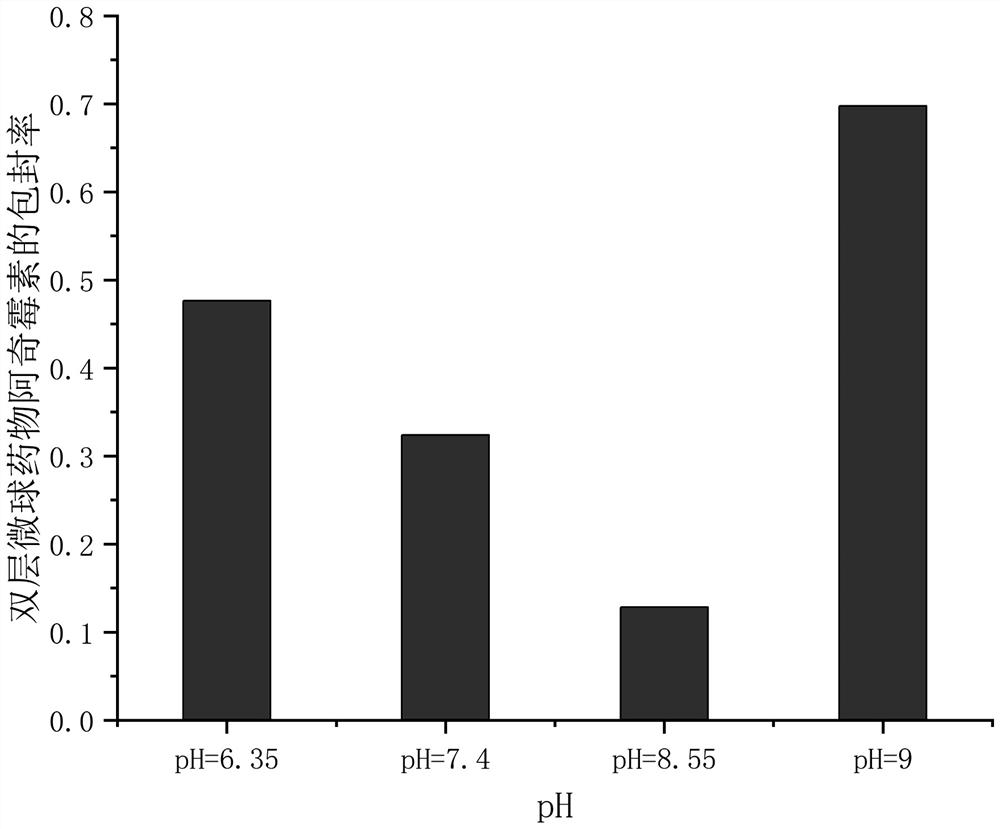
The invention belongs to the technical field of medical dressings for repairing burn skin wounds, and provides a double-sustained-release drug-loaded hydrogel dressing with double-layer microspheres entrapped by a semi-interpenetrating network. Acrylic acid is used as a pH-sensitive monomer. The pH / temperature dual-sensitive intelligent hydrogel takes methacrylic acid 2-ethyl ester and oligomeric (ethylene glycol) methyl ether methacrylate as temperature-sensitive monomers, and an anti-inflammatory drug gentamicin sulfate is entrapped; the preparation method comprises the following steps: preparing a calcium alginate core sphere entrapped with bovine serum albumin through a high-voltage electrostatic instillation method, coating the surface of the core sphere with a chitosan shell layer entrapped with an antibacterial drug azithromycin to form a double-layer microsphere, and loading the double-layer microsphere into the hydrogel to obtain the hydrogel dressing capable of precisely and slowly releasing two drugs and one protein. According to the pH / temperature change of a wound surface microenvironment, the hydrogel can achieve accurate controlled release of drugs and improve the utilization rate of the drugs, and is a novel double-sustained-release drug-loaded hydrogel dressing with a semi-interpenetrating network combined double-layer microsphere structure and good comprehensive performance.
Owner:TAIYUAN UNIV OF TECH
Purified PEG human growth hormone conjugates and preparation thereof
ActiveCN101385858AQuality improvementEasy to usePeptide/protein ingredientsPharmaceutical non-active ingredientsHuman growth hormoneDrugs preparations
The invention relates to a high-purity pegylation human growth hormone (HGH) conjugate which is especially suitable for drug uses and has a rate of purity no less than 96 percent. In addition, the invention also relates to a drug preparation containing the conjugate, as well as a preparation method and drug uses the conjugate.
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com