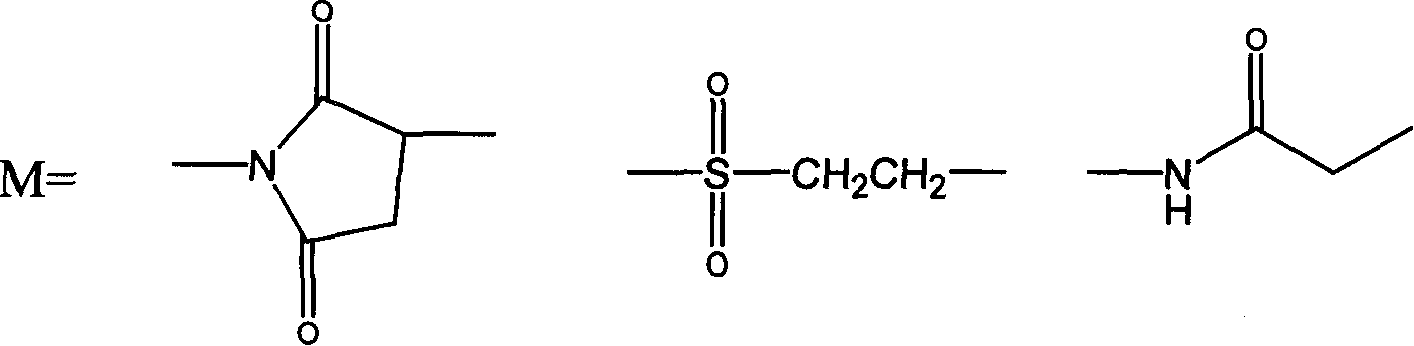
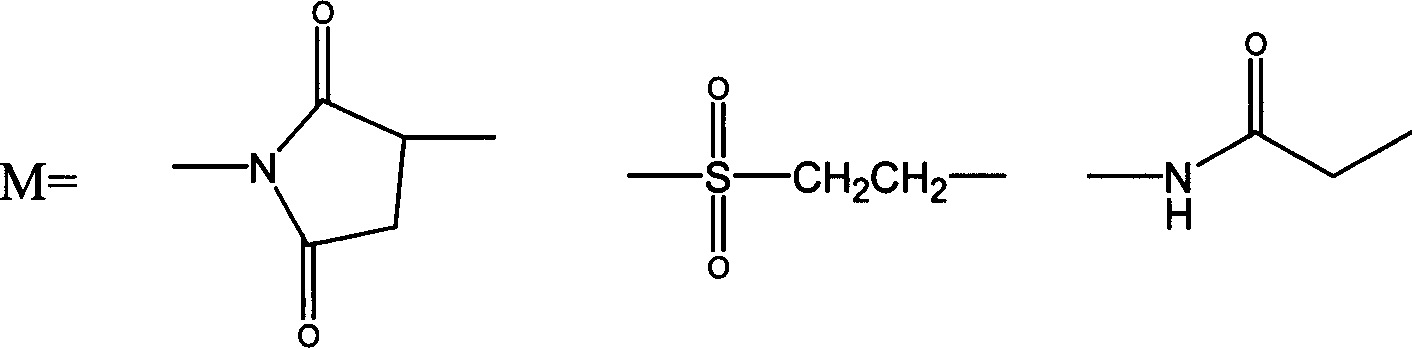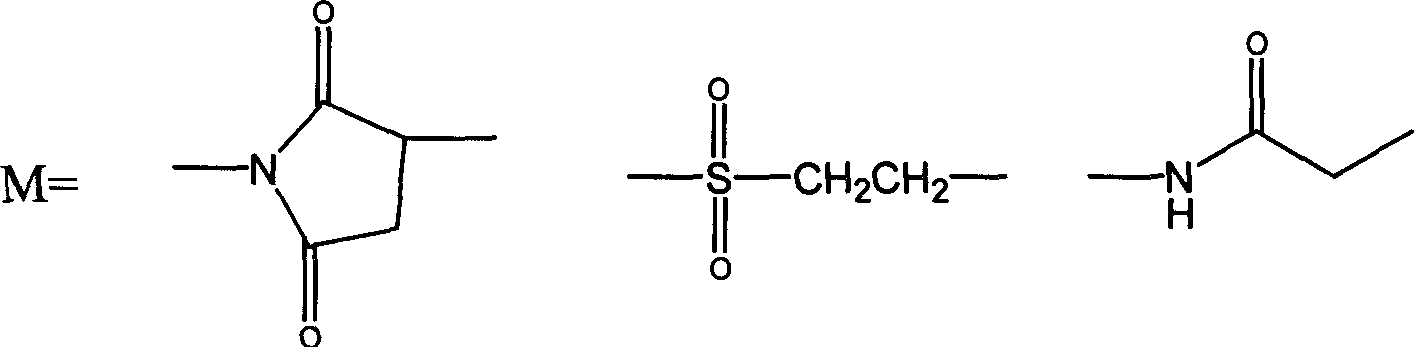Thymosin alpha 1 active segment cyclicpeptide analogue and its poly glycol derivative
A technology of peptide derivatives and compounds, applied in the field of related diseases, can solve the problems of long cycle, large dosage and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0550] wxya 2 CH 2 OCH 2 Synthesis of COOH: BocNHCH 2 OCH 2 COOH
[0551]
[0552] ●BocNHCH 2 CH 2 Synthesis of OH
[0553] Add 30ml of water into the 250ml reactor, NH 2 CH 2 CH 2 OH6.1g (100mmol) was added into the reactor and cooled to 0°C. Under ice bath conditions, (Boc) 2 O2 1.82g (100mmol) was dissolved in 30ml of dioxane and added to the reactor, the temperature was controlled below 0°C, 100ml of 1mol / L NaOH solution was added dropwise, and after 40min, the dropwise addition was completed, and it was naturally raised to room temperature, and the reaction was continued for 12 hours. (Boc) 2 O disappears, the reaction is stopped, and the plate is detected. The reaction solution is extracted three times with ethyl acetate, washed with saturated NaCl solution, and washed with anhydrous NaCl solution. 2 SO 4 After drying and evaporating to dryness under reduced pressure, 15.6 g of a colorless oil was obtained with a yield of 97.2%.
[0554] ●BocNHCH 2...
Embodiment 2
[0557] Cys-Glu-Val-Val-Glu-SCH 2 CH 2 CONH 2 Synthesis:
[0558] 350mg MBHA resin (loading capacity: 0.57mmol / g, 0.2mmol), suspended in DMF, sequentially add 0.12gHOBt, 150μL 3-mercaptopropionic acid (1.2mmol), 160mg DCC, add a small amount of DCM to dissolve fully, and magnetically Stir for 4 hours, wash twice with DMF, DCM, MeOH, and DCM, each for two minutes. Ninhydrin test was negative. Then mix and dissolve DMF with 50mg cysteine methyl ester hydrochloride (0.2mmol), 100mg (0.2mmol) triphenylphosphine and 120 μ IDIEA (0.2mmol), add in the resin after five minutes, react at room temperature for two hours, use DMF, DCM, MeOH, and DCM were washed twice each, mixed with 4 times the amount of BOC-protected amino acids, BOP, and 6 times the amount of DIEA, dissolved in DMF, and the mixture was added to the reactor after five minutes, and reacted at room temperature for 2 hours. Wash with DMF, DCM, MeOH, DCM, and use qualitative Ellman test to detect. Negative solution re...
Embodiment 3
[0559] Embodiment three: the synthesis of ring (CEVVE):
[0560] 350mg MBHA resin (loading capacity: 0.57mmol / g, 0.2mmol), suspended in DMF, sequentially add 0.12gHOBt, 150μL 3-mercaptopropionic acid (1.2mmol), 160mg DCC, add a small amount of DCM to dissolve fully, and magnetically Stir for 4 hours, wash twice with DMF, DCM, MeOH, and DCM, each for two minutes. Ninhydrin test was negative. Then mix and dissolve DMF with 50mg cysteine methyl ester hydrochloride (0.2mmol), 100mg (0.2mmol) triphenylphosphine and 120μl DIEA (0.2mmol), add in the resin after five minutes, react at room temperature for two hours, use DMF, DCM, MeOH, and DCM were washed twice each, mixed with 4 times the amount of BOC-protected amino acids, BOP, and 6 times the amount of DIEA, dissolved in DMF, and the mixture was added to the reactor after five minutes, and reacted at room temperature for 2 hours. Wash with DMF, DCM, MeOH, DCM, and use qualitative Ellman test to detect. Negative solution resin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com